Abstract
The ever-evolving market of electronic cigarettes (e-cigarettes) presents a challenge for analyzing and characterizing the harmful products they can produce. Earlier we reported that e-cigarette aerosols can deliver high levels of reactive free radicals; however, there are few data characterizing the production of these potentially harmful oxidants. Thus, we have performed a detailed analysis of the different parameters affecting the production of free radical by e-cigarettes. Using a temperature-controlled e-cigarette device and a novel mechanism for reliably simulating e-cigarette usage conditions, including coil activation and puff flow, we analyzed the effects of temperature, wattage, and e-liquid solvent composition of propylene glycol (PG) and glycerol (GLY) on radical production. Free radicals in e-cigarette aerosols were spin-trapped and analyzed using electron paramagnetic resonance. Free radical production increased in a temperature-dependent manner, showing a nearly 2-fold increase between 100 and 300 °C under constant-temperature conditions. Free radical production under constant wattage showed an even greater increase when going from 10 to 50 W due, in part, to higher coil temperatures compared to constant-temperature conditions. The e-liquid PG content also heavily influenced free radical production, showing a nearly 3-fold increase upon comparison of ratios of 0:100 (PG:GLY) and 100:0 (PG:GLY). Increases in PG content were also associated with increases in aerosol-induced oxidation of biologically relevant lipids. These results demonstrate that the production of reactive free radicals in e-cigarette aerosols is highly solvent dependent and increases with an increase in temperature. Radical production was somewhat dependent on aerosol production at higher temperatures; however, disproportionately high levels of free radicals were observed at ≥100 °C despite limited aerosol production. Overall, these findings suggest that e-cigarettes can be designed to minimize exposure to these potentially harmful products.
Graphical Abstract
INTRODUCTION
Nearly 20% of deaths in the United States can be attributed to tobacco smoking. The 2010 Surgeon General’s Report identified oxidative stress due to free radical and oxidant generation in tobacco smoke as playing a critical role in the development of smoking-related diseases.1 High concentrations of reactive oxygen species (ROS) and reactive nitrogen species (RNS) are found in cigarette smoke (>1016 molecules/puff).2–4 Oxidative stress due to free radicals in cigarette smoke can damage numerous cellular macromolecules and impact important pathways involved in inflammation, metabolism, and cell survival and proliferation. This resulting oxidative damage is thought to play a key role in the development of many tobacco-related diseases such as chronic obstructive pulmonary disease (COPD), cardiovascular disease, and cancer.5–8 While knowledge of free radicals formed in cigarette smoke is fairly well established, the factors underlying the generation of free radicals in e-cigarette aerosols have only recently been described. In previous studies performed by our laboratory, we have observed high levels of reactive free radicals in e-cigarette aerosols by electron paramagnetic resonance (EPR).9 Though this has set the groundwork for free radicals found in e-cigarette aerosols, a full understanding of the different factors affecting radical generation is still needed.
E-Cigarettes, e-pipes, and other electronic nicotine delivery systems are battery-powered devices that deliver nicotine without the burning of tobacco. These devices consist mainly of a battery, a heating element, and a nicotine or nicotine-free solution (e-liquid) that generates an aerosol when the e-liquid is heated. This new market is evolving rapidly with a multitude of different heating elements, heating profiles, and e-liquids.10–14 Most recently, temperature control devices have emerged, which purportedly provide the user with a better taste, a decrease in the levels of hazardous substances, and improved battery life.15
All e-liquids utilize solvents such as propylene glycol (PG) and glycerol (GLY) in addition to optional nicotine and flavorings. PG and GLY are both “generally recognized as safe” when consumed orally by the U.S. Food and Drug Administration (21CFR 182.1320); however, the thermal breakdown of these and other e-liquid components in e-cigarette aerosols has not yet been fully evaluated, particularly in a toxicological context. Recently, a number of studies have found a variety of different toxic agents in e-cigarette aerosols, including diethylene glycol, numerous reactive organic compounds, nitrosamines, and heavy metals.16–21
In this study, we systematically evaluated the effects of different solvents, wattages, and temperatures on free radical production in e-cigarette aerosols under laboratory conditions. To evaluate these factors, we utilized a newer generation of e-cigarette device that has a temperature control mode. The use of this device also serves a secondary purpose of evaluating the effects of constant-temperature and constant-wattage modes on the coil temperature on free radical generation in the e-cigarette aerosols.
MATERIALS AND METHODS
Temperature Control Devices.
Temperature control devices utilize the thermal coefficient of resistivity of the coil material (α), the initial temperature (TA), the initial coil resistance at TA (ρA), and the coil resistance as current is flowing (ρB) to indirectly measure the temperature of the coil (TB).22 Equation 1 shows how by monitoring resistance, one can show temperature control e-cigarettes can achieve a target temperature (TB).
