Abstract
Human neuroimaging research on cognitive aging has brought significant advances to our understanding of the neural mechanisms underlying age-related cognitive decline and successful aging. However, interpreting age-related changes and differences in brain structure, activation, and functional connectivity is an ongoing challenge. Ambiguous terminology is a major source of this challenge. For example, the terms ‘compensation,’ ‘maintenance,’ and ‘reserve’ are used in different ways and researchers disagree about the kinds of evidence or patterns of results required to interpret findings related to these concepts. As such inconsistencies can impede theoretical and empirical progress, we here aim to clarify these key terms and to propose consensual definitions of maintenance, reserve, and compensation.
Introduction
Around the world, the older segment of the adult population is rapidly expanding in number, proportion, or both.1 Advances in medicine and public health measures, rising standards of living, and improvements in education and nutrition have lengthened the human life span. Cohort comparisons suggest that the debilitating effects of senescence are increasingly delayed to later ages.2 Nevertheless, advancing adult age continues to be associated with cognitive decline and major challenges remain in our efforts to understand the mechanisms of cognitive loss and optimal aging, defined as the process of preserving cognitive abilities throughout aging.
Aging impacts neurobiological functions at multiple levels,3 including: genetics and gene expression,4–7 cellular and molecular function8–12, and neurotransmission13–17. In recent decades, the advent and availability of magnetic resonance imaging (MRI) methods has significantly advanced our understanding how brain change with advancing adult age at gross anatomical and functional levels.18–20 For example, magnetic resonance imaging (MRI) studies have shown that normal aging is associated with gray matter volume reductions and functional alterations in several regions critical for higher cognitive function: prefrontal, medial temporal and parietal cortices.21–24 Diffusion MRI methods have shown age-related changes in white matter connectivity between prefrontal and posterior cortical regions, and within posterior sensory cortices.25–27 These age-related declines in brain structure and function are associated with cognitive decline in a variety of domains.28,29
The field of cognitive neuroscience of aging24 seeks to understand the neural mechanisms of age-related cognitive decline, as well as those of optimal aging. We assume that mechanisms, which refer to putative causal explanations of age-related changes, exist at multiple levels of analysis (genetic, cellular, system, etc.). With the growth of the field, there has also been an introduction of specific terms, i.e. ‘reserve’30–32, ‘maintenance’33, and ‘compensation’,34–39 which have been used to both describe qualitative and quantitative differences in brain structure and function with age, and to advance current theories of brain aging and cognition. However, over the years, these terms have been used inconsistently, creating confusion and slowing progress. To address this terminological confusion, the authors of this article met in 2017 and worked to sharpen the definitions of some of the popular terms associated with aging. Some differences in opinion about the definitions persist; however, we emphasize here the points of agreement. We focus on the use of the terms ‘reserve’, ‘maintenance’ and ‘compensation’ in structural and functional neuroimaging studies in healthy humans, although other related terms and methods are also discussed. The terms maintenance, reserve, and compensation could be applied to other aspects of aging (e.g., bone changes) beyond the brain and cognition, and hence, it is important to qualify them. Given our interest in brain-behavior mappings, a possible qualifier would be “neurocognitive.” However, to avoid repeating “neurocognitive”, henceforth, we just use maintenance, reserve, and compensation with the understanding they refer to the neurocognitive phenomena. Except for Box 2, we do not consider how the mechanisms of reserve, maintenance, and compensation interact with pathological processes. This is of course an important question because these three mechanisms may support not only enhancement of healthy processes but also attenuation of pathological processes.40
Box 2. Maintenance, reserve, and compensation in Alzheimer’s Disease and Mild Cognitive Impairment.
The trajectory of Alzheimer’s disease (AD), which progresses from normal cognitive performance to mild cognitive impairment (MCI) to full-blown dementia,91 is by definition an example of poor brain maintenance. However, the trajectory from healthy aging to AD is modulated by reserve and compensation31,92,93. Studies have reported that β-amyloid deposition—a putative biomarker of AD —is lower in older adults with higher scores on reserve proxies, such as education32 and in those who participate in cognitively stimulating activities across the lifespan.94 Even in individuals in whom biomarkers of AD are present, higher scores on reserve proxies are associated with a lower risk of progression from normal cognition to the onset of clinical symptoms.95 The neural bases of these protective effects remain to be identified.
FMRI studies have shown that individuals with MCI96–98 and carriers of the apolipoprotein E ε4 allele (APOε4), a known risk factor for late-onset AD, 99 show increased task-related activity in brain regions first affected by AD, i.e. the hippocampus, cingulate and precuneus100,101. Some studies have associated greater activity in these regions with better cognition in individuals with MCI,96,98 consistent with compensation. Furthermore, some cognitively normal older individuals with high beta amyloid levels have shown greater activity in superior and lateral parietal cortex and in occipital cortex, that was related to better memory, compared to older adults with low beta amyloid levels, consistent with compensation.102 In some cases, however, hyper-activation may reflect the underlying neuropathology102 and excitotoxicity rather than compensation. It has been suggested that poor clearance of β-amyloid and tau proteins in the brain contribute to the accumulation of amyloid plaques and neurofibrillary tangles, respectively, and in turn may increase the production of, and inhibit the recapture of, glutamate103,104, leading to hyper-excitation. Consistent with this hypothesis, a study found that low doses of an antiepileptic drug reduced hippocampal hyperactivity and improved memory in individuals with MCI.80 An intriguing possibility is that hyper-activation is, at first, compensatory but reflects excitotoxicity at a later stage.97 Compensatory processes thus might characterize the early course of AD and contribute to its long prodrome. Thus, compensatory non-pharmacological interventions could be used to reduce cognitive symptoms. In turn, hyper-activation has the potential to contribute to an early signature of AD. Considering the possibility of excitotoxic hyper-activation, approaches might be developed to reduce hippocampal hyperactivity or to promote reliance on unimpaired brain networks.105
One of the most fundamental and urgent goals of research into the cognitive neuroscience of aging is to understand why some individuals decline faster than others during healthy aging, defined here as aging in individuals who are apparently free of brain disease.41 Inter-individual variability in cognitive aging is striking. In fact, some 80-year olds can perform as well as or better than some 40-year olds, even when considering functions generally impaired by aging such as episodic memory.42 When investigating individual differences among older adults, however, cross-sectional designs have several limitations compared to longitudinal designs. For example, whereas older participants are typically recruited only from the sub-set of people who aged successfully, young adult samples are more heterogeneous. Also, cross-sectional designs can be contaminated by birth cohort effects, including inter-generational IQ increases (the ‘Flynn Effect’).43,44 Moreover, cross-sectional designs cannot distinguish between age-invariant and age-related differences, which is important as a large proportion of variance in cognitive performance among older adults existed already when they were children.45 Although they have their own limitations,46 longitudinal designs avoid these problems and can consistently demonstrate large individual differences in rates of age-related decline.47,48
