Abstract
The study of lymphangiogenesis is an emerging science that has revealed the lymphatic system as a central player in many pathological conditions including cancer metastasis, lymphedema, and organ graft rejection. A thorough understanding of the mechanisms of lymphatic growth will play a key role in the development of therapeutic strategies against these conditions. Despite the known potential of this field, the study of lymphatics has historically lagged behind that of hemangiogenesis. Until recently, significant strides in lymphatic studies were impeded by a lack of lymphatic-specific markers and suitable experimental models compared to those of the more immediately visible blood vasculature. Lymphangiogenesis has also been shown to be a key phenomenon in developmental biological processes, such as cell proliferation, guided migration, differentiation, and cell-to-cell communication, making lymphatic-specific visualization techniques highly desirable and desperately needed. Imaging modalities including immunohistochemistry and in-situ hybridization are limited by the need to sacrifice animal models for tissue harvesting at every experimental time point. Moreover, the processes of mounting and staining harvested tissues may introduce artifacts that can confound results. These traditional methods for investigating lymphatic and blood vasculature are associated with several problems including animal variability (e.g., between mice) when replicating lymphatic growth environments and the cost concerns of prolonged, labor-intensive studies, all of which complicate the study of dynamic lymphatic processes. With the discovery of lymphatic-specific markers, researchers have been able to develop several lymphatic and blood vessel-specific, promoter-driven, fluorescent-reporter transgenic mice for visualization of lymphatics in vivo and in vitro. For instance, GFP, mOrange, tdTomato, and other fluorescent proteins can be expressed under control of a lymphatic-specific marker like Prospero-related homeobox 1 (Prox1), which is a highly conserved transcription factor for determining embryonic organogenesis in vertebrates that is implicated in lymphangiogenesis as well as several human cancers. Importantly, Prox1-null mouse embryos develop without lymphatic vessels. In human adults, Prox1 maintains lymphatic endothelial cells and upregulates proteins associated with lymphangiogenesis (e.g., VEGFR-3) and downregulates angiogenesis-associated gene expression (e.g., STAT6). To visualize lymphatic development in the context of angiogenesis, dual fluorescent-transgenic reporters, like Prox1-GFP/Flt1-DsRed mice, have been bred to characterize lymphatic and blood vessels simultaneously in vivo. In this review, we discuss the trends in lymphatic visualization and the potential usage of transgenic breeds in hemangiogenesis and lymphangiogenesis research to understand spatial and temporal correlations between vascular development and pathological progression.
Keywords: Angiogenesis, Lymphangiogenesis, Transgenic mice, Fluorescent reporter
The importance and challenges of lymphatic visualization, in vitro and in vivo
The main function of lymphatic vessels is to return leaked blood vessel plasma from the interstitium back into circulation. Macromolecules and white blood cells, including extravasated leukocytes and antigen-presenting cells that initiate immune responses in lymph nodes, can be found in lymph, which eventually drains into the venous system in the jugular area[1,2]. Similar to how veins function, the contractions of surrounding smooth and skeletal muscles around collecting lymphatic vessels propel lymph forward, while lymphatic valves prevent backflow. Despite the known importance of lymphatics in fluid homeostasis and the immune system, molecular markers specific for the lymphatic system have only recently been discovered. Since the discovery of these lymphatic-specific markers, developments in vascular biology have included insight into the complex mechanism underlying lymphangiogenesis– the generation of new lymphatic vessels from pre-existing ones– during which lymphatic endothelial cells proliferate, migrate, form sprouts and then become tubes [3].
Lymphangiogenesis is a key player in many prominent pathological contexts, including cancer metastasis, particularly that of carcinoma, which is the most common cancer cell lineage, and organ graft rejection[2,4–6]. The growth of new lymphatic vessels provides alternative conduits for the spread of cancer cells and anti-graft immune cells. Therefore, elucidating the mechanisms and regulation of lymphangiogenesis is vital to the discovery of therapeutic targets[7]. However, imaging and quantifying lymphatic vasculature in vivo has historically been a challenge. Unlike blood, which is densely packed with red blood cells and readily observed in exposed tissue, lymph is composed of mostly water, electrolytes, proteins, and leukocytes. Lymphatic vessels are thus relatively more difficult to visualize than their sanguineous counterparts are. Blood vessels and their involvement in tumor metastasis, atherosclerosis, myocardial infarction, and other common diseases are routinely studied using magnetic resonance imaging (MRI) and computed tomography (CT) angiography, as well as Doppler ultrasound, none of which facilitate convenient lymphatic visualization[8].
Both in vitro and in vivo methods have been used to visualize angiogenesis and lymphangiogenesis under a myriad of experimental conditions. Several two-dimensional culture systems are available to study lymphatic growth, including those that utilize lymphatic endothelial cells (LECs) taken from various sources (e.g., lymphatic-rich lesions induced by injecting Freund’s adjuvant, thoracic duct fragment explants, embryoid bodies, and immune-purified primary or immortalized human dermal LECs)[9]. While these assays are useful for investigating cell proliferation and migration, they continue to be burdened by their limitations in fully encapsulating the natural progression of lymphangiogenesis. On the other hand, some in vitro models, such as the lymphatic ring assay, Bruyere’s adaptation of the aortic ring assay, successfully demonstrate the three-dimensional, capillary-like sprouting of lymphatic vessels upon stimulation and also allow quantification of lymphatic growth via computer-assisted imaging[9]. Most in vitro models including the lymphatic ring assay, however, require not only a prolonged study period but also the harvesting of a large number of mice for tissue. Moreover, in vitro models cannot fully simulate the vast network of growth factors, stabilizing niches, and other regulatory factors that contribute to lymphatic growth in vivo.
In vivo clinical imaging, which includes lymphangiography, lymphoscintigraphy, contrast-enhanced MR lymphangiography (MRL), CT, positron emission tomography (PET) scans, and near-infrared indocyanine green fluorescence imaging, has been used to visualize gross features of lymphatics, including structure, filling defects, and obstruction in vessels and nodes[8]. Gross visualization can be aided by the use of dyes, including Evans Blue and India ink, which are readily taken up by the lymphatic system[10]. Fluorescent molecules such as FITC-dextran and nanoparticles or magnetic contrast agents provide higher resolution than dyes. Moreover, they can be visualized by lymphangiography or MRL, lymphatic analogs to angiography and MRA[8]. However, these modalities often exclude examination of endothelial heterogeneity, which may play a key role in regulating lymphangiogenesis, as well as real-time visualization of endothelial change in response to pathological stimuli.
These same methodologies have been adapted for use in animal studies; unfortunately, they are bound by many limitations (for a comprehensive review of these methodologies, refer to the following examples)[11]. Most often, the introduction of fluorescent compounds is restricted to injection sites proximal to the tissue of study (as opposed to simple tail injections for angiography), which can preclude global lymphatic vessel visualization. Visualization of deep lymphatic structures and lymph nodes is possible with the use of a near-infrared dye such as indocyanine green (ICG) and near infrared (NIR) fluorescence imaging. However, the visualization of deep lymphatic structures using near-infrared dyes is limited by specificity among others concerns[11].
The use of LEC-specific markers, including Prospero-related homeobox 1 (Prox1), lymphatic vessel endothelial hyaluronic acid receptor 1 (LYVE1), vascular endothelial growth factor 3 (VEGFR3), or podoplanin, has been instrumental in designing various in vivo models of lymphangiogenesis. Some examples are: (1) the overexpression of VEGF-C or -D in tumor cells[12–14], and transgenic mice[15,16]; (2) corneal micropocket containing growth factor pellets[17,18]; (3) intraperitoneal injection of incomplete Freund’s adjuvant to induce lymphatic hyperplasia[19,20]; and (4) stimulation of lymphangiogenesis using avian chorioallantoic membrane (CAM)[21]. Majumder et al. recently developed the ‘directed in vivo lymphangiogenesis assay’ (DIVLA), an adaptation of the ‘directed in vivo angiogenesis assay’ (DIVAA), to accurately quantify lymphangiogenesis and address the drawbacks of some in vivo models (e.g., Freund’s adjuvant can cause a baseline increase in inflammatory factors)[22].
The study of a process as dynamic and complex as lymphangiogenesis often necessitates the use of in vivo live imaging. Coupled with the power of transgenic protein reporter mice, in vivo live imaging of transgenic mice allows for greater specificity in visualizing lymphatic-specific processes. Moreover, bypassing the need to sacrifice mice at individual time points allows for continuity in imaging, which can greatly augment our understanding of various facets of lymphangiogenesis, ranging from its interplay with inflammation and angiogenesis to the screening of potential therapies. This review, then, continues with a discussion of in vivo live imaging with a focus on transgenic fluorescent reporter mice, their utility in various discoveries in the field of lymphangiogenesis, and their most recent applications.
Development of transgenic mice models to study lymphatics
The discoveries of Prox1, LYVE1, and podoplanin have significantly advanced the field of lymphangiogenesis by enabling lymphatic-specific labeling with fluorochrome dyes, which has helped elucidate important lymphangiogenic processes. For example, McElroy et al. demonstrated real-time trafficking of tumor cells tagged with red fluorescent protein within lymphatic vessels labeled with monoclonal LYVE-1 antibody conjugated to a green fluorophore and documented tumor cell invasion into lymph nodes[23]. Furthermore, transgenic mice engineered using a gene-targeted bacterial artificial chromosome (BAC) have been designed to express green fluorescent protein (GFP), mOrange, or tdTomato under Prox1 control. Under the same principle, many other transgenic models have been designed using Prox1-GFP, VEGFR3-YFP, Prox1-tdTomato, Flk1-mcherry[24,25], Flk1-GFP, Flt1-tdsRed[26], Flk1-Nano-lantern[27] and Flt1-DsRed for the visualization of hemangiogenesis and lymphangiogenesis in vivo and in vitro. Table 1 and 2 displays the result of a comprehensive literature search for recent transgenic models used in the studies of angiogenesis and lymphangiogenesis, respectively.
Table 1.
Promoter genes used in generating transgenic mouse lines with inherently fluorescent blood endothelial cells.
| Marker | Mouse line | Fluorescent Protein | First author | Year | Title/Reference | |
|---|---|---|---|---|---|---|
| Blood endothelial cells | Flk1 | Tg(Flk1::myr-mCherry) | mCherry | Larina | 2009 | A membrane-associated mCherry fluorescent reporter line for studying vascular remodeling and cardiac function during murine embryonic development [28]. |
| Flk1 | Tg(Flk1::H2B-EYFP) | EYFP | Fraser | 2005 | Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice [29]. | |
| Flk1 | Flk1+/EGFP mice | GFP | Ema | 2006 | Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors; [30]. | |
| Flk1 | Flk1-GFP BAC Tg mice | GFP | Ishitobi | 2010 | Flk1-GFP BAC Tg mice: an animal model for the study of blood vessel development [31]. | |
| Flt1 | Flk1-tdsRed BAC Tg mice | tdsRed | Matsumoto | 2012 | Study of normal and pathological blood vessel morphogenesis in Flt1-tdsRed BAC Tg mice[26]. | |
| Flk1 | Flk1-Nano-lantern BAC tg mice | Nano-lantern | Matsushita | 2017 | Fluorescence and Bioluminescence Imaging of Angiogenesis in Flk1-Nano-lantern Transgenic Mice [27]. | |
| Flt1 | Flt‐1/eGFP‐anillin | eGFP-anillin | Herz | 2018 | Visualization of endothelial cell cycle dynamics in mouse using the Flt‐1/eGFP‐anillin system [32]. | |
| Tie2 | Tie2-GFP | GFP | Motoike | 2000 | Universal GFP reporter for the study of vascular development [33]. | |
| Tie2 | Tie2-GFP | GFP | Glaser | 2014 | Specialized mouse embryonic stem cells for studying vascular development [34]. | |
| Tie1 | Tie1-GFP | GFP | Iljin | 2002 | A fluorescent Tie1 reporter allows monitoring of vascular development and endothelial cell isolation from transgenic mouse embryos [35]. | |
| Ephrin B2 |
Ephrin-B2-H2BGFP | GFP | Davy | 2006 | Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome [36]. | |
| Claudin5 | Tg eGFP-claudin5 (fusion protein) |
GFP | Knowland | 2014 | Stepwise recruitment of transcellular and paracellular pathways underlies blood–brain barrier breakdown in stroke [37]. | |
| VE-cadherin | VE-cadherin-EGFP | EGFP | Winderlich | 2009 | VE-PTP controls blood vessel development by balancing Tie-2 activity [38]. |
Table 2.