| (1) |
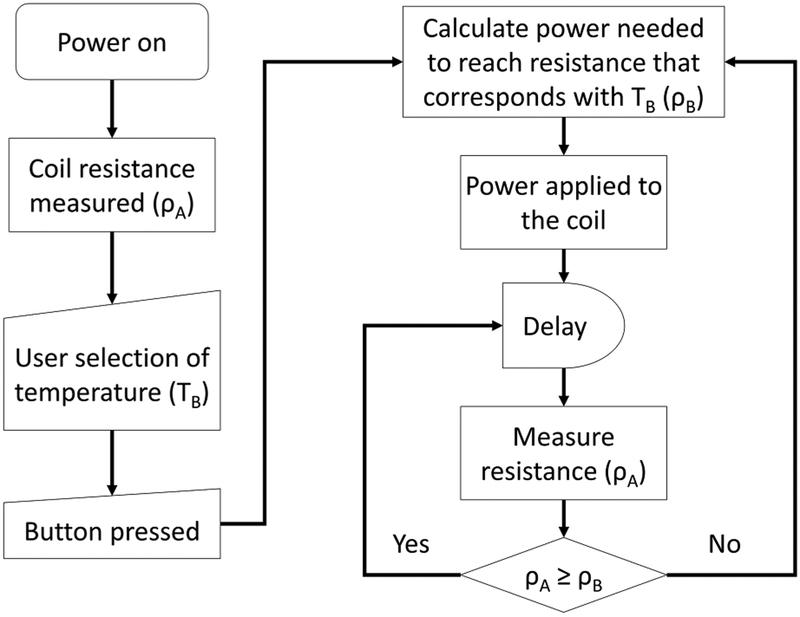
The thermal coefficient of resistivity constant is dependent on the material from which the coil is made. For example, pure nickel coils (Ni200) have a temperature coefficient of 0.006 °C−1, meaning that the coil resistance will increase by 0.006 Ω for every 1 °C increase in temperature.22 Newer temperature control mode e-cigarettes have these coefficients preprogrammed into the device so the user simply selects the type of coil they are using. This allows the device the ability to indirectly monitor the temperature by monitoring the change in resistance. These devices typically have a temperature sensor built into the circuitry to provide a reference temperature for the coil’s resistance when it is first installed. To achieve the target temperature, when the button is pressed, the device will measure the starting resistance of the coil and then calculate and apply the voltage needed to increase the resistance of the coil that corresponds to the target temperature. After a time interval set by the device’s manufacturer, if the coil resistance or temperature is lower than the set point, the device will then measure the new resistance of the coil and calculate and apply a new voltage to achieve the target temperature. If the temperature is too high, the device will not apply voltage to the coil until the next time point. The flow diagram in Figure 1 outlines this process.
Figure 1.
Logic flow diagram for a temperature control e-cigarette.
E-Cigarette, Coil, and Atomizer Tank.
The type of e-cigarette used for this study was a Wismec Reuleaux RX200S Mod (MyVaporStore.com), which has both constant-wattage and -temperature control modes. This specific device can generate consistent temperatures of 100–315 °C at 1–200 W. This device uses three high-amperage 18650 type batteries; for our experiments, we used Samsung INR18650–25R, 2500 mAh, 3.7 V batteries. Batteries were recharged after 250 puffs were taken from the device. The heating element was a commercially available 0.15 Ω nickel alloy coil (Ni200) Uwell Crown Coil (MyVaporStore.com) with an organic cotton wick. A 4 mL stainless steel and glass atomizer tank was used for all experiments (Uwell Crown Tank, MyVaporStore.com).
Reagents.
Propylene glycol (PG), glycerol (GLY), phenyl-N-tert-butylnitrone (PBN), 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO), hexane, tert-benzene, arachidonic acid (AA), cis-4,7,10,13,16,19-docosahexaenoic acid (DHA), cis-5,8,11,14,17-eicosapentaenoic acid (EPA), and tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl) were all purchased from Sigma-Aldrich (St. Louis, MO) and used as received.
E-Cigarette Setup.
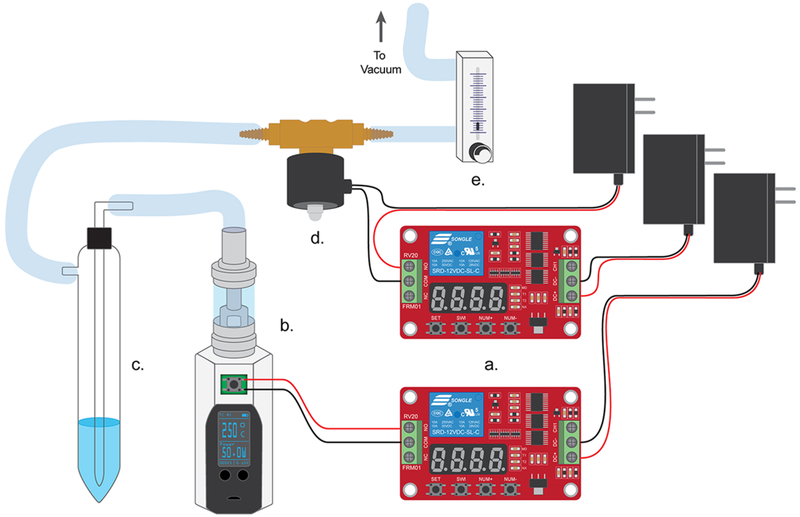
To ensure reproducibility in our experiments, the button to the e-cigarette device was modified by soldering two wires onto either side of the button terminal. The wires connected to the button of the e-cigarette were connected to a 12 V relay timer switch (SainSmart, Amazon.com). When the relay was switched on, the circuit to the e-cigarette was closed and the e-cigarette device registered it as the button being pressed. A second relay switch was connected to a dc 12 V solenoid valve (RioRand, Amazon.com). One end of the solenoid valve was connected to the impinger, and the other end was connected to a flow meter. The flow meter was connected to the house vacuum and adjusted to a flow rate of 500 mL/min. This setup is shown in Figure 2.
Figure 2.
E-Cigarette setup. (a) The relay timer switches the e-cigarette on and opens the solenoid valve. (b) The e-cigarette’s pushbutton switch has been shorted enabling it to be turned on when the relay switch is closed. (c) The e-cigarette aerosol is pulled into the impinger that is filled with a spin trap to capture free radicals. (d) The solenoid valve opens, which pulls a vacuum when the relay switch is closed allowing the aerosol to be pulled through the impinger. (e) The flow meter is adjusted to ensure that the volume pulled for each puff is consistent.
Solvent Components, Temperature, and Wattage Factors.
