Abstract
Background
Liver resection is a major surgery with significant mortality and morbidity. Specialists have tested various methods in attempts to limit blood loss, transfusion requirements, and morbidity during elective liver resection. These methods include different approaches (anterior versus conventional approach), use of autologous blood donation, cardiopulmonary interventions such as hypoventilation, low central venous pressure, different methods of parenchymal transection, different methods of management of the raw surface of the liver, different methods of vascular occlusion, and different pharmacological interventions. A surgeon typically uses only one of the methods from each of these seven categories. The optimal method to decrease blood loss and transfusion requirements in people undergoing liver resection is unknown.
Objectives
To assess the effects of different interventions for decreasing blood loss and blood transfusion requirements during elective liver resection.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and Science Citation Index Expanded to September 2015 to identify randomised clinical trials. We also searched trial registers and handsearched the references lists of identified trials.
Selection criteria
We included only randomised clinical trials (irrespective of language, blinding, or publication status) comparing different methods of decreasing blood loss and blood transfusion requirements in people undergoing liver resection.
Data collection and analysis
Two review authors independently identified trials and collected data. We assessed the risk of bias using Cochrane domains. We conducted a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in WinBUGS 1.4, following the guidelines of the National Institute for Health and Care Excellence Decision Support Unit guidance documents. We calculated the odds ratios (OR) with 95% credible intervals (CrI) for the binary outcomes, mean differences (MD) with 95% CrI for continuous outcomes, and rate ratios with 95% CrI for count outcomes, using a fixed‐effect model or random‐effects model according to model‐fit. We assessed the evidence with GRADE.
Main results
We identified 67 randomised clinical trials involving a total of 6197 participants. All the trials were at high risk of bias. A total of 5771 participants from 64 trials provided data for one or more outcomes included in this review. There was no evidence of differences in most of the comparisons, and where there was, these differences were in single trials, mostly of small sample size. We summarise only the evidence that was available in more than one trial below. Of the primary outcomes, the only one with evidence of a difference from more than one trial under the pair‐wise comparison was in the number of adverse events (complications), which was higher with radiofrequency dissecting sealer than with the clamp‐crush method (rate ratio 1.85, 95% CrI 1.07 to 3.26; 250 participants; 3 studies; very low‐quality evidence). Among the secondary outcomes, the only differences we found from more than one trial under the pair‐wise comparison were the following: blood transfusion (proportion) was higher in the low central venous pressure group than in the acute normovolemic haemodilution plus low central venous pressure group (OR 3.19, 95% CrI 1.56 to 6.95; 208 participants; 2 studies; low‐quality evidence); blood transfusion quantity (red blood cells) was lower in the fibrin sealant group than in the control (MD −0.53 units, 95% CrI −1.00 to −0.07; 122 participants; 2; very low‐quality evidence); blood transfusion quantity (fresh frozen plasma) was higher in the oxidised cellulose group than in the fibrin sealant group (MD 0.53 units, 95% CrI 0.36 to 0.71; 80 participants; 2 studies; very low‐quality evidence); blood loss (MD −0.34 L, 95% CrI −0.46 to −0.22; 237 participants; 4 studies; very low‐quality evidence), total hospital stay (MD −2.42 days, 95% CrI −3.91 to −0.94; 197 participants; 3 studies; very low‐quality evidence), and operating time (MD −15.32 minutes, 95% CrI −29.03 to −1.69; 192 participants; 4 studies; very low‐quality evidence) were lower with low central venous pressure than with control. For the other comparisons, the evidence for difference was either based on single small trials or there was no evidence of differences. None of the trials reported health‐related quality of life or time needed to return to work.
Authors' conclusions
Paucity of data meant that we could not assess transitivity assumptions and inconsistency for most analyses. When direct and indirect comparisons were available, network meta‐analysis provided additional effect estimates for comparisons where there were no direct comparisons. However, the paucity of data decreases the confidence in the results of the network meta‐analysis. Low‐quality evidence suggests that liver resection using a radiofrequency dissecting sealer may be associated with more adverse events than with the clamp‐crush method. Low‐quality evidence also suggests that the proportion of people requiring a blood transfusion is higher with low central venous pressure than with acute normovolemic haemodilution plus low central venous pressure; very low‐quality evidence suggests that blood transfusion quantity (red blood cells) was lower with fibrin sealant than control; blood transfusion quantity (fresh frozen plasma) was higher with oxidised cellulose than with fibrin sealant; and blood loss, total hospital stay, and operating time were lower with low central venous pressure than with control. There is no evidence to suggest that using special equipment for liver resection is of any benefit in decreasing the mortality, morbidity, or blood transfusion requirements (very low‐quality evidence). Radiofrequency dissecting sealer should not be used outside the clinical trial setting since there is low‐quality evidence for increased harm without any evidence of benefits. In addition, it should be noted that the sample size was small and the credible intervals were wide, and we cannot rule out considerable benefit or harm with a specific method of liver resection.
Keywords: Humans; Bayes Theorem; Blood Loss, Surgical; Blood Loss, Surgical/prevention & control; Blood Transfusion; Blood Transfusion/statistics & numerical data; Catheter Ablation; Catheter Ablation/methods; Fibrin Tissue Adhesive; Fibrin Tissue Adhesive/administration & dosage; Hemostasis, Surgical; Hemostasis, Surgical/methods; Hepatectomy; Hepatectomy/adverse effects; Hepatectomy/methods; Randomized Controlled Trials as Topic; Suction; Suction/instrumentation; Suction/methods
Plain language summary
Surgical methods to decrease blood loss during liver surgery
Background
Many cancerous and non‐cancerous growths that develop in the liver are treated by removing part of the liver (liver resection), which is major surgery with high risk of complications, including blood loss during division of the liver tissue. Specialists have tested several methods to decrease blood loss during liver resection. These include lowering the pressure in the liver veins (low central venous pressure) or decreasing the amount of air that enters and leaves the lungs (hypoventilation), again aimed at decreasing central venous pressure; different ways of cutting the liver, for example, without any special equipment or using ultrasound waves or high‐frequency (radiofrequency); applying glue to decrease bleeding from the cut surface; blocking the blood supply to the liver during the operation, a process known as vascular occlusion, which could be performed continuously or intermittently. In addition, medical treatments that improve clotting of blood can be given to decrease blood loss. A surgeon typically uses one or more methods to decrease blood loss during liver surgery. The optimal method is unknown. We sought to identify the best methods of decreasing blood loss during liver surgery by performing a literature search that included all studies reported until September 2015. We used special statistical methods, so‐called network meta‐analyses. to compare the different treatments simultaneously as compared to the traditional Cochrane method of comparing two treatments at a time as there are multiple treatment strategies.
Study characteristics
We identified 67 randomised clinical trials involving a total of 6197 participants that met our inclusion criteria. However, we were only able to include 5771 participants from 64 trials since investigators either did not include the remaining participants in the analysis or did not report any outcomes of interest.
Source of funding: 24 trials (35.8%) were funded by parties with no financial interest in obtaining positive results for the treatment being evaluated. The remaining trials received funding from either parties who would gain financially from the results of the study or did not report the funding.
Quality of evidence
All the trials were at high risk of bias, that is, investigators may have overestimated the benefits or underestimated the harms of one method or the other because of the way that the studies were conducted. Many trials included few participants, and there was a good chance of arriving at the wrong conclusions because of this. The overall quality of evidence was low or very low.
Key results
There was no evidence of differences in most of the comparisons, and where there was, these differences were in single trials, mostly of small sample size. Such evidence is unreliable. So, we mention only the evidence that was available in more than one trial. Of the primary outcomes, the only one where there was evidence of difference was in the number of adverse events, which was higher with radiofrequency dissecting sealer than with clamp‐crush method. Among the secondary outcomes, the only evidence of difference was in the following:
Blood transfusion (percentage): higher in the low central venous pressure group than in the acute normovolemic haemodilution (diluting the blood by giving fluids during operation) plus low central venous pressure group.
Blood transfusion amount: lower in the fibrin sealant group (a type of glue applied to the cut surface of the liver) than in the control.
Blood transfusion (fresh frozen plasma − a component of blood): higher in the oxidised cellulose (another type of glue applied to the cut surface of the liver) group than in the fibrin sealant group.
Blood loss, total hospital stay, and operating time: lower with the low central venous pressure group than control.
For other comparisons, the evidence for difference was based on single small trials, or there was no evidence of differences. None of the trials reported health‐related quality of life or time needed to return to work. There is no evidence to suggest that using special equipment for liver resection is of any benefit.
Summary of findings
for the main comparison.
| Methods to decrease blood loss during liver resection: a network meta‐analysis. Primary outcomes | |||||||
|
Patient or population: people undergoing liver resection Settings: secondary or tertiary setting Intervention and control: various treatments Follow‐up: until discharge or 1 month (except for mortality (long‐term follow‐up) which was reported at 1 year | |||||||
| Outcomes | Anterior approach versus conventional approach | Autologous blood donation versus control | Cardiopulmonary interventions | Methods of parenchymal transection | Methods of dealing with cut surface | Methods of vascular occlusion | Pharmacological interventions |
|
Treatments The first treatment listed is the control. The remaining are interventions. |
|
|
|
|
|
|
|
| Link for detailed 'Summary of Findings tables' | Table 2 | Table 3 | Table 4 | Table 5 | Table 6 | Table 7 | Table 8 |
| Mortality (perioperative) | There was no evidence of differences in perioperative mortality between the 2 groups. Quality of evidence = very low1,2,3. |
There was no evidence of differences in perioperative mortality between the two groups. Quality of evidence = very low1,2,3. |
There was no evidence of differences in perioperative mortality for any of the comparisons. Quality of evidence = very low1,2,3. |
There was no evidence of differences in perioperative mortality for any of the comparisons. Quality of evidence = very low1,2,3. |
There was no evidence of differences in perioperative mortality for any of the comparisons Quality of evidence = very low1,2,3. |
There was no evidence of differences in perioperative mortality for any of the comparisons. Quality of evidence = very low1,2,3. |
There was no evidence of differences in perioperative mortality for any of the comparisons. Quality of evidence = very low1,2,3. |
| Mortality (longest follow‐up) | None of the trials reported this outcome. | There was no evidence of differences in mortality at 1 year between the 2 groups. Quality of evidence = very low)1,2,3. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. |
| Serious adverse events (proportion) | There was no evidence of differences in the proportion of participants experiencing serious adverse events between the 2 groups. Quality of evidence = very low1,2,3. |
None of the trials reported this outcome. | There was no evidence of differences in the proportion of participants experiencing serious adverse events (for any of the comparisons Quality of evidence = very low1,2,3. |
There was no evidence of differences in the proportion of participants experiencing serious adverse events for any of the comparisons Quality of evidence = very low1,2,3. |
There was no evidence of differences in the proportion of participants experiencing serious adverse events for any of the comparisons Quality of evidence = very low1,2,3. |
The proportion of participants experiencing serious adverse eventsa was lower in continuous selective portal triad clamping than continuous portal triad clamping
There was no evidence of differences in other comparisons. Quality of evidence = very low1,2,3 |
There was no evidence of differences in the proportion of participants experiencing serious adverse events for any of the comparisons Quality of evidence = very low1,2,3. |
| Serious adverse events (number) | None of the trials reported this outcome. | None of the trials reported this outcome. | There was no evidence of differences in the number of serious adverse events for any of the comparisons Quality of evidence = very low1,2,3. |
The number of serious adverse events was higher in radiofrequency dissecting sealer than clamp‐crush method.
There was no evidence of differences in other comparisons. Quality of evidence = very low1,2,3. |
The number of serious adverse events was higher in fibrin sealant than argon beam.
There was no evidence of differences in other comparisons. Quality of evidence = very low1,2,3. |
The number of serious adverse events was lower in intermittent portal triad clamping than continuous portal triad clamping.
There was no evidence of differences in other comparisons Quality of evidence = very low1,2,3. |
There was no evidence of differences in the number of serious adverse events for any of the comparisons Quality of evidence = very low1,2,3. |
| Health‐related quality of life | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome at any time point. | None of the trials reported this outcome at any time point. | None of the trials reported this outcome at any time point. | None of the trials reported this outcome at any time point. | None of the trials reported this outcome at any time point. |
| CrI: credible intervals; OR: odds ratio. | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
1 Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2 Sample size was low (total number of participants fewer than 400 for continuous outcomes and fewer than 300 events in total in both groups for other outcomes) (downgraded by 1 point). 3 Credible intervals spanned no effect and clinically significant effect (20% relative risk reduction for binary outcomes; standardised mean difference of 0.5 for health‐related quality of life) (downgraded by 1 point). a Network meta‐analysis was performed for this outcome because of the availability of direct and indirect comparisons in the network. The remaining outcomes were analysed by direct comparisons.
14. Detailed 'Summary of findings' table: anterior approach vs conventional approach.
| Outcomes | Illustrative comparative risks* (95% CrI) | Relative effect (95% CrI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Mortality (perioperative) | 76 per 1000 | 19 per 1000 (2 to 82) | OR 0.23 (0.03 to 1.08) | 185 (2 studies) | ⊕⊝⊝⊝ Very low1,2,3 |
| Mortality (longest follow‐up) | None of the trials reported this outcome. | ||||
| Serious adverse events (proportion) | 125 per 1000 | 154 per 1000 (40 to 457) | OR 1.27 (0.29 to 5.89) | 65 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 |
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life (30 days, 3 months) | None of the trials reported this outcome. | ||||
| Health‐related quality of life (maximal follow‐up) | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion. The corresponding risk (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). Network meta‐analysis was not performed for any of the outcomes since there were only two treatments. CrI: credible intervals; OR: odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2 Sample size was low (total number of participants fewer than 400 for continuous outcomes and fewer than 300 events in total in both groups for other outcomes) (downgraded by 1 point). 3 Credible intervals spanned no effect and clinically significant effect (20% relative risk reduction for binary outcomes; standardised mean difference of 0.5 for health‐related quality of life) (downgraded by 1 point).
15. Detailed 'Summary of findings' table: autologous blood donation vs control.
| Outcomes | Illustrative comparative risks* (95% CrI) | Relative effect (95% CrI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Mortality (perioperative) | There was no mortality in either group. | 28 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Mortality (longest follow‐up): reported at 1 year | There was no mortality in either group. | 28 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number) | None of the trials reported this outcome. | ||||
| Health‐related quality of life (30 days, 3 months) | None of the trials reported this outcome. | ||||
| Health‐related quality of life (longest follow‐up) | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion. The corresponding risk (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). Network meta‐analysis was not performed for any of the outcomes since there were only two treatments. CrI: credible intervals; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2 Sample size was low (total number of participants fewer than 400 for continuous outcomes and fewer than 300 events in total in both groups for other outcomes) (downgraded by 1 point). 3Credible intervals spanned no effect and clinically significant effect (20% relative risk reduction for binary outcomes; standardised mean difference of 0.5 for health‐related quality of life) (downgraded by 1 point).
16. Detailed 'Summary of findings' table: cardiopulmonary interventions.
| Outcomes | Illustrative comparative risks* (95% CrI) | Relative effect (95% CrI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Mortality (perioperative) | |||||
| Hypoventilation vs control | There was no mortality in either group. |
79 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Low central venous pressure vs control | There was no mortality in either group. |
85 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Mortality (longest follow‐up) | None of the trials reported this outcome. | ||||
| Serious adverse events (proportion) | |||||
| Hypoventilation vs control | 26 per 1000 |
60 per 1000 (5 to 679) |
OR 2.41 (0.18 to 80.4) |
79 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Low central venous pressure vs acute normovolemic haemodilution plus low CVP | 302 per 1000 |
284 per 1000 (157 to 460) |
OR 0.92 (0.43 to 1.97) |
63 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Serious adverse events (number) | |||||
| Low central venous pressure vs control | 100 per 1000 |
0 per 1000 (0 to 2) |
Rate ratio 0.00 (0 to 0.02) |
42 (1 study) |
⊕⊝⊝⊝ Very lowa,b,c |
| Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 103 per 1000 |
77 per 1000 (15 to 287) |
Rate ratio 0.73 (0.13 to 3.53) |
78 (1 study) |
⊕⊝⊝⊝ Very lowa,b,c |
| Health‐related quality of life (30 days, 3 months) | None of the trials reported this outcome. | ||||
| Health‐related quality of life (longest follow‐up) | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion. The corresponding risk (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). Network meta‐analysis was not performed for any of the outcomes because of the lack of availability of direct and indirect comparisons in the network. CrI: credible intervals; OR: odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1aRisk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2 Sample size was low (total number of participants fewer than 400 for continuous outcomes and fewer than 300 events in total in both groups for other outcomes) (downgraded by 1 point). 3Credible intervals spanned no effect and clinically significant effect (20% relative risk reduction for binary outcomes; standardised mean difference of 0.5 for health‐related quality of life) (downgraded by 1 point).
17. Detailed 'Summary of findings' table: methods of parenchymal transection.
| Outcomes | Illustrative comparative risks* (95% CrI) | Relative effect (95% CrI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Mortality (perioperative) | |||||
| CUSA vs clamp‐crush method | 23 per 1000 |
6 per 1000 (0 to 54) |
OR 0.24 (0.01 to 2.41) |
172 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Radiofrequency dissecting sealer vs clamp‐crush method | 10 per 1000 |
16 per 1000 (4 to 65) |
OR 1.60 (0.43 to 6.7) |
390 (5 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Sharp transection method vs clamp‐crush method | There was no mortality in either group. |
82 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Stapler vs clamp‐crush method | 31 per 1000 |
67 per 1000 (12 to 375) |
OR 2.26 (0.39 to 18.93) |
130 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Hydrojet vs CUSA | 55 per 1000 |
54 per 1000 (9 to 258) |
OR 0.98 (0.16 to 6.04) |
111 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Radiofrequency dissecting sealer vs CUSA | 44 per 1000 |
28 per 1000 (3 to 166) |
OR 0.61 (0.07 to 4.28) |
90 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Stapler vs CUSA | There was no mortality in either group. |
79 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Radiofrequency dissecting sealer vs hydrojet | 80 per 1000 |
9 per 1000 (0 to 145) |
OR 0.10 (0 to 1.95) |
50 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Mortality (longest follow‐up) | None of the trials reported this outcome. | ||||
| Serious adverse events (proportion) | |||||
| CUSA vs clamp‐crush method | 93 per 1000 |
31 per 1000 (6 to 110) |
OR 0.31 (0.06 to 1.2) |
172 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Radiofrequency dissecting sealer vs clamp‐crush method | 58 per 1000 |
49 per 1000 (15 to 145) |
OR 0.83 (0.24 to 2.74) |
240 (3 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Sharp transection method vs clamp‐crush method | 49 per 1000 |
106 per 1000 (20 to 502) |
OR 2.31 (0.39 to 19.69) |
82 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Hydrojet vs CUSA | 100 per 1000 |
124 per 1000 (61 to 238) |
OR 1.27 (0.58 to 2.81) |
61 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Radiofrequency dissecting sealer vs CUSA | 50 per 1000 |
30 per 1000 (3 to 180) |
OR 0.58 (0.06 to 4.16) |
40 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Stapler vs CUSA | 246 per 1000 |
246 per 1000 (6 to 931) |
OR 1.00 (0.02 to 41.22) |
130 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Serious adverse events (number) | |||||
| CUSA vs clamp‐crush method | 45 per 1000 |
29 per 1000 (3 to 166) |
Rate ratio 0.63 (0.07 to 4.17) |
132 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Radiofrequency dissecting sealer vs clamp‐crush method | 61 per 1000 |
190 per 1000 (75 to 474) |
Rate ratio 3.64 (1.25 to 13.97) |
130 (2 studies) |
⊕⊕⊝⊝ Low1,2 |
| Hydrojet vs CUSA | 80 per 1000 |
121 per 1000 (20 to 546) |
Rate ratio 1.59 (0.24 to 13.83) |
50 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Radiofrequency dissecting sealer vs CUSA | 80 per 1000 |
121 per 1000 (20 to 546) |
Rate ratio 1.59 (0.24 to 13.83) |
50 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Stapler vs CUSA | 180 per 1000 |
230 per 1000 (109 to 424) |
Rate ratio 1.36 (0.56 to 3.36) |
100 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Radiofrequency dissecting sealer vs hydrojet | 120 per 1000 |
120 per 1000 (23 to 445) |
Rate ratio 1.00 (0.17 to 5.88) |
50 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Health‐related quality of life (30 days, 3 months) | None of the trials reported this outcome. | ||||
| Health‐related quality of life (maximal follow‐up) | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion. The corresponding risk (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). Network meta‐analysis was not performed for any of the outcomes because of the lack of availability of direct and indirect comparisons in the network. CrI: credible intervals; CUSA: cavitron ultrasonic surgical aspirator; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2 Sample size was low (total number of participants fewer than 400 for continuous outcomes and fewer than 300 events in total in both groups for other outcomes) (downgraded by 1 point). 3 Credible intervals spanned no effect and clinically significant effect (20% relative risk reduction for binary outcomes; standardised mean difference of 0.5 for health‐related quality of life) (downgraded by 1 point).
18. Detailed 'Summary of findings' Table: methods of dealing with cut surface.
| Outcomes | Illustrative comparative risks* (95% CrI) | Relative effect (95% CrI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Mortality (perioperative) | |||||
| Fibrin sealant vs control | 11 per 1000 |
41 per 1000 (10 to 253) |
OR 4.03 (0.9 to 31.72) |
380 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Fibrin sealant and collagen vs control | 13 per 1000 |
45 per 1000 (10 to 268) |
OR 3.48 (0.74 to 27.03) |
300 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Fibrin sealant vs argon beam | 53 per 1000 |
72 per 1000 (25 to 198) |
OR 1.39 (0.46 to 4.45) |
227 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Fibrin sealant vs collagen | 33 per 1000 |
30 per 1000 (7 to 123) |
OR 0.91 (0.2 to 4.14) |
256 (3 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Oxidised cellulose vs fibrin sealant | 56 per 1000 |
31 per 1000 (1 to 565) |
OR 0.54 (0.01 to 22.09) |
50 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Plasmajet vs fibrin sealant | 103 per 1000 |
65 per 1000 (7 to 332) |
OR 0.60 (0.06 to 4.31) |
58 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Mortality (longest follow‐up) | None of the trials reported this outcome. | ||||
| Serious adverse events (proportion) | |||||
| Fibrin sealant vs control | 186 per 1000 |
191 per 1000 (128 to 275) |
OR 1.03 (0.64 to 1.66) |
457 (3 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Fibrin sealant vs argon beam | 269 per 1000 |
183 per 1000 (78 to 360) |
OR 0.61 (0.23 to 1.53) |
106 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Fibrin sealant vs collagen | 258 per 1000 |
356 per 1000 (205 to 547) |
OR 1.59 (0.74 to 3.47) |
127 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Oxidised cellulose vs fibrin sealant | 444 per 1000 |
309 per 1000 (113 to 603) |
OR 0.56 (0.16 to 1.9) |
50 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Plasmajet vs fibrin sealant | 207 per 1000 |
25 per 1000 (0 to 165) |
OR 0.10 (0 to 0.76) |
58 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Serious adverse events (number) | |||||
| Fibrin sealant vs control | 486 per 1000 |
470 per 1000 (307 to 640) |
Rate ratio 0.94 (0.47 to 1.88) |
70 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Fibrin sealant & collagen vs control | 147 per 1000 |
186 per 1000 (116 to 286) |
Rate ratio 1.33 (0.76 to 2.33) |
300 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Fibrin sealant vs argon beam | 65 per 1000 |
249 per 1000 (107 to 547) |
Rate ratio 4.81 (1.73 to 17.5) |
121 (1 study) |
⊕⊕⊝⊝ Low1,2 |
| Fibrin sealant vs collagen | 323 per 1000 |
369 per 1000 (266 to 488) |
Rate ratio 1.23 (0.76 to 2) |
189 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Fibrin sealant vs cyanoacrylate | 67 per 1000 |
67 per 1000 (2 to 733) |
Rate ratio 1.01 (0.03 to 38.36) |
30 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Oxidised cellulose vs cyanoacrylate | 67 per 1000 |
277 per 1000 (46 to 921) |
Rate ratio 5.37 (0.67 to 163.2) |
30 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Oxidised cellulose vs fibrin sealant | 67 per 1000 |
278 per 1000 (46 to 926) |
Rate ratio 5.40 (0.67 to 174.86) |
30 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Health‐related quality of life (30 days, 3 months) | None of the trials reported this outcome. | ||||
| Health‐related quality of life (longest follow‐up) | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion. The corresponding risk (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). Network meta‐analysis was not performed for any of the outcomes because of the lack of availability of direct and indirect comparisons in the network. CrI: credible intervals; OR: odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2 Sample size was low (total number of participants fewer than 400 for continuous outcomes and fewer than 300 events in total in both groups for other outcomes) (downgraded by 1 point). 3Credible intervals spanned no effect and clinically significant effect (20% relative risk reduction for binary outcomes; standardised mean difference of 0.5 for health‐related quality of life) (downgraded by 1 point).
19. Detailed 'Summary of findings' table: methods of vascular occlusion.
| Outcomes | Illustrative comparative risks* (95% CrI) | Relative effect (95% CrI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Mortality (perioperative) | |||||
| Continuous portal triad clamping vs control | There was no mortality in either group. |
15 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Intermittent portal triad clamping vs control | 26 per 1000 |
15 per 1000 (3 to 60) |
OR 0.60 (0.13 to 2.42) |
392 (4 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Continuous portal triad clamping vs continuous hepatic vascular exclusion | 1 per 1000 |
5 per 1000 (4 to 15) |
OR 4.91 (3.68 to 15.64) |
170 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | There was no mortality in either group. |
160 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Continuous selective portal triad clamping vs continuous portal triad clamping | There was no mortality in either group. |
120 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Intermittent portal triad clamping vs continuous portal triad clamping | 67 per 1000 |
10 per 1000 (0 to 70) |
OR 0.14 (0 to 1.05) |
121 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Intermittent portal triad clamping vs continuous selective portal triad clamping | There was no mortality in either group. |
80 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Intermittent selective portal triad clamping vs intermittent portal triad clamping | 1 per 1000 |
2 per 1000 (0 to 69) |
OR 2.27 (0.17 to 74) |
138 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Mortality (longest follow‐up) | None of the trials reported this outcome. | ||||
| Serious adverse events (proportion)* | |||||
| Continuous hepatic vascular exclusion vs control | 99 per 1000 |
200 per 1000 (19 to 785) |
Rate ratio 2.27 (0.18 to 33.05) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Continuous portal triad clamping vs control | 99 per 1000 |
135 per 1000 (30 to 439) |
Rate ratio 1.42 (0.28 to 7.09) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Continuous selective hepatic vascular exclusion vs control | 99 per 1000 |
15 per 1000 (0 to 325) |
Rate ratio 0.14 (0 to 4.37) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Continuous selective portal triad clamping vs control | 99 per 1000 |
55 per 1000 (11 to 226) |
Rate ratio 0.53 (0.1 to 2.65) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Intermittent portal triad clamping vs control | 99 per 1000 |
113 per 1000 (56 to 217) |
Rate ratio 1.16 (0.54 to 2.51) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Continuous portal triad clamping vs continuous hepatic vascular exclusion | 50 per 1000 |
32 per 1000 (2 to 412) |
Rate ratio 0.63 (0.03 to 13.31) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Continuous selective hepatic vascular exclusion vs continuous hepatic vascular exclusion | 50 per 1000 |
3 per 1000 (0 to 442) |
Rate ratio 0.06 (0 to 15.06) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Continuous selective portal triad clamping vs continuous hepatic vascular exclusion | 50 per 1000 |
12 per 1000 (1 to 209) |
Rate ratio 0.23 (0.01 to 5.02) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Intermittent portal triad clamping vs continuous hepatic vascular exclusion | 50 per 1000 |
26 per 1000 (2 to 288) |
Rate ratio 0.51 (0.03 to 7.68) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | 139 per 1000 |
16 per 1000 (0 to 724) |
Rate ratio 0.10 (0 to 16.28) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Continuous selective portal triad clamping vs continuous portal triad clamping | 139 per 1000 |
56 per 1000 (6 to 374) |
Rate ratio 0.37 (0.04 to 3.7) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Intermittent portal triad clamping vs continuous portal triad clamping | 139 per 1000 |
117 per 1000 (22 to 439) |
Rate ratio 0.82 (0.14 to 4.86) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Continuous selective portal triad clamping vs continuous selective hepatic vascular exclusion | As there were no serious adverse events in either group, the credible intervals were extremely wide. This is equivalent to not estimable in direct comparisons. |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Intermittent portal triad clamping vs continuous selective hepatic vascular exclusion | As there were no serious adverse events in either group, the credible intervals were extremely wide. This is equivalent to not estimable in direct comparisons. |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Intermittent portal triad clamping vs continuous selective portal triad clamping | 130 per 1000 |
247 per 1000 (51 to 665) |
Rate ratio 2.19 (0.36 to 13.26) |
815 (6 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Serious adverse events (number) | |||||
| Intermittent portal triad clamping vs control | 80 per 1000 |
119 per 1000 (36 to 358) |
Rate ratio 1.55 (0.43 to 6.4) |
100 (1 study) |
⊕⊝⊝⊝ Very lowa,b,c |
| Continuous portal triad clamping vs continuous hepatic vascular exclusion | 179 per 1000 |
36 per 1000 (2 to 218) |
Rate ratio 0.17 (0.01 to 1.28) |
52 (1 study) |
⊕⊝⊝⊝ Very lowa,b,c |
| Intermittent portal triad clamping vs continuous portal triad clamping | 190 per 1000 |
21 per 1000 (0 to 116) |
Rate ratio 0.09 (0 to 0.56) |
86 (1 study) |
⊕⊕⊝⊝ Lowa,b |
| Intermittent selective portal triad clamping vs intermittent portal triad clamping | 134 per 1000 |
165 per 1000 (76 to 328) |
Rate ratio 1.27 (0.53 to 3.15) |
138 (2 studies) |
⊕⊝⊝⊝ Very lowa,b,c |
| Health‐related quality of life (30 days, 3 months) | None of the trials reported this outcome. | ||||
| Health‐related quality of life (longest follow‐up) | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion. The corresponding risk (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). Network meta‐analysis was not performed for any of the outcomes other than serious adverse events (proportion) because of the lack of availability of direct and indirect comparisons in the network. CrI: credible intervals; OR: odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2 Sample size was low (total number of participants fewer than 400 for continuous outcomes and fewer than 300 events in total in both groups for other outcomes) (downgraded by 1 point). 3 Credible intervals spanned no effect and clinically significant effect (20% relative risk reduction for binary outcomes; standardised mean difference of 0.5 for health‐related quality of life) (downgraded by 1 point).
20. Detailed 'Summary of findings' table: pharmacological interventions.
| Outcomes | Illustrative comparative risks* (95% CrI) | Relative effect (95% CrI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Mortality (perioperative) | |||||
| Recombinant factor VIIa vs control | 51 per 1000 |
33 per 1000 (7 to 158) |
OR 0.63 (0.13 to 3.51) |
185 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Tranexamic acid vs control | There was no mortality in either group. |
214 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
||
| Mortality (longest follow‐up) | None of the trials reported this outcome. | ||||
| Serious adverse events (proportion) | |||||
| Anti‐thrombin III vs control | 273 per 1000 |
312 per 1000 (67 to 761) |
OR 1.21 (0.19 to 8.49) |
24 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Recombinant Factor VIIa vs control | 376 per 1000 |
396 per 1000 (256 to 555) |
OR 1.09 (0.57 to 2.07) |
432 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Serious adverse events (number) | |||||
| Recombinant Factor VIIa vs control | 81 per 1000 |
120 per 1000 (68 to 217) |
Rate ratio 1.55 (0.83 to 3.16) |
432 (2 studies) |
⊕⊝⊝⊝ Very low1,2,3 |
| Tranexamic acid vs control | 75 per 1000 |
65 per 1000 (23 to 164) |
Rate ratio 0.85 (0.29 to 2.41) |
214 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 |
| Health‐related quality of life (30 days, 3 months) | None of the trials reported this outcome. | ||||
| Health‐related quality of life (maximal follow‐up) | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion. The corresponding risk (and its 95% credible interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). Network meta‐analysis was not performed for any of the outcomes because of the lack of availability of direct and indirect comparisons in the network. CrI: credible intervals; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2 Sample size was low (total number of participants fewer than 400 for continuous outcomes and fewer than 300 events in total in both groups for other outcomes) (downgraded by 1 point). 3 Credible intervals spanned no effect and clinically significant effect (20% relative risk reduction for binary outcomes; standardised mean difference of 0.5 for health‐related quality of life) (downgraded by 1 point).
Background
Description of the condition
Liver resection refers to removal of part of the liver. Every year, an average of 2400 people undergo liver resections in England (HSCIC 2015), 11,000 in the USA (Asiyanbola 2008), and 7200 in France (Farges 2012). In the West, the main indication for liver resection is colorectal liver metastases. Colorectal cancer is the third most common cancer in the world. Approximately 1.36 million people develop colorectal cancer each year (IARC 2012), and 50% to 60% will have colorectal liver metastases (Garden 2006). Liver resection, the only curative option for people with colorectal liver metastases, is indicated in 20% to 30% of people in whom the metastasis is confined to the liver (Garden 2006). Five‐year survival for people with colorectal liver metastases who undergo liver resection is about 45% (Garden 2006; Nordlinger 2013).
The second most common reason for liver resection is hepatocellular carcinoma. Hepatocellular carcinoma is one of the most common cancers, with a worldwide annual incidence of 780,000 people (IARC 2012). Most hepatocellular carcinomas develop in cirrhotic livers (Llovet 2005). Liver resection and liver transplantation are the main curative treatments (Llovet 2005; Taefi 2013). Of people who present with hepatocellular carcinoma, about 5% are candidates for liver resection (Chen 2006). Survival after surgery depends on the stage of cancer and the severity of the underlying chronic liver disease. People with early‐stage disease (cancers smaller than 5 cm) have a five‐year survival of about 50%, whereas people with more advanced disease have a five‐year survival of about 30% (Chen 2006; Navadgi 2016). Screening programmes in theory should lead to a diagnosis at an earlier stage, when surgery is feasible and associated with better outcomes.
Liver resection may also be performed for benign liver tumours (Belghiti 1993). The liver can be subdivided into eight segments (Couinaud 1999), which can be removed individually or by right hemi‐hepatectomy (Couinaud segments 5 to 8), left hemi‐hepatectomy (segments 2 to 4), right trisectionectomy (segments 4 to 8), or left trisectionectomy (segments 2 to 5 and 8 ± 1) (Strasberg 2000). Although every liver resection is considered major surgery, only resection of three or more segments is considered a major liver resection (Belghiti 1993).
Blood loss during liver resection is an important factor affecting complications and mortality in people undergoing liver resection (Shimada 1998; Yoshimura 2004; Ibrahim 2006). Estimates of blood loss have ranged from 200 mL to 2 L per patient (Gurusamy 2009a). Major blood loss during surgery or in the immediate postoperative period may result in death of the patient. Major blood loss can be defined based on the Advanced Trauma Life Support (ATLS definition of class 3 or class 4 shock, where there is a loss of 30% or more of blood volume) (ATLS 2008). During liver resection, the liver parenchyma is transected at the plane of resection. The blood vessels and the bile duct branches in the plane of resection (cut surface) are then sealed by different methods to prevent blood or bile leakage.
Description of the intervention
Specialists have tested various interventions in attempts to decrease blood loss during liver resection. These interventions include anterior approach as compared to the standard (conventional) surgical approach (Capussotti 2012); autologous blood donation with an aim of decreasing the use of others' blood (heterologous blood transfusion) (Kajikawa 1994), various cardiopulmonary interventions such as acute normovolemic haemodilution (ANH), low central venous pressure (central venous pressure), and hypoventilation that can be used either alone or in combination to decrease blood loss (Gurusamy 2012; Table 9); different methods of liver parenchymal transection (the way that the liver parenchyma is divided), such as the clamp‐crush method, the cavitron ultrasonic surgical aspirator, or the radiofrequency dissecting sealer (Gurusamy 2009b; Table 10); different methods of management of the cut surface of the liver (the way that the resection plane of the remnant liver is managed), such as use of fibrin sealant, argon beamer, or electrocautery and suture material (Frilling 2005; Table 11); temporary occlusion of the blood vessels that supply the liver (Gurusamy 2009a; Table 12); and various pharmacological interventions such as recombinant factor VIIa, antithrombin III, and tranexamic acid (Gurusamy 2009c).
1. Different methods of cardiopulmonary interventions.
| Acute normovolemic haemodilution (ANH) |
| Low central venous pressure (central venous pressure) |
| Hypoventilation |
| Combination of ANH with central venous pressure or hypotension |
2. Different methods of parenchymal transection.
| Finger‐fracture method |
| Clamp‐crush method |
| Cavitron ultrasonic surgical aspirator |
| Sharp dissection |
| Radiofrequency dissecting sealer |
| Ultrasonic shears |
| Stapler |
| Waterjet (Hydrojet) |
3. Different methods of dealing with raw surface.
| Suturing for large and medium vessels and ducts and performing electrocauterisation of small vessels and ducts |
| Suturing for large vessels and performing ultrasonic shears for medium‐sized and small vessels and ducts |
| Suturing and argon beam coagulator |
| Suturing and fibrin sealant |
| Suturing and collagen |
| Suturing and oxidised cellulose |
| Suturing and cyanoacrylate |
| Suturing and combination of fibrin sealant with collagen or oxidised cellulose |
4. Different methods of vascular occlusion.
| No vascular occlusion |
| Portal triad clamping (continuous) (occlusion of inflow alone) |
| Portal triad clamping (intermittent) (occlusion of inflow alone) |
| Hepatic vascular exclusion (occlusion of inflow and outflow) (continuous or intermittent) |
| Selective portal trial clamping (occlusion of inflow to the hemi‐liver that is being resected) (continuous or intermittent) |
| Selective hepatic vascular exclusion (occlusion of inflow to the hemi‐liver and outflow from the hemi‐liver that is being resected) (continuous or intermittent) |
Interventions selected to decrease blood loss can be used alone or in various combinations. Usually surgeons at different centres follow their own protocol for decreasing blood loss. The finger‐fracture and clamp‐crush techniques do not involve specialist equipment. The minimum and standard method of managing the cut surface involves electrocautery for sealing small vessels and suturing larger vessels. Altogether, the goal of these interventions is to decrease blood loss and the associated morbidity and mortality.
How the intervention might work
Temporarily occluding the vessels that supply blood to the liver may reduce the blood loss from the cut vessels. Different methods of liver transection are used to identify major vessels and allow them to be sutured and divided. This might result in clear visualisation of the blood vessels, which can be clamped and then divided. Different topical methods of managing the cut surface attempt to seal the blood vessels on the resection plane, preventing blood loss. Cardiopulmonary interventions decrease the amount of blood lost by dilution of blood or reducing the pressure in the hepatic veins (low central venous pressure). Autologous blood donation involves venesection of the patient prior to surgery and storage of blood which can be replaced if required during or after surgery with the aim of reducing homologous blood transfusion. Pharmacological interventions work by increasing the clotting of blood with a view to decreasing the blood loss. The anterior approach is a surgical technique that involves occluding the inflow and outflow vessels and performing parenchymal transection prior to mobilisation of the right liver (Liu 2006). The potential advantage of anterior approach over the conventional approach, in which liver is mobilised first, is that inadvertent injury to the blood vessels and the resulting bleeding can be avoided since the blood vessels are occluded before liver mobilisation in the anterior approach. Blood vessels may also be occluded first in conventional approach if one of the methods of vascular occlusion is used.
Why it is important to do this review
Liver resection is a major surgical procedure with significant mortality (estimated at 3.5%) and morbidity (estimated around 40%) (Finch 2007; Reissfelder 2011). Interventions that decrease blood loss may improve outcomes of liver resection. Previous systematic reviews have assessed some of the categories of interventions (Gurusamy 2009a; Gurusamy 2009b; Gurusamy 2009c; Gurusamy 2012). We also performed a network meta‐analysis assessing the combination of a method of vascular occlusion, parenchymal transection, and method of dealing with raw surface as a package (Simillis 2014). However, in that review, we found that most authors did not report the different aspects of the method of liver resection other than the factor being randomised or allowed surgeons to choose how to deal with the other factors according to their preference. Since that review excluded such trials, reviewers could only include a few studies. In this updated review, we have covered all the different aspects of the methods to decrease blood loss and blood transfusion requirements during liver resection. We included trials where at least one of the methods to decrease blood loss and blood transfusion requirements during liver resection was included in a randomised comparison with the other aspects either not reported or allowed to vary according to surgeons' preference. This systematic review is intended as a useful guide for patients and healthcare providers as they seek to understand the role of different methods in decreasing blood loss and blood transfusion requirements in people undergoing elective liver resection.
Objectives
To assess the effects of different interventions for decreasing blood loss and blood transfusion requirements during elective liver resection.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised clinical trials for this network meta‐analysis. We excluded studies of other designs.
Types of participants
We included randomised clinical trials in which participants underwent elective liver resection using different types of vascular occlusion or no vascular occlusion, irrespective of the method of vascular occlusion or the nature of the background liver (i.e. normal or cirrhotic), different types of parenchymal transection, different types of management of cut surface, or whether pharmacological interventions were used. We excluded randomised clinical trials in which participants underwent liver resection combined with other major surgical procedures (e.g. one‐stage liver and bowel resection for synchronous metastases from colorectal tumours).
Types of interventions
We included randomised clinical trials that assessed one or more of the following interventions in this review.
Anterior approach versus conventional approach.
Autologous blood donation versus control.
Cardiopulmonary interventions.
Methods of liver parenchymal transection.
Methods of management of the raw surface (resection plane) of the liver.
Methods of vascular occlusion (including no vascular occlusion).
Pharmacological interventions.
The surgeon (and hence the trialists) may use a particular combination of each of the above. For example, one surgeon may perform liver resection using intermittent vascular occlusion, clamp‐crush technique as the method of liver parenchymal transection, and a fibrin sealant on the cut surface, while another surgeon may perform liver resection without using any method of vascular occlusion, with the cavitron ultrasonic surgical aspirator as the method of liver parenchymal transection, without any fibrin sealant on the cut surface, or any additional pharmacological intervention.
Commonly used surgical techniques under each of the above categories are listed in Table 9, Table 10, Table 11, and Table 12. In practice, surgeons can use any intervention in Table 9 in combination with an intervention from Table 10, Table 11, or Table 12. Any intervention in Table 10 can be used in combination with an intervention from Table 11 or Table 12. Any intervention in Table 11 can be used in combination with an intervention in Table 12. Any of these combinations can be used in combination with anterior or conventional approach, with autologous blood donation, and with or without a pharmacological intervention.
Types of outcome measures
We assessed the comparative effectiveness of available treatment strategies that aimed to decrease blood loss during liver resection for the following outcomes.
Primary outcomes
-
Mortality.
Peri‐operative (30‐day mortality or postoperative mortality). We used in‐hospital mortality as defined in the included trials.
Long‐term (at longest follow‐up).
-
Adverse events. We defined an adverse event as any untoward medical occurrence not necessarily having a causal relationship with the treatment but resulting in a dose reduction or discontinuation of treatment (ICH‐GCP 1997). We considered a serious adverse event to be any event that would increase mortality; was life‐threatening; required inpatient hospitalisation; resulted in persistent or significant disability; might have jeopardised the person; or required intervention to prevent it. Serious adverse events correspond approximately to grade III or above of the Clavien‐Dindo classification ‐ the only validated system for classifying postoperative complications (Dindo 2004; Clavien 2009;Table 13). In cases where the authors did not classify the severity of adverse events, we followed the criteria provided in Table 13 to classify the severity. We analysed the following information.
Proportion of participants experiencing serious adverse events.
Number of serious adverse events.
Proportion of participants experiencing adverse events.
Number of adverse events.
-
Quality of life as defined in the included trials.
Short‐term (30 days, three months).
Long‐term (longest follow‐up).
5. Clavien‐Dindo classification of postoperative complications.
| Grades | Definitions | Examples |
| I | Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic, or radiological interventions | Drugs such as antiemetics, antipyretics, analgesics, diuretics, and electrolytes; physiotherapy; wound infections opened at the bedside |
| II | Requiring pharmacological treatment with drugs other than those allowed for grade I complications | Blood transfusions, total parenteral nutrition |
| III | Requiring surgical, endoscopic, or radiological intervention | Bile leak requiring endoscopic stent; re‐operation for any cause; drainage of infected intra‐abdominal collection |
| IV | Life‐threatening complication requiring high dependency or intensive care management | Dialysis |
| V | Death of patient | — |
| Suffix d | If the patient suffers from a complication at the time of discharge and needs further follow‐up to evaluate the complication fully | — |
Adapted from Dindo 2004; Clavien 2009.
Secondary outcomes
-
Blood transfusion requirements.
Number of participants who required red blood cells or whole blood heterologous blood transfusion.
Quantity of blood transfusion (heterologous red blood cells or whole blood product, platelet, or fresh frozen plasma).
Total operative blood loss.
Number of participants who had major operative blood loss.
-
Hospital stay.
Length of total hospital stay (including re‐admissions).
Intensive therapy unit stay.
Operating time.
Time needed to return to work.
Search methods for identification of studies
Electronic searches
We aimed to identify all relevant randomised clinical trials regardless of language or publication status (published, unpublished, in press, or in progress) (Royle 2003).
We searched the following databases up to 23 September 2015.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 9) in the Cochrane Library.
MEDLINE via PubMed (from 1947).
EMBASE via Ovid SP (from 1974).
Science Citation Index Expanded via Web of Science (from 1975).
We also searched the World Health Organization International Clinical Trials Registry Platform search portal (www.who.int/ictrp), which searches various trial registers, including ISRCTN and ClinicalTrials.gov, to identify further trials (searched 23 September 2015). Because existing Cochrane systematic reviews have comprehensively assessed subsets of all available interventions on this topic, we also used these reviews as a way to identify trials(Gurusamy 2009a; Gurusamy 2009b). We present full search strategies in Appendix 1.
Searching other resources
We searched the references of the identified trials for additional trials eligible for inclusion.
Data collection and analysis
Selection of studies
Two review authors (EM and KG) independently screened the titles and abstracts of all records retrieved. We sought full text for any references that at least one of the authors identified as potentially eligible. We assessed the full text for inclusion and listed the reasons for the excluding trials in the Characteristics of excluded studies tables. We listed any ongoing trials in Characteristics of ongoing studies for further follow‐up in updates of the reviews. We resolved discrepancies through discussion.
Data extraction and management
Two review authors (KG and EM) independently extracted the following data.
Year and language of publication.
Country in which investigators recruited the participants.
Year(s) in which the trial took place.
Inclusion and exclusion criteria.
Participant characteristics such as age, sex, underlying disease, comorbidity, number and proportion of participants with cirrhosis, and number and proportion of participants undergoing major versus minor liver resection.
Details of the intervention and treatment strategy that aimed to decrease blood loss and blood transfusion requirements (e.g. surgical technique, procedure and co‐intervention, concurrent surgery, and medications).
Outcomes (Primary outcomes; Secondary outcomes).
Follow‐up time points.
Risk of bias (Assessment of risk of bias in included studies).
We sought unclear or missing information by contacting the authors of the individual trials. If there had been any doubt whether trials shared the same participants – completely or partially (by identifying common authors and centres) – we would have contacted the authors of the trials to clarify whether the trial report was duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the guidance in the Cochrane Handbook for Systematic Reviews of Intervention and those described in the Cochrane Hepato‐Biliary Group Module to assess the risk of bias in included studies (Higgins 2011; Gluud 2013). Specifically, we assessed the risk of bias in included trials for the following domains (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savovic 2012a; Savovic 2012b).
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if an independent adjudicator performed them.
Uncertain risk of bias: authors described the trial as randomised but did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not, or may not have been, random. Quasi‐randomised studies (those using dates, names, or admittance numbers to allocate participants) were inadequate, and we excluded them for the assessment of benefits butof harms.
Allocation concealment
Low risk of bias: allocation was controlled by a central and independent randomisation unit and involved sequentially numbered, opaque, sealed envelopes, or something similar, so that neither participants nor investigators could have foreseen intervention allocations in advance of or during enrolment.
Uncertain risk of bias: authors described the trial as randomised but did not describe the method used to conceal the allocation, so participants or operators may have been able to foresee intervention allocations in advance of, or during, enrolment.
High risk of bias: the investigators who assigned participants were aware of the allocation sequence, or the study was quasi‐randomised. We excluded quasi‐randomised studies for assessment of benefits but not of harms.
Blinding of participants and personnel
Low risk of bias: blinding was performed adequately, or the outcome measurement was not likely to be influenced by lack of blinding.
Uncertain risk of bias: information was insufficient to allow assessment of whether the type of blinding used was likely to induce bias on the estimate of effect.
High risk of bias: no blinding or incomplete blinding and the outcome or the outcome measurements were likely to be influenced by lack of blinding.
Blinding of outcome assessors
Low risk of bias: blinding was performed adequately, or the outcome measurement was not likely to be influenced by lack of blinding.
Uncertain risk of bias: information was insufficient to allow assessment of whether the type of blinding used was likely to induce bias on the estimate of effect.
High risk of bias: no blinding or incomplete blinding and the outcome or the outcome measurements were likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: the underlying reasons for missing data were unlikely to make treatment effects depart from plausible values, or proper methods were employed to handle missing data.
Uncertain risk of bias: information was insufficient to allow assessment of whether the missing data mechanism in combination with the method used to handle missing data was likely to induce bias on the estimate of effect.
High risk of bias: the crude estimate of effects (e.g. complete case estimate) were clearly biased because of the underlying reasons for missing data, and the methods used to handle missing data were unsatisfactory.
Selective outcome reporting
Low risk of bias: authors reported pre‐defined or clinically relevant and reasonably expected outcomes (mortality and serious adverse events).
Uncertain risk of bias: authors did not fully report all pre‐defined or clinically relevant and reasonably expected outcomes, or it was unclear whether authors recorded data on these outcomes.
High risk of bias: authors failed to report one or more clinically relevant and reasonably expected outcomes; data on these outcomes were likely to have been recorded.
Vested interest bias
Low risk of bias: a party with no vested interests in the outcome (i.e. a party that would not benefit from the results of the trial) conducted the trial.
Uncertain risk of bias: it was not clear if those conducting the trial had a vested interest in its outcome.
High risk of bias: a party with vested interests in the outcome of the trial (such as a drug manufacturer) conducted the trial.
We considered a trial to be at low risk of bias if we assessed it as being at low risk of bias for all domains. We considered a trial at low risk of bias for an outcome if we assessed it as being at low risk of bias for all study level domains, as well as for outcome‐specific domains (e.g. blinding, incomplete outcome data). Otherwise, we considered trials with uncertain or high risk of bias regarding one or more domains to be trials at high risk of bias.
Measures of treatment effect
For dichotomous variables (short‐term mortality, serious adverse events, participants requiring blood transfusion), we calculated the odds ratio (OR) with 95% credible interval (CrI). For continuous variables, such as quantity of blood transfused, blood loss, hospital stay, and operating time, we calculated the mean difference (MD) with 95% CrI. When trials reported the blood transfusion as mL or L rather than units, we converted these into units by considering that each unit of whole blood or red blood cell transfusion was 400 mL and each unit of fresh frozen plasma was 250 mL. We planned to use MD and 95% CrI for time needed to return to work, but we did not use this because none of the included trials reported this outcome. We planned to use standardised mean difference (SMD) with 95% CrI for quality of life if trials used different scales, but we did not plan to combine the quality of life at different time points. For time‐to‐event data, such as long‐term survival, we planned to use the hazard ratio (HR) with 95% CrI.
Relative ranking
We estimated the probabilities for each intervention of being at each possible rank. Then we obtained a treatment hierarchy using the probability of each intervention being the best treatment by using the surface under the cumulative ranking curve (SUCRA) (Salanti 2011).
Unit of analysis issues
The unit of analysis was the person undergoing elective liver resection according to the intervention group to which they were randomly assigned.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992). Otherwise, we used data that were available to us (e.g. a trial may have reported only per protocol analysis results). As per protocol analyses may be biased, we planned to conduct best‐worst case scenario and worst‐best case scenario analyses as sensitivity analyses, if there was a possibility that authors could have judged a treatment as effective because of attrition bias.
For continuous outcomes, we imputed the standard deviation from P values according to guidance in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). If the data were likely to be normally distributed and the mean was not available, we used the median for meta‐analysis. If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we imputed the standard deviation using the largest standard deviation in other trials for that outcome. This form of imputation may decrease the weight of the study for calculation of mean differences and may bias the effect estimate to no effect for calculation of SMDs (Higgins 2011).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. Major sources of clinical heterogeneity included cirrhotic compared to non‐cirrhotic livers and major compared to minor liver resections. In addition, we anticipated considerable heterogeneity in the way the intervention was performed. For example, surgeons may perform intermittent portal triad clamping with different time periods of occlusion and non‐occlusion. In addition, they may use different doses of fibrin sealant. Different study design and risk of bias may contribute to methodological heterogeneity.
We used the residual deviance and Deviance Information Criteria (DIC) for assessing between‐study heterogeneity as per the guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) Technical Support Documents (Dias 2012b; Dias 2013a). We also calculated the between‐trial standard deviation and reported this if we used a random‐effects model. See Data synthesis for further details regarding residual deviance, DIC, and choice of model.
If we identified substantial heterogeneity ‐ clinical, methodological, or statistical ‐ we planned to explore and address it in a subgroup analysis (see section on Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We planned to use visual asymmetry on a funnel plot to explore reporting bias in case at least 10 trials were included for the outcome (Egger 1997; Macaskill 2001). In the presence of heterogeneity that we could explain by subgroup analysis, we planned to perform the funnel plot for each subgroup in the presence of the adequate number of trials. We planned to perform the linear regression approach described by Egger 1997 to determine the funnel plot asymmetry in the presence of at least 10 trials for the direct comparison. However, we did not perform this because there were not enough trials.
We also considered selective reporting as evidence of reporting bias.
Data synthesis
We applied classifications described in Table 9, Table 10, Table 11, and Table 12 to categorise cardiopulmonary interventions, parenchymal transection methods, methods of dealing with cut surface, and different vascular occlusion methods. Each category in the table is broadly defined to encompass a relatively homogeneous group of interventions, although we noted variations in the way each method is carried out. For example, surgeons may perform intermittent portal triad clamping with different time periods of occlusion and non‐occlusion. We categorised them under intermittent portal triad clamping regardless of the time intervals. Likewise, we did not distinguish different maximum periods for continuous vascular occlusion (Clavien 1996). These practice variations might be a source of heterogeneity; however, evidence was insufficient to suggest that they could affect the outcome. For the comparisons of anterior approach versus conventional approach and autologous blood donation versus control, there are only two treatments for each comparison. For pharmacological interventions, we treated each pharmacological treatment as a separate category.
In liver resection, a surgeon typically uses one item each from Table 9, Table 10, Table 11, and Table 12. Liver resection is usually performed using conventional approach without autologous blood donation or any pharmacological agent. Compared to the previous version of the review (Simillis 2014), where we considered a combination of one method each from Table 10, Table 11, and Table 12 as a treatment strategy, in this review, we considered each of these interventions (different methods of cardiopulmonary interventions, parenchymal transection methods, methods of dealing with raw surface, vascular occlusion methods, and pharmacological interventions) as separate networks. This approach was in response to the lack of information on the details of co‐interventions in the trials and the design of the trials, which limited the number of trials included in the previous analysis. In many of the trials, the surgeons involved were allowed to choose their method of liver resection apart from the factor being randomised, based on the assumption that the factors are independent of each other (i.e. there is no interaction between the factors, or the choice of one factor is independent of the choice of other factors). There is no evidence to support or refute this assumption. However, if we had included only trials that reported all the intervention variables adequately, and none were left to the choice of the surgeons, this would have resulted in inclusion of fewer trials than the previous version, as we have now included all the interventions aimed at decreasing blood loss and blood transfusion requirements during liver resection.
Direct comparison
We performed pair‐wise meta‐analyses using WinBUGS by Bayesian analysis using the same codes and methods described immediately below in the network meta‐analysis section (i.e. same burn‐in, number of simulations, choice of initial values, and choice of models). In addition, we performed the meta‐analysis using frequentist methods with Review Manager 5 (RevMan 2014), in accordance with recommendations of Higgins 2011 and those described in the Cochrane Hepato‐Biliary Group Module (Gluud 2013). For frequentist analyses, we presented the results of the model that was used for Bayesian analysis (which was determined by the model fit).
Network meta‐analysis
We conducted network meta‐analyses to compare multiple interventions simultaneously for each of the outcomes listed in the Types of outcome measures section. Network meta‐analysis combines direct evidence within trials and indirect evidence across trials (Mills 2012).
We obtained a network plot to ensure that the trials were connected by treatments using Stata/IC 11 (StataCorp LP). We performed a network meta‐analysis only when it was possible to compare the direct and indirect estimates. This is because one cannot assess whether there is consistency between the direct and indirect estimates unless both are available. We planned to exclude any trials that were not connected to the network. We conducted a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in WinBUGS 1.4. We modelled the treatment contrast (e.g. log OR for binary outcomes, MD for continuous outcomes) for any two interventions ('functional parameters') as a function of comparisons between each individual intervention and an arbitrarily selected reference group ('basic parameters') (Lu 2006). We used inconsistency models to assess this consistency assumption (Dias 2013e). The reference groups selected for the different comparisons are as follows.
Anterior approach versus conventional approach: conventional approach.
Autologous blood donation versus control: inactive control.
Cardiopulmonary interventions: inactive control.
Methods of parenchymal transection: clamp‐crush method.
Methods of dealing with raw surface: inactive control.
Methods of vascular occlusion: no vascular occlusion.
Pharmacological interventions: inactive control.
We performed the network analysis as per the guidance from the NICE DSU documents (Dias 2013a; Dias 2013c). Further details of the codes used, the raw data, and the technical details of how we performed the analysis are in Appendix 2, Appendix 3, and Appendix 4. We tested the codes on simulated data (Appendix 5) using predetermined effect estimates with no inconsistency between direct and indirect comparisons. This simulation testing demonstrated that the codes produced similar effect estimates as the predetermined effect estimates (allowing for some variability because of simulation) and that the effect estimates obtained using these codes were almost identical to the effect estimates obtained by direct estimates using RevMan (Appendix 6).
The codes allow handling of trials with multiple arms to be dealt in the same way as two‐armed trials, that is, one can enter the data from all the intervention arms in a trial as number of events and the number of people exposed to the event for binary outcomes; for continuous outcomes, one can enter the mean and standard error for all intervention arms in the trial. The choice between the fixed‐effect model and random‐effects model was based on the model fit as per the guidelines of the NICE TSU (a difference of three to five for deviance information criterion (DIC)) is important (Dias 2013a; Dias 2013c); we used a difference of three as important). We reported the treatment contrasts (i.e. log ORs for binary outcomes and MDs for continuous outcomes) of the different treatments in relation to the reference treatment, the deviance residuals, the number of effective parameters, and DIC for the fixed‐effect model and random‐effects model for each outcome. We also reported the parameters used to assess the model fit (i.e. deviance residuals, number of effective parameters, and DIC) for the inconsistency model in Table 14, Table 15, and Table 16.
6. Cardiopulmonary interventions: choice of model results.
| Blood transfusion (red blood cell) (units) | |||
| Treatment number | Treatment name | ||
| 1 | Control | ||
| 2 | Acute normovolemic haemodilution | ||
| 3 | Acute normovolemic haemodilution plus hypotension | ||
| 4 | Acute normovolemic haemodilution plus low central venous pressure | ||
| 5 | Low central venous pressure | ||
| Fixed‐effect model | Random‐effects model | Inconsistency model | |
| Dbara | 2.68 | −8.90 | −9.80 |
| pDb | 10.05 | 12.67 | 11.96 |
| DICc | 12.73 | 3.77 | 2.17 |
| d[2]d | −1.23 (95% CrI −1.74 to −0.73) | −1.26 (95% CrI −4.92 to 2.39) | — |
| d[3]e | −1.65 (95% CrI −2.06 to −1.25) | −1.68 (95% CrI −5.33 to 1.98) | — |
| d[4]f | 0.15 (95% CrI −0.10 to 0.40) | −0.57 (95% CrI −3.35 to 1.88) | — |
| d[5]g | −0.81 (95% CrI −1.33 to −0.30) | −1.08 (95% CrI −3.43 to 1.13) | — |
| Between‐study standard deviation | — | 1.446 | |
| Model used | Random‐effects model | ||
| Evidence of inconsistency | There is no evidence of inconsistency since the difference in DIC between consistency and inconsistency models was not significant. | ||
| Blood loss (litres) | |||
| Treatment number | Treatment name | ||
| 1 | Control | ||
| 2 | Acute normovolemic haemodilution | ||
| 3 | Acute normovolemic haemodilution plus hypotension | ||
| 4 | Acute normovolemic haemodilution plus low central venous pressure | ||
| 5 | Hypoventilation | ||
| 6 | Low central venous pressure | ||
| Fixed‐effect model | Random‐effects model | Inconsistency model | |
| Dbara | −24.73 | −36.06 | −36.65 |
| pDb | 14.00 | 17.77 | 18.26 |
| DICc | −10.73 | −18.29 | −18.39 |
| d[2]d | 0.00 (95% CrI −0.10 to 0.10) | 0.00 (95% CrI −0.95 to 0.96) | — |
| d[3]e | −0.25 (95% CrI −0.37 to −0.13) | −0.25 (95% CrI −1.20 to 0.71) | — |
| d[4]f | 0.01 (95% CrI −0.04 to 0.07) | −0.10 (95% CrI −0.88 to 0.46) | — |
| d[5]g | 0.00 (95% CrI −1.12 to 1.12) | −0.01 (95% CrI −1.44 to 1.43) | — |
| d[6]h | −0.29 (95% CrI −0.40 to −0.18) | −0.32 (95% CrI −0.86 to 0.09) | — |
| Between‐study standard deviation | — | 0.3734 | — |
| Model used | Random‐effects model | ||
| Evidence of inconsistency | There is no evidence of inconsistency since the difference in DIC between consistency and inconsistency models was not significant. | ||
aDbar = posterior mean of deviance. bpD = effective number of parameters. cDIC = deviance information criterion. dd[2] indicates effect estimate (mean difference) of treatment 2 versus treatment 1. ed[3] indicates effect estimate (mean difference) of treatment 3 versus treatment 1. fd[4] indicates effect estimate (mean difference) of treatment 4 versus treatment 1. gd[5] indicates effect estimate (mean difference) of treatment 5 versus treatment 1. hd[6] indicates effect estimate (mean difference) of treatment 6 versus treatment 1.
7. Parenchymal transection methods: choice of model results.
| Adverse events (proportion) | |||
| Treatment number | Treatment name | ||
| 1 | Clamp‐crush method | ||
| 2 | Cavitron ultrasonic surgical aspirator | ||
| 3 | Hydrojet | ||
| 4 | Radiofrequency dissecting sealer | ||
| 5 | Sharp transection method | ||
| 6 | Stapler | ||
| Fixed‐effect model | Random‐effects model | Inconsistency model* | |
| Dbara | 95.62 | 80.26 | 81.67 |
| pDb | 13.05 | 17.04 | 16.71 |
| DICc | 108.67 | 97.30 | 98.37 |
| d[2]d | 0.32 (95% CrI −0.28 to 0.92) | 0.76 (95% CrI −2.18 to 4.69) | — |
| d[3]e | −0.99 (95% CrI −2.76 to 0.54) | −0.56 (95% CrI −6.84 to 6.60) | — |
| d[4]f | 0.11 (95% CrI −0.46 to 0.68) | 0.19 (95% CrI −2.95 to 3.50) | — |
| d[5]g | 0.10 (95% CrI −0.79 to 1.00) | 0.1 (95% CrI −5.59 to 5.80) | — |
| d[6]h | 0.06 (95% CrI −0.63 to 0.76) | 0.06 (95% CrI −5.59 to 5.76) | — |
| Between‐study standard deviation | — | 2.436 | — |
| Model used | Random‐effects model | ||
| Evidence of inconsistency | There is no evidence of inconsistency since the difference in DIC between consistency and inconsistency models was not significant. | ||
| Adverse events (number) | |||
| Treatment number | Treatment name | ||
| 1 | Clamp‐crush method | ||
| 2 | Cavitron ultrasonic surgical aspirator | ||
| 3 | Hydrojet | ||
| 4 | Radiofrequency dissecting sealer | ||
| 5 | Sharp transection method | ||
| 6 | Stapler | ||
| Fixed‐effect model | Random‐effects model | Inconsistency model* | |
| Dbara | 80.99 | 80.94 | 79.59 |
| pDb | 11.93 | 11.88 | 14.76 |
| DICc | 92.92 | 92.83 | 94.35 |
| d[2]d | 0.47 (95% CrI −0.08 to 1.03) | 0.47 (95% CrI −0.08 to 1.03) | — |
| d[3]e | 0.34 (95% CrI −0.71 to 1.29) | 0.33 (95% CrI −0.71 to 1.28) | — |
| d[4]f | 0.61 (95% CrI 0.12 to 1.12) | 0.61 (95% CrI 0.12 to 1.11) | — |
| d[5]g | 0.12 (95% CrI −0.56 to 0.81) | 0.12 (95% CrI −0.56 to 0.81) | — |
| d[6]h | 0.62 (95% CrI −0.21 to 1.48) | 0.62 (95% CrI −0.20 to 1.45) | — |
| Between‐study standard deviation | — | 2.499 | — |
| Model used | Fixed‐effect model | — | |
| Evidence of inconsistency | There is no evidence of inconsistency since the difference in DIC between consistency and inconsistency models was not significant. | ||
| Blood transfusion (proportion) | |||
| Treatment number | Treatment name | ||
| 1 | Clamp‐crush method | ||
| 2 | Cavitron ultrasonic surgical aspirator | ||
| 3 | Hydrojet | ||
| 4 | Radiofrequency dissecting sealer | ||
| 5 | Sharp transection method | ||
| Fixed‐effect model | Random‐effects model | Inconsistency model* | |
| Dbara | 72.41 | 71.86 | 72.23 |
| pDb | 11.91 | 13.99 | 14.98 |
| DICc | 84.33 | 85.85 | 87.21 |
| d[2]d | 0.39 (95% CrI −0.62 to 1.42) | 0.42 (95% CrI −1.09 to 1.96) | — |
| d[3]e | 0.55 (95% CrI −0.75 to 1.83) | 0.60 (95% CrI −1.47 to 2.83) | — |
| d[4]f | 0.09 (95% CrI −0.50 to 0.68) | 0.14 (95% CrI −0.77 to 1.32) | — |
| d[5]g | −0.22 (95% CrI −1.16 to 0.71) | −0.22 (95% CrI −2.21 to 1.75) | — |
| Between‐study standard deviation | — | 0.6464 | — |
| Model used | Fixed‐effect model | — | |
| Evidence of inconsistency | There is no evidence of inconsistency since the difference in DIC between consistency and inconsistency models was not significant. | ||
aDBar = posterior mean of deviance. bpD = effective number of parameters. cDIC = deviance information criterion. dd[2] indicates log transformed effect estimate (odds ratio or rate ratio) of treatment 2 versus treatment 1. ed[3] indicates log transformed effect estimate (odds ratio or rate ratio) of treatment 3 versus treatment 1. fd[4] indicates log transformed effect estimate (odds ratio or rate ratio) of treatment 4 versus treatment 1. gd[5] indicates log transformed effect estimate (odds ratio or rate ratio) of treatment 5 versus treatment 1. hd[6] indicates log transformed effect estimate (odds ratio or rate ratio) of treatment 6 versus treatment 1.
