Abstract
Purpose of the Review:
The goal of this paper is to review current literature on nutritional ketosis within the context of weight management and metabolic syndrome—namely insulin resistance, lipid profile and cardiovascular disease risk, and development of non-alcoholic fatty liver disease. We provide background on the mechanism of ketogenesis and describe nutritional ketosis.
Recent Findings:
Nutritional ketosis has been found to improve metabolic and inflammatory markers, including lipids, HbA1c, high-sensitivity CRP, fasting insulin and glucose levels, and aid in weight management. We discuss these findings and elaborate on potential mechanisms of ketones for promoting weight loss, decreasing hunger, and increasing satiety.
Summary:
Humans have evolved with the capacity for metabolic flexibility and the ability to use ketones for fuel. During states of low dietary carbohydrate intake, insulin levels remain low and ketogenesis takes place. These conditions promote breakdown of excess fat stores, sparing of lean muscle, and improvement in insulin sensitivity.
Keywords: Nutritional ketosis, metabolic syndrome, ketogenic diet, insulin resistance, weight loss, low carbohydrate diet, ketone bodies, glucose metabolism, ketogenesis
INTRODUCTION
Obesity and the related Metabolic Syndrome are epidemics in the Western world. Worldwide, obesity has nearly tripled since 1975. In 2016, the WHO reported worldwide adult overweight and obesity rates in excess of 39% and 13%, respectively. In the United States, these staggering statistics include two billion adults, and account for over $149 billion health care dollars per year.1 In parallel, 40% of the population over 60 years old has metabolic syndrome, which is defined by a constellation of symptoms and biomarkers, including: obesity (waist circumference), elevated fasting blood sugar with insulin resistance, hypertriglyceridemia, low HDL cholesterol, and hypertension.2 Metabolic syndrome is a systemic inflammatory state associated with a 5-fold increased risk of diabetes and a 2-fold risk of cardiovascular disease (CVD), both of which are increasingly common causes of morbidity and mortality.3
Intriguingly, the five main components of Metabolic Syndrome—obesity, fasting blood sugar, high triglycerides (TGs), low HDL cholesterol, and hypertension—are all improved by carbohydrate restriction, which suggests that carbohydrate intolerance is a common thread. With industrialization of the food supply over the past few centuries, we have enjoyed a surplus of calories and food products with processed sugar and carbohydrate. In conjunction, we have seen the rates of obesity and overweight skyrocket along with worsening metabolic fitness.
By restricting dietary carbohydrates, insulin secretion can be stabilized at lower levels. When insulin levels are low, stored fat in adipose tissue undergoes lipolysis via hormone sensitive lipase. Once liberated, free fatty acids undergo beta-oxidation in hepatic mitochondria to produce acetyl CoA for the generation of ketone bodies. This process can induce a state of nutritional ketosis, which can result in a shift in metabolism. 4,5 Decreased insulin release promotes a metabolic shift toward lipid oxidation and utilization of fatty acids and ketones for energy.6
Ketones – An Alternative Fuel Source
As a species, we have metabolic flexibility with the capability to rely on alternative fuel sources for energy. Humans are not dependent on exogenous sources of glucose for optimal function; rather, we have evolved over millennia to adjust to changing conditions and adapt to both scarcity and abundance. As Dr. Randle explained in the Lancet in 1963, “Substrate metabolism in the normal human body is flexible. Our bodies have evolved to utilize different fuel sources depending on their availability.”7
As an alternative to glucose utilization, the body can metabolically flex into a state of ketosis, which relies on fat-derived ketones produced in the liver to provide fuel to nearly every cell in the body (See Figure 1). Ketones—acetoacetate, beta-hydroxybutyrate, and acetone—are water-soluble molecules produced by the liver from fatty acids when blood glucose and liver glycogen stores have been minimized. Glycogen depletion occurs and ketone levels rise during periods of fasting, low carbohydrate intake, intense exercise, starvation, or due to complete lack of insulin in untreated type I diabetes.
Figure 1.
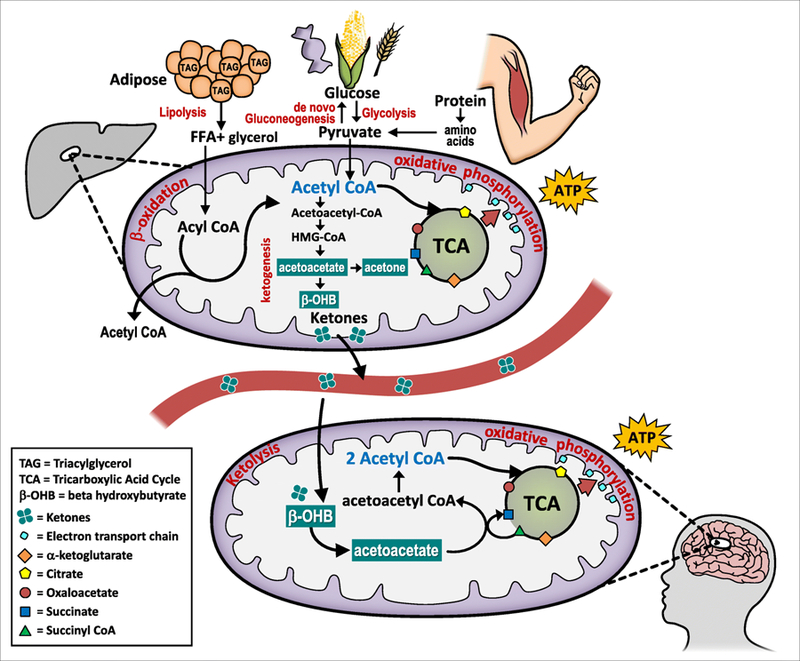
Ketogenesis, the production of ketones for fuel, is a normal, physiologic process that occurs via hepatic beta-oxidation of free fatty acids in the mitochondria of liver cells. Energy stored as fat in adipose tissue is liberated to acetyl-CoA and converted to ketones. Extra-hepatic tissues are able to undergo ketolysis and convert ketones back to acetyl-CoA which enters the TCA cycle and is used by the mitochondria to generate ATP for energy.
