Abstract
B7 family members have been associated with the signaling transduction pathways underlying tumor immune evasion in hepatocellular carcinoma. In the present study, associations between the clinical characteristics of patients with hepatocellular carcinoma (HCC) and the expression of B7-H2 and B7-H3 were analyzed. A total of 63 formalin-fixed and paraffin-embedded HCC tissues were collected to be used as a tissue microarray. Following this, the association between B7-H2/B7-H3 and the prognosis of patients with HCC was analyzed using Pearson's χ2 test, the Kaplan-Meier method and receiver operating characteristic curve analysis. The results demonstrated that the expression of B7-H2 was significantly associated with recurrence (within 1 year) in patients with HCC (P<0.01), and that the expression of B7-H3 was associated with recurrence (within 1 year), metastasis and 2-year overall survival rate in patients with HCC (P<0.01, P=0.036 and P=0.016, respectively). In addition, the combined expression of B7-H2 and B7-H3 was associated with prognostic factors, including recurrence (within 1 year) and survival rate (within 2 years), in patients with HCC. In particular, an increased area under the curve was achieved when the combined expression of B7-H2 and B7-H3 was considered, compared with that for α-fetoprotein. Taken together, these results indicated that B7-H2- and/or B7-H3-positive expression indicates a poor clinical outcome for patients, and the combination of B7-H2 and B7-H3 may be a preferential prognostic biomarker in patients with HCC.
Keywords: B7-H2, B7-H3, immunohistochemistry, hepatocellular carcinoma, prognostic factor
Introduction
The incidence of hepatocellular carcinoma (HCC), which is the fifth most common cancer and the third most common cause of cancer-associated mortality worldwide, has been observed to have an increasing trend over the last decade. Although surgery is the most efficient therapeutic method at present (1,2), the 5-year survival rate of HCC remains <5% due to the high rates of recurrence and metastasis (3,4). Therefore, determination of the risks and prognostic biomarkers is required to tailor drug therapy and ultimately improve patient prognosis following surgical therapies.
It has been indicated that the recurrence and metastasis of tumors are associated with the tumor cells evading immune surveillance by T cells in HCC (5). In particular, B7 family molecules, as types of peripheral membrane proteins, can produce a co-stimulatory or co-inhibitory signal to enhance or decrease the activity of major histocompatibility complex-T cell receptor signaling (6–10), which may subsequently induce T cell dysfunction or apoptosis upon antigen recognition. Under pathological conditions, B7 family molecules mainly interact with the receptor on activating T cells, which provides subsequent signals to weaken or even terminate the T-cell immune response (11–14). Therefore, disorder in the co-stimulatory or co-inhibitory signaling pathway serves an important role in tumor immune evasion (5).
In previous decades, novel members of the B7 family, including B7-1, B7-2, B7-H1, B7-H2, B7-H3, B7-H4 and B7-DC, have been identified. Accumulating data have demonstrated that the abnormal expression of B7 molecules is associated with the development of HCC. In particular, Tu et al (15) determined that the upregulation of B7-H2 can lead to an immunosuppressive HCC microenvironment and result in unfavorable prognostic outcomes for patients with HCC. B7-H3 is reported to promote the aggression and invasion of HCC via the Janus kinase 2/signal transducer and activator of transcription 3/Slug signaling pathway (8). Therefore, B7-H2 and B7-H3 molecules may useful as biological markers for predicting the prognosis of patients with HCC in clinical practice (3).
As B7-H2 and B7-H3 are likely to exert synergistic effects on HCC, as they are from the same family of B7 molecules and have similar immune regulatory functions in HCC, it is considered necessary to evaluate the expression of B7-H2 and B7-H3 in the same HCC cohort to determine whether their combined expression provides enhanced sensitivity and specificity of the co-stimulatory molecules in predicting the prognosis of patients with HCC. In the present study, investigations were performed on the expression signatures of B7-H2 and B7-H3 in the same HCC tissue set. It was revealed that the combination of B7-H2 with B7-H3 may be a preferable prognostic biomarker for predicting recurrence and overall survival rates in patients with HCC.
Materials and methods
Antibodies and reagents
Mouse anti-human ICOS (B7-H2) ligand monoclonal antibody (cat. no. ab189052) and mouse anti-human CD276 (B7-H3) monoclonal antibody (cat. no. ab105922) were obtained from Abcam (Cambridge, UK). HRP-conjugated secondary antibodies (anti-mouse IgG; cat. no. HS201-01) were purchased from Transgen Biotech Co., Ltd. (Beijing, China).
Patients and tissue samples
A total of 63 formalin-fixed and paraffin-embedded HCC tissues from patients who underwent curative resection between December 2002 and December 2009 at the First Affiliated Hospital of Fujian Medical University (Fujian, China) were retrieved for immunohistochemical staining. All patients were diagnosed as negative for hepatitis C virus [HCV(−)].
The fresh HCC tissues and adjacent tissues were collected at the time of surgery from patients, and were formalin fixed and embedded for immunohistochemistry. Clinical information was collected retrospectively from the electronic record of the First Affiliated Hospital of Fujian Medical University. The clinical information collected for each patient included information on etiological factors, HCC recurrence and metastasis, mortality (and the cause of mortality) and prognostic clinicopathological characteristics, including tumor differentiation stage, presence of vascular invasion, the number of lesions, the largest tumor size and pre-surgical α-fetoprotein (AFP) levels. Written consent was obtained from all participants prior to surgery. The clinical and pathological diagnoses of patients with HCC met the diagnostic criteria of the American Association for the Study of Liver Diseases (16). The study was approved by the Ethics Committee of the Mengchao Hepatobiliary Hospital of Fujian Medical University (Fujian, China).