8. Vascular occlusion methods: choice of model results.
| Serious adverse events (proportion) | |||
| Treatment number | Treatment name | ||
| 1 | Control | ||
| 2 | ConHVE | ||
| 3 | ConPTC | ||
| 4 | ConSelectiveHVE | ||
| 5 | ConSelectivePTC | ||
| 6 | IntPTC | ||
| Fixed‐effect model | Random‐effects model | Inconsistency model | |
| Dbara | 64.25 | 63.57 | 64.03 |
| pDb | 12.54 | 14.37 | 14.83 |
| DICc | 76.79 | 77.95 | 78.86 |
| d[2]d | 0.82 (95% CrI −1.70 to 3.50) | 0.62 (95% CrI −5.00 to 5.89) | — |
| d[3]e | 0.35 (95% CrI −1.26 to 1.96) | 0.16 (95% CrI −3.87 to 3.71) | — |
| d[4]f | −1.98 (95% CrI −8.24 to 1.48) | −2.25 (95% CrI −9.99 to 3.38) | — |
| d[5]g | −0.63 (95% CrI −2.29 to 0.97) | −1.01 (95% CrI −5.35 to 2.36) | — |
| d[6]h | 0.15 (95% CrI −0.61 to 0.92) | −0.07 (95% CrI −2.53 to 1.85) | — |
| Between‐study standard deviation | — | 1.216 | — |
| Model used | Fixed‐effect model | ||
| Evidence of inconsistency | There is no evidence of inconsistency since the difference in DIC between consistency and inconsistency models was not significant. | ||
| Adverse events (proportion) | |||
| Treatment number | Treatment name | ||
| 1 | Control | ||
| 2 | ConHVE | ||
| 3 | ConPTC | ||
| 4 | ConSelectiveHVE | ||
| 5 | ConSelectivePTC | ||
| 6 | IntPTC | ||
| 7 | IntSelectivePTC | ||
| Fixed‐effect model | Random‐effects model | Inconsistency model | |
| Dbara | 120.82 | 118.76 | 119.07 |
| pDb | 18.10 | 21.01 | 21.93 |
| DICc | 138.92 | 139.77 | 141.00 |
| d[2]d | 0.95 (95% CrI −0.21 to 2.12) | 0.90 (95% CrI −1.12 to 2.84) | — |
| d[3]e | 0.83 (95% CrI 0.00 to 1.69) | 0.78 (95% CrI −0.58 to 2.09) | — |
| d[4]f | 0.05 (95% CrI −1.19 to 1.27) | 0.00 (95% CrI −2.05 to 1.96) | — |
| d[5]g | 0.10 (95% CrI −0.81 to 1.01) | 0.07 (95% CrI −1.42 to 1.50) | — |
| d[6]h | 0.24 (95% CrI −0.19 to 0.68) | 0.18 (95% CrI −0.66 to 0.88) | — |
| d[7]i | 0.09 (95% CrI −0.75 to 0.93) | 0.04 (95% CrI −1.37 to 1.35) | — |
| Between‐study standard deviation | — | 0.4825 | — |
| Model used | Fixed‐effect model | ||
| Evidence of inconsistency | There is no evidence of inconsistency since the difference in DIC between consistency and inconsistency models was not significant. | ||
| Blood transfusion (proportion) | |||
| Treatment number | Treatment name | ||
| 1 | Control | ||
| 2 | ConHVE | ||
| 3 | ConPTC | ||
| 4 | ConSelectiveHVE | ||
| 5 | ConSelectivePTC | ||
| 6 | IntPTC | ||
| 7 | IntSelectivePTC | ||
| Fixed‐effect model | Random‐effects model | Inconsistency model | |
| Dbara | 139.87 | 120.00 | 120.10 |
| pDb | 19.04 | 25.25 | 25.72 |
| DICc | 158.91 | 145.25 | 145.82 |
| d[2]d | −2.55 (95% CrI −3.80 to −1.36) | −2.88 (95% CrI −7.47 to 1.47) | — |
| d[3]e | −0.77 (95% CrI −1.56 to 0.01) | −1.11 (95% CrI −3.72 to 1.28) | — |
| d[4]f | −1.46 (95% CrI −2.58 to −0.36) | −1.79 (95% CrI −6.38 to 2.53) | — |
| d[5]g | −0.26 (95% CrI −1.18 to 0.67) | −0.48 (95% CrI −3.83 to 2.72) | — |
| d[6]h | −0.34 (95% CrI −0.84 to 0.16) | −0.47 (95% CrI −2.32 to 1.28) | — |
| d[7]i | −0.92 (95% CrI −1.96 to 0.08) | −0.97 (95% CrI −4.24 to 2.24) | — |
| Between study standard deviation | — | 1.613 | — |
| Model used | Random‐effects model | ||
| Evidence of inconsistency | There is no evidence of inconsistency since the difference in DIC between consistency and inconsistency models was not significant. | ||
| Blood transfusion (red blood cell) (units) | |||
| Treatment number | Treatment name | ||
| 1 | Control | ||
| 2 | ConHVE | ||
| 3 | ConPTC | ||
| 4 | ConSelectiveHVE | ||
| 5 | ConSelectivePTC | ||
| 6 | IntPTC | ||
| 7 | IntSelectivePTC | ||
| Fixed‐effect model | Random‐effects model | Inconsistency model | |
| Dbara | −1.55 | −1.05 | 0.24 |
| pDb | 15.99 | 17.36 | 19.34 |
| DICc | 14.44 | 16.32 | 19.58 |
| d[2]d | −1.65 (95% CrI −3.96 to 0.67) | −1.56 (95% CrI −4.18 to 1.14) | — |
| d[3]e | −1.25 (95% CrI −2.39 to −0.10) | −1.18 (95% CrI −2.54 to 0.31) | — |
| d[4]f | −2.45 (95% CrI −4.08 to −0.82) | −2.37 (95% CrI −4.33 to −0.30) | — |
| d[5]g | −1.45 (95% CrI −2.59 to −0.31) | −1.41 (95% CrI −2.86 to 0.12) | — |
| d[6]h | −1.36 (95% CrI −2.48 to −0.23) | −1.35 (95% CrI −2.69 to 0.01) | — |
| d[7]i | −1.43 (95% CrI −2.61 to −0.24) | −1.43 (95% CrI −3.01 to 0.08) | — |
| Between‐study standard deviation | — | 0.3149 | — |
| Model used | Fixed‐effect model | ||
| Evidence of inconsistency | There is no evidence of inconsistency since the difference in DIC between consistency and inconsistency models was not significant. | ||
| Blood loss (litres) | |||
| Treatment number | Treatment name | ||
| 1 | Control | ||
| 2 | ConHVE | ||
| 3 | ConPTC | ||
| 4 | ConSelectiveHVE | ||
| 5 | ConSelectivePTC | ||
| 6 | IntPTC | ||
| 7 | IntSelectivePTC | ||
| Fixed‐effect model | Random‐effects model | Inconsistency model | |
| Dbara | −45.73 | −61.66 | −63.13 |
| pDb | 22.01 | 29.37 | 30.58 |
| DICc | −23.72 | −32.29 | −32.55 |
| d[2]d | −0.36 (95% CrI −0.50 to −0.23) | −0.37 (95% CrI −0.94 to 0.22) | — |
| d[3]e | −0.02 (95% CrI −0.12 to 0.07) | −0.14 (95% CrI −0.52 to 0.14) | — |
| d[4]f | −0.27 (95% CrI −0.54 to −0.01) | −0.39 (95% CrI −1.16 to 0.27) | — |
| d[5]g | 0.09 (95% CrI −0.04 to 0.21) | 0.00 (95% CrI −0.57 to 0.45) | — |
| d[6]h | 0.01 (95% CrI −0.05 to 0.07) | −0.06 (95% CrI −0.39 to 0.17) | — |
| d[7]i | 0.00 (95% CrI −0.21 to 0.2) | −0.18 (95% CrI −0.84 to 0.30) | — |
| Between‐study standard deviation | — | 0.2539 | — |
| Model used | Random‐effects model | ||
| Evidence of inconsistency | There is no evidence of inconsistency since the difference in DIC between consistency and inconsistency models was not significant. | ||
| Con: continuous; Int: intermittent; HVE: hepatic vascular exclusion; PTC: portal triad clamping. | |||
aDBar = posterior mean of deviance. bpD = effective number of parameters. cDIC = deviance information criterion. dd[2] indicates effect estimate (mean difference) of treatment 2 versus treatment 1. ed[3] indicates effect estimate (mean difference) of treatment 3 versus treatment 1. fd[4] indicates effect estimate (mean difference) of treatment 4 versus treatment 1. gd[5] indicates effect estimate (mean difference) of treatment 5 versus treatment 1. hd[6] indicates effect estimate (mean difference) of treatment 6 versus treatment 1. id[7] indicates effect estimate (mean difference) of treatment 7 versus treatment 1.
We reported estimates of treatment effects (ORs for binary outcomes, MDs for continuous outcomes, and rate ratios for count outcomes). We calculated the 95% credible intervals of treatment effects (e.g. odd ratios for binary outcomes, mean differences for continuous outcomes, and so on) in the Bayesian meta‐analysis and indicate that the average effect in the population lies within the credible intervals with 95% probability. We used the posterior median as the point estimate of treatment effect, the posterior 2.5 percentile as the lower bounds of its 95% credible interval, and the 97.5 percentile as the upper bounds, and we reported the effect estimates and associated 95% credible intervals for each pair‐wise comparison in a table. We presented these in Table 17, Table 18, and Table 19. We also presented the cumulative probability of the treatment ranks (i.e. the probability that the treatment is within the top two, top three, etc.) in SUCRA graphs (Salanti 2011). We also plotted the probability of each rank for each treatment (rankograms), which are generally considered more informative (Salanti 2011; Dias 2012a; Dias 2013b).
9. Cardiopulmonary interventions: pair‐wise comparisonsa,b.
| Blood transfusion (red blood cell) (units) | ||||
| Acute normovolemic haemodilution | Acute normovolemic haemodilution plus hypotension | Acute normovolemic haemodilution plus low central venous pressure | Low central venous pressure | |
| Control | MD −1.26; 95% CrI −4.92 to 2.39 | MD −1.68; 95% CrI −5.33 to 1.98 | MD −0.57; 95% CrI −3.35 to 1.88 | MD −1.08; 95% CrI −3.43 to 1.13 |
| Acute normovolemic haemodilution | — | MD −0.42; 95% CrI −5.59 to 4.75 | MD 0.69; 95% CrI −3.80 to 5.18 | MD 0.18; 95% CrI −4.12 to 4.49 |
| Acute normovolemic haemodilution plus hypotension | — | — | MD 1.11; 95% CrI −3.39 to 5.60 | MD 0.60; 95% CrI −3.71 to 4.91 |
| Acute normovolemic haemodilution plus low central venous pressure | — | — | — | MD −0.51; 95% CrI −3.97 to 2.96 |
| Blood loss (litres) | ||||
| Acute normovolemic haemodilution | Acute normovolemic haemodilution plus hypotension | Acute normovolemic haemodilution plus low central venous pressure | Hypoventilation | |
| Control | MD 0.00; 95% CrI −0.95 to 0.96 | MD −0.25; 95% CrI −1.20 to 0.71 | MD −0.10; 95% CrI −0.88 to 0.46 | MD −0.01; 95% CrI −1.44 to 1.43 |
| Acute normovolemic haemodilution | — | MD −0.25; 95% CrI −1.60 to 1.10 | MD −0.11; 95% CrI −1.27 to 1.06 | MD −0.01; 95% CrI −1.73 to 1.71 |
| Acute normovolemic haemodilution plus hypotension | — | — | MD 0.14; 95% CrI −1.02 to 1.31 | MD 0.24; 95% CrI −1.48 to 1.96 |
| Acute normovolemic haemodilution plus low central venous pressure | — | — | — | MD 0.10; 95% CrI −1.49 to 1.68 |
| Hypoventilation | — | — | — | — |
aThe table provides the effect estimate of each pair‐wise comparison. To identify the effect estimate of a comparison (e.g. A versus B), look at the cell that occupies the column corresponding to treatment A and the row corresponding to treatment B. This gives the information directly. If that cell is empty (indicated by a '—', you have to look at column corresponding to treatment B and row corresponding to treatment A. You will have to take the inverse of this number (i.e. 1/number) to get the treatment effect. bTreatment effects with evidence of difference are shown by italics (not applicable).
10. Parenchymal transection methods: pair‐wise comparisonsa,b.
| Adverse events (proportion) | ||||
| Cavitron ultrasonic surgical aspirator | Hydrojet | Radiofrequency dissecting sealer | Sharp transection method | |
| Clamp‐crush method | OR 2.15; 95% CrI 0.11 to 108.74 | OR 0.57; 95% CrI 0.00 to 732.89 | OR 1.20; 95% CrI 0.05 to 33.05 | OR 1.11; 95% CrI 0.00 to 331.29 |
| Cavitron ultrasonic surgical aspirator | — | OR 0.27; 95% CrI 0.00 to 501.34 | OR 0.56; 95% CrI 0.01 to 62.38 | OR 0.52; 95% CrI 0.00 to 398.54 |
| Hydrojet | — | — | OR 2.12; 95% CrI 0.00 to 3638.36 | OR 1.94; 95% CrI 0.00 to 12959.09 |
| Radiofrequency dissecting sealer | — | — | — | OR 0.92; 95% CrI 0.00 to 638.06 |
| Sharp transection method | — | — | — | ‐ |
| Adverse events (number) | ||||
| Cavitron ultrasonic surgical aspirator | Hydrojet | Radiofrequency dissecting sealer | Sharp transection method | |
| Clamp‐crush method | rate ratio 1.60; 95% CrI 0.92 to 2.79 | rate ratio 1.40; 95% CrI 0.49 to 3.63 | rate ratio 1.84; 95% CrI 1.13 to 3.06 | rate ratio 1.13; 95% CrI 0.57 to 2.24 |
| Cavitron ultrasonic surgical aspirator | — | rate ratio 0.88; 95% CrI 0.28 to 2.75 | rate ratio 1.15; 95% CrI 0.54 to 2.42 | rate ratio 0.71; 95% CrI 0.29 to 1.71 |
| Hydrojet | — | — | rate ratio 1.31; 95% CrI 0.43 to 4.01 | rate ratio 0.81; 95% CrI 0.24 to 2.71 |
| Radiofrequency dissecting sealer | — | — | — | rate ratio 0.62; 95% CrI 0.26 to 1.44 |
| Sharp transection method | — | — | — | — |
| Blood transfusion (proportion) | ||||
| Cavitron ultrasonic surgical aspirator | Hydrojet | Radiofrequency dissecting sealer | Sharp transection method | |
| Clamp‐crush method | OR 1.48; 95% CrI 0.54 to 4.13 | OR 1.73; 95% CrI 0.47 to 6.25 | OR 1.09; 95% CrI 0.61 to 1.97 | OR 0.80; 95% CrI 0.31 to 2.03 |
| Cavitron ultrasonic surgical aspirator | — | OR 1.17; 95% CrI 0.23 to 6.05 | OR 0.74; 95% CrI 0.23 to 2.39 | OR 0.54; 95% CrI 0.14 to 2.15 |
| Hydrojet | — | — | OR 0.63; 95% CrI 0.15 to 2.61 | OR 0.46; 95% CrI 0.09 to 2.27 |
| Radiofrequency dissecting sealer | — | — | — | OR 0.73; 95% CrI 0.24 to 2.21 |
aThe table provides the effect estimate of each pair‐wise comparison. To identify the effect estimate of a comparison (e.g. A versus B), look at the cell that occupies the column corresponding to treatment A and the row corresponding to treatment B. This gives the information directly. If that cell is empty (indicated by a '—', you have to look at column corresponding to treatment B and row corresponding to treatment A. You will have to take the inverse of this number (i.e. 1/number) to get the treatment effect. bTreatment effects with evidence of difference are shown by italics (not applicable).
11. Vascular occlusion methods: pair‐wise comparisonsa,b.
| Serious adverse events (proportion) | |||||
| ConHVE | ConPTC | ConSelectiveHVE | ConSelectivePTC | IntPTC | |
| Control | OR 2.27; 95% CrI 0.18 to 33.05 | OR 1.42; 95% CrI 0.28 to 7.09 | OR 0.14; 95% CrI 0.00 to 4.37 | OR 0.53; 95% CrI 0.10 to 2.65 | OR 1.16; 95% CrI 0.54 to 2.51 |
| ConHVE | — | OR 0.63; 95% CrI 0.03 to 13.31 | OR 0.06; 95% CrI 0.00 to 15.06 | OR 0.23; 95% CrI 0.01 to 5.02 | OR 0.51; 95% CrI 0.03 to 7.68 |
| ConPTC | — | — | OR 0.10; 95% CrI 0.00 to 16.28 | OR 0.37; 95% CrI 0.04 to 3.70 | OR 0.82; 95% CrI 0.14 to 4.86 |
| ConSelectiveHVE | — | — | — | Not estimable | Not estimable |
| ConSelectivePTC | — | — | — | — | OR 2.19; 95% CrI 0.36 to 13.26 |
| Adverse events (proportion) | |||||
| ConHVE | ConPTC | ConSelectiveHVE | ConSelectivePTC | IntPTC | |
| Control | OR 2.58; 95% CrI 0.81 to 8.30 | OR 2.30; 95% CrI 1.00 to 5.41 | OR 1.06; 95% CrI 0.31 to 3.58 | OR 1.11; 95% CrI 0.45 to 2.75 | OR 1.28; 95% CrI 0.83 to 1.97 |
| ConHVE | — | OR 0.89; 95% CrI 0.21 to 3.75 | OR 0.41; 95% CrI 0.08 to 2.22 | OR 0.43; 95% CrI 0.10 to 1.88 | OR 0.49; 95% CrI 0.14 to 1.71 |
| ConPTC | — | — | OR 0.46; 95% CrI 0.10 to 2.04 | OR 0.48; 95% CrI 0.14 to 1.67 | OR 0.55; 95% CrI 0.21 to 1.43 |
| ConSelectiveHVE | — | — | — | OR 1.05; 95% CrI 0.23 to 4.84 | OR 1.21; 95% CrI 0.33 to 4.45 |
| ConSelectivePTC | — | — | — | — | OR 1.15; 95% CrI 0.42 to 3.16 |
| IntPTC | — | — | — | — | — |
| Blood transfusion (proportion) | |||||
| ConHVE | ConPTC | ConSelectiveHVE | ConSelectivePTC | IntPTC | |
| Control | OR 0.06; 95% CrI 0.00 to 4.33 | OR 0.33; 95% CrI 0.02 to 3.59 | OR 0.17; 95% CrI 0.00 to 12.59 | OR 0.62; 95% CrI 0.02 to 15.18 | OR 0.63; 95% CrI 0.10 to 3.59 |
| ConHVE | — | Not estimable | Not estimable | Not estimable | Not estimable |
| ConPTC | — | — | OR 0.51; 95% CrI 0.00 to 83.52 | Not estimable | OR 1.89; 95% CrI 0.09 to 41.17 |
| ConSelectiveHVE | — | — | — | Not estimable | Not estimable |
| ConSelectivePTC | — | — | — | — | OR 1.01; 95% CrI 0.02 to 42.32 |
| IntPTC | — | — | — | — | — |
| Blood transfusion (red blood cell) | |||||
| ConHVE | ConPTC | ConSelectiveHVE | ConSelectivePTC | IntPTC | |
| Control | MD −1.65; 95% CrI −3.96 to 0.67 | MD −1.25; 95% CrI −2.39 to −0.10 | MD −2.45; 95% CrI −4.08 to −0.82 | MD −1.45; 95% CrI −2.59 to −0.31 | MD −1.36; 95% CrI −2.48 to −0.23 |
| ConHVE | — | MD 0.40; 95% CrI −2.18 to 2.98 | MD −0.80; 95% CrI −3.64 to 2.03 | MD 0.20; 95% CrI −2.39 to 2.78 | MD 0.29; 95% CrI −2.29 to 2.86 |
| ConPTC | — | — | MD −1.20; 95% CrI −3.20 to 0.79 | MD −0.20; 95% CrI −1.82 to 1.42 | MD −0.11; 95% CrI −1.72 to 1.50 |
| ConSelectiveHVE | — | — | — | MD 1.00; 95% CrI −0.99 to 2.99 | MD 1.09; 95% CrI −0.89 to 3.07 |
| ConSelectivePTC | — | — | — | — | MD 0.09; 95% CrI −1.51 to 1.70 |
| IntPTC | — | — | — | — | — |
| Blood loss | |||||
| ‐ | ConHVE | ConPTC | ConSelectiveHVE | ConSelectivePTC | IntPTC |
| Control | MD −0.37; 95% CrI −0.94 to 0.22 | MD −0.14; 95% CrI −0.52 to 0.14 | MD −0.39; 95% CrI −1.16 to 0.27 | MD 0.00; 95% CrI −0.57 to 0.45 | MD −0.06; 95% CrI −0.39 to 0.17 |
| ConHVE | — | MD 0.23; 95% CrI −0.44 to 0.90 | MD −0.02; 95% CrI −0.94 to 0.90 | MD 0.37; 95% CrI −0.41 to 1.14 | MD 0.31; 95% CrI −0.34 to 0.95 |
| ConPTC | — | — | MD −0.25; 95% CrI −1.04 to 0.54 | MD 0.14; 95% CrI −0.47 to 0.74 | MD 0.08; 95% CrI −0.35 to 0.52 |
| ConSelectiveHVE | — | — | — | MD 0.39; 95% CrI −0.49 to 1.26 | MD 0.33; 95% CrI −0.44 to 1.10 |
| ConSelectivePTC | — | — | — | — | MD −0.06; 95% CrI −0.64 to 0.52 |
| IntPTC | — | — | — | — | — |
| Con: continuous; Int: intermittent; HVE: hepatic vascular exclusion; PTC: portal triad clamping. | |||||
aThe table provides the effect estimate of each pair‐wise comparison. To identify the effect estimate of a comparison (e.g. A versus B), look at the cell that occupies the column corresponding to treatment A and the row corresponding to treatment B. This gives the information directly. If that cell is empty (indicated by a ' —', you have to look at column corresponding to treatment B and row corresponding to treatment A. You will have to take the inverse of this number (i.e. 1/number) to get the treatment effect. bTreatment effects with evidence of difference are shown by italics.
Sample size calculations and imprecision
To control for the risk of random errors, we interpreted the information with caution when the accrued sample size in the meta‐analysis was less than the required sample size (required information size). For calculation of the required information size, please see Appendix 7. We considered a 20% relative risk reduction as the minimal clinically important difference for binary outcomes and count outcomes. For continuous outcomes, we used or planned to use the following minimal clinically important differences: a standardised mean difference of 0.5 for health‐related quality of life, a mean difference of one unit for blood transfusion quantity, a mean difference of 500 mL for blood loss, a mean difference of one day of hospital stay and time‐to‐return to activity, and a mean difference of 15 minutes for operating time.
Subgroup analysis and investigation of heterogeneity
We planned to assess the differences in the effect estimates between the following subgroups using meta‐regression with the help of the WinBUGS code if we included a sufficient number of trials (Appendix 8). We planned to use study level co‐variates for meta‐regression.
Trials at low risk of bias compared to trials at
high risk of bias.
Participants with cirrhosis compared to those without cirrhosis.
Participants undergoing major liver resections compared to those undergoing minor liver resections.
We planned to calculate the interaction term (Dias 2012b; Dias 2013d). If the 95% credible intervals of the interaction term did not cross zero, we planned to consider this statistically significant. We did not perform any of the above because of the paucity of data.
Sensitivity analysis
We performed a sensitivity analysis when we imputed the mean, the standard deviation, or both.
Summary of findings table
We presented a 'Summary of findings' table, similar to the ones used in direct comparisons. We modified the table from the original format because of the presence of many comparisons and many outcomes. We presented only the comparisons in which there was evidence of differences with the illustrative examples. For other comparisons, we simply mentioned that there was no evidence of differences. This is to ensure that the most important information is available in the table. We provided links in the table to specific tables using more a traditional format.
In addition to this 'Summary of findings' table, we also provided the 'Summary of findings' table for network meta‐analysis in a graphical format (in the form of forest plots along with the quality of evidence), in which we used the methodology of grading the quality of evidence in network meta‐analysis suggested by the GRADE Working group (Puhan 2014). The first step was to estimate the evidence from direct and indirect effect estimates. Further steps included rating the quality of evidence from direct and indirect effect estimates, presenting the estimate combined from the direct estimate and indirect estimate, and rating the quality of the network meta‐analysis effect estimates (Puhan 2014). Although codes are available for node splitting, they resulted in numerical errors because of the data. So we calculated the direct estimates (including only the trials which compared the specific intervention and control) and indirect estimates (after removing the trials which compared the specific intervention and control).
Results
Description of studies
Results of the search
We identified 2938 references through electronic searches of CENTRAL (N = 342), MEDLINE (N = 1431), Embase (N = 445), Science Citation Index Expanded (N = 641), WHO ICTRP (N = 47), and ClinicalTrials.gov (N = 32). We excluded 893 duplicates and 1883 clearly irrelevant references through screening titles and reading abstracts. We retrieved 162 references for further assessment. We did not identify any references by scanning reference lists of the identified randomised trials. We excluded 76 references (67 studies) for the reasons listed in the Characteristics of excluded studies table. In total, 83 references for 67 completed randomised clinical trials met the inclusion criteria. Two references were for ongoing studies (Schmidt 2008; Chen 2015). We were unable to obtain one reference (Franceschi 2006). We included three studies under 'Studies awaiting classification' because there were no separate data for people who underwent liver resection, that is, the studies included a number of different surgical procedures, and information on people who underwent liver resection was not available (Chapman 2006; Bochicchio 2015; Wright 2015). This is summarised in the study flow diagram (Figure 1).
1.
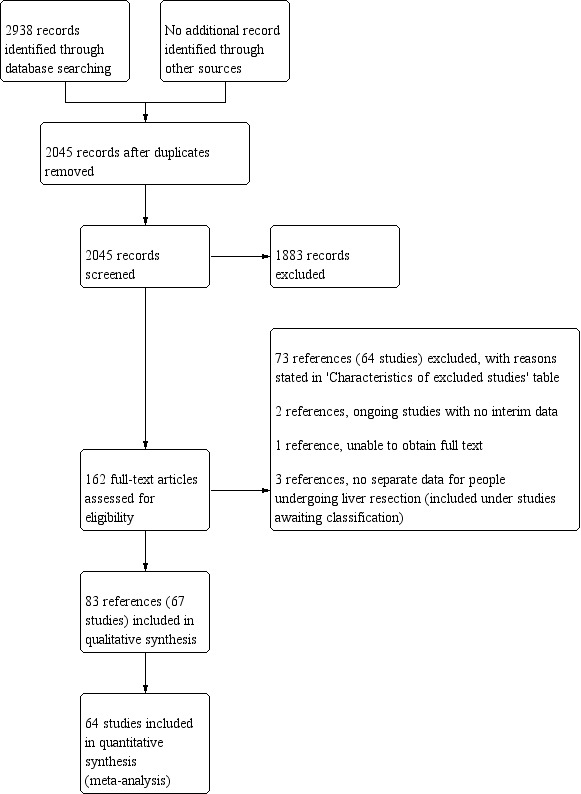
Study flow diagram.
Included studies
We describe the treatments used in the 67 randomised clinical trials in the Characteristics of included studies table and in Table 20.
12. Intervention and control (ordered by category and comparisons).
| Study | Intervention | Co‐interventions | ||||||||
| Intervention | Control | Other information | Type of intervention | Vascular occlusion | Parenchymal transection method | Raw surface | Pharmacological methods | Cardiopulmonary methods | Autologous transfusion | |
| Capussotti 2012 | Anterior approach | Control | — | Anterior approach | Not stated | Clamp‐crush, bipolar dissecting sealer | Not stated | Not stated | Not stated | Not stated |
| Liu 2006 | Anterior approach | Control | — | Anterior approach | Not stated | Cavitron ultrasonic surgical aspirator | Not stated | Not stated | Not stated | Not stated |
| Kajikawa 1994 | Autologous blood donation | Control | Note: autologous blood donation group was further randomised to recombinant erythropoietin and no erythropoietin | Autologous transfusion | Not stated | Not stated | Not stated | Not stated | Not stated | Factor being randomised |
| Kostopanagiotou 2007 | Autologous blood donation | Control | Autologous blood donation: 2 units of blood were withdrawn before surgery | Autologous transfusion | Hepatic vascular exclusion | Not stated | Not stated | Not stated | Not stated | Factor being randomised |
| Guo 2013 | Acute normovolemic haemodilution plus low central venous pressure | Control | Acute normovolemic dilution plus low central venous pressure: blood withdrawn to a target of 28% haematocrit and replaced with fluid. Target for central venous pressure was not reported | Cardiopulmonary methods | Not stated | Not stated | Not stated | Not stated | Factor being randomised | Not stated |
| Jarnagin 2008 | Acute normovolemic haemodilution plus low central venous pressure | Low central venous pressure | Acute normovolemic haemodilution: blood was withdrawn and replaced by colloids and crystalloids to reach a haematocrit target of 8 gm/dL. Low central venous pressure was maintained < 5 H20 using fluid restriction and pharmacologic manipulation | Cardiopulmonary methods | Intermittent portal triad clamping | Not stated | Not stated | Not stated | Factor being randomised | Not stated |
| Matot 2002 | Acute normovolemic haemodilution plus low central venous pressure | Low central venous pressure | Acute normovolemic haemodilution: blood was withdrawn and replaced by colloids to reach a haematocrit target of 24%. Low central venous pressure was achieved by fluid restriction | Cardiopulmonary methods | Not stated | Not stated | Not stated | Not stated | Factor being randomised | Not stated |
| Yao 2006 | Acute normovolemic haemodilution | Acute normovolemic haemodilution with hypotension 3rd group: control | Acute normovolemic haemodilution: withdrawal of blood and replacement with fluids to maintain a target haematocrit of 30%. Acute normovolemic haemodilution with controlled hypotension: in addition to acute normovolemic haemodilution, sodium nitroprusside was used. Target blood pressure not known. | Cardiopulmonary methods | Not stated | Not stated | Not stated | Not stated | Factor being randomised | Not stated |
| Hasegawa 2002 | Hypoventilation | Control | — | Cardiopulmonary methods | Intermittent portal triad clamping or selective occlusion | Clamp crush or cavitron ultrasonic surgical aspirator | Not stated | Not stated | Factor being randomised | None |
| Choi 2007 | Low central venous pressure | Control | Low central venous pressure: by restricting flow from legs | Cardiopulmonary methods | Not stated | Not stated | Not stated | Not stated | Factor being randomised | Not stated |
| El‐Kharboutly 2004 | Low central venous pressure | Control | Low central venous pressure: nitroglycerine | Cardiopulmonary intervention | Intermittent portal triad clamping | Not stated | Not stated | Not stated | Factor being randomised | Not stated |
| Kato 2008 | Low central venous pressure | Control | Low central venous pressure: by inferior IVC clamping | Cardiopulmonary methods | Intermittent portal triad clamping | Cavitron ultrasonic surgical aspirator | Fibrin glue used | Not stated | Factor being randomised | Not stated |
| Wang 2006 | Low central venous pressure | Control | Low central venous pressure: by limiting fluid, nitroglycerine, and furosemide | Cardiopulmonary methods | Varied | Clamp‐crush | Not stated | Not stated | Factor being randomised | Not stated |
| Guo 2014 | Low central venous pressure | Low central venous pressure + acute normovolemic haemodilution. 3rd group: control | Low central venous pressure: fluid restriction and nitroglycerine. Acute normovolemic haemodilution plus low central venous pressure: withdrawal of blood to a target haematocrit of 30% and replacement with colloids | Cardiopulmonary methods | Not stated | Not stated | Not stated | Not stated | Factor being randomised | Not stated |
| Rahbari 2014 | Stapler | Clamp‐crush method | Stapler: Autosuture EndoGIA stapler (Covidien) | Parenchumal transection | Variable | Factor being randomised | Variable | Not stated | Low central venous pressure | Not stated |
| Koo 2005 | Cavitron ultrasonic surgical aspirator | Clamp‐crush method | — | Parenchymal transection | No vascular occlusion | Factor being randomised | Not stated | Not stated | Not stated | Not stated |
| Takayama 2001 | Cavitron ultrasonic surgical aspirator | Clamp‐crush method | — | Parenchymal transection | Intermittent total or selective portal triad clamping | Factor being randomised | Fibrin glue used | Not stated | Not stated | Not stated |
| Doklestic 2012 | Cavitron ultrasonic surgical aspirator | Clamp‐crush method 3rd group: radiofrequency dissecting sealer | Ultrasonic dissector: cavitron ultrasonic surgical aspirator. Radiofrequency dissecting sealer: Ligasure | Parenchymal transection | Intermittent portal triad clamping | Factor being randomised | Not stated | Not stated | Low central venous pressure | Not stated |
| Rau 2001 | Cavitron ultrasonic surgical aspirator | Hydrojet | Hydrojet: Jet Cutter | Parenchymal transection | Portal triad clamping | Factor being randomised | Variable | Not stated | Not stated | Not stated |
| Savlid 2013 | Cavitron ultrasonic surgical aspirator | Stapler | Stapler: Endostapler (Covidien) | Parenchymal transection | Variable | Factor being randomised | Not stated | Not stated | Not stated | Not stated |
| Lesurtel 2005 | Cavitron ultrasonic surgical aspirator | Radiofrequency dissecting sealer. 3rd group: hydrojet | Radiofrequency dissecting sealer: Tissue Link Hydrojet: Helix Hydro‐Jet A 4th group with clamp‐crush and vascular occlusion was excluded since there was difference in the co‐intervention between the groups | Parenchymal transection | No vascular occlusion | Factor being randomised | Not stated | Not stated | Not stated | Not stated |
| Ikeda 2009 | Radiofrequency dissecting sealer | Clamp‐crush method | Radiofrequency dissecting sealer: Ligasure | Parenchymal transection | Intermittent portal triad clamping or hemihepatic occlusion | Factor being randomised | Not stated | Not stated | Not stated | No |
| Lupo 2007 | Radiofrequency dissecting sealer | Clamp‐crush method | Radiofrequency dissecting sealer: Radionics needles | Parenchymal transection | No vascular occlusion | Factor being randomised | Not stated | Not stated | Not stated | Not stated |
| Muratore 2014 | Radiofrequency dissecting sealer | Clamp‐crush method | Radiofrequency dissecting sealer: Ligasure (Covidien) | Parenchymal transection | Not stated | Factor being randomised | No fibrin glue used | Not stated | Low central venous pressure | Not stated |
| Arita 2005 | Radio‐frequency dissecting sealer | Clamp‐crush method | Radio‐frequency dissecting sealer: Tissue Link (Valley Lab) | Parenchymal transection | Variable | Factor being randomised | Not stated | Not stated | Not stated | Not stated |
| Smyrniotis 2005 | Sharp transection | Clamp‐crush method | Sharp transection: using scalpel | Parenchymal transection | Selective hepatic vascular exclusion | Factor being randomised | Not stated | Not stated | Low central venous pressure | Not stated |
| Shimada 1994 | Anti‐thrombin III concentrate | Control | Anti‐thrombin concentrate: 1500 IU IV over 30 min: immediately before the operation, just before hepatic division, and immediately after operation | Pharmacological methods | Not stated | Not stated | Not stated | Factor being randomised | Not stated | Not stated |
| Lentschener 1997 | Aprotinin | Control | Aprotinin: Loading dose: 2 X 106 kIU of aprotinin over a 20 min period after induction of anaesthesia. Continuous infusion: 5 x 105 kIU per hour administered by an infusion pump until skin closure Additional bolus: 5 X 105 KIU of aprotinin was infused every three transfused red b10od cell (red blood cell) packs Control: placebo | Pharmacological methods | Intermittent portal triad clamping | Kelly clamp | Fibrin glue used | Factor being randomised | None | Not stated |
| Wong 2003 | Desmopressin | Control | Desmopressin: 30 mcg/kg shortly after induction Control: placebo | Pharmacological methods | Varied | Cavitron ultrasonic surgical aspirator | Not stated | Factor being randomised | Not stated | Not stated |
| Lodge 2005 | Recombinant factor VIIa | Control | Recombinant factor VIIa: 1st dose: slow intravenous injection (20 mcg/kg or 80 mcg/kg) within 5 min before incision. 2nd dose: identical dose was given 5 h after incision if the surgery time was anticipated to exceed 6 hours Control: placebo | Pharmacological methods | Mixture of methods | Not stated | No fibrin glue used | Factor being randomised | Not stated | No |
| Shao 2006 | Recombinant factor VIIa | Control | Recombinant factor VIIa: brand not stated Dose: 50 or 100 mcg/kg before skin incision over 2 minutes and repeated every 2 hours until a maximum of 4 doses Control: placebo | Pharmacological methods | Not stated | Not stated | Not stated | Factor being randomised | Not stated | Not stated |
| Wu 2006 | Tranexamic acid | Control | Tranexamic acid: 500 mg just before the surgery followed by 250 mg 4x/day for 3 days | Pharmacological methods | Varied | Clamp‐crush method | Not stated | Factor being randomised | Not stated | Not stated |
| Chapman 2000 | Collagen | Fibrin sealant | Collagen: Instat (Johnson & Johnson) Fibrin sealant: Costasis (Cohesion Technologies) ‐ bovine thrombin and collagen combined with patient's own plasma | Raw surface | Not stated | Not stated | Factor being randomised | Not stated | Not stated | Not stated |
| Franceschi 2006 | Collagen | Fibrin sealant | Collagen: Instat (Ethicon) Fibrin sealant: CryoSeal FS | Raw surface | Not stated | Not stated | Factor being randomised | Not stated | Not stated | Not stated |
| Kohno 1992 | Collagen | Fibrin sealant | Collagen: Avitene (Alcon Inc). Fibrin sealant: Beriplast P (Beringwerke AB) | Raw surface | Not stated | Not stated | Factor being randomised | Not stated | Not stated | Not stated |
| Moench 2014 | Collagen | Fibrin sealant | Collagen: Sangustop fleece (Aesculap AG). Fibrin‐based haemostat: Tachosil (Nycomed) | Raw surface | Not stated | A number of parenchymal transection techniques | Factor being randomised | None | Not stated | Not stated |
| Fischer 2011 | Fibrin sealant | Argon beam coagulator | Fibrin sealant: Tacchosil (Nycomed) | Raw surface | A mixture of approaches | A mixture of approaches | Factor being randomised | Not stated | Not stated | Not stated |
| Frilling 2005 | Fibrin sealant | Argon beam coagulator | Fibrin sealant: Tacchosil | Raw surface | Not stated | A mixture of approaches | Factor being randomised | Not stated | Not stated | Not stated |
| Bektas 2014 | Fibrin sealant | Control | Fibrin sealant: TISSEEL (Baxter Health Corporation) Spray; 5 mL of fibrinogen with synthetic aprotinin and 5 mL of thrombin (500 IU/mL) | Raw surface | Intermittent portal triad clamping | Different types of liver resection | Factor being randomised | Not stated | Not stated | Not stated |
| De Boer 2012 | Fibrin sealant | Control | Fibrin sealant: Quixil (Johnson & Johnson Medical) spray; 5 mL of fibrinogen and tranexamic acid and 5 mL of thrombin | Raw surface | With and without inflow occlusion | Clamp‐crush, cavitron ultrasonic surgical aspirator, electric coagulation based, combined | Factor being randomised | Not stated | Not stated | Not stated |
| Liu 1993 | Fibrin sealant | Control | Fibrin sealant: name not available | Raw surface | Not stated | Not stated | Factor being randomised | Not stated | Not stated | Not stated |
| Noun 1996 | Fibrin sealant | Control | Fibrin sealant: Biocol | Raw surface | Varied | Clamp‐crush method or cavitron ultrasonic surgical aspirator | Factor being randomised | Not stated | Not stated | Not stated |
| Porte 2012 | Fibrin sealant | Gelatin | Fibrin sealant: Fibrocaps (ProFibrix) | Raw surface | Not stated | Not stated | Factor being randomised | Not stated | Not stated | Not stated |
| Genyk 2014 | Fibrin sealant | Oxidised cellulose | Fibrin sealant: Tacchosil Oxidised cellulose: Surgicel | Raw surface | Not stated | Not stated | Factor being randomised | Not stated | Not stated | Not stated |
| Koea 2013 | Fibrin sealant | Oxidised cellulose | Fibrin sealant: Fibrin Pad Oxidised cellulose: no further details | Raw surface | Not stated | Not stated | Factor being randomised | Not stated | Not stated | Not stated |
| Ollinger 2013 | Fibrin sealant | Oxidised cellulose | Fibrin sealant: Tachosil (Nycomed) Oxidised cellulose: Veriset (Covidien) | Raw surface | Varied | Not stated | Factor being randomised | Not stated | Not stated | Not stated |
| Kakaei 2013 | Fibrin sealant | Oxidised cellulose 3rd group: cyanoacrylate | Oxidised cellulose: Surgicel (Ethicon Inc) Cyanoacrylate: Glubran 2 (GEM SRL) Fibrin sealant: Tachosil (Takeda Pharmaceuticals) | Raw surface | Not stated | Clamp‐crush method | Factor being randomised | Not stated | Not stated | Not stated |
| Gugenheim 2011 | Fibrin sealant | PlasmaJet coagulator | Fibrin sealant: fibrin glue (no further details) | Raw surface | Not stated | Cavitron ultrasonic surgical aspirator | Factor being randomised | Not stated | Not stated | Not stated |
| Figueras 2007 | Fibrin sealant plus collagen | Control | Fibrin sealant spray: Tissucol Collagen: collagen sponge (Johnson & Johnson) Note: In both groups, bleeding from raw surface was controlled using argon beam coagulator or Tissuelink | Raw surface | Intermittent portal triad or selective clamping | Cavitron ultrasonic surgical aspirator | Factor being randomised | Not stated | Not stated | Not stated |
| Belghiti 1996 | Continuous portal triad clamping | Continuous hepatic vascular exclusion | Hepatic vascular exclusion by encircling the entire retrohepatic inferior vena cava | Vascular occlusion | Factor being randomised | Clamp‐crush or cavitron ultrasonic surgical aspirator | Fibrin glue used | Not stated | Not stated | Not stated |
| Chen 2006 | Continuous portal triad clamping | Continuous hepatic vascular exclusion | Hepatic vascular exclusion by encircling the entire infrahepatic inferior vena cava | Vascular occlusion | Factor being randomised | Clamp‐crush method | Not stated | Not stated | Not stated | Not stated |
| Si‐Yuan 2014 | Continuous portal triad clamping | Continuous selective hepatic vascular exclusion | — | Vascular occlusion | Factor being randomised | Not stated | Not stated | Not stated | Low central venous pressure | Not stated |
| Ni 2013 | Continuous portal triad clamping | Continuous selective portal triad clamping | — | Vascular occlusion | Factor being randomised | Clamp‐crush method | Not stated | Not stated | Low central venous pressure | Not stated |
| Chouker 2004 | Continuous portal triad clamping | Control | — | Vascular occlusion | Factor being randomised | Not stated | Not stated | Not stated | Not stated | Not stated |
| Clavien 1996 | Continuous portal triad clamping | Control | Note: After every 1 hour of continuous portal triad clamping (or 30 minutes for cirrhotic patients), the clamp was released for 10 minutes before reclamping | Vascular occlusion | Factor being randomised | Not stated | Not stated | Not stated | Not stated | Not stated |
| Dayangac 2010 | Continuous portal triad clamping | Control | — | Vascular occlusion | Factor being randomised | Not stated | Not stated | Not stated | Not stated | Not stated |
| Pietsch 2010 | Continuous portal triad clamping | Control | — | Vascular occlusion | Factor being randomised | Not stated | Not stated | Not stated | Not stated | Not stated |
| Belghiti 1999 | Continuous portal triad clamping | Intermittent portal triad clamping | Continuous portal triad clamping: until end of transection Intermittent portal triad clamping: 15 minutes on and 5 minutes off until hepatectomy | Vascular occlusion | Factor being randomised | Cavitron ultrasonic surgical aspirator | Not stated | Not stated | Low central venous pressure | Not stated |
| Capussotti 2003 | Continuous portal triad clamping | Intermittent portal triad clamping | Intermittent portal triad clamping: 15 minutes on and 5 minutes off | Vascular occlusion | Factor being randomised | Clamp‐crush | Fibrin glue used | Not stated | Not stated | Not stated |
| Liang 2009 | Continuous selective portal triad clamping | Intermittent portal triad clamping | Intermittent portal triad clamping: 20 minutes on and 5 minutes off | Vascular occlusion | Factor being randomised | Clamp crush | Not stated | None | Not stated | Not stated |
| Capussotti 2006 | Intermittent portal triad clamping | Control | Intermittent portal triad clamping: 15 minutes on and 5 minutes off | Vascular occlusion | Factor being randomised | Clamp‐crush or bipolar dissecting sealer | Not stated | Not stated | Low central venous pressure | Not stated |
| Lee 2012 | Intermittent portal triad clamping | Control | Intermittent portal triad clamping: 15 minutes on and 5 minutes off | Vascular occlusion | Factor being randomised | Cavitron ultrasonic surgical aspirator | Fibrin glue used | Not stated | Low central venous pressure | Not stated |
| Man 1997 | Intermittent portal triad clamping | Control | Intermittent portal triad clamping: 20 minutes on and 5 minutes off | Vascular occlusion | Factor being randomised | Cavitron ultrasonic surgical aspirator | Not stated | Not stated | Not stated | Not stated |
| Man 2003 | Intermittent portal triad clamping | Control | Intermittent portal triad clamping: 20 minutes on and 5 minutes off (until resection is completed or a maximum of 6 cycles) | Vascular occlusion | Factor being randomised | Not stated | Not stated | Not stated | Not stated | Not stated |
| Park 2012 | Intermittent portal triad clamping | Control | Intermittent portal triad clamping: 15 minutes on and 5 minutes off | Vascular occlusion | Factor being randomised | Not stated | Not stated | Not stated | Not stated | Not stated |
| Figueras 2005 | Intermittent portal triad clamping | Intermittent selective portal triad clamping | Intermittent clamping: 15 minutes on and 5 minutes off | Vascular occlusion | Factor being randomised | Not stated | Not stated | Not stated | Not stated | Not stated |
| Wu 2002 | Intermittent portal triad clamping | Intermittent selective portal triad clamping | Intermittent portal triad clamping: 15 minutes on and 5 minutes off Intermittent selective portal triad clamping: 30 minutes on and 5 minutes off | Vascular occlusion | Factor being randomised | Clamp‐crush method | Not stated | Not stated | Not stated | Not stated |
Two trials compared anterior approach versus conventional approach (Liu 2006; Capussotti 2012). Two trials compared autologous blood donation versus control (Kajikawa 1994; Kostopanagiotou 2007). Ten trials compared different methods of cardiopulmonary interventions (Hasegawa 2002; Matot 2002; El‐Kharboutly 2004; Wang 2006; Yao 2006; Choi 2007; Jarnagin 2008; Kato 2008; Guo 2013; Guo 2014). Twelve trials different compared methods of parenchymal transection (Takayama 2001; Rau 2001; Arita 2005; Koo 2005; Lesurtel 2005; Smyrniotis 2005; Lupo 2007; Ikeda 2009; Doklestic 2012; Savlid 2013; Muratore 2014; Rahbari 2014). Seventeen trials compared different methods of dealing with raw surface (Kohno 1992; Liu 1993; Noun 1996; Chapman 2000; Frilling 2005; Franceschi 2006; Figueras 2007; Fischer 2011; Gugenheim 2011; De Boer 2012; Porte 2012; Kakaei 2013; Koea 2013; Ollinger 2013; Bektas 2014; Genyk 2014; Moench 2014). Eighteen trials compared different methods of vascular occlusion (Belghiti 1996; Clavien 1996; Man 1997; Belghiti 1999; Wu 2002; Capussotti 2003; Man 2003; Chouker 2004; Figueras 2005; Capussotti 2006; Chen 2006; Liang 2009; Dayangac 2010; Pietsch 2010; Lee 2012; Park 2012; Ni 2013; Si‐Yuan 2014). Six trials compared different pharmacological interventions (Shimada 1994; Lentschener 1997; Wong 2003; Lodge 2005; Shao 2006; Wu 2006).
All the trials assessed different methods of open liver resection. Four trials were three‐armed trials (Yao 2006; Doklestic 2012; Kakaei 2013; Guo 2014), one trial was a four‐armed trial of which we included three arms (Lesurtel 2005), and the remaining trials were two‐armed trials. The 67 trials involved a total of 6197 participants. After exclusion of 133 participants after randomisation and 293 participants in three trials that did not provide any information about the outcomes included in this review (Franceschi 2006; Porte 2012; Koea 2013), we included 5771 participants who contributed to one or more outcomes of interest in this review.
Excluded studies
Of the 64 excluded studies, we excluded 6 because they were comments on included or excluded studies (Gonzalez 2009; Petras 2009; Schilling 2009; Strobel 2012; Strobel 2014; Hamady 2015); 19 because they were not randomised clinical trials (Le Treut 1995; Man 2002; Yin 2003; Azoulay 2005; Arru 2007; Kim 2008; Nagano 2009; Wang 2010; Wang 2011; Bellolio 2012; Beppu 2012; Narita 2012; NCT01651182; Palibrk 2012; Yang 2012; Dominioni 2014; Vlad 2014; Li 2015; Takatsuki 2015); 7 because of inadequate randomisation (Rau 1995; Smyrniotis 2002; Smyrniotis 2003a; Smyrniotis 2003b; Richter 2009; Obiekwe 2014; Shu 2014); 6 because they were comparisons of interventions that were not of interest to this review (Figueras 2003; Grobmyer 2009; Harimoto 2011; Levit 2012; Correa‐Gallego 2015; Feldheiser 2015); 18 since they were trials comparing variations within the treatments included in this review (for example, different periods of intermittent vascular occlusion or different methods of achieving low central venous pressure) (Standl 1998; Esaki 2006; Saiura 2006; Chapman 2007; Hashimoto 2007; Kim 2007; Torzilli 2008; El‐Moghazy 2009; Ryu 2010; Broek 2011; Rahbari 2011; Dello 2012; Zhu 2012; Frankel 2013; Kaibori 2013; Yang 2013; Saiura 2014; Zhang 2014); and 8 because the co‐interventions were not used equally in the intervention and control (Schwartz 2004; Petrowsky 2006; Smyrniotis 2006; Si‐Yuan 2011; Li 2013; Lu 2014; Gotohda 2015; Hanyong 2015).
Risk of bias in included studies
We summarise the risk of bias in the included trials in Figure 2 and Figure 3. Overall, we judged all trials to be at high risk of bias. The risk of bias according to the type of comparison is shown in Table 21.
2.
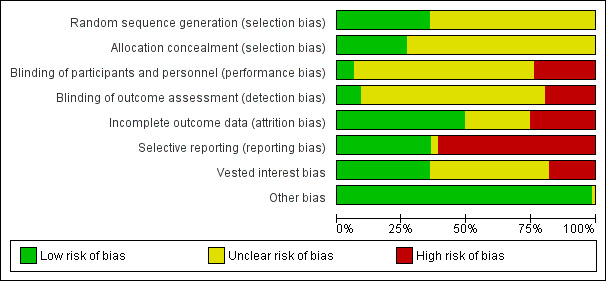
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
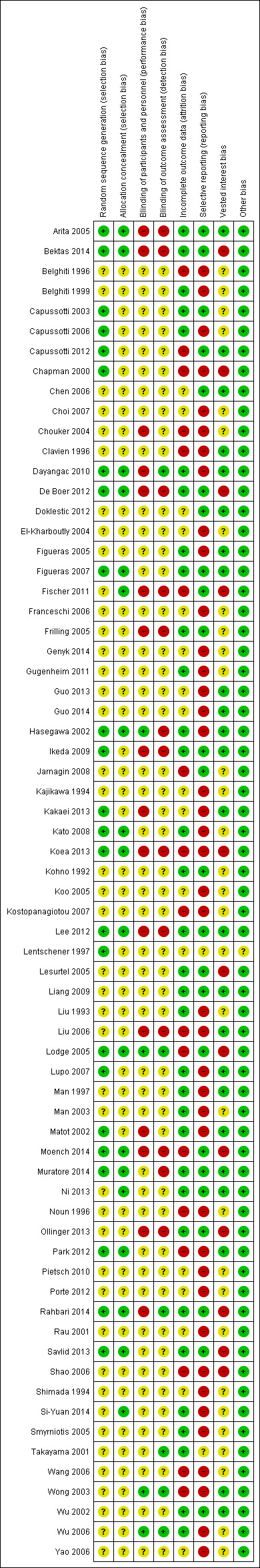
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
13. Risk of bias (ordered by category and comparisons).
| Study | Intervention | Control | Sequence generation | Allocation concealment | Blinding of participants and healthcare providers | Blinding of outcome assessors | Missing outcome bias | Selective reporting bias | Source of funding bias | Other bias | Overall risk of bias |
| Capussotti 2012 | Anterior approach | Control | Low | Unclear | Unclear | Unclear | High | Low | Low | Low | Unclear or high |
| Liu 2006 | Anterior approach | Control | Unclear | Unclear | High | High | High | High | Low | Low | Unclear or high |
| Kajikawa 1994 | Autologous blood donation | Control | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | Unclear or high |
| Kostopanagiotou 2007 | Autologous blood donation | Control | Unclear | Unclear | Unclear | Unclear | High | High | Unclear | Low | Unclear or high |
| Guo 2013 | Acute normovolemic haemodilution plus low central venous pressure | Control | Unclear | Unclear | Unclear | Unclear | Unclear | High | Low | Low | Unclear or high |
| Jarnagin 2008 | Acute normovolemic haemodilution plus low central venous pressure | Low central venous pressure | Unclear | Unclear | Unclear | Unclear | High | Low | Unclear | Low | Unclear or high |
| Matot 2002 | Acute normovolemic haemodilution plus low central venous pressure | Low central venous pressure | Low | Unclear | High | Unclear | Low | High | Low | Low | Unclear or high |
| Yao 2006 | Acute normovolemic haemodilution | Acute normovolemic haemodilution with hypotension 3rd group: control | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | Unclear or high |
| Hasegawa 2002 | Hypoventilation | Control | Low | Low | Low | High | Low | High | Low | Low | Unclear or high |
| Choi 2007 | Low central venous pressure | Control | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | Unclear or high |
| El‐Kharboutly 2004 | Low central venous pressure | Control | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | Unclear or high |
| Kato 2008 | Low central venous pressure | Control | Low | Low | Unclear | Unclear | Low | High | Unclear | Low | Unclear or high |
| Wang 2006 | Low central venous pressure | Control | Unclear | Unclear | Unclear | Unclear | High | High | Unclear | Low | Unclear or high |
| Guo 2014 | Low central venous pressure | Low central venous pressure + acute normovolemic haemodilution. 3rd group: control | Unclear | Unclear | Unclear | Unclear | Unclear | High | Low | Low | Unclear or high |
| Rahbari 2014 | Stapler | Clamp‐crush method | Low | Low | High | Low | Low | Low | High | Low | Unclear or high |
| Koo 2005 | Cavitron ultrasonic surgical aspirator | Clamp‐crush method | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | Unclear or high |
| Takayama 2001 | Cavitron ultrasonic surgical aspirator | Clamp‐crush method | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Low | Unclear or high |
| Doklestic 2012 | Cavitron ultrasonic surgical aspirator | Clamp‐crush method. 3rd group: radiofrequency dissecting sealer | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Unclear or high |
| Rau 2001 | Cavitron ultrasonic surgical aspirator | Hydrojet | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | Unclear or high |
| Savlid 2013 | Cavitron ultrasonic surgical aspirator | Stapler | Low | Low | Unclear | Unclear | Low | Low | High | Low | Unclear or high |
| Lesurtel 2005 | Cavitron ultrasonic surgical aspirator | Radiofrequency dissecting sealer. 3rd group: hydrojet | Unclear | Unclear | Unclear | Unclear | Low | Low | High | Low | Unclear or high |
| Ikeda 2009 | Radiofrequency dissecting sealer | Clamp‐crush method | Low | Unclear | High | High | Low | Low | Low | Low | Unclear or high |
| Lupo 2007 | Radiofrequency dissecting sealer | Clamp‐crush method | Low | Unclear | Unclear | Unclear | Low | High | Low | Low | Unclear or high |
| Muratore 2014 | Radiofrequency dissecting sealer | Clamp‐crush method | Low | Low | Unclear | High | Low | Low | Low | Low | Unclear or high |
| Arita 2005 | Radio‐frequency dissecting sealer | Clamp‐crush method | Low | Low | High | High | Low | Low | Low | Low | Unclear or high |
| Smyrniotis 2005 | Sharp transection | Clamp‐crush method | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | Low | Unclear or high |
| Shimada 1994 | Anti‐thrombin III concentrate | Control | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | Unclear or high |
| Lentschener 1997 | Aprotinin | Control | Low | Unclear | Unclear | Low | High | High | High | Low | Unclear or high |
| Wong 2003 | Desmopressin | Control | Unclear | Unclear | Low | Low | High | High | Low | Low | Unclear or high |
| Lodge 2005 | Recombinant factor VIIa | Control | Low | Low | Low | Low | High | Low | High | Low | Unclear or high |
| Shao 2006 | Recombinant factor VIIa | Control | Unclear | Unclear | Unclear | Unclear | High | High | High | Low | Unclear or high |
| Wu 2006 | Tranexamic acid | Control | Unclear | Unclear | Low | Low | Low | High | Unclear | Low | Unclear or high |
| Chapman 2000 | Collagen | Fibrin sealant | Low | Unclear | Unclear | Unclear | High | High | High | Low | Unclear or high |
| Franceschi 2006 | Collagen | Fibrin sealant | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | Unclear or high |
| Kohno 1992 | Collagen | Fibrin sealant | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Low | Unclear or high |
| Moench 2014 | Collagen | Fibrin sealant | Low | Low | High | High | High | Low | High | Low | Unclear or high |
| Fischer 2011 | Fibrin sealant | Argon beam coagulator | Unclear | Low | High | High | High | Low | High | Low | Unclear or high |
| Frilling 2005 | Fibrin sealant | Argon beam coagulator | Unclear | Unclear | High | High | Low | Low | Unclear | Low | Unclear or high |
| Bektas 2014 | Fibrin sealant | Control | Low | Low | High | High | Low | Low | High | Low | Unclear or high |
| De Boer 2012 | Fibrin sealant | Control | Low | Low | High | High | Low | Low | High | Low | Unclear or high |
| Liu 1993 | Fibrin sealant | Control | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | Low | Unclear or high |
| Noun 1996 | Fibrin sealant | Control | Unclear | Unclear | Unclear | Unclear | High | High | Unclear | Low | Unclear or high |
| Porte 2012 | Fibrin sealant | Gelatin | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | Unclear or high |
| Genyk 2014 | Fibrin sealant | Oxidised cellulose | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | Unclear or high |
| Koea 2013 | Fibrin sealant | Oxidised cellulose | Low | Low | High | High | High | High | High | Low | Unclear or high |
| Ollinger 2013 | Fibrin sealant | Oxidised cellulose | Unclear | Unclear | High | High | Low | Low | High | Low | Unclear or high |
| Kakaei 2013 | Fibrin sealant | Oxidised cellulose 3rd group: cyanoacrylate | Low | Unclear | High | Unclear | Unclear | High | Low | Low | Unclear or high |
| Gugenheim 2011 | Fibrin sealant | PlasmaJet coagulator | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | Low | Unclear or high |
| Figueras 2007 | Fibrin sealant plus collagen | Control | Low | Low | Unclear | Unclear | Low | Low | Low | Low | Unclear or high |
| Belghiti 1996 | Continuous portal triad clamping | Continuous hepatic vascular exclusion | Unclear | Unclear | Unclear | Unclear | High | High | Unclear | Low | Unclear or high |
| Chen 2006 | Continuous portal triad clamping | Continuous hepatic vascular exclusion | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Unclear or high |
| Si‐Yuan 2014 | Continuous portal triad clamping | Continuous selective hepatic vascular exclusion | Unclear | Low | Unclear | Unclear | Low | High | Unclear | Low | Unclear or high |
| Ni 2013 | Continuous portal triad clamping | Continuous selective portal triad clamping | Unclear | Low | Unclear | Unclear | Low | Low | Low | Low | Unclear or high |
| Chouker 2004 | Continuous portal triad clamping | Control | Unclear | Unclear | High | Unclear | High | High | Unclear | Low | Unclear or high |
| Clavien 1996 | Continuous portal triad clamping | Control | Unclear | Unclear | Unclear | Unclear | High | High | Low | Low | Unclear or high |
| Dayangac 2010 | Continuous portal triad clamping | Control | Low | Low | High | Low | Low | High | Low | Low | Unclear or high |
| Pietsch 2010 | Continuous portal triad clamping | Control | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | Unclear or high |
| Belghiti 1999 | Continuous portal triad clamping | Intermittent portal triad clamping | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | Low | Unclear or high |
| Capussotti 2003 | Continuous portal triad clamping | Intermittent portal triad clamping | Low | Unclear | Unclear | Unclear | Low | Low | Unclear | Low | Unclear or high |
| Liang 2009 | Continuous selective portal triad clamping | Intermittent portal triad clamping | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Low | Unclear or high |
| Capussotti 2006 | Intermittent portal triad clamping | Control | Low | Unclear | Unclear | Unclear | Low | High | Unclear | Low | Unclear or high |
| Lee 2012 | Intermittent portal triad clamping | Control | Low | Low | High | High | Low | Low | Low | Low | Unclear or high |
| Man 1997 | Intermittent portal triad clamping | Control | Unclear | Unclear | Unclear | Unclear | Low | High | Low | Low | Unclear or high |
| Man 2003 | Intermittent portal triad clamping | Control | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | Low | Unclear or high |
| Park 2012 | Intermittent portal triad clamping | Control | Low | Low | Unclear | Unclear | High | High | Low | Low | Unclear or high |
| Figueras 2005 | Intermittent portal triad clamping | Intermittent selective portal triad clamping | Unclear | Unclear | Unclear | Unclear | Low | High | Low | Low | Unclear or high |
| Wu 2002 | Intermittent portal triad clamping | Intermittent selective portal triad clamping | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Low | Unclear or high |
Allocation
Twenty‐four trials (35.8%) were at low risk of bias in the 'sequence generation' domain (Lentschener 1997; Chapman 2000; Hasegawa 2002; Matot 2002; Capussotti 2003; Arita 2005; Lodge 2005; Capussotti 2006; Figueras 2007; Lupo 2007; Kato 2008; Ikeda 2009; Dayangac 2010; Capussotti 2012; De Boer 2012; Lee 2012; Park 2012; Kakaei 2013; Koea 2013; Savlid 2013; Bektas 2014; Moench 2014; Muratore 2014; Rahbari 2014). Eighteen trials (26.9%) were at low risk of bias in the 'allocation concealment' domain (Hasegawa 2002; Arita 2005; Lodge 2005; Figueras 2007; Kato 2008; Dayangac 2010; Fischer 2011; De Boer 2012; Lee 2012; Park 2012; Koea 2013; Ni 2013; Savlid 2013; Bektas 2014; Moench 2014; Muratore 2014; Rahbari 2014; Si‐Yuan 2014). Fifteen trials (22.4%) were at low risk of bias in the 'both sequence generation and allocation concealment' domains and were free from selection bias (Hasegawa 2002; Arita 2005; Lodge 2005; Figueras 2007; Kato 2008; Dayangac 2010; De Boer 2012; Lee 2012; Park 2012; Koea 2013; Savlid 2013; Bektas 2014; Moench 2014; Muratore 2014; Rahbari 2014).
Blinding
Four trials (6.0%) were at low risk of bias in the 'blinding of participants and healthcare providers' domain (Hasegawa 2002; Wong 2003; Lodge 2005; Wu 2006). Six trials (9.0%) were at low risk of bias in the 'blinding of outcome assessors' domain (Lentschener 1997; Wong 2003; Lodge 2005; Wu 2006; Dayangac 2010; Rahbari 2014). Three trials (4.5%) were at low risk of bias in both the 'blinding of participants and healthcare providers' and 'blinding of outcome assessors' domains and were free from performance and detection bias (Wong 2003; Lodge 2005; Wu 2006).
Incomplete outcome data
Thirty‐three trials (49.3%) were at low risk of bias in the 'missing outcome bias' domain (Kohno 1992; Liu 1993; Man 1997; Belghiti 1999; Takayama 2001; Hasegawa 2002; Matot 2002; Wu 2002; Capussotti 2003; Man 2003; Arita 2005; Figueras 2005; Frilling 2005; Lesurtel 2005; Smyrniotis 2005; Capussotti 2006; Wu 2006; Figueras 2007; Lupo 2007; Kato 2008; Ikeda 2009; Liang 2009; Dayangac 2010; Gugenheim 2011; De Boer 2012; Lee 2012; Ni 2013; Ollinger 2013; Savlid 2013; Bektas 2014; Muratore 2014; Rahbari 2014; Si‐Yuan 2014).
Selective reporting
Twenty‐five trials (37.3%) reported mortality and serious adverse events and hence were considered to be at low risk of bias in the 'selective reporting bias' domain (Kohno 1992; Takayama 2001; Wu 2002; Capussotti 2003; Arita 2005; Frilling 2005; Lesurtel 2005; Lodge 2005; Chen 2006; Figueras 2007; Jarnagin 2008; Ikeda 2009; Liang 2009; Fischer 2011; Capussotti 2012; De Boer 2012; Doklestic 2012; Lee 2012; Ni 2013; Ollinger 2013; Savlid 2013; Bektas 2014; Moench 2014; Muratore 2014; Rahbari 2014).
Other potential sources of bias
Twenty‐four trials (35.8%) were at low risk of bias in the 'source of funding bias' domain (Clavien 1996; Man 1997; Hasegawa 2002; Matot 2002; Wu 2002; Wong 2003; Arita 2005; Figueras 2005; Chen 2006; Liu 2006; Figueras 2007; Lupo 2007; Ikeda 2009; Liang 2009; Dayangac 2010; Capussotti 2012; Doklestic 2012; Lee 2012; Park 2012; Guo 2013; Kakaei 2013; Ni 2013; Guo 2014; Muratore 2014). We did not identify any other bias in the trials.
Effects of interventions
See: Table 1
We provide the data used in network meta‐analysis in Appendix 3; the data used for direct comparisons in Data and analyses; and the overall results in Table 1, Appendix 9, and Appendix 10. We present the data in the following format for each comparison.
-
Outcome.
-
Different methods of measuring the outcome.
Direct comparison.
Network meta‐analysis (when applicable).
Differences between direct comparison and network meta‐analysis (when applicable).
-
Differences between Bayesian and frequentist meta‐analysis.
An overall summary for the comparison.
In addition, we also provide an overall summary for each outcome across all interventions at the end.
Anterior approach versus conventional approach
Two trials compared anterior approach versus conventional approach (Liu 2006; Capussotti 2012). Since this comparison only involved two treatments, we did not perform network meta‐analysis.
Quality of evidence
The quality of evidence was very low for all the outcomes. This was because of high risk of bias in the trials (downgraded by one point), imprecision due to small sample size (downgraded by one point), and wide credible intervals for all outcomes (downgraded by one point) as well as considerable heterogeneity for blood transfusion (proportion) and major blood loss (proportion) (downgraded by two points).
Mortality
Mortality (perioperative)
Two trials reported perioperative mortality (Liu 2006; Capussotti 2012). The unadjusted proportions of perioperative mortality are as follows.
Conventional approach: 7/92 (7.6%).
Anterior approach: 2/93 (2.2%).
Based on the DIC, we chose the fixed‐effect model. There was no evidence of differences in perioperative mortality between the two groups (OR 0.23, 95% CrI 0.03 to 1.08; 185 participants; 2 studies).
Mortality (longest follow‐up)
None of the trials reported this outcome.
Adverse events
Serious adverse events (proportion)
One trial reported serious adverse events as a proportion of participants who experienced one or more (Capussotti 2012). The unadjusted proportions of serious adverse events are as follows.
Conventional approach: 4/32 (12.5%).
Anterior approach: 5/33 (15.2%).
There was no evidence of differences in the proportion of participants experiencing serious adverse events between the two groups (OR 1.27, 95% CrI 0.29 to 5.89; 65 participants; 1 study).
Serious adverse events (number)
None of the trials reported this outcome.
Adverse events (proportion)
Two trials reported adverse events as a proportion (Liu 2006; Capussotti 2012). The unadjusted proportions of adverse events are as follows.
Conventional approach: 33/92 (35.9%).
Anterior approach: 31/93 (33.3%).
Based on the DIC, we chose the fixed‐effect model. There was no evidence of differences in the proportion of participants experiencing adverse events between the two groups (OR 0.89, 95% CrI 0.48 to 1.64; 185 participants; 2 studies).
Adverse events (number)
One trial reported the number of adverse events (Capussotti 2012). The unadjusted rates of adverse events (number) are as follows.
Conventional approach: 18/32 (56.3 per 100 participants).
Anterior approach: 17/33 (51.5 per 100 participants).
There was no evidence of differences in the number of adverse events between the two groups (rate ratio 0.91, 95% CrI 0.47 to 1.78; 65 participants; 2 studies).
Health‐related quality of life
None of the trials reported this outcome at any time point.
Blood transfusion requirements
Blood transfusion (proportion)
Two trials reported blood transfusion as a proportion of participants requiring one (Liu 2006; Capussotti 2012). The unadjusted proportions of participants receiving a blood transfusion are as follows.
Conventional approach: 20/92 (21.7%).
Anterior approach: 10/93 (10.8%).
Based on the DIC, we chose the random‐effects model. The between‐study standard deviation was 2.60. There was no evidence of differences in the proportion of participants receiving a blood transfusion between the two groups (OR 0.57, 95% CrI 0.01 to 50.91; 185 participants; 2 studies).
Blood transfusion (quantity)
None of the trials reported the quantity of blood transfusion in red blood cells, platelets, fresh frozen plasma, or cryoprecipitate.
Blood loss
Two trials reported blood loss (Liu 2006; Capussotti 2012). The median blood loss reported for each treatment in the two trials are as follows.
Conventional approach: 0.5 L and 1 L.
Anterior approach: 0.437 L and 0.8 L.
We did not perform meta‐analysis since both trials reported the median blood loss rather than the mean and standard deviation of blood loss. There was no evidence of differences in blood loss in either trial (Liu 2006; Capussotti 2012).
Major blood loss (proportion)
Two trials reported major blood loss as a proportion of participants experiencing it (Liu 2006; Capussotti 2012). One trial defined major blood loss as more than one litre of blood loss (Capussotti 2012), while the other trial defined it as more than two litres (Liu 2006). The unadjusted proportions of major blood loss (proportion) are as follows.
Conventional approach: 22/92 (23.9%).
Anterior approach: 12/93 (12.9%).
Based on the DIC, we chose the random‐effects model. The between‐study standard deviation was 2.3. There was no evidence of differences in the proportion of participants experiencing major blood loss between the two groups (OR 0.54, 95% CrI 0.01 to 34.54; 185 participants; 2 studies).
Hospital stay
Total hospital stay
Two trials reported hospital stay (Liu 2006; Capussotti 2012). The median hospital stay reported for each treatment in the two trials are as follows.
Conventional approach: 11.5 days (d) and 12.5 d.
Anterior approach: 10 d and 11 d.
We did not perform meta‐analysis since both trials reported the median hospital stay rather than the mean and standard deviation of hospital stay. There was no evidence of differences in hospital stay in either trial (Liu 2006; Capussotti 2012).
Intensive therapy unit (ITU) stay
One trial reported ITU stay (Liu 2006). The median ITU stay reported for each treatment is as follows.
Conventional approach: 2 d.
Anterior approach: 1.5 d.
