Abstract
The conservation of architectural heritage is a big challenge in times with increasing air pollution with aggressive gases. A second major threat to buildings is the combination of water and air contaminants which may be used by microorganisms for their metabolism. Hence, myriads of different bacteria and fungi populate stone surfaces and penetrate into the fine pores and cracks. Whereas epoxid-based paintings (or other paintings) may protect the coated surfaces from water and aggressive gases, these chemicals seal the stone surface and prevent also the evaporation of vapor from the inside of the buildings. Here, we tested a natural, fungal protein-based coating method. Fungi use small, amphiphilic proteins to turn their surfaces hydrophobic. We found that Aspergillus nidulans hydrophobin DewA and Trichoderma reesei HFBI confer hydrophobicity to stones but keep their pores open. The effect resembles “Gore-tex” fabric material.
Subject terms: Fungi, Biomaterials
Introduction
One of the most important challenges in the conservation of architectural elements made of stone is the protection against water1. Multiple freeze/thawing cycles of water inside the stone bulk are mainly responsible for stone decay. Over the last decades, several treatments have been proposed to reduce water absorption by increasing the hydrophobicity of stone surfaces. One way to achieve water repellency is by increasing the roughness of the substrate surface2,3. The use of a plasma treatment, despite being successful4, has been rejected due to the special equipment and complex control processes involved5. Other methods are the application of different organic compounds like synthetic and natural waxes6, acrylic and siloxane resins7,8 and perfluoropolyethers9 which form water repellent films on the surface. In the last years, the addition of different nanoparticles to protective products was heavily investigated. Inorganic silica (SiO2) and titanium dioxide (TiO2) nanoparticles were added to PMMA (polymethylmethacrylate), polyalkylsiloxane10,11 or PDMS (polydimethylsiloxane)12 to further increase the polymer hydrophobicity or to confer self-cleaning properties to the coatings.
A class of organic “nanoparticles” heavily investigated in the past 15 years are hydrophobins. They are small amphiphilic proteins secreted by fungi to reduce water surface tension or to increase the hydrophobicity of aerial hyphae or conidiospores13–15. Hydrophobins can self-assemble into stable monolayers on hydrophilic and hydrophobic surfaces and change their wettability properties13,16,17. They can be divided into two classes [I and II] differing in size and monolayer stability. While class I hydrophobins are resistant to detergents and high temperatures, class II hydrophobins can be removed from surfaces with ethanol, detergents or pressure18–20. So far hydrophobins have been applied in surface coating and modification, foam and emulsion stabilization and the increase of enzyme activity21–25. The application involving water at neutral pH as non-toxic solvent and the possibility of complete removal from the surface make hydrophobins an interesting candidate for stone protection with respect to the Restauration Charters26,27 and requirements to protectives imposed by several authors28,29.
In this study we applied the class I hydrophobin DewA from Aspergillus nidulans and the class II hydrophobin HFBI from Trichoderma reesei on three different lithotypes. Obernkirchen sandstone, Balegem limestone and Carrara marble were coated with either hydrophobin and we characterized surface binding properties, penetration depth and water repellency. Additionally, vapor permeability and coating stability of the treatments were tested.
