Abstract
Background:
Chemotherapy-induced peripheral neuropathy (CIPN) is a common and chronic complication associated with cancer treatment. Prior investigations have demonstrated the presence of subclinical peripheral neuropathy in patients with colorectal cancer even before the patients had received chemotherapy.
Objective:
To investigate subclinical peripheral neuropathy of the upper limbs in patients with squamous cell carcinoma (SCC) of the head and neck which developed before their exposure to neurotoxic anticancer agents.
Study Design:
Retrospective analysis.
Methods:
With the use of our quantitative sensory testing (QST) data bank, we retrospectively assessed the afferent fiber function of 25 patients with SCC of the head and neck before they had received chemotherapy (the patient group) and compared our findings with those from 23 healthy control patients. Skin temperature, sensorimotor function, sharpness detection, thermal detection, and touch detection (using both von Frey monofilaments and the Bumps detection test) were measured.
Results:
Touch thresholds were statistically higher in the patient group than in the healthy volunteer group at the palm (mean [± SD], 0.54 g [± 0.07 g] and 0.27 g [± 0.05 g], respectively [P < 0.01]) and at the forearm (0.74 g [± 0.12 g] and 0.41 g [± 0.08 g] [P < 0.05]). There was also a clear deficit in touch sensation as indicated by a Bumps detection threshold in patients of 6.5 μm ± 0.8 μm and in controls of 3.7 μm ± 0.5 μm. This yields an elevation in threshold to 165% in the patients relative to that of the control volunteers. The grooved pegboard test showed delayed completion times for patients compared with controls, with differences of 18.65 seconds in the dominant hand and of 23.36 seconds in the nondominant hand. The sharpness detection thresholds did not differ between patients and volunteers.
Limitations:
Inadequacies in the original data acquisition and documentation of the QST and the medical records could not be addressed due to the retrospective nature of the study. In addition, based on available information, we did not find an objective parameter able to correlate the QST findings with pre-pain levels.
Conclusion:
Patients with SCC were found to have deficits in sensory function before undergoing treatment, suggesting that cancer itself alters peripheral nerve function and may contribute to the development of CIPN. These results confirm the sensitivity of the Bumps detection test and highlight its potential role in early detection of peripheral neuropathy, especially in cancer patients for whom chemotherapies associated with CIPN have been prescribed.
Keywords: Peripheral neuropathy, head and neck cancer, quantitative sensory testing
Peripheral nerve dysfunction, most commonly caused by chemotherapy-induced nerve damage, is an important issue for cancer patients. Although well recognized and frequently studied, this phenomenon, known as chemotherapy-induced peripheral neuropathy (CIPN), continues to impede cancer treatment secondary to severe pain and neurologic symptoms (1). Before chemotherapy is administered, it is impossible to predict which patients will develop treatment-limiting CIPN.
To better understand factors that predispose individuals to CIPN, Dougherty et al (2) used quantitative sensory testing (QST) to assess the specific function of the various classes of primary afferents fibers. Von Frey filaments and a newer, more sensitive Bumps detection method were used to measure touch detection via large myelinated A-β fibers (3–6). Sharp pain detection via A-δ fibers was assessed with a sharpness detection threshold technique. Finally, C-fiber function was evaluated by a thermal detection strategy.
Thorough QST has revealed that CIPN occurs in a “stocking and glove” distribution, regardless of the chemotherapeutic agent. Furthermore, 3 distinct regions of symptoms have been described: an area of pain on the glabrous surfaces of the fingertips and toes, a more proximal “border zone” area of sometimes painful numbness in the palms and soles of the feet, and symptom-free skin above the wrists and ankles. Examination over time has shown persistent pain at 1 to 3 years after treatment (4).
Perhaps of more interest, QST evaluations have revealed sensory function deficits in patients with colorectal cancer before they underwent chemotherapy, compared with healthy control patients (7). This surprising finding suggests that cancer itself might contribute to CIPN. Such a subclinical peripheral neuropathy could be explained by systemic effects of tumor-derived factors or by cancer-induced changes in immunologic function, essentially leading to a paraneoplastic peripheral neurologic syndrome (7).
Further work has also identified subclinical peripheral neuropathy before chemotherapy in patients with multiple myeloma (8). These findings lead to the question of whether deficits in peripheral nerve function occur in the presence of other types of cancers as well. To address this question, QST was conducted in groups of patients with head and neck cancers before initiation of chemotherapy.
