Abstract
Anti-phospholipid syndrome (APS) is a systematic autoimmune disease that is associated with presence of antiphospholipid antibodies (aPL), recurrent thrombosis, and fetal morbidity in pregnancy. Toll-like receptor-4 (TLR-4), a member of TLR family, is known to have a fundamental role in pathogen recognition and activation of innate immunity. The β2-glycoprotein I (β2GPI), a protein circulating in the blood at a high concentration, is able of scavenging lipopolysaccharide (LPS) and clear unwanted anionic cellular remnants, such as microparticles, from the circulation. Our previous study demonstrated that TLR-4 and its signaling pathways contribute to the upregulation of pro-coagulant factors and pro-inflammatory cytokines in monocytes induced by anti-β2GPI in vitro. The present study aimed to define the roles of TLR-4 in vivo. C3H/HeN mice (TLR-4 intact) and C3H/HeJ mice (TLR-4 defective) were stimulated with an intraperitoneal injection with anti-β2GPI-immunoglobulin G(IgG), then peritoneal macrophages and vascular endothelial cells (VECs) were extracted from treated mice, and analyses were conducted on the expression profiles of pro-inflammatory cytokines and adhesion molecules. The results demonstrated that the expression of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6, in the peritoneal macrophages, and adhesion molecules, including intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin, in VECs of C3H/HeN mice (TLR-4 intact) were significantly higher than those of C3H/HeJ mice (TLR-4 defective). The phosphorylation levels of p38 mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) p65 in peritoneal macrophages and VECs from C3H/HeN mice stimulated with anti-β2GPI–IgG were significantly increased compared with those from C3H/HeJ mice (TLR-4 defective). The isotype control antibody (NR-IgG) had no such effects on peritoneal macrophages and VECs. Furthermore, the inhibitors of TLR-4, p38 MAPK and NF-κB may significantly reduce the anti-β2GPI–IgG-induced TNF-α, IL-1β and IL-6 mRNAs expression in the peritoneal macrophages from TLR-4 intact mice. The results indicated that a TLR-4 signal transduction pathway is involved in anti-β2GPI–IgG-induced activation of peritoneal macrophages and VECs. This study has provided a basis for subsequent investigations to elucidate the pathological mechanisms underlying anti-phospholipid syndrome.
Keywords: Toll-like receptor-4, anti-β2-glycoprotein I antibody, β2-glycoprotein I, inflammatory cytokines, adhesion molecules
Introduction
Anti-phospholipid syndrome (APS), also known as anti-phospholipid antibody syndrome, is a non-organ-specific autoimmune and hypercoagulable disease. APS is characterized by the presence of anti-phospholipid antibodies (aPL), venous and/or arterial thrombosis, thrombocytopenia and recurrent fetal loss (1,2). The aPL have been reported to have an important role in the etiology of thrombosis (3,4), the formation of a blood clot inside a blood vessel, leading to the obstruction of the blood flow through the circulatory system, which, in turn, can lead to a number of complications, including heart attack, stroke and miscarriage. In addition to the anionic phospholipids, phospholipid-binding proteins may be target antigens. β2-glycoprotein I (β2GPI), also known as apolipoprotein H, is a 38–50 kDa multifunctional plasma apolipoprotein that clears lipopolysaccharide (LPS) and dead cell remnants via interactions with phospholipids. In fact, the aPLs including anti-β2GPI antibodies are known to be involved in the pathogenesis of thrombosis (5,6). Anti-β2GPI antibodies are a heterogeneous group of antibodies and their common recognition of a single β2GPI domain I epitope around amino acids G40-R43 is associated with the observed clinical manifestation of APS (7).
Mononuclear cells (or simply monocytes) and vascular endothelial cells are the primary effector cells involved in thrombus formation. In these cells, the coagulation cascade is activated by high expression of tissue factor (8). Mononuclear cells can be activated to have a pro-inflammatory role via the secretion of various inflammatory cytokines [including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 (9)], inhibition of physiological anti-coagulant system, activation of platelets and endothelial cells, and further promotion of thrombosis (10,11). Endothelial cells can be activated in vivo by enhancing the expression of adhesion molecules, and enhancing the adhesion of leukocytes and/or platelets (9,12,13).
While the cellular and molecular mechanisms by which anti-phospholipid antibodies lead to thromboembolic events are still not entirely clear, Toll-like receptor-4 (TLR-4) has been suggested to have an important role in thrombosis. For instance, previous studies have reported that a β2GPI/anti-β2GPI complex is able of inducing the expression of inflammatory cytokines (TNF-α, IL-1β and IL-6) in monocytes via the activation of TLR-4 (14). In vitro studies have also demonstrated that anti-β2GPI can activate endothelial cells via TLR-4, as determined by measuring the increased expression levels of adhesion molecules, including intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin (15). These previous studies suggest the important role of TLR-4 in aPL/anti-β2GPI-mediated pathogenesis of thrombosis.
TLR-4 belongs to the TLR family type I transmembrane receptors. TLR-4 is widely expressed in various cell types, including macrophages, endothelial cells, lymphocytes and dendritic cells, and it actively participates in immune defense responses (16–18). Following activation by pathogen-associated molecular pattern (PAMPs), a signal transduction cascade is initiated. TLR-4 is an important receptor involved in mediating the responses to lipopolysaccharides (LPS), a polysaccharide composed of O-antigen that is present in the outer membrane of Gram-negative bacteria and elicits strong immune responses in animals. Upon binding to TLR-4, the β2GPI/anti-β2GPI-immunoglobulin G (IgG) complex activates a signaling cascade, which is characterized by phosphorylation of p38 mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB), the key factors involved in inflammatory responses. Activation of TLR-4 mainly induces the expression of inflammatory factors and chemokines (14,19,20). Myeloid differentiation primary response gene 88 (Myd88) and TIR-domain-containing adapter-inducing interferon-β (TRIF) are the adapters that respond to the activation of TLRs. Myd88 and TRIF induce intracellular signal transduction following TLR-4 activation (21). Thus, the role of TLR-4 in the aPL/anti-β2GPI-mediated pathogenesis of thrombosis deserves to be further investigated.
Currently, there is limited understanding of whether TLR-4 has the same roles in vivo. In the current study, it was aimed to further elucidate the roles of TLR-4 in aPL/anti-β2GPI-mediated pathogenesis of thrombosis by determining whether TLR4 is involved in anti-β2GPI–IgG-mediated activation of endothelial cells and macrophages in vivo. The effects of anti-β2GPI on the expression levels of inflammatory factors (TNF-α, IL-1β and IL-6) and adhesion molecules (ICAM-1, VCAM-1 and E-selectin) were examined and compared in TLR-4 intact C3H/HeN mice and TLR-4 defective C3H/HeJ mice.
Materials and methods
Animals
96 male C3H/HeN mice (TLR4-intact) were purchased from Vital River Laboratory Animal Technology (Beijing, China) and 24 male C3H/HeJ mice (TLR-4-defective) were obtained from the Model Animal Research Center of Nanjing University (Nanjing, China). C3H/HeJ mice carry a mutant, nonfunctional TLR-4 and thus, are hyporesponsive to the lethal effects of LPS. The body weights of mice were 20–25 g and the mice were used at 8–12 weeks of age. The animals were bred in the Laboratory Animal Research Center of Jiangsu University (Zhenjiang, China) at standardized specific pathogen free conditions (12-h light/dark cycle, with 22±2°C temperature and 50±10% humidity). All the experiments involving animals were approved by the Laboratory Animal Administration Committee of Jiangsu University and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (2011) published by the US National Institutes of Health (Bethesda, MD, USA) (22).
Treatment of animals with IgGs
The polyclonal anti-β2GPI antibodies were purified from sera of New Zealand rabbits immunized with human β2GPI peptide sequence (35GYVSRGGMRKFICPLTG51) according to our previous methods (23). The purified anti-β2GPI IgG could recognize human and mouse β2GPI as demonstrated by western blotting and ELISA (23). Our previous study demonstrated that this anti-β2GPI IgG could induce an APS mouse model. The isotype control antibodies (NR-IgG) from sera of normal rabbits were purified by Protein G Sepharose columns (GE Healthcare, Chicago, IL, USA). All the IgG samples and reagents were subjected to Detoxi-Gel™ (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to remove endotoxin contamination (<0.03 EU/ml) using the Limulus amebocyte lysate assay (Associates of Cape Cod, Inc., Falmouth, MA, USA).
