Summary.
Background:
Platelets from patients with X-linked chronic granulomatous disease or mice deficient in nicotinamide adenine dinucleotide (phosphate) (NAD(P)H) oxidase isoform NOX2 exhibit diminished reactive oxygen species (ROS) generation and platelet activation. Binding of Rac1 GTPase to p67phox plays a critical role in NOX2 activation by facilitating the assembly of the NOX2 enzyme complex.
Objective:
We tested the hypothesis that Phox-I, a rationally designed small molecule inhibitor of Rac–p67phox interaction, may serve as an antithrombosis agent by suppressing ROS production and platelet activation.
Results:
Collagen-related peptide (CRP) induced ROS generation in a time-dependent manner. Platelets from Rac1−/− mice or human platelets treated with NSC23766, a specific Rac inhibitor, produced significantly less ROS in response to CRP. Treatment of platelets with Phox-I inhibited diverse CRP-induced responses, including: (i) ROS generation; (ii) release of P-selectin; (iii) secretion of ATP; (iv) platelet aggregation; and (v) phosphorylation of Akt. Similarly, incubation of platelets with Phox-I inhibited thrombin-induced: (i) secretion of ATP; (ii) platelet aggregation; (iii) rise in cytosolic calcium; and (iv) phosphorylation of Akt. In mouse models, intraperitoneal administration of Phox-I inhibited: (i) collagen-induced platelet aggregation without affecting the tail bleeding time and (ii) in vivo platelet adhesion/accumulation at the laser injury sites on the saphenous vein without affecting the time for complete cessation of blood loss.
Conclusions:
Small molecule targeting of the Rac1–p67phox interaction may present an antithrombosis regimen by preventing GPVI- and non-GPVI-mediated NOX2 activation, ROS generation and platelet function without affecting the bleeding time.
Keywords: NADPH oxidase, platelet activation, Rac1 GTP-binding protein, reactive oxygen species, thrombosis
Introduction
Superoxide anion (O2−) and its derivatives, collectively called reactive oxygen species (ROS), are generated in platelets and contribute to signaling events involved in platelet activation [1–4]. The exact mechanisms of ROS generation and signaling mechanisms involved in ROS-mediated platelet activation are still being elucidated. Although diverse biochemical reactions contribute to ROS generation in platelets, nicotinamide adenine dinucleotide (phosphate) (NAD(P)H) oxidases (NOX) have emerged as critical sources of agonist-induced ROS generation. Six homologs of the cytochrome subunit of phagocyte NADPH oxidase (also known as NOX2 or gp91phox), namely NOX1, NOX3, NOX4, NOX5, DUOX1 and DUOX2, have been characterized in various cells [5]. Human platelets appear to possess NOX1 [6], NOX2 [6,7], NOX4 [8] and NOX5 [9]. Platelets also contain cytosolic cofactors, namely p22phox, p67phox [10] and p47phox, and Rac GTPase [11,12]. NOX2 is constitutively associated with p22phox and its activation requires translocation of p47phox, p67phox and p40phox as well as RacGTP to the NOX2/p22phox complex. The assembled NOX2 complex generates superoxide by transferring an electron from NADPH in the cytosol to oxygen in the extracellular space.
With the emergent role of NOX in platelet activation [13–15], it has been suggested that NOX2 may be a novel target for antithrombotic treatment [3,8]. A direct link between NOX2 activity, ROS generation and platelet activation has been shown in patients with X-linked chronic granulomatous disease (X-CGD). These patients are genetically deficient in NOX2 and exhibit diminished ROS generation and CD40 ligand expression in response to collagen or thrombin [12]. Moreover, plasma levels of soluble CD40 ligand and soluble P-selectin, two markers of in vivo platelet activation, are significantly diminished in X-CGD patients [16]. Recently, the role of NOX2 in platelet activation and thrombosis has also been reported using mice genetically deficient in NOX2 [8].
Rac1 GTPase plays a critical role in regulation of both NOX2 [17–19] and NOX1 [20,21]. The binding of Rac1-GTP to p67phox facilitates its binding to NOX2 and its activation [18], whereas binding of Rac1-GTP to NOXA1 enhances its binding to NOX1 and its activation. We have shown earlier that a rationally designed small molecule inhibitor of Rac1–p67phox interaction (Phox-I) inhibits ROS generation [22]. In this study, we tested our hypothesis that if Rac1–p67phox complex formation is critical in ROS generation, then interrupting this complex formation by Phox-I should inhibit ROS generation and consequently platelet activation. Here we report that Phox-I, by inhibiting Rac1–p67phox interaction in platelets, prevents GPVI- and non-GPVI-dependent NOX2 activation and the consequent ROS generation, as well as the in vivo and in vitro platelet activation without affecting the hemostatic response, presenting small molecule targeting of the Rac1-NOX2 interaction as a useful antithrombosis regimen.
Materials and methods
Materials
Chemicals and reagents were purchased either from Sigma-Aldrich (St. Louis, MO, USA), Chrono-Log Corporation (Havertown, PA, USA) or from specifically noted sources. Phox-I was custom synthesized as reported earlier [22]. Antibodies for Akt, p-Akt, ERK, p-ERK, P38-MAPK, p-P38-MAPK and β-tubulin were obtained from Cell Signaling (Boston, MA, USA).
Collection of blood and preparation of washed human platelet suspensions
All experiments using human blood from healthy volunteers were performed according to the protocols approved by the Institutional Review Board at Ohio University (Protocol # 08X126), Athens, Ohio, or Cincinnati Children’s Hospital Research Foundation (Protocol # 2010–1855), Cincinnati, Ohio. Procedures for drawing human blood, isolation of platelet-rich plasma (PRP) and preparation of washed platelet suspensions are the same as reported earlier [23,24]. The platelet count was adjusted to 3 × 108 per mL for aggregation studies.
Rac1 knockout mice
Conditional Rac1 knockout mice, Mx-Cre;Rac1loxP/loxP, inducible deletion of the Rac1gene by poly I:C induction, and blood platelet harvest, were described previously [23,25]. All animal maintenance and procedures were approved by Cincinnati Children’s Institution Animal Care and Utility Committee (Protocol # 1E05054).
ROS generation
Reactive oxygen species generation was quantified as reported earlier [4]. Briefly, washed platelets were incubated with 2′7′-dichlorofluorescein (dcf-da10 μM) for 15 min at 37 °C, washed once more to remove extracellular dye and ROS was detected by flow cytometry. ROS generation is expressed as a % of ROS in stimulated platelets. The mean fluorescence intensity (MFI) or the mean percentage of dcf-positive platelets were used to calculate ROS generation.
Assessment of P-selectin release, ATP secretion and platelet aggregation
P-selectin release from the α-granules was quantified by flow cytometry as described earlier [26]. Secretion of ATP from the dense granules was assessed by a luminescence method using a luciferin/luciferase kit from Chrono-Log Corporation [23]. The luciferin/luciferase reagent was added to platelets 1 min prior to addition of collagen-related peptide (CRP) or thrombin. Platelet aggregation was monitored by a standard optical density method [27] using an Aggregometer from Chrono-Log Corporation.
Measurement of platelet cytosolic calcium
Platelet cytosolic calcium levels were quantified using Fura2/AM loaded platelets. Washed human platelets were incubated with 3 μM Fura2/AM at room temperature for 30 min. After incubation, platelets were washed twice and resuspended in a modified Tyrode’s solution, pH 7.4. Fluorescence was recorded with a Hitachi F-2000 (Hitachi Medical Systems, Twinsburg, OH, USA) fluorescence spectrophotometer (Hitachi Medical Systems, Twinsburg, OH, USA) and calcium levels were quantified as described earlier [24].
