Abstract
Background
Screening hysteroscopy in infertile women with unexplained infertility, or prior to intrauterine insemination (IUI) or in vitro fertilisation (IVF) may reveal intrauterine pathology that may not be detected by routine transvaginal ultrasound. Hysteroscopy, whether purely diagnostic or operative may improve reproductive outcomes.
Objectives
To assess the effectiveness and safety of screening hysteroscopy in subfertile women undergoing evaluation for infertility, and subfertile women undergoing IUI or IVF.
Search methods
We searched the Cochrane Gynaecology and Fertility Group Specialised Register, CENTRAL CRSO, MEDLINE, Embase, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (September 2018). We searched reference lists of relevant articles and handsearched relevant conference proceedings.
Selection criteria
Randomised controlled trials comparing screening hysteroscopy versus no intervention in subfertile women wishing to conceive spontaneously, or before undergoing IUI or IVF.
Data collection and analysis
We independently screened studies, extracted data, and assessed the risk of bias. The primary outcomes were live birth rate and complications following hysteroscopy. We analysed data using risk ratio (RR) and a fixed‐effect model. We assessed the quality of the evidence by using GRADE criteria.
Main results
We retrieved 11 studies. We included one trial that evaluated screening hysteroscopy versus no hysteroscopy, in women with unexplained subfertility, who were trying to conceive spontaneously. We are uncertain whether ongoing pregnancy rate improves following a screening hysteroscopy in women with at least two years of unexplained subfertility (RR 4.30, 95% CI 2.29 to 8.07; 1 RCT; participants = 200; very low‐quality evidence). For a typical clinic with a 10% ongoing pregnancy rate without hysteroscopy, performing a screening hysteroscopy would be expected to result in ongoing pregnancy rates between 23% and 81%. The included study reported no adverse events in either treatment arm. We are uncertain whether clinical pregnancy rate is improved (RR 3.80, 95% CI 2.31 to 6.24; 1 RCT; participants = 200; very low‐quality evidence), or miscarriage rate increases (RR 2.80, 95% CI 1.05 to 7.48; 1 RCT; participants = 200; very low‐quality evidence), following screening hysteroscopy in women with at least two years of unexplained subfertility.
We included ten trials that included 1836 women who had a screening hysteroscopy and 1914 women who had no hysteroscopy prior to IVF. Main limitations in the quality of evidence were inadequate reporting of study methods and higher statistical heterogeneity. Eight of the ten trials had unclear risk of bias for allocation concealment.
Performing a screening hysteroscopy before IVF may increase live birth rate (RR 1.26, 95% CI 1.11 to 1.43; 6 RCTs; participants = 2745; I² = 69 %; low‐quality evidence). For a typical clinic with a 22% live birth rate, performing a screening hysteroscopy would be expected to result in live birth rates between 25% and 32%. However, sensitivity analysis done by pooling results from trials at low risk of bias showed no increase in live birth rate following a screening hysteroscopy (RR 0.99, 95% CI 0.82 to 1.18; 2 RCTs; participants = 1452; I² = 0%).
Only four trials reported complications following hysteroscopy; of these, three trials recorded no events in either group. We are uncertain whether a screening hysteroscopy is associated with higher adverse events (Peto odds ratio 7.47, 95% CI 0.15 to 376.42; 4 RCTs; participants = 1872; I² = not applicable; very low‐quality evidence).
Performing a screening hysteroscopy before IVF may increase clinical pregnancy rate (RR 1.32, 95% CI 1.20 to 1.45; 10 RCTs; participants = 3750; I² = 49%; low‐quality evidence). For a typical clinic with a 28% clinical pregnancy rate, performing a screening hysteroscopy would be expected to result in clinical pregnancy rates between 33% and 40%.
There may be little or no difference in miscarriage rate following screening hysteroscopy (RR 1.01, 95% CI 0.67 to 1.50; 3 RCTs; participants = 1669; I² = 0%; low‐quality evidence).
We found no trials that compared a screening hysteroscopy versus no hysteroscopy before IUI.
Authors' conclusions
At present, there is no high‐quality evidence to support the routine use of hysteroscopy as a screening tool in the general population of subfertile women with a normal ultrasound or hysterosalpingogram in the basic fertility work‐up for improving reproductive success rates.
In women undergoing IVF, low‐quality evidence, including all of the studies reporting these outcomes, suggests that performing a screening hysteroscopy before IVF may increase live birth and clinical pregnancy rates. However, pooled results from the only two trials with a low risk of bias did not show a benefit of screening hysteroscopy before IVF.
Since the studies showing an effect are those with unclear allocation concealment, we are uncertain whether a routine screening hysteroscopy increases live birth and clinical pregnancy, be it for all women, or those with two or more failed IVF attempts. There is insufficient data to draw conclusions about the safety of screening hysteroscopy.
Keywords: Female; Humans; Pregnancy; Reproductive Techniques, Assisted; Fertilization in Vitro; Hysteroscopy; Hysteroscopy/adverse effects; Hysteroscopy/methods; Infertility, Female; Infertility, Female/diagnosis; Live Birth; Pregnancy Rate; Randomized Controlled Trials as Topic
Plain language summary
Screening hysteroscopy in subfertile women trying to conceive spontaneously, and before in vitro fertilisation
Review question
To assess the safety and usefulness of performing a screening hysteroscopy on reproductive outcomes in women trying to conceive spontaneously, and those undergoing in vitro fertilisation (IVF).
Background
In women with an unexplained problem in becoming pregnant, or those seeking advanced fertility treatment, such as intrauterine insemination or IVF, it has been suggested that performing a hysteroscopy (visualisation of the inside of the womb, using a telescope) may help improve success. The routine ultrasound done during the work‐up may miss smaller abnormalities inside the womb, which may be detected and treated simultaneously by performing a hysteroscopy. It may also increase success by facilitating the subsequent insemination or embryo transfer, by widening the passage to the womb (cervical dilatation), or because of a scratching effect on the endometrium (lining of the womb), which may help to improve embryo implantation (adherence to lining of womb).
Study characteristics
For women wishing to become pregnant spontaneously, we found one trial (200 women). For women undergoing IVF, we included ten trials (3750 women). All trials evaluated the effects of screening hysteroscopy compared to no hysteroscopy. The evidence is current to September 2018.
Key results
In women wishing to become pregnant spontaneously, hysteroscopy was associated with a higher chance for an ongoing and clinical pregnancy in one study at high risk of bias. The trial reported no adverse events following hysteroscopy. The miscarriage rate was higher following hysteroscopy.
In women undergoing IVF, the included studies suggested that performing a screening hysteroscopy first, improved the chances of live birth or clinical pregnancy. However, adverse events following hysteroscopy were poorly reported, and therefore, we were unable to assess the safety of this intervention. For women at a typical clinic with a 22% live birth rate, performing a screening hysteroscopy would be expected to result in live birth rates between 25% and 32%. There was no increased risk of miscarriage following hysteroscopy.
We found no trials with women who were seeking intrauterine insemination.
Quality of the evidence
There was very low‐quality evidence from one study in women who were trying to become pregnant spontaneously.
There was low‐quality evidence that a screening hysteroscopy, performed prior to IVF, may increase the chance of live birth or clinical pregnancy, and very low‐quality evidence about adverse events following hysteroscopy. The quality of the evidence was reduced because of risk of bias and statistical heterogeneity.
Summary of findings
Summary of findings for the main comparison. Screening hysteroscopy versus no hysteroscopy in women wishing to conceive spontaneously.
| Hysteroscopy compared with no hysteroscopy in women wishing to conceive spontaneously | ||||||
|
Patient or population: women with unexplained subfertility wishing to conceive spontaneously Settings: Arafa Hospital, Fayoum, Egypt Intervention: screening hysteroscopy Comparison: no hysteroscopy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no hysteroscopy | Risk with hysteroscopy | |||||
| Live birth | 100 per 1000 | 430 per 1000 (229 to 807) |
RR 4.30 (2.29 to 8.07) |
200 (1 RCT) |
⊕⊕⊝⊝ very lowa,b | |
| Adverse events | 0 per 1000 | 0 per 1000 (0 to 0) |
not estimable | (1 RCT) | ⊕⊝⊝⊝ very lowa,b.c | |
| Clinical pregnancy | 150 per 1000 | 570 per 1000 (347 to 936) |
RR 3.80 (2.31 to 6.24) |
200 (1 RCT) |
⊕⊕⊝⊝ very lowa,b | |
| Miscarriage | 50 per 1000 | 140 per 1000 (52 to 374) |
RR 2.80 (1.05 to 7.48) |
200 (1 RCT) |
⊕⊝⊝⊝ very lowa,b,c | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aVery serious risk of bias, downgraded by two levels: unclear random sequence generation and allocation concealment ‐ high risk of selective outcome reporting bSerious risk of indirectness, downgraded by one level; only one single‐centre study cSerious risk of imprecision, downgraded by one level; no or low number of events
Summary of findings 2. Screening hysteroscopy versus no hysteroscopy in women before IVF.
| Screening hysteroscopy versus no hysteroscopy in women before IVF | ||||||
| Patient or population: women before IVF treatment Setting: academic and private clinics Intervention: screening hysteroscopy Comparison: no hysteroscopy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no hysteroscopy | Risk with hysteroscopy | |||||
| Live birth | 221 per 1000 | 279 per 1000 (245 to 316) | RR 1.26 (1.11 to 1.43) | 2745 (6 RCTs) | ⊕⊕⊝⊝ lowa,b | |
| Adverse events | 0 per 1000 | 0 per 1000 (0 to 0) | Peto OR 7.47 (0.15 to 376.42) | 1872 (4 RCTs) | ⊕⊕⊝⊝ very lowa,c | |
| Clinical pregnancy | 278 per 1000 | 368 per 1000 (334 to 404) | RR 1.32 (1.20 to 1.45) | 3750 (10 RCTs) | ⊕⊕⊝⊝ lowa,d | |
| Miscarriage | 53 per 1000 | 53 per 1000 (35 to 79) | RR 1.01 (0.67 to 1.50) | 1669 (3 RCTs) | ⊕⊕⊝⊝ lowa,e | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aSerious risk of bias, downgraded by one level: only two studies had low risk of bias for all the domains. The rest of the studies had unclear risk for allocation concealment. One study was categorised at high risk for 'other' domain. There is a likely chance of overestimating the treatment. bSerious inconsistency, downgraded by one level: there is a statistical heterogeneity of 69%, which is substantial. cVery serious risk of imprecision, downgraded by two levels: due to wide confidence interval; number of events is too low. dSerious inconsistency, downgraded by one level: there is a statistical heterogeneity of 49%, which is moderate. eSerious risk of imprecision, downgraded by one level: due to wide confidence interval.
Background
Description of the condition
According to the International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization's (WHO) revised glossary of assisted reproductive technology, subfertility is “a disease of the reproductive system, defined by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse, or due to an impairment of a person's capacity, either as an individual, or with his or her partner” (Zegers‐Hochschild 2017). It is estimated that 72.4 million women are subfertile, and that 40.5 million of these are currently seeking fertility treatment (Boivin 2007). A basic subfertility evaluation comprises tests for ovulation, tubal patency, and a transvaginal ultrasound to rule out uterine or ovarian pathology for the female partner, and semen analysis for the male partner (ASRM 2016). The evaluation of the uterine cavity could be considered an important step in the investigation of all subfertile women, as the uterine cavity and its inner layer, the endometrium, are assumed to be important for implantation of the human embryo.
In women undergoing in vitro fertilisation (IVF), despite numerous technological advances, live‐birth rates are between 21% and 25% (Mansour 2014; EIM/ESHRE 2016). Even after transferring euploid embryos following pre‐implantation genetic screening, pregnancy rate is only around 64% (Fiorentino 2014). Embryo implantation remains one of the crucial steps that determines the success of an IVF cycle. The successful implantation is dependent on embryo‐uterine 'cross talk', which is mediated through various factors, such as cytokines, growth factors, and adhesion molecules (Singh 2011; Zhang 2013). Uterine factors, such as polyps and adhesions, may negatively impact the outcomes of IVF (Taylor 2008).
Description of the intervention
Hysteroscopy can both evaluate the uterine cavity for pathology, and either during the same procedure or in a further procedure, treat polyps, adhesions, septa, and fibroids. Screening hysteroscopy is carried out in asymptomatic women, with no detectable uterine cavity abnormalities on pelvic imaging. Hysteroscopy is a commonly performed gynaecological procedure with low complication rates (0.1% to 0.95% (Jansen 2000)). It can be carried out in an outpatient setting without general or regional anaesthesia. Various methods of pain relief are used, such as local, oral, or intravenous analgesia, either alone or in combination (Ahmad 2017). It is considered the gold standard for the diagnosis of uterine cavity pathology (Taylor 2008;Bosteels 2015).
Hysteroscopy allows the direct visualisation of the uterine cavity through a rigid, semi‐rigid, or flexible endoscope. During hysteroscopy, the instrument is passed through the cervix into the uterine cavity. For optimal visualisation, a distension medium, commonly saline, is used to expand the uterine cavity. The hysteroscope consists of a rigid telescope with a proximal eyepiece, and a distal objective lens that can be angled at 0° to allow direct viewing, or offset at various angles to provide a forward‐oblique view. The total working diameters of modern diagnostic hysteroscopes are typically 2.5 to 4.0 mm. Operative hysteroscopy requires adequate visualisation through a continuous fluid circulation using an in‐ and outflow channel. The outer diameters of modern operative hysteroscopes have been reduced to a diameter of between 4.0 and 5.5 mm. The sheath system contains one or two 1.6 to 2.0 mm working channels for the insertion of small biopsy forceps, scissors, retraction loops and morcellators, or unipolar or bipolar electro diathermy instruments.
In clinical practice, evaluation of the uterine cavity is usually done with a transvaginal ultrasound scan (TVS) prior to IVF. Due to the perceived advantages of hysteroscopy over TVS, such as the potential for simultaneous detection and treatment of intrauterine pathologies, use of a pre‐IVF screening hysteroscopy has gained widespread acceptance (Campo 2014).
How the intervention might work
It is assumed that uterine cavity abnormalities may interfere with factors that regulate the embryo‐endometrium interplay, for example, hormones and cytokines, reducing the possibility of pregnancy. Many hypotheses have been formulated in the literature as to how endometrial polyps, submucous fibroids, intrauterine adhesions, and uterine septa may impair implantation of the human embryo; nevertheless, the precise mechanisms of the action through which each one of these cavity abnormalities affects this essential reproductive process are poorly understood.
Screening hysteroscopy in woman prior to IVF may reveal intrauterine pathology that may not be detected by routine TVS. The reported rate of intrauterine pathology is 12% in women undergoing first IVF (Smit 2016), and 27% in women with recurrent implantation failure (RIF) (El‐Toukhy 2016). Hysteroscopy allows detection and treatment of many of these intrauterine pathologies, which may improve IVF outcomes (Oliveira 2003). Cervical dilation during pre‐IVF hysteroscopy may facilitate subsequent embryo transfers, which could possibly improve outcomes. Another proposed mechanism to help improve IVF outcomes following hysteroscopy is local endometrial injury caused during the invasive procedure. The inflammatory reaction following endometrial injury leads to a release of cytokines and growth factors, which may help implantation and improve clinical pregnancy rates following IVF (Barash 2003; Nastri 2015).
Why it is important to do this review
Although detection of intrauterine pathologies in women with normal TVS prior to IVF is perceived as one of the benefits of performing hysteroscopy, we wish to evaluate whether treating these pathologies improves outcomes following IVF (Oliveira 2003; Pundir 2014; Smit 2016). Current guidelines do not advocate the routine use of screening hysteroscopy during the initial infertility work‐up (Crosignani 2000; NICE 2013). Due to uncertainty about the role of screening hysteroscopy in women with normal TVS during infertility work‐up, and prior to IVF, it is important to conduct a systematic appraisal of the current evidence.
Objectives
To assess the effectiveness and safety of screening hysteroscopy in subfertile women undergoing evaluation for infertility and subfertile women undergoing intrauterine insemination (IUI) or in vitro fertilisation (IVF).
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCT) were eligible for inclusion. We excluded non‐randomised studies and quasi‐randomised trials.
Types of participants
Subfertile women, with otherwise unexplained infertility, in whom routine imaging did not show uterine cavity abnormalities, who wished to conceive spontaneously
Subfertile women, in whom routine imaging did not show uterine cavity abnormalities, and before treatment with IUI.
Women in whom routine imaging did not show uterine cavity abnormalities, and before treatment with IVF.