C3H/HeN and C3H/HeJ mice (n=8 per treatment group) were twice injected intraperitoneally with anti-β2GPI (100 µg) or NR-IgG (100 µg) at 0 and 48 h. Surgical procedures to obtain peritoneal macrophages or aortas were performed at 72 h after the first injection. In addition, other groups of C3H/HeN and C3H/HeJ mice (n=8 in each group) were challenged with LPS (1 µg/g body weight; E. coli serotype O111:B4; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) 2 h before surgical procedures as the positive control.
Preparation of mouse peritoneal macrophages
Both C3H/HeN mice and C3H/HeJ mice were sacrificed via cervical dislocation at 72 h after the first injection. Peritoneal macrophages of the mice were obtained by flushing the peritoneal cavity of the mice with 10 ml PBS solution for 5 min. The peritoneal cells were centrifuged at 400 × g, 25°C, for 5 min. The cells were collected and washed twice with PBS, and suspended in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 1% penicillin/streptomycin and 10% (v/v) fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). The cells (2×106 cells/well) were seeded into 6-well culture plates and incubated at 37°C in a humid atmosphere of 5% CO2. Following incubation for 4 h, the non-adherent cells were removed and fresh RPMI-1640 was added, then the remaining cells were used as mouse peritoneal macrophages for the subsequent experiments.
Immunofluorescence staining for inflammatory cytokines in macrophages
The cultured peritoneal macrophages were fixed in 4% paraformaldehyde for 20 min and then washed with PBS three times. Following permeabilization with ice-cold 0.3% Triton X-100 for 10 min, the cells were blocked in 5% (m/v) bovine serum albumin (BSA, cat. no. A1993; Sigma-Aldrich; Merck KGaA) for 1 h at room temperature and then incubated with primary rabbit anti-mouse antibodies as follows: TNF-α (diluted 1:200 with PBS/5% BSA; cat. no. BS6000; Bioworld Technology, Inc., St. Louis Park, MN, USA), IL-1β (diluted 1:50 with PBS/5% BSA; cat. no. 31202; Cell Signaling Technology, Inc., Danvers, MA, USA), IL-6 (diluted 1:200 with PBS/5% BSA; cat. no. 12912; Cell Signaling Technology, Inc.) overnight at 4°C. Subsequently, cells were washed with PBS three times, followed by incubation with corresponding secondary phycoerythrin-conjugated goat anti-rabbit IgG (1:200 diluted with PBS/5% BSA; cat. no. sc-3739; Santa Cruz Biotechnology, Inc. Dallas, TX, USA) for 1 h at room temperature. For nuclear staining, cells were covered with 10 µg/ml DAPI for 2 min at room temperature. The stained cells were visualized under a fluorescence microscope at ×400 magnification with green excitation light. Different groups of images were taken in the same software settings.
Harvesting of aortas
The mice were sacrificed via cervical dislocation, and the midline of the chest was incised to expose the heart and lungs. The abdominal aorta was cut to release the blood. The aorta was dissected from the aortic arch. The fat tissues and connecting tissue were dissociated from aorta under the microscope, and washed with PBS three times.
Immunohistochemistry analysis of aortas
The aortas were fixed with 10% formalin at 4°C for 48 h, embedded in paraffin and sectioned transversely. For immunohistochemistry analysis, paraffin sections of 4 µm were deparaffinized, rehydrated, incubated with 0.3% H2O2 in PBS for 20 min at 25°C, and then blocked with 10% goat serum for 30 min at room temperature. Subsequently, tissue sections were washed with PBS and incubated with rabbit anti-mouse polyclonal antibody: ICAM-1 (diluted 1:150 with PBS; cat. no. BS7138; Bioworld Technology, Inc.), VCAM-1 (diluted 1:150 with PBS; cat. no. BS6005; Bioworld Technology, Inc.) or E-selectin (diluted 1:200 with PBS; cat. no. ab18981; Abcam, Cambridge, MA, USA) overnight at 4°C. Following washing with PBS three times, antibody reactivity was detected using peroxidase-conjugated goat anti-rabbit IgG (diluted 1:2,000 with PBS; cat. no. TA130017; OriGene Technologies, Inc., Beijing, China). The sections were developed with 50 and 100% diaminobenzidine solution (Sigma-Aldrich; Merck KGaA) diluted in ethanol, for 15 min each at 25°C. The tissue sections were visualized under a light microscope at ×40 magnification and analyzed using ImageJ software (ver.1.51J8; National Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was performed according to established protocols (24). In brief, aortas and peritoneal macrophages from C3H/HeN mice and C3H/HeJ mice (treated as described above) were homogenized in TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.). For inhibitory assays, the peritoneal macrophages from C3H/HeN mice were treated with 5 µM TAK-242 (TLR-4 inhibitor; Invitrogen; Thermo Fisher scientific, Inc.) or 20 µM pyrrolidine dithiocarbamate (PDTC; NF-κB inhibitor; Sigma-Aldrich; Merck KGaA) or 10 µM SB203508 (p38 MAPK inhibitor; Sigma-Aldrich; Merck KGaA) for 2 h, then stimulated with NR-IgG (100 µg/ml), anti-β2GPI–IgG (100 µg/ml) or LPS (500 ng/ml) for 6 h. Total RNA was isolated using TRIzol® and cDNA synthesis was performed using the Vazyme Reverse Transcription System (Vazyme, Piscataway, NJ, USA): HiScript II qRT SuperMix II was added into the RNA and reverse transcribed using the following temperature protocol: 25°C for 10 min, 42°C for 30 min and 85°C for 5 min. The primer sequences used for RT-qPCR are listed in Table I. mRNA levels of mouse TNF-α, IL-1β, IL-6, ICAM-1, VCAM-1 and E-selectin were measured by RT-qPCR using cDNA obtained from the RT reactions as the templates, with SYBR-Green I dye (Vazyme, Piscataway). RT-qPCR was conducted using the following parameters: Denaturation at 94°C for 5 min, followed by 40 cycles at 75°C for 30 sec each, and a final cycle at 72°C for 10 min. Amplification of cDNA for GAPDH was used as an internal control. The relative mRNA expressions of target genes compared to GAPDH were calculated by 2−ΔΔCq method (25).
Table I.
Sequences of primers used for reverse transcription-quantitative polymerase chain reaction analyses.
