Abstract
Current evidence links oxidative stress (OS) to male infertility, reduced sperm motility, sperm DNA damage and increased risk of recurrent abortions and genetic diseases. A review of PubMed, Medline, Google Scholar, and Cochrane review databases of published articles from years 2000–2018 was performed focusing on physiological and pathological consequences of reactive oxygen species (ROS), sperm DNA damage, OS tests, and the association between OS and male infertility, pregnancy and assisted reproductive techniques outcomes. Generation of ROS is essential for reproductive function, but OS is detrimental to fertility, pregnancy, and genetic status of the newborns. Further, there is a lack of consensus on selecting OS test, type, and duration of antioxidants treatment as well as on the target patients group. Developing advanced diagnostic and therapeutic options for OS is essential to improve fertility potential and limit genetic diseases transmitted to offspring.
KEYWORDS: Antioxidants, male infertility, oxidative stress, sperm DNA damage
INTRODUCTION
Infertility is defined as the inability to achieve pregnancy after 1 year of regular unprotected sexual intercourse.[1] The global prevalence of infertility varies between 2.5%–15%, correlating to at least 30 million infertile men worldwide.[2] Infertility has been linked to several emotional, physical, and sociocultural problems.[3] One of the mechanisms proposed for idiopathic male infertility is oxidative stress (OS). Male infertility accounts for about 40% of all cases and it is known that some conditions such as varicocele, cryptorchidism, hypogonadism, and genetic factors can cause infertility. However, no underlying cause can be identified for primary or secondary infertility in approximately 25% of couples which is termed idiopathic infertility.[4] One of the proposed mechanisms for idiopathic infertility is OS and reactive oxygen species (ROS).
Increased ROS along with decreased antioxidant defense result in redox imbalance, reduced sperm motility and sperm DNA damage. Spermatozoa are highly susceptible to the deleterious effects of ROS due to the large amounts of unsaturated fatty acids found in their cell membranes. Reactive oxygen species promote peroxidation of lipids, resulting in intracellular oxidative burden. The sequence of events involves lipid peroxidation, loss of membrane integrity with increased permeability, reduced sperm motility, structural DNA damage, and apoptosis.[5,6,7] Several intrinsic and extrinsic factors have been associated with increased OS in the male reproductive system.
The World Health Organization (WHO) has published references values for seminal fluid analysis parameters.[8] Decreased sperm concentration – defined as < 15 ×106 sperm/ml – is termed oligozoospermia; whereas asthenozoospermia corresponds to progressive sperm motility of <32% or total sperm mobility under 40%. Teratospermia is defined as normal sperm motility of <4% using Kruger's strict criteria.[9] The combination of all these abnormalities is termed oligoasthenoteratozoospermia.
Currently, there is a lack of agreement on which patients should be tested for OS, as well as which test to perform. There are also controversies on types, dose, and duration of antioxidants treatment of in patients with excessive ROS levels.[10] Therefore, the aim of this review is to provide an update on current evidence regarding ROS production, tests and the association between OS and male infertility as well as pregnancy and assisted reproductive techniques (ART) outcomes.
METHODS
This review of literature included a systematic search strategy performed in electronic scientific databases PUBMED, Medline, Google Scholar, and Cochrane review to include published articles from years 2000 to 2018. The search involved keywords including combinations of search terms “oxidative stress,” “reactive oxygen species,” “semen parameters,” “male infertility,” “sperm function,” “antioxidants,” “semen analysis,” “oxidative stress tests,” “pregnancy outcomes,” and “assisted reproductive techniques.” Articles were perused, and their reference lists were checked for relevant publications. We included articles published in English only.
OXIDATIVE STRESS DEFINITION
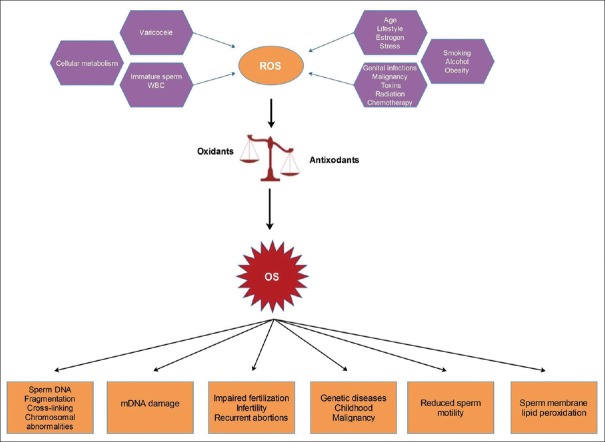
OS is defined as an imbalance between the production of reactive oxygen species (ROS) and the scavenging capacity of available antioxidants resulting in redox paradox.[11] Sperm cells are vulnerable to ROS because of the abundance of polyunsaturated fatty acids in their plasma membrane and cytoplasm[12] and limited antioxidant capacity and DNA repair system.[13] Certain levels of ROS are required for maturation of spermatozoa, acrosome reaction, capacitation, hyperactivation, and sperm-oocyte fusion.[14] Excessive ROS production, however, overwhelms the neutralizing capability of antioxidants (enzymatic and nonenzymatic) in the seminal plasma. ROS are formed as natural byproducts of oxygen during metabolism and have important roles in cell signaling and homeostasis.[15] Sources of ROS can be endogenous or exogenous [Figure 1] and body antioxidant defense mechanism aims to neutralize the harmful effects of these pro-oxidants molecules.
Figure 1.
Sources of reactive oxygen species in the body and their pathological consequences on semen, fertility and health. ROS = Reactive oxygen species, OS = Oxidative stress
SOURCES OF REACTIVE OXYGEN SPECIES
Intrinsic
Redox reactions in aerobic metabolism yield ROS as byproducts. In mitochondria, these reactions require nicotinamide adenine dinucleotide (NADH) as an electron donor and acceptor in the electron transport chain, which allows synthesis of adenosine triphosphate (ATP)[16] [Figure 1]. Seminal fluid ROS can also originate from cytoplasmic glucose-6-phosphate dehydrogenase.[17] Varicocele, the most common etiology of male infertility, has been linked with the increased oxidative burden and ROS-induced sperm DNA damage;[14] as well as with increased scrotal temperature.[18] These findings have been corroborated with evidence describing reduced levels of seminal lipid peroxidation and sperm DNA damage after varicocelectomy.[18] Moreover, increased temperature has also been related to increased ROS production and negative effects on other seminal fluid parameters.[19,20] In particular, elevated ROS have been associated with male accessory gland infection, including the urethra, prostate, deferent ducts, seminal vesicles, epididymis, or testes.[21] This has been attributed to the capacity of seminal leukocytes to produce 1000-fold more ROS and free radicals than any other cell with aerobic metabolism.[22] Infections have also been independently associated with increased ROS.[23,24] Furthermore, hyperglycemia is another important factor for male infertility, as strong correlations have been identified between prediabetes and diabetes mellitus with increased OS and altered sperm parameters.[25]
Extrinsic
Extrinsic factors such as smoking, alcohol intake, and exposure to radiation and industrial heavy metals have been associated with increased ROS and male infertility [Figure 1]. Smoking has been associated with reduced sperm concentration, motility, and altered morphology.[26] Smoking also elicits a chronic inflammatory response which recruits leukocytes to the genital tract and causes a substantial increase in seminal ROS levels,[27] as well as increased sperm DNA damage.[13]
Seminal fluid abnormalities have been associated with excessive alcohol intake, including decreased spermatogenesis, abnormal sperm morphology, decreased seminal fluid volume, low levels of testosterone, and increased OS.[28] Indeed, alcohol abuse results in increased production of acetaldehyde which promotes the generation of ROS due to its interactions with proteins and lipids.[11]
Altered sperm function and increased DNA damage have been associated with industrial exposure to heavy metals such as lead, cadmium, iron, and copper, as well as exposure to phthalates, pesticides, and pollution.[20,29] Malignancies are another important extrinsic source of ROS, along with the accompanying exposure to radiation and chemotherapy. Men treated with chemotherapy medications such as cisplatin, doxorubicin, or cyclophosphamide have been linked to increased OS.[30] Radiotherapy has also been associated with increased OS, while low-level radiation therapy appears to modulate NADH oxidase activity promoting sperm death.[31,32]
ANTIOXIDANTS IN SEMEN
Both enzymatic and nonenzymatic antioxidant protective systems have been identified in semen, which positively interact with each other to counteract the adverse effects of ROS.
