Abstract
Manipulations capable of breaking host tolerance to induce tissue-specific T cell-mediated inflammation are of central importance to tumor immunotherapy and our understanding of autoimmunity. We demonstrate that androgen ablative therapy induces profuse T cell infiltration of benign glands and tumors in human prostates. T cell infiltration is readily apparent after 7–28 days of therapy and is comprised predominantly of a response by CD4+ T cells and comparatively fewer CD8+ T cells. Also, T cells within the treated prostate exhibit restricted TCR Vβ gene usage, consistent with a local oligoclonal response. Recruitment/activation of antigen-presenting cells in treated prostate tissues may contribute to local T cell activation. The induction of T cell infiltration in prostate tissues treated with androgen ablation may have implications for the immunotherapeutic treatment of prostate cancer as well as other hormone-sensitive malignancies, including breast carcinoma.
Keywords: adenocarcinoma of the prostate‖CD4+ and/or CD8 + T cell‖cytotoxic T cell‖antigen-presenting cell‖hormone therapy
Distinct from most other organs, the prostate undergoes its functional differentiation after the onset of puberty (1, 2). This metamorphosis from a predominantly fibrous organ to one that is largely replaced by glandular epithelial cells is developmentally regulated by androgen (3). Interestingly, despite this sudden and relatively late intrusion by specialized epithelial cells elaborating prostate-specific proteins, the host immune system remains largely indifferent to the prostate. Although the mechanism governing this unresponsive state is unknown, several lines suggest that the prostate is precariously balanced between a tolerigenic state and a state in which it becomes an immunologic target. For instance, the high incidence of inflammatory disorders of the prostate that occur in the absence of an identifiable pathogen has led some to conclude that the prostate is predisposed to autoimmune disease (4, 5). Further, that responses against cancerous and/or normal prostate tissues in both animals (6–11) and humans (12–14) can be immunotherapeutically induced suggests that immune unresponsiveness to the prostate is governed by less stringent and reversible mechanisms for maintaining tolerance, such as T cell anergy or ignorance.
Our current study, which examines the potential of androgen withdrawal (androgen ablation) to induce T cell-mediated responses within the prostate, has been prompted by several observations. Androgen ablation, a standard palliative treatment for patients with advanced prostate cancer, induces rapid involution of normal and hormone-dependent cancerous prostate tissues at both primary and metastatic sites. Given that this regression has been attributed to the death or injury of androgen-dependent epithelial cells (15, 16), it was of interest to explore whether such events might be sufficient to prime a T cell-mediated response that ultimately targets prostate cells of epithelial origin. Supporting this possibility, mononuclear cell infiltration has been reported to be a consistent finding in cancerous prostates removed after 3–9 months of androgen ablative treatment (17–19). Burrows and Kennaway (20) and others (21, 22) have additionally shown that administration of estrogen, which ultimately abrogates host androgen production, induces inflammation in the rat prostate. To date, however, it has not been determined whether inflammation that arises within the prostate during androgen ablation represents a nonspecific response to tissue injury or, perhaps, a restricted response by T cells.
In the present study, we show that androgen ablation induces prominent T cell infiltration of the human prostate, particularly at glandular (normal epithelial) and tumor sites. T cell infiltration is readily apparent 7–28 days after treatment and is comprised predominantly of CD4+ T cells and comparatively fewer numbers of CD8+ T cells. Moreover, prostate-infiltrating T cells exhibit restricted TCR Vβ usage, consistent with a local oligoclonal response. This induction of restricted T cell responses within prostate tissues might encompass a repeal of host tolerance to hormone-sensitive tissues triggered on hormone withdrawal. Such T cell-mediated inflammation raised by hormone manipulation might have significant implications for the development of immunotherapeutic strategies to treat prostate cancer as well as other hormone-sensitive tumors, including breast carcinoma.
Materials and Methods
Patient Accrual and Treatment.
Patients in this prospective and randomized study were accrued from the general urologic population of Loyola University Medical Center between April 1999 and April 2000. The purpose of this Institutional Review Board-approved study is to examine immunologic, molecular, and histologic changes in the prostate after short-term androgen ablative therapy. Patients were selected on the basis of their ability to meet study inclusion criteria, including: age of <70, biopsy-proven clinical stages T1–T2b prostate adenocarcinoma, no prior hormone therapy, and no prior history of immunosuppressive medications or disease. After informed consent and by using a rotating randomization scheme, patients were assigned to receive 0, 7, 14, 21, or 28 days of androgen ablative therapy before radical prostatectomy. Androgen ablative treatment included the administration of two standard agents; an androgen receptor blocker (flutamide, 250 mg orally three times per day), and a single i.m. injection of a gonadotropin-releasing hormone supragonist (leuprolide acetate 7.5 mg depot), which inhibits testosterone production for 1 month by down-regulating pituitary luteinizing hormone secretion. During treatment, patients were monitored for compliance and drug-related toxicities on a weekly basis.
