Abstract
Background:
Lifestyle intervention decreases diabetes risk in prediabetic subjects, but the impact of passive notification of prediabetes status on glycemia or health behavior is unclear.
Methods:
The POP-ABC (Pathobiology of Prediabetes in A Biracial Cohort) study followed normoglycemic African American (AA) and European American (EA) offspring of parents with T2DM for incident prediabetes. During 5.5 years of follow-up (mean 2.62 years), 101 of 343 subjects developed prediabetes and were notified, without any interventions. Participants were recalled 18 months post-study. Here, we compared data from participants with incident prediabetes or normoglycemia (control) during POP-ABC who underwent re-testing 18 months post-study.
Results:
There were 73 subjects (46 F, 27 M; 36 AA, 37 EA) in the prediabetes group and 73 subjects (48 F, 25 M; 35 AA, 38 EA) in the control group. The mean (± SEM) enrollment age was 48.7±0.96 y vs. 48.3±1.06 y (P=0.37) and BMI was 31.1 ± 0.70 kg/m2 vs. 29.2 ± 0.69 kg/m2 (P=0.04) for prediabetes vs. control groups. The 18-mo changes (prediabetes vs,. control) were: FPG (−8.01±1.11 vs. 2.02 ± 0.64 mg/dl), 2hrPG (−8.21±3.34 vs. 8.53±3.17 8.53±3.17 mg/dl), weight (−0.54±0.72 vs. 2.77±1.25 2.77±1.25 kg) and waist circumference (−1.07±0.78 vs. 1.78±0.85 1.78±0.85 cm) (P=0.03 - <0.0001). The interval changes in FPG were significantly correlated with changes in weight and waist circumference (r=0.2, P=0.01). The prediabetes group reported improved dietary and exercise habits compared with control.
Conclusion:
Communication of prediabetes status is associated with improvements in glucose tolerance, glycemia, and adiposity, probably via self-directed lifestyle modification.
Keywords: Impaired Fasting Glucose, Impaired Glucose Tolerance, Race/Ethnicity, Screening, Health behavior
Introduction
The Department of Health and Human Services (HHS) and the American Diabetes Association (ADA) drew attention to prediabetes at a joint press conference: “HHS and the ADA are using the new term “pre-diabetes” to describe an increasingly common condition in which blood glucose levels are higher than normal but not yet diabetic - known in medicine as impaired glucose tolerance or impaired fasting glucose. Studies have shown that most people with this condition go on to develop type 2 diabetes within 10 years (1).” The Centers for Disease Control and Prevention (CDC) estimates that 84.1 million U.S. adults aged 18 years or older (~34% of the adult U.S. population) had prediabetes in 2015 (2). Worldwide, more than 300 million people are estimated to have prediabetes (3).
The diagnosis of prediabetes is established by the documentation of impaired fasting glucose (IFG), indicated by a fasting plasma glucose (FPG) level of 100–125 mg/dL (5.6 – 6.9 mmol/L) or impaired glucose tolerance (IGT), indicated by a 2-hr plasma glucose (2hrPG) level of 140–199 mg/dl (7.8 – 11.0 mmol/L) during a 75-g oral glucose tolerance test (OGTT) (4,5). Hemoglobin A1c (HbA1c) levels of 5.7– 6.4% are also diagnostic of prediabetes (4). Besides the risk of developing type 2 diabetes mellitus (T2DM), people with prediabetes are at increased risks for heart disease, stroke, neuropathy and other microvascular complications (6–8).
Several landmark clinical trials have demonstrated that lifestyle modification decreases blood glucose levels and prevents progression to type 2 diabetes (T2DM) in persons with prevalent prediabetes (9–12). In those studies, teams of dietitians, exercise physiologists and other clinicians executed the standard lifestyle intervention protocols that led to the improvement in glucose tolerance and successful prevention of T2DM in study participants (9–11, 13). Compared to the placebo group, individuals assigned to lifestyle intervention experienced 40%−58% relative reduction in the risk of developing T2DM (9–11). Notably, the participants in the landmark diabetes prevention studies all had prevalent prediabetes determined during cross-sectional population screening and, therefore, of unknown duration at enrollment (9–11).
