Abstract
Background
Caesarean sections (CS) are the most frequent major surgery in the world. A transient impairment of bowel motility is expected after CS. Although this usually resolves spontaneously within a few days, it can cause considerable discomfort, require symptomatic medication and delay hospital discharge, thus increasing costs. Chewing gum in the immediate postoperative period is a simple intervention that may be effective in enhancing recovery of bowel function in other types of abdominal surgeries.
Objectives
To assess the effects of chewing gum to reduce the duration of postoperative ileus and to enhance postoperative recovery after a CS.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (20 June 2016), LILACs (20 June 2016), ClinicalTrials.gov (20 June 2016), WHO International Clinical Trials Registry Platform (ICTRP) (20 June 2016) and the reference lists of retrieved studies.
Selection criteria
All randomised controlled trials comparing chewing gum versus usual care, for women in the first 24 hours after a CS. We included studies published in abstract form only.
Quasi‐randomised, cross‐over or cluster‐randomised trials were not eligible for inclusion in this review.
Data collection and analysis
Two review authors independently selected the studies for inclusion, extracted data and assessed the risk of bias following standard Cochrane methods. We present dichotomous outcome results as risk ratio (RR) with 95% confidence intervals (CI) and continuous outcome results as mean differences (MD) and 95% CI. We pooled the results of similar studies using a random‐effects model in case of important heterogeneity. We used the GRADE approach to assess the overall quality of evidence.
Main results
We included 17 randomised trials (3149 participants) conducted in nine different countries. Seven studies (1325 women) recruited exclusively women undergoing elective CS and five studies (833 women) only included women having a primary CS. Ten studies (1731 women) used conventional feeding protocols (nil by mouth until the return of intestinal function). The gum‐chewing regimen varied among studies, in relation to its initiation (immediately after CS, up to 12 hours later), duration of each session (from 15 to 60 minutes) and number of sessions per day (three to more than six). All the studies were classified as having a high risk of bias due to the nature of the intervention, women could not be blinded and most of the outcomes were self‐reported.
Primary outcomes of this review: for the women that chewed gum, the time to passage of first flatus was seven hours shorter than those women in the 'usual care' control group (MD ‐7.09 hours, 95% CI ‐9.27 to ‐4.91 hours; 2399 women; 13 studies; random‐effects Tau² = 14.63, I² = 95%, very low‐quality evidence). This effect was consistent in all subgroup analyses (primary and repeat CS, time spent chewing gum per day, early and conventional feeding protocols, elective and non‐elective CS and time after CS when gum‐chewing was initiated). The rate of ileus was on average over 60% lower in the chewing‐gum group compared to the control (RR 0.39, 95% CI 0.19 to 0.80; 1139 participants; four studies; I² = 39%, low‐quality evidence). Tolerance to gum‐chewing appeared to be high. Three women in one study complained about the chewing gum (but no further information was provided) and none of the studies reported adverse effects (eight studies, 925 women, low‐quality evidence).
Secondary outcomes of this review: the time to passage of faeces occurred on average nine hours earlier in the intervention group (MD ‐9.22 hours, 95% CI ‐11.49 to ‐6.95 hours; 2016 participants; 11 studies; random‐effects Tau² = 12.53, I² = 93%, very low‐quality evidence). The average duration of hospital stay was shorter in the intervention compared to the control group (MD ‐0.36 days, 95% CI ‐0.53 to ‐0.18 days; 1489 participants; seven studies; random‐effects Tau² = 0.04, I² = 92%). The first intestinal sounds were heard earlier in the intervention than in the control group (MD ‐4.56 hours, 95% CI ‐6.18 to ‐2.93 hours; 1729 participants; nine studies; random‐effects Tau² = 5.41, I² = 96%). None of the studies assessed women's satisfaction in relation to having to chew gum. The need for analgesia or antiemetic agents did not differ between the intervention and control groups (average RR 0.50, 95% CI 0.12 to 2.13; 726 participants; three studies; random‐effects Tau² = 0.79, I² = 69%).
Authors' conclusions
This review found 17 randomised controlled trials (involving 3149 women). We downgraded the quality of the evidence for time to first passage of flatus and of faeces and for adverse effects/intolerance to gum chewing because of the high risk of bias of the studies (due to lack of blinding and self‐report). For time to first flatus and faeces, we downgraded the quality of the evidence further because of the high heterogeneity in these meta‐analyses and the potential for publication bias based on the visual inspection of the funnel plots. The quality of the evidence for adverse effects/tolerance to gum chewing and for ileus was downgraded because of the small number of events. The quality of the evidence for ileus was further downgraded due to the unclear risk of bias for the assessors evaluating this outcome.
The available evidence suggests that gum chewing in the immediate postoperative period after a CS is a well tolerated intervention that enhances early recovery of bowel function. However the overall quality of the evidence is very low to low.
Further research is necessary to establish the optimal regimen of gum‐chewing (initiation, number and duration of sessions per day) to enhance bowel function recovery and to assess potential adverse effects of and women's satisfaction with this intervention. New studies also need to assess the compliance of the participants to the recommended gum‐chewing instructions. Future large, well designed and conducted studies, with better methodological and reporting quality, will help to inform future updates of this review and enhance the body of evidence for this intervention.
Plain language summary
Does chewing gum after a caesarean section lead to quicker recovery of bowel function?
What is the issue?
Many women deliver by caesarean section (CS) nowadays. The proportion of women who deliver by CS ranges from 15% to over 50%, in some countries. After a CS it is common for the bowel to stop working for several hours or days. Although this usually resolves by itself in a few days, it may be very uncomfortable. The retained gases and stools can cause the mother's belly to become swollen and painful with cramps and she may feel nauseated and vomit so she is not able to eat. She may need additional medications to ease these symptoms and her hospital discharge may be delayed. The use of medications that relieve pain during labour and painkillers following the surgery can also delay bowel function.
Although early feeding after a CS can stimulate the gut, it could also lead to vomiting. That is why many obstetricians still withhold food until bowel sounds are detected and there is passage of gas, or flatus. Chewing gum can help the bowels to function again earlier, as shown with other types of surgeries. We wanted to see if it also worked after a CS. Chewing gum in the first 24 hours after the surgery is a simple and cheap intervention.
What evidence did we find?
We included randomised controlled studies published up to June 2016.
We found 17 studies, with 3149 women who had just delivered by CS. In these studies, a group of women chewed gum and a second group did not, receiving usual care. The studies were conducted in nine countries (mostly low‐ to middle‐income countries) and were different in many aspects. For instance, some studies included only women having their first baby and others included women with a previous CS; some studies included only elective (pre‐scheduled) CS and others also included emergency CS. The way that gum was given also differed in the studies; in some the women started chewing gum right after the CS and in others they waited for up to 12 hours. Also, the women could not be blinded to receiving the gum. The combination of the results (in a meta‐analysis) of these studies showed that the women who chewed gum after a CS had an earlier return of their bowel function. On average, they passed gas seven hours earlier (13 studies, 2399 women). This effect was consistent for first versus repeat CS, time spent chewing gum per day, early feeding versus nothing by mouth until the return of intestinal function, elective versus non‐elective or emergency CS, and length of time after CS when gum‐chewing was initiated. The quality of the evidence for this outcome was very low. The women chewing gum were at least half as likely to have 'ileus' (a combination of symptoms such as bloating, cramping, nausea, vomiting and inability to defecate) than the women who did not chew gum (four studies, 1139 women, low‐quality evidence). Gum chewing reduced the time to first defecation to about nine hours earlier (11 studies, 2016 women, very low‐quality evidence) and the time to hospital discharge by some eight hours (seven studies, 1489 women). Only three out of 925 women complained about having to chew gum and there were no reports of adverse effects associated with gum‐chewing (eight studies, 925 women, low‐quality evidence). None of the studies assessed women's satisfaction in relation to chewing gum.
The overall quality of the evidence was low to very low, mostly due to lack of blinding of the participants (the women knew they were chewing gum) and heterogeneity between the studies.
What does this mean?
The available evidence suggests that gum‐chewing in the first 24 hours after a CS is a well‐tolerated simple, low‐cost, safe and easy intervention that enhances early recovery of bowel function, improves maternal comfort and potentially reduces hospital costs. Further research is necessary to establish the optimal regimen of gum‐chewing (when to start, number and duration of sessions per day) to enhance bowel function recovery and to assess potential adverse effects and women's satisfaction with this intervention.
Summary of findings
Summary of findings for the main comparison. Chewing gum compared to control for enhancing early recovery of bowel function after caesarean section.
| Chewing gum compared to control for enhancing early recovery of bowel function after caesarean section | ||||||
| Patient or population: women in the immediate postpartum period (within the first 24 hours) after having had a caesarean section Settings: all studies except one were conducted hospitals in low‐ and middle‐income countries Intervention: chewing gum Comparison: control (usual care in the post‐partum period) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Chewing gum | |||||
| Time to first passage of flatus In hours | The mean time to first passage of flatus in the control group was 30.36 hours | The mean time to first passage of flatus in the intervention groups was 7.09 h shorter than in the control group (9.27 to 4.91 h shorter) | Not estimable | 2399 (13 studies) | ⊕⊝⊝⊝ very low1,2,3 | 11 of these studies were conducted in Asia and 2 in Africa. |
| Proportion of participants with ileus | 11 per 100 | 5 per 100 (3 to 7) | RR 0.39 (0.19 to 0.80) | 1139 (4 studies) | ⊕⊕⊝⊝ low,4,5 | 3 of these studies were conducted in Asia and 1 in Africa |
| Number of participants with adverse effects or intolerance to gum | See comments | 3 of 925 participants in the intervention group had intolerance to gum | Not estimable | 1888 (8 studies) | ⊕⊕⊝⊝ low1,4 | 7 studies were conducted in Asia and 1 in the US. No events in the control group since it was not exposed to the intervention |
|
Time to passage of faeces in hours |
The mean time to first passage of faeces in the control group was 50.62 h | The mean time to first passage of faeces in the intervention groups was 9.22 h shorter than in the control group (11.49 to 6.95 h shorter) | Not estimable | 2016 (11 studies) |
⊕⊝⊝⊝ very low1,2,3 | 9 studies were conducted in Asia and 2 in Africa |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High risk of bias in studies; participants were not blinded to the intervention and self‐reported this outcome 2 High heterogeneity (I2 = 95%) 3 Possibility of publication bias (funnel plot asymmetry) 4 Low number of events (lower than 300)
5 Three of four studies with unclear risk of bias for assessors evaluating this outcome
Background
Description of the condition
Caesarean sections (CS) are the most frequent major surgery in the world with an estimated 18.5 million procedures being performed each year (WHO 2013). According to the latest official estimates, 15% of all deliveries in the world occur though CS, with large variations between and within countries, ranging from less than 5% in some African regions to over 50% in several Latin American countries and in China (Betrán 2016; WHO 2013).
A transient impairment of bowel motility is expected after any major surgery, including CS (Bauer 2004; Holte 2000). This condition, known as postoperative paralytic ileus (or 'ileus') is defined as the functional inhibition of propulsive bowel activity (Livingston 1990). Ileus resolution is often defined by the passage of flatus (gas) or stool, or both. These are signs that intestinal function is returning to normal and are traditionally considered the end points of postoperative ileus (Holte 2000; Kehlet 2001; Livingston 1990; Mattei 2006; Vather 2013). Usually the stomach and small intestines regain regular motility within 12 to 24 hours after major surgery, while it takes 48 to 72 hours for the colon to regain its propulsive function (Condon 1995; Waldhausen 1990). Although in most cases, ileus resolves spontaneously within two to three days of surgery, it is an important cause of discomfort and increased costs (Behm 2003; Iyer 2009; Johnson 2009; Kehlet 2001). There is no internationally accepted standardised clinical definition for ileus but its main clinical manifestations are intestinal distension, absence of bowel sounds or movements, and lack of flatus or stool passage. People with ileus usually complain of nausea, vomiting, abdominal cramping or pain and inability to tolerate diet (Holte 2000; Luckey 2003; Vather 2013). Other adverse effects of ileus include increased postoperative pain, delayed postoperative ambulation, increased risk of pulmonary complications such as pneumonia, pulmonary embolism, and atelectasis (collapse of air sacs in the lung), prolonged hospitalisation and decreased patient satisfaction (Behm 2003; Kehlet 2001).
The exact pathogenesis of postoperative ileus is complex, multifactorial and incompletely understood. It apparently involves stimulation of pain fibres, excessive sympathetic tone caused by the stress of surgical trauma and the release of inhibitory neurotransmitters and inflammatory mediators due to bowel manipulation and peritoneal irritation caused, for instance, by blood spillage (Bauer 2004; Behm 2003; Holte 2000; Johnson 2009; Luckey 2003). Although there are no specific studies on the mechanisms of post‐caesarean ileus, we can presume that they are similar to other abdominal surgeries. Risk factors for ileus include longer total surgery time, extensive manipulations of the abdominal cavity and blood loss (Artinyan 2008). Since these conditions are more frequent in women with one or more previous CS, it might be expected that these women would have more risk of developing postoperative ileus than those undergoing a primary CS. The use of opioids during labour and analgesic medication in the postoperative period also contribute to delayed bowel function (Delaney 2004; LaRosa 1993). The incidence of post CS ileus ranges from 0.9% to 21.5%, depending on the definition used by the authors (Craciunas 2014).