METHODS
Study Setting and Population
For the past 12 years, an open protocol has collected longitudinal QST data from patients receiving chemotherapy for cancer as well as from healthy volunteers. This database also contains demographic information and data regarding comorbid diseases and current cancer treatment. This protocol was reviewed and approved by the Institutional Review Board (IRB protocol # ANS 00–339) at MD Anderson Cancer Center. Patient participation was voluntary, and informed consent was obtained before patients were enrolled in the study. Patient information was stored in a secure database, and access to the database for secondary projects such as the current report required a separate approval by the IRB, which was obtained.
Selection of Cases
Patients in the QST data bank were identified and invited to patients in the QST assessment after a routine screening process of patients registered for cancer treatment. Patients were being treated in various oncology subspecialties. No cancer, stage, or chemotherapy agent was excluded. Recruitment procedures and QST protocols were described in their entirety to the patients. All patients who were invited to participate had given written informed consent before testing and were free to withdraw from the QST studies at any time. To participate, all patients had to understand the QST study modalities and had to be able to consent to, participate in, and report the testing thresholds. No financial incentive was offered to patients.
Our study population consisted of a subgroup of individuals from the QST data bank with a diagnosis of squamous cell carcinoma (SCC) of the head and neck who had undergone QST assessment at 1 month or more before starting treatment with neurotoxic agents. No patients had clinical evidence of peripheral neuropathy of the upper limbs.
QST
QST testing was performed at the QST laboratory at MD Anderson Cancer Center. All sensory tests were performed at 3 sites, including the tip of the index finger, the thenar eminence, and the volar surface of the forearm. As a general metric of autonomic tone in the extremities, a radiometer to measure skin temperature was placed gently against the skin at each site for approximately 2 seconds. This was followed by the QST battery, which consisted of the following 5 measurements.
Touch Detection Thresholds
Touch detection thresholds were determined with use of von Frey monofilaments in the up/down method (9).The fibers were applied perpendicularly to a skin area for approximately 1 second starting at a bending force of 0.05 g. If the patient failed to detect the stimulus, a monofilament at the next higher force was applied to the same skin location. When the patient was able to detect the stimulus, the next lower monofilament was applied. The filament detected 4 times was assigned as the touch detection threshold.
Bumps Detection
The second method involved detection of small particles (bumps) of varying height on a smooth surface by use of a fingertip. The Bumps device consisted of 3 etched glass plates, each of which contained 12, 1.5- x 1.5-inch squares. Within each square were 5 flat circles, each a different color. Located over one of the circles within each square was a bump that was 550 μm in diameter. Bumps on plate 1 of the 3-plate series varied from 2.5 to 8.0 μm in height; bumps on plate 2 varied from 8.5 to 14.0 μm in height; and bumps on plate 3 ranged from 14.5 to 26.0 μm in height. Patients began each session by being presented with bumps that ranged from 8.5 to 14 μm. Patients were instructed to use the index finger of the dominant hand to explore the circles within each square, beginning with the upper left square and moving from left to right and top to bottom. Bump location was not visible but was reported by the patient based on touch perception only. Correct responses were recorded during testing. Correct identification of bumps on plate 2 led to progression to plate 1 (2.5–8.0 μm). If unable to detect the bumps, patients were presented with plate 3 (14.5–26.0 μm). The Bumps detection threshold was defined as the smallest bump correctly identified in sequence to the next 2 higher bumps (8).
Grooved Pegboard Test
Patients were instructed to fill a 5-by-5 slotted pegboard in an ordered fashion, either across rows or down columns, to assess sensorimotor function and manual dexterity. The time required to complete the task was measured for both the dominant and nondominant hands.
Sharpness Detection Threshold
The sharpness detection threshold was determined with use of a weighted needle device (10). A blunted 30-gauge needle (200-μm diameter) was engaged to a calibrated brass weight fitted into the Luer connection. The assembly was placed inside the cylinder of a 10 mL syringe so that the weighted assembly moved freely within the syringe, from which a needle could emerge from the tip. When the needle was applied to the skin, a consistent force was applied. The forces used were 8, 10, 16, 20, 32, 64, and 128 g. Each stimulus was applied for about 1 second, in order of ascending weight. The patients were instructed to indicate whether the stimulus was perceived as touch, pressure, sharp, or pain. Each trial terminated with the report of sharp or pain sensation. The sharpness detection threshold was defined as the mean calculated from three trials randomly separated by 30 to 90 seconds.
