Abstract
Behavioral inhibition (BI) is an early temperamental precursor of anxiety disorders, characterized by withdrawal from novel situations. Some but not all young children with BI go on to display anxiety disorders. Neural correlates, such as frontal alpha asymmetry or event-related negativity (ERN), could moderate the relations between early BI and later anxiety. The goal of this longitudinal study was to test frontal alpha asymmetry as a potential moderator of the relation between BI and later anxiety, and of the relation between BI and the social-effect ERN. 100 children were assessed for BI at ages 2 and 3, and we collected EEG during resting state and the social Flanker task at age 12. Frontal alpha asymmetry did not correlate with BI or anxiety, nor did it moderate the relation between early BI and later anxiety. However, frontal alpha asymmetry did moderate the relation between BI and the social-effect ERN. This suggests that, in adolescents who previously manifested BI, a pattern of resting EEG associated with avoidance predicts hypersensitivity to errors in a social context.
Keywords: behavioral inhibition, EEG, error-related negativity, frontal alpha asymmetry
Introduction
Behavioral inhibition (BI) is an early temperamental precursor of anxiety disorders, characterized by withdrawal from novel situations or unfamiliar adults/peers (Fox et al., 2005; Kagan et al., 1984). Not all children with high BI will eventually develop an anxiety disorder, so it is important to study which factors increase the risk for anxiety disorders. One line of research focuses on neural correlates, such as the error-related negativity (ERN), as potential moderators in the relation between BI and later anxiety (Lahat et al., 2014; McDermott et al., 2009). Frontal alpha asymmetry is another neural correlate that has often been studied in relation to BI and anxiety separately. Here, we investigate whether frontal alpha asymmetry could act as a moderator of the relation between early BI and later anxiety symptoms. In addition, in prior research the ERN and frontal alpha asymmetry were correlated in healthy adults (Nash et al., 2012) and preschoolers (Begnoche et al., 2016), but no prior research considers whether frontal alpha asymmetry moderates the previously reported relation between BI and ERN. Therefore, the second goal of this study tests whether frontal alpha asymmetry moderates the relation between BI and ERN.
Frontal alpha asymmetry is typically measured as the difference in alpha power (8–13 Hz) between the left and right frontal hemispheres (Allen et al., 2004). Frontal alpha asymmetry is hypothesized to reflect approach and avoidance tendencies in the brain, with greater right frontal activity reflecting avoidance or withdrawal and greater left frontal activity reflecting approach (Davidson, 1992, 1998; Harmon-Jones and Gable, 2018). As greater right frontal activity has been related to avoidance, it has previously been studied in relation to BI, temperament and social behavior. Some cross-sectional studies have shown greater right frontal activity in children with high BI from 14 months to 4 years (Fox et al., 2001) and in children who showed solitary-passive and reticent behavior (Henderson et al., 2004). Others found no relation between frontal alpha asymmetry and social reticence or shyness (Fox et al., 1995; Schmidt et al., 1999; Theall-Honey and Schmidt, 2006). Frontal alpha asymmetry is relatively stable over time in infancy (Brooker et al., 2017), childhood (Poole et al., 2018; Tang et al., 2018) and adolescence (Schneider et al., 2016), which would suggest that the tendency to avoid (as measured by greater right frontal activity) could be a trait-like feature. Longitudinal research is necessary to study if frontal alpha asymmetry increases the risk of developing anxiety symptoms in children with high BI as a potential moderator. Longitudinal studies have shown that high reactive children with greater right frontal activity at 9 months show more social wariness at 48 months (Henderson et al., 2001), but right frontal activity at 4.5 years did not predict anxiety symptoms at 9 years (Hannesdottir et al., 2010). Only one study focused on the influence of early temperament on later frontal alpha asymmetry. Early temperament did not predict frontal alpha asymmetry at 10–12 years, but children that were high reactive at 4 months and highly fearful at 2 years were most likely to shown right frontal activity at 10–12 years (McManis et al., 2002). The first goal of this study was to investigate whether frontal alpha asymmetry during adolescence could be seen as a moderator of the relation between early BI and later anxiety symptoms.
The ERN, an early event-related potential in response to errors (Falkenstein et al., 1990; Gehring et al., 1993; Gehring et al., 2013), has been studied in relation to BI and anxiety (Buzzell et al., 2017b; Lahat et al., 2014; McDermott et al., 2009). In the current sample, BI was related to an increased ERN when being watched by peers compared to a non-social condition. This social-effect ERN and subsequent post-error reaction time slowing mediated the relation between early BI and social anxiety symptoms and diagnosis at age 12 (Buzzell et al., 2017b). Frontal alpha asymmetry could provide more insight in the relation between BI and the social-effect ERN, since previous studies have shown that greater right frontal activity predicts an increased ERN in healthy adults (Nash et al., 2012) and preschoolers (Begnoche et al., 2016). In addition, right frontal activity is related to avoidance (Davidson, 1992, 1998; Harmon-Jones and Gable, 2018), which is likely to influence reactivity to errors in social situations. Hence, the second goal of the current study was to investigate whether frontal alpha asymmetry moderates the relation between BI and the social-effect ERN.
