Abstract
Background: Griffonia simplicifolia Baill. (Caesalpiniaceae) is a medicinal plant whose seeds are widely used in traditional medicine for their high content of 5-hydroxy-l-tryptophan (5-HTP), a direct precursor and enhancer of the activity of the brain hormone serotonin (5-HT). The plant extracts are used in dietary supplements aimed to alleviate serotonin-related disorders. Methods: In order to characterize the chemical components of G. simplicifolia seeds and their identity, we used a combined methodology by using HPLC-DAD-ESI-MS/MS for the qualitative and quantitative determination of the N-containing compounds, GC-FID and GC-MS for the characterization of the major fatty acids, and DNA fingerprinting based on PCR–RFLP for the unequivocal identification of the plant. Results: 5-HTP was the most representative compound, followed by lower percentages of the β-carboline alkaloid derivative griffonine and other alkaloids. Fatty acids were dominated by the unsaturated fatty acids linoleic acid and oleic acid, followed by the saturated fatty acids stearic and palmitic acids. PCR analysis of the internal transcribed spacer amplified sequence showed a major band at about 758 bp, whereas the PCR–RFLP analysis of this sequence using three different restriction enzymes (MspI, HhaI, and HaeIII) generated a specific fingerprinting useful for the plant identification. Conclusions: The combined chemical and molecular analysis of G. simplicifolia provided an interesting integrated approach for the unequivocal identification of commercial G. simplicifolia seeds.
Keywords: Griffonia simplicifolia, Caesalpiniaceae, 5-hydroxy-l-tryptophan (5-HTP), fatty acids, PCR–RFLP, internal transcribed spacer (ITS), DNA fingerprinting
1. Introduction
The legume plant Griffonia simplicifolia Baill. (Caesalpiniaceae) (also known with the alternate incorrect name Bandeiraea simplicifolia) is a perennial woody shrub which grows in the tropical rain forest of West and Central Africa, with sites of cultivation present in Ghana, Ivory Coast, and Togo. The seed chemical constituents, including lectins, 5-hydroxy-l-tryptophan (5-HTP), and fatty acids have been studied intensively since 1960 [1,2], whereas leaves may contain lectin II—a legume lectin with GlcNAc binding specificity resulting in insecticidal activity [3].
Seed extracts from G. simplicifolia are rich in 5-HTP [4]—a direct precursor and enhancer of the activity of the brain hormone serotonin (5-hydroxytryptamine, 5-HT). After entering the central nervous system, 5-HTP is converted to 5-HT by the enzyme tryptophan decarboxylase [5]. The administration of 5-HTP to animals increases 5-HT levels in the central nervous system (CNS) [6]; however, in humans 5-HTP stimulates 5-HT receptors in the CNS only after conversion to 5-HT [7]. A medical food formulation comprised of G. simplicifolia containing high concentrations of 5-HTP is thought to be effective for serotonin-related disorders [8,9], including depression [10] and young subjects with high levels of romantic stress [11]. Further uses of G. simplicifolia seed extracts include the treatment of insomnia, migraine, headache, and the regulation of appetite leading to weight reduction in obese patients, as well as the regulation of mood, memory, and many other functions [12,13]. Other constituents of this plant are the β-carboline alkaloid derivative griffonine and other alkaloids, some of which show potential in vitro activity against HepG2 cells [14].
The UV absorption spectra of 5-HTP, tryptamine, tryptophan, and 5-hydroxytryptamine are very similar, with strong absorption between 230 and 300 nm, which makes UV spectrophotometric assay of 5-HTP in seed material almost impossible [15]. Therefore, HPLC methods coupled to mass spectrometry have been developed [15,16,17]. Moreover, the regioselective hydroxylation of tryptophan via chemical approaches is not easy, and this is why G. simplicifolia seeds are used for the extraction and the commercial production of 5-HTP. The recent development of metabolic engineering and protein engineering in combination with fundamental genetics and biochemistry has allowed the production of 5-HTP from tryptophan in heterologous systems [18,19]. A common sophistication of G. simplicifolia is the addition of 5-HTP derived from transgenic bacteria to the plant extract, in order to increase the 5-HTP content. However, the bacterial transformation may lead to tryptophan by-products (e.g., peak E as 1,1′-ethylidenebis(l-tryptophan) and peak UV-5 as 3-(phenylamino)alanine) that have been associated to diseases such as eosinophilia–myalgia syndrome [20,21,22].
Existing assay methods for the authentication of G. simplicifolia seeds are laborious, time-consuming, and sensitive to interference from co-occurring materials. In order to ensure reliable supplies of appropriate G. simplicifolia seed material, manufacturers require an efficient, dependable, and relatively rapid method for assessing the quality of seeds before export. Along with mass spectrometry-based analytical methods, the biomolecular characterization of food and medicinal plants offers a potent tool for their unequivocal identification [23]. Among techniques, internal transcribed spacer (ITS) is widely used in plant molecular systematics at the generic and species levels because of its potentially high resolution of inter- and intraspecific relationships [24].
