Abstract
A highly enantioselective catalytic method for the synthesis of quaternary α-fluoro derivatives of 3-oxo esters is described. The reaction uses europium (III) triflate and commercially available chiral pybox-type C2-symmetric ligand. Excellent results in terms of yields and enantioselectivities were assured using the electrophilic NFSI reagent under mild reaction conditions.
Keywords: europium, fluorination, enantioselectivity, pybox, keto ester
1. Introduction
Fluorine has drawn great interest from the scientific community. It has been used for a broad variety of purposes in all fields of chemistry, from the polymer industry, with hydrophobic plastics such as Teflon, to the nuclear power industry, or in the production of refrigerants and new solvents [1,2,3,4]. Of special interest is its use in pharmaceutical chemistry, where about 20% of all pharmaceuticals contain fluorinated moieties (30% in the case of agrochemicals) [5,6,7,8,9,10,11,12,13]. It is well known that the exchange of a hydrogen by a fluorine atom in a therapeutic molecule can produce a dramatic change on its physical and chemical properties, along with its biological activity. Generally, the addition of fluorine containing substituents in a drug enhances its metabolic stability and allows modulating its lipophilicity and bioavailability [12,13]. Thus, due to its importance in the pharmaceutical industry, the enantioselective synthesis of fluorinated molecules [14,15,16] is a great challenge in synthetic organic chemistry, especially under catalytic conditions.
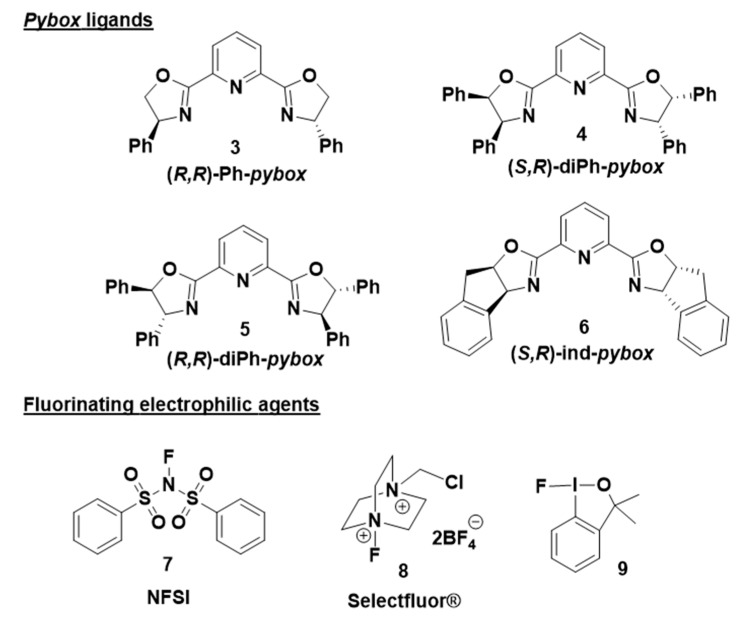
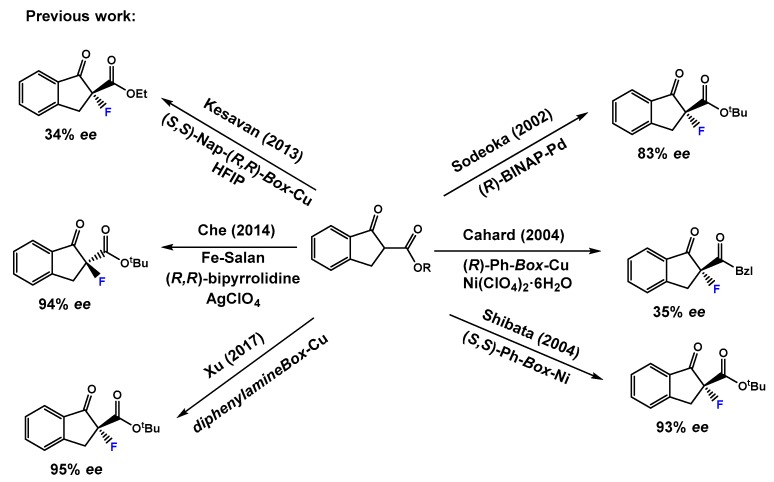
Optically active α-fluorinated cyclic β-keto esters are attractive compounds. On the one hand, they are regarded as non-enolizable and configurationally stable β-keto esters. Moreover, since the ketone is easily converted to other functional groups, they are versatile synthetic precursors. The development of stable sources of electrophilic fluorinating agents (Figure 1) such as N-fluorobenzensulfonimide [17] (NFSI), Selectfluor® [18] and 1-fluoro-1,3-dihydro-3,3-dimethyl-1,2-benziodoxole, have had a great impact on catalytic enantioselective electrophilic fluorination. An important breakthrough in this chemistry occurred when Togni and Hintermann [19] described the catalytic α-fluorination reactions of α-substituted acyclic β-keto esters using a taddol-titanium complex and Selectfluor®, yielding the fluorinated compounds in 33–90% ee. Remarkable contributions of the group of Sodeoka [20,21,22] using palladium-BINAP chiral complexes; Togni [23,24] using Ru(II) chiral complexes; and Cahard [25] using Cu(II) bis(oxazoline) (Box) chiral complexes merit also to be mentioned. Much effort has been devoted to the α-fluorination of alkyl 1-indanone-2-carboxylates under metal catalyst (Scheme 1). Sodeoka’s group using a BINAP-Pd(II) complex in the reaction of tert-butyl 1-indanone-2-carboxylate and NFSI afforded the α-fluorination product in 83% ee. [20] Then, Cahard and collaborators described [25] the reaction of benzyl 1-indanone-2-carboxylate and NFSI under (R)-Ph-Box-Cu(II) catalysis obtaining 35% ee (1 example). In 2004, Shibata’s group performed a study using the combination of Ni(ClO4)2·6H2O and Ph-Box-Cu(II) as catalyst and they succeeded with obtaining a 93% ee in the reaction of tert-butyl 1-indanone-2-carboxylate and NFSI [26]. Moreover, using Nap-Box-Cu(II) and NFSI as fluorinating reagent, Kesavan’s group obtained 34% ee in the α-fluorination of ethyl 1-indanone-2-carboxylate [27]. The use of iron(III)-salan complexes, NFSI, and AgClO4 has also been described, affording a large scope of α-fluorinated alkyl 1-indanone-2-carboxylates in good to excellent ee values up to 97%. [28] Moreover, Xu and collaborators [29] have reported a fast and highly enantioselective fluorination of different alkyl 1-indanone-2-carboxylates catalyzed by a chiral non commercial diphenylamineBox and Cu(OTf)2. The reactions were conducted using a ball mill apparatus [29] (Fritsch Planetary Micro Mill model “Pulverisette 7”) in the absence of solvent and yielding the fluorinated compounds with enantioselectivities up to 99% ee (19 examples, 27–99% ee). However, the obtained ee’s depended on the milling period and rotational speed, and normally in this kind of chemistry, contamination of products can occur as a result of wear and tear principally from the balls, and partially from the casing, which can be a problem, for example, in preparing building blocks for pharmaceuticals. To overcome these disadvantages, and due to our consolidated experience in the enantioselective α-amination of β-keto esters catalyzed by the combination of lanthanides and pybox ligands [30,31,32,33], herein we report the results on catalytic enantioselective electrophilic α-fluorination reaction of alkyl 1-indanone-2-carboxylates employing fully commercially available reagents.
