Abstract
Hand, foot, and mouth disease (HFMD) commonly produces herpangina, but fatal neurological complications have been observed in children. Enterovirus 71 (EV-A71) and Coxsackievirus 16 (CV-A16) are the predominant viruses causing HFMD worldwide. With rising concern about HFMD outbreaks, there is a need for an effective vaccine against EV-A71 and CV-A16. Although an inactivated vaccine has been developed against EV-A71 in China, the inability of the inactivated vaccine to confer protection against CV-A16 infection and other HFMD etiological agents, such as CV-A6 and CV-A10, necessitates the exploration of other vaccine platforms. Thus, the antigenic peptide-based vaccines are promising platforms to develop safe and efficacious multivalent vaccines, while the monoclonal antibodies are viable therapeutic and prophylactic agents against HFMD etiological agents. This article reviews the available information related to the antigenic peptides of the etiological agents of HFMD and their neutralizing antibodies that can provide a basis for the design of future therapies against HFMD etiological agents.
Keywords: vaccine, antigenic peptide, neutralizing antibodies
1. Introduction
Hand, foot, and mouth disease (HFMD) is a prevalent paediatric infectious disease that is caused by a variety of enteroviruses [1,2,3,4]. HFMD has become a major public health threat that leads to serious morbidity and mortality in Asia-Pacific. In 2017, a total of 1,952,435 HFMD cases were recorded in China [5]. Enterovirus 71 (EV-A71) and Coxsackievirus 16 (CV-A16) are the main HFMD causative agents of mild HFMD. However, some EV-A71 strains have been known to be associated with fatal neurological complications [1,2]. In recent years, many HFMD outbreaks are associated with emerging enteroviruses, including CV-A6, CV-A8, and CV-A10 [3,4,6]. Together with EV-A71 and CV-A16, these enteroviruses have been shown to co-circulate in many HFMD outbreaks, which may result in viral co-infections and genomic recombination [7,8].
With rising concern about HFMD outbreaks, there is an urgent need for a vaccine against EV-A71 to be produced that is approved by the United States Food and Drug Administration (FDA) or European Medicines Agency (EMEA). The inactivated EV-A71 vaccine has typically been used as the main strategy for vaccine design [9]. China’s FDA has issued drug certificates and production licenses for the inactivated vaccines from 3 companies, namely, Sinovac, Vigoo, and Chinese Academy of Medical Sciences (CAMS) [9]. Sinovac reported that the efficacy of their inactivated vaccine against EV-A71-associated HFMD cases was 94.8% during the 12-month surveillance period [10]. Besides that, CAMS and Vigoo reported 97.4% and 90.0% vaccine efficacy against EV-A71-associated HFMD, respectively [11,12]. Despite the promising results revealed by the safety and efficacy studies, the licensed vaccines are incapable of eliciting sufficient cross-neutralizing antibodies against the other HFMD causative agents, such as CV-A16, CV-A6 and CV-A10 [9]. Therefore, it is imperative to explore other vaccine strategies to produce a safe and effective vaccine against multiple HFMD etiological agents.
A comprehensive understanding of the antigenic peptides that could elicit neutralizing antibodies will greatly help in the design of an effective vaccine. We discuss the structural analysis of EV-A71 viral particles to identify possible foreign peptide insertion sites to develop a multivalent vaccine based on a virus-like particle (VLP) platform. Lastly, we suggest some strategies to develop multivalent vaccines against multiple viruses that can cause HFMD.
2. Etiological Agents of HFMD
HFMD etiological agents (EV-A71, CV-A16, CV-A6, and CV-A10) belong to the Picornaviridae family, within species A of the Enterovirus genus [13]. The Enterovirus A species possesses non-enveloped virus particles with symmetrical 20–30 nm icosahedral capsids. The viral particle contains a single-stranded positive sense 7.4 Kb ribonucleic acid (RNA) with a single open reading frame, which is flanked by a 5′-untranslated region (5′ UTR) and a 3′ UTR with a poly(A) tail. Enterovirus A encodes a polyprotein of approximately 2100 amino acids that will be further cleaved to form three precursor proteins, termed as P1, P2, and P3. Structural viral proteins, named as VP1, VP2, VP3, and VP4, are the products of P1 cleavage. Besides that, P2 and P3 cleavage form seven non-structural proteins, namely 2A, 2B, and 2C, which are derived from P2, and 3A, 3B, 3C, and 3D, which are derived from P3. Enterovirus A viral capsids are formed by 60 protomers of the structural viral proteins; each protomer consists of VP1, VP2, VP3, and VP4 [14]. The external capsid surface is formed by VP1, VP2, and VP3, while VP4 is located inside the capsid [14]. VP1, VP2, and VP3 are structurally similar to one another, each consisting of eight-stranded β-barrels.
Comprehensive structural and biochemical studies reported five recognizable viral particles throughout the Enterovirus A lifecycle. They are the putative pro-capsid, the pro-virion, the mature infectious virus, the “A-particle”, and the empty capsid. Particle assembly commences in the cytoplasm where the protomer is formed by viral structural proteins VP0, VP1, and VP3. Five protomers will then assemble to form the pentameric subunit, which ultimately self-assembles to form the 60 protomer pro-capsid. The pro-capsid will acquire the viral genome to form the pro-virion. After the formation of the pro-virion, VP0 will be autocatalytically cleaved into VP2 and VP4 to form the mature virions that are released from infected cells. The mature virions will bind to neighbouring host receptors on the cell surface and begin the two-step uncoating process, which are (1) the formation of an expanded and altered particle, termed as “A-particle”, which is primed for genome release, and (2) the release of RNA into the host cell generating an intact empty capsid. VP4 is essential during the uncoating process as it is inserted into the membrane to form a channel in which the RNA can be released into the host cell cytoplasm [15,16,17,18,19].
The mature virus particles and the empty capsid are predominantly produced during EV-A71 natural infection. These two particles can be separated using continuous sucrose gradient ultra-centrifugation. The empty capsids have ~5% larger diameter than the mature virus particles [19]. Crucially, both viral particles displayed distinct antigenic properties, whereby the empty capsids are hypothesised to act as baits for host immune evasion [20].
3. Neutralizing Epitope Mapping
3.1. EV-A71 Neutralizing Epitope Mapping
Over the years, a number of linear neutralizing epitopes in the enteroviruses causing HFMD have been reported by many research groups. These reported linear and conformational epitopes were mostly mapped to the enterovirus structural proteins VP1, VP2, VP3, and VP4. We have summarized the reported linear neutralizing epitopes from EV-A71 and CV-A16 in Table 1.
Table 1.
Experimentally mapped linear epitopes in the structural proteins of EV-A71 and CV-A16.
| Virus | Region | Name and Position | Peptide Sequences | Epitopes | Ref. |
|---|---|---|---|---|---|
| EV-A71 | VP1 | PEP23 (aa 41–55) | TGEVPALQAAEIGAS | IgG | [25] |
| VP1–15 (aa 43–54) | KVPALQAAEIGA | IgG | [26] | ||
| VP1–14 (aa 40–51) | DTGEVPALQAAE | IgM | [26] | ||
| VP1 | PEP27 (aa 142–156) | PTGEVVPQLLQYMFV | IgM | [25] | |
| VP1 | SP55 (aa 163–177) | PESRESLAWQTATNP | IgG | [22] | |
| VP1 | SP70 (aa 208–222) | YPTFGEHKQEKDLEYC | IgM and IgG | [22] | |
| VP1 (aa 208–222) | YPTFGEHKQEKDLEYC | IgG | [24] | ||
| VP1–43 (aa 211–220) | FGEHKQEKDL | IgG | [23] | ||
| VP2 | VP2–28 (aa 136–150) | AGGTGTEDSHPPYKQ | IgG | [23] | |
| 7C7 (aa 142–146) | EDSHP | IgG | [28] | ||
| VP2 (aa 141–155) | TEDSHPPYKQTQPGA | IgG | [27] | ||
| VP4 | VP4N20 (aa 1–20) | GSQVSTQRSGSHENSNSATE | Neutralizing | [31] | |
| CV-A16 | VP1 | PEP32 (aa 94–108) | TMPTMGTQNTDGYAN | IgG | [34] |
| PEP37 (aa 109–123) | WDIDLMGYAQLRRKC | IgG | [34] | ||
| PEP55 (aa 163–177) | PTSRDSFAWQTATNP | IgG | [34] | ||
| PEP63 (aa 187–201) | PAQVSVPFMSPASAY | IgG | [34] | ||
| PEP71 (aa 211–225) | FGEHLQANDLDYGQC | IgG | [34] | ||
| PEP91 (aa 271–285) | YLFKTNPNYKGNDIK | IgG | [34] | ||
| VP3 | VP3–41 (aa 176–190) | HYRAHARAGYFDYYT | IgG | [32] | |
| VP4 | VP4N20 (aa 1–20) | GSQVSTQRSGSHENSNSASE | Neutralizing | [35] |
aa = amino acid.
