Abstract
Plant-derived smoke has effects on plant growth. To find the molecular mechanism of plant-derived smoke on maize, a gel-free/label-free proteomic technique was used. The length of root and shoot were increased in maize by plant-derived smoke. Proteomic analysis revealed that 2000 ppm plant-derived smoke changed the abundance of 69 proteins in 4-days old maize shoot. Proteins in cytoplasm, chloroplast, and cell membrane were altered by plant-derived smoke. Catalytic, signaling, and nucleotide binding proteins were changed. Proteins related to sucrose synthase, nucleotides, signaling, and glutathione were significantly increased; however, cell wall, lipids, photosynthetic, and amino acid degradations related proteins were decreased. Based on proteomic and immunoblot analyses, ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) was decreased; however, RuBisCO activase was not changed by plant-derived smoke in maize shoot. Ascorbate peroxidase was not affected; however, peroxiredoxin was decreased by plant-derived smoke. Furthermore, the results from enzyme-activity and mRNA-expression analyses confirmed regulation of ascorbate peroxidase and the peroxiredoxinin reactive oxygen scavenging system. These results suggest that increases in sucrose synthase, nucleotides, signaling, and glutathione related proteins combined with regulation of reactive oxygen species and their scavenging system in response to plant-derived smoke may improve maize growth.
Keywords: proteomics, maize, plant-derived smoke, shoot
1. Introduction
Maize is highly commercial crop, being a major source of food, feed, biofuel, and industrial products [1]. It is the most diverse crop analyzed at morphological and molecular levels [2]. Maize is an ideal crop for genomic studies because it exhibits a high level of genetic diversity and many structural variations [3]. Structural changes and genetic diversity play a key role in the morphology of maize [4]. Maize plant has a very large size genome with a complex organization [5,6]. These findings indicate the importance of maize genetic diversity for the manipulation of new resistant and high yielding varieties.
Fire is documented as ecological factor in ecosystems, because many forest plant species’ life cycles depend on fire [7]. Fire products, which are heat, chemicals, ash, and smoke, have been widely identified as germination cues for different species from both fire-prone and fire-free ecosystems [8]. Plant-derived smoke contains active compounds to promote seed germination of crops [9]. The karrikins and cyanohydrins are identified as germination stimulants present in smoke [9]. These compounds have extensive implications for horticulture, weed control, conservation, and restoration [10]. Plant-derived smoke is a plant growth stimulant obtained from burning of wide variety of biotic sources including leaf, shoot, and straw [11]. These results indicate that plant-derived smoke and compounds isolated from smoke are vital stimulants for germination and plant growth.
Plant-derived smoke stimulated seed germination in 1200 plant species from more than 80 genera of different families [10] including crops [12], medicinal plants [13], and fruit [14]. The promotive effects of plant-derived smoke are independent of seed size, shape, and type [11]. In addition to stimulating seed germination, plant-derived smoke enhanced the seedling length/weight of different crops [15] and pollen germination/tube elongation of flowers belonging to different plant families [16]. Plant-derived smoke induces many changes in seeds, including sensitivity of seeds to phytohormones [17] and increased permeability by softening the seed coat [18]. These findings highlight the promotive effects of plant-derived smoke on plant morphology.
Different physio-chemical contents of plants have been reported to be increased by plant-derived smoke solution [19]. This solution has stimulatory effects on photosynthesis in Isatis indigotica seedlings by enhancing carbon dioxide fixation, the transpiration rate, gaseous exchange, stomatal conductance, and photochemical activities [20]. In addition, total soluble proteins, chlorophyll a/b, total carotenoids, and total nitrogen contents were also increased in smoke treated rice seedlings [21]. Positive effects of plant-derived smoke were also observed on seed germinating enzyme activities in different grasses [22]. This smoke improved the plant-defense system by increasing flavonoids, tannins contents, and level of phenolics [23]. It is presumed that the stimulatory effects of plant-derived smoke are due to its close relation with plant growth regulator [24]. Despite these findings, the mechanism underlying these physiological changes remains unclear and needs an in-depth study to find plant-derived smoke effects on the physiological processes of plants.
Various morphological and physiological studies were conducted to analyze the effects of plant-derived smoke on plants growth; however, its mechanism of action on plant growth has not been investigated yet. The present study is focused on investigating the effects of plant-derived smoke on the initial seedling-stage of maize. Morphological analysis was performed on maize seedlings raised from smoke treated seeds. Based on these morphological results, a gel-free/label-free proteomic analysis was applied to assess the effects of plant-derived smoke solution on maize-shoot. It attempted to explore some clues about the response mechanism of plants towards plant-derived smoke solution at molecular level. In order to further validate and peep into the results at the molecular level, immunoblot, enzyme-activity, and mRNA-expression analyses were performed in a continuation of proteomic results.
2. Results
2.1. Morphological Effects of Plant-Derived Smoke on Maize Growth
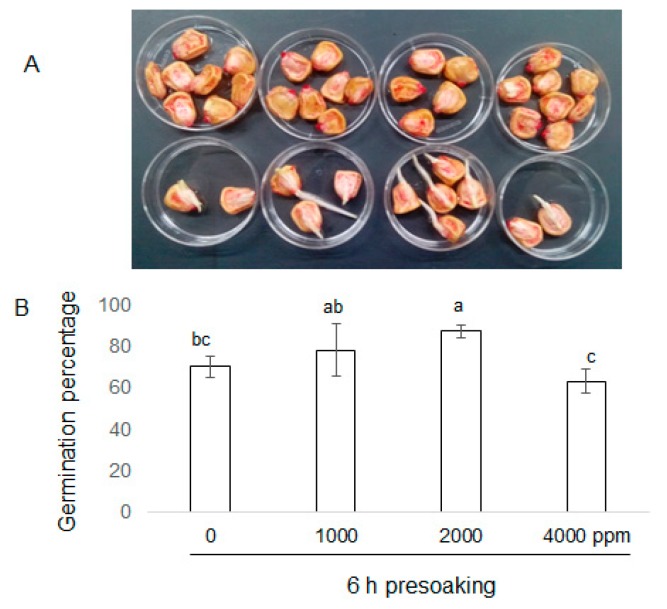
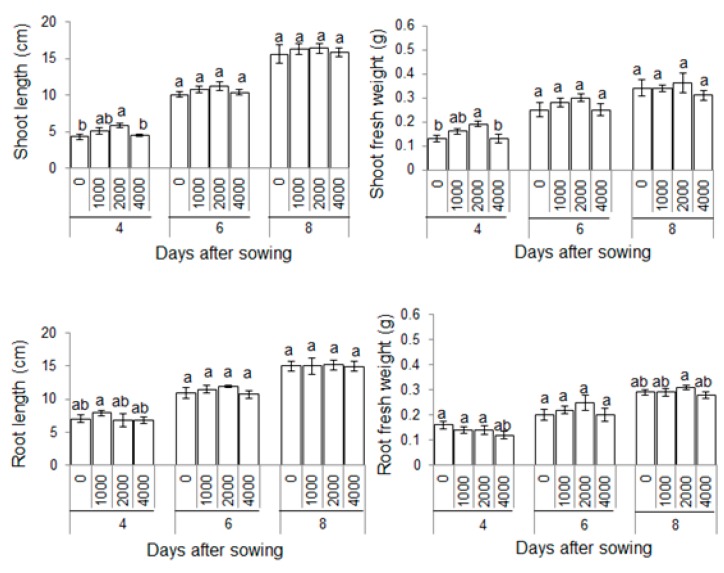
To investigate the effects of plant-derived smoke on maize growth, morphological analysis was performed. Seeds were soaked without or with 1000 ppm, 2000 ppm, and 4000 ppm plant-derived smoke for 6 h. Length and fresh weight of shoot and root were measured for 4, 6, and 8 days after sowing (Figure 1). The seed germination percentage was significantly increased by 2000 ppm plant-derived smoke as compared to control while 4000 ppm plant-derived smoke did not affect germination percentage (Figure 2). Length and fresh weight of shoot were increased by treatments of 1000 ppm and 2000 ppm plant-derived smoke 4 days after sowing (Figure 3). The length of the root was increased more by the 1000 ppm treatment than by the control as indicated in Figure 3; and the increase was not significant with the 2000 ppm treatment.
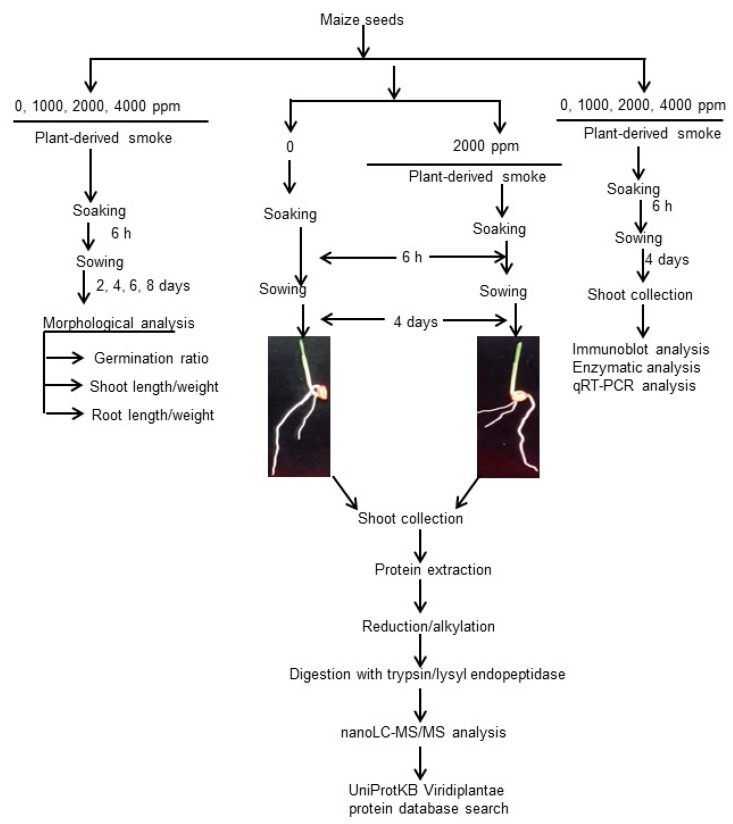
Figure 1.