To investigate the thermal degradation of solvents at each temperature, we used the following PG:GLY (v:v) ratios for our experiments: 0:100, 25:75, 50:50, 75:25, and 100:0. To investigate the effects of constant-temperature modes on free radical generation in the different solvent ratios, we conducted each experiment in 100 °C intervals (100, 200, and 300 °C). To investigate the effects of constant-wattage mode on free radical generation, we looked at three different wattages (10, 25, and 50W).
Generation of e-Eigarette Aerosols.
There is no typical or standardized e-cigarette vaping profile. Newer e-cigarette devices, such as the one used in this study, allow users a great deal of flexibility and allow for the generation of high levels of aerosols by taking larger puff volumes. For the purpose of this study, aerosols were generated using protocols previously established in the laboratory with a goal of generating aerosols representative of human usage without dry puffing, while still remaining within the limit of detection for free radicals.9,23 Careful consideration was given to avoid dry puffing during a session by preloading the cartomizer 15 min prior to vaping, limiting the number of puffs to 40, and ensuring that the tank is filled and aerosol generation is visible. Using the setup outlined in Figure 2, puffs were simulated using the following puffing topography: puff duration, 5 s; interpuff interval, 30 s; flow rate, 500 mL/min; number of puffs, 40. Cartomizers were weighed before and after puffing to measure the solvent consumption and aerosol production.
Spin Trapping of Free Radicals in e-Eigarette Aerosols.
Free radicals were trapped using the nitrone spin trap phenyl-N-tert-butylnitrone (PBN), which has been used extensively for reactive radical detection in cigarette smoke.24 The e-cigarette aerosols generated were passed through a 25 mL impinger containing 6 mL of PBN in hexane. After each experiment, the hexane was evaporated and the remaining residue was reconstituted in 500 μL of tert-benzene. High-purity quartz electron paramagnetic resonance (EPR) tubes were filled with 200 μL of the reconstituted tert-benzene solution and deoxygenated using a freeze–pump–thaw technique with a Schlenk line.25 Samples were deoxygenated as described previously.9 In brief, the samples were subjected to three freeze–pump–thaw argon cycles before being blanketed with argon.
EPR Measurements.
ERP spectroscopy has been used extensively to directly determine the free radical content in cigarette smoke and e-cigarette aerosols.9,26 The spectra derived from PBN radical adducts were recorded using a Bruker eScan R spectrometer (Bruker-Biospin, Billerica, MA) operating in X-band. The EPR parameters were as follows: microwave frequency, 9.7 GHz; modulation frequency, 86.0 kHz; microwave power, 6.00 mW; scan range, 60 G; modulation amplitude, 2.04 G; sweep time, 5.24 s; time constant, 10.24 ms; conversion time, 10.24 ms. All measurements were taken at room temperature (21.5 ± 0.5 °C).
Free radical quantitation was performed using peak height, and concentrations of radicals were compared against that of a stable radical standard, 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO), as described previously.4 All measurements were taken in triplicate, and solvent ratios were compared to each other using an ANOVA coupled with Tukey’s multiple-comparison test using GraphPad (San Diego, CA) Prism. Significant differences were identified at the p < 0.05 level.
Coil-Temperature Measurements.
To evaluate the effects of constant-temperature and constant-wattage modes on coil temperature, the wicks were removed from two different coils (0.15 Ω Ni200 and 0.5 Ω SS316 Uwell Crown). The temperature was measured using a Digi-Sense Type-K thermocouple from Cole-Parmer (Vernon Hills, IL) connected to a traceable thermometer and data acquisition software (Fisher Scientific). The thermocouple was placed at the top of the coils for all measurements. Temperatures were measured in triplicate for both constant-temperature and -wattage modes.
Lipid Peroxidation Analysis.
An impinger was filled with 50 μg/mL AA, DHA, and EPA in 0.1 M Tris-HCl (pH 7.4), and aerosols were generated using different PG:GLY ratios (25:75, 50:50, and 75:25) at a constant temperature of 200 °C as done previously for the EPR measurements. The 75:25 sample was also vaped at 80 and 120 puffs. The treated lipid mixture was analyzed for oxidative decomposition products using a thiobarbituric acid-reactive substances (TBARS) assay kit (Cayman, Ann Arbor, MI). The impinger solution was also analyzed for 8-isoprostane formation via an 8-isoprostane enzyme-linked immunosorbent assay (ELISA) (Eagle Biosciences, Inc., Nashua, NH). TBARS and 8-isoprostane samples were compared to a control that received the same puffing treatment but in the absence of the e-cigarette.
RESULTS
Effects of Solvent at Constant Temperature or Constant Wattage on Radical Production.
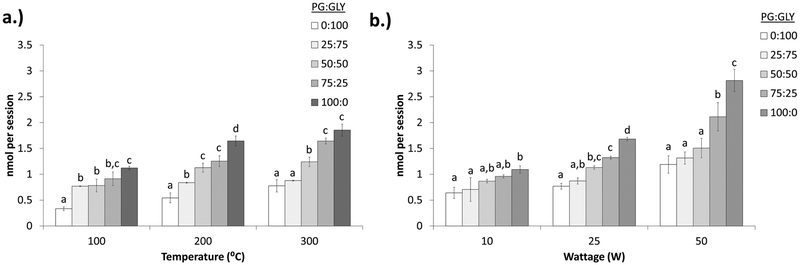
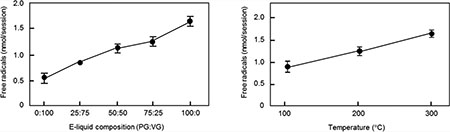
Under constant-temperature mode conditions, the radical yield increases with PG content (Figure 3a). At 100 °C, levels of radicals generated from 100% GLY solutions (0:100) were significantly lower than those of all other solutions, while levels of radicals from 100% PG solutions (0:100) were significantly higher than those of the 25:75 and 50:50 solutions. At 200 °C, all of the different solutions were significantly different from one another with the exception of those with the 50:50 and 75:25 ratios. At 300 °C, levels of radicals generated from solutions containing lower levels of PG (0:100 and 25:75) were significantly lower than those of the other solutions. Conversely, solutions with higher concentrations of PG (75:25 and 100:0) had levels of radicals significantly higher than those of the other solutions.