For many decades, usual care after a CS consisted of feeding only after a period of fasting (conventional feeding protocol) to allow bowel movements to resume. In the late 1990s 'early feeding' protocols, which consist of feeding usually less than six to eight hours after CS, became popular (Mangesi 2002). There is good‐quality evidence that early postoperative feeding may stimulate gut motility and avoid prolonged ileus after a CS (Mangesi 2002). However, because of concerns that early feeding could lead to vomiting with subsequent aspiration pneumonia and wound dehiscence (rupture), many obstetricians still withhold postoperative oral intake until the resolution of ileus, that is, when bowel sounds are detected and there is passage of flatus (Kramer 1996; Mangesi 2002; Soriano 1996).
Description of the intervention
Chewing gum is a confectionery that dates back to the Neolithic period and was originally made from latex sap (chicle) or resins of trees (Gustaitis 1998; Matthews 2009). Modern versions consist of a synthetic rubber polymer (polyethylene and polyvinyl acetate) mixed with sugar or artificial sweeteners, dyes, softeners such as glycerin or vegetable oil that help prevent the gum from becoming hard or stiff, and other substances which provide different flavours at the time of mastication (Hendrickson 1990). Historically, chewing gum was used to clean the teeth and freshen the breath. Chewing gum containing xylitol has been shown to reduce tooth cavities and dental plaque (Deshpande 2008; Milgrom 2006).
How the intervention might work
Chewing gum has been tested to accelerate the recovery from postoperative ileus for over a decade (Asao 2002). This intervention has been shown to be effective in the postoperative period of gastrointestinal surgery (Asao 2002; Griffiths 2007; Ho 2014; Parnaby 2009; Vasquez 2009; Wallström 2014; Wang 2013; Yin 2013) and there are some studies showing that it can also be effective after a CS (Craciunas 2014; Zhu 2014). Most of the studies that tested chewing gum to enhance post‐caesarean recovery of bowel function used between three to 12 pieces of gum per day and chewing times ranging from 15 to 60 minutes per session (Craciunas 2014; Zhu 2014).
There are several mechanisms that can explain how gum chewing can increase gut motility and ameliorate ileus: firstly, it is a form of sham feeding and therefore induces bowel movement (Jepsen 1989; Soffer 1992; Stern 1989); secondly, it produces vagal cholinergic stimulation, which in turn increases the production of hormones associated with intestinal motility (Griffiths 2007; Kellow 1999; Konturek 1986); and thirdly, it increases the secretion of pancreatic juice, salivation and swallowing (Stern 1989). Finally, it has been suggested that the artificial sweeteners used in sugar‐free gums could have a role in the recovery of bowel function due to their laxative effects (Ravry 1980; Tandeter 2009).
Although gum chewing is generally considered safe, it can lead to jaw muscle fatigue and pain (Christensen 1996) and sorbitol as well as other sweeteners in sugar‐free gums cause diarrhoea in a dose‐dependent manner as well headaches and vasculitis in susceptible individuals (Fitzgerald 2009; Ravry 1980).
Why it is important to do this review
Caesarean section is the most frequent major surgery in the world and rates are increasing in most high‐ and middle‐income countries (Betrán 2016; WHO 2013). Ileus is an expected consequence of any abdominal surgery, including CS. Although ileus resolves spontaneously in a few days in most cases, it can cause uncomfortable symptoms (e.g. nausea, vomiting, abdominal distension and pain) that can distress women postpartum and increase the duration of her hospital stay and even lead to serious morbidity (Behm 2003; Kehlet 2001; Mattei 2006). Chewing gum is a simple, low‐cost, safe and easy intervention that could reduce the duration of ileus and thus improve maternal comfort and potentially reduce hospital costs (Keenahan 2014; Lafon 2012; Yeh 2009; Yin 2013).
Several studies published in the last five years have examined the effect of chewing gum in the postoperative period following a CS. Two recently published systematic reviews assessed the findings of some of these trials and concluded that this intervention improves recovery of bowel function (Craciunas 2014; Zhu 2014). However, these two reviews included only studies published up to early 2013 and did not assess important outcomes such as patient satisfaction or adverse effects related to gum chewing as part of their objectives. There was also large heterogeneity in all the meta‐analyses performed in these reviews but this was not further explored by the authors. Both reviews had several methodological limitations, including the lack of searches for studies registered in trial platforms, thus increasing the potential for publication bias, and no subgroup analyses. Zhu 2014 excluded studies published in languages other than English, did not report if study selection was performed in duplicate and did not asses the quality of the evidence presented. Craciunas 2014 did not clearly define the outcomes of interest a priori, did not state whether 'Risk of bias' assessment was performed in duplicate, did not assess publication bias, did not present a list of the excluded studies, and provided no explanation for downgrading the quality of evidence. These limitations may compromise the completeness and reliability of these systematic reviews and therefore a degree of caution is required when interpreting their conclusions.
The findings of this review could help to inform millions of women and their physicians about a simple, non‐pharmacological intervention that could help to make the postoperative period of CS more comfortable.
Objectives
To assess the effects of chewing gum to reduce the duration of postoperative ileus and to enhance postoperative recovery after a caesarean section.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials comparing chewing gum versus other forms of treatment (usual care) for women in the postoperative period of a caesarean section (CS). We also included studies published in abstract form.
Quasi‐randomised, cross‐over or cluster‐randomised trials were not eligible for inclusion in this review.
Types of participants
Women in the immediate postpartum period (within the first 24 hours) after a CS.
Types of interventions
Chewing gum, at least once daily, in the immediate postoperative period (within the first 24 hours) of a CS, in any doses or time intervals. Studies with gums that contained an active therapeutic agent were not be included unless the agent was also given to the control group.
The intervention was compared to usual care (conventional feeding protocol, i.e. feeding after a period of fasting to allow bowel movements to resume, or early feeding protocol, i.e. feeding usually less than six to eight hours after CS) without gum chewing.
Types of outcome measures
Primary outcomes
Time to the first passage of flatus, in hours
Proportion of women with ileus as defined by the study authors or symptoms and signs of gastrointestinal disturbance such as nausea, vomiting, abdominal cramping or abdominal distension, within the first 72 hours after CS
Tolerance to gum and adverse effects of gum chewing (such as jaw pain), within the first 72 hours after CS
Secondary outcomes
Time to passage of faeces, in hours
Duration of hospital stay, in days
Woman's satisfaction as reported by the authors
Need for analgesia or antiemetic agents within the first 72 hours after CS
Time to first hearing of normal intestinal sounds, in hours
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting their Information Specialist (20 June 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition we searched
LILACS database (from inception to 20 June 2016). The search terms we used are given in Appendix 1;
ClinicalTrials.gov for planned, ongoing or unpublished trials (on 20 June 2016) (Appendix 2);
International Clinical Trials Registry Platform (ICTRP) for planned, ongoing or unpublished trials (on 20 June 2016) (Appendix 3).
Searching other resources
We handsearched the reference lists of all the studies identified.
We did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors (EPGM, VSV) independently screened all the citations identified through the search strategy, for potential inclusion in the review. The review authors resolved any disagreement through discussion. In case of persistent disagreement, a third author (MRT) was asked to arbitrate.
The authors created a study flow diagram to map out the number of records identified, included and excluded.
If any studies published in an abstract form satisfied the inclusion criteria we included them. We contacted the authors of included studies for additional information.
Data extraction and management
For eligible studies, two review authors (EPGM and ASP) independently extracted data using the form specifically created for this review. We resolved discrepancies through discussion or by consulting a third author (MRT). The same two review authors entered data into Review Manager (RevMan) (RevMan 2014) and checked accuracy. When information regarding the methods or results of the study was unclear, we contacted the authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (EPGM and ASP) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third review author (MRT), if needed.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes, but reported the overall estimate.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes, but reported overall estimates. Time to the first passage of flatus, participant‐reported tolerance to gum, time to passage of faeces and woman's satisfaction were considered subjective outcomes which could not be blinded. Proportion of participants with ileus, time to first hearing of intestinal sounds, need for analgesia or antiemetic agents and duration of hospital stay were categorised as objective outcomes which could, in theory, be blinded if the outcome assessors were not aware of whether the woman had chewed gum or not.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that we would have expected it to report);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We marked explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison: chewing gum versus control.
Time to the first passage of flatus, in hours
Proportion of participants with ileus as defined by the study authors, or symptoms and signs of gastrointestinal disturbance such as nausea, vomiting, abdominal cramping or abdominal distension, within the first 72 hours after CS
Tolerance to gum and adverse effects of gum chewing (such as jaw pain), within the first 72 hours after CS
Time to first passage of faeces, in hours
GRADEpro Guideline Development Tool was used to import data from RevMan 5.3 (RevMan 2014) in order to create 'Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we used the mean difference (MD) if outcomes were measured in the same way between trials. In future updates, we will use the standardised mean difference, where appropriate, to combine trials that measured the same outcome, but used different methods. When the data were reported only as median, range, or interquartile interval, and we did not obtain data from the study authors, we did not transform these data into mean and standard deviation.
Unit of analysis issues
The unit of analysis was the individual woman.
Cluster‐randomised trials
Cluster‐randomised trials were not eligible for inclusion in this review.
Cross‐over trials
Cross‐over trials were not eligible for inclusion in this review.
Other unit of analysis issues
There were no other unit of analysis issues.
Dealing with missing data
We noted levels of attrition in included studies. We explored the impact of including studies with high levels of missing data (more than 20% for primary or secondary outcomes) in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We examined heterogeneity between the results of different studies by inspecting the scatter plot in the data points on the graphs and the overlap in their CIs and, more formally, by checking the Chi2, the Tau2 and the I2 statistics. We regarded heterogeneity as substantial if an I² statistic was greater than 50% and either a Tau² statistic was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We investigated reporting biases (such as publication bias) visually, by assessing the funnel plots created for meta‐analyses with at least 10 studies. A visual asymmetry observed in a funnel plot was considered as indicative of a possible publication bias.
Data synthesis
We carried out statistical analysis using Review Manager 5.3 software (RevMan) (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: that is, where trials were examining the same intervention, and the populations and methods were judged sufficiently similar. In case of clinical heterogeneity we used random‐effects meta‐analysis to produce an overall summary. Where we used random‐effects analyses, we have presented the results as the average treatment effect with 95% CI, and the Tau² and I² statistics.
Subgroup analysis and investigation of heterogeneity
We planned to investigate substantial heterogeneity using subgroup analyses and sensitivity analyses.
-
We carried out the following planned subgroup analyses.
Primary (first CS) versus repeated CS
Time spent chewing gum: up to 60 minutes versus more than 60 minutes per day
Comparator group: early feeding versus conventional feeding protocol
-
In addition, at the review stage, we also added the following subgroup analyses to further explore heterogeneity.
Type of CS: elective versus non‐elective CS
Time to initiation of gum chewing: immediately after CS, versus two to five hours after CS, versus six or more hours after CS
We used the following outcomes in subgroup analysis.
Time to the first passage of flatus, in hours
Proportion of women with ileus as defined by the study authors or symptoms and signs of gastrointestinal disturbance such as nausea, vomiting, abdominal cramping or abdominal distension, within the first 72 hours after CS
We assessed subgroup differences by interaction tests available in RevMan (RevMan 2014). We investigated differences between two or more subgroups by a standard method using I2 statistic for heterogeneity across subgroup results (rather than across individual study results) (Higgins 2011). As random‐effects models were used for the analysis within each subgroup, then these statistics related to variation in the mean effects in the different subgroups.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made during the review process regarding the eligibility for end analysis. These analyses tested whether the review conclusions differed if:
eligibility was restricted to studies without high risk of selection or detection bias;
studies with high rates of attrition (more than 20%) had been excluded.
The first sensitivity analysis was not possible because all studies were judged to have a high risk of detection bias due to the nature of the intervention. This will not be carried out in future updates. We succeeded in performing the second sensitivity analysis. We analysed whether the pooled estimates changed if we excluded studies judged to be at high or at unclear risk of attrition bias, due to the amount, nature or handling of incomplete outcome data, as described in the Assessment of risk of bias in included studies section.
Assessing the quality of the evidence and 'Summary of findings' table
We used the five GRADE criteria (risk of bias, inconsistency, imprecision, indirectness and publication bias) to evaluate the quality of the body of evidence as it relates to the studies that contribute data to the meta‐analyses for pre‐defined outcomes. We used methods and recommendations proposed in Section 8.5 (Higgins 2011) and Chapter 12 (Schünemann 2011) of the Cochrane Handbook for Systematic Reviews of Interventions using GRADEpro software. We clarified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we added comments to help readers' understanding of the review when necessary.
We created a 'Summary of findings' table using the primary outcomes.
