Abstract
A new series of pyrazole 4–7 and pyrazolo[1,5-a]pyrimidine 8–13 were synthesized by using a simple, efficient procedure, and screened for their in-vitro antimicrobial and antitumor activities. Symmetrical and asymmetrical 3,6-diarylazo-2,5,7-triaminopyrazolo[1,5-a]pyrimidine were synthesized by the conventional method and also subjected to microwave irradiation and under ultrasound conditions. The biological results revealed that most of the tested compounds proved to be active as antibacterial and antifungal agents. The antitumor activity of the synthesized compounds was evaluated against human cancer cell lines, MCF-7, HCT-116, and HepG-2, as compared with Doxorubicin as a control.
Keywords: pyrazole; 3,5-diaminopyrazole; pyrazolo[1,5-a]pyrimidine; microwave; antimicrobial; antitumor activities
1. Introduction
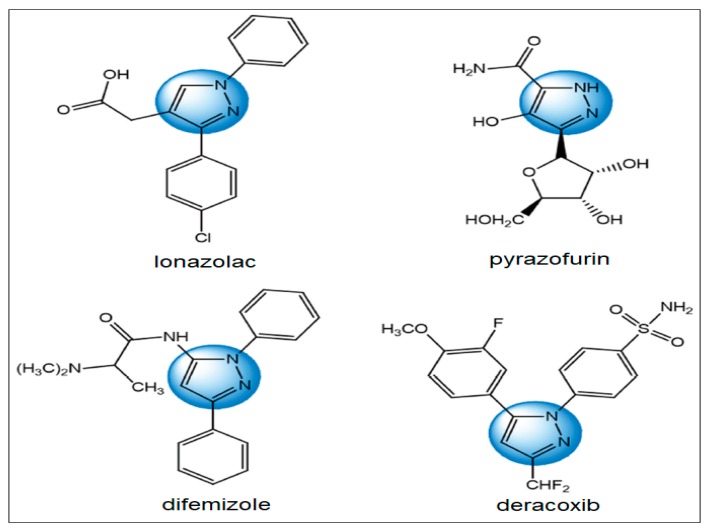
Pyrazole, a heterocyclic five-membered ring system, is one of the most significant heterogeneous compounds. Pyrazole derivatives have been found to possess a broad spectrum of biological properties including antitumor activity [1,2]. Pyrazoles have attracted much attention from researchers due to their high antibacterial and antifungal values [3]. In addition, pyrazoles exhibit anti-inflammatory [4], anti-HIV [5], antiviral [6], anti-diabetic [7], and anti-tubercular activities [8]. It gained great attention since the privileged structure was commonly found as an active constituent in commercial drugs (Figure 1), such as lonazolac nonsteroidal anti-inflammatory drug (NSAID), pyrazofurin (anticancer), difenamizole (analgesic), and deracoxib (NSAID). A great deal of interest has been paid to pyrazole derivatives in the last few years in agrochemical and chemical industries [9], as some pyrazole derivatives have exhibited insecticidal [10] and herbicidal activities [11]. The great importance of pyrazole is due to its ability to construct fused pyrazole compounds that have been used as intermediates for the synthesis of dyestuffs mainly for heterocyclic azo pyrazole disperse dyes [12]. Also, it has gained considerable attention from researchers in the past few decades since it is a major component in some marketing drugs such as Ocinaplon and Lorediplon [12]. Based on the above facts, the purpose of this paper is to design and synthesize new compounds containing pyrazole moieties and study their antimicrobial activity.
Figure 1.
Several commercial drugs containing pyrazole ring.
2. Results and Discussion
2.1. Chemistry
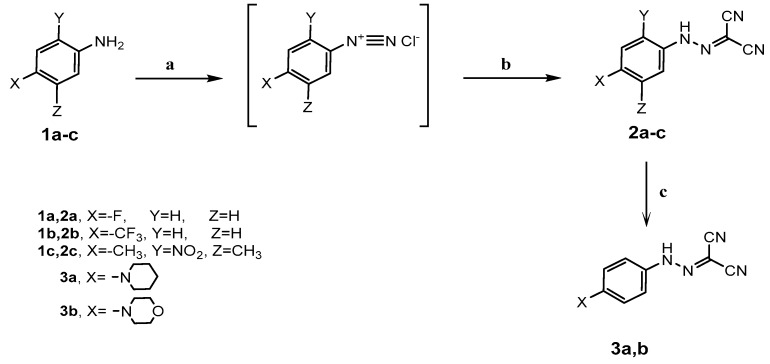
2-Arylazomalononitriles 2a–c were synthesized by diazotization of aniline derivatives 1a–c followed by coupling with malononitrile according to the reported method [13] (Scheme 1). The structure of 2-[4-(trifluoromethyl) phenylazo]malononitrile (2b) was established by analytical and spectral data. The IR spectrum of (2b) revealed the presence of absorption bands at 3141 and 2227 cm−1 characteristic for NH and CN groups, respectively. The 1H-NMR spectrum revealed two doublets at δ 7.64 and 7.76 ppm assignable to the aromatic protons and a broad signal at δ 10.36 ppm indicating the presence of the NH2 group, please find more detailed data in the Supplementary Materials.
Scheme 1.
Synthetic route for 2-arylazomalononitrile derivatives 2a–c and 3a,b. Reagents and Conditions: (a) NaNO2/HCl/stirring at 0–5 °C 1 h; (b) CH2(CN)2/CH3COONa/50% eq. EtOH/stirring at 0–5 °C 2 h; (c) 2a/piperidine or morpholine/EtOH/reflux 1 h.
Refluxing of arylazomalononitrile 2a with piperidine or morpholine in ethanol afforded the amino analogs 3a,b (Scheme 1). The structures for 3a,b were confirmed by spectral data. IR spectra of compounds 3a,b showed the appearance of NH at 3301–3303 cm−1 and CN stretch at 2179–2184 cm−1. The 1H and 13C-NMR spectrum of compound 3b revealed the presence of two characteristic signals aliphatic groups N-(CH2)2 and O-(CH2)2 of morpholine moiety.
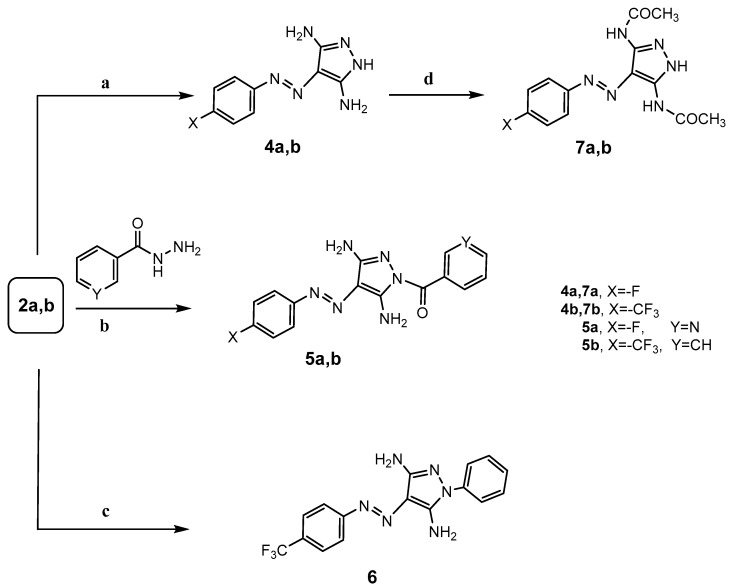
Several publications in recent years reported that various pyrazole derivatives were afforded by the reaction of arylazomalononitriles with different hydrazines [14]. The reactivity of the arylazomalononitriles 2a,b with hydrazine hydrate, phenylhydrazine, and acid hydrazide derivatives were investigated. Thus, treatment of arylazomalononitriles 2a,b with hydrazine hydrate or hydrazide derivatives—namely, nicotinohydrazide or benzohydrazide in ethanol—provided the corresponding 3,5-diaminopyrazole derivatives 4a,b or N-substituted diaminopyrazole derivatives 5a,b, respectively (Scheme 2). Furthermore, condensation of 2-[4-(trifluoromethyl)phenylazo]malononitrile (2b) with phenylhydrazine in refluxing ethanol furnished the corresponding 3,5-diamino-N-phenyl-4-[4-(trifluoromethyl)phenylazo]-1H-pyrazole (6) (Scheme 2). Spectral data confirmed the assignment of 3,5-diamino-4-[4-(trifluoromethyl)phenylazo]-1H-pyrazole (4b). The IR spectrum shows the absence of the characteristic absorption band of CN and the appearance of absorption bands at 3465, 3389, and 3296 corresponding to NH2 groups. Its 1H-NMR revealed the presence of NH2 signal at δ 6.29 ppm, a pair of doublets at δ 7.71 and 7.83 ppm corresponding to aromatic protons, and NH signal at δ 10.87 ppm. Also, the mass spectrum showed a molecular ion peak at m/z 270 (M+, 100%) which confirmed its molecular formula C10H9F3N6.
Scheme 2.
Synthetic route for 3,5-diaminopyrazole derivatives 4–7. Reagents and Conditions: (a) N2H4·H2O/EtOH-pyridine/reflux 2 h; (b) EtOH-pyridine/reflux 15 h; (c) 2b/PhNHNH2/EtOH-pyridine/reflux 3 h; (d) AcOH/reflux 10 h.
Acetylation of 3,5-diamino-1H-pyrazole derivatives 4a,b in refluxing acetic acid afforded the corresponding 4-arylazo-3,5-diacetamidopyrazole derivatives 7a,b; (Scheme 2). The structures of 1H-pyrazole derivatives 7a,b were supported by elemental and spectral data. The 1H-NMR spectrum for compound 7a exhibited two singlet signals at δ 2.12 and 2.17 ppm corresponding to the presence of methyl protons and two broad signals at δ 6.56 and 10.27 ppm related to two (NH) protons, while its mass spectrum revealed a molecular ion peak at m/z 304 (M+, 100%).
