Abstract
Introduction:
Manganese (Mn) is an essential nutrient but also a toxicant at high exposures, when it can induce oxidative stress (OS). Mn uptake is inversely correlated with iron status, therefore anemic individuals may be more susceptible to Mn overload induced-OS, which can manifest as changes in mitochondrial DNA copy number (mtDNA CN). Our objectives were to: 1) determine stage-specific associations of prenatal Mn exposure with cord blood MtDNA CN; and 2) investigate effect modification by maternal anemia, ferritin, and mean corpuscular volume (MCV).
Materials and Methods:
We measured whole blood Mn, hemoglobin, serum ferritin, and MCV in the 2nd and 3rd trimester, in maternal blood at birth, and in cord blood from a prospective birth cohort in Mexico City, Mexico (n = 485). We then extracted DNA from cord blood leukocytes to determine mtDNA CN. We used robust regression to measure associations between Mn and mtDNA CN at each trimester and at birth. Anemia (hemoglobin ≤ 11g/dL), iron deficiency (ferritin ≤ 15 ng/mL) and MCV (stratified at median), were examined as effect modifiers.
Results:
Mn levels increased throughout pregnancy, and Mn was inversely correlated with ferritin. We observed a positive association between Mn in the 3rd trimester and Mn in cord blood and mtDNA CN (β = 0.04 – 0.05; 95% CI = 0.01, 0.08). Anemia significantly modified the association between mtDNA CN and Mn in the 2nd trimester. We found a positive association between 2nd trimester Mn and mtDNA CN in mothers with normal hemoglobin, and a negative association in those with low hemoglobin. (βhigh = 0.06; 95% CI = 0.01, 0.11; p = 0.01 and βlow = −0.06; 95% CI = 0.03, −0.13; p = 0.06). No associations were detected between anemia, iron deficiency and MCV and mtDNA CN.
Conclusions:
Maternal blood Mn in the 3rd trimester and in cord blood was positively associated with mtDNA CN, suggesting that higher late pregnancy prenatal Mn exposures can impact newborn mitochondria by promoting OS. Furthermore, 2nd trimester Mn was positively associated with mtDNA in non-anemic mother-child pairs but inversely associated in anemic individuals, indicating potential interactions between Mn and chronic anemia.
Keywords: Prenatal Exposure, Manganese, Anemia, Iron Deficiency, Mitochondria, Mitochondrial DNA
1. Introduction
Manganese (Mn) is a transition element that is crucial for normal development and cellular function. It plays a particularly important role in defense against oxidative stress (OS) as a cofactor for superoxide dismutase, an enzyme found in mitochondria that detoxifies superoxide anions (Aguirre and Culotta, 2012). Mn is also vital to a number of other processes, such as calcium homeostasis, carbohydrate metabolism, and protein metabolism (Freeland-Graves et al., 2014). However, in situations of Mn overload, Mn can itself induce OS replacing iron in the Fenton reaction, a catalytic reaction via transition metals that converts hydrogen peroxide to free radicals, such as oxygen super anion, within the mitochondria. Elevated Mn exposure can thus induce detrimental health effects via OS, including neurotoxicity, hepatoxicity, and cardiovascular toxicity (Crossgrove and Zheng, 2004). Mn is ubiquitous and present naturally in soil, and can be released into the environment from anthropogenic activities (U.S. EPA, 2003). Exposure occurs primarily through the diet; however, airborne Mn can also be a significant source (U.S. EPA, 2003).
Mn levels in blood naturally rise in the mother throughout pregnancy. Mn crosses the placenta via an active transport mechanism allowing it to concentrate in the neonate when comparing maternal and cord blood levels at delivery (Krachler et al., 1999; Spencer, 1999). Furthermore, Mn also crosses the developing blood brain barrier to accumulate in the neonate brain, particularly in regions rich in dopaminergic neurons, such as the basal ganglia (Aschner, 2000). Although Mn is classified as a nutrient, elevated prenatal blood Mn has been associated with adverse neurodevelopmental effects, including lower child cognitive, language, and motor scores (Henn et al., 2010; Lin et al., 2013; Muñoz-Rocha et al., 2017; Takser et al., 2003).
Mn toxicity is often attributed to its ability to catalyze redox reactions generating reactive oxygen species (ROS). Mitochondria are the main site of oxidative phosphorylation and the dominant cellular site of ROS generation. We hypothesized that mitochondrial replication or loss, both of which are cellular reactions to oxidative stress, may play a role in Mn toxicity given these properties. Mitochondria are primarily responsible for cellular bioenergetics, producing over 90% of the cell’s energy (Chance and Williams, 2006). In the mitochondria, Mn acts as a cofactor for the anti-oxidant enzyme Mn-SOD under homeostasis, but can function as a toxicant under high exposures. Multiple studies have demonstrated interference of excess Mn with energy production in vitro (Fernandes et al., 2017; Gavin et al., 1992; Gunter et al., 2010; Zwingmann et al., 2003) and in vivo (Cordova et al., 2012; Zhang et al., 2003). Cells generally contain 102-104 copies of mitochondrial DNA (mtDNA) (Legros et al., 2004) and small variations in mtDNA content have been detected depending on mitochondrial morphology, energy demands, and physiological environmental conditions (Lee and Wei, 2005). mtDNA is highly susceptible to oxidative damage (Kazak et al., 2012). Short term, oxidative stress increases mtDNA CN while chronic OS reduces mtDNA CN. Under OS and following chemical exposure, mtDNA copy number (mtDNA CN) increases, possibly as a compensatory mechanism to decreased energy production (Lee et al., 2000; Lee and Wei, 2005; Meyer et al., 2013). However, under conditions of reduced antioxidant capacity, such as those resulting from chronic stress, OS can overwhelm the mitochondria resulting in apoptosis and reduced mtDNA CN (Lee and Wei, 2005). Low mtDNA CN has been linked to decreased cognition, strength, and overall health in human populations (Mengel-From et al., 2014). Furthermore, low mtDNA CN and mutations in mtDNA resulting from oxidative damage have been shown to persist and accumulate over time (Golden and Melov, 2001; Mengel-From et al., 2014; Sondheimer et al., 2011), suggesting that early exposures may impact later mitochondrial health. Thus, both elevated and reduced mtDNA CN can result from OS depending on the mechanisms leading to OS and its chronicity.
In addition to damaging effects on mitochondria, excess Mn interacts with other essential metals, especially iron. Mn is a transition metal like iron (Fe) and has similar valence states, but has a higher reduction potential. In excess, however, Mn can replace iron in chemical reactions, such as the Fenton reaction. Several studies have demonstrated that low iron levels increase Mn accumulation likely due to iron deficiency induced upregulation of shared transport/absorption proteins that bind both Mn and Fe (Finley, 1999; Henn et al., 2010; Smith et al., 2013). The joint presence of iron deficiency and excess Mn exposure could raise the risks of Mn toxicity (Seo et al., 2013). Iron requirements triple during pregnancy, and the prevalence of iron deficiency worldwide is high (World Health Organization, 2001). Prenatal iron deficiency anemia is also neurotoxic and can decrease cognitive performance, growth, and energy in infants and children (World Health Organization, 2001). Increased Mn absorption that arises from iron deficiency may play a role in the neurotoxicity of iron deficiency anemia, especially if Mn levels begin to exceed physiologic norms.