The aim of this study was to combine the seed chemical composition with DNA fingerprinting of commercial G. simplicifolia seeds. Chemical analyses included that of fatty acids, the characterization of 5-HTP and other tryptophan-derived compounds, the β-carboline alkaloid derivative griffonine and other alkaloids, as well as the search for the so-called peaks E and UV-5. To provide reliable DNA fingerprinting, DNA restriction fragment length polymorphism (PCR–RFLP) analysis was performed on the G. simplicifolia ITS. To our knowledge, there are no data on ITS characterization and on the combined use of ITS and chemical data for the chemical and molecular characterization of G. simplicifolia. The combination of chemical and molecular data provided an interesting integrated approach for the unequivocal identification of commercial G. simplicifolia seeds.
2. Results and Discussion
2.1. 5-HTP Is the Major Nitrogen-Containing Compound of G. simplicifolia
The seeds of G. simplicifolia showed a 9.18% (±0.18) weight loss and a 3.66% (±0.20) ashes content. The total amount of N-containing compounds was about 18% on a dry weight basis (Table 1). The main compound of the soluble fraction was 5-HTP. The content of this compound was about 16% of the seed weight (156 mg g−1) (Table 1), which is consistent with the literature data reporting concentrations ranging from 10% to 20%, obtained through different analytical methods and different solvents [15,25,26]. These data are in contrast with claims of frequently found 5-HTP concentrations in G. simplicifolia seed extracts of 98% [26]. As shown in Table 1, these high percentages can be obtained if the relative percentage is calculated based only on the total identified compounds. Another possibility for the high percentage found in G. simplicifolia extracts might be the sophistication with 5-HTP from genetically engineered bacterial fermentation, as frequently reported [27], or by filtration of culture media through a reverse osmosis membrane to remove chemicals with a molecular weight higher than 1000 [20]. One way to determine the quality of G. simplicifolia extracts is to evaluate the presence and content of their characteristic alkaloids [14]. The alkaloid hyrtioerectine B was present at about 7 mg g−1 on a seed dry weight basis (Table 1). This compound is also present in the marine sponge Hyrtios erectus and showed moderate cytotoxicity against HeLa cancer cells with an IC50 of 5.0 μg mL−1 [28] and was found to inhibit HepG2 cell lines with IC50 values of 9.6 μmol L−1 [14]. The 3-carboxy-6-hydroxy-β-carboline content was close to 3 mg g−1, whereas 5-hydroxytryptamine was present at lower concentrations (Table 1). These two compounds were found to be ineffective against HepG2 cell lines [14]. Minor contents were found for griffonine and hyrtiosulawesine. These two compounds were potentially active against the HepG2 cancer cell line in vitro [14]. Other minor compounds were 1H-indole-3-carboxylic acid and 5-hydroxy-3-(2-hydroxyethyl)indole (Table 1).
Table 1.
Chemical composition of nitrogen-containing compounds extracted from seeds of Griffonia simplicifolia.
| Compound | MW | [M + H]+ | m/z | mg g−1 d.wt. | SD | Relative Percentage | ||
|---|---|---|---|---|---|---|---|---|
| 1H-indole-3-carboxylic acid | 161.0 | 162.0 | 143.8 | 115.9 | 0.08 | 0.006 | 0.04 | |
| 5-hydroxytryptamine | 176.0 | 177.0 | 158.8 | 135.8 | 117.0 | 1.15 | 0.066 | 0.64 |
| 5-hydroxy-3-(2-hydroxyethyl)indole | 177.0 | 178.0 | 159.8 | 132.9 | 115.0 | 0.27 | 0.006 | 0.15 |
| 5-hydroxy-l-tryptophan (5-HTP) | 220.0 | 221.0 | 204.0 | 161.9 | 156.48 | 8.320 | 86.48 | |
| 3-carboxy-6-hydroxy-β-carboline | 228.0 | 229.0 | 210.0 | 183.0 | 101.1 | 2.69 | 0.120 | 1.49 |
| hyrtioerectine B | 246.0 | 247.0 | 229.9 | 203.9 | 174.0 | 6.75 | 0.320 | 3.73 |
| griffonine | 329.0 | 330.0 | 167.9 | 0.75 | 0.030 | 0.41 | ||
| hyrtiosulawesine | 343.0 | 344.0 | 228.9 | 200.9 | 182.9 | 0.9 | 0.030 | 0.50 |
| 1,1′-ethylidenebis(l-tryptophan) | 434.0 | 435.0 | 231.0 | n.d. | n.d. | n.d. | ||
| 3-(phenylamino)alanine | 180.0 | 181.0 | 106.0 | 88.0 | 70.0 | n.d. | n.d. | n.d. |
| 4,5-tryptophan-dione | 234.0 | 235.0 | 217.0 | 162.0 | 181.0 | 11.87 | 0.92 | 6.56 |
| TOTAL | 181.10 | 16.10 | 100.00 | |||||
SD: standard deviation; n.d.: not detectable (i.e., below the limit of detection).
