Abstract
Clonogenic multipotent mouse hematopoietic stem cells (HSCs) and progenitor cells are contained within the c-kit+ (K) lineage−/lo (L) Sca-1+ (S) population of hematopoietic cells; long-term (LT) and short-term (ST) HSCs are Thy-1.1lo. c-kit is a member of the receptor tyrosine kinase family, a class of receptors that are important in the proliferation and differentiation of hematopoietic cells. To establish whether the Flk-2/Flt3 receptor tyrosine kinase was expressed on the most primitive LT-HSCs, we sorted highly purified multipotent stem and progenitor cells on the basis of Flk-2 surface expression and used them in competitive reconstitution assays. Low numbers of Flk-2− HSCs gave rise to long-term multilineage reconstitution in the majority of recipients, whereas the transfer of Flk-2+ multipotent cells resulted in mostly short-term multilineage reconstitution. The KLS subset of adult mouse bone marrow was analyzed for Flk-2 and Thy-1.1 expression. Three phenotypically and functionally distinct populations were isolated: Thylo Flk-2− (LT-HSCs), Thylo Flk-2+ (ST-HSCs), and Thy− Flk-2+ multipotent progenitors. The loss of Thy-1.1 and gain of Flk-2 expression marks the loss of self-renewal in HSC maturation. The addition of Flk-2 antibody to the lineage mix allows direct isolation of LT-HSC from adult bone marrow as c-kit+ lin− Sca-1+ Flk-2− from many strains of mice. Fetal liver HSCs are contained within Flk-2− and Flk-2+ KTLS cells.
Highly purified hematopoietic stem cells (HSCs) can be isolated by fluorescence-activated cell sorting with the cell surface markers c-kit+ Thy-1.1lo lineage−/lo Sca-1+ (KTLS). Long-term HSCs (LT-HSCs) are defined by their ability to give rise to the lymphoid and myeloerythroid lineages for life after transplantation into lethally irradiated recipients. Short-term HSCs (ST-HSCs) have more limited self-renewal capacity and are capable of giving rise to these lineages for 8–12 weeks. The HSC pool has been shown to be heterogeneous in size (1), cell cycle status (2), rhodamine staining (3), and surface molecule expression (4–6). Further division of the stem cell pool based on these characteristics has allowed for separation of HSCs, providing long- or short-term engraftment.
The receptor tyrosine kinase Flk-2 has been shown to have heterogeneous expression on the stem cell pool. Long-term reconstituting activity has been demonstrated in both the Flk-2-positive and -negative fractions (7, 8); the Flk-2− fraction of adult bone marrow (BM) is hypothesized to contain the most primitive HSCs (8). Flk-2 was cloned initially from a highly purified fetal liver HSC library and shows a high degree of homology to the c-kit and c-fms receptors (9, 10). Flk-2 stimulated by the flt3 ligand has been implicated as both a survival (11) and a proliferative signal (12) of primitive hematopoietic progenitors, acting in synergy with the receptors for steel factor, IL-6, IL-11, granulocyte colony-stimulating factor, or thrombopoietin in enhancing the expansion of both murine (11, 13–16) and human (17–20) primitive progenitors.
To date, Flk-2 receptor expression has been assayed on cells enriched for HSC activity but not on highly purified HSCs. In this study we analyzed the functional differences in cells of HSC phenotype that do or do not express Flk-2. In adult BM, Flk-2− Thy-1.1lo KLS cells are LT-HSCs, Flk-2+ Thy-1.1lo KLS cells are ST-HSCs, and Flk-2+ Thy-1.1− KLS cells are multipotent progenitors. In fetal liver the phenotypes are similar except that the Flk-2+ Thy-1.1lo KLS subset contains some LT-HSCs as well. The addition of Flk-2 to the lineage mix allows isolation of highly purified HSCs from many mouse strains using three-color fluorescence-activated cell sorting capability.
Methods
Mouse Strains.
The C57BL/Ka-Thy-1.1 (Thy-1.1, Ly5.1), C57BL/Ka-Thy-1.2 (Thy-1.2, Ly5.1), C57BL/Ka-Ly5.2 (Thy1.2, Ly5.2), and C57BL/Ka-Thy-1.1/Ly5.2 (Thy-1.1, Ly5.2) mouse strains were bred and maintained at the Stanford University Laboratory Animal Facility. All mice were maintained routinely on acidified water (pH 2.5). Donors of purified HSCs and progenitors were 6–10 weeks of age. Irradiated recipient mice were greater than 8 weeks old at the time of irradiation.
Antibodies.