The complex biology between Mn homeostasis, mitochondria, OS, and anemia/iron homeostasis suggests that maternal anemia might interact with Mn to affect newborn OS, which could manifest as changes in mtDNA CN. Low iron is the most common cause of anemia worldwide, and approximately 30% of pregnant women in Latin America are anemic (Balarajan et al., 2011). Iron deficiency anemia (IDA) is a risk factor for low birth weight and preterm delivery (Allen, 2000). Mitochondria are crucial for iron and Mn transport and metabolism (Stehling and Lill, 2013; Zhao and Enns, 2012). Iron and Mn are both redox reactive metals, and can induce OS via the Fenton reaction (Koskenkorva-Frank et al., 2013) but Mn deficiency is extremely rare, whereas iron deficiency is very common. The most common synergistically toxic scenario therefore is Mn excess combined with iron deficiency, a combination that is rarely studied.
To date, few studies have investigated the role of excess Mn on mitochondria in humans, and to our knowledge, no studies have examined the contribution of iron deficiency and anemia to this association. In the current study, we leverage data from a prospective birth cohort to examine the association of maternal blood Mn levels and maternal iron deficiency and anemia with newborn mtDNA CN. The objective of this research was to: 1) determine the stage specific effects of prenatal Mn exposure on child mtDNA CN; and 2) investigate the effect modification of this relationship by maternal anemia, serum ferritin, and mean corpuscular volume (MCV). We hypothesized that excess Mn would generate OS within the mitochondria, leading to mitochondrial toxicity and increased mtDNA CN as a compensatory response. Furthermore, we hypothesized that anemia and iron deficiency would exacerbate the effects of Mn via three mechanisms: 1) through increased Mn uptake from divalent metal transporters; 2) through independent generation of chronic OS, leaving the cell more susceptible to Mn toxicity; and 3) through Mn replacement of iron in the Fenton reaction.
2. Methods
2.1. Study Population
Pregnant women receiving prenatal care from the Mexican Social Security Institute (Instituto Mexicano del Seguro Social) clinics were enrolled before 20 weeks of gestation into the Programming Research in Obesity, Growth Environment and Social Stress (PROGRESS) birth study in Mexico City, Mexico. Enrollment details have been described elsewhere (Braun et al., 2014; Burris et al., 2013). We enrolled 948 women who delivered a live baby. Women who reported smoking during pregnancy were removed from the analysis (n = 6), due to the reported effect of smoking on mtDNA CN (Pirini et al., 2017). mtDNA CN was measured in cord blood of 531 newborns and only individuals with complete exposure, outcome and covariate data were included in the analysis (n= 485 – 424). There were no significant differences between the characteristics of the participants used in this study with the whole PROGRESS study population (Data not shown). Procedures were approved by institutional review boards at the Columbia University, Icahn School of Medicine at Mount Sinai, and the Mexican National Institute of Public Health. All women provided written informed consent for themselves and their newborn.
2.2. Manganese Measurement
Manganese was measured in maternal whole blood at the 2nd trimester (n = 894), 3rd trimester (n = 745), at birth (n = 732), and in cord blood (n = 515). Maternal blood was collected by trained research staff and stored at −20°C. Mn was analyzed as previously described for lead with the appropriate quality controls (Renzetti et al., 2017). In brief, blood was digested in concentrated HNO3 and 30% H2O2 and analyzed via ICP-MS/MS on an Agilent 8800 ICP Triple Quad (ICP-QQQ) instrument (Agilent technologies, Inc., Delaware, USA) with Indium as the internal standard. Lead (Pb) values were measured with the same method for sensitivity analyses. The quantitation limit of detection (LOD) ranged between 0.02 and 0.08 μg/dL. None of the measured values were below the LOD.
2.3. Maternal Anemic Blood parameter measurement
Hemoglobin, mean corpuscular volume (MCV), and red blood cell distribution width (RDW) at the 2nd trimester, 3rd trimester, and in maternal blood at birth were measured as part of a standard complete blood count (CBC) by trained research staff. A CBC was only performed on a small subset of cord blood samples to estimate neutrophil, lymphocyte, and platelet counts (N=302). Serum was collected by centrifugation, and serum ferritin was measured using an ELISA chemi-luminescence assay on an Immulite 100 (Siemens, Munich, Germany).
2.4. Relative mitochondrial DNA copy Number measurement
Venous blood was collected from cord blood of 531 of the 948 newborns and mtDNA CN was measured as previously described (Rosa et al., 2017). In brief, the first 260 whole blood samples were extracted using a QIAamp DNA Blood Kit (QIAGEN) at stored at −80°C, while the next 271 samples were extracted by conventional phenol–chloroform method by a second laboratory and stored at 4°C. The ratio of abundance of mtDNA 12S ribosomal RNA to nuclear DNA (nDNA) as measured by multiplexed quantitative real-time polymerase chain reaction (qPCR) was used to calculate mtDNA CN. The primers for qPCR analysis of mtDNA were: mtF805 5’-CCACGGGAAACAGCAGTGATT-3’and mtR927 5’-CTATTGACTTGGGTTAATCGTGTGA-3’ and a commercial kit was used to quantify nDNA (TaqMan RNase P Control Reagents Kit, Applied Biosystems). The PCRs were performed on a Bio-Rad CFX96 Real-time PCR detection system (BioRad, Hercules, CA) as follows: 95°C for 15 minutes, 39 cycles of 95°C for 15 seconds, 60°C for 1 minute, and melting curve 65°C. To ensure quality control, each plate included 2 negative controls, each sample was run in triplicate and each plate contained five interplate controls. A pool of 300 DNA samples was used to construct a reference for mtDNA/nDNA and a ratio of 1 would indicate that the ratio of mtDNA/nDNA of the test sample was equal to the reference sample. The within-run and between-run coefficients of variation of this assay were 5% and 7%, respectively.
2.5. Statistics
To adjust for any effect of mtDNA CN that was due to the extraction method, we normalized values by a method as previously described (Rosa et al., 2017) and square root transformed the results. We then natural log transformed Mn values to approximate a normal distribution and averaged the 2nd trimester and 3rd trimester values to estimate average exposure during pregnancy.
Three types of blood parameters were used as indicators of maternal anemia: hemoglobin, serum ferritin, and MCV. Hemoglobin levels are influenced by atmospheric oxygen tension (i.e. elevation) and are positively correlated with elevation. Thus, we subtracted 1.3 g/dL from our values to reflect the elevation of Mexico City at 7,382ft (Dirren et al., 1994). We then determined anemia according to the World Health Organization guidelines for pregnancy: 11 g/dL hemoglobin (World Health Organization, 2001). We considered serum ferritin values less than 15 ng/mL as low (World Health Organization, 2001). MCV is a measurement of red blood cell size and is associated with both iron deficiency anemia and the genetic condition, Thalassemia trait, which can be common in some populations (Van Vranken, 2010). According to the American Association of Family Physicians, anemic individuals with an MCV of less than 95 fL and a serum ferritin of less than 30 ng/mL can be classified as having iron deficiency anemia (Short and Domagalski, 2013). Microcytosis, small red blood cells (MCV ≤ 80 fL), can also be an indicator of anemia or of the Thalassemia trait, whereas macrocytosis (MCV ≥ 100 fL) can be used to diagnose the type of anemia. The red blood cell width distribution (RDW) is another parameter used to diagnose Thalassemia. Using the AAFP guidelines to diagnose Thalessemia (MCV ≤ 80 fL, RDW ≥ 15 fL, and ferritin ≥ 100 ng/mL), we identified no individuals within our population with Thalessemia (Muncie and Campbell, 2009). Furthermore, in the diagnosis of microcytosis, only 8 individuals in the study population of 485 mothers had MCV values ≤ 80 fL, and within those with anemia, only 6% and 8% had low MCV in the 2nd trimester and 3rd trimester respectively. Thus, we opted to dichotomize MCV at the median for the purpose of this analysis (Table 2). Henceforth, all discussion of anemia herein will be referring explicitly to the WHO hemoglobin guidelines.
Table 2.