Assessment of platelet spreading on immobilized fibrinogen
Platelet spreading on fibrinogen was assayed as previously described [4]. Briefly, glass cover slips were coated with fibrinogen overnight at 4 °C. Non-specific binding was blocked by incubating cover slips with bovine serum albumin (BSA, 1%) in Tyrode’s-HEPES buffer at 37 °C. Cover slips were rinsed with Tyrode’s-HEPES buffer after removing BSA. Aspirin (1 mM)-treated washed human platelets containing apyrase (3 U Ml−1) were layered over cover slips in the presence or absence of Phox-I. After 10 min incubation at 37 °C the cover slips were rinsed with phosphate buffered saline (PBS) to remove free platelets. Platelets on cover slips were then fixed with 4% paraformaldehyde for 10 min, rinsed with PBS twice and permeabilized with 0.1% Triton X-100 for 60 s. After two rinses with PBS, platelets were stained with Alexa 594-phalloidin to visualize F-actin. A Carl Zeiss LSM-510 confocal Axioplan 200 microscope and a Plan-Neofluar 100×/1.45 oil objective were used to generate platelet images. Digital images were processed using Zen 2007 software from Carl Zeiss (Thornwood, NY, USA).
Phosphorylation of Akt, ERK and P38-MAPK
Washed human platelets were stimulated with CRP or thrombin for a specified time and the reactions were terminated by the addition of 4× sample buffer. Western blotting of total and phospho-Akt, phospho-ERK, phospho-P38-MAPK and β-tubulin was carried out as reported earlier [26].
Tail bleeding time measurement
Tail bleeding time measurements were performed as described earlier [23]. Mice were anesthetized with and kept under a constant flow of 2.5% isoflurane and 0.35% of oxygen. The tail was cut at 5 mm from the tip and immediately immersed in saline at 37 °C. The bleeding time was defined as the time needed for the cessation of a visible blood stream for 1 min [28]. Monitoring of the bleeding times was stopped at 10 min by cauterizing the tails to prevent excessive loss of blood.
Intravital microscopy of platelet adhesion/accumulation at the laser injury sites at the saphenous vein
Mice (12–16 weeks of age) were given intraperitoneal injections of either 12 mg kg−1 Phox-I or dimethylsulfoxide (DMSO) 30 min before laser injury. These mice were then anesthetized with injection of ketamine/xylazine (100 and 10 mg kg−1, respectively) (Med-Vet International, Mettawa, IL, USA). Alexa488-conjugated α-GPIX antibody (clone Xia.B4, Emfret, Eibelstadt, Germany) was injected via retro-orbital injection. The saphenous vein was surgically exposed and pulsed once with a 532-nm laser (70 μJ; 100 Hz, 0.3 ms duration) (Ablate photoablation system; Intelligent Imaging Innovations, Denver, CO, USA). The mice were maintained on a heated observation stage and platelet response at the injury site was recorded as previously described [29]. Acquired images were analyzed with Slidebook 6.0 (Intelligent Imaging Innovations).
Statistical analysis
Data are expressed as means ± standard deviation (SD) or standard error (SE) as described in the figure legends. A P value of < 0.05 indicates statistically significant difference between the control and test samples.
Results
Gene targeting or inhibition of Rac GTPase diminished CRP-induced ROS generation in platelets
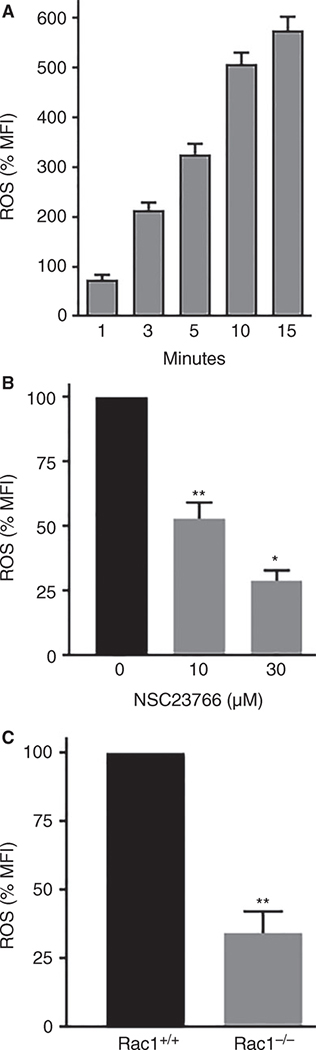
Rac1/2 GTPases have been shown to play a critical role in ROS generation by NOX2 in human and murine neutrophils [22]. Rac1 is the dominant Rac isoform in platelets [30] and if Rac1 is critical for ROS generation by NOX2 in blood platelets, then genetic deficiency or inhibition of Rac1 GTPase should inhibit ROS generation. To test this possibility, we first established that platelets stimulated with CRP generate ROS in a time-dependent manner. Addition of CRP (0.5 μg mL−1) to washed human platelets increased ROS in a time-dependent manner (Fig. 1A). Next, we investigated the role of Rac1 GTPase in CRP-induced ROS generation in human platelets treated with NSC23766, a small molecule inhibitor of Rac GTPase [23,31,32], and in platelets from Rac1-deficient mice [25,33]. Addition of NSC23766 to human platelets 2 min prior to stimulation with CRP significantly blocked ROS production (Fig. 1B). As shown in Fig. 1(C), Rac1−/− platelets exhibited significantly diminished ROS generation upon stimulation with CRP. These data suggest that Rac1 is essential for GPVI-mediated ROS generation by NOX2.
Fig. 1.
Gene targeting of Rac1 GTPase or small molecule inhibition of Rac1 diminished CRP-induced ROS generation in platelets. (A) CRP (0.5 μg mL−1) was added to washed human platelets and ROS was measured at the indicated time-points. CRP increased ROS generation in a time-dependent manner. (B) NSC23766, a Rac GTPase inhibitor [23], was added to platelets 2 min before stimulation with CRP (0.5 μg mL−1) and ROS was recorded at 10 min. (C) CRP (0.5 μg mL−1) was added to platelets from conditional Rac1 knockout mice [23] and ROS was measured at 10 min. Generation of ROS in dcf-da loaded platelets was monitored by flow cytometry as detailed in the Methods section. The data are mean ± SE, n = 4. *P < 0.0001, **P < 0.001. CRP, collagen-related peptide; MFI, mean fluorescence intensity; ROS, reactive oxygen species; SE, standard error.
