Abstract
Voltage-gated potassium (K+) channel sub-family B member 1 (KCNB1, Kv2.1) is known to undergo oxidation-induced oligomerization during aging but whether this process affects brain’s physiology was not known. Here, we used 10, 16 and 22 month-old transgenic mice overexpressing a KCNB1 variant that does not oligomerize (Tg-C73A) and as control, mice overexpressing the wild type (Tg-WT) channel and non-transgenic (non-Tg) mice to elucidate the effects of channel’s oxidation on cognitive function. Aging mice in which KCNB1 oligomerization is negligible (Tg-C73A), performed significantly better in the Morris Water Maze (MWM) test of working memory compared to non-Tg or Tg-WT mice. KCNB1 and synapsin-1 co-immunoprecipitated and the cognitive impairment in the MWM was associated with moderate loss of synapsin-1 in pre-synaptic structures of the hippocampus, whereas neurodegeneration and neuronal loss were not significantly different in the various genotypes.
We conclude that moderate oxidation of the KCNB1 channel during aging can affect neuronal networks by affecting synaptic function.
INTRODUCTION
The voltage-gated K+ channel KCNB1 is known to undergo oxidation in neurons of the cortex and hippocampus, through a process conserved from rodents to humans [1, 2]. Oxidizing conditions promote the cross-linking of KCNB1 subunits to each other, through disulfide bridges involving Cys73. KCNB1 oligomers can affect the function of the neurons in several ways [2]. They do not conduct current and thus promote hyperexcitability [3]. Upon robust oxidative conditions they activate a toxic pathway, mediated by Focal Adhesion Kinase (FAK), Src tyrosine kinases and c-Jun N-terminal kinases (JNK) among others, that generates more ROS and eventually apoptosis [4, 5]. Accordingly, mouse models of Traumatic Brain Injury (TBI) and Alzheimer’s disease (AD) engineered to overexpress a dominant-negative KCNB1 variant (C73A) that does not oligomerize, exhibit decreased neuronal loss and improved cognitive function [1, 6]. Moreover, a FDA-approved Src inhibitor, Dasatinib, moderates neuronal loss and cognitive deficit by directly impinging on the apoptotic pathway activated in response to KCNB1 oxidation [1, 6].
KCNB1 is evolutionarily conserved. One of its ancestors is the voltage-gated K+ channel KVS-1, which is expressed in sensory neurons of the worm Caenorhabditis elegans [7–10]. Oxidation of conserved Cys113 in KVS-1 during aging, causes sensorial decline in the worm [11]. KCNB1 oligomers are present in the brains of elder humans and mice but whether they affect the function of the brain was not known. Thus, to gain insight into the role of KCNB1 oxidation during aging we took advantage of transgenic mice in which oligomerization of KCNB1 is suppressed (Tg-C73A) or increased (Tg-WT) compared to non-Tg control that we characterized previously [1, 5, 6]. Overall, our studies indicate that oxidation of KCNB1 is associated with moderate cognitive loss via a mechanism that involves the impairment of the function of the synapses at their pre-synaptic sites.
METHODS AND MATERIALS
Transgenic Mice
We used Tg-WT, Tg-C73A and non-Tg mice (B6XCBA background) that was previously characterized [6]. Briefly these mice overexpress human KCNB1 tagged to the human influenza hemagglutinin (HA) tag in the C-terminus [12] under the Thy1.2 cassette in cortex and hippocampus. Homozygous Tg-WT mice exhibit developmental delays. Therefore we used only mice heterozygous in hKCNB1 in this study. All experimental protocols involving animals were approved by the Rutgers University IACUC Committee.