Promoter genes used in generating transgenic mouse lines with inherently fluorescent lymphatic cells.
| Marker | Mouse line | Fluorescent Protein | First author | Year | Title/Reference | |
|---|---|---|---|---|---|---|
|
Lymphatic endothelial cells |
Prox1 | prox1-mOrange2-pA-BAC | mOrange 2 | Hagerling | 2011 | Intravital two-photon microscopy of lymphatic vessel development and function using a transgenic Prox1 promoter-directed mOrange2 reporter mouse [39]. |
| Prox1 | BACTg(Prox1-EGFP) | EGFP | Choi | 2011 | Visualization of lymphatic vessels by Prox1- promoter directed GFP reporter in a bacterial artificial chromosomebased transgenic mouse [40]. | |
| Prox1 | ProxTom | tdTomato | Truman | 2012 | ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets [41]. | |
| Prox1 | Prox1-Cre-tdTomato | tdTomato | Bianchi | 2015 | A Transgenic Prox1-Cre-tdTomato Reporter Mouse for Lymphatic Vessel Research [42]. | |
| Prox1 | Prox1-EGFP BAC | EGFP | Jung | 2017 | Development and Characterization of A Novel Prox1-EGFP Lymphatic and Schlemm’s Canal Reporter Rat [43]. | |
| Prox1 | Prox1-GFP | GFP | Kang | 2015 | Intravital Imaging Reveals Dynamics of Lymphangiogenesis and Valvulogenesis [44]. | |
| Prox1 | Prox1-tdTomato | tdTomato | Hong | 2016 | Efficient Assessment of Developmental, Surgical and Pathological Lymphangiogenesis Using a Lymphatic Reporter Mouse and Its Embryonic Stem Cells [45]. | |
| LYVE1 | Lyve1CreERT2tdT | tdTomato | Connor | 2016 | Lymphatic endothelial lineage assemblage during corneal lymphangiogenesis [46]. | |
| VEGFR3 | Vegfr3EGFPLUC mice | EGFPluciferase | Olmeda | 2017 | Whole-body imaging of lymphovascular niches identifies premetastatic roles of midkine [47]. | |
| VEGFR3 | Vegfr3EGFPLucKnockin | dual reporter (EGFP-LUC) | Martinez- Corral | 2012 | In vivo imaging of lymphatic vessels in development, wound healing, inflammation, and tumor metastasis [48]. | |
| VEGFR3 | VEGFR3-YFP | YFP | Calvo | 2011 | Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis [49]. |
Fluorescent reporter mice that have successfully been used to image vascular development have also been shown to be applicable to studying lymphatic development. The Tie2-GFP transgenic mouse model was one of the first lines to be used for fluorescence-based vascular research. The fluorescence tag allows for visual isolation of endothelial cells as well as observation of vascular morphogenesis. Dash et al. suggested three requirements for successful use of fluorescent tags within in vivo experiments: 1) the fluorescent protein should be expressed efficiently as a monomer and without toxicity; 2) it should be bright enough to obtain adequate signal above auto-fluorescence levels and should have inherent photostability for the signal to be maintained for the duration of the experimental imaging period; and 3) there should be negligible cross-bleeding in the excitation and emission channels in experiments utilizing multiple fluorescence proteins[50]. Furthermore, as some transgenic mice tend to lose transgene expression through epigenetic silencing over several generations, it is important to have a number of reporter lines to preserve transgene expression over several generations.
Lymphatic imaging using transgenic fluorescent reporter mice models
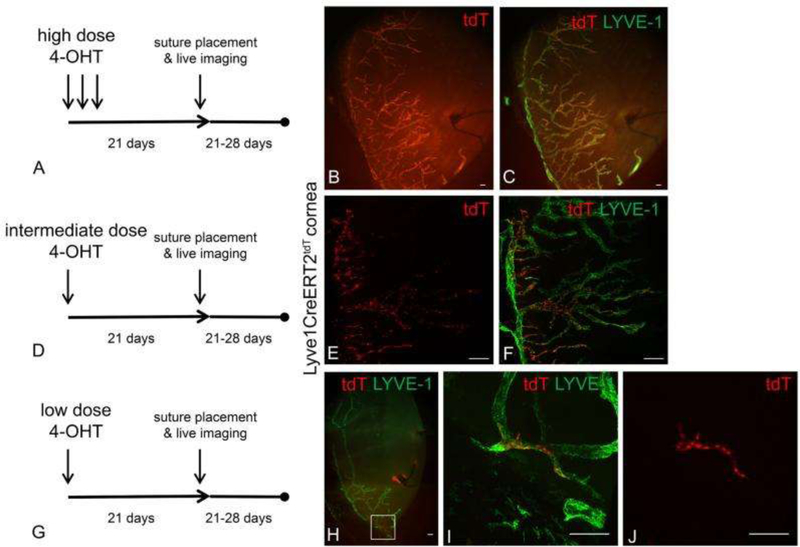
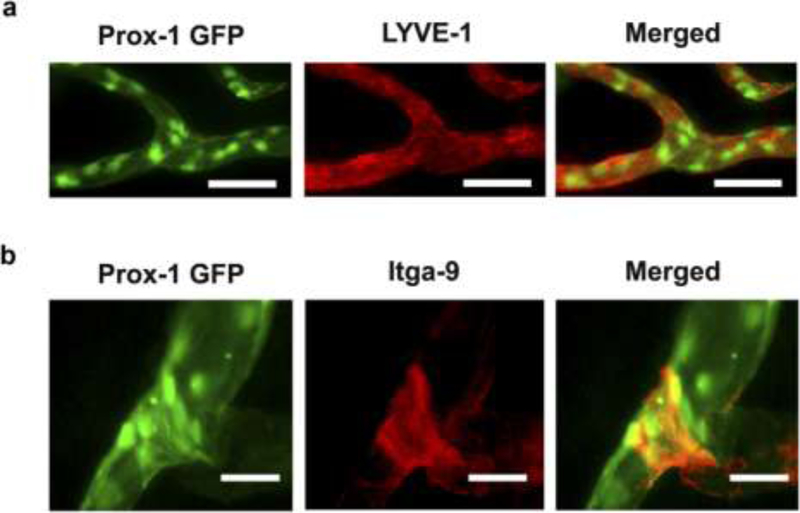
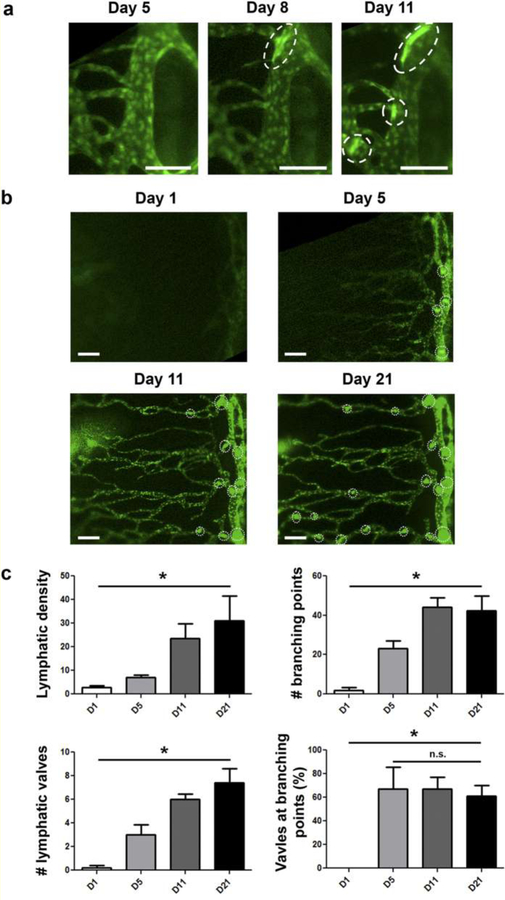
Various transgenic fluorescent reporter mouse models of corneal lymphangiogenesis have been instrumental in delineating the dynamic sequence of events that occur during lymphangiogenesis. Via live imaging in a reporter strain expressing tdTomato in LYVE1+ LECs upon 4-hydroxytamoxifen challenge, Connor et al. directly tracked the lineage of LEC progeny after corneal injury to demonstrate lymphatic vessel growth via a clustering maneuver dubbed “lineage assemblage.” This challenges the common assumption that LEC progeny distribute diffusely throughout the newly forming lymphatic vessel. Figure 1 depicts the technique used by Conner et al. to track the lineage of LEC progeny from tdTomato+ precursors on the background of tdTomato- lymphatic vessels in reporter mice after administration of low, intermediate, or high doses of 4-hydoxytamoxifen[46]. In addition, Kang et al. used a prox1-GFP mouse model and intravital imaging of the cornea after transplantation to clearly demonstrate the time course of lymphangiogenesis and valvulogenesis (the generation of lymphatic valves responsible for the unidirectional flow of lymph within lymphatic vessels). Previously difficult to measure with immunohistochemical staining, the lymphangiogenic sequence they directly observed is as follows: 1) valvulogenesis occurs initially within pre-existing lymphatic vessels; 2) vessel elongation occurs with lateral migration of stalk cells; 3) valvulogenesis continues within elongating vessels; 4) pruning takes place during early lymphangiogenesis; and 5) regression occurs during late lymphangiogenesis. Figure 2 shows the experiments of Kang et al. visualizing the co-localization of GFP with LYVE-1 and Itga-9, markers present on newly formed lymphatic vessels and valves, respectively, in response to corneal injury. Moreover, Figure 3 illustrates the spatial and temporal sequence and architecture of lymphatic valve and vessel formation after corneal transplantation[44].
Figure 1. Tracking of the lineage of LEC progeny from tdTomato+ precursors on the background of tdTomato- lymphatic vessels in reporter mice after administration of low, intermediate, or high doses of 4-hydoxytamoxifen.
Differences in the 4-OHT dose and schedule resulted in a range of tdT expression patterns from nearly 100% to very few tdT+ LYVE-1+ LECs. After Lyve1CreERT2tdT mice were treated with high, intermediate, or low 4-OHT dosing schedules, they were rested for 3 weeks ensure the completion of tdT conversion and stability. Corneal lymphangiogenesis was stimulated using the suture-induced model of corneal inflammation. Epifluorescence microscopy revealed that after administration of high 4-OHT dosing, nearly all Lyve-1+ LEC progenies were tdT+ (A-C). After administration of the intermediate 4-OHT dosing, some of the Lyve-1+ LEC progenies were tdT+ as shown by MIP confocal microscopy (D-F). After administration of the low 4-OHT dosing, very few of the Lyve-1+ LEC progeny were tdT+ as shown by MIP confocal microscopy (G-J). In these conditions, LYVE-1+ tdT+ LEC progeny were distributed in a contiguous and relatively linear manner within a newly synthesized lymphatic vessel (J). Scale bars, 100 μm. Reprinted with permission from [46].
