Abstract
The reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) protects against redox stress by providing reducing equivalents to antioxidants such as glutathione and thioredoxin. NADPH levels decline with aging in several tissues, but whether this is a major driving force for the aging process has not been well established. Global or neural overexpression of several cytoplasmic enzymes that synthesize NADPH have been shown to extend lifespan in model organisms such as Drosophila suggesting a positive relationship between cytoplasmic NADPH levels and longevity. Mitochondrial NADPH plays an important role in the protection against redox stress and cell death and mitochondrial NADPH-utilizing thioredoxin reductase 2 levels correlate with species longevity in cells from rodents and primates. Mitochondrial NADPH shuttles allow for some NADPH flux between the cytoplasm and mitochondria. Since a decline of nicotinamide adenine dinucleotide (NAD+) is linked with aging and because NADP+ is exclusively synthesized from NAD+ by cytoplasmic and mitochondrial NAD+ kinases, a decline in the cytoplasmic or mitochondrial NADPH pool may also contribute to the aging process. Therefore pro-longevity therapies should aim to maintain the levels of both NAD+ and NADPH in aging tissues.
Keywords: NADPH, aging, redox, lifespan, longevity, mitochondrial, NADP, nicotinamide adenine dinucleotide phosphate, glutathione, thioredoxin, Drosophila, C. elegans
1. NADPH and the Redox Theories of Aging
The reduced form of nicotinamide adenine dinucleotide phosphate, NADPH, protects cells from redox stress and is required for the synthesis of fatty acids, cholesterol, and deoxynucleotides. NADPH is also required for steroid biosynthesis and protects cells from toxic metabolites by providing reducing power to cytochrome P450 enzymes. NADPH levels decrease with age [1,2] due to both aging-related loss of nicotinamide adenine dinucleotide (NAD+) [3], a precursor for its synthesis, and to the aging related increase in the [NADP+]/[NADPH] ratio that occurs as a result of oxidative stress due to increased mitochondrial electron transport chain dysfunction [4] and inflammation [5]. Therapies that increase NAD+ levels combined with therapies that increase NADPH levels could greatly benefit subjects with aging-related disorders. This type of combination therapy has already been shown to be better than either monotherapy alone in an animal model of ischemic stroke [6]. The major objectives of this review are to discuss the mechanisms contributing to the aging-related loss of NADPH, to detail the mechanisms regulating the cytoplasmic and mitochondrial [NADP+]/[NADPH], and to give an update on enzymes of the NADPH-powered redox systems that have been identified to modulate lifespan.
The mitochondrial free radical theory of aging (MFRTA) has been a leading theory of aging for nearly 50 years [7] and states that increased levels of mitochondrial reactive oxygen species (ROS) stimulate aging. It therefore suggests that antioxidants and NADPH, which is used for the recycling of many antioxidants, will protect cells from mitochondrial generated ROS to delay tissue and organismal aging. Over the past two decades data has shown that ROS-mediated signaling events in young and healthy organisms can lead to lifespan extension, instead of lifespan decline as was originally proposed by the MFRTA [8]. There are also many experimental results that appear to refute the MFRTA [9]. For example, overexpression of antioxidant enzymes, such as superoxide dismutase (SOD) [10] or catalase [11], or administration of antioxidant supplements, such as vitamins C (ascorbate) [12] or E (tocopherols) [13], do not extend lifespan in several model organisms, although there are exceptions to this statement [14,15,16], the most notable being expression of mitochondrial-targeted catalase extending the lifespan of mice [17].
The fact that many supplemented antioxidants or overexpression of SODs or catalase do not generally extend lifespan could be explained if the activities of the NADPH-fueled redox systems are limiting factors for the protection against lifespan-shortening redox stress and that the activities of SODs and catalase are not limiting. However, it is acknowledged that SOD1 or SOD2 must first detoxify the superoxide produced from the mitochondrial electron transport chain (ETC) to H2O2 before the NADPH-linked redox systems can detoxify the H2O2 to water. In addition it is evident that SOD2 plays an essential role in ROS detoxification in newborns due to the death of SOD2 knockout mice shortly after birth [18]. NADPH is required for optimal catalase activity as it binds and stabilizes the catalase tetramer [19]. Antioxidant vitamins such as vitamins C or E that rely upon NADPH to be recycled to their active reduced forms would be expected to provide little longevity benefit in aged organisms where NADPH levels have declined to such an extent to prevent their efficient recycling. The Redox Stress Theory of Aging [20] and the Redox Theory of Aging [21] suggest that lifespan is regulated by redox changes, including alterations in the [NADP+]/[NADPH], GSSG/GSH (oxidized glutathione disulfide/reduced glutathione), and oxidized thioredoxin/reduced thioredoxin ratios. These theories also highlight the ability of redox changes to influence aging by not only altering oxidative damage, but by altering cell signaling. Examples of this include the activation of the lifespan-extending transcriptional regulators DAF-16/FOXO and SKN-1/Nrf2 in C. elegans by compounds that stimulate ROS production [22,23].
Much data obtained over the past two decades greatly support the MFRTA-derived redox-based theories of aging including the strong negative correlation between the rate of mitochondrial superoxide generation and lifespan in closely related species [24], the strong positive correlation between phospholipid fatty acid saturation levels and lifespan, and the negative correlation between the frequency of cysteine residues in mitochondrial electron transport chain transmembrane spanning regions and lifespan [25]. The higher fatty acid saturation in longer lived species likely evolved to prevent the ROS-mediated oxidation of fatty acid double bonds [26], while the depletion of mitochondrial inner transmembrane cysteine residues likely evolved to prevent thiyl radical formation and potentially lifespan shortening protein crosslinking that can occur when superoxide reacts with protein sulfhydryl groups [27]. The mitochondrial inner membrane is enriched with the phospholipid cardiolipin, which is essential for ETC function and ADP/ATP transport and due to its high degree of fatty acid unsaturation is especially vulnerable to ROS-mediated damage [28].
2. Loss of NAD+ as a Major Cause for Loss of NADPH With Aging
One cause for the aging-related loss of NADPH and increase in oxidative stress with aging is the decrease in the levels of cellular NAD+ [3], the immediate precursor for the synthesis of NADP+ by NAD+ kinases. NAD+ levels decline with aging in mammals for several reasons, one of which is the aging-related decrease in the salvage pathway of NAD+ synthesis as a result of decreased expression of nicotinamide phosphoribosyl transferase (NAMPT) [29], a committed step in this pathway. There is also an increase in NAD+ degradation with aging. The decreased NAD+ levels may be a cause of sirtuin protein deacetylase-dependent [30,31] or sirtuin-independent alterations in mitochondrial ETC activity that results in increased ROS production and increased nuclear DNA damage that activates poly-ADP-ribose polymerase (PARP) in several aged tissues including liver, heart, kidney, and lung [32]. This PARP activation together with the aging-related increase in expression and activity of the NAD+ and NADP+ hydrolyzing enzyme CD38 [33] lead to increased hydrolysis of NAD+ and NADP+ in aged tissues. CD38 was shown to have greater activity (6-fold lower Km and 2-fold higher Vmax) using NADP+ as a substrate than NAD+ [34,35]. PARP activation also leads to decreased NADPH levels as PARP inhibits hexokinase, the first enzyme of glycolysis also required for glucose flux into the NADPH-generating pentose phosphate pathway (PPP) [36].
In brain, SARM1 is another NADase that contributes to the loss of NAD+ under pathological conditions [37]. But whether or not SARM1 is activated in aged brain has yet to be studied in mammals. There are no homologs of CD38 present in the genomes of the aging models Drosophila or C. elegans, but both genomes encode homologs of PARP and SARM1 [38]. Expression of the Drosophila homolog of SARM1 increased during aging or mitochondrial ETC inhibition and was shown to play a role in inducing a pro-inflammatory state [39]. NADP+ phosphatase activities, resulting in the degradation of NADP+ to NAD+, have also been observed in rat liver mitochondrial and Golgi extracts [40,41], but the proteins responsible these activities or any aging-related changes in enzyme activity levels have yet to be identified.
Nematodes and insects, as with other invertebrates, lack NAMPT homologs and the two-step NAD+ salvage pathway present in vertebrates, but instead possess a four-step salvage pathway. In this pathway, nicotinamide is first deaminated to nicotinic acid by a nicotinamidase and then the 3-step Preiss–Handler pathway for NAD+ salvage synthesis from nicotinic acid is employed [42,43]. In addition, like mammals, C. elegans can synthesize NAD+ through a de novo pathway from tryptophan [44]. The Drosophila genome lacks a 3-hydroxyanthanilic acid dioxygenase gene encoding an enzyme that synthesizes quinolinic acid in the de novo NAD+ synthesis pathway [45]. Therefore Drosophila appears to strictly rely upon the salvage pathway for NAD+ synthesis.
Several lines of evidence suggest that loss of NAD+ and NADPH may play a role in the aging of Drosophila and C. elegans. The NAD+/NADH ratio declines with aging in both species [46,47], while in C. elegans this loss was driven almost entirely by a decrease in NAD+ levels. Since greater than 80% of pyridine nucleotides are bound to proteins and not free in solution [48], the (free) [NAD+]/[NADH] as measured by the pyruvate/lactate ratio is a much better functional indicator than measuring total levels. In aging C. elegans a loss of (free) [NAD+]/[NADH] also paralleled the loss in total NAD+/NADH [46]. Addition of NAD+ (10 μM–1 mM) [49], nicotinamide (0.2 mM), or nicotinamide riboside (0.5 mM) [50] to the culture medium extended lifespan in C. elegans, even though the C. elegans genome apparently lacks a nicotinamide riboside kinase gene to convert nicotinamide riboside to nicotinamide mononucleotide. But C. elegans possesses homologs to purine nucleoside phosphorylase (PNP) and methylthioadenosine phosphorylase (MTAP) that can likely catabolize nicotinamide riboside to nicotinamide for synthesis of NAD+ synthesis through the salvage pathway [51].
The decreased nuclear NAD+ levels with aging in mouse muscle lead to stabilization of hypoxia inducible factor-1α (HIF-1α) and decreased c-Myc-induced expression of mitochondrial targeted genes, including mitochondrial transcription factor A (TFAM), required for mitochondrial ETC function [52]. Decreased ETC complex I function with aging increases mitochondrial NADH and decreases mitochondrial NAD+ levels, which likely results in decreased phosphorylation of NAD+ to NADP+ by mitochondrial NAD+ kinase 2 (NADK2). Decreased ETC function with aging also reduces the proton-motive force across the mitochondrial inner membrane, which decreases mitochondrial nicotinamide nucleotide transhydrogenase (NNT)-mediated synthesis of NADPH and NAD+ from NADP+ and NADH in the matrix space.
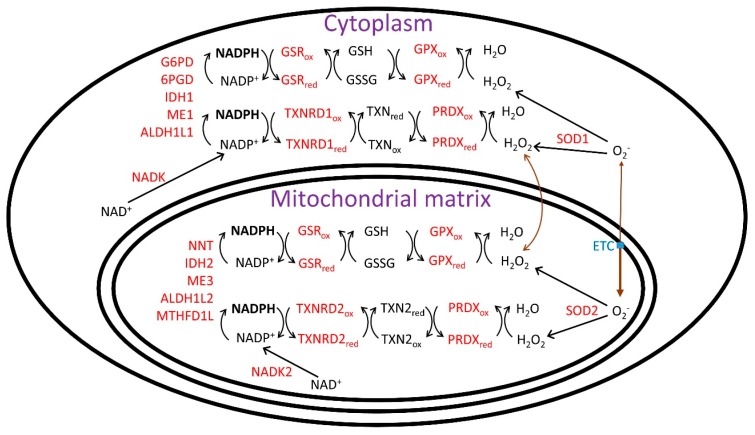
The increase in cytoplasmic and mitochondrial [NADP+]/[NADPH] with aging decreases the activities of cytoplasmic and mitochondrial glutathione reductase (GSR) and thioredoxin reductases (cytoplasmic TXNRD1 and mitochondrial TXNRD2), which lead to more oxidized states of the glutathione redox couple (GSSG/GSH) and thioredoxins (cytoplasmic TXN and mitochondrial TXN2), resulting in decreased activities of glutathione peroxidases (GPX) and peroxiredoxins (also called thioredoxin peroxidases). See Figure 1 for an overview of the cytoplasmic and mitochondrial NADPH-linked redox systems. This oxidation of the NADPH-linked redox systems with aging also causes the oxidation of glutaredoxins, ascorbate (vitamin C), and tocopherols (vitamin E) [53] and eventually leads to oxidation of lipids, nucleic acids, and amino acids and the cell and tissue dysfunction that accompanies the aging process.
Figure 1.
Cytoplasmic and mitochondrial reduced nicotinamide adenine dinucleotide phosphate (NADPH)-linked redox systems. NADK: nicotinamide adenine dinucleotide (NAD+) kinase; ETC: electron transport chain; GSR: glutathione reductase; GSH: reduced glutathione; GSSG: oxidized glutathione disulfide; GPX: glutathione peroxidase; TXN: thioredoxin; TXNRD: thioredoxin reductase; PRDX: peroxiredoxin; H2O2: hydrogen peroxide; O2−: superoxide; G6PD: glucose-6-phosphate dehydrogenase; 6PGD: 6-phosphogluconate dehydrogenase; IDH: isocitrate dehydrogenase; ME: malic enzyme ALDH: aldehyde dehydrogenase; MTHFD: methylenetetrahydrofolate dehydrogenase; NNT: nicotinamide nucleotide transhydrogenase.
3. Aging is Associated with Redox Imbalance
All multicellular organisms that have been studied thus far, with the possible exception of extremely long-lived naked mole rats [54], show oxidizing redox changes with aging accompanied by oxidative damage to macromolecules. Redox stress appears to contribute to almost all aging-related disorders including diabetes, heart disease, and neurodegenerative disease. The [NADP+]/[NADPH] redox couple is the most important redox couple in the fight against aging-induced cellular oxidation. The redox potentials of the free cytoplasmic and mitochondrial [NADP+]/[NADPH] are roughly −400 to −20 mV and are the most negative redox potentials in the cell [55,56]. The cytoplasmic and the mitochondrial [NAD+]/[NADH] couples [57], which are roughly 99.9% and 87% oxidized, respectively, in healthy cells [56,58] become more reduced with aging, while the cytoplasmic and most likely also the mitochondrial [NADP+]/[NADPH] couple, which are both roughly 99% reduced in healthy cells [56,58] become more oxidized with aging. For example, the energy of the cytoplasmic NADP+/NADPH couple became 35 mV less negative with aging corresponding to a 23% loss of brain NADPH levels from 10 to 21 months of age in mice [2]. The initial mechanisms involved in this redox imbalance remain unclear, but at least in the brain the aging-induced redox imbalances may result from the decreased expression of NAMPT and NNT [2].
The GSH/GSSG ratio in cells depends heavily on the [NADP+]/[NADPH], but appears not to be in equilibrium due to the moderate expression level of glutathione reductase, the enzyme that links these couples. The GSH/GSSG is approximately 20:1 to 100:1 (95–99% reduced) [59], while during times of oxidative stress this ratio may drop to 5:1 (80% reduced) or even 1:1 (50% reduced) [60]. Cytoplasmic and mitochondrial GSH levels are 1 to 10 mM, two to three orders of magnitude higher than cytoplasmic and mitochondrial [NADPH] [61,62], due in part to the fact that the vast majority of cellular NADP(H) is bound and not free [63]. In addition in rat liver 59% of the total cellular NADP(H) was reported to be present in mitochondria [58], although mitochondria only make up 10–15% of the cell volume. Therefore it may take many hours to days for cells to completely recover from oxidation of GSH if the cells are able to recover at all [64].
Opposite to the oxidizing effects of aging on the plasma GSSG/GSH and cystine/cysteine ratios [65], the NADP+/NADPH ratio in human plasma has been reported to become more reduced with aging due to increased levels of NADPH, even though NADP+ levels declined [66]. Inhibiting cellular export or stimulating cellular uptake of different forms of vitamin B3 (niacin) may be a strategy to maintain cell and tissue NADPH levels with aging. In this regard supplementation with nicotinamide riboside has been shown to be beneficial to increase plasma NAD+ levels [67]. No reports of plasma membrane transport of NADP(H) could be found, but intraperitoneal or tail vain injection of NADPH in mice was shown to increase the levels of NADPH in plasma and brain, respectively [68].
In Drosophila the NADP+/NADPH increased in late adulthood in parallel with the decline in the NAD+/NADH [47]. To the best of our knowledge total NADP+ and NADPH levels have not been measured in young and aged C. elegans. The isocitrate/alpha-ketoglutarate ratio as a measure of the cytoplasmic [NADP+]/[NADPH] in young and old worms has also yet to be determined. As only one malic enzyme gene is present in C. elegans and it contains a mitochondrial targeting signal [69], measurement of the nematode malate/pyruvate ratio will likely not be indicative of the cytoplasmic [NADP+]/[NADPH] as it is in mammals, which possess a cytoplasmic NADP+-dependent malic enzyme (ME1) [56].
4. Reductive Stress Can Influence the Rate of Aging
Although the study of oxidative stress is common, the study of reductive stress is somewhat rare, especially as it pertains to aging. The terms oxidative and reductive stress are somewhat unclear [70] as they do not specifically refer to the [NADP+]/[NADPH] or [NAD+]/[NADH] couple. Most investigators utilize the term oxidative stress to refer to a state where the [NADP+]/[NADPH], GSSG/GSH, and protein cysteine thiols are more oxidized than the normal healthy condition and reductive stress to mean that these redox couples are more reduced than normal.
Quantification of oxidative stress in lysed cell, tissue, or isolated mitochondrial extracts is frequently performed by measurements of GSSG/GSH. The cytoplasmic or mitochondrial [NADP+]/[NADPH] is slightly more difficult to measure as one must either use a fluorescent probe in intact cells [71] or measure the ratios of specific metabolites that are in equilibrium with the [NADP+]/[NADPH] in lysed cells or tissues [56,58]. Lysed cell measurements of total NADP+/NADPH (or NAD+/NADH) are not indicative of the free levels as bound nucleotides are released into solution upon lysis [56].
The oxidation state of protein thiols in live cells can be measured using the genetically encoded fluorescent probes roGFP or roGFP2 [72]. High levels of glutaredoxins can equilibrate roGFP or roGFP2 with the redox state of the glutathione pool. Therefore, a roGFP2-glutaredoxin fusion protein was developed and expressed in Drosophila to measure the GSSG/GSH with aging [73]. This probe showed a stable, highly reduced cytoplasmic GSSG/GSH with aging, while there was heterogeneous oxidation of the mitochondrial GSSG/GSH in both young and aged fat body, hemocytes, and Malpighian tubules, but a stable reduced GSSG/GSH in young and aged mitochondria from muscles and gut enterocytes [74]. Feeding N-acetylcysteine (NAC) to flies led to reductive stress characterized by slightly decreased cytoplasmic H2O2 in gut and increased mitochondrial H2O2 in fat, gut, and Malpighian tubules [74].
