The chromosome 22q11.2 deletion syndrome (22q11.2DS; MIM #188400) is the most common microdeletion syndrome with an estimated prevalence of 1:2,000–1:6,000 live births and 1:1,000 unselected fetuses (McDonald-McGinn et al., 2015). Based on the 2010 U.S. Census and employing a prevalence of 1:2,000, there should be ~150,000 Americans with the 22q11.2DS, including ~35,000 children and adolescents, which begs the question—where are these individuals?—perhaps awaiting diagnosis, including in the perinatal period. This is notable as 22q11.2DS is an important cause of morbidity and mortality across the lifespan, as outlined in this special issue, and despite development of a qPCR-based newborn screening test (NBS) designed specifically for 22q11.2DS earlier this decade (Tomita-Mitchell et al., 2010). Routine NBS for 22q11.2DS has not yet been initiated by any government healthcare agency across the globe, perpetuating reports of families continuing to traverse a diagnostic odyssey, in many instances for a protracted period of time and often with adverse consequence, as discussed in the manuscript contributed by Palmer et al. (2018). The importance of early diagnosis, inclusive of the chromosome 22q11.2 deletion, as well as, consequential associated features resulting in better outcomes, is highlighted herein.
Most frequently, the result of a ~2.5 Mb hemizygous 22q11.2 deletion, this condition provides insight into key developmental genes and pathways that may improve our understanding of major birth defects and disorders of later onset, as reviewed by Bernice Morrow et al. in both Molecular genetics of 22q11.2 deletion syndrome and Variance of IQ is partially dependent on deletion type among 1,427 22q11.2 deletion syndrome subjects, as well as, in Variable Immune Deficiency Related to Deletion Size in Chromosome 22q11.2 Deletion Syndrome as reported by Kathleen Sullivan et al., in particular as 22q11.2DS is the most common cause of syndromic palatal anomalies, the most common cause of schizophrenia, and the second most common cause of congenital heart disease and developmental delay after Down syndrome. In fact, as Marta Unolt et al. describes in Congenital Heart Diseases and Cardiovascular Abnormalities in 22q11.2DS, the chromosome 22q11.2 deletion is a more common cause of tetralogy of Fallot than Down syndrome and, as emphasized by Schindewolf et al. (2018) should always be considered as a cause of conotruncal cardiac anomalies in the prenatal setting. This is likewise true for other less commonly associated anomalies, including congenital diaphragmatic hernia and club foot, and related functional problems such as polyhydramnios.
Historically, the combination of findings now known to be most frequently caused by a chromosome 22q11.2 deletion included the neonatal co-occurrence of thymic aplasia and hypoparathyroidism. These features, described initially by Sedlackova (1955), Lobdell (1959), and most memorably by DiGeorge (1965;Figure 1) were subsequently referred to as DiGeorge syndrome (DGS). Thereafter, recognition of congenital heart disease, especially involving the outflow tract by the Japanese as a key feature of DGS contributed to the theory that a mechanism leading to perturbation of neural crest cell migration, particularly affecting the third and fourth pharyngeal arches, may be involved (Takao, Ando, Cho, Kinouchi, & Murakami, 1980). Cytogenetically visible chromosome 22q11.2 deletions were first reported in the early 1980s (de la Chapelle, Herva, Koivisto, & Aula, 1981; Kelley et al., 1982). In the early 1990s, fluorescence in situ hybridization (FISH) studies using probes within the commonly deleted region identified submicroscopic 22q11.2 deletions as the most frequent cause of DGS (Driscoll et al., 1992; Scambler et al., 1991). This preceded recognition that several seemingly unrelated conditions with overlapping phenotypic features were also caused by a 22q11.2 deletion, including velocardiofacial (VCFS; Driscoll et al., 1993), conotruncal anomaly face syndrome (CTAF; Burn et al., 1993; Matsuoka et al., 1994), and subsets of patients with Opitz G/BBB syndrome (McDonald-McGinn et al., 1995), and as reported by the Italians, Cayler Cardiofacial syndrome (Giannotti, Digilio, Marino, Mingarelli, & Dallapiccola, 1994), all described by clinicians concentrating on specific areas of expertise, for example: DGS by an endocrinologist, VCFS by a speech pathologist, and CTAF by a cardiologist (McDonald-McGinn, Low, & Zackai, 1997; Figure 2). Today, the term DGS is reserved for instances when the etiology of clinical features is not caused by a 22q11.2 deletion, for example: in patients with a CHD7 mutation “resulting in CHARGE syndrome,” as a result of a mutation in TBX1, in offspring of diabetic mothers, or as a result of retinoic acid embryopathy. Otherwise, the broad phenotypic spectrum, with or without features of classic DGS, is referred to by the underlying cytogenetic nomenclature, the 22q11.2DS providing a key unifying diagnosis for families, caregivers, payors, and support organizations (Bassett et al., 2011; McDonald-McGinn et al., 2015).
