Abstract
Both nonsteroidal anti-inflammatory drugs, such as ibuprofen, and the prototypical selective cyclooxygenase (Cox)-2 inhibitors DuP-697 and NS-398 block the inhibition of Cox-1 by aspirin in vitro. However, clinical studies have shown that the Cox-2 selective drugs (or coxibs) rofecoxib and etoricoxib, at therapeutic doses, do not interfere with the antiplatelet effect of aspirin, in contrast to ibuprofen. Here, we have evaluated the relative potential of ibuprofen and various coxibs to interfere with the inactivation of Cox-1 by aspirin by using purified enzyme and calcium ionophore-activated human platelets. The irreversible inactivation of Cox-1 by aspirin can be antagonized by ibuprofen and coxibs, albeit with widely different potencies. The rank order of potencies for this process (ibuprofen > celecoxib > valdecoxib > rofecoxib > etoricoxib) parallels that obtained for the inhibition of Cox-1-mediated thromboxane B2 production by calcium ionophore-stimulated platelets. The antagonism of aspirin therefore likely involves a competition at the enzyme active site. The EC50 value for the antagonism against 10 μM aspirin for each drug is ≈10- to 40-fold lower than the corresponding IC50 value for inhibition of platelet Cox-1 activity, consistent with the much weaker initial binding of aspirin to Cox-1 as compared with arachidonic acid. These results show that a low affinity for Cox-1 and a high degree of Cox-2 selectivity confers a low potential to block aspirin inhibition of platelet Cox-1, consistent with the results of clinical studies.
The two cyclooxygenase (Cox) isozymes (or prostaglandin H synthases), which share ≈60% sequence identity, perform the first committed steps in the prostaglandin pathway by catalyzing the oxygenation of arachidonic acid to PGG2 (Cox activity) and the reduction of PGG2 to PGH2 (peroxidase activity) (1–3). Cox-1 is constitutively expressed in most cell types, including platelets, whereas Cox-2 is absent from most healthy tissues but is induced by proinflammatory or proliferative stimuli. Cox-1 plays a role in the production of prostaglandins involved in protection of the gastric mucosal layer and thromboxanes (TX) in platelets. Cox-2 generally mediates elevations of prostaglandins associated with inflammation, pain, and pyresis (2). Nonsteroidal antiinflammatory drugs (NSAIDs) such as aspirin and ibuprofen are generally nonselective inhibitors of Coxs. This lack of selectivity has been linked to their propensity to cause gastrointestinal side effects. The new Cox-2 selective inhibitors, or coxibs, show the same antiinflammatory, analgesic, and antipyretic effects as nonselective NSAIDs but have reduced side-effect profiles (4).
The use of low-dose aspirin (50–325 mg/day) is currently indicated for cardiovascular prophylaxis (5). The mechanism of this cardioprotective effect is because of the irreversible inactivation of platelet Cox-1, resulting in a reduced production of the proaggregatory TXA2. As platelets are anuclear, the function of the platelets is inhibited for the remainder of their 8- to 10-day lifespan. The two currently approved coxibs (celecoxib and rofecoxib) show no effect on platelet aggregation even at supratherapeutic doses (6, 7). Thus, coxibs are not substitutes for aspirin for cardiovascular prophylaxis. As the cardioprotective effects of conventional nonselective NSAIDs have not been definitively established, coadministration of low-dose aspirin is recommended for high-risk patients taking NSAIDs for the treatment of inflammatory disorders (8). However, in vitro enzyme and human studies performed in the early 1980s showed that the NSAIDs ibuprofen and indomethacin can block aspirin inactivation of Cox-1 (9–12). Very recently, a clinical study confirmed that ibuprofen does block platelet Cox-1 inhibition by low-dose aspirin, suggesting that that the coadministration of the two drugs may compromise the cardioprotective effect of aspirin (42). The Cox-2 selective inhibitors NS-398 and DuP-697 also have been reported to block the aspirin-mediated inhibition of isolated and cellular Cox-1 (13, 14). However, human studies with rofecoxib showed no interference with the anti-platelet effect of aspirin (15, 42). Similar results also were obtained with etoricoxib.† Together, these studies suggest that weakly selective Cox-2 inhibitors, which show significant potency against Cox-1, might have a greater ability to block the aspirin inhibition of Cox-1 than highly Cox-2 selective inhibitors.
X-ray crystallography and other studies have shown that aspirin causes the irreversible acetylation of a serine (Ser-530 in Cox-1) in the substrate channel leading to the COX active site (16). Ibuprofen and indomethacin are able to block this acetylation as they also occupy this channel. Because selective Cox-2 inhibitors have been shown to also bind Cox-1, albeit comparatively weakly (17, 18), the possibility exists that these inhibitors can also block the acetylation of Ser-530 by aspirin by occupying the active site channel. The aims of this study were to investigate the mechanism of the antagonism of aspirin inhibition of Cox-1 by ibuprofen and coxibs and to examine the relationship between aspirin antagonism and potency against Cox-1.
Materials and Methods
Celecoxib, valdecoxib, etoricoxib, and rofecoxib were synthesized by the Medicinal Chemistry Department of Merck Frosst Canada. Unless stated, all centrifugations were performed at room temperature. Data fitting was performed with grafit software (Erithacus Software, Horley, U.K.), except for linear regression, which was performed with kaleidagraph software. IC50 values represent the inflection point of the curves generated when the data were fitted to a four-parameter equation with weighting according to the estimated error in each point.
Purified Ovine Cox-1 (oCox-1) Assays.