| Gene | Primer sequences | Product length (bp) | Annealing temperature (°C) |
|---|---|---|---|
| TNF-α | F:ATTATGGCTCAGGGTCCAAC | 197 | 60 |
| R:GACAGAGGCAACCTGACCAC | |||
| IL-1β | F:GCTGCTTCCAAACCTTTGACC | 110 | 56 |
| R:AGCCACAATGAGTGATACTGCC | |||
| IL-6 | F:GACTTCCATCCAGTTGCCTT | 150 | 59 |
| R:ATGTGTAATTAAGCCTCCGACT | |||
| ICAM-1 | F:CTCACTTGCAGCACTACGG | 138 | 59 |
| R:TTCATTCTCAAAACTGACAGGC | |||
| VCAM-1 | F:GCCACCCTCACCTTAATTGCT | 188 | 61 |
| R:GCACACGTCAGAACAACCGAA | |||
| E-selectin | F:ATAACGAGACGCCATCATGC | 191 | 58 |
| R:TGTCCACTGCCCTTGTGC | |||
| GAPDH | F:GGCATTGCTCTCAATGACAA | 200 | 58 |
| R:TGTGAGGGAGATGCTCAGTG |
TNF-α, tumor necrosis factor-α; F, forward primer; R, reverse primer; IL-interleukin; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
Western blot analysis
The mice were treated with different stimulants as described above. Protein samples were isolated from peritoneal macrophages and homogenate of aortas using a proteome extraction kit (radioimmunoprecipitation assay; Thermo Fisher Scientific Inc.), and the protein concentration of samples were measured using Pierce™ bicinchoninic acid protein assay kit (Thermo Fisher Scientific Inc.). The proteins and respective primary antibodies used in this assay were as follows: β-actin (cat. no. 4970), TNF-α (cat. no. 11948), IL-1β (cat. no. 31202), IL-6 (cat. no. 12912; all from Cell Signaling Technology, Inc.), ICAM-1 (cat. no. ab179707; Abcam), VCAM-1 (cat. no. 39036; Cell Signaling Technology, Inc.), E-selectin (cat. no. ab18981; Abcam), p38-MAPK (cat. no. 8690, p-p38-MAPK (cat. no. 4511), NF-κB (cat. no. 8242) and NF-κB phosphorylation (cat. no. 3033) (all from Cell Signaling Technology, Inc.). Equal amounts of protein (5 µg/well) from the samples under different experimental conditions were electrophoresed by SDS-PAGE on 12% gels. The gels were transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were blocked in PBS containing 5% (m/v) non-fat milk powder for 1 h at room temperature, washed with Tris aminomethane-buffered saline (TBS)/0.1% Triton X-100 (TBS/T) three times, and probed with corresponding rabbit anti-mouse antibodies described above (1:1,000 diluted with TBS/T) at 4°C overnight. Following three washes with TBS/T, the membranes were exposed to horseradish peroxidase-conjugated goat anti-rabbit antibodies (1:4,000 diluted with TBS/T; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) at room temperature for 1 h. Finally, the immunoblots were exposed and visualized with an Amersham Typhoon 9400 Fluor-S MultiImager (GE Healthcare Life Sciences, Little Chalfont, UK) using enhanced chemiluminescence western blotting detection reagents (GE Healthcare Life Sciences). Densitometry analysis was performed using ImageJ v1.8.0 software (National Institutes of Health).
Statistical analysis
Normally distributed variables were expressed as the mean ± standard deviation. One-way analyses of variance (ANOVA) with Newman-Keuls post-hoc test was used to compare three or more groups, and two-factor treatment results were analyzed by two-way ANOVA using SPSS statistical software package (version 20.0; IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
TLR-4 mediates the expression of inflammatory cytokines in peritoneal macrophages induced by anti-β2GPI–IgG
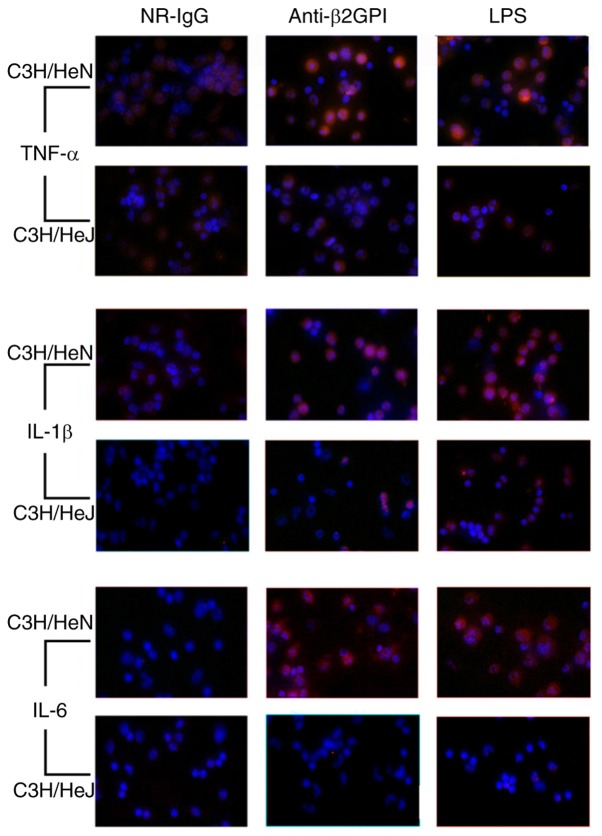
A previous study demonstrated the role of TLR-4 in the anti-β2GPI/β2GPI-induced expression of inflammatory cytokines in THP-1 cells and monocytes (14). The current study focused on examining the effect of TLR-4 in the activation of peritoneal macrophages in mice stimulated with anti-β2GPI–IgG. As demonstrated in Fig. 1, immunostaining revealed that anti-β2GPI–IgG significantly increased expression levels of TNF-α, IL-1β, and IL-6 in peritoneal macrophages isolated from C3H/HeN mice as compared with those of the NR-IgG group, as no significant fluorescence was detected in the isotype control. Notably, the increased expression levels of these cytokines were even higher than those of the LPS-stimulated positive control groups. However, in peritoneal macrophages isolated from C3H/HeJ mice (TLR-4-defective) stimulated with anti-β2GPI–IgG, the intensity of red fluorescence was far weaker than those of peritoneal macrophages derived from C3H/HeN received the same stimuli.
Figure 1.
TLR-4 mediates the expression of inflammatory cytokines in peritoneal macrophages from C3H/HeN mice induced by anti-β2GPI–IgG. C3H/HeN mice (TLR-4 intact; n=8 in each group) and C3H/HeJ mice (TLR-4 defective; n=8 in each group) were treated by intraperitoneal injection of NR-IgG (100 µg antibody per injection), anti-β2GPI–IgG (100 µg antibody per injection) at 0 and 48 h. Peritoneal macrophages were collected at 72 h after the first injection. Peritoneal macrophages in the positive control group were challenged with LPS (1 µg/g body-weight) 2 h before the experiment. The expression levels of inflammatory factors (TNF-α, IL-1β and IL-6) in peritoneal macrophages were detected by immunofluorescence with corresponding antibodies. The images are representative of three separated experiments with similar results. Magnification, ×400. TLR-4, Toll-like receptor-4; NR-IgG, isotype control antibody; anti-β2GPI, anti-β2-glycoprotein I; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α; IL, interleukin.
TLR-4 mediates the expression of adhesion molecules in vascular endothelial cells induced by anti-β2GPI–IgG
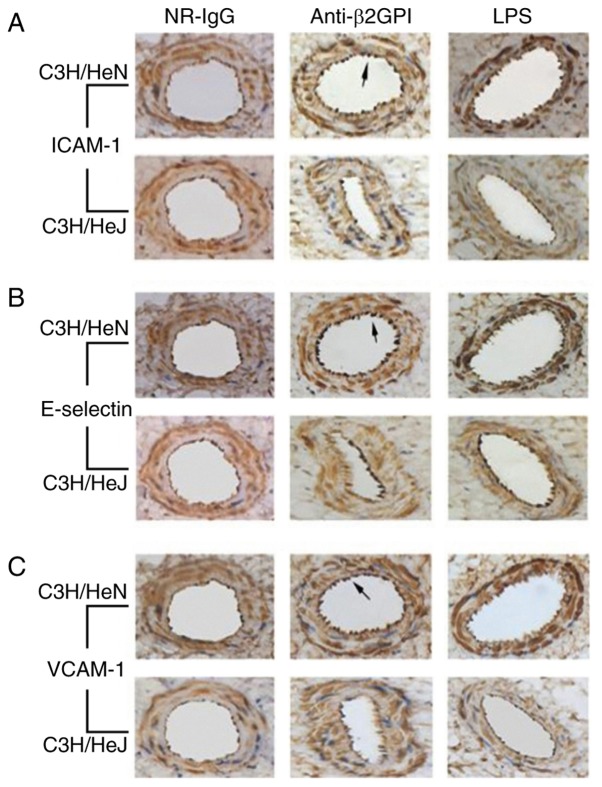
It has been reported that aPLs are able to enhance the adhesion of white blood cells to endothelial cells (26,27); thus, in the current study it was determined whether the expression levels of ICAM-1, E-selectin and VCAM-1 in mouse arteries were affected by anti-β2GPI–IgG injection. As presented in Fig. 2, brown granular staining on the aorta intima was produced using antibodies against ICAM-1, E-selectin and VCAM-1 from C3H/HeN mice treated with anti-β2GPI–IgG. In addition, endothelial cells of C3H/HeN mice stimulated with anti-β2GPI–IgG displayed more intense of brown color compared to those of the NR-IgG group, as no significant brown granular particles were detected in the isotype control. By contrast, endothelial cells derived from C3H/HeJ (TLR-4-defective) mice injected with anti-β2GPI–IgG exhibited lower expression of ICAM-1, E-selectin, and VCAM-1, as indicated by the lower intensity of brown granular particles on their intimal, compared with those derived from anti-β2GPI–IgG injected C3H/HeN mice.