Heat and Cold Detection Thresholds
The threshold for temperature (heat and cold) pain was determined by using a computer-controlled Peltier device in a Marstok method (11–13). A radiometer was used at the outset of testing to ascertain the baseline skin temperature at the body testing sites. The baseline temperature was set at 32°C (89.6°F), and the probe was either gradually cooled at a rate of 0.50°C/s or heated at a rate of 0.3°C/s. Patients were instructed to signal when a change in temperature (cooler or warmer) was first detected and then when the stimulus was perceived as painful. No correction was made for reaction time delay. If a patient failed to perceive heat or cold pain before the cutoff temperature of 51.5°F or 3°C held for 10 seconds, respectively, this temperature was recorded as the default value. The final threshold value was determined by averaging the results of 3 heat/cold ramp trials randomly separated by 30 to 90 seconds.
Statistical Analysis
Continuous variables and categorical variables were summarized by using means ± standard deviations and by medians and counts (percentages), respectively. Patient demographics and QST results were compared between patients with a diagnosis of SCC of the head and neck before they received chemotherapy agents and healthy volunteers, using a 2-sample t-test or Wilcoxon rank-sum test for continuous variables and chi-square test or Fisher exact test for categorical variables. P-values of less than 0.05 indicated statistical significance. SAS version 9.4 (SAS Institute INC, Cary, NC) was used for data analysis.
Results
Patient groups
In a data bank of 209 patients who underwent QST testing, 47 charts were selected. Of these, 22 were healthy individuals used as control patients, and 25 had received a diagnosis of SCC of the head and neck and had QST before initiation of chemotherapy. At the time of testing, neither patients nor healthy controls reported pain, numbness, tingling, or any other neuropathic symptoms. No skin or nail or other trophic abnormalities were reported.
Demographics
Among the 47 patients included in the analysis, the mean age was 60 years, with a range of 42 to 84 years. There were 13 women and 34 men. Most patients (34 of 47) were caucasian. None of the patients had a history of diabetes mellitus, alcoholism, or AIDS that might have contributed to the development of neuropathy. At the QST assessment, no patient had clinical evidence of peripheral neuropathy of the upper limbs.
QST Results
Skin temperatures were, on average, lower in the study patients at both the fingertip and the forearm. At the forearm, the average temperature was 0.94°C cooler (P < 0.05).
Touch Detection Thresholds
This test, a gauge of A-β fiber function, most especially Merkel disk function (14,15), was first performed with use of von Frey filaments, which were found to be elevated at all 3 tested sites in the patient group (Fig. 1). These differences were significant at the palm, where the patient group (mean [± SD]) touch detection threshold was 0.54 g ± 0.07 g, whereas in the healthy volunteer group this threshold was 0.27 g ± 0.05 g (P < 0.01). At the forearm, these values were also significantly higher, with the patient group mean threshold at 0.74 g ± 0.12 g and the healthy volunteer group mean threshold at 0.41 g ± 0.08 g (P < 0.05) (Fig. 1).
Fig. 1.
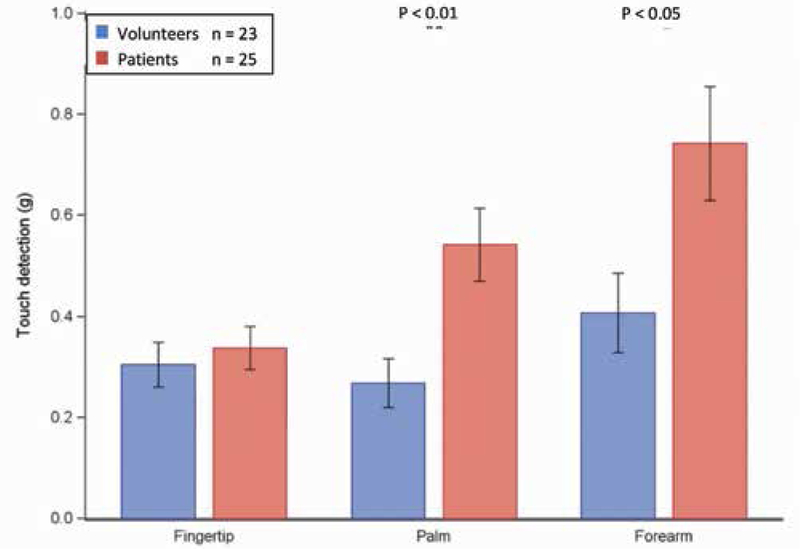
Touch detection thresholds. A Wilcoxon Rank Sum test to compare measurements between 2 groups of patients. Bar graphs show the means ± standard error (black line) of the touch detection thresholds (bending force in grams) at the fingertip, palm, and forearm in the healthy volunteer group (n = 23 in blue) and the patient group (n = 25 in red). The skin test sites show a statistically significant difference between the groups that is more pronounced at the palm and forearm (P < 0.05).