The current study investigated relations among BI, frontal alpha asymmetry and the social-effect ERN in a 12-year longitudinal sample. Children were recruited when they were 4 months old, their BI was assessed at ages 2 and 3, and they visited the lab again when they were 12 years old. During the 12-year visit, we measured frontal alpha asymmetry during resting state and the ERN in response to errors in social and non-social conditions of the Flanker task (Barker, 2016; Buzzell et al., 2017b; Eriksen and Eriksen, 1974). The ERN data have been published previously (Buzzell et al., 2017b), so this study adds to the existing literature by examining the relations between the social-effect ERN and frontal alpha asymmetry. In addition, EEG and MRI data from previous time points in this longitudinal study have been reported (Lahat et al., 2016; Lahat et al., 2014; Lamm et al., 2014; McDermott et al., 2009). Since previous studies have found mixed results on the relation between BI and frontal alpha asymmetry (Fox et al., 2001; Fox et al., 1995; Hannesdottir et al., 2010; Henderson et al., 2001; Henderson et al., 2004; McManis et al., 2002; Schmidt, 1999; Theall-Honey and Schmidt, 2006), we expected that greater right frontal activity in adolescence might act as a moderator in the relation between early BI and later anxiety symptoms. Furthermore, we expected that frontal alpha asymmetry would moderate the relation between BI and the social-effect ERN (Begnoche et al., 2016; Nash et al., 2012).
Materials and Methods
Participants
This study was part of a longitudinal study on BI. 291 participants (134 boys) were recruited at 4 months (Hane et al., 2008), and BI was measured for 268 children at 2 and 3 years of age (Fox et al., 2001; Kagan and Snidman, 1991). The continuous BI composite consisted of behavioral observations (during three novel situations: robot, tunnel and stranger) and a parent-report questionnaire (Toddler Behavior Assessment Questionnaire; (Goldsmith, 1996), as used in previous studies (Lahat et al., 2014; Lamm et al., 2014). All variables were standardized and averaged across measures and time points.
Useable EEG data were collected during resting state for 123 of these children at age 12 (61 boys; Mage = 13.16, SD = 0.62, range 12.08–15.29 years), of whom 100 children also had useable EEG data during the Flanker task at age 12 (46 boys; Mage = 13.23, SD = 0.63, range 12.11–15.29 years). These children with complete data at age 12 did not differ from children without complete data in gender and BI, ps > 0.68. 81% of the mothers of children with complete data were Caucasian, 10% African American, 4% Hispanic, 3% Asian, and 2 % ‘other’. All parents provided written informed consent, and all children provided assent. The study was approved by the University of Maryland-College Park institutional review board.
Anxiety symptoms
Anxiety symptoms were measured using the Screen for Child Anxiety Related Disorders (SCARED), which has shown to be a reliable questionnaire for symptoms of DSM-IV anxiety disorders (Muris et al., 2004). Children and parents filled out the questionnaire separately during the visit at age 12. We used the total score on the questionnaire, only complete questionnaires were used.
Procedure
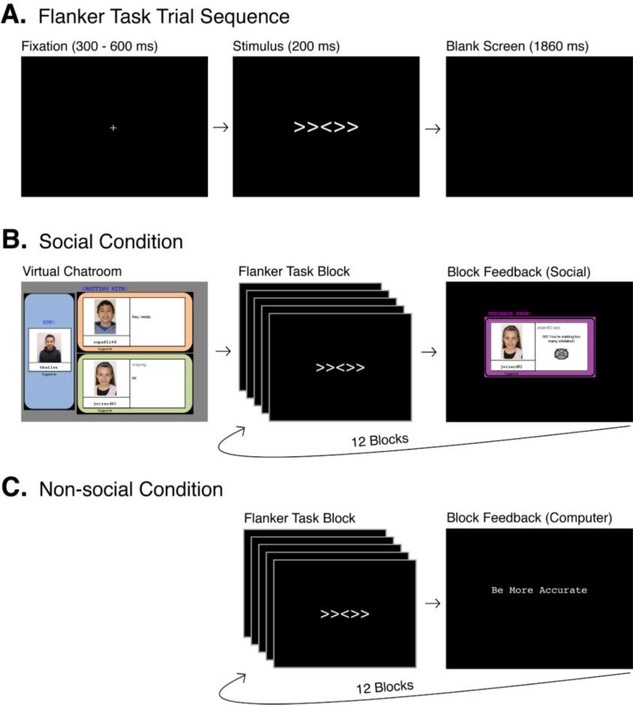
Resting state EEG was recorded during 3 blocks of 30 seconds with eyes closed, and 3 blocks of 30 seconds with eyes open (C-O-C-O-C-O). A research assistant told the children to open and close their eyes. Children also performed a Flanker task in a social and non-social condition (Barker, 2016; Buzzell et al., 2017b; Eriksen and Eriksen, 1974) (Figure 1). In this task, children had to respond as fast as possible to an arrow head in the middle of congruent arrow heads (>>>>>) or incongruent arrow heads (<<><<). During the social condition, participants believed that their performance was monitored through a webcam and evaluated after each block by a peer (this was not actually the case). During the non-social condition, participants were not monitored and received computer-generalized feedback (Buzzell et al., 2017b). Children completed the Flanker task in counter-balanced order, and resting state EEG was always recorded before the non-social condition. Order did not influence the results (see Supplementary data).
