Abstract
Oxidation of methionine to methionine sulfoxide is a type of posttranslational modification reversed by methionine sulfoxide reductases (Msrs), which present an exceptionally high number of gene copies in plants. The side-form general antioxidant function-specific role of each Msr isoform has not been fully studied. Thirty homologous genes of Msr type A (MsrA) and type B (MsrB) that originate from the genomes of Arabidopsis thaliana, Populus trichocarpa, and Oryza sativa were analyzed in silico. From 109 to 201 transcription factors and responsive elements were predicted for each gene. Among the species, 220 and 190 common transcription factors and responsive elements were detected for the MsrA and MsrB isoforms, respectively. In a comparison of 14 MsrA and 16 MsrB genes, 424 transcription factors and responsive elements were reported in both types of genes, with almost ten times fewer unique elements. The transcription factors mainly comprised plant growth and development regulators, transcription factors important in stress responses with significant overrepresentation of the myeloblastosis viral oncogene homolog (MYB) and no apical meristem, Arabidopsis transcription activation factor and cup-shaped cotyledon (NAC) families and responsive elements sensitive to ethylene, jasmonate, sugar, and prolamine. Gene Ontology term-based functional classification revealed that cellular, metabolic, and developmental process terms and the response to stimulus term dominated in the biological process category. Available experimental transcriptomic and proteomic data, in combination with a set of predictions, gave coherent results validating this research. Thus, new manners Msr gene expression regulation, as well as new putative roles of Msrs, are proposed.
Keywords: gene expression, methionine sulfoxide, responsive element, transcription factor
1. Introduction
The oxidation of methionine (Met) to methionine sulfoxide (MetSO) is inevitable for organisms living in an aerobic environment. The enzyme that catalyzes the reduction of MetSO to Met is methionine sulfoxide reductase (Msr). According to BRaunschweig ENzyme DAtabase (BRENDA), which is a comprehensive enzyme classification system, peptide-methionine (R)-S-oxide reductase (EC 1.8.4.12) is an enzyme that is classified as an oxidoreductase that acts on a sulfur group of donors, with disulfide as an acceptor. There are three known types of Msr: type A (MsrA), type B (MsrB), and type C, which is specific to eubacteria and unicellular eukaryotes [1,2,3]. Although MsrA is a reductase that is specific to the MetSO S epimer, and MsrB is specific to the MetSO R epimer, both reduce free and protein-bound MetSO [4]. More Msr studies are related to animals, although an extremely high number of Msr gene copies has been found in plants. For example, Arabidopsis and rice have fourteen and six Msr genes, respectively [5]. Studies have identified nine MsrB genes in Arabidopsis [6] and four MsrB genes in poplar [7]. Excluding review papers, over 200 experiments that are linked to human diseases and over 200 Msr studies in other animals can be found in the National Center for Biotechnology Information (NCBI) database. Approximately 60 experimental studies have been conducted on plants and algae. The most recent review demonstrated that nearly half of the studies were conducted on Arabidopsis, and only a few of the studies were conducted on rice and poplar [8]. Based on experimental data, the role of Msrs in redox homeostasis and signaling in plants was elucidated, providing many examples of the physiological functions of plant Msrs under oxidative, abiotic, and biotic stress conditions in different species. Most of the MsrA and MsrB genes encode proteins, with masses of approximately 25 kDa and 15 kDa, respectively. There is little or no sequence homology between the MsrA and MsrB proteins [9]. Within each MsrA and MsrB plant family, the sequence identity among isoforms ranges between 1% and 15% [7]. The increased production of reactive oxygen species and the subsequent oxidative modification of proteins accompanies important physiological transitions in plants. MetSO studies in Arabidopsis under oxidative stress led to the identification of approximately 400 proteins containing this reversible posttranslational modification that are potential targets of Msrs [10]. Thus, the multiplicity of plant Msr isoforms allowed for us to suppose that the Msr system might be involved in the regulation of many aspects of plant life.
Experimental studies have revealed several factors, mainly stress factors, regulating the expression of Msr genes in Arabidopsis. Few experiments have been performed on poplar and rice; thus, less information is known regarding the regulation of Msr expression in these plants than in other species. In previous studies, oxidative stress affected the expression of MsrB Arabidopsis genes [11] and the synthesis of AtMSRA2 [12], AtMSRA4 [12,13], AtMsrB7 [14], AtMSRB9 [15], and OsMSRB5 proteins. High light intensity modified the level of AtMSRA4 [12,13]. UV treatment induced the expression of MSRB7 and MSRB8 genes [16]. Low temperatures impacted the levels of AtMSRB3 [17], AtMSRB1 [18], and AtMSRB2 plastid proteins [12,18], and OsMSRA4.1 and OsMSRB1.1 transcripts [19]. High temperature induced and repressed the AtMSRA4 and AtMSRA2 protein levels, respectively [18], and modified OsMSRA4.1 and OsMSRB1.1 gene expression [19]. High salinity modified the levels of AtMSRA4 [12] and all OsMSR transcripts [20], particularly OsMSRA4.1 and OsMSRB1.1 [19]. Dehydration stress enhanced the synthesis of the AtMSRB9 protein [15]. Heavy metal stress increased the accumulation of the mRNA of all AtMsrs [21] and the OsMSRB5 protein [22]. Pathogen-induced changes in Arabidopsis MSRB7 and MSRB8 expression [16,23] and the poplar pMsrA and MsrB2 protein levels [18] have also been reported. Treatment with NO upregulated the AtMsrB7 [24] and OsMSR transcripts [20]. Mannitol, methyl viologen, and abscisic acid (ABA) treatments induced OsMSRA4.1 and OsMSRB1.1 transcription [19]. Combined ABA and NaCl treatment increased the AtMSRA4 levels [12]. Interestingly, AtMsrB7 was found to be responsive to linolenic acid, which is the precursor of jasmonic acid (JA) [25]. PMSR2 partially regulates A. thaliana development [26]; however, no data are available regarding the involvement of specific Msrs in the regulation of basic biological processes, leading to plant growth and development. Microarray data analyses have revealed that the expression levels of Msrs are organ-specific. The highest abundance of the AtMSRA4 transcript was detected in fully developed leaves, while AtMSRA2 and AtMSRA3 were dominant in the roots, and stamen and pollen contained mostly AtMSRA3 transcripts [7,11]. For the B-type genes, AtMSRB1, AtMSRB2, and AtMSRB6 mRNAs have been reported to be abundant in leaves, whereas AtMSRB5, AtMSRB7, AtMSRB8, and AtMSRB9 have been reported to be abundant in roots [7,11,13]. In rice, OsMSRA4.1 was constitutively expressed in all tissues, whereas OsMSRB1.1 was more abundant in leaves, flowers, and calli than in the roots and stems.
The above experimental results certainly do not present all the possibilities of Msrs gene expression regulation. To determine which compounds and factors may affect Msrs gene expression, in silico predictions that are based on the servers and programs listed in the bioinformatics resources portal, which was shown to be statistically reliable by previous publications, may provide interesting hints for further experimental studies. The region of DNA that was located upstream of a particular gene, called a promoter, mainly regulates gene expression by proteins that are known as transcription factors (TFs), which recognize specific DNA sequences within the promoter region and specific activators binding to responsive elements (REs). A genome-wide search for putative promoters revealed that Arabidopsis, poplar, and rice contain 17,896, 17,645, and 22,258 genes, respectively, with a transcription start site (TSS) ≥ 1, comprising over 99% of the genes in each genome [27]; these results emphasize the complexity of gene expression regulation. Here, we predominantly focus on the above three model species, because their Msr genes are homological, and the promoter regions of their Msr isoforms are well defined. TSS selection depends on the cellular and tissue context, developmental and physiological stage, environmental conditions, and the set of accessible TFs [27]. For a single gene, the proximal promoter (200–300 bp upstream of the TSS), together with the distal promoter, might contain hundreds of consensus sequences for different TFs [28]. Thus, the protein-DNA complex can upregulate or downregulate gene expression. TFs recognize primary, secondary, or even additional motifs that differ from each other, emphasizing the importance of TFs in the complex regulation of gene expression [29]. TFs representing the following families have been identified in plants: v-myb avian myeloblastosis viral oncogene homolog (MYB), basic Helix-Loop-Helix (bHLH), tryptophan, arginine, lysine and tyrosine domain (WRKY), no apical meristem, Arabidopsis transcription activation factor and cup-shaped cotyledon (NAC), and basic leucine zipper (bZIP). The multifunctionality of these TFs in the regulation of biological processes, including plant growth and development (MYB, NAC, bZIP, bHLH) and various stress responses (MYB, NAC, bZIP, bHLH, WRKY), has been extensively reviewed [30]. In particular, the participation of TFs from MYB and NAC families in the regulation of plant growth and development and stress responses has been well documented [31,32]. MYB is among the largest families of plant TFs that control various plant processes, such as development, differentiation, metabolism, defense, and stress responses. R2R3-MYB is a plant-specific class of MYB groups that contains over one hundred members in the genomes of different plant species [31]. NACs belong to a plant-specific family of TFs that is involved in various plant stress responses [32]. The extensive action of NAC TFs is derived from the large number of NAC genes in genomes. For example, there are 117 NAC genes in Arabidopsis, 163 NAC genes in poplar, and 151 NAC genes in rice [32]. The expression of ANAC2, NAC001, NAC004, NAC005, NAC013, and NAC019 was shown to be strictly connected to various stress conditions ([32] and references therein). In particular, ANAC013 functions in the regulation of the oxidative stress response [33], and NAC103 [34] and NAC87 modulate ROS [35]. The MYB-NAC regulatory network assures many developmental transitions in plants. For example, the interaction of MYB108 with the ANAC003 promoter region controls leaf senescence in Arabidopsis [36]. The cooperation of MYB and NAC TFs was expected, since both of the family members have been experimentally shown to be involved in osmotic stress and oxidative stress responses.
