Abstract
Reversal of immunodeficiency in the lung by gene therapy is limited in part by the difficulty of transfecting lung cells in vivo. Many options exist for successfully transfecting cells in vitro, but they are not easily adapted to the in vivo condition. To overcome this limitation, we transduced macrophages in vitro with the murine IFN-γ (mIFN-γ) gene and intratracheally delivered the macrophages to express mIFN-γ in vivo. A recombinant retroviral vector pSF91 system was modified to encode mIFN-γ and enhanced green fluorescent protein (EGFP). A murine macrophage cell line J774A.1 transduced with the retroviral supernatant increased secretion from undetectable levels to 131.6 ± 4.2 μg/ml mIFN-γ at 24 h in vitro. The mIFN-γ-producing macrophages were intratracheally instilled into mechanically ventilated scid mice. mIFN-γ levels in the bronchoalveolar lavage increased from undetectable levels at baseline to 158.8 ± 5.1 pg/ml at 48 h (P < 0.001). Analysis of the lavaged cells for EGFP expression revealed that EGFP expression was directly proportional to the number of transduced macrophages instilled into the lung. Immune function was partially restored in the alveolar spaces of scid mice with evidence of enhanced MHC class II antigen expression and increased phagocytosis (P < 0.05). Tumor necrosis factor α was increased from undetectable at baseline to 103.5 ± 11.4 pg/ml. In contrast, i.p. administration of the engineered macrophages did not enhance IFN-γ levels in the lung. Our study suggests airway delivery of genetically engineered macrophages expressing mIFN-γ gene can partially restore significant immune activity in the lungs of immunodeficient mice.
The alveolar immune response in the lung is often compartmentalized with reference to the systemic response (1). The alveolar compartment has an extremely large surface area that is continuously exposed to the external environment; and thus, during systemic immunosuppression the lungs are at high risk for development of opportunistic infection (2, 3). Alveolar macrophages (AMs) are the first cellular response in host defense of the lung. During immunosuppression, the functional activity of AMs diminishes together with loss of other immune activity and the risk of infection increases (4, 5).
IFN-γ, a potent lymphokine, is secreted by T lymphocytes and natural killer (NK) cells in the blood (6) and by macrophages and epithelial cells in the airway (7). IFN-γ is often diminished in the lungs of immunodeficient animals, and its absence is frequently implicated in the susceptibility of the lung to infection (8, 9). IFN-γ has intrinsic antiviral activity, up-regulates expression of major histocompatibility class I and II molecules, activates macrophages and NK cells, and has an important regulatory role in T helper (Th) cell proliferation. Others have used direct airway delivery of IFN-γ (10–13) or the IFN-γ gene in an attempt to reverse the immune deficiency in the lung (14, 15). The former method is prohibitively expensive, and the latter method suffers from the many problems associated with conventional gene therapy to the lung.
Gene therapy has been progressing at a fast pace over the past decade as an alternative therapeutic approach to control a variety of respiratory diseases, including genetic disorders, infectious diseases, immune deficiencies, and cancers (16, 17). Despite initial enthusiasm, gene therapy to the lung has been fraught with several limitations, including inadequate gene delivery to target cells, low level of expression, instability of expression, and induction of persistent inflammation (18–21). There are several in vitro methods of gene transfer that result in excellent gene expression (22–25). If these efficient in vitro methods could be adapted for gene transfer in vivo, this would represent a new approach to facilitate gene expression in the lung.
The purpose of this study was to find an alternative approach to enhance the immune function in the lungs of immunodeficient animals. We hypothesized that ex vivo gene transfer of the IFN-γ gene to macrophages with subsequent airway delivery of the macrophages would reverse alveolar immune deficiency. This approach resulted in a marked increase in murine IFN-γ (mIFN-γ) production in scid (severe combined immunodeficient) mice. Furthermore, AMs revealed enhanced MHC class II expression and increased phagocytic activity. Thus our study suggests that ex vivo transduction of macrophages to overexpress cytokines such as IFN-γ with subsequent airway delivery to the lung can effectively enhance immune activity in the alveolar spaces of immunodeficient animals.
Methods
Cell Culture.
Phoenix-Ampho, GP+E86, viral packaging cell lines were obtained from the American Type Culture Collection (ATCC), and cultured in high-glucose (0.45%) DMEM (BioWhittaker), 10% FBS, 1% l-glutamine, 1 mM sodium pyruvate, 100 units/ml penicillin, and 100 μg/ml streptomycin. The J774A.1 macrophage cell line obtained from ATCC was cultured in DMEM with 10% FBS, 1% l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Plasmid Construction.