VP1 is a capsid protein comprising 297 amino acids and is located at the most external part of the viral capsid. VP1 was the first EV-A71 antigen found to contain important epitopes that contribute to the neutralization of the virus. Initially, Wu et al. (2001) found that immunization using a recombinant VP1 protein of EV-A71 expressed in E. coli conferred protection against lethal EV-A71 infection in neonatal mice [21]. Subsequently, Foo et al. (2007) tested the neutralizing capabilities of the antisera elicited by ninety-five diphtheria toxoid-conjugated synthetic peptides encompassing the entire VP1 region [22]. Two of the synthetic peptides designated SP55 spanning amino acids 163–177 of VP1 and SP70 spanning amino acids 208–222 of VP1 elicited high titres of neutralizing antibodies belonging to the IgG1 subtype against EV-A71. The SP55 epitope was a weaker immunogen, as the specific IgG response in mice was relatively low. Studies carried out by Xu et al. (2015) and Liu et al. (2011) confirmed SP70 as a neutralizing linear epitope of EV-A71 [23,24]. In another study, sixty-three synthetic peptides were synthesized and used to map the EV-A71 linear B-cell epitopes [25]. This study revealed the synthetic peptide designated PEP27 spanning amino acids 142–156 of VP1 carried the EV-A71 IgM-specific immune-dominant epitope. In addition, another epitope denoted as PEP23 spanning amino acids 41–55 of VP1 was identified as the EV-A71 IgG cross-reactive immune-dominant epitope. Gao et al. (2012) also identified the same region spanning amino acids 43–54 of VP1 as the IgG epitope [26]. In addition, they identified the region spanning amino acids 40–51 of VP1 as the EV-A71 IgM epitope [26].
VP2 is another capsid protein consisting of 254 amino acids. Epitope mapping studies revealed that VP2 has one neutralization epitope located in the region spanning amino acids 134–155. Liu et al. (2011) reported the region corresponding to amino acids 136–150 of VP2 (designated as VP2-28 or SP28) was able to bind an EV-A71 cross-neutralizing antibody MAB979 [23]. Subsequently, Kiener et al. (2012) reported that the region spanning amino acids 141–155 of VP2 is a cross-neutralizing epitope [27]. They identified a single amino acid mutation within the region (S144T) that led to the loss of VP2 antigenicity against EV-A71 strain C4. The same region (amino acids 141-155 of VP2) was also identified by Xu et al. (2014) to be a cross-neutralizing epitope [28].
EV-A71 VP3 protein comprises 245 amino acids. Unlike other viral structural proteins, no linear neutralizing epitope has been mapped in VP3. There is a conformational epitope within the VP3 protein known as the “Knob” region, which was identified during neutralizing monoclonal antibody screening in mice [29]. The conformational epitope spanning amino acids 55 to 69 is highly conserved among EV-A71 sub-genotypes and was recognized by monoclonal antibody 10D3. In addition, another study that immunized mice with EV-A71 sub genotype B4 mapped a conformational epitope to the highly conserved amino acid 74 of VP3, whereby this region was found to be recognized by 5H7, a therapeutic IgG antibody [30].
VP4 protein consists of 69 amino acids. VP4 adopts an extended conformation and is localized inside the virion. It is the most conserved viral structural protein. Hence, there were some studies that focussed on mapping neutralization epitopes within the VP4 protein to produce a universal vaccine against all EV-A71 sub-genotypes. Zhao et al. (2013) identified the N-terminal residues 1–20 of EV-A71 VP4 designated as VP4N20 to be a neutralizing epitope [31]. In their study, they fused VP4N20 to hepatitis B core antigen. The chimeric fusion protein was expressed in E. coli and was found to spontaneously assemble into chimeric virus-like-particles (VLPs). Immunization with the chimeric VLPs presenting VP4N20 was able to elicit anti-VP4N20 antibody response. The anti-chimeric VLP sera were able to confer protection against EV-A71 challenge in neonatal mice.
3.2. CV-A16 Epitope Mapping
Unlike EV-A71, much less is known about neutralizing linear epitopes in CV-A16. Similar to EV-A71, most of the experimentally proven neutralizing linear epitopes were mapped to the VP1 protein. Chong et al. (2012) performed the first study to map linear neutralizing epitopes in CV-A16 viral particles [32]. They identified a single linear immune-dominant epitope localized in the VP3 protein of CV-A16, spanning amino acids 176–190. However, the epitope was weakly recognized by mouse anti-sera raised against formalin-inactivated CV-A16 [32]. The failure to map more linear neutralizing epitopes in this study could be the result of the formalin treatment that changed the native surface exposed epitopes of formalin-inactivated CV-A16. The altered surface could lead to either (1) the formalin-inactivated CV-A16 losing its immunogenicity, and thus the failure to elicit antibodies, or (2) the antibodies elicited against the formalin-inactivated CV-A16 altered surface could not recognize the native amino acids of the peptides that were screened. Indeed, an earlier study performed by Liu et al. (2012) reported immunization with CV-A16 VLPs consisting of VP0, VP1, and VP3 potently elicited CV-A16 specific antibody responses. The anti-VLP sera were able to confer protection against lethal CV-A16 challenge in mice, suggesting the presence of neutralizing epitopes in the structural viral proteins [33]. Subsequently, using a set of 95 synthetic peptides encompassing the entire VP1 for reactivity with neutralizing murine antisera raised against CV-A16 VLPs, they identified six linear neutralizing epitopes localized in the VP1 protein of CV-A16 [34]. The six peptides were PEP32 (amino acids 94–108), PEP37 (amino acids 109–123), PEP55 (amino acids 163–177), PEP63 (amino acids 187–201), PEP71 that corresponded to SP70 in EV-A71 (amino acids 211–225), and PEP91 (amino acids 271–285) [34]. Other than VP1 and VP3, a neutralizing epitope was experimentally mapped to the N-terminal region of VP4 corresponding to the VP4N20 peptide in EV-A71 [35].
3.3. CV-A6 and CV-A10 Epitope Mapping
Apart from EV-A71 and CV-A16, other coxsackieviruses, such as CV-A6 and CV-A10, have been reported as emerging viruses to cause large scale outbreaks of HFMD [3,4]. Since these viruses are the emerging causative agents for HFMD, studies related to these viruses were reported more recently [36,37,38,39]. In one of the studies, Xu et al. (2017) determined the near-atomic resolution structure of CV-A6 A-particle in complex with a neutralizing antibody [38]. Detailed inspection of the structure mapped an immune-dominant neutralizing epitope to the surface loops of VP1, including the BC, DE, EF, and HI loops. Besides that, another study provided further insight into the structural basis of CV-A10 in complex with a neutralizing antibody designated 2G8 [36]. The atomic complex structure revealed that the Fab of 2G8 binds to a border region across three capsid proteins, VP1, VP2, and VP3. Unlike EV-A71 and CV-A16, no linear epitope mapping experiments were performed for CV-A6 and CV-A10. Due to their overall similarities, it is possible that the amino acids in CV-A6 and CV-A10 corresponded to the linear neutralizing epitopes identified in EV-A71 and CV-A16.
4. Antigenic Peptide-Based Vaccine
Linear peptides are attractive vaccine candidates as they may induce highly targeted immune responses, albeit its usage in isolation are often weakly immunogenic [40]. Due to their relatively small size, peptide vaccine manufacturing is relatively safe and cost effective in comparison to conventional vaccines, such as the live-attenuated and the whole-inactivated vaccines [40]. In the case of HFMD, many studies were performed to develop linear neutralizing peptide-based vaccines against the main etiological agent of HFMD, such as EV-A71 and CV-A16. Although EV-A71 formaldehyde-inactivated vaccines were recently licensed in China, the inability of these inactivated vaccines to confer protection against other HFMD causative agents, such as CV-A16, CV-A6, and CV-A10, necessitates the exploration of other vaccine platforms, such as multi-epitope peptide vaccines.
Another advantage of peptide-based vaccine is the possibility of diminishing the antibody-dependent enhancement (ADE) of viral infection. ADE refers to sub-neutralizing or non-neutralizing antibodies produced from previous primary infections or through maternal-foetal transfer that could worsen subsequent infections leading to enhanced disease severity. During secondary viral infection, the binding of pre-existing non-neutralizing antibodies to the virus enhances the entry of the virus; through the interaction between an antibody bound virus with Fc receptors (FcR) of myeloid cells, such as natural killer cells, macrophages, and B lymphocytes [41]. In the context of EV-A71 infections, ADE has been observed in both clinical and experimental settings [42,43,44]. In a study describing human IgG subclass response to EV-A71 infections, Cao et al. (2013) found the neutralizing activity of human intravenous immunoglobulin is mainly mediated by the IgG1 subclass [45]. Besides that, they found that IgG2 subclass also displayed neutralizing activity, albeit to a lesser extent as compared to IgG1. In contrast, IgG3 was found not to have neutralizing activity. Furthermore, they demonstrated that the IgG3 subclass enhanced EV-A71 infection [45]. These findings are critical for the rational design of peptide-based vaccines that only induce high levels of the neutralizing antibody responses (e.g., IgG1 and IgG2), but not the non-neutralizing antibody responses (e.g., IgG3), towards EV-A71 or other HFMD etiological agents.
4.1. Peptide-Based Vaccines Against EV-A71
Several studies reported the efficacies of linear antigenic peptide-based vaccines against EV-A71. For instance, a diphtheria toxoid-conjugated synthetic peptide comprising the EV-A71 SP70 neutralizing epitope was able to confer 80% passive protection in mice upon lethal challenge and elicited cross protective neutralizing antibodies (1:32) against EV-A71 sub-genotypes B2, B5, C2, and C4 [46]. Subsequently, Tian et al. (2012) reported the incorporation of the EV-A71 SP70 epitope within surface-exposed domains of the adenovirus type 3 hexon [47]. The construct was able to elicit humoral responses specific to the SP70 epitope. They also found that antisera raised against the construct were able to confer 70% protection of mice upon lethal EV-A71 challenge. Even though the protection was less than the diphtheria toxoid-conjugated EV-A71 SP70, this study demonstrated the possibility of using adenovirus to display the EV-A71 neutralizing epitope.