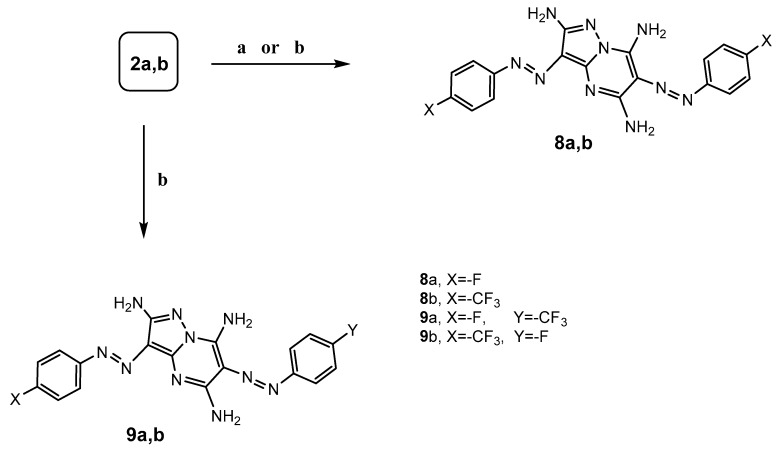
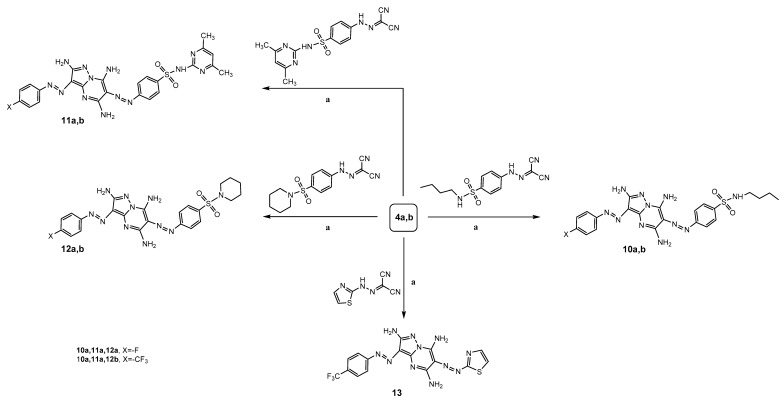
3,5-Diaminopyrazoles 4 was employed as a key intermediate for the synthesis of several poly substituted fused pyrazolopyrimidines. Pyrazolo[1,5-a]pyrimidines are of considerable pharmacological important as purine analogs [15,16,17]. Thus, it was important to study the reactivity of diaminopyrazole derivatives 4a,b toward a variety of nucleophilic reagents. Symmetrical 3,6-diarylazo-2,5,7-triaminopyrazolo[1,5-a]pyrimidine (8a,b) were synthesized by the reaction of hydrazone 2a,b [13,18] with hydrazine hydrate in the molar ratio 2:1 in ethanol containing 0.5 mL of pyridine under reflux for 4–5 h, or via the reaction of 3,5-diaminopyrazole 4a,b with the equivalent from the hydrazone derivatives 2a,b (Scheme 3). Asymmetrical 2,5,7-triaminopyrazolo[1,5-a]pyrimidine 9a,b were synthesized by the cyclization of diaminopyrazole 4a,b with equimolar of different hydrazone derivatives 2a,b in ethanol containing 0.5 mL of pyridine under reflux for 6–7 h (Scheme 3).
Scheme 3.
Synthetic route for symmetrical and asymmetrical 3,6-diarylazo-pyrazolo[1,5-a] pyrimidine derivatives 8a,b and 9a,b. Reagents and Conditions: (a) 2-arylazomalononitrile 2a,b: N2H4·H2O (2:1)/EtOH-pyridine/reflux 5 h; (b) 2-arylazomalononitrile 2a,b: 5-diaminopyrazoles 4a,b (1:1)/EtOH-pyridine/reflux 4–7 h or heated under MW at 140 °C 2 min or sonicated at room temperature 1 h.
Moreover, the synthesis of pyrazolopyrimidine derivatives 8a,b and 9a,b were achieved under microwave irradiation and ultrasound reactions conditions. TLC monitored the progress of the reactions, and the yields of the products were noted. Comparisons between conventional, microwave, and ultrasound techniques are given in (Table 1).
Table 1.
Comparison between methods for the synthesis of compounds 8a,b and 9a,b.
| Compound No. | Reflux | MW | US | |||
|---|---|---|---|---|---|---|
| Time | Yield% | Time | Yield% | Time | Yield% | |
| 8a | 300 min. | 62 | 2 min. | 74 | 60 min. | 52 |
| 8b | 240 min. | 60 | 2 min. | 71 | 60 min. | 40 |
| 9a | 360 min. | 63 | 2 min. | 76 | 60 min. | 60 |
| 9b | 420 min. | 67 | 2 min. | 78 | 60 min. | 43 |
The analytical and spectral data of the newly synthesized compounds 8a,b and 9a,b were compatible with their structures. For instance, IR spectrum for 6-[(4-trifluoromethyl)phenylazo]-3-(4-fluorophenylazo)-2,5,7-triaminopyrazolo[1,5-a] pyrimidine (9a) showed absorption bands at 3474, 3276, and 3123 cm−1, representing the presence of the amino groups, while 1H-NMR spectrum showed signals at δ 6.98 and 8.22 ppm referring to two amino groups, and its mass spectrum showed a molecular ion peak at m/z 458 (M+, 100%), which corresponds with its molecular formula C19H14F4N10.
Sulfonamide group can be considered as the main component in some drugs used in clinical treatment. It is reported that many of the sulfonamide derivatives act as antibacterial, antifungal, antihypertensive, anticancer, antimalarial, and antiprotozoal agents [13]. Thus, the reaction of 3,5-diaminopyrazole 4a,b with several hydrazones of sulfa drugs (N-[4-(N-butylsulfamoyl)phenyl]carbonohydrazonoyl dicyanide, N-{4-[N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl}carbono-hydrazonoyl dicyanide, and N-[4-(piperidin-1-ylsulfonyl)phenyl]carbono-hydrazonoyl dicyanide) in ethanol under reflux in the presence of a catalytic amount of pyridine afforded pyrazolo[1,5-a]pyrimidine derivatives 10a,b–12a,b, respectively (Scheme 4). Aiming to modify the yields and reduce the reactions time, we applied microwave irradiation conditions to resynthesize the targeted compounds 10a,b–12a,b, and the comparison of the reactions times and yields between the conventional and microwave irradiation conditions are summarized in Table 2.
Scheme 4.
Synthetic route for 2,5,7-triaminopyrazolo[1,5-a]pyrimidine derivatives 10–13. Reagents and Conditions: (a) EtOH-pyridine/reflux 7–12 h or heated under MW at 140 °C 3–8 min.
Table 2.
Comparison between methods for the synthesis of compounds 10a,b–13.
| Compound No. | Reflux | MW | ||
|---|---|---|---|---|
| Time | Yield% | Time | Yield% | |
| 10a | 540 min. | 70 | 5 min. | 76 |
| 10b | 480 min. | 78 | 5 min. | 84 |
| 11a | 360 min. | 88 | 3 min. | 92 |
| 11b | 420 min. | 72 | 3 min. | 78 |
| 12a | 420 min. | 73 | 3 min. | 80 |
| 12b | 420 min. | 75 | 7 min. | 81 |
| 13 | 720 min. | 69 | 8 min. | 78 |
The structures of 10a,b–12a,b were confirmed by their elemental analysis and spectral data (MS, IR, 1H-NMR, and 13C-NMR). The IR spectrum revealed an absorption band at 3474–3207 cm−1 that corresponds to NH and NH2 groups and at 2960–2835 and 1369–1347 cm−1 characteristic to aliphatic CH2 and SO2 groups, respectively. 1H-NMR spectrum of (12b), as an example, revealed signals at δ 1.36, 1.55, and 2.91 which refer to the presence of (3CH2) and (2NCH2) groups, respectively, while its 13C-NMR spectrum exhibited signals representing the presence of piperidine protons at δ 22.85, 24.66, and 46.58. Mass spectrum of (12b), as an example, gives the molecular ion peak at m/z 587 (M+; 44%), corresponding to the molecular formula C24H24FN11O2S.
Also, 6-(thiazol-2-yl-azo)-3-(4-(trifluoromethyl)phenylazo)-2,5,7-triamino pyrazolo [1,5-a]pyrimidine (13) was synthesized via the treatment of 3,5-diamine-4-[4-(trifluoromethyl)phenylazo]-1H-pyrazole (4b) with N-(thiazol-2-yl) carbonohydrazonoyl dicyanide in ethanol under reflux or microwave irradiation (Scheme 4).
1H-NMR spectrum of 13 showed singlet signals at δ 7.47 and 7.76 ppm due to the presence of two protons of thiazole ring, as well as signals at δ 7.11 and 8.24 ppm for amino groups. The mass spectrum revealed a molecular ion peak at 447 (M+; 100%), which confirms its molecular formula C16H12F3N11S.
2.2. Biological Evaluation
2.2.1. In Vitro Antimicrobial Evaluation
The newly synthesized compounds were evaluated for their antimicrobial activity toward six bacterial strains, three Gram-positive (Staphylococcus aureus, Bacillus subtilis, and Staphylococcus mutants), and three Gram-negative (Enterococcus faecalis, Proteus vulgaris, and Escherichia coli), using the standard antibiotic Gentamycin (5 mg/mL) as a reference. Also, their antifungal activity was evaluated against three fungal strains (Aspergillus fumigates, Aspergillus flavus, and Candida albicans) to determine the zone of inhibition using the standard antibiotic Ketoconazole (5 mg/mL). Their antibacterial activities were tested for their activities at a concentration of (5 mg/mL) using inhibition zone diameter in mm as a criterion for the antimicrobial activity [19,20], and the results are shown in (Table 3).
Table 3.