To verify whether different types and isoforms of Msrs are required for a range of distinct physiological states and responses to different stress conditions, the promoter regions of 30 homological Msr genes that represent 14 A-type isoforms and 16 B-type isoforms were analyzed in a search for TF binding sites, the specific TFs recognizing these sites and REs. The pool of predicted TFs and REs was investigated to determine whether a group of species-specific, type-specific, or isoform-specific TFs and REs could be distinguished. Based on the PLANTCARE database, 23 cis elements were predicted in the promoters of all Arabidopsis A-type Msr genes [13]. While suspecting that Msr promoters contain more REs, we performed a search using MatInspector software [37] that was based on the Plant cis-acting regulatory DNA elements (PLACE) database [38] because the MatInspector library contains 634 matrices, representing the largest library that is available for TF binding site searches.
2. Results
2.1. Prediction of Transcription Factor Binding Sites
Known and homologous MsrA and MsrB genes that originate from the genomes of Arabidopsis (Arabidopsis thaliana), poplar (Populus trichocarpa), and rice (Oryza sativa) were analyzed. The promoter region sequence of each gene was analyzed with a search of the REs and binding sites for plant-specific TFs to establish the potential physiological and environmental factors that regulate Msr gene expression. In total, 30 gene sequences were analyzed, and the number, length, and location of promoter regions and the number of coding transcripts were specified, as in Table S1. Two or more coding transcripts were established for 18 Msr genes, Table S1A–C. AtMsrB5 was assigned the highest number of coding transcripts (five). Over two hundred TF binding sites were predicted for each gene, as in Table 1. The largest numbers of TF binding sites and REs were predicted for MsrB9 originating from Arabidopsis, MsrB3.2 originating from poplar, and MsrA5 originating from rice. As the consensus core sequences were repeated within each promoter region, the final number of specific nonredundant TFs and REs ranged from 109–201 per gene.
Table 1.
Total number of transcription factor binding sites (TFBS) and responsive elements (REs), followed by the number of redundant transcription factors (TFs) and REs predicted for 30 homological genes of methionine sulfoxide reductase type A (MsrA) and B (MsrB) originating from the Arabidopsis thaliana (At), Populus trichocarpa (Pt), and Oryza sativa (Os) genomes.
| Gene Name | TFBS + REs | TFs + REs |
|---|---|---|
| AtPMSR1 | 249 | 163 |
| AtPMSR2 | 325 | 186 |
| AtPMSR3 | 310 | 174 |
| AtPMSR4 | 249 | 158 |
| AtMsrA5 | 254 | 162 |
| AtMsrB1 | 267 | 157 |
| AtMsrB2 | 294 | 169 |
| AtMsrB3 | 255 | 155 |
| AtMsrB4 | 210 | 144 |
| AtMsrB5 | 286 | 174 |
| AtMsrB6 | 362 | 170 |
| AtMsrB7 | 298 | 175 |
| AtMsrB8 | 283 | 147 |
| AtMsrB9 | 368 | 178 |
| total | 4010 | 438 |
| PtMsrA2.1 | 327 | 168 |
| PtMsrA2.2 | 298 | 130 |
| PtMsrA4.1 | 320 | 149 |
| PtMsrA4.2 | 252 | 152 |
| PtMsrA5 | 285 | 154 |
| PtMsrB1 | 258 | 160 |
| PtMsrB3.1 | 289 | 150 |
| PtMsrB3.2 | 366 | 144 |
| PtMsrB5 | 241 | 145 |
| total | 2636 | 382 |
| OsMsrA2.1 | 272 | 170 |
| OsMsrA2.2 | 266 | 109 |
| OsMsrA4 | 277 | 172 |
| OsMsrA5 | 464 | 201 |
| OsMsrB1 | 208 | 146 |
| OsMsrB3 | 248 | 141 |
| OsMsrB5 | 223 | 138 |
| total | 1958 | 409 |
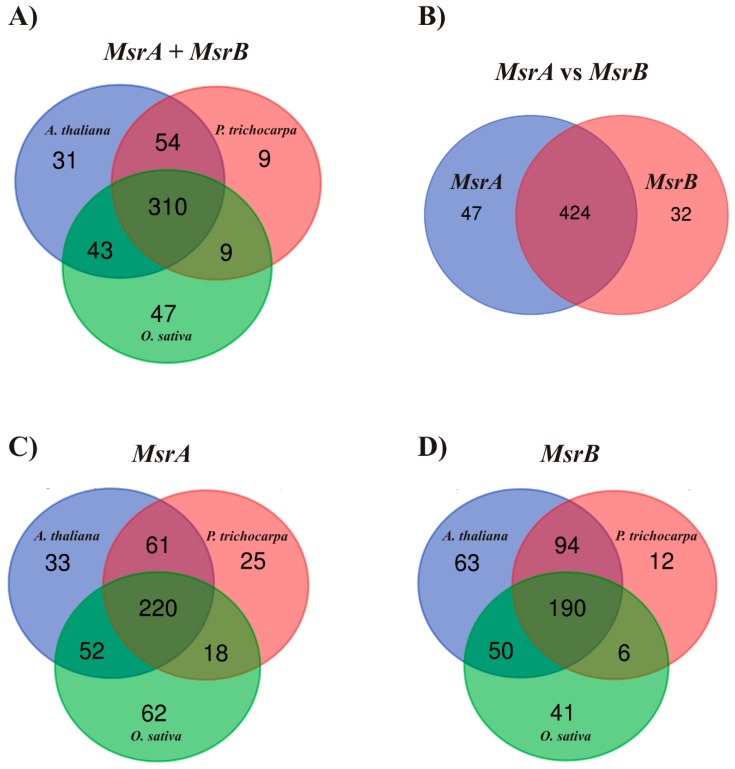
The TFs and REs also repeated among Msr isoforms and types; thus, in total, approximately 400 potential TFs and REs were predicted. A total of 310 TFs and REs overlapped among all 30 Msrs, as in Figure 1. A total of 220 common TFs and REs were detected for A-type Msrs and 190 common TFs and REs were detected for B-type Msrs, as in Figure 1. Arabidopsis and poplar shared the largest number of identical TFs and REs, Figure 1. Slightly fewer TFs and REs were shared between Arabidopsis and rice, while poplar and rice had the least common elements. Poplar was characterized by the lowest number of TFs and REs unique to the Msrs of this species. When comparing the 14 MsrAs and 16 MsrBs, a total of 424 TFs and REs were reported in both types of Msrs, and almost ten times fewer TFs and REs were unique to each type. The intersections were also calculated between each gene of the group of 30 TFs and REs that can potentially regulate most of the analyzed Msrs, as well as TFs and REs that are unique to each isoform of each type in each species, as in Table S2.
Figure 1.
Venn diagrams comparing the transcription factor binding sites and responsive elements predicted for 30 homological genes of methionine sulfoxide reductase type A (MsrA) and B (MsrB) originating from the Arabidopsis thaliana, Populus trichocarpa, and Oryza sativa genomes. The three-set Venn diagrams show species-dependent (A), species-dependent and A-type-dependent (C), and species-dependent and B-type-dependent (D) interactions. The two-set Venn diagram shows type-dependent intersections (B), irrespective of species.
2.2. The Common TFs and REs
Among all the identified TFs, 73 different MYB-type TFs, 39 different NAC-type TFs, 26 zinc finger TFs, 21 homeobox domain TFs, and 16 WRKY family TFs were identified. In general, the members of the MYB and NAC families were overrepresented. Thirty-five specific MYB TFs, Table S2C, and 29 NAC-containing domain proteins were predicted to bind to the Msr promoter regions of all tested species, as in Table S2C. Beyond the known TFs, several response elements and motifs are recognized as activated by plant hormones (JA, abscisic acid (ABA), gibberelins, ethylene), sugar, prolamines, nitrogen, iron, microRNAs, and environmental factors, such as light, heat, and cold. The majority of genes were reported to contain a core consensus sequence for binding TFs assigned to the regulation of different growth and physiological stages, such as plant morphogenesis, as well as a group of TFs that are functionally assigned as sensitive to environmental factors, such as light, heat, drought, osmotic stress, oxidative stress, and multiple abiotic stresses, regulating plant defenses and responses to biotic and abiotic stress. The two above groups are linked because of the detection of numerous TFs acting in response to auxin, salicylic acid, JA, ethylene, auxin, ABA, and cytokinins, which have important signaling roles in both plant growth and development, as well as in response to stress conditions.
