Abstract
Papillomaviruses (PVs) are found in many species and infect epithelial cells at both mucosal and cutaneous sites. PVs are generally species-specific and cause benign epithelial proliferations, often forming papillomas or plaques. Rarely, these infections can persist, allowing progression to in situ and invasive cancers. We describe herein a case of multiple cutaneous pigmented plaques from a California sea lion (Zalophus californianus) that progressed to in situ and invasive squamous cell carcinoma (SCC). The lesions were characterized by epithelial hyperplasia, hyperkeratosis, and hypergranulosis that bordered more dysplastic areas, and, at one site, bordered an invasive SCC. Immunohistochemistry for papillomavirus antigen revealed strong nuclear immunoreactivity within keratinocytes in the hyperplastic epidermis. PCR was performed using degenerate and specific primers to detect papillomavirus DNA. Specific primers were used to amplify Zalophus californianus papillomavirus 1 (ZcPV-1), the only sea lion papillomavirus known to date. We detected ZcPV-1 DNA within the pigmented plaque, and in both in situ and invasive SCC samples.
Keywords: California sea lion, papillomavirus, pigmented viral plaque, squamous cell carcinoma, Zalophus californianus.
Papillomaviruses (PVs) are double-stranded circular DNA viruses that infect epithelial cells at both mucosal and cutaneous sites.14 PVs are generally species-specific viruses that cause benign epithelial proliferations, often forming papillomas or plaques.14 Rarely, these infections can persist, allowing the lesions to progress to in situ and invasive cancers.2,3,10,12,14 PVs have been identified in a wide variety of species, including marine mammals.8,9,11,13 Only one PV has been identified thus far in sea lions, Zalophus californianus papillomavirus 1 (ZcPV-1; family
Papillomaviridae, subfamily Firstpapillomavirinae, genus Dyonupapillomavirus, species Dyonupapillomavirus 1).11 It was identified from proliferative lesions on the axilla and prepuce of 2 adult California sea lions.11 The lesions in both animals were benign and regressed spontaneously after 2–5 mo.11 In contrast to these previous cases, we describe herein multiple proliferative epithelial lesions on the skin of a sea lion that were associated with ZcPV-1 and that progressed to in situ and invasive squamous cell carcinoma (SCC).
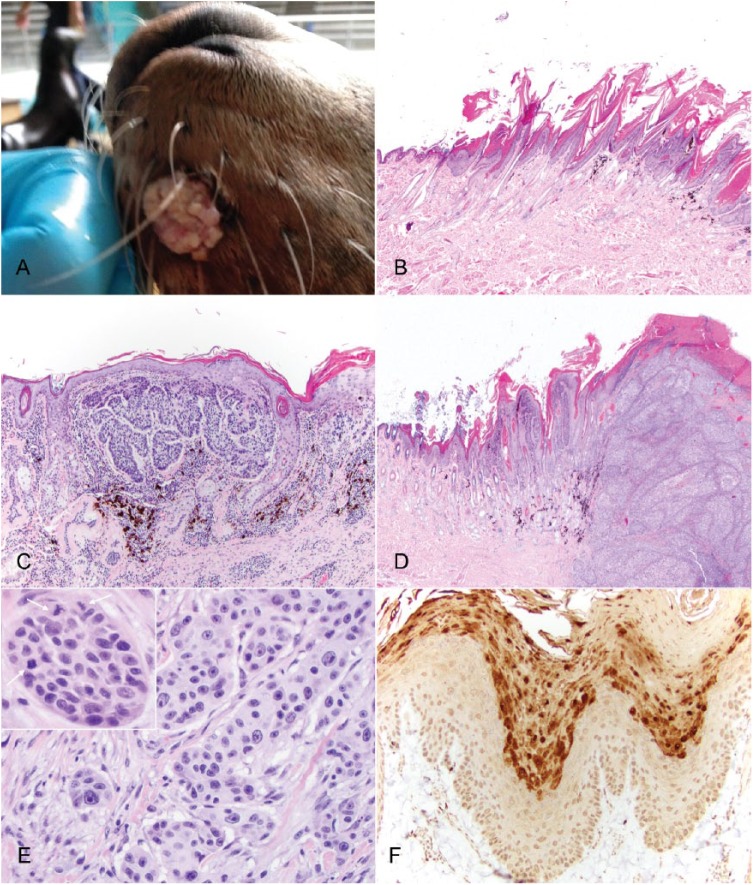
A 23-y-old, castrated male, captive-born California sea lion housed at an aquarium institution died after a 3-mo period of intermittent anorexia, hematuria, and right elbow swelling. Additional medical history included chronic gastric ulceration, noted clinically as intermittent gastric discomfort with hyporexia. The sea lion additionally had a history of seasonal chronic superficial dermatitis with parakeratotic hyperkeratosis and acanthosis on the ventral abdomen and thorax, which appeared grossly as miliary-to-papular skin nodules with scaling on the animal’s ventral surface, primarily the central caudal abdomen. Significant findings at autopsy that could have contributed to death were septic arthritis of the right elbow, gastric and duodenal ulcers, chronic urinary tract inflammation, and loss of body condition. Incidentally, 3 discrete alopecic and slightly raised, flat, and roughened lesions were identified on the head: a 1.5 cm diameter mass on the left lateral side of the muzzle (Fig. 1A) and two ~1 cm diameter lesions on the right dorsal aspect of the muzzle and right dorsal aspect of the cranium. The lesion on the left side of the muzzle had been present at least 2 mo prior to death, initially appearing as an exophytic, pink, verrucous mass. The lesions on the right side of the muzzle and right dorsal aspect of the head had been present for at least 3 y, and had been growing slowly over time.