Bumps Detection Test
Similar to the touch detection threshold, the Bumps detection test was used to measure A-β fiber function (16) (Fig. 2). There were significant differences in mean (± SD) in Bumps detection thresholds between patients and healthy volunteers. In the patient group, this threshold was 6.5 μm ± 0.8 μm, whereas in control volunteers it was 3.7 μm ± 0.5 μm. This yields an elevation in threshold to 165% in the patients relative to that of the volunteers (Fig. 2).
Fig. 2.
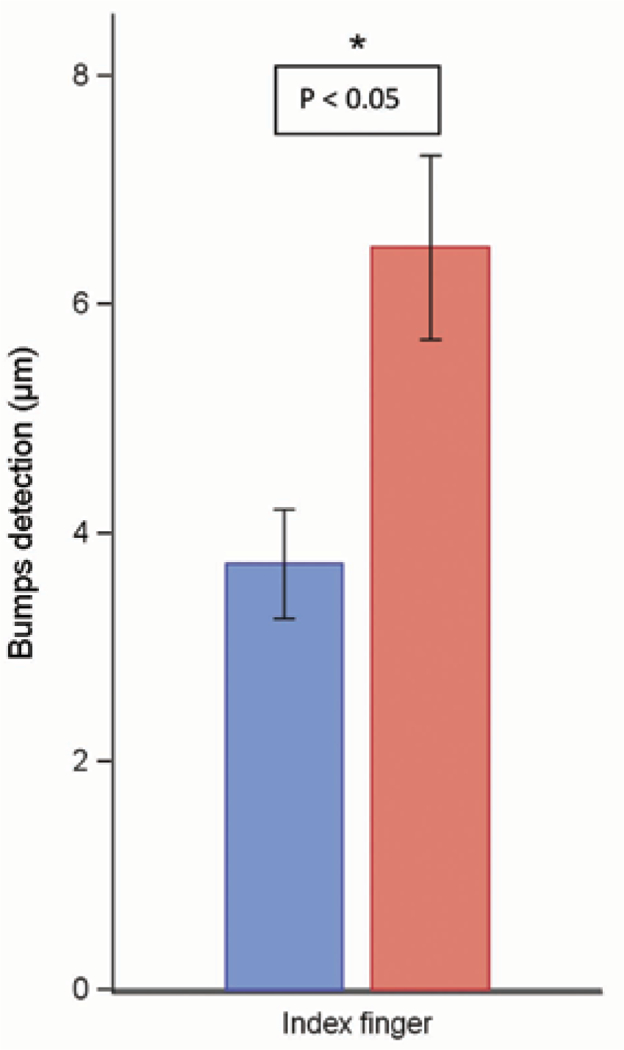
Bumps detection test. A Wilcoxon Rank Sum test was used to compare measurements between 2 groups of patients. Bar graphs show the means ± standard error (black line) of the Bumps detection test (μm in diameter) at the index finger in the healthy volunteer group (n = 23 in blue) and the patient group (n = 25 in red). The skin test site shows a statistically significant difference between the groups (P < 0.05).
Grooved Pegboard Test
This sensorimotor task, like the von Frey and Bumps detection, depends on large afferent fibers (17). As shown in Fig. 3, the patients had delayed completion times, both for the dominant (P < 0.05) and the nondominant hands (P < 0.05) when compared with completion times for healthy volunteer controls. The time differences between patients and controls was 18.65 seconds in the dominant hand and 23.36 seconds in the nondominant hand (Fig. 3).
Fig. 3.
