Abstract
Interventions that improve health are often associated with longevity. Reduced growth hormone signaling has been shown to increase life span in mice by over 50%. Similarly, reductions in dietary intake of methionine, in rats and mice, result in life-span extension. Many factors affect metabolic health, mitochondrial function, and resistance to stressors, each of which influence aging and life span. This paper presents a comparison of these two interventions, as well as the results of a study combining these interventions, to understand potential mechanisms underlying their effectiveness in enhancing healthy aging.
Keywords: growth hormone, diet restriction, methionine, metabolism, longevity
Dietary interventions significantly affect health, aging, and life span. It is well known that reducing caloric intake, or dietary restriction (DR; including calorie restriction and intermittent feeding), regardless of content, extends health span and life span in rodents, non-human primates, and other species.1 Plasma hormone levels are influenced by these nutritional interventions and exert downstream effects on physiological systems. For example, insulin levels decrease under DR protocols, in turn, affecting systemic glucose metabolism. The consistent lowering of plasma insulin-like growth factor 1 (IGF-1) in mammals is thought to be one of the major mechanisms by which DR exerts effects on aging processes and ultimately life span, although the mechanisms are still unclear. Altering specific components of the diet, such as the amino acid methionine, has also been shown to lower IGF-1 and increase longevity in rodents. Our laboratory and others have been conducting studies to better understand how a single amino acid can affect complex systems (e.g., the endocrine system) and lead to changes in metabolic pathways influencing health and aging.
Orentreich and colleagues2–4 demonstrated that rats fed low levels of methionine lived longer than those fed normal chow. Mice have also been shown to live longer when subjected to reductions in methionine intake.5 Extended health and longevity are also associated with altered plasma hormone levels. For example, reduced growth hormone (GH) signaling by either GH deficiency or resistance leads to life-span extension in mice.6–8 Although Ames and Snell dwarf mice lack prolactin and thyrotropin in addition to GH, the reduced GH signaling is thought to be the primary driver of the improved health and longevity extensions observed in these mice.9–12 In contrast, the over-expression of GH (high plasma levels) shortens the life span of GH transgenic mice by 50%.13 Similarly, dietary methionine in high levels is considered to be toxic;14–18 excessive methionine can be converted to toxic metabolites and cause oxidative injuries to the liver, hepatic encephalopathy, and splenic hemosiderosis. Interestingly, the methionine pathway is differentially affected in mice with altered GH status. Ames dwarf mice exhibit atypical methionine metabolism in comparison to GH-sufficient wild-type mice.19,20 Animals resistant to GH, namely, GH receptor knock-out (GHRKO) mice, display differences in this amino acid pathway with respect to gene and protein expression, as do Ames mice but to a lesser degree.21 Dwarf mice treated with GH, and GH transgenic mice, exhibit decreased expression and activities of components of this metabolic pathway, again indicating the relationship of this hormonal pathway with methionine metabolism.22
There are several shared characteristics between Ames dwarf mice and animals fed methionine-restricted diets, and these similarities likely contribute to the extended health and life spans observed in these mice. Both dwarf and methionine-restricted mice exhibit improvements in metabolic health and mitochondrial function when compared to wild-type and normal chow–fed mice, respectively. Physiological aspects that contribute to metabolic health include insulin sensitivity/resistance, body weight maintenance, body composition, endocrine status, and lipid parameters. A comparative summary of these features in dwarf versus methionine-restricted animals are shown in Table 1.
Table 1.
Phenotypic characteristics of GH signaling mutant dwarf mice and methionine restricted animals. Parameters measured in liver unless otherwise indicated. Arrows indicate direction of change compared to respective wild type or normal chow–fed controls.
↑↓↔ = no difference from controls.
| Genotype Characteristic | Ames Dwarf Snell Dwarf | Methionine restriction Mice, rats |
|---|---|---|
| Lifespan extension | ↑ | ↑ |
| Plasma insulin | ↓ | ↓ |
| Plasma glucose | ↓ | ↓ |
| Insulin sensitivity | ↑ | ↑ |
| Plasma IGF1 | ↓ | ↓ |
| Plasma Leptin | ↔ | ↓ |
| Plasma adiponectin | ↑ | ↑ |
| Serum cholesterol | ↓ | ↓ |
| Serum triglycerides | ↓ | ↓ |
| Body weight | ↓ | ↓ |
| Fat mass | ↓ | ↓ |
| Lean mass | ↓ | ↔ |
| Food consumption | ↑ | ↑ |
| mTOR | ↓ | ↔ |
| AMPK | ↑ | ↔ |
| Fatty acid oxidation | ↑ | ↓ |
| Respiratory control ratio (liver) | ↓ | ↓ |
| Mitochondrial H2O2 generation | ↓ | ↓ |
| Oxidative damage (protein, DNA) | ↓ | ↓ |
| Glutathione | ↑ | ↓ |
| Antioxidative enzymes (catalase, superoxide dismutase, glutathione peroxidase) | ↑ | ↔ |
| Cellular stress resistance | ↑ | ↔ |
Ames dwarf mice begin adult life at about one-third the body size of a wild-type sibling, typically weighing 10–12 g as adults.23 With regard to body composition, Bartke’s laboratory showed that lean and fat mass were significantly decreased in dwarf mice when compared to wild-type mice at young, adult, and old ages.24 The percent fat mass was also lower in dwarf animals than in corresponding adult and old wild-type mice. Ames mice consume more food per gram of body weight than their wild-type counterparts.25 Available data from studies using methionine-restricted diets in both rats and mice indicate similar responses among the reports. The studies in rodents used diets reducing methionine by approximately 80% compared to the levels in normal chow (0.17 vs. 0.86 g/kg, respectively; based on early work of Orentreich).2 Rodents were lighter in weight following placement on these diets, whether the food was introduced at 1 month of age or later (12 months).2,5,26 Fat mass is routinely decreased in studies of methionine restriction, whereas few differences are observed in lean mass between methionine-restricted rodents and those fed normal chow.27–29 Miller and coworkers reported that mice fed a control diet (0.43% methionine) consumed 3.8 g of food per day, whereas those receiving the methionine-restricted diets ate 4.3 g of food per day, representing a significant increase.5 Both groups started at the same body weight at the beginning of the food consumption study. Similar increases in food consumption have been reported in rats on methionine-restricted diets when food intake was expressed in terms of body weight.2,30 Thus, both Ames mice and rodents fed methionine-restricted diets consume more food per gram of body weight yet maintain lower body weight as adults, when compared to their respective controls.