Another study focussed on the design of a tandem multi-linear neutralizing peptide vaccine. Li et al. (2014) designed a novel recombinant tandem multi-linear neutralizing epitope of EV-A71 designated as mTLNE [48]. The mTLNE comprised three linear neutralizing epitopes of SP55, SP70, and SP28, which were sequentially connected by a (Gly4Ser)3 linker. The mTLNE also contained thioredoxin (Trx) at the N-terminal to improve solubility and immunogenicity. Additionally, six histidine (His) were added at the C-terminal for affinity chromatography purification of the E. coli expressed proteins. Following immunization in adult mice, the mTLNE elicited EV-A71 specific IgG antibodies. Passive transfer of the anti-mTLNE sera conferred 100% protection against lethal EV-A71 challenge in mice. This is a marked improvement in comparison to the 80% protection conferred by the anti-diphtheria toxoid-conjugated EV-A71 SP70 sera and 70% protection conferred by antisera raised against recombinant adenovirus carrying the SP70 epitope in previous lethal challenge studies [46,47]. Although the neutralizing titres elicited by SP55, SP70, and the mTLNE peptide vaccine were relatively low in comparison to EV-A71 VLPs and the formaldehyde-inactivated EV-A71, the advantage of this approach should be examined. For instance, immunization studies in mice revealed that SP55, SP70, and the mTLNE peptide vaccine induced robust IgG1 response and not IgG3 [22,48]. This indicated these peptides would not contribute to the ADE phenomenon if incorporated into future peptide-based vaccine development.
4.2. EV-A71/CV-A16 Peptide-Based Bivalent Vaccines
Consistent with the low amino acid sequence identity of 20.7% between EV-A71 and CV-A16 capsid proteins (VP1, VP2, VP3, and VP4), vaccine candidates that were able to confer protection against EV-A71 were weakly cross-protective against CV-A16 infection [49,50]. For instance, VLPs and inactivated EV-A71 or CV-A16 monovalent vaccines failed to provide sufficient cross protection against their enteroviral counterparts [50]. To address this issue, an EV-A71/CV-A16 bivalent vaccine was produced by mixing equivalent doses of EV-A71 and CV-A16 VLPs or the inactivated EV-A71 and CV-A16 viruses. The EV-A71/CV-A16 bivalent vaccine was able to elicit cross-neutralizing antibodies and the immune sera from vaccinated animals could confer passive protection against EV-A71 and CV-A16 lethal challenges. However, the large-scale growth and production of either of the viruses or the VLPs constituting the bivalent vaccine will not be cost effective. Moreover, chemical treatments have been shown to alter the antigenic surface of CV-A16 [32,51]. Considering these problems, it is better to produce a multi-epitope antigen or a chimeric antigen that can elicit neutralizing antibodies against both EV-A71 and CV-A16.
Based on the 80% sequence identity between SP70 and PEP71 epitopes, coupled with structural studies that revealed these epitopes were displayed on the virion surface, Zhao et al. (2015) designed a peptide-based EV-A71/CV-A16 bivalent vaccine [52,53,54,55]. They constructed a chimeric EV-A71 VLP designated as ChiEV-A71 VLP, in which the SP70 of EV-A71 was replaced with PEP71 of CV-A16 [52]. The yeast expressed ChiEV-A71 VLPs were found to induce robust Th1/Th2-dependent immune responses against EV-A71 and CV-A16 in mice. Besides that, passive immunization with anti-ChiEV-A71 VLP sera conferred full protection against lethal EV-A71 and CV-A16 challenges. Subsequent studies provided insights into the structural basis of EV-A71 and CV-A16 neutralizations by ChiEV-A71 VLPs [56]. The substitution of the SP70 segment in EV-A71 with the corresponding PEP71 region from CV-A16 led to surface charge potential of the modified region to shift from negative to neutral. The surface charge potential shifts coupled with the amino acid changes (K215L, E217A, K218N, E221D) most likely accounted for ChiEV-A71 VLP dual neutralization capabilities of EV-A71 and CV-A16. Importantly, this is the first study utilizing an antigenic peptide to produce a bivalent vaccine in the form of a chimeric VLP.
In another effort to produce a bivalent vaccine, Xu et al. (2015) used hepatitis B virus core protein to construct a bivalent chimeric VLP presenting the SP70 epitope and VP2 epitope spanning amino acids 141 to 155 of EV-A71 [24]. They discovered that the chimeric VLP designated as HBc-E1/2 elicited high IgG and neutralization titres against both EV-A71 and CV-A16. Furthermore, passive immunization with HBc-E1/2 protected neonatal mice against lethal EV-A71 and CV-A16 challenges. The VP2 epitope (amino acids 141 to 155) was shown to be immune-dominant in both EV-A71 and CV-A16. They found that the anti-VP2 epitope elicited antibodies that could bind to both EV-A71 and CV-A16 viral particles, whereas antibodies from the anti-SP70 epitope did not bind to CV-A16 viral particles. These studies highlighted the possibility of employing linear epitopes identified from both EV-A71 and CV-A16 to produce a bivalent vaccine against both viruses.
4.3. Peptide Insertion Sites in EV-A71 VLP
To date, VLP is one the most suitable and widely used platform technologies to display antigenic peptides for vaccine development [57]. Advantages of VLP as a vaccine platform include: (1) its 20–100 nm diameter size which allows easy entry into the lymphatic vessels, passive drainage to the subcapsular lymph nodes, and optimal uptake by antigen presenting cells; and (2) the geometry that contributes to their ability to activate B-cells as the epitopes presented on the repetitive and rigid structures of VLPs could extensively interact with antigen presenting cells, leading to stimulation of B-cells and elicitation of robust antibody responses. Besides directly substituting the corresponding regions in a VLP with another region from a different virus to form a bivalent vaccine, regions in the VLP that were exposed or externalized on the viral capsid could be exploited to display B-cell epitopes from other enteroviruses to form multivalent vaccines. A recent study has reported repeated administration of an EV-A71 VLP vaccine which were well tolerated in rabbits and immunogenic. This further supported the use of EV-A71 VLP as a vaccine platform to display antigenic peptides [58].
In EV-A71 research, Arita et al. (2007) firstly identified that a histidine tag could be inserted into the BC-loop of VP1 between amino acids 100 and 101 without affecting the infectivity of the engineered EV-A71 [59]. Subsequently, Lyu et al. (2013) inserted nucleating peptides at the same position to produce a thermostable attenuated EV-A71 vaccine [60]. The insertion of peptides endowed the virus with the capacity to generate an exterior calcium phosphate shell. Intriguingly, the modified surface enabled the virus to exhibit improved thermostability and immunogenicity. They also determined the structures of two naturally occurring empty EV-A71 particles, one from a clinical C4 strain and the other from the recombinant virus containing a foreign peptide in the BC loop of VP1 [55]. Detailed inspection of the structures revealed the inserted foreign peptide was well displayed on the viral particle surface without significant structural perturbations of the viral capsid. In addition, comparison of the uncoating intermediates of EV-A71 and recombinant EV-A71 virus demonstrated that the foreign peptide insertion did not affect the virus uncoating process. Furthermore, the crystal structure of yeast-produced EV-A71 revealed that the VLP produced using the yeast expression system (PDB code: 4YVS) shared very high structural similarity with the empty particles (PDB code: 4RQP). Superimposition using PyMOL of the VP1, VP3, and VP0 onto the corresponding viral structural proteins on EV-A71 empty virus particle resulted in a calculated root mean square deviation (r.m.s.d) of 0.504 Å over 560 Cα atoms. Based on the structural and functional evidence, the BC-loop of EV-A71 was identified to be a suitable peptide insertion site to develop multivalent vaccines.
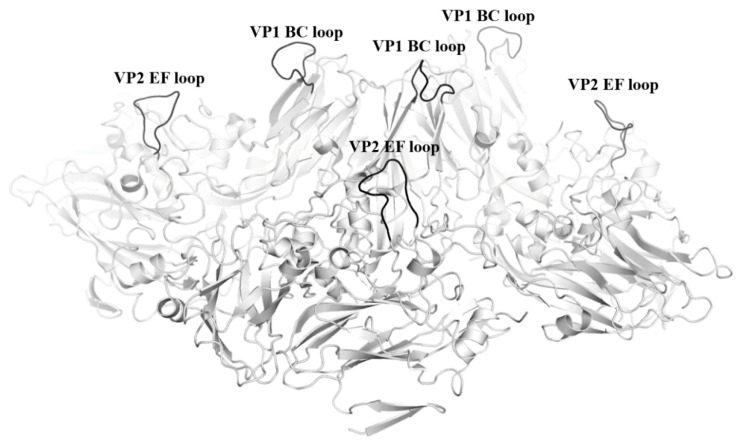
In addition to the BC-loop, other insertion sites should be identified to produce trivalent or tetravalent HFMD vaccines against other HFMD causative agents. Based on the available EV-A71 structures in the protein database, the EF loop of VP0 (or VP2) corresponding to amino acids 202 to 214 might be another suitable site for foreign peptide insertion (Figure 1). This region is located at the surface of the viral capsid. As demonstrated previously by Lyu et al. (2015), the surface-exposed loop was an ideal site for foreign linear peptide insertion [55]. However, peptide insertions might affect the ability of recombinant protein to assemble into VLPs. Thus, structural and functional validations using techniques such as X-ray crystallography and Cryo-electron microscopy are crucial to ensure that the peptide insertions do not perturb the overall structure of the VLP.
Figure 1.
Position of VP1 BC loop and VP2 EF loop on the EV-A71 viral capsid. Representation of the EV-A71 VLP viral capsid. VP1, VP2 and VP3 structural proteins are shown in grey. The VP1 BC loop and VP2 EF loop are shown in black and labelled. PDB code: 4RQP.
4.4. Tetravalent Peptide-Based HFMD Vaccine
Previous attempts were made to use the traditional VLP-based platform to produce a tetravalent HFMD vaccine. For instance, Zhang et al. (2018) produced each single VLP of EV-A71, CV-A16, CV-A6, and CV-A10 using a baculovirus-insect cell expression system, and then combined them to formulate a tetravalent HFMD vaccine [61]. They found the tetravalent vaccine elicited antigen specific and long lasting antibody responses in addition to passive protection conferred by the tetravalent vaccine-immunized sera against single or mixed infections with EV-A71, CV-A16, CV-A6, and CV-A10 in mice. However, large scale productions of the four VLPs will be costly and time-inefficient in comparison to the production of a single VLP carrying peptide epitopes from the four different viruses.