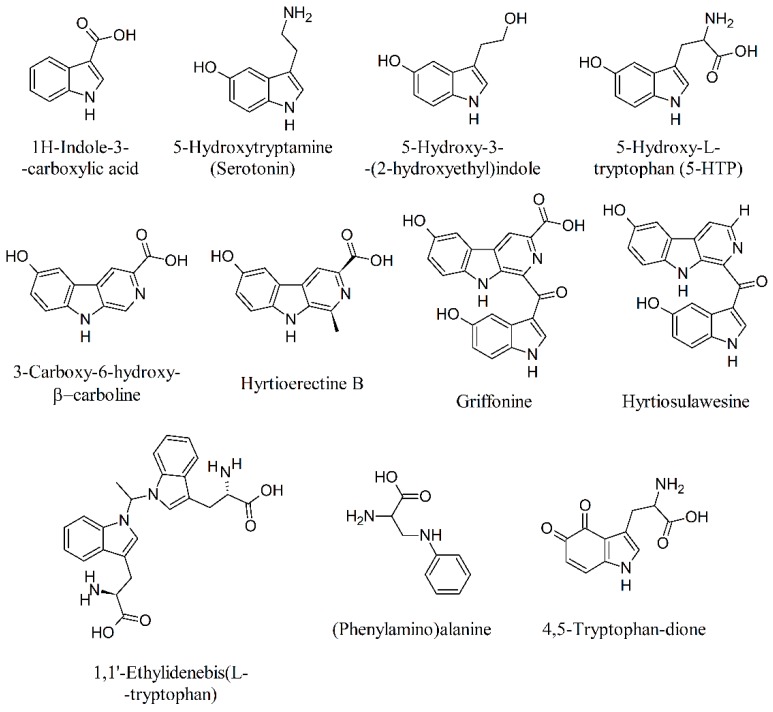
The search for the neurotoxic compounds 1,1′-ethylidenebis(l-tryptophan) (peak E) and 3-(phenylamino)alanine (peak UV-5) was ineffective, demonstrating that these two compounds are typical markers of engineered bacterial fermentation [20,29]. On the other hand, peak X1 (4,5-tryptophan-dione)—the oxidation product of 5-HTP [27]—was present at a concentration of about 12 mg g−1 on a dry weight basis (Table 1). By considering that the suggested recommended doses are around 10–50 mg of 5-HTP/day [30], this would provide about 0.7–3.7 mg 4,5-tryptophan-dione. Although the direct toxicity of this compound is unknown at present, possible reactions with reduced glutathione cannot be excluded [27]. Figure 1 shows the chemical structure of the compounds listed in Table 1.
Figure 1.
Structures of different isolated N-containing compounds from G. simplicifolia.
The data reported here, and a review of the literature [14,15,25,26], suggest that an original and natural G. simplicifolia extract should contain a 5-HTP:alkaloid ratio of about 6–7:1. Higher ratios might indicate a different 5-HTP origin or the use of fractionation methods during extraction.
As a proof of concept, we analyzed a commercial sample of a G. simplicifolia extract claiming a 95% 5-HTP content. After the chemical characterization, we found that the real content of 5-HTP was about 65%, that the ratio between 5-HTP and other alkaloids was 14:1, and the presence of peak E was about 6 mg g−1, which indicates a possible bacterial fermentation process (see Supplementary data S2).
2.2. Griffonia simplicifolia Seeds Show a High Content of Unsaturated Fatty Acids
The lipid composition of G. simplicifolia seeds was characterized by the presence of unsaturated and saturated fatty acids, as is typical for a south thermophilic plant. The total content of the major fatty acids was about 165 (±11) mg g−1 on a dry weight basis (Table 2). Among unsaturated fatty acids, linoleic acid (55%) and oleic acid (12%) were the most abundant compounds, whereas the remaining fatty acids were represented by saturated fatty acids, with stearic acid (20%) and palmitic acid (11%) being the most abundant (Table 2). The results reported here are in line with literature data, confirming the prevalence of unsaturated fatty acids in G. simplicifolia seeds [1].
Table 2.