Exposures measured in the 2nd trimester, 3rd trimester, in maternal blood at birth, and cord blood.
| Measurement Time | Exposure variable | N | Mean ± SD | Median (10th, 90th percentile) |
|---|---|---|---|---|
| 2nd Trimester | Manganese (μg/dL) | 485 | 1.46 ± 0.49 | 1.39 (0.95, 2.05) |
| Adj. hemoglobin (g/dL)a | 485 | 11.59 ± 0.88 | 11.6 (10.6, 12.66) | |
| Serum ferritin (ng/mL) | 475 | 39.24 ± 33.39 | 31 (10.5, 79.44) | |
| MCV (fL) | 485 | 91.59 ± 4.49 | 91.9 (86.3, 96.3) | |
| RDW (fL) | 482 | 13.99 ± 1.48 | 13.7 (12.81, 15.1) | |
| 3rd Trimester | Manganese (μg/dL) | 424 | 1.88 ± 0.63 | 1.81 (1.18, 2.62) |
| Adj. hemoglobin (g/dL) | 424 | 11.53 ± 0.92 | 11.6 (10.3, 12.67) | |
| Serum ferritin (ng/mL) | 222 | 18.74 ± 17.53 | 14 (5.05, 35.56) | |
| MCV (fL) | 424 | 92.38 ± 5.02 | 92.75 (86.43, 98) | |
| RDW (fL) | 424 | 13.69 ± 0.93 | 13.5 (12.7, 14.8) | |
| Maternal at Birth | Manganese (μg/dL) | 479 | 2.47 ± 0.95 | 2.29 (1.43, 3.6) |
| Adj. hemoglobin (g/dL) | 451 | 11.43 ± 1.68 | 11.5 (9.5, 13.3) | |
| Serum ferritin (ng/mL) | 457 | 37.28 ± 74.86 | 25.7 (8.6, 66.04) | |
| MCV (fL) | 411 | 91.48 ± 6.27 | 91.3 (84.8, 98.1) | |
| RDW (fL) | 344 | 14.48 ± 3.21 | 14 (12.7, 16.4) | |
| Cord Blood | Manganese (μg/dL) | 452 | 4.86 ± 2.33 | 4.43 (2.86, 7.03) |
N: Number; SD: Standard Deviation; Adj: Adjusted
Hemoglobin values after adjustment for elevation.
We calculated Pearson’s correlation coefficients between Mn and effect modifiers at each trimester and at birth. Differences between participant characteristics in the 2nd trimester, 3rd trimester, and at birth were determined with mixed effects models.
Information on covariates was collected from baseline questionnaires at the 2nd trimester and field measurements at birth. We included covariates in the models based on statistical criteria (significant association with outcome, change in effect estimate by greater than 10%), and based on biological significance. Ultimately, statistical covariates were: date of visit, socioeconomic status (low, middle or high), and mtDNA isolation method (2 batches-remained significantly associated after correction); while biological covariates were environmental tobacco smoke (ETS; present or absent in home) (Stangenberg et al., 2015), parity (primi- or multiparous), and maternal age (Mengel-From et al., 2014).
We examined the associations between Mn, Hb, and ferritin with mtDNA CN using generalized linear models (GAMs) to determine nonlinearity. However, GAMs as indicated that all associations were linear (Data not shown). Robust regressions were used to estimate the association between Mn and mtDNA CN, as the tails of the distribution of residuals were long on either side. We generated three types of robust regression models for the association of cord blood mtDNA CN with Mn: 1) Models adjusted for the modifier as a covariate; 2) Models stratified by effect modifier status; and 3) Models with an interaction term between Mn and the dichotomized effect modifier. We applied these models to Mn and anemia measurements taken at the 2nd trimester, 3rd trimester, and at birth in maternal blood and cord blood, and repeated them for hemoglobin, ferritin and MCV. Models with cord blood Mn were analyzed in relation to maternal hemoglobin, ferritin and MCV measured at birth, as not enough cord blood CBC’s were available for analysis. All associations were assessed in crude unadjusted models and models adjusted for all covariates.
As a sensitivity analysis, we elected to include child birthweight as a covariate in our adjusted models as it has been shown to be associated with mtDNA CN (Zota et al., 2009). Furthermore, Pb has also been shown to contribute to anemia (Kopp et al., 2012), and to ensure that concurrent Pb exposure was not influencing our results, we adjusted for it as a covariate in our models. Cell type composition of blood, and platelets in particular, has been shown to influence mtDNA CN estimates in some studies (Banas et al., 2004; Moore et al., 2018), while others have reported no association of cord blood cell type on mtDNA CN (Janssen et al., 2012). Furthermore, anemia, iron deficiency, MCV and ferritin are frequently associated with changes in cell type composition, suggesting that they may be mediating the observed associations. In order to test for this in our analysis, we performed a second sensitivity analysis adjusting for platelet, neutrophil, and lymphocyte cell counts in cord blood of a subset of individuals (N=302) (Peng et al., 2017; Zhong et al., 2016). The α-level for all statistical tests of significance was set at 5%. R version 3.4.0 was used for all statistical analyses.
3. Results
3.1. Study Participant Characteristics
The average maternal age at enrollment was 28.11 years with the majority of low or medium socioeconomic status (87%), and an average BMI of 26.25 kg/m2 (Table 1). Mothers were primarily multiparous (63%). About half of infants were male (54%) with an average birthweight of 3.08 kg and a gestational age of approximately 38 weeks. The ratio of mtDNA to nDNA (mtDNA CN) was 1.21 ± 0.36.
Table 1.
| Characteristic | Mean ± SD or N (%) | Median (10th, 90th percentile) |
|---|---|---|
| Relative Cord blood mtDNA CN | 1.21 ± 0.36 | 1.17 (0.86, 1.56) |
| Maternal age (years) | 28.11 ± 5.52 | 27.61 (21.09, 36.27) |
| Maternal BMI (kg/m2) | 26.25 ± 4.26 | 25.64 (21.37, 31.79) |
| Gestational age (weeks) | 38.31 ± 1.89 | 39 (36, 40) |
| Birthweight (kg) | 3.05 ± 0.48 | 3.06 (2.55, 3.61) |
| Child Sex | ||
| Male | 265 (54.6) | |
| Female | 220 (45.4) | |
| Parity | ||
| Primiparous | 176 (36.3) | |
| Multiparous | 309 (63.7) | |
| Socioeconomic Status | ||
| Low | 240 (49.5) | |
| Medium | 189 (38.9) | |
| High | 56 (11.6) | |
| Environmental Tobacco Smoke | ||
| No | 344 (70.9) | |
| Yes | 141 (29.1) |
N: Number; SD: Standard Deviation; CN: Copy number; BMI: Body Mass Index
2nd trimester measurements: maternal age, BMI, parity, socioeconomic status, environmental tobacco smoke.
At birth measurements: gestational age, birthweight, child sex
3.2. Maternal Manganese and Blood Parameters
Maternal Mn blood levels increased significantly throughout pregnancy. Furthermore, Mn concentrated approximately 2-fold in cord blood in comparison to maternal blood at birth (4.86 ± 2.33 μg/dL vs 2.47 ± 0.95 μg/dL, respectively) (Table 2). Approximately 28.86% of the women were anemic (Hb ≤ 11g/dL) in the 2nd trimester, 30.86% were anemic in the 3rd trimester, and 35% were anemic at birth (Table 3). The average maternal Hb was higher in the 2nd trimester than at birth (11.59 ± 0.88 g/dL and 11.43 ± 1.68 g/dL, respectively) (Table 2). Iron deficiency anemia was present in 9.28% of women in the 2nd trimester, 11.78% of women in the 3rd trimester, and 21% at birth. Serum ferritin levels were significantly lower in the 3rd trimester in comparison to the 2nd trimester (18.74 ± 17.53 ng/mL and 39.24 ± 33.39 ng/mL, respectively), but increased again to 37.28 ± 74.86 ng/mL at birth. MCV values were also significantly higher in the 3rd trimester, but lower in maternal blood at birth, while RDW values increased significantly throughout pregnancy (Table 2).