As mentioned previously, the endocrine system plays a multifaceted role in metabolic health, in which glucose metabolism is a key component. Appropriate glucose and insulin levels in fasted and fed states lead to optimal levels of insulin sensitivity and general well-being. In humans, insulin resistance is a well-documented risk factor for diabetes and cardiovascular disease, among several other age-related diseases.31 Ames dwarf mice exhibit low plasma glucose and insulin levels throughout life when compared to normal, wild-type mice.32,33 Plasma insulin levels rise acutely in response to a glucose bolus or to refeeding after fasting, and insulin injection suppresses plasma glucose levels greater, in Ames mice compared to normal mice.33 Recent hyperinsulinemic–euglycemic clamp studies indicate that the extreme insulin sensitivity in Ames mice is mediated by enhanced hepatic, skeletal muscle, and adipose tissue insulin action and improved insulin signaling.34,35 Anthony and Gietzen36 demonstrated that plasma insulin levels drop within 7 days of feeding methionine-restricted diets and are fourfold lower after 2 months. Serum glucose levels are also lower in methionine-restricted rodents.5,30 These data, among data from other studies, strongly suggest that insulin sensitivity is also enhanced in rodents maintained on methionine-restricted diets.27,37,38
Reduced GH/IGF-1 signaling in mammals and invertebrates has been shown to increase longevity, indicating the evolutionary significance of this endocrine pathway.9,39–41 Ames mice lack plasma GH, resulting in circulating IGF-1 levels that are extremely low.23 The low IGF-1 levels are reflected in the delayed onset of cancer, lower incidence of tumors and tumor burden, as well as reductions in mammalian target of rapamycin (mTOR) and downstream effects on protein translation and in organ-specific alterations in apoptosis.42–45 Similarly, plasma IGF-1 levels decrease significantly (40%), and lower levels are maintained in mice and rats following introduction to low-methionine diets.5,30,46 Although IGF-1 levels are markedly reduced, no advantage of consuming methionine-restricted diets has been observed for neoplastic disease, when compared to control mice.5 There is some evidence suggesting that low methionine in combination with choline deficiency increases hepatic steatosis and hepatocellular carcinoma.47,48 Further evidence suggests that low levels of methionine, S-adenosylmethionine, or glutathione (GSH) can lead to liver injury and disease.49 In contrast, several studies also indicate that methionine deficiency may improve cancer outcomes by depriving tumor cells of this essential amino acid, and thus increasing their vulnerability to chemotherapeutic agents.50–55 Thus, reductions in IGF-1 concentrations in methionine restriction may or may not affect cancer development, as this essential amino acid influences other contributing pathways.
Hormones involved in lipid metabolism are affected by dietary methionine. For example, adiponectin and fibroblast growth factor 21 are increased by methionine restriction, and each contributes to enhanced insulin sensitivity.30 Plasma leptin levels are lower, likely due to the reduction in fat mass initiated by the restricted diets, while serum cholesterol and triglycerides are also decreased in rats on methionine-restricted diets compared to animals fed normal chow.30,55 Similarly, Ames and Snell dwarf mice exhibit higher plasma adiponectin levels and low cholesterol, triglyceride, and free fatty acid levels.44,56–59 Adiponectin levels remained higher in dwarf mice even following GH treatment.44 The level of circulating leptin was not found to be different between genotypes in young females or adult males, but leptin was lower in adult and old female dwarfs compared to age-matched wild-type females.24 Two other research groups also measured plasma leptin in Ames and wild-type mice and found no significant differences, possibly reflecting age and sex differences in the animals used.44,56 Collectively, these reports suggest that the endocrine system of dwarf and methionine-restricted rodents appear to exhibit somewhat similar profiles with respect to metabolic health.
