Abstract
Recognition of altered self-antigens in tumor cells by lymphocytes forms the basis for antitumor immune responses. The effector cells in most experimental tumor systems are CD8+ T cells that recognize MHC class I binding peptides derived from molecules with altered expression in tumor cells. Although the need for CD4+ helper T cells in regulating CD8+ T cells has been documented, their target epitopes and functional impact in antitumor responses remain unclear. We examined whether broadly expressed wild-type molecules in murine tumor cells eliciting humoral immunity contributed to the generation of CD8+ T cells and protective antitumor immune responses to unrelated tumor-specific antigens [mutated ERK2 (mERK2) and c-erbB2/HER/neu (HER2)]. The immunogenic wild-type molecules, presumably dependent on recognition by CD4+ helper T cells, were defined by serological analysis of recombinant cDNA expression libraries (SEREX) using tumor-derived λ phage libraries screened with IgG antibodies of hosts bearing transplanted 3-methylchoranthrene-induced tumors. Coimmunization of mice with plasmids encoding SEREX-defined murine wild-type molecules and mERK2 or HER2 led to a profound increase in CD8+ T cells specific for mERK2 or HER2 peptides. This heightened response depended on CD4+ T cells and copresentation of SEREX-defined molecules and CD8+ T cell epitopes. In tumor protection assays, immunization with SEREX-defined wild-type molecules and mERK2 resulted in an inhibition of pulmonary metastasis, which was not achieved by immunization with mERK2 alone.
The original observation by Gross (1), subsequently confirmed by many investigators (2–6), demonstrated that specific resistance to tumor transplants could be induced in syngeneic systems by active immunization with tumor cells or fractions derived from them. The role of CD8+ and CD4+ T cells in these tumor systems has been the object of intense interest (7–9). CD8+ T cells from specifically immunized mice are capable of destroying tumor target cells in vitro (10), and adoptive transfer of CD8+ T cells from immunized donors confers resistance to tumor transplants to naive mice (7, 8, 11). In addition, anti-CD8 antibodies abolish resistance to tumor transplants in preimmunized mice (12–14). Over the past decade, a wide range of MHC class I binding peptides derived from tumor cells of mice and humans and recognized by CD8+ T cells have been defined (15, 16). In contrast, the requirement for CD4+ T cells has been less well documented and the nature of the antigen molecules recognized by these cells and their functional impact in antitumor immune responses are still largely unknown (8, 9, 17–19). Clearly, the goal is to understand how CD8+ T and CD4+ T cells, antigen-presenting cells (APCs), and other components of the immune system, such as B cells, antibodies, and complement, interact to achieve a state of effective tumor immunity.
Although antibodies have been generally relegated to a minor role in antitumor effector functions, there is a growing recognition that some tumor antigens, e.g., NY-ESO-1 (20–22), elicit a strong integrated immune response involving both cellular and humoral immunity. The analysis of the humoral immune response to human and murine tumors has been revolutionized by the introduction of an approach involving the serological analysis of recombinant cDNA expression libraries (SEREX) (23–26). A public database has been established to serve as a repository for information about SEREX-defined human tumor antigens (www.licr.org/SEREX.html). High-titered antibodies to a range of tumor cell products have been identified, and it has been surprising to find that the great majority of these molecules show no evident mutations or other structural abnormalities (24, 25). As high-titered antibody to SEREX-defined antigens implies recognition by CD4+ helper T cells, the SEREX repertoire can be considered to be a reflection of the CD4+ T cell repertoire.
In this report, we investigate whether immunogenic wild-type molecules defined by SEREX can intensify the response of CD8+ T cells to unrelated tumor-specific peptides through stimulation of a CD4+ helper T cell response.
Materials and Methods
Tumors and Tumor Cell Lines.
Established cell lines were derived from the following transplanted 3-methylchoranthrene-induced sarcomas of BALB/c origin: CMS5, CMS2, CMS7, CMS8, and CMS13 (27). CMS5a and CMS5m are subcloned cell lines obtained from CMS5. CMS5mHE is a cell line derived from CMS5a stably transfected with c-erbB-2/HER2/neu (HER2) cDNA. P1.HTR is a subline of P815 mastocytoma cell line of DBA/2 origin (28). UT-7/TPO is a human megakaryoblastic leukemia cell line (29).
Peptides.
ERK2–9m peptide, QYIHSANVL (30), and HER2 p63–71 (T) peptide, TYLPTNASL (31), have been described. They were synthesized at Takara Shuzo (Otsu, Japan).