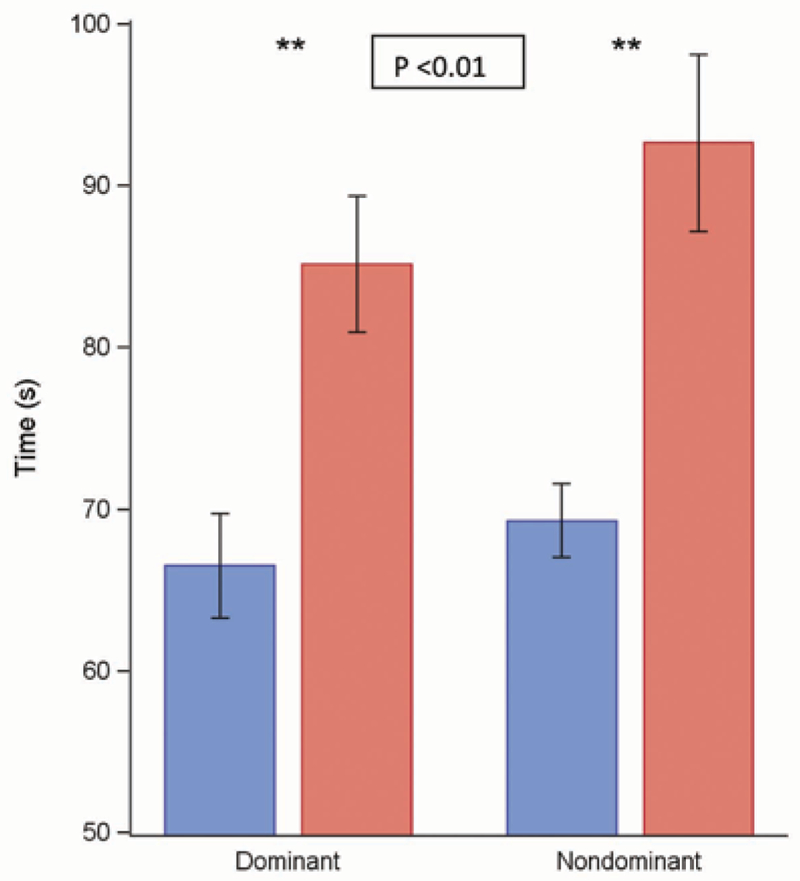
Grooved pegboard test. A Wilcoxon Rank Sum test was used to compare measurements between 2 groups of patients. Bar graphs show the means ± standard error (black line) of the grooved pegboard test (in seconds) at the dominant and nondominant hand in the healthy volunteer group (n = 23 in blue) and the patient group (n = 25 in red). The skin test sites show a statistically significant difference control between the groups (P < 0.05).
Sharpness Detection
Sharpness detection thresholds, largely mediated by A-δ fiber function, did not differ between patients and volunteers. Specifically, threshold values in the volunteers were 38.54 g at the fingertip, 30.68 g at the palm, and 18.92 g at the forearm, whereas these values in the patient group were 40.89 g, 37.36 g, and 28.81 g, respectively.
Heat and Cold Detection Thresholds
The cool detection threshold and cold-induced pain are both functions of the A-δ fiber. The warmth detection threshold and heat-induced pain are both functions of the C fiber (10). Patients showed numerous deficits in mean (± SD) ability to detect warmth, but they showed decreased sensitivity to cool and cold pain (Fig. 4). The ability to detect skin warming was impaired at the palm, with 38.3°C ± 4.9°C in the patient group and 36.3°C ± 1.33°C in the healthy volunteers (P < 0.05), and at the forearm, with 37.7°C ± 2.08°C in the patient group and 36.5°C ± 1.96°C in healthy volunteers (P < 0.05) and trended toward impairment at the fingertip. The heat pain threshold was not significantly different at any site but trended toward elevated in the patients. The skin cold pain thresholds did not differ between patients and volunteers, but of interest, detection of skin cooling was impaired at the palm (23.3°C ± 2.71°C in the patient group and 25.2°C ± 3.21°C in the healthy volunteer group) (P < 0.05) and at the forearm (23°C ± 2.64°C and 25.3°C ± 3.46°C, respectively) (P < 0.05) (Fig. 4).
Fig. 4.
