Abstract
The insulin-like growth factors (IGF-I and IGF-II) and their receptors are widely expressed in nervous tissue from early embryonic life. They also cross the blood brain barriers by active transport, and their regulation as endocrine factors therefore differs from other tissues. In brain, IGFs have paracrine and autocrine actions that are modulated by IGF-binding proteins and interact with other growth factor signalling pathways. The IGF system has roles in nervous system development and maintenance. There is substantial evidence for a specific role for this system in some neurodegenerative diseases, and neuroprotective actions make this system an attractive target for new therapeutic approaches. In developing new therapies, interaction with IGF-binding proteins and other growth factor signalling pathways should be considered. This evidence is reviewed, gaps in knowledge are highlighted, and recommendations are made for future research.
Keywords: IGF, IGFBP, nervous system, neurodegenerative disease
Introduction
There is evidence that the insulin-like growth factors (IGF-I and IGF-II) have key roles in nervous system development and function. This system is phylogenetically related to insulin and its receptors, and the IGF/insulin system is evolutionarily conserved.1 The IGFs cross the blood-brain barriers2 and have endocrine roles in brain. They bind with high affinity to a family of IGF-binding proteins (IGFBPs) which regulate availability of IGFs to interact with their receptors.3 It is well known that the action and expression of each IGFBP is cell- and tissue-specific.4 Therefore, an understanding of IGFBPs in nervous tissue is essential to understanding the paracrine/autocrine roles as well as the actions of endocrine IGFs in normal physiology and diseases of the nervous system. Neurodegenerative disorders are increasing in prevalence, and a knowledge of the IGF system is likely to be important in finding therapeutic targets.
The aim of this review is to present a broad perspective of current knowledge about the role of IGFs and IGFBPs in the nervous system. Articles included were retrieved through PubMed using a combination of the MeSH search term ‘Nervous System’ and the search term ‘IGF’ in all fields. Papers published between January 2014 and September 2018 were retrieved and the abstracts scanned for relevant papers. Key contributions to the field predate 2014 and therefore, in addition, the author’s own EndNote™ database of IGF papers prior to 2014 was searched using the term ‘Nervous System’. References within the articles obtained by these methods were also used to retrieve key papers. The field is dominated by experimental studies in rodents and this may be a limitation in identifying relevant therapeutic targets for human disease. Where possible, publications that focus on the human IGF system are presented in this review.
An overview of the IGF system and its expression and action, with a focus on the nervous system, will first be presented. This will set the scene for a discussion of the role of the IGF system in nervous system disorders, and the potential of this system in therapeutics. Signposting to future research will be included in the concluding section.
IGF System Overview
The IGF system has general roles in growth and metabolism, and ageing, that are evolutionarily conserved.1 Insulin-like growth factor 1 and IGF-II are evolutionarily related to proinsulin and share structural similarity so that all three bind to type 1 IGF receptors (IGF1R) and insulin receptors (IR), which also share structural similarity.5 There are two isoforms of IR, IRA and IRB, that can form heterodimers, and each isoform can form heterodimers with IGF1R subunits.6,7 All of these receptors are activated through ligand-induced autophosphorylation and subsequent phosphorylation of other tyrosine-containing substrates and enzyme cascades, including the phosphatidyl-inositol-3 kinase (PI3K)-protein kinase B (Akt) pathway.8 While IGFs have a higher affinity than insulin for IGF1R and are therefore likely to have important physiological roles through that pathway, the physiological roles of the IR isoforms and their hybrids are not fully established, and are likely to be influenced by differing affinities for IGF-II.6 IRA homodimers and IRA/IGF1R hybrids have high affinity for IGF-II and have a role in cancer cell growth.7 The IGF-II/mannose-6-phosphate receptor (IGF2R) is a structurally distinct cell-surface receptor that plays a role in internalising IGF-II and not IGF-I, as well as trafficking lysosomal enzymes.9 The IGF-II binding domain of IGF2R is also present in the circulation and can block IGF-II-induced cell growth.9 A key characteristic of the IGFs, not shared with insulin, is a high affinity for members of a family of IGFBPs10 that have distinct functional roles. They can stimulate or inhibit IGF actions and have IGF-independent effects, depending on the IGFBP, post-translational modifications and cellular milieu.11,12
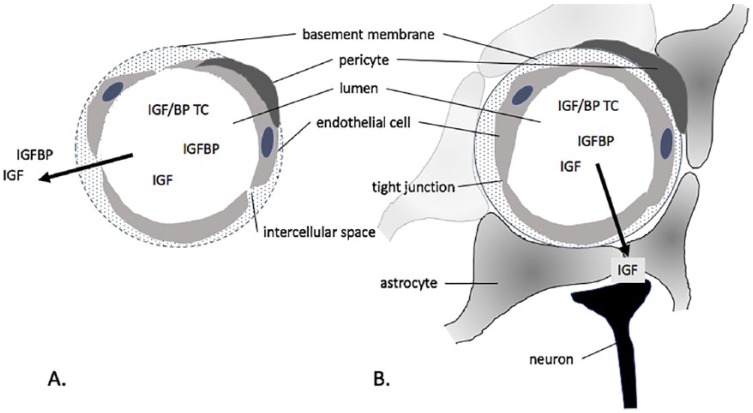
Our understanding of the IGF system is founded on the original work of Salmon and Daughaday13 who discovered the existence in the circulation of pituitary-dependent mediators of tissue growth. Later, this view expanded to encompass paracrine/autocrine roles.3 The endocrine IGF system will be discussed first. The liver plays a central role in the production of endocrine IGFs, secreting a ternary complex of approximately 140 kDa that has a long circulating half-life (hours-days).14,15 Hepatic IGF and an acid-labile subunit are produced by hepatocytes and enter the circulation in association with IGFBP-3 or IGFBP-5, produced by non-parenchymal cells. Circulating IGF-I and IGF-II are mainly associated in these ternary complexes, which have long circulating half-lives. Since IGF-I and the acid-labile subunit are both growth hormone (GH)-dependent, IGF-I is an ideal biomarker of GH, which is secreted in pulsatile bursts by the pituitary and has a short circulating half-life (minutes).16 The insulin-like growth factor I is also positively regulated by total caloric and protein intake and by insulin,15 so that the IGF-I in the circulation is also a marker of nutritional status. While most IGF-I and IGF-II in the circulation is associated in ternary complexes, IGF (~7 kDa) also associates in binary complexes with IGFBPs (~25-45 kDa) in the circulation and at the tissue level, and a proportion is ‘free’ to interact with cell surface receptors.12 Thus, circulating IGFs and IGFBPs are part of a dynamic system, crossing the endothelium of fenestrated and sinusoid capillaries rapidly (minutes-hours), alone or associated in binary complexes (Figure 1A).
Figure 1.
IGFs and IGFBPs that are not associated in a ~140 kDa ternary complex (TC) with an acid-labile subunit readily cross the endothelium of fenestrated capillaries (A) unbound or in binary complexes. Passage across the blood-brain barrier into brain parenchyma (B) involves active transport of IGFs that are not in binary or ternary complexes.
IGFs and IGFBPs are ubiquitously produced and have paracrine and autocrine roles.3 Each member of the family of six high-affinity IGFBPs (IGFBP-1 to IGFBP-6) has a distinctive pattern of tissue expression.4 The effect of IGFBPs on IGF availability to receptors depends on IGF-binding affinity,7 interaction with other proteins, and a variety of post-translational modifications of the IGFBPs. Limited proteolysis of IGFBPs by tissue proteases reduces affinity and therefore increases IGF activity, while association with matrix proteins can stabilise IGF near cell surface receptors, which also enhances activity.4 Human neuroblastoma cells, for example, secrete IGFBP-2 that is able to associate with cell membranes.17 These cells rely on autocrine stimulation by IGFs and, when exposed to fibroblast growth factor (FGF) a protease is induced that cleaves IGFBP-2 and results in increased IGF activity. In addition, the IGFBPs have IGF-independent actions, for example the interaction of IGFBP-2 with the α5β1 integrin via an arginyl-glycyl-aspartyl (RGD) domain promotes glioma cell migration.18 The IGFBPs are structurally related to a superfamily of proteins19 which do not bind IGFs and are beyond the scope of this review.
GH/IGF expression and regulation in the nervous system
Insulin-like growth factor I and IGF-II are widely expressed in nervous tissue from early embryonic life.20 Understanding of the tempo-spatial expression of IGFs in brain is primarily derived from studies in rodents. Insulin-like growth factor I is widely expressed in brain, in neurons and glial cells.21 At all stages of development, higher levels of IGF-I expression are associated with proliferating neural precursors.20 Insulin-like growth factor II is predominantly expressed in mesenchymal tissues. In rodents, IGF-II expression is highest during embryonic development, declines with age and is restricted to the meninges and choroid plexus in the adult.22 Circulating GH is produced by the anterior part of the pituitary that derives embryonically from the ectoderm and is linked functionally to the nervous system by a system of capillary loops and sinusoids, known as the hypophyseal-portal circulation. GH-releasing hormone (GHRH) and somatostatin, which are secreted by hypothalamic neurons into the hypophyseal-portal circulation, are important peptides regulating the synthesis and pulsatile release of pituitary GH.16 GH crosses the blood-brain barrier and IGF-I expressed in brain may be regulated by GH in a region-specific manner. In adult male rats, GH administration increases IGF-I expression in hypothalamus, cerebellum and hippocampus.23 Cell- and tissue-specific effects are observed, with no change in IGF-I expression in cerebral cortex in response to GH in that study. In addition to pituitary, GH is expressed in nervous tissues. GH immunoreactivity has been detected in the rat amygdaloid nucleus and hypothalamus and increases after hypophysectomy.24 Growth hormone and IGF-I are both expressed in the hippocampus of GH-deficient mice.25 However overexpression of GH in mouse hippocampus is associated with only a modest change in local IGF-I expression.26
Insulin-like growth factor inhibits GH secretion through endocrine negative feedback, crossing the fenestrated sinusoid capillaries of the anterior pituitary, and inhibiting spontaneous and GHRH-stimulated GH release by somatotrophs.16 It is also possible that there is negative feedback by IGF-I on GHRH in the hypothalamus. The blood-brain barriers regulate passage of substances, including IGFs,2 through specific transport mechanisms, from the systemic circulation into brain parenchyma (Figure 1B) and from the choroid plexuses into cerebrospinal fluid (CSF). IGF uptake into CSF appears to be independent of IGF1R and IGFBPs27 and, although also produced locally, brain IGF-I levels are determined to some extent by circulating concentrations.28 Insulin-like growth factor passage into brain is triggered by local neuronal activity through a mechanism that includes vasodilatation and increased IGFBP-3 protease activity generating fragments with lower affinity for IGFs.29 Insulin-like growth factor is then more available to interact with the endothelial transporter low-density lipoprotein-related receptor (LRP)1. It has been shown that LRP2 (megalin), which participates in brain uptake of β-amyloid carrier proteins, also has a role in IGF-I transport across the choroid plexus and mediates IGF-I-induced clearance of β-amyloid.30,31 Insulin-like growth factor II is also expressed in adult human brain.32 Cerebrospinal fluid provides a proliferative niche for supraventricular neural progenitors,33 and there is evidence that IGF-II acting via IGF1R is an important determinant of CSF activity on these stem cells.34 In songbirds (canaries and zebrafinches), IGF-II is expressed in neurons in areas of brain responsible for song, and correlates with neuronal plasticity.35
Studies of transgenic and knockout mice have indicated that IGF-I and IGF-II have distinct nervous system functions.36 Igf1 overexpressing mice have increased postnatal brain growth,37 while Igf2 overexpression appears to have no effect on brain growth.38 Mice with igf1 deficiency have impaired neuronal somatic and dendritic growth39 but no evidence of neurological dysfunction and a degree of myelination that is proportionate to brain mass,40 while igf2-/- mice have no apparent changes in brain morphology41 and are less susceptible to hippocampal neurodegeneration42 compared to controls. Insulin-like growth factor I is naturally cleaved in brain32 and in the circulation43 to a variant that lacks the N-terminal tripeptide glycine-proline-glutamate (GPE) and has reduced affinity for IGFBPs.44 IGFBP inhibits this cleavage of IGF-I.45 Centrally, the GPE tripeptide can also cross the blood-brain barrier to reach the CSF, where it has a longer half-life than in plasma associated with reduced susceptibility to proteolytic degradation.43 In retinal glial cells, both the truncated IGF-I variant and the cleaved tripeptide have mitogenic activity.46 GPE stimulates potassium-induced acetylcholine release in rat cortical slices47 and has neuroprotective effects in hippocampus and striatum.43,48,49 GPE inhibits gonadotrophin-releasing hormone secretion through antagonism at N-methyl-d-aspartate (NMDA) receptors.45
IGF receptors and signalling in the nervous system
The effects of IGFs on cell growth/apoptosis and metabolism are through IGF1R and IR which are ubiquitously expressed in the nervous system.50,51 IGF1R null mice have generalised growth retardation including brain, characterised by reduced neuronal fibres and neuroglial cell cytoplasm but increased nerve cell number.52 Mice with neuron-specific deletion of IR have normal brain size and development, but develop obesity and mild insulin resistance.53 This central metabolic effect may be due to the action of local insulin which is also expressed in brain.50,54 IGF2R is widely distributed in brain55; however, role in regulating IGF-II availability in human brain has not been elucidated.
