Abstract
Background: Imbalance of homocysteine (Hcy) metabolism links with several pathologies; nevertheless, it is poorly characterized in pediatric populations. This study investigated the impact of age on plasma concentrations of Hcy and relevant biomarkers along with correspondent genotype interactions. Methods: A healthy pediatric cohort aged 9 (n = 195) and 17 (n = 128) years old (yo) was studied. Immunoassays and GC-MS-SIM-mode quantified plasma levels of Hcy and biomarkers. PCR-RFLP or quantitative-PCR assays assessed common variations in related genes. Results: Age impacted on levels of Hcy and metabolic markers: older children presented with the lowest folates and total-cobalamin (tCbl), while with the highest Hcy concentrations, whereas methylmalonic acid (MMA) and holotranscobalamin (Holo-TC) levels remained similar in 9-yo and 17-yo children. The relationships between B-vitamins and metabolic markers were also dependent on age. Only in the older children, MMA correlated with tCbl and Holo-TC, and MMA levels were markedly higher in the 17-yo subjects presenting with the lowest quartiles of Holo-TC concentrations. Lastly, age also impacted on the correlations between genotype and biomarkers. In the 17-yo group, however not in the 9-yo children, tHcy differed between MTHFR 677 genotypes, with subjects who had the MTHFR 677TT genotype displaying the highest tHcy concentrations. Conclusions: Age impacts on the Hcy metabolism dynamics in a pediatric population.
Keywords: vitamin B12, methylmalonic acid, folate, homocysteine, MTHFR
1. Introduction
The homocysteine (Hcy) metabolism is a metabolic network centered on the folate and methionine cycles in which one-carbon (1-C) groups are transferred, supporting multiple physiological processes, including nucleotide biosynthesis, amino acid homeostasis, epigenetic maintenance and redox defense [1,2]. Consequently, a disturbed Hcy metabolism is associated with common pathologies, such as neurodegenerative and cardiovascular (CVD) diseases and cancer [3,4,5,6,7,8,9,10,11].
The metabolisms of Hcy, folate and vitamin B12 (or cobalamin, Cbl) are biochemically linked sharing several metabolic intermediates [9]. Hcy is formed from the essential amino acid methionine. Once formed, Hcy may be conserved and remethylated back to methionine by methionine synthase (MS). This enzyme requires the presence of methylcobalamin (methylCbl), as a cofactor, to carry on its function. In turn, the synthesis of methylCbl needs the intervention of 5-methyltetrahydrofolate (5-methyl-THF), the main circulating form of folate [7]. Then, 5-methyl-THF transfers the methyl group to Cbl, thus releasing tetrahydrofolate (THF) that will sustain several 1-C transfer reactions. Transcobalamin II (TC), a plasma protein, binds Cbl and transports it to cells. Transcobalamin-bound vitamin B12 is designated as holotranscobalamin (Holo-TC) [12]. The common term total Cbl (tCbl) also includes other circulating forms of Cbl, of which its functions are unclear. However only the Holo-TC fraction enters into the cells and releases the Cbl [12]. Consequently, insufficient levels of methylCbl may be associated with a conditional folate deficiency [7,13], impairing the remethylation of Hcy, which will accumulate in plasma [7,9,13,14], reflecting the insufficient Cbl and folate intracellular status. Moreover, Cbl is also converted to adenosylCbl, which is used for the metabolism of methylmalonyl-CoA [15,16]. Therefore, an insufficient B12/Cbl cell status leads to the build-up of plasma Hcy and methylmalonic acid (MMA), formed from methylmalonyl-CoA [17,18,19,20]. A more comprehensive description of the Hcy metabolism can be found in references [8,9,10].
Genetic background may also impact on the levels of plasma Hcy. Gene variants coding for enzymes involved in Hcy metabolism may modulate the correspondent enzyme activities [2]. MTHFR participates in the remethylation of Hcy to methionine. The MTHFR 677C>T polymorphism (p.A222V) results in a thermolabile variant of MTHFR with a decreased enzyme activity [21,22,23,24] and is a well-established genetic determinant of elevated plasma tHcy (total homocysteine; all the circulating forms of Hcy) levels [25,26,27]. Other common gene variations, which are also involved in the Hcy remethylation, have been reported, albeit with less consistent effects on Hcy circulating levels [2,27,28]. These include the MTHFR 1298A>C (p.E429A) [29] and the MTR 2756A>G (p.D919G) [30] and MTRR 66A>G, the last two being related with MS activity [31]. Further, variations in the TC encoding gene, or TCN2, may also affect the circulating concentrations of tHcy. The TCN2 776C>G (p.P259R) and 67A>G (p.I23V) variants diminish the TC’s ability to bind and transport cobalamin to tissues, causing an accumulation of Hcy due to deficient remethylation [32,33].
Despite the pivotal importance of Hcy metabolism for cellular homeostasis, data are scarce in the pediatric population, which is particularly vulnerable due to the higher requirements of nutrients for healthy growth and development. Adequate reference values for Hcy and related metabolic biomarkers in the pediatric population are important in clinical decision-making, with possible health impacts. Nevertheless, providing reference values in school-age children is challenging, and most of the available reference values for Hcy are obtained from adults. Another question in establishing pediatric reference values is to consider the potential effect of specific ages on Hcy metabolism. For instance, differences in physical size, organ maturity, fluid body compartments, immune and hormone responsiveness are likely to affect the concentrations of Hcy in children and youth. Here, we investigated the Hcy metabolism in 9 and 17 year-old (yo) subjects by exploring the impact of age, gender and genotype on the levels of tHcy, MMA and B-vitamins.
2. Materials and Methods
2.1. Subjects
Two groups of a pediatric population from Madeira Island, Portugal: 128 adolescents aged 17-yo (71 females, 57 males) and 195 children aged 9-yo (88 females, 107 males) were studied. Subjects were enrolled from schools that were selected from different areas of the island. All participants were Caucasian. Data on dietary and lifestyle habits were collected and used as a basis for the selection of eligible individuals. All subjects were under a Mediterranean diet and the intake of protein and vegetables/fruit followed the recommended dose/age. Dietary intake was evaluated by application of a semi-quantitative Food Frequency Questionnaire. Acute and chronic illnesses were the exclusion criteria. All selected individuals presented an irrelevant anamnesis and did not take drugs and vitamin supplementation. For all participants, written informed consents were obtained. All procedures were conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects and the study was approved by the Scientific and Research Committee of our Institute at Madeira University.
2.2. Anthropometric Data
All participants were assessed for Body Mass Index (BMI), waist circumference, systolic and diastolic pressure using standard methods.
2.3. Blood Sample Collection and Biochemical Analyses
Overnight fasting blood samples were collected by venipuncture in EDTA containing tubes. Part of the blood was used for DNA extraction (as described below) and the remaining blood was immediately centrifuged at 4000 g for 10 min at 4 °C. Plasma was collected, divided into several aliquots and stored at −80 °C until analysis.
