Abstract
The hydroferrate fluid MRN-100, an iron-based compound with potent antioxidant characteristics, was examined to identify its possible anti-inflammatory effects on human dendritic cells (DCs) in vitro. Human monocyte–derived DCs were treated with MRN-100 at two concentrations (50 and 100 μL/mL) for 24 h and then stimulated with or without lipopolysaccharides (LPS). The expression of DC maturation markers was assessed by flow cytometry and the production of cytokines was determined by enzyme-linked immunosorbent assay (ELISA). Functional assay was performed by co-culturing MRN-100-treated and untreated DCs with allogeneic naïve CD4+ T cells and assaying the T cells’ cytokine production. Results show that treatment with MRN-100 significantly upregulated the co-stimulatory molecules CD80 and CD86 and increased human leukocyte antigen-DR (HLA-DR) though not significantly. MRN-100 treatment also significantly increased the production of the anti-inflammatory cytokine interleukin (IL)-10. On the other hand, MRN-100 significantly induced the secretion of pro-inflammatory cytokines such as IL-6 only at high concentrations. Furthermore, DCs pretreated with MRN-100 and either stimulated or not with LPS were able to prime CD4+ T cells to secrete significant amounts of IL-10 while inhibiting the secretion of pro-inflammatory cytokine tumor necrosis factor (TNF)-α. These results indicate that MRN-100 is a powerful anti-inflammatory agent that promotes the generation of an anti-inflammatory immune response in vitro. MRN-100 could be beneficial for treating patients with inflammatory diseases, including arthritis and type 1 diabetes, and its potential benefits should be examined in clinical trials.
Keywords: anti-inflammatory, CD4+ T cells, dendritic cells, hydroferrate fluid, MRN-100
Introduction
Inflammation has been defined as the way a body tries to heal itself after sustaining an injury; defend itself against viruses, bacteria, and other foreign invaders; and repair any damaged tissue. Several types of inflammatory diseases have been identified and are becoming a more significant health problem globally, including rheumatoid arthritis; inflammatory bowel diseases like Crohn’s disease and ulcerative colitis; and type 1 diabetes.1–3 These are chronic, progressive, and often disabling disorders with no cure. Frequently immunosuppressive agents are used to control inflammation and maintain remission, and individuals with these diseases often struggle to find long-term success with any one therapeutic agent.4,5 In addition, the traditional therapeutic modalities for oral inflammatory periodontitis disease have shown limited success,6 and treatments for modulating neuroinflammation in the central and peripheral nervous system are all associated with risks and potential complications.7,8
Inflammatory responses in the body occur in parallel with cellular responses by the adaptive immune system. The activation of T cells to secrete cytokines is regulated by dendritic cells (DCs). DCs are the professional antigen-presenting cells,9 up-taking antigens and processing them to present to T cells and activate adaptive immunity.10 DCs possess several types of receptors to sense and respond to pathogens and other threats.11 Once a threat is sensed, DCs capture the antigens via phagocytosis, endocytosis, and so on. Since the next phase requires DCs to present antigens to T cells, the antigen capture by DCs along with signaling by pathogen recognition receptors (PRRs) causes maturation of DCs by upregulation of co-stimulatory CD80, CD86, CD40, and major histocompatibility complex (MHC) molecules and by secretion of inflammatory and anti-inflammatory cytokines. Adaptive immune cells such as CD4+ and CD8+ T cells cause destruction by a mechanism that involves their secretion of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-1.12 Two other cytokines that have been studied in relation to inflammation are IL-6 (pro-inflammatory) and IL-10 (anti-inflammatory). IL-6 is a multifunctional cytokine that plays a key role in inflammation and tissue injury, and regulates humoral and cellular responses. IL-6 levels positively correlate with many inflammatory diseases,13 though IL-6 has different functions and at times can have anti-inflammatory and pro-resolution properties.14 IL-10 is a pleiotropic cytokine with established anti-inflammatory properties.15 IL-10 treatment was associated with an increase in the percentage of CD4+ CD25+ regulatory T cells.16
In addition, mature DCs express the chemokine receptor CCR-7, allowing them to migrate to lymph nodes to prime CD4 and CD8 T cell responses, and they secrete cytokines such as IL-15, IL-12, and type I IFNs.17,18 The cytokines secreted by matured DCs influence the polarization of Th cell responses to Th1, Th2, Treg, and Th17.19 For example, IL-12 promotes differentiation of Th1 cells that produce pro-inflammatory cytokines for killing intracellular parasites, while Th2 mainly protects against helminthic infections;19 transforming growth factor (TGF)-β, IL-1β, IL-6, and IL-23 have been responsible for the Th17 polarization required for clearance of fungal infections; in addition to Th cells, cytokine secretion by matured DCs also primes cytotoxic CD8 T and natural killer (NK) cell responses via IL-12, IL-18, and type I IFN.20,21
Nonsteroidal anti-inflammatory drugs (NSAIDs) are drugs that relieve pain. These pain relievers can also curb inflammation at prescription doses. However, NSAIDs are associated with side effects including stomach ulcers and bleeding and an increased risk of cardiovascular complications by cyclooxygenase (COX)-2 inhibition.22–24 Therefore, there is an urgent and compelling need for finding safe and nontoxic anti-inflammatory agents.