In addition to feedback inhibition of GH, IGF-I acts directly to increase insulin sensitivity at the post-receptor level.15 IGFs also act in concert with other growth factors to influence nervous system function. The effect of FGF-2 withdrawal in promoting neuronal differentiation from stem cells is mediated by IGF-I,56 and pre-treatment with FGF-2 increases IGF1R expression.57 In rodent astrocytes, IGF-I secretion is stimulated by epidermal growth factor (EGF) and IGF1R blockade reduces the action of EGF on cell replication.58,59 In a human neuroblastoma cell line, IGF-II stimulates cell growth in the presence of EGF.60 Insulin-like growth factor I signalling pathways interact with those of sex steroids in the neuroendocrine hypothalamus and also in the hippocampus in the control of neurogenesis and synaptic plasticity.61 The effect of IGF-I on oestrogen signalling in brain is cell type-specific and oestrogen receptor isoform-specific.62 When the IGF-I gene is delivered to the medial basal hypothalamus in female rats, serum luteinising hormone levels are higher, probably due to enhanced oestrogen positive feedback on GnRH production, and ovarian function is prolonged.63 The IGF system interfaces with the brain-derived neurotrophic factor (BDNF) system. In rats, the exercise-induced increase in learning recall, and hippocampal BDNF expression and signalling is prevented when IGF1R-blocking antibody is delivered to the hippocampus.64
IGFBPs in the nervous system
In studies with transgenic mice, early null mutations of IGFBPs appeared to have no brain phenotype, and it was suggested that this indicated ‘redundancy’ in the system.36 Earlier studies of the overexpression of IGFBPs have shown little or inconsistent effects on the nervous system36 However, there are exceptions. Transgenic mice overexpressing IGFBP-1 in brain have impaired brain growth and reduced glial cell proliferation in response to injury.65,66 While IGFBP-1 is not normally expressed in brain, endocrine IGFBP-1 can have an effect on brain development, with reduced cortex and hippocampus development in mice with liver-specific overexpression of IGFBP-1 during foetal life.67 When IGFBP-6 is overexpressed in brain, mice have reduced cerebellum size and weight,68 dysregulation of energy homeostasis and obesity.69
Despite their importance in regulating IGF action, the roles of IGFBPs in brain are less well studied than IGF-I. Insulin-like growth factor binding protein 5 is one of the major IGFBPs expressed in brain. It is found in neurons throughout the cerebral cortex, colocalised with cells that secrete kallikreins that proteolyse IGFBP-5.70 There is also evidence that IGFBP-2 has an important role in the nervous system. Insulin-like growth factor binding protein 2 is abundant in brain and is highly expressed by astrocytes in the cortex.71 During depolarisation, IGFBP-2 expression is upregulated in astrocytes.72 NMDA receptors may be responsible for this upregulation. Along with IGF-II, IGFBP-2 is synthesised and secreted by meningeal cells.73 While IGFBP-2 has been shown to inhibit oligodendrocyte precursor cell survival and differentiation in vitro,74 there is evidence that cell membrane-associated IGFBP-2 can increase IGF activity.17 It has been suggested that IGFBP-4 is involved in the maintenance of cerebellar plasticity75 and in microtubule functions in astrocytes.76 In transgenic mice overexpressing tumour necrosis factor-alpha (TNF-α), changes in the IGF system are seen consistent with reduced IGF availability with increases in IGFBP-3 and IGFBP-4 protein expression, along with reduced IGFBP-5 and IGF-I in radial glial and Purkinje cells.77
Cell lines from neuroblastomas, which are malignant childhood tumours derived from neural crest stem cells, are often used as models for exploring the role of IGFs and IGFBPs. Insulin like growth factor I and IGF-II act as paracrine/autocrine signals via IGF1R in human neuroblastoma cell lines,78 including those comprised of epithelial Schwann cells,60 and may stimulate growth of primary tumours in concert with other growth factors. IGFBP-278 and IGFBP-579 are also expressed in neuroblastoma cells and can stimulate or inhibit cell growth depending on their concentration or the presence of IGFs79 or proteases that alter IGF binding affinity.17 IGFBP-2 is recognised as an oncogene in a variety of human cancers80 including those of the nervous system: gliomas81–86 and meningiomas87,88. Interaction of IGFBP-2 with the α5β1 integrin via its RGD domain has been implicated in glioma progression81 and migration.18 Higher serum IGFBP-5 levels are associated with glioblastoma recurrence.89
Normal Development and Ageing
The IGF system has an essential role in normal growth, development and maintenance of the nervous system.20,33,90,91 From week 3 of embryonic life, neural stem cells proliferate, migrate from the subventricular zone, and differentiate in a highly complex manner, producing neurotransmitter and neurotrophic factors and processes (axons and dendrites) that allow synaptic interconnections. Apoptotic cell death is an important mechanism for eliminating neural progenitor cells with a transient role in nervous system development. In the postnatal period, neuronal production and migration is largely complete; however, neurogenesis continues throughout adulthood in specific regions of the brain: the dentate gyrus of the hippocampus (important for learning and memory), the supraventricular zone (cells migrate to the olfactory bulb), and the striatum (voluntary motor control).92,93 Glial cell (oligodendrocytes, astrocytes and microglia) proliferation, migration and maturation continues throughout childhood and glial progenitors persist in adult brain and can differentiate in response to injury, and glial cell apoptosis continues into postnatal life.94
IGFs in development and maintenance of the nervous system
Local paracrine/autocrine sources of IGFs are essential for normal nervous system development. Children with reduced endocrine IGF-I due to GH insensitivity generally have normal cognitive function,95 despite craniofacial abnormalities,96,97 while those with IGF-I deletion98 or IGF-I receptor mutations99 and therefore reduced paracrine/autocrine IGF-I activity, have microencephaly and cognitive impairment. Nevertheless endocrine sources of IGFs also have important roles. In pre-term infants, circulating levels of IGF-I and IGFBP-3 postnatally are positively associated with brain volumes.100 Early treatment of children with GH insensitivity with IGF-I is reported to prevent cochlear hearing loss.101 Less is known about the role of IGF-II in nervous system development. Maternally imprinted, IGF-II gene hypermethylation has been identified as a potential risk factor for neural tube defects.102 Paternal folate deficiency in rats has also been shown to influence brain IGF-II methylation despite adequate maternal folate during gestation.103
Insulin like growth factor I and IGF1R are expressed early in development throughout the brain.20 In rats, neonatal undernutrition increases expression of IGF-I and IGF1R in cerebellum and hypothalamus, and decreases IGFBP-2 in hypothalamus in the perinatal period.104 In this way, in the face of reduced endocrine IGF-I production, the paracrine/autocrine availability of IGF-I at a time of rapid brain growth and development is likely to be optimised. There is substantial evidence from mutant mouse models36 that IGF-I promotes neuron numbers, through increased proliferation and reduced apoptosis, as well as process outgrowth and synaptogenesis, throughout nervous system development. Overexpression of IGF-I in the striatum of adult rat brain, for example, induces migration of adult neuronal precursor cells.105 There is evidence that the proliferating effect of IGF-1 is via RAF/MEK/ERK signalling, while the differentiating effects involve PI3K/Akt pathways.106 Insulin like growth factor I signalling interacts with other growth factor pathways that are important in the nervous system. These include growth factors (eg, FGFs, EGF and vascular endothelial growth factor [VEGF]) and neurotrophic factors (eg, BDNF), which together maintain proliferation of neural stem cells, and neurotransmitters and transcriptional factors, which regulate the neurogenic process.56,107,108 Studies in rodents suggest that prenatal exposure to steroids109,110 and neonatal repetitive maternal separation111 alters IGF system expression in developing brain in ways that may increase susceptibility to cell damage. These studies have potential implication for management of pre-term infants.
Oligodendrocyte differentiation is associated with increased myelin expression and the production of trophic factors that are important for neuronal survival and axonal integrity. Insulin like growth factor I enhances oligodendrocyte progenitor cell differentiation and therefore myelination.112,113 There is substantial evidence that IGFs play a role in oligodendrocyte differentiation and survival, and myelin synthesis36 as well as Schwann cell survival and motility.114,115 Microglia are the innate immune cells of the brain.116 Following an epileptic seizure, IGF-I expression in microglia is upregulated117 and may play a role in minimising cell damage. Astrocytes provide physical and nutrient support, and participate in maintaining blood-brain barriers and modulating synaptic transmission.94 Insulin like growth factor I is increased in activated astrocytes118 and regulation of mitochondrial function and redox status by IGF-I is essential in the maintenance of astrocyte function.119
Ageing and cognitive function
Insulin/IGF signalling pathways are phylogenetically conserved120 and are central to the ageing process.121 Reduced function of these pathways has been shown to extend survival in rodents.122,123 There is increasing evidence that changes in activity of splicing factors are involved in the ageing phenotype. Exercise-induced changes in the IGF-I splice variant mechano growth factor (MGF) have been shown to decrease with age.124 IGF1R variants have been described that are more prevalent in Ashkenazi Jewish centenarians125 and which are reduced-function mutations.126
IGF signalling is involved in adult hippocampal neurogenesis.127,128 Hippocampal neuroblasts decline with age, however this decrease is less pronounced in humans, compared to mice93 and the cognitive decline may be largely due to changes in neural stem cell activity rather than number.129 Glial cell numbers do not appear to decline with age.130 With ageing there is a decline in endocrine IGF-I,131 which is a candidate frailty biomarker.131,132 Brain IGF-I and IGF signalling is also reduced during ageing.131,133 In addition to the GH/IGF system, other age-related changes in growth factors have been linked to changes in neurogenesis, including loss of FGF-2 and VEGF.134,135 Studies in rodents have demonstrated close links between IGF-I, hippocampal neurogenesis and cognitive function. Intracerebroventricular infusion of IGF-I ameliorates age-related decline of hippocampal neurogenesis in rats.136 It has been suggested that reduced hippocampal neurogenesis contributes to the pathophysiology of depression.90 Mice with specific knockout of hippocampal IGF-I have been shown to have a depressive phenotype that is not rescued by endocrine IGF-I.137
In mice, the effects of physical activity on hippocampal neurogenesis and cognition are associated with circulating IGF-I levels.138 In rats, there is evidence that aerobic and resistance training increase learning and spatial memory through divergent molecular pathways: resistance training acts via the IGF-I/IGF1R/Akt pathway in hippocampus.139 Physical activity also increases brain uptake of endocrine IGF-I.140 In adolescent humans exercise increases both IGF-I and BDNF.141 In adults increased temporal lobe functional connectivity in response to exercise is associated with increases in circulating IGF-I, BDNF and VEGF.142 There is experimental evidence that hippocampal increases in BDNF are more important that changes in peripheral levels of IGF-I and BDNF,143 and that IGF-I interacts with BDNF and VEGF signalling pathways in exercise-related changes to hippocampal function.64,144,145
In humans, studies of the relationship between serum IGF-I and cognitive function or decline in cognitive function are conflicting. In a large prospective study, higher levels of serum IGF-I were associated with better cognitive performance in women but not men.146 Insulin like growth factor I treatment in postmenopausal women has no effect on memory.147 Overall, serum IGF-I is not considered a useful biomarker of cognitive decline in the ageing brain,148 and there may be a U-shaped relationship between IGF-I and cognitive function. In females with exceptional longevity, lower serum IGF-I is associated with better cognition.149 In adult patients with GH deficiency, however, cognitive impairment which contributes to reduced quality of life is ameliorated by GH replacement.150 Rodent models with GH deficiency or resistance have a delayed age-induced decline in memory retention.151,152 In adult rats, peripheral administration of GH stimulates hippocampal neurogenesis both in the presence153 and absence154 of GH deficiency. It seems likely that this effect of GH is mediated by endocrine IGF-I; peripheral administration of IGF-I also stimulates hippocampal neurogenesis.155
Insulin and IGF-I are nutrient-sensitive signalling pathways and have key roles in energy metabolism, including that of neural stem cells.156,157 In rodents, IGF-I regulates glucose metabolism in developing158 and aged159 brain. Brain is also an important target for insulin actions with effects on neuronal survival and synaptic plasticity, particularly in the hippocampus where IR are abundant.160 There is also evidence that IGF-II, given subcutaneously, is neuroprotective in ageing rats.161 Compared to IGF-I, differences in affinity for IGF1R/IR of IGF-II and its production by the choroid plexus indicate that it might have a distinct role in the nervous system.162 The role this plays in the choroid plexus alongside IGF-I, expression of which declines with ageing163 should be further explored. Insulin like growth factor II is also expressed in the leptomeninges and parenchymal vasculature.164 Expressed by neural stem cells, it has been suggested that IGF-II from these cells and from the choroid plexus has an important role in maintaining neurogenesis in the supraventricular zone.162 Insulin like growth factor II may also play a key role in maintenance of neurogenesis in the hippocampus. An effect of IGF-II on memory enhancement is supported by experimental evidence.165 Interestingly, IGF2R overexpression is associated with increased β-amyloid generation.166 While this is likely due to an effect on endocytic pathways, the role of increased IGF-II disposal has not been explored.167
Neurodegenerative Disorders
Brain regions with the capacity for neurogenesis are prone to neurodegenerative disease. Loss of neurons and their functions, particularly cholinergic and dopaminergic neurons, results in impairments ranging from cognitive abilities to coordination and mobility.168–170 Alzheimer disease (AD), Parkinson disease (PD), and Huntington disease (HD) all cause a dementia that is distinct from the physiological decline that occurs with ageing. Despite different distinct pathological processes, many of the hallmark features are identical, eg, depression and anxiety, loss of cognitive function and olfactory dysfunction. There is compelling evidence that inflammation is key to the aetiology or pathogenesis of these neurodegenerative disorders.169 In each, protein misfolding and aggregation lead to activation of neuroinflammatory processes.121 Activated glial cells produce a microenvironment of reactive oxygen species (ROS) and pro-inflammatory mediators contribute to neuronal damage and death in a vicious cycle.169 Reduced IGFBP expression in lipopolysaccharide-activated microglia171 might play an important role in increasing paracrine/autocrine IGF availability. While neurotrophic factors, including IGF-I, are increased in activated astrocytes, this may be insufficient to exert the required neuroprotective effect.118
Obesity is associated with a chronic inflammatory state that is considered a contributor to the prevalence of neurodegenerative disorders, with IGF/insulin resistance being the possible link.169 There is substantial evidence that IGF/insulin signalling and cross-talk with other signalling pathways are involved in the processes of neurodegeneration.121,127,172,173 The regions of brain with neurogenic capacity are highly vascular. In rodents there is evidence that IGF-I is required for vascular remodelling in adult brain.174 Age-related cerebrovascular changes that also contribute to the neurodegenerative pathology.175 These regions are highly vascular and multiple systemic factors including IGF-I and IGF-II may play a role.176
Alzheimer disease
Alzheimer disease is the most common of the neurodegenerative dementias. The two hallmarks of the disease are neuritic plaques, formed by the extracellular accumulation of abnormal β-amyloid protein,177 and intracellular neurofibrillary tangles, composed of hyperphosphorylated tau protein.178 Plaques and neurofibrillary tangles both contribute to glial cell activation and neuroinflammation that influence AD pathogenesis and neuronal loss.170,179 Glutamate is the most important excitatory neurotransmitter and is involved in neuronal growth and synaptic plasticity.180 Glutamate influences β-amyloid production, and β-amyloid is itself an activator of glutamatergic receptors of the NMDA type that are essential for both long-term potentiation (LTP) and long-term depression (LTD) and are therefore crucial in learning and memory. It is argued that interference with NMDA receptors by abnormal accumulation of β-amyloid and the ability of β-amyloid to increase tau phosphorylation underpin synaptic loss and cognitive decline in AD patients.181
The β-amyloid precursor protein (APP) is cleaved by membrane-bound β- and γ-secretases into β-amyloid, the longer forms of which are more likely to be deposited. Monomeric forms of β-amyloid are less toxic and are able to activate insulin/IGF pathways.182 While IGF-I has been shown to rescue rat hippocampal neurons from the toxicity induced oligomeric forms of β-amyloid in vitro,183,184 it also increases the extracellular concentration of β-amyloid by promoting its secretion and inhibiting its degradation.185 Neuronal death in AD is strongly associated with mitochondrial dysfunction186 including increased ROS production, decreased mitochondrial enzymes and increased oxidative damage. It has been argued that ageing, the most important non-genetic risk factor for AD development, does so largely via mitochondrial dysfunction, though levels of β-amyloid degrading enzymes also decline with age.187
There is an increased prevalence of AD in type 2 diabetes mellitus in humans188; however, this association is likely to be confounded by the presence of cerebrovascular pathology which reduces the number of AD lesions required for the manifestation of clinical dementia.189 Insulin signalling appears to be involved in both β-amyloid peptide deposition and tau phosphorylation,190,191 and defective insulin signalling is thought to play a key role in disease pathogenesis.192,193 The finding of altered brain expression of insulin and IGFs, and their receptors has led to the suggestion that AD be labelled ‘type 3 diabetes’.194 Indeed functional proteomics suggests that the link between AD and diabetes relates to insulin/IGF signalling. Using tissue samples from brains of patients with AD, compared to tissue from normal individuals, insulin resistance in hippocampus and cerebral cortex was found to be associated with IGF-I resistance and cognitive decline.195 Expressions of IGF1R and IR are increased in AD neurons in the temporal cortex while that of IR substrate (IRS)-1 and IRS-2 are decreased.196 Some studies have proposed a relationship between endocrine IGF-I and the risk of AD; however, in a meta-analysis of nine studies comprising 1639 individuals, no link between serum IGF-I and AD was demonstrated.197 There is one report of an association between an IGF-I polymorphism and late-onset AD in a Chinese population.198
