Abstract
Background
The association of plasma interleukin-8 (IL-8), or IL-8 genetic variants, with pediatric acute respiratory distress syndrome (PARDS) in children with acute respiratory failure at risk for PARDS has not been examined. The purpose of this study was to examine the association of early and sequential measurement of plasma IL-8 and/or its genetic variants with development of PARDS and other clinical outcomes in mechanically ventilated children with acute respiratory failure.
Methods
This was a prospective cohort study of children 2 weeks to 17 years of age with acute airways and/or parenchymal lung disease done in 22 pediatric intensive care units participating in the multi-center clinical trial, Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE). Plasma IL-8 levels were measured within 24 h of consent and 24 and 48 h later. DNA was purified from whole blood, and IL-8 single nucleotide polymorphisms, rs4073, rs2227306, and rs2227307, were genotyped.
Results
Five hundred forty-nine patients were enrolled; 480 had blood sampling. Plasma IL-8 levels ranged widely from 4 to 7373 pg/mL. Highest IL-8 levels were observed on the day of intubation with subsequent tapering. Levels of IL-8 varied significantly across primary diagnoses with the highest levels occurring in patients with sepsis and the lowest levels in those with asthma. Plasma IL-8 was strongly correlated with oxygenation defect and severity of illness. IL-8 was consistently higher in PARDS patients compared to those without PARDS; levels were 4–12 fold higher in non-survivors compared to survivors. On multivariable analysis, IL-8 was independently associated with death, duration of mechanical ventilation, and PICU length of stay on all days measured, but was not associated with PARDS development. There was no association between the IL-8 single nucleotide polymorphisms, rs4073, rs2227306, and rs2227307, and PARDS development or plasma IL-8 level.
Conclusions
When measured sequentially, plasma IL-8 was robustly associated with multiple, relevant clinical outcomes including mortality, but was not associated with PARDS development. The wide range of plasma IL-8 levels exhibited in this cohort suggests that further study into the heterogeneity of this patient population and its impact on individual responses to PARDS treatment is warranted.
Electronic supplementary material
The online version of this article (10.1186/s13054-019-2342-8) contains supplementary material, which is available to authorized users.
Keywords: Biomarkers, Acute respiratory distress syndrome, Genetic variants, Critical illness, ARDS, PARDS, SNP
Background
Approximately 3% of children admitted to the pediatric intensive care unit are diagnosed with pediatric acute respiratory distress syndrome (PARDS) [1], which currently carries a mortality rate of 17–51% in children [1–4] and 35–60% in adults [5, 6]. The pathogenesis of both adult and pediatric ARDS (PARDS) involves the release of pro-inflammatory cytokines and injury to both the lung vascular endothelium and alveolar epithelium. As damage progresses, proteins leak into the alveolar space, further stimulating the influx of neutrophils and macrophages into the area, propagating the inflammatory response [7, 8]. The influx of neutrophils is triggered by the increased expression and release of the chemoattractant, interleukin-8 (IL-8). The expression of IL-8 is stimulated by the classic pro-inflammatory cytokines tumor necrosis factor (TNF) and IL-1 which are released early in the inflammatory response. IL-8 is produced by multiple cell types including, but not limited to, monocytes, macrophages, fibroblasts, and endothelial cells [9]. Plasma IL-8 levels are almost immeasurable in the healthy child and are specifically elevated under conditions which trigger inflammation [8, 10–15].
In adults with ARDS, elevated levels of IL-8 in both plasma and bronchoalveolar lavage fluid have been associated with increased risk of death and multiple organ system failures [16–18]. In pediatrics, initial investigations indicate that elevations in plasma IL-8 measured at the start of PARDS correlate with worse clinical outcomes and mortality [8]. The association of IL-8 with outcomes in children with acute respiratory failure at risk for PARDS, or on multiple days after established PARDS, has not been examined, nor has the association of genetic variants of IL-8 with PARDS been examined. We hypothesized that plasma IL-8 or specific IL-8 variants would be associated with PARDS and with worse clinical outcomes both in children at risk for PARDS and in children with PARDS. The objective of this study was to determine whether plasma IL-8 levels, measured on sequential days in pediatric patients with acute respiratory failure, can identify children at increased risk of developing PARDS or correlate with clinically relevant outcomes in children with acute respiratory failure.
Methods
This study, Genetic Variation and Biomarkers in Children with Acute Lung Injury (BALI), was an ancillary study to the multi-site clinical trial, Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE; U01 HL086622) [19]. BALI was designed to examine the association of specific plasma protein and genetic biomarkers with PARDS in prospectively enrolled children with acute respiratory failure. Details of the study methodology have been published previously [20]. Twenty-two of the 31 pediatric intensive care units (PICUs) participating in RESTORE also participated in this study. The study was approved by the Institutional Review Boards at all participating sites. Blood samples were taken within 24 h of consent and again 24 and 48 h later. Plasma IL-8 was assayed in duplicate by ELISA (R&D Systems, #D800C). The reported limit of detection of the assay is 7.5 pg/ml; intra- and inter-assay coefficients of variations are reported to be 5.8% and 7.7%, respectively.
The primary analyses examined the association of plasma or genetic biomarkers with the presence of PARDS. For plasma IL-8, analyses examining the association with the presence of PARDS were done both across days and on each individual day. PARDS was defined using oxygenation index (OI) or oxygen saturation index (OSI) as described by the Pediatric Acute Lung Injury Consensus Conference [3] except that all patients defined as having PARDS also had bilateral infiltrates within 2 days before or 1 day after meeting OI or OSI criteria for PARDS. These chest radiograph criteria were applied as RESTORE and BALI were both conceived when the American European Consensus Conference (AECC) definition [21] was used to identify PARDS. Consequently, chest radiograph data only included the presence or absence of bilateral infiltrates. Individual chest radiographs were not collected as part of either study. Secondary analyses examined the association of biomarkers with oxygenation defect (OI, PaO2/FiO2) and other relevant clinical outcomes including duration of mechanical ventilation, PICU length of stay in survivors, and 90-day in-hospital mortality. Duration of mechanical ventilation was defined as done in RESTORE [19] with patients assigned 28 days if they remained intubated or were transferred or died prior to day 28, therefore making this outcome equivalent to ventilator-free days. Evaluation of the impact of the primary diagnosis associated with acute respiratory failure was conducted in two ways. First, diagnostic categories were evaluated based on categories reported in the parent RESTORE trial (bronchiolitis, aspiration, sepsis, pneumonia, asthma, and others). In addition, the primary diagnoses were trichotomized into infectious (bronchiolitis, laryngotracheobronchitis, pertussis, pneumonia, sepsis), non-infectious (thoracic trauma, aspiration, edema, and transfusion-associated), and indeterminant (asthma, chronic lung disease, acute respiratory failure post bone marrow transplantation, pulmonary hemorrhage, and acute chest syndrome) categories.