The monoclonal antibodies used in immunofluorescence staining for HSC and progenitor isolation included 2B8 (anti-c-kit, Allo-phycocyanin), 19XE5 (anti-Thy-1.1, FITC conjugate), E13 (anti-Sca-1, Ly6A/E, Texas red conjugate), and A2F10 (anti-Flk-2/Flt3, CD135, phycoerythrin conjugate, Becton Dickenson–PharMingen). Lineage marker antibodies included 6B2 (anti-B220), KT31.1 (anti-CD3), GK1.5 (anti-CD4), 53-7.3 (anti-CD5), 53-6.7 (anti-CD8), Ter119 (anti-erythrocyte-specific antigen), 8C5 (anti-Gr-1), and M1/70 (anti-Mac-1). Unconjugated lineage antibodies were used in conjunction with anti-rat Cy5PE (Caltag, South San Francisco, CA). Directly labeled phycoerythrin and Cy5PE (eBioscience, San Diego, CA) lineage antibodies were sometimes used. Biotinylated 3C11 (anti-c-kit) was used for the enrichment of c-kit+ cells; 3C11 and 2B8 recognize distinct, nonoverlapping epitopes of c-kit. A20.1 (anti-Ly5.1, FITC-conjugated, Becton Dickenson–PharMingen), and AL1–4A2 (anti-Ly5.2, Texas red conjugate) were used to analyze donor cells after reconstitution.
Cell Preparation and Staining.
Adult KTLS cells were isolated as described (6). c-kit-positive cells were enriched by positive selection using streptavidin-conjugated magnetic beads and an autoMACS cell separator (Miltenyi Biotec, Auburn, CA) according to manufacturer instructions. Fetal livers were collected at embryonic day 14.5 and dispersed by drawing them several times through a 26-gauge needle. Fetal liver preparations were as described above except Mac-1 was not included in the lineage mixture. Fetal liver cells were depleted of lineage+ cells by labeling with purified lineage antibodies followed by sheep anti-rat magnetic beads (Dynal, Great Neck, NY) per manufacturer instructions. All cells used in this study were double-sorted for purity.
Competitive Reconstitution.
Recipient mice were lethally irradiated with a split dose of 950 rad as described (6). Sorted cells were transferred by retroorbital injection along with a radioprotective dose of 3 × 105 recipient type whole BM (WBM) cells. For intrathymic injections, 6-week-old congenic recipients were sublethally irradiated with a single dose of 600 rad. Twenty sorted cells were injected directly into a single thymic lobe with 105 carrier donor type thymocytes as described (21, 22).
Methylcellulose Assays.
Stem and progenitor cells were sorted at 1 cell per well with an automated cell deposition unit device (Becton Dickenson) into 96-well plates containing Methylcult H4100 (StemCell Technologies, Vancouver) supplemented with FCS and the following cytokines; stem cell factor, Flt-3, IL-6, IL-11, granulocyte/macrophage colony-stimulating factor, IL-3, human thrombopoietin, and human erythropoietin. The final concentration of growth factors was 10 ng/ml except for erythropoietin (at 4 units/ml).
Results
Phenotypic and Functional Heterogeneity of Flk-2 Expression by HSCs.
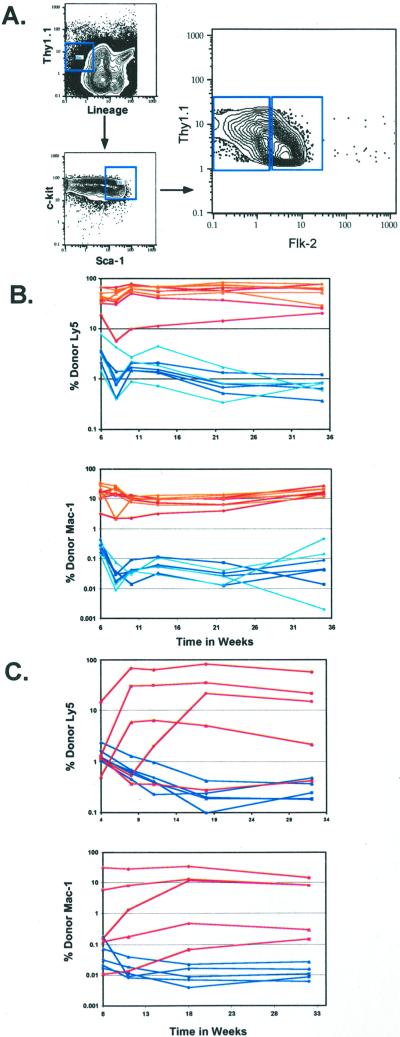
To determine the level of Flk-2 expression by purified HSCs, we analyzed staining of highly enriched KTLS HSCs with an anti-Flk-2 monoclonal antibody by flow cytometry. As shown in Fig. 1, Flk-2 staining was heterogeneous. To determine whether there were functional differences between Flk-2+ and Flk-2− KTLS cells, we double-sorted both populations and tested their long-term repopulating ability in lethally irradiated congenic hosts (Fig. 1 B and C). Low numbers of stem cells (20) were transferred intravenously along with a radioprotective dose of host-type WBM. Peripheral blood was analyzed periodically for donor lymphoid and myeloid cells. Flk-2− KTLS HSCs provided donor-derived long-term multilineage reconstitution in the majority of recipients (Table 1). Whether 20, 50, or 100 Flk-2+ HSCs were transferred, reconstitution ability was largely transient (Table 1, Fig. 1B). In the few individuals that showed long-term repopulation after Flk-2+ HSC reconstitution, the level of reconstitution was very low: ≤1%. These findings indicate that long-term repopulating HSCs are Flk-2−, whereas Flk-2+ HSCs are transiently repopulating stem cells with short-term repopulating ability.