This study examines the ability of a hydroferrate fluid, MRN-100, to activate DCs in regard to phenotypic changes and the ability of MRN-100-stimulated DCs to activate CD4+ T cells. The study includes measuring the kind of cytokines secreted and the mechanisms that underlie MRN-100’s effect. Being an iron-based compound, MRN-100 contains bivalent and trivalent ferrates isolated from phytosin.25 The antioxidant effect of MRN-100 has been studied in several animal models and in in vitro studies, where it has been shown to protect against age-associated oxidative stress,26 chemical carcinogens,27 and oxidative stress–induced apoptosis in lymphocytes.28 The findings of this study indicate that the natural product MRN-100 has the ability to activate human DCs and modify cytokine activity, including a significant increase in IL-10 expression. MRN-100 can therefore be considered as a strong adjuvant with the capability to provoke an effective immunological response against inflammatory diseases.
Materials and methods
Antibodies and reagents
This study used the following antibodies and reagents: human leukocyte antigen-DR (HLA-DR) PerCP (Clone L243 (G46-6)), CD80 PE (Clone L307.4), CD86 PE (Clone 2331 (FUN-1)), and CD11c APC (Clone B-ly6). All reagents were purchased from BD Biosciences (San Jose, CA). The negative control was an isotype antibody from BD Biosciences. Escherichia coli lipopolysaccharide (LPS) was purchased from InvivoGen (San Diego, CA).
Hydroferrate fluid (MRN-100)
MRN-100 was prepped for use in distilled water (DW) with the concentration of Fe2+ and Fe3+ ions at about 2 × 10−12 mol/L. MRN-100 was acquired from phytosin, a plant extract, and is composed of iron and neutral lipid compounds. It is a compound found in plants, including wheat, radish seeds, and rice. The following is the method of extraction for MRN-100: phytosin (1 unit) was dissolved in 100 mL DW before adding FeCl3·6H2O. A liquid–liquid extraction technique was then employed to remove lipid compounds. This technique was then followed by filtering the remaining liquid using No. 5 filter paper. The filtrate was subsequently evaporated and condensed in a water bath. In order to generate MRN-100, the iron compound that resulted was subjected to fractional determination with respect to bivalent ferrate and trivalent ferrate. In order to reduce Fe (III) to Fe (II), hydroxylamine-HCl (10%) was then added to the sample liquid. To determine the quantity of Fe (II), the o-phenanthroline method was employed. All of the ferrate quantities were then determined, in addition to those of Fe (III). Finally, the acquired iron compounds were bivalent and trivalent ferrates.16 MRN-100 was obtained from ACM Co., Ltd, Japan.
Complete medium
Complete medium consists of RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 1 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin.
Isolation and culture of human monocyte–derived DCs
We prepped monocyte-derived DCs in the manner previously described.29,30 To summarize, blood was obtained from normal healthy donors (approved by the Institutional Review Board, Charles Drew University). Ficoll-Hypaque density gradient centrifugation was used to separate peripheral blood mononuclear cells before allowing the cells to attach onto culture plates for 2 h. We removed any cells not adhering to plates, then cultured the monocytes adhering to plates for 6 days within a humidified atmosphere containing 5% CO2 at 37°C in RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin, 1 mM glutamine, 100 μg/mL streptomycin, human granulocyte-macrophage colony stimulating factor (GM-CSF) at 50 ng/mL (PeproTech, Rocky Hill, NJ), and 10 ng/mL recombinant human IL-4 (PeproTech). Subsequently, every 2 days, we discarded half of the culturing medium and replaced it with fresh medium containing GM-CSF and IL-4. DCs were collected after 6 days, after which we measured the purity of the DCs obtained as >95%. We pulsed DCs with MRN-100 (50 and 100 μL/mL) for 24 h and with 1 μg/mL E. coli LPS K12 (InvivoGen). For a positive control, we pulsed DCs with 1 μg/mL E. coli LPS but without MRN-100 for 24 h.
DC phenotyping
By employing flow cytometry, we determined the expression of cell surface markers. Fluorescence-activated cell sorting (FACS) analysis-flow cytometry was conducted using FACSCalibur (Becton-Dickenson, San Jose, CA) and analyzed with FlowJo software (TreeStar). Gated CD11c+ HLA-DR+ DCs for the expression of CD80, CD86, and HLA-DR were analyzed. Appropriate antibodies were acquired from BD Pharmingen (San Diego, CA). DC viability was tested using Trypan blue and more than 95% of cells were found to be viable.