Morris Water Maze
The detailed protocols for the Morris Water Maze were previously described [6]. Briefly, mice were acclimated to the paradigm and tested for baseline response using a visible platform test 4 days prior to injury. The animals were placed in a circular pool of water containing non-toxic white paint and a clear platform for escape. To assess learning, mice were trained using a hidden platform fixed in one of 4 quadrants for 6 consecutive days starting at 2 days post injury (4 trials/day). Black and white distal cues were placed on the walls. The quadrant in which the mouse was placed was pseudo-randomly varied throughout training and the time to locate the platform was recorded. Maximum trial time was 60 sec and the mouse remained or was placed on the platform for 15 sec and warmed for 10 min between trials. To assess memory retention, the day after the last training session, the animals were be subjected to a 60 sec probe trial with the platform removed and the time spent in the target quadrant was measured [13]. Data was recorded using a video-tracking system (EthoVision XT; Noldus Information Technology, Leesburg, VA).
Co-immunoprecipitations
The detailed procedures were previously described [6]. Briefly, frozen, half sagittal brains of three-month old-animals were homogenized with a glass tissue grinder in lysis buffer (0.32 M sucrose, 5 mM Tris-Cl pH 6.8, 0.5 mM EDTA, 1 mM PMSF) and protease inhibitor cocktail. Samples were centrifuged at 2,000 rpm for 10 min and the supernatant was used for biochemical analysis. Protein content was quantified with the Bradford colorimetric assay (Sigma). 1 mg of brain lysates were incubated at 4°C overnight with 8 μg of synapsin-1 antibody (clone AB1543. Millipore, Billerica, MA) or 10 μg of KCNB1 antibody (NeuroMab clone K89/34, UC Davis/NIH CA) or 10 μg of IgG-R Rhodamine conjugated antibody for control (clone sc-2091, Santa Cruz Biotechnology, Dallas, TX). Then, protein A/G agarose beads (30 μl of 50% bead slurry) were added and incubated for 2 hours at 4°C. Samples were centrifuged for 30 seconds at 4°C and the pellet was washed five times in cell lysis buffer. The pellet was resuspended in 50 μl 2× SDS Laemmli buffer, heated at 100°C for 10 minutes and centrifuged for 1 minute at 14,000× g. The sample was loaded on 8% SDS-PAGE gel and immunoblotted with either synapsin-1 or KCNB1 antibody.
Immunohistochemistry
The detailed immunohistochemical procedures were previously described [6]. Briefly, mice were transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. The brains were cryoprotected in 30% sucrose and 20–80 μm frozen sections were prepared.
Silver staining Sections were stained with FD NeuroSilver Kit II (FD Neurotechnologies, Columbia, MD) according to manufacturer’s instruction and were analyzed and photographed with an Olympus BX61 microscope equipped with a digital camera. Synapsin-1 staining Sections were incubated with synapsin-1 antibody (1:250) overnight and followed by 1:500 application of goat anti-rabbit IgG-R Rhodamine conjugated secondary antibody (clone sc-2091, Santa Cruz Biotechnology, Dallas, TX). All slides were mounted in VECTASHIELD Antifade Mounting Medium with DAPI mounting buffer (Vector Laboratories, Burlingame, CA) and stored at 4°C. Sections were analyzed and photographed with an Olympus FV1000MPE multi-photon microscope with full capacity for confocal microscopy (including 405nm, multiline-argon, 559nm, 635nm lasers) and dedicated software.
Statistical analysis
Quantitative data are presented as mean ± standard error of the mean (SEM). The level of significance, assumed at the 95% confidence limit or greater (P<0.05), was calculated using one-way ANOVA with a Tukey post hoc test (http://astatsa.com/OneWay_Anova_with_TukeyHSD).
RESULTS
KCNB1 oxidation contributes to cognitive impairment in aging mouse.