Most of our understanding of the role of insulin and IGFs in AD has come from studies in rodents. Intracerebrospinal streptozotocin in mice induces AD-like changes in pathology and behaviour and is associated with reduced brain expression of insulin, IGFs and reduced IGF1R binding and signalling.199,200 In mice, brain-specific IGF-I knockout is associated with hyperphosphorylation of tau protein,201 while blockade of IGF1R function in the choroid plexus of rats is associated with AD-like neuropathology.202 Transgenic models of AD have been developed including mutations that target APP or the tau protein.203,204 When mice expressing mutant APP are crossed with those genetically predisposed to diabetes, development of cognitive dysfunction is accelerated.205 In these animals, in addition to reduced brain insulin signalling, marked vascular inflammation was observed despite no change in β-amyloid deposition. Presenilin, a crucial component of the γ-secretase complex, also controls IR expression.206 Mice overexpressing pancreatic β-cell IGF-II develop hyperinsulinaemia, and co-expression of mutations of both APP and presenilin-1 genes exacerbates the development of peripheral insulin resistance, with no increase in brain insulin or β-amyloid deposition.207 Mice expressing mutant APP have reduced CSF/serum IGF-I ratio and low serum IGF-I is an early biomarker of AD onset.208 When mutations of both APP and presenilin-1 genes are combined with endocrine IGF-I deficiency due to targeted deletion of hepatic IGF-I, amyloid plaque formation occurs earlier.209 On the other hand, reduction in serum IGF-I through protein restriction, is associated with reduced AD neuropathology in mice expressing mutant APP, presenilin-1 and tau proteins.210
As has been observed in human brain tissue, brain slices from mice expressing mutant APP have increased IGF1R expression and reduced Akt response to IGF-I211 and, when crossed with igf1r +/-, a reduction in β-amyloid-associated behavioural impairment associated with the sequestration of β-amyloid aggregates of lower toxicity has been observed.212 The protective effect of neuronal IGF-I resistance is supported by the observation that, in a neuron-targeted IGF1R knockout combined with the APP mutation, APP processing is decreased and β-amyloid accumulation is reduced213 and, when combined with mutant APP and presenilin-1, there is improved spatial memory, fewer amyloid plaques and less neuroinflammation.214 Paradoxically, in the same model, systemic delivery of IGF-I ameliorates the AD-like changes and increases transport of β-amyloid/carrier protein complexes through the choroid plexus barrier.215 Taken together, these studies suggest that, while reduced IGF action centrally is associated with improved AD pathology, increased peripheral IGF availability is neuroprotective through increased β-amyloid clearance. In ageing mice with a targeted deletion of hepatic IGF-I, and therefore reduced endocrine IGF-I, there is a premature increase in brain β-amyloid, and administration of IGF-I increases clearance and reduces β-amyloid levels.216 In this research, IGF-I was found to affect the permeability of the blood-brain barrier to carrier proteins such as albumin and transthyretin. However other studies, using multiple in vivo models including APP-overexpressing mice, have shown no impact of peripheral IGF-I on brain β-amyloid levels or the phosphorylation state of tau.217 Furthermore in rats intracerebroventricular IGF-I prevents the deleterious effect of coadministered β-amyloid on the somatostatinergic system in the temporal cortex.218 The N-terminal tripeptide also has protective effects on the somatostatin system in temporal cortex of β-amyloid treated rats, through modulation of calcium and glycogen synthase kinase 3β (GSK3β) signalling.219
In addition to considerations of endocrine versus tissue IGFs, an understanding of the factors regulating expression and action in different cell types is required in order to unravel the role of the system in AD. IGF-I and insulin stimulate neuronal secretion of β-amyloid and reduce its degradation,185 while also having a neuroprotective role,183 however expression and action of IGF-I and insulin are reduced in AD. As the AD pathology progresses, astrocytes also have reduced expression of insulin and IGF signalling pathways particularly in individuals expressing the APOEε4 allele.220 Insulin reduces APP levels in individuals without the APOEε4 allele.221 In a co-culture system, impaired IGF-I signalling in human astrocytes is associated with reduced ability to protect neurons from oxidative stress.222 Oxidative stress has been identified as an important link between AD and insulin resistance,223 with Forkhead box class O (FoxO) transcriptions factors as candidates for the molecular integrative link.224 Insulin like growth factor I inactivates and displaces FoxO3 from calcineurin in activated astrocytes, with reduced inflammatory signalling associated with reduced AD phenotype in mice with mutations of both APP and presenilin-1 genes.225
Parkinson disease
Parkinson disease is a neurodegenerative disorder characterised by significant motor impairments, including bradykinesia, muscular rigidity, tremor and postural instability. However non-motor signs and symptoms, such as impaired olfaction, cognitive impairment and depression, may precede the classical motor signs by many years226 and indicate early involvement of the olfactory bulb and hippocampus in the disease. The hallmark of PD is the gradual, selective loss of dopaminergic neurons of the substantia nigra pars compacta region and the aggregation of misfolded α-synuclein protein forming insoluble cytoplasmic inclusions (Lewy Bodies).227 Individuals with the rare familial forms have mutations of α-synuclein.228 Misfolded α-synuclein specifically induces free radical production in dopaminergic neurons, triggering apoptosis,229 and there is also a strong association between PD and mitochondrial dysfunction.230 Other genes associated with PD encode proteins involved in cellular trafficking and protein turnover.231 Chronic exposure of human neuroblastoma cells to rotenone, an inhibitor of complex I of the mitochondrial electron transport chain, induces many of the biochemical features of PD.232 Interestingly, in peripheral lymphocytes, IGF-I has a protective effect on rotenone-induced apoptosis.233
A meta-analysis of five studies with 166 patients showed that IGF-I levels were higher in drug naive patients with PD compared to 323 healthy controls.234 However, in patients with PD, lower circulating IGF-I concentrations are associated with poor cognitive performance235,236 and have been shown to predict decline in cognitive function after a 2 year follow-up.237 Nevertheless it is clear that confounding factors, such as age and obesity limit the use of IGF-I as a predictive marker.238 Association of an IGF-I gene polymorphism with PD has been demonstrated in a Chinese population239 and is the same polymorphism as that associated with AD in the same population.198 In postmortem brain tissue, IGF-I expression is increased in frontal cortex in PD compared to controls, while insulin, IGF-II, IR, IGF1R and IGF2R are reduced in white matter and amygdala.240
Dopamine-denervated striatum, using 6-hydroxydopamine delivered unilaterally, induces a Parkinson’s-like disease in rats. Using this model, IGF-I, combined with FGF, improves dopamine neuron survival and behavioural outcome in response to transplants of human foetal tissue strands.241 Insulin like growth factor I expression using a lentiviral vector had neuroprotective effects in vitro; however, after intra-striatal delivery to 6-hydroxydopamine treated rats, no effect on survival of dopaminergic cells or behaviour was observed.242 This may have been due to insufficient concentrations of delivered IGF-I. Using high concentrations of dopamine to induce neurotoxicity in vitro, apoptosis is significantly reduced by IGF-I in primary rat cerebellar cells and a human neuroblastoma cell line.243
Later studies have demonstrated that the PI3K/Akt pathway is critical for the in vivo action of IGF-I and also mediates the protective effect of oestrogen on dopaminergic neurons in PD rat models.244 Peripheral administration of the N-terminal tripeptide, that has been shown to modulate GSK3β219 in a model of AD, also improves functional deficits in PD rats.245 The involvement of PI3K/Akt/GSK3β signalling pathways in PD has recently been reviewed.246
Huntington disease
Huntington disease is an autosomal progressive neurodegenerative disease characterised by chorea, abnormal voluntary movements, and cognitive and psychological dysfunction.247,248 A key characteristic of the disorder is the aggregation of mutant huntingtin protein in intranuclear inclusions in the GABAergic medium spiny neurons of the striatum.249 This is due to an expanding CAG triplet repeat in the gene, the length of which contributes approximately 70% of the variance in age of onset of symptoms.250
In a longitudinal study of patients with HD, higher levels of total circulating IGF-I at baseline were associated with a higher degree of cognitive impairment and predicted decreases in cognitive scores over a 3.5-year follow-up.251 While higher insulin levels were also associated with lower cognitive scores, they were not predictive of change in cognitive function. On the other hand, in humans, concentrations of the acid labile subunit of the ternary complex are reduced.252
Rodent models of HD, including striatal lesioning using mitochondrial toxins or quinolinic acid and mice expressing a mutant huntingtin transgene (eg, R6/1, R6/2, N171-82Q and YAC128), have been used to further explore the role of the IGF system. In R6/1 HD mice, histone deacetylase has been identified as a switch between neuroprotection and neuronal death with IGF-I inhibiting the neurotoxic effect.253 When combined with heterozygous Igf-1 knockout, there are some beneficial effects on the HD phenotype in female N171-82Q HD mice, but some detrimental effects in males, and no effect on survival.254 In R6/1 HD mice running-induced hippocampal neurogenesis is associated with reduced Akt signalling despite increased serum IGF-I.255 On the other hand, intranasal IGF-I rescues the YAC128 phenotype.256 The neuroprotective effect of cannabigerol in R6/2 and in mice given the mitochondrial toxin 3-nitropropionate is associated with modest improvements in striatal expression of BDNF and IGF-I.257 Ablation of caspase-6 in YAC128 HD mice reverses the HD phenotype and is associated with weight loss and reduced serum IGF-I.258 Administration of the N-terminal IGF-I tripeptide also prevents HD neuropathology in rats with lesions of the striatum induced by quinolinic acid.48 Atypical diabetes develops in 70% of R6/2 HD mice259 and is associated with dysregulated gene expression and intranuclear inclusions in pancreas.260,261 Blood glucose levels are restored by IGF-I infusion in these mice.262 Patients with HD are more likely to develop diabetes, and have impaired insulin secretion and peripheral insulin resistance.261
In studies using transfected striatal neurons in vitro, it was found that IGF-I blocks mutant huntingtin-induced cell death and decreased formation of intranuclear inclusions.263 BDNF, which also reduced apoptosis, did not block the formation of intranuclear inclusions.264 Striatal cell lines and primary cortical cultures derived from huntingtin knock-in mice have mitochondrial dysfunction that is ameliorated by insulin and IGF-I.265,266 Impaired mitochondrial function appears also to have an important pathological role in HD in peripheral tissues. In lymphoblasts derived from HD patients, reduced energy metabolism and mitochondrial dysfunction are associated with reduced Akt and ERK activation and can be rescued with IGF-I or insulin.267
Neuroprotective and Neurotrophic Roles
While there is convincing evidence that the IGF system has specific roles in the neurogenerative dementias through effects on hippocampal neurogenesis, it has been suggested that more general neurotrophic and neuroprotective effects of IGF-I might be important in a range of other disorders.268 HIV is associated with dementia that relates to TNFα released by activated macrophages: IGF-I has an antiapoptotic effect on neurons exposed to medium from infected macrophages.269 It is likely that the increase in IGF-I and BDNF after retinal stem cell transplantation in a rat model of glaucoma had a neuroprotective role.270 A potential role for IGF-I as a therapeutic approach has been considered for amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), cerebrovascular disease and following trauma, and these are therefore reviewed briefly here.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis is a degenerative disease of upper and lower motor neurons that leads to progressive weakness in limb and bulbar muscle with ultimately respiratory failure. The pathogenesis of ALS remains unclear, but a number of factors have been suggested including evidence of oxidative damage to proteins,271, lipids272 and DNA.273 In common with other neurodegenerative disorders, mitochondrial dysfunction274 and protein aggregation involving superoxide dismutase 1 (SOD1)275,276 and TDP-43277,278 have been associated with ALS. The first proven cause of ALS was identified in individuals with mutations in SOD1279 which involve a toxic gain of dysfunction280 but beyond involvement in protein aggregation, the mechanism remains unclear. In an animal model of ALS, the SOD1G93A mouse, IGF1R is increased in reactive astrocytes in the central nervous system.281 Retrograde viral delivery of IGF-I from muscle to motor neurons prolongs life and delays disease progress.282 Neuroinflammatory responses are also implicated in ALS.283 In the SOD1G93A mouse, intrathecal treatment with IGF-I decreases macrophage invasion and activation of TNFα production in sciatic nerves and delivery of a vector to knockdown IGF-I increases sciatic nerve inflammation.284 In the same mouse model, intraparenchymal spinal cord delivery of adeno-associated IGF-I partially rescues lumbar spinal cord motor neurons.285 VEGF and IGF-I gene transfer in to cellular components of the ventricular system have similar, non-additive effect to delay motor decline and prolong survival.286 Administration of IGF-I in another animal model with ALS features, the wobbler mouse, results in significant improvements in muscle strength and histopathology, although no changes in motor neurone numbers were observed.287
Patients with ALS have reduced circulating IGFs and insulin, and increased IGFBPs288; however, the potential for use of IGFs and IGFBPs in therapy is still considered worthwhile.289,290 While there have been randomised controlled trials involving the use of IGF-I in humans, these do not provide strong evidence supporting its effectiveness.6 There is a possibility that upregulation of IGFBP-5 might play a role in the response to IGF-I in these disorders.291
Multiple sclerosis
Multiple sclerosis is a demyelinating disease that has a variable clinical course from a relapsing-remitting disease to one that is relentlessly progressive.292 While an immune-mediated inflammatory process is considered central to the pathogenesis, anti-inflammatory therapies have limited effectiveness in promoting remyelination.293 Insulin like growth factor I has been considered as a possible therapeutic approach to MS. Insulin like growth factor I promotes myelin production by oligodendrocytes.112,294,295 Insulin like growth factor I and IGF1R are upregulated at the edges of demyelinated plaques296; however, it has been shown that oligodendrocytes within MS lesions have reduced IGF1R expression.297 Mice overexpressing IGF-I are protected from cuprizone-induced demyelination,298 while ablation of brain IGF1R prevents remyelination in this animal model.299 However, in mice with experimental autoimmue encephalomyelitis, a transient improvement in clinical indices and remyelination in response to IGF-I, delivered using osmotic subcutaneous pumps, is lost in the chronic phase of the disease.300
In patients with MS, IGF-I concentrations in the circulation301 and CSF302 are no different to a control group; however, it is possible that the observed increases in IGFBP expression296,301,302 or reduced oligodendrocyte IGF1R expression,297 modulate IGF bioactivity. A 6-month pilot study found no impact of IGF-I on magnetic resonance imaging or clinical measures of disease activity.303 In this study, IGF-I was delivered subcutaneously, and it remains to be seen whether alternative approaches that target oligodendrocyte IGF signalling pathways are effective.
Cerebrovascular disease
Recent data from the Framingham study indicate that, during mean follow-up of 10.2 years, individuals in the lowest quintile of serum IGF-I concentrations have a 2.3-fold higher risk of incident ischaemic stroke304; however, it is not known whether this is a causal relationship and studies of the predictive role IGF-I in patients who have sustained strokes are equivocal.127
In a rat model of unilateral hypoxic-ischaemic brain injury, IGF-I accumulates in the damaged hemisphere within 5 hours of severe injury, and at 3 days there is increased IGF-I production by microglia and increased IGFBP-2 expression in perineuronal reactive astrocytes throughout the hemisphere.305 This was associated with reduced expression of IGFBP-3 and IGFBP-5 expression in reactive microglia and neurones in the injured hippocampus, increased expression of IGFBP-6 in choroid plexus, ependyma and reactive glia and no change in IGF1R.
In animal studies of ischaemic brain injury, there is convincing evidence of a protective effect of IGF-I on cortical neurons. In foetal lambs, IGF-I delivered into a lateral cerebral ventricle 2 hours after a hypoxic ischemic insult induced by transient carotid artery occlusion in utero reduces neuronal loss and incidence of seizures.306 In rats, after unilateral hypoxic-ischaemic injury following transient middle cerebral artery occlusion, intramuscular IGF-I injection decreases neuronal apoptosis and improves motor function, effects that are eliminated by co-administration of an inhibitor of IGF1R.307Using this model of cerebral ischaemia, the benefit of physical activity on function recovery and enhanced neurogenesis is associated with increased IGF-I expression in the peri-infarct region.308 IGF-I promotes receptor-mediated anchorage of endothelial cells, stabilising the microvascular cytoskeleton under these conditions.309 In another model of transient focal cerebral ischaemia using endothelin-1 in conscious rats, subcutaneous IGF-I treatment reduced infarct volumes and increased motor-sensory functions.310 Using the same model in hypertensive rats, infarct size was greater and IGF-I was less protective, but significantly reduced microglial activation, not seen in normotensive animals.