Cytokine production by DCs
Monocyte-derived DCs were incubated with MRN-100 at the concentrations of 50 and 100 μL/mL for 24 h. Supernatants were collected and then stored at −70°C until being analyzed. We used specific enzyme-linked immunosorbent assay (ELISA) kits to measure cytokines IL-6, IL-10, and TNF-α (BD Pharmingen) in the supernatants following manufacturer’s instructions.
DC-CD4+ T cells
A magnetic bead–based kit (Stem Cell Technologies, Vancouver, Canada) was used to purify allogeneic CD4+ T cells by negative selection. We subsequently cultured allogeneic CD4+ T cells with DCs previously treated with MRN-100 (50 and 100 μL/mL) for 24 h as previously described. For 5 days, we co-cultured the DC-CD4+ T cells in a U-bottom 96-well plate. The DC:CD4+ T cell ratio was 1:5 (2 × 104:1 × 105). At the end of 5 days, we collected the supernatants and kept them at −70°C. Then, we employed a specific ELISA kit (BD Pharmingen) to detect cytokines IFN-γ, IL-10, and TNF-α. Cell viability was tested by Trypan blue, and more than 95% cells were alive.
Statistics
We repeated all experiments in this study with samples from 5–7 individual subjects. We used the two-tailed t-test for paired samples to test the level of significant difference between the mean values of two experimental groups. The level of significance was set at P < 0.05 and statistical analysis for bar graphs was performed using GraphPad Prism software (version 5).
Results
MRN-100 activated DCs through upregulation of co-stimulatory molecules
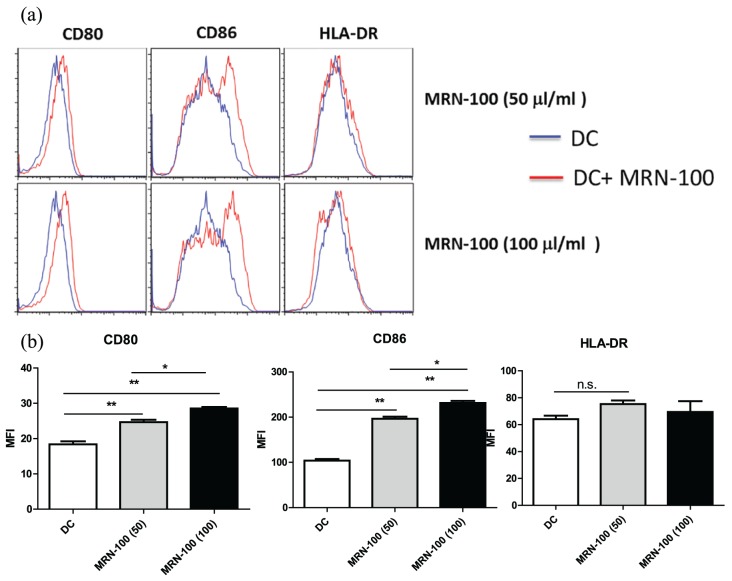
We treated monocyte-derived DCs for 24 h with MRN-100 (50 and 100 μL/mL), using DCs treated instead with isotype antibodies as a negative control. Flow cytometry was used to determine the expression of cell surface markers. Results in Figure 1(a) and (b) show that treatment with MRN-100 resulted in a significant increase in the expression of DC surface co-stimulatory and maturation markers CD80 and CD86, while there was an increase in HLA-DR but not significant. The resulting increase in CD80 and CD86 was dose dependent; it was detected at a concentration of 50 μL/mL, with a further increase at 100 μL/mL. Figure 1(a) shows one representative cytofluorograph from four individual experiments, and Figure 1(b) shows the density of mean florescent intensity (MFI) of CD80, CD86, and HLA-DR in DCs in the presence or absence of MRN-100. Data represent the mean ± standard error (SE) of four experiments for P < 0.05 compared to DCs alone.
Figure 1.
Upregulation of co-stimulatory and maturation molecules CD80, CD86, and HLA-DR on MRN-100-treated DCs: (a) a representative cytofluorograph from one experiment; (b) the density of mean florescent intensity (MFI) of CD80, CD86, and HLA-DR in DCs post treatment with MRN-100. Data represent the mean ± SE of four experiments. Values are considered significant for P < 0.05 as compared to DCs alone.
