Abstract
Non-alcoholic fatty liver disease (NAFLD) is likely to be associated with elevated plasma branched-chain amino acids (BCAAs) and may precede the development of type 2 diabetes (T2D). We hypothesized that BCAAs may be involved in the pathogenesis of T2D attributable to NAFLD and determined the extent to which plasma BCAAs influence T2D development in NAFLD. We evaluated cross-sectional associations of NAFLD with fasting plasma BCAAs (nuclear magnetic resonance spectroscopy), and prospectively determined the extent to which the influence of NAFLD on incident T2D is attributable to BCAA elevations. In the current study, 5791 Prevention of REnal and Vascular ENd-stage Disease (PREVEND) cohort participants without T2D at baseline were included. Elevated fatty liver index (FLI) ≥60, an algorithm based on triglycerides, gamma-glutamyltransferase, body mass index (BMI) and waist circumference, was used as proxy of NAFLD. Elevated FLI ≥ 60 was present in 1671 (28.9%) participants. Cross-sectionally, BCAAs were positively associated with FLI ≥ 60 (β = 0.208, p < 0.001). During a median follow-up of 7.3 years, 276 participants developed T2D, of which 194 (70.2%) had an FLI ≥ 60 (log-rank test, p < 0.001). Cox regression analyses revealed that both FLI ≥60 (hazard ratio (HR) 3.46, 95% CI 2.45–4.87, p < 0.001) and higher BCAA levels (HR 1.19, 95% CI 1.03–1.37, p = 0.01) were positively associated with incident T2D. Mediation analysis showed that the association of FLI with incident T2D was in part attributable to elevated BCAAs (proportion mediated 19.6%). In conclusion, both elevated FLI and elevated plasma BCAA levels are associated with risk of incident T2D. The association of NAFLD with T2D development seems partly mediated by elevated BCAAs.
Keywords: branched-chain amino acids, type 2 diabetes, non-alcoholic fatty liver disease, fatty liver index, insulin resistance
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is emerging as the most common cause of chronic liver disease in the Western world [1]. NAFLD is characterized by hepatic steatosis in the absence of alcohol abuse and its spectrum ranges from simple steatosis to non-alcoholic steatohepatitis (NASH), fibrosis and cirrhosis [1]. NAFLD and insulin resistance are closely related [2]. NAFLD frequently coincides with the metabolic syndrome (MetS) and type 2 diabetes (T2D). While MetS and T2D may precede NAFLD [2,3], two recent meta-analyses have demonstrated that NAFLD, irrespective of whether it is diagnosed by elevated liver enzymes, by radiological abnormalities or by histological abnormalities, may in fact precede T2D [4,5]. Moreover, an elevated fatty liver index (FLI), as proxy of NAFLD and assessed by an algorithm based on obesity measures, plasma triglycerides and gamma-glutamyltransferase (GGT), has been shown to predict T2D in two independent European populations [6,7]. Taken together, these findings [2,4,5,6,7] point to an intricate relationship between NAFLD, MetS and T2D, which are indeed considered as manifestations of a common cardio-metabolic multisystem disorder [8,9,10,11].
Branched-chain amino acids (BCAAs) are amino acids with non-linear aliphatic side-chains, and include the essential amino acids leucine, valine and isoleucine [12]. Although not fully understood, BCAAs may contribute to the development of obesity-associated insulin resistance [13,14]. Moreover, in the clinical setting, disturbances in BCAA metabolism have been described in insulin-resistant states, including MetS and T2D [15,16,17,18,19,20,21]. Conversely, a decrease in BCAA levels may result in an improvement in glucose metabolism [22]. Additionally, high plasma BCAA levels have been shown to be associated with an increased risk of T2D development [15].
Fasting plasma BCAAs may be elevated in (obesity-associated) NAFLD [13,23,24,25,26], and may coincide with abnormalities in BCAA catabolic enzymes in liver and adipose tissue [27]. Such abnormalities in (hepatic) BCAA metabolism conceivably affect carbon substrate oxidation that, together with impairment of antioxidant defence, may contribute to the generation of reactive oxygen species [13,19]. In turn, mitochondrial dysfunction in NAFLD, in particular in the context of hepatic inflammation, is likely to convey impaired BCAA metabolism conceivably resulting in plasma BCAA elevations [28,29,30]. Thus, it seems plausible to hypothesize that NAFLD and impaired hepatic BCAA metabolism jointly impact on deteriorating glucose tolerance.
Despite continued clinical interest concerning the impact of NAFLD and increased circulating BCAAs on the development of T2D, no large-scale population-based studies have yet reported on the relative contributions of NAFLD and plasma BCAAs on incident T2D in initially diabetes-naïve NAFLD subjects. Therefore, we initiated the present study to determine the extent to which BCAAs influence T2D development in the context of NAFLD. To this end, we carried out cross-sectional and prospective analyses among 5791 subjects participating in the Prevention of REnal and Vascular ENd-stage Disease (PREVEND) cohort, comprising a large and well-characterized population from the North of the Netherlands.
2. Materials and Methods
2.1. Study Population
The study was performed among participants of the Prevention of REnal and Vascular ENd-stage Disease (PREVEND) cohort study [31,32]. The PREVEND study was approved by the Medical Ethics Committee of the University Medical Center Groningen and performed in accordance with the Declaration of Helsinki guidelines [31,32]. All participants gave written informed consent. PREVEND is a large prospective general population-based study that was initiated to investigate cardiovascular and renal disease with a focus on albuminuria. All inhabitants (28–75 years old) of Groningen, The Netherlands were sent a questionnaire on demographics and cardiovascular morbidity and were asked to supply an early morning urine specimen. Pregnant women, type 1 diabetic subjects and T2D subjects using insulin were not allowed to participate. All participants with a urinary albumin concentration of ≥10 mg/L were invited to our clinic together with randomly selected subjects with a urinary albumin concentration of <10 mg/L. The initial study population of the PREVEND study was comprised of 8592 subjects who completed the total study screening program.
For the present study, we conducted a post-hoc analysis, using data of participants who completed the second screening round (n = 6893). We excluded all subjects with missing values of BCAA concentrations, pre-existing T2D, non-fasting subjects, and subjects in which the clinical and biochemical variables required to calculate the fatty liver index (FLI), a proxy of NAFLD, were not available, leaving a study population of 5791 participants with complete information for analysis.
2.2. Measurements and Definitions
During two outpatient visits, baseline data were collected on demographics, lifestyle factors, anthropometric measurements, medical history, parental history of T2D and medication use. Information on medication use was combined with information from a pharmacy-dispensing registry, which had complete information on the drug usage of >95% of subjects in the PREVEND study. Height and weight were measured in standing position without shoes and heavy outer garments. Body mass index (BMI) was calculated as weight (kg) divided by height squared (meter). Waist circumference was measured as the smallest girth between the rib cage and iliac crest. The waist/hip ratio was determined as the waist circumference divided by the largest girth between waist and thigh [31]. Blood pressure was measured using an automatic device, where the last two recordings of the second outpatient visit were averaged for analysis. Alcohol consumption was recorded with one alcoholic drink being assumed to contain 10 g of alcohol. Smoking was categorized into current and never/former smokers. Past cardiovascular history included hospitalization for myocardial ischemia, obstructive coronary artery disease or revascularization procedures. Homeostasis Model Assessment (HOMA)-IR was calculated as follows:
| Fasting plasma insulin (mU/L) × fasting plasma glucose (mmol/L)/22.5. | (1) |
HOMA-β was calculated as follows:
| 20 × Fasting plasma insulin (mU/L)/(fasting plasma glucose (mmol/L) − 3.5, | (2) |
where HOMA-β represents the relative β-cell function (expressed as a percentage).