Table 3.
Percentage of maternal study population with different anemic parameters in the 2nd trimester, 3rd trimester and at birth.
| Anemia Classification | 2nd Trimester (% (N)) | 3rd Trimester (% (N)) | At Birth (% (N)) |
|---|---|---|---|
| Anemia | 28.86 (106/485) | 30.86 (100/424) | 34.66 (166/451) |
| Low Serum Ferritin | 19.65 (78/475) | 52.25 (106/222) | 22.55 (108/452) |
| Iron Deficiency Anemia | 9.28 (45/485) | 11.78 (47/399) | 20.97 (82/391) |
| Microcytosis | 1.44 (7/485) | 1.89 (8/424) | 2.19 (9/411) |
| Macrocytosis | 1.65 (8/485) | 4.72 (20/424) | 1.46 (6/411) |
Pearson’s correlation tests between exposures reveal high correlations between maternal 2nd trimester MCV, 3rd trimester MCV, and MCV at birth (Pearson’s r approximately 0.85) and moderate correlations between time points of ferritin and hemoglobin (Hb approx. 0.65; ferritin approx. 0.55) (Figure 1). Interestingly, maternal Mn in the 2nd trimester, 3rd trimester, and at birth were moderately correlated (Pearson’s r Mn = 0.60, 0.39 and 0.43), however, Mn in cord blood was weakly but significantly correlated with maternal blood (r approximately 0.19). Maternal Mn was not correlated with Hb, and was negatively correlated with serum ferritin in the 2nd and 3rd trimesters and in maternal blood at birth (r = −0.31, −0.27, and −0.15 respectively).
Figure 1.
Correlation plot of Pearson’s R values between 2nd trimester (2T) Mn, 3rd trimester (3T) Mn, and anemic effect modifiers. Color gradient indicates degree of correlation and asterisks indicate level of statistical significance (* p ≤ 0.05; ** p ≤ 0.001, *** p ≤ 0.0001).
3.3. Associations of Maternal blood parameters with cord blood mtDNA CN
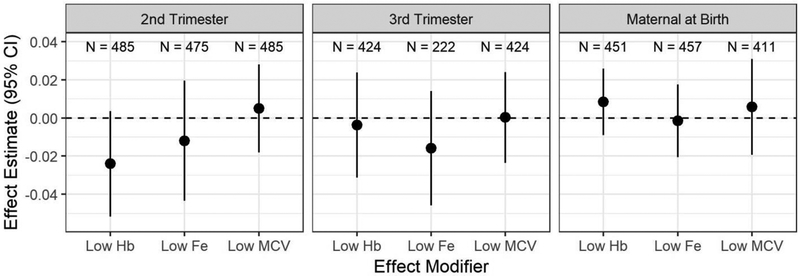
There were no significant associations between each effect modifier and cord mtDNA CN on either adjusted or unadjusted models at p ≤ 0.05 (Figure 2).
Figure 2.
Effect estimates with 95% confidence intervals of categorical anemic blood parameter effect modifiers measured in the 2nd trimester, 3rd trimester, and in maternal blood at birth on relative cord blood mtDNA CN in models adjusted for Mn, parity, socioeconomic status, maternal age, ETS, date, and batch.
In models investigating the relationship between blood Mn and cord blood mtDNA CN in the full population, 3rd trimester maternal Mn and cord blood Mn were positively associated with cord blood mtDNA CN (βfull = 0.05; 95% CI = 0.01, 0.08; p = 0.02; and βfull = 0.04; 95% CI = 0.01, 0.06; p = 0.01; respectively) (Figure 3). No relationship was observed in maternal blood measured at birth.
Figure 3.
Effect estimates with 95% confidence intervals of manganese (Mn) measured in 2nd trimester maternal blood, 3rd trimester maternal blood, and in maternal blood at birth on relative cord blood mtDNA CN in models adjusted for parity, socioeconomic status, maternal age, ETS, date, and batch.
In analyses incorporating maternal anemia, iron deficiency or MCV, 2nd trimester maternal Mn was not significantly associated with cord blood mtDNA CN in the full population (Figure 4A). However, when maternal Mn was stratified by anemic status, a significant positive association was detected between 2nd trimester Mn and cord blood mtDNA CN in mothers with normal Hb (βhigh = 0.06; 95% CI = 0.01, 0.11; p = 0.01 and βlow = −0.06; 95% CI = −0.13, 0.03; p = 0.06), and in mothers with higher MCV (βhigh = 0.06; 95% CI = 0.01, 0.10; p = 0.03 and βlow = −0.01; 95% CI =−0.06, 0.03; p = 0.82). There was a significant interaction between maternal Mn and Hb status in the second trimester (interaction p = 0.004). However, no other effect modifier was significant in the 2nd trimester.
Figure 4.
Effect modification of hemoglobin ferritin, and mean corpuscular volume on the association between blood Mn and relative mtDNA CN in the (A) 2nd trimester, (B) 3rd trimester, (C) cord blood and (D) maternal blood at birth. Effect estimates with 95% confidence intervals are provided for the full population, in the strata of the population with low values and the strata of the population with high values. The p-value for the interaction in the adjusted model is given in each plot
In the 3rd trimester, maternal Mn was significantly positively associated with cord blood mtDNA CN in the full population when adjusted for each effect modifier (βfull = 0.04 – 0.05; 95% CI = 0.01, 0.08; p = 0.02 – 0.03) (Figure 4B). This association was maintained in mothers with normal Hb (βhigh = 0.05; 95% CI = 0.00, 0.09; p = 0.04) and low ferritin (βlow = 0.08; 95% CI = 0.02, 0.15; p = 0.01) in the adjusted and unadjusted models.
Cord blood Mn was positively associated with cord blood mtDNA CN in full population models when adjusted for each effect modifier (βfull = 0.03 – 0.04; 95% CI = 0.001, 0.07; p = 0.02 – 0.04) (Figure 4C). Cord blood Mn was also significantly positively associated with cord blood mtDNA CN in individuals with low ferritin (βlow = 0.06; 95% CI = 0.004, 0.11; p = 0.04) and high MCV (βlow = 0.06; 95% CI = 0.01, 0.10; p = 0.02), however, no effect modification was observed.
Finally, Mn in maternal blood at birth was not associated with cord blood mtDNA CN in full models or models including maternal Hb or MCV as effect modifiers (Figure 4D). However, a significant interaction between maternal Mn and ferritin at birth was observed, as maternal Mn was positively associated with cord mtDNA CN in individuals with low ferritin at birth (βhigh = −0.004; 95% CI = −0.04, 0.03; p = 0.79 and βlow = 0.08; 95% CI = 0.02, 0.15; p = 0.02; Adj. int. p = 0.03). Results of unadjusted models can be found in supplemental Tables 1 and 2.