Hormonal activation of lipolysis and ketogenesis is mediated by epinephrine and glucagon and opposed by insulin. With minimal dietary carbohydrate, insulin is low and glucagon increases. In addition to stimulating glycogenolysis in the liver, glucagon stimulates lipolysis to release stored fatty acids from adipose tissue.8 In addition to forming ketones, fatty acids can be converted to acetyl CoA—an intermediary substrate between fatty acid oxidation and metabolism of glucose—that enters the citric acid cycle and then undergoes oxidative phosphorylation for ATP generation. Conversely, in response to high blood glucose (i.e. after a high carbohydrate meal), insulin levels rise and shut off ketogenesis in favor of de novo lipogenesis (fat storage). Thus, ketosis signifies a shift from an insulin-mediated glucose dependent state to an increased ability to use dietary fat and adipose stores for fuel.
Through this process, fat-derived energy is generated in the liver and then shipped throughout the body to supply energy to the brain, renal cortex, heart, and skeletal muscles.6 Ketones can supply up to 60% of ATP required by the body;9 the remainder is derived from endogenous gluconeogenesis that utilizes glycerol form triglycerides and glucogenic amino acids from protein for glucose production. Ketones cross the blood brain barrier and replace glucose as the primary energy source for the brain. Pioneering work by George Cahill using models of starvation ketosis revealed that the brain has metabolic flexibility and can switch from being a glucose dependent organ (~150g/day) to one that derives over 2/3rd of its energy from ketones.9,10
Nutritional ketosis
Nutritional ketosis can be defined as the intentional restriction of dietary carbohydrate intake to accelerate the production of ketones and induce a metabolic effect that stabilizes blood sugar, minimizes insulin release, and thereby mitigates the downstream anabolic and tumorigenic effects of longstanding insulin resistance. As described by Volek and Phinney, a “well-formulated” ketogenic diet is composed of 5–10% carbohydrates (<20–50g/day), adequate protein (1–1.5g/kg/day), and fat until satiated. The hallmark of nutritional ketosis is blood ketone levels of 0.5 to 3 mg/dL.11 This is in stark contrast to, and should not be confused with, the pathophysiologic state of type 1 diabetic ketoacidosis (DKA). Despite similar sounding names, they are two distinct metabolic processes. The production of endogenous insulin is protective against the occurrence of DKA; the range of ketones present in DKA is 5–10 fold greater than the levels achieved during nutritional ketosis. Additionally, while in nutritional ketosis, the body is able to maintain normal blood glucose levels and maintain a normal pH, as opposed to extremely elevated blood sugars and acidic pH associated with DKA.
After many weeks, “keto-adaptation” occurs, which signifies the bodies ability to adapt and respond to primarily using ketones for fuel. One of the potential reasons this occurs is secondary to upregulated transcription of genes that encode the metabolic machinery resulting in increased mitochondrial density in oxidative tissues like the brain and muscle. Mouse studies suggest that this may occur via increased mitogenesis or decreased mitochondrial damage.12,13 Additionally, ketones are capable of inducing epigenetic regulation via histone deacetylation inhibition indicating that they are a signaling molecule in addition to an energy source.14
Origins of the ketogenic diet as a medical therapy
Original research into a ketogenic diet began in the early 1900s as a way to manage epilepsy and minimize seizure activity. By inducing ketosis, patients had mitigation of seizure activity and improvements in cognitive function, highlighting the capacity for ketones to provide energy to the brain.15 From the 1960s onwards, very low carbohydrate ketogenic diets (VLCKD) have become more commonly known as a methods for obesity treatment. Recent work over the last few decades has provided evidence for the therapeutic potential of ketogenic diets in many pathological conditions, including diabetes, PCOS, acne, neurologic diseases (epilepsy, Alzheimer’s, CVA), cancer, and the amelioration of respiratory and cardiovascular disease risk factors.15 The possibility that modifying food intake can reduce or eliminate need for medications, which often carry significant side effects, calls for serious investigation. Dietary carbohydrate intake has been studied with variable findings, which is often due to lack of standardization of carbohydrate intake and inability to confirm ketosis without checking blood ketone levels.
According to Volek and Phinney,5,16 the primary feature of ketogenic diets is the establishment of ketosis and stabilization of insulin levels, which addresses the biomarkers of metabolic syndrome. By reducing insulin elevations, lipids are released from storage and oxidized.17 As such, ketosis can be used as an indicator of lipolysis.16 Thus, when looking at studies comparing “low carbohydrate” diets, it is important to evaluate whether the diet is ketogenic. This body of nutrition literature ranges broadly in its definition of what constitutes “low” carbohydrate intake, with some considering 35% calories from carbs or less than 140g/d as the metric. Thus, it is important when evaluating the impact of a VLCKD that the research is done using <50g of carbohydrate per day (<10% kcal) in order to achieve ketosis. Many of the beneficial physiological effects of a ketogenic diet are attributed to the action of the ketones and the metabolic shift to using ketones instead of glucose for fuel. A lack of demonstrable difference between non-ketogenic “low carbohydrate” diets and a low-fat diet, should not be extrapolated to ketogenic diets.18
This review will highlight the current understanding of the role of ketogenic diets for weight and metabolic management, diabetes, coronary vascular disease, and non-alcoholic fatty liver disease (NAFLD).