Urinary albumin excretion (UAE) was measured as described in two 24 h urine collections and the results were averaged for analysis [31]. Estimated glomerular filtration rate (eGFR) was calculated applying the combined creatinine cystatin C-based Chronic Kidney Disease Epidemiology Collaboration equation.
For the diagnosis of NAFLD, the algorithm of the FLI was used [33]. The FLI was calculated according to the following formula:
| [e (0.953 × loge (triglycerides + 0.139 × BMI + 0.718 × loge (GGT) + 0.053 × waist circumference − 15.745)]/[1 + e (0.953 × loge (triglycerides) + 0.139 × BMI + 0.718 × loge (GGT) + 0.053 × waist circumference − 15.745)] × 100. |
(3) |
The optimal cut-off value for the FLI is documented to be 60 with an accuracy of 84%, a sensitivity of 61% and a specificity of 86% for detecting NAFLD as determined by ultrasonography [33]. FLI ≥60 was therefore used as proxy of NAFLD. The FLI is currently considered as one of the best-validated steatosis scores for larger scale screening studies [34]. Alternatively, we used the hepatic steatosis index (HSI) [35]. The HSI is defined as follows:
| HSI = 8 × ALT/AST ratio + BMI (+2, if diabetes; +2, if female), | (4) |
where ALT is alanine aminotransferase and AST is aspartate aminotransferase. The cut-off value of the HSI for detecting NAFLD is 36 [35].
The MetS was defined according to the revised National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP) III criteria [36]. Participants were categorized with MetS when at least three out of the following five criteria were present: waist circumference >102 cm for men and >88 cm for women; plasma triglycerides ≥1.7 mmol/L; high-density lipoprotein (HDL) cholesterol <1.0 mmol/L for men and <1.3 mmol/L for women; hypertension (blood pressure ≥130/85 mm Hg or the use of antihypertensive medication); hyperglycaemia (fasting glucose ≥5.6 mmol/L or the use of glucose-lowering drugs).
2.3. Laboratory Methods
Venous blood samples were drawn after an overnight fast while participants rested for 15 min. Heparinized plasma and serum samples were obtained by centrifugation at 1400× g for 15 min at 4 °C. Plasma and serum samples were stored at −80 °C until analysis. EDTA plasma valine, leucine and isoleucine concentrations were measured using a Vantera Clinical Analyzer (LabCorp., Morrisville, NC, USA), a fully automated, high-throughput, 400 MHz proton (1H) nuclear magnetic resonance (NMR) spectroscopy platform. Plasma samples were prepared on board the instrument, and automatically delivered to the flow probe in the NMR spectrometer’s magnetic field. The validation of the use of NMR for quantification of BCAAs has been previously described [20,21]. Data acquisition on the Vantera and the spectra data processing have been reported in greater detail elsewhere [37]. Fasting plasma glucose was measured by dry chemistry (Eastman Kodak, Rochester, NY, USA) directly after blood collection. Insulin was measured with an immunoturbidometric assay (Diazyme Laboratories, Poway, CA, USA). Plasma total cholesterol, triglycerides and HDL cholesterol were measured as previously described [31,32]. Non-HDL cholesterol was calculated as the difference between total cholesterol and HDL cholesterol. Low-density lipoprotein (LDL) cholesterol was calculated by the Friedewald formula if triglycerides were <4.5 mmol/L. Serum ALT and AST were measured using the standardized kinetic method with pyridoxal phosphate activation (Roche Modular P, Roche Diagnostics, Mannheim, Germany). Serum GGT was assayed by an enzymatic colorimetric method (Roche Modular P, Roche Diagnostics, Mannheim, Germany). Standardization of ALT, AST and GGT was performed according to the International Federation of Clinical Chemistry guidelines [38,39,40]. High sensitivity C-reactive protein (hsCRP) was assayed by nephelometry (Dade Behring Diagnostic, Marburg, Germany). Serum creatinine was measured by an enzymatic method on a Roche Modular analyzer (Roche Diagnostics, Mannheim, Germany). Serum cystatin C was measured using Gentian Cystatin C Immunoassay (Gentian AS, Moss, Norway) reagents on a modular analyzer (Roche Diagnostics). Urinary albumin was measured by nephelometry (Dade Behring Diagnostic, Marburg, Germany).
2.4. T2D Development
Participants were followed from baseline outpatient visit until end of the follow-up period. Incident T2D was established if one or more of the four criteria were met during follow-up:
(1) Blood glucose ≥7.0 mmol/L (126 mg/dL); (2) Random sample plasma glucose ≥11.1 mmol/L (200 mg/dL); (3) Self-report of a physician diagnosis; (4) Initiation of glucose-lowering medication according to the central pharmacy registry follow-up data, which was completed on 1 January 2011.
2.5. Statistical Analysis
IBM SPSS software (version 23.0, IBM Corp., Armonk, NY, USA) was used for cross-sectional data analysis. R version 3.4.2 (Boston, MA, USA) and STATA version 13.1 (StataCorp LP, College Station, TX, USA) were used for prospective analyses. Cross-sectional data are expressed as mean ± standard deviation (SD), median with interquartile range (IQR) or as numbers (percentages). HOMA-IR and HOMA-β were loge transformed for multivariable regression analyses. Normality of distribution was assessed and checked for skewness. Between-group differences in variables were determined by unpaired t-tests for normally distributed and loge transformed variables, by Mann–Whitney U tests for non-normally distributed variables or by chi-squared tests for categorical variables where appropriate. Multivariable linear regression analyses were carried out to disclose the independent associations of BCAA levels with an elevated FLI or HSI while taking into account clinical covariates and laboratory parameters. Results from multivariable linear regression analyses are presented as standardized regression coefficients.
For the prospective analyses, we plotted cumulative Kaplan–Meier curves for T2D development during follow-up according to quartiles of plasma total BCAAs. Time-to-event Cox proportional hazards models were used to assess the hazard ratio (HR) with 95% confidence intervals (CI) of incident T2D.
A mediation analysis was performed to disclose the extent to which the plasma total BCAA concentration was a possible mediator between an elevated FLI and HSI, as proxies of NAFLD, and incident T2D, following the procedures as advocated by Preacher and Hayes [41,42]. The significance of the mediation effect was tested by computing bias-corrected bootstrap CIs with 2000 repetitions. Finally, the magnitude of mediation was calculated by dividing the coefficient of the indirect effect by the total effect. Significance of mediation was proved with p < 0.05 if zero was not between the lower and upper bound of the 95% CI of the indirect effect. Interaction terms were considered to be statistically significant at two-sided p-values <0.10 [43]. Otherwise, two-sided p-values <0.05 were considered significant.