Experimental design for the effects of plant-derived smoke on maize growth. Maize seeds were soaked without or with 1000 ppm, 2000 ppm, and 4000 ppm plant-derived smoke for 6 h and grown in sand. For morphological analysis, germination ratio, shoot/root length, and weight were measured at 2, 4, 6, and 8 days. For proteomic analysis, maize seeds were soaked with 2000 ppm plant-derived smoke for 6 h. Shoot was collected after 4 days. Proteins were extracted, reduced, alkylated, digested, and analyzed by nano LC-MS/MS. For western blotting, maize seeds were soaked without or with 1000 ppm, 2000 ppm, and 4000 ppm plant-derived smoke for 6 h and grown in sand. Shoot was collected and proteins were extracted after 4 days for immune blotting analysis.
Figure 2.
Germination percentage of maize seeds treated with plant-derived smoke. Maize seeds were soaked without or with 1000 ppm, 2000 ppm, and 4000 ppm plant-derived smoke for 6 h (A,B). The images in (A) correspond to data bars in (B). (A) The above row shows seeds, just after presoaking, while below row shows seeds, 2 days after presoaking. (B) Germination percentage was recorded after 2 days. The data are presented as the mean ± S.D. from 4 independent biological replicates. Different letters indicate that the change is significant as determined by one-way ANOVA followed by Tukey’s multiple comparison (p ˂ 0.05).
Figure 3.
Morphological changes in maize treated with plant-derived smoke. Maize seeds were soaked without or with 1000 ppm, 2000 ppm, and 4000 ppm plant-derived smoke for 6 h and sown in sand for 4, 6, and 8 days. The length and fresh weight of shoot and root were measured. The data are shown as means ± S.D. from 4 independent biological replicates. Different letters indicate that the change is significant as determined by one-way ANOVA followed by Tukey’s multiple comparison (p ˂ 0.05).
The fresh weight of the root was not significantly changed by 1000 ppm or 2000 ppm treatment compared to untreated plant (Figure 3). The length and fresh weight of root were not affected by plant-derived smoke concentrations 6 days after sowing. Treatment with 4000 ppm plant-derived smoke did not affect THE length and fresh weight of shoot and root compared to an untreated plant.
2.2. Functional Classification of Differentially Abundant Proteins in Maize Treated with Plant-Derived Smoke
Maize seeds were treated with 2000 ppm plant derived smoke solution and were grown for 4 days. Proteins were extracted from the 4-days old maize shoot and proteomic response was then investigated. The criteria for significantly changed proteins were 2 or more than 2 matched peptides with a p-value less than 0.05. Gel-free/label-free proteomic analysis revealed that a total of 69 proteins were significantly changed by 2000 ppm plant-derived smoke (Table 1). Results showed that in the biological process category, metabolic related proteins were prominently changed as compared to other categories (Figure S1). For the cellular compartment, cytoplasm localized proteins were significantly changed by plant-derived smoke (Figure S1). For the molecular function, the proteins with catalytic activity were more significantly changed than the other categories (Figure S1).
Table 1.
List of proteins altered by plant-derived smoke solution in maize shoot.
| No | Accession | Description | Fold Change | Functional Category | Biological Process | Cellular Component | Molecular Function |
|---|---|---|---|---|---|---|---|
| 1 | B4FTX3 | Profilin | 7.22 | Cell | not assigned | cell part | not assigned |
| 2 | B9RWF4 | Elongation factor 1-alpha | 6.79 | Protein | not assigned | cell part | binding |
| 3 | A0A1D6BQY9 | Tubulin beta chain | 4.91 | Cell | not assigned | not assigned | not assigned |
| 4 | C5XR12 | Uncharacterized protein | 4.89 | Not assigned | not assigned | not assigned | catalytic activity |
| 5 | K7TFA7 | Aldo/keto reductase family protein | 4.17 | Redox | biosynthetic process | cell part | catalytic activity |
| 6 | C9VWQ3 | Actin | 3.99 | Cell | not assigned | not assigned | not assigned |
| 7 | A0A1D6DZR9 | AICARFT/IMPCHase bienzyme | 3.11 | Nucleotide metabolism | not assigned | not assigned | not assigned |
| 8 | C5YEF7 | Uncharacterized protein | 2.91 | Not assigned | not assigned | cell part | adenyl nucleotide binding |
| 9 | B6T289 | Glucan endo-1,3-β-glucosidase 7 | 2.55 | Not assigned | carbohydrate metabolic process | not assigned | catalytic activity |
| 10 | A0A1B6QAM8 | Uncharacterized protein | 2.36 | Major CHO metabolism | not assigned | not assigned | not assigned |
| 11 | B4FTR5 | Uncharacterized protein | 1.95 | Not assigned | cell communication | cell part | not assigned |
| 12 | C8ZK16 | Malic enzyme | 1.37 | Tricarboxylic acid cycle | not assigned | not assigned | not assigned |
| 13 | C0PG78 | Monocopper oxidase-like protein SKU5 | 0.91 | Development | not assigned | not assigned | binding |
| 14 | A0A1D6M373 | Mannosylglycoprotein endo-beta-mannosidase | 0.91 | Miscellaneous | not assigned | not assigned | not assigned |
| 15 | A0A0P0X334 | Os07g0176900 protein | 0.89 | Photosynthesis | not assigned | not assigned | not assigned |
| 16 | B4FH75 | 4-Hydroxy-tetrahydrodipicolinate reductase 2 | −0.51 | Amino acid metabolism | amine biosynthetic process | cell part | binding |
| 17 | B4FAW7 | Histidinol-phosphate aminotransferase 2 | −0.70 | Amino acid metabolism | amine biosynthetic process | not assigned | binding |
| 18 | A0A1D6KWD0 | Lysine–tRNA ligase | −0.85 | Protein | not assigned | not assigned | not assigned |
| 19 | A0A1D6LT87 | Ras-related protein | −0.89 | Signaling | not assigned | not assigned | not assigned |
| 20 | O24574 | RuBisCO small chain | −1.05 | Photosynthesis | biosynthetic process | cell part | carbon-carbon lyase activity |
| 21 | Q5I204 | Brain acid soluble protein | −1.08 | RNA | not assigned | cell part | binding |
| 22 | A0A1Q0YQ12 | Oil body-associated protein 1B | −1.25 | Not assigned | not assigned | not assigned | not assigned |
| 23 | A0A1D6I8U5 | Flowering time control protein FPA | −1.31 | RNA | not assigned | not assigned | not assigned |
| 24 | K7UK47 | Clathrin interactor EPSIN | −1.69 | Not assigned | not assigned | not assigned | not assigned |
| 25 | A0A0E0CRT7 | Uncharacterized protein | −1.80 | Cell | not assigned | not assigned | not assigned |
| 26 | A0A0D9XRV3 | Adenosylhomocysteinase | −1.88 | Amino acid metabolism | not assigned | not assigned | not assigned |
| 27 | A0A1D6MQK0 | Rab escort protein 1 | −1.89 | Signaling | not assigned | not assigned | not assigned |
| 28 | B6TBI9 | Pyridoxamine 5-phosphate oxidase | −1.95 | Not assigned | not assigned | not assigned | binding |
| 29 | A0A0E0JEZ9 | Uncharacterized protein | −1.96 | Not assigned | not assigned | not assigned | not assigned |
| 30 | B4FTH5 | Xyloglucan endotransglucosylase/hydrolase | −2.06 | Cell wall | carbohydrate metabolic process | apoplast | catalytic activity |
| 31 | B4FZJ2 | β-Glucosidase 11 | −2.13 | Misce | carbohydrate metabolic process | not assigned | catalytic activity |
| 32 | B6TUP8 | Zinc finger homeodomain protein 1 | −2.21 | RNA | not assigned | not assigned | binding |
| 33 | A0A1D6H8U6 | 3-Oxoacyl-[acyl-carrier-protein] reductase | −2.31 | Lipid metabolism | not assigned | not assigned | not assigned |
| 34 | A0A1D6EZ65 | Uncharacterized protein | −2.38 | Not assigned | not assigned | not assigned | not assigned |
| 35 | A0A1D6GLX4 | DEK domain-containing chromatin associated protein | −2.54 | Not assigned | not assigned | not assigned | not assigned |
| 36 | B6TQH7 | THA4 | −2.63 | Not assigned | cellular process | cell part | protein transporter activity |
| 37 | C5WUG0 | Mitogen-activated protein kinase | −2.75 | Signaling | not assigned | not assigned | adenyl nucleotide binding |