Figure 1.
Fluorinating agents and pybox C2-symmetry ligands used in the optimization of the electrophilic enantioselective fluorination reaction.
Scheme 1.
Previous relevant work on enantioselective α-fluorination of alkyl 1-indanone-2-carboxylates using NFSI under metal catalyst.
2. Results and Discussion
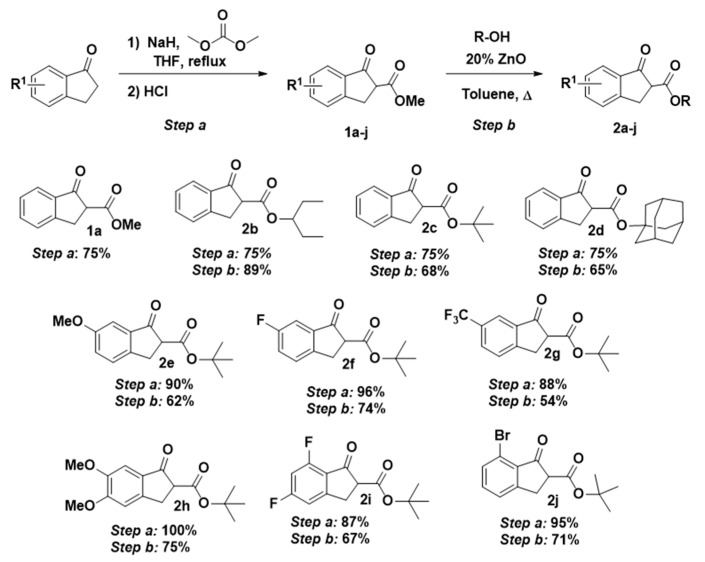
In the first part of this present work, we prepared a series of β-keto esters 2 derived from the corresponding 1-indanones. The acylation reaction of 1-indanones was carried out using sodium hydride as a base and dimethylcarbonate as electrophile in THF, obtaining compounds 1a–h and 1j in excellent yields (Scheme 2, 75–100%). In the case of 1i (87% yield), methyl 1H-imidazole-1-carboxylate was used as acylating reagent; otherwise, exchange of the fluorine atom in 5 position by methoxide was observed [33]. Next, following the reported methodology of our research group [34], we performed the transesterification reaction to successfully afford the secondary and tertiary β-keto esters 2 shown in Scheme 2 (54–89%).
Scheme 2.
Preparation of alkyl 1-indanone-2-carboxylates 1 and 2.
Our previous experience suggested that the use of β-keto esters bearing a bulkier OR group might be useful to achieve enantioinduction, and secondary 3-pentyl group fulfills this requirement. Therefore, we selected 2b to investigate the effect of different combinations of lanthanides and pybox ligands in the α-fluorination process. In the first attempt, we used europium (III) triflate and (R,R)-Ph-pybox (3) as catalyst and NFSI (7) as the fluorinating agent (Table 1, entry 1). Selection of NFSI was based in previous reported enantioselective fluorination reactions [25,28]. Under these conditions, product 10b was afforded in a 76% yield but a negligible enantiomeric ratio. Next, we changed the metal source, moving to lanthanum and ytterbium and obtaining in both cases 10b in nearly racemic form. At this point, we decided to change the chiral ligand. From our previous experience [31,32], we envisioned good results using more sterically hindered pybox ligands 4 and 5. They are non-commercially available and were prepared as previously described [31,35]. The ee’s improved but not enough (30–56 % ee, entries 4–7 of Table 1). Then, we studied the commercially available (S,R)-ind-pybox, 6, and the results improved in a great extend, achieving the highest yield (87%) in combination with La(III) and affording enhanced enantioselective ratios specially in the case of Eu(III) (78% ee) (entries 8–10 of Table 1). Selectfluor® (8) and 1-fluoro-1,3-dihydro-3,3-dimethyl-1,2-benziodoxole (9) were assayed giving worse results in terms of yield and ee (entries 11 and 12, Table 1). The hypervalent iodine fluorinated reagent 9 produces the oxidation of the α-position, affording the corresponding α-hydroxylated β-keto ester as the only detected product. This oxidation process has previously been described in the electrophilic cyanation of β-keto esters using 1-cyano-1,3-dihydro-3,3-dimethyl-1,2-benziodoxole [36]. So, we proceed again with the optimized reaction, using NFSI, but now at a low temperature (−30 °C). The reaction of 2b and 1a catalyzed by ind-pybox-La(III) gave rise to the fluorinating products in good yields (60 and 80% respectively) and moderate ee’s (64 and 63% respectively). Additionally, performing the reaction of 2c (tert-butyl ester) in the same conditions, product 10c was obtained in an enhanced 76% ee (entry 15, Table 1). Finally, changing to ind-pybox-Eu(III) compound 10c was achieved in an excellent 96% ee (entry 16, Table 1).