Energy expenditure has been studied in detail in both of these long-living groups of animals. Methionine restriction reduces growth and the deposition of fat by increasing energy expenditure.38 Since neither lean body mass nor activity appears to be affected by methionine restriction, various methods to calculate energy expenditure have provided similar answers. Overall, it appears that DR of methionine increases energy expenditure by increasing the energy costs of maintenance and growth.27,36–38 With regard to substrate utilization, the data to date suggest that metabolic flexibility is enhanced by this dietary intervention.27,38 Ames dwarf mice exhibit increases in oxygen consumption per unit body weight and significant reductions in respiratory quotient, suggesting that energy utilization is shifted from growth and reproduction to maintenance and repair.60–62 There are several reports indicating increased fatty acid oxidation in dwarf mice, further supporting these results.57,63–67 Hepatic fatty acid oxidation, however, appears to be downregulated in methionine-restricted animals, indicating a significant deviation from that observed in dwarf mice.68
Nutrient signaling and growth factor signaling pathways are integrated. Nutrient sensing is controlled by several factors, including GH/IGF-1, insulin, AMP-activated protein kinase (AMPK), mTOR, and sirtuin1, and levels of amino acids are detected by these nutrient sensors. For example, low concentrations of specific amino acids are detected by mTOR, an intracellular sensor of nutrient status and regulator of protein synthesis, cell growth, and proliferation. Excess methionine is also sensed and is sometimes toxic because of the inability of the system to redistribute excess amino acids effectively. TORC1, one of two mTOR effectors, has multiple functions related to coordinating anabolic and catabolic processes in response to growth, nutrients, and energy status.69 TORC1 is activated by insulin, IGF-1, and nutrients and is repressed by AMPK, a sensor of cellular energy status.70–72 The actions of mTOR and mTORC1 are diminished in multiple tissues of dwarf (Ames, Snell, and GHRKO) mice.12,44,45,73,74 It has been shown that mTORC2 activity is enhanced in liver tissue of dwarf (Snell) and GHRKO mice, indicating a potential compensatory response to low mTORC1 levels; the functions of mTORC2 are much less clear than mTORC1.12 In animals fed methionine-restricted diets, phosphorylated mTOR remains unaltered when compared to animals on diets containing normal levels of methionine; this contrasts with the findings in DR and in dwarf (Ames and Snell) and GHRKO mice showing decreased mTOR levels, indicating another difference between these long-living mice.12,26,44,54,73–75 AMPK levels are elevated in dwarf and GHRKO mice and may result from the higher levels of adiponectin.57,76 In addition, since AMPK is a mediator of fatty acid oxidation, elevated levels are indicative of the enhanced beta-oxidation reported in dwarf mice. In contrast, dietary methionine restriction does not appear to alter AMPK levels and may explain the described downregulation of components of fatty acid oxidation.77
Mitochondria contribute to numerous aspects of health and aging. Tissues from animals consuming methionine-restricted diets exhibit increases in energy expenditure owing to increased uncoupled respiration.27,37 Similarly, liver mitochondria from Ames dwarf mice are more uncoupled in comparison to wild-type tissue and produce less H2O2 (Brown-Borg, unpublished data).78 Many components of the electron transport chain are upregulated (mRNA, protein, or activity) in Ames mice, while in mice fed methionine-restricted diets, many of these oxidative phosphorylation components are decreased or unchanged (Brown-Borg, unpublished observations).58,79–81 In methionine-restricted human fibroblasts, mild uncoupling of respiration (decreased RCR) is observed along with decreased ROS production and a decrease in complex IV activity because of a decrease in the mitochondrially encoded complex IV subunit COX1.82
Dietary methionine restriction decreases mitochondrial ROS generation and oxidative damage to proteins and DNA.80,81,83–85 Similarly, GSH is decreased in liver tissues of animals fed low-methionine diets.3,84 Oxidative stress is also reduced with methionine restriction but not because of changes in the activities of antioxidative enzymes, as these activities are unaltered. Ames dwarf mice exhibit similar decreases in mitochondrial ROS production and oxidative damage to proteins and DNA, but antioxidative enzymes such as catalase, GSH peroxidase, and superoxide dismutase are upregulated (activities, protein, and mRNA).86 In addition, liver GSH levels are elevated in dwarf animals at 3, 12, and 24 months of age when compared to age-matched wild-type controls. These findings contrast with those reported in methionine-restricted rodents. Therefore, these two interventions result in longer living animals using potentially different mechanisms underlying mitochondrial function.
It has been widely reported that enhanced stress resistance is common among long-living species. Miller and colleagues have studied cellular resistance, using skin fibroblasts, in a variety of long-living animals and have shown that these animals tend to share an enhanced capacity to withstand various types of stress.87,88 Cells from Ames and Snell dwarf mice exhibit elevated resistance to more than one of the following: cadmium, heat, ultraviolet light, H2O2, paraquat, and some carcinogenic agents.87,88 Studies using other compounds have confirmed and extended this evidence, suggesting various mechanisms for the elevated responses observed in long-living mice, including differences in the expression of Nrf2, other ARE genes, FOXO, heat shock proteins, peroxisome proliferator activated receptors, glutathione S-transferases, ATF4, autophagy, among others.10,63,89–96 In sharp contrast, fibroblasts from mice on methionine-restricted diets do not exhibit resistance to cytotoxic agents in vitro.97 However, hepatocytes from these mice were resistant to the effects of acetaminophen, whereas dwarf mouse cells were not, suggesting that while cellular stress resistance was common to long-living mice, the tissue, type of resistance, and thus, the mechanism may vary.
With respect to other health span parameters, there are differences between GH-deficient and methionine-restricted mice. Ames dwarf mice exhibit a delayed occurrence of fatal neoplastic and non-neoplastic disease, including glomerulonephritis.42,98 Less data are available on neoplastic disease in methionine-restricted animals. Mice fed methionine-restricted diets were shown to exhibit the same types of illnesses as control mice but at slightly later ages (45 days), while others have shown that methionine restriction delays the development of prostate cancer in an animal model of this disease and inhibits chemically induced colon cancer in the rat.5,54,99 Both Snell dwarf and methionine-restricted mice exhibit a retardation in age-dependent T cell subset patterns, as well as a delay in the development of aging-related eye lens opacity.5,100,101 Overall, some similarities in health span parameters have been observed in animals with GH deficiency and methionine restriction, but the corresponding measures are not always the same across studies, making direct comparisons difficult. Collectively, these interventions appear to delay many age-sensitive endpoints.