We excluded subfertile women with suspected uterine cavity abnormalities (present on any imaging techniques), as this topic is covered by another Cochrane review (Bosteels 2015).
Types of interventions
We included the following three randomised comparisons:
A routine screening hysteroscopy, with or without treatment of any detected uterine cavity abnormalities, versus no hysteroscopy, in subfertile women wishing to conceive spontaneously.
A routine screening hysteroscopy, with or without treatment of any detected uterine cavity abnormalities, versus no hysteroscopy, before intrauterine insemination (IUI).
A routine screening hysteroscopy, with or without treatment of any detected uterine cavity abnormalities, versus no hysteroscopy, before in vitro fertilisation (IVF).
Types of outcome measures
Primary outcomes
Live birth or (in studies that do not report live birth) ongoing pregnancy. The live‐birth delivery rate (whether or not after assisted reproduction) was defined as delivery of a live foetus after 20 completed weeks of gestational age. We counted the delivery of singleton, twin, or multiple pregnancies as one live birth. The ongoing pregnancy rate (whether or not after assisted reproduction) was defined as evidence of a gestational sac with foetal heart motion at 12 weeks, confirmed by ultrasound. We counted multiple gestational sacs as one ongoing pregnancy. We used ongoing pregnancy as a surrogate outcome for live birth.
Adverse events: the incidence of complications due to the hysteroscopy procedure, analysed as a composite measure of any adverse events (including perforation, infection, vasovagal attacks).
Secondary outcomes
Clinical pregnancy rate (whether or not after assisted reproduction), defined as ultrasound evidence of a gestational sac.
Miscarriage rate (whether or not after assisted reproduction), defined as the spontaneous loss of a clinical pregnancy that occurred before 20 completed weeks of gestation (18 weeks post‐fertilisation) or, if gestational age was unknown, the loss of an embryo or foetus of less than 400 grams.
We did not exclude studies on the basis of their reported outcome measures. We reported any lack of data for key outcomes in the final results and discussion.
Search methods for identification of studies
We searched for all published and unpublished RCTs of routine hysteroscopy in infertile women, without language restriction, and in consultation with the Cochrane Gynaecology and Fertility Group (CGF) Information Specialist.
Electronic searches
We searched the following electronic databases, trial registers, and web sites from inception to 05 September 2018:
Cochrane CGF Specialised Register, ProCite platform, searched 05 September 2018 (Appendix 1);
Cochrane Central Register of Studies (CENTRAL CRSO), Web platform, searched 05 September 2018 (Appendix 2);
MEDLINE, Ovid platform, searched from 1946 to 05 September 2018 (Appendix 3);
Embase, Ovid platform, searched from 1980 to 05 September 2018(Appendix 4);
PsycINFO, Ovid platform, searched from 1806 to 05 September 2018 (Appendix 5);
CINAHL (Cumulative Index to Nursing and Allied Health Literature), EBSCO platform, searched from 1961 to 05 September 2018 (Appendix 6);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization International Trials Registry Platform (www.who.int/trialsearch/Default.aspx);
DARE (Database of Abstracts of Reviews of Effects) on the Cochrane Library for reference lists from relevant non‐Cochrane reviews (onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html);
Web of Knowledge (another source of trials and conference abstracts (wokinfo.com/));
OpenGrey for unpublished literature from Europe (www.opengrey.eu/);
LILACS (Latin American and Caribbean Health Science Information database) (regional.bvsalud.org/php/index.php?lang=en);
PubMed and Google for recent trials not yet indexed in MEDLINE.
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials, which appears in Section 6.4.11 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebre 2011).
We combined the Embase, PsycINFO, and CINAHL searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN (www.sign.ac.uk/search‐filters.html)).
Searching other resources
Four review authors (JB, SS for non‐IVF comparisons; MSK, SKS for IVF comparison) handsearched reference lists of articles retrieved by the search, and contacted experts in the field to obtain additional data. We also handsearched relevant journals and conference abstracts that were not covered in the GFG register, in liaison with the Information Specialist.
Data collection and analysis
Selection of studies
Two review authors (MSK, SKS) conducted an initial screen of titles and abstracts identified by the search, after which we retrieved the full texts of all potentially eligible studies. Four review authors (SS, JB, SKS, MSK) independently examined these full‐text articles for compliance with the inclusion criteria, and selected studies eligible for inclusion in the review. We corresponded with study investigators as required to clarify study eligibility. Disagreements as to study eligibility were resolved by discussion or by a fifth review author (BWJM). We documented the selection process with a PRISMA flow chart.
Data extraction and management
Four review authors (JB, MSK, SKS, SS) independently extracted data from eligible studies using a data extraction form designed and pilot‐tested by the review authors. Any disagreement was resolved by discussion or by a fifth review author (BWJM). We extracted data that included study characteristics and outcome data (Appendix 7). We corresponded with study investigators for further data on methods or results, or both, as required. We included studies irrespective of whether outcomes were reported in a 'usable' way.
Assessment of risk of bias in included studies
Four review authors (JB, SS for non‐IVF comparisons; MSK, SKS for IVF comparison) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). We assessed the following items: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. Disagreements were resolved by discussion or by a fifth review author. We described all judgements fully, and presented the conclusions in the 'Risk of bias' table, which were incorporated into the interpretation of review findings by means of sensitivity analyses (see Sensitivity analysis). Selective reporting is a type of reporting bias that affects the internal validity of an individual study. It refers to the selective reporting of some outcomes (e.g. positive outcomes) and the failure to report others (e.g. adverse events). We took care to search for within‐trial selective reporting, such as trials failing to report obvious outcomes, or reporting them in insufficient detail to allow inclusion. We compared the outcomes between the published protocol and the final published study. Where identified studies failed to report the primary outcome of live birth, but did report interim outcomes, such as clinical pregnancy, we undertook informal assessment as to whether the interim values (e.g. pregnancy rates) were similar to those reported in studies that also reported live birth.
Measures of treatment effect
For dichotomous data (e.g. live‐birth rates), we used the number of events in the control and intervention groups of each study to calculate risk ratios (RR). We used Peto odds ratio (OR) for outcomes with low event rates. We reversed the direction of effect of individual studies, if required, to ensure consistency across trials. We presented 95% confidence intervals (CI) for all outcomes. We compared the magnitude and direction of effect reported by studies with how they were presented in the current review, taking account of legitimate differences.
Unit of analysis issues
The primary analysis was per woman randomised; we included per pregnancy data for some outcomes (for the outcome miscarriage). We counted multiple live births (e.g. twins or triplets) as one live‐birth event.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis to the greatest degree possible, and attempted to obtain missing data from the original authors. We assumed live births or clinical pregnancies would not be present in women without a reported outcome. For other outcomes, we analysed only the available data.
For Imputated data, we had planned to conduct sensitivity analysis (see Sensitivity analysis).
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity using the I² statistic. An I² measurement greater than 50% indicated substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the review authors aimed to minimise the potential impact of these biases by ensuring a comprehensive search for eligible studies, and by being alert for duplication of data. Since there were fewer than 10 studies in each population, we did not use a funnel plot.
Data synthesis
Four review authors (JB, SS for non‐IVF comparisons; MSK, SKS for IVF comparison) entered the data and performed the statistical analysis using Review Manager 5 (RevMan 2014). We combined the data using a fixed‐effect model for the following comparisons.
Hysteroscopy versus no hysteroscopy for subfertile women wishing to conceive spontaneously
Hysteroscopy versus no hysteroscopy for subfertile women undergoing IUI
Hysteroscopy versus no hysteroscopy for women undergoing IVF
We displayed an increase in the odds of a particular outcome, which may be beneficial (e.g. live birth) or detrimental (e.g. adverse effects of the hysteroscopy) graphically in the meta‐analyses to the right of the centre‐line, and a decrease in the odds of an outcome to the left of the centre‐line.
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analysis:
For women undergoing IVF:
First IVF versus two or more IVF failures.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcome live birth and an important secondary outcome (clinical pregnancy rate) to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
eligibility had been restricted to studies without high or unclear risk of bias in any domain;
a random‐effects model had been adopted;
alternative imputation strategies had been implemented;
the summary effect measure had been odds ratio rather than risk ratio;
the primary outcome had been limited to live birth.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro GDT and Cochrane methods (GRADEpro GDT; Higgins 2011). This table evaluated the overall quality of the body of evidence for all review outcomes (live birth, adverse events, clinical pregnancy, and miscarriage), for the following comparisons: a screening hysteroscopy versus no hysteroscopy in subfertile women wishing to conceive spontaneously; and a screening hysteroscopy versus no hysteroscopy in women before IVF. We assessed the quality of the evidence using GRADE criteria (risk of bias, consistency of effect, imprecision, indirectness, and publication bias). Two review authors independently made judgements about evidence quality (high, moderate, low, or very low), with any disagreements resolved by discussion. They justified, documented, and incorporated the judgments into report of results for each outcome.
We extracted study data, formatted our comparisons in data tables, and prepared a 'Summary of findings' table before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
We ran our electronic search on 05 September 2018. The targeted search resulted in 761 records, out of which 227 were duplicate records. Two teams of review authors (JB and SS; MSK and SKS) screened the records simultaneously and independently, and examined the titles and abstracts to identify potentially eligible studies among the remaining 534 records.
After independent assessment, two authors (JB, SS) found five potentially eligible studies for the randomised comparisons in subfertile women wishing to conceive spontaneously, or before intrauterine insemination (IUI); we only included one trial (Seyam 2015). One study is awaiting classification (Moramezi 2012). We excluded three trials for not addressing the research question of interest (Brown 2000; El‐Khayat 2015; Shokeir 2016). See the 'Characteristics of excluded studies' tables.
Two authors (MSK, SKS) independently assessed studies that evaluated screening hysteroscopy before in vitro fertilisation (IVF), and found 24 records of potentially eligible studies. We excluded seven trials (Fatemi 2010; Wang 2011; Kasius 2013; Kamel 2015; Zhang 2015; Hebeisha 2018; Siristatidis 2017), and included ten trials in the review (Demirol 2004; Rama Raju 2006; El‐Nashar 2011; Aghahosseini 2012; Shawki 2012; Elsetohy 2015; El‐Toukhy 2016; Smit 2016; Alleyassin 2017; Juul Hare 2018). For two included trials, there were two published protocols and one conference abstract (Smit 2016; El‐Toukhy 2016). We identified four ongoing trials (NCT02245750; NCT03173404; PACTR201402000691997; UMIN000025679). See the 'Characteristics of ongoing studies' tables.
When combining all the populations evaluated under this review, we found 26 potentially eligible studies, out of which we included 11 trials for quantitative synthesis. The search results are summarized in the PRISMA flow chart (Figure 1).
1.

Study flow diagram
Included studies
See 'Characteristics of included studies' tables.
Design
We included a total of 11 randomised controlled trials (RCT) in the review. Out of these 11 included trials, eight were completed and published as full articles (Demirol 2004; Rama Raju 2006; Shawki 2012; Elsetohy 2015; Seyam 2015; El‐Toukhy 2016; Smit 2016; Alleyassin 2017), and three were conference abstracts (El‐Nashar 2011; Aghahosseini 2012; Juul Hare 2018). Three were multicentre trials, conducted in the Netherlands (Smit 2016), European centres (El‐Toukhy 2016), and Denmark (Juul Hare 2018). The remaining eight trials were single centre trials. Two were conducted in Iran (Aghahosseini 2012; Alleyassin 2017), four in Egypt (El‐Nashar 2011; Shawki 2012; Elsetohy 2015; Seyam 2015), one in India (Rama Raju 2006), and one in Turkey (Demirol 2004).
One of the studies was partly funded by the European Society of Human Reproduction and Embryology, the European Society for Gynaecological Endoscopy (ESGE), and the Karl Storz Company provided the hysteroscopy equipment for all centres (El‐Toukhy 2016). The Dutch Organisation for Health Research and Development (ZonMW) funded the other multicentre trial (Smit 2016). The rest of the trials did not acknowledge any funding support.
Participants
Seyam 2015 enrolled 200 women with unexplained subfertility who attended a single centre in Egypt. The basic work‐up included a hysterosalpingogram (HSG) and a transvaginal ultrasound to screen for uterine pathology and tubal patency. The authors of this RCT did not report whether or not a prior hysteroscopy or a concomitant endometrial biopsy were done. We could not obtain further clarification.
The characteristics of the participants among the IVF population are shown in Table 3. Three trials included subfertile women undergoing their first IVF (Elsetohy 2015; Smit 2016; Alleyassin 2017). Four trials included women with two or more IVF failures (Demirol 2004; Rama Raju 2006; Aghahosseini 2012; El‐Toukhy 2016). Two trials included an unselected IVF population (El‐Nashar 2011; Shawki 2012). One trial included women with one IVF failure who were undergoing their second IVF treatment (Juul Hare 2018). In six trials, participants had an additional radiological procedure, in the form of a HSG, as an inclusion criteria (Demirol 2004; Rama Raju 2006; Aghahosseini 2012; Shawki 2012; Elsetohy 2015; Alleyassin 2017). Two trials excluded participants with a history of previous hysteroscopy (Smit 2016; Alleyassin 2017); and one trial included women with a previous history of hysteroscopy in both the intervention and control arms (El‐Toukhy 2016).
1. Cycle characteristics of included trials.
| Study ID | Population | Previous HSG or hysteroscopy | Intervention | Additional endometrial biopsy | Detected abnormalities treated | Timing of hysteroscopy |
| Seyam 2015 | Unexplained infertility of 2 years duration | Normal HSG | Hysteroscopy | No | Yes | Follicular phase |
| Aghahosseini 2012 | Two or more IVF failures | Normal HSG; no hysteroscopy in previous 2 months | Hysteroscopy | No | No details | No details available |
| Alleyassin 2017 | First IVF | Normal HSG; women with history of hysteroscopy excluded. | Hysteroscopy | No | Yes | Preceding cycle; luteal phase |
| Demirol 2004 | Two or more IVF failures | Normal HSG; no details of history of hysteroscopy | Hysteroscopy | No | Yes | Preceding cycle; follicular phase |
| El‐Nashar 2011 | Unselected IVF population | No details of HSG; no details of history of hysteroscopy | Hysteroscopy | Yes | Yes | No details |
| Elsetohy 2015 | First IVF | Normal HSG; no details of history of hysteroscopy | Hysteroscopy | No | Yes | Within 3 months of IVF; follicular phase |
| El‐Toukhy 2016 | Two or more IVF failures | Hysteroscopy done within two months were excluded; Included women with previous history of hysteroscopy (45% in hysteroscopy group vs 44% in control) | Hysteroscopy | No | Yes; (only one partial septum not treated). |
Preceding cycle; follicular phase |
| Juul Hare 2018 | One IVF failure | No details available | Hysteroscopy | Yes | No details available | No details available |
| Rama Raju 2006 | Two or more IVF failures | Normal HSG; no details of history of hysteroscopy | Hysteroscopy | Yes | Yes | Preceding cycle; follicular phase |
| Shawki 2012 | Unselected IVF population | Normal HSG; no details of history of hysteroscopy | Hysteroscopy | Those with suspicious lesion after injecting methylene blue were biopsied. | Yes | No details available |
| Smit 2016 | First IVF | Excluded those with previous hysteroscopy | Hysteroscopy | No | Yes; (31/43 abnormalities treated; 5 septum and 2 submucous fibroids not treated) | 1 to 3 months before IVF; follicular phase |
IVF: in vitro fertilisation
HSG: hysterosalpingography
Interventions
Seyam 2015 randomly compared office microhysteroscopy versus no hysteroscopy in women with unexplained subfertility, for a mean duration of two years. Hysteroscopy was done in the follicular phase. When pathology was detected, treatment was done, including hysteroscopic resection of endometrial polyps and submucous fibroids, and excision of a uterine septum.
Cycle characteristics of included studies of an IVF population are shown in (Table 3). Among the IVF population, the hysteroscopy was performed in the luteal phase in one trial (Alleyassin 2017), and in the follicular phase in five trials (Demirol 2004; Rama Raju 2006; Elsetohy 2015; El‐Toukhy 2016; Smit 2016). In four trials, the IVF was performed in the immediate cycle following hysteroscopy (Demirol 2004; Rama Raju 2006; El‐Toukhy 2016; Alleyassin 2017); while in two trials, IVF was performed within one to three months after hysteroscopy (Elsetohy 2015; Smit 2016). No information on timing of hysteroscopy was available for four trials (El‐Nashar 2011; Aghahosseini 2012; Shawki 2012; Juul Hare 2018).