Tissue Procurement and Handling.
On procurement in the operating room, prostatectomy specimens were immediately transferred into a sterile ice-cold slurry of normal saline. Specimens were accessioned by surgical pathology and then processed within a biohazard cabinet by using sterile technique. Initial processing of the specimen included marking the fresh prostate for orientation with sterile inks. Prostates were cut transaxially at 0.5-cm intervals. Alternating prostate slices, including the apex and bladder-neck margin, were retained by surgical pathology for completion of staging. Remaining prostate tissues were divided equally and then immediately snap-frozen in liquid nitrogen to facilitate immunohistochemical analysis. Processing of all tissues was typically completed within 30 min of prostate removal. Tissues were maintained at 4°C until final processing.
Immunohistochemical Staining of Lymphocyte Markers and Cytokines in Prostate Tissues.
Prostate cryosections (5 μm, −20°C) were mounted on Superfrost Plus slides (Fisher), air dried, and fixed in ice-cold acetone. Before staining, sections were blocked for 30 min with 3% serum (VectaStain, Vector Laboratories) and 0.1% BSA in PBS. After blocking, serial sections were incubated for 1 h with monoclonal antibodies (typically mouse anti-human IgG) specific for human T cell CD3, CD4, CD8, macrophage CD68, B cell CD19 (B-D, San Jose, CA), cytotoxic granule-associated protein TIA-1 (Immunotech-Coulter, Miami, Fl), interferon (IFN)-gamma, IFN-γ (R & D Systems), interleukin-4, IL-4, B7.1 costimulatory ligand CD80 (B-D), IL-2 receptor CD25 (Dako), activated dendritic cell CD83 or B7.2 costimulatory ligand CD86 (PharMingen), or corresponding concentrations of irrelevant isotype-matched (control) antibody. After washing, sections were incubated for 30 min with corresponding biotin-conjugated secondary antibody, washed, and then incubated for another 30 min with horseradish peroxidase–streptavidin complex. After a final wash step, sections were developed in amino ethyl carbazole (Sigma) and counterstained with hematoxylin. Hematoxylin and eosin (H&E) staining of cryosections to facilitate routine light microscopic examination of prostate tissues was performed by using standard methods.
Scoring of Prostate Tissues for Levels of Marker- and Cytokine-Positive Cells.
Scoring of prostate tissues was performed by a panel of tissue reviewers (M.M., B.K.B., E.S.Y.P., and E.D.K.), including one pathologist (E.M.W.), all of whom were totally blinded to patient treatment. After immunohistochemical staining, numbers of marker- or cytokine-positive immune cells in prostate tissues were determined by randomly photographing 10 nonoverlapping 20 × fields in each of three separate prostate specimens obtained from the left, right, and middle posterior portion (peripheral zone) of each patient's prostate. Three spatially separated prostate specimens were used to ensure adequate representation of tumor and benign tissues for comparison and to reduce sampling error. Hence, mean numbers of marker- or cytokine-positive cells for each patient were calculated from a total of 30 photographed regions and these values converted to cells/mm2 prostate tissue. To determine numbers of infiltrating cells at tumor sites, cryosections were first stained by H&E to confirm the presence of tumor within a particular tissue specimen. Subsequently, serial sections were stained and numbers of CD3+, CD4+, or CD8+ cells within tumor sites quantified by using the method described above. Percentages of T cells expressing CD25 (IL-2 receptor), TIA-1, IFN-γ or IL-4, were estimated by counting marker-positive cells within a specified CD3-infiltrated region, and then dividing that number by the total number CD3+ cells in that same region.
Vβ CDR3 Size Spectratyping of Prostate-Infiltrating and Circulating T Cells.
To assess the TCR Vβ repertoire of T cells within the prostate, T cell-infiltrated regions within cryosectioned prostatic tissues were first isolated by laser capture microdissection. Laser-captured tissues were used, in lieu of whole prostate tissues, to reduce background that might arise from the inclusion of T cells circulating within the prostate. Briefly, regions heavily infiltrated by T cells were identified by CD3+ staining of an initial prostate cryosection specimen. Corresponding infiltrated regions were captured from 10 consecutive tissue sections by using a PixCell infrared diode laser capture microscope (Arcturus, Mountain View, CA). RNA extracted from these lymphocyte specimens was amplified by using a high-fidelity method (23). RNA from infiltrating lymphocytes as well as peripheral blood mononuclear cells from the same patient was reverse transcribed by using oligo(dT) primer. PCR amplification of cDNAs used [32P]-labeled constant primer and primers specific for Vβ families 1, 2, and 3 (24). A total of 30 cycles of amplification were performed by using hot-start PCR. PCR products were separated in a 6% denaturing sequencing gel and visualized/quantified by PhosphorImager analysis. Vβ heterogeneity and relative amounts of V(D)J (CDR3) recombination products for infiltrating T cells were compared against circulating T cells by normalizing all CDR3 peak intensities against the most frequent CDR3 product in a given Vβ family from T cells in the blood. For each set of patient samples analyzed (i.e., prostate tissue and patient-matched peripheral blood mononuclear cell), Vβ analyses were routinely performed by using triplicate assays repeated on three separate occasions. Additionally, for each prostatectomy specimen analyzed, individual spectratype profiles were generated from T cells recovered from at least four widely spaced locations within each specimen (typically emanating from the anterior, central, and left and right posterior zones of each prostate).