A list of TFs that are predicted to bind the promoter region in over 75% of the analyzed genes was created, as shown in Table 2. The vast majority of TFs, including homeodomain proteins (WUSCHEL and GLABROUS1), homeobox proteins (HB32 and HB34), two repressors (BELLRINGER and AS1/AS2), MYB proteins (DIVARICATA 1, ODO1), members of the KANADI class, TESMIN/TSO1-like CXC 2 (TCX2), stomatal carpenter 1 (DOF5.7), G2-like family protein (GLK), SBF-1, protodermal factor 2 (PDF2), Yabby transcription factor CRABS CLAW, and late elongated hypocotyl I (LHY), were associated with the regulation of growth and development. Other TFs, including MYB96, NAC proteins (GmNAC30, GmNAC81), class I GATA factors, trihelix transcription factor GT-1, storekeeper (STK), and bZIP protein G-Box binding factor 1 (GBF1), were involved in the stress responses. Development-related and stress-related groups of TFs included two ethylene-responsive factors (RAP2.2 and RAP2.7) and jasmonate-responsive elements. Apart from the typical TFs, the majority of the analyzed Msrs also contained nodulin consensus sequences, GAAA motifs that are involved in pollen-specific transcriptional activation, motifs of different sugar-responsive genes, and prolamin boxes conserved in cereal seed storage protein gene promoters.
Table 2.
List of TFs predicted to interact with the promoter region of at least 75% of 30 homological genes of methionine sulfoxide reductase type A (MsrA) and B (MsrB) originating from the Arabidopsis thaliana (At), Populus trichocarpa (Pt), and Oryza sativa (Os) genomes. The core consensus sequence of each TF is underlined.
| TF | Consensus Sequence | Not Detected in the Promoter of |
|---|---|---|
| Protodermal factor 2 | aaattgcaaATTCtagg | OsMsrA2.2 |
| SBF-1 | ggatttagttTTAAaattt | OsMsrA2.2, OsMsrA5, OsMsrB3 |
| Homeodomain protein WUSCHEL | gacgccATTAacacgtggc | OsMsrA2.2, PtMsrA2.2, PtMsrB5 |
| Homeodomain GLABROUS 1 | cacgtgttAATGgcgtc | AtPMSR1, OsMsrA2.2, OsMsrB1 |
| TESMIN/TSO1-like CXC 2 | atcaatctctcattCAAAatctcattctctc | AtMsrB4, OsMsrA2.2, OsMsrA5, PtMsrA4 |
| KANADI | tcGAATaacaaat | AtMsrB3, OsMsrA2.2, OsMsrB3, OsMsrB5, PtMsrB3.1 |
| G2-like family protein | caatagATTCctt | OsMsrA2.2, OsMsrB1, OsMsrB3, OsMsrB5, PtMsrA4.2 |
| Stomatal Carpenter 1 (DOF5.7) | ttAGTTaacca | AtMsrB7, OsMsrA4, OsMsrB3, PtMsrA2.2, PtMsrA4.2 |
| DIVARICATA 1 | tgctcgtTATCttcagccc | AtMsrB6, OsMsrA2.1, OsMsrA2.2, OsMsrA5, OsMsrB1 |
| Trihelix transcription factor GT-1 | gaacatttgGTTAactaaa | AtMsrB4, OsMsrA2.2, OsMsrA5, OsMsrB3, OsMsrB5 |
| Transcriptional repressor BELLRINGER | tcacaaaATTAattcttct | AtMsrB2, AtMsrB5, AtPMSR4, OsMsrA2.2, OsMsrB1 |
| RAP2.2 | atATCTaacaa | AtMsrB1, AtMsrB8, OsMsrA2.2, OsMsrA5, OsMsrB1, OsMsrB3 |
| DNA-binding storekeeper protein-related transcriptional regulator (AT4G00250) | aaaattGATCcaaagct | AtMsrB2, AtMsrB6, AtMsrB7, AtPMSR3, OsMsrA2.2, PtMsrA4.1 |
| Class I GATA factors | aagatGATAaatgtgtg | AtMsrB6, AtPMSR2, OsMsrA2.2, OsMsrA5, OsMsrB1, PtMsrB5 |
| Homeobox protein 32 | tagatttaTTATttgtatc | AtPMSR1, OsMsrA2.2, OsMsrA4, OsMsrB3, OsMsrB5, PtMsrB1, PtMsrB5 |
| Myb-like protein of Petunia hybrid (ODO1) | ttaggattTAGTtttaaaatt | AtMsrB3, AtMsrB4, OsMsrA2.1, OsMsrA4, OsMsrA5, PtMsrA2.2, PtMsrB3.2 |
| Heterodimer of NAC-domain transcription factors GmNAC30 and GmNAC81 | TGTGttg | AtMsrB2, OsMsrA2.1, OsMsrA2.2, OsMsrB1, OsMsrB5, PtMsrA4.2, PtMsrB5 |
| Myb domain protein 96 MYBCOV1 | ttcgtatttAGTTaaccaaat | AtMsrB3, AtMsrB4, OsMsrA2.2, OsMsrA4, OsMsrA5, PtMsrB3.2, PtMsrB5 |
| Late elongated hypocotyl 1 | tgtgtaTATAttttgga | AtMsrA5, AtMsrB1, AtMsrB4, OsMsrA2.2, OsMsrA5, OsMsrB3, PtMsrB5 |
| Target of early activation tagged 1 (RAP2.7) | tgacATTAaaa | AtMsrB4, AtMsrB6, AtMsrB8, AtPMSR4, OsMsrA2.1, OsMsrA2.2, OsMsrB1 |
| Yabby transcription factor CRABS CLAW | aagaTGATaaatg | AtPMSR3, OsMsrA2.2, OsMsrA4, OsMsrA5, OsMsrB1, OsMsrB3, PtMsrB5 |
| AS1/AS2 repressor | gctTTGAct | AtMsrB1, AtPMSR1, OsMsrA2.2, OsMsrA4, OsMsrB1, OsMsrB3, PtMsrA4.2 |
| NAC with transmembrane motif 1-like 6 (NTL6/NTM1) | tttgttagatatatTAAGaaagg | AtMsrA5, AtMsrB2, OsMsrA2.2, OsMsrB3, OsMsrB5, PtMsrA4.2, PtMsrA5, PtMsrB3.2 |
| Storekeeper (STK), plant specific DNA binding protein | cccacaTATCcactatt | AtMsrB3, AtMsrB4, AtPMSR4, OsMsrA2.1, OsMsrA2.2, OsMsrA5, PtMsrA2.1, PtMsrB5 |
| Homeobox protein 34 | ataagactTAATgaaaaca | AtMsrA5, AtMsrB1, AtMsrB7, AtPMSR4, OsMsrA2.2, OsMsrA5, OsMsrB1, OsMsrB3 |
| bZIP protein G-Box binding factor 1 | tttcacCACGtcactgctt | AtMsrA5, AtMsrB4, AtMsrB4, AtPMSR1, OsMsrA5, OsMsrB5, PtMsrA5, PtMsrB3.1, PtMsrB5 |
2.3. Type-Specific and Species-Specific TFs and REs
Forty-seven TFs and REs that were specific to MsrA and 32 TFs and REs specific to MsrB were recognized, Table S2A. Both types of Msr contained representatives of the main plant TF families; however, 12 TFs and REs responsive to ethylene were uniquely found in the A-type group. The list of species-specific TFs and REs is given in Table S2B. Species-specific TFs and Res that are predicted for rice included 17 ethylene-responsive TFs, five TCP TFs, three dehydration-responsive elements, and no MYB or DOF family members. Species-specific TFs and REs that were predicted for Arabidopsis included nine NAC TFs and three TFs in the WRKY family. Only nine species-specific TFs were predicted for poplar; these TFs included three zinc finger proteins in the DOF family, two MYB TFs, and no TFs from the NAC and WRKY families. Both type-specific and species-specific groups of TFs and REs were relatively numerous and varied; thus, we searched for more pronounced differences at the isoform-specific level.
2.4. Isoform-Specific TFs and REs
By developing the intersections of all 30 genes (Table S2C), unique TFs and REs were found for each gene, as in Table 3. It was predicted that specific members of MYB, NAC, DOF, and WRKY families that are different from these listed in Table 2 might regulate the Msr genes. Interestingly, four different ethylene-responsive TFs were found to interact singularly with OsMsrA5. AtPMSR3 was predicted to be cooperatively regulated by two hormones ethylene and JA. The expression of OsMsrA2.2 was reported to be iron-dependent. OsMsrA4 was described as containing unique redox-responsive elements.
Table 3.