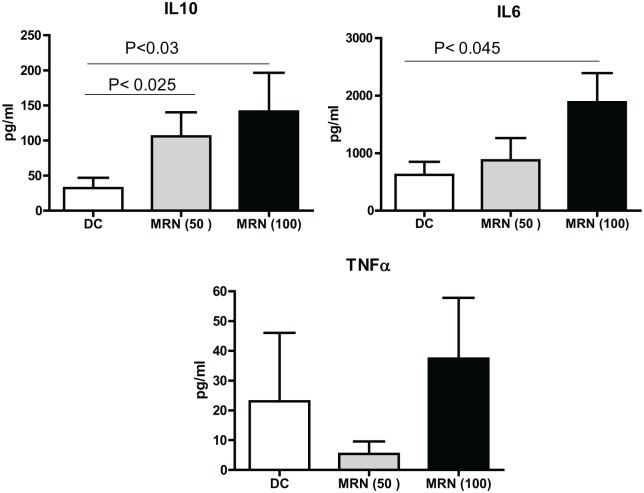
MRN-100 induces cytokine production by DCs
Monocyte-derived DCs were cultured with MRN-100 for 24 h and the production of pro- and anti-inflammatory cytokines was examined by ELISA. Data in Figure 2 also demonstrate that MRN-100 treatment increased IL-10 production. It increased the production of IL-10 at 50 µL/mL (P < 0.05), with a larger increase at 100 µL/mL. MRN-100 activated IL-6 production only at high concentrations, while TNF-α production was not significantly affected post treatment with MRN-100.
Figure 2.
MRN-100 induces cytokine production by DCs. Results are expressed as mean ± SE from seven individual experiments; values are considered significant for P < 0.05 as compared to DCs alone.
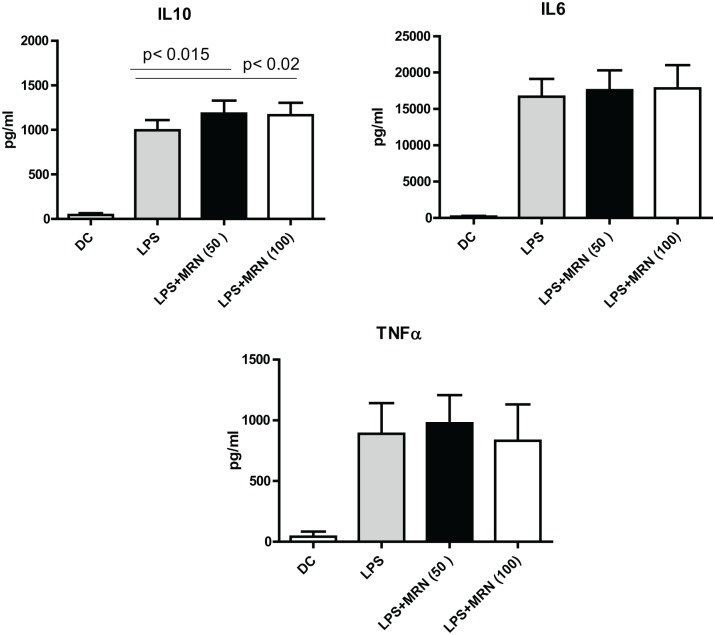
MRN-100 enhances IL-10 secretion of LPS-stimulated DCs
Data in Figure 3 show cytokine levels for DCs stimulated with LPS alone and with both LPS and MRN-100. In comparison with DCs stimulated with LPS alone, DCs treated with MRN-100 following LPS stimulation did not change IL-6 and TNF-α release but they did significantly improve IL-10 production (P < 0.05).
Figure 3.
MRN-100 enhances cytokine secretion on LPS stimulation. Data represent the mean ± SE of 10 experiments; values are considered significant for P < 0.05 as compared to DCs alone.
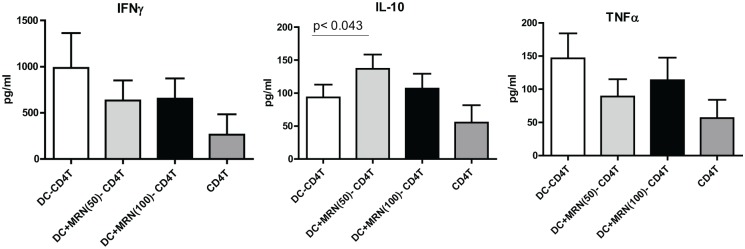
MRN-100-treated DCs prime CD4+ T cells to secrete significant amounts of IL-10
Allogeneic T cells were co-cultured with MRN-100-pretreated DCs and the production of pro- and anti-inflammatory cytokines by the T cells was measured via ELISA. Figure 4 shows secretion levels for IFN-γ, IL-10, and TNF-α. IL-10 secretion from T cells primed by MRN-100-treated DCs was significantly higher than the secretion from T cells primed by untreated DCs (DC-T, P < 0.05). In contrast, the production of pro-inflammatory cytokines IFN-γ and TNF-α was substantially reduced in T cells primed by MRN-100-treated DCs as compared to T cells primed with untreated DCs.