Table 1.
Optimization of the enantioselective α-fluorination reaction.

| Entry | 2 | Metal | Ligand | Fluorinating Reagent | Yield (%) | ee (%)[a] |
|---|---|---|---|---|---|---|
| 1 | 2b | Eu+3 | 3 | NFSI | 76 | 2 |
| 2 | 2b | La+3 | 3 | NFSI | 74 | 5 |
| 3 | 2b | Yb+3 | 3 | NFSI | 68 | 2 |
| 4 | 2b | La+3 | 4 | NFSI | 81 | 56 |
| 5 | 2b | Eu+3 | 4 | NFSI | 45 | 30 |
| 6 | 2b | Yb+3 | 4 | NFSI | 78 | 54 |
| 7 | 2b | La+3 | 5 | NFSI | 85 | 50 |
| 8 | 2b | La+3 | 6 | NFSI | 87 | 60 |
| 9 | 2b | Eu+3 | 6 | NFSI | 72 | 78 |
| 10 | 2b | Yb+3 | 6 | NFSI | 70 | 60 |
| 11 | 2b | La+3 | 6 | 8 | 59 | 14 |
| 12 | 2b | La+3 | 6 | 9 | 0 | n.d. |
| 13[b] | 1b | La+3 | 6 | NFSI | 60 | 64 |
| 14[b] | 1a | La+3 | 6 | NFSI | 80 | 63 |
| 15[b] | 2c | La+3 | 6 | NFSI | 86 | 76 |
| 16[b] | 2c | Eu+3 | 6 | NFSI | 78 | 96 |
[a] The racemic compounds 10 were prepared by reaction of the alkyl 1-indanone-2-carboxylates with Selectfluor® (1 equiv.) in CH3CN at 50 °C. The ee’s were determined by using chiral HPLC (see supplementary information). [b] Reaction done at −30 °C.
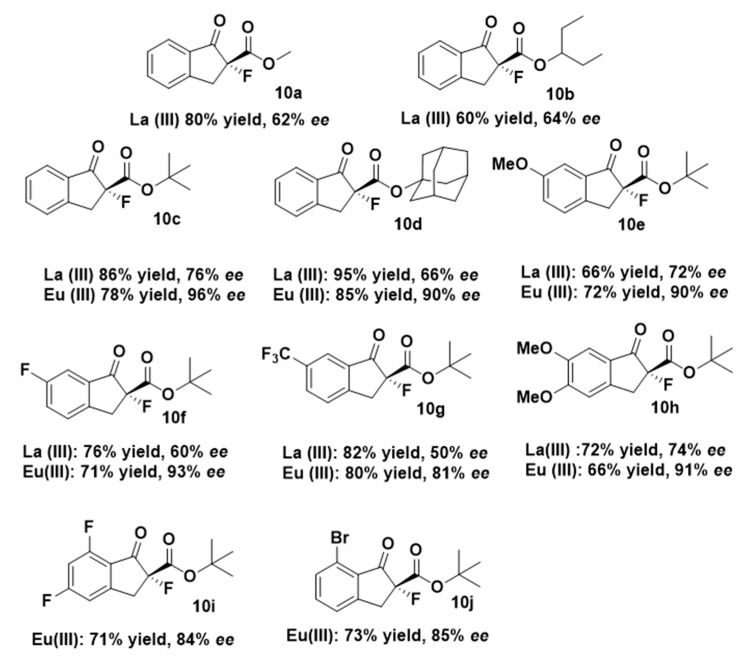
Therefore, as the best performance in terms of selectivity was obtained using sterically hindered tert-butyl 1-oxo-indanecarboxylate 2c (entry 16, Table 1), we also performed the reaction with adamantan-1-yl 1-oxo-2-indanecarboxylate in the same conditions, obtaining 10d in a slightly lower 90% ee (Figure 2). Thus, we focused our attention on other tert-butyl 1-indanone-2-carboxylates possessing different groups in the aromatic ring. α-Fluorinated compounds 10e–j were achieved in high yields (66–85%) and enantiomeric excesses up to 93% when using ind-pybox-Eu(III) combination. Lanthanium gave lower selectivities in all the studied substrates (Figure 2). First, we selected three different substituents in 6 positions of the aromatic ring. The change from OMe (10e, 90% ee) to CF3 (10g, 81% ee) lessen the ee and the reaction time from 24h to 8h. Addition of an extra OMe group in 5 position (10h) did not improve the ee (91%). Compounds 10i and 10j were obtained in 84 and 85% ee respectively. In general, the presence of electron withdrawing groups harmed the ee (10g, 10i and 10j, Figure 2), except in the case of 10f (F in 6 position), which was obtained in an excellent 93% ee.
Figure 2.
Scope of the reaction of alkyl 1-indanone-2-carboxylates 2 with different groups in aromatic positions. All the reactions were carried out at the same conditions (−30 °C), see experimental part.
The assignment of the absolute configuration of compounds 10 was done by comparison of the sign of the optical rotation values already described for 10c–f [28]. For new compounds 10b and 10g–j the assignment was based on the comparison of the circular dichroism (CD) (all studied compounds show a negative Cotton Effect, see supplementary information). In all cases, the absolute configuration was assigned to be R. The advantage of this method is that we could also have access to (S)-10 enantiomers by using commercially available (R,S)-ind-pybox.