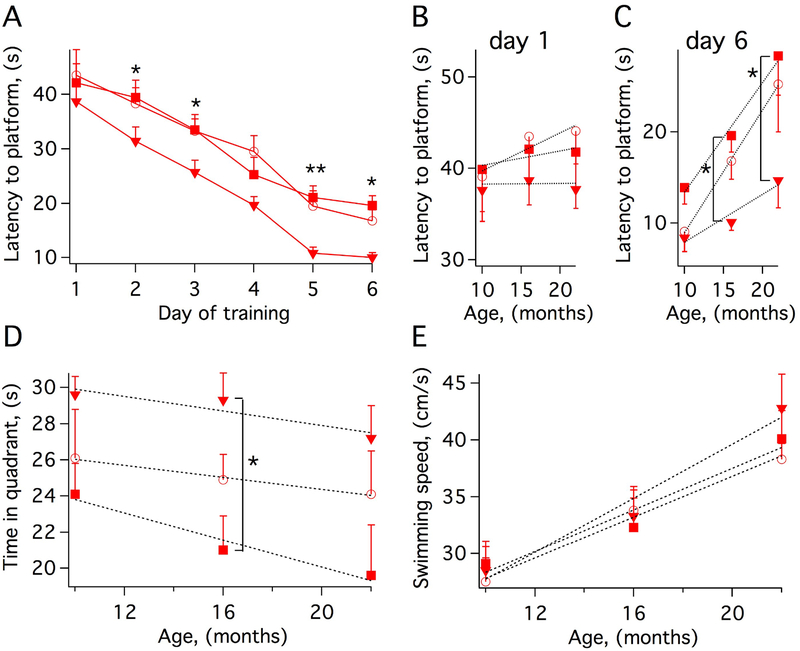
The hippocampus plays important roles in the consolidation of working and spatial memory. These cognitive functions in rodents are generally assessed in the Morris Water Maze (MWM). This test relies on distal cues to navigate from start locations around the perimeter of an open swimming pool to locate a submerged escape platform. Spatial learning is assessed across repeated trials (fours trials per day for 6 days) as the time to locate the platform (latency to platform, Figs. 1A–1C) and reference memory is determined by preference for the platform area when the platform is absent (time spent in target quadrant, Fig. 1D). Non-transgenic (non-Tg) mice and transgenic mice overexpressing in hippocampus and cortex the C73A variant of human KCNB1 that does not oligomerize (Tg-C73A) or the WT channel (Tg-WT) [5, 6] exhibited a progressive loss of spatial learning during aging, as expected. However, the Tg-C73A mice performed better than the other genotypes during both training and post-training. To illustrate this point, Fig. 1B shows latency to platform at the beginning (day 1) Fig. 1C shows latency and end (day 6) of training as a function of age. While at day 1 it took approximately all the genotypes the same amounts of time to find the hidden platform (~38 s for Tg-C73A compared to ~40 s of Tg-WT), at day 6, Tg-C73A mice had learned to find the platform progressively faster (~14 s for Tg-C73A compared to ~28 s of Tg-WT). Mice exhibited a progressive loss of muscular function (swimming speed, Fig. 1E) which is partially responsible for the delay in the latency to platform in old animals. However this affected all genotypes to the same extent, and thus swimming speed alone cannot account for the differences observed between Tg-C73A and the other genotypes.
Figure 1. Cognitive deficit is delayed in Tg-C73A mice.
A) Latency to reach the hidden platform of 16 month-old non-Tg (circles), Tg-WT (squares) and Tg-C73A (triangles) mice.
B-C) Latencies at day 1 (B) and day 6 (C) as a function of age for the indicated genotypes.
D) Time spent in the target quadrant (platform removed) at day 7 as a function of age for the indicated genotypes.
E) Swimming speed as a function of age for the indicated genotypes.
N=groups of 9–11 mice genotype/age. Statistically significant pair-wise comparisons between genotypes at each time point (Tukey’s post hoc) are indicated with *P<0.05 and **P<0.01. For simplicity, in A) are shown only comparisons between WT and C73A.