Statistics
The statistical analysis was performed using SAS version 9.4. Development of PARDS, death, duration of mechanical ventilation, and length of PICU stay in survivors were the outcomes examined for association with plasma IL-8 levels across all days and if significant with IL-8 levels on each individual day. Due to the skewed distribution of plasma IL-8 levels, a natural log transformation was used for the analyses. Basic univariate (including frequency distributions or means, medians and standard deviations, analysis of variance, Wilcoxon rank-sum tests) and bivariate (including chi-square, Fisher’s exact, t tests, Pearson correlations, or longitudinal models, as appropriate) analyses of all demographic measures, clinical measures, and outcomes were conducted across all study days and on each individual study day (0, 1, 2, and 3). Comparisons with respect to these variables used generalized estimating equations (GEE) to account for correlations within individuals. Study hypotheses were evaluated using longitudinal models across days and if significant, with separate models for each day using logistic regression models for binary outcomes, Poisson regression models for the duration of mechanical ventilation, and linear regression for the length of PICU stay. The results of the bivariate analyses and forward stepwise linear models were used to develop the final multivariate models. The potential covariates of age, gender, race, ethnicity, prior medical co-morbidity (including the history of prematurity, asthma, seizure disorder, neurological disorder, cancer, or immune deficiency), primary diagnosis associated with acute respiratory failure, and PRISM III score were considered for the multivariate models. For the final models, each outcome was modeled as a function of age, PRISM III score, diagnosis of sepsis, and IL-8 level, with study day included when longitudinal models across all days were evaluated. The diagnosis of sepsis was correlated to a past medical history of cancer and of immune deficiency; therefore, only sepsis was included in the final model. All multivariable logistic regression models of the binary outcomes accounted for individual clustering using GEE and adjusted for the covariates to assess the association of IL-8 across all days with outcomes. Final models for each individual day included IL-8 as a fixed covariate, whereas when examining IL-8 across all days, IL-8 was included as a time-varying covariate. The GEE analysis across all days included only patients with two (n = 143) or three (n = 208) IL-8 measurements within the time frame of interest; 73 patients with only one measurement were not included in the analysis. The total number of patients included in analyses done on each day is indicated in the figures, or is shown in table legends, and includes the 73 patients that were dropped from the analyses done across all days. We reported odds ratios with 95% confidence intervals. All statistical tests were two-tailed, with a significance level of 0.05.
Genotyping and genetic association analysis
DNA was extracted from whole blood using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI). Ninety-two percent of the samples (n = 481) had sufficient DNA for genotyping which was done using a custom Illumina Golden gate panel which included the three IL-8 SNPs of interest, rs4073, rs2227306, and rs2227307. SNP call rates were 100%, and genotypes did not deviate from Hardy-Weinberg equilibrium. Genotyping was done blinded to the clinical status of individuals. The association of these genetic variants with PARDS was examined as described previously [20]. Briefly, the cohort was stratified by race and ethnicity into three major subgroups, non-Hispanic Caucasians, Hispanic Caucasians, and African Americans (52%, 20%, and 17% of the cohort, respectively). Association of IL-8 SNPs with PARDS within each group was examined, and then, results of the individual subgroups were combined and examined by meta-analysis using the program METAL [22].
Results
Five hundred forty-nine patients were enrolled in the study with 480 having plasma samples. Clinical characteristics of the entire BALI cohort as well as those with and without PARDS have been described previously [20]. In brief, the patients enrolled in BALI were of mixed race and ethnicity with the major groups being non-Hispanic White (52%, n = 287), Hispanic White (20%, n = 113), and African American (17%, n = 92). The most frequent primary diagnoses associated with acute respiratory failure were pneumonia (37%, n = 203), bronchiolitis (20%, n = 107), and acute respiratory failure related to sepsis (19%, n = 104). Prior medical co-morbidities included history of asthma (16%, n = 89), prematurity (13%, n = 72), seizure disorder (10%, n = 56), neurological disorder (8% n = 45), cancer (7%, n = 37), and immune deficiency (2%, n = 13). There were no statistically significant differences in any of the clinical characteristics of the patients that consented for the BALI study who did, or did not, have plasma IL-8 or IL-8 genetic variants tested. Mortality in the BALI cohort was 9%, with a median duration of mechanical ventilation of 7.1 days (IQR, 4.0–13.6) and a median PICU length of stay in survivors of 10.6 days (IQR, 6.6–18.4).
Plasma IL-8 levels were highest on the day of intubation (day 0) and tapered over the days to follow with a statistically significant difference in median values across the indicated days (p < 0.001, Table 1). There was no association between plasma IL-8 level and age or gender (data not shown). IL-8 was significantly elevated in those with a past medical history of cancer (p < 0.05 on each day) or immune deficiency (p < 0.05 on days 1, 2, 3) as described in Additional file 1. IL-8 was also significantly higher in children with a primary diagnosis of sepsis (Additional file 1: Figure S1). The frequency of a primary diagnosis of sepsis was 38% and 41% in those with a history of immune deficiency or cancer, respectively.
Table 1.