Similar to EV-A71 and CV-A16, other Enterovirus A serotypes were found to carry the SP70 motif. Based on sequence analysis of several Enterovirus A serotypes, the SP70 motif can be denoted as YPTFGX1HPX2X3X4X5X6X7Y (Figure 2). The motif consists of the highly conserved amino acids Tyr, Pro, Thr, Phe, Gly (YPTFG), followed by X1 (which can either be polar uncharged amino acids or negatively charged amino acids), the highly conserved His and Pro, followed by X2, X3, X4, X5, X6, X7, and ending with a highly conserved Tyr. Amino acids X2 and X3 can be any amino acid, X4 and X7 can be any of the polar uncharged or charged amino acids, X5 can either be an Asp or an Asn, while X6 can be any hydrophobic amino acid (Leu, Val, Phe, Ile).
Figure 2.
Multiple sequence alignment of SP70 motif from various Enterovirus A viruses. The SP70 motif of Enterovirus A species can be denoted as YPTFGX1HPX2X3X4X5X6X7Y. The consensus amino acid is highlighted in grey and in bold.
Based on previous findings of the bivalent EV-A71/CV-A16 vaccine, the SP70 linear epitope from EV-A71, CV-A16, CV-A6, and CV-A10 could be incorporated into a VLP to produce a tetravalent HFMD vaccine [52]. Inspection of the viral particle structures of EV-A71, CV-A16, CV-A6, and CV-A10 revealed all viral particles to be structurally very similar. Crucially, the SP70 motif was present within the GH loop of each of the viral particles, hence, it is possible to insert the SP70 motif of each virus into a single VLP. For example, the EV-A71 VLP is a suitable VLP to be used to construct the HFMD tetravalent vaccine, as it is the most widely studied amongst all the enteroviral VLPs. In addition to the substitution of the SP70 motif of EV-A71 with the corresponding CV-A16 epitope in the EV-A71 VLP, the SP70 motif from CV-A6 could also be inserted into the BC-loop insertion site and another SP70 motif from CV-A10 could be inserted into the site in the EF loop of VP0 or VP2. The insertion site should be experimentally and structurally validated to ensure that the SP70 peptide insertion does not disrupt the global structure of the EV-A71 VLP. Furthermore, studies should be performed to confirm that the SP70 motifs of both CV-A6 and CV-A10 are able to elicit neutralization antibodies, similar to the SP70 motif present in either EV-A71 or CV-A16.
Another approach that should be considered is to expand the tandem multi-linear neutralizing epitope (mTLNE) vaccine strategy to produce a multivalent vaccine. It is possible to design a peptide vaccine containing multiple SP70 epitopes from EV-A71, CV-A16, CV-A6, and CV-A10 that are linked together to produce a tetravalent HFMD peptide-based vaccine that is protective against the four viruses. Besides that, the mTLNE vaccine approach could be presented on nanoparticles or on VLPs which might improve the immunogenicity and efficacy of the tetravalent vaccine.
Besides EV-A71 VLP, hepatitis B core antigen virus-like-particle (HBcAg-VLP) is an attractive alternative VLP to display multiple epitopes of HFMD etiological agents. HBcAg-VLP is one of the best characterized VLPs. It possesses three possible peptide or antigen insertion sites, which are located at the N terminus, the C terminus, and the immune-dominant loop region (between Asp79 and Pro79) [62]. For the N and C termini insertions, a maximum of 100 amino acids can be inserted into each insertion site [62]. Although the inserted antigens at the C and N termini will be immunogenic, HBcAg-VLP will retain its strong intrinsic immunogenicity as immune responses would target the immune-dominant loop region. Therefore, to achieve the highest immunogenicity, the insertion at the immune-dominant loop region is most favourable. Insertion of an antigen within the immune-dominant loop region will abrogate HBcAg-VLP intrinsic immunogenicity and directs immune responses towards the inserted foreign antigen. Besides that, the antigen insertion capacity of this region is very high, as demonstrated by the successful insertion of the green fluorescent protein (238 aa) and the dimeric outer surface lipoprotein C of Borrelia burgdorferi (~210 aa) [63,64].
Crucially, Wu et al. (2017) demonstrated that HBcAg-VLP inserted with two linear epitopes from EV-A71 (SP70 epitope and amino acids 141-155 of VP2) and one epitope from glycoprotein E (amino acids 121–135) of varicella-zoster virus (VCV) in a tandem manner was able to self-assemble into a VLP [65]. The VLP designated as HBc-V/1/2 elicited a balanced antibody response towards the three epitopes. Importantly, the HBc-V/1/2 antisera could neutralize EV-A71, CV-A16, and VCV. Moreover, the HBc-V/1/2 antisera were able to protect neonatal mice against lethal challenges of EV-A71 and CV-A16. Based on these studies, the combination of the HBcAg-VLP platform with the mTLNE strategy might yield an effective tetravalent HFMD vaccine against EV-A71, CV-A16, CV-A6, and CV-A10. For instance, a highly immunogenic linear epitope, such as the SP70 epitope from each of the four HFMD etiological agents, could be linked together to form a mTLNE. The mTLNE can be inserted into the immune-dominant region of HBcAg-VLP to develop a multivalent HFMD vaccine.
Theoretically, there are many advantages of using HBcAg-VLP than EV-A71 VLP to produce a multivalent HFMD vaccine. Firstly, the use of HBcAg-VLP is more cost-effective, as HBcAg-VLP could be produced in a bacterial expression system such as E. coli. This system will incur lower production cost than yeast, insect, or mammalian cell expression systems that are required to produce the EV-A71 VLP. Secondly, HBcAg-VLP has a higher peptide insertion capacity (238 aa could be inserted in its immune-dominant loop region) in comparison to EV-A71 VLP that could only receive a peptide of around 20 amino acids in its BC loop region. Nonetheless, the suitability of HBcAg-VLP and EV-A71 VLP to carry multiple epitopes as an effective multivalent HFMD vaccine should be further tested.
5. Neutralizing Antibodies Against HFMD Etiological Agents
5.1. EV-A71 Neutralizing Antibodies
Neutralizing antibodies play major roles in immune-protection against viral infections. In EV-A71 infection, robust induction of EV-A71-specific IgM and IgG were detected during the first week of infection and the responses peaked four to seven days after the onset of symptoms [66]. Passive transfer of neutralizing anti-sera was reported to protect mice against lethal EV-A71 challenge, indicating the importance of neutralizing antibodies in anti-EV-A71 immunity [46]. Importantly, comprehensive understanding of the molecular basis of EV-A71 neutralization by antibodies would aid in the rational design of an effective vaccine against EV-A71. In order to understand neutralizing antibodies elicited by EV-A71, many groups generated neutralizing murine monoclonal antibodies (mAbs) using immunogens, such as inactivated EV-A71, live EV-A71, EV-A71 VLPs, or the linear neutralizing epitopes derived from EV-A71 (Table 2).
Table 2.
Neutralizing monoclonal antibodies against EV-A71, CV-A16, CV-A6, and CV-A10.
| Virus | Name | Epitope Type | Epitope/Region Recognised | Isotype | Ref. |
|---|---|---|---|---|---|
| EV-A71 | N1 | Linear | Recognized a section of the SP70 epitope. Neutralized 2 subgenotypes tested (B4 and B5). | IgG2a | [67] |
| N3 | Linear | Recognized a section of the SP70 epitope. Neutralized 2 subgenotypes tested (B4 and B5). | IgG2a | [67] | |
| N4 | Linear | Recognized a section of the SP70 epitope. Neutralized 2 subgenotypes tested (B4 and B5). | IgG2a | [67] | |
| N6 | Linear | Recognized a section of the SP70 epitope. Neutralized 2 subgenotypes tested (B4 and B5). | IgG2a | [67] | |
| BB1A5 | Linear | Recognized amino acids 136–155 in VP2 (AGGTGTEDSHPPYKQTQPGA). Neutralized B3, B4, C2 and C5 subgenotypes. | IgG2a | [28] | |
| 3D1 | Unknown | Recognized VP1. Neutralized all 8 subgenotypes tested (C1, C2, C3, C4, C5, B3, B4, and B5). | IgM | [68] | |
| 4E8 | Unknown | Recognized two peptides P25 (aa2 40–250) and P26 (aa 250–260) of VP1. Neutralized C4 subgenotype (only subgenotype tested). | IgG | [69] | |
| 10D3 | Conformational | Recognized the knob region in VP3. Neutralized all 11 subgenotypes of EV-A71. | IgM | [29] | |
| mAb51 | Linear | Recognized a portion of the SP70 epitope (KQEKD). Neutralized all 11 subgenotypes of EV-A71. | IgM | [70] | |
| 2G8 | Linear | Recognized the SP70 epitope. Neutralized C4 subgenotype. | IgM | [71] | |
| MA28-7 | Conformational | Recognized the region comprising Gly145, Glu98, Lys242 and Lys244 in VP1. Neutralized 6 genotypes tested (A, B1, B3, B4, and C2). | N/A | [72] | |
| D6 | Unknown | Neutralized subgenotype C4 (only subgenotype tested). | N/A | [53] | |
| A9 | Unknown | Neutralized subgenotype C4 (only subgenotype tested). | N/A | [53] | |
| 5H7 | Conformational | Recognized conformational epitope harbouring Ser74 in VP3. Neutralized all 11 subgenotypes tested. | IgG | [30] | |
| D4 | Unknown | Neutralized subgenotype C4 (only subgenotype tested). | IgG | [73] | |
| G12 | Unknown | Neutralized subgenotype C4 (only subgenotype tested). | IgG | [73] | |
| 22A12 | Linear | Neutralized subgenotype C4 (only subgenotype tested). | IgG | [74] | |
| VP4N20 mAb | Linear | Recognized the VP4N20 epitope in VP4. Neutralized EV-A71 tested (genogroup A and subgenotype C4) and CV-A16. | N/A | [35] | |
| CV-A16 | VP4N20 mAb | Linear | Recognized the VP4N20 epitope in VP4. Neutralized EV-A71 tested (genogroup A and subgenotype C4) and CV-A16. | N/A | [35] |
| C33 | Unknown | Recognized the SP70 epitope. Neutralized genotype B of CV-A16 (only one tested). | N/A | [53] | |
| CV-A6 | 1D5 | Conformational | Recognized a region in VP1 comprising BC, DE, EF, and HI loops. | IgG | [38,39] |
| CV-A10 | 2G8 | Conformational | Recognized the VP1 C-terminus, VP2 EF loop, and VP3 AB loop. | IgG | [36] |
N/A: Not reported.