We did not perform meta‐analysis since the trial reported the median ITU stay rather than the mean and standard deviation of ITU stay. There was no evidence of differences in ITU stay in this trial (Liu 2006).
Operating time
Two trials reported operating time (Liu 2006; Capussotti 2012). The median operating times reported for each treatment are as follows.
Conventional approach: 312.8 minutes (min) and 415 min.
Anterior approach: 295.8 min and 420 min.
We did not perform meta‐analysis since both trials reported the median operating time rather than the mean and standard deviation of operating time. There was no evidence of differences in operating time in either trial.
Time needed to return to work
None of the trials reported this outcome.
Difference between Bayesian and frequentist meta‐analysis
The interpretation of information and conclusions did not alter by using the frequentist meta‐analysis.
Overall summary
There was no evidence of differences between the anterior approach and conventional approach in any of the reported outcomes of interest for this review.
Autologous blood donation versus control
Two trials compared autologous blood donation versus control (Kajikawa 1994; Kostopanagiotou 2007). As this comparison only included two treatments, we did not perform network meta‐analysis.
Quality of evidence
The quality of evidence was very low for all the outcomes and comparisons unless specifically indicated within the results. This was because of unclear or high risk of bias in the trials (downgraded by one point), imprecision due to small sample size (downgraded by one point), and wide credible intervals (downgraded by one point) for all outcomes with very low quality of evidence.
Mortality
Mortality (perioperative)
One trial (28 participants) reported perioperative mortality (Kostopanagiotou 2007); there was none in either group.
Mortality (longest follow‐up)
One trial (28 participants) reported mortality at longest follow‐up (Kostopanagiotou 2007). There was no mortality in either group after a follow‐up period of one year.
Adverse events
Serious adverse events (proportion)
None of the trials reported this outcome.
Serious adverse events (number)
None of the trials reported this outcome.
Adverse events (proportion)
One trial reported adverse events as a proportion of participants experiencing at least one (Kostopanagiotou 2007). The unadjusted proportions of participants experiencing an adverse event are as follows.
Control: 5/13 (38.5%).
Autologous blood donation: 5/15 (33.3%).
There was no evidence of differences in the proportion of participants experiencing adverse events between groups (OR 0.79, 95% CrI 0.15 to 3.98; 28 participants; 1 study).
Adverse events (number)
None of the trials reported this outcome.
Health‐related quality of life
None of the trials reported this outcome at any time point.
Blood transfusion requirements
Blood transfusion (proportion)
One trial reported the proportion of participants requiring a blood transfusion (Kajikawa 1994). The unadjusted proportions are as follows.
Control: 13/21 (61.9%).
Autologous blood donation: 5/21 (23.8%).
The proportion of participants requiring a blood transfusion was lower in the autologous blood donation group than in the control (OR 0.18, 95% CrI 0.04 to 0.66; 42 participants; 1 study; low‐quality evidence: downgraded one point for unclear or high risk of bias and one point for small sample size).
Blood transfusion (red blood cells)
One trial reported blood transfusion quantity in red blood cells (Kostopanagiotou 2007). The mean blood transfusion quantities reported for each treatment are as follows.
Control: 1.7 units.
Autologous blood donation: 1.6 units.
There was no evidence of differences in blood transfusion quantity (red blood cells) between the groups (MD −0.10 units, 95% CrI −0.59 to 0.38; 28 participants; 1 study).
Blood transfusion (platelets)
None of the trials reported this outcome.
Blood transfusion (fresh frozen plasma)
None of the trials reported this outcome.
Blood transfusion (cryoprecipitate)
None of the trials reported this outcome.
Blood loss
Two trials reported blood loss (Kajikawa 1994; Kostopanagiotou 2007). The mean blood loss reported for each treatment are as follows.
Control: 0.78 L and 1.193 L
Autologous blood donation: 0.68 L and 1.272 L
Based on the DIC, we chose the fixed‐effect model. There was no evidence of differences in blood loss between the groups (MD −0.02 L, 95% CrI −0.37 to 0.34; 70 participants; 2 studies).
Major blood loss (proportion)
One trial reported the proportion of participants experiencing major blood loss, defined as the loss of more than two litres (Kajikawa 1994). The unadjusted proportions of participants with major blood loss are as follows.
Control: 2/21 (9.5%).
Autologous blood donation: 4/21 (19.0%).
There was no evidence of differences in the proportion of participants experiencing major blood loss between the groups (OR 2.44, 95% CrI 0.39 to 21.5; 42 participants; 1 study).
Hospital stay
Total hospital stay
One trial reported total hospital stay (Kostopanagiotou 2007). The mean hospital stays reported for each treatment are as follows.
Control: 10 d.
Autologous blood donation: 11 d.
There was no evidence of differences in hospital stay between the groups (MD 0.99 d, 95% CrI −0.92 to 2.91; 28 participants; 1 study).
ITU stay
None of the trials reported this outcome.
Operating time
Two trials reported operating time (Kajikawa 1994; Kostopanagiotou 2007). The mean operating times reported for each treatment are as follows.
Control: 190 min and 290 min.
Autologous blood donation: 175 min and 318 min.
Based on the DIC, we chose the fixed‐effect model. There was no evidence of differences in operating times between the groups (MD 1.78 min, 95% CrI −28.13 to 31.68; 70 participants; 2 studies).
Time needed to return to work
None of the trials reported this outcome.
Difference between Bayesian and frequentist meta‐analysis
The interpretation of information and conclusions did not alter by using the frequentist meta‐analysis.
Overall summary
There was no evidence of difference between autologous blood donation and control in any of the reported outcomes of interest for this review other than the proportion of people who required blood transfusion, which was lower in the autologous blood donation group than control (OR 0.18, 95% CrI 0.04 to 0.66; 42 participants; 1 study).
Cardiopulmonary interventions
Ten trials compared different methods of cardiopulmonary interventions (Hasegawa 2002; Matot 2002; El‐Kharboutly 2004; Wang 2006; Yao 2006; Choi 2007; Jarnagin 2008; Kato 2008; Guo 2013; Guo 2014). We performed network meta‐analysis only for blood transfusion quantity (red blood cells) and blood loss since direct comparison and indirect comparison effect estimates (which would enable assessment of inconsistency) were available only for these outcomes. We present only direct comparison results for other outcomes.
Quality of evidence
The quality of evidence was very low for all the outcomes and comparisons unless specifically indicated within the results. This was because of unclear or high risk of bias in the trials (downgraded by one point), imprecision due to small sample size (downgraded by one point), and wide credible intervals (downgraded by one point) for all outcomes with very low quality of evidence.
Mortality
Mortality (perioperative)
Four trials reported perioperative mortality (Hasegawa 2002; Matot 2002; Jarnagin 2008; Kato 2008). These studies used four treatments in 372 participants. The unadjusted proportions of perioperative mortality are as follows.
Control: 0/81 (0.0%).
Acute normovolemic haemodilution plus low central venous pressure: 1/102 (1.0%).
Hypoventilation: 0/40 (0.0%).
Low central venous pressure: 3/149 (2.0%).
There was no evidence of differences in perioperative mortality for any of the comparisons.
Mortality (longest follow‐up)
None of the trials reported this outcome.
Adverse events
Serious adverse events (proportion)
Two trials reported the proportion of participants experiencing serious adverse events (Hasegawa 2002; Jarnagin 2008). A total of four treatments were used in a total of 209 participants in these studies. The unadjusted proportions of participants with serious adverse events are as follows.
Control: 1/39 (2.6%).
Acute normovolemic haemodilution plus low central venous pressure: 19/63 (30.2%).
Hypoventilation: 2/40 (5.0%).
Low central venous pressure: 19/67 (28.4%).
There was no evidence of differences in the proportion of participants experiencing serious adverse events for any of the comparisons.
Serious adverse events (number)
Two trials reported the total number of serious adverse events (Matot 2002; El‐Kharboutly 2004). These studies used three treatments in 118 participants. The unadjusted rates of serious adverse events (number) are as follows.
Control: 2/20 (10.0 per 100 participants).
Acute normovolemic haemodilution plus low central venous pressure: 4/39 (10.3 per 100 participants).
Low central venous pressure: 3/59 (5.1 per 100 participants).
There was no evidence of differences in the number of serious adverse events observed for any of the comparisons.
Adverse events (proportion)
Four trials reported the proportion of participants experiencing adverse events (Hasegawa 2002; Matot 2002; Wang 2006; Jarnagin 2008). These studies used four treatments in 337 participants. The unadjusted proportions of participants experiencing adverse events are as follows.
Control: 19/64 (29.7%).
Acute normovolemic haemodilution plus low central venous pressure: 37/102 (36.3%).
Hypoventilation: 16/40 (40.0%).
Low central venous pressure: 35/131 (26.7%).
There was no evidence of differences in the proportion of participants experiencing adverse events for any of the comparisons.
Adverse events (number)
Two trials reported adverse events (number) (Matot 2002; El‐Kharboutly 2004). These studies used three treatments in 118 participants. The unadjusted rates of adverse events (number) are as follows.
Control: 6/20 (30.0 per 100 participants).
Acute normovolemic haemodilution plus low central venous pressure: 12/39 (30.8 per 100 participants).
Low central venous pressure: 15/59 (25.4 per 100 participants).
There was no evidence of differences in adverse events (number) for any of the comparisons.
Health‐related quality of life
None of the trials reported this outcome at any time point.
Blood transfusion requirements
Blood transfusion (proportion)
Six trials reported the proportion of participants requiring a blood transfusion (Hasegawa 2002; Matot 2002; El‐Kharboutly 2004; Wang 2006; Jarnagin 2008; Kato 2008). These studies used four treatments in 462 participants. The unadjusted proportions of participants requiring a blood transfusion are as follows.
Control: 29/126 (23.0%).
Acute normovolemic haemodilution plus low central venous pressure: 12/102 (11.8%).
Hypoventilation: 3/40 (7.5%).
Low central venous pressure: 48/194 (24.7%).
Based on the DIC, we chose the fixed‐effect model. The proportion of participants requiring a blood transfusion was higher in the low central venous pressure group than in the group receiving acute normovolemic haemodilution plus low central venous pressure (OR 3.19, 95% CrI 1.56 to 6.95; 208 participants; 2; low‐quality evidence: downgraded by one point for unclear or high risk of bias in the trials and one more point for small sample size). There was no evidence of differences in other comparisons.
Blood transfusion (red blood cells)
Six trials reported blood transfusion quantity (as red blood cells) (Matot 2002; El‐Kharboutly 2004; Wang 2006; Yao 2006; Jarnagin 2008; Guo 2013), testing five treatments in 358 participants. The median and range of the mean blood transfusion quantity (red blood cells) reported for each treatment are as follows.
Control: 1.38 units (range 0.88 to 3.22).
Acute normovolemic haemodilution: 0.17 units (range 0.17 to 0.17).
Acute normovolemic haemodilution plus hypotension: 0.00 units (range 0.00 to 0.00).
Acute normovolemic haemodilution plus low central venous pressure: 0.44 (range 0.00 to 1.15).
Low central venous pressure: 0.61 (range 0.00 to 1.31).
Direct comparison
Based on the DIC, we chose the fixed‐effect model. The blood transfusion quantity (in red blood cells) was lower in the group receiving acute normovolemic haemodilution (MD −1.25 units, 95% CrI −1.75 to −0.74; 20 participants; 1 study; low‐quality evidence: downgraded by one point for unclear or high risk of bias in the trials and one more point for small sample size) and acute normovolemic haemodilution plus hypotension (MD −1.67 units, 95% CrI −2.06 to −1.32; 20 participants; 1 study; low‐quality evidence: downgraded by one point for unclear or high risk of bias in the trials and one more point for small sample size) than control.The blood transfusion quantity (red blood cells) was higher inthe acute normovolemic haemodilution plus low central venous pressure group than in the control group (MD 0.27 units, 95% CrI 0.01 to 0.52; 30 participants; 1 study). There was no evidence of differences in other comparisons. We imputed either the mean or standard deviation in two trials (Matot 2002; Jarnagin 2008). Excluding these trials did not alter the conclusions.
Network meta‐analysis
We present the network plots in Figure 4. Based on the DIC, we chose the random‐effects model. There was no evidence of differences in blood transfusion quantity (red blood cells) for any of the comparisons. Excluding the trials in which we imputed the mean or standard deviation (Matot 2002; Jarnagin 2008), we could not assess whether the direct and indirect evidence was consistent. We show the probability of each treatment being best, second best, third best, and so on in Figure 5 and the cumulative probability of a treatment being best in Figure 6.
4.
The network plot showing the comparisons in the trials included in the comparison of cardiopulmonary interventions in which network meta‐analysis was performed. The size of the node (circle) provides a measure of the number of trials in which the particular treatment was included as one of the arms. The thickness of the line provides a measure of the number of direct comparisons between two nodes (treatments).
ANH: acute normovolemic haemodilution; CVP: central venous pressure; RBC: red blood cells.
5.
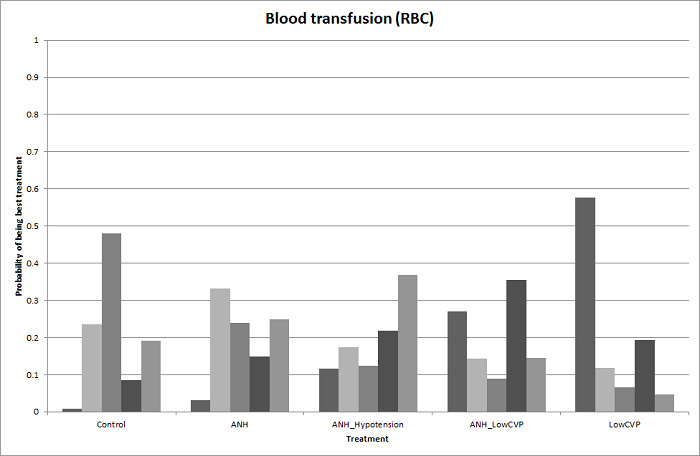
Probability of best treatment: probability of being best, second best, third best, etc. for each treatment for blood transfusion (red blood cells) (cardiopulmonary interventions). A probability of more than 90% is a reliable indicator that a treatment is best with regards to the specific outcome. A probability of less than 90% is less reliable. None of the treatments have a 90% probability of being best treatment.
ANH: acute normovolemic haemodilution; CVP: central venous pressure; RBC: red blood cells.
6.
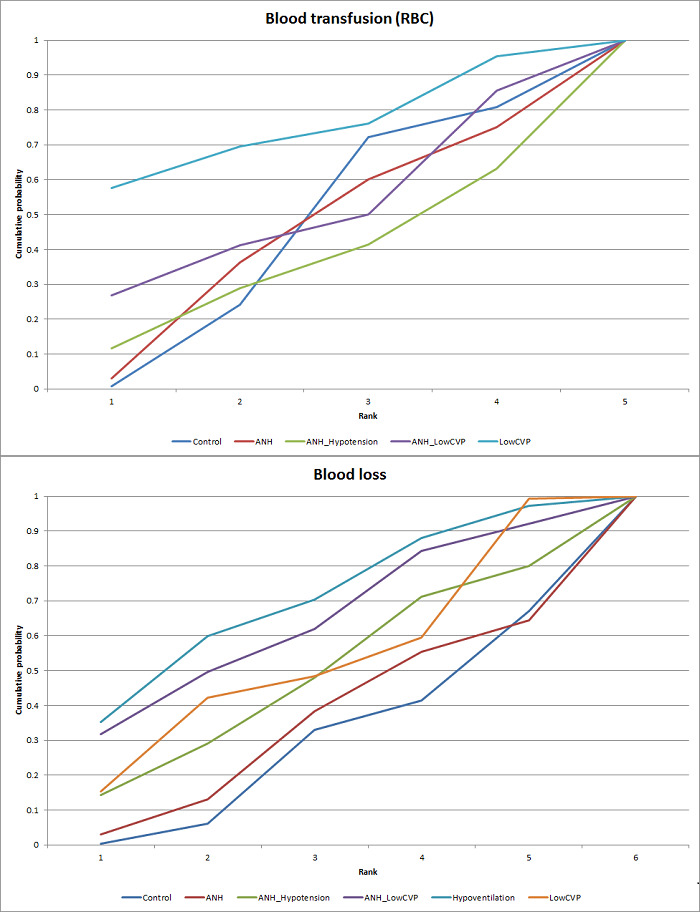
Cumulative probability of being best treatment: cumulative probability of being best for each treatment for cardiopulmonary interventions. Rank 1 indicates the probability that a treatment is best, rank 2 indicates the probability that a treatment is in the two best treatments, rank 3 indicates the probability that a treatment is in the three best treatments, and so on.
ANH: acute normovolemic haemodilution; CVP: central venous pressure; RBC: red blood cells.
Direct evidence compared to network meta‐analysis
We compare the information on direct evidence to network meta‐analysis in Figure 7. The mean effect goes in opposite directions in the indirect and direct estimates, suggesting that there may be discrepancies (incongruence or inconsistency) between direct and indirect estimates. Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
7.
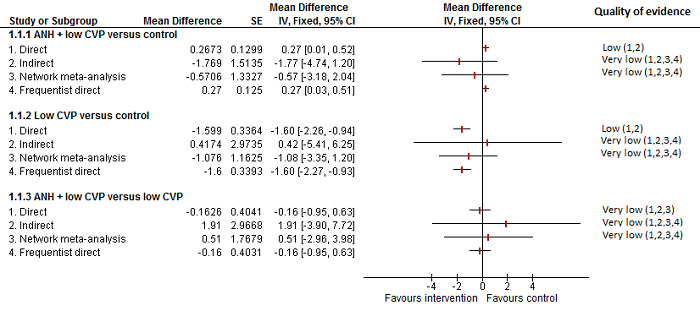
Cardiopulmonary intervention: blood transfusion (red blood cells)
Forest plot of the comparisons in which direct and indirect estimates were available. The mean effect is in opposite directions in the indirect estimate and the direct estimates, thus suggesting that there may be discrepancies between direct and indirect estimates. Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
1 Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2 Sample size was low (downgraded by 1 point). 3Confidence intervals spanned no effect and clinically significant effect (downgraded by 1 point). 4There was substantial or considerable heterogeneity (downgraded by 2 points).
Blood transfusion (platelets)
None of the trials reported this outcome.
Blood transfusion (fresh frozen plasma)
Two trials reported blood transfusion quantity (as fresh frozen plasma) (Wang 2006; Jarnagin 2008), testing three interventions in 180 participants. The mean blood transfusion quantities (fresh frozen plasma) reported for each treatment are as follows.
Control: 4.23 units.
Acute normovolemic haemodilution plus low central venous pressure: 0.17 units.
Low central venous pressure: 0.28 and 1.75 units.
The blood transfusion quantity (fresh frozen plasma) was lower in the low central venous pressure group than the control group (MD −2.48 units, 95% CrI −3.58 to −1.37; 50 participants; 1 study; low‐quality evidence: downgraded by one point for unclear or high risk of bias in the trials and one more point for small sample size). There was no evidence of differences in the other comparison (low central venous pressure versus acute normovolemic haemodilution plus low central venous pressure) (MD 0.11 units, 95% CrI −0.79 to 1.01; 130 participants; 1 study). We imputed the standard deviation in one of the trials (Jarnagin 2008). Excluding this trial did not alter the outcome.
Blood transfusion (cryoprecipitate)
One trial reported blood transfusion quantity (cryoprecipitate) (Hasegawa 2002). The mean blood transfusion quantities (cryoprecipitate) are as follows.
Control: 0.076 units.
Hypoventilation: 0.052 units.
There was no evidence of differences in blood transfusion quantity (cryoprecipitate) between the groups (MD −0.02 units, 95% CrI −0.12 to 0.07; 79 participants; 1 study).
Blood loss
Nine trials reported blood loss (Hasegawa 2002; Matot 2002; El‐Kharboutly 2004; Wang 2006; Yao 2006; Choi 2007; Jarnagin 2008; Kato 2008; Guo 2013),testing six interventions in 584 participants. The median and range of the mean blood loss reported for each treatment are as follows.
Control: 0.711 L (range 0.584 to 2.329).
Acute normovolemic haemodilution: 0.654 L (one trial only).
Acute normovolemic haemodilution plus hypotension: 0.404 L (one trial only).
Acute normovolemic haemodilution plus low central venous pressure: 0.75 L (range 0.735 to 0.8).
Hypoventilation: 0.63 L (one trial only).
Low central venous pressure: 0.6445 L (range 0.49 to 0.904).
Direct comparison
Based on the DIC, we chose the fixed‐effect model. The blood loss was lower in the acute normovolemic haemodilution plus hypotension group (MD −0.25 L; 95% CrI −0.37 to −0.13; 20 participants; 1 study) and the low central venous pressure group than in the control (MD −0.34 L, 95% CrI −0.46 to −0.22; 237 participants; 4 studies).The blood loss was lower for acute normovolemic haemodilution plus hypotension than for acute normovolemic haemodilution (MD −0.25 L; 95% CrI −0.40 to −0.10; 20 participants; 1 study). There was no evidence of differences in other comparisons. We imputed either the mean or standard deviation in four trials (Hasegawa 2002; Matot 2002; Jarnagin 2008; Kato 2008). Excluding these trials did not alter the conclusions.
Network meta‐analysis
We present the network plots in Figure 4. Based on the DIC, we chose the random‐effects model. There was no evidence of differences in blood loss for any of the comparisons. Excluding the trials in which we imputed the mean or standard deviation (Hasegawa 2002; Matot 2002; Jarnagin 2008; Kato 2008) meant that there would be no evidence from direct and indirect evidence, which would allow the assessment of whether the direct and indirect evidence was consistent. We show the probability of each treatment being the best, second best, third best, and so on in Figure 8. The cumulative probability of a treatment being best is shown in Figure 6.
8.
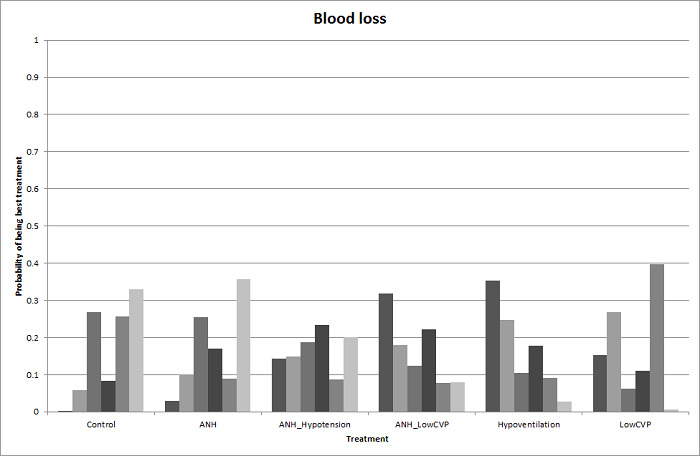
Probability of best treatment: probability of being best, second best, third best, etc. for each treatment for blood loss (cardiopulmonary interventions). A probability of more than 90% is a reliable indicator that a treatment is best with regards to the specific outcome. A probability of less than 90% is less reliable. None of the treatments have a 90% probability of being best treatment.
ANH: acute normovolemic haemodilution; CVP: central venous pressure.
Direct evidence compared to network meta‐analysis
We show the information on direct evidence compared to network meta‐analysis in Figure 9. There does not appear to be any discrepancy between the direct and indirect estimates, although the indirect estimates have wide credible intervals. Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
9.
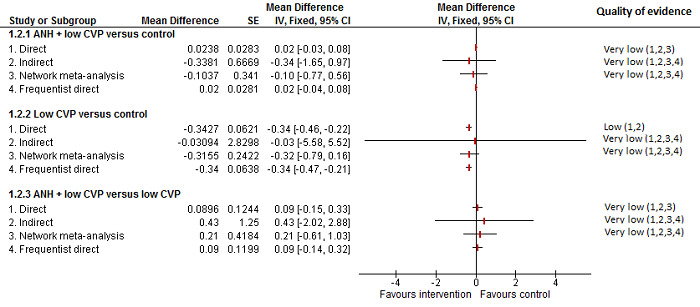
Cardiopulmonary intervention: blood loss
Forest plot of the comparisons in which direct and indirect estimates were available. There does not appear to be any discrepancy between the direct and indirect estimates, although the indirect estimates have wide credible intervals.
Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
ANH: acute normovolemic haemodilution; CVP: central venous pressure.
1Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2Sample size was low (downgraded by 1 point). 3Confidence intervals spanned no effect and clinically significant effect (downgraded by 1 point). 4There was substantial or considerable heterogeneity (downgraded by 2 points).
Major blood loss (proportion)
One trial reported the proportion of participants experiencing major blood loss (Jarnagin 2008), defined as more than 0.8 L. The unadjusted proportions of of participants experiencing major blood loss are as follows.
Acute normovolemic haemodilution plus low central venous pressure: 33/63 (52.4%).
Low central venous pressure: 29/67 (43.3%).
There was no evidence of differences in the proportion of participants experiencing major blood loss between the groups (OR 0.69, 95% CrI 0.34 to 1.38; 130 participants; 1 study).
Hospital stay
Total hospital stay
Five trials reported hospital stay (Hasegawa 2002; Wang 2006; Choi 2007; Jarnagin 2008; Kato 2008). They used four treatments in 406 participants. The median length and range of the mean or median hospital stay reported for each treatment are as follows.
Control: 21 d (range 14 to 30).
Acute normovolemic haemodilution plus low central venous pressure: 7 d (one trial only).
Hypoventilation: 20 d (one trial only).
Low central venous pressure: 15 d (range 7 to 26).
Based on the DIC, we chose the fixed‐effect modelwhen there were two or more trials under the comparison. The total hospital stay was lower in the low central venous pressure group than in the control group (MD −2.42 d, 95% CrI −3.91 to −0.94; 197 participants; 3 studies). There was no evidence of differences in the remaining comparisons. In three trials, either the mean or the standard deviation was not available (Hasegawa 2002; Jarnagin 2008; Kato 2008), so we did not perform a meta‐analysis. Exclusion of these three trials did not alter the conclusions.
ITU stay
None of the trials reported this outcome.
Operating time
Seven trials reported operating time (Hasegawa 2002; Matot 2002; El‐Kharboutly 2004; Wang 2006; Choi 2007; Jarnagin 2008; Guo 2014). They used four treatments in 499 participants. The median and range of the mean operating times reported for each treatment are as follows.
Control: 246 min (range 190 to 498).
Acute normovolemic haemodilution plus low central venous pressure: 255 min (range 179 to 293).
Hypoventilation: 498 min (one trial only).
Low central venous pressure: 244 min (range 164 to 321).
Based on the DIC, we chose the fixed‐effect model. The operating time was lower in the low central venous pressure group than in the control group (MD −15.32 min, 95% CrI −29.03 to −1.69; 192 participants; 4 studies). There was no evidence of differences in other comparisons. Two trials failed to report the mean, standard deviation, or both (Hasegawa 2002; Jarnagin 2008). Excluding these trials did not alter the conclusions.
Time needed to return to work
None of the trials reported this outcome.
Difference between Bayesian and frequentist meta‐analysis
The interpretation of information and conclusions did not alter by using the frequentist meta‐analysis.
Overall summary
There was no evidence of differences between different cardiopulmonary interventions in any of the reported outcomes of interest for this review other than the following.
The proportion of participants requiring a blood transfusion was higher in those receiving low central venous pressure than in those receiving acute normovolemic haemodilution plus low central venous pressure (OR 3.19, 95% CrI 1.56 to 6.95; 208 participants; 2 studies).
The blood transfusion quantity (red blood cells) was lower in the acute normovolemic haemodilution group (MD −1.25 units, 95% CrI −1.75 to −0.74; 20 participants; 1 study) and the acute normovolemic haemodilution plus hypotension group (MD −1.67 units, 95% CrI −2.06 to −1.32; 20 participants; 1 study) than in the control group. The blood transfusion quantity (red blood cells) was higher in the acute normovolemic haemodilution plus low central venous pressure group than in the control group (MD 0.27 units, 95% CrI 0.01 to 0.52; 30 participants; 1 study).
The blood transfusion quantity (fresh frozen plasma) was lower for low central venous pressure than for control (MD −2.48 units, 95% CrI −3.58 to −1.37; 50 participants; 1 study).
The blood loss was lower in the acute normovolemic haemodilution plus hypotension group (MD −0.25 L; 95% CrI −0.37 to −0.13; 20 participants; 1 study) and the low central venous pressure group than in the control (MD −0.34 L, 95% CrI −0.46 to −0.22; 237 participants; 4 studies). The blood loss was lower in the acute normovolemic haemodilution plus hypotension group than in the acute normovolemic haemodilution group (MD −0.25; 95% CrI −0.40 to −0.10; 20 participants; 1 study).
The total hospital stay was lower in the low central venous pressure group than in the control (MD −2.42 d, 95% CrI −3.91 to −0.94; 197 participants; 3 studies).
The operating time was lower in the low central venous pressure group than in the control (MD −15.32 min, 95% CrI −29.03 to −1.69; 192 participants; 4 studies).
Methods of parenchymal transection
Twelve trials compared different methods of parenchymal transection (Rau 2001; Takayama 2001; Arita 2005; Koo 2005; Lesurtel 2005; Smyrniotis 2005; Lupo 2007; Ikeda 2009; Doklestic 2012; Savlid 2013; Muratore 2014; Rahbari 2014). We performed network meta‐analysis only for adverse events (proportion), adverse events (number), and proportion requiring blood transfusion, since direct comparison and indirect comparison effect estimates (which would enable assessment of inconsistency) were available only for these outcomes. We present only direct comparison results for other outcomes.
Quality of evidence
The quality of evidence was very low for all the outcomes and comparisons unless specifically indicated within the results. This was because of unclear or high risk of bias in the trials (downgraded by one point), imprecision due to small sample size (downgraded by one point), and wide credible intervals (downgraded by one point) for all outcomes with very low‐quality of evidence. In addition, we downgraded the outcome of blood transfusion (proportion) by two points because of the presence of substantial or considerable heterogeneity in the pair‐wise comparison or in the network.
Mortality
Mortality (perioperative)
Eleven trials reported perioperative mortality (Rau 2001; Takayama 2001; Arita 2005; Lesurtel 2005; Smyrniotis 2005; Lupo 2007; Ikeda 2009; Doklestic 2012; Savlid 2013; Muratore 2014; Rahbari 2014). They used six treatments in 990 participants. The unadjusted proportions of perioperative mortality are as follows.
Clamp‐crush method: 4/368 (1.1%).
Cavitron ultrasonic surgical aspirator: 3/191 (1.6%).
Hydrojet: 3/56 (5.4%).
Radiofrequency dissecting sealer: 4/219 (1.8%).
Sharp transection method: 0/41 (0.0%).
Stapler: 4/115 (3.5%).
Based on the DIC, the fixed‐effect model was chosen for all comparisons involving two or more trials. There was no evidence of differences in perioperative mortality for any of the comparisons.
Mortality (longest follow‐up)
None of the trials reported this outcome.
Adverse events
Serious adverse events (proportion)
Seven trials reported the proportion of participants experiencing serious adverse events (Rau 2001; Takayama 2001; Arita 2005; Smyrniotis 2005; Ikeda 2009; Doklestic 2012; Rahbari 2014). They used six treatments in 665 participants. The unadjusted proportions of participants experiencing serious adverse events are as follows.
Clamp‐crush method: 28/292 (9.6%).
Cavitron ultrasonic surgical aspirator: 6/116 (5.2%).
Hydrojet: 2/31 (6.5%).
Radiofrequency dissecting sealer: 6/120 (5.0%).
Sharp transection method: 4/41 (9.8%).
Stapler: 19/65 (29.2%).
Based on the DIC, we chose the fixed‐effect model for all comparisons involving two or more trials. There was no evidence of differences in serious adverse events (proportion) for any of the comparisons.
Serious adverse events (number)
Five trials reported the number of serious adverse events (Takayama 2001; Arita 2005; Lesurtel 2005; Lupo 2007; Savlid 2013). They used five treatments in 437 participants. The unadjusted rates of serious adverse events (number) are as follows.
Clamp‐crush method: 7/132 (5.3 per 100 participants).
Cavitron ultrasonic surgical aspirator: 13/141 (9.2 per 100 participants).
Hydrojet: 3/25 (12.0 per 100 participants).
Radiofrequency dissecting sealer: 16/89 (18.0 per 100 participants).
Stapler: 12/50 (24.0 per 100 participants)..
Based on the DIC, we chose the fixed‐effect model for all comparisons involving two or more trials. The number of serious adverse events was higher in the radiofrequency dissecting sealer group than in the clamp‐crush method group (rate ratio 3.64, 95% CrI 1.25 to 13.97; 130 participants; 2 studies; low‐quality evidence: downgraded by one point for unclear or high risk of bias in the trials and one more point for small sample size). There was no evidence of differences in other comparisons.
Adverse events (proportion)
Eight trials reported the proportion of participants experiencing adverse events (Rau 2001; Takayama 2001; Arita 2005; Koo 2005; Smyrniotis 2005; Doklestic 2012; Muratore 2014; Rahbari 2014). They used six treatments in 695 participants. The unadjusted proportions of participants experiencing adverse events are as follows.
Clamp‐crush method: 116/307 (37.8%).
Cavitron ultrasonic surgical aspirator: 60/141 (42.6%).
Hydrojet: 3/31 (9.7%).
Radiofrequency dissecting sealer: 37/110 (33.6%).
Sharp transection method: 17/41 (41.5%).
Stapler: 31/65 (47.7%).
Direct comparison
Based on the DIC, we chose the fixed‐effect model for all comparisons involving two or more trials. There was no evidence of differences in adverse events (proportion) for any of the comparisons.
Network meta‐analysis
We show the network plots in Figure 10. Based on the DIC, we chose the random‐effects model. The between‐study standard deviation was 2.44. There was no evidence of differences in the proportion of participants experiencing adverse events for any of the comparisons. We show the probability of each treatment being best, second best, third best, and so on in Figure 11 and the cumulative probability of a treatment being best in Figure 12.
10.
The network plot showing the comparisons in the trials included in the comparison of methods for parenchymal transection in which network meta‐analysis was performed. The size of the node (circle) provides a measure of the number of trials in which the particular treatment was included as one of the arms. The thickness of the line provides a measure of the number of direct comparisons between two nodes (treatments).
CUSA: cavitron ultrsonic surgical aspirator; RFDS: radiofrequency dissecting sealer.
11.
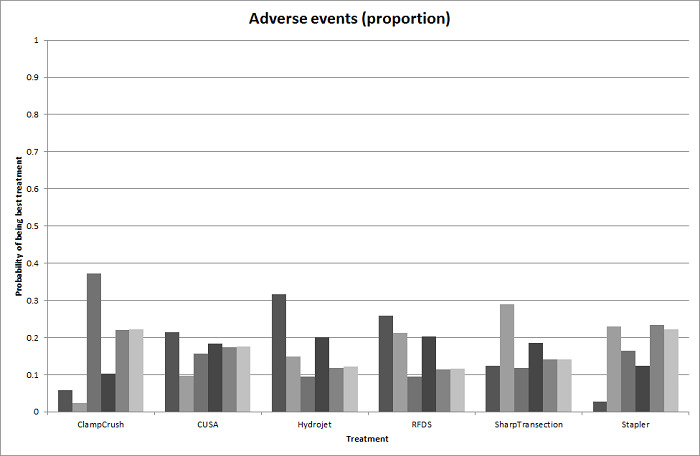
Probability of best treatment: probability of being best, second best, third best, etc. for each treatment for adverse events (proportion) (parenchymal transection methods). A probability of more than 90% is a reliable indicator that a treatment is best with regards to the specific outcome. A probability of less than 90% is less reliable. None of the treatments have a 90% probability of being best treatment.
CUSA: cavitron ultrsonic surgical aspirator; RFDS: radiofrequency dissecting sealer.
12.
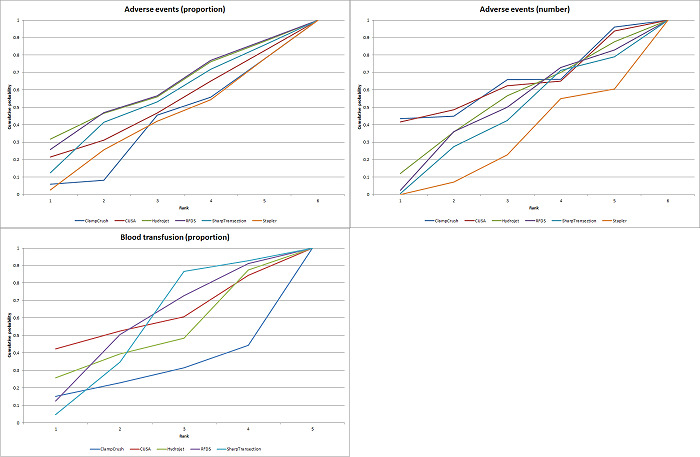
Cumulative probability of being best treatment: cumulative probability of being best for each treatment for parenchymal transection methods. Rank 1 indicates the probability that a treatment is best, rank 2 indicates the probability that a treatment is in the two best treatments, rank 3 indicates the probability that a treatment is in the three best treatments, and so on.
CUSA: cavitron ultrsonic surgical aspirator; RFDS: radiofrequency dissecting sealer.
Direct evidence compared to network meta‐analysis
Figure 13 shows the information on direct evidence compared to network meta‐analysis. There does not appear to be any discrepancy between the direct and indirect estimates, although the indirect estimates have wide credible intervals. Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
13.
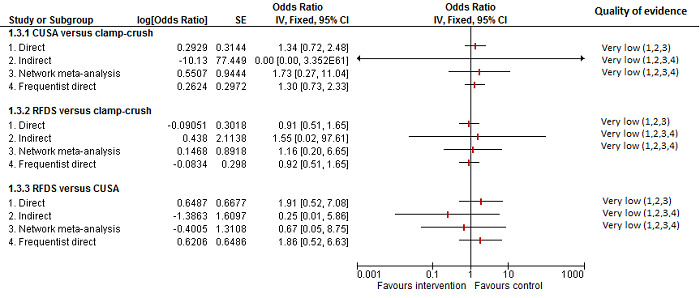
Parenchymal transection: adverse events (proportion)
Forest plot of the comparisons in which direct and indirect estimates were available. There does not appear to be any discrepancy between the direct and indirect estimates, although the indirect estimates have wide credible intervals.
Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
CUSA: cavitron ultrsonic surgical aspirator; RFDS: radiofrequency dissecting sealer.
1Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2Sample size was low (downgraded by 1 point). 3Confidence intervals spanned no effect and clinically significant effect (downgraded by 1 point). 4There was substantial or considerable heterogeneity (downgraded by 2 points).
Adverse events (number)
Seven trials reported the number of adverse events (Takayama 2001; Arita 2005; Lesurtel 2005; Smyrniotis 2005; Lupo 2007; Ikeda 2009; Savlid 2013). They used six treatments in 639 participants. The unadjusted rates of adverse events (number) are as follows.
Clamp‐crush method: 52/233 (22.3 per 100 participants).
Cavitron ultrasonic surgical aspirator: 52/141 (36.9 per 100 participants).
Hydrojet: 7/25 (28.0 per 100 participants).
Radiofrequency dissecting sealer: 45/149 (30.2 per 100 participants).
Sharp transection method: 18/41 (43.9 per 100 participants)
Stapler: 22/50 (44.0 per 100 participants).
Direct comparison
Based on the DIC, we chose the fixed‐effect model for all comparisons involving two or more trials. There was evidence for a higher adverse events (number) with radiofrequency dissecting sealer than with the clamp‐crush method (rate ratio 1.85, 95% CrI 1.07 to 3.26; 250 participants; 3 studies). There was no evidence of differences in the number of adverse events for any of the comparisons.
Network meta‐analysis
Figure 10 shows the network plots. Based on the DIC, we chose the fixed‐effect model. There was evidence of more adverse events (number) with the radiofrequency dissecting sealer method than with the clamp‐crush method (rate ratio 1.84, 95% CrI 1.13 to 3.06). There was no evidence of differences in other comparisons. Figure 14 shows the probability of each treatment being best, second best, third best, and so on, and Figure 12 shows the cumulative probability of a treatment being best.
14.
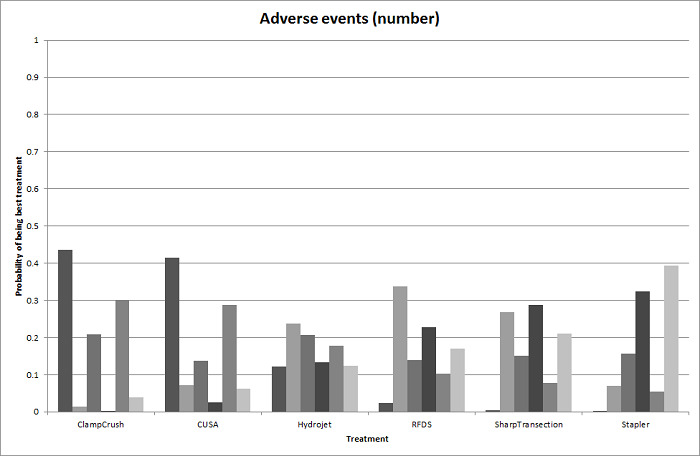
Probability of best treatment: probability of being best, second best, third best, etc. for each treatment for adverse events (number) (parenchymal transection methods). A probability of more than 90% is a reliable indicator that a treatment is best with regards to the specific outcome. A probability of less than 90% is less reliable. None of the treatments have a 90% probability of being best treatment.
CUSA: cavitron ultrsonic surgical aspirator; RFDS: radiofrequency dissecting sealer.
Direct evidence compared to network meta‐analysis
Figure 15 shows the information on direct evidence compared to network meta‐analysis. There does not appear to be any discrepancy between the direct and indirect estimates. Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
15.

Parenchymal transection: adverse events (number)
Forest plot of the comparisons in which direct and indirect estimates were available. There does not appear to be any discrepancy between the direct and indirect estimates.
Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
CUSA: cavitron ultrsonic surgical aspirator; RFDS: radiofrequency dissecting sealer.
1Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2Sample size was low (downgraded by 1 point). 3Confidence intervals spanned no effect and clinically significant effect (downgraded by 1 point).
Health‐related quality of life
None of the trials reported this outcome at any time point.
Blood transfusion requirements
Blood transfusion (proportion)
Eight trials reported the proportion of participants requiring a blood transfusion (Takayama 2001; Arita 2005; Lesurtel 2005; Smyrniotis 2005; Lupo 2007; Ikeda 2009; Doklestic 2012; Muratore 2014). They used five treatments in 699 participants. The unadjusted proportions of blood transfusion (proportion) are as follows.
Clamp‐crush method: 46/303 (15.2%).
Cavitron ultrasonic surgical aspirator: 12/111 (10.8%).
Hydrojet: 8/25 (32.0%).
Radiofrequency dissecting sealer: 37/219 (16.9%).
Sharp transection method: 13/41 (31.7%).
Direct comparison
Based on the DIC, we chose the fixed‐effect model for comparisons involving two or more trials. There was no evidence of differences in the proportion of participants requiring a blood transfusion for any of the comparisons.
Network meta‐analysis
Figure 10 shows the network plots. Based on the DIC, we chose the fixed‐effect model. There was no evidence of differences in the proportion of participants requiring a blood transfusion for any of the comparisons. Figure 16 shows the probability of each treatment being best, second best, third best, and so on. Figure 12 shows the cumulative probability of a treatment being best.
16.
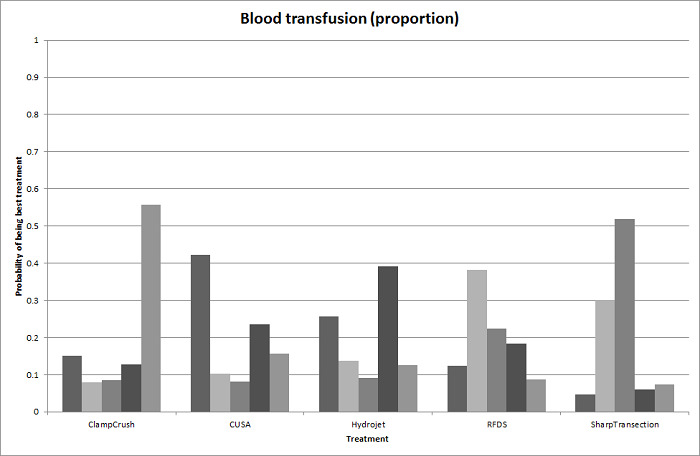
Probability of best treatment: probability of being best, second best, third best, etc. for each treatment for blood transfusion (proportion) (parenchymal transection methods). A probability of more than 90% is a reliable indicator that a treatment is best with regards to the specific outcome. A probability of less than 90% is less reliable. None of the treatments have a 90% probability of being best treatment.
CUSA: cavitron ultrsonic surgical aspirator; RFDS: radiofrequency dissecting sealer.
Direct evidence compared to network meta‐analysis
Figure 17 shows the information on direct evidence compared to network meta‐analysis. There does not appear to be any discrepancy between the direct and indirect estimates, although the indirect estimates have wide credible intervals for some comparisons. There was little apparent difference in the quality of evidence between direct, indirect estimates, and network meta‐analysis; so, we could not choose one estimate over the others based on the quality of evidence.
17.
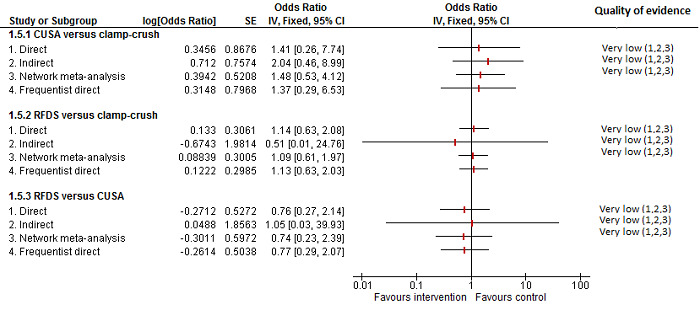
Parenchymal transection:blood transfusion (proportion)
Forest plot of the comparisons in which direct and indirect estimates were available. There does not appear to be any discrepancy between the direct and indirect estimates, although the indirect estimates have wide credible intervals for some comparisons. There was little apparent difference in the quality of evidence between direct, indirect estimates, and network meta‐analysis; so, we could not choose one estimate over the others based on the quality of evidence.
CUSA: cavitron ultrsonic surgical aspirator; RFDS: radiofrequency dissecting sealer.
1Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2Sample size was low (downgraded by 1 point). 3Confidence intervals spanned no effect and clinically significant effect (downgraded by 1 point).
Blood transfusion (red blood cells)
Four trials reported blood transfusion quantity (in red blood cells) (Rau 2001; Smyrniotis 2005; Savlid 2013; Rahbari 2014). They used five treatments in 373 participants. The median or mean blood transfusion quantity (red blood cells) reported for each treatment are as follows.
Clamp‐crush method: 0.00 and 1.20 units (two trials only).
Cavitron ultrasonic surgical aspirator: 2.48 and 4.00 units (two trials only).
Hydrojet: 1.50 units (one trial only).
Sharp transection method: 0.00 units (one trial only).
Stapler: 1.10 and 4.00 units (two trials only).
The blood transfusion quantity (red blood cells) was lower in the hydrojet group than in the cavitron ultrasonic surgical aspirator group (MD −0.98 units, 95% CrI −1.90 to −0.06; 61 participants; 1 study). There was no evidence of difference in blood transfusion quantity (red blood cells) in the remaining comparisons. Either mean or standard deviation or both were not available in two trials (Smyrniotis 2005; Savlid 2013). Excluding these two trials did not change the conclusion.
Blood transfusion (platelets)
None of the trials reported this outcome.
Blood transfusion (fresh frozen plasma)
One trial reported blood transfusion quantity (fresh frozen plasma) (Rahbari 2014). It used two treatments in 130 participants in these studies. The mean blood transfusion quantity (fresh frozen plasma) reported for each treatment are as follows.
Clamp‐crush method: 0.5 units.
Stapler: 0.3 units.
There was no evidence of differences in blood transfusion quantity (fresh frozen plasma) between the groups (MD −0.20 units, 95% CrI −0.66 to 0.26; 130 participants;1 study).
Blood transfusion (cryoprecipitate)
None of the trials reported this outcome.
Blood loss
Ten trials reported blood loss (Rau 2001; Takayama 2001; Arita 2005; Koo 2005; Smyrniotis 2005; Ikeda 2009; Doklestic 2012; Savlid 2013; Muratore 2014; Rahbari 2014). They used six treatments in 915 participants. The median or mean blood loss reported for each treatment are as follows.
Clamp‐crush method: 0.56 L (range 0.2 to 1.05).
Cavitron ultrasonic surgical aspirator: 0.875 L (range 0.15 to 1.797).
Hydrojet: 1.479 L (range 1.479 to 1.479).
Radiofrequency dissecting sealer: 0.47 L (range 0.15 to 0.665).
Sharp transection method: 0.5 L (range 0.5 to 0.5).
Stapler: 0.9625 L (range 0.925 to 1).
Of the 10 trials, 8 did not provide either the mean, the standard deviation or both (Takayama 2001; Arita 2005; Smyrniotis 2005; Ikeda 2009; Doklestic 2012; Savlid 2013; Muratore 2014; Rahbari 2014), so we performed the analysis only for two trials (Rau 2001; Koo 2005). There was no evidence of differences in blood loss for any of the comparisons.
Major blood loss (proportion)
None of the trials reported this outcome.
Hospital stay
Total hospital stay
Ten trials reported hospital stay (Doklestic 2012; Takayama 2001; Arita 2005; Lesurtel 2005; Smyrniotis 2005; Lupo 2007; Ikeda 2009; Savlid 2013; Muratore 2014; Rahbari 2014). They used six treatments in 929 participants. The mean and range of the mean hospital stays reported for each treatment are as follows.
Clamp‐crush method: 11 d (range 7 to 18).
Cavitron ultrasonic surgical aspirator: 11.95 d (range 8.5 to 17).
Hydrojet: 9 d (one trial only).
Radiofrequency dissecting sealer: 10.5 d (range 8 to 16).
Sharp transection method: 11 d (one trial only).
Stapler: 10 to 14.9 d (two trials only).
All 10 trials failed to provide the mean, standard deviation or both. There was no evidence of differences in total hospital stay for any of the comparisons.
ITU stay
Four trials reported ITU stay (Lesurtel 2005; Smyrniotis 2005; Doklestic 2012; Rahbari 2014). They used six treatments in 347 participants. The median ITU stays reported for each treatment are as follows.
Clamp‐crush method: 1 d (range 0 to 1.5).
Cavitron ultrasonic surgical aspirator: 0 and 1 d (two trials only).
Hydrojet: 1 d (one trial only).
Radiofrequency dissecting sealer: 1 d (two trials only).
Sharp transection method: 1 d (one trial only).
Stapler: 0 d (one trial only).
Either the mean, the standard deviation, or both were not available in all the four trials. There was no evidence of differences in ITU stay for any of the comparisons.
Operating time
Six trials reported operating time (Koo 2005; Smyrniotis 2005; Lupo 2007; Doklestic 2012; Savlid 2013; Rahbari 2014). They used five treatments in 472 participants. The median or mean operating time reported for each treatment are as follows.
Clamp‐crush method: 231 min (range 211 to 278).
Cavitron ultrasonic surgical aspirator: 270 min (range 259 to 298).
Radiofrequency dissecting sealer: 292 and 295 min (two trials only).
Sharp transection method: 205 min (one trial only).
Stapler: 190 and 272 min (two trials only).
Based on the DIC, we chose the fixed‐effect model when there were two or more studies in a comparison. There was no evidence of differences in operating time in any of the comparisons. We imputed either the mean or the standard deviation in two trials (Lupo 2007; Doklestic 2012). Excluding this trial did not alter the results.
Time needed to return to work
None of the trials reported this outcome.
Difference between Bayesian and frequentist meta‐analysis
The interpretation of information and conclusions did not change upon use of the frequentist meta‐analysis except for the following.
Adverse events (number): the number of adverse events was higher in the radiofrequency dissecting sealer group than in the group receiving the clamp‐crush method with Bayesian meta‐analysis (rate ratio 1.85, 95% CrI 1.07 to 3.26; 250 participants; 3 studies), while there was no evidence of difference in adverse events (number) in any comparisons by frequentist meta‐analysis (rate ratio 1.67, 95% CI 0.95 to 2.94; 250 participants; 3 studies).
Operating time: there was no evidence of difference in operating time in any comparisons by Bayesian meta‐analysis (stapler resection versus clamp‐crush method: MD −27.99 min, 95% CrI −56.91 to 1.02; 130 participants; 1 study), while the operating time was lower in stapler resection than clamp‐crush method with frequentist meta‐analysis (MD −31.00 min, 95% CI −60.40 to −1.60; 130 participants; 1 study).
Overall summary
There was no evidence of differences between different parenchymal transection methods in any of the reported outcomes of interest for this review other than the following.
The adverse events (number) was higher with the radiofrequency dissecting sealer than with the clamp‐crush method (rate ratio 1.85, 95% CrI 1.07 to 3.26; 250 participants; 3 studies) (Bayesian analysis only: both direct and network meta‐analysis).
The blood transfusion quantity (red blood cells) was lower in the hydrojet group than with the cavitron ultrasonic surgical aspirator group (MD −0.98 units, 95% CrI −1.90 to −0.06; 61 participants; 1 study).
The operating time was lower with stapler resection than with the clamp‐crush method with frequentist meta‐analysis (MD −31.00 min, 95% CI −60.40 to −1.60; 130 participants; 1 study) (frequentist analysis only).
Methods of dealing with cut surface
Seventeen trials compared different methods of dealing with cut surface (Kohno 1992; Liu 1993; Noun 1996; Chapman 2000; Frilling 2005; Franceschi 2006; Figueras 2007; Fischer 2011; Gugenheim 2011; De Boer 2012; Porte 2012; Kakaei 2013; Koea 2013; Ollinger 2013; Bektas 2014; Genyk 2014; Moench 2014). We did not perform network meta‐analysis since direct comparison and indirect comparison effect estimates (which would enable assessment of inconsistency) were not available for any of the outcomes.
Quality of evidence
The quality of evidence was very low for all the outcomes and comparisons unless specifically indicated within the results. This was because of unclear or high risk of bias in the trials (downgraded by one point), imprecision due to small sample size (downgraded by one point), and wide credible intervals (downgraded by one point) for all outcomes with very low quality of evidence. In addition, some of the pair‐wise comparisons in blood transfusion proportion and blood transfusion (red blood cells) were downgraded by two points because of the presence of substantial or considerable heterogeneity.
Mortality
Mortality (perioperative)
Ten trials reported perioperative mortality (Kohno 1992; Chapman 2000; Frilling 2005; Figueras 2007; Fischer 2011; Gugenheim 2011; De Boer 2012; Ollinger 2013; Bektas 2014; Moench 2014). They used seven interventions in 1271 participants. The unadjusted proportions of perioperative mortality are as follows.
Control: 4/339 (1.2%).
Argon beam: 6/114 (5.3%).
Collagen: 4/122 (3.3%).
Fibrin sealant: 23/485 (4.7%).
Fibrin sealant plus collagen: 6/150 (4.0%).
Oxidised cellulose: 1/32 (3.1%).
Plasmajet: 2/29 (6.9%).
Based on the DIC, we chose the fixed‐effect model when there were two or more trials. There was no evidence of differences in perioperative mortality for any of the comparisons.
Mortality (longest follow‐up)
None of the trials reported this outcome.
Adverse events
Serious adverse events (proportion)
Seven trials reported the proportion of participants experiencing serious adverse events (Noun 1996; Fischer 2011; Gugenheim 2011; De Boer 2012; Ollinger 2013; Bektas 2014; Moench 2014). They used six interventions in 798 participants. The unadjusted proportions of serious adverse events (proportion) are as follows.
Control: 43/231 (18.6%).
Argon beam: 14/52 (26.9%).
Collagen: 16/62 (25.8%).
Fibrin sealant: 90/392 (23.0%).
Oxidised cellulose: 10/32 (31.3%).
Plasmajet: 1/29 (3.4%).
Based on the DIC, we chose the fixed‐effect model when there were two or more trials. There was no evidence of differences in serious adverse events (proportion) for any of the comparisons.
Serious adverse events (number)
Six trials reported the number of serious adverse events (Kohno 1992; Frilling 2005; Figueras 2007; Kakaei 2013; Bektas 2014; Moench 2014). They used seven interventions in 725 participants. The unadjusted rates of serious adverse events (number) are as follows.
Control: 39/185 (21.1 per 100 participants).
Argon beam: 4/62 (6.5 per 100 participants).
Collagen: 30/93 (32.3 per 100 participants).
Cyanoacrylate: 1/15 (6.7 per 100 participants).
Fibrin sealant: 72/205 (35.1 per 100 participants).
Fibrin sealant plus collagen: 29/150 (19.3 per 100 participants).
Oxidised cellulose: 4/15 (26.7 per 100 participants).
Based on the DIC, we chose the fixed‐effect model when there were two or more trials. The serious adverse events (number) was higher in the fibrin sealant group than in the argon beam group (rate ratio 4.81, 95% CrI 1.73 to 17.5; 121 participants; 1 study; low‐quality evidence: downgraded one point for unclear or high risk of bias in the trial and one more point for small sample size). There was no evidence of differences in other comparisons.
Adverse events (proportion)
Nine trials reported the proportion of participants experiencing adverse events (Noun 1996; Frilling 2005; Figueras 2007; Fischer 2011; De Boer 2012; Ollinger 2013; Bektas 2014; Genyk 2014; Moench 2014). They used six interventions in 1385 participants. The unadjusted proportions of adverse events (proportion) are as follows.
Control: 166/381 (43.6%).
Argon beam: 52/114 (45.6%).
Collagen: 38/62 (61.3%).
Fibrin sealant: 227/536 (42.4%).
Fibrin sealant plus collagen: 35/150 (23.3%).
Oxidised cellulose: 27/142 (19.0%).
Based on the DIC, we chose the fixed‐effect model when there were two or more trials. There was no evidence of differences in adverse events (proportion) for any of the comparisons.
Adverse events (number)
Five trials reported the number of adverse events (Kohno 1992; Frilling 2005; Kakaei 2013; Bektas 2014; Moench 2014). They used six interventions in 425 participants. The unadjusted rates of adverse events (number) are as follows.
Control: 89/35 (254.3 per 100 participants).
Argon beam: 47/62 (75.8 per 100 participants).
Collagen: 135/93 (145.2 per 100 participants).
Cyanoacrylate: 2/15 (13.3 per 100 participants).
Fibrin sealant: 302/205 (147.3 per 100 participants).
Oxidised cellulose: 7/15 (46.7 per 100 participants).
Based on the DIC, we chose the fixed‐effect model when there were two or more trials. There was no evidence of differences in adverse events (number) for any of the comparisons.
Health‐related quality of life
None of the trials reported this outcome at any time point.
Blood transfusion requirements
Blood transfusion (proportion)
Four trials reported the proportion of participants requiring a blood transfusion (Noun 1996; Figueras 2007; De Boer 2012; Kakaei 2013). They used five interventions in 737 participants. The unadjusted proportions of participants requiring a blood transfusion are as follows.
Control: 62/348 (17.8%).
Cyanoacrylate: 2/15 (13.3%).
Fibrin sealant: 38/209 (18.2%).
Fibrin sealant plus collagen: 40/150 (26.7%).
Oxidised cellulose: 4/15 (26.7%).
Based on the DIC, we chose the fixed‐effect model when there were two or more trials. There was no evidence of differences in blood transfusion (proportion) for any of the comparisons.
Blood transfusion (red blood cells)
Five trials reported blood transfusion (red blood cells) (Liu 1993; Noun 1996; Figueras 2007; Kakaei 2013; Ollinger 2013). They used five interventions in 517 participants. The median and range of the mean blood transfusion (red blood cells) reported for each treatment are as follows.
Control: 3.50 units (range 0.31 to 8.13).
Cyanoacrylate: 2.13 units (one trial only).
Fibrin sealant: 4.30 units (range 3.00 to 5.94).
Fibrin sealant plus collagen: 0.30 units (one trial only).
Oxidised cellulose: 1.86 and 4.35 units (two trials only).
Based on the DIC, we chose the fixed‐effect model for the comparison of fibrin sealant versus control and the random‐effects model for the comparison of oxidised cellulose versus fibrin sealant. The remaining comparisons had only one trial. The blood transfusion quantity (red blood cells) was lower in the fibrin sealant group than in the control (MD −0.53 units, 95% CrI −1.00 to −0.07; 122 participants; 2 studies). The blood transfusion quantity (red blood cells) was higher in the fibrin sealant group than the cyanoacrylate group (MD 2.20 units; 95% CrI 1.59 to 2.81; 30 participants; 1 study; low‐quality evidence: downgraded one point for unclear or high risk of bias in the trial and one more point for small sample size). There was no evidence of differences in other comparisons.
Blood transfusion (platelets)
None of the trials reported this outcome.
Blood transfusion (fresh frozen plasma)
Two trials reported blood transfusion quantity (fresh frozen plasma) (Kakaei 2013; Ollinger 2013). They used three treatments in 95 participants. The median blood transfusion quantities (fresh frozen plasma) reported for each treatment are as follows.
Cyanoacrylate: 0.80 units (one trial only).
Fibrin sealant: 0.00 and 17.64 units (two trials only).
Oxidised cellulose: 0.53 and 20.12 units (two trials only).
Based on the DIC, we chose the fixed‐effect model when there were two or more trials. The blood transfusion quantity (fresh frozen plasma) was lower in the fibrin sealant group than in the cyanoacrylate group (MD −0.81 units, 95% CrI −1.04 to −0.62; 30 participants; 1 study). The blood transfusion quantity (fresh frozen plasma) was higher with oxidised cellulose than with fibrin sealant (MD 0.53 units, 95% CrI 0.36 to 0.71; 80 participants; 2 studies). There was no evidence of differences in other comparisons.
Blood transfusion (cryoprecipitate)
None of the trials reported this outcome.
Blood loss
Five trials reported blood loss (Kohno 1992; Liu 1993; Figueras 2007; De Boer 2012; Kakaei 2013). They usedsix interventions in 757 participants. The median and range of the mean blood loss reported for each treatment are as follows.
Control: 0.82 L (range 0.55 to 4.052).
Collagen: 1.027 L (one trial only).
Cyanoacrylate: 0.653 L (one trial only).
Fibrin sealant: 0.9325 L (range 0.675 to 3.047).
Fibrin sealant plus collagen: 0.884 L (one trial only).
Oxidised cellulose: 0.573 L (one trial only).
Based on the DIC, we chose the fixed‐effect model when there were two or more trials. There was no evidence of differences in blood loss for any of the comparisons. Excluding the trial for which the mean and standard deviation were not available did not alter the conclusions (De Boer 2012).
Major blood loss (proportion)
None of the trials reported this outcome.
Hospital stay
Total hospital stay
Four trials reported hospital stay (Noun 1996; Figueras 2007; Kakaei 2013; Ollinger 2013). They used five interventions in 477 participants. The median and range of the mean hospital stay reported for each treatment are as follows.
Control: 11.3 d and 12.6 d (two trials only).
Cyanoacrylate: 8.8 d (one trial only).
Fibrin sealant: 10.8 d (range 7.5 to 18.5).
Fibrin sealant plus collagen: 13.3 d (one trial only).
Oxidised cellulose: 8.1 d, 15.2 d (two trials only).
Based on the DIC, we chose the fixed‐effect model when there were two or more trials. There was no evidence of differences in hospital stay for any of the comparisons.
ITU stay
One trial (50 participants) reported ITU stay (Ollinger 2013). The median ITU stay reported for each treatment are as follows.
Fibrin sealant: 2.2 d (one trial only).
Oxidised cellulose: 2.8 d (one trial only).
There was no evidence of differences in ITU stay for any of the comparisons.
Operating time
Five trials reported operating time (Kohno 1992; Liu 1993; Noun 1996; Figueras 2007; Ollinger 2013). They used five interventions in 534 participants. The median and range of the mean operating time reported for each treatment are as follows.
Control: 263 min (range 258 to 343).
Collagen: 169 min (one trial only).
Fibrin sealant: 245 min (range 165 to 295).
Fibrin sealant plus collagen: 282 min (one trial only).
Oxidised cellulose: 253 min (one trial only).
Based on the DIC, we chose the fixed‐effect model when there were two or more trials. The operating time was higher in the group receiving fibrin sealant and collagen than in the control group (MD 19.72 min, 95% CrI 2.93 to 36.57; 300 participants; 1 study). There was no evidence of differences in other comparisons.
Time needed to return to work
None of the trials reported this outcome.
Difference between Bayesian and frequentist meta‐analysis
The interpretation of information and conclusions did not alter by using the frequentist meta‐analysis.
Overall summary
There was no evidence of differences between different methods of dealing with cut surface in any of the reported outcomes of interest for this review other than the following.
The serious adverse events (number) was higher in the fibrin sealant group than in the argon beam group (rate ratio 4.81, 95% CrI 1.73 to 17.5; 121 participants; 1 study).
The blood transfusion quantity (red blood cells) was lower in the fibrin sealant group than in the control (MD −0.53 units, 95% CrI −1.00 to −0.07; 122 participants; 2 studies). The blood transfusion quantity (red blood cells) was higher in fibrin sealant than cyanoacrylate (MD 2.20 units; 95% CrI 1.59 to 2.81; 30 participants; 1 study).
The blood transfusion quantity (fresh frozen plasma) was lower with fibrin sealant than with cyanoacrylate (MD −0.81 units, 95% CrI −1.04 to −0.62; 30 participants; 1 study). The blood transfusion quantity (fresh frozen plasma) was higher with oxidised cellulose than with fibrin sealant (MD 0.53 units, 95% CrI 0.36 to 0.71; 80 participants; 2 studies).
The operating time was higher with fibrin sealant and collagen than with control (MD 19.72 min, 95% CrI 2.93 to 36.57; 300 participants; 1 study).
Methods of vascular occlusion
Eighteen trials compared different methods of vascular occlusion (Belghiti 1996; Clavien 1996; Man 1997; Belghiti 1999; Wu 2002; Capussotti 2003; Man 2003; Chouker 2004; Figueras 2005; Capussotti 2006; Chen 2006; Liang 2009; Dayangac 2010; Pietsch 2010; Lee 2012; Park 2012; Ni 2013; Si‐Yuan 2014). We performed network meta‐analysis only for serious adverse events (proportion), adverse events (proportion), blood transfusion (proportion), and blood transfusion quantity (red blood cells) since direct comparison and indirect comparison effect estimates (which would enable assessment of inconsistency) were not available for the other outcomes. We present only direct comparison results for other outcomes.
Quality of evidence
The quality of evidence was very low for all the outcomes and comparisons unless specifically indicated within the results. This was because of unclear or high risk of bias in the trials (downgraded by one point), imprecision due to small sample size (downgraded by one point), and wide credible intervals (downgraded by one point) for all outcomes with very low quality of evidence. In addition, we downgraded the evidence for blood transfusion quantity (red blood cells), blood loss, and operating time by two points because of the presence of substantial or considerable heterogeneity in the pair‐wise comparison or in the network.
Mortality
Mortality (perioperative)
Fourteen trials reported perioperative mortality (Belghiti 1996; Clavien 1996; Man 1997; Belghiti 1999; Wu 2002; Capussotti 2003; Man 2003; Figueras 2005; Capussotti 2006; Chen 2006; Liang 2009; Lee 2012; Ni 2013; Si‐Yuan 2014). They used seven treatments in 1196 participants. The unadjusted proportions of perioperative mortality are as follows.
Control: 5/203 (2.5%).
Continuous hepatic vascular exclusion: 0/88 (0.0%).
Continuous portal triad clamping: 6/290 (2.1%).
Continuous selective hepatic vascular exclusion: 0/80 (0.0%).
Continuous selective portal triad clamping: 0/100 (0.0%).
Intermittent portal triad clamping: 3/364 (0.8%).
Intermittent selective portal triad clamping: 1/71 (1.4%).
Based on the DIC, we chose the fixed‐effect model for all comparisons with two or more trials. There was no evidence of differences in perioperative mortality for any of the comparisons.
Mortality (longest follow‐up)
None of the trials reported this outcome.
Adverse events
Serious adverse events (proportion)
Eight trials reported the proportion of participants experiencing serious adverse events (Capussotti 2003; Capussotti 2006; Chen 2006; Liang 2009; Lee 2012; Park 2012; Ni 2013; Si‐Yuan 2014). They used six treatments in 815 participants. The unadjusted proportions of participants experiencing serious adverse events are as follows.
Control: 15/151 (9.9%).
Continuous hepatic vascular exclusion: 3/60 (5.0%).
Continuous portal triad clamping: 30/216 (13.9%).
Continuous selective hepatic vascular exclusion: 0/80 (0.0%).
Continuous selective portal triad clamping: 13/100 (13.0%).
Intermittent portal triad clamping: 23/208 (11.1%).
Direct comparison
Based on the DIC, we chose the fixed‐effect model for all comparisons with two or more trials. The serious adverse events (proportion) was lower in the group receiving continuous selective portal triad clamping than in the continuous portal triad clamping group (OR 0.42, 95% CrI 0.18 to 0.96; 120 participants; 1 study). There was no evidence of differences in other comparisons.
Network meta‐analysis
The network plots are shown in Figure 18. Based on the DIC, we chose the fixed‐effect model. There was no evidence of differences in adverse events (proportion) for any of the comparisons. Figure 19 shows the probability of each treatment being best, second best, third best, and so on. Figure 20 shows the cumulative probability of a treatment being best.
18.