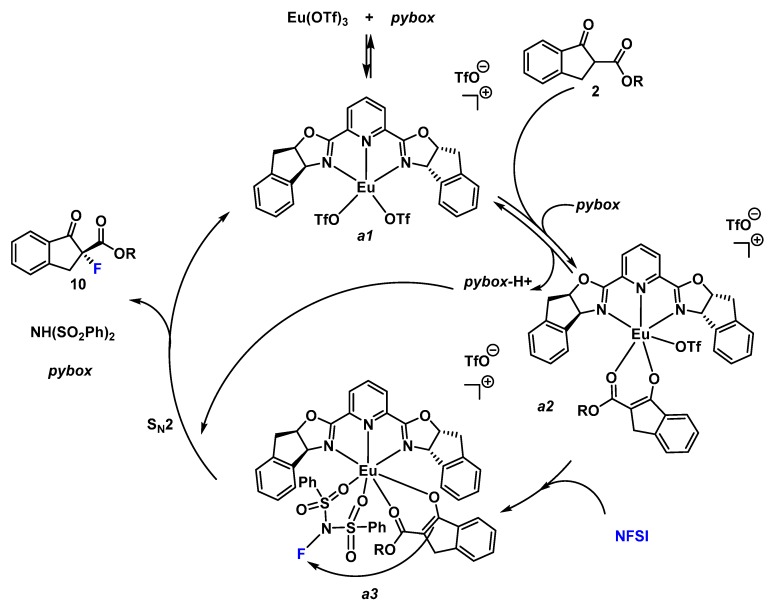
Furthermore, a plausible catalytic cycle is proposed for this enantioselective fluorination reaction (Scheme 3) [30,33]. First, we propose the formation of the [pyboxEu(OTf)2]+ (a1) complex that can exchange the labile triflate ligand by the enolate form of the β-keto ester affording the complex [pyboxEu(OTf)(enolate-2]+ (a2). Similar complexes were detected by ESI Mass Spectometry when mixing La(OTf)3 and ip-pybox [30]. The pybox ligand is the responsible base for the formation of the enolate-2. Abstraction of the intercarbonylic proton can be produced directly from 2 (as indicated in Scheme 3); or once the β-keto ester is coordinated to the europium enhancing its acidity. Next, a coordination of the NFSI, through the donor oxygen atom, to the europium forming a ternary complex is proposed. The known large coordination number of lanthanides makes that possible. The enantioselectivity of the reaction stems from the efficient blockage of one of the diastereotopic faces of the Eu(III) enolate by one unit of the C2-symmetric ligand. Subsequent reaction affords the formation of the stereogenic C–F bond through a SN2 mechanism.
Scheme 3.
Plausible catalytic cycle.
3. Materials and Methods
General transesterification procedure: In a round-bottomed flask, the corresponding 1-indanone (1 eq.), ZnO (0.2 eq.) and the corresponding alcohol (10 equivalents) were dissolved in toluene (5 mL/mol 1-indanone). The reaction mixture was heated up to 140 °C under a distillation setup until total conversion of the 1-indanone was observed by TLC (from 6 to 8 h). If necessary, more toluene was added. Afterwards, the reaction mixture was filtered through Celite® and the solvent was removed under reduced pressure. The product obtained was purified by column chromatography on silica-gel.
Pentan-3-yl 1-oxo-2,3-dihydro-1H-indene-2-carboxylate (2b): Following the general procedure, 1.00 g (5.26 mmol, 1 eq) of 1a was allowed to react with 3-pentanol (52.7 mmol, 10 eq.) and ZnO (0.09 g, 1.06 mmol, 0.2 eq.) in 6 mL of toluene for 5 h. After purification by column chromatography on silica-.gel (hexanes (95): AcOEt (5)) a brown oil was obtained in 90% yield (1.17 g). IR (ATR) ν = 2968, 1711 (C=O), 1608, 1572, 1207, 1092 cm−1; 1H-NMR (360 MHz [D]CDCl3, 298 K, TMS) δ = 10.50 (bs, 0.08H, OH, enol), 7.79 (d, 3J(H,H) = 7.5 Hz, 1H, ArH), 7.64 (t, 3J(H,H) = 7.1 Hz, 1H, ArH), 7.52 (d, 3J(H,H) = 7.5 Hz, 1H, ArH), 7.50–7.44 (m, 0.17H, ArH, enol), 7.41 (3J(H,H), J = 7.5 Hz, 1H, ArH), 4.98 (quint, 3J(H,H) = 6.1 Hz, 0.16H, OCH, enol), 4.86 (quint, 3J(H,H) = 6.3 Hz, 1H, OCH,), 3.74 (dd, 3J(H,H) = 4.2 Hz, 3J(H,H) = 8.7 Hz, 1H, CH2CH), 3.59 (d, 3J(H,H) = 4.2 Hz, 0.42H, CH2C, enol), 3.55 (d, 3J(H,H) = 3.7 Hz, 1H, CH2CH), 3.40 (dd, 3J(H,H) = 8.3 Hz, J = 17.5 Hz, 1H, CH2CH), 1.70 (m, 1H, CH2CH3, enol), 1.65 (m, 4H, CH2CH3), 1.02–0.86 ppm (m, 7.36, CH2CH3); 13C-NMR (101 MHz, [D]CDCl3, 298 K, TMS) δ = 199.6, 169.0, 153.6, 143.1, 137.0, 135.3, 135.2, 129.1, 127.7, 126.7, 126.5, 124.6, 124.6, 120.6, 102.7, 78.3, 76.4, 53.6, 32.52, 30.3, 26.6, 26.4, 9.6, 9.4 ppm; HRMS (ESI): (M + Na)+ Calcd for C15H18O3Na 269.1148; found 269.1144.
