Abstract
Macrophage polarization serves an important role in immune regulation that is regulated by T-cell immunoglobulin-mucin-3 (Tim-3). The objective of the present study was to explore the role of 1,25-dihydroxy-vitamin D3 [1,25(OH)2D3] in macrophage polarization. Plasmid transfection techniques were applied to prepare RAW264.7 cells with silenced or overexpressed Tim-3 gene. ELISAs were used to examine the level of inflammatory factors secreted by macrophages. Proteins levels were determined by western blot analysis. mRNAs expression levels were assessed using reverse transcription quantitative polymerase chain reaction. It was identified that 1,25(OH)2D3 upregulated Tim-3 levels and promoted the secretion of interleukin (IL)-10. 1,25(OH)2D3 was also observed to increase the level of transforming growth factor-β and to inhibit tumor necrosis factor-α and IL-6. The results also suggested that Tim-3 gene silencing induced macrophages polarization to classically activated macrophages (M1), and that overexpression of the Tim-3 gene induced macrophage polarization to alternatively activated macrophages (M2). 1,25(OH)2D3 treatment upregulated the expression level of Tim-3 in macrophages, which promoted cell polarization to M2 and inhibited polarization to M1. The data from the present study indicated that Tim-3 may induce macrophage polarization to M2, and that 1,25(OH)2D3 produced immunosuppressive effects by upregulating Tim-3.
Keywords: macrophages; 1,25-Dihydroxy-vitamin D3; T-cell immunogobulin-mucin-3; immunosuppression
Introduction
Autoimmune disorders are mediated by self-cells; that is, induced by the immune response to autoantigens (1). Though common autoimmune disorders have high incidence rates, the pathogeneses of these diseases remain incompletely characterized (2). At present, to the best of our knowledge, no drug has been identified to be sufficiently effective for the treatment of autoimmune diseases, and clinical therapy is largely based on symptomatic treatment and targeting the control of disease progression (3–5). Therefore, exploring the pathogenesis of these diseases is of great significance in developing effective drugs and clinical strategies for the treatment of autoimmune diseases.
Vitamin D3 is a derivative of cholesterol and the most active form of Vitamin D3 is 1,25-dihydroxy-vitamin D3 [1,25(OH)2D3], formed by the action of hepatic microsomal 25-hydroxylase and kidney mitochondrial 1α hydroxylase (6). Previous studies have demonstrated that abnormal levels of 1,25(OH)2D3 are closely associated with various autoimmune diseases (7,8). At present, studies investigating 1,25(OH)2D3 and immune function have attracted much attention. It has been identified that 1,25(OH)2D3 blocked the differentiation and maturation of dendritic cells (DCs) to promote T cell responses and differentiation (9,10). 1,25(OH)2D3 also promotes B lymphocyte-programmed death and inhibits the differentiation of activated B cells into plasma cells and memory B lymphocytes, which secrete immunoglobulins (11,12). However, only a limited number of studies have examined the macrophage polarization promoted by 1,25(OH)2D3.
It has been identified that 1,25(OH)2D3 promotes macrophage polarization: Macrophages are induced by tumor necrosis factor α (TNF-α) or interleukin-10 (IL-10) (13,14), and macrophage polarization to classically activated macrophages (M1) may assist in virus clearance and participate in inflammatory reactions; however, polarization of macrophages to alternatively activated macrophages (M2) may promote tumor development and inhibit inflammatory responses (15,16).
The polarization mechanism of macrophages has been studied extensively. T-cell Immunoglobulin-mucin-3 (Tim-3) was identified as a novel immunomodulatory protein (17). Tim-3 has the function of regulating natural immune cells (18). At present, previous studies have identified that Tim-3 may promote macrophage polarization to M2; however, its mechanism has not been fully described (19,20). Tim-3 belongs to the Tim protein family (21). Previous studies have suggested that Tim-3 is significantly upregulated in chronic infectious diseases, including chronic Hepatitis C virus infection (22), Human immunodeficiency virus infection (23) and tuberculosis (24) and downregulated in autoimmune diseases, including multiple sclerosis (25) and rheumatoid arthritis (17). Therefore, Tim-3 is considered to be included in a novel generation of immunomodulatory targets.