Plasma interleukin-8 levels decrease over time
| Day | N | Mean (SD) | Median (range) |
|---|---|---|---|
| 0 | 57 | 759 (1797) | 54 (4, 7373) |
| 1 | 239 | 344 (1035) | 44 (0, 6900) |
| 2 | 362 | 252 (872) | 39 (0, 8162) |
| 3 | 325 | 221 (822) | 37 (0, 7706) |
IL-8 levels are shown as picogram per milliliter. Day 0 is the day of intubation. The n is the number of plasma samples analyzed. Plasma IL-8 levels were log transformed for analysis due to skewness. Medians are significantly different across days, p < 0.001 using generalizing estimating equations to account for correlations within individuals
Plasma IL-8 and PARDS development
Of the 549 patients enrolled in BALI, 69% (n = 378) developed PARDS. Within the PARDS cohort, 83% met the diagnosis for PARDS at the time of intubation (day 0) and 94% by day 1. Plasma IL-8 levels were higher in children with PARDS compared to those children without PARDS on all days measured, but when examining each day, achieved statistical significance only on day 2 (p = 0.02 on day 2, Fig. 1a). Multivariable analyses adjusted for age, PRISM III, and sepsis diagnosis demonstrated that plasma IL-8 was not independently associated with PARDS across all days or on any specific day (Additional file 1: Table S3).
Fig. 1.
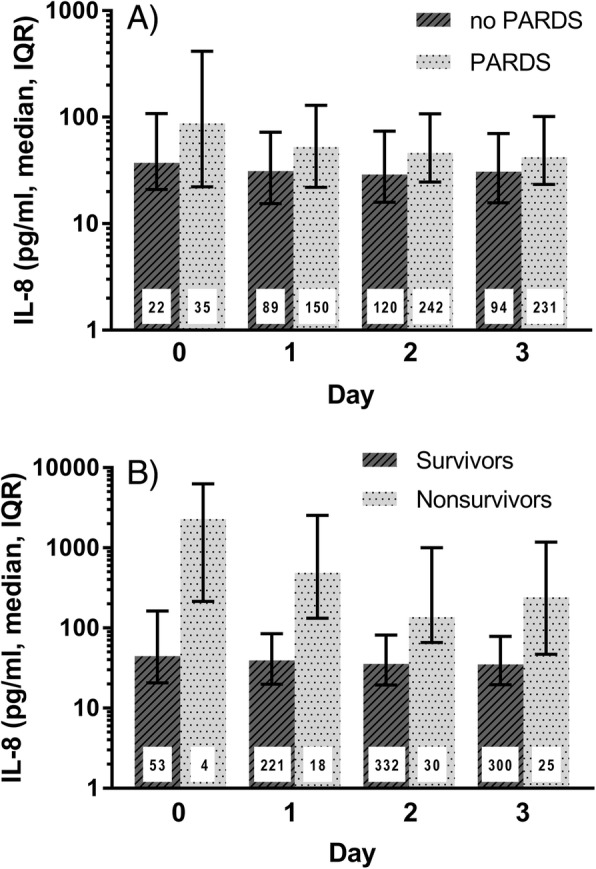
Plasma interleukin-8 is higher in children with pediatric acute respiratory distress syndrome and in non-survivors. a Comparison of plasma IL-8 in children with or without PARDS. The group with PARDS included children meeting the criteria for PARDS on the indicated day or any previous day. Plasma IL-8 was significantly higher in patients with PARDS across all days (p < 0.0001 using GEE methods as described in the “Methods” section), but when examining each day only achieved significance on day 2 (p = 0.02). b Comparison of plasma IL-8 in children who died or survived. IL-8 is significantly higher in non-survivors on each day (p < 0.05). Day 0 is the day of intubation. The number inside each bar indicates the number of plasma samples analyzed from the indicated days. IL-8 interleukin-8, PARDS pediatric acute respiratory distress syndrome
Analyses of plasma IL-8 and relevant clinical outcomes
On bivariate analysis of this cohort of children with acute respiratory failure, plasma IL-8 was significantly higher in non-survivors on each day measured (Fig. 1b, p < 0.05) with median IL-8 levels 4–12 fold higher in non-survivors than survivors. As IL-8 has been reported to be associated with death in children with PARDS, the cohort was stratified by PARDS to examine whether PARDS patients were responsible for the higher IL-8 levels observed in non-survivors. As shown in Fig. 2a, the levels of IL-8 were significantly higher in survivors with PARDS compared to survivors without PARDS; however, the levels of IL-8 were not significantly different in non-survivors with and without PARDS (Fig. 2b) but are higher than those seen in survivors with or without PARDS (Fig. 2b vs a) suggesting that IL-8 is associated with death in patients with acute respiratory failure independent of whether the child has PARDS. On multivariate analysis with adjustment for severity of illness (PRISM III), age, and sepsis diagnosis, plasma IL-8 was independently associated with death on days 1, 2, and 3 (Table 2). When PARDS was added to the model, IL-8 was still independently associated with death (Additional file 1: Table S4) again suggesting that IL-8 is associated with death both in the whole cohort and in patients without PARDS.
Fig. 2.
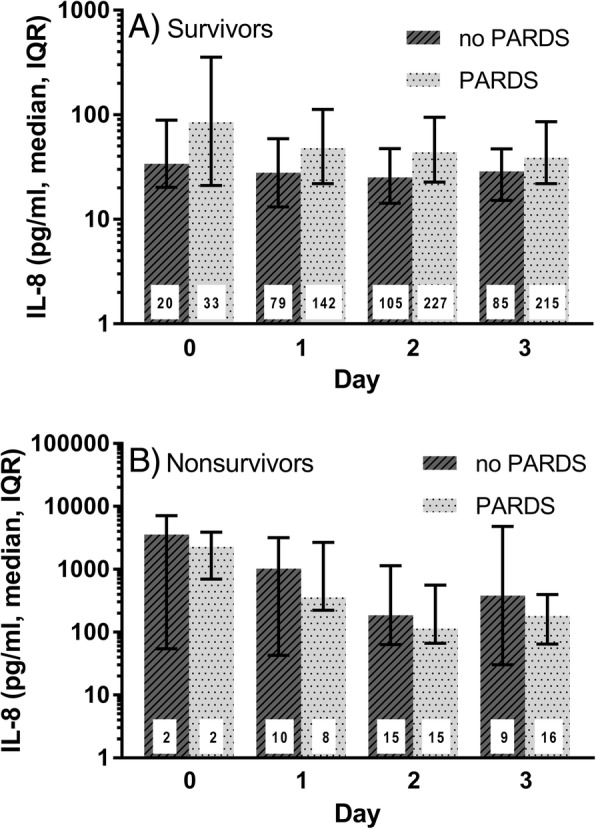
Comparison of plasma interleukin-8 levels in survivors and non-survivors with or without pediatric acute respiratory distress syndrome. a Comparison of plasma IL-8 in survivors with acute respiratory failure with or without PARDS. IL-8 is significantly higher in survivors with PARDS on each day (p < 0.05). b Comparison of plasma IL-8 in non-survivors with acute respiratory failure with or without PARDS. In non-survivors, IL-8 levels are not significantly different in children with or without PARDS. Day 0 is the day of intubation. The number inside each bar indicates the number of plasma samples analyzed from the indicated days. IL-8 interleukin-8, PARDS pediatric acute respiratory distress syndrome
Table 2.