KCNB1 oxidation is associated with loss of synapsin-1 in old mice
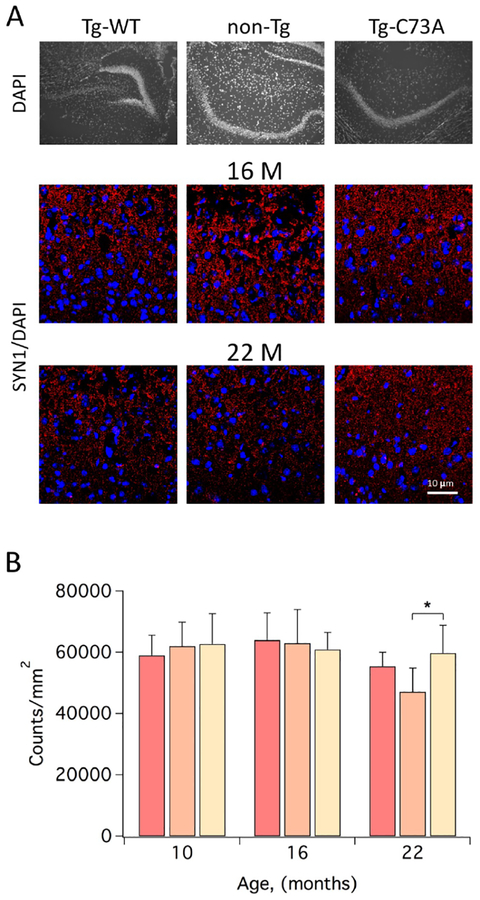
It is general opinion that long-term potentiation (LTP), a strengthening of synapses induced by patterns of repetitive electrical activity, is a major cellular mechanism of spatial learning and memory formation in the hippocampus. LTP is determined by a parallel increase of pre- and postsynaptic structures known as dendritic spines [14–16]. We therefore used synapsin-1, which plays a major role in short-term synaptic plasticity [17, 18], as a proxy to determine whether the differences in the performance in the MWM could be attributed to altered synaptic function. In hippocampal sections cut from the brains of the same 10 and 16 month old mice that had previously underwent MWM testing, the number of pre-synaptic structures reactive for synapsin-1 was fairly stable and comparable in all genotypes (Figs. 2A and 2B). In contrast 22 month-old Tg-WT, and to a lesser extent non-Tg mice, exhibited a decrease in the number of reactive pre-synapses which was not observed in Tg-C73A (Figs. 2A and 2B).
Figure 2. Oxidation of KCNB1 associates with loss of synapsin-1 in aging mice.
A) Representative low magnification Nomarski images of DAPI staining and confocal images of synapsin-1 (SYN1, red) and DAPI (blue) staining of coronal hippocampal sections cut from the brains of 16 and 22 month-old Tg-WT, non-Tg and Tg-C73A mice. B) Mean number of synapses reactive to synapsin-1 of hippocampal sections of non-Tg (red color), Tg-WT (beige color) and Tg-C73A (sand color) mice at the indicated time points. N=2 brains/genotype, 5 technical replicates/brain. *P<0.05.
KCNB1 channels co-immunoprecipitate with synapsin-1 in the brain
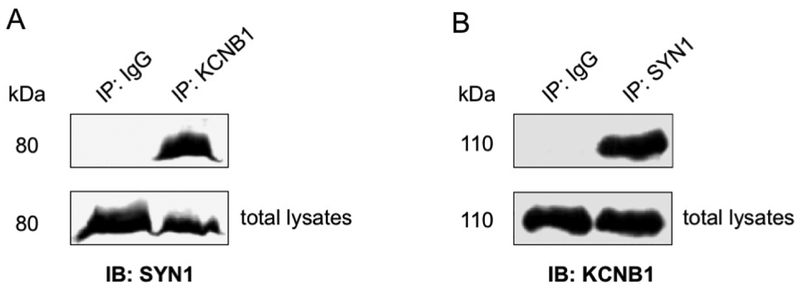
To determine whether synapsin-1 and KCNB1 interact in the brain, proteins were immunoprecipitated (IP) with an antibody that detects KCNB1 and immunoblotted (IB) with an antibody that detects synapsin-1 or vice versa. Representative Western blots of coimmunoprecipitation (co-IP) experiments from brain lysates of a three month-old non-Tg mouse are shown in Fig. 3. Thus, KCNB1 pulled down synapsin-1 (Fig. 3A. N=2 experiments) and similarly, synapsin-1 pulled down KCNB1 channels (Fig. 3B. N=2 experiments). KCNB1 and synapsin-1 similarly co-immunoprecipitated from the brain lysates of Tg-WT and Tg-C73A mice (N=1 experiment/genotype, data not shown). Together these data suggest that KCNB1 and synapsin-1 are part of macromolecular complexes present in pre-synaptic terminals of neurons of the hippocampus.