Figure 3.
Effects of solvent on radical production. (a) Radical production under constant-temperature mode at different temperatures and different solvent compositions. (b) Radical production under constant-wattage mode at different temperatures and different solvent ratios. Different letters indicate significant differences within the temperature or wattage group.
Under constant-wattage mode conditions, the radical content also appears to be PG-dependent (Figure 3b). At 10 W, levels of radicals generated from 100% PG solutions (100:0) were significantly higher than those of solutions that contained lower levels of PG (0:100 and 25:75) but not significantly higher than those of the 50:50 and 75:25 solutions. At 25 W, levels of radicals generated from 100% GLY solutions (0:100) were significantly lower than those of 50:50, 75:25, and 100:0 solutions. Conversely, levels of radicals generated from 100% PG solutions (100:0) were significantly higher than those of all other solutions. Similarly, levels of radicals generated from 100% PG solutions (100:0) were also significantly higher than those of all other solutions at 50 W, as were the levels of radicals generated from the 75:25 solution.
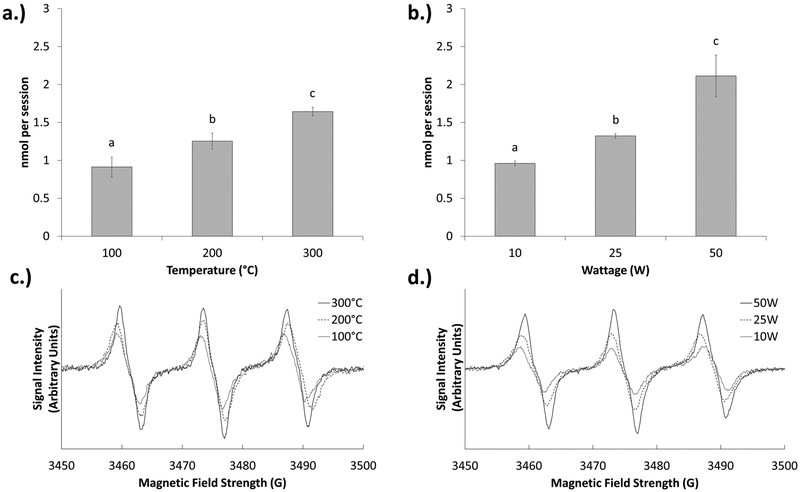
Effects Temperature and Wattage on Radical Production.
Using a 75:25 (PG:GLY) ratio as a sample ratio for most common e-liquids, as the temperature increased, statistically significant increases in radical production were also seen. (Figure 4a,c) This suggests that the formation of radicals is largely temperature-dependent. Similarly, statistically significant increases in radical production were also seen with increasing wattages being applied (Figure 4b,d).
Figure 4.
Effects of constant temperature and constant wattage on radical production for a typical e-liquid solvent (75:25). (a) Effects of different constant temperatures on radical generation in a 75:25 (PG:GLY) solution. (b) Effects of different constant wattages on radical generation in a 75:25 PG/GLY solution. Different letters indicate significantly different values. (c) Sample PBN spectra of the different constant temperatures. (d) Sample PBN spectra of the different constant wattages.
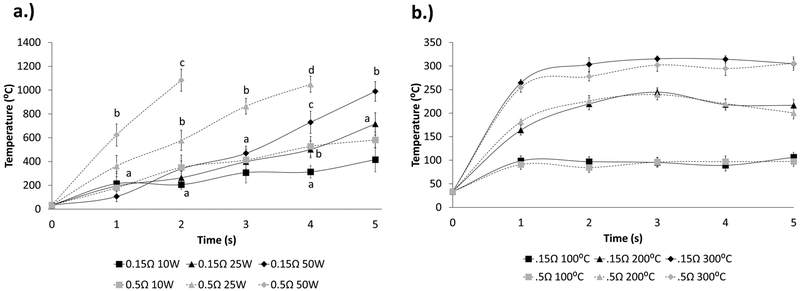
Coil Temperatures.
To evaluate the thermal conditions of the different e-cigarette device modes, the coil temperatures were measured. Under constant-wattage modes, the temperature increases in a time-dependent manner (Figure 5a). As the wattage increases, so does the rate at which the temperature increases. The more resistant coils (0.5 Ω) reach temperatures that are significantly higher than those of the less resistant coils (0.15 Ω) at the same wattage. For 0.5 Ω coils at 25 and 50 W, the temperature reached was higher than could be detected by the temperature monitor. This difference in coil resistance disappears when the e-cigarette device is used in constant-temperature mode (Figure 5b). In constant-temperature mode, the 0.5 and 0.15 Ω coils showed no significant differences between one another at their set temperature.
Figure 5.
Coil temperatures based on modes. (a) Temperature changes based on different coil resistances and different wattages using a constant-wattage mode. (b) Temperature changes based on different coil resistances and different set temperatures using a constant-temperature mode. Different letters indicate significant differences.
Solvent Consumption.