WEIGHT LOSS
In a systematic review with meta-analysis, Bezerra Bueno et al. reviewed 13 randomized controlled trials (RCT), which included a total of 1569 subjects and compared VLCKD (<50g carb/day) with low fat diet (LFD, <30% kcal from fat) in overweight and obese adults.19 The included RCTs had a minimum follow-up of 12 months. The primary outcome for their meta-analysis was body weight; secondary outcomes included lipid profile (triglycerides, HDL, LDL), systolic and diastolic blood pressure, fasting blood sugar, insulin, HbA1c, and CRP. Analysis revealed that VLCKD achieved greater long-term reductions in body weight, triglycerides, and diastolic blood pressure, and greater increases in HDL and LDL cholesterol compared to a low-fat diet.19 Similar findings were reached in another meta-analysis performed by Santos et al.20 One proposed mechanism for the beneficial effect of a VLCKD on body weight may be due to modulation of resting energy expenditure. Ebbeling et al. assessed the effects of dietary composition on energy expenditure during weight loss and maintenance. Under isoenergetic conditions, they found that a carbohydrate-restricted diet was better than LFD for maintaining basal metabolic rate.21 Likewise, Westman et al. attributed some of the beneficial appetite suppressant and satiating effects of a VLCKD to reduction in insulin levels;10 several studies have shown that insulin may increase food intake.22,23 In insulin sensitive individuals, insulin may act as a satiety hormone; however, in insulin resistant states, the transiently high levels of insulin stimulated in response to a meal may illicit a compensatory hypoglycemic response that ultimately leads to increased consumption of food. Or, alternatively, may not be adequate to stimulate satiety centers in the brain that normally act as an adiposity signal to determine food intake.24 Thus, foods with high insulin responses may be less satiating in this population;25 to further this supposition, suppression of insulin with octreotide has been shown to lead to weight loss.10,20 Not surprisingly, the relationship between dietary carbohydrate intake and metabolic syndrome is complexly intertwined due to the important hormonal and regulatory roles performed by insulin, lipoproteins, and inflammatory markers (eg. adiponectin, leptin).26,27
In a meta-analysis comparing low-carbohydrate and LFD interventions for overweight and obese adults, Sackner-Bernstein and colleagues reviewed 17 RCTs (total of 1,797 subjects). Baseline demographics, caloric intake, and completion rates were similar between the different groups. For the low-carb group, the mean daily intake of macronutrients was 60g of carbohydrate (95% CI: 44, 76), 90g of fat (95% CI: 77, 104), and 106g of protein (95% CI: 77, 104). The low-fat group was composed of 205g of carbs (95% CI: 186, 225), 37g of fat (95% CI: 32, 42), and 70g of protein (95% CI: 64, 76). Each diet was associated with significant weight loss and reduction in predicted risk of coronary events, but the low carbohydrate group had a statistically significantly greater improvement in both.28
One of the benefits of nutritional ketosis for weight loss may be attributed to the appetite suppressant effects of ketone bodies.29 Several studies have shown a spontaneous reduction in calorie intake on VLCKD with carbohydrate intake restricted to 5–10% of kcal (approximately 20–50g).29–31 A controlled feeding study assessing hunger levels found that despite significant caloric restriction, severely obese participants on a VLCKD reported hunger levels similar to participants consuming approximately 1000 kcal more on a low-fat diet.32 Another study by Johnstone et al., used the Eating Inventory, a validated questionnaire that focuses on hunger and cognitive restraint from eating, and reported that hunger was reduced by 50% after one week of a low carb diet.33 This phenomenon is corroborated by studies that link diet macronutrient composition to ad libitum energy intake. Laboratory-based and free-living studies have identified protein as a potentially more satiating macronutrient. To evaluate the impact of ketogenesis on diets with equal protein content, Johnstone et al. placed participants on either a high-protein, low carb ketogenic diet or a high-protein, moderate carb, non-ketogenic diet for four weeks; participants felt significantly less hungry on the ketogenic diet (p=0.014).29 In addition to decreased hunger, the high-protein, low-carbohydrate group had significantly lower average ad lib energy intake (p=0.02) and greater weight loss (6.34 kg vs. 4.35 kg, p=0.006). The authors posited that the effects may be due to the increased metabolic costs of gluconeogenesis and thermic effects of proteins.34–37
Participants on ketogenic diets have a tendency to maintain lean body mass with preferential loss of fat mass, independent of exercise.** (36) Due to this observation, there is interest into the use of ketogenic diets for shifting body composition and application during exercise. The majority of studies that have attempted to look at the role of low carbohydrate diets and exercise performance have not examined truly ketogenic diets (carbohydrate intake too high to promote ketone synthesis), they have not been long enough to allow for muscle metabolism to adapt to ketone utilization, they focused solely on endurance and not on resistance training, or they were under-powered (refer to article by Wilson et al. 2017 for more detailed information).38,39 Further, the existing studies that have investigated the relationship between a ketogenic diet and exercise have suggested that there may be a performance advantage to the ability to use fat oxidation for fuel in those who also trained in a chronically low insulin state, like the conditions of nutritional ketosis.40 Clearly, this is an area that needs further investigation. In regards to endurance, Phinney et al. showed that highly trained cyclists maintained their physical ability following four weeks on a ketogenic diet. Likewise, similar results were obtained in an obese cohort.30,41 These participants were able to increase fat oxidation during exercise while ona ketogenic diet compared to a high-carbohydrate, standard diet. In a recently published experiment, Wilson et al. investigated the effects of a ketogenic diet compared to a Western diet on adaptions to resistance training and found favorable body composition changes in the ketogenic diet group with similar increases in muscle strength and power as compared to the Western diet.38 This group also performed a 6-week study looking at muscle glycogen in a rodent model using ketogenic vs. Western diets and found no significant difference in muscle glycogen content despite significantly lower intake of carbohydrate in the ketogenic group.42 Conflicting reports on muscle glycogen while on ketogenic diets may be confounded by relatively short intervention periods that don’t allow for the metabolic adaptations that occur over extended periods of nutritional ketosis and may impact muscle glycogen maintenance.43,44Research is ongoing and needed in this area before conclusive recommendations can be made for ketogenic diets and exercise performance, but findings thus far are intriguing.