Figure 2. Immunofluorescent microscopic analysis confirming Prox-1 GFP signals identify lymphatic vessels and valves in the cornea.
(a) Immunofluorescent microscopic images showing co-localization of Prox-1 GFP signal (green) and LYVE-1 staining (red) along newly formed lymphatic vessels in the inflamed cornea. Scale bars, 50 μm. (b) Immunofluorescent microscopic images showing co-localization of Prox-1 GFP signal (green) and Itga-9 staining (red) on the luminal valve of the lymphatic vessel. Scale bars, 25 μm. Reprinted with permission from [44].
Figure 3. Intravital time course imaging showing the initiative and progressive processes of lymphangiogenesis and valvulogenesis after corneal transplantation.
(a) Initiation and progression of valve formation within limbal vessels. Scale bars, 100 μm. (b) Progressive lymphangiogenesis in parallel with valvulogenesis into the central cornea after transplantation. Scale bars, 150 μm. The vertical vessel on the right of the panel is a limbal vessel; dotted circles indicate newly formed valves. (c) Quantified data showing increase of lymphatic density, branching points, and valves over time. *P < 0.05. Reprinted with permission from [44].
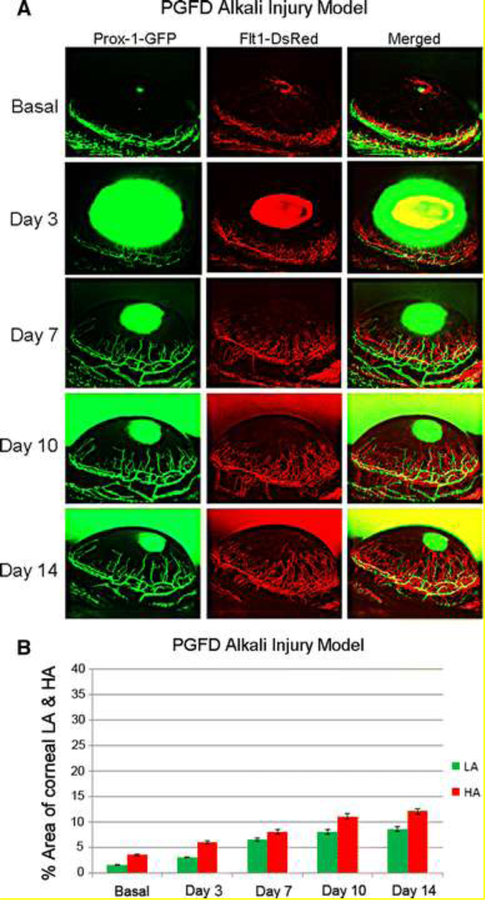
Finally, Zhong et al. developed Prox1-GFP/Flt1-DsRed (PGFD) mice for refined tracking of both blood and lymphatic vascular changes over time, particularly the monitoring of corneal hemangiogenesis and lymphangiogenesis within individual mice throughout a 14-day period after corneal injury [51]. Using confocal microscopy and two-photon imaging, they rapidly visualized fluorescent three-dimensional blood and lymphatic vessel patterns up to 150 and 400 µm deep, respectively, in large, unprocessed specimens from many tissues. Figure 4 provides a prime representation of this imaging technique in an alkali injury model of the cornea in PGFD mice.
Figure 4. Axiozoom stereo microscopy images of alkali burn-induced corneal HA and LA in PGFD mice.
Vascular (red) and lymphatic (green) vessels were imaged within the same cornea at each time point. (a) Blood and lymphatic vessel growth were quantitatively compared according to the percent area of the cornea occupied by each vessel type. (b) The percent areas occupied by alkali burn-induced corneal HA and LA were calculated over the experimental time course. Reprinted with permission from [51].
Together these studies highlight the utility of transgenic mice models in understanding lymphangiogenesis as a comprehensive cellular process and, in the case of PGFD mice, understanding its relation to hemangiogenesis. Only with a comprehensive understanding of the natural pathobiological progression by which lymphangiogenesis goes awry and results in pathological disease states, such as the obstruction of lymph flow and the inflammatory transport of self-antigens, can we begin to treat the complexity of lymphatic diseases. Live imaging within genetic fluorescent protein reporter animal models will greatly augment our knowledge of known lymphangiogenesis-regulating compounds and aid in the screening of anti-and pro-angiogenic/lymphangiogenic drugs. Moreover, animal models such as these hold the potential to inform the timing and strategy of lymphangiogenesis-targeting drug administration to balance interventional benefits with risks. For example, optimizing lymph flow to decrease interstitial fluid pressure could improve drug delivery, resolve inflammation in acute transplant settings, or limit the transport of self-antigens in the context of chronic graft rejection. On the other hand, mice have been used to observe the effects of photodynamic therapy (PDT)-induced ablation of vasculature[52]. Figure 5 shows lymphatic-specific ablation by verteporfin-PDT. Immunofluorescence for LYVE-1 effectively shows the injection site, drainage, as well as the eventual destruction of the lymphatic endothelium, demonstrating the possible application of PDT in the ablation of peritumoral lymphatics[52]. Only by studying the natural dynamic progression of lymphangiogenesis in intravital imaging can we hope to develop therapeutic insights and objectives that are currently on the horizon.
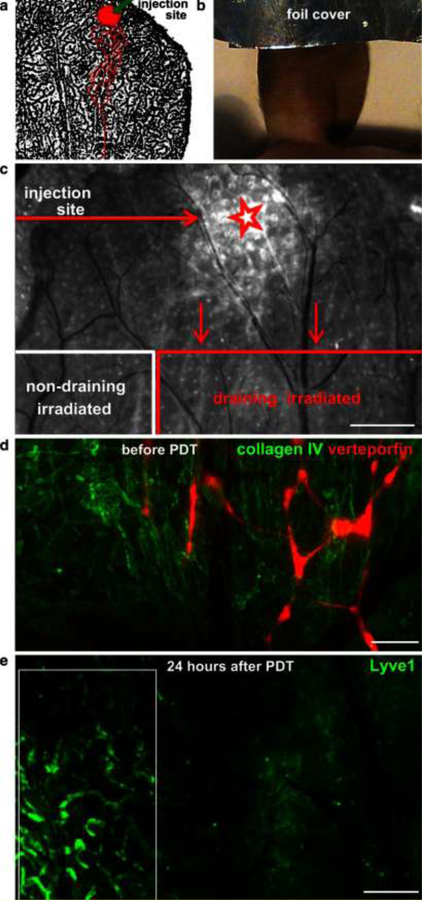
Figure 5. Fluorescence-based observation of lymphatic-specific ablation by verteporfin-PDT in the mouse ear.
(a) Schematic of the injection site and draining lymphatic vessels (red outline) over LYVE-1 staining of the mouse ear lymphatic vessels (black). (b) The injection site was covered with foil before irradiation of the rest of the ear. (c) Merged image of verteporfin fluorescence and brightfield view of the exposed dorsal ear dermis 24 h after PDT. Shown are the site of verteporfin injection (star) and the verteporfin-draining and non-draining (control) regions in the dorsal ear dermis after removal of the ventral skin and intermediate cartilage. Scale bar, 0.5 mm. (d) Fluorescence image showing verteporfin (red) draining within lymphatic vessels in the exposed dorsal skin after immunostaining for collagen IV (green). (e) Destruction of lymphatic endothelium was seen 24 h after PDT by intravital immunofluorescence staining for LYVE-1. Intact lymphatic vessels were seen in the control (non-draining) region (white box). Scale bar, 500 μm. Reprinted with permission from [52].
We must note, however, that aside from these advantages, live imaging in genetic fluorescent reporter mice (e.g., the PGFD mouse model) is limited by several factors: 1) genotyping is required to maintain the mouse strain; 2) Prox1-GFP expression enables visualization of lymphatic growth but is not solely limited to LECs; 3) similarly, Flt1-DsRed expression is not solely limited to blood endothelial cells; 4) breeding with other transgenic or knockout mice may not be possible if the target gene is localized to the same chromosome as Prox1-GFP or Flt1-DsRed; 5) without additional markers or methods, arteries and veins currently cannot be distinguished using Flt1-DsRed; 6) the Axiozoom V16 imaging time is limited to the duration under which the mouse remains under anesthesia to avoid any positional shift during Z-stack imaging; 7) in postmortem studies, diffusion of the fluorescent proteins prior to organ imaging may reduce the image quality; and 8) light excitation may cause phototoxicity and yield difficulty in distinguishing GFP fluorescence from autofluorescence[27]. There have also been reports on the drawbacks of podoplanin-reporter mice. In a podoplanin-Cre mouse line, it was recently observed that podoplanin is also prominently found on fibroblastic reticular cells of lymph nodes and of the white pulp of the spleen, where these cells play a major role in the activation of T cells [53]. This characteristic makes the podoplanin-Cre line unsuitable for lymphatic vessel imaging and lymph node analysis, because lymphogenic fluorescence could be confused with fluorescence from non-lymphogenic sources (i.e., thereby implicitly breaking Dash et al.’s second rule for using fluorescent markers within in vivo studies as the signal would not originate solely from the structure of interest)[50,42]. As a result, researchers have avoided this potential problem by generating Prox1-Cre lines. Figure 6 presents one such example of an inducible Prox1-Cre-tdTomato line for the visualization of lymphatic vessels in various organs[42]. Figure 7 shows another successful example of a similar usage of tdTomato reporter mice strains.
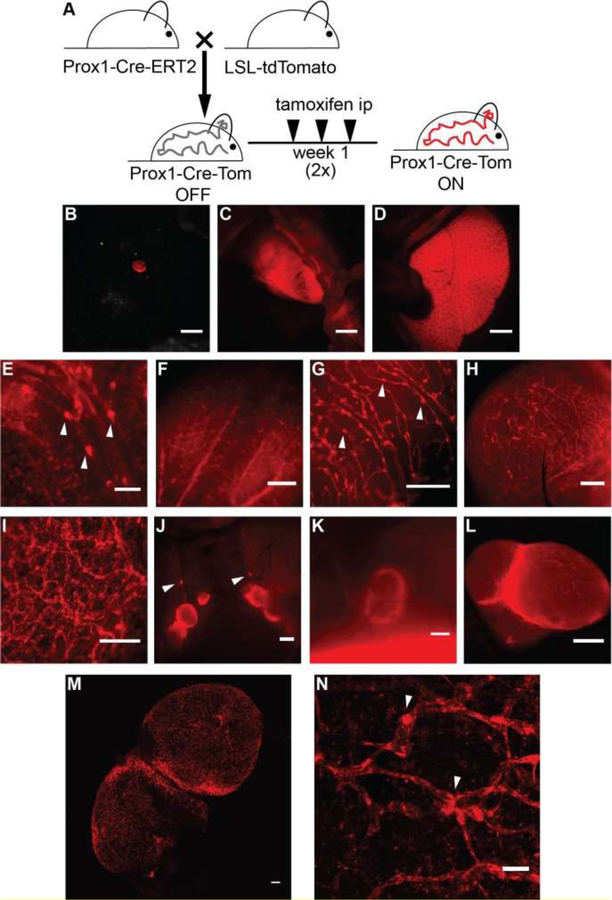
Figure 6. TdTomato expression in Prox1-positive cells upon crossing of the reporter mice with a Prox1-Cre-ERT2 line.