Results
Coating of different lithotypes
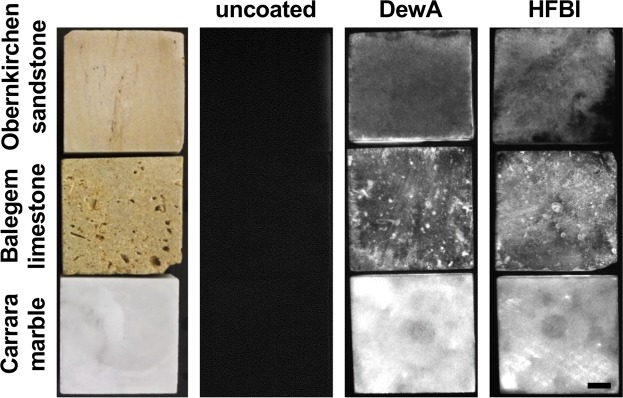
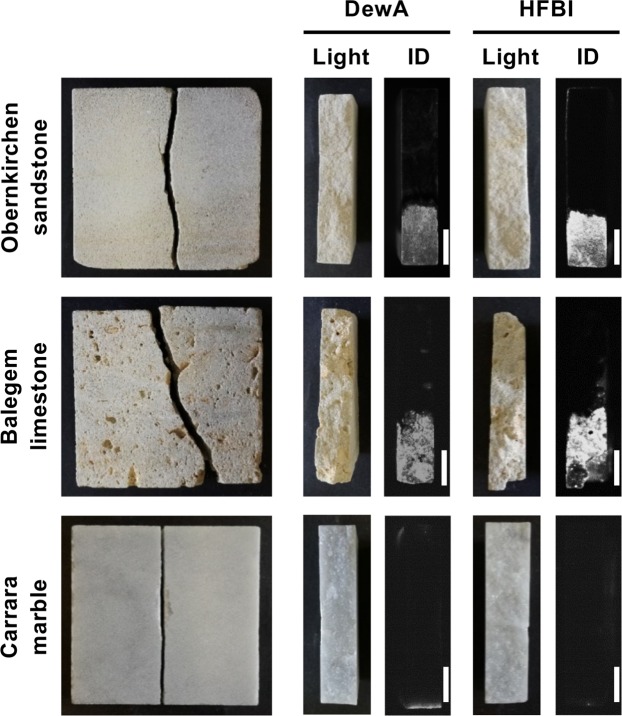
Nanoparticles are increasingly exploited in many research fields, one which is the conservation of deteriorated stones. Hydrophobin DewA from A. nidulans and HFBI from T. reesei, allocable to nanoparticles due to their small size, were produced in E. coli as described previously30. Samples of Obernkirchen sandstone, Balegem limestone and Carrara white marble were treated with solutions containing 100 µg/ml of the 6xHis-tagged hydrophobins DewA or HFBI. It has been shown before, that 100 µg/ml is a good concentration to obtain homogenous coatings. Higher hydrophobin concentrations lead to aggregations of the protein30,31. Positive hydrophobin coating was visualized by immunodetection and compared to untreated stone samples. Both hydrophobins were able to coat the three different lithotypes (Fig. 1). The coating appeared homogenous. To test how deep hydrophobins can penetrate the stone, 5 cm × 5 cm × 1 cm stone specimens were placed vertically into a hydrophobin solution for two hours. After the incubation and complete drying, the specimens were split and hydrophobin penetration depth was visualized by immunodetection. DewA and HFBI penetrated ca. 1 cm in Obernkirchen sandstone and up to 1.5 cm in Balegem limestone. Hydrophobins were unable to permeate significantly into marble (Fig. 2).
Figure 1.
Coating of Obernkirchen sandstone, Balegem limestone and Carrara marble with hydrophobins DewA and HFBI. The left row shows specimens of the three lithotypes (5 × 5 × 1 cm blocks) and imaged in bright field. The three following rows show uncoated stone samples and with DewA and HFBI coated specimen after immune detection of hydrophobin. The successful coating of stone with hydrophobin is proven by light emission whereas the uncoated samples did not emit any light and appear dark. Scale bar = 1 cm.
Figure 2.
Penetration depth of hydrophobin coating. Stone specimens were placed with the narrow side into the hydrophobin solution and incubated for 2 h. After several washing steps and complete drying, the stone plates were cracked and hydrophobins visualized by immunodetection. Shown are the cracked stones in light and the immunodetection images (ID). Scale bar = 1 cm.
