Abstract
The detection of allergen-specific immunoglobulin (Ig)E is an important method for the diagnosis of IgE-mediated allergic diseases. The sensitivity of the indirect IgE-ELISA method against allergen extracts is limited by interference from high IgG titers and low quantities of effectual allergen components in extracts. To overcome these limitations, a novel capture IgE-ELISA based on a recombinant Der f 1/Der f 2 fusion protein (rDer f 1/2) was developed to enhance the sensitivity to IgEs that bind allergens from the house dust mite (HDM) species Dermatophagoides farina. pET28-Der f 1/2 was constructed and expressed in Escherichia coli BL21 (DE3) pLysS. The purified fusion protein was evaluated by IgE western blotting, IgE dot blotting and indirect IgE-ELISA. Capture-ELISA was performed by coating wells with omalizumab and incubating in series with sera, biotinylated Der f 1/2, horseradish peroxidase-conjugated streptavidin and 3,3,5,5-tetramethylbenzidine. The relative sensitivities of indirect-ELISA and capture-ELISA for HDM allergen-specific IgE binding were determined; sera from non-allergic individuals were used as the control group. rDer f 1/2 was expressed in the form of inclusion bodies comprising refolded protein, which were then purified. It exhibited increased IgE-specific binding (24/28, 85.8%) than rDer f 1 (21/28, 75.0%) or rDer f 2 (22/28, 78.6%) with HDM-allergic sera. Furthermore, in a random sample of HDM-allergic sera (n=71), capture-ELISA (71/71, 100%) was more sensitive than indirect-ELISA (68/71, 95.8%) for the detection of HDM-specific IgEs (P<0.01), indicating that this novel method may be useful for the diagnosis of HDM allergy.
Keywords: house dust mite, recombinant allergen, recombinant Der f 1/2 fusion protein, capture immunoglobulin E-ELISA
Introduction
Allergic diseases, including allergic asthma, allergic rhinitis and allergic dermatitis, affect 30–40% of the world population; furthermore, incidence and mortality rates associated with allergic diseases are increasing, particularly within young populations (1). At present, >50% of allergic diseases are induced by house dust mites (HDMs), a common source of inhaled allergens (2). A total of 37 HDM allergen groups have been denominated (http://www.allergen.org); of these, the allergen groups 1 and 2 are the most clinically relevant, with >80 and >90% of patients with HDM allergies exhibiting an immunoglobulin (Ig)E response to groups 1 and 2 HDM allergens, respectively (3).
IgE is an important pathogenic mediator of allergic immune responses. The detection of allergen-specific IgE is an effective diagnostic method and anti-IgE therapy is used to treat IgE-mediated allergic diseases (4). The indirect IgE-ELISA method is frequently used to detect allergen-specific IgE in serum samples, due to its simplicity and low cost compared with automated ImmunoCAP® systems; however, there are certain limitations (5). The normal range of the human serum levels of IgE is 50–300 ng/ml, which is notably low compared with that of IgG (~10 mg/ml) (6). In addition, the levels of HDM allergen-specific IgE, even in sera from patients with HDM allergies, are markedly decreased compared with the normal range of total IgE (7). Thus, the sensitivity of the indirect ELISA method is reduced by high titers of IgG that compete to bind with coated antigens (8). Furthermore, indirect ELISA frequently uses an HDM extract mixture as a coated antigen; the sensitivity of this method is reduced by the low amounts of effective allergen components in these mixtures.
Recombinant allergens are increasingly used in the diagnosis and treatment of allergic diseases, due to their high purity and consistency (9). Howard et al (10) defined the evolution of IgE responses to 112 recombinant or native allergen components during childhood, which may aid the identification of better diagnostic and prognostic biomarkers of allergic diseases. Mas et al (11) reported the use of the recombinant protein Salsola Kali in the diagnosis of allergic disease induced by Salsola kali. The combined expression of various antigens or major antigen epitopes as a fusion protein may increase the sensitivity of detection of antibodies targeted against allergens from a particular organism. He et al (12) engineered a recombinant antigen with epitopes from four hepatitis C viral fragments to aid the detection of anti-hepatitis C antibodies. Dai et al (13) constructed and overexpressed a fusion gene comprising three Mycobacterium tuberculosis antigen proteins; using the fusion polyprotein as an immunogen, multi-target antibodies were produced that exhibited significantly increased sensitivity for the clinical diagnosis of tuberculosis than mono-target antibodies reactive to the three respective antigens.
Omalizumab is a recombinant DNA-derived humanized IgG monoclonal antibody that suppresses allergic symptoms by binding to human IgE (14); thus, free IgE levels are reduced, preventing interactions between IgE and immune cells and decreasing the serum levels of inflammatory mediators (15). The detection of allergen-specific IgE is required for the diagnosis and management of IgE-mediated allergic disease. In the present study, two fusion allergens derived from the major allergenic HDM species Dermatophagoides farina (Der f), Der f 1 and Der f 2, were cloned and expressed. Subsequently, a novel capture IgE-ELISA was developed using a recombinant Der f 1/2 fusion protein (rDer f 1/2), which was designed to improve the sensitivity of HDM allergen-specific IgE detection. The capture ELISA method involved the coating of wells with omalizumab to enrich serum IgE and reduce interference from IgG. Preliminary experiments were conducted using the assay to determine its reliability for accurate anti-allergen detection and test the potential of rDer f 1/2 fusion protein-based ELISAs for the diagnosis of HDM-allergic disease.
Materials and methods
Serum samples, reagents and antibodies
All serum samples from HDM-sensitive individuals (HDM-allergic sera) and non-allergic individuals (control sera) were provided by The First Affiliated Hospital of Guangzhou Medical College (Guangzhou, China) between March 2013 and July 2015. A total of 28 subjects (13 males and 15 females, 18–55 years old) were enrolled in the present study. The patients were subjected to a skin prick test using dust mite allergen extract, serum samples were separated by centrifugation at 500 × g for 15 min at room temperature for the detection of IgE using the ImmunoCAP allergen detection system (Phadia AB; Thermo Fisher Scientific, Inc., Waltham, MA, USA). IgE levels were defined as follows: Level 3, 3.5–17.5 IU/ml; level 4, 17.5–50.0 IU/ml; level 5, 50.0–100 IU/ml and level 6, >100 IU/ml. The clinicopathological characteristics of the patients are presented in Table I. A random cohort of 71 HDM-allergic serum samples (37 males and 34 females, 18–55 years old) were used to determine the sensitivity of capture IgE-ELISA and indirect IgE-ELISA. In total, 20 non-allergic individuals (8 males and 12 females, 18–55 years old) were used as negative controls. Ethical approval was obtained from The First Affiliated Hospital of Guangzhou Medical College and patients provided informed consent.