Figure 3. Synapsin-1 co-immunoprecipitates with KCNB1 channels.
A) KCNB1 (IP: KCNB1) or IgG immunoprecipitates (IP: IgG) and total lysates form the brain of a three month-old non-Tg mouse were immunoblotted (IB) with synapsin-1 (SYN1) antibody.
B) SYN1 (IP: SYN1) or IgG immunoprecipitates (IP: IgG) and total lysates form the brain of a three month-old non-Tg mouse were immunoblotted (IB) with KCNB1 antibody.
KCNB1 oxidation during aging does not promote neurodegeneration
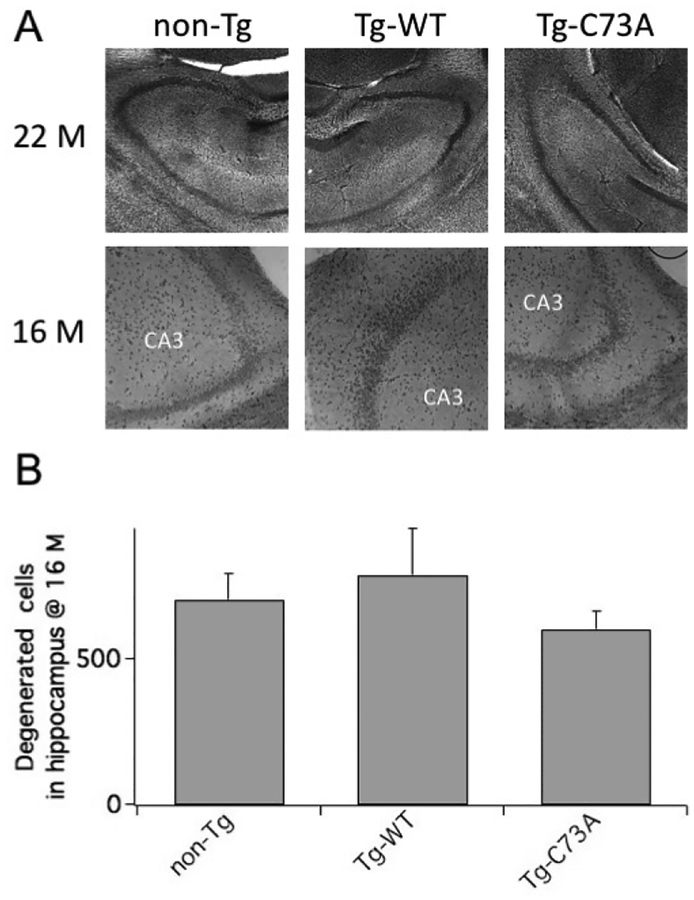
Under conditions of abundant oxidative stress such as those following a trauma or in AD, oxidation of KCNB1 causes neurodegeneration and apoptosis, that are reflected in marked behavioral deficit and cellular lesions [1, 6]. To determine whether oxidation of KCNB1 also promotes neurodegeneration during aging we used silver staining to assess neurodegeneration in the hippocampus of 22 month-old mice that had underwent MWM testing. The results of those experiments, illustrated in Fig. 4 showed that neurodegeneration was substantially comparable across genotypes.
Figure 4. KCNB1 oxidation does not promotes neurodegeneration in aging mice.
A) Representative images of coronal hippocampal sections stained with silver. Top: 22 month-old mice (4× magnification). Bottom: 16 month-old mice (10× magnification). B) Mean number of degenerated cells in the stratum radiatum and statum lacunoso (underlying the CA1 and CA3 pyramidal layers) of 22 month-old mice. N=2 brains/genotype with 2 technical replicates/brain.
