Abstract
The current opioid crisis has reinvigorated interest in development of therapeutics for opioid use disorder, and the choice of preclinical translational endpoints is an essential consideration. Anti-opioid immunopharmacotherapies (e.g. conjugate vaccines) that sequester drug peripherally, preventing opioids from reaching targeted receptors in the brain have recently emerged as promising therapeutics.
Keywords: opioid, vaccine, hapten, drug self-administration, choice
Unmet needs in the treatment of opioid use disorder
The United States is in the midst of a public health crisis involving opioid abuse and overdose deaths. Opioid abuse can be categorized from mild to severe based on eleven diagnostic criteria and treated by the medical community as an opioid use disorder (OUD) [1]. In response to this crisis, the National Institutes of Health recently outlined scientific strategies to focus efforts in developing treatments for OUD and overdose prevention and reversal [2]. Current Food and Drug Administration (FDA)-approved medications for OUD include opioid agonists (e.g. methadone or buprenorphine) and opioid antagonists (e.g. naltrexone). Another opioid antagonist naloxone (Narcan©) is FDA-approved for opioid overdose reversal. All currently approved medications work by binding to opioid receptors in the brain and either activate (e.g. methadone and buprenorphine) these receptors similar to the abused opioid or block (e.g. naltrexone and naloxone) these receptors from being activated by the abused opioid. Despite clinicians having these pharmacological tools at their disposal to treat OUD and opioid overdose, there are both regulatory and societal barriers to the broad utilization of these medications [3]. Furthermore, not all patients respond positively to currently available medications [3]. Thus, there is a critical need for preclinical and clinical research to develop more effective and readily available medications.
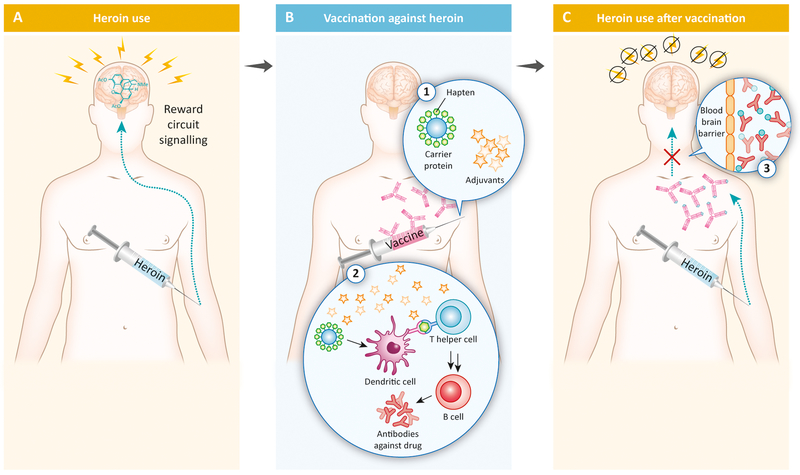
One category of candidate medication currently under development to address this unmet clinical need is immunopharmacotherapy. Immunopharmacotherapy is defined as the use highly specific antibodies, raised passively or actively, to sequester drugs of interest in the bloodstream. An example of an immunopharmacotherapy would be a conjugate vaccine that elicits an immune response to the abused opioid (e.g. heroin) to produce antibodies that would bind heroin in the periphery and prevent heroin from crossing the blood brain barrier and activating reward circuit signaling (Figure 1). There are three potential clinical advantages of this type of treatment for OUD over current FDA-approved treatments. First, immunopharmacotherapies would lack abuse potential, in contrast to the opioid agonists methadone and buprenorphine. Second, immunopharmacotherapies are selective for the targeted drug compared to the nonselective antagonism of all opioid receptors with naltrexone or naloxone. This specificity may be especially relevant for clinical use of opioid agonists in pain management situations. Finally, immunopharmacotherapies may have a longer duration of therapeutic effectiveness compared to current FDA-approved treatments and thus require less frequent dosing intervals.
Figure 1:
Mechanism of action for conjugate vaccines as candidate immunopharmacotherapies for opioid use disorder (OUD) and overdose adapted with permission from [18]. Panel A shows an individual injecting heroin intravenously to activate reward circuit signaling. Panel B shows intramuscular administration of a vaccine composed of the opioid hapten conjugated to a carrier protein (e.g. tetanus toxoid) and clinically available adjuvants elicits an antibody response against the targeted abused opioid (e.g. heroin). Panel C shows the presence of antibodies preventing the abused opioid from entering the brain, binding to opioid receptors, and activating reward circuit signaling.