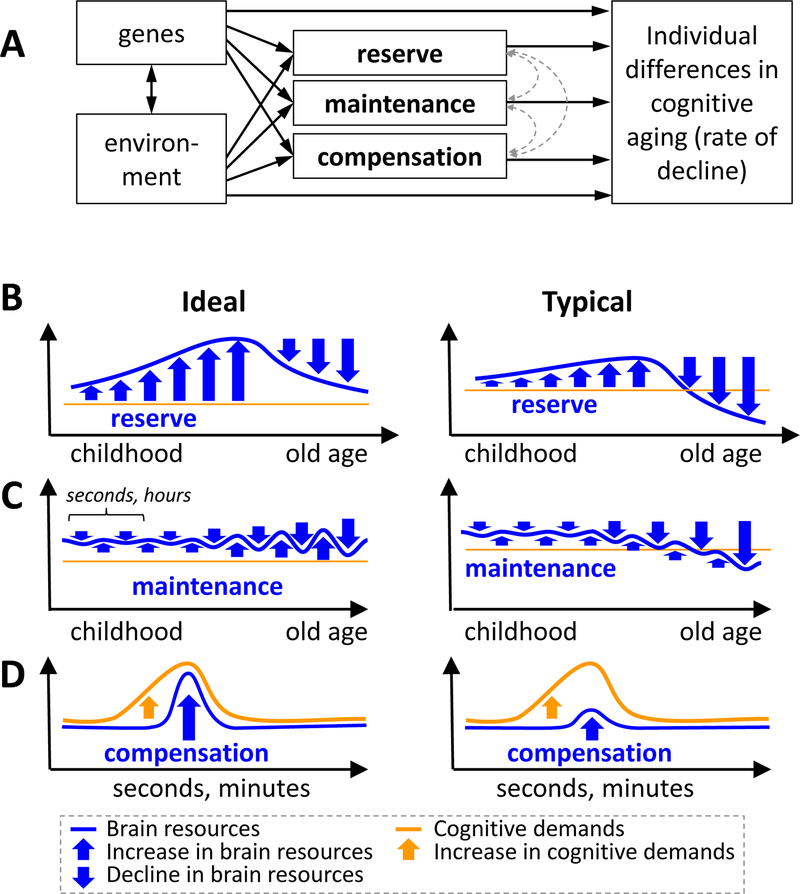
Individual differences in age-related cognitive decline undoubtedly reflect a complex interaction between genetic and environmental factors, whose actions could be partly mediated by three interacting mechanisms known as reserve, maintenance, and compensation (FIG. 1–A). In the case of reserve and maintenance (FIGS. 1–B/C), processes of age-related ‘neural decline’ (such as brain atrophy, synaptic loss, white-matter degradation, etc.) are countered by processes of ‘neural enhancement,’ that is, the creation, replenishment, and repair of neural resources (brain anatomy and physiology mediating cognitive processes). In the case of compensation (FIG. 1–D), heightened cognitive demands due to the combined effects of task difficulty and age-related cognitive decline are counteracted by recruiting additional neural resources, including those established by neural enhancement. It is important to emphasize that the mechanisms of reserve, maintenance, and compensation are not static but dynamic and modifiable, and they are likely not only responsible for modifying behavior, but are in turn modified by such changes.
Figure 1.
A. Individual differences in cognitive aging have been attributed to three interacting mechanisms, reserve, maintenance, and compensation mechanisms, which are all affected by genetic and environmental factors. B-D. Schematic charts illustrate hypothesized changes in brain resources and cognitive demand as a result of reserve, maintenance and compensation mechanisms. In the ideal scenario, these mechanisms completely eliminate age effects, whereas in the typical scenario, they only attenuate age effects. Reserve, maintenance, and compensation are hypothesized to involve an increase in brain resources (blue upward arrows) but differ regarding (1) the order of the brain resource increase in relation to brain decline (blue downward arrow), (2) the time-scale of the changes (x-axes of the graphs), and (3) the relation to cognitive demands. B. In the case of reserve, neural resources accumulate beyond what it is required to satisfy current cognitive demands, so that when these resources start to decline in old age, cognitive decline is attenuated. It is important to note that although the graph shows resources accumulating during childhood and young adulthood, cognitive reserve can continue growing in old age. C. In the case of maintenance, processes of neural decline are continuously offset by processes of neural enhancement. Given that neural decline increases in old age, greater maintenance is also required to maintain the same level of performance. The figure shows neural decline (downward arrows) and neural enhancement processes in alternation for illustration purposes only, as these processes can occur simultaneously. D. In the case of compensation, a task-related increase in cognitive demands is counteracted by the recruitment of additional neural resources.
We propose here one way of defining and linking the terms of reserve, maintenance, and compensation in order to provide greater coherence in an evolving field. Nevertheless, we recognize that these concepts are the subject of continuing debate and our goal is therefore to initiate an open dialogue about what these three concepts mean. Note that while, for the sake of simplicity, we use below examples involving single brain regions, each of the concepts are equally applicable to measures that account for covariance among multiple brain regions, including functional connectivity and multivariate activation patterns.49
Reserve
Reserve refers to a cumulative improvement in neural resources due to genetic and/or environmental factors that mitigate the effects of neural decline caused by aging or age-related diseases. Reserve is hypothesized to accumulate before neural resources demonstrate age-related decreases, over a period of years. A good example of a factor that promotes reserve is education, which improves neural resources during childhood and young adulthood, possibly by enhancing synaptic density 50, and then attenuates age-related cognitive decline. 51,52 The beneficial effect of education on cognitive performance might be moderated partly by its effects on a range of other outcomes (e.g.: health, stress, profession, lifestyle). In the ideal case, accumulated reserve is enough to completely offset age-related neural decline, whereas in the typical case, it only attenuates this decline. Presumably reserve accumulates most during childhood and young adulthood, but it may also build up in older age,9 which arguably underscores the importance of intellectual engagement throughout the lifespan.
Reserve has been defined as a cumulative increase in neural resources that mitigate the effects of brain decline due to aging or age-related disease.53 Some authors have used the more specific terms brain reserve to refer to aspects of reserve that are easily quantified in anatomical brain images and cognitive reserve to refer to aspects that are difficult to detect in anatomical images and either require functional imaging measures or cannot be delineated at the neural level given current technology.53,54 Given that cognition depends on the brain, we believe that the distinction between brain and cognitive reserve is somewhat artificial and prefer to use only the term reserve, with the understanding that various aspects of reserve require different technologies for their measurement and that some aspects cannot be assessed with current technology but could be measurable in the future.
Genetic55 and environmental factors,54,56 including longer education,32 greater physical activity,57 active participation in demanding leisure activities58 and bilingualism59,60 impact individual differences in reserve. Given that it is not possible to measure reserve directly, most studies of reserve have focused on a particular proxy and how it relates to the functioning brain. For example, functional neuroimaging studies have compared individuals who are high vs. low in one proxy (e.g, IQ, education, occupational attainment) to identify differences in brain activity. 61 For instance, one study found that greater cognitive reserve, as measured using IQ and education-occupation as proxies, was associated with lower activity during cognitive processing, suggesting more effective use of cerebral networks.30 When using functional neuroimaging to investigate reserve it is important to distinguish between across-individual activity differences that are related to reserve vs. compensation. Differences related to reserve might be expected to manifest as ‘trait-like’ effects that are evident across a range of different cognitive domains, and to correlate with independent proxies of reserve, such as IQ or educational level. Individual differences reflecting compensation, in contrast, might be expected to differ according to the nature of the cognitive challenge, and to correlate more with individual differences in task performance than with proxy measures of reserve (see Compensation section). Complicating the distinction, reserve and the capacity for compensation may interact. For example, highly educated individuals may show different activation patterns than individuals with lower educational attainment because their greater reserve allows them to deploy more effective compensatory processes.