In three trials, hysteroscopy was combined with endometrial biopsy in the intervention arm, but no procedure was done in the control arm (Rama Raju 2006; El‐Nashar 2011; Juul Hare 2018). In six trials, hysteroscopy was done in the intervention arm, while no intervention was done in control arm (Demirol 2004; Aghahosseini 2012; Elsetohy 2015; El‐Toukhy 2016; Smit 2016; Alleyassin 2017). In one trial, investigators performed an endometrial biopsy of suspicious lesions after injecting methylene blue in the hysteroscopy arm, while no intervention was done in the control arm (Shawki 2012).
In six trials, investigators performed a hysteroscopy and treated all of the detected intracavitary abnormalities prior to IVF in the intervention arm (Demirol 2004; Rama Raju 2006; El‐Nashar 2011; Shawki 2012; Elsetohy 2015; Alleyassin 2017). In two trials, some of the intracavitary abnormalities detected during hysteroscopy were treated in the intervention arm, while some were not treated (El‐Toukhy 2016; Smit 2016). In two trials, it was not clear if detected abnormalities were treated (Aghahosseini 2012; Juul Hare 2018).
Outcomes
Seyam 2015 did not report the primary outcome of live birth; the study report indicated the assessment of the cumulative ongoing pregnancy rate, although ongoing pregnancy was not defined. We used the data for this outcome as a surrogate for live birth. This study assessed an outcome measure ‐ patient compliance ‐ which was not of interest to this Cochrane Review.
Among the IVF population, six trials reported the primary outcome of live birth (Rama Raju 2006; Aghahosseini 2012; Elsetohy 2015; El‐Toukhy 2016; Smit 2016; Juul Hare 2018). Four trials reported the primary outcome of adverse events (Elsetohy 2015; El‐Toukhy 2016; Smit 2016; Juul Hare 2018). All ten trials reported clinical pregnancy rate as an outcome.
Excluded studies
We excluded three trials for not addressing the research questions of interest for the randomised comparisons in subfertile women wishing to conceive spontaneously or before IUI (Brown 2000; El‐Khayat 2015; Shokeir 2016). See 'Characteristics of excluded studies' tables.
Among the IVF population, we excluded seven trials in ten records (Fatemi 2010; Wang 2011; Kasius 2013; Kamel 2015; Zhang 2015; Hebeisha 2018; Siristatidis 2017). Two trials were non‐randomised, and hence, excluded (Kamel 2015; Siristatidis 2017). One was a prevalence study (Fatemi 2010), and another was a cost analysis study (Kasius 2013). One study was excluded due to a different study population (women with endometritis (Wang 2011)), and another study was excluded because hysteroscopy was performed in both the intervention and control arms, and those with intracavitary abnormalities were excluded (Zhang 2015). In another trial, the investigators mainly evaluated the role of endometrial scratch before Intracytoplasmic sperm injection (ICSI), and excluded women who were found to have intracavitary abnormalities during hysteroscopy (Hebeisha 2018).
Risk of bias in included studies
We assessed the included studies for methodological quality using the Cochrane ’Risk of bias’ tool (Higgins 2011). See the ’Risk of bias’ graph (Figure 2), and ’Risk of bias’ summary (Figure 3).
2.
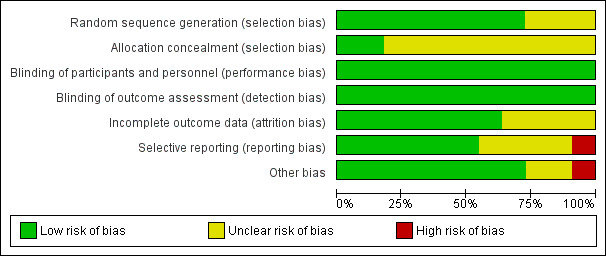
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
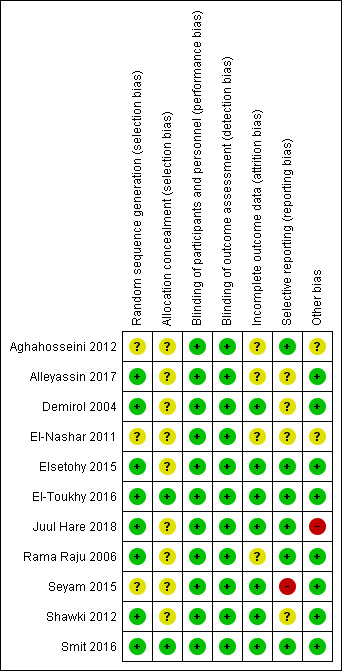
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Generation of random sequence
Eight studies used adequate methods for random sequence generation and were at low risk of selection bias (Demirol 2004; Rama Raju 2006; Shawki 2012; Elsetohy 2015; El‐Toukhy 2016; Smit 2016; Alleyassin 2017; Juul Hare 2018). Two multicentre trials among these seven studies used an intended third party trial management system (El‐Toukhy 2016), and web‐based randomisation (Smit 2016). The remaining three studies did not report the method used for randomisation clearly, and we categorised them as unclear risk of bias (El‐Nashar 2011; Aghahosseini 2012; Seyam 2015).
Allocation concealment
Only two studies clearly stated the method of allocation concealment and we rated them at low risk of bias (El‐Toukhy 2016; Smit 2016). The remaining nine trials did not state the method of allocation concealment clearly, and we assessed them as unclear risk of bias (Demirol 2004; Rama Raju 2006; El‐Nashar 2011; Aghahosseini 2012; Shawki 2012; Elsetohy 2015; Seyam 2015; Alleyassin 2017; Juul Hare 2018).
Blinding
Most of the studies did not report blinding of either clinician or participant. One study mentioned blinding of clinician and embryologist (Alleyassin 2017), and one study mentioned blinding of embryologist and researcher for allocated group (El‐Toukhy 2016). Since the outcomes (live birth, complications following hysteroscopy, ongoing and clinical pregnancy) were objective, we did not downgrade the quality of the studies for lack of blinding. Further, It was unlikely that the hysteroscopies were not performed according to the required standards, due to a lack of blinding. For these stated reasons, we deemed all the included studies to be at low risk of this bias.
Incomplete outcome data
Seven studies were deemed to be at low risk of attrition bias (Demirol 2004; Shawki 2012; Elsetohy 2015; Seyam 2015; El‐Toukhy 2016; Smit 2016; Juul Hare 2018). Four studies did not mention about dropouts, and not enough information was available to make a judgement, hence, we deemed them as unclear risk of bias (Rama Raju 2006; El‐Nashar 2011; Aghahosseini 2012; Alleyassin 2017).
Selective reporting
We judged Seyam 2015 to be at high risk of selective outcome reporting (live birth, primary outcome of interest not reported, even though study duration was long enough (seven years), giving sufficient time for authors to capture live birth data). We judged six trials evaluating women undergoing IVF at low risk of bias for selective reporting (Rama Raju 2006; Aghahosseini 2012; Elsetohy 2015; El‐Toukhy 2016; Smit 2016; Juul Hare 2018), and four trials as unclear risk of bias, since live birth was not reported (Demirol 2004; El‐Nashar 2011; Shawki 2012; Alleyassin 2017).
Other potential sources of bias
Two trials, published as conference abstracts, did not have enough information available for us to judge, hence, we deemed both of these studies as unclear risk of bias (El‐Nashar 2011; Aghahosseini 2012). One of these trials had an uneven distribution of randomised participants (142 versus 211), with substantially higher control numbers, with no clear available explanation (Aghahosseini 2012). We deemed another trial, which was also only available as a conference abstract, as high risk for this domain, due to premature termination of the trial. The authors made the decision due to slow recruitment (Juul Hare 2018). We did not observe any potential source of bias in the remaining eight trials, and deemed them to be at low risk (Demirol 2004; Rama Raju 2006; Shawki 2012; Elsetohy 2015; Seyam 2015; El‐Toukhy 2016; Smit 2016; Alleyassin 2017).
Effects of interventions
1. A routine screening hysteroscopy, including hysteroscopic treatment of any detected uterine cavity abnormalities, versus no hysteroscopy, in subfertile women wishing to conceive spontaneously
Primary outcomes
1.1 Live birth rate
Seyam 2015 reported data on ongoing pregnancies: we used these data as a surrogate outcome for the primary outcome of effectiveness, the live birth rate. There is very low‐quality evidence, and we are uncertain whether ongoing pregnancy rate improves following screening hysteroscopy in women with at least two years of unexplained subfertility compared to no hysteroscopy (risk ratio (RR) 4.30, 95% confidence interval (CI) 2.29 to 8.07; 1 RCT; participants = 200; Analysis 1.1; Figure 4). If 10% of women achieve an ongoing pregnancy without hysteroscopy, the evidence suggests that 43% of women (95% CI 23% to 81%) will achieve an ongoing pregnancy after hysteroscopy.
1.1. Analysis.
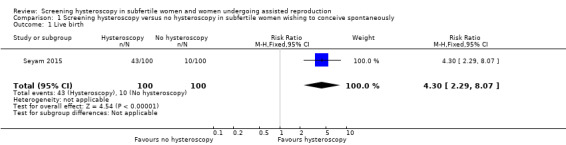
Comparison 1 Screening hysteroscopy versus no hysteroscopy in subfertile women wishing to conceive spontaneously, Outcome 1 Live birth.
4.
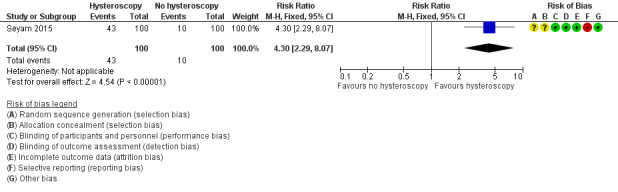
Forest plot of comparison 1. Screening hysteroscopy versus no hysteroscopy in subfertile women. Outcome 1.1. Ongoing pregnancy was used as a surrogate outcome for live birth
1.2 Adverse events
Seyam 2015 reported no adverse events in either treatment arm. The effect estimate is not estimable (Analysis 1.2).
1.2. Analysis.
Comparison 1 Screening hysteroscopy versus no hysteroscopy in subfertile women wishing to conceive spontaneously, Outcome 2 Adverse outcomes.
Secondary outcomes
1.3 Clinical pregnancy rate
There is very low‐quality evidence, and we are uncertain whether clinical pregnancy rate improves following screening hysteroscopy in women with at least two years of unexplained subfertility compared to no hysteroscopy (RR 3.80, 95% CI 2.31 to 6.24; 1 RCT; participants = 200; Analysis 1.3). If 15% of women achieve a clinical pregnancy without hysteroscopy, the evidence suggests that 57% of women (95% CI 35% to 94%) will achieve an clinical pregnancy after hysteroscopy.
1.3. Analysis.
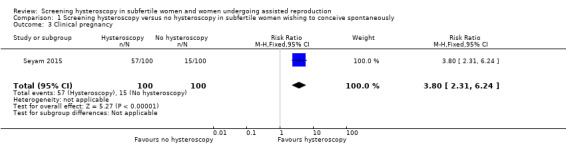
Comparison 1 Screening hysteroscopy versus no hysteroscopy in subfertile women wishing to conceive spontaneously, Outcome 3 Clinical pregnancy.
1.4 Miscarriage rate
There is very low‐quality evidence, and we are uncertain whether miscarriage rate increases following screening hysteroscopy compared to no hysteroscopy in women with at least two years of unexplained subfertility compared to no hysteroscopy (RR 2.80, 95% CI 1.05 to 7.48; 1 RCT; participants = 200; Analysis 1.4).
1.4. Analysis.
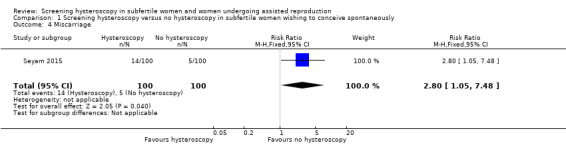
Comparison 1 Screening hysteroscopy versus no hysteroscopy in subfertile women wishing to conceive spontaneously, Outcome 4 Miscarriage.
Sensitivity analyses
Sensitivity analyses on the choice of the summary effect measure (OR versus RR versus RD) or the analysis model (random‐effects versus fixed‐effect model) did not demonstrate differences of the direction of the treatment effect or the statistical significance tests for the outcomes live birth and clinical pregnancy.
2. Hysteroscopy versus no hysteroscopy for subfertile women undergoing intrauterine insemination (IUI)
We found no trials that investigated this comparison.
3. A routine screening hysteroscopy, including hysteroscopic treatment of any detected uterine cavity abnormalities, versus no hysteroscopy before in vitro fertilisation (IVF)
We pooled results from ten trials (3750 women) for this comparison (Demirol 2004; Rama Raju 2006; El‐Nashar 2011; Aghahosseini 2012; Shawki 2012; Elsetohy 2015; El‐Toukhy 2016; Smit 2016; Alleyassin 2017). Investigators of three trials reported performing endometrial biopsy along with hysteroscopy in the intervention arm (Rama Raju 2006; El‐Nashar 2011; Juul Hare 2018).
Primary outcomes
3.1 Live birth rate
Six trials reported live birth rate. Low quality‐evidence indicates that performing screening hysteroscopy before IVF may increase live birth rate compared to no hysteroscopy (RR 1.26, 95% CI 1.11 to 1.43; 6 RCTs; participants = 2745, I² = 69%; Analysis 2.1; Figure 5). For a typical clinic with 22% live birth rate, performing a screening hysteroscopy would be expected to result in live birth rates between 25% and 32%.
2.1. Analysis.
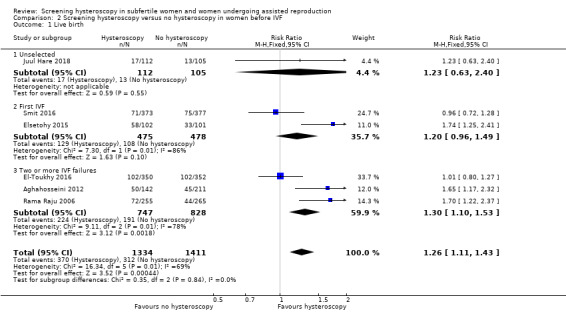
Comparison 2 Screening hysteroscopy versus no hysteroscopy in women before IVF, Outcome 1 Live birth.
5.
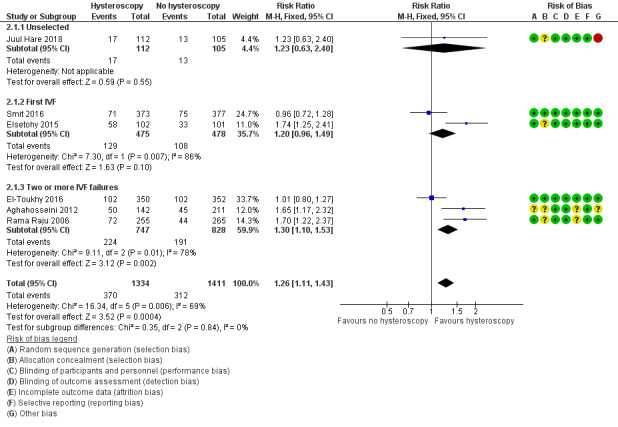
Forest plot of comparison 2. Screening hysteroscopy versus no hysteroscopy in women under IVF. Outcome 2.1. Live birth.
Sensitivity analysis
We performed sensitivity analysis, and after removing two trials in which an additional procedure (endometrial biopsy) was performed along with hysteroscopy, the result did not change (Rama Raju 2006; Juul Hare 2018): there was an increase in live birth rate in the intervention group (RR 1.19, 95% CI 1.03 to 1.37; 4 RCTs; participants = 2008; I² = 77%). When we removed four trials with unclear or high risk for bias for any domain (Rama Raju 2006; Aghahosseini 2012; Elsetohy 2015; Juul Hare 2018), the pooled result showed no increase in live birth rate following screening hysteroscopy (RR 0.99, 95% CI 0.82 to 1.18; 2 RCTs; participants = 1452; I² = 0%). Sensitivity analyses on the choice of the summary effect measure (OR versus RR ), or the analysis model (fixed‐effect versus random‐effects model) did not demonstrate differences in the direction of the treatment effect, or the statistical significance tests.
Subgroup analysis
We conducted a priori subgroup analyses based on the number of IVF attempts for: an unselected IVF population, before first IVF attempt, and after two or more IVF failures. It showed no evidence of a difference between the subgroups; test for subgroup differences: Chi² = 0.35, df = 2 (P = 0.84), I² = 0%.