Construction of tissue microarrays (TMAs)
The HCC samples and the adjacent tissues were collected for the construction of TMAs. Two 1-mm cores were obtained from the tumorous region and two 1-mm cores were obtained from the corresponding adjacent tissue region via a manual tissue arrayer method. The regions of vital tumor and adjacent tissues were marked by experienced pathologists using a Hematoxylin and Eosin staining kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and the examination was performed using a routine light microscope (Zeiss AG, Oberkochen, Germany). Subsequently, the wax block (containing HCC tissues) was mixed together with a fresh wax block at 52°C, cooled at room temperature for 30 min and maintained at 4°C ready for further use. The cooled wax block was fixed on an auto tissue slicer and sliced into 15 sections, each section was 4-µm in thickness. The sections were then immersed in distilled water for 2 min, and were finally adhered to slides.
Immunohistochemical staining
Immunohistochemical staining of B7-H2 and B7-H3 was performed using a two-step Elivision plus staining technique. Briefly, the 4-µm sections on the TMAs were dried at 60°C for 24 h, de-paraffinized in xylene I for 10 min and de-paraffinized in xylene II for another 10 min, and then rehydrated in graded ethanol (100% ethanol for 5 min, 95% ethanol for 3 min, 80% ethanol for 3 min and 75% ethanol for 3 min). The slides were then incubated in 1 mol/l citric acid (OriGene Technologies, Inc., Beijing, China) for antigen retrieval under 111.6°C and 150 KPa for 3 min, and cooled to room temperature, followed by three washes with PBS (HyClone; GE Healthcare Life Sciences, Logan, UT, USA). Subsequently, the slides were incubated in 3% hydrogen peroxide (OriGene Technologies, Inc.) for 10 min to block endogenous peroxidase activity and then rehydrated in PBS. The slides were incubated with primary antibodies against B7-H2 (1:200 dilution) and B7-H3 (1:1,000 dilution) at 37°C for 1 h, rinsed several times with PBS, and incubated with intensifier (OriGene Technologies, Inc.) at 37°C for another 20 min. Following three rinses with PBS, the slides were incubated with the HRP-conjugated secondary antibodies (goat anti-mouse IgG, respectively) at 37°C for 30 min. Finally, the slides were subjected to diaminobenzidine coloration (OriGene Technologies, Inc.) for 5 min; following rinsing with distilled water for 3 min, restaining with hematoxylin was performed.
The results were assessed by two double-blinded pathologists independently. All tissues, at ×200 magnification, were manually scored using the following criteria: i) According to the proportion of positive cells (cytoplasm, cell membrane or nucleus dyed yellow, brown or sepia), scored as 0 (≤5%), 1 (6–25%), 2 (26–50%), 3 (51–75%) and 4 (≥76%); and ii) according to the intensity of staining, scored as 0 (blue), 1 (faint yellow), 2 (pale brown) and 3 (sepia). The final score was the score of the first criterion multiplied by that of the second criterion. A final score of 0–2 was considered as negative expression, whereas a score of 3–12 was considered as positive expression. If the result of a specimen contradicted between assessors, then a third pathologist made the final decision. The histopathological examination was performed using an orthotopic microscope (Zeiss AG, Oberkochen, Germany).
Statistical analysis
All statistical analyses were performed using SPSS software version 19.0 (IBM Corp., Armonk, NY, USA). Association analyses of clinicopathological factors was performed with Pearson's χ2 test. Survival curves were estimated using the Kaplan-Meier method, and differences in survival rates were detected using the log-rank test. Univariate and multivariate analyses were used to identify prognostic factors in the patients with HCC. Covariates with P<0.05 in univariate analysis were further analyzed by multivariate analysis. In all tests, P<0.05 was considered to indicate a statistically significant difference. Receiver operating characteristic (ROC) curves were used to compare the diagnostic accuracy of the test molecules as prognostic factors in patients with HCC.
Results
Expression of B7-H2 and B7-H3 in patients with HCC
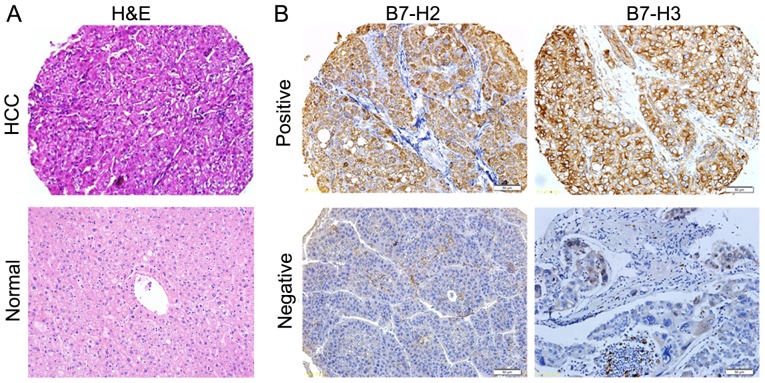
To determine the expression of B7-H2 and B7-H3 in patients with HCC, TMAs were constructed of 63 HCC specimens and matched adjacent tissues. As presented in Fig. 1, B7-H2 and B7-H3 were positively expressed in the HCC tissues. Considering the clinical history of the patients, the association between the expression of B7-H2 or B7-H3 and various clinical parameters was evaluated. As shown in Table I, 29/40 cases that recurred within 1 year were B7-H2+, whereas only 6/23 cases that recurred after >1 year were B7-H2+ (P<0.01); 39/40 cases that recurred within 1 year were B7-H3+, whereas only 14/23 cases that recurred after >1 year were B7-H3+ (P<0.01); 96.0% (24/25) of the patients presenting with metastasis were positive for B7-H3, whereas the positive expression rate for non-metastatic cases was 76.3% (29/38; P=0.036); and 94.3% (33/35) of the patients who had a survival time of <2 years were positive for B7-H3, whereas the positive expression rate in patients who had a survival time >2 years was 71.4% (20/28; P=0.016). These results suggested that the positive expression of B7-H2 was associated with recurrence within 1 year in patients with HCC, and that the positive expression of B7-H3 was associated with recurrence within 1 year, metastasis and a survival time of <2 years in patients with HCC.
