Abstract Abstract
Defining and recording the loss of species diversity is a daunting task, especially if identities of species under threat are not fully resolved. An example is the Pontocaspian biota. The mostly endemic invertebrate faunas that evolved in the Black Sea – Caspian Sea – Aral Sea region and live under variable salinity conditions are undergoing strong change, yet within several groups species boundaries are not well established. Collection efforts in the past decade have failed to produce living material of various species groups whose taxonomic status is unclear. This lack of data precludes an integrated taxonomic assessment to clarify species identities and estimate species richness of Pontocaspian biota combining morphological, ecological, genetic, and distribution data. In this paper, we present an expert-working list of Pontocaspian and invasive mollusc species associated to Pontocaspian habitats. This list is based on published and unpublished data on morphology, ecology, anatomy, and molecular biology. It allows us to (1) document Pontocaspian mollusc species, (2) make species richness estimates, and (3) identify and discuss taxonomic uncertainties. The endemic Pontocaspian mollusc species richness is estimated between 55 and 99 species, but there are several groups that may harbour cryptic species. Even though the conservation status of most of the species is not assessed or data deficient, our observations point to deterioration for many of the Pontocaspian species.
Keywords: Aral Sea, bivalves, Black Sea, Caspian Sea, conservation, gastropods, nomenclature, taxonomy
Introduction
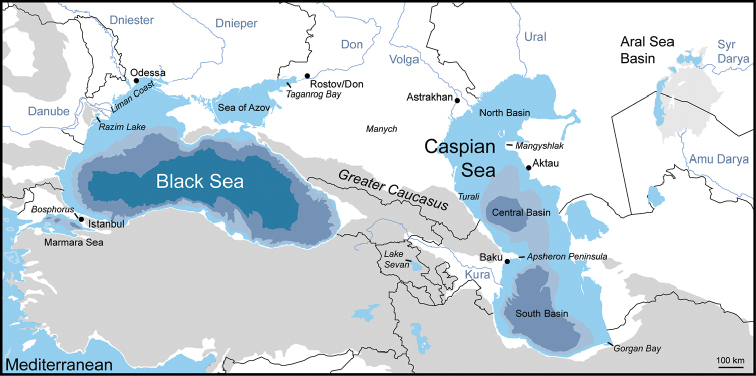
The aquatic Pontocaspian (or Ponto-Caspian) biota is constituted by taxa that evolved in saline water bodies in the Caspian Sea – Black Sea – Aral Sea region and surrounding rivers in the past few million years. They include diverse groups such as diatoms, dinoflagellates, foraminiferans, crustaceans, molluscs, as well as fish and the Caspian seal. Major Pontocaspian habitats are located in the northern coastal zone of the Black Sea (mostly confined to the Romanian and Ukrainian coasts) and the Sea of Azov (mostly in the Taganrog Bay), cover the entire Caspian Sea and, until recently, the Aral Sea (Fig. 1). However, Pontocaspian habitats are impacted by human activities such as pollution, habitat modification and introduction of invasive species (Bologa et al. 1995, Zolotarev 1996, Zaitsev and Mamaev 1997, Gomoiu et al. 2002, Grigorovich et al. 2003, Occhipinti-Ambrogi and Savini 2003, Barannik et al. 2004, Shalovenkov 2005, UNEP 2006, Stolberg et al. 2006, Selifonova 2008a, b, Popa et al. 2009), as well as the entire obliteration of environments in the case of the Aral Sea in the second half of the 20th century (Mainguet and Létolle 1997, Andreeva and Andreev 2003, Plotnikov et al. 2016).
Figure 1.
Map of the Pontocaspian region with the indication of major basins, rivers, regions, and cities referred to in the text.
Faunas in the Pontocaspian region have strongly changed in the past century. Pontocaspian species that were abundant only a century ago, such as Dreissenaelata and D.caspia in the Caspian Sea, have vanished in the mid-20th century (Kosarev and Yablonskaya 1994). For the Aral Sea, the faunas appear to have largely disappeared with the demise of the lake system since the 1950s (Andreeva and Andreev 2003). Abundances of several other species in the Caspian Sea and Black Sea Basin have severely declined (Bologa et al. 1995, Zaitsev and Mamaev 1997, Barannik et al. 2004, Popa et al. 2012).
However, we cannot evaluate the extent or nature of biodiversity loss as there is no general agreement on the species that it might concern. Much of the diversity in Pontocaspian mollusc groups is contained within a limited number of genera. Changing taxonomic approaches through time (e.g., Zhadin 1952, Logvinenko and Starobogatov 1969, Alexenko and Starobogatov 1987, Sitnikova and Starobogatov 1999, Munasypova-Motyash 2006a, b, Anistratenko 2007b, Kijashko in Bogutskaya et al. 2013, Vinarski and Kantor 2016, Neubauer et al. 2018) combined with large morphological variability and few diagnostic characters in certain groups, as well as the paucity of living material and partial disappearance of type material, has precluded critical reassessment of species boundaries and thus species richness. For the Caspian Sea, multiple efforts to collect fresh material in the past decade failed to produce sufficient living material to elucidate these taxonomic matters for most of the groups. Sampling efforts include coastal sampling around Turali, Russia (FW, 2003); northern Azerbaijan (FW, 2016), middle and southern Azerbaijan (VA, ML, AFS, TW, 2017); Mangyshlak region coastal areas, Kazakhstan (OA, VA, 2016, 2017); the transition of the northern to middle Caspian Sea Basin in Kazakhstan (PRIDE expedition, 2017); and the Gorgan Bay in Iran (AFS, 2018). A faunal inventory of the deep-water southern Caspian Sea Basin (> 200 m water depth) of southern Azerbaijan was published lately by Mirzoev and Alekperov (2017). We are uncertain whether it concerns living material nor can we assess the species identities. Their records are mentioned below but require further confirmation. We did find some living endemic species ourselves, and from coastal areas low numbers of such species have been reported elsewhere (e.g., Latypov 2015). Yet, many species and even groups of species (e.g., Turricaspia species) have not been encountered alive despite our attempts. Our inability to collect life specimens for several groups has made a combined molecular-morphological approach to delineate species impossible. As a result, a reliable estimate of the number of species involved is lacking, and therefore the potential magnitude of the biodiversity decline is speculative. Hence, we need an alternative approach to outline the species boundaries and estimate the numbers affected.
By pooling all insights, data (published and unpublished) and expert opinions on the Pontocaspian mollusc species through taxonomists we aim to provide a list of Pontocaspian mollusc species that can serve as a base for further research. We use molluscs as a model group since they are (1) an important, representative and well-known part of the Pontocaspian fauna, (2) have a number of taxonomic specialists available, and (3) can often be identified based on their shell characters even when living populations have vanished. The Pontocaspian aquatic mollusc species list will highlight uncertainties in species complexes as to give guidance to further research in resolving taxonomic matters. The aim of this work is to compile a list of Pontocaspian mollusc species with the underlying arguments why we consider these species as (likely) valid species, to outline taxonomic uncertainties and to provide an updated estimate of species richness.
Materials and methods
A preliminary Pontocaspian mollusc species list was assembled during a PRIDE program workshop in Giessen, Germany, in May 2018. The PRIDE (“Drivers of Pontocaspian Rise and biodiversity Demise”) program is an EU funded Innovative Training Network that studies the drivers of the rise and demise of Pontocaspian faunas. Using listings in Vinarski and Kantor (2016) supplemented with further information from the participants, this initial list was then circulated among a wider community of taxonomic workers for further updates and comments. Data on distribution and type material were derived from Vinarski and Kantor (2016) and further completed and amended. The systematic order above the species level follows Bouchet et al. (2017) and MolluscaBase (2018a). In cases where we deviate from the supraspecific classification, arguments are discussed below.
The list comprises aquatic Holocene Pontocaspian mollusc faunas. A substantial number of Pontocaspian species has been described from empty shells from beach material or derive from grab samples. Such samples typically are dominated by time-averaged Holocene shell assemblages, which may or may not yield living specimens and in very rare occasions also contain older (Pleistocene) material (see, e.g., Leroy et al. 2018). For the Black Sea Basin, the Holocene time interval largely coincides with the date of the marine flooding through the Bosphorus and subsequent marginalisation of Pontocaspian species to the NW coastal zone (Danube Delta to Dnieper Estuary) and the Sea of Azov (Mordukhay-Boltovskoy 1960). For the Caspian Sea, the time interval corresponds to the so-called Novocaspian period that started after the very deep Mangyshlak regression 8 ka (Fedorov 1953, Nevesskaja 1958, 2007, Yanina 2005). The time interval contains the earliest impact of humans on native faunas, such as the introduction of Cerastodermaglaucum in the Caspian Basin during the early Holocene (Fedorov 1957, Yanina 2009). It also contains the large faunal changes of the 20th century related to pollution, invasive species, and obliteration of habitats (Kosarev and Yablonskaya 1994).
One of the greatest difficulties is to establish the identities of taxa reported as geographic subspecies. Many species have forms, varieties, and subspecies described from the Aral Sea, the Caspian Sea Basin, and the Black Sea Basin (including the Azov Sea). Often, such distinctions are made based on the geographical isolation alone or on a range of morphological characters whose variation seems to be overlapping in geographical subpopulations. In order to assess whether the geographical populations are indeed species, we need combined morphological, ecological, and molecular data, but only few studies produced this information to date (e.g., Popa et al. 2012 for Black Sea Basin Monodacna). For the Aral Sea, we expect difficulties to obtain fresh material of almost all species for molecular analyses due to the obliteration of most of the lake and its fauna in the 20th century (Andreeva and Andreev 2003, Plotnikov et al. 2016). To date, hardly any molecular data on closely related species that are (potentially) shared between the Caspian and Black Sea have been published with the exceptions of Dreissenagrimmi/D.bugensis (e.g., Therriault et al. 2004, Stepien et al. 2013) and Ecrobiamaritima/E.grimmi (Haase et al. 2010). For several potentially shared species, ecological tolerances and preferences between Caspian and Black Sea Basin populations are overlapping, but in some cases (like for D.grimmi/D.bugensis) they are not. We have adopted a conservative approach, and as long as no additional arguments (morphological, ecological, or genetic differences) were found, we consider the Aral, Caspian and Black Sea varieties/subspecies synonyms. Another difficulty in especially Caspian taxa is the erection of so-called “bathymetric” subspecies, which seem to be distinguished mostly based on their depths of occurrence. As long as no other (morphological, genetic) arguments are available, we synonymise such bathymetrical forms.
A listing of synonyms and important past misidentifications from the literature is given. The list is not exhaustive and intended to show major shifts in taxonomic thinking about Pontocaspian and invasive species. The format of synonymy lists follows mostly suggestions of Matthews (1973). Asterisks in front of a record indicate valid first descriptions, a superscript “o” a prior yet invalidly introduced synonym. The status of each species is defined according to criteria outlined in Table 1.
Table 1.
Definitions we use to characterise the status of species.
| Pontocaspian | Centre of evolutionary history in Pontocaspian lakes |
|---|---|
| Native | Present in the Pontocaspian region today and in the Quaternary (not introduced by man) but centre of evolution not necessarily in that region: e.g., planorbid species with a Palearctic distribution, Cerastodermaglaucum. |
| Introduced | Species introduced in the Pontocaspian from elsewhere, usually anthropogenic: some Pontocaspian species have migrated between Pontocaspian basins and their status is explained in detail there (e.g., Monodacnacolorata/Dreissenabugensis: introduced in Caspian from natural ranges in Black Sea Basin). |
| Invasive | Species that have become disruptive in the ecosystem after introduction. |
Systematic catalogue
Bivalvia
Remarks. Within the endemic bivalve species groups, a general lack of combined molecular, morphological, and ecological approaches has led to partially unresolved taxonomy, especially within the genera Monodacna and Dreissena. Much of the bivalve taxonomy follows the latest review of Caspian bivalves by Kijashko in Bogutskaya et al. (2013), and we discuss deviations from his schedule. The list of Aral bivalves published by Vinarski and Kantor (2016) is based chiefly on Andreeva and Andreev (2003), and it is used here as a base with appropriate changes in nomenclature.
Family Mytilidae Rafinesque, 1815
Mytilasterminimus (Poli, 1795)
*1795 Mytilusminimus Poli: 209–210, pl. 32, fig. 1.
1932 Mytilasterlineatus (Gmelin, 1790). – Bogachev: 38, pl. 1, figs 5–11 [nonMytiluslineatus Gmelin, 1791].
1952 Mytilasterlineatus (Gmelin, 1789). – Zhadin: 285, fig. 248 [non Gmelin, 1791].
1969 Mytilasterlineatus (Gmel.). – Logvinenko and Starobogatov: 311–312, figs 339a, b, pl. 5, figs 1, 2 [non Gmelin, 1791].
1969 Mytilasterlineatus (Gmelin, 1790). – Vekilov: 155–157, pl. 35, figs 1–25 [non Gmelin, 1791].
2013 Mytilasterlineatus (Gmelin, 1791). – Kijashko in Bogutskaya et al.: 316, fig. 104 [non Gmelin, 1791].
Status. Native to Black Sea Basin, invasive in Caspian Sea, introduced in Aral Sea but extinct there.
Type locality. Sicily, Italy.
Distribution. Native to the Mediterranean and Black Sea. Introduced in the Caspian Sea between 1917 and 1919 (Grigorovich et al. 2003).
Taxonomic notes. This species has commonly been mentioned as Mytilasterlineatus (Gmelin, 1791), but the Caspian-Aral species lacks the ribbing typical for that species. The attribution to M.minimus is based on shell morphology but confirmation from molecular analyses is required.
Remarks.Mytilasterminimus has successfully replaced Dreissenacaspia and D.elata between 1938 and 1957 (Kostianoy and Kosarev 2005) in the Caspian Sea. Logvinenko and Starobogatov (1969) reported this species from the southern areas of the northern Caspian Sea, in the middle and the southern Caspian Sea down to 35–50 m water depth. Rarely, small individuals were found at depths down to 100 m. The species does not tolerate salinities below 7–8‰. This species was mentioned from depths between 200 and 600 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as M.lineatus). These deep records are unusual given other records and will require confirmation.
Conservation status. Not assessed.
Family Cardiidae Lamarck, 1809
Remarks. For the genus Cerastoderma, the species status of Pontocaspian material is subject of debate where morphological and increasingly molecular arguments show the possibility of sibling species occurrences (Sromek et al. 2016). The genus Didacna is relatively well established, however much uncertainty exists over distinction between the genera Monodacna, Adacna, and Hypanis. The generic concepts have shifted through time. Only lately, Kijashko in Bogutskaya et al. (2013) treated Monodacna as a subgenus of Adacna. Büyükmeriç and Wesselingh (2018) discussed the distinction between the three genera and considered Monodacna, Adacna, and Hypanis as valid.
Adacnalaeviuscula (Eichwald, 1829)
*1829 G. [lycymeris] laeviuscula Eichwald: 279, pl. 5, fig. 1a, b.
1838 AdacnaLaeviuscula m. – Eichwald: 170–171.
1841 Adacnalaeviuscula. – Eichwald: 281–282, pl. 39, fig. 1a–d.
1905 Adacnalaeviuscula (Eichwald, 1829). – Ostroumov: pl. 2, fig. E.
1907 Adacnalaeviuscula. – Ostroumov: 25, text fig., pl. 4, figs 6–8.
1952 Adacna (Adacna) laeviuscula (Eichwald, 1829). – Zhadin: 353–354, pl. 9, fig. 331.
1958 Adacna (Adacna) laeviuscula (Eichwald), 1829. – Nevesskaja: 49–50, pl. 9, figs 15–18.
1969 Hypanislaeviusculalaeviuscula (Eichw.). – Logvinenko and Starobogatov: 337, fig. 353(5).
1973 Hypanislaeviusculalaeviuscula Eichwald, 1829. – Grossu: 144–145, text fig. 29.
2013 Adacnalaeviuscula (Eichwald, 1829). – Kijashko in Bogutskaya et al.: 377, fig. 154, photo 48.
2016 Adacna (Adacna) laeviuscula (Eichwald, 1829). – Vinarski and Kantor: 64.
Status. Pontocaspian species, endemic to Caspian Sea and possibly Black Sea Basin.
Type locality. Azerbaijan, Caspian Sea, Gulf of Baku is the type locality given by Vinarski and Kantor (2016) and this is written on the label of the type material. However, the type description reads “Hab. australem ripam maris caspii, in sinu Astrabadensi” [southern border of the Caspian Sea, in bight of Astrabad (= Gorgan, Iran)]. Further research on the labels and documentation is required to assess whether a new lectotype or even neotype must be assigned for Adacnalaeviuscula.
Distribution. Caspian Sea; limans, coastal lakes, and Danube Delta in Black Sea Basin (in case A.fragilis will be shown to be a synonym of A.laeviuscula).
Taxonomic notes. See discussion under A.fragilis.
Remarks.Kijashko in Bogutskaya et al. (2013) list the presence of this species at 30–60 m water depth in the Caspian Sea from muddy, sandy-mud, and rarely sandy bottoms. Logvinenko and Starobogatov (1969) reported the species from the northern, middle, and southern Caspian Sea basins down to 80–85 m water depth. In the Caspian Sea, the species has not been found in areas with salinities below 4‰. However, the common occurrence of fresh (paired) specimens on beaches seen at Turali (Dagestan, Russia) and northern Azerbaijan indicates this species maintains viable populations in foreshore and possibly even shoreface habitats.
Conservation status. Not assessed.
Adacnafragilis Milaschewitsch, 1908
*1908 Adacnafragilis Milaschewitch: 992–993.
1973 Hypanislaeviusculafragilis Milaschevitsch, 1916. – Grossu: 145.
?2006b Hypanis (Adacna) laeviusculafragilis (Milachevitch, 1908). – Munasypova-Motyash: 522.
2009 Adacna (Adacna) fragilis Milaschevich, 1908. – Popa et al.: 13, fig. 5.
2016 Adacna (Adacna) fragilis Milaschewitsch, 1908. – Vinarski and Kantor: 64.
Status. Pontocaspian species, Black Sea Basin, status uncertain.
Type locality. Odessa region, Dniester liman and Katlabhuk Lake (Ukraine: Vinarski and Kantor 2016).
Distribution. Danube Delta region and NW Black Sea Basin coastal areas of Ukraine.
Taxonomic notes. We are uncertain about the status of Adacnafragilis Milaschewitch, 1908. The Black Sea Basin material has a wide variety of shapes and often is thinner and sometimes more elliptical than the Caspian A.laeviuscula. Both forms were synonymised by Graf and Cummings (2018) and indicated as a possible synonym in MolluscaBase (2018b). However, the Black Sea Basin occurrences are recorded from (coastal) lakes and small rivers suggesting little or only partial overlap in the ecological (and especially salinity) preferences of A.laeviuscula (e.g., Munasypova-Motyash 2006a, b, Popa et al. 2009). We are uncertain if A.fragilis might constitute a geographical subspecies (a status advocated by Grossu 1973), and further molecular analyses are needed to clarify the status of the Black Sea taxon.
Remarks. The species has been reported alive by Popa et al. (2009) from the Razim Lake complex on the Romanian Black Sea coast.
Conservation status. Not assessed.
Adacnaminima Ostroumov, 1907
*1907 Adacnaminima Ostroumov: 23, text fig., pl. 4, figs 1–5.
1952 Adacna (Adacna) vitreavar.minima (Ostroumoff, 1907). – Zhadin: 353.
1967 Hypanisminimaostroumovi Logvinenko and Starobogatov: 233.
1969 Hypanisminimaostroumovi Logv. et Star. – Logvinenko and Starobogatov: 338, fig. 354(3).
1973 Hypanisminimaostroumovi Logvinenko et Starobogatov, 1968. – Grossu: 146, text fig. 31.
?1974 Hypanisminimasidorovi Starobogatov: 246, fig. 213.
2003 Hypanisminimaminima (Ostroumov, 1907). – Andreeva and Andreev: 88, fig. 5.1(3, 4).
?2009 Hypania [sic] minima (Ostroumoff, 1907). – Filippov and Riedel: 75, fig. 4s, t.
2013 Adacnaminimaostroumovi (Logvinenko et Starobogatov, 1967). – Kijashko in Bogutskaya et al.: 378, fig. 146.
2016 Adacna (Adacna) minimaminima (Ostroumov, 1907). – Vinarski and Kantor: 64.
2016 Adacna (Adacna) minimaostroumovi Logvinenko et Starobogatov, 1967. – Vinarski and Kantor: 64.
Status. Pontocaspian species, endemic to Caspian Sea and Aral Sea; likely disappeared from the latter.
Type locality. The northern Caspian Sea and the Aral Sea (Vinarski and Kantor 2016).
Distribution. Aral Sea (probably extinct there; Andreeva and Andreev 2003), Caspian Sea.
Taxonomic notes.Graf and Cummings (2018) consider this species as a synonym of A.vitrea, but Kijashko in Bogutskaya et al. (2013) regards it as a valid species. The latter considers A.minimaminima from the Aral Sea and A.minimaostroumovi syn. n. from the Caspian Sea as distinct geographical subspecies. The likely disappearance of the species from the Aral Sea makes a molecular assessment of their distinctness very difficult and given the lack of other arguments we synonymise both. Furthermore, we are uncertain about the status of the subspecies Hypanisminimasidorovi Starobogatov, 1974 from the western Aral Sea. Without further data we assume it concerns a form that falls within the wide morphological variation of A.minima. We moreover are very uncertain as to the status of Hypanisminima from Holocene deposits of Aral Sea as illustrated by Filippov and Riedel (2009, fig. 4s, t). The juvenile specimen has relatively strong cardinal teeth, onset of clear ribs, and a general shape that more resembles Monodacnacaspia.
Remarks. The species has been recorded mostly from the middle and southern Caspian Sea and more rarely from the eastern areas in the northern Caspian Sea down to 35 m water depth (Logvinenko and Starobogatov 1969) as well as from the Aral Sea from where it may have disappeared.
Conservation status. Not assessed.
Adacnavitrea (Eichwald, 1829)
*1829 G. [lycymeris] vitrea Eichwald: 279, pl. 5, fig. 3.
1838 Adacnavitrea m. – Eichwald: 172–173.
1841 Adacnavitrea. – Eichwald: 282–283, pl. 39, fig. 2a, b.
1905 Adacnaglabra Ostroumov: 18–19.
1932a Adacnavitrea (Eichwald, 1829). – Bogachev: pl. 1, figs 3, 4, 11.
1932b Adacnavitrea (Eichwald, 1829). – Bogachev: 33, pl. 3, figs 13–16, 28–29.
1952 Adacna (Adacna) vitrea (Eichwald, 1829). – Zhadin: 352–353, fig. 330.
1958 Adacna (Adacna) vitrea (Eichwald), 1838. – Nevesskaja: 47–48, pl. 9, figs 19–22.
1969 Hypanisvitreavitrea (Eichw.). – Logvinenko and Starobogatov: 337, fig. 354(1), pl. 5, fig. 11.
1969 Hypanisvitreaglabra (Ostr.). – Logvinenko and Starobogatov: 338, fig. 354(2).
1973 Hypanisvitreavitrea Eichwald, 1829. – Grossu: 145–146, text fig. 30A.
1973 Hypanisvitreaglabra Ostroumoff, 1905. – Grossu: 146, text fig. 30B.
2003 Hypanisvitreabergi Starobogatov, 1974. – Andreeva and Andreev: 86, fig. 5.1(1, 2).
2013 Adacna (Adacna) vitreavitrea (Eichwald, 1829). – Kijashko in Bogutskaya et al.: 378, fig. 148.
2013 Adacna (Adacna) vitreaglabra Ostroumoff, 1905. – Kijashko in Bogutskaya et al.: 379, fig. 149.
2016 Adacna (Adacna) vitreavitrea (Eichwald, 1829). – Vinarski and Kantor: 65.
2016 Adacna (Adacna) vitreaglabra Ostroumov, 1905. – Vinarski and Kantor: 65.
2016 Adacna (Adacna) vitreabergi (Starobogatov, 1974). – Vinarski and Kantor: 65.
Status. Pontocaspian species, endemic to Caspian Sea Basin, Black Sea Basin, and Aral Sea Basin.
Type locality. “Australem oram caspii maris, Astrabadensem” [southern coast of Caspian Sea, near Astrabad (= Gorgan, Iran)].
Distribution. Black Sea Basin (also in Azov Sea and adjacent lower Don River), Caspian Sea Basin, and Aral Sea (including delta of Amu-Darya River). The Aral populations may have gone extinct in the 1980s (Andreeva and Andreev 2003).
Taxonomic notes. The species has been subdivided into three geographical subspecies which were not recognised by Graf and Cummings (2018). It concerns a species with thin shells that yield very few diagnostic characters that show overlap. Here, we synonymise the subspecies pending molecular assessments of their status.
Conservation status. Not assessed.
Cerastodermaglaucum (Bruguière, 1789) s.l.
*1789 Cardiumglaucum Bruguière: 221–222.
1789 CardiumGlaucum Poiret: 13–15.
1869 Cardiumisthmicus Issel: 74–76.
1952 Cardiumedule L., 1758. – Zhadin: 344–345, fig. 318 [nonCardiumedule Linnaeus, 1758].
2003 Cerastodermaisthmicum (Issel, 1869). – Andreeva & Andreev: 54, 62, figs 6.1(b), 6.7.
2013 Cerastodermaglaucum (Poiret, 1789). – Kijashko in Bogutskaya et al.: 342, fig. 126, photo 39.
2016 Cerastodermaglaucum (Bruguière, 1789). – Vinarski and Kantor: 69.
2016 Cerastodermaisthmicus (Issel, 1869). – Vinarski and Kantor: 70.
Status. Native Pontocaspian species (Black Sea Basin), Holocene invasive in Caspian Sea and Aral Sea.
Type locality. French Mediterranean.
Distribution. NE Atlantic, Baltic Sea, Mediterranean, Black Sea Basin, Caspian Sea Basin, Aral Sea, isolated Saharan lakes (Plaziat 1991).
Taxonomic notes. DNA studies have shown a strong structuring between Atlantic–western Mediterranean, Ionian, and Aegean-Pontocaspian populations of C.glaucum (Nikula and Väinölä 2003, Sromek et al. 2016). According to Sromek et al. (2016: 515), the “strong genetic differentiation and the occurrence of private alleles may hint at the presence of cryptic species within C.glaucum”. For a discussion on the authority of C.glaucum, see Vinarski and Kantor (2016: 69–70).
Remarks. The arrival of Cerastodermaglaucum in the Caspian Sea circa 8000 years ago has been linked to human settlement expansion through the Manych corridor (Fedorov 1957, Yanina 2009). It would be among the earliest human-mediated dispersal events for invertebrate species known to date.
Conservation status. Not assessed.
Cerastoderma sp. A [non C.rhomboides (Lamarck, 1819)]
1916 Cardiumedulevar.nuciformis Milaschewitch: 257–259, pl. 7, figs 7, 8 [nonCardiumnuciforme d’Orbigny, 1850].
2003 Cerastodermarhomboidesrhomboides (Lamarck, 1819). – Andreeva and Andreev: 93, fig. 6.1(A) [nonCardiumrhomboides Lamarck, 1819].
2013 Cerastodermarhomboides (Lamarck, 1819). – Kijashko in Bogutskaya et al.: 343, fig. 127, photo 40 [non Lamarck, 1819].
2016 Cerastodermarhomboides (Lamarck, 1819). – Vinarski and Kantor: 70 [non Lamarck, 1819].
Status. Native Pontocaspian species (Black Sea Basin), introduced to Caspian Sea and Aral Sea.
Distribution. Black Sea (including Sea of Azov), Caspian Sea, Aral Sea, Aegean.
Taxonomic notes. This concerns a common rhomboid-shaped species in the Pontocaspian region whose name is uncertain. It has a short ligament in common with C.glaucum and the persistent occurrence of ribs on the posterior margin, the well-defined character of the ribs and the regular occurrence of scales in common with western European C.edule. This form has been often referred to as C.rhomboides (Lamarck, 1819) that has been described from the Italian Pliocene but that concerns a typical glaucum form (Fig. 2), not the rhomboid form of the Pontocaspian Cerastoderma. The species has been named Cardiumedulevar.nuciformis by Milaschewitch (1916), but that name is a junior primary homonym of Cardiumnuciforme d’Orbigny, 1850. Even though some morphological features mentioned in the description of C.lamarcki (Reeve, 1845) may resemble those of the Pontocaspian species, the former has been traced to southern Great Britain from where molecular analyses only show the presence of C.glaucum (Nikula and Väinölä 2003).
Figure 2.
Syntype of Cerastodermarhomboides (Lamarck, 1819), stored in the Muséum national d’Histoire naturelle Paris (MNHN.F.A50142), Pliocene, Tuscany, Italy. Photograph by E Porez. https://science.mnhn.fr/institution/mnhn/collection/f/item/a50142?lang=fr_FR
Conservation status. Not assessed.
Didacnabaeri (Grimm, 1877)
Fig. 3a
Figure 3.
Didacnabaeri versus D.eichwaldi from Holocene (Novocaspian) deposits of Turali Lagoon (Dagestan, Russia). a RGM.961899, Didacnabaeri (Grimm, 1877) b RGM.961900, Didacnaeichwaldi (Krynicki, 1837), same locality. Scale bar: 1 cm.
*1877 CardiumBaeri Grimm: 51–54, pl. 8, figs 2, 3.
1914 DidacnaBaeri (Grimm, 1877). – Nalivkin & Anisimov: 4, pl. 1, figs 4, 5.
1932 DidacnaBaeri (Grimm, 1877). – Bogachev: 29, pl. 3, figs 1–7.
1933 DidacnaBaeri (Grimm, 1877). – Zhizhchenko: 34, pl. 2, figs 5–8.
1952 Didacnabaeri (Grimm, 1877). – Zhadin: 347–348, figs 321, 322.
1953 Didacnabaeri (Grimm, 1877). – Fedorov: 129, pl. 20, figs 10, 11.
1968 Didacnabaeri (Grimm, 1877). – Gadzhiev: 76–77, pl. 1, figs 1, 2.
1969 Didacnabaeri (Grimm). – Logvinenko & Starobogatov: 324, fig. 344(2).
1969 Didacnabaeri (Grimm, 1877). – Vekilov: 139–144, pl. 25, figs 1–8.
1973 Didacnabaeri Grimm, 1877. – Grossu: 131, text fig. 7.
1983 Didacnabaeri (Grimm, 1877). – Popov: 180, pl. 16, figs 20–23.
1988 Didacnabaeri (Grimm, 1877). – Yanina & Svitoch: 129, pl. 3, figs 7–13.
2005 Didacnabaeri (Grimm, 1877). – Yanina: 242–244, pl. 14, figs 12–15.
2007 Didacnabaeri (Grimm, 1877). – Nevesskaja: 940–941, pl. 23, figs 11–17.
2013 Didacnabaeri (Grimm, 1877). – Kijashko in Bogutskaya et al.: 352, fig. 136, photo 41 [pars, excluding synonymy of Didacnacrassa].
2016 Didacnabaeri (Grimm, 1877). – Vinarski & Kantor: 71 [pars, excluding synonymy of Didacnacrassa].
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. Caspian Sea, offshore Turkmenistan, station 132, 40°32'N, 52°23'E.
Distribution.Logvinenko and Starobogatov (1969) reported Didacnabaeri from the southern basin (mostly on the eastern side) and from the middle basin down to 60 m water depth.
Taxonomic notes. In recent works (e.g., Kijashko in Bogutskaya et al. 2013), the species Didacnacrassa (Eichwald, 1829) [= D.eichwaldi (Krynicki, 1837)] has been considered a synonym of D.baeri. However, both species can be distinguished. Didacnabaeri has a less extended, more roundish shell, a less developed keel, and a low top with less projecting beak and in general more ribs than D.eichwaldi (Fig. 3). Didacnabaeri occurred for the first time in the Novocaspian transgressive deposits whereas D.crassa already occurred in the late Khvalynian (Late Pleistocene). Both became very common during the Novocaspian.
Conservation status. Not assessed.
Didacnabarbotdemarnii (Grimm, 1877)
*1877 Cardium Barbot-de-Marnii Grimm: 56–58, pl. 8, figs 5, 6.
1952 Didacnabarbot-de-marnyi [sic] (Grimm, 1877). – Zhadin: 348, fig. 323.
1969 Didacnabarbotdemarnyi [sic] (Grimm). – Logvinenko and Starobogatov: 326–327, fig. 346, pl. 5, fig. 8.
1973 Didacnabarbotdemarnyi [sic] Grimm, 1877. – Grossu: 133, text fig. 10.
2007 Didacnabarbotdemarnyi [sic] (Grimm, 1877). – Nevesskaja: 941–943, pl. 24, figs 10–14.
2013 Didacnabarbotdemarnii (Grimm, 1877). – Kijashko in Bogutskaya et al.: 353, fig. 139, photo 42.
2016 Didacnabarbotdemarnii (Grimm, 1877). – Vinarski and Kantor: 71.
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. Caspian Sea, station 116, 44°17'N, 50°22'E.
Distribution. Southern, middle, and southern part of the northern Caspian Sea down to 40 m water depth, preferentially on sandy sediments (Logvinenko and Starobogatov 1969).
Conservation status. Not assessed.
Didacnaeichwaldi (Krynicki, 1837)
Fig. 3b
°1829 C. [ardium] crassum Eichwald: 283 [nonCardiumcrassum Gmelin, 1791].
*1837 CardiumEichwaldi Krynicki: 61 [nom. nov. pro C.crassum Eichwald, 1829, non Gmelin, 1791].