Tert-butyl-5,7-difluoro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (2i): Following the general procedure, 0.50 g (2.21 mmol, 1 eq) of 1i was allowed to react with tert-butanol (22.1 mmol, 10 eq.) and ZnO (0.04 g, 0.45 mmol, 0.2 eq.) in 5 mL of toluene for 5 h. After purification by column chromatography on silica-.gel (hexanes (95): AcOEt (5)) a purple oil was obtained in 64% yield (0.38 g). IR (ATR) ν = 2961, 1758 (C=O), 1722, 1617, 1283, 1045 cm−1; 1H-NMR (360 MHz [D]CDCl3, 298 K, TMS) δ = 10.62 (bs, 0.07H, OH, enol), 6.97 (d, 3J(H,F) = 7.3 Hz, 1H, ArH), 6.74 (t, 3J(H,F) = 9.1 Hz, 1H, ArH), 3.64 (dd, 3J(H,H) = 3.9 Hz, 3J(H,H) = 8.2 Hz, 1H, CH2CH), 3.48 (dd, 3J(H,H) = 4.4 Hz, 3J(H,H) = 16.7 Hz, CH2CH), 3.30 (dd, 3J(H,H) = 8.3 Hz, J = 17.6 Hz, 1H, CH2CH), 1.47 (s, 9H, C(CH3)3); 19F-NMR (235 MHz [D]CDCl3, 298 K, TMS) δ = −99.9 (d, 3J(F,F) = 13.7 Hz, 1F), −111.3 ppm (d, 3J(F,F) = 13.7 Hz, 1F); 13C-NMR (91 MHz, [D]CDCl3, 298 K, TMS) δ = 194.5 (d, 3J(C,F) = 2.2 Hz), 167.8 (dd, 3J(C,F) = 3.9 Hz, 1J(C,F) = 259.5 Hz), 167.5 (s), 160.0 (dd, 3J(C,F) = 14.2 Hz, 1J(C,F) = 267.5 Hz), 157.7 (m), 120.1 (m), 109.5 (dd,4J(C,F) = 4.2 Hz, 2J(C,F) = 22.5 Hz), 103.9 (dd, 2J(C,F) = 22.7 Hz, 2J(C,F) = 24.7 Hz), 82.5 (s), 55.0 (s), 27.8 (s) ppm; HRMS (ESI): (M + Na)+ Calcd for C14H14F2O3Na 291.0803; found 291.0805.
General procedure for the enantioselective electrophilic fluorinations: In a 10 mL Schlenk flask in presence of 4 Å MS, Ln(OTf)3 (0.13 eq.) and the desired pybox ligand (0.15 eq.) were dissolved with dry ACN (5 mL). The colorless reaction mixture was left stirring at room temperature under argon atmosphere overnight. Next, the corresponding indanone derivative 2 (80 mg; 1 eq.) was added to the reaction mixture and left stirring at room temperature for 1 h. Then, the reaction mixture was cooled down until −30 °C and, once at this temperature, NFSI (1.15 eq.) was added to the mixture in one portion. The reaction mixture was left at this temperature under argon atmosphere until complete conversion of the reagent. Afterwards, the solvent was removed under reduced pressure and the product was purified by column chromatography on silica gel, yielding the α-fluorinated compound.
Pentan-3-yl (R)-2-fluoro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (10b): Following the general procedure using La(OTf)3 and (S,R)-ind-pybox, 79 mg (0.29 mmol, 60% yield) of 10b were obtained as a tan oil from 0.37 mmol of 2b. It was purified by column chromatography on silica-gel (hexane/AcOEt 5:1). IR (ATR) ν = 2970, 1760 (C=O), 1607 (C=O), 1464, 1287, 1214, 1191, 1156, 1100 cm−1; 1H-NMR (250 MHz [D]CDCl3, 298 K, TMS) δ = 7.85 (d, 3J(H,H) = 8.1 Hz, 1H, ArH), 7.71 (t, 3J(H,H) = 7.4 Hz, 1H, ArH), 7.57 (m, 2H, ArH), 4.90 (quint, 3J(H,H) = 6.3 Hz, 1H, OCH), 3.78 (dd, 2J(H,H) = 11.4 Hz, 3J(H,F) = 17.8 Hz, 1H, CH2CF), 3.40 (dd, 2J(H,H) = 22.8 Hz, 3J(H,F) = 25.7 Hz, 1H, CH2CF), 1.55 (m, 4H, CH2CH3), 0.84 (m, 6H); 13C-NMR (91 MHz [D]CDCl3, 298 K, TMS) δ = 195.5 (d, 2J(C,F) = 18.2 Hz), 167.1 (d, 2J(C,F) = 27.8 Hz), 150.9 (d, 3J(H,F) = 3.7 Hz), 136.4 (d, J = 3.0 Hz, Ar-C), 133.4 (s), 128.6 (s), 126.5 (d, 4J(C,F) = 1.2 Hz), 125.5, 94.5 (d, 1J(C,F) = 201.2 Hz), 79.6 (s), 38.4 (d, 2J(C,F) = 24.1 Hz), 26.4 (s), 26.3 (s), 9.5 (s), 9.3 (s). 19F-NMR (235 MHz [D]CDCl3, 298 K, TMS) δ = −164.8 ppm (s, 1F); HPLC: Daicel Chiralpack AD-H, Hexane/iPrOH=99.5:0.5, 0.75 mL/min, 254 nm, tr(minor) = 33.4 min, tr(major) = 40.7 min (64% ee); [α]20D: −10.3 (c=5.9, CHCl3). HRMS (ESI): (M + Na)+ Calcd for C15H17O3FNa 287.1054; found 287.1059.