(A) Schematic representation of the breeding to Prox1-Cre-ERT2 mice and the tamoxifen administration regimen utilized (1 μg/g body weight in sunflower seed oil, intraperitoneally (ip), three times a week for 2 weeks). TdTomato expression was viewed under a stereomicroscope and detected in the eye (B), heart (C) and liver (D). TdTomato expression was visible in lymphatic structures in the mesentery (E), tongue (F), uterus (G), bladder (H), ear skin (ripped in half, I), lymph nodes of the neck area (J), and the inguinal lymph node (K). (L) Ex vivo image of an inguinal lymph node. (M) Confocal image of tdTomato autoflorescence showing lymphatic structures in a freshly isolated lymph node. Maximal intensity projection of a tile scan, z-stack of the lymph node is shown. (N) Confocal image (maximal intensity projection of a z-stack) of tdTomato autofluorescence showing lymphatic structures in a freshly isolated split ear sample. Arrowheads indicate lymphatic valves. Scale bars, 2000 μm (D-C), 1000 μm (J, K), 500 μm (E-I, L), and 100 μm (M-N). Reprinted with permission from [42].
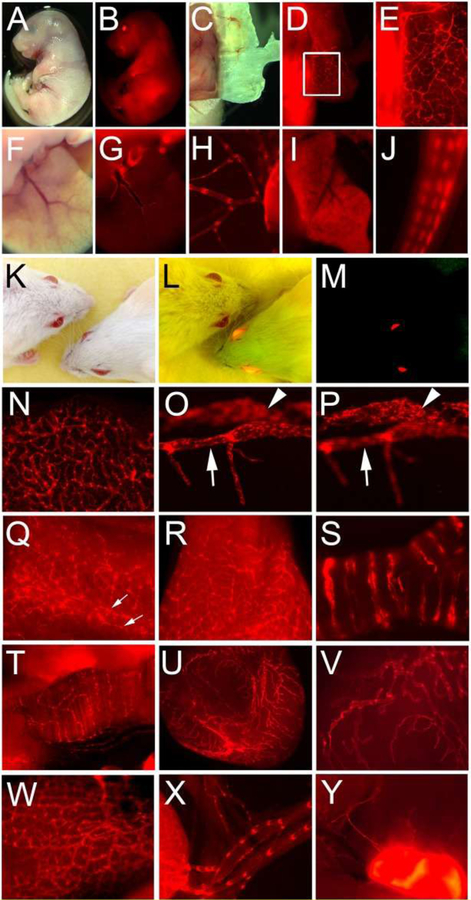
Figure 7. Prox1-tdTomato expression in reporter mouse embryos and adults.
(A-J) Embryonic tissues: bright field (A) and fluorescence (B) images of the Prox1-tdTomato embryo (E17.5). Distinct lymphatic networks shown in the embryonic skin (C-E). Panel E shows an enlarged image of the boxed area in panel D. Lymphatic vessels in the embryonic liver (F,G) and mesentery membrane (H). Note that hepatocytes (I) and tail nerves (J) were also positive for tdTomato. (K-Y) Adult tissues: headshots of adult wild-type and Prox1-tdTomato transgenic mice taken under a bright light (K), bright and fluorescent light (L) and fluorescent light (M). Lymphatic vessels were easily detectable in the ear (N), eye (O,P), tail (Q), tongue (R), trachea (S), diaphragm muscle (T), bladder (U,V), intestine (W), mesentery (X) and lymph node (Y). Corneal limbal lymphatic (arrow) and Schlemm’s canal (arrowhead) of the eye are shown in two consecutive focal planes (O,P). Bilateral lymphatic collectors in the tail were marked with two arrows (Q). Reprinted with permission from [45].
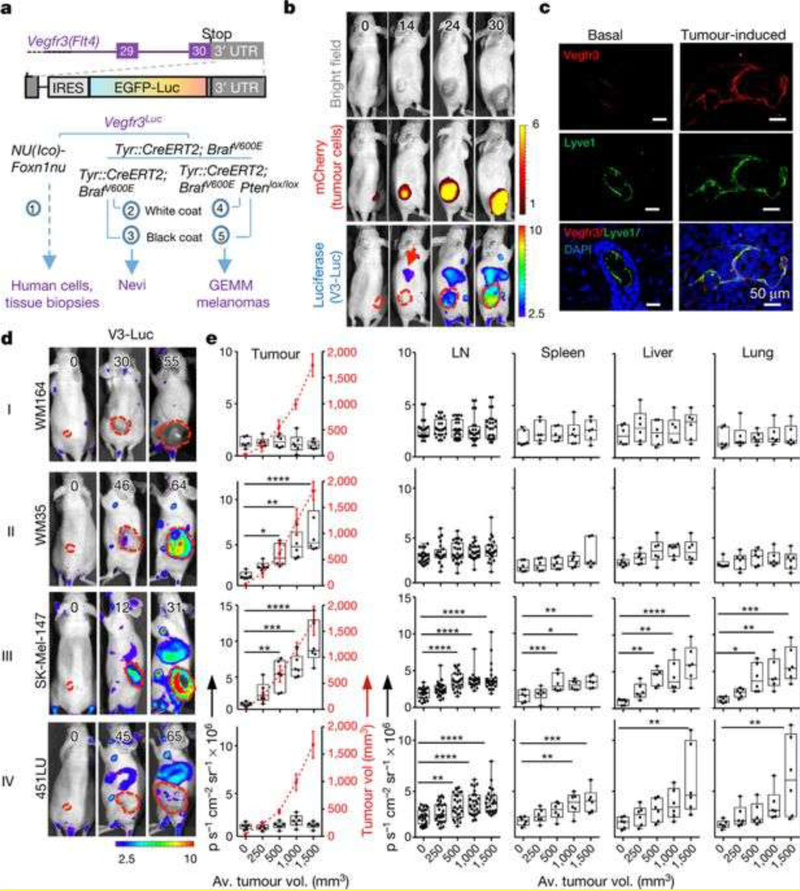
Recently, whole-body bioluminescent imaging of tumor lymphangiogenesis in transgenic mouse models has been adopted to understand lymphangiogenesis-driven metastasis due to the ability to image distant metastatic sites without the limitations of GFP. By analyzing the course of enhanced GFP (EGFP)-luciferase VEGFR3 reporter mice after implantation with nine different mCherry-labeled melanoma lines, Olmeda et al. found that early local and systemic VEGFR3- luc activation, and not VEGFC expression or VEGFR3-luc restricted to primary tumors, positively correlated with increased lymphatic vessel density at lymph nodes and tumor metastasis[47]. Moreover, surgical removal of tumors led to a decrease in systemic VEGFR3- luc. This led to the discovery of the novel pro-lymphangiogenic role of the heparin-binding factor midkine, which is expressed not only in melanoma but also in other tumors, indicating that tumors secrete midkine to establish pre-metastatic niches in distant lymphatic vessels and lymph nodes. Figure 8 illustrates a VEGFR3-Luc construct, the 9 different melanoma cell lines in which it was used, and the four main patterns of VEGFR3-Luc expression (I. no VEGFR3-Luc,II. VEGFR3-Luc localized to primary tumor, III. VEGFR3-Luc expression locally and systemically, IV. systemic VEGFR3-Luc expression with or without local primary tumor expression) observed as well as their correlations to tumoral metastasis to lymph nodes or distant organs[47].
Figure 8. Vegfr3Luc reporter mice for whole-body analysis of benign and malignant melanocytic lesions.
(a) Five strains of Vegfr3Luc mice generated. (b) Xenografts of mCherry–SK-Mel-147 cells, imaged as indicated. (c) Confocal immunomicroscopy of VEGFR3 (red) and Lyve1 (green) in normal skin or xenografts of SK -Mel-147 cells. (d) Four main patterns (I–IV) of V3-Luc emission identified by whole-body bioluminescence of mice bearing xenografts of the indicated melanoma cell lines. Numbers represent days after implantation and dotted lines show tumor area. (e) V3-Luc emission at the indicated locations and tumor volumes. LN, lymph nodes. Data are mean ± s.d. Fluorescence: p s−1 cm−2 sr−1 × 109; bioluminescence: p s−1 cm−2 per sr × 106. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Reprinted with permission from [47].
The limitations of GFP as a reporter include phototoxicity and background autofluorescence in mammalian cells due to exposure to the excitation frequency (i.e., thereby violating Dash et al.’s first and second criteria)[54,55,27]. Despite this, Prox1-GFP mice may still be a useful animal model. For example, Prox1-GFP mice were used to discover Prox1+ ECs (which may be closely related but distinct from LECs) in Schlemm’s canal with possible applications for glaucoma research and therapy[56]. Figure 9 reveals the location of these cells at the iridocorneal angle. Nevertheless, new bioluminescent proteins without the same drawbacks of GFP are on the horizon[56]. Recently, Matsushita et al. circumvented this problem by using Flk1-Nano-lantern BAC transgenic mice, which display bioluminence without light excitation or phototoxicity. Nano-lantern is a chimeric protein composed of an enhanced Renilla luciferase and Venus, a bioluminescent protein, while Flk1 (VEGFR2) is expressed in both blood lymphatic endothelial cells and forms a heterodimer with Flt4 (VEGFR3) in LECs to drive lymphangiogenesis [54,27]. Flk1-Nano-lantern BAC transgenic mice harboring lung carcinoma cells and given coelenterazine exhibited bioluminence due to tumor vessel growth days later that was detected with high sensitivity. Figure 10 demonstrates that blood and lymphatic vessel visualization in Flk1-Nano-lantern mice is comparable in quality to that in Flk1-GFP mice[27]. Thus, future experiments with Nano-lantern expression driven under the expression of VEGFR3 may serve as a useful transgenic animal model for the screening of lymphangiogenesis-targeting drugs.
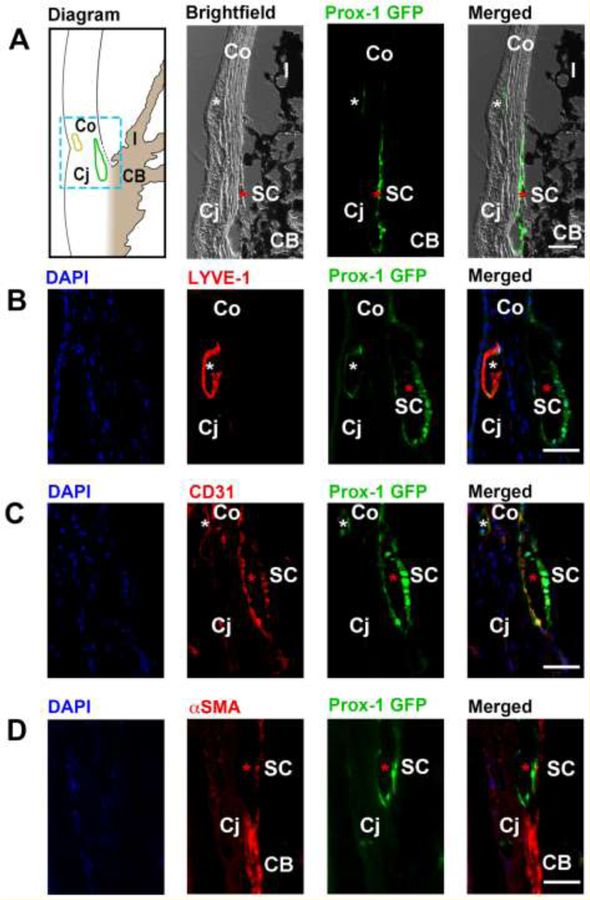
Figure 9. Cross-sectional immunohistochemical analysis of the iridocorneal angle of adult Prox-1-GFP mice.