Figure 1.
Overview of the Flanker task with social and non-social conditions. Reprinted from Journal of the American Academy of Child & Adolescent Psychiatry, 56, Buzzell, G.A., Troller-Renfree, S.V., Barker, T.V., Bowman, L.C., Chronis-Tuscano, A., Henderson, H.A., Kagan, J., Pine, D.S., & Fox, N.A., A neurobehavioral mechanism linking behaviorally inhibited temperament and later adolescent social anxiety, 1097–1105, Copyright (2017), with permission from Elsevier.
EEG collection and processing
EEG was recorded using a 128-channel HydroCel Geodesic Sensor Net and EGI software (Electrical Geodesic, Inc., Eugene, OR). This is a high input-impedance system, so electrode impedances were kept below 50 kΩ. Sampling rate was 250 Hz and data were online referenced to the vertex (Cz) electrode.
EEG was analyzed using the EEGLAB toolbox (Delorme and Makeig, 2004) and custom written MATLAB scripts (The MathWorks, Natick, MA). We corrected for systematic marker offsets of the EGI system (constant 36 ms offset) and E-Prime computer (constant 15 ms offset). Data were high-pass filtered at 0.3 Hz and low-pass filtered at 45 Hz. Bad channels were identified and removed using the EEGLAB plug-in FASTER (Nolan et al., 2010). To remove ocular artifacts and generic noise, we created a copy of the dataset and performed independent component analysis (ICA) on the copied dataset. This copied dataset was high-pass filtered at 1-Hz and segmented into arbitrary 1000 ms epochs. Epochs were removed from this copied dataset if the amplitude was +/− 1000 μV or if power in the 20–40 Hz band (after Fourier analysis) was greater than 30 dB. Additionally, if more than 20% of the epochs in a given channel were removed, that channel was excluded from both the ICA copied dataset and the original dataset. Then, ICA was performed on the copied dataset and the ICA weights were copied back to the original continuous dataset (high-pass filtered at 0.3 Hz). The ADJUST toolbox (Mognon et al., 2011) was used to automatically identify artefactual independent components (ICs) in the original dataset, and ICs were also visually inspected. All artifactual ICs were removed from the data. Then, data were segmented (differently for resting state and Flanker, see below). Epochs exceeding a voltage threshold of +/− 100 μV were automatically rejected. If more than 20% of the data were rejected due to one channel, this channel was rejected. Finally, all rejected channels were interpolated using a spherical spline and data were re-referenced to an average reference.
Frontal alpha asymmetry.
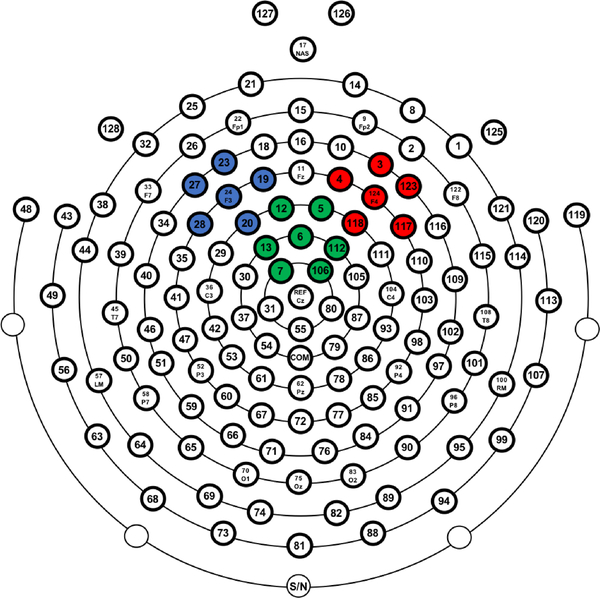
Resting state EEG data were epoched in segments of 3 seconds with 1.5 second (50%) overlap. The average number of used epochs was 52.78 (SD = 7.35, range: 14–57). A Fast Fourier Transformation (FFT) with a 3-second Hamming window was applied to the epoched data. Spectral power (μV2) was computed for alpha power (8–13 Hz) and for the 1–40 Hz frequency band. Relative alpha power was calculated as the proportion of alpha power relative to the total power (1–40 Hz). Alpha power was then averaged over electrode clusters approximating international 10–20 locations F3 and F4 (F3: 19, 20, 23, 24, 27, 28; F4: 3, 4, 117, 118, 123, 124; see Figure 2). These analyses were performed separately for the eyes open and eyes closed condition, but only the eyes closed data are reported (as alpha power is strongest when eyes are closed; Barry et al. (2009); Barry et al. (2007)). Frontal alpha asymmetry scores were computed by subtracting natural log-transformed relative alpha power in the left hemisphere (F3 cluster) from natural log-transformed relative alpha power in the right hemisphere (F4 cluster). Negative frontal alpha asymmetry scores are related to greater right frontal activity, as alpha power is inversely related to activity (Allen et al., 2004).
Figure 2.
128-channel EEG net with the F3 (left frontal), F4 (right frontal) and Fcz (frontocentral) clusters highlighted in respectively blue, red and green.