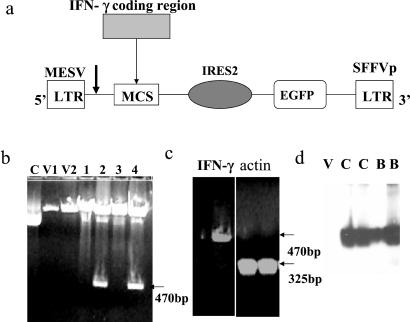
Cloning of mIFN-γ was done by PCR with a template plasmid pORF5-mIFN-γ (InvivoGen, San Diego). mIFN-γ oligo-primers (5′-primer, 5′-CGG-AAT-TCC-ATG-GCC-AAC-GCT-ACA-C and 3′-primer 3′-ACT-GTC-GAC-CCG-AAT-CAG-CAG-CGA-CTC containing added EcoRI and SalI sites, respectively) were used to perform a PCR. The PCR product was cloned into the corresponding sites in plasmid pSF91–RE (provided by Christopher Baum of the University of Hamburg, Hamburg, Germany; ref. 26), a vector derived from murine embryonic stem cell virus (MESV) retrovirus and modified to express enhanced green fluorescent protein (EGFP; Fig. 1a) (27). Its sequence and insertion were confirmed by sequencing as well as by agarose gel electrophoresis.
Figure 1.
Construction of mIFN-γ-producing vector on MESV backbone. (a) Diagram of MESV retroviral vector for producing IFN-γ. From 5′-end: long terminal repeat (LTR), multiple clone sites (MCS) with the mIFN-γ insert, internal ribosome entry site (IRES), EGFP, and 3′-end LTR. The insertion of mIFN-γ was done with a PCR method at the EcoRI and SalI sites. The arrow indicates the transcription start site. The transcript contains IFN-γ, the vector's splice donor and splice acceptor sites along with the EGFP driven by IRES promoter. (b) Agarose gel analysis for the vector construct pSF91-EGFP-mIFN-γ. The positive E. coli HB101 clones expressing mIFN-γ demonstrated a band at 470 bp of EcoRI–SalI fragment corresponding to the mIFN-γ gene. Lanes: C, clone 1 (no digestion); V1, V2, vector; 1–4, clones of mIFN-γ constructs. (c) Reverse transcription PCR of mIFN-γ. (Left) mIFN-γ bands assessed with mIFN-γ primers. Left lane, vector control; right lane, engineered macrophages. (Right) Actin bands assessed with actin primers. Left lane, vector control; right lane, engineered macrophages. (d) Western blot of mIFN-γ in cultured cells and BAL cells assessed by monoclonal antibodies against mIFN-γ (16 kDa). Lanes: V, vector control; C, cultured engineered macrophages; B, BAL cells of scid mice receiving engineered macrophages.
Stable and Transient Viral Production.
Phoenix-Ampho cells were transfected with the retroviral constructs by using Lipofectamine reagent according to the manufacturer's instructions (Life Technologies). The viral supernatant collected from the transfected Phoenix-Ampho cells was used to infect the GP+E86 cells (28). The EGFP-expressing cells were selected by FACS sorting (FACStar Plus, Becton Dickinson). Transient retroviral supernatant derived from Phoenix-Ampho (titers of ≈1.5 × 104 colony-forming units per ml) was also used to directly infect murine macrophage J774A.1 by using the procedures described previously (29). The EGFP-positive J774A.1 macrophages were isolated by FACS sorting. Expression of mIFN-γ in the cells was assessed by Northern blotting and Western blotting using 1:2000 dilution of anti-IFN-γ antibody (Sigma-Aldrich) as previously described (30).
Instillation of IFN-γ-Expressing Macrophages into Mouse Lungs.
Recipient female, 7- to 8-week-old BALB/c scid and C57BL/6 mice were purchased from Harlan Laboratories (Haslett, MI) and housed in pathogen-free conditions. IFN-γ knockout mice were obtained from The Jackson Laboratory. Mice were anesthetized with 0.15 mg ketamine i.m., tracheotomized by using a 20-gauge angiocath, and ventilated by using a small animal ventilator for 10 min (Analytical Specialties), as previously described (31). Transduced or control J774A.1 macrophages (0.5–6 × 106 in 50 μl) were instilled into the airway and ventilated for 10 min. Mice were maintained for 1, 2, 4, 7, 14, and 28 days before being killed to analyze gene expression and immune function in bronchoalveolar lavage (BAL) fluid and cells. In some experiments, J774A.1 macrophages were administered intraperitoneally to compare with the intratracheal instillation.
Quantitation of Murine Cytokine ELISAs.
Tumor necrosis factor (TNF)-α, mIFN-γ, and IL-4 in BAL and blood of various animals (scid, IFN-γ knockout, and C57BL/6) were quantified by using sandwich ELISA Quantikine kits (R&D Systems).
Detection of Class II Antigen Expression.
BAL cells were washed with cold buffer (PBS with 1% FBS and 0.1% sodium azide), and the cells were incubated in glass tubes with R-phycoerythrin (PE)-labeled MHC class II antibodies I-A/I-E (1/200, PharMingen) or with PE-labeled rat isotype Ig as controls in an antibody reaction buffer (2% FBS in PBS at pH 7.5) at 4°C for 2 h. Cells were washed twice with the same buffer followed by centrifugation at 200 × g for 5 min. Class II antigen expression was verified by FACS and fluorescent microscopy.
Phagocytosis Assay.
Phagocytic activity of macrophages was assessed in vitro by using Texas red-labeled Escherichia coli-coated particles (Molecular Probes). Phagocytosis was performed by incubating BAL cells from scid mice that received control or engineered macrophages with 1 × 106 Texas red-labeled E. coli-coated particles for 1–2 h at room temperature. Phagocytosis was quantified by FACS and expressed as percentage of cells with positive fluorescence compared with total cells.