Plasma levels of total cholesterol, HDL-cholesterol, LDL-cholesterol, triacylglycerols, creatinine and uric acid were quantified by standard routine methods (Unicel DXC, Beckman Coulter Inc., Brea, CA, USA).
Plasma tHcy and folate were evaluated, respectively, by a fluorescence polarization immunoassay and an ion-capture enzyme immunoassay from IMx Systems (Abbott Laboratories, Chicago, IL, USA), according to the manufacturer’s instructions. Plasma tCbl and Holo-TC were determined by a microparticle enzyme immunoassay using an AxSYM analyser (Abbott Laboratories, Chicago, IL, USA). Plasma MMA concentration was determined by gas chromatography coupled with mass spectrometry in a selected ion mode (GC-MS-SIM) following a laboratory procedure adapted from a previously published method [34].
2.4. Genotype Analyses
Genomic DNA was isolated from peripheral blood leucocytes (Puregene, DNA purification system, Gentra Systems) and was stored at 4 °C until use. Identification of the allelic variants MTHFR 677C>T, MTHFR 1298A>C, MTR 2756A>G, TCN2 67A>G and TCN2 776C>G was performed by PCR-RFLP assay using HinfI, MboII, HaeIII, RsaI and ScrFI endonucleases, respectively [16,30,35]. After restriction enzyme digestion, PCR products were evaluated by gel electrophoresis analysis. Allelic discrimination of MTRR 66A>G variants was performed by real-time PCR (iQ5 Real-Time PCR Thermocycler, BioRad, California, USA) using TaqMan probes (TaqMan® SNP Genotyping Assays, Applied Biosystems, Waltham, MA, USA).
2.5. Statistical Analysis
All statistical analyses were performed using the program IBM SPSS Statistics 22.0. Normality of data and homogeneity of variances were evaluated, respectively, by Kolmogorov-Smirnov and Levene tests. Data were expressed as the mean ± standard deviation (SD). Continuous variables were compared by independent samples T-test and by one-way analysis of variance (ANOVA). Spearman correlation coefficients were calculated to determine the relationship between all metabolites and vitamins. A p-value lower than <0.05 was considered statistically significant.
3. Results
3.1. Anthropometric and Biochemical Parameters
Anthropometric and routine biochemical parameters (data not shown) of the studied population were stratified according to age and gender. All the analyzed parameters were within the normal range according to age and gender. Between genders, significant differences were observed in the 9-yo group only for the systolic pressure (p < 0.05), which was higher in females. In the 17-yo group, males presented significantly higher systolic pressure (p < 0.01), waist circumference (p < 0.001) and creatinine (p < 0.001), however lower HDL-cholesterol (p = 0.001) than females. All the included individuals were eutrophic according to BMI cut-off values of the World Health Organization (WHO) Growth Reference Data 2007.
3.2. Age and Plasma Biomarkers
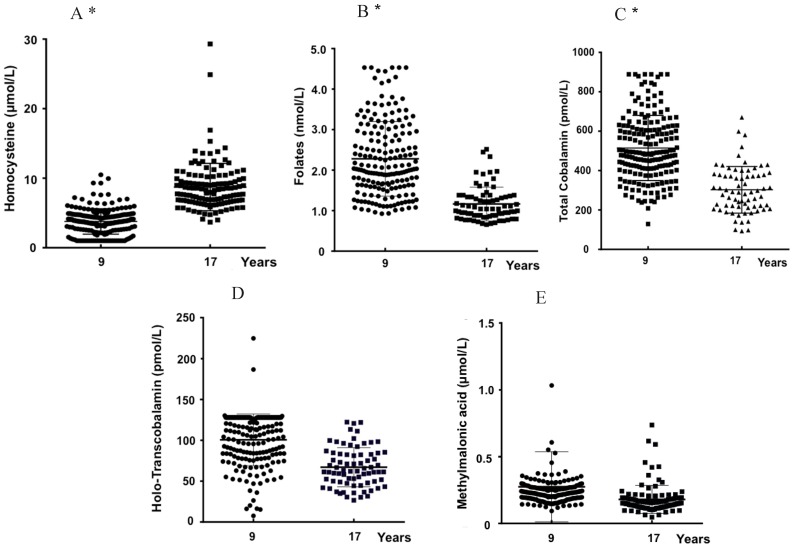
The levels (mean ± SD) of plasma biomarkers according to age and gender are displayed in Table 1. Figure 1A–E represents the distribution of the same biomarkers in the 9- (n = 195) and 17-yo (n = 128) individuals.
Table 1.
Plasma concentrations (mean ± SD) of vitamins and metabolic markers in the studied population, stratified according to gender and age.
| Males | Females | p ϕ | p Ψ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 9-Year-Old | N | 17-Year-Old | p 1 | N | 9-Years-Old | N | 17-Year-Old | p 2 | |||
| tHcy (µM) | 107 | 3.8 ± 1.8 | 52 | 10.2 ± 4.0 | <0.001 | 88 | 3.8 ± 1.9 | 67 | 7.5 ± 2.2 | <0.001 | 0.811 | <0.001 |
| Folates (nM) | 96 | 22.9 ± 9.1 | 39 | 11.1 ± 3.5 | <0.001 | 80 | 22.8 ± 9.5 | 37 | 12.2 ± 4.7 | <0.001 | 0.969 | 0.229 |
| tCbl (pM) | 104 | 502.1 ± 163.1 | 35 | 286.5 ± 97.2 | <0.001 | 83 | 530.6 ± 168.2 | 36 | 318.0 ± 135.3 | <0.001 | 0.243 | 0.265 |
| Holo-TC (pM) | 99 | 103.2 ± 28.5 | 38 | 69.9 ± 25 | <0.001 | 80 | 97.4 ± 35.1 | 37 | 62.4 ± 24.8 | <0.001 | 0.226 | 0.196 |
| MMA (µM) | 99 | 0.30 ± 0.31 | 48 | 0.21 ± 0.14 | 0.082 | 85 | 0.25 ± 0.19 | 62 | 0.18 ± 0.16 | <0.001 | 0.211 | 0.087 |
1 Independent samples T-test between age-groups within males; 2 Independent samples T-test between age-groups within females. ϕ Independent samples T-test between 9-yo males and females; Ψ Independent samples T-test between 17-yo males and females. Total Homocysteine (tHcy); total cobalamin (tCbl); holo-transcobalamin (Holo-TC); methylmalonic acid (MMA).
Figure 1.
Distribution of the concentrations of total homocysteine (A), folates (B), cobalamin (C), holo-transcobalamin (D) and methylmalonic acid (E) for the 9-yo (n = 195) and 17-yo (n = 128) children. * p < 0.05, Student T test 9-yo versus 17-yo.
Concerning the putative influence of gender within each age-group (Table 1), no significant differences were observed except for tHcy concentrations, which were significantly higher in 17-yo males (Hcy: 10.2 ± 4.0 µM) than in same-age females (Hcy: 7.5 ± 2.2 µM). When comparing the 17-yo subjects with the 9-yo individuals within the same gender, plasma concentrations of tHcy (µM) increased significantly with age, while those of folate, tCbl and Holo-TC decreased significantly. Moreover, MMA levels decreased significantly with age in females, however not in males.