Cox activity over the first 30 s of the reaction was determined by using a spectrophotometric assay (OD610 nm) using 50 nM oCox-1 (Cayman Chemicals, Ann Arbor, MI) in 100 mM sodium phosphate, pH 6.5/0.5 μM hematin/10 μM N,N,N′,N′-tetramethyl-p-phenylenediamine/1 mg/ml gelatin (final volume 200 μl). Inhibitors were added from 50-fold concentrated solutions in DMSO, and the reaction was initiated with 100 μM arachidonic acid/100 μM tetramethyl-p-phenylenediamine (in 50% ethanol). For experiments with aspirin alone, the percentage remaining enzyme activities were fit to a first-order equation, y = a + b exp(−kobst) to give values of kobs, the observed pseudofirst-order inactivation rate constant. The kobs values were fit to an equation describing a two-step inactivation process, kobs = kinact[I]/(Ki + [I]) (19).
Preparation of Washed Platelets.
Fresh peripheral blood from healthy volunteers who had not taken NSAIDs for 7 days was collected in vacutainer tubes without heparin and was mixed with 10% (vol/vol) anticoagulant solution (65 mM citric acid/85 mM sodium citrate/2% glucose, pH 7.4). The blood was centrifuged (210 × g, 10 min), and the platelet-rich plasma was mixed with 50% Hanks' balanced salt solution supplemented with 25 mM Hepes (HHBSS)/30% anticoagulant solution and further centrifuged (760 × g, 10 min). The platelet pellet was resuspended in fresh HHBSS/10% anticoagulant and centrifuged again (760 × g, 10 min), and the pellet was resuspended in HHBSS/10% anticoagulant.
Assays of TXB2 Production.
Aliquots of platelets from individual donors (200 μl at 108 cells/ml) were incubated with inhibitors (diluted from 400-fold concentrated solutions in DMSO, or for aspirin 95% HBSS/5% DMSO) for 25 min at 37°C, before the addition of 2 μM calcium ionophore (A23187). After 10 min at 37°C, the reactions were terminated with 100 μl of methanol. After centrifugation, TXB2 in the supernatant was determined by enzyme immunoassay (EIA, Assay Designs, Ann Arbor, MI). To determine inhibitor reversibility, after incubation with inhibitors for 25 min, the platelets were centrifuged (760 × g, 10 min) and washed twice with HHBSS/10% anticoagulant. The platelets were then resuspended in 200 μl of HHBSS/10% anticoagulant, challenged with 2 μM A23187 and TXB2 production determined as above. To determine the effect of coxibs and ibuprofen on aspirin inhibition of platelet Cox-1, inhibitors were preincubated with the platelets for 5 min before addition of 10 μM or 100 μM aspirin and incubated for an additional 20 min. The cells were washed as above and challenged. Platelets treated with 10 μM aspirin alone were inhibited ≈85%, whereas those treated with 100 μM aspirin were completely inhibited. The values shown in Fig. 3 A and B were calculated as % inhibition = [(Cont − (ASA + inhib)]/(Cont − ASAalone) * 100, where Cont represents TXB2 produced with no inhibitors, (ASA + inhib) represents TXB2 produced in the presence of aspirin plus reversible inhibitor, and ASAalone represents TXB2 produced in the presence of aspirin. TXB2 production from platelets from different donors untreated and treated with ionophore (no inhibitors) averaged 0.38 ng/2 × 107 cells (range: undetectable to 1.7 ng) and 21 ng/2 × 107 cells (range: 8 to 40 ng), respectively. The background (no ionophore) TXB2 production was subtracted from each value obtained in the presence of ionophore. Titrations with platelets from each donor were performed in duplicate.
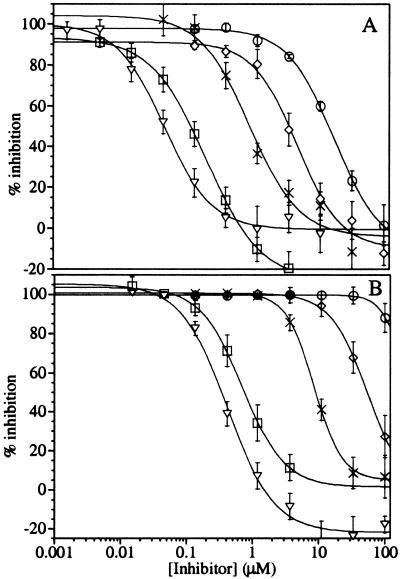
Figure 3.
Antagonism of the aspirin inhibition of platelet Cox-1 by ibuprofen and coxibs. Platelets were treated with 0–100 μM ibuprofen (▿), celecoxib (□), valdecoxib (×), rofecoxib (◊), and etoricoxib (○) for 5 min before the addition of 10 μM (A) or 100 μM (B) aspirin. After a 20-min incubation, the platelets were washed twice and challenged with calcium ionophore. After 10 min, the reactions were quenched, and the amount of TXB2 produced was determined by EIA. The data represent the average of (n) titrations from at least three different donors: (A) ibuprofen (10), celecoxib (9), valdecoxib (8), rofecoxib (8), and etoricoxib (6); (B) ibuprofen (11), celecoxib (12), valdecoxib (8), rofecoxib (8), and etoricoxib (6). For celecoxib, only data between 0.005 and 3.7 μM are plotted, as higher concentrations showed inhibition from celecoxib alone.
Labeling of Platelet Proteins with [14C]Acetyl Aspirin.