Fatty acid composition of G. simplicifolia seeds.
| Fatty Acid | Kovat’s Index | mg/g | SD | Relative Percentage |
|---|---|---|---|---|
| palmitic acid | 1942 | 18.22 | 0.93 | 11.06% |
| linoleic acid | 2095 | 91.24 | 5.74 | 55.38% |
| oleic acid | 2113 | 20.24 | 1.13 | 12.28% |
| stearic acid | 2187 | 32.12 | 2.25 | 19.50% |
| arachidic acid | 2359 | 10.39 | 0.65 | 6.31% |
| behenic acid | 2400 | 4.65 | 0.32 | 2.82% |
| TOTAL | 164.76 | 11.09 | 100.00% |
2.3. DNA Fingerprinting of Griffonia simplicifolia Using PCR–RFLP Analysis
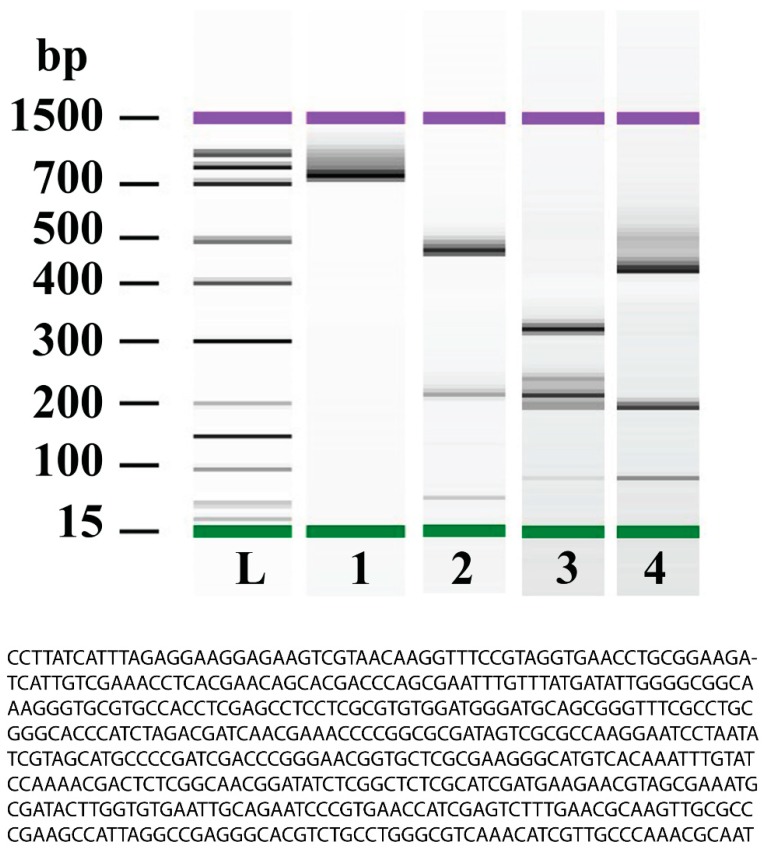
In order to provide molecular fingerprinting of G. simplicifolia seeds, ITS-1 coupled with ITS-4 was used for PCR amplification. Figure 2 shows the nucleotide sequence of the ITS region of G. simplicifolia. In general, the ITS-amplified sequence was about 758 bp long (NCBI GenBank Accession No MH707248) (Figure 2 lane 1). In order to provide a better-defined DNA fingerprint, a PCR–RFLP method was applied. Three different restriction enzymes (MspI, HhaI, and HaeIII) were used to selectively cleave the resulting amplicon. Digestion of the PCR product with MspI generated three fragments: one major fragment of about 471 bp, and two minor fragments of about 57 and 213 bp (Figure 2 lane 2). An HhaI site could also be identified in the ITS region, generating five distinct fragments: the major fragment gave a band of about 319 bp, followed by a band of about 215 bp. Two minor bands were found at about 198 and 240 bp, and there was a faint band at 86 bp (Figure 2 lane 3). Finally, digestion with HaeIII generated three bands: a major band of about 431 bp, followed by two bands of minor concentration of about 198 and 86 bp (Figure 2 lane 4). Supplementary data S1 provides the electropherograms and the concentration and molarity of both the ITS region and PCR–RFLP products. To our knowledge, this is the first report on the DNA fingerprinting of G. simplicifolia.
Figure 2.
Electrophoresis representation of the Agilent Bioanalyzer 2100 gel-electropherograms of PCR products generated by primers flanking the internal transcribed spacer (ITS) region of ITS-1 and ITS-4 and PCR–restriction fragment length polymorphism (RFLP) products from G. simplicifolia. Lane 1, PCR product of the spacer region produced a band of about 758 bp; Lane 2, PCR–RFLP analysis using MspI G. simplicifolia digested PCR products gave three bands of about 471, 312, and 57 bp; Lane 3, PCR–RFLP analysis using HhaI G. simplicifolia digested PCR products produced five bands of about 319, 240, 215, 198, and 86 bp; Lane 4, PCR–RFLP analysis using HaeIII G. simplicifolia digested PCR products generated three bands of about 431, 198, and 86 bp. L = bp markers. The green bar indicates the lower marker (15 bp), whereas the violet band indicates the upper marker (1500 bp). See Supplementary data S1 for more information. The figure also shows the nucleotide sequences of the ITS gene spacer region of G. simplicifolia.