Statistical Evaluations.
Mean values for marker/cytokine-positive cells in prostate tissues treated for 7, 14, 21, or 28 days with androgen ablation were compared against untreated (0 day) control prostates by independent Student's t test analysis (graphpad instat, Ver. 3.01, GraphPad, San Diego, CA). Similarly, t test analysis was used to compare the mean frequency of TCR Vβ clonotypes for T cells within prostate tissues against those in the blood of androgen-ablated subjects. P values of ≤0.05 were considered significant.
Results
Patient Information.
A total of 35 patients were randomized into the current study. Two patients were subsequently excluded because of intervening medical conditions that precluded surgery. Of the remaining 33 patients, 7 were randomized to the no-treatment control group. The remaining 26 patients were randomized to receive preoperative androgen ablative therapy for 7 (n = 7), 14 (n = 7), 21 (n = 5), or 28 (n = 7) days. All patients completed therapy per protocol.
Androgen Ablative Therapy Results in Prostate Benign Gland and Tumor Infiltration by CD4+ and CD8+ T Cells and Antigen-Presenting Cells (APCs).
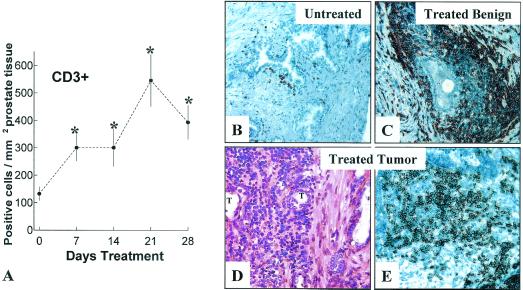
The mean number of CD3+ T cells in untreated (control) prostate specimens analyzed in our current study is 127 ± 18 (CD3+ cells/mm2 ± SD). This control value is in close agreement with a previously reported mean value for T cell amounts within untreated cancer-containing prostatectomy specimens (25). As depicted in Fig. 1A, mean CD3+ T cell levels within prostate tissues treated with androgen ablation for 7 or 14 days are >2-fold greater than the control value of T cells (P = 0.027) within untreated prostate tissues (Fig. 1B). At day 21 of therapy, mean T cell numbers within the prostate exceed the mean control value by nearly 5-fold (P = 0.0013). At day 28 of therapy, T cell levels appear to decline slightly from the 21-day value but remain well above the control value (P = 0.035). That androgen ablative therapy induces a primarily T cell-mediated inflammatory process within the prostate is further supported by the complete absence of neutrophils and near absence of CD19+ (Fig. 1B) cells in treated tissues (<2 cells/mm2 for all tissues studied; data not shown). Finally, inspection of H&E-stained sections of both treated and untreated prostatic urethra and the seminal vesicles reveals essentially no evidence of mononuclear cell infiltration, suggesting that androgen ablative therapy does not raise inflammatory responses in these nonprostatic tissues.
Figure 1.
Androgen ablation induces CD3+ T cell infiltration of the prostate at glandular and intratumoral sites. (A) Plot of CD3+ T cells in prostate tissues from patients treated for 0, 7, 14, 21, or 28 days with androgen ablation. T cells levels within the prostate were quantified as described in Materials and Methods. The vertical axis represents of CD3+ cells per mm2 prostate tissue (mean ± SD). The horizontal axis represents days of treatment. *, P < 0.05, treated tissues compared with untreated (0 day) controls. (B) CD3+ cells within an untreated prostate adjacent to a benign gland. (C) Prominent accumulation of CD3+ cells around a benign gland after 21 days of therapy. (D) H&E section of androgen-ablated tissues reveals profuse mononuclear cell infiltration of tumor sites (T) at 21 days of therapy. (E) Immunochemical staining of similar tumor site reveals intense infiltration by CD3+ cells. (×400.)