Many of the EV-A71 neutralizing antibodies were generated by immunizing mice with live EV-A71. For instance, Chang et al. (2011) inoculated mice with the live EV-A71/E59 genotype B4 strain to generate mAbs [67]. The study identified four mAbs belonging to the IgG2a subclass designated as N1, N3, N4, and N6. From epitope mapping studies, the four mAbs were found to recognize amino acids 211 to 225 of the VP1 protein corresponding to the SP70 epitope. The mAbs were able to fully cross-neutralize EV-A71 sub-genotypes B4 and B5 in the rhabdomyosarcoma (RD) cells and it was noted that these mAbs could work synergistically, as complete inhibition was achieved at a markedly lower concentration of the pooled mAbs in comparison to a single mAb in RD cells. In addition, Xu et al. (2014) generated a mAb designated as BB1A5 belonging to the IgG2a isotype by immunizing mice with the live EV-A71 strain 52-3 [28]. BB1A5 recognized a linear epitope spanning amino acids 136–155 in the VP2, and passive transfer of BB1A5 was able to provide 100% protection for the new-born mice challenged with a mouse-adapted EV-A71 strain. Besides that, another group identified a mAb designated as 3D1 belonging to the IgM isotype generated by immunizing mice with a mouse-adapted EV-A71 strain [68]. They demonstrated that passive immunization using 3D1 prevented central nervous system (CNS) infection. However, the exact binding site of 3D1 was unknown.
Additionally, inactivated EV-A71 was utilized to generate mAbs against EV-A71. For instance, Chang et al. (2010) identified mAb 4E8, which was generated by immunizing mice with an inactivated EV-A71 Henan2 (Hn2) genotype C4 strain [69]. Using synthetic peptide mapping, they identified mAb 4E8 to recognize peptides containing amino acids 240–250 and 250–260 of VP1 by ELISA. In the in vivo protection study, mAb 4E8 was able to provide partial protection against EV-A71 lethal challenge in mice. In another study, Kiener et al. (2014) immunized mice with a binary ethyleneimine (BEI)-inactivated EV-A71-B4 strain to generate mAb 10D3 belonging to the IgM isotype [29]. Dot blot assay coupled with mutational analysis revealed that mAb 10D3 recognized a conformational epitope at the highly conserved knob region of VP3. MAb 10D3 was able to confer in vivo passive protection against EV-A71 infection. Moreover, neutralizing mAb51 belonging to the IgM isotype was generated by immunizing mice with binary BEI-inactivated EV-A71 [70]. The mAb51 was able to provide 100% in vivo passive protection in lethal challenge with EV-A71 in mice. Epitope mapping revealed that the mAb51 targeted amino acids 215–219 in VP1, which is part of the SP70 epitope. Furthermore, Deng et al. (2015) generated IgM isotype mAb designated as 2G8 by immunizing mice with heat-inactivated EV-A71 [71]. The mAb was able to neutralize EV-A71 infection at the attachment stage. Biochemical assays mapped the 2G8 epitope to the SP70 epitope. They also demonstrated that the L220A mutation within the SP70 epitope completely abolished the ability of 2G8 to bind and neutralize EV-A71. Another mAb designated as MA28-7 was generated by immunizing mice with formalin-inactivated EV-A71 strain 1095 [72]. Structural studies of MA28-7 in complex with EV-A71 revealed that the mAb recognized a conformational epitope comprising several amino acids in VP1, including Gly145, Glu98, Lys242, and Lys244. D6 and A9 were two other mAbs that were generated by immunization of mice using inactivated EV-A71 [53]. Both mAbs were able to protect RD cells from EV-A71 infection. Most recently, a mAb designated as 5H7 belonging to the IgG isotype was generated by immunizing mice with inactivated EV-A71-B4 strain [30]. Epitope mapping revealed that 5H7 recognized a conformational epitope within the VP3 protein harboring the highly conserved amino acid Ser74. Interestingly, the highly conserved Ser74 is adjacent to the highly conserved knob region of VP3, which was recognized by 10D3. MAb 5H7 was able to neutralize major EV-A71 genogroups, including A, B4, C2, and C4. Chimeric 5H7 mAb was constructed by fusing the VH and VL regions of murine mAb 5H7 to human kappa and IgG1 constant regions. Passive immunization using the resulting mouse-human chimeric 5H7 protected 100% of mice challenged with EV-A71 B4 strain prophylactically and therapeutically.
Other than live and inactivated viruses, VLPs have been used to generate mAbs. For example, Lin et al. (2015) immunized mice with VLPs of EV-A71 produced in yeast [73]. From the immunization, two mAbs were isolated, designated as D4 and G12. The mAbs bound to the VP1 of EV-A71, however, the exact binding site of the mAbs was unknown. In an in vitro neutralization assay, both mAbs were able to protect RD cells from CPE upon EV-A71 infection.
Several mAbs were generated by immunizing mice with synthetic peptides. For example, Li et al. (2009) immunized mice using SP70 and SP55 peptides to isolate twelve IgG mAbs specific for SP70 and SP55 [74]. Out of the twelve mAbs, only mAb 22A12, which specifically recognized SP70 peptide, provided complete protection of RD cells from EV-A71 viral infection at a neutralizing titre of 1:16, albeit this titre was quite low in comparison to the 1:48 neutralization titre produced by antiserum from rabbit immunized with the heat-inactivated virus [74]. In another study, a phage display technique was used to produce a recombinant mAb targeting VP4N20 epitope in the VP4 protein [35]. In vitro neutralizing assays revealed that the VP4N20 mAb prevented the development of cytopathic effects in RD cells infected with either EV-A71 or CV-A16. This is consistent with the 90% amino acid identity of VP4N20 epitope in both EV-A71 and CV-A16.
5.2. CV-A16, CV-A6 and CV-A10 Neutralizing Antibodies
Unlike EV-A71, few neutralization mAbs have been generated and identified against CV-A16, CV-A6, and CV-A10. In a study performed by Ren et al. (2015), mAb C33 was generated by immunizing mice with inactivated CV-A16 [53]. The mAb C33 was able to provide complete protection against the development of cytopathic effects in Vero cells infected with CV-A16. For CV-A6, Yang et al. (2016) immunized mice using live CV-A6 to generate mAbs [39]. They identified mAb 1D5 that could fully protect mice from lethal CV-A6 challenge. The mAb also displayed good therapeutic effects in the mice infected with CV-A6. Structural studies using cryo-electron microscopy revealed that mAb 1D5 bound to the immune-dominant antigenic site within VP1 comprising the BC, DE, EF, and HI loops [38]. For CV-A10, a neutralizing mAb designated as 2G8 was obtained by immunizing mice with CV-A10 mature virions [36]. The mAb displayed high binding efficiency and potent neutralization activity against CV-A10 infection based on ELISA and in vitro neutralization in RD cells. In vivo experiments in the murine model revealed that 2G8 exhibited strong prophylactic and therapeutic properties, whereby all mice administered with 2G8 before or after the CV-A10 lethal challenge survived. Structural characterization using cryo-electron microscopy revealed that 2G8 recognized the VP1 C-terminus, VP2 EF loop, and VP3 AB loop.
5.3. Neutralizing Antibodies as Potential Prophylactic or Therapeutic Agents against Enteroviruses Causing HFMD
Antibodies can be administered as a therapeutic or prophylactic agent. Monoclonal antibody therapy is crucial for immunocompromised individuals, as vaccinations are only suitable for individuals with an immune system capable of eliciting responses against the administered vaccine [75]. The antibodies to be used in mAb therapy should have high neutralizing efficacy coupled with broad neutralizing spectrum against all or most of the viral subgenotypes. Ideally, the antibody therapy should be able to neutralize multiple viruses causing the disease for it to be effective against multiple etiological agents of HFMD.
As shown in Table 2, only four mAbs (3D1, 10D3, mAb51, and 5H7) were experimentally proven to display broad neutralizing spectrum. Interestingly, three of the four mAbs, with the exception of 5H7, belonged to the IgM isotype. This observation is consistent with other studies and observations made by several research groups that suggested antibody neutralizing capabilities were dependent on their isotypes [71,75]. For example, Lim et al. (2012) reported that in contrast to the IgM isotype that possessed neutralizing activity in vitro, IgG1 did not have neutralizing activity [70]. Besides that, Liu et al. (2011) reported that IgG2a mAb displayed only modest neutralization activity against EV-A71 [23]. Thus, it can be speculated that neutralizing capability of these mAbs is isotype-dependent. In addition, three of the universally neutralizing mAbs, 10D3, 5H7, and mAb51, recognized a region or a sequence that was highly conserved in all 11 subgenotypes, which was the knob region in VP3, the highly conserved Ser74 adjacent to the knob region in VP3, and a short sequence in the SP70 epitope (KQEKD), respectively [29,30,70]. This most likely contributes to their universal neutralizing capability. Based on the evidence, it is possible that other mAbs, such as mAb VP4N20, which recognized an epitope that is highly conserved in EV-A71 subgenotypes and in the CV-A16, might have a broad neutralizing spectrum. However, neutralization by the mAb was only tested against limited EV-A71 subgenotypes. Therefore, it is imperative to further characterize the neutralizing spectrum of all the mAbs identified so far to establish a comprehensive understanding of neutralizing mAbs against the etiological agents of HFMD.