Further elegant studies using cytoplasmic and mitochondrial targeted roGFP have shown that increasing the reduction of cytoplasmic GSSG/GSH and protein thiols to induce reductive stress as measured by cytoplasmic roGFP leads to production of ROS in mitochondria and oxidative stress as evidenced by oxidation of mitochondrial roGFP [75]. Reducing power in the cytoplasm can be transmitted to the mitochondrial matrix by both the direct transport of GSH into the mitochondrial matrix [76,77] as well as by mitochondrial NADPH shuttles that can transfer NADPH reducing equivalents [78]. A major NADPH shuttle system uses cytoplasmic isocitrate dehydrogenase 1 (IDH1), the mitochondrial tricarboxylate (citrate) carrier, and mitochondrial isocitrate dehydrogenase 2 (IDH2). Once the reducing equivalents are transported into the matrix space, the reduced [NADP+]/NADPH] in the matrix space activates glutathione reductase to reduce the GSSG/GSH and activates thioredoxin reductase to reduce thioredoxin. However, once the mitochondrial GSSG/GSH and thioredoxin pools become highly reduced, superoxide and hydrogen peroxide are generated by the mitochondrial glutathione reductase and thioredoxin reductase enzymes as flavins in these two enzymes produce ROS in the absence of their GSSG and oxidized thioredoxin substrates [79].
Adding 2-deoxyglucose to C. elegans culture medium or knocking down expression of the glycolytic enzyme glucose-6-phosphate isomerase, gpi-1, to inhibit glycolysis led to lifespan extension [23]. The mechanism of lifespan extension has not been fully established, but the authors showed a transient increase in respiration and ROS production following inhibition of glycolysis and showed that lifespan extension could be blocked by supplemented antioxidants [23]. Therefore, they concluded that a ROS signal mediates lifespan extension. However, others have suggested that the addition of antioxidants may have blocked lifespan extension by inducing reductive stress instead of by quenching ROS [80]. It has also been shown that inhibiting glycolysis stimulates metabolic flux through the PPP to increase NADPH production [81], so the lifespan extension by glycolytic inhibition may also have been due in part to a reduced cytoplasmic [NADP+]/[NADPH] and increased redox defenses that followed the transient increase in ROS production. Studies using yeast where the glycolytic enzyme triose phosphate isomerase was deleted showed a reduced NADP+/NADPH ratio likely due to increased PPP flux, but decreased replicative lifespan [82], perhaps in part due to reductive stress.
In mice reductive stress due to increased NADPH production by the PPP enzyme glucose-6-phosphate dehydrogenase (G6PD) has been shown to be associated with protein aggregation and cardiomyopathy [83]. Reductive stress could also potentially lower the already low levels of cytoplasmic [NADP+] (1–100 nM) to decrease the activity of enzymes that rely upon it to drive metabolism. As cytoplasmic oxidative stress appears to be much more prevalent than reductive stress, especially in aged tissues, it does not appear likely that cytoplasmic reductive stress is a cause of mitochondrial oxidative stress in aging tissues or major aging-related diseases.
5. Could the Low Levels of Cytoplasmic Glutathione Peroxidase Activity in Long-Lived Species Function in the Preservation of [NADP+]/[NADPH] and GSSG/GSH with Aging to Promote Longevity?
Since the redox theories of aging are gaining traction, more detailed studies of the roles of the cytoplasmic and mitochondrial NADPH-glutathione reductase and NADPH-thioredoxin reductase systems in aging are needed. There is also a dearth of knowledge on the mechanisms that cause the mitochondrial ETC of short-lived species to generate more superoxide than that of long-lived species and the mechanisms through which the ETC generates increased levels of superoxide with age [8]. Although compounds that block mitochondrial superoxide production from complex I [84] and complex III [85] without affecting oxidative phosphorylation have recently been identified as potential therapeutics, compounds that stabilize the cytoplasmic and mitochondrial [NADP+]/[NADPH] redox couples to maintain redox defense systems with aging are also desperately needed for clinical trials for aging-related diseases.
Naked mole rats have a mean lifespan over 30 years, at least ten times longer than similarly sized mice, and surprisingly have 70-fold less cellular glutathione peroxidase activity than mice [86]. Glutathione peroxidase detoxifies hydrogen peroxide to water through the oxidation of GSH. Therefore, it is possible that increases in specific types of ROS, such as specific types of peroxides in the cytoplasm, are compatible with increased longevity [86]. An alternative to this hypothesis is that the NADPH-thioredoxin reductase-thioredoxin-peroxiredoxin detoxification system for peroxides is upregulated in naked mole rats to compensate for the low activity of the NADPH-glutathione reductase-GSH-glutathione peroxidase system.
The extremely low glutathione peroxidase activity of naked mole rats may even contribute to their extreme longevity through the preservation of reduced cytoplasmic GSSG/GSH and [NADP+]/[NADPH]. If this is true, the maintained high energy of the [NADP+]/[NADPH] couple can then be used to stimulate longevity through increasing the activities of NADPH-utilizing enzymes such as glutathione reductase and thioredoxin reductase to combat other types of redox stress, such as that induced by peroxynitrite [87], which if left unchecked could decrease longevity. The increased lifespan of mice heterozygous for glutathione peroxidase-4 [88] and the strong inverse relationship between cellular glutathione peroxidase activity and lifespan in seven different rodent species [86] support a role for maintaining reduced cytoplasmic GSSG/GSH and [NADP+]/[NADPH] for increased longevity. If only cytoplasmic, but not mitochondrial glutathione peroxidase activity is low in long-lived species, mitochondrial shuttles and glutathione transporters could be used for the increased detoxification of mitochondrial peroxides as a mechanism responsible for the longevity of these species.
Unexpectedly, long lived Drosophila strains did not have more GSH than short-lived strains [89]. Likewise, in rodents and in birds there is no correlation between GSSG/GSH in liver and longevity and even a negative relationship was found between liver GSH and longevity [90]. This data suggests that the cytoplasmic NADPH-glutathione reductase-GSH redox system does not limit longevity. As possible compensation for their low GSH levels, liver from naked mole rats contain much higher levels of the cytoplasmically localized thioredoxin reductase (TXNRD1) and major peroxiredoxin (PRDX1) than short-lived guinea pigs [91]. This may be an indication of higher Nrf2 transcriptional activity in naked mole rats [92].
As mentioned above there is also a negative correlation between mitochondrial ETC-produced superoxide production and longevity [8]. Therefore, long-lived species appear to have evolved more efficient ETC machinery that plays an important role in maintaining a reduced intracellular environment. Consistent with this, it was also reported that naked mole rats have greatly decreased levels of proteins for ETC complex I, an important source of ROS, compared to short-lived guinea pigs [91]. However, another report indicated no difference in ETC complex I activity between naked mole rats and mice, but decreased ETC complex II activity in naked mole rats [93]. It was also shown that isolated mitochondria from naked mole rats have a greatly enhanced ability to detoxify hydrogen peroxide compared to those from mice, largely due to higher activity of the mitochondrial NADPH-glutathione reductase-GSH-glutathione peroxidase system [93].
To help resolve some of the current controversies in the field, future studies could focus on measurements of [NADP+]/[NADPH] and GSSG/GSH in hypothalamus and white adipose tissue in closely related species with different maximal lifespans. These tissues appear have a greater influence on organismal aging than tissues or cells where past measurements have been made [94,95]. Measurements using mitochondria isolated from these tissues would also be important, as only roughly 10–15% of cellular GSH is present in mitochondria [96]. Importantly NADPH has been shown to bind to the 39 kD subunit of ETC complex I to stabilize the complex [97,98] and ETC complex I activity declines with aging [99,100].
6. The NADPH-Thioredoxin Reductase Redox System and Aging
Thioredoxin reductases use NADPH to reduce oxidized thioredoxin. In the cytoplasm TXNRD1 reduces TXN and in mitochondria TXNRD2 reduces TXN2 [101]. Double heterozygous knock out mice (TXN+/−/TXN2+/−) were surprisingly long-lived [102] suggesting that TXN or TXN2 levels do not limit lifespan, although follow up studies should be performed to determine if compensatory reductions of other redox couples such as the NADP+/NADPH or GSSG/GSH are responsible for the increased longevity. Transgenic TXN or TXN2 mice show increased mean, but not maximal lifespan [102,103]. Overexpression of TXN and TXN2 together surprisingly led to decreased lifespan [101] suggesting that redox signaling occurs between the cytoplasmic and mitochondrial compartments.
Therapies that maintain a reduced [NADP+]/[NADPH] in the cytoplasm or mitochondria of aged organisms, but without inducing excessive reductive stress are promising longevity therapies. Longevity studies using mice overexpressing TXNRD1 or TXNRD2 would aid our understanding of the role that these proteins play in mammalian longevity. Mitochondrial TXNRD2 is an especially promising candidate to overexpress or deplete in mice for aging studies because protein levels and activities in fibroblasts positively correlate with longevity across multiple rodent and primate species [104]. Overexpression of the Drosophila homolog of TXNRD2, Trxr-2, extended median lifespan [104], while overexpression of the mostly cytoplasmic, but partially mitochondrial Drosophila thioredoxin reductase Trxr-1 did not extend lifespan during normoxia [105], but it did during hyperoxia [106]. Further evidence for a role of the mitochondrial TXNRD2-TXN2 system in enhanced longevity is that growth hormone downregulates the expression of these two genes in liver and growth-hormone deficient Ames dwarf mice are long-lived [107].
In rat brain mitochondria that naturally lack catalase, roughly 75% of the hydrogen peroxide generated was shown to be detoxified by the NADPH-TXNRD2-TXN2-peroxiredoxin system, while only 25% was detoxified by the NADPH-glutathione reductase-GSH-glutathione peroxidase system [108]. This result was quite surprising given that GSH is present at roughly 100-1,000 fold higher levels than Txn2 in mitochondria [109]. In addition to reducing oxidized thioredoxin, thioredoxin reductases can reduce dehydroascorbate [110], ubiquinone [111], cytochrome c [112], lipoamide, lipoic acid, [113], and lipid hydroperoxides [114], which also may play a role in the regulation of lifespan.
Knockdown of C. elegans cytoplasmic thioredoxin reductase trxr-1 [115,116] or mitochondrial thioredoxin reductase trxr-2 [117] by RNA interference decreased lifespan. However, deletion of trxr-1 did not affect lifespan [117], while deletion of trx-2 decreased lifespan at 25 °C, a temperature at the high end of the normal culture range, but not at 20 °C, the most typical culture temperature [117]. TRXR-2 was found to be protective against beta-amyloid toxicity in a C. elegans Alzheimer’s disease model [118], while TRXR-1 was found to be protective against 6-hydroxydopamine toxicity in a nematode Parkinson’s disease model [119].
C. elegans peroxiredoxin prdx-2, homologous to human PRDX1, was required for the lifespan extension that occurs in daf-2 insulin receptor-deficient worms [120] or when metformin was added to the culture medium [121]. The prdx-2 gene was also required for the longevity that was induced by exposing C. elegans larvae to a 25 °C temperature when the culture temperature was otherwise kept at 15 °C [122]. However PRDX-2 did not become oxidized during the normal aging process in C. elegans [123], so its role in lifespan extension could be independent of redox changes.
Perhaps the redox enzyme with the strongest connection with longevity in invertebrates is methionine sulfoxide reductase A (MSRA). During oxidative stress, methionine is oxidized to methionine sulfoxide. MSRA catalyzes the reduction of methionine sulfoxide back to methionine. But MSRA must be reduced by the NADPH-thioredoxin reductase-thioredoxin system to be recycled for use [124]. There are two types of genes that encode isoforms of methione sulfoxide reductase, A and B, that each reduces one of the two isomers of methione sulfoxide. MSRA has been more closely linked with lifespan extension and uses both free methionine sulfoxide and the methionine sulfoxide present in proteins as substrates, while MSRB can only the use methionine-sulfoxide present in proteins [125]. MSRA is present in the cytoplasm, mitochondria and nucleus [126], while there are 3 genes in mammals encoding MSRBs, where the gene products localize to the cytoplasm, mitochondria, nucleus, and ER [126].
Knockdown of the C. elegans homolog of MSRA called msra-1 decreased lifespan [127], likely due to an increase in protein unfolding and proteotoxicity when methionine sulfoxide cannot be repaired. Msra-1 is induced by the pro-longevity transcription factor DAF-16/FOXO that becomes activated when insulin signaling is blocked. Knockdown of msra-1 in long-lived daf-2 insulin receptor mutant worms decreased lifespan. Drosophila that simultaneously lack both MSRA and MSRB are short-lived [128], while overexpression of the Drosophila homolog of MSRA, mostly in neurons, extended lifespan [129] and stimulated nuclear translocation of dFOXO [130]. However, in mice overexpression of MSRA in either the cytoplasm or mitochondria did not extend lifespan [131]. This may be due to the fact that Drosophila MSRA lacks methionine oxidase activity while mammalian MSRA contains methionine oxidase activity [132].
7. The NADPH-Glutathione Reductase Redox System and Aging
By analyzing data from multiple rodent and primate species, a positive association between glutathione reductase (GSR) protein levels (and activity) and lifespan in fibroblasts was found [104]. But a negative correlation between brain glutathione reductase activity and lifespan was found in another study [133]. Drosophila lacks a glutathione reductase gene and its function is replaced by the two thioredoxin reductase genes [134]. However, C. elegans studies of the NADPH-glutathione reductase redox system and aging have been performed. Like mammals C. elegans contain only one glutathione reductase gene gsr-1 that generates both cytoplasmic and mitochondrial protein variants [135]. Lifespan extension of 18% occurred following overexpression [136]. Knockout of gsr-1 was lethal as it is required for molting [137]. Transgenic overexpression of the cytoplasmic isoform but not the mitochondrial isoform rescued lethality [135]. Knockdown of gsr-1 by RNAi resulted in decreased lifespan and increased sensitivity to the superoxide generator juglone. Decreased GSR-1 levels may decrease lifespan in part through decreasing autophagy and increasing proteotoxicity [138].
Global low level overexpression of the catalytic subunit of glutamate-cysteine ligase (GCLc), the rate-limiting step in glutathione synthesis, or high level overexpression in neurons has also been shown to increase lifespan in Drosophila by up to 50%, while overexpression of the regulatory subunit of GCL increased lifespan by up to 24% [139,140]. Administering a recombinant glutaredoxin (small protein reduced by GSH) to C. elegans culture medium was shown to extend lifespan dependent upon upregulation of the pro-longevity transcriptional regulator heat shock factor-1 (HSF-1) [141]. Deletion of either of the yeast glutaredoxin genes Grx1 or Grx2 decreased chronological longevity by increasing ROS production that activated Ras-protein kinase A signaling. Therefore, altered redox status and glutaredoxin function modulates lifespan through the activation of longevity regulating signaling pathways.
Surprisingly, young naked mole rats have been shown to have a more oxidized liver GSSG/GSH than mice [142], even though the naked mole rats live much longer. But the naked mole rats did not show increased levels of oxidative damage with aging as was shown in mice [54]. In mice and rats, important redox couples have been shown to be maintained at distinct, non-equilibrium potentials in different subcellular compartments [143]. The mitochondrial [NADP+]/[[NADPH] couple has been estimated to be around −419 mV compared to −400 mV for the cytoplasmic couple [58]. The mitochondrial GSSG/GSH couple was estimated to be between −280 and -330 mV [144,145,146], while the cytoplasmic couple was estimated to be between −200 and −260 mV [145]. So the existing data on GSSG/GSH ratios cannot be used to estimate cytoplasmic or mitochondrial [NADP+]/[NADPH]. Therefore, the cytoplasmic and/or mitochondrial [NADP+]/[NADPH] may not decline with aging in naked mole rats as they do in mice to drive longevity through maintaining thioredoxin and glutathione reductase activities in one or both of these compartments. Since several markers of oxidative damage, such as urinary isoprostanes (marker of lipid peroxidation) and liver malondialdehyde (marker of lipid damage) are higher in aged naked mole rats than in aged mice [54], naked mole rat redox potential is likely only maintained with aging in one or more specific subcellular compartments and not the entire cell as a whole.
Identifying the redox events that drive the aging process is a high priority. Measurements using isolated mitochondria may be required if the redox events that regulate aging are mostly confined to this organelle [107]. Some data suggest that the mitochondrial [NADP+]/[NADPH] redox systems may play a role in longevity regulation, but further studies manipulating the mitochondrial [NADP+]/[NADPH], GSSG/GSH, and oxidized thioredoxin/reduced thioredoxin ratios and monitoring lifespan are required to obtain a better understanding of how the activity of the NADPH-powered mitochondrial redox systems influence lifespan.
8. Evidence for the Regulation of Longevity by the NADPH-Powered Redox Systems in Drosophila
Data supporting the importance of the NADPH-powered redox networks in the regulation of Drosophila longevity include overexpression of glucose-6-phosphate dehydrogenase (G6PD), an NADPH-generating enzyme of the oxidative PPP, increasing lifespan by up to 50% [147]. Expression from a neuronal-specific promoter was able to extend lifespan by up to 40%. Longer-lived strains of Drosophila were shown to have higher G6PD activity than shorter-lived strains [148]. Knockdown of ribose-5-phosphate isomerase, an enzyme of the non-oxidative portion of the PPP, increased G6PD and NADPH levels and lifespan by over 30% [149]. Overexpression of the NADPH-generating cytoplasmic malic enzyme (Men) [150] extended lifespan in Drosophila as well [151,152]. Overexpression of Men, either throughout lifespan or only during larval development, was able to stimulate longevity, while adulthood only expression in the fat body did not extend lifespan. Surprisingly ROS production was increased when overexpression occurred during larval development that increased stress resistance during adulthood. The data suggest that cytoplasmic reductive stress may have been responsible for the increased mitochondrial ROS production during larval development resulting in the lifespan extension. Men, cytoplasmic isocitrate dehydrogenase (Idh), and G6PD have been shown to form a network maintaining cytoplasmic NADPH levels stable with Men having a slightly larger role than either of the other two enzymes [153].
It will be important to determine if overexpression of G6PD only during larval development increases cytoplasmic reductive stress and mitochondrial ROS production during this time period to increase lifespan. Also, it will be important to determine if overexpression of Pug/Pugilist, a cytoplasmic NADPH-generating methylenetetrahydrofolate dehydrogenase enzyme [154,155] extends lifespan. However, these results may be difficult to interpret as targeted overexpression of Nmdmc, a mitochondrial NADH-generating methylenetetrahydrofolate dehydrogenase gene, in the fat body also extended lifespan [156]. Lastly, much information could be gained by determining the effects of overexpression of Drosophila mitochondrial NADPH-generating malic enzyme (Men-b) on lifespan. The Drosophila genome also contains two other uncharacterized malic enzyme-like genes Menl-1 and Menl-2 whose gene products also likely have a mitochondrial localization [157].
Further data linking NADPH levels with increased lifespan in Drosophila include the association between longevity and a specific allele of the cytoplasmic NADPH-generating isocitrate dehydrogenase gene Idh [158] and flies that were selected for a long lifespan showing increased activities of several NADPH-generating enzymes [159]. Interestingly, Drosophila lacks homologs to mammalian mitochondrial NADPH-generating isocitrate dehydrogenase (IDH2) and NADPH-generating mitochondrial nicotinamide nucleotide transhydrogenase (NNT), while homologs of both are present in C. elegans. However, a proteomics experiment identified the mostly cytoplasmic Idh protein as being present in Drosophila mitochondria [160]. Therefore, Drosophila likely depends heavily on malic enzyme (Men-b) and the mitochondrial localized fraction of Idh for mitochondrial NADPH production.