| 38 | A0A0K9PCU0 | RPM1-interacting protein 4 | −2.80 | Not assigned | not assigned | not assigned | not assigned |
| 39 | B6TIG8 | Protein arginine N-methyltransferase 1 | −2.80 | Miscellaneous | cellular macromolecule metabolic process | not assigned | catalytic activity |
| 40 | B6TR82 | Thioredoxin F-type | −2.81 | Redox | biological regulation | not assigned | catalytic activity |
| 41 | K3Y2G0 | Uncharacterized protein | −2.83 | Redox | catabolic process | cell part | not assigned |
| 42 | A0A0D3GFF5 | Uncharacterized protein | −2.85 | Stress | not assigned | not assigned | not assigned |
| 43 | B6TYK8 | Putative uncharacterized protein | −2.87 | Not assigned | not assigned | not assigned | not assigned |
| 44 | A0A1D6FY68 | SIT4 phosphatase-associated | −2.95 | Metal handling | not assigned | not assigned | not assigned |
| 45 | A0A1E5V130 | Uncharacterized protein | −3.01 | Not assigned | not assigned | not assigned | not assigned |
| 46 | A0A0N7KSP9 | Os11g0247300 protein | −3.18 | Cell | not assigned | not assigned | not assigned |
| 47 | A0A1D6JR65 | Alcohol dehydrogenase-like 2 | −3.22 | Miscellaneous | not assigned | not assigned | not assigned |
| 48 | B6SW97 | Putative uncharacterized protein | −3.28 | Protein | not assigned | not assigned | not assigned |
| 49 | B6U581 | Ribosome-like protein | −3.31 | Protein | biosynthetic process | cell part | structural constituent of ribosome |
| 50 | K4BGM2 | Uncharacterized protein | −3.40 | Protein | biological regulation | cell part | binding |
| 51 | B5QSJ9 | Acetyl-coenzyme A carboxylase | −3.53 | Lipid metabolism | biosynthetic process | not assigned | acetyl-CoA carboxylase activity |
| 52 | A0A1D6L558 | Plasmodesmata callose-binding protein 2 | −3.67 | Miscellaneous | not assigned | not assigned | not assigned |
| 53 | A0A0K9NNM0 | Cysteine proteinase cathepsin F | −3.69 | Protein | not assigned | not assigned | not assigned |
| 54 | C0P3K6 | Aspartic proteinase A1 | −3.75 | Protein | lipid metabolic process | not assigned | aspartic-type endopeptidase activity |
| 55 | J3LDT9 | Uncharacterized protein | −3.76 | Not assigned | anatomical structure morphogenesis | cell part | not assigned |
| 56 | B9MSV5 | Tubulin alpha chain | −3.77 | Cell | cellular component assembly | cell part | binding |
| 57 | A0A068UVK8 | Chlorophyll a-b binding protein | −3.84 | Photosynthesis | cellular macromolecule metabolic process | cell part | binding |
| 58 | B6SZR1 | Chlorophyll a-b binding protein | −3.97 | Photosynthesis | cellular macromolecule metabolic process | cell part | binding |
| 59 | A0A1D6LJZ2 | 30S ribosomal protein S16 | −4.00 | Protein | not assigned | not assigned | not assigned |
| 60 | B4FV94 | Chlorophyll a-b binding protein | −4.06 | Photosynthesis | cellular macromolecule metabolic process | cell part | binding |
| 61 | E9KIP1 | Photosystem I P700 chlorophyll apoprotein A1 | −4.13 | Photosynthesis | cellular macromolecule metabolic process | cell part | 4 iron, 4 sulfur cluster binding |
| 62 | A0A061EVS4 | Nascent polypeptide-associated complex beta | −4.23 | RNA | biological regulation | not assigned | not assigned |
| 63 | A0A1D6IIC3 | Nuclear transport factor 2 | −4.38 | Protein | not assigned | not assigned | not assigned |
| 64 | A0A1D5WFY2 | Small ubiquitin-related modifier | −4.40 | Protein | not assigned | not assigned | not assigned |
| 65 | A0A1D6PYA1 | 60S ribosomal protein L17 | −4.61 | Protein | not assigned | not assigned | not assigned |
| 66 | Q8H6N0 | Tubulin beta chain | −4.76 | Cell | cellular component assembly | cell part | binding |
| 67 | A0A1D5AHD9 | RuBisCO large chain | −4.79 | Photosynthesis | not assigned | not assigned | not assigned |
| 68 | A0A1D1Z067 | Elongation factor 1-alpha | −6.21 | Protein | not assigned | not assigned | not assigned |
| 69 | A0A097PJF2 | Structural maintenance of chromosomes protein 1 | −9.99 | Cell | cell cycle process | cell part | adenyl nucleotide binding |
Accession, according to UniProtKB Viridiplantae protein database; Fold change, relative abundance of identified proteins in maize shoot raised from seeds treated with 2000 ppm plant-derived smoke solution; Functional category, protein function categorized using MapMan bin codes. Abbreviations are as follows: cell, cell division/organization/vesicle; transport; CHO, carbohydrate; protein, protein synthesis/degradation/post-translational modification/targeting; RNA, RNA processing/ transcription/binding; Redox, redox homeostasis; DNA, nucleotide binding, and metal ion binding.
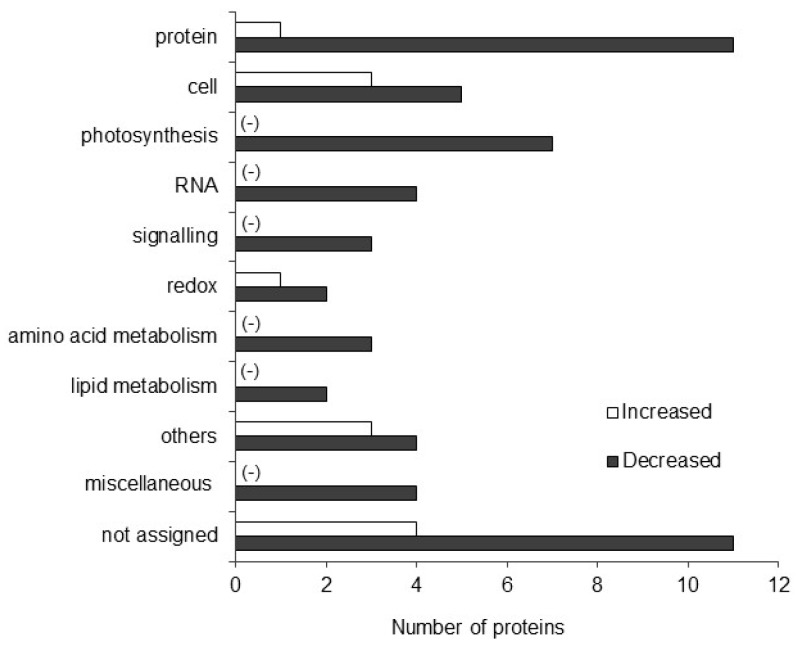
To determine the function of proteins in response to the plant-derived smoke solution, functional classification of identified proteins was performed using MapMan bin codes (Figure 4). It was found that 1 and 11 proteins involved in protein synthesis, degradation, post-translational modification, targeting and folding were increased and decreased, respectively, in response to plant-derived smoke solution. Plant-derived smoke treatment increased one and decreased two proteins related to redox homeostasis. Three proteins related to signaling and 4 transport related proteins were also increased, while RNA, amino acid metabolism and lipid metabolism related proteins were decreased in maize shoot in response to the plant-derived smoke solution (Figure 4).
Figure 4.
Categorization of identified proteins on the basis of their function in maize shoot in response to plant derived smoke solution. Maize seeds were treated without or with 2000 ppm plant-derived smoke solution for 6 h and grown for 4 days. Proteins were extracted from shoot and identified using a gel-free/label-free proteomic technique. Mapman bin code software was used to categories all the identified 69 proteins. Categories containing zero proteins are marked with (−). Abbreviations: protein, protein synthesis/degradation/ post-translational modification/targeting/folding; cell, cell division/organization/vesicle transport; RNA, RNA processing/ transcription/binding; Redox, redox homeostasis; The “Others” category includes proteins related to N-metabolism, cellular homeostasis, response to stimulus, cytoplasm, fermentation, DNA, nucleotide binding, and metal ion binding.
2.3. Pathway Analysis of Identified Proteins in Maize Treated with Plant-Derived Smoke
The plant-derived smoke treatment significantly changed some proteins. To visualize these proteomic results in the context of pathways and processes, MapMan software was used (Figure S2). Proteins associated with starch/sucrose, glutathione and nucleotide biosynthesis were significantly increased; while, ascorbate, photosynthetic, lipids, and cell wall related proteins were decreased in response to plant-derived smoke (Figure S2).
2.4. Immuno-Blot Analysis of Proteins Involved in Redox Homeostasis and Photosynthesis
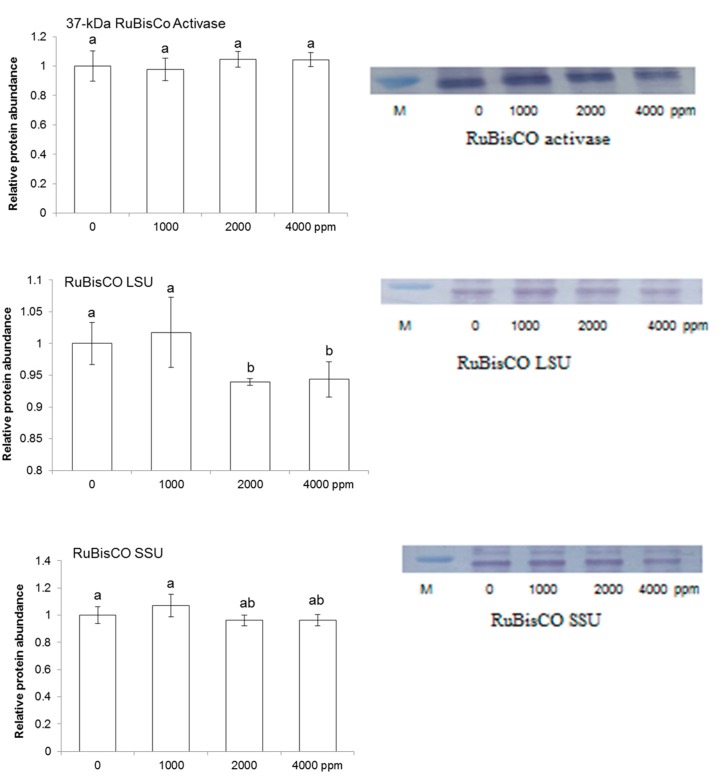
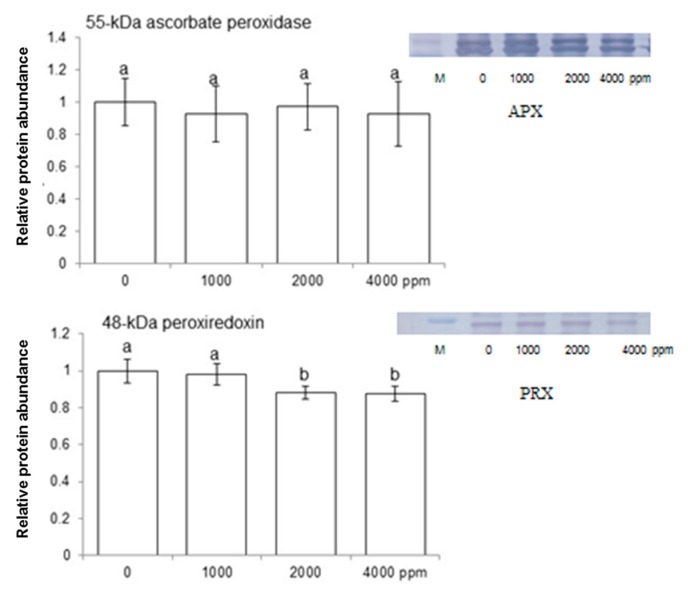
To find out abundance level of large and small RuBisCO subunits, RuBisCO activase, ascorbate peroxidase, and peroxiredoxin in maize shoot, immunoblot analysis was performed. Maize seeds were treated without or with 1000 ppm, 2000 ppm, and 4000 ppm plant-derived smoke and proteins were extracted from shoot after 4 days of sowing (Figure 1). Plant-derived smoke did not affect the abundance of 37-kDa RuBisCO activase while decreasing the abundance of RuBisCO large subunit in shoot of 4-day-old plants treated by 2000 ppm and 4000 ppm (Figure 5). Abundance of 55 kDa ascorbate peroxidase was not affected by different treatments of plant-derived smoke; while, 2000 ppm and 4000 ppm plant-derived smoke very slightly decreased the abundance of 37-kDa peroxiredoxin compared to 1000 ppm and the control (Figure 6).