Time to the first passage of flatus, in hours
Rate of ileus or symptoms and signs of gastrointestinal disturbance such as nausea, vomiting, abdominal cramping or abdominal distension, within the first 72 hours after CS
Tolerance to gum and adverse effects of gum chewing (such as jaw pain), within the first 72 hours after CS
Results
Description of studies
Results of the search
The search retrieved 74 records from the electronic databases and we obtained seven additional records from other sources (Dehcheshmeh 2011; Kamalimanesh 2015; Liang 2007; Lu 2010; Luo 2010; Rashad 2013; Wang 2011). We excluded one duplicate and checked the titles and abstracts of the remaining 80 records. At this stage, we excluded 51 records and selected 29 records for full‐text assessment. After reading these full texts, we excluded two records, referring to two studies (Cevik 2016; Sahin 2015) because they were not randomised controlled trials (RCTs) and we also excluded five records because they were protocols of five ongoing trials (Abd‐El‐Maeboud 2010; Ellaithy 2015; El‐Sharkawy 2015; Kamalimanesh 2015; Yilmaz 2015). We included a total of 22 records reporting 17 trials in the review (Figure 1). Twelve of these 17 studies were full‐text publications with complete data (Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Akhlaghi 2008; Jakkaew 2013; Kafali 2010; Ledari 2012; Liang 2007; Lu 2010; Luo 2010; Rashad 2013; Shang 2010; Wang 2011) and five of the 17 included studies had limited numerical and methodological information because they were either conference abstracts (Garshasbi 2010; Satij 2006; Zamora 2012) or English abstracts of full‐text articles published in Farsi (Abasi 2014; Dehcheshmeh 2011) which we could not translate. One of the 17 included studies (Ledari 2012) had four reports (one protocol of the trial and three publications describing different aspects of the same study); another two studies (Dehcheshmeh 2011; Jakkaew 2013), had two reports each (a protocol of the trial and a publication of the final study). All studies included in this review except Abasi 2014 (one report) and Jakkaew 2013 (two reports) provided data that could be included in one or more of our meta‐analyses.
1.
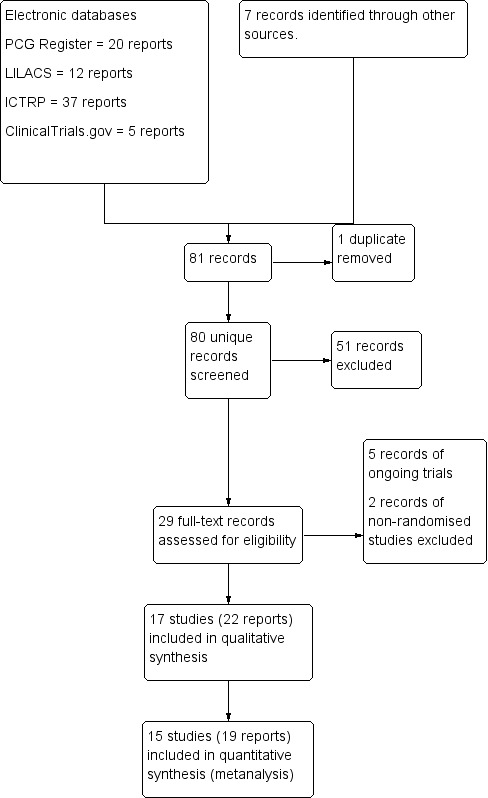
Study flow diagram of the process of study selection.
Included studies
The 17 included studies involved a total of 3149 women, ranging from 32 (Satij 2006) to 500 (Garshasbi 2010) women per study.
Design
All included studies were parallel‐design RCTs.
Setting
The 17 studies were conducted in nine different countries, mostly low‐ to middle‐income countries: China (Liang 2007; Lu 2010; Luo 2010; Shang 2010; Wang 2011), Egypt (Abd‐El‐Maeboud 2009), Iran (Abasi 2014; Akhlaghi 2008; Dehcheshmeh 2011; Garshasbi 2010; Ledari 2012), Nigeria (Ajuzieogu 2014), The Philippines (Zamora 2012), Saudi Arabia (Rashad 2013), Thailand (Jakkaew 2013), Turkey (Kafali 2010), and USA (Satij 2006). All studies except one were conducted in single hospitals. The Nigerian study (Ajuzieogu 2014) was multicentric and involved a university teaching hospital and three satellite specialised obstetric hospitals located in the same geographic region.
Types of participants
The mean age of the women participating in the studies ranged from 25 to 31.2 years.
Parity varied among the studies: three studies included only nulliparas (Dehcheshmeh 2011; Luo 2010; Wang 2011), six studies did not provide any information at all about the parity of their participants (Akhlaghi 2008; Garshasbi 2010; Liang 2007; Lu 2010; Satij 2006; Zamora 2012) and the other eight studies included both nulliparas and multiparas. Among these eight studies that included multiparas (Abasi 2014; Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Jakkaew 2013; Kafali 2010; Ledari 2012; Rashad 2013; Shang 2010), five were unclear or did not specify how many of their participants had had a previous caesarean section (CS) (Abasi 2014; Kafali 2010; Ledari 2012; Rashad 2013; Shang 2010). One study (Ajuzieogu 2014) included only women without any previous CS, another study had less than half of the participants with a previous CS (Jakkaew 2013) and one study (Abd‐El‐Maeboud 2009) had almost 60% of its participants with at least one previous CS. For our subgroup analyses, we categorised this last study in the "Repeat CS" subgroup since over half of the participants had a previous CS.
The type of CS (moment when the surgery was performed) varied among the 17 studies. Seven studies (Abasi 2014; Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Akhlaghi 2008; Dehcheshmeh 2011; Ledari 2012; Wang 2011) included only women delivered by elective CS. Four studies (Jakkaew 2013; Rashad 2013; Shang 2010; Zamora 2012) reported that their studies included a mixed population comprising women submitted to elective as well as intrapartum or emergency CS. Finally, six studies (Garshasbi 2010; Kafali 2010; Liang 2007; Lu 2010; Luo 2010; Satij 2006) were unclear or provided no information on the number of women submitted to elective or non‐elective CS.
Nine studies (Ajuzieogu 2014; Dehcheshmeh 2011; Jakkaew 2013; Ledari 2012; Liang 2007; Luo 2010; Shang 2010; Wang 2011; Zamora 2012) reported that all or most of their participants received regional anaesthesia, that is, spinal or epidural or both. Two studies (Abd‐El‐Maeboud 2009; Rashad 2013) reported that all or most of their participants received general anaesthesia. One study (Kafali 2010) reported that half of its participants received general anaesthesia and half received regional anaesthesia. Finally, five studies (Abasi 2014; Akhlaghi 2008; Garshasbi 2010; Lu 2010; Satij 2006) did not report the type of anaesthesia used.
Types of interventions
Fourteen studies used different brands of sugar‐free gum, some of which were flavoured (Abasi 2014; Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Akhlaghi 2008; Dehcheshmeh 2011; Jakkaew 2013; Kafali 2010; Ledari 2012; Liang 2007; Luo 2010; Rashad 2013; Shang 2010; Wang 2011; Zamora 2012) while three studies (Garshasbi 2010; Lu 2010; Satij 2006) provided no details on the type of gum used in the intervention group. Most studies reported that the women were asked to chew one stick per session.
In eight studies (Akhlaghi 2008; Dehcheshmeh 2011; Garshasbi 2010; Jakkaew 2013; Liang 2007; Rashad 2013; Satij 2006; Shang 2010) women started chewing gum immediately after the CS; in five studies (Abd‐El‐Maeboud 2009; Kafali 2010; Lu 2010; Luo 2010; Wang 2011) they started two hours after surgery; in two studies (Ajuzieogu 2014; Ledari 2012) they started after six hours; and in one study (Zamora 2012) they started only 12 hours after their CS. One study (Abasi 2014) did not provide information on the exact moment when women started the intervention.
In six studies (Abd‐El‐Maeboud 2009; Dehcheshmeh 2011; Liang 2007; Luo 2010; Wang 2011; Zamora 2012) the duration of each chewing session was 10 to 15 minutes, in another six studies it lasted 30 minutes (Ajuzieogu 2014; Garshasbi 2010; Jakkaew 2013; Lu 2010; Rashad 2013) or at least 30 minutes (Shang 2010); in one study (Akhlaghi 2008) it lasted 45 minutes and in three studies (Abasi 2014; Kafali 2010; Ledari 2012) each session lasted 60 minutes. One study (Satij 2006) did not state the duration of each session.
Most (N = 13) of the studies reported that women chewed gum three or four times per day (Abasi 2014; Ajuzieogu 2014; Akhlaghi 2008; Dehcheshmeh 2011; Garshasbi 2010; Jakkaew 2013; Kafali 2010; Ledari 2012; Liang 2007; Luo 2010; Rashad 2013; Satij 2006; Shang 2010). The authors of three studies (Abd‐El‐Maeboud 2009; Lu 2010; Wang 2011) instructed the participants to chew gum every two hours during the daytime, which resulted in five to six sessions of gum chewing per day. Zamora 2012 did not specify the number of gum‐chewing sessions per day.
The overall duration of gum chewing per day ranged from 45 minutes (Liang 2007) to 180 minutes (Abasi 2014; Kafali 2010; Ledari 2012). Only three studies (Dehcheshmeh 2011; Liang 2007; Luo 2010) had a daily total duration of gum chewing lasting 60 minutes or less. In two studies (Satij 2006; Zamora 2012) the information provided by the authors was insufficient to ascertain the total daily duration of gum chewing.
Most studies (N = 10, 1731 women) used conventional feeding protocols, which consisted of nothing by mouth until the return of intestinal function, defined according to study authors as a series of events such as passage of flatus or return of bowel sounds (Abasi 2014; Abd‐El‐Maeboud 2009; Akhlaghi 2008; Dehcheshmeh 2011; Jakkaew 2013; Ledari 2012; Lu 2010; Shang 2010; Wang 2011; Zamora 2012). Two studies (Kafali 2010; Luo 2010) used early feeding protocols (i.e. oral intake of liquids or food, or both, before there were signs of the return of bowel function) and five studies were unclear or did not describe the feeding protocol used in the control groups (Ajuzieogu 2014; Garshasbi 2010; Liang 2007; Rashad 2013; Satij 2006).
Types of outcome measures
The time to first passage of flatus was reported by 15 studies but only 13 could be used in this meta‐analysis (Garshasbi 2010 and Jakkaew 2013 provided these data as median and range).
Abasi 2014 stated in the study protocol that time to first flatus was one of the outcomes of interest but did not report it in the publication. Satij 2006 was unclear about the definition of their only outcome "return to bowel function"; we contacted the study authors to obtain more details but they did not answer.
Four studies reported the number of participants with postoperative ileus as one of their outcomes (Abd‐El‐Maeboud 2009; Garshasbi 2010; Shang 2010; Zamora 2012) and we included them in our meta‐analyses. Jakkaew 2013 was not included because this study reported the median incidence of participants with ileus in each group. We contacted the study authors to obtain the exact number of participants with ileus in each group but did not obtain an answer.
Tolerance related to gum chewing was reported by eight trials (Abd‐El‐Maeboud 2009; Akhlaghi 2008; Garshasbi 2010; Kafali 2010; Ledari 2012; Liang 2007; Satij 2006; Shang 2010).
Time to first passage of faeces was reported by 12 studies (Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Akhlaghi 2008; Dehcheshmeh 2011; Garshasbi 2010; Ledari 2012; Liang 2007; Lu 2010; Luo 2010; Rashad 2013; Shang 2010; Zamora 2012) but one of them (Garshasbi 2010) reported this as median and range and it could not be included in this meta‐analysis.
The duration of hospital stay was reported by eight studies (Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Akhlaghi 2008; Dehcheshmeh 2011; Jakkaew 2013; Kafali 2010; Shang 2010; Zamora 2012) but one of them (Jakkaew 2013) reported this in median values and could not be pooled in our meta‐analysis. Garshasbi 2010 did not provide numerical data but stated that there was "virtually no difference" for duration of hospital stay between the groups. We contacted the authors of both studies for additional information but obtained no response.
None of the studies assessed women's satisfaction with gum chewing. The Ajuzieogu 2014 study stated (in the Methods) that "patients in the gum group were interviewed on their satisfaction with the technique to rate it using a visual analogue scale", but in the Results they present the scores for both the gum and the control group; therefore, we judged that this study did not actually assess satisfaction with gum chewing.
The need for additional antiemetic or analgesic medication was reported by Abd‐El‐Maeboud 2009; Kafali 2010 and Shang 2010.
The time to first hearing of normal intestinal sounds was reported by 11 studies (Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Akhlaghi 2008; Dehcheshmeh 2011; Garshasbi 2010; Kafali 2010; Ledari 2012; Luo 2010; Rashad 2013; Shang 2010; Wang 2011).
Excluded studies
Two studies were excluded because they were not RCTs.
Cevik 2016 was a study involving 120 women randomised to usual care, chewing gum or oral hydration after a CS. We did not include it because the method described by the study authors to randomise the participants did not ensure that each woman had an equal chance of being randomised to one of the three groups. We therefore classified it as a quasi‐randomised trial, which was part of our exclusion criteria.
Sahin 2015 described the study as a randomised trial involving 240 women divided into eight arms of 30 women each: seven intervention arms and an eighth arm that was the control group. The seven intervention arms tested three interventions (chewing gum, oral hydration or exercise) alone or in combination with each other. The eighth arm of the study (the control group) consisted of women who received usual care after their CS. We did not include this study because the control group was not randomised but consisted of a convenience sample that included women whose doctors did not allow them to be included in the trial.
Ongoing studies
Five studies are ongoing trials (Abd‐El‐Maeboud 2010; Ellaithy 2015; Kamalimanesh 2015; El‐Sharkawy 2015; Yilmaz 2015). See Characteristics of ongoing studies for more details.
Risk of bias in included studies
Details of the risk of bias for each of the 17 individual studies are presented in the Characteristics of included studies table and in Figure 2 In Figure 3 we present the risk of bias as percentages across all included studies.