Water repellency
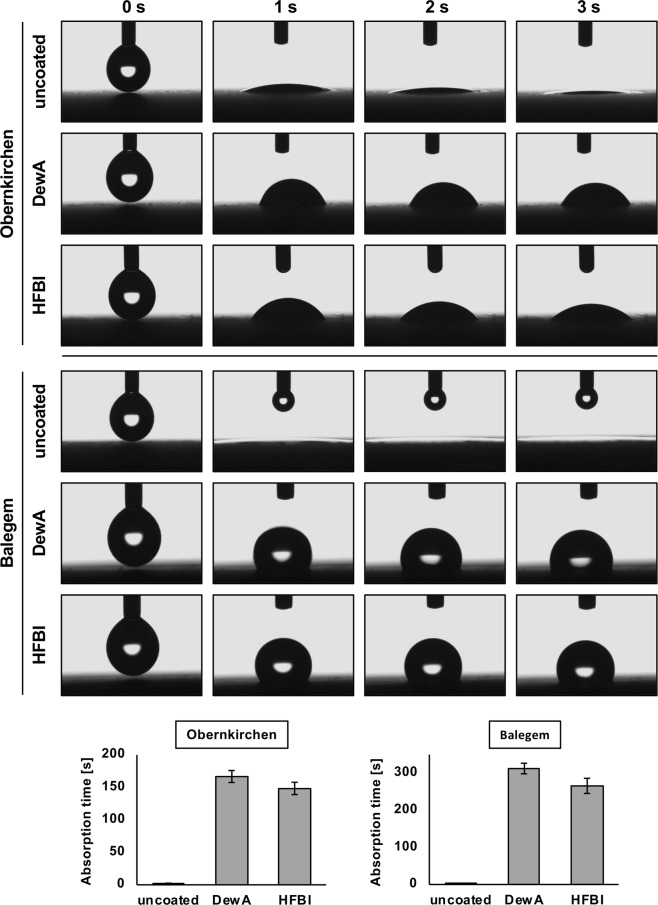
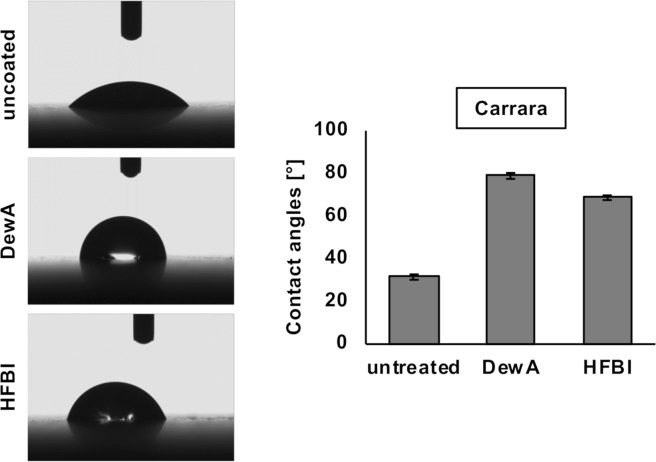
To test if the hydrophobin coatings can confer hydrophobicity to different lithotypes, droplets of deionized water were put on top of the treated samples and water absorption was followed visually. The droplets remained on the surface for 2.3 ± 0.2 s on untreated Obernkirchen and for 1.7 ± 0.5 s on untreated Balegem. On DewA-coated Obernkirchen, the droplets remained on the surface for 166.7 ± 9.2 s, whereas on HFBI coated sandstone the droplets stayed for 148.3 ± 9.4 s. Balegem coated with DewA showed the strongest water repellency with droplet retention times of 312.3 ± 14.3 s, reduced to 265.7 ± 20.4 s for HFBI (Fig. 3). White Carrara marble did not absorb water droplets. Water contact angles were calculated to further characterize hydrophobin coatings. Contact angles of untreated marble were 31.4 ± 1.3°. The measured contact angles for DewA-coated marble were 78.8 ± 1.4° and for HFBI 68.6 ± 1.1° (Fig. 4). Because the droplets on non-treated stones of the other two lithotypes were absorbed so quickly contact angles could not be determined. Generally, both hydrophobins rendered the three tested lithotypes hydrophobic, with DewA being more effective than HFBI.
Figure 3.
Reduced water absorption of hydrophobin coated stone. Water droplets were applied on the stone surface and absorption of the droplets was documented with a camera until complete absorption. Single frames from the droplets touching the stone surface are shown after 1 s, 2 s and 3 s contact. Data for absorption time are shown as mean ± standard deviation (n = 5).
Figure 4.
Water contact angles of DewA and HFBI treated Carrara marble. Deionized water droplets were put on the marble surface and imaged with a CCD camera. The results of the water contact angles are shown as mean ± standard deviation (n = 10).