Lung Harvesting for Histological Examination.
At designated time points, mice were killed by i.p. injection of Euthanasia Solution containing pentobarbital (Schering–Plough). Lungs were removed and were homogenized in 1 ml of protease inhibitor (Roche Molecular Biochemicals) by using a tissue homogenizer. After centrifugation, supernatants were collected, passed through a 0.45-μm filter (Gelman), then stored at −70°C for assessment of cytokine levels. For cryosections, the lungs were inflated with Tissue-Tek OCT (Sakura Finetek USA, Torrance, CA) in 0.9% NaCl (1:1). Cryosections of 8 μm for histologic examination were made by using a Leica CM1850 cryostat microtome. Typically, only one lung was lavaged for cytokine analysis; the other lung was used for histological examination of EGFP-expressing cells.
Statistical Analysis.
All experiments were performed three times in triplicate, and animal experiments were carried out in a group of three mice versus control group comprising the same numbers of animals. Data were presented as the mean ± SEM, and data were compared by using Student's t test. All calculations were performed by using the SIGMASTAT statistical program (Jandel Scientific Software).
Results
Production of Cells Expressing mIFN-γ.
A vector expressing mIFN-γ was constructed by inserting the mIFN-γ coding region into revised MESV retroviral vector pSF91. The insertion was performed by means of PCR subcloning of mIFN-γ into the multiple cloning site of the vector near the 5′-end of the IRES2 promoter as an EcoRI/SalI fragment (Fig. 1a) and confirmed by agarose gel electrophoresis. Clones were selected from transformed plates showing the mIFN-γ fragment (Fig. 1b). Insertion of mIFN-γ into the pSF91-EGFP vector and its orientation were confirmed by DNA sequencing analysis.
Northern blot analysis of RNA isolated from cells infected with mIFN-γ-expressing viral supernatant demonstrated mIFN-γ mRNA of the expected size (data not shown). Consistent with the above results, reverse transcription PCR analysis of the mIFN-γ gene during four passages of mIFN-γ provided no evidence of deletion and mutation (Fig. 1c). Expression of the mIFN-γ protein in cell culture and BAL was confirmed by Western blotting (Fig. 1d) and by ELISA.
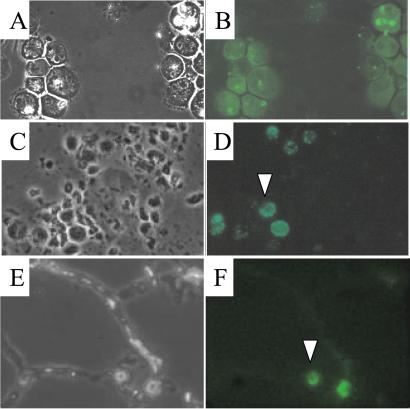
Flow cytometry sorting selection was used to obtain cells of which more than 95% were expressing EGFP (Fig. 2 A and B). Only 30–40% of unselected cells expressed EGFP. mIFN-γ-expressing macrophage cells were present in mouse lung BAL cell populations 4–7 days after intratracheal instillation as detected by microscopy (Fig. 2 C and D). Furthermore, positive EGFP-expressing cells were present in lung cryosections of scid mice (Fig. 2 E and F).
Figure 2.
J774A.1 macrophages expressing mIFN-γ identified by EGFP in vitro and in vivo. (Top) A representative (clone 10) of selected EGFP-positive J774A.1 clones after FACS sorting and cloning (over 90% cells are EGFP-positive), viewed with phase-contrast (A) and fluorescent (B) microscopy. (×800.) (Middle) Detection of J774A.1 macrophages expressing EGFP in scid mouse BAL cells 48 h after instillation by using phase-contrast (C) and fluorescent (D) microscopy. (×400.) Note that residential AMs have a different morphology, and are smaller compared with J774A.1 cells. (Bottom) EGFP-expressing cells in the lung 24 h after instillation, viewed by phase-contrast (E) and fluorescent (F) microscopy. (×400.) A typical J774A.1 cell is shown with an arrowhead in D and F.
Expression of mIFN-γ in Vitro and in Vivo.