Knowing that the ultimate outcome of these two interventions, reduced GH signaling and dietary methionine restriction, was similar, we designed studies to determine whether combining these strategies would produce an additional longevity benefit, as has been demonstrated in dietary-restricted Ames dwarf mice.102 We found that, in marked contrast to animals with sufficient plasma GH and GH signaling, Ames dwarf and GHRKO mice do not live longer when fed a methionine-restricted diet (80% restriction).103 Wild-type mice from each of these lines responded with increased longevity, similar to previously reported observations in rodents. We also tested two other levels of methionine representing a 50% restriction and a 50% supplementation. Ames and GHRKO mice did not exhibit any differences in longevity on these diets when compared to dwarf and knock-out mice on an 80% restricted diet or to their wild-type counterparts. Therefore, the absence of GH signaling prevents these animals from discriminating levels of amino acid in their diet. This inability to detect amino acid abundance may result, in part, from the low levels of mTOR in these animals. Short-living mice expressing high levels of GH (average life span of 12 months) and fed a methionine-restricted diet displayed a 50% increase in life span (18 months) over those on low- or high-methionine diets, further supporting that the presence of GH is necessary to respond to diets low in methionine.103
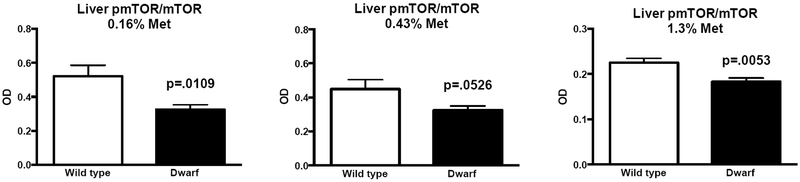
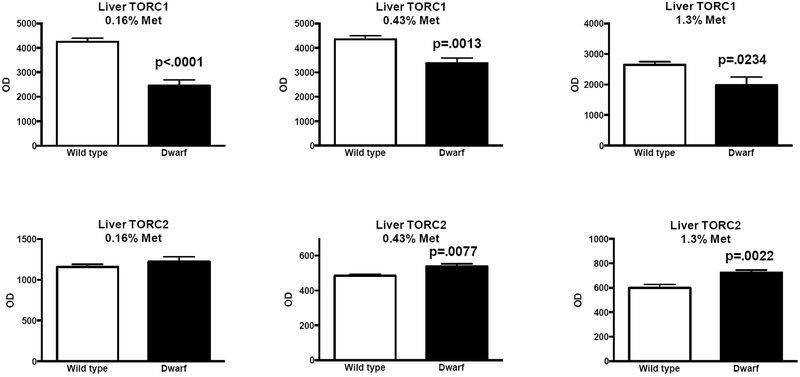
In further studies assessing the biochemical responses to methionine restriction, we found that, in general, Ames dwarf mice demonstrated an overall lack of responsiveness to the different diets when compared to their wild-type controls.46 Metabolite expression was examined in liver tissue, and gene and protein expression were examined in liver and other tissues of dwarf and normal mice consuming 0.16%, 0.43%, or 1.3% methionine. The body weights of dwarf mice were maintained on these diets, while the high-methionine diets increased body weights of wild-type mice. Methionine pathway and transsulfuration enzymes were elevated in dwarf mice regardless of methionine intake. Ames mice were also able to maintain high levels of GSH on methionine-restricted diets. Elevations in measures of fatty acid oxidation as well as coenzyme A were also observed in dwarf mice, which were unaffected by diet.46 Using liver tissue generated from this published study, we examined mTOR and show here that phosphorylated mTOR/total mTOR protein levels were decreased in Ames mice regardless of diet (Fig. 1), similar to previous reports in Ames dwarf mice fed with normal rodent chow. When diets and genotypes were compared by two-way ANOVA, only genotype was significant (interaction, P = 0.6919; diet, P = 0.4188; genotype, P = 0.0118; n = 4/diet/genotype; data not shown). In addition, TORC1 protein expression was found to be decreased in dwarf mice on each diet (0.16%, 0.43%, and 1.3% methionine), while TORC2 levels were elevated in animals on diets containing 0.43% and 1.3% methionine when compared to corresponding wild-type mice (Fig. 2), indicating that the differential methionine contents in the diets did not override the genotype differences established on normal rodent chow.
Figure 1.
Phosphorylated mTOR/mTOR protein expression (optical density (OD) units) in dwarf and wild-type mice consuming 0.16%, 0.43%, or 1.3% methionine for 8 weeks, beginning at 8 weeks of age (n = 11–13/genotype/diet). Data are presented as means ± SEM. (See Ref. 46 for information on the experimental conditions, including diets, tissue collection, and protein isolation.) Phospho-mTOR and mTOR antibodies were obtained from Cell Signaling Technology (Beverly, MA). Standard techniques that have been previously developed and published were employed.22,93 Chemiluminescence (Bio-Rad, Hercules, CA) and densitometry (Omega-Lum, Aplegen) were used for the detection and analysis of protein levels. Unpaired Student’s t-tests were used to determine significant differences among means.
Figure 2.