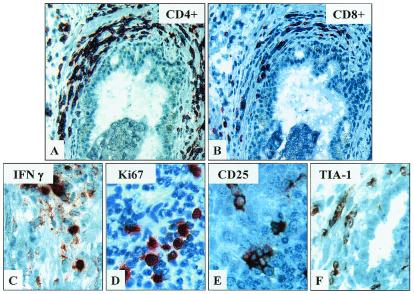
Analysis of epithelial sites within prostatic tissues reveals that androgen ablation not only induces T cell infiltration around benign acinar glands (Fig. 1C) but also promotes T cell infiltration of prostate tumor sites (Fig. 1 D and E). Independent analysis of malignant sites within treated and untreated prostate tissues demonstrates that androgen ablation increases tumor levels of T cells by >6-fold (mean number of tumor infiltrating T cells/mm2 of prostate tissue ± SD: untreated tissues, 54 ± 7 versus 21-day treated tissues, 339 ± 15; P = 0.009). In contrast, CD3+ T cell numbers within the prostatic stroma remain relatively low and unaffected by treatment (data not shown). T cell subset staining further reveals that androgen ablation induces a response predominantly by CD4+ T cells (Fig. 2A) and a lesser response by CD8+ T cells (Fig. 2B). By 21 days of treatment, CD4+ levels increase by ≈5-fold, and CD8+ levels increase by ≈2-fold in treated tissues compared with untreated prostate tissues (≈90 CD4+ and ≈40 CD8+ T cells per mm2 of untreated prostate tissue). Hence, by 21 days of treatment, the ratio of CD4/CD8 T cells is >5:1. In contrast, the ratio of CD4/CD8+ T cells in untreated prostatic tissues is ≈2:1, similar to that for T cells in circulation.
Figure 2.
T cells infiltrates within treated prostate tissues are comprised predominantly of CD4+ and a lesser amount of CD8+ T cells, including cells exhibiting markers of activation and cytotoxic potential. Immunochemical staining of CD3+ T cell-rich prostate infiltrates within treated prostate tissues demonstrates relatively greater numbers of CD4+ (A) compared with CD8+ T cells (B). Figures are at final ×400. Further staining of these sites reveals moderate numbers of cells expressing activation markers, IFN-γ (C), proliferative marker, Ki67 (D), IL2 receptor, CD25 (E), and cytotoxic granule-associated protein, TIA-1 (F). (×800.)
Further immunochemical analysis of CD3+ T cell-rich infiltrates within treated prostate tissues reveals that ≈65% of mononuclear cells comprising these infiltrates express IFN-γ (Fig. 2C). Additionally, a high proportion of these cells express the proliferative marker, Ki67, activation marker CD25 (IL2 receptor) and/or cytotoxic marker, TIA-1 (Fig. 2 D–F, respectively). In contrast, <1% of mononuclear cells within either treated or untreated prostate tissues express IL-4, and little or no CD25, TIA-1, or Ki67 is expressed by mononuclear cells residing within untreated tissues (data not shown). Collectively, these data indicate that androgen ablation not only triggers infiltration of the prostate by predominantly CD4+ and a lesser number of CD8+ T cells but also promotes a locally proliferative cellular inflammatory response that appears polarized toward IFN-γ production.
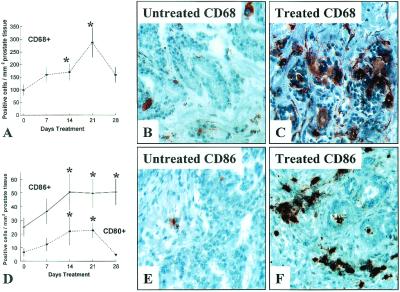
Given that APCs play a central role in the induction of antigen-specific T cell activation (26–28), prostate tissues were additionally examined for levels of two APCs, macrophages, and dendritic cells (DC). As is depicted in Fig. 3A, androgen ablative therapy causes an increase in levels of CD68+ macrophages (Fig. 3C) as well as CD83+ DC (data not shown) within treated prostate tissues relative to untreated tissues (Fig. 3B). At day 21 of therapy, the mean level of CD68+ macrophages in treated prostate tissues exceeds the control value by ≈3-fold (Fig. 3A, P = 0.024). Prostate levels of cells expressing costimulatory ligands CD80+ (B7.1) (Fig. 3D) and CD86+ (B7.2) (Fig. 3 D–F) also increase in response to treatment consistent with an increased presence of competent (activated) APCs within treated tissues. Together, these data indicate a potential role for APCs in mediating T cell activation within the prostate.
Figure 3.
Androgen ablation induces infiltration of prostate tissues by APCs. Immunochemical staining reveals an increase in numbers of CD68+ macrophages (A) within androgen ablation-treated prostate tissues. CD68+ macrophages in control tissues (B) and prostate tissues treated for 21 days (C). Further staining reveals increased numbers of cells expressing CD80+ (B7.1) and CD86+ (B7.2) within treated prostate tissues (D) relative to untreated control tissues. CD86+ cells in control tissues (E) and prostate tissues treated for 21 days (F). The vertical axes represents mean ± SD of CD68+, CD80+, or CD86+ cells per mm2 of tissue. The horizontal axis represents duration of androgen ablative treatment. *, P < 0.05 for treated tissues compared with untreated (0 day) control tissues. (×400.)