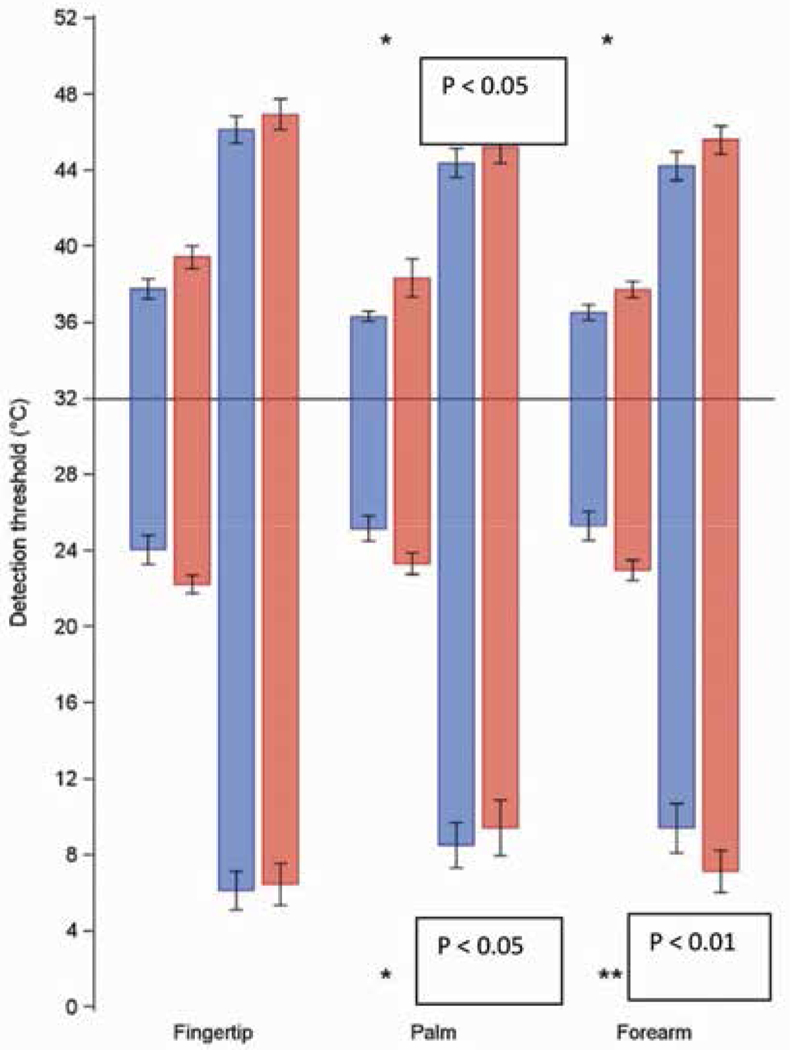
Heat and cold detection thresholds. A Wilcoxon Rank Sum test was used to compare measurements between 2 groups of patients. Bar graphs show the means ± standard error (black line) of the warmth detection and heat pain thresholds in degrees Celsius (short and long bars above the horizontal axis, respectively) as well as the cool detection and cold pain thresholds in degrees celsius (short and long bars below the horizontal axis, respectively) in the healthy volunteer group (n = 23 in blue) and the patient group (n = 25 in red). The ability to detect skin warming was impaired at the palm and forearm compared with healthy volunteers (P < 0.05) and trended toward impairment at the fingertip. The heat pain thresholds were not significantly different at any site. The cold pain thresholds did not significantly differ between patients and volunteers, but of interest, detection of skin cooling was impaired at the palm and the forearm, (P <0.05).
DISCUSSION
The main finding in this study was that subclinical deficits in sensory detection are a common occurrence in patients with head and neck cancer before they undergo chemotherapy. These deficits were most prominent in low-threshold mechanical stimuli shown by the touch detection threshold (von Frey filament) and Bumps detection test. Sensorimotor (grooved pegboard test) and thermal stimuli deficits were also unrecognized by the patients. The pattern of subclinical sensory deficits was most notable distally and less pronounced proximally, which parallels that in patients with multiple myeloma and colorectal cancer.
In light of prior studies demonstrating subclinical neuropathies in patients with multiple myeloma and colorectal cancer, our findings further suggest that cancer itself can alter peripheral nerve function. Although treatment-related neuropathy symptoms also develop in patients without obvious baseline neuropathy, increasing evidence suggests that patients with pretreatment neuropathy are at increased risk of developing CIPN (17–20). Previous nerve conduction (20) and QST (7,21) studies have shown subclinical sensory deficits in a surprisingly large percentage of patients with colorectal cancer or multiple myeloma before any therapy. It is possible that these subclinical deficits may contribute to the development of CIPN after initiation of chemotherapy. Patients with preexisting neuropathy may develop more severe sensory impairment during active chemotherapy, resulting in more severe CIPN. Vichaya et al (22) showed that subclinical pretreatment sensory deficits appear to predict the development of pain and numbness in patients with multiple myeloma undergoing chemotherapy. During the maintenance phase, patients with warmth-detection deficits (n = 5, 38% of the sample) reported more severe pain and numbness, and those with skin temperature deficits (n = 7, 47% of maintenance sample) reported more severe pain (P < 0.05). The warmth detection (OR: 4.9, P = 0.036) and skin temperature (OR: 5.0, P = 0.031) deficits were independent prognostic factors for the development of painful sensory disturbances. In addition, sharpness detection (OR: 0.1, P = 0.032) and warmth detection (OR: 4.8, P = 0.021) deficits were independent prognostic factors for the development of no painful sensory disturbances.