TORC1 and TORC2 protein expression (optical density (OD) units) in dwarf and wild-type mice consuming 0.16%, 0.43%, or 1.3% methionine for 8 weeks, beginning at 8 weeks of age (n = 11–13/genotype/diet). Data are presented as means ± SEM. (See Ref. 46 for information on the experimental conditions, including diets, tissue collection, and protein isolation.) TORC1 and TORC2 antibodies were obtained from Proteintech (Chicago, IL). Standard techniques that have been previously developed and published were employed.22,93 Chemiluminescence (Bio-Rad, Hercules, CA) and densitometry (Omega-Lum, Aplegen) were used for the detection and analysis of protein levels. Unpaired Student’s t-tests were used to determine significant differences among means.
As a point of comparison, there has been one study showing that DR extends life span, and one study showing that methionine restriction does not extend life span, in Ames mice.102,103 An additional report by Ikeno and colleagues98 compared pathology of Ames mice on DR and ad libitum fed Ames and DR wild-type mice, and found that Ames and DR mice have both independent and shared mechanisms underlying their extended longevity. In methionine-restricted (0.16%) Ames mice, we evaluated pathology on a gross level and found that these mice had fewer liver tumors compared to dwarf mice on a diet containing higher levels of methionine (0.43% and 1.3%), but no comparison was made to chow-fed Ames.103
In conclusion, many similarities exist between two longevity interventions, reduced GH signaling and dietary methionine restriction. The observed differences in some of the measures between the two interventions may result from the species studied, the background strains within species, as well as tissue specificities, as many reports focus on single-tissue responsiveness. Both interventions were shown to result in improved metabolic health, reduced oxidative stress and damage, and some indices of mitochondrial function and stress resistance, each of which contribute to the observed extensions of health span and life span. The research to date suggests that intact GH signaling is necessary to “sense” differences in dietary amino acid levels. The mechanisms underlying these responses have been partially identified and require further study.
Acknowledgments
This work was supported by NIH Grants RO1 AG034206 and KO2 AG038509, and the Ellison Medical Foundation.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Ingram DK & Roth GS. 2015. Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res. Rev 20: 46–62. [DOI] [PubMed] [Google Scholar]
- 2.Orentreich N, Matias JR, DeFelice A & Zimmerman JA. 1993. Low methionine ingestion by rats extends lifespan. J. Nutr 123: 269–274. [DOI] [PubMed] [Google Scholar]
- 3.Richie JP, Leutzinger Y Jr., Parthasarathy S, et al. 1994. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 8: 1302–1307. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman JA, Malloy V, Krajcik R & Orentreich N. 2003. Nutritional control of aging. Exp. Geron 38: 47–52. [DOI] [PubMed] [Google Scholar]
- 5.Miller RA, Buehner G, Chang Y, et al. 2005. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown-Borg HM, Borg KE, Meliska CJ & Bartke A. 1996. Dwarf mice and the ageing process. Nature 384: 33. [DOI] [PubMed] [Google Scholar]
- 7.Coschigano KT, Clemmons D, Bellush LL & Kopchick JJ. 2000. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology 141: 2608–2613. [DOI] [PubMed] [Google Scholar]
- 8.Sun LY, Spong A, Swindell WR, et al. 2013. Growth hormone-releasing hormone disruption extends lifespan and regulated response to caloric restriction in mice. Elife 2: e01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartke A, Sun LY & Longo V. 2013. Somatotropic signaling: trade-off between growth, reproductive development and longevity. Physiol. Rev 93: 571–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panici JA, Harper JM, Miller RA, et al. 2010. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 24: 5073–5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junnila RK, List EO, Berryman DE, et al. 2013. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol 9: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominick G, Berryman DE, List EO, et al. 2015. Regulation of mTOR activity in Snell dwarf and GH receptor gene-disrupted mice. Endocrinology 156: 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steger RW, Bartke A & Cecim M. 1993. Premature aging in transgenic mice expressing growth hormone genes. J. Reprod. Fertil. Suppl 46: 61–75. [PubMed] [Google Scholar]
- 14.Garlick PJ 2006. Toxicity of methionine in humans. J. Nutr 136: 1722S–1725S. [DOI] [PubMed] [Google Scholar]
- 15.Yalçinkaya S, Unlüçerçi Y, Giriş M, et al. 2009. Oxidative and nitrositive stress and apoptosis in the liver of rats fed on high methionine diet: protective effect of taurine. Nutrition 25: 436–444. [DOI] [PubMed] [Google Scholar]
- 16.Harper AE, Benevenga NJ & Wohlhueter RM. 1970. Effects of ingestion of disproportionate amounts of amino acids. Physiol. Rev 50: 428–558. [DOI] [PubMed] [Google Scholar]
- 17.Fukagawa NK 2006. Sparing of methionine requirements: evaluation of human data takes sulfur amino acids beyond protein. J. Nutr 136: 1676S–1681S. [DOI] [PubMed] [Google Scholar]
- 18.Yamada H, Akahoshi N, Kamata S, et al. 2012. Methionine excess in diet induces acute lethal hepatitis in mice lacking cystathionine γ-lyase, an animal model of cystathioninuria. Free Radic. Biol. Med 52: 1716–1726. [DOI] [PubMed] [Google Scholar]