There are seven peroxiredoxin genes in Drosophila. Overexpression of three different Drosophila peroxiredoxin (thioredoxin peroxidase) genes has been shown to extend lifespan. When expressed in neurons dPrx1/Jafrac1, homologous to mammalian PRDX2, increased lifespan up to 29% [161]. Drosophila dPrx5 is endogenously localized to multiple subcellular compartments including the cytoplasm, nucleus, and mitochondria. Global overexpression increased lifespan by more than 30% [162]. Overexpression of dPrx5 specifically in mitochondria also extended lifespan [163]. dPrx3 is exclusively expressed in mitochondria. Although dPrx3 overexpression did not lead to lifespan extension, a dPrx3/dPrx5 double knockout showed premature aging phenotypes [164,165]. dPrx4 is endogenously localized to the ER, and moderate global overexpression or high level overexpression in neurons increased lifespan by greater than 30% [166]. Therefore, the NADPH-thioredoxin reductase-thioredoxin-peroxiredoxin network plays an important role in regulating aging in Drosophila.
9. Evidence for the Regulation of Longevity by the NADPH-Powered Redox Systems in Mammals
The study of NADPH synthesis in mammalian cells and tissues is complex as it synthesis is shared among at least 5 cytoplasmic enzymes including G6PD, 6PGD (6-phosphogluconate dehydrogenase), IDH1 (isocitrate dehydrogenase 1), ME1 (malic enzyme 1), and ALDH1L1 (10-formyltetrahydrofolate dehydrogenase) and 5 mitochondrial enzymes NNT, IDH2, ME3 (malic enzyme 3), ALDH1L2 (10-formyltetrahydrofolate dehydrogenase), and MTHFD1L (methylenetetrahydrofolate dehydrogenase 1 like) [167] (Figure 1). ALDH1L1, ALDH1L2, and MTHFD1L play a role in one-carbon and folate metabolism [167], and are an important source of NADPH in proliferating cells [168], where ribonucleotide reductase-mediated deoxynucleotide synthesis utilizes reducing equivalents from the NADPH-thioredoxin reductase-thioredoxin redox system. Overexpression of G6PD increased the mean lifespan of female mice [169].
Levels and activities of NADPH generating enzymes are altered by aging. For example, G6PD levels were shown to decline with aging in mouse brain [170], rat liver [171,172] and human erythrocytes [173], but not lymphocytes [174]. Activity of the PPP enzyme 6PGD was shown to decline by 26% with aging due to lysine oxidation, while malic enzyme activity declined 36% with aging due to histidine oxidation [175]. Activities of IDH1 and IDH2 decreased with aging in rat kidney, but increased with aging in rat testes [176]. Both of these changes were prevented by lifespan-extending calorie restriction (CR). The C57B/6J strain of mice was identified to contain a mutation in the NNT gene, but this strain has a longer lifespan than many other inbred strains. So it appears that the mice are able to compensate for the loss of NNT function and synthesize sufficient NADPH for proper mitochondrial function, at least under normal, non-stressed conditions [177].
Reducing the cytoplasmic [NADP+]/[NADPH] may not be beneficial for maintaining a reduced cellular redox environment in all cell types as NADPH oxidase enzymes use NADPH to produce superoxide, and sometimes also hydrogen peroxide [178]. For example, NADPH oxidase Nox2 complexes are present at high levels in macrophages and microglia and function to increase superoxide production. Most NADPH oxidase isoforms are not constitutively active, but require a signal such as increased Ca2+ levels for activation. However, the Nox4 isoform is widely expressed, constitutively active, and present in intracellular membranes such as those of mitochondria, ER, and the nucleus. Nox4 produces mostly hydrogen peroxide that is membrane permeable instead of superoxide that is not [179]. Nox4 has a Km for NADPH of 55 μM [180], while human glutathione reductase has a Km for NADPH of ~9 μM [181], and human cytoplasmic thioredoxin reductase (TXNRD1) has a Km for NADPH of 18 μM. Cytoplasmic NADPH has been measured to be at a concentration of 3 μM in transformed cells, while mitochondrial NADPH was measured to be 37 μM in the same cells [61]. Therefore Nox4 activity is likely to be low under normal conditions, but would increase if Nox4 localized to the inner mitochondrial membrane with the active site facing the matrix space where increased levels of NADPH are present.
Stress increases Nox4 levels and increased Nox4 levels can lead to ETC complex I instability [182]. In addition dominant-negative Nox4 expression caused cytoplasmic reductive stress and increased mitochondrial ROS production, while Nox4 overexpression led to cytoplasmic oxidative stress. Although Nox4 appears to regulate mitochondrial ETC function and ROS production in heart and endothelial cells [183,184], Nox4 knockout mice have a normal lifespan [185]. This is likely due to the low levels of mitochondrial Nox4 in many tissues under normal, healthy conditions [186]. But increased Nox4 expression has been shown to contribute to hydrogen peroxide-induced senescence of endothelial cells [183].
10. Evidence for the Regulation of Longevity by the NADPH-Powered Redox Systems in Budding Yeast
When S. cerevisiae cells are cultured with glucose as the carbon source the majority of cytoplasmic NADPH was produced by the PPP enzyme Zwf1 (G6PD) and acetaldehyde dehydrogenase Ald6 [187], although the PPP 6PGD enzymes Gnd1 and Gnd2 also contributed to a certain extent [188]. When the yeast cells were cultured using a nonfermentable carbon source instead of glucose, levels of cytoplasmic isocitrate dehydrogenase Idp2 increased and it became an important source of cytoplasmic NADPH [189]. Deletion of Ald6 was shown to increase the chronological longevity of yeast [190].
Most mitochondrial NADPH in yeast is generated by the mitochondrial NADH kinase Pos5. Pos5 also contains an NAD+ kinase activity and the Pos5 NAD+ kinase activity followed by the activity of the NADPH-generating Ald4 acetaldehyde dehydrogenase, homologous to human ALDH2, was also shown to play a role in mitochondrial NADPH production. Deletion of Ald4 in yeast increased chronological lifespan [191]. Unlike multicellular eukaryotes, yeast mitochondrial malic enzyme Mae1 and mitochondrial NADP+-dependent isocitrate dehydrogenase Idp1 only appeared to play minor roles in mitochondrial NADPH generation [187]. Like Drosophila, budding yeast lack a homolog to mammalian mitochondrial NNT. In yeast, mitochondrial transporters of NAD+ (Ndt1 and Ndt2) provide the vast majority of NAD+ for mitochondrial NADP(H) synthesis. Ndt1/Ndt2 double deletion mutants showed decreased mitochondrial and cellular NAD(H) levels and increased chronological lifespan, while Ndt1 overexpression resulted in increased mitochondrial and cellular NAD(H) levels and decreased chronological lifespan [192].
In chronologically aged S. cerevisiae cells, NADPH levels and levels of reduced cytoplasmic thioredoxin reductase drop before the majority of cysteine oxidation in proteins and before increased ROS production, while anti-aging dietary restriction (DR) delayed the oxidation of NADPH and thioredoxin reductase and extended lifespan [193]. Therefore, the drop in NADPH levels could be a key driving force in postmitotic cell aging that leads to redox stress. Consistent with this data, deletion of Zwf1 [194] or the cytoplasmic thioredoxin reductase Trr1 decreased chronological longevity when using a strain of the α haploid mating type (MATα), which may be a result of the inverse relationship between thioredoxin reductase activity and the activity of longevity controlling TORC1 kinase [195]. The chronologically short-lived MATα Zwf1 deletion strain had both increased NADP+/NADPH and increased NAD+/NADH demonstrating that the pro-aging effects of NADPH depletion were dominant over the pro-longevity effects of increased NAD+/NADH in this context [194]. However, deletion of the Zwf1 gene increased chronological lifespan when a strain of the a mating type (MATa) was used [196]. Since deletion of NADPH-generating enzymes in yeast generally led to increased longevity, a lifespan-extending signaling pathway appears to be activated by increased NADP+/NADPH. Other studies in yeast have shown that the heat shock-induced increase in replicative longevity was due to a transient increase in superoxide levels that signaled for the redirection of metabolic flux from glycolysis to the PPP to increase NADPH synthesis and GSH levels [197].
There are three different NADPH-generating isocitrate dehydrogenase genes in S. cerevisiae. Idp1 is mitochondrial, Idp2 is cytoplasmic as mentioned above, and Idp3 is peroxisomal. Deletion of each of these 3 genes increased replicative lifespan in a MATα strain [198,199], while deletion of Idp2 or Idp3 decreased replicative lifespan in a MATa strain. Deletion of Idp1 increased chronological longevity in a MATa strain [196], while deletion of Idp2 decreased chronological longevity in a MATa strain [191]. As a comparison, deletions of Idh1 or Idh2 subunits of the mitochondrial NADH-generating isocitrate dehydrogenase complex led to increased replicative lifespans when using either MATa or MATα strains [198,199]. Since these replicative lifespan analyses were performed on glucose-containing media, where the NADPH-generating isocitrate dehydrogenases do not greatly control NADPH levels, the effects on lifespan are likely due to changes in citric acid cycle flux that may alter the NAD+/NADH. Studies like these using a nonfermentable carbon source instead of glucose would be more informative regarding the influence of [NADP+]/[NADPH] on yeast replicative lifespan.
11. Evidence for the Regulation of Longevity by NADPH-Powered Redox Systems in C. Elegans Nematodes
The most commonly studied model of lifespan extension in C. elegans is the daf-2 insulin receptor-deficient strain [200], which requires the DAF-16/FOXO transcriptional regulator and to a lesser extent the SKN-1/Nrf2 transcriptional regulator for the enhanced longevity [201]. The PPP enzyme GSPD-1/G6PD and the cytoplasmic NADP+-dependent isocitrate dehydrogenase IDH-1 likely provide the vast majority of cytoplasmic NADPH in C. elegans, while the PPP enzyme T25B9.9/6PGD also likely contributes. Knockdown of gspd-1 in an idh-1 mutant strain led to severe growth, locomotion, and molting defects, although individual RNAi-mediated knockdown of gspd-1 or individual knockout of idh-1 had no effect on these phenotypes. The growth defect in gspd-1 and idh-1 double deficient animals was possibly mediated by alterations in amino acid metabolism as a result of NADPH depletion [202]. Decreased glutamate levels were found in these NADPH-deficient worms that may have been due to decreased glutamate dehydrogenase (GDH-1) activity, which relies upon NAD(P)H as a cofactor. However, GDH-1 is predicted to be localized to mitochondria [69], where GDH-1 would be less affected by GSPD-1 and IDH-1 deficiency than if it were localized to the cytoplasm. Folate metabolism could also contribute to a minor extent to cytoplasmic NADPH levels as C. elegans possesses two homologs to human MTHFD1 (NADP+-dependent methylenetetrahydrofolate dehydrogenase 1), K07E3.4 and dao-3, the latter being transcriptionally regulated by DAF-16/FOXO [203].
Long-lived daf-2 mutants showed increased IDH-1 activity [204], GSPD-1 levels [204,205], and levels of the mitochondrial citrate carrier (K11H3.3), a component of the mitochondrial NADPH shuttle [204]. The increase in IDH-1 activity may be especially important for longevity as we and others have shown that IDH-1 protein levels (as well as cytoplasmic aconitase (ACO-1) levels) decline greatly with aging [46,206]. Therefore, decreased cytoplasmic NADPH levels or decreased mitochondrial NADPH shuttle activity could contribute to aging phenotypes.
There are likely three major enzymes that contribute to mitochondrial NADPH generation in C. elegans, nicotinamide nucleotide transhydrogenase-1 (NNT-1), isocitrate dehydrogenase-2 (IDH-2), and malic enzyme (MEN-1) [69,157]. However, the localization of MEN-1 has yet to be confirmed experimentally. Nnt-1 gene expression is induced mainly in the intestine and neurons by DAF-16 in the long-lived daf-2 mutant worms [207,208]. Since knockdown of nnt-1 by RNAi decreased lifespan by 18–20% in both N2 control and daf-2 mutant worms [207], NNT-1 is likely a major regulator of mitochondrial NADPH levels. NADPH-generating malic enzyme (MEN-1) levels were not altered in long-lived daf-2 mutants [206].
The increased longevity of C. elegans daf-2 mutants required increased expression of icl-1 (gei-7) [209], the bifunctional mitochondrial glyoxylate shunt enzyme possessing isocitrate lyase and malate synthase activity [210]. Increased ICL-1 activity decreases flux through mitochondrial IDH-2 and the heterotrimeric (IDHA-1, IDHB-1, and IDHG-1/IDHG-2) mitochondrial NADH-generating isocitrate dehydrogenase. ICL-1 expression levels also correlate with longevity in eat-2 mutants that undergo dietary restriction and long-lived mitochondrial ETC mutants [211]. However, this correlation could be uncoupled for ETC deficiency, where knockout of the transcriptional regulator nhr-49 prevented increased icl-1 expression, but did not prevent increased lifespan [212]. With aging there is a strong decrease in the abundance of the IDHG-2 subunit of the NADH-generating isocitrate dehydrogenase complex [206] and a decrease in the NAD+/NADH [46] that would likely increase flux through IDH-2 and counter aging-induced increases in the mitochondrial NADP+/NADPH.
Knockdown of the C. elegans mitochondrial citric acid cycle genes aconitase (aco-2) [213], isocitrate dehydrogenase subunit idha-1 [213], or alpha-ketoglutarate dehydrogenase subunits ogdh-1 [214] or dld-1 (dihydrolipoamide dehydrogenase) [215] extended lifespan. Knockdown of these enzymes could lead to accumulation of mitochondrial citrate, transport of citrate into the cytoplasm, its conversion into isocitrate (by ACO-1) and then into α-ketoglutarate by IDH-1 to reduce cytoplasmic [NADP+]/[NADPH] and increase lifespan.
C. elegans GSPD-1/G6PD may be a very important source of NADPH for removing old cuticle for molting during larval development [137]. Consistent with its important role in NADPH synthesis a gspd-1 knockout is inviable [216]. Unexpectedly, knocking down each of four cytoplasmic PPP enzymes 6PGD (T25B9.9), transaldolase (tald-1), transketolase (tkt-1) [217], or gspd-1 (G6PD) [205] or knocking down idh-1 [213] extended lifespan. The lifespan extension may have been a result of upregulated expression of compensatory NADPH generating enzymes, through ROS-mediated activation of SKN-1/Nrf2 and/or through activation of the mitochondrial unfolded protein response (UPRmt) [217,218]. UPRmt is a stress response pathway that frequently, but not always, associates with lifespan extension [219] and is associated with activation of the transcriptional regulators ATFS-1 (ATF4, ATF5, and CHOP in mammals), DVE-1, and SKN-1/Nrf2 [220]. During larval development ATFS-1 was activated in ETC complex I nuo-6 mutants that resulted in DAF-16/FOXO and HIF-1 activation and increased longevity [221].
In Drosophila, mutations that decreased Idh and malic enzyme (Men) activities were accompanied by a compensatory upregulation of G6PD activity [153,222], while in mice decreased G6PD activity leads to a compensatory increase in IDH1 levels [223]. G6PD is also allosterically activated by NADP+ [224,225]. Perhaps due to these responses, knockdown of C. elegans idh-1 [213], gspd-1 (G6PD) [205] or T25B9.9 (6PGD) [217] led to lifespan extension. Knockdown of the PPP gene tald-1, beginning from the L1 larval stage extended lifespan, but not when knockdown was initiated during adulthood [217]. In contrast, knockdown of gcs-1, the rate limiting step of glutathione synthesis, had no effect on lifespan when initiated at the L1 larval stage, but extended lifespan when initiated at the L4 larval stage [226]. Low levels of the glutathione depleting compound diethyl maleate increased lifespan through a DAF-16 and SKN-1 dependent mechanism, but high diethyl maleate levels decreased lifespan [226]. These compensatory effects that modulate longevity partly driven by DAF-16 and SKN-1 make aging studies where the cytoplasmic redox state is oxidized in young individuals difficult to interpret unless detailed redox measurements are made in parallel.
A C. elegans RNAi screen identified 41 genes including gspd-1, other PPP genes, and glutathione reductase (gsr-1) as the strongest inducers of the SKN-1/Nrf2 target gene gcs-1 [218]. SKN-1 is activated by oxidative stress and regulates expression of hundreds of genes. It decreases expression of insulin-like peptides to activate DAF-16/FOXO [227]. Together DAF-16 and SKN-1 induce several NADPH-generating enzymes and antioxidant enzymes. However, DAF-16 and SKN-1 activity greatly decline with aging [228], which may contribute to the increased NADP+/NADPH with aging. Juglone, an oxidant that induces robust activation of the SKN-1 reporter Pgst-4::gfp in young worms was completely ineffective in activating the reporter by day 10 of the lifespan [228], while daf-2 knockdown failed to extend lifespan when initiated at day 8 of adulthood or later [229]. Consistent with their important roles in longevity, skn-1 or daf-16 knockdown can slightly decrease normal lifespan [230].
Compensatory responses to NADPH depletion also likely occur in humans as G6PD deficiency is associated with long life [231]. As NADPH is a known activator of the class I histone deacetylases (HDACs) HDAC1 and HDAC2 [232], part of this compensatory response may involve decreased activity of class I HDACs, which extends lifespan in model organisms [233].
Some data do not support a role for NADPH levels to be limiting for lifespan. For example, deletion of the mitochondrial nnt-1 in C. elegans increased the GSSG/GSH ratio, but did not decrease lifespan [234]. However, unexpectedly as referred to above, RNAi knockdown of nnt-1 in C. elegans decreased lifespan by 18% [207]. The reasons for the different effects between knockout and knockdown of nnt-1 on lifespan are not known. Completely knocking out nnt-1 may lead to greater ROS production and activation of SKN-1 and DAF-16-mediated protective pathways than incompletely knocking it down. Not only does ROS-mediated SKN-1/Nrf2 activation induce expression of NADPH-generating enzymes to partially compensate for decreased NADPH levels [235], it also increases expression of other antioxidants including superoxide dismutase [236], catalase [237], and enzymes of glutathione metabolism [238]. Activation of SKN-1 by knocking out its endogenous inhibitor WDR-23 extended lifespan, but unexpectedly increased the GSSG/GSH ratio [239], yet another example of how the cellular GSSG/GSH ratio is not always predictive of lifespan. Determining the effects of SKN-1 activation or DAF-16 activation on cytoplasmic and mitochondrial [NADP+]/[NADPH] may help to decipher the mechanisms through which each extends lifespan.
12. Calorie Restriction May Extend Lifespan In Part through the NADPH-Driven Redox Systems
Calorie restriction (CR) extends lifespan in the vast majority of eukaryotic organisms from yeasts to humans [240] and studies using mice have shown that the methionine restriction (MR) induced by the CR diet provides much of this longevity effect through decreasing mitochondrial ROS production [241] and increasing hydrogen sulfide production through the transulfuration pathway [242]. Flux from methionine degradation through the transulfuration pathway results in cysteine production. The majority of cysteine from the diet is oxidized to cystine and requires NADPH oxidation to reduce it to cysteine once it is taken up by cells [243]. Reduction of the extracellular cystine/cysteine has been shown to increase the intracellular levels of NADPH and GSH [244]. Therefore, use of cystine for cysteine synthesis decreases cellular NADPH levels and use of the transulfuration pathway for cysteine production conserves NADPH. Somewhat paradoxically, CR or MR increases expression of transulfuration pathway enzymes [245]. Long-lived Ames dwarf mice also show increased transulfuration pathway activity [246]. The increased cysteine production through this pathway likely leads to decreased cellular cystine uptake, decreased oxidation of NADPH to reduce cystine, and stabilization of the [NADP+]/[NADPH] with aging leading to lifespan extension.