Enzymatic antioxidants
Superoxide dismutase, catalase, and glutathione (GSH) peroxidase form the main antioxidant system in semen.[33] These metalloenzymes are present in both the intracellular and extracellular space. Superoxide dismutase catalyzes the dismutation of the superoxide anion, utilizing the copper and zinc molecules in its active center.[34] There are two main isoforms of superoxide dismutase (SOD) enzyme: SOD-1, with about 75% of the antioxidant, and SOD-3, with the remaining 25%.[35] Seminal SOD activity has been positively correlated with sperm concentration and motility.[36]
Catalase acts on hydrogen peroxide, resulting in its decomposition to water and molecular oxygen. The heme system with an iron atom in the center is the prominent characteristic of this enzyme, which can be found in the cytoplasm, endoplasmic reticulum, and various other organelles.[37] This enzyme is synthesized in the prostrate and catalase activity involves capacitation of nitric oxide and involvement of hydrogen peroxide.[33,38] Catalase level has been positively correlated with progressive sperm motility in normospermic individuals.[39] GSH peroxidase is responsible for the catalytic reduction of hydrogen peroxide and organic peroxides, including the peroxides of phospholipids.[34] This enzyme contains selenium in its active site and is primarily located in the mitochondrial matrix of spermatozoa. A specific isoform protects sperm DNA from oxidative damage and chromatin condensation.[40] GSH peroxidase activity was reduced in individuals diagnosed with severe asthenozoospermia, oligozoospermia, and teratozoospermia.[41]
Nonenzymatic antioxidants
Numerous nonenzymatic antioxidants are present in semen, including Vitamins A, E, C, and B complex, GSH, coenzyme Q10, carnitine, and minerals such as zinc, copper, selenium, and chromium.[42] GSH is a tripeptide thiol with a wide array of biological functions, including the preservation of the intracellular redox status and detoxification of exogenous and endogenous compounds. Structurally, it stems from a combination of three amino acids; cysteine, glycine, and glutamine. GSH possesses an innate reducing power that protects cells against OS, in particular, due to its sulfhydryl group (SH). GSH is found in both its oxidized and reduced forms. The antioxidant mechanisms of GSH are mediated by associated enzymes such as GSH peroxidase and GSH reductase.[43]
Vitamin E or alpha-tocopherol is a fat-soluble molecule found in almonds, avocados, spinach, and sweet potatoes. It has potent antioxidant properties, by neutralizing free radicals and inhibiting ROS damage to cell membranes; resulting in prevention of lipid peroxidation and enhancement of other antioxidants. According to the European Commission Directive 2008/100/EC, the recommended daily intake of Vitamin E is 12 mg.[44] In an interventional placebo-controlled trial on infertile men conducted by Greco et al.,[45] minimal sperm DNA damage was observed after 2-month supplementation of Vitamin E (1 g/day) and Vitamin C (1 g/day).
Vitamin C is a water-soluble vitamin with antioxidant properties found in citric fruits and fresh berries. Numerous studies have evaluated the effect of Vitamin C supplementation on sperm function. A study performed on male rats showed ascorbic acid could revert testicular OS induced by cyclophosphamide.[46] In another study, reduced levels of Vitamin C and increased ROS levels were detected in the seminal fluid of men with asthenozoospermia.[47] Carotenoids are a group of organic compounds found in orange, red, yellow, and pink vegetable dyes, which act as precursors for Vitamin A, whose integral component is retinol. These are naturally occurring antioxidants, necessary for maintaining the integrity of cell membranes. They are also involved in the regulation of spermatogenesis. Carotenoid deficiency can lead to decreased sperm motility and male infertility.[48]
Zinc is the second-most abundant metal in the human body and a cofactor for various enzymes involved in DNA transcription and protein synthesis, playing a pivotal role in reproduction. Zinc participates in various reproductive processes such as steroidogenesis, testicular development, gonadal differentiation, production of luteinizing hormone and follicle stimulating hormone, formation and maturation of spermatozoa, acrosome reaction, and fertilization.[49,50] The WHO currently estimates zinc deficiency to affect one-third of the population worldwide. Zinc, along with various antioxidant enzymes, may improve sperm parameters and increase the likelihood of pregnancy for men with oligoasthenoteratozoospermia.[51] Selenium is another essential trace element, which intervenes in sperm formation and testosterone synthesis.[52] At least 25 selenoproteins have been identified in humans and animals, involved in the maintenance of sperm structural integrity. Several randomized clinical trials have tried selenium in combination with other antioxidants with promising results.[53,54]
PHYSIOLOGICAL ROLE OF REACTIVE OXYGEN SPECIES IN MALE FERTILITY
The development of male germ cells yields significant amounts of ROS which constitutes a principal source of OS in spermatozoa.[55] Reactive oxygen species modulate sperm chromatin condensation by adjusting the number of germ cells and inducing apoptosis or proliferation of spermatozoa.[56] ROS are also involved in the processes of capacitation, acrosome reaction, mitochondrial stability, and sperm motility in mature sperm. ROS can function as messengers, by modulating the NADPH oxidase enzyme complex in the cell membrane, and intervening in the respiratory chain within mitochondria. In spermatozoa, superoxide anion metabolism is regulated by the NADH oxidoreductase enzyme, which works in close conjunction with the mitochondrial respiratory chain and xanthine oxidase found in sperm and seminal plasma.[33] Immature spermatozoa with cytoplasmic residues show increased production of ROS when compared to sperm with normal morphology.[17,57]
Seminal leukocytes are another source of ROS, producing 1000 times more of these molecules than sperm cells under physiological conditions. This is because seminal leukocytes represent the first line of defense against offending infectious agents, using primarily oxidative and inflammatory mechanisms.[22] However, this can become a double-edged sword, as an imbalance between oxidants and antioxidants could result in cellular injury. Indeed, ROS generated to counteract infectious agents can also damage host cells, which can result in the disintegration of the cell membrane or sperm DNA damage.
PATHOLOGICAL EFFECTS OF REACTIVE OXYGEN SPECIES ON MALE FERTILITY
The rationale behind the use of antioxidants for the treatment of male infertility relies on excessive levels of ROS and free radicals cause altered sperm function and sperm DNA damage. A study Desai et al. found that sperm characteristics were significantly lower in infertile men with high levels of ROS in semen as assessed through chemiluminescence.[32] Reactive oxygen species alter DNA integrity in the sperm nucleus by inducing breakage of DNA strands, base modifications, and chromatin cross-linking [Figure 1].[58] Moreover, spermatozoa have limited defense mechanisms against ROS-induced DNA damage.