3.1.1 Live birth rate in unselected population
There was no evidence of a difference in live birth rate following screening hysteroscopy versus no hysteroscopy in an unselected IVF population (RR 1.23, 95% CI 0.63 to 2.40; 1 RCT; participants = 217; I² = not applicable; Analysis 2.1; Figure 5).
3.1.2 Live birth rate before first IVF
There was no evidence of a difference in live birth rate following screening hysteroscopy versus no hysteroscopy in women undergoing their first IVF (RR 1.20, 95% CI 0.96 to 1.49; 2 RCTs; participants = 953; I² = 86%; Analysis 2.1; Figure 5).
2.1.3 Live birth rate after two or more IVF failures
There was an increase in live birth rate following screening hysteroscopy versus no hysteroscopy in women with two or more IVF failures (RR 1.30, 95% CI 1.10 to 1.53; 3 RCTs; participants = 652; I² = 78%; Analysis 2.1; Figure 5).
3.2 Adverse events
Four trials reported adverse events. Three trials did not report any adverse events following hysteroscopy (Elsetohy 2015; El‐Toukhy 2016; Juul Hare 2018). One trial reported a case of endometritis in the hysteroscopy arm (Smit 2016). There is very low‐quality evidence, and we are uncertain whether screening hysteroscopy is associated with higher adverse events versus no hysteroscopy (Peto odds ratio (OR) 7.47, 95% CI 0.15 to 376.42; 4 RCTs; participants = 1872; I² = not applicable; Analysis 2.2).
2.2. Analysis.
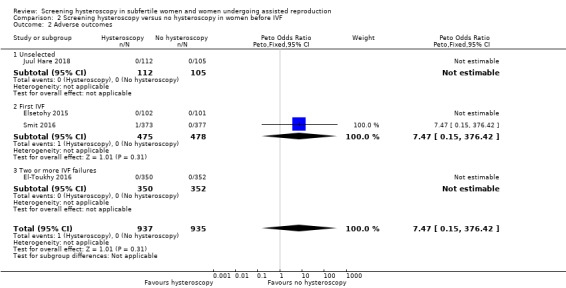
Comparison 2 Screening hysteroscopy versus no hysteroscopy in women before IVF, Outcome 2 Adverse outcomes.
Secondary outcomes
3.3 Clinical pregnancy rate
Ten trials reported clinical pregnancy rate. Low‐quality evidence indicates that performing screening hysteroscopy before IVF may increase clinical pregnancy rate compared to no hysteroscopy (RR 1.32, 95% CI 1.20 to 1.45; 10 RCTs; participants = 3750; I² = 49%; Analysis 2.3; Figure 6). For a typical clinic with 28% clinical pregnancy rate, performing a screening hysteroscopy would be expected to result in clinical pregnancy rates between 33% and 40%.
2.3. Analysis.
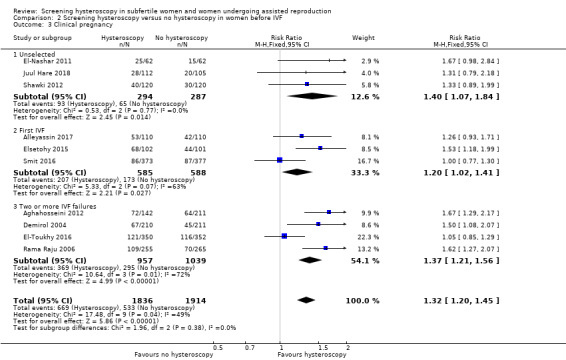
Comparison 2 Screening hysteroscopy versus no hysteroscopy in women before IVF, Outcome 3 Clinical pregnancy.
6.
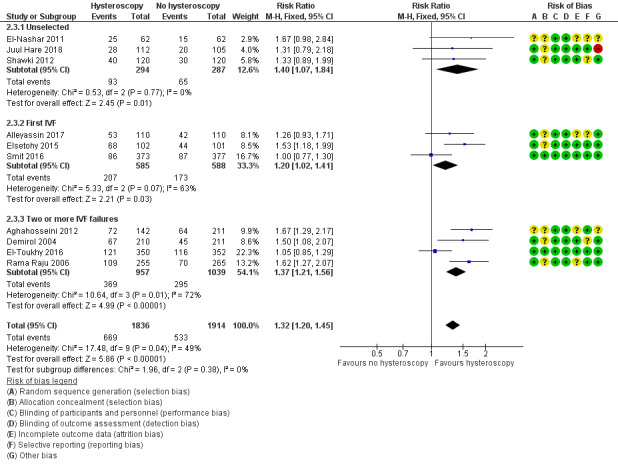
Forest plot of comparison 2. Screening hysteroscopy versus no hysteroscopy in women under IVF. Outcome 2.3. Clinical pregnancy
Sensitivity analysis
We conducted a sensitivity analysis by removing eight trials with unclear or high risk for bias for any domain (Demirol 2004; Rama Raju 2006; El‐Nashar 2011; Aghahosseini 2012; Shawki 2012; Elsetohy 2015; Alleyassin 2017; Juul Hare 2018), which showed no increase in clinical pregnancy rate following screening hysteroscopy (RR 1.03, 95% CI 0.87 to 1.21; 2 RCTs; participants = 1452; I² = 0%). Sensitivity analyses on the choice of the summary effect measure (OR versus RR ), or the analysis model (fixed‐effect versus random‐effects model), did not demonstrate differences in the direction of the treatment effect, or the statistical significance tests.
Subgroup analysis
We conducted subgroup analysis according to the number of IVF attempts. It showed no evidence of a difference between the subgroups: test for subgroup differences: Chi² = 1.96, df = 2 (P = 0.38), I² = 0%.
2.3.1 Clinical pregnancy rate in unselected IVF population
There was an increase in clinical pregnancy rate following screening hysteroscopy in an unselected IVF population (RR 1.40, 95% CI 1.07 to 1.84; 3 RCTs; participants = 581; I² = 0%; Analysis 2.3; Figure 6).
2.3.2 Clinical pregnancy rate before first IVF
There was an increase in clinical pregnancy rate following screening hysteroscopy before first IVF (RR 1.20, 95% CI 1.02 to 1.41; 3 RCTs; participants = 1173; I² = 63%; Analysis 2.3; Figure 6).
2.3.3 Clinical pregnancy rate after two or more IVF failures
There was an increase in clinical pregnancy rate following screening hysteroscopy in women with two or more IVF failures (RR 1.37, 95% CI 1.21 to 1.56; 4 RCTs; participants = 1996; I² = 72%; Analysis 2.3; Figure 6).
3.4 Miscarriage rate per woman randomised
Three trials reported miscarriage rate. Low‐quality evidence indicates that there may be little or no difference in miscarriage rate following screening hysteroscopy versus no hysteroscopy (RR 1.01, 95% CI 0.67 to 1.50; 3 RCTs; participants = 1669; I² = 0%; Analysis 2.4). There may be little or no difference in miscarriage rate per pregnancy between the two groups (RR 0.97, 95% CI 0.67 to 1.40).
2.4. Analysis.
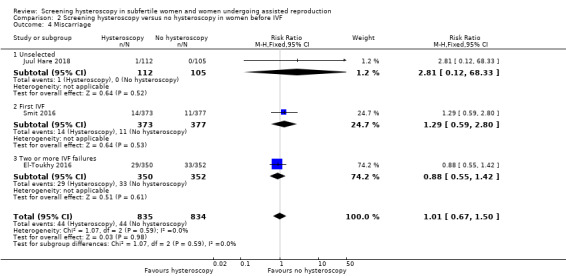
Comparison 2 Screening hysteroscopy versus no hysteroscopy in women before IVF, Outcome 4 Miscarriage.
Subgroup analysis, according to the number of IVF attempts, showed no evidence of a difference between the subgroups; test for subgroup differences: Chi² = 1.07, df = 2 (P = 0.59), I² = 0%.
In the subgroup analysis, there was one trial each under unselected population, first IVF, and two or more IVF failures, and we noted little or difference between the miscarriage rate between the two groups.
Discussion
Summary of main results
Screening hysteroscopy versus no hysteroscopy in subfertile women wishing to conceive spontaneously
We retrieved only one single‐centre study, which reported ongoing pregnancy as a surrogate outcome for live birth. There was very low‐quality evidence, and we are uncertain whether a screening hysteroscopy improves the ongoing or clinical pregnancy rates in women with at least two years of unexplained subfertility compared to no hysteroscopy (Table 1). The included trial reported no adverse events (complications following hysteroscopy) in either comparison arm. Evidence was very low‐quality, and we are uncertain whether screening hysteroscopy increases miscarriage rate compared to no hysteroscopy.
Screening hysteroscopy versus no hysteroscopy before intrauterine insemination (IUI)
We found no studies that investigated this comparison.
Sreening hysteroscopy versus no hysteroscopy before in vitro fertilisation (IVF)
There was low‐quality evidence that indicates that performing a screening hysteroscopy before IVF may increase the live birth and clinical pregnancy rates compared to no hysteroscopy (Table 2). Sensitivity analysis performed by excluding those studies in which hysteroscopy was combined with endometrial biopsy, showed similar estimates for live birth and clinical pregnancy rates between the two groups. Importantly, sensitivity analysis done by pooling only trials with low risk of bias showed no improvement in live birth and clinical pregnancy rates following screening hysteroscopy. With very low‐quality evidence, we are uncertain whether screening hysteroscopy is associated with higher adverse events (complications related to hysteroscopy) versus no hysteroscopy. Low‐quality evidence indicates little or no difference in miscarriage rate following screening hysteroscopy versus no hysteroscopy.
Overall completeness and applicability of evidence
At present, there is no high‐quality evidence to support the routine use of hysteroscopy as a screening tool in the general subfertile population, for improving reproductive outcomes. We retrieved only one trial, that provided very low‐quality evidence, and assessed the effects of screening hysteroscopy in women with unexplained subfertility trying to conceive spontaneously.
The current review suggests that screening hysteroscopy may increase live birth and clinical pregnancy rates compared to no intervention, in women undergoing IVF. However, the applicability of the evidence may have some limitations and may vary according to different subpopulations. The subgroup analysis suggests that screening hysteroscopy may benefit women with two or more IVF failures.
We observed the presence of variations in eligibility criteria among individual trials in terms of additional radiological procedures done (e.g. hysterosalpingogram (HSG)) before 2D ultrasound versus only 2D ultrasound, hysteroscopy naive versus previous history of hysteroscopy, hysteroscopy alone versus hysteroscopy and endometrial biopsy, and treatment of all detected intracavitary abnormalities versus selective treatment (Table 3).The literature suggests that the diagnostic accuracy of 2D transvaginal ultrasound is suboptimal in terms of detecting intrauterine pathologies, such as polyps and adhesions, compared to other diagnostic procedures, such as sonohysterography or hysterosalpingography (Salle 1999; Ragni 2005). Trials including additional radiological procedures (e.g. HSG) would screen out more women with abnormalities, compared to trials relying solely on 2D transvaginal ultrasound before IVF, thereby, introducing some degree of heterogeneity. Further, there is supportive evidence of benefit of endometrial scratching (a procedure similar to endometrial biopsy) before IVF, and the addition of the same, along with screening hysteroscopy, may influence the true estimate (Nastri 2015). There is evidence of improved reproductive outcomes following treatment of intracavitary uterine abnormalities, such as a uterine septum and polyp (Bosteels 2015; ASRM 2016). The current review included a trial in which few women who were detected to have intracavitary abnormalities did not undergo correction in the intervention arm, and this could be a possible source of heterogeneity (Table 3). There could be some clinical heterogeneity among studies due to these stated variations in trials' protocols, as well as inclusion and exclusion criteria for recruited participants. In the IVF population, due to the high statistical heterogeneity for outcomes, such as live birth (69%) and clinical pregnancy (49%), these results should be interpreted with caution.
The optimum timing to perform hysteroscopy is still not clear, since the time interval between screening hysteroscopy and IVF varied between one and three months, although most included studies performed the hysteroscopy in the preceding menstrual cycle. Further, since the review included only studies involving fresh IVF cycles, the evidence cannot be extrapolated to other types of ART treatments, such as frozen cycles. Importantly, the complications associated with hysteroscopy were reported by very few studies.
Quality of the evidence
The current review had three comparisons
Hysteroscopy versus no hysteroscopy for subfertile women wishing to conceive spontaneously
Hysteroscopy versus non hysteroscopy for subfertile women undergoing IUI.
Hysteroscopy versus no hysteroscopy for women undergoing IVF.
In the comparison for subfertile women with unexplained subfertility wishing to conceive spontaneously, we reported ongoing pregnancy (as a surrogate for live birth) rate, adverse events (complications following hysteroscopy), clinical pregnancy rate, and miscarriage rate. The quality of the evidence was very low for the outcomes ongoing pregnancy and clinical pregnancy rates. Only one trial was included, and we downgraded by two levels for risk of bias and one level for indirectness. We assessed the evidence to be very low‐quality for miscarriage rate, downgrading by one level each for risk of bias, indirectness, and imprecision.
We did not find any trials that compared screening hysteroscopy versus no hysteroscopy before IUI.
For the comparison with the greatest number of studies, i.e. studying an IVF population, we reported on live birth, adverse events (complications following hysteroscopy), clinical pregnancy rate, and miscarriage rate. Overall, the evidence for live birth and clinical pregnancy was of low quality. We found the majority of the studies with unclear descriptions of allocation concealment, hence, we downgraded by one level for risk of bias. We found substantial statistical heterogeneity (49% to 69%), which could not be explained on the basis of differences in population or intervention, hence, we downgraded the evidence by a further one level for the live birth and clinical pregnancy outcomes. The adverse events outcome, which comprised of complications following hysteroscopy, was poorly reported, and we graded it as very low‐quality evidence (downgraded risk of bias by one, plus two levels for imprecision). We graded the miscarriage rate outcome as low‐quality evidence due to risk of bias (downgraded by one level), and imprecision (downgraded by one level).
Potential biases in the review process
We aimed to search for and identify all the studies eligible for this review. The search was comprehensive, and included identifying ongoing studies through trial registries. We also tried contacting authors for additional information and clarification regarding their published data. However, for conference abstracts, it was difficult to contact authors because of the absence of contact addresses or information. We requested clarification regarding data from nine authors of the included studies, and got a satisfactory reply from three authors. Additionally, two authors of this review (BWM and FB) were investigators and authors of one of the included trials (Smit 2016). However, both these authors did not participate in selection of the studies or in extracting data from that study.
Agreements and disagreements with other studies or reviews
One systematic review evaluated the role of diagnostic hysteroscopy in infertile couples and women undergoing IVF (Di Spiezio Sardo 2016). For subfertile women wishing to conceive spontaneously, the findings are in accordance with the present Cochrane Review: there is only a limited body of evidence on the role of hysteroscopy as a screening tool in the early assessment of infertile women. More research is needed.
An earlier systematic review evaluated the role of office hysteroscopy in women undergoing IVF, and included both randomised (N = 2) and non‐randomised trials (N = 3 (El‐Toukhy 2008)).The included studies had women who were both undergoing their first IVF, and those with two or more IVF failures. The authors found a significant increase in clinical pregnancy following hysteroscopy after pooling the results from both the randomised and non‐randomised trials (RR = 1.75, 95% CI 1.51 to 2.03). The authors suggested benefit could be due to the treatment of intracavitary abnormalities; negotiation of the cervical canal, thus, facilitating the subsequent embryo transfer; or inadvertent endometrial injury during hysteroscopy. These results are in agreement with current review findings, where we found a possible benefit of screening hysteroscopy, especially in women with two or more IVF failures.
Another systematic review evaluated the role of routine hysteroscopy before the first IVF, and included one RCT and five non‐randomised trials (Pundir 2014). The authors reported significantly higher clinical pregnancy rate after pooling the results (RR, 1.44, 95% CI 1.08 to 1.92). The pooled RR for live birth was 1.30 (95% CI 1.00 to 1.67). The authors suggested improved IVF outcomes following routine hysteroscopy in women undergoing their first IVF. In the subgroup population of women undergoing their first IVF, the current review did not find an increase in live birth or clinical pregnancy following screening hysteroscopy. The reason for the difference from the earlier review could be due to the inclusion of non‐randomised trials, which often overestimate the treatment effect.