Progress in development of anti-opioid conjugate vaccines as candidate medications
Anti-opioid conjugate vaccines represent one promising research area for OUD treatment, including relapse and overdose. Mechanistically unique to current FDA-approved opioid receptor therapeutics (methadone, buprenorphine, naltrexone, naloxone), conjugate vaccines prompt an individual’s immune system to generate high affinity, anti-opioid antibodies (Figure 1B). These antibodies bind to the target opioid in the blood and form a complex that is too large to enter the brain and thus prevent the opioid from activating reward circuit signaling (Figure 1C). To successfully train the immune system, vaccines must incorporate three components: a hapten, immunogenic carrier, and adjuvant(s). Opioid agonists (e.g. heroin) are blind to the immune system due their size. As a result, researchers have developed a strategy where opioid analogues, termed haptens, are chemically linked to immunogenic carriers (e.g. adenoviruses, nanoparticles, or foreign proteins). Coupled with adjuvants, or molecules that strengthen an immune response, the hapten-protein complexes are recognized by antigen presenting cells of the immune system as foreign invaders. These immune cells engulf the complex, digest the protein into peptides, and present hapten-peptide antigens to immune cells, which produce antibodies specifically tuned to the opioid hapten (Figure 1B). The crux of vaccine development for OUD is raising specific, high affinity, anti-opioid antibodies in high concentration. For example, a cocaine vaccine that failed to raise and maintain both high levels and exquisite affinity antibodies amongst all clinical trial participants also demonstrated poor clinical effectiveness as measured by cocaine negative urine samples [4]. Subsequent preclinical research efforts have addressed these limitations by improving antibody concentration, affinity and specificity through hapten, immunogenic carrier, and adjuvant optimization (for review, see [5]).
Vaccines targeting heroin, fentanyl, oxycodone, and hydrocodone are currently in various stages of preclinical development. For example, the Janda Laboratory at the Scripps Research Institute has developed an anti-heroin vaccine that reduced heroin potency >15-fold in mice, >10-fold in rats, and >4-fold in monkeys [5]. Crucial to clinical translation, these heroin vaccines maintained high affinity anti-heroin antibody concentrations and blunted heroin challenges on a timescale of months. Conjugate anti-heroin and anti-oxycodone vaccines have been also been developed by researchers at Walter Reed Army Institute of Research [6] and Minneapolis Medical Research Foundation [7]. Anti-fentanyl vaccines are in earlier stages of development, primarily resulting from the drug’s recent emergence in illicit drug use as a contaminant in the United States heroin supply. The Janda Laboratory also reported the first vaccine for fentanyl, which demonstrated a 30-fold reduction in fentanyl potency in mice. Moreover, the vaccine protected against lethal fentanyl doses, alluding to the possibility that the vaccine could have overdose reversal utility [8].
Challenges in the development of candidate immunopharmacotherapies for OUD
Efforts to develop new medications for treating OUD will require translational research involving both laboratory animals (i.e. preclinical) and humans. However, the diverse endpoints used for candidate medication assessment both within and across laboratory animals and humans have been one confounding factor that has impeded the drug development process [3, 9]. For example, clinical trials assessing candidate treatments for OUD use negative urine samples as the primary endpoint because the FDA requires evidence of discontinued drug use or abstinence for approval considerations [3]. Human laboratory studies assess candidate treatment effectiveness by measuring drug-taking behavior using a drug vs. money choice procedure and drug choice as the primary endpoint [10]. An effective treatment should attenuate or eliminate opioid choices and correspondingly increase money or other nondrug reinforcer choices as shown in Figure 2 [10]. Preclinical (i.e. rat or monkey) laboratory studies also assess candidate treatment effectiveness by measuring drug-taking behavior. However, in contrast to human laboratory studies, the primary endpoint is typically rates of drug-taking behavior and not behavioral allocation (i.e. choice) between a drug and nondrug alternative reinforcer [9]. The identification and utilization of endpoints that are clinically relevant and translatable across multiple species could facilitate preclinical-to-clinical translation and enhance the success in OUD drug discovery. Preclinical measures of behavioral allocation between nondrug and drug reinforcers could serve this purpose because six of the eleven diagnostic criteria for OUD [1] are based on the allocation of behavior towards the procurement and use of the abused opioid compared with other behaviors maintained by more adaptive alternative reinforcers (Figure 2).
Figure 2:
A conceptual framework for how opioid use disorder (OUD) manifests clinically based on diagnostic criteria (Panel A) [1] and how an effective treatment should change behavior maintained by abused drugs (e.g. heroin) and nondrug reinforcers (e.g. food, money, social commendation) (Panel B) adapted with permission from [9]. In a natural environment, a human allocates his/her behavior (or makes “choices”) based on numerous environmental, pharmacological, and biological factors. For most individuals, available nondrug reinforcers (e.g. food, money, social commendation) are effective to minimize or eliminate behavior directed towards the procurement and use of heroin. However, some individuals misallocate their behavior by choosing (red arrow) heroin at the expense of these nondrug reinforcers and this may lead to an OUD diagnosis (Panel A). This misallocation of behavior may be due to environmental, pharmacological, and biological factors that remain to be fully understood. Candidate OUD treatments should not only decrease behavior maintained by heroin (faded red arrow), but also increase behavior (thicker black arrows) maintained by socially adaptive, nondrug reinforcers (Panel B). The utilization of shared endpoints between preclinical and clinical studies related to this misallocation of behavior should strengthen the translational bridge in the development of candidate medications for OUD.