METABOLIC SYNDROME
Lipid profile & CVD
Reduction of carbohydrates to induce nutritional ketosis can result in significant improvements to blood lipid profiles, even in the setting of increased saturated fat intake. The main improvements in lipid biomarkers include: marked reduction in plasma triglyceride levels, significant positive effects on total cholesterol reduction, increased HDL cholesterol, and a shift in size and volume of LDL particles. 15,45–47 Smaller, dense LDL particles are more atherogenic and associated with worse cardiovascular risk parameters. In a 12-week study that randomized 40 overweight subjects with atherogenic dyslipidemia to either a carbohydrate-restricted (%carb:fat:protein = 12:59:28) or a low-fat diet (56:24:20), Volek et al. demonstrated that subjects who followed the carbohydrate-restricted diet had greater reductions in their fasting triglyceride levels and higher HDL, with a more pronounced HDL effect seen in women.5 Furthermore, those following the carbohydrate-restricted diet demonstrated lower fasting triglyceride levels after an oral fat load consisting primarily of saturated fats.5 The objective was to evaluate the impact on parameters of the metabolic syndrome and associated CVD risks. The carbohydrate-restricted group consistently demonstrated weight loss (−10%) and decreased adiposity (−14%), reduction in fasting glucose (−12%) and insulin (−50%), reduction in triglycerides (−51%), and an increase in HDL(+13%).5 Perhaps most interesting was the effect on lipoproteins; they observed a shift from smaller, more dense LDL particles that are more atherogenic, to larger LDL particles after a carbohydrate-restricted diet (mean LDL particle size increased by both PAGE and NMR).5 They also showed a decrease in RBP4 (retinol binding protein 4), a marker that is thought to be closely related to insulin resistance,5,48 which further supports their proposition of insulin flux being the primary mediator of metabolic syndrome. Many of the changes in lipid profile were thought to be independent of fat loss and were attributed to a reduction in insulin.5 Limitations of this study include its small sample size and short duration. A multi-center, RCT spanning 2 years compared a low-carbohydrate diet to a LFD in approximately 300 participants. The low-carbohydrate group exhibited greater reductions in triglyceride level and VLDL level at 6 months, when compared to the low-fat group. In addition, they demonstrated a more significant increase in HDL that was sustained throughout the 2-year trial.49 Further studies have shown promising results of the impact of ketogenic diets on parameters of metabolic syndrome,19,50 triglycerides and HDL. 51–54
As mentioned above, several studies have demonstrated a correlation between carbohydrate intake and size and density of LDL particles, a relationship that appears even more robust in individuals with diabetes.5,55 Smaller and denser LDL particles are known to be directly associated with a significant increase in risk of cardiovascular disease. The positive lipid effects of VLCKD are explained by the principles and driving forces behind lipoprotein turnover. Elevated plasma triglyceride levels are correlated with a shift towards smaller, dense LDL particles via cholesterol esterification of abundant VLDL particles.46,55 In controlled amounts over short periods of time, insulin normally acts to suppress VLDL production; however, with prolonged secretion in the setting of persistent hyperglycemia, hyperinsulinemia leads to hepatic insulin resistance and an increase in triglyceride-rich VLDL particles. This subsequently decreases HDL and promotes small, dense atherogenic LDL particles.55–57 Not only does hyperinsulinemia contribute to triglyceride-rich lipoprotein production, but it also stimulates hepatic de-novo lipogenesis, triglyceride synthesis, and can inhibit VLDL clearance.5,58 Furthermore, insulin plays a role in the synthesis of endogenous cholesterol, via direct diet-related activation of HMGCoA reductase. Increase in plasma glucose and insulin in response upregulates the activity of HMGCoA reductase, thereby increasing endogenous cholesterol synthesis.15 On the contrary, a reduction in dietary carbohydrate has the opposite effect on HMGCoA reductase, and in concert with the negative feedback inhibition provided by dietary cholesterol and fats, may be the mechanism through which nutritional ketosis can improve lipid profiles. In the setting of low insulin and acetyl CoA abundance, mitochondrial HMGCoA lyase is able to divert HMGCoA toward the production of ketone bodies instead of cholesterol synthesis (See Figure 1).