A major issue regarding the potential of using mAb clinically is that most of the mAbs were derived from mice. Such mouse-derived mAbs would lead to immune rejection in humans, as they might be recognized as foreign antigens by the human immune system [76]. Besides that, murine mAbs were unable to induce downstream human immune responses due to the inability of human immune cells, such as macrophages, to recognize the Fc region of murine mAbs. To circumvent this problem, the humanization of murine mAbs is mandatory prior to passive immunization using mouse-derived mAbs in humans. Taking dengue virus mAb513 as an example, which is currently undergoing clinical trials in Singapore, few amino acid changes were made, including Thr33Val mutation and Ser26 deletion, which resulted in the murine mAb 4E11 to be humanized [77]. In another example, complementary determining region (CDR) grafting and variable (V) region replacement were performed to convert the influenza virus subtype H5N1 antibody m8A8 to humanized 8A8 with minimal murine immunogenicity [78]. Based on this evidence, it is possible to humanize murine mAbs of enteroviruses for future medical applications (Table 2).
The design of an effective prophylactic mAb to prevent HFMD needs to account for the multiple etiological agents that could be involved in HFMD outbreaks. The multi-domain antibody approach could be an attractive approach to target several etiological agents of HFMD. Recently, there has been one research group that designed a multi-domain antibody against multiple strains of influenza virus. Laursen et al. (2018) linked multiple antibodies to design a prophylactic antibody against influenza A and B strains designated as MD3606 [79]. MD3606 was able to confer protection in mice against influenza A and influenza B infections. Thus, it may be possible to link four mAbs that were able to neutralize each of the HFMD etiological agents (EV-A71, CV-A16, CV-A6, and CV-A10) to design a tetravalent HFMD prophylactic antibody.
6. Conclusions
The global burden of HFMD is increasing and the trend will continue until an effective vaccine against multiple HFMD causative agents is developed. Although there are three formaldehyde-inactivated EV-A71 vaccines licensed by the China FDA, the inactivated monovalent vaccines are only protective against EV-A71 and not the other HFMD causative agents. Apart from developing a bivalent EV-A71 and CV-A16 vaccine, research efforts are now focussed on developing a multivalent HFMD vaccine. Comprehensive evaluations of humoral responses elicited by epitopes from EV-A71, CV-A16, CV-A6, and CV-A10 are mandatory for a successful multivalent HFMD vaccine development. The multivalent vaccine should provide protection against significant HFMD Enterovirus A serotypes in a balanced manner and should ideally avoid the ADE effect. Future studies to utilize other vaccine platforms, such as live-attenuated, recombinant protein subunit, viral vectored, and peptide-based vaccines are needed, and will definitely open up new avenues to develop fully efficacious and safe vaccines against HFMD. The multi epitope peptide-based HFMD vaccines are gaining attention due to their safety, easy large-scale production, and low cost. With the rising concern about the current HFMD outbreaks in Asia, the future will see more development of the HFMD tetravalent vaccines, especially on a peptide-based multivalent HFMD vaccine platform, if low immunogenicity of such vaccines could be overcome. Finally, further identification and generation of neutralizing antibodies against etiological agents of HFMD are warranted, as prophylactic and therapeutic antibodies against viruses are gaining popularity, as demonstrated in the development of mAb therapies against dengue and influenza viruses.
Abbreviations
| ADE | Antibody dependent enhancement |
| BEI | Binary ethyleneimine |
| CDR | Complementary Determining Region |
| CNS | Central Nervous System |
| CV-A16 | Coxsackievirus 16 |
| EMEA | European Medicine Agency |
| EV-A71 | Enterovirus A 71 |
| Fab | Fragment antigen-binding |
| FcR | Fc Receptor |
| FDA | Food and Drug Administration |
| HFMD | Hand, Foot and Mouth Disease |
| mTLNE | Multiple tandem linear neutralizing epitope |
| RD | Rhabdomyosarcoma |
| r.m.s.d | Root Mean Square Deviation |
| RNA | Ribonucleic Acid |
| Trx | Thioredoxin |
| UTR | Untranslated Region |
| VCV | Varicella-zoster virus |
| VLP | Virus-Like-Particle |
Author Contributions
M.I.A. wrote the manuscript and C.L.P. provided critical revision and final approval of the manuscript
Funding
This work was funded by the Sunway University Research Centre Grant (2018) to the Centre for Virus and Vaccine Research
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Solomon T., Lewthwaite P., Perera D., Cardosa M.J., McMinn P., Ooi M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010;10:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 2.Mao Q., Wang Y., Yao X., Bian L., Wu X., Xu M., Liang Z. Coxsackievirus A16: Epidemiology, diagnosis, and vaccine. Hum. Vaccin. Immunother. 2014;10:360–367. doi: 10.4161/hv.27087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M., He S., Yan Q., Xu X., Wu W., Ge S., Zhang S., Chen M., Xia N. Severe hand, foot and mouth disease associated with coxsackievirus A10 infections in Xiamen, China in 2015. J. Clin. Virol. 2017;93:20–24. doi: 10.1016/j.jcv.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Anh N.T., Nhu L.N.T., Van H.M.T., Hong N.T.T., Thanh T.T., Hang V.T.T., Ny N.T.H., Nguyet L.A., Phuong T.T.L., Nhan L.N.T., et al. Emerging coxsackievirus A6 causing hand, foot and mouth disease, Vietnam. Emerg. Infect. Dis. 2018;24:654–662. doi: 10.3201/eid2404.171298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yee P.T.I., Poh C.L. T cell immunity to enterovirus 71 infection in humans and implications for vaccine development. Int. J. Med. Sci. 2018;15:1143–1152. doi: 10.7150/ijms.26450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L., Yang H., Wang C., Yao X.J., Zhang H.L., Zhang R.L., He Y.Q. Genomic characteristics of coxsackievirus A8 strains associated with hand, foot, and mouth disease and herpangina. Arch. Virol. 2016;161:213–217. doi: 10.1007/s00705-015-2646-1. [DOI] [PubMed] [Google Scholar]
- 7.Liu W., Wu S., Xiong Y., Li T., Wen Z., Yan M., Qin K., Liu Y., Wu J. Co-circulation and genomic recombination of coxsackievirus A16 and enterovirus 71 during a large outbreak of hand, foot, and mouth disease in Central China. PLoS ONE. 2014;9:e96051. doi: 10.1371/journal.pone.0096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomqvist S., Klemola P., Kaijalainen S., Paananen A., Simonen M.L., Vuorinen T., Roivainen M. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J. Clin. Virol. 2010;48:49–54. doi: 10.1016/j.jcv.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Mao Q., Wang Y., Bian L., Xu M., Liang Z. EV-A71 vaccine licensure: A first step for multivalent enterovirus vaccine to control HFMD and other severe diseases. Emerg. Microbes Infect. 2016;5:e75. doi: 10.1038/emi.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu F., Xu W., Xia J., Liang Z., Liu Y., Zhang X., Tan X., Wang L., Mao Q., Wu J., et al. Efficacy, Safety, and Immunogenicity of an Enterovirus 71 Vaccine in China. N. Engl. J. Med. 2014;370:818–828. doi: 10.1056/NEJMoa1304923. [DOI] [PubMed] [Google Scholar]
- 11.Zhu F.C., Meng F.Y., Li J.X., Li X.L., Mao Q.Y., Tao H., Zhang Y.T., Yao X., Chu K., Chen Q.H., et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381:2024–2032. doi: 10.1016/S0140-6736(13)61049-1. [DOI] [PubMed] [Google Scholar]
- 12.Liu L., Mo Z., Liang Z., Zhang Y., Li R., Ong K.C., Wong K.T., Yang E., Che Y., Wang J., et al. Immunity and clinical efficacy of an inactivated enterovirus 71 vaccine in healthy Chinese children: A report of further observations. BMC Med. 2015;13:226. doi: 10.1186/s12916-015-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown B.A., Pallansch M.A. Complete nucleotide sequence of enterovirus 71 is distinct from poliovirus. Virus Res. 1995;39:195–205. doi: 10.1016/0168-1702(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 14.Plevka P., Perera R., Cardosa J., Kuhn R.J., Rossmann M.G. Crystal structure of human enterovirus 71. Science. 2012;336:1274. doi: 10.1126/science.1218713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shingler K.L., Yoder J.L., Carnegie M.S., Ashley R.E., Makhov A.M., Conway J.F., Hafenstein S. The enterovirus 71 A-particle forms a gateway to allow genome release: A cryoEM study of picornavirus uncoating. PLoS Pathog. 2013;9:e1003240. doi: 10.1371/annotation/e92d19e0-996a-4bfa-afdd-20dce770ed75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belnap D.M., Filman D.J., Trus B.L., Cheng N., Booy F.P., Conway J.F., Curry S., Hiremath C.N., Tsang S.K., Steven A.C., et al. Molecular tectonic model of virus structural transitions: The putative cell entry states of poliovirus. J. Virol. 