Methionine synthesis in yeast requires 3 NADPH molecules. Studies have shown that a Zwf1 (G6PD) mutant yeast strain becomes a methionine auxotroph as it is not able to produce the NADPH needed for methionine synthesis [247]. Genes of methionine metabolism appear to be co-regulated with PPP enzymes [248]. Methionine auxotrophs show extended replicative [249] and chronological longevity, while methionine supplementation decreases chronological longevity [250,251]. MR may provide an optimal level of cellular NADPH for longevity by minimizing methionine oxidation to methionine sulfoxide, which requires NADPH oxidation to repair, while maintaining high enough levels of methionine where methionine synthesis does not deplete NADPH levels. Arginine and lysine synthesis in yeast also require NADPH and synthesis becomes impaired when NADPH levels are low [252,253]. But, lysine auxotrophy does not increase chronological longevity [250].
Some of the largest consumers of NADPH include the enzymes for the biosynthesis of fatty acids, cholesterol, steroids, and deoxyribonucleotides [48]. During CR, AMP kinase (AMPK) becomes activated [254], prominently in the hypothalamus [255]. AMPK phosphorylates acetyl-CoA carboxylase to inhibit fatty acid synthesis [255]. This inhibition of fatty acid synthesis leads to the activation of fatty acid beta-oxidation (except in neurons that lack these enzymes) to provide cellular energy and prevent a futile cycle of fatty acid synthesis and breakdown. The inhibition of fatty acid synthesis preserves NADPH levels and contributes to the increased levels of redox defenses associated with the longevity benefits of CR. Stimulating AMPK using the AMP analog AICAR (5-Aminoimidazole-4-carboxamide ribonucleotide) increased the cellular NAD+/NADH to activate the NAD+-dependent sirtuin deacetylase SIRT1 [256], which deacetylates and activates the master regulator of mitochondrial gene expression PGC-1α to stimulate mitochondrial biogenesis and increase expression of SOD2 and catalase. Blocking mitochondrial fatty acid uptake with etomoxovir blocked the change in NAD+/NADH [256]. The reduced [NADP+]/[NADPH] induced by AMPK activation functions together with the increased expression of SOD2 and catalase to maintain redox status in the presence of increased mitochondrial ETC function.
The effect of the anti-aging CR diet in rodents on antioxidant systems has been reviewed [257]. Overall, the majority of studies showed no overall change in mitochondrial ROS production or antioxidant enzyme levels by CR. However, GSH was increased, and the GSSG/GSH and oxidative damage were decreased in the vast majority of studies supporting the redox stress theory of aging. The Nrf2 transcriptional regulator was not required for lifespan extension by CR [258]. Two major mechanisms likely drive the reduced GSSG/GSH and decreased oxidative damage in CR mice. These include the CR-mediated increase in mitochondrial SIRT3 levels and the increased activity of FOXO transcriptional regulators due to decreased insulin signaling during CR. SIRT3 has been shown to deacetylate and activate mitochondrial IDH2 to increase mitochondrial NADPH production [259], while the FOXO3 transcriptional regulator increases PPP flux to increase cytoplasmic NADPH [260]. Neural progenitor cells from FOXO3 knockout mice showed increased NADP+/NADPH and GSSG/GSH ratios due to decreased glucose uptake and PPP pathway activity. Furthermore SIRT1 overexpression, which mimics the increased expression of SIRT1 during CR in muscle and white adipose tissue [261], stimulated the formation of a FOXO3/PGC-1α complex that was shown to induce expression of the mitochondrial redox factors TXNRD2, TXN2, peroxiredoxin 3 (PRDX3), and peroxiredoxin 5 (PRDX5) [262].
CR was also shown to blunt the aging-related loss of cytoplasmic TXNRD1 and TXN protein levels in rat kidney [263]. In contrast, in rat cardiac and skeletal muscle aging and CR had no effect on TXNRD1 levels, while CR decreased the aging-related loss of TXN [264]. In muscle, mitochondria-localized TXNRD2 decreased with aging, while CR blunted this effect. Muscle mitochondrial TXN2 levels increased with aging with no effect of CR [264]. CR was also shown to decrease the skeletal muscle levels of TXNIP, an inhibitor of TXN, to enhance TXN function [265]. Extension of lifespan by DR in yeast also required the peroxiredoxin Tsa1 and increased expression of a sulfiredoxin gene to decrease the oxidation of Tsa1 with aging [266]. In C. elegans, lifespan extension by DR required the thioredoxin trx-1 [267]. Global protein cysteine levels were shown not to be oxidized during aging in Drosophila, although 24 hours of fasting caused a major oxidation of protein cysteines [268], most likely by decreasing PPP flux and NADPH generation. However, the use of tissue-specific redox probes for H2O2 and GSSG/GSH were able to measure an aging-induced oxidation of the cytoplasm of midgut enterocytes [74]. Surprisingly long-lived chico (insulin receptor substrate) heterozygotes showed increased, not decreased midgut oxidation.
Some portion of the disease delaying effects of CR may be mediated by the increased levels of ketone bodies that occur as a result of the CR diet [55], as a ketogenic diet increased the mean lifespan of mice [269,270]. Excitingly, exogenous ketone body supplementation partially mimicked the protective redox changes induced by CR in the brain (i.e. reduced GSSG/GSH [257]) as exogenous ketone bodies were able to reduce the cytoplasmic [NADP+]/[NADPH] in the cerebral cortex of 3XTgAD Alzheimer’s mice, but not in their hippocampus [271]. However, oxidative damage as measured by protein carbonyl levels (2,4-dintrophenylhydrazine reactivity) and lipid peroxidation (4-hydroxynonenal levels) was decreased by ketone body treatment in the hippocampus, but not the cortex of these animals. The data suggest that hippocampal NADPH was oxidized to prevent oxidative damage in this region of the brain and this oxidation of NADPH was the likely mechanism through which no ketone body-induced change in the [NADP+]/[NADPH] was found. However, the cerebral cortex of these mice may have lacked high enough NADPH-coupled redox enzyme levels to utilize the reduced [NADP+]/[NADPH] for the prevention of oxidative damage. The exogenous ketone treatment also resulted in increased cytoplasmic [NAD+]/[NADH] in both brain regions similar to CR and increased mitochondrial [NAD+]/[NADH] only in the hippocampus [271].
13. Regulation of Aging by NADPH and the Circadian Clock
It has been clearly demonstrated in several model organisms that robust circadian rhythms are associated with longevity and that disrupting circadian oscillations in humans and other models is associated with aging and aging-related disorders [272,273]. It was found that 43% of mouse protein coding genes oscillated in expression in a circadian manner in at least one of twelve tissues studied [274], while the levels of roughly half of the mRNAs for these proteins oscillated [275] suggesting post-transcriptional events that regulate the circadian changes in the other half of these proteins. The NAD+/NADH and the NADP+/NADPH ratios oscillate over a 24 hour cycle [276,277] due in part to circadian regulation of NAMPT [278,279], NADK [280], and the PPP [281,282]. Therefore, loss of circadian rhythms could play an important role in the decreased NAD+ [3] and NADPH [1,2] levels observed in aged tissues. Disruption of the PPP through the administration of 6-aminonicotinic acid was described to lengthen the circadian period in one study [282], while disruption of the PPP with the inhibitors diphenyleneiodonium (DPI) or dehydroepiandrosterone (DHEA) was described to alter the phase and amplitude of the circadian changes without affecting the period in another [281]. To limit the potential side effects of small molecule inhibitors, future studies could express the highly specific NADPH-oxidizing enzyme triphosphopyridine nucleotide oxidase (TPNOX) [283], an engineered mutant of Lactobacillus brevis NADH oxidase, in cells and target it to the mitochondria or to the cytoplasm to determine the compartment-specific role of changes in the [NADP+]/[NADPH] on the circadian clock.
CR may extend longevity in part by restoring the loss of circadian rhythms. This may occur through the increased NAD+/NADH and reduced NADP+/NADPH conferred by CR, as dimerization of the circadian clock proteins CLOCK and BMAL1 is regulated by redox state [284]. Also, NADPH binds the circadian transcriptional regulator NPAS2 and modulates its dimerization with BMAL1 and DNA binding of the heterodimer [285,286]. In addition, circadian oscillations, especially in genes involved with metabolism, occur in C. elegans [287], Drosophila S2 cells [288], and budding yeast [289], organisms that lack essential components of the well-characterized circadian clock, suggesting an evolutionarily ancient uncharacterized circadian system regulating metabolism. It will be important to determine the influence of changes in the [NAD+]/[NADH] and [NADP+]/[NADPH] on this ancient circadian system.
14. NAD+ Kinases as Possible Regulators of NADPH Levels and Longevity
The human cytoplasmic NAD+ kinase (NADK) was identified in 2001 [290] and has been fairly well-characterized since then, including its regulation by Ca2+/calmodulin-dependent protein kinases [291] and the control of expression by the circadian clock [280]. Mice lacking NADK are not viable [292]. The Drosophila melanogaster genome contains two homologous genes, while the C. elegans genome contains no homologs of human NADK. So up until the year 2012, it was unknown how C. elegans obtained NADP(H). In 2012 a pivotal discovery of the human mitochondrial NAD+ kinase (NADK2) was made [293,294]. The C. elegans genome contains two homologous uncharacterized genes, while the fruit fly genome contains one. Recently, studies using genetically encoded iNap fluorescent NADPH sensors have verified the exchange of NADPH equivalents between the cytoplasmic and mitochondrial compartments in mammalian cells [61]. Overexpression of cytoplasmic NADK resulted in a 4-fold increase in cytoplasmic [NADPH] and a 57% increase in mitochondrial [NADPH].
Like other eukaryotes, yeast rely on cytoplasmic and mitochondrial NAD(H) kinases to provide NADP(H). The two yeast cytoplasmic NAD(H) kinases, Yef1 and Utr1 appear to prefer NAD+ as a substrate unlike mitochondrial Pos5, which prefers NADH [187]. In the filamentous fungus Podospora anserine, loss of a mitochondrial NADH kinase gene extended lifespan by apparently inducing expression of an ETC alternative oxidase that decreased ROS production [295].
15. Pharmacological Modulation of Cellular Redox Status to Prevent Cellular Senescence and Delay Aging
Methylene blue is a redox active compound that has been demonstrated to delay cellular senescence [296] and showed promise in a phase II clinical trial for Alzheimer’s disease [297]. It has been shown to accept electrons from NADH or reduced flavins and pass them to ETC complex III [298] bypassing superoxide generation from ETC complexes I or II [299]. Presumably these redox properties are responsible at least in part for the many other protective cellular responses including inhibition of the NLRP3 inflammasome [300], activation of the proteasome [301], and stabilization of HSF1 [302]. Methylene blue treatment was shown to extend the lifespan of C. elegans [303] and female mice [304], but not Drosophila [305].
A screen of 2684 compounds identified the redox-active compounds violuric acid (VA) and 1-naphthoquinone-2-monoxime (N2N1) as the top hits that delayed cellular senescence [306]. These compounds were then shown to increase the lifespan of C. elegans and mice. VA greatly reduced the level of cellular protein disulfides by facilitating electron transfer from NADH (and NADPH to a lesser extent) to GSSG to increase GSH and the cellular NAD+/NADH. VA also facilitated the transfer of electrons from NADH to H2O2 to form water, but did not transfer electrons from NADH to cytochrome c. N2N1 was shown to pass electrons from NADH (and NADPH to a lesser extent) to CoQ10 and to cytochrome c with the NQO1 (NAD(P)H: quinone oxidoreductase 1) redox system as a major target. Consistent with these results, overexpression of NQO1 together with another NADH-oxidizing redox protein, cytochrome b5 reductase 3 (CYB5R3), extended lifespan in mice and mimicked some phenotypes of CR [307]. However, invertebrates such as C. elegans lack the NQO1 system, so the longevity effects of N2N1 and methylene blue in nematodes may be due to stimulation of electron transfer from NADH to ETC complex III or to the C. elegans CYB5R3 homologs HPO-19 or T05H4.4. Increasing the NAD+/NADH ratio is likely key to the longevity effects of these compounds, but VA may also stabilize the NADP+/NADPH to provide longevity benefits. Compounds that facilitate the selective transfer of electrons from NADH to GSSG, H2O2, oxidized thioredoxin, and other electron acceptors could increase the NAD+/NADH with aging and would therefore be compounds for the potential treatment of aging-related disorders. Compounds that facilitate the selective transfer of electrons from NADPH to these electron acceptors may also be beneficial at low levels, but at high levels they also have the potential to increase the [NADP+]/[NADPH] leading to oxidative stress.
16. Summary and Future Perspectives
Evidence from model organisms strongly suggests that redox changes influence the rate of aging. However, both oxidizing and reducing changes can lead to increased longevity making redox measurements in both cytoplasmic and mitochondrial compartments especially important in when determining the mechanism for lifespan extension. In S. cerevisiae and C. elegans, deleting or knocking down NADPH-generating enzymes increased longevity more frequently than decreasing it, likely due to the active stress response pathways that have evolved in young cells to compensate for the loss of NADPH. Manipulations that lead to a reduction of the [NADP+]/[NADPH] in the cytoplasm are especially linked with increased longevity. But reducing the redox potential of the cytoplasm frequently leads to reductive stress characterized by mitochondrial oxidation and ROS production. Mitochondrial ROS production and oxidation is linked with increased longevity when it occurs in young organisms capable of mounting robust longevity enhancing stress responses. The theory that mild reductive stress in the cytoplasm during development leads to increased mitochondrial superoxide production and lifespan extension should be tested in model organisms.
Another clear trend in the literature is the protective effects of the mitochondrial NADPH-linked redox systems. It would be helpful to explore potential molecular mechanisms behind the positive association between mitochondrial thioredoxin reductase activity and increased longevity. This could be accomplished in part through overexpression studies and measurements of mitochondrial superoxide or hydrogen peroxide production, GSSG/GSH, and [NADP+]/[NADPH] measurements. However, overexpression of mitochondrial thioredoxin reductase on its own would be expected to have little effect on longevity, as was verified in studies using Drosophila [104], without a corresponding reduction of the mitochondrial [NADP+]/[NADPH] that drives its enzymatic activity. Since little is known regarding the effects of a more reduced mitochondrial redox environment on longevity, future important experiments will be to overexpress a mitochondrial NADPH-generating enzyme, such as Men-b or mitochondrial targeted Idh in Drosophila, to determine the effects of reduced mitochondrial [NADP+]/[NADPH] on lifespan.
Acknowledgments
I would like to thank the pioneers of research in the field of redox biology and aging including Dean Jones, Rajindar Sohal, and William Orr.
Abbreviations
ADP: adenosine diphosphate; AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; ALDH1L1, cytoplasmic 10-formyltetrahydrofolate dehydrogenase; ALDH1L2, mitochondrial 10-formyltetrahydrofolate dehydrogenase; AMP, adenosine monophosphate; AMPK, AMP kinase; ATP, adenosine triphosphate; CR, calorie restriction; CYB5R3, cytochrome b5 reductase 3; DAF-16, dauer formation-16; DAF-2, dauer formation-2; DHEA, dehydroepiandrosterone; DPI, diphenyleneiodonium; DR, dietary restriction; ER, endoplasmic reticulum; ETC, Electron transport chain; FOXO, forkhead box O; G6PD, glucose-6-phosphate dehydrogenase; GCL, glutamate-cysteine ligase; GDH-1, glutamate dehydrogenase-1; GPI-1, glucose-6-phosphate isomerase-1; GPX, glutathione peroxidase; GSH, glutathione; GSSG, glutathione disulfide; GSR, glutathione reductase; HDAC, histone deacetylase; HSF-1, heat shock factor-1; IDH1, isocitrate dehydrogenase 1 (cytoplasmic); IDH-1, C. elegans isocitrate dehydrogenase-1; IDH2, isocitrate dehydrogenase 2 (mitochondrial); IDH-2, C. elegans isocitrate dehydrogenase-2; Idh, Drosophila isocitrate dehydrogenase; ICL-1, isocitrate lyase-1; Km, Michaelis constant; MATa, haploid mating type a; MATα, haploid mating type α; Men, Drosophila cytoplasmic malic enzyme; Men-b, Drosophila mitochondrial malic enzyme; MEN-1, C. elegans malic enzyme; ME1, malic enzyme 1; ME3, malic enzyme 3; MFRTA, Mitochondrial free radical theory of aging; MSRA, methionine sulfoxide reductase A; MSRB, methionine sulfoxide reductase B; MTAP, methylthioadenosine phosphorylase; MTHFD1L, methylenetetrahydrofolate dehydrogenase 1 like; mV, milliVolt; mM, milliMolar; N2N1, 1-naphthoquinone-2-monoxime; NAC, N-acetylcysteine; NAD+, nicotinamide adenine dinucleotide (oxidized form); NADK, NAD+ kinase; NADK2, mitochondrial NAD+ kinase; NADH, nicotinamide adenine dinucleotide (reduced form); NADP+, nicotinamide adenine dinucleotide phosphate (oxidized form); NADPH, nicotinamide adenine dinucleotide phosphate (reduced form); NAMPT, nicotinamide phosphoribosyl transferase; NQO1, NAD(P)H: quinone oxidoreductase 1; NNT, nicotinamide nucleotide transhydrogenase; Nox4, NADPH oxidase 4; Nrf2, Nuclear factor (erythroid-derived 2)-like 2; PARP, poly-ADP-ribose polymerase; 6PGD, 6-phosphogluconate dehydrogenase; PNP, purine nucleoside phosphorylase; PPP, pentose phosphate pathway; PRDX, peroxiredoxin; ROS, reactive oxygen species; SARM1, Sterile alpha and TIR motif containing 1; SKN-1, skinhead-1; SOD, superoxide dismutase; TFAM, mitochondrial transcription factor A; TPNOX, triphosphopyridine nucleotide oxidase; TRXR-1, C. elegans cytoplasmic thioredoxin reductase; TRXR-2, C. elegans mitochondrial thioredoxin reductase; TXN, cytoplasmic thioredoxin; TXN2, mitochondrial thioredoxin, TXNRD1, cytoplasmic thioredoxin reductase; TXNRD2, mitochondrial thioredoxin reductase; UPRmt, mitochondrial unfolded protein response; VA, violuric acid; Vmax, maximal velocity.
Funding
This research was funded by The National Institutes of Health grant number AG059096.
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the writing of the manuscript or in the decision to publish.