In our first sensitivity analysis, birthweight was significantly associated with cord blood mtDNA CN in all models (β approximately 0.014), but it did not alter the association of Mn with mtDNA CN, nor did it change the effect modification by anemia (Results not shown). Adjustment for Pb as a covariate in our models did not alter the associations of the anemic factors with cord blood mtDNA CN, nor did it alter the associations between Mn and mtDNA CN at each time (Results not shown). When cell type in cord blood (as assessed by CBC) was included as a covariate in unadjusted and adjusted models, no alterations were observed in associations between maternal blood parameters and cord blood mtDNA CN (Supplemental Figure 1). The trends for associations between maternal 2nd trimester Mn and cord blood mtDNA CN remained the same, however, the interaction between Mn and Hb was no longer significant, likely due to large confidence intervals (Supplemental Figure 2A). Effect modification by maternal MCV was detected at this time point (βhigh = 0.08; 95% CI = 0.006, 0.13; p = 0.006 and βlow = −0.02; 95% CI = −0.08, 0.05; p = 0.61; interaction p = 0.03), which had been borderline significant in the main analysis. When adjusting for maternal blood cell type in the 3rd trimester, Mn was positively associated with cord blood mtDNA CN in mother’s with low Hb (βhigh = 0.13; 95% CI = 0.21, 0.14; p = 0.001) (Supplemental Figure 2B). Adjustment for cell type decreased effect estimates for the association between cord blood Mn and cord blood mtDNA CN in models with Hb and ferritin, however, similar to 2nd trimester Mn, effect modification by MCV was detected. No changes were observed in the effects of maternal blood at birth.
4. Discussion
Our findings demonstrate developmental stage-specific effects of maternal blood Mn levels and anemia on newborn mtDNA CN. We detected a positive association between 3rd trimester maternal blood Mn and cord blood mtDNA CN, as well as between cord blood Mn and cord blood mtDNA CN. This association was modified by 2nd trimester maternal anemia (Hb ≤ 11 g/dL) – 2nd trimester maternal Mn was negatively associated with cord blood mtDNA CN in anemic mothers, and positively associated with cord blood mtDNA CN in mothers with normal Hb levels. Furthermore, cord blood mtDNA CN was associated with maternal Mn at birth only in individuals with low ferritin, suggesting that excess Mn may either be modified by iron deficiency and/or result from iron deficiency. We observed no significant effect modification by MCV on the association between Mn and mtDNA CN. Maternal anemia, iron deficiency and MCV were not independently associated with cord blood mtDNA CN.
Mn and Iron are both transition metals and have similar chemical properties. Because they bind to similar proteins, including those involved in absorption and transport, we observed a significant inverse correlation between maternal Mn and serum ferritin as expected. In a small study of 26 young women fed a Mn heavy diet for 60 days, those with low serum ferritin absorbed significantly more Mn than those with normal ferritin (Finley, 1999). Iron status was significantly inversely associated with 1–6 year old child blood Mn, and blood Mn concentrations were higher in children with iron deficiency anemia (Smith et al., 2013). In another study in Mexico City, child blood Mn at 12 and 24 months was inversely associated with hemoglobin and serum ferritin levels (Henn et al., 2010). However, we observed little correlation between maternal Mn and Hb levels in the current study.
Approximately 28–35% of mothers in this study were anemic during pregnancy, which is comparable to estimated levels of anemia in pregnant women in Latin America (Balarajan et al., 2011). The most common cause of anemia in pregnancy is iron deficiency, accounting for approximately 75% of cases (Di Renzo et al., 2015), and we assume that the majority of anemic women in PROGRESS were iron deficient, which may not be accurately portrayed by serum ferritin values. We did not observe associations between maternal Hb, ferritin, or MCV status with cord blood mtDNA CN. Experimental studies suggest that both iron deficiency anemia and iron overload are capable of inducing OS and mitochondrial toxicity. For instance, knockdown on iron regulatory proteins resulted in mitochondrial dysfunction and lower mtDNA CN (Li et al., 2018), while mtDNA fragmentation increased in severely iron deficient anemic rats (Walter et al., 2002). However, rats fed a high iron diet also had mitochondrial dysfunction induced by OS (Volani et al., 2017). The degree of iron deficiency anemia or iron overload may not be high enough in the current population to induce these effects, which could explain our null results.
Blood Mn levels rise throughout pregnancy in order to provide the fetus with adequate supplies (Krachler et al., 1999; Spencer, 1999). In the present study, we observed an approximate 150% increase in maternal Mn throughout pregnancy, and an approximate 2-fold increase in Mn values in cord blood in relation to maternal blood. These values are comparable to other studies comparing maternal and cord blood Mn (Krachler et al., 1999; Rudge et al., 2009; Zota et al., 2009). This increased blood Mn concentration over levels seen outside pregnancy might disrupt the normal homeostasis that regulates basal OS. Mn plays a complex role in maintaining OS homeostasis. In the mitochondria, Mn acts as a cofactor in the antioxidant protein Mn-SOD. However, unbound Mn is able to replace iron the Fenton reaction, which produces a major source of ROS. This is further complicated by the relationship between iron deficiency and Mn absorption, in which iron deficient subjects hyper-absorb Mn. Both Mn and iron accumulate in the mitochondria as they are cofactors to mitochondrial enzymes including Mn-SOD (Aguirre and Culotta, 2012; Maynard and Cotzias, 1955). mtDNA is highly susceptible to oxidative damage as it is the site of oxidative phosphorylation and lacks nucleotide excision repair pathways (Kazak et al., 2012).
In our analyses of 2nd trimester Mn, maternal Mn was positively associated with cord blood mtDNA CN in individuals with normal Hb and inversely associated with cord blood mtDNA CN in anemic individuals – an effect modification that was robust to our sensitivity analyses. The mitochondrial response to OS is complex and depends on the mechanisms leading to OS and its chronicity. Both higher and lower mtDNA CN are reflective of increased OS, with lower mtDNA believed to arise from chronic OS. In contrast, small increases in mtDNA CN, such as those observed in the present study, are indicative of an adaptive response to acute oxidative damage. Iron itself is a redox reactive element, capable of generating OS and mitochondrial toxicity at high concentrations (Koskenkorva-Frank et al., 2013; Volani et al., 2017). The joint impact of higher Mn and higher iron (i.e. women with higher hemoglobin levels likely have higher iron stores than women who are anemic), may induce more acute OS to provide the fetus with a higher load of transition elements leading to increased mitochondrial damage.
In the situation of iron deficiency anemia, there is increased OS that arises from the anemia state, which develops over the course of weeks to months prior to the 2nd trimester measurement. This situation arises slowly and would induce chronic OS. In addition, iron deficiency will induce increased absorption of Mn into the maternal blood stream, which would be available to the fetus. The higher Mn might further exacerbate the chronic OS of iron deficiency anemia resulting in lowered mtDNA CN that reflects the chronic nature of iron deficiency.
Later in pregnancy, during the 3rd trimester and in cord blood, we detected a positive association between maternal Mn and newborn mtDNA CN regardless of anemic or iron status. Mn concentrations were elevated at these measurement times and OS has been previously shown to increase mtDNA CN in human cells (Lee et al., 2000). This positive association suggests that Mn may generate OS to increase protein, lipid and DNA damage leading to a decrease mitochondrial respiratory function (Lee and Wei, 2005). The cell then increases mitochondrial biogenesis to compensate for the reduction in energy output, resulting in a corresponding adaptive increase in mtDNA CN (Lee and Wei, 2005). These effects were specific to 3rd trimester blood Mn and cord blood Mn, which are by definition subacute exposures and more likely to induce increased mtDNA CN as an adaptive response. This relationship was not observed when Mn was measured in maternal blood at birth, which may be in part due to the low correlation between maternal Mn levels at birth and cord blood Mn levels (r = 0.19). This finding is comparable to previous studies, which have demonstrated correlation coefficients between maternal Mn at birth and cord blood Mn ranging between 0.19 and 0.38. Collectively, these results suggest that maternal Mn values at birth may not be the best biomarker for public health research.
Increased adult mtDNA CN was associated with childhood adversity, depressive disorder, and anxiety disorder (Tyrka et al., 2016). In a cross-sectional study of 63 adult Italian men, airborne Mn exposure was not associated with mtDNA CN in whole blood (Hou et al., 2010). However, this analysis was in adults, with a different exposure pathway, to which mitochondria may not be as susceptible. Whole blood Mn measurements are indicative of recent exposures (Crossgrove and Zheng, 2004), suggesting that the increase in mtDNA CN may be transient and influenced by recent exposure.