Human ejaculate contains sperm cells with various degrees of maturity, along with leukocytes, epithelial cells and round cells from different stages spermatogenesis. Among these cells, peroxidase-positive leukocytes and immature spermatozoa produce significant amount of free radicals.[59] Spermatozoa are especially susceptible to oxidative damage due to the presence of abundant polyunsaturated fatty acids in their plasma membrane. These fatty acids are important as they provide membrane fluidity, a key feature for several membrane fusion events such as acrosome reaction and sperm-egg interactions. However, these unsaturated fatty acids render them vulnerable to free radical attacks and ongoing lipid peroxidation.[60]
Nevertheless, in around 85% of cases, the sperm genome is protected from free radical damage as it is bound to central nucleoprotamines.[13] Deficient protamination has been observed in infertile men, representing yet another source of ROS-induced DNA damage[61] which is compounded by the limited capacity for sperm DNA repair seen during spermatogenesis.[62] ROS-mediate disruption of mitochondrial membranes leads to caspase activation, resulting in apoptosis. The apoptotic pathways involve cytochrome c release, which augments the levels of ROS, DNA damage, and apoptosis.[63]
DNA bases are also prone to OS-induced damage with base modifications, strand-breaks, and chromatin cross-linking. Indeed, OS and apoptosis are key events involved in causing DNA damage in the germ line.[64] The major role of ROS in the etiology of sperm DNA damage in infertile men has been corroborated in multiple studies.[65,66,67,68]
Spermatozoa carry a complete haploid genome to the ovum to form a new individual. Condensation of the nuclear material in the sperm nucleus is essential for this process to be successful. This condensation is promoted by the unique process of protamination, which involves the replacement of histones by positively charged protamines, which in turn form tight toroidal complexes. This is essential, as chromatin organization is necessary for fertilization and early embryonic development.[69] However, normal sperm appears to possess varying degrees of fragmented DNA; although, infertile men appear to have larger proportions of fragmented DNA.[70]
Both extrinsic and intrinsic factors are involved in the pathogenesis of fragmented DNA. The latter include poor chromatin structure and limited repair capacity. Intrinsic factors include abortive apoptosis and defective maturation.[71,72] Accumulating evidence suggests extrinsic factors are responsible for the increased DNA fragmentation found in the epididymis and ejaculated sperm in comparison to testicular sperm.[73] Recent research posits OS as another extrinsic cause of sperm DNA fragmentation (SDF),[74] as ROS can surpass the limited antioxidant mechanisms of sperm and damage polyunsaturated fatty acids in membranes, resulting in SDF.[75,76]
SPERM DNA DAMAGE INDUCED BY REACTIVE OXYGEN SPECIES
Although ROS seem to play a physiological role in the acrosome reaction, normal sperm function, activation, motility, and capacitation;[77] their potentially deleterious effects cannot be overlooked. Spermatozoa are especially vulnerable to ROS as they contain large amounts of polyunsaturated fatty acids in their plasma membrane and cytoplasm. OS could induce a rapid loss of intracellular ATP, resulting in axonemal damage with decreased sperm viability and mobility and increased mid-piece structural defects, with deleterious effects on sperm capacitation and the acrosome reaction. Lipid peroxidation of the sperm membrane is a key mediator of ROS-induced sperm damage, leading to infertility [Figure 1].[78,79]
Hydrogen peroxide is the principal ROS in human spermatozoa, while excessive production of ROS by abnormal spermatozoa or leukocytes appears to be associated with male infertility.[75] Moderately elevated concentrations of hydrogen peroxide cause sperm immobilization, mostly through depletion of intracellular ATP and reduced phosphorylation of axonemal proteins, with no impact on viability. In contrast, higher concentrations of hydrogen peroxide promote lipid peroxidation and cell death.[80,81]
In a study by Pasqualotto et al.,[82] the levels of antioxidants in seminal plasma from infertile men were significantly lower than in fertile controls, and the levels of ROS produced by spermatozoa were negatively correlated with sperm quality. In semen of infertile men, pathological levels of ROS are likely to be the result of increased ROS production and impaired antioxidant capacity.[83]
Exogenous or endogenous sources of ROS can induce sperm DNA damage that in turn may cause childhood diseases such as autosomal dominant disorders, neuropsychiatric disorders, and childhood cancers like retinoblastoma.[84,85] OS tends to target on telomeres, which are key genome protectors. Telomeres erode faster when exposed to OS, resulting in telomere dysfunction, chromosome instability, and apoptosis; all of which have been related to aging and carcinogenesis.[86]
This form of DNA damage could be particularly important in recurrent spontaneous abortion (RSA). Various paternal factors have been linked to RSA, including ROS-induced sperm DNA damage. In a study on 25 couples with idiopathic RSA and 25 proven fertile controls, ROS levels and DNA damage were significantly higher among the men in the RSA group.[87] Mitochondrial dysfunction and OS have been associated with cancer, cellular senescence, apoptosis and aging; as well as with isolated cases of asthenozoospermia.[88] Antioxidants may prevent telomere loss and promote genomic stability in cells with mitochondrial dysfunction, corroborating the association with OS. Furthermore, nuclear transfer protected the genomes from telomere dysfunction and reconstitution of the mitochondria, thereby promoting cell survival.[89]
Lipid peroxidation cascade contributes to the production of free radicals and induces the production of lipid aldehydes such as acrolein, 4-hydorxynonenal (4-HNE), and malondialdehyde (MDA).[12] These have been linked with OS and damage to nuclear and mitochondrial DNA, with shorter telomeres, formation of the base product 8-hydroxy-deoxyguanine (8-OHdG), and fragmentation of mitochondrial DNA. They can also affect sperm plasma membranes, thus affecting their motility and ability to fuse with the oocyte. Production of 8-OHdG facilitates DNA damage by limiting the repairing capacity of spermatozoa.[90] Because fragmented DNA carries a high mutagenic potential, the oocyte may skip the base-excision repair and correction of 8-OHdG-associated changes, resulting in genomic hypermutability and instability, as well as infertility.[91] A high incidence of genetic aberrations in embryos have been attributed to ROS-induced OS in the male germ line; in association with conditions such as childhood cancers, neuropsychiatric disorders such as autism and schizophrenia, and dominant gene mutations such as Apert syndrome and achondroplasia.[13]
ANTIOXIDANT THERAPY IN MALE INFERTILITY
The rationale for oral antioxidant therapy is because seminal OS is due to increased ROS production and/or decreased levels of seminal antioxidants.[60] The different oral antioxidants available belong to the exogenous antioxidant category and they include Vitamin C, Vitamin E, coenzyme Q10, N-acetyl cysteine, carnitines, trace elements such as zinc, selenium, pentoxifylline, and a combination of these oral antioxidants. Numerous studies have been conducted to assess the effectiveness of oral antioxidant supplementation for the treatment of male infertility. Most of the studies showed an improvement in one or more of seminal fluid parameters,[92,93] whereas some studies reported no positive effect [Table 1].[94,95,96]
Table 1.
Antioxidant therapy in male infertility
| Author, years | Groups/number of participants | Controlled | Type of antioxidant and dose | Intervention period | Results |
|---|---|---|---|---|---|
| Safarinejad, 2011[97] | Idiopathic oligoasthenoteratozoospermia/211 | Yes | EPA and DHA acids, 1.84 g/day versus placebo | 32 weeks | Increase in total sperm count and concentration. Both EPA and DHA positively correlated with plasma superoxide dismutase and catalase activity |
| Wirleitner et al., 2012[98] | Oligoasthenoteratozoospermia and nonoligoasthenoteratozoospermia/147 | Yes | Fertilovit M-Plus Vitamin C-100 mg Vitamin E-100 mg Folic acid-500 µg Zinc-25 mg Selenium-100 µg N-acetyl L-cysteine 50 mg L-carnitine 300 mg Citrulline 300 mg GSH reductase 50 mg Lycopene 4 mg Co-enzyme Q10 15 mg twice daily |
2 months | Increased sperm concentration and motility. No significant improvement in morphology |
| Safarinejad et al., 2012[99] | Idiopathic oligoasthenoteratozoospermia/228 | Yes | Coenzyme Q10-200 mg per day | 26 weeks | Increased sperm density, motility and morphology. Decreased follicle stimulating hormone activity |