Figure 4.
MRN-100-stimulated DCs activate CD4+ T cells to secrete a higher amount of IL-10. Results are expressed as mean ± SE from seven individual experiments; values are considered significant for P < 0.05 as compared to DC-CD4+ T cells alone.
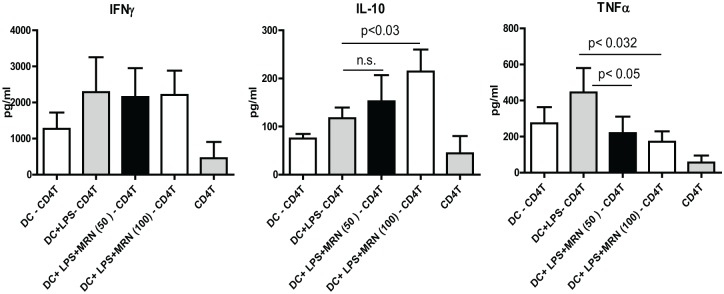
MRN-100 enhances IL-10 secretion in LPS-stimulated DC-CD4+ T cells
DCs were activated with LPS with or without MRN-100 for 24 h before being co-cultured with allogeneic CD4+ T cells for a total of 5 days. We then collected culture supernatants and assayed for cytokines by ELISA. Data in Figure 5 show the levels of secretion for IFN-γ, IL-10, and TNF-α. DCs treated with both MRN-100 and LPS caused a significant increase in IL-10 production by T cells, when compared with DC-CD4+ T cells alone (P < 0.05). In contrast, the production of pro-inflammatory cytokine TNF-α was significantly inhibited by MRN-100- and LPS-treated DCs (P < 0.05). However, MRN-100 did not modulate the induction of IFN-γ by LPS-treated DCs.
Figure 5.
MRN-100-stimulated DCs prime CD4+ T cells to secrete IL-10 but not IFN-γ or TNF-α. Data represent the mean ± SE from five individual experiments; values are considered significant for P < 0.05.
Discussion
DCs play an essential role in the link between the innate as well as the adaptive immune systems by mounting powerful T-cell responses. This adaptive immune response clears pathogens and limits tissue damage resulting from inflammation. This study shows that the iron-based compound MRN-100, which is composed of both bivalent and trivalent ferrates, is a modulator of human DC maturation and function. It increased significantly the secretion levels of anti-inflammatory cytokine IL-10 in DCs and in LPS-activated DCs. In addition, MRN-100-treated DCs exerted differential effects on pro- and anti-inflammatory cytokines: it enhanced anti-inflammatory IL-10 but attenuated pro-inflammatory TNF-α induction in T cells.
Several studies have shown that many natural anti-inflammatory products modulate inflammatory cytokines TNF-α and IL-6 and anti-inflammatory IL-10 production by DCs. Our previous work has shown that Biobran/MGN-3 (an arabinoxylan rice bran), PFT (a novel kefir product), and Marina Crystal Minerals (MCM; a mixture of crystallized minerals and trace elements extracted from seawater) all promote the production of inflammatory and anti-inflammatory cytokines.27,31,32 Biobran and PFT also stimulate DC-primed CD4+ T cell proliferation and their production of IL-10, and MCM causes the upregulation of co-stimulatory molecules CD86, CD80, and HLA-DR. In addition, DPV576 (a dispersed aqueous mixture composed of nanodiamond and nanoplatinum) activates human DCs and upregulates the levels of DC-secreted cytokine IL-10,33 and we have recently observed that exposure of human CD4+ T lymphocytes to DPV576 inhibits the activity of the transient receptor potential vanilloid channel TRPV1 in CD4+ T lymphocytes, suggesting that it has the potential for use in pain management.34 Work of others on the anti-inflammatory activity of several natural agents has also shown effects on IL-10 and DCs. Lippia sidoides, an aromatic shrub, can induce IL-10 production in THP-1 monocytes;35 PureCell Complex (PCT)-233, an active molecular complex from Spinacia oleracea, increased IL-10 production but did not significantly modulate TNF release in alveolar macrophages treated with PCT-233 and budesonide after LPS stimulation;36 and a complex mixture of herbal extracts has been shown to modulate the innate responsiveness of murine DCs.37
While we do not fully understand the underlying mechanisms of MRN-100’s activation of DCs, MRN-100 could act by binding to DC surface receptors or by being phagocytized, ultimately triggering the signaling mechanisms for DC activation and cytokine secretion. One possible mechanism could be the involvement of PRRs which are abundantly expressed on DCs. Through these PRRs, DCs recognize bacteria, fungi, viruses, and carbohydrate molecules.38,39 Exposing DCs to ligands and agonists of these PRRs results in the production of cytokines and chemokines; this ultimately shapes the T helper cell response, such as via Th1, Th2, Tfh, Treg, and Th17 cells.29,30,40,41