List and number (nr) of TFs and REs predicted to interact with the promoter region of one specific MsrA or MsrB gene originating from the Arabidopsis thaliana (At), Populus trichocarpa (Pt), and Oryza sativa (Os) genomes. The predictions were made using MatInspector software, and intersections of the lists of elements were calculated while using the Venn web tool.
| Gene Name | Nr | TFs and REs |
|---|---|---|
| AtPMSR1 | 1 | NAC domain containing protein 3 (NAC3) |
| AtPMSR3 | 3 | WRKY transcription factor 8 (WRKY8), R2R3-type myb-like transcription factor IIG-type binding site (MYB0), Cooperatively regulated by ethylene and jasmonate 1 (CEJ1) |
| AtPMSR4 | 2 | Myb domain protein 116 (MYB116), RY and Sph motifs conserved in seed-specific promoters |
| AtMsrA5 | 1 | Heat stress transcription factor C-1 (HSFC1) |
| AtMsrB1 | 1 | NAC domain containing protein 45 (NAC045) |
| AtMsrB2 | 4 | Transcription factor TGA3 (TGA3), Arabidopsis NAC domain containing protein 81 (NAC081), ATAF2 WRKY transcription factor 55 (WRKY55) |
| AtMsrB6 | 1 | Indeterminate (ID)-domain 11 (IDD11) |
| AtMsrB7 | 2 | REVEILLE 5 (RVE5), Dof zinc finger protein DOF4.7 AT4G38000 (DOF4.7) |
| AtMsrB8 | 2 | Indeterminate (ID)-domain 5 protein RAVEN (IDD5), ABA-responsive element binding protein 3 (AREB3) |
| AtMsrB9 | 1 | Phytochrome-interacting fator 5 (PIL6) |
| PtMsrA2.1 | 2 | Dof zinc finger protein DOF5.1 AT5G02460 (DOF5.1), Dof zinc finger protein DOF2.2 AT2G28810 (DOF2.2) |
| PtMsrA4.1 | 1 | C-repeat-binding factor 3 (DREB1A) |
| PtMsrA4.2 | 1 | Myb domain protein 118 Plant Growth Activator 37 (MYB118) |
| PtMsrA5 | 2 | B3 domain-containing transcription factor NGA4 (NGA4), Myb domain protein 111 (MYB111) |
| PtMsrB3.1 | 2 | C-repeat-binding factor 2 (DREB1C,) Dof zinc finger protein DOF5.8 AT5G66940 (DOF5.8) |
| OsMsrA2.2 | 2 | Iron-dependent regulatory sequence, TCP class II transcription factor (BRC1) |
| OsMsrA4 | 3 | NAC domain containing protein 96 (NAC096), Ethylene-responsive transcription factor 105 (ERF105), Redox responsive transcription factor 1 (RRTF1) |
| OsMsrA5 | 8 | Ethylene-responsive transcription factor 2 (ERF2), Rice bHLH protein (bHLH39), Ethylene-responsive transcription factor 10 (ERF10), Nodulin consensus sequence 3, Ethylene-responsive transcription factor 115 (ERF115), C-repeat-binding factor 2 (DREB1B), Ethylene-responsive transcription factor 5 (ERF5), TCP domain protein 21 AT5G08330 (TCP21) |
| OsMsrB1 | 4 | Dehydration-responsive element-binding protein 2C (DREB2C), LIM domain protein binding to a PAL-box like sequence (WLIM1), E2F transcription factor 3 (E2F3), BBES1/BZR1-like protein 2 AT4G36780 (BEH2) |
| OsMsrB3 | 1 | Transcription factor TCP15 AT1G69690 (TCP15) |
| OsMsrB5 | 1 | Heat stress transcription factor A-1b (HSF3) |
2.5. GO Classification
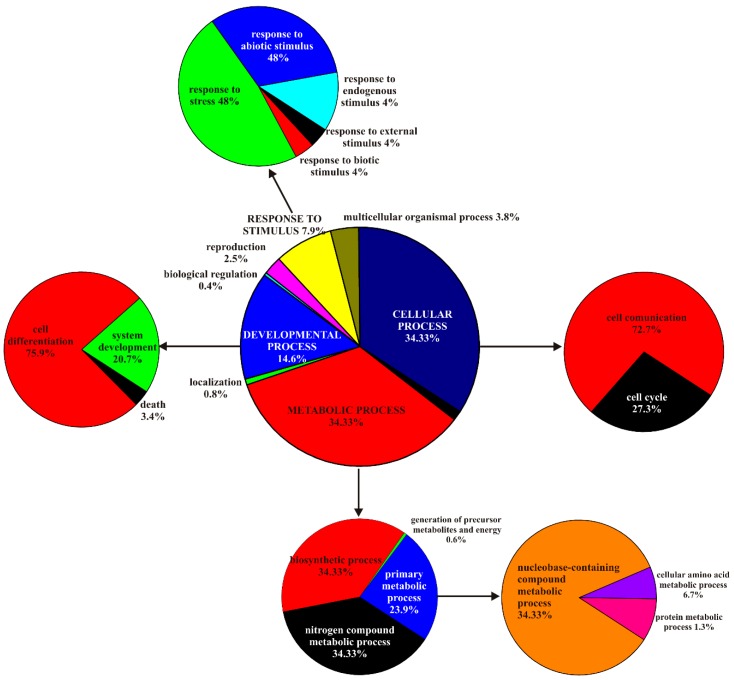
To establish the participation of all detected TFs in specific biological processes, the list of gene IDs of transcription factors was functionally analyzed based on Gene Ontology (GO) terms using the protein annotation through an evolutionary relationship (PANTHER) classification system [39]. Among these TFs, 347 genes were annotated with GOs and 251 hits were represented in the biological process category, as in Figure 2. Two categories were equally overrepresented: cellular process and metabolic process. The term developmental processes comprised 14.6% of the analyzed genes, and the term response to stimulus comprised 7.9% of the analyzed genes. The four main categories were analyzed in greater detail. The cellular process category contained members of cell communication and cell cycle, which represented 72.7% and 27.3% of all hits, respectively. Within the metabolic process category, two terms, biosynthetic process and nitrogen compound metabolic process, were equally (37.8%) overrepresented. The primary metabolic process term constituted 23.9% of the hits and it represented the nucleobase-containing compound metabolic process. The developmental process terms were mainly cell differentiation and system development. Nearly half of the terms in the response to stimulus category were composed of the response to stress members. The response to abiotic, biotic, endogenous, and external stimuli terms complemented this category.
Figure 2.
PANTHER biological process analysis based on gene ontology terms. Functional classification of genes encoding TFs predicted to interact with the promoter regions of MsrA and MsrB originating from the Arabidopsis thaliana, Populus trichocarpa, and Oryza sativa genomes.
3. Discussion
The possible presence of up to five coding transcripts and up to 201 putative TFs and REs in a single Msr gene makes the regulation of Msr gene expression in plants highly complex. Irrespective of species-dependent or Msr type-dependent analyses, we demonstrated that up to ten times more common than unique TFs and REs might bind to the Msr promoter regions. Thus, the TFs and REs comprising the group of common elements appear to contain the most likely candidates regulating the expression of Msr genes.
3.1. Putative Involvement of Msrs in Regulation of Plant Growth and Development
Among the TFs and REs that were predicted to bind the majority of analyzed Msr genes, the bulk were assigned to the regulation of plant growth and development processes, suggesting the accompanying expression of Msrs during these processes. The majority of the experimentally proven functions of particular TFs comes from Arabidopsis studies. In summary, 11 of the TFs (PDF2, SBF, WUSCHEL, TESMIN/TSO1-like CXC, KANADI1, GLK, DOF5.7, LHY, repressor AS1/AS2, GBF1, STK) that were predicted to bind the promoter regions of most of the analyzed Msrs act in plant growth and regulation. Studies have demonstrated that the above TFs are involved in shoot development [40], lateral root formation and development [41], embryo development [40], leaf development [42,43], stomata patterning [44], chloroplast development [45], the development of reproductive tissues [46], early embryogenesis [47], seedling development [48], senescence [49], the regulation of circadian rhythms [50], and cell-cycle-dependent transcription-enabling plant growth [47]. Interestingly, seven of the TFs that are listed in Table 2 (WUSCHEL, DIVARICITA, BELLRINGER, HB32 and HB34, RAP2.7, CRABS CLAW, ODO1) control the flowering process by regulating floral development [51,52], fragrance [53], and nectary development [54] and the initiation and repression of flowering [47,49,55,56]. In this context, it can be hypothesized that Msrs are activated by specific TFs and they mediate this important developmental transition in plants. This hypothesis is supported by the fact that the biological process analysis that is based on GO terms confirmed that the predicted TFs are involved in developmental processes that include cell differentiation and the system development subcategory, which consists of plant organ development, root system development, and shoot system development child terms in the GO classification system; furthermore, this hypothesis is supported by literature data that show that PMSR2 partially regulates Arabidopsis development [26].
3.2. Predictions vs. Experimental Data
3.2.1. The Involvement of MYB and NAC Family TFs in the Regulation of Msr Gene Expression
Both EFM [57] and MYB12 [58] mediate plant responses to light stimulus, and they both were found to bind promoters of MsrA in Arabidopsis. It is possible that the two MYB TFs are involved in changes in PMSR activity detected during light and dark periods [26]. Tissue-specific gene expression is regulated by TFs [59]. Similarly, MYB factors might mediate the organ-specific expression of Msrs. MYB77 was predicted to bind to the MsrA2, MsrB4, and MsrB9 promoters, whereas MYB93 was predicted to bind to the MsrB8 promoter. Interestingly, MYB77 [60] and MYB93 [61] are specific to lateral root growth and development, and the expression of MsrA2, MsrB4, MsrB9, and MsrB8 was demonstrated to be specific to roots [7,11,13].
The members of the MYB and NAC families can be mediators in both the increased synthesis of AtMSRB9 proteins during dehydration stress [15] and the upregulation of PMSRA4 under oxidative stress conditions [13], because the PMSR4 promoter contains 34 binding sites for MYB-type TFs and 17 binding sites for NAC-type TFs. Similarly, the promoter of AtMsrB9 contains 42 binding sites for MYB-type TFs, including binding sites for MYB49, MYB52, and MYB96, which act under water stress [62,63,64], and 35 binding sites for NAC-type TFs, including dehydration-related NAC096 [65]. The NAC-type TFs IDEF1/IDEF2, which are similar to MYB58 [66], are linked to iron deficiency in plants [67]. Therefore, Msrs might be susceptible players in the regulation of redox-sensitive elements. This speculation is likely true, because OsMSRB5 was shown in rice seedlings to be involved in defenses against copper toxicity [22], and cadmium upregulated the entire Msr system in Arabidopsis [21]; in addition, MYB62 plays a role in the regulation of phosphate starvation responses [68].