| Safarinejad, 2012[100] | Idiopathic oligoasthenoteratozoospermia/287 | No | Coenzyme Q10-300 mg twice daily | 12 months | Increased mean sperm concentration, progressive motility and normal morphology |
| Busetto et al., 2012[101] | Idiopathic asthenoteratozoospermia/114 | No | L-carnitine-145 mg Acetyl L carnitine-64 mg, fructose 250 mg, citric acid 50 mg Selenium 50 µg CoQ10 20 mg Zinc 10 mg Ascorbic acid 90 mg Cyanocobalamine 1.5 µg Folic acid 200 mcg/once a day |
4 months | Increased progressive sperm motility, and no significant improvement in sperm concentration and morphology |
| Chen et al., 2012[102] | Oligozoospermia and asthenozoospermia/64 and 42 | Yes | Oligospermia Tamoxifen 10 mg bid Tamoxifen 10 mg + Vitamin E 100 mg tid Asthenospermia Levocarnitine 1 bottle bid Levocarnitine 1 bottle bid, Vitamin E 100 mg tid |
3 months | Progressive increase in sperm motility in oligospermic men. There was nonsignificant improvement in sperm motility in asthenozoospermic individuals |
| Cavallini, 2006[8] | Idiopathic oligoasthenoteratozoospermia/55 | Yes | L-carnitine 1 g bid L-carnitine 500 mg bid +30 mg cinnoxicam every 4 days |
3 months | Improvement in morphology and number of spermatozoa. Increased percentage of pregnancy following ICSI. Nonsignificant improvement in the number of fertilised oocytes and embryos transferred |
| Abad et al., 2013[103] | Asthenoteratozoospermia/20 | Yes | L-carnitine-1500 mg Vitamin C-60 mg Coenzyme Q10-20 mg Vitamin E-10 mg Zinc-10 mg Vitamin B9-200 µg Vitamin B12-1 µg Selenium-50 µg |
0 h, 2 h, 6 h, 8 h and 24 h | Increase in sperm concentration, motility, vitality and morhological parameters |
| Nadjarzadeh et al., 2014[104] | Idiopathic oligoasthenoteratozoospermia/60 | Yes | 200 mg/day CoQ10 or placebo |
3 months | Improved semen parameters |
| Raigani et al., 2014[105] | Oligoasthenoteratozoospermia/83 | Yes | Folic acid (5 mg/day) Zinc sulfate (220 mg/day) or placebo |
16 weeks | Increased sperm concentration with combined treatment |
| Hadwan et al., 2014[106] | Asthenozoospermia/60 | Yes | Zinc sulfate (220 mg/day) bid | 3 months | Increased in semen volume, sperm count and forward motility |
| Cyrus et al., 2015[107] | Clinical varicocele/115 | Yes | Vitamin C (250 mg) Bid or placebo |
3 months | No effect on sperm count but improved sperm motility and morphology |
| Haghighian et al., 2015[108] | Idiopathic asthenozoospermia/44 | Yes | Alpha lipoic acid (600 mg) or placebo | 12 weeks | Sperm count, concentration, and motility were significantly improved |
| ElSheikh et al., 2015[94] | Idiopathic oligoasthenozoospermia/90 | Yes | I: Vitamin E (400 mg/day) II. Clomiphene citrate (25 mg/day) III: Combination of drugs with same dosage |
6 months | There was no significant increase in sperm concentration but only in the Vitamin E group. Combination therapy showed increased sperm concentration and motility |
| Bozhedomov et al., 2017[109] | Oligo or astheno or teratozoospermia/173 | Yes | L-carnitine fumarate (1 g), acetyl L-carnitine (0.5 g) twice daily, combination of Vitamins A, E, C, selenium, zinc, clomiphene (25 mg) bid | 3-4 months | Improve in the concentration of spermatozoa but no effect on sperm morphology, motility and pregnancy rates |
EPA=Eicosapentaenoic acid, DHA=Docosahexaenoic acid, ICSI=Intracytoplasmic sperm injection, GSH=Glutathione
TESTS TO MEASURE REACTIVE OXYGEN SPECIES IN SEMEN
Assessment of sperm ROS levels among infertile men can aid in determining which individuals who may benefit from antioxidant therapy. Various tests have been developed to detect seminal ROS levels which can be classified into direct and indirect assays [Tables 2 and 3]. Currently, there are no infertility guidelines that recommend routine ROS measurement and there is still an ongoing debate on which type of patients have to be tested for the oxidative burden. Asthenozoospermia in a semen sample is probably a marker of ROS.[11] Hyperviscosity has also been suggestive of increased OS because it is attributed to increased malondialdehyde levels. Increased leukocytes or round cells which is one of the principal sources of ROS may suggest further testing for OS. Abnormal sperm morphology due to cytoplasmic residues also correlates with high levels of ROS.[10] The hypo-osmotic swelling test suggests membrane damage in the sperm due to lipid peroxidation and this might imply higher levels of ROS in semen. Besides, some studies recommend ROS testing in individuals with idiopathic infertility.[110]
Table 2.
Direct assays of oxidative stress
| Test | Method of measurement | Function | Advantages | Disadvantages |
|---|---|---|---|---|
| Chemilum-inescence assay | Charged or uncharged probes undergo oxidation/reduction with generation of light as by-product | Deduce oxidation or reduction through the generation of light | High sensitivity and specificity. Robust test | Large and expensive equipment Time consuming Requires higher sample volume Interfering variables like semen age, volume, and temperature control |
| Flow cytometry | When excited by light of differing wavelengths, the incubation with the dye emits fluorescence | Measurement of ROS | It requires a small number of spermatozoa - patients with low sperm count- and can measure multiple markers simultaneously | Expensive tool that is not practical for widespread clinical use |
| Electron spin resonance | Obtains the absorption spectra of spin energy among unpaired electrons in an applied magnetic field | Detection of free radicals | Broad usage covering various parameters like: Observation of free radicals Analysis of free radicals characteristics Quantitative analysis of free radicals Kinetic analysis Good for high levels of ROS production |
Free radicals can react with another molecule other than spin-trapping agent Interference factors like neutralization |
| Cytochrome c reduction | Superoxide radicals and reduced ferricytochrome c are identified | Evaluation of ROS on the cell membrane | Good in detecting high levels of ROS Quantifies superoxide released during respiratory burst of neutrophils or enzymes |
If enzyme activity is less, relative insensitivity to the detection of NADPH oxidase activity |
| Nitroblue tetrazolium test | Nitroblue tetrazolium turns yellow to purple/blue when exposed to ROS | Localization of reaction between leukocytes or sperm cells and superoxide ions | Detects neutrophils at a concentration of 0.5×106/mL or higher Easy and cost-effective |
Subjective interpretation of a positive or negative neutrophil |
| Thiobarbituric acid assay | Based on the reaction of a chromogenic agent, 2-thiobarbituric acid with MDA | Used to evaluate the resistance of sperm to oxidative stress | Can assess sperm MDA levels Needs expensive microplate readers |
Expensive equipment is required |
| Xylenol orange-based assay | Oxidants in semen samples oxidise the ferrous ion-o-dianisidine complex to ferric ion. Ferric ion forms a colored compound with xylenol that can be detected using a spectrophotometer | Colorimetric automated assay | Measures the net oxidative imbalance between ROS production and antioxidant concentration | Limited widespread utility due to cost |
| FITC-labelled lectins | Used to detect the sperm acrosome status | Used to detect sperm peroxidase using plant lectins labeled with a fluorescent agent (FITC) to detect a group of sperm peroxidases | Detects sperm acrosome status | Difficult to detect true and false acrosome reaction Cannot detect sperm viability and acrosomal reaction status in one picture Fluorescent signal can fade sometimes |
ROS=Reactive oxygen species, MDA=Malondialdehyde, NADPH=Nicotinamide adenine dinucleotide phosphate, FITC=Fluorescein isothiocyanate
Table 3.
Indirect assays of oxidative stress
| Test | Method of measurement | Function | Advantages | Disadvantages |
|---|---|---|---|---|
| Myeloperoxidase or Endtz test | Peroxidase positivity is assessed through staining using benzidine as a buffer | Detection of granulocytes in semen | Specifically distinguishes WBC’s especially producing granulocytes from immature germ cells in semen | Cannot be used to detect ROS production in spermatozoa |
| Lipid peroxidation levels | Detection of MDA and toxic 4-HNE through colorimetric and thiobarbituric acid assays | Identification of by-products of lipid peroxidation | MDA is a colored substance that can be measured by fluorometry or spectrophotometry. Low sperm concentration of MDA can be measured through sensitive HPLC equipment or spectrofluorometric measurement of iron-based promoters | Not a widely-used test in clinic practice |
| MiOXSYs | Assessment of electron transfer in millivolts from a reducing agent to the oxidant using a galvanostat-based system | Measurement of oxidation-reduction potential | Easy to employ in a clinical setting Can be used in patients with low semen volume |