A major concern from opponents of the ketogenic diet stems from the faulty theory that a significant increase in dietary fat consumption leads to a negative impact on blood lipid levels. In the 1970s, the “diet-heart hypothesis” was introduced, which suggested that dietary saturated fat consumption correlated with plasma cholesterol and increased risk of cardiovascular disease.59 However, to date, there has been no proven causation effect between saturated fat and cardiovascular disease.60 This compelling argument against critics of the ketogenic diet was illustrated by Feinman et al. in a systematic review of the dietary impact of nutritional ketosis on type 2 diabetes in which they pointed out the weak association between dietary fat intake and risk for cardiovascular disease.58 Furthermore, the macronutrient composition of these diets, including the type of fat consumed (eg. saturated, monounsaturated, polyunsaturated, etc.) and protein intake, varies from study to study which makes comparisons challenging.61 Several studies have demonstrated an increase in LDL levels with a low-carbohydrate diet, likely related to the shift in particle size.49 It is important not to make conclusions based on these studies that did not characterize the type of LDL particles (i.e. small dense vs. large), which impacts their atherogenic potential.
Glycemic control, insulin sensitivity, & diabetes
The management of plasma glucose and modulation of insulin’s effects on lipid metabolism are important targets in the treatment of diabetes and the prevention of comorbid conditions, including systemic microvascular complications. Insulin resistance is associated with increased hepatic lipid accumulation, production of VLDL, and gluconeogenesis.57,62 Insulin resistance is a complex metabolic state that affects energy utilization and stimulates “ectopic” fat deposition in non-adipose organs, notably skeletal muscle, the heart, and the pancreas.52,61,63 At the level of skeletal muscle, it hinders the ability to take up plasma glucose, which results in glucose diversion to the liver where it is converted to and stored as fat.15,62 In contrast, nutritional ketosis reduces insulin levels thereby suppressing lipogenesis.15
In addition to the positive impact on weight management, nutritional ketosis has can improve glycemic control and reduce medication usage. As early as 1983, Phinney et al. conducted a RCT that demonstrated the ability of VLCKD to reduce serum glucose and improve overall glucose metabolism.64 Saslow et al. randomized 34 overweight and obese individuals to a VLCKD or moderate-carbohydrate, calorie-restricted low fat (MCCR) diet. At 12 months, participants on VLCKD demonstrated a greater reduction in hemoglobin A1c compared to the MCCR group, and VLCKD were able to reduce anti-diabetic medications.65 Dashti et al. placed 64 obese diabetic and non-diabetic subjects on a VLCKD and observed a sustained reduction in blood glucose levels after 56 weeks.52 A similar study on VLCKD in 66 obese individuals with either high or normal cholesterol demonstrated a significant decrease in blood glucose at 56 weeks.51 Another small study of 10 obese, diabetic patients who followed a low-carbohydrate diet for 2 weeks demonstrated lower fasting plasma glucose, improved insulin sensitivity, and a decrease in diabetes medication requirements. 53 This trial was limited by small sample size, short duration, and inability to extrapolate the results as the study was performed in the inpatient setting. A larger study of 363 overweight and obese patients, including 102 diabetics and 261 non-diabetics, compared a low-calorie diet to a VLCKD and demonstrated a significantly greater reduction in blood glucose and hemoglobin A1c at 24 weeks in the VLCKD group.66 In a systematic review and meta-analysis of 20 RCTs (over 3000 participants), Ajala et al. compared four diet groups (low-carbohydrate, low glycemic index, Mediterranean, and high-protein diets) and observed a significant decrease in hemoglobin A1c in the low-carbohydrate group compared to the other diets.54 This study, however, had its own limitations including heterogeneity and confounding as a result of variable control diets for comparison, differing baseline patient characteristics, and wide range in study durations.
In type 2 diabetic patients, VLCKD are associated with decreased need for exogenous insulin; increasing ketone levels are inversely related to levels of hepatic glucose generation, suggesting that higher levels of ketones are associated with improved glycemic control. Accurso et al. showed that type 2 diabetes patients on a VLCKD (<20g/d) compared to usual high carb diet, had much less insulin released in response to a meal and required less insulin to achieve and maintain a lower blood glucose level. Together, this implies that the VLCKD resulted in greater insulin sensitivity and better glycemic control despite needing less insulin to reach the targeted blood sugar. Significantly, over half of the patients in this trial were able to stop or decrease their diabetic medications after switching to the VLCKD intervention.67 Similarly, McKenzie et al. enrolled 262 subjects with diabetes in an outpatient program with nutrition counseling, behavioral modification, digital education, and physician-assisted medication management. After 10 weeks, re-evaluation was notable for consistent carbohydrate restriction as evidenced by mean beta-hydroxybutyrate levels, reduction in hemoglobin A1c, and decrease in dose and number of diabetic medications.68 In a prospective, 1-year open label, non-randomized, controlled study, Bhanpuri et al. evaluated a continuous care intervention for diabetes using nutritional ketosis as compared to usual care (262 and 87 participants, respectively). The nutritional ketosis group had improved biomarkers of CVD risk at 1 year, including improvements in lipid profile and LDL particle size, decreased blood pressure, and inflammation.69