3.2.2. Hormone-Dependent Regulation of Msr Gene Expression
Experimental data showed that Msr expression in Arabidopsis is ABA- [12] and JA-dependent [25]. JA signaling is important in the responses to biotic and abiotic stresses and in plant development [69]. The JA response element was in 29 promoters of the 30 analyzed Msr genes. The exception with no JA response element was OsMsrA2.2, which had a promoter containing elements that were uniquely responsive to ABA and ethylene. AtPMSR3 seems to be especially regulated by JA, because AtPMSR3 was the only Msr gene containing the responsive element that is cooperatively regulated by ethylene and jasmonate. The prediction of abscisic acid-responsive element-binding factor 2 in promoters of OsMsrA4, OsMsrB1, and AtPMSR4 is consistent with experimental data showing the ABA-induced expression of OsMsrA4.1, OsMsrB1.1 [19], and AtMSRA4 [12]. Moreover, salt stress and NO in an ABA-dependent manner increased the expression of OsMSRA4, OsMSRA5, OsMSRB1.1, OsMSRB3, and OsMSRB5, whereas OsMSRA2.1 was modulated independent of ABA [20]. MYB44, which was demonstrated to confer ABA-dependent resistance to abiotic stresses [70], was predicted to interact with the MsrB5 homolog in poplar and rice. The effect of ethylene on Msr expression has not been experimentally tested in Arabidopsis, poplar, or rice. Our analyses revealed that the ethylene-responsive elements are abundant and characteristic of A-type Msrs. Ethylene strongly induced tomato SlMSRA1 [71], indicating that other isoforms of Msrs might also be upregulated through ethylene-mediated responses. In particular, four additional ethylene-responsive factors that were unique to the OsMsrA5 isoform were found. Therefore, the ethylene-dependent regulation of Msr expression is worth consideration. TFs classified as the GA-regulated myb gene from barley were identified in 21 promoters of the 30 analyzed genes, and GA-inducible regulatory elements were identified in 11 promoters, including five isoforms different from the group of 21. There are no experimental data on GA-dependent Msr expression in the literature; however, cross-talk between hormones is well known. For example, MYB96, which is the TF interacting with the majority of the promoters of analyzed Msr genes, mediates the cross-talk between ABA and JA [62]. Cis-regulatory elements that are involved in salicylic acid induction of secretion-related genes via NPR1 were detected in 17 different Msr genes, including types A2-A5. In tomato, SlMsrA2, SlMsrA3, and SlMsrA5 transcripts increased upon salicylic acid treatment, whereas SlMsrA4 transcripts decreased [71], underlying the fact that the expression of Msr genes is hormone-sensitive. The DOF family is crucial in response to salicylic acid [72]. DOF5.7 commonly regulates Msrs. Moreover, AtMsrB7 is the only Msr-containing DOF4.7 binding site, and DOF5.1 and DOF2.2 can singularly bind to the PtMsrA2.1 promoter, while DOF5.8 can bind to the PtMsrB3.1 promoter. Auxin response elements were recognized in the promoters of 18 analyzed Msr genes. Auxins might indirectly affect Msr expression, possibly through reactions that are linked to MYB TFs, as MYB12 [58], MYB77 [60], and MYB88 [73] modulate the response to auxin, and therefore growth and developmental processes.
3.2.3. Seed Traits Control
Among the Arabidopsis Msrs, two genes, i.e., AtMsrB2 and AtMsrB6, have been identified as organ-specific genes in seeds [7]. The promoter regions of both genes contained binding sites for WUSCHEL, NAC2, and GLABBOROUS TFs, which were demonstrated to be involved in seed morphogenesis [74,75] and seedling development [48], respectively. The AtMsrB6 promoter also contained binding sites that are specific to MYB56, which regulates seed size [76], and MYB107, which affects seed coat suberin assembly [77]. The AtMsrB2 promoter included binding sites for MYB33 and MYB65, which promote protein storage reserve utilization during seed germination [78], and it uniquely contained a binding site for NAC081, which is required for normal seed development and morphology [75]. The above information again supports the compatibility between the in silico predictions and experimental evidence. We speculate that, based on the large group of predicted TFs, the Msr system is also important for other seed-associated traits, such as desiccation tolerance, seed development and dormancy, germination, and responses to oxidative stress. This speculation might be supported by experimental results in which the accumulation of MsrB was related to desiccation tolerance in seeds [79] and the fact that MSRB5 was upregulated after oxidative stress in response to the sulfur nutrition of Arabidopsis seeds [80]. A-type Msr expression was also reported during the morphogenesis and maturation of Acer platanoides seeds [81]. Interestingly, FUSCA3, which can bind to the AtPMSR4 promoter, acts as a major regulator of seed maturation in Arabidopsis [82]. Thus, MsrA4 might impact seed development and seed aging by regulating redox homeostasis, because the rice MsrA4 promoter can only interact with redox-responsive transcription factor 1. Experimental data confirmed that the Msr system is involved in seed longevity [83].
3.3. Perspectives
The sugar-dependent regulation of Msr expression definitely requires investigation in subsequent studies, because no data on this subject are available in the literature. The predicted involvement of Msrs in the regulation of plant growth and development, particularly during flowering and seed production, requires experimental confirmation. In the future, relatively simple experimental research will be required to understand the effects of plant hormones on Msr expression at the mRNA and protein levels. Among all of the predicted TFs, MYBs and NACs seem to be the most influential factors in the regulation of Msr gene expression, and some speculations regarding the perspectives of possible Msr functions can be proposed. It was experimentally proven that MYBs are involved in the regulation of many biological processes and stress responses, such as secondary wall biosynthesis [84] and thickening [85], stamen maturation [86], programmed cell death [78], microsporogenesis [87], wounding [88], trichome formation [89], and xylem development [90], which have not yet been linked to the corresponding Msr genes and proteins; these processes and responses are worth further attention. Our meta-analysis was definitely limited by the fact that not all of the plant genomes are sequenced, and the isoforms of Msrs genes are not characterized fully enough to study their promoter regions. However, this study provides a useful reference for researchers who are interested in the Msr system. The selection of homologous genes was planned to avoid species-specific TFs and REs. Data in the literature shows that, among the hundreds of TFs and Rest that are currently identified, 15 TFs and four REs found in the promoter region of a specific Msr gene can affect its expression in a manner that is specific to the TF or RE. The fact that our predictions coincided with experimental data makes our results more reliable. However, further experimental evidence is needed to confirm that our findings are linked to the regulation of Msr gene expression and the new putative roles of Msrs in plants.
4. Materials and Methods
4.1. Material
A total of 30 MsrA and MsrB gene sequences were analyzed. Five isoforms of MsrA (PMSR1, PMSR2, PMSR3, PMSR4, PMsrA5) and nine isoforms of MsrB (MsrB1, PMsrB2, MsrB3, MsrB4, MsrB5, MsrB6, MsrB7, MsrB8, MsrB9) genes originating from the Arabidopsis thaliana genome were analyzed. Gene sequences were derived from the Arabidopsis genome using the TAIR 10 database (https://www.arabidopsis.org/). Table S1A provides additional information, including Arabidopsis Msr descriptions. Five isoforms of MsrA (MsrA2.1, MsrA2.2, MsrA4.1, MsrA4.2, MsrA5) and four isoforms of MsrB (MsrB1, MsrB3.1, MsrB3.2, MsrB5) genes originating from the Populus trichocarpa genome were analyzed. Gene sequences were derived from the poplar genome using the Poptr 2.0 database (https://phytozome.jgi.doe.gov/pz/portal.html#info?alias=Org_Ptrichocarpa). Table S1B provides additional information, including poplar Msr descriptions. Four isoforms of MsrA (MsrA2.1, MsrA2.2, MsrA4, MsrA5) and three isoforms of MsrB (MsrBl, MsrB3, MsrB5) genes originating from the Oryza sativa genome were analyzed. Gene sequences were derived from the rice genome while using the MSU release 7 database (http://www.plantedb.org/OsGDB/).
4.2. The Search for Transcription Factor Binding Sites and Responsive Elements
The search for TF binding sites and REs was performed for 30 Msr genes (Table S1) while using Matlnspector software [40]. For each gene, all of the functional promoters were extracted directly from the ElDorado genome database (https://www.genomatix.de/online_help/help_eldorado/introduction.html). The location and length of each promoter were accompanied by the number of coding transcripts, and their accession numbers were specified; these numbers are given for each analyzed gene in Table S1. The promoter sequences were searched for TFBS using a comparison of gene identification numbers (IDs) against predefined IUPAC library plant TF sites based on the Plant cis-acting regulatory DNA elements (PLACE) database [41]. The Matlnspector library represents the largest library available for TF binding site searches.
4.3. GO-Based Functional Classification
The PANTHER classification system (http://www.pantherdb.org/) was used to assess the involvement of the genes of the predicted TFs annotated with GO terms in the biological process and molecular function categories [42]. The gene list analysis was performed using a list of gene identification numbers (ID) corresponding to the predicted TFs, Table S3. All of the IDs were derived from the NCBI gene database (https://www.ncbi.nlm.nih.gov/), specifically from the Arabidopsis genome, which was queried by the search terms.