3. Results
3.1. Clinical and Laboratory Characteristics of the Study Population
The study population consisted of 5791 participants free of T2D at baseline. There were 1671 participants (28.9%) with an FLI ≥60. Table 1 shows the clinical characteristics and laboratory data of the study population according to FLI categorization. Subjects with an FLI ≥60 were older, more likely to be men (67.8% vs. 32.2%) and more likely to be classified with MetS, history of cardiovascular disease and parental history of T2D. Consequently, subjects with an FLI ≥60 used antihypertensive medication and lipid-lowering drugs more frequently. Alcohol consumption ≥10 g/day was recorded in subjects with an elevated FLI more frequently, but cigarette smoking was not significantly different. BMI, waist circumference, waist/hip ratio, systolic and diastolic blood pressure, glucose, insulin, HOMA-IR, HOMA-β, hsCRP, transaminases, ALP, GGT, UAE, total cholesterol, non-HDL cholesterol, LDL cholesterol and triglycerides were higher in subjects with FLI ≥60, whereas eGFR and HDL cholesterol were lower in subjects with an elevated FLI (Table 1). Plasma total BCAAs as well as valine, leucine and isoleucine concentrations were all higher in subjects with an FLI ≥60 (Table 1).
Table 1.
Clinical and laboratory characteristics including plasma branched-chain amino acids in 4120 subjects with a fatty liver index (FLI) < 60 and 1671 subjects with an FLI ≥60.
| FLI < 60, n = 4120 (71.1%) | FLI ≥ 60, n = 1671 (28.9%) | p-Value | |
|---|---|---|---|
| Age (years), median (IQR) | 49.6 (41.9–59.3) | 56.0 (47.7–65.6) | <0.001 |
| Sex (men/women), n (%) | 1707 (41.4)/2413 (58.6) | 1133 (67.8)/538 (32.2) | <0.001 |
| MetS, n (%) | 296 (7.2) | 946 (56.6) | <0.001 |
| History of cardiovascular disease, n (%) | 169 (4.1) | 155 (9.3) | <0.001 |
| Parental history of T2D, n (%) | 577 (14.0) | 266 (15.9) | 0.061 |
| Current smokers, n (%) | 1167 (28.3) | 457 (27.3) | 0.454 |
| Alcohol ≥10 g/day, n (%) | 137 (3.4) | 109 (6.6) | <0.001 |
| Antihypertensive medication, n (%) | 560 (13.6) | 556 (33.3) | <0.001 |
| Lipid-lowering drugs, n (%) | 249 (6.0) | 234 (14.0) | <0.001 |
| Systolic blood pressure (mm Hg), mean ± SD | 121 ± 17 | 134 ± 18 | <0.001 |
| Diastolic blood pressure (mm Hg), mean ± SD | 71 ± 8 | 77 ± 9 | <0.001 |
| BMI (kg/m2), mean ± SD | 24.7 ± 2.8 | 30.8 ± 4.0 | <0.001 |
| Waist circumference, mean ± SD | 85.8 ± 9.1 | 104.8 ± 8.9 | <0.001 |
| Waist/hip ratio, mean ± SD | 0.87 ± 0.07 | 0.96 ± 0.07 | <0.001 |
| Glucose (mmol/L), mean ± SD | 4.71 ± 0.58 | 5.10 ± 0.67 | <0.001 |
| Insulin (mU/L), median (IQR) | 6.80 (5.1–9.2) | 12.50 (9.2–18.1) | <0.001 |
| HOMA-IR (mU mmol/L2/22.5), median (IQR) | 1.42 (1.04–1.98) | 2.86 (2.00–4.17) | <0.001 |
| HOMA-β (%), median (IQR) | 25.55 (18.61–35.31) | 46.92 (33.29–66.68) | <0.001 |
| hsCRP (mg/L), median (IQR) | 1.00 (0.48–2.26) | 2.35 (1.17–4.25) | <0.001 |
| ALT (U/L), median (IQR) | 15 (12–20) | 23 (17–32) | <0.001 |
| AST (U/L), median (IQR) | 21 (19–25) | 25 (21–29) | <0.001 |
| ALP (U/L), mean ± SD | 63 ± 19 | 72 ± 22 | <0.001 |
| GGT (U/L), median (IQR) | 19 (13–27) | 39 (27–60) | <0.001 |
| eGFR (mL/min/1.73 m2), median (IQR) | 95.9 (84.2–105.7) | 88.4 (76.4–99.4) | <0.001 |
| UAE (mg/24 h), median (IQR) | 7.4 (5.6–11.0) | 9.7 (6.6–17.3) | <0.001 |
| Total cholesterol (mmol/L), mean ± SD | 5.31 ± 1.00 | 5.71 ± 1.03 | <0.001 |
| Non-HDL cholesterol (mmol/L), mean ± SD | 3.96 ± 0.98 | 4.60 ± 1.00 | <0.001 |
| LDL cholesterol (mmol/L), mean ± SD | 3.49 ± 0.90 | 3.79 ± 0.92 | <0.001 |
| HDL cholesterol (mmol/L), mean ± SD | 1.35 ± 0.30 | 1.11 ± 0.24 | <0.001 |
| Triglycerides (mmol/L), median (IQR) | 0.94 (0.71–1.26) | 1.66 (1.26–2.19) | <0.001 |
| Total BCAAs (µM), mean ± SD | 356.90 ± 62.58 | 425.48 ± 67.78 | <0.001 |
| Valine (µM), mean ± SD | 197.22 ± 33.20 | 229.74 ± 35.48 | <0.001 |
| Leucine (µM), mean ± SD | 119.78 ± 23.64 | 144.48 ± 27.63 | <0.001 |
| Isoleucine (µM), mean ± SD | 39.74 ± 13.47 | 51.19 ± 15.99 | <0.001 |
Data are given in number with percentages (%), mean ± standard deviation (SD) for normally distributed data or median with interquartile ranges (IQR) for non-normally distributed data. Abbreviations: ALP, alkaline phosphatase; ALT, aminotransferase; AST, aspartate aminotransferase; BCAA, branched-chain amino acids; BMI, body mass index; FLI, fatty liver index; eGFR, estimated glomerular filtration rate; GGT, gamma-glutamyltransferase; HOMA, Homeostasis Model Assessment; HDL, high-density lipoproteins; hsCRP, high sensitivity C-reactive protein; IR, insulin resistance; LDL, low-density lipoproteins; MetS, metabolic syndrome; T2D, type 2 diabetes; UAE, urinary albumin excretion. LDL cholesterol was calculated by the Friedewald formula.
3.2. Cross-Sectional Associations of BCAA with an Elevated FLI and HSI
Multivariable linear regression analyses were subsequently performed in order to establish the extent to which plasma BCAA concentrations were independently associated with an elevated FLI (Table 2). In an age- and sex-adjusted analysis, a positive association of plasma BCAA concentrations with an elevated FLI was found (Table 2, Model 1, β = 0.326, p < 0.001). This positive association of plasma BCAA concentrations with an elevated FLI was also demonstrated after further adjustment for family history of T2D (Table 2, Model 2, β = 0.324, p < 0.001), alcohol intake and current smoking (Table 2, Model 3, β = 0.323, p < 0.001), eGFR, UAE, use of antihypertensive medication and lipid-lowering drugs (Table 2, Model 4, β = 0.318, p < 0.001) and, finally, after additional adjustment for HOMA-IR and HOMA-β (Table 2, Model 5, β = 0.208, p < 0.001). In alternative analyses with an elevated HSI instead of an elevated FLI, similar independent positive associations of plasma BCAA concentrations with an elevated HSI were found (Table S1, Supplementary Materials, all models p < 0.001).
Table 2.