Multivariable analysis of association of interleukin-8 with death
| Day | Variable | Odds ratio | 95% Confidence interval | p a |
|---|---|---|---|---|
| 0 | IL-8 | 2.05 | 0.56–7.46 | 0.28 |
| Age | 1.00 | 0.83–1.20 | 0.99 | |
| PRISM III | 0.96 | 0.68–1.31 | 0.73 | |
| Sepsis | 1.79 | 0.27–12.1 | 0.55 | |
| 1 | IL-8 | 2.33 | 1.51–3.60 | < 0.001 |
| Age | 1.14 | 1.04–1.25 | 0.007 | |
| PRISM III | 0.97 | 0.88–1.06 | 0.50 | |
| Sepsis | 0.76 | 0.20–2.99 | 0.70 | |
| 2 | IL-8 | 1.90 | 1.39–2.60 | < 0.001 |
| Age | 1.13 | 1.06–1.22 | < 0.001 | |
| PRISM III | 1.00 | 0.93–1.08 | 0.96 | |
| Sepsis | 1.09 | 0.38–3.13 | 0.88 | |
| 3 | IL-8 | 2.19 | 1.48–3.24 | < 0.001 |
| Age | 1.14 | 1.06–1.22 | < 0.001 | |
| PRISM III | 0.98 | 0.91–1.06 | 0.58 | |
| Sepsis | 0.87 | 0.24–3.11 | 0.83 |
ap value using a multivariable logistic regression model with adjustment for covariates. The n for days 0–3 is 57, 239, 362, and 325, respectively. Day 0 is the day of intubation. IL-8 interleukin-8, PRISM pediatric risk of mortality
Bivariate analysis also indicated that plasma IL-8 was significantly correlated with duration of mechanical ventilation on each day measured and with PICU length of stay on days 1, 2, and 3 (Additional file 1: Table S5). When duration of mechanical ventilation was dichotomized into 7 or more ventilator days versus fewer than 7 days (median duration of mechanical ventilation was 7.1 days, IQR, 4.0–13.6), plasma IL-8 was significantly higher in patients with a duration of mechanical ventilation ≥ 7 days on each day measured (p < 0.01, Fig. 3a). Using 14 days to dichotomize PICU length of stay (median PICU length of stay was 10.6 days, IQR, 6.6–18.4), IL-8 was significantly elevated on each day measured in those patients requiring a prolonged PICU stay (14 or more days vs fewer than 14 days, p < 0.01 on each day, Fig. 3b). Using Poisson regression and controlling for age, severity of illness, and sepsis diagnosis, plasma IL-8 was independently associated with prolonged duration of mechanical ventilation on each day measured (p ≤ 0.01 on each day). Finally, plasma IL-8 was associated with prolonged PICU length of stay in survivors on each day measured when adjusted for age and severity of illness and was also associated with longer length of stay on days 0 (p = 0.01), 2 (p = 0.02), and 3 (p = 0.03) when sepsis diagnosis was also added to the model (data not shown).
Fig. 3.
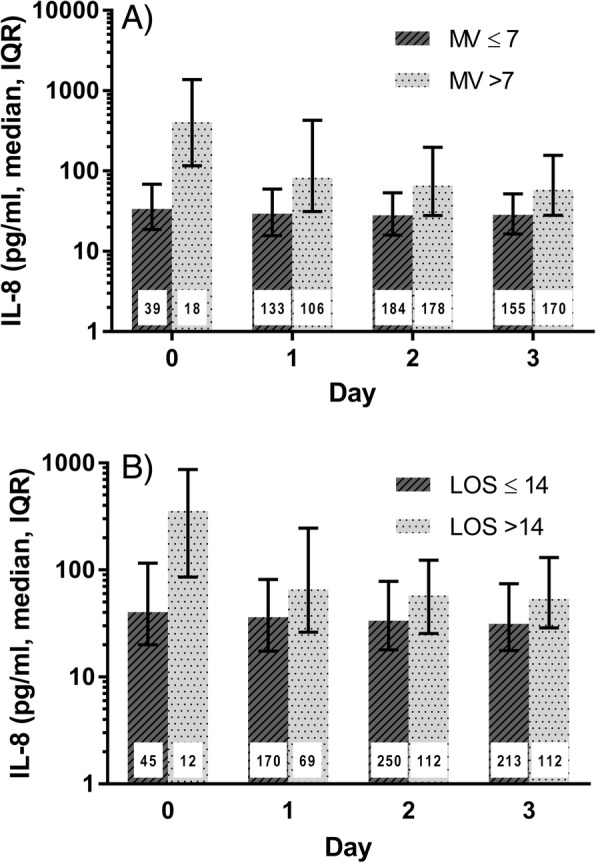
Plasma interleukin-8 is higher in children with a longer duration of mechanical ventilation or length of stay. a Comparison of plasma IL-8 in children with acute respiratory failure who were mechanically ventilated for ≤ 7 days to those mechanically ventilated for > 7 days; p < 0.01 on each day. b Comparison of plasma IL-8 in children with acute respiratory failure who were in the PICU for ≤ 14 days to those in the PICU for > 14 days; p < 0.01 on each day. Day 0 is the day of intubation. The number inside each bar indicates the number of plasma samples analyzed from the indicated days. IL-8 interleukin-8, LOS length of stay in the PICU, MV mechanical ventilation, PICU pediatric intensive care unit
Genetic association studies for IL-8
Three IL-8 SNPs previously reported to be associated with severity in cystic fibrosis (rs4073, rs2227306, rs2227307) or with the length of mechanical ventilation in adults with ARDS (rs4073) were examined for association with PARDS. The cohort was stratified into non-Hispanic Caucasians, Hispanic Caucasians, and African Americans for analyses which were done with adjustment for population structure (as described under the “Methods” section). None of the variants examined showed a significant association with PARDS within any of the subgroups or when the three subgroups were combined in a meta-analysis (n = 412). In addition, these variants were not associated with plasma levels of IL-8.