DISCUSSION
To determine whether the physiological oxidation of KCNB1 that occurs during aging is a contributing factor to cognitive decline we assessed non-transgenic and transgenic mice in the MWM test of working memory and we then used their brains for biochemical assessment of synaptic marker synapsin-1. Our result show that KCNB1 and synapsin-1 are part of a macromolecular complex and that oxidation of KCNB1 causes moderate cognitive impairment in aging mice and loss of synapsin-1 in pre-synaptic structures. We conclude that moderate oxidation of KCNB1 channels, such as that occurring during normal aging [2], can impair hippocampal function via a mechanism that affects the synapses.
KCNB1 channels form macromolecular complexes with integrins in the brain [1, 5]. Robust oxidation of KCNB1 channels is translated by their integrin partners into a toxic pathway, mediated by FAK, Src and JNK kinases among others, that generates more ROS and eventually apoptosis [4, 5, 19–21]. What causes integrins to initiate apoptosis is not known but it is likely that they may interpret the formation of KCNB1 oligomers as detachment from the extracellular matrix [2, 4], a general requirement for cell survival [22, 23], and thus initiate apoptosis in response. While in mouse model of AD oxidized KCNB1 channels are ~70% of the total channels, in non-Tg mice they are only ~35% [2], a fraction that does not seem to be sufficient to trigger apoptosis, as neurodegeneration and neuronal loss were comparable in all genotypes. However, in aging Tg-WT mice where oxidation of KCNB1 is increased compared to control (heterozygous mice overexpresses ~30% more KCNB1 protein than non-Tg) [1, 5, 6] synapsin-1 levels were moderately decreased whereas in Tg-C73A brains they remained stable. The ability to form synaptic connections is a fundamental function of neurons and spine morphogenesis and plasticity depend upon dynamic remodeling of the actin cytoskeleton [24]. Integrins have an established importance in determining these mechanisms [25–36] but also synapsins, which play a major role in neurotransmitter release, interact with actin [37, 38]. Indeed KCNB1 and synapsin-1 coimmunoprecipitated. This underscores the existence of functional interactions between KCNB1 channels and synapsin-1 as they are appear to be part of macromolecular complexes that probably act to transduce events at the surface into inside signals. Within this broad picture it is possible that moderate oxidation of KCNB1 affects synaptic function via mechanisms that range from altered neurotransmission to spine morphogenesis. Our findings are in agreement with a previous study that suggested that KCNB1 and synapsin-1b are part of a macromolecular complex containing the dopamine transporter (DAT) in presynaptic terminals of dopaminergic neurons in the substantia nigra [39, 40].
Voltage-gated K+ channels play important roles in learning and memory formation in the hippocampus. For example, rapidly inactivating K+ channel KCND2 (Kv4.2), contributes to boost Ca2+ influx--a crucial step for the establishment of LTP, by promoting local increase in dendritic excitability via a mechanism known as action potential back-propagation [41–44]. The data reported here suggest that voltage-gated K+ channels can also transduce membrane excitability into intracellular signals that affect LTP via mechanisms that affect the function and/or the structure of the synapses.
Highlights.
Determination of voltage-gated potassium channel KCNB1 induced behavioral deficit in mice during aging.
Identification of KCNB1-associated loss of synapsin-1 in aging mice.
Formation of stable complexes between KCNB1 and synapsin-1 in the mouse’s brain.
ACKNOWLEDGEMENTS
We thank Ms. Tatiana Popovitchenko and Dr. Mladen-Roko Rasin for help with the confocal microscope. This work was supported by NSF grant (#1456675) and NIH grants (R01AG060919 and R21NS096619) to FS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are not conflicts of interest.