One analytical approach to measuring the neural correlates of reserve, and other concepts that cannot be directly measured due to current technological limitations, is to regress out (control for) the effects of cognitive performance on neural variables known to affect cognitive decline (including volume, white-matter hyper-intensities, etc.) and then to correlate the residual neural measures with a hypothesized proxy of reserve (or other concept of interest, i.e. maintenance or compensation)62–64 For example, one study decomposed variance in episodic memory performance into a component predicted by demographics, a component predicted by pathology (as measured by structural MRI), and a residual “reserve” component, which was then shown to moderate cognitive decline.64 It is worth noting that if reserve is defined merely as “the factor that individuals with greater reserve have,” and then this factor is used to explain why some individuals have greater reserve, the argument is clearly circular. To address this issue, it is critical to specify the proxy factors and mechanisms that are assumed to build and constitute reserve a priori.
In addition, it is likely that proxy measures of reserve may engage different neural mechanisms, each important for a different aspect reserve, such neural capacity (the total amount of neural resources available for cognition) or neural efficiency (using less neural resources—often operationalized as neural activity—to perform a cognitive task).54,56 One example of how increased capacity is associated with a proxy measure of reserve is the aforementioned effect of education on synaptic density.50 An example of an increase in neural efficiency is the development of expertise in a particular domain through training. The development of expertise is associated with the presence of richer and more differentiated conceptual representations, which can attenuate age-related decline in the domain of expertise.54,65–67 This idea can perhaps explain why older individuals can remain highly effective in their specific domains of professional expertise.68 However, it has been observed that when adults with high levels of reserve, as indicated by one or more reserve proxies, do eventually display cognitive decline, they do so at a rapid rate 69. Possibly, at some level, the burden of pathology becomes great enough to overcome this protective mechanism, resulting in rapid cognitive decline31,53.
Maintenance
Maintenance refers to the preservation of neural resources, which entails ongoing repair of the brain.33 Maintenance occurs throughout the lifespan but may become more critical in old age, as neural deterioration becomes more severe. The time-scale of maintenance processes likely varies depending on the neural level at which it takes place (molecules, cells or systems). In the optimal case, repair processes fully counteract decline. In the typical scenario, however, repair processes do not completely offset neural deterioration, leading to a gradual process of age-related neural deterioration. However, some individuals may be relatively spared from detrimental brain changes in the first place resulting in a high likelihood of displaying maintenance regardless of the capacity for repair. Thus, the efficacy of maintenance depends both on the magnitude of decline and the efficacy of repair. The concepts of reserve and maintenance are clearly related but here we highlight what distinguishes them: reserve is about enhancing and augmenting resources, whereas maintenance is about their up-keep. We acknowledge that reserve has an impact on later maintenance, and vice versa, as the accumulated reserve must be maintained.
In principle, it is possible to distinguish different forms of maintenance. For example, maintenance can refer to different aspects of the brain, such as gray matter, white matter (e.g., myelin), or neurotransmitter functions, or to different brain regions, such as the hippocampus or the prefrontal cortex (PFC). For example, given the link between exercise and white-matter integrity,70 it is possible that individuals who exercise regularly maintain white-matter integrity better than other aspects of the brain. However, given the close interaction between different brain structures and processes, it is also possible to talk about general brain maintenance. Given that maintenance is operationalized as a relative lack of decline in one or more neural measures, and not in absolute terms, “brain maintainers” include individuals who start with above-average brain measures in early adulthood (e.g., larger hippocampus) and maintain these high levels into older age, as well as individuals who start below average (e.g., smaller hippocampus) and maintaining functioning at that lower level. It is theoretically possible that the extent to which different maintenance mechanisms operate depends on initial brain measures.
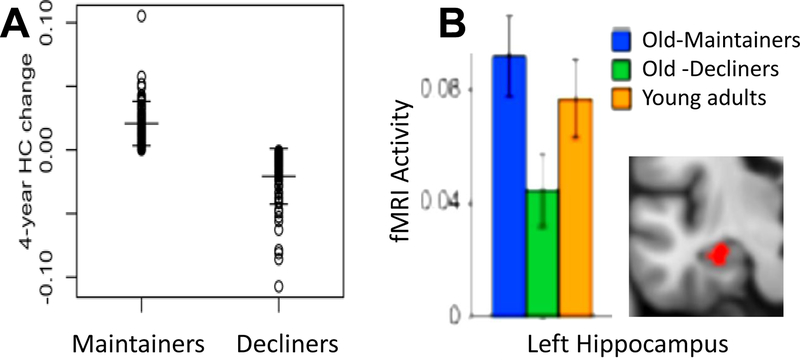
The notion of maintenance is consistent with evidence that adults who display stable cognitive performance as they age tend to show minimal brain decline or pathology.33 For example, a longitudinal structural MRI study from the Swedish Betula project71 found that individuals aged 65 years or older, with little or no episodic memory decline over a 15-year period, showed less hippocampal atrophy over 4 years than individuals with substantial memory decline (FIG. 2–A, see also ref.72). Similarly, in a longitudinal fMRI study73 hippocampal activity during an episodic memory task was significantly higher in people whose memory function was stable over two decades than in people whose memory abilities declined over this period (FIG. 2–B). Whereas hippocampal maintenance has been associated with episodic memory maintenance, the maintenance of other brain regions is likely to be associated with the preservation of other cognitive abilities33. For example, PFC maintenance could be associated with the maintenance of cognitive control. To reduce the number of multiple comparisons when investigating the maintenance of different brain regions using neuroimaging, it is advisable to propose beforehand specific hypotheses about the relationship between specific brain measures (e.g., volume) from particular brain regions (e.g., hippocampus) and specific cognitive measures (e.g., episodic free recall). At the same time, the high degree of covariance among changes in different cognitive abilities suggests that maintenance in one functional domain is likely to be related to maintenance in another domain48.
Figure 2. Maintenance.
A. Consistent with the idea stable cognitive performance is associated with maintenance,33 individuals aged 65–80 yrs who showed minimal episodic memory decline on verbal immediate free recall and delayed cued recall tasks during a 4-year period (maintainers) also showed less hippocampal volume decline, as measured by FreeSurfer using the Desikan-Killiany atlas, over 4 years compared to decliners (from REF.71). B. Also consistent with maintenance, over a period of two decades, Old-maintainers (mean age = 68.8 yrs) who showed no significant episodic memory decline on verbal immediate free recall and delayed cued recall tasks compared to young adults (mean age = 35.3), also displayed comparable levels of hippocampal activity during an fMRI study of face-name associative encoding, compared to young adults. In contrast, Old-decliners showed longitudinal episodic memory decline in the aforementioned verbal tasks, and exhibited significantly lower hippocampal activity during associative encoding, compared to young adults and Old-maintainers (from REF.73). Consistent with the idea that there can be high-maintenance and low-maintenance, in these studies, maintainers and decliners were defined independently of their absolute levels of memory and hippocampal activity. It is impossible in these studies to know if maintenance involved repair or just absence of decline.