Figure 1.
Representative expression of B7-H2 and B7-H3 in HCC tissues. (A) histopathological examination of HCC tissues using H&E staining (magnification, ×200). (B) The typical expression profiles of B7-H2 and B7-H3 were observed in HCC tissues using immunohistochemistry (magnification, ×200). HCC, hepatocellular carcinoma; H&E, hematoxylin and eosin.
Table I.
Associations between clinical parameters and the expression of B7-H2 and B7-H3 in patients with hepatocellular carcinoma.
| B7-H2 | B7-H3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical parameter | − | + | χ2-value | P-value | − | + | χ2-value | P-value |
| Sex | 1.210 | 0.078 | 0.627 | |||||
| Male | 23 | 32 | 9 | 46 | ||||
| Female | 5 | 3 | 1 | 7 | ||||
| Age (years) | 0.556 | 0.332 | 1.027 | 0.289 | ||||
| >60 | 5 | 9 | 1 | 13 | ||||
| ≤60 | 23 | 26 | 9 | 40 | ||||
| Size (cm) | 0.423 | 0.350 | 1.189 | 0.230 | ||||
| ≤5 | 11 | 11 | 5 | 17 | ||||
| >5 | 17 | 24 | 5 | 36 | ||||
| Differentiation | 0.944 | 0.624 | 2.772 | 0.153 | ||||
| Poor | 10 | 9 | 2 | 17 | ||||
| Well | 12 | 19 | 4 | 27 | ||||
| Moderate | 6 | 7 | 4 | 9 | ||||
| AFP (µg/l) | 0.213 | 0.437 | 1.310 | 0.238 | ||||
| <200 | 6 | 9 | 1 | 14 | ||||
| >200 | 22 | 25 | 9 | 38 | ||||
| Liver cirrhosis | 0.001 | 0.644 | 1.981 | 0.177 | ||||
| − | 4 | 5 | 0 | 9 | ||||
| + | 24 | 30 | 10 | 44 | ||||
| HBV infection | 1.210 | 0.344 | 0.078 | 0.627 | ||||
| − | 5 | 3 | 1 | 7 | ||||
| + | 23 | 32 | 9 | 46 | ||||
| TNM stage | 0.082 | 0.489 | 0.374 | 0.392 | ||||
| I–II | 17 | 20 | 5 | 32 | ||||
| III–IV | 11 | 15 | 5 | 21 | ||||
| Recurrence | 12.740 | 0.007b | 14.674 | <0.001b | ||||
| <1 year | 11 | 29 | 1 | 39 | ||||
| >1 year | 17 | 6 | 9 | 14 | ||||
| Metastasis | 0.004 | 0.582 | 2.594 | 0.036a | ||||
| − | 18 | 20 | 9 | 29 | ||||
| + | 10 | 15 | 1 | 24 | ||||
| Survival | 3.291 | 0.059 | 6.086 | 0.016a | ||||
| <2 year | 12 | 23 | 2 | 33 | ||||
| >2 year | 16 | 12 | 8 | 20 | ||||
Values in bold indicate significance.
P<0.05
P<0.01. AFP, α-fetoprotein; HBV, hepatitis B virus; TNM, tumor-node-metastasis.
Positive expression of B7-H2 and B7-H3 is associated with poor prognosis in HCC
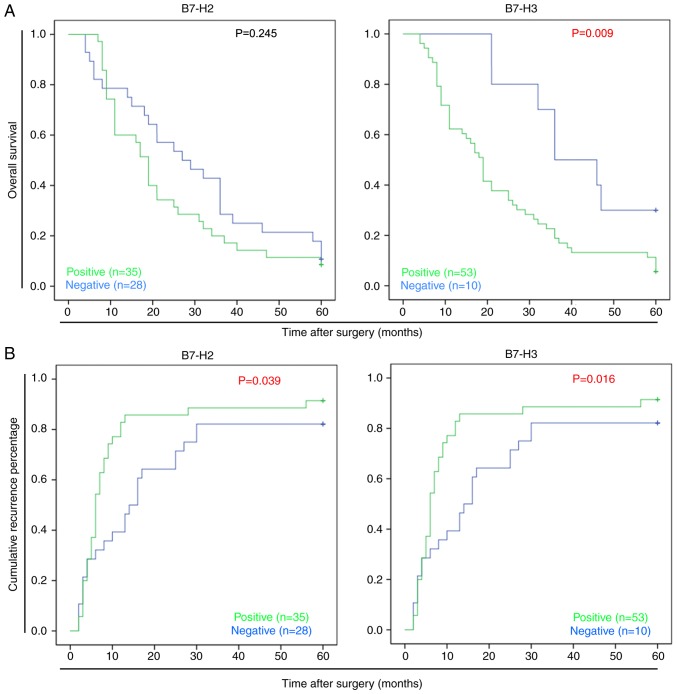
To evaluate the association between the expression of B7-H2 or B7-H3 and the prognosis of HCC, the expression data on B7-H2 and B7-H3 were analyzed further by considering disease-related survival events. As shown in Fig. 2A and B, a Kaplan-Meier plot indicated that the B7-H3− patients had significantly longer survival rates following surgery than the B7-H3+ patients (P=0.009); the median survival time of the B7-H3+ patients was 19.2 months compared with 37.3 months for the B7-H3− patients. In addition, it was identified that B7-H3 may promote HCC recurrence; the median disease-free survival time of the B7-H3+ patients was 5.9 months, compared with 18.1 months for the B7-H3− patients (P=0.016). Similar results were observed in the B7-H2+ patients, for whom the median disease-free survival time was 6.8 months, compared with 12.6 months for the B7-H2− patients (P=0.039). These data suggested that the positive expression of B7-H2 or B7-H3 was indicative of poor survival rates in patients with HCC.
