Abstract
In recent years, several studies have reported monocyte lymphocyte ratio (MLR) to predict prognosis in various tumors. Our study was performed to evaluate the association between preoperative MLR between prognostic variables in urothelial carcinoma patients. Systematic literature search was conducted in PubMed, Embase, Web of science. The correlation between preoperative MLR and overall survival (OS), cancer specific survival (CSS), disease free survival (DFS)/relapse free survival (RFS), progression free survival(PFS) was evaluated in urothelial carcinoma patients. Meanwhile, the association between MLR and clinicopathological characteristics was assessed. Finally, 12 comparative studies comprising a total of 6209 patients were included for pooled analysis. The hazard ratios (HRs), odds ratios (ORs)and 95% confidence intervals (CIs) were further analyzed as effect measures. The pooled results demonstrated that elevated preoperative MLR indicated unfavorable OS (HR = 1.29, 95%CI = 1.18-1.39, I2 = 33.6%), DFS/RFS (HR = 1.42, 95%CI = 1.30–1.55, I2 = 0.0%) and CSS (HR = 1.41, 95%CI = 1.29–1.52, I2 = 0.0%). Moreover, the pooled results also suggested that elevated preoperative MLR was correlated with high tumor stage (OR = 1.22, 95%CI = 1.07–1.37, I2 = 0.0%) in urothelial carcinoma patients. No significant association was found between preoperative MLR and PFS in upper urinary tract urothelial carcinoma (UUTUC) patients. Collectively, elevated preoperative MLR predicted poor prognosis in urothelial carcinoma and have the potential to be a feasible and cost-effective prognostic predictor for management of urothelial carcinoma.
Subject terms: Tumour biomarkers, Tumour biomarkers, Prognostic markers, Prognostic markers, Tumour biomarkers
Introduction
Urothelial carcinoma is defined as the malignancy derived from the mucosal surface of urinary system. Urothelial carcinoma of bladder (UCB) was the most common malignancy in urothelial carcinoma, followed by UUTUC accounting for 5–10% of all urothelial malignancies1,2. Urothelial carcimona always means a large toll on human health and huge economic burden for patients or health care systems3, due to its high recurrence and malignancy. In spite of advanced surgical techniques, growing expertise, emerging new treatments in recent years, the improvement of long-term survival have barely changed and the treatment decision-making for urothelial carcinoma is still oftentimes challenging1,4–6. Therefore, it is essential to develop and validate the potential biomarkers to establish the accurate preoperative risk stratifications and predict the prognosis after treatment.
Inflammation can affect immune surveillance and responses to the treatments for tumors, and there has been increasing evidence indicating that systemic inflammatory responses play an important role in the development, progression and metastasis of malignancies in past decades7–9. Many inflammation related biomarkers have been evaluated as prognostic indicators in multiple malignancies, such as platelet to lymphocyte ratio (PLR)10, c-reactive protein (CRP)11, tumor associated macrophages (TAMs)12 and so on. Recently, the lymphocyte to monocyte ratio (LMR) has been reported as a predictor in various tumors13–15. Since 2014, emerging studies have reported the preoperative MLR as a potential predictor in UCB or UUTUC patients16–18, and Yoshida’s study reported preoperative MLR could be a better predictor in UCB patients, comparing with NLR19. However, the role of preoperative MLR is still in controversy for urothelial malignancies, and needs to be validated due to the inevitable discrepancy among the studies. Therefore, we performed this pooled analysis to assess the potential impact of preoperative MLR in patients with urothelial malignancies. Moreover, relationships between preoperative MLR and the clinicopathological characteristics was also assessed.
Results
Literature selection
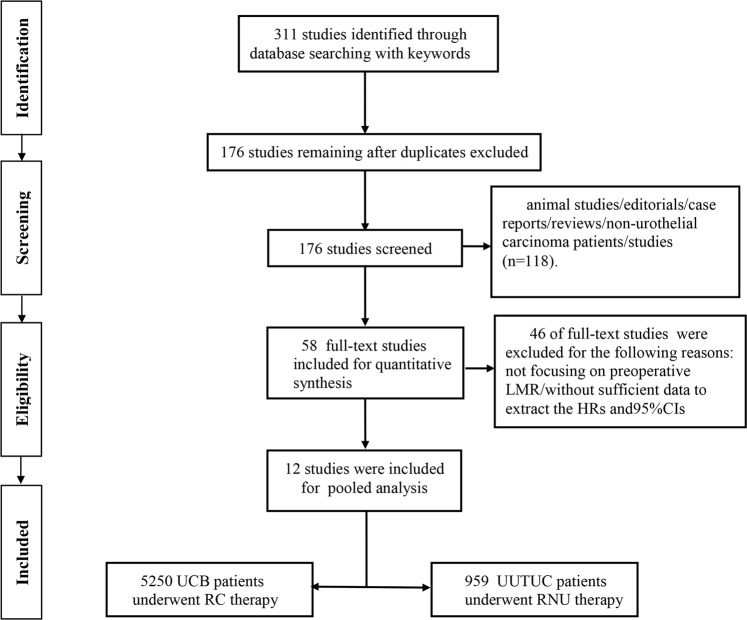
There databases (Pubmed, Embase and Web of science) were systematically searched, and 311 studies were initially identified. 176 studies remained after removing 135 duplicates. Subsequent to the screening of titles and abstracts of the remaining 176 studies, 118 studies were excluded for the following reasons: animal studies, editorials, case reports, reviews, non-urothelial carcinoma patients’ studies. Therefore, 58 full-text studies were evaluated for eligibility, and 46 studies were further excluded for not focusing on preoperative MLR or without sufficient data to extract the HRs and 95%CI for prognostic variables. Finally, 12 studies comprising a total of 6209 patients were included in this pooled analysis16–28. The flow diagram for literature selection was shown in Fig. 1.