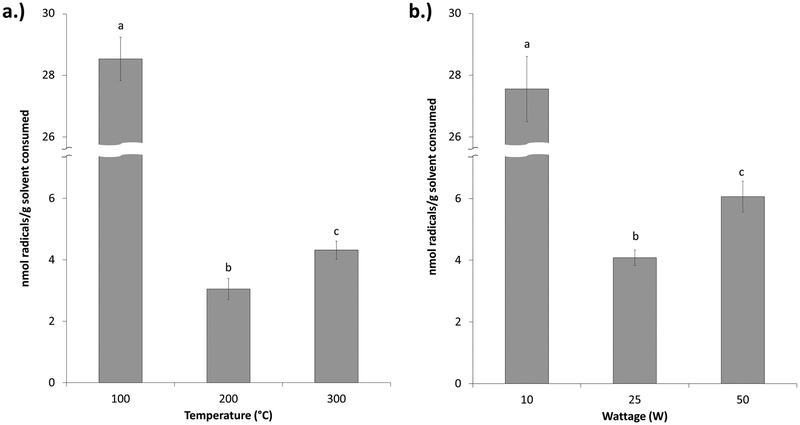
To examine whether the increases in radical production were simply the result of increased aerosol production, radicals were normalized to the amount of solvent consumed. In constant-temperature mode, paradoxically, radical production on a per gram solvent basis resulted in significantly higher levels at 100 °C for the 75:25 PG/GLY solution (Figure 6a). A significant increase in radical production on a per gram solvent basis was observed at 300 °C as compared to 200 °C. In constant-wattage mode, a similar trend was observed with radical production on a per gram solvent basis with the 75:25 PG/GLY solution (Figure 6b). At 10 W, levels of radicals on a per gram solvent basis were significantly higher than under the 25 or 50 W condition. At 50 W, a significant increase in levels of radicals per gram of solvent was observed as compared to that under the 25 W condition. No significant differences in solvent consumption as a result of different solvents were seen (Figure S1).
Figure 6.
Effects of constant-temperature and constant-wattage radical production on a per gram solvent consumption basis for a typical e-liquid solvent (75:25). (a) Effects of different constant temperatures on radical generation in a 75:25 PG/GLY solution on a per gram solvent consumed basis. (b) Effects of different constant wattages on radical generation in a 75:25 PG/GLY solution on a per gram solvent consumed basis. Different letters indicate significantly different values between temperatures and wattages only.
Lipid Peroxidation.
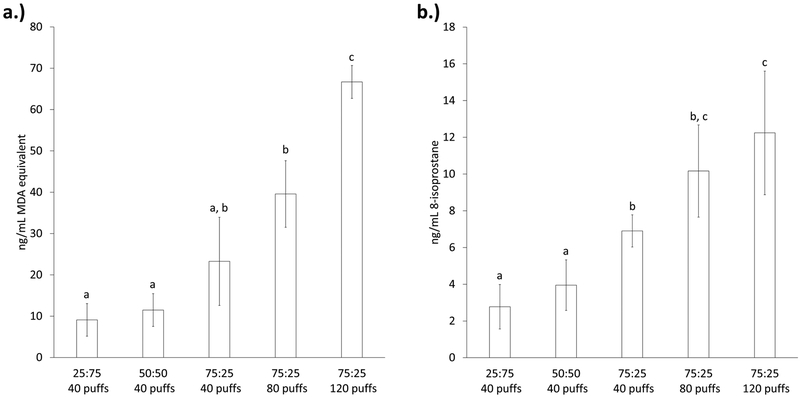
To examine the biological effects of the aerosol radicals caused by PG:GLY solvent ratios, we measured the lipid oxidation products of biologically relevant lipids (AA, DHA, and EPA) in a model system. In constant-temperature mode at 200 °C, lipid oxidation product formation was measured using TBARS (Figure 7a). While the extent of lipid oxidation did increase with increasing concentrations of PG, the differences were not significant. When the number of puffs was increased for the 75:25 sample, an increase in the level of lipid oxidation also occurred, with that for the 120-puff samples being significantly higher than that of the 40-puff samples. The formation of 8-isoprostane from the free radical-catalyzed peroxidation of AA was also measured (Figure 7b). The level of 8-isoprostane formation was significantly increased in the 75:25 samples compared to those at the lower PG concentrations (25:75 and 50:50). Increasing the number of puffs for the 75:25 ratio also resulted in an increase in the level of 8-isoprostane formation, with that of the 120-puff sample being significantly higher than that of the 40-puff sample.
Figure 7.
Effects of solvent on lipid peroxidation markers under constant-temperature conditions (200 °C). (a) Thiobarbituric acid-reactive substances (TBARS) formed by different PG:GLY ratios and numbers of puffs. (b) 8-Isoprostane formation from different PG:GLY ratios and numbers of puffs. Different letters indicate significantly different values.
DISCUSSION
While e-cigarettes are commonly thought to be a safer alternative to combustible tobacco products, additional research is required to identify potential health consequences associated with their use. Recent studies have identified a number of toxic constituents generated in the process of e-cigarette vaping, including our finding of high levels of reactive free radicals in e-cigarette aerosols.9,16–21 e-Cigarettes and e-liquids available on the market vary widely in their design and usage. In this study, we have examined the impact of different e-cigarette temperatures and wattages and e-liquid solvents on free radical production. Our results indicate that free radical production is highly dependent on the PG:GLY ratio in the e-liquid. The relationship between increasing coil temperature and wattage on both aerosol and radical generation suggests a complex relationship in which radicals are not solely dependent on aerosol generation as disproportionately high levels of ROS were produced at the lowest temperature and power settings. These relationships are particularly important given the growing usage of more powerful e-cigarette designs.