1841 Didacnacrassa. – Eichwald: 273, pl. 39, fig. 6a, b.
1876 Cardiumcrassum Eichwald, 1829. – Grimm: 136–138, pl. 6, fig. 3.
1905 Didacnacrassa (Eichwald, 1829). – Ostroumov: 15, 69, pl. 2(A).
1932 Didacnaaff.crassa (Eichwald, 1829). – Bogachev: 27, pl. 2, figs 11–14.
1952 Didacnacrassa Eichwald, 1841. – Zhadin: 349, fig. 325.
1953 Didacnacrassa (Eichwald, 1829). – Fedorov: 130, pl. 20, figs 8, 9, 12, 13.
1958 Didacnacrassacrassa Eichwald, 1829. – Nevesskaja: 39–40, pl. 7, figs 8, 9.
1969 Didacnacrassa (Eichwald, 1829). – Vekilov: 134–139, pl. 24, figs 1–6, pl. 27, figs 1, 2.
1988 Didacnacrassacrassa (Eichwald, 1829). – Yanina and Svitoch: pl. 12, figs 8, 9, pl. 13, figs 1–5.
2005 Didacnacrassa (Eichwald, 1829). – Yanina: 242, pl. 14, figs 3–6.
2007 Didacnacrassa (Eichwald, 1829). – Nevesskaja: 939–940, pl. 23, figs 1–5.
2013 Didacnabaeri (Grimm, 1877). – Kijashko in Bogutskaya et al.: 352 [pars, non fig. 136, photo 41, nonCardiumbaeri Grimm, 1877].
2016 Didacnabaeri (Grimm, 1877). – Vinarski and Kantor: 71 [pars, non Grimm, 1877].
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. “Caspium mare” (Caspian Sea) (for C.crassum Eichwald, 1829).
Distribution. Caspian Sea. Didacnaeichwaldi is known from the middle and southern Caspian Sea basins down to 35 m water depth and cannot tolerate lowered salinities.
Taxonomic notes. In recent works (Kijashko in Bogutskaya et al. 2013), the species Didacnacrassa (Eichwald, 1829) [= D.eichwaldi (Krynicki, 1837)] has been considered a synonym of D.baeri. However, we see morphological discontinuities in our extensive material from the northern Caspian Sea Basin that implies that D.eichwaldi with its protruding umbo and shouldered appearance is distinct from D.baeri that is characterised by a rounded umbo (see discussion above under D.baeri). Despite being in common usage, the name Didacnacrassa is invalid as it is a junior homonym of Cardiumcrassum Gmelin, 1791; Krynicki (1837) introduced Cardiumeichwaldi as replacement name.
Conservation status. Not assessed.
Didacnalongipes (Grimm, 1877)
*1877 Cardiumlongipes Grimm: 54–56, pl. 8, fig. 4a–c.
1952 Didacnalongipes (Grimm, 1877). – Zhadin: 349–350, fig. 326.
1969 Didacnalongipes (Grimm). – Logvinenko and Starobogatov: 326, fig. 345.
1973 Didacnalongipes Grimm, 1877. – Grossu: 132, text fig. 9, pl. 1, fig. 2.
?2007 Didacnacarinata Nevesskaja: 943, pl. 24, figs 15–19.
2013 Didacnalongipes (Grimm, 1877). – Kijashko in Bogutskaya et al.: 354, fig. 137, photo 43.
2016 Didacnalongipes (Grimm, 1877). – Vinarski and Kantor: 71.
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. Caspian Sea, offshore Azerbaijan, approximately 40°39'N, 50°26'E.
Distribution. Southern and middle Caspian Sea basins and southern part of the northern Caspian Sea down to 30–40 m water depth. The species often co-occurs with D.barbotdemarnii.
Remarks. We are uncertain about the status of Didacnacarinata Nevesskaja, 2007. The overall outline resembles that of D.barbotdemarnii, but the former species appears smaller and thinner. Kijashko in Bogutskaya et al. (2013) considered D.carinata as a synonym of D.longipes.
Conservation status. Not assessed.
Didacnaparallela Bogachev, 1932
*1932a Didacnaparallela Bogachev: pl. 2, figs 2, 3.
1932b Didacnaparallela Bogachev: 44, pl. 5, figs 1–7, 9.
1953 Didacnaparallella [sic] Bogatchev, 1932. – Fedorov: 126, pl. 17, figs 1–11.
1969 Didacnaparallella [sic] Bog. – Logvinenko and Starobogatov: 324–325, fig. 344(3).
1969 Didacnaparallella [sic] Bogatchev, 1932. – Vekilov: 117–120, pl. 21, figs 1–8.
1973 Didacnaparallella [sic] Bogatchev, 1922 [sic]. – Grossu: 131, text fig. 8, pl. 1, fig. 4.
2005 Didacnaparallella [sic] Bogatchev, 1932. – Yanina: 237–238, pl. 12, figs 1–8.
2007 Didacnaparallella [sic] Bogatchev, 1932. – Nevesskaja: 933–935, pl. 21, figs 1–5.
2013 Didacnaparallela Bogachev, 1932. – Kijashko in Bogutskaya et al.: 355–356, fig. 138.
2016 Didacnaparallela Bogachev, 1932. – Vinarski and Kantor: 72.
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. Khala, Apsheron Peninsula, Azerbaijan (early Khvalynian, Late Pleistocene).
Distribution. Caspian Sea, southern basin and western part of middle basin between 50–85 m water depth (Logvinenko and Starobogatov 1969). This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017), but we are not certain whether it concerns living specimens.
Remarks.Didacnaparallela has been considered as extinct by Nevesskaja (2007) but was nevertheless treated in Kijashko in Bogutskaya et al. (2013). Live records are known at least until 1986 and we have no particular reason to assume it is extinct.
Conservation status. Not assessed.
Didacnapraetrigonoides Nalivkin & Anisimov, 1914
*1914 Didacnapraetrigonoides Nalivkin & Anisimov: 5–6, 16–17, pl. 1, figs 1, 2.
1932a Didacnapraetrigonoides Nalivkin & Anisimov, 1914. – Bogachev: pl. 2, fig. 1.
1932b Didacnapraetrigonoides Nalivkin & Anisimov, 1914. – Bogachev: 42, pl. 4, figs 1–8, pl. 5, fig. 8.
1948 Didacnapraetrigonoides Nal. – Fedorov: pl. 2, figs 10–13.
1953 Didacnapraetrigonoides Nalivkin & Anisimov, 1914. – Fedorov: 128, pl. 18, figs 1–6, pl. 19, figs 1–6.
1958 Didacnapraetrigonoides Nalivkin & Anisimov, 1914. – Nevesskaja: 17–20, pl. 1, figs 1–14.
1969 Didacnatrigonoidespraetrigonoides Nal. & Anis. – Logvinenko and Starobogatov: 324, fig. 343(2).
1969 Didacnapraetrigonoides Nalivkin & Anisimov, 1914. – Vekilov: 120–128, pl. 22, figs 1–9.
1973 Didacnatrigonoidespraetrigonoides Nalivkin & Anisimov, 1915. – Grossu: 129, text fig. 5.
1983 Didacnapraetrigonoidespraetrigonoides Nalivkin & Anisimov, 1914. – Popov: 195, pl. 15, figs 1, 2.
1988 Didacnapraetrigonoides Nalivkin & Anisimov, 1914. – Yanina and Svitoch: pl. 8, figs 4–7.
2005 Didacnapraetrigonoides Nalivkin & Anisimov, 1914. – Yanina: 241, pl. 14, figs 1, 2.
2007 Didacnapraetrigonoidespraetrigonoides Nalivkin & Anisimov, 1914. – Nevesskaja: 927, pl. 19, figs 9, 10.
Status. Pontocaspian species, endemic to Caspian Sea. Possibly extinct.
Type locality. Apsheron Peninsula, Azerbaijan, Quaternary.
Distribution. Caspian Sea. Logvinenko and Starobogatov (1969) reported the species from the southern Caspian Sea Basin and the southern part of the middle Caspian Sea Basin down to 60 m water depth. The species has been collected from Holocene deposits and beach occurrences the western part of the middle Caspian Sea Basin as well (FW, pers. obs.). The species is reportedly extinct, not mentioned in Kijashko in Bogutskaya et al. (2013).
Remarks. The first appearance of Didacnapraetrigonoides is in lower Khvalynian deposits, it became widespread during the late Khvalynian and was rare during the Novocaspian.
Conservation status. Not assessed. Didacnapraetrigonoides has been reported to occur ‘rarely in the modern Caspian Sea’ (Nevesskaja 2007: 927), but material from recent assemblages has not been found.
Didacnaprofundicola Logvinenko & Starobogatov, 1966
*1966a Didacnaprofundicola Logvinenko & Starobogatov: 13–14, fig. 1.
1969 Didacnaprofundicola Logv. & Star. – Logvinenko and Starobogatov: 328–329, fig. 349.
1973 Didacnaprofundicola Logvinenko & Starobogatov, 1966. – Grossu: 134, text fig. 13.
2007 Didacnaprofundicola Logvinenko & Starobogatov, 1966. – Nevesskaja: 944, pl. 20, fig. 28a–c.
2013 Didacnaprofundicola Logvinenko & Starobogatov. – Kijashko in Bogutskaya et al.: 356, fig. 140, photo 45.
2016 Didacnaprofundicola Logvinenko & Starobogatov. – Vinarski and Kantor: 72.
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. Central part of the Caspian Sea, 39°38'N, 52°02'E(offshore Turkmenistan).
Distribution. Middle and southern basins of Caspian Sea between 75 and 409 m water depth (Logvinenko and Starobogatov 1969). This species was mentioned from depths between 200 and 600 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Conservation status. Not assessed.
Didacnaprotracta (Eichwald, 1841)
*1841 Adacnaprotracta Eichwald: 280, pl. 40, figs 10, 11 [non figs 9, 10 as indicated in the text].
1877 Cardiumcatillus Eichw. – Grimm: 58, pl. 8, figs 7, 8 [nonMonodacnacatillus Eichwald, 1841].
1910 Didacnaprotracta (Eichwald, 1841). – Andrusov: 67, pl. 8, figs 22, 33, pl. 9, figs 1–9.
1952 Didacnaprotracta (Eichwald, 1841). – Zhadin: 348–349, fig. 324.
1953 Didacnaprotracta (Eichwald, 1829). – Fedorov: 127, pl. 14, figs 12–15, pl. 15, figs 1–16.
1967 Didacnaprotracta Eichwald, 1841. – Svitoch: 42–43, pl. 6, figs 6–9, pl. 7, figs 1, 2.
1969 Didacnaprotractaprotracta (Eichw.). – Logvinenko and Starobogatov: 327, fig. 347.
1973 Didacnaprotractaprotracta Eichwald, 1841. – Grossu: 133, text fig. 11.
1973 Didacnaprotractasubmedia Andrusov, 1911. – Grossu: 133–134, text fig. 12.
1999 Didacnaprotracta (Eichwald, 1829). – Fedorov: pl. 12, figs 4–7.
2005 Didacnaprotracta (Eichwald, 1829). – Yanina: 238–239, pl. 12, figs 9–19.
2007 Didacnaprotractaprotracta (Eichwald, 1829). – Nevesskaja: 938–939, pl. 22, figs 4–13.
2013 Didacnaprotracta (Eichwald, 1829). – Kijashko in Bogutskaya et al.: 356, fig. 141.
2013 Didacnaprotractasubmedia Andrusov, 1910. – Kijashko in Bogutskaya et al.: 356, fig. 142.
2016 Didacnaprotracta (Eichwald, 1841). – Vinarski and Kantor: 72.
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. The type series (?Recent, Caspian Sea) was reported as lost by Nevesskaja (2007) who introduced a neotype from the Elton Lake surroundings in the northern Caspian plains, Russia (early Khvalynian, Late Pleistocene).
Distribution. Middle and southern Caspian Sea basins; it is most common in the middle basin at 25–85 m water depth (Logvinenko and Starobogatov 1969).
Taxonomic notes. According to Logvinenko and Starobogatov (1969), two subspecies occur in the Caspian Sea at different depth ranges: D.protractaprotracta at 25–50 m and D.protractasubmedia Andrusov, 1910 at 50–85 m. The latter differs from D.p.protracta by the relative posterior location of the umbo that is furthermore subdued. Both forms of Didacnaprotracta are widespread in the Khvalynian deposits of the Caspian Sea and Manych depression. According to Kijashko in Bogutskaya et al. (2013) morphological differences characteristic for the subspecies of Didacnaprotracta are due to allometric growth. The mere difference in depth distribution, with overlapping depths and intermediate forms, does not provide any argument to maintain these subspecies. Didacnaprotracta is the type species of the subgenus Protodidacna Logvinenko & Starobogatov, 1966.
Remarks. The authorship attribution of this species to Eichwald (1829) as proposed by several authors was rejected in Vinarski and Kantor (2016). According to them, Cardiumprotractum Eichwald, 1829, described from the western Ukraine, probably refers to a different species.
Conservation status. Not assessed.
Didacnapyramidata (Grimm, 1877)
*1877 Cardiumpyramidatum Grimm: 46–49, pl. 8, fig. 1a–d.
1932 Didacnapyramidata (Grimm, 1877). – Bogachev: 28–29, pl. 2, figs 15, 16.
1952 Didacnapyramidata (Grimm, 1877). – Zhadin: 347, fig. 320.
1969 Didacnapyramidata (Grimm). – Logvinenko and Starobogatov: 324, fig. 344(1).
1969 Didacnapyramidata (Grimm, 1877). – Vekilov: 144–147, pl. 26, figs 1–5.
1973 Didacnapyramidata Grimm, 1877. – Grossu: 130, text fig. 6, pl. 1, fig. 1.
2007 Didacnapyramidata (Grimm, 1877). – Nevesskaja: 940, pl. 23, figs 6–10.
2013 Didacnapyramidata (Grimm, 1877). – Kijashko in Bogutskaya et al.: 357, fig. 135, photo 47.
2016 Didacnapyramidata (Grimm, 1877). – Vinarski and Kantor: 73.
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. Caspian Sea, offshore Azerbaijan, 39°47'N, 49°59'30"E (Kijashko in Bogutskaya et al. 2013).
Distribution. Caspian Sea: southern basin and southern part of the middle basin at depths between 30–100 m (Logvinenko and Starobogatov 1969).
Conservation status. Not assessed.
Didacnatrigonoides (Pallas, 1771)
*1771 Cardiumtrigonoides Pallas: 478.
1831 Cardiumtrigonoides (Pallas, 1771). – Eichwald: 282.
1838 Didacnatrigonoides n. – Eichwald: 166–167.
1841 Didacnatrigonoides. – Eichwald: 271–272, pl. 39, fig. 5a–c.
1876 Cardiumtrigonoides, Pall. – Grimm: 138–140, pl. 6, fig. 2.
1914 Didacnatrigonoides (Pallas, 1771). – Kalitskiy: pl. 3, figs 1, 2.
1914 Didacnatrigonoides (Pallas, 1771). – Nalivkin and Anisimov: 6, pl. 1, fig. 3.
1932a Didacnatrigonoides (Pallas, 1771). – Bogachev: pl. 1, figs 5, 6.
1932b Didacnatrigonoides (Pallas, 1771). – Bogachev: 25, pl. 2, figs 1–9.
1933 Didacnatrigonoides (Pallas, 1771). – Zhizhchenko: 35–36, pl. 2, figs 9, 10.
1950 Didacnatrigonoides (Pallas, 1771). – Pravoslavlev: 21–22, figs 1–4.
1952 Didacnatrigonoides (Pallas, 1771). – Zhadin: 346, fig. 319.
1953 Didacnatrigonoides (Pallas, 1771). – Fedorov: 129, pl. 20, figs 7–9.
1969 Didacnatrigonoidestrigonoides (Pall.). – Logvinenko and Starobogatov: 323, fig. 343(1), pl. 5, fig. 7.
1969 Didacnatrigonoides (Pallas, 1771). – Vekilov: 128–134, pl. 23, figs 1–9, pl. 27, fig. 6.
1973 Didacnatrigonoidestrigonoides Pallas, 1771. – Grossu: 129, text fig. 4, pl. 1, fig. 3.
1977 Didacnatrigonoidestuzetae Tadjalli-Pour: 97, pl. 1, fig. 3.
1983 Didacnatrigonoides (Pallas, 1771). – Popov: 204, pl. 16, fig. 19.
1986 Didacnatrigonoides (Pallas, 1771). – Yakhimovich et al.: 79, pl. 10, fig. 1.
1988 Didacnatrigonoides (Pallas, 1771). – Yanina and Svitoch: pl. 9, figs 7–12.
2005 Didacnatrigonoides (Pallas, 1771). – Yanina: 244–245, pl. 14, figs 7–11.
2007 Didacnatrigonoides (Pallas, 1771). – Nevesskaja: 941, pl. 24, figs 1–9.
2013 Didacnatrigonoides (Pallas, 1771). – Kijashko in Bogutskaya et al.: 358, fig. 134.
2016 Didacnatrigonoides (Pallas, 1771). – Vinarski and Kantor: 70.
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. Caspian Sea, a neotype has been designated based on a specimen from Chechen Island by Nevesskaja (2007, pl. 24, fig. 4).
Distribution. Caspian Sea, mostly eastern part of northern Caspian Sea Basin (Logvinenko and Starobogatov 1969). Furthermore found in living position in Novocaspian deposits near Turali, Dagestan (western part middle basin; FW).
Remark. Genetic data are available through Albrecht et al. (2014).
Conservation status. Not assessed.
Hypanisplicata (Eichwald, 1829)
*1829 G. [lycymeris] plicata Eichwald: 279, pl. 5, fig. 2a, b.
1838 Adacne [sic] plicata m. – Eichwald: 171–172.
1916 Adacnarelicta Milaschewitch: 274–276, pl. 8, figs 10–13 [non figs 10–12 as indicated in the text].
1926 Adacnarelictavar.dolosmiana Borcea: 468–469, pl. 18, figs 156–158, pl. 21, fig. 2.
1952 Adacna (Hypanis) plicata (Eichwald, 1829). – Zhadin: 354–355, fig. 332.
1958 Adacna (Hypanis) plicata (Eichwald), 1829. – Nevesskaja: 50–51, pl. 9, figs 9–14.
1969 Hypanisplicataplicata (Eichw.). – Logvinenko and Starobogatov: 331–332, fig. 350.
1973 Hypanisplicataplicata Eichwald, 1829. – Grossu: 136, text fig. 14, pl. 1, fig. 5.
1973 Hypanisplicatarelicta Milaschevitsch, 1916. – Grossu: 136, text fig. 15, pl. 1, figs. 6, 20–23.
1973 Hypanisdolosmaniana [sic] Borcea, 1826. – Grossu: 136, text fig. 16, pl. 1, figs 16–19.
1977 Hypanisplicatagolbargae Tadjalli-Pour: 99, pl. 1, fig. 5.
2006a Hypanisplicatarelicta (Milachevitch, 1916). – Munasypova-Motyash: 45–46.
2009 Adacna (Hypanis) plicatarelicta Milaschevich, 1916. – Popa et al. 12, fig. 4.
2013 Hypanisplicata (Eichwald, 1829). – Kijashko in Bogutskaya et al.: 387, fig. 164, photo 56.
2016 Hypanisplicataplicata (Eichwald, 1829). – Vinarski and Kantor: 73.
2016 Hypanisplicatarelicta (Milaschewitsch, 1916). – Vinarski and Kantor: 74.
Status. Pontocaspian species, endemic to Caspian Sea Basin and Black Sea Basin.
Type locality. “Sinum Astrabadensem” [Caspian Sea near Astrabad (= Gorgan, Iran)].
Distribution. Caspian Sea, western liman coast Black Sea Basin.
Taxonomic notes. The Black Sea populations of H.plicata show a large range of morphological variation with elongated specimens that cannot be distinguished from Caspian H.plicata to severely stunted and irregularly shaped specimens that have been considered as a subspecies (H.plicatarelicta) or as distinct species (H.dolosmiana) (e.g., Munasypova-Motyash 2006a). These forms have intermediates indicating that the Black Sea Basin specimens are a single species that should be attributed to H.plicata even though the latter appear to have lived under lower salinities than their Caspian counterparts. Molecular studies are required to elucidate the status of the Black Sea Basin material.
Conservation status. Not assessed. Fresh shells (including paired specimens) have been found at several beaches around the Caspian Sea (Turali, Dagestan, Russia; Şuraabad, Azerbaijan; FW). The species has been reported alive from the Razim lake complex of the Romanian Black Sea coast by Popa et al. (2009).
Monodacnaacuticosta (Logvinenko & Starobogatov, 1967)
*1967 Hypanisacuticosta Logvinenko & Starobogatov: 232.
1969 Hypanisangusticostataacuticosta Logvinenko & Starobogatov: 334, fig. 353(1).
1973 Hypanisangusticostataacuticosta Logvinenko et Starobogatov, 1967. – Grossu: 141, fig. 23.
2013 Adacna (Monodacna) acuticosta (Logvinenko & Starobogatov, 1967). – Kijashko in Bogutskaya et al.: 379, fig. 160, photo 50.
2016 Adacna (Monodacna) acuticosta (Logvinenko & Starobogatov, 1967). – Vinarski and Kantor: 66.
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. “Northern Caspian Sea on the central part of the slope” (Vinarski and Kantor 2016: 66), which likely refers to northern slope of the middle Caspian Sea Basin.
Distribution. Caspian Sea (middle Caspian Sea Basin).
Conservation status. Not assessed.
Monodacnaalbida (Logvinenko & Starobogatov, 1967)
*1967 Hypanisalbida Logvinenko & Starobogatov: 232.
1969 Hypanisalbida Logv. & Star. – Logvinenko and Starobogatov: 336, fig. 353(3).
1973 Hypanisalbida Logvinenko & Starobogatov, 1967. – Grossu: 144, text fig. 28.
2013 Adacna (Monodacna) albida (Logvinenko & Starobogatov, 1967). – Kijashko in Bogutskaya et al.: 380, fig. 162, photo 51.
2016 Adacna (Monodacna) albida (Logvinenko & Starobogatov, 1967). – Vinarski and Kantor: 66.
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. “Western Caspian Sea southeastwards from Derbent” (Vinarski and Kantor 2016: 66).
Distribution. Caspian Sea (middle and southern Caspian Sea Basin). This species was mentioned from depths between 200 and 400 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Hypanisalbida).
Taxonomic notes. This species is part of a group of Caspian Monodacna with relative flat and wedge-shaped shells with low and sometimes poorly defined ribs (M.albida, M.polymorpha). Like for the Monodacnacaspia group (see below), we are in need of studies to assess whether these taxa might form ecomorphs of a single species.
Conservation status. Not assessed.
Monodacnacaspia (Eichwald, 1829)
*1829 C.[orbula] caspia Eichwald: 281, pl. 5, fig. 6a, b.
1841 Monodacnacaspia. – Eichwald: 274, pl. 39, fig. 4a–c.
1905 Monodacnacaspia (Eichwald, 1829). – Ostroumov: pl. 3, fig. C.
1932a Monodacnacaspia (Eichwald, 1829). – Bogachev: pl. 1, figs 10, 13.
1932b Monodacnacaspia (Eichwald, 1829). – Bogachev: 30, pl. 3, figs 21–27.
1952 Monodacnaedentula(Pallas, 1771)var.caspia Eichwald, 1841. – Zhadin: 350, fig. 327B.
1958 Monodacnacaspia (Eichwald), 1829. – Nevesskaja: 44–46, pl. 9, figs 1–8.
1963 Monodacnacaspiacaspia (Eichwald, 1829). – Nevesskaja: 66, pl. 8, figs 1–4.
1965 Monodacnacaspiacaspia (Eichwald). – Nevesskaja: 187–198, pl. 9, figs 6–15, 17–19, 23–26, 29.
1969 Monodacnacaspia (Eichwald, 1829). – Vekilov: 147–150, pl. 31, figs 9–11.
1973 Hypaniscaspiacaspia Eichwald, 1829. – Grossu: 139, text fig. 19B.
1977 Hypaniscaspiaassalae Tadjalli-Pour: 99, pl. 1, fig. 4.
1977 Hypaniscaspianahali Tadjalli-Pour: 99, pl. 1, fig. 6.
2013 Adacna (Monodacna) caspiacaspia (Eichwald, 1829). – Kijashko in Bogutskaya et al.: 380, fig. 154.
2016 Adacna (Monodacna) caspiacaspia (Eichwald, 1829). – Vinarski and Kantor: 67.
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. “Caspium mare” [Caspian Sea].
Distribution. Caspian Sea.
Taxonomic notes. The Monodacnacaspia group (M.caspia, M.filatovae, and M.knipowitschi) comprises three (sub-) species that all share the relatively convex and rounded shell and well-defined ribbing. These species have been described from different areas and habitats in the Caspian Sea and have been morphologically characterised by Kijashko in Bogutskaya et al. (2013). However, neither morphological analyses of intermediate populations nor genetic analyses have been performed to clarify if the three taxa are distinct or ecomorphs of a single species. We are therefore uncertain whether M.filatovae and M.knipowitschi should be maintained.
Conservation status. Not assessed.
Monodacnacolorata (Eichwald, 1829)
*1829 G. [lycymeris] colorata Eichwald: 279–280, pl. 5, fig. 4a, b.
1838 Adacnacolorata m. – Eichwald: 169–170.
?1838 Monodacnapontica Eichwald: 168–169.
1926 Monodacnacoloratavar.ialpugensis Borcea: 452, pl. 15, fig. 16.
1926 Monodacnacoloratavar.angusticostata Borcea: 452–453, pl. 15, figs 27, 28, pl. 16, figs 90, 91, pl. 18, figs 143, 169, 173, pl. 21, fig. 7.
1926 Adacna Luciae Borcea: 469–471, pl. 18, figs 146, 148–149, 151–153, pl. 21, figs 8, 9.
1952 Monodacnacolorata (Eichwald, 1829). – Zhadin: 351, fig. 328.
?1972 Hypaniscaspiagrossui Scarlato and Starobogatov: 214, pl. 4, fig. 1a, b.
1973 Hypaniscaspiagrossui Scarlato & Starobogatov, 1971. – Grossu: 140, text fig. 21, pl. 1, fig. 8.
1973 Hypanisangusticostataangusticostata Borcea, 1926. – Grossu: 141, pl. 1, fig. 12.
1973 Hypanisluciae Borcea, 1926. – Grossu: 138, text fig. 18.
1973 Hypanisialpugensis Borcea, 1926. – Grossu: 142, fig. 24, pl. 1, figs 9, 10.
1973 Hypaniscolorata Eichwald, 1829. – Grossu: 142–143, fig. 25, pl. 1, figs 13–15.
1973 Hypanispontica Eichwald, 1838. – Grossu: 143, fig. 26, pl. 1, fig. 11.
2006a Hypaniscolorata (Eichwald, 1829). – Munasypova-Motyash: 42–43.
?2006a Hypanispontica (Eichwald, 1838). – Munasypova-Motyash: 43–44.
?2006a Hypanisangusticostataangusticostata (Borcea, 1926). – Munasypova-Motyash: 44.
2009 Monodacnapontica Eichwald, 1838. – Popa et al.: 10, text fig. 2.
2009 Monodacnacolorata Eichwald, 1829. – Popa et al.: 10–11, text fig. 3.
2012 Hypaniscolorata (Eichwald, 1829). – Popa et al.: 153, 154.
2012 Hypanisangusticostata (Borcea, 1926). – Popa et al.: 153, 154.
2013 Adacna (Monodacna) colorata (Eichwald, 1829). – Kijashko in Bogutskaya et al.: 383, fig. 158.
2016 Adacna (Monodacna) angusticostata (Borcea, 1926). – Vinarski and Kantor: 66.
2016 Adacna (Monodacna) grossui (Scarlato et Starobogatov, 1972). – Vinarski and Kantor: 67.
2016 Adacna (Monodacna) ialpugensis (Borcea, 1926). – Vinarski and Kantor: 68.
Status. Pontocaspian species, native to Black Sea Basin (including lower Danube River), invasive in Caspian Sea and Volga River.
Type locality. “Hypanin fluvium, ad nigrum usque mare” [Lower course of the Yuzhnyi Bug River, all the way to the Black Sea, Ukraine].
Distribution. Native to all Black Sea Basin Pontocaspian habitats and lower courses of adjacent rivers such as the Danube, Dnieper, and Dniester; invasive in Caspian Sea Basin and lower Volga, as well as Lake Balkhash (Kazakhstan). Occurs hundreds of kilometres upstream in major tributaries (Danube: Popa et al. 2009; recent observations in Volga River upstream Volgograd by MV and AFS).
Taxonomic notes.Monodacnacolorata appears to be a morphologically very variable species. Here, we propose to synonymise several local Black Sea species with this taxon. Given the difficulty to distinguish relatively flat shells typically associated with M.colorata from the more convex shells typically associated with M.pontica in, e.g., Lake Razim (Romania) and the apparent lack of genetic differentiation of convex specimens from M.colorata we assume that M.pontica is a synonym of M.colorata. Shell differences have been attributed to substrate differences. Further investigations to confirm the synonymy are required. Monodacnaangusticostata was synonymised by Popa et al. (2012) based on molecular evidence, even though some morphological distinction was reported from M.colorata, which they attributed to differential habitat preference (sediment type).
Conservation status. Not assessed.
Monodacnafilatovae (Logvinenko & Starobogatov, 1967)
1876 Cardiumcaspium, Eichw. – Grimm: 134–136 [pars].
*1967 Hypaniscaspiafilatovae Logvinenko and Starobogatov: 231.
1973 Hypaniscaspiafilatovae Logvinenko & Starobogatov, 1967. – Grossu: 139, text fig. 19a.
2013 Adacna (Monodacna) caspiafilatovae (Logvinenko & Starobogatov, 1967). – Kijashko in Bogutskaya et al.: 381, fig. 155, photo 52.
2016 Adacna (Monodacna) caspiafilatovae (Logvinenko & Starobogatov, 1967). – Vinarski and Kantor: 67.
Status. Pontocaspian species, endemic to Caspian Sea. Uncertain whether it concerns a morph of M.caspia.
Type locality. Gulf of Baku, Caspian Sea, Azerbaijan.
Distribution. Southern Caspian Sea Basin.
Taxonomic notes. See remarks under Monodacnacaspia above for uncertain status of M.filatovae.
Conservation status. Not assessed.
Monodacnaknipowitschi (Logvinenko & Starobogatov, 1966)
*1966a Hypaniscaspiaknipowitschi Logvinenko & Starobogatov: 15, fig. 2.
1973 Hypaniscaspiaknipowitschi Logvinenko & Starobogatov, 1967. – Grossu: 140, text fig. 20.
2013 Adacna (Monodacna) caspiaknipowitschi (Logvinenko & Starobogatov, 1966). – Kijashko in Bogutskaya et al.: 381–382, figs 152, 153, photo 53.
2016 Adacna (Monodacna) caspiaknipowitschi (Logvinenko & Starobogatov, 1966). – Vinarski and Kantor: 67.
Status. Pontocaspian species, endemic to Caspian Sea. Uncertain whether it concerns a morph of M.caspia.
Type locality. Middle Caspian Sea Basin.
Distribution. Caspian Sea (middle and southern basins). This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Hypaniscaspiaknipowitchi).
Taxonomic notes. See remarks under Monodacnacaspia above for uncertain status of M.knipowitschi.
Conservation status. Not assessed.
Monodacnapolymorpha (Logvinenko & Starobogatov, 1967)
*1967 Hypanisangusticostatapolymorpha Logvinenko & Starobogatov, 1967: 232.
1973 Hypanisangusticostatapolymorpha Logvinenko & Starobogatov, 1967. – Grossu: 141, fig. 22, pl. 1, fig. 7.
2013 Adacna (Monodacna) polymorpha (Logvinenko & Starobogatov, 1967). – Kijashko in Bogutskaya et al.: 383–384, fig. 159, photo 54.
2016 Adacna (Monodacna) polymorpha (Logvinenko & Starobogatov, 1967). – Vinarski and Kantor: 68.
Status. Pontocaspian species, endemic to Caspian Sea. Status uncertain.
Type locality. Central part of northern Caspian Sea.
Distribution. Northern Caspian Sea.
Taxonomic notes. See remarks under M.albida for uncertain species status.
Conservation status. Not assessed.
Monodacnasemipellucida (Logvinenko & Starobogatov, 1967)
*1967 Hypanissemipellucida Logvinenko & Starobogatov: 232–233.
1973 Hypanissemipellucida Logvinenko & Starobogatov, 1967. – Grossu: 144, text fig. 27.
2013 Adacna (Monodacna) semipellucida (Logvinenko & Starobogatov, 1967). – Kijashko in Bogutskaya et al.: 384, fig. 161, photo 55.
2016 Adacna (Monodacna) semipellucida (Logvinenko & Starobogatov, 1967). – Vinarski and Kantor: 68–69.
Status. Pontocaspian species, endemic to Caspian Sea.
Type locality. Off Tokmak Cape (also as Toqmaq Müyis), southern Kazakhstan, Caspian Sea.
Distribution. Middle Caspian Sea.
Conservation status. Not assessed.
Family Semelidae Stoliczka, 1870
Abrasegmentum (Récluz, 1843)
°1836 Erycinaovata Philippi: 13, pl. 1 fig. 13 [nonErycinaovata Gray, 1825].
*1843 Syndosmyasegmentum Récluz: 365–366.
1969 Abraovata (Phil.). – Logvinenko and Starobogatov: 339, fig. 355, pl. 5, fig. 12.
2013 Abrasegmenta (Récluz, 1843). – Kijashko in Bogutskaya et al.: 391, fig. 165.
2015 Abraovata (Philippi, 1836). – Latypov: 240.
Status. Invasive Pontocaspian species.