When both genders were considered together (Figure 1), plasma concentrations of tHcy (µM) increased significantly (3.8 ± 1.8 to 8.7 ± 3.4; p < 0.001) with age, while those of folate (nM) (22.8 ± 9.3 to 11.6 ± 4.2; p < 0.001) and tCbl (pM) (302.5 ± 118.3 to 286.5 ± 97.2; p < 0.001) decreased significantly. No significant differences were observed for Holo-TC and MMA plasma levels.
Taking all subjects into account, as expected, tHcy showed a significant negative linear association with folate, Holo-TC and tCbl (Spearman correlation coefficient, r = −0.625; −0.380 and −0.519, respectively, p < 0.01). Moreover, a positive linear association was observed between Holo-TC and tCbl levels (r = 0.426, p < 0.01).
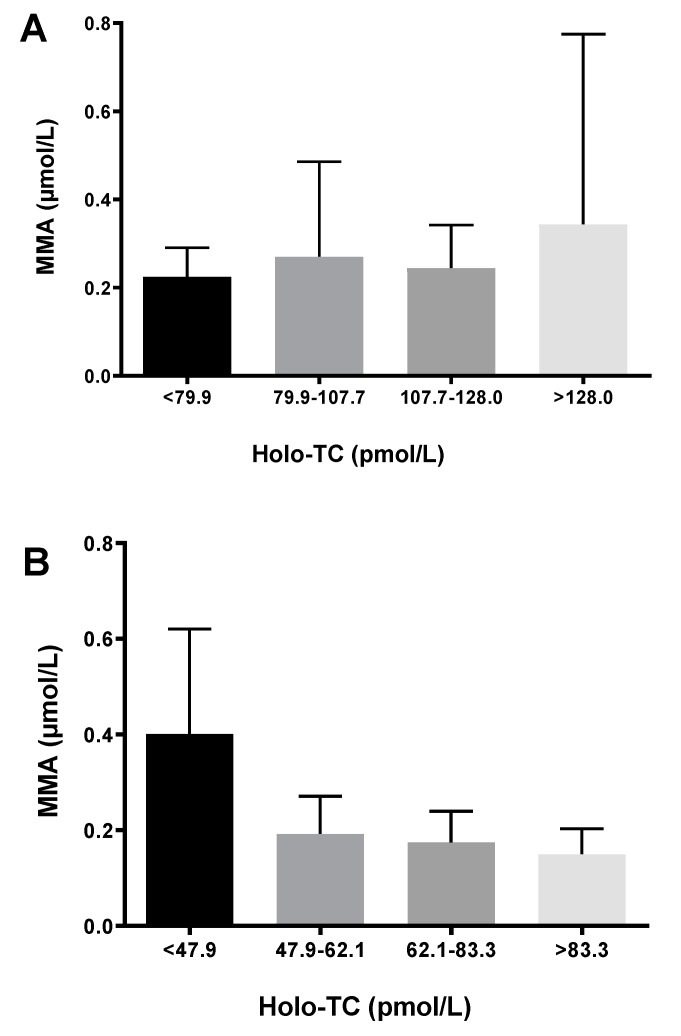
Plasma MMA levels were significantly correlated with tCbl (r = −0.335, p < 0.05) and with Holo-TC (r = −0.515, p < 0.01) in the 17-yo subjects, however not in the 9-yo group. Moreover, MMA concentrations for each age group were plotted according to Holo-TC quartiles (Figure 2), and only in the 17-yo group, the highest MMA levels were associated with the lowest Holo-TC levels. Taken together, these observations confirm MMA as a good predictive metabolic marker of cobalamin status in the older children, however also suggest that MMA concentrations do not reflect the cobalamin status in the younger children.
Figure 2.
Methylmalonic acid (MMA) concentrations by quartiles of holo-transcobalamin (Holo-TC) in the 9-yo (A) and 17-yo (B) children.
3.3. Genotypes, Age, and Biomarkers
Genotype and allele frequencies for the whole group are displayed in Table 2, with all genotype frequencies consistent with the Hardy-Weinberg equilibrium.
Table 2.
Genotypic and allelic frequencies of single nucleotide polymorphisms (SNPs) in MTHFR, TCNII, MTR and MTRR genes in the whole studied group (n = 323).
| SNP | Genotype | Allele Frequency (%) | |||
|---|---|---|---|---|---|
| MTHFR | CC | CT | TT | C | T |
| C677T (n = 565) | 295 (52.2%) | 218 (38.6%) | 52 (9.2%) | 71.5 | 28.5 |
| MTHFR | AA | AC | CC | A | C |
| A1298C (n = 565) | 301 (53.3%) | 196 (34.7%) | 68 (12.0%) | 70.6 | 29.4 |
| TCNII | AA | AG | GG | A | G |
| A67G (n = 301) | 252 (83.7%) | 43 (14.3%) | 6 (2%) | 90.9 | 9.1 |
| TCNII | CC | CG | GG | C | G |
| C776G (n = 292) | 83 (28.4%) | 141 (48.3%) | 68 (23.3%) | 52.6 | 47.4 |
| MTR | AA | AG | GG | A | G |
| A2756G (n = 218) | 128 (58.7%) | 82 (37.6%) | 8 (3.7%) | 77.5 | 22.5 |
| MTRR | AA | AG | GG | A | G |
| A66G (n = 246) | 75 (30.5%) | 114 (46.3%) | 57 (23.2%) | 53.7 | 46.3 |
The plasma concentrations of vitamins and metabolic markers were stratified according to the various single nucleotide polymorphisms (SNPs), and the results are summarized in Table 3 (9-yo group) and 4 (17-yo group). In the 9-yo group, no significant differences were observed for any of the evaluated biomarkers among the different genotypes. However, in the 17-yo group, significant differences (p < 0.05) were observed for tHcy levels among the MTHFR 677 genotypes and tCbl concentrations among MTR 2756 genotypes.
Table 3.