Blood from individual donors was pooled and platelets were prepared as described above, except that the pH of the anticoagulant solution was not adjusted. Platelets (1.26 ml, 1.5 × 1010 cells) were treated with either 10 μM ibuprofen or vehicle for 5 min at 37°C before addition of 100 μM [14C]acetyl aspirin (55 mCi/mmol, American Radiolabeled Chemicals, St. Louis). After 30 min, the cells were centrifuged (760 × g) and washed twice before being sonicated; the lysates were then centrifuged (100,000 × g, 1.5 h, 4°C). The pellet was resuspended in 0.4 ml of HHBSS/10% anticoagulant, recentrifuged, and solubilized with 1% vol/vol octylglucoside for 1 h at 4°C. After centrifugation, the S100 (approximately 1 mg of protein) was electrophoresed on 10-well 4–20%, 1.5-mm thickness, denaturing SDS/PAGE gels (Novex). After fixation with acetic acid/methanol, the gels were cut in 3-mm horizontal fractions and mixed with 10 ml of scintillation fluid. The gel fractions were incubated for 1 h at 37°C and then transferred to a fresh 10-ml aliquot of scintillation fluid and counted. The background counts from scintillation fluid alone (30 cpm) were subtracted from each sample.
Results
Effects of Selective Cox-2 Inhibitors on Inactivation of Ovine Cox-1 by Aspirin.
Initial experiments were conducted with purified oCox-1 to determine the effects of ibuprofen and selective Cox-2 inhibitors on the inactivation of oCox-1 by aspirin. The time dependency and potency of inhibition of oCox-1 by aspirin was examined in assays in which the enzyme was treated with 0–10 mM aspirin for 0–30 min before the addition of 100 μM arachidonic acid and measurement of the Cox activity. This spectrophotometric assay follows the oxidation of the reducing agent cosubstrate tetramethyl-p-phenylenediamine by the Cox peroxidase activity and has been shown to accurately reflect the conversion of arachidonic acid to PGG2 (20). The time-dependent loss of oCox-1 activity at each aspirin concentration followed a pseudofirst-order process, with inhibition reaching completion in the presence of 200 μM aspirin after a preincubation period of 30 min (data not shown). The observed rate constants for the inactivation process increased with higher aspirin concentration in a hyperbolic fashion. Fitting the data to an equation describing an initial reversible enzyme–inhibitor association with dissociation constant Ki, followed by an irreversible inactivation with a rate constant kinact, gave estimated values of 27 ± 6 mM and 12 ± 2 min−1, respectively. The high dissociation constant shows that aspirin has a very weak initial intrinsic affinity for purified oCox-1. The values of kinact/Ki and Ki obtained here (0.44 mM−1⋅min−1 and 27 mM) are similar (0.3 mM−1⋅min−1 and 14 mM, respectively) to those determined by using microsomal Cox-1 (19).
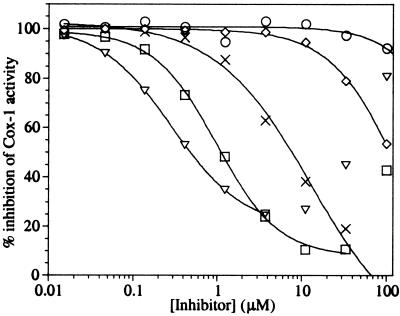
The conditions of 200 μM aspirin and a 30-min preincubation were chosen to evaluate whether ibuprofen, celecoxib, valdecoxib, rofecoxib, and etoricoxib can interfere with the irreversible inactivation of oCox-1 by aspirin. Thus, oCox-1 was coincubated with 200 μM aspirin and different concentrations of each inhibitor for 30 min, before the addition of 100 μM arachidonic acid substrate and determination of the Cox activity by using the spectrophotometric assay. The results (Fig. 1 and Table 1) confirm previous studies and show that under these conditions, ibuprofen can block the aspirin inactivation of purified oCox-1 with an EC50 value of ≈0.29 μM. The results also show that celecoxib has an EC50 value (1.0 μM) close to that of ibuprofen. In contrast, valdecoxib, rofecoxib, and etoricoxib show much weaker abilities to block the irreversible inactivation of oCox-1 by aspirin (EC50 values of 13, ≈100, and >100 μM, respectively). Using the same spectrophotometric assay with 100 μM arachidonic acid, each of these compounds was a weak inhibitor of Cox-1 activity, with IC50 values of ≈70 μM for ibuprofen and celecoxib and greater than 100 μM for the other coxibs. This finding is consistent with the competitive, reversible nature of the inhibition of Cox-1 by ibuprofen and coxibs and the high substrate concentration used in this assay. The large differences in the EC50 values for aspirin antagonism and the IC50 values for inhibition of Cox activity for each inhibitor ensured that the interpretation of the aspirin antagonism experiments was not complicated by inhibition from the reversible inhibitor itself. Nonetheless, inhibition of Cox activity at high concentrations of ibuprofen (>5 μM) and celecoxib (>30 μM) can be observed in Fig. 1. For ibuprofen, inhibition from the compound itself may have affected the slope of the EC50 curve, indicating that the true EC50 is somewhat less than 0.29 μM.
Figure 1.
Antagonism of the aspirin inhibition of oCox-1 by ibuprofen and coxibs. Purified oCox-1 was treated with 200 μM aspirin and 0–100 μM ibuprofen (▿), celecoxib (□), valdecoxib (×), rofecoxib (◊), and etoricoxib (○) for 30 min before the addition of substrate and the determination of the remaining Cox activity. Each point represents the average of duplicate determinations. The complete data were fit to the four-parameter IC50 equation for each inhibitor with the exceptions of ibuprofen and celecoxib, where data from the ranges 0.015 to 3.7 μM and 0.015 to 33.3 μM, respectively, were fit. Higher concentrations of these inhibitors resulted in Cox-1 inhibition from these agents themselves.