FIGURE 1.
Dr. Angelo DiGeorge (center) was born on April 15, 1921 in Philadelphia, PA. He died on October 11, 2009 also in Philadelphia, where he was educated, practiced pediatric endocrinology, performed breakthrough research, and taught students at St. Christopher’s Hospital for Children and Temple University. Dr. DiGeorge is seen here, in 2006, on the occasion of the dedication of the Angelo M. DiGeorge teaching Center at St. Christopher’s Hospital for Children, pictured with his close collaborators and friends, Donna M. McDonald-McGinn, MS, LCGC (left) and Elaine H. Zackai, MD (right), from the Children’s Hospital of Philadelphia and Perelman School of Medicine of the University of Pennsylvania
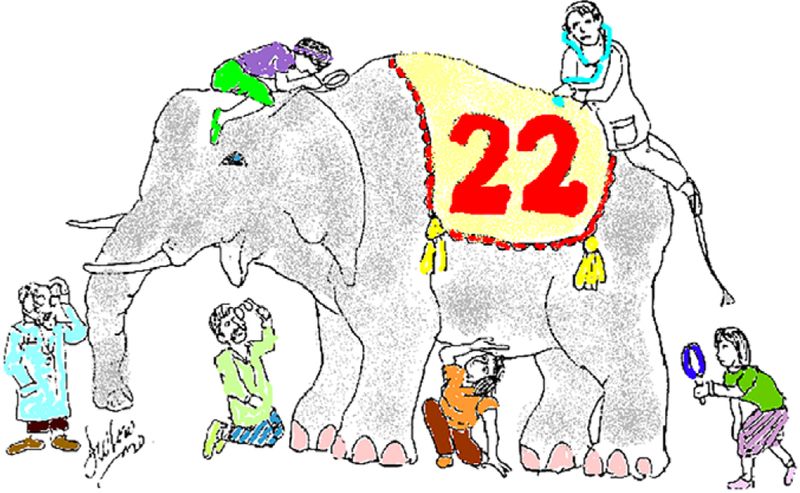
FIGURE 2.
Our colorized version of the image previously published in 1997 in this journal (McDonald-McGinn etal., 1997) illustrating the old adage of the nearsighted veterinarians trying to describe an elephant by each examining a separate part. So too was the case of the chromosome 22q11.2 deletion syndrome when reported by individual subspecialists concentrating on specific areas of expertise, for example, endocrinology, cardiology, and speech pathology. It was not until the development of FISH studies in the early 1990s that investigators were able to see the big picture and recognize that many previously described clinical conditions were actually caused by the 22q11.2 deletion
Fast forward to 2018, and the 22q11.2 deletion is now known to cause additional birth defects, such as club foot as reported by Jelle Homans et al. in Orthopaedic Manifestations within the 22q11.2 Deletion Syndrome; later onset conditions including autoimmune disease and malignancies as described by Michele Lambert et al. in The 22q11.2 Deletion Syndrome: Cancer Predisposition, Platelet Abnormalities and Cytopenias; cognitive delays as outlined by Ann Swillen et al. in Neurodevelopmental Outcome in 22q11.2 DS and Management; behavioral differences as elucidated by Ania Fiksinski et al. in Pediatric Psychiatry; neurologic issues in children and adults as reported by Sarah Hopkins et al. in Neurologic Challenges in 22q11.2 Deletion Syndrome and Nancy Butcher et al. in Adult Movement Disorders respectively; and psychiatric illness as explained by Sunny Tang et al. in Adult Neurology and Schizophrenia. All far exceeding the original description of DGS and delineated in great detail by several additional authors in this issue. Moreover, as reported by Jennifer Cohen et al., in 22q and Two: 22q11.2 Deletion Syndrome and Coexisting Conditions, given the frequency of 22q11.2DS, some atypical features may now be ascribed to the co-occurrence of an additional diagnosis by chance alone, for example, 22q11.2 deletion in a child with Severe Combined Immunodeficiency (SCID), or as a result of a mutation in an important developmental gene on the other allele unmasking an autosomal recessive condition, for example in, GP1BB resulting in Bernard–Soulier syndrome and in SNAP29 causing CEDNIK syndrome.