The methodologies used to generate and measure e-cigarette aerosols have varied greatly in the literature.11,27,28 Some have used heating chambers for vapor generation, while others have used dc power supplies to deliver a constant voltage to the coils. In studies that used e-cigarette devices themselves for the generation of aerosols, most have relied upon the researcher manually pressing the e-cigarette button to activate the coils or do not describe how the device was activated during the vaping period.29–31 To avoid this variability inherent in previously used protocols, we utilized a novel mechanism to reliably simulate usage parameters, including coil activation and puff flow, to provide consistent puffs over the course of a vaping session. In addition to being highly reliable and reproducible, this method also provides enough flexibility to allow for the evaluation of any e-cigarette device as it requires only rewiring the firing button. With this method, future devices can be easily evaluated as long as they have a physical button that can be modified.
Our finding that free radical production was highly dependent on the PG:GLY ratio provides insight into potential mechanisms of radical formation. This relationship also seems to be independent of aerosol production as there were no significant differences in solvent consumption as a result of different PG:GLY ratios within the same constant-temperature settings. While some radicals appear to be formed due to glycerol, our data suggest that the majority of radicals found in e-cigarette aerosols are generated from propylene glycol in both constant-temperature and constant-wattage modes. This would align well with recent papers that found degradation products of propylene glycol were the result of α- and β-hydroxyl-alkyl radical-mediated pathways involving O2 insertion and hydrogen abstraction even at moderate temperatures (127–227 °C).30,32 The limited information about the degradation of glycerol suggests that it is relatively stable at temperatures below its boiling point, but at higher temperatures, it begins to undergo decomposition.30,33 Glycerol’s main oxidation product, glyceraldehyde, is believed to be formed from radical-mediated reactions as a result of hydrogen abstraction.30,33 For our studies, we utilized PBN in excess as a spin trap as it is capable of forming long-lived adducts with a variety of different radical species.34 As we were interested mainly in quantitation, PBN was chosen for its relative stability over time. A major disadvantage of PBN is that its nitrone spin-trapping orientation it makes it particularly hard to gain structural information from the spectra.34 Future studies will need to be performed to identify the species of these radicals.
Many of the newer e-cigarettes can be operated in a constant-wattage or constant-temperature mode. We show that under constant-wattage usage, coil resistance and e-liquid solvent composition appear to be critical factors for free radical production. Under constant-temperature mode, levels of radicals increased as the temperature was increased in a manner independent of the PG:GLY ratio, suggesting a temperature-dependent mechanism for the formation of radicals. However, the nature of this temperature dependence and its relationship with aerosol production are likely complex and are likely to vary at lower versus higher temperatures. This relationship is also independent of aerosol production as at higher temperatures and wattages, solvent consumption does not differ significantly. The increase in the level of radical formation as wattage increases also suggests a temperature dependence of the formation of radicals as the increase in power output would increase the final temperature. This aligns with what was discussed earlier as the degradation of propylene glycol and the degradation of glycerol are both affected by temperature.30,32,33 While the exact temperatures of formation for different carbonyls are still being debated in the literature, many of the studies agree that lower levels of reactive carbonyls such as formaldehyde, acetaldehyde, acrolein, and acetone are produced at lower temperatures.19,27,31
Even though 100 °C and 10 W settings in these powerful e-cigarette devices will likely not be used by vapers due to the very low levels of aerosol generation, it is interesting that free radicals are still being generated. Follow-up work will have to investigate the heating coils, wicking material, and solvent interactions as potential contributors to radical production at these low temperatures. To date, we have observed free radical production in all the different generations (first through fourth) of e-cigarettes tested (unpublished data). However, the current investigations of temperature and wattage have been tested only in a single temperature-controlled device. Future studies will need to replicate the findings in other such devices.
Recent studies with non-temperature-controlled devices also found increased production of carbonyls, including form-aldehyde and acetaldehyde, with increasing temperature.29,31 Further, they found that with successive puffs, overall coil temperatures were increased, suggesting that non-temperature-controlled devices can reach temperatures higher than those of temperature-controlled units, leading to increased carbonyl production. However, these studies did not look at radical generation as a function of temperature. In constant-wattage mode, we saw that coil temperatures increase in a time-dependent manner, reaching well over 600 °C under some conditions. While our use of a K type thermocouple allowed us to measure only a specific part of the coil, it demonstrates that the coil will continue to get hotter the longer the button is pressed. While “dry puffing” conditions are likely to occur at these very high temperatures, when a wick and solvent are present, it is likely that the maximum temperatures achieved will be substantially lower. To avoid “dry puffing” conditions in our study, we ensured that the atomizer tank remained full of solvent. All puffs at the higher temperatures and wattages produced visible vapors, suggesting that “dry puffing” was kept to a minimum. However, we cannot eliminate the possibility that undetectable low levels of “dry puffing” may be occurring.
Previous studies have also identified other toxic agents, including diethylene glycol, numerous reactive organic compounds, nitrosamines, and heavy metals, in e-cigarette aerosols.16–21 Many of these studies utilized earlier-generation e-cigarette devices that produce temperatures lower than those of newer models currently being sold. A recent study testing different tanks on high-power e-cigarette devices found high levels of formaldehyde and acetaldehyde production on a per gram solvent basis with increases in wattage for three of the five devices tested.35 While these high levels could be, in part, explained by excessive “dry puffing” resulting from the vaping protocols used, the finding does suggest that increased temperatures and solvents effects can impact the overall chemical toxicity of the e-liquid aerosols.36
When free radicals were expressed on a per gram aerosol basis, differential results were observed at lower versus higher temperatures, suggesting that the relationship between radical production and aerosol formation may be temperature-dependent. At lower temperatures, it would appear that radical production is rather independent of aerosol formation. It is important to note that the mechanism of radical generation remains unknown but may involve a number of different factors in addition to aerosol production. For example, radicals may be generated from the solvent without aerosol formation (e.g., at lower temperatures), materials in the coil itself, the wicking material, or a complex interaction of all three. From the perspective of the consumer, it might appear that these findings would suggest that vaping a consistent volume of e-liquid at 100 °C may result in a higher level of exposure to radicals than vaping the same volume at a higher temperature such as 200 °C. However, this is unlikely as it would require the consumer to vape nearly 10 times longer at 100 °C than at 200 °C. Given the very low level of aerosol production at 100 °C, it is not likely a relevant condition for human usage. The lowest level of radical production per volume vaped was observed at 200 °C, likely a more relevant temperature for common use.