The impact of awareness of incident prediabetes status on glycemic trajectories among previously normoglycemic persons is unknown, and cross-sectional surveys show conflicting reports regarding the impact of such awareness on self-directed health behavior (14, 15). The POP-ABC (Pathobiology of Prediabetes in A Biracial Cohort) (16,17) study enrolled normoglycemic African American (AA) and European American (EA) offspring of parents with T2DM and followed them for the occurrence of incident prediabetes, defined as impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) (4,5). All POP-ABC study participants completed a screening oral glucose tolerance test (OGTT) prior to enrollment. The first subject was enrolled in September 2006 and the POP-ABC study ended in February 2012 after the final follow-up visits. During ~5years of follow-up, 101 of 343 subjects developed prediabetes and were duly notified, without any active intervention or counseling, as POP-ABC was a natural history study.
Eighteen months after the study ended, POP-ABC study participants were invited to return for OGTT and clinical examination, as part of the screening procedures for a newly-funded PROP-ABC (Pathobiology and Reversibility of Prediabetes in A Biracial Cohort) study, whose aims include intensive lifestyle intervention to reverse prediabetes (ClinicalTrials.gov ID: NCT02027571). Here, we report temporal changes in glucose and other metabolic measures between the end of POP-ABC and the beginning of PROP-ABC (18 months later) in participants with incident prediabetes during POP-ABC (Pre-DM group) compared with a matched group of POP-ABC participants who did not develop prediabetes.
Methods
Study Subjects
The study subjects were participants in the POP-ABC study (16,17). Eligibility criteria for the POP-ABC study included age 18–65 years; self-reported non-Hispanic white (European American) or non-Hispanic black (African American) race/ethnicity status; one or both biological parents with T2DM; no evidence of diabetes; normal FPG (< 100 mg/dL{<5.6 mmol/L}) and/or normal 2hrPG (< 140 mg/dL {<7.8 mmol/L}) during a 75-g OGTT; and good overall health, as previously described (16,17). None of the participants was using any medications known to alter glucose or lipid metabolism. Enrollment in behavioral, pharmacological, or other active weight loss program or a history of liposuction or bariatric surgery, were additional exclusion criteria (16, 17). The primary outcome was the occurrence of prediabetes (IFG and/or IGT) or diabetes, defined by the American Diabetes Association criteria (4). A confirmatory test was performed within 6 weeks of each endpoint occurrence. The 75-g OGTT was the method of confirmation. All endpoints were independently adjudicated by the Institutional Data and Safety Officer (Murray Heimberg, MD, PhD). The study protocols were approved by the University of Tennessee Institutional Review Board; all participants gave written informed consent before initiation of study procedures.
Chronology and Outcome
The first subject was enrolled in September 2006 and the POP-ABC study ended in February 2012 after the final follow-up visits (16,17). During 5.5years of follow-up (mean 2.62 years), 101 of 343 participants developed incident prediabetes (17). Per study protocol, a copy of the confirmatory OGTT result was provided to all participants reaching the endpoint. However, no intervention or counseling was provided, as POP-ABC was a purely natural history study. In September 2013, 18 months after close-out of the POP-ABC study, funding became available for a continuation study, the Pathobiology and Reversibility of Prediabetes in a Biracial Cohort (PROP-ABC), whose goal is to provide intensive lifestyle intervention to the POP-ABC participants with incident prediabetes (ClinicalTrials.gov ID: NCT02027571). Eligibility for PROP-ABC thus was limited to the original POP-ABC participants, all of whom were invited to join the new study. Interested participants underwent a standard OGTT, anthropometry and clinical examination, as part of the baseline re-enrollment procedures. To test the hypothesis that awareness of incident prediabetes status triggers self-directed health behaviors that improve metabolic endpoints, we compared the temporal trends in OGTT, anthropometric and lipid data between the end of POP-ABC and the initiation of PROP-ABC study in participants with incident prediabetes during the POP-ABC phase and an age-, gender- and ethnicity-matched control group. We also compared changes in self-reported dietary and physical activity behaviors between the two groups of participants.
Figure 1 summarizes the time intervals from the beginning until the end of the POP-ABC study to re-screening for the extension PROP-ABC study.
Figure 1.