2.
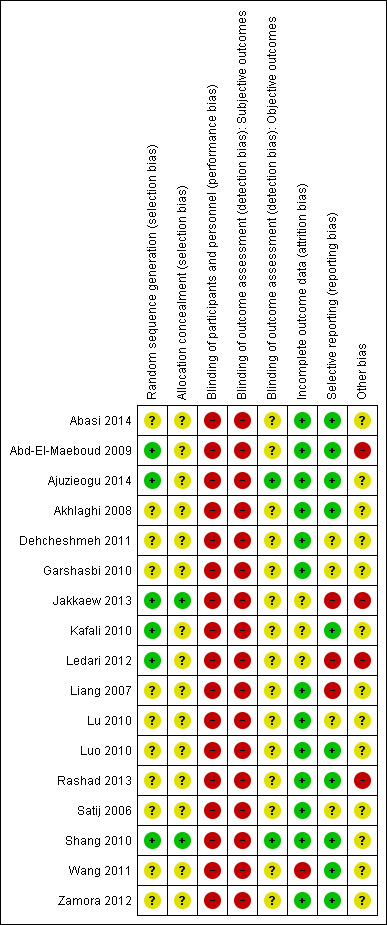
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
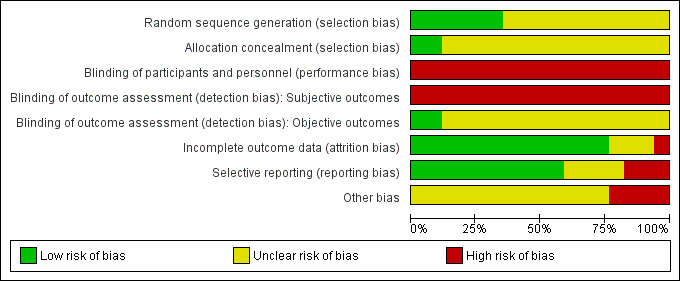
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation was judged to be at low risk of bias in six studies (Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Jakkaew 2013; Kafali 2010; Ledari 2012; Shang 2010). A computer‐generated sequence was used by five studies (Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Jakkaew 2013; Ledari 2012; Shang 2010) and one study (Kafali 2010) used sequential, randomised card‐pulling. All other studies were classified as having an unclear risk of bias for this domain because of lack of information.
Allocation concealment was judged to be at low risk in two studies (Jakkaew 2013; Shang 2010) and all other studies were assessed as unclear, because they provided no information to allow judgment.
Blinding
The performance bias was considered high in all 17 studies because, due to the nature of the intervention, it was not possible to blind the participants and personnel.
We separated the outcomes into two categories: subjective and objective. Time to first flatus, tolerance or adverse effects to gum, time to passage of stools and woman's satisfaction were considered subjective outcomes since they were reported directly by the participants. Since the assessors for these outcomes were the women themselves, and since they could not be blinded to the intervention (chewing gum), we considered the assessment of these outcomes as having a high risk of bias in all 17 studies. Diagnosis of ileus, need for additional analgesics or antiemetics, time to first bowel sounds, and duration of hospital stay were considered objective outcomes because they were assessed or determined by the medical personnel who could, in theory, be blinded to the group to which the participant had been allocated. Only two studies (Ajuzieogu 2014; Shang 2010) were classified as having a low risk of bias for objective outcome assessors because they clearly described that the personnel assessing these events were blinded to the participants' group (gum‐chewing or control). The other 15 studies were classified as having an unclear risk of bias for objective outcome assessors due to the lack of information.
Incomplete outcome data
Thirteen studies (Abasi 2014; Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Akhlaghi 2008; Dehcheshmeh 2011; Garshasbi 2010; Liang 2007; Lu 2010; Luo 2010; Rashad 2013; Satij 2006; Shang 2010; Zamora 2012) were classified as having a low risk of attrition bias because they either had no losses or the number of dropouts was small and balanced among the two groups.
Wang 2011 reported that approximately 22% of the participants in the intervention and in the control groups were excluded after randomisation due to complications during or after surgery (e.g. excessive bleeding, abdominal adhesions, extensive resection, surgery duration more than two hours, postoperative fever or use of pethidine). Although exclusions were balanced in the two groups, we classified this study as being at high risk of attrition bias because losses were more than 20%.
We classified Jakkaew 2013 as having an unclear risk for attrition bias because, although the authors state in the publication that "no participant was excluded from the analysis", the sample size in the study protocol was 100 women, while the number of participants described in the published paper was only 50, and the study authors did not reply to our email inquiring about this difference.
Kafali 2010 had a small unbalance in the number of dropouts in the intervention and control groups (5.4% and 3.9%, respectively), did not present the results of these women and did not reply to our email asking for this information; we therefore classified this study as having an unclear risk of attrition bias. Finally, we also classified Ledari 2012 as having an unclear risk of attrition bias because the study authors did not provide any information about the number of dropouts per group and did not reply to our email asking about this information.
Selective reporting
Ten studies were judged as having a low risk of bias for this domain (Abasi 2014; Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Akhlaghi 2008; Kafali 2010, Luo 2010; Rashad 2013; Shang 2010; Wang 2011; Zamora 2012) because all pre‐specified outcomes proposed in the study protocol or the methods section of the publication were reported in the results section. Three studies (Jakkaew 2013; Ledari 2012; Liang 2007) were categorised as having a high risk of bias for this domain because they described outcomes in the study protocols or methods section that were not reported in the results section (see details in the risk of bias tables for the individual studies). We assessed the other four studies (Dehcheshmeh 2011; Garshasbi 2010; Lu 2010; Satij 2006) as having an unclear risk of bias for selective reporting because of lack of information in the publications, and the study authors did not respond to our emails.
Other potential sources of bias
We judged four studies as having a high risk of bias for this domain. Abd‐El‐Maeboud 2009 and Rashad 2013 reported that the duration of surgery was significantly longer in the intervention than in the control groups. Jakkaew 2013 described in the protocol that the planned sample size was of 100 participants but reported the results of only 50 participants in their publication, without providing any explanation for this fact which raises the possibility of partial reporting. We contacted the study authors for more information but they did not reply. Ledari 2012 was categorised as having a high risk of bias for this domain because of several deviations from the study protocol, including the duration of gum‐chewing sessions (planned as 15 minutes bur reported as 60 minutes), initiation of intervention (planned as immediately after CS but reported as six hours after) and type of participants (planned only elective CS but reported that 14% were not elective). All other studies were classified as having an unclear risk of bias for this domain.
Effects of interventions
See: Table 1
Chewing gum versus control
Primary outcomes
Time to first passage of flatus, in hours
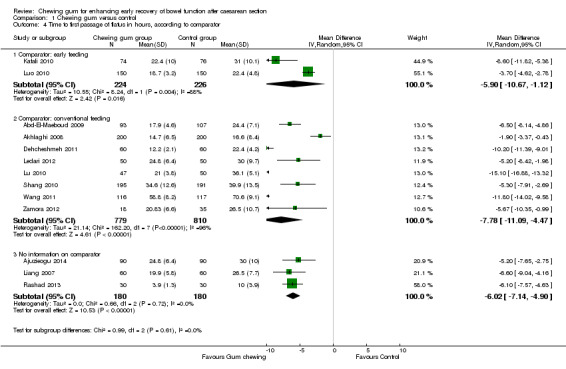
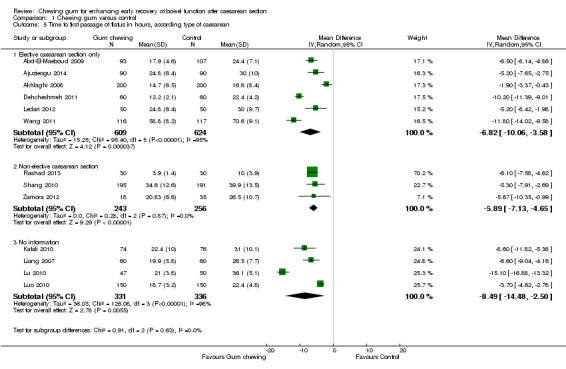
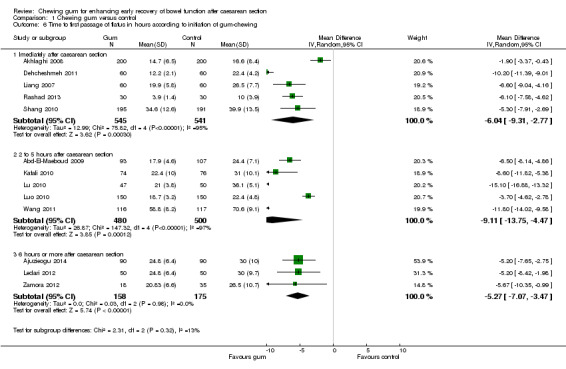
A meta‐analysis of 13 studies (Abd‐El‐Maeboud 2009; Ajuzieogu 2014; Akhlaghi 2008; Dehcheshmeh 2011; Kafali 2010; Ledari 2012; Liang 2007; Lu 2010; Luo 2010; Rashad 2013; Shang 2010; Wang 2011; Zamora 2012) showed that the time to first passage of flatus was on average seven hours shorter in the group of women who chewed gum compared to the control group (mean difference (MD) ‐7.09 hours, 95% confidence interval (CI) ‐9.27 to ‐4.91 hours; 2399 women; 13 studies; random‐effects Tau2 = 14.63, I2 = 95%) (Analysis 1.1). All of the studies had effects in the same direction but they were extremely variable. Visual inspection of the funnel plot suggests the possibility of publication bias (Figure 4). Using the GRADE approach, the quality of the evidence for this outcome was graded as very low because of potential bias in most studies (participants and personnel were not blinded to the intervention and the outcome was self‐reported), high heterogeneity (although it is more likely to be due to difference in size of effect and not to direction of effect) and possibility of publication bias (see Table 1).
1.1. Analysis.
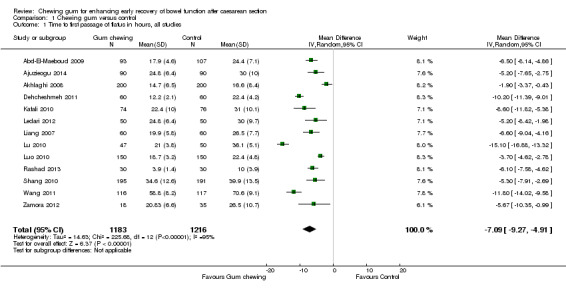
Comparison 1 Chewing gum versus control, Outcome 1 Time to first passage of flatus in hours, all studies.
4.
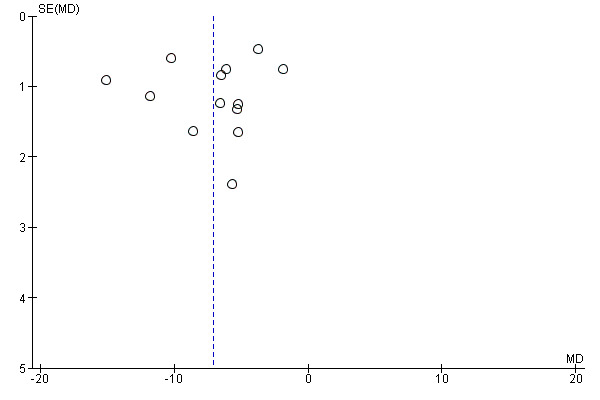
Funnel plot of comparison: 1 Chewing gum versus control, outcome: 1.1 Time to first passage of flatus in hours, all studies.
The reduction in the time to passage of flatus was observed across all of the subgroups that we examined (see Subgroup analyses).
Proportion of participants with ileus
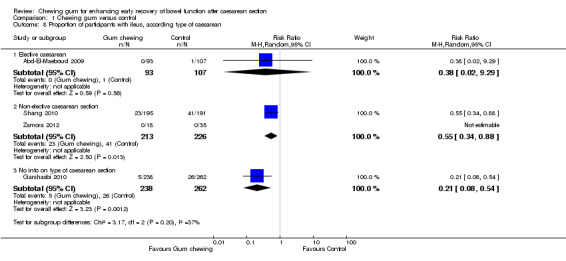
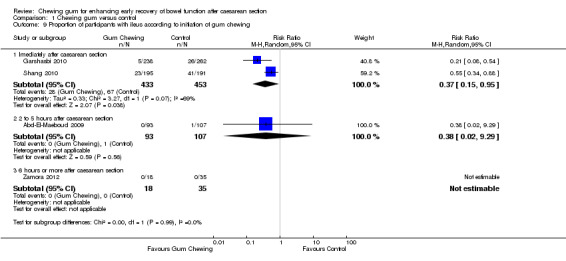
This meta‐analysis (Analysis 1.7), which included four studies (Abd‐El‐Maeboud 2009; Garshasbi 2010; Shang 2010; Zamora 2012), showed that the rate of ileus (as defined by the authors) was over 60% lower in the group that chewed gum compared to the control group, with low heterogeneity (risk ratio (RR) 0.39, 95% CI 0.19 to 0.80; four studies, 1139 participants; I2 = 39%). The quality of the evidence for this outcome was graded as low because of potential bias in most studies (no information on blinding of outcome assessors in three studies) and low number of events (Table 1).