(A) Left panel, diagram of anterior chamber angle showing location of Schlemm’s canal (green) at the corneoscleral junction. Yellow, limbal lymphatic vessels. Middle to right panels: brightfield, green fluorescent, and merged micrographs corresponding to the boxed region of interest in the diagram. White asterisks: limbal lymphatics; red asterisks: Schlemm’s canal. (B) Representative images showing the LYVE-1+Prox-1+ limbal lymphatic vessel (white asterisk) located between the cornea and conjunctiva, and the LYVE- 1−Prox1+ Schlemm’s canal (red asterisk) located nearby. Blue: DAPI for nuclear staining; red: LYVE-1; green: Prox-1. (C) Representative images showing that both limbal lymphatics (white asterisk) and the Schlemm’s canal (red asterisk) expressed CD31, a panendothelial cell marker. Blue: DAPI; red: CD31; green: Prox-1. (D) Representative images showing absence of α-smooth muscle actin (α-SMA) expression on the Prox-1+ Schlemm’s canal (red asterisk), but expression of α-SMA on adjacent positive control tissue of the ciliary body. Blue: DAPI; red: αSMA; green: Prox-1. Scale bars, 50 µm (A–D). SC, Schlemm’s canal; Co, cornea; Cj, conjunctiva; I, iris; CB: ciliary body. Reprinted with permission from [56].
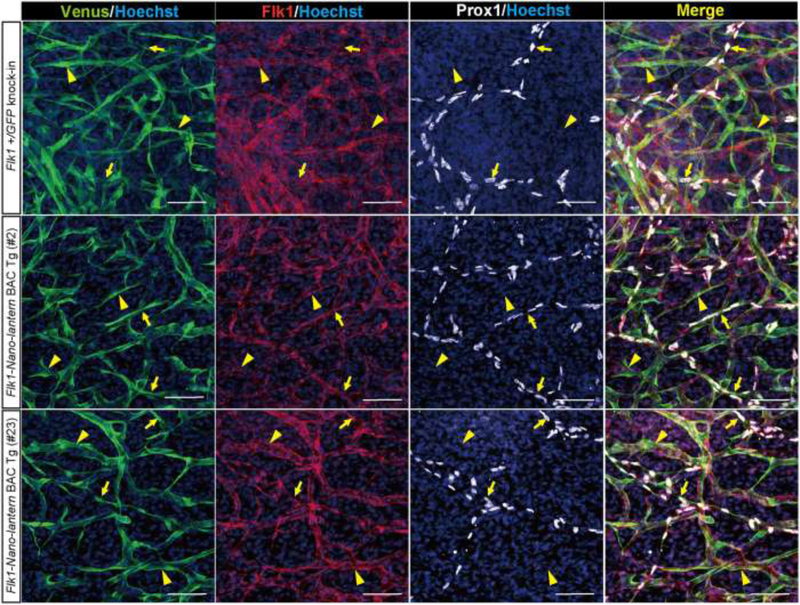
Figure 10. Expression of Flk1-Nano-lantern in lymphatic ECs.
Immunohistochemical analysis of the back skin of Flk1-Nano-lantern BAC Tg mice with anti-GFP, -Flk1 and –Prox1 antibodies. Arrows and arrowheads indicate Prox1-positive LECs and Prox1-negative VECs, respectively. Scale bars, 50 μm. Reprinted with permission from [27].
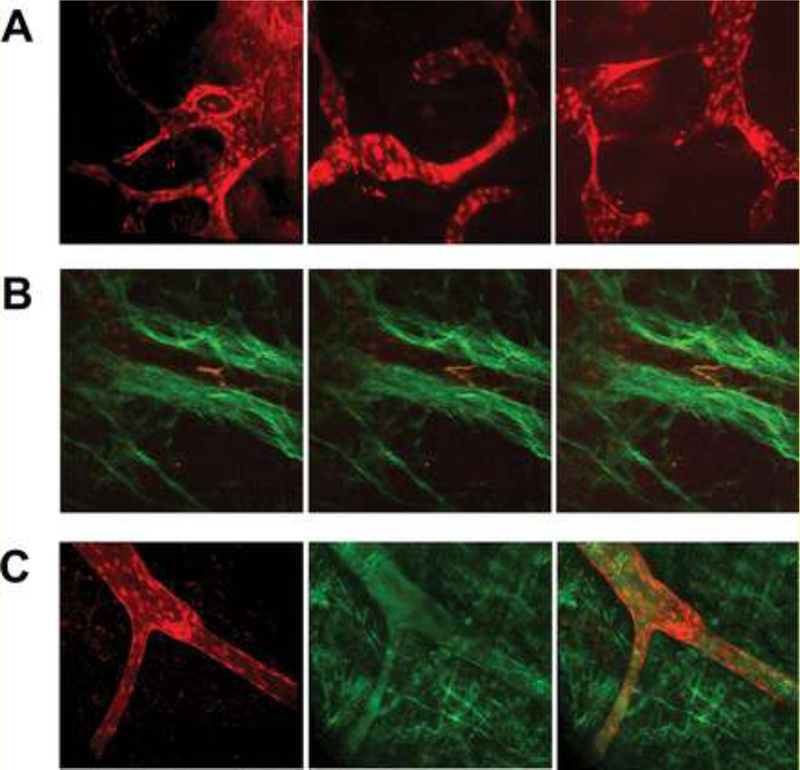
While traditional fluorescence or confocal microscopy may not penetrate deep tissue layers sufficiently to visualize lymphatics in other animal models, including mice, 2P-LSM (two-photon laser-scanning microscopy) has been successfully used in transgenic, prox1-mOrange2-pA-BAC mice to visualize vascular structures in live tissue [39]. NIR (near-IR) waves provided by femtosecond pulsed TiSa (titanium sapphire) lasers typically scatter less and penetrate deeper into tissue due to a shortage of endogenous chromophores that absorb NIR. Figure 11 shows intravital two-photon imaging of the lymphatic vasculature in fetal mouse skin using 2P-LSM, which successfully captured the intricate lymphatic vasculature structure as well as valvular movement in live mice.
Figure 11. Intravital two-photon imaging of lymphatic vessels.
(A) Superficial lymphatic vasculature of the fetal skin (E16.5) imaged using 2P-LSM. Superficial lymphatics (marked by mOrange2 expression) showed different developmental and remodeling stages of fetal lymphangiogenesis. (B) 2P-LSM intravital microscopy of lymphatic valve action. Image series shows the opening of a lymphatic valve upon lymph flow in an adult mouse. Lymphatic vessels were marked by mOrange2 expression, and the surrounding tissue was visualized through second harmonic signals from the extracellular matrix. (C) Lymphatic valve architecture and lymph flow after FITC injection. Two-photon images show a lymphatic collecting vessel including a saddle-shaped lymphatic valve (left-hand panel, red) and FITC uptake into lymphatic vessels (central panel, green content within the vessel) embedded in abundant connective tissue (right-hand panel, merged image showing FITC-filled lymphatic vessel and surrounding tissue). Reprinted with permission from [39].
Zebrafish have also been a popular transgenic model for in vivo imaging of vascular development. Taking advantage of the optical clarity of zebrafish embryos, investigators have been able to observe a wide range of vascular phenomena, including sprouting, remodeling, endothelial proliferation, and lumen formation, which are more difficult to study in the opaque embryos of mice. In addition, many new anti-lymphangiogenic compounds have been discovered using this model[57]. Zebrafish are especially useful for live imaging of deep lymphatic structures, such as the thoracic duct. In 2006, Yaniv et al. conducted one of the first studies to directly confirm the venous origin of lymphatic cells that form the developing thoracic duct through in vivo imaging. Utilizing a transgenic zebrafish expressing cytosolic EGFP and EGFP localized to the nuclei of endothelial cells under the control of fli1, a marker of vasculogenesis (Tg(fli1:EGFP) y1 and Tg(fli1:nEGFP)y7, respectively), this group continuously observed the formation of a parachordal “vessel” (referred to as parachordal lymphangioblasts in subsequent studies) from the posterior cardinal vein. These cells migrated ventrally to the dorsal aorta to form the thoracic duct, which previously had only been hypothesized and indirectly supported but not directly measured[58]. Other transgenic zebrafish lines with fluorescent vasculature are reviewed extensively by Okuda et al[57].
In addition to being a tool for studying vascular development, zebrafish provide a powerful in vivo preclinical drug-screening platform. Current preclinical cancer drug screening models depend on the use of in vitro human cancer cell line models, which precludes a complete investigation of pharmacokinetics, pharmacodynamics, or organ-specific toxicity. However, the advent of transgenic lines has allowed for high-resolution, readily quantifiable, real-time imaging of vessels during the development of zebrafish embryos. Identification of zebrafish genes, i.e., kdrl in blood endothelial cells and lyve1b in veins and LECs, and the tagging of these genes with various fluorophores have made for a robust experimental model that can be easily used for in vivo live observations of vascular development. For instance, this model has been used to screen for anti-lymphangiogenic compounds against cancers[57].
Discovery of other lymphatic endothelial markers
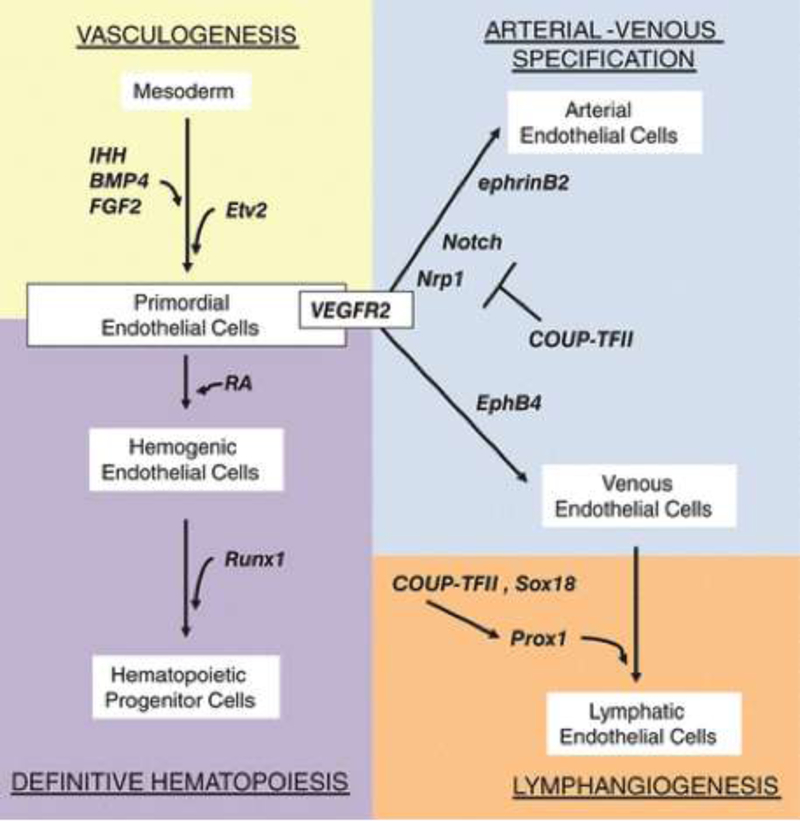
During embryologic development, angioblasts differentiate de novo from mesoderm into arteries and veins through a process called vasculogenesis (Figure 12). Subsequently, veins eventually give rise to lymphangioblasts that form the lymphatic vascular network (initially, the thoracic duct and its branches from the cardinal vein) through strictly regulated lymphangiogenesis (Figure 13). As previously discussed, however, there are a few exceptions currently known to this general rule such as for the lymphatics associated with the heart[59], skin[60], mesentery[61], and perhaps other organ-associated lymphatics that arise from non-venous precursors. The heterogeneity of the lymphatic vascular network was recently reviewed[62]). The steps of vasculogenesis, angiogenesis (i.e., the sprouting of blood vessels from pre-existing ones), and lymphangiogenesis give rise to the circulatory system. The coordination of several molecular signals, expressed at various points in embryo development and specific to each of these steps, modulate the complex development of the vascular network. Fibroblast growth factor-2 (FGF-2), for example, has been shown to be an important signaling molecule in the differentiation of mesoderm into angioblasts[63]. Meanwhile, Indian hedgehog (IHH) was shown to be crucial for the formation of both blood islands in yolk sac vessel development [64], and vascular tubes in vasculogenesis[65]. Figure 12 summarizes the roles of a few of the many critical and highly regulated genes involved in vasculogenesis.