The anti-inflammatory effect of iron may offer another mechanism by which MRN-100 induces an anti-inflammatory response, since MRN-100 is an iron-based hydroferrate fluid that is made up of bivalent and trivalent ferrates. We have shown in a previous study that MRN-100 can reverse oxidative stress associated with age in aged rats by a mechanism involving MRN-100’s ability to enhance the levels of plasma iron and ferritin concentration and of transferrin saturation.26 This suggests that the presence of iron in MRN-100 may be responsible for the induction of functional changes in DCs. Iron is required in numerous essential proteins and enzymes, and its deficiency can significantly decrease the response of lymphocytes to mitogens and antigens as well as decreasing the ability of polymorphonuclear leukocytes to efficiently destroy ingested bacteria and fungi.42 Furthermore, human lactoferrin, an iron-binding multifunctional cationic glycoprotein, inhibits inflammation.43
Reactive oxygen species (ROS) can induce remarkable effects on cellular functions which could result in tissue damage under inflammatory conditions.44 The mitochondrial respiration of oxygen produces ROS as a byproduct, which may damage tissues. Under inflammatory conditions, oxidative stress facilitates migration of inflammatory cells across the endothelium and may cause tissue damage.44 The anti-inflammatory effect of many natural agents may involve their ability to protect tissues against oxidative stress–induced damage. Our earlier studies have shown MRN-100 to be a potent antioxidant agent. This characteristic enables MRN-100 to act as a protector in several in vitro and in vivo animal models. MRN-100 treatment has been shown to reverse the age-associated oxidative stress in aged rats by decreasing the levels of oxidative stress biomarkers including nitric oxide, malondialdehyde, protein carbonyl groups, and total free radicals and by enhancing the values of glutathione (GSH), total thiol contents, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) in the blood, liver, and brain in aged rats.26 Treatment with MRN-100 has been shown to provide protection to blood and stomach tissues in rats with methylnitronitrosoguanidine-induced esophageal and gastric cancer by the mechanism involved in increasing the levels of GSH; antioxidant enzymes CAT, SOD, and GPx; and total antioxidant capacity level, with an accompanying reduction in the total free radical and malondialdehyde levels.27 Finally, MRN-100 has been shown to protect against oxidative stress–induced apoptosis of murine lymphocytes in vitro by preventing H2O2-induced downregulation of Bcl-2 and upregulation of Bax.28 Other studies have also shown the protective effects of natural antioxidant agents: Myrtus communis extracts protected rats against liver injury and fibrosis following biliary obstruction via its antioxidant activities,45 and mushrooms have been shown to support anti-inflammatory actions and, in turn, offer protection against obesity-related hypertension and dyslipidemia.46
The data in this study complement previous work showing MRN-100’s positive antioxidant effects, in addition to previous studies showing its immunological ability to activate NK cells in humans.47–49 Here, MRN-100 is shown to stimulate DCs and prime CD4+ T cells to secrete significant amounts of IL-10. Furthermore, MRN-100 enhanced IL-10 secretion by LPS-activated DC-CD4+ T cells. Taken together, these findings present MRN-100 as a potentially potent immune modulator that has the ability to activate various facets of the immune system.
This study suggests that MRN-100 is a powerful natural dietary adjuvant that effectively modulates human DCs’ maturation and function. Results suggest the use of MRN-100 as an anti-inflammatory agent for patients with inflammatory diseases in clinical trials.
Acknowledgments
We would like to thank our colleagues Dr S. Gollapudi (UC Irvine) for guidance in this study and Dr B. J. Winjum (UCLA) for editorial assistance.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The work reported in this study was supported by a grant from ACM Co., Ltd, Tokyo, Japan.
ORCID iD: Mamdooh H Ghoneum  https://orcid.org/0000-0002-1087-7127
https://orcid.org/0000-0002-1087-7127
References
- 1. Cross M, Smith E, Hoy D, et al. (2014) The global burden of rheumatoid arthritis: Estimates from the Global Burden of Disease 2010 study. Annals of the Rheumatic Diseases 73(7): 1316–1322. [DOI] [PubMed] [Google Scholar]
- 2. Molodecky NA, Soon IS, Rabi DM, et al. (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142: 46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 3. Guariguata L. Estimating the worldwide burden of type 1 diabetes. Available at: https://www.idf.org/component/attachments/attachments.html?id=275&task=download (accessed 16 March 2019).