5. Conclusions
Overall, the antioxidant role and the role in stress-related responses of different Msr types and isoforms have been experimentally proven, and therefore have high confidence. Here, new putative roles and regulatory mechanisms were predicted for Msrs in silico. Based on our research, we suggest that Msrs are involved in the regulation of many plant growth and development processes, including flowering. Among the studied phytohormones, Msrs are very sensitive to JA and ethylene, and ethylene principally regulates the A-type Msrs. Msrs are also under the control of gibberellins and auxins. Therefore, Msrs might mediate important hormone-dependent developmental transitions in plants and stress-related responses. Mainly MYB and NAC TFs control Msr expression. In particular, MYBs might determine the organ-specific expression of Msrs. Presumably, the Msr system is also significant for seed-associated traits. The predicted and described TFs might individually bind to Msr promoters, while considering the cellular and tissue context, developmental and physiological stage, environmental conditions, and pool of accessible TFs required for the selection of a particular TSS. Thus, the Msr system might participate in important biological processes and stress responses in plants, exhibiting a protective role.
Abbreviations
| ID | identification number |
| GO | gene ontology |
| JA | jasmonic acid |
| MetSO | methionine sulfoxide |
| Msr | methionine sulfoxide reductase |
| MYB | v-myb avian myeloblastosis viral oncogene homolog |
| NAC | no apical meristem, Arabidopsis transcription activation factor and cup-shaped cotyledon |
| NCBI | National Center for Biotechnology Information |
| REs | Responsive elements |
| TFs | Transcription factors |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/6/1309/s1.
Author Contributions
E.M.K. designed and run the meta-analysis and prepared figures and tables. E.S. wrote the Introduction section, E.M.K. wrote other manuscript sections. All authors read and approved the final manuscript.
Funding
This research was funded by the National Center of Science (Poland) grant No. 2015/18/E/NZ9/00729.
Conflicts of Interest
None of the authors have any competing interests.
References
- 1.Brot N., Weissbach L., Werth J., Weissbach H. Enzymatic reduction of protein-bound methionine sulfoxide. Proc. Natl. Acad. Sci. USA. 1981;78:2155–2158. doi: 10.1073/pnas.78.4.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimaud R., Ezraty B., Mitchell K.J., Lafitte D., Briand C., Derrick P.J., Barras F. Repair of oxidized proteins. Identification of a new methionine sulfoxide reductase. J. Biol. Chem. 2001;276:48915–48920. doi: 10.1074/jbc.M105509200. [DOI] [PubMed] [Google Scholar]
- 3.Lin Z., Johnson L.C., Weissbach H., Brot N., Lively M.O., Lowther T.W. Free methionine-(R)-sulfoxide reductase from Escherichia coli reveals a new GAF domain function. Proc. Natl. Acad. Sci. USA. 2007;104:9597–9602. doi: 10.1073/pnas.0703774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rousseau F., Vandekerckhove J., Gevaert K. Redox proteomics of protein-bound methionine oxidation. Mol. Cell. Proteom. 2011;10:M110.006866. doi: 10.1074/mcp.M110.006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarrago L., Laugier E., Rey P. Protein-repairing methionine sulfoxide reductases in photosynthetic organisms: Gene organization, reduction mechanisms, and physiological roles. Mol. Plant. 2009;2:202–217. doi: 10.1093/mp/ssn067. [DOI] [PubMed] [Google Scholar]
- 6.Moskovitz J. Methionine sulfoxide reductases: Ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim. Biophys. Acta. 2005;1703:213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Rouhier N., Vieira Dos Santos C., Tarrago L., Rey P. Plant methionine sulfoxide reductase A and B multigenic families. Photosynth. Res. 2006;89:247–262. doi: 10.1007/s11120-006-9097-1. [DOI] [PubMed] [Google Scholar]
- 8.Rey P., Tarrago L. Physiological Roles of Plant Methionine Sulfoxide Reductases in Redox Homeostasis and Signaling. Antioxidants. 2018;7:114. doi: 10.3390/antiox7090114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boschi-Muller S., Olry A., Antoine M., Branlant G. The enzymology and biochemistry of methionine sulfoxide reductases. Biochim. Biophys. Acta. 2005;1703:231–238. doi: 10.1016/j.bbapap.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Jacques S., Ghesquière B., De Bock P.J., Demol H., Wahni K., Willems P., Messens J., Van Breusegem F., Gevaert K. Protein Methionine Sulfoxide Dynamics in Arabidopsis thaliana under Oxidative Stress. Mol. Cell. Proteom. 2015;14:1217–1229. doi: 10.1074/mcp.M114.043729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C.W., Lee S.H., Chieh P.S., Lin C.S., Wang Y.C., Chan M.T. Arabidopsis root-abundant cytosolic methionine sulfoxide reductase B genes MsrB7 and MsrB8 are involved in tolerance to oxidative stress. Plant Cell Physiol. 2012;53:1707–1719. doi: 10.1093/pcp/pcs114. [DOI] [PubMed] [Google Scholar]
- 12.Oh J.E., Hong S.W., Lee Y., Koh E.K., Kim K., Seo Y.W., Chung N., Jeong M., Jang C.S., Lee B., et al. Modulation of gene expressions and enzyme activities of methionine sulfoxide reductases by cold, ABA or high salt treatments in Arabidopsis. Plant Sci. 2005;169:1030–1036. doi: 10.1016/j.plantsci.2005.05.033. [DOI] [Google Scholar]
- 13.Romero H.M., Pell E.J., Tien M. Expression profile analysis and biochemical properties of the peptide methionine sulfoxide reductase A (PMSRA) gene family in Arabidopsis. Plant Sci. 2006;170:705–714. doi: 10.1016/j.plantsci.2005.10.005. [DOI] [Google Scholar]
- 14.Danon A., Coll N.S., Apel K. Cryptochrome-1-dependent execution of programmed cell death induced by singlet oxygen in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2006;103:17036–17041. doi: 10.1073/pnas.0608139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigo M.J., Moskovitz J., Salamini F., Bartels D. Reverse genetic approaches in plants and yeast suggest a role for novel, evolutionarily conserved, selenoprotein-related genes in oxidative stress defense. Mol. Genet. Genom. 2002;267:613–621. doi: 10.1007/s00438-002-0692-3. [DOI] [PubMed] [Google Scholar]
- 16.Roy S., Nandi A.K. Arabidopsis thaliana methionine sulfoxide reductase B8 influences stress-induced cell death and effector-triggered immunity. Plant Mol. Biol. 2017;93:109–120. doi: 10.1007/s11103-016-0550-z. [DOI] [PubMed] [Google Scholar]
- 17.Kwon S.J., Kwon S.I., Bae M.S., Cho E.J., Park O.K. Role of the methionine sulfoxide reductase MsrB3 in cold acclimation in Arabidopsis. Plant Cell Physiol. 2007;48:1713–1723. doi: 10.1093/pcp/pcm143. [DOI] [PubMed] [Google Scholar]
- 18.Vieira Dos Santos C., Cuiné S., Rouhier N., Rey P. The Arabidopsis plastidic methionine sulfoxide reductase B proteins. Sequence and activity characteristics, comparison of the expression with plastidic methionine sulfoxide reductase A, and induction by photooxidative stress. Plant Physiol. 2005;138:909–922. doi: 10.1104/pp.105.062430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo X., Wu Y., Wang Y., Chen Y., Chu C. OsMSRA4.1 and OsMSRB1.1, two rice plastidial methionine sulfoxide reductases, are involved in abiotic stress responses. Planta. 2009;230:227–238. doi: 10.1007/s00425-009-0934-2. [DOI] [PubMed] [Google Scholar]
- 20.Hsu Y.T., Lee T.M. Abscisic acid-dependent nitric oxide pathway and abscisic acid-independent nitric oxide routes differently modulate NaCl stress induction of the gene expression of methionine sulfoxide reductase A and B in rice roots. J. Plant Physiol. 2018;231:374–382. doi: 10.1016/j.jplph.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Méndez A.A., Pena L.B., Benavides M.P., Gallego S.M. Priming with NO controls redox state and prevents cadmium-induced general up-regulation of methionine sulfoxide reductase gene family in Arabidopsis. Biochimie. 2016;131:128–136. doi: 10.1016/j.biochi.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Xiao T., Memgmeng M., Wang G., Quian M., Chen Y., Zheng L., Zhang H., Hu Z., Shen Z., Xia Y. A methionine-R-sulfoxide reductase, OsMSRB5, is required for rice defense against copper toxicity. Environ. Exp. Bot. 2018;153:45–53. doi: 10.1016/j.envexpbot.2018.04.006. [DOI] [Google Scholar]
- 23.Sadanandom A., Poghosyan Z., Faibairn D.J., Murphy D.J. Differential regulation of plastidial and cytosolic izoforms of peptide methionine sulfoxide reductase in Arabidopsis. Plant Physiol. 2000;123:255–264. doi: 10.1104/pp.123.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begara-Morales J.C., Sánchez-Calvo B., Luque F., Leyva-Pérez M.O., Leterrier M., Corpas F.J., Barroso J.B. Differential transcriptomic analysis by RNA-Seq of GSNO-responsive genes between Arabidopsis roots and leaves. Plant Cell Physiol. 2014;55:1080–1095. doi: 10.1093/pcp/pcu044. [DOI] [PubMed] [Google Scholar]