Figure 1.
Flow diagram.
Characteristics of the included publications
The MLR data were collected before surgery in the included studies. The number of patients ranged from 68 to 4198, with 959 UUTUC patients and 5250 UCB patients included in this pooled analysis. All the patients underwent radical surgery, radical cystectomy was chosen for UCB patients and UUTUC patients were performed with radical nephroureterectomy. MLR was defined as absolute monocyte counts divided by lymphocyte counts, and the cut-off values for MLR ranged from 0.25 to 0.50, while Bhindi’s study reported per 1-log unit as the cut-off value. As for Temraz’s study, there were two kinds of cut-off values to evaluate the potential role of MLR in DFS or OS, respectively16. The studies were designed retrospectively and published between 2014 and 2018. With regard to prognostic outcomes assessed, OS was investigated as prognostic endpoint in 10 studies, DFS/RFS in 5 studies and CSS in 5 studies, and PFS in 3 studies. Regarding the quality of included studies, the mean NOS score was 7.3. The detailed characteristics were summarized in Table 1.
Table 1.
Charateristics of the included studies.
| First author(year) | Design | geographical region | Cases number | diagnosis | therapy | Cut-off value | Outcome | Analysis methods | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Temraz S, et al.(2014) | Retrospective | Asian | 68 | UCB | RC | 0.36a,0.35b | DFS,OS | K | 6 |
| Hutterer GC, et al.(2015) | Retrospective | Australasian | 182 | UUTUC | RNU | 0.50 | OS | M | 8 |
| Zhang GB, et al.(2015) | Retrospective | Asian | 124 | UCB | RC | 0.25 | OS | U | 7 |
| Yoshida T, et al.(2015) | Retrospective | Asian | 181 | UCB | RC | 0.28 | OS | M | 8 |
| Bhindi B, et al.(2016) | Retrospective | North American | 418 | UCB | RC | per 1-log unitc | RFS,CSS,OS | U | 7 |
| Song X, et al.(2016) | Retrospective | Asian | 140 | UUTUC | RNU | 0.28 | DFS,PFS | M | 7 |
| D’Andrea D, et al.(2017) | Retrospective | European | 4198 | UCB | RC | 0.29 | RFS,CSS,OS | M | 8 |
| Altan M, et al.(2017) | Retrospective | Asian | 113 | UUTUC | RNU | 0.34 | DFS,PFS | M | 6 |
| Miyake M, et al.(2017) | Retrospective | Asian | 117 | UCB | RC | 0.3 | OS,DSS | U | 7 |
| Rajwa P, et al.(2018) | Retrospective | European | 144 | UCB | RC | 0.41 | OS,CSS | M | 8 |
| Jan HC, et al.(2018) | Retrospective | Asian | 424 | UUTUC | RNU | 0.4 | OS,CSS,PFS | M | 8 |
| Zhang XK, et al.(2018) | Retrospective | Asian | 100 | UUTUC | RNU | 0.33 | OS | M | 8 |
UUTUC: upper urinary tract urothelial carcinoma; UCB: urothelial carcinoma of bladder; OS: overall survival; DFS: disease free survival; RFS: recurrence free survival; CSS: cancer specific survival; PFS: progression free survival; RNU: radical nephroureterectomy; RC: radical cystectomy; M: multivariate analysis; U:univariate analysis; K: Kaplan-meier curve; a: cut-off value for OS; b: cut-off value for DFS; c: Log-transformed.