- 4. Furfaro F, Fiorino G, Allocca M, et al. (2016) Emerging therapeutic targets and strategies in Crohn’s disease. Expert Review of Gastroenterology & Hepatology 10(6): 735–744. [DOI] [PubMed] [Google Scholar]
- 5. Singh JA, Saag KG, Bridges SL, Jr, et al. (2016) 2015. American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis & Rheumatology 68(1): 1–25. [DOI] [PubMed] [Google Scholar]
- 6. Hasturk H, Kantarci A. (2015) Activation and resolution of periodontal inflammation and its systemic impact. Periodontology 2000 69(1): 255–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stüve O, Zettl U. (2014) Neuroinflammation of the central and peripheral nervous system: An update. Clinical and Experimental Immunology 175(3): 333–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rommer PS, Zettl UK, Kieseier B, et al. (2014) Requirement for safety monitoring for approved multiple sclerosis therapies: An overview. Clinical and Experimental Immunology 175(3): 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steinman L. (1991) Prospects for immunotherapy directed to the T cell receptor in human autoimmune disease. Annals of the New York Academy of Sciences 636: 147–153. [DOI] [PubMed] [Google Scholar]
- 10. Banchereau J, Steinman RM. (1998) Dendritic cells and the control of immunity. Nature 392(6673): 245–252. [DOI] [PubMed] [Google Scholar]
- 11. Niu J, Ren Y, Zhang T, et al. (2014) Retrospective comparative study of the effects of dendritic cell vaccine and cytokine-induced killer cell immunotherapy with that of chemotherapy alone and in combination for colorectal cancer. BioMed Research International 2014: 214727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Padgett LE, Broniowska KA, Hansen PA, et al. (2013) The role of reactive oxygen species and pro-inflammatory cytokines in type 1 diabetes pathogenesis. Annals of the New York Academy of Sciences 1281: 16–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fisman EZ, Tenenbaum A. (2010) The ubiquitous interleukin-6: A time for reappraisal. Cardiovascular Diabetology 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones SA. (2005) Directing transition from innate to acquired immunity: Defining a role for IL-6. Journal of Immunology 175(6): 3463–3468. [DOI] [PubMed] [Google Scholar]
- 15. Moore KWM, de Waal RL, Coffman O’Garra A. (2001) Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology 19: 683. [DOI] [PubMed] [Google Scholar]
- 16. Goudy KS, Burkhardt BR, Wasserfall C, et al. (2003) Systemic overexpression of IL-10 induces CD4+CD25+ cell populations in vivo and ameliorates type 1 diabetes in nonobese diabetic mice in a dose-dependent fashion. Journal of Immunology 171(5): 2270–2278. [DOI] [PubMed] [Google Scholar]
- 17. Pulendran B, Tang H, Denning TL. (2008) Division of labor, plasticity, and crosstalk between dendritic cell subsets. Current Opinion in Immunology 20(1): 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Legitimo A, Consolini R, Failli A, et al. (2014) Dendritic cell defects in the colorectal cancer. Human Vaccines & Immunotherapeutics 10(11): 3224–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koido S, Ohkusa T, Homma S, et al. (2013) Immunotherapy for colorectal cancer. World Journal of Gastroenterology 19: 8531–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mailliard RB, Son YI, Redlinger R, et al. (2003) Dendritic cells mediate NK cell help for Th1 and CTL responses: Two-signal requirement for the induction of NK cell helper function. Journal of Immunology 171(5): 2366–2373. [DOI] [PubMed] [Google Scholar]
- 21. Borg C, Jalil A, Laderach D, et al. (2004) NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood 104(10): 3267–3275. [DOI] [PubMed] [Google Scholar]
- 22. Fosslien E. (2005) Cardiovascular complications of non-steroidal anti-inflammatory drugs. Annals of Clinical and Laboratory Science 35(4): 347–385. [PubMed] [Google Scholar]
- 23. Hermann M, Ruschitzka F. (2006) Coxibs, non-steroidal anti-inflammatory drugs and cardiovascular risk. Internal Medicine Journal 36(5): 308–319. [DOI] [PubMed] [Google Scholar]
- 24. Pang L, Cai Y, Tang EH, et al. (2016) Cox-2 inhibition protects against hypoxia/reoxygenation-induced cardiomyocyte apoptosis via Akt-dependent enhancement of iNOS expression. Oxidative Medicine and Cellular Longevity 2016: 3453059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghoneum M, Elbaghdady HA, El-Shebly AA, et al. (2013) Protective effect of hydroferrate fluid, MRN-100, against lethality and hematopoietic tissue damage in γ-radiated Nile tilapia, Oreochromis niloticus. Journal of Radiation Research 54: 852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Badr El-Din NK, Noaman E, Fattah SM, et al. (2010) Reversal of age-associated oxidative stress in rats by MRN-100, a hydro-ferrate fluid. In Vivo 24(4): 525–533. [PubMed] [Google Scholar]