Small molecule targeting of Rac1–p67phox interaction blocked CRP-induced ROS generation and platelet activation
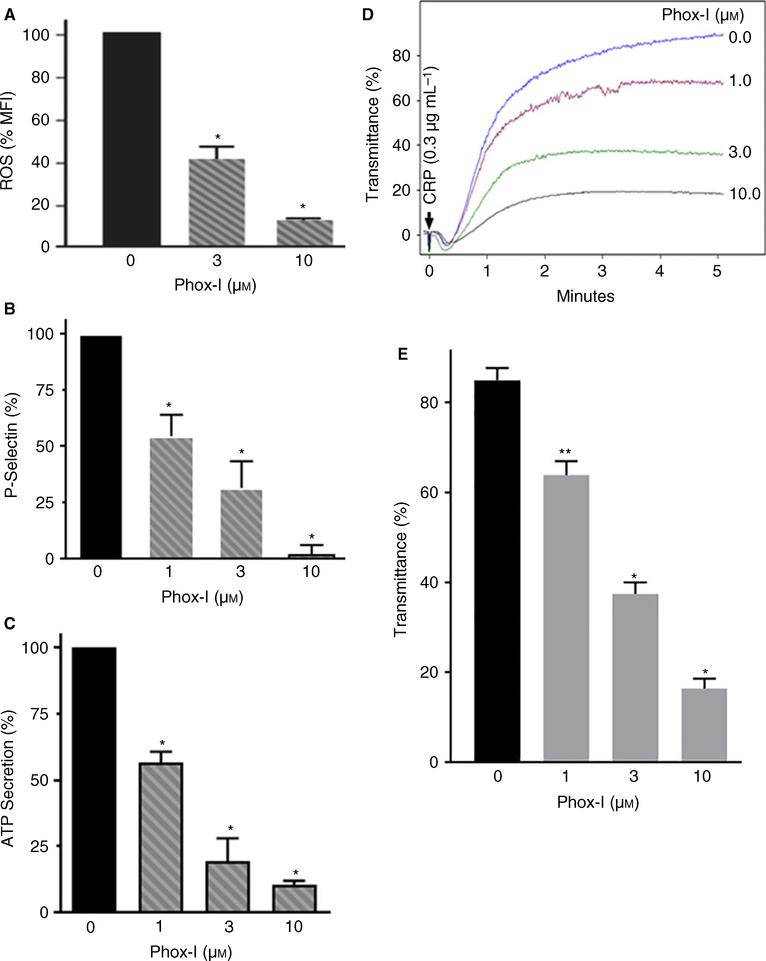
Rac1 GTPase binds to P67phox and facilitates its interaction with NOX2. This is a critical step for ROS generation by NOX2 [5]. The Arg38 and Arg102 residues of P67phox create a deep binding pocket necessary for its binding to Rac1 GTPase. We have shown earlier that Phox-I, a small molecule, binds to the Rac1 GTPase binding pocket of P67phox and prevents Rac1–P67phox interaction and consequently inhibits NOX2 and ROS generation [22]. We investigated the possibility that if Phox-I inhibits NOX2 activation then it should block CRP-induced ROS generation and platelet activation. Addition of Phox-I to human platelets 2 min before stimulation with CRP inhibited ROS generation in a concentration-dependent manner (Fig. 2A). Treatment of platelets with Phox-I blocked CRP-induced release of p-selectin from α-granules (Fig. 2B), secretion of ATP from the dense granules (Fig. 2C) and platelet aggregation (Fig. 2D–E) in a concentration-dependent manner. These data show that interrupting Rac1–P67phox interaction inhibits GPVI-dependent ROS production by NOX2 and thereby blocks platelet activation. Recently, Delaney et al. [8] have reported that platelets from mice genetically deficient in NOX2 exhibit diminished ROS generation and platelet aggregation in response to CRP. Our findings that inhibition of NOX2 by Phox-I prevents CRP-induced ROS generation and platelet activation concur with their findings that NOX2 plays a critical role in platelet ROS generation and activation.
Fig. 2.
Phox-I inhibited CRP-induced ROS generation, release of p-selectin, secretion of ATP and platelet aggregation. (A) Phox-I, a Rac1-p67phox inhibitor [22], was added to washed human platelets 2 min before addition of CRP and ROS generation was measured at 10 min. Phox-I inhibited ROS generation in a concentration-dependent manner (n = 4, mean ± SE *P < 0.0001). (B) Phox-I was added to washed human platelets 2 min prior to addition of CRP and the release of p-selectin from platelet α-granules was quantified by flow cytometry in aspirin-treated (1 mM) washed platelets, containing 0.2% bovine serum albumin and apyrase (0.4 U mL−1) as detailed in the Methods section. Phox-I inhibited the release of p-selectin in a concentration-dependent manner (mean ± SE, n = 4, *P < 0.0001). (C) CRP was added to washed human platelets 2 min after addition of Phox-I and ATP secretion was recorded using the Chrono-Log Lumi-Aggregometer (Havertown, PA). Phox-I inhibited ATP secretion (mean ± SE, n = 4, *P < 0.0001). (D) CRP was added to washed human platelets 2 min after addition of Phox-I and platelet aggregation was recorded using the Chrono-Log Lumi-Aggregometer. Addition of Phox-I to platelets inhibited aggregation in a concentration-dependent manner. (E) Platelet aggregation was analyzed by quantifying percent transmittance (mean ± SE, n = 4, *P < 0.0001, **P < 0.001). CRP, collagen-related peptide; ROS, reactive oxygen species; SE, standard error. [Color figure can be viewed at wileyonlinelibrary.com]
Inhibition of NOX2 by Phox-I blocked thrombin-induced platelet secretion, aggregation and calcium mobilization
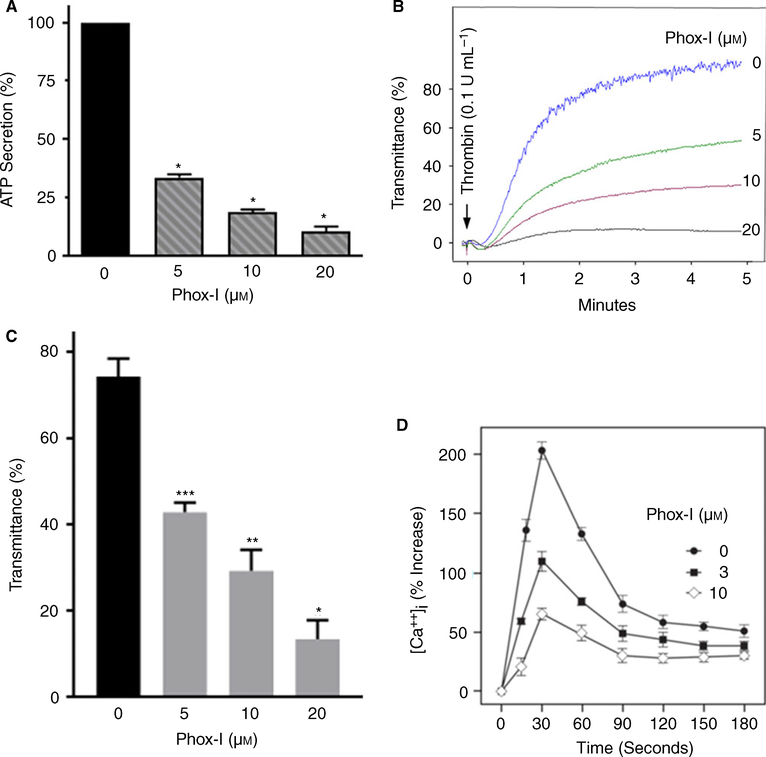
Thrombin, via activation of PI3kinase, regulates translocation of p67phox to the plasma membrane [34] and thereby regulates endogenous ROS production by NOX2. More recently, thrombin-induced platelet activation has been shown to be diminished in platelets from NOX2-deficient mice [8], implying a role for NOX2 in non-GPVI-mediated ROS generation and platelet activation. We have shown earlier that thrombin-induced ROS generation is diminished in Rac1-deficient murine platelets or human platelets treated with NSC23766 or Phox-I [4]. These findings suggest that Rac1 is essential for ROS generation by non-GPVI-mediated activation of NOX2 and inhibition of the Rac1–p67phox interaction by Phox-I blocks NOX2 activation. Here we investigated the possibility that Phox-I, by preventing Rac1–p67phox interaction, would block thrombin-induced platelet activation. Human platelets were incubated with Phox-I for 2 min and then challenged with thrombin. Phox-I inhibited thrombin-induced secretion of ATP (Fig. 3A), platelet aggregation (Fig. 3B–C) and rise in platelet cytosolic calcium in a concentration-dependent manner (Fig. 3D). These findings suggest that NOX2 is involved in non-GPVI-mediated ROS generation and platelet activation.