Tert-butyl (R)-2-fluoro-1-oxo-6-(trifluoromethyl)-2,3-dihydro-1H-indene-2-carboxylate (10g): Following the general procedure, using Eu(OTf)3 and (S,R)-ind-pybox, 67 mg (0.21 mmol, 80% yield) of 10g were obtained as a brown oil from 0.31 mmol of the starting material. It was purified by column chromatography on silica-gel (hexane/AcOEt 7:3). IR (ATR) ν = 2916, 2849, 1736 (C=O), 1467, 1330, 1259, 1179 cm−1; 1H-NMR (360 MHz [D]CDCl3, 298 K, TMS) δ = 8.11 (s, 1H, ArH), 7.95 (d, 3J(H,H) = 7.9 Hz, 1H, ArH), 7.68 (d, 3J(H,H) = 7.9 Hz, 1H, ArH), 3.82 (dd, 2J(H,H) = 10.7 Hz, 3J(H,F) = 18.5 Hz, 1H, CH2CF), 3.47 (dd, 2J(H,H) = 18.2 Hz, 3J(H,F) = 21.8 Hz, 1H, CH2CF), 1.45 (s, 9H, OC(CH3)3); 19F-NMR (235 MHz [D]CDCl3, 298 K, TMS) δ = −63.2 (s, 3F, CF3), -163.9 pm (s, 1F, CF); 13C-NMR (91 MHz [D]CDCl3, 298 K, TMS) δ = 194.7 (d, 2J(C,F) = 18.6 Hz), 165.6 (d, 2J(C,F) = 27.5 Hz), 154.7 (d, 3J(C,F) = 3.6 Hz), 133.9 (s), 132.8 (q, 4J(C,F) = 3.2 Hz), 131.4 (q, 2J(C,F) = 33.5 Hz), 127.4 (s), 123.4 (q, 1J(C,F) = 272.5 Hz), 122.5 (q, 3J(C,F) = 4.0 Hz), 94.2 (d, 1J(C,F) = 203.5 Hz), 84.7 (s), 38.3 (d, 2J(C,F) = 24.2 Hz), 27.2 (s) ppm; HPLC: Daicel Chiralpack AD-H, Hexane/iPrOH=99.9:0.1 0.7 mL/min, 254 nm, tR(minor) = 20.2 min, tS(major) = 23.1 min (81% ee); [α]20D: −5.6 (c=6.2, CHCl3). HRMS (ESI): (M + Na)+ Calcd for C15H14O3F4Na 341.0771; found 341.0772.
Tert-butyl (R)-2,5,7-trifluoro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (10i): Following the general procedure, using Eu(OTf)3 and (S,R)-ind-pybox, 45 mg (0.15 mmol, 71% yield) of 10i were obtained as a colorless oil from 0.22 mmol of 2i. It was purified by column chromatography on silica-gel (hexane/AcOEt 9:1). IR (ATR) ν = 2921, 1751 (C=O), 1721, 1484, 1298, 1149,1040 cm−1; 1H-NMR (400 MHz [D]CDCl3, 298 K, TMS) δ = 7.01 (d, 3J(H,F) = 6.5 Hz, 1H, ArH), 6.86 (t, 3J(H,F) = 8.2 Hz, 1H, ArH), 3.74 (dd, 2J(H,H) = 10.0, 3J(H,F) = 17.6 Hz, 1H), 3.42 (dd, 2J(H,H) = 15.5, 3J(H,F) = 23.9 Hz, 1H), 1.48 ppm (s, 9H, OC(CH3)3); 19F-NMR (376 MHz [D]CDCl3, 298 K, TMS) δ = −94.9 (d, 4J(F,F) = 14.7 Hz, 1F, ArF), −106.4 (d, 4J(F,F) = 14.7 Hz, 1F, ArF), −162.5 ppm (s, 1F, CF); 13C-NMR (101 MHz [D]CDCl3, 298 K, TMS) δ = 193.2 (d, 2J(C,F) = 18.2 Hz), 167.8 (dd, 3J(C,F) = 3.9 Hz, 1J(C,F) = 259.5 Hz), 165.8 (d, 2J(C,F) = 24.5 Hz), 160.0 (dd, 3J(C,F) = 14.2 Hz, 1J(C,F) = 267.5 Hz), 153.4 (m), 120.1, 109.8 (dd,4J(C,F) = 4.2 Hz, 2J(C,F) = 22.5 Hz), 95.4 (d, 1J(C,F) = 202.4 Hz), 82.5 (s), 38.9 (d, 2J(C,F) = 24.1 Hz), 27.8 (s) ppm; HPLC: Daicel Chiralpack AD-H, Hexane/iPrOH=97:3, 1.0 mL/min, 254 nm, tr(major) = 8.7 min, tr(minor) = 12.9 min (84% ee); [α]20D: −10.7 (c=6.8, CHCl3). HRMS (ESI): (M + Na)+ Calcd for C14H13F3O3Na 309.0709; found 309.0709.
Tert-butyl (R)-2-fluoro-7-bromo-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (10j): Following the general procedure, using Eu(OTf)3 and (S,R)-ind-pybox, 48 mg (0.14 mmol, 73% yield) of 10j were obtained as a colorless oil from 0.20 mmol of 2j. It was purified by column chromatography on silica-gel (hexane/AcOEt 9:1). 1H-NMR (360 MHz [D]CDCl3, 298 K, TMS) δ = 7.61 (d, 3J(H,H) = 7.6 Hz, 1H, ArH), 7.51 (t, 3J(H,H) = 7.6 Hz, 1H, ArH), 7.45 (d, 3J(H,H) = 7.6 Hz, 1H, ArH), 3.69 (dd, 2J(H,H) = 11.2, 3J(H,F) = 17.6 Hz, 1H), 3.37 (dd, 2J(H,H) = 17.6, 3J(H,F) = 22.9 Hz, 1H), 1.44 ppm (s, 9H, OC(CH3)3); 19F-NMR (235 MHz [D]CDCl3, 298 K, TMS) δ = −163.8 ppm (s, 1F); 13C-NMR (91 MHz [D]CDCl3, 298 K, TMS) δ = 193.2 (d, 2J(C,F) = 19.2 Hz), 165.8 (d, 2J(C,F) = 27.6 Hz), 153.4 (d, 3J(C,F) = 3.6 Hz), 136.7 (s), 133.4 (s), 131.3 (s), 125.4 (s), 121.2 (s), 94.5 (d, 1J(C,F) = 202.4 Hz), 84.5 (s), 37.4 (d, 2J(C,F) = 24.1 Hz), 27.8 (s) ppm; HPLC: Daicel Chiralpack AD-H, Hexane/iPrOH=97:3, 1.0 mL/min, 254 nm, tr(major) = 12.1 min, tr(minor) = 18.9 min (85% ee); [α]20D: −34.7 (c=6.3, CHCl3). HRMS (ESI): (M + Na)+ Calcd for C14H14BrFO3Na 351.0003; found 351.0005.