Although common, lack of recognition of the condition and/or lack of familiarity with current testing methods to identify patients with atypical nested deletions—those excluding the LCR22A–LCR22B region inclusive of FISH probes (N25 and TUPLE) and the important developmental gene TBX1—as reported by Ian Campbell et al. in What’s New with 22q? An update from the 22q and You Center at the Children’s Hospital of Philadelphia, together with wide variability, often delays diagnosis which could improve outcomes. In fact, adults with 22q11.2DS may only come to attention following the birth of a child with related congenital anomalies, most often heart disease (McDonald-McGinn et al., 2001). While most typical (LCR22A–LCR22D) 22q11.2 deletions are de novo (>90%), as a result of nonallelic homologous recombination because of the presence of low copy repeats, once present the 50% recurrence risk for this contiguous gene deletion syndrome, as first described by the late Roy Schmickel (Schmickel, 1986), becomes a major focus for adults with 22q11.2DS, and their parents. Moreover, the 22q11.2DS is not related to maternal age so young woman are equally likely to have a child with 22q11.2DS as women of advanced maternal age.
Importantly, as highlighted by Alisdair McNeill et al. in The psychosocial impact of 22q11 deletion syndrome on patients and families—a systematic review, the overall prevalence of significant medical problems varies by age at ascertainment and the focus changes over time. In fact, in early infancy there is attention to birth defects, associated medical issues, motor milestones, and speech and language. By school age, families and caregivers shift the emphasis to academics and peer relations, whereas in adolescents and adulthood, all focus shifts primarily to discussions surrounding independence, behavioral differences, and recurrence risk. So, how can healthcare providers in this age of extraordinarily complex and fast paced medicine address these time intensive and multifaceted issues? Well, familiarity with the condition is the first step, and while the availability of pediatric (Bassett et al., 2011) and adult (Fung et al., 2015) healthcare guidelines has already provided essential consensus in caring for these patients, the details afforded by this special issue are unprecedented, particularly in emphasizing the need to “treat what is treatable” as early diagnosis and effective management improves outcome—from a physical, neurocognitive and behavioral perspective, as illustrated by Katheryn Grand et al. in Endocrine Manifestations and the Impact of Hypocalcemia on FSIQ in Patients with 22q11.2 DS and Buijs, Bassett, and Boot (2018). We hope you find this issue as valuable a resource as we intended!
Footnotes
CONFLICT OF INTEREST
Donna McDonald-McGinn has given lectures on 22q11.2 Deletion Syndrome for Natera, Inc.
REFERENCES
- Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, … International 22q11.2 deletion syndrome consortium. (2011). Practical guidelines for managing patients with 22q11.2 deletion syndrome. The Journal of Pediatrics, 159, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs PCM, Bassett AS, & Boot E (2018). Non-pharmacological treatment of psychiatric disorders in individuals with 22q11.2 deletion syndrome: A systematic review. American Journal of Medical Genetics. Part A, 176, 1742–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn J, Takao A, Wilson D, Cross I, Momma K, Scambler P, & Goodship J (1993). Conotruncal anomaly face syndrome is associated with a deletion within chromosome 22q11. Journal of Medical Genetics, 30, 822–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Chapelle A, Herva R, Koivisto M, & Aula P (1981). A deletion in chromosome 22 can cause DiGeorge syndrome. Human Genetics, 57, 253–256. [DOI] [PubMed] [Google Scholar]
- DiGeorge A (1965). Discussion on a new concept of the cellular immunology. The Journal of Pediatrics, 67, 907–908. [Google Scholar]