Figure 5.
Effect of plant-derived smoke on RuBisCO subunits and activase enzymes in maize. Maize seeds were treated without or with 1000, 2000, and 4000 ppm plant-derived smoke for 6 h and sown in sand. Proteins were extracted from 4-days old maize shoot. ImageJ software was used to determine relative proteins intensities. Data are shown as means ± S.D. Different alphabets showing the statistical level of significance as determined by one-way ANOVA followed by Tukey’s multiple comparison (p ˂ 0.05). In images, ‘M’ is used for marker.
Figure 6.
Effect of plant-derived smoke on redox-homeostasis related proteins in maize. Maize seeds were treated without or with 1000, 2000, and 4000 ppm plant-derived smoke for 6 h and grown for 4 days. Proteins extracted from shoot were separated by SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidine difluoride membranes. The membranes were incubated with anti-RuBisCO large/small subunits and anti-RuBisCO activase antibodies. The relative intensities of bands were calculated using ImageJ software. Data are shown as means ± S.D. from 3 independent biological replicates. Different letters indicate that the change is statistically significant as determined by one-way ANOVA followed by Tukey’s multiple comparison (p ˂ 0.05). In images, “M” is used for marker.
2.5. Effect of Plant-Derived Smoke on Enzymatic Activities and Gene Expression of Maize
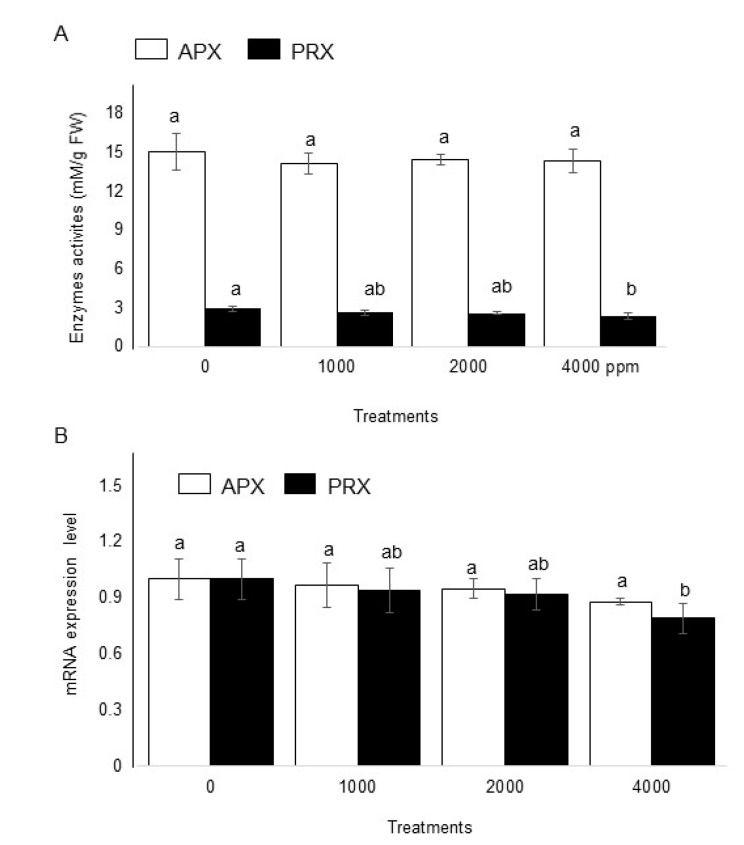
To investigate the effects of plant-derived smoke on ROS scavenging enzymes, enzymatic analysis was performed. Seeds were soaked in without or with 1000 ppm, 2000 ppm, and 4000 ppm plant-derived smoke for 6 h and maize shoot was collected 4 days after sowing (Figure 1). Results showed that 1000 ppm, 2000 ppm, and 4000 ppm plant-derived smoke did not change activity of ascorbate peroxidase as compared to the control (Figure 7A). On the other hand, treatments 1000 ppm and 2000 ppm and 4000 ppm slightly, but significantly affected the activity of peroxiredoxin with a significant decrease compared to the control (Figure 7A).
Figure 7.
Effect of plant-derived smoke on enzymatic analysis and expression level of ascorbate peroxidase and peroxiredoxin gene in maize shoot. Maize seeds were treated without or with 1000 ppm, 2000 ppm, and 4000 ppm plant-derived smoke for 6 h and sown. Activities of ascorbate peroxidase (APX) and peroxiredoxin (PRX) were measured in 4-day old maize shoot (A). For gene expression level, RNAs extracted from maize shoot at 3rd day of treatment were analyzed by qRT-PCR (B). Relative mRNA abundances of ascorbate peroxidase and peroxiredoxin were normalized against 18S rRNA abundance. The data are shown as means ± S.D. from 4 independent biological replicates. White and black bars indicate ascorbate peroxidase (APX) and peroxiredoxin (PRX), respectively. Different letters indicate that the change is significant as determined by one-way ANOVA followed by Tukey’s multiple comparison (p ˂ 0.05).
To check the specific expression pattern of genes encoding ascorbate peroxidase and peroxiredoxin, their mRNA levels in maize shoot were analyzed. Shoot total RNA was extracted, and qRT-PCR was performed. The 18S rRNA was used as an internal control on qRT-PCR. Results revealed that 1000 ppm, 2000 ppm and 4000 ppm plant-derived smoke did not significantly affect the expression level of ascorbate peroxidase (Figure 7B); however, the expression level of peroxiredoxin was regulated with decreased expression at the transcript level in response to plant-derived smoke (Figure 7B).
3. Discussion
3.1. Effect of Plant-Derived Smoke on Morphology of Maize
Plant-derived smoke increased seed germination in different plant species [25]. Soós et al. [26] reported that plant-derived smoke enhanced the post-germination growth of maize. In this study, 2000 ppm plant-derived smoke significantly increased the seed germination percentage; furthermore, shoot/root length/fresh weight were also increased in maize. Aslam et al. [27] reported that plant-derived smoke increased the germination percentage, shoot/root length, and fresh weight of maize. Furthermore, plant-derived smoke treated maize developed vigorous seedling and fresh weight [28]. Abu et al. [29] reported that priming seeds in low concentrations of plant-derived smoke increased the germination percentage and seedling length in wild rye. On the other hand, high concentrations of plant-derived smoke reduced germination and shoot/root growth in lettuce [30]. In this study, 4000 ppm plant-derived smoke did not affect maize seed germination and shoot/root length/fresh weight (Figure 2 and Figure 3). This finding is similar to previous results revealing that plant-derived smoke inhibited seed germination percentage at higher concentrations.
Plants treated with 2000 ppm plant-derived smoke and 10−8 M karrikins produced vigorous shoot and increased leaf area in Eucomis autumnalis [31]. The vigorous shoot and high leaf area enhanced photosynthetic activities in plants [31]. Flematti et al. [32] reported that plant-derived smoke containing karrikins enhanced germination percentage and shoot length/fresh weight in Solanum orbiculatum. Plant-derived smoke improved the shoot length and fresh weight in several plants, including acacia [33], onion [34], and milk thistle [35]. Our results are in accordance with previous findings suggesting that plant-derived smoke increases shoot length and fresh weight. Based on these results, 4-days old shoot raised from maize seeds treated by 2000 ppm plant-derived smoke was selected for gel-free/label free proteomic analysis.
3.2. Positive Effect of Plant-Derived Smoke on Cytoskeleton Related Proteins in Maize
With treatment of plant-derived smoke, the fold change of cytoskeleton related proteins such as profilin and actin was significantly increased compared to protein abundance of untreated plant (Table 1). The plant cytoskeleton plays an important role in many biological processes, including cell division, expansion, organogenesis, tip growth, and intracellular signaling [36]. The actin cytoskeleton proteins are concerned with the establishment and maintenance of cell polarity, and responses to numerous environmental stimuli [37]. Profilin promoted F-actin elongation which played an important role in the mobility and cell contraction during cell division [38]. Profilins are multifunctional proteins according to their abundance and locations [39]. Furthermore, profilin is associated with plasma membrane during development of microspores, pollens, and playing vital role in signal transduction [40]. The present results confirmed that seed priming in plant-derived smoke promoted shoot cytoskeleton proteins, which are necessary for cell division, signaling, and pollens development.
3.3. Effect of Plant-Derived Smoke on Photosynthetic Proteins in Maize
Plant derived smoked changed 8 photosynthesis related proteins with different regulation. RuBisCO is the most abundant protein on earth playing key role in photosynthesis having CO2 fixation function [41]. Strong reduction was observed in activity of RuBisCO under drought stress [42]. Khodadadi et al. [43] observed that the activity of RuBisCO was reduced under drought stress. Another study reported that RuBisCO is responsive to stresses and the rapid loss of its activity caused by drought in sunflower [44]. Beside drought, cold stress also reduced the activity of RuBisCO, suggesting damage to the chloroplasts and a decrease in the rate of photosynthesis under these conditions. In plant-smoke derived compounds, karrikins also decreased RuBisCO in Arabidopsis [45]. The present immunoblot result revealed that RuBisCO activase is not affected by plant-derived smoke; however, a RuBisCO large subunit was decreased by plant-derived smoke. It may be possible that plant-derived smoke plays an inhibitory role at higher concentrations. It is also possible that this short period of presoaking in plant-derived smoke is not enough to activate the RuBisCO activase enzyme.