4. Conclusions
In conclusion, we have established a catalytic method for the highly enantioselective α-fluorination of a series of alkyl 1-oxo-indanecarboxylates, using europium (III) triflate and (S,R)-ind-pybox as pre-catalyst, and NFSI as electrophilic fluorinating agent in acetonitrile at −30 °C. Selectivities up to 96% ee could be obtained in mild conditions, and using a commercially available chiral ligand. Results reveal a dependence of the enantiocontrol on the sterically hindrance of the ester in substrates. Access to both enantiomers of the α-fluorinated oxo ester is guaranteed by the commercial availability of both (R,S) and (S,R) ind-pybox C2-symmetric ligands.
Acknowledgments
We are thankful for financial support from Spain’s MICINN (CTQ2014-53662-P) and MEC (CTQ2016-81797-REDC) and DURSI -Generalitat de Catalunya (2017 SGR 465).
Supplementary Materials
The following are available online: synthesis and characterization of starting materials 1a-j and 2a-j, and pybox ligands 4 and 5. Spectra, HPLC and CD of compounds 10a-j.
Author Contributions
Conceptualization, A.V.; methodology, A.G. and P.S.; formal analysis, A.G. and P.S.; investigation, A.G. and P.S.; resources, A.V.; data curation, A.G.; writing—original draft preparation, A.V. and A.G.; writing—review and editing, A.V.; supervision, A.V. and A.G.; funding acquisition, A.V.
Funding
This research was funded by Spain’s MICINN (CTQ2014-53662-P) and MEC (CTQ2016-81797-REDC) and DURSI -Generalitat de Catalunya (2017 SGR 465).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Kirsch P. Modern Fluoroorganic Chemistry. Wiley-VCH; Weinheim, Germany: 2004. [Google Scholar]
- 2.Gladysz J.A., Curran D.E., Horváth I.T. Handbook of Fluorous Chemistry. Wiley-VCH; Weinheim, Germany: 2004. [Google Scholar]
- 3.Liang T., Neumann C.N., Ritter T. Introduction of Fluorine and Fluorine-Containing Functional Groups. Angew. Chem. Int. Ed. 2013;52:8214–8264. doi: 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]
- 4.Smart B.E. Fluorine Substituent Effects (on Bioactivity) J. Fluor. Chem. 2001;109:3–11. doi: 10.1016/S0022-1139(01)00375-X. [DOI] [Google Scholar]
- 5.O’Hagan D. Understanding Organofluorine Chemistry. An Introduction to the C–F Bond. Chem. Soc. Rev. 2008;37:308–319. doi: 10.1039/B711844A. [DOI] [PubMed] [Google Scholar]
- 6.Isanbor C., O’Hagan D. Fluorine in Medicinal Chemistry: A Review of Anti-cancer Agents. J. Fluor. Chem. 2006;127:303–319. doi: 10.1016/j.jfluchem.2006.01.011. [DOI] [Google Scholar]
- 7.Müller K., Faeh C., Diederich F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 8.Purser S., Moore P.R., Swallow S., Gouverneur V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008;37:320–330. doi: 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- 9.Ojima I. Fluorine in Medicinal Chemistry and Chemical Biology. Wiley-Blackwell; Hobpken, NY, USA: 2009. [Google Scholar]
- 10.O’Hagan D. Fluorine in Health Care: Organofluorine Containing Blockbuster Drugs. J. Fluor. Chem. 2010;131:1071–1081. doi: 10.1016/j.jfluchem.2010.03.003. [DOI] [Google Scholar]
- 11.Ilardi E.A., Vitaku E., Njardarson J.T. Data-Mining for Sulfur and Fluorine: An Evaluation of Pharmaceuticals To Reveal Opportunities for Drug Design and Discovery. J. Med. Chem. 2014;57:2832–2842. doi: 10.1021/jm401375q. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Sanchez-Roselló M., Aceña J.L., del Pozo C., Sorochinsky A.E., Soloshonok V.A., Liu H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011) Chem. Rev. 2014;114:2432–2506. doi: 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh A., Nakanishi T. Frontiers of Solvent-free Functional Molecular Liquids. Chem. Comm. 2017;53:10344–10357. doi: 10.1039/C7CC05883G. [DOI] [PubMed] [Google Scholar]
- 14.Pihko P. Enantioselective α-Fluorination of Carbonyl Compounds: Organocatalysis or Metal Catalysis? Angew. Chem. Int. Ed. 2006;45:544–547. doi: 10.1002/anie.200502425. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y., Han J., Wang J., Shibata N., Sodeoka M., Soloshonok V.A., Coelho J.A.S., Toste F.D. Modern Approaches for Asymmetric Construction of Carbon–Fluorine Quaternary Stereogenic Centers: Synthetic Challenges and Pharmaceutical Needs. Chem. Rev. 2018;118:3887–3964. doi: 10.1021/acs.chemrev.7b00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen X., Zhang W., Zhang L., Luo T., Wan X., Gu Y., Hu J. Enantioselective Synthesis of Cyclopropanes that Contain Fluorinated Tertiary Stereogenic Carbon Centers: A Chiral α-Fluoro Carbanion Strategy. Angew. Chem. Int. Ed. 2012;51:6966–6970. doi: 10.1002/anie.201202451. [DOI] [PubMed] [Google Scholar]
- 17.Differding E., Ofner H. N-Fluorobenzenesulfonimide: A Practical Reagent for Electrophilic Fluorinations. Synlett. 1991:187–189. doi: 10.1055/s-1991-20673. [DOI] [Google Scholar]