Table I.
Clinicopathological factors of the patients.
| Patient no. | Gender/age | ImmunoCAP HDM-specific IgE testa | Clinical history |
|---|---|---|---|
| 1 | F/45 | 3+ | AD |
| 2 | M/32 | 3+ | AR |
| 3 | F/18 | 3+ | – |
| 4 | F/50 | 3+ | AD |
| 5 | M/53 | 3+ | BA |
| 6 | M/34 | 3+ | – |
| 7 | F/31 | 3+ | BA |
| 8 | F/55 | 4+ | AR + BA |
| 9 | M/42 | 4+ | AR |
| 10 | F/24 | 4+ | AD |
| 11 | F/37 | 4+ | BA |
| 12 | F/52 | 4+ | BA |
| 13 | M/18 | 4+ | – |
| 14 | M/29 | 4+ | AR |
| 15 | F/35 | 5+ | – |
| 16 | M/37 | 5+ | AD |
| 17 | F/28 | 5+ | AR |
| 18 | M/53 | 5+ | AD + AR |
| 19 | F/48 | 5+ | AR |
| 20 | F/21 | 5+ | AD |
| 21 | M/46 | 5+ | BA |
| 22 | F/33 | 6+ | −b |
| 23 | M/41 | 6+ | AR |
| 24 | F/54 | 6+ | AD + BA |
| 25 | M/34 | 6+ | AR |
| 26 | M/21 | 6+ | AD |
| 27 | F/44 | 6+ | BA |
| 28 | F/27 | 6+ | AD + AR |
IgE levels were determined by the ImmunoCAP system with Dermatophagoides farina antigen.
-, data not shown. IgE levels were defined as follows: Level 3, 3.5–17.5 IU/ml; level 4, 17.5–50.0 IU/ml; level 5, 50.0–100 IU/ml and level 6, >100 IU/ml. AR, allergic rhinitis; AD, atopic dermatitis; BA, bronchial asthma; HDM, house dust mite; Ig, immunoglobulin.
The restriction enzymes XhoI, NdeI and pET28 plasmid were purchased from Takara Biotechnology Co., Ltd. (Dalian, China). E. coli BL21(DE3)plysS cells were purchased from Invitrogen (Thermo Fisher Scientific, Inc.). Isopropyl-β-D-thiogalactopyranoside (IPTG) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). A Pierce™ 3′-diaminobenzidine (DAB) kit was purchased from Pierce (Thermo Fisher Scientific, Inc.). Biotinylated mouse anti-human IgE antibody (cat. no. 9160-08), streptavidin-labeled horseradish peroxidase (HRP; cat. no. 7100-05) and mouse anti-human IgE HRP-labeled antibody (cat. no. 9160-05) were purchased from SouthernBiotech (Birmingham, AL, USA).
Construction of the rDer f 1/2 fusion expression vector
The pET28-Der f 1 and pET28-Der f 2 (Takara Biotechnology Co., Ltd.) expression vectors were constructed as previously described (16,17). Overlapping polymerase chain reactions were performed to splice the Der f 1 and Der f 2 genes. The construction of pET28-rDer f 1/2 plasmid was confirmed by DNA Sanger sequencing (Sangon Biotech Co., Ltd., Shanghai, China). The pET28-Der f 1/2 expression vector was engineered using NdeI and XhoI restriction sites. The fusion sequence encoding Der f 1/2 was reported in GenBank (accession no. MF074325.1; http://www.ncbi.nlm.nih.gov/nuccore/MF074325.1). Der f 1 amino acid residues 19–321 were linked to Der f 2 residues 18–146 via a GGGSS linker. The resultant recombinant protein contained a hexahistidine (6×His) tag in its C-terminus.
Expression and purification of rDer f 1, rDer f 2 and rDer f 1/2 proteins
In total, 5 µl recombinant plasmid pET28-Der f 1/2 was transformed into 50 µl Escherichia coli BL21(DE3) pLysS cells using heat shock method (18). Cells were inoculated in Luria-Bertani medium (Merck KGaA, Darmstadt, Germany) containing 0.01 mg/ml kanamycin at 37°C for overnight. IPTG (1 mM)-induced expression was observed following growth for 3 h at 37°C, at which time cells were harvested by centrifugation at 8,000 × g for 2 min at 4°C. Pellets were resuspended in buffer (20 mM Tris-HCl, 150 mM NaCl, pH 8.0) and the cells were lysed by ultrasonic homogenization. The supernatant and precipitate were collected and analyzed via SDS-PAGE (described below). Gels were stained with Coomassie G-250 Brilliant Blue for protein analysis as previously described (19). rDer f 1, rDer f 2 and rDer f 1/2 in inclusion body fractions were solubilized with 6 M guanidine hydrochloride in 100 mM Tris (pH 8.0) for 2 h at room temperature (20). The proteins were purified by nickel affinity chromatography (Ni Sepharose 6 Fast Flow; cat. no. 17531801; GE Healthcare, Chicago, IL, USA) under denaturing conditions as previously described (21). Inclusion body surfaces frequently contain DNA, endotoxins and heteroproteins; washing with low concentrations of denaturing agent reduces impurities, thereby improving inclusion body protein purity (22). Deagglomeration of inclusion bodies into free loose structures with high concentrations of guanidine hydrochloride enables the material to be stored in buffer in a soluble state. The solution was treated with 20 mM β-mercaptoethanol for 30 min prior to diluting renaturation. Denatured proteins were refolded and diluted 10-fold with 0.5 M L-arginine (pH 8.0) (23). Arginine buffer was added to the denatured protein solution slowly (flow rate of 0.5 ml/min) and incubated overnight at 4°C. The solution was then dialyzed for 24 h using buffer containing 20 mM Tris and 150 mM NaCl. Following renaturation, protein concentrations were determined by the Bradford method.