The mechanism behind tumor-induced subclinical neuropathy is unclear, but Kosturakis et al (8) showed that in patients with multiple myeloma with sensory and sensorimotor deficits before undergoing treatment, the subclinical neuropathy seemed to be due to tumor-related decreases in peripheral innervation density. Patients showed that the decreased density of Meissner Corpuscles (MC) was correlated with their ability to detect small bumps in the Bumps detection test. These data suggest that a decrease in tactile sensitivity is well correlated with MC density as visualized by in vivo confocal imaging and is consistent with studies comparing Bumps threshold with MC density using quantified skin biopsy (9).
Previous observations in multiple myeloma were not without precedent since this tumor is associated with paraneoplastic syndrome and invades nerves; thus, neuropathy is often a presenting or co-occurring sign. Colorectal cancer is an adenocarcinoma not associated with a paraneoplastic syndrome or particularly with the invasion of nerves. Head and neck cancer is often an SCC and has a predilection to run along nerves, but here the deficit is distal. This increases the likelihood of an immunologic basis of the neuropathy.
The clinical suggestions of this study are limited by its retrospective nature. It takes into account missing or incomplete data that is contingent on the quality and accuracy of provider documentation in the medical records and the QST data collected. In addition, the uniformity of the findings described suggest a pattern that might differ in magnitude alone. Furthermore, the basis for the etiology of this subclinical neuropathy remains unknown, and therefore the association between our findings and the development of symptoms might still be circumstantial. Finally, we did not find an objective clinical parameter able to correlate the QST findings.
Long-term studies are needed to follow outcomes in patients to determine whether the degree of baseline sensory impairment correlates with severity of CIPN. However, our work does suggest that a simple method of screening patients for subclinical neuropathy with administration of the Bumps detection test would easily identify patients who are at potentially heightened risk. This information could be used by oncologists to increase vigilance in screening for neuropathic symptoms in those patients or even to preemptively dose-adjust certain high-risk chemotherapeutics. Furthermore, patients identified with subclinical neuropathy before treatment could be referred to pain management specialists and considered for prophylactic initiation of pharmacotherapy aimed at preventing the development of nerve-related pain. These strategies could potentially decrease the rates of treatment delays secondary to CIPN.
CONCLUSION
Our analysis found that patients with head and neck cancers are affected by a mild peripheral neuropathy even before initiation of chemotherapy. These findings have identified a third type of cancer to be associated with subclinical neuropathy and suggest that the malignancy itself alters peripheral nerve function. Patients may benefit from testing of peripheral nerve function before initiation of chemotherapy in the hopes of preventing or modifying the development of CIPN.
Acknowledgments
Disclaimer: The study was funded by NIH grant ANS 00–339 (PMD). The statistical analysis work was supported in part by the NIH/NCI Cancer Center Support Grant (NCI Grant P30CA016672). Additional personnel funds were provided by The University of Texas MD Anderson Cancer Center Pain Medicine Research Fellowship.
References
- 1.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014; 155:2461–2470. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty PM, Cata JP, Burton AW, Vu K, Weng HR. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Pain Symptom Manage 2007; 33:166–179. [DOI] [PubMed] [Google Scholar]
- 3.Boyette-Davis JA, Cata JP, Zhang H, Driver LC, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients. J Pain 2011; 12:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyette-Davis JA, Cata JP, Driver LC, Novy DM, Bruel BM, Mooring DL, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Persistent chemoneuropathy in patients receiving the plant alkaloids paclitaxel and vincristine. Cancer Chemother Pharmacol 2013; 71:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroder S, Beckmann K, Franconi G, Meyer-Hamme G, Friedemann T, Greten HJ, Rostock M, Efferth T. Can medical herbs stimulate regeneration or neuroprotection and treat neuropathic pain in chemotherapy-induced peripheral neuropathy? Evid Based Complement Alternat Med 2013; 2013:423713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder A, Stengel M, Maag R, Wasner G, Schoch R, Moosig F, Schommer B, Baron R. Pain in oxaliplatin-induced neuropathy-sensitization in the peripheral and central nociceptive system. Eur J Cancer 2007; 43:2658–2663. [DOI] [PubMed] [Google Scholar]