Social-effect ERN.
The ERN data have been reported previously (Buzzell et al., 2017b) and we used the data from the previous paper. Task data were epoched around the response markers (−500 to 1000 ms), and baseline corrected (−200 – 0 ms). Mean amplitude of the ERN was calculated within the first 100 ms after errors from a frontocentral cluster (Fcz, EGI electrodes 12, 5, 6, 13, 112, 7, and 106; see Figure 2); only incongruent trials were used (Buzzell et al., 2017b). A minimum of 6 artifact-free incongruent-error trials were used (Buzzell et al., 2017a; Jentzsch and Dudschig, 2009), the average number of used trials was 39.19 (SD = 17.84, range: 7–114) in the social condition and 40.14 (SD = 19.98, range: 6–125) in the non-social condition. The social-effect ERN was calculated by subtracting the ERN in the non-social condition from the social condition (Buzzell et al., 2017b).
Statistical analysis
First, we computed correlations between frontal alpha asymmetry, BI and self-reported and parent-reported anxiety (using the SCARED (Muris et al., 2004)). The first goal of the current study was to investigate frontal alpha asymmetry as a potential moderator in the relation between early BI and later anxiety symptoms. We started by examining the influence of early BI on later anxiety symptoms (child-reported and parent-reported separately), with age and gender as independent variables in the first step and BI in the second step. Then, a regression analysis was performed with anxiety symptoms as the dependent variable, age and gender as independent variables in the first step, frontal alpha asymmetry and BI in the second step, and the interaction between frontal alpha asymmetry and BI in the third step. The second goal was to investigate frontal alpha asymmetry as a potential moderator in the relation between BI and the social-effect ERN. A regression analysis was performed with the social-effect ERN as the dependent variable, with gender and age entered as independent variables in the first step, frontal alpha asymmetry and BI in the second step, and the interaction between frontal alpha asymmetry and BI in the third step. A significant interaction term would be followed up with regression analyses separately for children with high and low BI scores (median split) with the social-effect ERN as the dependent variable, and gender, age and frontal alpha asymmetry as independent variables. Outliers (+/− 3 SD) were removed for BI, frontal alpha asymmetry, and ERN data, and alpha was set at 0.05. We repeated all analyses for delta-beta correlation, as a different line of studies have focused on this other resting state EEG measure (Harrewijn et al., 2017). Delta-beta correlation was not related to BI, nor to the social-effect ERN (see Supplementary data).
Results
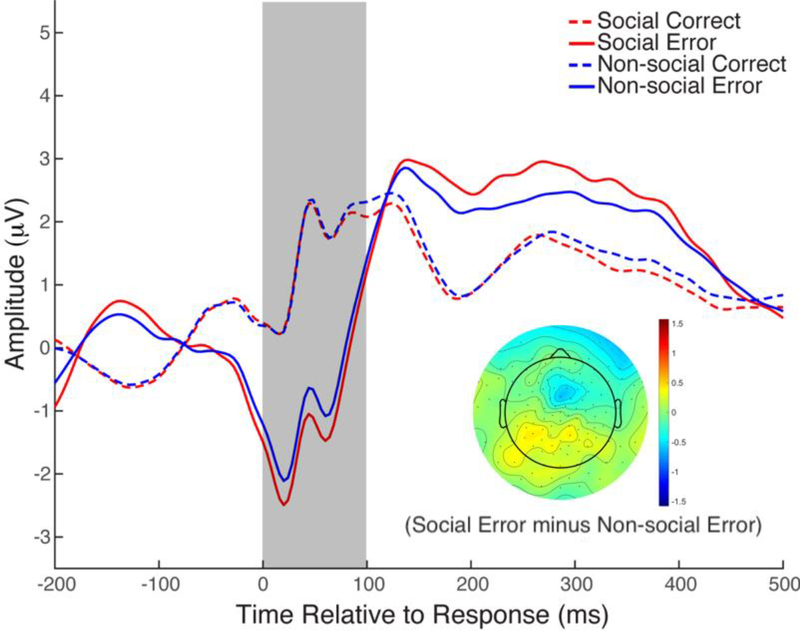
Frontal alpha asymmetry was not correlated with BI, anxiety or any of the other variables, all ps > 0.20 (Table 1). The social-effect ERN data have been reported previously (Buzzell et al., 2017b), showing that the ERN was more negative in the social compared to the non-social condition (Figure 3). In addition, BI predicted an increased social-effect ERN (an increased ERN in the social compared to the non-social condition) (Buzzell et al., 2017b).
Table 1.
Means, standard deviations, range and correlations with frontal alpha asymmetry for all variables.
| n | M | SD | Range | Correlation with frontal alpha asymmetry | |
|---|---|---|---|---|---|
| Left frontal alpha power (relative) | 123 | −1.52 | 0.59 | −3.06 – −0.42 | |
| Right frontal alpha power (relative) | 123 | −1.52 | 0.58 | −2.86 – −0.48 | |
| Frontal alpha asymmetry | 123 | 0.003 | 0.17 | −0.46 – 0.46 | |
| BI | 123 | 0.01 | 0.77 | −1.64 – 2.30 | −0.03 |
| Social-effect ERN | 100 | −0.38 | 1.50 | −3.93 – 3.88 | 0.13 |
| Social-effect post-error slowing | 100 | 0.38 | 3.44 | −7.22 – 15.98 | 0.15 |
| SCARED total self-report | 98 | 17.10 | 11.61 | 0 – 62 | −0.06 |
| SCARED total parent-report | 103 | 9.14 | 8.08 | 0 – 46 | 0.08 |
Figure 3.