Multivariable linear regression analysis demonstrating the positive association of plasma branched-chain amino acids with an elevated fatty liver index (FLI) (≥60) after adjustment for clinical and laboratory covariates in 5791 subjects.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | β | p | |
| Age | 0.015 | 0.173 | 0.014 | 0.199 | 0.010 | 0.339 | −0.032 | 0.035 | −0.046 | 0.002 |
| Sex (men vs. women) | 0.424 | <0.001 | 0.426 | <0.001 | 0.426 | <0.001 | 0.431 | <0.001 | 0.431 | <0.001 |
| FLI ≥60 vs. <60 | 0.326 | <0.001 | 0.324 | <0.001 | 0.323 | <0.001 | 0.318 | <0.001 | 0.208 | <0.001 |
| Family history of T2D (yes/no) | 0.045 | <0.001 | 0.048 | <0.001 | 0.046 | <0.001 | 0.033 | 0.002 | ||
| Alcohol intake (≥10 g/day) | 0.007 | 0.495 | 0.011 | 0.325 | 0.014 | 0.207 | ||||
| Current smoking (yes/no) | −0.043 | <0.001 | −0.044 | <0.001 | −0.030 | 0.005 | ||||
| eGFR (mL/min/1.73 m2) | −0.057 | <0.001 | −0.046 | 0.002 | ||||||
| UAE (mg/24 h) | −0.015 | 0.165 | −0.021 | 0.058 | ||||||
| Use of antihypertensive medication | 0.009 | 0.478 | −0.011 | 0.376 | ||||||
| Use of lipid-lowering drugs | 0.026 | 0.026 | 0.010 | 0.395 | ||||||
| HOMA-IR | 0.271 | <0.001 | ||||||||
| HOMA-β | −0.043 | 0.089 | ||||||||
β: standardized regression coefficients. HOMA-IR and HOMA-β were loge transformed for analyses. eGFR, estimated glomerular filtration rate; FLI, fatty liver index; HOMA, Homeostasis Model Assessment; IR, insulin resistance; T2D, type 2 diabetes; UAE; urinary albumin excretion. Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, family history of type 2 diabetes. Model 3: adjusted for age, sex, family history of type 2 diabetes, alcohol intake and current smoking. Model 4: adjusted for age, sex, family history of type 2 diabetes, alcohol intake, current smoking, estimated glomerular filtration rate, urinary albumin excretion and use of antihypertensive medication and lipid-lowering drugs. Model 5: adjusted for age, sex, family history of type 2 diabetes, alcohol intake, current smoking, estimated glomerular filtration rate, urinary albumin excretion, use of antihypertensive medication and lipid-lowering drugs, HOMA-IR and HOMA-β.
3.3. Prospective Analyses of FLI and BCAA with Incident T2D
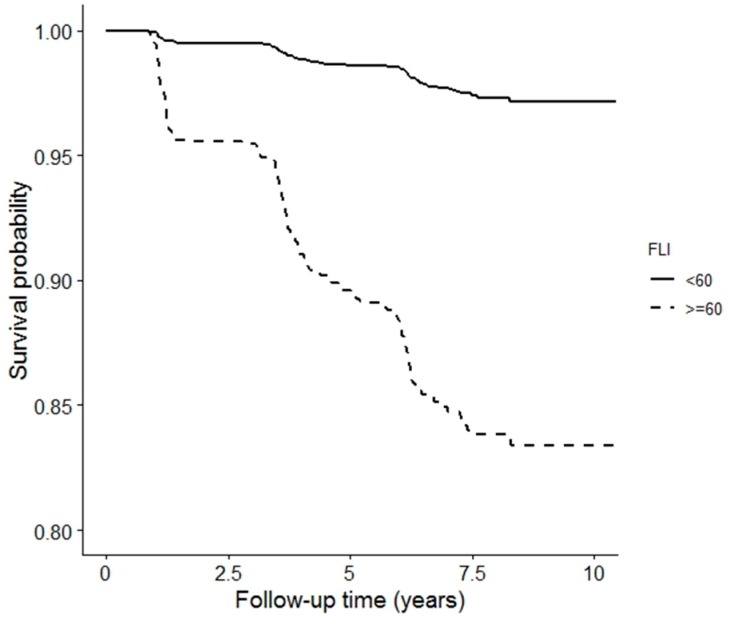
During a median follow-up of 7.3 years (IQR 5.7–7.7), a total of 276 participants developed T2D, of which 194 (70.2%) subjects had an elevated FLI (Table 3). Figure 1 shows the Kaplan–Meier curves for incident T2D survival according to non-elevated FLI (<60) and elevated FLI (≥60), which shows a significantly increased risk of incident T2D in subjects with an elevated FLI (log-rank test p < 0.001). Comparing associations of non-elevated and elevated FLI with incident T2D in Cox regression analyses showed an HR of 5.84 (95% CI 4.46–7.66, p < 0.001) when adjusted for age and sex (Table 3). This positive association of elevated FLI with risk of T2D incidence remained present after further adjustment for family history of T2D, HR 5.72 (95% CI 4.37–7.50, p < 0.001); alcohol intake and smoking, HR 5.64 (95% CI 4.31–7.39, p < 0.001); eGFR, UAE, antihypertensive medication and lipid-lowering drugs, HR 5.09 (95% CI 3.77–6.88, p < 0.001); HOMA-IR and HOMA-β, HR 3.84 (95% CI 2.76–5.36, p < 0.001); and, finally, for BCAAs, HR 3.46 (95% CI 2.45–4.87, p < 0.001) (Table 3). Similar Cox regression analyses were performed to address the relationship between BCAA (per 1 SD increment) and incident T2D (Table 4), which showed a similar pattern with T2D risk. However, in the fully adjusted model 6, additional adjustment for an elevated FLI attenuated the hazard ratio to 1.19 (95% CI 1.03–1.37, p = 0.01) (Table 4). There was no significant interaction of elevated FLI with age (p = 0.11) and there was a marginally significant interaction with sex (p = 0.07). In Cox regression analyses in which an elevated HSI was used instead, an elevated HSI was similarly associated with incident T2D, which remained associated with incident T2D independent of adjustment for plasma total BCAAs, whereas in analyses with plasma total BCAAs as determinant of incident T2D, the association of plasma total BCAAs with incident T2D was reduced after adjustment for an elevated HSI (Tables S2 and S3, Supplementary Materials). There was no significant interaction of sex with an elevated HSI on incident T2D (p = 0.36 for sex interaction) and there was a marginally significant interaction of elevated HSI with age (p = 0.07).
Table 3.
Prospective associations of FLI with incident type 2 diabetes.
| Non-Elevated FLI (<60) | Elevated FLI (≥60) | ||
| Participants, n | 4120 | 1671 | |
| Incident T2D, n (%) | 82 (2.0) | 194 (11.6) | |
| HR (95% CI) | p -Value | ||
| Crude Model | (ref) | 6.78 (5.23–8.79) | <0.001 |
| Model 1 | (ref) | 5.84 (4.46–7.66) | <0.001 |
| Model 2 | (ref) | 5.72 (4.37–7.50) | <0.001 |
| Model 3 | (ref) | 5.64 (4.31–7.39) | <0.001 |
| Model 4 | (ref) | 5.09 (3.77–6.88) | <0.001 |
| Model 5 | (ref) | 3.84 (2.76–5.36) | <0.001 |
| Model 6 | (ref) | 3.46 (2.45–4.87) | <0.001 |
Data are presented as hazard ratio (HR) with 95% confidence interval (CI). FLI, fatty liver index; BCAA, branched-chain amino acids; T2D, type 2 diabetes. Model 1: adjusted for age and sex. Model 2: adjusted for age, sex and family history of type 2 diabetes. Model 3: adjusted for age, sex, family history of type 2 diabetes, alcohol intake and current smoking. Model 4: adjusted for age, sex, family history of type 2 diabetes, alcohol intake, current smoking, eGFR, UAE, antihypertensive medication and lipid-lowering drugs. Model 5: adjusted for age, sex, family history of type 2 diabetes, alcohol intake, current smoking, eGFR, UAE, antihypertensive medication, lipid-lowering drugs, HOMA-IR and HOMA-β. Model 6: adjusted for age, sex, family history of type 2 diabetes, alcohol intake, current smoking, eGFR, UAE, antihypertensive medication, lipid-lowering drugs, HOMA-IR, HOMA-β and BCAAs.