In this section we have focused on examples from longitudinal studies. This is because maintenance mechanisms are ideally investigated using longitudinal data, as only within-person assessments can truly quantify change, or, in case of maintenance, lack thereof.74 Critically, this does not imply that cross-sectional brain data cannot be informative. For example, successful maintenance could explain cross-sectional findings that the brains of high-performing older adults look similar in anatomy and physiology to young brains, whereas the brains of low-performing older adults look different to young brains.33 However, when considering cross-sectional studies it is important to remember that some differences between young and older adults could be due to cohort effects, such as early life influences, and not aging per se, as such effects have been noted in the brain75. In summary, we see maintenance as a dynamic process that engages neural mechanisms of cellular repair and may overlap to a large degree with mechanisms of brain plasticity in adulthood76. Similar to the concept of reserve, the mechanisms contributing to maintenance are also likely to have both genetic77 and environmental origins, with the latter including factors such as diet, exercise, and cognitive and social engagement33,57,78. Behavioral genetic studies suggest that genetic and environmental contributions to maintenance become increasingly correlated with advancing age.77 The specific mechanisms remain to be determined, but likely include both neural (e.g., neurogenesis) and non-neural (e.g. vascular) components.
However, what may most differentiate reserve and maintenance are the mechanisms by which these factors influence healthy brain aging and cognition. In the case of reserve, these factors cumulative influence neural capacity and neural efficiency (and other mechanisms not yet identified due to limits of technology). In the case of maintenance, these factors influence neural mechanisms of repair and plasticity (and others not yet identified). Therefore, although the concepts of reserve and maintenance are similar, we view them as complementary perspectives on how environmental and biological factors influence brain aging and cognition.
Compensation
Compensation refers to the cognition-enhancing recruitment of neural resources in response to relatively high cognitive demand. Compensation is temporally linked to variations in cognitive demands and can occur rapidly, in a matter of seconds. As explained below, we reserve the term compensation for neural recruitment that enhances cognitive performance. In the ideal case, the cognition-enhancing recruitment is sufficient to meet the task-demands, whereas in the typical scenario, it is insufficient to match the demands.
In functional neuroimaging studies, compensation is often used to describe a situation in which brain activity, or functional connectivity, is greater, or more widespread, in older adults than in younger adults. Greater brain activity or connectivity is sometimes interpreted as being beneficial to older adults, without any additional supporting evidence. However, we believe that two basic criteria must be fulfilled in order to attribute greater activity/connectivity in older adults to compensation.
First, it is not just “compensation” but “compensation for”, and hence, evidence should be available that the activation is directly or indirectly related to some insufficiency or gap between available neural resources (“supply”) and task demands (supply-demand gap)76,79. This gap may be due to an age-related reduction in neural resources (e.g., brain atrophy, reduced blood flow, neurotransmitter deficits, reduced neural specificity, etc.), due to an increase in task demands, or both. To explain this with a metaphor, using eyeglasses for reading compensates for an insufficiency in visual acuity, and the magnitude of the supply-demand gap depends both on the visual deficit of the individual (supply) and the size of the letters (demand). In the context of aging, it is more appropriate to emphasize the loss in neural resources. That is, we assume that due to age-related neural decline, some older adults have difficulty implementing cognitive operations that would not have taxed their younger selves.
Second, evidence should be available that the enhanced activation in older adults is related to a beneficial effect on cognitive performance. Using the same metaphor, to be compensatory, the use of eyeglasses should be associated with better reading performance than when eyeglasses are not used. This is a point on which our view departs from some uses of the term compensation in the literature, where the term is often applied to any age-related increase in brain activity, or to the recruitment of additional brain regions in older but not young adults, regardless of relationship with performance. In our view, without a link to performance, these findings should be simply described as age-related differences (increases or decreases) in activity, and not as compensatory activity. Linking compensation to successful performance (Box 1) helps distinguish compensation from activation differences due to inefficiency, dedifferentiation, or pathology (Box 2)80.
Box 1. Linking compensatory activity to successful cognitive performance.
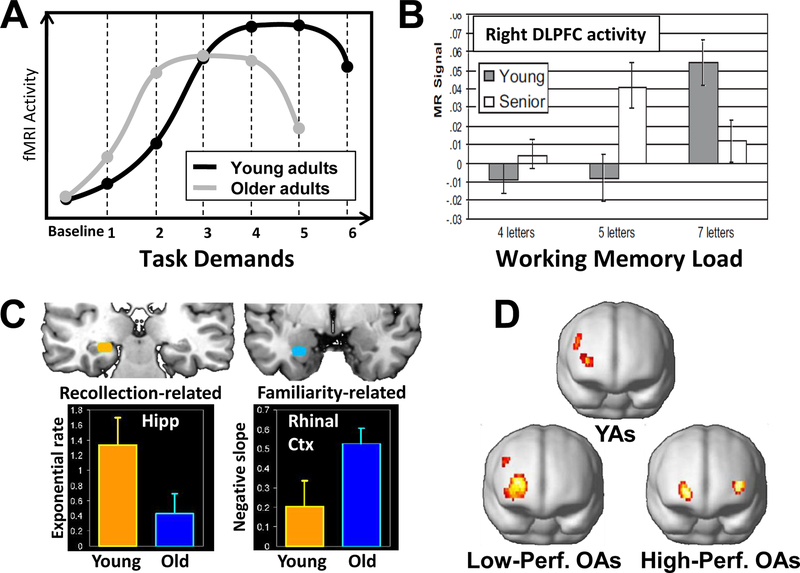
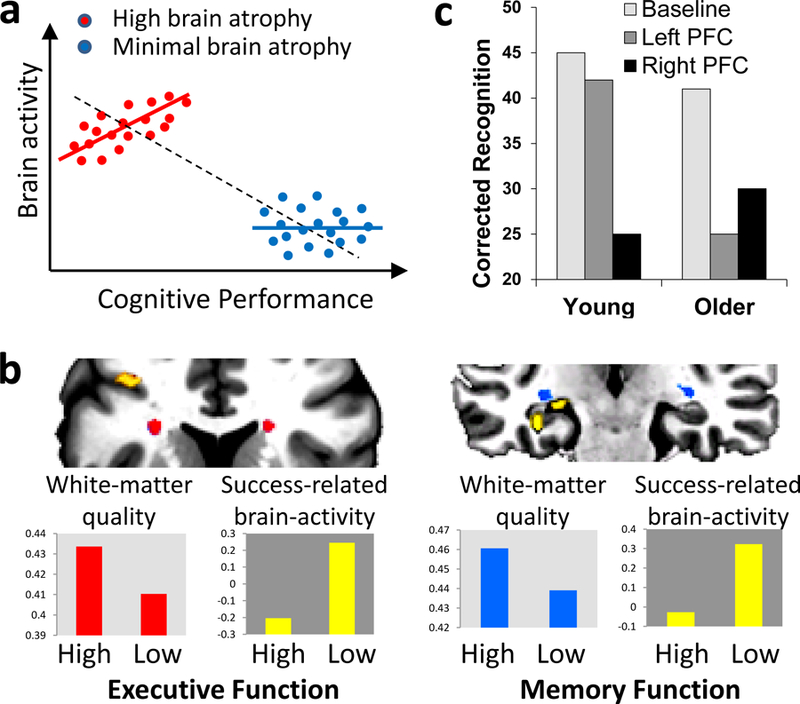
There are three basic methods for linking functional neuroimaging activations to successful cognitive performance. (1) Correlation across participants. This is the most common approach, and the only one available when using blocked fMRI designs. If the activity of a brain region that is recruited to a greater extent by older adults than by younger adults is positively correlated with performance in older adults, the finding is consistent with compensation. A potential problem of correlation across-participants is Simpson’s paradox: the direction of association at the population level may be different in the subgroups or the individuals comprising the population.88 For example, if one hypothesizes that increased activity compensates for brain atrophy, then a positive activity-cognition correlation should be expected in individuals with high brain atrophy; however, if one calculates the correlation using all individuals, including those with minimal brain atrophy) a negative correlation could be found (part a adapted from REF88). Thus, correlations should be conducted within the group in which compensation is assumed to take place. (2) Correlation within participants. This approach, which requires an event related design (using fMRI or EEG), bypasses Simpson’s paradox but it requires the reasonable assumption that compensatory processes vary from trial to trial. The activity-performance association should be stronger in individuals with greater brain decline (that is, those in which there is a supply-demand gap). Consistent with this idea, a study found greater success-related fMRI activity in older adults with low white-matter integrity. This link was found both for executive function in PFC and for memory function in MTL (part b from REF.89). (3) Noninvasive brain stimulation. If a brain region is engaged during a task by older but not younger adults, then disrupting or enhancing the function of this region using brain stimulation should have a greater impact on task performance in older than younger adults. For example, a study found that rTMS of right PFC disrupted episodic memory retrieval regardless of age, whereas rTMS of left PFC disrupted retrieval in older adults but not in younger adults, suggesting that this region contributed to retrieval only in older adults (part c, from REF.90). Brain stimulation goes beyond correlations by establishing a causal link between localized brain activity and performance.