- 25.Mata-Pérez C., Sánchez-Calvo B., Begara-Morales J.C., Luque F., Jiménez-Ruiz J., Padilla M.N., Fierro-Risco J., Valderrama R., Fernández-Ocaña A., Corpas F.J., et al. Transcriptomic profiling of linolenic acid-responsive genes in ROS signaling from RNA-seq data in Arabidopsis. Front. Plant Sci. 2015;6:122. doi: 10.3389/fpls.2015.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bechtold U., Murphy D.J., Mullineaux P.M. Arabidopsis peptide methionine sulfoxide reductase2 prevents cellular oxidative damage in long nights. Plant Cell. 2004;16:908–919. doi: 10.1105/tpc.015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahmuradov I.A., Umarov R.K., Solovyev V.V. TSSPlant: A new tool for prediction of plant Pol II promoters. Nucleic Acids Res. 2017;45:e65. doi: 10.1093/nar/gkw1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porto M.S., Pinheiro M.P., Batista V.G., dos Santos R.C., Filho Pde A., de Lima L.M. Plant promoters: An approach of structure and function. Mol. Biotechnol. 2014;56:38–49. doi: 10.1007/s12033-013-9713-1. [DOI] [PubMed] [Google Scholar]
- 29.Franco-Zorrilla J.M., López-Vidriero I., Carrasco J.L., Godoy M., Vera P., Solano R. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc. Natl. Acad. Sci. USA. 2014;111:2367–2372. doi: 10.1073/pnas.1316278111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan S.A., Li M.Z., Wang S.M., Yin H.J. Revisiting the Role of Plant Transcription Factors in the Battle against Abiotic Stress. Int. J. Mol. Sci. 2018;19:1634. doi: 10.3390/ijms19061634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambawat S., Sharma P., Yadav N.R., Yadav R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants. 2013;19:307–321. doi: 10.1007/s12298-013-0179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuruzzaman M., Sharoni A.M., Kikuchi S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 2013;4:248. doi: 10.3389/fmicb.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Clercq I., Vermeirssen V., Van Aken O., Vandepoele K., Murcha M.W., Law S.R., Inzé A., Ng S., Ivanova A., Rombaut D., et al. The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell. 2013;25:3472–3490. doi: 10.1105/tpc.113.117168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niu F., Wang B., Wu F., Yan J., Li L., Wang C., Wang Y., Yang B., Jiang Y.Q. Canola (Brassica napus L.) NAC103 transcription factor gene is a novel player inducing reactive oxygen species accumulation and cell death in plants. Biochem. Biophys. Res. Commun. 2014;454:30–35. doi: 10.1016/j.bbrc.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 35.Yan J., Tong T., Li X., Chen Q., Dai M., Niu F., Yang M., Deyholos M.K., Yang B., Jiang Y.Q. A Novel NAC-Type Transcription Factor, NAC87, from Oilseed Rape Modulates Reactive Oxygen Species Accumulation and Cell Death. Plant Cell Physiol. 2018;59:290–303. doi: 10.1093/pcp/pcx184. [DOI] [PubMed] [Google Scholar]
- 36.Chou M.L., Liao W.Y., Wei W.C., Li A.Y., Chu C.Y., Wu C.L., Liu C.L., Fu T.H., Lin L.F. The Direct Involvement of Dark-Induced Tic55 Protein in Chlorophyll Catabolism and Its Indirect Role in the MYB108-NAC Signaling Pathway during Leaf Senescence in Arabidopsis thaliana. Int. J. Mol. Sci. 2018;19:1854. doi: 10.3390/ijms19071854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cartharius K., Frech K., Grote K., Klocke B., Haltmeier M., Klingenhoff A., Frisch M., Bayerlein M., Werner T. MatInspector and beyond: Promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21:2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- 38.Higo K., Ugawa Y., Iwamoto M., Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mi H., Muruganujan A., Casagrande J.T., Thomas P.D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa E., Yamada Y., Sezaki N., Kosaka S., Kondo H., Kamata N., Abe M., Komeda Y., Takahashi T. ATML1 and PDF2 Play a Redundant and Essential Role in Arabidopsis Embryo Development. Plant Cell Physiol. 2015;56:1183–1192. doi: 10.1093/pcp/pcv045. [DOI] [PubMed] [Google Scholar]
- 41.Hawker N.P., Bowman J.L. Roles for Class III HD-Zip and KANADI genes in Arabidopsis root development. Plant Physiol. 2004;135:2261–2270. doi: 10.1104/pp.104.040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curaba J., Herzog M., Vachon G. GeBP, the first member of a new gene family in Arabidopsis, encodes a nuclear protein with DNA-binding activity and is regulated by KNAT1. Plant J. 2003;33:305–317. doi: 10.1046/j.1365-313X.2003.01622.x. [DOI] [PubMed] [Google Scholar]
- 43.Fu Y., Xu L., Xu B., Yang L., Ling Q., Wang H., Huang H. Genetic interactions between leaf polarity-controlling genes and ASYMMETRIC LEAVES1 and 2 in Arabidopsis leaf patterning. Plant Cell Physiol. 2007;48:724–735. doi: 10.1093/pcp/pcm040. [DOI] [PubMed] [Google Scholar]
- 44.Castorina G., Fox S., Tonelli C., Galbiati M., Conti L. A novel role for STOMATAL CARPENTER 1 in stomata patterning. BMC Plant Biol. 2016;16:172. doi: 10.1186/s12870-016-0851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitter D.W., Martin D.J., Copley M.J., Scotland R.W., Langdale J.A. GLK gene pairs regulate chloroplast development in diverse plant species. Plant J. 2002;31:713–727. doi: 10.1046/j.1365-313X.2002.01390.x. [DOI] [PubMed] [Google Scholar]
- 46.Andersen S.U., Algreen-Petersen R.G., Hoedl M., Jurkiewicz A., Cvitanich C., Braunschweig U., Schauser L., Oh S.A., Twell D., Jensen E.Ø. The conserved cysteine-rich domain of a tesmin/TSO1-like protein binds zinc in vitro and TSO1 is required for both male and female fertility in Arabidopsis thaliana. J. Exp. Bot. 2007;58:3657–3670. doi: 10.1093/jxb/erm215. [DOI] [PubMed] [Google Scholar]
- 47.Ashe M., de Bruin R.A., Kalashnikova T., McDonald W.H., Yates J.R., 3rd, Wittenberg C. The SBF- and MBF-associated protein Msa1 is required for proper timing of G1-specific transcription in Saccharomyces cerevisiae. J. Biol. Chem. 2008;283:6040–6049. doi: 10.1074/jbc.M708248200. [DOI] [PubMed] [Google Scholar]
- 48.Maurya J.P., Sethi V., Gangappa S.N., Gupta N., Chattopadhyay S. Interaction of MYC2 and GBF1 results in functional antagonism in blue light-mediated Arabidopsis seedling development. Plant J. 2015;83:439–450. doi: 10.1111/tpj.12899. [DOI] [PubMed] [Google Scholar]
- 49.Nietzsche M., Guerra T., Alseekh S., Wiermer M., Sonnewald S., Fernie A.R., Börnke F. STOREKEEPER RELATED1/G-Element Binding Protein (STKR1) Interacts with Protein Kinase SnRK1. Plant Physiol. 2018;176:1773–1792. doi: 10.1104/pp.17.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizoguchi T., Wheatley K., Hanzawa Y., Wright L., Mizoguchi M., Song H.R., Carré I.A., Coupland G. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev. Cell. 2002;2:629–641. doi: 10.1016/S1534-5807(02)00170-3. [DOI] [PubMed] [Google Scholar]
- 51.Galego L., Almeida J. Role of DIVARICATA in the control of dorsoventral asymmetry in Antirrhinum flowers. Genes Dev. 2002;16:880–891. doi: 10.1101/gad.221002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan Q.K., Irish V.F. The Arabidopsis zinc finger-homeodomain genes encode proteins with unique biochemical properties that are coordinately expressed during floral development. Plant Physiol. 2006;140:1095–1108. doi: 10.1104/pp.105.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verdonk J.C., Haring M.A., van Tunen A.J., Schuurink R.C. ODORANT1 regulates fragrance biosynthesis in petunia flowers. Plant Cell. 2005;17:1612–1624. doi: 10.1105/tpc.104.028837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gross T., Broholm S., Becker A. CRABS CLAW Acts as a Bifunctional Transcription Factor in Flower Development. Front. Plant Sci. 2018;29:835. doi: 10.3389/fpls.2018.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bao X., Franks R.G., Levin J.Z., Liu Z. Repression of AGAMOUS by BELLRINGER in floral and inflorescence meristems. Plant Cell. 2004;16:1478–1489. doi: 10.1105/tpc.021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aukerman M.J., Sakai H. Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell. 2003;15:2730–2741. doi: 10.1105/tpc.016238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan Y., Shen L., Chen Y., Bao S., Thong Z., Yu H. A MYB-domain protein EFM mediates flowering responses to environmental cues in Arabidopsis. Dev. Cell. 2014;30:437–448. doi: 10.1016/j.devcel.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Stracke R., Ishihara H., Huep G., Barsch A., Mehrtens F., Niehaus K., Weisshaar B. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007;50:660–677. doi: 10.1111/j.1365-313X.2007.03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yanagisawa S., Sheen J. Involvement of maize Dof zinc finger proteins in tissue-specific and light-regulated gene expression. Plant Cell. 1998;10:75–89. doi: 10.1105/tpc.10.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin R., Burch A.Y., Huppert K.A., Tiwari S.B., Murphy A.S., Guilfoyle T.J., Schachtman D.P. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell. 2007;19:2440–2453. doi: 10.1105/tpc.107.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gibbs D.J., Coates J.C. AtMYB93 is an endodermis-specific transcriptional regulator of lateral root development in arabidopsis. Plant Signal. Behav. 2014;9:e970406. doi: 10.4161/15592316.2014.970406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seo P.J., Park C.M. MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis in Arabidopsis. New Phytol. 2010;186:471–483. doi: 10.1111/j.1469-8137.2010.03183.x. [DOI] [PubMed] [Google Scholar]