In the present study, genetic interference techniques were applied to explore the effect of 1,25(OH)2D3 on macrophages polarization, and its mechanism. The expression levels of polarization-associated factors with or without Tim-3 expression were detected. The results of the present study enrich the current understanding of the mechanisms underlying autoimmune diseases, and suggest alternative approaches for disease treatment.
Materials and methods
Cells culture
The mouse macrophage RAW264.7 cell line was purchased from the American Type Culture Collection (Rockville, MD, USA). Cells were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) that contained 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) with penicillin (100 U/ml) and streptomycin sulfate (100 µg/ml) at 37°C in an incubator with 5% CO2. Culture reagents were purchased from Gibco (Thermo Fisher Scientific, Inc.).
Cell transfection
The recombinant Tim-3 overexpression plasmid (pTim-3), empty vector (pScramble) and small interfering (si)Tim-3 were purchased from Promega Corporation (Madison, WI, USA). The sequences of siTim-3 were Sense 5′:GGUGUCUUAAUCCUUAAAU, and Antisense 3′:CCACAGAAUUAGGAAUUUA. The cells were seeded at a density of 1×104 cells/well into a 24-well plate. The cells were then starved overnight by incubating them in DMEM without FBS prior to cell transfection. When the confluence reached 60–70%, the cells were transfected with pTim-3 or siTim-3 (50 pmol) using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the protocol of the manufacturer. The time interval between transfection and subsequent experimentation was 24 h. For screening the positive clones, the RAW264.7 cell line with the stable knockdown or Tim-3 overexpression plasmid was cultured in the aforementioned complete medium with 200 and 700 µg/ml G418 respectively, at 37°C for 24 h. According to data from previous studies (26–28), 10−8 or 10−7 M 1,25(OH)2D3 was used to treat the RAW264.7 cells for 3 days. Cell morphology was observed using a laser confocal microscope, with magnifications ×100 and ×200 (Leica TCS SP8; Leica Microsystems, Wetzlar, Germany).
ELISAs
ELISAs were used to measure the concentrations of TNF-α, IL-6, IL-10 and transforming growth factor-β (TGF-β). The kits were Mouse TNF-α Quantikine ELISA Kit (MTA00B, R&D Systems, Inc., Minneapolis, MN, USA), Mouse IL-6 Quantikine ELISA Kit (M6000B, R&D), Mouse IL-10 Quantikine ELISA Kit (M1000B, R&D), and Mouse/Rat/Porcine/Canine TGF-beta 1 Quantikine ELISA Kit (MB100B, R&D). Following washing, blocking solution was added at 4°C and incubated for 2 h. Next, the primary antibody was added at 4°C and incubated overnight. The secondary antibody was then added and incubated for 1 h at room temperature. Horseradish peroxidase was added dropwise for 30 min at room temperature, and then tetramethylbenzidine was added for 10 min. The absorbance value was measured at 450 nm using a microplate reader (ELX 800; Bio-Tek Instruments, Winooski, VT, USA) and the concentration was calculated following the standard curve.
Western blot analysis
The expression levels of Tim-3, nitric oxide synthase, inducible (NOS2) and macrophage mannose receptor 1 (COD206) proteins were detected using western blot analysis. Cells were lysed with liquid nitrogen and radioimmunoprecipitation assay lysis buffer (Abmole Bioscience Inc., Houston, TX, USA). Next, the cells were cleaved with 1% phenylmethyl sulfonyl fluoride mixed with phosphatase inhibitors (Abmole Bioscience Inc) for 30 min at 4°C. The supernatant was collected using centrifugation at 13,000 × g at 4°C for 15 min. A standard curve was drawn using the BCA method to determine the protein concentration. Then, proteins (20 µg/lane) were separated using 10% SDS-PAGE gels, and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using a Trans-Blot Transfer Slot (Bio-Rad Laboratories, Inc.). The PVDF membranes were then blocked with 5% skimmed milk