Figure 1.
Heterogeneous expression of Flk-2 on the HSC population. (A) Flk-2 expression was analyzed on the HSC population; Lin−/lo Thy-1.1lo c-kit+ Sca-1+ of c-kit enriched BM. HSCs that were either Flk-2+ or Flk-2− were sorted. Lethally irradiated recipients were competitively reconstituted with 50–100 (B) or 20 (C) Flk-2+ or Flk-2− HSCs plus host-type radioprotective WBM. (B) Fifty Flk-2+ HSCs are shown in blue, 100 flk-2+ HSCs are in light blue, 50 Flk-2− HSCs are in red, and 100 Flk-2− HSCs are in orange. (C) Twenty Flk-2+ HSCs are shown in blue, and 20 Flk-2− HSCs are in red. Peripheral blood was analyzed for donor Ly5 B, T, and myeloid contribution. Each curve represents an individual animal. Because myeloid cells are present in the blood for a short period, myeloid activity is shown as a good indicator of stem cell activity. The background is 0.1%.
Table 1.
Summary of reconstitution profiles of mice reconstituted with Flk-2+/− HSCs
| Percentage of mice exhibiting indicated reconstitution
profiles
|
Frequency observed in long-term reconstituted
animals
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| KTLS | n | Long-term B+T+M | Transient B+T+M | B+T | B+M | B | None | Mean | Range |
| 50 Flk-2+ | 12 | 33 | 41.7 | 8.3 | 8.3 | 8.3 | 0 | 0.75 | 0.24–1.34 |
| 50 Flk-2− | 11 | 100 | 0 | 0 | 0 | 0 | 0 | 24.36 | 0.21–88.2 |
| 20 Flk-2+ | 12 | 16.7 | 50 | 25 | 8.3 | 0 | 33.3 | 0.84 | 0.56–1.12 |
| 20 Flk-2− | 12 | 66.7 | 25 | 0 | 8.3 | 0 | 0 | 23.30 | 0.28–84.2 |
Analysis of competitive repopulation by Flk-2+ or Flk-2− HSCs. The donor repopulation profiles are given as a percentage of transplanted mice. The presence of donor B, T, and myeloid cells lasting longer than 16 weeks are designated long-term B+T+M. Transient B+T+M indicates recipients that lost myeloid contribution by 10–12 weeks. B+T, B+M, and B indicate recipients with donor contribution only to the lineages indicated. None indicates no donor cells were detectable above background levels. The frequency of repopulation is the total donor white blood cell count divided by the total live cell count of a given blood sample.
Characterization of a Thy-1.1− Population with Multilineage Differentiation Potential.
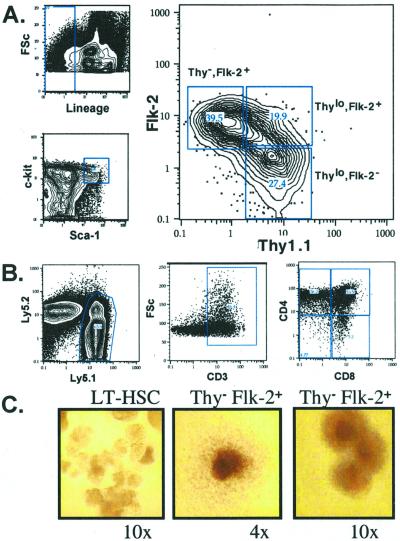
Phenotypic characterization of Flk-2 expression by HSCs indicated that the expression of Thy-1.1 was lower on Flk-2+ HSCs than on Flk-2− HSCs (Fig. 1A). Simultaneous analysis of Thy-1.1 and Flk-2 expression by a c-kit+ Lin−/lo Sca-1+ fraction of WBM identified three distinct populations (Fig. 2A): Thy-1.1lo Flk-2− (LT-HSC), Thy-1.1lo Flk-2+ (ST-HSC), and a Thy-1.1− Flk-2+ population. To characterize further the Thy-1.1− Flk-2+ (KLS) population, lethally irradiated mice were repopulated competitively with 50 or 100 Thy-1.1− Flk-2+ KLS cells (Table 2). All mice showed a low level of multilineage donor reconstitution by 6 weeks. However, by 8 weeks most had only donor lymphoid contribution, and by 15 weeks no donor myeloid cells were detectable above background levels in any of the mice.
Figure 2.