Table 1.
Potencies of ibuprofen and coxibs on Cox-1 activity and ability to block aspirin inactivation of Cox-1
| Purified oCox-1 EC50 antagonism of 200 μM aspirin μM | Platelet Cox-1
|
|||
|---|---|---|---|---|
| IC50 inhibition of TXB2 synthesis μM | EC50 antagonism of 10 μM aspirin μM | EC50 antagonism of 100 μM aspirin μM | ||
| Ibuprofen | ≤0.29 | 1.4 ± 0.4 | 0.048 ± 0.005 | 0.42 ± 0.05 |
| Celecoxib | 1.0 ± 0.07 | 2.2 ± 0.3 | 0.21 ± 0.01 | 0.76 ± 0.11 |
| Valdecoxib | 13 ± 4 | 28 ± 9 | 0.70 ± 0.20 | 8.6 ± 0.4 |
| Rofecoxib | ≈100 | >100 | 5.3 ± 0.8 | 42 ± 8 |
| Etoricoxib | >100 | >100 | 19 ± 4 | >100 |
Reversible Inhibition of Platelet Cox-1 by Ibuprofen and Coxibs.
The effects of the above inhibitors on the activity of platelet Cox-1 were then evaluated. In these assays, washed human platelets were treated with aspirin, ibuprofen, and the four coxibs individually for 25 min before the stimulation of TXB2 production with 2 μM calcium ionophore. After 10 min, the cells were lysed, and the TXB2 produced was quantitated by EIA. The titrations show that aspirin, ibuprofen, celecoxib, valdecoxib, rofecoxib, and etoricoxib inhibited the ionophore-stimulated production of TXB2 in platelets with IC50 values of 1.3 ± 0.5 μM, 1.4 ± 0.4 μM, 2.2 ± 0.3 μM, 28 ± 9 μM, >100 μM, and >100 μM, respectively (Fig. 2).
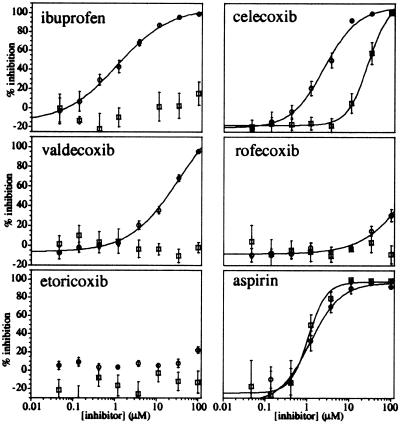
Figure 2.
Titration and reversibility of inhibition of platelet Cox-1 by ibuprofen, celecoxib, valdecoxib, rofecoxib, etoricoxib, and aspirin. Washed platelets were treated with inhibitor for 25 min before the stimulation of Cox activity with calcium ionophore. After a 10-min reaction, the amount of TXB2 produced was determined by EIA (○). To determine inhibitor reversibility, platelets were treated with each inhibitor for 25 min, and the platelets were then washed twice with fresh buffer before ionophore stimulation and TXB2 production as above (□). The data represent the average of titrations from at least three blood donors, except for aspirin (n = 2). The error bars represent the SEM of each value.
The abilities of ibuprofen and the coxibs to block aspirin inactivation of purified oCox-1 were readily demonstrated, as the inhibition resulting from these competitive, reversible compounds themselves was competed out by the subsequent addition of the high concentration of substrate. In contrast, for experiments with isolated platelets, any antagonism of aspirin inactivation of platelet Cox-1 by a second inhibitor could potentially be masked by its own inhibition. However, as these drugs are rapidly reversible inhibitors of purified Cox-1, whereas aspirin is an irreversible inhibitor, their effects on aspirin inactivation of platelet Cox-1 could be determined if the reversible inhibitor were washed from the cells. To determine inhibitor reversibility, platelets were treated with each inhibitor for 25 min and then centrifuged and resuspended in fresh buffer. This washing step was repeated before challenging the cells with ionophore as for the above titrations. The results show that the inhibitory effects of ibuprofen, valdecoxib, and rofecoxib were completely removed by washing of the platelets (Fig. 2). Etoricoxib showed no inhibition under both conditions. In contrast, the effects of aspirin were not reversible (IC50 of 1.1 ± 0.2 μM), as expected for an irreversible inhibitor. The inhibitory effect of celecoxib appeared only partially reversible under the washing conditions used, an apparent IC50 of 27 ± 3 μM being obtained after the washing steps compared with an IC50 value of 2.2 ± 0.3 μM obtained with no washing. As celecoxib has been described as a reversible inhibitor of purified human Cox-1 (21), the above effects may be caused by a relatively slow release of the compound from the platelets.
Differential Effects of Ibuprofen and Coxibs on the Inactivation of Platelet Cox-1 by Aspirin.
Experiments were then conducted to determine the relative abilities of ibuprofen and the four coxibs to block aspirin inactivation of platelet Cox-1. Platelets were pretreated with each of the above drugs for 5 min before the addition of 10 μM or 100 μM aspirin and further incubated for 20 min. The cells were then centrifuged and washed twice, as described above, to remove the reversible inhibitors and then challenged for TXB2 production with ionophore. The results (Fig. 3A) show that ibuprofen, celecoxib, valdecoxib, rofecoxib, and etoricoxib block the inactivation of platelet Cox-1 by 10 μM aspirin with EC50 values of 0.048 ± 0.005, 0.21 ± 0.01, 0.7 ± 0.2, 5.3 ± 0.8, and 19 ± 4 μM, respectively. On the order of 10-fold, higher concentrations of each drug were required (Table 1) to block the inactivation caused by 100 μM aspirin (Fig. 3B). The results show that the same rank order of potencies was obtained for the antagonism of aspirin with both purified and platelet Cox-1.