IgE-western blotting and IgE-dot blotting
In total, 20 µg of rDer f 1/2 fusion protein was loaded in each well. Proteins were separated by 12% SDS-PAGE and then transferred to polyvinylidene difluoride membranes for western blotting. For dot blotting, 2 µl of the allergens (rDer f 1, rDer f 2 and rDer f 1/2) was separately spotted onto nitrocellulose membranes at a concentration of 1 µg/µl. The membranes were blocked with 5% Difco™ Skim Milk (DSM; BD Biosciences, San Jose, CA, USA) diluted in TBS containing 0.05% Tween 20 (TBST) at 4°C overnight. Serum samples from 15 HDM-allergic patients and 15 control subjects were also analyzed and incubated for 2 h at 37°C. Subsequently, mouse anti-human IgE Fc-HRP antibody was applied (1:2,000 in TBST with 1% DSM) for 1 h at 37°C. Antigen-antibody complexes on membranes were visualized using a Pierce™ DAB kit.
Preparation of biotinylated Der f 1/2 fusion protein
rDer f 1/2 contained a 6×His tag attached to its C-terminus. It was biotinylated using EZ-Link Sulfo-NHS-Biotin reagents (Thermo Fisher Scientific, Inc.), which enable simple and efficient molecular labeling (24). Fusion protein dissolved in PBS (pH 7.2) was incubated with 10 mM biotin reagent solution at room temperature for 30 min. Upon demonstration of protein labeling, the labeled fusion protein was purified by desalting column (HiTrap Desalting; GE Healthcare) in preparation for capture ELISA.
Indirect and capture IgE-ELISA
The IgE binding activities of recombinant proteins were detected by indirect and capture IgE-ELISAs. For indirect IgE-ELISA, 96-well plates were coated with recombinant antigen (100 ng/well, diluted in carbonate buffer, pH 9.6) at 4°C overnight. The plates were then blocked with 5% (w/v) DSM in PBS containing 0.05% Tween 20 (PBST) for 3 h at 37°C. Following washing, the plates were incubated with serum from HDM-allergic patients (1:5) for 2 h at 37°C, followed by incubation with mouse anti-human IgE biotin-labeled (1:2,000) for 1.5 h at 37°C. The plates were washed and subsequently incubated with streptavidin-HRP (1:4,000) for 30 min at 37°C. The plates were washed with PBST. Bound biotinylated-labeled antibody was detected by adding 100 µl of 3,3′,5,5′-tetramethylbenzidine (TMB; 1 mM); the reaction was stopped with 50 µl of H2SO4 (2 M). For capture IgE-ELISA, wells were coated with omalizumab (500 ng/well) overnight at 4°C. The wells were blocked with 5% DSM for 3 h at 37°C and HDM-allergic human serum (1:5) was subsequently added to the wells prior to incubation for 2 h at 37°C. To select an optimal concentration, a standard serial dilution (50, 100, 200 and 400 ng/well) of biotinylated rDer f 1/2 was initially employed for 1 h at 37°C; 50 ng/well was selected for subsequent experiments. Each protein solution was incubated in wells with streptavidin-HRP for 0.5 h at 37°C, prior to addition of TMB. The TMB reaction was conducted for 10 min at 37°C prior to the application of H2SO4. For indirect and capture IgE-ELISAs, the absorbance was measured at 450 nm by a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The experimental data were presented as the mean ± standard error of the mean. Data were analyzed using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA). Differences between groups were determined by analysis of one-way variance followed by Dunnett's t-test for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Linear IgE B cell epitopes of Der f 1 and Der f 2
B cell epitopes are antigenic regions in proteins recognized by B cells and are considered to be important for the diagnosis of allergies via the serum (25). The literature was investigated to identify linear IgE B cell epitopes in Der f 1 and Der f 2; it was revealed that Der f 1 (GenBank accession no. EF139428.1) and Der f 2 (GenBank accession no. FJ436110) separately possess three B cell epitopes. The amino acid sequences of the epitopes in Der f 1 and Der f 2 (26–28) are presented in Fig. 1. It was hypothesized that Der f 1/2, a fusion protein comprising these B cell epitopes, would bind with a broader range of specific IgEs, rendering it suitable for the diagnosis of HDM allergy.
Figure 1.

Reported linear B cell epitopes located in Der f 1 and Der f 2. Boxes indicate the locations of epitopes in (A) Der f 1 (GenBank accession no. MF074325.1) and (B) Der f 2 (GenBank accession no. FJ436110). The epitope peptide sequences for Der f 1 were 113LDLRSLRTVTPIRMQGGCG131, 151NTSLDLSEQELVDCASQHGC170 and 216CQIYPPDVKQIREALTQ232, whereas those for Der f 2 were 18DQVDVKDCANNEIKKVMVDGCHGS41, 42DPCII46 and 74GIDTNACHYIKCPLVKGQQYDIKY97. Der f, allergen from Dermatophagoides farina.
Expression, purification and IgE binding activity of rDer f 1/2
A schematic diagram of the rDer f 1/2 construct is presented in Fig. 2A. The rDer f 1/2 protein was expressed in the form of inclusion bodies, solubilized with guanidine hydrochloride and identified by SDS-PAGE (Fig. 2B). Purification of rDer f 1/2 by nickel affinity chromatography resulted in the presence of a single band migrating at a theoretical molecular weight of 46 kDa (Fig. 2C). IgE western blot analysis revealed that rDer f 1/2 bound to IgEs when incubated with serum samples from 15 patients with diagnosed HDM allergies, but did not bind to IgEs in sera obtained from the 15 non-allergic control subjects (Fig. 2D). These results indicated that rDer f 1/2 exhibited a selective, strong binding affinity for IgE in HDM-allergic sera.
Figure 2.