T Cells Infiltrating the Androgen-Ablated Prostate Exhibit Restricted TCR Vβ Gene Usage.
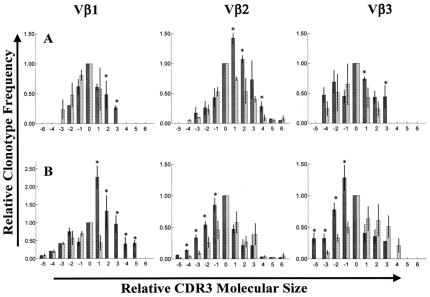
That T cells represent the predominant inflammatory cell within the treated prostate, and that many of these cells appear activated, suggest that androgen ablation might promote local antigen-specific T cell activation. To test this possibility, we used TCR Vβ spectratyping to compare Vβ gene usage by T cells from the prostate and blood of the same individual. This technique generates a profile or “spectratype” of the V(D)J (CDR3)-region recombination products that collectively define a given Vβ-chain family and that individually determine the antigen-specificity of a particular Vβ subunit. Therefore, populations of T cells responding to specific antigen(s) within the prostate might exhibit restricted Vβ gene usage relative to T cells in the blood.
To date, we have performed Vβ spectratyping on T cells recovered from prostate and blood specimens of six androgen-ablated subjects accrued into this study. Because of restricted tissue availability, our present study was limited to an evaluation of TCR Vβ families 1, 2, and 3, because these families are typically well represented in the bloods of most individuals. By using our spectratyping approach, we have established that individual clonotype (CDR3) values within a given tissue sample (analyzed in triplicate and on three separate occasions) vary by ≈±10% (SD), supporting that our method is capable of producing reasonably consistent spectratypes (Fig. 4A). Moreover, prostate T cell spectratypes from all six patients analyzed to date have revealed “skewing” of Vβ clonotype frequencies relative to Vβ clonotype frequencies exhibited by patient-matched circulating T cells (Fig. 4A). For all six patient tissue sets analyzed to date, statistically significant skewing of spectratypes occurred in at least one of the three Vβ families surveyed. Most significantly, in all six patients (six of six), spectratypes of T cells recovered from widely separated locations within the same prostatectomy specimen (i.e., T cells from the anterior, central, and left and right posterior peripheral zones of the prostate) yielded highly concordant patterns of spectratype skewing (Fig. 4B). Collectively, these data support that T cells infiltrating treated prostate tissues exhibit restricted Vβ gene usage, suggesting the induction by androgen ablation of oligoclonal T cell responses uniformly throughout the prostate.
Figure 4.
(A and B) Comparison of TCR Vβ clonotype frequencies for prostate-infiltrating and circulating T cells recovered from patients treated with androgen ablative therapy. Histograms in A and B depict the relative frequency of TCR clonotypes (CDR3 products) within Vβ-chain families 1, 2, and 3 for prostate-infiltrating T cells (black bars) relative to patient-matched circulating T cells (hatched bars). Vertical axes represent mean frequencies of Vβ clonotypes (±SD) normalized against a predominant clonotype within each Vβ family for circulating T cells (arbitrarily assigned an x value of 0.0 and y value of 1.0). Horizontal axes represent relative molecular size of CDR3 products within each Vβ family. Data are mean values generated from triplicate assays repeated on three separate occasions. For all histograms, * denotes prostate T cell clonotypes that are significantly increased (P < 0.05) relative to corresponding circulating T cell clonotypes. A demonstrates significant skewing of Vβ spectratypes, relative to circulating T cell spectratypes, for T cells within a single prostate specimen from a representative androgen-ablated subject. B represents averaged Vβ spectratypes for T cells recovered from four widely separated locations (central, anterior, and left and right posterior) within the prostatectomy specimen of a second androgen ablated subject. Fairly concordant patterns of spectratype skewing throughout the prostate are evidenced by the relatively small variation (SD) in individual clonotype frequencies. Similarly, concordant patterns of prostate T cell spectratype skewing for Vβs 1, 2, and/or 3 have also been observed throughout the prostate tissues of 5/5 additional androgen ablated subjects analyzed to date.
Discussion
Systematic studies examining immune responses within regressing human hormone-sensitive tumors or tissues have not been previously reported. This is likely because there is rarely, if ever, an occasion to examine such tissues after initiation of hormone treatment.