for 2 h at room temperature. Primary antibodies against Tim-3 (cat. no. ab185703; 1:800; Abcam, Cambridge, MA, USA), NOS2 (cat. no. 14-5920-80; 1:800; eBioscience; Thermo Fisher Scientific, Inc.), CD206 (cat. no. 141703; 1:600; BioLegend, Inc., San Diego, CA, USA) were then added according to the manufacturers' protocols. The samples were agitated at room temperature for 2 h and then incubated at 4°C for 12 h. Rabbit anti-mouse IgG (cat. no. ab99697; 1:9,000 Abcam), mouse anti-rabbit IgG (cat. no. BA1034; 1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.) and goat anti-mouse IgG (cat. no. ab6785; 1:8,000; Abcam) HRP-conjugated secondary antibodies were added and incubated at room temperature for 1.5 h. Chemiluminescence detection was performed using ECL reagent (EMD Millipore, Billerica, MA, USA). The densitometric analysis was performed using Bio-Rad ChemiDoc™ XRS+ System with Image Lab™ Software version 4.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis
RT-qPCR was performed to determine the mRNA levels of Tim-3, NOS2 and COD206. The cells were triturated and lysed by TRIzol® (Thermo Fisher Scientific, Inc.) at 0°C for 5 min. RNA was then extracted using CCl3 (Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China) and dissolved in diethyl pyrocarbonate-treated water (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). RNA concentration was measured using a UV NanoDrop One Microvolume UV–Vis spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE, USA). Reverse transcription assays were performed on RNA samples to synthesize cDNA using a reverse transcription kit (Takara Biotechnology, Co., Ltd., Dalian, China). The samples were incubated at 37°C for 15 min, followed by heat inactivation at 85°C for 15 sec. RT-qPCR experiments were performed using the SYBR Prellix Ex Taq™ Real-Time PCR kit (Takara Biotechnology, Co., Ltd.). qPCR was performed by activating the DNA polymerase at 95°C for 5 min, which was followed by 40 cycles of a two-step PCR (at 95°C for 10 sec and at 60°C for 30 sec) with a final extension step at 75°C for 10 min. Samples were then held at 4°C. β-actin was used as an internal control. All primers were purchased from Genewiz, Inc., (Suzhou, Jiangsu China), and are summarized in Table I. The 2−ΔΔCq method was used to quantify the gene expression levels (29).
Table I.
Sequences of primers.
| Primer name | Sequence (5′-3′) | Product size, bp |
|---|---|---|
| Tim-3-forward | CTCCAAGAACCCTAACCACG | |
| Tim-3-reverse | AGCCCATGTGGAAATTTTTG | 288 |
| NOS2-forward | CAGCTGGGCTGTACAAACCTT | |
| NOS2-reverse | CATTGGAAGTGAAGCGTTTCG | 245 |
| CD206-forward | TTCGGACACCCATC-GGAATTT | |
| CD206-reverse | CACAAGCGCTGCGTGGAT | 262 |
| β-actin-forward | GCTGCGTGTGGCCCCTGAG | |
| β-actin-reverse | ACGCAGGATGGCATGAGGGA | 252 |
Tim-3, T-cell Immunoglobulin-mucin-3; NOS2, nitric oxide synthase, inducible; CD206, macrophage mannose receptor 1.
Statistical analysis
All experimental data are presented as means ± standard deviation. Statistical analysis was performed using SPSS 20 (IBM Corp., Armonk, NY, USA). The one-way analysis of variance followed by Tukey's multiple comparison post-hoc test was performed to analyze the differences among experimental groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Confocal microscopy
The viable RAW264.7 macrophages were normal at magnifications, ×100 and ×200 (Fig. 1).
Figure 1.
Confocal microscopy imaging. The morphology of live RAW264.7 cells at magnifications, ×100 and ×200 were observed using confocal microscopy.
1,25(OH)2D3 regulates the secretion of inflammatory cytokines in RAW264.7 cell
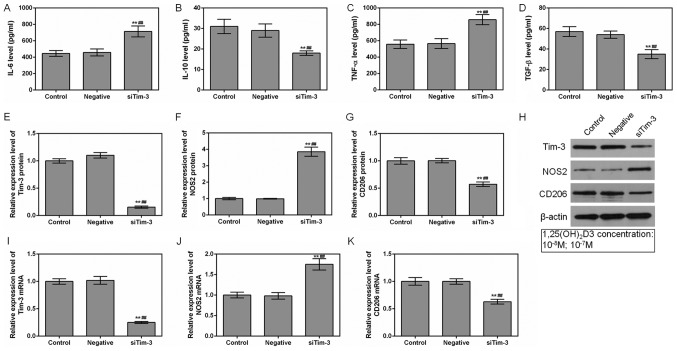
It was identified that the levels of TNF-α and IL-6 secreted by RAW264.7 cells were decreased in the presence of 1,25(OH)2D3. However, the levels of IL-10 and TGF-β were increased (P<0.01; Fig. 2A-D). These differences were the most significant at concentrations of 10−7 M (P<0.05).