Figure 12.
Vasculogenesis, the de novo formation of vessels from mesoderm, is dependent on early gene expression of factors that specify precursor cells toward an endothelial cell lineage. Fibroblast growth factor 2 (FGF2), Indian hedgehog (IHH), and bone morphogenic protein 4 (BMP4), for example, are critical for the differentiation of mesoderm into endothelial and hematopoietic cells. VEGF-A, a well-studied angiogenic factor, is also a key regulator of vasculogenesis through its receptors Flt-1 (VEGFR1) and Flk-1 (VEGFR2), as well as receptors Neuropilin 1 and 2 (Nrp-1/2). Angiopoietin-1 (Ang-1) and its receptor, Tie-2, have been shown to promote angiogenesis and recruitment of pericytes to the endothelium[66].
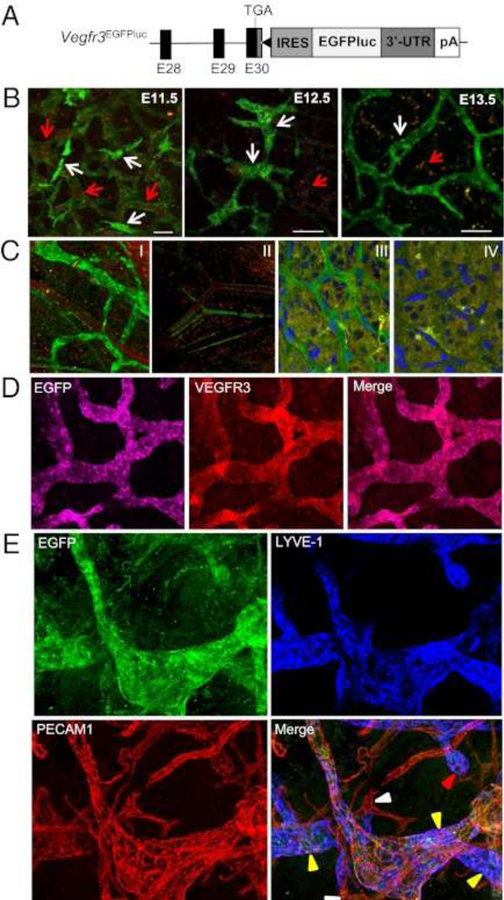
Figure 13. Vegfr3 expression shifts during early development from VECs to LECs.
(A) Schematic structure of the 3′ region of the Vegfr3EGFPLuc KI allele. The last three coding exons of the Vegfr3 gene (E28 to E30) are represented by black boxes. The 3′-UTR (dark gray box), the position of the stop codon (TGA), and the Vegfr3 polyadenilation signal (pA) are also shown. The black triangle represents an frt site remaining after Flp-mediated excision of the neomycin resistance cassette used for gene targeting. (B) Whole-body confocal images (EGFP) of Vegfr3-expressing cells during lymphatic vessel development in E11.5-E13.5 Vegfr3EGFPLuc (KI/KI) embryos. EGFP expression is seen in blood vessels (identified by the autofluorescence of the erythrocytes [red arrows]) at E11.5, while at E13.5 EGFP expression becomes restricted to the lymphatic vessels. Scale bar, 50 μm. (C) Confocal overlay of red and green channels of the ear skin (I and II) and retina (III and IV) from 3-week- and 7-day-old animals, respectively. Vegfr3EGFPLuc (KI/KI) mice (I and III). Wild-type mice (II and IV). Nuclei are stained with DAPI (II and IV). (D) Confocal images of EGFP and VEGFR3 whole-mount immunofluorescence staining of the ear skin from 2-week-old mice. (E) Expression of EGFP, LYVE-1 and PECAM1 in lymphatic vessels in the skin. Initial lymphatic capillaries (yellow arrowheads), some with blunt endings (red arrowhead), are EGFP+ and LYVE-1+. In PECAM1+ blood vessels (white arrowheads), only autofluorescence of red blood cells was detected. Reprinted with permission from [48].
In the study of hemangiogenesis and lymphangiogenesis in postnatal tissue, there is considerable overlap in gene expression in blood and lymphatic vessels, owing to their common developmental origin. In theory, mutually exclusive endothelial markers for blood and lymphatic endothelium can be powerful tools for investigating the molecular and cellular mechanisms responsible for angiogenesis and lymphangiogenesis, respectively. However, due to the diversity and heterogeneity of vascular gene expression in various tissues, discovery of these exclusive markers has been challenging. For instance, Hirakawa et al. developed a technique to purify LECs from endothelial cells in skin, based on findings that LYVE-1 is expressed exclusively in the lymphatics of postnatal murine skin, while CD34 is expressed only on blood vessels in the same region. However, it was also reported that the lymphatic marker podoplanin and CD34 can be found together in a subpopulation of endothelial cells in normal human skin [67], suggesting that CD34 may be expressed on lymphatic vasculature as well. Similarly, VE-cadherin, which was once thought to be an endothelial marker, has been identified on fetal hematopoietic stem cells. Hirakawa et al. further conducted comparative gene array analyses of cultured blood vessel endothelial cells and LECs to develop an extensive list of genes with overlapping expression in both cell types[67]. For instance, they confirmed with quantitative real-time RT-PCR that Prox-1 expression was up to 113-fold greater in LECs than in blood endothelial cells. While many mechanisms remain unclear in the study of lymphangiogenesis, several genes have been implicated in regulating this process. Figure 13 summarizes key regulators expressed on venous endothelial cells that are destined for the lymphatic endothelial lineage.
Many regulatory molecules that were originally thought to be exclusively expressed on endothelial cells have since been shown to be expressed in hematopoietic stem cells. Thus, as more of these putative endothelial markers are identified in other tissues or stem cells, the list of markers that can potentially be used to isolate blood and lymphatic vessels continues to shorten. Nevertheless, many investigators have found success in isolating lymphatic vessels using Prox1, podoplanin, and LYVE1, three markers used widely for the study of lymphatics today. Hirakawa et al. demonstrated that Prox1 and LYVE1 are completely absent from blood vessels and that both markers are expressed on LECs, thus enabling them to successfully isolate lymphatics for immunofluorescence experiments[67]. The discovery of reliable endothelial markers may not be solely contingent on specificity, however. A reliable tracer should also be expressed in the majority of cells being isolated. In addition, when observing cellular changes in the progression of disease, an optimal marker expression in diseased tissue should be present to allow for accurate measurement[70]. Importantly, lymphatic-specific markers have already shown promise in allowing imaging during lymphatic development during embryogenesis. Figure 13 shows experiments with Vegfr3EGFPLuc embryos depicting the shift in Vegfr3-driven EGFP expression in LEC precursors from VECs during early development to LECs specifically in 2-week-old Vegfr3EGFPLuc mice[48].
Concluding Remarks and Future Directions
The emerging field of lymphangiogenesis has defined fundamental mechanisms for lymphatic growth in both physiological and pathological contexts. This may eventually provide a novel opportunity for using molecular targets expressed by lymphatic vessels to intervene in diseased states, including tumor metastasis. The discovery of lymphatic-specific markers, including Prox1, LYVE1, and podoplanin, immediately advanced the study of lymphangiogenesis, which has historically been troubled by difficulty visualizing the “colorless” lymphatic vessels. In the past decade, many in vitro and in vivo models for lymphangiogenesis studies have enabled researchers to monitor the growth patterns of lymphatic vessels in response to certain stimuli, such as VEGFs. In vitro models have struggled to mimic the full range of key physiological conditions and stimuli that contribute to the three-dimensional growth of lymph vessels. In combination with experimental animal models, lymphatic fluorescent transgenic reporter mice have made visualization of experimental injury-induced lymphatic growth easy and reproducible. Meanwhile, modalities including lymphangiography, lymphoscintigraphy, and MRL have provided new methods for studying the gross structural changes of lymphatics in vivo. Recently, the study of lymphangiogenesis has also benefited from the use of transgenic reporter mouse models.
This review summarized various transgenic reporter mouse models that have been used for visualizing angiogenesis and lymphangiogenesis. Fluorescent reporter transgenic mice can also be used to study interaction between endothelial cells and other cell type (neuron, neural progenitors, etc) [71,72]. It also described the relative advantages and disadvantages of various markers as well as how the use of Prox1, LYVE1, and podoplanin in transgenic mouse has been quintessential in this nascent field. These early techniques have paved the way for the design of many transgenic models using multiple markers, such as the pairings Prox1-GFP, VEGFR3-YFP, Prox1-tdTomato, Flk1-mcherry, Flk1-GFP, Flk1-Nano-lantern, and Flt1-DsRed, for the visualization of hemangiogenesis and lymphangiogenesis in vivo and in vitro. For example, in vivo simultaneous visualization of angiogenesis and lymphangiogenesis is now possible using Prox1-GFP/Flt1-DsRed (PGFD) transgenic mice, enabling easy differentiation between blood vessels and lymph vessels. These new mouse models provide opportunities for future research not only in mice but also in other animal models based on genomic conservation between species (e.g., genomic sequences used to create transgenic Prox1-EGFP mice are highly conserved in other species)[73]. For example, Jung et al. developed a transgenic rat model for studying surgical physiology along with convenient structural and functional analysis of lymphatic vessels expressing Prox1[43].
With the availability of these models, it is now possible to explore the effects of various promoters and inhibitors of hemangiogenesis and lymphangiogenesis in vivo and in vitro. Studies in these models will also elucidate the molecular mechanisms involved in the formation of vasculature in order to better understand the spatial and temporal correlations between vascular development and pathological progression. For example, observing coronary vasculature formation according to the expression patterns of Flk1 and Flt1 might provide a new strategy for repairing infarcted hearts. Another model, the Prox1-Cre-tdTomato mouse model, offers lymphatic vessels that express bright red fluorescence and thus represents a useful model for studying the functions of lymphatic vessels in immune cell trafficking, inflammation, and cancer metastasis. In addition, PGFD transgenic mice can be bred with specific gene knockout mice to analyze the effects of gene knockouts on injury-induced angiogenesis and lymphangiogenesis. The use of such models in future studies of hemangiogenesis and lymphangiogenesis is expected to facilitate breakthroughs needed for the development of novel treatments ranging from cancer therapy to ocular injury remediation. Furthermore, imaging of functional and architectural lymphatic changes in animal models of cancer can lead to new discoveries regarding the roles of the lymphatics in cancer progression and metastasis. These models will lay the foundation for several vital drugs that will advance medical science to a new level.