Effect of Ibuprofen on the Acetylation of Platelet Cox-1.
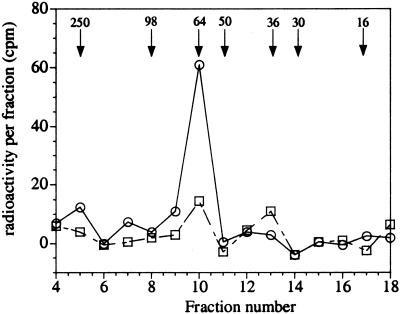
Previous work has shown that incubation of acetyl-radiolabeled aspirin with intact platelets leads to labeling of a single microsomal protein that was identified as Cox-1 (22). Ibuprofen was chosen here as a model compound to determine whether the mechanism of its protection against aspirin inactivation of platelet Cox-1 involves the blocking of acetylation. The potency of ibuprofen to inhibit ionophore-stimulated TXB2 production was not shifted by the 150-fold higher density of platelets required for these experiments. In contrast, the IC50 of celecoxib was increased at least 10-fold, precluding its use in these experiments. Platelets were treated with 10 μM ibuprofen or vehicle for 5 min and then 100 μM [14C]acetyl aspirin for an additional 30 min. The cells were then washed and sonicated, and the microsomal proteins were electrophoresed on SDS/PAGE gels. The gels were sliced into fractions, and the radioactivity in each was measured by scintillation counting. In platelets treated with [14C]acetyl aspirin, a single peak of radioactivity was obtained (Fig. 4) at an approximate molecular mass corresponding to that of Cox-1 (72 kDa). When the platelets were treated with 10 μM ibuprofen and 100 μM [14C]acetyl aspirin, the radioactivity peak area was almost completely abolished. These results are therefore consistent with the antagonism of ibuprofen against aspirin in platelets involving an inhibition of the irreversible acetylation of Cox-1.
Figure 4.
Effect of ibuprofen on the incorporation of [14C]acetyl aspirin radioactivity into microsomal platelet proteins. Washed platelets were treated with vehicle (○) or 10 μM ibuprofen (□) for 5 min before the addition of 100 μM [14C]acetyl aspirin. After 30 min, the cells were washed and lysed, and the microsomal fraction was solubilized with octylglucoside before electrophoresis on denaturing gels. Horizontal fractions were excised and counted in scintillation fluid. Fraction 4 represents the beginning of the separating gel. The mobility of molecular mass markers (in kDa) are shown by arrows. The data shown are representative of multiple results obtained with ibuprofen (n = 2) and vehicle-treated platelets (n = 4).
Discussion
Previous human studies have shown that clinical doses of the highly Cox-2-selective inhibitors rofecoxib and etoricoxib, in contrast to the nonselective NSAID ibuprofen, do not block the antiplatelet effects of low-dose aspirin. In a study with healthy subjects, individuals received placebo or rofecoxib (50 mg) for 10 days and aspirin (81 mg) from days 4 to 10. Rofecoxib alone had no effect on serum TXB2 levels or platelet aggregation (day 4), whereas both platelet parameters were equally inhibited when aspirin was coadministered with placebo or rofecoxib (15). Similar results were obtained with etoricoxib (120 mg) and aspirin (81 mg) (J. Wagner, personal communication). In a 6-day study, healthy subjects received low-dose aspirin (81 mg) 2 h before or after ibuprofen (400 mg) or rofecoxib (25 mg). Serum TXB2 levels and platelet aggregation were fully inhibited 24 h after the sixth dose in the subjects taking aspirin before the other drugs, as well as in those taking aspirin after rofecoxib. In those subjects taking ibuprofen before aspirin, platelet function and serum TXB2 levels were inhibited 2 h postdosing, but after 24 h, both parameters had fully recovered (42). This observed transient inhibition of platelet Cox-1 is likely caused by ibuprofen itself, which has a short t1/2 (2 h), rather than aspirin (23). Similar results were obtained in a single-dose study in which the coadministration of ibuprofen (5 mg/kg) and aspirin (650 mg) resulted in lower inhibition of platelet Cox-1 as compared with aspirin alone (11).
These clinical studies show that rofecoxib and etoricoxib, at their normal therapeutic doses, can selectively inhibit Cox-2 without interfering with the inactivation of Cox-1 by aspirin. However, in vitro data obtained with the prototypical Cox-2-selective inhibitors NS-398 and DuP-697 show that these compounds can block aspirin inhibition of Cox-1 (13, 14). The present in vitro study was aimed to determine whether all clinically used coxibs show a similar, low potential to antagonize aspirin or whether their potency in this process is related to their affinity for Cox-1 and their Cox-2 selectivity.
The present results obtained with purified oCox-1 and human platelets show that the nonselective NSAID ibuprofen, and also the selective Cox-2 inhibitors celecoxib, valdecoxib, rofecoxib, and etoricoxib, can block the inactivation of Cox-1 by aspirin in vitro. However, the relative potency of each coxib varies considerably and is related to their differences in inhibitory potencies for Cox-1. The least potent Cox-1 inhibitor etoricoxib was only able to block the aspirin inactivation of platelet Cox-1 under the most sensitive conditions (10 μM aspirin). In contrast, ibuprofen, the most potent Cox-1 inhibitor tested here, was also the most potent at blocking the aspirin inactivation of Cox-1 (Table 1). This antagonism of aspirin by ibuprofen in platelets, and reasonably also the coxibs, can be attributed to the blocking of the acetylation of Cox-1 as shown by the present radiolabeling results (Fig. 4).