Figure 1.
Kaplan–Meier curves for incident type 2 diabetes survival according to FLI score. Log-rank test (p < 0.001). FLI, fatty liver index.
Table 4.
Prospective associations of plasma total branched chain amino acids with incident type 2 diabetes.
| BCAA Per 1 SD Increment | p-Value | |
|---|---|---|
| Participants, n | 5791 | |
| Incident T2D, n (%) | 276 (4.8) | |
| HR (95% CI) | ||
| Crude Model | 1.68 (1.57–1.81) | <0.001 |
| Model 1 | 1.65 (1.52–1.79) | <0.001 |
| Model 2 | 1.63 (1.50–1.77) | <0.001 |
| Model 3 | 1.64 (1.50–1.79) | <0.001 |
| Model 4 | 1.64 (1.49–1.81) | <0.001 |
| Model 5 | 1.35 (1.20–1.53) | <0.001 |
| Model 6 | 1.19 (1.03–1.37) | 0.01 |
Data are presented as hazard ratio (HR) with 95% confidence interval (CI). FLI, fatty liver index; BCAA, branched-chain amino acids; T2D, type 2 diabetes. Model 1: adjusted for age and sex. Model 2: adjusted for age, sex and family history of type 2 diabetes. Model 3: adjusted for age, sex, family history of type 2 diabetes, alcohol intake and current smoking. Model 4: adjusted for age, sex, family history of type 2 diabetes, alcohol intake, current smoking, eGFR, UAE, antihypertensive medication and lipid-lowering drugs. Model 5: adjusted for age, sex, family history of type 2 diabetes, alcohol intake, current smoking, eGFR, UAE, antihypertensive medication, lipid-lowering drugs, HOMA-IR and HOMA-β. Model 6: adjusted for age, sex, family history of type 2 diabetes, alcohol intake, current smoking, eGFR, UAE, antihypertensive medication, lipid-lowering drugs, HOMA-IR, HOMA-β and FLI elevated (yes/no).
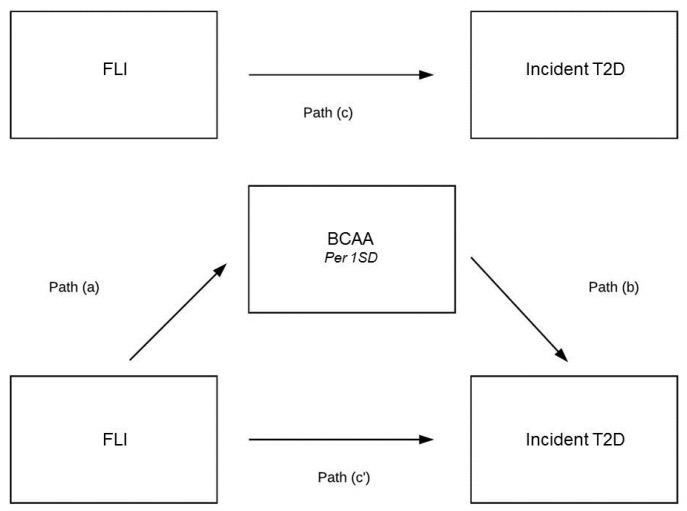
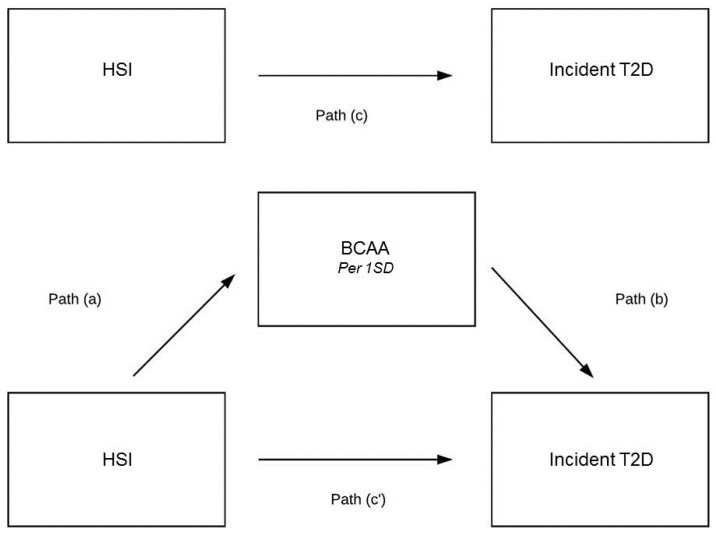
Additionally, mediation analyses were performed to disclose the extent to which the plasma total BCAA concentration was a possible mediator between elevated FLI or HSI and incident T2D. BCAAs appeared to be a mediator in the association of FLI with incident T2D (Figure 2). The indirect pathway was significant (B = 0.040, 95% CI 0.027–0.054, p-indirect < 0.001) and the magnitude of mediation was 19.6% (Table 5). In alternative analyses, BCAAs appeared to be a mediator in the association of HSI with incident T2D (Figure 3). The indirect pathway was significant (B = 0.033, 95% CI 0.026–0.041, p-indirect < 0.001) and the magnitude of mediation was 22.6% (Table 6).
Figure 2.
Mediation analysis on the association of FLI with incident type 2 diabetes. (a)–(c) are the regression coefficients between variables. The indirect effect is calculated as a × b. Total effect (c) is a × b + c’. Magnitude of mediation is calculated as indirect effect divided by total effect.
Table 5.
Mediating effect of BCAAs on the association of FLI with incident type 2 diabetes.
| Coefficient (95% CI) * | Proportion Mediated | |
|---|---|---|
| Indirect pathway (ab path) | B = 0.040 (95% CI 0.027–0.054) | 19.6% ** |
| Total effect (ab + c’ path) | B = 0.204 (95% CI 0.174–0.232) |
Analyses were performed according to Preacher and Hayes Procedure. B: unstandardized regression coefficient. Coefficients are adjusted for age and sex. * 95% CIs were bias-corrected confidence intervals after running 2000 bootstrap samples. ** The size of the significant mediated effect is calculated as the standardized indirect effect divided by the standardized total effect multiplied by 100.
Figure 3.
Mediation analysis on the association of HSI with incident type 2 diabetes. (a)–(c) are the regression coefficients between variables. The indirect effect is calculated as a × b. Total effect (c) is a × b + c’. Magnitude of mediation is calculated as indirect effect divided by total effect.
Table 6.
Mediating effect of BCAAs on the association of HSI with incident type 2 diabetes.
| Coefficient (95% CI) * | Proportion Mediated | |
|---|---|---|
| Indirect pathway (ab path) | B = 0.033 (95% CI 0.026–0.041) | 22.6% ** |
| Total effect (ab + c’ path) | B = 0.146 (95% CI 0.115–0.176) |
Analyses were performed according to Preacher and Hayes Procedure. B: unstandardized regression coefficient. Coefficients are adjusted for age and sex. * 95% CIs were bias-corrected confidence intervals after running 2000 bootstrap samples. ** The size of the significant mediated effect is calculated as the standardized indirect effect divided by the standardized total effect multiplied by 100.