Discussion
Identification of children within the at-risk population with a higher likelihood of developing PARDS using either specific biomarker levels and/or specific genetic variations provides a unique insight into PARDS pathogenesis and may identify novel therapeutic targets allowing future studies to stratify patients so that they might benefit from targeted therapies. This is the first study in children examining whether IL-8 is associated with the development of PARDS. Plasma IL-8 is significantly elevated in pediatric acute respiratory failure and was even higher in those who develop PARDS; however, multivariable analysis adjusting for relevant covariates indicates that plasma IL-8 is not independently associated with PARDS.
Our data indicate a very strong association of elevated IL-8 levels with mortality in this cohort of children with acute respiratory failure independent of whether the child had PARDS. IL-8 levels were not significantly different in non-survivors with or without PARDS. Plasma IL-8 levels in non-survivors are 4–12 fold higher than in survivors, and this elevation is present for several days after onset of acute respiratory failure. Further, the association of IL-8 remained independently associated with death even after adjusting for many key covariates: severity of underlying disease (PRISM III), sepsis diagnosis, development of PARDS, and others. Plasma IL-8 elevation was also strongly associated with prolonged mechanical ventilation, PICU length of stay, and degree of pulmonary morbidity (as measured by oxygenation defect).
Persistent elevation of IL-8 has been reported in adults with ARDS and also appears to be related to the differential impact of ventilator strategy or steroid responsiveness in randomized controlled trials of adult ARDS patients [23–25]. To date, pediatric studies have largely focused on IL-8 elevations at the onset of disease [8, 26]. The persistence of inflammation after the onset of pediatric acute respiratory failure in general, and in PARDS specifically, offers a unique opportunity to determine whether measuring plasma IL-8 over time can be used as an indicator of therapeutic effectiveness and/or disease progression. If so, real-time IL-8 measurement to guide management strategies in randomized clinical trials, as well as routine clinical practice, appears promising if technological advances allowing the measurement of cytokines in real-time were available.
IL-8 levels were highly variable, especially on the day of intubation (day 0) where levels ranged from 4 to 7373 pg/ml. This degree of variability may have profound implications for future therapeutic trials targeting the inflammatory cascade in either pediatric respiratory failure and/or PARDS, particularly since elevated IL-8 is one of the biomarkers shown to be able to distinguish between the hyperinflammatory and hypoinflammatory subphenotypes in adults ARDS [17, 27, 28]. The observed degree of variability in IL-8 levels together with adult data strongly suggest the need for future studies to determine whether subphenotypes are also present in PARDS patients and whether subphenotypes differ between children and adults.
Although age does impact the development of the immune system and levels of cytokines have been reported to vary with age, in this cohort of children with acute respiratory failure and/or PARDS, plasma IL-8 was independent of age. Indeed, age was not associated with plasma IL-8 levels on any day measured. This lack of an association of age with IL-8 levels may be because these children are critically ill and in the midst of a very marked inflammatory response and because of the high degree of variability. The lack of an impact of age on the association of IL-8 levels with these outcome measures underscores important similarities between the inflammatory pathophysiology exhibited in ARDS in adult and pediatric patients. Our data support the inclusion of both adult and pediatric patients into future trial design aimed at impacting the inflammatory cascades during early ARDS. However, it remains imperative for any biological marker which will potentially be used for bedside risk stratification for clinical or research purposes to be tested for associations with age and to be evaluated in both adults and children.
Our study has some limitations. The definition of PARDS was changed during the study enrollment period. For the analysis, PARDS was defined using the more recent PALICC criteria. However, the parent RESTORE trial and the BALI study were designed using AECC criteria to diagnose ARDS; consequently, chest radiograph interpretation was limited to the presence or absence of new bilateral infiltrates on each study day. Lastly, while the majority of patients met the criteria for PARDS on the day of intubation (day 0), only 16% of patients had blood drawn for plasma biomarkers on day 0. This is noteworthy as it limited our ability to assess whether plasma IL-8 was higher before the onset of PARDS in patients who went on to develop PARDS. As a result, we were unable to determine whether IL-8 levels are significantly higher in those who do not meet the criteria for PARDS but are destined to develop PARDS subsequently.
Conclusions
In conclusion, plasma IL-8 is remarkably elevated over several days after the onset of acute respiratory failure and PARDS. These elevations are robust and are independently associated with multiple relevant clinical outcomes, most importantly death, but are not independently associated with PARDS. The wide range of plasma IL-8 seen in our investigation suggests the potential existence of subphenotypes in children with PARDS which should be investigated further as subphenotypes can significantly impact future therapeutic trial design. Additional investigation is warranted to determine the utility of serial IL-8 measurement in corroborating disease progression and/or treatment effectiveness in pediatric acute respiratory failure and PARDS.