3.4. Effect of Plant-Derived Smoke on Scavenging Activity through the Ascorbate/Glutathione Pathway in Maize
Ascorbate and glutathione are main antioxidants in the leaves of maize [46]. The fundamental role of these compounds is linked to scavenging a broad range of reactive oxygen species generated under stress conditions in plants [47]. It participates in scavenging of hydrogen peroxide [48]. Selote and Chopra [49] reported that drought stress increased the amount of glutathione in rice. It is reported that ascorbate peroxidase was decreased under flooding conditions [50]. Present results revealed that ascorbate is decreased while glutathione is increased at the proteomic level. These results suggest that plant-derived smoke seed pretreatment may have positive effects on glutathione pool in the maize seedlings that might be helpful in achieving better performance during stress responses.
3.5. Effect of Plant-Derived Smoke on Enzymatic Activities of Maize
Ascorbate peroxidase and peroxiredoxin are important enzymes that are present in the plant kingdom [51]. The exposure of plants to unfavorable environmental conditions increases the production of reactive oxygen species such as hydrogen peroxide, and hydroxyl radical [52]. The reactive oxygen species detoxification process in plants is essential and occurs due to different enzymes like ascorbate peroxidase and peroxiredoxin in plant cells and their organelles [52]. Ascorbate peroxidase utilizes ascorbate as a specific electron donor to reduce hydrogen peroxide to water [53]. Experimental evidence has proven that during the metabolism process, antioxidant enzymes are also produced to balance metabolism [54]. On the other hand, peroxiredoxins constitute the most recently identified group of hydrogen peroxide -decomposing antioxidant enzymes [55]. It is reported that plant-derived smoke decreased scavenging enzymes activity in maize [56]. Another redox homeostasis enzymes, thioredoxins (Trxs) was also affected by plant-derived smoke treatments. It is a small and widely distributed protein with a conserved active site, which controls the redox status of target proteins through thiol-disulfide exchange reactions [57]. In plants, it has a fundamental role in a number of cellular processes, including seed germination, carbon assimilation, lipid metabolism, hormone metabolism, redox signaling, and stress response [57]. The present results are in accordance with the previous reports and showed that plant-derived smoke did not significantly affect the activities of ascorbate peroxidase and that peroxiredoxin activities were decreased, which revealed that plant-derived smoke is a growth promoter and stress suppressor, which decreases the production of stress responsive hormones.
Antioxidants are mostly expressed to cope with stressed situations and their expression also has positive effects on the activation of enzymes related to plant growth [58,59]. In the present study, plant-derived smoke is behaving as a growth promoter and thus did not affected ascorbate peroxidase enzyme levels (Figure 7A). Auxins might not affect or could promote increases in the activity of antioxidant enzymes regulating ROS levels, which could be associated with the activation of embryo/organogenesis [60]. It might be suggested from the results that treatment with plant-derived smoke could markedly enhance the self-capacity of defense against oxidative damage in normal growth conditions, thus not affecting the production of antioxidant enzymes in maize seedlings significantly.
3.6. Effect of Plant-Derived Smoke on Expression of Ascorbate Peroxidase and Peroxiredoxin in Maize
Plant-derived smoke did not affect ascorbate peroxidase gene expression level, whereas gene expression of peroxiredoxin was decreased at higher concentrations (Figure 7B). Ascorbate peroxidase is an important enzyme of plant scavenging system. It uses ascorbate as a specific electron donor for the conversion of hydrogen peroxide into water [61]. Besides this, it also improves the stress resistance capacity in plants against various stresses [62,63]. Ascorbate peroxidase also plays a key role in balancing the homeostasis of ascorbate and glutathione, and maintaining high photosynthetic rate in unfavorable conditions [64]. In addition, ascorbate is involved in other functions such as plant growth, gene regulation, modulation of some enzymes, and redox regulation of membrane-bound antioxidant compounds [65]. The present results revealed that ascorbate peroxidase related gene was not significantly altered in maize seedlings due to plant-derived smoke. The present results are consistent with immunoblotting results showing no effect on the abundance of ascorbate peroxidase. Decreased peroxiredoxin abundance in maize shoot in response to plant derived smoke reflects the resource economy phenomena of all living organisms, including plants. Peroxiredoxin might be present in maize plants, taking part in plant defense system against stresses. There is close interaction between plant-derived smoke and plant growth hormones [24]. It is also possible that plant immune systems might be strengthened by plant-derived smoke, resulting in a decreased level of peroxiredoxin level so as to economize plant resources. These results are in agreement with El-Gaied et al. [59] who clearly demonstrated the decreased antioxidant enzymes level in the tomato plant in response to plant growth promoting hormones.
4. Materials and Methods
4.1. Preparation of Plant-Derived Smoke Solution
Smoke solution was prepared from aerial semi dried parts of Cymbopogon jawarncusa [66]. Dry plant material weighing 333 g was taken and placed in a burner [67]. An electric heater was adjusted beneath the burner until all the plant material was converted into ash. Smoke was bubbled though 1 L of distilled water, resulting into 1 L of concentrated plant-derived smoke solution. It was further diluted to 1000 ppm, 2000 ppm, and 4000 ppm and used for seed treatment in the experiment. The seeds treated with distilled water were used as the control.
4.2. Plant Material and Treatment
Seeds of maize (Zea mays L. cv. Azam) were sterilized with 3% sodium hypochlorite solution. The sterilized seeds were primed with 1000 ppm, 2000 ppm, and 4000 ppm plant-derived smoke for 6 h, and then sown in seedling case (150 mm × 60 mm × 100 mm) supplied with water. The seeds treated with distilled water were used as control. Maize was grown in growth chamber illuminated with white fluorescent light (160 μmol·m−2·s−1, 16 h light period/day) at 25 °C with 60% humidity. Germination percentage was recorded after two days of sowing. Fifteen seeds were sown for each treatment and 4 independent replicates were used for morphological analysis.
For proteomics and further investigation through immunoblot analysis, enzymatic analysis and qRT-PCR, maize seeds (Zea mays L. cv. Honey Bantam) were sterilized using 3% sodium hypochlorite solution, soaked in 2000 ppm plant-derived smoke for 6 h and grown in 450 mL silica sands with water in seedling case (150 mm × 60 mm × 100 mm). The seeds treated with distilled water were used as control. Conditions in growth chamber were illumination with white fluorescent light (160 μmol·m−2·s−1, 16 h light period/day) at 25 °C with 60% humidity. Shoot was collected on the 4th day after sowing from 3 biological replicates. Shoot raised from the seeds treated with distilled water served as control. Biological replicates mean that maize was sown on different days.
4.3. Protein Extraction
A portion (300 mg) of maize shoot was cut into small pieces and ground 60 times in 2 mL tube. It was ground 30 times after adding 50 µL of lysis buffer containing 7 M urea, 2 M thiourea, 5% CHAPS, and 2 mM tributylphosphine. Furthermore, 50 µL of lysis buffer was added and ground for 30 times. Suspension was incubated for 2 min at 25 °C and centrifuged at 15,000× g for 2 min at 25 °C. Afterwards, the filter cartridge was removed and supernatant was collected as total proteins.
4.4. Protein Enrichment, Reduction, Alkylation, and Digestion
Extracted proteins (100 µg) in lysis buffer were adjusted to a final volume of 100 µL. Methanol (400 µL) was added to each sample and mixed before the addition of 100 µL of chloroform and 300 µL of water. After mixing and centrifugation at 20,000× g for 10 min to achieve phase separation, the upper phase was discarded and 300 µL of methanol was added to the lower phase, and then centrifuged at 20,000× g for 10 min. The pellet was collected as the soluble fraction [68].
Proteins were resuspended in 50 mM NH4HCO3, reduced with 50 mM dithiothreitol for 30 min at 56 °C, and alkylated with 50 mM iodoacetamide for 30 min at 37 °C in the dark. Alkylated proteins were digested with trypsin and lysyl endopeptidase (Wako, Osaka, Japan) at a 1:100 enzyme/protein ratio for 16 h at 37 °C. Peptides were desalted with Mono Spin C18 Column (GL Sciences, Tokyo, Japan). Peptides were acidified with formic acid (pH < 3) and analyzed by nano-liquid chromatography (LC) mass spectrometry (MS)/MS.
4.5. Measurement of Protein and Peptide Concentrations
The method of Bradford [69] was used to determine the protein concentration with bovine serum albumin used as the standard.
4.6. Protein Identification Using NanoLC-MS/MS
The peptides were loaded onto the LC system (EASY-nLC 1000; Thermo Fisher Scientific, San Jose, CA, USA) equipped with a trap column (Acclaim PepMap 100 C18 LC column, 3 µm, 75 µm ID × 20 mm; Thermo Fisher Scientific) equilibrated with 0.1% formic acid and eluted with a linear acetonitrile gradient (0–35%) in 0.1% formic acid at a flow rate of 300 nL/min. The eluted peptides were loaded and separated on the column (Easy-Spray C18 LC column, 3 µm, 75 µm ID × 150 mm; Thermo Fisher Scientific) with a spray voltage of 2 kV (Ion Transfer Tube temperature: 275 °C). The peptide ions were detected using MS (Orbitrap Fusion EDT MS; Thermo Fisher Scientific) in the data-dependent acquisition mode with the installed Xcalibur software (version 4.0; Thermo Fisher Scientific). Full-scan mass spectra were acquired in the MS over 375–1500 m/z with resolution of 120,000. The most intense precursor ions were selected for collision-induced fragmentation in the linear ion trap at a normalized collision energy of 35%. Dynamic exclusion was employed within 90 s to prevent repetitive selection of peptides [70].
4.7. MS Data Analysis
The MS/MS searches were carried out using the Mascot (version 2.6.1, Matrix Science, London, UK) and SEQUEST HT search algorithms against the UniProtKB Viridiplantae protein database (2017-07) using Proteome Discoverer 2.1 (version 2.1.1.21; Thermo Fisher Scientific). The workflow for both algorithms included a spectrum selector, Mascot, SEQUEST HT search nodes, percolator, ptmTS, event detector, and precursor ion area detector nodes. Oxidation of methionine was set as a variable modification and carbamidomethylation of cysteine was set as a fixed modification. MS and MS/MS mass tolerance were set to 10 ppm and 0.6 Da, respectively. Trypsin was specified as the protease and a maximum of one missed cleavage was allowed. Target-decoy database searches used for calculation of false discovery rate (FDR) and for peptide identification FDR was set at 1%. Label-free quantification was also performed with Proteome Discoverer 2.1 using precursor ions area detector nodes.