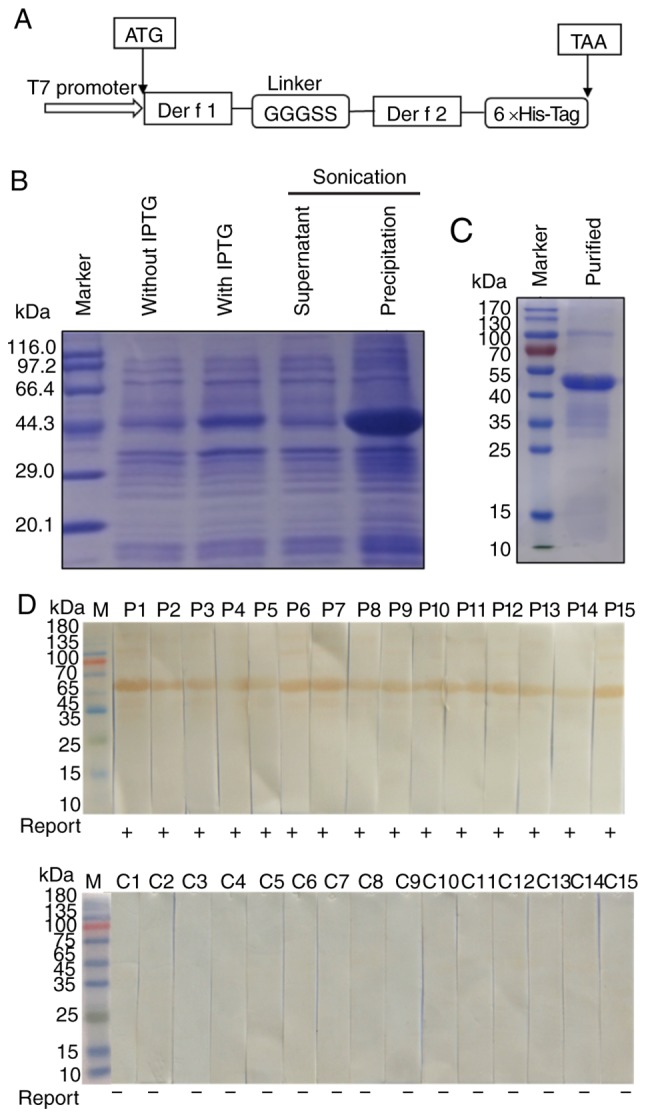
Expression, purification and IgE binding activity of rDer f 1/2. (A) Schematic diagram of the Der f 1/2 fusion protein construct. (B) SDS-PAGE of rDer f 1/2 following expression in Escherichia coli BL21(DE3) pLysS. (C) SDS-PAGE of rDer f 1/2 following purification by nickel affinity chromatography. Western blotting was conducted to determine the binding of rDer f 1/2 to specific IgE in the sera from (D) 15 house dust mite-allergic patients or (C) 15 non-allergic controls. 6×His-Tag, hexahistidine tag; Der f, allergen from Dermatophagoides farina; Ig, immunoglobulin; IPTG, isopropyl-β-D-thiogalactopyranoside; r, recombinant.
Analysis of the binding of specific IgE to rDer f 1, rDer f 2 and rDer f 1/2
SDS-PAGE of purified E. coli-expressed rDer f 1, rDer f 2 and rDer f 1/2 indicated that rDer f 1 and rDer f 2 possessed molecular weights of ~32 and 15 kDa, respectively (Fig. 3A). In a previous study, 6×His-tagged antibody assay revealed two bands for Der f 1, which may be due to minor differences in molecular weight or structural differences during the expression process, potentially occurring at the site of dissolution and degradation (16). Non-denaturing PAGE (without SDS or β-mercaptoethanol) indicated that rDer f 2 forms a dimer (data not shown) (29). Indirect ELISAs, in which the three recombinant proteins were incubated separately with HDM-allergic (n=28) and control sera (n=10), yielded mean optical density (OD) values of 1.293±0.241 for Der f 1, 1.377±0.26 for Der f 2 and 1.795±0.31 for Der f 1/2 (Fig. 3B). Using a cutoff OD value of 0.7, the reported IgE-positive rates for rDer f 1, rDer f 2 and rDer f 1/2 were 21/28 (75.0%), 22/28 (78.6%) and 24/28 (85.8%), respectively (Table II). All OD values for ELISAs performed with Der f 1/2 were increased compared with those of rDer f 1 or rDer f 2 when using HDM-allergic sera (Fig. 3C). A dot-blot assay revealed a stronger signal for rDer f 1/2 than rDer f 1, or rDer f 2 following incubation with an HDM-allergic serum pool from 3 individual patients; negative results were observed for all three proteins when incubated with pooled control serum (Fig. 3D). Collectively, the results indicated that rDer f 1/2 exhibited improved binding to HDM allergen-specific IgEs compared with rDer f 1 or rDer f 2.
Figure 3.
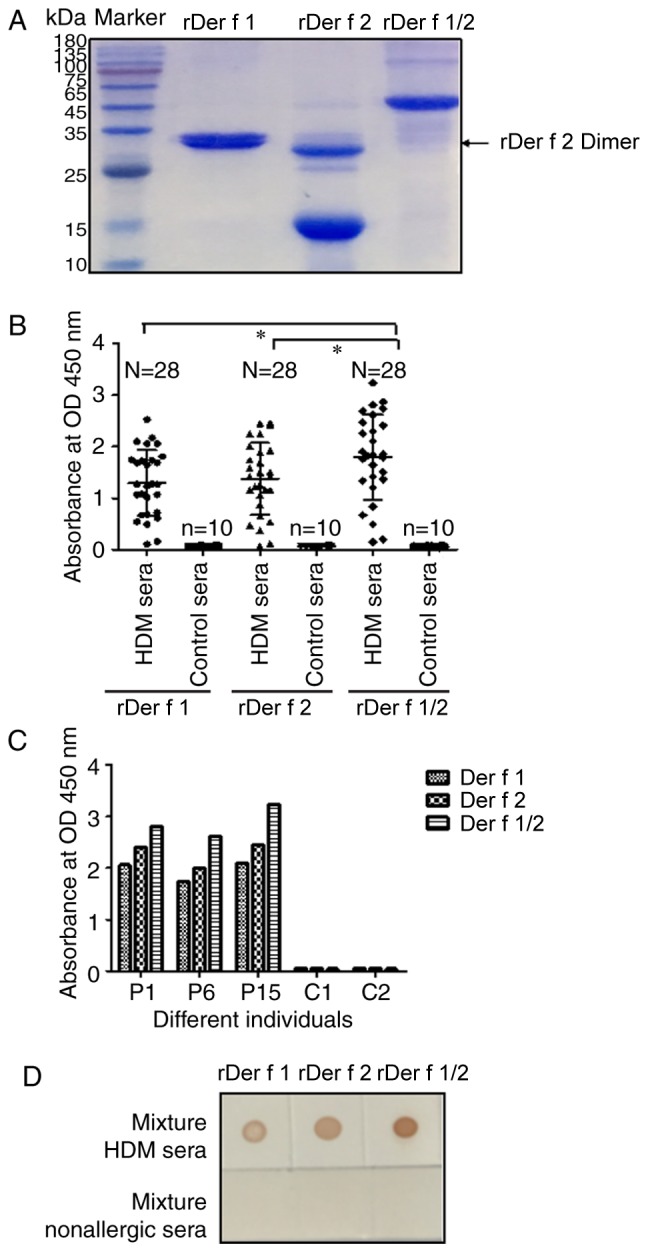
Analysis of the binding of specific IgE to rDer f 1, rDer f 2 and rDer f 1/2 in HDM sera. (A) SDS-PAGE of purified recombinant proteins. (B) Levels of binding of rDer f 1, rDer f 2 and rDer f 1/2 to IgE in 28 HDM and 10 control sera as determined by indirect ELISA. (C) Binding of rDer f 1, rDer f 2, and rDer f 1/2 to specific IgE in sera from individuals with HDM allergies (P1, P6 and P15) and non-allergic controls (C1 and C2) as determined by indirect ELISA. (D) Recombinant proteins were dotted onto nitrocellulose strips and incubated with mixed sera from 3 HDM-allergic patients or 3 non-allergic individuals. Data are presented as the mean ± standard error of the mean. *P<0.05. Control sera, sera from non-allergic control individuals; Der f, allergen from Dermatophagoides farina; HDM, house dust mite; HDM sera, sera from patients with HDM allergies; Ig, immunoglobulin; OD, optical density; r, recombinant.