Plasma concentrations of vitamins and metabolic markers (tHcy, Folate, Holo-TC, tCbl, MMA; mean ± SD) according to the MTHFR, TCN II, MTR and MTRR genotypes in the 9-yo group.
| 9-Year-Old | |||||
|---|---|---|---|---|---|
| SNP | tHcy (µmol/L) | Folate (nmol/L) | Holo-TC (pmol/L) | tCbl (pmol/L) | MMA (µmol/L) |
| MTHFR C677T | |||||
| CC | 3.7 ± 1.9 (n = 115) | 23.1 ± 9.0 (n = 109) | 101.8 ± 30.2 (n = 108) | 502.2 ± 156.3 (n = 110) | 0.274 ± 0.300 (n = 107) |
| CT | 3.7 ± 1.7 (n = 65) | 23.4 ± 9.7 (n = 60) | 100.0 ± 33.7 (n = 59) | 527.6 ± 178.6 (n = 63) | 0.270 ± 0.198 (n = 62) |
| TT | 4.5 ± 1.8 (n = 15) | 17.4 ± 7.1 (n = 14) | 92.7 ± 35.41 (n = 12) | 555.3 ± 176.2 (n = 14) | 0.295 ± 0.210 (n = 15) |
| MTHFR A1298C | |||||
| AA | 3.7 ± 1.8 (n = 101) | 23.4 ± 10.1 (n = 97) | 101.3 ± 33.7 (n = 93) | 511.1 ± 157.8 (n = 98) | 0.284 ± 0.252 (n = 97) |
| AC | 3.8 ± 1.8 (n = 59) | 22.1 ± 8.4 (n = 52) | 98.2 ± 27.2 (n = 53) | 544.5 ± 186.9 (n = 57) | 0.225 ± 0.599 (n = 56) |
| CC | 3.8 ± 2.0 (n = 35) | 22.1 ± 7.8 (n = 34) | 102.6 ± 33.0 (n = 33) | 472.9 ± 141.2 (n = 32) | 0.334 ± 0.448 (n = 31) |
| TCN2 A67G | |||||
| AA | 3.9 ± 1.8 (n = 146) | 22.8 ± 9.5 (n = 137) | 101.2 ± 29.9 (n = 117) | 523.0 ± 168.2 (n = 127) | 0.277 ± 0.238 (n = 139) |
| AG | 3.2 ± 2.0 (n = 37) | 22.1 ± 7.9 (n = 35) | 92.1 ± 26.4 (n = 33) | 489.8 ± 178.8 (n = 35) | 0.221 ± 0.054 (n = 35) |
| GG | 3.7 ± 2.4 (n = 5) | 26.1 ± 8.3 (n = 5) | 87.9 ± 40.6 (n = 3) | 561.2 ± 100.3 (n = 3) | 0.153 ± 0.043 (n = 4) |
| TCN2 C776G | |||||
| CC | 4.0 ± 1.8 (n = 59) | 22.5 ± 8.7 (n = 54) | 96.8 ± 29.2 (n = 48) | 496.8 ± 171.8 (n = 56) | 0.241 ± 0.063 (n = 55) |
| CG | 3.6 ± 1.9 (n = 74) | 21.5 ± 9.4 (n = 70) | 101.2 ± 29.8 (n = 64) | 525.1 ± 160.7 (n = 69) | 0.281 ± 0.306 (n = 70) |
| GG | 3.8 ± 2.0 (n = 42) | 23.8 ± 8.9 (n = 40) | 98.0 ± 29.7 (n = 61) | 529. 8 ± 182.1 (n = 40) | 0.287 ± 0.267 (n = 40) |
| MTR A2756G | |||||
| AA | 3.9 ± 1.5 (n = 70) | 23.8 ± 10.2 (n = 64) | 102.2 ± 25.4 (n = 63) | 538.9 ± 188.8 (n = 68) | 0.251 ± 0.084 (n = 67) |
| AG | 3.7 ± 1.7 (n = 44) | 23.6 ± 9.4 (n = 43) | 99.0 ± 33.7 (n = 44) | 531.0 ± 152.8 (n = 39) | 0.325 ± 0.403 (n = 42) |
| GG | 4.8 ± 3.3 (n = 2) | 21.7 ± 7.6 (n = 2) | 87.4 ± 28.2 (n = 2) | 467.7 ± 33.4 (n = 2) | 0.234 ± 0.044 (n = 2) |
| MTRR A66G | |||||
| AA | 3.9 ± 1.8 (n = 36) | 24.8 ± 9.8 (n = 33) | 109.0 ± 32.0 (n = 35) | 530.8 ± 180.8 (n = 34) | 0.257 ± 0.159 (n = 35) |
| AG | 3.8 ± 1.6 (n = 60) | 21.8 ± 9.1 (n = 55) | 100.4 ± 34.7 (n = 55) | 537.0 ± 165.7 (n = 56) | 0.262 ± 0.242 (n = 56) |
| GG | 4.0 ± 1.8 (n = 30) | 22.9 ± 9.1 (n = 27) | 100.4 ± 25.8 (n = 26) | 513.8 ± 183.8 (n = 28) | 0.354 ± 0.461 (n = 29) |
p values for differences between means of the three genotypes were tested with one-way analysis of variance (ANOVA). p values < 0.05 were considered statistically significant. No statistically significant differences were observed for any of the evaluated biomarkers among the different genotypes. Total Homocysteine (tHcy); total cobalamin (tCbl); holo-transcobalamin (Holo-TC); methylmalonic acid (MMA).
4. Discussion
Disruption of Hcy metabolism leads to hyperhomocysteinemia, which has been associated with different pathological conditions, including vascular and neurodegenerative diseases [11,36,37,38]. The causes of hyperhomocysteinemia are multifactorial and include insufficient levels of the B-vitamins, particularly folates and Cbl, which are cofactors and co-substrates in Hcy metabolism [36,37,39]. European dietary surveys disclosed a widespread prevalence of suboptimal plasma concentrations of folates and tCbl in various age groups, despite apparent adequate intakes [37,39,40]. This fact is of particular concern in young age groups in which it has been related to developmental delay and irreversible neurological damage [41,42,43,44,45]. Nevertheless, few studies have examined the Hcy metabolism in pediatric populations.
This study was conducted in a healthy pediatric population of children (aged 9-yo) and adolescents (aged 17-yo). The impact of age on Hcy-related metabolism biomarkers and gender differences were evaluated along with that of common variants in genes (MTHFR, MTR and MTRR) encoding enzymes involved in the folate/Cbl dependent Hcy remethylation cycle, or in the TCN2 gene, which encodes the protein that delivers Cbl to cells.
The results showed an increase of tHcy concentration and a decrease of folate and Cbl levels with age (Figure 1 and Table 1). Moreover, gender differences were found, however only in the 17-yo group, for tHcy level, with a higher mean value in males. These observations concur with previously published data [46,47,48,49]. The plasma tHcy values are influenced not only by environmental and genetic factors, however also by nutritional ones, including different fortification policies worldwide [47,48]. Therefore, differences between the dietary habits and lifestyle between the younger children and adolescents may explain the observed decrease of folate and Cbl levels, which harm Hcy metabolism, contributing to Hcy accumulation. The increase in tHcy concentrations is also influenced not only by hormonal factors, which support gender differences, however also by the increment of body mass, along with healthy development, which demands a high ratio of creatine synthesis. This metabolic reaction is the leading consumer of the methyl groups donated by S-adenosylmethionine, which is formed during the conversion of methionine to Hcy [9].
The 9-yo group displays a tHcy plasma mean level (3.8 µmol/L) (Table 1) that is lower than those reported for Dutch children aged 6–10 yo (6.2 µmol/L) [49]; for Belgian children aged 5–9 and 10–14 yo (6.2 to 7.1 µmol/L, respectively) [46]; and for American children aged 6–11 yo (5.0 to 5.4 µmol/L) [48]. The observed difference may be attributable to the Mediterranean diet, characterized by a high consumption of fresh vegetables and fruits, which, at this age, is still under family control, therefore contributing to the high levels of plasma vitamins observed and its lowering effect on tHcy concentrations.