In our effort to reach consensus about the use of the term compensation, we found it necessary to distinguish among three different mechanisms or forms of compensation (all incorporating the two criteria above): upregulation, selection, and recruitment of additional processes (see FIG 3). We posit that these forms of compensation are not mutually exclusive such that one or more may co-occur within or across individuals.
Figure 3. Compensation.
A. Hypothetical demand-activity function in young and old adults: as task demands increase activity first rises, then asymptotes, and finally declines. Because of reduced neural resources, this demand-activity function is shifted to the left in older adults, and hence they tend to show greater activity in the same regions as younger adults at lower levels of task difficulty (e.g., level 2) but lower activity at higher levels of task difficulty.82 B. Example of compensation by upregulation (consistent with the hypothetical function in panel A): in an fMRI study, older adults showed greater working memory activity in the right DLPFC than younger adults at lower levels of task demands (working memory load of 5 letters) but less activity at higher levels of task demands higher working memory load (working memory load of 7 letters, from REF.85) . C. Example of compensation by selection. This fMRI study compared age effects on the rich form of memory known as “recollection” and the less precise form of memory known as “familiarity,” measured in the same recognition memory task.86 Compared to younger adults, older adults showed reduced recollection-related activity in the hippocampus but increased familiarity-related activity in rhinal cortex . Thus, older adults compensated for deficits in an optimal but demanding process (recollection) by recruiting a suboptimal but less demanding process (familiarity). D. Example of compensation by reorganization. During an episodic memory retrieval task, young adults and low-performing older adults showed unilateral frontal activity whereas high-performing older adults showed bilateral frontal activity, suggesting a reorganization of the episodic retrieval network (from REF.87).
Compensation by upregulation refers to the enhancement of cognitive performance by boosting a neural process in response to task demands. In such cases, the processes recruited by older adults would be the same as those engaged by younger adults, with the primary difference being quantitative: older adults would engage the process to a greater extent than younger adults. Compensatory upregulation could explain the frequent finding that at least some age-related activity increases are evident within the same brain regions that younger adults recruit during the same task.81 Although greater activity in older adults may merely reflect inefficiency,82 it is difficult to interpret greater activity as inefficient when it correlates positively with cognitive performance, which is one of our requirements for applying the term compensation.
It is worth noting that young adults may also upregulate activity in response to increased task demands, but that the demand threshold for upregulation may be higher in young, compared to older adults24,36. As task difficulty increases, neural activity (particularly in frontal regions) tends to increase up to a certain level33, beyond which activation asymptotes and ultimately declines (FIG. 3–A)36,37. It is assumed that the asymptote reflects available neural resources, and the final decline reflects the breakdown in cognitive performance when these resources are exceeded. Several studies36,38–41 have found that, consistent with reduced neural resources, older adults show a greater increase in activity, a lower asymptote, and an earlier decline than younger adults (FIG. 3–A, example in FIG. 3–B). Because age differences in brain activity can depend on task difficulty, researchers should ideally investigate multiple levels of task demands.
Compensation by selection occurs when older adults recruit neural circuitry associated with cognitive processes that are available to but not engaged by young adults under the same objective task conditions. For example, older adults may engage a less effective but also a less demanding process, whereas younger adults prefer a more effective but more demanding one. To explain this idea with a metaphor, during a swimming competition an older adult may prefer to breaststroke, which is slower but easier, whereas a younger adult may choose freestyle, which is faster but harder. (Note that selecting a neural implementation of a behavioral strategy need not be as deliberate as choosing a swimming stroke.) The important point is that the process selected by older adults is also available to young adults, but less likely to be the one that supports their performance. An experimental example of compensation by selection is shown in Figure 3–C (see caption).
Unlike compensation by upregulation, compensation by selection involves a qualitative difference in the cognitive processes engaged by older and younger individuals, and hence it is likely to be associated with the recruitment of different brain regions, rather than recruitment of the same region with differential levels of activity (as in upregulation). Note that without a parametric manipulation of task demands, one could mistakenly attribute an over-activation in older adults to selection rather than upregulation. Also, older adults may show activation in a different region than young adults, but this region could be a different component of the same functional network recruited by younger adults, suggesting compensation by upregulation rather than selection. One possible method to distinguish compensation by upregulation vs. selection is to use repetitive transcranial magnetic stimulation (see Box 1). Finally, we note that this form of compensation is related to individual differences in reserve because some older adults may have a larger repertoire of alternative neural implementation of a given behavior than others, reflecting differences in accumulated reserve.
Finally, compensation by reorganization occurs when older adults use an alternative neural mechanism to respond to aging-induced losses that is not available to younger individuals76. The closest analogy to this type of compensation would be the development of new neural mechanisms following brain damage. For example, there is evidence that recovery from aphasia following a left-hemisphere stroke is associated with the recruitment of right hemispheric regions that do not support language processes in the normal brain83. Although these alternative circuits may be less effective than the original ones, their recruitment may still benefit performance. In the case of aging, reorganization could underlie the well-established finding that older adults often show more bilateral patterns of brain activity than younger adults43–45 (see example in FIG. 3–D). If the new regions engaged by older adults are sometimes recruited by younger adults in other similar or more difficult conditions, then the finding would be more consistent with compensation by selection. It is worth noting that although we compared reorganization due to aging and due to brain damage, they differ in several ways, including the fact that the time-course of aging is slow whereas the course of brain injury is fast. It is not clear which time-course is better for reorganization: a slow change gives the brain more time to adapt, but a fast change provides a clear trigger for reorganization. Clarification of the issue awaits future research.