1.7. Analysis.
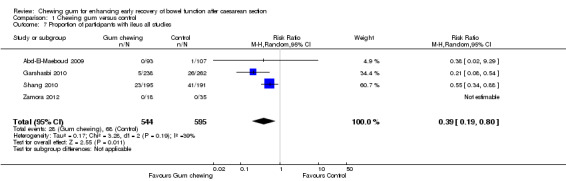
Comparison 1 Chewing gum versus control, Outcome 7 Proportion of participants with ileus all studies.
The definition of ileus varied in these four studies. Garshasbi 2010 and Zamora 2012, both short congress abstracts, provided no specific definition for this condition; Garshasbi 2010 stated that the rate of "mild ileus" was 2% in the gum‐chewing group compared to 10% in the control group, while Zamora 2012 stated that there were "no recorded post‐operative ileus symptoms in either group". Abd‐El‐Maeboud 2009 described no cases of ileus in the gum‐chewing group and one case of "severe ileus" in the control group, which was a woman with non‐passage of flatus or bowel movement, abdominal distension and more than three episodes of vomiting, who responded to conservative management including the placement of a nasogastric tube for two days. Shang 2010 had no cases of "severe ileus symptoms" but stated that 12% of the women in the gum‐chewing group, compared to 21% in the control group, developed "mild ileus" defined by the study author as mild anorexia, abdominal cramps and non‐persistent nausea or vomiting".
Due to lack of data, we did not carry out all planned subgroup analyses for this outcome; there was no difference in the results in the subgroups that we examined (see Subgroup analyses).
Tolerance to gum and adverse effects of gum chewing
None of the 17 studies included in this review reported any specific adverse effects associated with the intervention (although this outcome was not listed as a pre‐specified outcome within the trial protocols or the methods sections).
Eight studies, involving 925 women in the intervention group, reported tolerance to gum chewing as one of their outcomes (Abd‐El‐Maeboud 2009; Akhlaghi 2008; Garshasbi 2010; Kafali 2010; Ledari 2012; Liang 2007; Satij 2006; Shang 2010). Seven of these stated that all their participants tolerated the intervention well, while one study (Shang 2010) reported that three of their 195 participants (1.6%) were dissatisfied with having to chew gum but nonetheless completed the intervention until first passage of stool. We wrote to the authors of this study to obtain more details about the specific reasons for this lack of satisfaction but they did not reply (Analysis 1.14). The overall rate of intolerance to gum chewing, based on these eight studies, was 0.3% (3/925).
1.14. Analysis.
Comparison 1 Chewing gum versus control, Outcome 14 Number of participants with adverse effects or intolerance to gum.
| Number of participants with adverse effects or intolerance to gum | |||
|---|---|---|---|
| Study | Number of participants | N with adverse effects or intolerance to gum | Details |
| Abd‐El‐Maeboud 2009 | 93 | 0 | no details |
| Akhlaghi 2008 | 200 | 0 | no details |
| Garshasbi 2010 | 238 | 0 | no details |
| Kafali 2010 | 74 | 0 | no details |
| Ledari 2012 | 50 | 0 | no details |
| Liang 2007 | 60 | 0 | no details |
| Satij 2006 | 15 | 0 | no details |
| Shang 2010 | 195 | 3 | No information on the exact symptoms.Three women complained about gum chewing but completed their course until first passage of stool. |
Due to lack of data, we did not carry out any subgroup analyses for this outcome.
Subgroup analyses
Time to first passage of flatus, in hours
There was no difference in results between subgroups according to:
previous caesarean (first versus repeated CS) (Chi2 0.29, df 2 (P = 0.87), I2 0%) Analysis 1.2;
time spent chewing (more than 1 hour/day versus up to 1 hour/day) (Chi2 0.34, df 2 (P = 0.84), I2 0%, Analysis 1.3;
comparator (early versus conventional feeding) (Chi2 0.99, df 2 (P = 0.61), I2 0%), Analysis 1.4;
type of cesarean (elective versus non‐elective) (Chi2 0.91, df 2 (P = 0.63), I2 0%), Analysis 1.5;
initiation of gum chewing (immediately after, versus two to five hours after CS, versus six or more hours after CS) (Chi2 2.31, df 2 (P = 0.32), I2 13.4%) Analysis 1.6.
1.2. Analysis.
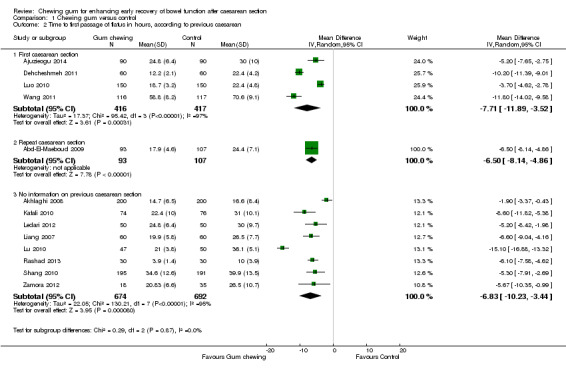
Comparison 1 Chewing gum versus control, Outcome 2 Time to first passage of flatus in hours, according to previous caesarean.
1.3. Analysis.
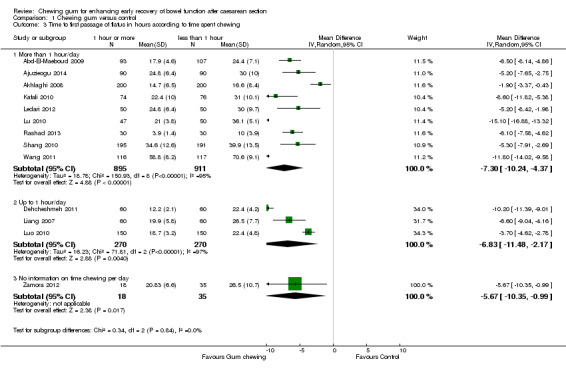
Comparison 1 Chewing gum versus control, Outcome 3 Time to first passage of flatus in hours according to time spent chewing.
1.4. Analysis.
Comparison 1 Chewing gum versus control, Outcome 4 Time to first passage of flatus in hours, according to comparator.
1.5. Analysis.
Comparison 1 Chewing gum versus control, Outcome 5 Time to first passage of flatus in hours, according type of caesarean.
1.6. Analysis.
Comparison 1 Chewing gum versus control, Outcome 6 Time to first passage of flatus in hours according to initiation of gum‐chewing.
Proportion of participants with ileus
There was no difference in results between subgroups according to:
type of cesarean (elective versus non‐elective) (Chi2 3.17, df 2 (P = 0.20), I2 36.9%) Analysis 1.8; and
initiation of gum chewing (immediately after, versus two to five hours after CS, versus six or more hours after CS) (Chi2 0.00, df 1 (P = 0.99), I2 0%) Analysis 1.9.
1.8. Analysis.
Comparison 1 Chewing gum versus control, Outcome 8 Proportion of participants with ileus, according type of caesarean.
1.9. Analysis.
Comparison 1 Chewing gum versus control, Outcome 9 Proportion of participants with ileus according to initiation of gum chewing.
Sensitivity analysis
We planned to perform a sensitivity analysis for the primary outcomes 'Time to first passage of flatus' and 'Proportion of participants with ileus' by excluding studies with high or unclear risk of selection or detection bias, however this was not possible because all studies were judged to have a high risk of detection bias due to the nature of the intervention.
After the exclusion of the studies with unclear (Kafali 2010; Ledari 2012) or high (Wang 2011) risk of attrition bias, the pooled results of the remaining studies showed that chewing gum reduced the time to first passage of flatus by an average of six to seven hours (MD ‐6.65 hours, 95% CI ‐9.15 to ‐4.16 hours, 1916 participants, 10 studies; I2 = 96%) (Analysis 2.1).
2.1. Analysis.
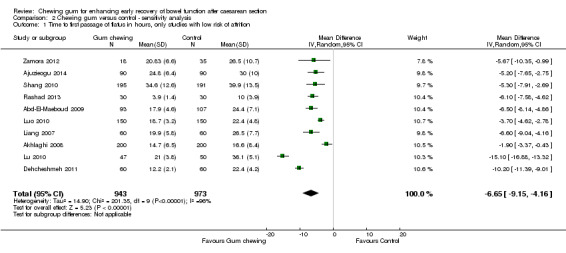
Comparison 2 Chewing gum versus control ‐ sensitivity analysis, Outcome 1 Time to first passage of flatus in hours, only studies with low risk of attrition.
We could not perform a sensitivity analysis for rate of ileus excluding studies with high attrition because all four studies with this outcome had a low risk for attrition bias.
Secondary outcomes
Time to passage of faeces
Eleven studies provided data on the time to passage of stools, that could be pooled into a meta‐analysis. The average time to passage of stools was over nine hours shorter in the intervention compared to the control group (MD ‐9.22, 95% CI ‐11.49 to ‐6.95; 2016 participants; 11 studies; random‐effects Tau2 = 12.53, I2 = 93%) (Analysis 1.10), with high statistical heterogeneity. The funnel plot suggests the possibility of publication bias (Figure 5). Garshasbi 2010 stated, in their congress abstract, that the median time to passage of stools was significantly shorter in the gum‐chewing group compared to the control group (19.8 hours versus 27.3 hours, P < 0.001).
1.10. Analysis.
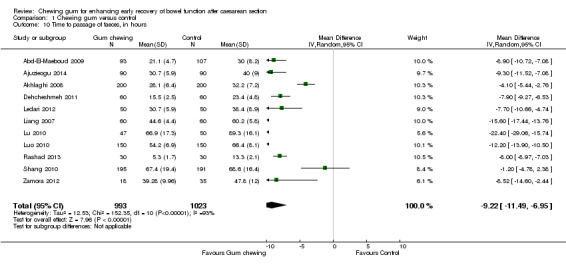
Comparison 1 Chewing gum versus control, Outcome 10 Time to passage of faeces, in hours.
5.
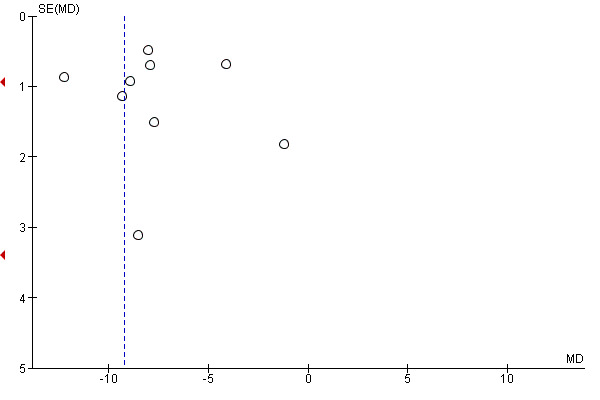
Funnel plot of comparison: 1 Chewing gum versus control, outcome: 1.10 Time to passage of faeces, in hours.
Duration of hospital stay
Seven studies assessed this outcome and could be combined in a meta‐analysis (Analysis 1.11). The average duration of hospital stay was about eight hours shorter (0.36 days) in the group that chewed gum compared to the control group (MD ‐0.36 days, 95% CI ‐0.53 to ‐0.18 days; 1489 participants; seven studies; random‐effects Tau2 = 0.04, I2 = 92%). Jakkaew 2013 reported this outcome as median and concluded that duration of hospital stay did not differ significantly between the groups (2.92 days in both groups; P = 0.99). Garshasbi 2010 did not provide any numerical data on this, in a congress abstract, but stated that there was "virtually no difference between the groups".
1.11. Analysis.
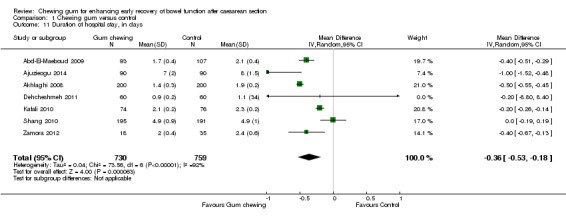
Comparison 1 Chewing gum versus control, Outcome 11 Duration of hospital stay, in days.
Woman's satisfaction
None of the studies assessed women's satisfaction with having to chew gum in the postoperative period. Ajuzieogu 2014 stated in Methods that "patients in the gum group were interviewed on their satisfaction with the technique to rate it using a visual analogue scale" but in the Results they present the scores for both the gum and the control group; therefore, we judged that this study did not actually assess satisfaction related to gum chewing. Jakkaew 2013 stated in the study protocol that this was one of the pre‐specified outcomes but it was not reported in the publication.
Need for analgesia or antiemetic agents within the first 72 hours after CS
Abd‐El‐Maeboud 2009 reported no differences in the proportion of women who required additional pethidine in the post‐CS period in the gum and control groups (13/93 versus 15/107). Two other studies (Kafali 2010; Shang 2010) reported on the use of additional anti‐emetic medication in the postoperative period. The meta‐analysis of these three studies showed no clear difference between the intervention and control group (average RR 0.50, 95% CI 0.12 to 2.13; 726 women; three studies; random‐effects Tau2 = 0.79, I2 = 69%) (Analysis 1.12).
1.12. Analysis.
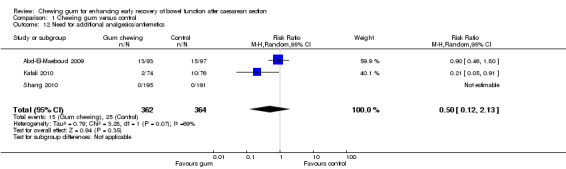
Comparison 1 Chewing gum versus control, Outcome 12 Need for additional analgesics/antiemetics.