The isomerization of L-methylmalonyl coenzyme A by methylmalonyl coenzyme A mutase, using AdoCbl as a cofactor, and its independence from folate metabolism, have made MMA an attractive biomarker of Cbl deficiency [41]. In this study, despite a decrease in tCbl and Holo-Cbl levels with age, MMA plasma concentrations in the 9- and 17-yo groups were not significantly different (Figure 1 and Table 1). This observation agrees with the NHANES study, which states that circulating MMA is generally stable at an age below 20 years and only increases later, with an upward trend after 40 years of age [50]. In the present report, MMA levels did not correlate with tCbl or with Holo-TC levels in the 9-yo group, while significant correlations were observed in the adolescents’ group. This observation suggests that, in pediatric populations, the usefulness of MMA as a metabolic biomarker of cobalamin status needs to be further investigated. It should be taken into account that several factors may cause variability of plasma MMA, among them alterations in the gut microbiota [51], as well as in the metabolism of odd-chain fatty acids and amino acids precursors of MMA (such as methionine, isoleucine and threonine) [18,20,41,50]. Moreover, genetic background may also influence plasma MMA concentrations. Recently, in an Irish population, a common polymorphism in the HIBCH gene, encoding 3-hydroxyisobutyryl-CoA hydrolase, was strongly associated with elevated MMA concentrations independently of tCbl or Holo-TC levels [52].
The frequency of the MTHFR 677TT genotype was 9.2% (Table 2), similar to other European populations, including the Netherlands (8.2%) [49], Germany (9.7%) [25] and Czech Republic (10%) [53,54], however was slightly lower than the one reported for Northern Ireland (13.5%) [55], France (11.8%) and Spain (11.8%) [56]. Moreover, MTHFR 1298CC, MTR 2756GG, MTRR 66GG, TCN2 776GG and TCN2 67GG genotype frequencies were also comparable to those reported for other Caucasian European populations [53,54,55,57].
MTHFR 677C>T is a well-established genetic determinant of elevated plasma tHcy [25,26,27], and lower folate concentrations significantly enhance this effect. Accordingly, in the 9-yo group (Table 3), which presents high plasma folate levels, tHcy plasma concentrations did not differ significantly among the MTHFR 677 genotypes, suggesting that folate levels modulate the expected correlations between genotype and metabolic markers. Supporting that correlation, in the 17-yo group (Table 4), subjects bearing the MTHFR 677TT genotype displayed significantly higher tHcy concentrations than those bearing the wild-type genotype. A similar situation was observed concerning the MTR2756GG genotype, since it was associated with significantly decreased tCbl plasma levels in 17-yo adolescents (Table 4).
Table 4.
Plasma concentrations of vitamins and metabolic markers (tHcy, Folate, Holo-TC, tCbl, MMA; mean ± SD) according to the MTHFR, TCN II, MTR and MTRR genotypes in the 17-yo group.
| 17-Year-Old | |||||
|---|---|---|---|---|---|
| SNP | tHcy (µmol/L) | Folate (nmol/L) | Holo-TC (pmol/L) | tCbl (pmol/L) | MMA (µmol/L) |
| MTHFR C677T | |||||
| CC | 8.4 ± 2.4 (n = 62) * | 11.9 ± 3.4 (n = 34) | 71.9 ± 26.3 (n = 33) | 307.6 ± 114.2 (n = 32) | 0.170 ± 0.101 (n = 55) |
| CT | 8.8 ± 3.9 (n = 48) * | 11.8 ± 5.1 (n = 34) | 63.8 ± 24.3 (n = 34) | 305.4 ± 124.2 (n = 32) | 0.175 ± 0.092 (n = 39) |
| TT | 11.3 ± 5.0 (n = 12) * | 9.0 ± 9.2 (n = 7) | 51.4 ± 19.1 (n = 7) | 250.6 ± 124.4 (n = 6) | 0.252 ± 0.161 (n = 10) |
| MTHFR A1298C | |||||
| AA | 9.4 ± 3.0 (n = 58) | 11.6 ± 4.3 (n = 35) | 69.5 ± 27.9 (n = 35) | 320.4 ± 131.6 (n = 33) | 0.190 ± 0.114 (n = 47) |
| AC | 8.5 ± 4.0 (n = 46) | 11.2 ± 3.7 (n = 25) | 63.2 ± 19.1 (n = 25) | 292.0 ± 100.7 (n = 23) | 0.162 ± 0.067 (n = 41) |
| CC | 7.9 ± 3.1 (n = 18) | 12.2 ± 4.9 (n = 15) | 63.7 ± 28.5 (n = 14) | 273.5 ± 115.9 (n = 14) | 0.197 ± 0.155 (n = 16) |
| TCN2 A67G | |||||
| AA | 8.7 ± 3.5 (n = 105) | 12.0 ± 4.4 (n = 62) | 66.2 ± 25.5 (n = 61) | 298.8 ± 119.6 (n = 58) | 0.183 ± 0.113 (n = 90) |
| AG | 10.3 ± 2.5 (n = 6) | 9.6 ± 2.6 (n = 4) | 68.1 ± 30.1 (n = 4) | 286.1 ± 71.5 (n = 4) | 0.146 ± 0.010 (n = 5) |
| GG | (n = 1) | - | - | - | (n = 1) |
| TCN2 C776G | |||||
| CC | 8.9 ± 2.4 (n = 24) | 9.6 ± 4.5 (n = 13) | 73.2 ± 28.8 (n = 13) | 292.0 ± 107.8 (n = 13) | 0.154 ± 0.073 (n = 21) |
| CG | 8.8 ± 3.2 (n = 66) | 12.5 ± 4.5 (n = 44) | 65.8 ± 25.1 (n = 44) | 314.5 ± 125.2 (n = 41) | 0.194 ± 0.128 (n = 55) |
| GG | 8.9 ± 5.0 (n = 26) | 10.4 ± 3.5 (n = 14) | 63.7 ± 24.5 (n = 13) | 279.8 ± 131.0 (n = 12) | 0.175 ± 0.074 (n = 23) |
| MTR A2756G | |||||
| AA | 8.6 ± 2.4 (n = 57) | 11.9 ± 4.5 (n = 35) | 68.7 ± 28.8 (n = 34) | 333.0 ± 126.3 (n = 31) * | 0.188 ± 0.124 (n = 50) |
| AG | 8.6 ± 2.7 (n = 38) | 11.6 ± 4.2 (n = 22) | 67.1 ± 20.8 (n = 22) | 283.1 ± 96.9 (n = 22) * | 0.165 ± 0.062 (n = 31) |
| GG | 10.1 ± 7.3 (n = 6) | 10.5 ± 3.0 (n = 5) | 61.1 ± 31.8 (n = 5) | 200.2 ± 112.3 (n = 5) * | 0.230 ± 0.193 (n = 6) |
| MTRR A66G | |||||
| AA | 8.8 ± 3.8 (n = 38) | 11.3 ± 3.9 (n = 24) | 64.3 ± 27.8 (n = 24) | 337.4 ± 138.6 (n = 22) | 0.193 ± 0.141 (n = 33) |
| AG | 8.6 ± 2.5 (n = 54) | 12.0 ± 4.7 (n = 33) | 68.9 ± 27.5 (n = 32) | 282.8 ± 110.2 (n = 31) | 0.178 ± 0.076 (n = 48) |
| GG | 8.8 ± 2.4 (n = 27) | 11.4 ± 3.8 (n = 16) | 65.6 ± 17.3 (n = 16) | 297.4 ± 106.2 (n = 15) | 0.165 ± 0.107 (n = 22) |
* p value statistically significant. p values for differences between means of the three genotypes were tested with one-way analysis of variance (ANOVA). p values < 0.05 were considered statistically significant. Significantly different plasma concentrations were found for tHcy between MTHFR 677 three genotypes (p = 0.027) and for tCbl between MTR 2756 genotypes (p = 0.041). Total Homocysteine (tHcy); total cobalamin (tCbl); holo-transcobalamin (Holo-TC); methylmalonic acid (MMA).