Fig. 3.
Phox-I blocked thrombin-induced ATP secretion, platelet aggregation and rise in platelet cytosolic calcium. (A–C) Addition of thrombin to washed human platelets induced ATP secretion and platelet aggregation. A 2 min pre-incubation with Phox-I inhibited ATP secretion (mean ± SE, n = 4, *P < 0.0001) and platelet aggregation (n = 4, *P < 0.0001, **P < 0.001, ***P < 0.05) in a concentration-dependent manner. A Lumi-Aggregometer from Chrono-Log-Corporation was used to monitor platelet ATP secretion and aggregation. (D) Addition of thrombin to washed human platelets increased the cytosolic calcium level. Treatment of platelets with Phox-I 2 min before addition of thrombin inhibited the rise in calcium in a concentration-dependent manner. Changes in calcium levels were quantified in Fura2/AM loaded platelets as detailed in the methods section. [Color figure can be viewed at wileyonlinelibrary.com]
Phox-I inhibition of platelet aggregation is surmountable
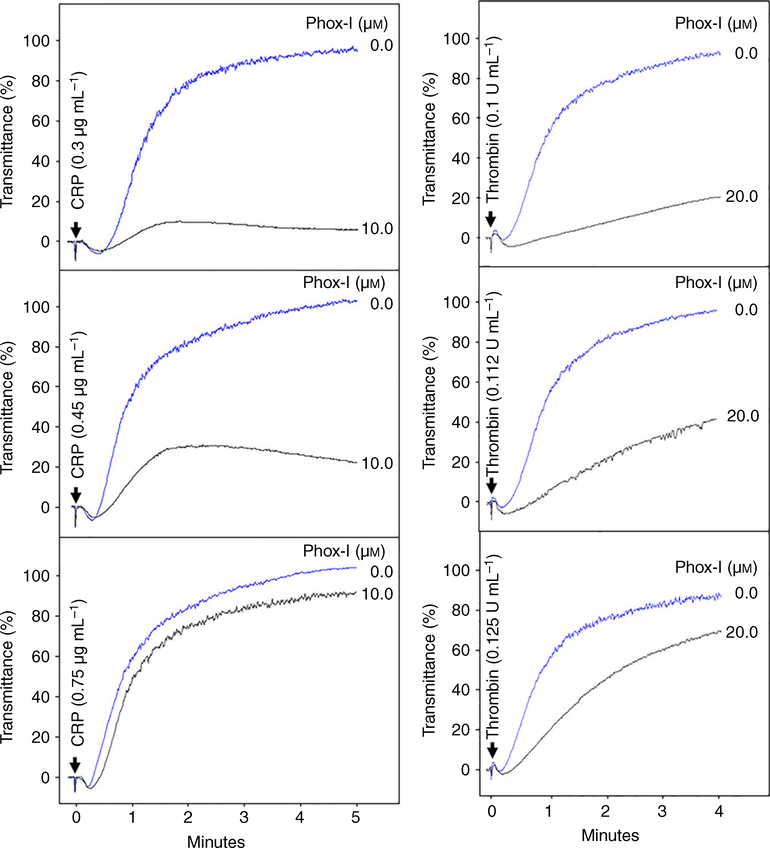
Next, we investigated whether the inhibitory effect of Phox-I is permanent in nature or can be overcome by increasing the agonist concentration. Addition of Phox-I (10 μM) to washed human platelets 2 min prior to addition of CRP (0.3 μg mL−1) or thrombin (0.1 U mL−1) inhibited platelet aggregation (Fig. 4). Addition of higher concentrations of CRP (Fig. 4 left panels) or thrombin (Fig. 4 right panels) gradually reversed the inhibition of platelet aggregation. These data show that Phox-I inhibition of platelet aggregation can be overcome by a high dose of agonists.
Fig. 4.
Phox-I inhibition of platelet aggregation is surmountable. Phox-I was added to platelets 2 min prior to addition of CRP (left panels) or thrombin (right panels) and aggregation was recorded using a Chrono-Log Aggregometer. Phox-I inhibited aggregation induced by CRP or thrombin. Increase in CRP or thrombin concentrations gradually reversed the inhibitory effect of Phox-I. The aggregation tracings are representative of three experiments. CRP, collagen-related peptide. [Color figure can be viewed at wileyonlinelibrary.com]
Platelet spreading on immobilized fibrinogen is diminished by Phox-I
Spreading of platelets on immobilized fibrinogen offers a readout of outside-in signaling [35]. We investigated the effect of Phox-I on platelet spreading on fibrinogen to determine if ROS inhibition affects the integrin outside-in signaling. Platelet spreading in the absence (Fig. 5A) and presence (Fig. 5B) of Phox-I was assessed by confocal microscopy. Platelets treated with Phox-I exhibited diminished platelet spreading on immobilized fibrinogen (Fig. 5C). This finding suggests that Phox-I inhibits integrin outside-in signaling.
Fig. 5.
Phox-I diminished platelet spreading on immobilized fibrinogen. Washed human platelets in the absence (A) or presence of (B) Phox-I (10 μM) were layered over fibrinogen (5 μg mL−1) coated cover slips in the presence of apyrase (3 U mL−1) for 10 min. The cover slips were washed and adherent platelets were processed for immuno-fluorescence confocal microscopy as detailed in the methods section. Platelets treated with Phox-I (B), as compared with DMSO (A), exhibited diminished spreading on immobilized fibrinogen. (C) The bar graph shows that spreading of Phox-I, as compared with DMSO, is diminished (***P < 0.05). Spreading of washed platelets on fibrinogen was quantified using Image J software (http://rsbweb.nih.gov/ij). DMSO, dimethylsulfoxide. [Color figure can be viewed at wileyonlinelibrary.com]
Phox-I inhibited CRP-induced phosphorylation of ERK and P38-MAPK
The extracellular signal-regulated kinase (ERK) has been linked with collagen-induced platelet aggregation [36] and thrombin-induced phosphorylation of ERK and P38-MAPK has been shown to be diminished in NOX2 knockout mice [37]. We investigated the possibility that Phox-I inhibits ROS-mediated platelet activation by inhibiting phosphorylation of ERK and or P38-MAPK. Phox-I added to washed human platelets 2 min before addition of CRP blocked phosphorylation of ERK and P38-MAPK (Figure S2). These findings suggest inhibition of ROS generation by NOX2 by the small molecule inhibitor inhibits platelet activation by inhibiting ERK/P38-MAPK activation.