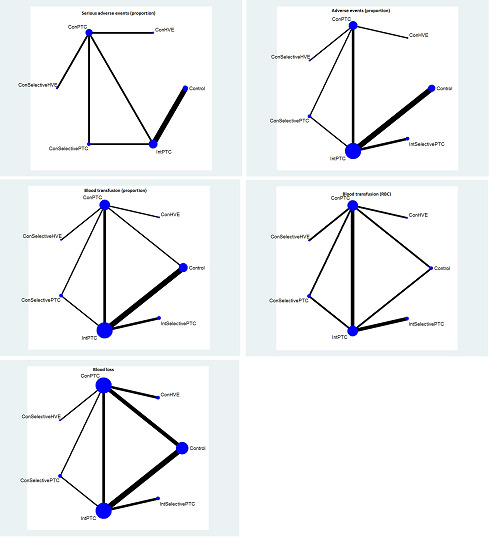
The network plot showing the comparisons in the trials included in the comparison of methods for vascular occlusion in which network meta‐analysis was performed. The size of the node (circle) provides a measure of the number of trials in which the particular treatment was included as one of the arms. The thickness of the line provides a measure of the number of direct comparisons between two nodes (treatments).
Con: continuous; Int: intermittent; HVE: hepatic vascular exclusion; PTC: portal triad clamping; RBC: red blood cells.
19.
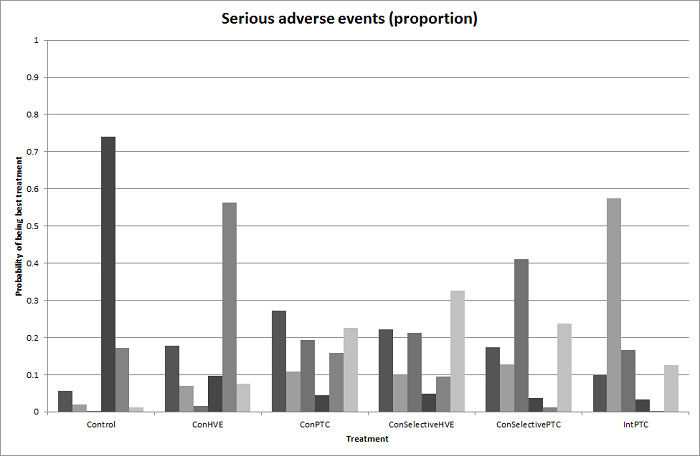
Probability of best treatment: probability of being best, second best, third best, etc. for each treatment for serious adverse events (proportion) (vascular occlusion methods). A probability of more than 90% is a reliable indicator that a treatment is best with regards to the specific outcome. A probability of less than 90% is less reliable. None of the treatments have a 90% probability of being best treatment.
Con: continuous; Int: intermittent; HVE: hepatic vascular exclusion; PTC: portal triad clamping.
20.
Cumulative probability of being best treatment: cumulative probability of being best for each treatment for vascular occlusion methods. Rank 1 indicates the probability that a treatment is best, rank 2 indicates the probability that a treatment is in the two best treatments, rank 3 indicates the probability that a treatment is in the three best treatments, and so on.
Con: continuous; Int: intermittent; HVE:hepatic vascular exclusion; PTC: portal triad clamping.
Direct evidence compared to network meta‐analysis
Figure 21 shows the information on direct evidence compared to network meta‐analysis. Although there is overlap of credible intervals, the mean indirect estimate seems to be quite different from the direct estimate (sometimes suggesting an opposite effect), thus suggesting that there may be discrepancies between direct and indirect estimates. There was little apparent difference in the quality of evidence between direct, indirect estimates, and network meta‐analysis; so, we could not choose one estimate over the others based on the quality of evidence.
21.
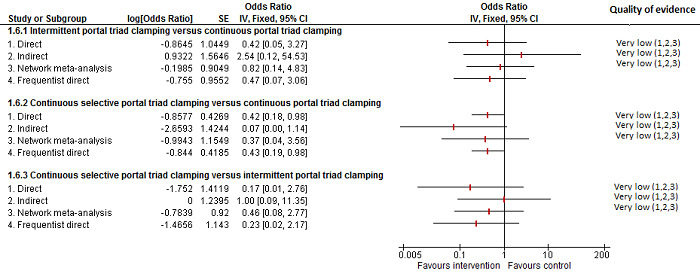
Methods of vascular occlusion: serious adverse events (proportion)
Forest plot of the comparisons in which direct and indirect estimates were available. Although there is overlap of confidence intervals, the mean indirect estimate seems to be quite different from the direct estimate (sometimes, suggesting an opposite effect), thus suggesting that there may be discrepancies between direct and indirect estimates.
There was little apparent difference in the quality of evidence between direct, indirect estimates, and network meta‐analysis; so, we could not choose one estimate over the others based on the quality of evidence.
1Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2Sample size was low (downgraded by 1 point). 3Confidence intervals spanned no effect and clinically significant effect (downgraded by 1 point).
Serious adverse events (number)
Five trials reported the number of serious adverse events (Belghiti 1996; Man 1997; Belghiti 1999; Wu 2002; Figueras 2005). They used five treatments in 376 participants. The unadjusted rates of serious adverse events (number) are as follows.
Control: 4/50 (8.0 per 100 participants).
Continuous hepatic vascular exclusion: 5/28 (17.9 per 100 participants).
Continuous portal triad clamping: 9/66 (13.6 per 100 participants).
Intermittent portal triad clamping: 16/161 (9.9 per 100 participants).
Intermittent selective portal triad clamping: 12/71 (16.9 per 100 participants).
Based on the DIC, we chose the fixed‐effect model for all comparisons with two or more trials. The number of serious adverse events was lower in the intermittent portal triad clamping group than in the continuous portal triad clamping group (rate ratio 0.09, 95% CrI 0.00 to 0.56; 86 participants; 1 study; low‐quality evidence: downgraded one point for unclear or high risk of bias in trial and one more point for small sample size). There was no evidence of differences in other comparisons.
Adverse events (proportion)
Twelve trials reported the proportion of participants experiencing adverse events (Man 1997; Belghiti 1999; Wu 2002; Capussotti 2003; Man 2003; Figueras 2005; Capussotti 2006; Chen 2006; Liang 2009; Lee 2012; Ni 2013; Si‐Yuan 2014). They used seven treatments in 1129 participants. The unadjusted proportions of adverse events (proportion) are as follows.
Control: 55/196 (28.1%).
Continuous hepatic vascular exclusion: 19/60 (31.7%).
Continuous portal triad clamping: 75/258 (29.1%).
Continuous selective hepatic vascular exclusion: 9/80 (11.3%).
Continuous selective portal triad clamping: 22/100 (22.0%).
Intermittent portal triad clamping: 109/364 (29.9%).
Intermittent selective portal triad clamping: 22/71 (31.0%).
Direct comparison
Based on the DIC, we chose the fixed‐effect model for comparisons with two or more studies. The proportion of participants experiencing adverse events was lower in the continuous selective portal triad clamping group than in the continuous portal triad clamping group (OR 0.41, 95% CrI 0.18 to 0.90; 120 participants; 1 study). There was no evidence of differences in other comparisons.
Network meta‐analysis
Figure 18 shows the network plots. Based on the DIC, we chose the fixed‐effect model. There was no evidence of differences in the proportion of participants experiencing adverse events for any of the comparisons. Figure 22 shows the probability of each treatment being best, second best, third best, and so on. Figure 20 shows the cumulative probability of a treatment being best.
22.
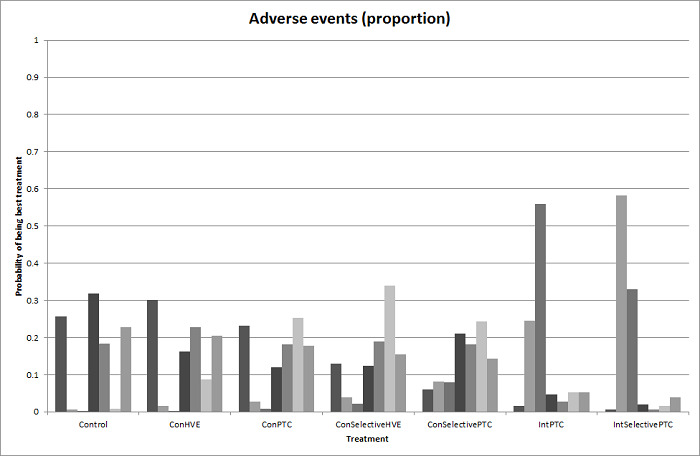
Probability of best treatment: probability of being best, second best, third best, etc. for each treatment for adverse events (proportion) (vascular occlusion methods). A probability of more than 90% is a reliable indicator that a treatment is best with regards to the specific outcome. A probability of less than 90% is less reliable. None of the treatments have a 90% probability of being best treatment.
Con: continuous; Int: intermittent; HVE: hepatic vascular exclusion; PTC: portal triad clamping.
Direct evidence compared to network meta‐analysis
Figure 23 shows the information on direct evidence compared to network meta‐analysis. There do not appear to be any discrepancies between direct and indirect estimates. Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
23.
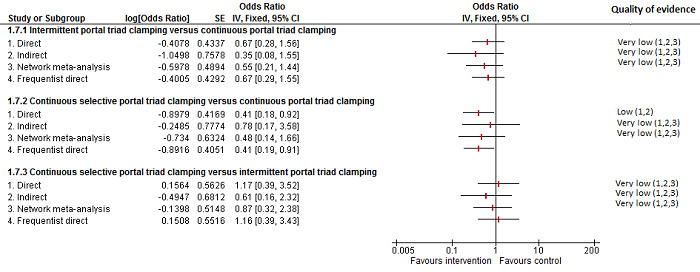
Methods of vascular occlusion: adverse events (proportion)
Forest plot of the comparisons in which direct and indirect estimates were available. There does not appear to be any discrepancies between direct and indirect estimates. Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
1Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2Sample size was low (downgraded by 1 point). 3Confidence intervals spanned no effect and clinically significant effect (downgraded by 1 point).
Adverse events (number)
Six trials reported the number of adverse events (Belghiti 1996; Man 1997; Belghiti 1999; Wu 2002; Figueras 2005; Lee 2012). They used five in 502 participants. The unadjusted rates of adverse events (number) are as follows.
Control: 47/113 (41.6 per 100 participants).
Continuous hepatic vascular exclusion: 19/28 (67.9 per 100 participants).
Continuous portal triad clamping: 28/66 (42.4 per 100 participants).
Intermittent portal triad clamping: 97/224 (43.3 per 100 participants).
Intermittent selective portal triad clamping: 36/71 (50.7 per 100 participants).
Based on the DIC, we chose the fixed‐effect model for comparisons with two or more studies. There was no evidence of differences in adverse events (number) for any of the comparisons.
Health‐related quality of life
None of the trials reported this outcome at any time point.
Blood transfusion requirements
Blood transfusion (proportion)
Thirteen trials reported the proportion of participants requiring a blood transfusion (Man 1997; Belghiti 1999; Wu 2002; Capussotti 2003; Man 2003; Chouker 2004; Figueras 2005; Capussotti 2006; Chen 2006; Liang 2009; Lee 2012; Ni 2013; Si‐Yuan 2014). They used seven treatments in 1163 participants. The unadjusted proportions of participants requiring a blood transfusion are as follows.
Control: 64/211 (30.3%).
Continuous hepatic vascular exclusion: 8/60 (13.3%).
Continuous portal triad clamping: 71/277 (25.6%).
Continuous selective hepatic vascular exclusion: 13/80 (16.3%).
Continuous selective portal triad clamping: 21/100 (21.0%).
Intermittent portal triad clamping: 101/364 (27.7%).
Intermittent selective portal triad clamping: 11/71 (15.5%).
Direct comparison
Based on the DIC, we used the random‐effects model for comparisons with two or more studies for intermittent portal triad clamping versus continuous portal triad clamping and the fixed‐effect model for the remaining comparisons with two or more studies. The proportion of participants requiring a blood transfusion was lower in the continuous portal triad clamping group than in the control (OR 0.06, 95% CrI 0.00 to 0.49; 34 participants; 1 study; low‐quality evidence: downgraded one point for unclear or high risk of bias in trial and one more point for small sample size). The blood transfusion (proportion) was higher in continuous portal triad clamping than continuous hepatic vascular exclusion (OR 5.90, 95% CrI 2.45 to 15.58; 118 participants; 1 study; low‐quality evidence: downgraded one point for unclear or high risk of bias in trial and one more point for small sample size). There was no evidence of differences in other comparisons.
Network meta‐analysis
Figure 18 shows the network plots. Based on the DIC, we chose the random‐effects model. There was no evidence of differences in the proportion of participants requiring a blood transfusion for any of the comparisons. Figure 24 shows the probability of each treatment being best, second best, third best, and so on. Figure 20 shows the cumulative probability of a treatment being the best.
24.
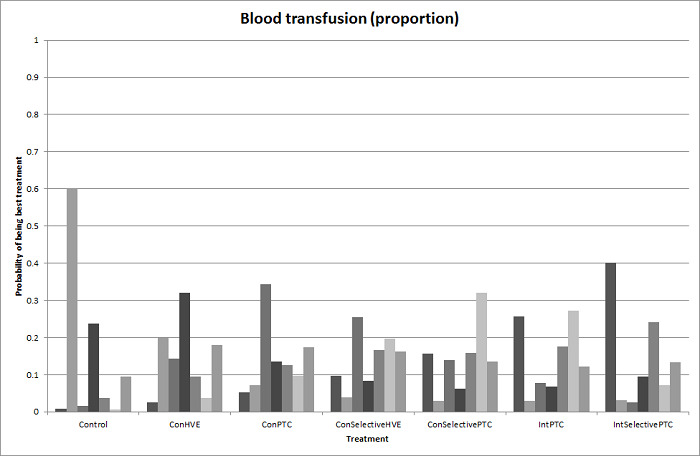
Probability of best treatment: probability of being best, second best, third best, etc. for each treatment for blood transfusion (proportion) (vascular occlusion methods). A probability of more than 90% is a reliable indicator that a treatment is best with regards to the specific outcome. A probability of less than 90% is less reliable. None of the treatments have a 90% probability of being best treatment.
Con: continuous; Int: intermittent; HVE: hepatic vascular exclusion; PTC: portal triad clamping.
Direct evidence compared to network meta‐analysis
Figure 25 shows the information on direct evidence compared to network meta‐analysis. Although the credible intervals overlap, there appears to be some discrepancies between direct and indirect estimates for continuous portal triad clamping versus control, intermittent portal triad clamping versus control, and intermittent portal triad clamping versus continuous portal triad clamping. Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
25.
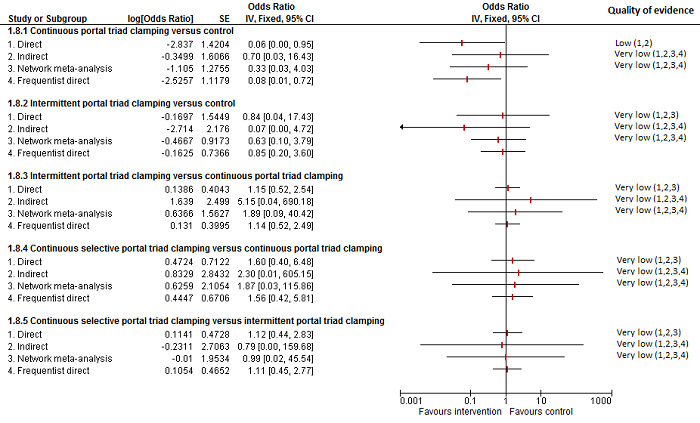
Methods of vascular occlusion: blood transfusion (proportion)
Forest plot of the comparisons in which direct and indirect estimates were available. Although the confidence intervals overlap, there appear to be some discrepancies between direct and indirect estimates for continuous portal triad clamping versus control, intermittent portal triad clamping versus control, and intermittent portal triad clamping versus continuous portal triad clamping. Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
1Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2Sample size was low (downgraded by 1 point). 3Confidence intervals spanned no effect and clinically significant effect (downgraded by 1 point). 4There was substantial or considerable heterogeneity (downgraded by 2 points).
Blood transfusion (red blood cells)
Ten trials reported blood transfusion quantity (red blood cells) (Belghiti 1996; Clavien 1996; Man 1997; Belghiti 1999; Wu 2002; Capussotti 2003; Figueras 2005; Liang 2009; Ni 2013; Si‐Yuan 2014). They usedseven treatments in 786 participants. The median and range of the mean blood transfusion quantity (red blood cells) reported for each treatment are as follows.
Control: 1.50 units and 1.90 units (two trials only).
Continuous hepatic vascular exclusion: 2.50 units (one trial only).
Continuous portal triad clamping: 1.80 units (range 0.50 to 30).
Continuous selective hepatic vascular exclusion: 1.00 unit (one trial only).
Continuous selective portal triad clamping: 1.20 units and 1.37 units (two trials only).
Intermittent portal triad clamping: 0.99125 units (range 0.00 to 2.54).
Intermittent selective portal triad clamping: 0.34 units, 2.24 units (two trials only).
Direct comparison
Based on the DIC, we chose the fixed‐effect model for comparisons with two or more studies. The blood transfusion quantity (red blood cells) was lower in the group receiving intermittent portal triad clamping than in the control (−1.50, 95% CrI −2.75 to −0.26; 100 participants; 1 study). The blood transfusion quantity (red blood cells) was lower in the group receiving continuous selective hepatic vascular exclusion than in the continuous portal triad clamping group (MD −1.20 units, 95% CrI −2.37 to −0.04; 160 participants; 1 study). The blood transfusion quantity (red blood cells) was lower in the continuous selective portal triad clamping group than in the continuous portal triad clamping group (MD −0.20 units, 95% CrI −0.31 to −0.09; 120 participants; 1 study). There was no evidence of differences in other comparisons. Exclusion of four trials in which we calculated the mean, standard deviation, or both did not change the conclusions (Man 1997; Belghiti 1999; Wu 2002; Si‐Yuan 2014).
Network meta‐analysis
Figure 18 shows the network plots. Based on the DIC, we chose the fixed‐effect model. Compared with the control group, there was evidence for a lower blood transfusion quantity (red blood cells) with continuous portal triad clamping (MD −1.25 units, 95% CrI −2.39 to −0.10), continuous selective hepatic vascular exclusion (MD −2.45 units, 95% CrI −4.08 to −0.82), continuous selective portal triad clamping (MD −1.45 units, 95% CrI −2.59 to −0.31), intermittent portal triad clamping (MD −1.36 units, 95% CrI −2.48 to −0.23), and intermittent selective portal triad clamping (MD −1.43 units, 95% CrI −2.61 to −0.24). There was no evidence of differences in other comparisons. On excluding the trials in which either mean or standard deviation was not available, there was no evidence of differences in any of the comparisons. Figure 26 shows the probability of each treatment being best, second best, third best, and so on. Figure 20 shows the cumulative probability of a treatment being best.
26.
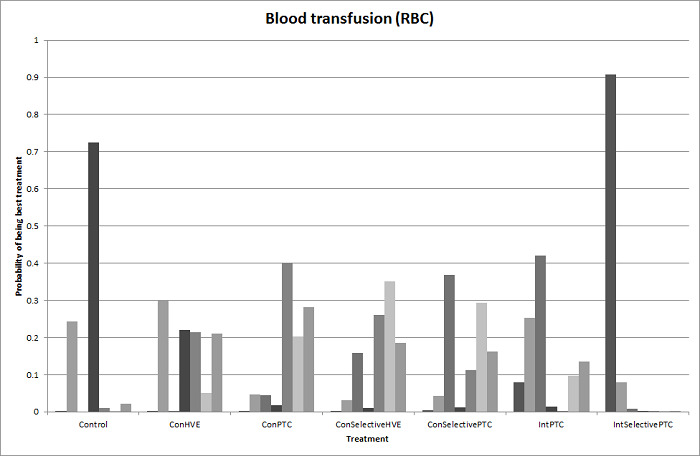
Probability of best treatment: probability of being best, second best, third best, etc. for each treatment for blood transfusion (red blood cells) (vascular occlusion methods). A probability of more than 90% is a reliable indicator that a treatment is best with regards to the specific outcome. A probability of less than 90% is less reliable. Intermittent selective portal triad clamping has about 90% probability of being best treatment. However, other random and systematic errors make this finding unreliable.
Con: continuous; Int: intermittent; HVE: hepatic vascular exclusion; PTC: portal triad clamping.
Direct evidence compared to network meta‐analysis
Figure 27 shows the information on direct evidence compared to network meta‐analysis. There do not appear to be any discrepancies between direct and indirect estimates, although the credible intervals are different (the direct evidence had narrower credible intervals in four of the five comparisons above) resulting in the differences in the comparisons in which there was evidence for difference. Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence for the comparison 'continuous selective portal triad clamping versus continuous portal triad clamping'. Indirect evidence and network meta‐analysis appear to be preferable over direct evidence for the comparison 'continuous portal triad clamping versus control'. Direct evidence and network meta‐analysis appear to be preferable over indirect evidence for the comparison 'intermittent portal triad clamping versus control'. There was little apparent difference in the quality of evidence between direct, indirect estimates, and network meta‐analysis; so, we could not choose one estimate over the others based on the quality of evidence.
27.
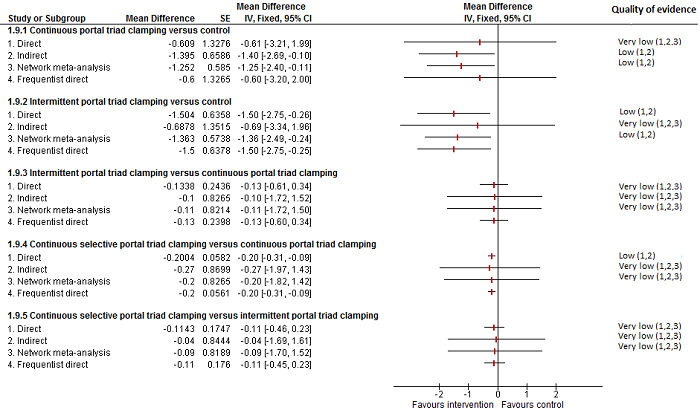
Methods of vascular occlusion:blood transfusion (red blood cells)
Forest plot of the comparisons in which direct and indirect estimates were available. There do not appear to be any discrepancies between direct and indirect estimates, although the credible intervals are different (the direct evidence had narrower credible intervals in four of the five comparisons above) resulting in the differences in the comparisons in which there was evidence for difference. Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence for the comparison 'continuous selective portal triad clamping versus continuous portal triad clamping'. Indirect evidence and network meta‐analysis appear to be preferable over direct evidence for the comparison 'continuous portal triad clamping versus control'. Direct evidence and network meta‐analysis appear to be preferable over indirect evidence for the comparison 'intermittent portal triad clamping versus control'. There was little apparent difference in the quality of evidence between direct, indirect estimates, and network meta‐analysis; so, we could not choose one estimate over the others based on the quality of evidence.
1Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2Sample size was low (downgraded by 1 point). 3Confidence intervals spanned no effect and clinically significant effect (downgraded by 1 point).
Blood transfusion (platelets)
None of the trials reported this outcome.
Blood transfusion (fresh frozen plasma)
None of the trials reported this outcome.
Blood transfusion (cryoprecipitate)
None of the trials reported this outcome.
Blood loss
Sixteen trials reported blood loss (Belghiti 1996; Man 1997; Belghiti 1999; Wu 2002; Capussotti 2003; Chouker 2004; Figueras 2005; Capussotti 2006; Chen 2006; Liang 2009; Dayangac 2010; Pietsch 2010; Lee 2012; Park 2012; Ni 2013; Si‐Yuan 2014). They used seven interventions in 1322 participants. The median and range of the mean blood loss reported for each treatment are as follows.
Control: 0.489 L (range 0.204 to 2.17).
Continuous hepatic vascular exclusion: 0.42 L and 1.195 L (two trials only).
Continuous portal triad clamping: 0.77 L (range 0.2 to 1.38).
Continuous selective hepatic vascular exclusion: 0.529 L (one trial only).
Continuous selective portal triad clamping: 0.3 L and 0.649 L (two trials only).
Intermittent portal triad clamping: 0.671 L (range 0.184 to 1.685).
Intermittent selective portal triad clamping: 0.735 L and 1.159 L (two trials only)..
Direct comparison
Based on the DIC, we chose the fixed‐effect model for intermittent portal triad clamping versus continuous portal triad clamping and the random‐effects model for the remaining comparisons with two or more studies. There was no evidence of differences in blood loss for any of the comparisons. Either the mean, the standard deviation, or both were not available in six trials (Man 1997; Wu 2002; Capussotti 2006; Pietsch 2010; Ni 2013; Si‐Yuan 2014). Excluding these trials did not alter the conclusions.
Network meta‐analysis
Figure 18 shows the network plots. Based on the DIC, we chose the random‐effects model. There was no evidence of differences in blood loss for any of the comparisons. Excluding the six trials in which either the mean, the standard deviation, or both were not available did not alter the results (Man 1997; Wu 2002; Capussotti 2006; Pietsch 2010; Ni 2013; Si‐Yuan 2014). Figure 28 shows the probability of each treatment being best, second best, third best, and so on. Figure 20 shows the cumulative probability of a treatment being best.
28.
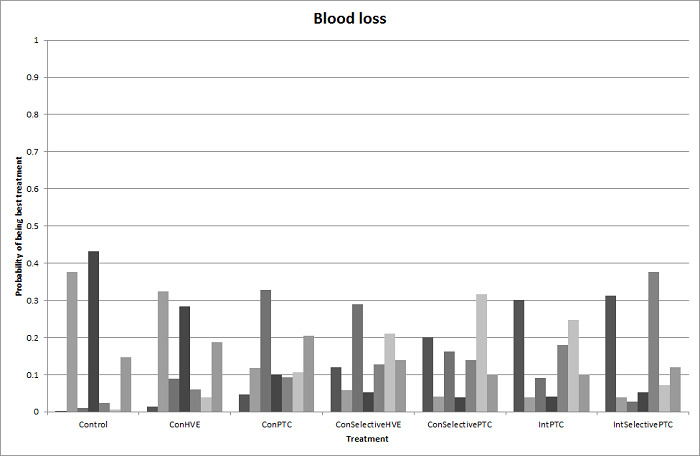
Probability of best treatment: probability of being best, second best, third best, etc. for each treatment for blood loss (vascular occlusion methods). A probability of more than 90% is a reliable indicator that a treatment is best with regards to the specific outcome. A probability of less than 90% is less reliable. None of the treatments have a 90% probability of being best treatment.
Con: continuous; Int: intermittent; HVE: hepatic vascular exclusion; PTC: portal triad clamping.
Direct evidence compared to network meta‐analysis
Figure 29 shows the information on direct evidence compared to network meta‐analysis. There do not appear to be any discrepancies between direct and indirect estimates, although the credible intervals are different (the direct evidence had narrower credible intervals in three of the five comparisons above). Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
29.
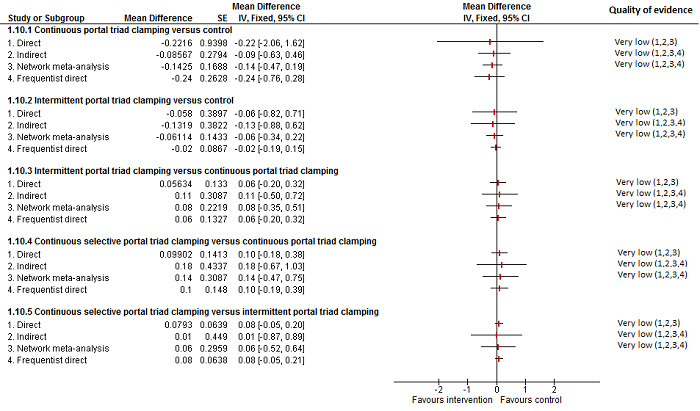
Methods of vascular occlusion:blood loss
Forest plot of the comparisons in which direct and indirect estimates were available. There does not appear to be any discrepancies between direct and indirect estimates, although the credible intervals are different (the direct evidence had narrower credible intervals in three of the five comparisons above). Direct evidence appears to be preferable over indirect evidence and network meta‐analysis based on the quality of evidence.
1Risk of bias was unclear or high in the trial(s) (downgraded by 1 point). 2 Sample size was low (downgraded by 1 point). 3Confidence intervals spanned no effect and clinically significant effect (downgraded by 1 point).Ç 4There was substantial or considerable heterogeneity (downgraded by 2 points).
Major blood loss (proportion)
Three trials reported the proportion of participants experiencing major blood loss (Lee 2012; Ni 2013; Si‐Yuan 2014), defined as more than one litre in Lee 2012 and Ni 2013 and as more than two litres in Si‐Yuan 2014. The trials used five interventions in 406 participants. The unadjusted proportions of participants experiencing major blood loss are as follows.
Control: 4/63 (6.3%).
Continuous portal triad clamping: 8/140 (5.7%).
Continuous selective hepatic vascular exclusion: 2/80 (2.5%).
Continuous selective portal triad clamping: 0/60 (0.0%).
Intermittent portal triad clamping: 5/63 (7.9%).
There was only one trial for each comparison. There was no evidence of differences in major blood loss (proportion) for any of the comparisons.
Hospital stay
Total hospital stay
Ten trials reported total hospital stay (Belghiti 1996; Man 1997; Belghiti 1999; Wu 2002; Figueras 2005; Capussotti 2006; Liang 2009; Lee 2012; Park 2012; Si‐Yuan 2014). They used seven treatments in 918 participants. The medians and ranges of the mean hospital stay reported for each treatment are as follows.
Control: 9 d (range 7 to 19).
Continuous hepatic vascular exclusion: 22 d (one trial only).
Continuous portal triad clamping: 14 d (range 13 to 14).
Continuous selective hepatic vascular exclusion: 10 d (one trial only).
Continuous selective portal triad clamping: 10 d (one trial only).
Intermittent portal triad clamping: 10 d (range 8 to 16).
Intermittent selective portal triad clamping: 8 d and 16 d (two trials only)..
Based on the DIC, we chose the fixed‐effect model for comparisons with two or more studies. The total hospital stay was lower in the continuous portal triad clamping group than in the continuous hepatic vascular exclusion group (MD −8.00 d, 95% CrI −13.03 to −2.95; 52 participants; 1 study; low‐quality evidence: downgraded one point for unclear or high risk of bias in trial and one more point for small sample size). The total hospital stay was lower in the continuous selective hepatic vascular exclusion group than in the continuous portal triad clamping group (MD −2.80 d, 95% CrI −4.13 to −1.47; 160 participants; 1 study; low‐quality evidence: downgraded 1 point for unclear or high risk of bias in trial and one more point for small sample size). There was no evidence of differences in other comparisons. Either the mean, the standard deviation, or both were not available in four trials (Man 1997; Wu 2002; Capussotti 2006; Lee 2012). Excluding these trials did not alter the conclusions except for intermittent portal triad clamping versus control. We excluded three of the four trials under this comparison because of the lack of availability of either the mean, the standard deviation, or both (Man 1997; Capussotti 2006; Lee 2012). Excluding these trials, the hospital stay was shorter in the intermittent portal triad clamping group than in the control (MD −3.51 d, 95% CrI −6.85 to −0.16; 50 participants; 1 study).
ITU stay
One trial reported ITU stay (Si‐Yuan 2014); the mean ITU stays reported for each treatment are as follows.
Continuous portal triad clamping: 1.5 d.
Continuous selective hepatic vascular exclusion: 1.2 d.
The ITU stay was lower in the continuous selective hepatic vascular exclusion group than in the continuous portal triad clamping group (MD −0.30 d, 95% CrI −0.55 to −0.06; 160 participants; 1 study).
Operating time
Twelve trials reported operating time (Belghiti 1996; Clavien 1996; Wu 2002; Capussotti 2003; Figueras 2005; Chen 2006; Liang 2009; Pietsch 2010; Lee 2012; Park 2012; Ni 2013; Si‐Yuan 2014). They used seven treatments in 919 participants. The medians and ranges of the mean operating times reported for each treatment are as follows.
Control: 292 min (range 239 to 339).
Continuous hepatic vascular exclusion: 133 min and 366 min (two trials only).
Continuous portal triad clamping: 200 min (range 116 to 301).
Continuous selective hepatic vascular exclusion: 131 min (one trial only).
Continuous selective portal triad clamping: 136 min and 236 min (two trials only).
Intermittent portal triad clamping: 241 min (range 204 to 409).
Intermittent selective portal triad clamping: 219 min and 399 min (two trials only).
Based on the DIC, we chose the fixed‐effect model for continuous portal triad clamping versus control and intermittent selective portal triad clamping versus intermittent portal triad clamping, and we used the random‐effects model for the remaining comparisons with two or more studies. The operating time was lower in the intermittent portal triad clamping group than in the continuous selective portal triad clamping group (MD −30.53 min, 95% CrI −49.68 to −11.29; 80 participants; 1 study). There was no evidence of differences in other comparisons. Either the mean, the standard deviation, or both were not available in four trials (Wu 2002; Pietsch 2010; Lee 2012; Si‐Yuan 2014). Excluding these trials did not alter the conclusions except for intermittent portal triad clamping versus control. We excluded Lee 2012 from this two‐trial comparison because no mean or standard deviation were available (Lee 2012; Park 2012). Excluding this trial, the operating time was longer in the intermittent portal triad clamping group than in the control (MD 49.63 min, 95% CrI 26.72 to 72.55; 50 participants; 1 study; low‐quality evidence: downgraded one point for unclear or high risk of bias in trial and one more point for small sample size).
Time needed to return to work
None of the trials reported this outcome.
Difference between Bayesian and frequentist meta‐analysis
The interpretation of information and conclusions did not alter by using the frequentist meta‐analysis.
Overall summary
There was no evidence of differences between the tested methods of vascular occlusion in any of the reported outcomes of interest for this review other than the following − and they all ought to be considered of low or very low quality .
The proportion of participants experiencing serious adverse events was lower in the continuous selective portal triad clamping group than in the continuous portal triad clamping group (OR 0.42, 95% CrI 0.18 to 0.96; 120 participants; 1 study).
The number of serious adverse events was lower in the intermittent portal triad clamping group than in the continuous portal triad clamping group (rate ratio 0.09, 95% CrI 0.00 to 0.56; 86 participants; 1 study).
The proportion of participants experiencing adverse events was lower in the continuous selective portal triad clamping group than in the continuous portal triad clamping group (OR 0.41, 95% CrI 0.18 to 0.90; 120 participants; 1 study).
The proportion of participants requiring a blood transfusion was lower in the continuous portal triad clamping group than in the control (OR 0.06, 95% CrI 0.00 to 0.49; 34 participants; 1 study). The proportion of participants requiring a blood transfusion was higher in the continuous portal triad clamping group than in the continuous hepatic vascular exclusion group (OR 5.90, 95% CrI 2.45 to 15.58; 118 participants; 1 study).
The blood transfusion quantity (red blood cells) was lower with continuous portal triad clamping than in the control (MD −1.25 units, 95% CrI −2.39 to −0.10; network meta‐analysis: 786 participants; 10 studies). The blood transfusion quantity (red blood cells) was lower in the intermittent portal triad clamping group than in the control (−1.50, 95% CrI −2.75 to −0.26; 100 participants; 1 study). The blood transfusion quantity (red blood cells) was lower in the continuous selective hepatic vascular exclusion group than in the continuous portal triad clamping group(MD −1.20 units, 95% CrI −2.37 to −0.04; 160 participants; 1 study). The blood transfusion quantity (red blood cells) was lower in the continuous selective portal triad clamping group than in the continuous portal triad clamping group (MD −0.20, 95% CrI −0.31 to −0.09; 120 participants; 1 study).
The hospital stay was lower in the continuous portal triad clamping group than in the continuous hepatic vascular exclusion group (MD −8.00 d, 95% CrI −13.03 to −2.95; 52 participants; 1 study). The hospital stay was lower in the continuous selective hepatic vascular exclusion group than in the continuous portal triad clamping group (MD −2.80 d, 95% CrI −4.13 to −1.47; 160 participants; 1 study).
The ITU stay was lower in the continuous selective hepatic vascular exclusion group than in the continuous portal triad clamping group (MD −0.30 d, 95% CrI −0.55 to −0.06; 160 participants; 1 study).
The operating time was lower in the intermittent portal triad clamping group than in the continuous selective portal triad clamping group (MD −30.53 min, 95% CrI −49.68 to −11.29; 80 participants; 1 study).
Pharmacological interventions
Six trials compared different pharmacological interventions (Shimada 1994; Lentschener 1997; Wong 2003; Lodge 2005; Shao 2006; Wu 2006). We did not perform network meta‐analysis since direct comparison and indirect comparison effect estimates (which would enable assessment of inconsistency) were not available for any of the outcomes.
Quality of evidence
The quality of evidence was very low for all the outcomes and comparisons unless specifically indicated within the results. This was because of unclear or high risk of bias in the trials (downgraded by one point), imprecision due to small sample size (downgraded by one point), and wide credible intervals (downgraded by one point) for all outcomes with very low quality of evidence. In addition, we downgraded the quality for blood transfusion (as a proportion of participants requiring one) by two points because of the presence of substantial or considerable heterogeneity in the pair‐wise comparison or in the network.
Mortality
Mortality (perioperative)
Two trials reported perioperative mortality (Lodge 2005; Wu 2006). They used three treatments in 399 participants. The unadjusted proportions of perioperative mortality are as follows.
Control: 3/165 (1.8%).
Recombinant factor VIIa: 4/126 (3.2%).
Tranexamic acid: 0/108 (0.0%).
There was no evidence of differences in perioperative mortality for any of the comparisons.
Mortality (longest follow‐up)
None of the trials reported this outcome.
Adverse events
Serious adverse events (proportion)
Three trials reported the proportion of participants experiencing serious adverse events (Shimada 1994; Lodge 2005; Shao 2006). They used three treatments in 456 participants. The unadjusted proportions of participants experiencing serious adverse events are as follows.
Control: 59/160 (36.9%).
Anti‐thrombin III: 4/13 (30.8%).
Recombinant factor VIIa: 111/283 (39.2%).
There was no evidence of differences in the proportion of participants experiencing serious adverse events for any of the comparisons.
Serious adverse events (number)
Three trials reported the number of serious adverse events (Lodge 2005; Shao 2006; Wu 2006). They used three treatments in 646 participants. The unadjusted rates of serious adverse events (number) are as follows.
Control: 20/255 (7.8 per 100 participants).
Recombinant factor VIIa: 35/283 (12.4 per 100 participants).
Tranexamic acid: 7/108 (6.5 per 100 participants).
There was no evidence of differences in the number of serious adverse events for any of the comparisons.
Adverse events (proportion)
Three trials reported the proportion of participants experiencing adverse events (Shimada 1994; Shao 2006; Wu 2006). A total of four treatments were used in a total of 470 participants in these studies. The unadjusted proportions of adverse events (proportion) are as follows.
Control: 98/198 (49.5%)
Anti‐thrombin III: 4/13 (30.8%)
Recombinant factor VIIa: 142/151 (94.0%)
Tranexamic acid: 14/108 (13.0%).
There was no evidence of differences in the proportion of participants experiencing adverse events for any of the comparisons.
Adverse events (number)
Three trials reported the number of adverse events (number) (Lodge 2005; Shao 2006; Wu 2006). They used three treatments in 646 participants. The unadjusted rates of adverse events (number) are as follows.
Control: 467/255 (183.1 per 100 participants).
Recombinant factor VIIa: 824/283 (291.2 per 100 participants).
Tranexamic acid: 19/108 (17.6 per 100 participants).
There was no evidence of differences in the number of adverse events reported for any of the comparisons.
Health‐related quality of life
None of the trials reported this outcome at any time point.
Blood transfusion requirements
Blood transfusion (proportion)
Five trials reported the proportion of participants requiring a blood transfusion (Lentschener 1997; Wong 2003; Lodge 2005; Shao 2006; Wu 2006). They used five treatments in 787 participants. The unadjusted proportions of participants requiring a blood transfusion (proportion) are as follows.
Control: 93/320 (29.1%).
Aprotinin: 8/48 (16.7%).
Desmopressin: 3/30 (10.0%).
Recombinant factor VIIa: 104/281 (37.0%).
Tranexamic acid: 0/108 (0.0%).
The the proportion of participants requiring a blood transfusion was lower in the aprotinin group (OR 0.31, 95% CrI 0.11 to 0.78; 97 participants; 1 study; low‐quality evidence: downgraded one point for unclear or high risk of bias in trial and one more point for small sample size) and in the tranexamic acid group than in the control (OR 0.01, 95% CrI 0.00 to 0.13; 214 participants; 1 study; low‐quality evidence: downgraded one point for unclear or high risk of bias in trial and one more point for small sample size). There was no evidence of differences in other comparisons.
Blood transfusion (red blood cells)
Four trials reported blood transfusion quantity (red blood cells) (Shimada 1994; Lentschener 1997; Lodge 2005; Shao 2006). They used four interventions in 537 participants. The median and range of the mean blood transfusion quantity (red blood cells) reported for each treatment are as follows.
Control: 2.07 units (range 0.00 to 4.40).
Anti‐thrombin III: 4.80 units (one trial only).
Aprotinin: 0.63 units (one trial only).
Recombinant factor VIIa: 0.40 and 3.00 units (two trials only).
We did not perform meta‐analysis since none of the studies provided both the mean and the standard deviation. The blood transfusion quantity (red blood cells) was lower in the aprotinin group than in the control (MD −0.94 units; P = 0.015; 97 participants; 1 study). There was no evidence of differences in other comparisons.
Blood transfusion (platelets)
Two trials reported blood transfusion quantity (platelets) (Lentschener 1997; Shao 2006). They used three treatments in 328 participants. No participants received a platelets transfusion in Lentschener 1997 (aprotinin versus control). The median platelets transfused was 0 in both groups in the other trial (Shao 2006; recombinant factor VIIa versus control).
Blood transfusion (fresh frozen plasma)
Three trials reported blood transfusion quantity (fresh frozen plasma) (Lentschener 1997; Wong 2003; Shao 2006). They used four treatments in 388 participants. The median and range of the mean or median blood transfusion quantity (fresh frozen plasma) reported for each treatment are as follows.
Control: 0.45 units (range 0.00 to 0.80).
Aprotinin: 0.04 units (one trial only).
Desmopressin: 0.20 units (one trial only).
Recombinant factor VIIa: 0.00 units (one trial only).
We did not perform meta‐analysis since either mean or standard deviation was not available in two trials (Lentschener 1997; Shao 2006). There was no evidence of differences in blood transfusion quantity (fresh frozen plasma) for any of the comparisons.
Blood transfusion (cryoprecipitate)
None of the trials reported this outcome.
Blood loss
Six trials reported blood loss (Shimada 1994; Lentschener 1997; Wong 2003; Lodge 2005; Shao 2006; Wu 2006). They used six treatments in 810 participants. The median and range of the mean blood loss reported for each treatment are as follows.
Control: 1.10 L (range 0.50 to 1.65).
Anti‐thrombin III: 1.86 L (one trial only).
Aprotinin: 1.22 L (one trial only).
Desmopressin: 0.83 L (one trial only).
Recombinant factor VIIa: 0.65 L and 1.23 L (two trials only).
Tranexamic acid: 0.30 L (one trial only).
We did not perform meta‐analysis since we imputed the mean, standard deviation, or both in five trials (Shimada 1994; Wong 2003; Lodge 2005; Shao 2006; Wu 2006). The blood loss was lower in the tranexamic acid group than in the control (difference in median: −0.30 L, P < 0.001; 214 participants; 1 study). There was no evidence of any difference in other comparisons.
Major blood loss (proportion)
None of the trials reported this outcome.
Total hospital stay
Hospital stay
One trial (214 participants) reported hospital stay (Wu 2006). The median hospital stays reported for each treatment are as follows.
Control: 9 d (one trial only).
Tranexamic acid: 8 d (one trial only).
There was no evidence of difference in median hospital stay between the groups.
ITU stay
None of the trials reported this outcome.
Operating time
Five trials reported operating time (Shimada 1994; Lentschener 1997; Wong 2003; Lodge 2005; Wu 2006). They used six treatments in 580 participants. The medians and ranges of the mean operating times reported for each treatment are as follows.
Control: 261 min (range 233 to 435).
Anti‐thrombin III: 233 min (one trial only).
Aprotinin: 232 min (one trial only).
Desmopressin: 405 min (one trial only).
Recombinant factor VIIa: 230 min (one trial only).
Tranexamic acid: 254min (one trial only).
The mean, standard deviation or both were not available from four studies (Shimada 1994; Wong 2003; Lodge 2005; Wu 2006). The operating time was lower in the tranexamic acid group than in the control group (difference in medians −52.20 min; P = 0.003; 214 participants; 1 study; low‐quality evidence: downgraded one point for unclear or high risk of bias in trial and one more point for small sample size). There was no evidence of differences in other comparisons.
Time needed to return to work
None of the trials reported this outcome.
Difference between Bayesian and frequentist meta‐analysis
The interpretation of information and conclusions did not alter by using the frequentist meta‐analysis.
Overall summary
There was no evidence of differences between different pharmacological interventions in any of the reported outcomes of interest for this review other than the following.
The proportion of participants requiring a blood transfusion was lower in the aprotinin group (OR 0.31, 95% CrI 0.11 to 0.78; 97 participants; 1 study) and in the tranexamic acid group (OR 0.01, 95% CrI 0.00 to 0.13; 214 participants; 1 study) than in the control.
The blood transfusion quantity (red blood cells) was lower in the aprotinin group than in the control (MD −0.94 units; P = 0.015; 97 participants; 1 study).
The blood loss was lower in the tranexamic acid group than in the control (difference in median: −0.3 L, P < 0.001; 214 participants; 1 study).
The operating time was lower in the tranexamic acid group than in the control (difference in medians −52.20 min; P = 0.003; 214 participants; 1 study).
Overall summary across all interventions
Mortality (perioperative)
There was no evidence of differences in perioperative mortality for any of the comparisons for which this information was available.
Mortality at longest follow‐up
There was no evidence of differences in mortality at longest follow‐up for any of the comparisons for which this information was available.
Serious adverse events (proportion)
The proportion of participants experiencing serious adverse events was lower in the continuous selective portal triad clamping group than in the continuous portal triad clamping group (OR 0.42, 95% CrI 0.18 to 0.96; 120 participants; 1 study).
There was no evidence of differences in other comparisons for which this information was available.
Serious adverse events (number)
The number of serious adverse events was higher in the fibrin sealant group than in the argon beam group (rate ratio 4.81, 95% CrI 1.73 to 17.5; 121 participants; 1 study).
The number of serious adverse events was lower in the intermittent portal triad clamping group than in the continuous portal triad clamping group (rate ratio 0.09, 95% CrI 0.00 to 0.56; 86 participants; 1 study).
There was no evidence of differences in other comparisons for which this information was available.
Adverse events (proportion)
The proportion of participants experiencing adverse events was lower in the continuous selective portal triad clamping group than in the continuous portal triad clamping group (OR 0.41, 95% CrI 0.18 to 0.90; 120 participants; 1 study).
There was no evidence of differences in other comparisons for which this information was available.
Adverse events (number)
The number of adverse events was higher with radiofrequency dissecting sealer than with the clamp‐crush method (rate ratio 1.85, 95% CrI 1.07 to 3.26; 250 participants; 3 studies) (Bayesian analysis only: both direct and network meta‐analysis).
There was no evidence of differences in other comparisons for which this information was available.
Health‐related quality of life
None of the trials reported this outcome.
Blood transfusion (proportion)
The proportion of participants requiring a blood transfusion was lower in the group receiving an autologous blood donation than in the control (OR 0.18, 95% CrI 0.04 to 0.66; 42 participants; 1 study).
The proportion of participants requiring a blood transfusion was higher in the low central venous pressure group than in the acute normovolemic haemodilution plus low central venous pressure group (OR 3.19, 95% CrI 1.56 to 6.95; 208 participants; 2 studies).
The proportion of participants requiring a blood transfusion was lower in the continuous portal triad clamping group than in the control (OR 0.06, 95% CrI 0.00 to 0.49; 34 participants; 1 study). The proportion of participants requiring a blood transfusion was higher in the continuous portal triad clamping group than in the continuous hepatic vascular exclusion group (OR 5.90, 95% CrI 2.45 to 15.58; 118 participants; 1 study).
The proportion of participants requiring a blood transfusion was lower in the aprotinin group (OR 0.31, 95% CrI 0.11 to 0.78; 97 participants; 1 study) and in the tranexamic acid group than in the control (OR 0.01, 95% CrI 0.00 to 0.13; 214 participants; 1 study).
There was no evidence of differences in other comparisons for which this information was available.
Blood transfusion (red blood cells)
Compared to control, the blood transfusion quantity (red blood cells) was lower in the acute normovolemic haemodilution group (MD −1.25 units, 95% CrI −1.75 to −0.74; 20 participants; 1 study) and in the acute normovolemic haemodilution plus hypotension group (MD −1.67 units, 95% CrI −2.06 to −1.32; 20 participants; 1 study). The blood transfusion quantity (red blood cells) was higher in the acute normovolemic haemodilution plus low central venous pressure group than in the control (MD 0.27 units, 95% CrI 0.01 to 0.52; 30 participants; 1 study).
The blood transfusion quantity (red blood cells) was lower in the hydrojet group than in the cavitron ultrasonic surgical aspirator group (MD −0.98 units, 95% CrI −1.90 to −0.06; 61 participants; 1 study).
The blood transfusion quantity (red blood cells) was lower in the fibrin sealant group than in the control (MD −0.53 units, 95% CrI −1.00 to −0.07; 122 participants; 2 studies). The blood transfusion quantity (red blood cells) was higher in the fibrin sealant group than in the cyanoacrylate group (MD 2.20 units; 95% CrI 1.59 to 2.81; 30 participants; 1 study).
The blood transfusion quantity (red blood cells) was lower with continuous portal triad clamping than control (MD −1.25 units, 95% CrI −2.39 to −0.10; network meta‐analysis: 786 participants; 10 studies). The blood transfusion quantity (red blood cells) was lower in the intermittent portal triad clamping group than in the control (−1.50, 95% CrI −2.75 to −0.26; 100 participants; 1 study). The blood transfusion quantity (red blood cells) was lower in the continuous selective hepatic vascular exclusion group than in the continuous portal triad clamping group (MD −1.20 units, 95% CrI −2.37 to −0.04; 160 participants; 1 study). The blood transfusion quantity (red blood cells) was lower in the continuous selective portal triad clamping group than in the continuous portal triad clamping group (MD −0.20, 95% CrI −0.31 to −0.09; 120 participants; 1 study).
The blood transfusion quantity (red blood cells) was lower in the aprotinin group than in the control (MD −0.94; P = 0.015; 97 participants; 1 study).
There was no evidence of differences in other comparisons for which this information was available.
Blood transfusion (platelets)
There was no evidence of differences in blood transfusion quantity (platelets) in any of the comparisons for which this information was available.
Blood transfusion (fresh frozen plasma)
The blood transfusion quantity (fresh frozen plasma) was lower in the low central venous pressure group than in the control (MD −2.48 units, 95% CrI −3.58 to −1.37; 50 participants; 1 study).
The blood transfusion quantity (fresh frozen plasma) was lower in the fibrin sealant group than in the cyanoacrylate group (MD −0.81 units, 95% CrI −1.04 to −0.62; 30 participants; 1 study). The blood transfusion quantity (fresh frozen plasma) was higher in the oxidised cellulose group than in the fibrin sealant group (MD 0.53 units, 95% CrI 0.36 to 0.71; 80 participants; 2 studies).
There was no evidence of differences in other comparisons for which this information was available.
Blood transfusion (cryoprecipitate)
There was no evidence of differences in blood transfusion quantity (cryoprecipitate) in any of the comparisons for which this information was available.
Blood loss
The blood loss was lower in the acute normovolemic haemodilution plus hypotension group (MD −0.25 L; 95% CrI −0.37 to −0.13; 20 participants; 1 study) and in the low central venous pressure group than in the control (MD −0.34 L, 95% CrI −0.46 to −0.22; 237 participants; 4 studies). The blood loss was lower in the acute normovolemic haemodilution plus hypotension group than in the acute normovolemic haemodilution group (MD −0.25; 95% CrI −0.40 to −0.10; 20 participants; 1 study).
The blood loss was lower in the tranexamic acid group than in the control (difference in median: −0.3 L, P < 0.001; 214 participants; 1 study).
There was no evidence of differences in other comparisons for which this information was available.
Major blood loss (proportion)
There was no evidence of differences in the proportion of participants experiencing major blood loss in any of the comparisons for which this information was available.
Hospital stay
The total hospital stay was lower in the low central venous pressure group than in the control (MD −2.42 d, 95% CrI −3.91 to −0.94; 197 participants; 3 studies).
The total hospital stay was lower in the continuous portal triad clamping group than in the continuous hepatic vascular exclusion group (MD −8.00 d, 95% CrI −13.03 to −2.95; 52 participants; 1 study). The total hospital stay was lower in the continuous selective hepatic vascular exclusion group than in the continuous portal triad clamping group (MD −2.80 d, 95% CrI −4.13 to −1.47; 160 participants; 1 study).
There was no evidence of differences in other comparisons for which this information was available.
ITU stay
The ITU stay was lower in the continuous selective hepatic vascular exclusion group than in the continuous portal triad clamping group (MD −0.30 d, 95% CrI −0.55 to −0.06; 160 participants; 1 study).
There was no evidence of differences in other comparisons for which this information was available.
Operating time
The operating time was lower in the low central venous pressure group than in the control (MD −15.32 min, 95% CrI −29.03 to −1.69; 192 participants; 4 studies).
The operating time was lower in the stapler resection group than in the clamp‐crush method group with frequentist meta‐analysis (MD −31.00 min, 95% CI −60.40 to −1.60; 130 participants; 1 study) (frequentist analysis only).
The operating time was higher in the fibrin sealant and collagen group than in the control (MD 19.72 min, 95% CrI 2.93 to 36.57; 300 participants; 1 study).
The operating time was lower in the intermittent portal triad clamping group than in the continuous selective portal triad clamping group (MD −30.53 min, 95% CrI −49.68 to −11.29; 80 participants; 1 study).
The operating time was lower in the tranexamic acid group than in the control (difference in medians −52.20 min; P = 0.003; 214 participants; 1 study).
There was no evidence of differences in other comparisons for which this information was available.
Time needed to return to work
None of the trials reported this outcome.
Subgroup analysis
We did not perform subgroup analyses because of the paucity of data.
Reporting bias
For outcomes with 10 or more trials, we explored reporting bias using funnel plots. There were nine comparisons with at least 10 trials. Of these, there was no evidence of funnel plot asymmetry on visualisation for perioperative mortality for methods of parenchymal transection, methods of dealing with cut surface, or methods of vascular occlusion. There was funnel plot asymmetry in the remaining six comparisons, all of which fall under the comparison of different methods of vascular occlusion: adverse events (proportion), blood transfusion (proportion), blood transfusion (red blood cells), blood loss, hospital stay, and operating time. The funnels plots of blood transfusion (proportion), blood transfusion (red blood cells), and blood loss are shown in Figure 30, Figure 31, and Figure 32.
30.

Funnel plot of blood transfusion (proportion): The funnel plot shows funnel plot asymmetry (i.e. some trials with large variance with large effects favouring one treatment were not matched by other trials with similarly large variance with large effects favouring the other treatment). This may be evidence of reporting bias or could be because of heterogeneity between the studies.
31.
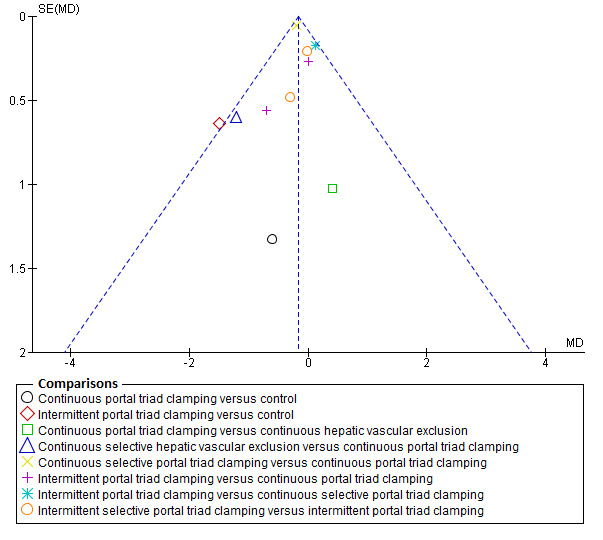
Funnel plot of blood transfusion (red blood cells): The funnel plot shows funnel plot asymmetry (i.e. some trials with large variance with large effects favouring one treatment were not matched by other trials with similarly large variance with large effects favouring the other treatment). This may be evidence of reporting bias or could be because of heterogeneity between the studies.
32.
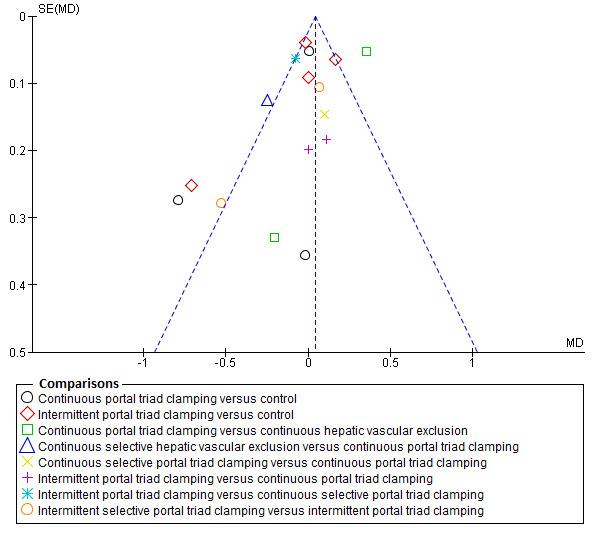
Funnel plot of blood loss: The funnel plot shows funnel plot asymmetry (i.e. some trials with large variance with large effects favouring one treatment were not matched by other trials with similarly large variance with large effects favouring the other treatment). This may be evidence of reporting bias or could be because of heterogeneity between the studies.
Since none of the comparisons had 10 or more trials, we did not perform Egger's test to assess the funnel plot asymmetry.
Discussion
Summary of main results
In this updated network meta‐analysis, we compared all the interventions aimed at decreasing blood loss and blood transfusion requirements in people undergoing liver resection. We included 67 randomised clinical trials involving 6197 participants in this review. A total of 5771 participants from 64 trials provided data for one or more outcomes assessed.
In order to perform a network meta‐analysis, it is necessary to satisfy the transitivity assumption, that is, the participants had to be sufficiently similar across the pair‐wise comparisons. While some trials restricted their participant recruitment to those with cirrhotic livers or those who were undergoing major liver resections, others did not. Although there is no clear evidence for an interaction between the presence of cirrhosis or extent of liver resection and the treatment effect, lack of evidence supporting an interaction does not mean that one does not exist. For example, experimental research has shown that cirrhotic livers are more susceptible to ischaemia than normal livers (Figueras 1997; Jang 2008). So vascular occlusion may be beneficial in limiting blood loss in people without cirrhosis while the same treatment may be harmful in people with cirrhotic liver. When different trials use different types of participants (with regards to the presence of cirrhosis), this may lead to problems with clinical heterogeneity in pair‐wise comparisons and undermine the transivitiy assumption in network meta‐analysis. Similarly, a method of treating the cut surface may be more beneficial in people undergoing major liver resections with larger cut surfaces than in those undergoing minor liver resections with smaller cut surfaces that bleed. In the presence of sufficient data, we could have assessed the interaction between the treatment effects and the presence of cirrhosis and the extent of liver resection; however, this was not possible because of paucity of data. So we are unable to comment on the transitivity assumption. We performed network meta‐analyses only when direct and indirect effect estimates for one of more comparisons in a network. This allowed us to evaluate inconsistency in the network. Although we did not find any inconsistency in the networks, lack of evidence of inconsistency did not indicate that the results were consistent. With the paucity of data due to few trials and few participants under each comparison, we were unable to make any firm conclusions about inconsistency. Likewise, the paucity of data decreases the confidence in the results of the network meta‐analysis. As a result of these limitations, readers should interpret our network meta‐analysis with caution. Nevertheless, these results provide relative estimates between treatments that have not been compared in head‐to‐head comparisons.
We present the summary of findings in the Table 1, Appendix 9, and Appendix 10, as well as in the Results section. There was no evidence of differences in most of the comparisons, and where such differences existed, they were in single trials, mostly of small sample size. Without confirmation of the findings in additional trials, combined with lack of reporting in some (possibly because of selective outcome reporting), the evidence from these single trials is not reliable. So we discuss only the evidence that was available in more than one trial below. Of the primary outcomes, the only comparison showing evidence of a difference was in the number of adverse events, which was higher with radiofrequency dissecting sealer than with the clamp‐crush method (rate ratio 1.85, 95% CrI 1.07 to 3.26; 250 participants; 3 studies). However, even for this comparison, the credible intervals overlap a clinically non‐significant difference (i.e. < 20% difference). So, there is significant uncertainty in the difference in the number of adverse events between those operated on with the radiofrequency dissecting sealer compared to the clamp‐crush method due to imprecision in addition to the uncertainty caused by the risk of bias in the trials.
There was no evidence of a reduction in mortality for any of the interventions. Major blood loss may cause multiorgan failure leading to sepsis and death. Mortality was generally low in all the groups compared to that reported in previous studies (Finch 2007). This may be because of the careful selection of participants included in randomised clinical trials compared to a consecutive patient series, which report the results of all liver resections. We have provided the sample size calculations based on the mortality observed in the control groups of 1.8%. To demonstrate a significant 20% relative reduction in mortality (20% relative risk reduction) from 1.8% to 1.4%, approximately 38,000 participants are required for a single direct comparison with one intervention. As shown in the Appendix 7, the effective sample size in an indirect comparison involving just three treatments is only a fraction of the number of participants included in the trials. For example, 10,000 participants included in the indirect comparisons is equivalent to fewer than 2000 'direct' participants in the absence of heterogeneity and fewer than 1000 'direct' participants in the presence of moderate heterogeneity. Even without these complicated calculations, one can easily observe that the credible intervals were very wide, meaning that we cannot rule out a significant benefit or harm for different treatments in terms of mortality. Approximately 16.7% of people in the control group (as defined above) developed serious adverse events. To demonstrate a significant 20% relative reduction in serious adverse events (20% relative risk reduction) from 16.7% to 13.4%, approximately 3592 participants are required for a single direct comparison with a specific intervention. This critical mass of information has not been reached, and there is a significant risk of both type I (alpha) and type II (beta) random errors, that is, there is a significant risk of making false positive and false negative conclusions. Given the number of participants required to show a significant benefit of treatment with relation to mortality and serious adverse events, it is unlikely that trials of the adequate magnitude will be funded.
Of the secondary outcomes, the main outcome measure of the included trials was blood loss and transfusion requirement. The only comparisons with more than one trial where there was evidence of difference were the following: the proportion of participants requiring a blood transfusion was higher in the low central venous pressure group than in the acute normovolemic haemodilution plus low central venous pressure group; blood transfusion (red blood cells) was lower in the fibrin sealant group than in the control; blood transfusion (fresh frozen plasma) was higher in the oxidised cellulose group than in the fibrin sealant group; and blood loss, total hospital stay, and operating time were lower with low central venous pressure than in the control. Trials measured blood loss in different ways. Most reports did not specify whether they measured the amount of blood obtained in the suction, weighed the swabs, or measured the decrease in haemoglobin. In any case, this is only important if the intervention decreases the blood transfusion requirements, operating time, or serious adverse events. Except for low central venous pressure, which decreases blood loss, operating time, and hospital stay, none of the interventions consistently lowered the blood transfusion requirements or improved other clinical outcomes.
Approximately 21.8% of people in the control group required a blood transfusion. Decreasing this need can reduce transfusion‐related anaphylactic reactions and transmission of transfusion‐related diseases. In addition, there are significant costs associated with blood transfusion, so this is an important outcome. To demonstrate a (significant) 20% relative reduction in serious adverse events (20% relative risk reduction) from 21.8% to 17.4%, approximately 2600 participants are required for a single direct comparisonwith a specific intervention. This critical mass of information has not been reached, and there is significant risk of both alpha and beta random errors in secondary outcomes also.
None of the trials reported quality of life, which is an important outcome used to assess the cost‐effectiveness of a treatment in a state‐funded healthcare system. Given that the quality of life would depend upon various factors including perioperative complications, length of hospital stay, and time to return to work, it is likely to be easier to demonstrate a significant difference in quality of life if the treatment is effective than to demonstrate a difference in mortality or serious adverse events. Future randomised clinical trials should use a validated quality of life measure as one of the outcomes. Serious adverse events are likely to result in decreased quality of life for patients and increased costs to the healthcare provider and are, therefore, more important endpoints than a modest decrease in blood transfusion. Length of total hospital stay and intensive therapy unit stay are important to the patients, their carers, and the healthcare funders. These should be reported in future trials assessing interventions to decrease blood loss or blood transfusion requirements. None of the trials reported time taken to return to work, which is an important outcome for the patient and their carers in the absence of significant sickness benefit and is an important outcome for the healthcare provider in a state‐funded healthcare system with significant sickness benefits.
The major purpose of using different methods of liver resection is to limit blood loss and blood transfusion requirements. Some methods do not require any additional equipment (e.g. vascular occlusion), while other methods do (e.g. cavitron ultrasonic surgical aspirator or radiofrequency dissecting sealer). None of the interventions that require special equipment were better than the clamp‐crush method in terms of blood transfusion requirements or other important patient‐oriented outcomes and hence cannot be recommended over the standard. However, as mentioned previously, there is a significant risk of random errors because of the small sample sizes and possibly important benefits or harms.
Overall completeness and applicability of evidence
The participants included in this trial underwent elective open liver resection and were generally anaesthetically fit. The findings of this review are applicable only to such patients.
Quality of the evidence
The overall quality of evidence was low or very low as shown in Table 1, Appendix 9, and Appendix 10. The risk of bias was high in many of the domains in the trials. Using appropriate methods of randomisation and reporting the method of randomisation adequately will decrease selection bias. While surgeons who perform the surgery cannot be blinded to the treatments, it is possible to blind the surgeons who are involved in the day‐to‐day postoperative management of the patient. While it may be difficult to blind the anaesthetist to the treatment groups, using objective criteria for transfusion may overcome the problem of bias due to lack of blinding with regards to intraoperative blood transfusion (NHS Blood and Transplant 2007). The intensivist involved in the postoperative care of the patient can be easily blinded. Objective criteria for detection of complications along with the postoperative management of the patient by a healthcare team not involved in the operation can decrease detection and performance bias. Even if blinding of participants and healthcare providers was excluded as a criterion to classify a trial as being at low risk of bias (i.e. even if we considered that trials were at low risk of bias if they were classified as low risk of bias in all domains other than blinding of participants and healthcare providers), we would not have classified any of the trials as being at low risk of bias. With regards to dropouts, randomising the participants after confirming that the tumour can be removed can avoid postrandomisation dropouts due to metastatic spread identified at the time of laparotomy. This can decrease attrition bias. Reporting all the important clinical outcomes can decrease selective reporting bias.
There was heterogeneity in some of the comparisons, which resulted in downgrading the level of evidence, but we did not observe heterogeneity in most of the comparisons in which there were two or more trials. However, it was not possible to assess the consistency of evidence in many comparisons because of the presence of single trials.
The effect estimates were wide with the credible intervals spanning either 0.80 (a 20% reduction) or 1.20 (a 20% increase), which both can be considered clinically significant effects. The total number of participants included in the analysis was only a small fraction of the required sample size even without adjustment for heterogeneity. These findings indicate that there is significant risk of imprecision in all the comparisons. Future trials should be adequately powered to decrease the risk of random errors. There was no indirectness of evidence for any of the outcomes. Although we did not find any reporting bias since the paucity of trials precluded the creation of funnel plots, many of the trials did not adequately report a number of important outcomes. Only 25 trials (37.3%) reported mortality and serious adverse events, although these outcomes ought to be routinely measured in trials comparing interventions aimed at limiting blood loss. This suggests indirect evidence of reporting bias.
Potential biases in the review process
We selected a range of databases without any language restrictions and conducted the meta‐analysis according to the NICE TSU (Dias 2012a; Dias 2012b; Dias 2012c; Dias 2013a; Dias 2013b; Dias 2013c; Dias 2013d; Dias 2013e). We performed network meta‐analysis only when the treatments were connected to each other and only when it was possible to obtain the direct and indirect estimates for a comparison. This allowed us to evaluate the quality of evidence of direct estimates, indirect estimates, and network meta‐analysis estimates, choosing the estimates with the best quality of evidence. These are the strengths of the review process.
The major potential source of bias was that we considered each of these interventions (different methods of cardiopulmonary interventions, parenchymal transection methods, methods of dealing with raw surface, vascular occlusion methods, and pharmacological interventions) as separate networks. This was due to the lack of sufficient information in the trials (which resulted in very few trials in the previous version) and the design of the trials. In many of the trials, the surgeons involved in the trial were allowed to choose their method of liver resection apart from the factor being randomised. This design is based on the assumption that the other factors are independent of each other, that is, there is no interaction between the factors, or the choice of one factor is not dependent upon the choice of another factor. There is no evidence to support or refute this assumption. However, if we planned to include only trials in which all the factors were included, we would not even have been able to include as many trials as we did in the previous version, as we have now included all the interventions aimed at limiting blood loss and blood transfusion requirements during liver resection. Each of the factors are independent of other, i.e. the method of parenchymal transection does not affect the method of vascular occlusion that the surgeons use. However, it is quite possible that there were interactions between the different methods. For example, when a parenchymal transection method with high blood loss was chosen, additional interventions such as fibrin glue may have been used to deal with the cut surface (although there is currently no evidence that fibrin glue is effective). Such use may not necessarily mean that there was an interaction unless there was a systematic difference in the use of the other methods for limiting blood loss between the intervention and control. However, it is only possible to assess this if there are details about all the methods to decrease blood loss from the trial report. Future trials should describe the methods used for reducing blood loss even if it was not the factor being randomised. It is only possible to assess the presence of interaction (i.e. the intervention is more effective or less effective depending upon the presence or absence of a second factor) in well‐designed factorial trials. However, the sample size required to detect interaction is much higher than the usual primary analysis of the 'margins'. It is highly unlikely that trials powered to measure interactions can be conducted because of this very large sample size.