3. Materials and Methods
3.1. Plant Material
Seeds of Griffonia simplicifolia Baill. (Caesalpiniaceae) were kindly provided by Demar Srl Cesena (FC), Italy, batch 171/10/17/722, and were collected from plants originating from Ivory Coast (North-West Africa). Seeds were stored in the dark at 4 °C before extraction. At least three technical replicates were done for each lot of seeds. The dry weight of the seeds was obtained after placing the seeds overnight in an oven at 105 °C, whereas ashes were obtained by placing the seeds in a muffle at 600 °C for 6 h.
3.2. Extraction of 5-HTP and Other N-Containing Compounds
For 5-HTP and alkaloid analysis, ground seeds of G. simplicifolia were extracted by maceration in an 1:10 w/v ethanol:water 70:20 v/v solution for 3 days in the dark at room temperature. Extraction provided a soluble and an insoluble fraction. The supernatant soluble fraction was recovered after centrifugation (10 min at 10,000× g, at 4 °C) and filtration through a Millex HV 0.45 μm filter (Millipore, Billerica, MA, USA). In order to guarantee an exhaustive extraction, pellets were re-extracted twice. After centrifugation and filtration, the supernatants were combined and stored at −80 °C until analysis.
Lipophilic extracts of G. simplicifolia seeds were obtained by Soxhlet extraction for 6 h with cyclohexane (1:10, w/v). After extraction, the solvent was removed with a nitrogen flow and the extract stored at −20 °C until analysis.
3.3. Identification and Quantification of 5-HTP and Other N-Containing Compounds
The HPLC system consisted of an Agilent Technologies 1200 coupled to a DAD and a 6330 Series Ion Trap LC-MS System (Agilent Technologies, Santa Clara, CA, USA) equipped with an electrospray ionization (ESI) source. The chromatographic separation was carried out at constant flow rate (0.2 mL min−1). The column was a reverse-phase C18 Luna column (3.00 μm, 150 mm × 3.0 mm i.d., Phenomenex, Torrance, CA, USA) maintained at 25 °C by an Agilent 1100 HPLC G1316A Column Compartment. The binary solvent system for the analysis of N-containing compounds was MilliQ H2O acidified with 0.1% v/v formic acid (Solvent A) and acetonitrile acidified with 0.1% v/v formic acid (Solvent B). The samples were separated by an isocratic gradient of 97% A and 3% B. In order to clean the column from other interferences before the next injection, the concentration of B was slowly raised and maintained at 3% for 5 min. Sample injection volume was set to 5 μL. The UV–VIS spectra were recorded between 220 and 500 nm and the chromatographic profiles were registered at 230 and 270 nm. Tandem mass spectrometry analyses were performed by operating in positive mode. The nitrogen flow rate was set at 5.0 mL min−1 and maintained at 325 °C, whereas the capillary voltage was set at 1.5 kV. Helium was used as a collision gas. Compound identification was carried out by comparison of the retention time and UV–VIS/MS spectra with those of authentic reference compounds or using literature data. Limit of detection (LOD) and limit of quantification (LOQ) for each compound was determined as previously described [31].
3.4. Identification and Quantification of Fatty Acids
Lipophilic extracts of G. simplicifolia seeds were esterified with boron tri-fluoride (10% w/v in methanol). An internal standard of 50 μg heptadecanoic acid (C17:0) was added. The fatty acid methyl esters (FAMEs) were obtained by acid catalysis according to Christie and Han [32] and were dehydrated with anhydrous MgSO4. FAME identification and quantification was performed by GC-MS (5975T, Agilent Technologies, USA) and by GC-FID (GC-2010 Plus, SHIMADZU, Kyoto, Japan), respectively, as previously described [33]. Compounds were identified through comparison of mass fragmentation spectra with reference NIST 98 spectra or by comparison of Kovats indexes and internal standard co-injection of pure standards (Sigma-Aldrich, St Louis, MO, USA). FAME quantification was obtained by internal standard. At least three technical replicates were run for each lot of G. simplicifolia seeds.