Figure 2.
TLR-4 mediates the expression of adhesion molecules in vascular endothelial cells from mice induced by anti-β2GPI–IgG. C3H/HeN mice (TLR-4 intact; n=8 in each group) and C3H/HeJ mice (TLR-4 defective; n=8 in each group) were treated by intraperitoneal injection of NR-IgG (100 µg antibody per injection), anti-β2GPI–IgG (100 µg antibody per injection) at time 0 and 48 h. Aortas were collected at 72 h after the first injection. Mice in positive control group were challenged with LPS (1 µg/g body-weight) 2 h before the experiment. The expression levels of (A) ICAM-1, (B) E-selectin and (C) VCAM-1 were detected by immunohistochemistry with corresponding antibodies. The images are representative of three separate experiments with similar results. Magnification, ×40. TLR-4, Toll-like receptor-4; NR-IgG, isotype control antibody; Anti-β2GPI, anti-β2-glycoprotein I; LPS, lipopolysaccharide; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
TLR-4 mediates the mRNA expression levels of inflammatory cytokines in peritoneal macrophages, and adhesion molecules in vascular endothelial cells derived from mice induced by anti-β2GPI–IgG
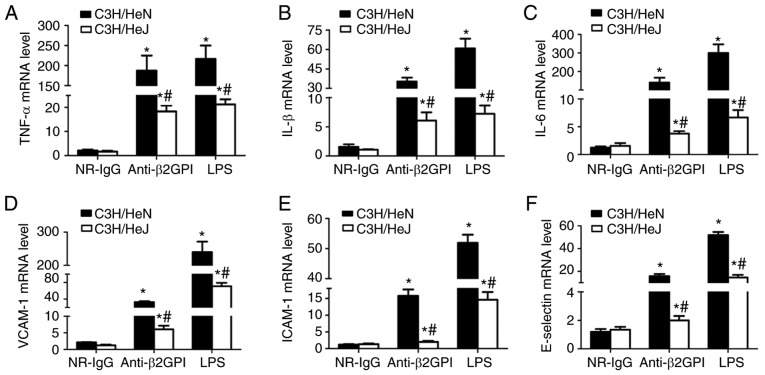
To investigate the roles of TLR-4 in the anti-β2GPI–IgG-induced expression of inflammatory cytokines and adhesion molecules, the mRNA levels of inflammatory cytokines (TNF-α, IL-1β and IL-6) were measured in peritoneal macrophages, and adhesion molecules (ICAM-1, E-selectin, and VCAM-1) were measured in artery endothelial cells with RT-qPCR. As presented in Fig. 3, NR-IgG-treated mice exhibited the basal expression levels of TNF-α, IL-1β, and IL-6 and ICAM-1, VCAM-1 and E-selectin, with very low levels. Treatment of C3H/HeN mice with anti-β2GPI–IgG or with LPS significantly enhanced the mRNA expression levels of all these effector molecules; however, in C3H/HeJ (TLR-4-defective) mice treated with anti-β2GPI–IgG or LPS, the mRNA expression levels of these effector molecules were significantly lower than those from C3H/HeN mice treated with the same stimuli.
Figure 3.
TLR-4 mediates the mRNA expression levels of inflammatory cytokines in peritoneal macrophages and adhesion molecules in vascular endothelial cells from mice induced by anti-β2GPI–IgG. C3H/HeN mice (TLR-4 intact; n=8 in each group) and C3H/HeJ mice (TLR-4 defective; n=8 in each group) were treated by intraperitoneal injection of NR-IgG (100 µg antibody per injection), anti-β2GPI–IgG (100 µg antibody per injection) at time 0 and 48 h. The peritoneal macrophages and aortas were collected at 72 h after the first injection, and the positive control group was challenged with LPS (1 µg/g body weigh) 2 h before the experiments. Total RNA was extracted from peritoneal macrophages (2×106) and mRNA expression levels of (A) TNF-α, (B) IL-1β and (C) IL-6 were detected by RT-qPCR. The total RNA was extracted from vascular tissue (aortas) and the mRNA levels of (D) VCAM-1, (E) ICAM-1 and (F) E-selectin were detected by RT-qPCR. The data are representative of three experiments. *P<0.05 vs. control NR-IgG; #P<0.05 vs. corresponding C3H/HeN stimulation group. TLR-4, Toll-like receptor-4; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; NR-IgG, isotype control antibody; Anti-β2GPI, anti-β2-glycoprotein I; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α; IL, interleukin; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
TLR-4 mediates the protein expression levels of inflammatory cytokines in peritoneal macrophages, and adhesion molecules in vascular endothelial cells from mice induced by anti-β2GPI–IgG
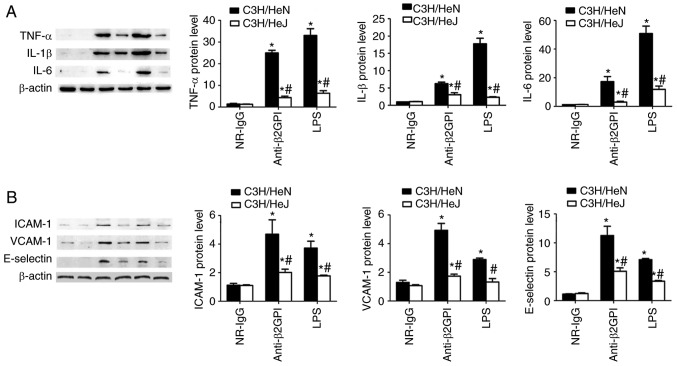
To further demonstrate the roles of TLR-4 in the anti-β2GPI–IgG-induced expression of inflammatory cytokines and adhesion molecules, the protein expression levels of inflammatory cytokines (TNF-α, IL-1β and IL-6) were examined in peritoneal macrophages, and adhesion molecules (ICAM-1, E-selectin and VCAM-1) were examined in artery endothelial cells via western blot analysis with corresponding antibodies. As demonstrated in Fig. 4, the protein levels of inflammatory cytokines (TNF-α, IL-1β and IL-6) and adhesion molecules (ICAM-1, E-selectin and VCAM-1) are basically consistent with their mRNA levels. NR-IgG-treated mice exhibited low basal levels of all above-mentioned molecules. Treatment of C3H/HeN mice with anti-β2GPI–IgG or with LPS significantly enhanced the protein expression level of these effector molecules; however, in C3H/HeJ mice treated with anti-β2GPI–IgG or LPS, the protein expression levels of these effector molecules were significantly lower than those from C3H/HeN mice treated with the same stimuli.
Figure 4.