We excluded studies that compared variations in the methods listed in Table 9, Table 10, Table 11, and Table 12 and treated variations in the method as single treatment. For example, we included intermittent portal triad clamping of differing durations as a single treatment and did not include comparisons of different methods of intermittent portal triad clamping, unless trials compared them with a different method of vascular occlusion. Hence, this review does not provide information on whether one variation is better than another. We imputed the standard deviations when they were not available from the trials. We performed a sensitivity analysis in all these situations, and there were no changes in results.
Another major limitation of the review was the paucity of data. Many of the networks had few closed loops (i.e. where direct and indirect evidence was available for a particular comparison). Along with this, there were few trials included under each comparison. This also makes the assessment of inconsistency underpowered. Lack of evidence of inconsistency should not be considered the same as lack of inconsistency. This paucity of data decreases the confidence in the results of the network meta‐analysis.
Different interventions may have different effects based on on the extent of liver resection and whether the underlying liver was diseased. However, we were unable to assess this because of paucity of data.
We included only randomised clinical trials in this review. While this is the best way to prevent arriving at biased false conclusions on the benefits of a treatment, the harms of treatment may not be fully captured. This is because of the highly selected group of people who enter into randomised clinical trials compared to clinical practice. In addition, randomised clinical trials may not report rare or late serious adverse events, simply due to their generally small sample size and short duration of follow‐up.
Agreements and disagreements with other studies or reviews
This is an update of ourfirst network meta‐analysis on methods to reduce blood loss during liver resection from 2014 (Simillis 2014). In that review, we concluded that liver resection using a radiofrequency dissecting sealer without vascular occlusion or fibrin sealant may increase serious adverse events. In that review as well, we highlighted the paucity of data. Previously, we also compared individual components included in this review and concluded that intermittent vascular occlusion and the clamp‐crush method may decrease blood loss (Gurusamy 2009a; Gurusamy 2009b). In this review, we concluded that there is no evidence for any significant advantage of different methods of liver resection with regards to blood loss. The differences in conclusion may be because of the decreased importance that we have given to single trials of small sample size and inclusion of trials in which the methods were not reported or when the other aspects of liver resection other than the component being compared were chosen in a non‐random manner.
Authors' conclusions
Implications for practice.
Paucity of data meant that we could not assess the transitivity assumption or inconsistency for most analyses. When direct and indirect comparisons were available, network meta‐analysis provided additional effect estimates for comparisons where there were no direct comparisons. However, the paucity of data decreases the confidence in the results of the network meta‐analysis. Low‐quality evidence suggests that liver resection using a radiofrequency dissecting sealer may be associated with more adverse events than with the clamp‐crush method. Low‐quality evidence also suggests that the proportion of participants requiring a blood transfusion was higher in the groups receiving low central venous pressure than in those receiving acute normovolemic haemodilution plus low central venous pressure; very low‐quality evidence suggests that blood transfusion quantity (red blood cells) was lower in the fibrin sealant group than in the control; blood transfusion quantity (fresh frozen plasma) was higher in the oxidised cellulose group than in the fibrin sealant group; and blood loss, total hospital stay, and operating time were lower with low central venous pressure than control. There is no evidence to suggest that using special equipment for liver resection is of any benefit in decreasing the mortality, morbidity, or blood transfusion requirements (very low‐quality evidence). Radiofrequency dissecting sealer should not be used outside the clinical trial setting since there is low‐quality evidence for increased harm without any evidence of benefits. In addition, it should be noted that the sample size was small and the credible intervals were wide, and considerable benefit or harm with a specific method of liver resection cannot be ruled out.
Implications for research.
Trials need to be conducted and reported according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement (www.spirit‐statement.org/) and the CONSORT (Consolidated Standards for Reporting of Trials) statement (www.consort‐statement.org). Future randomised clinical trials ought to include people at higher anaesthetic risk eligible for liver resection and to blind outcome assessors.
What's new
| Date | Event | Description |
|---|---|---|
| 18 July 2016 | New citation required and conclusions have changed | The conclusions changed from "Very low quality evidence suggested that liver resection using a radiofrequency dissecting sealer without vascular occlusion or fibrin sealant may increase serious adverse events, and this should be evaluated in further randomised clinical trials. The risk of serious adverse events with liver resection using no special equipment compared with more complex methods requiring special equipment was uncertain due to the very low quality of the evidence. The credible intervals were wide and considerable benefit or harm with a specific method of liver resection cannot be ruled out" into "Low‐quality evidence suggests that liver resection using a radiofrequency dissecting sealer may be associated with more adverse events than with the clamp‐crush method. Low‐quality evidence also suggests that the proportion of participants requiring a blood transfusion was higher in the groups receiving low central venous pressure than in those receiving acute normovolemic haemodilution plus low central venous pressure; very low‐quality evidence suggests that blood transfusion quantity (red blood cells) was lower in the fibrin sealant group than in the control; blood transfusion quantity (fresh frozen plasma) was higher in the oxidised cellulose group than in the fibrin sealant group; and blood loss, total hospital stay, and operating time were lower with low central venous pressure than control. There is no evidence to suggest that using special equipment for liver resection is of any benefit in decreasing the mortality, morbidity, or blood transfusion requirements (very low‐quality evidence). Radiofrequency dissecting sealer should not be used outside the clinical trial setting since there is low‐quality evidence for increased harm without any evidence of benefits. In addition, it should be noted that the sample size was small and the credible intervals were wide, and considerable benefit or harm with a specific method of liver resection cannot be ruled out." |
| 18 July 2016 | New search has been performed | We performed a new search on 23 September 2015. Because of the revised inclusion criteria, we could include 67 trials, compared to 9 trials in the previous version. |
| 16 July 2016 | Amended | We revised the inclusion criteria and methods. This allowed the inclusion of 67 trials, compared to 9 trials in the previous version. This also led to changes in the conclusions. |
Notes
Considerable overlap is evident in the Background and Methods sections of this review and those of several other reviews written by the same group of authors.
Author order was changed in August 2013 as follows: Constantinos Simillis, Tianjing Li, Jessica Vaughan, Lorne Becker, Brian Davidson, Kurinchi Gurusamy.
Author order was changed in October 2016 as follows: Elisabetta Moggia, Benjamin Rouse, Constantinos Simillis, Tianjing Li, Jessica Vaughan, Brian Davidson, Kurinchi Gurusamy.
Acknowledgements
Peer reviewers of the current version: Lifeng Lin, USA; Yong Chen, USA; Silvio Nadalin, Germany; Theis Lange, Denmark. Contact editor: Janus Christian Jakobsen, Denmark. Sign‐off editor: Christian Gluud, Denmark.
We thank the Cochrane Comparing of Multiple Interventions Methods Group and the Cochrane Hepato‐Biliary Group for their support and advice. We thank the Cochrane Central Editorial Unit for their advice, which has improved the review. We thank the copy‐editors for their advice and efforts to improve the review.
We thank the authors who provided additional information.
Peer reviewers of first version of the review: Emmanouil Giorgakis, UK; Aleksander Krag, Denmark. Peer reviewers of protocol: Christopher Schmid, USA; Kristian Thorlund, Canada.
We also acknowledge Lorne A Becker, who contributed to the protocol and to the previous version of the review.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of The Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Central Register of Controlled Trials (CENTRAL) | 2015, Issue 9 | 1. Blood loss OR bleeding OR hemorrhage OR haemorrhage OR hemorrhages OR haemorrhages OR hemostasis OR haemostasis OR transfusion
2. MeSH descriptor Hemorrhage explode all trees
3. MeSH descriptor Blood Transfusion explode all trees
4. (#1 OR #2 OR #3)
5. Liver OR hepatic OR hepato*
6. MeSH descriptor Liver explode all trees
7. (5 OR 6)
8. Resection OR resections OR segmentectomy OR segmentectomies
9. (7 AND 8)
10. Hepatectomy OR hepatectomies 11. MeSH descriptor Hepatectomy explode all trees 12. (9 OR 10 OR 11) 13. (4 AND 12) |
| MEDLINE (PubMed) | January 1947 to September 2015 | (Blood loss OR bleeding OR hemorrhage OR haemorrhage OR hemorrhages OR haemorrhages OR hemostasis OR haemostasis OR transfusion OR "Hemorrhage" [MeSH] OR "Blood Transfusion" [MeSH]) AND (((liver OR hepatic OR hepato* OR "liver" [MeSH]) AND (resection OR resections OR segmentectomy OR segmentectomies)) OR hepatectomy OR hepatectomies OR "hepatectomy" [MeSH]) AND ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) |
| Embase (OvidSP) | January 1974 to September 2015 | 1. (Blood loss or bleeding or hemorrhage or haemorrhage or hemorrhages or haemorrhages or hemostasis or haemostasis or transfusion).af
2. Exp bleeding/or exp blood transfusion/
3 .1 or 2
4. (Liver or hepatic or hepato*).af
5. (Resection or resections or segmentectomy or segmentectomies).af
6. 4 and 5
7. (Hepatectomy or hepatectomies).af
8. Exp Liver Resection/
9. 6 or 7 or 8
10. 3 and 9
11. Exp crossover‐procedure/or exp double‐blind procedure/or exp randomized controlled trial/or single‐blind procedure/ 12. (Random* OR factorial* OR crossover* OR cross over* OR cross‐over* OR placebo* OR double* adj blind* OR single* adj blind* OR assign* OR allocat* OR volunteer*).af 13. 11 OR 12 14. 10 AND 13 |
| Science Citation Index Expanded (Web of Science) | January 1945 to September 2015 | 1. TS=(Blood loss OR bleeding OR hemorrhage OR haemorrhage OR hemorrhages OR haemorrhages OR hemostasis OR haemostasis OR transfusion) 2. TS=((liver OR hepatic OR hepato*) AND (resection OR resections OR segmentectomy OR segmentectomies) OR hepatectomy OR hepatectomies) 3. TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) 4. 1 AND 2 AND 3 |
| World Health Organization International Clinical Trials Registry Platform Search Portal (www.who.int/ictrp) | September 2015 | Liver resection OR hepatectomy |
Appendix 2. WinBUGS code
Binary outcome
Binary outcome ‐ fixed‐effect model
# Binomial likelihood, logit link # Fixed effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH trials mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dbin(p[i,k],n[i,k]) # binomial likelihood # model for linear predictor logit(p[i,k]) <‐ mu[i] + d[t[i,k]] ‐ d[t[i,1]] # expected value of the numerators rhat[i,k] <‐ p[i,k] * n[i,k] #Deviance contribution dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k])) + (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) } totresdev <‐ sum(resdev[]) # Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } # pair wise ORs and LORs for all possible pair‐wise comparisons, if nt>2 for (c in 1:(nt‐1)) { for (k in (c+1):nt) { or[c,k] <‐ exp(d[k] ‐ d[c]) lor[c,k] <‐ (d[k]‐d[c]) } } # ranking on relative scale for (k in 1:nt) { # rk[k] <‐ nt+1‐rank(d[],k) # assumes events are “good” rk[k] <‐ rank(d[],k) # assumes events are “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Binary outcome ‐ random‐effects model
# Binomial likelihood, logit link # Random effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH trials w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dbin(p[i,k],n[i,k]) # binomial likelihood logit(p[i,k]) <‐ mu[i] + delta[i,k] # model for linear predictor rhat[i,k] <‐ p[i,k] * n[i,k] # expected value of the numerators #Deviance contribution dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k])) + (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific LOR distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of LOR distributions (with multi‐arm trial correction) md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of LOR distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment for multi‐arm randomised clinical trialss w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) # Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # pair wise ORs and LORs for all possible pair‐wise comparisons, if nt>2 for (c in 1:(nt‐1)) { for (k in (c+1):nt) { or[c,k] <‐ exp(d[k] ‐ d[c]) lor[c,k] <‐ (d[k]‐d[c]) } } # ranking on relative scale for (k in 1:nt) { # rk[k] <‐ nt+1‐rank(d[],k) # assumes events are “good” rk[k] <‐ rank(d[],k) # assumes events are “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Binary outcome ‐ inconsistency model (random‐effects)
# Binomial likelihood, logit link, inconsistency model # Random effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH trials delta[i,1]<‐0 # treatment effect is zero in control arm mu[i] ˜ dnorm(0,.0001) # vague priors for trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dbin(p[i,k],n[i,k]) # binomial likelihood logit(p[i,k]) <‐ mu[i] + delta[i,k] # model for linear predictor #Deviance contribution rhat[i,k] <‐ p[i,k] * n[i,k] # expected value of the numerators dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k])) + (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific LOR distributions delta[i,k] ˜ dnorm(d[t[i,1],t[i,k]] ,tau) } } totresdev <‐ sum(resdev[]) # Total Residual Deviance for (c in 1:(nt‐1)) { # priors for all mean treatment effects for (k in (c+1):nt) { d[c,k] ˜ dnorm(0,.0001) } } sd ˜ dunif(0,5) # vague prior for between‐trial standard deviation var <‐ pow(sd,2) # between‐trial variance tau <‐ 1/var # between‐trial precision } # *** PROGRAM ENDS
Continuous outcome (mean difference)
Continuous outcome (mean difference) ‐ fixed‐effect model
# Normal likelihood, identity link # Fixed effect model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH trials mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS var[i,k] <‐ pow(se[i,k],2) # calculate variances prec[i,k] <‐ 1/var[i,k] # set precisions y[i,k] ˜ dnorm(theta[i,k],prec[i,k]) # model for linear predictor theta[i,k] <‐ mu[i] + d[t[i,k]] ‐ d[t[i,1]] #Deviance contribution dev[i,k] <‐ (y[i,k]‐theta[i,k])*(y[i,k]‐theta[i,k])*prec[i,k] } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for control arm # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } # ranking on relative scale for (k in 1:nt) { rk[k] <‐ rank(d[],k) # assumes lower is better # rk[k] <‐ nt+1‐rank(d[],k) # assumes lower outcome is worse best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Continuous outcome (mean difference) ‐ random‐effects model
# Normal likelihood, identity link # Random effects model for multi‐arm trials model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH trials w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS var[i,k] <‐ pow(se[i,k],2) # calculate variances prec[i,k] <‐ 1/var[i,k] # set precisions y[i,k] ˜ dnorm(theta[i,k],prec[i,k]) theta[i,k] <‐ mu[i] + delta[i,k] # model for linear predictor #Deviance contribution dev[i,k] <‐ (y[i,k]‐theta[i,k])*(y[i,k]‐theta[i,k])*prec[i,k] } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific MD distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of MD distributions, with multi‐arm trial correction md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of MD distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment, multi‐arm randomised clinical trialss w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for control arm # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # ranking on relative scale for (k in 1:nt) { rk[k] <‐ rank(d[],k) # assumes lower is better # rk[k] <‐ nt+1‐rank(d[],k) # assumes lower outcome is worse best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Continuous outcome (mean difference) ‐ inconsistency model (random‐effects)
# Normal likelihood, identity link # Random effects model for multi‐arm trials model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH trials delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS var[i,k] <‐ pow(se[i,k],2) # calculate variances prec[i,k] <‐ 1/var[i,k] # set precisions y[i,k] ˜ dnorm(theta[i,k],prec[i,k]) # binomial likelihood theta[i,k] <‐ mu[i] + delta[i,k] # model for linear predictor #Deviance contribution dev[i,k] <‐ (y[i,k]‐theta[i,k])*(y[i,k]‐theta[i,k])*prec[i,k] } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific MD distributions delta[i,k] ˜ dnorm(d[t[i,1],t[i,k]] ,tau) } } totresdev <‐ sum(resdev[]) # Total Residual Deviance for (c in 1:(nt‐1)) { # priors for all mean treatment effects for (k in (c+1):nt) { d[c,k] ˜ dnorm(0,.0001) } } sd ˜ dunif(0,5) # vague prior for between‐trial standard deviation tau <‐ pow(sd,‐2) # between‐trial precision } # *** PROGRAM ENDS
Continuous outcome (standardised mean difference)
We will calculate the standardised mean difference and its standard error for each treatment comparison using the statistical algorithms used by RevMan 2014.
Continuous outcome (standardised mean difference) ‐ fixed‐effect model
# Normal likelihood, identity link # Trial‐level data given as treatment differences # Fixed effects model model{ # *** PROGRAM STARTS for(i in 1:ns2) { # LOOP THROUGH 2‐ARM trials y[i,2] ˜ dnorm(delta[i,2],prec[i,2]) # normal likelihood for 2‐arm trials #Deviance contribution for trial i resdev[i] <‐ (y[i,2]‐delta[i,2])*(y[i,2]‐delta[i,2])*prec[i,2] } for(i in (ns2+1):(ns2+ns3)) { # LOOP THROUGH THREE‐ARM trials for (k in 1:(na[i]‐1)) { # set variance‐covariance matrix for (j in 1:(na[i]‐1)) { Sigma[i,j,k] <‐ V[i]*(1‐equals(j,k)) + var[i,k+1]*equals(j,k) } } Omega[i,1:(na[i]‐1),1:(na[i]‐1)] <‐ inverse(Sigma[i,,]) #Precision matrix # multivariate normal likelihood for 3‐arm trials y[i,2:na[i]] ˜ dmnorm(delta[i,2:na[i]],Omega[i,1:(na[i]‐1),1:(na[i]‐1)]) #Deviance contribution for trial i for (k in 1:(na[i]‐1)){ # multiply vector & matrix ydiff[i,k]<‐ y[i,(k+1)] ‐ delta[i,(k+1)] z[i,k]<‐ inprod2(Omega[i,k,1:(na[i]‐1)], ydiff[i,1:(na[i]‐1)]) } resdev[i]<‐ inprod2(ydiff[i,1:(na[i]‐1)], z[i,1:(na[i]‐1)]) } for(i in 1:(ns2+ns3)){ # LOOP THROUGH ALL trials for (k in 2:na[i]) { # LOOP THROUGH ARMS var[i,k] <‐ pow(se[i,k],2) # calculate variances prec[i,k] <‐ 1/var[i,k] # set precisions delta[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] } } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } # ranking on relative scale for (k in 1:nt) { rk[k] <‐ nt+1‐rank(d[],k) # assumes higher HRQoL is “good” #rk[k] <‐ rank(d[],k) # assumes higher outcome is “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Continuous outcome (standardised mean difference) ‐ random‐effects model
# Normal likelihood, identity link # Trial‐level data given as treatment differences # Random effects model model{ # *** PROGRAM STARTS for(i in 1:ns2) { # LOOP THROUGH 2‐ARM trials y[i,2] ˜ dnorm(delta[i,2],prec[i,2]) # normal likelihood for 2‐arm trials #Deviance contribution for trial i resdev[i] <‐ (y[i,2]‐delta[i,2])*(y[i,2]‐delta[i,2])*prec[i,2] } for(i in (ns2+1):(ns2+ns3)) { # LOOP THROUGH THREE‐ARM trials for (k in 1:(na[i]‐1)) { # set variance‐covariance matrix for (j in 1:(na[i]‐1)) { Sigma[i,j,k] <‐ V[i]*(1‐equals(j,k)) + var[i,k+1]*equals(j,k) } } Omega[i,1:(na[i]‐1),1:(na[i]‐1)] <‐ inverse(Sigma[i,,]) #Precision matrix # multivariate normal likelihood for 3‐arm trials y[i,2:na[i]] ˜ dmnorm(delta[i,2:na[i]],Omega[i,1:(na[i]‐1),1:(na[i]‐1)]) #Deviance contribution for trial i for (k in 1:(na[i]‐1)){ # multiply vector & matrix ydiff[i,k]<‐ y[i,(k+1)] ‐ delta[i,(k+1)] z[i,k]<‐ inprod2(Omega[i,k,1:(na[i]‐1)], ydiff[i,1:(na[i]‐1)]) } resdev[i]<‐ inprod2(ydiff[i,1:(na[i]‐1)], z[i,1:(na[i]‐1)]) } for(i in 1:(ns2+ns3)){ # LOOP THROUGH ALL trials w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm for (k in 2:na[i]) { # LOOP THROUGH ARMS var[i,k] <‐ pow(se[i,k],2) # calculate variances prec[i,k] <‐ 1/var[i,k] # set precisions } for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific SMD distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of random effects distributions, with multi‐arm trial correction md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of random effects distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment, multi‐arm randomised clinical trialss w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # ranking on relative scale for (k in 1:nt) { rk[k] <‐ nt+1‐rank(d[],k) # assumes higher HRQoL is “good” # rk[k] <‐ rank(d[],k) # assumes higher outcome is “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Continuous outcome (standardised mean difference) ‐ inconsistency model (random‐effects)
# Normal likelihood, identity link # Trial‐level data given as treatment differences # Random effects model model{ # *** PROGRAM STARTS for(i in 1:ns2) { # LOOP THROUGH 2‐ARM trials y[i,2] ˜ dnorm(delta[i,2],prec[i,2]) # normal likelihood for 2‐arm trials #Deviance contribution for trial i resdev[i] <‐ (y[i,2]‐delta[i,2])*(y[i,2]‐delta[i,2])*prec[i,2] } for(i in (ns2+1):(ns2+ns3)) { # LOOP THROUGH THREE‐ARM trials for (k in 1:(na[i]‐1)) { # set variance‐covariance matrix for (j in 1:(na[i]‐1)) { Sigma[i,j,k] <‐ V[i]*(1‐equals(j,k)) + var[i,k+1]*equals(j,k) } } Omega[i,1:(na[i]‐1),1:(na[i]‐1)] <‐ inverse(Sigma[i,,]) #Precision matrix # multivariate normal likelihood for 3‐arm trials y[i,2:na[i]] ˜ dmnorm(delta[i,2:na[i]],Omega[i,1:(na[i]‐1),1:(na[i]‐1)]) #Deviance contribution for trial i for (k in 1:(na[i]‐1)){ # multiply vector & matrix ydiff[i,k]<‐ y[i,(k+1)] ‐ delta[i,(k+1)] z[i,k]<‐ inprod2(Omega[i,k,1:(na[i]‐1)], ydiff[i,1:(na[i]‐1)]) } resdev[i]<‐ inprod2(ydiff[i,1:(na[i]‐1)], z[i,1:(na[i]‐1)]) } for(i in 1:(ns2+ns3)){ # LOOP THROUGH ALL trials for (k in 2:na[i]) { # LOOP THROUGH ARMS var[i,k] <‐ pow(se[i,k],2) # calculate variances prec[i,k] <‐ 1/var[i,k] # set precisions } for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific SMD distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of random effects distributions md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] # precision of random effects distributions taud[i,k] <‐ tau *2*(k‐1)/k } } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) } # *** PROGRAM ENDS
Count outcome
Count outcome ‐ fixed‐effect model
# Poisson likelihood, log link # Fixed effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH trials mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dpois(theta[i,k]) # Poisson likelihood theta[i,k] <‐ lambda[i,k]*E[i,k] # failure rate * exposure # model for linear predictor log(lambda[i,k]) <‐ mu[i] + d[t[i,k]] ‐ d[t[i,1]] #Deviance contribution dev[i,k] <‐ 2*((theta[i,k]‐r[i,k]) + r[i,k]*log(r[i,k]/theta[i,k])) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) } totresdev <‐ sum(resdev[]) #Total Residual Deviance d[1]<‐0 # treatment effect is zero reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } # pair wise RRs and LRRs for all possible pair‐wise comparisons, if nt>2 for (c in 1:(nt‐1)) { for (k in (c+1):nt) { rater[c,k] <‐ exp(d[k] ‐ d[c]) lrater[c,k] <‐ (d[k]‐d[c]) } } # ranking on relative scale for (k in 1:nt) { # rk[k] <‐ nt+1‐rank(d[],k) # assumes events are “good” rk[k] <‐ rank(d[],k) # assumes events are “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Count outcome ‐ random‐effects model
# Poisson likelihood, log link # Random effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH trials w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dpois(theta[i,k]) # Poisson likelihood theta[i,k] <‐ lambda[i,k]*E[i,k] # failure rate * exposure # model for linear predictor log(lambda[i,k]) <‐ mu[i] + d[t[i,k]] ‐ d[t[i,1]] #Deviance contribution dev[i,k] <‐ 2*((theta[i,k]‐r[i,k]) + r[i,k]*log(r[i,k]/theta[i,k])) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific LOR distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of LOR distributions (with multi‐arm trial correction) md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of LOR distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment for multi‐arm randomised clinical trialss w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) # Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # pair wise ORs and LORs for all possible pair‐wise comparisons, if nt>2 for (c in 1:(nt‐1)) { for (k in (c+1):nt) { or[c,k] <‐ exp(d[k] ‐ d[c]) lor[c,k] <‐ (d[k]‐d[c]) } } # ranking on relative scale for (k in 1:nt) { # rk[k] <‐ nt+1‐rank(d[],k) # assumes events are “good” rk[k] <‐ rank(d[],k) # assumes events are “bad” best[k] <‐ equals(rk[k],1) #calculate probability that treat k is best for (h in 1:nt){ prob[h,k] <‐ equals(rk[k],h) } # calculates probability that treat k is h‐th best } } # *** PROGRAM ENDS
Count outcome ‐ inconsistency model (random‐effects)
# Poisson likelihood, log link # Random effects model model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH trials delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dpois(theta[i,k]) # Poisson likelihood theta[i,k] <‐ lambda[i,k]*E[i,k] # failure rate * exposure # model for linear predictor log(lambda[i,k]) <‐ mu[i] + d[t[i,k]] ‐ d[t[i,1]] #Deviance contribution dev[i,k] <‐ 2*((theta[i,k]‐r[i,k]) + r[i,k]*log(r[i,k]/theta[i,k])) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific LOR distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of LRR distributions (without multi‐arm trial correction) md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] # precision of LOR distributions (without multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k } } totresdev <‐ sum(resdev[]) # Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment # vague priors for treatment effects for (k in 2:nt){ d[k] ˜ dnorm(0,.0001) } sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) } # *** PROGRAM ENDS
Appendix 3. Raw data
Legend
Binary outcomes
# ns= number of studies; nt=number of treatments; t[,1] indicates control and t[,2] indicates intervention. In a three‐arm trial, t[,3] indicates the second intervention. r[,1] indicates the number with events in the control group; n[,1] indicates the total number of people in the control group. r[,2], n[,2], r[,3], and n[,3] indicate the corresponding numbers for intervention and second intervention. In two‐arm trials, r[,3] and n[,3] will be entered as 'NA' to indicate empty cells. na[] indicates the number of arms in the trial. Study indicates the study name and is for reference only.
# Continuous outcomes
# ns= number of studies; nt=number of treatments; t[,1] indicates control and t[,2] indicates intervention. In a three‐arm trial, t[,3] indicates the second intervention. y[,1] indicates the mean in the control group; se[,1] indicates the standard error in the control group. y[,2], se[,2], y[,3], and se[,3] indicate the corresponding numbers for intervention and second intervention. In two‐arm trials, y[,3] and se[,3] will be entered as 'NA' to indicate empty cells. na[] indicates the number of arms in the trial. Study indicates the study name and is for reference only.
# Count outcomes
# ns= number of studies; nt=number of treatments; t[,1] indicates control and t[,2] indicates intervention. In a three‐arm trial, t[,3] indicates the second intervention. r[,1] indicates the number of events in the control group; E[,1] indicates the total number of people in the control group. r[,2], E[,2], r[,3], and E[,3] indicate the corresponding numbers for intervention and second intervention. In two‐arm trials, r[,3] and E[,3] will be entered as 'NA' to indicate empty cells. na[] indicates the number of arms in the trial. Study indicates the study name and is for reference only.
| Cardiopulmonary interventions | ||||||||||
| #Blood_transfusion_red blood cell; treatment codes: 1 = Control; 2 = ANH; 3 = ANH_Hypotension; 4 = ANH_Lowcentral venous pressure; 5 = Lowcentral venous pressure. | ||||||||||
| list(nt=5,ns=6) | ||||||||||
| y[,1] | se[,1] | y[,2] | se[,2] | y[,3] | se[,3] | t[,1] | t[,2] | t[,3] | na[] | #study |
| 1.6625 | 0.2 | 0.4175 | 0.16 | 0 | 0.01 | 1 | 2 | 3 | 3 | #Yao 2006 |
| 0.8775 | 0.05 | 1.145 | 0.12 | NA | NA | 1 | 4 | NA | 2 | #Guo 2013 |
| 2.75 | 0.4 | 1.3 | 0.075 | NA | NA | 1 | 5 | NA | 2 | #El‐Kharboutly 2004 |
| 3.215 | 0.58 | 1.3125 | 0.12 | NA | NA | 1 | 5 | NA | 2 | #Wang 2006 |
| 0.44 | 0.37 | 0.7 | 0.35 | NA | NA | 4 | 5 | NA | 2 | #Jarnagin 2008 |
| 0 | 0.47 | 0 | 0.47 | NA | NA | 4 | 5 | NA | 2 | #Matot 2002 |
| END | ||||||||||
| #Blood_loss; treatment codes: 1 = Control; 2 = ANH; 3 = ANH_Hypotension; 4 = ANH_Lowcentral venous pressure; 5 = Hypoventilation; 6 = Lowcentral venous pressure. | ||||||||||
| list(nt=6,ns=9) | ||||||||||
| y[,1] | se[,1] | y[,2] | se[,2] | y[,3] | se[,3] | t[,1] | t[,2] | t[,3] | na[] | #study |
| 0.651 | 0.01 | 0.654 | 0.05 | 0.404 | 0.06 | 1 | 2 | 3 | 3 | #Yao 2006 |
| 0.711 | 0.02 | 0.735 | 0.02 | NA | NA | 1 | 4 | NA | 2 | #Guo 2013 |
| 0.63 | 0.41 | 0.63 | 0.4 | NA | NA | 1 | 5 | NA | 2 | #Hasegawa 2002 |
| 0.783 | 0.08 | 0.589 | 0.07 | NA | NA | 1 | 6 | NA | 2 | #Choi 2007 |
| 1.021 | 0.07 | 0.49 | 0.06 | NA | NA | 1 | 6 | NA | 2 | #El‐Kharboutly 2004 |
| 0.584 | 0.1 | 0.499 | 0.1 | NA | NA | 1 | 6 | NA | 2 | #Kato 2008 |
| 2.329 | 0.51 | 0.904 | 0.04 | NA | NA | 1 | 6 | NA | 2 | #Wang 2006 |
| 0.8 | 0.09 | 0.7 | 0.09 | NA | NA | 4 | 6 | NA | 2 | #Jarnagin 2008 |
| 0.75 | 0.41 | 0.89 | 0.41 | NA | NA | 4 | 6 | NA | 2 | #Matot 2002 |
| END | ||||||||||
| Methods of parenchymal transection | ||||||||||
| #Adverse_events_proportion; treatment codes: 1 = ClampCrush; 2 = cavitron ultrasonic surgical aspirator; 3 = Hydrojet; 4 = RFDS; 5 = SharpTransection; 6 = Stapler. | ||||||||||
| list(nt=6,ns=8) | ||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | #study |
| 15 | 20 | 7 | 20 | 10 | 20 | 1 | 2 | 4 | 3 | #Doklestic 2012 |
| 17 | 25 | 25 | 25 | NA | NA | 1 | 2 | NA | 2 | #Koo 2005 |
| 14 | 66 | 20 | 66 | NA | NA | 1 | 2 | NA | 2 | #Takayama 2001 |
| 7 | 40 | 9 | 40 | NA | NA | 1 | 4 | NA | 2 | #Arita 2005 |
| 17 | 50 | 18 | 50 | NA | NA | 1 | 4 | NA | 2 | #Muratore 2014 |
| 16 | 41 | 17 | 41 | NA | NA | 1 | 5 | NA | 2 | #Smyrniotis 2005 |
| 30 | 65 | 31 | 65 | NA | NA | 1 | 6 | NA | 2 | #Rahbari 2014 |
| 8 | 30 | 3 | 31 | NA | NA | 2 | 3 | NA | 2 | #Rau 2001 |
| END | ||||||||||
| #Adverse_events_number; treatment codes: 1 = ClampCrush; 2 = Cavitron ultrasonic surgical aspirator; 3 = Hydrojet; 4 = RFDS; 5 = SharpTransection; 6 = Stapler. | ||||||||||
| list(nt=6,ns=7) | ||||||||||
| r[,1] | E[,1] | r[,2] | E[,2] | r[,3] | E[,3] | t[,1] | t[,2] | t[,3] | na[] | #study |
| 16 | 66 | 25 | 66 | NA | NA | 1 | 2 | NA | 2 | #Takayama 2001 |
| 7 | 40 | 9 | 40 | NA | NA | 1 | 4 | NA | 2 | #Arita 2005 |
| 11 | 60 | 15 | 60 | NA | NA | 1 | 4 | NA | 2 | #Ikeda 2009 |
| 2 | 26 | 12 | 24 | NA | NA | 1 | 4 | NA | 2 | #Lupo 2007 |
| 16 | 41 | 18 | 41 | NA | NA | 1 | 5 | NA | 2 | #Smyrniotis 2005 |
| 8 | 25 | 7 | 25 | 9 | 25 | 2 | 3 | 4 | 3 | #Lesurtel 2005 |
| 19 | 50 | 22 | 50 | NA | NA | 2 | 6 | NA | 2 | #Savlid 2013 |
| END | ||||||||||
| #Blood_transfusion_proportion; treatment codes: 1 = ClampCrush; 2 = Cavitron ultrasonic surgical aspirator; 3 = Hydrojet; 4 = RFDS; 5 = SharpTransection. | ||||||||||
| list(nt=5,ns=8) | ||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | #study |
| 2 | 20 | 3 | 20 | 4 | 20 | 1 | 2 | 4 | 3 | #Doklestic 2012 |
| 1 | 66 | 1 | 66 | NA | NA | 1 | 2 | NA | 2 | #Takayama 2001 |
| 0 | 40 | 2 | 40 | NA | NA | 1 | 4 | NA | 2 | #Arita 2005 |
| 2 | 60 | 2 | 60 | NA | NA | 1 | 4 | NA | 2 | #Ikeda 2009 |
| 13 | 26 | 8 | 24 | NA | NA | 1 | 4 | NA | 2 | #Lupo 2007 |
| 13 | 50 | 16 | 50 | NA | NA | 1 | 4 | NA | 2 | #Muratore 2014 |
| 15 | 41 | 13 | 41 | NA | NA | 1 | 5 | NA | 2 | #Smyrniotis 2005 |
| 8 | 25 | 8 | 25 | 5 | 25 | 2 | 3 | 4 | 3 | #Lesurtel 2005 |
| END | ||||||||||
| Methods of vascular occlusion | ||||||||||
| #Serious_adverse_events_proportion; treatment codes: 1 = Control; 2 = ConHVE; 3 = ConPTC; 4 = ConSelectiveHVE; 5 = ConSelectivePTC; 6 = IntPTC. | ||||||||||
| list(nt=6,ns=8) | ||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | #study |
| 4 | 63 | 2 | 63 | NA | NA | 1 | 6 | NA | 2 | #Capussotti 2006 |
| 9 | 63 | 14 | 63 | NA | NA | 1 | 6 | NA | 2 | #Lee 2012 |
| 2 | 25 | 1 | 25 | NA | NA | 1 | 6 | NA | 2 | #Park 2012 |
| 3 | 60 | 2 | 58 | NA | NA | 2 | 3 | NA | 2 | #Chen 2006 |
| 2.5 | 81 | 0.5 | 81 | NA | NA | 3 | 4 | NA | 2 | #Si‐Yuan 2014 |
| 22 | 60 | 12 | 60 | NA | NA | 3 | 5 | NA | 2 | #Ni 2013 |
| 4 | 18 | 2 | 17 | NA | NA | 3 | 6 | NA | 2 | #Capussotti 2003 |
| 1 | 40 | 4 | 40 | NA | NA | 5 | 6 | NA | 2 | #Liang 2009 |
| END | ||||||||||
| #Adverse_events_proportion; treatment codes: 1 = Control; 2 = ConHVE; 3 = ConPTC; 4 = ConSelectiveHVE; 5 = ConSelectivePTC; 6 = IntPTC; 7 = IntSelectivePTC. | ||||||||||
| list(nt=7,ns=12) | ||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | #study |
| 16 | 63 | 21 | 63 | NA | NA | 1 | 6 | NA | 2 | #Capussotti 2006 |
| 15 | 63 | 26 | 63 | NA | NA | 1 | 6 | NA | 2 | #Lee 2012 |
| 15 | 50 | 13 | 50 | NA | NA | 1 | 6 | NA | 2 | #Man 1997 |
| 9 | 20 | 5 | 20 | NA | NA | 1 | 6 | NA | 2 | #Man 2003 |
| 19 | 60 | 17 | 58 | NA | NA | 2 | 3 | NA | 2 | #Chen 2006 |
| 17 | 80 | 9 | 80 | NA | NA | 3 | 4 | NA | 2 | #Si‐Yuan 2014 |
| 24 | 60 | 13 | 60 | NA | NA | 3 | 5 | NA | 2 | #Ni 2013 |
| 13 | 42 | 11 | 44 | NA | NA | 3 | 6 | NA | 2 | #Belghiti 1999 |
| 4 | 18 | 2 | 17 | NA | NA | 3 | 6 | NA | 2 | #Capussotti 2003 |
| 9 | 40 | 8 | 40 | NA | NA | 5 | 6 | NA | 2 | #Liang 2009 |
| 15 | 39 | 12 | 41 | NA | NA | 6 | 7 | NA | 2 | #Figueras 2005 |
| 8 | 28 | 10 | 30 | NA | NA | 6 | 7 | NA | 2 | #Wu 2002 |
| END | ||||||||||
| #Blood_transfusion_proportion; treatment codes: 1 = Control; 2 = ConHVE; 3 = ConPTC; 4 = ConSelectiveHVE; 5 = ConSelectivePTC; 6 = IntPTC; 7 = IntSelectivePTC. | ||||||||||
| list(nt=7,ns=13) | ||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | #study |
| 6 | 15 | 1 | 19 | NA | NA | 1 | 3 | NA | 2 | #Chouker 2004 |
| 1 | 63 | 8 | 63 | NA | NA | 1 | 6 | NA | 2 | #Capussotti 2006 |
| 9 | 63 | 14 | 63 | NA | NA | 1 | 6 | NA | 2 | #Lee 2012 |
| 29 | 50 | 18 | 50 | NA | NA | 1 | 6 | NA | 2 | #Man 1997 |
| 19 | 20 | 12 | 20 | NA | NA | 1 | 6 | NA | 2 | #Man 2003 |
| 8 | 60 | 27 | 58 | NA | NA | 2 | 3 | NA | 2 | #Chen 2006 |
| 22 | 80 | 13 | 80 | NA | NA | 3 | 4 | NA | 2 | #Si‐Yuan 2014 |
| 4 | 60 | 6 | 60 | NA | NA | 3 | 5 | NA | 2 | #Ni 2013 |
| 12 | 42 | 14 | 44 | NA | NA | 3 | 6 | NA | 2 | #Belghiti 1999 |
| 5 | 18 | 5 | 17 | NA | NA | 3 | 6 | NA | 2 | #Capussotti 2003 |
| 15 | 40 | 14 | 40 | NA | NA | 5 | 6 | NA | 2 | #Liang 2009 |
| 4 | 39 | 6 | 41 | NA | NA | 6 | 7 | NA | 2 | #Figueras 2005 |
| 12 | 28 | 5 | 30 | NA | NA | 6 | 7 | NA | 2 | #Wu 2002 |
| END | ||||||||||
| #Blood_transfusion_red blood cell; treatment codes: 1 = Control; 2 = ConHVE; 3 = ConPTC; 4 = ConSelectiveHVE; 5 = ConSelectivePTC; 6 = IntPTC; 7 = IntSelectivePTC. | ||||||||||
| list(nt=7,ns=10) | ||||||||||
| y[,1] | se[,1] | y[,2] | se[,2] | y[,3] | se[,3] | t[,1] | t[,2] | t[,3] | na[] | #study |
| 1.9 | 1.02 | 1.3 | 0.85 | NA | NA | 1 | 3 | NA | 2 | #Clavien 1996 |
| 1.5 | 0.45 | 0 | 0.45 | NA | NA | 1 | 6 | NA | 2 | #Man 1997 |
| 2.5 | 0.64 | 2.9 | 0.8 | NA | NA | 2 | 3 | NA | 2 | #Belghiti 1996 |
| 2.2 | 0.42 | 1 | 0.42 | NA | NA | 3 | 4 | NA | 2 | #Si‐Yuan 2014 |
| 1.4 | 0.05 | 1.2 | 0.03 | NA | NA | 3 | 5 | NA | 2 | #Ni 2013 |
| 3 | 0.4 | 2.3 | 0.39 | NA | NA | 3 | 6 | NA | 2 | #Belghiti 1999 |
| 0.5 | 0.02 | 0.5 | 0.27 | NA | NA | 3 | 6 | NA | 2 | #Capussotti 2003 |
| 1.3675 | 0.09 | 1.4825 | 0.15 | NA | NA | 5 | 6 | NA | 2 | #Liang 2009 |
| 0.36 | 0.16 | 0.34 | 0.14 | NA | NA | 6 | 7 | NA | 2 | #Figueras 2005 |
| 2.5425 | 0.26 | 2.24 | 0.4 | NA | NA | 6 | 7 | NA | 2 | #Wu 2002 |
| END | ||||||||||
| #Blood_loss; treatment codes: 1 = Control; 2 = ConHVE; 3 = ConPTC; 4 = ConSelectiveHVE; 5 = ConSelectivePTC; 6 = IntPTC; 7 = IntSelectivePTC. | ||||||||||
| list(nt=7,ns=16) | ||||||||||
| y[,1] | se[,1] | y[,2] | se[,2] | y[,3] | se[,3] | t[,1] | t[,2] | t[,3] | na[] | #study |
| 2.17 | 0.22 | 1.38 | 0.16 | NA | NA | 1 | 3 | NA | 2 | #Chouker 2004 |
| 0.32 | 0.05 | 0.328 | 0.02 | NA | NA | 1 | 3 | NA | 2 | #Dayangac 2010 |
| 0.671 | 0.32 | 0.65 | 0.16 | NA | NA | 1 | 3 | NA | 2 | #Pietsch 2010 |
| 0.204 | 0.02 | 0.184 | 0.03 | NA | NA | 1 | 6 | NA | 2 | #Capussotti 2006 |
| 0.489 | 0.06 | 0.488 | 0.07 | NA | NA | 1 | 6 | NA | 2 | #Lee 2012 |
| 1.99 | 0.18 | 1.28 | 0.18 | NA | NA | 1 | 6 | NA | 2 | #Man 1997 |
| 0.324 | 0.03 | 0.486 | 0.06 | NA | NA | 1 | 6 | NA | 2 | #Park 2012 |
| 1.195 | 0.21 | 0.989 | 0.26 | NA | NA | 2 | 3 | NA | 2 | #Belghiti 1996 |
| 0.42 | 0.03 | 0.77 | 0.04 | NA | NA | 2 | 3 | NA | 2 | #Chen 2006 |
| 0.777 | 0.09 | 0.529 | 0.09 | NA | NA | 3 | 4 | NA | 2 | #Si‐Yuan 2014 |
| 0.2 | 0.1 | 0.3 | 0.1 | NA | NA | 3 | 5 | NA | 2 | #Ni 2013 |
| 1.18 | 0.12 | 1.29 | 0.14 | NA | NA | 3 | 6 | NA | 2 | #Belghiti 1999 |
| 0.733 | 0.12 | 0.732 | 0.15 | NA | NA | 3 | 6 | NA | 2 | #Capussotti 2003 |
| 0.649 | 0.04 | 0.57 | 0.05 | NA | NA | 5 | 6 | NA | 2 | #Liang 2009 |
| 0.671 | 0.09 | 0.735 | 0.06 | NA | NA | 6 | 7 | NA | 2 | #Figueras 2005 |
| 1.685 | 0.17 | 1.159 | 0.22 | NA | NA | 6 | 7 | NA | 2 | #Wu 2002 |
| END | ||||||||||
Appendix 4. Technical details of network meta‐analysis
The posterior probabilities (effect estimates or values) of the treatment contrast (i.e., log odds ratio or mean difference) may vary depending upon the priors and initial values to start the simulations.
We used non‐informative priors for all distributions. For distributions of effect estimates for different studies and different treatments, normal distribution with mean = 0 and variance = 10,000 were used. For between‐study standard deviation in random‐effects models, a uniform distribution with limits of 0 and 5 was used for all analyses. The only exception was adverse events proportion in the comparison of parenchymal transection methods, where we chose the random‐effects model based on the fit, but the posterior distribution was determined by the prior distribution. For this comparison, the distribution for between‐study standard deviation was changed to a uniform distribution with limits of 0 and 2.
In order to control the random error due to the choice of initial values, we performed the network analysis for three different initial values (priors) as per the guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) documents (Dias 2013a). If the results from three different initial values ('chains') are similar (convergence), then the results are reliable. It is important to discard the results of the initial simulations as they can be significantly affected by the choice of the initial values and only include the results of the simulations obtained after the convergence. The discarding of the initial simulations is called 'burn in'. We ran the models for all outcomes for 30,000 simulations for 'burn in' for three different chains (a set of initial values). We ran the models for another 100,000 simulations to obtain the effect estimates. We obtained the effect estimates from the results of all the three chains (different initial values). We ensured that the results in the three different chains were similar in order to control for random error due to the choice of initial values. This was done in addition to the visual inspection of convergence obtained after simulations in the burn in. The mean effect estimate and 95% credible intervals were the median and 2.5% percentile and 97.5% credible intervals.
We ran three different models for each outcome. Fixed‐effect model assumes that the treatment effect is the same across studies. The random‐effects consistency model assumes that the treatment effect is distributed normally across the studies but assumes that the transitivity assumption is satisfied (i.e., the population studied, the definition of outcomes, and the methods used were similar across studies and that there is consistency between the direct comparison and indirect comparison). A random‐effects inconsistency model does not assume transitivity assumption. If the inconsistency model resulted in a better model fit than the consistency model, the results of the network meta‐analysis can be unreliable and so should be interpreted with extreme caution. If there was evidence of inconsistency, we planned to identify areas in the network where substantial inconsistency might be present in terms of clinical and methodological diversities between trials and, when appropriate, limit network meta‐analysis to a more compatible subset of trials.
The choice of the model between fixed‐effect model and random‐effects model was based on the model fit as per the guidelines of the NICE TSU (Dias 2013a). The model fit was assessed by deviance residuals and Deviance Information Criteria (DIC) according to NICE TSU guidelines (Dias 2013a). A difference of three or five in the DIC is not generally considered important (Dias 2012b). We used the simpler model, that is, fixed‐effect model was used if the DIC were similar between the fixed‐effect model and random‐effects model. We used the random‐effects model if it resulted in a better model fit as indicated by a DIC lower than that of fixed‐effect model by at least three.
We have calculated the effect estimates of the treatment and the 95% credible intervals using the formulae for calculating the effect estimates in indirect comparisons (Bucher 1997):
ln(ORAC) = ln(ORAB) ‐ ln(ORCB) and
Var(ln ORAC) = Var (ln ORAB) + Var (ln ORCB)
where ln indicates natural logarithm; OR indicates odds ratio; Var indicates variance; and A, B, and C are three different treatments.
Appendix 5. Simulated data
| #Simulation used for analysis; treatments 1,2,3,4; ln effect estimates: 2 vs 1 = 0, 3 vs 1 = 0.1, 4 vs 1 =‐ 0.15, 3 vs 2 = 0.1, 4 vs 2 = ‐0.15; 4 vs 3 = 0.25). | ||||||||||
| Methods of simulating data: We have simulated the data using Excel. For this purpose, we have fixed the ln (natural logarithm) odds of the comparisons at the predetermined values. We have then added or subtracted a random value between ‐0.25 and 0.25 from the resulting odds ratio to determine the odds ratio of the individual study. We simulated the odds ratio for 15 studies. We then performed the network meta‐analysis using the codes provided in Appendix 2. We also performed a meta‐analysis of the simulated data using frequentist meta‐analysis in RevMan; this showed the effect estimates obtained by the frquentist estimates included the predetermined effect estimate and was close but not the same to the predetermined effect estimate. | ||||||||||
| list(nt=4,ns=15) | ||||||||||
| r[,1] | n[,1] | r[,2] | n[,2] | r[,3] | n[,3] | t[,1] | t[,2] | t[,3] | na[] | #study |
| 22 | 23 | 22 | 23 | NA | NA | 1 | 2 | NA | 2 | #1 |
| 12 | 30 | 20 | 60 | NA | NA | 1 | 2 | NA | 2 | #2 |
| 4 | 20 | 7 | 40 | NA | NA | 1 | 2 | NA | 2 | #3 |
| 12 | 22 | 13 | 22 | NA | NA | 1 | 2 | NA | 2 | #4 |
| 20 | 24 | 19 | 24 | NA | NA | 1 | 3 | NA | 2 | #5 |
| 24 | 26 | 24 | 26 | NA | NA | 1 | 3 | NA | 2 | #6 |
| 16 | 20 | 16 | 20 | NA | NA | 1 | 4 | NA | 2 | #7 |
| 9 | 22 | 9 | 22 | NA | NA | 1 | 4 | NA | 2 | #8 |
| 5 | 26 | 6 | 26 | NA | NA | 1 | 4 | NA | 2 | #9 |
| 27 | 28 | 27 | 28 | NA | NA | 1 | 4 | NA | 2 | #10 |
| 9 | 21 | 9 | 21 | NA | NA | 1 | 4 | NA | 2 | #11 |
| 4 | 20 | 4 | 20 | NA | NA | 2 | 3 | NA | 2 | #12 |
| 18 | 22 | 18 | 22 | NA | NA | 2 | 4 | NA | 2 | #13 |
| 5 | 27 | 11 | 54 | NA | NA | 3 | 4 | NA | 2 | #14 |
| 5 | 27 | 13 | 54 | NA | NA | 3 | 4 | NA | 2 | #15 |
| END | ||||||||||
Appendix 6. Results of simulation
| Frequentist direct1 | Network (fixed‐effect model)2 | Network (random‐effects model)2 |
| 0.89 [0.48, 1.66] | 0.90 [0.51,1.58] | 0.90 [0.49,1.67] |
| 0.83 [0.26, 2.72] | 0.83 [0.40,1.69] | 0.84 [0.39,1.81] |
| 1.05 [0.56, 1.99] | 1.04 [0.60,1.81] | 1.05 [0.58,1.92] |
| 1.00 [0.21, 4.71] | 0.93 [0.41,2.08] | 0.93 [0.39,2.20] |
| 1.00 [0.22, 4.63] | 1.16 [0.57,2.36] | 1.16 [0.53,2.54] |
| 1.26 [0.55, 2.86] | 1.25 [0.65,2.48] | 1.25 [0.60,2.56] |
|
Footnotes: 1Mean estimate and 95% confidence intervals 2Mean estimate and 95% credible intervals | ||
Appendix 7. Sample size calculation
The overall mortality in the control groups (conventional approach in the comparison 'anterior approach versus conventional approach'; no autologous blood transfusion in the comparison autologous blood transfusion in the comparison 'autologous blood transfusion versus control'; no active intervention or control group in the 'cardiopulmonary interventions'; 'clamp‐crush method' for 'parenchymal transection methods'; no active intervention or control group in the 'methods of dealing with raw surface'; no vascular occlusion in the 'methods of vascular occlusion'; and no active intervention or control group in the 'pharmacological interventions'), in which mortality was reported, was 1.8% (21/1196). Based on this control group proportion, a relative risk reduction of 20% in the experimental group, type I error of 5%, and type II error of 20%, the required information size for the outcome measure of perioperative mortality was 38,614 participants. This is the sample size required in a meta‐analysis if there was no heterogeneity. In the presence of I2 of 25%, the required sample size is 38,614/(1‐0.25) = 51,485; In the presence of I2 of 50%, the required sample size is 38,614/(1‐0.5) = 77,228.
Network analyses may be more prone to the risk of random errors than direct comparisons (Del Re 2013). Accordingly, a greater sample size is required in indirect comparisons than direct comparisons (Thorlund 2012). The power and precision in indirect comparisons depends upon various factors such as the number of participants included under each comparison and the heterogeneity between the trials (Thorlund 2012). If there were no heterogeneity across the trials, the sample size in indirect comparisons would be equivalent to the sample size in direct comparisons. The effective indirect sample size can be calculated using the number of participants included in each direct comparison (Thorlund 2012). For example, a sample size of 2500 participants in the direct comparison A versus C (nAC) and a sample size of 7500 participants in the direct comparison B versus C (nBC) results in an effective indirect sample size of 1876 participants. However, in the presence of heterogeneity within the comparisons, the sample size required is higher. In the above scenario, for an I2 statistic for each of the comparisons A versus C (IAC2) and B versus C (IBC2) of 25%, the effective indirect sample size is 1407 participants. For an I2 statistic for each of the comparisons A versus C and B versus C of 50%, the effective indirect sample size is 938 participants (Thorlund 2012). We planned to calculate the effective indirect sample size using the following generic formula (Thorlund 2012):
((nAC x (1 ‐ IAC2)) x (nBC x (1‐IBC2))/((nAC x (1 ‐ IAC2)) + (nBC x (1‐IBC2)).
However, we did not perform this as the number of participants included in this network analysis is less than that needed in a direct comparison. In addition, there is currently no method to calculate the effective indirect sample size for a network analysis involving more than three treatment groups.
Sample size calculations for serious adverse events and blood transfusion (proportion) for a relative risk reduction of 20% in the experimental group, type I error of 5%, and type II error of 20% are shown below.
Control group proportion for serious adverse events = 16.7% (151/905)
Required information size for serious adverse events = 3592
Required information size for serious adverse events with I2 of 25% = 3592/(1‐0.25) = 4789
Required information size for serious adverse events with I2 of 50% = 3592/(1‐0.5) = 7184
Control group proportion for blood transfusion = 21.8% (327/1500)
Required information size for blood transfusion = 2602
Required information size for blood transfusion with I2 of 25% = 3592/(1‐0.25) = 3469
Required information size for blood transfusion with I2 of 50% = 3592/(1‐0.5) = 5204
Appendix 8. WinBUGS code for subgroup analysis
We have only shown the code for the random‐effects model for a binary outcome. The differences in the code are underlined. We planned to make similar changes for other outcomes.
# Binomial likelihood, logit link, subgroup # Random effects model for multi‐arm trials model{ # *** PROGRAM STARTS for(i in 1:ns){ # LOOP THROUGH trials w[i,1] <‐ 0 # adjustment for multi‐arm trials is zero for control arm delta[i,1] <‐ 0 # treatment effect is zero for control arm mu[i] ˜ dnorm(0,.0001) # vague priors for all trial baselines for (k in 1:na[i]) { # LOOP THROUGH ARMS r[i,k] ˜ dbin(p[i,k],n[i,k]) # binomial likelihood # model for linear predictor, covariate effect relative to treat in arm 1 logit(p[i,k]) <‐ mu[i] + delta[i,k] + (beta[t[i,k]]‐beta[t[i,1]]) * x[i] rhat[i,k] <‐ p[i,k] * n[i,k] # expected value of the numerators #Deviance contribution dev[i,k] <‐ 2 * (r[i,k] * (log(r[i,k])‐log(rhat[i,k])) + (n[i,k]‐r[i,k]) * (log(n[i,k]‐r[i,k]) ‐ log(n[i,k]‐rhat[i,k]))) } # summed residual deviance contribution for this trial resdev[i] <‐ sum(dev[i,1:na[i]]) for (k in 2:na[i]) { # LOOP THROUGH ARMS # trial‐specific LOR distributions delta[i,k] ˜ dnorm(md[i,k],taud[i,k]) # mean of LOR distributions (with multi‐arm trial correction) md[i,k] <‐ d[t[i,k]] ‐ d[t[i,1]] + sw[i,k] # precision of LOR distributions (with multi‐arm trial correction) taud[i,k] <‐ tau *2*(k‐1)/k # adjustment for multi‐arm randomised clinical trialss w[i,k] <‐ (delta[i,k] ‐ d[t[i,k]] + d[t[i,1]]) # cumulative adjustment for multi‐arm trials sw[i,k] <‐ sum(w[i,1:k‐1])/(k‐1) } } totresdev <‐ sum(resdev[]) # Total Residual Deviance d[1]<‐0 # treatment effect is zero for reference treatment beta[1] <‐ 0 # covariate effect is zero for reference treatment for (k in 2:nt){ # LOOP THROUGH TREATMENTS d[k] ˜ dnorm(0,.0001) # vague priors for treatment effects beta[k] <‐ B # common covariate effect } B ˜ dnorm(0,.0001) # vague prior for covariate effect sd ˜ dunif(0,5) # vague prior for between‐trial SD tau <‐ pow(sd,‐2) # between‐trial precision = (1/between‐trial variance) # treatment effect when covariate = z[j] for (k in 1:nt){ # LOOP THROUGH TREATMENTS for (j in 1:nz) { dz[j,k] <‐ d[k] + (beta[k]‐beta[1])*z[j] } } # *** PROGRAM ENDS
Appendix 9. Summary of findings (secondary outcomes): blood transfusion requirements
| Methods to decrease blood loss during liver resection: a network meta‐analysis: blood transfusion requirements | |||||||
|
Patient or population: people undergoing liver resection Settings: secondary or tertiary setting Intervention and control: various treatments Follow‐up: perioperative period | |||||||
| Outcomes | Anterior approach versus conventional approach | Autologous blood donation versus control | Cardiopulmonary interventions | Methods of parenchymal transection | Methods of dealing with raw surface | Methods of vascular occlusion | Pharmacological interventions |
|
Treatments The first treatment listed is the control. The remaining are interventions. |
|
|
|
|
|
|
|
| Blood transfusion (proportion) | There was no evidence of differences in blood transfusion (proportion) between the 2 groups (quality of evidence = very low)1,2,3,4. | The blood transfusion (proportion) was lower in autologous blood donation than control. Proportion requiring blood transfusion in control group: 619 per 1000 Proportion requiring blood transfusion in autologous blood donation group: 111 per 1000 (25 to 409) Relative effect: OR 0.18, 95% CrI 0.04 to 0.66 42 participants; 1. Quality of evidence = low1,2. | The blood transfusion (proportion) was higher in low central venous pressure than acute normovolemic haemodilution plus low central venous pressure. Proportion requiring blood transfusion in acute normovolemic haemodilution plus low central venous pressure: 118 per 1000 Proportion requiring blood transfusion in low central venous pressure group: 376 per 1000 (184 to 820) Relative effect: OR 3.19, 95% CrI 1.56 to 6.95 208 participants; 2. Quality of evidence = low1,2. There was no evidence of differences in other comparisons (quality of evidence = very low)1,2,3. | *There was no evidence of differences in blood transfusion (proportion) for any of the comparisons (quality of evidence = very low)1,2,3,4. | There was no evidence of differences in blood transfusion (proportion) for any of the comparisons (quality of evidence = very low)1,2,3,4. | * The blood transfusion (proportion) was lower in continuous portal triad clamping than control. Proportion requiring blood transfusion in control group: 300 per 1000 Proportion requiring blood transfusion in continuous portal triad clamping: 18 per 1000 (0 to 148) Relative effect: OR 0.06, 95% CrI 0.00 to 0.49 34 participants; 1. Quality of evidence = low1,2. The blood transfusion (proportion) was higher in continuous portal triad clamping than continuous hepatic vascular exclusion Proportion requiring blood transfusion in continuous hepatic vascular exclusion: 133 per 1000 Proportion requiring blood transfusion in continuous portal triad clamping group: 785 per 1000 (326 to 2072) Relative effect: OR 5.90, 95% CrI 2.45 to 15.58 118 participants; 1. Quality of evidence = low1,2. There was no evidence of differences in other comparisons (quality of evidence = very low)1,2,3,4. | The blood transfusion (proportion) was lower in aprotinin than control.
Proportion requiring blood transfusion in control group: 291 per 1000
Proportion requiring blood transfusion in aprotinin group: 90 per 1000 (32 to 227)
Relative effect: OR 0.31, 95% CrI 0.11 to 0.78.
97 participants; 1.
Quality of evidence = low1,2.
The blood transfusion (proportion) was lower in tranexamic acid than control. Proportion requiring blood transfusion in tranexamic acid group: 3 per 1000 (0 to 38) Relative effect: OR 0.01, 95% CrI 0.00 to 0.13. 214 participants; 1. Quality of evidence = low1,2. There was no evidence of differences in other comparisons (quality of evidence = very low)1,2,3. |
| Blood transfusion (red blood cells) | None of the trials reported this outcome. | There was no evidence of differences in blood transfusion quantity (red blood cells) between the groups (quality of evidence = very low)1,2,3. | * The blood transfusion quantity (red blood cells) was lower in acute normovolemic haemodilution. The mean blood transfusion quantity (red blood cells) in the control group was 1.38 units. The mean blood transfusion quantity (red blood cells) in the acute normovolemic haemodilution was 1.25 lower (1.74 to 0.75 lower). 20 participants; 1. Quality of evidence: very low)1,2,3. The mean blood transfusion quantity (red blood cells) in the acute normovolemic haemodilution plus hypotension was 1.66 lower (2.06 to 1.32 lower). 20 participants; 1. Quality of evidence: low1,2. The mean blood transfusion quantity (red blood cells) in the acute normovolemic haemodilution plus low central venous pressure was 0.27 higher (0.01 to 0.52 higher). 30 participants; 1. Quality of evidence: very low1,2,3. There was no evidence of differences in other comparisons (quality of evidence = very low)1,2,3. | The blood transfusion quantity (red blood cells) was lower in hydrojet than cavitron ultrasonic surgical aspirator. The mean blood transfusion quantity (red blood cell) in the cavitron ultrasonic surgical aspirator group was 2.48 units. The mean blood transfusion quantity (red blood cells) in the hydrojet group was 0.98 lower (1.90 to 0.06 lower). 61 participants; 1. Quality of evidence = very low1,2,3. There was no evidence of difference in blood transfusion quantity (red blood cells) in the remaining comparisons (quality of evidence = very low)1,2,3. | The blood transfusion quantity (red blood cells) was lower in fibrin sealant than control. The mean blood transfusion quantity (red blood cells) in the control group was 3.5 units. The mean blood transfusion quantity (red blood cells) in the fibrin sealant group was 0.53 lower (1.00 to 0.07 lower). 122 participants; 2. Quality of evidence = very low1,2,3. The blood transfusion quantity (red blood cells) was higher in fibrin sealant than cyanoacrylate. The mean blood transfusion quantity (red blood cells) in the cyanoacrylate group was 2.13 units. The mean blood transfusion quantity (red blood cells) in the fibrin sealant group was 2.20 higher (1.59 to 2.81 higher). 30 participants; 1. Quality of evidence = low1,2. There was no evidence of difference in blood transfusion quantity (red blood cells) in the remaining comparisons (quality of evidence =very low)1,2,3,4. | * The blood transfusion quantity (red blood cells) was lower in continuous portal triad clamping than control.
The mean blood transfusion quantity (red blood cells) in the control group was 1.7 units.
The mean blood transfusion quantity (red blood cells) in the intermittent portal triad clamping was 1.25 lower (2.39 to 0.10 lower).
(network meta‐analysis) 786 participants; 10. Quality of evidence = very low1,2,3. The blood transfusion quantity (red blood cells) was lower in intermittent portal triad clamping than control. The mean blood transfusion quantity (red blood cells) in the intermittent portal triad clamping was 1.50 lower (2.75 to 0.26 lower). 100 participants; 1. Quality of evidence = very low1,2,3. The blood transfusion quantity (red blood cells) was lower in continuous selective hepatic vascular exclusion than continuous portal triad clamping. The mean blood transfusion quantity (red blood cells) in the continuous portal triad clamping group was 1.125 units. The mean blood transfusion quantity (red blood cells) in the continuous selective hepatic vascular exclusion was 1.20 lower (2.37 to 0.04 lower). 160 participants; 1. Quality of evidence = very low1,2,3. The blood transfusion quantity (red blood cells) was lower in continuous selective portal triad clamping than continuous portal triad clamping. The mean blood transfusion quantity (red blood cells) in the continuous selective portal triad clamping was 0.20 lower (0.31 to 0.09 lower). 120 participants; 1. Quality of evidence = very low1,2,3. There was no evidence of difference in blood transfusion quantity (red blood cells) in the remaining comparisons (quality of evidence = very low)1,2,3,4. |
The blood transfusion quantity (red blood cells) was lower in aprotinin than control. The mean blood transfusion quantity (red blood cells) in the control group was 2.10 units. The mean blood transfusion quantity (red blood cells) in the aprotinin group was 0.94 lower (no information to calculate confidence intervals; P = 0.015). 97 participants; 1. Quality of evidence = very lowa,b,c. There was no evidence of difference in blood transfusion quantity (red blood cells) in the remaining comparisons (quality of evidence = very low)1,2,3. |
| Blood transfusion (platelets) | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. | There was no evidence of differences in blood transfusion quantity (platelets) between the groups (quality of evidence = very low)1,2,3. |
| Blood transfusion (fresh frozen plasma) | None of the trials reported this outcome. | None of the trials reported this outcome. | The blood transfusion quantity (fresh frozen plasma) was lower in low central venous pressure than control The mean blood transfusion quantity (fresh frozen plasma) in the control group was 4.23 units. The mean blood transfusion quantity (red blood cells) in the low central venous pressure was 2.48 lower (3.58 to 1.37 lower). 50 participants; 1. Quality of evidence = low1,2. There was no evidence of differences in the other comparison (quality of evidence = very low)1,2,3. | There was no evidence of differences in blood transfusion quantity (fresh frozen plasma) between the groups (quality of evidence = very low)1,2,3. | The blood transfusion quantity (fresh frozen plasma) was lower in fibrin sealant than cyanoacrylate. The mean blood transfusion quantity (fresh frozen plasma) in the cyanoacrylate group was 0.8 units. The mean blood transfusion quantity (fresh frozen plasma) in the fibrin sealant group was 0.81 lower (1.04 to 0.62 lower). 30 participants; 1. Quality of evidence = very low1,2,3. The blood transfusion quantity (fresh frozen plasma) was higher in oxidised cellulose than fibrin sealant. The mean blood transfusion quantity (fresh frozen plasma) in the fibrin sealant group was 8.8 units. The mean blood transfusion quantity (fresh frozen plasma) in the oxidised cellulose group was 0.53 higher (0.36 to 0.71 higher). 80 participants; 2. Quality of evidence = very low1,2,3. There was no evidence of difference in blood transfusion quantity (fresh frozen plasma) in the remaining comparisons (quality of evidence = very low)1,2,3. | None of the trials reported this outcome. | There was no evidence of differences in blood transfusion quantity (fresh frozen plasma) between the groups (quality of evidence = very low)1,2,3. |
| Blood transfusion (cryoprecipitate) | None of the trials reported this outcome. | None of the trials reported this outcome. | There was no evidence of differences in blood transfusion quantity (cryoprecipitate) between the groups (quality of evidence = very low)1,2,3. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. |
| Blood loss | There was no evidence of differences in blood loss between the groups (quality of evidence = very low)1,2,3. | There was no evidence of differences in blood loss between the groups (quality of evidence = very low)1,2,3. | * The blood loss was lower in acute normovolemic haemodilution plus hypotension than control The mean blood loss in the control group was 0.71 litres. The mean blood loss in the acute normovolemic haemodilution plus hypotension was 0.25 lower (0.37 to 0.13 lower). 20 participants; 1. Quality of evidence = very low1,2,3. The mean blood loss in the low central venous pressure was 0.34 lower (0.46 to 0.22 lower). 237 participants; 4. Quality of evidence = very low1,2,3. The mean blood loss in the acute normovolemic haemodilution group was 0.65 litres. The blood loss in acute normovolemic haemodilution plus hypotension was 0.25 lower (0.40 to 0.10 lower) 20 participants; 1. Quality of evidence = very low1,2,3. There was no evidence of differences in other comparisons (quality of evidence = very low)1,2,3. | There was no evidence of differences in blood loss between the groups (quality of evidence = very low)1,2,3. | There was no evidence of differences in blood loss between the groups (quality of evidence = very low)1,2,3. | There was no evidence of differences in blood loss between the groups (quality of evidence = very low)1,2,3,4. | The blood loss was lower in tranexamic acid than control (difference in median: ‐0.30 litres, P < 0.001; 214 participants; 1 study). The mean blood loss in the control group was 0.45 litres. The mean blood loss in the tranexamic acid was 0.30 lower (no information to calculate confidence intervals; P < 0.001). 214 participants; 1. Quality of evidence = low1,2. There was no evidence of difference in blood transfusion quantity (red blood cells) in the remaining comparisons (quality of evidence = very low)1,2,3. |
| Major blood loss (proportion) | There was no evidence of differences in major blood loss (proportion) between the 2 groups (quality of evidence = very low)1,2,3,4. | There was no evidence of differences in major blood loss (proportion) between the 2 groups (quality of evidence = very low)1,2,3. | There was no evidence of differences in major blood loss (proportion) between the groups (quality of evidence = very low)1,2,3. | None of the trials reported this outcome. | None of the trials reported this outcome. | There was no evidence of differences in major blood loss (proportion) between the groups (quality of evidence = very low)1,2,3. | None of the trials reported this outcome. |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
|
Footnotes 1 Risk of bias was unclear or high in the trial[s) (downgraded by 1 point). 2 Sample size was low (total number of participants fewer than 400 for continuous outcomes and fewer than 300 events in total in both groups for other outcomes) (downgraded by 1 point). 3 Credible intervals overlapped no effect and clinically significant effect (20% relative risk reduction for binary outcomes;1 unit of transfusion quantity; 500 ml blood loss) (downgraded by 1 point). 4 There was considerable or substantial heterogeneity in the pair‐wise comparison or at least 1 of the comparisons in the network (downgrade by 2 points). *Network meta‐analysis was performed for these outcome because of the availability of direct and indirect comparisons in the network. The remaining outcomes were analysed by direct comparisons. CrI: credible intervals; MD: mean difference; OR: odds ratio. | |||||||
Appendix 10. Summary of findings (secondary outcomes): operating time, hospital stay, and time needed to return to work
| Methods to decrease blood loss during liver resection: a network meta‐analysis: operating time, hospital stay, and time‐to‐return to work | |||||||
|
Patient or population: people undergoing liver resection Settings: secondary or tertiary setting Intervention and control: various treatments Follow‐up: peri‐operative period | |||||||
| Outcomes | Anterior approach versus conventional approach | Autologous blood donation versus control | Cardiopulmonary interventions | Methods of parenchymal transection | Methods of dealing with raw surface | Methods of vascular occlusion | Pharmacological interventions |
|
Treatments The first treatment listed is the control. The remaining are interventions. |
|
|
|
|
|
|
|
| Total hospital stay | There was no evidence of differences in hospital stay between the groups (quality of evidence = very low)1,2,3. | There was no evidence of differences in hospital stay between the groups (quality of evidence = very low)1,2,3. | The total hospital stay was lower in low central venous pressure than control. The mean hospital stay in the control group was 20.75 days. The mean hospital stay in the low central venous pressure was 2.42 lower (3.91 to 0.94 lower). 197 participants; 3. Quality of evidence = very low1,2,3. There were no evidence of differences in the remaining comparisons (quality of evidence = very low)a,b,c. | There was no evidence of differences in hospital stay between the groups (quality of evidence = very low)1,2,3. | There was no evidence of differences in hospital stay between the groups (quality of evidence = very low)1,2,3. | The total hospital stay was lower in continuous portal triad clamping than continuous hepatic vascular exclusion. The mean hospital stay in the continuous hepatic vascular exclusion group was 22 days. The mean hospital stay in the continuous portal triad clamping was 8.00 lower (13.03 to 2.95 lower). 52 participants; 1. Quality of evidence = low1,2. The mean hospital stay in the continuous portal triad clamping group was 14 days. The mean hospital stay in the continuous selective hepatic vascular exclusion was 2.80 lower (4.13 to 1.47 lower). 160 participants; 1. Quality of evidence = low1,2. There were no evidence of differences in the remaining comparisons (quality of evidence = very low)1,2,3. | There was no evidence of differences in hospital stay between the groups (quality of evidence = very low)1,2,3. |
| ITU stay | There was no evidence of differences in ITU stay between the 2 groups (quality of evidence = very low)1,2,3. | None of the trials reported this outcome. | None of the trials reported this outcome. | There was no evidence of differences in ITU stay between the 2 groups (quality of evidence = very low)1,2,3. | There was no evidence of differences in ITU stay between the 2 groups (quality of evidence = very low)1,2,3. | The ITU stay was lower in continuous selective hepatic vascular exclusion than continuous portal triad clamping.