Inhibition zone in (mm) as a criterion of antimicrobial activity of some newly synthesized compounds.
| Microorganism Inhibition Zone Diameter (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound No. | Gram +ve Bacteria | Gram -ve Bacteria | Fungi | ||||||
| Staphylococcus aureus | Bacillus subtilis | Strepotococcus mutants | Enterococcus faecalis | proteus vulgaris | Escherichia coli | Aspergillus fumigates | Aspergillus flavus | Candida Albicans | |
| 2a | 25 | 40 | 22 | 35 | 40 | 28 | 24 | 20 | 21 |
| 2c | 22 | 24 | 18 | 25 | 26 | 14 | NA | NA | NA |
| 4b | 23 | 23 | 24 | 22 | 23 | 20 | 20 | 13 | 15 |
| 5a | 12 | 8 | NA | 11 | 8 | 12 | NA | NA | NA |
| 5b | 18 | 25 | 20 | 22 | 28 | 20 | 15 | 10 | 16 |
| 7a | 14 | 16 | 14 | 13 | 20 | 16 | 15 | 9 | 11 |
| 7b | 15 | 17 | 19 | 18 | 16 | 13 | NA | NA | NA |
| 8a | NA | NA | NA | NA | NA | 10 | NA | NA | NA |
| 8b | NA | 8 | NA | NA | NA | NA | NA | NA | NA |
| 9a | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 9b | 10 | NA | NA | NA | NA | NA | NA | NA | NA |
| 10a | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 10b | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 11a | 18 | 12 | 9 | 13 | 9 | 11 | NA | NA | NA |
| 11b | 22 | 23 | 29 | 25 | 26 | 13 | 20 | 17 | 15 |
| 12a | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 12b | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 13 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Gentamycin | 24 | 26 | 20 | 26 | 25 | 30 | - | - | - |
| Ketoconazole | - | - | - | - | - | - | 17 | 16 | 20 |
NA = not active; Diameter of hole = 6 mm; data are expressed in the form of mean ± SD.
Compounds 2a, 2c, and 11b demonstrated more potent significant activity against tested Enterococcus faecalis and Proteus vulgaris with a diameter of inhibition zone between 25 and 40 mm; further, compound 2a showed high antimicrobial activity on Bacillus subtilis with a zone of inhibition of 40 mm, and these compounds had moderate antimicrobial activity on other bacterial strains with an inhibition zone ranging from 13 to 25 mm. Moreover, compound 2a has significant antifungal activity towards three fungal strains, and compound 11b showed a moderate effect on fungal strains, while compound 2c was not active on fungal strains. Also, compound 4b had a moderate effect on bacterial strains with an inhibition zone of 20–24 mm diameter, as well as on fungal strains with an inhibitory region ranging from 13 to 20 mm.
Furthermore, compounds 5a, 7a, 7b, and 11a showed mild antibacterial activity on the tested Gram-positive and Gram-negative bacterial strains with an inhibition zone of 9 to 18 mm, except compound 5a was NA on Streptococcus mutants strain. Meanwhile, compounds 5a, 7b, and 11a had no antifungal activity towards the three fungal strains.
Compounds 8a, 8b, 9a, 9b, 10a, 10b, 12a, 12b, and 13 were NA on all evaluated bacterial and fungal strains, except for compounds 8a, 8b, and 9b, which exhibited low antibacterial activity on Escherichia coli (10 mm), Bacillus subtilis (8 mm), and Staphylococcus aureus (10 mm), respectively.
2.2.2. In Vitro Cytotoxic Screening
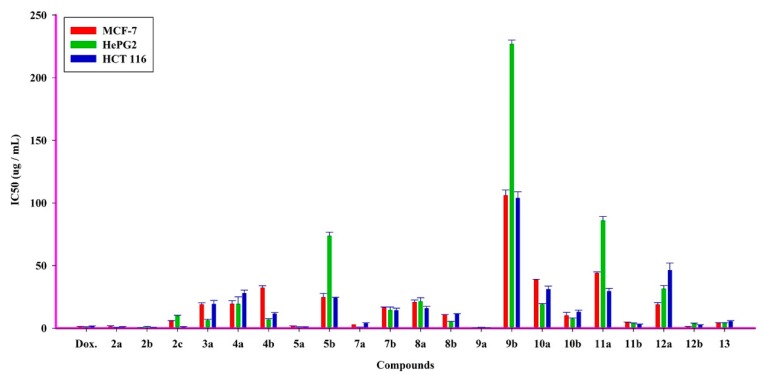
The antitumor activity of the target compounds was screened against three human cancer cell lines—breast adenocarcinoma (MCF-7), hepatocellular carcinoma (HepG2), and human colon carcinoma (HCT-116). The IC50 values of the tested compounds are listed in Table 4 and Figure 2.
Table 4.
The IC50 (µg) of the new tested chemicals compounds against different tumor cell lines.
| Compound | IC50 (μg/mL) | ||
|---|---|---|---|
| MCF-7 | HepG2 | HCT-116 | |
| 2a | 1.3 ± 0.7 | 0.5 ± 0.2 | 1.2 ± 0.1 |
| 2b | 0.5 ± 0.04 | 1.1 ± 0.3 | 0.5 ± 0.2 |
| 2c | 5.9 ± 0.2 | 9.8 ± 0.5 | 1.2 ± 0.12 |
| 3a | 18.8 ± 1.5 | 6.1 ± 1.1 | 19.1 ± 3.1 |
| 3b | 19.4 ± 2.6 | 19.3 ± 5.7 | 27.8 ± 2.6 |
| 4a | 32.1 ± 1.8 | 6.7 ± 0.9 | 11.5 ± 1.1 |
| 4b | 1.7 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.08 |
| 5a | 24.6 ± 3.2 | 73.6 ± 3.1 | 24.1 ± 0.7 |
| 5b | 2.8 ± 0.7 | 0.9 ± 0.1 | 3.7 ± 0.7 |
| 7a | 16.3 ± 0.6 | 14.3 ± 2.6 | 14.1 ± 1.9 |
| 7b | 20.8 ± 1.7 | 21.3 ± 3.1 | 15.7 ± 1.7 |
| 8a | 10.7 ± 0.3 | 4.9 ± 0.6 | 11.1 ± 0.5 |
| 8b | 0.3 ± 0.01 | 0.6 ± 0.2 | 0.4 ± 0.02 |
| 9a | 106 ± 4.3 | 226.9 ± 3.2 | 103.8 ± 5.1 |
| 9b | 38.5 ± 0.5 | 18.9 ± 0.8 | 31.1 ± 2.5 |
| 10a | 10.1 ± 2.6 | 7.5 ± 0.7 | 12.9 ± 1.6 |
| 10b | 43.9 ± 1.1 | 85.9 ± 3.4 | 29.4 ± 2.4 |
| 11a | 19.8 ± 1.9 | 26.6 ± 4.4 | 25.9 ± 1.7 |
| 11b | 4.5 ± 0.4 | 3.9 ± 0.4 | 2.7 ± 0.6 |
| 12a | 18.8 ± 1.7 | 31.4 ± 2.7 | 46.1 ± 5.9 |
| 12b | 1.4 ± 0.03 | 3.4 ± 0.6 | 2.4 ± 0.4 |
| 13 | 4.2 ± 0.2 | 4.1 ± 0.3 | 5.3 ± 0.8 |
| Doxorubicin | 1.2 ± 0.2 | 0.9 ± 0.3 | 1.6 ± 0.2 |
Figure 2.
The differences of IC50 % of new tested chemicals compounds effects on different tumor cells (MCF-7, HePG2, and HCT 116 cells).
From the obtained results, compounds 2a,b, 4b, 5b, 8b, and 12b showed the most potent cytotoxic profile on cancer cells (MCF-7, HepG2, and HCT-116) with IC50 values ranging from 0.3 to 3.4 µg while the compound 8b is most effective among all of them on all types of cancer cells.
Compounds 2c, 11b, and 13 have demonstrated a significant effect on MCF-7 and HePG2 cells with IC50 values ranging from 2.7 to 9.8 µg, and 2c and 11b compounds significantly increased on HCT 116 with IC50 of 1.2 μg and 2.4, respectively. Moreover, the compounds 4a, 8a, and 10a showed moderate effects on cancer cells with IC50 values ranging from 6.7 to 12.9 µg, while the compound 8a was the most significant on HePG2 cells with IC50 of 4.9 µg; on the other hand, the effect of the 4a compound was low on MCF-7 cells. In addition, the compounds 3a,b, 5a, 7a,b, 9b, 11a, and 12a had slightly significant effects with IC50 values ranging from 14.1 to 38.5 µg, the compound 3a indicated a slightly noticeable effect on HePG2 cells with IC50 of 6.1 µg, and the compound 12a had weak toxicity on HCT 116 cells with IC50 of 46.1 µg. Furthermore, the 10b compound had a low cytotoxic effect on MCF-7 and HePG2 cells with IC50 of 43.9 and 85.9 µg, respectively, and a moderate effect with IC50 of 29.4 µg. Compound 9a showed very low cellular toxicity with IC50 higher than 100 μg compared to all other compounds on cancer cells.
3. Materials and Methods
3.1. General Information
All melting points were determined with a Stuart Digital Melting Point apparatus SMP10 and are uncorrected. Elemental analyses were performed on a Perkin-Elmer 240 microanalyser, PE 2400 Series II CHNS/O Analyzer, carried out at the regional center for mycology and biotechnology, Al-Azhar University, cairo, Egypt. IR spectra were determined as KBr pellets on a Thermo Nicolet apparatus (Thermo Scientific, Madison, WI, USA) at Postgraduate campus for Girls at Lassan, King Khalid University, Abha, Saudi Arabia. The NMR spectra were recorded on a Bruker NMR spectrometer (Bruker, Billerica, MA, USA) in DMSO-d6, as solvent at 300, 500 MHz for 1H-NMR and 75, 125 MHz for 13C-NMR at Faculty of Science, Cairo University, Egypt and King Khalid University, Abha, respectively. The chemical shifts (δ) are reported in parts per million (ppm). Mass spectra were measured on GC/MS-QP5 spectrometer at regional center for mycology and biotechnology, Al-Azhar University, Egypt. Antimicrobial activity was measured at the regional center for mycology and biotechnology, Al-Azhar University, Egypt. Follow-up of the reactions and checking the purity of the compounds were carried out using TLC on silica gel-precoated aluminum sheets (Fluorescent indicator 254 nm, Fluka, Germany) and the spots were detected by exposure to UV lamp at λ 254/366 nm for a few seconds or under iodine vapor. Compounds 2-(4-fluorphenylazo)malononitrile 2a and 3,5-diamino-4-(4-fluorophenylazo)-1H-pyrazole 4b were prepared according to the reported procedure [21,22,23].