Type locality. Mediterranean coast near Taranto (Italy).
Distribution. Mediterranean, Black Sea coastal regions, Sea of Azov, Caspian Sea, Aral Sea.
Taxonomic notes. This species has been reported in much of the 20th century literature as Abraovata (Philippi, 1836), which is invalid since the original name (Erycinaovata Philippi, 1836) represents a junior primary homonym of Erycinaovata Gray, 1825.
Remarks. The first transfer of Abrasegmentum into the Caspian Sea occurred in 1947–1948, and the species has not been detected since 1955 (Latypov, 2015).
Conservation status. Not assessed.
Family Cyrenidae Gray, 1840
Corbiculafluminalis (Müller, 1774)
*1774 Tellinafluminalis Müller: 205–206.
1952 Corbiculafluminalis (Müller, 1774). – Zhadin: 317, fig. 283.
2012 Corbiculafluminalis (Müller, 1774). – Welter-Schultes: 15, unnumbered text figures.
2016 Corbiculafluminalis (O.F. Müller, 1774). – Nabozhenko and Nabozhenko: 62, text fig. 1(3, 4).
2016 Corbiculafluminalis (O.F. Müller, 1774). – Vinarski and Kantor: 80.
Status. Native/Invasive Pontocaspian species.
Type locality. Euphrates River.
Distribution. Native to large parts of western Asia (including southern Caspian river systems) and northern Africa, introduced in 1939 to southern North America and in 1980 from there to Europe (Seddon and Van Damme 2016). The species has been recently recorded from the Caspian Dagestan coast (Nabozhenko and Nabozhenko 2016).
Remarks. This species has been native to south Caspian rivers including the Kura river system (Zhadin 1952) and has expanded several times in the Late Pleistocene into the Caspian Sea, where in time intervals it survived in proximal lacustrine habitats. A recent introduction and expansion of the species has been recorded in the Kizlyarsky Gulf in Dagestan (Nabozhenko and Nabozhenko 2016) and the strong increase in fresh material found around the gulf in subsequent years, including whole specimens (AS Gasanova, Makhachkala, pers. comm.) suggests the species may have established there.
Conservation status. Least Concern (Seddon and Van Damme 2016).
Family Dreissenidae Gray, 1840
Remarks. Pontocaspian dreissenid taxonomy suffers from a lack of coordinated shell and DNA analyses. A large part of our considerations relies on the work of Rosenberg and Ludyanskiy (1994) who examined and illustrated all type material of Pontocaspian Dreissena.
Dreissenabugensis Andrusov, 1897
*1897 Dreissensiabugensis Andrusov: 285–286, pl. 15, figs 31–37.
1972 Dreissenarostriformisbugensis (Andrusov, 1897). – Scarlato and Starobogatov: 232–233, pl. 6, fig. 16.
1994 Dreissenabugensis (Andrusov, 1897). – Rosenberg and Ludyanskiy: 1479–1480, fig. 1a–e.
2013 Dreissenabugensis (Andrusov, 1897). – Kijashko in Bogutskaya et al.: 331, fig. 119.
2016 Dreissenabugensis (Andrusov, 1897). – Vinarski and Kantor: 78.
Status. Until mid-20th century endemic to northern Black Sea liman coast, since then invasive elsewhere in Black Sea Basin, Volga catchment, western Europe, and North America.
Type locality. Bug Liman near Nikolaev, Ukraine.
Distribution. Endemic to western Ukrainian liman coast, introduced in Danube Delta, Azov Sea, Volga catchment, western and central Europe, and North America (Orlova et al. 2005, Coughlan et al. 2017).
Taxonomic notes. This species has been considered as a subspecies of D.rostriformis (Deshayes, 1838) by some authors (e.g., Orlova et al. 2005), yet we follow the argumentation of Kijashko in Bogutskaya et al. (2013) to consider it as a distinct species. The proposed synonymy of Caspian D.rostriformis (= D.grimmi) and Black Sea D.bugensis by Stepien et al. (2013) is discussed below under D.grimmi.
Conservation status. Least Concern (von Rintelen and Van Damme 2011a).
Dreissenacaspia Eichwald, 1855
*1855 Dreissenacaspia Eichwald: 311–312, pl. 10, figs 19–21.
1969 Dreissenacaspia (Eichw.). – Logvinenko and Starobogatov: 316–318, fig. 341(2).
1994 Dreissenacaspia Eichwald, 1855. – Rosenberg and Ludyanskiy: 1482, fig. 3e, f.
2013 Dreissenacaspia Eichwald, 1855. – Kijashko in Bogutskaya et al.: fig. 109.
2016 Dreissena (Dreissena) caspiacaspia Eichwald, 1855. – Vinarski and Kantor: 76.
Status. Caspian endemic, probably extinct.
Type locality. Chistyi Bank and Cheleken Island, Caspian Sea, Russia.
Distribution. Caspian Sea and Aral Sea, probably extinct.
Taxonomic notes. The species is commonly subdivided into a Caspian subspecies (D.caspiacaspia) and an Aral Sea subspecies (D.caspiapallasi Andrusov, 1897). However, syntypes of the latter illustrated in Rosenberg and Ludyanskiy (1994, fig. 3f) show a broad and keeled Dreissena that has major morphological characters in common with D.polymorpha/elata rather than D.caspia. Filippov and Riedel (2009) reported Dreissenacaspia from Holocene core deposits of Aral Sea, but given the juvenile status of their material they noted they were uncertain whether it might comprise D.polymorpha. Dreissenacaspia was reported alive from the remaining “small Aral Sea” by Plotnikov et al. (2016). However, this latter record concerns more likely D.polymorpha and needs confirmation. Andreeva and Andreev (2003) mentioned that this subspecies has not been found in the Aral Sea since 1989.
Conservation status. Critically endangered, possibly extinct (von Rintelen and Van Damme 2011b).
Dreissenaelata Andrusov, 1897
*1897 Dreissensiapolymorphavar.elata Andrusov: 353, pl. 20, fig. 25.
1969 Dreissenaelata (Andr.). – Logvinenko and Starobogatov: 316, fig. 341(1).
1994 Dreissenaelata Andrusov, 1897. – Rosenberg and Ludyanskiy: 1482, fig. 3g.
2013 Dreissenaelata (Andrusov, 1897). – Kijashko in Bogutskaya et al.: fig. 108.
2016 Dreissena (Dreissena) elata (Andrusov, 1897). – Vinarski and Kantor: 76.
Status. Pontocaspian species, endemic to the Caspian Sea, probably extinct. Species status uncertain.
Type locality. Kuuli Cape, Dazmyk, Apsheron Peninsula, Azerbaijan (Vinarski and Kantor 2016).
Distribution. Caspian Sea. Probably extinct.
Taxonomic notes.Dreissenaelata has morphological features in common with D.polymorpha, including a relatively wide shell and a well-pronounced keel located close to the ventral margin. However, the D.elata shell is in general wider, flatter, and has a more rounded abapical margin even though shell characters are higly variable. Dreissenaelata has been reported from areas in the Caspian Sea with salinities well above 5 ‰, which is unusual for D.polymorpha elsewhere. We are uncertain whether D.elata might be a sibling species. Its apparently distinct morphology and autecological preferences suggest it is different from D.polymorpha, but it will require molecular comparison to investigate whether it concerns a mere morph that has undergone “ecological release” (Kohn 1972) or is a different species. However, no living specimens of D.elata have been recorded since 1957 (Kostianoy and Kosarev 2005) when its Caspian habitats were invaded by Mytilasterminimus.
Conservation status. Not assessed. It was reported as extinct by Kostianoy and Kosarev (2005, and references therein). If D.elata is accepted as a valid species, it might qualify for the same conservation status as D.caspia (critically endangered, possibly extinct; von Rintelen and Van Damme 2011b).
Dreissenagrimmi (Andrusov, 1890)
Fig. 4b
Figure 4.
Lectotype Dreissenarostriformis versus D.grimmi. aD.rostriformis Deshayes, 1838. Lectotype. Pliocene, Crimea. Reproduced from Archambault-Guezou (1976, pl. 6, fig 2a-2c) b RGM.961901, D.grimmi (Andrusov, 1890). Caspian Sea offshore Aktau, Kazakhstan, sample KAZ17-21, depth 44.3 m. Scale bar: 1 cm.
1877 DreyssenaBrardii var. caspia Grimm: 74–75 [nonDreissenacaspia Eichwald, 1855].
*1890 Dr. [eissena] Grimmi Andrusov: 233 [nom. nov. pro Dreissenacaspia Grimm, 1877, non Eichwald, 1855].
1897 DreissensiaGrimmi Andrus. – Andrusov: 279–282, pl. 16, figs 16–18.
1897 Dreissensiarostriformisvar.distincta Andrusov: 273–278, pl. 14, figs 18–24.
1897 Dreissensia Tschaudae var. pontocaspica Andrusov: 294–297, pl. 9, figs 27–32, pl. 15, figs 29, 30.
1966a Dreissenarostriformiscompressa Logvinenko and Starobogatov: 15–16, fig. 3.
1969 Dreissenarostriformisgrimmi Andr. – Logvinenko and Starobogatov: 318, fig. 341(3).
1969 Dreissenarostriformispontocaspica (Andr.). – Logvinenko and Starobogatov: 319, fig. 341(6).
1994 Dreissenarostriformis (Deshayes, 1838). – Rosenberg and Ludyanskiy: 1477–1479, figs 1f, 2a–j [nonMytilusrostriformis Deshayes, 1838].
2013 Dreissenarostriformis (Deshayes, 1838). – Kijashko in Bogutskaya et al.: 330 [non Deshayes, 1838].
2013 D. [reissena] rostriformis compressa Logvinenko & Starobogatov, 1966. – Kijashko in Bogutskaya et al.: 331, fig. 117a, photo 38.
2013 D. [reissena] rostriformis distincta (Andrusov, 1897). – Kijashko in Bogutskaya et al.: 331, fig. 117c.
2013 D. [reissena] rostriformis grimmi (Andrusov, 1890). – Kijashko in Bogutskaya et al.: 331, fig. 117b.
2013 D. [reissena] rostriformis pontocaspica (Andrusov, 1897). – Kijashko in Bogutskaya et al.: 331, fig. 117d.
Status. Caspian Sea endemic.
Type locality. Caspian Sea.
Distribution. Middle to southern Caspian Sea basins. This species was mentioned from depths between 200 and 400 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as D.rostriformiscompressa) and found living offshore Aktau (Kazakhstan) in 2017 below 20 m water depth.
Taxonomic notes. This Caspian species is very often cited as Dreissenarostriformis. Rosenberg and Ludyanskiy (1994: 1497) discuss the uncertainties of this attribution but state that “D. pontocaspica, D. distincta, D. compressa, and D. grimmi are synonyms of D. rostriformis” even though they find “some justification for maintaining a distinction between an extinct subspecies, D.rostriformisrostriformis and a living one, for which D.rostriformisgrimmi is the oldest name”. Their figure of the lectotype of D.rostriformis (Rosenberg and Ludyanskiy 1994: fig. 2a), which derives from Pliocene deposits of the Black Sea Basin, concerns a relative small, thick-shelled, and low Dreissena with a pointed beak and lacking a keel. On interior view, the shell area outside the pallial line is thick. Deshayes’s lectotype has several characters in common with modern Caspian D.rostriformis and the closely related Black Sea Basin D.bugensis. Yet, the Pliocene form has a broader umbonal area that results in a more subquadrangular shape, which is different from the modern Caspian Dreissena that have tear-drop to pear-shaped shells. The subquadrangular shape of Deshayes’s material is even more pronounced in the pallial line on the shell’s interior, a feature not seen in any modern Caspian material. The Pliocene Black Sea D.rostriformis has its general shape in common with Apsheronian (Early Pleistocene) Caspian dreissenids referred to as D.carinatocurvata as illustrated in Kolesnikov (1950, pl. 14, figs 14–16). Hence, we conclude that the recent Caspian species should be treated different from Pliocene D.rostriformis and the name D.grimmi should be applied instead.
Various subspecies have been attributed to Caspian Dreissenarostriformis (see, e.g., Kijashko in Bogutskaya et al. 2013 for a synonymy list). Even though morphological differences appear to be large, intermediates are known between the morphs. Stepien et al. (2013) reviewed molecular evidence for species boundaries within Dreissena. They concluded that (1) all Caspian Sea forms that have been mentioned in literature as (sub-) species of D.rostriformis (= D.grimmi) are one and the same species and (2) there is not enough molecular evidence and great difficulty in morphology to separate the Caspian species from the Black Sea Basin D.bugensis. We agree with the first point made by Stepien et al. (2013); all forms reported from the middle and southern Caspian Sea basins appear to be mere morphs of a single species, a feature also noted by Rosenberg and Ludyanskiy (1994). However, we disagree with their second proposal. Dreissenabugensis and D.grimmi have non-overlapping ecological tolerances and are separated geographically (Rosenberg and Ludyanskiy 1994). This fact together with the very limited but consistent genetic differentiation suggests that it may concern very recently evolved sister species. In the early 1980s, D.bugensis was introduced in the Volga (Zhulidov et al. 2005) and since then spread from there to central and western Europe and North America. So far, Dreissenabugensis has only been reported from the Volga itself and its delta but not from the northern Caspian Sea Basin. If it would be conspecific with the middle-southern Caspian species, which lives at higher salinities and deeper habitats, we would expect that the invasive populations in the north would have been blended with the Caspian population in the south. With no such intermediate populations found so far we consider both taxa as viable species.
Conservation status. Least Concern (for Dreissenarostriformis; von Rintelen and Van Damme 2011c).
Dreissenapolymorpha (Pallas, 1771) s.l.
*1771 Mytiluspolymorphus Pallas: 368, 435, 478.
1897 Dreissensia Andrusovi Andrusov: 374–376 pl. 18, figs 21–23.
1897 Dreissensia Pallasi Andrusov: 671–672, pl. 20, figs 33–35.
1897 Dreissensiapolymorphavar.aralensis Andrusov: 354–355.
1897 Dreissensiapolymorphavar.obtusecarinata Andrusov: 354.
1994 Dreissenapolymorpha (Pallas, 1771). – Rosenberg and Ludyanskiy: 1480–1482, fig. 3a, b.
1994 Dreissenapolymorphaaralensis Andrusov, 1897. – Rosenberg and Ludyanskiy: 1480, fig. 3c.
1994 Dreissenapolymorphaobtusecarinata Andrusov, 1897. – Rosenberg and Ludyanskiy: 1481, fig. 3d.
1994 Dreissenacaspiapallasi Andrusov, 1897. – Rosenberg and Ludyanskiy: 1482, fig. 3f.
2003 Dreissenacaspiapallasi (Andrusov, 1897). – Andreeva and Andreev: 80, fig. 4.1(7–9).
2003 Dreissenapolymorphaaralensis (Andrusov, 1897). – Andreeva and Andreev: 79, fig. 4.1(1–3).
2003 Dreissenaobtusecarinata (Andrusov, 1897). – Andreeva and Andreev: 80, fig. 4.1(4–6).
2013 Dreissena (Dreissena) polymorpha (Andrusov, 1897). – Kijashko in Bogutskaya et al.: 328, fig 118a [pars, status fig. 118b uncertain].
2016 Dreissena (Dreissena) polymorphapolymorpha (Andrusov, 1897). – Vinarski and Kantor: 75.
?2016 Dreissena (Dreissena) polymorphaandrusovi (Brusina in Andrusov, 1897). – Vinarski and Kantor: 75.
?2016 Dreissena (Dreissena) polymorphaaralensis (Andrusov, 1897). – Vinarski and Kantor: 75.
?2016 Dreissena (Dreissena) polymorphaobtusecarinata (Andrusov, 1897). – Vinarski and Kantor: 76.
?2016 Dreissena (Dreissena) caspiapallasi (Andrusov, 1897). – Vinarski and Kantor: 7.
Status. Native Pontocaspian species.
Type locality. Volga and Yaik (Ural) rivers, Caspian Sea.
Distribution. Eurasian (native and invasive), North America (invasive) rivers, lakes, estuaries, deltas (Rosenberg and Ludyanskiy 1994, Cummings and Graf 2015, Coughlan et al. 2017). Several unique forms/species within this group reported from the Pontocaspian region.
Taxonomic notes.Dreissenapolymorpha has been subject of intense DNA and ecological studies, but rarely were Caspian communities involved. Combined insights into the shell morphology, ecology, and molecular biology has to date not fully resolved several aspects of Pontocaspian records of this species. Occurrences in rivers and deltas of the Pontocaspian region are consistently attributed to Dreissenapolymorpha. However, slightly deviating morphs exist(ed) in salinities typically not favoured by D.polymorpha elsewhere in the Caspian and Aral seas. A particular form of Dreissenapolymorpha, documented by Kijashko in Bogutskaya et al. (2013), viz. D.polymorphaandrusovi (his figure 118b) will need further study as it has many morphological similarities with D.caspia (including general shape, location of semidiameter, and broad flat shape of hinge platform).
Conservation status. Least Concern (Van Damme 2014).
Mytilopsisleucophaeata (Conrad, 1831)
*1831 Mytilusleucophaeatus Conrad: 263–264, pl. 11, fig. 13.
2013 Mytilopsisleucophaeata (Conrad, 1831). – Kijashko in Bogutskaya et al.: 320, fig. 107.
Status. Invasive Pontocaspian species.
Type locality. Southern coast of eastern United States.
Distribution. Black Sea Basin, Caspian Sea, coasts of western Europe, Caribbean, and northern South America.
Remarks. The species, native to the southern coast of North America, was first introduced in Europe in 1835 (Heiler et al. 2010). In the Pontocaspian region, it first appeared in the northern Black Sea Basin in 2002 and was first collected in the Caspian Sea in 2009 (Heiler et al. 2010). It is easily distinguished from Pontocaspian dreissenids by the presence of an aphophysis near the hinge.
Conservation status. Least Concern (Cummings 2011).
Gastropoda
Family Neritidae Rafinesque, 1815
Theodoxusdanubialis (Pfeiffer, 1828)
*1828 Neritadanubialis Pfeiffer: 48, pl. 8, figs 17, 18.
2009 Theodoxusdanubialis (C. Pfeiffer, 1828). – Fehér et al.: figs 2a–k, 4a–c, 5a–c.
2012 Theodoxusdanubialis (Pfeiffer, 1828). – Welter-Schultes: 27, unnumbered text figures.
2016 Theodoxus (Theodoxus) danubialis (Pfeiffer, 1828). – Vinarski and Kantor: 156 [and synonyms therein].
Status. Accepted native species.
Type locality. Danube River, Vienna, Austria.
Distribution. Danube River catchment, central to south-eastern Europe, as well as northern Italy (Fehér et al. 2009).
Taxonomic notes. The latest phylogenetic data supports a sister relationship between Theodoxusdanubialis and the clade containing T.fluviatilis and T.velox (AFS, unpublished data). Some authors believe T.danubialis and T.prevostianus may represent different species given some level of genetic, ecological, and morphological differentiation (Fehér et al. 2009, Welter-Schultes 2012; but see also Bandel 2001). More recent unpublished results may suggest that the genetic level of differentiation between these species is more indicative of intraspecific diversity within a single species (AFS, unpublished data).
Conservation status. Least Concern (Tomovic et al. 2010).
Theodoxusfluviatilis (Linnaeus, 1758)
*1758 Neritafluviatilis Linnaeus: 777.
1865 Theodoxusfluviatilisvar.subthermalis Issel: 22–23.
1886 Neritinaeuxina Clessin: 55.
1908 Neritinadanubialisvar.danasteri Lindholm: 214–215.
?1972 Theodoxusdniestroviensis Put’: 80–82, text fig. 5.
?1999 Th.dniestroviensisPut’, 1972. – Anistratenko et al.: 19, figs 4, 8.
1999 Th.fluviatilis (Linnaeus, 1758). – Anistratenko et al.: 13–15, figs 3, 4.
2005 Theodoxusfluviatilis (Linnaeus, 1758). – Anistratenko: 7–8, text figs 3, 4.
2012 Theodoxuseuxinus (Clessin, 1886). – Welter-Schultes: 27, unnumbered text figures.
2012 Theodoxusfluviatilis (Linnaeus, 1758). – Welter-Schultes: 28, unnumbered text figures.
2015 Theodoxusfluviatilis (Linnaeus, 1758). – Glöer and Pešić: 88–91, figs 1, 3–5, 9, 13–34.
2016 Theodoxus (Theodoxus) fluviatilis (Linnaeus, 1758). – Vinarski and Kantor: 154–155 [pars, excluding synonyms sarmatica and velox].
2016 Theodoxus (Theodoxus) euxinus (Clessin, 1886). – Vinarski and Kantor: 155.
2016 Theodoxus (Theodoxus) subthermalis (Bourguignat in Issel, 1865). – Vinarski andKantor: 157–158.
Status. Accepted native species.
Type locality. Near Uppsala, Sweden. The lectotype was designated by Anistratenko (2005).
Distribution. Widely distributed all over Europe, Anatolia, and north-western Africa. Within the Pontocaspian region, it is a common component of the lower reaches of Black and Azov Sea drainages (specifically in Bulgaria, Romania, and Ukraine). Towards the east, the species extends at least as far as the Don River system in Russia and the coastal rivers of Georgia, but it is absent from the Caspian system. Records of this species from Iran and western Asia are likely misidentifications (AFS, unpublished data).
Taxonomic notes.Theodoxusfluviatilis exhibits considerable variation in shell colouration and shape (Glöer and Pešić 2015). Unpublished molecular data confirm the synonymy of a number of taxa such as Theodoxuseuxinus syn. n., T.danasteri, and T.subthermalis syn. n., and further suggest the inclusion of T.saulcyi and T.heldreichi (AFS, unpublished data). A final decision concerning the status of T.dniestroviensisPut’, 1972 described from the Dniester River (Rukhotyn village, Khotyn district, Chernivtsi region, Ukraine) is not possible at the moment. Despite appropriate efforts, we were unable to trace the type specimens of this species. Based on the original description and illustration (Put’ 1972) it was considered as a junior synonym of T.fluviatilis by Anistratenko et al. (1999) having an unusual colour pattern. Theodoxusmilachevichi was described as a subfossil from the Crimean coast. It closely resembles morphotypes of both T.fluviatilis and T.velox V. Anistratenko in O. Anistratenko et al., 1999 and might be synonym of either species (compare type material illustrated in Kantor and Sysoev 2006). However, the morphological variability of the taxa involved, as well as the lacking possibility of acquiring genetic data for T.milachevichi, complicates a decision on the independence or synonymy of this species.
Conservation status. Least Concern (Kebapçı and Van Damme 2012).
Theodoxuspallasi Lindholm, 1924
°1838 Neritinaliturata Eichwald: 156–157 [nonNeritinaliturata Schultze, 1826].
*1924 Theodoxuspallasi Lindholm: 33, 34 [nom. nov. pro Neritinaliturata Eichwald, 1838, non Schultze, 1826].
1947 Theodoxus (Theodoxus) pallasivar.nalivkini Kolesnikov: 106, 110.
1976 Theodoxuspallasi Lindholm, 1924. – Akramovskiy: 88, text fig. 23, pl. 1, figs 1, 2.
1994 Theodoxusastrachanicus Starobogatov in Starobogatov, Filchakov, Antonova and Pirogov: 8–9, fig. 1(1, 2).
1994 Theodoxusastrachanicus Starobogatov et al.: 8–9, fig. 1(1, 2).
2009 Theodoxuspallasi Lindholm, 1924. – Filippov and Riedel: 70, 72, 74, 76, fig. 4g–i.
2011 Theodoxusastrachanicus Starobogatov in Starobogatov, Filchakov, Antonova & Pirogov, 1994. – Anistratenko et al.: 54–55, fig. 1(6).
2012 Theodoxuspallasi Lindholm, 1924. – Welter-Schultes: 29, unnumbered text figures.
2016 Theodoxus (Theodoxus) astrachanicus Starobogatov in Starobogatov, Filchakov, Antonova & Pirogov, 1994. – Vinarski and Kantor: 155–156.
2016 Theodoxus (Theodoxus) pallasi (Lindholm, 1924). – Vinarski and Kantor: 156–157 [and synonyms therein].
2017 Theodoxuspallasi Lindholm, 1924. – Anistratenko et al.: 221, figs 4, 7, 10, 11.
2018 Theodoxuspallasi Lindholm, 1924. – Neubauer et al.: 48–51, fig. 4A–F.
Status. Accepted Pontocaspian species, name uncertain.
Type locality. “Inter Fucos littoris Derbendensis viva” (living among algae on the shores of Derbent), Dagestan, Russia.
Distribution. Present along the Caspian Sea shores, in the Volga River, and the Sea of Azov. Lived until the late 1980s in the Aral Sea but is possibly extinct there now (Andreev et al. 1992, Aladin et al. 1998, Micklin et al. 2014).
Taxonomic notes.Eichwald (1838) introduced the species Neritinaliturata based on material from the shores of Derbent (Dagestan, Russia, northwestern Caspian Sea). That name is invalid as it is a junior primary homonym of N.liturata Schultze, 1826; it was replaced by Lindholm (1924) with Theodoxuspallasi (see also Anistratenko et al. 2017). Theodoxuspallasi is a widely used name, but a major nomenclatural change might be due. Unpublished molecular data suggest that all Theodoxus from the Caspian Sea, Azov Sea, and Armenian lakes Sevan and Yerevan, as well as several mineral springs and streams in the Khorasan provinces of Iran, belong to a single species (AFS, unpublished results). The oldest name available for that group is Theodoxusmajor Issel, 1865, described from Lake Sevan in Armenia (originally as variety of the unavailable name T.schirazensis). Akramovskiy (1976) noted the similarity of T.pallasi and T.major and considered the latter as a morphotype of the former. Although he did not explicitly state it, he thereby suggested the two taxa to be synonymous. This view was adopted by Vinarski and Kantor (2016), who listed major in synonymy of pallasi, although Issel’s (1865) name has priority. The potential synonymy also involves T.schultzii. Despite the characteristic appearance of the syntypes, the presence of intermediate morphologies in samples taken on shores of Azerbaijan and Kazakhstan in 2016 and 2017 (pers. obs. OA, VA, FW) indicates a close relationship with T.pallasi. The radulae of these two species differ in the relative width of the central and marginal teeth (see Zettler 2007 and compare Anistratenko et al. 2017).
Unfortunately, the types of T.major, supposed to be in the Museo Regionale di Scienze Naturali, Torino, are inaccessible at the moment due to museum renovation (E Gavetti, pers. comm., Oct 2018). We refrain from a final conclusion on the synonymy of the species involved until information on the types of all taxa as well as published molecular data are available. For details on the taxonomic relationship between T.pallasi and T.astrachanicus, see discussion in Anistratenko et al. (2017).
Conservation status. Data Deficient (Van Damme and Kebapçı 2014).
Theodoxusschultzii (Grimm, 1877)
*1877 Neritina Schultzii Grimm: 77–78, pl. 7, fig. 5, pl. 8, fig. 16.
1909 Neritina (Ninnia) Schultzei [sic] Grimm. – Andrusov: 106–107, pl. 6, fig. 38.
?1947 Theodoxus (Ninnia) schultzi [sic] var.jukovi Kolesnikov: 106, 110.
1950 Theodoxus (Ninnia) schultzei [sic] (Grimm). – Kolesnikov: 215–216, pl. 26, figs 12, 13.
1969 Theodoxusschultzi [sic] (Grimm, 1877). – Logvinenko and Starobogatov: 344, fig. 357.
?1974 Theodoxuszhukovi [sic] Kolesnikov, 1947. – Starobogatov: 255, text fig. 223.
2007 Theodoxus (Theodoxus) schultzii (Grimm, 1877). – Zettler: 249, figs 2–5.
2016 Theodoxus (Theodoxus) schultzii (Grimm, 1877). – Vinarski and Kantor: 157.
Status. Pontocaspian species, status uncertain.
Type locality. Caspian Sea, in two localities, given by Grimm (1877) as 43°17'N, 01°03'E, 40 fathoms, and 42°48'N, 01°22'E, 48 fathoms. Since the longitude was calculated relative to the geographic position of Baku, situated approximately at 50E, the correct longitude should be about 51°00'E (Vinarski and Kantor 2016).
Distribution. Middle and southern Caspian Sea basins, between 15 and 100 m (Logvinenko and Starobogatov 1969).
Taxonomic notes. See discussion of T.pallasi for notes on the potential synonymy with T.major Issel, 1865. The status of T.jukovi still requires confirmation (Vinarski and Kantor 2016).
Conservation status. Not assessed.
Theodoxusvelox V. Anistratenko in O. Anistratenko et al., 1999
*1999 Th. [eodoxus] velox V. Anistratenko in O. Anistratenko et al.: 17–18, fig. 4(7).
Status. Pontocaspian species, name uncertain.
Type locality. Dnieper Delta, Zbur’ivka liman, Ukraine.
Distribution. This species was believed to be restricted to drainage systems of the northern Black Sea coast (even though the Oskol River lies far from the Black Sea coast), but unpublished molecular data suggest it may be distributed as far north as the eastern part of the Baltic Sea and as far south as Anatolia (AFS, unpublished data).
Taxonomic notes. The species was listed as junior synonym of T.fluviatilis by Vinarski and Kantor (2016). Theodoxusvelox is indeed challenging to differentiate from some regional morphotypes of that species given the overlap in shell patterns. Unpublished molecular data indicate however that T.velox belongs to a different molecular clade (AFS, unpublished data). The distribution range of that clade overlaps with the range of T.sarmaticus (Lindholm, 1901), which is widely accepted as a junior synonym of T.fluviatilis in the literature (e.g., Vinarski and Kantor 2016). A revision of the taxa involved and study of the type material is required to solve the synonymy issues.
Conservation status. Not assessed.
Family Cochliopidae Tryon, 1866
Eupaludestrinastagnorum (Gmelin, 1791)
*1791 Helixstagnorum Gmelin: 3653.
1975 Falsihydrobiastreletzkiensis Chukhchin: 121.
2012 Heleobiastagnorum (Gmelin, 1791). – Welter-Schultes: 39, unnumbered text figures.
2012 Semisalsastagnorum (Gemlin, 1791). – Kroll et al.: 1520.
Status. Accepted, native Pontocaspian or immigrant species.
Type locality. Kaasjeswater, Zierikzee, the Netherlands.
Distribution. Coastal areas of Europe and the Mediterranean region, extending to North Africa and east to Iran (Glöer 2002). Occurrence in Black Sea according to, e.g., Chukhchin (1975) and in the Caspian Sea (TW, unpublished data).
Taxonomic notes. We find the attribution of this species to the genus Eupaludestrina unsatisfactory, yet a further revision is required to establish and stabilise the generic attribution as there is considerable confusion. It is commonly classified in the South American genus Heleobia (e.g., Prié 2011), whereas Kroll et al. (2012) suggested that this species belongs to the genus Semisalsa, a group of European Cochliopidae distinct from Heleobia. However, Semisalsa is currently listed as junior synonym of Eupaludestrina Mabille, 1877 (type species: Hydrobiamacei Paladilhe, 1867, by subsequent designation by Kadolsky 2008). Following Kadolsky (2008), Eupaludestrinais currently ranked assubgenusofHeleobia in MolluscaBase (2018), but both the phylogenetic and geographic distinction of the European and American species suggest separation on the genus level.
Remarks. It is unclear whether the species is native to the Pontocaspian area or a recent immigrant.
Conservation status. Least Concern (Prié 2011).
Family Hydrobiidae Stimpson, 1865
Remarks. The Hydrobiidae form the most species-rich mollusc group in the Pontocaspian region. However, in general, useful shell characters are few and highly variable (Wilke and Delicado in press). Descriptions in the past have often been very general, and illustrations of types are notably poor for several of the endemic taxa. A strong tendency of naming large numbers of species has developed throughout the 20th century (e.g., Logvinenko and Starobogatov 1969), but for some groups where morphological and genetic analyses could be performed (e.g., Caspiohydrobia spp.) it has been demonstrated that actual species numbers were much lower than the number of species described (Haase et al. 2010). For many of the endemic species, especially in the genus Turricaspia, the apparent loss of types, combined with the lack of living material makes it impossible to assess their taxonomic status. Currently, a number of taxonomic works is in progress on the endemic Pontocaspian hydrobiid groups, and some different insights on the genus-level classifications exist. Here, we adopt a conservative approach, mostly based on Neubauer et al. (2018).
Subfamily Caspiinae Dybowski, 1913
Remarks. The distinction of the genera Caspia, Ulskia, and Clathrocaspia follows Neubauer et al. (2018). The three taxa are differentiated based on details of the protoconch and the expression of teleoconch sculpture. Caspia s. s. is characterised by a single distinct but fine spiral keel below the suture. It is usually smooth, yet within the type species some reticulate ornament can be found. Species of Clathrocaspia expose a distinctive, reticulate pattern on the teleoconch and a malleate protoconch with faint spiral threads. The aperture of Clathrocaspia often develops a distinct flat base. The discinction of the two genera is subject of current research. Ulskia also has a malleate protoconch but with more distinct spiral threads; teleoconch sculpture is occasionally present as minute elongate nodules.
Caspiabaerii Clessin & Dybowski in Dybowski, 1887
*1887 CaspiaBaerii Clessin & Dybowski in Dybowski: 36–37.
1888 [Caspia] Baerii n. sp. – Dybowski: 79, pl. 3, fig. 4a, b.