Of note, while most studies have been conducted in the type 2 diabetic population, there is no contraindication to pursuing nutritional ketosis for the management of type 1 diabetes. It is important, however, due to the different pathophysiology of these two diseases to embark upon a nutritional ketosis dietary intervention under the guidance of a medical professional. It is quite common to need decreasing levels on both bolus and basal insulin while in ketosis, so it is prudent to have appropriate monitoring to avoid the complications of unrecognized hypoglycemia.70 In a 2018 study of adult type 1 diabetics adhering to a ketogenic diet (as measured by plasma B-hydroxybutyrate levels) for a mean of 2.6 years, researchers concluded that a ketogenic diet for type 1 diabetics leads to excellent HbA1c (mean 5.3±0.4%) with minimal glycemic variability.70 However, they did note episodes of hypoglycemia as detected by continuous glucose monitoring, which highlights the need for adequate monitoring. Most trials looking at both low carbohydrate and ketogenic diets in type 2 diabetics note a decrease in HbA1c that reflects the lower glycemia values in response to both decreased intake of carbohydrate and increased insulin sensitivity (demonstrated by decreased excursions in blood sugar and lower glycemic variability),71 this would be expected to similarly occur in type 1 diabetics. A systematic review published in March 2018 surveyed the existing literature and demonstrated the need for well-designed intervention studies to evaluate the effects of ketogenic diets in type 1 diabetics. While the existing literature is promising, there are only a few trials that have examined the relationship in this population.72 Furthermore, evidence suggests that the cardiovascular side effects of type 1 diabetes may be secondary to upregulation of one of at least 10 ketone-sensitive enzymes, namely HMG-CoA Synthase 2, which may provide a protective mechanism by which ketosis can decrease incidence of CVD in type 1 diabetics.73 (As demonstrated in figure 1, HMG-CoA is one of the intermediate steps in the synthesis of ketones from acetyl CoA. Not depicted: During increased ketone synthesis, HMG-CoA synthase is activated, which in turn decreases the amount of HMG CoA available for the cholesterol synthesis pathway via HMG CoA reductase (i.e. target of statins).)
Hepatic steatosis (NAFLD)
NAFLD—the hepatic manifestation of metabolic syndrome—is characterized by hepatic steatosis in the absence of other well-known forms of hepatic injury such as excessive alcohol consumption, viral infection, autoimmune disease, or toxic (e.g. medication) insults.56 NAFLD encompasses a wide spectrum of hepatic diseases states from simple steatosis to advanced, fibrotic liver disease.62 Non-alcoholic steatohepatitis (NASH) is a more concerning form of NAFLD that is characterized by hepatocyte ballooning, inflammatory infiltrate, and various stages of fibrosis often leading to advanced liver disease (e.g. cirrhosis and hepatocellular carcinoma).56,57,62,74
NAFLD is thought to be a negative consequence of ectopic hepatic lipid accumulation, in the setting of insulin resistance, as discussed above.26,57,61 Steatosis—characterized by triglyceride deposition in hepatocytes—underscores the close connection between visceral adiposity, NAFLD, and the carb-laden, high-fat Western diet.62 Visceral adipose deposition stimulates inflammatory cytokine release and systemic insulin resistance, and diets high in saturated fat and simple carbohydrates lead to free fatty acid delivery to the liver, stimulating de novo lipogenesis, oxidative stress, and lipo-toxicity.57,62
A small, two-week clinical trial randomized 18 subjects with NAFLD to a carbohydrate-restricted diet or a calorie-restricted diet and witnessed a greater reduction in intra-hepatic triglyceride content in the carbohydrate-restricted arm.75 In a more extensive review, Yki-Jarvinen et al. evaluated recent studies comparing the impact of various diets on hepatic fat and insulin sensitivity, while controlling for calorie content. Results were inconclusive and showed a decrease in liver volume in the hypocaloric low-carbohydrate ketogenic diet when compared to standard diet. However, between isocaloric groups, a greater decrease in liver fat content was seen with low-fat, high-carbohydrate diets (16–23% fat, 57–65% carb) compared to low-carbohydrate, high-fat diets (43–55% fat, 27–38% carb).57 An important caveat to these results is that the low-carbohydrate group was not following a ketogenic diet, so it would be unwise to prematurely attribute this discordant result to dietary fat intake without conducting a similar experiment evaluating isocaloric nutritional ketosis.
Most of the studies reviewed were limited by small sample size and short duration of follow-up, which reinforces the scarcity of literature studying the association between diet and NAFLD and its long-term clinical implications. Furthermore, a variety of dietary strategies have shown improvements in hepatic steatosis, but some have simultaneously shown unfavorable effects on hepatic inflammation and fibrosis.26,61 An earlier study on morbidly obese subjects who were given a reduced calorie diet, followed by gastroplasty, demonstrated a reduction in hepatic fat content accompanied by a concerning increase in portal inflammation and fibrosis, which was hypothesized to be related to the rate of weight loss.76 In contrast, Weiner et al. observed biopsy-proven evidence of improvement in steatosis, inflammation, and fibrosis in 284 morbidly obese bariatric surgery patients.77 It remains unclear whether improvements in hepatic steatosis translates to a reduction in fibrosis and advanced liver disease.
CONCLUSION
Compelling evidence exists for the use of nutritional ketosis for the management of weight and the components of metabolic syndrome. Through the utilization of alternative fuel sources, namely ketones, we can capitalize on the antagonistic relationship of high glucagon and low insulin levels that promote breakdown of fat for fuel, sparing of glycogen in muscles, and de novo gluconeogenesis as needed. Further research is needed into long-term adherence and practicality of VLCKD, but the current results are promising for weight management, lipid profiles, and insulin sensitivity.