Larger cohort studies to establish the reference value are needed |
| Total antioxidant capacity | Evaluates the reductive ability of the antioxidants within the semen against an oxidative agent such as hydrogen peroxide and measures the effect on the substrate | Assesses the cumulative effect of antioxidants within the semen | Rapid colorimetric method Total antioxidants in seminal plasma can be measured |
Does not measure individual or enzymatic antioxidants. Requires expensive assay kit and microplate reader |
| Gpx activity | The activity of Gpx is measured by the decrease in GSH content after incubating the sample in the presence of H2O2 and NaN3 | Based on the principle that Gpx catalyzes the reaction between hydrogen peroxide and reduced GSH | Gpx protects the sperm from lipid peroxidation and the DNA damage can be significant if the Gpx levels are lower | Some studies show that Gpx activity does not correlate with sperm motility or concentration |
| Comet assay | Allows DNA migration in an agarose gel under an electric field. The loose DNA forms a pattern of migration that resembles a comet | Single-cell gel electrophoresis assay to assess DNA damage | Can detect extent of DNA damage equivalent to 50-single strand breaks per cell Can be employed in men with low sperm concentrations (requires only 100 cells for analysis) |
No consensus reached on the standardization protocol |
GSH=Glutathione, GPx=GSH peroxidase, 4-HNE=4-hydorxynonenal, MDA=Malondialdehyde, WBC’s=White blood cells, ROS=Reactive oxygen species, HPLC=High-performance liquid chromatography
REACTIVE OXYGEN SPECIES EFFECTS ON PREGNANCY LOSS AND OUTCOMES
The effects of SDF on natural pregnancy and pregnancy outcome have been recognized.[111] The Danish First Pregnancy Planner study illustrated a correlation between infertility and an SDF index of >30%. There was a significant association between a high sperm SDF and increased time to conceive naturally and lower fertility potential.[112] Similarly, in 500 couples with no infertility history who discontinued contraception for the purpose of pregnancy and they were enrolled in the Longitudinal Investigation of Fertility and the Environment study, SDF was associated with low fecundity.[113] A meta-analysis included 616 couples demonstrated that three studies showed an odds ratio of 7.01 suggestive of an association between high SDF and failure to achieve natural pregnancy.[114]
OS has become a growing concern for researchers and clinicians because of association with decreased fertilization, poor embryonic development, pregnancy loss, potential birth defects such as autism and cancers.[11] The chromatin in the sperm DNA is vulnerable to OS and there are base-pair modifications along with DNA fragmentation. Sperm and oocyte DNA damage may interfere with implantation and ultimately result in abortion. Evidence suggests that about 80% of the chromosomal aberrations are of paternal origin in humans.[115] Further, sperm DNA damage has been implicated in apoptosis, poor fertilization rate, higher frequency of miscarriage, and morbidity in offspring.[11]
Emerging evidence suggests that in ART, there is a correlation between high SDF and an increased risk of miscarriage. In a systematic review reported by Rilcheva et al., it was found that after in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), there were increased chances of pregnancy loss due to high SDF with a combined odds ratio of 2.48.[116] Another systematic review with 16 individual cohorts and 2969 couples corroborated the above results and it observed a 2.16-fold increase in the risk of pregnancy loss after IVF and ICSI with semen specimens with high SDF.[117] Both systematic reviews stated that the significant correlations between miscarriage rates and high SDF were independent of the method of fertilization used. A study involving 25 fertile sperm donors and 20 recurrent pregnancy loss (RPL) couples showed double-stranded DNA breaks analyzed by Comet assay among RPL sperm donors without any female factor.[118] A more recent study showed that there was a positive association between RSA and high SDF.[119]
REACTIVE OXYGEN SPECIES, FERTILITY CAPACITY AND ASSISTED REPRODUCTION TECHNIQUES OUTCOMES
Reactive oxygen species and oxidation-reduction potential (ORP) play significant roles in the fertility process and in the outcome of ART. In a prospective case-control study carried out in 1168 infertile and 100 fertile men, semen analysis parameters, the ORP and SDF were compared. The study concluded that infertile men had significant lower semen parameters and higher ORP and SDF levels, and that a significant positive correlation exists between ORP, SDF, and sperm head defects.[120] In a meta-analysis by Van Waart et al., pregnancy rates achieved with intrauterine insemination (IUI) were correlated with normal sperm morphology and it was found a significant improvement in the pregnancy rate associated with >4% sperm morphology, and hence, they concluded that sperm morphology assessment by strict criteria is a good predictor of IUI outcome.[121] However, more recent studies failed to show an association of ART outcomes in groups with or without isolated teratozoospermia is semen.[122] Hotaling et al., reported that in four retrospective studies, isolated teratozoospermia was not associated with lower pregnancy rates following IVF or ICSI. Hence, the predictive power of sperm morphology on ART outcomes is still debatable.[123,124]
The existing literature about the consequences of sperm DNA damage on ART outcomes is still controversial. Li et al. performed a meta-analysis and found that sperm DNA damage had a negative impact on IVF clinical pregnancy rates but did not affect the IVF and ICSI fertilization and the ICSI clinical pregnancy.[125] Another meta-analysis by Zini et al., evaluating the influence of sperm DNA damage on spontaneous pregnancy loss after IVF and ICSI, showed the detrimental effect of sperm DNA damage on ART outcomes and suggested a clinical test to evaluate the DNA damage before IVF or ICSI procedures.[126] However, the Practice Committee of the American Society for Reproductive Medicine concluded that the data in the existing literature does not support the adverse consequences of sperm DNA damage on ART and natural pregnancy outcomes, but recommends future research to validate the clinical utility of sperm integrity tests.[127] A more recent meta-analysis by Simon et al. including 41 studies showed that DNA damage in the sperm significantly correlated with adverse ART outcomes with IVF or ICSI techniques. The sperm DNA damage was evaluated using various tests such as Comet assay, sperm chromatin structure assay, terminal deoxyuridine nick end labeling assay, and sperm chromatin dispersion assay.[128] Therefore, despite that studies demonstrated correlation between sperm DNA damage and adverse ART events, the evidence is inconclusive as there is no standardization of the tests that has been used to detect sperm DNA damage.
CONCLUSION
Although reactive oxygen species are essential for some reproductive processes such as capacitation and acrosome reaction, increased ROS along with decreased antioxidant defense result in OS status which ultimately leads to sperm membrane lipid peroxidation, reduced motility, sperm DNA damage, poor pregnancy, and ART outcomes and increased risk of genetic diseases in offspring. Various diagnostic and therapeutic options have been developed for OS. However, there is lack of agreement on selecting OS test, type, and duration of antioxidants treatment as well as on defining the target patients group. Further studies are warranted to overcome these limitations, improve fertility potential and reduce the risk of genetic diseases and malignant tumors in newborns.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The author would like to show sincere gratitude for anonymous reviewers for their valuable help and support to accomplish this work.
REFERENCES
- 1.Rowe P, Comhaire FH, Hargreave T. WHO Manual for the Standardized Investigation of the Infertile Couple. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- 2.Agarwal A, Ahmad G, Sharma R. Reference values of reactive oxygen species in seminal ejaculates using chemiluminescence assay. J Assist Reprod Genet. 2015;32:1721–9. doi: 10.1007/s10815-015-0584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slade P, O'Neill C, Simpson AJ, Lashen H. The relationship between perceived stigma, disclosure patterns, support and distress in new attendees at an infertility clinic. Hum Reprod. 2007;22:2309–17. doi: 10.1093/humrep/dem115. [DOI] [PubMed] [Google Scholar]
- 4.Alahmar A. Effect of Vitamin C, Vitamin E, zinc, selenium, and coenzyme Q10 in infertile men with idiopathic oligoasthenozoospermia. Int J Infertil Fetal Med. 2017;8:45–9. [Google Scholar]