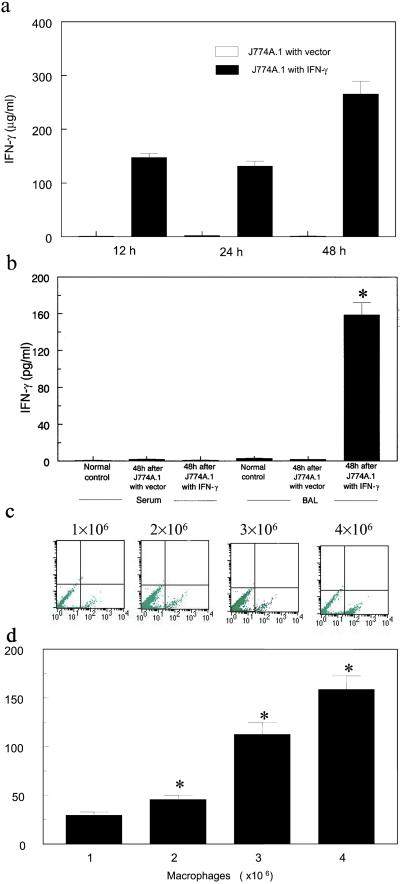
The culture supernatant from J774A.1 macrophages transduced with the mIFN-γ gene secreted 131.6 ± 9.2 μg/ml mIFN-γ at 24 h and peaked at ≈300 μg/ml at 48 h, whereas no mIFN-γ was detected in the culture supernatant from native J774A.1 (data not shown) and J774A.1 macrophages transduced with vector control (P < 0.001, Fig. 3a). mIFN-γ was not detectable in serum or BAL of normal BALB/c, BALB/c scid (Fig. 3b), and was also not detectable in normal C57BL/6, mIFN-γ knockout mice (data not shown). All subsequent studies using intratracheal instillation of J774A.1 macrophages were performed exclusively in scid mice. After instillation of J774A.1 macrophages containing the pSF91 vector, mIFN-γ in BAL from scid mice were still undetectable (Fig. 3b). However, after instillation of J774A.1 macrophages containing the mIFN-γ gene into scid mice, mIFN-γ in BAL fluid was markedly increased. For example, mIFN-γ in BAL at 48 h was increased from being undetectable at baseline to 158.8 ± 12.7 pg/ml (P < 0.001, Fig. 3b).
Figure 3.
Expression of mIFN-γ protein and EGFP in vitro and in vivo. (a) Time-dependent expression of mIFN-γ in cell culture from engineered J774A.1 macrophages determined by ELISA. (b) In vivo mIFN-γ expression in BALB/c scid mice including serum and BAL from normal controls or from mice 48 h after intratracheal instillation of J774A.1 macrophages with vector control or mIFN-γ gene (∗, P < 0.001, compared with control). (c and d) Effect of concentration of airway-delivered J774A.1 macrophages expressing mIFN-γ on number of EGFP-expressing BAL cells assessed by FACS (c, 24 h) and on mIFN-γ levels in BAL assessed by ELISA (d, 24 h). Expression of mIFN-γ correlates with the number of J774A.1 macrophages expressing mIFN-γ (*, P < 0.05).
Interestingly, the production of mIFN-γ in vivo depended on the concentration of instilled J774A.1 macrophages, which was demonstrated either by quantifying EGFP expression (Fig. 3c) or by quantifying mIFN-γ protein (Fig. 3d). This observation indicates that the production of mIFN-γ correlates with the number of J774A.1 macrophages containing mIFN-γ gene.
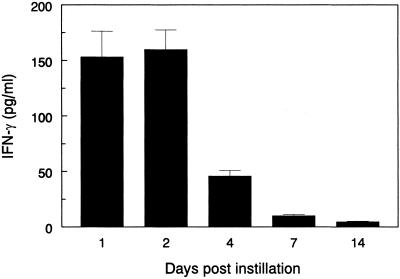
Expression of mIFN-γ was a result of IFN-γ produced from the J774A.1 macrophages transduced with mIFN-γ, as there was no evidence for a background or vector effect of the gene constructs. This expression was stable up to a week, but gradually diminished over the next 3 weeks (Fig. 4). Even so, mIFN-γ in BAL was detectable at 1 month after instillation of the J774A.1 macrophages transduced with the mIFN-γ gene.
Figure 4.
Time-dependent in vivo expression of mIFN-γ in BAL after intratracheal instillation of 106 mIFN-γ-expressing macrophages into scid mice. IFN-γ expression peaked at the first week and eventually tapered, but was detectable for a month.
Activation of Immune Function by Instillation of J774A.1 Expressing mIFN-γ.
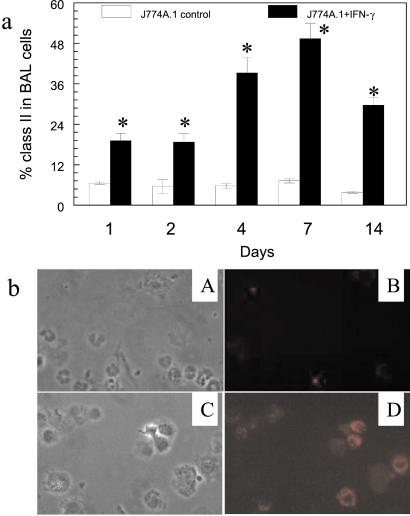
Overexpression of mIFN-γ in the lung enhanced immune function in the lungs of scid mice. For example, 48 h after instillation of mIFN-γ-expressing J774A.1 macrophages, expression of MHC class II antigen on AMs was 19.4 ± 1.7%, whereas, 48 h after instillation of vector control J774A.1 macrophages, expression of MHC class II antigen on AMs was only 6.5 ± 0.7% (Fig. 5a, P < 0.05). Expression of class II antigen remained at high levels up to 14 days, suggesting the persistence of immune stimulation by mIFN-γ. Similarly, there was morphological evidence of AM activation by staining AMs with PE after airway instillation of J774A.1 macrophages containing mIFN-γ as compared with vector control J774A.1 (Fig. 5b).
Figure 5.