The mechanism of selectivity of all of the Cox-2 selective inhibitors studied so far seems due to a common kinetic feature. Cox-2-selective inhibitors initially bind both Cox-1 and Cox-2 in a reversible manner, with similar affinities. For Cox-2 only, this complex then undergoes one or more kinetically distinct isomerizations to form a tightly bound complex that only slowly dissociates (17, 18, 21, 24). The formation of the final, slowly reversible complex with Cox-2 therefore provides the increased potency, resulting in the selectivity of inhibition. In contrast, ibuprofen binds in a rapidly reversible manner to both isozymes (25). The crystal structure of bromoacetylated Cox-1 shows salicylate bound in the active site channel near the site of serine acetylation (16), although the present results show that the initial binding of aspirin to purified Cox-1 is extremely weak (Ki ≈ 27 mM). Fluorescence quenching experiments with SC-299, a Cox-2-selective inhibitor structurally related to the four coxibs studied here, suggest that the reversible binding of this compound to Cox-1 involves an interaction at both the mouth of the channel leading to the active site as well as a second site further up the channel (24). The occupancy of either site would likely be competitive with aspirin binding, therefore providing a rationale for the antagonism of aspirin inactivation of Cox-1 by Cox-2 selective inhibitors.
The same rank order of potency is observed here for the EC50 values for aspirin antagonism in platelets (Table 1) and the Ki values of celecoxib (0.3 μM), valdecoxib (1.6 μM), rofecoxib (18 μM), and etoricoxib (167 μM) against purified human Cox-1 (26). Furthermore, the IC50 value of each drug for inhibition of platelet TXB2 production correlates extremely well with the EC50 values for protection of platelet Cox-1 activity against both 10 μM and 100 μM aspirin (r = 0.99). Therefore, the overall data are consistent with the antagonism of aspirin by coxibs and ibuprofen involving competition at the active site.
The EC50 values of the four coxibs and ibuprofen for the antagonism of aspirin inhibition of platelet Cox-1 (at 10 μM aspirin) are ≈10- to 40-fold lower than the respective IC50 values for inhibition of Cox-1 activity (see Table 1). This is now explainable, as the lower EC50 values reflect the competition of each drug with the weakly binding aspirin, whereas the higher IC50 values reflect the competition with arachidonic acid, which is released at high concentrations after ionophore challenge (27) and which binds Cox-1 strongly (28). This effect is exemplified by the results with purified Cox-1, where ibuprofen, celecoxib, and valdecoxib were able to significantly protect oCox-1 against aspirin inactivation at concentrations that showed no effect on Cox activity (Fig. 1).
The kinetic equations that describe the competition between a reversible inhibitor (i.e., ibuprofen or coxib) and an irreversible inhibitor (i.e., aspirin) show that as the exposure time or concentration of the irreversible inhibitor is increased, the degree of protection afforded by a reversible inhibitor will decrease (29). This effect was observed here, as ≈10-fold higher concentrations of ibuprofen or coxibs were required to block 100 μM aspirin in platelets as compared with 10 μM (Table 1). At low aspirin concentration, or exposure time, conditions that will result in less than complete inactivation of Cox-1, the EC50 value for the protection against inactivation will approach the inhibitor dissociation constant. In the present experiments, platelets treated with 10 μM aspirin alone for 20 min were inhibited ≈85%, indicating that the EC50 values obtained for the reversible inhibitors under these conditions represent the concentrations that give close to 50% occupancy of the Cox-1 active site.
Whole-blood assays of Cox-1 and Cox-2 inhibition have been used to relate clinically achieved drug serum concentrations to inhibitor side effects and efficacy, respectively (30–33). As the present experiments with platelets were performed in the absence of blood proteins, which can shift inhibitor potencies, it is not possible to directly predict the in vivo interactions of aspirin and coxibs or ibuprofen from drug serum levels and the EC50 values for the antagonism of aspirin inhibition of platelet Cox-1. However, the observation that the EC50 values of each drug for antagonism of 10 μM aspirin are on the order of 10- to 40-fold lower than their respective IC50 values for inhibition of ionophore-dependent platelet Cox-1 activity indicates that a low concentration of aspirin may be more readily antagonized in vivo by a weak Cox-1 inhibitor than would be apparent from the inhibition of serum TXB2 levels. Oral low-dose aspirin provides poor systemic bioavailability, and inactivation of platelet Cox-1 occurs in the presystemic circulation (34). There, the concentration and length of exposure are sufficiently low to only partially inactivate platelet Cox-1, and ≈4–5 days of treatment are needed to obtain >90% platelet inactivation (35–37). These conditions therefore would favor antagonism by a Cox-1 inhibitor. Furthermore, near complete platelet Cox-1 inactivation is required for the cardioprotective effect of aspirin, as platelets having 5–10% remaining activity still retain the ability to aggregate (38). The serum concentration of rofecoxib [≈1 μM at a dose of 25 mg qd (7)] is well below literature whole blood Cox-1 IC50 values [18.8 μM (26), 63 μM (39)]. In humans, rofecoxib showed no significant dose-related effects on serum TXB2 levels at doses of up to 375 mg (7.5 times the highest recommended dose) for 14 days (7) and at a single dose of 1,000 mg (33). Clearly, the serum concentrations of rofecoxib achieved at normal doses are well below that required to inhibit platelet Cox-1 activity and, as confirmed by the human studies, are also below that required to block Cox-1 inactivation by low-dose aspirin (15). Etoricoxib is an even weaker Cox-1 inhibitor (whole blood IC50 of 116 μM) and has a high degree of Cox-2 selectivity (26), consistent with its lack of interference with the antiplatelet effect of aspirin in humans (J. Wagner, personal communication). At the other extreme, ibuprofen gives serum levels of ≈50 μM at a dose of 200 mg (23), which is many-fold higher than Cox-1 whole blood IC50 values [4.8 μM (26), 7.6 μM (39)]. These data are consistent with the large, but transitory, antiplatelet effect of ibuprofen at therapeutic doses and its antagonism of the antiplatelet effect of aspirin (11, 42). The serum levels of celecoxib at a therapeutic dose of 200 mg twice a day are 1–2 μM (6) and are close to the range of literature whole blood IC50 values [1.2 μM (39), 6.7 μM (26), 6.67 μM (40)]. Consistent with this, in human studies, celecoxib showed ≈30% inhibition of serum TXB2 levels at doses of 600 mg twice a day (which gives serum levels of ≈3–6 μM) and 800 mg (6, 41). As the blockage of aspirin inactivation of platelet Cox-1 in vitro can occur at significantly lower drug concentrations than inhibition of platelet Cox-1 activity (see Table 1), the results suggest the possibility that celecoxib at therapeutic doses may antagonize the antiplatelet effects of aspirin.