3.1.1. General Procedure for the Synthesis of 2-Arylazomalononitrile 2a–c:
To a solution of aniline derivatives 1a–c (0.01 mol) in hydrochloric acid (6 mL), a solution of sodium nitrite (0.72 g, 0.0105 mol) in water (3 mL) was added portion wise with stirring at 0–5 °C for 1 h. The clear diazonium salt was added to a stirred solution of malononitrile (0.66 g, 0.01 mol) and sodium acetate (4.6 g) in aqueous ethanol 50% (50 mL) with continued stirring at 0–5 °C for 2 h. The reaction mixture was allowed to stand overnight at room temperature, then the precipitate that formed was filtered off, washed several times with water, dried, and recrystallized from ethanol to afford 2-arylazomalononitrile 2a–c.
2-[4-(Trifluoromethyl)phenylazo]malononitrile (2b): Yield: 93%; (orange powder): mp 179–180 °C; IR (KBr) νmax in cm−1: 3141 (NH), 2227 (C≡N), 1618 (C=N) 1H-NMR (500 MHz, DMSO-d6): δ 7.64 (d, 2H, Ar–H, J = 6.9 Hz), 7.76 (d, 2H, Ar–H, J = 7.1 Hz), 10.36 (s, br, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ 86.98 (C(CN)2), 109.52 (C≡N), 113.88, 116.69 (Ar–C), 123.03, 125.19 (q, C–F3, J = 270 Hz), 126.72, 127.35, 144.52 (Ar–C); MS (m/z), 239 (M+ + 1; 6%), 238 (M+; 10%), 94 (95%), 67 (100%). Anal. Calcd. for C10H5F3N4 (238.17): C, 50.43; H, 2.12; N, 23.52%. Found C, 50.47; H, 2.34; N, 23.64%.
2-(4,5-Dimethyl-2-nitrophenylazo)malononitrile (2c): Yield: 45%; (yellow powder): mp 160–162 °C; IR (KBr) νmax in cm−1: 3231 (NH), 2989, 2954 (C-H aliphatic), 2215 (C≡N), 1617 (C=N); 1H-NMR (500 MHz, DMSO-d6): δ 2.30, 2.36 (2s, 6H, 2CH3), 7.60 (s, 1H, Ar–H), 7.98 (s, 1H, Ar–H), 9.88 (s, br, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ 18.52, 19.69 (CH3), 89.05 (C(CN)2), 109.05 (C≡N), 113.34, 118.97, 125.61, 134.62, 135.11, 146.70 (Ar–C); MS (m/z), 244 (M+ + 1; 11%), 243 (M+; 25%), 61 (100%). Anal. Calcd. for C11H9N5O2 (243.23): C, 54.32; H, 3.73; N, 28.79%. Found C, 54.19; H, 3.59; N, 28.86%.
3.1.2. General Procedures for the Synthesis of 2-[(4-Substituted)arylazo] malononitrile 3a,b:
A mixture of 2-arylazomalononitrile 2a (0.01 mol) and piperidine or morpholine (0.01 mol) in ethanol (30 mL) was refluxed for 1 h and then left to cool to room temperature. The reaction mixture was poured onto cooled water with continuous stirring. The precipitate that formed was filtered, dried, and recrystallized from toluene to afford 2-[(4-substituted)arylazo] malononitrile 3a,b.
2-[4-(Piperidino)phenylazo]malononitrile (3a): Yield: 81%; (yellow plates): mp 197–199 °C; IR (KBr) νmax in cm−1: 3301 (NH), 2942, 2856 (C-H aliphatic), 2184 (C≡N), 1621 (C=N); 1H-NMR (300 MHz, DMSO-d6): δ 1.64 (m, 6H, 3CH2), 3.55 (m, 4H, 2N–CH2), 7.13 (d, 2H, Ar–H, J = 6.9 Hz), 7.50 (m, 3H, 2H Ar–H + NH); 13C-NMR (75 MHz, DMSO-d6): δ 23.61, 25.62 (CH2), 49.31 (N–CH2), 92.58 (C(CN)2), 115.12, 115.41 (C≡N), 116.95, 121.75, 121.86 (Ar–C); MS (m/z), 253 (M+; 7%), 242 (88%), 72 (100%). Anal. Calcd. for C14H15N5 (253.31): C, 66.38; H, 5.97; N, 27.65%. Found C, 66.33; H, 5.80; N, 27.54%.
2-(4-Morpholinophenylazo)malononitrile (3b): Yield: 81%; (yellow crystals): mp 166–167 °C; IR (KBr) νmax in cm−1: 3303 (NH), 2934, 2861 (C–H aliphatic), 2179 (C≡N), 1618 (C=N); 1H-NMR (300 MHz, DMSO-d6): δ 3.60 (t, 4H, 2N–CH2), 3.70 (t, 4H, 2O–CH2), 7.15 (d, 2H, 2 Ar–H, J = 4.8 Hz), 7.51(d, 2H, 2 Ar–H, J = 5.4 Hz), 7.69 (s, 1H, NH); 13C-NMR (75 MHz, DMSO-d6): δ 48.75 (N–CH2), 65.99 (O–CH2), 92.64 (C(CN)2), 115.19, 115.48 (C≡N), 116.87, 121.91, 122.02 (Ar–C); MS (m/z), 255 (M+; 21%), 111 (56%), 43 (100%). Anal. Calcd. for C13H13N5O (255.28): C, 61.17; H, 5.13; N, 27.43%. Found C, 61.28; H, 5.22; N, 27.48%.
3.1.3. General Procedure for the Synthesis of 4-Arylazo-3,5-diaminopyrazole 4a,b
To a solution of 2-arylazomalononitrile 2a,b (0.01 mol) in ethanol (30 mL), hydrazine hydrate (0.01 mol) and pyridine (0.5 mL) were added. The reaction mixture was heated under reflux for 2 h (monitored by TLC). After completion of the reaction, the precipitate that formed was filtered off, dried, and recrystallized from ethanol to afford 3,5-diamino-4-(arylazo)pyrazole 4a,b.
3,5-Diamino-4-[4-(trifluoromethyl)phenylazo]-1H-pyrazole (4b): Yield: 98%; (orange crystals): mp 231–232 °C; IR (KBr) νmax in cm−1: 3465, 3389, 3296 (NH2,NH), 1622 (C=N), 1421 (N=N); 1H-NMR (500 MHz, DMSO-d6): δ 6.29 (br, 4H, 2NH2), 7.71 (d, 2H, Ar–H, J = 8.5 Hz), 7.83 (d, 2H, Ar–H, J = 8.0 Hz), 10.87 (br, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ 115.61, 120.62, 120.94 (Ar–C), 123.47, 125.63 (q, C–F3, J = 270 Hz), 125.80, 125.83, 127.79 (Ar–C), 156.37 (C=N); MS (m/z), 271 (M+ + 1; 14%), 270 (M+; 100%). Anal. Calcd. for C10H9F3N6 (270.22): C, 44.45; H, 3.36; N, 31.10%. Found C, 44.39; H, 3.23; N, 31.19%.
3.1.4. General Procedure for the Synthesis of 4-Arylazo-3,5-diamino-N-substitutedpyrazole 5a,b
To a solution of 2-arylazomalononitrile 2a,b (0.01 mol) in ethanol (30 mL), hydrazide derivatives, namely nicotinohydrazide or benzohydrazide (0.01 mol) and pyridine (0.5 mL), were added. The reaction mixture was heated under reflux for 15 h (monitored by TLC). After completion of the reaction, the precipitate that formed was filtered off, dried, and recrystallized from ethanol to afford N-substituted-3,5-diamino-4-(arylazo)pyrazole 5a,b.
{3,5-Diamino-4-[(4-fluorophenyl)azo]-1H-pyrazol-1-yl}(pyridin-3-yl)methanone (5a): Yield: 69%; (orange plates): mp >3 00 °C; IR (KBr) νmax in cm−1: 3477, 3274, 3127 (NH2), 1672 (C=O), 1593 (C=N), 1423 (N=N); 1H-NMR (500 MHz, DMSO-d6): δ 6.92 (s, 2H, NH2), 7.28–7.32 (m, 4H, Ar–H), 7.72–7.75 (m, 2H, Ar–H), 7.95–7.98 (m, 2H, Ar–H), 8.08 (br, s, 2H, NH2); 13C-NMR (125 MHz, DMSO-d6): δ 107.82, 112.32, 120.54, 121.24, 125.82, 125.89, 126.92, 127.16, 128.35, 129.02, 131.04 (Ar–C), 153.15, 157.93 (C=N), 160.52 (C–F), 166.38 (C=O); MS (m/z), 327 (M+ + 2; 3.3%), 325 (M+; 12%), 95 (100%). Anal. Calcd. for C15H12FN7O (325.31): C, 55.38; H, 3.72; N, 30.14%. Found C, 55.28; H, 3.68; N, 30.24%.
{3,5-Diamino-4-[(4-(trifluoromethyl)phenylazo]-1H-pyrazol-1-yl}(phenyl)methanone (5b): Yield: 71%; (orange powder): mp 284–286 °C; IR (KBr) νmax in cm−1: 3506, 3353, (NH2), 1689 (C=O), 1618 (C=N), 1411 (N=N); 1H-NMR (500 MHz, DMSO-d6): δ 7.02 (s, 2H, NH2), 7.67–7.73 (m, 4H, Ar–H), 7.77–7.86 (m, 7H, Ar–H+NH2); 13C-NMR (125 MHz, DMSO-d6): δ 107.46, 121.02, 121.16, 121.65 (Ar–C), 123.63, 125.81 (q, C–F3, J = 272.5 Hz), 126.15, 127.02, 127.68, 149.08 (Ar–C), 150.11 (C=N), 167.31 (C=O); MS (m/z), 375 (M+ + 1; 5%), 374 (M+; 12%), 145 (100%). Anal. Calcd. for C17H13F3N6O (374.33): C, 54.55; H, 3.50; N, 22.45%. Found C, 54.75; H, 3.58; N, 22.35%.