The mean ITU stay in the continuous portal triad clamping group was 1.5 days.
The mean ITU stay in the continuous selective hepatic vascular exclusion group was 0.3 lower (0.55 to 0.06 lower).
160 participants; 1.
Quality of evidence = very low1,2,3. There was no evidence of differences in other comparisons (quality of evidence = very low)1,2,3. |
None of the trials reported this outcome. |
| Operating time | There was no evidence of differences in operating time between the 2 groups (quality of evidence = very low)1,2,3. | There was no evidence of differences in operating time between the 2 groups (quality of evidence = very low)1,2,3. | The operating time was lower in low central venous pressure than control. The mean operating time in the control group was 246 minutes. The mean operating time in the low central venous pressure was 15.32 lower (29.03 to 1.69 lower). 192 participants; 4. Quality of evidence = very low1,2,3. There was no evidence of differences in other comparisons (quality of evidence = very low)1,2,3. | There was no evidence of differences in operating time between the groups (quality of evidence = very low)1,2,3. | The operating time was higher in fibrin sealant & collagen than control. The mean operating time in the control group was 263 minutes. The mean operating time in the fibrin sealant & collagen was 19.72 higher (2.93 to 36.57 higher). 300 participants; 1. Quality of evidence = very low1,2,3. There was no evidence of differences in other comparisons (quality of evidence = very low)1,2,3. | The operating time was lower in intermittent portal triad clamping than continuous selective portal triad clamping. The mean operating time in the continuous selective portal triad clamping group was 236 minutes. The mean operating time in the intermittent portal triad clamping group was 30.53 lower (49.68 to 11.29 lower). 80 participants; 1. Quality of evidence = very low1,2,3. There was no evidence of differences in other comparisons (quality of evidence = very low)1,2,3,4. | The operating time was lower in tranexamic acid than control. The mean operating time in the control group was 261 minutes. The mean operating time in the tranexamic acid was 52.20 lower (no information to calculate confidence intervals; P = 0.003). 214 participants; 1. Quality of evidence = low1,2. There was no evidence of differences in other comparisons (quality of evidence = very low)1,2,3. |
| Time needed to return to work | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. | None of the trials reported this outcome. |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
|
Footnotes 1 Risk of bias was unclear or high in the trial[s) (downgraded by 1 point). 2 Sample size was low (total number of participants fewer than 400 for continuous outcomes and fewer than 300 events in total in both groups for other outcomes) (downgraded by 1 point). 3 Credible intervals overlapped no effect and clinically significant effect (20% relative risk reduction for binary outcomes; 1 day of hospital stay, intensive therapy unit stay, and time‐to‐return to work; 15 minutes of operating time) (downgraded by 1 point). 4 There was considerable or substantial heterogeneity in the pair‐wise comparison or at least 1 of the comparisons in the network (downgrade by 2 points). * Network meta‐analysis was not performed for any of the outcomes because of the lack of availability of direct and indirect comparisons in the network. CrI:credible intervals; ITU: intensive therapy unit;MD: mean difference; OR: odds ratio. | |||||||
Data and analyses
Comparison 1. Anterior approach vs conventional approach.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (perioperative) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Anterior approach vs conventional approach | 2 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.32] |
| 2 Serious adverse events (proportion) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Anterior approach vs conventional approach | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events (proportion) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Anterior approach vs conventional approach | 2 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.48, 1.64] |
| 4 Adverse events (number) | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 4.1 Anterior approach vs conventional approach | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Blood transfusion (proportion) | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Anterior approach vs conventional approach | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.05, 6.74] |
| 6 Major blood loss (proportion) | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Anterior approach vs conventional approach | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.09, 3.41] |
1.1. Analysis.
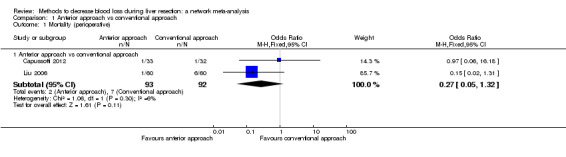
Comparison 1 Anterior approach vs conventional approach, Outcome 1 Mortality (perioperative).
1.2. Analysis.
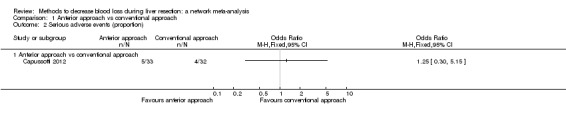
Comparison 1 Anterior approach vs conventional approach, Outcome 2 Serious adverse events (proportion).
1.3. Analysis.

Comparison 1 Anterior approach vs conventional approach, Outcome 3 Adverse events (proportion).
1.4. Analysis.
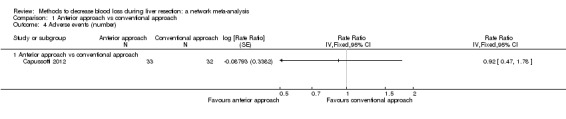
Comparison 1 Anterior approach vs conventional approach, Outcome 4 Adverse events (number).
1.5. Analysis.
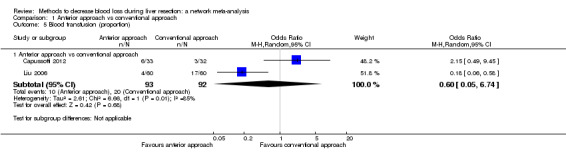
Comparison 1 Anterior approach vs conventional approach, Outcome 5 Blood transfusion (proportion).
1.6. Analysis.
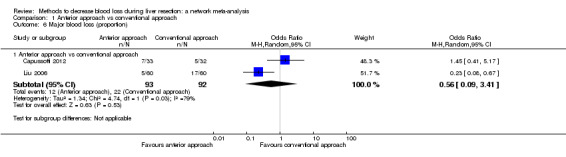
Comparison 1 Anterior approach vs conventional approach, Outcome 6 Major blood loss (proportion).
Comparison 2. Autologous blood donation vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events (proportion) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Autologous blood donation vs control | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Blood transfusion (proportion) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Autologous blood donation vs control | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Blood transfusion (red blood cell) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Autologous blood donation vs control | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Blood loss | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Autologous blood donation vs control | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.37, 0.34] |
| 5 Major blood loss (proportion) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Autologous blood donation vs control | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Total hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Autologous blood donation vs control | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Operating time | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Autologous blood donation vs control | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐3.79 [‐34.28, 26.70] |
2.1. Analysis.
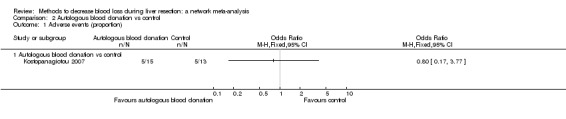
Comparison 2 Autologous blood donation vs control, Outcome 1 Adverse events (proportion).
2.2. Analysis.
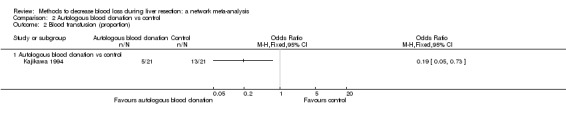
Comparison 2 Autologous blood donation vs control, Outcome 2 Blood transfusion (proportion).
2.3. Analysis.
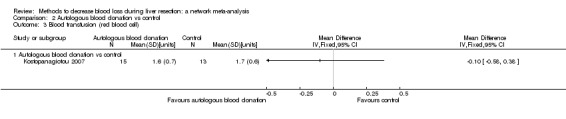
Comparison 2 Autologous blood donation vs control, Outcome 3 Blood transfusion (red blood cell).
2.4. Analysis.
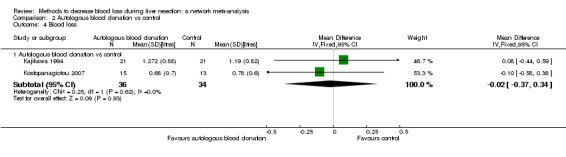
Comparison 2 Autologous blood donation vs control, Outcome 4 Blood loss.
2.5. Analysis.
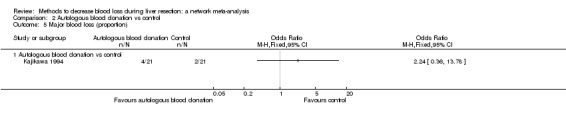
Comparison 2 Autologous blood donation vs control, Outcome 5 Major blood loss (proportion).
2.6. Analysis.
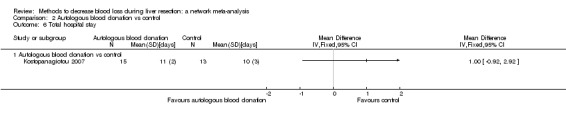
Comparison 2 Autologous blood donation vs control, Outcome 6 Total hospital stay.
2.7. Analysis.
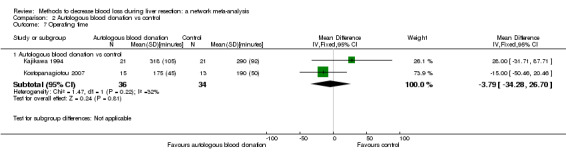
Comparison 2 Autologous blood donation vs control, Outcome 7 Operating time.
Comparison 3. Cardiopulmonary interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (perioperative) | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Hypoventilation vs control | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Low central venous pressure vs control | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.29, 28.70] |
| 2 Serious adverse events (proportion) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Hypoventilation vs control | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serious adverse events (number) | 2 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 3.1 Low central venous pressure vs control | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events (proportion) | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Hypoventilation vs control | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.53, 3.34] |
| 4.2 Low central venous pressure vs control | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.21, 3.03] |
| 4.3 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.37, 1.23] |
| 5 Adverse events (number) | 2 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 5.1 Low central venous pressure vs control | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Blood transfusion (proportion) | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Hypoventilation vs control | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.15, 3.40] |
| 6.2 Low central venous pressure vs control | 3 | 175 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.21, 1.13] |
| 6.3 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.09 [1.49, 6.42] |
| 7 Blood transfusion (red blood cell) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Acute normovolemic haemodilution vs control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.25 [‐1.74, ‐0.75] |
| 7.2 Acute normovolemic haemodilution plus hypotension vs control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.66 [‐2.05, ‐1.28] |
| 7.3 Acute normovolemic haemodilution plus low central venous pressure vs control | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [0.02, 0.51] |
| 7.4 Low central venous pressure vs control | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐2.26, ‐0.93] |
| 7.5 Acute normovolemic haemodilution plus hypotension vs acute normovolemic haemodilution | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.74, ‐0.10] |
| 7.6 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.63, 0.95] |
| 8 Blood transfusion (fresh frozen plasma) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Low central venous pressure vs control | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Blood transfusion (cryoprecipitate) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Hypoventilation vs control | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Blood loss | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Acute normovolemic haemodilution vs control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.10, 0.11] |
| 10.2 Acute normovolemic haemodilution plus hypotension vs control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.36, ‐0.14] |
| 10.3 Acute normovolemic haemodilution plus low central venous pressure vs control | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.03, 0.08] |
| 10.4 Hypoventilation vs control | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.12, 1.12] |
| 10.5 Low central venous pressure vs control | 4 | 237 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.47, ‐0.22] |
| 10.6 Acute normovolemic haemodilution plus hypotension vs acute normovolemic haemodilution | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.39, ‐0.11] |
| 10.7 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.32, 0.15] |
| 11 Major blood loss (proportion) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Hospital stay | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Hypoventilation vs control | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.79, 3.79] |
| 12.2 Low central venous pressure vs control | 3 | 197 | Mean Difference (IV, Fixed, 95% CI) | ‐2.43 [‐3.93, ‐0.94] |
| 12.3 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.96, 2.96] |
| 13 Operating time | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Acute normovolemic haemodilution plus low central venous pressure vs control | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐17.0 [‐42.78, 8.78] |
| 13.2 Hypoventilation vs control | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐88.21, 88.21] |
| 13.3 Low central venous pressure vs control | 4 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐17.41 [‐31.14, ‐3.67] |
| 13.4 Low central venous pressure vs acute normovolemic haemodilution plus low central venous pressure | 3 | 248 | Mean Difference (IV, Fixed, 95% CI) | 13.63 [‐4.11, 31.38] |
3.1. Analysis.
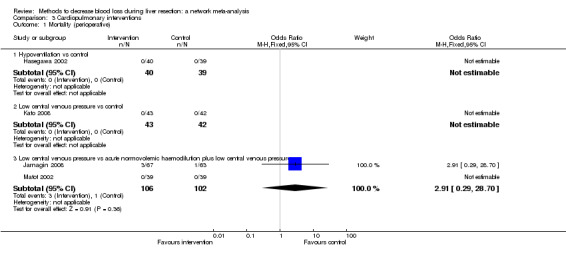
Comparison 3 Cardiopulmonary interventions, Outcome 1 Mortality (perioperative).
3.2. Analysis.
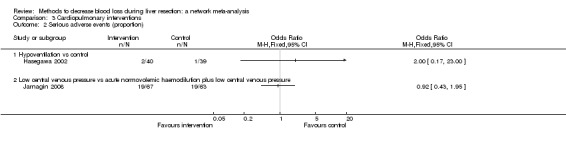
Comparison 3 Cardiopulmonary interventions, Outcome 2 Serious adverse events (proportion).
3.3. Analysis.
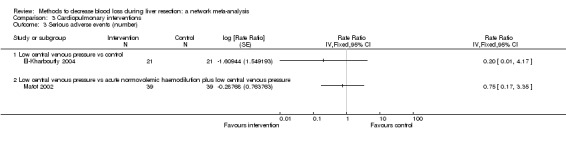
Comparison 3 Cardiopulmonary interventions, Outcome 3 Serious adverse events (number).
3.4. Analysis.
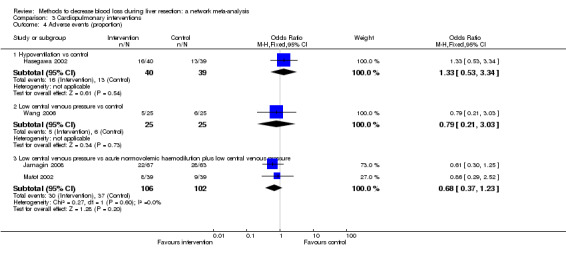
Comparison 3 Cardiopulmonary interventions, Outcome 4 Adverse events (proportion).
3.5. Analysis.

Comparison 3 Cardiopulmonary interventions, Outcome 5 Adverse events (number).
3.6. Analysis.
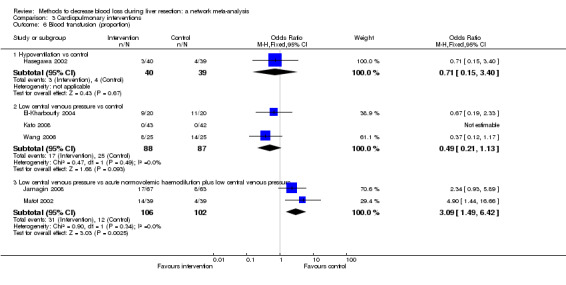
Comparison 3 Cardiopulmonary interventions, Outcome 6 Blood transfusion (proportion).
3.7. Analysis.
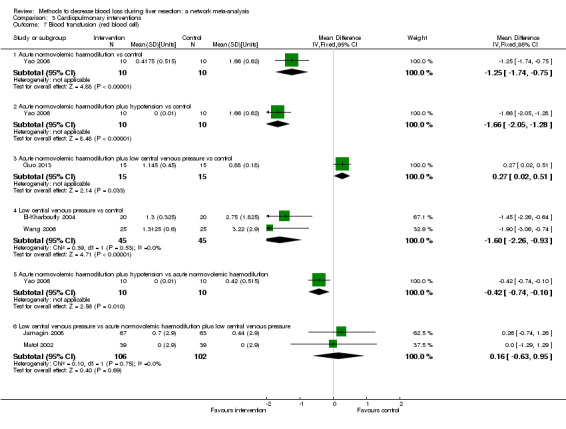
Comparison 3 Cardiopulmonary interventions, Outcome 7 Blood transfusion (red blood cell).
3.8. Analysis.

Comparison 3 Cardiopulmonary interventions, Outcome 8 Blood transfusion (fresh frozen plasma).
3.9. Analysis.
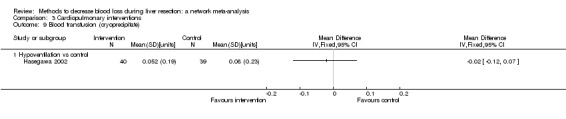
Comparison 3 Cardiopulmonary interventions, Outcome 9 Blood transfusion (cryoprecipitate).
3.10. Analysis.
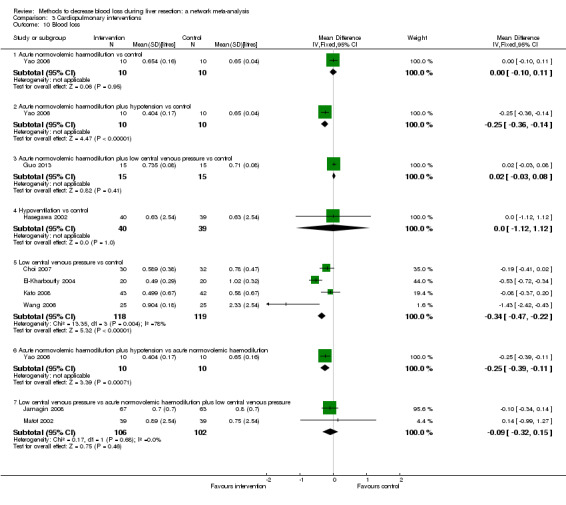
Comparison 3 Cardiopulmonary interventions, Outcome 10 Blood loss.
3.11. Analysis.
Comparison 3 Cardiopulmonary interventions, Outcome 11 Major blood loss (proportion).
3.12. Analysis.
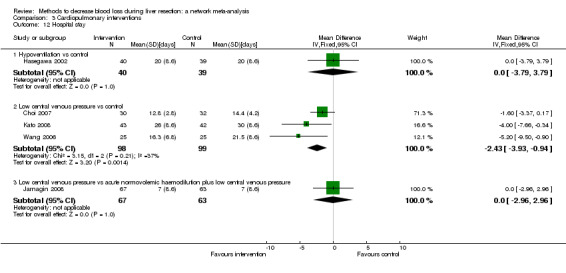
Comparison 3 Cardiopulmonary interventions, Outcome 12 Hospital stay.
3.13. Analysis.
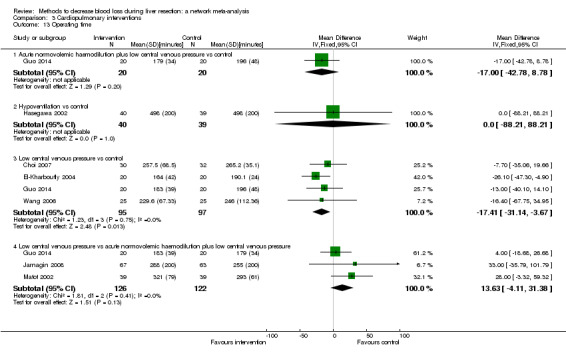
Comparison 3 Cardiopulmonary interventions, Outcome 13 Operating time.
Comparison 4. Methods of parenchymal transection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (perioperative) | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Cavitron ultrasonic surgical aspirator vs clamp‐crush method | 2 | 172 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.01] |
| 1.2 Radiofrequency dissecting sealer vs clamp‐crush method | 5 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.38, 8.97] |
| 1.3 Sharp transection method vs clamp‐crush method | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Stapler vs clamp‐crush method | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.36, 11.69] |
| 1.5 Hydrojet vs cavitron ultrasonic surgical aspirator | 2 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.19, 5.17] |
| 1.6 Radiofrequency dissecting sealer vs cavitron ultrasonic surgical aspirator | 2 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 4.05] |
| 1.7 Stapler vs cavitron ultrasonic surgical aspirator | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Radiofrequency dissecting sealer vs hydrojet | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.04] |
| 2 Serious adverse events (proportion) | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Cavitron ultrasonic surgical aspirator vs clamp‐crush method | 2 | 172 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.09, 1.35] |
| 2.2 Radiofrequency dissecting sealer vs clamp‐crush method | 3 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.63] |
| 2.3 Sharp transection method vs clamp‐crush method | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.36, 12.20] |
| 2.4 Stapler vs clamp‐crush method | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.58, 2.75] |
| 2.5 Hydrojet vs cavitron ultrasonic surgical aspirator | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.10, 4.00] |
| 2.6 Radiofrequency dissecting sealer vs cavitron ultrasonic surgical aspirator | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 17.18] |
| 3 Serious adverse events (number) | 5 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Cavitron ultrasonic surgical aspirator vs clamp‐crush method | 1 | 132 | Rate Ratio (Fixed, 95% CI) | 0.67 [0.11, 3.99] |
| 3.2 Radiofrequency dissecting sealer vs clamp‐crush method | 2 | 130 | Rate Ratio (Fixed, 95% CI) | 3.34 [1.08, 10.31] |
| 3.3 Hydrojet vs cavitron ultrasonic surgical aspirator | 1 | 50 | Rate Ratio (Fixed, 95% CI) | 1.50 [0.25, 8.98] |
| 3.4 Radiofrequency dissecting sealer vs cavitron ultrasonic surgical aspirator | 1 | 50 | Rate Ratio (Fixed, 95% CI) | 1.50 [0.25, 8.98] |
| 3.5 Stapler vs cavitron ultrasonic surgical aspirator | 1 | 100 | Rate Ratio (Fixed, 95% CI) | 1.33 [0.56, 3.16] |
| 3.6 Radiofrequency dissecting sealer vs hydrojet | 1 | 50 | Rate Ratio (Fixed, 95% CI) | 1.0 [0.20, 4.95] |
| 4 Adverse events (proportion) | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Cavitron ultrasonic surgical aspirator vs clamp‐crush method | 3 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.73, 2.34] |
| 4.2 Radiofrequency dissecting sealer vs clamp‐crush method | 3 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.51, 1.64] |
| 4.3 Sharp transection method vs clamp‐crush method | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.46, 2.68] |
| 4.4 Stapler vs clamp‐crush method | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.53, 2.12] |
| 4.5 Hydrojet vs cavitron ultrasonic surgical aspirator | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.24] |
| 4.6 Radiofrequency dissecting sealer vs cavitron ultrasonic surgical aspirator | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.52, 6.61] |
| 5 Adverse events (number) | 7 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Cavitron ultrasonic surgical aspirator vs clamp‐crush method | 1 | 132 | Rate Ratio (Fixed, 95% CI) | 1.56 [0.83, 2.93] |
| 5.2 Radiofrequency dissecting sealer vs clamp‐crush method | 3 | 250 | Rate Ratio (Fixed, 95% CI) | 1.67 [0.95, 2.94] |
| 5.3 Sharp transection method vs clamp‐crush method | 1 | 82 | Rate Ratio (Fixed, 95% CI) | 1.12 [0.57, 2.21] |
| 5.4 Hydrojet vs cavitron ultrasonic surgical aspirator | 1 | 50 | Rate Ratio (Fixed, 95% CI) | 0.88 [0.32, 2.41] |
| 5.5 Radiofrequency dissecting sealer vs cavitron ultrasonic surgical aspirator | 1 | 50 | Rate Ratio (Fixed, 95% CI) | 1.12 [0.43, 2.92] |
| 5.6 Stapler vs cavitron ultrasonic surgical aspirator | 1 | 100 | Rate Ratio (Fixed, 95% CI) | 1.16 [0.63, 2.14] |
| 5.7 Radiofrequency dissecting sealer vs hydrojet | 1 | 50 | Rate Ratio (Fixed, 95% CI) | 1.29 [0.48, 3.45] |
| 6 Blood transfusion (proportion) | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Cavitron ultrasonic surgical aspirator vs clamp‐crush method | 2 | 172 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.29, 6.59] |
| 6.2 Radiofrequency dissecting sealer vs clamp‐crush method | 5 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.63, 2.03] |
| 6.3 Sharp transection method vs clamp‐crush method | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.01] |
| 6.4 Hydrojet vs cavitron ultrasonic surgical aspirator | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.30, 3.28] |
| 6.5 Radiofrequency dissecting sealer vs cavitron ultrasonic surgical aspirator | 2 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.29, 2.09] |
| 6.6 Radiofrequency dissecting sealer vs hydrojet | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.15, 1.93] |
| 7 Blood transfusion (red blood cell) | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Sharp transection method vs clamp‐crush method | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Stapler vs clamp‐crush method | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Hydrojet vs cavitron ultrasonic surgical aspirator | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Stapler vs cavitron ultrasonic surgical aspirator | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Blood transfusion (fresh frozen plasma) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Stapler vs clamp‐crush method | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Blood loss | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Cavitron ultrasonic surgical aspirator vs clamp‐crush method | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Hydrojet vs cavitron ultrasonic surgical aspirator | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Operating time | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Cavitron ultrasonic surgical aspirator vs clamp‐crush method | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | 27.47 [‐2.87, 57.81] |
| 10.2 Radiofrequency dissecting sealer vs clamp‐crush method | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | 16.11 [‐11.45, 43.67] |
| 10.3 Sharp transection method vs clamp‐crush method | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐90.85, 78.85] |
| 10.4 Stapler vs clamp‐crush method | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐31.0 [‐60.40, ‐1.60] |
| 10.5 Radiofrequency dissecting sealer vs cavitron ultrasonic surgical aspirator | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 25.0 [‐96.48, 146.48] |
| 10.6 Stapler vs cavitron ultrasonic surgical aspirator | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐26.0 [‐87.12, 35.12] |
4.1. Analysis.
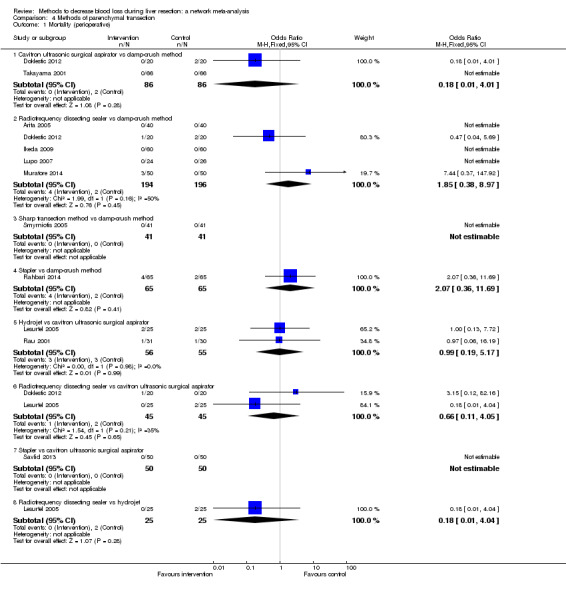
Comparison 4 Methods of parenchymal transection, Outcome 1 Mortality (perioperative).
4.2. Analysis.
Comparison 4 Methods of parenchymal transection, Outcome 2 Serious adverse events (proportion).
4.3. Analysis.
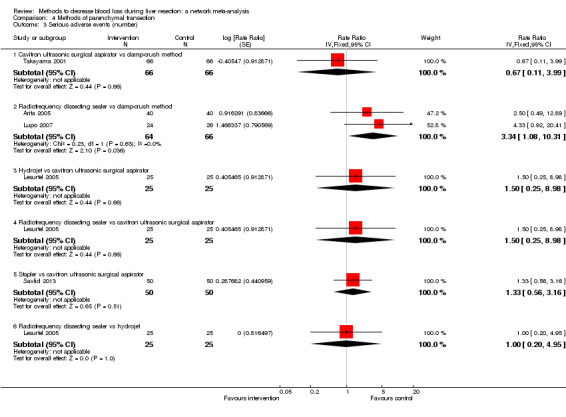
Comparison 4 Methods of parenchymal transection, Outcome 3 Serious adverse events (number).
4.4. Analysis.
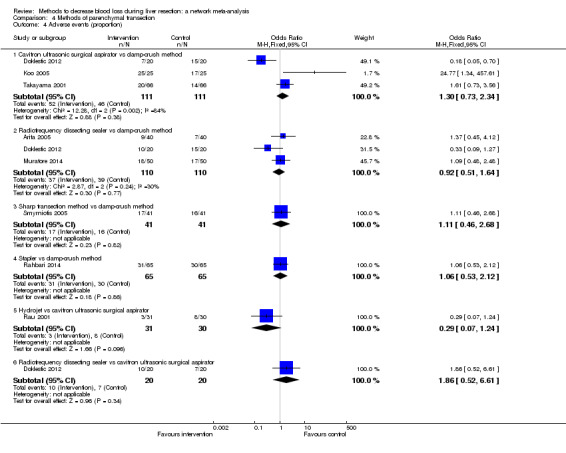
Comparison 4 Methods of parenchymal transection, Outcome 4 Adverse events (proportion).
4.5. Analysis.
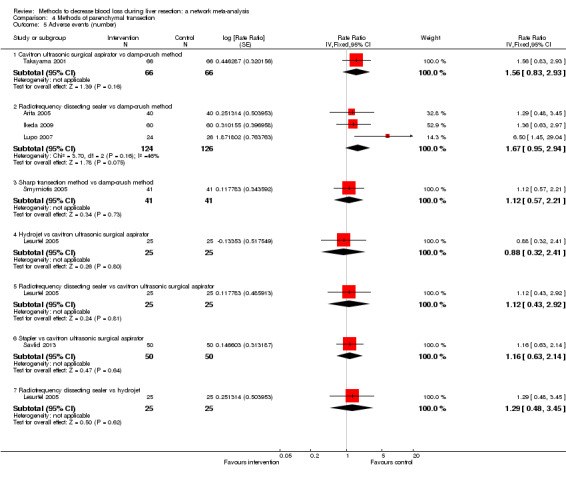
Comparison 4 Methods of parenchymal transection, Outcome 5 Adverse events (number).
4.6. Analysis.
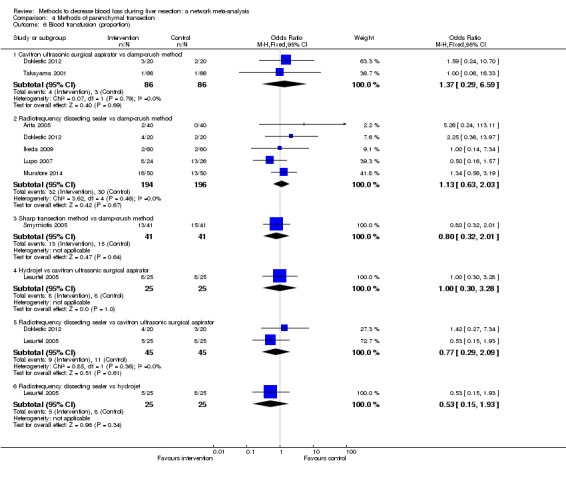
Comparison 4 Methods of parenchymal transection, Outcome 6 Blood transfusion (proportion).
4.7. Analysis.
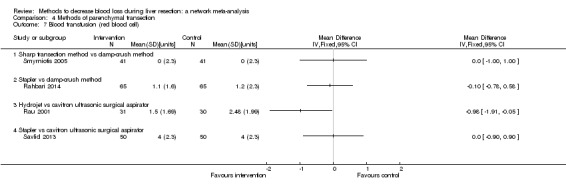
Comparison 4 Methods of parenchymal transection, Outcome 7 Blood transfusion (red blood cell).
4.8. Analysis.
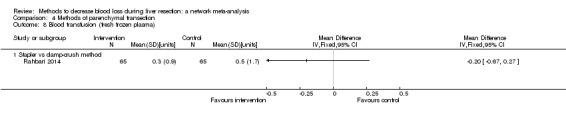
Comparison 4 Methods of parenchymal transection, Outcome 8 Blood transfusion (fresh frozen plasma).
4.9. Analysis.
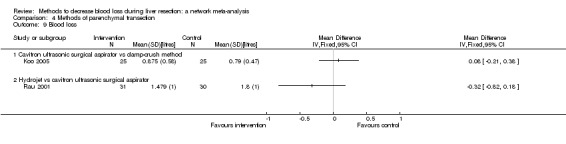
Comparison 4 Methods of parenchymal transection, Outcome 9 Blood loss.
4.10. Analysis.
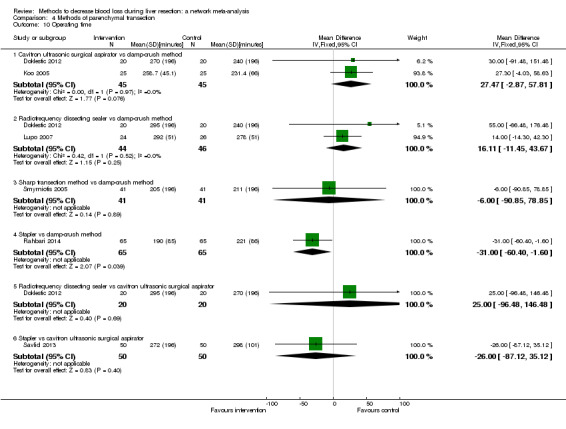
Comparison 4 Methods of parenchymal transection, Outcome 10 Operating time.
Comparison 5. Methods of dealing with cut surface.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (perioperative) | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fibrin sealant vs control | 2 | 380 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.56 [0.73, 17.35] |
| 1.2 Fibrin sealant and collagen vs control | 1 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.61, 15.53] |
| 1.3 Fibrin sealant vs argon beam | 2 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.46, 4.03] |
| 1.4 Fibrin sealant vs collagen | 3 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.24, 3.32] |
| 1.5 Oxidised cellulose vs fibrin sealant | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.03, 9.33] |
| 1.6 Plasmajet vs fibrin sealant | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.10, 4.16] |
| 2 Serious adverse events (proportion) | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Fibrin sealant vs control | 3 | 457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.64, 1.65] |
| 2.2 Fibrin sealant vs argon beam | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.25, 1.55] |
| 2.3 Fibrin sealant vs collagen | 1 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.73, 3.38] |
| 2.4 Oxidised cellulose vs fibrin sealant | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.17, 1.87] |
| 2.5 Plasmajet vs fibrin sealant | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.22] |
| 3 Serious adverse events (number) | 6 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Fibrin sealant vs control | 1 | 70 | Rate Ratio (Fixed, 95% CI) | 0.94 [0.48, 1.86] |
| 3.2 Fibrin sealant and collagen vs control | 1 | 300 | Rate Ratio (Fixed, 95% CI) | 1.32 [0.76, 2.29] |
| 3.3 Fibrin sealant vs argon beam | 1 | 121 | Rate Ratio (Fixed, 95% CI) | 4.47 [1.50, 13.27] |
| 3.4 Fibrin sealant vs collagen | 2 | 189 | Rate Ratio (Fixed, 95% CI) | 1.22 [0.76, 1.98] |
| 3.5 Fibrin sealant vs cyanoacrylate | 1 | 30 | Rate Ratio (Fixed, 95% CI) | 1.0 [0.06, 15.99] |
| 3.6 Oxidised cellulose vs cyanoacrylate | 1 | 30 | Rate Ratio (Fixed, 95% CI) | 4.00 [0.45, 35.79] |
| 3.7 Oxidised cellulose vs fibrin sealant | 1 | 30 | Rate Ratio (Fixed, 95% CI) | 4.00 [0.45, 35.79] |
| 4 Adverse events (proportion) | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Fibrin sealant versus control | 3 | 457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.55, 1.17] |
| 4.2 Fibrin sealant and collagen vs control | 1 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.59, 1.71] |
| 4.3 Fibrin sealant vs argon beam | 2 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.58, 1.64] |
| 4.4 Fibrin sealant vs collagen | 1 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.46, 1.93] |
| 4.5 Oxidised cellulose vs fibrin sealant | 2 | 274 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.30, 2.01] |
| 5 Adverse events (number) | 5 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Fibrin sealant vs control | 1 | 70 | Rate Ratio (Fixed, 95% CI) | 1.01 [0.75, 1.36] |
| 5.2 Fibrin sealant vs argon beam | 1 | 121 | Rate Ratio (Fixed, 95% CI) | 1.12 [0.75, 1.66] |
| 5.3 Fibrin sealant vs collagen | 2 | 189 | Rate Ratio (Fixed, 95% CI) | 1.13 [0.90, 1.42] |
| 5.4 Fibrin sealant vs cyanoacrylate | 1 | 30 | Rate Ratio (Fixed, 95% CI) | 1.50 [0.25, 8.98] |
| 5.5 Oxidised cellulose vs cyanoacrylate | 1 | 30 | Rate Ratio (Fixed, 95% CI) | 3.50 [0.73, 16.85] |
| 5.6 Oxidised cellulose vs fibrin sealant | 1 | 30 | Rate Ratio (Fixed, 95% CI) | 2.33 [0.60, 9.02] |
| 6 Blood transfusion (proportion) | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Fibrin sealant vs control | 2 | 392 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.61, 1.76] |
| 6.2 Fibrin sealant and collagen vs control | 1 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.88, 2.61] |
| 6.3 Fibrin sealant vs cyanoacrylate | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.25 [0.52, 20.37] |
| 6.4 Oxidised cellulose vs cyanoacrylate | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.36, 15.45] |
| 6.5 Oxidised cellulose vs fibrin sealant | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.15, 3.49] |
| 7 Blood transfusion (red blood cell) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Fibrin sealant vs control | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.00, ‐0.06] |
| 7.2 Fibrin sealant and collagen vs control | 1 | 300 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.16, 0.14] |
| 7.3 Fibrin sealant vs collagen | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Fibrin sealant vs cyanoacrylate | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 2.2 [1.59, 2.81] |
| 7.5 Oxidised cellulose vs cyanoacrylate | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.81, 0.27] |
| 7.6 Oxidised cellulose vs fibrin sealant | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.76 [‐2.00, 0.47] |
| 8 Blood transfusion (fresh frozen plasma) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Fibrin sealant vs cyanoacrylate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.01, ‐0.59] |
| 8.2 Oxidised cellulose vs cyanoacrylate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.55, 0.01] |
| 8.3 Oxidised cellulose vs fibrin sealant | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [0.35, 0.71] |
| 9 Blood loss | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Fibrin sealant vs control | 2 | 350 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.13, 0.33] |
| 9.2 Fibrin sealant and collagen vs control | 1 | 300 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.06, 0.19] |
| 9.3 Fibrin sealant vs collagen | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.54, 0.68] |
| 9.4 Fibrin sealant vs cyanoacrylate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.20, 0.43] |
| 9.5 Oxidised cellulose vs cyanoacrylate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.35, 0.19] |
| 9.6 Oxidised cellulose vs fibrin sealant | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.45, 0.06] |
| 10 Total hospital stay | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Fibrin sealant vs control | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.45, 1.45] |
| 10.2 Fibrin sealant and collagen vs control | 1 | 300 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐1.83, 3.23] |
| 10.3 Fibrin sealant vs cyanoacrylate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.34 [‐3.61, 0.93] |
| 10.4 Oxidised cellulose vs cyanoacrylate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐3.12, 1.78] |
| 10.5 Oxidised cellulose vs fibrin sealant | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐1.84, 2.33] |
| 11 ITU stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Oxidised cellulose vs fibrin sealant | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Operating time | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Fibrin sealant vs control | 2 | 122 | Mean Difference (IV, Fixed, 95% CI) | ‐14.55 [‐52.86, 23.76] |
| 12.2 Fibrin sealant and collagen vs control | 1 | 300 | Mean Difference (IV, Fixed, 95% CI) | 19.0 [2.09, 35.91] |
| 12.3 Fibrin sealant vs collagen | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐44.33, 36.33] |
| 12.4 Oxidised cellulose vs fibrin sealant | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 5.40 [‐70.13, 80.93] |
5.1. Analysis.
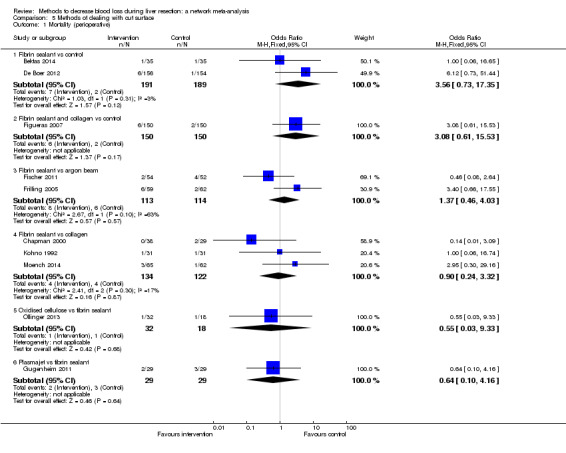
Comparison 5 Methods of dealing with cut surface, Outcome 1 Mortality (perioperative).
5.2. Analysis.

Comparison 5 Methods of dealing with cut surface, Outcome 2 Serious adverse events (proportion).
5.3. Analysis.

Comparison 5 Methods of dealing with cut surface, Outcome 3 Serious adverse events (number).
5.4. Analysis.
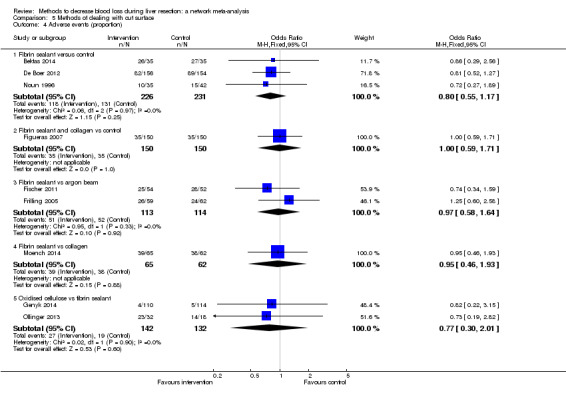
Comparison 5 Methods of dealing with cut surface, Outcome 4 Adverse events (proportion).
5.5. Analysis.
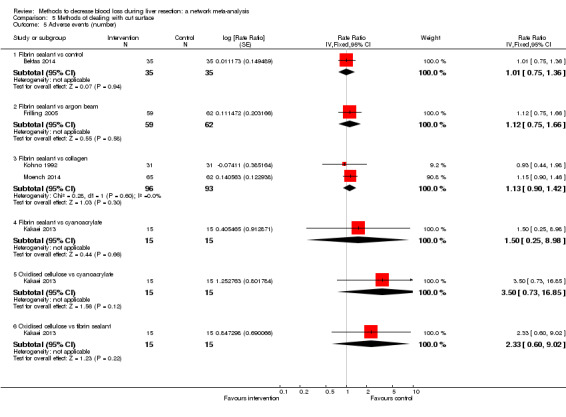
Comparison 5 Methods of dealing with cut surface, Outcome 5 Adverse events (number).
5.6. Analysis.
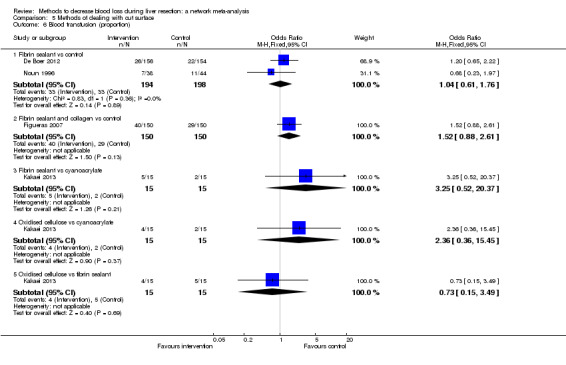
Comparison 5 Methods of dealing with cut surface, Outcome 6 Blood transfusion (proportion).
5.7. Analysis.
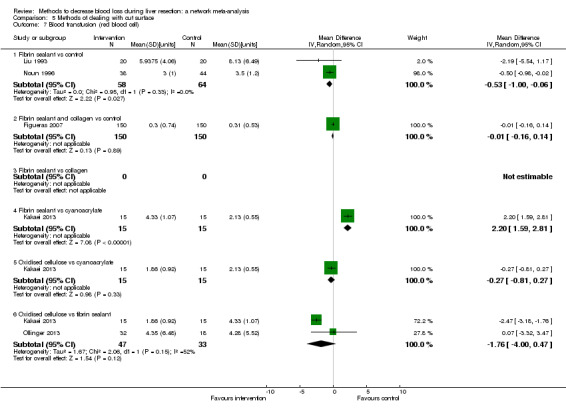
Comparison 5 Methods of dealing with cut surface, Outcome 7 Blood transfusion (red blood cell).
5.8. Analysis.
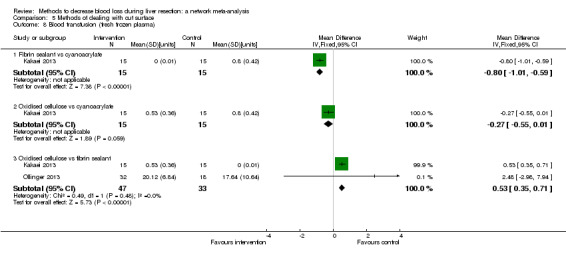
Comparison 5 Methods of dealing with cut surface, Outcome 8 Blood transfusion (fresh frozen plasma).
5.9. Analysis.

Comparison 5 Methods of dealing with cut surface, Outcome 9 Blood loss.
5.10. Analysis.
Comparison 5 Methods of dealing with cut surface, Outcome 10 Total hospital stay.
5.11. Analysis.
Comparison 5 Methods of dealing with cut surface, Outcome 11 ITU stay.
5.12. Analysis.
Comparison 5 Methods of dealing with cut surface, Outcome 12 Operating time.
Comparison 6. Methods of vascular occlusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (perioperative) | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Continuous portal triad clamping vs control | 1 | 15 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Intermittent portal triad clamping vs control | 4 | 392 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.16, 2.44] |
| 1.3 Continuous portal triad clamping vs continuous hepatic vascular exclusion | 2 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.39 [0.34, 33.33] |
| 1.4 Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | 1 | 160 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Continuous selective portal triad clamping vs continuous portal triad clamping | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Intermittent portal triad clamping vs continuous portal triad clamping | 2 | 121 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.64] |
| 1.7 Intermittent portal triad clamping vs continuous selective portal triad clamping | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Intermittent selective portal triad clamping vs intermittent portal triad clamping | 2 | 138 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 74.00] |
| 2 Serious adverse events (proportion) | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Intermittent portal triad clamping vs control | 3 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.55, 2.44] |
| 2.2 Continuous portal triad clamping vs continuous hepatic vascular exclusion | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.11, 4.22] |
| 2.3 Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | 1 | 160 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.13] |
| 2.4 Continuous selective portal triad clamping vs continuous portal triad clamping | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.98] |
| 2.5 Intermittent portal triad clamping vs continuous portal triad clamping | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.07, 2.96] |
| 2.6 Intermittent portal triad clamping vs continuous selective portal triad clamping | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.46, 40.61] |
| 3 Serious adverse events (number) | 5 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Intermittent portal triad clamping vs control | 1 | 100 | Rate Ratio (Fixed, 95% CI) | 1.50 [0.42, 5.32] |
| 3.2 Continuous portal triad clamping vs continuous hepatic vascular exclusion | 1 | 52 | Rate Ratio (Fixed, 95% CI) | 0.23 [0.03, 2.00] |
| 3.3 Intermittent portal triad clamping vs continuous portal triad clamping | 1 | 86 | Rate Ratio (Fixed, 95% CI) | 0.12 [0.01, 0.95] |
| 3.4 Intermittent selective portal triad clamping vs intermittent portal triad clamping | 2 | 138 | Rate Ratio (Fixed, 95% CI) | 1.26 [0.53, 2.99] |
| 4 Adverse events (proportion) | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Intermittent portal triad clamping vs control | 4 | 392 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.83, 1.94] |
| 4.2 Continuous portal triad clamping vs continuous hepatic vascular exclusion | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.96] |
| 4.3 Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | 1 | 160 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.20, 1.13] |
| 4.4 Continuous selective portal triad clamping vs continuous portal triad clamping | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.93] |
| 4.5 Intermittent portal triad clamping vs continuous portal triad clamping | 2 | 121 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.29, 1.56] |
| 4.6 Intermittent portal triad clamping vs continuous selective portal triad clamping | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.29, 2.52] |
| 4.7 Intermittent selective portal triad clamping vs intermittent portal triad clamping | 2 | 138 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.42, 1.75] |
| 5 Adverse events (number) | 6 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Intermittent portal triad clamping vs control | 2 | 226 | Rate Ratio (Fixed, 95% CI) | 1.19 [0.80, 1.76] |
| 5.2 Continuous portal triad clamping vs continuous hepatic vascular exclusion | 1 | 52 | Rate Ratio (Fixed, 95% CI) | 0.61 [0.29, 1.32] |
| 5.3 Intermittent portal triad clamping vs continuous portal triad clamping | 1 | 86 | Rate Ratio (Fixed, 95% CI) | 0.64 [0.31, 1.32] |
| 5.4 Intermittent selective portal triad clamping vs intermittent portal triad clamping | 2 | 138 | Rate Ratio (Fixed, 95% CI) | 1.17 [0.72, 1.91] |
| 6 Blood transfusion (proportion) | 13 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Continuous portal triad clamping vs control | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.80] |
| 6.2 Intermittent portal triad clamping vs control | 4 | 392 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.35] |
| 6.3 Continuous portal triad clamping vs continuous hepatic vascular exclusion | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.66 [2.29, 14.00] |
| 6.4 Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | 1 | 160 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.24, 1.11] |
| 6.5 Continuous selective portal triad clamping vs continuous portal triad clamping | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.42, 5.82] |
| 6.6 Intermittent portal triad clamping vs continuous portal triad clamping | 2 | 121 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.52, 2.49] |
| 6.7 Intermittent portal triad clamping vs continuous selective portal triad clamping | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.36, 2.23] |
| 6.8 Intermittent selective portal triad clamping vs intermittent portal triad clamping | 2 | 138 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.25, 1.36] |
| 7 Blood transfusion (red blood cell) | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Continuous portal triad clamping vs control | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.20, 2.00] |
| 7.2 Intermittent portal triad clamping vs control | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐2.75, ‐0.25] |
| 7.3 Continuous portal triad clamping vs continuous hepatic vascular exclusion | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.61, 2.41] |
| 7.4 Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.38, ‐0.02] |
| 7.5 Continuous selective portal triad clamping vs continuous portal triad clamping | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.31, ‐0.09] |
| 7.6 Intermittent portal triad clamping vs continuous portal triad clamping | 2 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.60, 0.34] |
| 7.7 Intermittent portal triad clamping vs continuous selective portal triad clamping | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.23, 0.46] |
| 7.8 Intermittent selective portal triad clamping vs intermittent portal triad clamping | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.45, 0.32] |
| 8 Blood loss | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Continuous portal triad clamping vs control | 3 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.76, 0.27] |
| 8.2 Intermittent portal triad clamping vs control | 4 | 402 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.19, 0.15] |
| 8.3 Continuous portal triad clamping vs continuous hepatic vascular exclusion | 2 | 170 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.35, 0.68] |
| 8.4 Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | 1 | 160 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.49, ‐0.00] |
| 8.5 Continuous selective portal triad clamping vs continuous portal triad clamping | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.19, 0.39] |
| 8.6 Intermittent portal triad clamping vs continuous portal triad clamping | 2 | 121 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.20, 0.32] |
| 8.7 Intermittent portal triad clamping vs continuous selective portal triad clamping | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.20, 0.05] |
| 8.8 Intermittent selective portal triad clamping vs intermittent portal triad clamping | 2 | 138 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.74, 0.39] |
| 9 Major blood loss (proportion) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Intermittent portal triad clamping vs control | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Continuous selective portal triad clamping vs continuous portal triad clamping | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Total hospital stay | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Intermittent portal triad clamping vs control | 4 | 402 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.64, 1.28] |
| 10.2 Continuous portal triad clamping vs continuous hepatic vascular exclusion | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐13.05, ‐2.95] |
| 10.3 Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐4.13, ‐1.47] |
| 10.4 Intermittent portal triad clamping vs continuous portal triad clamping | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.82, 4.82] |
| 10.5 Intermittent portal triad clamping vs continuous selective portal triad clamping | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐1.60, 1.06] |
| 10.6 Intermittent selective portal triad clamping vs intermittent portal triad clamping | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐2.40, 1.06] |
| 11 ITU stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Operating time | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Continuous portal triad clamping vs control | 2 | 40 | Mean Difference (IV, Random, 95% CI) | ‐45.87 [‐95.61, 3.87] |
| 12.2 Intermittent portal triad clamping vs control | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 25.66 [‐31.57, 82.89] |
| 12.3 Continuous portal triad clamping vs continuous hepatic vascular exclusion | 2 | 170 | Mean Difference (IV, Random, 95% CI) | ‐29.32 [‐82.75, 24.10] |
| 12.4 Continuous selective hepatic vascular exclusion vs continuous portal triad clamping | 1 | 160 | Mean Difference (IV, Random, 95% CI) | ‐7.20 [‐63.42, 49.02] |
| 12.5 Continuous selective portal triad clamping vs continuous portal triad clamping | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 20.0 [‐0.00, 40.00] |
| 12.6 Intermittent portal triad clamping vs continuous portal triad clamping | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 13.40 [‐41.28, 68.08] |
| 12.7 Intermittent portal triad clamping vs continuous selective portal triad clamping | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐32.17 [‐51.50, ‐12.84] |
| 12.8 Intermittent selective portal triad clamping vs intermittent portal triad clamping | 2 | 138 | Mean Difference (IV, Random, 95% CI) | 8.64 [‐10.16, 27.45] |
6.1. Analysis.
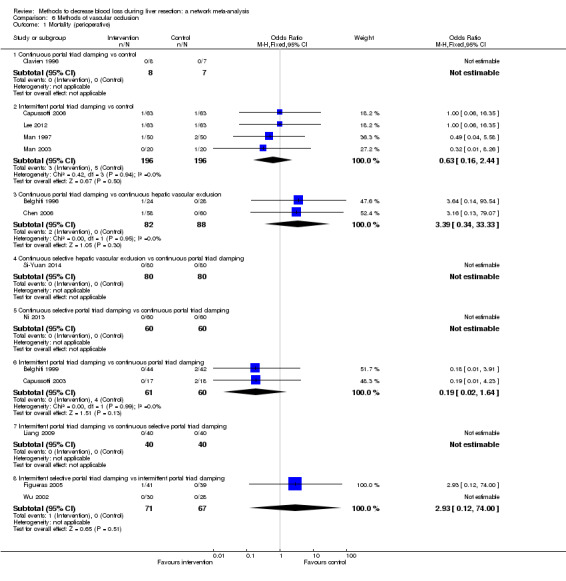
Comparison 6 Methods of vascular occlusion, Outcome 1 Mortality (perioperative).
6.2. Analysis.
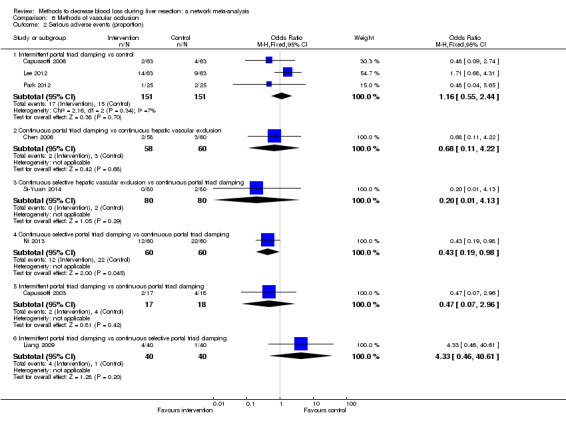
Comparison 6 Methods of vascular occlusion, Outcome 2 Serious adverse events (proportion).
6.3. Analysis.
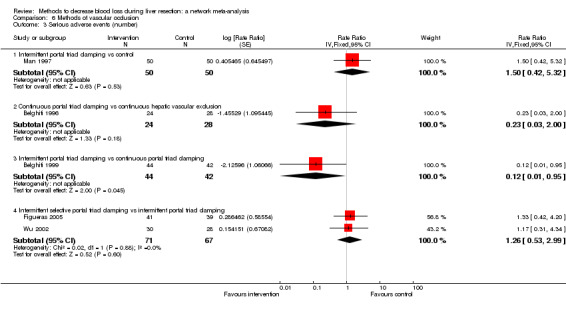
Comparison 6 Methods of vascular occlusion, Outcome 3 Serious adverse events (number).
6.4. Analysis.

Comparison 6 Methods of vascular occlusion, Outcome 4 Adverse events (proportion).
6.5. Analysis.
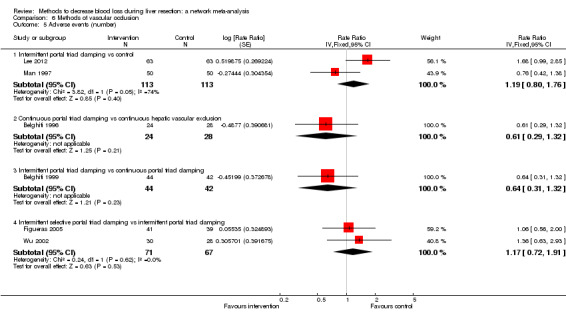
Comparison 6 Methods of vascular occlusion, Outcome 5 Adverse events (number).
6.6. Analysis.
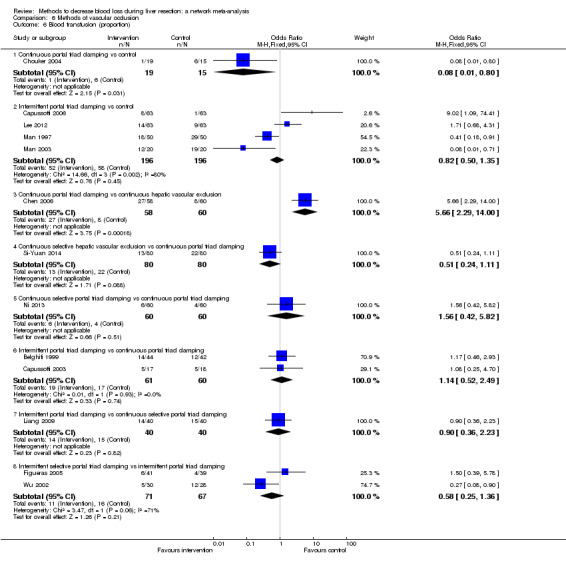
Comparison 6 Methods of vascular occlusion, Outcome 6 Blood transfusion (proportion).
6.7. Analysis.
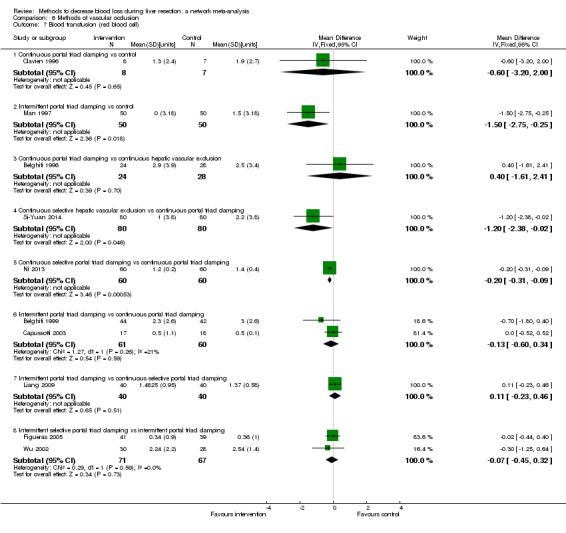
Comparison 6 Methods of vascular occlusion, Outcome 7 Blood transfusion (red blood cell).
6.8. Analysis.
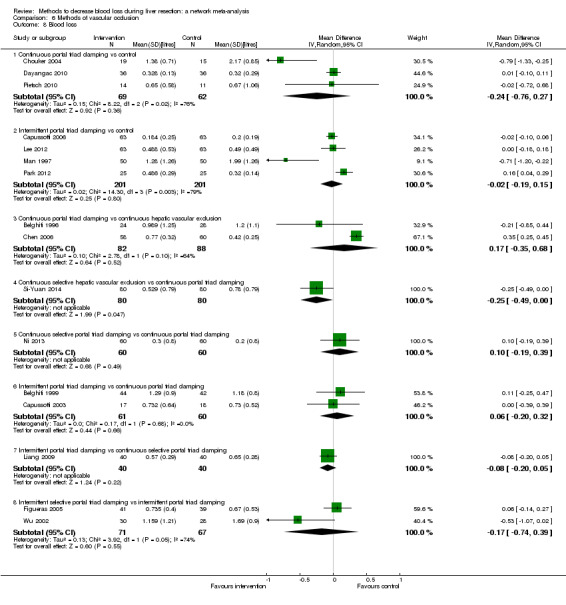
Comparison 6 Methods of vascular occlusion, Outcome 8 Blood loss.
6.9. Analysis.
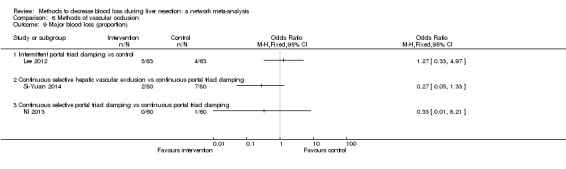
Comparison 6 Methods of vascular occlusion, Outcome 9 Major blood loss (proportion).
6.10. Analysis.
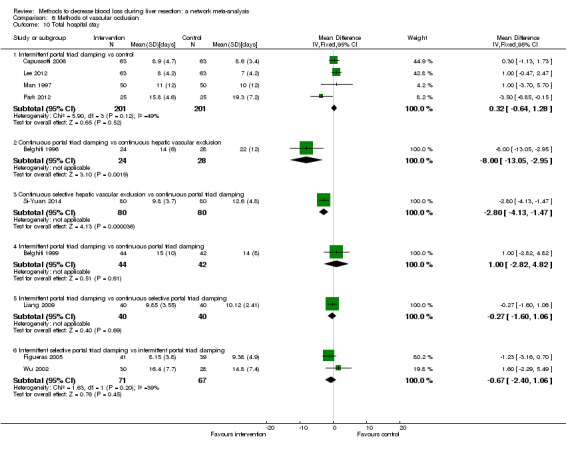
Comparison 6 Methods of vascular occlusion, Outcome 10 Total hospital stay.
6.11. Analysis.
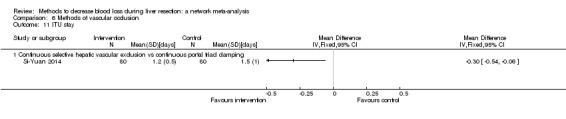
Comparison 6 Methods of vascular occlusion, Outcome 11 ITU stay.
6.12. Analysis.
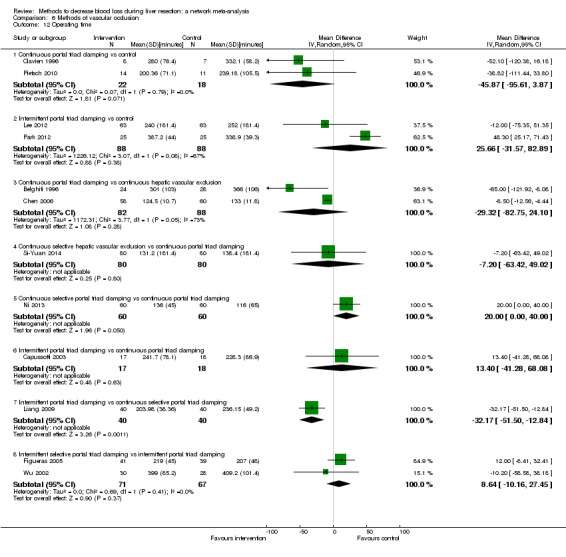
Comparison 6 Methods of vascular occlusion, Outcome 12 Operating time.
Comparison 7. Pharmacological interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (perioperative) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Recombinant factor VIIa vs control | 1 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.13, 2.83] |
| 1.2 Tranexamic acid vs control | 1 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious adverse events (proportion) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Anti‐thrombin III vs control | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.20, 6.99] |
| 2.2 Recombinant factor VIIa vs control | 2 | 432 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.58, 2.09] |
| 3 Serious adverse events (number) | 3 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Recombinant factor VIIa vs control | 2 | 432 | Rate Ratio (Fixed, 95% CI) | 1.46 [0.75, 2.84] |
| 3.2 Tranexamic acid vs control | 1 | 214 | Rate Ratio (Fixed, 95% CI) | 0.86 [0.31, 2.37] |
| 4 Adverse events (proportion) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Anti‐thrombin III vs control | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.10, 2.84] |
| 4.2 Recombinant factor VIIa vs control | 1 | 232 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.34, 3.21] |
| 4.3 Tranexamic acid vs control | 1 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.36, 1.67] |
| 5 Adverse events (number) | 3 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Recombinant factor VIIa vs control | 2 | 432 | Rate Ratio (Fixed, 95% CI) | 0.98 [0.87, 1.10] |
| 5.2 Tranexamic acid vs control | 1 | 214 | Rate Ratio (Fixed, 95% CI) | 0.78 [0.43, 1.42] |
| 6 Blood transfusion (proportion) | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Aprotinin vs control | 1 | 97 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.82] |
| 6.2 Desmopressin vs control | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.12, 2.57] |
| 6.3 Recombinant factor VIIa vs control | 2 | 416 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.62, 1.43] |
| 6.4 Tranexamic acid vs control | 1 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.40] |
| 7 Blood transfusion (fresh frozen plasma) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Desmopressin vs control | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.39, 0.19] |
| 8 Blood loss | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Aprotinin vs control | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.87, 0.00] |
| 9 Hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Tranexamic acid vs control | 1 | 214 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.06, 1.06] |
| 10 Operating time | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Aprotinin vs control | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐30.08, 28.08] |
7.1. Analysis.

Comparison 7 Pharmacological interventions, Outcome 1 Mortality (perioperative).
7.2. Analysis.
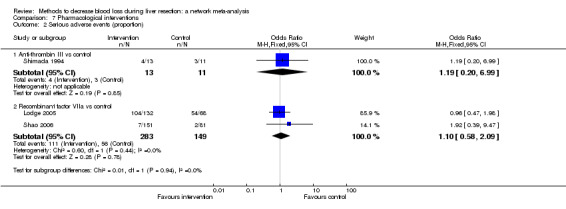
Comparison 7 Pharmacological interventions, Outcome 2 Serious adverse events (proportion).
7.3. Analysis.
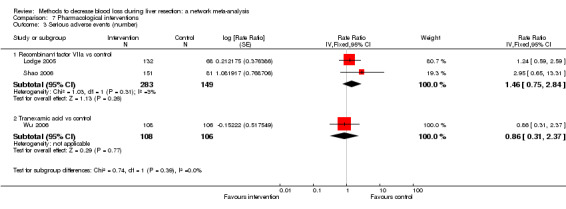
Comparison 7 Pharmacological interventions, Outcome 3 Serious adverse events (number).
7.4. Analysis.
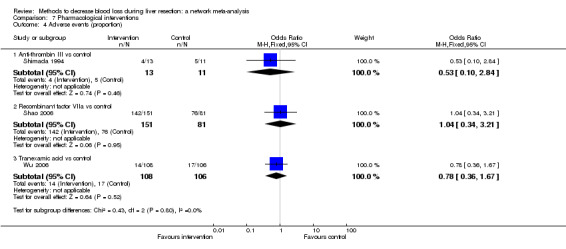
Comparison 7 Pharmacological interventions, Outcome 4 Adverse events (proportion).