In summary, the present study shows that the ability of coxibs to interfere with the aspirin inhibition of platelet and purified Cox-1 directly correlates with their potency against Cox-1. Thus, coxibs that have a high degree of selectivity and are weak Cox-1 inhibitors show a low ability to block the antiplatelet effect of aspirin, consistent with the available clinical data. The antagonism of coxibs and ibuprofen against aspirin likely involves a competition at the Cox-1 active site. As aspirin has an initial weak affinity for Cox-1, it is therefore more readily blocked than substrate arachidonic acid. Consequently, antagonism of aspirin may occur at drug concentrations that are lower than those that inhibit platelet Cox-1. A human study on the interaction of aspirin with NSAIDs and coxibs, which do not possess a high degree of Cox-2 selectivity, would determine whether this effect is clinically relevant.
Abbreviations
- Cox
cyclooxygenase
- HHBSS
Hanks' balanced salt solution supplemented with 25 mM Hepes
- NSAID
nonsteroidal antiinflammatory drug
- oCox-1
ovine cyclooxygenase-1
- TX
thromboxane
- EIA
enzyme immunoassay
Footnotes
Wagner, J. A., Kraft, W., Burke, J., Gleave, M., Wildonger, L., Ebel, D., Vanburen, S., Gottesdiener, K., Waldman, S. & Greenberg, H. (2001) Arthritis Rheum. 44, 498 (abstr.).
References
- 1.Marnett L J, Rowlinson S W, Goodwin D C, Kalgutkar A S, Lanzo C A. J Biol Chem. 1999;274:22903–22906. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- 2.Hawkey C J. Lancet. 1999;353:307–314. doi: 10.1016/s0140-6736(98)12154-2. [DOI] [PubMed] [Google Scholar]
- 3.Smith W L, DeWitt D L, Garavito R M. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 4.Hawkey C J, Jackson L, Harper S E, Simon T J, Mortensen E, Lines C R. Aliment Pharmacol Ther. 2001;15:1–9. doi: 10.1046/j.1365-2036.2001.00894.x. [DOI] [PubMed] [Google Scholar]
- 5.Patrono C. Am J Med. 2001;110:S62–S65. [Google Scholar]
- 6.Leese P T, Hubbard R C, Karim A, Isakson P C, Yu S S, Geis G S. J Clin Pharmacol. 2000;40:124–132. doi: 10.1177/00912700022008766. [DOI] [PubMed] [Google Scholar]
- 7.Depre M, Ehrich E, Van Hecken A, De Lepeleire I, Dallob A, Wong P, Porras A, Gertz B J, De Schepper P J. Eur J Clin Pharmacol. 2000;56:167–174. doi: 10.1007/s002280050736. [DOI] [PubMed] [Google Scholar]
- 8.Schafer A. Health News. 2000;6:10. [PubMed] [Google Scholar]
- 9.Parks W M, Hoak J C, Czervionke R L. J Pharmacol Exp Ther. 1981;219:415–419. [PubMed] [Google Scholar]
- 10.Livio M, Del Maschio A, Cerletti C, de Gaetano G. Prostaglandins. 1982;23:787–796. doi: 10.1016/0090-6980(82)90123-x. [DOI] [PubMed] [Google Scholar]
- 11.Rao G H, Johnson G G, Reddy K R, White J G. Arteriosclerosis. 1983;3:383–388. doi: 10.1161/01.atv.3.4.383. [DOI] [PubMed] [Google Scholar]
- 12.Cerletti C. Int J Tissue React. 1985;7:309–312. [PubMed] [Google Scholar]
- 13.Rosenstock M, Danon A, Rimon G. Biochim Biophys Acta. 1999;1440:127–137. doi: 10.1016/s1388-1981(99)00105-5. [DOI] [PubMed] [Google Scholar]
- 14.Rosenstock M, Danon A, Rubin M, Rimon G. Eur J Pharmacol. 2001;412:101–108. doi: 10.1016/s0014-2999(00)00931-6. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg H E, Gottesdiener K, Huntington M, Wong P, Larson P, Wildonger L, Gillen L, Dorval E, Waldman S A. J Clin Pharmacol. 2000;40:1509–1515. [PubMed] [Google Scholar]
- 16.Loll P J, Picot D, Garavito R M. Nat Struct Biol. 1995;2:637–643. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]
- 17.Copeland R A, Williams J M, Giannaras J, Nurnberg S, Covington M, Pinto D, Pick S, Trzaskos J M. Proc Natl Acad Sci USA. 1994;91:11202–11206. doi: 10.1073/pnas.91.23.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouellet M, Percival M D. Biochem J. 1995;306:247–251. doi: 10.1042/bj3060247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rome L H, Lands W E. Proc Natl Acad Sci USA. 1975;72:4863–4865. doi: 10.1073/pnas.72.12.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulmacz R J. Prostaglandins. 1987;34:225–240. doi: 10.1016/0090-6980(87)90246-2. [DOI] [PubMed] [Google Scholar]