- Driscoll DA, Salvin J, Sellinger B, McDonald-McGinn D, Zackai EH, & Emanuel BS (1993). Prevalence of 22q11 microdeletions in DGS and VCFS: Implications for genetic counseling and prenatal diagnosis. Journal of Medical Genetics, 30, 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll DA, Spinner NB, Budarf ML, McDonald-McGinn DM, Zackai EH, Goldberg RB, … Emanuel BS (1992). Deletions and microdeletions of 22q11.2 in velo-cardio-facial syndrome. American Journal of Medical Genetics, 44, 261–268. [DOI] [PubMed] [Google Scholar]
- Fung WL, Butcher NJ, Costain G, Andrade DM, Boot E, Chow EW, … Bassett AS (2015). Practical guidelines for managing adults with 22q11.2 deletion syndrome. Genetics in Medicine, 17, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti A, Digilio MC, Marino B, Mingarelli R, & Dallapiccola B (1994). Cayler cardiofacial syndrome and del 22q11: Part of the CATCH22 phenotype. American Journal of Medical Genetics, 53, 303–304. [DOI] [PubMed] [Google Scholar]
- Kelley RI, Zackai EH, Emanuel BS, Kistenmacher M, Greenberg F, & Punnett HH (1982). The association of the DiGeorge anomalad with partial monosomy of chromosome 22. The Journal of Pediatrics, 101, 197–200. [DOI] [PubMed] [Google Scholar]
- Lobdell DH (1959). Congenital absence of the parathyroid glands. American Medical Association Archives of Pathology, 67, 412–415. [PubMed] [Google Scholar]
- Matsuoka R, … Momma K (1994). Confirmation that the conotruncal anomaly face syndrome is associated with a deletion within 22q11.2. American Journal of Medical Genetics, 53, 285–289. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Driscoll DA, Bason L, Christensen K, Lynch D, Sullivan K, … Zackai EH (1995). Autosomal dominant “Opitz” GBBB syndrome due to a 22q11.2 deletion. American Journal of Medical Genetics, 59, 103–113. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Low D, & Zackai EH (1997). Letter to the editor: What’s in a name? The 22q11.2 deletion. American Journal of Medical Genetics, 72, 247. [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan K, Marino B, Philip N, Swillen A, Vortsman J, … Bassett A (2015). 22q11.2 deletion syndrome. Nature Reviews Disease Primers, 1, 15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Tonnesen MK, Laufer-Cahana A, Finucane B, Driscoll DA, Emanuel BS, & Zackai EH (2001). Phenotype of the 22q11.2 deletion in individuals identified through an affected relative: Cast a wide FISHing net! Genetics in Medicine, 3, 23–29. [DOI] [PubMed] [Google Scholar]
- Palmer LD, Butcher NJ, Boot E, Hodgkinson KA, Heung T, Chow EC, … Bassett AS (2018). Elucidating the diagnostic odyssey of 22q11.2 deletion syndrome. American Journal of Medical Genetics. Part A, 176, 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambler PJ, Carey AH, Wyse RKH, Roach S, Dumanski JP, Nordenskjold M, & Williamson R (1991). Microdeletions within 22q11 associated with sporadic and familial DiGeorge syndrome. Genomics, 10, 201–206. [DOI] [PubMed] [Google Scholar]
- Schindewolf E, Khalek N, Johnson MP, Gebb J, Coleman B, Crowley TB., … Moldenhauer JS (2018). Expanding the fetal phenotype: Prenatal sonographic findings and perinatal outcomes in a cohort of patients with a confirmed 22q11.2 deletion syndrome. American Journal of Medical Genetics, 176, 1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmickel RD (1986). Contiguous gene syndromes: A component of recognizable syndromes, 109, 231–241. [DOI] [PubMed] [Google Scholar]
- Sedlackova E (1955). Insufficiency of palatolaryngeal passage as a developmental disorder. Casopís Lékar̆ů Cφeských, 94, 1304–1307. [PubMed] [Google Scholar]
- Takao A, Ando M, Cho K, Kinouchi A, & Murakami Y (1980). In Van Praagh R & Takao A (Eds.), Etiologic categorization of common congenital heart disease. Mount Kisco, NY: Futura Publishing Company. [Google Scholar]
- Tomita-Mitchell A, Mahnke DK, Larson JM, Ghanta S, Feng Y, Simpson PM, … Mitchell ME (2010). Multiplexed quantitative real-time PCR to detect 22q11.2 deletion in patients with congenital heart disease. Physiological Genomics, 42A, 52–60. 10.1152/physiolgenomics.00073.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]