(A) Characterization of the Lin−/lo c-kit+ Sca-1+ population of c-kit-enriched murine WBM for Thy-1.1 and Flk-2 expression. (B) T cell read-out from 20 Thy-1.1− Flk-2+ cells injected intrathymically 3 weeks postinjection. (C) In vitro methylcellulose colonies derived from a single HSC or Thy-1.1− Flk-2+ progenitor. (Left) A single HSC forms many blast-like colonies at early time points (day 7). (Center and Right) Thy-1.1− Flk-2+ KLS cells do not form blast colonies. Although some form a single large colony of differentiated cell types, others form multifoci colonies.
Table 2.
Summary of reconstitution profiles of mice transplanted with Thy-1.1− Flk2+ cells
| No. of cells | Time, weeks | No.
of mice
|
Reconstitution
|
||||
|---|---|---|---|---|---|---|---|
| B+T+M | B+T | B+M | B | Mean | Range, % | ||
| 50–100* | 6 | 8/8 | 3.0 | 0.9–5.6 | |||
| 8 | 2/8 | 6/8 | 1.8 | 1.2–3.2 | |||
| 15 | 1/8 | 7/8 | 0.87 | 0.34–1.74 | |||
| 10† | 2 | 5/8 | 3/8 | ||||
| 20† | 2 | 7/8 | 1/8 | ||||
Mice were competitively transplanted with 50–100 Thy−, Flk-2+ cells (Lineage−/lo, c-kit+, Sca-1+). Transient donor profiles are indicated for three time points.
Mice were competitively transplanted with 10 or 20 Thy-Flk-2+ cells. Blood and bone marrow were assayed for B and myeloid lineages at 2 weeks posttransplant.
The Thy-1.1− Flk-2+ population yielded similar multilineage reconstitution at low (10–20) cell numbers (Table 2), although burst size was small. Because the burst size is minimal from the Thy-1.1− Flk-2+ population, recipient mice were killed at 2–3 weeks. Blood and BM were analyzed for the presence of donor B and myeloid cells.
We also tested the ability of the Thy-1.1− Flk-2+ population to give rise to T cells at low cell numbers (Fig. 2B). Twenty Thy-1.1− Flk-2+ cells were injected intrathymically into sublethally irradiated recipients along with a carrier population of host-type thymocytes. Thymuses were analyzed at 3 weeks. In all injected mice, the thymus was populated with donor-type thymocytes (both double- and single-positive for CD4 and CD8) in a pattern indicating normal T cell development from Thy-1.1− Flk-2+ cells. These data indicate that when transferred at low cell numbers, the Thy-1.1− Flk-2+ (KLS) population has transient in vivo multilineage potential.
Thy-1.1− Flk-2+ cells were sorted clonally into methylcellulose supplemented with growth factors. The Thy-1.1− Flk-2+ cells had a reduced plating efficiency of 30% as compared with the 75–80% plating efficiency of LT-HSCs. Approximately 70% of colonies arising from LT-HSCs first formed blast colonies before differentiating into mature cell types. The Thy-1.1− Flk-2+ population does not form blast colonies in vitro but rather mostly large more differentiated granulocyte/macrophage or macrophage colonies (Fig. 2C), indicating this population is likely to be less primitive than HSC.
Consistent with this hypothesis, the Thy-1.1− Flk-2+ population showed no radioprotective capability at doses ranging from 30 to 650 cells per recipient when transplanted into lethally irradiated recipients (data not shown). Similar to HSCs, the Thy− Flk-2+ population does not express the common lymphoid progenitor marker IL-7 receptor α chain (data not shown).
Flk-2 Allows for Simplified LT-HSC Isolation.
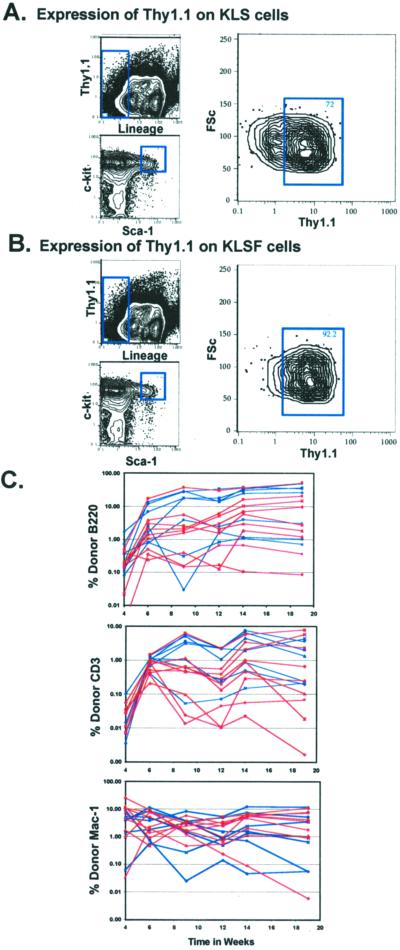
An important complication to the ability to isolate highly purified HSCs from multiple mouse lines is the lack of expression of Thy-1.1 by many strains. Although Thy1.2+ KLS cells are HSCs (23), the Thy1.2 level is lower than Thy-1.1 (24), and the Thy-1.2 very low cells bleed into the Thy-1.2− gates (25). However, the inverse relationship in the expression of Thy-1.1 and Flk-2 by the HSC population in C57BL/Ka-Thy-1.1 mice suggested that LT-HSCs might be isolated from Thy1.2 expressing strains by the addition of Flk-2 to the lineage mix. Fig. 3A shows the Thy-1.1 staining profile on the c-kit+ Lin− Sca-1+ fraction of WBM. By including antibodies to Flk-2 in the lineage mix (Fig. 3B) the Thy-1.1− subset was largely eliminated. Cells were sorted from C57BL/Ka Thy-1.1 mice by either our standard Thy-1.1lo protocol (c-kit+ Thy-1.1lo Lin− Sca-1+) or adding anti-Flk-2 to the phycoerythrin lineage mix (c-kit+ Lin− Sca-1+ Flk-2−). Lethally irradiated congenic recipients were reconstituted competitively with 10 cells of the above purified populations plus 3 × 105 radioprotective host-type WBM (Fig. 3C). The donor reconstitution profiles were indistinguishable between these two sorting protocols.