Prognostic significance of MLR in OS
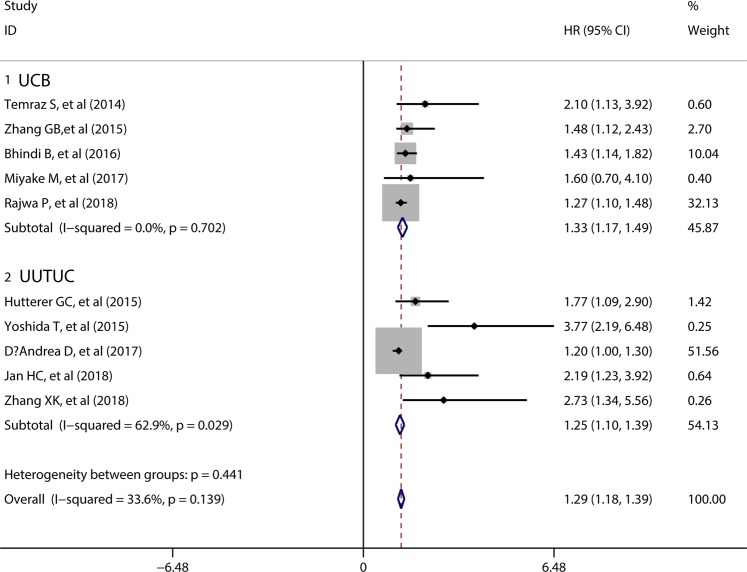
10 studies comprising 5956 patients reported the data of MLR and OS in urothelial carcinoma patients. Overall, the pooled results indicated elevated preoperative MLR was correlated with reduced OS (HR = 1.29, 95%CI = 1.18–1.39), without significant heterogeneity among the included studies (I2 = 33.6%, p = 0.139) (Fig. 2), Subgroup analyses regarding UCB and UUTUC types were conducted to detect the potential heterogeneity. The pooled results suggested elevated preoperative MLR predicted reduced OS in both UCB patients (HR = 1.33, 95%CI = 1.17–1.49) and UUTUC patients (HR = 1.25, 95%CI = 1.10–1.39). The details were illustrated in Fig. 2.
Figure 2.
Forest plot evaluating the association between MLR and OS.
Prognostic role of MLR in DFS/RFS
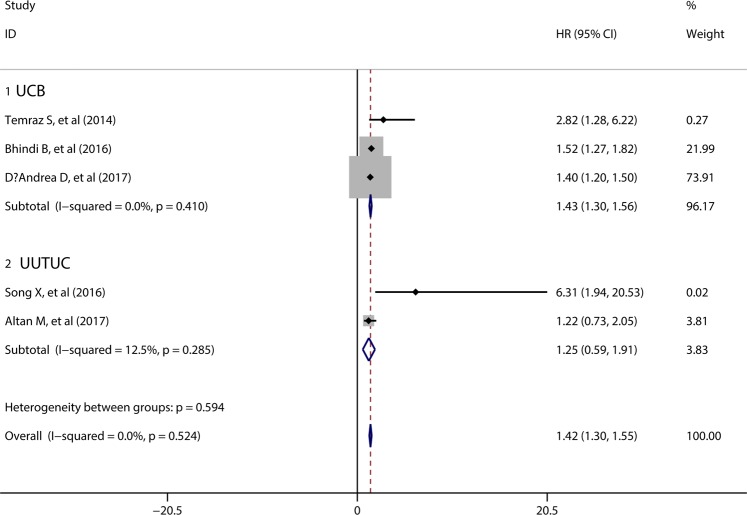
5 studies involved with 4937 patients assessed the potential role of preoperative MLR in DFS/RFS. The pooled results demonstrated that elevated preoperative MLR predicted poor DFS/RFS in urothelial maligancies (HR = 1.42, 95%CI = 1.30–1.55), without significant between-study heterogeneity studies (I2 = 0.0%, p = 0.417) (Fig. 3). Subgroup analysis indicated elevated preoperative MLR was significantly associated with poor DFS/RFS in UCB patients, and no significant association was found between MLR and DFS/RFS in UUTUC patients.
Figure 3.
Forest plot evaluating the association between MLR and DFS/RFS.