Water evaporation
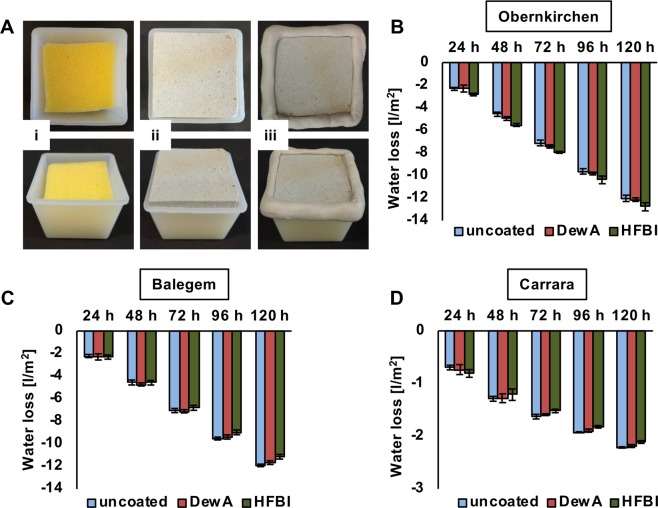
An important aspect of stone protection is the ability of water permeation even through coated stones to allow transpiration and therefore avoidance of water inclusion. With hydrophobins DewA and HFBI treated 5 cm × 5 cm × 1 cm stone samples were placed with the uncoated side on a sponge placed in a container filled with deionized water. The gap between stone and container was sealed air-tight with plastic-fermit and weight loss of the specimens was documented for 5 days (Fig. 5A). The untreated Obernkirchen sandstone lost 12.03 l/m2 water by evaporation whereas the samples coated with DewA (12.15 l/m2) and HFBI (12.78 l/m2) even lost slightly more water than the control (Fig. 5B). The untreated Balegem specimens lost 11.9 l/m2, the DewA coated 11.65 l/m2 and the HFBI coated 11.13 l/m2, which corresponds to 6.5% less evaporation compared to the uncoated samples (Fig. 5C). The marble specimens lost 2.21 l/m2 (untreated), 2.18 l/m2 (DewA coated) and 2.11 l/m2 (HFBI coated) by evaporation (Fig. 5D). The differences between untreated and hydrophobin coated stones were not significant.
Figure 5.
Water evaporation assay. (A) Experimental setup. Stone samples were placed on top of a sponge sitting in a small box filled with water (i, ii). Remaining gaps were sealed with sealing compound (iii) and weight loss of the specimens was documented over five days. Boxes are shown from the top (upper row) and from the side (lower row). The amount of evaporated water, which is equal to the weight loss, is shown for Obernkirchen sandstone (B), Balegem limestone (C) and Carrara marble (D). Data shown as mean ± standard deviation (n = 5).
Coating stability
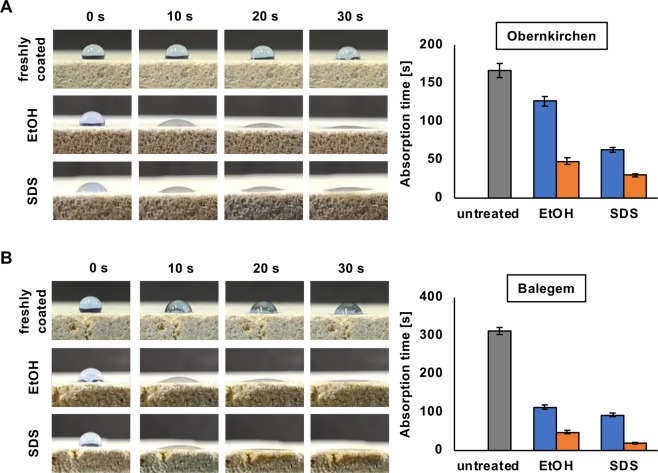
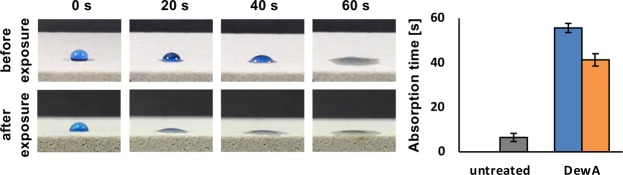
With DewA showing the best results in water repellency, the stability of the DewA hydrophobin coating on Obernkirchen sandstone and Balegem limestone was tested against alcohol (70% EtOH) and detergent (1% SDS). DewA-coated stone specimens were put in aqueous solutions of 70% EtOH or 1% SDS and water repellency was analyzed after 1 and 3 days, respectively. For Obernkirchen, the droplet absorption time was reduced to 126.3 ± 8.7 s after 1 day in 70% EtOH and to 63.0 ± 3.9 s after 1 day in 1% SDS. After an incubation for 3 days, absorption times were reduced to 48.3 ± 4.4 s for EtOH and to 29.7 ± 1.9 s for the SDS incubated samples (Fig. 6A). After 1 day, the droplet absorption time for Balegem was 113.7 ± 7.6 s for the sample in 70% EtOH and 93.3 ± 5.7 s for the sample incubated in 1% SDS. After 3 days, the absorption time was 48.3 ± 4.4 s for the alcohol specimen and 19.3 ± 2.4 s for the SDS one (Fig. 6B). Additionally, cubic specimens with 5 cm side length were treated with DewA and exposed outside on a rooftop to assess the coating stability under natural weather conditions. The freshly coated specimens showed an absorption time of 55.7 ± 21 s. After 3 months, the absorption time was reduced by 25.9% to 41.3 ± 2.8 s (Fig. 7).