Figure 3.
HSC purification by the addition of Flk-2 to the lineage mix. Characterization of Thy-1.1 expression on the KLS (c-kit+ Lin− Sca-1+) population, with the lineage mixture either including (B) or not including (A) anti-Flk-2. (C) Lethally irradiated recipients were reconstituted competitively with 10 Thy-1.1lo KTLS HSCs (c-kit+ Thy-1.1lo Lin− Sca-1+) or 10 Flk-2− KLSF HSCs (c-kit+ Lin− Sca-1+ Flk-2−). Flk-2− HSCs (KLSF) were sorted by including Flk-2 in the lineage mixture while omitting Thy-1.1 from the sort conditions. Mice reconstituted with KTLS cells are shown in blue, and KLSF cells are shown in red.
We also sorted Flk-2− HSCs from C57BL/Ka (Thy1.2) mice, which do not express the Thy-1.1 allele. Congenic recipients were transplanted as described above. Long-term reconstitution evident in four of five transplanted with 10 cells, seven of eight transplanted with 20 cells, and three of three with 50 cells (data not shown).
Phenotypic and Functional Analysis of Flk-2 on Fetal Liver HSCs.
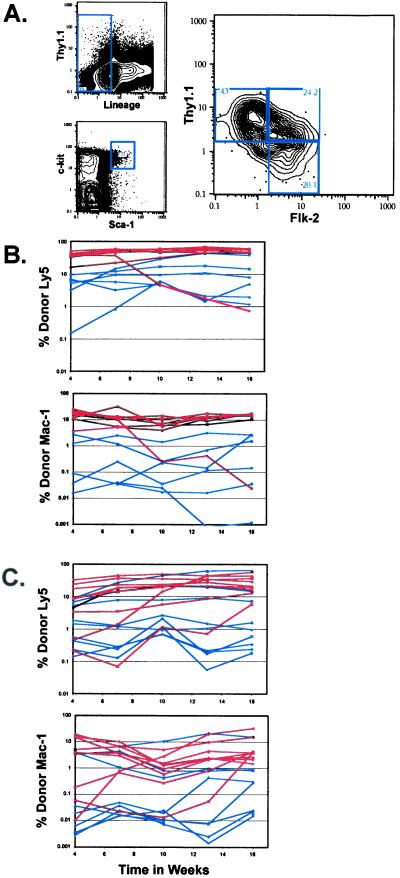
Several phenotypic and functional differences have been observed between adult BM and fetal liver HSCs. Because the flk-2 cDNA was isolated originally from primitive fetal HSCs, we wished to determine the characteristics of Flk-2 expression on the fetal liver HSC population. The c-kit+ Thy-1.1lo Lin−/lo Mac-1+ Sca-1+ fraction of fetal liver is highly enriched for LT-HSC (37). As observed in the adult BM HSC population, Flk-2 is expressed heterogeneously on fetal liver HSCs (Fig. 4A). To determine whether functional heterogeneity exists between the Thy-1.1lo Flk-2− or Flk-2+ fetal liver HSC populations, competitive reconstitution assays were performed with either 20 (Fig. 4B) or 50 (Fig. 4C) Flk-2− or Flk-2+ HSCs. Although more of the long-term repopulating activity was present in the Flk-2− subset, measurable activity was present also in the Flk-2+ subset of fetal liver HSCs. At both the 50- and 20-cell level Flk-2+ fetal liver HSCs read out at ≈70% of the Flk-2− fetal liver HSCs (Table 3). Similar to that in the adult BM, no long-term activity was found to be present in the Thy-1.1− fraction of lin− c-kit+ Sca-1+ fetal liver. The Thy-1.1− Flk-2+ population gave rise to both lymphoid and myeloid progeny after transplantation. Both the Thy-1.1-low and Thy-1.1-negative fractions of the c-kit+ Sca-1+ lineage− cells express the fetal stem cell marker AA4.1 (data not shown).