4.8. Differential Analysis of Proteins Using MS Data
For differential analysis of the relative abundance of peptides and proteins between samples, the freely software PERSEUS (version 1.6.0.7) [71] was used. Proteins and peptides intensities were transferred into log2 scale. Three biological replicates of each sample were grouped and a minimum of 3 valid values was required in at least one group. Normalization of the intensities was performed to subtract the median of each sample. Missing values were imputed based on a normal distribution (width = 0.3, down-shift = 2.2). Significance was assessed using student’s t-test analysis. Accession codes is as follows: For MS data, RAW data, peak lists and result files have been deposited in the ProteomeXchange Consortium [72] via the jPOST [73] partner repository under data-set identifiers PXD008315.
4.9. Functional Categorization
The protein sequences of the differentially changed proteins, based on the Lan10 strain, were subjected to a BLAST query against the Ami gene ontology database (http://amigo1.geneontology.org/cgi-bin/amigo/blast.cgi). The corresponding Ami gene ontology terms were extracted from the most homologous proteins using a Perl program. The Ami gene ontology database annotation results were plotted by the Web Gene Ontology Annotation Plot (http://wego.genomics.org.cn/) tool by uploading compiled Web Gene Ontology Annotation Plot native format files containing the obtained Ami gene ontology terms. Functional categorization of identified proteins was performed using MapMan bin codes (http://mapman.gabipd.org/) [74]. Visualization of protein abundance was performed using MapMan software (version 3.6.0 RC1, http://mapman.gabipd.org/web/guest/mapman) [75]. The software and mapping files of Gmax_109_peptide were also downloaded from the MapMan website.
4.10. Immunoblotting Analysis
Maize shoot (100 mg) sample was ground in an SDS-sample buffer consisting of 60 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, and 5% 2-mercaptoethanol using mortar and pestle [76]. The obtained mixture was centrifuged 2 times at 15,000× g for 10 min and protein was collected as a supernatant. SDS-polyacrylamide gel electrophoresis was used to separate protein (10 µg) in SDS-sample buffer. The separated proteins were shifted to polyvinylidene difluoride membrane using a semi-dry transfer blotter. A buffer containing 137 mM NaCl, 20 mM Tris-HCl (pH 7.5), 0.1% Tween-20, and a blocking solution (Wako) was used to block the blotted membrane for 1 h. Afterwards, different diluted (1:1000) anti-ascorbate peroxidase antibody [77], anti-peroxiredoxin antibody [78], anti-ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) large and small subunits antibodies [79], and anti-RuBisCO activase antibody [80] was used to incubate the membrane for 1 h. The membrane was washed 3 times with buffer containing 137 mM NaCl, 20 mM Tris-HCl (pH 7.5), and 0.1% Tween-20 and treated for 1 h with anti-rabbit IgG conjugated with horseradish peroxidase (Bio-Rad, Hercules, CA, USA) as secondary antibody. The membrane was incubated with TMB membrane peroxidase substrate system (KPL, Sylacauga, AL, USA). ImageJ software (version 1.46, https://imagej.nih.gov/ij/) was used to calculate the relative intensities of bands.
4.11. Enzymatic Analysis
For ascorbate peroxidase analysis, a sample (1 g) of maize shoot was ground in pestle and mortar with liquid nitrogen. This grinded mixture was homogenized in 50 mM potassium phosphate buffer (pH 7.0) containing 0.5 mM Ascorbic acid, 0.1 mM EDTA and 0.1 mM hydrogen peroxide [81]. The hydrogen peroxide-dependent oxidation of Ascorbic acid was followed by monitoring the decrease in absorbance at 290 nm assuming an absorption coefficient of 2.8 mM−1·cm−1. For peroxiredoxin analysis, the assay contains 100 mM potassium phosphate-buffer (pH 7.0), 0.3–3 µM peroxiredoxin, 100 µM hydrogen peroxide in a total volume of 1000 µL. The reaction was stopped with 800 µL oftrichloroacetic acid (12.5%) to an aliquot of 50 µL of assay solution. After the addition of 200 µL, 10 mM Fe (NH4)2(SO4)2 and 100 µL of 2.5 M KSCN, the absorbance at 480 nm was measured to quantify the hydrogen peroxide contents of the solution, and hydrogen peroxide reduction rates were calculated [82].
4.12. RNA Extraction and Reverse Transcription Polymerase Chain Reaction Analysis
A total of 100 mg maize shoot was ground in mortar and pestle using liquid nitrogen. Total RNAs was extracted by RNeasy plant mini kit (Qiagen, Valencia, CA, USA) from maize shoot powder and treated with RNase-free DNase I during extraction. cDNA was synthesized from the extracted RNAs by using RevertAid first strand cDNA synthesis Kit (Thermo Scientific) in reverse transcription polymerase chain reaction (qRT-PCR). Fast Real Time PCR system (7900HT; Applied Biosystems, Foster City, CA, USA) was used to perform a qRT-PCR reaction at the following conditions; 95 °C for 600 s, followed by 35 cycles of 95 °C for 15 s and 60 °C for 60 s. As an internal control, 18S rRNA was used to normalize the gene expression. For normalization of gene expression, 18S rRNA was used as an internal control. For quantification of specific gene, primers were designed for regions of interest using NCBI tool (primer blast) and Primer 3 online bioinformatics tools. Quantitative variation between different samples was calculated by the relative quantification method (2−ΔΔCt) [83].
4.13. Statistical Analysis
Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison among multiple groups using SPSS (version 22.0; IBM, Armonk, New York, USA). A p-value of less than 0.05 was considered to be statistically significant.
5. Conclusions
Gel-free/label-free proteomic technique was used to examine the effects of plant-derived smoke on maize growth after 6 h presoaking. The main results of this study are as follows: (i) Plant-derived smoke increased seed germination and seedling length/ fresh weight at low concentrations; (ii) Nucleotide, starch degradation, and glutathione related proteins were increased; (iii) Protein synthesis/degradation and cell division/organization proteins were changed; (iv) Cell wall, lipids, photosynthetic, and amino acid degradations related proteins ware decreased; (v) plant-derived smoke increased cytoskeleton proteins in maize; (vi) plant-derived smoke did not affect the activity of ascorbate peroxidase and decreased the activity of peroxiredoxin; (vii) gene expression level of peroxiredoxin was altered by plant-derived smoke. These results suggest that plant-derived smoke affects the proteins related to metabolic processes while inhibiting proteins related with lipids, proteins, and cell wall. Furthermore plant-derived smoke regulates the reactive oxygen species and their scavenging system. Although various studies have been conducted demonstrating the promoting effects of plant derived smoke solution on different growth parameters of plants, the molecular response of plant to plant derived smoke solution remained unknown. This study was carried out to fill the gap between various physiological processes regulated by a plant derived smoke solution and the possible mechanism of action behind it.
Acknowledgments
The authors thank Dr. A. Hashiguchi and Dr. X. Wang in University of Tsukuba for their useful discussion. M.M.A. was supported by scholarship from Higher Education Commission, Pakistan.
Abbreviations
| FDR | False discovery rate |
| LC | Liquid Chromatography |
| RuBisCO | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| MS | Mass Spectrometry |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/6/1319/s1. Figure S1. GO categories of proteins with differential abundance in maize treated with plant-derived smoke. Figure S2. Metabolic pathway of proteins identified in maize treated with plant-derived smoke.