- 63.Cui J., Jiang N., Zhou X., Hou X., Yang G., Meng J., Luan Y. Tomato MYB49 enhances resistance to Phytophthora infestans and tolerance to water deficit and salt stress. Planta. 2018;248:1487–1503. doi: 10.1007/s00425-018-2987-6. [DOI] [PubMed] [Google Scholar]
- 64.Park M.Y., Kang J.Y., Kim S.Y. Overexpression of AtMYB52 confers ABA hypersensitivity and drought tolerance. Mol. Cells. 2011;31:447–454. doi: 10.1007/s10059-011-0300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Z.Y., Kim S.Y., Hyeon do Y., Kim D.H., Dong T., Park Y., Jin J.B., Joo S.H., Kim S.K., Hong J.C., et al. The Arabidopsis NAC transcription factor ANAC096 cooperates with bZIP-type transcription factors in dehydration and osmotic stress responses. Plant Cell. 2013;25:4708–4724. doi: 10.1105/tpc.113.119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang F.P., Wang X.F., Zhang J., Ma F., Hao Y.J. MdMYB58 modulates Fe homeostasis by directly binding to the MdMATE43 promoter in plants. Plant Cell Physiol. 2018;59:2476–2489. doi: 10.1093/pcp/pcy168. [DOI] [PubMed] [Google Scholar]
- 67.Ogo Y., Kobayashi T., Nakanishi I.R., Nakanishi H., Kakei Y., Takahashi M., Toki S., Mori S., Nishizawa N.K. A novel NAC transcription factor, IDEF2, that recognizes the iron deficiency-responsive element 2 regulates the genes involved in iron homeostasis in plants. J. Biol. Chem. 2008;283:13407–13417. doi: 10.1074/jbc.M708732200. [DOI] [PubMed] [Google Scholar]
- 68.Devaiah B.N., Madhuvanthi R., Karthikeyan A.S., Raghothama K.G. Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol. Plant. 2009;2:43–58. doi: 10.1093/mp/ssn081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wasternack C. Action of jasmonates in plant stress responses and development—Applied aspects. Biotechnol. Adv. 2014;32:31–39. doi: 10.1016/j.biotechadv.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Jung C., Seo J.S., Han S.W., Koo Y.J., Kim C.H., Song S.I., Nahm B.H., Choi Y.D., Cheong J.J. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 2008;146:623–635. doi: 10.1104/pp.107.110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dai C., Wang M.H. Characterization and functional analysis of methionine sulfoxide reductase A gene family in tomato. Mol. Biol. Rep. 2012;39:6297–6308. doi: 10.1007/s11033-012-1451-0. [DOI] [PubMed] [Google Scholar]
- 72.Kang H.G., Singh K.B. Characterization of salicylic acid-responsive, arabidopsis Dof domain proteins: Overexpression of OBP3 leads to growth defects. Plant J. 2000;21:329–339. doi: 10.1046/j.1365-313x.2000.00678.x. [DOI] [PubMed] [Google Scholar]
- 73.Wang H.Z., Yang K.Z., Zou J.J., Zhu L.L., Xie Z.D., Morita M.T., Tasaka M., Frim J., Grotewold E., Beeckman T., et al. Transcriptional regulation of PIN genes by FOUR LIPS and MYB88 during Arabidopsis root gravitropism. Nat. Commun. 2015;6:8822. doi: 10.1038/ncomms9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zuo J., Niu Q.W., Frugis G., Chua N.H. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002;30:349–359. doi: 10.1046/j.1365-313X.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- 75.Kunieda T., Mitsuda N., Ohme-Takagi M., Takeda S., Aida M., Tasaka M., Kondo M., Nishimura M., Hara-Nishimura I. NAC family proteins NARS1/NAC2 and NARS2/NAM in the outer integument regulate embryogenesis in Arabidopsis. Plant Cell. 2008;20:2631–2642. doi: 10.1105/tpc.108.060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y., Liang W., Shi J., Xu J., Zhang D. MYB56 encoding a R2R3 MYB transcription factor regulates seed size in Arabidopsis thaliana. J. Integr. Plant Biol. 2013;55:1166–1178. doi: 10.1111/jipb.12094. [DOI] [PubMed] [Google Scholar]
- 77.Lashbrooke J., Cohen H., Levy-Samocha D., Tzfadia O., Panizel I., Zeisler V., Massalha H., Stern A., Trainotti L., Schreiber L., et al. MYB107 and MYB9 Homologs Regulate Suberin Deposition in Angiosperms. Plant Cell. 2016;28:2097–2116. doi: 10.1105/tpc.16.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alonso-Peral M.M., Li J., Li Y., Allen R.S., Schnippenkoetter W., Ohms S., White R.G., Millar A.A. The microRNA159-regulated GAMYB-like genes inhibit growth and promote programmed cell death in Arabidopsis. Plant Physiol. 2010;154:757–771. doi: 10.1104/pp.110.160630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng L., Sun Q., Xue H., Wang X. iTRAQ-based quantitative proteomic analysis reveals pathways associated with re-establishing desiccation tolerance in germinating seeds of Caragana korshinskii Kom. J. Proteom. 2018;179:1–16. doi: 10.1016/j.jprot.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 80.Higashi Y., Hirai M.Y., Fujiwara T., Naito S., Noji M., Saito K. Proteomic and transcriptomic analysis of Arabidopsis seeds: Molecular evidence for successive processing of seed proteins and its implication in the stress response to sulfur nutrition. Plant J. 2006;48:557–571. doi: 10.1111/j.1365-313X.2006.02900.x. [DOI] [PubMed] [Google Scholar]
- 81.Staszak A.M., Pawłowski T.A. Proteomic analysis of embryogenesis and the acquisition of seed dormancy in Norway maple (Acer platanoides L.) Int. J. Mol. Sci. 2014;15:10868–10891. doi: 10.3390/ijms150610868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang F., Perry S.E. Identification of direct targets of FUSCA3, a key regulator of Arabidopsis seed development. Plant Physiol. 2013;161:1251–1264. doi: 10.1104/pp.112.212282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Châtelain E., Satour P., Laugier E., Ly Vu B., Payet N., Rey P., Montrichard F. Evidence for participation of the methionine sulfoxide reductase repair system in plant seed longevity. Proc. Natl. Acad. Sci. USA. 2013;110:3633–3638. doi: 10.1073/pnas.1220589110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ko J.H., Jeon H.W., Kim W.C., Kim J.Y., Han K.H. The MYB46/MYB83-mediated transcriptional regulatory programme is a gatekeeper of secondary wall biosynthesis. Ann. Bot. 2014;114:1099–1107. doi: 10.1093/aob/mcu126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ko J.H., Yang S.H., Park A.H., Lerouxel O., Han K.H. ANAC012, a member of the plant-specific NAC transcription factor family, negatively regulates xylary fiber development in Arabidopsis thaliana. Plant J. 2007;50:1035–1048. doi: 10.1111/j.1365-313X.2007.03109.x. [DOI] [PubMed] [Google Scholar]
- 86.Mandaokar A., Browse J. MYB108 acts together with MYB24 to regulate jasmonate-mediated stamen maturation in Arabidopsis. Plant Physiol. 2009;149:851–862. doi: 10.1104/pp.108.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alves-Ferreira M., Wellmer F., Banhara A., Kumar V., Riechmann J.L., Meyerowitz E.M. Global expression profiling applied to the analysis of Arabidopsis stamen development. Plant Physiol. 2007;145:747–762. doi: 10.1104/pp.107.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Asahina M., Azuma K., Pitaksaringkarn W., Yamazaki T., Mitsuda N., Ohme-Takagi M., Yamaguchi S., Kamiya Y., Okada K., Nishimura T., et al. Spatially selective hormonal control of RAP2.6L and ANAC071 transcription factors involved in tissue reunion in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:16128–16132. doi: 10.1073/pnas.1110443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tian H., Wang X., Guo H., Cheng Y., Hou C., Chen J.G., Wang S. NTL8 Regulates Trichome Formation in Arabidopsis by Directly Activating R3 MYB Genes TRY and TCL1. Plant Physiol. 2017;174:2363–2375. doi: 10.1104/pp.17.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao J., Liu J.S., Meng F.N., Zhang Z.Z., Long H., Lin W.H., Luo X.M., Wang Z.Y., Zhu S.W. ANAC005 is a membrane-associated transcription factor and regulates vascular development in Arabidopsis. J. Integr. Plant Biol. 2016;58:442–451. doi: 10.1111/jipb.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.