The N-terminal tripeptide of IGF-I is also active after unilateral hypoxic-ischaemic brain injury in rats, preventing neuronal apoptosis, promoting astrocyte survival and inhibiting microglial proliferation following intravenous infusion.311 After cardiac arrest in rats, a modest neuroprotective effect of intracerebral ventricular infusion of N-terminal tripeptide was seen.312 After unilateral hypoxic-ischaemic brain injury, intracerebral ventricular infusion of des(1-3)IGF-I is less potent than IGF-I in preventing neuronal loss.313 It is possible that this is due to the additional effect of the N-terminal tripeptide, however co-administration of IGF-II blocked the effect of IGF-I and displacement from IGFBPs that play a targeting role was a suggested explanation. In mice exposed to cerebral hypoxic-ischaemic injury, the increased IGF-I expression around the injury is associated with IGFBP-2 expression in activated astrocytes, with evidence that IGF-I is an paracrine/autocrine mitogen for microglia/macrophages under these conditions.314 The role of IGFBPs as facilitators of brain IGF action and the role of IGF-II and IGF2R following cerebral ischaemia remain to be fully explored.
Traumatic nervous system injury
Traumatic brain injury during early development is an important cause of cognitive dysfunction and is associated with epigenetic changes.315 After traumatic brain injury in rat pups, hippocampal IGF-I expression is increased and associated with epigenetic modifications in the promoter region.316 A decrease in circulating IGF is also predictive of cognitive dysfunction from hippocampal damage.317 Increased IGF-I expression in response to traumatic injury is seen in both adult and 2 week old mice.318 A penetrating cerebral wound in adult rats leads to acute and transient increases in expression of IGF-I, IGF1R and IGFBP-2 in injury-responsive astrocytes and neurons and IGFBP-3 in microvascular endothelium, with IGFBP-4 and -5 expressed in astrocytes and neurons later in the wounding response.319 There appears to be a therapeutic window of at least 6 hours for central infusion of IGF-I to promote neurobehavioural recovery following traumatic brain injury in mice.320
Insulin like growth factor I and IGFBP-2 are likely to play a more general and widespread neuroprotective role in the nervous system. Increases in IGF-I and IGFBP-2 expression are seen in astrocytes following cryogenic spinal cord injury in adult rats321 and in the hippocampus in response to cytotoxic damage.322 IGF-I delivered subcutaneously or intracerebroventricularly partially rescues neurons and restores motor coordination in a rat model of cerebellar ataxia induced by 3-acetylpyridine.323 There may be unwanted effects of IGF-I in damaged peripheral nerves. Neutralising anti-IGF-I antibodies reduced collateral axonal sprouting after peripheral nerve lesion324 and an IGF1R antagonist reduced IGF-I-induced hyperalgesia in a mouse model of type 2 diabetes.325
IGF System As A Therapeutic Target
There is sufficient evidence for a specific role of the IGF/insulin system for it to be worth considering as a therapeutic target in AD and other neurodegenerative diseases.326,327 However, these disorders are characterised by IGF and insulin resistance, and directing therapy towards the endocrine IGF/insulin system are likely to have limited effectiveness. Systemic approaches that increase neurovascular coupling and increase transfer at the blood-brain barriers are worthy of consideration. Inhibitors of glycogen synthase kinase 3β, by modulation of megalin transport, increase brain IGF-I levels.328 Approaches that target neuronal IGF/insulin signalling are also appealing. Gene therapy would have advantages in meeting this goal, with the attendant challenges in reaching target areas in the nervous system.329 Genomic and proteomic approaches that identify the interaction of IGF-I with other growth factor pathways that prevent apoptosis, are likely to hold promise in identifying potential drug targets.252,330,331
In addition to diseases in which the IGF system is likely to have a specific role, the more general neuroprotective effects make it worthwhile considering for a range of other disorders. However, in a series of clinical trials of patients with ALS, the use of IGF-I delivered subcutaneously has not been promising, with no improvement in survival.332,333 In humans, several studies have demonstrated that GH improves neuron recovery and clinical outcome following traumatic brain injury.333 In the light of studies using IGF-I in rodents, described in preceding sections, it might be that approaches that combine the use GH and IGF-I are worthwhile trying in humans. The combination of GH and IGF-I delivered intravenously for two weeks improved metabolic and nutritional endpoints in patients after acute traumatic brain injury,334 however effects on neurological function were not reported.
Systemic administration of IGF-I is facilitated by approaches that prolong the half-life or promote IGF delivery. In trials of IGF-I for retinopathy of prematurity, IGF-I was delivered complexed with IGFBP-3.335 Early trials with this combination, however, have failed to show a positive effect on the prevention of retinopathy of prematurity.336 In a mouse model of motor neuron degeneration, IGF-I coupled with polyethylene glycol extend its circulating half-life, prolonged survival, maintained motor coordination, and rescued motor neurons from cell death.337 Microsphere formulations that provided controlled release from subcutaneous depots are associated with extended survival and enhanced motor co-ordination in a mouse model of spinocerebellar neurodegeneration.338 Use of an IGF-I analogue with high IGFBPs and no biological activity through IGF1R, increased availability of endogenous IGFs and had a neuroprotective effect in rat model of hypoxia-ischaemia.339
Therapeutic approaches that deliver IGFs directly to their target, or ones increasing IGF/insulin sensitivity within the nervous system deserve focus. Intranasal administration of insulin raises central nervous system levels without raising plasma levels340 and early clinical trials in humans were promising.341 Intranasal delivery of insulin improves some tests of memory in patients with AD without the APOEε4 allele.342 Success of these approaches raise the hypothesis that intranasal IGF-I might be an option for the treatment of depression,343–345 or for improving cognitive function in normal ageing. Peripheral IGF-I infusion improves spatial reference memory and working memory in healthy ageing rats.346 Studies of intracerebroventricular IGF-I gene therapy in ageing rats improves motor performance.347 and modulates relevant hippocampal genes.348 Intracerebroventricular FGF-2 also enhances neurogenesis in the hippocampus of aged rats.349 Therapeutic approaches that combine IGF-I with other growth factors might be effective.350 Since the ERK pathway is often coactivated with the PI3 K/Akt signalling pathway,351 the use of EGF might be considered. Insulin like growth factor I mediates resistance to anti-EGF therapy in glioblastoma cells352 and insulin and EGF have been shown to act synergistically to promote astrocyte survival and proliferation.353 There are connections between sphingolipid and IGF signalling354 and an effect of Klotho on IGF-I signalling355 that might have implications for the management of nervous system disease.
The possibility of generating the main cell types of the nervous system from multipotent neural stem cells is an important focus for regenerative medicine329,356 and the IGF system will play a key role, most likely in combination with other growth factors. Cell replacement therapy have been pursued for PD.168 Human neural progenitor cells produced to release IGF-I have improved survival and, when transplanted into the substantia nigra in the 6-hydroxydopamine rat model of PD, exert trophic effects on degenerating dopamine neurons.357 Combinations of IGF-I with FGF241 or BDNF and glial-derived neurotrophic factor have been used to prepare neural progenitor cells for transplantation.184
Conclusions and Recommendations
IGF system components are widely expressed in the nervous system where there is substantial evidence for neuroprotective and neurotrophic actions of IGF-I. Low IGF-I is associated with longevity and this apparent paradox is best understood when the complexity of the IGF system is taken into account. Association of IGF with IGFBP-2 and IGFBP-5 in the nervous system may promote local IGF action, while high concentrations or the presence of other IGFBPs may be inhibitory. Nutrition and insulin which are important regulators of IGF-I production, have other effects on the nervous system, through pathways that interact with the IGF1R. It is important to note that much of our understanding of the IGFs in the nervous comes from experimental studies in rodents where there are differences in neurogenesis compared to humans,33,93 with a focus on IGF-I and not IGF-II. Since the IRA isoform is expressed at significant concentrations in brain tissue,6 IRA/IGF1R hybrids are also present and IGF-II may therefore have a distinct role. These gaps in knowledge should be addressed in future research. In particular (a) the role of brain IGFBPs as regulators of local IGF actions, and what are their IGF-independent roles; (b) the role of IGF-II and, in particular is there a potential therapeutic role in human neurodegenerative disease? and (c) the effect of a combination approach to therapy; using other growth factors with IGF-I or IGF-II across the spectrum of nervous system disorders.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: ML and GB developed the structure and arguments for the paper, wrote and critically revised, and approved the final version.
Disclosure and Ethics: The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. There are no conflicts of interest to declare. The authors also confirm that this article is unique and not under consideration or published in any other publication, and no copyrighted material is reproduced.
ORCID iD: Moira S Lewitt  https://orcid.org/0000-0002-3859-1382
https://orcid.org/0000-0002-3859-1382
References
- 1. Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994;135:1753–1761. [DOI] [PubMed] [Google Scholar]
- 3. Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74. [DOI] [PubMed] [Google Scholar]
- 4. Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. [DOI] [PubMed] [Google Scholar]
- 5. Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev. 2001;22:818–835. [DOI] [PubMed] [Google Scholar]
- 6. Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. [DOI] [PubMed] [Google Scholar]
- 7. Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005;16:421–439. [DOI] [PubMed] [Google Scholar]
- 8. Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. [DOI] [PubMed] [Google Scholar]
- 9. Scott CD, Firth SM. The role of the M6P/IGF-II receptor in cancer: tumor suppression or garbage disposal? Horm Metab Res. 2004;36:261–271. [DOI] [PubMed] [Google Scholar]
- 10. Daza DO, Sundstrom G, Bergqvist CA, Duan C, Larhammar D. Evolution of the insulin-like growth factor binding protein (IGFBP) family. Endocrinology. 2011;152:2278–2289. [DOI] [PubMed] [Google Scholar]
- 11. Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 2014;14:329–341. [DOI] [PubMed] [Google Scholar]
- 12. Clemmons DR. Role of IGF binding proteins in regulating metabolism. Trends Endocrinol. Metab. 2016;27:375–391. [DOI] [PubMed] [Google Scholar]
- 13. Salmon WD, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957;49:825–836. [PubMed] [Google Scholar]
- 14. Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol. 2001;170:63–70. [DOI] [PubMed] [Google Scholar]
- 15. Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am. 2012;41:425–443, vii-viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev. 1999;79:511–607. [DOI] [PubMed] [Google Scholar]
- 17. Russo VC, Rekaris G, Baker NL, Bach LA, Werther GA. Basic fibroblast growth factor induces proteolysis of secreted and cell membrane-associated insulin-like growth factor binding protein-2 in human neuroblastoma cells. Endocrinology. 1999;140:3082–3090. [DOI] [PubMed] [Google Scholar]
- 18. Mendes KN, Wang GK, Fuller GN, Zhang W. JNK mediates insulin-like growth factor binding protein 2/integrin alpha5-dependent glioma cell migration. Int J Oncol. 2010;37:143–153. [DOI] [PubMed] [Google Scholar]
- 19. Grotendorst GR, Lau LF, Perbal B. CCN proteins are distinct from and should not be considered members of the insulin-like growth factor-binding protein superfamily. Endocrinology. 2000;141:2254–2256. [DOI] [PubMed] [Google Scholar]
- 20. O’Kusky J, Ye P. Neurodevelopmental effects of insulin-like growth factor signaling. Front Neuroendocrinol. 2012;33:230–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rotwein P, Burgess SK, Milbrandt JD, Krause JE. Differential expression of insulin-like growth factor genes in rat central nervous system. Proc Natl Acad Sci U S A. 1988;85:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bondy CA, Werner H, Roberts CT, Le Roith D. Cellular pattern of insulin-like growth factor-I (IGF-I) and type I IGF receptor gene expression in early organogenesis: comparison with IGF-II gene expression. Mol Endocrinol. 1990;4:1386–1398. [DOI] [PubMed] [Google Scholar]
- 23. Frago LM, Paneda C, Dickson SL, Hewson AK, Argente J, Chowen JA. Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin- like growth factor-I expression and activate intracellular signaling pathways involved in neuroprotection. Endocrinology. 2002;143:4113–4122. [DOI] [PubMed] [Google Scholar]
- 24. Hojvat S, Baker G, Kirsteins L, Lawrence AM. Growth hormone (GH) immunoreactivity in the rodent and primate CNS: distribution, characterization and presence posthypophysectomy. Brain Res. 1982;239:543–557. [DOI] [PubMed] [Google Scholar]
- 25. Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. [DOI] [PubMed] [Google Scholar]
- 26. Walser M, Sama MT, Wickelgren R, et al. Local overexpression of GH and GH/IGF1 effects in the adult mouse hippocampus. J Endocrinol. 2012;215:257–268. [DOI] [PubMed] [Google Scholar]
- 27. Pulford BE, Ishii DN. Uptake of circulating insulin-like growth factors (IGFs) into cerebrospinal fluid appears to be independent of the IGF receptors as well as IGF-binding proteins. Endocrinology. 2001;142:213–220. [DOI] [PubMed] [Google Scholar]
- 28. Yan H, Mitschelen M, Bixler GV, et al. Circulating IGF1 regulates hippocampal IGF1 levels and brain gene expression during adolescence. J Endocrinol. 2011;211:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishijima T, Piriz J, Duflot S, et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67:834–846. [DOI] [PubMed] [Google Scholar]
- 30. Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci. 2005;25:10884–10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lehtinen MK, Bjornsson CS, Dymecki SM, Gilbertson RJ, Holtzman DM, Monuki ES. The choroid plexus and cerebrospinal fluid: emerging roles in development, disease, and therapy. J Neurosci. 2013;33:17553–17559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carlsson-Skwirut C, Jörnvall H, Holmgren A, et al. Isolation and characterization of variant IGF-1 as well as IGF-2 from adult human brain. FEBS Lett. 1986;201:46–50. [DOI] [PubMed] [Google Scholar]
- 33. Bond AM, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lehtinen MK, Zappaterra MW, Chen X, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holzenberger M, Jarvis ED, Chong C, Grossman M, Nottebohm F, Scharff C. Selective expression of insulin-like growth factor II in the songbird brain. J Neurosci. 1997;17:6974–6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D’Ercole AJ, Ye P, O’Kusky JR. Mutant mouse models of insulin-like growth factor actions in the central nervous system. Neuropeptides. 2002;36:209–220. [DOI] [PubMed] [Google Scholar]