Study design and time intervals of data collection. Prediabetes outcome was assessed after ~5 years of follow-up of initially normoglycemic POP-ABC participants. Eighteen months later, POP-ABC study participants were re-screened for a newly funded extension PROP-ABC study. The present report compares the 18-month interval changes in glycemic and other measurements between participants who developed prediabetes during POP-ABC study and those who remained normoglycemic.
Assessments
Participants arrived at the University of Tennessee General Clinical Research Center (GCRC) after an overnight fast to undergo physical examination and scheduled assessments. Height was recorded using a stadiometer, weight was measured with a digital scale (Tanita WB-300 Plus Digital, Arlington Heights, IL), and body mas index (BMI) was calculated as weight (kg) divided by the square of the height in meters. A Gulick II tape was used to measure waist circumference, as previously described (16,17). Usual food intake was captured using the Food Habits Questionnaire (FHQ) (18) and habitual physical activity was recorded using the Modifiable Activity Questionnaire (MAQ) (19), as previously described (20). The responses to the MAQ were converted to metabolic equivalent (MET) based on the intensity of the reported activities and expressed as MET-hours/week (20). A standard OGTT was initiated between 0700 and 1100: venous blood specimens for glucose and insulin measurement were obtained before (0 min) and at 30 min and 120 min after ingestion of 75 grams flavored glucose (Trutol 75; Custom Laboratories, Baltimore, MD).
Biochemical Measurements
Plasma glucose was measured with a glucose oxidase method (Yellow Spring Instruments Co., Inc., Yellow Spring, OH). Plasma insulin levels were measured immunochemically in our Endocrine Research Laboratory, using commercial ELISA kits. Hemoglobin A1c (HbA1c) and fasting plasma lipid profiles were measured in a contract clinical laboratory.
Statistical analysis
Data were reported as means ± SEM. Differences between defined groups were analyzed using unpaired t-tests for continuous variables and chi-square test for categorical variables. Interval changes in glycemic, anthropometric and behavioral measures between the two defined testing periods were analyzed using paired t tests. General linear regression models were used to analyze the relationship between temporal changes in anthropometric and metabolic variables. All statistical analyses were performed with the use of SAS statistical software, version 9.3 (SAS Institute Inc., Cary, NC).
Results
Cohort description
The characteristics at enrollment of study subjects who developed prediabetes and were so informed during POP-ABC and age- and gender-matched participants who maintained normoglycemia during POP-ABC study (control) are shown in Table 1. As previously reported (17), participants who developed incident prediabetes had higher BMI, waist circumference, FPG and 2hrPG at enrollment, compared with participants who maintained normoglycemia.
Table 1.
Baseline Characteristics of POP-ABC Study Participants Who Subsequently Developed Incident Prediabetes or Maintained Normoglycemia (Control) during ~5 Years of Follow-up
| Characteristics | Prediabetes | Control | P Value |
|---|---|---|---|
| Number | 73 | 73 | |
| Age (yr) | 48.7 ± 0.96 | 48.3 ± 1.06 | 0.37 |
| Women/Men | 46 / 27 | 48 / 25 | 0.85 |
| Black/White | 36 / 37 | 35 / 38 | 0.84 |
| Weight (kg) | 88.7± 2.02 | 83.5 ± 2.19 | 0.09 |
| BMI (kg/m2) | 31.1 ± 0.70 | 29.2 ± 0.69 | 0.04 |
| Waist circumference (cm) | 97.9 ± 1.34 | 93.0 ± 1.71 | 0.02 |
| FPG | 94.4 ± 0.60 | 91.5 ± 0.72 | 0.002 |
| 2hrPG | 128 ± 2.80 | 120 ± 2.91 | 0.05 |
Data are mean ± SEM. FPG, fasting plasma glucose; 2hrPG, plasma glucose value at 2 hours during oral glucose tolerance test. To convert the values for glucose to millimoles per liter, multiply by 0.0555.