Upon examination of radical production on a per gram solvent basis, it is clear that these two events are somewhat independent of one another. In this regard, the temperature profile for radical production is very different from that of aerosol production. This is, in part, supported by our observation that unlike aerosol components, free radicals are not trapped by a Cambridge filter and are, thus, gas phase constituents (Figure S2). Paradoxically, the levels of radicals observed on a per gram solvent basis at lower temperatures and wattages (100 °C and 10 W, respectively) were significantly higher than those observed at the higher temperatures and wattages. This discrepancy is likely due to interactions of radicals with the aerosols themselves, as gas phase radicals have been shown to interact with the surfaces of aerosols.37 At higher temperatures and wattages, aerosol production is greater and provides for more possible interactions for the radicals, resulting in a dilution or blunting effect of radical populations. Higher temperatures also can result in a decrease in radical stability and as a result may be quenched before interacting with the PBN spin trap in our experiments.38 On the basis of our findings, it is clear that temperature influences both aerosol production and radical formation; however, these interactions are likely independent in nature. Further research is needed to elucidate the complex interactions between aerosol and radical generation and their temperature dependence.
In our studies with biologically relevant lipids, we found that as the PG content increased, so did the levels of markers of lipid oxidation, mimicking the results of our free radical measurements. Free radicals are known to be highly reactive with polyunsaturated fatty acids.39 A common byproduct of this oxidative damage is the formation of thiobarbituric acid-reactive substances (TBARS) from AA DHA and EPA. TBARS are commonly used as an indicator of oxidative stress in the body. Recent studies in mice found that e-cigarette aerosol exposure increased levels of TBARS in lung homogenate.40 Similarly, the free radical-catalyzed formation of 8-isoprostane from AA has long been used as a marker of oxidative stress.41 Previous studies with e-cigarette users found increased 8-isoprostane levels in the plasma after vaping.42 Our findings with TBARS and 8-isoprostane suggest these radicals are indeed biologically active and may pose a significant risk to the consumer.
Overall, our results demonstrate that free radicals are produced under normal usage conditions by e-cigarettes in a temperature-and aerosol-dependent manner with propylene glycol being the major contributor. This echoes other recent studies that found temperature and propylene glycol to be the major factors in the production of reactive carbonyls.18,19,27,29,31 Our findings show that the temperature control modes found in newer-generation e-cigarette devices can limit the temperatures the device and e-liquids reach and, thus, the number of radicals generated. The temperature control mode is also independent of the coil’s resistance, so it may provide an easier and potentially safer option for the consumer. To the best of our knowledge, this is the first time the inner workings of these temperature control devices have been described, and we are hopeful it will allow researchers to better understand their functionality. Finally, we find that e-liquid composition is also an important factor and those with lower propylene glycol levels may result in decreased delivery of radicals. Additional studies will be required to assess the overall harm associated with exposure to e-cigarette aerosol free radicals.
Supplementary Material
Funding
This work was supported in part by the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the U.S. Food and Drug Administration (under Grant P50-DA-036107).
ABBREVIATIONS
- AA
arachidonic acid
- COPD
chronic obstructive pulmonary disease
- DHA
cis-4,7,10,13,16,19-docosahexaenoic acid
- ELISA
enzyme-linked immunosorbent assay
- EPA
cis-5,8,11,14,17-eicosapentaenoic acid
- EPR
electron paramagnetic resonance
- GLY
glycerol
- PBN
phenyl-N-tert-butylnitrone
- PG
propylene glycol
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid-reactive substances
- TEMPO
2,2,6,6-tetramethyl-1-piperidinyloxyl
- Tris-HCl
tris-(hydroxymethyl)aminomethane hydrochloride
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemrestox.7b00116.
Solvent consumption and Cambridge filter figures and results (PDF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. (2010) How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Centers for Disease Control and Prevention, Atlanta. [PubMed] [Google Scholar]
- (2).Bartalis J, Chan WG, and Wooten JB (2007) A new look at radicals in cigarette smoke. Anal. Chem 79, 5103–5106. [DOI] [PubMed] [Google Scholar]
- (3).Dellinger B, Khachatryan L, Masko S, and Lomnicki S (2011) Free radicals in tobacco smoke. Mini-Rev. Org. Chem 8, 427–433. [Google Scholar]
- (4).Goel R, Bitzer Z, Reilly SM, Trushin N, Foulds J, Muscat J, Liao J, Elias RJ, and Richie JP Jr. (2017) Variation in Free Radical Yields from U.S. Marketed Cigarettes. Chem. Res. Toxicol 30, 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Pryor WA (1997) Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ. Health Perspect. 105, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Messner B, and Bernhard D (2014) Smoking and cardiovascular disease mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler., Thromb., Vasc. Biol 34, 509–515. [DOI] [PubMed] [Google Scholar]
- (7).Dekhuijzen P (2004) Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur. Respir. J 23, 629–636. [DOI] [PubMed] [Google Scholar]
- (8).MacNee W, and Rahman I (2001) Is oxidative stress central to the pathogenesis of chronic obstructive pulmonary disease? Trends Mol. Med 7, 55–62. [DOI] [PubMed] [Google Scholar]
- (9).Goel R, Durand E, Trushin N, Prokopczyk B, Foulds J, Elias RJ, and Richie JP Jr. (2015) Highly reactive free radicals in electronic cigarette aerosols. Chem. Res. Toxicol 28, 1675–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Yingst JM, Veldheer S, Hrabovsky S, Nichols TT, Wilson SJ, and Foulds J (2015) Factors associated with electronic cigarette users’ device preferences and transition from first generation to advanced generation devices. Nicotine Tob. Res 17, 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Baalbaki R, Saliba N, and Shihadeh A (2015) Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob. Res 17, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, and Voudris V (2015) Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci. Rep 4, 4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Brown CJ, and Cheng JM (2014) Electronic cigarettes: product characterisation and design considerations. Tobacco Control 23 (Suppl. 2), ii4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Foulds J, Veldheer S, and Berg A (2011) Electronic cigarettes (e-cigs): views of aficionados and clinical/public health perspectives. Int. J. Clin. Pract 65, 1037–1042. [DOI] [PubMed] [Google Scholar]
- (15).dicodes.de (2015) Application Note for Temperature Controlled Vaping.