- 7.Boyette-Davis JA, Eng C, Wang XS, Clee- land CS, Wendelschafer-Crabb G, Kennedy WR, Simone DA, Zhang H, Dougherty PM. Subclinical peripheral neuropathy is a common finding in colorectal cancer patients prior to chemotherapy. Clin Cancer Res 2012; 18:3180–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosturakis AK, He Z, Li Y, Boyette-Davis JA, Shah N, Thomas SK, Zhang H, Vichaya EG, Wang XS, Wendelschafer-Crabb G, Kennedy WR, Simone DA, Cleeland CS, Dougherty PM. Subclinical peripheral neuropathy in patients with multiple myeloma before chemotherapy is correlated with decreased fingertip innervation density. J Clin Oncol 2014; 32:3156–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy WR, Selim MM, Brink TS, Hodges JS, Wendelschafer-Crabb G, Foster SX, Nolano M, Provitera V, Simone DA. A new device to quantify tactile sensation in neuropathy. Neurology 2011; 76:1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelissen L, Donado C, Kim J, Chiel L, Zurakowski D, Logan DE, Meier P, Sethna NF, Blankenburg M, Zernikow B, Sundel RP, Berde CB. Pain hypersensitivity in juvenile idiopathic arthritis: A quantitative sensory testing study. Pediatr Rheumatol Online J 2014; 12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer S, Zims R, Simang M, Rüger L, Irnich D. Hypnotic relaxation results in elevated thresholds of sensory detection but not of pain detection. BMC Complement Altern Med 2014; 14:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knutti IA, Suter MR, Opsommer E. Testretest reliability of thermal quantitative sensory testing on two sites within the L5 dermatome of the lumbar spine and lower extremity. Neurosci Lett 2014; 579:157–162. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz U, Ciol MA, Berger RE, Yang CC. Sensory perception thresholds in men with chronic pelvic pain syndrome. Urology 2010; 75:34–37. [DOI] [PubMed] [Google Scholar]
- 14.Koga K, Furue H, Rashid H, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain 2005; 1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hübscher M, Moloney N, Leaver A, Rebbeck T, McAuley JH, Refshauge KM. Relationship between quantitative sensory testing and pain or disability in people with spinal pain-a systematic review and meta-analysis. Pain 2013; 154:1497–1504. [DOI] [PubMed] [Google Scholar]
- 16.Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia AS, McNamara JO. Neuroscience, 5th edition Sunderland, MA, Sinauer Associates, Inc, 2012: pp. 345–347–17. [Google Scholar]
- 17.Bruna J, Ale A, Velasco R, Jaramillo J, Navarro X, Udina E. Evaluation of preexisting neuropathy and bortezomib retreatment as risk factors to develop severe neuropathy in a mouse model. J Peripher Nerv Syst 2011; 16:199–212. [DOI] [PubMed] [Google Scholar]
- 18.Cavaletti G, Bogliun G, Marzorati L, Zincone A, Piatti M, Colombo N, Franchi D, La Presa MT, Lissoni A, Buda A, Fei F, Cundari S, Zanna C. Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann Oncol 2004; 15:1439–1442. [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos MA, Mateos MV, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, Kropff M, Spicka I, Palumbo A, Wu KL, Esseltine DL, Liu K, Deraedt W, Cakana A, Van De Velde H, San Miguel JF. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: Subanalysis of the phase 3 VISTA study. Eur J Haematol 2011; 86:23–31. [DOI] [PubMed] [Google Scholar]
- 20.Lanzani F, Mattavelli L, Frigeni B, Rossini F, Cammarota S, Petro D, Jann S, Cavaletti G. Role of a pre-existing neuropathy on the course of bortezomib-induced peripheral neurotoxicity. J Peripher Nerv Syst 2008; 13:267–274. [DOI] [PubMed] [Google Scholar]
- 21.Kosturakis AK, He Z, Li Y, Boyette-Da- vis JA, Shah N, Thomas SK, Zhang H, Vichaya EG, Wang XS, Wendelschafer-Crabb G, Kennedy WR, Simone DA, Cleeland CS, Dougherty PM. Subclinical peripheral neuropathy in patients with multiple myeloma before chemotherapy is correlated with decrease in fingertip innervation density. J Clin Oncol 2014; 32:3156–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vichaya EG, Wang XS, Boyette-Davis JA, Mendoza TR, He Z, Thomas SK, Shah N, Williams LA, Cleeland CS, Dougherty PM. Subclinical pretreatment sensory deficits appear to predict the development of pain and numbness in patients with multiple myeloma undergoing chemotherapy. Cancer Chemother Pharmacol 2013; 71: 1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]