Event-related potentials in response to correct responses and errors in the Flanker task in the social and non-social conditions, as published by Buzzell, Troller-Renfree, et al., 2017. Adapted from Journal of the American Academy of Child & Adolescent Psychiatry, 56, Buzzell, G.A., Troller-Renfree, S.V., Barker, T.V., Bowman, L.C., Chronis-Tuscano, A., Henderson, H.A., Kagan, J., Pine, D.S., & Fox, N.A., A neurobehavioral mechanism linking behaviorally inhibited temperament and later adolescent social anxiety, 1097–1105, Copyright (2017), with permission from Elsevier.
BI, frontal alpha asymmetry and anxiety
The first goal of the current study was to investigate frontal alpha asymmetry as a potential moderator in the relation between early BI and later anxiety symptoms. BI at 2–3 years predicted parent-reported anxiety symptoms at 12 years, β = 0.31, p = 0.002, but not child-reported anxiety symptoms, β = −0.03, p = 0.80. Frontal alpha asymmetry was not a moderator of the effect of early BI on later anxiety symptoms. There was no interaction between frontal alpha asymmetry and BI in predicting parent-reported anxiety symptoms, β = −0.02, p = 0.87 (Table 2).
Table 2.
Results of the moderation analysis with parent-reported anxiety symptoms as dependent variable and gender, age, frontal alpha asymmetry, BI and the interaction between frontal alpha asymmetry and BI as independent variables.
| B | SE B | β | p-value | ||
|---|---|---|---|---|---|
| Step 1 | Constant | −17.77 | 16.46 | 0.28 | |
| Gender | 2.65 | 1.69 | 0.16 | 0.12 | |
| Age | 1.73 | 1.31 | 0.14 | 0.19 | |
| Step 2 | Constant | −15.08 | 16.31 | 0.36 | |
| Gender | 1.56 | 1.64 | 0.10 | 0.34 | |
| Age | 1.66 | 1.30 | 0.13 | 0.20 | |
| Frontal alpha asymmetry | 6.12 | 4.46 | 0.13 | 0.17 | |
| BI | 3.21 | 0.97 | 0.32 | 0.001* | |
| Step 3 | Constant | −14.76 | 16.50 | 0.37 | |
| Gender | 1.59 | 1.66 | 0.10 | 0.34 | |
| Age | 1.63 | 1.31 | 0.13 | 0.22 | |
| Frontal alpha asymmetry | 6.09 | 4.49 | 0.13 | 0.18 | |
| BI | 3.20 | 0.97 | 0.32 | 0.001* | |
| Frontal alpha asymmetry * BI | −0.90 | 5.35 | −0.02 | 0.87 | |
BI, frontal alpha asymmetry and social-effect ERN
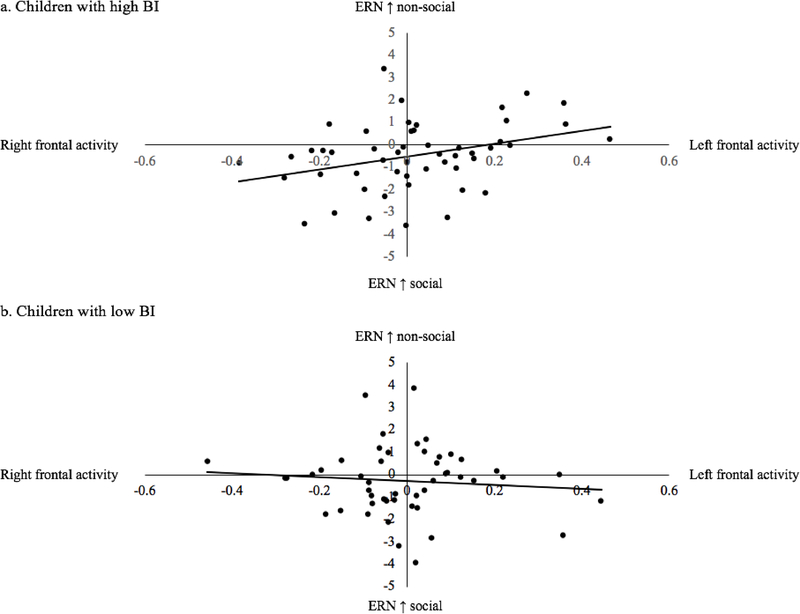
The second goal was to investigate whether frontal alpha asymmetry moderates the relation between BI and the social-effect ERN. Neither frontal alpha asymmetry nor BI predicted the social-effect ERN, although the effect of BI on the social-effect ERN was at trend-level, β = −0.17, p = 0.09. Interestingly, there was an interaction between frontal alpha asymmetry and BI in predicting the social-effect ERN, β = 0.19, p = 0.047 (Table 2). Follow-up regression analysis for children with high and low BI separately (median split), showed that frontal alpha asymmetry predicts the social-effect ERN for children with high BI, β = 0.27, p = 0.07, but not for children with low BI, β = −0.12, p = 0.42 (Figure 5). In children with high BI, more right frontal activity is related to a larger ERN in the social condition compared to the non-social condition. The interaction between frontal alpha asymmetry and BI was not statistically significant for the ERN in the social and non-social conditions separately, ps > 0.50, showing that the effect is specific for the social-effect ERN. It should be noted that the interaction between BI and frontal alpha asymmetry was not significant when we used current-source density (CSD) transformed data.