Figure 1.
Multiple pigmented plaques in a California sea lion (Zalophus californianus) with malignant transformation. A. Gross image of the proliferative mass on the left lateral aspect of the muzzle, which progressed to squamous cell carcinoma (SCC). B. Pigmented plaque from the dorsum of the head with characteristic features of epithelial hyperplasia, hyperkeratosis, and prominent keratohyalin granules. H&E. C. Pigmented plaque with in situ carcinoma from the right side of the muzzle. In addition to epithelial hyperplasia, hyperkeratosis, and prominent keratohyalin granules, there is also marked epithelial dysplasia and disorderly keratinocyte differentiation. H&E. D. SCC from the left side of the muzzle, with invasion of the dermis. H&E. E. Higher magnification of the SCC from the left side of the muzzle, which highlights invasion of the dermis and marked nuclear pleomorphism. H&E. Inset: invasive island of neoplastic cells containing 3 mitotic figures (arrows). H&E. F. Immunohistochemistry for papillomavirus antigen on sections from the left side of the muzzle. There is strong nuclear immunoreactivity within keratinocytes from the hyperplastic epidermis adjacent to sections of invasive SCC.
Tissue samples were fixed in 10% neutral-buffered formalin, processed, and sectioned for routine hematoxylin and eosin staining. Histologic examination of the stained sections from the right side of the muzzle identified a focally extensive area of hyperplastic and pigmented epidermis. The lesion was characterized by a moderately thickened epidermis that formed exophytic villous projections with moderate parakeratotic hyperkeratosis (Fig. 1B). All layers of the epidermis were hyperplastic with prominent hypergranulosis. Scattered individual apoptotic cells and koilocytes were identified. Small numbers of lymphocytes and plasma cells were present in the superficial dermis along with pigmentary dispersal. The masses from the dorsum of the head and the left side of the muzzle revealed similar changes, but included areas of marked epithelial dysplasia along the basal keratinocytes with loss of normal stratification and numerous mitotic figures (carcinoma in situ; Fig. 1C). Within the center of the lesion from the left side of the muzzle, the dysplastic foci merged with an area of invasive SCC (Fig. 1D, 1E). This region was characterized by an infiltrative neoplasm composed of large cuboidal-to-polygonal cells arranged in sheets, islands, and anastomosing trabeculae. Neoplastic cells at the periphery of islands and trabeculae palisaded along a moderately dense fibrovascular stroma and matured in a disorganized manner toward the centers with scattered dyskeratotic cells and frequent central necrosis. Neoplastic cells generally had indistinct cell borders, although sometimes with prominent desmosomes between cells, a large amount of eosinophilic cytoplasm, and large round-to-oval nuclei with finely stippled chromatin and 1–2 distinct nucleoli. There was moderate-to-marked anisocytosis and anisokaryosis, numerous apoptotic cells, and occasional bizarre mitotic figures. The mitotic rate was 0–3 per high-power field. The surface of the mass was necrotic and replaced by a thick hypereosinophilic layer of serum, cellular debris, hemorrhage, degenerate neutrophils, and bacterial colonies. At the periphery of the mass, the epidermis was hyperplastic with areas of dysplasia forming exophytic projections covered by a thick layer of para- and orthokeratotic hyperkeratosis. There were also areas of epidermal hyperplasia, mild dysplasia, and variable hyperkeratosis away from the mass.