Excess Mn in pregnancy has been associated with adverse health effects. In a different study population in Mexico City, 3rd trimester maternal blood Mn (mean 2.06 μg/dL) and cord blood Mn (mean 5.01 μg/dL) were significantly associated with decreased cognitive, language, and motor scores at 24 months (Muñoz-Rocha et al., 2017). However, the association was nonlinear and the lowest quartile of blood Mn was also associated with lower performance on the Bayley Scale of Infant Development, reflecting Mn’s role as a nutrient. Higher cord blood Mn was associated with lower cognitive and language scores at 24 months in a Taiwanese birth cohort, and interactions between cord blood Mn (mean 5.06 μg/dL) and Pb (mean 1.3 μg/dL) were detected on cognitive development (Lin et al., 2013). In a cohort of French mother-child pairs, cord blood Mn levels (geometric mean 2.04 μg/dL) were negatively associated with three recombined scores at 3 years: attention, non-verbal memory, and hand skill, but not a 6 years (Takser et al., 2003).
This study has several limitations. While useful as a biomarker of acute exposure, the measures of Mn in blood used in the present study are not indicative of chronic exposures. However, blood Mn values are correlated with environmental Mn exposure, and with Mn in the brain in human studies (Jiang et al., 2007; Ono et al., 1995). Furthermore, several studies have found associations between prenatal blood Mn levels and child neurodevelopment, and our use of blood measurements ensures that our work is comparable to previous research. Furthermore, we included Mn measurements at multiple time points in this analysis, strengthening our results. We did not observe enough individuals with microcytosis, macrocytosis or IDA to investigate the associations of these conditions with mtDNA CN or as effect modifiers; however, we were able to rule out the impact of Thalassemia trait on our results. Serum ferritin levels are susceptible to infection and changes in inflammation, and an additional measure of soluble transferrin receptor or hepcidin would have been ideal to assess iron deficiency (Di Renzo et al., 2015). We were also unable to account for other potential confounders during pregnancy, such as vitamin intake, deficient maternal nutrition, or other factors that may modify mtDNA, as this data was unavailable.
Our use of whole blood to estimate Mn exposure deserves comment. While there is no gold standard biomarker for Mn exposure, there is a large body of literature that suggests that the blood compartment reflects biologically active Mn (Claus Henn et al., 2017; Guan et al., 2014; Vigeh et al., 2008). Blood levels vary by age, sex, diet, and exposure with age and sex being two of the strongest predictors of levels (Henn et al., 2010; Takser et al., 2004; Zota et al., 2009). Because Mn is a nutrient physiologic metabolism tightly regulates levels in the blood, unlike lead which has no nutritional value and is not regulated physiologically. Blood Mn rises during pregnancy despite no evidence of increased exposure, likely from tissue mobilization. Thus, blood Mn may not have a half-life in the conventional pharmacologic sense, as it is an endogenous nutrient and not a xenobiotic. Nevertheless, slight increases and decreases in whole blood Mn have been linked to health status (Ashley-Martin et al., 2018; Eum et al., 2014; Henn et al., 2010; Zota et al., 2009).
This analysis also had several strengths, which included recruitment of women during pregnancy, high retention rate for prospective follow-up, and multiple measurements of Mn during pregnancy. We also analyzed a variety of blood parameters as effect modifiers, including serum ferritin, which although susceptible to infection and inflammation, is more stable than blood iron levels (Ulvik, 1984). Follow-up analyses would benefit from validation in an independent cohort and longitudinal measurement of child mtDNA CN and Mn to determine persistence of altered mtDNA CN. Furthermore, future studies will examine a potential mediation of prenatal Mn on child neurodevelopment by mtDNA CN.
5. Conclusions
We demonstrate a positive association between maternal 3rd trimester and cord blood Mn and newborn mtDNA CN. When measurements were taken in the 2nd trimester, this association was only observed in non-anemic mothers. This study furthers research suggesting that maternal exposures can impact child mtDNA and mitochondrial function. It provides new insight into the mechanisms behind Mn toxicity, and its complex relationship with maternal blood parameters. Future research will determine the significance of these effects on child health and neurodevelopment.
Supplementary Material
Highlights:
Mn in 3rd trimester blood and in cord blood was positively associated with mtDNA CN.
The relationship between 2nd trimester Mn and mtDNA CN was modified by anemia.
Mn in maternal blood at birth was not associated with cord blood mtDNA content.
Maternal anemia, serum ferritin, and MCV were not associated with mtDNA CN.
Acknowledgements
This work was supported by NIEHS grants: R01ES014930; R01ES013744 R01ES021357, P30ES009089, P30ES023515, and R24ES028522. Co-Investigators and staff at the INSP were also supported and received partial funding from the National Institute of Public Health/Ministry of Health of Mexico. The authors would like to thank the American British Cowdray Hospital in Mexico City, Mexico, for providing research facilities. MSG was financially supported by the Fundación Mé;xico en Harvard, A.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Aguirre JD, Culotta VC, 2012. Battles with Iron: Manganese in Oxidative Stress Protection. J Biol Chem 287, 13541–13548. 10.1074/jbc.R111.312181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LH, 2000. Anemia and iron deficiency: effects on pregnancy outcome. Am. J. Clin. Nutr 71, 1280S–4S. 10.1093/ajcn/71.5.1280s [DOI] [PubMed] [Google Scholar]
- Aschner M, 2000. Manganese: brain transport and emerging research needs. Environ Health Perspect 108, 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro GD, Fisher M, Monnier P, Morisset A-S, Fraser WD, Bouchard MF, 2018. Maternal and cord blood manganese (Mn) levels and birth weight: The MIREC birth cohort study. Int J Hyg Environ Health 221, 876–882. 10.1016/j.ijheh.2018.05.015 [DOI] [PubMed] [Google Scholar]
- Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian SV, 2011. Anaemia in low-income and middle-income countries. The Lancet 378, 2123–2135. 10.1016/S0140-6736(10)62304-5 [DOI] [PubMed] [Google Scholar]
- Banas B, Kost BP, Goebel FD, 2004. Platelets, a typical source of error in real-time PCR quantification of mitochondrial DNA content in human peripheral blood cells. Eur. J. Med. Res 9, 371–377. [PubMed] [Google Scholar]
- Braun JM, Wright RJ, Just AC, Power MC, Tamayo Y Ortiz M, Schnaas L, Hu H, Wright RO, Tellez-Rojo MM, 2014. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ Health 13, 50 10.1186/1476-069X-13-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Braun JM, Byun H-M, Tarantini L, Mercado A, Wright RJ, Schnaas L, Baccarelli AA, Wright RO, Tellez-Rojo MM, 2013. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics 5, 271–281. 10.2217/epi.13.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Williams GR, 2006. The Respiratory Chain and Oxidative Phosphorylation, in: Advances in Enzymology and Related Areas of Molecular Biology. Wiley-Blackwell, pp. 65–134. 10.1002/9780470122624.ch2 [DOI] [PubMed] [Google Scholar]