1969 Pyrgula (Caspia) baerii (Cless. & Dyb.). – Logvinenko and Starobogatov: 377, fig. 367(3).
2016 Caspiabaerii Clessin & W. Dybowski in W. Dybowski, 1888. – Vinarski and Kantor: 224.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Caspian Sea and possibly Danube Delta (Romania). This species was mentioned from depths between 200 and 400 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Turricaspiabaerii).
Taxonomic notes. The type material is stored in the von Baer collection of Caspian Sea molluscs in the Zoological Museum of Lviv University (Ukraine) and comprises more than a hundred syntypes (Anistratenko et al. 2018). The slender shell, the presence of a fine spiral keel below the suture, and the occasionally weakly reticulated surface distinguish this species from congeners.
Conservation status. Not assessed.
Caspiavalkanovi (Golikov & Starobogatov, 1966)
*1966 P. [yrgula] (Caspia) baeri [sic] valkanovi Golikov & Starobogatov: 354–355, fig. 1(9).
2006 Caspiavalkanovi (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 88, pl. 41, fig. N.
Status. Pontocaspian species, identity uncertain.
Type locality. Off Crimea, station 18, sample 173.
Distribution. Endemic to the Black Sea Basin.
Taxonomic notes. The identity and status of this subfossil taxon, described from phaseoline silt, are somewhat uncertain. The holotype illustrated in Kantor and Sysoev (2006) is poorly preserved and does not allow a proper assessment of its validity. The general shape and size are indicative of the genus Caspia and it looks like a variety that might even be a synonym of C.baerii. Furthermore, we are not entirely certain as to the stratigraphic age of the stratigraphic origin of this species. The phaseoline silt is a marine Holocene unit, yet it contains reworked Late Pleistocene Neoeuxinian (Pontocaspian) species (FW, pers. obs.).
Conservation status. Not assessed.
Clathrocaspiabrotzkajae (Starobogatov in Anistratenko & Prisyazhniuk, 1992)
*1992 Caspia (Clathrocaspia) brotzkajae Starobogatov in Anistratenko & Prisyazhniuk: 18–19, fig. 2a.
2016 Caspiabrotzkajae Starobogatov in Anistratenko & Prisyazhniuk, 1992. – Vinarski and Kantor: 224.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea shores of Dagestan, Russia, at ca. 60 m.
Distribution. Presently endemic to the Caspian Sea. The species was also recorded from the Holocene of Danube Delta, Ukraine (Anistratenko and Prisyazhniuk 1992).
Taxonomic notes. The species differs from its congeners in the bulbous shape, with a ratio of body whorl height/shell height of approx. 3/4, as well as regarding the expanded aperture.
Conservation status. Not assessed.
Clathrocaspiagmelinii (Clessin & Dybowski in Dybowski, 1887)
*1887 CaspiaGmelinii Clessin & Dybowski in Dybowski: 37–38.
1888 [Caspia] Gmelini [sic] n. sp. – Dybowski: 79, pl. 3, fig. 7a, b.
1969 Pyrgula (Caspia) gmelinii (Cless. & W. Dyb.). – Logvinenko and Starobogatov: 378, fig. 367(7).
?1969 Pyrgula (Caspia) sowinskyi Logvinenko and Starobogatov: 378, fig. 367(4).
?1977 Pyrgula (Caspia) gaillardi Tadjalli-Pour: 107, pl. 2, fig. 8.
2015 Caspiagmelinii Clessin & W. Dybowski, 1887. – Boeters et al.: 178, figs 1–6.
2016 Caspiagmelinii Clessin & W. Dybowski in W. Dybowski, 1888. – Vinarski andKantor: 224.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Endemic to the Caspian Sea, recorded from the middle and southern parts. This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Turricaspiagmelinii).
Taxonomic notes. The broad shell and the heavily reticulated surface distinguish this species from congeners. Pyrgulasowinskyi, from the middle and southern Caspian Sea, and P.gaillardi, from the Caspian Sea shore between Astara and Hashtpar (= Talesh), Iran, closely resemble C.gmelinii in terms of shell shape, the shape of the aperture, and the distinct reticulate teleoconch sculpture. Very likely, the two species are synonyms of C.gmelini. Since the type material of Logvinenko and Starobogatov (1969) has not been found, and the whereabouts of the material of Tadjalli-Pour (1977) is unknown, a re-examination of these species has to be postponed. Here, we suggest to treat them as nomina dubia until more information becomes available.
Conservation status. Data Deficient (same for P.sowinskyi; Son 2011a, Vinarski 2011o).
Clathrocaspiaisseli (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Caspia) isseli Logvinenko & Starobogatov: 378, fig. 367(6).
2016 Pyrgulaisseli Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 239.
Status. Pontocaspian species, identity uncertain.
Type locality. Southern Caspian Sea (no details), between 40–75 m water depth.
Distribution. Endemic to the Caspian Sea.
Taxonomic notes. This species hardly differs from C.pallasii and might be a junior synonym. Observations on Holocene material from the southern and northern Caspian Sea shores (VA, TN, FW) suggest that the minor differences range within intraspecific variability but further studies (preferentially involving DNA) are required to solve the identity of this taxon. The classification in Clathrocaspia is based on the reticulate sculpture typical of that genus.
Conservation status. Data Deficient (Vinarski 2011j).
Clathrocaspiaknipowitschii (Makarov, 1938)
*1938 Caspiagmelini [sic] var. Knipowitschii Makarov: 1058.
?1966 P. [yrgula] (Caspia) gmelini [sic] aluschtensis Golikov and Starobogatov: 354, fig. 1(8).
1966 P. [yrgula] (Caspia) makarovi Golikov and Starobogatov: 353–354, fig. 1(5).
?1987 Caspiagmeliniistanislavi Alexenko and Starobogatov: 33, fig. 1.
1992 Caspia (Clathrocaspia) knipowitchi Makarov, 1938. – Anistratenko and Prisyazhniuk: 19, fig. 2b.
2006 Caspiaknipowitchi [sic] Makarov, 1938. – Kantor and Sysoev: 87–88, pl. 41, fig. J.
2006 Caspiamakarovi (Golikov et Starobogatov, 1966). – Kantor and Sysoev: 88, pl. 41, fig. L.
2013 Caspiaknipowitchii [sic] Makarov, 1938. – Anistratenko: 53–55, figs 1A–I, 3A–D, 5A–D.
2013 Caspiamakarovi (Golikov & Starobogatov, 1966). – Anistratenko: 56–59, figs 2A–E, 3E.
2016 Caspiaknipowitchi [sic] Makarov, 1938. – Vinarski and Kantor: 224.
2016 Caspiamakarovi (Golikov & Starobogatov, 1966). – Vinarski and Kantor: 225.
?2016 Caspiastanislavi Alexenko & Starobogatov, 1987. – Vinarski and Kantor: 225.
Status. Accepted Pontocaspian species.
Type locality. Ukraine, in the Dniester River (exact locality not specified).
Distribution. Azov Sea and northern Black Sea Basin. Known from the Holocene of Danube Delta, Ukraine (Anistratenko and Prisyazhniuk 1992).
Taxonomic notes.Clathrocaspiaknipowitschii, C.makarovi, C.gmelinialuschtensis, and C.stanislavi were all described from the northern margin of the Black Sea. After detailed morphological comparison of C.knipowitschii and C.makarovi syn. n. and preliminary genetic analyses (TW, unpublished data), we conclude that both taxa should be considered synonyms. Very likely, also C.gmelinialuschtensis and C.stanislavi are synonyms of C.knipowitschii, but a final decision on that matter requires investigation of the type material.
Conservation status. Least Concern (same for C.makarovi; Son 2011b, c).
Clathrocaspialogvinenkoi (Golikov & Starobogatov, 1966)
*1966 P. [yrgula] (Caspia) logvinenkoi Golikov & Starobogatov: 354, fig. 1(7).
2006 Caspialogvinenkoi (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 88, pl. 41, fig. I.
2007a Caspia (Clathrocaspia) logvinenkoi (Golikov & Starobogatov, 1966). – Anistratenko: 25–26, fig. 2.
2016 Caspialogvinenkoi (Golikov & Starobogatov, 1966). – Vinarski and Kantor: 224–225.
Status. Accepted Pontocaspian species.
Type locality. Don Delta, Russia.
Distribution. Known only from the type locality.
Taxonomic notes. The species has distinctive shell characters: broad conical shape with a weak subsutural bulge and apically thickened peristome.
Remarks. The type material was collected by Mordukhay-Boltovskoy in 1937 and comprises two specimens, the holotype and the paratype. Three additional specimens were collected from the same region in 2006 (Anistratenko 2007a). The salinity at the type locality fluctuates between freshwater and ca. 1‰.
Conservation status. Not assessed. In the fifty years since the description of this species five specimens have been collected; this is likely evidence of its rarity. Known only from two close localities, C.logvinenkoi appears to have an extremely narrow distributional range in the Azov–Black Sea Basin, being endemic to the Taganrog province (e.g., Anistratenko 2007a).
Clathrocaspiamilae (Boeters, Glöer & Georgiev, 2015)
*2015 Caspiamilae Boeters, Glöer & Georgiev in Boeters et al.: 180–183, figs 9–21.
Status. Pontocaspian species, identity uncertain.
Type locality. Bulgaria, Danube Island Vardim (43°37'N, 25°28'E).
Distribution. Only known from type locality.
Taxonomic notes. This species closely resembles C.knipowitschii concerning shape, size, and sculpture. According to Boeters et al. (2015), the two species differ in the degree of cover of the umbilicus, the shape of the peristome and the size and number of whorls of the protoconch. Molecular and/or more in-depth morphological and anatomical studies are required to confirm that these apparently minor differences are sufficient to separate the species.
Remarks. If the species would be confirmed, it concerns a Pontocaspian species whose distribution currently is outside prime Pontocaspian habitat, yet Boeters et al. (2015) implied they would expect that several of the Caspia records from the lower Danube and Razim Lake complex might be attributed to C.milae as well. The Razim Lake complex is Pontocaspian habitat.
Conservation status. Not assessed.
Clathrocaspiapallasii (Clessin & Dybowski in Dybowski, 1887)
*1887 Caspia Pallasii Clessin & Dybowski in Dybowski: 37.
1888 Caspia Pallasii n. sp. – Dybowski: 79, pl. 3, fig. 3a, b.
1969 Pyrgula (Caspia) pallasii (Cless. & W. Dyb.). – Logvinenko and Starobogatov: 378, fig. 367(5).
2016 Pyrgulapallasii (Clessin & W. Dybowski in W. Dybowski, 1888). – Vinarski and Kantor: 241.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Endemic to the Caspian Sea.
Taxonomic notes. This species differs from the other Caspian species C.gmelinii in its very slender shape.
Conservation status. Not assessed.
Ulskiabehningi (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Ulskia) behningi Logvinenko & Starobogatov: 380, fig. 367(13).
2016 Pyrgulabehningi Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 236.
Status. Pontocaspian species, identity uncertain.
Type locality. Western part of the southern Caspian Sea, in the vicinity of the Kura River mouth, 39°05'N, 49°48'E, 120 m.
Distribution. Type locality only.
Taxonomic notes. The drawings provided by Logvinenko and Starobogatov (1969) sketch a broad and conical shell. As such, it differs from the more elongate and ovoid Ulskiaulskii (Neubauer et al. 2018). A revision is required to clarify its taxonomic status.
Conservation status. Data Deficient (Vinarski 2011f).
? Ulskiaderzhavini (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Ulskia) derzhavini Logvinenko & Starobogatov: 379, fig. 367(9).
2016 Pyrguladerzhavini Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 237.
Status. Pontocaspian species, identity uncertain.
Type locality. Middle and southern Caspian Sea, 45–81 m.
Distribution. Type locality only.
Taxonomic notes. The species differs from U.ulskii and U.behningi in the very slender elongate shape and the presence of a subsutural band; this suggests P.derzhavini might be likely a member of Caspia s.s. A revision is required to clarify its taxonomic status and generic placement.
Conservation status. Not assessed.
Ulskiaulskii (Clessin & Dybowski in Dybowski, 1887)
*1887 CaspiaUlskii Clessin & Dybowski in Dybowski: 38–39.
1888 [Caspia] Ulskii n. sp. – Dybowski: 79, pl. 3, fig. 8a, b.
1969 Pyrgula (Ulskia) nana Logvinenko and Starobogatov: 379–380, fig. 367(12).
1969 Pyrgula (Ulskia) schorygini Logvinenko and Starobogatov: 379, fig. 367(11).
2016 Pyrgulaulskii (Clessin & W. Dybowski in W. Dybowski, 1888). – Vinarski and Kantor: 244.
2018 Ulskiaulskii (Clessin & W. Dybowski in W. Dybowski, 1887). – Neubauer et al.: 52–54, fig. 5A–K [and synonyms therein].
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Western part of the Caspian Sea. This species was mentioned from depths between 200 and 400 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Turricaspiaulskii, T.schorgyni, and T.nana).
Taxonomic notes. This species was recently studied by Neubauer et al. (2018), who considered P.nana and P.schorygini as its junior synonyms.
Conservation status. Not assessed.
Subfamily Hydrobiinae Stimpson, 1865
Remarks. In addition to the taxa discussed below, the following species of Hydrobiinae have been mentioned from the Black Sea basin (updated statuses after MolluscaBase 2018a): Hydrobiaaciculina (Bourguignat, 1876), H.acuta (Draparnaud, 1805), H.euryomphala (Bourguignat, 1876), H.mabilli (Bourguignat, 1876) [currently accepted as Peringiamabilli], H.macei Paladilhe, 1867 [currently accepted as Heleobiamacei], H.procerula (Paladilhe, 1869) [currently considered a synonym of H.acuta] (Anistratenko et al. 2011). These species were described from the Western Mediterranean and their occurrence in the Black Sea region requires re-investigation; partly the records might be misidentifications of the species of Ecrobia listed below or Eupaludestrina (Cochliopidae) listed above.
Ecrobiagrimmi (Clessin in Dybowski, 1887)
*1887 Hydrobiagrimmi Clessin in Dybowski: 55–56.
1888 [Hydrobia] grimmi Clessin. – Dybowski: 79, pl. 3, fig. 2.
2009 Caspiohydrobiagrimmi (Clessin & Dybowski, 1888). – Filippov and Riedel: 70–72, 74–76, fig. 4a–d.
Status. Accepted native Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Caspian Sea; Aral Sea; salt lakes near Chelyabinsk, Russia (Shishkoedova 2010); Lake Sawa, Iraq (Haase et al. 2010); Arabian (Persian) Gulf (Glöer and Pešić 2012); possibly also northern and central Kazakhstan and Tajikistan (Vinarski and Kantor 2016), however, no molecular data are known to confirm the identity of the Central Asian snails. This species was mentioned from depths between 200 and 500 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Caspiohydrobiacurta and C.gemma).
Taxonomic notes. Most of the species that have been assigned to the genus Caspiohydrobia Starobogatov, 1970, including its type species, Pyrgohydrobiaeichwaldiana Golikov & Starobogatov, 1966, range within the morphological variability of E.grimmi. Previous examination of some Caspiohydrobia juvenile shells (Filippov and Riedel 2009, Anistratenko 2013, fig. 4A–C) as well as reproductive systems and radula did not find any criteria to support differentiation. Probably, all of the thirty Caspiohydrobia species listed by Kantor and Sysoev (2006) and Vinarski and Kantor (2016) for the Caspian Sea are morphotypes of a single species. Prelimary genetic analyses of Caspiohydrobia spp. from salt lakes near Chelyabinsk, Russia (TW, unpublished data) support this assumption.
Conservation status. Data Deficient (Vinarski 2011b).
Ecrobiamaritima (Milaschewitch, 1916)
*1916 Hydrobiamaritima Milaschewitch: 60–61, pl. 2, fig. 34.
1973 Hydrobiapontieuxini Radoman: 15–16.
1977 Ventrosiapontieuxini (Radoman, 1973). – Radoman: 210, pl. 21, figs 19, 20.
1992 Pseudopaludinellacygnea Anistratenko in Anistratenko and Prisyazhniuk: 17, fig. 1a.
1992 Pseudopaludinellainflata Anistratenko in Anistratenko and Prisyazhniuk: 17–18, fig. 1b.
1992 Pseudopaludinellaismailensis Anistratenko in Anistratenko and Prisyazhniuk: 18, fig. 1c.
2011 Pseudopaludinellapontieuxini (Radoman, 1973). – Anistratenko et al.: 78, pl. 3, fig. 4.
2015 Graecoanatolicayildirimi Glöer and Pešić: 49–50, figs 10–14.
Status. Accepted, Pontocaspian species.
Type locality. Black Sea, at Feodosiya and Adler (Crimea, Ukraine).
Distribution. Black Sea Basin; northern Aegean Sea; Lake Sarikum, Turkey; northern Adriatic Sea.
Taxonomic notes.Hydrobiapontieuxini, described from the Black Sea coast in Mangalia, Romania, has been considered a synonym of E.maritima based on molecular data (Kevrekidis et al. 2005). Herein, we also consider the Pseudopaludinella species introduced by Anistratenko and Prisyazhniuk (1992) as junior synonyms of E.maritima based on morphological similarities. A proper revision is still pending.
Conservation status. Not assessed.
Ecrobiaventrosa (Montagu, 1803)
*1803 Turboventrosus Montagu: 317, pl. 12, fig. 13.
2012 Ecrobiaventrosa (Montagu, 1803). – Kadolsky: 69–70.
2012 Hydrobiaventrosa (Montagu, 1803). – Welter-Schultes: 40, unnumbered text figures.
Status. Accepted, immigrant species.
Type locality. On the Kent coast (United Kingdom), at Folkstone and Sandwich.
Distribution. Widespread along the coastal zones of northern and western Europe, the Mediterranean Sea, the Russia White Sea; introduced into the western Black Sea.
Taxonomic notes. Unpublished genetic data (TW) suggest that most previous records of E.ventrosa in the Black Sea are likely misidentifications of E.grimmi. A notable exception is a recent, genetically confirmed record from Constanța, Romania (Osikowski et al. 2016). Probably, the French species Paludestrinaarenarum Bourguignat, 1876, P.leneumicra Bourguignat, 1876, P.paludinelliformis Bourguignat, 1876, and Ventrosiacissana Radoman, 1977, which have been listed for the Black Sea Basin (Anistratenko 1991, Anistratenko and Prisyazhniuk 1992, Anistratenko et al. 2011), are junior synonyms or misidentifications of this species.
Conservation status. Least Concern (Van Damme 2011a).
Subfamily Pyrgulinae Brusina, 1882
Remarks. The genus concepts of Pontocaspian Pyrgulinae follow the revision of Neubauer et al. (2018). Further change is expected in several of the keeled species here listed under ?Turricaspia (?T.aenigma, ?T.basalis, ?T.dimidiata, ?T.pseudobacuana, and ?T.pseudodimiata) that may be grouped in their own genus for which the name Trachycaspia Dybowski & Grochmalicki, 1917 (type species: Rissoadimidiata Eichwald, 1838) is available. However, such a decision will require further documentation.
Clessiniolavariabilis (Eichwald, 1838)
*1838 Paludinavariabilis Eichwald: 151–152.
1838 Paludina Triton Eichwald: 152.
1874 Bithynia? Eichwaldi Martens: 81.
?1887 CaspiaGrimmi Clessin and Dybowski in Dybowski: 39
?1888 [Caspia] Grimmi n. sp. – Dybowski: 79, pl. 3, fig. 5a, b.
1887 Clessinia Martensii Clessin and Dybowski in Dybowski: 43.
1888 Clessinia Martensii n. sp. – Dybowski: 79, pl. 2, fig. 5.
1902a Clessiniaahngeri Westerlund: 45–46.
1966 P.[yrgula] (Clessiniola) pseudotriton Golikov and Starobogatov: 356–357, fig. 2(3
?1969 Pyrgula (Caspiella) derbentina Logvinenko and Starobogatov: 374, fig. 366(8).
1969 Pyrgula (Caspiella) ovum Logvinenko and Starobogatov: 374, fig. 366(9).
1969 Pyrgula (Caspiella) trivialis Logvinenko and Starobogatov: 374–375, fig. 366(10).
1987 Turricaspia (Clessiniola) variabilis (Eichwald, 1838). – Alexenko and Starobogatov: 34, text fig. 5.
1987 Turricaspia (Clessiniola) triton (Eichwald, 1838). – Alexenko and Starobogatov: 34, text fig. 3.
1987 Turricaspia (Clessiniola) martensii (Clessin & Dybowski in Dybowski, 1888). – Alexenko and Starobogatov: 34, text fig. 4.
1987 Turricaspia (Clessiniola) bogensis (Küster, 1852). – Alexenko and Starobogatov: 34.
2006 Turricaspiavariabilis (Eichwald, 1838). – Kantor and Sysoev: 111, pl. 49, fig. J.
2011 Turricaspiamartensii (Clessin & W. Dybowski in W. Dybowski, 1888). – Anistratenko et al.: 86, fig. 3(17).
2011 Turricaspiatriton (Eichwald, 1838). – Anistratenko et al.: 85–86, fig. 3(16).
2011 Turricaspiavariabilis (Eichwald, 1838). – Anistratenko et al.: 85, fig. 3(15).
2014 Turricaspiavariabilis. – Taviani et al.: 4, fig. 3b.
?2016 Turricaspiaderbentina (Logvinenko & Starobogatov, 1968). – Vinarski and Kantor: 247.
2016 Turricaspiamartensii (Clessin & W. Dybowski in W. Dybowski, 1888). – Vinarski and Kantor: 248.
2016 Turricaspiaovum (Logvinenko & Starobogatov, 1968). – Vinarski and Kantor: 248–249.
2016 Turricaspiapseudotriton (Golikov & Starobogatov, 1966). – Vinarski and Kantor: 249.
2016 Turricaspiatriton (Eichwald, 1838). – Vinarski and Kantor: 250.
2016 Turricaspiatrivialis (Logvinenko & Starobogatov, 1968). – Vinarski and Kantor: 250–251.
2016 Turricaspiavariabilis (Eichwald, 1838).– Vinarski and Kantor: 251.
2018 Clessiniolavariabilis (Eichwald, 1838). – Neubauer et al.: 60–63, fig. 7A–I.
Status. Accepted Pontocaspian species.
Type locality. At the Volga River mouth near Astrakhan, and towards the Caspian Sea; also in recently lithified fossil limestone at the shores of Dagestan, Russia.
Distribution. Caspian Sea, Azov Sea, and northern Black Sea region. This species was mentioned in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Turricaspiavariabilis, T.derbentica, and T.trivialis).
Taxonomic notes.Neubauer et al. (2018) recently demonstrated the high variability of this species. Comparison of available illustrations and descriptions of the species listed in the synonymy list indicates that all of them range within this species’ variability. Consequently, we consider all of them as junior synonyms of C.variabilis. A more in-depth review of the type material of the species involved is required to confirm this approach.
The status of Paludinabogensis Dubois in Küster, 1852, which was listed as a valid species of Turricaspia by Anistratenko and Stadnichenko (1995), is still unclear. That species was described from the Zapadnyi Bug River in Poland and closely resembles C.variabilis. It is, however, unlikely that a Pontocaspian species typical of oligohaline conditions occurs so far away in a pure freshwater environment. “Paludinaeichwaldi Krynicki, 1837” found in the literature is a nomen nudum. Martens (1874) provided measurements and made the name available, but he listed Paludinavariabilis Eichwald, 1838 in synonymy, which has priority. Dybowski (1887) obviously overlooked this and considered Nematurellaeichwaldi Krynicki a valid species. We follow Vinarski and Kantor (2016) and consider the species as a junior synonym of Clessiniolavariabilis.
Conservation status. Least Concern (Cioboiu et al. 2011).
Laevicaspiaabichi (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Caspiella) abichi Logvinenko & Starobogatov: 372, fig. 366(3).
2016 Pyrgulaabichi Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 235.
Status. Accepted Pontocaspian species.
Type locality. Southern and western parts of the Middle Caspian Sea, 36–120 m.
Distribution. Middle and South Caspian Basin. This species was mentioned from depths between 200 and 400 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Turricaspiaabichi).
Taxonomic notes. The species differs from the L.cincta in its much larger size, the conical shape, the narrower subsutural band, and the larger aperture (compare Neubauer et al. 2018).
Conservation status. Data Deficient (Vinarski 2011e).
Laevicaspiacaspia (Eichwald, 1838)
*1838 Rissoacaspia Eichwald: 154–155.
non 1888 Micr. [omelania] caspia Eichw. sp. – Dybowski: 78, pl. 1, fig. 1.
?1896 B. [uliminus] (Napaeus?) goebeli Westerlund: 188.
1915 Micromelania (?) curta Nalivkin: 21–22, 31, pl. 6, figs 1, 2 [pars, non figs 3, 4, 7, 9–14].
1915 [Micromelania (?) curta] var. plano-convexa Nalivkin: 22, 31, pl. 6, figs 15–18.
non 1915 Micromelaniacaspia Eichw. – Nalivkin: 22, 31, pl. 6, figs 5, 6 [pars, non fig. 8].
non 1917 Micromelania (Turricaspia, Laevicaspia) caspia Eichw. – Dybowski and Grochmalicki: 5–8, 36–38, pl. 1, figs 1–3.
non 1969 Pyrgulacaspia (Eichw.). – Logvinenko and Starobogatov: 369–370, fig. 364(1).
2006 Turricaspiacaspia (Eichwald, 1838). – Kantor and Sysoev: 106, pl. 49, fig. M.
2016 Turricaspiacaspia (Eichwald, 1838). – Vinarski and Kantor: 246.
2018 Laevicaspiacaspia (Eichwald, 1838). – Neubauer et al.: 63–66, fig. 8A–K [and synonyms therein].
Status. Accepted Pontocaspian species.
Type locality. In fossil limestone of Dagestan, Russia.
Distribution. Endemic to the Caspian Sea. This species was mentioned from depths between 200 and 500 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Turricaspiacaspia and T.curta).
Taxonomic notes. For a detailed discussion about the identity of this species, its synonyms and former misidentifications, see Neubauer et al. (2018).
Conservation status. IUCN indicates “Least Concern” (Vinarski 2012), but the true status of this species is highly uncertain.
Laevicaspiacincta (Abich, 1859)
*1859 Rissoacincta Abich: 57, pl. 2, fig. 6.
?1887 CaspiaOrthii Clessin & Dybowski in Dybowski: 40.
?1888 [Caspia] Orthii n. sp. – Dybowski: 79, pl. 3, fig. 6.
1969 Pyrgula (Caspiella) cincta (Abich). – Logvinenko and Starobogatov: 372, fig. 366(4).
2006 Pyrgulacincta (Abich, 1859). – Kantor and Sysoev: 98, pl. 47, fig. L.
2016 Pyrgulacincta (Abich, 1859). – Vinarski and Kantor: 236–237.
2018 Laevicaspiacincta (Abich, 1859). – Neubauer et al.: 66–68, fig. 9A–H.
Status. Accepted Pontocaspian species.
Type locality. Gulf of Baku, Azerbaijan.
Distribution. Southern Caspian Sea (Logvinenko and Starobogatov 1969).
Taxonomic notes. For a detailed discussion about the identity of this species and its synonym, see Neubauer et al. (2018).
Conservation status. Data Deficient (Vinarski 2011g).
Laevicaspiaconus (Eichwald, 1838)
*1838 RissoaConus Eichwald: 155.
non 1876 Eulimaconus, Eichw?. – Grimm: 154–156, pl. 6, fig. 14.
non 2006 Turricaspiaconusconus (Eichwald, 1838). – Kantor and Sysoev: 106, pl. 48, fig. J.
2016 Turricaspiaconusconus (Eichwald, 1838). – Vinarski and Kantor: 246–247.
2018 Laevicaspiaconus (Eichwald, 1838). – Neubauer et al.: 69–71, fig. 9I–P [and synonyms therein].
Status. Accepted Pontocaspian species.
Type locality. In fossil limestone of Dagestan, Russia.
Distribution. Endemic to the Caspian Sea (Logvinenko and Starobogatov 1969). This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Turricaspiaconus).
Taxonomic notes. For a detailed discussion about the identity of this polymorphic species and previous misidentifications, see Neubauer et al. (2018).
Conservation status. Data Deficient (Vinarski 2011p).
? Laevicaspiaebersini (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Oxypyrgula) ebersini Logvinenko & Starobogatov: 368, fig. 363(7).
2016 Pyrgulaebersini Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 238.
Status. Pontocaspian species, identity uncertain.
Type locality. Western part of the middle Caspian Sea, 0–50 m water depth.
Distribution. Type locality only.
Taxonomic notes. We cannot verify the status of this species given the inadequate descriptions and illustrations and its general resemblance to other species that were described earlier.
Conservation status. Data Deficient (Vinarski 2011h).
? Laevicaspiaismailensis (Golikov & Starobogatov, 1966)
*1966 P. [yrgula] ismailensis Golikov & Starobogatov: 358, fig. 2(11).
2006 Turricaspiaismailensis (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 108, pl. 50, fig. A.
2016 Turricaspiaismailensis (Golikov & Starobogatov, 1966). – Vinarski and Kantor: 248.
Status. Accepted Pontocaspian species.
Type locality. Ukraine, Danube Delta, lakes Yalpug and Kugurlui.
Distribution. North-western Black Sea Basin (Anistratenko and Stadnichenko 1995).
Taxonomic notes. Based on the illustration of the holotype in Kantor and Sysoev (2006), we tentatively place the species in the genus Laevicaspia. A more detailed study is necessary to clarify its systematic position.
Conservation status. Vulnerable (Son and Cioboiu 2011).
Laevicaspiakolesnikoviana (Logvinenko & Starobogatov in Golikov & Starobogatov, 1966)
*1966 P. [yrgula] (Caspiella) kolesnikoviana Golikov & Starobogatov: 357–358, fig. 2(8–9).
1969 Pyrgula [(Caspiella)] kolesnikoviana Logv. & Star. – Logvinenko and Starobogatov: 372, fig. 366(1).
2006 Pyrgulakolesnikoviana Logvinenko & Starobogatov in Golikov & Starobogatov, 1966. – Kantor and Sysoev: 100, pl. 47, fig. N.
2016 Pyrgulakolesnikoviana Logvinenko & Starobogatov in Golikov & Starobogatov, 1966. – Vinarski and Kantor: 239.
2018 Laevicaspiakolesnikoviana (Logvinenko & Starobogatov in Golikov & Starobogatov, 1966). – Neubauer et al.: 71–73, fig. 10A–E, K, N.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea, northward of Apsheron Peninsula, north-westward from Kamni Dva Brata Island, 40°47'N, 49°42'E, 30 m water depth.
Distribution. Endemic to the Caspian Sea. This species was mentioned from depths between 200 and 400 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Turricaspiakolesnikoviana).
Taxonomic notes. For a detailed discussion about the identity of this species, see Neubauer et al. (2018).
Conservation status. Data Deficient (Vinarski 2011k).
Laevicaspiakowalewskii (Clessin & Dybowski in Dybowski, 1887)
*1887 CaspiaKowalewskii Clessin & Dybowski in Dybowski: 40–41.
1888 [Caspia] Kowalewskii n. sp. – Dybowski: 79, pl. 3, fig. 9a–c.
2006 Pyrgulakowalewskii (Clessin & W. Dybowski in W. Dybowski, 1888). – Kantor and Sysoev: 100, pl. 47, fig. M.
2016 Pyrgulakowalewskii (Clessin & W. Dybowski in W. Dybowski, 1888). – Vinarski and Kantor: 239–240.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Caspian Sea, recorded from southern basin (Logvinenko and Starobogatov 1969) and middle basin (personal observation based on material from Dagestan region, TAN, FW). This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Turricaspiakowalewskii).
Taxonomic notes. This species differs from L.kolesnikoviana in its bigger size, broader shape, and thinner peristome. Laevicaspiacincta can be distinguished based on the stouter shape and the presence of a narrow subsutural band.
Conservation status. Not assessed.
Laevicaspialencoranica (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Eurycaspia) lencoranica Logvinenko & Starobogatov: 357, fig. 358(14).
2016 Pyrgulalencoranica Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 240.
Status. Pontocaspian species, identity uncertain.
Type locality. Caspian Sea (no details).
Distribution. Caspian Sea (Logvinenko and Starobogatov 1969).
Taxonomic notes. Based on the illustrations provided in Kantor and Sysoev (2006), this species differs from L.cincta and L.kowalewskii in the conical shape and large body whorl. A revision is required to assure its status as distinct species.
Conservation status. Not assessed.
Laevicaspialincta (Milaschewitch, 1908)
*1908 Micromelanialincta Milaschewitch: 991.
?1966 P.[yrgula] (Caspiella) azovica Golikov and Starobogatov: 357, fig. 2(7).
?1966 P.[yrgula] (Caspiella) boltovskoji Golikov and Starobogatov: 357, fig. 2(4).
?1966 P.[yrgula] (Caspiella) crimeana Golikov and Starobogatov: 358, fig. 2(10).
?1966 P.[yrgula] (Caspiella) limanica Golikov and Starobogatov: 357, fig. 2(6).
?1966 P.[yrgula] (Caspiella) lindholmiana Golikov and Starobogatov: 357, fig. 2(5).
?1966 P.[yrgula] (Laevicaspia) iljinae Golikov and Starobogatov: 358–359, fig. 2(14).
?1966 P.[yrgula] (Laevicaspia) milachevitchi Golikov and Starobogatov: 359, fig. 2(15).
?1966 P.[yrgula] (Laevicaspia) ostroumovi Golikov and Starobogatov: 358, fig. 2(13).
?1966 P.[yrgula] (Turricaspia) borceana Golikov and Starobogatov: 359, fig. 2(16).