Additional file
Supplementary tables and figures for a prospective investigation of interleukin-8 levels in pediatric acute respiratory failure and acute respiratory distress syndrome. Supplementary tables (S1–S5) and figures (S1–S2) are found in the additional file. (DOCX 273 kb)
Acknowledgements
We would like to thank all the patients and guardians of those patients for their participation in the study and Nadine Halligan for sample storage and genotyping. We would also like to acknowledge the contribution of the subgroup of PALISI members who were BALI study investigators at the sites that participated in the RESTORE study including: Scot T. Bateman (University of Massachusetts Memorial Children's Medical Center, Worcester, MA), M. D. Berg (University of Arizona Medical Center, Tucson, AZ), Santiago Borasino (Children’s Hospital of Alabama, Birmingham, AL), G. Kris Bysani (Medical City Children's Hospital, Dallas, TX), Allison S. Cowl (Connecticut Children's Medical Center, Hartford, CT), Cindy Darnell Bowens (Children’s Medical Center of Dallas, Dallas, TX), E. Vincent S. Faustino (Yale-New Haven Children’s Hospital,New Haven, CT), Lori D. Fineman (University of California San Francisco Benioff Children’s Hospital at San Francisco, San Francisco, CA), A. J. Godshall (Florida Hospital for Children, Orlando, FL), Ellie Hirshberg (Primary Children’s Medical Center, Salt Lake City, UT), Aileen L. Kirby (Oregon Health & Science University Doernbecher Children's Hospital, Portland, OR), Gwenn E. McLaughlin (Holtz Children’s Hospital, Jackson Health System, Miami,FL), Shivanand Medar (Cohen Children's Medical Center of New York, Hyde Park, NY), Phineas P. Oren (St. Louis Children’s Hospital, St. Louis, MO), James B. Schneider (Cohen Children's Medical Center of New York, Hyde Park, NY), Adam J. Schwarz (Children’s Hospital of Orange County, Orange, CA), Thomas P. Shanley (C. S. Mott Children’s Hospital at the University of Michigan, Ann Arbor, MI), Lauren R. Sorce (Ann & Robert H. Lurie, Children’s Hospital of Chicago, Chicago, IL), Edward J. Truemper (Children’s Hospital and Medical Center, Omaha, NE), Michele A. Vander Heyden (Children's Hospital at Dartmouth, Dartmouth, NH), Kim Wittmayer (Advocate Hope Children’s Hospital, IL), Athena Zuppa (Children’s Hospital of Philadelphia, Philadelphia, PA) and the RESTORE data coordination center led by David Wypij, PhD (Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts; Department of Pediatrics, Harvard Medical School, Boston, Massachusetts; Department of Cardiology, Boston Children’s Hospital, Boston, Massachusetts).
Funding
This study was funded by a grant from the NIH awarded to Drs. Dahmer, Flori and Quasney (R01HL095410). The parent study was supported by grants from the NIH awarded to Drs. Curley and Wypij (U01HL086622, U01 HL086649).
Availability of data and materials
The data that support the findings of this study (deidentified participant data and data dictionary) are not yet currently available but will be made available after the publication of all primary papers associated with this study or within two years’ time from the date of this publication. All reasonable requests should be made concurrently to the primary author of this publication (MKD) along with the primary author (MAQC.) of the parent clinical trial.
Abbreviations
- AECC
American European Consensus Conference
- ARDS
Acute respiratory distress syndrome
- FiO2
Fraction of inspired oxygen
- GEE
Generalized estimating equations
- IL-8
Interleukin-8
- OI
Oxygenation index
- OSI
Oxygen saturation index
- PaO2
Partial pressure of oxygen
- PARDS
Pediatric acute respiratory distress syndrome
- PICU
Pediatric intensive care unit
- PRISM
Pediatric risk of mortality
- SNP
Single nucleotide polymorphism
- TNF
Tumor necrosis factor
Authors’ contributions
HF designed and conceived the project, enrolled the patients in the project, participated in the statistical analyses and interpretation of the results, wrote the first draft of the manuscript, and edited the manuscript. AS designed and conceived the project, enrolled the patients in the project, participated in the interpretation of the results, and edited the manuscript. MWQ designed and conceived the project, enrolled the patients in the project, participated in the interpretation of the results, and edited the manuscript. GG performed the statistical analyses, participated in the interpretation of the results and edited the manuscript. MAQC was the principal investigator of the clinical trial and liaison to this ancillary study, provided the clinical data for the analysis, participated in the interpretation of results, and edited the manuscript. MAM was a consultant on the design of the project, participated in the interpretation of results, and edited the manuscript. MKD designed and conceived the project, oversaw the day to day running of the project, participated in the statistical analyses and interpretation of the results, and performed the initial edit of the manuscript. All authors reviewed the manuscript and read and approved the final version.
Ethics approval and consent to participate
The study was approved by the Institutional Review Boards at all participating sites as described under Materials and Methods.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Heidi Flori, Email: heidiflo@med.umich.edu.
Anil Sapru, Email: ASapru@mednet.ucla.edu.
Michael W. Quasney, Email: mquasney@med.umich.edu
Ginny Gildengorin, Email: Ginny.Gildengorin@ucsf.edu.
Martha A. Q. Curley, Email: curley@nursing.upenn.edu
Michael A. Matthay, Email: MatthayM@anesthesia.ucsf.edu
Mary K. Dahmer, Phone: 734-647-1426, Email: mkdahmer@umich.edu
for the BALI and RESTORE Study Investigators, Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, Email: palisinet@gmail.com.