Time to first hearing of normal intestinal sounds
Nine studies (involving more than 1700 women) assessed this outcome. The first intestinal sounds were identified, on average, four to five hours earlier in the group of women who chewed gum than in the control group (MD ‐4.56 hours, 95% CI ‐6.18 to ‐2.93; 1729 participants; nine studies; random‐effects, Tau2 = 5.41, I2 = 96%) (Analysis 1.13).
1.13. Analysis.
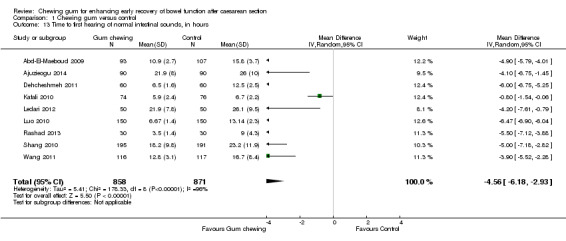
Comparison 1 Chewing gum versus control, Outcome 13 Time to first hearing of normal intestinal sounds, in hours.
Discussion
Summary of main results
This review identified 17 randomised trials (involving more than 3000 women) that assessed the effects of chewing gum to enhance the recovery of bowel function in the postoperative period after a caesarean section (CS). The intervention substantially reduced the time to first passage of flatus, on average, by approximately seven hours and this effect was consistent in all subgroup analyses (primary versus repeat CS, time spent chewing gum per day, early feeding versus conventional feeding, elective versus non‐elective CS and time after CS when gum‐chewing was initiated). The quality of the evidence for this outcome was classified as very low because of the high risk of bias of most studies (due to lack of blinding and self‐reporting of the outcome), high heterogeneity in the meta‐analysis and possibility of publication bias (Table 1).
Chewing gum also reduced the rate of ileus by over 60% in the intervention compared to the control group. The quality of the evidence for this outcome was judged to be low because of the low rate of events and the uncertain risk of bias for assessors evaluating this outcome in three of the four studies. The number of women with intolerance to the intervention was very low (less than 0.5%) and there were no reports of adverse effects associated with gum‐chewing in any of the studies included in this review. The quality of the evidence for this outcome was judged to be low because of the low number of events and the high risk of bias of most studies (due to lack of blinding of the participants and the fact that this outcome was self‐reported) (Table 1).
Chewing gum in the post‐caesarean period also shortened the time to passage of stools by approximately nine hours. The quality of the evidence was judged to be very low because participants were not blinded to the intervention and self‐reported this outcome, there was high heterogeneity in the meta‐analysis and the possibility of publication bias (Table 1). Chewing gum shortened the time to hearing of first bowel sounds by nearly five hours, and the duration of hospital stay by approximately eight hours. No study assessed the satisfaction of the women who were asked to chew gum.
Overall completeness and applicability of evidence
This review included 17 studies published between 2006 to 2014, conducted in many different countries (mostly low‐ to middle‐income countries) and involving 3149 women. The women had different reproductive histories (nulliparas and multiparas, with and without previous CS) and were undergoing caesarean delivery in different circumstances (elective, intrapartum or emergency), using different types of anaesthesia (regional or general) and these women received different doses and types of chewing gum, in association with different postoperative care and feeding protocols. Despite these differences, our overall and subgroup meta‐analyses show that chewing gum improves the recovery of bowel function. The effectiveness of the intervention under these very different contexts supports the external validity of our findings which indicate that gum chewing can contribute to the early recovery of bowel function after CS.
All of the studies had effects in the same direction but they were extremely variable for most of the outcomes assessed. Therefore, the major uncertainty about the effectiveness of gum chewing on the recovery of bowel function after CS is about the size of the effect rather than its direction. The differences in the size of the effects, and the ensuing high statistical heterogeneity in several of our meta‐analyses, can explained by intrinsic differences in the sample sizes of the studies, and in the characteristics of the participants and the intervention. For instance, the selection criteria of the women in each individual study varied considerably with respect to obstetric characteristics (e.g. inclusion or exclusion of women without one or more previous CS or other abdominal surgeries), type of anaesthesia used, pre‐surgical fasting or not, elective versus emergency CS, duration of the surgery, technique and skills of the surgeons and blood loss during the operation. There were also differences between the studies in the type of postoperative routine protocols used (e.g. time to ambulation, early versus conventional feeding protocol, type of food, routine prescription and type of analgesic and antiemetic medications). The 17 trials also had many differences in the intervention itself such as the time after surgery when gum‐chewing was initiated (e.g. immediately upon recovery from anaesthesia versus only 12 hours after the CS), the number of gum sticks given to chew per session, the exact composition of the gum (type and amount of artificial sweetener and flavouring), the number of chewing sessions per day, the duration of each chewing‐gum session, and consequently the total amount of time spent chewing gum per day. We tried to assess the potential effect of some of these characteristics by doing subgroup analyses for our primary outcomes but many studies did not provide detailed information on these aspects.
The existing data suggest that gum‐chewing may be a simple, effective and apparently safe intervention that could help to improve the recovery of bowel function after a CS.
Quality of the evidence
We used the GRADE approach to assess the quality of the evidence. As can be seen in Table 1 the quality of the evidence for time to first passage of flatus was very low. For the proportion of women with ileus and with intolerance to gum, the evidence was of low quality.
We downgraded the quality of the evidence for time to first passage of flatus and to first passage of faeces and also for adverse effects/intolerance to gum chewing because of the high risk of bias of the studies. Due to the nature of the intervention, it was not possible to blind the participants and they self‐reported these outcomes. For time to first flatus and faeces, we downgraded the quality of the evidence further because of the high heterogeneity in these meta‐analyses and the potential for publication bias based on the visual inspection of the funnel plots. The quality of the evidence for adverse effects/tolerance to gum chewing and for ileus was downgraded because of the small number of events. The quality of the evidence for ileus was further downgraded due to the unclear risk of bias for the assessors evaluating this outcome.
Potential biases in the review process
Although this review included 17 studies (3149 women), our results may be incomplete. Three of our included studies (Garshasbi 2010; Satij 2006; Zamora 2012), totaling 585 women were presented only as congress abstracts with very limited information; one of these trials (Garshasbi 2010) was the study with the largest sample size (500 participants). Moreover, two other trials (212 participants) were published in Farsi and we could only extract the information provided in the English abstracts (Abasi 2014; Dehcheshmeh 2011). This lack of information limited our ability to assess the risk of bias in several studies and to include more data in our meta‐analyses.
Another limitation of this review was that the results of two studies (Garshasbi 2010; Jakkaew 2013) could not be included in our meta‐analyses because the study authors presented their findings in medians and ranges and they did not respond to our emails. We decided not to make estimations and assumptions to extract data from these studies, to avoid introducing additional bias into our meta‐analyses. Two studies included in this review (Abasi 2014; Jakkaew 2013) did not provide any information that could be included in the meta‐analyses and we have presented their findings in a narrative format only.
There were no reports of adverse effects related to gum chewing in the 17 included studies. However, since none of them specifically stated that this was a pre‐specified outcome in their protocols or methods, we cannot be sure that gum‐chewing in the postoperative period of CS is devoid of adverse effects. Since there is some evidence suggesting that chewing gum can lead to jaw pain (Tabrizi 2014; Watemberg 2014), it is possible that this may have occurred in some of the participants, especially those who chewed gum for longer periods of time.
None of the studies assessed or reported adherence to gum‐chewing. Since the intervention is totally dependent on the participant, this is an important issue. It is possible that variation in adherence to the intervention (e.g. not following exactly the recommended interval between or duration of each gum‐chewing session) may have contributed to the large variation in the magnitude of the intervention seen in several of the outcomes.
The funnel plots of the meta‐analyses for time to first flatus and time to first bowel sounds indicate the possibility of publication bias.
We used a random‐effects model in all meta‐analyses except one because of the high heterogeneity between studies.
Agreements and disagreements with other studies or reviews
Our findings on the effectiveness of gum‐chewing to enhance early recovery of bowel function are in concordance with other existing systematic reviews on gum‐chewing for CS (Craciunas 2014; Hochner 2015; Huang 2015; Zhu 2014) and systematic reviews for other types of abdominal surgeries which also included some studies on CS (Li 2013; Short 2015; Yin 2013). Our effect estimates for time to first flatus, passage of stools, bowel sounds and length of hospital stay vary somewhat in relation to these other publications because of the smaller number of studies included in those reviews and methodological differences in study selection and data pooling.
Due to differences in the search strategy, language restrictions and the date when the searches were run, ours is the largest and most comprehensive review to date on gum chewing for CS, in terms of the number of included studies and total number of participants. Our review also included additional outcomes (tolerance/adverse effects and women's satisfaction regarding gum chewing) that are not part of the other reviews on gum for CS. Finally, unlike any of the previous reviews, we performed subgroup analyses to assess the effects of gum chewing according to previous CS and type of CS (elective or not), as well as time spent chewing gum per day and time after surgery when gum‐chewing started and also according to the type of feeding protocol used (conventional or early). We show that gum‐chewing is effective in improving recovery of bowel function in all of these subgroups.
Authors' conclusions
Implications for practice.
There is consistent evidence that chewing gum in the immediate postoperative period after a caesarean section (CS) enhances early recovery of bowel function. The overall quality of the evidence is low to very low mainly because of the high risk of bias in the included studies since the participants could not be blinded to the intervention and they self‐reported the outcomes, and also because of the high heterogeneity between the studies. All of the studies show effects in the same direction but they were extremely variable for most of the outcomes assessed. Therefore, the major uncertainty about the effectiveness of gum chewing on the recovery of bowel function after CS is about the size of the effect. The evidence indicates that chewing gum after CS is well tolerated by the vast majority of women and there is a lack of evidence on potential adverse effects related to this intervention. This simple and effective intervention could easily be integrated into routine post‐CS protocols.
Implications for research.
Future large, well‐designed and conducted studies, with better methodological and reporting quality, will help to improve the update of this review and increase the quality of the evidence for this intervention. These studies should be large enough to provide a relatively precise estimate of the treatment effect or clarify the difference in effects between subgroups, or both. Further research is also necessary to establish the optimal regimen of gum‐chewing (initiation, number and duration of sessions per day) to enhance bowel function recovery and to assess potential adverse effects of and women's satisfaction with this intervention. New studies should also assess the compliance of the participants to the recommended gum‐chewing instructions.
Acknowledgements
We are grateful to Cochrane Pregnancy and Childbirth for their help in the search process.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
We are grateful for Qian Long for help with the translation of Chinese texts.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We are grateful to the peer reviewers for helping us improve the quality of this manuscript.