Expression of MHC class II antigen (I–A/I–E) in BAL cells of scid mice receiving J774A.1 expressing mIFN-γ. (a) Expression of I–A/I–E class II antigen from 1–14 days, assessed by PE-labeled anti-IA/I-E antibodies. (∗, P < 0.05, compared with control.) (b) Fluorescent microscopy of BAL cells expressing class II antigen (I-A/I-E) 48 h after intratracheal instillation of the engineered J774A.1 macrophages expressing mIFN-γ. (A and B) Cells with vector control showed by phase-contrast (A) and PE fluorescent (B) microscopy. (×400.) (C and D) Cells expressing mIFN-γ as shown by phase-contrast (C) and PE fluorescent (D) microscopy. (×400.)
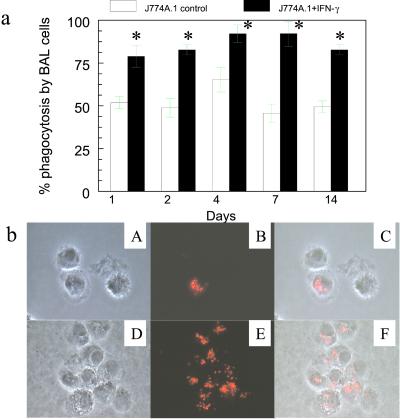
The ability of IFN-γ-expressing macrophages to restore AM immune function was confirmed by the enhanced phagocytosis activity by AMs after airway instillation of mIFN-γ-expressing cells. For example, phagocytic activity was markedly increased to 82.8 ± 2.9% in scid mice at 48 h after instillation of 2 × 106 mIFN-γ-expressing J774A.1 macrophages; however, phagocytic activity of AMs from scid mice after airway instillation of vector control J774A.1 at 48 h was only 48.8 ± 5.3% (P < 0.05, Fig. 6a). Consistent with the class II antigen expression, enhanced phagocytic activity was also detectable up to 14 days after instillation of mIFN-γ-producing J774A.1 macrophages. Furthermore, after airway instillation of mIFN-γ-expressing macrophages there was morphologic evidence that BAL cells were more phagocytic than control BAL cells (Fig. 6b).
Figure 6.
Phagocytosis by BAL cells after intratracheal instillation of the engineered J774A.1 macrophages expressing mIFN-γ into scid mice. (a) Phagocytic function of BAL cells determined by Texas red-labeled E. coli particles from BALB/c scid mice 1–14 days after receiving J774A.1 macrophages expressing mIFN-γ (∗, P < 0.05, compared with control). (b) Phase-contrast and fluorescent microscopy of BAL cells phagocytizing Texas red-labeled E. coli particles. (×400.) (Upper) BAL cells from mice receiving J774A.1 containing vector control shown by phase-contrast (A) and fluorescent (B) microscopy and merged images (C). (Lower) BAL cells from mice receiving J774A.1 expressing mIFN-γ as shown by phase-contrast (D) and fluorescent (E) microscopy and merged images (F).
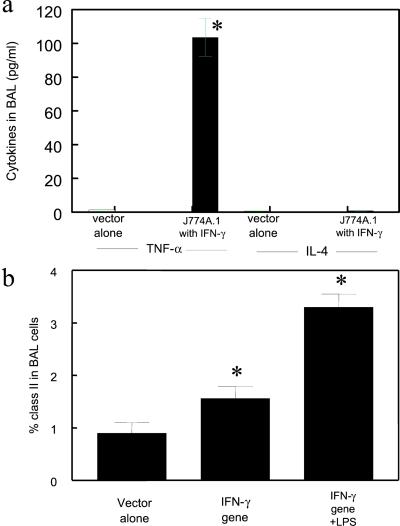
After instillation of mIFN-γ-expressing J774A.1 macrophages, BAL cells produced 103.5 ± 11.4 pg/ml of TNF-α; whereas after instillation of vector control macrophages, BAL cells produced 0.6 ± 0.1 pg/ml TNF-α (Fig. 7a, P < 0.001). Furthermore, lipopolysaccharide (LPS) was found to further stimulate the BAL cells of the mice that received macrophage-expressing mIFN-γ. After addition of 10 μg/ml LPS to the cultured BAL cells of mice receiving J774A.1 expressing mIFN-γ, the levels of MHC class II in the BAL cells was further enhanced by 2-fold compared with the control BAL cells with the engineered J774A.1 but without LPS (Fig. 7b). However, addition of LPS did not result in higher phagocytic activity compared with the control BAL cells receiving the engineered J774A.1 (data not shown).
Figure 7.
Cytokine expression in BAL from scid mice following intratracheal instillation of J774A.1 expressing mIFN-γ. (a) Cytokine expression in BAL 48 h after instillation of macrophages expressing mIFN-γ and vector control (∗, P < 0.01, compared with control). (b) MHC class II expression by BAL cells 24 h after intratracheal instillation of J774A.1 expressing mIFN-γ into scid mice. In the right-most column the BAL cells were stimulated with E. coli stereotype 026:B6 lipopolysaccharide (LPS) at 10 μg/ml for a further 24 h (∗, P < 0.05, compared with control).
Comparison of Airway Delivery and i.p. Delivery of mIFN-γ-Expressing Macrophages.