Plasmids.
cDNA encoding mutated mitogen-activated protein kinase, ERK2 (mERK2), and cDNA encompassing the N-terminal 147 aa of HER2 (147HER2) were cloned into pCAGGS-New, kindly provided by J. Miyazaki, Osaka University, Osaka, Japan (32). Chicken ovalbumin cDNA was a kind gift of M. J. Bevan, University of Washington, Seattle (33) and was cloned into pBK-CMV (Stratagene). SEREX-defined plasmids and randomly selected plasmids are listed in Table 1 and were cloned into pBK-CMV.
Table 1.
List of SEREX-defined and randomly selected plasmids encoding murine and human tumor cell antigens
| Antigens (accession number) | Size, bp | Source (cDNA expression libraries) |
|---|---|---|
| Murine molecules | ||
| Immunogenic* | ||
| Mus heat shock protein, Dna J-like 2 (AF055664) | 2,242 | CMS 5a and CMS 2 |
| Mus DNA ligase 1 (U19604) | 3,172 | CMS 13 |
| Mus galectin-8 (AF218069) | 1,086 | CMS 2 and CMS 7 |
| Mus poly(A)binding protein, cytoplasmic 1 (X65553) | 2,244 | CMS 8 |
| Nonimmunogenic† | ||
| Mus sorting nexin 1 (AB019214) | 2,007 | CMS 5a |
| Mus glucose-regulated protein (D78645) | 2,408 | CMS 5a |
| Mus Cctz-1 gene for chaperon containing TCP-1-zeta-1 subunit (AB022159) | 19,505 | CMS 5a |
| Human molecules | ||
| Immunogenic‡ | ||
| Homo sapiens HMBA-inducible (XM_008348) | 3,594 | UT-7/TPO |
| Human retinoic acid-responsive protein (U50383) | 2,520 | UT-7/TPO |
| H. sapiens hepatitis delta antigen interacting protein A (XM_006503) | 997 | UT-7/TPO |
| H. sapiens cDNA FLJ20644 fis, clone KATO2588 (AK000651) | 1,781 | UT-7/TPO |
HMBA, hexamethylene-bis-acetamide.
Detected by syngeneic antibody from tumor-bearing mice in SEREX analysis of cDNA expression libraries of the corresponding tumor.
Obtained by random selection of clones from the CMS5a cDNA expression library.
Detected by antibody from patients with idiopathic thrombocytopenic purpura in SEREX analysis of cDNA expression libraries of the human megakaryoblastic leukemia cell line.
Antibodies.
Neutralizing antibodies were produced in 8- to 10-week-old female BALB/c nude mice by injecting hybridoma cells producing anti-CD4 (GK1.5) or Lyt-2.1 (49, 1) antibodies as described (30).
Immunization by Gene Gun.
Seven-week-old female BALB/c mice received abdominal delivery of plasmid DNA-coated gold particles by using a Helios Gene Gun System (Bio-Rad) at a helium discharge pressure of 350–400 psi. Gold particles were prepared as described (34–36). Mice received a booster injection 2 weeks after the initial immunization, and spleen cells were harvested for analysis 7 days later.
SEREX.
SEREX was performed as originally described by Sahin et al. (23) with some modifications.
Enzyme-Linked Immunospot Assays.
Enzyme-linked immunospot assays were performed as described by Power et al. (37).
Analysis of Pulmonary Metastasis.
Seven-week-old female BALB/c mice were challenged with 1 × 106 CMS5m or CMS5mHE tumor cells in a total volume of 0.1 ml injected through the lateral tail vein. Twenty to 28 days later, mice were killed, and the number of pulmonary nodules were counted with a dissecting microscope. Lungs were also evaluated microscopically.
Results
Identification of Tumor-Derived Immunogenic Molecules by SEREX.