3.5. DNA Extraction, PCR Amplification, Subcloning, and Sequencing
Whole G. simplicifolia seeds were pulverized in liquid N2 using a mortar and pestle. Genomic DNA was extracted and quantified according to Capuzzo and Maffei [34]. Briefly, 20 ng of genomic DNA were used as a template for PCR amplification with specific primers for ITS-p5 (5’-CTTATCAYTTAGAGGAAGGAG-3’) and ITS-u4 (5’-RGTTTCTTTTCCTCCGCTTA-3’). PCR products were separated and purified as previously described [33]. Subcloning of the purified products was obtained by using the TOPO-TA Cloning Kit (Thermo Fisher Scientific, Waltham, MA, USA) followed by transformation in Escherichia coli sub-cloning DH5α Efficiency Competent Cells (Invitrogen, Paisley, UK). Colonies containing DNA inserts of the correct size were picked and grown overnight in 5 mL Luria-Bertani liquid medium. The mini-preparation of plasmid DNAs was carried out using NucleoSpin Plasmid Miniprep Kit (Macherey-Nagel, Düren, Germany). Plasmid DNAs were used as a template for sequencing (Macrogen, Wageningen, Holland). Both DNA strands were sequenced.
3.6. PCR–RFLP Analysis and DNA Fingerprinting
PCR products of the ITS gene were digested at 37 °C for 15 min with 10 U MspI, HhaI, or HaeIII (NEB, New England Biolabs, Ipswich, AM, USA) at 37 °C for 15 min. For each digestion reaction 1 μL was analyzed by capillary gel electrophoresis using the Agilent 2100 Bioanalyzer (Agilent Technologies) and the DNA 1000 LabChip Kit (Agilent Technologies) following the manufacturer’s instructions.
3.7. Statistical Analysis
Data are expressed as the mean of three technical replicates for each lot of seeds. ANOVA followed by Tukey–Kramer’s HSD post-hoc test (p < 0.05) was used to determine significant differences. All statistical analyses were performed by using the SYSTAT 10 software.
4. Conclusions
In conclusion, our data showed that natural G. simplicifolia seed extract was characterized by 5-HTP percentages that rarely exceeded 20% on a dry weight basis. Therefore, claims of 98% 5-HTP in G. simplicifolia seed extracts should be carefully considered. Moreover, natural G. simplicifolia extracts did not contain peaks E and UV-5, which are typical of transgenic bacterial fermentation used for the production of 5-HTP. We showed that a good indicator of G. simplicifolia natural extract is the ratio between 5-HTP and the other N-containing compounds. Our results also indicated that only analytical methods based on HPLC coupled to mass spectrometry allow the precise quantification and identification of N-containing compounds, and other methodologies (UV–VIS or sole HPLC) were insufficient to determine their identity and content. We also provided a molecular characterization of G. simplicifolia seeds by DNA fingerprinting using specific PCR–RFLP markers and our data provided a simple and unequivocal method for the plant identification. DNA fingerprinting offers the advantage of a fast and rather inexpensive method for the unequivocal identification of this plant species long before the chemical analysis is done. Moreover, being restricted to DNA, this analysis it is not affected by environmental factors that might affect the expression of those genes involved in 5-HTP production.
Acknowledgments
The authors are grateful to Demar Srl Cesena (FC), Italy, for providing seeds originating from Ivory Coast (North-West Africa).
Supplementary Materials
The following are available online, Supplementary data S1: Electrophoresis assay details illustrating the gel electropherograms obtained to plot Figure 2; Supplementary data S2: Chemical characterization of a Griffonia simplicifolia extract claiming a 95% content of 5-HTP.
Author Contributions
Conceptualization, M.E.M.; methodology, M.E.M., I.V., G.M.; formal analysis, I.V., G.M.; investigation, I.V., G.M.; resources, M.E.M.; data curation, M.E.M., I.V., G.M.; writing—original draft preparation, M.E.M., I.V., G.M.; writing—review and editing, M.E.M.; visualization, M.E.M., I.V., G.M.; supervision, M.E.M.; project administration, M.E.M.; funding acquisition, M.E.M.
Funding
This research was funded by the University of Turin, local research grant and by Biosfered srl.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the Griffonia simplicifolia extract are available from the authors.