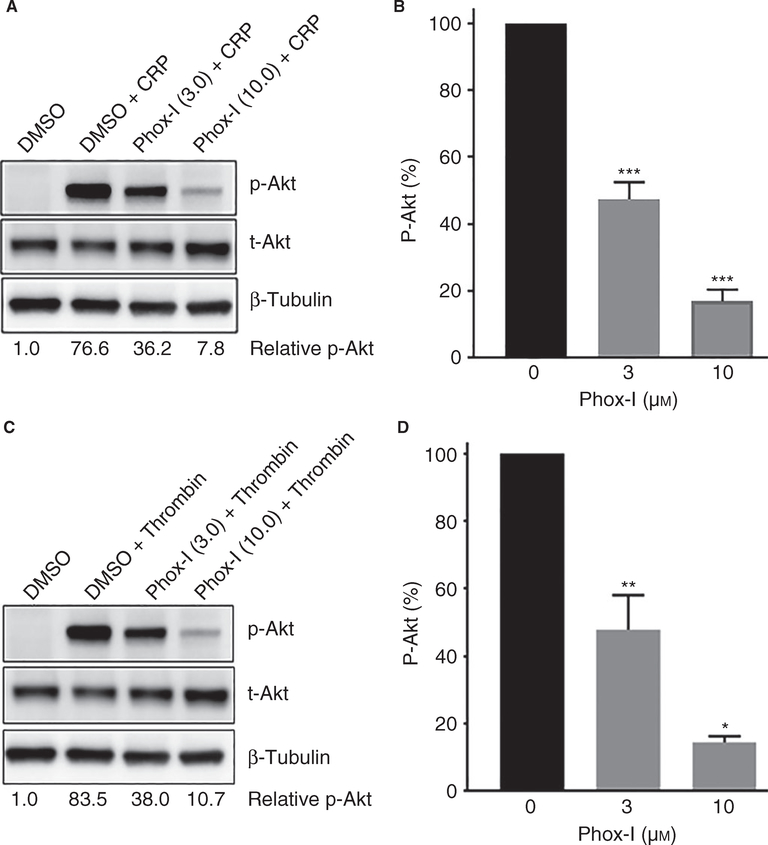
Inhibition of NOX2 by Phox-I blocked CRP or thrombin-induced phosphorylation of Akt
Platelet activation by diverse agonists induces activation of phosphoinositide 3-kinase (PI3K) isoforms, leading to calcium mobilization, platelet activation and thrombus formation [38,39]. PI3K activation leads to phosphorylation of Akt during inside-out and outside-in signaling [39–42]. We investigated the possibility that Phox-I inhibits platelet aggregation by downregulating PI3K. Addition of Phox-I to washed human platelets 2 min prior to stimulation with CRP (Fig. 6A–B) or thrombin (Fig. 6C–D) inhibited phosphorylation of Akt in a concentration-dependent manner. These data imply that inhibition of ROS generation by Phox-I blocks platelet aggregation, at least in part, by preventing PI3K activation.
Fig. 6.
Phox-I inhibited CRP or thrombin-induced phosphorylation of Akt. Phox-I was added to washed human platelets 2 min prior to addition of CRP or thrombin. The reactions were terminated at 5 min by adding 4× sample buffer and processed for western blotting and probed for Akt, p-Akt and β-tubulin. Phosphorylation was quantified by densitometry. The data in the bar graphs are mean ± SE from three experiments (*P < 0.0001, **P < 0.001, ***P < 0.05). CRP, collagen-related peptide; SE, standard error
Administration of Phox-I to mice inhibited collagen-induced platelet aggregation without affecting the murine tail bleeding time
The ability of Phox-I to inhibit in vitro platelet aggregation suggests that it may block platelet activation in vivo. To test this possibility, we investigated the effect of intraperitoneal injection of Phox-I on collagen-induced platelet aggregation. Collagen-induced aggregation in mice given Phox-I, as compared with DMSO, is inhibited in a dose-dependent manner (Fig. 7A–E). These data suggest that inhibition of Rac1–p67phox is effective in blocking ex vivo platelet aggregation.
Fig. 7.
Administration of Phox-I to wild-type mice blocked ex vivo platelet aggregation without altering the tail bleeding times. (A–D) Collagen-induced platelet aggregation in citrated platelet-rich plasma from mice administered Phox-I is completely blocked at the lower collagen concentrations and is partially recovered at the higher collagen concentrations. (E) Platelet aggregation was analyzed by quantifying percent transmittance (*P < 0.0001, **P < 0.001). (F) Tail bleeding times were assessed by cutting 5 mm off the tails of mice after 20 min of intraperitoneal administration of Phox-I. The dot plot shows that Phox-I administration did not affect the tail bleeding time. [Color figure can be viewed at wileyonlinelibrary.com]
Next, we investigated the possibility that Phox-I, by inhibiting platelet function, may also alter the hemostatic response. We measured the tail bleeding times in wildtype mice after 20 min of intraperitoneal administration of Phox-I or DMSO. The dot plot in Fig. 7(F) shows that the tail bleeding times in the Phox-I or DMSO-treated mice are essentially the same. These findings imply that pharmacologic targeting of NOX2 may be effective in diminishing platelet aggregation without prolonging the bleeding time.
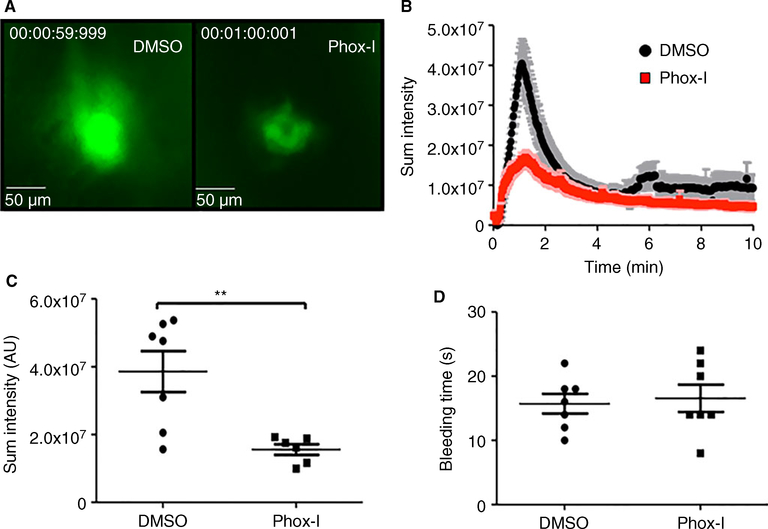
Administration of Phox-I to mice diminished platelet accumulation at the laser-induced injury site on the saphenous vein without affecting the time for cessation of blood loss
We further investigated the in vivo effectiveness of Phox-I by monitoring its effect on platelet accumulation at sites of laser injury to the saphenous vein [29]. DMSO or Phox-I was administered to wild-type mice intraperitoneally and platelet accumulation at sites of vascular injury was monitored by intravital fluorescence microscopy. Representative images of platelet accumulation in DMSO or Phox-I-treated mice are shown in Fig. 8(A); the quantification of platelet accumulation over time is shown in Fig. 8(B). In the DMSO-treated mice platelets rapidly accumulated upon laser injury to the saphenous vein. Platelets also accumulated rapidly at sites of injury in mice given Phox-I, but platelet accumulation was significantly diminished when compared with DMSO-treated controls (Fig. 8B–C). These data show that inhibition of NOX2 downregulates platelet-dependent thrombus formation in vivo.
Fig. 8.
Administration of Phox-I to wild-type mice diminished accumulation of platelets at the laser injury sites at the saphenous vein without affecting the bleeding time. Mice were administered Phox-I (12 mg kg−1) or DMSO vehicle control. (A) Representative images taken 1 min after laser injury. (B) Sum platelet intensity over time recorded at the injury sites is diminished in the Phox-I-treated mice. (C) Sum platelet intensity at 1 min after laser injury is significantly more diminished in the Phox-I-treated than control mice (**P < 0.001). (D) Time to complete stoppage of blood loss (in vivo bleeding time) after laser injury is essentially unaltered. [Color figure can be viewed at wileyonlinelibrary.com]
In parallel with the effects on thrombus formation, we also monitored the time to complete cessation of blood loss (in vivo bleeding time) following laser injury in the mice given DMSO or Phox-I. As shown in Fig. 8(D), the bleeding time was not altered by Phox-I administration.
Discussion
The findings in this study demonstrate that inhibition of Rac1–p67phox interaction by Phox-I, a rationally designed small molecule inhibitor of NOX2, prevents ROS generation and platelet activation in vitro and ex vivo and platelet adhesion/accumulation in vivo without affecting the hemostatic response to injury.