4. Discussion
In this large-scale study among a predominantly Caucasian population, who were free of T2D at the baseline evaluation, we have first cross-sectionally demonstrated that fasting total plasma BCAA levels are elevated in subjects with NAFLD and that this relationship of BCAAs with NAFLD is independent of a considerable number of clinical and laboratory covariates, including measures of insulin resistance and β-cell function. Second, we have longitudinally documented that an elevated FLI predicts the development of T2D, an association that was in part attributable to higher plasma total BCAAs as inferred from mediation analyses. Taken together, the current results not only indicate that there is an intricate relationship between NAFLD and elevated plasma BCAA levels, but also suggest that NAFLD and elevated circulating BCAAs could act together on T2D development. In this study, we have used the FLI [33] as proxy of NAFLD, which is in line with recommendations of international guidelines [34], advocating the use of biomarker-derived algorithms in order to categorize subjects with probable NAFLD in large-scale studies. Alternative analyses using the HSI as proxy of NAFLD [35] confirmed the findings, supporting the validity of our results.
The cross-sectional part of our study demonstrated that plasma BCAA elevations were independently associated with suspected NAFLD, irrespective of whether an elevated FLI or an elevated HSI was used as a proxy of NAFLD. In comparison, recent studies have also shown that plasma BCAAs are elevated in the context of (obesity-associated) NAFLD, and that BCAAs may aggravate mitochondrial dysfunction in NAFLD [13,19]. Obesity, MetS and T2D are all related to plasma BCAA elevations [24], and elevated BCAA levels in NAFLD are likely linked to increased insulin resistance and protein catabolism [13,24]. Accumulating evidence points to a key role of mitochondrial dysfunction in the pathophysiology and progression of NAFLD, possibly caused by increased mitochondrial β-oxidation of fatty acids in insulin-resistant states [28,29] and by impaired adaptation of hepatic mitochondrial function in NAFLD [30]. A dysfunctional hepatic tricarboxylic acid (TCA) cycle is regarded as a central feature of hepatic insulin resistance. BCAAs are essential to mediate the transport of carbon substrates for oxidation through the mitochondrial TCA cycle, and an impaired upregulation of BCAA-mediated TCA is thought to be a significant contributor of mitochondrial dysfunction in NAFLD [19]. Moreover, the hepatic accumulation of BCAAs is mainly regulated by the activities of transporters including the SLC43A1 (LAT3), which are responsible for controlling the efflux of BCAAs from the liver to the circulation [44]. In addition to the accumulation of BCAAs in hepatocytes, hepatic BCAA-degrading enzymes are downregulated during worsening of NAFLD [45]. Taken together, it seems plausible that hepatic fat accumulation and altered BCAA metabolism have a negative impact on each other in worsening of NAFLD and further deteriorating BCAA metabolism and their accumulation in the circulation. Notably, the association of plasma BCAA elevations with suspected NAFLD, as cross-sectional documented in the current study, was found to be independent of HOMA-IR and β-cell function. We did not adjust for BMI or waist circumference in the multivariable analysis because obesity indices comprise part of the FLI and HSI algorithms. Such an association of plasma BCAA elevations with suspected NAFLD would suggest that maladaptation in fatty liver may contribute to mitochondrial dysfunction [13], resulting in plasma BCAA elevations.
Our findings regarding the longitudinal association of an elevated FLI with incident T2D was anticipated. Of note, a similar association was found in alternative analysis using an elevated HSI instead. Two recent smaller-scaled studies among subjects of European ancestry, namely, the European Prospective Investigation into Cancer and Nutrition-Potsdam study and a cohort of Spanish adults with pre-diabetes, have demonstrated that an elevated FLI is associated with increased risk of T2D development [6,7]. We also anticipated that plasma BCAA elevations predicted T2D risk [15]. However, to the best of our knowledge, our study shows for the first time that the association of suspected NAFLD with incident T2D appears to be in part attributable to plasma BCAA elevations. In the current study, mediation analysis suggested that 19.6% of the association of an elevated FLI and, alternatively, 22.6% of the association of an elevated HSI with incident T2D was mediated by plasma BCAA concentrations. Hepatic fat accumulation has a deleterious role in T2D development [4,46], and recent evidence shows accumulation of BCAAs in the liver to take place in the context of progressive steatohepatitis [47]. Although our study supports the hypothesis that higher BCAA levels promote the development of T2D, it is clear that further research is needed regarding the mechanisms whereby altered BCAA metabolism and NAFLD may act together to deteriorate glucose tolerance, an effect which seems, at least in part, independent of insulin resistance as demonstrated here with respect to both elevated FLI- and BCAA-associations with incident T2D.
Our study has several strengths. To the best of our knowledge, this is the first study reporting on the joint contributions of NAFLD and higher total plasma BCAAs on the development of T2D. Furthermore, considering a sample size of over 5500 individuals, this is the largest study to date reporting on the association of both suspected NAFLD and plasma BCAA levels with T2D development, which enabled us to carry out sufficiently powered multivariable adjusted analyses. Furthermore, we also used a robust methodology, adjusting our results for relevant variables such as HOMA-IR to investigate the association between FLI and incident T2D. Several other methodological aspects and limitations also need to be addressed. First, the PREVEND cohort study mainly comprises individuals of European ancestry, which could limit extrapolation of our findings to other ethnicities. Second, the FLI is not an absolute measure of hepatic fat accumulation and thus some over- and underestimation of NAFLD could have occurred. However, the FLI is considered to have sufficient accuracy for NAFLD assessment and has been validated against magnetic resonance spectroscopy with moderate diagnostic accuracy for NAFLD [48]. Indeed, the use of the FLI is in line with international guidelines to apply biomarker scores in order to characterize NAFLD in larger-sized cohorts [33,34]. Additionally, liver biopsy, with well-known limitations with respect to invasiveness and sampling variability, or liver ultrasound were not feasible in the PREVEND cohort study, which recruited individuals from the general population. Moreover, the positive associations of plasma BCAA levels with suspected NAFLD and prospective associations with incident T2D were confirmed using the HSI as an alternative algorithm for NAFLD categorization [35]. Third, we did not have measurements of insulin and BCAA levels beyond baseline assessment, which limited us to evaluate the evolution of insulin resistance and regression dilution of BCAAs could not be excluded. Therefore, underestimation of the BCAA–incident T2D associations could have occurred. Fourth, the proportion of subjects using alcohol in excess of 30 g per day in the PREVEND is low (5.2%) [49], and we adjusted for alcohol consumption in all analyses. Finally, people with micro-albuminuria preferentially participated in the PREVEND cohort and therefore multivariable and prospective analyses were adjusted for eGFR and UAE, showing positive and independent associations of plasma BCAA levels in NAFLD and incident T2D development.
5. Conclusions
This large-scale population study cross-sectionally demonstrated that elevated plasma BCAA levels are positively associated with an elevated FLI, as a proxy of NAFLD. Furthermore, it was longitudinally shown that the association of NAFLD with T2D development is likely to be attributable in part to plasma BCAA elevations, which likely reflects abnormalities in BCAA metabolism.