TLR-4 mediated the protein expression of inflammatory cytokines in peritoneal macrophages and adhesion molecules in vascular endothelial cells from mice induced by anti-β2GPI–IgG. C3H/HeN mice (n=8 in each group) and C3H/HeJ mice (n=8 in each group) were treated by intraperitoneal injection of NR-IgG (100 µg antibody per injection), anti-β2GPI–IgG (100 µg antibody per injection) at time 0 and 48 h. The peritoneal macrophages and aortas were collected at 72 h after the first injection, and positive control group were challenged with LPS (1 µg/g body weigh) 2 h before the experiments. Protein samples were isolated from peritoneal macrophages and homogenate of aortas using a proteome extraction kit. The levels of (A) TNF-α, IL-1β, and IL-6 in peritoneal macrophages and (B) ICAM-1, VCAM-1, and E-selectin in vascular tissue were measured by western blotting using their corresponding antibodies. The data shown are the pooled data representative of three separated experiments. *P<0.05 vs. control NR-IgG; #P<0.05 vs. corresponding C3H/HeN stimulation group. TLR-4, Toll-like receptor-4; TNF-α, tumor necrosis factor-α; IL, interleukin; NR-IgG, isotype control antibody; Anti-β2GPI, anti-β2-glycoprotein I; LPS, lipopolysaccharide; ICAM-1, intercellular cell adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
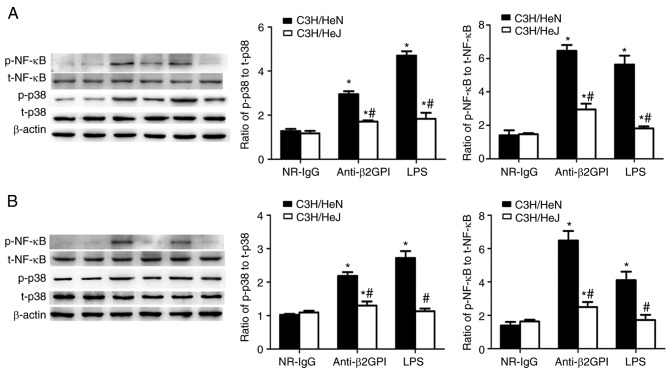
Role of TLR-4 in anti-β2GPI–IgG-stimulated phosphorylation of p38 MAPK and NF-κB p65
p38 MAPK and the p65 subunit of NF-κB are key molecules involved in triggering the expression of pro-inflammatory cytokines and pro-coagulant factors during the inflammatory response (28,29). Our previous experiments (19,20) demonstrated that p38 MAPK and NF-κB p65 were involved in inducing the expression of pro-inflammatory cytokines and pro-coagulant factors in human monocytes (THP-1 cells) stimulated by β2GPI/anti-β2GPI–IgG complex. Based on these preliminary experimental results, the effects of anti-β2GPI–IgG treatment on the phosphorylation status of p38 MAPK and NF-κB p65 in peritoneal macrophages and artery endothelial cells derived from C3H/HeN mice were examined. As presented in Fig. 5, treatment with anti-β2GPI–IgG or LPS (as the positive control) increased the phosphorylation of p38 MAPK and NF-κB p65 in peritoneal macrophages and artery endothelial cells from C3H/HeN mice, as compared with the NR-IgG treatment (as isotype control). Additionally, the phosphorylation of these two signaling molecules in peritoneal macrophages and artery endothelial cells derived from C3H/HeJ (TLR-4 defective) mice was significantly lower than in C3H/HeN mice, indicating the involvement of p38 MAPK and NF-κB p65 in the TLR-4-mediated anti-β2GPI–IgG-induced activation of mouse peritoneal macrophages and endothelial cells.
Figure 5.
Involvement of TLR-4 in anti-β2GPI–IgG-stimulated phosphorylation of p38 MAPK and NF-κB p65 during the expression of inflammatory cytokines and adhesion molecules. C3H/HeN mice (n=8 in each group) and C3H/HeJ mice (n=8 in each group) were treated by intraperitoneal injection of anti-β2GPI–IgG (100 µg antibody per injection) at time 0 and 48 h. The peritoneal macrophages and aortas were collected at 72 h after the first injection. The positive control group was challenged with LPS (1 µg/g body weigh) 2 h before the experiments. Protein samples were isolated from (A) peritoneal macrophages and (B) homogenate of aortas using a proteome extraction kit. The phosphorylation of p38 MAPK and the p65 subunit of NF-κB were measured by western blotting with their corresponding antibodies. The western blot and the quantitative data are representative of three separated experiments with the similar result. *P<0.05 vs. control NR-IgG; #P<0.05 vs. corresponding C3H/HeN stimulation group. TLR-4, Toll-like receptor-4; MAPK, mitogen-activated protein kinase; p-, phospho; NF-κB, nuclear factor-κB; t-, total; NR-IgG, isotype control antibody; Anti-β2GPI, anti-β2-glycoprotein I; LPS, lipopolysaccharide.
To further confirm the roles of a TLR-4/p38 MAPK/NF-κB signal transduction pathway in anti-β2GPI–IgG-induced activation of mouse peritoneal macrophages, the inhibitors of TLR-4, p38 MAPK and NF-κB were used in subsequent assays. As demonstrated in Fig. 6, TLR-4 inhibitor (TAK-242), NF-κB inhibitor (PDTC) and p38 MAPK inhibitor (SB203508) significantly decreased the expression of TNF-α, IL-6 and IL-1β mRNA in the peritoneal macrophages from anti-β2GPI–IgG and LPS-injected C3H/HeN mice (P<0.05 vs. untreated); however, none of these inhibitors could fully block the effects of anti-β2GPI–IgG or LPS on peritoneal macrophages, suggesting that TLR-4/p38 MAPK/NF-κB signal transduction pathway plays a part of roles in the process.
Figure 6.
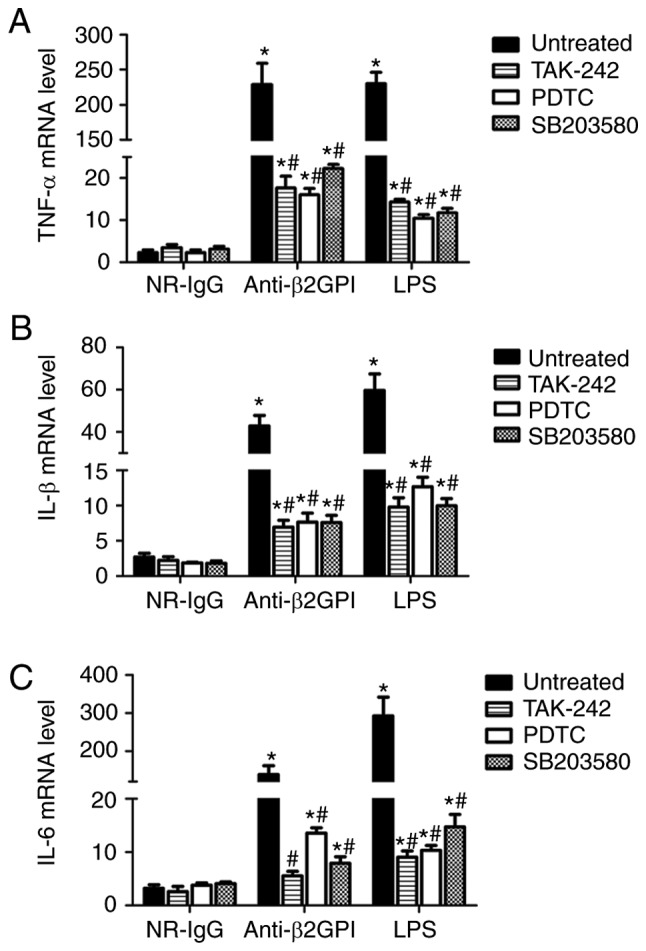
Inhibitors of TLR-4, p38 MAPK and NF-κB reduce the expression of TNF-α, IL-1β, and IL-6 mRNAs in peritoneal macrophages from C3H/HeN mice stimulated with anti-β2GPI–IgG. The peritoneal macrophages (2×106) from C3H/HeN mice (TLR-4 intact; n=8 in each group) were incubated with 5 µM TAK-242 (TLR-4 inhibitor) or 20 µM PDTC (NF-κB inhibitor) or 10 µM SB203508 (p38 MAPK inhibitor) for 2 h, then treated with NR-IgG (100 µg/ml), anti-β2GPI–IgG (100 µg/ml) or LPS (500 ng/ml) for 6 h. The total RNA were extracted from the macrophages and mRNA expression levels of (A) TNF-α, (B) IL-1β and (C) IL-6 were detected by reverse transcription-quantitative polymerase chain reaction. The data are representative of three separated experiments with the similar results. *P<0.05 vs. control NR-IgG; #P<0.05 vs. untreated. TLR-4, Toll-like receptor-4; TNF-α, tumor necrosis factor-α; PDTC, pyrrolidine dithiocarbamate; NR-IgG, isotype control antibody; Anti-β2GPI, anti-β2-glycoprotein I; LPS, lipopolysaccharide; IL, interleukin.