Figure 2.
Associations of B7-H2 and B7-H3 with survival and recurrence rates. Kaplan-Meier analysis of the association between the expression of B7-H2 or B7-H3 and (A) overall survival rate and (B) cumulative recurrence in patients with hepatocellular carcinoma.
Positive expression of B7-H2 and B7-H3 is associated with extrahepatic metastasis and early recurrence/metastasis
To further determine the prognostic factors affected by B7-H2 and B7-H3, the associations between the expression of B7-H2 or B7-H3 and postoperative clinical features were assessed. As shown in Table II, extrahepatic metastasis was associated with positive expression of B7-H2 (P=0.041) and B7-H3 (P=0.036). Specifically, 25 patients exhibited HCC metastasis, 17 of whom exhibited pulmonary metastasis in B7-H3+ patients (68%). In addition, time of recurrence in patients with HCC was analyzed, and the expression of B7-H2 or B7-H3 was assessed in the 23 cases of later recurrence/metastasis (>12 months) and the 40 cases of early recurrence/metastasis (≤12 months). As shown in Table III, positive expression of B7-H2 was detected in 29 of the ≤12 months cases (29/40, 72.5%), and positive expression of B7-H3 was detected in 39 of the ≤12 months cases (39/40, 97.5%). Notably, lower expression rates of B7-H2 (26.09%, P=0.007) and B7-H3 (60.86%, P=0.001) were observed in the >12 months cases than in the ≤12 months cases. These results indicated that the positive expression of B7-H2 or B7-H3 was linked with susceptibility to extrahepatic metastasis and early recurrence/metastasis (within 1 year).
Table II.
Associations between postoperative clinical features and expression of B7-H2 and B7-H3 in patients with hepatocellular carcinoma.
| B7-H2 expression | B7-H3 expression | |||||
|---|---|---|---|---|---|---|
| Clinical feature | − | + | P-value | − | + | P-value |
| Recurring type | NS | NS | ||||
| Regional recurring | 6 | 6 | 2 | 10 | ||
| Distant recurring | 11 | 18 | 4 | 25 | ||
| Histological grade | NS | NS | ||||
| Well-differentiated | 5 | 6 | 3 | 8 | ||
| Moderately differentiated | 13 | 19 | 5 | 27 | ||
| Poorly differentiated | 10 | 10 | 2 | 18 | ||
| Extrahepatic metastasis | 0.041a | 0.036a | ||||
| Yes | 8 | 17 | 1 | 24 | ||
| No | 20 | 18 | 9 | 29 | ||
P<0.05. NS, not significant.
Table III.
Associations between the time of postoperative R/M and the expression of B7-H2 and B7-H3 in patients with hepatocellular carcinoma.
| B7-H2 expression | B7-H3 expression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R/M time | N | − | + | Positive expression rate (%) | P-value | N | − | + | Positive expression rate (%) | P-value |
| ≤12 months | 40 | 11 | 29 | 72.50 | 0.007a | 40 | 1 | 39 | 97.50 | 0.001a |
| >12 months | 23 | 17 | 6 | 26.09 | 23 | 9 | 14 | 60.87 | ||
Values in bold indicate significance.
P<0.01. R/M, recurrence/metastasis.
Expression of B7-H2 is associated with tumor capsule presence
To further determine the association of potential tumor features with the expression of B7-H2 and B7-H3, associations were analyzed using Pearson's χ2 test. As shown in Table IV, the negative expression of B7-H2 in HCC tissues was significantly associated with tumor capsule presence (P=0.046). Additionally, larger tumor size and faster progression of liver cirrhosis were more frequently observed in the B7-H2- and B7-H3-positive expression groups, although not to statistical significance. These data suggested that the positive expression of B7-H2 was associated with the invasive features of HCC, as the absence of tumor encapsulation is more likely to lead to a further intrahepatic recurrence or metastasis.
Table IV.
Associations between tumor features and the expression of B7-H2 and B7-H3 in patients with hepatocellular carcinoma.
| B7-H2 expression | B7-H3 expression | |||||
|---|---|---|---|---|---|---|
| Tumor feature | − | + | P-value | − | + | P-value |
| Progression of cirrhosis | NS | NS | ||||
| Normal/early-stage | 26 | 33 | 9 | 50 | ||
| Advanced-stage | 2 | 2 | 1 | 3 | ||
| Dissemination to regional lymph nodes | NS | NS | ||||
| Yes | 3 | 1 | 1 | 3 | ||
| No | 25 | 34 | 3 | 50 | ||
| Tumor capsule | 0.046a | NS | ||||
| Absent | 23 | 34 | 9 | 48 | ||
| Present | 5 | 1 | 1 | 5 | ||
| Tumor boundaries | NS | NS | ||||
| Distinct | 24 | 32 | 10 | 46 | ||
| Indistinct | 4 | 3 | 0 | 7 | ||
| PVTT | NS | NS | ||||
| Yes | 16 | 15 | 6 | 25 | ||
| No | 12 | 20 | 4 | 28 | ||
| Intraoperative ascites | NS | NS | ||||
| Yes | 7 | 8 | 2 | 13 | ||
| No | 21 | 27 | 8 | 40 | ||
Values in bold indicate significance.
P<0.05. PVTT, portal vein tumor thrombosis; NS, not significant.