Figure 2.
Effects of 1,25(OH)2D3 on macrophage at 10−8 and 10−7 M concentrations. RAW264.7 cells were cultured at different 1,25(OH)2D3 concentrations (0, 10−8 and 10−7 M). ELISAs were used to detect the (A) IL-6, (B) IL-10, (C) TNF-α and (D) TGF-β protein expression levels among different groups. (E) Tim-3, (F) NOS2 and (G) CD206 protein levels were assessed by western blot analysis. (H) Representative western blot image. (I) Tim-3, (J) NOS2 and (K) CD206 mRNA levels were analyzed by reverse transcription quantitative polymerase chain reaction assays. *P<0.05 and **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. 10−8 M group. Data are presented as mean ± standard deviation (n=5). 1,25(OH)2D3, 1,25-dihydroxy-vitamin D3; IL, interleukin; TNF-α, tumor necrosis factor α; TGF-β, transforming growth factor; Tim-3, T-cell Immunoglobulin-mucin-3; NOS2, nitric oxide synthase, inducible; CD206, macrophage mannose receptor 1.
1,25(OH)2D3 upregulates Tim-3 expression
To investigate the mechanism of how 1,25 (OH)2D3 affects the expression levels of inflammatory factors, levels of Tim-3, and NOS2 and COD206, the markers of M1 and M2, were examined. The results indicated that the expression level of Tim-3 was gradually increased in the 10−8 M and 10−7 M groups. However, whilst 1,25 (OH)2D3 did not affect the expressions of the NOS2 and COD206, it decreased NOS2 mRNA levels (P<0.05; Fig. 2E-K). This observation indicated that 1,25(OH)2D3 promoted Tim-3 expression in a dose-dependent manner.
Effects of Tim-3 gene silencing on cytokines
Following silencing of the Tim-3 gene, the levels of TNF-α and IL-6 secreted by RAW264.7 cells were upregulated, while the levels of IL-10 and TGF-β were downregulated (P<0.01; Fig. 3A-D). Following the decrease of the Tim-3 gene expression, the mRNA and protein expression levels of NOS2 were observed increased compared with that of the NC and control groups, while the expression levels of CD206 mRNA and protein were significantly decreased (P<0.01; Fig. 3E-K). This phenomenon suggested that silence of Tim-3 gene promoted the polarization of macrophages to M1.
Figure 3.
Effects of Tim-3 gene silencing on macrophages. ELISAs were applied to detect the (A) IL-6, (B) IL-10, (C) TNF-α and (D) TGF-β levels in the control, NC and siTim-3 groups. (E) Tim-3, (F) NOS2 and (G) CD206 protein levels were assessed by western blot analysis. (H) Representative western blot image. (I) Tim-3, (J) NOS2 and (K) CD206 mRNA levels were analyzed by reverse transcription quantitative polymerase chain reaction assays. **P<0.01 vs. control group; ##P<0.01 vs. NC group. Data are presented as mean ± standard deviation (n=5). IL, interleukin; TNF-α, tumor necrosis factor α; TGF-β, transforming growth factor; Tim-3, T-cell Immunoglobulin-mucin-3; NOS2, nitric oxide synthase, inducible; CD206, macrophage mannose receptor 1; si, small interfering.
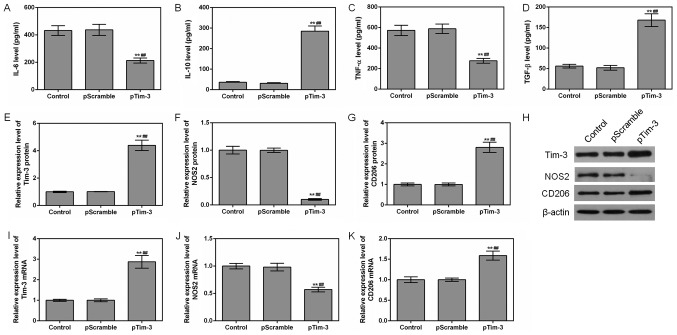
Effects of overexpression of Tim-3 on cytokines
The effects of the overexpression of Tim-3 on cytokines were also examined. It was identified that the levels of TNF-α and IL-6 in the pTim-3 group were decreased compared with those in the NC group, and that the levels of IL-10 and TGF-β were increased compared with those in the NC group (P<0.01; Fig. 4A-D). When Tim-3 was overexpressed, the mRNA and protein expression levels of NOS2 were decreased, while the expression levels of CD206 mRNA and protein were increased (P<0.01; Fig. 4E-K). This suggested that the overexpression of Tim-3 promoted the polarization of macrophages to M2.