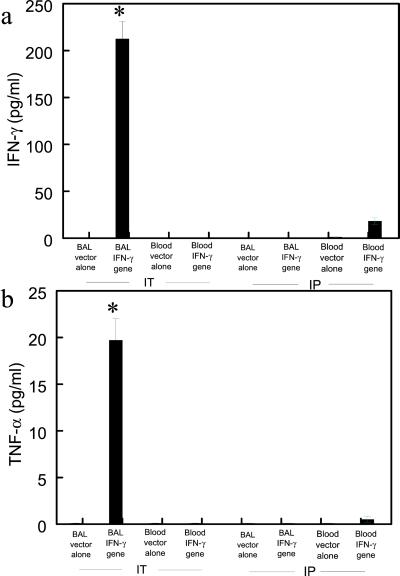
After i.p. administration of the J774A.1 macrophages expressing mIFN-γ, mIFN-γ was increased from 0 to 18.3 ± 3.2 pg/ml in the blood at 24 h, but was undetectable in the BAL fluid (Fig. 8a, P < 0.01). After i.p. administration of mIFN-γ-expressing macrophages, TNF-α was increased from 0 to 2.5 ± 0.3 pg/ml in the blood at 24 h, but was undetectable in the BAL fluid (Fig. 8b, P < 0.01). In contrast, after intratracheal instillation of the J774A.1 macrophages expressing mIFN-γ, mIFN-γ in the BAL increased significantly to 224.5 ± 18.7 pg/ml at 24 h, but was undetectable in the blood (Fig. 8a, P < 0.01). In addition, after intratracheal instillation of mIFN-γ-expressing macrophages, TNF-α in BAL increased to 21.4 ± 3.5 pg/ml, but was not detectable in the blood (Fig. 8b).
Figure 8.
Comparison of cytokine production between airway and i.p. delivery of J774A.1 expressing mIFN-γ. (a) mIFN-γ production in BAL from scid mice (24 h) receiving J774A.1 macrophages expressing mIFN-γ by airway (IT) and i.p. delivery (∗, P < 0.01, compared with control). (b) TNF-α production in BAL from scid mice (24 h) receiving J774A.1 macrophages expressing mIFN-γ by airway and i.p. delivery (∗, P < 0.01, compared with control).
Discussion
Our study offers an approach to reverse alveolar immunodeficiency even during ongoing systemic immunosuppression. Airway delivery of the genetically engineered J774A.1 macrophages expressing high levels of mIFN-γ resulted in a rapid increase in mIFN-γ levels in the lung with a subsequent physiological response by resident AMs. Delivery of the IFN-γ-expressing macrophages to the lower respiratory tract resulted in several positive outcomes: (i) production of mIFN-γ above physiological concentration in the BAL fluid of scid mice; (ii) expression of mIFN-γ in a concentration-dependent and time-dependent manner; (iii) increased MHC class II antigen; (iv) enhanced phagocytic activity; and (v) production of a proinflammatory cytokine (TNF-α), but no production of IL-4.
This report describes use of ex vivo gene transfer to macrophages to overexpress a specific protein in the lung. We used this approach to overexpress IFN-γ to help correct immunodeficiency, even when systemic immunosuppression persists. Others have engineered cells to overexpress cytokines or other genes in other organ systems (32, 33). For example, cancer cells have been engineered to express macrophage colony-stimulating factor to boost immune response against tumor cells (34, 35). Also, macrophages were engineered to overexpress β-galactosidase or IκBαM and were delivered to glomeruli to model experimental glomerulonephritis (36, 37).
The lung is uniquely well suited for successful delivery of macrophages engineered to overexpress proteins such as INF-γ. Intratracheal delivery of transduced macrophages resulted in high levels of mIFN-γ in the lung. Our study demonstrated a rapid increase in IFN-γ levels in the lung that tapered toward baseline over the next month. Expression of MHC class II antigen on AMs increased up to 4 days, and was maintained at high levels for 14 days. In addition, enhanced phagocytic activity in AMs occurred quickly and was maintained up to 14 days compared with control macrophages. This finding suggests that despite a decline in IFN-γ expression over time, the impact of both immune functions was sustainable. It is possible that the time-limited IFN-γ production in vivo was due to diminished survival of the instilled cultured macrophages. We assume that a more sustainable level of expression will occur if freshly isolated AMs are used for gene transduction, as AMs are likely to survive longer in the lower respiratory tract. However, it may be preferable to have expression of IFN-γ be limited by time. The physiological concentration of IFN-γ in this model may be sufficient for enhancing immune function sufficient to control an infection. However, prolonged expression of IFN-γ may also have potential untoward side effects. It was recently demonstrated that chronic expression of IFN-γ could be associated with development of pulmonary emphysema (38). Thus, time-limited expression of IFN-γ is advantageous. This may also be achieved by using a toggle promoter that can be regulated in response to an exogenous agent such as tetracycline (39).
A minimal inflammatory response was associated with instillation of IFN-γ-expressing macrophages (data not shown). This finding is in agreement with previous investigations by Kolls et al. (40), who demonstrated that there was no additional immune response to IFN-γ expression above the inflammation directed to the adenovirus vector control. One of the reasons for the minimal response in our study may be that the engineered macrophage J774A.1 were derived from the same strain as the recipient BALB/c scid mice.