Immunohistochemistry was performed on sections from all 3 cutaneous lesions from the head using a mix of 2 mouse monoclonal antibodies (anti–bovine papillomavirus 1 and anti–human papillomavirus 16, ab-2417, Abcam, Cambridge, MA) as described previously.5 A canine viral papilloma known to be immunoreactive was used as the positive control. Omission of the primary antibody served as the negative control. Strong nuclear immunoreactivity was identified within keratinocytes within the granular layer and overlying keratin (Fig. 1F) in the hyperplastic epidermis within all 3 locations. There was no staining associated with the cells within the regions of in situ carcinoma or invasive SCC. These areas, however, were immediately adjacent to the hyperplastic epidermis, supporting a papillomaviral etiology for the proliferative lesions.
In order to identify which PV was present, PCR was performed using a set of degenerate primers, MY09/MY11, known to amplify a wide variety of papillomaviruses. Amplification of GAPDH as a reference gene confirmed adequate quality of extracted DNA.6,7 Genomic (g)DNA was extracted using a commercial kit (DNeasy tissue kit, Qiagen, Valencia, CA) from two 25-μm thick scrolls of formalin-fixed, paraffin-embedded tissue sections from each lesion as described previously.6 Also included was a proliferative mass from the bladder of the sea lion. Precautions were taken to minimize cross-contamination between samples, including changing microtome blades. PCR was then performed using 100 ng of extracted gDNA as described previously.6 The negative control included the addition of water instead of gDNA. The positive control was extracted DNA from a canine viral pigmented plaque. The PCR products were run on a 1% agarose gel and visualized using GelRed (Phenix Research Products, Candler, NC). The MY09/MY11 primer set amplifies a 450-bp fragment, which was identified within the positive control and the sample from the dorsum of the head. The other samples were negative, but all samples successfully amplified GAPDH.
The MY09/MY11 amplicon from the dorsum of the head was extracted, purified (Wizard SV gel and PCR clean-up system, Promega, Madison, WI), and submitted for sequencing. An ~400-bp fragment was sequenced and compared to the nucleotide collection at the National Center for Biotechnology Information (NCBI) using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequence was 99% homologous to ZcPV-1 (GenBank accession NC_015325.1). Given that degenerate primers are not as sensitive as specific primers, we designed specific primers for ZcPV-1 to increase the sensitivity in detecting PV DNA. The ZcPV-1 forward primer was 5’-TTGGCTATTACTACCACGCCGAATC-3’ and the reverse primer was 5’-CTCTGCTGTCCGTGCTGCCAGTACC-3’, and amplified a 344-bp region of the L1 gene. PCR was performed using 5 µL of 10× PCR buffer, 1 μL of 10 nM dNTP mix, 0.25 μL of Taq polymerase, 1 μL of 10 μM forward and reverse primers, and 100 ng of extracted DNA in a 50-μL reaction mixture following the manufacturer’s recommended protocol (Hot Start Taq polymerase, Qiagen). The PCR conditions included an initial hold for 15 min at 95°C, followed by 40 cycles of 94°C for 30 s, 58°C for 45 s, and 72°C for 1 min, all followed by a final extension for 10 min at 72°C. The PCR products were electrophoresed through 1% agarose gel to visualize the amplicons. Amplicons were identified at the expected size from the samples from the head and right and left sides of the muzzle using these specific primers. The sample from the bladder and the water negative control were both negative. We performed an additional PCR in order to rule out a potential co-infection with otarine herpesvirus 1 (OtHV-1), a gammaherpesvirus, which is associated with proliferative lesions in the urogenital tract that can progress to cancer.1,4 Primers and PCR parameters were performed as described previously4 using DNA extracted from a sea lion genital carcinoma as the positive control. All samples in this sea lion were negative for OtHV-1 DNA.