References
- 1.Aw T.Y. Postnatal Changes in Pyridine Nucleotides in Rat Hepatocytes: Composition and O2 Dependence. Pediatr. Res. 1991;30:112–117. doi: 10.1203/00006450-199107000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh D., Levault K.R., Brewer G.J. Relative importance of redox buffers GSH and NAD(P)H in age-related neurodegeneration and Alzheimer disease-like mouse neurons. Aging Cell. 2014;13:631–640. doi: 10.1111/acel.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu X.H., Lu M., Lee B.Y., Ugurbil K., Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. USA. 2015;112:2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenaz G., D’Aurelio M., Merlo Pich M., Genova M.L., Ventura B., Bovina C., Formiggini G., Parenti Castelli G. Mitochondrial bioenergetics in aging. Biochim. Biophys. Acta. 2000;1459:397–404. doi: 10.1016/S0005-2728(00)00177-8. [DOI] [PubMed] [Google Scholar]
- 5.Baciou L., Masoud R., Souabni H., Serfaty X., Karimi G., Bizouarn T., Houee Levin C. Phagocyte NADPH oxidase, oxidative stress and lipids: Anti- or pro ageing? Mech. Ageing Dev. 2018;172:30–34. doi: 10.1016/j.mad.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Huang Q., Sun M., Li M., Zhang D., Han F., Wu J.C., Fukunaga K., Chen Z., Qin Z.H. Combination of NAD(+) and NADPH Offers Greater Neuroprotection in Ischemic Stroke Models by Relieving Metabolic Stress. Mol. Neurobiol. 2018;55:6063–6075. doi: 10.1007/s12035-017-0809-7. [DOI] [PubMed] [Google Scholar]
- 7.Harman D. The biologic clock: The mitochondria? J. Am. Geriatr. Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 8.Barja G. Updating the mitochondrial free radical theory of aging: An integrated view, key aspects, and confounding concepts. Antioxid. Redox Signal. 2013;19:1420–1445. doi: 10.1089/ars.2012.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gems D., Doonan R. Antioxidant defense and aging in C. elegans: Is the oxidative damage theory of aging wrong? Cell Cycle. 2009;8:1681–1687. doi: 10.4161/cc.8.11.8595. [DOI] [PubMed] [Google Scholar]
- 10.Jang Y.C., Perez V.I., Song W., Lustgarten M.S., Salmon A.B., Mele J., Qi W., Liu Y., Liang H., Chaudhuri A., et al. Overexpression of Mn superoxide dismutase does not increase life span in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez V.I., Van Remmen H., Bokov A., Epstein C.J., Vijg J., Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selman C., McLaren J.S., Meyer C., Duncan J.S., Redman P., Collins A.R., Duthie G.G., Speakman J.R. Life-long vitamin C supplementation in combination with cold exposure does not affect oxidative damage or lifespan in mice, but decreases expression of antioxidant protection genes. Mech. Ageing Dev. 2006;127:897–904. doi: 10.1016/j.mad.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Ernst I.M., Pallauf K., Bendall J.K., Paulsen L., Nikolai S., Huebbe P., Roeder T., Rimbach G. Vitamin E supplementation and lifespan in model organisms. Ageing Res. Rev. 2013;12:365–375. doi: 10.1016/j.arr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Desjardins D., Cacho-Valadez B., Liu J.L., Wang Y., Yee C., Bernard K., Khaki A., Breton L., Hekimi S. Antioxidants reveal an inverted U-shaped dose-response relationship between reactive oxygen species levels and the rate of aging in Caenorhabditis elegans. Aging Cell. 2017;16:104–112. doi: 10.1111/acel.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orr W.C., Sohal R.S. Does overexpression of Cu,Zn-SOD extend life span in Drosophila melanogaster? Exp. Gerontol. 2003;38:227–230. doi: 10.1016/S0531-5565(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 16.Shibamura A., Ikeda T., Nishikawa Y. A method for oral administration of hydrophilic substances to Caenorhabditis elegans: Effects of oral supplementation with antioxidants on the nematode lifespan. Mech. Ageing Dev. 2009;130:652–655. doi: 10.1016/j.mad.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Schriner S.E., Linford N.J., Martin G.M., Treuting P., Ogburn C.E., Emond M., Coskun P.E., Ladiges W., Wolf N., Van Remmen H., et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 18.Lebovitz R.M., Zhang H., Vogel H., Cartwright J., Jr., Dionne L., Lu N., Huang S., Matzuk M.M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkman H.N., Gaetani G.F. Catalase: A tetrameric enzyme with four tightly bound molecules of NADPH. Proc. Natl. Acad. Sci. USA. 1984;81:4343–4347. doi: 10.1073/pnas.81.14.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohal R.S., Orr W.C. The redox stress hypothesis of aging. Free Radic. Biol. Med. 2012;52:539–555. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Go Y.M., Jones D.P. Redox theory of aging: Implications for health and disease. Clin. Sci. (Lond. Engl. 1979) 2017;131:1669–1688. doi: 10.1042/cs20160897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidler T., Hartwig K., Daniel H., Wenzel U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology. 2010;11:183–195. doi: 10.1007/s10522-009-9239-x. [DOI] [PubMed] [Google Scholar]
- 23.Schulz T.J., Zarse K., Voigt A., Urban N., Birringer M., Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Barja G. Rate of generation of oxidative stress-related damage and animal longevity. Free Radic. Biol. Med. 2002;33:1167–1172. doi: 10.1016/S0891-5849(02)00910-3. [DOI] [PubMed] [Google Scholar]
- 25.Schindeldecker M., Stark M., Behl C., Moosmann B. Differential cysteine depletion in respiratory chain complexes enables the distinction of longevity from aerobicity. Mech. Ageing Dev. 2011;132:171–179. doi: 10.1016/j.mad.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Pamplona R., Barja G., Portero-Otin M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: A homeoviscous-longevity adaptation? Ann. N. Y. Acad. Sci. 2002;959:475–490. doi: 10.1111/j.1749-6632.2002.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 27.Moosmann B. Respiratory chain cysteine and methionine usage indicate a causal role for thiyl radicals in aging. Exp. Gerontol. 2011;46:164–169. doi: 10.1016/j.exger.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 28.Paradies G., Paradies V., Ruggiero F.M., Petrosillo G. Cardiolipin and mitochondrial function in health and disease. Antioxid. Redox Signal. 2014;20:1925–1953. doi: 10.1089/ars.2013.5280. [DOI] [PubMed] [Google Scholar]
- 29.Ma C., Pi C., Yang Y., Lin L., Shi Y., Li Y., Li Y., He X. Nampt Expression Decreases Age-Related Senescence in Rat Bone Marrow Mesenchymal Stem Cells by Targeting Sirt1. PLoS ONE. 2017;12:e0170930. doi: 10.1371/journal.pone.0170930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn B.H., Kim H.S., Song S., Lee I.H., Liu J., Vassilopoulos A., Deng C.X., Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemoto S., Fergusson M.M., Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 32.Braidy N., Guillemin G.J., Mansour H., Chan-Ling T., Poljak A., Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS ONE. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Camacho-Pereira J., Tarrago M.G., Chini C.C.S., Nin V., Escande C., Warner G.M., Puranik A.S., Schoon R.A., Reid J.M., Galina A., et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016;23:1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boslett J., Helal M., Chini E., Zweier J.L. Genetic deletion of CD38 confers post-ischemic myocardial protection through preserved pyridine nucleotides. J. Mol. Cell. Cardiol. 2018;118:81–94. doi: 10.1016/j.yjmcc.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vu C.Q., Lu P.J., Chen C.S., Jacobson M.K. 2′-Phospho-cyclic ADP-ribose, a calcium-mobilizing agent derived from NADP. J. Biol. Chem. 1996;271:4747–4754. [PubMed] [Google Scholar]
- 36.Andrabi S.A., Umanah G.K.E., Chang C., Stevens D.A., Karuppagounder S.S., Gagné J.-P., Poirier G.G., Dawson V.L., Dawson T.M. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc. Natl. Acad. Sci. USA. 2014;111:10209–10214. doi: 10.1073/pnas.1405158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Essuman K., Summers D.W., Sasaki Y., Mao X., DiAntonio A., Milbrandt J. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD(+) Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron. 2017;93:1334–1343.e5. doi: 10.1016/j.neuron.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrero E., Malavasi F. A Natural History of the Human CD38 Gene. In: Lee H.C., editor. Cyclic ADP-Ribose and NAADP: Structures, Metabolism and Functions. Springer; Boston, MA, USA: 2002. pp. 65–79. [Google Scholar]
- 39.Sur M., Dey P., Sarkar A., Bar S., Banerjee D., Bhat S., Mukherjee P. Sarm1 induction and accompanying inflammatory response mediates age-dependent susceptibility to rotenone-induced neurotoxicity. Cell Death Discov. 2018;4:114. doi: 10.1038/s41420-018-0119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navas P., Minnifield N., Sun I., Morre D.J. NADP phosphatase as a marker in free-flow electrophoretic separations for cisternae of the Golgi apparatus midregion. Biochim. Biophys. Acta. 1986;881:1–9. doi: 10.1016/0304-4165(86)90089-9. [DOI] [PubMed] [Google Scholar]
- 41.Richter C. NADP+ phosphatase: A novel mitochondrial enzyme. Biochem. Biophys. Res. Commun. 1987;146:253–257. doi: 10.1016/0006-291X(87)90718-2. [DOI] [PubMed] [Google Scholar]
- 42.Balan V., Miller G.S., Kaplun L., Balan K., Chong Z.Z., Li F., Kaplun A., VanBerkum M.F., Arking R., Freeman D.C., et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J. Biol. Chem. 2008;283:27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vrablik T.L., Huang L., Lange S.E., Hanna-Rose W. Nicotinamidase modulation of NAD+ biosynthesis and nicotinamide levels separately affect reproductive development and cell survival in C. elegans. Development. 2009;136:3637–3646. doi: 10.1242/dev.028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McReynolds M.R., Wang W., Holleran L.M., Hanna-Rose W. Uridine monophosphate synthetase enables eukaryotic de novo NAD+ biosynthesis from quinolinic acid. J. Biol. Chem. 2017;292:11147–11153. doi: 10.1074/jbc.C117.795344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breda C., Sathyasaikumar K.V., Sograte Idrissi S., Notarangelo F.M., Estranero J.G., Moore G.G., Green E.W., Kyriacou C.P., Schwarcz R., Giorgini F. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc. Natl. Acad. Sci. USA. 2016;113:5435–5440. doi: 10.1073/pnas.1604453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Copes N., Edwards C., Chaput D., Saifee M., Barjuca I., Nelson D., Paraggio A., Saad P., Lipps D., Stevens S.M., Jr., et al. Metabolome and proteome changes with aging in Caenorhabditis elegans. Exp. Gerontol. 2015;72:67–84. doi: 10.1016/j.exger.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sohal R.S., Arnold L., Orr W.C. Effect of age on superoxide dismutase, catalase, glutathione reductase, inorganic peroxides, TBA-reactive material, GSH/GSSG, NADPH/NADP+ and NADH/NAD+ in Drosophila melanogaster. Mech. Ageing Dev. 1990;56:223–235. doi: 10.1016/0047-6374(90)90084-S. [DOI] [PubMed] [Google Scholar]
- 48.Pollak N., Dolle C., Ziegler M. The power to reduce: Pyridine nucleotides—Small molecules with a multitude of functions. Biochem. J. 2007;402:205–218. doi: 10.1042/BJ20061638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hashimoto T., Horikawa M., Nomura T., Sakamoto K. Nicotinamide adenine dinucleotide extends the lifespan of Caenorhabditis elegans mediated by sir-2.1 and daf-16. Biogerontology. 2010;11:31–43. doi: 10.1007/s10522-009-9225-3. [DOI] [PubMed] [Google Scholar]
- 50.Mouchiroud L., Houtkooper R.H., Moullan N., Katsyuba E., Ryu D., Canto C., Mottis A., Jo Y.S., Viswanathan M., Schoonjans K., et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belenky P., Christensen K.C., Gazzaniga F., Pletnev A.A., Brenner C. Nicotinamide riboside and nicotinic acid riboside salvage in fungi and mammals. Quantitative basis for Urh1 and purine nucleoside phosphorylase function in NAD+ metabolism. J. Boil. Chem. 2009;284:158–164. doi: 10.1074/jbc.M807976200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomes A.P., Price N.L., Ling A.J., Moslehi J.J., Montgomery M.K., Rajman L., White J.P., Teodoro J.S., Wrann C.D., Hubbard B.P., et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren X., Zou L., Zhang X., Branco V., Wang J., Carvalho C., Holmgren A., Lu J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017;27:989–1010. doi: 10.1089/ars.2016.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andziak B., Buffenstein R. Disparate patterns of age-related changes in lipid peroxidation in long-lived naked mole-rats and shorter-lived mice. Aging Cell. 2006;5:525–532. doi: 10.1111/j.1474-9726.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- 55.Veech R.L., Bradshaw P.C., Clarke K., Curtis W., Pawlosky R., King M.T. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life. 2017;69:305–314. doi: 10.1002/iub.1627. [DOI] [PubMed] [Google Scholar]
- 56.Veech R.L., Eggleston L.V., Krebs H.A. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem. J. 1969;115:609–619. doi: 10.1042/bj1150609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Son M.J., Kwon Y., Son T., Cho Y.S. Restoration of Mitochondrial NAD(+) Levels Delays Stem Cell Senescence and Facilitates Reprogramming of Aged Somatic Cells. Stem Cells. 2016;34:2840–2851. doi: 10.1002/stem.2460. [DOI] [PubMed] [Google Scholar]
- 58.Tischler M.E., Friedrichs D., Coll K., Williamson J.R. Pyridine nucleotide distributions and enzyme mass action ratios in hepatocytes from fed and starved rats. Arch. Biochem. Biophys. 1977;184:222–236. doi: 10.1016/0003-9861(77)90346-0. [DOI] [PubMed] [Google Scholar]
- 59.Owen J.B., Butterfield D.A. Measurement of oxidized/reduced glutathione ratio. Methods Mol. Biol. 2010;648:269–277. doi: 10.1007/978-1-60761-756-3_18. [DOI] [PubMed] [Google Scholar]
- 60.Chai Y.C., Ashraf S.S., Rokutan K., Johnston R.B., Jr., Thomas J.A. S-thiolation of individual human neutrophil proteins including actin by stimulation of the respiratory burst: Evidence against a role for glutathione disulfide. Arch. Biochem. Biophys. 1994;310:273–281. doi: 10.1006/abbi.1994.1167. [DOI] [PubMed] [Google Scholar]
- 61.Tao R., Zhao Y., Chu H., Wang A., Zhu J., Chen X., Zou Y., Shi M., Liu R., Su N., et al. Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat. Methods. 2017;14:720–728. doi: 10.1038/nmeth.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yap L.P., Garcia J.V., Han D., Cadenas E. The energy-redox axis in aging and age-related neurodegeneration. Adv. Drug Deliv. Rev. 2009;61:1283–1298. doi: 10.1016/j.addr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canepa L., Ferraris A.M., Miglino M., Gaetani G.F. Bound and unbound pyridine dinucleotides in normal and glucose-6-phosphate dehydrogenase-deficient erythrocytes. Biochim. Biophys. Acta. 1991;1074:101–104. doi: 10.1016/0304-4165(91)90046-J. [DOI] [PubMed] [Google Scholar]
- 64.Booty L.M., Gawel J.M., Cvetko F., Caldwell S.T., Hall A.R., Mulvey J.F., James A.M., Hinchy E.C., Prime T.A., Arndt S., et al. Selective Disruption of Mitochondrial Thiol Redox State in Cells and In Vivo. Cell Chem. Boil. 2019 doi: 10.1016/j.chembiol.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones D.P., Mody V.C., Jr., Carlson J.L., Lynn M.J., Sternberg P., Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic. Biol. Med. 2002;33:1290–1300. doi: 10.1016/S0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 66.Clement J., Wong M., Poljak A., Sachdev P., Braidy N. The Plasma NAD(+) Metabolome Is Dysregulated in “Normal” Aging. Rejuvenation Res. 2018 doi: 10.1089/rej.2018.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dellinger R.W., Santos S.R., Morris M., Evans M., Alminana D., Guarente L., Marcotulli E. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD(+) levels in humans safely and sustainably: A randomized, double-blind, placebo-controlled study. Npj Aging Mech. Dis. 2017;3:17. doi: 10.1038/s41514-017-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Y., Wu J., Sheng R., Li M., Wang Y., Han R., Han F., Chen Z., Qin Z.H. Reduced Nicotinamide Adenine Dinucleotide Phosphate Inhibits MPTP-Induced Neuroinflammation and Neurotoxicity. Neuroscience. 2018;391:140–153. doi: 10.1016/j.neuroscience.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 69.Horton P., Park K.J., Obayashi T., Fujita N., Harada H., Adams-Collier C.J., Nakai K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gutteridge J.M.C., Halliwell B. Mini-Review: Oxidative stress, redox stress or redox success? Biochem. Biophys. Res. Commun. 2018;502:183–186. doi: 10.1016/j.bbrc.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 71.Zou Y., Wang A., Shi M., Chen X., Liu R., Li T., Zhang C., Zhang Z., Zhu L., Ju Z., et al. Analysis of redox landscapes and dynamics in living cells and in vivo using genetically encoded fluorescent sensors. Nat. Protoc. 2018;13:2362–2386. doi: 10.1038/s41596-018-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanson G.T., Aggeler R., Oglesbee D., Cannon M., Capaldi R.A., Tsien R.Y., Remington S.J. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 73.Gutscher M., Pauleau A.L., Marty L., Brach T., Wabnitz G.H., Samstag Y., Meyer A.J., Dick T.P. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods. 2008;5:553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- 74.Albrecht S.C., Barata A.G., Grosshans J., Teleman A.A., Dick T.P. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 2011;14:819–829. doi: 10.1016/j.cmet.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H., Limphong P., Pieper J., Liu Q., Rodesch C.K., Christians E., Benjamin I.J. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J. 2012;26:1442–1451. doi: 10.1096/fj.11-199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Booty L.M., King M.S., Thangaratnarajah C., Majd H., James A.M., Kunji E.R., Murphy M.P. The mitochondrial dicarboxylate and 2-oxoglutarate carriers do not transport glutathione. FEBS Lett. 2015;589:621–628. doi: 10.1016/j.febslet.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wadey A.L., Muyderman H., Kwek P.T., Sims N.R. Mitochondrial glutathione uptake: Characterization in isolated brain mitochondria and astrocytes in culture. J. Neurochem. 2009;109(Suppl. 1):101–108. doi: 10.1111/j.1471-4159.2009.05936.x. [DOI] [PubMed] [Google Scholar]
- 78.Blacker T.S., Duchen M.R. Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radic. Biol. Med. 2016;100:53–65. doi: 10.1016/j.freeradbiomed.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Korge P., Calmettes G., Weiss J.N. Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases. Biochim. Biophys. Acta. 2015;1847:514–525. doi: 10.1016/j.bbabio.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ralser M., Benjamin I.J. Reductive stress on life span extension in C. elegans. BMC Res. Notes. 2008;1:19. doi: 10.1186/1756-0500-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ralser M., Wamelink M.M., Kowald A., Gerisch B., Heeren G., Struys E.A., Klipp E., Jakobs C., Breitenbach M., Lehrach H., et al. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J. Boil. 2007;6:10. doi: 10.1186/jbiol61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Breitenbach M., Laun P., Dickinson J.R., Klocker A., Rinnerthaler M., Dawes I.W., Aung-Htut M.T., Breitenbach-Koller L., Caballero A., Nystrom T., et al. The role of mitochondria in the aging processes of yeast. Sub-Cell. Biochem. 2012;57:55–78. doi: 10.1007/978-94-007-2561-4_3. [DOI] [PubMed] [Google Scholar]