Table II.
Positive rate of IgE-ELISA for sera from patients with HDM-allergic disease as determined using rDer f 1, rDer f 2 and rDer f 1/2.
| Allergen | Positive rate [cutoff (P/N>2.1)] (%) | Positive rate (cutoff=0.7)a (%) |
|---|---|---|
| Der f 1 | 27/28 (96.4) | 21/28 (75.0) |
| Der f 2 | 27/28 (96.4) | 22/28 (78.6) |
| Der f 1/2 | 28/28 (100) | 24/28 (85.8) |
Artificial value for comparison. P/N, ratio of the optical density values of the positive samples (allergic patients) compared with the negative samples (healthy controls). Der f, allergen from Dermatophagoides farina; HDM, house dust mite; Ig, immunoglobulin; r, recombinant.
Enhanced sensitivity of capture IgE-ELISA based on rDerf1/2
Schematic diagrams of the capture and indirect IgE-ELISA methods employed for the detection of HDM-specific IgEs are presented in Fig. 4A and B, respectively. A preliminary assay performed to optimize the rDer f 1/2-biotin conjugate concentration used during the assay revealed that 0.5 µg/ml was optimal for capture IgE-ELISA, based upon the relative OD values following incubation with HDM-allergic and control sera (Fig. 5A).
Figure 4.
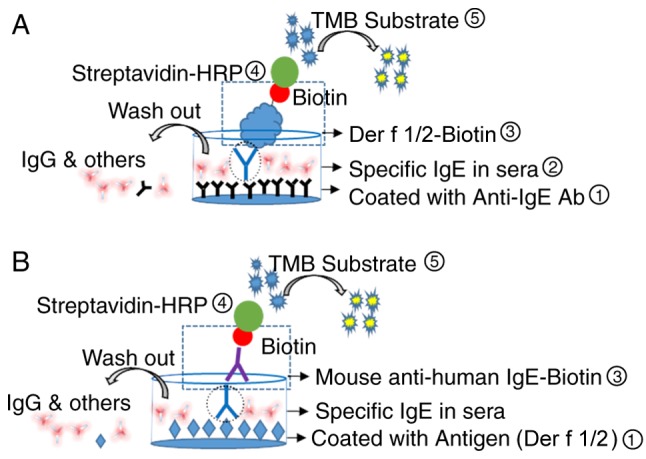
Schematic diagrams of direct capture IgE-ELISA and indirect IgE-ELISA. (A) Direct capture and (B) indirect IgE-ELISAs for the detection of specific IgEs in HDM-allergic sera. For capture IgE-ELISA, 96-well plates were coated with anti-IgE Ab (omalizumab), blocked and incubated with HDM-allergic sera containing HDM-specific IgE, prior to incubations with rDer f 1/2-Biotin and streptavidin-HRP. For indirect IgE-ELISA, plates were coated with rDer f 1/2, blocked and incubated with HDM-allergic sera, prior to incubations with biotinylated mouse anti-human IgE and streptavidin-HRP. For the two methods, TMB substrate was subsequently added; the reaction was terminated following the addition of 2 M H2SO4. Ab, antibody; Der f, allergen from Dermatophagoides farina; Der f 1/2-biotin, biotinylated Der f 1/2 protein; HDM, house dust mite; HDM-allergic sera, sera from patients with HDM allergies; HRP, horseradish peroxidase; Ig, immunoglobulin; r, recombinant; TMB, 3,3′,5,5′-tetramethylbenzidine.
Figure 5.