To identify immunogenic molecules in murine tumor cells, we screened λ phage libraries prepared from cDNA of several cell lines derived from 3-methylchoranthrene-induced sarcoma of BALB/c origin (27) by using sera from syngeneic mice bearing cognate tumor lines. Among the array of genes detected, four of the most frequently detected gene products were Mus heat shock protein Dna J-like 2 (Dna J-like 2) (AF055664), Mus DNA ligase 1 (ligase 1) (U19604), Mus galectin-8 (galectin-8) (AF218069), and Mus poly(A) binding protein cytoplasmic 1 [poly(A)] (X65553). Analyses of the coding sequences of these genes revealed no mutations as compared with the registered sequences in the GenBank (Table 1), and these highly immunogenic tumor antigens were selected for further study. In addition, three randomly selected cDNAs from the library, Mus sorting nexin 1 (sorting nexin) (AB019214), Mus glucose-regulated protein (glucose-regulated protein) (D78645), and Mus Cctz-1 gene for chaperon containing TCP-1-zeta-1 subunit (Cctz-1) (AB022159), which were consistently negative in repetitive SEREX screenings, i.e., no antibody response was detected, were included to represent nonimmunogenic molecules derived from tumor cells.
SEREX-Defined Wild-Type Molecules Enhance Generation of Tumor-Specific CD8+ T Cells.
We previously identified a 9-mer peptide, 9m, derived from mutated mitogen-activated protein kinase, ERK2, as the antigenic peptide recognized by a CD8+ Kd-restricted cytotoxic T lymphocyte clone specific for CMS5, a 3-methylchoranthrene-induced sarcoma of BALB/c origin (30). Vaccination of syngeneic animals with 9m peptide led to rejection of subsequent challenge with CMS5 sarcoma, indicating that 9m peptide is a tumor rejection antigen of CMS5 sarcoma (30).
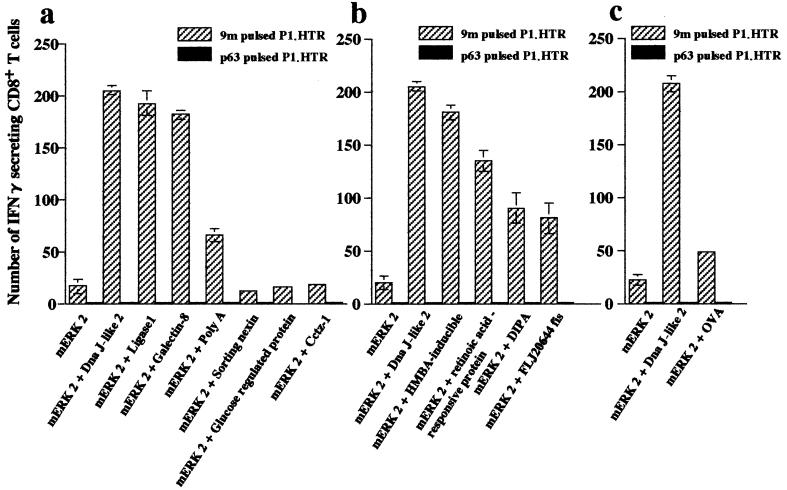
Gold particles for gene gun immunization were prepared by coating them with plasmids encoding mERK2 or a mixture of plasmids encoding mERK2 and plasmids encoding SEREX-defined molecules [Dna J-like 2, ligase 1, galectin-8, or poly(A)]. Mice immunized with mERK2 cDNA alone generated low levels of 9m peptide-specific CD8+ T cells (Fig. 1). In contrast, mice immunized with mERK2 and SEREX-defined antigens showed a striking increase in the number of 9m peptide-specific CD8+ T cells (Fig. 1a). The number of positive spots in these mice ranged from three to 10 times higher than in mice immunized with mERK2 cDNA alone. The specificity of the enzyme-linked immunospot assay was confirmed by tests with a control Kd binding peptide, p63–71 (T). In contrast, immunization with mERK2 plasmids mixed with plasmids encoding molecules not detected in repeated SEREX analyses, e.g., sorting nexin, glucose-regulated protein, or Cctz-1, showed no increase of 9m peptide-specific CD8+ T cells (Fig. 1a). Coimmunization with the control vector did not increase the number of 9m peptide-specific CD8+ T cells (Fig. 2a).
Figure 1.
Increased numbers of 9m peptide-specific CD8+ T cells generated by coimmunization with plasmids encoding mERK2 and SEREX-defined molecules. (a) Mice were immunized twice at a 2-week interval by using a gene gun containing gold particles coated with plasmids encoding mERK2, a mixture of plasmids encoding mERK2 and murine SEREX-defined molecules [Dna J-like 2, ligase 1, galectin-8, or poly(A)], or randomly selected plasmids encoding sorting nexin, glucose-regulated protein, or Cctz-1. The number of 9m peptide-specific CD8+ T cells in spleen cells of immunized mice was determined by enzyme-linked immunospot assay for IFN-γ-secreting cells 7 days after the second immunization. (b and c) Mice were immunized twice at a 2-week interval by using a gene gun containing gold particles coated with a mixture of plasmids for mERK2 and human SEREX-defined molecules [hexamethylene-bis-acetamide (HMBA)-inducible, retinoic acid responsive protein, delta antigen interacting protein A (DIPA), or FLJ20644 fis], or encoding chicken ovalbumin. Each bar represents the number of IFN-γ-secreting CD8+ T cells per 105 CD8+ T cells. Target cells were 9m-pulsed P1.HTR (hatched bars) or p63–71 (T) pulsed P1.HTR (solid bars) as a control. Data are mean ± SEM of three experiments.