- 37. Mathews LS, Hammer RE, Behringer RR, et al. Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology. 1988;123:2827–2833. [DOI] [PubMed] [Google Scholar]
- 38. van Buul-Offers SC, de Haan K, Reijnen-Gresnigt MG, et al. Overexpression of human insulin-like growth factor-II in transgenic mice causes increased growth of the thymus. J Endocrinol. 1995;144:491–502. [DOI] [PubMed] [Google Scholar]
- 39. Cheng CM, Mervis RF, Niu SL, et al. Insulin-like growth factor 1 is essential for normal dendritic growth. J Neurosci Res. 2003;73:1–9. [DOI] [PubMed] [Google Scholar]
- 40. Cheng CM, Joncas G, Reinhardt RR, et al. Biochemical and morphometric analyses show that myelination in the insulin-like growth factor 1 null brain is proportionate to its neuronal composition. J Neurosci. 1998;18:5673–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dikkes P, Hawkes C, Kar S, Lopez MF. Effect of kainic acid treatment on insulin-like growth factor-2 receptors in the IGF2-deficient adult mouse brain. Brain Res. 2007;1131:77–87. [DOI] [PubMed] [Google Scholar]
- 42. Dikkes PDBJ, B Jaffe D, Guo WH, et al. IGF2 knockout mice are resistant to kainic acid-induced seizures and neurodegeneration. Brain Res. 2007;1175:85–95. [DOI] [PubMed] [Google Scholar]
- 43. Baker AM, Batchelor DC, Thomas GB, et al. Central penetration and stability of N-terminal tripeptide of insulin-like growth factor-I, glycine-proline-glutamate in adult rat. Neuropeptides. 2005;39:81–87. [DOI] [PubMed] [Google Scholar]
- 44. Ballard FJ, Wallace JC, Francis GL, Read LC, Tomas FM. Des(1-3)IGF-I – a truncated form of insulin-like growth-factor-I. Int J Biochem Cell Biol. 1996;28:1085–1087. [DOI] [PubMed] [Google Scholar]
- 45. Bourguignon J, Gerard A. Role of insulin-like growth factor binding proteins in limitation of IGF-I degradation into the N-methyl-D-aspartate receptor antagonist GPE: evidence from gonadotrophin-releasing hormone secretion in vitro at two developmental stages. Brain Res. 1999;847:247–252. [DOI] [PubMed] [Google Scholar]
- 46. Ikeda T, Waldbillig RJ, Puro DG. Truncation of IGF-I yields two mitogens for retinal Muller glial cells. Brain Res. 1995;686:87–92. [DOI] [PubMed] [Google Scholar]
- 47. Sara VR, Carlsson-Skwirut C, Bergman T, et al. Identification of GLY-PRO-GLU (GPE), the aminoterminal tripeptide of insulin-like growth factor 1 which is truncated in brain, as a novel neuroactive peptide. Biochem Biophys Res Commun. 1989;165:766–771. [DOI] [PubMed] [Google Scholar]
- 48. Alexi T, Hughes PE, van Roon-Mom WM, et al. The IGF-I amino-terminal tripeptide glycine-proline-glutamate (GPE) is neuroprotective to striatum in the quinolinic acid lesion animal model of Huntington’s disease. Exp Neurol. 1999;159:84–97. [DOI] [PubMed] [Google Scholar]
- 49. Saura J, Curatolo L, Williams CE, et al. Neuroprotective effects of Gly-Pro-Glu, the N-terminal tripeptide of IGF-1, in the hippocampus in vitro. Neuroreport. 1999;10:161–164. [DOI] [PubMed] [Google Scholar]
- 50. Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63:2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baron-Van Evercooren A, Olichon-Berthe C, Kowalski A, Visciano G, Van Obberghen E. Expression of IGF-I and insulin receptor genes in the rat central nervous system: a developmental, regional, and cellular analysis. J Neurosci Res. 1991;28:244–253. [DOI] [PubMed] [Google Scholar]
- 52. Liu J-P, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and the type 1 IGF receptor (Igf1r). Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 53. Schubert M, Gautam D, Surjo D, et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101:3100–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A. Insulin in the brain: sources, localization and functions. Mol Neurobiol. 2013;47:145–171. [DOI] [PubMed] [Google Scholar]
- 55. Hawkes C, Kar S. Insulin-like growth factor-II/mannose-6-phosphate receptor: widespread distribution in neurons of the central nervous system including those expressing cholinergic phenotype. J Comp Neurol. 2003;458:113–127. [DOI] [PubMed] [Google Scholar]
- 56. Brooker GJ, Kalloniatis M, Russo VC, Murphy M, Werther GA, Bartlett PF. Endogenous IGF-1 regulates the neuronal differentiation of adult stem cells. J Neurosci Res. 2000;59:332–341. [DOI] [PubMed] [Google Scholar]
- 57. Aberg MA, Aberg ND, Palmer TD, et al. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24:23–40. [DOI] [PubMed] [Google Scholar]
- 58. Han VKM, Smith A, Myint W, Nygard K, Bradshaw S. Mitogenic activity of epidermal growth factor on newborn rat astroglia: interaction with insulin-like growth factors. Endocrinology. 1992;131:1134–1142. [DOI] [PubMed] [Google Scholar]
- 59. Chernausek SD. Insulin-like growth factor-I (IGF-I) production by astroglial cells: regulation and importance for epidermal growth factor-induced cell replication. J Neurosci Res. 1993;34:189–197. [DOI] [PubMed] [Google Scholar]
- 60. Leventhal PS, Randolph AE, Vesbit TE, Schenone A, Windebank AJ, Feldman EL. Insulin-like growth factor-II as a paracrine growth factor in human neuroblastoma cells. Exp Cell Res. 1995;221:179–186. [DOI] [PubMed] [Google Scholar]
- 61. Garcia-Segura LM, Sanz A, Mendez P. Cross-talk between IGF-I and estradiol in the brain: focus on neuroprotection. Neuroendocrinology. 2006;84:275–279. [DOI] [PubMed] [Google Scholar]
- 62. Ishunina TA, Sluiter AA, Swaab DF, Verwer RW. Transcriptional activity of human brain estrogen receptor-alpha splice variants: evidence for cell type-specific regulation. Brain Res. 2013;1500:1–9. [DOI] [PubMed] [Google Scholar]
- 63. Rodriguez SS, Schwerdt JI, Barbeito CG, et al. Hypothalamic IGF-I gene therapy prolongs estrous cyclicity and protects ovarian structure in middle-aged female rats. Endocrinology. 2013;154:2166–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. [DOI] [PubMed] [Google Scholar]
- 65. Ni W, Rajkumar K, Nagy JI, Murphy LJ. Impaired brain development and reduced astrocyte response to injury in transgenic mice expressing IGF binding protein-1. Brain Res. 1997;769:97–107. [DOI] [PubMed] [Google Scholar]
- 66. D’Ercole AJ, Dai Z, Xing Y, et al. Brain growth retardation due to the expression of human insulin like growth factor binding protein-1 in transgenic mice: an in vivo model for the analysis of IGF function in the brain. Brain Res Dev Brain Res. 1994;82:213–222. [DOI] [PubMed] [Google Scholar]
- 67. Doublier S, Duyckaerts C, Seurin D, Binoux M. Impaired brain development and hydrocephalus in a line of transgenic mice with liver-specific expression of human insulin-like growth factor binding protein-1. Growth Horm IGF Res. 2000;10:267–274. [DOI] [PubMed] [Google Scholar]
- 68. Bienvenu G, Seurin D, Grellier P, et al. IGFBP-6 transgenic mice: post-natal growth, brain development and reproduction abnormalities. Endocrinology. 2004;145:2412–2420. [DOI] [PubMed] [Google Scholar]
- 69. Bienvenu G, Seurin D, Le Bouc Y, Even P, Babajko S, Magnan C. Dysregulation of energy homeostasis in mice overexpressing insulin-like growth factor-binding protein 6 in the brain. Diabetologia. 2005;48:1189–1197. [DOI] [PubMed] [Google Scholar]
- 70. Iwadate H, Sugisaki T, Kudo M, Kizuki K. Actions of insulin-like growth factor binding protein-5 (IGFBP-5) are potentially regulated by tissue kallikrein in rat brains. Life Sci. 2003;73:3149–3158. [DOI] [PubMed] [Google Scholar]
- 71. Bonham LW, Geier EG, Steele NZR, et al. Insulin-like growth factor binding protein 2 is associated with biomarkers of Alzheimer’s disease pathology and shows differential expression in transgenic mice. Front Neurosci. 2018;12:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Holmin S, Mathiesen T, Langmoen IA, Sandberg Nordqvist AC. Depolarisation induced insulin-like growth factor binding protein-2 expression in vivo via NMDA receptor stimulation. Growth Horm IGF Res. 2001;11:399–406. [DOI] [PubMed] [Google Scholar]
- 73. Ishikawa K, Ohe Y, Tatemoto K. Synthesis and secretion of insulin-like growth factor (IGF)-II and IGF binding protein-2 by cultivated brain meningeal cells. Brain Res. 1995;697:122–129. [DOI] [PubMed] [Google Scholar]
- 74. Kuhl NM, De Keyser J, De Vries H, Hoekstra D. Insulin-like growth factor binding proteins-1 and -2 differentially inhibit rat oligodendrocyte precursor cell survival and differentiation in vitro. J Neurosci Res. 2002;69:207–216. [DOI] [PubMed] [Google Scholar]
- 75. Jiang X, Zhao J, Ju L, et al. Temporal expression patterns of insulin-like growth factor binding protein-4 in the embryonic and postnatal rat brain. BMC Neurosci. 2013;14:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chesik D, Wilczak N, De Keyser J. Insulin-like growth factor binding protein-4 interacts with centrosomes and microtubules in primary astrocytes. Neuroscience. 2004;125:381–390. [DOI] [PubMed] [Google Scholar]
- 77. Ye P, Price W, Kassiotis G, Kollias G, D’Ercole AJ. Tumor necrosis factor-alpha regulation of insulin-like growth factor-I, type 1 IGF receptor, and IGF binding protein expression in cerebellum of transgenic mice. J Neurosci Res. 2003;71:721–731. [DOI] [PubMed] [Google Scholar]
- 78. Kiess W, Koepf G, Christiansen H, Blum WF. Human neuroblastoma cells use either insulin–like growth factor–I or insulin–like growth factor–II in an autocrine pathway via the IGF–I receptor – variability of IGF, IGF binding protein (IGFBP) and IGF receptor gene expression and IGF and IGFBP secretion in human neuroblastoma cells in relation to cellular proliferation. Regul Pept. 1997;72:19–29. [DOI] [PubMed] [Google Scholar]
- 79. Tanno B, Negroni A, Vitali R, et al. Expression of insulin-like growth factor-binding protein 5 in neuroblastoma cells is regulated at the transcriptional level by c-Myb and B-Myb via direct and indirect mechanisms. J Biol Chem. 2002;277:23172–23180. [DOI] [PubMed] [Google Scholar]
- 80. Russo VC, Azar WJ, Yau SW, Sabin MA, Werther GA. IGFBP-2: the dark horse in metabolism and cancer. Cytokine Growth Factor Rev. 2015;26:329–346. [DOI] [PubMed] [Google Scholar]
- 81. Holmes KM, Annala M, Chua CY, et al. Insulin-like growth factor-binding protein 2-driven glioma progression is prevented by blocking a clinically significant integrin, integrin-linked kinase, and NF-kappaB network. Proc Natl Acad Sci U S A. 2012;109:3475–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zheng S, Houseman EA, Morrison Z, et al. DNA hypermethylation profiles associated with glioma subtypes and EZH2 and IGFBP2 mRNA expression. Neuro Oncol. 2011;13:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kulkarni A, Thota B, Srividya MR, et al. Expression pattern and prognostic significance of IGFBP isoforms in anaplastic astrocytoma. Pathol Oncol Res. 2012;18:961–967. [DOI] [PubMed] [Google Scholar]
- 84. Zumkeller W, Saaf M, Rahn T. Insulin-like growth factor (IGF)-I, (IGF)-II and IGF-binding proteins in the cyst fluid of a patient with astrocytoma. Childs Nerv Syst. 1993;9:100–103. [DOI] [PubMed] [Google Scholar]
- 85. Hsieh D, Hsieh A, Stea B, Ellsworth R. IGFBP2 promotes glioma tumor stem cell expansion and survival. Biochem Biophys Res Commun. 2010;397:367–372. [DOI] [PubMed] [Google Scholar]
- 86. Yuan ZS, Cao Y, Li ZY. IGFBP2 induces SPRY1 expression via NF-kappaB signaling pathway in glioblastoma multiforme (GBM). Eur Rev Med Pharmacol Sci. 2017;21:5072–5080. [DOI] [PubMed] [Google Scholar]
- 87. Nordqvist AC, Mathiesen T. Expression of IGF-II, IGFBP-2, -5, and -6 in meningiomas with different brain invasiveness. J Neurooncol. 2002;57:19–26. [DOI] [PubMed] [Google Scholar]
- 88. Nordqvist A, Peyrard M, Pettersson H, et al. A high ratio of insulin-like growth factor II: insulin-like growth factor binding protein 2 messenger RNA as a marker for anaplasia in meningiomas. Cancer Res. 1997;57:2611–2614. [PubMed] [Google Scholar]
- 89. Chinnaiyan P, Chowdhary S, Potthast L, et al. Phase I trial of vorinostat combined with bevacizumab and CPT-11 in recurrent glioblastoma. Neuro Oncol. 2012;14:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Baptista P, Andrade JP. Adult hippocampal neurogenesis: regulation and possible functional and clinical correlates. Front Neuroanat. 2018;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nieto-Estevez V, Defterali C, Vicario-Abejon C. IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front Neurosci. 2016;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. [DOI] [PubMed] [Google Scholar]
- 93. Ernst A, Frisen J. Adult neurogenesis in humans- common and unique traits in mammals. PLoS Biol. 2015;13:e1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jessen KR. Glial cells. Int J Biochem Cell Biol. 2004;36:1861–1867. [DOI] [PubMed] [Google Scholar]
- 95. Kranzler JH, Rosenbloom AL, Martinez V, Guevara-Aguirre J. Normal intelligence with severe insulin-like growth factor I deficiency due to growth hormone receptor deficiency: a controlled study in a genetically homogeneous population. J Clin Endocrinol Metab. 1998;83:1953–1958. [DOI] [PubMed] [Google Scholar]
- 96. Kornreich L, Horev G, Schwarz M, Karmazyn B, Laron Z. Craniofacial and brain abnormalities in Laron syndrome (primary growth hormone insensitivity). Eur J Endocrinol. 2002;146:499–503. [DOI] [PubMed] [Google Scholar]
- 97. Laron Z, Iluz M, Kauli R. Head circumference in untreated and IGF-I treated patients with Laron syndrome: comparison with untreated and hGH-treated children with isolated growth hormone deficiency. Growth Horm IGF Res. 2012;22:49–52. [DOI] [PubMed] [Google Scholar]
- 98. Woods KA, Camacho-Hubner C, Savage MO, Clark AJL. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335:1363–1367. [DOI] [PubMed] [Google Scholar]
- 99. Abuzzahab MJ, Schneider A, Goddard A, et al. IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med. 2003;349:2211–2222. [DOI] [PubMed] [Google Scholar]
- 100. Hansen-Pupp I, Hovel H, Hellstrom A, et al. Postnatal decrease in circulating insulin-like growth factor-I and low brain volumes in very preterm infants. J Clin Endocrinol Metab. 2011;96:1129–1135. [DOI] [PubMed] [Google Scholar]
- 101. Attias J, Zarchi O, Nageris BI, Laron Z. Cochlear hearing loss in patients with Laron syndrome. Eur Arch Otorhinolaryngol. 2012;269:461–466. [DOI] [PubMed] [Google Scholar]
- 102. Wu L, Wang L, Shangguan S, et al. Altered methylation of IGF2 DMR0 is associated with neural tube defects. Mol Cell Biochem. 2013;380:33–42. [DOI] [PubMed] [Google Scholar]
- 103. Kim HW, Kim KN, Choi YJ, Chang N. Effects of paternal folate deficiency on the expression of insulin-like growth factor-2 and global DNA methylation in the fetal brain. Mol Nutr Food Res. 2013;57:671–676. [DOI] [PubMed] [Google Scholar]
- 104. Chowen JA, Goya L, Ramos S, et al. Effects of early undernutrition on the brain insulin-like growth factor- I system. J Neuroendocrinol. 2002;14:163–169. [DOI] [PubMed] [Google Scholar]
- 105. Maucksch C, McGregor AL, Yang M, Gordon RJ, Yang M, Connor B. IGF-I redirects doublecortin-positive cell migration in the normal adult rat brain. Neuroscience. 2013;241:106–115. [DOI] [PubMed] [Google Scholar]
- 106. Yuan H, Chen R, Wu L, et al. The regulatory mechanism of neurogenesis by IGF-1 in adult mice. Mol Neurobiol. 2015;51:512–522. [DOI] [PubMed] [Google Scholar]
- 107. Gao C, Wang Q, Chung SK, Shen J. Crosstalk of metabolic factors and neurogenic signaling in adult neurogenesis: implication of metabolic regulation for mental and neurological diseases. Neurochem Int. 2017;106:24–36. [DOI] [PubMed] [Google Scholar]