Figure 4.
Effects of Tim-3 gene overexpression on macrophages. The levels of (A) IL-6, (B) IL-10, (C) TNF-α and (D) TGF-β in the control, NC and pTim-3 groups were detected using ELISAs. (E) Tim-3, (F) NOS2 and (G) CD206 protein levels were assessed by western blot analysis. (H) Representative western blot image. (I) Tim-3, (J) NOS2 and (K) CD206 mRNA levels were analyzed by reverse transcription quantitative polymerase chain reaction assays. **P<0.01 vs. control group; ##P<0.01 vs. NC group. Data are presented as mean ± standard deviation (n=5). IL, interleukin; TNF-α, tumor necrosis factor α; TGF-β, transforming growth factor; Tim-3, T-cell Immunoglobulin-mucin-3; NOS2, nitric oxide synthase, inducible; CD206, macrophage mannose receptor 1; pTim-3, overexpressing Tim-3 plasmid.
Effects of 1,25(OH)2D3 on cytokine under Tim-3 silencing or overexpression conditions
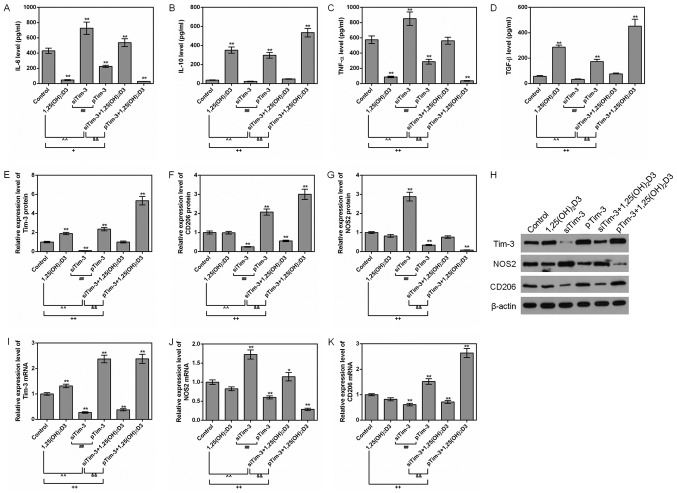
To additionally explore the effect of 1,25(OH)2D3 on the polarization of macrophages, RAW264.7 cells were cultured in the presence of 10−7 M 1,25(OH)2D3. The expression levels of inflammatory factors, and M1 and M2 marker proteins NOS2 and CD206, were detected.
It was identified that 1,25(OH)2D3 downregulated the levels of TNF-α and IL-6 and upregulated the level of TGF-β in siTim-3 cells. In addition, 1,25(OH)2D3 promoted the expression of Tim-3 and increased the expression levels of CD206 protein in siTim-3 cells. However, 1,25(OH)2D3 decreased the expression levels of NOS2. For RAW264.7 cells with stable overexpression of Tim-3, 1,25(OH)2D3 was observed to additionally decrease TNF-α and IL-6 levels and increase IL-10 and TGF-β levels, compared to the Tim-3 overexpression group. In addition, 1,25 (OH)2D3 increased the level of Tim-3 and the expression levels of CD206 in RAW264.7 cell with stable expression of Tim-3 gene (Fig. 5A-K). This indicated that 1,25(OH)2D3 promoted the polarization of macrophages to M2 and inhibited polarization of macrophages to M1 via upregulating the expression of Tim-3.
Figure 5.