Although a possible role for ROS in platelet activation has been implicated for over 40 years [43,44], a number of recent reports have shown that agonist-induced activation of NOX plays a major role in ROS generation and consequent platelet activation [15,45]. Collagen-related peptide-induced, GPVI-dependent ROS generation has been suggested to utilize both Syk-dependent and Syk-independent signaling [15]. In addition, TRAF4 has been reported to generate ROS via the NOX1/2-p47phox axis [14]. Thrombin-induced ROS generation has been reported to involve PAR4 and GPIbα [13]. Based on these and other reports including genetic evidence in human [16,46] and mice [8] studies, it has been suggested that NOX2 may serve as an antithrombotic target [3,8]. This study was conducted to investigate this possibility by investigating the effect of Phox-I on CRP and thrombin-induced ROS generation and platelet activation.
Rac1 GTPase is one of the components of the NOX2 complex and is essential for ROS generation by NOX2 [5]. Rac1 interacts with p67phox, a cytosolic component of NOX2, and facilitates its binding to the membrane-bound enzyme and consequent ROS generation. Our findings that CRP induces ROS generation in a time-dependent manner and platelets from Rac1−/− conditional knockout mice or human platelets treated with NSC23766, a Rac GTPase inhibitor [23,31], exhibit significantly diminished ROS generation (Fig. 1) clearly demonstrate that Rac1 GTPase is essential for CRP-GPVI-mediated ROS generation in platelets.
We have shown earlier that the Rac1 GTPase–p67phox interaction is critical for ROS generation by NOX2 and interrupting this interaction with Phox-I inhibits NOX2 activation and ROS generation in human and murine neutrophils [22]. Our observations that Phox-I not only inhibited ROS generation in platelets (Fig. 2A), but also blocked the release of P-selectin (Fig. 2B), secretion of ATP (Fig. 2C) and platelet aggregation (Fig. 2D–E), suggest that pharmacologic targeting of NOX2 is an effective means of downregulating platelet activation. This possibility is further supported by a recent report that platelets from NOX2-deficient mice are defective in CRP-induced ROS generation and platelet activation [8]. Taken together these genetic and pharmacologic findings suggest that ROS generation by NOX2 plays a critical role in platelet activation and therefore NOX2 may serve as a therapeutic target for controlling platelet activation by curtailing ROS supply.
The X-CGD patients, because of the genetic deficiency of NOX2, are susceptible to severe immunological diseases and infections. This raises the possibility that inhibition of NOX2 by Phox-I may result in clinically unacceptable immunological side-effects. We don’t anticipate, although we can’t rule out based on the present study, that the transient inhibition of NOX2 will have similar clinical manifestations to the permanent absence of NOX2, as the transient nature and dose dependencies of the Phox-I inhibitor are likely to be distinct from those of the genetic defect in the NOX2 gene.
Platelets from NOX2-deficient mice are defective not only in CRP-GPVI-induced, but also in thrombin-GPCR-induced ROS generation and platelet activation [8]. Similarly, our observations that genetic deficiency or inhibition of Rac GTPase by NSC23766 blocks thrombin-induced ROS generation [4] and platelet activation [23] suggest that Rac GTPase signaling plays a critical role in thrombin-induced ROS generation and platelet activation. Our observations that inhibition of the Rac1-p67phox interaction by Phox-I not only impairs thrombin-induced ROS generation [4], but also secretion of ATP (Fig. 3A) and platelet aggregation (Fig. 3B–C), demonstrate that Phox-I is effective in inhibiting NOX2 activation and consequent ROS generation and platelet activation via thrombin-GPCR signaling.
NOX2-deficient murine platelets have been shown to exhibit diminished aggregation in response to CRP [8] as well as thrombin [8,37]. We observed the same defects in CRP-induced ROS generation and aggregation in NOX2−/− platelets (Figure S1). Furthermore, we found that the addition of Phox-I to NOX2−/− platelets had only a nominal effect on CRP-induced ROS generation and aggregation (Figure S1). Inability of Phox-I to further diminish ROS generation or platelet activation suggests that Phox-I specifically inhibits NOX2. However, the possibility that Phox-I may also inhibit NOX1 cannot be ruled out from these data.
NOX-generated superoxide (O−) is converted to H2O2 by the superoxide dismutase, which has been linked to agonist-induced calcium mobilization [47,48]. Platelets from NOX2-deficient mice have been shown to be defective in thrombin-induced mobilization of stored calcium [8]. Our findings that Phox-I inhibited thrombin-induced rise in platelet calcium levels suggest that inhibition of NOX2 by Phox-I has a similar effect on calcium mobilization in thrombin-stimulated platelets (Fig. 3D).
Focal adhesion kinase (FAK) has been shown to be involved in GPVI-dependent ROS generation and platelet activation [49] and Delaney et al. have reported that collagen-induced activation of Syk and PLCɣ2 is diminished in platelets from NOX2 KO mice [8]. Our findings that Phox-I did not inhibit phosphorylation of Syk, FAK or PLCɣ2 and inhibitors of FAK (PF573228) and Syk (BAY61–3606) inhibited phosphorylation of Syk, FAK and PLCɣ2 (data not shown) suggest that Phox-I, an inhibitor known to interrupt Rac-p67phox interaction [22], inhibits NOX downstream of Syk, FAK and PLCɣ2. The initial ROS generation by GPVI has been suggested to be independent of the Syk activation [14]. It is possible that Phox-I, regardless of the signaling events leading to NOX2 activation, prevents ROS generation by interrupting the Rac–p67–phox interaction.
P38 mitogen-activated protein kinase (P38-MAPK) has been linked with collagen-induced platelet aggregation [36] and H2O2, a ROS byproduct, has been shown to activate P38-MAPK in different cell types, including platelets [4–6]. In our study, Phox-I inhibited CRP-induced phosphorylation of P38-MAPK as well as ERK in a concentration-dependent manner (Figure S2). These findings suggest that these MAPKs are involved in ROS-mediated platelet activation. However, the role of P38-MAPK and ERK in ROS-mediated platelet activation remains to be resolved at this time as NOX2−/− platelets have been reported to exhibit a decrease in [37] as well as no effect [8] on thrombin-induced phosphorylation of p38MAPK and ERK.
Agonist-induced PI3K activation has been linked with platelet aggregation [39] and there is evidence that class III PI3K regulates platelet NOX and platelet activation and thrombosis via PI(3)P [50]. Agonist-induced calcium mobilization in platelets appears to correlate with PI3K signaling, as indicated by phosphorylation of Akt, as well as ERK and P38-MAPK [7,9]. Our findings that inhibition of NOX2 signaling by Phox-I blocked activation of Akt, a PI3K activity marker, in CRP as well as thrombin-stimulated platelets (Fig. 6) suggest that inhibition of NOX2 prevents platelet aggregation, at least in part, by inhibiting PI3K signaling.
Our findings that Phox-I, a NOX2 inhibitor, inhibited ex vivo aggregation induced by collagen (Fig. 7) as well as in vivo platelet adhesion and accumulation at the laser injury site (Fig. 8), but did not affect the murine tail bleeding times, are in agreement with the report by Delaney et al. that NOX2-deficient mice exhibit defective platelet aggregation and impaired laser-induced thrombosis without affecting the tail bleeding times [8]. Inhibition of platelet function carries the risk of prolonging the bleeding time. However, the impaired ex vivo aggregation in mice given Phox-I did not affect tail bleeding times. For the hemostatic response, that is the arrest of bleeding, only a sufficient number of platelets need to aggregate to provide a surface for coagulation reactions to occur to convert fibrinogen into fibrin. It is the continuous perpetuation of platelet aggregation/secretion that helps to stabilize and grow the size of the thrombus. Phox-I, by diminishing the secretion/aggregation reactions, reduces the adhesion/accumulation of platelets at the laser injury site. As seen in Fig. 8, Phox-I diminished but did not completely abolish adhesion/accumulation of platelets at the laser injury site. The accumulated platelets appear to be sufficient to prevent bleeding.