5. Conclusions
Most available reference values for Hcy plasma concentrations have been established in adults. Therefore, the present study adds new information reporting plasma concentrations of tCbl, Holo-Cbl, tHcy and MMA in two groups, 9-yo and 17-yo, of a healthy pediatric cohort. The study of genetic variants related to Hcy metabolism in young pediatric populations may also allow for better knowledge of native phenotypes, as time was not enough for environmental factors to modify them substantially.
In conclusion, this work contributes to a better characterization of Hcy metabolism in pediatric populations. Moreover, it reinforces the notion that regarding the plasma concentrations of relevant biomarkers and their interactions with genotype, age powerfully impacts on Hcy metabolism within these populations. Extensive studies are needed to assess further knowledge concerning the impact of age on the modulation of Hcy metabolism and related pathologies, enabling the implementation of disease prevention measures.
Acknowledgments
We thank Elisa M. Alves for her excellent technical assistance.
Author Contributions
The authors’ responsibilities were as follows—H.C.-A., R.C. and I.T.d.A.: designed the research; H.C.-A., R.R. and C.F.: conducted the research; H.C.-A.: analyzed the data; I.R. and I.T.d.A.: critically revised the manuscript; H.C.-A. and R.C. wrote the manuscript and had responsibility for the final content; and all authors: read and approved the final manuscript.
Funding
Supported by FCT (Fundação para a Ciência e a Tecnologia) (project Pest-OE/QUI/UI0674/2013, CQM, Portuguese Government funds), Associação Regional para o Desenvolvimento da Investigação Tecnologia e Inovação (ARDITI) through the project M1420-01-0145-FEDER-000005—Centro de Química da Madeira - CQM+ (Madeira 14-20 Program) and by the Genetics and Metabolism Laboratory, Faculty of Pharmacy, University of Lisbon.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.Ducker G.S., Rabinowitz J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017;25:27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho V., Massey T.E., King W.D. Effects of methionine synthase and methylenetetrahydrofolate reductase gene polymorphisms on markers of one-carbon metabolism. Genes Nutr. 2013;8:571–580. doi: 10.1007/s12263-013-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacalini M.G., Friso S., Olivieri F., Pirazzini C., Giuliani C., Capri M., Santoro A., Franceschi C., Garagnani P. Present and future of anti-ageing epigenetic diets. Mech. Ageing Dev. 2014;136–137:101–115. doi: 10.1016/j.mad.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Fiorito G., Guarrera S., Valle C., Ricceri F., Russo A., Grioni S., Mattiello A., Di Gaetano C., Rosa F., Modica F., et al. B-vitamins intake, DNA-methylation of One Carbon Metabolism and homocysteine pathway genes and myocardial infarction risk: The EPICOR study. Nutr. Metab. Cardiovasc. Dis. 2014;24:483–488. doi: 10.1016/j.numecd.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Cascalheira J.F., Goncalves M., Barroso M., Castro R., Palmeira M., Serpa A., Dias-Cabral A.C., Domingues F.C., Almeida S. Association of the transcobalamin II gene 776C --> G polymorphism with Alzheimer’s type dementia: Dependence on the 5,10-methylenetetrahydrofolate reductase 1298A --> C polymorphism genotype. Ann. Clin. Biochem. 2015;52:448–455. doi: 10.1177/0004563214561770. [DOI] [PubMed] [Google Scholar]
- 6.Cascalheira J.F., Joao S.S., Pinhancos S.S., Castro R., Palmeira M., Almeida S., Domingues F.C. Serum homocysteine: Interplay with other circulating and genetic factors in association to Alzheimer’s type dementia. Clin. Biochem. 2009;42:783–790. doi: 10.1016/j.clinbiochem.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein J.D. Methionine metabolism in mammals. J. Nutr. Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 8.Selhub J. Homocysteine metabolism. Annu. Rev. Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 9.Castro R., Rivera I., Blom H.J., Jakobs C., Tavares de Almeida I. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: An overview. J. Inherit. Metab. Dis. 2006;29:3–20. doi: 10.1007/s10545-006-0106-5. [DOI] [PubMed] [Google Scholar]
- 10.Esse R., Barroso M., Tavares de Almeida I., Castro R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int. J. Mol. Sci. 2019;20:867. doi: 10.3390/ijms20040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handy D.E., Castro R., Loscalzo J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation. 2011;123:2145–2156. doi: 10.1161/CIRCULATIONAHA.110.956839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regec A., Quadros E.V., Platica O., Rothenberg S.P. The cloning and characterization of the human transcobalamin II gene. Blood. 1995;85:2711–2719. [PubMed] [Google Scholar]
- 13.Klee G.G. Cobalamin and folate evaluation: Measurement of methylmalonic acid and homocysteine vs. vitamin B(12) and folate. Clin Chem. 2000;46:1277–1283. [PubMed] [Google Scholar]
- 14.Cacciapuoti F. Hyper-homocysteinemia: A novel risk factor or a powerful marker for cardiovascular diseases? Pathogenetic and therapeutical uncertainties. J. Thromb. Thrombolysis. 2011;32:82–88. doi: 10.1007/s11239-011-0550-4. [DOI] [PubMed] [Google Scholar]
- 15.Fowler B., Schutgens R.B., Rosenblatt D.S., Smit G.P., Lindemans J. Folate-responsive homocystinuria and megaloblastic anaemia in a female patient with functional methionine synthase deficiency (cblE disease) J. Inherit. Metab. Dis. 1997;20:731–741. doi: 10.1023/A:1005372730310. [DOI] [PubMed] [Google Scholar]
- 16.Castro R., Barroso M., Rocha M., Esse R., Ramos R., Ravasco P., Rivera I., de Almeida I.T. The TCN2 776CNG polymorphism correlates with vitamin B(12) cellular delivery in healthy adult populations. Clin. Biochem. 2010;43:645–649. doi: 10.1016/j.clinbiochem.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Nexo E., Hoffmann-Lucke E. Holotranscobalamin, a marker of vitamin B-12 status: Analytical aspects and clinical utility. Am. J. Clin. Nutr. 2011;94:359S–365S. doi: 10.3945/ajcn.111.013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogiatzoglou A., Oulhaj A., Smith A.D., Nurk E., Drevon C.A., Ueland P.M., Vollset S.E., Tell G.S., Refsum H. Determinants of plasma methylmalonic acid in a large population: Implications for assessment of vitamin B12 status. Clin. Chem. 2009;55:2198–2206. doi: 10.1373/clinchem.2009.128678. [DOI] [PubMed] [Google Scholar]