- (16).Cheng T (2014) Chemical evaluation of electronic cigarettes. Tobacco Control 23 (Suppl. 2), ii11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Long GA (2014) Comparison of select analytes in exhaled aerosol from e-cigarettes with exhaled smoke from a conventional cigarette and exhaled breaths. Int. J. Environ. Res. Public Health 11, 11177–11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Jensen RP, Luo W, Pankow JF, Strongin RM, and Peyton DH (2015) Hidden formaldehyde in e-cigarette aerosols. N. Engl. J. Med 372, 392–394. [DOI] [PubMed] [Google Scholar]
- (19).Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, and Goniewicz ML (2014) Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob. Res 16, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Williams M, Villarreal A, Bozhilov K, Lin S, and Talbot P (2013) Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One 8, e57987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Uchiyama S, Ohta K, Inaba Y, and Kunugita N (2013) Determination of carbonyl compounds generated from the E-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal. Sci 29, 1219–1222. [DOI] [PubMed] [Google Scholar]
- (22).Moosbrugger C (2000) ASM Ready Reference: Electrical and Magnetic Properties of Metals, ASM International, Materials Park, OH. [Google Scholar]
- (23).Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, and Voudris V (2013) Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int. J. Environ. Res. Public Health 10, 2500–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Church DF (1994) Spin trapping organic radicals. Anal. Chem 66, 418A–427A. [Google Scholar]
- (25).Yu L-X, Dzikovski BG, and Freed JH (2012) A protocol for detecting and scavenging gas-phase free radicals in mainstream cigarette smoke. J. Visualized Exp, e3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Robinson EA, and Johnson JD (2011) Methods for analysis of free radicals in cigarette smoke. Mini-Rev. Org. Chem 8, 401–411. [Google Scholar]
- (27).Wang P, Chen W, Liao J, Matsuo T, Ito K, Fowles J, Shusterman D, Mendell M, and Kumagai K (2017) A Device-Independent Evaluation of Carbonyl Emissions from Heated Electronic Cigarette Solvents. PLoS One 12, e0169811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Zhao J, Pyrgiotakis G, and Demokritou P (2016) Development and characterization of electronic-cigarette exposure generation system (Ecig-EGS) for the physico-chemical and toxicological assessment of electronic cigarette emissions. Inhalation Toxicol. 28, 658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Geiss O, Bianchi I, and Barrero-Moreno J (2016) Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health 219, 268–277. [DOI] [PubMed] [Google Scholar]
- (30).Jensen RP, Strongin RM, and Peyton DH (2017) Solvent Chemistry in the Electronic Cigarette Reaction Vessel. Sci. Rep 7, 42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Talih S, Balhas Z, Salman R, Karaoghlanian N, and Shihadeh A (2016) ″Direct Dripping″: A High-Temperature, High-Form-aldehyde Emission Electronic Cigarette Use Method. Nicotine Tob. Res 18, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Díaz E, Sad ME, and Iglesia E (2010) Homogeneous Oxidation Reactions of Propanediols at Low Temperatures. Chem-SusChem 3, 1063–1070. [DOI] [PubMed] [Google Scholar]
- (33).Stein YS, Antal MJ, and Jones M (1983) A study of the gas-phase pyrolysis of glycerol. J. Anal. Appl. Pyrolysis 4, 283–296. [Google Scholar]
- (34).Hawkins CL, and Davies MJ (2014) Detection and characterisation of radicals in biological materials using EPR methodology. Biochim. Biophys. Acta, Gen. Subj 1840, 708–721. [DOI] [PubMed] [Google Scholar]
- (35).Gillman IG, Kistler KA, Stewart EW, and Paolantonio AR (2016) Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul. Toxicol. Pharmacol 75, 58–65. [DOI] [PubMed] [Google Scholar]
- (36).Nitzkin JL, Farsalinos K, and Siegel M (2015) More on hidden formaldehyde in e-cigarette aerosols. N. Engl. J. Med 372, 1575. [DOI] [PubMed] [Google Scholar]
- (37).Wiegel AA, Liu MJ, Hinsberg WD, Wilson KR, and Houle FA (2017) Diffusive confinement of free radical intermediates in the OH radical oxidation of semisolid aerosols. Phys. Chem. Chem. Phys 19, 6814–6830. [DOI] [PubMed] [Google Scholar]
- (38).Bawn C (1938) The stability of free radicals. Trans. Faraday Soc. 34, 598–607. [Google Scholar]
- (39).Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem 524, 13–30. [DOI] [PubMed] [Google Scholar]
- (40).Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, Pekosz A, and Biswal S (2015) Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 10, e0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Milne GL, Yin H, and Morrow JD (2008) Human biochemistry of the isoprostane pathway. J. Biol. Chem 283, 15533–15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, and Frati G (2016) Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest 150, 606–612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.