Figure 6.
Reversibility of hydrophobin coating. DewA coated Obernkirchen sandstone (A) and Balegem limestone (B) were incubated for one day (blue) or three days (orange) in 70% ethanol or 1% SDS. Absorption of water droplets was measured after the treatment and compared with the “untreated” data. Data shown as mean ± standard deviation (n = 3).
Figure 7.
Stability assay. DewA coated Obernkirchen sandstone was exposed for three months outside (at cologne cathedral or a rooftop near Karlsruhe, Germany) and water absorption documented before (blue) and after (orange) exposure. Data shown as mean ± standard deviation (n = 3).
Discussion
DewA and HFBI, typical representatives of class I and class II hydrophobins, were able to coat and to reduce the water absorption of different lithotypes, independent of their chemical nature and structure. Especially DewA generated a strong water repellency on all three lithotypes without decreasing the vapor permeability of the stone samples. The coating with hydrophobin, in comparison to various traditional treatments5,32, has not clogged the stone pores and allowed water evaporation through the stone. This feature is crucial for the protection of architectural heritage buildings, since water that accumulates inside the stone bulk will cause severe damage to the material. It has been shown before that hydropobin layers are permeable for vapor31. However, in the case of stones it is conceivable that the pores remain open because of their diameter and are not sealed with hydrophobin layers. The nanoparticle-sized hydrophobins were able to penetrate up to 2 cm in porous sand- and limestone specimens. Compared to traditional protective and consolidation formulations that only reach from a few mm up to 1 cm33–36, this is quite deep. This may be due to the characteristics of hydrophobins to change the polarity of coated surfaces and to reduce the water surface tension, both crucial parameters causing strong capillarity forces37. The hydrophobin coating was easily removable with alcohol and detergent, the main components of cleaning agents. This is in line with the restorer charter pointing out that every newly developed protective must be reversible, meaning removable from the stone surface without damaging the substrate26,27. As a protein, the long-term stability of hydrophobins when exposed in the environment seems however limited. Comparing the water contact angles of DewA coated marble (79°) with the results achieved by the commercially available product Silres® BS29A (140°)38, the use of hydrophobins as sole water-repellent protective seems not favorable. Another potential usage of hydrophobins in the field of stone consolidation could be their use together with consolidation products or as a pretreatment to enhance the penetration depth of the applied product. Also, the functionalization of DewA with small peptides that improve the polymerization of diammonium phosphate to hydroxyapatite is possible39. (NH4)2HPO4 reacts with calcium to solid hydroxyapatite and is widely used as consolidation product35,40,41. Even the fusion of antimicrobial peptides to hydrophobins is possible and could reduce microbial growth on stone surface42. This study provides first insights in the potential use of hydrophobins in stone conservation and may be a stimulus for further investigations in that aspect.
Methods
Lithotypes and hydrophobin production
Obernkirchen sandstone from Lower Saxony, Germany, is mainly composed of quartz and kaolinite and has an open porosity of 24.1%. Balegem limestone is a sedimentary rock with large siliceous clasts in a calcitic matrix with a compact texture and open porosity of 9.9%, found around Ghent, Belgium. Carrara marble is a metamorphic rock with almost purely calcific composition and highly compact texture with an open porosity of 0.7%. Hydrophobins DewA and HFBI were produced as described previously30. Shortly, DewA and HFBI, carrying a 6xHis tag, were expressed in E. coli, purified from inclusion bodies and freeze dried for long term storage. Freshly prepared solutions of 100 µg/ml hydrophobin in deionized water were used for stone coating.
Stone coating
Before coating, stone specimens were incubated for 6 hours in deionized water to remove residual salts and dried for 36 hours at 60 °C. Stone plates were placed 5 mm deep in 100 µg/ml DewA or HFBI solutions and incubated at 60 °C for two hours. 100 µg/ml hydrophobin were shown to form uniform monolayers/coatings on water-gas interfaces as well as on hard surfaces30,31. After three washing steps with deionized water for 15 minutes, the stones were dried over night at room temperature.