- 108. Erickson KI, Prakash RS, Voss MW, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Feng Y, Famuyide M, Bhatt AJ. Dexamethasone decreases insulin-like growth factor-I and -II via a glucocorticoid receptor dependent mechanism in developing rat brain. Neuro Endocrinol Lett. 2013;34:624–634. [PubMed] [Google Scholar]
- 110. Velayo C, Ito T, Chisaka H, Yaegashi N, Okamura K, Kimura Y. Effects of antenatal steroid therapy on neurodevelopment in an IUGR mouse model. Fetal Diagn Ther. 2010;28:79–86. [DOI] [PubMed] [Google Scholar]
- 111. Lee KY, Miki T, Yokoyama T, et al. Neonatal repetitive maternal separation causes long-lasting alterations in various neurotrophic factor expression in the cerebral cortex of rats. Life Sci. 2012;90:578–584. [DOI] [PubMed] [Google Scholar]
- 112. Cui QL, D’Abate L, Fang J, et al. Human fetal oligodendrocyte progenitor cells from different gestational stages exhibit substantially different potential to myelinate. Stem Cells Dev. 2012;21:1831–1837. [DOI] [PubMed] [Google Scholar]
- 113. Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;164:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cheng HL, Steinway M, Delaney CL, Franke TF, Feldman EL. IGF-I promotes Schwann cell motility and survival via activation of Akt. Mol Cell Endocrinol. 2000;170:211–215. [DOI] [PubMed] [Google Scholar]
- 115. Syroid DE, Zorick TS, Arbet-Engels C, Kilpatrick TJ, Eckhart W, Lemke G. A role for insulin-like growth factor-I in the regulation of Schwann cell survival. J Neurosci. 1999;19:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85:352–370. [DOI] [PubMed] [Google Scholar]
- 117. Choi YS, Cho HY, Hoyt KR, Naegele JR, Obrietan K. IGF-1 receptor-mediated ERK/MAPK signaling couples status epilepticus to progenitor cell proliferation in the subgranular layer of the dentate gyrus. Glia. 2008;56:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhu Y, Chen X, Liu Z, Peng YP, Qiu YH. Interleukin-10 protection against lipopolysaccharide-induced neuro-inflammation and neurotoxicity in ventral mesencephalic cultures. Int J Mol Sci. 2015;17:E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Logan S, Pharaoh GA, Marlin MC, et al. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-beta uptake in astrocytes. Mol Metab. 2018;9:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hesp K, Smant G, Kammenga JE. Caenorhabditis elegans DAF-16/FOXO transcription factor and its mammalian homologs associate with age-related disease. Exp Gerontol. 2015;72:1–7. [DOI] [PubMed] [Google Scholar]
- 121. Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci. 2008;9:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the aging process. Nature. 1996;384:33. [DOI] [PubMed] [Google Scholar]
- 123. Brown-Borg HM, Bartke A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012;67:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Deschenes M, Chabot B. The emerging role of alternative splicing in senescence and aging. Aging Cell. 2017;16:918–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Suh Y, Atzmon G, Cho MO, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tazearslan C, Huang J, Barzilai N, Suh Y. Impaired IGF1R signaling in cells expressing longevity-associated human IGF1R alleles. Aging Cell. 2011;10:551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gubbi S, Quipildor GF, Barzilai N, Huffman DM, Milman S. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171–T185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Agis-Balboa RC, Fischer A. Generating new neurons to circumvent your fears: the role of IGF signaling. Cell Mol Life Sci. 2014;71:21–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lugert S, Basak O, Knuckles P, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. [DOI] [PubMed] [Google Scholar]
- 130. Capilla-Gonzalez V, Herranz-Perez V, Garcia-Verdugo JM. The aged brain: genesis and fate of residual progenitor cells in the subventricular zone. Front Cell Neurosci. 2015;9:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ashpole NM, Sanders JE, Hodges EL, Yan H, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol. 2015;68:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cardoso AL, Fernandes A, Aguilar-Pimentel JA, et al. Towards frailty biomarkers: candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214–277. [DOI] [PubMed] [Google Scholar]
- 133. Muller AP, Fernandez AM, Haas C, Zimmer E, Portela LV, Torres-Aleman I. Reduced brain insulin-like growth factor I function during aging. Mol Cell Neurosci. 2012;49:9–12. [DOI] [PubMed] [Google Scholar]
- 134. Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. [DOI] [PubMed] [Google Scholar]
- 135. Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. [DOI] [PubMed] [Google Scholar]
- 137. Mitschelen M, Yan H, Farley JA, et al. Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: a potential model of geriatric depression. Neuroscience. 2011;185:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–411. [DOI] [PubMed] [Google Scholar]
- 139. Cassilhas RC, Lee KS, Fernandes J, et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. [DOI] [PubMed] [Google Scholar]
- 140. Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pareja-Galeano H, Brioche T, Sanchis-Gomar F, et al. Impact of exercise training on neuroplasticity-related growth factors in adolescents. J Musculoskelet Neuronal Interact. 2013;13:368–371. [PubMed] [Google Scholar]
- 142. Voss MW, Erickson KI, Prakash RS, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yau SY, Lau BW, Zhang ED, et al. Effects of voluntary running on plasma levels of neurotrophins, hippocampal cell proliferation and learning and memory in stressed rats. Neuroscience. 2012;222:289–301. [DOI] [PubMed] [Google Scholar]
- 144. Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci. 2013;15:189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Maass A, Duzel S, Brigadski T, et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage. 2016;131:142–154. [DOI] [PubMed] [Google Scholar]
- 146. Wennberg AMV, Hagen CE, Machulda MM, et al. The association between peripheral total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 and functional and cognitive outcomes in the Mayo Clinic Study of Aging. Neurobiol Aging. 2018;66:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Friedlander AL, Butterfield GE, Moynihan S, et al. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab. 2001;86:1496–1503. [DOI] [PubMed] [Google Scholar]
- 148. Frater J, Lie D, Bartlett P, McGrath JJ. Insulin-like Growth Factor 1 (IGF-1) as a marker of cognitive decline in normal ageing: a review. Ageing Res Rev. 2018;42:14–27. [DOI] [PubMed] [Google Scholar]
- 149. Perice L, Barzilai N, Verghese J, et al. Lower circulating insulin-like growth factor-I is associated with better cognition in females with exceptional longevity without compromise to muscle mass and function. Aging. 2016;8:2414–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Falleti MG, Maruff P, Burman P, Harris A. The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: a meta-analysis of the current literature. Psychoneuroendocrinology. 2006;31:681–691. [DOI] [PubMed] [Google Scholar]
- 151. Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72:653–660. [DOI] [PubMed] [Google Scholar]
- 152. Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39:277–284. [DOI] [PubMed] [Google Scholar]
- 153. Aberg ND, Johansson I, Aberg MA, et al. Peripheral administration of GH induces cell proliferation in the brain of adult hypophysectomized rats. J Endocrinol. 2009;201:141–150. [DOI] [PubMed] [Google Scholar]
- 154. Åberg DN, Lind J, Isgaard J, Georg Kuhn H. Peripheral growth hormone induces cell proliferation in the intact adult rat brain. Growth Horm IGF Res. 2010;20:264–269. [DOI] [PubMed] [Google Scholar]
- 155. Åberg MA, Aberg ND, Hedbäcker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog Neurobiol. 2011;93:182–203. [DOI] [PubMed] [Google Scholar]
- 157. Fidaleo M, Cavallucci V, Pani G. Nutrients, neurogenesis and brain ageing: from disease mechanisms to therapeutic opportunities. Biochem Pharmacol. 2017;141:63–76. [DOI] [PubMed] [Google Scholar]
- 158. Cheng CM, Reinhardt RR, Lee WH, Joncas G, Patel SC, Bondy C. Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proc Natl Acad Sci U S A. 2000;97:10236–10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lynch CD, Lyons D, Khan A, Bennett SA, Sonntag WE. Insulin-like growth factor-1 selectively increases glucose utilization in brains of aged animals. Endocrinology. 2001;142:506–509. [DOI] [PubMed] [Google Scholar]
- 160. De Felice FG. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest. 2013;123:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Castilla-Cortazar I, Garcia-Fernandez M, Delgado G, et al. Hepatoprotection and neuroprotection induced by low doses of IGF-II in aging rats. J Transl Med. 2011;9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ziegler AN, Levison SW, Wood TL. Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat Rev Endocrinol. 2015;11:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, Doetsch F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell. 2016;19:643–652. [DOI] [PubMed] [Google Scholar]
- 164. Logan A, Gonzalez AM, Hill DJ, Berry M, Gregson NA, Baird A. Coordinated pattern of expression and localization of insulin-like growth factor-II (IGF-II) and IGF-binding protein-2 in the adult rat brain. Endocrinology. 1994;135:2255–2264. [DOI] [PubMed] [Google Scholar]
- 165. Alberini CM, Chen DY. Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends Neurosci. 2012;35:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Mathews PM, Guerra CB, Jiang Y, et al. Alzheimer’s disease-related overexpression of the cation-dependent mannose 6-phosphate receptor increases Abeta secretion: role for altered lysosomal hydrolase distribution in beta-amyloidogenesis. J Biol Chem. 2002;277:5299–5307. [DOI] [PubMed] [Google Scholar]
- 167. Wang Y, MacDonald RG, Thinakaran G, Kar S. Insulin-like growth factor-II/cation-independent mannose 6-phosphate receptor in neurodegenerative diseases. Mol Neurobiol. 2017;54:2636–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J. Neurosci. 2011;33:1139–1151. [DOI] [PubMed] [Google Scholar]
- 169. Spielman LJ, Little JP, Klegeris A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J Neuroimmunol. 2014;273:8–21. [DOI] [PubMed] [Google Scholar]
- 170. Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011;6:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Chesik D, Glazenburg K, Wilczak N, Geeraedts F, De Keyser J. Insulin-like growth factor binding protein-1-6 expression in activated microglia. Neuroreport. 2004;15:1033–1037. [DOI] [PubMed] [Google Scholar]
- 172. Labandeira-Garcia JL, Rodriguez-Perez AI, Garrido-Gil P, Rodriguez-Pallares J, Lanciego JL, Guerra MJ. Brain renin-angiotensin system and microglial polarization: implications for aging and neurodegeneration. Front Aging Neurosci. 2017;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Bryan MR, Bowman AB. Manganese and the insulin-IGF signaling network in Huntington’s disease and other neurodegenerative disorders. Adv Neurobiol. 2017;18:113–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10:698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ihara Y, Nukina N, Miura R, Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. J Biochem. 1986;99:1807–1810. [DOI] [PubMed] [Google Scholar]
- 179. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Revett TJ, Baker GB, Jhamandas J, Kar S. Glutamate system, amyloid ß peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology. J Psychiatry Neurosci. 2013;38:6–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82:756–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Giuffrida ML, Tomasello F, Caraci F, Chiechio S, Nicoletti F, Copani A. Beta-amyloid monomer and insulin/IGF-1 signaling in Alzheimer’s disease. Mol Neurobiol. 2012;46:605–613. [DOI] [PubMed] [Google Scholar]
- 183. Dore S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci U S A. 1997;94:4772–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Kitiyanant N, Kitiyanant Y, Svendsen CN, Thangnipon W. BDNF-, IGF-1- and GDNF-secreting human neural progenitor cells rescue amyloid beta-induced toxicity in cultured rat septal neurons. Neurochem Res. 2012;37:143–152. [DOI] [PubMed] [Google Scholar]
- 185. Gasparini L, Gouras GK, Wang R, et al. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci. 2001;21:2561–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: implications for synaptic damage and cognitive decline. J Alzheimers Dis. 2010;20:S499–S512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Hellström-Lindahl E, Ravid R, Nordberg A. Age-dependent decline of neprilysin in Alzheimer’s disease and normal brain: inverse correlation with Abeta levels. Neurobiol Aging. 2008;29:210–221. [DOI] [PubMed] [Google Scholar]
- 188. Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia. 1996;39:1392–1397. [DOI] [PubMed] [Google Scholar]
- 189. Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer’s disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35:152–160. [DOI] [PubMed] [Google Scholar]
- 190. Ribe EM, Lovestone S. Insulin signalling in Alzheimer’s disease and diabetes: from epidemiology to molecular links. J Intern Med. 2016;280:430–442. [DOI] [PubMed] [Google Scholar]
- 191. Carro E, Torres-Aleman I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur J Pharmacol. 2004;490:127–133. [DOI] [PubMed] [Google Scholar]
- 192. Candeias E, Duarte AI, Carvalho C, et al. The impairment of insulin signaling in Alzheimer’s disease. IUBMB Life. 2012;64:951–957. [DOI] [PubMed] [Google Scholar]
- 193. Rani V, Deshmukh R, Jaswal P, Kumar P, Bariwal J. Alzheimer’s disease: is this a brain specific diabetic condition? Physiol Behav. 2016;164:259–267. [DOI] [PubMed] [Google Scholar]
- 194. Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. [DOI] [PubMed] [Google Scholar]
- 195. Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31:224–243. [DOI] [PubMed] [Google Scholar]
- 197. Ostrowski PP, Barszczyk A, Forstenpointner J, Zheng W, Feng ZP. Meta-analysis of serum insulin-like growth factor 1 in Alzheimer’s disease. PLoS ONE. 2016;11:e0155733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Wang W, Yu JT, Tan L, Liu QY, Wang HF, Ma XY. Insulin-like growth factor 1 (IGF1) polymorphism is associated with Alzheimer’s disease in Han Chinese. Neurosci Lett. 2012;531:20–23. [DOI] [PubMed] [Google Scholar]
- 199. Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2006;9:13–33. [DOI] [PubMed] [Google Scholar]
- 200. Salkovic-Petrisic M, Hoyer S. Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: an experimental approach. J Neural Transm Suppl. 2007:217–233. [DOI] [PubMed] [Google Scholar]
- 201. Cheng CM, Tseng V, Wang J, Wang D, Matyakhina L, Bondy CA. Tau is hyperphosphorylated in the insulin-like growth factor-I null brain. Endocrinology. 2005;146:5086–5091. [DOI] [PubMed] [Google Scholar]
- 202. Carro E, Trejo JL, Spuch C, Bohl D, Heard JM, Torres-Aleman I. Blockade of the insulin-like growth factor I receptor in the choroid plexus originates Alzheimer’s-like neuropathology in rodents: new cues into the human disease? Neurobiol Aging. 2006;27:1618–1631. [DOI] [PubMed] [Google Scholar]
- 203. Torres-Aleman I. Mouse models of Alzheimer’s dementia: current concepts and new trends. Endocrinology. 2008;149:5952–5957. [DOI] [PubMed] [Google Scholar]
- 204. Bilkei-Gorzo A. Genetic mouse models of brain ageing and Alzheimer’s disease. Pharmacol Ther. 2014;142:244–257. [DOI] [PubMed] [Google Scholar]