In the present study, we demonstrate that androgen ablative treatment of patients with prostate cancer triggers vigorous T cell-mediated inflammation within the prostate. Prostate T cell infiltration is readily apparent between 7 and 28 days of therapy and is comprised of a response predominantly by CD4+ T cells and comparatively fewer numbers of CD8+ T cells. Also, prostate-infiltrating T cells express proliferation/activation markers and exhibit similar patterns of restricted Vβ gene usage despite their recovery from disparate locations throughout the prostate. Collectively, the above observations implicate androgen ablative therapy as a means to induce oligoclonal T cell responses uniformly throughout prostate tissues. Evidence that this T cell response might be directed at cells of prostate epithelial origin is suggested by the preferential accumulation of T cells in proximity to both benign glands and tumor sites within the prostate as well as the absence of a similar inflammatory response within nonprostatic tissues including the prostatic urethra and seminal vesicles. Also, we have recently observed that one of three propagating bulk T cell lines (established from prostate tissues of androgen-ablated subjects) exhibits in vitro reactivity against HLA-matched prostate tumor cell targets providing some, albeit preliminary, evidence that T cells within treated prostate tissues can exhibit tumor-specific activity. Clearly, however, further studies will be required to establish the specificity of T cells that accumulate within the prostate in response to androgen ablative therapy.
A number of mechanisms may contribute to the T cell response that develops within the prostate during androgen ablation. For instance, it is generally accepted that androgen ablation promptly induces apoptosis and injury of hormone-dependent prostate tumor and epithelial cells (15, 16). Because apoptotic bodies are ready targets for phosphatidylserine pathway-mediated phagocytosis and can potentially serve as an efficient source of antigen to prime APC (29–32), androgen ablation might simply enhance in situ APC-mediated prostate antigen presentation. Consistent with this, we demonstrate that tissue levels of two APC types, macrophage and dendritic cells, increase with ablative therapy. This response is paralleled by a rise in CD80 (B7.1)- and CD86 (B7.2)-expressing cells that could conceivably function to induce prostate-specific T cell costimulatory activation. Hence, increasing sources for antigen combined with simultaneously rising levels of competent APC might converge to induce prostate-specific T cell activation. A number of additional nonexclusionary mechanisms whereby androgen withdrawal might facilitate antiprostate immune responses have been reported. These include: disruption of prostate tumor vessels (33) and normal prostate glandular architecture (17–19), which could permit greater immune access to cryptic or sequestered prostate antigens; modulation of locally produced cytokines (34, 35) to provide an environment favoring antigen-specific T cell activation; and/or down-regulation of immune-inhibitory substances, such as zinc, that accumulate in high concentrations within the androgen-intact prostate (36, 37). Finally, that adult prostate development begins at puberty—curiously coinciding with the onset of androgen-mediated regression of both thymic and marrow tissues—suggests a mechanism whereby autoimmune responses against the prostate might be down-regulated by androgen. Given that postpubertal withdrawal of androgen promotes restitution of cellular immune components in animals (38, 39) raises the possibility that restrictions on immune targeting of the prostate might be eliminated on androgen withdrawal. A similar paradigm for hormone-mediated down-regulation of maternal T cell-mediated immunity to protect developing fetal tissues has recently been proposed (40).
That androgen ablation also causes tumor regression at disseminated cancer sites may extend its potential to prime systemic responses against metastases. In support of this, several studies have reported correlation between host immune parameters and the response to androgen ablative treatment (41–44). For instance, increased levels of circulating lymphocytes (41) and reductions in serum anti-inflammatory cytokines (42) after initiation of treatment have both been reported to portend a more favorable outcome during androgen ablation therapy. Additionally, inverse relationships between rates of cancer recurrence and number of infiltrating T cells within tumor tissues (43), as well as macrophage numbers within prostate tumors versus tumor-grade severity (44), imply that host cell-mediated immune responses may act to suppress prostate cancer progression. Because a minor fraction of advanced prostate cancer patients experience exceptionally long intervals of survival after androgen ablative treatment, it will be of significant interest to determine whether longer survival for this subpopulation of prostate cancer patients results from prostate-specific T cell-mediated immunity that is induced by androgen ablative therapy.
The importance of CD4+ T cells (45), a type-1 (IFN-γ) intratumoral microenvironment (46), and tissue-specific immunity (47) for the induction of optimal responses to antitumoral immunotherapy collectively suggest that T cell responses raised by androgen ablation might be particularly amenable to immunotherapeutic potentiation. We and others have reported that manipulations of the T cell costimulatory pathway, particularly in vivo antibody-mediated blockade of the T cell inhibitory CTLA-4 receptor (CTLA-4 blockade), can potentiate T cell-mediated responses against a number of murine tumors (48) including TRansgenic Adenocarcinoma of the Mouse Prostate (TRAMP) tumors (9–11, 49, 50). It has further been shown that manipulations that boost host antigen presentation to facilitate tumor-specific costimulatory T cell activation (i.e., granulocyte/macrophase colony-stimulating factor tumor cell vaccination) act synergistically with CTLA-4 blockade (11, 51–54). That androgen ablative therapy promotes robust and restricted T cell-mediated responses within human prostate tissues suggests a potential for this form of therapy to prime prostate-specific T cell responses that might be synergistically enhanced by CTLA-4 blockade to treat prostate cancer.