- 83.Rajasekaran N.S., Connell P., Christians E.S., Yan L.J., Taylor R.P., Orosz A., Zhang X.Q., Stevenson T.J., Peshock R.M., Leopold J.A., et al. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brand M.D., Goncalves R.L., Orr A.L., Vargas L., Gerencser A.A., Borch Jensen M., Wang Y.T., Melov S., Turk C.N., Matzen J.T., et al. Suppressors of Superoxide-H2O2 Production at Site IQ of Mitochondrial Complex I Protect against Stem Cell Hyperplasia and Ischemia-Reperfusion Injury. Cell Metab. 2016;24:582–592. doi: 10.1016/j.cmet.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Orr A.L., Vargas L., Turk C.N., Baaten J.E., Matzen J.T., Dardov V.J., Attle S.J., Li J., Quackenbush D.C., Goncalves R.L., et al. Suppressors of superoxide production from mitochondrial complex III. Nat. Chem. Boil. 2015;11:834–836. doi: 10.1038/nchembio.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lewis K.N., Andziak B., Yang T., Buffenstein R. The naked mole-rat response to oxidative stress: Just deal with it. Antioxid. Redox Signal. 2013;19:1388–1399. doi: 10.1089/ars.2012.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Broniowska K.A., Diers A.R., Hogg N. S-nitrosoglutathione. Biochim. Biophys. Acta. 2013;1830:3173–3181. doi: 10.1016/j.bbagen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ran Q., Liang H., Ikeno Y., Qi W., Prolla T.A., Roberts L.J., 2nd, Wolf N., Van Remmen H., Richardson A. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:932–942. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- 89.Mockett R.J., Orr W.C., Rahmandar J.J., Sohal B.H., Sohal R.S. Antioxidant status and stress resistance in long- and short-lived lines of Drosophila melanogaster. Exp. Gerontol. 2001;36:441–463. doi: 10.1016/S0531-5565(00)00258-8. [DOI] [PubMed] [Google Scholar]
- 90.Lopez-Torres M., Perez-Campo R., Rojas C., Cadenas S., Barja G. Maximum life span in vertebrates: Relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, and basal and maximum aerobic capacity. Mech. Ageing Dev. 1993;70:177–199. doi: 10.1016/0047-6374(93)90047-U. [DOI] [PubMed] [Google Scholar]
- 91.Heinze I., Bens M., Calzia E., Holtze S., Dakhovnik O., Sahm A., Kirkpatrick J.M., Szafranski K., Romanov N., Sama S.N., et al. Species comparison of liver proteomes reveals links to naked mole-rat longevity and human aging. BMC Biol. 2018;16:82. doi: 10.1186/s12915-018-0547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hawkes H.J., Karlenius T.C., Tonissen K.F. Regulation of the human thioredoxin gene promoter and its key substrates: A study of functional and putative regulatory elements. Biochim. Biophys. Acta. 2014;1840:303–314. doi: 10.1016/j.bbagen.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 93.Munro D., Baldy C., Pamenter M.E., Treberg J.R. The exceptional longevity of the naked mole-rat may be explained by mitochondrial antioxidant defenses. Aging Cell. 2019:e12916. doi: 10.1111/acel.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perez L.M., Pareja-Galeano H., Sanchis-Gomar F., Emanuele E., Lucia A., Galvez B.G. ‘Adipaging’: Ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J. Physiol. 2016;594:3187–3207. doi: 10.1113/JP271691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang Y., Purkayastha S., Cai D. Hypothalamic microinflammation: A common basis of metabolic syndrome and aging. Trends Neurosci. 2015;38:36–44. doi: 10.1016/j.tins.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ribas V., García-Ruiz C., Fernández-Checa J.C. Glutathione and mitochondria. Front. Pharmacol. 2014;5:151. doi: 10.3389/fphar.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abdrakhmanova A., Zwicker K., Kerscher S., Zickermann V., Brandt U. Tight binding of NADPH to the 39-kDa subunit of complex I is not required for catalytic activity but stabilizes the multiprotein complex. Biochim. Biophys. Acta. 2006;1757:1676–1682. doi: 10.1016/j.bbabio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 98.Fiedorczuk K., Letts J.A., Degliesposti G., Kaszuba K., Skehel M., Sazanov L.A. Atomic structure of the entire mammalian mitochondrial complex I. Nature. 2016;538:406–410. doi: 10.1038/nature19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lesnefsky E.J., Hoppel C.L. Oxidative phosphorylation and aging. Ageing Res. Rev. 2006;5:402–433. doi: 10.1016/j.arr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Stefanatos R., Sanz A. Mitochondrial complex I: A central regulator of the aging process. Cell Cycle. 2011;10:1528–1532. doi: 10.4161/cc.10.10.15496. [DOI] [PubMed] [Google Scholar]
- 101.Cunningham G.M., Flores L.C., Roman M.G., Cheng C., Dube S., Allen C., Valentine J.M., Hubbard G.B., Bai Y., Saunders T.L., et al. Thioredoxin overexpression in both the cytosol and mitochondria accelerates age-related disease and shortens lifespan in male C57BL/6 mice. GeroScience. 2018;40:453–468. doi: 10.1007/s11357-018-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cunningham G.M., Roman M.G., Flores L.C., Hubbard G.B., Salmon A.B., Zhang Y., Gelfond J., Ikeno Y. The paradoxical role of thioredoxin on oxidative stress and aging. Arch. Biochem. Biophys. 2015;576:32–38. doi: 10.1016/j.abb.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 103.Flores L.C., Roman M.G., Cunningham G.M., Cheng C., Dube S., Allen C., Van Remmen H., Hubbard G.B., Saunders T.L., Ikeno Y. Continuous overexpression of thioredoxin 1 enhances cancer development and does not extend maximum lifespan in male C57BL/6 mice. Pathobiol. Aging Age Relat. Dis. 2018;8:1533754. doi: 10.1080/20010001.2018.1533754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pickering A.M., Lehr M., Gendron C.M., Pletcher S.D., Miller R.A. Mitochondrial thioredoxin reductase 2 is elevated in long-lived primate as well as rodent species and extends fly mean lifespan. Aging Cell. 2017;16:683–692. doi: 10.1111/acel.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Orr W.C., Mockett R.J., Benes J.J., Sohal R.S. Effects of overexpression of copper-zinc and manganese superoxide dismutases, catalase, and thioredoxin reductase genes on longevity in Drosophila melanogaster. J. Biol. Chem. 2003;278:26418–26422. doi: 10.1074/jbc.M303095200. [DOI] [PubMed] [Google Scholar]
- 106.Mockett R.J., Sohal R.S., Orr W.C. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. FASEB J. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- 107.Rojanathammanee L., Rakoczy S., Brown-Borg H.M. Growth hormone alters the glutathione S-transferase and mitochondrial thioredoxin systems in long-living Ames dwarf mice. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:1199–1211. doi: 10.1093/gerona/glt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Drechsel D.A., Patel M. Respiration-dependent H2O2 Removal in Brain Mitochondria via the Thioredoxin/Peroxiredoxin System. J. Boil. Chem. 2010;285:27850–27858. doi: 10.1074/jbc.M110.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Calabrese G., Morgan B., Riemer J. Mitochondrial Glutathione: Regulation and Functions. Antioxid. Redox Signal. 2017;27:1162–1177. doi: 10.1089/ars.2017.7121. [DOI] [PubMed] [Google Scholar]
- 110.May J.M., Mendiratta S., Hill K.E., Burk R.F. Reduction of dehydroascorbate to ascorbate by the selenoenzyme thioredoxin reductase. J. Biol. Chem. 1997;272:22607–22610. doi: 10.1074/jbc.272.36.22607. [DOI] [PubMed] [Google Scholar]
- 111.Xia L., Nordman T., Olsson J.M., Damdimopoulos A., Bjorkhem-Bergman L., Nalvarte I., Eriksson L.C., Arner E.S., Spyrou G., Bjornstedt M. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J. Biol. Chem. 2003;278:2141–2146. doi: 10.1074/jbc.M210456200. [DOI] [PubMed] [Google Scholar]
- 112.Nalvarte I., Damdimopoulos A.E., Spyrou G. Human mitochondrial thioredoxin reductase reduces cytochrome c and confers resistance to complex III inhibition. Free Radic. Biol. Med. 2004;36:1270–1278. doi: 10.1016/j.freeradbiomed.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 113.Arner E.S., Nordberg J., Holmgren A. Efficient reduction of lipoamide and lipoic acid by mammalian thioredoxin reductase. Biochem. Biophys. Res. Commun. 1996;225:268–274. doi: 10.1006/bbrc.1996.1165. [DOI] [PubMed] [Google Scholar]
- 114.Bjornstedt M., Hamberg M., Kumar S., Xue J., Holmgren A. Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocystine strongly stimulates the reaction via catalytically generated selenols. J. Biol. Chem. 1995;270:11761–11764. doi: 10.1074/jbc.270.20.11761. [DOI] [PubMed] [Google Scholar]
- 115.Jee C., Vanoaica L., Lee J., Park B.J., Ahnn J. Thioredoxin is related to life span regulation and oxidative stress response in Caenorhabditis elegans. Genes Cells. 2005;10:1203–1210. doi: 10.1111/j.1365-2443.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 116.Miranda-Vizuete A., Fierro Gonzalez J.C., Gahmon G., Burghoorn J., Navas P., Swoboda P. Lifespan decrease in a Caenorhabditis elegans mutant lacking TRX-1, a thioredoxin expressed in ASJ sensory neurons. FEBS Lett. 2006;580:484–490. doi: 10.1016/j.febslet.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 117.Li W., Bandyopadhyay J., Hwaang H.S., Park B.J., Cho J.H., Lee J.I., Ahnn J., Lee S.K. Two thioredoxin reductases, trxr-1 and trxr-2, have differential physiological roles in Caenorhabditis elegans. Mol. Cells. 2012;34:209–218. doi: 10.1007/s10059-012-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cacho-Valadez B., Munoz-Lobato F., Pedrajas J.R., Cabello J., Fierro-Gonzalez J.C., Navas P., Swoboda P., Link C.D., Miranda-Vizuete A. The characterization of the Caenorhabditis elegans mitochondrial thioredoxin system uncovers an unexpected protective role of thioredoxin reductase 2 in beta-amyloid peptide toxicity. Antioxid. Redox Signal. 2012;16:1384–1400. doi: 10.1089/ars.2011.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arodin L., Miranda-Vizuete A., Swoboda P., Fernandes A.P. Protective effects of the thioredoxin and glutaredoxin systems in dopamine-induced cell death. Free Radic. Biol. Med. 2014;73:328–336. doi: 10.1016/j.freeradbiomed.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 120.Oláhová M., Veal E.A. A peroxiredoxin, PRDX-2, is required for insulin secretion and insulin/IIS-dependent regulation of stress resistance and longevity. Aging Cell. 2015;14:558–568. doi: 10.1111/acel.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Haes W., Frooninckx L., Van Assche R., Smolders A., Depuydt G., Billen J., Braeckman B.P., Schoofs L., Temmerman L. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc. Natl. Acad. Sci. USA. 2014;111:E2501–E2509. doi: 10.1073/pnas.1321776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Henderson D., Huebner C., Markowitz M., Taube N., Harvanek Z.M., Jakob U., Knoefler D. Do developmental temperatures affect redox level and lifespan in C. elegans through upregulation of peroxiredoxin? Redox Boil. 2017;14:386–390. doi: 10.1016/j.redox.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thamsen M., Kumsta C., Li F., Jakob U. Is overoxidation of peroxiredoxin physiologically significant? Antioxid. Redox Signal. 2011;14:725–730. doi: 10.1089/ars.2010.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang B., Moskovitz J. The Functions of the Mammalian Methionine Sulfoxide Reductase System and Related Diseases. Antioxidants. 2018;7:122. doi: 10.3390/antiox7090122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee B.C., Lee H.M., Kim S., Avanesov A.S., Lee A., Chun B.-H., Vorbruggen G., Gladyshev V.N. Expression of the methionine sulfoxide reductase lost during evolution extends Drosophila lifespan in a methionine-dependent manner. Sci. Rep. 2018;8:1010. doi: 10.1038/s41598-017-15090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim H.Y., Gladyshev V.N. Methionine sulfoxide reductases: Selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem. J. 2007;407:321–329. doi: 10.1042/BJ20070929. [DOI] [PubMed] [Google Scholar]
- 127.Minniti A.N., Cataldo R., Trigo C., Vasquez L., Mujica P., Leighton F., Inestrosa N.C., Aldunate R. Methionine sulfoxide reductase A expression is regulated by the DAF-16/FOXO pathway in Caenorhabditis elegans. Aging Cell. 2009;8:690–705. doi: 10.1111/j.1474-9726.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- 128.Bruce L., Singkornrat D., Wilson K., Hausman W., Robbins K., Huang L., Foss K., Binninger D. In Vivo Effects of Methionine Sulfoxide Reductase Deficiency in Drosophila melanogaster. Antioxidants. 2018;7:155. doi: 10.3390/antiox7110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ruan H., Tang X.D., Chen M.L., Joiner M.L., Sun G., Brot N., Weissbach H., Heinemann S.H., Iverson L., Wu C.F., et al. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc. Natl. Acad. Sci. USA. 2002;99:2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chung H., Kim A.K., Jung S.A., Kim S.W., Yu K., Lee J.H. The Drosophila homolog of methionine sulfoxide reductase A extends lifespan and increases nuclear localization of FOXO. FEBS Lett. 2010;584:3609–3614. doi: 10.1016/j.febslet.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 131.Salmon A.B., Kim G., Liu C., Wren J.D., Georgescu C., Richardson A., Levine R.L. Effects of transgenic methionine sulfoxide reductase A (MsrA) expression on lifespan and age-dependent changes in metabolic function in mice. Redox Boil. 2016;10:251–256. doi: 10.1016/j.redox.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tarafdar S., Kim G., Levine R.L. Drosophila methionine sulfoxide reductase A (MSRA) lacks methionine oxidase activity. Free Radic. Biol. Med. 2018;131:154–161. doi: 10.1016/j.freeradbiomed.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barja G., Cadenas S., Rojas C., Lopez-Torres M., Perez-Campo R. A decrease of free radical production near critical targets as a cause of maximum longevity in animals. Comp. Biochem. Physiol. Part B. 1994;108:501–512. doi: 10.1016/0305-0491(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 134.Kanzok S.M., Fechner A., Bauer H., Ulschmid J.K., Muller H.M., Botella-Munoz J., Schneuwly S., Schirmer R., Becker K. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science. 2001;291:643–646. doi: 10.1126/science.291.5504.643. [DOI] [PubMed] [Google Scholar]
- 135.Mora-Lorca J.A., Saenz-Narciso B., Gaffney C.J., Naranjo-Galindo F.J., Pedrajas J.R., Guerrero-Gomez D., Dobrzynska A., Askjaer P., Szewczyk N.J., Cabello J., et al. Glutathione reductase gsr-1 is an essential gene required for Caenorhabditis elegans early embryonic development. Free Radic. Biol. Med. 2016;96:446–461. doi: 10.1016/j.freeradbiomed.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luersen K., Stegehake D., Daniel J., Drescher M., Ajonina I., Ajonina C., Hertel P., Woltersdorf C., Liebau E. The glutathione reductase GSR-1 determines stress tolerance and longevity in Caenorhabditis elegans. PLoS ONE. 2013;8:e60731. doi: 10.1371/journal.pone.0060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stenvall J., Fierro-Gonzalez J.C., Swoboda P., Saamarthy K., Cheng Q., Cacho-Valadez B., Arner E.S., Persson O.P., Miranda-Vizuete A., Tuck S. Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2011;108:1064–1069. doi: 10.1073/pnas.1006328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guerrero-Gomez D., Mora-Lorca J.A., Saenz-Narciso B., Naranjo-Galindo F.J., Munoz-Lobato F., Parrado-Fernandez C., Goikolea J., Cedazo-Minguez A., Link C.D., Neri C., et al. Loss of glutathione redox homeostasis impairs proteostasis by inhibiting autophagy-dependent protein degradation. Cell Death Differ. 2019 doi: 10.1038/s41418-018-0270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luchak J.M., Prabhudesai L., Sohal R.S., Radyuk S.N., Orr W.C. Modulating longevity in Drosophila by over- and underexpression of glutamate-cysteine ligase. Ann. N. Y. Acad. Sci. 2007;1119:260–273. doi: 10.1196/annals.1404.000. [DOI] [PubMed] [Google Scholar]
- 140.Orr W.C., Radyuk S.N., Prabhudesai L., Toroser D., Benes J.J., Luchak J.M., Mockett R.J., Rebrin I., Hubbard J.G., Sohal R.S. Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J. Biol. Chem. 2005;280:37331–37338. doi: 10.1074/jbc.M508272200. [DOI] [PubMed] [Google Scholar]
- 141.Li F., Ma X., Cui X., Li J., Wang Z. Recombinant buckwheat glutaredoxin intake increases lifespan and stress resistance via hsf-1 upregulation in Caenorhabditis elegans. Exp. Gerontol. 2018;104:86–97. doi: 10.1016/j.exger.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 142.Andziak B., O’Connor T.P., Qi W., DeWaal E.M., Pierce A., Chaudhuri A.R., Van Remmen H., Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 143.Go Y.M., Jones D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cai J., Jones D.P. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- 145.Kemp M., Go Y.M., Jones D.P. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free Radic. Biol. Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rebrin I., Sohal R.S. Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp. Gerontol. 2004;39:1513–1519. doi: 10.1016/j.exger.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Legan S.K., Rebrin I., Mockett R.J., Radyuk S.N., Klichko V.I., Sohal R.S., Orr W.C. Overexpression of glucose-6-phosphate dehydrogenase extends the life span of Drosophila melanogaster. J. Biol. Chem. 2008;283:32492–32499. doi: 10.1074/jbc.M805832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Luckinbill L.S., Riha V., Rhine S., Grudzien T.A. The role of glucose-6-phosphate dehydrogenase in the evolution of longevity in Drosophila melanogaster. Pt 1Heredity. 1990;65:29–38. doi: 10.1038/hdy.1990.66. [DOI] [PubMed] [Google Scholar]
- 149.Wang C.-T., Chen Y.-C., Wang Y.-Y., Huang M.-H., Yen T.-L., Li H., Liang C.-J., Sang T.-K., Ciou S.-C., Yuh C.-H., et al. Reduced neuronal expression of ribose-5-phosphate isomerase enhances tolerance to oxidative stress, extends lifespan, and attenuates polyglutamine toxicity in Drosophila. Aging Cell. 2012;11:93–103. doi: 10.1111/j.1474-9726.2011.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Farkas R., Danis P., Medved’ova L., Mechler B.M., Knopp J. Regulation of cytosolic malate dehydrogenase by juvenile hormone in Drosophila melanogaster. Cell Biochem. Biophys. 2002;37:37–52. doi: 10.1385/CBB:37:1:37. [DOI] [PubMed] [Google Scholar]