It has been suggested that it may be useful to distinguish between a narrow and a broad notion of compensation. According to this distinction, compensation by reorganization would meet the stringent criterion of compensation in the narrow sense because a new process is generated in response to a loss. In contrast, compensation by upregulation or by selection would only qualify as compensation in the broader sense because these forms of compensation rely on an already existing process (upregulation) or an already existing strategy (selection) and do not require the evolution of a new process or structure.
Conclusions
In this article, we have tried to elucidate three important concepts that are widely used in studies of brain and cognitive aging, namely: reserve, maintenance, and compensation. Reserve refers to the accumulation of brain resources during the lifespan; maintenance to the preservation of these resources via constant recovery and repair; and compensation to the deployment of these resources in response to task demands. In other words, reserve is about how much you have, maintenance is about how well you keep it, and compensation is about when and how you use it.
Although we have discussed reserve, maintenance, and compensation in separate sections, they can operate concurrently and affect each other. For example, if education augments reserve by increasing synaptic density, this can only go on to attenuate age-related cognitive decline if the new synapses are preserved via maintenance. Thus, when considering the potential effects of cognitive training on promoting reserve, one has to consider whether age-related deficits in maintenance will render these effects less effective. Likewise, it is not enough to accumulate reserve and maintain it, it is also necessary to deploy these resources during task performance in response to task demands, that is to engage in compensation. Future neuroimaging research should aim to link more directly predictions derived from maintenance, reserve, and functional compensation. Such studies will result in stronger models of successful cognitive aging, which are essential for the interpretation of findings from studies of pathological aging, most notably, Alzheimer’s disease (see Box 2).
In addition, we note that despite our focus on healthy aging, the concepts we have discussed also can be applied in other domains, including child development, acute brain injury, neurodegeneration, and psychiatric illness, and we hope the present paper will help promote consensus in these domains as well. That is, individuals with neurological disease or disorder may compensate for their disorder-related deficit in ways similar to those described here for healthy older adults. We also note with caution that most studies of cognitive aging to date have been limited to testing samples of high functioning, highly educated, and mostly Caucasian healthy older adults. In order to develop more representative models of cognitive and brain aging, we strongly recommend expanding inclusion criteria to encompass individuals from diverse backgrounds84. This will of course require funding sufficient to support the large, ideally longitudinal, studies that such research requires, with an emphasis on combining longitudinal observations with intervention studies to gauge long-term effects of physical exercise, cognitive training, and other variables. Such studies will allow researchers to better understand age-related changes in brain and cognition in terms of biological aging (senescence), variations in environmental and genetic factors for a given birth cohort, and the secular trends in health, education, and technology that will determine the aging trajectories of future generations.
Acknowledgements:
To address this topic the co-authors of this manuscript participated in a two-day symposium at McGill University, Montreal, Canada on May 31st – June 2, 2017, which was funded by the Canadian Institutes of Health Research (C.I.H.R.), Duke University (North Carolina, U.S.A) and Douglas Institute Research Centre (Montreal, Canada).
Footnotes
Competing interests
The authors declare no competing interests.
Contributor Information
Roberto Cabeza, Duke University.
Marilyn Albert, John Hopkins University.
Sylvie Belleville, University of Montreal.
Fergus Craik, Baycrest Centre and University of Toronto.
Audrey Duarte, Georgia Tech.
Cheryl Grady, Baycrest Centre and University of Toronto.
Ulman Lindenberger, Max Planck Institute for Human Development.
Lars Nyberg, Umea University, Sweden.
Denise Park, University of Texas, Dallas.
Patricia A. Reuter-Lorenz, University of Michigan
Michael D. Rugg, University of Texas, Dallas
Jason Steffener, University of Ottawa.
M. Natasha Rajah, McGill University.
References
- 1.Beard JR et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet 387, 2145–2154, doi: 10.1016/S0140-6736(15)00516-4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerstorf D et al. Secular Changes in Late-Life Cognition and Well-Being: Towards a Long Bright Future With a Short Brisk Ending? Psychol. Aging 30, 301–310, doi: 10.1037/pag0000016 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Raz N & Daugherty AM Pathways to Brain Aging and Their Modifiers: Free-Radical-Induced Energetic and Neural Decline in Senescence (FRIENDS) Model-A Mini-Review. Gerontology 64, 49–57 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller RA Age-related changes in T cell surface markers: A longitudinal analysis in genetically heterogeneous mice. Mech Ageing Dev 96, 181–196 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Roy AK et al. Impacts of transcriptional regulation on aging and senescence. Ageing Res Rev 1, 367–380 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Foster TC Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus 22, 656–669, doi: 10.1002/hipo.20935 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papenberg G, Salami A, Persson J, Lindenberger U & Backman L Genetics and functional imaging: effects of APOE, BDNF, COMT, and KIBRA in aging. Neuropsychol Rev 25, 47–62, doi: 10.1007/s11065-015-9279-8 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Campisi J Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes. Exp Gerontol 38, 5–11 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Musiek ES & Holtzman DM Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008, doi: 10.1126/science.aah4968 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hullinger R & Puglielli L Molecular and cellular aspects of age-related cognitive decline and Alzheimer’s disease. Behav Brain Res 322, 191–205, doi: 10.1016/j.bbr.2016.05.008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattson MP & Arumugam TV Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab 27, 1176–1199, doi: 10.1016/j.cmet.2018.05.011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoro A et al. Innate immunity and cellular senescence: The good and the bad in the developmental and aged brain. J Leukoc Biol 103, 509–524, doi: 10.1002/JLB.3MR0118-003R (2018). [DOI] [PubMed] [Google Scholar]
- 13.Backman L, Lindenberger U, Li SC & Nyberg L Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev 34, 670–677, 10.1016/j.neubiorev.2009.12.008 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Sampedro-Piquero P, Alvarez-Suarez P & Begega A Coping with Stress During Aging: The Importance of a Resilient Brain. Curr Neuropharmacol 16, 284–296, doi: 10.2174/1570159X15666170915141610 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosario ER, Chang L, Head EH, Stanczyk FZ & Pike CJ Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging 32, 604–613, doi: 10.1016/j.neurobiolaging.2009.04.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan DG The dopamine and serotonin systems during aging in human and rodent brain. A brief review. Prog Neuropsychopharmacol Biol Psychiatry 11, 153–157 (1987). [DOI] [PubMed] [Google Scholar]
- 17.Freeman GB & Gibson GE Dopamine, acetylcholine, and glutamate interactions in aging. Behavioral and neurochemical correlates. Ann N Y Acad Sci 515, 191–202 (1988). [DOI] [PubMed] [Google Scholar]