Combined expression of B7-H2 and B7-H3 is associated with recurrence and survival rates
Considering the similar expression characteristics of B7-H2 and B7-H3 in patients with HCC, the associations between clinical parameters and the combined expression of B7-H2 and B7-H3 were further investigated. As shown in Table V, the B7-H2+/B7-H3+ patients were more likely to exhibit recurrence within 1 year compared with the B7-H2−/B7-H3− patients (P<0.001); and the 2-year survival rate of the B7-H2+/B7-H3+ patients was 38.9%, which was lower than that of the B7-H2−/B7-H3− patients (77.8%; P=0.035). Kaplan-Meier analysis further suggested that the overall survival rate of the B7-H2−/B7-H3− patients was significantly higher than that of the B7-H2+/B7-H3−, B7-H2−/B7-H3+ and B7-H2+/B7-H3+ patients (P=0.014; Fig. 3A and B). Therefore, these data suggested that the combined expression of B7-H2 and B7-H3 was associated with recurrence and poor survival rate.
Table V.
Associations between clinical parameters and the combined expression of B7-H2 and B7-H3 in patients with hepatocellular carcinoma.
| Combination of B7-H2 and B7-H3 | ||||
|---|---|---|---|---|
| Clinical parameter | Both negative (n) | Other (n) | χ2-value | P-value |
| Sex | 0.064 | 0.680 | ||
| Male | 8 | 47 | ||
| Female | 1 | 7 | ||
| Age (years) | 0.075 | 0.354 | ||
| >60 | 1 | 13 | ||
| <60 | 8 | 41 | ||
| Size (cm) | 0.419 | 0.384 | ||
| <5 | 4 | 18 | ||
| >5 | 5 | 36 | ||
| Differentiation | 1.088 | 0.580 | ||
| Poor | 2 | 17 | ||
| Well | 4 | 27 | ||
| Moderate | 3 | 10 | ||
| AFP (µg/l) | 3.360 | 0.067 | ||
| <200 | 0 | 15 | ||
| >200 | 9 | 38 | ||
| Liver cirrhosis | 1.750 | 0.225 | ||
| − | 0 | 9 | ||
| + | 9 | 45 | ||
| HBV infection | 0.024 | 0.680 | ||
| − | 1 | 7 | ||
| + | 8 | 47 | ||
| TNM stage | 0.884 | 0.280 | ||
| I–II | 4 | 33 | ||
| III–IV | 5 | 21 | ||
| Recurrence | 18.261 | <0.001b | ||
| <1 year | 0 | 40 | ||
| >1 year | 9 | 14 | ||
| Metastasis | 2.063 | 0.146 | ||
| − | 8 | 35 | ||
| + | 1 | 19 | ||
| Survival | 4.725 | 0.035a | ||
| <2 years | 2 | 33 | ||
| >2 years | 7 | 21 | ||
Values in bold indicate significance.
P<0.05
P<0.01. AFP, α-fetoprotein; HBV, hepatitis B virus; TNM, tumor-node-metastasis.
Figure 3.
Kaplan-Meier analysis of the association between survival rate and the combined expression of B7-H2 and B7-H3 in patients with hepatocellular carcinoma. (A) Comparison between the overall survival rate of patients and the expression of B7-H2 and B7-H3. (B) Comparison of overall survival rate between B7-H2−/B7-H3− patients and B7-H2+/B7-H3+ or B7-H2+/B7-H3− or B7-H2−/B7-H3+ patients.
Combined expression of B7-H2 and B7-H3 is associated with prognostic factors
In order to determine the association of combined expression of B7-H2 and B7-H3 with prognosis, multivariate and univariate analyses were performed. As shown in Table VI, tumor size (P<0.001) and differentiation (P=0.003), tumor capsule presence (P=0.022) and, most notably, the combined expression of B7-H2 and B7-H3 (P=0.022) were significantly associated with overall survival rate, which indicated that the combined expression of B7-H2 and B7-H3 was among those factors associated with poor overall survival rate.
Table VI.
Analysis of prognostic factors based on Cox's proportional hazards model at 5 years of follow-up.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Clinical parameter | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
| Sex | ||||||
| Male/female | 0.611 | 0.274–1.360 | 0.227 | |||
| Tumor size | ||||||
| <5 cm/>5 cm | 2.982 | 1.662–5.349 | <0.001b | 4.343 | 2.153–8.763 | <0.001b |
| Differentiation | ||||||
| Poor/moderate/well | 0.578 | 0.403–0.829 | 0.003b | 0.576 | 0.385–0.863 | 0.007b |
| TNM stage | ||||||
| I–II/III–IV | 1.661 | 0.972–2.836 | 0.063 | |||
| Tumor capsule | ||||||
| Present/absent | 0.367 | 0.156–0.864 | 0.022a | 0.376 | 0.146–0.970 | 0.043a |
| B7-H2 and B7-H3 combination | ||||||
| Both low/other | 2.703 | 1.155–6.328 | 0.022a | 6.784 | 2.522–18.245 | <0.001b |
Values in bold indicate significance.
P<0.05
P<0.01. TNM, tumor-node-metastasis; CI, confidence interval.
Combined expression of B7-H2 and B7-H3 increases sensitivity and specificity in predicting HCC prognosis
On the basis of the results on associations with prognostic factors, ROC curves were evaluated for prognostic indicator (PI). PI was calculated as follows: PI = (β1 × score of combined B7-H2 and B7-H3) + (β2 × score of tumor size) + (β3 × score of tumor differentiation) + (β4 × score of tumor capsule). The values of β1–4 represented the regression coefficients of the these variables from the COX regression model. To further determine the sensitivity and specificity of combined expression in predicting 2-year survival rate, the recognized indicator of HCC, AFP, was used as the control. As shown in Fig. 4A and B, the area under the curve (AUC) for the combined expression of B7-H2 and B7-H3 was 0.910 [95% confidence interval (CI), 0.833–0.986, P<0.001], whereas the AUC for the AFP model was 0.858 (95% CI, 0.762–0.955, P<0.001), indicating greater sensitivity and specify for predicting HCC-related survival with use of the combined expression of B7-H2 and B7-H3. In addition, the samples were re-divided into two groups according to the value of PI: Group 1 [PI<Cutoff (0.977)] and Group 2 [PI>Cutoff (0.977)] to further confirm the prediction of HCC-related survival and recurrence rates following the use of the combined expression of B7-H2 and B7-H3. As shown in Fig. 4C and D, the combination of B7-H2 and B7-H3 predicted poor overall survival and recurrence rates of HCC. Therefore, these data demonstrated that the combined expression of B7-H2 and B7-H3 may be used to enhance the sensitivity and specificity of predicting prognosis in HCC.