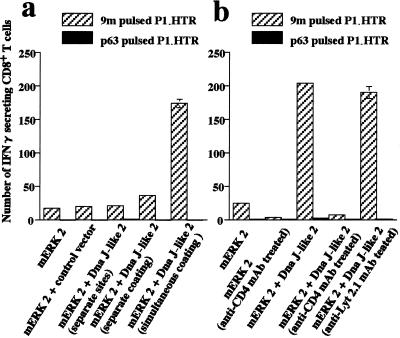
Figure 2.
Enhanced 9m peptide-specific CD8+ T cell response induced by coimmunization with SEREX-defined antigens requirement for copresentation and CD4+ T cells. (a) Mice were immunized twice with gold particles coated with plasmids encoding mERK2 alone, gold particles coated with a mixture of plasmids for mERK2 and Dna J-like 2 (simultaneous coating), or gold particles coated separately with plasmids encoding mERK2 or Dna J-like 2 and mixed together (separate coating). In another group, the separately coated particles were not mixed, but were administered at two distinct sites (separate sites). Increased numbers of 9m peptide-specific CD8+ T cells were observed only in animals immunized with gold particles cocoated with the plasmid mixtures. (b) Mice were immunized twice with gold particles coated with a mixture of plasmids encoding mERK2 and the SEREX-defined antigen Dna J-like 2. Anti-CD4 mAb (GK1.5) abolished the enhancing effect of coimmunization with mERK2 and the SEREX-defined antigen Dna J-like 2. Target cells were as shown in Fig. 1. Data are mean ± SEM of three experiments.
For comparison, similar experiments were performed by using cDNA encoding antigens of UT-7/TPO (a human megakaryoblastic leukemia cell line) (29) detected with sera from patients with idiopathic thrombocytopenic purpura. The following SEREX-defined human gene products were selected for study: Homo sapiens hexamethylene-bis-acetamide-inducible (XM_008348), human retinoic acid-responsive protein (retinoic acid-responsive protein) (U50383), H. sapiens hepatitis delta antigen interacting protein A (DIPA) (XM_006503), and H. sapiens cDNA FLJ20644 fis, clone KATO2588 (FLJ20644 fis) (AK000651). The cDNA encoding these immunogenic heterologous molecules also greatly increased 9m peptide-specific CD8+ T cell responses when presented together with mERK2 (Fig. 1b). In contrast, coimmunization with chicken ovalbumin resulted in only a marginal increase in specific CD8+ T cell reactivity (Fig. 1c).
Copresentation of SEREX-Defined Molecules and CD8+ T Cell Epitopes Is Required for an Enhanced CD8+ T Cell Response.
To determine whether the CD8+ T cell epitopes and SEREX-defined molecules need to be copresented on the same gold particle, mice were immunized with a mixture of gold particles individually coated with either mERK2 plasmids or Dna J-like 2 plasmids. Alternatively, mERK2 plasmids and Dna J-like 2 plasmids were injected on opposite sides of the abdomen. No increase in CD8+ T cells specific for 9m peptides was observed in either of these groups (Fig. 2a).
Enhanced CD8+ T Cell Response Depends on CD4+ T Cells.
We next asked whether CD4+ T cells were involved in facilitating induction of 9m peptide-specific CD8+ T cells by SEREX-defined antigens. No increase in 9m peptide-specific CD8+ T cells was observed when mice were pretreated with anti-CD4 antibody (GK1.5), but not with a control antibody (Lyt-2.1), before immunization with a mixture of plasmids encoding mERK2 and the SEREX-defined antigen, Dna J-like 2 (Fig. 2b).
Immunogenic Wild-Type Molecules also Enhance the Peptide-Specific CD8+ T Cell Response to HER2.