Figure 4.
(A) Analysis of Flk-2 and Thy-1.1 on the Lin−/lo c-kit+ Sca-1+ subset of fetal liver. Fetal liver HSCs (c-kit+ Thy-1.1lo Lin−/lo Sca-1+) that were either positive or negative for Flk-2 expression were sorted. Lethally irradiated recipients were reconstituted competitively with 50 (B) or 20 (C) Flk-2+ or Flk-2− fetal liver HSCs. Mice reconstituted with Flk-2+ fetal liver HSCs are shown in blue, and those reconstituted with Flk-2− fetal liver HSCs are in red. Peripheral blood was analyzed for donor B, T, or myeloid contribution.
Table 3.
Summary of reconstitution profiles of mice reconstituted with Flk-2+ and Flk-2− fetal liver HSCs
| Percentage of mice exhibiting indicated
reconstitution profiles
|
Frequency observed in
long-term reconstituted animals
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| KTLS | n | Long-term B+T+M | Transient B+T+M | B+T | B+M | B | None | Mean | Range |
| 50 Flk-2+ | 10 | 70 | 20 | 0 | 0 | 10 | 0 | 30.4 | 1.46–64.4 |
| 50 Flk-2− | 14 | 92.9 | 7.1 | 0 | 0 | 0 | 0 | 56.8 | 1.86–94.0 |
| 20 Flk-2+ | 13 | 46.2 | 15.4 | 15.4 | 0 | 0 | 23.1 | 19.7 | 1.51–63.9 |
| 20 Flk-2− | 14 | 78.6 | 21.4 | 0 | 0 | 0 | 0 | 34.9 | 0.75–86.9 |
Analysis of competitive reconstitution by Flk-2+ and Flk-2− fetal liver HSCs as described in the Table 1 legend.
Discussion
Flk-2 and Thy-1.1 Expression Define a Pathway of HSC Differentiation.
With these experiments we have found that Flk-2 and Thy-1.1 expression allows the segregation of the Lin−/lo c-kit+ Sca-1+ fraction of WBM into three functionally distinct populations with multilineage differentiation potential: Thy-1.1lo Flk-2−, Thy-1.1lo Flk-2+, and Thy-1.1− Flk-2+ (Fig. 2A). As described previously the Thy-1.1lo portion contains all the long-term reconstitution activity of adult BM (6, 26, 27). The Thy-1.1lo Flk-2− subset contains the LT-HSCs, and the Thy-1.1lo Flk-2+ subset contains predominantly ST-HSCs. The newly described Thy-1.1− Flk-2+ subset is enriched for very transient multilineage reconstituting potential. The Thy-1.1− Flk-2+ population is functionally more mature than the Mac-1lo CD-4lo (6) multipotent progenitor population and may be downstream of the multipotent progenitor. However, we cannot rule out that the Thy-1.1− Flk-2+ population is an alternative differentiation pathway from the ST-HSC. Unlike the multipotent progenitors that radioprotect 50% of recipients at 40-cell doses, the Thy-1.1− Flk-2+ progenitor population does not have radioprotective capacity at the cell doses transplanted. The Thy-1.1− Flk-2+ population also does not form HSC characteristic blast colonies at day 7 in methylcellulose but rather forms large multifoci (high proliferative potential) colonies of mature cell types. The myeloid committed oligopotent progenitor cell subset common myeloid progenitor do not form blast colonies and form smaller single-foci colonies. Thus the Thy-1.1− Flk-2+ population seems to have potential between these two subsets. The Thy-1.1− Flk-2+ subset also does not show commitment to the lymphoid pathway in that it does not express the common lymphoid progenitor marker, IL-7 receptor α chain. It shall be important to test whether the Thy-1.1− Flk-2+ population has any detectable self-renewal potential; its limited temporal production of myeloid cells in vivo rules out longer term self-renewal. Thus, as BM HSCs mature and begin to differentiate, Thy-1.1 is down-regulated and Flk-2 is up-regulated. Interestedly, Flk-2 expression is maintained by the down-stream oligopotent progenitors, the common lymphoid progenitor, and common myeloid progenitor (M. Manz, unpublished results). We propose that Flk-2 is an important negative indicator of BM stem cell self-renewal capacity, becomes up-regulated as stem cells become more mature, and may be important in expanding primitive cell types. The use of Flt3 ligand as an in vitro expansion factor of HSCs has been explored in several reports (14, 16). Because Flk-2 is not expressed by LT-HSCs, any measured expansion may be indirect or an expansion of progenitors down-stream of the LT-HSC.
Simplified LT-HSC Sorting.