3.1.5. Synthesis of 3,5-Diamino-N-phenyl-4-[4-(trifluoro-methyl)phenylazo]-1H-pyrazole (6)
Phenylhydrazine (0.98 mL, 0.01 mol) and pyridine (0.5 mL) were added to a solution of 2-arylazomalononitrile 2b (2.7 g, 0.01 mol) in ethanol (30 mL). The reaction mixture was heated under reflux for 3 h (monitored by TLC). After completion of the reaction, the precipitate that formed was filtered off, dried, and recrystallized from ethanol to give 3,5-diamino-1-phenyl-4-[4-(trifluoro-methyl)phenylazo]-1H-pyrazole 6. Yield: 71%; (yellow crystals): mp >300 °C; IR (KBr) νmax in cm−1: 3400, 3304, 3197 (NH2, NH), 1616 (C=N), 1423 (N=N); 1H-NMR (500 MHz, DMSO-d6): δ 6.99 (br, s, 2H, NH2), 7.33 (d, 2H, Ar–H, J = 7.5 Hz), 7.50 (d, 2H, Ar–H, J = 8.0 Hz), 7.53–7.76 (m, 5H, Ar–H), 7.95 (br, s, 2H, NH2); 13C-NMR (125 MHz, DMSO-d6): δ 101.00, 121.07, 121.26, 122.26 (Ar–C), 123.42, 125.59, 125.95 (q, C–F3, J = 270 Hz), 126.16, 126.41, 129.27, 149.07 (Ar–C), 156.07 (C=N). Anal. Calcd. for C16H13F3N6 (346.32): C, 55.49; H, 3.78; N, 24.27%. Found C, 55.68; H, 3.91; N, 24.22%.
3.1.6. Synthesis of 4-Arylazo-3,5-diacetamido-1H-pyrazole 7a,b
A solution of 3,5-diamine-4-arylazo-1H-pyrazole 4a,b (0.01 mol) in acetic acid (30 mL) was heated under reflux for 10 h (monitored by TLC). The reaction mixture was left to cool to room temperature, and then poured onto cooled water (50 mL) portion wise with continuous stirring for 1 h. The separated yellow compound was filtered off, washed with water, and, finally, dried and recrystallized from ethanol to afford 3,5-diacetamio-4-arylazo-1H-pyrazole 7a,b.
3,5-Diacetamido-4-[(4-fluorophenyl)azo]-1H-pyrazole (7a): Yield: 86%; (yellow powder): mp 234–236 °C; IR (KBr) νmax in cm−1: 3277, 3144 (NH), 1698 (C=O), 1605 (C=N), 1418 (N=N); 1H-NMR (300 MHz, DMSO-d6): δ 2.12, 2.17 (2s, 6H, 2CH3), 6.56 (br, 1H, NH), 7.77–7.92 (m, 5H, Ar–H+NH), 10.27 (br, 1H, NH); 13C-NMR (75 MHz, DMSO-d6): δ 23.12 (CH3), 115.82, 116.08, 120.56, 122.74, 122.85, 123.68, 123.80 (Ar–C), 149.12 (C=N), 161.04 (C–F), 169.24 (2C=O): MS (m/z), 305 (M+ + 1; 28%), 304 (M+; 100%), 42 (76%). Anal. Calcd. for C13H13FN6O2 (304.29): C, 51.31; H, 4.31; N, 27.62%. Found C, 51.38; H, 4.41; N, 27.53%.
3,5-Diacetamido-4-[4-(trifluoromethyl)phenylazo]-1H-pyrazole (7b): Yield: 80%; (Yellow plates): mp 236–238 °C; IR (KBr) νmax in cm−1: 3238 (NH), 1678 (C=O), 1595 (C=N), 1426 (N=N); 1H-NMR (300 MHz, DMSO-d6): δ 2.24 (s, 6H, 2CH3), 7.82–7.96 (m, 5H, Ar–H+NH), 8.40, 10.11 (2br, s, 2H, 2NH); 13C-NMR (125 MHz, DMSO-d6): δ 23.38 (CH3), 100.33, (Ar–C), 123.10, 125.26 (q, C–F3, J = 270 Hz), 126.07, 126.24, 126.26, 128.75, 129.00 (Ar–C), 155.61 (C=N), 167.33 (C=O): MS (m/z), 354 (M+; 19%), 312 (72%), 70 (100%). Anal. Calcd. for C14H13F3N6O2 (354.29): C, 47.46; H, 3.70; N, 23.72%. Found C, 47.57; H, 3.79; N, 23.76%.
3.1.7. General Procedure for the Synthesis of 3,6-Diarylazo-2,5,7-triaminopyrazolo[1,5-a]pyrimidine derivatives 8a,b
A. Under reflux Condition:
Method A: A mixture of 2-arylazomalononitrile 2a,b (0.02 mol) and hydrazine hydrate (0.01 mol) in ethanol (30 mL) in the presence of pyridine (0.5 mL) was heated under reflux for 5 h, (monitored by TLC). After completion of the reaction, the precipitates those formed were filtered off, dried, and recrystallized from 1,4-dioxane to afford 8a,b.
Method B: A mixture of 4-arylazo-3,5-diaminopyrazole 4a,b (0.01 mol) and 2-arylazomalononitrile 2a,b (0.01 mol) in ethanol (30 mL) and in the presence of pyridine (0.5 mL) was heated under reflux for 4–5 h, (monitored by TLC). After completion of the reaction, the precipitates those formed were filtered off, dried, and recrystallized from 1,4-dioxane to afford 8a and 8b, respectively.
B. Under Microwave Irradiation:
A mixture of 5-diamino-4-arylazopyrazole 4a,b (0.01 mol) and 2-arylazomalononitrile 2a,b (0.01 mol) was grinded carefully in a porcelain mortar using a pestle and transferred to a pyrex test tube; then, ethanol (4 mL) was added, followed by pyridine (0.5 mL). The reaction mixture was heated under microwave irradiation at 50% power for 2 min at 140 °C, monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature and the precipitated solid was filtered off, washed with MeOH, and recrystallized from dioxane to afford 8a and 8b, respectively.
C-Under Ultrasound Condition:
To a solution of 4-arylazo-3,5-diaminopyrazole 4a,b (0.01 mol) in ethanol (30 mL), 2-arylazomalononitrile 2a,b (0.01 mol) and pyridine (0.5 mL) was added. The reaction mixture was sonicated for 1 h at room temperature, (monitored by TLC). After completion of the reaction, the precipitate product was filtered off, dried, and recrystallized from dioxane to afford 8a and 8b respectively.
3,6-Bis[(4-fluorophenyl)azo]-2,5,7-triaminopyrazolo[1,5-a]pyrimidine (8a): Yield: 74%; (orange plates): mp >300 °C; IR (KBr) νmax in cm−1: 3422, 3274 (NH2), 1615 (C=N), 1420 (N=N); 1H-NMR (500 MHz, DMSO-d6): δ 6.92 (s, 2H, NH2), 7.29–7.32 (m, 4H, Ar–H), 7.72–7.75 (m, 4H, Ar–H), 7.96 (s, br, 2H, NH2), 8.10 (s, 2H, NH2); 13C-NMR (125 MHz, DMSO-d6): δ 107.81, 115.11, 115.66, 115.70, 115.84, 115.86, 122.34, 122.40, 123.72, 123.79, 147.22, 149.08, 149.10 (Ar–C), 150.05, 152.31 (C=N), 160.52, 161.18 (C–F); MS (m/z), 409 (M+ + 1; 30%), 408 (M+; 100%), 363 (54%), Anal. Calcd. for C18H14F2N10 (408.38): C, 52.94; H, 3.46; N, 34.30%. Found C, 52.83; H, 3.52; N, 34.22%.
3,6-Bis[4-(trifluoromethyl)phenylazo]-2,5,7-triaminopyrazolo[1,5-a]pyrimidine (8b): Yield: 71%; (orange crystals): mp >300 °C; IR (KBr) νmax in cm−1: 3460, 3259, 3103 (NH2), 1602 (C=N), 1417 (N=N); 1H-NMR (500 MHz, DMSO-d6): δ 7.06 (s, 2H, NH2; exchangeable with D2O), 7.77–7.86 (m, 10H, Ar–H+NH2; exchangeable with D2O), 8.19 (s, 2H, NH2; exchangeable with D2O); 13C-NMR (125 MHz, DMSO-d6): δ 108.98, 116.57, 121.82, 122.25 (Ar–C), 123.19, 125.35 (q, C–F3, J = 287.5 Hz), 125.49, 125.99, 126.02, 126.17, 126.20, 126.83, 127.08, 127.86, 128.11, 148.05 (Ar–C), 152.38, 154.71 (C=N); MS (m/z), 509 (M+ + 1; 25%), 508 (M+; 100%). Anal. Calcd. for C20H14F6N10 (508.39): C, 47.25; H, 2.78; F, 22.42; N, 27.55%. Found C, 47.34; H, 2.71; N, 27.44%.
3.1.8. General Procedure for the Synthesis of 3,6-Diarylazo-2,5,7-triaminopyrazolo[1,5-a]pyrimidine Derivatives 9a,b
A. Under Reflux Condition:
To a solution of 4-arylazo-3,5-diaminopyrazole 4a,b (0.01 mol) in ethanol (30 mL), 2-arylazomalononitrile 2a,b (0.01 mol) and pyridine (0.5 mL) were added. The reaction mixture was heated under reflux for 6–7 h (monitored by TLC). After completion of the reaction, the formed precipitates were filtered off, dried, and recrystallized from ethanol to afford 9a,b.
B. Under Microwave Irradiation:
A mixture of 5-diamino-4-(arylazo)pyrazole 4a,b (0.01 mol) and 2-arylazomalononitrile 2a,b (0.01 mol) was grinded carefully in a porcelain mortar using a pestle and transferred to a pyrex test tube, and ethanol (4 mL) was added, followed by pyridine (0.5 mL). The reaction mixture was heated under microwave irradiation at 50% power for 2 min at 140 °C, monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature and the precipitated solid was filtered off, washed with MeOH, and recrystallized from ethanol to afford 9a,b.
C-Under Ultrasound Condition:
To 5-diamino-4-(arylazo)pyrazole 4a,b (0.01 mol) in ethanol (30 mL), 2-arylazomalononitrile 2a,b (0.01 mol) and pyridine (0.5 mL) was added. The reaction mixture was sonicated for 1 h at room temperature (monitored by TLC). After completion of the reaction, the precipitate was filtered off, dried, and recrystallized from ethanol to afford 9a,b.