Glycemic, anthropometric and lipidemic changes
We assessed the interval changes in plasma glucose and other metabolic endpoints when progressors and nonprogressors to prediabetes during POP-ABC study were re-screened for enrollment in the extension study(PROP-ABC). As already noted, the POP-ABC study was an observational, natural history study that did not entail any drug intervention or lifestyle counseling to alter metabolic endpoints. During the 18-month interval between the end of POP-ABC study and re-screening for the PROP-ABC study, FPG levels decreased by 8.01 ± 1.11 mg/dl in the prediabetes group and by 2.02 ± 0.64 mg/dl in the control group (P<0.0001). During the same said interval, 2hrPG levels decreased by 8.21±3.34 mg/dl in the prediabetes group but increased by 8.53 ± 3.17 mg/dl) in the control group (P=0.0004) (Fig. 2 and Table 2). The corresponding interval changes (Prediabetes group vs. Control group) were −0.54 ± 0.72 kg vs. 2.77 ± 1.25 kg (P=0.006) for body weight, and −1.07 ± 0.78 cm vs. 1.78 ± 0.85 cm (P=0.001) for waist circumference (Fig. 2 and Table 2). The change in BMI was 0.23 ± 0.29 kg/m2 in the prediabetes group and 1.06 ± 0.46 kg/m2 in control (P=0.03). In regression analyses, the interval changes in FPG were correlated with interval changes in weight (r=0.18, P= 0.04) and waist circumference (r=0.20, P= 0.01).
Figure 2.
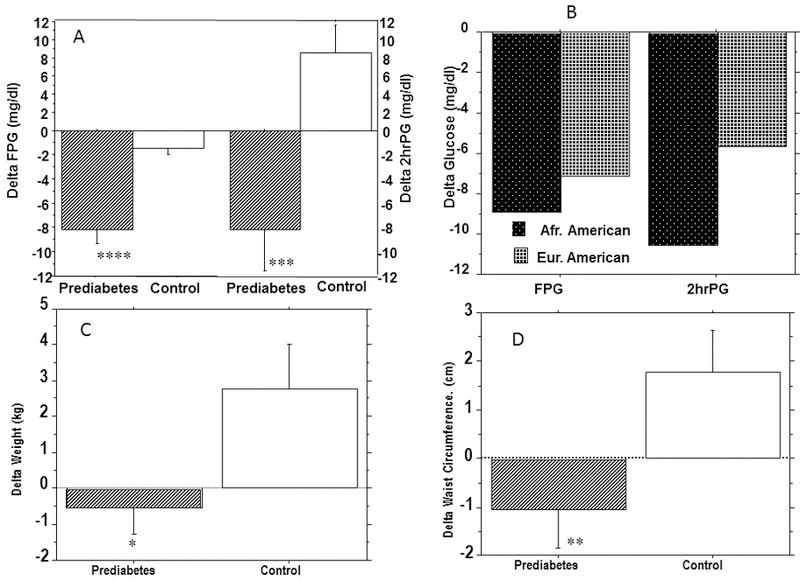
Interval changes in glycemia in prediabetes and control groups (A) and within the Prediabetes group by ethnicity (B), and interval changes in weight (C) and waist circumference (D) in the prediabetes and control groups. There were no significant ethnic differences in the interval changes in glycemia. * P=0.006, ** P= 0.001, ***P=0.0004, ****P<0.0001. To convert the values for glucose to millimoles per liter, multiply by 0.0555.
Table 2.
Interval Changes in Glycemic, Anthropometric and Behavioral Measures in Subjects with Incident Prediabetes or Normoglycemia (Control)
| Measures | Prediabetes | Control | P Value |
|---|---|---|---|
| FPG (mg/dl) | −8.01 ±1.11 | −2.02 ±0.64 | <0.0001 |
| 2hrPG (mg/dl) | −8.21 ± 3.34 | 8.53 ±3.17 | 0.0004 |
| Weight (kg) | −0.54 ± 0.72 | 2.77 ± 1.25 | 0.006 |
| BMI (kg/m2) | 0.23 ± 0.29 | 1.06 ± 0.46 | 0.03 |
| Waist (cm) | −1.07 ± 0.78 | 1.78 ± 0.85 | 0.001 |
| FHQ score | −0.23 ± 0.05 | −0.16 ± 0.04 | 0.002 |
| MAQ (MET-hr/wk) | 2.70 ± 3.45 | −3.30 ± 4.20 | 0.03 |
Data are mean ± SEM. FPG, fasting plasma glucose; 2hrPG, plasma glucose value at 2 hours during oral glucose tolerance test. To convert the values for glucose to millimoles per liter, multiply by 0.0555.