Immunodetection
For specific immunodetection of hydrophobin, untreated and hydrophobin-coated stone samples were blocked for 30 min with 10% milk in TBS (Tris-buffered saline) at room temperature. The primary α-His antibody (Thermo Fisher Scientific, Waltham, USA), diluted 1:3000 in 1% milk in TBS was applied for 1 hour. The stones were washed three times for 5 minutes in TBS and the secondary HRP-labelled antibody (Sigma-Aldrich, Hamburg, Germany) was applied in a 1:10000 dilution in 1% milk in TBS for one hour. After several washing steps in TBS and water, immunodetection was carried out with WesternBright ECL HRP solution (Advansta, Menlo Park, USA).
Determination of water absorption
8 µl water droplets colored with Remazol Brilliant Blue were applied on the stone surface and imaged with a camera. The time until the complete drop was absorbed in the stone was measured.
Determination of water contact angles
The static water contact angles of uncoated and coated marble were measured with an OCA20 and the software SCA 202 v3.12.11 (both DataPhysics Instruments GmbH). 4 μl deionized water droplets were put on the surfaces and imaged with a CCD camera (resolution of 768 × 576 px). An ellipse fit was chosen to approach the droplet form, followed by the determination of the contact angles.
Water evaporation
To measure the water evaporation of coated stone, a water-soaked sponge was placed in a box and the pot was filled half with deionized water. The stone samples were placed on top of the sponge and the remaining gap between stone and pot was closed with sealing compound. The weight loss of the specimen, equal to the amount of water evaporated through the stone, was monitored for five days on a micro scale. Statistics were carried out using the two-tailed student’s t-test assuming equal variance with an alpha level of 0.05. Data were tested for normality using the Anderson-Darling test.
Coating stability
Freshly coated stones were incubated for one or three days in alcohol (70% EtOH) and detergent (1% SDS), the main components of cleaning agents. After several rinsing steps with deionized water and complete drying of the specimens, the water absorption was measured to determine the reduction in water repellency, indicating the removal of the hydrophobins from the stone. For long term stability, DewA coated Obernkirchen sandstone cubes with 5 cm side length were exposed outside at the Cathedral Church of Saint Peter (Cologne, Germany) and a rooftop near Karlsruhe, Germany. The water absorption was measured before and after the exposure.
Acknowledgements
This project was funded within the European Commission program Horizon 2020 (Project ID 646178, “Nanomaterials for conservation of European architectural heritage developed by research on characteristic lithotypes”). We would like to thank Ulrike Brinkmann for providing several stone samples and Elisabeth Ghaffari and Johannes Weber for advices for measuring the evaporation through the stones.
Author Contributions
L.W. performed the experiments, prepared the figures and wrote the first draft of the manuscript. O.S. designed the experiments and supervised the project. R.F. designed the experiments and received funding for the project.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Poli T, et al. The protection of different Italian marbles with two partially fluorinated acrylic copolymers. Appl. Phys. A. 2004;79:347–351. doi: 10.1007/s00339-004-2530-4. [DOI] [Google Scholar]
- 2.Chau TT, et al. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid. Interface Sci. 2009;150(2):106–115. doi: 10.1016/j.cis.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Tserepi AD, et al. Nanotexturing of poly(dimethylsiloxane) in plasmas for creating robust super-hydrophobic surfaces. Nanotechnology. 2006;17:3977. doi: 10.1088/0957-4484/17/15/062. [DOI] [Google Scholar]
- 4.Karapanagiotis, I. & Hosseine, M. (eds) Superhydrophobic coatings for the protection of natural stone. Advanced Materials for the Conservation of Stone. Springer Cham. 1–25 (2018)
- 5.Manoudis PN, et al. Fabrication of super-hydrophobic surfaces for enhanced stone protection. Surf. Coat. Technol. 2009;203:10–11. doi: 10.1016/j.surfcoat.2008.10.041. [DOI] [Google Scholar]
- 6.Klingspore, M. & Kwiatowski, D. Protection of dense limestone and marble with microcrystalline waxes. Proceedings of the 7thInternational Congress on Deterioration and Conservation of Stone. 1157–1166 (1992).