Acknowledgments
J.E. Kootstra-Ros, Laboratory Center, University Medical Center Groningen, supervised the performance of the liver function tests.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/3/705/s1. Table S1: Multivariable linear regression analysis demonstrating the positive association of plasma branched-chain amino acids with an elevated hepatic steatosis index (HSI) (>36) after adjustment for clinical and laboratory covariates in 5791 subjects. Table S2: Prospective associations of HSI with incident type 2 diabetes. Table S3: Prospective associations of BCAAs with incident type 2 diabetes.
Author Contributions
Conceptualization, E.H.v.d.B., J.L.F.-G., S.J.L.B. and R.P.F.D.; Data curation, E.H.v.d.B., J.L.F.-G., E.G.G., M.H.d.B., J.W.-D. and M.A.C.; Formal analysis, E.H.v.d.B., J.L.F.-G., E.G.G. and R.P.F.D.; Investigation, E.H.v.d.B., J.L.F.-G., S.J.L.B. and R.P.F.D.; Methodology, E.H.v.d.B., J.L.F.-G., M.H.d.B., S.J.L.B. and R.P.F.D.; Supervision, S.J.L.B. and R.P.F.D.; Visualization, E.H.v.d.B., J.L.F.-G., S.J.L.B. and R.P.F.D.; Writing—Original draft, E.H.v.d.B., J.L.F.-G. and R.P.F.D.; Writing—Review and editing, E.H.v.d.B., J.L.F.-G., E.G.G., M.H.d.B., J.W.-D., M.A.C., S.J.L.B. and R.P.F.D.
Funding
The Dutch Kidney Foundation supported the infrastructure of the PREVEND program from 1997 to 2003 (Grant E.033). The University Medical Center Groningen supported the infrastructure from 2003 to 2006. This project received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 754425 (‘‘PROMINENT’’). Dade Behring, Ausam, Roche and Abbott financed laboratory equipment and reagents with which various laboratory determinations could be performed. BCAAs were measured at LabCorp, Morrisville, NC, USA, at no cost.
Conflicts of Interest
E.H.v.d.B., J.L.F.-G., E.G.G., M.H.d.B., S.J.L.B. and R.P.F.D. declare no conflict of interest with respect to this manuscript. J.W.-D. and M.A.C. are employees of LabCorp. However, J.W.-D. and M.A.C. had no role in the design of the study; in the data analyses or interpretation of data; in the writing of the initial draft of the manuscript; or in the decision to publish the results.
References
- 1.Loomba R., Sanyal A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Bugianesi E., McCullough A.J., Marchesini G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 3.Lonardo A., Nascimbeni F., Mantovani A., Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018;68:335–352. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Ballestri S., Zona S., Targher G., Romagnoli D., Baldelli E., Nascimbeni F., Roverato A., Guaraldi G., Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016;31:936–944. doi: 10.1111/jgh.13264. [DOI] [PubMed] [Google Scholar]
- 5.Musso G., Gambino R., Cassader M., Pagano G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 6.Jäger S., Jacobs S., Kröger J., Stefan N., Fritsche A., Weikert C., Boeing H., Schulze M.B. Association between the Fatty Liver Index and Risk of Type 2 Diabetes in the EPIC-Potsdam Study. PLoS ONE. 2015;10:e0124749. doi: 10.1371/journal.pone.0124749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franch-Nadal J., Caballería L., Mata-Cases M., Mauricio D., Giraldez-García C., Mancera J., Goday A., Mundet-Tudurí X., Regidor E., PREDAPS Study Group Fatty liver index is a predictor of incident diabetes in patients with prediabetes: The PREDAPS study. PLoS ONE. 2018;13:e0198327. doi: 10.1371/journal.pone.0198327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bril F., Sninsky J.J., Baca A.M., Superko H.R., Portillo Sanchez P., Biernacki D., Maximos M., Lomonaco R., Orsak B., Suman A., et al. Hepatic Steatosis and Insulin Resistance, But Not Steatohepatitis, Promote Atherogenic Dyslipidemia in NAFLD. J. Clin. Endocrinol. Metab. 2016;101:644–652. doi: 10.1210/jc.2015-3111. [DOI] [PubMed] [Google Scholar]
- 9.Nass K.J., van den Berg E.H., Faber K.N., Schreuder T.C.M.A., Blokzijl H., Dullaart R.P.F. High prevalence of apolipoprotein B dyslipoproteinemias in non-alcoholic fatty liver disease: The lifelines cohort study. Metab. Clin. Exp. 2017;72:37–46. doi: 10.1016/j.metabol.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Van den Berg E.H., Amini M., Schreuder T.C.M.A., Dullaart R.P.F., Faber K.N., Alizadeh B.Z., Blokzijl H. Prevalence and determinants of non-alcoholic fatty liver disease in lifelines: A large Dutch population cohort. PLoS ONE. 2017;12:e0171502. doi: 10.1371/journal.pone.0171502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunutsor S.K., Bakker S.J.L., Blokzijl H., Dullaart R.P.F. Associations of the fatty liver and hepatic steatosis indices with risk of cardiovascular disease: Interrelationship with age. Clin. Chim. Acta. 2017;466:54–60. doi: 10.1016/j.cca.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Nair K.S., Short K.R. Hormonal and signaling role of branched-chain amino acids. J. Nutr. 2005;135:1547S–1552S. doi: 10.1093/jn/135.6.1547S. [DOI] [PubMed] [Google Scholar]
- 13.Gaggini M., Carli F., Rosso C., Buzzigoli E., Marietti M., Della Latta V., Ciociaro D., Abate M.L., Gambino R., Cassader M., et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology. 2018;67:145–158. doi: 10.1002/hep.29465. [DOI] [PubMed] [Google Scholar]
- 14.Bloomgarden Z. Diabetes and branched-chain amino acids: What is the link? J. Diabetes. 2018;10:350–352. doi: 10.1111/1753-0407.12645. [DOI] [PubMed] [Google Scholar]
- 15.Wang T.J., Larson M.G., Vasan R.S., Cheng S., Rhee E.P., McCabe E., Lewis G.D., Fox C.S., Jacques P.F., Fernandez C., et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Würtz P., Soininen P., Kangas A.J., Rönnemaa T., Lehtimäki T., Kähönen M., Viikari J.S., Raitakari O.T., Ala-Korpela M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. 2013;36:648–655. doi: 10.2337/dc12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiehn O., Garvey W.T., Newman J.W., Lok K.H., Hoppel C.L., Adams S.H. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS ONE. 2010;5:e15234. doi: 10.1371/journal.pone.0015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman M.A., She P., Peroni O.D., Lynch C.J., Kahn B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 2010;285:11348–11356. doi: 10.1074/jbc.M109.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunny N.E., Kalavalapalli S., Bril F., Garrett T.J., Nautiyal M., Mathew J.T., Williams C.M., Cusi K. Cross-talk between branched-chain amino acids and hepatic mitochondria is compromised in nonalcoholic fatty liver disease. Am. J. Physiol. Endocrinol. Metab. 2015;309:E311–E319. doi: 10.1152/ajpendo.00161.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolak-Dinsmore J., Gruppen E.G., Shalaurova I., Matyus S.P., Grant R.P., Gegen R., Bakker S.J.L., Otvos J.D., Connelly M.A., Dullaart R.P.F. A novel NMR-based assay to measure circulating concentrations of branched-chain amino acids: Elevation in subjects with type 2 diabetes mellitus and association with carotid intima media thickness. Clin. Biochem. 2018;54:92–99. doi: 10.1016/j.clinbiochem.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Connelly M.A., Wolak-Dinsmore J., Dullaart R.P.F. Branched Chain Amino Acids Are Associated with Insulin Resistance Independent of Leptin and Adiponectin in Subjects with Varying Degrees of Glucose Tolerance. Metab. Syndr. Relat. Disord. 2017;15:183–186. doi: 10.1089/met.2016.0145. [DOI] [PubMed] [Google Scholar]