- 21.Gierse J K, Koboldt C M, Walker M C, Seibert K, Isakson P C. Biochem J. 1999;339:607–614. [PMC free article] [PubMed] [Google Scholar]
- 22.Roth G J, Majerus P W. J Clin Invest. 1975;56:624–632. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox S R, VanderLugt J T, Gumbleton T J, Smith R B. Clin Pharmacol Ther. 1987;41:510–521. doi: 10.1038/clpt.1987.66. [DOI] [PubMed] [Google Scholar]
- 24.Lanzo C A, Sutin J, Rowlinson S, Talley J, Marnett L J. Biochemistry. 2000;39:6228–6234. doi: 10.1021/bi992761o. [DOI] [PubMed] [Google Scholar]
- 25.Callan O H, So O Y, Swinney D C. J Biol Chem. 1996;271:3548–3554. doi: 10.1074/jbc.271.7.3548. [DOI] [PubMed] [Google Scholar]
- 26.Riendeau D, Percival M D, Brideau C, Charleson S, Dube D, Ethier D, Falgueyret J P, Friesen R W, Gordon R, Greig G, et al. J Pharmacol Exp Ther. 2001;296:558–566. [PubMed] [Google Scholar]
- 27.Riendeau D, Guay J, Weech P K, Laliberte F, Yergey J, Li C, Desmarais S, Perrier H, Liu S, Nicoll-Griffith D, et al. J Biol Chem. 1994;269:15619–15624. [PubMed] [Google Scholar]
- 28.Smith T, Leipprandt J, DeWitt D. Arch Biochem Biophys. 2000;375:195–200. doi: 10.1006/abbi.1999.1659. [DOI] [PubMed] [Google Scholar]
- 29.Kulmacz R J, Lands W E. J Biol Chem. 1985;260:12572–12578. [PubMed] [Google Scholar]
- 30.Patrono C, Ciabattoni G, Pinca E, Pugliese F, Castrucci G, De Salvo A, Satta M A, Peskar B A. Thromb Res. 1980;17:317–327. doi: 10.1016/0049-3848(80)90066-3. [DOI] [PubMed] [Google Scholar]
- 31.Patrignani P, Panara M R, Greco A, Fusco O, Natoli C, Iacobelli S, Cipollone F, Ganci A, Creminon C, Maclouf J, et al. J Pharmacol Exp Ther. 1994;271:1705–1712. [PubMed] [Google Scholar]
- 32.Brooks P, Emery P, Evans J F, Fenner H, Hawkey C J, Patrono C, Smolen J, Breedveld F, Day R, Dougados M, et al. Rheumatology (Oxford) 1999;38:779–788. doi: 10.1093/rheumatology/38.8.779. [DOI] [PubMed] [Google Scholar]
- 33.Ehrich E W, Dallob A, De Lepeleire I, Van Hecken A, Riendeau D, Yuan W, Porras A, Wittreich J, Seibold J R, De Schepper P, et al. Clin Pharmacol Ther. 1999;65:336–347. doi: 10.1016/S0009-9236(99)70113-X. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen A K, FitzGerald G A. N Engl J Med. 1984;311:1206–1211. doi: 10.1056/NEJM198411083111902. [DOI] [PubMed] [Google Scholar]
- 35.Burch J W, Stanford N, Majerus P W. J Clin Invest. 1978;61:314–319. doi: 10.1172/JCI108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patrignani P, Filabozzi P, Patrono C. J Clin Invest. 1982;69:1366–1372. doi: 10.1172/JCI110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jakubowski J A, Stampfer M J, Vaillancourt R, Deykin D. Br J Haematol. 1985;60:635–642. doi: 10.1111/j.1365-2141.1985.tb07467.x. [DOI] [PubMed] [Google Scholar]
- 38.Reilly I A, FitzGerald G A. Blood. 1987;69:180–186. [PubMed] [Google Scholar]
- 39.Warner T D, Giuliano F, Vojnovic I, Bukasa A, Mitchell J A, Vane J R. Proc Natl Acad Sci USA. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talley J J, Brown D L, Carter J S, Graneto M J, Koboldt C M, Masferrer J L, Perkins W E, Rogers R S, Shaffer A F, Zhang Y Y, et al. J Med Chem. 2000;43:775–777. doi: 10.1021/jm990577v. [DOI] [PubMed] [Google Scholar]
- 41.McAdam B F, Catella-Lawson F, Mardini I A, Kapoor S, Lawson J A, FitzGerald G A. Proc Natl Acad Sci USA. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Catella-Lawson, F., Reilly, M. P., Kapoor, S. C., Cucchiara, A. J., DeMarco, S., Tournier, B., Vyas, S. N. & FitzGerald, G. A. (2001) N. Engl. J. Med., in press. [DOI] [PubMed]