Figure 4.
Receiver operating characteristic analysis of the combined expression of B7-H2 and B7-H3 for indicating the survival rate of patients with HCC. (A) AUC for the combined expression of B7-H3 and B7-H2 in patients with HCC. (B) AUC for the expression of AFP in patients with HCC. (C) Confirmation of overall survival prediction in patients with HCC by the combined expression of B7-H3 and B7-H2 by Kaplan-Meier analysis. (D) Kaplan-Meier analysis of cumulative recurrence prediction in patients with HCC by the combination expression of B7-H3 and B7-H2. AUC, area under the curve; AFP, α-fetoprotein; HCC, hepatocellular carcinoma; CI, confidence interval; PI, prognostic indicator.
Discussion
A large proportion of patients with HCC present with intrahepatic recurrence or extra-hepatic metastasis within 5 years following curative liver resection performed according to the Milan criteria (17), and 50–70% of the recurrence/metastasis occurs within 1 year following the surgical procedure (18,19); the median survival time following presentation of recurrence/metastasis is only 13 months (20). Therefore, the determination of novel prognostic biomarkers is urgently required in order to enhance the prediction of the disease course of HCC at diagnosis. Previous findings have suggested that disorder in co-stimulatory or co-inhibitory signaling pathways serves a critical role in tumor cell evasion of adaptive immune surveillance (21–24). In particular, the dysregulation of B7-H2 and/or B7-H3 may deregulate T lymphocyte function in the HCC microenvironment, and even promote recurrence following surgery in patients with HCC (8,15). Considering the apparently similar pathological expression profiles of B7-H2 and B7-H3 in HCC, it was logical to investigate the combined expression of B7-H2 and B7-H3 in the same set of patients with HCC to assess whether this provided enhanced prediction of HCC prognosis. On evaluating the expression of B7-H2 and B7-H3 in the same group of patients with HCC, it was determined that the combination of B7-H2 and B7-H3 may serve as a potential prognostic indicator for the prediction of disease-associated survival events post-liver resection in HCC.
Although B7-H3 and B7-H2 are abundantly expressed in patients with HCC and have been indicated to possess similar immune regulation effects in the progression of HCC, there are several differences in their prognostic value in HCC. In the present study, the B7-H3+ patients were more likely to develop pulmonary metastasis (17/25, 68%), whereas the B7-H2+ patients were associated with absence of a tumor capsule, indicating an application in predicting intrahepatic recurrence or metastasis. However, it was determined that both B7-H3 and B7-H2 were associated with prognostic factors, including recurrence (within 1 year), metastasis and survival (within 2 years), indicating the accordance of B7-H3 and B7-H2 for predicting prognosis in HCC. On the basis of these results, the subsequent aim was to evaluate the associations between the combined expression of B7-H3 and B7-H2 and prognostic factors. As expected, it was identified that the combined expression of B7-H3 and B7-H2 was also associated with prognostic factors, including recurrence (within 1 year) and survival (within 2 years). Therefore, the combination of B7-H3 and B7-H2 may provide a novel prognostic biomarker in HCC.
Several important risk factors of HCC have been identified, including chronic hepatitis B virus (HBV) infection, chronic HCV, nonalcoholic steatohepatitis and excessive consumption of alcohol; however, the heterogeneity and the geographic variability of the incidence of HCC have been widely associated to the different distribution of HCV and HBV infections worldwide (25). In particular, it has been suggested that HBV accounts for ~80% of virus-infective HCC cases globally and is the main causal factor in regions with a high incidence of HCC, particularly in Africa and East Asia; whereas HCV is a major etiological factor of HCC in regions with a low incidence, including America and Western Europe (25–28). Considering chronic infections with HBV and HCV are key risk factors for patients with HCC, the association of the expression of B7-H3/B7-H2 with HBV and HCV was examined. In the present study, although there was no significant association between the expression of B7-H2/B7-H3 and HBV infection, it remained unclear whether the expression of B7-H3/B7-H2 was associated with the prognosis of HCV(+) patients with HCC, as all cases in the present study were HCV(−). Further investigations are necessary to ascertain this information.
In view of the fact that diabetes mellitus is another risk factor for patients with HCC, the association between the expression of B7-H3/B7-H2 and body mass index (BMI) was also investigated in the present study. Although there was no significant association between the expression of B7-H2/B7-H3 and BMI, whether the expression of B7-H3/B7-H2 was associated with other diabetes-related factors, including insulin resistance and expression of SH2 domain-containing inositol phosphatase 2, in patients with HCC remains unclear. Previously, it has been suggested that BMI and insulin resistance are risk factors for patients with HCC with HCV infection (27). In particular, an antidiabetic agent exhibited efficacy in reducing the spontaneous regression of HCC (28). Further investigations are necessary to determine the link between the expression of B7-H2/B7-H3 and diabetes mellitus in HCC, particularly in those patients with HCV infection.