Prognostic role of MLR in CSS
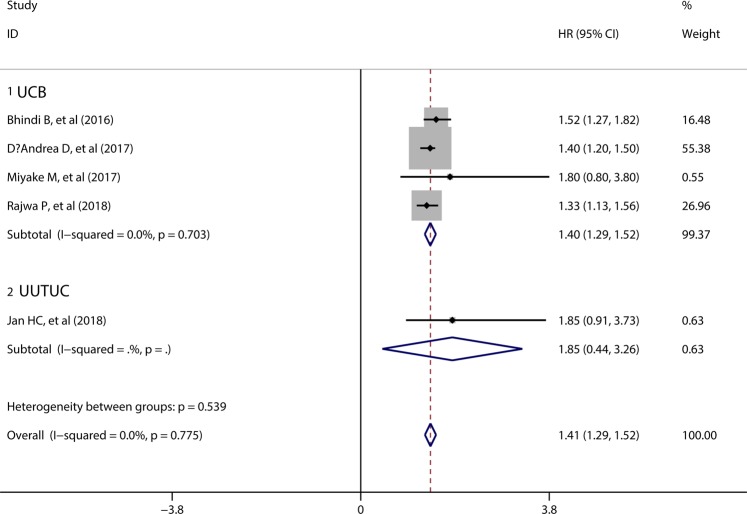
5 studies including 5301 patients presented the data about MLR and CSS, and 4 of 5 studies focused the relationship between MLR and CSS in UCB patients. The pooled results showed that elevated preoperative MLR was significantly associated with poor CSS in urothelial patients (HR = 1.41, 95%CI = 1.29–1.52), without significant heterogeneity among the 5 studies (I2 = 0.0%, p = 0.775). Only 1 of 5 studies reported the prognostic role of MLR in CSS in UUTUC patients (HR = 1.85, 95%CI = 0.91–3.73) (Fig. 4).
Figure 4.
Forest plot evaluating the correlation between MLR and CSS.
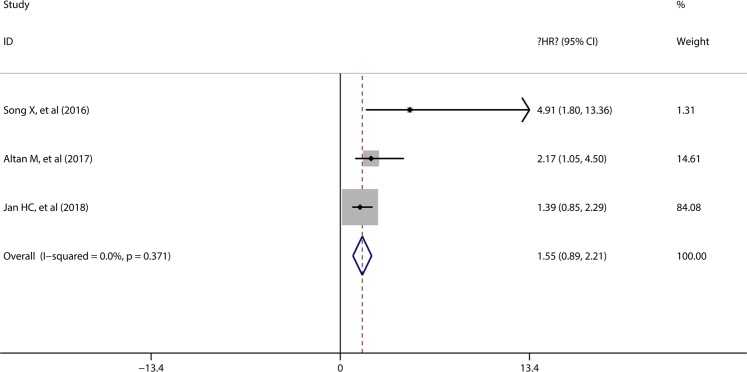
Prognostic role of MLR in PFS
3 studies including 677 patients presented the data about MLR and CSS, and all the patients were diagnosed as UUTUC patients. The pooled results showed that no significant association was found between preoperative MLR and PFS in UUTUC patients (HR = 1.55, 95%CI = 0.89–2.21), and significant heterogeneity was not found among the included studies (I2 = 0, p = 0.371) (Fig. 5).
Figure 5.
Forest plot evaluating the correlation between MLR and PFS.
Pooled analysis of MLR and clinicopathological characteristics
Raw data were extracted and calculated for the ORs combined with 95%CIs to assess the impact of preoperative MLR on clinicopathological characteristics in urothelial carcinoma patients (high MLR vs. low MLR). Our pooled results based on pooled analysis suggested elevated preoperative MLR predicted high Tumor stage (≥T2 vs. <T2) in UCB or UUTUC patients (OR = 1.22, 95%CI = 1.07–1.37), without significant heterogeneity (I2 = 0, p = 0.661). No significant correlation was found between preoperative MLR and Diabetes, tumor necrosis, multifocality, tumor grade and lymphovascular invasion (LVI). The detailed results were shown in Table 2.
Table 2.
The association between MLR and clinicopathological characteristics. MLR: high vs. low; Cis: carcinoma in situ; LVI: lymphovascular invasion.