The prediabetes group did not show significant alterations in plasma levels of LDL cholesterol ( 111 ± 3.61 mg/dl vs. 111 ± 3.79 mg/dl ), HDL cholesterol (49.2 ± 2.18 mg/dl vs. 50.5 ±1.65 mg/dl) or triglycerides (106 ± 6.71 mg/dl vs. 102 ±5.33 mg/dl) between the end of POP-ABC vs. follow-up examination 18 months later, nor did the control group: LDL cholesterol (106 ± 3.55 mg/dl vs. 106 ±3.31 mg/dl), HDL cholesterol (54.7 ± 1.73 mg/dl vs. 55.1 ±1.57 mg/dl ) and triglycerides (91.5 ± 5.85 vs. 94.2 ± 4.91).
Behavioral changes
Self-reported FHQ score (lower values indicate healthier eating pattern) decreased from 2.57 ± 0.51 to 2.34 ± 0.55 (−0.23; P=0.0003) in the Prediabetes group and from 2.51 ± 0.50 to 2.35 ± 0.52 (−0.16; P=0.0005) in the Control group. The absolute decrease in FHQ score was significantly greater in the Prediabetes group vs. Control group (−0.23 ± 0.05 vs. −0.16 ± 0.04, P=0.002). Self-reported MAQ score (higher values indicate greater physical activity) increased nominally from 13.8 ± 3.03 MET-hr/wk to 16.5 ± 2.47 MET-hr/wk in the Prediabetes group, but decreased nominally from 21.7 ± 3.39 MET-hr/wk to 18.4 ± 2.76 MET-hr/wk in the Control group. The absolute change in MAQ score was significantly greater in the Prediabetes group vs. Control group (2.70 ± 3.45 MET-hr/wk vs. −3.30 ± 4.20 MET-hr/wk, P=0.002) (Table 2).
Discussion
In the present report, we observed that POP-ABC participants who were notified that they had developed incident prediabetes showed decreases in FPG and 2hrPG levels, weight, and waist circumference, when reassessed 18 months later. These improvements were in comparison with a control group of age-, gender- and ethnicity-matched POP-ABC participants who had maintained normoglycemia during the same follow-up period. The POP-ABC study was a longitudinal natural history study of the transition from normal glucose regulation to prediabetes among initially normoglycemic African American and European American adults who have one or both biological parents with diagnosed T2DM (16,17). By design, no therapeutic intervention was offered to the participants who progressed to prediabetes. However, a written report of the confirmatory OGTT results indicating the development of impaired fasting glucose and/or impaired glucose tolerance was provided to participants reaching those endpoints. Thus, our present findings suggest that the mere notification of incident prediabetes status may trigger beneficial changes in glycemia and body size in African Americans and European Americans with parental history of T2DM.
The mechanism(s) for the observed benefits in glycemia, glucose tolerance, and adiposity following notification of incident prediabetes status are unclear, but could have involved self-directed lifestyle modification. To explore such a mechanism, we analyzed changes in self-reported dietary and physical activity behaviors in the two comparison groups of participants across the 18-month interval between conclusion of the POP-ABC study and initiation of the PROP-ABC study. We found that FHQ scores decreased in both the Prediabetes and Control groups (indicating healthier eating patterns), but the improvement was significantly greater in the Prediabetes group. Similarly, participants in the Prediabetes group reported a significant 6 MET-hr/wk greater physical activity than did those in the Control group. These behavioral alterations, if sustained, could explain the glycemic and weight benefits observed in the Prediabetes group. In addition to self-directed behavioral changes, it is possible that some participants may have discussed their prediabetes status with their primary care providers and may have received lifestyle counseling outside our study.
Did the Hawthorne Effect or regression to the mean play a role in our findings? Subjects enrolled in research studies may alter their behavior and produce unintended results, from the increased attention and encounters associated with research participation (Hawthorne effect) (21). Usually, the Hawthorne Effect dissipates within approximately four months or once study procedures cease (22,23). Notably, our POP-ABC study had a follow-up period of 5.5 years (mean 2.62 years) and the interval between end of study and re-testing of participants was 18 months. Thus, it is unlikely that our present findings could be explained by the Hawthorne effect. Moreover, there is no reason why any Hawthorne effect would be restricted to the Prediabetes group. The statistical phenomenon of regression to the mean describes the tendency of individuals with outlier values on a given measure to have spontaneously lower values upon re-testing, without any intervention. The use of contemporaneous measurements in a matched control group, as was done in the present study, usually obviates the risk of misinterpretation due to regression to the mean (24,25).