- 205. Takeda S, Sato N, Uchio-Yamada K, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107:7036–7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Maesako M, Uemura K, Kuzuya A, et al. Presenilin regulates insulin signaling via a gamma-secretase-independent mechanism. J Biol Chem. 2011;286:25309–25316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Hiltunen M, Khandelwal VK, Yaluri N, et al. Contribution of genetic and dietary insulin resistance to Alzheimer phenotype in APP/PS1 transgenic mice. J Cell Mol Med. 2012;16:1206–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Trueba-Saiz A, Cavada C, Fernandez AM, et al. Loss of serum IGF-I input to the brain as an early biomarker of disease onset in Alzheimer mice. Transl Psychiatry. 2013;3:e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Poirier R, Fernandez AM, Torres-Aleman I, Metzger F. Early brain amyloidosis in APP/PS1 mice with serum insulin-like growth factor-I deficiency. Neurosci Lett. 2012;509:101–104. [DOI] [PubMed] [Google Scholar]
- 210. Parrella E, Maxim T, Maialetti F, et al. Protein restriction cycles reduce IGF-1 and phosphorylated Tau, and improve behavioral performance in an Alzheimer’s disease mouse model. Aging Cell. 2013;12:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Zhang B, Tang XC, Zhang HY. Alternations of central insulin-like growth factor-1 sensitivity in APP/PS1 transgenic mice and neuronal models. J Neurosci Res. 2013;91:717–725. [DOI] [PubMed] [Google Scholar]
- 212. Cohen E, Paulsson JF, Blinder P, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Freude S, Hettich MM, Schumann C, et al. Neuronal IGF-1 resistance reduces Abeta accumulation and protects against premature death in a model of Alzheimer’s disease. FASEB J. 2009;23:3315–3324. [DOI] [PubMed] [Google Scholar]
- 214. Gontier G, George C, Chaker Z, Holzenberger M, Aid S. Blocking IGF signaling in adult neurons alleviates Alzheimer’s disease pathology through amyloid-beta clearance. J Neurosci. 2015;35:11500–11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Carro E, Trejo JL, Gerber A, et al. Therapeutic actions of insulin-like growth factor I on APP/PS2 mice with severe brain amyloidosis. Neurobiol Aging. 2006;27:1250–1257. [DOI] [PubMed] [Google Scholar]
- 216. Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-b levels. Nat Med. 2002;8:1390–1397. [DOI] [PubMed] [Google Scholar]
- 217. Lanz TA, Salatto CT, Semproni AR, et al. Peripheral elevation of IGF-1 fails to alter Abeta clearance in multiple in vivo models. Biochem Pharmacol. 2008;75:1093–1103. [DOI] [PubMed] [Google Scholar]
- 218. Aguado-Llera D, Arilla-Ferreiro E, Campos-Barros A, Puebla-Jimenez L, Barrios V. Protective effects of insulin-like growth factor-I on the somatostatinergic system in the temporal cortex of beta-amyloid-treated rats. J Neurochem. 2005;92:607–615. [DOI] [PubMed] [Google Scholar]
- 219. Burgos-Ramos E, Hervas-Aguilar A, Aguado-Llera D, et al. Somatostatin and Alzheimer’s disease. Mol Cell Endocrinol. 2008;286:104–111. [DOI] [PubMed] [Google Scholar]
- 220. Simpson JE, Ince PG, Shaw PJ, et al. Microarray analysis of the astrocyte transcriptome in the aging brain: relationship to Alzheimer’s pathology and APOE genotype. Neurobiol Aging. 2011;32:1795–1807. [DOI] [PubMed] [Google Scholar]
- 221. Craft S, Asthana S, Schellenberg G, et al. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer’s disease differ according to apolipoprotein-E genotype. Ann N Y Acad Sci. 2000;903:222–228. [DOI] [PubMed] [Google Scholar]
- 222. Ratcliffe LE, Vazquez Villasenor I, Jennings L, et al. Loss of IGF1R in human astrocytes alters complex I activity and support for neurons. Neuroscience. 2018;390:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Yang Y, Song W. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience. 2013;250:140–150. [DOI] [PubMed] [Google Scholar]
- 224. Manolopoulos KN, Klotz LO, Korsten P, Bornstein SR, Barthel A. Linking Alzheimer’s disease to insulin resistance: the FoxO response to oxidative stress. Mol Psychiatry. 2010;15:1046–1052. [DOI] [PubMed] [Google Scholar]
- 225. Fernandez AM, Jimenez S, Mecha M, et al. Regulation of the phosphatase calcineurin by insulin-like growth factor I unveils a key role of astrocytes in Alzheimer’s pathology. Mol Psychiatry. 2012;17:705–718. [DOI] [PubMed] [Google Scholar]
- 226. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. [DOI] [PubMed] [Google Scholar]
- 227. Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. [DOI] [PubMed] [Google Scholar]
- 228. Polymeropoulos MH, Higgins JJ, Golbe LI, et al. Mapping of a gene for Parkinson’s disease to chromosome 4q21-q23. Science. 1996;274:1197–1199. [DOI] [PubMed] [Google Scholar]
- 229. Xu J, Kao S-Y, Lee FJS, Song W, Jin L-W, Yankner BA. Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002;8:600–606. [DOI] [PubMed] [Google Scholar]
- 230. Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem. 2016;139:216–231. [DOI] [PubMed] [Google Scholar]
- 231. Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–216. [DOI] [PubMed] [Google Scholar]
- 232. Sherer TB, Betarbet R, Stout AK, et al. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered a-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Avila-Gomez IC, Velez-Pardo C, Jimenez-Del-Rio M. Effects of insulin-like growth factor-1 on rotenone-induced apoptosis in human lymphocyte cells. Basic Clin Pharmacol Toxicol. 2010;106:53–61. [DOI] [PubMed] [Google Scholar]
- 234. Li DH, He YC, Quinn TJ, Liu J. Serum insulin-like growth factor-1 in patients with De Novo, drug naive Parkinson’s disease: a meta-analysis. PLoS ONE. 2015;10:e0144755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Ma J, Jiang Q, Xu J, et al. Plasma insulin-like growth factor 1 is associated with cognitive impairment in Parkinson’s disease. Dement Geriatr Cogn Disord. 2015;39:251–256. [DOI] [PubMed] [Google Scholar]
- 236. Picillo M, Pivonello R, Santangelo G, et al. Serum IGF-1 is associated with cognitive functions in early, drug-naive Parkinson’s disease. PLoS ONE. 2017;12:e0186508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Pellecchia MT, Santangelo G, Picillo M, et al. Insulin-like growth factor-1 predicts cognitive functions at 2-year follow-up in early, drug-naive Parkinson’s disease. Eur J Neurol. 2014;21:802–807. [DOI] [PubMed] [Google Scholar]
- 238. Bernhard FP, Heinzel S, Binder G, et al. Insulin-like growth factor 1 (IGF-1) in Parkinson’s disease: potential as trait-, progression- and prediction marker and confounding factors. PLoS ONE. 2016;11:e0150552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Xiao Y, Cen L, Mo M, et al. Association of IGF1 gene polymorphism with Parkinson’s disease in a Han Chinese population. J Gene Med. 2017;19:e2949. [DOI] [PubMed] [Google Scholar]
- 240. Tong M, Dong M, de la Monte SM. Brain insulin-like growth factor and neurotrophin resistance in Parkinson’s disease and dementia with Lewy bodies: potential role of manganese neurotoxicity. J Alzheimers Dis. 2009;16:585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Clarkson ED, Zawada WM, Bell KP, et al. IGF-I and bFGF improve dopamine neuron survival and behavioral outcome in parkinsonian rats receiving cultured human fetal tissue strands. Exp Neurol. 2001;168:183–191. [DOI] [PubMed] [Google Scholar]
- 242. Lu-Nguyen NB, Broadstock M, Yanez-Munoz RJ. Efficient expression of Igf-1 from lentiviral vectors protects in vitro but does not mediate behavioral recovery of a parkinsonian lesion in rats. Hum Gene Ther. 2015;26:719–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Offen D, Shtaif B, Hadad D, Weizman A, Melamed E, Gil-Ad I. Protective effect of insulin-like-growth-factor-1 against dopamine-induced neurotoxicity in human and rodent neuronal cultures: possible implications for Parkinson’s disease. Neurosci Lett. 2001;316:129–132. [DOI] [PubMed] [Google Scholar]
- 244. Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev Neurobiol. 2008;68:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Krishnamurthi R, Stott S, Maingay M, et al. N-terminal tripeptide of IGF-1 improves functional deficits after 6-OHDA lesion in rats. Neuroreport. 2004;15:1601–1604. [DOI] [PubMed] [Google Scholar]
- 246. Yang L, Wang H, Liu L, Xie A. The role of insulin/IGF-1/PI3K/Akt/GSK3beta signaling in Parkinson’s disease dementia. Front Neurosci. 2018;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Cummings JL, Benson DF. Psychological dysfunction accompanying subcortical dementias. Annu Rev Med. 1988;39:53–61. [DOI] [PubMed] [Google Scholar]
- 248. Rothlind JC, Bylsma FW, Peyser C, Folstein SE, Brandt J. Cognitive and motor correlates of everyday functioning in early Huntington’s disease. J Nerv Ment Dis. 1993;181:194–199. [DOI] [PubMed] [Google Scholar]
- 249. Hsiao HY, Chern Y. Targeting glial cells to elucidate the pathogenesis of Huntington’s disease. Mol Neurobiol. 2010;41:248–255. [DOI] [PubMed] [Google Scholar]
- 250. Wexler NS, Lorimer J, Porter J, et al. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc Natl Acad Sci U S A. 2004;101:3498–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Salem L, Saleh N, Desamericq G, et al. Insulin-like growth factor-1 but not insulin predicts cognitive decline in Huntington’s disease. PLoS ONE. 2016;11:e0162890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Dalrymple A, Wild EJ, Joubert R, et al. Proteomic profiling of plasma in Huntington’s disease reveals neuroinflammatory activation and biomarker candidates. J Proteome Res. 2007;6:2833–2840. [DOI] [PubMed] [Google Scholar]
- 253. Bardai FH, Price V, Zaayman M, Wang L, D’Mello SR. Histone deacetylase-1 (HDAC1) is a molecular switch between neuronal survival and death. J Biol Chem. 2012;287:35444–35453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Corrochano S, Renna M, Osborne G, et al. Reducing Igf-1r levels leads to paradoxical and sexually dimorphic effects in HD mice. PLoS ONE. 2014;9:e105595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Ransome MI, Hannan AJ. Impaired basal and running-induced hippocampal neurogenesis coincides with reduced Akt signaling in adult R6/1 HD mice. Mol Cell Neurosci. 2013;54:93–107. [DOI] [PubMed] [Google Scholar]
- 256. Lopes C, Ribeiro M, Duarte AI, et al. IGF-1 intranasal administration rescues Huntington’s disease phenotypes in YAC128 mice. Mol Neurobiol. 2014;49:1126–1142. [DOI] [PubMed] [Google Scholar]
- 257. Valdeolivas S, Navarrete C, Cantarero I, Bellido ML, Munoz E, Sagredo O. Neuroprotective properties of cannabigerol in Huntington’s disease: studies in R6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics. 2015;12:185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Wong BKY, Ehrnhoefer DE, Graham RK, et al. Partial rescue of some features of Huntington Disease in the genetic absence of caspase-6 in YAC128 mice. Neurobiol Dis. 2015;76:24–36. [DOI] [PubMed] [Google Scholar]
- 259. Hunt MJ, Morton AJ. Atypical diabetes associated with inclusion formation in the R6/2 mouse model of Huntington’s disease is not improved by treatment with hypoglycaemic agents. Exp Brain Res. 2005;166:220–229. [DOI] [PubMed] [Google Scholar]
- 260. Andreassen OA, Dedeoglu A, Stanojevic V, et al. Huntington’s disease of the endocrine pancreas: insulin deficiency and diabetes mellitus due to impaired insulin gene expression. Neurobiol Dis. 2002;11:410–424. [DOI] [PubMed] [Google Scholar]
- 261. Lalic NM, Maric J, Svetel M, et al. Glucose homeostasis in Huntington disease: abnormalities in insulin sensitivity and early-phase insulin secretion. Arch Neurol. 2008;65:476–480. [DOI] [PubMed] [Google Scholar]
- 262. Duarte AI, Petit GH, Ranganathan S, et al. IGF-1 protects against diabetic features in an in vivo model of Huntington’s disease. Exp Neurol. 2011;231:314–319. [DOI] [PubMed] [Google Scholar]
- 263. Humbert S, Bryson EA, Cordelieres FP, et al. The IGF-1/Akt pathway is neuroprotective in Huntington’s disease and involves Huntingtin phosphorylation by Akt. Dev Cell. 2002;2:831–837. [DOI] [PubMed] [Google Scholar]
- 264. Saudou F, Finkbeiner S, Devys D, Greenberg ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95:55–66. [DOI] [PubMed] [Google Scholar]
- 265. Naia L, Ribeiro M, Rodrigues J, et al. Insulin and IGF-1 regularize energy metabolites in neural cells expressing full-length mutant huntingtin. Neuropeptides. 2016;58:73–81. [DOI] [PubMed] [Google Scholar]
- 266. Ribeiro M, Rosenstock TR, Oliveira AM, Oliveira CR, Rego AC. Insulin and IGF-1 improve mitochondrial function in a PI-3K/Akt-dependent manner and reduce mitochondrial generation of reactive oxygen species in Huntington’s disease knock-in striatal cells. Free Radic Biol Med. 2014;74:129–144. [DOI] [PubMed] [Google Scholar]
- 267. Naia L, Ferreira IL, Cunha-Oliveira T, et al. Activation of IGF-1 and insulin signaling pathways ameliorate mitochondrial function and energy metabolism in Huntington’s Disease human lymphoblasts. Mol Neurobiol. 2015;51:331–348. [DOI] [PubMed] [Google Scholar]
- 268. Lewis ME, Neff NT, Contreras PC, et al. Insulin-like growth factor-I: potential for treatment of motor neuronal disorders. Exp Neurol. 1993;124:73–88. [DOI] [PubMed] [Google Scholar]
- 269. Ying Wang J, Peruzzi F, Lassak A, et al. Neuroprotective effects of IGF-I against TNFalpha-induced neuronal damage in HIV-associated dementia. Virology. 2003;305:66–76. [DOI] [PubMed] [Google Scholar]
- 270. Zhou X, Xia XB, Xiong SQ. Neuro-protection of retinal stem cells transplantation combined with copolymer-1 immunization in a rat model of glaucoma. Mol Cell Neurosci. 2013;54:1–8. [DOI] [PubMed] [Google Scholar]
- 271. Shaw PJ, Ince PG, Falkous G, Mantle D. Oxidative damage to protein in sporadic motor neuron disease spinal cord. Ann Neurol. 1995;38:691–695. [DOI] [PubMed] [Google Scholar]
- 272. Simpson EP, Henry YK, Henkel JS, Smith RG, Appel SH. Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology. 2004;62:1758–1765. [DOI] [PubMed] [Google Scholar]
- 273. Bogdanov M, Brown RH, Matson W, et al. Increased oxidative damage to DNA in ALS patients. Free Radic Biol Med. 2000;29:652–658. [DOI] [PubMed] [Google Scholar]
- 274. Shi P, Gal J, Kwinter DM, Liu X, Zhu H. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2010;1802:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Bosco DA, Morfini G, Karabacak NM, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Shibata N, Hirano A, Kobayashi M, et al. Cu/Zn superoxide dismutase-like immunoreactivity in Lewy body-like inclusions of sporadic amyotrophic lateral sclerosis. Neurosci Lett. 1994;179:149–152. [DOI] [PubMed] [Google Scholar]
- 277. Mackenzie IRA, Bigio EH, Ince PG, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61:427–434. [DOI] [PubMed] [Google Scholar]
- 278. Kabashi E, Valdmanis PN, Dion P, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. [DOI] [PubMed] [Google Scholar]
- 279. Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364:362. [DOI] [PubMed] [Google Scholar]
- 280. Gurney ME, Pu H, Chiu AY, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. [DOI] [PubMed] [Google Scholar]
- 281. Chung YH, Joo KM, Shin CM, et al. Immunohistochemical study on the distribution of insulin-like growth factor I (IGF-I) receptor in the central nervous system of SOD1(G93A) mutant transgenic mice. Brain Res. 2003;994:253–259. [DOI] [PubMed] [Google Scholar]
- 282. Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. [DOI] [PubMed] [Google Scholar]
- 283. Zhao W, Beers DR, Appel SH. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J Neuroimmune Pharmacol. 2013;8:888–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Ji Y, Duan W, Liu Y, et al. IGF1 affects macrophage invasion and activation and TNF-alpha production in the sciatic nerves of female SOD1G93A mice. Neurosci Lett. 2018;668:1–6. [DOI] [PubMed] [Google Scholar]