References
- 1.Petkov G., Ramazanov Z. Fatty acids and sterols of Griffonia seeds oil. Grasas Aceites. 2003;54:30–31. doi: 10.3989/gya.2003.v54.i1.273. [DOI] [Google Scholar]
- 2.Irvine F.R. Woody Plants of Ghana. Oxford University Press; Oxford, UK: 1961. [Google Scholar]
- 3.Zhu-Salzman K., Shade R.E., Koiwa H., Salzman R.A., Narasimhan M., Bressan I.A., Hasegawa P.M., Murdock L.L. Carbohydrate binding and resistance to proteolysis control insecticidal activity of Griffonia simplicifolia lectin II. Proc. Natl. Acad. Sci. USA. 1998;95:15123–15128. doi: 10.1073/pnas.95.25.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fellows L.E., Bell E.A. 5-Hydroxy-l-tryptophan, 5-hydroxytryptamine and L-tryptophan-5-hydroxylase in Griffonia simplicifolia. Phytochemistry. 1970;9:2389–2396. doi: 10.1016/S0031-9422(00)85745-3. [DOI] [Google Scholar]
- 5.Freedman R.R. Treatment of menopausal hot flashes with 5-hydroxytryptophan. Maturitas. 2010;65:383–385. doi: 10.1016/j.maturitas.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitahama K., Jouvet A., Fujimiya M., Nagatsu I., Arai R. 5-hydroxytryptophan (5-HTP) uptake and decarboxylation in the kitten brain. J. Neural Transm. 2002;109:683–689. doi: 10.1007/s007020200057. [DOI] [PubMed] [Google Scholar]
- 7.Pranzatelli M.R., Galvan I., Tailor P.T. Human brainstem serotonin receptors: Characterization and implications for subcortical myoclonus. Clin. Neuropharmacol. 1996;19:507–514. doi: 10.1097/00002826-199619060-00004. [DOI] [PubMed] [Google Scholar]
- 8.Esposito M., Precenzano F., Sorrentino M., Avolio D., Carotenuto M. A medical food formulation of Griffonia simplicifolia/magnesium for childhood periodic syndrome therapy: An open-label study on motion sickness. J. Med. Food. 2015;18:916–920. doi: 10.1089/jmf.2014.0113. [DOI] [PubMed] [Google Scholar]
- 9.Muszynska B., Lojewski M., Rojowski J., Opoka W., Sulkowska-Ziaja K. Natural products of relevance in the prevention and supportive treatment of depression. Psychiatr. Polska. 2015;49:435–453. doi: 10.12740/PP/29367. [DOI] [PubMed] [Google Scholar]
- 10.Iovieno N., Dalton E.D., Fava M., Mischoulon D. Second-tier natural antidepressants: Review and critique. J. Affect. Disord. 2011;130:343–357. doi: 10.1016/j.jad.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Emanuele E., Bertona M., Minoretti P., Geroldi D. An open-label trial of L-5-hydroxytryptophan in subjects with romantic stress. Neuroendocrinol. Lett. 2010;31:663–666. [PubMed] [Google Scholar]
- 12.Carnevale G., Di Viesti V., Zavatti M., Zanoli P. Anxiolytic-like effect of Griffonia simplicifolia Baill. seed extract in rats. Phytomedicine. 2011;18:848–851. doi: 10.1016/j.phymed.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Carnevale G., Di Viesti V., Zavatti M., Benelli A., Zanoli P. Griffonia simplicifolia negatively affects sexual behavior in female rats. Phytomedicine. 2010;17:987–991. doi: 10.1016/j.phymed.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Wang X.Z., Wu F.H., Qu W., Liang J.Y. A new beta-carboline alkaloid from the seeds of Griffonia simplicifolia. Chin. J. Nat. Med. 2013;11:401–405. doi: 10.3724/SP.J.1009.2013.00401. [DOI] [PubMed] [Google Scholar]
- 15.Lemaire P.A., Adosraku R.K. An HPLC method for the direct assay of the serotonin precursor, 5-hydroxytrophan, in seeds of Griffonia simplicifolia. Phytochem. Anal. 2002;13:333–337. doi: 10.1002/pca.659. [DOI] [PubMed] [Google Scholar]
- 16.Koppisetti G., Siriki A., Sukala K., Subbaraju G.V. Estimation of L-5-hydroxytryptophan in rat serum and Griffonia seed extracts by liquid chromatography-mass spectrometry. Anal. Chim. Acta. 2005;549:129–133. doi: 10.1016/j.aca.2005.06.006. [DOI] [Google Scholar]
- 17.Kim K.S., Juliani H.R., Bucuk M., Acquaye D., Asante-Dartey J., Wu Q.L., Simon J.E. Quality control and 5-HTP (5-Hydroxy-L-tryptophan) Analysis of Griffonia (Griffonia simplicifolia (DC.) Baill.) seed accessions collected in Ghana. In: Juliani H.R., Simon J.E., Ho C.T., editors. African Natural Plant Products: New Discoveries and Challenges in Chemistry and Quality. Volume 1021. American Chemical Society; Washington, DC, USA: 2009. pp. 381–390. [Google Scholar]