- 7.Alessandrini G, et al. Comparative evaluation of fluorinated and un fluorinated acrylic copolymers as water-repellent coating materials for stone. J. Appl. Polym. Sci. 2000;76:962–977. doi: 10.1002/(SICI)1097-4628(20000509)76:6<962::AID-APP24>3.0.CO;2-Z. [DOI] [Google Scholar]
- 8.van Hees, R. P. J. et al. The assessment of the performance of surface treatments in the fields. Results of 60 case studies confronted with lab results. Proceedings of the 4thInternational Symposium on the Conservation of Monuments in the Mediterranean. 395–413 (1997).
- 9.Toniolo L, et al. Tailoring new fluorinated acrylic copolymers as protective coatings for marble. Journal of Cultural Heritage. 2002;3(4):309–316. doi: 10.1016/S1296-2074(02)01240-2. [DOI] [Google Scholar]
- 10.Manoudis PN, et al. Superhydrophobic composite films produced on various substrates. Langmuir. 2008;24(19):11225–11232. doi: 10.1021/la801817e. [DOI] [PubMed] [Google Scholar]
- 11.Manoudis PN, et al. Modification of the wettability of polymer surfaces using nanoparticles. Progress in Organic Coatings. 2014;77(2):331–338. doi: 10.1016/j.porgcoat.2013.10.007. [DOI] [Google Scholar]
- 12.Kapridaki C, Maravelaki-Kalaitzaki P. TiO2-SiO2-PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013;76:400–410. doi: 10.1016/j.porgcoat.2012.10.006. [DOI] [Google Scholar]
- 13.Wösten HA, Scholtmeijer K. Applications of hydrophobins: current state and perspectives. Appl. Microbiol. Biotechnol. 2015;99:1587–1597. doi: 10.1007/s00253-014-6319-x. [DOI] [PubMed] [Google Scholar]
- 14.Grünbacher, A. et al. Six hydrophobins are involved in hydrophobin rodlet formation in Aspergillus nidulans and contribute to hydrophobicity of the spore surface. PLoS One9 (2014) [DOI] [PMC free article] [PubMed]
- 15.Valsecchi, I. et al. Role of Hydrophobins in Aspergillus fumigatus. J. Fungi (Basel)4 (2017). [DOI] [PMC free article] [PubMed]
- 16.Wösten H, et al. Interfacial Self-Assembly of a Fungal Hydrophobin into a Hydrophobic Rodlet Layer. Plant Cell. 1993;5:1567–1574. doi: 10.1105/tpc.5.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kershaw MJ, Talbot NJ. Hydrophobins and repellents: proteins with fundamental roles in fungal morphogenesis. Fungal Genet. Biol. 1998;23:18–33. doi: 10.1006/fgbi.1997.1022. [DOI] [PubMed] [Google Scholar]
- 18.Wösten HA. Hydrophobins: multipurpose proteins. Annu. Rev. Microbiol. 2001;55:625–646. doi: 10.1146/annurev.micro.55.1.625. [DOI] [PubMed] [Google Scholar]
- 19.Wessels JG. Hydrophobins: proteins that change the nature of the fungal surface. Adv. Microb. Physiol. 1997;38:1–45. doi: 10.1016/s0065-2911(08)60154-x. [DOI] [PubMed] [Google Scholar]
- 20.Askolin S, et al. Interaction and comparison of a class I hydrophobin from Schizophyllum commune and class II hydrophobins from Trichoderma reesei. Biomacromolecules. 2006;7:1295–1301. doi: 10.1021/bm050676s. [DOI] [PubMed] [Google Scholar]
- 21.Fokina O, Fenchel A, Winandy L, Fischer R. Immobilization of LccC Laccase from Aspergillus nidulans on Hard Surfaces via Fungal Hydrophobins. Appl. Environ. Microbiol. 2016;82:6395–6402. doi: 10.1128/AEM.01413-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boeuf S, et al. Engineering hydrophobin DewA to generate surfaces that enhance adhesion of human but not bacterial cells. Acta Biomater. 2012;8:1037–1047. doi: 10.1016/j.actbio.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Janssen MI, et al. Promotion of fibroblast activity by coating with hydrophobins in the beta-sheet end state. Biomaterials. 2004;25:2731–2739. doi: 10.1016/j.biomaterials.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 24.Ribitsch D, et al. Enhanced cutinase-catalyzed hydrolysis of polyethylene terephthalate by covalent fusion to hydrophobins. Appl. Environ. Microbiol. 2015;81:3586–3592. doi: 10.1128/AEM.04111-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green AJ, et al. Formation and stability of food foams and aerated emulsions: Hydrophobins as novel functional ingredients. Curr. Opin. Colloid. In. 2013;18:292–301. doi: 10.1016/j.cocis.2013.04.008. [DOI] [Google Scholar]
- 26.Ministero della Pubblica Istruzione. Carta Italiana del Restauro; Circolare No. 117; Rome, Italy, 6 April (1972).