In a fourth systematic review, the authors pooled three trials under the diagnostic hysteroscopy group, and found increased live birth following hysteroscopy compared to control (RR1.48, 95% CI 1.20 to 1.81); the quality of the evidence was very low. The pooled results from seven trials, found an increase in clinical pregnancy following hysteroscopy (RR 1.45, 95% CI 1.26 to 1.67); the quality of the evidence was moderate (Di Spiezio Sardo 2016). The current review pooled results from an additional three trials (Smit 2016; Alleyassin 2017; Juul Hare 2018). However, the results from both these reviews are in broad agreement.
Authors' conclusions
Implications for practice.
At present, there is no high‐quality evidence to support the routine use of hysteroscopy as a screening tool in the general population of subfertile women with a normal uterine cavity on ultrasound or hysterosalpingogram in the basic fertility work‐up to improve reproductive success rates.
In women undergoing in vitro fertilisation (IVF), low‐quality evidence from all studies reporting these outcomes, suggests that performing a screening hysteroscopy before IVF may increase live birth and clinical pregnancy rates. However, pooled results from the only two trials at low risk of bias, did not show that a screening hysteroscopy before IVF provided any benefit. Since the studies showing an effect are those with unclear allocation concealment, we are uncertain whether a routine screening hysteroscopy increases live birth and clinical pregnancy, be it for all women or those with two or more failed IVF attempts. There is insufficient data to draw conclusions about the safety of screening hysteroscopy.
Implications for research.
High‐quality randomised controlled trials are needed to assess the effectiveness of hysteroscopy as a screening tool in the general population of women with fertility problems, whether planning to conceive spontaneously, or undergo IUI or IVF. . Future trials should also assess the cost‐effectiveness of hysteroscopy as a screening tool in both these populations.
Even after publication of large, adequately powered trials evaluating the role of screening hysteroscopy before IVF, the uncertainties about its role seem to persists. Indeed, if there is a possible beneficial effect of screening hysteroscopy, the mechanism is not clear. There is a need to conduct trials comparing therapeutic intervention for intra cavitary abnormalities detected during screening hysteroscopy versus no intervention, and screening hysteroscopy versus sham procedure, involving only cervical dilation, before IVF, which will help elucidate the mechanism and explore its true effect. Trials are also needed to explore the optimal timing of a screening hysteroscopy before IVF. It remains uncertain if hysteroscopy alone, or hysteroscopy along with endometrial biopsy or scratching is beneficial. There is also a need to evaluate whether inclusion of women who are hysteroscopy naive versus those with a history of previous hysteroscopy, impact the outcomes.
Future trials should also report live birth or ongoing clinical pregnancy rate as an outcome. Pain, discomfort, and procedural complications associated the hysteroscopy should be adequately reported as outcomes in the trials.
Recent years have seen an increasing interest in basic research into the complex molecular network of the implantation process of the human embryo. This has led to the identification of markers of endometrial receptivity to help improve the clinical outcomes of IVF. The comparative cost‐effectiveness of screening hysteroscopy versus the use of markers of endometrial receptivity is needed.
What's new
| Date | Event | Description |
|---|---|---|
| 17 April 2019 | Amended | Minor textual amendments for sense and clarity. |
Acknowledgements
Cochrane Gynaecology and Fertility Group (CGF). We wish to thank Helen Nagels, CGF Managing Editor, Marian Showell, CGF Information Specialist, and Professor Cindy Farquahar, CGF Co‐ordinating Editor. We also acknowledge the contributions of referees Dr Sarah Lensen, Dr Jack Wilkinson, and Professor Fulco van der Veen.
Biomedical Library Gasthuisberg, Catholic University, Leuven, Belgium. Many thanks to Jens De Groot for skilful assistance in developing the literature search strategy. Finally, we would like to express our gratitude to the following investigators, who provided essential information for the preparation of this review: Dr Rama Raju, Dr Kristine Juul Hare, Dr K Elsetohy, and Dr F Moeity.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGFG) Specialised Register search strategy
Searched 05 September 2018
Procite platform
Keywords CONTAINS "hysteroscopic "or "hysteroscope diameter" or "hysteroscope size" or "hysteroscopy" or "hysteroscopy, techniques" or "hysteroscopy‐second look" or "hysterscope" or "uterine cavity assessment" or "mini‐hysteroscopy" or "minihysteroscopy" or "endometrial polypectomy" or "endometrial polyps" or "endoscopy" or Title CONTAINS "hysteroscopic "or "hysteroscope diameter"or "hysteroscope size" or "hysteroscopy" or "hysteroscopy, techniques" or "hysteroscopy‐second look" or "hysteroscope" or "uterine cavity assessment" or "mini‐hysteroscopy" or "mini hysteroscopy" or "endometrial polypectomy" or "endometrial polyps" or "endoscopy"
AND
Keywords CONTAINS "IVF" or "ICSI" or "subfertility" or "in vitro fertilisation" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "assisted conception" or "assisted reproduction" or "ART" or "infertility" or "IUI" or "Intrauterine Insemination" or "artificial insemination" or "ovarian hyperstimulation" or "ovarian stimulation" or "ovulation induction" or "COH" or "controlled ovarian " or "insemination" or "insemination‐intrauterine" or "subfertility‐female" or "IUI" or "recurrent miscarriage" or "pregnancy" or Title CONTAINS "IVF" or "ICSI" or "subfertility" or "in vitro fertilisation" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "assisted conception" or "assisted reproduction" or "ART" or "infertility" or "IUI" or "Intrauterine Insemination" or "artificial insemination" or "ovarian hyperstimulation" or "ovarian stimulation" or "ovulation induction" or "COH" or "controlled ovarian " or "insemination" or "insemination‐intrauterine" or "subfertility‐female" or "IUI" or "pregnancy" (162 hits)
Appendix 2. CENTRAL via Central Register of Studies Online (CRSO)
Searched 05 September 2018
Web platform
#1 MESH DESCRIPTOR Hysteroscopy EXPLODE ALL TREES 355
#2 Hysteroscop*:TI,AB,KY 1056
#3 Uteroscop*:TI,AB,KY 0
#4 minihysteroscop*:TI,AB,KY 10
#5 (Uter* adj3 Endoscop*):TI,AB,KY 9
#6 #1 OR #2 OR #3 OR #4 OR #5 1063
#7 MESH DESCRIPTOR Infertility, Female EXPLODE ALL TREES 1228
#8 MESH DESCRIPTOR Reproductive Techniques, Assisted EXPLODE ALL TREES 2977
#9 (subfertil* or infertil*):TI,AB,KY 6142
#10 (IVF or ICSI):TI,AB,KY 4909
#11 (artificial insemination):TI,AB,KY 189
#12 (assisted reproducti*):TI,AB,KY 1030
#13 (intrauterine insemination):TI,AB,KY 801
#14 IUI:TI,AB,KY 641
#15 pregnancy:TI,AB,KY 35148
#16 conception:TI,AB,KY 1071
#17 fertility:TI,AB,KY 2490
#18 MESH DESCRIPTOR Abortion, Habitual EXPLODE ALL TREES 252
#19 miscarriage*:TI,AB,KY 1151
#20 (pregnancy loss):TI,AB,KY 359
#21 conceive:TI,AB,KY 280
#22 MESH DESCRIPTOR Gynatresia EXPLODE ALL TREES 16
#23 Gynatresia:TI,AB,KY 16
#24 (implant* adj3 failure*):TI,AB,KY 1056
#25 IVF‐ET:TI,AB,KY 441
#26 (ovulation induction):TI,AB,KY 2122
#27 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 41363
#28 #6 AND #27 324
Appendix 3. MEDLINE search strategy
Searched from 1946 to 05 September 2018
Ovid platform
1 exp Hysteroscopy/ (4409) 2 Hysteroscop$.tw. (6236) 3 Uteroscop$.tw. (13) 4 minihysteroscop$.tw. (22) 5 (Uter$ adj3 Endoscop$).tw. (86) 6 or/1‐5 (7237) 7 exp Infertility/ (61724) 8 subfertil$.tw. (4619) 9 (IVF or ICSI).tw. (24871) 10 artificial insemination.tw. (6081) 11 assisted conception.tw. (1121) 12 intrauterine insemination.tw. (2231) 13 iui.tw. (1583) 14 reproductive techniques, assisted/ or exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or in vitro oocyte maturation techniques/ or exp insemination, artificial/ or exp ovulation induction/ or exp superovulation/ (60827) 15 exp Infertility, Female/ (27010) 16 Infertil$.tw. (54744) 17 pregnancy.tw. (346819) 18 conception.tw. (27848) 19 fertility.tw. (69691) 20 Abortion, Habitual/ (6515) 21 miscarriage$.tw. (11922) 22 recurrent pregnancy loss$.tw. (1670) 23 conceive.tw. (4654) 24 Gynatresia/ (176) 25 (implant$ adj3 failure$).tw. (7800) 26 IVF‐ET.tw. (2164) 27 ovulation induction.tw. (3384) 28 or/7‐27 (519949) 29 randomized controlled trial.pt. (467803) 30 controlled clinical trial.pt. (92620) 31 randomized.ab. (420714) 32 randomised.ab. (84006) 33 placebo.tw. (196717) 34 clinical trials as topic.sh. (184694) 35 randomly.ab. (296498) 36 trial.ti. (186948) 37 (crossover or cross‐over or cross over).tw. (77529) 38 or/29‐37 (1228103) 39 exp animals/ not humans.sh. (4493304) 40 38 not 39 (1130506) 41 6 and 28 and 40 (227)
Appendix 4. Embase search strategy
Searched from 1980 to 05 September 2018
Ovid platform
1 exp Hysteroscopy/ (10545) 2 Hysteroscop$.tw. (10349) 3 Uteroscop$.tw. (20) 4 minihysteroscop$.tw. (42) 5 (Uter$ adj3 Endoscop$).tw. (113) 6 or/1‐5 (12891) 7 subfertil$.tw. (6172) 8 (IVF or ICSI).tw. (41929) 9 artificial insemination.tw. (5364) 10 assisted conception.tw. (1610) 11 intrauterine insemination.tw. (3295) 12 iui.tw. (2883) 13 Infertil$.tw. (74005) 14 pregnancy.tw. (402281) 15 conception.tw. (32699) 16 fertility.tw. (79564) 17 miscarriage$.tw. (19484) 18 recurrent pregnancy loss$.tw. (2820) 19 conceive.tw. (6360) 20 (implant$ adj3 failure$).tw. (10557) 21 IVF‐ET.tw. (2970) 22 ovulation induction.tw. (4680) 23 exp infertility/ or exp female infertility/ or exp infertility therapy/ (164673) 24 assisted reproducti$.tw. (19631) 25 exp intracytoplasmic sperm injection/ (18393) 26 exp artificial insemination/ (15778) 27 exp ovulation induction/ (13068) 28 exp superovulation/ (2593) 29 exp recurrent abortion/ (5281) 30 or/7‐29 (613203) 31 6 and 30 (5090) 32 Clinical Trial/ (939390) 33 Randomized Controlled Trial/ (506064) 34 exp randomization/ (79110) 35 Single Blind Procedure/ (32096) 36 Double Blind Procedure/ (148976) 37 Crossover Procedure/ (56068) 38 Placebo/ (307810) 39 Randomi?ed controlled trial$.tw. (183941) 40 Rct.tw. (29057) 41 random allocation.tw. (1783) 42 randomly allocated.tw. (30135) 43 allocated randomly.tw. (2330) 44 (allocated adj2 random).tw. (792) 45 Single blind$.tw. (21113) 46 Double blind$.tw. (182169) 47 ((treble or triple) adj blind$).tw. (803) 48 placebo$.tw. (269794) 49 prospective study/ (463707) 50 or/32‐49 (1904367) 51 case study/ (55585) 52 case report.tw. (348767) 53 abstract report/ or letter/ (1017866) 54 or/51‐53 (1413483) 55 50 not 54 (1855981) 56 31 and 55 (722)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 05 September 2018
Ovid platform
1 subfertil$.tw. (84) 2 (IVF or ICSI).tw. (558) 3 artificial insemination.tw. (251) 4 assisted conception.tw. (98) 5 intrauterine insemination.tw. (23) 6 iui.tw. (34) 7 Infertil$.tw. (3267) 8 pregnancy.tw. (35219) 9 conception.tw. (21249) 10 fertility.tw. (6592) 11 miscarriage$.tw. (1141) 12 recurrent pregnancy loss$.tw. (13) 13 conceive.tw. (2967) 14 (implant$ adj3 failure$).tw. (46) 15 IVF‐ET.tw. (17) 16 ovulation induction.tw. (21) 17 assisted reproducti$.tw. (871) 18 exp Infertility/ (2028) 19 exp Reproductive Technology/ (1716) 20 exp Spontaneous Abortion/ (786) 21 or/1‐20 (66886) 22 Hysteroscop$.tw. (17) 23 21 and 22 (10)
Appendix 6. CINAHL search strategy
Searched from 1961 to 05 September 2018
EBSCO platform
| # | Query | Results |
| S47 | S32 AND S46 | 86 |
| S46 | S33 OR S34 or S35 or S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 | 1,255,218 |
| S45 | TX allocat* random* | 9,040 |
| S44 | (MH "Quantitative Studies") | 20,289 |
| S43 | (MH "Placebos") | 10,837 |
| S42 | TX placebo* | 52,077 |
| S41 | TX random* allocat* | 9,040 |
| S40 | (MH "Random Assignment") | 50,529 |
| S39 | TX randomi* control* trial* | 153,082 |
| S38 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 972,341 |
| S37 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 201 |
| S36 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 201 |
| S35 | TX clinic* n1 trial* | 227,629 |
| S34 | PT Clinical trial | 86,040 |
| S33 | (MH "Clinical Trials+") | 244,152 |
| S32 | S6 AND S31 | 598 |
| S31 | S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 | 205,364 |
| S30 | TX ovulation induction | 741 |
| S29 | TX IVF‐ET | 86 |
| S28 | TX (implant* N3 fail*) | 2,614 |
| S27 | TX conceive | 1,066 |
| S26 | TX (recurrent pregnancy loss) | 289 |
| S25 | TX miscarriage | 2,880 |
| S24 | (MM "Abortion, Habitual") | 263 |
| S23 | TX fertility | 10,698 |
| S22 | TX conception | 25,694 |
| S21 | TX pregnancy | 180,252 |
| S20 | TX Infertil* | 10,835 |
| S19 | TX superovulation | 28 |
| S18 | (MM "Ovulation Induction") | 318 |
| S17 | TX (sperm injection* intracytoplasmic) | 423 |
| S16 | (MM "Fertilization in Vitro") | 1,971 |
| S15 | (MH "Reproduction Techniques+") | 7,812 |
| S14 | TX iui | 165 |
| S13 | TX intrauterine insemination | 241 |
| S12 | TX assisted conception | 376 |
| S11 | (MM "Insemination, Artificial") | 283 |
| S10 | TX artificial insemination | 551 |
| S9 | TX (IVF or ICSI) | 2,380 |
| S8 | TX subfertil* | 602 |
| S7 | (MM "Infertility") OR (MM "Embryo Transfer") | 5,152 |
| S6 | S1 OR S2 OR S3 OR S4 OR S5 | 1,576 |
| S5 | TX (Uter* N3 Endoscop*) | 30 |
| S4 | TX minihysteroscop* | 2 |
| S3 | TX Uteroscop* | 1 |
| S2 | TX Hysteroscop* | 1,555 |
| S1 | (MM "Hysteroscopy") | 677 |
Appendix 7. Data extraction form
| Study information | |||||||||
| 1. Ref ID | |||||||||
| 2. First author | |||||||||
| 3. Year | |||||||||
| 4. Published | q No | ||||||||
| 5. Language | |||||||||
| Criteria for eligibility | YES | NO | |||||||
| Patients: | Couples undergoing hysteroscopy prior to IVF/ICSI | q | q | ||||||
| Intervention | Screening/routine hysteroscopy a) Prior to the first IVF/ICSI cycle b) Prior to 2 or more failed IVF cycles |
q | q | ||||||
| Comparison | No hysteroscopy | q | q | ||||||
| Outcomes |
Primary: Live‐birth rate (per randomised couple) Secondary: Clinical pregnancy rate (per randomised couple; positive pregnancy test, gestational sac on ultrasound) Multiple pregnancy rate (per randomised couple) Miscarriage rate (per randomised couple) Congenital anomalies (per randomised couple) Additional: |
q q q q q q q q q |
q q q q q q q q q |
||||||
| Study characteristics | |||||||||
| Design | |||||||||
| 1. Study design | RCT Parallel (intervention vs control) Cross‐over (participants used as intervention and control group) ………………………………. Quotes: ……………………………………………………………………………………………………………………………………………………………………………………………………………………………… |
||||||||
| 2. Setting | Single‐centre | q Multicentre | |||||||
| Country: .……………………………………………………………………………………………………………… | |||||||||
| Participants: in‐ and exclusion | |||||||||
| 3. Study criteria for patient inclusion | ____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________ | ||||||||
| 4. Study criteria for patient exclusion | _____________________________________________________________________________________________________________________________________________________________________________________________ | ||||||||
| 5. Description control/ comparison treatment | ……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………… | ||||||||
| Baseline characteristics | |||||||||
| Previous IVF and/or ICSI treatment | Reported | q Not reported | |||||||
| Intervention | |||||||||
| Embryo transfer after IVF, ICSI | |||||||||
| 1. Time of randomisation during cycle | Prior to commencement of treatment cycle | ||||||||
| 2. Nature of intervention | Hysteroscopy No hysteroscopy |
||||||||
| 3. Timing of intervention | Late luteal phase in the preceding cycle Follicular phase in the preceding cycle |
||||||||
Data and analyses
Comparison 1. Screening hysteroscopy versus no hysteroscopy in subfertile women wishing to conceive spontaneously.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.3 [2.29, 8.07] |
| 2 Adverse outcomes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Clinical pregnancy | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.8 [2.31, 6.24] |
| 4 Miscarriage | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.8 [1.05, 7.48] |
Comparison 2. Screening hysteroscopy versus no hysteroscopy in women before IVF.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 6 | 2745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.11, 1.43] |
| 1.1 Unselected | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.63, 2.40] |
| 1.2 First IVF | 2 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.96, 1.49] |
| 1.3 Two or more IVF failures | 3 | 1575 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.10, 1.53] |
| 2 Adverse outcomes | 4 | 1872 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.47 [0.15, 376.42] |
| 2.1 Unselected | 1 | 217 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 First IVF | 2 | 953 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.47 [0.15, 376.42] |
| 2.3 Two or more IVF failures | 1 | 702 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Clinical pregnancy | 10 | 3750 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.20, 1.45] |
| 3.1 Unselected | 3 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.07, 1.84] |
| 3.2 First IVF | 3 | 1173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.02, 1.41] |
| 3.3 Two or more IVF failures | 4 | 1996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.21, 1.56] |
| 4 Miscarriage | 3 | 1669 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.50] |
| 4.1 Unselected | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 68.33] |
| 4.2 First IVF | 1 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.80] |
| 4.3 Two or more IVF failures | 1 | 702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.42] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aghahosseini 2012.