Our study demonstrated that IFN-γ-expressing macrophages could rapidly increase levels of IFN-γ and TNF-α in the BAL, but there was no increase in the levels of IL-4, indicating a typical T helper 1 (Th1) response. Cytokine therapy may help control underlying lung infections. Beck et al. (10) used aerosolized recombinant IFN-γ to reduce the intensity of Pneumocystis carinii pneumonia in mice; however, this strategy required repeated use of the cytokine up to 2 weeks and is prohibitively expensive as a potential therapeutic approach. Our laboratory has demonstrated that IFN-γ-primed macrophages enhance the killing of P. carinii by a TNF-α-dependent mechanism (41). Thus, modulation of cytokines to induce a vigorous Th1 response may help control opportunistic pulmonary infections.
A previous study showed that intrapulmonary delivery of TNF-α could reverse sepsis-induced impairment in mice better than i.p. administration of TNF-α (42). In our study, we also found that compartmentalized delivery and expression of IFN-γ-expressing macrophages significantly enhanced the IFN-γ expression in the lung, whereas i.p. administration of mINF-γ-expressing macrophages did not enhance mIFN-γ in the lung. This result suggests that targeting of mIFN-γ-expressing macrophages to the lung is more likely to correct the immune deficiency in the alveolar compartment during systemic immunosuppression.
Several vectors, including adenovirus, retrovirus, and liposome, have been used to modulate cytokine levels in the lung (40, 43). Use of adenoviral vector for gene transfer enhanced expression of IFN-γ or TNF-α, but was also accompanied by an adverse immune reaction toward the adenoviral vector (16, 21). Retroviral vectors are associated with lower immune response; however, retroviral vectors may have limited use for gene therapy in the lung because of their inactivation by AMs in the lung (44). We have achieved reasonably high titers of IFN-γ by intratracheal transfer of genetically modified macrophages. This approach may provide a new model of gene therapy in the lung.
Acknowledgments
We thank Dr. Christopher Baum of University of Hamburg for the pSF vector. We also thank Z. Zhu, J. R. Wagner, M. Brown, and T. Evanoff for their technical help. This work was supported by National Institutes of Health Grants RO1 AI48455 and NCI PO1-CA75426.
Abbreviations
- mIFN-γ
murine IFN-γ
- TNF-α
tumor necrosis factor α
- EGFP
enhanced green fluorescent protein
- BAL
bronchoalveolar lavage
- LPS
lipopolysaccharide
- PE
phycoerythrin
- AM
alveolar macrophage
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hunninghake G W, Crystal R G. N Engl J Med. 1981;305:429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- 2.Limper A H, Offord K P, Smith T F, Martin II W J. Am Rev Respir Dis. 1989;140:1024–1209. doi: 10.1164/ajrccm/140.5.1204. [DOI] [PubMed] [Google Scholar]
- 3.Pifer L L, Hughes T, Stagno S, Woods D. Pediatrics. 1978;62:35–41. [PubMed] [Google Scholar]
- 4.Reynolds H Y. Clin Chest Med. 1987;8:339–559. [PubMed] [Google Scholar]
- 5.Hunninghake G W, Fauci A S. J Immunol. 1977;118:146–150. [PubMed] [Google Scholar]
- 6.Krampera M, Tavecchia L, Benedetti F, Nadali G, Pizzolo G. Haematologica. 2000;85:675–679. [PubMed] [Google Scholar]
- 7.Fenton M J, Vermeulen M W, Kim S, Burdick M, Strieter R M, Kornfeld H. Infect Immun. 1997;65:5149–5156. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaffner A. J Clin Invest. 1985;76:1755–1764. doi: 10.1172/JCI112166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudmann D G, Preston A M, Moore M W, Beck J M. J Immunol. 1998;161:360–366. [PubMed] [Google Scholar]
- 10.Beck J M, Liggitt H D, Brunette E N, Fuchs H J, Shellito J E, Debs R J. Infect Immun. 1991;59:3859–3862. doi: 10.1128/iai.59.11.3859-3862.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffe H A, Buhl R, Mastrangeli A, Holroyd K J, Saltini C, Czerski D, Jaffe H S, Kramer S, Sherwin S, Crystal R G. J Clin Invest. 1991;88:297–302. doi: 10.1172/JCI115291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai H, Guo J, Choi H, Kurup V. J Infect Dis. 1995;172:1554–1560. doi: 10.1093/infdis/172.6.1554. [DOI] [PubMed] [Google Scholar]
- 13.Badger A, Meunier P C, Weiss R A, Bugelski P J. J Interferon Res. 1988;8:251–260. doi: 10.1089/jir.1988.8.251. [DOI] [PubMed] [Google Scholar]
- 14.Kolls J K, Lei D H, Nelson S, Summer W R, Shellito J E. Chest. 1997;111,(Suppl. 6):104S. doi: 10.1378/chest.111.6_supplement.104s. [DOI] [PubMed] [Google Scholar]