Discussion
The findings of the present study demonstrated that TLR-4 was involved in anti-β2GPI-induced activation of mouse peritoneal macrophages and vascular endothelial cells in vivo. These results indicate that anti-β2GPI antibodies stimulate the expression of pro-inflammatory molecules in macrophages and adhesion molecules in endothelial cells in mice. Furthermore, it was demonstrated that TLR-4 is involved in anti-β2GPI–IgG-stimulated phosphorylation of p38 MAPK and p65 NF-κB that mediates the induced expression of inflammatory cytokines in peritoneal macrophages, and adhesion molecules in vascular endothelial cells. This TLR-4/p38 MAPK/NF-κB signal transduction pathway has an important role in the anti-β2GPI–IgG-induced expression of pro-inflammatory molecules in macrophages.
The role of TLR-4 in anti-β2GPI-induced activation of mouse peritoneal macrophages and vascular endothelial cells in vivo is supported by the following findings. Firstly, the expression levels of inflammatory cytokines (TNF-α, IL-1β, and IL-6) were significantly upregulated at the mRNA and protein levels by anti-β2GPI–IgG in peritoneal macrophages derived from TLR-4-intact C3H/HeN mice, but were not induced in peritoneal macrophages derived from TLR-4-defective C3H/HeJ mice, as demonstrated by immunostaining, RT-qPCR analysis and immunoblot analysis. Additionally, the expression levels of adhesion molecules (ICAM-1, E-selectin and VCAM-1) were significantly upregulated by anti-β2GPI–IgG in vascular endothelial cells derived from TLR-4-intact C3H/HeN mice, but were not induced in vascular endothelial cells derived from TLR-4-defective C3H/HeJ mice, as indicated by immunostaining, RT-qPCR analysis and immunoblotting. These results clearly indicate that TLR-4 has a key role in mediating of the expression levels of inflammatory cytokines in mouse peritoneal macrophages, and adhesion molecules in vascular endothelial cells induced by anti-β2GPI–IgG. The results are consistent with the report by He et al (24), which demonstrated that TLR-4 was involved in the interaction of aPLs with endothelial cells in vivo and showed that anti-β2GPI thrombogenic activity was strongly reduced in mice expressing defective TLR4 (27). These results have also enriched the findings of our previous study (23), in which TLR-4 was involved in IgG-APS or anti-β2GPI-induced activation of peritoneal macrophages and vascular endothelium, as well as thrombosis induced by FeCl3.
The aPL/anti-β2GPI antibodies are known to contribute to thrombosis pathogenesis, pregnancy morbidity and accelerated atherosclerosis in patients with APS and systemic lupus erythematosus (30). By binding to Annexin A2 (ANX2), anti-β2GPI triggers activation of peritoneal macrophages and vascular endothelial cells, and regulates the production of pro-inflammatory molecules and pro-coagulant factors (31–36); however, as ANX2 is not a transmembrane protein, it is unable to recruit downstream signaling molecules. Instead, TLR-4 was considered as an ‘adaptor’ in cells expressing anti-β2GPI-induced effector molecules (35). Consistent with the proposed role of TLR-4 as an ‘adaptor’, the results of the current study further suggest the close co-operation between TLR-4 and anti-β2GPI antibody in mediating the pathogenesis of APS in vivo by inducing the expression of inflammatory cytokines and adhesion molecules.
In C3H/HeN mice, peritoneal macrophages and endothelial cells derived from anti-β2GPI treatment upregulated the expression of inflammatory cytokines (TNF-α, IL-1β and IL-6) and adhesion molecules (ICAM-1, VCAM-1 and E-selectin); however, this phenomenon was not been present in C3H/HeN mice (TLR-4 defective) treated with NR-IgG. Our previous study demonstrated that targeting β2GPI using F(ab)2 fragments, but not the Fc segment, of anti-β2GPI was affected by TLR-4 (31,34). A significant protective effect was observed in TLR-4 defective C3H/HeJ mice, i.e. the increased expression levels of inflammatory cytokines and adhesion molecules did not occur in C3H/HeJ mice treated with anti-β2GPI. These results clearly indicate that TLR-4 is involved in mediating the effects of anti-β2GPI in the pathogenesis of thrombosis in APS. Inflammatory cytokines (TNF-α, IL-1β and IL-6) inhibit the physiological anti-coagulant system, transforming endothelial cells from the anti-coagulant state into the pro-coagulant state (37). The increased expression of adhesion molecules (e.g. ICAM-1, VCAM-1 and E-selectin) can induce the adhesion of monocytes and platelets to endothelial cells, thus promoting the inflammation and coagulation responses. Increased adhesion of leukocytes to the endothelium of mouse cremaster muscle, an indication of activation of endothelial cells in vivo, and enhanced thrombosis in vivo was found to be closely associated with upregulated expression of ICAM-1, VCAM-1 and P-selectin on endothelial cells stimulated by aPL (13,27,38). Furthermore, ICAM-1, VCAM-1 and P-selectin were shown to be involved in mediating the activation of endothelial cells and enhanced thrombosis by aPL in vivo, as demonstrated by reduced adhesion of leukocytes in ICAM-1-defective mice and completely abrogated leukocyte adhesion in ICAM-1/P-selectin double defective mice treated with IgG-APS, compared with wild-type mice treated with IgG-APS (38). These observations indicate that increased expression levels of ICAM-1, VCAM-1 and E-selectin are involved in the thrombotic complications mediated by aPL antibodies.
LPS is an important TLR-4 ligand. In the current study, the expression levels of inflammatory cytokines and adhesion molecules in TLR-4-intact C3H/HeN mice stimulated with anti-β2GPI were higher than those stimulated by LPS, suggesting that in addition to TLR-4, there may be other receptors mediating the effects of anti-β2GPI in vivo. In addition, the expression levels of inflammatory cytokines and adhesion molecules were not fully abolished in TLR-4 defective C3H/HeJ mice. Furthermore, the TLR-4 inhibitor TAK-242 blocked the effects of anti-β2GPI or LPS on the expression of inflammatory cytokines in C3H/HeN mouse peritoneal macrophages, but did not fully abrogate the effects. These results suggested that other receptors may participate in the antibody binding and associated activation pathways. In fact, it was also reported that TLR-8, another member of TLR family, induced upregulation of TNF-α following aPL stimulation (39). Furthermore, TLR-2, which has a fundamental role in pathogen recognition and activation of innate immunity, was also reported to have a role in the pathogenesis of APS (40). Our previous study investigated the relationship between TLR-2 and the β2GPI/anti-β2GPI complex, and revealed that TLR-2 blockade could reduce TNF-α expression induced by β2GPI/anti-β2GPI complex in mouse peritoneal macrophages (41). Additionally, it has also been previously demonstrated that apolipoprotein E receptor (apoER) and platelet factor 4 (PF4) are able to induce the activation of endothelial cells and platelets (42); thus, more studies are required to define the precise roles of TLR-4, TLR-8, TLR-2, apoER, PF4 and other factors in mediating the pathogenesis of APS caused by anti-β2GPI.
In the present study, further experiments were performed to identify molecules downstream of TLR-4. It has been previous reported that the interaction between TLR-4 and anti-β2GPI aPLs led to the activation of Myd88-dependent and TRIF-dependent signaling pathways, which, in turn, induced phosphorylation of NF-κB p65 and p38 MAPK, and finally the upregulation of TNF-α, IL-1β, IFN-γ and IL-6 protein expression (43). Consistent with this finding, in the current study, the phosphorylated levels of p38 MAPK and NF-κB p65 in C3H/HeN cells stimulated by anti-β2GPI were significantly increased compared with those of C3H/HeJ mice, suggesting that p38 MAPK and NF-κB p65 are involved in TLR-4-mediated β2GPI-induced activation in peritoneal macrophages and vascular endothelial cells. Subsequently, it was demonstrated that NF-κB inhibitor, PDTC, and p38 MAPK inhibitor, SB203508, significantly decreased the expression of TNF-α, IL-1β and IL-6 mRNA in peritoneal macrophages of C3H/HeN mice. p38 MAPK and NF-κB p65 are the key factors involved in mediating inflammatory responses in several types of cells induced by different stimuli. For instance, transcriptional activation of NF-κB and TNF-α production in response to Borrelia burgdorferi antigens was found to be controlled by RelA phosphorylation, which was mediated by mitogen- and stress-activated protein kinase 1 (44). Activation of the p38 MAPK/NF-κB pathway has been reported to contribute to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells (45). TLR-4 is also involved in mediating anti-β2GPI/β2GPI-induced expression of tissue factor (TF) in THP-1 cells (34). Circulating levels of TF and pro-inflammatory cytokines, including IL-6, IL-6 receptor, TNF-α and interferon-γ, were reported to be higher in patients with primary APS or leprosy associated with aPLs. It is proposed that the imbalance of cytokines and upregulation of the TF pathway may be potential mechanisms of thrombosis in APS.