REFERENCES
- [1].Wei Y, Shin MR, Sesti F, Oxidation of KCNB1 channels in the human brain and in mouse model of Alzheimer’s disease, Cell Death Dis, 9 (2018) 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cotella D, Hernandez-Enriquez B, Wu X, Li R, Pan Z, Leveille J, Link CD, Oddo S, Sesti F, Toxic role of K+ channel oxidation in mammalian brain, J Neurosci, 32 (2012) 4133–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Frazzini V, Guarnieri S, Bomba M, Navarra R, Morabito C, Mariggio MA, Sensi SL, Altered Kv2.1 functioning promotes increased excitability in hippocampal neurons of an Alzheimer’s disease mouse model, Cell Death Dis, 7 (2016) e2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu X, Hernandez-Enriquez B, Banas M, Xu R, Sesti F, Molecular mechanisms underlying the apoptotic effect of KCNB1 K+ channel oxidation, J Biol Chem, 288 (2013) 4128–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yu W, Gowda M, Sharad Y, Singh SA, Sesti F, Oxidation of KCNB1 potassium channels triggers apoptotic integrin signaling in the brain, Cell Death Dis, 8 (2017) e2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yu W, Parakramaweera R, Teng S, Gowda M, Sharad Y, Thakker-Varia S, Alder J, Sesti F, Oxidation of KCNB1 Potassium Channels Causes Neurotoxicity and Cognitive Impairment in a Mouse Model of Traumatic Brain Injury, J Neurosci, 36 (2016) 11084–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bianchi L, Kwok SM, Driscoll M, Sesti F, A potassium channel-MiRP complex controls neurosensory function in Caenorhabditis elegans, J Biol Chem, 278 (2003) 12415–12424. [DOI] [PubMed] [Google Scholar]
- [8].Cai SQ, Hernandez L, Wang Y, Park KH, Sesti F, MPS-1 is a K(+) channel beta-subunit and a serine/threonine kinase, Nat. Neurosci, 8(11) (2005) 1503–1509. [DOI] [PubMed] [Google Scholar]
- [9].Cai SQ, Sesti F, A new mode of regulation of N-type inactivation in a Caenorhabditis elegans voltage-gated potassium channel, J Biol Chem, 282 (2007) 18597–18601. [DOI] [PubMed] [Google Scholar]
- [10].Rojas P, Garst-Orozco J, Baban B, de Santiago-Castillo JA, Covarrubias M, Salkoff L, Cumulative activation of voltage-dependent KVS-1 potassium channels, J Neurosci, 28 (2008) 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cai SQ, Sesti F, Oxidation of a potassium channel causes progressive sensory function loss during aging, Nat Neurosci, 12 (2009) 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cotella D, Hernandez-Enriquez B, Wu X, Li R, Pan Z, Leveille J, Link CD, Oddo S, Sesti F, Toxic role of k+ channel oxidation in Mammalian brain, The Journal of neuroscience: the official journal of the Society for Neuroscience, 32 (2012) 4133–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Longhi L, Saatman KE, Fujimoto S, Raghupathi R, Meaney DF, Davis J, McMillan BSA, Conte V, Laurer HL, Stein S, Stocchetti N, McIntosh TK, Temporal window of vulnerability to repetitive experimental concussive brain injury, Neurosurgery, 56 (2005) 364–374; discussion 364–374. [DOI] [PubMed] [Google Scholar]
- [14].Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K, Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo, Neuron, 38 (2003) 447–460. [DOI] [PubMed] [Google Scholar]
- [15].Colicos MA, Collins BE, Sailor MJ, Goda Y, Remodeling of synaptic actin induced by photoconductive stimulation, Cell, 107 (2001) 605–616. [DOI] [PubMed] [Google Scholar]
- [16].Bramham CR, Local protein synthesis, actin dynamics, and LTP consolidation, Curr Opin Neurobiol, 18 (2008) 524–531. [DOI] [PubMed] [Google Scholar]
- [17].Rosahl TW, Geppert M, Spillane D, Herz J, Hammer RE, Malenka RC, Sudhof TC, Short-term synaptic plasticity is altered in mice lacking synapsin I, Cell, 75 (1993) 661–670. [DOI] [PubMed] [Google Scholar]