- 18.Sperling R Potential of functional MRI as a biomarker in early Alzheimer’s disease. Neurobiol Aging 32 Suppl 1, S37–43, doi: 10.1016/j.neurobiolaging.2011.09.009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grady C The cognitive neuroscience of ageing. Nat Rev Neurosci 13, 491–505, doi: 10.1038/nrn3256 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tromp D, Dufour A, Lithfous S, Pebayle T & Despres O Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Res Rev 24, 232–262, doi: 10.1016/j.arr.2015.08.006 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Walhovd KB et al. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging 32, 916–932, doi: 10.1016/j.neurobiolaging.2009.05.013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajah MN, Maillet D, and Grady CL in The Wiley Handbook of Cognitive Neuroscience of Memory (ed Barense M Addis D, Duarte A) 347–361 (Wiley Publishers, 2015). [Google Scholar]
- 23.Nyberg L et al. Longitudinal evidence for diminished frontal cortex function in aging. Proceedings of the National Academy of Sciences USA 107, 22682–22686, 10.1073/pnas.1012651108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabeza R, Nyberg L & Park DC Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging Second Edition., (Oxford University Press, 2017). [Google Scholar]
- 25.Salat DH et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci 1064, 37–49 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Giorgio A et al. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage 51, 943–951, 10.1016/j.neuroimage.2010.03.004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madden DJ et al. Adult age differences in functional connectivity during executive control. Neuroimage 52, 643–657, 10.1016/j.neuroimage.2010.04.249 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craik FIM & Salthouse TA The Handbook of Aging and Cognition (Lawrence Erlbaum Associates, 2000). [Google Scholar]
- 29.Lindenberger U Human cognitive aging: Corriger la fortune? Science 346, 572–578 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Sole-Padulles C et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 30, 1114–1124, doi: 10.1016/j.neurobiolaging.2007.10.008 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Barulli D & Stern Y Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci 17, 502–509, doi: 10.1016/j.tics.2013.08.012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arenaza-Urquijo EM et al. Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurobiol. Aging 59, 72–79, doi: 10.1016/j.neurobiolaging.2017.06.016 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Nyberg L, Lovden M, Riklund K, Lindenberger U & Backman L Memory aging and brain maintenance. Trends Cogn Sci 16, 292–305, doi: 10.1016/j.tics.2012.04.005 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Grady CL Age-related changes in cortical blood flow activation during perception and memory. Annals of the New York Academy of Science 777, 14–21 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Cabeza R, Anderson ND, Locantore JK & McIntosh AR Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage 17, 1394–1402. (2002). [DOI] [PubMed] [Google Scholar]
- 36.Rajah MN & D’Esposito M Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain : a journal of neurology 128, 1964–1983, doi: 10.1093/brain/awh608 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Persson J & Nyberg L Altered brain activity in healthy seniors: what does it mean? Prog Brain Res 157, 45–56 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Reuter-Lorenz PA & Cappell KA Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci, 177–182 (2008). [Google Scholar]
- 39.Park DC & Reuter-Lorenz P The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol 60, 173–196, doi: 10.1146/annurev.psych.59.103006.093656 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arenaza-Urquijo EM & Vemuri P Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology 90, 695–703 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beard J, Officer A, Cassels A 59 (World Health Organization, Luxembourg, 2015).
- 42.Habib R, Nyberg L & Nilsson LG Cognitive and non-cognitive factors contributing to the longitudinal identification of successful older adults in the Betula study. Aging Neuropsychol. Cogn 14, 257–273, doi: 10.1080/13825580600582412 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Ronnlund M & Nilsson LG Flynn effects on sub-factors of episodic and semantic memory: parallel gains over time and the same set of determining factors. Neuropsychologia 47, 2174–2180, doi: 10.1016/j.neuropsychologia.2008.11.007 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Trahan LH, Stuebing KK, Fletcher JM & Hiscock M The Flynn effect: a meta-analysis. Psychol Bull 140, 1332–1360, doi: 10.1037/a0037173 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deary IJ, Whiteman MC, Starr JM, Whalley LJ & Fox HC The impact of childhood intelligence on later life: following up the Scottish mental surveys of 1932 and 1947. J Pers Soc Psychol 86, 130–147, doi: 10.1037/0022-3514.86.1.130 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Nyberg L, Pudas S & Lundquist A in Cogntiive Neuroscience of Aging, Second Edition (eds Cabeza R, Nyberg L, & Park DC) (Oxford University Press, 2016). [Google Scholar]
- 47.Raz N et al. Regional Brain Changes in Aging Healthy Adults: General Trends, Individual Differences and Modifiers. Cerebral cortex (2005). [DOI] [PubMed] [Google Scholar]
- 48.Ghisletta P, Rabbitt P, Lunn M & Lindenberger U Two thirds of the age-based changes in fluid and crystallized intelligence, perceptual speed, and memory in adulthood are shared. Intelligence 40, 260–268, doi: 10.1016/j.intell.2012.02.008 (2012). [DOI] [Google Scholar]
- 49.Stern Y, Gazes Y, Razlighi Q, Steffener J & Habeck C A task-invariant cognitive reserve network. Neuroimage 178, 36–45, doi: 10.1016/j.neuroimage.2018.05.033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piras F, Cherubini A, Caltagirone C & Spalletta G Education mediates microstructural changes in bilateral hippocampus. Hum Brain Mapp 32, 282–289, doi: 10.1002/hbm.21018 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stern Y Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord 20, 112–117, doi: 10.1097/01.wad.0000213815.20177.1900002093-200604000-00006[pii] (2006). [DOI] [PubMed] [Google Scholar]
- 52.Scarmeas N et al. Cognitive reserve-mediated modulation of positron emission tomographic activations during memory tasks in Alzheimer disease. Arch Neurol 61, 73–78, doi: 10.1001/archneur.61.1.7361/1/73[pii] (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stern Y Cognitive reserve. Neuropsychologia 47, 2015–2028, 10.1016/j.neuropsychologia.2009.03.004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barulli D & Stern Y Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn. Sci 17, 502–509, doi: 10.1016/j.tics.2013.08.012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soldan A et al. Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer’s disease. Hum Brain Mapp 36, 2826–2841, doi: 10.1002/hbm.22810 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bialystok E, Craik FIM & Luk G Bilingualism: consequences for mind and brain. Trends Cogn. Sci 16, 240–250, doi: 10.1016/j.tics.2012.03.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prakash RS, Voss MW, Erickson KI & Kramer AF in Annual Review of Psychology, Vol 66 Vol. 66 Annual Review of Psychology (ed Fiske ST) 769-+ (Annual Reviews, 2015). [DOI] [PubMed] [Google Scholar]
- 58.Scarmeas N & Stern Y Cognitive reserve and lifestyle. J Clin Exp Neuropsychol 25, 625–633, doi: 10.1076/jcen.25.5.625.14576 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bialystok E, Craik FIM & Freedman M Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia 45, 459–464, doi: 10.1016/j.neuropsychologia.2006.10.009 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Alladi S et al. Bilingualism delays age at onset of dementia, independent of education and immigration status. Neurology 81, 1938–1944, doi: 10.1212/01.wnl.0000436620.33155.a4 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Anthony M & Lin F A Systematic Review for Functional Neuroimaging Studies of Cognitive Reserve Across the Cognitive Aging Spectrum. Arch. Clin. Neuropsychol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reed BR et al. Cognitive Activities During Adulthood Are More Important than Education in Building Reserve. J. Int. Neuropsychol. Soc 17, 615–624, doi: 10.1017/s1355617711000014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zahodne LB et al. Quantifying Cognitive Reserve in Older Adults by Decomposing Episodic Memory Variance: Replication and Extension. J. Int. Neuropsychol. Soc 19, 854–862, doi: 10.1017/s1355617713000738 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reed BR et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain 133, 2196–2209, doi: 10.1093/brain/awq154 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y et al. Sound credit scores and financial decisions despite cognitive aging. Proc. Natl. Acad. Sci. U. S. A 112, 65–69, doi: 10.1073/pnas.1413570112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindenberger U, Kliegl R & Baltes PB PROFESSIONAL EXPERTISE DOES NOT ELIMINATE AGE-DIFFERENCES IN IMAGERY-BASED MEMORY PERFORMANCE DURING ADULTHOOD. Psychol. Aging 7, 585–593, doi: 10.1037/0882-7974.7.4.585 (1992). [DOI] [PubMed] [Google Scholar]