2000;74:1342–1354. doi: 10.1128/JVI.74.3.1342-1354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossmann M.G., Arnold E., Erickson J.W., Frankenberger E.A., Griffith J.P., Hecht H.J., Johnson J.E., Kamer G., Luo M., Mosser A.G., et al. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985;317:145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- 18.Tuthill T.J., Bubeck D., Rowlands D.J., Hogle J.M. Characterization of early steps in the poliovirus infection process: Receptor-decorated liposomes induce conversion of the virus to membrane-anchored entry-intermediate particles. J. Virol. 2006;80:172–180. doi: 10.1128/JVI.80.1.172-180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., Peng W., Ren J., Hu Z., Xu J., Lou Z., Li X., Yin W., Shen X., Porta C., et al. A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat. Struct. Mol. Biol. 2012;19:424–429. doi: 10.1038/nsmb.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu L., Xu K., Wang N., Cao L., Wu J., Gao Q., Fry E.E., Stuart D.I., Rao Z., Wang J., et al. Neutralization mechanisms of two highly potent antibodies against human enterovirus 71. mBio. 2018;9:e01013–e01018. doi: 10.1128/mBio.01013-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C.N., Lin Y.C., Fann C., Liao N.S., Shih S.R., Ho M.S. Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine. 2001;20:895–904. doi: 10.1016/S0264-410X(01)00385-1. [DOI] [PubMed] [Google Scholar]
- 22.Foo D.G., Alonso S., Phoon M.C., Ramachandran N.P., Chow V.T., Poh C.L. Identification of neutralizing linear epitopes from the VP1 capsid protein of Enterovirus 71 using synthetic peptides. Virus Res. 2007;125:61–68. doi: 10.1016/j.virusres.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Liu C.C., Chou A.H., Lien S.P., Lin H.Y., Liu S.J., Chang J.Y., Guo M.S., Chow Y.H., Yang W.S., Chang K.H., et al. Identification and characterization of a cross-neutralization epitope of Enterovirus 71. Vaccine. 2011;29:4362–4372. doi: 10.1016/j.vaccine.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Xu L., He D., Yang L., Li Z., Ye X., Yu H., Zhao H., Li S., Yuan L., Qian H., et al. A broadly cross-protective vaccine presenting the neighboring epitopes within the VP1 GH loop and VP2 EF loop of enterovirus 71. Sci. Rep. 2015;5:12973. doi: 10.1038/srep12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aw-Yong K.L., Sam I.C., Koh M.T., Chan Y.F. Immunodominant IgM and IgG epitopes recognized by antibodies induced in enterovirus A71-associated Hand, Foot and Mouth Disease patients. PLoS ONE. 2016;11:e0165659. doi: 10.1371/journal.pone.0165659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao F., Wang Y.P., Mao Q.Y., Yao X., Liu S., Li F.X., Zhu F.C., Yang J.Y., Liang Z.L., Lu F.M., et al. Enterovirus 71 viral capsid protein linear epitopes: Identification and characterization. Virol. J. 2012;9:26. doi: 10.1186/1743-422X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiener T.K., Jia Q., Lim X.F., He F., Meng T., Chow V.T., Kwang J. Characterization and specificity of the linear epitope of the enterovirus 71 VP2 protein. Virol. J. 2012;9:55. doi: 10.1186/1743-422X-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L., He D., Li Z., Zheng J., Yang L., Yu M., Yu H., Chen Y., Que Y., Shih J.W., et al. Protection against lethal enterovirus 71 challenge in mice by a recombinant vaccine candidate containing a broadly cross-neutralizing epitope within the VP2 EF loop. Theranostics. 2014;4:498–513. doi: 10.7150/thno.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiener T.K., Jia Q., Meng T., Chow V.T., Kwang J. A novel universal neutralizing monoclonal antibody against enterovirus 71 that targets the highly conserved “knob” region of VP3 protein. PLoS Negl. Trop. Dis. 2014;8:e2895. doi: 10.1371/journal.pntd.0002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia Q., Ng Q., Chin W., Meng T., Chow V.T.K., Wang C.I., Kwang J., He F. Effective in vivo therapeutic IgG antibody against VP3 of enterovirus 71 with receptor-competing activity. Sci. Rep. 2017;7:46402. doi: 10.1038/srep46402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao M., Bai Y., Liu W., Xiao X., Huang Y., Cen S., Chan P.K., Sun X., Sheng W., Zeng Y. Immunization of N terminus of enterovirus 71 VP4 elicits cross-protective antibody responses. BMC Microbiol. 2013;13:287. doi: 10.1186/1471-2180-13-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong P., Guo M.S., Lin F.H., Hsiao K.N., Weng S.Y., Chou A.H., Wang J.R., Hsieh S.Y., Su I.J., Liu C.C. Immunological and biochemical characterization of coxsackie virus A16 viral particles. PLoS ONE. 2012;7:e49973. doi: 10.1371/journal.pone.0049973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q., Yan K., Feng Y., Huang X., Ku Z., Cai Y., Liu F., Shi J., Huang Z. A virus-like particle vaccine for coxsackievirus A16 potently elicits neutralizing antibodies that protect mice against lethal challenge. Vaccine. 2012;30:6642–6648. doi: 10.1016/j.vaccine.2012.08.071. [DOI] [PubMed] [Google Scholar]
- 34.Shi J., Huang X., Liu Q., Huang Z. Identification of conserved neutralizing linear epitopes within the VP1 protein of coxsackievirus A16. Vaccine. 2013;31:2130–2136. doi: 10.1016/j.vaccine.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X., Sun C., Xiao X., Pang L., Shen S., Zhang J., Cen S., Yang B.B., Huang Y., Sheng W., et al. Phage Display-Derived Cross-Reactive Neutralizing Antibody against Enterovirus 71 and Coxsackievirus A16. Jpn. J. Infect. Dis. 2016;69:66–74. doi: 10.7883/yoken.JJID.2015.060. [DOI] [PubMed] [Google Scholar]
- 36.Zhu R., Xu L., Zheng Q., Cui Y., Li S., He M., Yin Z., Liu D., Li S., Li Z., et al. Discovery and structural characterization of a therapeutic antibody against coxsackievirus A10. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aat7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu L., Sun Y., Fan J., Zhu B., Cao L., Gao Q., Zhang Y., Liu H., Rao Z., Wang X. Structures of coxsackievirus A10 unveil the molecular mechanisms of receptor binding and viral uncoating. Nat. Commun. 2018;9:4985. doi: 10.1038/s41467-018-07531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu L., Zheng Q., Li S., He M., Wu Y., Li Y., Zhu R., Yu H., Hong Q., Jiang J., et al. Atomic structures of Coxsackievirus A6 and its complex with a neutralizing antibody. Nat. Commun. 2017;8:505. doi: 10.1038/s41467-017-00477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang L., Mao Q., Li S., Gao F., Zhao H., Liu Y., Wan J., Ye X., Xia N., Cheng T., et al. A neonatal mouse model for the evaluation of antibodies and vaccines against coxsackievirus A6. Antiviral Res. 2016;134:50–57. doi: 10.1016/j.antiviral.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 40.Li W., Joshi M.D., Singhania S., Ramsey K.H., Murthy A.K. Peptide vaccine: Progress and challenges. Vaccines. 2014;2:515–536. doi: 10.3390/vaccines2030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tirado S.M., Yoon K.J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- 42.Han J.F., Cao R.Y., Deng Y.Q., Tian X., Jiang T., Qin E.D., Qin C.F. Antibody dependent enhancement infection of enterovirus 71 in vitro and in vivo. Virol. J. 2011;8:106. doi: 10.1186/1743-422X-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S.M., Chen I.C., Su L.Y., Huang K.J., Lei H.Y., Liu C.C. Enterovirus 71 infection of monocytes with antibody-dependent enhancement. Clin. Vaccine Immunol. 2010;17:1517–1523. doi: 10.1128/CVI.00108-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen I.C., Wang S.M., Yu C.K., Liu C.C. Subneutralizing antibodies to enterovirus 71 induce antibody-dependent enhancement of infection in newborn mice. Med. Microbiol. Immunol. 2013;202:259–265. doi: 10.1007/s00430-013-0289-y. [DOI] [PubMed] [Google Scholar]
- 45.Cao R.Y., Dong D.Y., Liu R.J., Han J.F., Wang G.C., Zhao H., Li X.F., Deng Y.Q., Zhu S.Y., Wang X.Y., et al. Human IgG subclasses against enterovirus Type 71: Neutralization versus antibody dependent enhancement of infection. PLoS ONE. 2013;8:e64024. doi: 10.1371/journal.pone.0064024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foo D.G., Alonso S., Chow V.T., Poh C.L. Passive protection against lethal enterovirus 71 infection in newborn mice by neutralizing antibodies elicited by a synthetic peptide. Microbes Infect. 2007;9:1299–1306. doi: 10.1016/j.micinf.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Tian X., Su X., Li X., Li H., Li T., Zhou Z., Zhong T., Zhou R. Protection against enterovirus 71 with neutralizing epitope incorporation within adenovirus type 3 hexon. PLoS ONE. 2012;7:e41381. doi: 10.1371/annotation/2da932d4-8654-45c8-bf83-7b3b09dbc146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y.X., Zhao H., Cao R.Y., Deng Y.Q., Han J.F., Zhu S.Y., Ma J., Liu L., Qin E.D., Qin C.F. Recombinant tandem multi-linear neutralizing epitopes of human enterovirus 71 elicited protective immunity in mice. Virol. J. 2014;11:79. doi: 10.1186/1743-422X-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oberste M.S., Penaranda S., Maher K., Pallansch M.A. Complete genome sequences of all members of the species Human enterovirus A. J. Gen. Virol. 2004;85:1597–1607. doi: 10.1099/vir.0.79789-0. [DOI] [PubMed] [Google Scholar]