- 22.Iwasa M., Ishihara T., Mifuji-Moroka R., Fujita N., Kobayashi Y., Hasegawa H., Iwata K., Kaito M., Takei Y. Elevation of branched-chain amino acid levels in diabetes and NAFL and changes with antidiabetic drug treatment. Obes. Res. Clin. Pract. 2015;9:293–297. doi: 10.1016/j.orcp.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Haufe S., Witt H., Engeli S., Kaminski J., Utz W., Fuhrmann J.C., Rein D., Schulz-Menger J., Luft F.C., Boschmann M., et al. Branched-chain and aromatic amino acids, insulin resistance and liver specific ectopic fat storage in overweight to obese subjects. Nutr. Metab. Cardiovasc. Dis. 2016;26:637–642. doi: 10.1016/j.numecd.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D., Lien L.F., Haqq A.M., Shah S.H., Arlotto M., Slentz C.A., et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adeva M.M., Calviño J., Souto G., Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids. 2012;43:171–181. doi: 10.1007/s00726-011-1088-7. [DOI] [PubMed] [Google Scholar]
- 26.Lynch C.J., Adams S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014;10:723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.She P., Van Horn C., Reid T., Hutson S.M., Cooney R.N., Lynch C.J. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1552–E1563. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng K.-Y., Watt M.J., Rensen S., Greve J.W., Huynh K., Jayawardana K.S., Meikle P.J., Meex R.C.R. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res. 2018;59:1977–1986. doi: 10.1194/jlr.M085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begriche K., Igoudjil A., Pessayre D., Fromenty B. Mitochondrial dysfunction in NASH: Causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F., Herder C., Carstensen M., Krausch M., Knoefel W.T., et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Kappelle P.J.W.H., Gansevoort R.T., Hillege J.L., Wolffenbuttel B.H.R., Dullaart R.P.F., PREVEND Study Group Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein. J. Intern. Med. 2011;269:232–242. doi: 10.1111/j.1365-2796.2010.02323.x. [DOI] [PubMed] [Google Scholar]
- 32.Borggreve S.E., Hillege H.L., Wolffenbuttel B.H.R., de Jong P.E., Bakker S.J.L., van der Steege G., van Tol A., Dullaart R.P.F., PREVEND Study Group The effect of cholesteryl ester transfer protein -629C->A promoter polymorphism on high-density lipoprotein cholesterol is dependent on serum triglycerides. J. Clin. Endocrinol. Metab. 2005;90:4198–4204. doi: 10.1210/jc.2005-0182. [DOI] [PubMed] [Google Scholar]
- 33.Bedogni G., Bellentani S., Miglioli L., Masutti F., Passalacqua M., Castiglione A., Tiribelli C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–1140. doi: 10.1007/s00125-016-3902-y. [DOI] [PubMed] [Google Scholar]
- 35.Lee J.-H., Kim D., Kim H.J., Lee C.-H., Yang J.I., Kim W., Kim Y.J., Yoon J.-H., Cho S.-H., Sung M.-W., et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., Gordon D.J., Krauss R.M., Savage P.J., Smith S.C., et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 37.Matyus S.P., Braun P.J., Wolak-Dinsmore J., Jeyarajah E.J., Shalaurova I., Xu Y., Warner S.M., Clement T.S., Connelly M.A., Fischer T.J. NMR measurement of LDL particle number using the Vantera Clinical Analyzer. Clin. Biochem. 2014;47:203–210. doi: 10.1016/j.clinbiochem.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Schumann G., Bonora R., Ceriotti F., Férard G., Ferrero C.A., Franck P.F.H., Gella F.J., Hoelzel W., Jørgensen P.J., Kanno T., et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 4. Reference procedure for the measurement of catalytic concentration of alanine aminotransferase. Clin. Chem. Lab. Med. 2002;40:718–724. doi: 10.1515/CCLM.2002.124. [DOI] [PubMed] [Google Scholar]
- 39.Schumann G., Bonora R., Ceriotti F., Férard G., Ferrero C.A., Franck P.F.H., Gella F.J., Hoelzel W., Jørgensen P.J., Kanno T., et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clin. Chem. Lab. Med. 2002;40:725–733. doi: 10.1515/CCLM.2002.125. [DOI] [PubMed] [Google Scholar]
- 40.Schumann G., Bonora R., Ceriotti F., Férard G., Ferrero C.A., Franck P.F.H., Gella F.J., Hoelzel W., Jørgensen P.J., Kanno T., et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 6. Reference procedure for the measurement of catalytic concentration of gamma-glutamyltransferase. Clin. Chem. Lab. Med. 2002;40:734–738. doi: 10.1515/CCLM.2002.126. [DOI] [PubMed] [Google Scholar]
- 41.Preacher K.J., Hayes A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput. 2004;36:717–731. doi: 10.3758/BF03206553. [DOI] [PubMed] [Google Scholar]
- 42.Hayes A.F. Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Commun. Monogr. 2009;76:408–420. doi: 10.1080/03637750903310360. [DOI] [Google Scholar]
- 43.Selvin S. Statistical Analysis of Epidemiologic Data. Oxford University Press; Oxford, UK: 1996. [Google Scholar]
- 44.Bodoy S., Fotiadis D., Stoeger C., Kanai Y., Palacín M. The small SLC43 family: Facilitator system l amino acid transporters and the orphan EEG. Mol. Aspects Med. 2013;34:638–645. doi: 10.1016/j.mam.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Lake A.D., Novak P., Shipkova P., Aranibar N., Robertson D.G., Reily M.D., Lehman-McKeeman L.D., Vaillancourt R.R., Cherrington N.J. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids. 2015;47:603–615. doi: 10.1007/s00726-014-1894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotronen A., Juurinen L., Hakkarainen A., Westerbacka J., Cornér A., Bergholm R., Yki-Järvinen H. Liver fat is increased in type 2 diabetic patients and underestimated by serum alanine aminotransferase compared with equally obese nondiabetic subjects. Diabetes Care. 2008;31:165–169. doi: 10.2337/dc07-1463. [DOI] [PubMed] [Google Scholar]
- 47.Mardinoglu A., Agren R., Kampf C., Asplund A., Uhlen M., Nielsen J. Genome-scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non-alcoholic fatty liver disease. Nat. Commun. 2014;5:3083. doi: 10.1038/ncomms4083. [DOI] [PubMed] [Google Scholar]
- 48.Kahl S., Straßburger K., Nowotny B., Livingstone R., Klüppelholz B., Keßel K., Hwang J.-H., Giani G., Hoffmann B., Pacini G., et al. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS ONE. 2014;9:e94059. doi: 10.1371/journal.pone.0094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruppen E.G., Bakker S.J.L., James R.W., Dullaart R.P.F. Serum paraoxonase-1 activity is associated with light to moderate alcohol consumption: The PREVEND cohort study. Am. J. Clin. Nutr. 2018;108:1283–1290. doi: 10.1093/ajcn/nqy217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.