Author Contributions
S.K. conceived and designed the experiments; M.M.A. and S.K. performed the experiments; H.Y., K.H., and K.T. performed MS analysis and data analysis; S.K., A.K., X.L., Y.S., and H.M. contributed analysis tools; S.K., M.M.A., A.K., H.Y., K.H., K.T., X.L., Y.S., and H.M. wrote the paper. S.K., M.M.A., A.K., H.Y., K.H., K.T., X.L., Y.S., H.M., S.R., and M.J. read the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lawrence C.J., Walbot V. Translational genomics for bioenergy production from fuel stock grasses: Maize as the model species. Plant Cell. 2007;19:2091–2094. doi: 10.1105/tpc.107.053660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morari F., Meggio F., Lunardon A., Scudiero E., Forestan C., Farinati S., Varotto S. Time course of biochemical, physiological, and molecular responses to field-mimicked conditions of drought, salinity, and recovery in two maize lines. Front. Plant Sci. 2015;6:314. doi: 10.3389/fpls.2015.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao Y., Liu H., Wu L., Warburton M., Yan J. Genome-wide association studies in maize: Praise and stargaze. Mol. Plant. 2017;10:359–374. doi: 10.1016/j.molp.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Chia J.M., Song C., Bradbury P.J., Costich D., Leon N., Doebley J., Elshire R.J., Gaut B., Geller L., Glaubitz J.C., et al. Maize HapMap2 identifies extant variation from a genome in flux. Nat. Genet. 2012;44:803–807. doi: 10.1038/ng.2313. [DOI] [PubMed] [Google Scholar]
- 5.Schnable P.S., Ware D., Fulton R.S., Stein J.C., Wei F., Pasternak S., Liang C., Zhang J., Fulton L., Graves T.A., et al. The B73 Maize Genome: Complexity, Diversity, and Dynamics. Science. 2009;326:1112–1114. doi: 10.1126/science.1178534. [DOI] [PubMed] [Google Scholar]
- 6.Hake S., Ross-Ibarra J. The Natural History of Model Organisms: Genetic, evolutionary and plant breeding insights from the domestication of maize. eLife. 2015;4:e05861. doi: 10.7554/eLife.05861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradshaw S.D., Dixon K.W., Hopper S.D., Lambers H., Turner S.R. Little evidence for fire-adapted plant traits in Mediterranean climate regions. Trends Plant Sci. 2011;16:69–76. doi: 10.1016/j.tplants.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Nelson D.C., Flematti G.R., Ghisalberti E.L., Dixon K.W., Smith S.M. Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Annu. Rev. Plant Biol. 2012;63:107–130. doi: 10.1146/annurev-arplant-042811-105545. [DOI] [PubMed] [Google Scholar]
- 9.Flematti G.R., Waters M.T., Scaffidi A., Merritt D.M., Ghisalberti E.L., Kingsley W., Dixon K.W., Smith S.M. Karrikin and cyanohydrin smoke signals provide clues to new endogenous plant signaling compounds. Mol. Plant. 2013;6:29–37. doi: 10.1093/mp/sss132. [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni M.G., Light M.E., van Staden J. Plant derived smoke: Old technology with possibilities for economic applications in agriculture and horticulture. S. Afr. J. Bot. 2011;77:972–979. doi: 10.1016/j.sajb.2011.08.006. [DOI] [Google Scholar]
- 11.Dixon K.W., Roche S., Pate J.S. The promotive effect of smoke derived from burnt vegetation on seed germination of western Australian plants. Oecologia. 1995;101:185–192. doi: 10.1007/BF00317282. [DOI] [PubMed] [Google Scholar]
- 12.van Staden J., Sparg S.G., Kulkarni M.G., Light M.E. Post germination effects of the smoke-derived compound 3-methyl-2H-furo[2,3-c]pyran-2-one, and its potential as a preconditioning agent. Field Crop Res. 2006;98:98–105. doi: 10.1016/j.fcr.2005.12.007. [DOI] [Google Scholar]
- 13.Kulkarni M.G., Street R.A., van Staden J. Germination and seedling growth requirements for propagation of Dioscorea dregeana (Kunth) Dur. and Schinz-A tuberous medicinal plant. S. Afr. J. Bot. 2007;73:131–137. doi: 10.1016/j.sajb.2006.09.002. [DOI] [Google Scholar]
- 14.Mavi K., Light M.E., Demir I., van Staden J., Yasar F. Positive effect of smoke-derived butenolide priming on melon seedling emergence and growth. New Zeal. J. Crop Hort. 2010;38:147–155. doi: 10.1080/01140671.2010.482967. [DOI] [Google Scholar]
- 15.Moreira B., Tormo J., Estrelles E., Pausas J.G. Disentangling the role of heat and smoke as germination cues in Mediterranean Basin flora. Ann. Bot. 2010;105:627–635. doi: 10.1093/aob/mcq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumari A., Papenfus H.B., Kulkarni M.G., Posta M., van Staden J. Effect of smoke derivatives on in vitro pollen germination and pollen tube elongation of species from different plant families. Plant Biol. 2015;17:825–830. doi: 10.1111/plb.12300. [DOI] [PubMed] [Google Scholar]
- 17.Gardner M.J., Dalling K.J., Light M.E., Jager A.K., van Staden J. Does smoke substitute for red light in the germination of light-sensitive lettuce seeds by affecting gibberellin metabolism? S. Afr. J. Bot. 2001;67:636–640. doi: 10.1016/S0254-6299(15)31194-7. [DOI] [Google Scholar]
- 18.Egerton-Warburton L.M. A smoke-induced alteration of the sub-testa cuticle in seeds of the post-fire recruiter, Emmenanthe penduliflora Benth (Hydrophyllaceae) J. Exp. Bot. 1998;49:1317–1327. doi: 10.1093/jxb/49.325.1317. [DOI] [Google Scholar]
- 19.Kulkarni M.G., Amoo S.O., Kandari L.S., van Staden J. Seed germination and phytochemical evaluation in seedlings of Aloe arborescens Mill. Plant Biosyst. 2013;148:460–466. doi: 10.1080/11263504.2013.782901. [DOI] [Google Scholar]
- 20.Zhou J., Da Silva J.A.T., Ma G. Effects of smoke water and karrikin on seed germination of 13 species growing in China. Cent. Eur. J. Biol. 2014;9:1108–1116. doi: 10.2478/s11535-014-0338-6. [DOI] [Google Scholar]
- 21.Jamil M., Kanwal M., Aslam M.M., Shakir K.S., Malook I., Tu J., Rehman S. Effect of plant-derived smoke priming on physiological and biochemical characteristics of rice under salt stress condition. Aust. J. Crop Sci. 2014;8:159–170. [Google Scholar]
- 22.Kamran M., Latif K.A., Waqas M., Imran Q.M., Hamayun M., Kang S.-M., Kim Y.-H., Kim M.-J., Lee I.J. Effects of plant-derived smoke on the growth dynamics of Barnyard Grass (Echinochloa crus-galli) Acta Agric. Scand. Sect. B Soil Plant Sci. 2014;64:121–128. doi: 10.1080/09064710.2014.888468. [DOI] [Google Scholar]
- 23.Aremu A.O., Bairu M.W., Finnie J.F., van Staden J. Stimulatory role of smoke-water and karrikinolide on the photosynthetic pigment and phenolic contents of micro-propagated ‘Williams’ bananas. Plant Growth Regul. 2012;67:271–279. doi: 10.1007/s10725-012-9685-3. [DOI] [Google Scholar]
- 24.Chiwocha S.D.S., Dixon K.W., Flematti G.R., Ghisalberti E.L., Merritt D.J., Nelson D.C., Riseborough J.M., Smith S.M., Stevens J.C. Karrikins: A new family of plant growth regulators in smoke. Plant Sci. 2009;177:252–256. doi: 10.1016/j.plantsci.2009.06.007. [DOI] [Google Scholar]
- 25.Chou Y.-F., Cox R.D., Wester D.B. Smoke water and heat shock influence germination of shortgrass prairie species. Rangel. Ecol. Manag. 2012;65:260–267. doi: 10.2111/REM-D-11-00093.1. [DOI] [Google Scholar]
- 26.Soós V., Sebestyén E., Juhász A., Pintér J., Light M.E., van Staden J., Balázs E. Stress-related genes define essential steps in the response of maize seedlings to smoke-water. Funct. Integr. Genom. 2009;9:231–242. doi: 10.1007/s10142-008-0105-8. [DOI] [PubMed] [Google Scholar]
- 27.Aslam M.M., Jamil M., Khatoon A., Hendawy S.E., Suhaibani N.A., Malook I., Rehman S. Physiological and biochemical responses of maize (Zea mays L.) to plant derived smoke solution. Pak. J. Bot. 2017;49:435–443. [Google Scholar]
- 28.Sparg S.G., Kulkarni M.G., van Staden J. Aerosol smoke and smoke-water stimulation of seedling vigor of a commercial maize cultivar. Crop Sci. 2006;46:1336–1340. doi: 10.2135/cropsci2005.07-0324. [DOI] [Google Scholar]
- 29.Abu Y., Romo J.T., Bai Y., Coulman V. Priming seeds in aqueous smoke solutions to improve seed germination and biomass production of perennial forage species. Can. J. Plant Sci. 2016;96:551–563. doi: 10.1139/cjps-2015-0229. [DOI] [Google Scholar]
- 30.Light M.E., Gardner M.J., van Staden J. Dual regulation of seed germination by smoke solutions. Plant Growth Regul. 2002;37:135–141. doi: 10.1023/A:1020536711989. [DOI] [Google Scholar]
- 31.Aremu A.O., Plackova L., Novak O., Stirk W.A., Dolezal K., van Staden J. Cytokinin profiles in ex vitro acclimatized Eucomis autumnalis plants pre-treated with smoke-derived karrikinolide. Plant Cell Rep. 2016;35:227–238. doi: 10.1007/s00299-015-1881-y. [DOI] [PubMed] [Google Scholar]
- 32.Flematti G.R., Ghisalberti E.L., Dixon K.W., Trengove R.D. Identification of alkyl substituted 2H-furo[2,3-c]pyran-2-ones as germination stimulants present in smoke. J. Agric. Food Chem. 2009;57:9475–9480. doi: 10.1021/jf9028128. [DOI] [PubMed] [Google Scholar]
- 33.Kulkarni M.G., Sparg S.G., van Staden J. Germination and post-germination response of Acacia seeds to smoke-water and butenolide, a smoke-derived compound. J. Arid Environ. 2007;69:177–187. doi: 10.1016/j.jaridenv.2006.09.001. [DOI] [Google Scholar]
- 34.Kulkarni M.G., Ascough G.D., Verschaeve L., Baeten K., Arruda M.P., van Staden J. Effect of smoke-water and a smoke-isolated butenolide on the growth and genotoxicity of commercial onion. Sci. Hort. 2010;124:434–439. doi: 10.1016/j.scienta.2010.02.005. [DOI] [Google Scholar]
- 35.Abdollahi M.R., Mehrshad B., Moosavi S.S. Effect of method of seed treatment with plant derived smoke solutions on germination and seedling growth of milk thistle (Silybum marianum L.) Seed Sci. Technol. 2011;39:225–229. doi: 10.15258/sst.2011.39.1.22. [DOI] [Google Scholar]
- 36.Staiger C.J. Signalling to the actin cytoskeleton in plants. Annu. Rev. Plant Biol. 2000;51:257–288. doi: 10.1146/annurev.arplant.51.1.257. [DOI] [PubMed] [Google Scholar]
- 37.Schmelzer E. Cell polarization, a crucial process in fungal defense. Trends Plant Sci. 2002;7:411–415. doi: 10.1016/S1360-1385(02)02307-5. [DOI] [PubMed] [Google Scholar]