- 19.Uthus EO & Brown-Borg HM. 2003. Altered methionine metabolism in long living Ames dwarf mice. Exp. Gerontol 38: 491–498. [DOI] [PubMed] [Google Scholar]
- 20.Uthus EO & Brown-Borg HM. 2006. Methionine flux to transsulfuration is enhanced in the long living Ames dwarf mouse. Mech. Ageing Dev 127: 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown-Borg HM, Rakoczy SG, Sharma S & Bartke A. 2009. Long-living growth hormone receptor knock out mice: potential mechanisms of altered stress resistance. Exp. Gerontol 44: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown-Borg HM, Rakoczy SG & Uthus EO. 2005. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech. Ageing Dev 126: 389–398. [DOI] [PubMed] [Google Scholar]
- 23.Bartke A & Brown-Borg HM. 2004. Life extension in the dwarf mouse. Curr. Top. Dev. Biol 63: 189–225. [DOI] [PubMed] [Google Scholar]
- 24.Heiman M, Tinsley F, Mattison J, et al. 2003. Body composition of prolactin-, growth hormone-, and thyrotropin-deficient Ames dwarf mice. Endocrine 20: 149–154. [DOI] [PubMed] [Google Scholar]
- 25.Mattison JA, Wright C, Bronson RT, et al. 2001. Studies of aging in Ames dwarf mice: effects of caloric restriction. J. Am. Aging Assoc 23: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun L, Sadighi Akha AA, Miller RA & Harper JM. 2009. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J. Gerontol. A Biol. Sci. Med. Sci 64: 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasek BE, Stewart LK, Henegan TM, et al. 2010. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am. J. Physiol. Regul. Integr. Comp. Physiol 299: R728–R739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elshorbagy AK, Kozich V, Smith AD & Refsum H. 2012. Cysteineand obesity: consistency of the evidence across epidemiologic, animal and cellular studies. Curr. Opin. Clin. Nutr. Metab. Care 15: 49–57. [DOI] [PubMed] [Google Scholar]
- 29.Elshorbagy AK 2014. Body composition in gene knockouts of sulfur amino acid-metabolizing enzymes. Mamm. Genome 25: 455–463. [DOI] [PubMed] [Google Scholar]
- 30.Malloy VL, Krajcik RA, Bailey SJ, et al. 2006. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 5: 305–314. [DOI] [PubMed] [Google Scholar]
- 31.Facchini FS, Hua N, Abbasi F & Reaven GM. 2001. Insulin resistance as a predictor of age-related diseases. J. Clin. Endocrinol. Metab 86: 3574–3578. [DOI] [PubMed] [Google Scholar]
- 32.Borg KE, Brown-Borg HM & Bartke A. 1995. Assessment of the primary adrenal cortical and pancreatic hormone basal levels in relation to plasma glucose and age in the unstressed Ames dwarf mouse. Proc. Soc. Exp. Biol. Med 210: 126–133. [DOI] [PubMed] [Google Scholar]
- 33.Dominici FP, Hauck S, Argentino DP, et al. 2002. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate IRS-1 and IRS-2 in liver of Ames dwarf mice. J. Endocrinol 173: 81–94. [DOI] [PubMed] [Google Scholar]
- 34.Masternak MM, Panici JA, Bonkowski MS, et al. 2009. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J. Gerontol. A Biol. Sci. Med. Sci 64: 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiesenborn DS, Ayala JE, King E & Masternak MM. 2014. Insulin sensitivity in long-living Ames dwarf mice. Age (Dordr.) 36: 9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anthony TG & Gietzen DW. 2013. Detection of amino acid deprivation in the central nervous system. Curr. Opin. Clin. Nutr. Metab. Care 16: 96–101. [DOI] [PubMed] [Google Scholar]
- 37.Plaisance EP, Henagan TM, Echlin H, et al. 2010. Role of beta-adrenergic receptors in the hypoerphagic and hyper-metabolic responses to dietary methionine restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol 299: R740–R750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orgeron ML, Stone KP, Wanders D, et al. 2014. The impact of dietary methionine restriction on biomarkers of metabolic health. Prog. Mol. Biol. Transl. Sci 121: 351–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guarente L & Kenyon C. 2000. Genetic pathways that regulate ageing in model organisms. Nature 408: 255–262. [DOI] [PubMed] [Google Scholar]
- 40.Tatar M, Bartke A & Antebi A. 2003. The endocrine regulation of aging by insulin-like signals. Science 299: 1346–1351. [DOI] [PubMed] [Google Scholar]
- 41.McElwee JJ, Schuster E, Blanc E, et al. 2007. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 8: R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikeno Y, Bronson RT, Hubbard GB, et al. 2003. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J. Gerontol. A Biol. Sci. Med. Sci 58: 291–296. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy MA, Rakoczy SG & Brown-Borg HM. 2003. Long-living Ames dwarf mouse hepatocytes readily undergo apoptosis. Exp. Gerontol 38: 997–1008. [DOI] [PubMed] [Google Scholar]
- 44.Gesing A, Masternak MM, Wang F, et al. 2011. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J. Gerontol. A Biol. Sci. Med. Sci 66A: 1062–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharp ZD & Bartke A. 2005. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J. Gerontol. A Biol. Sci. Med. Sci 60: 293–300. [DOI] [PubMed] [Google Scholar]
- 46.Brown-Borg HM, Rakoczy S, Wonderlich JA, et al. 2014. Altered dietary methionine differentially impacts glutathione and methionine metabolism in long-living growth hormone-deficient Ames dwarf and wild-type mice. Longev. Healthspan 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakae D 1999. Endogenous liver carcinogenesis in the rat. Pathol. Int 49: 1028–1042. [DOI] [PubMed] [Google Scholar]
- 48.Kotiesh A & Diehl AM. 2001. Animal models of steatosis. Semin. Liver Dis 21: 89–104. [DOI] [PubMed] [Google Scholar]