?1966 P.[yrgula] (Turricaspia) nevesskae Golikov and Starobogatov: 359, fig. 2(17).
?1987 Turricaspiaabichiphaseolinica Alexenko and Starobogatov: 33.
?1987 Turricaspia (Caspiella) derbentinaborysthenica Alexenko adn Starobogatov: 34–35, fig. 6.
?1987 Turricaspia (Laevicaspia) grigorievi Alexenko and Starobogatov: 35, fig. 7.
?1987 Turricaspia (Laevicaspia) meneghinianaukrainica Alexenko and Starobogatov: 35, fig. 9.
?2006 Euxinipyrgulaazovica (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 95, pl. 44, fig. K.
?2006 Euxinipyrgulaborysthenica (Alexenko & Starobogatov, 1987). – Kantor and Sysoev: 95, pl. 44, fig. J.
?2006 Euxinipyrgulagrigorievi (Alexenko & Starobogatov, 1987). – Kantor and Sysoev: 95, pl. 44, fig. I.
?2006 Euxinipyrgulalimanica (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 95, pl. 44, fig. H.
2006 Euxinipyrgulalincta (Milaschewitsch, 1908). – Kantor and Sysoev: 95–96, pl. 45, fig. D.
?2006 Euxinipyrgulamilachevitchi (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 96, pl. 45, fig. C.
?2006 Euxinipyrgulaostroumovi (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 96, pl. 45, fig. B.
?2006 Euxinipyrgulaukrainica (Alexenko & Starobogatov, 1987). – Kantor and Sysoev: 95, pl. 45, fig. A.
?2006 Turricaspiaboltovskoji (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 105–106, pl. 48, fig. K.
?2006 Turricaspiaborceana (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 106, pl. 49, fig. B.
?2006 Turricaspiaconuslindholmiana (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 107, pl. 48, fig. L.
?2006 Turricaspiacrimeana (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 107, pl. 48, fig. C.
?2006 Turricaspiailjinae (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 108, pl. 49, fig. D.
?2006 Turricaspianevesskae (Golikov & Starobogatov, 1966). – Kantor and Sysoev: 109, pl. 49, fig. L.
Status. Accepted Pontocaspian species.
Type locality. Kotlabukh Lake, Odessa Region, Ukraine (approximately 45°25'35"N, 28°59'41"E).
Distribution. Limans and lower reaches of rivers Don, Dnieper, Dniester, and Southern Bug entering the northern Black Sea Basin and the Azov Sea (Taganrog Bay), as well as in coastal lakes Kotlabukh and Yalpug (Vinarski and Kantor 2016). The record of an undescribed subspecies of T.boltovskoji from the Caspian Sea mentioned by Anistratenko and Stadnichenko (1995) is probably based on a misidentification.
Taxonomic notes.Golikov and Starobogatov (1966) and Alexenko and Starobogatov (1987) introduced a plethora of names for morphologically similar species from the northern Black Sea Basin, partly deriving from subfossil horizons. They differ from Laevicaspialincta slightly in the number of whorls and outline shape, but overall range within its morphological variability. Here, we consider them tentatively all junior synonyms of L.lincta. Since Starobogatov’s type material is unknown, support for this approach requires collection of new material from the type localities of these taxa. Molecular data confirmed the conspecifity of L.lincta and L.milachevitchi (Wilke et al. 2007).
Conservation status. Least Concern (Son 2011e).
? Laevicaspiamarginata (Westerlund, 1902)
*1902a Nematurellamarginata Westerlund: 45.
2013 Pyrgulamarginata (Westerlund, 1902). – Vinarski et al.: 85, fig. 2F.
2016 Pyrgulamarginata (Westerlund, 1902). – Vinarski and Kantor: 240.
Status. Pontocaspian species, identity uncertain.
Type locality. Caspian Sea, “near Krasnojarsk” (Westerlund 1902a). This statement is clearly erroneous since Krasnojarsk is situated in Siberia. Most probably, Westerlund meant Krasnovodsk (nowadays Turkmenbashi) in Turkmenistan (Vinarski et al. 2013).
Distribution. Endemic to the Caspian Sea. This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Turricaspiamarginata).
Taxonomic notes. The status of this species is uncertain. The illustrations of the type material by Vinarski et al. (2013) suggest a tentative placement in the genus Laevicaspia. It shows close similarities with L.sieversii (Clessin in Dybowski, 1887). A careful revision of the species is required to clarify its taxonomic status and systematic placement.
Conservation status. Not assessed.
Laevicaspiasieversii (Clessin in Dybowski, 1887)
*1887 Nematurella Sieversii Clessin in Dybowski: 45–46.
1888 Nematurella Sieversi [sic] n. sp. – Dybowski: 78, pl. 2, fig. 1.
Status. Pontocaspian species, identity uncertain.
Type locality. Caspian Sea (no details).
Distribution. Endemic to the Caspian Sea.
Taxonomic notes. This species has not been found since its first description, its identity is unclear (Vinarski and Kantor 2016). Judging from the description and drawing in Dybowski (1887), we suggest a systematic placement in Laevicaspia. It might be related to L.conus (Eichwald, 1838).
Conservation status. Not assessed.
? Turricaspiaaenigma (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Celekenia) aenigma Logvinenko & Starobogatov: 375, fig. 366(12).
2016 Pyrgulaaenigma Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 235.
Status. Pontocaspian species, identity uncertain.
Type locality. Caspian Sea, northward of Apsheron Peninsula, 75 m.
Distribution. Type locality only.
Taxonomic notes. The identity of this species is unclear. The illustrations of the holotype in Kantor and Sysoev (2006) show a small shell with four whorls, of which the latter two bear a distinct keel. The small size and the relatively large protoconch suggest that the type specimen is a juvenile shell. More specimens (including adult material) are required to shed light on this species’ identity.
Conservation status. Not assessed.
Turricaspiaandrussowi (Dybowski & Grochmalicki, 1915)
*1915 Micromelania (Turricaspia) andrussowi Dybowski & Grochmalicki: 125–126, pl. 3, fig. 31a, b.
?1969 Pyrgula (Oxypyrgula) dubia Logvinenko and Starobogatov: 368, fig. 363(5).
?1969 Pyrgula (Oxypyrgula) turkmenica Logvinenko and Starobogatov: 368, fig. 363(6).
2006 Turricaspiaandrussowi (B. Dybowski & Grochmalicki, 1915). – Kantor and Sysoev: 104–105, pl. 48, fig. A [pars, excluding synonymy].
2016 Turricaspiaandrussowi (B. Dybowski & Grochmalicki, 1915). – Vinarski and Kantor: 245 [pars, excluding synonymy].
2018 Turricaspiaandrussowi (B. Dybowski & Grochmalicki, 1915). – Neubauer et al.: 74–76, fig. 11A, BB.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Endemic to the Caspian Sea. The two tentative synonyms were recorded from the western part of the middle Caspian Sea and the eastern part of the southern Caspian Sea, respectively. This species was mentioned from depths between 200 and 500 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as T.turkmenica, T.dubia, and T.andrussowi).
Taxonomic notes. The species was recently investigated by Neubauer et al. (2018). Pyrguladubia and P.turkmenica are tentatively considered juveniles and thus junior synonyms of this species.
Conservation status. Not assessed.
? Turricaspiabasalis (Dybowski & Grochmalicki, 1915)
*1915 Micromelaniadimidiatavar.basalis Dybowski & Grochmalicki: 131, pl. 3, fig. 36a, b.
1969 Pyrgula (Trachycaspia) laticarinata Logvinenko and Starobogatov: 359, fig. 359(3).
2006 Pyrgulabasalisbasalis (B. Dybowski & J. Grochmalicki, 1915). – Kantor and Sysoev: 97, pl. 46, fig. A.
2006 Pyrgulabasalislaticarinata Logvinenko & Starobogatov, 1968. – Kantor and Sysoev: 97, pl. 46, fig. B.
2016 Pyrgulabasalisbasalis (B. Dybowski & Grochmalicki, 1915). – Vinarski and Kantor: 236.
2016 Pyrgulabasalislaticarinata Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 236.
Status. Pontocaspian species, identity uncertain.
Type locality. Caspian Sea (no details).
Distribution. Middle and southern Caspian Sea (Logvinenko and Starobogatov 1969). This species was mentioned from depths between 200 and 400 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as T.laticarinata).
Taxonomic notes. The species is characterised by a massive keel near the lower suture. ?Turricaspiadimidiata is distinguished based on its more centrally placed keel. This distinction is tentative and only based on comparison of available illustrations; we are aware of the possibility that these differences might not be diagnostic. Moreover, the keel seems to become stronger with increasing water depth (Starobogatov 1968). Pyrgulalaticarinata Logvinenko & Starobogatov, 1969, which differs from T.basalis only in the strength of the keels, was considered a junior synonym by Neubauer et al. (2018).
Conservation status. Not assessed.
? Turricaspiabogatscheviana (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Oxypyrgula) bogatscheviana Logvinenko & Starobogatov: 367, fig. 363(2).
2016 Turricaspiabogatscheviana (Logvinenko & Starobogatov, 1968). – Vinarski and Kantor: 245.
Status. Pontocaspian species, identity uncertain.
Type locality. Western part of the Caspian Sea.
Distribution. Type locality only.
Taxonomic notes. The description and drawing of this species provided by Logvinenko and Starobogatov (1969) do not allow an evaluation whether it is a distinct species or synonym of a previously species.
Conservation status. Not assessed.
Turricaspiachersonica Alexenko & Starobogatov, 1987
*1987 Turricaspia (Oxypyrgula) chersonica Alexenko & Starobogatov: 35–36, fig. 10.
2016 Turricaspiachersonica Alexenko & Starobogatov, 1987. – Vinarski and Kantor: 246.
Status. Pontocaspian species, identity uncertain.
Type locality. Ukraine, in the Dnieper Delta.
Distribution. Type locality only.
Taxonomic notes. The status of this species is highly uncertain. The slender conical shell illustrated by Alexenko and Starobogatov (1987) suggest classification in the genus Turricaspia, which is otherwise only known from the Caspian Sea.
Conservation status. Data Deficient (Son 2011d).
Turricaspiacolumna (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Oxypyrgula) columna Logvinenko & Starobogatov: 368, fig. 363(8).
2016 Pyrgulacolumna Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 237.
Status. Pontocaspian species, identity uncertain.
Type locality. Western part of the southern Caspian Sea.
Distribution. Type locality only.
Taxonomic notes. The species has not been found since its first description, and the whereabouts of the type material is unknown. Logvinenko and Starobogatov (1969) illustrate a small slender shell with convex whorls. It might well be a juvenile of another species.
Conservation status. Not assessed.
Turricaspiaconcinna (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Turricaspia) concinna Logvinenko & Starobogatov: 365, fig. 362(3).
2016 Pyrgulaconcinna Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 237.
Status. Pontocaspian species, identity uncertain.
Type locality. Middle Caspian Sea, 25–80 m.
Distribution. Type locality only.
Taxonomic notes. The illustrations provided by Logvinenko and Starobogatov (1969) indicate a large conical shell with nine convex whorls and a large, slightly inflated last whorl. These features are reminiscent of T.meneghiniana (Issel, 1865). However, T.concinna has not been found since its first description. The type material has been very recently detected in the collections of ZIN and awaits further study.
Conservation status. Not assessed.
Turricaspiadagestanica (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Turricaspia) dagestanica Logvinenko & Starobogatov: 361, fig. 360(3).
2016 Turricaspiadagestanica (Logvinenko & Starobogatov, 1968). – Vinarski and Kantor: 247.
Status. Pontocaspian species, identity uncertain.
Type locality. Western shore of the middle Caspian Sea.
Distribution. Middle and south Basin of Caspian Sea. This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. The status of this species is highly uncertain. The illustrations of Logvinenko and Starobogatov (1969) show a slightly distorted shell with weakly convex whorls and a thin line below the suture. We are uncertain whether it might concern a growth aberration of a more common species.
Conservation status. Data Deficient (Vinarski 2011r).
Turricaspiadimidiata (Eichwald, 1838)
*1838 Rissoadimidiata Eichwald: 156.
?1947 Turricaspiabakuana Kolesnikov: 108, 112.
2006 Pyrguladimidiata (Eichwald, 1838). – Kantor and Sysoev: 99, pl. 46, fig. K.
?2006 Pyrgulabakuana (Kolesnikov, 1947). – Kantor and Sysoev: 97, pl. 47, fig. C.
2016 Pyrguladimidiata (Eichwald, 1838). – Vinarski and Kantor: 238.
2016 Pyrgulabakuana (Kolesnikov, 1947). – Vinarski and Kantor: 236–237.
Status. Accepted Pontocaspian species.
Type locality. In fossil limestone of Dagestan, Russia.
Distribution. Middle and southern Caspian Sea (Logvinenko and Starobogatov 1969). This species was mentioned from depths between 200 and 500 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. Although there is little doubt about the validity of this species, its true identity and possible synonyms are unclear. Eichwald’s (1838) description clearly indicates a slender shell with median keel. His type material is unfortunately unknown. The high number of keeled species complicates an evaluation what is the “true” T.dimidiata and what are synonyms. We tentatively consider Turricaspiabakuana Kolesnikov, 1947 a junior synonym of this species, based on its slender shell with median keel matching Eichwald’s description as well as the prevailing concept of T.dimidiata (compare Kantor and Sysoev 2006). More data are required to support this view.
Conservation status. Not assessed.
Turricaspiaeburnea (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Laevicaspia) eburnea Logvinenko & Starobogatov: 370, fig. 365(1).
2016 Turricaspiaeburnea (Logvinenko & Starobogatov, 1968). – Vinarski and Kantor: 247.
Status. Pontocaspian species, identity uncertain.
Type locality. Eastern part of the southern Caspian Sea.
Distribution. South Caspian Basin. This species was mentioned from depths between 200 and 500 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. The identity of this species is unclear. Its shell resembles T.lyrata (Dybowski & Grochmalicki, 1915) in terms of general shape and the large, flat protoconch; it differs from that species in the large size. The type material has been very recently found in the collection of ZIN and awaits further study. Until then, we refrain from a final decision on the species’ status, but we have severe doubt that Pyrgulaeburnea is a distinct species.
Conservation status. Not assessed.
Turricaspiaelegantula (Clessin & Dybowski in Dybowski, 1887)
*1887 Micromelaniaelegantula Clessin & Dybowski in Dybowski: 33.
1888 [Micromelania] elegantula n. sp. – Dybowski: 78, pl. 1, fig. 7a–c.
2016 Turricaspiaelegantula (Clessin & W. Dybowski in W. Dybowski, 1888). – Vinarski and Kantor: 247–248.
Status. Pontocaspian species, identity uncertain.
Type locality. Caspian Sea (no details).
Distribution. Endemic to the Caspian Sea. This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. There is considerable confusion about the identity of this species. Dybowski (1887) described and illustrated a very slender shell with a distinct whorl profile showing a straight-sided upper half and a convex lower half. In contrast, the illustrations in Logvinenko and Starobogatov (1969) suggest a similarly slender yet distorted shell with near almost sided whorls and expanded aperture. A restudy of the type material of T.elegantula show close similarities to T.spica. It differs from that species in the more slender outline and flattened whorls.
Conservation status. Not assessed.
Turricaspiaeulimellula (Dybowski & Grochmalicki, 1915)
*1915 Micromelania (Turricaspia) eulimellula Dybowski & Grochmalicki: 123–125, pl. 3, fig. 27a, b.
2006 Pyrgulaeulimellula (B. Dybowski & J. Grochmalicki, 1915). – Kantor and Sysoev: 99–100, pl. 46, fig. L.
2016 Pyrgulaeulimellula (B. Dybowski & Grochmalicki, 1915). – Vinarski and Kantor: 238–239.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Middle Caspian Sea Basin (Logvinenko and Starobogatov 1969). This species was mentioned from depths between 200 and 400 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. The nearly straight-sided, strongly attached whorls easily distinguish this species from most other Turricaspia species. Only Turricaspiagrimmi (Clessin & Dybowski in Dybowski, 1887) has a similar whorl arrangement, but its shell is slightly wider and the whorls are weakly stepped and bear a thin subsutural band.
Conservation status. Not assessed.
Turricaspiafedorovi (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Turricaspia) fedorovi Logvinenko & Starobogatov: 362, fig. 360(2).
2016 Pyrgulafedorovi Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 239.
Status. Pontocaspian species, identity uncertain.
Type locality. Western part of the middle Caspian Sea, 80 m.
Distribution. Middle and South Caspian Basin. This species was mentioned from depths between 200 and 400 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. The slender elongate shell with whorls slowly increasing in height distinguishes this species from its congeners. However, a proper assessment of the species’ status requires investigation. The whereabouts of the type material is unknown and no other records of this species are known, so we are not able to verify the status of this species.
Conservation status. Not assessed.
Turricaspiagrimmi (Clessin & Dybowski in Dybowski, 1887)
*1887 MicromelaniaGrimmi Clessin & Dybowski in Dybowski: 27–29.
1888 [Micromelania] Grimmi n. sp. – Dybowski: 78, pl. 1, fig. 2a–c.
2006 Pyrgulagrimmi (Clessin & W. Dybowski in W. Dybowski, 1888). – Kantor and Sysoev: 100, pl. 46, fig. L.
2016 Pyrgulagrimmi (Clessin & W. Dybowski in W. Dybowski, 1888). – Vinarski and Kantor: 239.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Southern Caspian Sea Basin (Logvinenko and Starobogatov 1969). This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. The peculiar morphology with straight-sided, weakly stepped whorls with a thin subsutural band is unique among Caspian Pyrgulinae. See above for a comparison with T.eulimellula.
Conservation status. Data Deficient (Vinarski 2011i).
Turricaspialyrata (Dybowski & Grochmalicki, 1915)
*1915 Micromelania (Turricaspia) spicavar.lyrata Dybowski & Grochmalicki: 117, pl. 2, fig. 18.
2006 Pyrgulalirata [sic] (B. Dybowski & J. Grochmalicki, 1915). – Kantor and Sysoev: 101, pl. 46, fig. E.
2016 Pyrgulalirata [sic] (B. Dybowski & Grochmalicki, 1915). – Vinarski and Kantor: 240.
2018 Turricaspialyrata (B. Dybowski & Grochmalicki, 1915). – Neubauer et al.: 77–79, fig. 12A–K [and synonyms therein].
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Endemic to the Caspian Sea (after Logvinenko and Starobogatov 1969); it occurs in the western part of the middle and southern Caspian Sea basins, but these authors used a slightly different concept of the species. This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Turricaspialirata).
Taxonomic notes. See Neubauer et al. (2018) for a detailed discussion of the species and its synonyms.
Conservation status. Not assessed.
Turricaspiamarisnigri Starobogatov in Alexenko & Starobogatov, 1987
*1987 Turricaspialiratamarisnigri Starobogatov in Alexenko & Starobogatov: 33.
Status. Pontocaspian species, identity uncertain.
Type locality. “Meotida” station 24, sample 229, near the coast of Crimea, in phaseoline silt (Holocene).
Distribution. Type locality only.
Taxonomic notes. The species can be distinguished based on its extremely slender shell with whorls slowly increasing in size. Still, clarification of its identity as well as its generic classification requires investigation of additional material.
Conservation status. So far only known from Holocene deposits of the type locality; species might be extinct. Within Holocene deposits in the Black Sea small amounts of reworked Late Pleistocene “Neoeuxinian” faunas are found (FW, pers. obs.), and therefore the stratigraphic origin of such Pontocaspian species is uncertain.
Turricaspiameneghiniana (Issel, 1865)
*1865 Bythinia Meneghiniana Issel: 21, pl. 1, figs 12, 13.
1902a Micromelaniasubulata Westerlund: 47.
?1969 Pyrgulacaspia (Eichw). – Logvinenko and Starobogatov: 369–370, fig. 364(1) [nonRissoacaspia Eichwald, 1838].
non 1987 T.[urricaspia] meneghiniana meneghiniana (Iss.). – Alexenko and Starobogatov: 35, fig. 8.
2006 Turricaspiameneghiniana (Issel, 1865). – Kantor and Sysoev: 109, pl. 49, fig. E.
2016 Turricaspiameneghiniana (Issel, 1865). – Vinarski and Kantor: 248.
2018 Turricaspiameneghiniana (Issel, 1865). – Neubauer et al.: 79–81, fig. 13A–K [and synonyms therein].
Status. Accepted Pontocaspian species.
Type locality. Baku, Azerbaijan; (sub?)fossil.
Distribution. Middle and southern Caspian Sea (Logvinenko and Starobogatov 1969).
Taxonomic notes. The species was recently discussed in detail by Neubauer et al. (2018), who also discussed previous misidentifications.
Conservation status. Not assessed.
Turricaspianossovi Kolesnikov, 1947
*1947 Turricaspianossovi Kolesnikov: 108, 111.
2006 Pyrgulanossovi (Kolesnikov, 1947). – Kantor and Sysoev: 101, pl. 45, fig. G.
2016 Pyrgulanossovi (Kolesnikov, 1947). – Vinarski and Kantor: 241.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Southern Caspian Sea (Logvinenko and Starobogatov 1969). This species was mentioned from depths between 200 and 500 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. The very slender shape and the characteristic, highly convex whorls that slowly and regularly increase in height distinguish the species from most congeners. Pyrgulavinogradovi Logvinenko & Starobogatov, 1969 and P.astrachanica Pirogov, 1971, which show very similar traits, might be junior synonyms. A more in-depth study is required to solve their statuses.
Conservation status. Data Deficient (Vinarski 2011l).
? Turricaspiaobventicia (Anistratenko in Anistratenko & Prisyazhniuk, 1992)
*1992 Caspia (Clathrocaspia) obventicia Anistratenko in Anistratenko & Prisyazhniuk: 19–20, fig. 2b.
Status. Uncertain Pontocaspian species.
Type locality. Well 37 near Kiliya, Izmail district, Odessa region, Ukraine (from Holocene sediments).
Distribution. Type locality only.
Taxonomic notes. This species was originally attributed to the genus Caspia due to its small shell. A study of the holotype of this species, specifically its protoconch characteristics, suggest placement in the genus Turricaspia. Further studies are required to assure its validity.
Remarks. The species is known only from the holotype. The occurrence of Turricaspia in the Black Sea Basin is unusual, as almost all other pyrguline Black Sea species are assigned to the genus Laevicaspia (but see remark at T.spica for another unusual occurrence).
Conservation status. So far only known from Holocene deposits of the type locality; species might be extinct.
? Turricaspiapseudobacuana (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Eurycaspia) pseudobacuana Logvinenko & Starobogatov: 358, fig. 358(16).
2016 Pyrgulapseudobacuana Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 241.
Status. Pontocaspian species, probably junior synonym.
Type locality. Southern Caspian Sea, 50–80 m.
Distribution. South Caspian Basin. This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. The slender shell with a keel near the lower suture is reminiscent of T.basalis (Dybowski & Grochmalicki, 1915). The short description and poor drawing precluded the verification of its status. The type material has been very recently detected in the collection of ZIN and awaits further study.
Conservation status. Not assessed.
? Turricaspiapseudodimidiata (Dybowski & Grochmalicki, 1915)
*1915 Micromelania (Turricaspia) pseudodimidiata Dybowski & Grochmalicki: 126–128, pl. 3, fig. 32a, b.
?1969 Pyrgula (Eurycaspia) pseudodimidiata (Dyb. et Gr.). – Logvinenko and Starobogatov: 357, fig. 358(15).
?2006 Pyrgulapseudodimidiata (B. Dybowski & Grochmalicki, 1915). – Kantor and Sysoev: 102, pl. 47, fig. G.
2016 Pyrgulapseudodimidiata (B. Dybowski & Grochmalicki, 1915). – Vinarski and Kantor: 241.
Status. Pontocaspian species, identity uncertain.
Type locality. Caspian Sea (no details).
Distribution. Southern Caspian Sea (Logvinenko and Starobogatov 1969). This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. The identity of this species is uncertain. Dybowski and Grochmalicki (1915) describe and illustrate a shell with eight convex whorls bearing a weak, hardly protruding, irregular shaped keel near the lower suture. According to these authors, the keel varies considerably between a thin thread, a blunt bulge, or a weak thickening at the suture. In contrast, the drawings provided by Logvinenko and Starobogatov (1969) and reproduced by Kantor and Sysoev (2006) suggest a shell with straight-sided whorls and a distinct keel. Inspection of the type material is required to clarify the status of this species.
Conservation status. Not assessed.
Turricaspiapseudospica (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Oxypyrgula) pseudospica Logvinenko & Starobogatov: 366, fig. 363(1).
2016 Pyrgulapseudospica Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 241–242.
Status. Pontocaspian species, identity uncertain.
Type locality. Middle and southern Caspian Sea, 15–75 m.
Distribution. Type locality only.
Taxonomic notes. The identity of this species is unclear. Judging from the drawing by Logvinenko and Starobogatov (1969), showing a small slender shell with ca. 6.5 convex whorls, the species might be based on a juvenile specimen. Moreover, it could be a junior synonym of the similarly shaped T.spica (Eichwald, 1855).
Conservation status. Not assessed.
Turricaspiapulla (Dybowski & Grochmalicki, 1915)
*1915 Micromelania (Turricaspia) caspiavar.pulla Dybowski & Grochmalicki: 111, pl. 1, fig. 6a.
1969 Pyrgula [(Turricaspia)] pulla (Dyb. et Gr.). – Logvinenko and Starobogatov: 361–
362, fig. 360(8).
2006 Pyrgulapulla (B. Dybowski & Grochmalicki, 1915). – Kantor and Sysoev: 102, pl. 46, fig. C.
2016 Pyrgulapulla (B. Dybowski & Grochmalicki, 1915). – Vinarski and Kantor: 242.
2018 Turricaspiapulla (B. Dybowski & Grochmalicki, 1915). – Neubauer et al.: 81–82, fig. 14A–J.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Endemic to the Caspian Sea, reported from the middle and southern Caspian Sea basins (Logvinenko and Starobogatov 1969). This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. The species can be easily distinguished from other Turricaspia species based on its relatively broad shell, the low-convex whorls, and its small size (Neubauer et al. 2018).
Conservation status. Data Deficient (Vinarski 2011m).
Turricaspiapullula (Dybowski & Grochmalicki, 1915)
*1915 Micromelania (Turricaspia) caspiavar.pullula Dybowski & Grochmalicki: 111–112, pl. 1, fig. 7.
1969 Pyrgula [(Turricaspia)] pullula (Dyb. et Gr.). – Logvinenko and Starobogatov:
366–367, fig. 363(3).
2006 Turricaspiapullula (B. Dybowski & Grochmalicki, 1915). – Kantor and Sysoev: 109, pl. 50, fig. B.
2016 Turricaspiapullula (B. Dybowski & Grochmalicki, 1915). – Vinarski and Kantor: 249.
2018 Turricaspiapullula (B. Dybowski & Grochmalicki, 1915). – Neubauer et al.: 82–84, fig. 14K–L.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Endemic to the Caspian Sea, reported from the western part of the middle Caspian Sea (Logvinenko and Starobogatov 1969).
Taxonomic notes. The very characteristic tripartite whorl profile allows an easy identification and discrimination from other Pontocaspian Pyrgulinae (Neubauer et al. 2018).
Conservation status. Data Deficient (Vinarski 2011s).
Turricaspiarudis (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Turricaspia) rudis Logvinenko & Starobogatov: 362, fig. 360(5).
2016 Pyrgularudis Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 242.
Status. Pontocaspian species, identity uncertain.
Type locality. Middle and southern Caspian Sea, 50–100 m.
Distribution. Type locality only.
Taxonomic notes. The status of this species is unclear. The drawing provided by Logvinenko and Starobogatov (1969) shows strong similarities to T.grimmi in terms of the nearly straight-sided whorls and the large aperture. Since the whereabouts of the type material is unknown, we refrain from a final conclusion on the potential synonymy.
Conservation status. Data Deficient (Vinarski 2011n).
Turricaspiasajenkovae (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Turricaspia) sajenkovae Logvinenko & Starobogatov: 361, fig. 360(4).
2016 Turricaspiasajenkovae (Logvinenko & Starobogatov, 1968). – Vinarski and Kantor: 249–250.
Status. Pontocaspian species, identity uncertain.
Type locality. Middle Caspian Sea.
Distribution. Type locality only.
Taxonomic notes. The available drawing of this species suggests a very slender shell with highly convex whorls bearing a subsutural band. The type material has not been found, and the identity of this species remains unclear.
Conservation status. Data Deficient (Vinarski 2011t).
Turricaspiasimilis (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Caspiella) similis Logvinenko & Starobogatov: 375, fig. 366(11).
2016 Pyrgulasimilis Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 243.
Status. Pontocaspian species, identity uncertain.
Type locality. Eastern part of the middle Caspian Sea, 20–50 m.
Distribution. Middle and southern Caspian Basin. This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. Judging from the drawing in Logvinenko and Starobogatov (1969), presenting a small slender shell with ca. 5.5 highly convex whorls, the species might be based on a juvenile specimen. It might be a junior synonym of the similarly shaped T.meneghiniana (Issel, 1865). Without investigating the type material, which has not been found in the ZIN collection, the identity of this species remains unclear.
Conservation status. Not assessed.
Turricaspiasimplex (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Oxypyrgula) simplex Logvinenko & Starobogatov: 367–368, fig. 363(4).
2016 Pyrgulasimplex Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 243.
Status. Pontocaspian species, identity uncertain.
Type locality. Middle Caspian Sea, 40–120 m.
Distribution. Middle and southern Caspian Sea. This species was mentioned from depths between 200 and 900 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. As for the previous species, it is highly uncertain whether this taxon is a distinct species. It might also be based on a juvenile and could be a synonym of an earlier described species, perhaps T.pulla or T.lyrata.
Conservation status. Not assessed.
Turricaspiaspasskii (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Turricaspia) spasskii Logvinenko & Starobogatov: 361, fig. 360(7).
2016 Turricaspiaspasskii (Logvinenko & Starobogatov, 1968). – Vinarski and Kantor: 250.
Status. Accepted Pontocaspian species.
Type locality. Western part of the middle Caspian Sea.
Distribution. Middle and southern Caspian Sea. This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. The fast growing whorls terminating in a large body whorl with expanded aperture are characteristic for this species and facilitate discrimination from other Turricaspia species.
Conservation status. Data Deficient (Vinarski 2011u).
Turricaspiaspica (Eichwald, 1855)
*1855 Paludinaspica Eichwald: 303–304, pl. 10, figs 8, 9.
?1992 Turricaspiaspica (Eichw.). – Anistratenko and Prisyazhniuk: 18, fig. 2d.
2006 Turricaspiaspica (Eichwald, 1855). – Kantor and Sysoev: 110, pl. 49, fig. F.
2009 Turricaspiacf.spica (Eichwald, 1855). – Filippov and Riedel: 70, 72, 74, 76, fig. 4e, f.
2016 Turricaspiaspica (Eichwald, 1855). – Vinarski and Kantor: 250.
Status. Accepted Pontocaspian species.
Type locality. Ostrov Chechen’ (island in NW Caspian Sea), Dagestan, Russia.
Distribution. Endemic to the Caspian Sea. Occurred also in the Aral Sea during the Holocene (Filippov and Riedel 2009) but now extinct there. It has been reported from the Holocene of Danube Delta (Anistratenko and Prisyazhniuk 1992) (see below).
Taxonomic notes. As the oldest described species presently attributed to Turricaspia, the validity of this species is without doubt. Its identity, however, is poorly known, given the limited information and poor drawing provided by Eichwald (1855), as well as the largely diverging concepts applied by later authors (see Neubauer et al. 2018 for a detailed discussion of the matter). We have a geographic record (Anistratenko and Prisyazhniuk 1992) that is outside the Caspian–Aral distribution range of this genus. Comparison of the Danube material with Caspian specimens suggests the identification might be correct, yet further detail study is required to assess whether the Danube record might actucally not be an unusual form of Laevicaspialincta.
Conservation status. Not assessed.
Turricaspiaturricula (Clessin & Dybowski in Dybowski, 1887)
*1887 Micromelaniaturricula Clessin & Dybowski in Dybowski: 34.
1888 [Micromelania] turricula n. sp. – Dybowski: 78, pl. 1, fig. 3a–c.
2006 Turricaspiaturricula (Clessin & W. Dybowski in W. Dybowski, 1888). – Kantor and Sysoev: 111, pl. 49, fig. I.
2016 Turricaspiaturricula (Clessin & W. Dybowski in W. Dybowski, 1888). – Vinarski and Kantor: 244.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Middle and southern Caspian Sea. This species was mentioned from depths between 200 and 500 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017).
Taxonomic notes. The species is characterised by a slender conical shell with weakly convex whorls with weak subsutural swelling and a slightly inflated body whorl with large aperture.
Conservation status. Not assessed.
Turricaspiauralensis (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Turricaspia) uralensis Logvinenko & Starobogatov: 359, fig. 360(1).
2016 Pyrgulauralensis Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 244.
Status. Pontocaspian species, identity uncertain.
Type locality. Eastern part of the northern Caspian Sea.
Distribution. Type locality only.
Taxonomic notes.Logvinenko and Starobogatov (1969) illustrated a comparably small shell with eight highly convex whorls, large body whorl, and large aperture. Reliable assessment of the species’ status requires investigation of the type material, which has only been discovered in ZIN in June 2018 and awaits further study.
Conservation status. Not assessed.
Turricaspiavinogradovi (Logvinenko & Starobogatov, 1969)
*1969 Pyrgula (Oxypyrgula) vinogradovi Logvinenko & Starobogatov: 368, fig. 363(9).