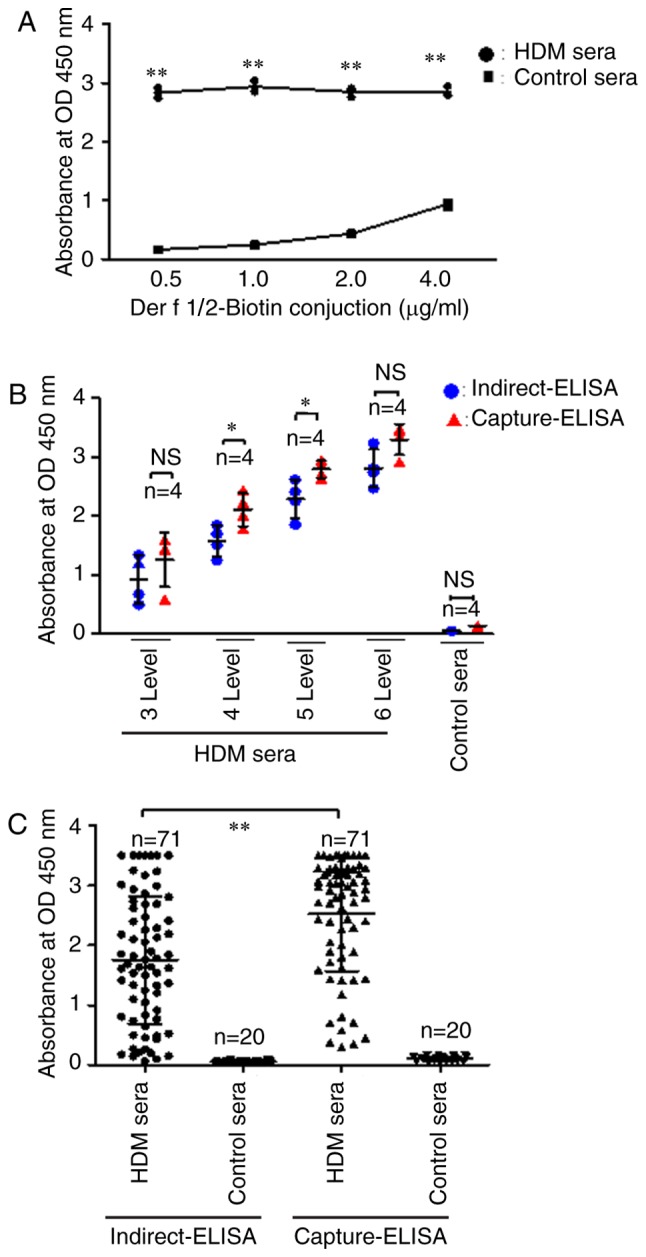
Capture-ELISA detection of specific IgEs in HDM sera. (A) Identification of optimal Der f1/2-biotin conjugate concentration for capture ELISA. (B) Sensitivity of indirect and capture ELISAs in the detection of allergen-specific IgE in HDM serum samples with varying levels of IgE as determined by ImmunoCAP. IgE levels were defined as follows: Level 3, 3.5–17.5 IU/ml; level 4, 17.5–50.0 IU/ml; level 5, 50.0–100 IU/ml and level 6, >100 IU/ml. (C) Sensitivity of indirect and capture ELISAs in the detection of allergen-specific IgE in a random cohort of HDM serum samples (n=71). Data are presented as the mean ± standard error of the mean. *P<0.05, **P<0.01. Control sera, sera from non-allergic control individuals; Der f, allergen from Dermatophagoides farina; Der f 1/2-biotin, biotinylated Der f 1/2 protein; HDM, house dust mite; HDM sera, sera from patients with HDM allergies; Ig, immunoglobulin; OD, optical density.
Subsequently, the relative sensitivity of rDer f 1/2-based capture IgE-ELISA compared with indirect IgE-ELISA for the detection of specific IgEs was determined using HDM-allergic serum samples containing various levels of IgE (as defined by ImmunoCAP). Significantly increased OD values were reported following capture IgE-ELISA compared with indirect IgE-ELISA using level 4 and 5 serum samples (P<0.05; Fig. 5B).
Finally, a random cohort of 71 HDM-allergic serum samples was used to determine the specific IgE detection reliability of the two ELISA methods. The mean OD value for rDer f 1/2-specific IgE detected by capture IgE-ELISA was significantly increased compared with indirect IgE-ELISA; indirect and capture ELISAs yielded mean OD values of 1.754±0.25 and 2.52±0.23, respectively (P<0.01; Fig. 5C). The P/N value was defined as the ratio of the optical density values of positive samples compared with the negative samples. The positive rates for specific IgE detection in the random HDM-allergic serum cohort were 68/71 (95.8%) for indirect IgE-ELISA and 71/71 (100%) for capture IgE-ELISA when the cutoff was set to P/N value >2.1 (Table III). Collectively, the results suggested that capture IgE-ELISA based on rDer f 1/2 protein exhibits good sensitivity for HDM allergen-specific IgEs.
Table III.
Positive rate of IgE-ELISA for sera from patients with HDM-allergic disease as determined using indirect-ELISA and capture-ELISA.
| Methods | Positive rate [cutoff (P/N>2.1)] (%) | Positive rate (cutoff=0.7)a (%) |
|---|---|---|
| Indirect ELISA | 68/71 (95.8) | 56/71 (78.9) |
| Capture ELISA | 71/71 (100) | 66/71 (93.0) |
Artificial value for comparison. P/N, ratio of the optical density values of the positive samples compared with the negative samples. HDM, house dust mite; Ig, immunoglobulin.
Discussion
The sensitivity of indirect ELISA for the detection of allergen-specific IgE is decreased by competition from high-titer IgG, reducing the accuracy of indirect ELISAs in determining the levels of allergen-specific IgE in the sera of patients (30). To overcome this limitation, capture ELISA was employed, using anti-IgE antibodies to capture total IgE antibodies in sera, thereby enhancing sensitivity for the detection of allergen-specific IgEs (31). In a random HDM-allergic serum cohort, positive rDer f 1/2-specific IgE detection rates of 68/71 (95.8%) and 71/71 (100%) were reported with indirect and capture ELISA, respectively, demonstrating the increased sensitivity of capture IgE-ELISA for allergen-specific IgE detection compared with indirect IgE-ELISA.
The sensitivity of the capture ELISA method reported in the present study was enhanced by the high efficiency of the rDer f 1/2 fusion protein in detecting IgE. Antigen coatings produced from HDM extract mixtures exhibit low sensitivity due to the inclusion of very small amounts of effective allergen components. Compared with natural antigen extraction, recombinant antigens possess the benefits of high antigenic consistency and low risk of contamination by impurities (32). Recombinant allergens mimic the properties of natural allergens or modified variants; however, they can be manipulated to improve safety or efficacy (33).
It was previously reported that Der f 1- and Der f 2-specific IgEs were detected in 86.2 and 89.4% of patients with HDM-sensitive respiratory allergy, respectively, whereas IgEs that bound to Der f 1 and Der f 2 were detected in 92.3% of the patient population (33). Multi-allergen fusion proteins produced by genetic engineering are a novel strategy for the diagnosis or treatment of allergies.
Allergen fusion proteins enable various allergen components to be combined into a single molecule, thereby simplifying molecular diagnostics. The development of a recombinant molecule comprising four major dog allergens for the diagnosis and vaccination of patients with dog allergies has been reported (34). Additionally, the two major allergens associated with Japanese cedar pollen were expressed as a fusion protein and conjugated to polyethylene glycol to improve solubility and create a safer vaccine (35). These advancements promote the replacement of cruder allergen extract mixtures with recombinant allergen proteins for the diagnosis of allergic diseases.