for the BALI and RESTORE Study Investigators, Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network:
Scot T. Bateman, M. D. Berg, Santiago Borasino, G. Kris Bysani, Allison S. Cowl, Cindy Darnell Bowens, E. Vincent S. Faustino, Lori D. Fineman, A. J. Godshall, Ellie Hirshberg, Aileen L. Kirby, Gwenn E. McLaughlin, Shivanand Medar, Phineas P. Oren, James B. Schneider, Adam J. Schwarz, Thomas P. Shanley, Lauren R. Sorce, Edward J. Truemper, Michele A. Vander Heyden, Kim Wittmayer, Athena Zuppa, and David Wypij
References
- 1.Khemani Robinder G, Smith Lincoln, Lopez-Fernandez Yolanda M, Kwok Jeni, Morzov Rica, Klein Margaret J, Yehya Nadir, Willson Douglas, Kneyber Martin C J, Lillie Jon, Fernandez Analia, Newth Christopher J L, Jouvet Philippe, Thomas Neal J, Abaleke Eugenia, Ackerman Kate G, Acuña Carlos, Adu-Darko Michelle, Affolter Jeremy T, Agbeko Rachel, Al Amoudi Ahmed, Alahmadti Ahmad, Aldairi Nedaa, Alibrahim Omar, Allen Kiona, Allen Christine, Al-Subu Awni, Althabe María, Alvear Jimena, Anil Ayse Berna, Anthony Heather, Aramburo Angela, Arjona Villanueva David, Ashtari Neda, Ávila Vera Antonio, Baines Paul, Bales Melissa, Barr Samantha, Barry Dana, Baudin Florent, Beca John, Belfield Holly, Beltramo Fernando, Benken Laura, Bhalla Anoopindar, Blom Andrea, Botta Priscila, Bourgoin Pierre, Brezmes Marta, Briassoulis George, Bridier Armelle, Brierley Joe, Brio Sanagustin Sonia, Broden Elizabeth, Butt Warwick, Bysani Kris, Camilo Cristina, Camporesi Anna, Campos-Miño Santiago, Can Fulya Kamit, Capocasa Patricia, Caro I Daniel, Carroll Christopher, Castellani Pablo, Castillo Andres E., Chen Yang, Chima Ranjit S., Chiusolo Fabrizio, Cinquegrani Karina, Coates Bria, Coronado-Munoz Alvaro, Cortéz Ambar, Cruces Romero Pablo, Cullimore Melissa, Cvijanovich Natalie, Dahmer Mary K., Deep Akash, Delzoppo Carmel, Di Nardo Matteo, Díaz Franco, Dijkstra Sandra, Dockery W. Keith, Dominguez Troy E., Dumitrascu Mariana, Dursun Oguz, Dwarakanathan Buvana, Elghuwael Ismail, Emeriaud Guillaume, Erickson Simon, Español Segundo Fernando, Estil Jim Brian, Feather Calandra, Feinstein Yael, Fernández Analía, Ferreyra Marcela, Flori Heidi, Fortini Yanina Vanesa, Fortune Peter-Marc, French Mary Ellen, Gaboli Mirella, Gale Helen, García Casas Paula, García González Maria, Gautam Richa, Gedeit Rainer, Genuini Mathieu, Gertz Shira, Giampieri Martin, Gil Escobar Carlos, Giuliano Jr John S., Godoy Mundaca Loreto, Goni Orayen Concepción, Gonzalez Gomez Jose Manuel, Govantes Beatriz, Guichoux Julie, Guzman Rivera Gustavo Alfredo, Haileselassie Bereketeab, Han Yong Y, Harrell Amy, Hartmann Silvia, Hazwani Tarek, Hefley Glenda, Henderson Grace, Hsing Deyin D., Hughes-Schalk Amber, Hume Janet, Ilia Stavroula, Inwald David, Iolster Thomas, Izquierdo Ledys María, Jafari-Namin Shirin, Jaimon Nancy, Jarillo Quijada Alberto E, Jarvis J. Dean, Jayachandran Chaandini, Jennings Claire, Jeyapalan Asumthia S., Jimenez Rivera Nestor Javier, Jones Dawn, Jouvet Philippe, Kasch Mary, Keary Jane't, Kelley Connor, Kessel Aaron, Khemani Robinder, Kida Yoshiko, King Caroline, Kneyber Martin, Kniola Allison, Krallman Kelli, Kubis Sherri, Kustka Lucinda, Kwok Jeni, Kyo Michihito, Landry Luis Martín, Latifi Samir, Lawton-Woodhall Angela, Lillie Jon, Lin John C., Llorente de la Fuente Ana M., Lopez Alarcón Yurika Paola, López Fernández Yolanda, Lopez-Herce Jesús, Lum Lucy Chai See, Macrae Duncan, Maddux Aline B., Madurga Revilla Paula, Mahapatra Sidharth, Maria Matthieu, Martínez Lidia, Martinez de Azagra Amelia, Martínez León Alejandro Fabio, Mazzillo Vega Liliana, McCorkell Jenni, McIntyre Karen, Medina Tania, Medina Alberto, Mellish Christie, Mendizabal Mikel, Merritt Courtney, Mildner Reinout, Milesi Christophe, Modesto I Alapont Vicent, Monjes Cecilia, Monjure Tracey, Montes María José, Morales Martinez Antonio, Morgan Ryan, Morzov Rica, Mourani Peter M., Murkowski Kathy, Murphy Marie, Napolitano Natalie, Nerheim Dan, Nett Sholeen T., Newth Christopher, Nofziger Ryan, Nunez Maria Jose, Ohshimo Shinichiro, Onate Vergara Eider, Ongun Ebru A, Orqueda Daniel, Oruganti Siva, Pagowska-Klimek Izabela, Palanca Arias Daniel, Pappachan Jon, Pardo Carrero Rosalba, Parker Margaret M., Parrilla Julio, Patankar Nikhil, Pávez Madrid Paula, Payen Valerie, Paziencia Fernando, Pedraza Claudia, Perez Lozano Germán, Pilar Orive Javier, Piñeres Olave Byron Enrique, Pintimalla Alyssa, Pinto Neethi, Plunkett Adrian, Pon Steve, Pons Odena Marti, Poterala Rossana, Qiao Haiping, Quiñonez Lopez Deyanira, Ralston Kimberly, Ramirez Cortez Grimaldo, Ratiu Anna, Rea Miriam, Reyes Dominguez Susana, Rodgers Chiara, Rodriguez Campoy Patricia, Ronan Laurie, Rosemary Deheza, Rowan Courtney, Sadasivam Kalaimaran, Sanchez Diaz Juan Ignacio, Sanders Ron, Santanelli James, Sapru Anil, Schneider James, Sforza Jesica, Shea Sara, Shein Steven L., Sherring Claire, Sheward Victoria, Shime Nobuaki, Shukla Avani, Siaba Serrate Alejandro, Sierra Yamila, Sikora Lindsay, Silvestre Catarina, Singleton Marcy, Sloniewsky Daniel, Smith Rebecca, Smith Lincoln, Song Hanqiu, Sousa Moniz Marta, Spaeder Michael, Spear Debbie, Spinella Philip, Starck Julie, Stoneman Erin, Su Felice, Subramanian Gayathri, Sullivan Erin, Sundararajan Santosh, Sweberg Todd, Sykes Kim, Tabata Yuichi, Tai Chian Wern, Tala Joana, Tang Swee Fong, Tantalean José, Taylor Ryan, Thomas Neal, Tibby Shane, Tieves Kelly S, Torero Luis, Torres Silvio Fabia, Totapally Balagangadhar, Travert Brendan, Truemper Edward, Turón Gonzalo, Typpo Katri, Valle Juan Ramón, Vargas G Sonia I, Vasquez Hoyos Pablo, Vasquez Miranda Daniel, Vavrina Martin, Vidal Nilda Águeda, Virk Manpreet, Walsh Laura, Wegner Araya Adriana, Weitz James, Wellisch Lawren, Wellman Paul, Willson Douglas, Woods Katherine, Yehya Nadir, Yerovi Rocio, Yunger Toni, Zuluaga Orrego Cesar, Zurek Jiri. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. The Lancet Respiratory Medicine. 2019;7(2):115–128. doi: 10.1016/S2213-2600(18)30344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta S, Sankar J, Lodha R, Lodha R, Kabra SK. Comparison of prevalence and outcomes of pediatric acute respiratory distress syndrome using pediatric acute lung injury consensus conference criteria and Berlin definition. Front Pediatr. 2018;6(Article 93):1–7. doi: 10.3389/fped.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong JJ, Jit M, Sultana R, Mok YH, Yeo JG, Koh JWJC, et al. Mortality in pediatric acute respiratory distress syndrome: a systematic review and meta-analysis. J Int Care Med. 2017. 10.1177/0885066617705109. [DOI] [PubMed]