Discussion
The goal of this longitudinal study was to investigate frontal alpha asymmetry as a potential moderator of the relation between early BI and later anxiety symptoms, as well as of the relation between BI and the social-effect ERN. BI was assessed when children were 2 and 3 years old, and resting state EEG data and the ERN in social and non-social conditions were collected when these children were 12 years old. Frontal alpha asymmetry was not related to BI or anxiety and did not moderate the relation between early BI and later parent-reported anxiety symptoms. Frontal alpha asymmetry was related to the social-effect ERN in children with high BI.
Specifically, in adolescents who previously manifested BI, greater right frontal activity was related to an increased ERN in the social compared to the non-social condition. Previous studies showed that BI longitudinally predicts the ERN (Lahat et al., 2014; McDermott et al., 2009), in that children with higher BI have higher ERN responses to errors. Furthermore, BI is related to the social-effect ERN during adolescence in this sample, interpreted as hypersensitivity to errors in a social context (Buzzell et al., 2017b). We add to the existing literature by showing that this relation between BI and the social-effect ERN is influenced by frontal alpha asymmetry. Because greater right frontal activity has been associated with withdrawal or avoidance (Davidson, 1992, 1998; Harmon-Jones and Gable, 2018), our results suggest that children with high BI and this tendency for avoidance are particularly sensitive to errors in a social, versus a non-social, context. Hypersensitivity to errors in a social context was associated with post-error slowing, and these two measures together explain the link between early BI and later to social anxiety symptoms (Buzzell et al., 2017b). Here, we have shown that frontal alpha asymmetry also plays a role in the mechanism from early BI to later social anxiety, by clarifying which children with BI have a larger social-effect ERN.
The relation between frontal alpha asymmetry and the social-effect ERN was in line with previous studies (Begnoche et al., 2016; Nash et al., 2012). However, the authors have interpreted the effect in the opposite direction, that is greater left frontal activity predicts reduced ERN. The authors suggested that approach motivation reduced reactivity to aversive events (Nash et al., 2012). This focus on left frontal activity is supported by a review concluding that more evidence has been found for the connection between greater left frontal activity and approach, than for the connection between greater right frontal activity and avoidance (Harmon-Jones and Gable, 2018). Following this focus on left frontal activity, our findings would suggest that greater left frontal activity is a protective factor for children with BI, as it relates to a decreased social-effect ERN. This might indicate that children with greater left frontal activity were less bothered by social evaluation and thus less at risk for later social anxiety. However, we interpreted our findings focusing on right frontal activity, because previous studies on BI have focused on right frontal activity when interpreting frontal alpha asymmetry findings (Fox et al., 2001; Henderson et al., 2001; Henderson et al., 2004; McManis et al., 2002). Therefore, we concluded that avoidance motivation increases reactivity to aversive events.
Frontal alpha asymmetry was not related to BI or anxiety and did not moderate the relation between early BI and later anxiety. Although studies in infancy and early childhood would predict a role of frontal alpha asymmetry this relation (Fox et al., 2001; Henderson et al., 2001; Henderson et al., 2004), studies in later childhood did not find a relation between constructs related to BI (such as high reactive temperament, social withdrawal, or shyness) and frontal alpha symmetry (Fox et al., 1995; Hannesdottir et al., 2010; McManis et al., 2002; Schmidt, 1999; Theall-Honey and Schmidt, 2006). Also, most studies in adults show that frontal alpha asymmetry is not related to social anxiety disorder (Harrewijn et al., 2017). Possibly, frontal alpha asymmetry is related to BI in infancy, but has not a longitudinal influence on anxiety.
This 12-year longitudinal study provides important insights into neural correlates during adolescence that are related to early BI. However, some limitations should be taken into account. First, resting state EEG and ERN were measured during the same visit, so we cannot draw any conclusions on the direction of this relation. Second, resting state EEG was administered either at the start of the EEG session, or after the social condition of the Flanker task (it was always before the non-social condition). The social condition could have influenced participant’s resting state, but there was no effect of order and results remained the same when we included order in the analysis (see Supplementary data). Third, a research assistant was present in the room during resting state to tell the participants to open and close their eyes, which could have affected the resting state EEG because the participants were not completely alone. Fourth, the average reference has been criticized as it might result in frontal alpha mirroring (Hagemann et al., 2001). However, this is mainly the problem when few electrodes are used, and we used 128 electrodes covering a large part of the head (Allen et al., 2004; Smith et al., 2017). Furthermore, we used the average reference to be consistent with most previous studies in BI and anxiety (Brooker et al., 2017; Fox et al., 2001; Fox et al., 1995; Hannesdottir et al., 2010; Harrewijn et al., 2016; Henderson et al., 2001; Henderson et al., 2004; Schmidt et al., 1999; Schneider et al., 2016). The interaction between BI and frontal alpha asymmetry in predicting the social-effect ERN was not significant when we used CSD transformed data. This underlines the influence of reference scheme on the calculation of frontal alpha asymmetry scores.