- Claus Henn B, Bellinger DC, Hopkins MR, Coull BA, Ettinger AS, Jim R, Hatley E, Christiani DC, Wright RO, 2017. Maternal and Cord Blood Manganese Concentrations and Early Childhood Neurodevelopment among Residents near a Mining-Impacted Superfund Site. Environ Health Perspect 125 10.1289/EHP925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova FM, Jr ASA, Peres TV, Lopes MW, Gonçalves FM, Remor AP, Lopes SC, Pilati C, Latini AS, Prediger RDS, Erikson KM, Aschner M, Leal RB, 2012. In Vivo Manganese Exposure Modulates Erk, Akt and Darpp-32 in the Striatum of Developing Rats, and Impairs Their Motor Function. PLOS ONE 7, e33057 10.1371/journal.pone.0033057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossgrove J, Zheng W, 2004. Manganese toxicity upon overexposure. NMR Biomed 17, 544–553. 10.1002/nbm.931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzo GC, Spano F, Giardina I, Brillo E, Clerici G, Roura LC, 2015. Iron Deficiency Anemia in Pregnancy. Womens Health (Lond Engl) 11, 891–900. 10.2217/whe.15.35 [DOI] [PubMed] [Google Scholar]
- Dirren H, Logman MH, Barclay DV, Freire WB, 1994. Altitude correction for hemoglobin. Eur J Clin Nutr 48, 625–632. [PubMed] [Google Scholar]
- Eum J-H, Cheong H-K, Ha E-H, Ha M, Kim Y, Hong Y-C, Park H, Chang N, 2014. Maternal blood manganese level and birth weight: a MOCEH birth cohort study. Environ Health 13, 31 10.1186/1476-069X-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J, Hao L, Bijli KM, Chandler JD, Orr M, Hu X, Jones DP, Go Y-M, 2017. From the Cover: Manganese Stimulates Mitochondrial H2O2 Production in SH-SY5Y Human Neuroblastoma Cells Over Physiologic as well as Toxicologic Range. Toxicol Sci 155, 213–223. 10.1093/toxsci/kfw196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JW, 1999. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am J Clin Nutr 70, 37–43. 10.1093/ajcn/70.1.37 [DOI] [PubMed] [Google Scholar]
- Freeland-Graves JH, Mousa TY, Sanjeevi N, 2014. Chapter 2:Nutritional Requirements for Manganese, in: Manganese in Health and Disease. pp. 34–75. 10.1039/9781782622383-00034 [DOI] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE, 1992. Mn2+ sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicology and Applied Pharmacology 115, 1–5. 10.1016/0041-008X(92)90360-5 [DOI] [PubMed] [Google Scholar]
- Golden TR, Melov S, 2001. Mitochondrial DNA mutations, oxidative stress, and aging. Mech. Ageing Dev 122, 1577–1589. [DOI] [PubMed] [Google Scholar]
- Guan H, Wang M, Li X, Piao F, Li Q, Xu L, Kitamura F, Yokoyama K, 2014. Manganese concentrations in maternal and umbilical cord blood: related to birth size and environmental factors. Eur J Public Health 24, 150–157. 10.1093/eurpub/ckt033 [DOI] [PubMed] [Google Scholar]
- Gunter TE, Gerstner B, Lester T, Wojtovich AP, Malecki J, Swarts SG, Brookes PS, Gavin CE, Gunter KK, 2010. An analysis of the effects of Mn2+ on oxidative phosphorylation in liver, brain, and heart mitochondria using state 3 oxidation rate assays. Toxicology and Applied Pharmacology 249, 65–75. 10.1016/j.taap.2010.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn BC, Ettinger AS, Schwartz J, Téllez-Rojo MM, Lamadrid-Figueroa H, Hernández-Avila M, Schnaas L, Amarasiriwardena C, Bellinger DC, Hu H, Wright RO, 2010. Early Postnatal Blood Manganese Levels and Children’s Neurodevelopment. Epidemiology 21, 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Zhu Z-Z, Zhang X, Nordio F, Bonzini M, Schwartz J, Hoxha M, Dioni L, Marinelli B, Pegoraro V, Apostoli P, Bertazzi PA, Baccarelli A, 2010. Airborne particulate matter and mitochondrial damage: a cross-sectional study. Environ Health 9, 48 10.1186/1476-069X-9-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BG, Munters E, Pieters N, Smeets K, Cox B, Cuypers A, Fierens F, Penders J, Vangronsveld J, Gyselaers W, Nawrot TS, 2012. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environ. Health Perspect. 120, 1346–1352. 10.1289/ehp.1104458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zheng W, Long L, Zhao W, Li X, Mo X, Lu J, Fu X, Li W, Liu S, Long Q, Huang J, Pira E, 2007. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: Search for biomarkers of manganese exposure. Neurotoxicology 28, 126–135. 10.1016/j.neuro.2006.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L, Reyes A, Holt IJ, 2012. Minimizing the damage: repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol 13, 659–671. 10.1038/nrm3439 [DOI] [PubMed] [Google Scholar]
- Kopp RS, Kumbartski M, Harth V, Brüning T, Käfferlein HU, 2012. Partition of metals in the maternal/fetal unit and lead-associated decreases of fetal iron and manganese: an observational biomonitoring approach. Arch Toxicol 86, 1571–1581. 10.1007/s00204-012-0869-4 [DOI] [PubMed] [Google Scholar]
- Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S, 2013. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radical Biology and Medicine 65, 1174–1194. 10.1016/j.freeradbiomed.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Krachler M, Rossipal E, Micetic-Turk D, 1999. Trace element transfer from the mother to the newborn — investigations on triplets of colostrum, maternal and umbilical cord sera. European Journal of Clinical Nutrition 53, 486–494. 10.1038/sj.ejcn.1600781 [DOI] [PubMed] [Google Scholar]
- Lee H-C, Wei Y-H, 2005. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol 37, 822–834. 10.1016/j.biocel.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Lee H-C, Yin P-H, Lu C-Y, Chi C-W, Wei Y-H, 2000. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J 348, 425–432. 10.1042/bj3480425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros F, Malka F, Frachon P, Lombès A, Rojo M, 2004. Organization and dynamics of human mitochondrial DNA. J Cell Sci 117, 2653–2662. 10.1242/jcs.01134 [DOI] [PubMed] [Google Scholar]
- Li H, Zhao H, Hao S, Shang L, Wu J, Song C, Meyron-Holtz EG, Qiao T, Li K, 2018. Iron regulatory protein deficiency compromises mitochondrial function in murine embryonic fibroblasts. Sci Rep 8 10.1038/s41598-018-23175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-C, Chen Y-C, Su F-C, Lin C-M, Liao H-F, Hwang Y-H, Hsieh W-S, Jeng S-F, Su Y-N, Chen P-C, 2013. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environmental Research 123, 52–57. 10.1016/j.envres.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Maynard LS, Cotzias GC, 1955. The Partition of Manganese Among Organs and Intracellular Organelles of the Rat. J. Biol. Chem 214, 489–495. [PubMed] [Google Scholar]
- Mengel-From J, Thinggaard M, Dalgård C, Kyvik KO, Christensen K, Christiansen L, 2014. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum. Genet 133, 1149–1159. 10.1007/s00439-014-1458-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Leung MCK, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS, 2013. Mitochondria as a Target of Environmental Toxicants. Toxicol Sci 134, 1–17. 10.1093/toxsci/kft102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AZ, Ding J, Tuke MA, Wood AR, Bandinelli S, Frayling TM, Ferrucci L, 2018. Influence of cell distribution and diabetes status on the association between mitochondrial DNA copy number and aging phenotypes in the InCHIANTI study. Aging Cell 17 10.1111/acel.12683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncie HL, Campbell J, 2009. Alpha and beta thalassemia. Am Fam Physician 80, 339–344. [PubMed] [Google Scholar]