- 5.Henkel R, Schill WB. Sperm separation in patients with urogenital infections. Andrologia. 1998;30(Suppl 1):91–7. doi: 10.1111/j.1439-0272.1998.tb02832.x. [DOI] [PubMed] [Google Scholar]
- 6.Sanocka-Maciejewska D, Ciupińska M, Kurpisz M. Bacterial infection and semen quality. J Reprod Immunol. 2005;67:51–6. doi: 10.1016/j.jri.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Schuppe HC, Meinhardt A, Allam JP, Bergmann M, Weidner W, Haidl G, et al. Chronic orchitis: A neglected cause of male infertility? Andrologia. 2008;40:84–91. doi: 10.1111/j.1439-0272.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 8.Cavallini G. Male idiopathic oligoasthenoteratozoospermia. Asian J Androl. 2006;8:143–57. doi: 10.1111/j.1745-7262.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 9.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 10.Wagner H, Cheng JW, Ko EY. Role of reactive oxygen species in male infertility: An updated review of literature. Arab J Urol. 2018;16:35–43. doi: 10.1016/j.aju.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod Biomed (Yazd) 2016;14:231–40. [PMC free article] [PubMed] [Google Scholar]
- 13.Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. [Last accessed on 2018 Jan 28];Nat Rev Urol. 2017 14:470–85. doi: 10.1038/nrurol.2017.69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28508879 . [DOI] [PubMed] [Google Scholar]
- 14.Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online. 2014;28:684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Liang R, Ghaffari S. Stem cells, redox signaling, and stem cell aging. Antioxid Redox Signal. 2014;20:1902–16. doi: 10.1089/ars.2013.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–16. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, Aitken RJ, et al. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: Correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J Androl. 1996;17:276–87. [PubMed] [Google Scholar]
- 18.Ni K, Steger K, Yang H, Wang H, Hu K, Zhang T, et al. A comprehensive investigation of sperm DNA damage and oxidative stress injury in infertile patients with subclinical, normozoospermic, and astheno/oligozoospermic clinical varicocoele. Andrology. 2016;4:816–24. doi: 10.1111/andr.12210. [DOI] [PubMed] [Google Scholar]
- 19.Rao M, Zhao XL, Yang J, Hu SF, Lei H, Xia W, et al. Effect of transient scrotal hyperthermia on sperm parameters, seminal plasma biochemical markers, and oxidative stress in men. Asian J Androl. 2015;17:668–75. doi: 10.4103/1008-682X.146967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tremellen K. Oxidative stress and male infertility – A clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 21.Depuydt CE, Bosmans E, Zalata A, Schoonjans F, Comhaire FH. The relation between reactive oxygen species and cytokines in andrological patients with or without male accessory gland infection. J Androl. 1996;17:699–707. [PubMed] [Google Scholar]
- 22.Plante M, de Lamirande E, Gagnon C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil Steril. 1994;62:387–93. doi: 10.1016/s0015-0282(16)56895-2. [DOI] [PubMed] [Google Scholar]
- 23.Henkel RR. Leukocytes and oxidative stress: Dilemma for sperm function and male fertility. Asian J Androl. 2011;13:43–52. doi: 10.1038/aja.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trum JW, Mol BW, Pannekoek Y, Spanjaard L, Wertheim P, Bleker OP, et al. Value of detecting leukocytospermia in the diagnosis of genital tract infection in subfertile men. Fertil Steril. 1998;70:315–9. doi: 10.1016/s0015-0282(98)00163-0. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira PF, Tomás GD, Dias TR, Martins AD, Rato L, Alves MG, et al. White tea consumption restores sperm quality in prediabetic rats preventing testicular oxidative damage. Reprod Biomed Online. 2015;31:544–56. doi: 10.1016/j.rbmo.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: A new meta-analysis examining the effect of the 2010 world health organization laboratory methods for the examination of human semen. Eur Urol. 2016;70:635–45. doi: 10.1016/j.eururo.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Nada EA, et al. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril. 2002;78:1215–24. doi: 10.1016/s0015-0282(02)04237-1. [DOI] [PubMed] [Google Scholar]
- 28.La Vignera S, Condorelli RA, Balercia G, Vicari E, Calogero AE. Does alcohol have any effect on male reproductive function? A review of literature. Asian J Androl. 2013;15:221–5. doi: 10.1038/aja.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Queiroz EK, Waissmann W. Occupational exposure and effects on the male reproductive system. Cad Saude Publica. 2006;22:485–93. doi: 10.1590/s0102-311x2006000300003. [DOI] [PubMed] [Google Scholar]
- 30.Tiligada E. Chemotherapy: Induction of stress responses. Endocr Relat Cancer. 2006;13(Suppl 1):S115–24. doi: 10.1677/erc.1.01272. [DOI] [PubMed] [Google Scholar]
- 31.Manda K, Ueno M, Moritake T, Anzai K. Alpha-lipoic acid attenuates x-irradiation-induced oxidative stress in mice. Cell Biol Toxicol. 2007;23:129–37. doi: 10.1007/s10565-006-0137-6. [DOI] [PubMed] [Google Scholar]
- 32.Desai N, Sharma R, Makker K, Sabanegh E, Agarwal A. Physiologic and pathologic levels of reactive oxygen species in neat semen of infertile men. Fertil Steril. 2009;92:1626–31. doi: 10.1016/j.fertnstert.2008.08.109. [DOI] [PubMed] [Google Scholar]
- 33.Fraczek M, Kurpisz M. The redox system in human semen and peroxidative damage of spermatozoa. Postepy Hig Med Dosw (Online) 2005;59:523–34. [PubMed] [Google Scholar]
- 34.Gałecka E, Jacewicz R, Mrowicka M, Florkowski A, Gałecki P. Antioxidative enzymes – structure, properties, functions. Pol Merkur Lekarski. 2008;25:266–8. [PubMed] [Google Scholar]
- 35.Peeker R, Abramsson L, Marklund SL. Superoxide dismutase isoenzymes in human seminal plasma and spermatozoa. Mol Hum Reprod. 1997;3:1061–6. doi: 10.1093/molehr/3.12.1061. [DOI] [PubMed] [Google Scholar]
- 36.Yan L, Liu J, Wu S, Zhang S, Ji G, Gu A, et al. Seminal superoxide dismutase activity and its relationship with semen quality and SOD gene polymorphism. J Assist Reprod Genet. 2014;31:549–54. doi: 10.1007/s10815-014-0215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scibior D, Czeczot H. Catalase: Structure, properties, functions. Postepy Hig Med Dosw (Online) 2006;60:170–80. [PubMed] [Google Scholar]
- 38.de Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod. 1997;3:175–94. doi: 10.1093/molehr/3.3.175. [DOI] [PubMed] [Google Scholar]
- 39.Macanovic B, Vucetic M, Jankovic A, Stancic A, Buzadzic B, Garalejic E, et al. Correlation between sperm parameters and protein expression of antioxidative defense enzymes in seminal plasma: A pilot study. Dis Markers. 2015;2015:436236. doi: 10.1155/2015/436236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeung CH, Cooper TG, De Geyter M, De Geyter C, Rolf C, Kamischke A, et al. Studies on the origin of redox enzymes in seminal plasma and their relationship with results of in vitro fertilization. Mol Hum Reprod. 1998;4:835–9. doi: 10.1093/molehr/4.9.835. [DOI] [PubMed] [Google Scholar]
- 41.Crisol L, Matorras R, Aspichueta F, Expósito A, Hernández ML, Ruiz-Larrea MB, et al. Glutathione peroxidase activity in seminal plasma and its relationship to classical sperm parameters and in vitro fertilization-intracytoplasmic sperm injection outcome. Fertil Steril. 2012;97:852–7. doi: 10.1016/j.fertnstert.2012.01.097. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: An overview of the literature. Reprod Biomed Online. 2004;8:616–27. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 43.Luberda Z. The role of glutathione in mammalian gametes. Reprod Biol. 2005;5:5–17. [PubMed] [Google Scholar]
- 44.Commission Directive 2008/100/EC of 28 October 2008 Amending Council Directive 90/496/EEC on Nutrition Labelling for Foodstuffs as Regards Recommended Daily Allowance, Energy Conversion Factors and Definitions. 2008 [Google Scholar]
- 45.Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J, et al. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005;26:349–53. doi: 10.2164/jandrol.04146. [DOI] [PubMed] [Google Scholar]
- 46.Das UB, Mallick M, Debnath JM, Ghosh D. Protective effect of ascorbic acid on cyclophosphamide – Induced testicular gametogenic and androgenic disorders in male rats. Asian J Androl. 2002;4:201–7. [PubMed] [Google Scholar]
- 47.Lewis SE, Simon L. Clinical implications of sperm DNA damage. Hum Fertil (Camb) 2010;13:201–7. doi: 10.3109/14647273.2010.528823. [DOI] [PubMed] [Google Scholar]
- 48.Hogarth CA, Griswold MD. The key role of Vitamin A in spermatogenesis. J Clin Invest. 2010;120:956–62. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Favier AE. The role of zinc in reproduction. Hormonal mechanisms. Biol Trace Elem Res. 1992;32:363–82. doi: 10.1007/BF02784623. [DOI] [PubMed] [Google Scholar]