- 49.Jung YS 2015. Metabolism of sulfur-containing amino acids in the liver: a link between hepatic injury and recovery. Biol. Pharm. Bull 38: 971–974. [DOI] [PubMed] [Google Scholar]
- 50.Lamb R, Harrison H, Smith DL, et al. 2015. Targeting tumor-initiating cells: eliminating anabolic cancer stem cells with inhibitors of protein synthesis or by mimicking caloric restriction. Oncotarget 6: 4585–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu H, Zhang W, Wang K, et al. 2015. Methionine and cysteine double deprivation stress suppresses glioma proliferation via inducing ROS/autophagy. Toxicol. Lett 232: 349–355. [DOI] [PubMed] [Google Scholar]
- 52.Cavuoto P & Fenech MF. 2012. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat. Rev 38: 726–736. [DOI] [PubMed] [Google Scholar]
- 53.Epner DE 2001. Can dietary methionine restriction increase the effectiveness of chemotherapy in treatment of advance cancer? J. Am. Coll. Nutr 20: 443S–449S. [DOI] [PubMed] [Google Scholar]
- 54.Sinha A, Cooper TK, Rogers CJ, et al. 2014. Dietary methionine restriction inhibits prostatic intraepithelial neopplasia in TRAMP mice. Prostate 74: 1663–1673. [DOI] [PubMed] [Google Scholar]
- 55.Elshorbagy AK, Valdivia-Garcia M, Mattocks DA, et al. 2011. Cysteine supplementation reverses methionine restriction effects on rat adiposity: significance of stearoylcoenzyme A desaturase. J. Lipid Res 52: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Al-Regaiey KA, Masternak MM & Bartke A. 2006. Adipocytokines and lipid levels in Ames dwarf and caloric restricted mice. J. Gerontol. A Biol. Sci. Med. Sci 61A: 323–331. [DOI] [PubMed] [Google Scholar]
- 57.Brooks NL, Trent CM, Raetzsch CF, et al. 2007. Low utilization of circulating glucose after food withdrawal in Snell dwarf mice. J. Biol. Chem 282: 35069–35077. [DOI] [PubMed] [Google Scholar]
- 58.Eto I 2013. Expression p27Kip1, a cell cycle repressor protein, is inversely associated with potential carcinogenic risk in the genetic rodent models of obesity and long-lived Ames dwarf mice. Metabolism 62: 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gesing A, Al-Regaiey KA, Bartke A & Masternak MM. 2014. Growth hormone abolishes beneficial effects of calorie restriction in long-lived Ames dwarf mice. Exp. Gerontol 58: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westbrook R, Bonkowski MS, Strader AD & Bartke A. 2009. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J. Gerontol. A Biol. Sci. Med. Sci 64:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartke A & Westbrook R. 2012. Metabolic characteristics of long-lived mice. Front. Genet 3: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bartke A 2011. Growth hormone, insulin and aging: the benefits of endocrine defects. Exp. Gerontol 46: 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corton JC & Brown-Borg HM. 2005. Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J. Gerontol. A Biol. Sci. Med. Sci 60: 1494–1509. [DOI] [PubMed] [Google Scholar]
- 64.Tsuchiya T, Dhahbi JM, Cui X, et al. 2004. Additive regulation of hepatic gene expression by dwarfism and caloric restriction. Physiol. Genomics 17: 307–315. [DOI] [PubMed] [Google Scholar]
- 65.Al-Regaiey KA, Masternak MM, Bonkowski M, et al. 2005. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor 1/insulin signaling and caloric restriction. Endocrinology 146: 851–860. [DOI] [PubMed] [Google Scholar]
- 66.Brown-Borg HM & Bartke A. 2012. GH and IGF1: role in energy metabolism of long-living GH mutant mice. J. Gerontol. A Biol. Sci. Med. Sci 67: 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stauber AJ,Brown-Borg H,Liu J,et al. 2005Constitutive expression of peroxisome proliferator-activated receptor alpha-regulated genes in dwarf mice. Mol. Pharmacol 67: 681–694. [DOI] [PubMed] [Google Scholar]
- 68.Perrone CE, Mattocks DA, Plummer JD, et al. 2012. Genomic and metabolic responses to methionine-restricted and methionine-restricted, cysteine-supplemented diets in Fischer 344 rate inguinal adipose tissue, liver and quadriceps muscle. J. Nutrigenet. Nutrigenomics 5: 132–157. [DOI] [PubMed] [Google Scholar]
- 69.Laplante M & Sabatini DM. 2012. mTOR signaling in growth control and disease. Cell 149: 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zoncu R, Efeyan A & Sabatini DM. 2011. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol 12: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J & Guan KL. 2011. Amino acid signaling in TOR activation. Annu. Rev. Biochem 80: 1001–1032. [DOI] [PubMed] [Google Scholar]
- 72.Kim SG, Buel GR & Blenis J. 2013. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol. Cells 35: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang M & Miller RA. 2012. Augmented autophagy pathways and mTOR modulation in fibroblasts from long-lived mutant mice. Autophagy 8: 1273–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schneider A, Zhi X, Moreira F, et al. 2014. Primordial follicle activation in the ovary of Ames dwarf mice. J. Ovarian Res 7: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gomez A, Gomez J, Lopez-Torres M, et al. 2015. Cysteine dietary supplementation reverses the decrease in mitochondrial ROS production at complex I induced by methionine restriction. J. Bioenerg. Biomembr 47: 199–208. [DOI] [PubMed] [Google Scholar]
- 76.Al-Regaiey KA, Masternak MM, Bonkowski MS, et al. 2007. Effects of caloric restriction and growth hormone resistance on insulin-related intermediates in the skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci 62: 18–26. [DOI] [PubMed] [Google Scholar]