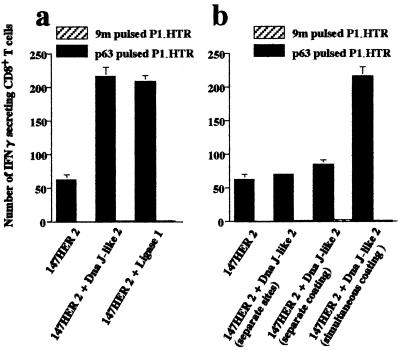
In the analysis of immune responses against HER2-expressing sarcomas, we defined a 9mer peptide, p63–71 (T), derived from HER2 as a target of CD8+ Kd-restricted cytotoxic T lymphocyte and also as a tumor rejection antigen of a syngeneic sarcoma, CMS17HE, stably transfected with HER2 cDNA (31). Coimmunization with plasmids encoding 147HER2 [which includes coding sequences for the p63–71 (T) peptide] and plasmids encoding the SEREX-defined molecule, Dna J-like 2, resulted in a striking increase in the number of p63–71 (T) peptide-specific CD8+ T cells (Fig. 3). Enhanced induction of p63–71 (T) peptide-specific CD8+ T cells was also observed when cDNA for the SEREX-defined antigen ligase 1 was used for coimmunization with 147HER2 (Fig. 3a).
Figure 3.
SEREX-defined molecules copresented with HER2 enhance p63–71 (T) peptide-specific CD8+ T cells. (a) Mice were immunized twice with plasmids encoding 147HER2 alone or with a mixture of plasmids encoding 147HER2 and Dna J-like 2 or ligase 1. Both SEREX-defined antigens enhanced the p63–71 (T) peptide-specific CD8+ T cell response. (b) Mice were immunized twice with gold particles coated with a mixture of plasmids encoding 147HER2 and Dna J-like 2 (simultaneous coating) or a mixture of particles separately coated with each plasmids (separate coating). Another group of mice were immunized with gold particles coated separately with 147HER2 or Dna J-like 2 plasmids and injected at two distant sites (separate sites). Enhanced numbers of p63–71 (T) peptide-specific CD8+ T cells were observed only in animals immunized with particles cocoated with 147HER2 and the SEREX-defined molecules. Target cells were as shown in Fig. 1. Data are mean ± SEM of three experiments.
Immunogenic Wild-Type Molecules also Augment in Vivo Tumor Rejection.
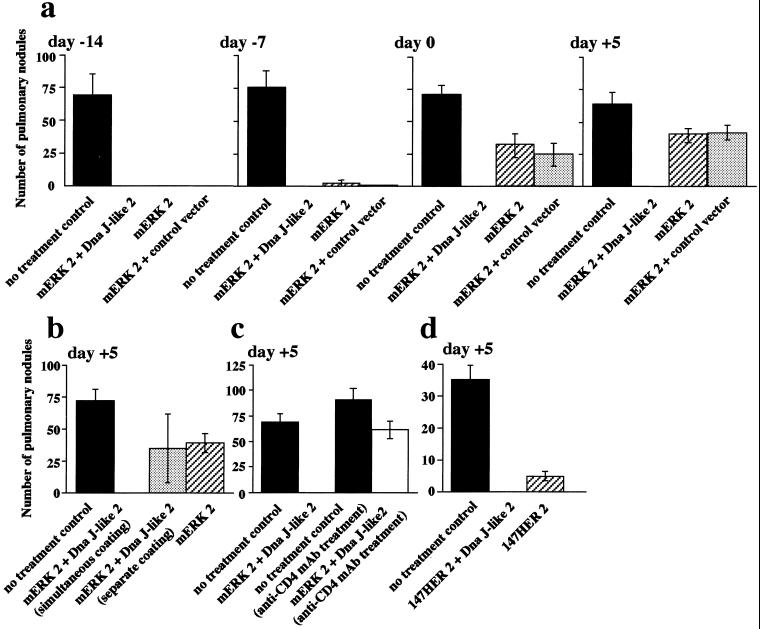
After i.v. injection, CMS5m establishes metastases in the lungs, leading to death of animals within 5–6 weeks. Two immunizations of mice with plasmids encoding mERK2 initiated 14 days before tumor challenge led to complete prevention of pulmonary metastases. This protective effect was lost when immunization was initiated 7 days before tumor challenge or after tumor challenge (Fig. 4a). In contrast, immunization using a combination of plasmids of mERK2 and Dna J-like 2 plasmids showed complete prevention of metastases even when initiated as late as 5 days after tumor challenge (Fig. 4a). Absence of metastasis was confirmed by histopathological examination. These therapeutic effects required copresentation of mERK2 and Dna J-like 2 on the same gold particle (Fig. 4b) and were CD4+ T cell-dependent (Fig. 4c). A comparable heightened therapeutic effect against CMS5mHE was demonstrated in mice coimmunized with plasmids encoding 147HER2 and Dna J-like 2 (Fig. 4d).