Anti-coagulant therapy has been the mainstay of management in APS; however, the therapeutic effects of the current treatments are not satisfactory (46). Therefore, effective alternative or additional strategies for treatment of APS are urgently needed. Blockade of TLR-4 signaling pathway with genetic and/or pharmacological approaches in combination with anti-inflammatory drugs and anti-coagulant agents may be a new strategy for management in APS. In term of pharmacological approaches, it was reported that resatorvid (or TAK-242), a small-molecule inhibitor, bound selectively to TLR-4 that interferes with interactions between TLR-4 and its adaptor molecules, can serve as an inhibitor for TLR-4 (47). TLR-4 activation is reported to be a potent inducer of signaling pathways in the nervous system, causing chronic pain, opioid tolerance and dependence. Small-molecule modulators of TLR-4, including MD2-I and tricyclic antidepressants, can be developed as therapeutic agents to target TLR-4-mediated neuroinflammation (48). Several other approaches, including inhibition of intracellular pathways, anti-cytokine therapies and hydroxychloroquine, have been also suggested (49).
TLR-4 is involved in the activation of peritoneal macrophages and endothelial cells stimulated by anti-β2GPI in vivo. TLR-4-mediated pro-inflammatory and pro-coagulant events via p38 MAPK/NF-κB pathway are involved in the aPL-mediated pathogenic effects in APS. Blockage of TLR-4/p38 MAPK/NF-κB signal transduction pathways may be a promising potential alternative or additional treatment strategy for APS.
Acknowledgements
The authors would like to acknowledge all members of Hong Zhou team for collaboration to the project.
Funding
This research was supported by National Natural Science Foundation of China (grant no. 81370614), a grant awarded to HZ.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
MW and HZ conceived and designed the experiments. MW, XK, YX and CH performed the experiments: MW, XK, HZ, TW and CH analyzed the data. MW, XK, YX, HZ, TW and CH contributed reagents/materials/analysis tools. MW, XK and HZ wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All the experiments involving animals were approved by the Laboratory Animal Administration Committee of Jiangsu University (Zhenjiang, China; UJS-LAER-2014072301).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chaturvedi S, McCrae KR. Diagnosis and management of the antiphospholipid syndrome. Blood Rev. 2017;31:406–417. doi: 10.1016/j.blre.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeil HP, Chesterman CN, Krilis SA. Immunology and clinical importance of antiphospholipid antibodies. Adv Immunol. 1991;49:193–280. doi: 10.1016/S0065-2776(08)60777-4. [DOI] [PubMed] [Google Scholar]
- 3.Willis R, Harris EN, Pierangeli SS. Pathogenesis of the antiphospholipid syndrome. Semin Thromb Hemost. 2012;38:305–321. doi: 10.1055/s-0032-1311827. [DOI] [PubMed] [Google Scholar]
- 4.Tanne D, Katzav A, Beilin O, Grigoriadis NC, Blank M, Pick CG, Landenberg Pv, Shoenfeld Y, Chapman J. Interaction of inflammation, thrombosis, aspirin and enoxaparin in CNS experimental antiphospholipid syndrome. Neurobiol Dis. 2008;30:56–64. doi: 10.1016/j.nbd.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 5.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: Beta 2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galli M, Comfurius P, Maassen C, Hemker HC, de Baets MH, van Breda-Vriesman PJ, Barbui T, Zwaal RF, Bevers EM. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335:1544–1547. doi: 10.1016/0140-6736(90)91374-J. [DOI] [PubMed] [Google Scholar]
- 7.de Laat B, Derksen RH, Urbanus RT, de Groot PG. IgG antibodies that recognize epitope Gly40-Arg43 in domain I of beta 2-glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood. 2005;105:1540–1545. doi: 10.1182/blood-2004-09-3387. [DOI] [PubMed] [Google Scholar]
- 8.Forastiero RR, Martinuzzo ME, de Larrañaga GF. Circulating levels of tissue factor and proinflammatory cytokines in patients with primary antiphospholipid syndrome or leprosy related antiphospholipid antibodies. Lupus. 2005;14:129–136. doi: 10.1191/0961203305lu2048oa. [DOI] [PubMed] [Google Scholar]
- 9.Clemens N, Frauenknecht K, Katzav A, Sommer C, von Landenberg P. In vitro effects of antiphospholipid syndrome-IgG fractions and human monoclonal antiphospholipid IgG antibody on human umbilical vein endothelial cells and monocytes. Ann NY Acad Sci. 2009;1173:805–813. doi: 10.1111/j.1749-6632.2009.04632.x. [DOI] [PubMed] [Google Scholar]
- 10.Levi M, van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med. 2005;15:254–259. doi: 10.1016/j.tcm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 11.ten Cate JW, van der Poll T, Levi M, ten Cate H, van Deventer SJ. Cytokines: Triggers of clinical thrombotic disease. Thromb Haemost. 1997;78:415–419. doi: 10.1055/s-0038-1657562. [DOI] [PubMed] [Google Scholar]
- 12.Meroni PL, Raschi E, Testoni C, Tincani A, Balestrieri G, Molteni R, Khamashta MA, Tremoli E, Camera M. Statins prevent endothelial cell activation induced by antiphospholipid (anti-beta2-glycoprotein I) antibodies: Effect on the proadhesive and proinflammatory phenotype. Arthritis Rheum. 2001;44:2870–2878. doi: 10.1002/1529-0131(200112)44:12<2870::AID-ART475>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 13.Pierangeli SS, Colden-Stanfield M, Liu X, Barker JH, Anderson GL, Harris EN. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation. 1999;99:1997–2002. doi: 10.1161/01.CIR.99.15.1997. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, Sheng L, Wang H, Xie H, Mu Y, Wang T, Yan J. Anti-β2GPI/β2GPI stimulates activation of THP-1 cells through TLR4/MD-2/MyD88 and NF-κB signaling pathways. Thromb Res. 2013;132:742–749. doi: 10.1016/j.thromres.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 15.Vega-Ostertag ME, Ferrara DE, Romay-Penabad Z, Liu X, Taylor WR, Colden-Stanfield M, Pierangeli SS. Role of p38 mitogen-activated protein kinase in antiphospholipid antibody-mediated thrombosis and endothelial cell activation. J Thromb Haemost. 2007;5:1828–1834. doi: 10.1111/j.1538-7836.2007.02680.x. [DOI] [PubMed] [Google Scholar]
- 16.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 17.Medzhitov R, Janeway CA., Jr Innate immunity: The virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/S0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 18.Werling D, Jungi TW. TOLL-like receptors linking innate and adaptive immune response. Vet Immunol Immunopathol. 2003;91:1–12. doi: 10.1016/S0165-2427(02)00228-3. [DOI] [PubMed] [Google Scholar]
- 19.Xia L, Zhou H, Hu L, Xie H, Wang T, Xu Y, Liu J, Zhang X, Yan J. Both NF-κB and c-Jun/AP-1 involved in anti-β2GPI/β2GPI-induced tissue factor expression in monocytes. Thromb Haemost. 2013;109:643–651. doi: 10.1160/TH12-09-0655. [DOI] [PubMed] [Google Scholar]
- 20.Zhou H, Chen D, Xie H, Xia L, Wang T, Yuan W, Yan J. Activation of MAPKs in the anti-β2GPI/β2GPI-induced tissue factor expression through TLR4/IRAKs pathway in THP-1 cells. Thromb Res. 2012;130:e229–e235. doi: 10.1016/j.thromres.2012.08.303. [DOI] [PubMed] [Google Scholar]