?1971 Pyrgulaastrachanica Pirogov: 249–251, fig. 1.
?2006 Turricaspiaastrachanica (Pirogov, 1971). – Kantor and Sysoev: 105, pl. 48, fig. B.
2006 Turricaspiavinogradovi (Logvinenko & Starobogatov, 1968). – Kantor and Sysoev: 111, pl. 50, fig. C.
2016 Turricaspiavinogradovi (Logvinenko & Starobogatov, 1968). – Vinarski and Kantor: 251.
Status. Pontocaspian species, identity uncertain.
Type locality. Northern Caspian Sea.
Distribution. Northern Caspian Sea and Volga Delta (Logvinenko and Starobogatov 1969).
Taxonomic notes. The species as illustrated by Logvinenko and Starobogatov (1969) is based on a slender shell with highly convex whorls. The same traits are also typical for Pyrgulaastrachanica; in fact, the type of T.vinogradovi could be a juvenile of that species. Moreover, both of them might be synonyms of Turricaspianossovi Kolesnikov, 1947. Since a part of the type material of the species involved is lacking and some of the taxa are based on incomplete or presumably juvenile specimens, the identities of Pyrgulaastrachanica and Turricaspiavinogradovi remain unresolved.
Conservation status.Turricaspiavinogradovi has not been assessed by the IUCN, T.astrachanica is marked as “Data Deficient” (Vinarski 2011q).
Hydrobiidae incertae sedis
Abeskunusbrusinianus (Clessin & Dybowski in Dybowski, 1887)
*1887 Zagrabica Brusiniana Clessin & Dybowski in Dybowski: 52–53.
1888 Zagrabica Brusiniana n. sp. – Dybowski: 79, pl. 2, fig. 7.
2006 Pseudamnicolabrusiniana (Clessin & W. Dybowski in W. Dybowski, 1888). – Kantor and Sysoev: 114, pl. 51, fig. J.
2016 Pseudamnicolabrusiniana (Clessin & W. Dybowski in W. Dybowski, 1888). – Vinarski and Kantor: 222.
2018 Abeskunusbrusinianus (Clessin & W. Dybowski in W. Dybowski, 1887). – Neubauer et al.: 87–88, fig. 16A–I.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Middle and southern Caspian Sea (Logvinenko and Starobogatov 1969, Parr et al. 2007). Mirzoev and Alekperov (2017) mention Pseudamnicolabrusinianus from depths between 200 and 400 m in the South Caspian Basin of Azerbaijan but we are not entirely certain whether these records might include other Abeskunus species as well.
Taxonomic notes. For a detailed description and discussion, see Neubauer et al. (2018).
Conservation status. Least Concern (Vinarski 2011c).
Abeskunusdepressispira (Logvinenko & Starobogatov, 1969)
*1969 Pseudamnicola (Abeskunus) depressispira Logvinenko & Starobogatov: 381, fig. 367(14).
2016 Pseudamnicoladepressispira Logvinenko & Starobogatov, 1968. – Vinarski and Kantor: 222–223.
Status. Accepted Pontocaspian species.
Type locality. Western part of the southern Caspian Sea, northward of Kuraginsky Kamen’ [= Kür Daşı] Island (approximately 39°01'05"N, 49°20'02"E), 81 m water depth.
Distribution. In addition to the type locality, specimens have been found in Holocene material retrieved near the Kura Delta, a few kilometres north of the type locality.
Taxonomic notes. Current investigations on recently collected Holocene material from the south-western Caspian Sea confirm that this species belongs to the genus Abeskunus. The finely ribbed, low trochiform shell facilitates distinction from its congeners. The species epithet is based on the Latin noun spira, spire, and is to be considered a noun in apposition (ICZN 1999, Art. 31.2.1.).
Conservation status. Data Deficient (Vinarski 2011d).
Abeskunusexiguus (Eichwald, 1838)
°1837 Lithoclypus [sic] Caspius m. – Krynicki: 58 (nomen nudum).
*1838 Paludinaexigua Eichwald: 152–153.
1863 Bithiniasphaerion Mousson: 409–410.
1874 Lithoglyphus? Caspius Krynicki. – Martens: 80.
1877 Lithoglyphuscaspius Grimm: 82–84, pl. 9, fig. 8.
1977 Pseudamnicola (Abeskunus) brusinianamichelae Tadjalli-Pour: 108, pl. 2, fig. 9.
2016 Pseudamnicolaexigua (Eichwald, 1838). – Vinarski and Kantor: 223.
2016 Pseudamnicolasphaerion (Mousson, 1863). – Vinarski and Kantor: 223.
Status. Accepted Pontocaspian species.
Type locality. In fossil (likely Pleistocene) limestone of Dagestan, Russia.
Distribution. Western Caspian Sea, known from northern and southern parts. Records from the eastern Caspian Sea by Logvinenko and Starobogatov (1969) could not be confirmed.
Taxonomic notes. An in-depth study of the literature suggests that the names Paludinaexigua, Bithiniasphaerion syn. n., and Lithoglyphuscaspius all refer to the same species. The name Lithoglyphuscaspius was made available by Martens (1874) by referring to the description and illustration of Eichwald’s species, rendering L.caspius a junior objective synonym of Abeskunusexiguus. All three taxa share the globular shape, short spire, and inflated last whorl. The subspecies Pseudamnicolabrusinianamichelae syn. n. from Iranian coasts of the Caspian Sea closely resembles A.exiguus and is herein considered a synonym as well. Abeskunusexiguus differs from A.brusinianus in the highly globular shell with small spire. A revision of the species is currently being prepared.
Conservation status. Not assessed.
Andrusoviaandrusovi Starobogatov, 2000
*2000 Andrusoviaandrusovi Starobogatov: 39–41, fig. 1B.
2016 Andrusoviaandrusovi Starobogatov, 2000. – Vinarski and Kantor: 214.
Status. Pontocaspian species, identity uncertain.
Type locality. Eastern part of the South Caspian Sea (39°05'N, 52°35'E).
Distribution. Middle and southern Caspian Sea (Starobogatov 2000).
Taxonomic notes. The species is very similar to the type species of Andrusovia, A.dybowskii, regarding the low spire. Investigation of the type material is required to clarify whether both taxa are distinct.
Remarks. Only recently, paratypes of this species were detected at the Zoological Museum of Moscow University. A study of the taxonomy of Andrusovia is currently under way.
Conservation status. Not assessed.
Andrusoviabrusinai Starobogatov, 2000
*2000 Andrusoviabrusinai Starobogatov: 41, fig. 1C.
2016 Andrusoviabrusinai Starobogatov, 2000. – Vinarski and Kantor: 214.
2018 Andrusoviabrusinai Starobogatov, 2000. – Neubauer et al.: 54–56, fig. 6F–K, M–N.
Status. Pontocaspian species, identity uncertain.
Type locality. Eastern part of the middle Caspian Sea (42°42.5'N, 51°32.5'E), at 80 m water depth.
Distribution. Northern, middle, and southern Caspian Sea (Starobogatov 2000, Neubauer et al. 2018).
Taxonomic notes. The species was recently described in detail by Neubauer et al. (2018). The species was distinguished from A.dybowskii and A.andrusovi by the higher spire, but this is a variable character. Currently, the taxonomy of Andrusovia species is the subject of further study.
Remarks.Starobogatov (2000) mentioned that the type material is housed in the ZIN collection, but we were unable to find the holotype and it is presumed lost. Only recently, paratypes of this species were detected at the Zoological Museum of Moscow University and are currently being studied.
Conservation status. Not assessed.
Andrusoviadybowskii Brusina in Westerlund, 1902b
*1902b AndrusoviaDybowskii Westerlund: 133.
? 2000 Andrusoviadybowskii Brusina in Westerlund, 1903. – Starobogatov: 39, fig. 1A.
2016 Andrusoviadybowskii Brusina in Westerlund, 1903. – Vinarski and Kantor: 214.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Middle and southern Caspian Sea (Starobogatov 2000).
Taxonomic notes. Apparently, Brusina considered both the more conical and flatter shells (“conoidea vel discoidea”) to belong to a single species. Starobogatov (2000) in turn referred only the flat type to as Andrusoviadybowskii and considered the conical ones to belong to separate species (A.brusinai and A.marina). The recently rediscovered type material represents the conico-globular type and is currently subject of study by V. Anistratenko and collaegues.
Conservation status. Not assessed.
Andrusoviamarina (Logvinenko & Starobogatov, 1969)
*1969 Horatia (Caspiohoratia) marina Logvinenko & Starobogatov: 382, fig. 367(18).
2000 Andrusoviamarina (Logvinenko & Starobogatov, 1969). – Starobogatov: 41–42, fig. 1D.
2016 Andrusoviamarina (Logvinenko & Starobogatov, 1968). – Vinarski and Kantor: 214–215.
Status. Pontocaspian species, identity uncertain.
Type locality. Northern slope of the middle Caspian Sea Basin, 43°32.5'N, 49°17.5'E, 60 m water depth.
Distribution. Middle and southern Caspian Sea (Starobogatov 2000). This species was mentioned from depths between 200 and 400 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Horatiamarina).
Taxonomic notes. According to Neubauer et al. (2018), this species might be a senior synonym of A.brusinai Starobogatov, 2000. Inspection of recently discovered type material appears to support that view, but more in-depth studies are required to evaluate the status of this species.
Remarks. The holotype is not traced and presumed lost. Only recently, paratypes of this species were detected at the Zoological Museum of Moscow University and are currently being studied.
Conservation status. Not assessed.
Family Lithoglyphidae Tryon, 1866
Lithoglyphusnaticoides (Pfeiffer, 1828)
*1828 Paludinanaticoides Pfeiffer: 45–46, pl. 8, figs 1, 2, 4.
2012 Lithoglyphusnaticoides (Pfeiffer, 1828). – Welter-Schultes: 41, unnumbered text figures.
2016 Lithoglyphusnaticoides (C. Pfeiffer, 1828). – Vinarski and Kantor: 253.
Status. Accepted native species.
Type locality. In the Danube at Vienna, Austria, and at Pesth (today part of Budapest), Hungary.
Distribution. Originally only in rivers entering the Black Sea, in the Danube up to Regensburg (Germany). After 1800, also introduced to Elbe and Rhine regions by artificial canals; after 1900 in France (Welter-Schultes 2012). Very common in the Volga Delta (Vinarski et al. 2018).
Conservation status. Least Concern (Van Damme 2011b).
Family Tateidae Thiele, 1925
Potamopyrgusantipodarum (Gray, 1843)
*1843 Amnicolaantipodarum Gray: 241.
1951 Potamopyrgusjenkinsi E. A. Smith 1889. – Grossu: 693–695, fig. 1a–d.
1966 P.[yrgula] (Trachycaspia?) grossui Golikov and Starobogatov: 359.
1991 Potamopyrguspolistchuki Anistratenko: 75, fig. 1(2).
1995 Potamopyrgusalexenkoae Anistratenko in Anistratenko and Stadnichenko: 92–93, fig. 69.
2012 Potamopyrgusantipodarum (Gray, 1843). – Welter-Schultes: 40, unnumbered text figures.
Status. Accepted species, invasive.
Type locality. New Zealand (no details).
Distribution. Originally from New Zealand, probably introduced in 1859 to England, in 1872 to Tasmania, in 1895 to mainland Australia, in ca. 1900 to European mainland (Ponder 1988), and in 1987 to North America (Zaranko et al. 1997).
Taxonomic notes. The two Black Sea species P.polistchuki syn. n. and P.alexenkoae syn. n. are here considered as junior synonyms of P.antipodarum, differing only very weakly in outline. Vinarski and Kantor (2016) listed Pyrgula (Trachycaspia?) grossui syn. n. Golikov & Starobogatov in the synonymy of T.dimidiata (Eichwald, 1838). Golikov and Starobogatov (1966) introduced this species as new name for the supposedly misidentified Potamopyrgusjenkinsi sensu Grossu (1951) from Razim Lake in Romania. The shell they later illustrated (Golikov and Starobogatov 1972) indeed shows similarities with T.dimidiata. The shell illustrated in Grossu (1951), however, is completely different and shows a keeled form of P.antipodarum.
Conservation status. Least Concern (Van Damme 2013).
Family Planorbidae Rafinesque, 1815
Gyrauluseichwaldi (Clessin & Dybowski in Dybowski, 1887)
°1876 Pl.[anorbis] Eichwaldi. – Grimm: 157 (nomen nudum).
*1887 PlanorbisEichwaldi Clessin & Dybowski in Dybowski: 49–52.
1888 PlanorbisEichwaldi Grimm. – Dybowski: 79, pl. 2, fig. 11a–c, pl. 3, fig. 10a–c.
?1966b Anisus (Andrusowia) [sic] eichwaldiinfundibularis Logvinenko and Starobogatov: 1472, fig. 4.
?1977 Anisusdjalali Tadjalli-Pour: 109, pl. 2, fig. 10.
2016 Gyraulus (Gyraulus) eichwaldi (Grimm in W. Dybowski, 1888). – Vinarski and Kantor, 2016: 378.
Status. Accepted Pontocaspian species.
Type locality. Caspian Sea (no details).
Distribution. Middle and southern Caspian Sea (Logvinenko and Starobogatov 1969). This species was mentionedfrom depths between 200 and 900 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Anisuseichwaldi).
Taxonomic notes. The species is characterised by a relatively large, asymmetrical shell. Anisuseichwaldiinfundibularis is probably a morphotype of G.eichwaldi. We are uncertain about the status of Anisusdjalali Tadjalli-Pour, 1977 as the description is very brief and the photographs are not very clear. It may be within the range of the morphological variability of G.eichwaldi.
Conservation status. Not assessed.
Gyraulusdybowskii (Kolesnikov, 1947)
*1947 Planorbiseichwaldivar.dybowskii Kolesnikov: 109, 112, fig. in tab. 1.
1966b Anisus (Andrusowia) [sic] kolesnikovi Logvinenko and Starobogatov: 1473, fig. 5.
1966b Anisus (Andrusowia) [sic] kolesnikovi sublittoralis Logvinenko and Starobogatov: 1472–1473, fig. 6.
2016 Gyraulus (Gyraulus) kolesnikovi (Logvinenko & Starobogatov, 1966). – Vinarski and Kantor, 2016: 379.
Status. Pontocaspian species, identity uncertain.
Type locality. Caspian Sea, 40°37'N, 50°52'E, 115 m.
Distribution. Middle and southern Caspian Sea (Logvinenko and Starobogatov 1969). This species was mentioned from depths between 200 and 300 m in the South Caspian Basin of Azerbaijan (Mirzoev and Alekperov 2017, who reported the species as Anisuscolesnikovi [sic]).
Taxonomic notes.Logvinenko and Starobogatov (1966b) considered this species and Andrusoviadybowskii Brusina in Westerlund, 1902b to belong in the same genus, Anisus (Andrusovia), rendering P. dybowskii Kolesnikov, 1947 a junior homonym. Therefore, they introduced A.kolesnikovi as replacement name. Since both taxa do clearly not belong to the same genus or even the same family, the replacement name is to be discarded.
The species resembles G.eichwaldi regarding the general habitus; it differs in the more pronounced angle at the transition between whorl flank and apical plane. A revision is required to investigate if the Caspian Gyraulus species are distinct species or morphotypes of G.eichwaldi. The generic placement follows Vinarski and Kantor (2016). Note that those authors listed the earlier described P. eichwaldi dybowskii Kolesnikov, 1947 as a synonym of G.kolesnikovi.
Conservation status. Least Concern (for Anisuskolesnikovi; Vinarski 2011a).
Gyraulussulcatus (Logvinenko & Starobogatov, 1966, non Hilgendorf, 1867)
*1966b Anisus (Andrusowia) [sic] sulcatus Logvinenko & Starobogatov: 1474, fig. 7.
2016 Gyraulus (Gyraulus) sulcatus (Logvinenko & Starobogatov, 1966). – Vinarski and Kantor, 2016: 382.
Status. Pontocaspian species, identity uncertain, name invalid.
Type locality. Caspian Sea, 42°45'N, 48°29'E, 79 m.
Distribution. Middle Caspian Sea (Logvinenko and Starobogatov 1969).
Taxonomic notes. The species in its present combination as Gyraulussulcatus (following Vinarski and Kantor 2016) is invalid as it is a secondary homonym of the Miocene Gyraulussulcatus (Hilgendorf, 1867). We refrain here from introducing a replacement name as the species’ status is uncertain. It resembles G.eichwaldi and G.kolesnikovi in outline shape and differs only in the more pronounced angle between whorl flank and apical plane and the shallow furrow on the apical side. An in-depth revision is required to clarify if Gyraulussulcatus is a distinct species or a mere morphotype of G.eichwaldi (Clessin & Dybowski in Dybowski, 1887).
Conservation status. Not assessed.
Discussion and conclusions
The annotated check-list presented here is a first attempt to assess the species diversity of the Pontocaspian molluscs by experts working in different countries and fields (neontology, palaeontology, biogeography, phylogenetics). Hitherto, progress has been limited by a number of factors: (1) fresh material for genetic studies is available only for few nominal species, and (2) the type series of many species are lost or at least have not yet been found. This concerns not only the species described by Eichwald or Grimm in the 19th century; the type specimens of many species established by Starobogatov and his co-workers in the 1960–2000s could not be traced in ZIN (Kantor and Sysoev 2006, Vinarski and Kantor 2016). Furthermore, progress has been limited by (3) a lack of representative shell samples to undertake quantitative statistical analyses of conchological variation, and (4) insufficient ecological and distribution data for many of the species.
Three species that have been reported from the Pontocaspian region are not included in this list. The bithyniid gastropod Alocinmacaspica (Westerlund, 1902) has been described from the east side of the Caspian Sea (Beriozkina et al. 1995 indicated this record is probably from the vicinity of Krasnovodsk, Turkmenistan). However, Starobogatov et al. (2004) argued the species lives in waterbodies of Bol’shoy Balkhan (Turkmenistan) and probably not in the Caspian Sea itself (Vinarski et al. 2013, Vinarski and Kantor 2016). Furthermore, two Pseudamnicola species have been described from Lake Razim in Romania (P.leontina Grossu, 1986 and P.razelmiana Grossu, 1986) that is prime Pontocaspian habitat. Like bithyniids, Pseudamnicola has not been reported as a Pontocaspian group elsewhere, and probably they are freshwater species that live in the surrounding streams or in springs. For now, we have excluded these species from the Pontocaspian species list.
This list contains 55 accepted and a further 44 uncertain endemic Pontocaspian mollusc species (Table 2), here defined as species that are considered to be endemic for at least one of the Pontocaspian basins. There are 14 native and three immigrant species (at least in one of the Pontocaspian basins), even though some species may be native or endemic in one of the basins and have become invasive in another of the Pontocaspian Basins. All species that have an uncertain status belong to the Pontocaspian category. The Caspian Sea Basin has the highest number of accepted endemic Pontocaspian species (48) but also poses the greatest taxonomic challenges, with a further 37 species whose status are unclear.
Table 2.
Pontocaspian mollusc species list. Abbreviations: Status: A – accepted, U – uncertain. Basins: AS – Aral Sea, BSB – Black Sea Basin, CSB – Caspian Sea Basin. Species are E – endemic, EX – extinct, IM – immigrant, IN – invasive, N – native (definitions in Table 1); *species encountered alive during the PRIDE program expeditions by participants; †very fresh material of species encountered, but not living specimens.
| Species | Status | BSB | CSB | AS |
|---|---|---|---|---|
| Mytilasterminimus (Poli, 1795)* | A | N | IN | IM/EX |
| Adacnalaeviuscula (Eichwald, 1829) | A | ? | E | |
| Adacnafragilis Milaschewitsch, 1908 | U | E | ||
| Adacnaminima Ostroumov, 1907 | A | E | E/EX? | |
| Adacnaminimaostroumovi (Logvinenko & Starobogatov, 1967) | U | E | ||
| Adacnavitrea (Eichwald, 1829) | A | E | E | E/EX? |
| Adacnavitreaglabra Ostroumov, 1905 | U | E | E | |
| Adacnavitreabergi (Starobogatov, 1974) | U | E/EX? | ||
| Cerastodermaglaucum (Bruguière, 1789) s.l.* | A | N | IN | IN? |
| Cerastoderma sp. A [non C.rhomboides (Lamarck, 1819)]* | A | N | IN | IN? |
| Didacnabaeri (Grimm, 1877) | A | E | ||
| Didacnabarbotdemarnii (Grimm, 1877)* | A | E | ||
| Didacnaeichwaldi (Krynicki, 1837) | A | E | ||
| Didacnalongipes (Grimm, 1877)* | A | E | ||
| Didacnaparallela Bogachev, 1932 | A | E | ||
| Didacnapraetrigonoides Nalivkin & Anisimov, 1914 | A | E/EX | ||
| Didacnaprofundicola Logvinenko & Starobogatov, 1966† | A | E | ||
| Didacnaprotracta (Eichwald, 1841) | A | E | ||
| Didacnapyramidata (Grimm, 1877) | A | E | ||
| Didacnatrigonoides (Pallas, 1771)* | A | E | ||
| Hypanisplicata (Eichwald, 1829) | A | E | E | |
| Monodacnaacuticosta (Logvinenko & Starobogatov, 1967) | A | E | ||
| Monodacnaalbida (Logvinenko & Starobogatov, 1967) | A | E | ||
| Monodacnacaspia (Eichwald, 1829) | A | E | ? | |
| Monodacnacolorata (Eichwald, 1829)* | A | E | IM | |
| Monodacnafilatovae (Logvinenko & Starobogatov, 1967) | U | E | ||
| Monodacnaknipowitschi (Logvinenko & Starobogatov, 1966) | U | E | ||
| Monodacnapolymorpha (Logvinenko & Starobogatov, 1967) | U | E | ||
| Monodacnasemipellucida (Logvinenko & Starobogatov, 1967) | A | E | ||
| Abrasegmentum (Récluz, 1843)* | A | N | IN | IN |
| Corbiculafluminalis (Müller, 1774) | A | N/IN | ||
| Dreissenabugensis Andrussov, 1897† | A | E/IN | IN | |
| Dreissenacaspia Eichwald, 1855 | A | E/EX | E/EX | |
| Dreissenaelata Andrusov, 1897 | U | E/EX | ||
| Dreissenagrimmi (Andrusov, 1890)* | A | E | ||
| Dreissenapolymorpha (Pallas, 1771) s.l.* | A | N | N | N |
| Mytilopsisleucophaeata (Conrad, 1831)* | A | IN | IN | |
| Theodoxusdanubialis (Pfeiffer, 1828)* | A | N | ||
| Theodoxusfluviatilis (Linnaeus, 1758) | A | N | ||
| Theodoxuspallasi Lindholm, 1924* | A | N | N | N/EX? |
| Theodoxusschultzii (Grimm, 1877)* | U | E | ||
| Theodoxusvelox V. Anistratenko in O. Anistratenko et al., 1999 | A | N | ||
| Eupaludestrinastagnorum (Gmelin, 1791) | A | N/IM | N/IM | |
| Caspiabaerii Clessin & Dybowski in Dybowski, 1887 | A | E? | E | |
| ?Caspiavalkanovi (Golikov & Starobogatov, 1966) | U | E | ||
| Clathrocaspiabrotzkajae (Starobogatov in Anistratenko & Prisyazhniuk, 1992) | A | ?E | E | |
| Clathrocaspiagmelinii (Clessin & Dybowski in Dybowski, 1887) | A | E | ||
| Clathrocaspiaisseli (Logvinenko & Starobogatov, 1969) | U | E | ||
| Clathrocaspiaknipowitschii (Makarov, 1938) | A | E | ||
| Clathrocaspialogvinenkoi (Golikov & Starobogatov, 1966) | A | E | ||
| Clathrocaspiamilae Boeters, Glöer & Georgiev, 2015 | U | E | ||
| Clathrocaspiapallasii (Clessin & Dybowski in Dybowski, 1887) | A | E | ||
| Ulskiabehningi (Logvinenko & Starobogatov, 1969) | U | E | ||
| ?Ulskiaderzhavini (Logvinenko & Starobogatov, 1969) | U | E | ||
| Ulskiaulskii (Clessin & Dybowski in Dybowski, 1887) | A | E | ||
| Ecrobiagrimmi (Clessin in Dybowski, 1887)* | A | N | N | |
| Ecrobiamaritima (Milaschewitsch, 1916)* | A | N | ||
| Ecrobiaventrosa (Montagu, 1803) | A | IM | ||
| Clessiniolavariabilis (Eichwald, 1838) | A | E | E | |
| Laevicaspiaabichi (Logvinenko & Starobogatov, 1969) | A | E | ||
| Laevicaspiacaspia (Eichwald, 1838) | A | E | ||
| Laevicaspiacincta (Abich, 1859) | A | E | ||
| Laevicaspiaconus (Eichwald, 1838) | A | E | ||
| ?Laevicaspiaebersini (Logvinenko & Starobogatov, 1969) | U | E | ||
| ?Laevicaspiaismailensis (Golikov & Starobogatov, 1966) | A | E | ||
| Laevicaspiakolesnikoviana (Logvinenko & Starobogatov in Golikov & Starobogatov, 1966) | A | E | ||
| Laevicaspiakowalewskii (Clessin & Dybowski in Dybowski, 1887) | A | E | ||
| Laevicaspialencoranica (Logvinenko & Starobogatov, 1969) | U | E | ||
| Laevicaspialincta (Milaschewitsch, 1908) | A | E | ||
| ?Laevicaspiamarginata (Westerlund, 1902) | U | E | ||
| Laevicaspiasieversii (Clessin in Dybowski, 1887) | U | E | ||
| ?Turricaspiaaenigma (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiaandrussowi (Dybowski & Grochmalicki, 1915) | A | E | ||
| ?Turricaspiabasalis (Dybowski & Grochmalicki, 1915) | U | E | ||
| ?Turricaspiabogatscheviana (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiachersonica Alexenko & Starobogatov, 1987 | U | E | ||
| Turricaspiacolumna (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiaconcinna (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiadagestanica (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiadimidiata (Eichwald, 1838) | A | E | ||
| Turricaspiaeburnea (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiaelegantula (Clessin & Dybowski in Dybowski, 1887) | U | E | ||
| Turricaspiaeulimellula (Dybowski & Grochmalicki, 1915) | A | E | ||
| Turricaspiafedorovi (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiagrimmi (Clessin & Dybowski in Dybowski, 1887) | A | E | ||
| Turricaspialyrata (Dybowski & Grochmalicki, 1915) | A | E | ||
| Turricaspiamarisnigri Starobogatov in Alexenko & Starobogatov, 1987 | U | E/EX? | ||
| Turricaspiameneghiniana (Issel, 1865) | A | E | ||
| Turricaspianossovi Kolesnikov, 1947 | A | E | ||
| ?Turricaspiaobventicia (Anistratenko in Anistratenko & Prisyazhniuk, 1992) | U | E | ||
| ?Turricaspiapseudobacuana (Logvinenko & Starobogatov, 1969) | U | E | ||
| ?Turricaspiapseudodimidiata (Dybowski & Grochmalicki, 1915) | U | E | ||
| Turricaspiapseudospica (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiapulla (Dybowski & Grochmalicki, 1915) | A | E | ||
| Turricaspiapullula (Dybowski & Grochmalicki, 1915) | A | E | ||
| Turricaspiarudis (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiasajenkovae (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiasimilis (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiasimplex (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiaspasskii (Logvinenko & Starobogatov, 1969) | A | E | ||
| Turricaspiaspica (Eichwald, 1855) | A | ?E | E | ?E |
| Turricaspiaturricula (Clessin & Dybowski in Dybowski, 1887) | A | E | ||
| Turricaspiauralensis (Logvinenko & Starobogatov, 1969) | U | E | ||
| Turricaspiavinogradovi (Logvinenko & Starobogatov, 1969) | U | E | ||
| Abeskunusbrusinianus (Clessin & Dybowski in Dybowski, 1887) | A | E | ||
| Abeskunusdepressispira (Logvinenko & Starobogatov, 1969) | A | E | ||
| Abeskunusexiguus (Eichwald, 1838) | A | E | ||
| Andrusoviaandrusovi Starobogatov, 2000 | U | E | ||
| Andrusoviabrusinai Starobogatov, 2000 | U | E | ||
| Andrusoviadybowskii Brusina in Westerlund, 1902 | A | E | ||
| Andrusoviamarina (Logvinenko & Starobogatov, 1969) | U | E | ||
| Lithoglyphusnaticoides (Pfeiffer, 1828)* | A | N | IM? | |
| Potamopyrgusantipodarum (Gray, 1843)* | A | IM | ||
| Gyrauluseichwaldi (Clessin & Dybowski in Dybowski, 1887)† | A | E | ||
| Gyraulusdybowskii (Kolesnikov, 1947) | U | E | ||
| Gyraulussulcatus (Logvinenko & Starobogatov, 1966) | U | E |
The species richness estimate reflects the current shift of molluscan systematics from morphology-based to integrated studies, with increasing contributions of molecular and statistical species delineation approaches (Vinarski 2018). It has recently been shown that many nominal taxa of fresh- and brackish-water snails and mussels described on the basis of their shell characters (the Pontocaspian molluscs rarely were described on the base of anatomical studies) lack a genetic support (with few exceptions such as e.g., Popa et al. 2012, Stepien et al. 2013) and thus do not represent evolutionary meaningful units. On the other hand, cryptic speciation is known within many taxa of molluscs in long-lived lakes (Albrecht et al. 2006), and the Pontocaspian biota may include some previously unrecognised species. Thus, we consider our check-list rather as a starting point for further integrated research, not a definitive and fixed inventorisation of the Pontocaspian molluscs.
Anyone who reads this list or works such as Logvinenko and Starobogatov (1969) or Vinarski and Kantor (2016) may think that the Caspian Sea still maintains its unique and species-rich mollusc fauna. However, the actual state of affairs is problematic as many species thought to be endemic to this large saline lake have not been found since their description, and recent attempts to obtain fresh material for genetic studies mostly failed. Clearly, the conservation status of Pontocaspian species is insufficiently known. With our working list we aim to assist in the necessary follow-up conservation assessments.
Most taxonomic difficulties were encountered for the bivalve genera Monodacna and Dreissena and the Pyrgulinae gastropods (especially genera Turricaspia and Laevicaspia). Furthermore, there is an urgent need to assess whether representatives of species complexes in the three main Pontocaspian basins (Aral Sea, Caspian Sea, Black Sea) concern separate species as several of these regional populations are in immediate danger of extinction or already extinct (for example with the disappearance of the Aral Sea). Combined methodological efforts will enable us to estimate the extent and characterise the nature of Pontocaspian faunal turnover, and this species list is a first attempt in the required uniform taxonomic base.
Acknowledegments
The PRIDE program has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 642973. TAN was supported by an Alexander-von-Humboldt Scholarship. Further support came from the German Research Foundation (DFG, grant no. WI1902/14) to TW. MV has financial support from the Russian Ministry of Higher Education and Science (project no. 6.1352.2017/4.6). TY was supported by the Russian Science Foundation (grant no. 16-17-10103). We thank Ana Bianca Pavel (Geoecomar, Constanța, Romania) and AS Gasanova (Makhachkala, Russia) for additional observations on living species occurrences. We are furthermore grateful to Dietrich Kadolsky and Mathias Harzhauser, as well as to the editor Eike Neubert and the technical editor Nathalie Yonow, for constructive comments.
Citation
Wesselingh FP, Neubauer TA, Anistratenko VV, Vinarski MV, Yanina T, ter Poorten JJ, Kijashko P, Albrecht C, Anistratenko OYu, D’Hont A, Frolov P, Gándara AM, Gittenberger A, Gogaladze A, Karpinsky M, Lattuada M, Popa L, Sands AF, van de Velde S, Vandendorpe J, Wilke T (2019) Mollusc species from the Pontocaspian region – an expert opinion list. ZooKeys 827: 31–124. https://doi.org/10.3897/zookeys.827.31365
References
- Abich H. (1859) Vergleichende chemische Untersuchungen der Wasser des Caspischen Meeres, Urmia- und Van-See’s. Mémoires de l’Académie impériale des sciences de St.-Pétersbourg, 6e série, Sciences mathématiques, physiques et naturelles 7: 1–57. https://biodiversitylibrary.org/page/46467180
- Akramovskiy NN. (1976) Fauna Armyanskoy SSR. Mollyuski (Mollusca). Izvestiya Akademii Nauk Armyanskoy SSR, Yerevan, 268 pp. [Google Scholar]
- Aladin NV, Filippov AA, Plotnikov IS, Orlova MI, Williams WD. (1998) Changes in the structure and function of biological communities in the Aral Sea, with particular reference to the northern part (Small Aral Sea), 1985–1994: A review. International Journal of Salt Lake Research 7(4): 301–343. 10.1023/A:1009009924839 [DOI] [Google Scholar]
- Albrecht C, Trajanovski S, Kuhn K, Streit B, Wilke T. (2006) Rapid evolution of an ancient lake species flock: freshwater limpets (Gastropoda: Ancylidae) in the Balkan Lake Ohrid. Organisms, Diversity & Evolution 6(4): 294–307. 10.1016/j.ode.2005.12.003 [DOI] [Google Scholar]
- Albrecht C, von Rintelen T, Sereda S, Riedel F. (2014) Evolution of ancient lake bivalves: the Lymnocardiinae (Cardiidae) of the Caspian Sea. Hydrobiologia 739(1): 85–94. 10.1007/s10750-014-1908-3 [DOI] [Google Scholar]
- Alexenko TL, Starobogatov YaI. (1987) Vidy Caspia i Turricaspia (Gastropoda, Pectinibranchia, Pyrgulidae) Azovo-Chernomorskogo basseyna. Vestnik Zoologii 21(3): 32–38. [Google Scholar]
- Andreev NI, Andreeva SI, Filippov AA, Aladin NV. (1992) The fauna of the Aral Sea in 1989. 1. The benthos. International Journal of Salt Lake Research 1(1): 103–110. 10.1007/BF02904954 [DOI] [Google Scholar]
- Andreeva SI, Andreev NI. (2003) Evolyutsionnyye preobrazovaniya dvustvorchatykh mollyuskov Aral’skogo morya v usloviyakh ekologicheskogo krizisa. Omskiy Gosudarstvennyy Pedagogicheskiy Universitet, Omsk, 382 pp http://herba.msu.ru/shipunov/school/books/andreevy2003_evol_moll_aral.pdf [Google Scholar]
- Andrusov N. (1890) Kerchenskiy izvestnyak i yego fauna. Zapiski Imperatorskago S.-Petersburgskago Mineralogicheskago Obshchestva (seriya 2) 26: 193–344.