Figure 4.
Immunization with mERK2 or 147HER2 and Dna J-like 2; In vivo preventive and therapeutic effects against pulmonary metastases. (a) 1.0 × 106 CMS5m were injected via the lateral tail vein. Biweekly immunization with mERK2 (hatched bars), mERK2 plus control vector (dotted bars), or mERK2 plus Dna J-like 2 (open bars) commenced 14 or 7 days before tumor challenge, on the day of tumor challenge, or 5 days after tumor challenge. (b and c) 1.0 × 106 CMS5m were injected via the lateral tail vein. Immunization with gold particles separately coated with mERK2 or Dna J-like 2, or treatment of mice with anti-CD4 mAb (GK1.5) abolished the therapeutic effect of immunization with gold particles cocoated with mERK2 and Dna J-like 2. (d) 1.0 × 106 CMS5mHE were injected via the lateral tail vein. Biweekly immunization with 147HER2 (hatched bar), or 147HER2 plus Dna J-like 2 (open bar) commenced 5 days after tumor challenge. Mice were killed 28 days (a–c) or 20 days (d) after tumor injection, and the number of pulmonary metastatic nodules were counted with a dissecting microscope and expressed as mean ± SEM of five mice in each group.
Discussion
A striking increase in the CD8+ T cell response to MHC class I peptides derived from two tumor antigens, mERK2 (30) and HER2 (31), can be induced by coimmunization with plasmids encoding these antigens and unrelated SEREX-derived antigens. The plasmids need to be copresented at the same site by the same gold particles, because immunization with gold particles prepared separately and injected together at the same site, or separately at distant sites, did not lead to enhanced CD8+ T cell responses. SEREX-defined human antigens were comparable to SEREX-defined murine antigens in showing augmentation of the CD8+ T cell response. CD4+ T cells are clearly involved in this phenomenon, as antibody-mediated CD4+ T cell depletion abolishes the augmenting effect of SEREX-defined antigens. This effect of SEREX-defined antigens on the specific CD8+ T cell response is not induced by nonimmunogenic tumor cell products; randomly selected cDNAs encoding tumor proteins that do not elicit antibodies are without augmenting activity. Tumor protection assays show that the potentiation of specific CD8+ T cell generation demonstrable in vitro is reflected in increased tumor resistance in vivo. Immunization with a combination of plasmids encoding a tumor-specific antigen and a SEREX-defined antigen results in a much greater degree of protection against lung metastases than immunization with the tumor antigen alone. Mice can be completely protected by the combined vaccine when injected as late as 5 days after tumor challenge.
Although both mERK2 and 147HER2 encode one or more helper epitopes (unpublished data), these are clearly not sufficient to generate the development of an optimal CD8+ T cell response. The basis for the strong “helper” function of SEREX-defined antigens in the CD8+ T cell response to these tumor rejection antigens is unknown. The requirement for copresentation on the same gold particle suggests that the tumor antigen and the SEREX-defined antigen must be taken up and presented together by the same APC. It might be expected that this effect could be reproduced by a mixture of separately prepared gold particles injected at the same site, but injection with this mixture was ineffective, indicating that physical proximity of the two plasmid populations is crucial for intracellular events in APCs. Another surprise is that heterologous SEREX-defined molecules are no more or less effective than SEREX-derived murine antigens in eliciting this phenomenon. In fact, ovalbumin has only a weakly augmenting effect.
A number of approaches to augment CD4+ T cell help have been investigated (9, 19), and these fall in one of three general categories. One involves modifying the immunizing antigen itself by, for instance, haptenizing the antigen (38) or linking heterologous immunogenic peptides directly to the antigen (39, 40). The second involves coimmunization with tumor antigens and molecules with strong helper determinants (41, 42), and viral vectors encoding tumor antigens (43) can serve this function. Finally, the discovery of a range of molecular signals, such as CD40 ligand (44–46) and other stimulatory and costimulatory signals (35) involved in the helper function of CD4+ T cells and in modulating the interaction of APCs with CD4+ T cells, provide other ways to augment the CD8+ T cell response.