The findings of the present study require further validation with increased sample sizes. Additionally, the efficacies of rDer f 1/2 and the capture IgE-ELISA method were only investigated compared with the fusion proteins Der f 1 and Der f 2, antigens from two highly dominant HDM allergen groups. The high sensitivity of the capture IgE-ELISA method reported in the present study indicated that it may be particularly useful for identifying minor allergens, such as HDM allergens from groups beyond groups 1 and 2. The sensitivity of this method for the detection of minor allergens requires further investigation.
In conclusion, rDer f 1/2 was successfully expressed and purified, and was demonstrated to exhibit high IgE-binding activity in HDM-allergic sera. The novel capture IgE-ELISA based upon the biotinylation of Der f 1/2 exhibited increased efficacy for the detection of HDM allergen-specific IgEs compared with indirect IgE-ELISA. Thus, it was demonstrated to be a reliable method for the accurate detection of specific anti-allergen IgEs in allergic sera. Further studies employing this method may aid to improve the diagnosis of HDM-induced allergic disease and the development of anti-allergy vaccines.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- Der f 1
Group 1 allergen of Dermatophagoides farina
- Der f 2
Group 2 allergen of Dermatophagoides farina
- rDer f 1/2
recombinant Der f 1 and Der f 2 fusion protein
- DSM
Difco skim milk
- HDM
house dust mite
- HDM sera/HDM-allergic sera
sera from HDM-sensitive individuals
- control sera
sera from non-allergic individuals
- HRP
horseradish peroxidase
- IPTG
isopropyl-β-D-thiogalactopyranoside
- PBST
phosphate-buffered saline containing 0.05% Tween 20
- TBST
Tris buffered saline containing 0.05% Tween 20
Funding
The present study was supported in part by research funding from the National Natural Science Foundation of China (grant no. 81571570), Guangdong Province (grant nos. 2014A030313563, 2016A020215176, 2016A030313039 and 2017A010105014), and Shenzhen City (grant no. JCYJ20150626141652681 and 2016 Biochemistry Discipline Construction).
Availability of data and materials
All data generated or analyzed in the present study are available from the corresponding author upon reasonable request.
Authors' contributions
ZZ performed the experiments and drafted the manuscript. ZC, YiH, JH, and YoH participated in the experiments. JC and KJ made substantial contributions to the design of the present study and wrote the manuscript. All the authors reviewed and approved the final version of the manuscript.
Ethics approval and consent to participate
Permission to conduct the present study was obtained from the Ethics Committee of The First Affiliated Hospital of Guangzhou Medical College (Guangzhou, China). All procedures involving human participants were in accordance with the ethical standards of the committee. Informed consent was obtained from all participants.
Patient consent for publication
All procedures involving human participants were in accordance with the ethical standards of the committee.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chan TF, Ji KM, Yim AK, Liu XY, Zhou JW, Li RQ, Yang KY, Li J, Li M, Law PT, et al. The draft genome, transcriptome, and microbiome of Dermatophagoides farinae reveal a broad spectrum of dust mite allergens. J Allergy Clin Immunol. 2015;135:539–548. doi: 10.1016/j.jaci.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 2.Elkady A. Allergy to Dermatophagoides pteronyssinus (Der p1) and Dermatophagoides farina (Der f1) in patients with atopic asthma. Int J Sci Res. 2015;4:1896–1902. [Google Scholar]
- 3.An S, Chen L, Long C, Liu X, Xu X, Lu X, Rong M, Liu Z, Lai R. Dermatophagoides farinae allergens diversity identification by proteomics. Mol Cell Proteomics. 2013;12:1818–1828. doi: 10.1074/mcp.M112.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang ML, Cui C, Liu YH, Pei LC, Shao B. Analysis of total immunoglobulin E and specific immunoglobulin E of 3,721 patients with allergic disease. Biomed Rep. 2015;3:573–577. doi: 10.3892/br.2015.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams P, Sewell WA, Pumphrey R, Read G, Jolles S. Clinical immunology review series: An approach to the use of the immunology laboratory in the diagnosis of clinical allergy. Clin Exp Immunol. 2010;153:10–18. doi: 10.1111/j.1365-2249.2008.03695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platts-Mills TA, Snajdr MJ, Ishizaka K, Frankland AW. Measurement of IgE antibody by an antigen-binding assay: Correlation with PK activity and IgG and IgA antibodies to allergens. J Immunol. 1978;120:1201–1210. [PubMed] [Google Scholar]
- 7.De Amici M, Ciprandi G. The age impact on serum total and allergen-specific IgE. Allergy Asthma Immunol Res. 2013;5:170–174. doi: 10.4168/aair.2013.5.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honigberg BM. Trichomonads parasitic in humans. J Parasitol. 1991;10 [Google Scholar]
- 9.Jeong KY, Hongb CS, Yong TS. Recombinant allergens for diagnosis and immunotherapy of allergic disorders, with emphasis on cockroach allergy. Curr Protein Pept Sci. 2006;7:57–71. doi: 10.2174/138920306775474112. [DOI] [PubMed] [Google Scholar]
- 10.Howard R, Belgrave D, Papastamoulis P, Simpson A, Rattray M, Custovic A. Evolution of IgE responses to multiple allergen components throughout childhood. J Allergy Clin Immunol. 2018;142:1322–1330. doi: 10.1016/j.jaci.2017.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mas S, Boissy P, Monsalve RI, Cuesta-Herranz J, Díaz-Perales A, Fernández J, Colás C, Rodríguez R, Barderas R, Villalba M. A recombinant Sal k 1 isoform as an alternative to the polymorphic allergen from Salsola kali pollen for allergy diagnosis. Int Arch Allergy Immunol. 2015;167:83–93. doi: 10.1159/000434680. [DOI] [PubMed] [Google Scholar]
- 12.He J, Xiu B, Wang G, Chen K, Feng X, Song X, Zhu C, Yang X, Bai G, Ling S, Zhang H. Construction, expression, purification and biotin labeling of a single recombinant multi-epitope antigen for double-antigen sandwich ELISA to detect hepatitis C virus antibody. Protein Pept Lett. 2011;18:839–847. doi: 10.2174/092986611795714014. [DOI] [PubMed] [Google Scholar]