- 67.Morrow D, Leirer V, Altieri P & Fitzsimmons C WHEN EXPERTISE REDUCES AGE-DIFFERENCES IN PERFORMANCE. Psychol. Aging 9, 134–148, doi: 10.1037/0882-7974.9.1.134 (1994). [DOI] [PubMed] [Google Scholar]
- 68.Vaci N, Gula B & Bilalic M Is age really cruel to experts? Compensatory effects of activity. Psychol Aging 30, 740–754, doi: 10.1037/pag0000056 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Stern Y, Albert S, Tang M & Tsai W Rate of memory decline in AD is related to education and occupation. Neurology 1, 1942–1947 (1999). [DOI] [PubMed] [Google Scholar]
- 70.Ten Brinke LF et al. Aerobic exercise increases cortical white matter volume in older adults with vascular cognitive impairment: A 6-month randomized controlled trial. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 11, P606 (2015). [Google Scholar]
- 71.Gorbach T et al. Longitudinal association between hippocampus atrophy and episodic-memory decline. Neurobiol. Aging 51, 167–176, doi: 10.1016/j.neurobiolaging.2016.12.002 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Persson J et al. Longitudinal structure–function correlates in elderly reveal MTL dysfunction with cognitive decline. Cerebral cortex 22, 2297–2304 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Pudas S et al. Brain characteristics of individuals resisting age-related cognitive decline over two decades. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 8668–8677, doi: 10.1523/JNEUROSCI.2900-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raz N & Lindenberger U Only time will tell: cross-sectional studies offer no solution to the age-brain-cognition triangle: comment on Salthouse (2011). Psychol Bull 137, 790–795, doi: 10.1037/a0024503 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kovari E, Herrmann FR, Bouras C & Gold G Amyloid deposition is decreasing in aging brains: an autopsy study of 1,599 older people. Neurology 82, 326–331, doi: 10.1212/WNL.0000000000000069 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Lovden M, Backman L, Lindenberger U, Schaefer S & Schmiedek F A Theoretical Framework for the Study of Adult Cognitive Plasticity. Psychol. Bull 136, 659–676, doi: 10.1037/a0020080 (2010). [DOI] [PubMed] [Google Scholar]
- 77.Beam CR & Turkheimer E Phenotype-environment correlations in longitudinal twin models. Dev. Psychopathol 25, 7–16, doi: 10.1017/s0954579412000867 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lovden M, Ghisletta P & Lindenberger U Social participation attenuates decline in perceptual speed in old and very old age. Psychol. Aging 20, 423–434 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Cabeza R & Dennis NA in Principles of frontal lobe function (eds Stuss Donald T. & Knight Robert T.) (Oxford University Press, 2013). [Google Scholar]
- 80.Bakker A et al. Reduction of Hippocampal Hyperactivity Improves Cognition in Amnestic Mild Cognitive Impairment. Neuron 74, 467–474 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spreng RN, Wojtowicz M & Grady CL Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neuroscience & Biobehavioral Reviews 34, 1178–1194 (2010). [DOI] [PubMed] [Google Scholar]
- 82.Reuter-Lorenz PA & Cappell KA Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science 17, 177–182 (2008). [Google Scholar]
- 83.Hope TMH et al. Right hemisphere structural adaptation and changing language skills years after left hemisphere stroke. Brain 140, 1718–1728, doi: 10.1093/brain/awx086 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Falk EB et al. What is a representative brain? Neuroscience meets population science. Proc. Natl. Acad. Sci. U. S. A 110, 17615–17622, doi: 10.1073/pnas.1310134110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cappell KA, Gmeindl L & Reuter-Lorenz PA Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex 46, 462–473, doi: 10.1016/j.cortex.2009.11.009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daselaar SM, Fleck M, Dobbins IG, Madden DJ & Cabeza R Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. . Cerebral cortex 16, 1771–1782 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cabeza R, Anderson ND, Locantore JK & McIntosh AR Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage 17, 1394–1402 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Kievit RA, Frankenhuis WE, Waldorp LJ & Borsboom D Simpson’s paradox in psychological science: a practical guide. Front. Psychol 4, 14, doi: 10.3389/fpsyg.2013.00513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daselaar SM et al. Less Wiring, More Firing: Low-Performing Older Adults Compensate for Impaired White Matter with Greater Neural Activity. Cerebral cortex 25, 983–990, doi: 10.1093/cercor/bht289 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rossi S et al. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 7939–7944 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albert MS et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers. Dement 7, 270–279, doi: 10.1016/j.jalz.2011.03.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Driscoll I & Troncoso J Asymptomatic Alzheimer’s disease: a prodrome or a state of resilience? Curr Alzheimer Res 8, 330–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brickman AM et al. White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging 32, 1588–1598, doi: 10.1016/j.neurobiolaging.2009.10.013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Landau SM et al. Association of Lifetime Cognitive Engagement and Low beta-Amyloid Deposition. Arch. Neurol 69, 623–629, doi: 10.1001/archneurol.2011.2748 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soldan A et al. Relationship of cognitive reserve and cerebrospinal fluid biomarkers to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Neurobiol. Aging 34, 2827–2834, doi: 10.1016/j.neurobiolaging.2013.06.017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dickerson BC et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol 56, 27–35 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huijbers W et al. Amyloid-beta deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain 138, 1023–1035, doi: 10.1093/brain/awv007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clement F & Belleville S Compensation and Disease Severity on the Memory-Related Activations in Mild Cognitive Impairment. Biological Psychiatry 68, 894–902, doi: 10.1016/j.biopsych.2010.02.004 (2010). [DOI] [PubMed] [Google Scholar]
- 99.Bookheimer SY et al. Patterns of brain activation in people at risk for Alzheimer’s disease. New England Journal of Medicine 343, 450–456 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rajah MN et al. Family history and APOE4 risk for Alzheimer’s disease impact the neural correlates of episodic memory by early midlife. Neuroimage Clin 14, 760–774, doi: 10.1016/j.nicl.2017.03.016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Celone KA et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 10222–10231 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elman JA et al. Neural compensation in older people with brain amyloid-beta deposition. Nat. Neurosci 17, 1316–1318, doi: 10.1038/nn.3806 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Esposito Z et al. Amyloid beta, Glutamate, Excitotoxicity in Alzheimer’s Disease: Are We on the Right Track? CNS Neurosci. Ther 19, 549–555, doi: 10.1111/cns.12095 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rudy CC, Hunsberger HC, Weitzner DS & Reed MN The Role of the Tripartite Glutamatergic Synapse in the Pathophysiology of Alzheimer’s Disease. Aging Dis 6, 131–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Belleville S et al. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain 134, 1623–1634, doi: 10.1093/brain/awr037 (2011). [DOI] [PubMed] [Google Scholar]