- 15.Steinmuller C, Franke-Ullmann G, Lohmann-Matthes M L, Emmendorffer A. Am J Respir Cell Mol Biol. 2000;22:481–490. doi: 10.1165/ajrcmb.22.4.3336. [DOI] [PubMed] [Google Scholar]
- 16.Crystal R G, MecElvaney N G, Rosenfeld M A, Chu C S, Mastrangeli A, Hay J G. Nat Genet. 1994;8:42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- 17.Curiel D T, Pilewsky J M, Albelda S M. Am J Respir Cell Mol Biol. 1996;14:1–18. doi: 10.1165/ajrcmb.14.1.8534480. [DOI] [PubMed] [Google Scholar]
- 18.Albelda S M, Wiewrodt R, Zuckerman J B. Ann Intern Med. 2000;132:649–660. doi: 10.7326/0003-4819-132-8-200004180-00008. [DOI] [PubMed] [Google Scholar]
- 19.Look D C, Brody S L. Am J Respir Cell Mol Biol. 1999;20:1103–1106. doi: 10.1165/ajrcmb.20.6.f150. [DOI] [PubMed] [Google Scholar]
- 20.Anderson W F. Science. 1992;256:808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- 21.Verma I M, Somia N. Nature (London) 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 22.Felgner P L, Gadek T R, Holm M, Roman R, Chan H W, Wenz M, Northrop J P, Ringold G M, Danielsen M. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debs R, Pian M, Gaensler K, Clements J, Friend D S, Dobbs L. Am J Respir Cell Mol Biol. 1992;7:406–413. doi: 10.1165/ajrcmb/7.4.406. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Huang L. Biochem Biophys Res Commun. 1991;179:280–285. doi: 10.1016/0006-291x(91)91366-k. [DOI] [PubMed] [Google Scholar]
- 25.Wu M, Kelley M R, Hansen W K, Martin W J, II. Am J Physiol. 2001;280:L755–L761. doi: 10.1152/ajplung.2001.280.4.L755. [DOI] [PubMed] [Google Scholar]
- 26.Hildinger M, Abel K L, Ostertag W, Baum C. J Virol. 1999;73:4083–4089. doi: 10.1128/jvi.73.5.4083-4089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobune M, Xu Y, Baum C, Kelley M R, Williams D A. Cancer Res. 2001;61:5116–5125. [PubMed] [Google Scholar]
- 28.Markowitz D G, Goff S P, Bank A. Virology. 1988;167:400–406. [PubMed] [Google Scholar]
- 29.Hanenberg H, Hashino K, Konishi H, Hock R A, Kato I, Williams D A. Hum Gene Ther. 1997;8:2193–2206. doi: 10.1089/hum.1997.8.18-2193. [DOI] [PubMed] [Google Scholar]
- 30.Wu M, Brown W L, Stockley P G. Bioconjugate Chem. 1995;6:587–595. doi: 10.1021/bc00035a013. [DOI] [PubMed] [Google Scholar]
- 31.Weaver T, Hall C L, Kachel D L, Ward R P, Williams M D, Perry D G, Wisniowski P, Martin W J, II. J Immunol Methods. 1996;193:149–156. doi: 10.1016/0022-1759(96)00031-2. [DOI] [PubMed] [Google Scholar]
- 32.Schadendorf D, Paschen A, Sun Y. Immunol Lett. 2000;74:67–74. doi: 10.1016/s0165-2478(00)00251-0. [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi T, Worgall S, Singh R, Moore M A, Crystal R G. Nat Med. 2000;6:1154–1159. doi: 10.1038/80498. [DOI] [PubMed] [Google Scholar]
- 34.Sone S, Yano S, Hanibuchi M, Nokihara H, Nishmura N, Miki T, Nishioka Y, Shinohara T. Cancer Chemother Pharmacol. 1999;43,Suppl.:S26–S31. doi: 10.1007/s002800051094. [DOI] [PubMed] [Google Scholar]
- 35.Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger J C, Hodi F S, Liebster L, Lam P, Mentzer S, et al. Proc Natl Acad Sci USA. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura M. J Am Soc Nephrol. 2000;11, Suppl. 16:S154–S158. [PubMed] [Google Scholar]
- 37.Kitamura M, Suto T S. Kidney Int. 1997;51:1274–1279. doi: 10.1038/ki.1997.174. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Zheng T, Zhu Z, Homer R J, Riese R J, Chapman H A J, Shapiro S D, Elias J A. J Exp Med. 2000;192:1587–1600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolls J K, Lei D H, Stoltz D, Zhang P, Schwarzenberger P O, Ye P, Bagby G J, Summer W R, Shellito J E, Steve N. Alcohol Clin Exp Res. 1998;22:157–162. [PubMed] [Google Scholar]
- 41.Downing J F, Kachel D L, Pasula R, Martin W J, II. Infect Immun. 1999;67:1347–1352. doi: 10.1128/iai.67.3.1347-1352.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen G H, Reddy R C, Newstead M W, Tateda K, Kyasapura B L, Standiford T J. J Immunol. 2000;165:6496–6503. doi: 10.4049/jimmunol.165.11.6496. [DOI] [PubMed] [Google Scholar]
- 43.Lei D H, Lancaster J R, Jr, Joshi M S, Nelson S, Stoltz D, Bagby G J, Odom G, Shellito J E, Kolls J K. Am J Physiol. 1997;272:L852–L859. doi: 10.1152/ajplung.1997.272.5.L852. [DOI] [PubMed] [Google Scholar]
- 44.McCray P B, Wang G, Kline J N, Zabner J, Chada S, Jolly D J, Chang S M, Davidson B L. Hum Gene Ther. 1997;8:1087–1093. doi: 10.1089/hum.1997.8.9-1087. [DOI] [PubMed] [Google Scholar]