- 50.Sun S., Jiang L., Liang Z., Mao Q., Su W., Zhang H., Li X., Jin J., Xu L., Zhao D., et al. Evaluation of monovalent and bivalent vaccines against lethal Enterovirus 71 and Coxsackievirus A16 infection in newborn mice. Hum. Vaccin. Immunother. 2014;10:2885–2895. doi: 10.4161/hv.29823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan C., Ye X., Ku Z., Kong L., Liu Q., Xu C., Cong Y., Huang Z. Beta-propiolactone inactivation of coxsackievirus A16 induces structural alteration and surface modification of viral capsids. J. Virol. 2017;91 doi: 10.1128/JVI.00038-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao H., Li H.Y., Han J.F., Deng Y.Q., Zhu S.Y., Li X.F., Yang H.Q., Li Y.X., Zhang Y., Qin E.D., et al. Novel recombinant chimeric virus-like particle is immunogenic and protective against both enterovirus 71 and coxsackievirus A16 in mice. Sci. Rep. 2015;5:7878. doi: 10.1038/srep07878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren J., Wang X., Zhu L., Hu Z., Gao Q., Yang P., Li X., Wang J., Shen X., Fry E.E., et al. Structures of coxsackievirus A16 capsids with native antigenicity: Implications for particle expansion, receptor binding, and immunogenicity. J. Virol. 2015;89:10500–10511. doi: 10.1128/JVI.01102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ren J., Wang X., Hu Z., Gao Q., Sun Y., Li X., Porta C., Walter T.S., Gilbert R.J., Zhao Y., et al. Picornavirus uncoating intermediate captured in atomic detail. Nat. Commun. 2013;4:1929. doi: 10.1038/ncomms2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lyu K., Wang G.C., He Y.L., Han J.F., Ye Q., Qin C.F., Chen R. Crystal structures of enterovirus 71 (EV71) recombinant virus particles provide insights into vaccine design. J. Biol. Chem. 2015;290:3198–3208. doi: 10.1074/jbc.M114.624536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyu K., He Y.L., Li H.Y., Chen R. Crystal structures of yeast-produced enterovirus 71 and enterovirus 71/coxsackievirus A16 chimeric virus-like particles provide the structural basis for novel vaccine design against hand-foot-and-mouth disease. J. Virol. 2015;89:6196–6208. doi: 10.1128/JVI.00422-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frietze K.M., Peabody D.S., Chackerian B. Engineering virus-like particles as vaccine platforms. Curr. Opin. Virol. 2016;18:44–49. doi: 10.1016/j.coviro.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salmons B., Lim P.Y., Djurup R., Cardosa J. Non-clinical safety assessment of repeated intramuscular administration of an EV-A71 VLP vaccine in rabbits. Vaccine. 2018;36:6623–6630. doi: 10.1016/j.vaccine.2018.09.062. [DOI] [PubMed] [Google Scholar]
- 59.Arita M., Nagata N., Iwata N., Ami Y., Suzaki Y., Mizuta K., Iwasaki T., Sata T., Wakita T., Shimizu H. An attenuated strain of enterovirus 71 belonging to genotype A showed a broad spectrum of antigenicity with attenuated neurovirulence in cynomolgus monkeys. J. Virol. 2007;81:9386–9395. doi: 10.1128/JVI.02856-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang G., Cao R.Y., Chen R., Mo L., Han J.F., Wang X., Xu X., Jiang T., Deng Y.Q., Lyu K., et al. Rational design of thermostable vaccines by engineered peptide-induced virus self-biomineralization under physiological conditions. Proc. Natl. Acad. Sci. USA. 2013;110:7619–7624. doi: 10.1073/pnas.1300233110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang W., Dai W., Zhang C., Zhou Y., Xiong P., Wang S., Ye X., Liu Q., Zhou D., Huang Z. A virus-like particle-based tetravalent vaccine for hand, foot, and mouth disease elicits broad and balanced protective immunity. Emerg. Microbes Infect. 2018;7:94. doi: 10.1038/s41426-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blokhina E.A., Kuprianov V.V., Stepanova L.A., Tsybalova L.M., Kiselev O.I., Ravin N.V., Skryabin K.G. A molecular assembly system for presentation of antigens on the surface of HBc virus-like particles. Virology. 2013;435:293–300. doi: 10.1016/j.virol.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 63.Kratz P.A., Böttcher B., Nassal M. Native display of complete foreign protein domains on the surface of hepatitis B virus capsids. Proc. Natl. Acad. Sci. USA. 1999;96:1915–1920. doi: 10.1073/pnas.96.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skamel C., Ploss M., Bottcher B., Stehle T., Wallich R., Simon M.M., Nassal M. Hepatitis B virus capsid-like particles can display the complete, dimeric outer surface protein C and stimulate production of protective antibody responses against Borrelia burgdorferi infection. J. Biol. Chem. 2006;281:17474–17481. doi: 10.1074/jbc.M513571200. [DOI] [PubMed] [Google Scholar]
- 65.Wu Y., Zhu R., Xu L., Li Y., Li S., Yu H., Li S., Zhu H., Cheng T., Xia N. A novel combined vaccine based on monochimeric VLP co-displaying multiple conserved epitopes against enterovirus 71 and varicella-zoster virus. Vaccine. 2017;35:2728–2735. doi: 10.1016/j.vaccine.2017.03.065. [DOI] [PubMed] [Google Scholar]
- 66.Huang K.Y., Lin J.J., Chiu C.H., Yang S., Tsao K.C., Huang Y.C., Lin T.Y. A Potent Virus-Specific Antibody-Secreting Cell Response to Acute Enterovirus 71 Infection in Children. J. Infect. Dis. 2015;212:808–817. doi: 10.1093/infdis/jiv094. [DOI] [PubMed] [Google Scholar]
- 67.Chang H.W., Liu C.C., Lin M.H., Ho H.M., Yang Y.T., Chow Y.H., Chong P., Sia C. Generation of murine monoclonal antibodies which cross-neutralize human enterovirus genogroup B isolates. J. Virol. Methods. 2011;173:189–195. doi: 10.1016/j.jviromet.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Tan S.H., Ong K.C., Perera D., Wong K.T. A monoclonal antibody to ameliorate central nervous system infection and improve survival in a murine model of human Enterovirus-A71 encephalomyelitis. Antiviral Res. 2016;132:196–203. doi: 10.1016/j.antiviral.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 69.Chang G.H., Luo Y.J., Wu X.Y., Si B.Y., Lin L., Zhu Q.Y. Monoclonal antibody induced with inactived EV71-Hn2 virus protects mice against lethal EV71-Hn2 virus infection. Virol. J. 2010;7:106. doi: 10.1186/1743-422X-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lim X.F., Jia Q., Khong W.X., Yan B., Premanand B., Alonso S., Chow V.T., Kwang J. Characterization of an isotype-dependent monoclonal antibody against linear neutralizing epitope effective for prophylaxis of enterovirus 71 infection. PLoS ONE. 2012;7:e29751. doi: 10.1371/journal.pone.0029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deng Y.Q., Ma J., Xu L.J., Li Y.X., Zhao H., Han J.F., Tao J., Li X.F., Zhu S.Y., Qin E.D., et al. Generation and characterization of a protective mouse monoclonal antibody against human enterovirus 71. Appl. Microbiol. Biotechnol. 2015;99:7663–7671. doi: 10.1007/s00253-015-6652-8. [DOI] [PubMed] [Google Scholar]
- 72.Lee H., Cifuente J.O., Ashley R.E., Conway J.F., Makhov A.M., Tano Y., Shimizu H., Nishimura Y., Hafenstein S. A strain-specific epitope of enterovirus 71 identified by cryo-electron microscopy of the complex with fab from neutralizing antibody. J. Virol. 2013;87:11363–11370. doi: 10.1128/JVI.01926-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin T., Xianyu L., Lyu S. Monoclonal neutralizing antibodies against EV71 screened from mice immunized with yeast-produced virus-like particles. Virol. Sin. 2015;30:208–213. doi: 10.1007/s12250-015-3573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X., Mao C., Ma S., Wang X., Sun Z., Yi Y., Guo M., Shen X., Sun L., Bi S. Generation of neutralizing monoclonal antibodies against Enterovirus 71 using synthetic peptides. Biochem. Biophys. Res. Commun. 2009;390:1126–1128. doi: 10.1016/j.bbrc.2009.09.103. [DOI] [PubMed] [Google Scholar]
- 75.Ng Q., He F., Kwang J. Recent Progress towards Novel EV71 Anti-Therapeutics and Vaccines. Viruses. 2015;7:6441–6457. doi: 10.3390/v7122949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Almagro J., Fransson J. Humanization of antibodies. Front. Biosci. 2008;13:1619–1633. doi: 10.2741/2786. [DOI] [PubMed] [Google Scholar]
- 77.Wong Y.H., Kumar A., Liew C.W., Tharakaraman K., Srinivasaraghavan K., Sasisekharan R., Verma C., Lescar J. Molecular basis for dengue virus broad cross-neutralization by humanized monoclonal antibody 513. Sci. Rep. 2018;8:8449. doi: 10.1038/s41598-018-26800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan Y., Ng Q., Jia Q., Kwang J., He F. A Novel Humanized Antibody Neutralizes H5N1 Influenza Virus via Two Different Mechanisms. J. Virol. 2015;89:3712–3722. doi: 10.1128/JVI.03014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laursen N.S., Friesen R.H.E., Zhu X., Jongeneelen M., Blokland S., Vermond J., van Eijgen A., Tang C., van Diepen H., Obmolova G., et al. Universal protection against influenza infection by a multidomain antibody to influenza hemagglutinin. Science. 2018;362:598–602. doi: 10.1126/science.aaq0620. [DOI] [PMC free article] [PubMed] [Google Scholar]