| Patient charateristics | Number of studies | Number of patients | Effect models | OR (95%CI) | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| Chi2 | I2 | P | |||||
| Gender (male vs. female) | 6 | 5168 | fixed | 0.87 (0.75–0.99) | 2.59 | 0 | 0.763 |
| Diabetes (yes vs. no) | 2 | 264 | fixed | 0.66 (0.16–1.17) | 0.29 | 0 | 0.587 |
| Hypertension (yes vs. no) | 2 | 264 | fixed | 0.61 (0.26–0.95) | 0.16 | 0 | 0.693 |
| Concomitant Cis (yes vs. no) | 2 | 4322 | fixed | 0.88 (0.77–0.98) | 0.01 | 0 | 0.913 |
| Tumor grade (high vs. low) | 7 | 5256 | fixed | 1.06 (0.82–1.30) | 1.93 | 0 | 0.926 |
| Tumor necrosis (yes vs. no) | 4 | 846 | fixed | 1.21 (0.70–1.72) | 1.20 | 0 | 0.753 |
| LVI (yes vs. no) | 4 | 4862 | Random | 1.00 (0.47–1.53) | 9.31 | 67.8 | 0.025 |
| Tumor stage (≥T2 vs. <T2) | 6 | 5096 | fixed | 1.22 (1.07–1.37) | 3.26 | 0 | 0.661 |
| Multifocality | 2 | 524 | fixed | 1.33(0.78–1.87) | 0.04 | 0 | 0.838 |
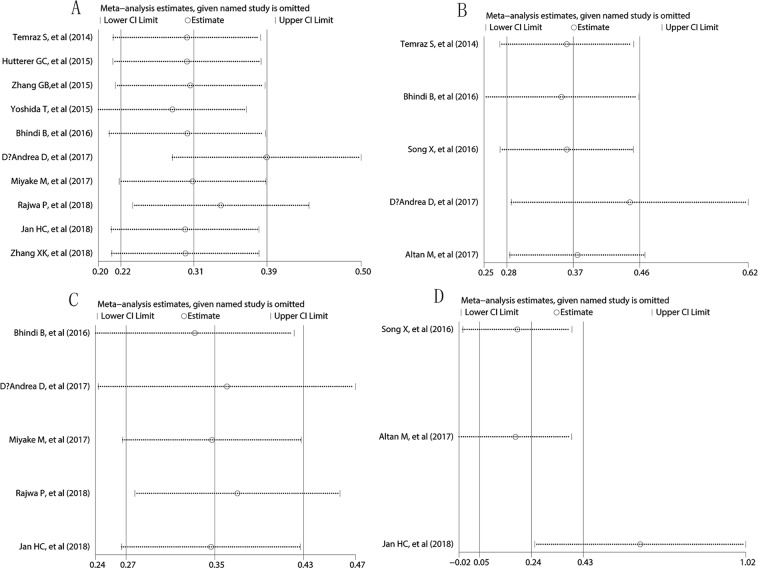
Sensitivity analysis
Sensitivity analysis was performed to evaluate the impact of each single study on the final results. The sensitivity analysis has not found any study contributed significantly to the origin of heterogeneity among the included studies: MLR and OS (Fig. 6A), MLR and DFS/RFS (Fig. 6B), MLR and CSS (Fig. 6C). However, the sensitivity analysis indicated Jan HC et al.’s study may be the main origin of heterogeneity regarding the pooled analysis about MLR and PFS. After removal of Jan HC et al.’s study, the pooled result also indicated there was no significant association between preoperative MLR and PFS (HR = 2.40, 95%CI = 0.74–4.05, I2 = 0.0%).
Figure 6.
Sensitivity analysis. (A) MLR with OS; (B) MLR with DFS/RFS; (C) MLR with CSS; (D) MLR with PFS. Circles: included studies; Dash line: 95% confidence interval.
Discussion
Urothelial carcinoma was the most common malignant tumors in urinary system, which derived from the lining surface epithelium termed as “urothelium” with the same embryologic origin1. UCB and UUTUC were the two main types in urothelial carcinoma with respect to anatomical location, however, the two types behave identically, and much of clinical decision-making could be similar between UCB and UUTUC29,30. In order to improve the management of urothelial carcinoma, it is pivotal to identify novel, economic and feasible predictors for individual therapy. Emerging evidences have shown immune cells were involved with cancer initiation, progression and invasion in multiple tumors31,32. The hematological inflammatory cells and factors have been widely investigated and identified as important prognostic indicators in urothelial carcinoma10,33. More recently, preoperative MLR, as an inflammation related marker, was reported as a potential predictor for urothelial maligancies in several studies. However, there was no related meta-analysis, which can increase the statistical power by methodologically combining the reported results from varying studies. To the best of our knowledge, this is the first pooled analysis to assess the preoperative MLR as a kind of predictor in urothelial maligancies.
According to the pooled analysis of comparative studies from meta-analysis, our results indicated elevated preoperative MLR was significantly associated with reduced OS, DFS/RFS and CSS in urothelial carcinoma patients. However, no significant association was found between preoperative MRL and PFS in urothelial carcinoma patients. Furthermore, subgroup analysis was performed according to the different sites of urothelial carcinoma(UCB, UUTUC), and the results also suggested elevated preoperative MLR predicted worse OS, DFS/RFS and CSS in UCB or UUTUC, respectively. The relationship between elevated preoperative MLR and selected clincopathological features was also assessed in this pooled analysis, and the results demonstrated elevated preoperative MLR was an independent risk for high tumor stage (≥T2 vs. <T2) in urothelial maligancies (OR = 1.201, 95%CI = 1.047–1.356, I2 = 0). Collectively, the pooled data from this meta-analysis showed that MLR may serve as a prognostic indicator in urothelial carcinoma patients.