Appendices
Appendix 1. LILACS search strategy
((PT:"randomized controlled trial" OR PT:"controlled clinical trial" OR PT:"multicenter study" OR MH:"randomized controlled trials as topic" OR MH:"controlled clinical trials as topic" OR MH:"multicenter study as topic" OR MH:"random allocation" OR MH:"double‐blind method" OR MH:"single‐blind method") OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animals OR MH:rabbits OR MH:rats OR MH:primates OR MH:dogs OR MH:cats OR MH:swine OR PT:"in vitro") AND (MH:D05.750.078.739.249$ OR MH:D09.698.700.249$ OR MH:D20.215.721.249.249$ OR MH:J02.500.140.200$ OR chiclete OR chicletes OR chiclet OR TW:"Chewing Gum" OR TW:"Goma de Mascar" OR TW:"Gomas de Mascar" OR TW:"Goma‐de‐Mascar" OR TW:"Gum‐Chewing" OR TW:"Chewing Gums" OR TW:"Chewing‐Gums" OR TW:"Chewing‐Gum" OR TW:"Gum‐Chewing" OR TW:"Gum Chewing" OR TW:"Gum" OR TW:"Gums" OR TW:"Chewing") AND (MH:E04.520.252.500$ OR TW:"Cesarean Section" OR TW:Cesárea OR TW:"Abdominal Delivery" OR MH:C06.405.469.531.492.500$ OR TW:"Intestinal Pseudo‐Obstruction" OR TW:"Seudoobstrucción Intestinal" OR TW:"Pseudo‐Obstrução Intestinal" TW:"Paralytic Ileus" OR TW:"Visceral Myopathy" OR TW:"Cesárea Repetida" OR TW:Recesariana OR TW:"Repeat Cesarean Section" OR MH:E04.520.252.500.150 OR MH:C06.405.469.531.492$ OR TW:"Ileus" OR MH:G10.261.326.310$ OR TW:"Gastrointestinal Motility" OR TW:"Motilidad Gastrointestinal" OR TW:"Motilidade Gastrointestinal"OR TW:"Intestinal Motility" OR MH:C23.888.821.360$ OR TW:"Flatulence" OR TW:"Flatulência" OR TW:Flatus OR MH:E02.760.731.700$ OR MH:E04.604.500$ OR MH:N02.421.585.722.700$ OR TW:"Postoperative Care" OR TW:"Cuidado Pós‐Operatório" OR MH:G01.910.857$ OR TW:"Time Factor" OR TW:"Factor de Tiempo" OR TW:"Factor de Tempo")
Appendix 2. Clinical Trials. gov search strategy
(cesarean OR caesarean) AND ("Bowel movement" OR "Bowel function" OR Gas OR Ileus OR "Intestinal pseudo‐obstruction" OR "intestinal obstruction" OR "Intestinal diseases" OR "Gastro intestinal diseases" OR "Digestive system diseases") AND (gum)
Appendix 3. International Clinical Trials Register search strategy
(cesarean AND gum AND ileus)
(caesarean AND gum AND ileus)
Data and analyses
Comparison 1. Chewing gum versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to first passage of flatus in hours, all studies | 13 | 2399 | Mean Difference (IV, Random, 95% CI) | ‐7.09 [‐9.27, ‐4.91] |
| 2 Time to first passage of flatus in hours, according to previous caesarean | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 First caesarean section | 4 | 833 | Mean Difference (IV, Random, 95% CI) | ‐7.71 [‐11.89, ‐3.52] |
| 2.2 Repeat caesarean section | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐6.5 [‐8.14, ‐4.86] |
| 2.3 No information on previous caesarean section | 8 | 1366 | Mean Difference (IV, Random, 95% CI) | ‐6.83 [‐10.23, ‐3.44] |
| 3 Time to first passage of flatus in hours according to time spent chewing | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 More than 1 hour/day | 9 | 1806 | Mean Difference (IV, Random, 95% CI) | ‐7.30 [‐10.24, ‐4.37] |
| 3.2 Up to 1 hour/day | 3 | 540 | Mean Difference (IV, Random, 95% CI) | ‐6.83 [‐11.48, ‐2.17] |
| 3.3 No information on time chewing per day | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐5.67 [‐10.35, ‐0.99] |
| 4 Time to first passage of flatus in hours, according to comparator | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Comparator: early feeding | 2 | 450 | Mean Difference (IV, Random, 95% CI) | ‐5.90 [‐10.67, ‐1.12] |
| 4.2 Comparator: conventional feeding | 8 | 1589 | Mean Difference (IV, Random, 95% CI) | ‐7.78 [‐11.09, ‐4.47] |
| 4.3 No information on comparator | 3 | 360 | Mean Difference (IV, Random, 95% CI) | ‐6.02 [‐7.14, ‐4.90] |
| 5 Time to first passage of flatus in hours, according type of caesarean | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Elective caesarean section only | 6 | 1233 | Mean Difference (IV, Random, 95% CI) | ‐6.82 [‐10.06, ‐3.58] |
| 5.2 Non‐elective caesarean section | 3 | 499 | Mean Difference (IV, Random, 95% CI) | ‐5.89 [‐7.13, ‐4.65] |
| 5.3 No information | 4 | 667 | Mean Difference (IV, Random, 95% CI) | ‐8.49 [‐14.48, ‐2.50] |
| 6 Time to first passage of flatus in hours according to initiation of gum‐chewing | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Imediately after caesarean section | 5 | 1086 | Mean Difference (IV, Random, 95% CI) | ‐6.04 [‐9.31, ‐2.77] |
| 6.2 2 to 5 hours after caesarean section | 5 | 980 | Mean Difference (IV, Random, 95% CI) | ‐9.11 [‐13.75, ‐4.47] |
| 6.3 6 hours or more after caesarean section | 3 | 333 | Mean Difference (IV, Random, 95% CI) | ‐5.27 [‐7.07, ‐3.47] |
| 7 Proportion of participants with ileus all studies | 4 | 1139 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.19, 0.80] |
| 8 Proportion of participants with ileus, according type of caesarean | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Elective caesarean | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 9.29] |
| 8.2 Non‐elective caesarean section | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.34, 0.88] |
| 8.3 No info on type of caesarean section | 1 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.08, 0.54] |
| 9 Proportion of participants with ileus according to initiation of gum chewing | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Imediately after caesarean section | 2 | 886 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.15, 0.95] |
| 9.2 2 to 5 hours after caesarean section | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 9.29] |
| 9.3 6 hours or more after caesarean section | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Time to passage of faeces, in hours | 11 | 2016 | Mean Difference (IV, Random, 95% CI) | ‐9.22 [‐11.49, ‐6.95] |
| 11 Duration of hospital stay, in days | 7 | 1489 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.53, ‐0.18] |
| 12 Need for additional analgesics/antiemetics | 3 | 726 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.12, 2.13] |
| 13 Time to first hearing of normal intestinal sounds, in hours | 9 | 1729 | Mean Difference (IV, Random, 95% CI) | ‐4.56 [‐6.18, ‐2.93] |
| 14 Number of participants with adverse effects or intolerance to gum | Other data | No numeric data |
Comparison 2. Chewing gum versus control ‐ sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to first passage of flatus in hours, only studies with low risk of attrition | 10 | 1916 | Mean Difference (IV, Random, 95% CI) | ‐6.65 [‐9.15, ‐4.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abasi 2014.
| Methods | Clinical trial, Bent‐al‐Hoda hospital Bojnoord, Iran. | |
| Participants | 92 women, mean age: 29 years, with uncomplicated elective CS, parity 1‐4, no information on previous CS, no information on type of anaesthesia. Exclusion criteria: bleeding > 80 mL. |
|
| Interventions |
Intervention group (N = 46): chewing gum for 1 h, 3 times/d at 8:00, 14:00 and 20:00 after the surgery (total 180 min/d). Control group (N = 46): "routine normal diet postoperative"; no information on exact type of feeding (early or conventional). |
|
| Outcomes | First passage of flatus and stool, first feeling of peristaltic movements. | |
| Notes | IRCT2012082610661N1. This study is in Farsi and we could not translate the full text. The extracted information was obtained exclusively from the English abstract, which provided no numerical data for any of the outcomes. This study could not be included in any of the meta‐analyses. We contacted the study authors for details but they did not respond. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information provided: "The subjects were randomly assigned into two groups". |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of the intervention did not allow blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus and stools. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not assessed. The proportion of participants with ileus, time to first hearing of intestinal sounds, need for analgesia or antiemetic agents and duration of hospital stay were not part of the outcomes of this study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses. |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in protocol were reported in the results. |
| Other bias | Unclear risk | No information. |
Abd‐El‐Maeboud 2009.
| Methods | RCT in Ain Shams University Maternity Hospital, Egypt. | |
| Participants | 200 women, parity 0‐7, most (64.5%) multiparas; 59.9% of participants with a previous CS; delivered by "elective CS under general anaesthesia" in the early morning period. Mean age: intervention group 26.2 (± 4.1), control group 26.4 (± 4.6) years. Exclusion criteria: "hysterectomy or other extensive intra‐abdominal surgery as a result of operative complications". |
|
| Interventions |
Intervention group (N = 93): women received "one stick of commercially available sugarless gum (Samarah Food, Cairo, Egypt)" to chew for 15 min every 2 h, starting 2 h "after surgery (performed in the early morning)" and every 2 h during day time (total chewing time = 210 min/d). This was stopped when the passage of flatus occurred. "Compliance was monitored by counting and recording the number of sticks remaining" with the women during recording of vital signs postoperatively. Control group (N = 107): traditional management (oral intake of clear fluids allowed after passage of flatus and regular diet with the passage of bowel movement). |
|
| Outcomes | Time to first passage of flatus, time to first hearing of normal intestinal sounds, time to first bowel movement, mild ileus symptoms "(vomiting or abdominal distension felt by the participant and seen on examination)", paralytic ileus ("a group of manifestations persisting longer than 24 h or requiring nasogastric tube placement", which include "absent or hypoactive bowel sounds, non‐passage of flatus or bowel movement, abdominal distension, more than 3 episodes of vomiting, with or without generalised crampy abdominal pain"), tolerance of gum chewing, time until discharge from the hospital, need for additional use of narcotics (pethidine). Additional outcomes: febrile morbidity (temperature > 38 C on 2 occasions 6 h apart), re‐operation, blood transfusion, hospital readmission. |
|
| Notes | Duration of surgery was longer in the intervention than in the control group (41.3 +/‐ 7.5 min versus 38.4 +/‐ 8.1 min, P < 0.001. No study protocol available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each enrolled subject was allocated the next available number in the concealed sequence in a computer‐generated randomisation plan." |
| Allocation concealment (selection bias) | Unclear risk | "The assigned intervention was revealed by the first author who played no role in patients’ enrolment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The nature of the study did not allow blinding after application of the assigned intervention postoperatively." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus and stools or tolerance to gum. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | "Auscultation for intestinal sounds was performed at 4 to 6‐hours intervals by two of the authors only." No information on blinding of the assessors. No information about blinding of assessors regarding hospital discharge or ileus. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses. All randomised participants were included in the results. |
| Selective reporting (reporting bias) | Low risk | All outcomes included in Methods were reported. |
| Other bias | High risk | Duration of surgery was longer in the intervention than in the control group (41.3 +/‐ 7.5 min versus 38.4 +/‐ 8.1 min, P < 0.001). |
Ajuzieogu 2014.
| Methods | A prospective, single‑blind RCT, in Enugu, Nigeria, 4 hospitals. | |
| Participants | 200 primiparous* (see Notes) women scheduled for elective CS under spinal anaesthesia were consecutively recruited, but only 180 women were randomised (see reasons in Notes). Inclusion criteria: age 18‐35, primigravida* (see Notes), and no allergy to mint. Exclusion criteria: women with loose teeth, using opioids, diabetic, with previous pelvic or abdominal surgeries ** (see Notes) or hypothyroid; and "if the surgery lasted more than 1 h". Mean of age: 25.0 (± 6.4) years and 25.5 (± 6.0) years in gum and control groups, respectively. |
|
| Interventions | "Commercially available sugar‑free gum (Orbit, Wrigley Company, Poland) was used for this study". Intervention group (N = 90): were given 1 stick of sugar‑free, "chewing gum 3 times daily (in the morning, afternoon and in the evening) from the 1st post‑operative day for 5 consecutive days with an instruction to chew for 30 minutes without swallowing the chewed gum. The gums were given to patients at a fixed interval to help monitor compliance". Total chewing time per day = 90 min. Control group (N = 90): "standard postoperative care was provided"; no detailed information on type of feeding protocol. |
|
| Outcomes | Time to the first passage of flatus, time to first intestinal sound, time to first defecation and "patient satisfaction concerning postoperative gum chewing"***. Duration of hospital stay also reported in Results. | |
| Notes | Reasons why 20 eligible participants were not randomised: they disclosed their group to the researchers, surgery lasted more than 1 h, or failed spinal anaesthesia. *Although authors state in Methods that only primiparous women were included, in the Results section table, mean parity is reported as 2 (+/‐0.7) and 1.8 (+/‐1.5) in gum and control groups. Therefore, we considered this as a study including both nulliparas and multiparas. ** Since authors describe that they excluded women with previous pelvic or abdominal surgeries, we concluded that none of the participants had a previous CS No study protocol available. *** Authors state in Methods that "patients in the gum group were interviewed on their satisfaction with the technique to rate it using a visual analogue scale".However, in Results they present mean satisfaction scores for both the gum and the control group. Therefore, we judged that this study did not actually assess satisfaction with gum‐chewing itself, but some other type of satisfaction. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All enrolled women were allocated using a computer generated random sequence from a statistics program into gum‑chewing (G group) and control (control group)." |
| Allocation concealment (selection bias) | Unclear risk | "The women were notified of their groups at the immediate postoperative period." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The nature of the study did not allow blinding of the subjects after assignment of the intervention postoperatively." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus and stools or satisfaction to gum chewing. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Time to first intestinal sound and duration of hospital stay "The researchers were blinded to the patients’ group allocation. Patients and nursing staff were also educated to keep the group allocation secret from the researchers". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses after randomisation. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in Methods were reported in Results. |
| Other bias | Unclear risk | No information. |
Akhlaghi 2008.
| Methods | RCT in 1 hospital, Mashhad Zeinab, Iran. | |
| Participants | 400 women, elective CS. No information on anaesthesia or parity or previous CS. Mean age: 27.3 years in intervention group and 26.6 years in control group. Exclusion criteria: complications during or after surgery. |
|
| Interventions |
Intervention group (N = 200): chewed sugar‐free and flavourless gum after regaining consciousness after surgery, for 45 min per session, 3 times/d at 8:00, 14:00 and 20:00 (total 135 min/d), until the regular diet was initiated. Control group (N = 200): routine care which consisted of "restriction of oral intake until the bowel function was returned" (conventional feeding). |
|
| Outcomes | Time of the first flatus passage, bowel sound*, defecation, duration of hospital stay and gum tolerability. Additional outcomes: time to ambulation post CS and to initiation regular diet. |
|
| Notes | *Time to bowel sounds not included in meta‐analysis because there was only mean value and no information on standard deviation. We wrote to authors but obtained no response. No study protocol available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The nature of the study did not allow blinding after application of the assigned intervention." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus and stools or tolerability to gum. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information on blinding of outcome assessors for hearing of bowel sounds and length of hospital stay, ileus or use of antiemetics or analgesics NA. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in Methods were reported in Results. |
| Other bias | Unclear risk | No information. |
Dehcheshmeh 2011.