- 285. Lepore AC, Haenggeli C, Gasmi M, et al. Intraparenchymal spinal cord delivery of adeno-associated virus IGF-1 is protective in the SOD1G93A model of ALS. Brain Res. 2007;1185:256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Dodge JC, Treleaven CM, Fidler JA, et al. AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Mol Ther. 2010;18:2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Hantai D, Akaaboune M, Lagord C, et al. Beneficial effects of insulin-like growth factor-I on wobbler mouse motoneuron disease. J Neurol Sci. 1995;129:122–126. [DOI] [PubMed] [Google Scholar]
- 288. Torres-Aleman I, Barrios V, Berciano J. The peripheral insulin-like growth factor system in amyotrophic lateral sclerosis and in multiple sclerosis. Neurology. 1998;50:772–776. [DOI] [PubMed] [Google Scholar]
- 289. Chesik D, Wilczak N, De Keyser J. The insulin-like growth factor system in multiple sclerosis. Int Rev Neurobiol. 2007;79:203–226. [DOI] [PubMed] [Google Scholar]
- 290. Chesik D, De Keyser J, Wilczak N. Insulin-like growth factor binding protein-2 as a regulator of IGF actions in CNS: implications in multiple sclerosis. Cytokine Growth Factor Rev. 2007;18:267–278. [DOI] [PubMed] [Google Scholar]
- 291. Rauskolb S, Dombert B, Sendtner M. Insulin-like growth factor 1 in diabetic neuropathy and amyotrophic lateral sclerosis. Neurobiol Dis. 2017;97:103–113. [DOI] [PubMed] [Google Scholar]
- 292. Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol. 2014;72:1–5. [DOI] [PubMed] [Google Scholar]
- 293. Ontaneda D, Thompson AJ, Fox RJ, Cohen JA. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389:1357–1366. [DOI] [PubMed] [Google Scholar]
- 294. Roth GA, Spada V, Hamill K, Bornstein MB. Insulin-like growth factor I increases myelination and inhibits demyelination in cultured organotypic nerve tissue. Brain Res Dev Brain Res. 1995;88:102–108. [DOI] [PubMed] [Google Scholar]
- 295. Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–740. [DOI] [PubMed] [Google Scholar]
- 296. Wilczak N, Chesik D, Hoekstra D, De Keyser J. IGF binding protein alterations on periplaque oligodendrocytes in multiple sclerosis: implications for remyelination. Neurochem Int. 2008;52:1431–1435. [DOI] [PubMed] [Google Scholar]
- 297. Gveric D, Cuzner ML, Newcombe J. Insulin-like growth factors and binding proteins in multiple sclerosis plaques. Neuropathol Appl Neurobiol. 1999;25:215–225. [DOI] [PubMed] [Google Scholar]
- 298. Mason JL, Ye P, Suzuki K, D’Ercole AJ, Matsushima GK. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J Neurosci. 2000;20:5703–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23:7710–7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Cannella B, Pitt D, Capello E, Raine CS. Insulin-like growth factor-1 fails to enhance central nervous system myelin repair during autoimmune demyelination. Am J Pathol. 2000;157:933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Wilczak N, Ramsaransing GS, Mostert J, Chesik D, De Keyser J. Serum levels of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 in relapsing and primary progressive multiple sclerosis. Mult Scler. 2005;11:13–15. [DOI] [PubMed] [Google Scholar]
- 302. Pirttila T, Vanhatalo S, Turpeinen U, Riikonen R. Cerebrospinal fluid insulin-like growth factor-1, insulin growth factor binding protein-2 or nitric oxide are not increased in MS or ALS. Acta Neurol Scand. 2004;109:337–341. [DOI] [PubMed] [Google Scholar]
- 303. Frank JA, Richert N, Lewis B, et al. A pilot study of recombinant insulin-like growth factor-1 in seven multiple sderosis patients. Mult Scler. 2002;8:24–29. [DOI] [PubMed] [Google Scholar]
- 304. Saber H, Himali JJ, Beiser AS, et al. Serum insulin-like growth factor 1 and the risk of ischemic stroke: the Framingham study. Stroke. 2017;48:1760–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Beilharz EJ, Russo VC, Butler G, et al. Co-ordinated and cellular specific induction of the components of the IGF/IGFBP axis in the rat brain following hypoxic-ischemic injury. Brain Res Mol Brain Res. 1998;59:119–134. [DOI] [PubMed] [Google Scholar]
- 306. Johnston BM, Mallard EC, Williams CE, Gluckman PD. Insulin-like growth factor-1 is a potent neuronal rescue agent after hypoxic-ischemic injury in fetal lambs. J Clin Invest. 1996;97:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Chang HC, Yang YR, Wang PS, Kuo CH, Wang RY. The neuroprotective effects of intramuscular insulin-like growth factor-I treatment in brain ischemic rats. PLoS ONE. 2013;8:e64015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Zhang L, Hu X, Luo J, et al. Physical exercise improves functional recovery through mitigation of autophagy, attenuation of apoptosis and enhancement of neurogenesis after MCAO in rats. BMC Neurosci. 2013;14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Bake S, Okoreeh A, Khosravian H, Sohrabji F. Insulin-like growth factor (IGF)-1 treatment stabilizes the microvascular cytoskeleton under ischemic conditions. Exp Neurol. 2018;311:162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. De Geyter D, Stoop W, Sarre S, De Keyser J, Kooijman R. Neuroprotective efficacy of subcutaneous insulin-like growth factor-I administration in normotensive and hypertensive rats with an ischemic stroke. Neuroscience. 2013;250:253–262. [DOI] [PubMed] [Google Scholar]
- 311. Guan J, Thomas GB, Lin H, et al. Neuroprotective effects of the N-terminal tripeptide of insulin-like growth factor-1, glycine-proline-glutamate (GPE) following intravenous infusion in hypoxic-ischemic adult rats. Neuropharmacology. 2004;47:892–903. [DOI] [PubMed] [Google Scholar]
- 312. Knapp J, Teschendorf P, Vogel P, Bruckner T, Bottiger BW, Popp E. Effects of intracerebroventricular application of insulin-like growth factor 1 and its N-terminal tripeptide on cerebral recovery following cardiac arrest in rats. Resuscitation. 2013;84:684–689. [DOI] [PubMed] [Google Scholar]
- 313. Guan J, Williams CE, Skinner S, Mallard EC, Gluckman PD. The effects of insulin-like growth factor (IGF)-1, IGF-2, and des-IGF-1 on neuronal loss after hypoxic-ischemic brain injury in adult rats: evidence for a role for IGF binding proteins. Endocrinology. 1996;137:893–898. [DOI] [PubMed] [Google Scholar]
- 314. O’Donnell SL, Frederick TJ, Krady JK, Vannucci SJ, Wood TL. IGF-I and microglia/macrophage proliferation in the ischemic mouse brain. Glia. 2002;39:85–97. [DOI] [PubMed] [Google Scholar]
- 315. Wong VS, Langley B. Epigenetic changes following traumatic brain injury and their implications for outcome, recovery and therapy. Neurosci Lett. 2016;625:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Schober ME, Ke X, Xing B, et al. Traumatic brain injury increased IGF-1B mRNA and altered IGF-1 exon 5 and promoter region epigenetic characteristics in the rat pup hippocampus. J Neurotrauma. 2012;29:2075–2085. [DOI] [PubMed] [Google Scholar]
- 317. Ozdemir D, Baykara B, Aksu I, et al. Relationship between circulating IGF-1 levels and traumatic brain injury-induced hippocampal damage and cognitive dysfunction in immature rats. Neurosci Lett. 2012;507:84–89. [DOI] [PubMed] [Google Scholar]
- 318. Li XS, Williams M, Bartlett WP. Induction of IGF-1 mRNA expression following traumatic injury to the postnatal brain. Brain Res Mol Brain Res. 1998;57:92–96. [DOI] [PubMed] [Google Scholar]
- 319. Walter HJ, Berry M, Hill DJ, Logan A. Spatial and temporal changes in the insulin-like growth factor (IGF) axis indicate autocrine/paracrine actions of IGF-I within wounds of the rat brain. Endocrinology. 1997;138:3024–3034. [DOI] [PubMed] [Google Scholar]
- 320. Carlson SW, Saatman KE. Central infusion of insulin-like growth factor-1 increases hippocampal neurogenesis and improves neurobehavioral function after traumatic brain injury. J Neurotrauma. 2018;35:1467–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Yao DL, West NR, Bondy CA, et al. Cryogenic spinal cord injury induces astrocytic gene expression of insulin-like growth factor I and insulin-like growth factor binding protein 2 during myelin regeneration. J Neurosci Res. 1995;40:647–659. [DOI] [PubMed] [Google Scholar]
- 322. Breese CR, D’Costa A, Rollins YD, et al. Expression of insulin-like growth factor-1 (IGF-1) and IGF-binding protein 2 (IGF-BP2) in the hippocampus following cytotoxic lesion of the dentate gyrus. J Comp Neurol. 1996;369:388–404. [DOI] [PubMed] [Google Scholar]
- 323. Fernandez AM, de la Vega AG, Torres-Aleman I. Insulin-like growth factor I restores motor coordination in a rat model of cerebellar ataxia. Proc Natl Acad Sci U S A. 1998;95:1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Streppel M, Azzolin N, Dohm S, et al. Focal application of neutralizing antibodies to soluble neurotrophic factors reduces collateral axonal branching after peripheral nerve lesion. Eur J Neurosci. 2002;15:1327–1342. [DOI] [PubMed] [Google Scholar]
- 325. Tang Z, Cao F, Zhang H, et al. Peripheral pain is enhanced by insulin-like growth factor 1 and its receptors in a mouse model of type 2 diabetes mellitus. J Diabetes. 2019:11:309–315. [DOI] [PubMed] [Google Scholar]
- 326. Zemva J, Schubert M. The role of neuronal insulin/insulin-like growth factor-1 signaling for the pathogenesis of Alzheimer’s disease: possible therapeutic implications. CNS Neurol Disord Drug Targets. 2014;13:322–337. [DOI] [PubMed] [Google Scholar]
- 327. Saez JM. Possible usefulness of growth hormone/insulin-like growth factor-I axis in Alzheimer’s disease treatment. Endocr Metab Immune Disord Drug Targets. 2012;12:274–286. [DOI] [PubMed] [Google Scholar]
- 328. Bolos M, Fernandez S, Torres-Aleman I. Oral administration of a GSK3 inhibitor increases brain insulin-like growth factor I levels. J Biol Chem. 2010;285:17693–17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Pardo J, Morel GR, Astiz M, et al. Gene therapy and cell reprogramming for the aging brain: achievements and promise. Curr Gene Ther. 2014;14:24–34. [DOI] [PubMed] [Google Scholar]
- 330. Maino B, Paparone S, Severini C, et al. Drug target identification at the crossroad of neuronal apoptosis and survival. Expert Opin Drug Discov. 2017;12:249–259. [DOI] [PubMed] [Google Scholar]
- 331. Ayyadevara S, Ganne A, Hendrix RD, Balasubramaniam M, Shmookler Reis RJ, Barger SW. Functional assessments through novel proteomics approaches: application to insulin/IGF signaling in neurodegenerative disease [published online ahead of print November 6, 2018]. J Neurosci Methods. doi: 10.1016/j.jneumeth.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Beauverd M, Mitchell JD, Wokke JH, Borasio GD. Recombinant human insulin-like growth factor I (rhIGF-I) for the treatment of amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2012;11:CD002064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Bianchi VE, Locatelli V, Rizzi L. Neurotrophic and neuroregenerative effects of GH/IGF1. Int J Mol Sci. 2017;18:E2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Hatton J, Kryscio R, Ryan M, Ott L, Young B. Systemic metabolic effects of combined insulin-like growth factor-I and growth hormone therapy in patients who have sustained acute traumatic brain injury. J Neurosurg. 2006;105:843–852. [DOI] [PubMed] [Google Scholar]
- 335. Ley D, Hansen-Pupp I, Niklasson A, et al. Longitudinal infusion of a complex of insulin-like growth factor-I and IGF-binding protein-3 in five preterm infants: pharmacokinetics and short-term safety. Pediatr Res. 2013;73:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Liegl R, Lofqvist C, Hellstrom A, Smith LE. IGF-1 in retinopathy of prematurity, a CNS neurovascular disease. Early Hum Dev. 2016;102:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Jablonka S, Holtmann B, Sendtner M, Metzger F. Therapeutic effects of PEGylated insulin-like growth factor I in the pmn mouse model of motoneuron disease. Exp Neurol. 2011;232:261–269. [DOI] [PubMed] [Google Scholar]
- 338. Carrascosa C, Torres-Aleman I, Lopez-Lopez C, et al. Microspheres containing insulin-like growth factor I for treatment of chronic neurodegeneration. Biomaterials. 2004;25:707–714. [DOI] [PubMed] [Google Scholar]
- 339. Loddick SA, Liu XJ, Lu ZX, et al. Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proc Natl Acad Sci U S A. 1998;95:1894–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Nedelcovych MT, Gadiano AJ, Wu Y, et al. Pharmacokinetics of Intranasal versus Subcutaneous Insulin in the Mouse. ACS Chem Neurosci. 2018;9:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Freiherr J, Hallschmid M, Frey WH, II, et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Reger MA, Watson GS, Frey WH, II, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451–458. [DOI] [PubMed] [Google Scholar]
- 343. Paslakis G, Blum WF, Deuschle M. Intranasal insulin-like growth factor I (IGF-I) as a plausible future treatment of depression. Med Hypotheses. 2012;79:222–225. [DOI] [PubMed] [Google Scholar]
- 344. Manev H, Manev R. New antidepressant drugs that do not cross the blood-brain barrier. Med Hypotheses. 2002;58:83–84. [DOI] [PubMed] [Google Scholar]
- 345. Mueller PL, Pritchett CE, Wiechman TN, Zharikov A, Hajnal A. Antidepressant-like effects of insulin and IGF-1 are mediated by IGF-1 receptors in the brain. Brain Res Bull. 2018;143:27–35. [DOI] [PubMed] [Google Scholar]
- 346. Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. [DOI] [PubMed] [Google Scholar]
- 347. Nishida F, Morel GR, Herenu CB, Schwerdt JI, Goya RG, Portiansky EL. Restorative effect of intracerebroventricular insulin-like growth factor-I gene therapy on motor performance in aging rats. Neuroscience. 2011;177:195–206. [DOI] [PubMed] [Google Scholar]
- 348. Pardo J, Abba MC, Lacunza E, et al. IGF-I gene therapy in aging rats modulates hippocampal genes relevant to memory function. J Gerontol A Biol Sci Med Sci. 2018;73:459–467. [DOI] [PubMed] [Google Scholar]
- 349. Rai KS, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur J Neurosci. 2007;26:1765–1779. [DOI] [PubMed] [Google Scholar]
- 350. Lauzon MA, Daviau A, Marcos B, Faucheux N. Growth factor treatment to overcome Alzheimer’s dysfunctional signaling. Cell Signal. 2015;27:1025–1038. [DOI] [PubMed] [Google Scholar]
- 351. Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773:1299–1310. [DOI] [PubMed] [Google Scholar]
- 352. Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti- epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62:200–207. [PubMed] [Google Scholar]
- 353. Jia M, Shi Z, Yan X, et al. Insulin and heparin-binding epidermal growth factor-like growth factor synergistically promote astrocyte survival and proliferation in serum-free medium. J Neurosci Methods. 2018;307:240–247. [DOI] [PubMed] [Google Scholar]
- 354. Jesko H, Stepien A, Lukiw WJ, Strosznajder RP. The cross-talk between sphingolipids and insulin-like growth factor signaling: significance for aging and neurodegeneration [published online ahead of print August 23, 2018]. Mol Neurobiol. doi: 10.1007/s12035-018-1286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Pavlatou MG, Remaley AT, Gold PW. Klotho: a humeral mediator in CSF and plasma that influences longevity and susceptibility to multiple complex disorders, including depression. Transl Psychiatry. 2016;6:e876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Vieira MS, Santos AK, Vasconcellos R, et al. Neural stem cell differentiation into mature neurons: mechanisms of regulation and biotechnological applications. Biotechnol Adv. 2018;36:1946–1970. [DOI] [PubMed] [Google Scholar]
- 357. Ebert AD, Beres AJ, Barber AE, Svendsen CN. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson’s disease. Exp Neurol. 2008;209:213–223. [DOI] [PubMed] [Google Scholar]