7.5. Analysis.
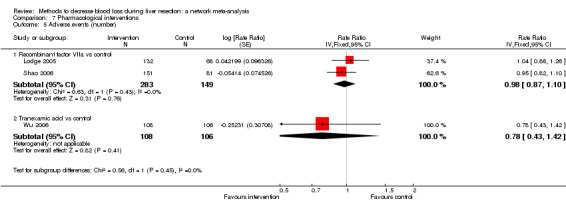
Comparison 7 Pharmacological interventions, Outcome 5 Adverse events (number).
7.6. Analysis.
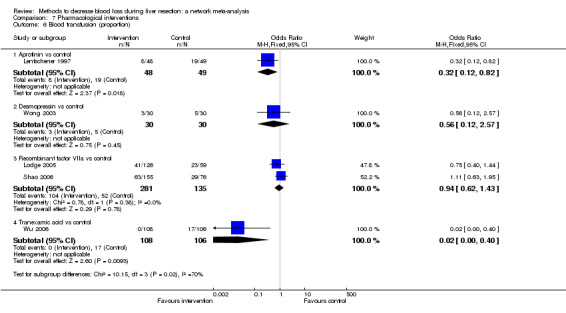
Comparison 7 Pharmacological interventions, Outcome 6 Blood transfusion (proportion).
7.7. Analysis.
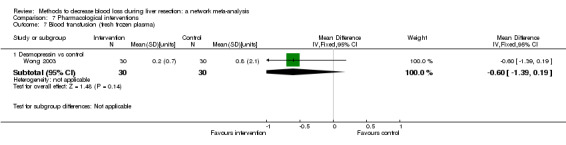
Comparison 7 Pharmacological interventions, Outcome 7 Blood transfusion (fresh frozen plasma).
7.8. Analysis.
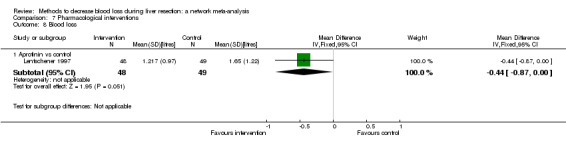
Comparison 7 Pharmacological interventions, Outcome 8 Blood loss.
7.9. Analysis.
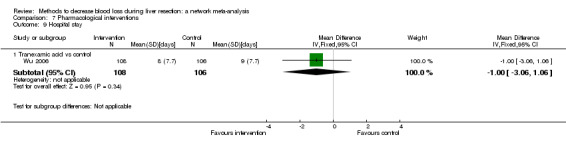
Comparison 7 Pharmacological interventions, Outcome 9 Hospital stay.
7.10. Analysis.
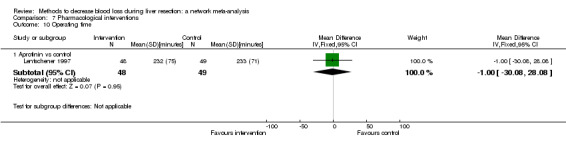
Comparison 7 Pharmacological interventions, Outcome 10 Operating time.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arita 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan
Number randomised: 80
Postrandomisation dropouts: 0 (0%)
Revised sample size: 80
Average age: 67 years
Women: 20 (25%)
Number of cirrhotics: 21 (26.3%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): not stated
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria: inflow occlusion at the hepatic hilum proved impossible at laparotomy |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: radiofrequency dissecting sealer (n = 40) Group 2: clamp‐crush method (n = 40) Radiofrequency dissecting sealer: Tissue Link (Valley Lab) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, number of serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, and length of hospital stay. | |
| Notes | Authors provided replies in March 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was done by the minimization procedure with stratification by age (less than 65 versus 65 years or more), indocyanine green retention rate at 15 min (ICG‐R15) (less than 20 versus 20 per cent or more) and type of resection (minor or major). Resection of two or more Couinaud segments was defined as 'major'". |
| Allocation concealment (selection bias) | Low risk | Quote: "The assignments were done by an internet‐accessed registration system administered by the independent randomization service University Hospital Medical Information Network in Japan". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (author replies): "Patients were informed just of a study plan, but did not know which cohort they belonged to. However, surgeons, of course, could not be blinded because of the nature of study". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The outcome assessors were not blinded". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Low risk | Quote: "This work was supported by a grant from the Kanae Foundation for Life‐Socio‐medical service". |
| Other bias | Low risk | Comment: no other bias |
Bektas 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany
Number randomised: 70
Postrandomisation dropouts: not stated
Revised sample size: 70
Average age: 57 years
Women: 31 (44.3%)
Number of cirrhotics: 2 (2.9%)
Number of major liver resections: 33 (47.1%)
Number of right hepatectomies: not stated
Follow‐up (months): 1
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria: arterial or venous bleeding |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = 35) Group 2: control (n = 35) Fibrin sealant: TISSEEL (Baxter Health Corporation) Spray; 5 mL of fibrinogen with synthetic aprotinin and 5 mL of thrombin (500 IU/mL) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, number of serious adverse events, proportion of people with any adverse events, and number of adverse events. | |
| Notes | Authors provided replies in March 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " Subjects were randomized at a ratio of 1:1 to receive either FS or MC according to a predetermined randomization scheme stratified by study center using the random number generator algorithm of Wichmann and Hill as modified by McLeod". Comment: FS: fibrin sealant; MC: manual compression. |
| Allocation concealment (selection bias) | Low risk | Quote: "On the day of surgery, the randomization envelope number was obtained from an electronic data capture system. The randomization envelope assigned was opened in the operating room after confirmation of the intraoperative eligibility criteria and clamping of the hilar vessels in the hepatoduodenal ligament (i.e., Pringle maneuver)". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (author reply): "the patient was blinded to the treatment administered. Blinding of the investigator (surgeon) was not possible due to the difference in procedures (spray administration of fibrin sealant vs. manual compression with a surgical gauze swab". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (author reply): "The investigator assessed intra‐operative time to hemostasis and other outcome measures, i.e., outcome was assessed unblinded". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all patients were included for the clinical outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | High risk | Quote: "This clinical research was sponsored by Baxter Innovations GmbH, Vienna, Austria". |
| Other bias | Low risk | Comment: no other bias |
Belghiti 1996.
| Methods | Randomised clinical trial | |
| Participants | Country: France
Number randomised: 52
Postrandomisation dropouts: 8 (15.4%)
Revised sample size: 44
Average age: 46 years
Women: 31 (70.5%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: 44 (100%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria: encasement of blood vessels . |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: continuous portal triad clamping (n = 24) Group 2: continuous hepatic vascular exclusion (n = 28) Hepatic vascular exclusion by encircling the entire retrohepatic inferior vena cava | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, number of adverse events, operative blood loss, quantity of blood transfused (red cell transfusion or whole blood), length of hospital stay, and operating time. | |
| Notes | Reasons for postrandomisation dropouts: cross‐over to other group (n = 4 in each group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Belghiti 1999.
| Methods | Randomised clinical trial | |
| Participants | Country: France
Number randomised: 86
Postrandomisation dropouts: 0 (0%)
Revised sample size: 86
Average age: 51 years
Women: 39 (45.3%)
Number of cirrhotics: not stated
Number of major liver resections: 39 (45.3%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: continuous portal triad clamping (n = 42) Group 2: intermittent portal triad clamping (n = 44) Continuous portal triad clamping: until end of transection Intermittent portal triad clamping: 15 min on and 5 min off until hepatectomy | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), and length of hospital stay. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Capussotti 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Number randomised: 35
Postrandomisation dropouts: not stated
Revised sample size: 35
Average age: 63 years
Women: 8 (22.9%)
Number of cirrhotics: 35 (100%)
Number of major liver resections: 8 (22.9%)
Number of right hepatectomies: 2 (5.7%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups Group 1: continuous portal triad clamping (n = 18) Group 2: intermittent portal triad clamping (n = 17) Intermittent portal triad clamping: 15 min on and 5 min off | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), and operating time | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization to the type of clamping was assigned by computer generated random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Capussotti 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Number randomised: 126
Postrandomisation dropouts: 0 (0%)
Revised sample size: 126
Average age: 64 years
Women: 51 (40.5%)
Number of cirrhotics: 19 (15.1%)
Number of major liver resections: 56 (44.4%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients with resectable liver tumours Exclusion criteria: patients requiring concomitant bowel or bile duct resection or total vascular exclusion |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: intermittent portal triad clamping (n = 63) Group 2: control (n = 63) Intermittent portal triad clamping: 15 min on and 5 min off | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, proportion of people requiring blood transfusion, and length of hospital stay. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization took place intraoperatively and was performed with a computerized random‐number generator". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Capussotti 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Number randomised: 66
Postrandomisation dropouts: 1 (1.5%)
Revised sample size: 65
Average age: 62 years
Women: 39 (60%)
Number of cirrhotics: 5 (7.7%)
Number of major liver resections: 65 (100%)
Number of right hepatectomies: 65 (100%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: anterior approach (n = 33) Group 2: control (n = 32) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people with major blood loss, proportion of people requiring blood transfusion, length of hospital stay, and operating time. | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random sequence was performed using a computerised random number generator". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Low risk | Quote: "there has been no significant financial support for this work that could have influenced its outcome". |
| Other bias | Low risk | Comment: no other bias |
Chapman 2000.
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Number randomised: 80
Postrandomisation dropouts: 13 (16.3%)
Revised sample size: 67
Average age: 58 years
Women: 38 (56.7%)
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients undergoing elective liver resection . |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = 38) Group 2: collagen (n = 29) Fibrin sealant: Costasis (Cohesion Technologies) ‐ bovine thrombin and collagen combined with patient's own plasma Collagen: Instat (Johnson & Johnson) | |
| Outcomes | The outcomes reported were: short‐term mortality, quantity of blood transfused (red cell transfusion or whole blood), and operating time. | |
| Notes | Reasons for postrandomisation dropouts: surgery cancelled (n = 8), study co‐ordinator not available (n = 1), other reasons (n = 4); 7 in intervention and 6 in control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Thus, separate computer generated randomization schedules of treatment group assignment placed in sealed envelopes were used for each clinical site and for each type of surgery". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Thus, separate computer generated randomization schedules of treatment group assignment placed in sealed envelopes were used for each clinical site and for each type of surgery". Comment: further details of sealed envelope were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: morbidity was not reported. |
| Vested interest bias | High risk | Quote: "This work was supported in part by Cohesion Technologies Inc, Palo Alto, Calif". |
| Other bias | Low risk | Comment: no other bias |
Chen 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: China
Number randomised: 118
Postrandomisation dropouts: not stated
Revised sample size: 118
Average age: 41 years
Women: 14 (11.9%)
Number of cirrhotics: 118 (100%)
Number of major liver resections: 102 (86.4%)
Number of right hepatectomies: 0 (0%)
Follow‐up (months): 1
Further details of methods of liver resection
Inclusion criteria: patients with cirrhosis and hepatocellular carcinoma undergoing minor or major right sided liver resections Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: continuous portal triad clamping (n = 58) Group 2: continuous hepatic vascular exclusion (n = 60) Hepatic vascular exclusion by encircling the entire infrahepatic inferior vena cava | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, proportion of people requiring blood transfusion, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Low risk | Quote: "This work was supported by the key clinical project fund [No. 321 (2001)] from the Chinese Ministry of Public Health". |
| Other bias | Low risk | Comment: no other bias |
Choi 2007.
| Methods | Randomised clinical trial | |
| Participants | Country: South Korea
Number randomised: 62
Postrandomisation dropouts: not stated
Revised sample size: 62
Average age: 55 years
Women: 18 (29%)
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients undergoing liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: low central venous pressure (n = 30) Group 2: control (n = 32) Low central venous pressure: by restricting flow from legs | |
| Outcomes | The outcomes reported were: operative blood loss, length of hospital stay, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Chouker 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany
Number randomised: 46
Postrandomisation dropouts: 12 (26.1%)
Revised sample size: 34
Average age: 61 years
Women: 11 (32.4%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: 8 (23.5%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: continuous portal triad clamping (n = 19) Group 2: control (n = 15) | |
| Outcomes | The outcomes reported were: operative blood loss and proportion of people requiring blood transfusion. | |
| Notes | Reasons for postrandomisation dropouts: patients in this trial were randomised to 3 groups out of which 2 are eligible for this review. The reason for dropout in the included groups was not available. There were 4 dropouts in intervention group and 8 dropouts in control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A blinded allocation of surgeons/anaesthesists was not feasible". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Clavien 1996.
| Methods | Randomised clinical trial | |
| Participants | Country: International multicentric trial
Number randomised: 17
Postrandomisation dropouts: 2 (11.8%)
Revised sample size: 15
Average age: 63 years
Women: 4 (26.7%)
Number of cirrhotics: 6 (40%)
Number of major liver resections: 15 (100%)
Number of right hepatectomies: 15 (100%)
Follow‐up (months): 3 months
Further details of methods of liver resection
Inclusion criteria: patients undergoing right hepatectomy |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: continuous portal triad clamping (n = 8) Group 2: control (n = 7) Note: after every 1 h of continuous portal triad clamping (or 30 min for cirrhotic patients), the clamp was released for 10 min before reclamping. | |
| Outcomes | The outcomes reported were: short‐term mortality, quantity of blood transfused (red cell transfusion or whole blood), and operating time. | |
| Notes | Reasons for postrandomisation dropouts: cardiac transplant patient (n = 1), haemodynamic instability during surgery (n = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: morbidity was not reported. |
| Vested interest bias | Low risk | Quote: "Supported by a grant from the Medical Research Council of Canada and by a special grant from the Toronto Hospital, University of Toronto, Toronto". |
| Other bias | Low risk | Comment: no other bias |
Dayangac 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: Turkey
Number randomised: 72
Postrandomisation dropouts: 0 (0%)
Revised sample size: 72
Average age: 39 years.
Women: not stated
Number of cirrhotics: not stated
Number of major liver resections: 72 (100%)
Number of right hepatectomies: 72 (100%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients undergoing right donor hepatectomy |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: continuous portal triad clamping (n = 36) Group 2: control (n = 36) | |
| Outcomes | The outcome reported was: operative blood loss. | |
| Notes | Authors provided replies in March 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (author reply): "The patients were randomly assigned by coin tossing". |
| Allocation concealment (selection bias) | Low risk | Quote: "Neither participants, nor investigators could foresee the assignment, because the coin tossing was performed by the chief operating room nurse at the time of incision". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (author reply): "Yes, all the patients and all of the transplant nurses, coordinators, and physicians (except the senior donor surgeon, who performed all hepatectomies) were blinded". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (author reply): "Yes, at the end of the study, I performed all the analyses on the prospectively collected data. As the outcome assessor, I was blinded until the end of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Low risk | Quote (author reply): "There was no direct or indirect financial support". |
| Other bias | Low risk | Comment: no other bias |
De Boer 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: Netherlands
Number randomised: 310
Postrandomisation dropouts: 0 (0%)
Revised sample size: 310
Average age: 62 years
Women: 151 (48.7%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: 160 (51.6%)
Number of right hepatectomies: not stated
Follow‐up (months): 1
Further details of methods of liver resection
Inclusion criteria: at least 1 liver segment or a nonanatomical resection Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = 156) Group 2: control (n = 154) Fibrin sealant: Quixil (Johnson & Johnson Medical) spray; 5 mL of fibrinogen and tranexamic acid and 5 mL of thrombin | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, and proportion of people requiring blood transfusion. | |
| Notes | Authors provided replies in March 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (author reply): "A statistician, who was not otherwise involved in the conduct of the study prepared the randomization list, using a computer random number generator". |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation employed a sequentially numbered opaque and sealed envelope system". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Surgeons could not be kept unaware of treatment allocation, but patients, local investigators responsible for data gathering, data analysts, and radiologists did remain unaware of the study group assignment". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Surgeons could not be kept unaware of treatment allocation, but patients, local investigators responsible for data gathering, data analysts, and radiologists did remain unaware of the study group assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all patients were included for the clinical outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | High risk | Quote: "This study was supported by the Fund for Medical Technology Assessment of the University Medical Center Groningen and by Johnson & Johnson Medical". |
| Other bias | Low risk | Comment: no other bias |
Doklestic 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: Serbia
Number randomised: 60
Postrandomisation dropouts: not stated
Revised sample size: 60
Average age: 58 years
Women: 40 (66.7%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: 20 (51.6%)
Number of right hepatectomies: not stated
Follow‐up (months): 1
Further details of methods of liver resection
Inclusion criteria: patients undergoing hepatectomy for benign or malignant tumours in patients with adequate functional reserve of the heart, lungs, and kidneys Exclusion criteria: cirrhosis |
|
| Interventions | Participants were randomly assigned to 3 groups. Group 1: clamp‐crush method (n = 20) Group 2: cavitron ultrasonic surgical aspirator (n = 20) Group 3: radiofrequency dissecting sealer (LIGASURE) (n = 20) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, proportion of people requiring blood transfusion, length of hospital stay, length of intensive therapy unit stay, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization was performed on the day prior to surgery using the sealed envelopes; each group consisted of 20 subjects". Comment: further details of sealed envelope method were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Low risk | Quote: "This study was supported by funding by funding from the Ministry of Education and Science of the Republic of Serbia". |
| Other bias | Low risk | Comment: no other bias |
El‐Kharboutly 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: Egypt
Number randomised: 40
Postrandomisation dropouts: not stated
Revised sample size: 40
Average age: 51 years
Women: 17 (42.5%)
Number of cirrhotics: 40 (100%)
Number of major liver resections: 25 (62.5%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: cirrhotic patients undergoing liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: control (n = 20) Group 2: low central venous pressure (n = 20) Low central venous pressure: nitroglycerine | |
| Outcomes | The outcomes reported were: number of serious adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly (closed envelope method)". Comment: further details of sealed envelope method were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Figueras 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain
Number randomised: 80
Postrandomisation dropouts: 0 (0%)
Revised sample size: 80
Average age: 62 years
Women: 21 (26.3%)
Number of cirrhotics: 39 (48.8%)
Number of major liver resections: 0 (0%)
Number of right hepatectomies: 0 (0%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients undergoing minor liver resection Exclusion criteria: patients requiring concomitant bowel resection or contralateral hepatic resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: intermittent portal triad clamping (n = 39) Group 2: intermittent selective portal triad clamping (n = 41) Intermittent clamping: 15 min on and 5 min off | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), length of hospital stay, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed using sealed envelopes". Comment: further details of sealed envelope method were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Low risk | Quote: "This is study was partially supported by a grant from 'August Pi i Sunyer Foundation', Ciutat Sanitaria i Universitaria de Bellvitge, Barcelona, Spain; and by a grant from 'Fundacio August Pi i Sunyer', Hospital Universitario de Bellvitge, Barcelona, Spain". |
| Other bias | Low risk | Comment: no other bias |
Figueras 2007.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain
Number randomised: 300
Postrandomisation dropouts: 0 (0%)
Revised sample size: 300
Average age: 61 years
Women: 195 (65%)
Number of cirrhotics: 21 (7%)
Number of major liver resections: 181 (60.3%)
Number of right hepatectomies: 112 (37.3%)
Follow‐up (months): 6
Further details of methods of liver resection
Inclusion criteria:patients undergoing liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant plus collagen (n = 150) Group 2: control (n = 150) Fibrin sealant spray: Tissucol Collagen: collagen sponge (Johnson & Johnson) Note: in both groups, bleeding from raw surface was controlled using argon beam coagulator or Tissuelink | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, proportion of people with any adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), length of hospital stay, and operating time. | |
| Notes | Authors provided replies in March 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (author reply): "Random list was generated by a computer". |
| Allocation concealment (selection bias) | Low risk | Quote (author reply): "For patient allocation among groups we used consecutive sealed opaque envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The patients were blinded as well as the healthcare providers. After finishing the liver resection the envelope was opened and the surgeon applied the technique of the allocated group". Comment: further details of blinding were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The data manager and assessors were also blinded". Comment: further details of blinding were not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Low risk | Quote (author reply): "Supported in part by a grant from Fundacio Bellvitge, Barcelona, Spain. The study was not funded because the hemostatic product was approved by the agencia española del medicamento". |
| Other bias | Low risk | Comment: no other bias |
Fischer 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: European multicentre trial
Number randomised: 119
Postrandomisation dropouts: 13 (10.9%)
Revised sample size: 106
Average age: 61 years
Women: 49 (46.2%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = 54) Group 2: argon beam coagulator (n = 52) Fibrin sealant: Tachosil (Nycomed) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events and proportion of people with any adverse events. | |
| Notes | Reasons for postrandomisation dropouts: lost to follow‐up or discontinued (6 in TachoSil group and 7 in control group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Clinical monitoring, centralized telephone randomization, data management, and statistics were done by Quintiles Ltd". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This trial was open, because blinding for surgeons and outcome assessors was not possible owing to the nature of the interventions and the primary end point". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This trial was open, because blinding for surgeons and outcome assessors was not possible owing to the nature of the interventions and the primary end point". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | High risk | Quote: "This study was fully sponsored by Nycomed". |
| Other bias | Low risk | Comment: no other bias |
Franceschi 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Number randomised: 153
Postrandomisation dropouts: not stated
Revised sample size: 153
Average age: not stated
Women: not stated
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): 1
Further details of methods of liver resection
Inclusion criteria: patients undergoing liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = not stated) Group 2: collagen (n = not stated) Fibrin sealant: CryoSeal FS Collagen: Instat (Ethicon) | |
| Outcomes | None of the outcomes of interest were reported | |
| Notes | Number of participants in each group was not stated. There were no significant difference in blood loss, operating time, hospital stay, or complications. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Frilling 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: European multicentre trial
Number randomised: 121
Postrandomisation dropouts: 0 (0%)
Revised sample size: 121
Average age: not stated
Women: not stated
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients undergoing elective liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = 59) Group 2: argon beam coagulator (n = 62) Fibrin sealant: Tachosil | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, proportion of people with any adverse events, and number of adverse events. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation was concealed by the use of sealed treatment code envelopes, which were opened when the patients had fulfilled the eligibility criteria". Comment: further details of sealed envelope method were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The trial was open, since the appearance of TachoSil precluded blinding". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The trial was open, since the appearance of TachoSil precluded blinding". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Genyk 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Number randomised: 224
Postrandomisation dropouts: not stated
Revised sample size: 224
Average age: not stated
Women: not stated
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients undergoing liver resection with minor to moderate bleeding from the resection area after primary control of arterial bleeding or major venous haemorrhage |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = 114) Group 2: oxidised cellulose (n = 110) Fibrin sealant: Tachosil Oxidised cellulose: Surgicel | |
| Outcomes | The outcomes reported were: proportion of people with any adverse events | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Gugenheim 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: France
Number randomised: 58
Postrandomisation dropouts: not stated
Revised sample size: 58
Average age: 62 years
Women: 31 (53.4%)
Number of cirrhotics: 9 (15.5%)
Number of major liver resections: 31 (53.4%)
Number of right hepatectomies: 20 (34.5%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = 29) Group 2: plasmajet coagulator (n = 29) Fibrin sealant: fibrin glue (no further details) | |
| Outcomes | The outcomes reported were: short‐term mortality and proportion of people with serious adverse events | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Random assignment was done by opening an envelope in which allotted treatment was hidden". Comment: further details of sealed envelope method were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: morbidity was not reported adequately. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Guo 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: China
Number randomised: 30
Postrandomisation dropouts: not stated
Revised sample size: 30
Average age: 65 years
Women: 8 (26.7%)
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: acute normovolemic haemodilution plus low central venous pressure (n = 15) Group 2: control (n = 15) Acute normovolemic dilution plus low central venous pressure: blood withdrawn to a target of 28% haemocrit and replaced with fluid; target for central venous pressure was not reported | |
| Outcomes | The outcomes reported were: operative blood loss and quantity of blood transfused (red cell transfusion or whole blood). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Low risk | Quote: "The study is supported by Ningbo Medical Technology Foundation 200612". |
| Other bias | Low risk | Comment: no other bias |
Guo 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: China
Number randomised: 60
Postrandomisation dropouts: not stated
Revised sample size: 60
Average age: 50 years
Women: 22 (36.7%)
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 groups. Group 1: control (n = 20) Group 2: low central venous pressure (n = 20) Group 3: low central venous pressure + acute normovolemic haemodilution (n = 20) Low central venous pressure: fluid restriction and nitroglycerine Acute normovolemic haemodilution plus low central venous pressure: withdrawal of blood to a target haematocrit of 30% and replacement with colloids | |
| Outcomes | The outcomes reported were: operating time | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Low risk | Quote: "The study is supported by Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai Grant no:PWR12013‐03 and funded by Disciplines Group Construction Project of Pudong Health Bureau of Shanghai Grant no:PWZxq2014‐06". |
| Other bias | Low risk | Comment: no other bias. |
Hasegawa 2002.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan
Number randomised: 80
Postrandomisation dropouts: 1 (1.3%)
Revised sample size: 79
Average age: 65 years
Women: not stated
Number of cirrhotics: 35 (44.3%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients scheduled to undergo hepatic resection for the removal of tumours were entered into this trial Exclusion criteria: patients with severe pulmonary dysfunction (< 70% vital capacity, or 1 second forced expiratory volume divided by forced vital capacity < 60%) |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: control (n = 39) Group 2: hypoventilation (n = 40) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (cryoprecipitate), length of hospital stay, and operating time. | |
| Notes | Authors provided replies in March 2016. Reasons for postrandomisation dropouts: did not undergo liver resection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In the operating room, eligible patients were randomly assigned to the normoventilation or hypoventilation groups by the minimization method.". |
| Allocation concealment (selection bias) | Low risk | Quote: "In the operating room, eligible patients were randomly assigned to the normoventilation or hypoventilation groups by the minimization method ". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (author reply): "Only 2 investigators (K.H. and R.O.), who were not involved in the hepatic resections, had seen the results of the randomization procedure, and they were able to decide to alter the respiratory conditions without consulting with the surgeon. The intervention of this study was hypoventilation during liver parenchyma division, while the control was normoventilation. Both are done by anesthesiologists, which could be blinded to the surgeons and the enrolled patients". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (author reply): "Outcome assessors were not blinded. The outcome measures including blood loss and central venous pressure were evaluated by nurses and anesthesiologists as the outcome assessors". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there was 1 postrandomisation dropout. This was because the patient did not undergo liver resection. This postrandomisation dropout is unlikely to affect the effect estimates for people undergoing liver resection. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Low risk | Quote: "This work was supported by a grant‐in‐aid for scientific research from the Ministry of Education, Science, and Culture of Japan (grant 12470252) (Drs Kubota and Makuuchi)". |
| Other bias | Low risk | Comment: no other bias |
Ikeda 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan
Number randomised: 120
Postrandomisation dropouts: 0 (0%)
Revised sample size: 120
Average age: 66 years
Women: 39 (32.5%)
Number of cirrhotics: 27 (22.5%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: radiofrequency dissecting sealer (n = 60) Group 2: clamp‐crush method (n = 60) Radiofrequency dissecting sealer: ligasure | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, and length of hospital stay. | |
| Notes | Authors provided replies in March 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the assignments were generated by an internet‐accessed randomization system supported by Mebix Inc.". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "In this study, results of assignment were not blinded". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "In this study, results of assignment were not blinded". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Low risk | Quote: "Supported by grants from the Public Trust Surgery Research Fund, the Japanese Clinical Oncology Fund, the Public Trust Haraguchi Memorial Cancer Research Fund, the JSPS Fujita Memorial Fund for Medical Research; and a grant‐in‐aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant 18790955)". |
| Other bias | Low risk | Comment: no other bias |
Jarnagin 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: USA
Number randomised: 135
Postrandomisation dropouts: 5 (3.7%)
Revised sample size: 130
Average age: 53 years
Women: 61 (46.9%)
Number of cirrhotics: not stated
Number of major liver resections: 130 (100%)
Number of right hepatectomies: 53 (40.8%)
Follow‐up (months): 3
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: acute normovolemic haemodilution plus low central venous pressure (n = 63) Group 2: low central venous pressure (n = 67) Acute normovolemic haemodilution: blood was withdrawn and replaced by colloids and crystalloids to reach a haemocrit target of 8 gm/dL Low central venous pressure was maintained < 5 H20 using fluid restriction and pharmacologic manipulation | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, proportion of people with major blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), quantity of blood transfused (fresh frozen plasma), length of hospital stay, and operating time. | |
| Notes | Reasons for postrandomisation dropouts: not clearly stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The generation of the randomization sequences was performed in the Office of Clinical Research at Memorial Sloan‐Kettering Cancer Center (MSKCC) by a statistician completely blinded to patient clinical data ". Comment: the method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Kajikawa 1994.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan
Number randomised: 42
Postrandomisation dropouts: not stated
Revised sample size: 42
Average age: not stated
Women: not stated
Number of cirrhotics: 42 (100%)
Number of major liver resections: 12 (28.6%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: cirrhotic patients undergoing liver resection for HCC |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: autologous blood donation (n = 21) Group 2: control (n = 21) Note: autologous blood donation group was further randomised to recombinant erythropoietin and no erythropoietin | |
| Outcomes | The outcomes reported were: operative blood loss, proportion of people with major blood loss, proportion of people requiring blood transfusion, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Kakaei 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: Iran
Number randomised: 45
Postrandomisation dropouts: not stated
Revised sample size: 45
Average age: 48 years
Women: 27 (60%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge.
Further details of methods of liver resection
Inclusion criteria: patients aged 18‐75 years old undergoing liver resection for resectable mass Exclusion criteria
Patients in need of resurgery due to bleeding or bile leak from liver other than resection site |
|
| Interventions | Participants were randomly assigned to 3 groups. Group 1: fibrin sealant (n = 15) Group 2: oxidised cellulose (n = 15) Group 3: cyanoacrylate (n = 15) Oxidised cellulose: Surgicel (Ethicon Inc) Fibrin sealant: Tachosil (Takeda Pharmaceuticals) Cyanoacrylate: Glubran 2 (GEM S.R.L.) | |
| Outcomes | The outcomes reported were: number of serious adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), quantity of blood transfused (fresh frozen plasma), and length of hospital stay. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to these 3 groups by a web‐based calculator available in this web address: http://www.randomizer.org". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Blinding for surgeons was not possible owing to the nature of the used materials’ consistency (spongy TachoSil knitted fabric Surgicel and liquid Glubran 2) and their packages". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The postoperative assessors were completely blinded to which agents were used for each patient". Comment: it is not clear how the assessment was done if the surgeons were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality was not reported. |
| Vested interest bias | Low risk | Quote: "This research was financially supported by the Vice Chancellor for Research, Tabriz University of Medical Sciences, Iran". |
| Other bias | Low risk | Comment: no other bias |
Kato 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan
Number randomised: 85
Postrandomisation dropouts: 0 (0%)
Revised sample size: 85
Average age: 66 years
Women: not stated
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): not stated
Further details of methods of liver resection
Inclusion criteria: patients undergoing liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: low central venous pressure (n = 43) Group 2: control (n = 42) Low central venous pressure: by inferior IVC clamping | |
| Outcomes | The outcomes reported were: short‐term mortality, operative blood loss, proportion of people requiring blood transfusion, length of hospital stay | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eighty‐five patients who underwent hepatic resection between June 2002 and May 2006 were randomly assigned to an IVC clamping or an IVC nonclamping group by the minimization method ". |
| Allocation concealment (selection bias) | Low risk | Quote: "Eighty‐five patients who underwent hepatic resection between June 2002 and May 2006 were randomly assigned to an IVC clamping or an IVC nonclamping group by the minimization method ". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: morbidity was not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Koea 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: European and Australian multicentre trial
Number randomised: 84
Postrandomisation dropouts: 0 (0%)
Revised sample size: 84
Average age: 65 years
Women: 36 (42.9%)
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: older than 18 years of age and required urgent or elective hepatic resection and were able to provide written, informed consent Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = 45) Group 2: oxidised cellulose (n = 39) Fibrin sealant: Fibrin Pad Oxidised cellulose: no further details | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Authors provided replies in March 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random allocation of patients to the FP or SoC groups was generated by a computer program and validated by a secondary statistician". Comment: FP: Fibrin Pad; SoC: standard of care. |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation was on sequentially numbered concealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (author reply): "The patients were blinded regarding the treatment, but health care providers can't be blinded given the obvious difference in the nature of the test products". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (author reply): "Outcomes assessor for outcomes not specific for research may include hospital staff which may not be aware of the research nor the treatment assignment. The collection of the outcomes information for analysis was done by research staff that is aware of the treatment assignment. However, the information collected is verified with the hospital source documents". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: although the authors call this intention‐to‐treat analysis, only an 'as‐treated' analysis is presented. |
| Selective reporting (reporting bias) | High risk | Comment: none of the outcomes of interest were presented for the randomised patients. |
| Vested interest bias | High risk | Quote: "Financial and product support was provided by Ethicon Inc, Sommervile, New Jersey, USA". |
| Other bias | Low risk | Comment: no other bias |
Kohno 1992.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan
Number randomised: 62
Postrandomisation dropouts: 0 (0%)
Revised sample size: 62
Average age: 62 years
Women: 14 (22.6%)
Number of cirrhotics: 46 (74.2%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): not stated
Further details of methods of liver resection
Inclusion criteria: patients undergoing liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: collagen (n = 31) Group 2: fibrin sealant (n = 31) Collagen: Avitene (Alcon Inc) Fibrin sealant: Beriplast P (Beringwerke AB) | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, number of adverse events, operative blood loss, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Koo 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: South Korea
Number randomised: 50
Postrandomisation dropouts: not stated
Revised sample size: 50
Average age: 53 years
Women: 14 (28%)
Number of cirrhotics: not stated
Number of major liver resections: 38 (76%)
Number of right hepatectomies: 27 (54%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: adults scheduled for elective hepatectomy Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: clamp‐crush method (n = 25) Group 2: cavitron ultrasonic surgical aspirator (n = 25) | |
| Outcomes | The outcomes reported were: proportion of people with any adverse events, operative blood loss, and operating time | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed by opening a sealed envelope before induction of anaesthesia". Comment: further information on sealed envelope system were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Kostopanagiotou 2007.
| Methods | Randomised clinical trial | |
| Participants | Country: Greece
Number randomised: 35
Postrandomisation dropouts: 7 (20%)
Revised sample size: 28
Average age: 52 years
Women: 11 (39.3%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: 16 (57.1%)
Number of right hepatectomies: 11 (39.3%)
Follow‐up (months): 12
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria: receiving immunosuppressive drugs |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: autologous blood donation (n = 15) Group 2: control (n = 13) Autologous blood donation: 2 units of blood were withdrawn before surgery | |
| Outcomes | The outcomes reported were: short‐term mortality, long‐term mortality, proportion of people with any adverse events, operative blood loss, quantity of blood transfused (red cell transfusion or whole blood), length of hospital stay, and operating time. | |
| Notes | Reasons for postrandomisation dropouts: requirement of allogenic transfusion in autologous group or did not require any transfusion (4 in intervention group and 3 in control group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Lee 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: Hong Kong, China
Number randomised: 126
Postrandomisation dropouts: 0 (0%)
Revised sample size: 126
Average age: 59 years
Women: 32 (25.4%)
Number of cirrhotics: 54 (42.9%)
Number of major liver resections: 62 (49.2%)
Number of right hepatectomies: 39 (31%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: adult patients (> 18 years) undergoing elective open liver resection. Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: intermittent portal triad clamping (n = 63) Group 2: control (n = 63) Intermittent portal triad clamping: 15 min on and 5 min off | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people with major blood loss, proportion of people requiring blood transfusion, length of hospital stay, operating time | |
| Notes | Authors provided replies in March 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The computer‐generated numbers were kept in sealed envelopes.". |
| Allocation concealment (selection bias) | Low risk | Quote (author reply): "The randomisation code was put in the sealed opaque envelops with consecutive number before the start of the study by a clerical staff not related to the study. An envelop was provided by research assistant consecutively and was brought to the theatre on day of surgery. The envelop was opened by the operation nurse or anesthetist independent to the study when and only if the surgical team confirm feasibility of proceeding to liver resection according to the study protocol intra‐operatively". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients and surgeons were not blinded to the randomization result". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (author reply): "The outcome assessors for blood loss were not blinded to the surgeons (because we felt operating surgeons should know about the degree of intra‐operative blood loss). But the actual recording procedure were performed by independent OT nurses and anaesthetists in the particular operation. The blood loss was measure by measuring all the blood collected in the suction bottle and weighing the gauzes in different phases of the operation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Low risk | Quote (author reply): "The study received no external funding. It was supported by the team's own private funding". |
| Other bias | Low risk | Comment: no other bias |
Lentschener 1997.
| Methods | Randomised clinical trial | |
| Participants | Country: France
Number randomised: 109
Postrandomisation dropouts: 12 (11%)
Revised sample size: 97
Average age: 54 years
Women: 45 (46.4%)
Number of cirrhotics: not stated
Number of major liver resections: 63 (64.9%)
Number of right hepatectomies: 34 (35.1%)
Follow‐up (months): not stated
Further details of methods of liver resection
Inclusion criteria: adult patients undergoing elective liver resection Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: aprotinin (n = 48) Group 2: control (n = 49) Aprotinin: loading dose: 2 X 106 kIU of aprotinin over a 20 min period after induction of anaesthesia Continuous infusion: 5 x 105 kIU per h administered by an infusion pump until skin closure Additional bolus: 5 X 105 kIU of aprotinin was infused every 3 transfused red blood cell packs Control: placebo | |
| Outcomes | The outcomes reported were: long‐term mortality, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), quantity of blood transfused (platelets), quantity of blood transfused (fresh frozen plasma), and operating time. | |
| Notes | Reasons for postrandomisation dropouts: tumour could not be removed (n = 6), wrong pre‐operative histological assessment (n = 5), and extension of incision to a thoracotomy (n = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " Patients were assigned in a double blind fashion by means of a computer‐generated code to receive either aprotinin or the equivalent volume of placebo". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: a placebo was used. It was not clear whether the anaesthetists and surgeons performing the surgery and the patients were aware of the groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "An identical‐appearing placebo was prepared by a nurse not involved in latter assessment. Each patient in the control group received equivalent volumes of the placebo (0.9% saline solution) at the respective times". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Quote: "This study was conducted independently of, but partially supported by, Assistance Publique‐Hopitaux de Paris, Bayer Pharma France and the Associations Claude Bernard and Mises au Point en Anesthesie‐Reanimation". |
| Other bias | Unclear risk | Comment: no other bias |
Lesurtel 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: Switzerland
Number randomised: 75
Postrandomisation dropouts: not stated
Revised sample size: 75
Average age: 57 years
Women: 34 (45.3%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: 45 (60%)
Number of right hepatectomies: 23 (30.6%)
Follow‐up (months): 3
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 groups. Group 1: radiofrequency dissecting sealer (n = 25) Group 2: cavitron ultrasonic surgical aspirator (n = 25) Group 3: hydrojet (n = 25) Radiofrequency dissecting sealer: Tissue Link Hydrojet: Helix Hydro‐Jet A fourth group with clamp‐crush and vascular occlusion was excluded since there was difference in the co‐intervention between the groups. | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, number of adverse events, proportion of people requiring blood transfusion, length of hospital stay, and length of intensive therapy unit stay. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | High risk | Quote: "Supported in an equivalent amount by Erbe (Tubingen, Germany), Tissuelink (Dover, NH), and Tyco Healthcare (Mansfield, MA). Dr. Selzner and Dr. Petrowsky are the recipients of the Novartis fellowship in HPB surgery and liver transplantation". |
| Other bias | Low risk | Comment: no other bias |
Liang 2009.
| Methods | Randomised clinical trial | |
| Participants | Country: China
Number randomised: 80
Postrandomisation dropouts: 0 (0%)
Revised sample size: 80
Average age: 49 years
Women: 22 (27.5%)
Number of cirrhotics: 36 (45%)
Number of major liver resections: 23 (28.8%)
Number of right hepatectomies: 6 (7.5%)
Follow‐up (months): 1
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria: patients requiring concomitant gastrointestinal procedures or bilioenteric anastomosis |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: continuous selective portal triad clamping (n = 40) Group 2: intermittent portal triad clamping (n = 40) Intermittent portal triad clamping: 20 min on and 5 min off | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), length of hospital stay, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Low risk | Quote: "The study was supported by the Basic Research Foundation of Sichuan Province of China (05JY29‐005‐3)". |
| Other bias | Low risk | Comment: no other bias |
Liu 1993.
| Methods | Randomised clinical trial | |
| Participants | Country: Taiwan
Number randomised: 40
Postrandomisation dropouts: 0 (0%)
Revised sample size: 40
Average age: 60 years
Women: 3 (7.5%)
Number of cirrhotics: 22 (55%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients undergoing liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = 20) Group 2: control (n = 20) Fibrin sealant: name not available | |
| Outcomes | The outcomes reported were: operative blood loss, quantity of blood transfused (red cell transfusion or whole blood), and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Liu 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: Hong Kong, China
Number randomised: 136
Postrandomisation dropouts: 16 (11.8%)
Revised sample size: 120
Average age: 52 years
Women: 17 (14.2%)
Number of cirrhotics: 38 (31.7%)
Number of major liver resections: 120 (100%)
Number of right hepatectomies: 120 (100%)
Follow‐up (months): 20
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: anterior approach (n = 60) Group 2: control (n = 60) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with any adverse events, operative blood loss, proportion of people with major blood loss, proportion of people requiring blood transfusion, length of hospital stay, length of intensive therapy unit stay, and operating time. | |
| Notes | Reasons for postrandomisation dropouts: 7 and 9 in intervention and control groups; Non‐HCC on histology (n = 8); segmentectomy (n = 1); palliative resection (n = 7) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A total of 136 patients were randomized initially to have either anterior approach hepatectomy (AA group) or conventional approach resection (CA group) by drawing consecutive sealed envelopes". Comment: further information on sealed envelope system were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The randomization was made known to the operating surgeon only when the disease was deemed suitable for curative resection". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "All patients received the same postoperative care by the same team of surgeons in the intensive care unit during the early postoperative course". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Low risk | Quote: "Supported by the Earmarked Research Grant of the Research Grants Council of Hong Kong". |
| Other bias | Low risk | Comment: no other bias |
Lodge 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: European multicentre trial
Number randomised: 204
Postrandomisation dropouts: 19 (9.3%)
Revised sample size: 185
Average age: 57 years
Women: 92 (49.7%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: recombinant factor viia (n = 126) Group 2: control (n = 59) Recombinant factor VIIa: first dose: slow intravenous injection (20 mcg/kg or 80 mcg/kg) within 5 min before incision. Second dose: identical dose was given 5 h after incision if the surgery time was anticipated to exceed 6 h Control: placebo | |
| Outcomes | The outcomes reported were: short‐term mortality, long‐term mortality, proportion of people with serious adverse events, number of serious adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), and operating time. | |
| Notes | Reasons for postrandomisation dropouts: did not receive drug (n = 4); did not undergo hepatectomy (n = 15) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer‐generated and was performed after patient eligibility assessments on the day of surgery by means of a central interactive voice response system set up by Novo Nordisk A/S". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was computer‐generated and was performed after patient eligibility assessments on the day of surgery by means of a central interactive voice response system set up by Novo Nordisk A/S". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The current randomized, controlled, double‐blind, multi‐national trial was designed to evaluate the efficacy and safety of rFVIIa in noncirrhotic patients undergoing major liver resection. To maintain blinding, an equal volume of trial drug per body weight was administered to all patients, irrespective of treatment group allocation". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The current randomized, controlled, double‐blind, multi‐national trial was designed to evaluate the efficacy and safety of rFVIIa in noncirrhotic patients undergoing major liver resection. To maintain blinding, an equal volume of trial drug per body weight was administered to all patients, irrespective of treatment group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | High risk | Quote: "The authors thank the patients and the hospital staff participating in the trial, as well as Allan Blemings, M.Sc. (Statistician), and Karsten Soendergaard, M.Sc. (Clinical Researcher), both at Novo Nordisk A/S, Copenhagen, Denmark". |
| Other bias | Low risk | Comment: no other bias |
Lupo 2007.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Number randomised: 51
Postrandomisation dropouts: 1 (2%)
Revised sample size: 50
Average age: 62 years
Women: 14 (28%)
Number of cirrhotics: 7 (14%)
Number of major liver resections: 21 (42%)
Number of right hepatectomies: 9 (18%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients undergoing potentially curative liver resection for primary or secondary liver cancers |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: radiofrequency dissecting sealer (n = 24) Group 2: clamp‐crush method (n = 26) Radiofrequency dissecting sealer: radionics needles | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, number of adverse events, proportion of people requiring blood transfusion, length of hospital stay, and operating time. | |
| Notes | Authors provided replies in March 2016. Reasons for postrandomisation dropouts: did not undergo liver resection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were assigned, in the operating room, by random‐number tables to undergo RF‐R (even numbers) or resection by the clamp‐crushing method (odd numbers)". Comment: RF‐R: radiofrequency radiation |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Authors replied that patients and healthcare providers were blinded". Comment: further information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Authors replied that outcome assessors were blinded". Comment: further information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there was 1 postrandomisation dropout. This was because the patient did not undergo liver resection. This postrandomisation dropout is unlikely to affect the effect estimates for people undergoing liver resection. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Low risk | Quote: "The authors replied that there was no external funding". |
| Other bias | Low risk | Comment: no other bias |
Man 1997.
| Methods | Randomised clinical trial | |
| Participants | Country: Hong Kong, China
Number randomised: 100
Postrandomisation dropouts: not stated
Revised sample size: 100
Average age: 56 years
Women: 19 (19%)
Number of cirrhotics: 29 (29%)
Number of major liver resections: 69 (69%)
Number of right hepatectomies: 14 (14%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria Adult patients undergoing liver resection Exclusion criteria Requiring concomitant bowel resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: intermittent portal triad clamping (n = 50) Group 2: control (n = 50) Intermittent portal triad clamping: 20 min on and 5 min off | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), and length of hospital stay. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Low risk | Quote: "Research Grant Council of Hong Kong in funding the study". |
| Other bias | Low risk | Comment: no other bias |
Man 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: Hong Kong, China
Number randomised: 40
Postrandomisation dropouts: 0 (0%)
Revised sample size: 40
Average age: 50 years
Women: 11 (27.5%)
Number of cirrhotics: not stated
Number of major liver resections: 26 (65%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients with resectable tumours. |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: intermittent portal triad clamping (n = 20) Group 2: control (n = 20) Intermittent portal triad clamping: 20 min on and 5 min off (until resection is completed or a maximum of 6 cycles) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with any adverse events, and proportion of people requiring blood transfusion. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Matot 2002.
| Methods | Randomised clinical trial | |
| Participants | Country: Israel
Number randomised: 78
Postrandomisation dropouts: not stated
Revised sample size: 78
Average age: 57 years
Women: 47 (60.3%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: 78 (100%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: acute normovolemic haemodilution + low central venous pressure (n = 39) Group 2: low central venous pressure (n = 39) Acute normovolemic haemodilution: blood was withdrawn and replaced by colloids to reach a haemocrit target of 24% Low central venous pressure was achieved by fluid restriction | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "On admission to the operating room, patients who met inclusion criteria were randomly assigned (random numbers) to one of two groups". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The anesthesiologist making decisions regarding transfusion was not blinded to patient group assignment". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Subsequent blood loss was estimated by assessment of the suction bottles, sponges, and the surgical drapes and gowns by an anesthesiologist who was not aware of the patient’s group assignment". Comment: Not clear whether other outcomes were assessed by a blinded observer. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Low risk | Quote: "Supported by a grant from the Joint Research Fund of the Hebrew University and Hadassah, Jerusalem, Israel". |
| Other bias | Low risk | Comment: no other bias |
Moench 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany
Number randomised: 128
Postrandomisation dropouts: 1 (0.8%)
Revised sample size: 127
Average age: 61 years
Women: 53 (41.7%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): 3
Further details of methods of liver resection
Inclusion criteria: non‐cirrhotic adult patients undergoing elective open liver resection Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: collagen (n = 62) Group 2: fibrin sealant (n = 65) Collagen: sangustop fleece (Aesculap AG) Fibrin sealant: Tachosil (Nycomed) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, number of serious adverse events, proportion of people with any adverse events, and number of adverse events. | |
| Notes | Authors provided replies in March 2016. Reasons for postrandomisation dropouts: the resection area was dry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Lists with a block size of 4 were generated for each participating center prior to the initiation of the study using the Software RandList of the DatInf GmbH (Tübingen, Germany)". |
| Allocation concealment (selection bias) | Low risk | Quote: "A1:1 intraoperative randomization was performed using identical looking, sealed, and numbered opaque envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "ESSCALIVER is a single‐blinded trial, i.e., patients were not informed about their assignment in order to increase reliability of secondary outcomes, assessed during the follow‐up visits". Comment: healthcare providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Due to the appearance of the products used and the differences in their application, blinding of the primary outcome assessor was not possible". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: the outcomes stated in the protocol wer reported. |
| Vested interest bias | High risk | Quote (author reply): "The study was sponsored by Aesculap AG (Tuttlingen , Germany). Clinical Monitoring and data management were contracted to Centrial GmbH (Tübingen, Germany). Statistical planning and analysis was performed by Dr.M.Koehler GmbH (Freiburg, Germany)". |
| Other bias | Low risk | Comment: no other bias |
Muratore 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy
Number randomised: 100
Postrandomisation dropouts: 0 (0%)
Revised sample size: 100
Average age: 65 years
Women: 38 (38%)
Number of cirrhotics: not stated
Number of major liver resections: 10 (10%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: clamp‐crush method (n = 50) Group 2: radiofrequency dissecting sealer (n = 50) Radiofrequency dissecting sealer: ligasure (Covidien) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with any adverse events, operative blood loss, proportion of people requiring blood transfusion, and length of hospital stay. | |
| Notes | Authors provided replies in March 2016. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to treatment at the ratio of 1:1 according to a computer‐generated randomization list by means of STATA software (version 10©; StataCorp LP, College Station, TX, USA)". |
| Allocation concealment (selection bias) | Low risk | Quote (author reply): "The details of the randomization series were unknown to any of the invesigators and were contained in sealed envelopes, each bearing outside the name of the hospital and a number. After the patient was deemed resectable in the operating room, the numbered envelope was opened at the central office and the card inside told if the patient was kellyclasia or ligasure group. This information was given to the surgeon performing the operation". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (author reply): "Patients and healthcare providers were blinded". Comment: further details were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (author reply): "Outcome assessors were not blinded". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There were no postrandomisation dropouts". |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Low risk | Quote (author reply): "The study was funded by the participating hospitals". |
| Other bias | Low risk | Comment: no other bias |
Ni 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: China
Number randomised: 120
Postrandomisation dropouts: 0 (0%)
Revised sample size: 120
Average age: 56 years
Women: 28 (23.3%)
Number of cirrhotics: 120 (100%)
Number of major liver resections: 15 (12.5%)
Number of right hepatectomies: 3 (2.5%)
Follow‐up (months): until discharge
Further details of methods of liver resection:
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: continuous portal triad clamping (n = 60) Group 2: continuous selective portal triad clamping (n = 60) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, proportion of people with major blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were randomly assigned to the Pringle manoeuvre group or to the hemi‐hepatic vascular inflow occlusion group by drawing sealed and opaque envelops from a box containing 120 prearranged envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Low risk | Quote: "This study was supported by the State Key Project on Infectious Diseases of China (2012ZX10002010, 2012ZX10002016), Nature Science Fund for Creative Research Groups, China (30921006,81221061,81201940) and Innovation Program of Shanghai Municipal Education Commission (09ZZ82)". |
| Other bias | Low risk | Comment: no other bias |
Noun 1996.
| Methods | Randomised clinical trial | |
| Participants | Country: France
Number randomised: 82
Postrandomisation dropouts: not stated
Revised sample size: 82
Average age: 51 years
Women: 39 (47.6%)
Number of cirrhotics: 7 (8.5%)
Number of major liver resections: 34 (41.5%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients undergoing elective liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = 38) Group 2: control (n = 44) Fibrin sealant: Biocol | |
| Outcomes | The outcomes reported were: proportion of people with serious adverse events, proportion of people with any adverse events, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), length of hospital stay, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: participants were excluded from complications because drains were not inserted or drainage data was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and severity of morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Ollinger 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: European multicentre trial
Number randomised: 50
Postrandomisation dropouts: not stated
Revised sample size: 50
Average age: 62 years
Women: 20 (40%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: 21 (42%)
Number of right hepatectomies: 15 (30%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: oxidised cellulose (n = 32) Group 2: fibrin sealant (n = 18) Oxidised cellulose: Veriset (Covidien) Fibrin sealant: Tachosil (Nycomed) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, quantity of blood transfused (red cell transfusion or whole blood), quantity of blood transfused (fresh frozen plasma), length of hospital stay, length of intensive therapy unit stay, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This study was a prospective, non‐inferiority, multicentre, twoarm, randomized, patient‐blinded study to compare a haemostatic patch (Veriset™) with a fibrinogenand thrombin‐coated collagen patch (TachoSil®; control) in the management of bleeding during hepatic surgery". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This study was a prospective, non‐inferiority, multicentre, twoarm, randomized, patient‐blinded study to compare a haemostatic patch (Veriset™) with a fibrinogenand thrombin‐coated collagen patch (TachoSil®; control) in the management of bleeding during hepatic surgery". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | High risk | Quote: "This study was sponsored by Covidien, Inc. ". |
| Other bias | Low risk | Comment: no other bias |
Park 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: South Korea
Number randomised: 53
Postrandomisation dropouts: 3 (5.7%)
Revised sample size: 50
Average age: 31 years
Women: 11 (22%)
Number of cirrhotics: 0 (0%)
Number of major liver resections: 50 (100%)
Number of right hepatectomies: 50 (100%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: donors underwent right hemihepatectomy and recipients received right hemiliver grafts Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: intermittent portal triad clamping (n = 25) Group 2: control (n = 25) Intermittent portal triad clamping: 15 min on and 5 min off | |
| Outcomes | The outcomes reported were: proportion of people with serious adverse events, operative blood loss, length of hospital stay, and operating time. | |
| Notes | Reasons for postrandomisation dropouts: graft‐to‐recipent body weight ratio < 0.9% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The donor‐recipient pairs were randomized (1:1) into 2 groups (IHIO and control groups) at the time of anesthesia induction for donors via the extraction of a black or white (but otherwise identical) stone from an unseen box". Comment: IHIO: intermittent hepatic inflow occlusion |
| Allocation concealment (selection bias) | Low risk | Quote: "The donor‐recipient pairs were randomized (1:1) into 2 groups (IHIO and control groups) at the time of anesthesia induction for donors via the extraction of a black or white (but otherwise identical) stone from an unseen box". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported adequately. |
| Vested interest bias | Low risk | Quote: "This study was funded by the Clinical Research Development Program (CRS1091811)". |
| Other bias | Low risk | Comment: no other bias |
Pietsch 2010.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany
Number randomised: 25
Postrandomisation dropouts: not stated
Revised sample size: 25
Average age: 56 years
Women: 11 (44%)
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients undergoing elective liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: continuous portal triad clamping (n = 14) Group 2: control (n = 11) | |
| Outcomes | The outcomes reported were: operative blood loss and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Porte 2012.
| Methods | Randomised clinical trial | |
| Participants | Country: Netherlands
Number randomised: 56
Postrandomisation dropouts: not stated
Revised sample size: 56
Average age: 61 years
Women: 20 (35.7%)
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients undergoing liver resection and having diffuse bleeding |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: fibrin sealant (n = 39) Group 2: gelatin (n = 17) Fibrin sealant: Fibrocaps (ProFibrix) | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Rahbari 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany
Number randomised: 130
Postrandomisation dropouts: 0 (0%)
Revised sample size: 130
Average age: 61 years
Women: 60 (46.2%)
Number of cirrhotics: 2 (1.5%)
Number of major liver resections: 73 (56.2%)
Number of right hepatectomies: 43 (33.1%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: clamp‐crush method (n = 65) Group 2: stapler resection (n = 65) Stapler: Autosuture EndoGIA stapler (Covidien) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, quantity of blood transfused (red cell transfusion or whole blood), quantity of blood transfused (fresh frozen plasma), length of hospital stay, length of intensive therapy unit stay, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A block randomisation list is generated by the Institute for Medical Biometrics and Informatics (IMBI) applying SAS (SAS™ Version 9.1., SAS Institute Inc., Cary, USA) ". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was carried out during surgery using consecutively numbered opaque and sealed envelopes, once the operating surgeon had confirmed resectability". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were blinded to the study intervention. Blinding of the staff in the operating room was not feasible". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Therefore, a third party blinded to the allocated treatment group assessed postoperative outcomes". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | High risk | Quote: "The trial was funded by the Department of General, Visceral and Transplant Surgery, University of Heidelberg, Germany. M.K., P.S., M.W.B and J.W. received speaker's honoraria from Covidien". |
| Other bias | Low risk | Comment: no other bias |
Rau 2001.
| Methods | Randomised clinical trial | |
| Participants | Country: Germany
Number randomised: 61
Postrandomisation dropouts: not stated
Revised sample size: 61
Average age: 62 years
Women: 25 (41%)
Number of cirrhotics: not stated
Number of major liver resections: 24 (39.3%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cavitron ultrasonic surgical aspirator (n = 30) Group 2: hydrojet (n = 31) Hydrojet: jet cutter | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, quantity of blood transfused (red cell transfusion or whole blood). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: severity of postoperative morbidity was not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Savlid 2013.
| Methods | Randomised clinical trial | |
| Participants | Country: Sweden
Number randomised: 100
Postrandomisation dropouts: 0 (0%)
Revised sample size: 100
Average age: 65 years
Women: 41 (41%)
Number of cirrhotics: 2 (2%)
Number of major liver resections: 71 (71%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cavitron ultrasonic surgical aspirator (n = 50) Group 2: stapler resection (n = 50) Stapler: Endostapler (Covidien) | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, number of adverse events, operative blood loss, quantity of blood transfused (red cell transfusion or whole blood), length of hospital stay, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was completed by the use of opaque, sealed envelopes with computer‐generated random numbers in blocks of 10 (5:5)". |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was completed by the use of opaque, sealed envelopes with computer‐generated random numbers in blocks of 10 (5:5)". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | High risk | Quote: "This study was supported by an unconditional research grant by Covidien Sweden AB ". |
| Other bias | Low risk | Comment: no other bias |
Shao 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: Asian multicentre trial
Number randomised: 235
Postrandomisation dropouts: 14 (6%)
Revised sample size: 221
Average age: 52 years
Women: 38 (17.2%)
Number of cirrhotics: 231 (104.5%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: cirrhotic patients (> 21 years of age) scheduled for partial hepatectomy as a result of liver cancer or benign tumors (> 5 cm, involving ≥ 2 segments or located centrally) Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: control (n = 76) Group 2: recombinant factor via (n = 155) Recombinant factor VIIa: brand not stated Dose: 50 or 100 mcg/kg before skin incision over 2 min and repeated every 2 h until a maximum of 4 doses Control: placebo | |
| Outcomes | The outcomes reported were: proportion of people with serious adverse events, number of serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), quantity of blood transfused (platelets), and quantity of blood transfused (fresh frozen plasma). | |
| Notes | Reasons for postrandomisation dropouts: did not receive intervention (n = 11); lost‐to follow‐up (n = 2); withdrew consent (n = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A multicenter, randomized, double‐blind, placebo‐controlled trial". Comment: further information on blinding was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "a multicenter, randomized, double‐blind, placebo‐controlled trial". Comment: further information on blinding was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality was not reported. |
| Vested interest bias | High risk | Comment: one of the co‐authors belonged to a pharmaceutical industry. |
| Other bias | Low risk | Comment: no other bias |
Shimada 1994.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan
Number randomised: 24
Postrandomisation dropouts: not stated
Revised sample size: 24
Average age: 63 years
Women: 4 (16.7%)
Number of cirrhotics: 13 (54.2%)
Number of major liver resections: 10 (41.7%)
Number of right hepatectomies: 9 (37.5%)
Follow‐up (months): until discharge
Further details of methods of liver resection 1. Vascular occlusion: not stated 2. Parenchymal transection: not stated 3. Fibrin glue: not stated 4. Pharmacological methods: factor being randomised 5. Cardiopulmonary methods: not stated 6. Autologous transfusion: not stated Inclusion criteria: patients with hepatocellular carcinoma undergoing liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: antithrombin iii (n = 13) Group 2: control (n = 11) Antithrombin concentrate: 1500 IU IV over 30 min: immediately before the operation, just before hepatic division, and immediately after operation | |
| Outcomes | The outcomes reported were: proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, quantity of blood transfused (red cell transfusion or whole blood), and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and severity of morbidity were nor reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Si‐Yuan 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: China
Number randomised: 160
Postrandomisation dropouts: 0 (0%)
Revised sample size: 160
Average age: 49 years
Women: 36 (22.5%)
Number of cirrhotics: 98 (61.3%)
Number of major liver resections: 112 (70%)
Number of right hepatectomies: 53 (33.1%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: continuous portal triad clamping (n = 80) Group 2: continuous selective hepatic vascular exclusion (n = 80) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, operative blood loss, proportion of people with major blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), length of hospital stay, length of intensive therapy unit stay, operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "All eligible patients were randomly assigned to the Pringle manoeuvre and selective hepatic vascular occlusion group by drawing sealed, consecutively numbered, and opaque envelopes after abdominal exploration had confirmed resectability". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Smyrniotis 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: Greece
Number randomised: 82
Postrandomisation dropouts: 0 (0%)
Revised sample size: 82
Average age: 64 years
Women: 17 (20.7%)
Number of cirrhotics: 12 (14.6%)
Number of major liver resections: 60 (73.2%)
Number of right hepatectomies: 31 (37.8%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients who underwent liver resection for benign or malignant tumours |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: sharp transection (n = 41) Group 2: clamp‐crush method (n = 41) Sharp transection: using scalpel | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), length of hospital stay, length of intensive therapy unit stay, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Takayama 2001.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan
Number randomised: 132
Postrandomisation dropouts: 0 (0%)
Revised sample size: 132
Average age: 62 years
Women: not stated
Number of cirrhotics: 45 (34.1%)
Number of major liver resections: 43 (32.6%)
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: cavitron ultrasonic surgical aspirator (n = 66) Group 2: clamp‐crush method (n = 66) | |
| Outcomes | The outcomes reported were: short‐term mortality, proportion of people with serious adverse events, number of serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, length of hospital stay. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Unclear risk | Quote: "This work was supported in part by a grant‐in‐aid for cancer research from the Ministry of Health and Welfare, Tokyo, Japan". Comment: only part of the funding information was available. |
| Other bias | Low risk | Comment: no other bias |
Wang 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: China
Number randomised: 52
Postrandomisation dropouts: 2 (3.8%)
Revised sample size: 50
Average age: 46 years
Women: 10 (20%)
Number of cirrhotics: 29 (58%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: patients with hepatocellular carcinoma undergoing liver resection |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: low central venous pressure (n = 25) Group 2: control (n = 25) Low central venous pressure: by limiting fluid, nitroglycerine, and furosemide | |
| Outcomes | The outcomes reported were: proportion of people with any adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), quantity of blood transfused (fresh frozen plasma), length of hospital stay, and operating time. | |
| Notes | Reasons for postrandomisation dropouts: hepatectomy was not performed because of cardiac arrest or because it was not possible to demarcate the tumour | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "By the sealed envelope method, the patients were blindly randomized into Lcentral venous pressure group (n = 25) and control group (n = 27) at the beginning of the operation". Comment: further details of sealed envelope method were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and severity of morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
Wong 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: Hong Kong, China
Number randomised: 60
Postrandomisation dropouts: 0 (0%)
Revised sample size: 60
Average age: 51 years
Women: 23 (38.3%)
Number of cirrhotics: 23 (38.3%)
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: adult patients scheduled for hepatectomy Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: desmopressin (n = 30) Group 2: control (n = 30) Desmopressin: 30 mcg/kg shortly after induction Control: placebo | |
| Outcomes | The outcomes reported were: operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (fresh frozen plasma), and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patient randomization was by drawing a sealed envelope specifying a prescription for either desmopressin or placebo, which was then prepared by an independent investigator and blinded to the patient, attending anesthesiologist and surgeon". Comment: further details of sealed envelope method were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patient randomization was by drawing a sealed envelope specifying a prescription for either desmopressin or placebo, which was then prepared by an independent investigator and blinded to the patient, attending anesthesiologist and surgeon". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patient randomization was by drawing a sealed envelope specifying a prescription for either desmopressin or placebo, which was then prepared by an independent investigator and blinded to the patient, attending anesthesiologist and surgeon". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 1 patient who had heavy bleeding in control group was excluded for blood loss. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Low risk | Quote: "This study was supported by a Hong Kong University CRCG grant (10202115/20013/20100/323/01)". |
| Other bias | Low risk | Comment: no other bias |
Wu 2002.
| Methods | Randomised clinical trial | |
| Participants | Country: Taiwan
Number randomised: 58
Postrandomisation dropouts: 0 (0%)
Revised sample size: 58
Average age: 55 years
Women: 10 (17.2%)
Number of cirrhotics: 58 (100%)
Number of major liver resections: 20 (34.5%)
Number of right hepatectomies: 0 (0%)
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria: cirrhotic patients who had no previous biliary operations and no preoperative therapies and whose main tumour was located at the central portion of the liver (defined as Couinaud segments 4, 5, and 8) without having directly invaded the hepatic hilar plate Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: intermittent portal triad clamping (n = 28) Group 2: intermittent selective portal triad clamping (n = 30) Intermittent portal triad clamping: 15 min on and 5 min off Intermittent selective portal triad clamping: 30 min on and 5 min off | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, quantity of blood transfused (red cell transfusion or whole blood), length of hospital stay, and operating time. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "If the tumour condition and procedures fulfilled the aforementioned criteria, randomization was performed by opening a sealed envelope after the abdomen was explored". Comment: further details of sealed envelope method were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity were reported. |
| Vested interest bias | Low risk | Quote: "This study was supported in part by grant NSC 902314‐075A‐018 from the National Science Council, Taipei, Taiwan". |
| Other bias | Low risk | Comment: no other bias |
Wu 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: Taiwan
Number randomised: 217
Postrandomisation dropouts: 3 (1.4%)
Revised sample size: 214
Average age: 60 years
Women: 57 (26.6%)
Number of cirrhotics: 110 (51.4%)
Number of major liver resections: 38 (17.8%)
Number of right hepatectomies: 18 (8.4%)
Follow‐up (months): until discharge
Further details of methods of liver resection 1. Vascular occlusion: varied 2. Parenchymal transection: clamp‐crush method 3. Fibrin glue: not stated 4. Pharmacological methods: factor being randomised 5. Cardiopulmonary methods: not stated 6. Autologous transfusion: not stated Inclusion criteria: patients undergoing liver resections |
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: tranexamic acid (n = 108) Group 2: control (n = 106) Tranexamic acid: 500 mg just before the surgery followed by 250 4 times a day for 3 d | |
| Outcomes | The outcomes reported were: short‐term mortality, number of serious adverse events, proportion of people with any adverse events, number of adverse events, operative blood loss, proportion of people requiring blood transfusion, length of hospital stay, and operating time. | |
| Notes | Reasons for postrandomisation dropouts: liver resection not completed because of presence of more extensive disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization was double‐blinded in a sealed envelope". Comment: further details of sealed envelope method were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither surgeons nor medical staffs knew whether patients were enrolled in group A or group B ". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither surgeons nor medical staffs knew whether patients were enrolled in group A or group B ". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: although there were 3 postrandomisation dropouts, this was because liver resection could not be carried out. |
| Selective reporting (reporting bias) | High risk | Comment: severity of morbidity was not reported. |
| Vested interest bias | Unclear risk | Quote: "Supported in part by a grant from National Science Council, Taiwan (No. 92‐2314‐B‐075A‐006) ". Comment: only part of the funding information was available. |
| Other bias | Low risk | Comment: no other bias |
Yao 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: China
Number randomised: 30
Postrandomisation dropouts: not stated
Revised sample size: 30
Average age: not stated
Women: 14 (46.7%)
Number of cirrhotics: not stated
Number of major liver resections: not stated
Number of right hepatectomies: not stated
Follow‐up (months): until discharge
Further details of methods of liver resection
Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 groups. Group 1: acute normovolemic haemodilution (n = 10) Group 2: acute normovolemic haemodilution with hypotension (n = 10) Group 3: control (n = 10) Acute normovolemic haemodilution: withdrawal of blood and replacement with fluids to maintain a target haematocrit of 30% Acute normovolemic haemodilution With controlled hypotension: in addition to acute normovolemic haemodilution, sodium nitroprusside was used; target blood pressure not known | |
| Outcomes | The outcomes reported were: operative blood lossand quantity of blood transfused (red cell transfusion or whole blood). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and morbidity were not reported. |
| Vested interest bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other bias |
ABO: blood group incompatible; ASA: American Society of Anesthesiologists; HCC: hepatocellular carcinoma; INR: international normalised ratio; IU: international unit; IVC: infrahepatic inferior vena cava; kIU: kilo international units.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arru 2007 | Not a randomised clinical trial |
| Azoulay 2005 | Not a randomised clinical trial |
| Bellolio 2012 | Not a randomised clinical trial |
| Beppu 2012 | Not a randomised clinical trial |
| Broek 2011 | Comparison of 2 methods of intermittent Pringle manoeuvre of different duration |
| Chapman 2007 | Variations of thrombin |
| Correa‐Gallego 2015 | Not an intervention targeted at decreasing blood loss |
| Dello 2012 | Comparison of 2 different methods of intermittent portal triad clamping |
| Dominioni 2014 | Not a randomised clinical trial |
| El‐Moghazy 2009 | Comparison of minor variations of same transection method |
| Esaki 2006 | Comparison of 2 different methods of intermittent portal triad clamping |
| Feldheiser 2015 | Not an intervention targeted at decreasing blood loss |
| Figueras 2003 | Not a comparison with main focus on blood loss |
| Frankel 2013 | Different methods of selection for acute normovolemic haemodilution |
| Gonzalez 2009 | Comment on Figueras 2007 |
| Gotohda 2015 | Different methods of treatment of raw surface were allowed in control group |
| Grobmyer 2009 | The intervention was started 1 day after operation and used only in selected patients undergoing surgery. |
| Hamady 2015 | Comment on an excluded trial (Rahbari 2011) |
| Hanyong 2015 | Vascular occlusion was used in only method of parenchymal transection |
| Harimoto 2011 | Different methods of suturing on the raw surface of the liver |
| Hashimoto 2007 | Different methods of autologous blood donation (pre‐operative or pre‐operative + intra‐operative) |
| Kaibori 2013 | Variations in cavitron ultrasonic surgical aspirator technique |
| Kim 2007 | Comparison of 2 different methods of intermittent portal triad clamping |
| Kim 2008 | Not a randomised clinical trial |
| Le Treut 1995 | Not a randomised clinical trial |
| Levit 2012 | Comparison of interventions that were not of interest for this review |
| Li 2013 | In the control group, 2 different forms of vascular occlusion were used |
| Li 2015 | Not a randomised clinical trial |
| Lu 2014 | Low central venous pressure was used in fast‐track group, but this was combined with a number of other measures in the intervention group only. |
| Man 2002 | Not a randomised clinical trial |
| Nagano 2009 | Not a randomised clinical trial |
| Narita 2012 | Not a randomised clinical trial |
| NCT01651182 | Not a randomised clinical trial |
| Obiekwe 2014 | Quasi‐randomised study (alternate assignment) |
| Palibrk 2012 | Not a randomised clinical trial |
| Petras 2009 | Comment on Richter 2009 |
| Petrowsky 2006 | Ischaemic preconditioning was applied only in 1 group |
| Rahbari 2011 | Different methods of achieving low central venous pressure |
| Rau 1995 | Started as a randomised clinical trial but did not continue because of problems with nozzles of jet cutter. So, the report consisted of non‐randomised patients. |
| Richter 2009 | In this randomised clinical trial, if the patients did not undergo liver resection, the envelopes were resealed and returned to the pool of sealed envelopes. The allocation concealment is not adequate in this trial. |
| Ryu 2010 | Comparison of different methods of low central venous pressure |
| Saiura 2006 | Comparison of variations in clamp‐crush method |
| Saiura 2014 | Comparison of variations in clamp‐crush method |
| Schilling 2009 | Comment on Richter 2009 |
| Schwartz 2004 | In the control group a number of topical haemostatic agents were used. |
| Shu 2014 | In this study, patients were divided into 4 groups ‐ people who received blood transfusion and ulinastatin, people who received blood transfusion but not ulinastatin, people who received ulinastatin but not blood transfusion, and people who did not receive blood transfusion or ulinastatin. Although the authors randomised patients to ulinastatin or control, they ensured that the number of patients in each group was the same, i.e. the number of people in ulinastatin group who received blood transfusion was 50% and the number of people in control group who received blood transfusion was 50%. This would have seriously impaired the randomisation to the extent that we feel that this is not a randomised clinical at all. |
| Si‐Yuan 2011 | Used continuous and intermittent portal triad clamping depending upon transection time with vascular occlusion being the factor randomised |
| Smyrniotis 2002 | Quasi‐randomised (random sequence generated by hospital number) |
| Smyrniotis 2003a | Quasi‐randomised (random sequence generated by hospital number) |
| Smyrniotis 2003b | Quasi‐randomised (random sequence generated by hospital number) |
| Smyrniotis 2006 | Ischaemic preconditioning was applied to only one of the groups |
| Standl 1998 | Variations in autologous blood donation |
| Strobel 2012 | Commentary on Lee 2012 |
| Strobel 2014 | Commentary on Rahbari 2014 |
| Takatsuki 2015 | Not a randomised clinical trial |
| Torzilli 2008 | Variations in clamp‐crush method |
| Vlad 2014 | Not a randomised clinical trial |
| Wang 2010 | Not a randomised clinical trial |
| Wang 2011 | Not a randomised clinical trial |
| Yang 2012 | Not a randomised clinical trial |
| Yang 2013 | Variations in selective hepatic vascular exclusion |
| Yin 2003 | Not a randomised clinical trial |
| Zhang 2014 | Variations in portal triad clamping |
| Zhu 2012 | Different methods of low central venous pressure |
Characteristics of studies awaiting assessment [ordered by study ID]
Bochicchio 2015.
| Methods | Randomised clinical trial |
| Participants | Patients undergoing different types of surgical procedures |
| Interventions | Fibrin sealant versus gelatin |
| Outcomes | Adverse events |
| Notes | Attempts were made to contact the authors in September 2016. |
Chapman 2006.
| Methods | Randomised clinical trial |
| Participants | Patients undergoing different types of surgical procedures |
| Interventions | Recombinant thrombin versus placebo |
| Outcomes | Adverse events |
| Notes | Attempts were made to contact the authors in September 2016. |
Wright 2015.
| Methods | Randomised clinical trial |
| Participants | Adult patients undergoing major oncologic surgery |
| Interventions | Pre‐operative tranexamic acid |
| Outcomes | Proportion requiring transfusion |
| Notes | We were unable to obtain further contact details of the author from the institution. |
Characteristics of ongoing studies [ordered by study ID]
Chen 2015.
| Trial name or title | Usefulness of BiClamp forceps for liver resection: a randomized clinical trial |
| Methods | Randomised clinical trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | BiClamp forceps versus clamp‐crush methods for liver parenchymal transection |
| Outcomes | Primary outcome: total intraoperative blood loss Secondary outcomes
|
| Starting date | 1 October 2014 |
| Contact information | Jiang‐ming Chen (email: chenjm10@126.com) |
| Notes | NCT02197481 |
Schmidt 2008.
| Trial name or title | Influence of two different resection techniques (conventional liver resection versus anterior approach) of liver metastases from colorectal cancer on hematogenous tumor cell dissemination ‐ prospective randomized multicenter trial |
| Methods | Randomised clinical trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Anterior approach versus conventional approach |
| Outcomes |
|
| Starting date | Not stated |
| Contact information | J Weitz (email: jeurgen.weitz@med.uni‐heidelberg.ed |
| Notes | ISN45066244 |
Differences between protocol and review
We calculated the odds ratios (OR) rather than the risk ratios (RR) since it is easier to model the OR for network meta‐analysis. Although ORs are more difficult to interpret than RRs, we overcame this problem by presenting the results as illustrative comparative risks for mortality, serious adverse events, and proportion of people requiring blood transfusion.