- 77.Perrone CE, Mattocks DA, Hristopoulos G, et al. 2008. Methionine restriction effects on 11-HSD1 activity and lipogenic/lipolytic balance in F344 rat adipose tissue. J. Lipid Res 49: 12–23. [DOI] [PubMed] [Google Scholar]
- 78.Brown-Borg H, Johnson WT, Rakoczy S & Romanick M. 2001. Mitochondrial oxidant generation and oxidative damage in Ames dwarf and GH transgenic mice. J. Am. Aging Assoc 24: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown-Borg HM, Johnson WT & Rakoczy SG. 2012. Expression of oxidative phosphorylation components in mitochondria of long-living Ames dwarf mice. Age (Dordr.) 34: 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanz A, Caro P, Ayala V, et al. 2006. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 20: 1064–1073. [DOI] [PubMed] [Google Scholar]
- 81.Caro P, Gómez J, López-Torres M, et al. 2008. Forty percent and eighty percent methionine restriction decrease meitochondrial ROS generation and oxidative stress in rat liver. Biogerontology 9: 183–196. [DOI] [PubMed] [Google Scholar]
- 82.Kozieł R, Ruckenstuhl C, Albertini E, et al. 2014. Methionine restriction slows down senescence in human diploid fibroblasts. Aging Cell 13: 1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lopez-Torres M & Barja G. 2008. Lowered methionine ingestion as responsible for the decrease in rodent mitochondrial oxidative stress in protein and dietary restriction possible implications for humans. Biochim. Biophys. Acta 1780: 1337–1347. [DOI] [PubMed] [Google Scholar]
- 84.Maddineni S, Nichenametla S, Sinha R, et al. 2013. Methionine restriction affects oxidative stress and glutathione-related redox pathways in the rat. Exp. Biol. Med 238: 392–399. [DOI] [PubMed] [Google Scholar]
- 85.Yang Y, Ji Y, Wu G, et al. 2015. Dietary l-methionine restriction decreases oxidative stress in porcine liver mitochondria. Exp. Gerontol 65: 35–41. [DOI] [PubMed] [Google Scholar]
- 86.Brown-Borg HM 2009. Hormonal control of aging in rodents: the somatotropic axis. Mol. Cell. Endocrinol 299: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murakami S, Salmon A & Miller RA. 2003. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 17: 1565–1566. [DOI] [PubMed] [Google Scholar]
- 88.Salmon AB, Murakami S, Bartke A, et al. 2005. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am. J. Physiol. Endocrinol. Metab 289: E23–E29. [DOI] [PubMed] [Google Scholar]
- 89.Bokov AF, Lindsey ML, Khodr C, et al. 2009. Long-lived Ames dwarf mice are resistant to chemical stressors. J. Gerontol. A Biol. Sci. Med. Sci 64: 819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leiser SF & Miller RA. 2010. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol. Cell. Biol 30: 871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schrag M, Sharma S, Brown-Borg H & Ghribi O. 2008. Hippocampus of Ames dwarf mice is resistant to beta-amyloid-induce tau hyperphosphorylation and changes in apoptosis-regulatory protein levels. Hippocampus 18: 239–244. [DOI] [PubMed] [Google Scholar]
- 92.Page MM, Salmon AB, Leiser SF, et al. 2009. Mechanisms of stress resistance in Snell dwarf mouse fibroblasts: enhanced antioxidant and DNA base excision repair capacity, but no differences in mitochondrial metabolism. Free Radic. Biol. Med 46:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rojanathammanee L, Rakoczy S & Brown-Borg HM. 2014. Growth hormone alters the glutathione S-transferase and mitochondrial thioredoxin systems in long-living Ames dwarf mice. J. Gerontol. A Biol. Sci. Med. Sci 69: 1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsieh CC & Papaconstaninou J. 2009. Dermal fibroblasts from long-lived Ames dwarf mice maintain their in vivo resistance to mitochondrial generated reactive oxygen species (ROS). Aging 1: 784–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang M & Miller RA. 2012. Augmented autophagy pathways and mTOR modulation in fibroblasts from long-lived mutant mice. Autophagy 8: 1273–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li W, Li X & Miller RA. 2014. ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell 13: 1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harper JM, Durkee SJ, Dysko RC, et al. 2006. Genetic modulation of hormone levels and life span in hybrids between laboratory and wild-derived mice. J. Gerontol. A Biol. Sci. Med. Sci 61: 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ikeno Y, Hubbard GB, Lee S, et al. 2013. Do Ames dwarf and calorie-restricted mice share common effects on age-related pathology? Pathobiol. Aging Age Relat. Dis 20: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kominou D, Leutzinger Y, Reddy BS & Richie JP Jr. 2006. Methionine restriction inhibits colon carcinogenesis. Nutr. Cancer 54: 202–208. [DOI] [PubMed] [Google Scholar]
- 100.Flurkey K, Papaconstantinou J, Miller RA, & Harrison DE. 2001. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. U.S.A 98: 6736–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vergara M, Smith-Wheelock M, Harper JM, et al. 2004. Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J. Gerontol. A Biol. Sci. Med. Sci 59: 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bartke A, Wright JC, Mattison JA, et al. 2001. Extending the lifespan of long-lived mice. Nature 414: 412. [DOI] [PubMed] [Google Scholar]
- 103.Brown-Borg HM, Rakoczy S, Wonderlich JA, et al. 2014. Growth hormone signaling is necessary for lifespan extension by dietary methionine. Aging Cell 36: 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]