- 19.Hannibal L., Lysne V., Bjorke-Monsen A.L., Behringer S., Grunert S.C., Spiekerkoetter U., Jacobsen D.W., Blom H.J. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front. Mol. Biosci. 2016;3:27. doi: 10.3389/fmolb.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey R.L., Durazo-Arvizu R.A., Carmel R., Green R., Pfeiffer C.M., Sempos C.T., Carriquiry A., Yetley E.A. Modeling a methylmalonic acid-derived change point for serum vitamin B-12 for adults in NHANES. Am. J. Clin. Nutr. 2013;98:460–467. doi: 10.3945/ajcn.113.061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frosst P., Blom H.J., Milos R., Goyette P., Sheppard C.A., Matthews R.G., Boers G.J., den Heijer M., Kluijtmans L.A., van den Heuvel L.P., et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 22.Trinh B.N., Ong C.N., Coetzee G.A., Yu M.C., Laird P.W. Thymidylate synthase: A novel genetic determinant of plasma homocysteine and folate levels. Hum. Genet. 2002;111:299–302. doi: 10.1007/s00439-002-0779-2. [DOI] [PubMed] [Google Scholar]
- 23.Dekou V., Gudnason V., Hawe E., Miller G.J., Stansbie D., Humphries S.E. Gene-environment and gene-gene interaction in the determination of plasma homocysteine levels in healthy middle-aged men. Thromb. Haemost. 2001;85:67–74. doi: 10.1055/s-0037-1612906. [DOI] [PubMed] [Google Scholar]
- 24.Kluijtmans L.A., van den Heuvel L.P., Boers G.H., Frosst P., Stevens E.M., van Oost B.A., den Heijer M., Trijbels F.J., Rozen R., Blom H.J. Molecular genetic analysis in mild hyperhomocysteinemia: A common mutation in the methylenetetrahydrofolate reductase gene is a genetic risk factor for cardiovascular disease. Am. J. Hum. Genet. 1996;58:35–41. [PMC free article] [PubMed] [Google Scholar]
- 25.Klerk M., Verhoef P., Clarke R., Blom H.J., Kok F.J., Schouten E.G., MTHFR Studies Collaboration Group MTHFR 677C-->T polymorphism and risk of coronary heart disease: A meta-analysis. JAMA. 2002;288:2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- 26.Chmurzynska A., Malinowska A.M., Twardowska-Rajewska J., Gawecki J. Elderly women: Homocysteine reduction by short-term folic acid supplementation resulting in increased glucose concentrations and affecting lipid metabolism (C677T MTHFR polymorphism) Nutrition. 2013;29:841–844. doi: 10.1016/j.nut.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Castro R., Rivera I., Ravasco P., Jakobs C., Blom H.J., Camilo M.E., de Almeida I.T. 5,10-Methylenetetrahydrofolate reductase 677C-->T and 1298A-->C mutations are genetic determinants of elevated homocysteine. QJM. 2003;96:297–303. doi: 10.1093/qjmed/hcg039. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang S., Li Y., Liu Z., Chang H., Wu J. Association between MTR A2756G and MTRR A66G polymorphisms and maternal risk for neural tube defects: A meta-analysis. Gene. 2013;515:308–312. doi: 10.1016/j.gene.2012.11.070. [DOI] [PubMed] [Google Scholar]
- 29.Weisberg I., Tran P., Christensen B., Sibani S., Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol. Genet. Metab. 1998;64:169–172. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 30.Harmon D.L., Shields D.C., Woodside J.V., McMaster D., Yarnell J.W., Young I.S., Peng K., Shane B., Evans A.E., Whitehead A.S. Methionine synthase D919G polymorphism is a significant but modest determinant of circulating homocysteine concentrations. Genet. Epidemiol. 1999;17:298–309. doi: 10.1002/(SICI)1098-2272(199911)17:4<298::AID-GEPI5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 31.Olteanu H., Munson T., Banerjee R. Differences in the efficiency of reductive activation of methionine synthase and exogenous electron acceptors between the common polymorphic variants of human methionine synthase reductase. Biochemistry. 2002;41:13378–13385. doi: 10.1021/bi020536s. [DOI] [PubMed] [Google Scholar]
- 32.Afman L.A., Lievers K.J., van der Put N.M., Trijbels F.J., Blom H.J. Single nucleotide polymorphisms in the transcobalamin gene: Relationship with transcobalamin concentrations and risk for neural tube defects. Eur. J. Hum. Genet. 2002;10:433–438. doi: 10.1038/sj.ejhg.5200830. [DOI] [PubMed] [Google Scholar]
- 33.Lievers K.J., Kluijtmans L.A., Boers G.H., Verhoef P., den Heijer M., Trijbels F.J., Blom H.J. Influence of a glutamate carboxypeptidase II (GCPII) polymorphism (1561C-->T) on plasma homocysteine, folate and vitamin B(12) levels and its relationship to cardiovascular disease risk. Atherosclerosis. 2002;164:269–273. doi: 10.1016/S0021-9150(02)00065-5. [DOI] [PubMed] [Google Scholar]
- 34.Yazdanpanah M., Chan P.C., Evrovski J., Romaschin A., Cole D.E. An improved assay for plasma methylmalonic acid using chemical ionization gas chromatography mass spectrometry. Clin. Biochem. 2003;36:617–620. doi: 10.1016/S0009-9120(03)00113-9. [DOI] [PubMed] [Google Scholar]
- 35.Brouns R., Ursem N., Lindemans J., Hop W., Pluijm S., Steegers E., Steegers-Theunissen R. Polymorphisms in genes related to folate and cobalamin metabolism and the associations with complex birth defects. Prenat Diagn. 2008;28:485–493. doi: 10.1002/pd.2006. [DOI] [PubMed] [Google Scholar]
- 36.Blom H.J., Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J. Inherit. Metab. Dis. 2011;34:75–81. doi: 10.1007/s10545-010-9177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doets E.L., Ueland P.M., Tell G.S., Vollset S.E., Nygard O.K., Van’t Veer P., de Groot L.C., Nurk E., Refsum H., Smith A.D., et al. Interactions between plasma concentrations of folate and markers of vitamin B(12) status with cognitive performance in elderly people not exposed to folic acid fortification: The Hordaland Health Study. Br. J. Nutr. 2014;111:1085–1095. doi: 10.1017/S000711451300336X. [DOI] [PubMed] [Google Scholar]