Effects of 1,25(OH)2D3 on macrophages with different Tim-3 expression levels. RAW264.7 macrophages with silenced Tim-3 expression and Tim-3 overexpression were cultured with 10−7 M 1,25(OH)2D3. ELISAs were used to assess the levels of (A) IL-6, (B) IL-10, (C) TNF-α and (D) TGF-β in the control group. (E) Tim-3, (F) CD206 and (G) NOS2 protein levels were assessed by western blot analysis. (H) Representative western blot image. (I) Tim-3, (J) NOS2 and (K) CD206 mRNA levels were analyzed by reverse transcription quantitative polymerase chain reaction assays. *P<0.05 and **P<0.01 vs. control group; +P<0.05; ++P<0.01; ##P<0.01; &&P<0.01. Data are presented as mean ± standard deviation (n=5). 1,25(OH)2D3, 1,25-dihydroxy-vitamin D3; IL, interleukin; TNF-α, tumor necrosis factor α; TGF-β, transforming growth factor; Tim-3, T-cell Immunoglobulin-mucin-3; NOS2, nitric oxide synthase, inducible; CD206, macrophage mannose receptor 1; pTim-3, overexpressing Tim-3 plasmid; si, small interfering.
Discussion
Macrophages may be induced to polarize to M1 or M2 subtypes by different cytokines, and also secrete different cytokines to promote or suppress the immune response (30). Macrophage cells may also maintain the homeostasis and serve an important role in the formation and treatment of diseases (31).
It has been identified that Vitamin D3 has immunomodulatory effects on chicken macrophages (32). Yin et al (33) also revealed that Vitamin D may induce macrophage polarization by activating CYP27A1 to prevent atherosclerosis in hypercholesterolemic animals. The present study demonstrated that 1,25(OH)2D3 inhibited TNF-α and IL-6 and promoted the secretion of IL-10 and TGF-β. TNF-α induces macrophage polarization to M1 and the secretion of TNF-α and IL-6. IL-10 induces macrophage polarization to M2 and secretion of IL-10 and TGF-β (13,14). The results of the present study indicated that 1,25(OH)2D3 decreased the level of NOS2 mRNA, but did not produce a significant effect on NOS2 protein expression or CD206 protein and mRNA expression. However, the expression of Tim-3 was increased by 1,25(OH)2D3. Tim-3 serves a role in modulating immunity (34), through inducing macrophage polarization. This suggests that 1,25(OH)2D3 may regulate macrophage polarization through Tim-3. Therefore, the effect of 1,25(OH)2D3 on macrophages was investigated through silencing or overexpressing Tim-3. The results demonstrate that silencing of the Tim-3 gene may promote the secretion of TNF-α and IL-6 but inhibit the secretion of IL-10 and TGF-β. In addition, downregulation of Tim-3 increased the protein and mRNA levels of NOS2 and decreased the protein and mRNA levels of the M2 marker CD206. By contrast, overexpression of the Tim-3 gene exerted the opposite effects This is consistent with previous data (17,22–25), indicating that a high expression level of Tim-3 gene promotes macrophage polarization to M2 and produces immunosuppressive effects. Therefore, we hypothesized that 1,25(OH)2D3 may induce cell polarization to M2 by upregulating Tim-3 expression and exerting immunosuppressive effects.
The role of 1,25(OH)2D3 in macrophages polarization was also explored. The data indicated that the decreased levels of IL-6 and TNF-α and the increased IL-10 and TGF-β were reversed in the presence of siTim-3. However, the effect of 1,25(OH)2D3 was enhanced by the overexpression of Tim-3. This suggested that 1,25(OH)2D3 produced an immunosuppressive effect by promoting Tim-3 protein. It was consistent with a previous study, in which 1,25(OH)2D3 was identified to induce macrophage polarization to M2, leading to immunosuppression (33).
However, the mechanism underlying the regulation of Tim-3 protein levels by 1,25(OH)2D3 remains unclear. An additional limitation of the present study is that RAW264.7 is a cancerous macrophage cell line, as opposed to a primary cell line. Therefore, additional investigation in primary cells or in vivo experiments would strengthen the results of the present study. Altogether, the mechanism for the regulation of M1/M2 conversion and inflammation by Tim-3 remains to be determined.