- 151.Kim G.H., Lee Y.E., Lee G.H., Cho Y.H., Lee Y.N., Jang Y., Paik D., Park J.J. Overexpression of malic enzyme in the larval stage extends Drosophila lifespan. Biochem. Biophys. Res. Commun. 2015;456:676–682. doi: 10.1016/j.bbrc.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 152.Paik D., Jang Y.G., Lee Y.E., Lee Y.N., Yamamoto R., Gee H.Y., Yoo S., Bae E., Min K.J., Tatar M., et al. Misexpression screen delineates novel genes controlling Drosophila lifespan. Mech. Ageing Dev. 2012;133:234–245. doi: 10.1016/j.mad.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Merritt T.J.S., Kuczynski C., Sezgin E., Zhu C.-T., Kumagai S., Eanes W.F. Quantifying interactions within the NADP(H) enzyme network in Drosophila melanogaster. Genetics. 2009;182:565–574. doi: 10.1534/genetics.109.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rong Y.S., Golic K.G. A targeted gene knockout in Drosophila. Genetics. 2001;157:1307–1312. doi: 10.1093/genetics/157.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tremblay G.B., Sohi S.S., Retnakaran A., MacKenzie R.E. NAD-dependent methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase is targeted to the cytoplasm in insect cell lines. FEBS Lett. 1995;368:177–182. doi: 10.1016/0014-5793(95)00640-U. [DOI] [PubMed] [Google Scholar]
- 156.Yu S., Jang Y., Paik D., Lee E., Park J.J. Nmdmc overexpression extends Drosophila lifespan and reduces levels of mitochondrial reactive oxygen species. Biochem. Biophys. Res. Commun. 2015;465:845–850. doi: 10.1016/j.bbrc.2015.08.098. [DOI] [PubMed] [Google Scholar]
- 157.Gaudet P., Livstone M.S., Lewis S.E., Thomas P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform. 2011;12:449–462. doi: 10.1093/bib/bbr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Da Cunha G.L., de Oliveira A.K. Citric acid cycle: A mainstream metabolic pathway influencing life span in Drosophila melanogaster? Exp. Gerontol. 1996;31:705–715. doi: 10.1016/S0531-5565(96)00056-3. [DOI] [PubMed] [Google Scholar]
- 159.Arking R., Burde V., Graves K., Hari R., Feldman E., Zeevi A., Soliman S., Saraiya A., Buck S., Vettraino J., et al. Forward and reverse selection for longevity in Drosophila is characterized by alteration of antioxidant gene expression and oxidative damage patterns. Exp. Gerontol. 2000;35:167–185. doi: 10.1016/S0531-5565(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 160.Alonso J., Rodriguez J.M., Baena-Lopez L.A., Santaren J.F. Characterization of the Drosophila melanogaster mitochondrial proteome. J. Proteome Res. 2005;4:1636–1645. doi: 10.1021/pr050130c. [DOI] [PubMed] [Google Scholar]
- 161.Lee K.S., Iijima-Ando K., Iijima K., Lee W.J., Lee J.H., Yu K., Lee D.S. JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends life span. J. Biol. Chem. 2009;284:29454–29461. doi: 10.1074/jbc.M109.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Radyuk S.N., Michalak K., Klichko V.I., Benes J., Rebrin I., Sohal R.S., Orr W.C. Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila. Biochem. J. 2009;419:437–445. doi: 10.1042/BJ20082003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Odnokoz O., Nakatsuka K., Klichko V.I., Nguyen J., Solis L.C., Ostling K., Badinloo M., Orr W.C., Radyuk S.N. Mitochondrial peroxiredoxins are essential in regulating the relationship between Drosophila immunity and aging. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:68–80. doi: 10.1016/j.bbadis.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Orr W.C., Radyuk S.N., Sohal R.S. Involvement of redox state in the aging of Drosophila melanogaster. Antioxid. Redox Signal. 2013;19:788–803. doi: 10.1089/ars.2012.5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Radyuk S.N., Rebrin I., Klichko V.I., Sohal B.H., Michalak K., Benes J., Sohal R.S., Orr W.C. Mitochondrial peroxiredoxins are critical for the maintenance of redox state and the survival of adult Drosophila. Free Radic. Biol. Med. 2010;49:1892–1902. doi: 10.1016/j.freeradbiomed.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Klichko V.I., Orr W.C., Radyuk S.N. The role of peroxiredoxin 4 in inflammatory response and aging. Biochim. Biophys. Acta. 2016;1862:265–273. doi: 10.1016/j.bbadis.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fernandez-Marcos P.J., Nóbrega-Pereira S. NADPH: New oxygen for the ROS theory of aging. Oncotarget. 2016;7:50814–50815. doi: 10.18632/oncotarget.10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fan J., Ye J., Kamphorst J.J., Shlomi T., Thompson C.B., Rabinowitz J.D. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nobrega-Pereira S., Fernandez-Marcos P.J., Brioche T., Gomez-Cabrera M.C., Salvador-Pascual A., Flores J.M., Vina J., Serrano M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016;7:10894. doi: 10.1038/ncomms10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dietzmann K. Histochemical demonstration of enzyme activity changes in the regio postcentralis of the mouse brain under the effect of the aging process. J. Hirnforsch. 1981;22:405–408. [PubMed] [Google Scholar]
- 171.Kaliman P.A., Konovalova E.O. Age-dependent characteristics of the regulation of cytoplasmic NADP+-dehydrogenases in the liver of rats on different diets. Ukrainskii Biokhimicheskii Zhurnal (1978) 1985;57:38–42. [PubMed] [Google Scholar]
- 172.Lemeshko V.V., Nikitchenko Iu V., Kaliman P.A. Enzymes of the antioxidant system of rat liver during aging. Ukrainskii Biokhimicheskii Zhurnal (1978) 1983;55:523–528. [PubMed] [Google Scholar]
- 173.Maurya P.K., Kumar P., Chandra P. Age-dependent detection of erythrocytes glucose-6-phosphate dehydrogenase and its correlation with oxidative stress. Arch. Physiol. Biochem. 2016;122:61–66. doi: 10.3109/13813455.2015.1136648. [DOI] [PubMed] [Google Scholar]
- 174.Gartler S.M., Hornung S.K., Motulsky A.G. Effect of chronologic age on induction of cystathionine synthase, uroporphyrinogen I synthase, and glucose-6-phosphate dehydrogenase activities in lymphocytes. Proc. Natl. Acad. Sci. USA. 1981;78:1916–1919. doi: 10.1073/pnas.78.3.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Machado A., Ayala A., Gordillo E., Revilla E., Santa Maria C. Relationship between enzymatic activity loss and post-translational protein modification in aging. Arch. Gerontol. Geriatr. 1991;12:187–197. doi: 10.1016/0167-4943(91)90027-N. [DOI] [PubMed] [Google Scholar]
- 176.Kil I.S., Lee Y.S., Bae Y.S., Huh T.L., Park J.W. Modulation of NADP(+)-dependent isocitrate dehydrogenase in aging. Redox Rep. 2004;9:271–277. doi: 10.1179/135100004225006056. [DOI] [PubMed] [Google Scholar]
- 177.Wong N., Blair A.R., Morahan G., Andrikopoulos S. The deletion variant of nicotinamide nucleotide transhydrogenase (Nnt) does not affect insulin secretion or glucose tolerance. Endocrinology. 2010;151:96–102. doi: 10.1210/en.2009-0887. [DOI] [PubMed] [Google Scholar]
- 178.Serrander L., Cartier L., Bedard K., Banfi B., Lardy B., Plastre O., Sienkiewicz A., Fórró L., Schlegel W., Krause K.-H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nisimoto Y., Diebold B.A., Cosentino-Gomes D., Lambeth J.D. Nox4: A hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53:5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nisimoto Y., Jackson H.M., Ogawa H., Kawahara T., Lambeth J.D. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry. 2010;49:2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Worthington D.J., Rosemeyer M.A. Glutathione reductase from human erythrocytes. Catalytic properties and aggregation. Eur. J. Biochem. 1976;67:231–238. doi: 10.1111/j.1432-1033.1976.tb10654.x. [DOI] [PubMed] [Google Scholar]
- 182.Koziel R., Pircher H., Kratochwil M., Lener B., Hermann M., Dencher N.A., Jansen-Durr P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem. J. 2013;452:231–239. doi: 10.1042/BJ20121778. [DOI] [PubMed] [Google Scholar]
- 183.Goy C., Czypiorski P., Altschmied J., Jakob S., Rabanter L.L., Brewer A.C., Ale-Agha N., Dyballa-Rukes N., Shah A.M., Haendeler J. The imbalanced redox status in senescent endothelial cells is due to dysregulated Thioredoxin-1 and NADPH oxidase 4. Exp. Gerontol. 2014;56:45–52. doi: 10.1016/j.exger.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 184.Handy D.E., Loscalzo J. Responses to reductive stress in the cardiovascular system. Free Radic. Boil. Med. 2017;109:114–124. doi: 10.1016/j.freeradbiomed.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rezende F., Schürmann C., Schütz S., Harenkamp S., Herrmann E., Seimetz M., Weißmann N., Schröder K. Knock out of the NADPH oxidase Nox4 has no impact on life span in mice. Redox Boil. 2016;11:312–314. doi: 10.1016/j.redox.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hirschhauser C., Bornbaum J., Reis A., Bohme S., Kaludercic N., Menabo R., Di Lisa F., Boengler K., Shah A.M., Schulz R., et al. NOX4 in Mitochondria: Yeast Two-Hybrid-Based Interaction with Complex I Without Relevance for Basal Reactive Oxygen Species? Antioxid. Redox Signal. 2015;23:1106–1112. doi: 10.1089/ars.2014.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Miyagi H., Kawai S., Murata K. Two sources of mitochondrial NADPH in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2009;284:7553–7560. doi: 10.1074/jbc.M804100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.He W., Wang Y., Liu W., Zhou C.Z. Crystal structure of Saccharomyces cerevisiae 6-phosphogluconate dehydrogenase Gnd1. BMC Struct. Boil. 2007;7:38. doi: 10.1186/1472-6807-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Minard K.I., McAlister-Henn L. Sources of NADPH in yeast vary with carbon source. J. Biol. Chem. 2005;280:39890–39896. doi: 10.1074/jbc.M509461200. [DOI] [PubMed] [Google Scholar]
- 190.Garay E., Campos S.E., Gonzalez de la Cruz J., Gaspar A.P., Jinich A., Deluna A. High-resolution profiling of stationary-phase survival reveals yeast longevity factors and their genetic interactions. PLoS Genet. 2014;10:e1004168. doi: 10.1371/journal.pgen.1004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Laschober G.T., Ruli D., Hofer E., Muck C., Carmona-Gutierrez D., Ring J., Hutter E., Ruckenstuhl C., Micutkova L., Brunauer R., et al. Identification of evolutionarily conserved genetic regulators of cellular aging. Aging Cell. 2010;9:1084–1097. doi: 10.1111/j.1474-9726.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Orlandi I., Stamerra G., Vai M. Altered Expression of Mitochondrial NAD(+) Carriers Influences Yeast Chronological Lifespan by Modulating Cytosolic and Mitochondrial Metabolism. Front. Genet. 2018;9:676. doi: 10.3389/fgene.2018.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brandes N., Tienson H., Lindemann A., Vitvitsky V., Reichmann D., Banerjee R., Jakob U. Time line of redox events in aging postmitotic cells. eLife. 2013;2:e00306. doi: 10.7554/eLife.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kwolek-Mirek M., Maslanka R., Molon M. Disorders in NADPH generation via pentose phosphate pathway influence the reproductive potential of the Saccharomyces cerevisiae yeast due to changes in redox status. J. Cell. Biochem. 2018 doi: 10.1002/jcb.28140. [DOI] [PubMed] [Google Scholar]
- 195.Picazo C., Matallana E., Aranda A. Yeast thioredoxin reductase Trr1p controls TORC1-regulated processes. Sci. Rep. 2018;8:16500. doi: 10.1038/s41598-018-34908-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Matecic M., Smith D.L., Pan X., Maqani N., Bekiranov S., Boeke J.D., Smith J.S. A microarray-based genetic screen for yeast chronological aging factors. PLoS Genet. 2010;6:e1000921. doi: 10.1371/journal.pgen.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Musa M., Peric M., Bou Dib P., Sobocanec S., Saric A., Lovric A., Rudan M., Nikolic A., Milosevic I., Vlahovicek K., et al. Heat-induced longevity in budding yeast requires respiratory metabolism and glutathione recycling. Aging. 2018;10:2407–2427. doi: 10.18632/aging.101560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Managbanag J.R., Witten T.M., Bonchev D., Fox L.A., Tsuchiya M., Kennedy B.K., Kaeberlein M. Shortest-path network analysis is a useful approach toward identifying genetic determinants of longevity. PLoS ONE. 2008;3:e3802. doi: 10.1371/journal.pone.0003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Smith E.D., Tsuchiya M., Fox L.A., Dang N., Hu D., Kerr E.O., Johnston E.D., Tchao B.N., Pak D.N., Welton K.L., et al. Quantitative evidence for conserved longevity pathways between divergent eukaryotic species. Genome Res. 2008;18:564–570. doi: 10.1101/gr.074724.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 201.Tullet J.M., Hertweck M., An J.H., Baker J., Hwang J.Y., Liu S., Oliveira R.P., Baumeister R., Blackwell T.K. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yang H.C., Yu H., Liu Y.C., Chen T.L., Stern A., Lo S.J., Chiu D.T. IDH-1 deficiency induces growth defects and metabolic alterations in GSPD-1-deficient Caenorhabditis elegans. J. Mol. Med. 2019;366:461–464. doi: 10.1007/s00109-018-01740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yu H., Larsen P.L. DAF-16-dependent and independent expression targets of DAF-2 insulin receptor-like pathway in Caenorhabditis elegans include FKBPs. J. Mol. Biol. 2001;314:1017–1028. doi: 10.1006/jmbi.2000.5210. [DOI] [PubMed] [Google Scholar]
- 204.Depuydt G., Xie F., Petyuk V.A., Smolders A., Brewer H.M., Camp D.G., 2nd, Smith R.D., Braeckman B.P. LC-MS Proteomics Analysis of the Insulin/IGF-1-Deficient Caenorhabditis elegans daf-2(e1370) Mutant Reveals Extensive Restructuring of Intermediary Metabolism. J. Proteome Res. 2014;13:1938–1956. doi: 10.1021/pr401081b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Martell J., Seo Y., Bak D.W., Kingsley S.F., Tissenbaum H.A., Weerapana E. Global Cysteine-Reactivity Profiling during Impaired Insulin/IGF-1 Signaling in C. elegans Identifies Uncharacterized Mediators of Longevity. Cell Chem. Boil. 2016;23:955–966. doi: 10.1016/j.chembiol.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Walther D.M., Kasturi P., Zheng M., Pinkert S., Vecchi G., Ciryam P., Morimoto R.I., Dobson C.M., Vendruscolo M., Mann M., et al. Widespread Proteome Remodeling and Aggregation in Aging C. elegans. Cell. 2015;161:919–932. doi: 10.1016/j.cell.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pinkston-Gosse J., Kenyon C. DAF-16/FOXO targets genes that regulate tumor growth in Caenorhabditis elegans. Nat. Genet. 2007;39:1403–1409. doi: 10.1038/ng.2007.1. [DOI] [PubMed] [Google Scholar]
- 208.Zhang P., Judy M., Lee S.J., Kenyon C. Direct and indirect gene regulation by a life-extending FOXO protein in C. elegans: Roles for GATA factors and lipid gene regulators. Cell Metab. 2013;17:85–100. doi: 10.1016/j.cmet.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Murphy C.T., McCarroll S.A., Bargmann C.I., Fraser A., Kamath R.S., Ahringer J., Li H., Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 210.Erkut C., Gade V.R., Laxman S., Kurzchalia T.V. The glyoxylate shunt is essential for desiccation tolerance in C. elegans and budding yeast. eLife. 2016;5:e13614. doi: 10.7554/eLife.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gallo M., Park D., Riddle D.L. Increased longevity of some C. elegans mitochondrial mutants explained by activation of an alternative energy-producing pathway. Mech. Ageing Dev. 2011;132:515–518. doi: 10.1016/j.mad.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 212.Zuryn S., Kuang J., Tuck A., Ebert P.R. Mitochondrial dysfunction in Caenorhabditis elegans causes metabolic restructuring, but this is not linked to longevity. Mech. Ageing Dev. 2010;131:554–561. doi: 10.1016/j.mad.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 213.Hamilton B., Dong Y., Shindo M., Liu W., Odell I., Ruvkun G., Lee S.S. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 2005;19:1544–1555. doi: 10.1101/gad.1308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chin R.M., Fu X., Pai M.Y., Vergnes L., Hwang H., Deng G., Diep S., Lomenick B., Meli V.S., Monsalve G.C., et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510:397–401. doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kim Y., Sun H. Functional genomic approach to identify novel genes involved in the regulation of oxidative stress resistance and animal lifespan. Aging Cell. 2007;6:489–503. doi: 10.1111/j.1474-9726.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 216.Chen T.L., Yang H.C., Hung C.Y., Ou M.H., Pan Y.Y., Cheng M.L., Stern A., Lo S.J., Chiu D.T. Impaired embryonic development in glucose-6-phosphate dehydrogenase-deficient Caenorhabditis elegans due to abnormal redox homeostasis induced activation of calcium-independent phospholipase and alteration of glycerophospholipid metabolism. Cell Death Dis. 2017;8:e2545. doi: 10.1038/cddis.2016.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bennett C.F., Kwon J.J., Chen C., Russell J., Acosta K., Burnaevskiy N., Crane M.M., Bitto A., Vander Wende H., Simko M., et al. Transaldolase inhibition impairs mitochondrial respiration and induces a starvation-like longevity response in Caenorhabditis elegans. PLoS Genet. 2017;13:e1006695. doi: 10.1371/journal.pgen.1006695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang J., Robida-Stubbs S., Tullet J.M., Rual J.F., Vidal M., Blackwell T.K. RNAi screening implicates a SKN-1-dependent transcriptional response in stress resistance and longevity deriving from translation inhibition. PLoS Genet. 2010;6:e1001048. doi: 10.1371/journal.pgen.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bennett C.F., Kaeberlein M. The mitochondrial unfolded protein response and increased longevity: Cause, consequence, or correlation? Exp. Gerontol. 2014;56:142–146. doi: 10.1016/j.exger.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Melber A., Haynes C.M. UPR(mt) regulation and output: A stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018;28:281–295. doi: 10.1038/cr.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu Z., Senchuk M.M., Dues D.J., Johnson B.K., Cooper J.F., Lew L., Machiela E., Schaar C.E., DeJonge H., Blackwell T.K., et al. Mitochondrial unfolded protein response transcription factor ATFS-1 promotes longevity in a long-lived mitochondrial mutant through activation of stress response pathways. BMC Biol. 2018;16:147. doi: 10.1186/s12915-018-0615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rzezniczak T.Z., Merritt T.J. Interactions of NADP-reducing enzymes across varying environmental conditions: A model of biological complexity. G3 Genes Genomes Genet. 2012;2:1613–1623. doi: 10.1534/g3.112.003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.White K., Kim M.J., Ding D., Han C., Park H.J., Meneses Z., Tanokura M., Linser P., Salvi R., Someya S. G6pd Deficiency Does Not Affect the Cytosolic Glutathione or Thioredoxin Antioxidant Defense in Mouse Cochlea. J. Neurosci. 2017;37:5770–5781. doi: 10.1523/JNEUROSCI.0519-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Au S.W., Gover S., Lam V.M., Adams M.J. Human glucose-6-phosphate dehydrogenase: The crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency. Structure (Lond. Engl. 1993) 2000;8:293–303. doi: 10.1016/s0969-2126(00)00104-0. [DOI] [PubMed] [Google Scholar]