- 50.Freedman LP. Anatomy of the steroid receptor zinc finger region. Endocr Rev. 1992;13:129–45. doi: 10.1210/edrv-13-2-129. [DOI] [PubMed] [Google Scholar]
- 51.Arcaniolo D, Favilla V, Tiscione D, Pisano F, Bozzini G, Creta M, et al. Is there a place for nutritional supplements in the treatment of idiopathic male infertility? Arch Ital Urol Androl. 2014;86:164–70. doi: 10.4081/aiua.2014.3.164. [DOI] [PubMed] [Google Scholar]
- 52.Flohé L. Selenium in mammalian spermiogenesis. Biol Chem. 2007;388:987–95. doi: 10.1515/BC.2007.112. [DOI] [PubMed] [Google Scholar]
- 53.Moslemi MK, Tavanbakhsh S. Selenium-Vitamin E supplementation in infertile men: Effects on semen parameters and pregnancy rate. Int J Gen Med. 2011;4:99–104. doi: 10.2147/IJGM.S16275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, et al. Sperm oxidative stress and the effect of an oral Vitamin E and selenium supplement on semen quality in infertile men. Arch Androl. 2003;49:83–94. doi: 10.1080/01485010390129269. [DOI] [PubMed] [Google Scholar]
- 55.Fisher HM, Aitken RJ. Comparative analysis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. J Exp Zool. 1997;277:390–400. doi: 10.1002/(sici)1097-010x(19970401)277:5<390::aid-jez5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 56.Aitken RJ. The amoroso lecture? The human spermatozoon – a cell in crisis. J Reprod Fertil. 1999;115:1–7. doi: 10.1530/jrf.0.1150001. [DOI] [PubMed] [Google Scholar]
- 57.Abd-Aziz N, Chatterjee C, Durairajanayagam D. Corticosterone-induced oxidative stress alters epididymal sperm fertility in rats. ASM Sci J. 2014;8:117–24. [Google Scholar]
- 58.Said TM, Agarwal A, Sharma RK, Thomas AJ, Jr, Sikka SC. Impact of sperm morphology on DNA damage caused by oxidative stress induced by beta-nicotinamide adenine dinucleotide phosphate. Fertil Steril. 2005;83:95–103. doi: 10.1016/j.fertnstert.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 59.Hendin BN, Kolettis PN, Sharma RK, Thomas AJ, Jr, Agarwal A. Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol. 1999;161:1831–4. [PubMed] [Google Scholar]
- 60.Zini A, San Gabriel M, Baazeem A. Antioxidants and sperm DNA damage: A clinical perspective. J Assist Reprod Genet. 2009;26:427–32. doi: 10.1007/s10815-009-9343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oliva R. Protamines and male infertility. Hum Reprod Update. 2006;12:417–35. doi: 10.1093/humupd/dml009. [DOI] [PubMed] [Google Scholar]
- 62.Marcon L, Boissonneault G. Transient DNA strand breaks during mouse and human spermiogenesis new insights in stage specificity and link to chromatin remodeling. Biol Reprod. 2004;70:910–8. doi: 10.1095/biolreprod.103.022541. [DOI] [PubMed] [Google Scholar]
- 63.Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang JA, Clark AM, et al. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod. 2009;24:2061–70. doi: 10.1093/humrep/dep214. [DOI] [PubMed] [Google Scholar]
- 64.Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: A clinical approach. BJU Int. 2005;95:503–7. doi: 10.1111/j.1464-410X.2005.05328.x. [DOI] [PubMed] [Google Scholar]
- 65.Angelopoulou R, Plastira K, Msaouel P. Spermatozoal sensitive biomarkers to defective protaminosis and fragmented DNA. Reprod Biol Endocrinol. 2007;5:36. doi: 10.1186/1477-7827-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tunc O, Tremellen K. Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J Assist Reprod Genet. 2009;26:537–44. doi: 10.1007/s10815-009-9346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song GJ, Norkus EP, Lewis V. Relationship between seminal ascorbic acid and sperm DNA integrity in infertile men. Int J Androl. 2006;29:569–75. doi: 10.1111/j.1365-2605.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 68.Bui AD, Sharma R, Henkel R, Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. 2018;50:e13012. doi: 10.1111/and.13012. [DOI] [PubMed] [Google Scholar]
- 69.Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014;29:2402–12. doi: 10.1093/humrep/deu228. [DOI] [PubMed] [Google Scholar]
- 70.Simon L, Aston KI, Emery BR, Hotaling J, Carrell DT. Sperm DNA damage output parameters measured by the alkaline Comet assay and their importance. Andrologia. 2017;49(2) doi: 10.1111/and.12608. [DOI] [PubMed] [Google Scholar]
- 71.Sakkas D, Manicardi G, Bianchi PG, Bizzaro D, Bianchi U. Relationship between the presence of endogenous nicks and sperm chromatin packaging in maturing and fertilizing mouse spermatozoa. Biol Reprod. 1995;52:1149–55. doi: 10.1095/biolreprod52.5.1149. [DOI] [PubMed] [Google Scholar]
- 72.Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, Bianchi U, et al. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:31–7. doi: 10.1530/ror.0.0040031. [DOI] [PubMed] [Google Scholar]
- 73.Esteves SC, Sánchez-Martín F, Sánchez-Martín P, Schneider DT, Gosálvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015;104:1398–405. doi: 10.1016/j.fertnstert.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 74.Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg HR, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83:635–42. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 75.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–50. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 76.Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989;41:183–97. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- 77.Makker K, Agarwal A, Sharma R. Oxidative stress and male infertility. Indian J Med Res. 2009;129:357–67. [PubMed] [Google Scholar]
- 78.Alvarez JG, Storey BT. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995;42:334–46. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- 79.de Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl. 1992;13:368–78. [PubMed] [Google Scholar]
- 80.Kemal Duru N, Morshedi M, Oehninger S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril. 2000;74:1200–7. doi: 10.1016/s0015-0282(00)01591-0. [DOI] [PubMed] [Google Scholar]
- 81.Misro MM, Choudhury L, Upreti K, Gautam D, Chaki SP, Mahajan AS, et al. Use of hydrogen peroxide to assess the sperm susceptibility to oxidative stress in subjects presenting a normal semen profile. Int J Androl. 2004;27:82–7. doi: 10.1046/j.0105-6263.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 82.Pasqualotto FF, Sharma RK, Nelson DR, Thomas AJ, Agarwal A. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil Steril. 2000;73:459–64. doi: 10.1016/s0015-0282(99)00567-1. [DOI] [PubMed] [Google Scholar]
- 83.Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in semen of infertile patients: Levels of superoxide dismutase – And catalase-like activities in seminal plasma and spermatozoa. Int J Androl. 1993;16:183–8. doi: 10.1111/j.1365-2605.1993.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 84.Wiener-Megnazi Z, Auslender R, Dirnfeld M. Advanced paternal age and reproductive outcome. Asian J Androl. 2012;14:69–76. doi: 10.1038/aja.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl. 2014;16:31–8. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rima D, Shiv BK, Bhavna CH, Shilpa B, Saima KH. Oxidative stress induced damage to paternal genome and impact of meditation and yoga – Can it reduce incidence of childhood cancer? Asian Pac J Cancer Prev. 2016;17:4517–25. [PubMed] [Google Scholar]
- 87.Shamsi MB, Venkatesh S, Pathak D, Deka D, Dada R. Sperm DNA damage and oxidative stress in recurrent spontaneous abortion (RSA) Indian J Med Res. 2011;133:550–1. [PMC free article] [PubMed] [Google Scholar]
- 88.Nowicka-Bauer K, Lepczynski A, Ozgo M, Kamieniczna M, Fraczek M, Stanski L, et al. Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated asthenozoospermia. J Physiol Pharmacol. 2018;69(3) doi: 10.26402/jpp.2018.3.05. [DOI] [PubMed] [Google Scholar]
- 89.Liu L, Trimarchi JR, Smith PJ, Keefe DL. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell. 2002;1:40–6. doi: 10.1046/j.1474-9728.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 90.Hekmatdoost A, Lakpour N, Sadeghi MR. Sperm chromatin integrity: Etiologies and mechanisms of abnormality, assays, clinical importance, preventing and repairing damage. Avicenna J Med Biotechnol. 2009;1:147–60. [PMC free article] [PubMed] [Google Scholar]
- 91.Aitken RJ. DNA damage in human spermatozoa; important contributor to mutagenesis in the offspring. Transl Androl Urol. 2017;6:S761–4. doi: 10.21037/tau.2017.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imamovic Kumalic S, Pinter B. Review of clinical trials on effects of oral antioxidants on basic semen and other parameters in idiopathic oligoasthenoteratozoospermia. Biomed Res Int. 2014;2014:426951. doi: 10.1155/2014/426951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alahmar AT. The effects of oral antioxidants on the semen of men with idiopathic oligoasthenoteratozoospermia. Clin Exp Reprod Med. 2018;45:57–66. doi: 10.5653/cerm.2018.45.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.ElSheikh MG, Hosny MB, Elshenoufy A, Elghamrawi H, Fayad A, Abdelrahman S, et al. Combination of Vitamin E and clomiphene citrate in treating patients with idiopathic oligoasthenozoospermia: A prospective, randomized trial. Andrology. 2015;3:864–7. doi: 10.1111/andr.12086. [DOI] [PubMed] [Google Scholar]