The underlying mechanisms involved with the prognostic role of hematological MLR in urothelial carcinoma remain to be addressed. Mounting studies have indicated tumor infiltrating lymphocytes (TILs) were associated with prognosis of urothelial carcinoma34,35, and the prognostic role may vary according to the subpopulations of TILs35. Low lymphocyte counts can lead to the deficiency of host immune response, which may cause the progression and metastasis of cancer cells and result in poor survival in malignancies36,37. Circulating monocytes can be recruited into tumor microenviroment and further polarize into tumor associated macrophages (TAMs). Elevated tumor infiltrating TAMs were always involved with worse survival in various tumors38,39. Taken together, we postulated that elevated preoperative MLR could reflect the disorders of immune cells in tumor miroenvironment, which generated poor survival in urothelial carcinoma patients.
This pooled analysis also had some disadvantages. First, considerable heterogeneity was found in our pooled analysis. In order to detect and decrease the potential heterogeneity, we have performed sensitivity analysis and subgroup analyses. Second, the number of included studies was limited, and the pooled results should be cautiously interpreted. Moreover, 8 included studies had reported HRs and 95%CIs derived from multivariate analysis, while HRs and 95%CIs in 4 studies were extracted from univariate analysia, which may overestimate the prognostic role of MLR. In addition, all the included studies were retrospective design. In future, large-scale prospective studies are still needed to validate our results in this pooled analysis.
Collectively, our priamry results derived from pooled analysis suggested elevated preoperative MLR was significantly correlated with poor survival, which may contribute to the risk stratifications before surgery and clinical decision-making for individual therapeutic strategies in urothelial carcinoma patients.
Materials and Methods
This pooled analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA)40, and the PRISMA checklist was detailed in Supplementary Table S1.
Search Strategies
There databases (Pubmed, Embase and Web of science) were systematically searched for eligible studies involved with preoperative MLR in urotheilal malignancies, up to November 30th, 2018. The selected keywords were confined in the followings: (urothelial cancer OR bladder cancer OR bladder malignancies OR transitional cancer OR ureteral cancer OR renal cancer) AND (MLR OR monocyte to lymphocyte ratio OR monocyte lymphocyte ratio). References listed in identified studies were also went through to expand the scope of search.
Study selection
Inclusion criteria
The studies were included according to the criteria listed in the following: (1) patients were pathologically diagnosed as UCB or UUTUC. (2) MLR was collected before surgery. (3) the studies provided HRs or Kaplan-meier curve for evaluating the role of MLR in prognostic variables. (4) studies reported cut-off values for stratified analysis.
Exclusion criteria
The studies were excluded if they met the following criteria: (1) animal studies, editorials, case reports, reviews, comments; (2) duplicates identified by Endnote X7 version; (3) the studies did not focused on the relationship between preoperative MLR and urothelial maligancies or without sufficient data to extract HRs and 95% CIs.
Data management and quality assessment
Data from the included studies were summarized via a kind of predefined form: Acronym of first author (year of publication), design, geographical region, cases number, diagnosis, therapy, cut-off value, outcome and analysis methods. The related HRs and 95%CIs were extracted to further analyze the association between preoperative MLR and urothelial malignancies. OS, CSS, DFS/RFS and PFS were taken as prognostic outcomes for pooled analysis. The clinicopathological characteristics included gender, diabetes, hypertension, concomitant carcinoma in situ, multifocality, tumor grade, tumor necrosis, lymphovascular invasion and tumor stage.
The Newcastle-Ottawa Scale (NOS) system was used to evaluate the quality of included studies41. The maximum score is 9, which was involved with the evaluation of subject selection, comparability of groups, and clinical outcome. If the NOS scored more than 7 (included 7) for a study, it was usually taken as a high-quality study. All the processes about data management and quality assessment were reviewed independently by two authors, and any disagreement was settled through discussion with a senior author.
Statistical analysis
The pooled HRs or ORs were analyzed with Stata version 15.0 (StatCorp, College Station, TX, USA). ORs and 95%CIs regarding the association between preoperative MLR and chinicopathological characteristics were calculated from raw data reported in the included studies. Heterogeneity among the studies was reported via the statistic effects, such as Chi-squared tests, p value and I-square. A fixed-effect model was preferentially used for this pooled analysis, unless there was significant heterogeneity among the studies(I2 > 50%). Otherwise, a random-effect model was adopted as an alternative method. An observed HR >1, combined with 1 not included in its 95%CIs, indicated elevated preoperative MLR was associated with poor prognosis in urothelial carcinoma patients. We did not perform Begg’s and Egger’s tests to evaluate the publication bias for that the number of included studies was less than 10 in this pooled analysis42–44. However, we conducted sensitivity analysis to validate the pooled results by excluding each study.