Acknowledgements:
The authors would like to thank Robin Noel for her technical assistance in the creation of the graphics for the figure.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Victoria M. Gershuni, Stephanie L. Yan, and Valentina Medici declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
REFERENCES
- 1.Obesity and Overweight Fact Sheet Vol. 2018 (World Health Organization, 2017). [Google Scholar]
- 2.Kaur J A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014, 943162 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Wilson PW, D’Agostino RB, Parise H, Sullivan L & Meigs JB Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 112, 3066–3072 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Saslow LR, et al. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PloS one 9, e91027 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volek JS, et al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 44, 297–309 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Laffel L Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 15, 412–426 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Randle PJ, Garland PB, Hales CN & Newsholme EA The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 (1963). [DOI] [PubMed] [Google Scholar]
- 8.Sato K, et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J 9, 651–658 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Veech RL, Chance B, Kashiwaya Y, Lardy HA & Cahill GF Jr. Ketone bodies, potential therapeutic uses. IUBMB Life 51, 241–247 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Westman EC, et al. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr 86, 276–284 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Volek JS & Phinney SD The Art and Science of Low Carbohydrate Living, (Beyond Obesity, LLC, Miami, FL, USA: ). [Google Scholar]
- 12.Bough KJ, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol 60, 223–235 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Ahola-Erkkila S, et al. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum Mol Genet 19, 1974–1984 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Newman JC & Verdin E beta-hydroxybutyrate: much more than a metabolite. Diabetes Res Clin Pract 106, 173–181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.••Paoli A, Rubini A, Volek JS & Grimaldi KA Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr 67, 789–796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent review that explains the role of physiologic ketosis and possible mechanisms for reversing chronic disease.
- 16.Volek JS & Feinman RD Carbohydrate restriction improves the features of Metabolic Syndrome. Metabolic Syndrome may be defined by the response to carbohydrate restriction. Nutr Metab (Lond) 2, 31 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volek JS, Fernandez ML, Feinman RD & Phinney SD Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog Lipid Res 47, 307–318 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Sacks FM, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 360, 859–873 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bueno NB, de Melo IS, de Oliveira SL & da Rocha Ataide T Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr 110, 1178–1187 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Santos FL, Esteves SS, da Costa Pereira A, Yancy WS Jr. & Nunes JP Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev 13, 1048–1066 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Ebbeling CB, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 307, 2627–2634 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardillo S, Seshadri P & Iqbal N The effects of a low-carbohydrate versus low-fat diet on adipocytokines in severely obese adults: three-year follow-up of a randomized trial. Eur Rev Med Pharmacol Sci 10, 99–106 (2006). [PubMed] [Google Scholar]
- 23.Seshadri P, et al. Adipocytokine changes caused by low-carbohydrate compared to conventional diets in obesity. Metab Syndr Relat Disord 3, 66–74 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Woods SC, Lutz TA, Geary N & Langhans W Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci 361, 1219–1235 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodin J, Wack J, Ferrannini E & DeFronzo RA Effect of insulin and glucose on feeding behavior. Metabolism 34, 826–831 (1985). [DOI] [PubMed] [Google Scholar]
- 26.Asrih M & Jornayvaz FR Diets and nonalcoholic fatty liver disease: the good and the bad. Clinical nutrition (Edinburgh, Scotland) 33, 186–190 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Cox PJ, et al. Nutritional Ketosis Alters Fuel Preference and Thereby Endurance Performance in Athletes. Cell Metab 24, 256–268 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Sackner-Bernstein J, Kanter D & Kaul S Dietary Intervention for Overweight and Obese Adults: Comparison of Low-Carbohydrate and Low-Fat Diets. A Meta-Analysis. PLoS One 10, e0139817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnstone AM, Horgan GW, Murison SD, Bremner DM & Lobley GE Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 87, 44–55 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Phinney SD, Bistrian BR, Wolfe RR & Blackburn GL The human metabolic response to chronic ketosis without caloric restriction: physical and biochemical adaptation. Metabolism 32, 757–768 (1983). [DOI] [PubMed] [Google Scholar]
- 31.Bistrian BR Recent developments in the treatment of obesity with particular reference to semistarvation ketogenic regimens. Diabetes Care 1, 379–384 (1978). [DOI] [PubMed] [Google Scholar]
- 32.Samaha FF, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 348, 2074–2081 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Johnstone AM, Murison SD, Duncan JS, Rance KA & Speakman JR Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr 82, 941–948 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Veldhorst MA, Westerterp KR & Westerterp-Plantenga MS Gluconeogenesis and protein-induced satiety. Br J Nutr 107, 595–600 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Veldhorst MA, Westerterp-Plantenga MS & Westerterp KR Gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet. Am J Clin Nutr 90, 519–526 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Veldhorst M, et al. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav 94, 300–307 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Veldhorst MA, Westerterp KR, van Vught AJ & Westerterp-Plantenga MS Presence or absence of carbohydrates and the proportion of fat in a high-protein diet affect appetite suppression but not energy expenditure in normal-weight human subjects fed in energy balance. Br J Nutr 104, 1395–1405 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Wilson JM, et al. The Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Males. J Strength Cond Res (2017). [DOI] [PubMed] [Google Scholar]