Acknowledgments
We thank Donna Enge, Linda Dee, Patricia Simms, and Jeff Panella for expert technical assistance. This work has been supported, in part, by funding provided by National Institutes of Health/National Cancer Institute Grant CA 82185 (E.D.K.), Department of Defense Grants PC991568 (E.D.K) and PC970131 (W.M.K), and by CaPCURE (E.D.K.). We also thank TAP Pharmaceuticals and Schering-Plough for providing medications to support our studies. E.D.K. is a past American Foundation for Urologic Disease Scholar and American Cancer Society Clinical Oncology Fellow.
Abbreviations
- APC
antigen-presenting cell
- IFN
interferon
- H&E
hematoxylin/eosin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Andrews G S. J Anat. 1951;85:44–54. [PMC free article] [PubMed] [Google Scholar]
- 2.Glenister T W. J Anat. 1962;96:443. [PMC free article] [PubMed] [Google Scholar]
- 3.Partin A W, Coffey D S. In: Cambell's Urology. 7th Ed. Walsh P C, Retik A B, Vaughan E D Jr, Wein A J, editors. Philadelphia, PA: Saunders; 1998. [Google Scholar]
- 4.Alexander R B, Brady F, Ponniah S. Urology. 1997;50:893–899. doi: 10.1016/S0090-4295(97)00456-1. [DOI] [PubMed] [Google Scholar]
- 5.Ponniah S, Arah I, Alexander R B. Prostate. 2000;44:49–54. doi: 10.1002/1097-0045(20000615)44:1<49::aid-pros7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Sanda M G, Ayyagari S R, Jaffee E M, Epstein J I, Clift S L, Cohen L K, Dranoff G, Pardoll D M, Mulligan R C, Simons J W. J Urol. 1994;151:622–628. doi: 10.1016/s0022-5347(17)35032-2. [DOI] [PubMed] [Google Scholar]
- 7.Vieweg J, Rosenthal F M, Bannerji R, Heston W D W, Rair W R, Gansbacher B, Gilboa E. Cancer Res. 1994;54:1760–1765. [PubMed] [Google Scholar]
- 8.Fong L, Ruegg C L, Brockstedt D, Engleman E G, Laus R. J Immunol. 1997;159:3113–3117. [PubMed] [Google Scholar]
- 9.Kwon E D, Hurwitz A A, Foster B A, Madias C, Greenberg N M, Burg M B, Allison J P. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon E D, Foster B A, Hurwitz A A, Madias C, Allison J P, Greenberg N M, Burg M B. Proc Natl Acad Sci USA. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurwitz A A, Foster B A, Kwon E D, Truong T, Choi E M, Greenberg N M, Burg M B, Allison J P. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 12.Simons J W, Mikhak B, Chang J F, DeMarzo A M, Carducci M A, Lim M, Weber C E, Baccala A A, Goemann M A, Clift S M, Ando D G, et al. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 13.Small E J, Reese D M, Um B, Whisenant S, Dixon S C, Figg W D. Clin Cancer Res. 1999;5:1738–1744. [PubMed] [Google Scholar]
- 14.Salgaller M L, Tjoa B A, Lodge P A, Ragde H, Kenny G, Boynton A, Murphy G P. Crit Rev Immunol. 1998;18:109–119. doi: 10.1615/critrevimmunol.v18.i1-2.120. [DOI] [PubMed] [Google Scholar]
- 15.Isaacs J T, Furuya Y, Berges R. Cancer Biol. 1994;5:391–400. [PubMed] [Google Scholar]
- 16.Montironi R, Pomante R, Diamanti L, Magi-Galluzzi C. Urol Int. 1998;60:25–30. doi: 10.1159/000056542. [DOI] [PubMed] [Google Scholar]
- 17.Armas O A, Aprikian A G, Melamed J, Cordon-Cardo C, Cohen D W, Erlandson R, Fair W R, Reuter V E. Am J Surg Pathol. 1994;18:979–991. doi: 10.1097/00000478-199410000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Grignon D J, Sakr W A. Cancer. 1995;75:1837–1841. [Google Scholar]
- 19.Montironi R, Schulman C C. J Clin Pathol. 1998;51:5–12. doi: 10.1136/jcp.51.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burrows H, Kennaway N M. Am J Cancer. 1934;20:48. [Google Scholar]
- 21.Robinette C L. Prostate. 1998;12:271–286. doi: 10.1002/pros.2990120310. [DOI] [PubMed] [Google Scholar]
- 22.Naslund M J, Strandberg J D, Coffey D S. J Urol. 1988;140:1049–1053. doi: 10.1016/s0022-5347(17)41924-0. [DOI] [PubMed] [Google Scholar]
- 23.Wang E, Miller L, Ohnmacht G, Liu E T, Marincola F M. Nat Biotechnol. 2000;18:457–459. doi: 10.1038/74546. [DOI] [PubMed] [Google Scholar]
- 24.Gorski J, Yassai M, Zhu X, Kissella B, Keever C, Flomenberg N. J Immunol. 1994;152:5109–5119. [PubMed] [Google Scholar]