Supplementary information
Acknowledgements
This study was supported by the grant from Technological Innovation Guidance Plan (no. 2017sk50123) of Hunan Province. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Conceived and designed the analysis: X.K.Z., Y.H.W. Literature searching and data collection: S.Q.W., X.Z., Z.H.Z. and L.Z. Performed the analysis: X.Z., S.Q.W. and X.K.Z. Prepared the manuscript: X.Z., R.X. and S.Q.W. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuan Zhu and Shui-Qing Wu contributed equally.
Contributor Information
Yin-Huai Wang, Email: wangyinhuai@csu.edu.cn.
Xiao-Kun Zhao, Email: xiaokunzhao@csu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42781-y.
References
- 1.Miyazaki J, Nishiyama H. Epidemiology of urothelial carcinoma. Int. J. Urol. 2017;24:730–734. doi: 10.1111/iju.13376. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.González del Alba A, et al. Recent advances in genitourinary tumors: A review focused on biology and systemic treatment. Crit Rev Oncol Hematol. 2017;113:171–190. doi: 10.1016/j.critrevonc.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z, et al. Down-regulation of HMGB1 expression by shRNA constructs inhibits the bioactivity of urothelial carcinoma cell lines via the NF-κB pathway. Sci. Rep. 2015;5:12807. doi: 10.1038/srep12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canter D, et al. Clinicopathological outcomes after radical cystectomy for clinical T2 urothelial carcinoma: Further evidence to support the use of neoadjuvant chemotherapy. BJU Int. 2011;107:58–62. doi: 10.1111/j.1464-410X.2010.09442.x. [DOI] [PubMed] [Google Scholar]
- 6.Farina, M. S., Lundgren, K. T. & Bellmunt, J. Immunotherapy in Urothelial Cancer: Recent Results and Future Perspectives. Drugs. 4 (2017). [DOI] [PubMed]
- 7.Rollins BJ. Inflammatory chemokines in cancer growth and progression. Eur. J Cancer. 2006;42:760–767. doi: 10.1016/j.ejca.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Wu S, et al. Inflammatory myofibroblastic tumor of renal pelvis presenting with iterative Hematuria and abdominal pain: A case report. Oncol Lett. 2015;10:3847–3849. doi: 10.3892/ol.2015.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761–773. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S, et al. Systematic review and meta-analysis of the prognostic value of preoperative platelet-to-lymphocyte ratio in patients with urothelial carcinoma. Oncotarget. 2017;8:91694–91702. doi: 10.18632/oncotarget.21162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou, L. et al. Prognostic Role of C-Reactive Protein In Urological Cancers: A Meta-Analysis. Sci Rep. 5 12733 (2015). [DOI] [PMC free article] [PubMed]
- 12.Cao J, et al. Prognostic role of tumour-associated macrophages and macrophage scavenger receptor 1 in prostate cancer: A systematic review and meta-analysis. Oncotarget. 2017;5:1–9. doi: 10.18632/oncotarget.18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song W, Tian C, Wang K, Zhang R, Zou S. The pretreatment lymphocyte to monocyte ratio predicts clinical outcome for patients with hepatocellular carcinoma: A meta-analysis. Sci. Rep. 2017;7:46601. doi: 10.1038/srep46601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teng JJ, Zhang J, Zhang TY, Zhang S, Li BS. Prognostic value of peripheral blood lymphocyte- to-monocyte ratio in patients with solid tumors: a meta-analysis. Onco Targets Ther. 2015;9:37–47. doi: 10.2147/OTT.S94458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41:971–978. doi: 10.1016/j.ctrv.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Temraz S, et al. Preoperative lymphocyte-to-monocyte ratio predicts clinical outcome in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder: a retrospective analysis. BMC Urol. 2014;14:1–6. doi: 10.1186/1471-2490-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutterer GC, et al. Pretreatment lymphocyte – monocyte ratio as a potential prognostic factor in a cohort of patients with upper tract urothelial carcinoma. J Clin Pathol. 2015;68:351–5. doi: 10.1136/jclinpath-2014-202658. [DOI] [PubMed] [Google Scholar]
- 18.Bhindi B, et al. Identification of the best complete blood count-based predictors for bladder cancer outcomes in patients undergoing radical cystectomy. Br J Cancer. 2016;114:207–212. doi: 10.1038/bjc.2015.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida T, Kinoshita H, Yoshida K, Mishima T. Prognostic impact of perioperative lymphocyte – monocyte ratio in patients with bladder cancer undergoing radical cystectomy. Tumor Biology. 2016;37:10067–74. doi: 10.1007/s13277-016-4874-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G-M, et al. Preoperative lymphocyte-monocyte and platelet-lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumour Biol. 2015;36:8537–8543. doi: 10.1007/s13277-015-3613-x. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida T, et al. A novel risk stratification model, involving preoperative lymphocyte – monocyte ratio and standard pathological factors, for overall survival in patients with bladder cancer undergoing radical cystectomy. Jpn J Clin Oncol. 2015;45:1162–1167. doi: 10.1093/jjco/hyv057. [DOI] [PubMed] [Google Scholar]