- 38.Sun T., Li S., Ren H. Profilin as a regulator of the membrane-actin cytoskeleton interface in plant cells. Front. Plant Sci. 2013 doi: 10.3389/fpls.2013.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pruitt K.D., Tatusova T., Maglott D.R. NCBI reference sequences (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007;35:61–65. doi: 10.1093/nar/gkl842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Witsch N., Baluska F.C., Staiger J., Volkmann D. Profilin is associated with the plasma membrane in microspores and pollen. Eur. J. Cell Biol. 1998;77:303–312. doi: 10.1016/S0171-9335(98)80089-7. [DOI] [PubMed] [Google Scholar]
- 41.Feller U., Anders I., Mae T. Rubiscolytics: Fate of Rubisco after its enzymatic function in a cell is terminated. J. Exp. Bot. 2008;59:1615–1624. doi: 10.1093/jxb/erm242. [DOI] [PubMed] [Google Scholar]
- 42.Bota J., Medrano H., Flexas J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004;162:671–681. doi: 10.1111/j.1469-8137.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- 43.Khodadadi E., Fakheria B.A., Aharizad S., Emamjomeha A., Norouzic M., Komatsu S. Leaf proteomics of drought-sensitive and -tolerant genotypes of fennel. Biochim. Biophys. Acta. 2017;1865:1433–1444. doi: 10.1016/j.bbapap.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Tezara W., Mitchell V., Driscoll S.P., Lawlor D.W. Effects of water deficit and its interaction with CO2 supply on the biochemistry and physiology of photosynthesis in sunflower. J. Exp. Bot. 2002;53:1781–1791. doi: 10.1093/jxb/erf021. [DOI] [PubMed] [Google Scholar]
- 45.Baldrianová J., Černý M., Novák J., Jedelský P.L., Divíšková E., Brzobohatý B. Arabidopsis proteome responses to the smoke-derived growth regulator karrikins. J. Proteom. 2015;120:7–20. doi: 10.1016/j.jprot.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Sanahuja G., Farré G., Bassie L., Zhu C., Christou P., Capell T. Ascorbic acid synthesis and metabolism in maize are subject to complex and genotype-dependent feedback regulation during endosperm development. Biotechnol. J. 2013;8:1221–1230. doi: 10.1002/biot.201300064. [DOI] [PubMed] [Google Scholar]
- 47.Li Z., Su D., Lei B., Wang F., Geng W., Pan G., Cheng F. Transcriptional profile of genes involved in ascorbate-glutathione cycle in senescing leaves for an early senescence leaf (esl) rice mutant. J. Plant Physiol. 2015;176:1–15. doi: 10.1016/j.jplph.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Pandey P., Singh J., Achary V.M., Reddy M.K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Environ. Sci. 2015;3:25. doi: 10.3389/fenvs.2015.00025. [DOI] [Google Scholar]
- 49.Selote D.S., Chopra P.K. Drought acclimation confers oxidative stress tolerance by inducing co-ordinated antioxidant defense at cellular and subcellular level in leaves of wheat seedlings. Physiol. Plant. 2006;127:494–506. doi: 10.1111/j.1399-3054.2006.00678.x. [DOI] [Google Scholar]
- 50.Kausar R., Hossain Z., Makino T., Komatsu S. Characterization of ascorbate peroxidase in soybean under flooding and drought stresses. Mol. Biol. Rep. 2012;39:10573–10579. doi: 10.1007/s11033-012-1945-9. [DOI] [PubMed] [Google Scholar]
- 51.Scandalios J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005;38:995–1014. doi: 10.1590/S0100-879X2005000700003. [DOI] [PubMed] [Google Scholar]
- 52.Apel K., Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 53.Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Asada K. Ascorbate peroxidase: A hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant. 1992;85:235–241. doi: 10.1111/j.1399-3054.1992.tb04728.x. [DOI] [Google Scholar]
- 55.Baier M., Dietz K.J. Chloroplasts as source and target of cellular redox regulation: A discussion on chloroplast redox signals in the context of plant physiology. J. Exp. Bot. 2005;56:1449–1462. doi: 10.1093/jxb/eri161. [DOI] [PubMed] [Google Scholar]
- 56.Waheed M.A., Jamil M., Khan M.D., Shakir S.K., Rehman S.U. Effect of plant-derived smoke solutions on physiological and biochemical attributes of maize (Zea mays L.) under salt stress. Pak. J. Bot. 2016;48:1763–1774. [Google Scholar]
- 57.Naranjo B., Diaz-Espejo A., Lindahl M., Cejudo F.J. Type-f thioredoxins have a role in the short-term activation of carbon metabolism and their loss affects growth under short-day conditions in Arabidopsis thaliana. J. Exp. Bot. 2016;67:1951–1964. doi: 10.1093/jxb/erw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bharwana S.A., Ali S., Farooq M.A., Iqbal N., Abbas F., Ahmad M.S.A. Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J. Bioremed. Biodeg. 2013;4:187. doi: 10.4172/2155-6199.1000187. [DOI] [PubMed] [Google Scholar]
- 59.El-Gaied L.F., Abu El-Heba G.A., El-Sherif N.A. Effect of growth hormones on some antioxidant parameters and gene expression in tomato. GM Crops Food. 2013;4:67–73. doi: 10.4161/gmcr.24324. [DOI] [PubMed] [Google Scholar]
- 60.Pasternak T.P., Potters G., Caubergs R., Jansen M.A.K. Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. J. Exp. Bot. 2005;56:1991–2001. doi: 10.1093/jxb/eri196. [DOI] [PubMed] [Google Scholar]
- 61.Correa-Aragunde N., Foresi N., Delledonne M., Lamattina L. Auxin induces redox regulation of ascorbate peroxidase 1 activity by S-nitrosylation/denitrosylation balance resulting in changes of root growth pattern in Arabidopsis. J. Exp. Bot. 2013;64:3339–3349. doi: 10.1093/jxb/ert172. [DOI] [PubMed] [Google Scholar]
- 62.Hernández J.A., Ferrer M.A., Jiménez A., Ros-Barceló A., Sevilla F. Antioxidant systems and O2−/H2O2 production in the apoplast of Pisum sativum L. leaves: Its relation with NaCl-induced necrotic lesions in minor veins. Plant Physiol. 2001;127:817–831. doi: 10.1104/pp.010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diaz-Vivancos P., Faize M., Barba-Espin G., Faize L., Petri C., Hernández J.A., Burgos L. Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums. Plant Biotechnol. J. 2013;11:976–985. doi: 10.1111/pbi.12090. [DOI] [PubMed] [Google Scholar]
- 64.Foyer C.H., Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011;155:93–100. doi: 10.1104/pp.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L., Song Y., Li S., Zhang L., Zou C., Yu D. The role of WRKY transcription factors in plant abiotic stresses. Biochim. Biophys. Acta. 2012;1819:120–128. doi: 10.1016/j.bbagrm.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 66.de Lange J.H., Boucher C. Auto ecological studies on Audinia capitate (Bruniaceaae), plant-derived smoke as a germination cue. S. Afr. J. Bot. 1990;56:188–202. doi: 10.1016/S0254-6299(16)31009-2. [DOI] [Google Scholar]
- 67.Tieu A., Dixon K.A., Sivasithamparam K., Plummer J.A. Germination of four species of native Western Australian plant using plant-derived smoke. Aust. J. Bot. 1999;47:207–219. doi: 10.1071/BT96119. [DOI] [Google Scholar]
- 68.Komatsu S., Han C., Nanjo Y., Altaf-Un-Nahar M., Wang K., He D., Yang P. Label-free quantitative proteomic analysis of abscisic acid effect in early-stage soybean under flooding. J. Proteome Res. 2013;12:4769–4784. doi: 10.1021/pr4001898. [DOI] [PubMed] [Google Scholar]
- 69.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y., Wen Z., Washburn M.P., Florens L. Effect of dynamic exclusion duration on spectral count based quantitative proteomics. Anal. Chem. 2009;81:6317–6326. doi: 10.1021/ac9004887. [DOI] [PubMed] [Google Scholar]
- 71.Tyanova S., Temu T., Siniteyn P., Carlson A., Hein Y., Gieger T., Mann M., Cox J. The Perseus computational platform for comprehensive analysis of proteomics data. Nat. Methods. 2016;13:731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
- 72.Vizcaíno J.A., Côté R.G., Csordas A., Dianes J.A., Fabregat A., Foster J.M., Griss J., Alpi E., Birim M., Contell J., et al. The proteomics identifications (PRIDE) database and associated tools: Status in 2013. Nucleic Acids Res. 2013;41:D1063–D1069. doi: 10.1093/nar/gks1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okuda S., Watanabe Y., Moriya Y., Kawano S., Yamamoto T., Matsumoto M., Takami T., Kobayashi D., Araki N., Yoshizawa A.C., et al. jPOSTrepo: An international standard data repository for proteomes. Nucleic Acids Res. 2017;45:D1107–D1111. doi: 10.1093/nar/gkw1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Usadel B., Nagel A., Thimm O., Redestig H., Blaesing O.E., Palacios-Rofas N., Selbig J., Hannemann J., Piques M.C., Steinhauser D., et al. Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes and comparison with known responses. Plant Physiol. 2005;138:1195–1204. doi: 10.1104/pp.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Usadel B., Poree F., Nagel A., Lohse M., Czedik-Eysenberg A., Stitt M. A guide to using MapMan to visualize and compare Omics data in plants: A case study in the crop species, maize. Plant Cell Environ. 2009;32:1211–1229. doi: 10.1111/j.1365-3040.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- 76.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 77.Komatsu S., Yamamoto A., Nakamura T., Nouri M.Z., Nanjo Y., Nishizawa K., Furukawa K. Comprehensive analysis of mitochondria in roots and hypocotyls of soybean under stress using proteomics and metabolomics techniques. J. Proteome Res. 2011;10:3993–4004. doi: 10.1021/pr2001918. [DOI] [PubMed] [Google Scholar]
- 78.Nishizawa K., Komatsu S. Characteristics of soybean 1-cys peroxiredoxin and its behavior in seedlings under flooding stress. Plant Biotechnol. J. 2011;28:83–88. doi: 10.5511/plantbiotechnology.10.1006a. [DOI] [Google Scholar]
- 79.Hashimoto M., Komatsu S. Proteomic analysis of rice seedlings during cold stress. Proteomics. 2007;7:1293–1302. doi: 10.1002/pmic.200600921. [DOI] [PubMed] [Google Scholar]
- 80.Komatsu S., Masuda T., Abe K. Phosphorylation of a protein (pp56) is related to the regeneration of rice cultured suspension cells. Plant Cell Physiol. 1996;37:748–753. doi: 10.1093/oxfordjournals.pcp.a029009. [DOI] [PubMed] [Google Scholar]
- 81.Nakano Y., Asada K. Hydrogen-peroxide is scavenged by ascorbate-specific peroxidase in spinachchloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 82.Horling F., König J., Dietz K.J. Type II peroxiredoxin C, a member of the peroxiredoxin family of Arabidopsis thaliana: Its expression and activity in comparison with other peroxiredoxins. Plant Physiol. Biochem. 2002;40:491–499. doi: 10.1016/S0981-9428(02)01396-7. [DOI] [Google Scholar]
- 83.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.