6-[(4-Trifluoromethyl)phenylazo]-3-[(4-fluorophenyl)azo]-2,5,7-triaminopyrazolo [1,5-a]pyrimidine (9a): Yield: 76%; (orange plates): mp >300 °C; IR (KBr) νmax in cm−1: 3474, 3276, 3123 (NH2), 1616 (C=N), 1423 (N=N); 1H-NMR (500 MHz, DMSO-d6): δ 6.98 (s, 2H, NH2), 7.31–7.35 (m, 4H, Ar–H), 7.76–7.82 (m, 6H, Ar–H+NH2), 8.22 (s, 2H, NH2); MS (m/z), 408 (45%), 458 (M+; 100%), 459 (M+ + 1; 22%). Anal. Calcd. for C19H14F4N10 (458.13): C, 49.79; H, 3.08; N, 30.56. Found C, 49.65; H, 3.19; N, 30.45%.
3-[(4-Trifluoromethyl)phenylazo]-6-[(4-fluorophenyl)azo]-2,5,7-triaminopyrazolo [1,5-a]pyrimidine (9b): Yield: 78%; (orange plates): mp >300 °C; IR (KBr) νmax in cm−1: 3468, 3266, 3124 (NH2), 1594 (C=N), 1419 (N=N); 1H-NMR (500 MHz, DMSO-d6): δ 7.06 (s, 2H, NH2), 7.28–7.32 (m, 4H, Ar–H), 7.80 (d, 2H, Ar–H, J = 7.5 Hz), 8.10 (s, br, 2H, NH2), 8.13 (s, 2H, NH2), 7.84 (d, 2H, Ar–H, J = 7.5 Hz); 13C-NMR (125 MHz, DMSO-d6): δ 108.98, 116.48, 121.82, 122.25 (Ar–C), 123.19, 125.35 (q, C–F3, J = 270 Hz), 125.52, 125.99, 126.02, 126.17, 126.20, 126.90, 127.68, 128.11, 148.05 (Ar–C), 152.83, 154.90 (C=N), 161.27 (C–F). Anal. Calcd. for C19H14F4N10 (458.13): C, 49.79; H, 3.08; N, 30.56%. Found C, 49.50; H, 3.21; N, 30.46%.
3.1.9. General Procedure for the Synthesis of 3,6-Diarylazo-2,5,7-triaminopyrazolo[1,5-a] pyrimidine Derivatives 10a,b
A. Under reflux Condition:
To a solution of 4-arylazo-3,5-diaminopyrazole 4a,b (0.01 mol) in ethanol (30 mL), N-[4-(N-butylsulfamoyl)phenyl]carbonohydrazonoyl dicyanide (3.05 g, 0.01 mol) and pyridine (0.5 mL) was added. The reaction mixture was heated under reflux for 8–9 h (monitored by TLC). After completion of the reaction, the precipitated product that formed was filtered off, dried, and recrystallized from toluene to afford 3-arylazo-2,5,7-triaminopyrazolo[1,5-a] pyrimidine 10a,b.
B. Under Microwave Irradiation:
A mixture of 5-diamino-4-(arylazo)pyrazole 4a,b (0.01 mol) and N-[4-(N-butylsulfamoyl)phenyl]carbonohydrazonoyl dicyanide (3.05 g, 0.01 mol) was grinded carefully in a porcelain mortar using a pestle and transferred to a pyrex test tube, and ethanol (4 mL) was added, followed by pyridine (0.5 mL). The reaction mixture was heated under microwave irradiation at 50% power for 5 min at 140 °C, monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature and the precipitated solid was filtered off, washed with MeOH, and recrystallized from ethanol to afford 10a,b.
N-Butyl-4-{[2,5,7-triamino-3-(4-fluorophenylazo)pyrazolo[1,5-a]pyrimidin-6-yl]azo}benzenesulfonamide (10a): Yield: 76%; (orange plates): mp >300 °C; IR (KBr) νmax in cm−1 3474, 3279 (NH2), 2932, 2870 (C–H aliphatic), 1611 (C=N), 1420 (N=N), 1369 (SO2); 1H-NMR (500 MHz, DMSO-d6): δ 0.80 (t, 3H, CH3), 1.22–1.27 (m, 2H, CH2), 1.34–1.37 (m, 2H, CH2), 2.75–2.77 (t, 2H, CH2), 6.94 (s, 2H, NH2), 7.29–7.33 (m, 4H, Ar–H), 7.60 (br, s, 1H, NH), 7.74–7.76 (m, 4H, Ar–H), 7.83 (s, 2H, NH2), 8.18 (s, 2H, NH2); 13C-NMR (125 MHz, DMSO-d6): δ 13.43 (CH3), 19.19, 31.02 (CH2), 42.20 (N–CH2), 108.95, 115.28, 115.73, 115.91, 116.65, 120.81, 122.06, 122.43, 122.50, 123.91, 127.44, 127.68, 139.29, 147.51, 150.01 (Ar–C), 152.58, 154.48 (C=N), 160.63 (C–F); MS (m/z), 525 (M+; 11%), 526 (M+ + 1; 4%), 403 (100%). Anal. Calcd. for C22H24FN11O2S (525.57): C, 50.28; H, 4.60; N, 29.32; S, 6.10%. Found C, 50.39; H, 4.78; N, 29.39; S, 6.01%.
N-Butyl-4-{[2,5,7-triamino-3-((4-(trifluoromethyl)phenyl)azo)pyrazolo[1,5-a]pyrimidin-6-yl]azo}benzene-sulfonamide (10b): Yield: 84%; (reddish brown powder): mp 289–291 °C; IR (KBr) νmax in cm−1: 3446, 3315 (NH2) 2960, 2870 (C-H aliphatic), 1611 (C=N), 1412 (N=N), 1366 (SO2); 1H-NMR (500 MHz, DMSO-d6): δ 0.79 (t, 3H, CH3), 1.23–1.26 (m, 2H, CH2), 1.34–1.37 (m, 2H, CH2), 2.76–2.77 (t, 2H, CH2), 7.07 (s, 2H, NH2), 7.61 (br, s, 1H, NH), 7.82–7.89 (m, 8H, Ar–H), 8.21 (br, s, 4H, 2NH2); 13C-NMR (125 MHz, DMSO-d6): δ 13.40 (CH3), 19.19, 31.01 (2CH2), 42.19 (N–CH2), 109.06, 116.58, 120.72, 120.89, 121.03 (Ar–C), 123.32, 125.48 (q, C–F3, J = 270 Hz), 126.22, 126.85, 127.10, 127.45, 127.69, 139.47, 148.04 (Ar–C), 152.40, 154.41 (C=N); MS (m/z), 576 (M+ + 1; 3%), 575 (M+; 9%), 398 (100%). Anal. Calcd. for C23H24F3N11O2S (575.58): C, 48.00; H, 4.20; N, 26.77%. Found C, 48.24; H, 4.14; N, 26.86%.
3.1.10. General Procedure for the Synthesis of 3,6-Diarylazo-2,5,7-triaminopyrazolo[1,5-a] pyrimidine Derivatives 11a,b
A. Under Reflux Condition:
To a solution of 5-diamino-4-(arylazo)pyrazole 4a,b (0.01 mol) in ethanol (30 mL), N-{4-[N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl} carbonohydrazonoyl dicyanide (3.55 g, 0.01 mol) and pyridine (0.5 mL) were added. The reaction mixture was heated under reflux for 6–7 h, (monitored by TLC). After completion of the reaction, the precipitated product that formed was filtered off, dried, and recrystallized from toluene to afford 3-arylazo-2,5,7-triamino pyrazolo[1,5-a]pyrimidine 11a,b.
B. Under Microwave Irradiation:
A mixture of 5-diamino-4-(arylazo)pyrazole 4a,b (0.01 mol) and N-{4-[N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl}carbonohydrazonoyl dicyanide (3.55 g, 0.01 mol) was grinded carefully in a porcelain mortar using a pestle and transferred to a pyrex test tube, and ethanol (4 mL) was added, followed by pyridine (0.5 mL). The reaction mixture was heated under microwave irradiation at power 50% for 3 min at 140 °C, monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature and the precipitated solid was filtered off, washed with MeOH, and recrystallized from ethanol to afford 11a,b.
N-(4,6-Dimethylpyrimidin-2-yl)-4-{[2,5,7-triamino-3-(4-fluorophenylazo)pyrazolo [1,5-a]pyrimidin-6-yl]azo}benzenesulfonamide (11a): Yield: 92%; (reddish-brown powder): mp > 300 °C; IR (KBr) νmax in cm−1: 3403, 3307 (NH2), 2959 (C–H aliphatic), 1616 (C=N), 1421 (N=N), 1347 (SO2); 1H-NMR (500 MHz, DMSO-d6): δ 2.26 (s, 6H, 2CH3), 6.75 (s, 1H, CH–pyrimidine), 6.94 (s, 2H, NH2), 7.20–7.32 (m, 5H, Ar–H+NH), 7.73–7.76 (m, 4H, Ar–H), 8.03, 8.13 (2br, s, 4H, 2NH2); MS (m/z), 575 (M+; 41%), 408 (100%). Anal. Calcd. for C24H22FN13O2S (575.59): C, 50.08; H, 3.85; N, 31.64. Found C, 50.27; H, 3.97; N, 31.51%.
N-(4,6-Dimethylpyrimidin-2-yl)-4-{[2,5,7-triamino-3-(4-(trifluoromethyl) phenylazo)pyrazolo[1,5-a]pyrimidin-6-yl]azo}benzenesulfonamide (11b): Yield: 78%; (reddish orange powder): mp >300 °C; IR (KBr) νmax in cm−1: 3418, 3324 (NH2), 1600 (C=N), 1418 (N=N), 1355 (SO2); 1H-NMR (500 MHz, DMSO-d6): δ 2.26 (s, 6H, 2CH3), 6.75 (s, 1H, CH–pyrimidine), 7.07 (s, 2H, NH2), 7.80 (d, 2H, Ar–H, J = 8.5 Hz), 7.85 (d, 2H, Ar–H, J = 8.5 Hz), 8.04 (d, 2H, Ar–H, J = 7.5 Hz), 8.14 (d, 2H, ArvH, J = 7.5 Hz), 8.24 (br, 4H, 2NH2), 9.5 (br, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ 22.73 (CH3), 109.08, 113.25, 116.52, 120.70, 121.03, 121.36, 122.26 (Ar–C), 123.32, 125.48 (q, C–F3, J = 270 Hz), 126.20, 126.22, 126.86, 127.11, 127.64, 128.98, 139.73, 148.03 (Ar–C), 152.40, 154.50, 155.80, 156.08 (C=N). Anal. Calcd. C25H22F3N13O2S (625.60): C, 48.00; H, 3.54; N, 29.11. Found C, 48.18; H, 3.46; N, 29.08%.