- 21.Xie HX, Zhou H, Wang HB, Chen DD, Wang T, Zhang XM, Xia LF, Mu Y. The activation of TRIF-dependent signaling pathway in THP-1 cells induced by β2 GPI/anti-β2 GPI antibodies complex. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011;27:1280–1283, 1287. (In Chinese) [PubMed] [Google Scholar]
- 22.Ethics and Animal Use, corp-author. Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press (US); Washington (DC): 2011. [Google Scholar]
- 23.Xie H, Kong X, Zhou H, Xie Y, Sheng L, Wang T, Xia L, Yan J. TLR4 is involved in the pathogenic effects observed in a murine model of antiphospholipid syndrome. Clin Immunol. 2015;160:198–210. doi: 10.1016/j.clim.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 24.He C, Zhang G, Zhou H, Cheng S, Farwa A. Effects of Toll-like receptor 4 on β2-glycoprotein I-induced splenic T cell subsets differentiation. Immunol Lett. 2018;198:17–25. doi: 10.1016/j.imlet.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Brandt KJ, Kruithof EK, de Moerloose P. Receptors involved in cell activation by antiphospholipid antibodies. Thromb Res. 2013;132:408–413. doi: 10.1016/j.thromres.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Pierangeli SS, Vega-Ostertag ME, Raschi E, Liu X, Romay-Penabad Z, De Micheli V, Galli M, Moia M, Tincani A, Borghi MO, et al. Toll-like receptor and antiphospholipid mediated thrombosis: In vivo studies. Ann Rheum Dis. 2007;66:1327–1333. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao MY, Chen L, Yang L, Yu X, Kou JP, Yu BY. Berberine inhibits LPS-induced TF procoagulant activity and expression through NF-κB/p65, Akt and MAPK pathway in THP-1 cells. Pharmacol Rep. 2014;66:480–484. doi: 10.1016/j.pharep.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Zheng Z, Li X, Ma X. Unfractionated heparin inhibits lipopolysaccharide-induced inflammatory response through blocking p38 MAPK and NF-κB activation on endothelial cell. Cytokine. 2012;60:114–121. doi: 10.1016/j.cyto.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Willis R, Pierangeli SS. Anti-β2-glycoprotein I antibodies. Ann NY Acad Sci. 2013;1285:44–58. doi: 10.1111/nyas.12080. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H, Wolberg AS, Roubey RA. Characterization of monocyte tissue factor activity induced by IgG antiphospholipid antibodies and inhibition by dilazep. Blood. 2004;104:2353–2358. doi: 10.1182/blood-2004-01-0145. [DOI] [PubMed] [Google Scholar]
- 32.Zhou H, Wang H, Li N, Yu Y, Huang H, Yan Y, Wang T. Annexin A2 mediates anti-beta 2 GPI/beta 2 GPI-induced tissue factor expression on monocytes. Int J Mol Med. 2009;24:557–562. doi: 10.3892/ijmm_00000265. [DOI] [PubMed] [Google Scholar]
- 33.Zhou H, Ling S, Yu Y, Wang T, Hu H. Involvement of Annexin A2 in anti-beta2GPI/beta2GPI-induced tissue factor expression on monocytes. Cell Res. 2007;17:737–739. doi: 10.1038/cr.2007.33. [DOI] [PubMed] [Google Scholar]
- 34.Zhou H, Yan Y, Xu G, Zhou B, Wen H, Guo D, Zhou F, Wang H. Toll-like receptor (TLR)-4 mediates anti-β2GPI/β2GPI-induced tissue factor expression in THP-1 cells. Clin Exp Immunol. 2011;163:189–198. doi: 10.1111/j.1365-2249.2010.04291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie H, Zhou H, Wang H, Chen D, Xia L, Wang T, Yan J. Anti-β(2)GPI/β(2)GPI induced TF and TNF-α expression in monocytes involving both TLR4/MyD88 and TLR4/TRIF signaling pathways. Mol Immunol. 2013;53:246–254. doi: 10.1016/j.molimm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Hurst J, Lorenz M, Prinz N, von Landenberg P. The roll of Toll-like receptors in the antiphospholipid syndrome. Curr Rheumatol Rep. 2010;12:58–63. doi: 10.1007/s11926-009-0079-0. [DOI] [PubMed] [Google Scholar]
- 37.Zelaya H, Rothmeier AS, Ruf W. Tissue factor at the crossroad of coagulation and cell signaling. J Thromb Haemost. 2018;16:1941–1952. doi: 10.1111/jth.14246. [DOI] [PubMed] [Google Scholar]
- 38.Pierangeli SS, Espinola RG, Liu X, Harris EN. Thrombogenic effects of antiphospholipid antibodies are mediated by intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and P-selectin. Circ Res. 2001;88:245–250. doi: 10.1161/01.RES.88.2.245. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh TK, Mickelson DJ, Solberg JC, Lipson KE, Inglefield JR, Alkan SS. TLR-TLR cross talk in human PBMC resulting in synergistic and antagonistic regulation of type-1 and 2 interferons, IL-12 and TNF-alpha. Int Immunopharmacol. 2007;7:1111–1121. doi: 10.1016/j.intimp.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Benhamou Y, Bellien J, Armengol G, Brakenhielm E, Adriouch S, Iacob M, Remy-Jouet I, Le Cam-Duchez V, Monteil C, Renet S, et al. Role of Toll-like receptors 2 and 4 in mediating endothelial dysfunction and arterial remodeling in primary arterial antiphospholipid syndrome. Arthritis Rheumatol. 2014;66:3210–3220. doi: 10.1002/art.38785. [DOI] [PubMed] [Google Scholar]
- 41.Yu Y, Zhou H, Xia L, Kong X, Xie Y, Xie H, He C, Cheng S. TLR2 blockade reduces TNF-α expression induced by β2GP1/anti-β2GP1 complex in mouse peritoneal macrophages. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32:446–450, 456. (In Chinese) [PubMed] [Google Scholar]
- 42.Sikara MP, Routsias JG, Samiotaki M, Panayotou G, Moutsopoulos HM, Vlachoyiannopoulos PG. {beta}2 Glycoprotein I ({beta}2GPI) binds platelet factor 4 (PF4): Implications for the pathogenesis of antiphospholipid syndrome. Blood. 2010;115:713–723. doi: 10.1182/blood-2009-03-206367. [DOI] [PubMed] [Google Scholar]
- 43.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Olson CM, Hedrick MN, Izadi H, Bates TC, Olivera ER, Anguita J. p38 mitogen-activated protein kinase controls NF-kappaB transcriptional activation and tumor necrosis factor alpha production through RelA phosphorylation mediated by mitogen- and stress-activated protein kinase 1 in response to Borrelia burgdorferi antigens. Infect Immun. 2007;75:270–277. doi: 10.1128/IAI.01412-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo RM, Xu WM, Lin JC, Mo LQ, Hua XX, Chen PX, Wu K, Zheng DD, Feng JQ. Activation of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol Med Rep. 2013;8:603–608. doi: 10.3892/mmr.2013.1554. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Irastorza G, Khamashta MA. Lupus and pregnancy: Integrating clues from the bench and bedside. Eur J Clin Invest. 2011;41:672–678. doi: 10.1111/j.1365-2362.2010.02443.x. [DOI] [PubMed] [Google Scholar]
- 47.Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79:34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Csakai A, Jin J, Zhang F, Yin H. Therapeutic developments targeting Toll-like receptor-4-mediated neuroinflammation. ChemMedChem. 2016;11:154–165. doi: 10.1002/cmdc.201500188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Comarmond C, Cacoub P. Antiphospholipid syndrome: From pathogenesis to novel immunomodulatory therapies. Autoimmun Rev. 2013;12:752–757. doi: 10.1016/j.autrev.2012.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.