| Methods | RCT Single centre Iran |
|
| Participants | Inclusion: women undergoing IVF with at least two implantation failures (recurrent ART failure) younger than 38 years, BMI less than 35 kg/m², no hysteroscopy in 2 months, normal HSG. Exclusion: not mentioned |
|
| Interventions | Intervention group (N = 142) underwent hysteroscopy prior to ART. No mention of any additional procedures during hysteroscopy, or whether abnormalities found were treated. Control group (N = 211) did not undergo hysteroscopy. |
|
| Outcomes | Clinical pregnancy rate, live birth rate, abnormal and normal hysteroscopy findings | |
| Notes | This was published as a conference abstract. Authors did not respond to emails for clarification regarding participants and data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors use 'randomized' in the abstract. However, information provided was insufficient for making a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence performance bias. Hence, absence of blinding was categorized as low risk for performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence the findings for our primary and secondary outcomes, hence, categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided regarding losses to follow‐up or dropouts. Insufficient information for making a judgement |
| Selective reporting (reporting bias) | Low risk | No published protocol. However, all the prespecified outcomes of interest (including live birth) were reported. |
| Other bias | Unclear risk | The numbers randomised in both groups were uneven (142 vs 211) and control group numbers were substantially higher. No clear explanation was available for the unbalanced numbers since this is a conference abstract and authors did not respond to emails for clarification. |
Alleyassin 2017.
| Methods | RCT Single centre Iran |
|
| Participants | Inclusion: women undergoing first ICSI treatment normal TVS scan and normal HSG Exclusion: women with recurrent miscarriages undergone hysteroscopy earlier |
|
| Interventions | Intervention group (N = 110): hysteroscopy done; mid‐luteal phase and down, regulation done using buserelin; rigid hysteroscopy was used, vaginoscopic approach. Intracavitary uterine abnormalities noticed were treated in the same sitting. No additional procedure, such as endometrial biopsy, were mentioned. Control group (N =110): no hysteroscopy before ICSI treatment |
|
| Outcomes | Clinical pregnancy rate, miscarriage rate, multiple pregnancy rate, normal and abnormal hysteroscopy findings | |
| Notes | Authors were contacted for data, however, authors did not respond to emails. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The physician performing embryo transfer and embryologist were blinded for group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | While physician performing embryo transfer and embryologist were blinded, it was not clear if outcome assessors were blinded. However, blinding was unlikely to influence the findings for our primary and secondary outcomes, hence, categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | While all the randomised participants were included in the analysis, no dropouts were mentioned. |
| Selective reporting (reporting bias) | Unclear risk | The published protocol for this study was not available. While prespecified outcomes were reported, primary outcome (live birth) was not reported. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. Source of funding was not mentioned. |
Demirol 2004.
| Methods | RCT Single centre Turkey |
|
| Participants | Women who had undergone two or more failed IVF cycles, in which two or more good quality embryos were transferred All the participants had normal HSG (normal intrauterine cavity and bilaterally patent tubes) Age group 24 to 40 years All women had primary infertility |
|
| Interventions | Intervention group (N = 210) had office hysteroscopic evaluation of uterine cavity and cervix with intrauterine lesions treated during the office procedure. No additional procedure, such as endometrial biopsy, was mentioned. Hysteroscopy was performed in the early proliferative phase, using saline distension medium All office hysteroscopies were performed 2 to 6 months after the last failed IVF cycle, by the same physician All IVF treatments were carried out on the menstrual cycles after office hysteroscopy Control group (N = 211) did not have hysteroscopy prior to IVF. |
|
| Outcomes | Clinical pregnancy rate, first trimester miscarriage rate, normal and abnormal hysteroscopy findings | |
| Notes | Authors were contacted for clarification regarding data, however authors did not respond to emails. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized into two groups using computer generated random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence performance bias. Hence, absence of blinding was categorised as low risk for performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not mentioned.However, absence of blinding is unlikely to influence the findings for our primary and secondary outcomes, hence categorized under " low risk" for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants and losses to follow‐up were mentioned and appeared to be balanced. Intention‐to‐treat analysis was done. |
| Selective reporting (reporting bias) | Unclear risk | The published protocol for this study was not available. While the prespecified outcomes were reported, the primary outcome (live birth) was not reported. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. Instituitional ethical board clearance and source of funding was not mentioned. |
El‐Nashar 2011.
| Methods | RCT Single centre Egypt |
|
| Participants | Included women with primary infertility undergoing ICSI cycle; no other criteria mentioned Exclusion criteria: not mentioned |
|
| Interventions | Intervention group (N = 62) underwent hysteroscopy and directed endometrial biopsy before ICSI. Any uterine abnormalities found were corrected. Control group (N = 62): no hysteroscopy |
|
| Outcomes | Clinical pregnancy rate, normal and abnormal hysteroscopy findings | |
| Notes | This was published as a conference abstract. There was no response from authors to email queries regarding participants and data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors use "randomized" in the abstract. However, information provided was insufficient for making a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence performance bias. Hence, absence of blinding was categorised as low risk for performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence the findings for our primary and secondary outcomes, hence, categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided regarding losses to follow‐up or dropouts. Insufficient information for making a judgement |
| Selective reporting (reporting bias) | Unclear risk | No published protocol. While all the prespecified outcomes were reported, primary outcome (live birth) was not reported. |
| Other bias | Unclear risk | There was not enough information to make a judgement. This was a conference abstract. |
El‐Toukhy 2016.
| Methods | RCT Multicenter European centres |
|
| Participants | Included women aged < 38 years who had undergone at least two, three, or four fresh IVF or frozen cycles without a pregnancy Normal ultrasound assessment of the uterine cavity At least 8 oocytes retrieved in the previous IVF cycle Exclusion Less than two, or more than four failed IVF cycles ending in an embryo transfer Hysteroscopy less than two months before randomisation Submucous or intramural fibroids diagnosed by ultrasound found to be distorting the uterine cavity Untreated tubal hydrosalpinges BMI > 35 kg/m² |
|
| Interventions | Intervention group (N = 350 ) had outpatient hysteroscopy. Hysteroscopy was performed in the early proliferative phase using saline distension medium. intracavitary abnormalities, such as endometrial polyps, septums, and submucosal fibroids were treated. No additional procedure, such as endometrial biopsy, was done All IVF treatments were carried out on the menstrual cycles after office hysteroscopy Control group (N = 352 ) had no hysteroscopy. |
|
| Outcomes | Live birth rate, positive pregnancy rate, clinical pregnancy rate, implantation rate, miscarriage rate, normal and abnormal findings of hysteroscopy, adverse events following hysteroscopy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned using independent third party trial management system" |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was done to mask the researchers to the order of group assignment at randomisation and recruitment". The minimisation procedure, with a computer‐based algorithm from the integrated trial management system, incorporates allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | While the embryologists were not aware of the allocated group, the physicians performing the embryo transfer were not blinded. However, absence of blinding was unlikely to influence performance bias. Hence, absence of blinding was categorised as low risk for performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The researchers were not aware of the allocated group. Secondary outcome assessors and physicians were not blinded. Further, absence of blinding was unlikely to influence the findings for our primary and secondary outcomes, hence, categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants and losses to follow‐up were mentioned and appeared to be balanced. Intention‐to‐treat analysis was done. |
| Selective reporting (reporting bias) | Low risk | Published protocol was available. All the prespecified outcomes (including live birth) were reported in the final analysis. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. Details regarding source of funding and ethical clearance were mentioned. |
Elsetohy 2015.
| Methods | RCT Egypt Single centre |
|
| Participants | Included women undergoing first IVF/ICSI treatment Normal transvaginal ultrasound apart from intramural myomas without uterine cavity deformity HSG done in the past one year Exclusion criteria: Uterine factor infertility History of recurrent miscarriage Abnormal HSG Abnormal transvaginal ultrasound Previous uterine surgery Contraindication for hysteroscopy |
|
| Interventions | Intervention group (N = 102) had office hysteroscopic evaluation of uterine cavity and cervix; intrauterine lesions treated during the office procedure. Hysteroscopy was performed in the early mid‐follicular phase of a menstrual cycle (day 3 to12) with a vaginoscopic approach without anaesthesia, using saline infusion medium. No additional procedure, such as endometrial biopsy, was done All IVF/ ICSI treatments were carried out within three months of hysteroscopic examination. Control group (N = 101) had no hysteroscopy. |
|
| Outcomes | Clinical pregnancy rate Live birth rate Adverse events |
|
| Notes | Authors were contacted for clarification regarding data (clinical pregnancy rate) and response recorded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a computer generated table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence performance bias. Hence, absence of blinding was categorised as low risk for performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence the findings for our primary and secondary outcomes, hence, categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants and losses to follow‐up were mentioned, and appeared to be balanced. However, intention‐to‐treat analysis not done. |
| Selective reporting (reporting bias) | Low risk | The published protocol for this study was not available, however, prespecified outcomes of interest (including live birth) were reported. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. Source of funding was not mentioned. |
Juul Hare 2018.
| Methods | Randomized controlled trial Denmark Two centres |
|
| Participants | Inclusion criteria: Age: 18 to 40 years Women submitted to IVF or ISCI treatment with previous one IVF failure (before 2nd IVF cycle)
Exclusion Criteria:
|
|
| Interventions | Intervention: hysteroscopy with endometrial biopsy (N = 112) before 2nd IVF cycle. Control: no hysteroscopy (N = 105) |
|
| Outcomes | Clinical pregnancy, miscarriage rate, live birth rate, complication rates | |
| Notes | This was published as a conference abstract. The authors were contacted through email; authors responded and gave clarification regarding data and methods used in the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a computer generated randomization", as stated by authors |
| Allocation concealment (selection bias) | Unclear risk | Author responded "computer randomization and SAS program". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not done. However, absence of blinding was unlikely to influence performance bias. Hence, absence of blinding was categorised as low risk for performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not done. However, absence of blinding was unlikely to influence the findings for our primary and secondary outcomes, hence, categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants and losses to follow‐up were mentioned. Intention‐to‐treat analysis was done. |
| Selective reporting (reporting bias) | Low risk | Published protocol was available. All the prespecified outcomes (including live birth) were reported in the final analysis. |
| Other bias | High risk | Original sample size for the trial was 300. However, the trial was stopped prematurely due to slow recruitment. |
Rama Raju 2006.
| Methods | RCT Single centre India |
|
| Participants | Included women who had undergone two or more failed IVF cycles, in which two or more good quality embryos were transferred per procedure Normal HSG (normal intrauterine cavity) Informed consent obtained before study entry Patient aged between 26 and 30 years All patients had primary infertility No exclusion criteria mentioned |
|
| Interventions | Intervention group (N = 255) had outpatient hysteroscopy and sampling of the endometrium for histological evaluation (endometrial biopsy). Hysteroscopy was performed in the early proliferative phase using glycine. Abnormalities detected by hysteroscopy were corrected in the same sitting. After hysteroscopy, down regulation was initiated. Control group (N = 265) had no hysteroscopy. |
|
| Outcomes | Live birth rate, clinical pregnancy rate, miscarriage rate, abnormal and normal hysteroscopy findings. | |
| Notes | Authors were contacted for clarification regarding data (clinical pregnancy and live birth rate) and response recorded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized "using computer generated numbers". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence performance bias. Hence, absence of blinding was categorised as low risk for performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence the findings for our primary and secondary outcomes, hence, categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No clear information on dropouts after randomisation. It was unclear if intention‐to‐treat analysis was done. |
| Selective reporting (reporting bias) | Low risk | The published protocol for this study was not available, however, prespecified outcomes of interest (including livebirth) were reported. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. Source of funding was not mentioned. |
Seyam 2015.