Two previous national surveys had reported discordant findings on the impact of awareness of prediabetes status on health behavior (14, 15). Data from the 2006 National Health Interview Survey (NHIS) showed that of the ~ 4% of U.S. adults who had been told that they had prediabetes, 68% reported active attempts to lose or control weight, 55% reported increased physical activity, 60% reported less fat consumption, and 42% reported trying all three approaches (14). In a different report, investigators analyzed data on 24-h dietary recall, self-reported diabetes and prediabetes awareness status, and FPG and HbA1c values from the 2005–2010 National Health and Nutrition Examination Survey (NHANES) (15). Persons unaware of diabetes and prediabetes were identified by FPG <126 mg/dL or HbA1c <6.5% and FPG 100–125 mg/dL or HbA1c of 5.7%–6.4%, respectively (15). People with diagnosed diabetes reported consumption of less carbohydrates and more protein compared to those with undiagnosed diabetes (15). However, the authors observed no significant differences in macronutrient intake by awareness of prediabetes status (15). The conclusion from the 2005–2010 NHANES data was that knowledge of glycemic status induced healthier dietary patterns for people with diabetes but not those with prediabetes. Unlike the cross-sectional surveys based on self-reported prediabetes status, our prospective study employed rigorous ascertainment of incident prediabetes status, using OGTT and independent adjudication. Thus, the mechanism of becoming aware of incident prediabetes in POP-ABC study was the documentation that a participant’s FPG and/or 2hrPG values had drifted upward from normal glucose regulation to prediabetes (IFG and/or IGT). Our findings support a beneficial impact of prediabetes awareness on health behavior and metabolic endpoints. We argue that awareness of incident prediabetes status through direct communication of measured glucose values, as was done in the POP-ABC study, was impactful in triggering behavioral change.
Remarkably, the vast majority of people with prediabetes in the general primary care population remain undiagnosed and unaware of their condition (2, 14, 15, 26). In the 2006 National Health Interview Survey, only an estimated 4% of U.S. adults had been told they had prediabetes (15). Although ~34% of U.S. adults had prediabetes in 2015, only 11.6% self-reported that they had been diagnosed with prediabetes by a health care worker (2). Awareness of and response to prediabetes among health care providers is even much lower. Analyzing HbA1c data from the 2012 National Ambulatory Medical Care Survey that targeted adults aged >45 years without diagnosed diabetes who had an HbA1c test within 90 days (N = 11,167,004 weighted visits), the prevalence of prediabetes was found to be 33.6% (26). However, <1% of patients with HbA1c results consistent with prediabetes had formal diagnosis and documentation of prediabetes in the medical records (26). Clearly, there is need for greater awareness and early action regarding the diagnosis and management of prediabetes. Once diagnosis, using FPG, OGTT or HbA1c values, the clinical significance of prediabetes needs to be communicated clearly to all affected patients (6–8), as the present report has shown that notification of prediabetes status (even without active intervention) may trigger beneficial self-directed lifestyle modifications. In addition, current guidelines by the American Diabetes Association recommend referring people with prediabetes for intensive lifestyle modification (4).
Acknowledgments
We are indebted to the participants who volunteered for this study.
Role of Funding Sources: The funding sources had no role in the design and execution of the POP-ABC study, or analysis and publication of the data obtained from the study.
Funding Statement: This study was supported by Grant R01 DK067269 from the National Institutes of Health and Grant 7-07-MN-13 from the American Diabetes Association, both awarded to SD-J. The funding sources had no role in the design and execution of the study, or analysis and publication of the data obtained from the study.
Footnotes
Conflicting and Competing Interest: The authors have no conflicting or competing interest to disclose with regard to the content of this manuscript.