- 38.Joseph J., Handy D.E., Loscalzo J. Quo vadis: Whither homocysteine research? Cardiovasc. Toxicol. 2009;9:53–63. doi: 10.1007/s12012-009-9042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novakovic R., Cavelaars A.E., Bekkering G.E., Roman-Vinas B., Ngo J., Gurinovic M., Glibetić M., Nikolić M., Golesorkhi M., Medina M.W., et al. Micronutrient intake and status in Central and Eastern Europe compared with other European countries, results from the EURRECA network. Public Health Nutr. 2013;16:824–840. doi: 10.1017/S1368980012004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strand T.A., Taneja S., Kumar T., Manger M.S., Refsum H., Yajnik C.S., Bhandari N. Vitamin B-12, folic acid, and growth in 6- to 30-month-old children: A randomized controlled trial. Pediatrics. 2015;135:e918–e926. doi: 10.1542/peds.2014-1848. [DOI] [PubMed] [Google Scholar]
- 41.Green R., Allen L.H., Bjorke-Monsen A.L., Brito A., Gueant J.L., Miller J.W., Molloy A.M., Nexo E., Stabler S., Toh B.H., et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers. 2017;3:17040. doi: 10.1038/nrdp.2017.40. [DOI] [PubMed] [Google Scholar]
- 42.Hogeveen M., van Beynum I., van Rooij A., Kluijtmans L., den Heijer M., Blom H. Methylmalonic acid values in healthy Dutch children. Eur. J. Nutr. 2008;47:26–31. doi: 10.1007/s00394-007-0692-5. [DOI] [PubMed] [Google Scholar]
- 43.Iglesia I., Doets E.L., Bel-Serrat S., Roman B., Hermoso M., Pena Quintana L., García-Luzardo M.R., Santana-Salguero B., García-Santos Y., Vucic V., et al. Physiological and public health basis for assessing micronutrient requirements in children and adolescents. The EURRECA network. Matern. Child Nutr. 2010;6:84–99. doi: 10.1111/j.1740-8709.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mensink G.B., Fletcher R., Gurinovic M., Huybrechts I., Lafay L., Serra-Majem L., Szponar L., Tetens I., Verkaik-Kloosterman J., Baka A., et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013;110:755–773. doi: 10.1017/S000711451200565X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roman Vinas B., Ribas Barba L., Ngo J., Gurinovic M., Novakovic R., Cavelaars A., de Groot L.C., van’t Veer P., Matthys C., Serra Majem L. Projected prevalence of inadequate nutrient intakes in Europe. Ann. Nutr. Metab. 2011;59:84–95. doi: 10.1159/000332762. [DOI] [PubMed] [Google Scholar]
- 46.De Laet C., Wautrecht J.C., Brasseur D., Dramaix M., Boeynaems J.M., Decuyper J., Kahn A. Plasma homocysteine concentration in a Belgian school-age population. Am. J. Clin. Nutr. 1999;69:968–972. doi: 10.1093/ajcn/69.5.968. [DOI] [PubMed] [Google Scholar]
- 47.Jacques P.F., Rosenberg I.H., Rogers G., Selhub J., Bowman B.A., Gunter E.W., Wright J.D., Johnson C.L. Serum total homocysteine concentrations in adolescent and adult Americans: Results from the third National Health and Nutrition Examination Survey. Am. J. Clin. Nutr. 1999;69:482–489. doi: 10.1093/ajcn/69.3.482. [DOI] [PubMed] [Google Scholar]
- 48.Must A., Jacques P.F., Rogers G., Rosenberg I.H., Selhub J. Serum total homocysteine concentrations in children and adolescents: Results from the third National Health and Nutrition Examination Survey (NHANES III) J. Nutr. 2003;133:2643–2649. doi: 10.1093/jn/133.8.2643. [DOI] [PubMed] [Google Scholar]
- 49.van Beynum I.M., den Heijer M., Thomas C.M., Afman L., Oppenraay-van Emmerzaal D., Blom H.J. Total homocysteine and its predictors in Dutch children. Am. J. Clin. Nutr. 2005;81:1110–1116. doi: 10.1093/ajcn/81.5.1110. [DOI] [PubMed] [Google Scholar]
- 50.Ganji V., Kafai M.R. Population Reference Values for Serum Methylmalonic Acid Concentrations and Its Relationship with Age, Sex, Race-Ethnicity, Supplement Use, Kidney Function and Serum Vitamin B12 in the Post-Folic Acid Fortification Period. Nutrients. 2018;10:74. doi: 10.3390/nu10010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jimenez L., Stamm D.A., Depaula B., Duggan C.P. Is Serum Methylmalonic Acid a Reliable Biomarker of Vitamin B12 Status in Children with Short Bowel Syndrome: A Case Series. J. Pediatr. 2018;192:259–261. doi: 10.1016/j.jpeds.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molloy A.M., Pangilinan F., Mills J.L., Shane B., O’Neill M.B., McGaughey D.M., Velkova A., Abaan H.O., Ueland P.M., McNulty H., et al. A Common Polymorphism in HIBCH Influences Methylmalonic Acid Concentrations in Blood Independently of Cobalamin. Am. J. Hum. Genet. 2016;98:869–882. doi: 10.1016/j.ajhg.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Candito M., Rivet R., Herbeth B., Boisson C., Rudigoz R.C., Luton D., Journel H., Oury J.F., Roux F., Saura R., et al. Nutritional and genetic determinants of vitamin B and homocysteine metabolisms in neural tube defects: A multicenter case-control study. Am. J. Med. Genet. A. 2008;146A:1128–1133. doi: 10.1002/ajmg.a.32199. [DOI] [PubMed] [Google Scholar]
- 54.Freitas A.I., Mendonca I., Guerra G., Brion M., Reis R.P., Carracedo A., Brehm A. Methylenetetrahydrofolate reductase gene, homocysteine and coronary artery disease: The A1298C polymorphism does matter. Inferences from a case study (Madeira, Portugal) Thromb. Res. 2008;122:648–656. doi: 10.1016/j.thromres.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Kluijtmans L.A., Young I.S., Boreham C.A., Murray L., McMaster D., McNulty H., Strain J.J., McPartlin J., Scott J.M., Whitehead A.S. Genetic and nutritional factors contributing to hyperhomocysteinemia in young adults. Blood. 2003;101:2483–2488. doi: 10.1182/blood.V101.7.2483. [DOI] [PubMed] [Google Scholar]
- 56.Wilcken B., Bamforth F., Li Z., Zhu H., Ritvanen A., Renlund M., Stoll C., Alembik Y., Dott B., Czeizel A.E., et al. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): Findings from over 7000 newborns from 16 areas world wide. J. Med. Genet. 2003;40:619–625. doi: 10.1136/jmg.40.8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaughan D.J., Kluijtmans L.A., Barbaux S., McMaster D., Young I.S., Yarnell J.W., Evans A., Whitehead A.S. The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis. 2001;157:451–456. doi: 10.1016/S0021-9150(00)00739-5. [DOI] [PubMed] [Google Scholar]