HSCs have been enriched by several means (1, 3, 26–35). Further purification and isolation of highly enriched populations of HSCs have been achieved by using cell surface antibody staining and fluorescence-activated cell sorting (6, 36). We have shown that Flk-2 can replace Thy-1.1 for purifying LT-HSCs from lines of mice that do not express Thy-1.1. This strategy allows for LT-HSC isolation with only three color-sorting parameters, which opens up additional parameters for analysis of other potential markers on the LT-HSC population. This finding will allow for the analysis of stem cell activity in knockout or transgenic lines, which usually are in backgrounds without Thy-1.1 expression. Also, many currently used mouse models are not on a Thy-1.1 background.
Long-Term Activity Is Found in both the Flk-2+ and Flk-2− Subsets of Fetal Liver HSCs.
Unlike the BM, where Flk-2+ HSCs gave only short-term reconstitution, Flk-2+ mouse fetal liver HSCs show some long-term reconstitution ability. This finding is not surprising in that fetal liver HSCs have been demonstrated to differ from their adult counterparts in both phenotype and function. Phenotypically, the fetal liver HSCs express the cell surface markers Mac-1 and AA4.1, which are not expressed on the long-term repopulating HSCs in adult BM (37, 38). Fetal liver HSCs cycle more rapidly and give a more robust reconstitution than adult HSCs (37–40). Most of the stem cell pool of adult HSCs is composed of short-term and multipotent progenitors, whereas most stem cells in the fetal liver are thought to be LT-HSCs (41). Several lineages of specialized lymphoid cells arise during fetal hematopoiesis derived from fetal HSCs that cannot be generated by adult BM HSCs (42–44).
The Flk-2 expression pattern on the HSC pool allows further definition of the HSC population. Expression of the Flk-2 receptor on BM HSC correlates with loss of long-term activity. By using Flk-2 antibody to separate the long-term from short-term multilineage potential, HSC isolation becomes more accessible, allowing purification from more mouse strains and adaptation to additional mouse model systems. Further investigation into Flk-2 receptor activity will give insight into how this receptor functions in progenitor differentiation and expansion.
While this manuscript was in preparation we learned that another group found that Flk-2 (Flt3) is a marker of adult BM short-term myeloid progenitors with sustained production of lymphocytes (45).
Acknowledgments
We thank A. Wagers for helpful discussions and critical reading of the manuscript, L. Jerabek for laboratory management and assistance in intrathymic injections, V. Braunstein and S. Smith for antibody preparation, and L. Hidalgo and B. Lavarro for animal care. This work was supported by National Institutes of Health Grants CA42551 and DK53074. J.L.C. is supported by the National Institutes of Health Training Grant 5T32AI07290-16 in molecular and cellular immunobiology.
Abbreviations
- HSC
hematopoietic stem cell
- KTLS
c-kit+ Thy-1.1lo lineage−/lo Sca-1+
- LT-HSC
long-term HSC
- ST-HSC
short-term HSC
- BM
bone marrow
- WBM
whole BM
References
- 1.Jones R J, Wagner J E, Celano P, Zicha M S, Sharkis S J. Nature (London) 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 2.Fleming W H, Alpern E J, Uchida N, Ikuta K, Spangrude G J, Weissman I L. J Cell Biol. 1993;122:897–902. doi: 10.1083/jcb.122.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spangrude G J, Johnson G R. Proc Natl Acad Sci USA. 1990;87:7433–7437. doi: 10.1073/pnas.87.19.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesmann A, Phillips R L, Mojica M, Pierce L J, Searles A E, Spangrude G J, Lemischka I. Immunity. 2000;12:193–199. doi: 10.1016/s1074-7613(00)80172-7. [DOI] [PubMed] [Google Scholar]
- 5.Morel F, Galy A, Chen B, Szilvassy S J. Exp Hematol (Charlottesville, Va) 1998;26:440–448. [PubMed] [Google Scholar]
- 6.Morrison S J, Weissman I L. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 7.Zeigler F C, Bennett B D, Jordan C T, Spencer S D, Baumhueter S, Carroll K J, Hooley J, Bauer K, Matthews W. Blood. 1994;84:2422–2430. [PubMed] [Google Scholar]
- 8.Orlic D, Fischer R, Nishikawa S, Nienhuis A W, Bodine D M. Blood. 1993;82:762–770. [PubMed] [Google Scholar]
- 9.Rosnet O, Marchetto S, deLapeyriere O, Birnbaum D. Oncogene. 1991;6:1641–1650. [PubMed] [Google Scholar]
- 10.Matthews W, Jordan C T, Wiegand G W, Pardoll D, Lemischka I R. Cell. 1991;65:1143–1152. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- 11.Banu N, Deng B, Lyman S D, Avraham H. Cytokine. 1999;11:679–688. doi: 10.1006/cyto.1998.0477. [DOI] [PubMed] [Google Scholar]