- 18.Lin Y.H., Sun X.X., Yuan Q.P., Yan Y.J. Engineering bacterial phenylalanine 4-hydroxylase for microbial synthesis of human neurotransmitter precursor 5-hydroxytryptophan. ACS Synth. Biol. 2014;3:497–505. doi: 10.1021/sb5002505. [DOI] [PubMed] [Google Scholar]
- 19.Hara R., Kino K. Enhanced synthesis of 5-hydroxy-L-tryptophan through tetrahydropterin regeneration. AMB Express. 2013;3 doi: 10.1186/2191-0855-3-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belongia E.A., Hedberg C.W., Gleich G.J., White K.E., Mayeno A.N., Loegering D.A., Dunnette S.L., Pirie P.L., Macdonald K.L., Osterholm M.T. An investigation of the cause of the eosinophilia-myalgia syndrome associated with tryptophan use. N. Engl. J. Med. 1990;323:357–365. doi: 10.1056/NEJM199008093230601. [DOI] [PubMed] [Google Scholar]
- 21.Mayeno A.N., Lin F., Foote C.S., Loegering D.A., Ames M.M., Hedberg C.W., Gleich G.J. Characterization of Peak-E, a novel amino-acid associated with eosinophilia-myalgia-syndrome. Science. 1990;250:1707–1708. doi: 10.1126/science.2270484. [DOI] [PubMed] [Google Scholar]
- 22.Goda Y., Suzuki J., Maitani T., Yoshihira K., Takeda M., Uchiyama M. 3-Anilino-l-alanine, structural determination of UV-5, a contaminant in EMS-associated l-tryptophan samples. Chem. Pharm. Bull. 1992;40:2236–2238. doi: 10.1248/cpb.40.2236. [DOI] [PubMed] [Google Scholar]
- 23.Gnavi G., Bertea C.M., Maffei M.E. PCR, sequencing and PCR-RFLP of the 5S-rRNA-NTS region as a tool for the DNA fingerprinting of medicinal and aromatic plants. Flavour Fragr. J. 2010;25:132–137. doi: 10.1002/ffj.1970. [DOI] [Google Scholar]
- 24.Cheng T., Xu C., Lei L., Li C.H., Zhang Y., Zhou S.L. Barcoding the kingdom Plantae: New PCR primers for ITS regions of plants with improved universality and specificity. Mol. Ecol. Res. 2016;16:138–149. doi: 10.1111/1755-0998.12438. [DOI] [PubMed] [Google Scholar]
- 25.Coelho A.G., Aguiar F.P.C., de Jesus D.P. A Rapid and simple method for determination of 5-hydroxytryptophan in dietary supplements by capillary electrophoresis. J. Braz. Chem. Soc. 2014;25:783–787. doi: 10.5935/0103-5053.20140029. [DOI] [Google Scholar]
- 26.Babu S.K., Ramakrishna T., Subbaraju G.V. HPLC estimation of 5-hydroxytryptophan in Griffonia simplicifolia extracts. Asian J. Chem. 2005;17:506–510. [Google Scholar]
- 27.Klarskov K., Johnson K.L., Benson L.M., Cragun J.D., Gleich G.J., Wrona M., Jiang X.R., Dryhurst G., Naylor S. Structural characterization of a case-implicated contaminant, “Peak X,” in commercial preparations of 5-hydroxytryptophan. J. Rheumatol. 2003;30:89–95. [PubMed] [Google Scholar]
- 28.Youssef D.T.A. Hyrtioerectines A-C, cytotoxic alkaloids from the Red Sea sponge Hyrtios erectus. J. Nat. Prod. 2005;68:1416–1419. doi: 10.1021/np050142c. [DOI] [PubMed] [Google Scholar]
- 29.Sato F., Hagiwara Y., Kawase Y. Subchronic toxicity of 3-Phenylamino alanine, an impurity in l-Tryptophan reported to be associated with eosinophilia-myalgia-syndrome. Arch. Toxicol. 1995;69:444–449. doi: 10.1007/s002040050197. [DOI] [PubMed] [Google Scholar]
- 30.Murray M.T. 5-HTP: The Natural Way to Overcome Depression, Obesity, and Insomnia. Bantam Books; New York, NY, USA: 1998. [Google Scholar]
- 31.Mannino G., Occhipinti A., Maffei M.E. Quantitative determination of 3-O-Acetyl-11-Keto-beta-Boswellic Acid (AKBA) and other boswellic acids in Boswellia sacra Flueck (syn. B. carteri Birdw) and Boswellia serrata Roxb. Molecules. 2016;21:1329. doi: 10.3390/molecules21101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christie W.W., Han X. Lipid Analysis. 4th ed. Woodhead Publishing; Oxford, UK: 2010. [Google Scholar]
- 33.Mannino G., Gentile C., Maffei M.E. Chemical partitioning and DNA fingerprinting of some pistachio (Pistacia vera L.) varieties of different geographical origin. Phytochemistry. 2019;160:40–47. doi: 10.1016/j.phytochem.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Capuzzo A., Maffei M.E. Molecular fingerprinting of some Mentha species by sequencing and RFLP analysis of the 5S-rRNA non-transcribed spacer region. Plant Biosyst. 2014;148:683–690. doi: 10.1080/11263504.2013.790853. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.