We calculated the mean difference (MD) and 95% credible interval (CrI) for quantity of blood transfused rather than the standardised mean difference (SMD) and 95% CrI. We expected some authors to report quantity of blood transfused in litres transfused and others to report this as number of units transfused. However, all the trials included in this review reported the quantity of blood transfused in units enabling us to calculate the MD and 95% CrI, which is easier to interpret than SMD.
We planned to calculate the rate ratio with 95% CrI. However, the trials reported the proportion of people with serious adverse events. So we calculated the OR with 95% CrI rather than the rate ratio with 95% CrI.
We used the residual deviance and Deviance Information Criteria (DIC) for assessing between‐study heterogeneity as per the guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) Technical Support Documents (Dias 2012b; Dias 2013a).
We reported the network meta‐analysis on all the outcomes although we planned to perform the network analysis for the primary outcomes and one secondary outcome on blood transfusion requirements. This was to obtain and report the maximum information from the available data.
We planned to report the random‐effects model for network meta‐analysis. However, we decided to report the fixed‐effects model or random‐effects model based on residual deviance and DIC statistics as recommended by the NICE DSU Technical Support Documents (Dias 2013a).
We did not fit the inconsistency model that uses the design‐by‐treatment approach proposed by Higgins and White (Higgins 2012; White 2012), since we used the assessment of inconsistency using the approach suggested by NICE DSU.
We did not first calculate all pair‐wise meta‐analysis estimates and then compare them with indirect comparison estimates (Bucher 1997) for each loop, as the method that we used is an extension of the Bucher et al. (Bucher 1997) method to assess inconsistency (Dias 2012c; Dias 2013e).
We did not perform the direct comparison. This was because of the exclusion of many trials that might have been suitable for direct comparison but were unsuitable for the overview.
Differences between first version and second version (current version)
We included all the interventions aimed at limiting blood loss and blood transfusion requirements. This was because of requests for this information by stakeholders, which resulted in a directly commissioned report that included all interventions aimed at decreasing blood loss and blood transfusion requirements.
We included the outcome 'any adverse event' in addition to the serious adverse events since it was not possible to assess the severity of the outcomes in many trials, for example, bile leak could be a mild adverse event or a serious adverse event depending upon whether an additional intervention was needed to resolve it.
Unlike in the previous version, where we considered a combination of one method from each of Table 10, Table 11, and Table 12 as a treatment strategy, in this review, we considered each of these interventions (different methods of cardiopulmonary interventions, parenchymal transection methods, methods of dealing with raw surface, vascular occlusion methods, and pharmacological interventions) as separate networks. This approach was in response to the lack of information on the details of co‐interventions in the trials and the design of the trials, which limited the number of trials included in the previous analysis. In many of the trials, the surgeons involved in the trial were allowed to choose their method of liver resection apart from the factor being randomised. This is based on an assumption that the factors are independent of each other, that is, there is no interaction between the factors, or the choice of one factor is not dependent on the choice of another factor. There is no evidence to support or refute this assumption. However, if we planned to include only trials in which all the intervention variables were adequately reported and none were left to the choice of the surgeons, we would not even have been able to include as many trials as we did in the previous version, as we have now included all the interventions aimed at decreasing blood loss and blood transfusion requirements during liver resection.
We performed a network meta‐analysis only when it was possible to compare the direct and indirect estimates because one cannot assess consistency between the direct and indirect estimates unless both are available.
We presented the direct estimates as those performed using Bayesian and frequentist analyses. For frequentist analysis, we presented the results of the model that was used for Bayesian analysis (which was determined by the model fit).
We planned to perform subgroup analysis using WinBUGS rather than RevMan.
We did not perform sensitivity analysis considering some adverse events as serious and mild, since we included 'any adverse events' as an outcome. This captured the adverse events for which we were unable to assess the severity.
We modified the 'Summary of findings' table from the original format because of the presence of many comparisons and many outcomes. We presented only the comparisons in which there was evidence of differences with the illustrative examples. For other comparisons, we simply mentioned that there was no evidence of differences. This is to ensure that the most important information is available in the 'Summary of findings' table.
We have provided links in the 'Summary of findings' table to tables with a more traditional 'Summary of findings' format.
In addition to this 'Summary of findings' table, we also provided the 'Summary of findings' table for network meta‐analysis in a graphical format (in the form of forest plots along with the quality of evidence), in which we used the methodology of grading the quality of evidence in network meta‐analysis suggested by the GRADE Working group (Puhan 2014). The first step is to estimate the evidence from direct and indirect effect estimates. Further steps included rating the quality of evidence from direct and indirect effect estimates, presenting the estimate combined from the direct estimate and indirect estimate, and rating the quality of the network meta‐analysis effect estimates (Puhan 2014). Although codes are available for node splitting, they resulted in numerical errors because of the data,so we calculated the direct estimates (including only the trials that compared the specific intervention and control) and indirect estimates (after removing the trials that compared the specific intervention and control).
We provided the minimal clinically important differences that we used or planned to use in an explicit manner. We considered a 20% relative risk reduction as minimal clinically important differences for binary outcomes and count outcomes. For continuous outcomes, we used or planned to use the following minimal clinically important differences: a standardised mean difference of 0.5 for health‐related quality of life, a mean difference of one unit for blood transfusion quantity, a mean difference of 500 mL for blood loss, a mean difference of one day of hospital stay and time‐to‐return to activity, and a mean difference of 15 min for operating time.
Contributions of authors
Elisabetta Moggia identified the studies, extracted the data, and completed sections of the review. Benjamin Rousse re‐analysed the network meta‐analysis and revised the errors in the analysis. Constantinos Simillis identified the studies, extracted the data, performed part of the analysis, and drafted the previous version of review (Simillis 2014). Tianjing Li critically reviewed the content, particularly in relation to the network meta‐analysis. Brian R Davidson critically commented on the review. Kurinchi S Gurusamy performed the analysis and revised the review. All review authors agreed on this review version before publication.
Sources of support
Internal sources
University College London, UK.
External sources
-
National Institute for Health Research, UK.
National Institute for Health Research, the health research wing of the UK Government Department of Health funds K Gurusamy to complete this review.
Award number: Directly commissioned Incentive Award 15/65/01
Declarations of interest
Review authors perform research related to decreasing blood loss in liver resection. This includes clinical studies. No other conflicts of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Arita 2005 {published data only}
- Arita J, Hasegawa K, Kokudo N, Sano K, Sugawara Y, Makuuchi M. Randomized clinical trial of the effect of a saline‐linked radiofrequency coagulator on blood loss during hepatic resection. British Journal of Surgery 2005;92(8):954‐9. [DOI] [PubMed] [Google Scholar]
Bektas 2014 {published data only}
- Bektas H, Nadalin S, Schmidt J, Szabo I, Ploder B, Sharkhawy M. Hemostatic efficacy of latest generation fibrin sealant after hepatic resection; a randomized controlled clinical study. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2013;15:6‐7. [DOI] [PubMed] [Google Scholar]
- Bektas H, Nadalin S, Szabo I, Ploder B, Sharkhawy M, Schmidt J. Hemostatic efficacy of latest‐generation fibrin sealant after hepatic resection: a randomized controlled clinical study. Langenbecks Archives of Surgery 2014;399(7):837‐47. [DOI] [PubMed] [Google Scholar]
Belghiti 1996 {published data only}
- Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Annals of Surgery 1996;224(2):155‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belghiti 1999 {published data only}
- Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Annals of Surgery 1999;229(3):369‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capussotti 2003 {published data only}
- Capussotti L, Nuzzo G, Polastri R, Giuliante F, Muratore A, Giovannini I. Continuous versus intermittent portal triad clamping during hepatectomy in cirrhosis. Results of a prospective, randomized clinical trial. Hepato‐Gastroenterology 2003;50(52):1073‐7. [PubMed] [Google Scholar]
Capussotti 2006 {published data only}
- Capussotti L, Muratore A, Ferrero A, Massucco P, Ribero D, Polastri R. Randomized clinical trial of liver resection with and without hepatic pedicle clamping. British Journal of Surgery 2006;93(6):685‐9. [DOI] [PubMed] [Google Scholar]
- Ferrero A, Russolillo N, Vigano L, Lo Tesoriere R, Muratore A, Capussotti L. Does Pringle maneuver affect survival in patients with colorectal liver metastases?. World Journal of Surgery 2010;34(10):2418‐25. [DOI] [PubMed] [Google Scholar]
Capussotti 2012 {published data only}
- Capussotti L, Ferrero A, Russolillo N, Langella S, Lo Tesoriere R, Vigano L. Routine anterior approach during right hepatectomy: results of a prospective randomised controlled trial. Journal of Gastrointestinal Surgery 2012;16(7):1324‐32. [DOI] [PubMed] [Google Scholar]
Chapman 2000 {published data only}
- Chapman WC, Clavien PA, Fung J, Khanna A, Bonham A. Effective control of hepatic bleeding with a novel collagen‐based composite combined with autologous plasma ‐ results of a randomized controlled trial. Archives of Surgery 2000;135(10):1200‐4. [DOI] [PubMed] [Google Scholar]
- Chapman WC, Wren SM, Lebovic GS, Malawer M, Sherman R, Block JE. Effective management of bleeding during tumor resection with a collagen‐based hemostatic agent. American Surgeon 2002;68(9):802‐7. [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen XP, Zhang ZW, Zhang BX, Chen YF, Huang ZY, Zhang WG, et al. Modified technique of hepatic vascular exclusion: effect on blood loss during complex mesohepatectomy in hepatocellular carcinoma patients with cirrhosis. Langenbeck's Archives of Surgery/Deutsche Gesellschaft fur Chirurgie 2006;391(3):209‐15. [DOI] [PubMed] [Google Scholar]
Choi 2007 {published data only}
- Choi SH, Ban SY, Jun NH, Jun DB, Nam SH, Kil HK. Central venous pressure and its effect on blood loss during hepatic lobectomy. Korean Journal of Anesthesiology 2007;52(6):663‐8. [Google Scholar]
Chouker 2004 {published data only}
- Chouker A, Schachtner T, Schauer R, Dugas M, Lohe F, Martignoni A, et al. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. British Journal of Anaesthesia 2004;93(2):204‐11. [DOI] [PubMed] [Google Scholar]
Clavien 1996 {published data only}
- Clavien PA, Camargo CA, Gorczynski R, Washington MK, Levy GA, Langer B, et al. Acute reactant cytokines and neutrophil adhesion after warm ischemia in cirrhotic and noncirrhotic human livers. Hepatology 1996;23(6):1456‐63. [DOI] [PubMed] [Google Scholar]
Dayangac 2010 {published data only}
- Dayangac M, Taner BC, Balci D, Duran C, Akin B, Killi R, et al. Prospective randomized trial of intermittent portal triad clamping vs. no clamping in donor right hepatectomy. American Journal of Transplantation 2010;10:102‐3. [Google Scholar]
De Boer 2012 {published data only}
- Boer MT, Klaase JM, Verhoef C, Dam RM, Gulik TM, Molenaar IQ, et al. Fibrin sealant for prevention of resection surface‐related complications after liver resection. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2012;14:109. [Google Scholar]
- Boer MT, Klaase JM, Verhoef C, Dam RM, Gulik TM, Molenaar IQ, et al. Fibrin sealant for prevention of resection surface‐related complications after liver resection: a randomized controlled trial. Annals of Surgery 2012;256(2):229‐34. [DOI] [PubMed] [Google Scholar]
Doklestic 2012 {published data only}
- Doklestic K, Karamarkovic A, Stefanovic B, Milic N, Gregoric P, Djukic V, et al. The efficacy of three transection techniques of liver resection: a randomized clinical trial. Hepato‐Gastroenterology 2012;59(117):1501‐6. [DOI] [PubMed] [Google Scholar]
El‐Kharboutly 2004 {published data only}
- El‐Kharboutly WS, El‐Wahab MA. The role of adoption of low central venous pressure in hepatic resection with Pringle manoeuvre in reducing blood loss and improving operative outcome. Egyptian Journal of Anaesthesia 2004;20(4):369‐76. [Google Scholar]
Figueras 2005 {published data only}
- Figueras J, Llado L, Ruiz D, Ramos E, Busquets J, Rafecas A, et al. Complete versus selective portal triad clamping for minor liver resections: a prospective randomized trial. Annals of Surgery 2005;241(4):582‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Figueras 2007 {published data only}
- Figueras J, Llado L, Miro M, Ramos E, Torras J, Fabregat J, et al. Application of fibrin glue sealant after hepatectomy does not seem justified: results of a randomized study in 300 patients. Annals of Surgery 2007;245(4):536‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer 2011 {published data only}
- Fischer L, Seiler CM, Broelsch CE, Hemptinne B, Klempnauer J, Mischinger HJ, et al. Hemostatic efficacy of TachoSil in liver resection compared with argon beam coagulator treatment: an open, randomized, prospective, multicenter, parallel‐group trial. Surgery 2011;149(1):48‐55. [DOI] [PubMed] [Google Scholar]
Franceschi 2006 {published data only}
- Franceschi D, Madsen T, Weatherford C, Kumar V, Chapman J. Clinical evaluation of fibrin sealant produced by CryoSeal® FS system in patients undergoing liver resection: a multicenter randomized clinical trial. Vox Sanguinis 2006;91(Suppl 3):21. [Google Scholar]
Frilling 2005 {published data only}
- Frilling A, Stavrou GA, Mischinger HJ, Hemptinne B, Rokkjaer M, Klempnauer J, et al. Effectiveness of a new carrier‐bound fibrin sealant versus argon beamer as haemostatic agent during liver resection: a randomised prospective trial. Langenbecks Archives of Surgery 2005;390(2):114‐20. [DOI] [PubMed] [Google Scholar]
Genyk 2014 {published data only}
- Genyk Y, Kato T, Pomposelli JJ, Lophaven KW, Chapman WC. TachoSil® versus Surgicel® original for the secondary treatment of local bleeding in adult patients undergoing hepatic resection. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2014;16(Suppl S1):27. [Google Scholar]
Gugenheim 2011 {published data only}
- Gugenheim J, Bredt LC, Iannelli A. A randomized controlled trial comparing fibrin glue and PlasmaJet on the raw surface of the liver after hepatic resection. Hepato‐Gastroenterology 2011;58(107‐8):922‐5. [PubMed] [Google Scholar]
Guo 2013 {published data only}
- Guo JR, Jin XJ, Yu J, Xu F, Zhang YW, Shen HC, et al. Acute normovolemic hemodilution effects on perioperative coagulation in elderly patients undergoing hepatic carcinectomy. Asian Pacific Journal of Cancer Prevention 2013;14(8):4529‐32. [DOI] [PubMed] [Google Scholar]
- Guo JR, Yu J, Jin XJ, Du JM, Guo W, Yuan XH. Effects of acute normovolemic hemodilution on perioperative coagulation and fibrinolysis in elderly patients undergoing hepatic carcinectomy. Chinese Academy of Medical Sciences 2010;25(3):146‐50. [DOI] [PubMed] [Google Scholar]
Guo 2014 {published data only}
- Guo JR, Shen HC, Liu Y, Xu F, Zhang YW, Zhang JP, et al. Effect of acute normovolemic hemodilution combined with controlled low central venous pressure on cerebral oxygen metabolism of patients with hepalobectomy. Hepato‐Gastroenterology 2014;61(136):2321‐5. [PubMed] [Google Scholar]
Hasegawa 2002 {published data only}
- Hasegawa K, Takayama T, Orii R, Sano K, Sugawara Y, Imamura H, et al. Effect of hypoventilation on bleeding during hepatic resection ‐ a randomized controlled trial. Archives of Surgery 2002;137(3):311‐5. [DOI] [PubMed] [Google Scholar]
Ikeda 2009 {published data only}
- Ikeda M, Hasegawa K, Sano K, Imamura H, Beck Y, Sugawara Y, et al. The vessel sealing system (LigaSure) in hepatic resection: a randomized controlled trial. Annals of Surgery 2009;250(2):199‐203. [DOI] [PubMed] [Google Scholar]
Jarnagin 2008 {published data only}
- Camilo CG, Gonen M, Fischer M, Grant F, Kemeny NE, Kingham TP, et al. Effect of perioperative complications on recurrence and survival after resection of hepatic colorectal metastases: analysis of data from a randomized controlled trial. Journal of Clinical Oncology 2012;30(15 Suppl):3620. [Google Scholar]
- Jarnagin WR, Gonen M, Maithel SK, Fong Y, D'Angelica MI, Dematteo RP, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Annals of Surgery 2008;248(3):360‐9. [DOI] [PubMed] [Google Scholar]
Kajikawa 1994 {published data only}
- Kajikawa M, Nonami T, Kurokawa T, Hashimoto S, Harada A, Nakao A, et al. Autologous blood transfusion for hepatectomy in patients with cirrhosis and hepatocellular carcinoma: use of recombinant human erythropoietin. Surgery 1994;115(6):727‐34. [PubMed] [Google Scholar]
Kakaei 2013 {published data only}
- Kakaei F, Seyyed Sadeghi MS, Sanei B, Hashemzadeh S, Habibzadeh A. A randomized clinical trial comparing the effect of different haemostatic agents for haemostasis of the liver after hepatic resection. HPB Surgery 2013;2013:587608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kato 2008 {published data only}
- Kato M, Kubota K, Kita J, Shimoda M, Rokkaku K, Sawada T. Effect of infra‐hepatic inferior vena cava clamping on bleeding during hepatic dissection: a prospective, randomized, controlled study. World Journal of Surgery 2008;32(6):1082‐7. [DOI] [PubMed] [Google Scholar]
Koea 2013 {published data only}
- Koea JB, Batiller J, Patel B, Shen J, Hammond J, Hart J, et al. A phase III, randomized, controlled, superiority trial evaluating the fibrin pad versus standard of care in controlling parenchymal bleeding during elective hepatic surgery. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2013;15(1):61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohno 1992 {published data only}
- Kohno H, Nagasue N, Chang YC, Taniura H, Yamanoi A, Nakamura T. Comparison of topical hemostatic agents in elective hepatic resection: a clinical prospective randomized trial [Discussion 70]. World Journal of Surgery 1992;16(5):966‐9. [DOI] [PubMed] [Google Scholar]
Koo 2005 {published data only}
- Koo BN, Kil HK, Choi JS, Kim JY, Chun DH, Hong YW. Hepatic resection by the cavitron ultrasonic surgical aspirator increases the incidence and severity of venous air embolism. Anesthesia and Analgesia 2005;101(4):966‐70, table of contents. [DOI] [PubMed] [Google Scholar]
Kostopanagiotou 2007 {published data only}
- Kostopanagiotou G, Pandazi A, Matsota P, Arkadopoulos N, Dalamanga N, Politou M, et al. Effect of packed red blood cells transfusion on plasma fibronectin during liver resection. Transfusion Medicine 2007;17(2):115‐8. [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Cheung YS, Lee KF, Wong J, Wong SWJ, Chong CNC, Lai BSP. Open hepatectomy with or without Pringle maneuver: a prospective randomized trial. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2011;13:115. [Google Scholar]
- Lee KF, Cheung YS, Wong J, Chong CC, Wong JS, Lai PB. Randomized clinical trial of open hepatectomy with or without intermittent Pringle manoeuvre. British Journal of Surgery 2012;99(9):1203‐9. [DOI] [PubMed] [Google Scholar]
Lentschener 1997 {published data only}
- Lentschener C, Benhamou D, Mercier FJ, Boyer Neumann C, Naveau S, Smadja C, et al. Aprotinin reduces blood loss in patients undergoing elective liver resection. Anesthesia and Analgesia 1997;84(4):875‐81. [DOI] [PubMed] [Google Scholar]
- Lentschener C, Li H, Franco D, Mercier FJ, Lu H, Soria J, et al. Intraoperatively‐administered aprotinin and survival after elective liver resection for colorectal cancer metastasis: a preliminary study. Fibrinolysis and Proteolysis 1999;13(1):39‐45. [Google Scholar]
Lesurtel 2005 {published data only}
- Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Annals of Surgery 2005;242(6):814‐22, discussion 22‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2009 {published data only}
- Liang G, Wen T, Yan L, Bo L, Wu G, Yang J, et al. A prospective randomized comparison of continuous hemihepatic with intermittent total hepatic inflow occlusion in hepatectomy for liver tumors. Hepato‐Gastroenterology 2009;56(91‐92):745‐50. [PubMed] [Google Scholar]
Liu 1993 {published data only}
- Liu M, Lui WY. The use of fibrin adhesive for hemostasis after liver resection. Chinese Medical Journal 1993;51(1):19‐22. [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Liu CL, Fan ST, Cheung ST, Lo CM, Ng IO, Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Annals of Surgery 2006;244(2):194‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lodge 2005 {published data only}
- Lodge JP, Jonas S, Oussoultzoglou E, Malago M, Jayr C, Cherqui D, et al. Recombinant coagulation factor VIIa in major liver resection: a randomized, placebo‐controlled, double‐blind clinical trial. Anesthesiology 2005;102(2):269‐75. [DOI] [PubMed] [Google Scholar]
Lupo 2007 {published data only}
- Lupo L, Gallerani A, Panzera P, Tandoi F, Palma G, Memeo V. Randomized clinical trial of radiofrequency‐assisted versus clamp‐crushing liver resection. British Journal of Surgery 2007;94(3):287‐91. [DOI] [PubMed] [Google Scholar]
Man 1997 {published data only}
- Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Annals of Surgery 1997;226(6):704‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man K, Fan ST, Ng IOL, Lo CM, Liu CL, Yu WC, et al. Tolerance of the liver to intermittent Pringle maneuver in hepatectomy for liver tumors. Archives of Surgery 1999;134(5):533‐9. [DOI] [PubMed] [Google Scholar]
Man 2003 {published data only}
- Man K, Lo CM, Liu CL, Zhang ZW, Lee TK, Ng IO, et al. Effects of the intermittent Pringle manoeuvre on hepatic gene expression and ultrastructure in a randomized clinical study. British Journal of Surgery 2003;90(2):183‐9. [DOI] [PubMed] [Google Scholar]
Matot 2002 {published data only}
- Matot I, Scheinin O, Jurim O, Eid A. Effectiveness of acute normovolemic hemodilution to minimize allogeneic blood transfusion in major liver resections. Anesthesiology 2002;97(4):794‐800. [DOI] [PubMed] [Google Scholar]
Moench 2014 {published data only}
- Moench C, Bechstein WO, Hermanutz V, Hoexter G, Knaebel H. Comparison of the collagen haemostat Sangustop® versus a carrier‐bound fibrin sealant during liver resection; ESSCALIVER‐Study. Trials 2010;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench C, Mihaljevic AL, Hermanutz V, Thasler WE, Suna K, Diener MK, et al. Randomized controlled multicenter trial on the effectiveness of the collagen hemostat Sangustop® compared with a carrier‐bound fibrin sealant during liver resection (ESSCALIVER study, NCT00918619). Langenbeck's Archives of Surgery/Deutsche Gesellschaft fur Chirurgie 2014;399(6):725‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muratore 2014 {published data only}
- Muratore A, Mellano A, Tarantino G, Marsanic P, Simone M, Benedetto F. Radiofrequency vessel‐sealing system versus the clamp‐crushing technique in liver transection: results of a prospective randomized study on 100 consecutive patients. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2014;16(8):707‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ni 2013 {published data only}
- Junsheng N, Zhou W, Lau WY. A prospective randomized controlled trial to compare Pringle manoeuvre with hemi‐hepatic vascular inflow occlusion in liver resection for hepatocellular carcinoma with cirrhosis. Journal of Hepatology 2014;60(1 Suppl 1):S384. [DOI] [PubMed] [Google Scholar]
- Ni JS, Lau WY, Yang Y, Pan ZY, Wang ZG, Liu H, et al. A prospective randomized controlled trial to compare Pringle manoeuvre with hemi‐hepatic vascular inflow occlusion in liver resection for hepatocellular carcinoma with cirrhosis. Journal of Gastrointestinal Surgery 2013;17(8):1414‐21. [DOI] [PubMed] [Google Scholar]
- Ni JS, Lau WY, Yang Y, Pan ZY, Wang ZG, Liu H, et al. A prospective randomized controlled trial to compare Pringle manoeuvre with hemihepatic vascular inflow occlusion in liver resection for hepatocellular carcinoma with cirrhosis. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2014;16(Suppl S2):548. [Google Scholar]
Noun 1996 {published data only}
- Noun R, Elias D, Balladur P, Bismuth H, Parc R, Lasser P, et al. Fibrin glue effectiveness and tolerance after elective liver resection: a randomized trial. Hepato‐Gastroenterology 1996;43(7):221‐4. [PubMed] [Google Scholar]
Ollinger 2013 {published data only}
- Ollinger R, Mihaljevic AL, Schuhmacher C, Bektas H, Vondran F, Kleine M, et al. A multicentre, randomized clinical trial comparing the verisett haemostatic patch with fibrin sealant for the management of bleeding during hepatic surgery. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2013;15(7):548‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi RI, Bektas H, Pratschke J, Topal B, Buchler M, Schuhmacher CP, et al. A prospective, multi‐center, randomized, single‐blind study to compare the verisett hemostatic patch to fibrin sealant (Tachosil) in subjects undergoing hepatic surgery. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2012;14:104‐5. [Google Scholar]
Park 2012 {published data only}
- Park JB, Joh JW, Kim SJ, David Kwon CH, Min Chun J, Man Kim J, et al. Effect of intermittent hepatic inflow occlusion with the Pringle maneuver during donor hepatectomy in adult living donor liver transplantation with right hemiliver grafts: a prospective, randomized controlled study. Liver Transplantation 2012;18(1):130‐8. [DOI] [PubMed] [Google Scholar]
Pietsch 2010 {published data only}
- Pietsch UC, Herrmann ML, Uhlmann D, Busch T, Hokema F, Kaisers UX, et al. Blood lactate and pyruvate levels in the perioperative period of liver resection with Pringle maneuver. Clinical Hemorheology and Microcirculation 2010;44(4):269‐81. [DOI] [PubMed] [Google Scholar]
Porte 2012 {published data only}
- Porte RJ, Verhoef C, Wilt JHW, Rijken AM, Klaase JM, Ayez N, et al. Fibrocapstm, a novel fibrin sealant, for bleeding during hepatic resection: results of a phase 2, randomized, controlled study. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2012;14:133. [Google Scholar]
- Verhoef C, Singla N, Moneta G, Muir W, Rijken A, Lockstadt H, et al. Fibrocaps for surgical hemostasis: two randomized, controlled phase II trials. Journal of Surgical Research 2015;194(2):679‐87. [DOI] [PubMed] [Google Scholar]
Rahbari 2014 {published data only}
- Rahbari NN, Elbers H, Koch M, Bruckner T, Vogler P, Striebel F, et al. Clamp‐crushing versus stapler hepatectomy for transection of the parenchyma in elective hepatic resection (CRUNSH)‐‐a randomized controlled trial (nct01049607). BMC Surgery 2011;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbari NN, Elbers H, Koch M, Vogler P, Striebel F, Bruckner T, et al. Randomized clinical trial of stapler versus clamp‐crushing transection in elective liver resection. British Journal of Surgery 2014;101(3):200‐7. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Elbers H, Vogler P, Striebel F, Bruckner T, Mehrabi A, et al. Stapler hepatectomy versus the clamp‐crushing technique in elective liver resection: a randomized controlled trial. Langenbeck's Archives of Surgery/Deutsche Gesellschaft fur Chirurgie 2014;399(3):396. [Google Scholar]
Rau 2001 {published data only}
- Rau HG, Wichmann MW, Schinkel S, Buttler E, Pickelmann S, Schauer R, et al. [Surgical techniques in hepatic resections: ultrasonic aspirator versus jet‐cutter. A prospective randomized clinical trial]. Zentralblatt für Chirurgie 2001;126(8):586‐90. [DOI] [PubMed] [Google Scholar]
Savlid 2013 {published data only}
- Savlid M, Strand AH, Jansson A, Agustsson T, Soderdahl G, Lundell L, et al. Transection of the liver parenchyma with an ultrasound dissector or a stapler device: results of a randomized clinical study. World Journal of Surgery 2013;37(4):799‐805. [DOI] [PubMed] [Google Scholar]
Shao 2006 {published data only}
- Shao YF, Yang JM, Chau GY, Sirivatanauksorn Y, Zhong SX, Erhardtsen E, et al. Safety and hemostatic effect of recombinant activated factor vii in cirrhotic patients undergoing partial hepatectomy: a multicenter, randomized, double‐blind, placebo‐controlled trial. American Journal of Surgery 2006;191(2):245‐9. [DOI] [PubMed] [Google Scholar]
Shimada 1994 {published data only}
- Shimada M, Matsumata T, Kamakura T, Hayashi H, Urata K, Sugimachi K. Modulation of coagulation and fibrinolysis in hepatic resection: a randomized prospective controlled study using antithrombin iii concentrates. Thrombosis Research 1994;74(2):105‐14. [DOI] [PubMed] [Google Scholar]
Si‐Yuan 2014 {published data only}
- Si‐Yuan F, Yee LW, Yuan Y, Sheng‐Xian Y, Zheng‐Guang W, Gang H, et al. Pringle manoeuvre versus selective hepatic vascular exclusion in partial hepatectomy for tumours adjacent to the hepatocaval junction: a randomized comparative study. International journal of surgery 2014;12(8):768‐73. [DOI] [PubMed] [Google Scholar]
Smyrniotis 2005 {published data only}
- Smyrniotis V, Arkadopoulos N, Kostopanagiotou G, Farantos C, Vassiliou J, Contis J, et al. Sharp liver transection versus clamp crushing technique in liver resections: a prospective study. Surgery 2005;137(3):306‐11. [DOI] [PubMed] [Google Scholar]
Takayama 2001 {published data only}
- Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, et al. Randomized comparison of ultrasonic vs clamp transection of the liver. Archives of Surgery 2001;136(8):922‐8. [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World Journal of Gastroenterology 2006;12(6):935‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2003 {published data only}
- Wong AYC, Irwin MG, Hui TWC, Fung SKY, Fan ST, Ma ESK. Desmopressin does not decrease blood loss and transfusion requirements in patients undergoing hepatectomy. Canadian Journal of Anesthesia 2003;50(1):14‐20. [DOI] [PubMed] [Google Scholar]
Wu 2002 {published data only}
- Wu CC, Yeh DC, Ho WM, Yu CL, Cheng SB, Liu TJ, et al. Occlusion of hepatic blood inflow for complex central liver resections in cirrhotic patients ‐ a randomized comparison of hemihepatic and total hepatic occlusion techniques. Archives of Surgery 2002;137(12):1369‐76. [DOI] [PubMed] [Google Scholar]
Wu 2006 {published data only}
- Wu CC, Ho WM, Cheng SB, Yeh DC, Wen MC, Liu TJ, et al. Perioperative parenteral tranexamic acid in liver tumor resection: a prospective randomized trial toward a "blood transfusion"‐free hepatectomy. Annals of Surgery 2006;243(2):173‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yao 2006 {published data only}
- Yao XH, Wang B, Xiao ZK, Zhou P, Chen CY, Qing ZH. Acute normovolemic hemodilution combined with controlled hypotension in patients undergoing liver tumorectomy. Nan Fang Yi Ke Da Xue Xue Bao [Journal of Southern Medical University] 2006;26(6):828‐30. [PubMed] [Google Scholar]
References to studies excluded from this review
Arru 2007 {published data only}
- Arru M, Pulitano C, Aldrighetti L, Catena M, Finazzi R, Ferla G. A prospective evaluation of ultrasonic dissector plus harmonic scalpel in liver resection. American Surgeon 2007;73(3):256‐60. [PubMed] [Google Scholar]
Azoulay 2005 {published data only}
- Azoulay D, Eshkenazy R, Andreani P, Castaing D, Adam R, Ichai P, et al. In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Annals of Surgery 2005;241(2):277‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellolio 2012 {published data only}
- Bellolio C, Montedeonico C, Hernandez A, Harguindeguy M, Leites A, Rando K. Hemodynamic changes in liver surgery with intrathecal morphine versus epidural local anesthetics: a prospective comparative study. British Journal of Anaesthesia 2012;108:ii246‐ii7. [Google Scholar]
Beppu 2012 {published data only}
- Beppu T, Ishiko T, Chikamoto A, Komori H, Masuda T, Hayashi H, et al. Liver hanging maneuver decreases blood loss and operative time in a right‐side hepatectomy. Hepato‐Gastroenterology 2012;59(114):542‐5. [DOI] [PubMed] [Google Scholar]
Broek 2011 {published data only}
- Broek MA, Bloemen JG, Dello SA, Poll MC, Olde Damink SW, Dejong CH. Randomized controlled trial analyzing the effect of 15 or 30 min intermittent Pringle maneuver on hepatocellular damage during liver surgery. Journal of Hepatology 2011;55(2):337‐45. [DOI] [PubMed] [Google Scholar]
Chapman 2007 {published data only}
- Chapman WC, Singla N, Genyk Y, McNeil JW, Renkens Jr KL, Reynolds TC, et al. A phase 3, randomized, double‐blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. Journal of the American College of Surgeons 2007;205(2):256‐65. [DOI] [PubMed] [Google Scholar]
Correa‐Gallego 2015 {published data only}
- Correa‐Gallego C, Tan KS, Arslan‐Carlon V, Gonen M, Denis SC, Langdon‐Embry L, et al. Goal‐directed fluid therapy using stroke volume variation for resuscitation after low central venous pressure‐assisted liver resection: a randomized clinical trial. Journal of the American College of Surgeons 2015;221(2):591‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dello 2012 {published data only}
- Dello S, Reisinger K, Jong M, Dam R, Damink SO, Bemelmans M, et al. Intermittent Pringle manoeuvre is associated with gut injury in patients undergoing liver resection. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2011;13:48. [Google Scholar]
- Dello SA, Reisinger KW, Dam RM, Bemelmans MH, Kuppevelt TH, Broek MA, et al. Total intermittent Pringle maneuver during liver resection can induce intestinal epithelial cell damage and endotoxemia. PLOS ONE 2012;7(1):e30539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger K, Dello S, Dam R, Bemelmans MHA, Damink SO, Poeze M, et al. Intermittent Pringle manoeuvre is associated with gut injury in patients undergoing liver resection. Gastroenterology 2011;140(5 Suppl):S395. [Google Scholar]
- Broek M, Bloemen J, Dello S, Poll M, Damink SO, Dejong C. Randomized controlled trial analyzing the effect of 15 or 30 minutes intermittent Pringle manoeuvre on hepatocellular damage during liver surgery in man. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2011;13:6. [Google Scholar]
- Broek MA, Bloemen JG, Dello SA, Poll MC, Olde Damink SW, Dejong CH. Randomized controlled trial analyzing the effect of 15 or 30 min intermittent Pringle maneuver on hepatocellular damage during liver surgery. Journal of Hepatology 2011;55(2):337‐45. [DOI] [PubMed] [Google Scholar]
Dominioni 2014 {published data only}
- Dominioni T, Vigano J, Cobianchi L, Peloso A, Ferrario J, D'Addiego A, et al. The slippery slope of cross clamping vs open surgery: is the choice obvious?. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2014;16:62‐3. [Google Scholar]
El‐Moghazy 2009 {published data only}
- El‐Moghazy WM, Hedaya MS, Kaido T, Egawa H, Uemoto S, Takada Y. Two different methods for donor hepatic transection: cavitron ultrasonic surgical aspirator with bipolar cautery versus cavitron ultrasonic surgical aspirator with radiofrequency coagulator‐a randomized controlled trial. Liver Transplantation 2009;15(1):102‐5. [DOI] [PubMed] [Google Scholar]
Esaki 2006 {published data only}
- Esaki M, Sano T, Shimada K, Sakamoto Y, Takahashi Y, Wakai IC, et al. Randomized clinical trial of hepatectomy using intermittent pedicle occlusion with ischaemic intervals of 15 versus 30 minutes. British Journal of Surgery 2006;93(8):944‐51. [DOI] [PubMed] [Google Scholar]
Feldheiser 2015 {published data only}
- Feldheiser A, Pavlova V, Weimann K, Hunsicker O, Stockmann M, Koch M, et al. Haemodynamic optimization by oesophageal doppler and pulse power wave analysis in liver surgery: a randomised controlled trial. PlLOS ONE 2015;10(7):e0132715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Figueras 2003 {published data only}
- Figueras J, Lopez‐Ben S, Llado L, Rafecas A, Torras J, Ramos E, et al. Hilar dissection versus the "glissonean" approach and stapling of the pedicle for major hepatectomies: a prospective, randomized trial. Annals of Surgery 2003;238(1):111‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frankel 2013 {published data only}
- Frankel TL, Fischer M, Grant F, Krone J, D'Angelica MI, Dematteo RP, et al. Selecting patients for acute normovolemic hemodilution during hepatic resection: a prospective randomized evaluation of nomogram‐based allocation. Journal of the American College of Surgeons 2013;217(2):210‐20. [DOI] [PubMed] [Google Scholar]
Gonzalez 2009 {published data only}
- Gonzalez HD, Figueras Felip J. Topical hemostatic devices in surgery: between science and marketing [Hemostáticos tópicos en cirugía: entre la cienciay el marketing]. Cirugia Espanola 2009;85(Suppl 1):23‐8. [DOI] [PubMed] [Google Scholar]
Gotohda 2015 {published data only}
- Gotohda N, Yamanaka T, Saiura A, Uesaka K, Hashimoto M, Konishi M, et al. Impact of energy devices during liver parenchymal transection: a multicenter randomized controlled trial. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2015;17(Suppl S2):60. [DOI] [PubMed] [Google Scholar]
- Gotohda N, Yamanaka T, Saiura A, Uesaka K, Hashimoto M, Konishi M, et al. Impact of energy devices during liver parenchymal transection: a multicenter randomized controlled trial. World Journal of Surgery 2015;39(6):1543‐9. [DOI] [PubMed] [Google Scholar]
Grobmyer 2009 {published data only}
- Grobmyer SR, Hemming AW, Harris N, Behrns K, Logan H, Kim RD, et al. A pilot prospective randomized trial of postoperative epoetin alfa in patients undergoing major operation for upper gastrointestinal malignancy. American Journal of Clinical Oncology 2009;32(6):570‐3. [DOI] [PubMed] [Google Scholar]
Hamady 2015 {published data only}
- Hamady Z, Toogood G. Infrahepatic inferior vena cava clamping for reduction of central venous pressure and blood loss during hepatic resection: a randomized controlled trial. Annals of Surgery 2015;261(1):E8. [DOI] [PubMed] [Google Scholar]
Hanyong 2015 {published data only}
- Hanyong S, Wanyee L, Siyuan F, Hui L, Yuan Y, Chuan L, et al. A prospective randomized controlled trial: comparison of two different methods of hepatectomy. European Journal of Surgical Oncology 2015;41(2):243‐8. [DOI] [PubMed] [Google Scholar]
Harimoto 2011 {published data only}
- Harimoto N, Shirabe K, Abe T, Yukaya T, Tsujita E, Gion T, et al. Prospective randomized controlled trial investigating the type of sutures used during hepatectomy. World Journal of Gastroenterology 2011;17(18):2338‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashimoto 2007 {published data only}
- Hashimoto T, Kokudo N, Orii R, Seyama Y, Sano K, Imamura H, et al. Intraoperative blood salvage during liver resection: a randomized controlled trial. Annals of Surgery 2007;245(5):686‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaibori 2013 {published data only}
- Kaibori M, Matsui K, Ishizaki M, Sakaguchi T, Matsushima H, Matsui Y, et al. A prospective randomized controlled trial of hemostasis with a bipolar sealer during hepatic transection for liver resection. Surgery 2013;154(5):1046‐52. [DOI] [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim YI, Fujita S, Hwang YJ, Chun JM, Song KE, Chun BY. Successful intermittent application of the Pringle maneuver for 30 minutes during human hepatectomy: a clinical randomized study with use of a protease inhibitor. Hepato‐Gastroenterology 2007;54(79):2055‐60. [PubMed] [Google Scholar]
Kim 2008 {published data only}
- Kim KH, Lee SG. Usefulness of Kelly clamp crushing technique during hepatic resection. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2008;10(4):281‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le Treut 1995 {published data only}
- Treut YP, Christophe M, Banti JC, Berthet B, Bricot R. Pedicular clamping in major hepatectomies: clamping "of principle" or "of necessity"? A comparative study [Le clampage pediculaire au cours des hepatectomies majeures: clampage "de principe" ou "de necessite"? Une etude comparative]. Journal de Chirurgie 1995;132(2):55‐60. [PubMed] [Google Scholar]
Levit 2012 {published data only}
- Levit D, Levit A. Different combinations of colloids and crystalloids in major abdominal surgery: hemodynamics effects. Intensive Care Medicine 2012;38:S152. [Google Scholar]
Li 2013 {published data only}
- Li M, Zhang W, Li Y, Li P, Li J, Gong J, et al. Radiofrequency‐assisted versus clamp‐crushing parenchyma transection in cirrhotic patients with hepatocellular carcinoma: a randomized clinical trial. Digestive Diseases and Sciences 2013;58(3):835‐40. [DOI] [PubMed] [Google Scholar]
Li 2015 {published data only}
- Li M, Zhang C, Zhang T, Wang L, Ding Y, Niu Z, et al. Outcome using selective hemihepatic vascular occlusion and Pringle maneuver for hepatic resection of liver cavernous hemangioma. World Journal of Surgical Oncology 2015;13:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2014 {published data only}
- Lu H, Fan Y, Zhang F, Li G, Zhang C, Lu L. Fast‐track surgery improves postoperative outcomes after hepatectomy. Hepato‐Gastroenterology 2014;61(129):168‐72. [PubMed] [Google Scholar]
Man 2002 {published data only}
- Man K, Liang TB, Lo CM, Liu CL, Ng IO, Yu WC, et al. Hepatic stress gene expression and ultrastructural features under intermittent Pringle manoeuvre. Hepatobiliary and pancreatic diseases international 2002;1(2):249‐57. [PubMed] [Google Scholar]
Nagano 2009 {published data only}
- Nagano H, Kishimoto S, Kobayashi S, Marubashi S, Eguchi H, Takeda Y, et al. A safe protocol of intermittent hilar vascular clamping for hepatic resection in cirrhosis. Hepato‐Gastroenterology 2009;56(94‐95):1439‐44. [PubMed] [Google Scholar]
Narita 2012 {published data only}
- Narita M, Oussoultzoglou E, Fuchshuber P, Chenard MP, Rosso E, Yamamoto K, et al. Prolonged portal triad clamping increases postoperative sepsis after major hepatectomy in patients with sinusoidal obstruction syndrome and/or steatohepatitis. World Journal of Surgery 2012;36(8):1848‐57. [DOI] [PubMed] [Google Scholar]
NCT01651182 {published data only}
- NCT01651182. Tranexamic acid versus placebo for blood to reduce perioperative bleeding post‐liver resection [Open label, non‐randomized, study to evaluate the pharmacokinetics of tranexamic acid in patients undergoing major liver resection]. clinicaltrials.gov/ct2/show/NCT01651182 (first received 24 July 2012).
Obiekwe 2014 {published data only}
- Obiekwe SR, Quintaine L, Khannaz A, Laurent C, Saric J. To Pringle or not to Pringle: is pedicle clamping a necessity in liver resection?. Hepato‐Gastroenterology 2014;61(133):1402‐14. [PubMed] [Google Scholar]
- Quintane L, Obiekwe S, Laurent C, Saric J. To Pringle or not to Pringle. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2012;14:23. [Google Scholar]
Palibrk 2012 {published data only}
- Palibrk I, Milicic B, Stojiljkovic L, Manojlovic N, Dugalic V, Bumbasirevic V, et al. Clamp‐crushing vs. radiofrequency‐assisted liver resection: changes in liver function tests. Hepato‐Gastroenterology 2012;59(115):800‐4. [DOI] [PubMed] [Google Scholar]
Petras 2009 {published data only}
- Petras P, Kontostolis V, Sheen AJ, Siriwardena AK. Randomized clinical trial of efficacy and costs of three dissection devices in liver resection (Br J Surg 2009; 96: 593‐601). British Journal of Surgery 2009;96(10):1223. [DOI] [PubMed] [Google Scholar]
Petrowsky 2006 {published data only}
- Petrowsky H, McCormack L, Trujillo M, Selzner M, Jochum W, Clavien PA. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Annals of Surgery 2006;244(6):921‐8; discussion 8‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahbari 2011 {published data only}
- Rahbari NN, Koch M, Zimmermann JB, Elbers H, Bruckner T, Contin P, et al. Infrahepatic inferior vena cava clamping for reduction of central venous pressure and blood loss during hepatic resection: a randomized controlled trial. Annals of Surgery 2011;253(6):1102‐10. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Zimmermann JB, Koch M, Bruckner T, Schmidt T, Elbers H, et al. IVC CLAMP: infrahepatic inferior vena cava clamping during hepatectomy‐‐a randomised controlled trial in an interdisciplinary setting. Trials 2009;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rau 1995 {published data only}
- Rau HG, Schardey HM, Buttler E, Reuter C, Cohnert TU, Schildberg FW. A comparison of different techniques for liver resection: blunt dissection, ultrasonic aspirator and jet‐cutter. European Journal of Surgical Oncology 1995;21(2):183‐7. [DOI] [PubMed] [Google Scholar]
Richter 2009 {published data only}
- Richter S, Kollmar O, Schuld J, Moussavian MR, Igna D, Schilling MK. Randomized clinical trial of efficacy and costs of three dissection devices in liver resection. British Journal of Surgery 2009;96(6):593‐601. [DOI] [PubMed] [Google Scholar]
Ryu 2010 {published data only}
- Ryu HG, Nahm FS, Sohn HM, Jeong EJ, Jung CW. Low central venous pressure with milrinone during living donor hepatectomy. American Journal of Transplantation 2010;10(4):877‐82. [DOI] [PubMed] [Google Scholar]
Saiura 2006 {published data only}
- Saiura A, Yamamoto J, Koga R, Sakamoto Y, Kokudo N, Seki M, et al. Usefulness of LigaSure for liver resection: analysis by randomized clinical trial. American Journal of Surgery 2006;192(1):41‐5. [DOI] [PubMed] [Google Scholar]
Saiura 2014 {published data only}
- Saiura A, Arita J, Takahashi Y, Inoue Y, Ono Y, Takahashi M, et al. Faster liver transection using LigaSure combined with clamp crush technique. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2014;16(Suppl S2):719. [Google Scholar]
Schilling 2009 {published data only}
- Schilling MK, Richter S. Authors' reply: randomized clinical trial of efficacy and costs of three dissection devices in liver resection (Br J Surg 2009; 96: 593‐601). British Journal of Surgery 2009;96(10):1223. [DOI] [PubMed] [Google Scholar]
Schwartz 2004 {published data only}
- Schwartz M, Madariaga J, Hirose R, Shaver TR, Sher L, Chari R, et al. Comparison of a new fibrin sealant with standard topical hemostatic agents. Archives of Surgery 2004;139(11):1148‐54. [DOI] [PubMed] [Google Scholar]
Shu 2014 {published data only}
- Shu H, Liu K, He Q, Zhong F, Yang L, Li Q, et al. Ulinastatin, a protease inhibitor, may inhibit allogeneic blood transfusion‐associated pro‐inflammatory cytokines and systemic inflammatory response syndrome and improve postoperative recovery. Blood Transfusion 2014;12(Suppl 1):s109‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Si‐Yuan 2011 {published data only}
- Si‐Yuan FU, Yee LW, Guang‐Gang L, Qing‐He T, Ai‐Jun LI, Ze‐Ya PAN, et al. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. American Journal of Surgery 2011;201(1):62‐9. [DOI] [PubMed] [Google Scholar]
Smyrniotis 2002 {published data only}
- Smyrniotis VE, Kostopanagiotou GG, Gamaletsos EL, Vassiliou JG, Voros DC, Fotopoulos AC, et al. Total versus selective hepatic vascular exclusion in major liver resections. American Journal of Surgery 2002;183(2):173‐8. [DOI] [PubMed] [Google Scholar]
Smyrniotis 2003a {published data only}
- Smyrniotis V, Kostopanagiotou G, Lolis E, Theodoraki K, Farantos C, Andreadou I, et al. Effects of hepatovenous back flow on ischemic‐ reperfusion injuries in liver resections with the Pringle maneuver. Journal of the American College of Surgeons 2003;197(6):949‐54. [DOI] [PubMed] [Google Scholar]
Smyrniotis 2003b {published data only}
- Smyrniotis VE, Kostopanagiotou GG, Contis JC, Farantos CI, Voros DC, Karmas DC, et al. Selective hepatic vascular exclusion versus Pringle maneuver in major liver resections: prospective study. World Journal of Surgery 2003;27(7):765‐9. [DOI] [PubMed] [Google Scholar]
Smyrniotis 2006 {published data only}
- Smyrniotis V, Theodoraki K, Arkadopoulos N, Fragulidis G, Condi‐Pafiti A, Plemenou‐Fragou M, et al. Ischemic preconditioning versus intermittent vascular occlusion in liver resections performed under selective vascular exclusion: a prospective randomized study. American Journal of Surgery 2006;192(5):669‐74. [DOI] [PubMed] [Google Scholar]
Standl 1998 {published data only}
- Standl T, Burmeister MA, Horn EP, Wilhelm S, Knoefel WT, Esch JSA. Bovine haemoglobin‐based oxygen carrier for patients undergoing haemodilution before liver resection. British Journal of Anaesthesia 1998;80(2):189‐94. [DOI] [PubMed] [Google Scholar]
- Standl T, Wilhelm S, Horn EP, Burmeister M, Gundlach M, Esch JSA. Acute haemodynamic effects of preoperative haemodilution with bovine haemoglobin for liver surgery. Anaesthesist 1997;46(9):763‐70. [DOI] [PubMed] [Google Scholar]
Strobel 2012 {published data only}
- Strobel O, Buchler MW. Intermittent Pringle manoeuvre: no reduction of blood loss according to recent randomised clinical trials. Der Chirurg; Zeitschrift fur Alle Gebiete der Operativen Medizen 2012;83(11):994. [DOI] [PubMed] [Google Scholar]
Strobel 2014 {published data only}
- Strobel O, Buchler MW. Stapler vs clamp‐crushing transection for liver resection: a randomized clinical trial [Parenchymdurchtrennungmittels stapler vs. clamp‐crushingbei leberresektion: eine randomisierte kontrollierte studie]. Der Chirurg 2014;85(4):349. [DOI] [PubMed] [Google Scholar]
Takatsuki 2015 {published data only}
- Takatsuki M, Soyama A, Hidaka M, Kinoshita A, Adachi T, Kitasato A, et al. Prospective study of the safety and efficacy of intermittent inflow occlusion (Pringle maneuver) in living donor left hepatectomy. Hepatology Research 2015;45(8):856‐62. [DOI] [PubMed] [Google Scholar]
Torzilli 2008 {published data only}
- Torzilli G, Donadon M, Marconi M, Procopio F, Palmisano A, Fabbro D, et al. Monopolar floating ball versus bipolar forceps for hepatic resection: a prospective randomized clinical trial. Journal of Gastrointestinal Surgery 2008;12(11):1961‐6. [DOI] [PubMed] [Google Scholar]
Vlad 2014 {published data only}
- Vlad N, Gouillat C, Moldovanu R, Lupascu C, Georgescu S, Tarcoveanu E. Radiofrequency ablation device assisted liver resection for hepatocellular carcinoma. Chirurgia 2014;109(4):500‐6. [PubMed] [Google Scholar]
- Vlad N, Tarcoveanu E, Gouillat C, Lupascu C, Georgescu S, Moldovanu R. Hepatic resection for hepatocellular carcinoma using a radiofrequency ablation device. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2012;14:163. [Google Scholar]
Wang 2010 {published data only}
- Wang CC, Jawade K, Yap AQ, Concejero AM, Lin CY, Chen CL. Resection of large hepatocellular carcinoma using the combination of liver hanging maneuver and anterior approach. World Journal of Surgery 2010;34(8):1874‐8. [DOI] [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Wang CC, Yap AQ, Chen CL, Concejero AM, Lin YH. Comparison of major hepatectomy performed under intermittent Pringle maneuver versus continuous Pringle maneuver coupled with in situ hypothermic perfusion. World Journal of Surgery 2011;35(4):842‐9. [DOI] [PubMed] [Google Scholar]
Yang 2012 {published data only}
- Yang Y, Fu SY, Lau WY, Lai E, Li AJ, Pan ZY, et al. Selective main portal vein clamping to minimize the risk of recurrence after curative liver resection for hepatocellular carcinoma. Hepato‐Gastroenterology 2012;59(117):1560‐5. [DOI] [PubMed] [Google Scholar]
Yang 2013 {published data only}
- Yang Y, Lai EC, Fu SY, Gu FM, Li PP, Lau WY, et al. A prospective randomized controlled trial to compare two methods of selective hepatic vascular exclusion in partial hepatectomy. European Journal of Surgical Oncology 2013;39(2):125‐30. [DOI] [PubMed] [Google Scholar]
Yin 2003 {published data only}
- Yin ZY, Wang XM, Yu RX, Zhang BM, Yu KK, Li N, et al. Total vascular exclusion technique for resection of hepatocellular carcinoma. World Journal of Gastroenterology 2003;9(10):2194‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang B, Chen X. Ligating the corresponding inflow and outflow vessels during hepatectomy: a prospective randomized controlled trial and animal study. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2014;16(Suppl S2):528. [Google Scholar]
Zhu 2012 {published data only}
- Zhu P, Lau WY, Chen YF, Zhang BX, Huang ZY, Zhang ZW, et al. Randomized clinical trial comparing infrahepatic inferior vena cava clamping with low central venous pressure in complex liver resections involving the Pringle manoeuvre. British Journal of Surgery 2012;99(6):781‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bochicchio 2015 {published data only}
- Bochicchio GV, Gupta N, Porte RJ, Renkens KL, Pattyn P, Topal B, et al. The finish‐3 trial: a phase 3, international, randomized, single‐blind, controlled trial of topical fibrocaps in intraoperative surgical hemostasis. Journal of the American College of Surgeons 2015;220(1):70‐81. [DOI] [PubMed] [Google Scholar]
Chapman 2006 {published data only}
- Chapman WC, Lockstadt H, Singla N, Kafie FE, Lawson JH. Phase 2, randomized, double‐blind, placebo‐controlled, multicenter clinical evaluation of recombinant human thrombin in multiple surgical indications [6]. Journal of Thrombosis and Haemostasis 2006;4(9):2083‐5. [DOI] [PubMed] [Google Scholar]
Wright 2015 {published data only}
- Wright G, Waldherr TL, Ritz‐Holland D, Lane BR, Chung MH. Reducing transfusion rates in major oncologic surgery: preliminary results of a randomized, double‐blind, placebo‐controlled trial using preoperative tranexamic acid. Annals of Surgical Oncology 2015;22(1 Suppl 1):S179. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Chen 2015 {published data only}
- Chen JM, Geng W, Liu FB, Zhao HC, Xie SX, Hou H, et al. BiClamp® forcep liver transection versus clamp crushing technique for liver resection: study protocol for a randomized controlled trial. Trials 2015;16:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2008 {published data only}
- Schmidt T, Koch M, Antolovic D, Reissfelder C, Schmitz‐Winnenthal FH, Rahbari NN, et al. Influence of two different resection techniques (conventional liver resection versus anterior approach) of liver metastases from colorectal cancer on hematogenous tumor cell dissemination ‐ prospective randomized multicenter trial. BMC Surgery 2008;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Asiyanbola 2008
- Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, et al. Operative mortality after hepatic resection: are literature‐based rates broadly applicable?. Journal of Gastrointestinal Surgery 2008;12(5):842‐51. [DOI] [PubMed] [Google Scholar]
ATLS 2008
- American College of Surgeons Committee on Trauma. ATLS Student Course Manual. 8th Edition. American College of Surgeons, 2008. [Google Scholar]
Belghiti 1993
- Belghiti J, Kabbej M, Sauvanet A, Vilgrain V, Panis Y, Fekete F. Drainage after elective hepatic resection: a randomized trial. Annals of Surgery 1993;218(6):748‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Clavien 2009
- Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Annals of Surgery 2009;250(2):187‐96. [DOI] [PubMed] [Google Scholar]
Couinaud 1999
- Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Digestive Surgery 1999;16(6):459‐67. [DOI] [PubMed] [Google Scholar]
Del Re 2013
- Re AC, Spielmans GI, Fluckiger C, Wampold BE. Efficacy of new generation antidepressants: differences seem illusory. PLOS ONE 2013;8(6):e63509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2012a
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: introduction to evidence synthesis for decision making, 2012. www.nicedsu.org.uk/TSD1%20Introduction.final.08.05.12.pdf (accessed 27 March 2014).
Dias 2012b
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 3: heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment, 2012. www.nicedsu.org.uk/TSD3%20Heterogeneity.final%20report.08.05.12.pdf (accessed 27 March 2014).
Dias 2012c
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials, 2012. www.nicedsu.org.uk/TSD4%20Inconsistency.final.08.05.12.pdf (accessed 27 March 2014). [PubMed]
Dias 2013a
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta‐analysis of randomised controlled trials, 2013. www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%20Mar2013.pdf (accessed 27 March 2014). [PubMed]
Dias 2013b
- Dias S, Welton NJ, Sutton AJ, Ades AE. Evidence synthesis for decision making 1: introduction. Medical Decision Making 2013;33(5):597‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2013c
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta‐analysis of randomized controlled trials. Medical Decision Making 2013;33(5):607‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2013d
- Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity‐‐subgroups, meta‐regression, bias, and bias‐adjustment. Medical Decision Making 2013;33(5):618‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2013e
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Medical Decision Making 2013;33(5):641‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farges 2012
- Farges O, Goutte N, Bendersky N, Falissard B. Incidence and risks of liver resection: an all‐inclusive French nationwide study. Annals of Surgery 2012;256(5):697‐704. [DOI] [PubMed] [Google Scholar]
Figueras 1997
- Figueras J, Farran L, Benasco C, Ribas Y, Ramos E, Borobia FG, et al. Vascular occlusion in hepatic resections in cirrhotic rat livers: an experimental study in rats. Liver Transplantation and Surgery 1997;3(6):617‐23. [DOI] [PubMed] [Google Scholar]
Finch 2007
- Finch RJ, Malik HZ, Hamady ZZ, Al‐Mukhtar A, Adair R, Prasad KR, et al. Effect of type of resection on outcome of hepatic resection for colorectal metastases. British Journal of Surgery 2007;94(10):1242‐8. [DOI] [PubMed] [Google Scholar]
Garden 2006
- Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut 2006;55(Suppl 3):iii1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2013
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 7. Art. No.: LIVER.
Gurusamy 2009a
- Gurusamy KS, Kumar Y, Ramamoorthy R, Sharma D, Davidson BR. Vascular occlusion for elective liver resections. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007530] [DOI] [PubMed] [Google Scholar]
Gurusamy 2009b
- Gurusamy KS, Pamecha V, Sharma D, Davidson BR. Techniques for liver parenchymal transection in liver resection. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006880] [DOI] [PubMed] [Google Scholar]
Gurusamy 2009c
- Gurusamy KS, Li J, Sharma D, Davidson BR. Pharmacological interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008085] [DOI] [PubMed] [Google Scholar]
Gurusamy 2012
- Gurusamy KS, Li J, Vaughan J, Sharma D, Davidson BR. Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD007338.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2012
- Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
HSCIC 2015
- Health & Social Care Information Centre. Hospital Episode Statistics. Admitted Patient Care ‐ England, 2014 ‐ 2015: main procedures and interventions. www.hscic.gov.uk/catalogue/PUB19124/hosp‐epis‐stat‐admi‐proc‐2014‐15‐tab.xlsx (accessed 6 June 2016).
IARC 2012
- International Agency for Research on Cancer (World Health Organization). GLOBOCAN 2012. Estimated cancer incidence, mortality, and prevalence worldwide in 2012. globocan.iarc.fr (accessed on 6 June 2016).
Ibrahim 2006
- Ibrahim S, Chen CL, Lin CC, Yang CH, Wang CC, Wang SH, et al. Intraoperative blood loss is a risk factor for complications in donors after living donor hepatectomy. Liver Transplantation 2006;12(6):950‐7. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Jang 2008
- Jang JH, Kang KJ, Kang Y, Lee IS, Graf R, Clavien PA. Ischemic preconditioning and intermittent clamping confer protection against ischemic injury in the cirrhotic mouse liver. Liver Transplantation 2008;14(7):980‐8. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Llovet 2005
- Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Seminars in Liver Disease 2005;25(2):181‐200. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447‐59. [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Mills 2012
- Mills EJ, Ioannidis JP, Thorlund K, Schunemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Navadgi 2016
- Navadgi S, Chang CC, Bartlett A, McCall J, Pandanaboyana S. Systematic review and meta‐analysis of outcomes after liver resection in patients with hepatocellular carcinoma (HCC) with and without bile duct thrombus. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2016;18(4):312‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
NHS Blood and Transplant 2007
- NHS Blood and Transplant. Handbook of Transfusion Medicine, 2007. www.transfusionguidelines.org.uk/index.aspx?Publication=HTM&Section=9 (accessed 27 March 2014).
Nordlinger 2013
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long‐term results of a randomised, controlled, phase 3 trial. Lancet Oncology 2013;14(12):1208‐15. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical Research Ed.) 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
Reissfelder 2011
- Reissfelder C, Rahbari NN, Koch M, Kofler B, Sutedja N, Elbers H, et al. Postoperative course and clinical significance of biochemical blood tests following hepatic resection. British Journal of Surgery 2011;98(6):836‐44. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
Savovic 2012a
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savovic 2012b
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Shimada 1998
- Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, et al. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. British Journal of Surgery 1998;85(2):195‐8. [DOI] [PubMed] [Google Scholar]
Strasberg 2000
- Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB Surgery 2000;2(3):333‐9. [Google Scholar]
Taefi 2013
- Taefi A, Abrishami A, Nasseri‐Moghaddam S, Eghtesad B, Sherman M. Surgical resection versus liver transplant for patients with hepatocellular carcinoma. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD006935.pub2] [DOI] [PubMed] [Google Scholar]
Thorlund 2012
- Thorlund K, Mills EJ. Sample size and power considerations in network meta‐analysis. Systematic Reviews 2012;1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Research Synthesis Methods 2012;3(2):111‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
WinBUGS 1.4 [Computer program]
- Imperial College and MRC, UK. WinBUGS with DoodleBUGS. Version 1.4.3. Imperial College and MRC, UK, 2007.
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshimura 2004
- Yoshimura Y, Kubo S, Shirata K, Hirohashi K, Tanaka H, Shuto T, et al. Risk factors for postoperative delirium after liver resection for hepatocellular carcinoma. World Journal of Surgery 2004;28(10):982‐6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Simillis 2014
- Simillis C, Li T, Vaughan J, Becker LA, Davidson BR, Gurusamy KS. Methods to decrease blood loss during liver resection: a network meta‐analysis. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD010683.pub2] [DOI] [PubMed] [Google Scholar]