In summary, Tim-3 induces macrophage polarization to M2. 1,25(OH)2D3 exhibited immunosuppressive effects by upregulating Tim-3 protein levels. The present study suggested that 1,25(OH)2D3 may serve as an immunosuppressive drug and that Tim-3 may be a potential target in treating autoimmune diseases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
SL and JC did all of the experiments, YL analyzed the data, RY designed the study, and SL wrote the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, Mortensen PB. Autoimmune diseases and severe infections as risk factors for mood disorders: A nationwide study. JAMA Psychiatry. 2013;70:812–820. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- 2.Chen SW, Zhong XS, Jiang LN, Zheng XY, Xiong YQ, Ma SJ, Qiu M, Huo ST, Ge J, Chen Q. Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. Behav Brain Res. 2016;296:61–69. doi: 10.1016/j.bbr.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 3.Iyer A, Elsone L, Appleton R, Jacob A. A review of the current literature and a guide to the early diagnosis of autoimmune disorders associated with neuromyelitis optica. Autoimmunity. 2014;47:154–161. doi: 10.3109/08916934.2014.883501. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen PR, Benros ME, Dalsgaard S. Associations between autoimmune diseases and attention-deficit/hyperactivity disorder: A nationwide study. J Am Acad Child Adolesc Psychiatry. 2017;56:234–240.e231. doi: 10.1016/j.jaac.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Andersson NW, Gustafsson LN, Okkels N, Taha F, Cole SW, Munk-Jorgensen P, Goodwin RD. Depression and the risk of autoimmune disease: A nationally representative, prospective longitudinal study. Psychol Med. 2015;45:3559–3569. doi: 10.1017/S0033291715001488. [DOI] [PubMed] [Google Scholar]
- 6.Ota K, Dambaeva S, Kim MW, Han AR, Fukui A, Gilman-Sachs A, Beaman K, Kwak-Kim J. 1,25-Dihydroxy-vitamin D3 regulates NK-cell cytotoxicity, cytokine secretion, and degranulation in women with recurrent pregnancy losses. Eur J Immunol. 2015;45:3188–3199. doi: 10.1002/eji.201545541. [DOI] [PubMed] [Google Scholar]
- 7.Alhassan Mohammed H, Saboor-Yaraghi AA, Mirshafiey A, Vahedi H, Shiri-Shahsavar MR, Mousavi Nasl Khameneh A. Immunomodulatory and immunosuppressive roles of 1α,25(OH)2D3 in autoimmune diseases. Scand J Immunol. 2017;85:95–103. doi: 10.1111/sji.12512. [DOI] [PubMed] [Google Scholar]
- 8.Fitch N, Becker AB, HayGlass KT. Vitamin D [1,25(OH)2D3] differentially regulates human innate cytokine responses to bacterial versus viral pattern recognition receptor stimuli. J Immunol. 2016;196:2965–2972. doi: 10.4049/jimmunol.1500460. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira GB, Gysemans CA, Demengeot J, da Cunha JP, Vanherwegen AS, Overbergh L, Van Belle TL, Pauwels F, Verstuyf A, Korf H, Mathieu C. 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J Immunol. 2014;192:4210–4220. doi: 10.4049/jimmunol.1302350. [DOI] [PubMed] [Google Scholar]
- 10.Ria F, Penna G, Adorini L. Th1 cells induce and Th2 inhibit antigen-dependent IL-12 secretion by dendritic cells. Eur J Immunol. 1998;28:2003–2016. doi: 10.1002/(SICI)1521-4141(199806)28:06<2003::AID-IMMU2003>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Sergeev IN, Pletsityi KD, Rusnak FI, Spirichev VB. 1,25-Dihydroxyvitamin D3 receptors in lymphocytes and T- and B-lymphocyte count in patients with glomerulonephritis. Vopr Med Khim. 1989;35:117–121. (In Russian) [PubMed] [Google Scholar]
- 12.Geldmeyer-Hilt K, Heine G, Hartmann B, Baumgrass R, Radbruch A, Worm M. 1,25-dihydroxyvitamin D3 impairs NF-kappaB activation in human naive B cells. Biochem Biophys Res Commun. 2011;407:699–702. doi: 10.1016/j.bbrc.2011.03.078. [DOI] [PubMed] [Google Scholar]