Our work is the first to demonstrate that a reversible inhibitor of NOX2 is capable of inhibiting platelet function in vitro and in vivo without altering the hemostatic response. Intravital imaging studies, in our unique model of laser injury to the saphenous vein [29], to evaluate platelet adhesion at sites of vascular injury show that platelet accumulation is reduced by Phox-I (i.e. the hemostatic plugs are much smaller in the presence of the inhibitor) (Fig. 8). We extrapolate from these findings that thrombosis (i.e. the formation of occlusive thrombi) would be strongly impaired by the presence of Phox-1, similar to what is observed in mice treated with the P2Y12 inhibitor, clopidogrel [29,51]. Based on these data we conclude that pharmacologic targeting of NOX2 may effectively downregulate platelet activation without disrupting the normal hemostatic response.
Supplementary Material
Data S1. Materials and methods.
Fig. S1. The effects of Phox-I on CRP-induced ROS generation and aggregation in platelets from NOX2-deficient mice.
Fig. S2. Phox-I inhibited CRP induced phosphorylation of ERK and P38-MAPK.
Essentials.
Reactive oxygen species (ROS) generation by NOX2 plays a critical role in platelet activation.
Rac1 regulation of NOX2 is important for ROS generation.
Small molecule inhibitor of the Rac1-p67phox interaction prevents platelet activation.
Pharmacologic targeting of Rac1-NOX2 axis can be a viable approach for antithrombotic therapy.
Acknowledgements
This work was supported by NIH grants R01 HL134617 and R01 CA193350 (to Y. Zheng), AHA grant 14EIA18910004 and NIH grant HL130404 (to W. Bergmeier) and a grant from the OUHCOM (to H. Akbar). S. Saleem was supported by a post-doctoral fellowship from the OUHCOM.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Krotz F, Sohn HY, Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler Thromb Vasc Biol 2004; 24: 1988–96. [DOI] [PubMed] [Google Scholar]
- 2.Begonja AJ, Gambaryan S, Geiger J, Aktas B, Pozgajova M, Nieswandt B, Walter U. Platelet NAD(P)H-oxidase-generated ROS production regulates alphaIIbbeta3-integrin activation independent of the NO/cGMP pathway. Blood 2005; 106: 2757–60. [DOI] [PubMed] [Google Scholar]
- 3.Violi F, Pignatelli P. Platelet NOX, a novel target for antithrombotic treatment. Thromb Haemost 2014; 111: 817–23. [DOI] [PubMed] [Google Scholar]
- 4.Akbar H, Duan X, Saleem S, Davis AK, Zheng Y. RhoA and Rac1 GTPases differentially regulate agonist-receptor mediated reactive oxygen species generation in platelets. PLoS ONE 2016; 11: e0163227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007; 87: 245–313. [DOI] [PubMed] [Google Scholar]
- 6.Vara D, Campanella M, Pula G. The novel NOX inhibitor 2-acetylphenothiazine impairs collagen-dependent thrombus formation in a GPVI-dependent manner. Br J Pharmacol 2013; 168: 212–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chlopicki S, Olszanecki R, Janiszewski M, Laurindo FR, Panz T, Miedzobrodzki J. Functional role of NADPH oxidase in activation of platelets. Antioxid Redox Signal 2004; 6: 691–8. [DOI] [PubMed] [Google Scholar]
- 8.Delaney MK, Kim K, Estevez B, Xu Z, Stojanovic-Terpo A, Shen B, Ushio-Fukai M, Cho J, Du X. Differential roles of the NADPH-oxidase 1 and 2 in platelet activation and thrombosis. Arterioscler Thromb Vasc Biol 2016; 36: 846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartimoccia S, Carnevale R, Sanguigni V, De Falco E, Frati G, Loffredo L, Plebani A, Soresina A, Pignatelli P, Violi F. NOX 5 is expressed in platelets from patients with chronic granulomatous disease. Thromb Haemost 2016; 116: 198–200. [DOI] [PubMed] [Google Scholar]
- 10.Seno T, Inoue N, Gao D, Okuda M, Sumi Y, Matsui K, Yamada S, Hirata KI, Kawashima S, Tawa R, Imajoh-Ohmi S, Sakurai H, Yokoyama M. Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb Res 2001; 103: 399–409. [DOI] [PubMed] [Google Scholar]
- 11.Krotz F, Sohn HY, Gloe T, Zahler S, Riexinger T, Schiele TM, Becker BF, Theisen K, Klauss V, Pohl U. NAD(P)H oxidasedependent platelet superoxide anion release increases platelet recruitment. Blood 2002; 100: 917–24. [DOI] [PubMed] [Google Scholar]
- 12.Pignatelli P, Sanguigni V, Lenti L, Ferro D, Finocchi A, Rossi P, Violi F. gp91phox-dependent expression of platelet CD40 ligand. Circulation 2004; 110: 1326–9. [DOI] [PubMed] [Google Scholar]
- 13.Carrim N, Arthur JF, Hamilton JR, Gardiner EE, Andrews RK, Moran N, Berndt MC, Metharom P. Thrombin-induced reactive oxygen species generation in platelets: a novel role for protease-activated receptor 4 and GPIbalpha. Redox Biol 2015; 6: 640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao J, Arthur JF, Gardiner EE, Andrews RK, Zeng L, Xu K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol 2018; 14: 126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arthur JF, Qiao J, Shen Y, Davis AK, Dunne E, Berndt MC, Gardiner EE, Andrews RK. ITAM receptor-mediated generation of reactive oxygen species in human platelets occurs via Syk-dependent and Syk-independent pathways. J Thromb Haemost 2012; 10: 1133–41. [DOI] [PubMed] [Google Scholar]
- 16.Carnevale R, Loffredo L, Sanguigni V, Plebani A, Rossi P, Pignata C, Martire B, Finocchi A, Pietrogrande MC, Azzari C, Soresina AR, Martino S, Cirillo E, Martino F, Pignatelli P, Violi F. Different degrees of NADPH oxidase 2 regulation and in vivo platelet activation: lesson from chronic granulomatous disease. J Am Heart Assoc 2014; 3: e000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol 2001; 2: 211–5. [DOI] [PubMed] [Google Scholar]
- 18.Koga H, Terasawa H, Nunoi H, Takeshige K, Inagaki F, Sumimoto H. Tetratricopeptide repeat (TPR) motifs of p67(phox) participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J Biol Chem 1999; 274: 25051–60. [DOI] [PubMed] [Google Scholar]
- 19.Lapouge K, Smith SJ, Walker PA, Gamblin SJ, Smerdon SJ, Rittinger K. Structure of the TPR domain of p67phox in complex with Rac.GTP. Mol Cell 2000; 6: 899–907. [DOI] [PubMed] [Google Scholar]
- 20.Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem 2006; 281: 17718–26. [DOI] [PubMed] [Google Scholar]
- 21.Miyano K, Koga H, Minakami R, Sumimoto H. The insert region of the Rac GTPases is dispensable for activation of superoxide-producing NADPH oxidases. Biochem J 2009; 422: 373–82. [DOI] [PubMed] [Google Scholar]
- 22.Bosco EE, Kumar S, Marchioni F, Biesiada J, Kordos M, Szczur K, Meller J, Seibel W, Mizrahi A, Pick E, Filippi MD, Zheng Y. Rational design of small molecule inhibitors targeting the Rac GTPase-p67(phox) signaling axis in inflammation. Chem Biol 2012; 19: 228–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akbar H, Kim J, Funk K, Cancelas JA, Shang X, Chen L, Johnson JF, Williams DA, Zheng Y. Genetic and pharmacologic evidence that Rac1 GTPase is involved in regulation of platelet secretion and aggregation. J Thromb Haemost 2007; 5: 1747–55. [DOI] [PubMed] [Google Scholar]