The translational concordance of candidate medication effects in preclinical drug vs. food choice procedures was recently highlighted in the evaluation of buspirone as a candidate medication for cocaine use disorder. For example, repeated buspirone treatment decreased rates of cocaine-taking behavior [11], but failed to attenuate cocaine choice and increase food choice in nonhuman primates [12]. Buspirone treatment has also been evaluated in both a clinical trial [13] and a human laboratory drug self-administration study [14]. Buspirone failed to significantly decrease cocaine-positive urine samples and cocaine choice under both clinical settings. The utilization of shared endpoints should strengthen the translational bridge between preclinical and clinical studies in the development of candidate medications for OUD, such as immunopharmacotherapies.
Remaining challenges to develop immunopharmacotherapies for OUD
Two significant challenges remain for translation of these preclinical anti-opioid vaccine results to clinical trials. One challenge is the choice of translational endpoints for assessing vaccine effectiveness in preclinical studies. Although anti-opioid vaccines decrease rates of opioid-taking behavior in preclinical studies [15–17], anti-opioid conjugate vaccine effectiveness to decrease opioid choice and increase food choice is currently unknown. As highlighted above with the recent translational failure of buspirone, the utilization of shared endpoints between preclinical and clinical studies should enhance translational concordance. Second, successful clinical translation of anti-opioid vaccines will require the navigation of alternative funding mechanisms. Limited funding for continued preclinical evaluation, especially safety profiling and large-scale vaccine production, has delayed potential clinical trial start dates. The National Institute on Drug Abuse has funded the bulk of the preclinical work described above, but their financial contribution alone is insufficient to see these vaccines through clinical trial translation. Unfortunately, industry investment, which often propels late stage drug candidates for other diseases or mental health disorders, has largely been absent. Despite the present economic situation, the field is better poised than ever before to deliver a clinically-relevant opioid vaccine to address the current opioid crisis.
Acknowledgements
The authors and portions of the research described are supported by the National Institutes of Health grants F32DA044692 and UH3DA041146. The National Institute on Drug Abuse had no role in the writing or decision to submit the manuscript for publication. The manuscript content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
References
- 1.American Psychiatric Association. (2013) Diagnositc and Statistical Manual of Mental Disorders, Fifth Edition, American Psychiatric Association. [Google Scholar]
- 2.Volkow ND and Collins FS (2017) The Role of Science in Addressing the Opioid Crisis. N Engl J Med 377 (4), 391–394. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND et al. (2018) Medication development in opioid addiction: Meaningful clinical end points. Sci Trans Med 10 (434). [DOI] [PubMed] [Google Scholar]
- 4.Kosten TR et al. (2014) Vaccine for cocaine dependence: A randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend 140, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremer PT et al. (2017) Development of a Clinically Viable Heroin Vaccine. J Am Chem Soc 139 (25), 8601–8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sulima A et al. (2018) A Stable Heroin Analogue That Can Serve as a Vaccine Hapten to Induce Antibodies That Block the Effects of Heroin and Its Metabolites in Rodents and That Cross-React Immunologically with Related Drugs of Abuse. J Med Chem 61 (1), 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raleigh MD et al. (2018) Opioid Dose- and Route-Dependent Efficacy of Oxycodone and Heroin Vaccines in Rats. J Pharmacol Exp Ther 365 (2), 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bremer PT et al. (2016) Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew Chem Int Ed Engl55 (11), 3772–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banks ML and Negus SS (2017) Insights from Preclinical Choice Models on Treating Drug Addiction. Trends Pharmacol Sci 38, 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comer SD et al. (2008) The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend 96 (1–2), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mello NK et al. (2013) Effects of Chronic Buspirone Treatment on Cocaine Self-Administration. Neuropsychopharmacology 38 (3), 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John WS et al. (2015) Effects of buspirone and the dopamine D3 receptor compound PG619 on cocaine and methamphetamine self-administration in rhesus monkeys using a food-drug choice paradigm. Psychopharmacology 232 (7), 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winhusen TM et al. (2014) Multisite, randomized, double-blind, placebo-controlled pilot clinical trial to evaluate the efficacy of buspirone as a relapse-prevention treatment for cocaine dependence. J Clin Psychiatry 75 (7), 757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolin BL et al. (2016) Buspirone reduces sexual risk-taking intent but not cocaine self-administration. Exp Clin Psychopharmacol 24 (3), 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raleigh MD et al. (2014) Pharmacokinetic Correlates of the Effects of a Heroin Vaccine on Heroin Self-Administration in Rats. PLOS ONE 9 (12), e115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlosburg JE et al. (2013) Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Nat Acad Sci USA 110 (22), 9036–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonese KF et al. (1974) Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 252 (5485), 708–710. [DOI] [PubMed] [Google Scholar]
- 18.Olson ME and Janda KD (2018) Vaccines to combat the opioid crisis. Vaccines that prevent opioids and other substances of abuse from entering the brain could effectively treat addiction and abuse. EMBO Rep 19 (1), 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]