The improved molecular understanding of lymphatic vessels in pathological situations opens up new strategies for lymphatic therapy[11]. Proteins that act as growth factors or inhibitors of lymphatic function may be manipulated to suit the requirements of certain conditions. For example, the lymphatic system can be used to deliver drugs to collecting lymphatic vessels or draining lymph nodes. Also, lymphatic-specific ligands such as LYVE-1 could increase the specificity of a drug by targeting it to lymphatic vessels and lymph nodes. Only with animal models that are specially designed for visualizing these lymphatic vessels, however, can we hope to achieve these goals toward a deeper understanding of lymphangiogenesis in health and disease and the development of therapeutic strategies toward a myriad of human conditions.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health [EY10101 (D.T.A.),EY027912 (MIR), EY021886, I01 BX002386 (J.H.C), and EY01792] and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Footnotes
CONFLICTS OF INTEREST - The authors declare that they have no competing financial interests
REFERENCES
- 1.Karpanen T, Alitalo K (2008) Molecular biology and pathology of lymphangiogenesis. Annual review of pathology 3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515 [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Oliver G (2010) Current views on the function of the lymphatic vasculature in health and disease. Genes & development 24 (19):2115–2126. doi: 10.1101/gad.1955910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG (2014) Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 14 (3):159–172. doi: 10.1038/nrc3677 [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki H, Yoshimatsu Y, Akatsu Y, Mishima K, Fukayama M, Watabe T, Miyazono K (2014) Expression of platelet-derived growth factor receptor beta is maintained by Prox1 in lymphatic endothelial cells and is required for tumor lymphangiogenesis. Cancer science 105 (9):1116–1123. doi: 10.1111/cas.12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alitalo K (2011) The lymphatic vasculature in disease. Nature medicine 17 (11):1371–1380. doi: 10.1038/nm.2545 [DOI] [PubMed] [Google Scholar]
- 6.Yang JF, Walia A, Huang YH, Han KY, Rosenblatt MI, Azar DT, Chang JH (2016) Understanding lymphangiogenesis in knockout models, the cornea, and ocular diseases for the development of therapeutic interventions. Survey of ophthalmology 61 (3):272–296. doi: 10.1016/j.survophthal.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamakawa M, Doh SJ, Santosa SM, Montana M, Qin EC, Kong H, Han KY, Yu C, Rosenblatt MI, Kazlauskas A, Chang JH, Azar DT (2018) Potential lymphangiogenesis therapies: Learning from current antiangiogenesis therapies-A review. Medicinal research reviews doi: 10.1002/med.21496 [DOI] [PMC free article] [PubMed]
- 8.Sevick-Muraca EM, Kwon S, Rasmussen JC (2014) Emerging lymphatic imaging technologies for mouse and man. The Journal of clinical investigation 124 (3):905–914. doi: 10.1172/JCI71612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruyere F, Melen-Lamalle L, Blacher S, Roland G, Thiry M, Moons L, Frankenne F, Carmeliet P, Alitalo K, Libert C, Sleeman JP, Foidart JM, Noel A (2008) Modeling lymphangiogenesis in a three-dimensional culture system. Nature methods 5 (5):431–437. doi: 10.1038/nmeth.1205 [DOI] [PubMed] [Google Scholar]
- 10.Adamczyk LA, Gordon K, Kholova I, Meijer-Jorna LB, Telinius N, Gallagher PJ, van der Wal AC, Baandrup U (2016) Lymph vessels: the forgotten second circulation in health and disease. Virchows Archiv : an international journal of pathology 469 (1):3–17. doi: 10.1007/s00428-016-1945-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proulx ST, Luciani P, Dieterich LC, Karaman S, Leroux JC, Detmar M (2013) Expansion of the lymphatic vasculature in cancer and inflammation: new opportunities for in vivo imaging and drug delivery. Journal of controlled release : official journal of the Controlled Release Society 172 (2):550–557. doi: 10.1016/j.jconrel.2013.04.027 [DOI] [PubMed] [Google Scholar]
- 12.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature medicine 7 (2):192–198. doi: 10.1038/84643 [DOI] [PubMed] [Google Scholar]
- 13.Majumder M, Tutunea-Fatan E, Xin X, Rodriguez-Torres M, Torres-Garcia J, Wiebe R, Timoshenko AV, Bhattacharjee RN, Chambers AF, Lala PK (2012) Co-expression of alpha9beta1 integrin and VEGF-D confers lymphatic metastatic ability to a human breast cancer cell line MDA-MB-468LN. PloS one 7 (4):e35094. doi: 10.1371/journal.pone.0035094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG (2001) VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nature medicine 7 (2):186–191. doi: 10.1038/84635 [DOI] [PubMed] [Google Scholar]
- 15.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS (2001) Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. The EMBO journal 20 (4):672–682. doi: 10.1093/emboj/20.4.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopfstein L, Veikkola T, Djonov VG, Baeriswyl V, Schomber T, Strittmatter K, Stacker SA, Achen MG, Alitalo K, Christofori G (2007) Distinct roles of vascular endothelial growth factor- D in lymphangiogenesis and metastasis. The American journal of pathology 170 (4):1348–1361. doi: 10.2353/ajpath.2007.060835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y (2004) PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer cell 6 (4):333–345. doi: 10.1016/j.ccr.2004.08.034 [DOI] [PubMed] [Google Scholar]
- 18.Cao R, Lim S, Ji H, Zhang Y, Yang Y, Honek J, Hedlund EM, Cao Y (2011) Mouse corneal lymphangiogenesis model. Nature protocols 6 (6):817–826. doi: 10.1038/nprot.2011.359 [DOI] [PubMed] [Google Scholar]
- 19.Mancardi S, Stanta G, Dusetti N, Bestagno M, Jussila L, Zweyer M, Lunazzi G, Dumont D, Alitalo K, Burrone OR (1999) Lymphatic endothelial tumors induced by intraperitoneal injection of incomplete Freund’s adjuvant. Experimental cell research 246 (2):368–375. doi: 10.1006/excr.1998.4270 [DOI] [PubMed] [Google Scholar]
- 20.Nakamura ES, Koizumi K, Kobayashi M, Saiki I (2004) Inhibition of lymphangiogenesis-related properties of murine lymphatic endothelial cells and lymph node metastasis of lung cancer by the matrix metalloproteinase inhibitor MMI270. Cancer science 95 (1):25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh SJ, Jeltsch MM, Birkenhager R, McCarthy JE, Weich HA, Christ B, Alitalo K, Wilting J (1997) VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Developmental biology 188 (1):96–109. doi: 10.1006/dbio.1997.8639 [DOI] [PubMed] [Google Scholar]
- 22.Majumder M, Xin X, Lala PK (2013) A practical and sensitive method of quantitating lymphangiogenesis in vivo. Laboratory investigation; a journal of technical methods and pathology 93 (7):779–791. doi: 10.1038/labinvest.2013.72 [DOI] [PubMed] [Google Scholar]
- 23.McElroy M, Hayashi K, Garmy-Susini B, Kaushal S, Varner JA, Moossa AR, Hoffman RM, Bouvet M (2009) Fluorescent LYVE-1 antibody to image dynamically lymphatic trafficking of cancer cells in vivo. The Journal of surgical research 151 (1):68–73. doi: 10.1016/j.jss.2007.12.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu J, Dugas-Ford J, Chang M, Purta P, Han KY, Hong YK, Dickinson ME, Rosenblatt MI, Chang JH, Azar DT (2015) Simultaneous in vivo imaging of blood and lymphatic vessel growth in Prox1-GFP/Flk1::myr-mCherry mice. The FEBS journal 282 (8):1458–1467. doi: 10.1111/febs.13234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang JH, Putra I, Huang YH, Chang M, Han K, Zhong W, Gao X, Wang S, Dugas-Ford J, Nguyen T, Hong YK, Azar DT (2016) Limited versus total epithelial debridement ocular surface injury: Live fluorescence imaging of hemangiogenesis and lymphangiogenesis in Prox1-GFP/Flk1::Myr-mCherry mice. Biochimica et biophysica acta 1860 (10):2148–2156. doi: 10.1016/j.bbagen.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto K, Azami T, Otsu A, Takase H, Ishitobi H, Tanaka J, Miwa Y, Takahashi S, Ema M (2012) Study of normal and pathological blood vessel morphogenesis in Flt1-tdsRed BAC Tg mice. Genesis 50 (7):561–571. doi: 10.1002/dvg.22031 [DOI] [PubMed] [Google Scholar]
- 27.Matsushita J, Inagaki S, Nishie T, Sakasai T, Tanaka J, Watanabe C, Mizutani KI, Miwa Y, Matsumoto K, Takara K, Naito H, Kidoya H, Takakura N, Nagai T, Takahashi S, Ema M (2017) Fluorescence and Bioluminescence Imaging of Angiogenesis in Flk1-Nano-lantern Transgenic Mice. Scientific reports 7:46597. doi: 10.1038/srep46597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larina IV, Shen W, Kelly OG, Hadjantonakis AK, Baron MH, Dickinson ME (2009) A membrane associated mCherry fluorescent reporter line for studying vascular remodeling and cardiac function during murine embryonic development. Anatomical record 292 (3):333–341. doi: 10.1002/ar.20821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraser ST, Hadjantonakis AK, Sahr KE, Willey S, Kelly OG, Jones EA, Dickinson ME, Baron MH (2005) Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice. Genesis 42 (3):162–171. doi: 10.1002/gene.20139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ema M, Takahashi S, Rossant J (2006) Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood 107 (1):111–117. doi: 10.1182/blood-2005-05-1970 [DOI] [PubMed] [Google Scholar]
- 31.Ishitobi H, Matsumoto K, Azami T, Itoh F, Itoh S, Takahashi S, Ema M (2010) Flk1-GFP BAC Tg mice: an animal model for the study of blood vessel development. Experimental animals 59 (5):615–622 [DOI] [PubMed] [Google Scholar]
- 32.Herz K, Becker A, Shi C, Ema M, Takahashi S, Potente M, Hesse M, Fleischmann BK, Wenzel D (2018) Visualization of endothelial cell cycle dynamics in mouse using the Flt-1/eGFP-anillin system. Angiogenesis 21 (2):349–361. doi: 10.1007/s10456-018-9601-1 [DOI] [PubMed] [Google Scholar]
- 33.Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, Richardson CD, Kawate T, Kuno J, Weinstein BM, Stainier DY, Sato TN (2000) Universal GFP reporter for the study of vascular development. Genesis 28 (2):75–81 [DOI] [PubMed] [Google Scholar]
- 34.Glaser DE, Burns AB, Hatano R, Medrzycki M, Fan Y, McCloskey KE (2014) Specialized mouse embryonic stem cells for studying vascular development. Stem cells and cloning : advances and applications 7:79–88. doi: 10.2147/SCCAA.S69554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iljin K, Petrova TV, Veikkola T, Kumar V, Poutanen M, Alitalo K (2002) A fluorescent Tie1 reporter allows monitoring of vascular development and endothelial cell isolation from transgenic mouse embryos. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 16 (13):1764–1774. doi: 10.1096/fj.01-1043com [DOI] [PubMed] [Google Scholar]