| Methods | Parallel‐group randomised controlled trial Single centre, Arafa Hospital, Fayoum, Egypt Protocol approved by EC/IRB: yes Study protocol registration: not reported Statistical power calculation: not reported Funding: not reported Conflicts of interest reported: no | |
| Participants | Number recruited: 200
Number randomly assigned: 200 women
Number excluded: 0 women
200 infertile women, previously diagnosed as unexplained infertility, were recruited for the study between 2006 and 2013. All patients had a transvaginal ultrasound scanning performed in the office prior to the procedure, to screen for uterine pathology, including uterine anomalies, and intramural or subserosal myomas, as well as to assess uterine position. The basic infertility work‐up included a HSG to evaluate the uterine cavity and tubal patency. Inclusion criteria: • not reported Exclusion criteria: • not reported Study duration: 84 months |
|
| Interventions |
Office microhysteroscopy (intervention: N = 100) vs no office microhysteroscopy (control: N = 100)
The participants were randomised using a computer software into two groups: (A) study group including 100 infertile women who were short‐listed for the studied office microhysteroscopic procedure, and (B) control group including 100 women with unexplained infertility who were followed up without the proposed office microhysteroscopic intervention. All office microhysteroscopies were performed using a malleable 0 degree diagnostic and 30 degrees operative 2 mm fibreoptic microhysteroscope (Circon, Germany) with an operative channel for the use of grasping forceps, scissors, or coaxial bipolar electrode. Instruments were placed through the built‐in operative channel when needed for treatment of pathology, after the diagnostic portion had been completed. Typically, less than 1 L of normal saline was used as the distention media for procedures, except with myomectomies, which occasionally required larger volumes. Operative procedures, including hysteroscopic resection of endometrial polyps and submucous myomas, excision of intrauterine septum, and postoperative management plan for bicornuate uterus were performed, where another conventional operative session for bicornuate uterus was arranged by another team. For those longer cases, fluid balance was monitored by ancillary staff throughout the procedure. Diagnostic findings, operative outcomes, complications, and patient tolerance during the procedure were noted. The coaxial bipolar electrode surgical system (Versapoint, Gynecare, NJ) was used for myomectomies. Power settings were from 60 W (desiccation) to 130 W (cutting). Office microhysteroscopies were performed during the early postmenstrual period. Patients received oral premedication with midazolam (Sigma, Egypt), intramuscular analgesia with diclofenac (Epico, Egypt), and a paracervical uterine block with 1% lidocaine (Kahira, Egypt). Five patients requested conscious sedation with intravenous fentanyl (Cid, Egypt) and midazolam in place of the above regimen. All women were discharged immediately after the procedure, except those who were discharged after 2 hours, due to prolonged operative indications. There were no data on the timing of the hysteroscopy with respect to the menstrual cycle. For a 12‐month follow‐up period, pregnancy outcome were evaluated after the office microhysteroscopic procedure in A and B groups, for spontaneous pregnancy without any intervention, while each pregnancy developed after the microhysteroscopic procedure was correlated to each uterine abnormality diagnosed and treated during the microhysteroscopic procedure. Early pregnancy complications were evaluated for both groups, and some of the successful ongoing pregnancies were recorded as well. |
|
| Outcomes | No explicit prioritisation of outcomes by the primary study authors. Main outcome: according to the abstract, the total developing cumulative spontaneous pregnancy rate after one year of follow‐up was measured. Pregnancy was not defined in the manuscript. In the methods section, the authors mentioned that the successful ongoing pregnancies were recorded as well. In the results section, table 2 reports data for the cumulative pregnancy rate and the ongoing pregnancy rate. We could not obtain further clarification from the primary study authors. Other outcomes: patient compliance |
|
| Notes | Not reported if participants had already had a hysteroscopy prior to the fertility assessment or not. No information whether endometrial biopsy was performed at the time of the screening hysteroscopy. We repeatedly contacted the corresponding author (Dr Emaduldin Mostafa Seyam), but failed to obtain further clarification. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ”The participants were randomized using a computer software into two groups: A. study group including 100 infertile women who were short‐listed for the studied office microhysteroscopic procedure, and B. control group including 100 women with unexplained infertility who were followed up without the proposed office microhysteroscopic intervention". Comment: We could not obtain further clarification from the primary study authors on the specific computer software used to randomise. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment not reported ‐ no further clarification obtained from the primary study authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women were not blinded, but this did not affect the main outcome measures of cumulative or ongoing pregnancy rate. No further clarification obtained from the primary study authors. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was not clear who did the outcome assessment. No further clarification obtained from the primary study authors. The main outcomes (cumulative or ongoing pregnancy rates) were very likely not influenced by a lack of blinding of the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 200 women randomised ‐ all data were available for analysis |
| Selective reporting (reporting bias) | High risk | Protocol not available. Primary outcome of interest not reported (live birth), even though study duration was long enough (seven years), giving sufficient time for authors to collect live birth data. |
| Other bias | Low risk | No differences in the baseline characteristics. No co‐treatment. |
Shawki 2012.
| Methods | RCT Single centre Egypt |
|
| Participants | Included women undergoing ICSI cycle. Mixed group consisting of women undergoing first ICSI, or after one, two, or more failures. All women underwent HSG 2 to 3 months prior to IVF. Exclusion: Abnormal HSG Abnormal TVS Intrauterine surgery history Contraindication for hysteroscopy |
|
| Interventions | Intervention group (N = 120): vaginoscopic approach hysteroscopy done. For normal hysteroscopy findings, methylene blue injected to identify endometrial pathology, such as endometrial hyperplasia or endometritis, and biopsy taken. Abnormal hysteroscopy findings were recorded and treated. Control group (N = 120): no hysteroscopy |
|
| Outcomes | Implantation rate, clinical pregnancy rate, normal and abnormal hysteroscopy findings | |
| Notes | Authors were contacted for data, however, authors did not respond to emails. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Containing computer generated random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Authors mention 'sealed envelopes' containing random numbers. Authors did not describe actual allocation concealment, specifically did not mention consecutively numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence performance bias. Hence, absence of blinding was categorised as low risk for performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence the findings for our primary and secondary outcomes, hence, categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants and losses to follow‐up were mentioned, and appeared to be balanced. However, intention‐to‐treat analysis not done. |
| Selective reporting (reporting bias) | Unclear risk | The published protocol for this study was not available. While prespecified outcomes of interest were reported, primary outcome (live birth) was not reported. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. Source of funding was mentioned. |
Smit 2016.
| Methods | RCT Multicentre The Netherlands |
|
| Participants | Included infertile women undergoing first IVF, and normal transvaginal ultrasound Excluded women with two or more miscarriages, intermenstrual blood loss, previously undergone hysteroscopy |
|
| Interventions | Intervention group (N = 373): hysteroscopy done; mid follicular phase, 1 to 3 months before IVF. Uterine abnormalities noticed were treated during same sitting, or in some cases, at subsequent sitting. No additional procedure, such as endometrial biopsy, was done. Control group (N = 377): no hysteroscopy was done |
|
| Outcomes | Live birth rate, ongoing pregnancy rate, implantation rate, miscarriage rate, abnormal and normal hysteroscopy findings | |
| Notes | Authors were contacted for data, and they provided relevant data (clinical pregnancy and live birth rate after first IVF cycle) for inclusion in the review. In a few cases in hysteroscopy group, uterine abnormalities, such as septum (n = 5), and fibroids (n = 2), did not undergo therapeutic intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Web‐based randomisation" |
| Allocation concealment (selection bias) | Low risk | Web‐based randomisation incorporates allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not mentioned. However, absence of blinding was unlikely to influence performance bias. Hence, absence of blinding was categorised as low risk for performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Absence of blinding was unlikely to influence the findings for our primary and secondary outcomes, hence, categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants and losses to follow‐up were mentioned, and appeared to be balanced. Intention‐to‐treat analysis was done. |
| Selective reporting (reporting bias) | Low risk | Published protocol was available. All the prespecified outcomes (including live birth) were reported in the final analysis. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. Details regarding source of funding and ethical clearance were mentioned. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brown 2000 | Not addressing the PICO research questions of the Cochrane Review. Parallel group randomised trial comparing the diagnostic accuracy, pain scores, and procedure length of outpatient hysteroscopy, hysterosalpingography (HSG), and saline infusion hystero sonography (SIS) for evaluation of the uterine cavity of infertile women. |
| El‐Khayat 2015 | Not addressing the PICO research questions of the Cochrane Review. Parallel group randomised trial comparing office hysteroscopy with endometrial scratch versus office hysteroscopy on intrauterine insemination outcome. The aim of the trial was to evaluate the role of endometrial injury in the cycle preceding ovarian stimulation for intrauterine insemination (IUI) cycle on the clinical pregnancy rate. |
| Fatemi 2010 | Not a randomised controlled trial; prevalence study |
| Hebeisha 2018 | Randomised trial included women undergoing IVF, and investigated role of endometrial scratch before ICSI. The trial had three arms: first arm underwent endometrial scratch, second arm underwent hysteroscopy and endometrial scratch, and control arm underwent direct IVF. Investigators excluded women who were found to have intracavitary abnormalities during hysteroscopy (as communicated by authors). Since women who had normal ultrasound but intracavitary abnormalities were excluded from hysteroscopy, we excluded this trial, since current review focus was on effectiveness of screening hysteroscopy with or without treatment of intracavitary abnormalities. |
| Kamel 2015 | Not a randomised controlled trial |
| Kasius 2013 | Cost‐effectiveness analysis study |
| Shokeir 2016 | RCT on the effectiveness of local endometrial injury To evaluate the efficacy of a hysteroscopic site‐specific local endometrial injury (LEI) in a group of women with unexplained infertility (UI), undergoing expectant management with no fertility treatment versus no intervention. |
| Siristatidis 2017 | Non randomised trial |
| Wang 2011 | Different population; women with endometritis |
| Zhang 2015 | Diagnostic hysteroscopy was performed for both intervention and control group to rule out intracavitary pathologies. Those with intracavitary abnormalities on hysteroscopy were excluded. |
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Moramezi 2012.
| Methods | Parallel‐group randomised controlled trial Single centre, Fertility, Infertility and Perinatology Research Center, School of Medicine, Ahvaz Jundishapour University of Medical Sciences, Ahvaz, Iran Protocol approved by EC/ IRB: yes Study protocol registration: not reported Statistical power calculation: not reported Funding: supported by a research grant from the Ahvaz Jundishapour University of Medical Sciences, Ahvaz, Iran Conflicts of interest reported: no |
| Participants | Number recruited: not reported
Number randomly assigned: 110 women
Number excluded: 0 women
110 healthy women, between the ages of 22 and 44 years, candidate IUI cycles, were randomly assigned to one of two groups from the start of the cycle. Patient assessment included demographic information, as well as medical and gynaecological history taking, with physical examination and routine laboratory screening (including BMI, CBC, PAP smear, TSH, PRL, and viral serology). Comment: uncertain if all women were screened by transvaginal ultrasound before entering the trial. We could not obtain clarification from the primary study authors. Inclusion criteria: • healthy women Exclusion criteria: • sexually transmitted disease • pelvic inflammatory disease • pregnancy • active vaginal bleeding Study duration: 10 months. |
| Interventions |
Hysteroscopy (intervention: N = 55) vs no hysteroscopy (control: N = 55) before IUI.
The women of group 1 (intervention, N = 55) underwent hysteroscopy to rule out pathology of the endometrial cavity. During this procedure, the endometrial cavity was examined for the presence of polyps, or submucosal myoma, or other pathologic conditions. Any projection inside the uterine cavity was observed, with special attention to its shape and echo, whether it was of polypoid‐like structure, or type of myomas. No data on the instrumentation used, the timing and technique of the hysteroscopic intervention, or the type of anaesthesia. In case of surgical treatment of unsuspected uterine cavity abnormalities, IUI was started after 2 or 3 cycles, whereas IUI was done in the next cycle when hysteroscopy was normal. The women of group 2 (control, N = 55) were treated with IUI without prior hysteroscopy. Clomifen (50 to 100 mg per day) followed by HMG (75 U per day) were given for ovarian stimulation. Transvaginal ultrasonography was done between cycle day 12 and 14. A single dose of human chorionic gonadotrophin (hCG) was used to induce ovulation if the follicles were about 18 to 20 mm. Semen specimens were washed using the swim‐up method, and a single IUI using a volume of 0.3 mL was done 36 hours after hCG injection. Pregnancy was documented by the serum hCG level, 2 weeks after IUI. When pregnant, a transvaginal ultrasound examination was carried out 2 to 4 weeks later. |
| Outcomes | No explicit prioritisation of outcomes by the primary study authors. Main outcome: pregnancy rate. Pregnancy was not defined. In the abstract, the authors mention clinical pregnancy rates. According to the methods section, pregnancy was documented by a serum hCG level, 2 weeks after IUI. When positive, a transvaginal examination was scheduled 2 to 4 weeks later. We assume that only clinical pregnancies, defined by positive findings at transvaginal ultrasound after positive hCG testing, were counted as the main outcome measure. The time point at which the main outcome was measured (clinical pregnancy) was not reported. We judged that the clinical pregnancy rates were measured after one IUI cycle, since the number of women treated was 110 and the number of IUI cycles was 114,±,2.07 in the control group and 106,±,1.92 in the intervention group. Other outcomes: abortion rate ‐ not defined, and hysteroscopy complications. |
| Notes | Did not report if participants had already had a hysteroscopy prior to the fertility assessment or not. No information on endometrial biopsy or not at the time of the screening hysteroscopy. We repeatedly contacted the corresponding author (Dr Masoud Hemadi) but failed to obtain further clarification. |
hCG =
Characteristics of ongoing studies [ordered by study ID]
NCT02245750.
| Trial name or title | Value of routine hysteroscopy prior to IVF/ICSI cycles |
| Methods | Randomized controlled trial |
| Participants | Women with recurrent implantation failure planned for IVF Inclusion Criteria: patient's age ranged from 20 to 40 years; normal appearance of the uterine cavity on hysterosalpingography; patients prepared for IVF/ICSI cycle. Exclusion Criteria: Patients who have any contraindications for hysteroscopy. (menstruation, pregnancy, severe vaginitis or cervicitis, endometrial infection, and history of pelvic inflammatory diseases); patients with uterine cavity pathology previously known to the examiner; patients with previous uterine surgery, such as myomectomy; patients with abnormal HSG |
| Interventions | Hysteroscopy with endometrial injury |
| Outcomes | Live birth, clinical pregnancy rates |
| Starting date | August 2014 |
| Contact information | ahmadmarzok85@gmail.com |
| Notes | We tried contacting the authors but did not get any response regarding status of the trial. |
NCT03173404.
| Trial name or title | Benefits of hysteroscopy prior to performing a cycle of in vitro fertilization/intracytoplasmic sperm injection |
| Methods | Randomized controlled trial |
| Participants | Women scheduled for their first or second IVF/ICSI cycle and with no abnormality detected in transvaginal ultrasound examination Inclusion criteria:
Exclusion criteria:
|
| Interventions | Hysteroscopy before IVF |
| Outcomes | Biochemical pregnancy, ongoing pregnancy, and live birth rates |
| Starting date | 2014 |
| Contact information | La Paz University, Madrid. No contact email address or author name provided |
| Notes | We could not identify author names or email ID to contact for status of the study. |
PACTR201402000691997.
| Trial name or title | Role of hysteroscopy before first trial ICSI: a prospective randomized controlled trial |
| Methods | Randomized controlled trial |
| Participants | Women undergoing first IVF Inclusion criteria: no previous IVF/ICSI cycle Exclusion criteria: antral follicle count (AFC) 4 Anti‐mullarian hormone (AMH) 0.7 Detectable uterine pathology by ultrasound Age minimum: 20 years Age maximum: 40 years |
| Interventions | Hysteroscopy before IVF |
| Outcomes | Clinical pregnancy, miscarriage rate |
| Starting date | June 2013 |
| Contact information | hassanmaghraby@gmail.com |
| Notes | Status: ongoing recruitment on trial registry. We contacted one of the authors, but could not get confirmation on status of the trial. |
UMIN000025679.
| Trial name or title | Technique with intrauterine fiberscope and curettage of the endometrium (IFCE) improves the pregnancy rate for infertile patients with repeated embryo implantation failures ‐ a randomized controlled trial |
| Methods | Randomized controlled trial |
| Participants | Women with recurrent implantation failure planned for IVF Women age: 18 to 50 years BMI ‐ 18.5 to 30 Normal ovarian reserve: AFC = 8 ; FSH < 8 Exclusion: severe male factor |
| Interventions | Hysteroscopy with endometrial scratch |
| Outcomes | Clinical pregnancy rate |
| Starting date | 2014 |
| Contact information | funabiki_m@oakclinic‐group.com |
| Notes | We contacted the authors for data. The authors were currently doing data reanalysis and could not provide the necessary data for inclusion in the current review. |
Differences between protocol and review
We renamed the first category of randomised comparisons 'A routine, screening hysteroscopy, including hysteroscopic treatment of any detected uterine cavity abnormalities versus no hysteroscopy in subfertile women wishing to conceive spontaneously'.
Introduction of a second category of randomised comparisons, namely 'A routine, screening hysteroscopy, including hysteroscopic treatment of any detected uterine cavity abnormalities versus no hysteroscopy before intrauterine insemination (IUI)'. The inclusion of this category made the review more comprehensive.
We used ongoing pregnancy as a surrogate outcome for live birth when data for live birth were not available.
In the protocol, the planned sensitivity analysis was restricted to studies without high risk of bias (not at high risk of bias in any domain and at low risk for randomisation procedures). We changed the eligibility to studies at low risk of bias (without high or unclear risk of bias in any domain) as per recent Cochrane editorial guidelines.
In the protocol, the sensitivity analysis was planned only for primary outcomes but in the review, we conducted a sensitivity analysis for a clinically important secondary outcome (clinical pregnancy rate). This was done to test the robustness of our findings.
In the protocol, the planned analyses were to have been done using a random‐effects model. In this review, we conducted analyses using a fixed‐effect model.
Contributions of authors
MSK and JB screened the studies, extracted and analysed data, and wrote the review; SS and SKS contributed to screening, and data extraction, and commented on the review draft.
TDH, SW, BWM, and FB gave clinical and methodological advice, reviewed and commented on the review.
Sources of support
Internal sources
-
Cochrane South Asia, Prof BV Moses Centre for Evidence‐Informed Health Care and Health Policy, Christian Medical College, Vellore, India.
Protocol development and review completion workshops
External sources
None, Other.
Declarations of interest
Mohan S Kamath, Jan Bosteels, Srividya Seshadri, Steven Weyers, and Sesh Kamal Sunkara have no conflicts of interest to declare.