- Andrusov N. (1897) Fossile und lebende Dreissenidae Eurasiens. Tipografiya M. Merkusheva, St. Petersburg, 683 pp https://biodiversitylibrary.org/page/35041266 [atlas] [Google Scholar]
- Andrusov N. (1909) Beiträge zur Kenntnis des Kaspischen Neogen. Pontischen Schichten des Schemachinischen Distriktes. Mémories du Comité Géologique, nouvelle série 40: 1–177. [Google Scholar]
- Andrusov N. (1910) Studien über die Brackwassercardiden. Didacna (erste Hälfte). Lieferung II. Mémoires de l’Académie impériale des sciences de St.-Pétersbourg. VIIIe série. Classe physico-mathématique 25(8): 1–84. https://biodiversitylibrary.org/page/36733928 [Google Scholar]
- Anistratenko OYu, Starobogatov YaI, Anistratenko VV. (1999) Mollyuski roda Theodoxus (Gastropoda, Pectinibranchia, Neritidae) Azovo-Chernomorskogo basseyna. Vestnik Zoologii 33(3): 11–19. [Google Scholar]
- Anistratenko VV. (1991) Mollyuski gruppy Hydrobia sensu lato Chernogo i Azovskogo morey. Byulleten’ Moskovskogo Obshchestva Ispytateley Prirody, Otdel biologicheskiy 96(6): 73–81. [Google Scholar]
- Anistratenko VV. (2005) Lectotypes for Tricoliapullus, Gibbuladivaricata and Theodoxusfluviatilis revisited. Vestnik Zoologii 39(6): 3–10. [Google Scholar]
- Anistratenko VV. (2007a) Finding of the extremely rare hydrobiid Caspialogvinenkoi (Mollusca: Gastropoda) in the estuary of the River Don and its zoogeographical significance. Mollusca 25(1): 23–26. [Google Scholar]
- Anistratenko VV. (2007b) Novyye dannyye o sostave, strukture i genezise Ponto-Kaspiyskoy fauny bryukhonogikh mollyuskov v Azovo-Chernomorskom basseyne. Zoologicheskiy Zhurnal 86(7): 793–801. [Google Scholar]
- Anistratenko VV. (2013) On the taxonomic status of the highly endangered Ponto-Caspian gastropod genus Caspia (Gastropoda: Hydrobiidae: Caspiinae). Journal of Natural History 47(1–2): 51–64. 10.1080/00222933.2012.742934 [DOI] [Google Scholar]
- Anistratenko VV, Anistratenko OYu, Shydlovskyy IV. (2018) Karl E. von Baer’s collection of Caspian Sea mollusks stored in the Zoological Museum of Lviv University, Ukraine. Part 1. Catalogue and general description. Archiv für Molluskenkunde 147(2): 223–236. 10.1127/arch.moll/147/223-236 [DOI] [Google Scholar]
- Anistratenko VV, Khaliman IA, Anistratenko OYu. (2011) Mollyuski Azovskogo morya. Naukova dumka, Kiev, 171 pp http://ashipunov.info/shipunov/school/books/anistratenko2011_moll_azovsk_morja.pdf [Google Scholar]
- Anistratenko VV, Prisyazhniuk VA. (1992) Novyye dannyye o mollyuskakh golotsenovykh otlozheniy Chernogo morya na Ukraine. Vestnik Zoologii 5: 15–21. [Google Scholar]
- Anistratenko VV, Stadnichenko AP. (1995) Fauna Ukraine. Vol. 29: Mollusca. Fasc. 1. B. 2: Orders Littoriniformes, Rissoiformes. Naukova dumka, Kiev, 175 pp. [Google Scholar]
- Anistratenko VV, Zettler ML, Anistratenko OYu. (2017) On the taxonomic relationship between Theodoxuspallasi and T.astrachanicus (Gastropoda: Neritidae) from the Ponto-Caspian region. Archiv für Molluskenkunde 146(2): 213–226. 10.1127/arch.moll/146/213-226 [DOI] [Google Scholar]
- Bandel K. (2001) The history or Theodoxus and Neritina connected with the description and systematic evaluation of related Neritimorpha (Gastropoda). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg 85: 65–164. http://www.paleoliste.de/bandel/bandel_2001e.pdf [Google Scholar]
- Barannik V, Borysova O, Stolberg F. (2004) The Caspian Sea Region: Environmental Change. AMBIO: A Journal of the Human Environment 33(1): 45–51. 10.1579/0044-7447-33.1.45 [DOI] [PubMed] [Google Scholar]
- Beriozkina GV, Levina OV, Starobogatov YaI. (1995) Revision of Bithyniidae from European Russia and Ukraine. Ruthenica 5(1): 27–38. [Google Scholar]
- Boeters HD, Glöer P, Georgiev D, Dedov I. (2015) A new species of Caspia Clessin et W. Dybowski, 1887 (Gastropoda: Truncatelloidea: Hydrobiidae) in the Danube of Bulgaria. Folia Malacologica 23: 177–186. 10.12657/folmal.023.014 [DOI] [Google Scholar]
- Bogachev VV. (1932a) Geologicheskiye ekskursii v okrestnostyakh Baku. Aznefteizdat, Baku, 88 pp. [Google Scholar]
- Bogachev VV. (1932b) Vedushchiye iskopayemyye razreza Apsheronskogo poluostrova i prilegayushchikh rayonov. Chast’ 1. Trudy Azerbaidzhanskogo Neftyanogo Instituta 4: 1–92. [Google Scholar]
- Bologa AS, Bodeanu N, Petran A, Tiganus V, Zaitsev YuP. (1995) Major modifications of the Black Sea benthic and planktonic biota in the last three decades. In: Briand F. (Ed.) Les Mers Tributaires de Mediterranée. Bulletin de l’Institut oceanographique, Monaco, no.spécial 15: 85–110. http://ciesm.org/online/monographs/CSS-1/CSS_1_85_110.pdf
- Borcea I. (1926) Quelques remarques sur les Adacnides et principalement sur les Adacnides des Lacs Razelm. Analele Stiintifice ale Universitatii din Iasi 13(3–4): 449–485. [Google Scholar]
- Bouchet P, Rocroi J-P, Hausdorf B, Kaim A, Kano Y, Nützel A, Parkhaev P, Schrödl M, Strong EE. (2017) Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 61(1–2): 1–526. 10.4002/040.061.0201 [DOI] [Google Scholar]
- Bourguignat JR. (1876) Species novissimae Molluscorum in Europaeo systemati detectae, notis diagnosticis succinctis breviter descriptae. Paul Klincksieck, Paris, 80 pp 10.5962/bhl.title.10357 [DOI] [Google Scholar]
- Bruguière JG. (1789) Encyclopedie methodique. Histoire naturelle des Vers. Tome sixième. Panckoucke & Plomteux, Paris & Liege, 344 pp. [Google Scholar]
- Brusina S. (1882) Le Pyrgulinae dell’Europa orientale. Bollettino della Società Malacologica Italiana 7(13–19): 229–292.https://biodiversitylibrary.org/page/39283992 [Google Scholar]
- Büyükmeriç Y, Wesselingh FP. (2018) New cockles (Bivalvia: Cardiidae: Lymnocardiinae) from Late Pleistocene Lake Karapinar (Turkey): Discovery of a Pontocaspian refuge? Quaternary International 465(A): 37–45. 10.1016/j.quaint.2016.03.018 [DOI]
- Chukhchin VD. (1975) Sistematicheskoye polozheniye i ekologiya chernomorskikh Hydrobiidae. In: Mollyuski, ikh sistema, evolyutsiya i rol’ v prirode. In: 5 Vsesoyuznoye soveshchaniye po izucheniyu mollyukov. Avtoreferaty dokladov. Nauka Publishers, Leningrad, 120–122.
- Cioboiu O, Son M, von Rintelen T. (2011) Turricaspiavariabilis The IUCN Red List of Threatened Species 2011: e.T155608A4807675. 10.2305/IUCN.UK.2011-2.RLTS.T155608A4807675.en [accessed on 05 December 2018]. [DOI]
- Clessin S. (1886) Binnenmollusken aus Rumänien. Malakozoologische Blätter (Neue Folge) 8: 49–56. https://biodiversitylibrary.org/page/35594868 [Google Scholar]
- Conrad TA. (1831) Description of Fifteen New Species of Recent, and three of Fossil Shells, chiefly from the coast of the United States. Journal of the Academy of Natural Sciences of Philadelphia 6: 256–268. https://biodiversitylibrary.org/page/24677674 [Google Scholar]
- Coughlan NE, Stevens AL, Kelly TC, Dick JTA, Jansen MAK. (2017) Zoochorous dispersal of freshwater bivalves: an overlooked vector in biological invasions? Knowledge & Management of Aquatic Ecosystystems 418: article number 42. 10.1051/kmae/2017037 [DOI]
- Cummings K. (2011) Mytilopsisleucophaeata The IUCN Red List of Threatened Species 2011: e.T155623A4809971. 10.2305/IUCN.UK.2011-2.RLTS.T155623A4809971.en [Accessed on 05 December 2018] [DOI]
- Cummings KS, Graf DL. (2015) Class Bivalvia. In: Thorp JH, Rogers DC. (Eds) Thorp and Covich’s Freshwater Invertebrates (Fourth Edition).Academic Press, Elsevier, Boston, 423–506. 10.1016/B978-0-12-385026-3.00019-X [DOI]
- d’Orbigny A. (1850) Prodrome de Paléontologie. Stratigraphique universelle des animaux mollusques et rayonnés faisant suitre au cours élémentaire de paléontologie et de géologie stratigraphique. Deuxième volume. Victor Masson, Paris, 427 pp https://biodiversitylibrary.org/page/41091877 [Google Scholar]
- Draparnaud JPR. (1805) Histoire naturelle des Mollusques terrestres et fluviatiles de la France. Plassan, Renaud, Paris/Montpellier, 134 pp https://biodiversitylibrary.org/page/12898711 [Google Scholar]
- Dybowski B. (1913) Ueber Kaspische Schnecken aus der Abteilung Turricaspiinae subfam. nova, zum Vergleich mit den Turribaicaliinae subfam. nova. Bulletin de l’Académie Impériale des Sciences de St. -Pétersbourg, sixième série 7(16): 905–906. https://biodiversitylibrary.org/page/4183344 [Google Scholar]
- Dybowski B, Grochmalicki J. (1915) Ueber kaspische Schnecken aus der Abteilung “Turricaspiinae” subfam. nova zum Vergleich mit den Turribaikalina nobis. Petrograd, 34 pp. [Numbered 103–136]
- Dybowski B, Grochmalicki J. (1917) Studien über die turmförmigen Schnecken des Baikalsees und des Kaspimeeres (Turribaicaliinae – Turricaspiinae). Abhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 9(3): 1–55. https://biodiversitylibrary.org/page/5550074 [Google Scholar]
- Dybowski W. (1887–1888) Die Gasteropoden-Fauna des Kaspischen Meeres. Nach der Sammlung des Akademikers Dr. K. E. v. Baer. Malakozoologische Blätter (Neue Folge) 10(1–3): 1–64 [1, 1887], 65–79 [2, 1888], pl. 1–3 [3, 1888]. https://biodiversitylibrary.org/page/35483241
- Eichwald E. (1829) Zoologia specialis quam expositis animalibus tum vivis, tum fossilibus potissimum Rossiae in universum et Poloniae in specie, in usum lectionum publicarum in Universitate Caesarea Vilnensi habendarum. Pars prior propaedeuticam zoologiae atque specialem heterozoorum expositionem continens. Joseph Zawadzki, Vilnius, 314 pp https://biodiversitylibrary.org/page/35882071 [Google Scholar]
- Eichwald E. (1838) Faunae Caspii Maris primitiae. Bulletin de la Société Impériale des Naturalistes de Moscou 11(2): 125–174. https://biodiversitylibrary.org/page/41342125 [Google Scholar]
- Eichwald E. (1841) Fauna Caspio-Caucasica nonnullis observationibus novis illustravit. Nouveaux Mémoires de la Société Impériale des Naturalistes de Moscou 7: 1–290. https://biodiversitylibrary.org/page/33163057 [Google Scholar]
- Eichwald E. (1855) Zur Naturgeschichte des Kaspischen Meeres. Nouveaux Mémoires de la Société Impériale des Naturalistes de Moscou 10: 283–323. https://biodiversitylibrary.org/page/33191120 [Google Scholar]
- Fedorov PV. (1948) Kaspiyskiye mollyuski Zapadnoy Turkmenii. Byulleten’ Komissii po Izucheniyu Chetvertichnogo Perioda 13: 54–66. http://ginras.ru/library/pdf/13_1948_bull_quatern_comission.pdf [Google Scholar]
- Fedorov PV. (1953) Kaspiyskiye chetvertichnyye mollyuski roda Didacna Eichwald i ikh stratigraficheskoye znacheniye. In: [Unknown] Stratigrafiya chetvertichnykh otlozheniy i noveyshaya tektonika Prikaspiyskoy nizmennosti. Akademiya Nauk SSSR, Moskva, 112–130.
- Fedorov PV. (1957) Stratigrafiya chetvertichnykh otlozheniy i istoriya razvitiya Kaspiyskogo Morya. Trudy Geologicheskogo Instituta Akademii Nauk SSSR 10: 1–298. http://www.ginras.ru/library/pdf/10_1957_fedorov_quaternary_caspian.pdf [Google Scholar]
- Fedorov PV. (1999) Ot Kaspiya do Evksina. Zapiski geologa. GEOS, Moskva, 220 pp. [Google Scholar]
- Fehér Z, Zettler ML, Boszó M, Szabó K. (2009) An attempt to reveal the systematic relationship between Theodoxusprevostianus (C. Pfeiffer, 1828) and Theodoxusdanubialis (C. Pfeiffer, 1828) (Mollusca, Gastropoda, Neritidae). Mollusca 27(2): 95–107. [Google Scholar]
- Filippov A, Riedel F. (2009) The late Holocene mollusc fauna of the Aral Sea and its biogeographical and ecological interpretation. Limnologica 39(1): 67–85. 10.1016/j.limno.2008.04.003 [DOI] [Google Scholar]
- Gadzhiev TM. (1968) Izmenchivost’ Didacnabaeri Grimm i nekotoryye novyye vidy Didacna novokaspiyskikh otlozheniy ostrovov Bakinskogo arkhipelaga. Paleontologicheskiy Sbornik 1(5): 75–85. [Google Scholar]
- Glöer P. (2002) Die Tierwelt Deutschlands, 73. Teil: Die Süßwassergastropoden Nord- und Mitteleuropas. Bestimmungsschlüssel, Lebensweise, Verbreitung. ConchBooks, Hackenheim, 327 pp. [Google Scholar]
- Glöer P, Pešić V. (2012) The freshwater snails (Gastropoda) of Iran, with descriptions of two new genera and eight new species. ZooKeys 219: 11–61. 10.3897/zookeys.219.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glöer P, Pešić V. (2015) Two new freshwater mollusk species of the genus Graecoanatolica Radoman, 1973 from Turkey (Gastropoda: Hydrobiidae). Ecologica Montenegrina 4: 46–51. [Google Scholar]
- Gmelin JF. (1774) Reise durch Rußland zur Untersuchung der drey Natur-Reiche. Dritter Theil. Reise durch das nordliche Persien, in den Jahren 1770, 1771, bis im April 1772. Kayserl. Acad. der Wißenschaften, St. Petersburg, 508 pp https://gdz.sub.uni-goettingen.de/id/PPN63264706X [Google Scholar]
- Gmelin JF. (1791) Caroli a Linné, systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima tertia, aucta, reformata. Tomus I. Pars VI: Vermes Georg Emanuel Beer, Lipsiae, 3021–3910. https://biodiversitylibrary.org/page/25743606
- Golikov AN, Starobogatov YaI. (1966) Ponto-kaspiyskiy bryukhonogiye mollyuski v Azovo-Chernomorskom basseyne. Zoologicheskiy Zhurnal 45(3): 352–362. [Google Scholar]
- Golikov AN, Starobogatov YaI. (1972) Klass bryukhonogiye mollyuski. In: Mordukhay-Boltovskoy FD. (Ed.) Opredelitel’ fauny Chernogo i Azovskogo morey: Svobodnozhivushchiye bespozvonochnyye.T.3. Chlenistonogiye (krome rakoobraznykh), mollyuski, iglokozhiye, shchetinkochelyustnyye, khordovyye. Naukova dumka, Kiev, 65–166.
- Gomoiu M-T, Alexandrov B, Shadrin N, Zaitsev Yu. (2002) The Black Sea – A Recipient, Donor and Transit Area for Alien Species. In: Leppäkoski E, Gollasch S, Olenin S. (Eds) Invasive Aquatic Species of Europe.Distribution, Impacts and Management. Springer, Dordrecht, 341–350. 10.1007/978-94-015-9956-6_35 [DOI]
- Graf DL, Cummings KS. (2018) The Freshwater Mussels (Unionoida) of the World (and other less consequential bivalves), updated 9 August 2018. MUSSEL Project Web Site. http://www.mussel-project.net
- Gray JE. (1825) A List and Description of some Species of Shells not taken Notice of by Lamarck. Annals of Philosophy, new series 9: 134–140. https://biodiversitylibrary.org/page/15880862 [Google Scholar]
- Gray JE. (1840) A manual of the land and freshwater shells of the British Islands, with figures of each of the kinds. By William Turton, MD A new edition, thoroughly revised and much enlarged. Longman, Orme, Brown, Green, and Longmans, Paternoster Row, London, 324 pp https://biodiversitylibrary.org/page/18243759 [Google Scholar]
- Gray JE. (1843) Catalogue of the Species of Mollusca and their Shells, which have hitherto been recorded as found at New Zealand, with the Description of some lately discovered Species. In: Dieffenbach E. (Ed.) Travels in New Zealand; with contributions to the geography, geology, botany, and natural history of that country.Vol. II. John Murray, London, 228–265. https://biodiversitylibrary.org/page/20760205
- Grigorovich IA, Therriault TW, MacIsaac HJ. (2003) History of aquatic invertebrate invasions in the Caspian Sea. Biological Invasions 5: 103–115. 10.1023/A:1024050824073 [DOI] [Google Scholar]
- Grimm OA. (1876) Kaspiyskoye more i yego fauna. Tetrad’ 1. Trudy Aralo-Kaspiyskoy Ekspeditsii 2: 1–168. http://www.cawater-info.net/library/rus/hist/2_grimm.pdf [Google Scholar]
- Grimm OA. (1877) Kaspiyskoye more i yego fauna. Tetrad’ 2. Trudy Aralo-Kaspiyskoy Ekspeditsii 2(2): 1–105. http://www.cawater-info.net/library/rus/hist/2_grimm.pdf [Google Scholar]
- Grossu AV. (1951) Potamopyrgusjenkinsi, gasteropod nou pentru apele continentale ale Republicii Populare Romine. Comunicarile Academiei Republicii Populare Române 1(7): 593–596. [Google Scholar]
- Grossu AV. (1973) Les Limnocardiides actuelles du Bassin Ponto-Caspique. Informations de la Société Belge de Malacologie 2(7–8): 123–152. [Google Scholar]
- Grossu AV. (1986) Le genre Pseudamnicola Paulaci, 1868 en Roumanie et description de quelques nouvelles espèces (Prosobranchia, Hydrobiidae). Apex. Informations scientifiques de la Société Belge de Malacologie 1(1): 7–17. http://www.biodiversitylibrary.org/item/129784#page/23/mode/1up [Google Scholar]
- Haase M, Naser MD, Wilke T. (2010) Ecrobiagrimmi in brackish Lake Sawa, Iraq: indirect evidence for long-distance dispersal of hydrobiid gastropods (Caenogastropoda: Rissooidea) by birds. Journal of Molluscan Studies 76: 101–105. 10.1093/mollus/eyp051 [DOI] [Google Scholar]
- Heiler KCM, Nahavandi N, Albrecht C. (2010) A New Invasion Into an Ancient Lake – The Invasion History of the Dreissenid Mussel Mytilopsisleucophaeata (Conrad, 1831) and Its First Record in the Caspian Sea. Malacologia 53(1): 185–192. 10.4002/040.053.0112 [DOI] [Google Scholar]
- Hilgendorf F. (1867) Über Planorbismultiformis im Steinheimer Süßwasserkalk. Monatsberichte der Königlich-Preussischen Akademie der Wissenschaften zu Berlin 1866: 474–504. [Google Scholar]
- ICZN (1999) International Code of Zoological Nomenclature. International Trust for Zoological Nomenclature, London, 306 pp http://www.nhm.ac.uk/hosted-sites/iczn/code/index.jsp [Google Scholar]
- Issel A. (1865) Catalogo dei molluschi raccolti dalla missione italiana in Persia aggiuntavi la descrizione delle specie nuove o poco note. Stamperia Reale, Torino, 55 pp http://magteca-fi-ese.inera.it/unifi/opac/unifi/scheda.jsp?pid=mag:13023 [Google Scholar]
- Kadolsky D. (2008) Mollusks from the Late Oligocene of Oberleichtersbach (Rhön Mountains, Germany). Part 2: Gastropoda. Neritimorpha and Caenogastropoda. Courier Forschungsinstitut Senckenberg 260: 103–137. [Google Scholar]
- Kadolsky D. (2012) Nomenclatural comments on non-marine molluscs occurring in the British Isles. Journal of Conchology 41(1): 65–90. [Google Scholar]
- Kalitskiy KP. (1914) Neftyanaya gora (Zakaspiyskaya oblast’). Trudy Geologicheskogo Komiteta 95: 1–78. [Google Scholar]
- Kantor YuI, Sysoev AV. (2006) Morskiye i solonovatovodnyye bryukhonogiye mollyuski Rossii i sopredel’nykh stran: illyustrirovannyy katalog. KMK Scientific Press, Moscow, 372 pp. [140 pls] [Google Scholar]
- Kebapçı U, Van Damme D. (2012) Theodoxusfluviatilis (errata version published in 2017). The IUCN Red List of Threatened Species 2012: e.T165352A113400624. 10.2305/IUCN.UK.2012-1.RLTS.T165352A1081028.en [Accessed on 05 December 2018] [DOI]
- Kevrekidis T, Wilke T, Mogias A. (2005) When DNA puts ecological works back on the right track: genetic assessment and distribution patterns of mudsnail populations in the Evros Delta lagoons. Archiv für Hydrobiologie 162(1): 19–35. 10.1127/0003-9136/2005/0162-0019 [DOI] [Google Scholar]
- Kijashko PV. (2013) Glava 5. Mollyuski kaspiyskogo morya. In: Bogutskaya NG, Kijashko PV, Naseka AM, Orlova MI (Eds) Opredelitel’ ryb i bespozvonochnykh Kaspiyskogo morya. T. 1. Ryby i mollyuski. KMK Scientific Press Ltd., St. Petersburg, Moscow.
- Kohn AJ. (1972) Conusmiliaris at Easter Island – ecological release of diet and habitat in an isolated population. American Zoologist 12: 712.
- Kolesnikov VP. (1947) Tablitsa dlya opredeleniya kaspiyskikh gastropod. Byulleten’ Moskovskogo Obshchestva Ispytateley Prirody, otdel geologicheskiy 22(1): 105–112. [Google Scholar]
- Kolesnikov VP. (1950) Paleontologiya SSSR. Tom X, Chast’ 3, Vyp. 12: Akchagyl’skie i apsheronskie mollyuski. Izdatel’stvo Akademii nauk SSSR, Moskva, Leningrad, 259 pp. [Google Scholar]
- Kosarev AN, Yablonskaya EA. (1994) The Caspian Sea. SPB Academic Publishing, The Hague, 259 pp. [Google Scholar]
- Kostianoy AG, Kosarev AN. (2005) The Caspian Sea Environment. Springer, Berlin, 271 pp 10.1007/b138238 [DOI] [Google Scholar]
- Krijgsman W, Tesakov A, Yanina T, Lazarev S, Danukalova G, Van Baak CGC, Agustí J, Alçiçek MC, Aliyeva E, Bista D, Bruch A, Büyükmeriç Y, Bukhsianidze M, Flecker R, Frolov P, Hoyle TM, Jorissen EL, Kirscher U, Koriche SA, Kroonenberg SB, Lordkipanidze D, Oms O, Rausch R, Singarayer J, Stoica M, van de Velde S, Titov VV, Wesselingh FP. (2019) Quaternary time scales for the Pontocaspian domain: Interbasinal connectivity and faunal evolution. Earth-Science Reviews. 10.1016/j.earscirev.2018.10.013 [DOI]
- Kroll O, Hershler R, Albrecht C, Terrazas EM, Apaza R, Fuentealba C, Wolff C, Wilke T. (2012) The endemic gastropod fauna of Lake Titicaca: correlation between molecular evolution and hydrographic history. Ecology and Evolution 2(7): 1517–1530. 10.1002/ece3.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynicki AJ. (1837) Conchylia tam terrestria, quam fluviatilia et e maribus adjacentibus Imperii Rossici indigena, quae pro mutua offeruntur historiae naturalis cultoribus commutatione. Bulletin de la Société Impériale des Naturalistes de Moscou 10(2): 50–64. https://biodiversitylibrary.org/page/5521585 [Google Scholar]
- Küster HC. (1852–1853) Die Gattungen Paludina, Hydrocaena und Valvata. In Abbildungen nach der Natur mit Beschreibungen. Systematisches Conchylien-Cabinet von Martini und Chemnitz 1(21). Bauer & Raspe, Nürnberg, 96 pp https://biodiversitylibrary.org/page/34226358 [Google Scholar]
- Lamarck J-BPAdMd. (1809) Philosophie Zoologique, ou exposition des considérations relatives à l’histoire naturelle des Animaux; à la diversité de leur organisation et des facultés qu’ils en obtiennent; aux causes physiques qui maintiennent en eux la vie et donnent lieu aux mouvements qu’ils exécutent; enfin à celles qui produisent les unes le sentiment, et les autres l’intelligence de ceux qui en sont doués. Dentu, Paris, 428 pp [Vol. 1]; 475 pp [Vol. 2].
- Lamarck J-BPAdMd. (1819) Histoire naturelle des animaux sans vertèbres, présentant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres, et la citation des principales espèces qui s’y rapportent; précédée d’une introduction offrant la détermination des caractères essentiels de l’animal, sa distinction du végétal et des autres corps naturels, enfin, l’exposition des principes fondomentaux de la zoologie. Tome sixième, première partie. Privately published, Paris, 343 pp https://biodiversitylibrary.org/page/13181542 [Google Scholar]
- Latypov YuYa. (2015) The Bivalve Mollusc Abraovata: Role in Succession of Soft Bottom Communities on Newly Flooded Area of the Caspian Sea. American Journal of Climate Change 4: 239–247. 10.4236/ajcc.2015.43019 [DOI] [Google Scholar]
- Leroy SAG, Chalié F, Wesselingh F, Sanjani S, Lahijani HAK, Athersuch J, Struck U, Plunkett G, Reimer PJ, Habibi P, Kabiri K, Haghani S, Naderi Beni A, Arpe K. (2018) Multiproxy indicators in a Pontocaspian system: a depth transect of surface sediment in the S-E Caspian Sea. Geologica Belgica 21(3–4): 143–165. 10.20341/gb.2018.008 [DOI] [Google Scholar]
- Lindholm VA. (1901) Beiträge zur Kenntniss der Weichthierfauna Süd-Russlands. Nachrichtsblatt der Deutschen Malakozoologischen Gesellschaft 33(11–12): 161–186. https://biodiversitylibrary.org/page/15598704 [Google Scholar]
- Lindholm VA. (1908) Materialien zur Molluskenfauena [sic] von Südwestrussland, Polen und der Krim. Zapiski Novorossijskago Obshchestva Estestvoispytatelej 31: 199–232. [Google Scholar]
- Lindholm VA. (1924) K nomenklature nekotorykh kaspiyskikh gastropod. Russkiy Gidrobiologicheskiy Zhurnal 3(1–2): 32–34. [Google Scholar]
- Linnaeus C. (1758) Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. Laurentius Salvius, Holmiae, 824 pp https://biodiversitylibrary.org/page/726886 [Google Scholar]
- Logvinenko BM, Starobogatov YaI. (1966a) Novyye dvustvorchatyye mollyuski iz kaspiyskoy profundali. Nauchnyye doklady vysshey shkoly. Biologicheskiye nauki 2: 13–16. [Google Scholar]
- Logvinenko BM, Starobogatov YaI. (1966b) Mollyuski semeystva Planorbidae Kaspiya. Zoologicheskiy Zhurnal 45(10): 1467–1475. [Google Scholar]
- Logvinenko BM, Starobogatov YaI. (1967) K izucheniyu vidovogo sostava fauny dvustvorchatykh mollyuskov tanatotsenozov podvodnogo sklona Azerbaydzhanskogo poberezh’ya Kaspiya. In: Kudritskiy DM. (Ed.) Opyt geologo-geomorfologicheskikh i gidrobiologicheskikh issledovaniy beregovoy zony morya.Nauka, Leningrad, 225–235.
- Logvinenko BM, Starobogatov YaI. (1969) Mollusca. In: Birshtein YaA, Vinogradov LG, Kondakov NN, Kuhn MS, Astakhova TV, Romanova NN. (Eds) Atlas bespozvonochnykh Kaspiyskogo morya.Pishchevaya Promyshlennost (Vsesoyuznyi Nauchno-issledovatel’skii Institut Morskogo Rybnogo Khozyaistva i Okeanografii), Moskva, 308–385.