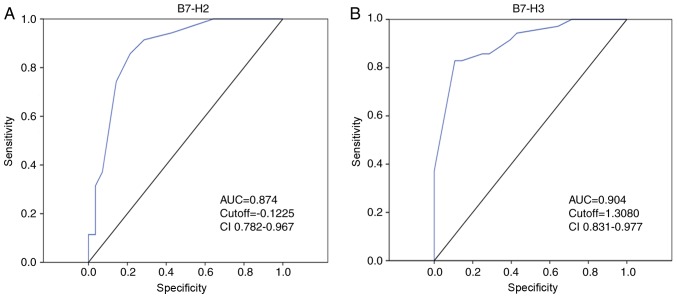
At present, AFP, having been established as an indicator for HCC, is widely used in the diagnosis of HCC. However, its specificity and sensitivity are below what is usually required in clinical practice, with the result being that it is responsible for a high rate of misdiagnosis in HCC. More recently, combination with B7 family molecules has been used to enhance the sensitivity and specificity of prediction. Previous studies have determined that the combination of B7-H3 and B7-H4 may be used as a novel prognostic marker for esophageal cancer (29), and that T lymphocytes plus enoblituzumab (B7-H3 antibody) can inhibit tumor growth in renal and bladder carcinoma xenografts (30). Furthermore, the B7-H2 antibody JTX-2011 has shown success in the phase 1 ICONIC clinical trial (NCT02904226) (31). Therefore, in the present study, ROC analysis was used to evaluate the prediction value of the combination of B7-H3 and B7-H2. Notably, an increased AUC was observed for B7-H3 or B7-H2 alone compared with that for AFP alone (Fig. 5A and B), suggesting greater specificity and sensitivity for predicting prognosis in HCC. More notably, the higher specificity and sensitivity was further improved using the combination of B7-H3 and B7-H2.
Figure 5.
Receiver operating characteristic analysis of B7-H2 and B7-H3. (A) AUC for expression of B7-H2 in patients with HCC (AUC=0.874). (B) AUC for expression of B7-H3 in patients with HCC (AUC=0.904). AUC, area under the curve; HCC, hepatocellular carcinoma; CI, confidence interval.
To conclude, TMA-based immunohistochemistry staining was applied in the present large-scale cohort study, and it was observed that combined expression of B7-H2 and B7-H3 was significantly associated with the prognosis of HCC. Based on these findings, the combination of B7-H2 and B7-H3 may be a potential prognostic biomarker for predicting recurrence and overall survival rates in HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science Foundation of China (grant nos. 81472830, 31600616 and 81672376), the Natural Science Foundation of Fujian Province (grant nos. 2017J 05142 and 2016J01417), the Youth Research Project of Fujian Provincial Health and Family Planning Commission (grant no. 2018-1-93), the Scientific Foundation of Fuzhou Health Department (grant no. 2015-s-143-19), the Medical Innovation Project of Fujian Province (grant no.2016-CX-48) and the Fuzhou Health and Family Planning Science and Technology Project (grant no.2017-S-wt2).
Availability of data and materials
All data generated and/or analyzed during this study are included in this published article.
Authors' contributions
YZ and NL were involved in study design and drafted the manuscript. YWu and JG were involved in TMA. ZL, WL and YWa were involved in the statistical analysis. ML, XL and LC were involved in clinical data collection. WZ and BZ were involved in the study design, financial support and proof-reading of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experiments were approved by the Ethics Committee of the Mengchao Hepatobiliary Hospital of Fujian Medical University. Patients provided informed consent and agreed to the use of their tissues for research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pan R, Zhu M, Yu C, Lv J, Guo Y, Bian Z, Yang L, Chen Y, Hu Z, Chen Z, et al. Cancer incidence and mortality: A cohort study in China, 2008–2013. Int J Cancer. 2017;141:1315–1323. doi: 10.1002/ijc.30825. [DOI] [PubMed] [Google Scholar]
- 2.Chen WQ, Zheng RS, Zhang SW. Liver cancer incidence and mortality in China, 2009. Chin J Cancer. 2013;32:162–169. doi: 10.5732/cjc.013.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forner A, Bruix J. Biomarkers for early diagnosis of hepatocellular carcinoma. Lancet Oncol. 2012;13:750–751. doi: 10.1016/S1470-2045(12)70271-1. [DOI] [PubMed] [Google Scholar]
- 4.Braillon A. Hepatocellular carcinoma. Lancet. 2012;380:469. doi: 10.1016/S0140-6736(12)61282-3. author reply 470, 471. [DOI] [PubMed] [Google Scholar]
- 5.Worns MA, Galle PR. Immune oncology in hepatocellular carcinoma-hype and hope. Lancet. 2017;389:2448–2449. doi: 10.1016/S0140-6736(17)31044-9. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Shen J, Wang MH, Yi T, Yu Y, Zhu Y, Chen B, Chen J, Li L, Li M, et al. Comprehensive molecular profiling of the B7 family of immune-regulatory ligands in breast cancer. Oncoimmunology. 2016;5:e1207841. doi: 10.1080/2162402X.2016.1207841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong B, Qian Y, Zhang H, Sang YW, Cheng LF, Wang Q, Gao S, Zheng M, Yao HP. Expression of B7-H4 and hepatitis B virus X in hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol. 2016;22:4538–4546. doi: 10.3748/wjg.v22.i18.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang FB, Wang L, Jia HC, Li D, Li HJ, Zhang YG, Sun DX. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. doi: 10.1186/s12935-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung J, Suh WK. The CD28-B7 Family in anti-tumor immunity: Emerging concepts in cancer immunotherapy. Immune Netw. 2014;14:265–276. doi: 10.4110/in.2014.14.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 11.Mao Y, Chen L, Wang F, Zhu D, Ge X, Hua D, Sun J. Cancer cell-expressed B7-H3 regulates the differentiation of tumor-associated macrophages in human colorectal carcinoma. Oncol Lett. 2017:6177–6183. doi: 10.3892/ol.2017.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung HI, Jeong D, Ji S, Ahn TS, Bae SH, Chin S, Chung JC, Kim HC, Lee MS, Baek MJ. Overexpression of PD-L1 and PD-L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res Treat. 2017;49:246–254. doi: 10.4143/crt.2016.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C1, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay D, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: Relationship with clinical and pathological features. Hepatology. 2016;64:2038–2046. doi: 10.1002/hep.28710. [DOI] [PubMed] [Google Scholar]