- 25.Troy A, Davidson P, Atkinson C, Hart D. J Urol. 1998;160:214–219. [PubMed] [Google Scholar]
- 26.Lenschow D J, Walunas T L, Bluestone J A. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 27.Grewal I S, Flavell R A. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 28.Chambers C A, Allison J P. Curr Opin Cell Biol. 1999;11:203–210. doi: 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 29.Rovere P, Vallinoto C, Bondanza A, Crosti M C, Rescigno M, Ricciardi-Castagnoli P, Rugarli C, Manfredi A A. J Immunol. 1998;161:4467–4471. [PubMed] [Google Scholar]
- 30.Albert M L, Sauter B, Bhardwaj N. Nature (London) 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 31.Albert M L, Pearch S F A, Francisco L M, Sauter B, Roy P, Silverstein R L, Bhardwaj N. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronchetti A, Rovere P, Iezzi G, Galati G, Heltai S, Protti M P, Garancini M P, Manfredi A A, Rugarli C, Bellone M. J Immunol. 1999;163:130–136. [PubMed] [Google Scholar]
- 33.Jain R K, Safabakhsh N, Sckell A, Chen Y, Jiang P, Benjamin L, Yuan F, Keshet E. Proc Natl Acad Sci USA. 1998;95:10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wikstrom P, Bergh A, Damber J E. Scand J Urol Nephrol. 2000;34:85–94. doi: 10.1080/003655900750016689. [DOI] [PubMed] [Google Scholar]
- 35.Harris M T, Feldberg R S, Lau K M, Lazarus N H, Cochrane D E. Prostate. 2000;44:19–25. doi: 10.1002/1097-0045(20000615)44:1<19::aid-pros3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 36.Stankova L, Drach G W, Hicks T, Zukoski C F, Chvapil M. J Lab Clin Med. 1976;88:640–648. [PubMed] [Google Scholar]
- 37.Leissner K H, Fjelkegard B, Tisell L E. Invest Urol. 1980;18:32–35. [PubMed] [Google Scholar]
- 38.Grossman C J. Science. 1985;227:257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- 39.Ellis T M, Moser M T, Le P T, Flanigan R C, Kwon E D. Int Immunol. 2001;13:553–558. doi: 10.1093/intimm/13.4.553. [DOI] [PubMed] [Google Scholar]
- 40.Tibbetts T A, DeMayo F, Rich S, Conneely O M, O'Malley B W. Proc Natl Acad Sci USA. 1999;96:12021–12026. doi: 10.1073/pnas.96.21.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliver R T D, Gallagher C J G. Cancer Surv. 1995;23:191–207. [PubMed] [Google Scholar]
- 42.Wise G J, Marella V K, Talluri G, Shirazian D. J Urol. 2000;164:722–725. doi: 10.1097/00005392-200009010-00024. [DOI] [PubMed] [Google Scholar]
- 43.Vesalainen S, Lipponen P, Talja M, Syrjanen K. Eur J Cancer. 1994;30A:1797–1803. doi: 10.1016/0959-8049(94)e0159-2. [DOI] [PubMed] [Google Scholar]
- 44.Shimura S, Yang G, Ebara S, Wheeler T M, Frolov A, Thompson TC. Cancer Res. 2000;60:5857–5861. [PubMed] [Google Scholar]
- 45.Mokyr M B, Kalinichenko T, Gorelik L, Bluestone J A. Cancer Res. 1998;58:5301–5304. [PubMed] [Google Scholar]
- 46.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pardoll D M. Proc Natl Acad Sci USA. 1999;96:5340–5342. doi: 10.1073/pnas.96.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leach D R, Krummel M F, Allison J P. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg N M, DeMayo F, Finegold M J, Medina D, Tilley W D, Aspinall J O, Cunha G R, Donjacour A A, Matusik R J, Rosen J M. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurwitz A A, Foster B A, Allison J P, Greenberg N M, Kwon E D. In: Current Protocols in Immunology. Kruisbeek A M, editor. New York: Current Protocol and Wiley; 2001. , in press. [Google Scholar]
- 51.Hurwitz A A, Yu T F-Y, Leach D R, Allison J P. Proc Natl Acad Sci USA. 1998;95:10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Elaas A, Hurwitz A A, Allison J P. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allison J P, Hurwitz A A, Elsas A V, Kwon E D, Sullivan T, Foster B A, Greenberg N M. In: Principles and Practice of the Biologic Therapy of Cancer. 3rd Ed. Rosenberg S A, editor. Lippincott Williams & Wilkins; 2000. pp. 890–895. [Google Scholar]
- 54.Hurwitz A A, Kwon E D, Elsas A V. Curr Opin Immunol. 2000;12:589–596. doi: 10.1016/s0952-7915(00)00147-3. [DOI] [PubMed] [Google Scholar]