The results of histology, immunohistochemistry, and PCR suggest progression of a PV-induced precursor lesion to in situ and invasive SCC; however, a causal association cannot be proven definitively, because that would require assessment of the transforming abilities of the viral oncogenes, dysfunction of tumor suppressor genes, and presence of viral RNA within the tumor cells. Even so, PVs in humans have a well-established causal association with cancer.14 Mucosal PVs cause virtually all cases of cervical cancer, and cutaneous PVs are associated with skin cancer in some immunodeficient patients3; therefore, it seems plausible that this sea lion developed chronic PV infections that eventually progressed to cancer. The previous reports of ZcPV-1 infections occurred in adult, healthy animals, and in those cases, the lesions regressed spontaneously, most likely because of activation of the host immune response to viral infection. In our case, the lesions first developed when the sea lion was healthy and had no other underlying diseases to cause an immune deficiency. It is unknown why the lesions in this case did not spontaneously regress and instead progressed to cancer, although the presence of other comorbidities may have been a contributing factor. However, our report highlights the potential significance of these infections in California sea lions.
Acknowledgments
We thank Denise Long and Ione Jackman for histology. This manuscript constitutes Sea Research Foundation publication 269.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Buckles EL, et al. Otarine herpesvirus-1, not papillomavirus, is associated with endemic tumours in California sea lions (Zalophus californianus). J Comp Pathol 2006;135:183–189. [DOI] [PubMed] [Google Scholar]
- 2. Burger B, Itin PH. Epidermodysplasia verruciformis. Curr Probl Dermatol 2014;45:123–131. [DOI] [PubMed] [Google Scholar]
- 3. Connolly K, et al. Papillomavirus-associated squamous skin cancers following transplant immunosuppression: one Notch closer to control. Cancer Treat Rev 2014;40:205–214. [DOI] [PubMed] [Google Scholar]
- 4. King DP, et al. Otarine herpesvirus-1: a novel gammaherpesvirus associated with urogenital carcinoma in California sea lions (Zalophus californianus). Vet Microbiol 2002;86:131–137. [DOI] [PubMed] [Google Scholar]
- 5. Linder KE, et al. Generalized papillomatosis in three horses associated with a novel equine papillomavirus (EcPV8). Vet Dermatol 2018;29:72–e30. [DOI] [PubMed] [Google Scholar]
- 6. Luff JA, et al. Detection of six novel papillomavirus sequences within canine pigmented plaques. J Vet Diagn Invest 2012;24:576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manos M, et al. The use of polymerase chain reaction amplification for the detection of genital human papillomaviruses In: Furth M, Greaves M, eds. Molecular Diagnostics of Human Cancer. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, 1989:209–214. [Google Scholar]
- 8. Rector A, et al. Genomic characterization of novel dolphin papillomaviruses provides indications for recombination within the Papillomaviridae. Virology 2008;378:151–161. [DOI] [PubMed] [Google Scholar]
- 9. Rehtanz M, et al. Isolation and characterization of the first American bottlenose dolphin papillomavirus: Tursiops truncatus papillomavirus type 2. J Gen Virol 2006;87:3559–3565. [DOI] [PubMed] [Google Scholar]
- 10. Reusser NM, et al. HPV carcinomas in immunocompromised patients. J Clin Med 2015;4:260–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rivera R, et al. Characterization of a novel papillomavirus species (ZcPV1) from two California sea lions (Zalophus californianus). Vet Microbiol 2012;155:257–266. [DOI] [PubMed] [Google Scholar]
- 12. Smola S. Human papillomaviruses and skin cancer. Adv Exp Med Biol 2014;810:192–207. [PubMed] [Google Scholar]
- 13. Van Bressem MF, et al. A review of virus infections of cetaceans and the potential impact of morbilliviruses, poxviruses and papillomaviruses on host population dynamics. Dis Aquat Organ 1999;38:53–65. [DOI] [PubMed] [Google Scholar]
- 14. zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol 1994;48:427–447. [DOI] [PubMed] [Google Scholar]