To conclude, greater right frontal activity was related to increased social-effect ERN in adolescents who previously manifested BI, suggesting that a tendency for avoidance predicts hypersensitivity to errors in a social context in these children. Given the relations between BI, the ERN and anxiety, frontal alpha asymmetry may also play a role in the mechanism from early BI to later social anxiety, by showing which children with high BI have an increased social-effect ERN.
Supplementary Material
Figure 4.
Effect of frontal alpha asymmetry on the social-effect ERN separately for children with high and low BI.
Table 3.
Results of the regression analysis with social-effect ERN as dependent variable and gender, age, frontal alpha asymmetry, BI and the interaction between frontal alpha asymmetry and BI as independent variables.
| B | SE B | β | p-value | ||
|---|---|---|---|---|---|
| Step 1 | Constant | 7.54 | 3.16 | 0.02* | |
| Gender | 0.54 | 0.31 | 0.18 | 0.09 | |
| Age | −0.66 | 0.25 | −0.28 | 0.01* | |
| Step 2 | Constant | 6.22 | 3.25 | 0.06 | |
| Gender | 0.60 | 0.31 | 0.20 | 0.06 | |
| Age | −0.57 | 0.26 | −0.24 | 0.03* | |
| Frontal alpha asymmetry | 0.56 | 0.89 | 0.06 | 0.53 | |
| BI | −0.34 | 0.19 | −0.18 | 0.08 | |
| Step 3 | Constant | 5.61 | 3.21 | 0.08 | |
| Gender | 0.55 | 0.31 | 0.18 | 0.08 | |
| Age | −0.52 | 0.25 | −0.22 | 0.04 | |
| Frontal alpha asymmetry | 0.63 | 0.87 | 0.07 | 0.48 | |
| BI | −0.33 | 0.19 | −0.17 | 0.09 | |
| Frontal alpha asymmetry * BI | 2.03 | 1.01 | 0.19 | 0.047* | |
Note: R2 = 0.08 for Step 1, R2 = 0.11 for Step 2, R2 = 0.15 for Step 3, Δ R2 = 0.04 for Step 3 (p = 0.047).
p < 0.05
Acknowledgements
This work was supported by the National Institute of Mental Health (R37HD17899 and U01MH093349) to Nathan A. Fox and the Intramural Research Program at the National Institute of Mental Health (NCT00018057) supporting D.S. Pine and E. Leibenluft.
Footnotes
Declarations of interest: none
References
- Allen JJB, Coan JA, Nazarian M, 2004. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biol. Psychol 67, 183–218. [DOI] [PubMed] [Google Scholar]
- Barker TV, 2016. Social influences of error monitoring. University of Maryland, College Park. [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, Brown CR, 2009. EEG differences in children between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol 120, 1806–1811. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA, 2007. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol 118, 2765–2773. [DOI] [PubMed] [Google Scholar]
- Begnoche JP, Brooker RJ, Vess M, 2016. EEG asymmetry and ERN: Behavioral outcomes in preschoolers. PLoS One 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Canen MJ, Davidson RJ, Goldsmith HH, 2017. Short- and long-term stability of alpha asymmetry in infants: Baseline and affective measures. Psychophysiology 54, 1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell GA, Beatty PJ, Paquette NA, Roberts DM, McDonald CG, 2017a. Error-induced blindness: Error detection leads to impaired sensory processing and lower accuracy at short response-stimulus intervals. J. Neurosci 37, 2895–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell GA, Troller-Renfree SV, Barker TV, Bowman LC, Chronis-Tuscano A, Henderson HA, Kagan J, Pine DS, Fox NA, 2017b. A neurobehavioral mechanism linking behaviorally inhibited temperament and later adolescent social anxiety. Journal of the American Academy of Child & Adolescent Psychiatry 56, 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, 1992. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 20, 125–151. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, 1998. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion 12, 307–330. [Google Scholar]
- Delorme A, Makeig S, 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW, 1974. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics 16, 143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, 1990. Effects of errors in choice reaction tasks on the ERP under focused and divided attention, in: Brunia CHM, Gaillaird AWK, Kok A (Eds.), Psychophysiological brain research, vol. 1 Tilburg University Press, Tilburg, The Netherlands, pp. 192–195. [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM, 2005. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annu. Rev. Psychol 56, 235–262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA, 2001. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development 72, 1–21. [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, Long JM, Stewart S, 1995. Frontal activation asymmetry and social competence at four years of age. Child Development 66, 1770–1784. [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E, 1993. A neural system for error-detection and compensation. Psychol. Sci 4, 385–390. [Google Scholar]
- Gehring WJ, Liu Y, Orr JM, Carp J, 2013. The error-related negativity (ERN/Ne), in: Luck SJ, Kappenman ES (Eds.), The Oxford handbook of event-related potential components. Oxford University Press, New York, NY. [Google Scholar]
- Goldsmith HH, 1996. Studying temperament via construction of the toddler behavior assessment questionnaire. Child Development 67, 218–235. [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF, 2001. The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology 38, 847–857. [PubMed] [Google Scholar]