- 27.De Naeyer, A. et al. Krakow Charter 2000: Principles for Conservation and Restoration of Built Heritage; Bureau Krakow, Poland (2000).
- 28.Della Volpe C, et al. The combined effect of roughness and heterogeneity on contact angles: The case of polymer coating for stone protection. J. Adhes. Sci. Technol. 2000;14:273–299. doi: 10.1163/156856100742555. [DOI] [Google Scholar]
- 29.Kourkoulis, S. K. (ed.) Fracture and failure of natural building stones: applications in the restauration of ancient monuments. Springer Netherlands. 557–559 (2006).
- 30.Winandy L, Hilpert F, Schlebusch O, Fischer R. Comparative analysis of surface coating properties of five hydrophobins from Aspergillus nidulans and Trichoderma reesei. Sci. Rep. 2018;8(1):12033. doi: 10.1038/s41598-018-29749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, X. et al. The SC3 hydrophobin self-assembles into a membrane with distinct mass transfer properties (2005). [DOI] [PMC free article] [PubMed]
- 32.Vicini S, et al. Frontal polymerization of acrylic monomers for the consolidation of stone. Polym. Adv. Technol. 2005;16:293–298. doi: 10.1002/pat.584. [DOI] [Google Scholar]
- 33.Delgado Rodrigues, J. Consolidation of decayed stones: a delicated problem with few practical solutions. Historical Constructions Lourenço, P. B., Roca, P. (eds), Universidade do Minho, Guimarães, Portugal (2001).
- 34.Slavíková M, et al. X-ray radiography and tomography for monitoring the penetration depth of consolidants in Opuka - the building stone of Prague monuments. J Cult Herit. 2012;13(4):357–364. doi: 10.1016/j.culher.2012.01.010. [DOI] [Google Scholar]
- 35.Sassoni E, et al. Consolidation of calcareous and siliceous sandstones by hydroxyapatite: Comparison with a TEOS-based consolidant. J Cult Herit. 2013;14(3):103–108. doi: 10.1016/j.culher.2012.11.029. [DOI] [Google Scholar]
- 36.Delgado Rodrigues J, Ferreira Pinto AP, Nogueira R, Gomes A. Consolidation of lime mortars with ethyl silicate, nanolime and barium hydroxide. Effectiveness assessment with microdrilling data. J Cult Herit. 2018;29:43–53. doi: 10.1016/j.culher.2017.07.006. [DOI] [Google Scholar]
- 37.Richards LA. Capillary conduction of liquids through porous mediums. Physics. 1931;1:318–333. doi: 10.1063/1.1745010. [DOI] [Google Scholar]
- 38.Aslanidou D, Karapanagiotis I, Lampakis D. Waterborne superhydrophobic and superoleophobic coatings for the protection of marble and sandstone. Materials. 2018;11(4):585–598. doi: 10.3390/ma11040585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melcher M, et al. Accelerated nucleation of hydroxyapatite using an engineered hydrophobin fusion protein. Biomacromolecules. 2016;17(5):1716–1726. doi: 10.1021/acs.biomac.6b00135. [DOI] [PubMed] [Google Scholar]
- 40.Naidu S, Sassoni E, Scherer GW. New treatment for corrosion-resistant coatings for marble and consolidation of limestone. Proc of Jardins de Pierres - Conservation of Stone in Parks, Gardens and Cemeteries. 2011;1:289–294. [Google Scholar]
- 41.Sassoni E, Naidu S, Scherer GW. The use of hydroxyapatite as a new inorganic consolidant for damaged carbonate stones. J Cult Herit. 2011;12(4):346–355. doi: 10.1016/j.culher.2011.02.005. [DOI] [Google Scholar]
- 42.Wang X, et al. Design of antibacterial biointerfaces by surface modification of poly (ε-caprolactone) with fusion protein containing hydrophobin and PA-1. Colloids Surf B Biointerfaces. 2017;151:255–263. doi: 10.1016/j.colsurfb.2016.12.019. [DOI] [PubMed] [Google Scholar]