- 22.Song X, et al. Comparison of preoperative neutrophil – lymphocyte, lymphocyte – monocyte, and platelet – lymphocyte ratios in patients with upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy. Onco Targets Ther. 2016;9:1399–407. doi: 10.2147/OTT.S97520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Andrea D, et al. Lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio as biomarkers for predicting lymph node metastasis and survival in patients treated with radical cystectomy. J Surg Oncol. 2017;115:455–461. doi: 10.1002/jso.24521. [DOI] [PubMed] [Google Scholar]
- 24.Altan M, et al. A critical prognostic analysis of neutrophil–lymphocyte ratio for patients undergoing nephroureterectomy due to upper urinary tract urothelial carcinoma. International Journal of Clinical Oncolog. 2017;22:964–971. doi: 10.1007/s10147-017-1150-x. [DOI] [PubMed] [Google Scholar]
- 25.Miyake M, et al. Integrative assessment of pretreatment inflammation-, nutrition-, and muscle-based prognostic markers in patients with muscle-invasive bladder cancer undergoing radical cystectomy. Oncology (Switzerland) 2017;93:259–269. doi: 10.1159/000477405. [DOI] [PubMed] [Google Scholar]
- 26.Rajwa, P. et al. Evaluation of the prognostic value of LMR, PLR, NLR, and dNLR in urothelial bladder cancer patients treated with radical cystectomy, 22, 3027–3037 (2018). [DOI] [PubMed]
- 27.Jan, HC. et al. Combination of the Preoperative Systemic Immune-Inflammation Index and Monocyte-Lymphocyte Ratio as a Novel Prognostic Factor in Patients with Upper-Tract Urothelial Carcinoma. Annals of Surgical Oncology. Epub ahead of print (2018). [DOI] [PubMed]
- 28.Zhang X, et al. Preoperative Low Lymphocyte-to-Monocyte Ratio Predicts Poor Clinical Outcomes for Patients with Urothelial Carcinoma of the Upper Urinary Tract. Urol J. 2018;15:348–354. doi: 10.22037/uj.v0i0.4120. [DOI] [PubMed] [Google Scholar]
- 29.Catto JWF, et al. Behavior of Urothelial Carcinoma With Respect to Anatomical Location. J. Urol. 2007;177:1715–1720. doi: 10.1016/j.juro.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Green DA, et al. Urothelial carcinoma of the bladder and the upper tract: Disparate twins. J. Urol. 2013;189:1214–1221. doi: 10.1016/j.juro.2012.05.079. [DOI] [PubMed] [Google Scholar]
- 31.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2011;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S, et al. Assessment of the potential diagnostic role of anaplastic lymphoma kinase for inflammatory myofibroblastic tumours: A meta-analysis. PLoS One. 2015;10:1–12. doi: 10.1371/journal.pone.0125087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masson-lecomte A, Rava M, Real FX, Hartmann A. Inflammatory Biomarkers and Bladder Cancer Prognosis: A Systematic Review. Eur Urol. 2014;66:1078–1091. doi: 10.1016/j.eururo.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, et al. CD103 D Tumor Infiltrating Lymphocytes Predict a Favorable Prognosis in Urothelial Cell Carcinoma of the Bladder. J. Urol. 2015;194:556–562. doi: 10.1016/j.juro.2015.02.2941. [DOI] [PubMed] [Google Scholar]
- 35.Horn T, et al. The prognostic effect of tumour-infiltrating lymphocytic subpopulations in bladder cancer. World J. Urol. 2016;34:181–187. doi: 10.1007/s00345-015-1615-3. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann K, Dworacki G, Meidenbauer N, Johnson JT. Spontaneous Apoptosis of Circulating T Lymphocytes in Patients with Head and Neck Cancer and Its Clinical Importance 1. Clin. Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 37.Väyrynen JP, et al. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br J Cancer. 2013;109:1839–1847. doi: 10.1038/bjc.2013.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu P, et al. Inverse role of distinct subsets and distribution of macrophage in lung cancer prognosis: a meta-analysis. Oncotarget. 2016;7:40451–40460. doi: 10.18632/oncotarget.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Q, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells G. et al. Newcastle -Ottawa Quality Assessment Scale Case Control Studies. Ottawa Hospital Research Institute http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2013).
- 42.Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics. 1994;50:1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 43.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical. BMJ. 1997;13(315):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu L, et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget. 2016;7:31926–31942. doi: 10.18632/oncotarget.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.