- 13.Dai Z, Liu Z, Xiu B, Yang X, Zhao P, Zhang X, Duan C, Que H, Zhang H, Feng X. A multiple-antigen detection assay for tuberculosis diagnosis based on broadly reactive polyclonal antibodies. Iran J Basic Med Sci. 2017;20:360–367. doi: 10.22038/IJBMS.2017.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babu KS, Polosa R, Morjaria JB. Anti-IgE-emerging opportunities for Omalizumab. Expert Opin Biol Ther. 2013;13:765–777. doi: 10.1517/14712598.2013.782391. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi N, Tsukamoto Y, Sallas WM, Lowe PJ. A mechanism-based binding model for the population pharmacokinetics and pharmacodynamics of omalizumab. Br J Clin Pharmacol. 2007;63:548–561. doi: 10.1111/j.1365-2125.2006.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai Y, Ji K, Liu Z, Cai C. Polymorphic analysis of gene encoding Der f 1 allergen and identification on the bioactivity of its prokaryotic expression products. Chin J Zoonoses. 2007;23:156–160. (In Chinese) [Google Scholar]
- 17.Zhu JQ, Liu ZG, Gao B, Ji KM, Xing M. Cloning, expression, purification, and identification of Der f II gene and its immunological characteristics. Immunol J. 2006;24:213–216. (In Chinese) [PubMed] [Google Scholar]
- 18.Froger A, Hall JE. Transformation of plasmid DNA into E. coli using the heat shock method. J Visual Exp. 2007;6:253. doi: 10.3791/253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2010;25:1327–1333. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee S, Weber M, Blatt K, Swoboda I, Focke-Tejkl M, Valent P, Valenta R, Vrtala S. Conversion of Der p 23, a new major house dust mite allergen, into a hypoallergenic vaccine. J Immunol. 2014;192:4867–4875. doi: 10.4049/jimmunol.1400064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asturias JA, Ibarrola I, Arilla MC, Vidal C, Ferrer A, Gamboa PM, Viñuela JE, Sanz ML, Andreu C, Martínez A. Engineering of major house dust mite allergens Der p 1 and Der p 2 for allergen-specific immunotherapy. Clin Exp Allergy. 2009;39:1088–1098. doi: 10.1111/j.1365-2222.2009.03264.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang Z, Zhang L, Zhang Y, Zhang T, Feng Y, Lu X, Lan W, Wang J, Wu H, Cao C, Wang X. Highly efficient production of soluble proteins from insoluble inclusion bodies by a two-step-denaturing and refolding method. PLoS One. 2011;6:e22981. doi: 10.1371/journal.pone.0022981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsumoto K, Umetsu M, Kumagai I, Ejima D, Philo JS, Arakawa T. Role of arginine in protein refolding, solubilization, and purification. Biotechnol Prog. 2004;20:1301–1308. doi: 10.1021/bp0498793. [DOI] [PubMed] [Google Scholar]
- 24.Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, Perez-Sanchez J, Boudreau D, Wang B, Dumas L, et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. 2013;19:1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gieras A, Linhart B, Roux KH, Dutta M, Khodoun M, Zafred D, Cabauatan CR, Lupinek C, Weber M, Focke-Tejkl M, et al. IgE epitope proximity determines immune complex shape and effector cell activation capacity. J Allergy Clin Immunol. 2016;137:1557–1565. doi: 10.1016/j.jaci.2015.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Halleux S, Stura E, VanderElst L, Carlier V, Jacquemin M, Saint-Remy JM. Three-dimensional structure and IgE-binding properties of mature fully active Der p 1, a clinically relevant major allergen. J Allergy Clin Immunol. 2006;117:571–576. doi: 10.1016/j.jaci.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 27.Jeannin P, Didierlaurent A, Gras-Masse H, Elass AA, Delneste Y, Cardot E, Joseph M, Tartar A, Vergoten G, Pestel J. Specific histamine release capacity of peptides selected from the modelized Der p I protein, a major allergen of Dermatophagoides pteronyssinus. Mol Immunol. 1992;29:739–749. doi: 10.1016/0161-5890(92)90184-Y. [DOI] [PubMed] [Google Scholar]
- 28.Takai T, Yuuki T, Okumura Y, Mori A, Okudaira H. Determination of the N- and C-terminal sequences required to bind human IgE of the major house dust mite allergen Der f 2 and epitope mapping for monoclonal antibodies. Mol Immunol. 1997;34:255–261. doi: 10.1016/S0161-5890(97)00020-5. [DOI] [PubMed] [Google Scholar]
- 29.Takeda A, Wu JJ, Maizel AL. Evidence for monomeric and dimeric forms of CD45 associated with a 30-kDa phosphorylated protein. J Biol Chem. 1992;267:16651–16659. [PubMed] [Google Scholar]
- 30.Nilsson OB, Neimert-Andersson T, Bronge M, Grundström J, Sarma R, Uchtenhagen H, Kikhney A, Sandalova T, Holmgren E, Svergun D, et al. Designing a multimer allergen for diagnosis and immunotherapy of dog allergic patients. PLoS One. 2014;9:e111041. doi: 10.1371/journal.pone.0111041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waritani T, Chang J, Mckinney B, Terato K. An ELISA protocol to improve the accuracy and reliability of serological antibody assays. MethodsX. 2017;4:153–165. doi: 10.1016/j.mex.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidal C, Lojo S, Juangorena M, Gonzalezquintela A. Association between asthma and sensitization to allergens of Dermatophagoides pteronyssinus. J Investig Allergol Clin Immunol. 2016;26:304–309. doi: 10.18176/jiaci.0048. [DOI] [PubMed] [Google Scholar]
- 33.Jeong KY, Lee JY, Son M, Yi MH, Yong TS, Shin JU, Lee KH, Kim YJ, Park KH, Park HJ, et al. Profiles of IgE sensitization to Der f 1, Der f 2, Der f 6, Der f 8, Der f 10, and Der f 20 in Korean house dust mite allergy patients. Allergy Asthma Immunol Res. 2015;7:483–488. doi: 10.4168/aair.2015.7.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodfolk JA. T-cell responses to allergens. J Allergy Clin Immunol. 2007;119:280–294. doi: 10.1016/j.jaci.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Little SA, Warner JO. Improved diagnosis of ABPA using gp66 (formerly antigen 7) of Aspergillus fumigatus for specific IgE detection. J Allergy Clin Immunol. 1996;98:55–63. doi: 10.1016/S0091-6749(96)70226-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in the present study are available from the corresponding author upon reasonable request.