- 4.Pediatric Acute Lung Injury Consensus Conference Group Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. New Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 6.McNicholas B, Rooney G, Laffey J. Lessons to learn from epidemiologic studies in ARDS. Curr Opin Crit Care. 2018;24:41–48. doi: 10.1097/MCC.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 7.Sapru A, Flori H, Quasney MW, Dahmer MK. Pathobiology of acute respiratory distress syndrome. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S6–22. doi: 10.1097/PCC.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 8.Zinter MS, Orwoll BE, Spicer AC, Alkhouli MF, Calfee CS, Matthay MA, et al. Incorporating inflammation into mortality risk in pediatric acute respiratory distress syndrome. Crit Care Med. 2017;45:858–866. doi: 10.1097/CCM.0000000000002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roux J, McNicholas CM, Carles M, Goolaerts A, Houseman BT, Dickinson DA, et al. IL-8 inhibits cAMP-stimulated alveolar epithelial fluid transport via a GRK2/PI3K-dependent mechanism. FASEB J. 2013;27:1095–1106. doi: 10.1096/fj.12-219295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haugen J, Chandyo RK, Brokstad KA, Mathisen M, Ulak M, Basnet S, et al. Cytokine concentrations in plasma from children with severe and non-severe community acquired pneumonia. PLoS One. 2015;10:e0138978. doi: 10.1371/journal.pone.0138978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orwoll BE, Sapru A. Biomarkers in pediatric ARDS: future directions. Front Pediatr. 2016;4:55. doi: 10.3389/fped.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer JE, Benn A, Harbarth S, Nadal D, Fanconi S. Diagnostic accuracy of G-CSF, IL-8, and IL-1ra in critically ill children with suspected infection. Int Care Med. 2002;28:1324–1331. doi: 10.1007/s00134-002-1423-2. [DOI] [PubMed] [Google Scholar]
- 13.Wong HR, Cvijanovich N, Wheeler DS, Bigham MT, Monaco M, Odoms K, et al. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Respir Crit Care Med. 2008;178:276–282. doi: 10.1164/rccm.200801-131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine levels in the serum of healthy subjects. Mediat Inflamm. 2013;2013:434010. doi: 10.1155/2013/434010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sack U, Burkhardt U, Borte M, Schadlich H, Berg K, Emmrich F. Age-dependent levels of select immunological mediators in sera of healthy children. Clin Diagn Lab Immunol. 1998;5:28–32. doi: 10.1128/cdli.5.1.28-32.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147:1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware L, Koyama T, Zhao Z, Janz DR, Wickersham N, Bernard GR, et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013;17:R253. doi: 10.1186/cc13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calfee CS, Thompson BT, Parsons PE, Ware LB, Matthay MA, Wong HR. Plasma interleukin-8 is not an effective risk stratification tool for adults with vasopressor-dependent septic shock. Crit Care Med. 2010;38:1436–1441. doi: 10.1097/CCM.0b013e3181de42ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curley MA, Wypij D, Watson RS, Grant MJ, Asaro LA, Cheifetz IM, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313:379–389. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahmer MK, Quasney M, Sapru A, Gildengorin G, Curley MAQ, Matthay MA, et al. Interleukin-1 receptor antagonist is associated with PARDS and worse outcomes in children with acute respiratory failure. Pediatr Crit Care Med. 2018; Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 21.Bernard G, Artigas A, Brigham K, Carlet J, Falke K, Hudson L, et al. The North American-European Consensus Conference on ARDS. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 22.Willer C, Li Y, Abecasis G. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meduri G, Healey S, Kohler G, Stentz F, Tolley E, Umberger R, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 24.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Acute Respiratory Distress Syndrome Network et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 25.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.CCM.0000149854.61192.DC. [DOI] [PubMed] [Google Scholar]
- 26.Todd DA, Marsh MJ, George A, Henderson NG, Barr H, Sebastian S, et al. Surfactant phospholipids, surfactant proteins, and inflammatory markers during acute lung injury in children. Pediatr Crit Care Med. 2010;11:82–91. doi: 10.1097/PCC.0b013e3181ae5a4c. [DOI] [PubMed] [Google Scholar]
- 27.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195:331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bos L, Schouten L, van Vught L, Wiewel MA, Ong DSY, Cremer O, et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax. 2017;72:876–883. doi: 10.1136/thoraxjnl-2016-209719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables and figures for a prospective investigation of interleukin-8 levels in pediatric acute respiratory failure and acute respiratory distress syndrome. Supplementary tables (S1–S5) and figures (S1–S2) are found in the additional file. (DOCX 273 kb)
Data Availability Statement
The data that support the findings of this study (deidentified participant data and data dictionary) are not yet currently available but will be made available after the publication of all primary papers associated with this study or within two years’ time from the date of this publication. All reasonable requests should be made concurrently to the primary author of this publication (MKD) along with the primary author (MAQC.) of the parent clinical trial.