3.1.11. General Procedure for the Synthesis of 3,6-Diarylazo-2,5,7-triaminopyrazolo[1,5-a]pyrimidine Derivatives 12a,b
A. Under Reflux Condition:
N-[4-(Piperidin-1-ylsulfonyl)phenyl]carbonhydrazonoyl dicyanide (3.17 g, 0.01 mol) and pyridine (0.5 mL) were added to a solution of 4-arylazo-3,5-diaminopyrazole 4a,b (0.01 mol) in ethanol (30 mL). The reaction mixture was heated under reflux for 7 h (monitored by TLC). After completion of the reaction, the precipitates which formed were filtered off, dried, and recrystallized from ethanol to afford 3-arylazo-2,5,7-triaminopyrazolo[1,5-a]pyrimidine 12a,b.
B. Under Microwave Irradiation:
A mixture of 4-arylazo-3,5-diaminopyrazole 4a,b (0.01 mol) and N-[4-(piperidin-1-ylsulfonyl)phenyl]carbonhydrazonoyl dicyanide (3.17 g, 0.01 mol) was grinded carefully in a porcelain mortar using a pestle and transferred to a pyrex test tube, and ethanol (4 mL) was added, followed by pyridine (0.5 mL). The reaction mixture was heated under microwave irradiation at power 50% for 3–7 min at 140 °C (monitored by TLC). After completion of the reaction, the reaction mixture was cooled to room temperature and the precipitated solid was filtered off, washed with MeOH, and recrystallized from ethanol to afford 12a,b.
3-[(4-Fluorophenyl)azo]-6-[4-(piperidin-1-ylsulfonyl)phenylazo]-2,5,7-triamino pyrazolo[1,5-a]pyrimidine (12a): Yield: 80%; (orange plates): mp >300 °C; IR (KBr) νmax in cm−1: 3404, 3266 (NH2), 2940, 2835 (C-H aliphatic), 1611 (C=N), 1420 (N=N), 1371 (SO2); 1H-NMR (500 MHz, DMSO-d6): δ 1.36–1.37 (m, 2H, CH2), 1.54–1.55 (m, 4H, 2CH2), 2.91–2.93 (m, 4H, 2NCH2), 6.95 (s, 2H, NH2), 7.29–7.33 (m, 4H, Ar–H), 7.74–7.77 (m, 6H, Ar–H+NH2), 8.21 (br, s, 2H, NH2); 13C-NMR (125 MHz, DMSO-d6): δ 22.85 (CH2), 24.67 (CH2), 46.58 (N–CH2), 109.17, 115.30, 115.74, 115.92, 120.88, 122.14, 122.45, 122.51, 128.38, 128.67, 134.07, 147.54, 149.99 (Ar–C), 152.62, 154.97 (C=N), 160.64 (C–F). Anal. Calcd. for C23H24FN11O2S (537.58): C, 51.39; H, 4.50; N, 28.66. Found C, 51.32; H, 4.73; N, 28.49%.
6-[(4-Piperidin-1-ylsulfonyl)phenylazo]-3-[4-(trifluoromethyl)phenylazo]-2,5,7-triaminopyrazolo[1,5-a]pyrimidine (12b): Yield: 81%; (reddish brown crystals): mp > 300 °C; IR (KBr) νmax in cm−1: 3439, 3273 (NH2), 2941, 2855 (C-H aliphatic), 1607 (C=N), 1409 (N=N), 1361 (SO2); 1H-NMR (500 MHz, DMSO-d6): δ 1.36 (m, 2H, CH2), 1.55 (m, 4H, 2CH2), 2.91 (m, 4H, 2N–CH2), 7.07 (s, 2H, NH2), 7.76–7.87 (m, 10H, Ar–H+NH2), 8.23 (br, s, 2H, NH2); 13C-NMR (125 MHz, DMSO-d6): δ 22.85 (CH2), 24.66 (CH2), 46.58 (N–CH2), 109.27, 116.59, 120.62, 120.97, 121.06, 122.24 (Ar–C), 123.33, 125.50, (q, C–F3, J = 271.2 Hz), 126.22, 126.25, 126.87, 127.13, 128.38, 128.66, 134.28, 148.10 (Ar–C), 152.43, 154.90 (C=N); MS (m/z), 588 (M+ + 1; 21%), 587 (M+; 44%), 442 (100%), 54 (99%). Anal. Calcd. for C24H24F3N11O2S (587.59): C, 49.06; H, 4.12; N, 26.22. Found C, 49.21; H, 4.32; N, 26.41%.
3.1.12. Synthesis of 6-(Thiazol-2-yldiazenyl)-3-(4-(trifluoro methyl)phenylazo)-2,5,7-triaminopyrazolo[1,5-a] pyrimidine (13)
A. Under reflux Condition:
N-(Thiazol-2-yl)carbonohydrazonoyl dicyanide (1.77 g, 0.01 mol) and pyridine (0.5 mL) were added to a solution of 3,5-diamino-4-[4-(trifluoromethyl)phenylazo]-1H-pyrazole (4b) (2.7 g, 0.01 mol) in ethanol (30 mL). The reaction mixture was heated under reflux for 12 h (monitored by TLC). After completion of the reaction, the precipitate which formed was filtered off, dried, and recrystallized from ethanol to afford 6-(thiazol-2-yldiazenyl)-3-(4-(trifluoromethyl)phenylazo)-2,5,7-triaminopyrazolo[1,5-a] pyrimidine (13).
B. Under Microwave Irradiation:
A mixture of 3,5-diamino-4-[4-(trifluoromethyl)phenylazo]-1H-pyrazole (4b) (2.7 g, 0.01 mol) and N-(thiazol-2-yl)carbonohydrazonoyl dicyanide (1.77 g, 0.01 mol) was grinded carefully in a porcelain mortar using a pestle and transferred to a pyrex test tube, and ethanol (4 mL) was added, followed by pyridine (0.5 mL). The reaction mixture was heated under microwave irradiation at 50% power for 8 min at 140 °C, monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature and the precipitated solid was filtered off, washed with MeOH, and recrystallized from ethanol to give 13.
Yield: 78%; (brown powder): mp > 300 °C; IR (KBr) νmax in cm−1: 3415, 3309 (NH2), 1608 (C=N), 1419 (N=N); 1H-NMR (500 MHz, DMSO-d6): δ 7.11 (s, 2H, NH2), 7.46 (d, 1H, CH–thiazole, J = 3.5 Hz), 7.65 (d, 1H, CH–thiazol, J = 3.5 Hz), 7.77–7.89 (m, 6H, Ar–H+NH2), 8.24 (br, s, 2H, NH2); 13C-NMR (125 MHz, DMSO-d6): δ 109.10, 109.20, 116.78, 119.63, 121.15, 121.34, 122.43 (Ar–C), 123.30, 125.46 (q, C–F3, J = 270 Hz), 126.04, 126.24, 126.27, 142.47 (Ar–C), 152.76, 155.58, (2C=N), 167.33 (C=N thiazole); MS (m/z), 448 (M+ + 1; 24%), 447 (M+; 100%). Anal. Calcd. for C16H12F3N11S (447.40): C, 42.95; H, 2.70; N, 34.44. Found C, 42.81; H, 2.49; N, 34.38%.
3.2. Biological Evaluation
3.2.1. In Vitro Antimicrobial Evaluation
Antimicrobial activities were carried out against highly pathogenic strains—three strains of Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis, and Staphylococcus mutants), three strains of Gram-negative bacteria (Enterococcus faecalis, proteus vulgaris, and Escherichia coli) using the standard antibiotic Gentamycin (5 mg/mL) as reference drugs, and fungi (Aspergillus fumigates, Aspergillus flavus, and Candida Albicans) using the standard antibiotic Ketoconazol (5 mg/mL). Antimicrobial activity of the tested samples was determined by using the agar diffusion method using Mueller–Hinton agar medium for bacteria and Sabouraud’s agar medium for fungi [19,20].
The tested microorganisms were obtained from the Regional Center for Mycology and Biotechnology (RCMP), Al-Azhar University. The assayed collection included Gram-positive (Staphylococcus aureus, Bacillus subtilis, and Staphylococcus mutants), Gram-negative bacteria (Enterococcus faecalis, proteus vulgaris, and Escherichia coli) using the standard antibiotic Gentamycin (5 mg/mL) as reference drugs, and fungi (Aspergillus fumigates, Aspergillus flavus, and Candida Albicans) using the standard antibiotic Ketoconazole (5 mg/mL). The mean zone of inhibition in mm ± standard deviation beyond the diameter (6 mm) was determined using a 5 µg/mL concentration of the tested compounds. The inhibitory effects of the synthetic compounds against these organisms are listed in Table 3.
3.2.2. In Vitro Cytotoxic Screening
Cell culture: Human hepatocellular carcinoma cell line (HepG-2), colorectal adenocarcinoma cell line (HCT-116), and breast adenocarcinoma cell line (MCF-7 cell) were obtained from the American type culture collection (ATCC). Cells were maintained in RPMI-1640 supplemented with (100 μg/mL), penicillin (100 units/mL), and heat-inactivated fetal bovine serum (10% v/v) in a humidified, 5% (v/v) CO2 atmosphere at 37 °C [24].
Cytotoxicity assessment: The cytotoxicity of different compounds was tested against human tumor cells using Sulphorhodamine B assay (SRB). Healthy growing cells were cultured in a 96-well tissue culture plate (3000 cells/well) for 24 h before treatment with the synthesized compounds to allow attachment of the cells to the plate. Cells were exposed to the five different concentrations of tested compounds (0.01, 0.1, 1, 10, and 100 μM/mL); untreated cells (control) were added. Triplicate wells were incubated with the different concentrations for 72 h and subsequently fixed with TCA (10% w/v) for 1 h at 4 °C. After several washing, cells were stained by 0.4% (w/v) SRB solution for 10 min in a dark place. Excess stain was washed with 1% (v/v) glacial acetic acid. After drying overnight, the SRB-stained cells were dissolved with tris–HCl and the color intensity was measured in a microplate reader at 540 nm. The linear relation between viability percentage of each tumor cell line and extracts concentrations were analyzed to get the IC50 (dose of the drug which reduces survival to 50%) using Sigma Plot 12.0 software [25].