- [18].De Camilli P, Cameron R, Greengard P, Synapsin I (protein I), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections, J Cell Biol, 96 (1983) 1337–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E, Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels, J Neurosci, 23 (2003) 4798–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McCord MC, Aizenman E, Convergent Ca2+ and Zn2+ signaling regulates apoptotic Kv2.1 K+ currents, Proc Natl Acad Sci U S A, 110 (2013) 13988–13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Redman PT, He K, Hartnett KA, Jefferson BS, Hu L, Rosenberg PA, Levitan ES, Aizenman E, Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1, Proc Natl Acad Sci U S A, 104 (2007) 3568–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Meredith JE Jr., Fazeli B, Schwartz MA, The extracellular matrix as a cell survival factor, Mol Biol Cell, 4 (1993) 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Frisch SM, Francis H, Disruption of epithelial cell-matrix interactions induces apoptosis, J Cell Biol, 124 (1994) 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bosch M, Hayashi Y, Structural plasticity of dendritic spines, Curr Opin Neurobiol, 22 (2012) 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wu GY, Deisseroth K, Tsien RW, Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway, Proc Natl Acad Sci U S A, 98 (2001) 2808–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wu GY, Deisseroth K, Tsien RW, Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology, Nat Neurosci, 4 (2001) 151–158. [DOI] [PubMed] [Google Scholar]
- [27].Webb DJ, Zhang H, Majumdar D, Horwitz AF, alpha5 integrin signaling regulates the formation of spines and synapses in hippocampal neurons, J Biol Chem, 282 (2007) 6929–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shi Y, Ethell IM, Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization, J Neurosci, 26 (2006) 1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D’Souza S, Wong TP, Taghibiglou C, Lu J, Becker LE, Pei L, Liu F, Wymann MP, MacDonald JF, Wang YT, Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons, Neuron, 38 (2003) 611–624. [DOI] [PubMed] [Google Scholar]
- [30].Sui L, Wang J, Li BM, Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex, Learn Mem, 15 (2008) 762–776. [DOI] [PubMed] [Google Scholar]
- [31].Patel R, Sesti F, Oxidation of ion channels in the aging nervous system, Brain Res, 1639 (2016) 174–185. [DOI] [PubMed] [Google Scholar]
- [32].Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G, Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation, J Cell Biol, 186 (2009) 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Murakoshi H, Wang H, Yasuda R, Local, persistent activation of Rho GTPases during plasticity of single dendritic spines, Nature, 472 (2011) 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY, Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways, J Neurosci, 25 (2005) 11288–11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Akama KT, McEwen BS, Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway, J Neurosci, 23 (2003) 2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lynch MA, Long-term potentiation and memory, Physiol Rev, 84 (2004) 87–136. [DOI] [PubMed] [Google Scholar]
- [37].Greengard P, Benfenati F, Valtorta F, Synapsin I, an actin-binding protein regulating synaptic vesicle traffic in the nerve terminal, Adv Second Messenger Phosphoprotein Res, 29 (1994) 31–45. [DOI] [PubMed] [Google Scholar]
- [38].Bloom O, Evergren E, Tomilin N, Kjaerulff O, Low P, Brodin L, Pieribone VA, Greengard P, Shupliakov O, Colocalization of synapsin and actin during synaptic vesicle recycling, J Cell Biol, 161 (2003) 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maiya R, Ponomarev I, Linse KD, Harris RA, Mayfield RD, Defining the dopamine transporter proteome by convergent biochemical and in silico analyses, Genes Brain Behav, 6 (2007) 97–106. [DOI] [PubMed] [Google Scholar]
- [40].Nirenberg MJ, Chan J, Pohorille A, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM, The dopamine transporter: comparative ultrastructure of dopaminergic axons in limbic and motor compartments of the nucleus accumbens, J Neurosci, 17 (1997) 6899–6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Aniksztejn L, Ben-Ari Y, Novel form of long-term potentiation produced by a K+ channel blocker in the hippocampus, Nature, 349 (1991) 67–69. [DOI] [PubMed] [Google Scholar]
- [42].Watanabe S, Hoffman DA, Migliore M, Johnston D, Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons, Proc Natl Acad Sci U S A, 99 (2002) 8366–8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA, Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons, Neuron, 54 (2007) 933–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Frick A, Magee J, Johnston D, LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites, Nat Neurosci, 7 (2004) 126–135. [DOI] [PubMed] [Google Scholar]