References
- 1.US Department of Health and Human Services, American Diabetes Association. Press release 27 March 2002. Washington, DC: DHHS, 2002 http://wayback.archive-it.org/3926/20130930184836/http://archive.hhs.gov/news/press/2002pres/20020327.htm (accessed August 2018). [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017 https://www.cdc.gov/diabetes/data/statistics/statistics-report.html (accessed August 2018) [Google Scholar]
- 3.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017;128:40–50 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Standards of medical care in diabetes—2018. Diabetes Care 2018;41(Suppl.1):S1–S15529222369 [Google Scholar]
- 5.World Health Organization 1985 Diabetes Mellitus: report of a WHO Study Group Geneva, World Health Organization; (Tech. Rep. Ser. 1985, no. 727) [PubMed] [Google Scholar]
- 6.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B; American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–759 [DOI] [PubMed] [Google Scholar]
- 7.Brannick B, Wynn A, Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med (Maywood) 2016;241:1323–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brannick B, Dagogo-Jack S. Prediabetes and Cardiovascular Disease: Pathophysiology and Interventions for Prevention and Risk Reduction. Endocrinol Metab Clin North Am 2018;47:33–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–480 [DOI] [PubMed] [Google Scholar]
- 11.Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 12.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE; Diabetes Prevention Program Research Group. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diabetes Prevention Program Research Group. Achieving weight and activity goals among Diabetes Prevention Program lifestyle participants. Obes Res 2004;12:1426–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Self-reported prediabetes and risk-reduction activities -- United States, 2006. MMWR 2008;57:1203–1205 https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5744a3.htm (accessed August 2018) [PubMed] [Google Scholar]
- 15.Bardenheier BH, Cogswell ME, Gregg EW, Williams DE, Zhang Z, Geiss LS. Does knowing one’s elevated glycemic status make a difference in macronutrient intake? Diabetes Care 2014;37:3143–3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dagogo-Jack S, Edeoga E, Ebenibo S, Chapp-Jumbo E. Pathobiology of Prediabetes in a Bi-racial Cohort (POP-ABC) Study: Baseline characteristics of enrolled subjects. J Clin Endocrinol Metab 98:120–128, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagogo-Jack S, Edeoga C, Ebenibo S, Nyenwe E, Wan J; for the Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) Research Group. Lack of racial disparity in incident prediabetes and glycemic progression among black and white offspring of parents with type 2 Diabetes: The Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) Study. J Clin Endocrinol Metab 2014;99:E1078–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krista AR, Shattuck AL, Henry HJ. Patterns of dietary behavior associated with selecting diets low in fat: reliability and validity of a behavioral approach to dietary assessment. J Am Diet Assoc 1990; 90:214–220 [PubMed] [Google Scholar]
- 19.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, Bennett PH, Kuller LH. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care 1990; 13:401–411 [DOI] [PubMed] [Google Scholar]
- 20.Boucher AB, Adesanya EAO, Owei I, Gilles AK, Ebenibo S, Wan J, Edeoga C, Dagogo-Jack S. Dietary habits and leisure-time physical activity in relation to adiposity, dyslipidemia, and incident dysglycemia in the Pathobiology of Prediabetes in A Biracial Cohort Study. Metabolism 2015;64:1060–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsons HM. What Happened at Hawthorne?: New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science 1974;183:922–1932 [DOI] [PubMed] [Google Scholar]
- 22.Cizza G, Piaggi P, Rother KI, Csako G; Sleep Extension Study Group. Hawthorne effect with transient behavioral and biochemical changes in a randomized controlled sleep extension trial of chronically short-sleeping obese adults: implications for the design and interpretation of clinical studies. PLoS One 2014. August 20;9(8):e104176. doi: 10.1371/journal.pone.0104176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol 2014;3:267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu R, Chen L. The need to control for regression to the mean in social psychology studies. Front Psychol 2015. January 8;5:1574. doi: 10.3389/fpsyg.2014.01574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linden A Assessing regression to the mean effects in health care initiatives. BMC Med Res Methodol 2013. September 28;13:119. doi: 10.1186/1471-2288-13-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mainous AG, Tanner RJ, Baker R. Prediabetes diagnosis and treatment in primary care. J Am Board Fam Med 2016;29:283–285 [DOI] [PubMed] [Google Scholar]