- 27. Ghoneum MH, Badr El-Din NK, Abdel Fattah SM, et al. (2015) Hydroferrate fluid, MRN-100, provides protection against chemical-induced gastric and esophageal cancer in Wistar rats. International Journal of Biological Sciences 11(3): 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghoneum M, Matsuura M, Gollapudi S. (2009) An iron-based beverage, HydroFerrate fluid (MRN-100), alleviates oxidative stress in murine lymphocytes in vitro. Nutrition Journal 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agrawal S, Agrawal A, Doughty B, et al. (2003) Cutting edge: Different toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. Journal of Immunology 171(10): 4984–4989. [DOI] [PubMed] [Google Scholar]
- 30. Agrawal S, Dillon S, Banerjee K, et al. (2006) Yeast zymosan, a stimulus for TLR2 and dectin-1, induce regulatory antigen-presenting cells and immunological tolerance. Journal of Clinical Investigation 116: 916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghoneum M, Agrawal S. (2011) Activation of human monocyte-derived dendritic cells in vitro by the biological response modifier arabinoxylan rice bran (MGN-3/Biobran). International Journal of Immunopathology and Pharmacology 24(4): 941–948. [DOI] [PubMed] [Google Scholar]
- 32. Ghoneum MH, Ogura T, Gimzewski JK, et al. (2018) Marina crystal minerals (MCM) activate human dendritic cells to induce CD4+ and CD8+ T cell responses in vitro. International Journal of Immunopathology and Pharmacology 32: 2058738418797768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghoneum M, Ghoneum A, Gimzewski J. (2010) Nanodiamond and nanoplatinum liquid, DPV576, activates human monocyte-derived dendritic cells in vitro. Anticancer Research 30(10): 4075–4079. [PubMed] [Google Scholar]
- 34. Ghoneum MH, Gimzewski JK, Ghoneum A, et al. (2018. b) Inhibition of TRPV1 channel activity in human CD4+ T cells by nanodiamond and nanoplatinum liquid, DPV576. Nanomaterials 8(10): E770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rajgopal A, Rebhun JF, Burns CR, et al. (2015) Immunomodulatory effects of Lippia sidoides extract: Induction of IL-10 through cAMP and p38 MAPK-dependent mechanisms. Journal of Medicinal Food 18(3): 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bissonnette EY, Proulx LI, Turmel V, et al. (2004) PCT-233, a novel modulator of pro- and anti-inflammatory cytokine production. Clinical and Experimental Immunology 135(3): 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miller AK, Benson JM, Muanza DN, et al. (2011) Anti-inflammatory effects of natural product formulations on murine dendritic cells. Journal of Dietary Supplements 8(1): 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takeuchi O, Akira S. (2010) Pattern recognition receptors and inflammation. Cell 140(6): 805–820. [DOI] [PubMed] [Google Scholar]
- 39. Geijtenbeek TB, Gringhuis SI. (2009) Signalling through C-type lectin receptors: Shaping immune responses. Nature Reviews Immunology 9(7): 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Agrawal S, Gupta S, Agrawal A. (2010) Human dendritic cells activated via dectin-1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PLoS ONE 5(10): e13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iwasaki A, Medzhitov R. (2010) Regulation of adaptive immunity by the innate immune system. Science 327(5963): 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chandra RK. (1985) Goldsmith Award lecture. Trace element regulation of immunity and infection. Journal of the American College of Nutrition 4(1): 5–16. [DOI] [PubMed] [Google Scholar]
- 43. Rosa L, Cutone A, Lepanto MS, et al. (2017) Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis. International Journal of Molecular Sciences 18(9): E1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mittal M, Siddiqui MR, Tran K, et al. (2014) Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling 20(7): 1126–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sen A, Ozkan S, Recebova K, et al. (2016) Effects of Myrtus communis extract treatment in bile duct ligated rats. The Journal of Surgical Research 205(2): 359–367. [DOI] [PubMed] [Google Scholar]
- 46. Ganesan K, Xu B. (2018) Anti-obesity effects of medicinal and edible mushrooms. Molecules 23(11): E2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ghoneum M, Kijima Y. (1996) Induction of human natural killer (NK) cell activity by pi-water (MRN-100). In: Proceedings of the annual conference on clinical immunology, New Orleans, LA, 31 May – 3 June. [Google Scholar]
- 48. Ghoneum M, Namatalla G, Kijima Y. (1997) Phenotypic analysis of human lymphocyte sub-populations post treatment with pi-water (MRN-100). In: Proceedings of the 88th annual meeting of the American Association for Cancer Research, San Diego, CA, 12–16 April. [Google Scholar]
- 49. Ghoneum M. (1998) NK immunorestoration in cancer patients by MRN-100, an iron based compound derived from bivalent and trivalent ferrate. In: Proceedings of the fourth international symposium on predictive oncology and therapy, Nice, France, 24–27 October. [Google Scholar]