- 225.Pandolfi P.P., Sonati F., Rivi R., Mason P., Grosveld F., Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995;14:5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Urban N., Tsitsipatis D., Hausig F., Kreuzer K., Erler K., Stein V., Ristow M., Steinbrenner H., Klotz L.O. Non-linear impact of glutathione depletion on C. elegans life span and stress resistance. Redox Boil. 2017;11:502–515. doi: 10.1016/j.redox.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Okuyama T., Inoue H., Ookuma S., Satoh T., Kano K., Honjoh S., Hisamoto N., Matsumoto K., Nishida E. The ERK-MAPK pathway regulates longevity through SKN-1 and insulin-like signaling in Caenorhabditis elegans. J. Biol. Chem. 2010;285:30274–30281. doi: 10.1074/jbc.M110.146274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Przybysz A.J., Choe K.P., Roberts L.J., Strange K. Increased age reduces DAF-16 and SKN-1 signaling and the hormetic response of Caenorhabditis elegans to the xenobiotic juglone. Mech. Ageing Dev. 2009;130:357–369. doi: 10.1016/j.mad.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dillin A., Crawford D.K., Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- 230.Edwards C., Canfield J., Copes N., Rehan M., Lipps D., Bradshaw P.C. D-β-hydroxybutyrate extends lifespan in C. elegans. Aging (Albany Ny) 2014;6:621–644. doi: 10.18632/aging.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schwartz A.G., Pashko L.L. Dehydroepiandrosterone, glucose-6-phosphate dehydrogenase, and longevity. Ageing Res. Rev. 2004;3:171–187. doi: 10.1016/j.arr.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 232.Vogelauer M., Krall A.S., McBrian M.A., Li J.Y., Kurdistani S.K. Stimulation of histone deacetylase activity by metabolites of intermediary metabolism. J. Biol. Chem. 2012;287:32006–32016. doi: 10.1074/jbc.M112.362467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pasyukova E.G., Vaiserman A.M. HDAC inhibitors: A new promising drug class in anti-aging research. Mech. Ageing Dev. 2017;166:6–15. doi: 10.1016/j.mad.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 234.Arkblad E.L., Tuck S., Pestov N.B., Dmitriev R.I., Kostina M.B., Stenvall J., Tranberg M., Rydstrom J. A Caenorhabditis elegans mutant lacking functional nicotinamide nucleotide transhydrogenase displays increased sensitivity to oxidative stress. Free Radic. Biol. Med. 2005;38:1518–1525. doi: 10.1016/j.freeradbiomed.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 235.Wu K.C., Cui J.Y., Klaassen C.D. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol. Sci. 2011;123:590–600. doi: 10.1093/toxsci/kfr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yang W., Hekimi S. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell. 2010;9:433–447. doi: 10.1111/j.1474-9726.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 237.Dues D.J., Schaar C.E., Johnson B.K., Bowman M.J., Winn M.E., Senchuk M.M., Van Raamsdonk J.M. Uncoupling of oxidative stress resistance and lifespan in long-lived isp-1 mitochondrial mutants in Caenorhabditis elegans. Free Radic. Biol. Med. 2017;108:362–373. doi: 10.1016/j.freeradbiomed.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.An J.H., Blackwell T.K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tang L., Choe K.P. Characterization of skn-1/wdr-23 phenotypes in Caenorhabditis elegans; pleiotrophy, aging, glutathione, and interactions with other longevity pathways. Mech. Ageing Dev. 2015;149:88–98. doi: 10.1016/j.mad.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 240.Sohal R.S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sanchez-Roman I., Barja G. Regulation of longevity and oxidative stress by nutritional interventions: Role of methionine restriction. Exp. Gerontol. 2013;48:1030–1042. doi: 10.1016/j.exger.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 242.Hine C., Mitchell J.R. Calorie restriction and methionine restriction in control of endogenous hydrogen sulfide production by the transsulfuration pathway. Exp. Gerontol. 2015;68:26–32. doi: 10.1016/j.exger.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Eriksson S., Prigge J.R., Talago E.A., Arner E.S., Schmidt E.E. Dietary methionine can sustain cytosolic redox homeostasis in the mouse liver. Nat. Commun. 2015;6:6479. doi: 10.1038/ncomms7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ghosh D., Brewer G.J. External cys/cySS redox state modification controls the intracellular redox state and neurodegeneration via Akt in aging and Alzheimer’s disease mouse model neurons. J. Alzheimers Dis. 2014;42:313–324. doi: 10.3233/JAD-132756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.McIsaac R.S., Lewis K.N., Gibney P.A., Buffenstein R. From yeast to human: Exploring the comparative biology of methionine restriction in extending eukaryotic life span. Ann. N. Y. Acad. Sci. 2016;1363:155–170. doi: 10.1111/nyas.13032. [DOI] [PubMed] [Google Scholar]
- 246.Uthus E.O., Brown-Borg H.M. Methionine flux to transsulfuration is enhanced in the long living Ames dwarf mouse. Mech. Ageing Dev. 2006;127:444–450. doi: 10.1016/j.mad.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Slekar K.H., Kosman D.J., Culotta V.C. The Yeast Copper/Zinc Superoxide Dismutase and the Pentose Phosphate Pathway Play Overlapping Roles in Oxidative Stress Protection. J. Boil. Chem. 1996;271:28831–28836. doi: 10.1074/jbc.271.46.28831. [DOI] [PubMed] [Google Scholar]
- 248.Campbell K., Vowinckel J., Keller M.A., Ralser M. Methionine Metabolism Alters Oxidative Stress Resistance via the Pentose Phosphate Pathway. Antioxid. Redox Signal. 2016;24:543–547. doi: 10.1089/ars.2015.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lee B.C., Kaya A., Ma S., Kim G., Gerashchenko M.V., Yim S.H., Hu Z., Harshman L.G., Gladyshev V.N. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat. Commun. 2014;5:3592. doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Johnson J.E., Johnson F.B. Methionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cells. PLoS ONE. 2014;9:e97729. doi: 10.1371/journal.pone.0097729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ruckenstuhl C., Netzberger C., Entfellner I., Carmona-Gutierrez D., Kickenweiz T., Stekovic S., Gleixner C., Schmid C., Klug L., Sorgo A.G., et al. Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet. 2014;10:e1004347. doi: 10.1371/journal.pgen.1004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wipf B., Leisinger T. Compartmentation of arginine biosynthesis in Saccharomyces cerevisiae. Fems Microbiol. Lett. 1977;2:239–242. doi: 10.1111/j.1574-6968.1977.tb00950.x. [DOI] [Google Scholar]
- 253.Zabriskie T.M., Jackson M.D. Lysine biosynthesis and metabolism in fungi. Nat. Prod. Rep. 2000;17:85–97. doi: 10.1039/a801345d. [DOI] [PubMed] [Google Scholar]
- 254.Canto C., Auwerx J. Calorie restriction: Is AMPK a key sensor and effector? Physiol. 2011;26:214–224. doi: 10.1152/physiol.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Iio W., Tokutake Y., Koike H., Matsukawa N., Tsukahara T., Chohnan S., Toyoda A. Effects of chronic mild food restriction on behavior and the hypothalamic malonyl-CoA signaling pathway. Anim. Sci. J. 2015;86:181–188. doi: 10.1111/asj.12255. [DOI] [PubMed] [Google Scholar]
- 256.Canto C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C., Elliott P.J., Puigserver P., Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Walsh M.E., Shi Y., Van Remmen H. The effects of dietary restriction on oxidative stress in rodents. Free Radic. Boil. Med. 2014;66:88–99. doi: 10.1016/j.freeradbiomed.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pearson K.J., Lewis K.N., Price N.L., Chang J.W., Perez E., Cascajo M.V., Tamashiro K.L., Poosala S., Csiszar A., Ungvari Z., et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc. Natl. Acad. Sci. USA. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Someya S., Yu W., Hallows W.C., Xu J., Vann J.M., Leeuwenburgh C., Tanokura M., Denu J.M., Prolla T.A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yeo H., Lyssiotis C.A., Zhang Y., Ying H., Asara J.M., Cantley L.C., Paik J.H. FoxO3 coordinates metabolic pathways to maintain redox balance in neural stem cells. EMBO J. 2013;32:2589–2602. doi: 10.1038/emboj.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Chen D., Bruno J., Easlon E., Lin S.J., Cheng H.L., Alt F.W., Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Olmos Y., Sanchez-Gomez F.J., Wild B., Garcia-Quintans N., Cabezudo S., Lamas S., Monsalve M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1alpha complex. Antioxid. Redox Signal. 2013;19:1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cho C.G., Kim H.J., Chung S.W., Jung K.J., Shim K.H., Yu B.P., Yodoi J., Chung H.Y. Modulation of glutathione and thioredoxin systems by calorie restriction during the aging process. Exp. Gerontol. 2003;38:539–548. doi: 10.1016/S0531-5565(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 264.Rohrbach S., Gruenler S., Teschner M., Holtz J. The thioredoxin system in aging muscle: Key role of mitochondrial thioredoxin reductase in the protective effects of caloric restriction? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R927–R935. doi: 10.1152/ajpregu.00890.2005. [DOI] [PubMed] [Google Scholar]
- 265.Johnson M.L., Distelmaier K., Lanza I.R., Irving B.A., Robinson M.M., Konopka A.R., Shulman G.I., Nair K.S. Mechanism by Which Caloric Restriction Improves Insulin Sensitivity in Sedentary Obese Adults. Diabetes. 2016;65:74–84. doi: 10.2337/db15-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Molin M., Yang J., Hanzen S., Toledano M.B., Labarre J., Nystrom T. Life span extension and H(2)O(2) resistance elicited by caloric restriction require the peroxiredoxin Tsa1 in Saccharomyces cerevisiae. Mol. Cell. 2011;43:823–833. doi: 10.1016/j.molcel.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 267.Fierro-Gonzalez J.C., Gonzalez-Barrios M., Miranda-Vizuete A., Swoboda P. The thioredoxin TRX-1 regulates adult lifespan extension induced by dietary restriction in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2011;406:478–482. doi: 10.1016/j.bbrc.2011.02.079. [DOI] [PubMed] [Google Scholar]
- 268.Menger K.E., James A.M., Cocheme H.M., Harbour M.E., Chouchani E.T., Ding S., Fearnley I.M., Partridge L., Murphy M.P. Fasting, but Not Aging, Dramatically Alters the Redox Status of Cysteine Residues on Proteins in Drosophila melanogaster. Cell Rep. 2015;11:1856–1865. doi: 10.1016/j.celrep.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Newman J.C., Covarrubias A.J., Zhao M., Yu X., Gut P., Ng C.P., Huang Y., Haldar S., Verdin E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017;26:547–557.e8. doi: 10.1016/j.cmet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Roberts M.N., Wallace M.A., Tomilov A.A., Zhou Z., Marcotte G.R., Tran D., Perez G., Gutierrez-Casado E., Koike S., Knotts T.A., et al. A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab. 2017;26:539–546.e5. doi: 10.1016/j.cmet.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Pawlosky R.J., Kemper M.F., Kashiwaya Y., King M.T., Mattson M.P., Veech R.L. Effects of a dietary ketone ester on hippocampal glycolytic and tricarboxylic acid cycle intermediates and amino acids in a 3xTgAD mouse model of Alzheimer’s disease. J. Neurochem. 2017;141:195–207. doi: 10.1111/jnc.13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.De Nobrega A.K., Lyons L.C. Aging and the clock: Perspective from flies to humans. Eur. J. Neurosci. 2018 doi: 10.1111/ejn.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Froy O. Circadian rhythms, aging, and life span in mammals. Physiology. 2011;26:225–235. doi: 10.1152/physiol.00012.2011. [DOI] [PubMed] [Google Scholar]
- 274.Zhang R., Lahens N.F., Ballance H.I., Hughes M.E., Hogenesch J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Mauvoisin D., Wang J., Jouffe C., Martin E., Atger F., Waridel P., Quadroni M., Gachon F., Naef F. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc. Natl. Acad. Sci. USA. 2014;111:167–172. doi: 10.1073/pnas.1314066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mendez I., Vazquez-Martinez O., Hernandez-Munoz R., Valente-Godinez H., Diaz-Munoz M. Redox regulation and pro-oxidant reactions in the physiology of circadian systems. Biochimie. 2016;124:178–186. doi: 10.1016/j.biochi.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 277.Peek C.B., Affinati A.H., Ramsey K.M., Kuo H.Y., Yu W., Sena L.A., Ilkayeva O., Marcheva B., Kobayashi Y., Omura C., et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Nakahata Y., Sahar S., Astarita G., Kaluzova M., Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ramsey K.M., Yoshino J., Brace C.S., Abrassart D., Kobayashi Y., Marcheva B., Hong H.K., Chong J.L., Buhr E.D., Lee C., et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Goodman R.P., Calvo S.E., Mootha V.K. Spatiotemporal compartmentalization of hepatic NADH and NADPH metabolism. J. Boil. Chem. 2018;293:7508–7516. doi: 10.1074/jbc.TM117.000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Putker M., Crosby P., Feeney K.A., Hoyle N.P., Costa A.S.H., Gaude E., Frezza C., O’Neill J.S. Mammalian Circadian Period, But Not Phase and Amplitude, Is Robust Against Redox and Metabolic Perturbations. Antioxid. Redox Signal. 2018;28:507–520. doi: 10.1089/ars.2016.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rey G., Valekunja U.K., Feeney K.A., Wulund L., Milev N.B., Stangherlin A., Ansel-Bollepalli L., Velagapudi V., O’Neill J.S., Reddy A.B. The Pentose Phosphate Pathway Regulates the Circadian Clock. Cell Metab. 2016;24:462–473. doi: 10.1016/j.cmet.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Cracan V., Titov D.V., Shen H., Grabarek Z., Mootha V.K. A genetically encoded tool for manipulation of NADP(+)/NADPH in living cells. Nat. Chem. Boil. 2017;13:1088–1095. doi: 10.1038/nchembio.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sandbichler A.M., Jansen B., Peer B.A., Paulitsch M., Pelster B., Egg M. Metabolic Plasticity Enables Circadian Adaptation to Acute Hypoxia in Zebrafish Cells. Cell. Physiol. Biochem. 2018;46:1159–1174. doi: 10.1159/000489058. [DOI] [PubMed] [Google Scholar]
- 285.Rutter J., Reick M., Wu L.C., McKnight S.L. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 286.Yoshii K., Tajima F., Ishijima S., Sagami I. Changes in pH and NADPH regulate the DNA binding activity of neuronal PAS domain protein 2, a mammalian circadian transcription factor. Biochemistry. 2015;54:250–259. doi: 10.1021/bi5008518. [DOI] [PubMed] [Google Scholar]
- 287.Goya M.E., Romanowski A., Caldart C.S., Benard C.Y., Golombek D.A. Circadian rhythms identified in Caenorhabditis elegans by in vivo long-term monitoring of a bioluminescent reporter. Proc. Natl. Acad. Sci. USA. 2016;113:E7837–E7845. doi: 10.1073/pnas.1605769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Rey G., Milev N.B., Valekunja U.K., Ch R., Ray S., Silva Dos Santos M., Nagy A.D., Antrobus R., MacRae J.I., Reddy A.B. Metabolic oscillations on the circadian time scale in Drosophila cells lacking clock genes. Mol. Syst. Biol. 2018;14:e8376. doi: 10.15252/msb.20188376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Causton H.C., Feeney K.A., Ziegler C.A., O’Neill J.S. Metabolic Cycles in Yeast Share Features Conserved among Circadian Rhythms. Curr. Biol. 2015;25:1056–1062. doi: 10.1016/j.cub.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lerner F., Niere M., Ludwig A., Ziegler M. Structural and functional characterization of human NAD kinase. Biochem. Biophys. Res. Commun. 2001;288:69–74. doi: 10.1006/bbrc.2001.5735. [DOI] [PubMed] [Google Scholar]
- 291.Love N.R., Pollak N., Dolle C., Niere M., Chen Y., Oliveri P., Amaya E., Patel S., Ziegler M. NAD kinase controls animal NADP biosynthesis and is modulated via evolutionarily divergent calmodulin-dependent mechanisms. Proc. Natl. Acad. Sci. USA. 2015;112:1386–1391. doi: 10.1073/pnas.1417290112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Shianna K.V., Marchuk D.A., Strand M.K. Genomic characterization of POS5, the Saccharomyces cerevisiae mitochondrial NADH kinase. Mitochondrion. 2006;6:94–101. doi: 10.1016/j.mito.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 293.Ohashi K., Kawai S., Murata K. Identification and characterization of a human mitochondrial NAD kinase. Nat. Commun. 2012;3:1248. doi: 10.1038/ncomms2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhang R. MNADK, a novel liver-enriched mitochondrion-localized NAD kinase. Boil. Open. 2013;2:432–438. doi: 10.1242/bio.20134259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.El-Khoury R., Sainsard-Chanet A. Deletion of the mitochondrial NADH kinase increases mitochondrial DNA stability and life span in the filamentous fungus Podospora anserina. Exp. Gerontol. 2010;45:543–549. doi: 10.1016/j.exger.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 296.Xiong Z.M., O’Donovan M., Sun L., Choi J.Y., Ren M., Cao K. Anti-Aging Potentials of Methylene Blue for Human Skin Longevity. Sci. Rep. 2017;7:2475. doi: 10.1038/s41598-017-02419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gura T. Hope in Alzheimer’s fight emerges from unexpected places. Nat. Med. 2008;14:894. doi: 10.1038/nm0908-894. [DOI] [PubMed] [Google Scholar]
- 298.Gureev A.P., Shaforostova E.A., Popov V.N., Starkov A.A. Methylene blue does not bypass Complex III antimycin block in mouse brain mitochondria. FEBS Lett. 2019 doi: 10.1002/1873-3468.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wen Y., Li W., Poteet E.C., Xie L., Tan C., Yan L.J., Ju X., Liu R., Qian H., Marvin M.A., et al. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J. Biol. Chem. 2011;286:16504–16515. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ahn H., Kang S.G., Yoon S.I., Ko H.J., Kim P.H., Hong E.J., An B.S., Lee E., Lee G.S. Methylene blue inhibits NLRP3, NLRC4, AIM2, and non-canonical inflammasome activation. Sci. Rep. 2017;7:12409. doi: 10.1038/s41598-017-12635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Medina D.X., Caccamo A., Oddo S. Methylene blue reduces abeta levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 2011;21:140–149. doi: 10.1111/j.1750-3639.2010.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Huang C., Wu J., Xu L., Wang J., Chen Z., Yang R. Regulation of HSF1 protein stabilization: An updated review. Eur. J. Pharm. 2018;822:69–77. doi: 10.1016/j.ejphar.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 303.Copes N. High-Throughput Screening of Age-Related Changes in Caenorhabditis elegans. University of South Florida; Tampa, FL, USA: 2015. [Google Scholar]
- 304.Harrison D.E., Strong R., Allison D.B., Ames B.N., Astle C.M., Atamna H., Fernandez E., Flurkey K., Javors M.A., Nadon N.L., et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Massie H.R., Aiello V.R., Williams T.R. Influence of photosensitizers and light on the life span of Drosophila. Mech. Ageing Dev. 1993;68:175–182. doi: 10.1016/0047-6374(93)90149-L. [DOI] [PubMed] [Google Scholar]
- 306.Vatolin S., Radivoyevitch T., Maciejewski J.P. New drugs for pharmacological extension of replicative life span in normal and progeroid cells. Npj Aging Mech. Dis. 2019;5:2. doi: 10.1038/s41514-018-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Diaz-Ruiz A., Lanasa M., Garcia J., Mora H., Fan F., Martin-Montalvo A., Di Francesco A., Calvo-Rubio M., Salvador-Pascual A., Aon M.A., et al. Overexpression of CYB5R3 and NQO1, two NAD(+) -producing enzymes, mimics aspects of caloric restriction. Aging Cell. 2018;17:e12767. doi: 10.1111/acel.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]