SEREX analysis of human and murine tumors has identified a vast array of tumor products that elicit humoral immune responses in tumor-bearing hosts (20–26). The basis for the immunogenicity of these products is unknown. Structural alterations through mutations or other changes appear to be the exception and not the rule with SEREX-defined antigens. Although some SEREX antigens show restricted tumor expression in normal tissues, e.g., cancer/testis antigens and melanocyte differentiation antigens, most SEREX-defined antigens are ubiquitously expressed. The current hypothesis is that amplified expression of the tumor product is the immunogenic stimulus for eliciting humoral immunity.
Given the magnitude and the frequency of humoral immune responses to the tumor antigen revealed by SEREX, it is appropriate to ask whether these responses are beneficial, detrimental, or insignificant to the tumor-bearing host. It is likely that the phenomena that we have described in the mouse of heightened CD8+ T on cell responses to tumor antigens by corecognition of SEREX-defined antigens has its counterpart in humans, occurring as a consequence of simultaneous uptake of complex antigenic mixtures from disintegrating tumor cells by APCs. As coimmunization with SEREX antigens and tumor antigens also results in heightened resistance to tumor challenge in the mouse, this approach is an attractive strategy for human cancer immunotherapy and one that will be facilitated by the extensive base of information about SEREX-defined human tumor antigens.
Acknowledgments
This work was partly supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Abbreviations
- SEREX
serological analysis of recombinant cDNA expression libraries
- APC
antigen-presenting cell
- mERK2
mutated ERK2
- HER2
c-erbB2/HER2/neu
References
- 1.Gross L. Cancer Res. 1943;3:326–333. [Google Scholar]
- 2.Foley E J. Cancer Res. 1953;13:835–837. [PubMed] [Google Scholar]
- 3.Prehn R T, Main J M. J Natl Cancer Inst. 1957;18:769–778. [PubMed] [Google Scholar]
- 4.Klein G, Sjögren H O, Klein E, Hellström K E. Cancer Res. 1960;20:1561–1572. [PubMed] [Google Scholar]
- 5.Old L J, Boyse E A, Clarke D A, Carswell E A. Ann NY Acad Sci. 1962;101:80–106. [Google Scholar]
- 6.Globerson A, Feldman M. J Natl Cancer Inst. 1964;32:1229–1243. doi: 10.1093/jnci/32.6.1229. [DOI] [PubMed] [Google Scholar]
- 7.North R J. Adv Immunol. 1984;35:89–155. doi: 10.1016/s0065-2776(08)60575-1. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg P D. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 9.Pardoll D M, Topalian S L. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 10.Wagner H, Pfizenmaier K, Röllinghoff M. Adv Cancer Res. 1980;31:77–124. doi: 10.1016/s0065-230x(08)60657-0. [DOI] [PubMed] [Google Scholar]
- 11.Melief C J M. Adv Cancer Res. 1992;58:143–175. doi: 10.1016/s0065-230x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama E, Uenaka A. J Exp Med. 1985;161:345–355. doi: 10.1084/jem.161.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu X G, Schmitt M, Hiasa A, Nagata Y, Ikeda H, Sasaki Y, Akiyoshi K, Sunamoto J, Nakamura H, Kuribayashi K, Shiku H. Cancer Res. 1998;58:3385–3390. [PubMed] [Google Scholar]
- 14.Noguchi Y, Richards E C, Chen Y-T, Old L J. Proc Natl Acad Sci USA. 1994;92:2219–2223. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boon T, Cerottini J C, van den Eynde B, van der Bruggen P, van Pel A. Annu Rev Immunol. 1994;12:337–366. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg S A. Immunity. 1999;10:281–287. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 17.Bennett S R, Carbone F R, Karamalis F, Miller J F A P, Heath W R. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung K, Hayashi R, Walker A L, Lowenstein C, Pardoll D M, Levitsky H. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang R F. Trends Immunol. 2001;5:269–276. doi: 10.1016/s1471-4906(01)01896-8. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y-T, Scanlan M J, Sahin U, Tureci O, Gure A O, Tsang B, Williamson B, Stockert E, Pfreundschuh M, Old L J. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jager E, Jager D, Karbach J, Chen Y-T, Ritter G, Nagata Y, Gnjatic S, Stockert E, Arand M, Old L J, Knuth A. J Exp Med. 2000;187:625–630. doi: 10.1084/jem.191.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jager E, Gnjatic S, Nagata Y, Stockert E, Jager D, Karbach J, Neumann A, Rieckenberg J, Chen Y-T, Ritter G, et al. Proc Natl Acad Sci USA. 2000;97:12198–12203. doi: 10.1073/pnas.220413497. . (First Published October 10, 2000; 10.1073/pnas.220413497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Proc Natl Acad Sci USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Old L J, Chen Y-T. J Exp Med. 1998;187:1163–1167. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y-T, Scanlan M J, Obata Y, Old L J. In: Principles and Practice of the Biologic Therapy of Cancer. Rosenberg S A, editor. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 557–570. [Google Scholar]
- 26.Ono T, Sato S, Tanaka M, Shibuya A, Old L J, Nakayama E. Int J Cancer. 2000;88:845–851. doi: 10.1002/1097-0215(20001215)88:6<845::aid-ijc1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 27.DeLeo A B, Shiku H, Takahashi T, John M, Old L J. J Exp Med. 1977;146:720–734. doi: 10.1084/jem.146.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Pel A, De Plaen E, Boon T. Somatic Cell Genet. 1985;11:467–475. doi: 10.1007/BF01534840. [DOI] [PubMed] [Google Scholar]
- 29.Komatsu N, Kunitama M, Yamada M, Hagiwara T, Kato T, Miyazaki H, Eguchi M, Yamamoto M, Miura Y. Blood. 1996;87:4552–4560. [PubMed] [Google Scholar]
- 30.Ikeda H, Ohta N, Furukawa K, Miyazaki H, Wang L, Furukawa K, Kuribayashi K, Old L J, Shiku H. Proc Natl Acad Sci USA. 1997;94:6375–6379. doi: 10.1073/pnas.94.12.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagata Y, Furugen R, Hiasa A, Ikeda H, Ohta N, Furukawa K, Nakamura H, Furukawa K, Kanamatsu T, Shiku H. J Immunol. 1997;159:1336–1343. [PubMed] [Google Scholar]
- 32.Niwa H, Yamamura K, Miyazaki J. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 33.Carbone F R, Bevan M J. J Exp Med. 1989;169:603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D. Nat Med. 1997;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 35.Porgador A, Irvine K R, Iwasaki A, Barber B H, Restifo N P, Germain R N. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klinman D, Sechler J M G, Conover J, Gu M, Rosenberg S A. J Immunol. 1998;160:2388–2392. [PubMed] [Google Scholar]
- 37.Power C A, Grand C L, Ismail N, Peters N C, Yurkowski D P, Bretscher P A. J Immunol Methods. 1999;227:99–107. doi: 10.1016/s0022-1759(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 38.Mizushima Y, Fujiwara H, Takai Y, Shearer G M, Hamaoka T. J Natl Cancer Inst. 1985;74:1269–1273. [PubMed] [Google Scholar]
- 39.Chesnut R W, Sette A, Celis E, Wentworth P, Kubo R T, Alexander J, Ishioka G, Vitiello A, Grey H M. In: Vaccine Design. Powell M F, Newman M J, editors. New York: Plenum; 1995. pp. 847–874. [DOI] [PubMed] [Google Scholar]
- 40.Rice J, Elliott T, Buchan S, Stevenson F K. J Immunol. 2001;167:1558–1565. doi: 10.4049/jimmunol.167.3.1558. [DOI] [PubMed] [Google Scholar]
- 41.Romieu R, Baratin M, Kayibanda M, Lacabanne V, Ziol M, Guillet J-D, Viguier M. J Immunol. 1998;161:5133–5137. [PubMed] [Google Scholar]
- 42.Casares N, Lasarte J J, Lopez-Diaz de Cerio A, Sarobe P, Ruiz M, Melero I, Prieto J, Borras-Cuesta F. Eur J Immunol. 2001;31:1780–1789. doi: 10.1002/1521-4141(200106)31:6<1780::aid-immu1780>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 43.Wang M, Bronte V, Chen P W, Gritz L, Panicali D, Rosenberg S A, Restifo N P. J Immunol. 1995;154:4685–4692. [PMC free article] [PubMed] [Google Scholar]
- 44.Ridge J P, Rosa F D, Matzinger P. Nature (London) 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 45.Bennett S R M, Carbone F R, Karamalis F, Flavell R A, Miller J F A P, Heath W R. Nature (London) 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 46.Schoenberger S P, Toes R E M, van der Voort E I H, Offringa R, Melief C J M. Nature (London) 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]