- Muñoz-Rocha TV, Tamayo y Ortiz M, Romero M, Pantic I, Schnaas L, Bellinger D, Claus-Henn B, Wright R, Wright RO, Téllez-Rojo MM, 2017. Prenatal co-exposure to manganese and depression and 24-months neurodevelopment. NeuroToxicology. 10.1016/j.neuro.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono J, Harada K, Kodaka R, Sakurai K, Tajiri H, Takagi Y, Nagai T, Harada T, Nihei A, Okada A, Okada S, 1995. Manganese Deposition in the Brain During Long-Term Total Parenteral Nutrition. Journal of Parenteral and Enteral Nutrition 19, 310–312. 10.1177/0148607195019004310 [DOI] [PubMed] [Google Scholar]
- Peng C, Cayir A, Sanchez-Guerra M, Di Q, Wilson A, Zhong J, Kosheleva A, Trevisi L, Colicino E, Brennan K, Dereix AE, Dai L, Coull BA, Vokonas P, Schwartz J, Baccarelli AA, 2017. Associations of Annual Ambient Fine Particulate Matter Mass and Components with Mitochondrial DNA Abundance. Epidemiology 28, 763 10.1097/EDE.0000000000000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirini F, Goldman LR, Soudry E, Halden RU, Witter F, Sidransky D, Guerrero-Preston R, 2017. Prenatal exposure to tobacco smoke leads to increased mitochondrial DNA content in umbilical cord serum associated to reduced gestational age. Int J Environ Health Res 27, 52–67. 10.1080/09603123.2016.1268677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzetti S, Just AC, Burris HH, Oken E, Amarasiriwardena C, Svensson K, Mercado-García A, Cantoral A, Schnaas L, Baccarelli AA, Wright RO, Téllez-Rojo MM, 2017. The association of lead exposure during pregnancy and childhood anthropometry in the Mexican PROGRESS cohort. Environmental Research 152, 226–232. 10.1016/j.envres.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MJ, Just AC, Guerra MS, Kloog I, Hsu H-HL, Brennan KJ, García AM, Coull B, Wright RJ, Téllez Rojo MM, Baccarelli AA, Wright RO, 2017. Identifying sensitive windows for prenatal particulate air pollution exposure and mitochondrial DNA content in cord blood. Environ Int 98, 198–203. 10.1016/j.envint.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge CV, Röllin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JØ, 2009. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J. Environ. Monit 11, 1322–1330. 10.1039/B903805A [DOI] [PubMed] [Google Scholar]
- Seo YA, Li Y, Wessling-Resnick M, 2013. Iron depletion increases manganese uptake and potentiates apoptosis through ER stress. Neurotoxicology 38, 67–73. 10.1016/j.neuro.2013.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short MW, Domagalski JE, 2013. Iron deficiency anemia: evaluation and management. Am Fam Physician 87, 98–104. [PubMed] [Google Scholar]
- Smith EA, Newland P, Bestwick KG, Ahmed N, 2013. Increased whole blood manganese concentrations observed in children with iron deficiency anaemia. Journal of Trace Elements in Medicine and Biology 27, 65–69. 10.1016/j.jtemb.2012.07.002 [DOI] [PubMed] [Google Scholar]
- Sondheimer N, Glatz CE, Tirone JE, Deardorff MA, Krieger AM, Hakonarson H, 2011. Neutral mitochondrial heteroplasmy and the influence of aging. Hum Mol Genet 20, 1653–1659. 10.1093/hmg/ddr043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer A, 1999. Whole blood manganese levels in pregnancy and the neonate. Nutrition 15, 731–734. 10.1016/S0899-9007(99)00144-6 [DOI] [PubMed] [Google Scholar]
- Stangenberg S, Nguyen LT, Chen H, Al-Odat I, Killingsworth MC, Gosnell ME, Anwer AG, Goldys EM, Pollock CA, Saad S, 2015. Oxidative stress, mitochondrial perturbations and fetal programming of renal disease induced by maternal smoking. The International Journal of Biochemistry & Cell Biology 64, 81–90. 10.1016/j.biocel.2015.03.017 [DOI] [PubMed] [Google Scholar]
- Stehling O, Lill R, 2013. The role of mitochondria in cellular iron-sulfur protein biogenesis: mechanisms, connected processes, and diseases. Cold Spring Harb Perspect Biol 5, a011312. 10.1101/cshperspect.a011312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takser L, Lafond J, Bouchard M, St-Amour G, Mergler D, 2004. Manganese levels during pregnancy and at birth: relation to environmental factors and smoking in a Southwest Quebec population. Environmental Research 95, 119–125. 10.1016/j.envres.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G, 2003. Manganese, Monoamine Metabolite Levels at Birth, and Child Psychomotor Development. NeuroToxicology, Eighth International Symposium on Neurobehavioral Methods and Effects in Occupational and Environmental Health, Brescia, Italy, June 23–26, 2002 24, 667–674. 10.1016/S0161-813X(03)00058-5 [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Price LH, Kao H-T, Porton B, Philip NS, Welch ES, Carpenter LL, 2016. Alterations of Mitochondrial DNA Copy Number and Telomere Length With Early Adversity and Psychopathology. Biological Psychiatry 79, 78–86. 10.1016/j.biopsych.2014.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvik RJ, 1984. Stability of serum ferritin in healthy subjects. Haematologia (Budap) 17, 433–438. [PubMed] [Google Scholar]
- U.S. EPA, 2003. Health Effects Support Document for Manganese (No. EPA 822-R-03–003). Office of Water (4304T); Health and Ecological Criteria Division, Washington D.C. [Google Scholar]
- Van Vranken M, 2010. Evaluation of microcytosis. Am Fam Physician 82, 1117–1122. [PubMed] [Google Scholar]
- Vigeh M, Yokoyama K, Ramezanzadeh F, Dahaghin M, Fakhriazad E, Seyedaghamiri Z, Araki S, 2008. Blood manganese concentrations and intrauterine growth restriction. Reprod. Toxicol 25, 219–223. 10.1016/j.reprotox.2007.11.011 [DOI] [PubMed] [Google Scholar]
- Volani C, Doerrier C, Demetz E, Haschka D, Paglia G, Lavdas AA, Gnaiger E, Weiss G, 2017. Dietary iron loading negatively affects liver mitochondrial function. Metallomics 9, 1634–1644. 10.1039/C7MT00177K [DOI] [PubMed] [Google Scholar]
- Walter PB, Knutson MD, Paler-Martinez A, Lee S, Xu Y, Viteri FE, Ames BN, 2002. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. PNAS 99, 2264–2269. 10.1073/pnas.261708798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2001. Iron Deficiency Anaemia: Assessment, Prevention, and Control: A Guide For Programme Managers. WHO, Geneva, Switzerland. [Google Scholar]
- Zhang S, Zhou Z, Fu J, 2003. Effect of manganese chloride exposure on liver and brain mitochondria function in rats. Environmental Research 93, 149–157. 10.1016/S0013-9351(03)00109-9 [DOI] [PubMed] [Google Scholar]
- Zhao N, Enns CA, 2012. Iron Transport Machinery of Human Cells: Players and Their Interactions. Curr Top Membr 69, 67–93. 10.1016/B978-0-12-394390-3.00003-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Cayir A, Trevisi L, Sanchez-Guerra M, Lin X, Peng C, Bind M-A, Prada D, Laue H, Brennan KJM, Dereix A, Sparrow D, Vokonas P, Schwartz J, Baccarelli AA, 2016. Traffic-Related Air Pollution, Blood Pressure, and Adaptive Response of Mitochondrial Abundance. Circulation 133, 378–387. 10.1161/CIRCULATIONAHA.115.018802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, Wright RO, 2009. Maternal Blood Manganese Levels and Infant Birth Weight. Epidemiology 20, 367 10.1097/EDE.0b013e31819b93c0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwingmann C, Leibfritz D, Hazell AS, 2003. Energy Metabolism in Astrocytes and Neurons Treated with Manganese: Relation among Cell-Specific Energy Failure, Glucose Metabolism, and Intercellular Trafficking Using Multinuclear NMR-Spectroscopic Analysis. J Cereb Blood Flow Metab 23, 756–771. 10.1097/01.WCB.0000056062.25434.4D [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.