- 14.Vigdorovich V, Ramagopal UA, Lazar-Molnar E, Sylvestre E, Lee JS, Hofmeyer KA, Zang X, Nathenson SG, Almo SC. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure. 2013;21:707–717. doi: 10.1016/j.str.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu JF, Ding YH, Ying XH, Wu FZ, Zhou XM, Zhang DK, Zou H, Ji JS. Regulatory T cells, especially ICOS+ FOXP3+ regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci Rep. 2016;6:35056. doi: 10.1038/srep35056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pahwa A, Beckett K, Channual S, Tan N, Lu DS, Raman SS. Efficacy of the American association for the study of liver disease and Barcelona criteria for the diagnosis of hepatocellular carcinoma. Abdom Imaging. 2014;39:753–760. doi: 10.1007/s00261-014-0118-9. [DOI] [PubMed] [Google Scholar]
- 17.Hwang M, Jayakrishnan TT, Green DE, George B, Thomas JP, Groeschl RT, Erickson B, Pappas SG, Gamblin TC, Turaga KK. Systematic review of outcomes of patients undergoing resection for colorectal liver metastases in the setting of extra hepatic disease. Eur J Cancer. 2014;50:1747–1757. doi: 10.1016/j.ejca.2014.03.277. [DOI] [PubMed] [Google Scholar]
- 18.Ng KM, Yan TD, Black D, Chu FC, Morris DL. Prognostic determinants for survival after resection/ablation of a large hepatocellular carcinoma. HPB (Oxford) 2009;11:311–320. doi: 10.1111/j.1477-2574.2009.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi GH, Han DH, Kim DH, Choi SB, Kang CM, Kim KS, Choi JS, Park YN, Park JY, Kim DY, et al. Outcome after curative resection for a huge (>or=10 cm) hepatocellular carcinoma and prognostic significance of gross tumor classification. Am J Surg. 2009;198:693–701. doi: 10.1016/j.amjsurg.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg. 2007;94:320–326. doi: 10.1002/bjs.5622. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Wang YR, Dingi GH, Yang TS, Jiang SL, Wang L, Xun LJ, Song RM, Song ZS, Zhou B. Influence of surgical resection of hepatocellular carcinoma(HCC) for hematogenous dissemination of HCC cells and its effect on recurrence and metastasis: 3 years prospective study. Neoplasma. 2015;62:635–640. doi: 10.4149/neo_2015_076. [DOI] [PubMed] [Google Scholar]
- 22.Xu B, Cai Z, Zeng Y, Chen L, Du X, Huang A, Liu X, Liu J. Alpha-Methylacyl-CoA racemase (AMACR) serves as a prognostic biomarker for the early recurrence/metastasis of HCC. J Clin Pathol. 2014;67:974–979. doi: 10.1136/jclinpath-2014-202378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang YF, Yang ZH, Hu JQ. Recurrence or metastasis of HCC: Predictors, early detection and experimental antiangiogenic therapy. World J Gastroenterol. 2000;6:61–65. doi: 10.3748/wjg.v6.i1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 25.Petruzziello A. Epidemiology of hepatitis B virus (HBV) and hepatitis C virus (HCV) related hepatocellular carcinoma. Open Virol J. 2018;12:26–32. doi: 10.2174/1874357901812010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu CJ, Kao JH. Hepatitis B virus-related hepatocellular carcinoma: Epidemiology and pathogenic role of viral factors. J Chin Med Assoc. 2007;70:141–145. doi: 10.1016/S1726-4901(09)70346-6. [DOI] [PubMed] [Google Scholar]
- 27.Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thorav F. The burden of liver disease in Europe: A review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Sumie S, Kawaguchi T, Komuta M, Kuromatsu R, Itano S, Okuda K, Taniguchi E, Ando E, Takata A, Fukushima N, et al. Significance of glucose intolerance and SHIP2 expression in hepatocellular carcinoma patients with HCV infection. Oncol Rep. 2007;18:545–552. [PubMed] [Google Scholar]
- 29.Kawaguchi T, Nakano D, Okamura S, Shimose S, Hayakawa M, Niizeki T, Koga H, Torimura T. Spontaneous regression of hepatocellular carcinoma with reduction in angiogenesis-related cytokines after treatment with sodium-glucose cotransporter 2 inhibitor in a cirrhotic patient with diabetes mellitus. Hepatol Res. 2018 4 Sep; doi: 10.1111/hepr.13247. Doi: 10.1111/hepr.13247. [DOI] [PubMed] [Google Scholar]
- 30.Chen L, Xie Q, Wang Z, Shi L, Wu C, Jiang J. Assessment of combined expression of B7-H3 and B7-H4 as prognostic marker in esophageal cancer patients. Oncotarget. 2016;7:77237–77243. doi: 10.18632/oncotarget.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burris HA, Callahan MK, Tolcher AW, Kummar S, Falchook GS, Pachynski RK, Tykodi SS, Gibney GT, Seiwert TY, Gainor JF, et al. Phase 1 safety of ICOS agonist antibody JTX-2011 alone and with nivolumab (nivo) in advanced solid tumors; predicted vs. observed pharmacokinetics (PK) in ICONIC. J Clin Oncol. 2017;15:3033–3033. doi: 10.1200/JCO.2017.35.15_suppl.3033. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and/or analyzed during this study are included in this published article.