- 95.Akmal M, Qadri JQ, Al-Waili NS, Thangal S, Haq A, Saloom KY, et al. Improvement in human semen quality after oral supplementation of Vitamin C. J Med Food. 2006;9:440–2. doi: 10.1089/jmf.2006.9.440. [DOI] [PubMed] [Google Scholar]
- 96.Vahidinia A, Rahbar AR, Shakoori Mahmoodabadi MM. Effect of astaxanthin, Vitamin E, and Vitamin C in combination with calorie restriction on sperm quality and quantity in male rats. J Diet Suppl. 2017;14:252–63. doi: 10.1080/19390211.2016.1211783. [DOI] [PubMed] [Google Scholar]
- 97.Safarinejad MR. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: A double-blind, placebo-controlled, randomised study. Andrologia. 2011;43:38–47. doi: 10.1111/j.1439-0272.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 98.Wirleitner B, Vanderzwalmen P, Stecher A, Spitzer D, Schuff M, Schwerda D, et al. Dietary supplementation of antioxidants improves semen quality of IVF patients in terms of motility, sperm count, and nuclear vacuolization. Int J Vitam Nutr Res. 2012;82:391–8. doi: 10.1024/0300-9831/a000136. [DOI] [PubMed] [Google Scholar]
- 99.Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: A double-blind, placebo controlled, randomized study. J Urol. 2012;188:526–31. doi: 10.1016/j.juro.2012.03.131. [DOI] [PubMed] [Google Scholar]
- 100.Safarinejad MR. The effect of coenzyme Q10 supplementation on partner pregnancy rate in infertile men with idiopathic oligoasthenoteratozoospermia: An open-label prospective study. Int Urol Nephrol. 2012;44:689–700. doi: 10.1007/s11255-011-0081-0. [DOI] [PubMed] [Google Scholar]
- 101.Busetto GM, Koverech A, Messano M, Antonini G, De Berardinis E, Gentile V, et al. Prospective open-label study on the efficacy and tolerability of a combination of nutritional supplements in primary infertile patients with idiopathic astenoteratozoospermia. Arch Ital Urol Androl. 2012;84:137–40. [PubMed] [Google Scholar]
- 102.Chen XF, Li Z, Ping P, Dai JC, Zhang FB, Shang XJ, et al. Efficacy of natural Vitamin E on oligospermia and asthenospermia: A prospective multi-centered randomized controlled study of 106 cases. Zhonghua Nan Ke Xue. 2012;18:428–31. [PubMed] [Google Scholar]
- 103.Abad C, Amengual MJ, Gosálvez J, Coward K, Hannaoui N, Benet J, et al. Effects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNA. Andrologia. 2013;45:211–6. doi: 10.1111/and.12003. [DOI] [PubMed] [Google Scholar]
- 104.Nadjarzadeh A, Shidfar F, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Effect of coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: A double-blind randomised clinical trial. Andrologia. 2014;46:177–83. doi: 10.1111/and.12062. [DOI] [PubMed] [Google Scholar]
- 105.Raigani M, Yaghmaei B, Amirjannti N, Lakpour N, Akhondi MM, Zeraati H, et al. The micronutrient supplements, zinc sulphate and folic acid, did not ameliorate sperm functional parameters in oligoasthenoteratozoospermic men. Andrologia. 2014;46:956–62. doi: 10.1111/and.12180. [DOI] [PubMed] [Google Scholar]
- 106.Hadwan MH, Almashhedy LA, Alsalman AR. Study of the effects of oral zinc supplementation on peroxynitrite levels, arginase activity and NO synthase activity in seminal plasma of Iraqi asthenospermic patients. Reprod Biol Endocrinol. 2014;12:1. doi: 10.1186/1477-7827-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cyrus A, Kabir A, Goodarzi D, Moghimi M. The effect of adjuvant Vitamin C after varicocele surgery on sperm quality and quantity in infertile men: A double blind placebo controlled clinical trial. Int Braz J Urol. 2015;41:230–8. doi: 10.1590/S1677-5538.IBJU.2015.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haghighian HK, Haidari F, Mohammadi-Asl J, Dadfar M. Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil Steril. 2015;104:318–24. doi: 10.1016/j.fertnstert.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 109.Bozhedomov VA, Lipatova NA, Bozhedomova GE, Rokhlikov IM, Shcherbakova EV, Komarina RA, et al. Using L – And acetyl-L-carnintines in combination with clomiphene citrate and antioxidant complex for treating idiopathic male infertility: A prospective randomized trial. Urologiia. 2017;7:22–32. doi: 10.18565/urol.2017.3.22-32. [DOI] [PubMed] [Google Scholar]
- 110.Mayorga-Torres BJ, Camargo M, Cadavid ÁP, du Plessis SS, Cardona Maya WD. Are oxidative stress markers associated with unexplained male infertility? Andrologia. 2017;49(5) doi: 10.1111/and.12659. [DOI] [PubMed] [Google Scholar]
- 111.Agarwal A, Cho CL, Esteves SC. Should we evaluate and treat sperm DNA fragmentation? Curr Opin Obstet Gynecol. 2016;28:164–71. doi: 10.1097/GCO.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 112.Spanò M, Bonde JP, Hjøllund HI, Kolstad HA, Cordelli E, Leter G, et al. Sperm chromatin damage impairs human fertility. The Danish first pregnancy planner study team. Fertil Steril. 2000;73:43–50. doi: 10.1016/s0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 113.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney A, Lynch CD, Kim S, et al. Semen quality and time to pregnancy: The longitudinal investigation of fertility and the environment study. Fertil Steril. 2014;101:453–62. doi: 10.1016/j.fertnstert.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57:78–85. doi: 10.3109/19396368.2010.515704. [DOI] [PubMed] [Google Scholar]
- 115.González-Marín C, Gosálvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012;13:14026–52. doi: 10.3390/ijms131114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rilcheva V, Ayvazova N, Ilieva L, Ivanova S, Konova E. Sperm DNA integrity test and assisted reproductive technology (Art) outcome. J Biomed Clin Res. 2016;9:21–9. [Google Scholar]
- 117.Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil Steril. 2014;102:998–1005.e8. doi: 10.1016/j.fertnstert.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 118.Ribas-Maynou J, García-Peiró A, Fernandez-Encinas A, Amengual MJ, Prada E, Cortés P, et al. Double stranded sperm DNA breaks, measured by comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS One. 2012;7:e44679. doi: 10.1371/journal.pone.0044679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khadem N, Poorhoseyni A, Jalali M, Akbary A, Heydari ST. Sperm DNA fragmentation in couples with unexplained recurrent spontaneous abortions. Andrologia. 2014;46:126–30. doi: 10.1111/and.12056. [DOI] [PubMed] [Google Scholar]
- 120.Majzoub A, Arafa M, Mahdi M, Agarwal A, Al Said S, Al-Emadi I, et al. Oxidation-reduction potential and sperm DNA fragmentation, and their associations with sperm morphological anomalies amongst fertile and infertile men. Arab J Urol. 2018;16:87–95. doi: 10.1016/j.aju.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Van Waart J, Kruger TF, Lombard CJ, Ombelet W. Predictive value of normal sperm morphology in intrauterine insemination (IUI): A structured literature review. Hum Reprod Update. 2001;7:495–500. doi: 10.1093/humupd/7.5.495. [DOI] [PubMed] [Google Scholar]
- 122.Blanchard M, Haguenoer K, Apert A, Poret H, Barthélémy C, Royère D, et al. Sperm morphology assessment using David's classification: Time to switch to strict criteria? Prospective comparative analysis in a selected IVF population. Int J Androl. 2011;34:145–52. doi: 10.1111/j.1365-2605.2010.01066.x. [DOI] [PubMed] [Google Scholar]
- 123.Hotaling JM, Smith JF, Rosen M, Muller CH, Walsh TJ. The relationship between isolated teratozoospermia and clinical pregnancy after in vitro fertilization with or without intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil Steril. 2011;95:1141–5. doi: 10.1016/j.fertnstert.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 124.Lockwood GM, Deveneau NE, Shridharani AN, Strawn EY, Sandlow JI. Isolated abnormal strict morphology is not a contraindication for intrauterine insemination. Andrology. 2015;3:1088–93. doi: 10.1111/andr.12098. [DOI] [PubMed] [Google Scholar]
- 125.Li Z, Wang L, Cai J, Huang H. Correlation of sperm DNA damage with IVF and ICSI outcomes: A systematic review and meta-analysis. J Assist Reprod Genet. 2006;23:367–76. doi: 10.1007/s10815-006-9066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: Systematic review and meta-analysis. Hum Reprod. 2008;23:2663–8. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 127.Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: A guideline. Fertil Steril. 2013;99:673–7. doi: 10.1016/j.fertnstert.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 128.Simon L, Zini A, Dyachenko A, Ciampi A, Carrell DT. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl. 2017;19:80–90. doi: 10.4103/1008-682X.182822. [DOI] [PMC free article] [PubMed] [Google Scholar]