Thomas M D’Hooghe, MD, PhD, is a Professor in Reproductive Medicine, Department of Development and Regeneration, University of Leuven (KU Leuven), Belgium, and Professor Adjunct, Department of Obstetrics and Gynecology, Yale University, New Haven, USA. Since October 2015, he has been Vice‐President and Head of Global Medical Affairs Fertility, Merck KGaA, Darmstadt, Germany. His participation in this publication is part of his academic work. Merck KGaA is not involved in the development or marketing of products related to hysteroscopy. Professor D'Hooghe's employment by Merck is not in breach of Cochrane's Commercial Sponsorship Policy (clause 2), as he does not have a real or potential financial interest in the outcome of this review. This matter was referred to Cochrane's Funding Arbiter for advice.
Ben Willem J Mol has received consultancy fees from ObsEva Geneva, Guerbet, and Merck; payment for review preparation from European Journal of Obstetrics and Gynecology and Reproductive Biology; and travel, accommodation, and meeting expenses for various non‐commercial scientific meetings. Ben Willem J Mol was author of a trial included in the review (Smit 2016). He did not participate in selection of the studies or in extracting data from that study.
Frank J Broekmans has received monetary compensation for the following: member of the external advisory board for Merck Serono and Ferring, the Netherlands; educational activities for Ferring BV, the Netherlands; consultancy work for Gedeon Richter, Belgium; strategic co‐operation with Roche on automated anti‐Müllerian hormone (AMH) assay development; and research co‐operation with Ansh Labs. Frank K Broekmans was author of a trial included in the review (Smit 2016). He did not participate in selection of the studies or in extracting data from that study.
Edited (no change to conclusions)
References
References to studies included in this review
Aghahosseini 2012 {published data only}
- Aghahosseini M, Ebrahimi N, Mahdavi A, Aleyasin A, Safdarian L, Sina S. Hysteroscopy prior to assisted reproductive technique in women with recurrent implantation failure improves pregnancy likelihood. Fertility and Sterility 2012;98(Suppl 3):S4. [Google Scholar]
Alleyassin 2017 {published data only}
- Alleyassin A, Abiri A, Agha‐Hosseini M, Sarvi F. The value of routine hysteroscopy before the first intracytoplasmic sperm injection treatment cycle. Gynecologic and Obstetric Investigation 2017;82(2):125‐30. [DOI] [PubMed] [Google Scholar]
Demirol 2004 {published data only}
- Demirol A, Gurgan T. Effect of treatment of intrauterine pathologies with office hysteroscopy in patients with recurrent IVF failure. Reproductive Biomedicine Online 2004;8(5):590‐4. [DOI] [PubMed] [Google Scholar]
El‐Nashar 2011 {published data only}
- El‐nashar IH, Nasr A. The role of hysteroscopy before intracytoplasmic sperm injection (ICSI): a randomized controlled trial. Fertility and Sterility 2011;96(Suppl 3):S266. [Google Scholar]
Elsetohy 2015 {published data only}
- Elsetohy KA, Askalany AH, Hassan M, Dawood Z. Routine office hysteroscopy prior to ICSI vs. ICSI alone in patients with normal transvaginal ultrasound: a randomized controlled trial. Archives of Gynecology and Obstetrics 2015;291(1):193‐9. [DOI] [PubMed] [Google Scholar]
El‐Toukhy 2016 {published data only}
- El‐Toukhy T, Campo R, Khalaf Y, Tabanelli C, Gianaroli L, Gordts SS, et al. Hysteroscopy in recurrent in‐vitro fertilisation failure (TROPHY): a multicentre, randomised controlled trial. Lancet 2016;387(100382):614‐21. [DOI] [PubMed] [Google Scholar]
- El‐Toukhy T, Campo R, Sunkara SK, Khalaf Y, Coomarasamy A. A multi‐centre randomised controlled study of pre‐IVF outpatient hysteroscopy in women with recurrent IVF implantation failure: Trial of Outpatient Hysteroscopy ‐ (TROPHY) in IVF. Reproductive Health 2009;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juul Hare 2018 {unpublished data only}
- NCT01743391. Hysteroscopy before in vitro fertilization ‐ does it improve the outcome?. clinicaltrials.gov/ct2/show/NCT01743391 (first posted 6 December 2012).
Rama Raju 2006 {published data only}
- Rama Raju GA, Shashi Kumari G, Krishna KM, Prakash GJ, Madan K. Assessment of uterine cavity by hysteroscopy in assisted reproduction programme and its influence on pregnancy outcome. Archives of Gynecology and Obstetrics 2006;274(3):160‐4. [DOI] [PubMed] [Google Scholar]
Seyam 2015 {published data only}
- Seyam EEM, Hassan MM, Gad MT, Mahmoud HS, Ibrahim MG. Pregnancy outcome after office microhysteroscopy in women with unexplained infertility. International Journal of Fertility & Sterility 2015;9(2):168‐75. [PUBMED: 26246874 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shawki 2012 {published data only}
- Shawki HE, Elmorsy M, Eissa MK. Routine office hysteroscopy prior to ICSI and its impact on assisted reproduction program outcome: a randomized controlled trial. Middle East Fertility Society Journal 2012;17(1):14‐21. [DOI: 10.1016/j.mefs.2011.04.005] [DOI] [Google Scholar]
Smit 2016 {published data only}
- Smit J, Kasius JC, Eijkemans MJC, Torrance HL, Mol BW, Broekmans FJM. The effectiveness of hysteroscopy prior to IVF: a randomized controlled trial (inSIGHT study). Human Reproduction 2015;30:i305. [Google Scholar]
- Smit JG, Kasius JC, Eijkemans MJ, Koks CA, Golde R, Oosterhuis JG, et al. The inSIGHT study: costs and effects of routine hysteroscopy prior to a first IVF treatment cycle. A randomised controlled trial. BMC Women's Health 2012;12:22. [DOI: 10.1186/1472-6874-12-22.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit JG, Kasius JC, Eijkemans MJ, Koks CA, Golde R, Nap AW, et al. Hysteroscopy before in‐vitro fertilisation (inSIGHT): a multicentre, randomised controlled trial. Lancet 2016;387(10038):2622‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brown 2000 {published data only}
- Brown SE, Coddington CC, Schnorr J, Toner JP, Gibbons W, Oehninger S. Evaluation of outpatient hysteroscopy,saline infusion hysterosonography, and hysterosalpingography in infertile women: a prospective, randomized study. Fertility and Sterility 2000;74(5):1029‐34. [DOI] [PubMed] [Google Scholar]
El‐Khayat 2015 {published data only}
- El‐Khayat W, Elsadek M, Saber W. Comparing the effect of office hysteroscopy with endometrial scratch versus office hysteroscopy on intrauterine insemination outcome: a randomized controlled trial. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2015;194:96‐100. [DOI: 10.1016/j.ejogrb.2015.08.025; PUBMED: 26344351] [DOI] [PubMed] [Google Scholar]
Fatemi 2010 {published data only}
- Fatemi HM, Kasius JC, Timmermans A, Disseldorp J, Fauser BC, Devroey P, et al. Prevalence of unsuspected uterine cavity abnormalities diagnosed by office hysteroscopy prior to in vitro fertilization. Human Reproduction 2010;25(8):1959‐65. [DOI] [PubMed] [Google Scholar]
Hebeisha 2018 {published data only}
- Hebeisha SA, Moiety FS, Samir M, Hussein M. Effect of endometrial injury on implantation and pregnancy rates: a randomised controlled trial. Clinical and Experimental Obstetrics and Gynecology 2018;45(1):105‐8. [Google Scholar]
Kamel 2015 {published data only}
- Kamel M, Elsedeek M, Mohamed K. Should office hysteroscopy be done routinely before first ICSI trial?. Journal of Minimally Invasive Gynecology 2015;22(6S):S111‐2. [DOI] [PubMed] [Google Scholar]
Kasius 2013 {published data only}
- Kasius JC, Eijkemans RJ, Mol BW, Fauser BC, Fatemi HM, Broekmans FJ. Cost‐effectiveness of hysteroscopy screening for infertile women. Reproductive Biomedicine Online 2013;26(6):619‐26. [DOI] [PubMed] [Google Scholar]
Shokeir 2016 {published data only}
- Shokeir T, Ebrahim M, El‐Mogy H. Hysteroscopic‐guided local endometrial injury does not improve natural cycle pregnancy rate in women with unexplained infertility: randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2016;42(11):1553‐7. [DOI: 10.1111/jog.13077; PUBMED: 27363928 ] [DOI] [PubMed] [Google Scholar]
Siristatidis 2017 {published data only}
- Siristatidis C, Kreatsa M, Koutlaki N, Galazios G, Pergialiotis V, Papantoniou N. Endometrial injury for RIF patients undergoing IVF/ICSI: a prospective non‐randomized controlled trial. Gynecology Endocrinology 2017;33(4):297‐300. [DOI] [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Wang MM, Hao CF, Bao HC. Clinical efficacy of Chinese herbal retention enema combined with intrauterine douching for patients with endometritis. Zhongguo Zhong Xi Yi Jie He Za Zhi 2011;31(5):639‐42. [PubMed] [Google Scholar]
Zhang 2015 {published data only}
- Zhang XL, Fu YL, Kang Y, Qi C, Zhang QH, Kuang YP. Clinical observations of sequential therapy with Chinese medicine and hysteroscopic mechanical stimulation of the endometrium in infertile patients with repeated implantation failure undergoing frozen‐thawed embryo transfer. Chinese Journal of Integrative Medicine 2015;21(4):249‐53. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Moramezi 2012 {published data only}
- Moramezi F, Barati M, Mohammadjafari R, Barati S, Hemadi M. Effect of hysteroscopy before intra uterine insemination on fertility in infertile couples. Pakistan Journal of Biological Sciences: PJBS 2012;15(19):942‐6. [PUBMED: 24159691] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02245750 {unpublished data only}
- NCT02245750. Value of routine hysteroscopy prior to IVF/ICSI cycles. clinicaltrials.gov/ct2/show/NCT02245750 (first posted 22 September 2014).
NCT03173404 {unpublished data only}
- NCT03173404. Benefits of hysteroscopy prior to performing a cycle of in vitro fertilization/intracytoplasmic sperm injection. clinicaltrials.gov/ct2/show/NCT03173404 (first posted 1 June 2017).
PACTR201402000691997 {unpublished data only}
- PACTR201402000691997. Role of hysteroscopy before first trial ICSI: A prospective randomized controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201402000691997 (registered 29 October 2013).
UMIN000025679 {unpublished data only}
- UMIN000025679. The evaluation for IFCR (intrauterine fiberscope and curettage of the endometrium) by a prospective randomized controlled trial. upload.umin.ac.jp/cgi‐open‐bin/icdr_e/ctr_view.cgi?recptno=R000029541 (last modified 14 January 2017).
Additional references
Ahmad 2017
- Ahmad G, Saluja S, O'Flynn H, Sorrentino A, Leach D, Watson A. Pain relief for outpatient hysteroscopy. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD007710.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
ASRM 2016
- Practice Committee of the American Society for Reproductive Medicine (ASRM). Uterine septum: a guideline. Fertility and Sterility 2016;106(3):530‐40. [DOI] [PubMed] [Google Scholar]
Barash 2003
- Barash A, Dekel N, Fieldust S, Segal I, Schechtman E, Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertility and Sterility 2003;79(6):1317‐22. [DOI] [PubMed] [Google Scholar]
Boivin 2007
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment‐seeking: potential need and demand for infertility medical care. Human Reproduction 2007;22(6):1506‐12. [DOI] [PubMed] [Google Scholar]
Bosteels 2015
- Bosteels J, Kasius J, Weyers S, Broekmans FJ, Mol BW, D'Hooghe TM. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD009461.pub3] [DOI] [PubMed] [Google Scholar]
Campo 2014
- Campo R, Meier R, Dhont N, Mestdagh G, Ombelet W. Implementation of hysteroscopy in an infertility clinic: the one‐stop uterine diagnosis and treatment. Facts, Views & Vision in ObGyn 2014;6(4):234‐9. [PMC free article] [PubMed] [Google Scholar]
Crosignani 2000
- Crosignani PG, Rubin BL. Optimal use of infertility diagnostic tests and treatments. The ESHRE Capri Workshop Group. Human Reproduction 2000;15(3):723‐32. [DOI] [PubMed] [Google Scholar]
Di Spiezio Sardo 2016
- Spiezio Sardo A, Carlo C, Minozzi S, Spinelli M, Pistotti V, Alviggi C, et al. Efficacy of hysteroscopy in improving reproductive outcomes of infertile couples: a systematic review and meta‐analysis. Human Reproduction Update 2016;22(4):479‐96. [DOI] [PubMed] [Google Scholar]
EIM/ESHRE 2016
- European IVF‐Monitoring Consortium (EIM), European Society of Human Reproduction and Embryology (ESHRE), Kupka MS, D'Hooghe T, Ferraretti AP, Mouzon J, Erb K, Castilla JA, et al. Assisted reproductive technology in Europe, 2011: results generated from European registers by ESHRE. Human Reproduction 2016;31(2):233‐48. [DOI] [PubMed] [Google Scholar]
El‐Toukhy 2008
- El‐Toukhy T, Sunkara SK, Coomarasamy A, Grace J, Khalaf Y. Outpatient hysteroscopy and subsequent IVF cycle outcome: a systematic review and meta‐analysis. Reproductive Biomedicine Online 2008;16(5):712‐9. [DOI] [PubMed] [Google Scholar]
Fiorentino 2014
- Fiorentino F, Bono S, Biricik A, Nuccitelli A, Cotroneo E, Cottone G, et al. Application of next‐generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Human Reproduction 2014;29(12):2802‐13. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT [GRADEpro Guideline Development Tool [Software].]. Version accessed 4 November 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jansen 2000
- Jansen FW, Vredevoogd CB, Ulzen K, Hermans J, Trimbos JB, Trimbos‐Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstetrics & Gynecology 2000;96(2):266‐70. [DOI] [PubMed] [Google Scholar]
Lefebre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Mansour 2014
- Mansour R, Ishihara O, Adamson GD, Dyer S, Mouzon J, Nygren KG, et al. International Committee for Monitoring Assisted Reproductive Technologies World Report: Assisted Reproductive Technology 2006. Human Reproduction 2014;29:1536‐51. [DOI] [PubMed] [Google Scholar]
Nastri 2015
- Nastri CO, Lensen SF, Gibreel A, Raine‐Fenning N, Ferriani RA, Bhattacharya S, et al. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD009517.pub3] [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence (NICE). Fertility: assessment and treatment for people with fertility problems. NICE guideline (CG156). www.nice.org.uk/guidance/cg156 Last updated: September 2017.
Oliveira 2003
- Oliveira FG, Abdelmassih VG, Diamond MP, Dozortsev D, Nagy ZP, Abdelmassih R. Uterine cavity findings and hysteroscopic interventions in patients undergoing in vitro fertilization‐embryo transfer who repeatedly cannot conceive. Fertility and Sterility 2003;80(6):1371‐5. [DOI] [PubMed] [Google Scholar]
Pundir 2014
- Pundir J, Pundir V, Omanwa K, Khalaf Y, El‐Toukhy T. Hysteroscopy prior to the first IVF cycle: a systematic review and meta‐analysis. Reproductive Biomedicine Online 2014;28(2):151‐61. [DOI] [PubMed] [Google Scholar]
Ragni 2005
- Ragni G, Diaferia D, Vegetti W, Colombo M, Arnoldi M, Crosignani PG. Effectiveness of sonohysterography in infertile patient work‐up: a comparison with transvaginal ultrasonography and hysteroscopy. Gynecologic and Obstetric Investigation 2005;59(4):184‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salle 1999
- Salle B, Gaucherand P, Saint Hilaire P, Rudigoz RC. Transvaginal sonohysterographic evaluation of intrauterine adhesions. Journal of Clinical Ultrasound 1999;27(3):131‐4. [DOI] [PubMed] [Google Scholar]
Singh 2011
- Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. Journal of Endocrinology 2011;210(1):5‐14. [DOI] [PubMed] [Google Scholar]
Taylor 2008
- Taylor E, Gomel V. The uterus and fertility. Fertility and Sterility 2008;89:1‐16. [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2017
- Zegers‐Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertility and Sterility 2017;108(3):393‐406. [DOI] [PubMed] [Google Scholar]
Zhang 2013
- Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant RD. Physiological and molecular determinants of embryo implantation. Molecular Aspects of Medicine 2013;34(5):939‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]