- 39.Egan B & D’Agostino DP Fueling Performance: Ketones Enter the Mix. Cell Metab 24, 373–375 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Noakes T, Volek JS & Phinney SD Low-carbohydrate diets for athletes: what evidence? Br J Sports Med 48, 1077–1078 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Phinney SD, et al. Capacity for moderate exercise in obese subjects after adaptation to a hypocaloric, ketogenic diet. J Clin Invest 66, 1152–1161 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts MD, et al. A putative low-carbohydrate ketogenic diet elicits mild nutritional ketosis but does not impair the acute or chronic hypertrophic responses to resistance exercise in rodents. J Appl Physiol (1985) 120, 1173–1185 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Phinney SD Ketogenic diets and physical performance. Nutr Metab (Lond) 1, 2 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volek JS, et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism 65, 100–110 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Larosa JC, Fry AG, Muesing R & Rosing DR Effects of high-protein, low-carbohydrate dieting on plasma lipoproteins and body weight. Journal of the American Dietetic Association 77, 264–270 (1980). [PubMed] [Google Scholar]
- 46.Volek JS, Sharman MJ & Forsythe CE Modification of lipoproteins by very low-carbohydrate diets. The Journal of nutrition 135, 1339–1342 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Forsythe CE, et al. Limited effect of dietary saturated fat on plasma saturated fat in the context of a low carbohydrate diet. Lipids 45, 947–962 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham TE, et al. Retinol-Binding Protein 4 and Insulin Resistance in Lean, Obese, and Diabetic Subjects. New England Journal of Medicine 354, 2552–2563 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Foster GD, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Annals of internal medicine 153, 147–157 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steckhan N, et al. Effects of different dietary approaches on inflammatory markers in patients with metabolic syndrome: A systematic review and meta-analysis. Nutrition 32, 338–348 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Dashti HM, et al. Long term effects of ketogenic diet in obese subjects with high cholesterol level. Molecular and cellular biochemistry 286, 1–9 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Dashti HM, et al. Beneficial effects of ketogenic diet in obese diabetic subjects. Molecular and cellular biochemistry 302, 249–256 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Boden G, Sargrad K, Homko C, Mozzoli M & Stein TP Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Annals of internal medicine 142, 403–411 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Ajala O, English P & Pinkney J Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. The American journal of clinical nutrition 97, 505–516 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Gerber PA & Berneis K Regulation of low-density lipoprotein subfractions by carbohydrates. Current opinion in clinical nutrition and metabolic care 15, 381–385 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Chalasani N, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology (Baltimore, Md.) 55, 2005–2023 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Yki-Jarvinen H Nutritional Modulation of Non-Alcoholic Fatty Liver Disease and Insulin Resistance. Nutrients 7, 9127–9138 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feinman RD, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition (Burbank, Los Angeles County, Calif.) 31, 1–13 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Ramsden CE, et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ 353, i1246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Souza RJ, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 351, h3978 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kosinski C & Jornayvaz FR Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 9(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tiniakos DG, Vos MB & Brunt EM Nonalcoholic fatty liver disease: pathology and pathogenesis. Annual review of pathology 5, 145–171 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Hokanson JE & Austin MA Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. Journal of cardiovascular risk 3, 213–219 (1996). [PubMed] [Google Scholar]
- 64.Phinney SD, Bistrian BR, Evans WJ, Gervino E & Blackburn GL The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 32, 769–776 (1983). [DOI] [PubMed] [Google Scholar]
- 65.•Saslow LR, et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr Diabetes 7, 304 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Long-term, randomized human-subject dietary interention comparing very low carb ketogenic diet to moderate carb low fat diet. Demonstrating greater improvements in blood sugar (Hb a1c) and weight loss, despite reducing need for hypoglycemic medications in the very low carbohydrate ketogenic diet group.
- 66.Hussain TA, et al. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 28, 1016–1021 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Accurso A, et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr Metab (Lond) 5, 9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McKenzie AL, et al. A Novel Intervention Including Individualized Nutritional Recommendations Reduces Hemoglobin A1c Level, Medication Use, and Weight in Type 2 Diabetes. JMIR Diabetes 2, e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.•Bhanpuri NH, et al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: an open label, non-randomized, controlled study. Cardiovasc Diabetol 17, 56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Examination of coronary vascular disease risk factors in a cohort of patients who participated in a long-term human-subject dietary intervention evaluating the use of a ketogenic diet vs. standard care in a continous care model for type 2 diabetes. Nutritional ketosis was associated with improvement in most biomarkers of CVD risk after 1 year. An increase in LDL-C was limited to the large LDL subfraction with incresed particle size. Inflammation and blood pressure decreased.
- 70.Leow ZZX, Guelfi KJ, Davis EA, Jones TW & Fournier PA The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with Type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet Med (2018). [DOI] [PubMed] [Google Scholar]
- 71.Snorgaard O, Poulsen GM, Andersen HK & Astrup A Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care 5, e000354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turton JL, Raab R & Rooney KB Low-carbohydrate diets for type 1 diabetes mellitus: A systematic review. PLoS One 13, e0194987 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shukla SK, et al. HMGCS2 is a key ketogenic enzyme potentially involved in type 1 diabetes with high cardiovascular risk. Sci Rep 7, 4590 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schugar RC & Crawford PA Low-carbohydrate ketogenic diets, glucose homeostasis, and nonalcoholic fatty liver disease. Current opinion in clinical nutrition and metabolic care 15, 374–380 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Browning JD, et al. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr 93, 1048–1052 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andersen T, Gluud C, Franzmann MB & Christoffersen P Hepatic effects of dietary weight loss in morbidly obese subjects. Journal of hepatology 12, 224–229 (1991). [DOI] [PubMed] [Google Scholar]
- 77.Weiner RA Surgical treatment of non-alcoholic steatohepatitis and non-alcoholic fatty liver disease. Digestive diseases (Basel, Switzerland) 28, 274–279 (2010). [DOI] [PubMed] [Google Scholar]