- 24.Perveen R, Funk K, Thuma J, Wulf Ridge S, Cao Y, Akkerman JW, Chen X, Akbar H. A novel small molecule 1,2,3,4,6-penta-O-galloyl-alpha-D-glucopyranose mimics the antiplatelet actions of insulin. PLoS ONE 2011; 6: e26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med 2005; 11: 886–91. [DOI] [PubMed] [Google Scholar]
- 26.Akbar H, Shang X, Perveen R, Berryman M, Funk K, Johnson JF, Tandon NN, Zheng Y. Gene targeting implicates Cdc42 GTPase in GPVI and non-GPVI mediated platelet filopodia formation, secretion and aggregation. PLoS ONE 2011; 6: e22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huzoor A, Wang W, Kornhauser R, Volker C, Stock JB. Protein prenylcysteine analog inhibits agonist-receptor-mediated signal transduction in human platelets. Proc Natl Acad Sci USA 1993; 90: 868–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cambien B, Bergmeier W, Saffaripour S, Mitchell HA, Wagner DD. Antithrombotic activity of TNF-alpha. J Clin Invest 2003; 112: 1589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Getz TM, Piatt R, Petrich BG, Monroe D, Mackman N, Bergmeier W. Novel mouse hemostasis model for real-time determination of bleeding time and hemostatic plug composition. J Thromb Haemost 2015; 13: 417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VL, Machesky LM, Watson SP. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem 2005; 280: 39474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akbar H, Cancelas J, Williams DA, Zheng J, Zheng Y. Rational design and applications of a Rac GTPase-specific small molecule inhibitor. Methods Enzymol 2006; 406: 554–65. [DOI] [PubMed] [Google Scholar]
- 32.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 2004; 101: 7618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 2003; 302: 445–9. [DOI] [PubMed] [Google Scholar]
- 34.Clutton P, Miermont A, Freedman JE. Regulation of endogenous reactive oxygen species in platelets can reverse aggregation. Arterioscler Thromb Vasc Biol 2004; 24: 187–92. [DOI] [PubMed] [Google Scholar]
- 35.Durrant TN, van den Bosch MT, Hers I. Integrin alphaIIbbeta3 outside-in signaling. Blood 2017; 130: 1607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saklatvala J, Rawlinson L, Waller RJ, Sarsfield S, Lee JC, Morton LF, Barnes MJ, Farndale RW. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J Biol Chem 1996; 271: 6586–9. [DOI] [PubMed] [Google Scholar]
- 37.Kim K, Li J, Tseng A, Andrews RK, Cho J. NOX2 is critical for heterotypic neutrophil-platelet interactions during vascular inflammation. Blood 2015; 126: 1952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilio K, Munnix IC, Mangin P, Cosemans JM, Feijge MA, van der Meijden PE, Olieslagers S, Chrzanowska-Wodnicka MB, Lillian R, Schoenwaelder S, Koyasu S, Sage SO, Jackson SP, Heemskerk JW. Non-redundant roles of phosphoinositide 3-kinase isoforms alpha and beta in glycoprotein VI-induced platelet signaling and thrombus formation. J Biol Chem 2009; 284: 33750–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S, Mangin P, Dangelmaier C, Lillian R, Jackson SP, Daniel JL, Kunapuli SP. Role of phosphoinositide 3-kinase beta in glycoprotein VI-mediated Akt activation in platelets. J Biol Chem 2009; 284: 33763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Brien KA, Gartner TK, Hay N, Du X. ADP-stimulated activation of Akt during integrin outside-in signaling promotes platelet spreading by inhibiting glycogen synthase kinase-3beta. Arterioscler Thromb Vasc Biol 2012; 32: 2232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Battram AM, Durrant TN, Agbani EO, Heesom KJ, Paul DS, Piatt R, Poole AW, Cullen PJ, Bergmeier W, Moore SF, Hers I. The Phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) Binder Rasa3 Regulates Phosphoinositide 3-kinase (PI3K)-dependent Integrin alphaIIbbeta3 Outside-in Signaling. J Biol Chem 2017; 292: 1691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho MJ, Pestina TI, Steward SA, Lowell CA, Jackson CW, Gartner TK. Role of the Src family kinase Lyn in TxA2 production, adenosine diphosphate secretion, Akt phosphorylation, and irreversible aggregation in platelets stimulated with gammathrombin. Blood 2002; 99: 2442–7. [DOI] [PubMed] [Google Scholar]
- 43.Handin RI, Karabin R, Boxer GJ. Enhancement of platelet function by superoxide anion. J Clin Invest 1977; 59: 959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcus AJ, Silk ST, Safier LB, Ullman HL. Superoxide production and reducing activity in human platelets. J Clin Invest 1977; 59: 149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh TG, Berndt MC, Carrim N, Cowman J, Kenny D, Metharom P. The role of Nox1 and Nox2 in GPVI-dependent platelet activation and thrombus formation. Redox Biol 2014; 2: 178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pignatelli P, Carnevale R, Di Santo S, Bartimoccia S, Sanguigni V, Lenti L, Finocchi A, Mendolicchio L, Soresina AR, Plebani A, Violi F. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler Thromb Vasc Biol 2011; 31: 423–34. [DOI] [PubMed] [Google Scholar]
- 47.Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F. Hydrogen peroxide is involved in collagen-induced platelet activation. Blood 1998; 91: 484–90. [PubMed] [Google Scholar]
- 48.Pastori D, Pignatelli P, Carnevale R, Violi F. Nox-2 up-regulation and platelet activation: novel insights. Prostaglandins Other Lipid Mediat 2015; 120: 50–5. [DOI] [PubMed] [Google Scholar]
- 49.Carrim N, Walsh TG, Consonni A, Torti M, Berndt MC, Metharom P. Role of focal adhesion tyrosine kinases in GPVI-dependent platelet activation and reactive oxygen species formation. PLoS ONE 2014; 9: e113679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Hu M, Luo D, Yue M, Wang S, Chen X, Zhou Y, Wang Y, Cai Y, Hu X, Ke Y, Yang Z, Hu H. Class III PI3K positively regulates platelet activation and thrombosis via PI(3) P-directed function of NADPH oxidase. Arterioscler Thromb Vasc Biol 2017; 37: 2075–86. [DOI] [PubMed] [Google Scholar]
- 51.Stolla M, Stefanini L, Andre P, Ouellette TD, Reilly MP, McKenzie SE, Bergmeier W. CalDAG-GEFI deficiency protects mice in a novel model of Fcgamma RIIA-mediated thrombosis and thrombocytopenia. Blood 2011; 118: 1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Materials and methods.
Fig. S1. The effects of Phox-I on CRP-induced ROS generation and aggregation in platelets from NOX2-deficient mice.
Fig. S2. Phox-I inhibited CRP induced phosphorylation of ERK and P38-MAPK.