- Mabille J. (1877) Catalogue des Paludestrines des côtes de France. Revue et Magasin de Zoologie, 3e Série 5: 214–222. https://biodiversitylibrary.org/page/33779087
- Mainguet M, Létolle R. (1997) The Ecological Crisis of the Aral Sea Basin in the Frame of a New Time Scale: The “Anthropo-Geological Scale”. Naturwissenschaften 84(8): 331–339. 10.1007/s001140050406 [DOI] [Google Scholar]
- Makarov AK. (1938) Rasprostraneniye nekotorykh rakoobraznykh (Mysidacea, Cumacea) i limannykh mollyuskov v ust’yakh i otkrytykh limanakh Severnogo Prichernomor’ya. Zoologicheskiy Zhurnal 17(6): 1055–1062. [Google Scholar]
- Martens Ev. (1874) Ueber vorderasiatische Conchylien nach den Sammlungen des Prof. Hausknecht. Novitates conchologicae. Supplement 5: 1–127. https://biodiversitylibrary.org/page/12980992 [Google Scholar]
- Matthews SC. (1973) Notes on open Nomenclature and on Synonymy Lists. Palaeontology 16(4): 713–719. https://www.palass.org/sites/default/files/media/publications/palaeontology/volume_16/vol16_part4_pp713-719.pdf [Google Scholar]
- Micklin P, Aladin NV, Plotnikov I. (2014) The Aral Sea. The Devastation and Partial Rehabilitation of a Great Lake. Springer, Berlin, 453 pp. [Google Scholar]
- Milaschewitch KO. (1908) Mollyuski, sobrannyye vo vremya ekskursii S.A. Zernova na minonostse No. 264 na r. Dunay s 28 iyunya po 3 iyulya 1907 goda. Bulletin de l’Académie Impériale des Sciences de St. -Pétersbourg, sixième série 2(12): 991–996. [Google Scholar]
- Milaschewitch KO. (1916) Mollyuski russkikh morey. Tom 1. Mollyuski Chernago i Azovskago morey. Imperatorskaya Akademiya Nauk, Zoologicheskiy Muzey, Petrograd, 312 pp http://www.biodiversitylibrary.org/item/44223 [Google Scholar]
- Mirzoev GS, Alekperov IH. (2017) Zoobenthos distribution patterns in the deepwater horizon of Azerbaijan sector of the Caspian Sea. International Journal of Zoological Studies 2: 43–48. http://www.zoologyjournals.com/download/74/1-7-29-696.pdf [Google Scholar]
- MolluscaBase (2018a) MolluscaBase. http://www.molluscabase.org [Accessed on 2018-09-28]
- MolluscaBase (2018b) Adacnafragilis Milaschewitch, 1908. World Register of Marine Species. http://marinespecies.org/aphia.php?p=taxdetails&id=381870 [Accessed on 2018-09-05]
- Montagu G. (1803) Testacea Britannica, or natural history of British shells, marine, land, and fresh-water, including the most minute: systematically arranged and embellished with figures. White, London, 606 pp https://biodiversitylibrary.org/page/24430071 [Google Scholar]
- Mordukhay-Boltovskoy FD. (1960) Kaspiyskaya fauna v Azovo-Chernomorskom basseyne. Izdatel’stvo Akademii Nauk SSSR, Leningrad, 228 pp. [Google Scholar]
- Mousson A. (1863) Coquilles terrestres et fluviatiles, recueillies dans l’Orient par M le Dr Alex Schläfli. Vierteljahrsschrift der Naturforschenden Gesellschaft in Zürich 8: 275–320, 368–426. https://biodiversitylibrary.org/page/8403222
- Müller OF. (1773–1774) Vermium terrestrium et fluviatilium historia, seu animalium Infusoriorum, Helminthicorum et Testaceorum non marinorum succincta historia. Volumen alterum. Heineck & Faber, Havniae et Lipsiae, 214 pp https://biodiversitylibrary.org/page/32096857 [Google Scholar]
- Munasypova-Motyash IA. (2006a) O sovremennoy faune dvustvorchatykh mollyuskov podsemeystva Limnocardiinae (Bivalvia, Cardiidae) Severo-Zapadnogo Prichernomor’ya. Vestnik Zoologii 40(1): 41–48. http://dspace.nbuv.gov.ua/bitstream/handle/123456789/9419/03_Munasipova.pdf?sequence=3 [Google Scholar]
- Munasypova-Motyash IA. (2006b) Morfometricheskiye priznaki rakoviny dvustvorchatykh mollyuskov podsemeystva Limnocardiinae (Bivalvia, Cardiidae) i ikh znacheniye v taksonomii gruppy. Vestnik Zoologii 40(6): 521–527. http://www.v-zool.kiev.ua/pdfs/2006/6/05_Munasypova.pdf [Google Scholar]
- Nabozhenko MV, Nabozhenko SV. (2016) Corbiculafluminalis (O.F. Müller, 1774) – novyy dlya rossiyskogo sektora kaspiyskogo basseyna vid dvustvorchatykh mollyuskov. Nauka Yuga Rossii (Vestnik Yuzhnogo Nauchnogo Tsentra) 12(1): 61–64. http://www.ssc-ras.ru/ckfinder/userfiles/files/61-64_Nabozhenko_1_2016.pdf [Google Scholar]
- Nalivkin DV. (1915) Mollyuski gory Bakinskogo yarusa. Trudy Geologicheskogo Komiteta, novaya seriya 116: 1–32. [Google Scholar]
- Nalivkin DV, Anisimov A. (1914) Opisaniye glavneyshikh mestnykh form roda Didacna Eichwald iz postpliotsena Apsheronskogo poluostrova. Trudy Geologicheskogo Komiteta, novaya seriya 117: 1–22. [Google Scholar]
- Neubauer TA, van de Velde S, Yanina TA, Wesselingh FP. (2018) A late Pleistocene gastropod fauna from the northern Caspian Sea with implications for Pontocaspian gastropod taxonomy. ZooKeys 770: 43–103. 10.3897/zookeys.770.25365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevesskaja LA. (1958) Chetvertichnyye morskiye mollyuski Turkmenii. Trudy Paleontologicheskogo Instituta 65: 1–82. [Google Scholar]
- Nevesskaja LA. (1963) Opredelitel’ dvustvorchatykh mollyuskov morskikh chetvertichnykh otlozheniy Chernomorskogo basseyna. Trudy Paleontologicheskogo Instituta 96: 1–211. [Google Scholar]
- Nevesskaja LA. (1965) Pozdnechetvertichnyye dvustvorchatyye mollyuski Chernogo morya, ikh sistematika i ekologiya. Trudy Paleontologicheskogo Instituta 105: 1–391. [Google Scholar]
- Nevesskaja LA. (2007) History of the Genus Didacna (Bivalvia: Cardiidae). Paleontological Journal 41(9): 861–949. 10.1134/S0031030107090018 [DOI] [Google Scholar]
- Nikula R, Väinölä R. (2003) Phylogeography of Cerastodermaglaucum (Bivalvia: Cardiidae) across Europe: a major break in the Eastern Mediterranean. Marine Biology 143(2): 339–350. 10.1007/s00227-003-1088-6 [DOI] [Google Scholar]
- Occhipinti-Ambrogi A, Savini D. (2003) Biological invasions as a component of global change in stressed marine ecosystems. Marine Pollution Bulletin 46(5): 542–551. 10.1016/S0025-326X(02)00363-6 [DOI] [PubMed] [Google Scholar]
- Orlova MI, Muirhead JR, Antonov PI, Shcherbina GKh, Starobogatov YaI, Biochino GI, Therriault TW, MacIsaac HJ. (2005) Range expansion of quagga mussels Dreissenarostriformisbugensis in the Volga River and Caspian Sea basin. Aquatic Ecology 38(4): 561–573. 10.1007/s10452-005-0311-6 [DOI] [Google Scholar]
- Osikowski A, Hofman S, Georgiev D, Kalcheva S, Falniowski A. (2016) Aquatic snails Ecrobiamaritima (Milaschewitsch, 1916) and E.ventrosa (Montagu, 1803) (Caenogastropoda: Hydrobiidae) in the East Mediterranean and Black Sea. Annales Zoologici 66(3): 477–486. 10.3161/00034541ANZ2016.66.3.012 [DOI] [Google Scholar]
- Ostroumov AA. (1905) Poyezdka na Kaspiy. Trudy Obshchestva yestestvoispytateley pri Kazanskom universitete 39(6): 1–84. [Google Scholar]
- Ostroumov A. (1907) O mollyuskakh Aralskago morya. Izvestiya Turkestanskogo otdela Imperatorskogo Russkogo Geograficheskogo Obshchestva 4: 20–26. https://lib.rgo.ru/reader/flipping/Resource-9382/RuPRLIB12047877/index.html [Google Scholar]
- Paladilhe A. (1867) Nouvelles Miscellanées malacologiques. Revue et magasin de zoologie pure et appliquée, deuxième série 19: 88–95. https://biodiversitylibrary.org/page/2704268 [Google Scholar]
- Paladilhe A. (1869) Descriptions de quelques Paludinées, Assiminidées et Mélanidées. Revue et Magasin de Zoologie Pure et Appliquée, deuxième série 21: 225–237, 273–284, 316–325, 379–383. https://biodiversitylibrary.org/page/33749249
- Pallas PS. (1771) Reise durch verschiedene Provinzen des Rußischen Reichs. Erster Theil. Kayserliche Academie der Wissenschaften, St. Petersburg, 504 pp http://resolver.sub.uni-goettingen.de/purl?PPN329913735 [Google Scholar]
- Pfeiffer C. (1828) Naturgeschichte deutscher Land- und Süsswasser-Mollusken. Dritte Abtheilung. Landes-Industrie-Comptoir, Weimar, 84 pp https://opacplus.bsb-muenchen.de/search?oclcno=229922635&db=100&View=default [Google Scholar]
- Philippi RA. (1836) Enumeratio Molluscorum Siciliae cum viventium tum in tellure teriaria fossilium, quae in itinere suo observavit. Vol. 1. Simon Schropp, Berlin, 267 pp http://reader.digitale-sammlungen.de/de/fs2/object/display/bsb10231737_00007.html [Google Scholar]
- Pirogov VV. (1971) O nakhozhdenii novogo vida mollyuska iz roda Pyrgula Crist. et Jan. v avandel’tye reki Volgi. Trudy Astrakhanskogo Zapovednika imeni V.I. Lenina 13: 249–253. [Google Scholar]
- Plaziat J-C. (1991) Paleogeographic significance of the Cardium, Potamids and Foraminifera living in intra-continental salt lakes of North Africa (Sahara Quaternary, Egypt Present lakes). Journal of African Earth Sciences (and the Middle East) 12(1–2): 383–389. 10.1016/0899-5362(91)90087-F [DOI] [Google Scholar]
- Plotnikov IS, Ermakhanov ZK, Aladin NV, Micklin P. (2016) Modern state of the Small (Northern) Aral Sea fauna. Lakes & Reservoirs: Science, Policy and Management for Sustainable Use 21(4): 315–328. 10.1111/lre.12149 [DOI] [Google Scholar]
- Poiret JLM. (1789) Voyage en Barbarie, ou Lettres écrites de l’Ancienne Numidie. Pendant les années 1785 & 1786, sur la Religion, les Contumes & les Moeurs des Maures des Arabes-Bédouins; avec un Essai sur l’Histoire Naturelle de ce pays. Seconde partie. JBF Née de la Rochelle, Paris, 319 pp https://biodiversitylibrary.org/page/13528290 [Google Scholar]
- Poli IX. (1795) Testacea utriusque Siciliae eorumque historia et anatome tabulis aeneis illustrata. Tomus secundus. Regio Typographeio, Parma, 75–264. [pls 19–39] http://gallica.bnf.fr/ark:/12148/bpt6k98950s/f1.image
- Ponder WF. (1988) Potamopyrgusantipodarum – a molluscan coloniser of Europe and Australia. Journal of Molluscan Studies 54(3): 271–285. 10.1093/mollus/54.3.271 [DOI] [Google Scholar]
- Popa L, Popa O, Iorgu E, Kelemen B, Murariu D. (2012) Molecular insights into the taxonomy of Hypanis (Bivalvia, Cardiidae, Lymnocardiinae) in the Black Sea lagoons. Helgoland Marine Research 66(2): 153–158. 10.1007/s10152-011-0256-1 [DOI] [Google Scholar]
- Popa OP, Sárkány-Kiss A, Kelemen B, Iorgu EI, Murariu D, Popa LO. (2009) Contributions to the knowledge of the present Limnocardiinae fauna (Mollusca: Bivalvia) from Romania. Travaux du Muséum National d’Histoire Naturelle “Grigore Antipa” 52: 7–15. [Google Scholar]
- Popov GI. (1983) Pleystotsen Chernomorsko-Kaspiyskikh prolivov (stratigrafiya, korrelyatsiya, paleofaunistika, geologicheskaya istoriya). Nauka, Moskva, 216 pp. [Google Scholar]
- Pravoslavlev PA. (1950) Nekotoryye zamechaniya o gruppe sovremennykh Didacnatrigonoides Pall. Uchenyye zapiski Leningradskogo Gosudarstvennogo Universiteta, seriya geologichekskikh nauk 102(1): 20–27. [Google Scholar]
- Prié V. (2011) Heleobiastagnorum The IUCN Red List of Threatened Species 2011: e.T155989A4879741. 10.2305/IUCN.UK.2011-2.RLTS T155989A4879741.en [Accessed on 05 December 2018] [DOI]
- Put’ AL. (1972) Do vyvchennya lunkovykh (Neritidae) Ukrayini. Dopovidi Akademii Nauk Ukrainskoi SSR, Seriya B, Geologiya, Khimiya, Biologiya 1: 78–83. [Google Scholar]
- Radoman P. (1973) New classification of fresh and brakish water Prosobranchia from the Balkans and Asia Minor. Prirodnjacki Muzej u Beogradu, Posebna Izdanja 32: 3–30. [Google Scholar]
- Radoman P. (1977) Hydrobiidae auf der Balkanhalbinsel und in Kleinasien. Archiv für Molluskenkunde 107(4/6): 203–223.
- Rafinesque CS. (1815) Analyse de la nature ou tableau de l’univers et des corps organisés. Privately published by author, Palermo, 223 pp http://gallica.bnf.fr/ark:/12148/bpt6k98061z [Google Scholar]
- Récluz CA. (1843) Monographie du genre Syndosmya. Revue Zoologique, par la Société Cuvierienne 6: 359–369. http://biodiversitylibrary.org/page/2271434 [Google Scholar]
- Reeve LA. (1844–1845) Monograph of the genus Cardium. In: Reeve LA (Ed.) Conchologia Iconica: or, Illustrations of the Shells of Molluscous Animals. Vol. II. Privately published, London, 22 pp https://biodiversitylibrary.org/page/8937393 [Google Scholar]
- Rosenberg G, Ludyanskiy ML. (1994) A Nomenclatural Review of Dreissena (Bivalvia: Dreissenidae), with Identification of the Quagga Mussel as Dreissenabugensis. Canadian Journal of Fisheries and Aquatic Sciences 51: 1474–1484. 10.1139/f94-147 [DOI] [Google Scholar]
- Scarlato OA, Starobogatov YaI. (1972) Klass dvustvorchatyye mollyuski. In: Mordukhay-Boltovskoy FD. (Ed.) Opredelitel’ fauny Chernogo i Azovskogo morey: Svobodnozhivushchiye bespozvonochnyye.T.3. Chlenistonogiye (krome rakoobraznykh), Mollyuski, Iglokozhiye, Shchetinkochelyustnyye, Khordovyye. Naukova dumka, Kiev, 178–249.
- Schultze FTS. (1826) Catalog der Conchylien-Sammlung des verstorbenen Herrn Ober-Einnehmers Freiherrn von der Malsburg, in deren Besitz sich jetzt befindet der Herr Kammerherr Baron v. d. Malsburg zu Escheberg bei Cassel in Cur-Hessen. Berlin, 199 pp.
- Seddon MB, Van Damme D. (2016) Corbiculafluminalis The IUCN Red List of Threatened Species 2016: e.T98201936A98201989. 10.2305/IUCN.UK.2016-3.RLTS.T98201936A98201989.en [Accessed on 20 October 2018] [DOI]
- Selifonova JP. (2008a) Functioning of the Sea of Azov ecosystem. Inland Water Biology 1(3): 199–203. 10.1134/S1995082908030012 [DOI] [Google Scholar]
- Selifonova ZhP. (2008b) Taxonomic composition and interannual variations in numerical density of meroplankton in the Sea of Azov. Russian Journal of Marine Biology 34(5): 263–269. 10.1134/S1063074008050015 [DOI] [Google Scholar]
- Shalovenkov N. (2005) Restoration of Some Parameters in the Development of Benthos After Reduction of Anthropogenous Loading in the Ecosystem of the Sevastopol Bay in the Black Sea. Mitigation and Adaptation Strategies for Global Change 10(1): 105–113. 10.1007/s11027-005-7833-z [DOI] [Google Scholar]
- Shishkoedova OS. (2010) Pervaya nakhodka mollyuskov roda Caspiohydrobia (Mollusca: Gastropoda) v Chelyabinskoy oblasti. [Ecology: from the southern mountains to the northern seas. Materials of the young scientists’ meeting, 19–23 April, 2010, Yekaterinburg.] Goschchitsky Publisher, Yekaterinburg, 210–213. [in Russian]
- Sitnikova TYa, Starobogatov YaI. (1999) Novyy rod semeystva Pyrgulidae (Gastropoda, Pectinibranchia) iz presnykh vod Azovo-Chernomorskogo basseyna (v svyazi s voprosom o Ponto-Kaspiyskikh vidakh v Azovo-Chernomorskom basseyne). Zoologicheskiy Zhurnal 78(2): 158–163. [Google Scholar]
- Son M. (2011a) Caspiagmelinii The IUCN Red List of Threatened Species 2011: e.T155474A4782113. 10.2305/IUCN.UK.2011-1.RLTS.T155474A4782113.en [Accessed on 05 December 2018] [DOI]
- Son M. (2011b) Caspiaknipowitchi The IUCN Red List of Threatened Species 2011: e.T156116A4900657. 10.2305/IUCN.UK.2011-1.RLTS.T156116A4900657.en [Accessed on 05 December 2018] [DOI]
- Son M. (2011c) Caspiamakarovi The IUCN Red List of Threatened Species 2011: e.T155680A4822960. 10.2305/IUCN.UK.2011-1.RLTS.T155680A4822960.en [Accessed on 05 December 2018] [DOI]
- Son M. (2011d) Turricaspiachersonica The IUCN Red List of Threatened Species 2011: e.T155738A4835520. 10.2305/IUCN.UK.2011-1.RLTS.T155738A4835520.en [Downloaded on 05 December 2018] [DOI]
- Son M. (2011e) Turricaspialincta The IUCN Red List of Threatened Species 2011: e.T155627A4811075. 10.2305/IUCN.UK.2011-1.RLTS.T155627A4811075.en [Accessed on 05 December 2018] [DOI]
- Son M, Cioboiu O. (2011) Turricaspiaismailensis The IUCN Red List of Threatened Species 2011: e.T155600A4806726. 10.2305/IUCN.UK.2011-1.RLTS.T155600A4806726.en [Accessed on 05 December 2018] [DOI]
- Sromek L, Forcioli D, Lasota R, Furla P, Tarnowska-Marini K, Wolowicz M, Chenuil A. (2016) Strong genetic structuring of the cockle Cerastodermaglaucum across Europe: new insights from an intronic marker and multivariate analysis. Journal of Molluscan Studies 82(4): 515–524. 10.1093/mollus/eyw019 [DOI] [Google Scholar]
- Starobogatov YaI. (1968) Prakticheskiye priyomy sistematiki i vopros o kriterii vida. Zoologicheskiy Zhurnal 47(6): 875–886. [Google Scholar]
- Starobogatov YaI. (1970) Fauna mollyuskov i zoogeographicheskoye rayonirovaniye kontinental’nykh vodoemov zemnogo shara. Nauka, Leningrad, 372 pp. [Google Scholar]
- Starobogatov YaI. (1974) Phylum Mollusca. In: Mordukhay-Boltovskoy FD. (Ed.) Atlas bespozvonochnykh Aral’skogo Morya.Pishchevaya Promyshlennost’, Moscow, 237–257.
- Starobogatov YaI. (2000) Caspian endemic genus Andrusovia (Gastropoda Pectinibranchia Horatiidae). Ruthenica 10(1): 37–42. [Google Scholar]
- Starobogatov YaI, Filchakov VA, Antonova LA, Pirogov VV. (1994) Novyye dannyye o mollyuskakh i vysshikh rakoobraznykh delty Volgi. Vestnik Zoologii 4–5: 8–12.
- Starobogatov YaI, Prozorova LA, Bogatov VV, Sayenko EM. (2004) Mollyuski. In: Tsalolikhin SJ. (Ed.) Opredelitel’ presnovodnykh bespozvonochnykh Rossii i sopredel’nykh territoriy.T. 6. Mollyuski, Polikhety, Nemertiny. Izdatel’stvo “Nauka”, St. Petersburg, 9–491.
- Stepien CA, Grigorovich IA, Gray MA, Sullivan TJ, Yerga-Woolwine S, Kalayci G. (2013) Evolutionary, Biogeographic, and Population Genetic Relationships of Dreissenid Mussels, with Revision of Component Taxa. In: Nalepa TF, Schloesser DW. (Eds) Quagga and Zebra Mussels: Biology, Impacts, and Control.2nd edition. CRC Press, Boca Raton, 403–444. 10.1201/b15437-33 [DOI]
- Stimpson W. (1865) Researches upon the Hydrobiinae and allied forms: chiefly made from materials in the Museum of the Smithsonian Institution. Smithsonian Miscellaneous Collections 7: 1–59. https://biodiversitylibrary.org/page/8817453 [Google Scholar]
- Stolberg FV, Borysova O, Mitrofanov I, Barannik V, Eghtesadi P. (2006) Global International Water Assessment 23. Caspian Sea. University of Kalmar (on behalf of United Nations Environment Programme). Kalmar, 71 pp.
- Stoliczka F. (1870–1871) Cretaceous fauna of southern India. The Pelycopoda, with a review of all known Genera of this class, fossil and recent. Palaeontologia Indica, being figures and description s of the organic remains procured during the progress of the Geological Survey of India. Memoirs of the Geological Survey of India 6(3): 1–538. [Google Scholar]
- Svitoch AA. (1967) Atlas-opredelitel’ mollyuskov roda Didacna Eichwald iz chetvertichnykh otlozheniy Tsentral’nogo Prikaspiya. Nedra, Moskva, 87 pp. [Google Scholar]
- Tadjalli-Pour M. (1977) Les Mollusques marins des côtes Iraniennes de la Mer Caspienne (Astara-Hachtpar). Journal de Conchyliologie 114(3–4): 87–117. [Google Scholar]
- Taviani M, Angeletti L, Çagatay MN, Gasperini L, Polonia A, Wesselingh FP. (2014) Sedimentary and faunal signatures of the post-glacial marine drowning of the Pontocaspian Gemlik “lake” (Sea of Marmara). Quaternary International 345: 11–17. 10.1016/j.quaint.2014.05.045 [DOI] [Google Scholar]
- Therriault TW, Docker MF, Orlova MI, Heath DD, MacIsaac HJ. (2004) Molecular resolution of the family Dreissenidae (Mollusca: Bivalvia) with emphasis on Ponto-Caspian species, including first report of Mytilopsisleucophaeata in the Black Sea basin. Molecular Phylogenetics and Evolution 30(3): 479–489. 10.1016/S1055-7903(03)00240-9 [DOI] [PubMed] [Google Scholar]
- Thiele J. (1925–1926) Mollusca = Weichtiere. In: Kükenthal W, Krumbach T. (Eds) Handbuch der Zoologie.De Gruyter, Berlin & Leipzig, 15–266.
- Tomovic J, Bodon M, Giusti F, Manganelli G, Cioboiu O, Beran L. (2010) Theodoxusdanubialis. The IUCN Red List of Threatened Species 2010: e.T165349A6005150. [Accessed on 05 December 2018]
- Tryon GW. (1866) [Book review of] Researches upon the Hydrobiinae and allied forms by Dr. Wm. Stimpson, 8 vol. Smithsonian Institution, Washington DC, August 1865, 58 p. American Journal of Conchology 2(2): 152–158. https://biodiversitylibrary.org/page/6660366 [Google Scholar]
- UNEP [United Nations Environment Programme] (2006) Annual Report. https://wedocs.unep.org/bitstream/handle/20.500.11822/7476/-UNEP%202006%20Annual%20Report-2007755.pdf
- Van Damme D. (2011a) Hydrobiaventrosa The IUCN Red List of Threatened Species 2011: e.T155734A4834019. 10.2305/IUCN.UK.2011-2.RLTS.T155734A4834019.en [Accessed on 05 December 2018] [DOI]
- Van Damme D. (2011b) Lithoglyphusnaticoides The IUCN Red List of Threatened Species 2011: e.T155563A4798694. 10.2305/IUCN.UK.2011-2.RLTS.T155563A4798694.en [Accessed on 05 December 2018] [DOI]
- Van Damme D. (2013) Potamopyrgusantipodarum The IUCN Red List of Threatened Species 2013: e.T155980A738398. 10.2305/IUCN.UK.2013-2.RLTS.T155980A738398.en [Accessed on 05 December 2018] [DOI]
- Van Damme D. (2014) Dreissenapolymorpha The IUCN Red List of Threatened Species 2014: e.T155495A42428801. 10.2305/IUCN.UK.2014-3.RLTS.T155495A42428801.en. [Accessed on 05 December 2018] [DOI]
- Van Damme D, Kebapçı U. (2014) Theodoxuspallasi The IUCN Red List of Threatened Species 2014: e.T165355A42421481. 10.2305/IUCN.UK.2014-3.RLTS.T165355A42421481.en [Accessed on 05 December 2018] [DOI]
- Vekilov BG. (1969) Antropogenovyye otlozheniya severo-vostochnogo Azerbaydzhana. Elm, Baku, 260 pp. [Google Scholar]
- Vinarski MV. (2011a) Anisuskolesnikovi The IUCN Red List of Threatened Species 2011: e.T189408A8727730. 10.2305/IUCN.UK.2011-2.RLTS.T189408A8727730.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011b) Caspiohydrobiagrimmi The IUCN Red List of Threatened Species 2011: e.T189337A8717931. 10.2305/IUCN.UK.2011-2.RLTS.T189337A8717931.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011c) Pseudamnicolabrusiniana The IUCN Red List of Threatened Species 2011: e.T189051A8685851. 10.2305/IUCN.UK.2011-2.RLTS.T189051A8685851.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011d) Pseudamnicoladepressispira The IUCN Red List of Threatened Species 2011: e.T189477A8737258. 10.2305/IUCN.UK.2011-2.RLTS.T189477A8737258.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011e) Pyrgulaabichi The IUCN Red List of Threatened Species 2011: e.T189305A8713559. 10.2305/IUCN.UK.2011-2.RLTS.T189305A8713559.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011f) Pyrgulabehningi The IUCN Red List of Threatened Species 2011: e.T189386A8724662. 10.2305/IUCN.UK.2011-2.RLTS.T189386A8724662.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011g) Pyrgulacincta The IUCN Red List of Threatened Species 2011: e.T189385A8724527. 10.2305/IUCN.UK.2011-2.RLTS.T189385A8724527.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011h) Pyrgulaebersini The IUCN Red List of Threatened Species 2011: e.T189454A8734554. 10.2305/IUCN.UK.2011-2.RLTS.T189454A8734554.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011i) Pyrgulagrimmi The IUCN Red List of Threatened Species 2011: e.T189124A8688657. 10.2305/IUCN.UK.2011-2.RLTS.T189124A8688657.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011j) Pyrgulaisseli The IUCN Red List of Threatened Species 2011: e.T189070A8673755. 10.2305/IUCN.UK.2011-2.RLTS.T189070A8673755.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011k) Pyrgulakolesnikoviana The IUCN Red List of Threatened Species 2011: e.T189244A8705915. 10.2305/IUCN.UK.2011-2.RLTS.T189244A8705915.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011l) Pyrgulanossovi The IUCN Red List of Threatened Species 2011: e.T189508A8741457. 10.2305/IUCN.UK.2011-2.RLTS.T189508A8741457.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011m) Pyrgulapulla The IUCN Red List of Threatened Species 2011: e.T189458A8735062. 10.2305/IUCN.UK.2011-2.RLTS.T189458A8735062.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011n) Pyrgularudis The IUCN Red List of Threatened Species 2011: e.T188922A8662920. 10.2305/IUCN.UK.2011-2.RLTS.T188922A8662920.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011o) Pyrgulasowinskyi The IUCN Red List of Threatened Species 2011: e.T189266A8708984. 10.2305/IUCN.UK.2011-2.RLTS.T189266A8708984.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011p) Turricaspiaconus The IUCN Red List of Threatened Species 2011: e.T189262A8708478. 10.2305/IUCN.UK.2011-2.RLTS.T189262A8708478.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011q) Turricaspiaastrachanica The IUCN Red List of Threatened Species 2011: e.T188872A8655897. 10.2305/IUCN.UK.2011-2.RLTS.T188872A8655897.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011r) Turricaspiadagestanica The IUCN Red List of Threatened Species 2011: e.T189097A8680601. 10.2305/IUCN.UK.2011-2.RLTS.T189097A8680601.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011s) Turricaspiapullula The IUCN Red List of Threatened Species 2011: e.T189467A8736127. 10.2305/IUCN.UK.2011-2.RLTS.T189467A8736127.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011t) Turricaspiasajenkovae The IUCN Red List of Threatened Species 2011: e.T189280A8710891. 10.2305/IUCN.UK.2011-2.RLTS.T189280A8710891.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2011u) Turricaspiaspasskii The IUCN Red List of Threatened Species 2011: e.T189404A8727214. 10.2305/IUCN.UK.2011-2.RLTS.T189404A8727214.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2012) Turricaspiacaspia The IUCN Red List of Threatened Species 2012: e.T189493A1927211. 10.2305/IUCN.UK.2012-1.RLTS.T189493A1927211.en [Accessed on 05 December 2018] [DOI]
- Vinarski MV. (2018) The species question in freshwater malacology: from Linnaeus to the present day. Folia Malacologica 26(1): 39–52. 10.12657/folmal.026.005 [DOI] [Google Scholar]
- Vinarski MV, Karimov AV, Litvinov KV, Podoliako SA. (2018) Presnovodnaya malakofauna Astrakhanskogo zapovednika: Vzglyad iz 21-go veka. Trudy Astrakhanskogo Gosudarstvennogo Prirodnogo Biosfernogo Zapovednika. Astrakhan’ 17: 65–87. [Google Scholar]
- Vinarski MV, Kantor YuI. (2016) Analytical catalogue of fresh and brackish water molluscs of Russia and adjacent countries. A.N. Severtsov Institute of Ecology and Evolution of RAS, Moscow, 544 pp. [Google Scholar]
- Vinarski MV, Nekhaev IO, Glöer P, Proschwitz Tv. (2013) Type materials of freshwater gastropod species described by CA Westerlund and accepted in current malacological taxonomy: a taxonomic and nomenclatorial study. Ruthenica 23(2): 79–108. http://www.ruthenica.com/documents/VOL23_Vinarski_et_al_79-108_standard.pdf [Google Scholar]
- von Rintelen T, Van Damme D. (2011a) Dreissenabugensis The IUCN Red List of Threatened Species 2011: e.T188911A8661357. 10.2305/IUCN.UK.2011-2.RLTS.T188911A8661357.en [Accessed on 05 December 2018] [DOI]
- von Rintelen T, Van Damme D. (2011b) Dreissenacaspia The IUCN Red List of Threatened Species 2011: e.T188971A8669278. 10.2305/IUCN.UK.2011-2.RLTS.T188971A8669278.en [Accessed on 06 September 2018] [DOI]
- von Rintelen T, Van Damme D. (2011c) Dreissenarostriformis The IUCN Red List of Threatened Species 2011: e.T189369A8722237. 10.2305/IUCN.UK.2011-2.RLTS.T189369A8722237.en [Accessed on 05 December 2018] [DOI]
- Welter-Schultes FW. (2012) European non-marine molluscs, a guide for species identification. Planet Poster Editions, Göttingen, 679 pp. [Google Scholar]
- Westerlund CA. (1896) Neue centralasiatische Mollusken. Ezhegodnik Zoologicheskogo Muzeya Imperatorskoy Akademii Nauk 1(3): 181–198.https://biodiversitylibrary.org/page/9006596 [Google Scholar]
- Westerlund CA. (1902a) Malacologische Bemerkungen und Beschreibungen. Nachrichtsblatt der Deutschen Malakozoologischen Gesellschaft 34: 35–47. https://biodiversitylibrary.org/page/15598774 [Google Scholar]
- Westerlund CA. (1902b) Methodus dispositionis Conchyliorum extramarinorum in Regione palaearctica viventium, familias, genera, subgenera et stirpes sistens. Rad Jugoslavenske Akademije Znanosti i Umjetnosti 151: 82–139. http://dizbi.hazu.hr/object/view/km6Jc2154m [Google Scholar]
- Wilke T, Albrecht C, Anistratenko VV, Sahin SK, Yildirim MZ. (2007) Testing biogeographical hypotheses in space and time: faunal relationships of the putative ancient Lake Egirdir in Asia Minor. Journal of Biogeography 34: 1807–1821. 10.1111/j.1365-2699.2007.01727.x [DOI] [Google Scholar]
- Wilke T, Delicado D [in press] Hydrobiidae Stimpson, 1865. In: Lydeard C, Cummings KS (Eds) Freshwater Mollusks of the World. A Distribution Atlas. John Hopkins University Press, Baltimore.
- Yakhimovich VL, Nemkova VK, Dorofeev PI, Popova-Lvova MG, Suleimanova FI, Khabibullina GA, Alimbekova LI, Latypova EK. (1986) Pleystotsen nizhnego techeniya r. Ural. BFAN SSSR, Ufa, 135 pp. [Google Scholar]
- Yanina TA. (2005) Didakny Ponto-Kaspiya. Majenta, Smolensk-Moskva, 300 pp. [Google Scholar]
- Yanina TA. (2009) Paleogeografiya basseynov Ponto-Kaspiya v pleystotsene po rezul’tatam malakofaunisticheskogo analiza. Moscow State University, Moscow. http://www.geogr.msu.ru/structure/labs/notl/personal/Abstracts/Yanina_avtoreferat.2009.pdf
- Yanina TA, Svitoch AA. (1988) Pleystotsenovyye mollyuski Dagestana (opredelitel’ roda Didacna Eichwald). VINITI, Moscow, 180 pp. [Google Scholar]
- Zaitsev Yu, Mamaev V. (1997) Marine Biological Diversity in the Black Sea: A Study of Change and Decline. United Nations Publications, New York, 208 pp. [Google Scholar]
- Zaranko DT, Farara DG, Thompson FG. (1997) Another exotic mollusc in the Laurentian Great Lakes: the New Zealand native Potamopyrgusantipodarum (Gray 1843) (Gastropoda, Hydrobiidae). Canadian Journal of Fisheries and Aquatic Sciences 54(4): 809–814. 10.1139/f96-343 [DOI] [Google Scholar]
- Zettler ML. (2007) A redescription of Theodoxusschultzii (Grimm, 1877), an endemic neritid gastropod of the Caspian Sea. Journal of Conchology 39(3): 245–251. [Google Scholar]
- Zhadin VI. (1952) Mollyuski presnykh i solonovatykh vod SSSR. Izdatel’stvo Akademii Nauk SSSR, Moskva, Leningrad, 376 pp. [Google Scholar]
- Zhizhchenko BP. (1933) Fauna kaspiyiskikh terras. In: Archangelsky AD, Davitashvili LS. (Eds) Rukovodyashchiye iskopayemyye neftyenosnykh rayonov Krymsko-Kavkazskoy oblasti, XV.Trudy Gosudarstvennogo Issledovatel’skogo Neftyanogo Instituta 1933: 30–36.
- Zhulidov AV, Zhulidov DA, Pavlov DF, Nalepa TF, Gurtovaya TYu. (2005) Expansion of the invasive bivalve mollusk Dreissenabugensis (quagga mussel) in the Don and Volga River Basins: Revisions based on archived specimens. Ecohydrology & Hydrobiology 5(2): 127–133. https://www.glerl.noaa.gov/pubs/fulltext/2005/20050013.pdf [Google Scholar]
- Zolotarev V. (1996) The Black Sea ecosystem changes related to the introduction of new mollusc species. Marine Ecology 17(1–3): 227–236. 10.1111/j.1439-0485.1996.tb00504.x [DOI] [Google Scholar]