- 13.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado Jde D, Popovich PG, Partida-Sanchez S, Guerau-de-Arellano M. Novel markers to delineate murine M1 and M2 macrophages. PLoS One. 2015;10:e0145342. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cucak H, Grunnet LG, Rosendahl A. Accumulation of M1-like macrophages in type 2 diabetic islets is followed by a systemic shift in macrophage polarization. J Leukoc Biol. 2014;95:149–160. doi: 10.1189/jlb.0213075. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Park EJ, Noh JW, Hwang JW, Bae EK, Ahn JK, Koh EM, Cha HS. Underexpression of TIM-3 and blunted galectin-9-induced apoptosis of CD4+ T cells in rheumatoid arthritis. Inflammation. 2012;35:633–637. doi: 10.1007/s10753-011-9355-z. [DOI] [PubMed] [Google Scholar]
- 18.Kuchroo VK, Meyers JH, Umetsu DT, DeKruyff RH. TIM family of genes in immunity and tolerance. Adv Immunol. 2006;91:227–249. doi: 10.1016/S0065-2776(06)91006-2. [DOI] [PubMed] [Google Scholar]
- 19.Jiang X, Zhou T, Xiao Y, Yu J, Dou S, Chen G, Wang R, Xiao H, Hou C, Wang W, et al. Tim-3 promotes tumor-promoting M2 macrophage polarization by binding to STAT1 and suppressing the STAT1-miR-155 signaling axis. Oncoimmunology. 2016;5:e1211219. doi: 10.1080/2162402X.2016.1211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, Huang Q, Li S, Meng F, Li X, Gong X. miR-330-5p/Tim-3 axis regulates macrophage M2 polarization and insulin resistance in diabetes mice. Mol Immunol. 2018;95:107–113. doi: 10.1016/j.molimm.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu Z, Wang H, Xu F, Shi L. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J Immunol. 2015;194:1239–1251. doi: 10.4049/jimmunol.1402088. [DOI] [PubMed] [Google Scholar]
- 22.Moorman JP, Wang JM, Zhang Y, Ji XJ, Ma CJ, Wu XY, Jia ZS, Wang KS, Yao ZQ. Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J immunol. 2012;189:755–766. doi: 10.4049/jimmunol.1200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tandon R, Giret MT, Sengupta D, York VA, Wiznia AA, Rosenberg MG, Kallas EG, Ndhlovu LC, Nixon DF. Age-related expansion of Tim-3 expressing T cells in vertically HIV-1 infected children. PLoS One. 2012;7:e45733. doi: 10.1371/journal.pone.0045733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu Y, Chen J, Liao H, Zhang Y, Wang H, Li S, Luo Y, Fang D, Li G, Zhou B, et al. Tim-3-expressing CD4+ and CD8+ T cells in human tuberculosis (TB) exhibit polarized effector memory phenotypes and stronger anti-TB effector functions. PLoS Pathog. 2012;8:e1002984. doi: 10.1371/journal.ppat.1002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koguchi K, Anderson DE, Yang L, O'Connor KC, Kuchroo VK, Hafler DA. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med. 2006;203:1413–1418. doi: 10.1084/jem.20060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villaggio B, Soldano S, Cutolo M. 1,25-dihydroxyvitamin D3 downregulates aromatase expression and inflammatory cytokine production in human macrophages. Clin Exp Rheumatol. 2012;30:934–938. [PubMed] [Google Scholar]
- 27.Chambers ES, Suwannasaen D, Mann EH, Urry Z, Richards DF, Lertmemongkolchai G, Hawrylowicz CM. 1α,25-dihydroxyvitamin D3 in combination with transforming growth factor-β increases the frequency of Foxp3+ regulatory T cells through preferential expansion and usage of interleukin-2. Immunology. 2014;143:52–60. doi: 10.1111/imm.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karmali R, Hewison M, Rayment N, Farrow SM, Brennan A, Katz DR, O'Riordan JL. 1,25(OH)2D3 regulates c-myc mRNA levels in tonsillar T lymphocytes. Immunology. 1991;74:589–593. [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, Cheng SC, Ratter J, Berentsen K, van der Ent MA, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shojadoost B, Behboudi S, Villanueva AI, Brisbin JT, Ashkar AA, Sharif S. Vitamin D3 modulates the function of chicken macrophages. Res Vet Sci. 2015;100:45–51. doi: 10.1016/j.rvsc.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Yin K, You Y, Swier V, Tang L, Radwan MM, Pandya AN, Agrawal DK. Vitamin D protects against atherosclerosis via regulation of cholesterol efflux and macrophage polarization in hypercholesterolemic swine. Arterioscler Thromb Vasc Biol. 2015;35:2432–2442. doi: 10.1161/ATVBAHA.115.306132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.