- 12.Lyman S D, James L, Vanden Bos T, de Vries P, Brasel K, Gliniak B, Hollingsworth L T, Picha K S, McKenna H J, Splett R R, et al. Cell. 1993;75:1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- 13.Hirayama F, Lyman S D, Clark S C, Ogawa M. Blood. 1995;85:1762–1768. [PubMed] [Google Scholar]
- 14.Ku H, Hirayama F, Kato T, Miyazaki H, Aritomi M, Ota Y, D'Andrea A D, Lyman S D, Ogawa M. Blood. 1996;88:4124–4131. [PubMed] [Google Scholar]
- 15.Hannum C, Culpepper J, Campbell D, McClanahan T, Zurawski S, Bazan J F, Kastelein R, Hudak S, Wagner J, Mattson J, et al. Nature (London) 1994;368:643–648. doi: 10.1038/368643a0. [DOI] [PubMed] [Google Scholar]
- 16.Yonemura Y, Ku H, Lyman S D, Ogawa M. Blood. 1997;89:1915–1921. [PubMed] [Google Scholar]
- 17.Haylock D N, Horsfall M J, Dowse T L, Ramshaw H S, Niutta S, Protopsaltis S, Peng L, Burrell C, Rappold I, Buhring H J, Simmons P J. Blood. 1997;90:2260–2272. [PubMed] [Google Scholar]
- 18.Namikawa R, Muench M O, Firpo M T, Humeau L, Xu Y, Menon S, Roncarolo M G. Exp Hematol (Charlottesville, Va) 1999;27:1029–1037. doi: 10.1016/s0301-472x(99)00034-x. [DOI] [PubMed] [Google Scholar]
- 19.Xiao M, Oppenlander B K, Plunkett J M, Dooley D C. Exp Hematol (Charlottesville, Va) 1999;27:916–927. doi: 10.1016/s0301-472x(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 20.Rappold I, Ziegler B L, Kohler I, Marchetto S, Rosnet O, Birnbaum D, Simmons P J, Zannettino A C, Hill B, Neu, et al. Blood. 1997;90:111–125. [PubMed] [Google Scholar]
- 21.Akashi K, Weissman I L. Immunity. 1996;5:147–161. doi: 10.1016/s1074-7613(00)80491-4. [DOI] [PubMed] [Google Scholar]
- 22.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman I L. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 23.Muller-Sieburg C E, Whitlock C A, Weissman I L. Cell. 1986;44:653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- 24.Spangrude G J, Brooks D M. Blood. 1992;80:1957–1964. [PubMed] [Google Scholar]
- 25.Uchida N. Stanford University Thesis. Palo Alto, CA: Stanford Univ. Press; 1992. pp. 56–82. [Google Scholar]
- 26.Spangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 27.Uchida N, Weissman I L. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visser J W, Bauman J G, Mulder A H, Eliason J F, de Leeuw A M. J Exp Med. 1984;159:1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ploemacher R E, Brons N H. Exp Hematol (Charlottesville, Va) 1988;16:27–32. [PubMed] [Google Scholar]
- 30.Jurecic R, Van N T, Belmont J W. Blood. 1993;82:2673–2683. [PubMed] [Google Scholar]
- 31.Li C L, Johnson G R. J Exp Med. 1992;175:1443–1447. doi: 10.1084/jem.175.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodell M A, Brose K, Paradis G, Conner A S, Mulligan R C. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones R J, Collector M I, Barber J P, Vala M S, Fackler M J, May W S, Griffin C A, Hawkins A L, Zehnbauer B A, Hilton J, Colvin O M, Sharkis S J. Blood. 1996;88:487–491. [PubMed] [Google Scholar]
- 34.Storms R W, Trujillo A P, Springer J B, Shah L, Colvin O M, Ludeman S M, Smith C. Proc Natl Acad Sci USA. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szilvassy S J, Cory S. Blood. 1993;81:2310–2320. [PubMed] [Google Scholar]
- 36.Osawa M, Hanada K, Hamada H, Nakauchi H. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 37.Morrison S J, Hemmati H D, Wandycz A M, Weissman I L. Proc Natl Acad Sci USA. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordan C T, McKearn J P, Lemischka I R. Cell. 1990;61:953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- 39.Holyoake T L, Nicolini F E, Eaves C J. Exp Hematol (Charlottesville, Va) 1999;27:1418–1427. doi: 10.1016/s0301-472x(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 40.Rebel V I, Miller C L, Eaves C J, Lansdorp P M. Blood. 1996;87:3500–3507. [PubMed] [Google Scholar]
- 41.Harrison D E, Zhong R K, Jordan C T, Lemischka I R, Astle C M. Exp Hematol (Charlottesville, Va) 1997;25:293–297. [PubMed] [Google Scholar]
- 42.Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien Y H, Weissman I L. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- 43.Mebius R E, Streeter P R, Michie S, Butcher E C, Weissman I L. Proc Natl Acad Sci USA. 1996;93:11019–11024. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayakawa K, Hardy R R, Herzenberg L A. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adolfsson J, Borge O J, Bryder D, Theilgaard-Mönch K, Åstrand-Grundström I, Sitnicka E, Sasaki Y, Jacobsen S E. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]