4. Conclusions
In conclusion, 4-arylazo-3,5-diaminopyrazole 4–6 was synthesized and used as a building block for the synthesis of pyrazolopyrimidine derivatives such as 3,6-diarylazo-2,5,7-triaminopyrazolo[1,5-a]pyrimidine, 8 and 9, 3,6-Diarylazo-2,5,7-triaminopyrazolo[1,5-a]pyrimidine 10–12 and 13. The structures of newly synthesized compounds were confirmed by spectral data (IR, 1H-NMR, 13C-NMR, and mass spectra) and elemental analysis. Most of the newly synthesized compounds were assessed as antimicrobial agents against a number of Gram-positive, Gram-negative, and fungal strains. Some of the tested compounds showed excellent antimicrobial activity compared to the standard antibiotics. The obtained data reflected that compound 2b exhibited the best antimicrobial activity against most tested microorganisms and the rest of the compounds were moderately active or inactive for all the microorganisms. Most of the synthesized compounds exhibited good cytotoxic activity towards the examined three cell lines. From the obtained results, compounds 2a,b, 4b, 5b, 8b, and 12b showed the most potent cytotoxic profile on cancer cells (MCF-7, HepG2, and HCT-116) with IC50 values ranging from 0.3 to 3.4 µg, while the compound 8b is most effective among all of them on all types of cancer cells.
Acknowledgments
The authors would like to gratefully acknowledge for King Abdulaziz City for Science and Technology to support this research.
Supplementary Materials
The Supplementary Materials are available online.
Author Contributions
The listed authors contributed to this work as described in the following. E.H.A. carried out the synthetic work; E.H.A., A.M.F. and H.-A.S.A. gave the concepts of the work, interpreted the results, and prepared the manuscript. A.A.S., M.Y.A. and S.E.I.E. carried and interpreted the results of the cytotoxic activities. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: All the compounds are available from the authors.
References
- 1.Kamal A., Shaik A.B., Jain N., Kishor C., Nagabhushana A., Supriya B., Kumar G.B., Chourasiya S.S., Suresh Y., Mishra R.K., et al. Design and synthesis of pyrazole–oxindole conjugates targeting tubulin polymerization as new anticancer agents. Eur. J. Med. Chem. 2015;92:501–513. doi: 10.1016/j.ejmech.2013.10.077. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto S., Tomita N., Suzuki Y., Suzaki T., Kaku T., Hara T., Yamaoka M., Kanzaki N., Hasuoka A., Baba A., et al. Design, synthesis, and biological evaluation of 4-arylmethyl-1-phenylpyrazole and 4-aryloxy-1-phenylpyrazole derivatives as novel androgen receptor antagonists. Bioorg. Med. Chem. 2012;20:2338–2352. doi: 10.1016/j.bmc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Alam M., Zaman M.S., Alam M.M., Arora K., Ahmad A., Husain A. Synthesis and antimicrobial activities of new pyrazolopyridazine derivatives. Int. J. Pharma Sci. Res. 2015;6:495–501. [Google Scholar]
- 4.Viveka S.D., Shama P., Nagaraja G.K., Ballav S., Kerkar S. Design and synthesis of some new pyrazolyl-pyrazolines as potential antiinflammatory, analgesic and antibacterial agents. Eur. J. Med. Chem. 2015;101:442–451. doi: 10.1016/j.ejmech.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Mizuhara T., Kato T., Hirai A., Kurihara H., Shimada Y., Taniguchi M., Maeta H., Togami H., Shimura K., Matsuoka M., et al. Structure–activity relationship study of phenylpyrazole derivatives as a novel class of anti-HIV agents. Bioorg. Med. Chem. Lett. 2013;23:4557–4561. doi: 10.1016/j.bmcl.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Tantawy A.S., Nasr M.N.A., El-Sayed M.A.A., Tawfik S.S. Synthesis and antiviral activity of new 3-methyl-1,5-diphenyl-1H-pyrazole derivatives. Med. Chem. Res. 2012;21:4139–4149. doi: 10.1007/s00044-011-9960-2. [DOI] [Google Scholar]
- 7.Yoshida T., Akahoshi F., Sakashita H., Kitajima H., Nakamura M., Sonda S., Takeuchi M., Tanaka Y., Ueda N., Sekiguchi S., et al. Discovery and preclinical profile of teneligliptin (3-[(2S,4S)-4-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl]thiazolidine): A highly potent, selective, long-lasting and orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg. Med. Chem. 2012;20:5705–5719. doi: 10.1016/j.bmc.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt J.D., Chudasama C.J., Patel K.D. Pyrazole clubbed triazolo[1,5-a]pyrimidine hybrids as an anti-tubercular agents: Synthesis, in vitro screening and molecular docking study. Bioorg. Med. Chem. 2015;23:7711–7716. doi: 10.1016/j.bmc.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Mert S., Kasimogullari R., Ok S. A Short review on pyrazole derivatives and their applications. J. Postdoc. Resch. J. 2014;2:64–72. [Google Scholar]
- 10.Mao M., Li Y., Liu Q., Zhou Y., Zhang X., Xiong L., Li Y., Li Z. Synthesis and insecticidal evaluation of novel N-pyridylpyrazolecarboxamides containing cyano substituent in the ortho-position. Bioorg. Med. Chem. Lett. 2013;23:42–46. doi: 10.1016/j.bmcl.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 11.Ma H.J., Li Y.H., Zhao Q.F., Zhang T., Xie R.L., Mei X.D., Ning J. Synthesis and Herbicidal Activity of Novel N-(2,2,2)-trifluoroethylpyrazole derivatives. J. Agric. Food. Chem. 2010;58:4356–4360. doi: 10.1021/jf9042166. [DOI] [PubMed] [Google Scholar]
- 12.Pericherla K., Shelke G.M., Kothari Y.C., Kumar A. Yb(OTf)3 catalyzed Mannich reaction of imidazo[1,2-a]pyridine and pyrazolo[1,5-a]pyrimidines. Indian J. Chem. 2015;54B:290–300. doi: 10.1002/chin.201545070. [DOI] [Google Scholar]
- 13.Abbas H.S., Abd El-Karim S.S., Ahmed E.M., Eweas A.F., El-Awdan S.A. Synthesis, biological evaluation and molecular docking studies of aromatic sulfonamide derivatives as anti-inflammatory and analgesic agents. Acta. Pol. Pharm. 2016;73:1163–1180. [PubMed] [Google Scholar]
- 14.Anwar H.F., Elnagdi M.H. Recent developments in aminopyrazole chemistry. ARKIVOC. 2009;2009:198–250. [Google Scholar]
- 15.Hassan A.S., Hafez T.S., Osman S.A.M., Ali M.M. Synthesis and in vitro cytotoxic activity of novel pyrazolo[1,5-a]pyrimidines and related Schiff bases. Turk. J. Chem. 2015;39:1102–1113. doi: 10.3906/kim-1504-12. [DOI] [Google Scholar]
- 16.Compton D.R., Sheng S., Carlson K.E., Rebacz N.A., Lee I.Y., Katzenellenbogen B.S., Katzenellenbogen J.A. Pyrazolo[1,5-a]pyrimidines: Estrogen receptor ligands possessing estrogen receptor antagonist activity. J. Med. Chem. 2004;47:5872–5893. doi: 10.1021/jm049631k. [DOI] [PubMed] [Google Scholar]
- 17.El-Gaby M.S.A., Atalla A.A., Gaber A.M., Abd Al-Wahab K.A. Studies on aminopyrazoles: Antibacterial activity of some novel pyrazolo[1,5-a]pyrimidines containing sulfonamido moieties. II Farmaco. 2000;55:596–602. doi: 10.1016/S0014-827X(00)00079-3. [DOI] [PubMed] [Google Scholar]
- 18.Al-Sehemi A.G., Irfan A., Fouda A.M. Synthesis, characterization and density functional theory investigations of the electronic, photophysical and charge transfer properties of donor–bridge–acceptor triaminopyrazolo[1,5-a]pyrimidine dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013;111:223–229. doi: 10.1016/j.saa.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller M.A., Burmeister L., Bartlett M.S., Rinaldi M.G. Multicenter evaluation of four methods of yeast inoculum preparation. J. Clin. Microbiol. 1988;26:1437–1441. doi: 10.1128/jcm.26.8.1437-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krystof V., Cankar P., Frysova I., Slouka J., Kontopidis G., Dzubak P., Hajduch M., Srovnal J., de Azevedo W.F., Jr., Orsag M.J., et al. 4-Arylazo-3,5-diamino-1H-pyrazole CDK inhibitors: SAR study, crystal structure in complex with CDK2, selectivity, and cellular effect. J. Med. Chem. 2006;49:6500–6509. doi: 10.1021/jm0605740. [DOI] [PubMed] [Google Scholar]
- 22.Kumar V., Kaur G.K.G.K., Singh R. 4-Fluorophenylhydrazones as potential COX-2 inhibitors: A novel, efficient, one pot solid phase synthesis, docking study and pharmacological evaluation. Med. Chem. Res. 2013;22:5890–5901. doi: 10.1007/s00044-013-0566-8. [DOI] [Google Scholar]
- 23.Aggarwal R., Gupta V.K.G.K., Kumar V. Synthesis of some new 3,5-diamino-4-(4′-fluorophenylazo)-1-aryl/heteroarylpyrazoles as antimicrobial agents. Med. Chem. Res. 2013;22:3566–3574. doi: 10.1007/s00044-012-0343-0. [DOI] [Google Scholar]
- 24.Mahmoud A.M., Al-Abd A.M., Lightfoot D.A., El-Shemy H.A. Anti-cancer characteristics of mevinolin against three different solid tumor cell lines was not solely p53-dependent. J. Enzyme Inhib. Med. Chem. 2012;27:673–679. doi: 10.3109/14756366.2011.607446. [DOI] [PubMed] [Google Scholar]
- 25.Alahdal A.M., Asfour H.Z., Ahmed S.A., Noor A.O., Al-Abd A.M., Elfaky M.A., Elhady S.S. Anti-helicobacter, antitubercular and cytotoxic activities of scalaranes from the red sea sponge hyrtios erectus. Molecules. 2018;23:978. doi: 10.3390/molecules23040978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.