- 18.Nyffeler P.T., Gonzalez S., Burkart M.D., Vicent S.P., Wong C.-H. Selectfluor: Mechanistic Insight and Applications. Angew. Chem. Int. Ed. 2005;44:192–202. doi: 10.1002/anie.200400648. [DOI] [PubMed] [Google Scholar]
- 19.Hintermann L., Togni A. Catalytic Enantioselective Fluorination of β-Ketoesters. Angew. Chem. Int. Ed. 2000;39:4359–4362. doi: 10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Hamashima Y., Yagi K., Takano H., Tamás L., Sodeoka M. An Efficient Enantioselective Fluorination of Various β-Ketoesters Catalyzed by Chiral Palladium Complexes. J. Am. Chem. Soc. 2002;124:14530–14531. doi: 10.1021/ja028464f. [DOI] [PubMed] [Google Scholar]
- 21.Hamashima Y., Takano H., Hotta D., Sodeoka M. Immobilization and Reuse of Pd Complexes in Ionic Liquid: Efficient Catalytic Asymmetric Fluorination and Michael Reactions with β-Ketoesters. Org. Lett. 2003;5:3225–3228. doi: 10.1021/ol035053a. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T., Goto T., Hamashima Y., Sodeoka M. Enantioselective Fluorination of tert-Butoxycarbonyl Lactones and Lactams Catalyzed by Chiral Pd(II)-Bisphosphine Complexes. J. Org. Chem. 2007;72:246–250. doi: 10.1021/jo062048m. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim H., Togni A. Enantioselective Halogenation Reactions. Chem. Commun. 2004;0:1147–1155. doi: 10.1039/b317004g. [DOI] [Google Scholar]
- 24.Huber D.P., Stanek K., Togni A. Consecutive Catalytic Electrophilic Fluorination/Amination of β-Keto esters: Toward α-Fluoro-α-amino Acids? Tetrahedron-Asymmetr. 2006;17:658–664. doi: 10.1016/j.tetasy.2006.01.035. [DOI] [Google Scholar]
- 25.Ma J.-A., Cahard D. Copper(II) Triflate-bis(oxazoline)-catalysed Enantioselective Electrophilic Fluorination of β-Ketoesters. Tetrahedron-Asymmetr. 2004;15:1007. doi: 10.1016/j.tetasy.2004.01.014. [DOI] [Google Scholar]
- 26.Shibata N., Ishimaru T., Nagai T., Kohno J., Toru T. First Enantio-Flexible Fluorination Reaction Using Metal-Bis(oxazoline) Complexes. Synlett. 2004;10:1703–1706. doi: 10.1055/s-2004-829571. [DOI] [Google Scholar]
- 27.Balaraman K., Vasanthan R., Kesavan V. Enantioselective Fluorination of β-Ketoesters using Tartrate Derived Bidentate Bisoxazoline-Cu(II) Complexes. Tetrahedron-Asymmetr. 2013;24:919–924. doi: 10.1016/j.tetasy.2013.07.004. [DOI] [Google Scholar]
- 28.Gu X., Zhang Y., Xu Z.-J., Che C.-M. Iron(III)–salan complexes catalysed highly enantioselective fluorination and hydroxylation of β-keto esters and N-Boc oxindoles. Chem. Commun. 2014;50:7870–7873. doi: 10.1039/c4cc01631a. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Wang H., Jiang Y., Zhang C., Shao J., Xu D. Fast, solvent-free and highly enantioselective fluorination of β-keto esters catalyzed by chiral copper complexes in a ball mill. Green Chem. 2017;19:1674–1677. doi: 10.1039/C6GC03306G. [DOI] [Google Scholar]
- 30.Comelles J., Pericas A., Moreno-Mañas M., Vallribera A., Drudis-Solé G., Lledós A., Parella T., Roglans A., García-Granda S., Roces-Fernández L. Highly Enantioselective Electrophilic Amination and Michael Addition of Cyclic β-Ketoesters Induced by Lanthanides and (S,S)-ip-pybox: The Mechanism. J. Org. Chem. 2007;72:2077–2087. doi: 10.1021/jo0622678. [DOI] [PubMed] [Google Scholar]
- 31.Pericas A., Shafir A., Vallribera A. Asymmetric Synthesis of l-Carbidopa Based on a Highly Enantioselective α-Amination. Org. Lett. 2013;15:1448–1451. doi: 10.1021/ol400136y. [DOI] [PubMed] [Google Scholar]
- 32.Pericas A., Jiménez R., Granados A., Shafir A., Vallribera A., Roglans A., Molins E. Lanthanides–pybox: An Excellent Combination for Highly Enantioselective Electrophilic α-Amination of Acyclic β-Keto Esters. Isolation of Ternary Pybox/Ln/β-Keto Ester Complexes. ChemistrySelect. 2016;1:4305–4312. doi: 10.1002/slct.201600820. [DOI] [Google Scholar]
- 33.Granados A., del Olmo A., Peccati F., Billard T., Sodupe M., Vallribera A. Fluorous l-Carbidopa Precursors: Highly Enantioselective Synthesis and Computational Prediction of Bioactivity. J. Org. Chem. 2018;83:303–313. doi: 10.1021/acs.joc.7b02685. [DOI] [PubMed] [Google Scholar]
- 34.Pericas A., Shafir A., Vallribera A. ZnO-Catalyzed Transesterification of.β-Keto Esters. Tetrahedron. 2008;64:9258–9263. doi: 10.1016/j.tet.2008.07.034. [DOI] [Google Scholar]
- 35.Meng J.-C., Fokin V.V., Finn M.G. Kinetic resolution by copper-catalyzed azide-alkyne cycloaddition. Tetrahedron Lett. 2005;46:4543–4546. doi: 10.1016/j.tetlet.2005.05.019. [DOI] [Google Scholar]
- 36.Wang Y.-F., Qiu J., Kong D., Gao Y., Lu F., Karmarker P.G., Chen F.-X. The direct electrophilic cyanation of β-keto esters and amides with cyano benziodoxole. Org. Biomol. Chem. 2015;13:365–368. doi: 10.1039/C4OB02032D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.