- 36.Davy A, Bush JO, Soriano P (2006) Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS biology 4 (10):e315. doi: 10.1371/journal.pbio.0040315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Barres BA, Nimmerjahn A, Agalliu D (2014) Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 82 (3):603–617. doi: 10.1016/j.neuron.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winderlich M, Keller L, Cagna G, Broermann A, Kamenyeva O, Kiefer F, Deutsch U, Nottebaum AF, Vestweber D (2009) VE-PTP controls blood vessel development by balancing Tie-2 activity. The Journal of cell biology 185 (4):657–671. doi: 10.1083/jcb.200811159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagerling R, Pollmann C, Kremer L, Andresen V, Kiefer F (2011) Intravital two-photon microscopy of lymphatic vessel development and function using a transgenic Prox1 promoter-directed mOrange2 reporter mouse. Biochemical Society transactions 39 (6):1674–1681. doi: 10.1042/BST20110722 [DOI] [PubMed] [Google Scholar]
- 40.Choi I, Chung HK, Ramu S, Lee HN, Kim KE, Lee S, Yoo J, Choi D, Lee YS, Aguilar B, Hong YK (2011) Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood 117 (1):362–365. doi: 10.1182/blood-2010-07-298562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Truman LA, Bentley KL, Smith EC, Massaro SA, Gonzalez DG, Haberman AM, Hill M, Jones D, Min W, Krause DS, Ruddle NH (2012) ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. The American journal of pathology 180 (4):1715–1725. doi: 10.1016/j.ajpath.2011.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bianchi R, Teijeira A, Proulx ST, Christiansen AJ, Seidel CD, Rulicke T, Makinen T, Hagerling R, Halin C, Detmar M (2015) A transgenic Prox1-Cre-tdTomato reporter mouse for lymphatic vessel research. PloS one 10 (4):e0122976. doi: 10.1371/journal.pone.0122976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung E, Gardner D, Choi D, Park E, Jin Seong Y, Yang S, Castorena-Gonzalez J, Louveau A, Zhou Z, Lee GK, Perrault DP, Lee S, Johnson M, Daghlian G, Lee M, Jin Hong Y, Kato Y, Kipnis J, Davis MJ, Wong AK, Hong YK (2017) Development and Characterization of A Novel Prox1-EGFP Lymphatic and Schlemm’s Canal Reporter Rat. Scientific reports 7 (1):5577. doi: 10.1038/s41598-017-06031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang GJ, Ecoiffier T, Truong T, Yuen D, Li G, Lee N, Zhang L, Chen L (2016) Intravital Imaging Reveals Dynamics of Lymphangiogenesis and Valvulogenesis. Scientific reports 6:19459. doi: 10.1038/srep19459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong M, Jung E, Yang S, Jung W, Seong YJ, Park E, Bramos A, Kim KE, Lee S, Daghlian G, Seo JI, Choi I, Choi IS, Koh CJ, Kobielak A, Ying QL, Johnson M, Gardner D, Wong AK, Choi D, Hong YK (2016) Efficient Assessment of Developmental, Surgical and Pathological Lymphangiogenesis Using a Lymphatic Reporter Mouse and Its Embryonic Stem Cells. PloS one 11 (6):e0157126. doi: 10.1371/journal.pone.0157126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connor AL, Kelley PM, Tempero RM (2016) Lymphatic endothelial lineage assemblage during corneal lymphangiogenesis. Laboratory investigation; a journal of technical methods and pathology 96 (3):270–282. doi: 10.1038/labinvest.2015.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olmeda D, Cerezo-Wallis D, Riveiro-Falkenbach E, Pennacchi PC, Contreras-Alcalde M, Ibarz N, Cifdaloz M, Catena X, Calvo TG, Canon E, Alonso-Curbelo D, Suarez J, Osterloh L, Grana O, Mulero F, Megias D, Canamero M, Martinez-Torrecuadrada JL, Mondal C, Di Martino J, Lora D, Martinez-Corral I, Bravo-Cordero JJ, Munoz J, Puig S, Ortiz-Romero P, Rodriguez-Peralto JL, Ortega S, Soengas MS (2017) Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature 546 (7660):676–680. doi: 10.1038/nature22977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez-Corral I, Olmeda D, Dieguez-Hurtado R, Tammela T, Alitalo K, Ortega S (2012) In vivo imaging of lymphatic vessels in development, wound healing, inflammation, and tumor metastasis. Proceedings of the National Academy of Sciences of the United States of America 109 (16):6223–6228. doi: 10.1073/pnas.1115542109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calvo CF, Fontaine RH, Soueid J, Tammela T, Makinen T, Alfaro-Cervello C, Bonnaud F, Miguez A, Benhaim L, Xu Y, Barallobre MJ, Moutkine I, Lyytikka J, Tatlisumak T, Pytowski B, Zalc B, Richardson W, Kessaris N, Garcia-Verdugo JM, Alitalo K, Eichmann A, Thomas JL (2011) Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes & development 25 (8):831–844. doi: 10.1101/gad.615311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dash AK, Yende AS, Tyagi RK (2017) Novel Application of Red Fluorescent Protein (DsRed-Express) for the Study of Functional Dynamics of Nuclear Receptors. Journal of fluorescence 27 (4):1225–1231. doi: 10.1007/s10895-017-2109-z [DOI] [PubMed] [Google Scholar]
- 51.Zhong W, Gao X, Wang S, Han K, Ema M, Adams S, Adams RH, Rosenblatt MI, Chang JH, Azar DT (2017) Prox1-GFP/Flt1-DsRed transgenic mice: an animal model for simultaneous live imaging of angiogenesis and lymphangiogenesis. Angiogenesis 20 (4):581–598. doi: 10.1007/s10456-017-9572-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kilarski WW, Muchowicz A, Wachowska M, Mezyk-Kopec R, Golab J, Swartz MA, Nowak-Sliwinska P (2014) Optimization and regeneration kinetics of lymphatic-specific photodynamic therapy in the mouse dermis. Angiogenesis 17 (2):347–357. doi: 10.1007/s10456-013-9365-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onder L, Scandella E, Chai Q, Firner S, Mayer CT, Sparwasser T, Thiel V, Rulicke T, Ludewig B (2011) A novel bacterial artificial chromosome-transgenic podoplanin-cre mouse targets lymphoid organ stromal cells in vivo. Frontiers in immunology 2:50. doi: 10.3389/fimmu.2011.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saito K, Chang YF, Horikawa K, Hatsugai N, Higuchi Y, Hashida M, Yoshida Y, Matsuda T, Arai Y, Nagai T (2012) Luminescent proteins for high-speed single-cell and whole-body imaging. Nature communications 3:1262. doi: 10.1038/ncomms2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajar BT, Wang ES, Lam AJ, Kim BB, Jacobs CL, Howe ES, Davidson MW, Lin MZ, Chu J (2016) Improving brightness and photostability of green and red fluorescent proteins for live cell imaging and FRET reporting. Scientific reports 6:20889. doi: 10.1038/srep20889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Truong TN, Li H, Hong YK, Chen L (2014) Novel characterization and live imaging of Schlemm’s canal expressing Prox-1. PloS one 9 (5):e98245. doi: 10.1371/journal.pone.0098245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okuda KS, Lee HM, Velaithan V, Ng MF, Patel V (2016) Utilizing Zebrafish to Identify Anti-(Lymph)Angiogenic Compounds for Cancer Treatment: Promise and Future Challenges. Microcirculation 23 (6):389–405. doi: 10.1111/micc.12289 [DOI] [PubMed] [Google Scholar]
- 58.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM (2006) Live imaging of lymphatic development in the zebrafish. Nature medicine 12 (6):711–716. doi: 10.1038/nm1427 [DOI] [PubMed] [Google Scholar]
- 59.Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dube KN, Bollini S, Matsuzaki F, Carr CA, Riley PR (2015) Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 522 (7554):62–67. doi: 10.1038/nature14483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez-Corral I, Ulvmar MH, Stanczuk L, Tatin F, Kizhatil K, John SW, Alitalo K, Ortega S, Makinen T (2015) Nonvenous origin of dermal lymphatic vasculature. Circ Res 116 (10):1649–1654. doi: 10.1161/CIRCRESAHA.116.306170 [DOI] [PubMed] [Google Scholar]
- 61.Stanczuk L, Martinez-Corral I, Ulvmar MH, Zhang Y, Lavina B, Fruttiger M, Adams RH, Saur D, Betsholtz C, Ortega S, Alitalo K, Graupera M, Makinen T (2015) cKit Lineage Hemogenic Endothelium-Derived Cells Contribute to Mesenteric Lymphatic Vessels. Cell Rep doi: 10.1016/j.celrep.2015.02.026 [DOI] [PubMed]
- 62.Ulvmar MH, Makinen T (2016) Heterogeneity in the lymphatic vascular system and its origin. Cardiovascular research 111 (4):310–321. doi: 10.1093/cvr/cvw175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cox CM, Poole TJ (2000) Angioblast differentiation is influenced by the local environment: FGF-2 induces angioblasts and patterns vessel formation in the quail embryo. Developmental dynamics : an official publication of the American Association of Anatomists 218 (2):371–382. doi: [DOI] [PubMed] [Google Scholar]
- 64.Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH (2001) Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development 128 (10):1717–1730 [DOI] [PubMed] [Google Scholar]
- 65.Byrd N, Becker S, Maye P, Narasimhaiah R, St-Jacques B, Zhang X, McMahon J, McMahon A, Grabel L (2002) Hedgehog is required for murine yolk sac angiogenesis. Development 129 (2):361–372 [DOI] [PubMed] [Google Scholar]
- 66.Adams RH, Alitalo K (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nature reviews Molecular cell biology 8 (6):464–478. doi: 10.1038/nrm2183 [DOI] [PubMed] [Google Scholar]
- 67.Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M (2003) Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. The American journal of pathology 162 (2):575–586. doi: 10.1016/S0002-9440(10)63851-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marcelo KL, Sills TM, Coskun S, Vasavada H, Sanglikar S, Goldie LC, Hirschi KK (2013) Hemogenic endothelial cell specification requires c-Kit, Notch signaling, and p27-mediated cell-cycle control. Developmental cell 27 (5):504–515. doi: 10.1016/j.devcel.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oliver G, Srinivasan RS (2010) Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development 137 (3):363–372. doi: 10.1242/dev.035360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lokmic Z (2016) Isolation, Identification, and Culture of Human Lymphatic Endothelial Cells. Methods in molecular biology 1430:77–90. doi: 10.1007/978-1-4939-3628-1_5 [DOI] [PubMed] [Google Scholar]
- 71.Okabe K, Kobayashi S, Yamada T, Kurihara T, Tai-Nagara I, Miyamoto T, Mukouyama YS, Sato TN, Suda T, Ema M, Kubota Y (2014) Neurons limit angiogenesis by titrating VEGF in retina. Cell 159 (3):584–596. doi: 10.1016/j.cell.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 72.Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, Sawamoto K (2010) Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells 28 (3):545–554. doi: 10.1002/stem.306 [DOI] [PubMed] [Google Scholar]
- 73.Elsir T, Smits A, Lindstrom MS, Nister M (2012) Transcription factor PROX1: its role in development and cancer. Cancer metastasis reviews 31 (3–4):793–805. doi: 10.1007/s10555-012-9390-8 [DOI] [PubMed] [Google Scholar]