- Hane AA, Fox NA, Henderson HA, Marshall PJ, 2008. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology 44, 1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesdottir DK, Doxie J, Bell MA, Ollendick TH, Wolfe CD, 2010. A longitudinal study of emotion regulation and anxiety in middle childhood: Associations with frontal EEG asymmetry in early childhood. Dev. Psychobiol 52, 197–204. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, 2018. On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: An updated review of the evidence. Psychophysiology 55. [DOI] [PubMed] [Google Scholar]
- Harrewijn A, Schmidt LA, Westenberg PM, Tang A, Van der Molen MJW, 2017. Electrocortical measures of information processing biases in social anxiety disorder: A review. Biol. Psychol 129, 324–348. [DOI] [PubMed] [Google Scholar]
- Harrewijn A, Van der Molen MJW, Westenberg PM, 2016. Putative EEG measures of social anxiety: Comparing frontal alpha asymmetry and delta-beta cross-frequency correlation. Cognitive Affective & Behavioral Neuroscience 16, 1086–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Fox NA, Rubin KH, 2001. Temperamental contributions to social behavior: The moderating roles of frontal EEG asymmetry and gender. J. Am. Acad. Child Adolesc. Psychiatr 40, 68–74. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Marshall PJ, Fox NA, Rubin KH, 2004. Psychophysiological and behavioral evidence for varying forms and functions of nonsocial behavior in preschoolers. Child Development 75, 251–263. [DOI] [PubMed] [Google Scholar]
- Jentzsch I, Dudschig C, 2009. Why do we slow down after an error? Mechanisms underlying the effects of posterror slowing. Quarterly Journal of Experimental Psychology 62, 209–218. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garciacoll C, 1984. Behavior-inhibition to the unfamiliar. Child Development 55, 2212–2225. [Google Scholar]
- Kagan J, Snidman N, 1991. Infant predictors of inhibited and uninhibited profiles. Psychol. Sci 2, 40–44. [Google Scholar]
- Lahat A, Benson BE, Pine DS, Fox NA, Ernst M, 2016. Neural responses to reward in childhood: Relations to early behavioral inhibition and social anxiety. Social Cognitive and Affective Neuroscience nsw 122, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA, 2014. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. J. Am. Acad. Child Adolesc. Psychiatr 53, 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Walker OL, Degnan KA, Henderson HA, Pine DS, McDermott JM, Fox NA, 2014. Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: An ERP study. Developmental Science 17, 667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA, 2009. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry 65, 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManis MH, Kagan J, Snidman NC, Woodward SA, 2002. EEG asymmetry, power, and temperament in children. Dev. Psychobiol 41, 169–177. [DOI] [PubMed] [Google Scholar]
- Mognon A, Jovicich J, Bruzzone L, Buiatti M, 2011. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology 48, 229–240. [DOI] [PubMed] [Google Scholar]
- Muris P, Dreessen L, Bogels S, Weckx M, van Melick M, 2004. A questionnaire for screening a broad range of DSM-defined anxiety disorder symptoms in clinically referred children and adolescents. Journal of Child Psychology and Psychiatry 45, 813–820. [DOI] [PubMed] [Google Scholar]
- Nash K, Inzlicht M, McGregor I, 2012. Approach-related left prefrontal EEG asymmetry predicts muted error-related negativity. Biol. Psychol 91, 96–102. [DOI] [PubMed] [Google Scholar]
- Nolan H, Whelan R, Reilly RB, 2010. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. J. Neurosci. Methods 192, 152–162. [DOI] [PubMed] [Google Scholar]
- Poole KL, Santesso DL, Van Lieshout RJ, Schmidt LA, 2018. Trajectories of frontal brain activity and socio-emotional development in children. Dev. Psychobiol 60, 353–363. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, 1999. Frontal brain electrical activity in shyness and sociability. Psychol. Sci 10, 316–320. [Google Scholar]
- Schmidt LA, Fox NA, Schulkin J, Gold PW, 1999. Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Dev. Psychobiol 35, 119–135. [PubMed] [Google Scholar]
- Schneider M, Chau L, Mohamadpour M, Stephens N, Arya K, Grant A, 2016. EEG asymmetry and BIS/BAS among healthy adolescents. Biol. Psychol 120, 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Reznik SJ, Stewart JL, Allen JJB, 2017. Assessing and conceptualizing frontal EEG asymmetry: An updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. Int. J. Psychophysiol 111, 98–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A, Miskovic V, Lahat A, Tanaka M, MacMillan H, Van Lieshout RJ, Schmidt LA, 2018. Trajectories of resting frontal brain activity and psychopathology in female adolescents exposed to child maltreatment. Dev. Psychobiol 60, 67–77. [DOI] [PubMed] [Google Scholar]
- Theall-Honey LA, Schmidt LA, 2006. Do temperamentally shy children process emotion differently than nonshy children? Behavioral, psychophysiological, and gender differences in reticent preschoolers. Dev. Psychobiol 48, 187–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.