| Methods | RCT conducted in 1 hospital in Iran. | |
| Participants | 120 primiparas scheduled for elective CS under spinal anaesthesia. Exclusion criterion: previous abdominal surgeries. Mean age: 25.63 ± 4.53 years. Range: 17‐38 years. |
|
| Interventions | Intervention group (N = 60): chewed sugar‐free gum (manufactured by Saghez Sazi Kurdestan, Iran) for 15 min 4 times daily, for 1 d as soon as they recovered from anaesthesia (total 60 min/d). Control group (N = 60): "received routine postoperative dietary" regimen, i.e. nothing by mouth until first bowel sounds (conventional feeding protocol). | |
| Outcomes | Time to first passage of flatus, time to defecation, time to first intestinal sounds and duration of hospital stay. Other outcomes pre‐specified in protocol: sensation of bowel movement, nausea, vomiting*. | |
| Notes | IRCT138902222265N1. This study is in Farsi and we could not translate the full text. The extracted information was obtained from the English abstract and the study protocol. We contacted the study authors for details but they did not reply. *Nausea and vomiting not reported in results of Abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of the study did not allow blinding after application of the assigned intervention. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus and stools. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information on blinding of outcome assessors for hearing of bowel sounds and length of hospital stay. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the results. |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes pre‐specified in study protocol were not reported in the Abstract (nausea, vomiting). However, since we could not read the full text, we cannot judge whether these outcomes were reported or not. |
| Other bias | Unclear risk | No information available. |
Garshasbi 2010.
| Methods | Prospective RCT conducted in 1 hospital, Shahid Mostafa Khomeini, Tehran, Iran. | |
| Participants | 500 women, CS (no information on parity, previous CS or if CS was elective or not); no information on type of anaesthesia. | |
| Interventions |
Intervention group (N = 238): "Patients in the gum‐chewing group chewed gum" (no info on type) 3 x/d at least 30 min each time immediately after surgery until regular diet was initiated. Total 90 min/d. Control group (N = 262): no information available about diet protocol in control group. |
|
| Outcomes | Time to first postoperative passage of flatus (in median), time to first bowel movement (in median), length of hospital stay (no numerical data), time to first hearing bowel sounds (no standard deviation), tolerance to gum and rate of ileus. | |
| Notes | This study was published only as a congress abstract. We wrote to the study author for additional information but got no response. Due to the form the data were presented (median, no standard deviation, no numerical data), we could not use the time to passage of flatus or stools, nor duration of hospital stay, nor time to first bowel sounds in our meta‐analyses. We only used rate of ileus and tolerance to gum. No study protocol available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information: "500 pregnant women who underwent a CS were randomly divided into two groups...". |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of the study did not allow blinding participants. No information provided about blinding personnel. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus and stools and tolerance to gum. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information on blinding of outcome assessors for hearing of bowel sounds, ileus and length of hospital stay. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported. |
| Selective reporting (reporting bias) | Unclear risk | None of the outcomes reported in the results were pre‐specified in the methods section. This study was published only as a congress abstract and no protocol was available. |
| Other bias | Unclear risk | No information available. |
Jakkaew 2013.
| Methods | RCT, 1 hospital in Thailand (Chiang Mai University). | |
| Participants | 50 women randomised after CS; > 80% were non‐elective CS, > 50% of the participants were having a 1ary CS; > 80% under regional anaesthesia. Participants included both nulliparas and multiparas; mean of age 31.2 ± 6.33 and 29.48 ± 5.91 years in control and gum group. Inclusion criterion: "at least 15 years of age". Exclusion criteria: hysterectomy, "surgical management of severe postpartum haemorrhage, perioperative hyperalimentation, previous bowel surgery (except for appendectomy), bowel obstruction, history of inflammatory bowel diseases, recent chemotherapy (less than 1 week), previous abdominal or pelvic radiation, postoperative endotracheal or naso/orotracheal intubation, and postoperative admission to intensive care unit". |
|
| Interventions |
Intervention group (N = 25): "Chew two tablets of artificial fresh mint flavoured sugarless gum (LotteXylitol®, Thai Lotte Co., Ltd., Chonburi, Thailand) for 30 minutes four times a day (morning, noon, evening, and before bed time) starting since the regain of consciousness and normal vital sign until the first passage of flatus (total chewing time = 120 minutes/day). For those who were allowed to receive diet but had not had first passage of flatus, they were asked to continue gum chewing for 30 minutes before each meal and at bedtime until the first passage of flatus. To promote compliance, the gums were provided to participants by ward nurses at specific times". Control group (N = 25): "conventional feeding protocol, i.e. not given anything by mouth after surgery until at least 2 of the following signs": bowel sound, feeling of hunger, passage of flatus or defecation. "Then sips of water were allowed. Subsequently, the feeding schedule proceeded to liquid diet for the next meal. Soft diet was given on the next day given good tolerance to the liquid diet. Once the passage of flatus occurred, diet was advanced to regular diet." |
|
| Outcomes | Time of the first passage of flatus (in median), duration of hospital stay (in median), rate of participants with symptoms and signs of gastrointestinal disturbances (nausea, abdominal cramping, abdominal distension, rate of vomiting)*. Additional outcomes: time to the first meal; level of feeding satisfaction; time to the first regular diet; tolerance to the first meal; rate of postoperative complications such as fever, pneumonia, wound infection and lung atelectasis. |
|
| Notes | Due to the form the authors used to present the results (median and ranges), we could not use the time to passage of flatus or duration of hospital stay in our meta‐analyses. *The number of women with vomiting episodes is reported for day 1 and day 2 (as N, %), but we do not know if it was the same woman or not. We wrote to the study authors for additional information and obtained no answer. Therefore, this study was not included in any of our meta‐analyses. IRCT NCT01131416. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The random sequence was generated by a computer‐based program. Randomization was stratified according to the type of anaesthesia (regional anaesthesia and general anaesthesia)." |
| Allocation concealment (selection bias) | Low risk | "Participants were randomly assigned into two groups by central telephone assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of intervention did not allow blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | "The symptoms and signs of gastrointestinal disturbance were evaluated daily by the outcome assessor who was blinded to the study allocation." No information on blinding of outcome assessors for duration of hospital stay. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "No participant was excluded from analysis." However the sample size reported in the paper (50) is only half of the size proposed in the protocol (100). We contacted the study authors for more details but they did not reply. |
| Selective reporting (reporting bias) | High risk | Passage of stools, rate of ileus and woman's satisfaction were described as outcomes in the protocol but not reported in the paper. |
| Other bias | High risk | In the protocol, the sample size was described as 100 participants (50 in each group); the publication describes only 50 participants (25 in each group). It is possible that the study was ended before the proposed time. |
Kafali 2010.
| Methods | RCT in Fatih University Medical Hospital, Turkey. | |
| Participants | 157 women randomly assigned to chew group (N = 78) or not to chew gum group (N = 79) after their operation; but 7 were excluded after randomisation because they needed blood transfusions; final analyses were conducted in 74 women in gum‐chewing group and 76 in control group. Both nulliparas and multiparas were included; no information on previous CS; unclear about type of CS (elective or not); half of participants used general anaesthesia. Exclusion criteria: women that "needed bowel preparation, premedication, nasogastric tubes and drains and those with medical disorders of pregnancy, antepartum haemorrhage, postpartum haemorrhage necessitating blood transfusion, delay in recovery from anaesthesia and need for intensive care postoperatively". Mean age: intervention group: 29.3 (± 3.8) years and control group: 29.2 (± 4.8) years. |
|
| Interventions | Women in both the study and control groups were managed according to their early oral hydration and ambulation protocols: "oral fluids were initiated within 6 h after surgery, irrespective of return of bowel sounds. The women were actively encouraged to increase their oral intake so as to ensure a minimum of 500 mL oral fluid intake in the first 24 h". All received 3 L of intravenous fluids in the first 12 h. "Solid food was allowed after 24 h on detection of bowel sounds on auscultation". Intervention group (N = 74): "Women began gum chewing 2 hours postoperatively. Patients chewed sugarless gum (one stick) 3 times daily in the morning, afternoon, and evening. Each episode of gum chewing lasted 1 hour except the initial one which lasted 15 minutes". Total chewing time = 3 h 15 min. Control group (N = 76): early oral hydration within 6 h of surgery (early feeding protocol). All women received similar anaesthetic agents and antibiotics. "Most patients received intravenous mefenamic acid for postoperative pain management." |
|
| Outcomes | Time to first flatus, time to first bowel sound and duration of hospital stay; need for additional analgesics and antiemetics, tolerance to gum. | |
| Notes | We wrote to authors for more details about study but got no answer. No study protocol available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was achieved by using a sequential, randomized card‐pull design." |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of intervention did not allow blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus and tolerance to gum. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | The patients’ assistant evaluated length of hospital stay, bowel sounds and postoperative analgesic and antiemetic requirement. No information available about blinding of assistant. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5.4% dropout rates in study group (N = 4) and 3.9% dropouts in control group (N = 3) because they needed blood transfusions. Data from these women were not considered in the final analysis of outcomes. Since we cannot be sure about the potential impact of these dropouts on the outcomes, we considered the risk of bias as unclear. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes in Methods were reported in Results. |
| Other bias | Unclear risk | No information available. |
Ledari 2012.
| Methods | A single‐blind RCT. Study conducted in 1 hospital, in Babol, Iran. | |
| Participants | 110 eligible participants enrolled but 10 excluded because "the surgeon considered them inadequate for the study". Data provided for 100 women. Both nulliparas and multiparas were included, no information on proportion of nulliparas or of participants with a previous CS, most CS were elective (86%). Anaesthesia: spinal. Inclusion criteria: candidates for CS with local anaesthesia. Exclusion criteria: "women with history of drug consumption, especially opioids, water and electrolyte disturbances, pancreatitis or peritonitis, history of abdominal surgery except cesarean section, postoperative complications, inability to chew gum, diabetes, pre‐eclampsia, prolonged rupture of membranes, hypothyroidism, muscular and neurological disorders". Mean age: 27.9 (± 6.4) and 28.5 (± 6.2) years in gum and control groups, respectively. Mean number of pregnancies: 1.1 (1.0) and 2.1 (1.2) in gum and control groups, respectively. |
|
| Interventions |
Intervention group (N = 50): "... chewed sugar‐free gum for at least one hour, three times daily starting 6 hours after surgery (after recovery from anesthesia) until being discharged. Commercially available sugar‐free gum (Wrigley Company, Poland) was used...". Total chewing time = 180 min/d. Control group (N = 50): conventional feeding protocol (oral intake only after at least 2 of the following: bowel sounds, feeling of hunger or passage of flatus). |
|
| Outcomes | First bowel sounds, first passage of flatus, first defecation, tolerance to gum. Additional outcomes: first feeling of hunger; postoperative complications. |
|
| Notes | A total of 110 women were enrolled, but the available data for outcomes are 100 women. This study has 3 publications, including 1 that presents only the results of the nulliparas. IRCT201008093902N2: (see deviations from protocol in Other risk of bias). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All enrolled women were allocated using a computer‐generated random sequence from a statistics program." |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of the study did not allow blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus or stool or tolerance to gum. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information on blinding of outcome assessors for auscultation of bowel sounds. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Rate of dropouts = 9.1%: 110 women were enrolled, but the available data for outcomes are from 100 women. No information on the number of dropouts per group. |
| Selective reporting (reporting bias) | High risk | Rate of complications pre‐specified in Methods but not reported in Results. |
| Other bias | High risk | Additional deviations from protocol: only elective CS were to be included, duration of gum chewing session was planned as 15 minutes (not 60 minutes) per session, initiation of gum chewing was supposed to occur immediately after surgery (not 6 hours after); in published paper the authors added a new outcome in Methods (complications) but did not report it in Results. |
Liang 2007.
| Methods | RCT, Tongji University Hospital, Shanghai, China. | |
| Participants | 120 parturients, 21‐38 years, mean age 28.4 years, undergoing CS under epidural anaesthesia, no information on parity or previous CS or elective/non‐elective CS. | |
| Interventions | Intervention group (N = 60): chewed gum (xylitol sugar‐free) for 15 min at 2‐h intervals, up to 3 times, starting immediately after surgery (total time = 45 min/d). Control group (N = 60): no information provided on feeding protocol. | |
| Outcomes | First passage of flatus, first defecation time, bowel sounds (not reported in Results but specified in Methods), nausea or vomiting or abdominal distention in the first 6‐24 hours post surgery. Tolerance reported in Results but not specified in Methods. | |
| Notes | We wrote to the study authors for additional information but got no response. No study protocol available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of the study did not allow blinding after assignment of the intervention postoperatively. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus or stools or tolerance. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information about blinding of outcome assessors regarding hearing of bowel sounds or vomiting. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were included in results. |
| Selective reporting (reporting bias) | High risk | Bowel sounds specified in Methods but not reported. |
| Other bias | Unclear risk | No information available. |
Lu 2010.
| Methods | RCT, Guangxi Liuzhou Railway Central Hospital, Liuzhou, China. | |
| Participants | 97 women who underwent CS, no information on type (elective or not), parity, previous CS or anaesthesia. Mean age range: 20‐35 years. | |
| Interventions | Intervention group (N = 47): participants had the same perioperative care as the control group except that they chewed 1‐2 pieces of gum (no information on type) for 30‐40 min, at 2‐h intervals, from 2 h postoperatively until first flatus. (Total chewing time = more than 6 h, judging from mean time to flatus data). After chewing gum, participants were provided with traditional Chinese medicinal food (radish, astralagus, tangerine peel, lean pork, chicken essence and salt). Control group (N = 50): participants were given intravenous fluid, anti‐infective drugs if needed, and were observed for uterine contractions. Participants lay flat on the bed without a pillow for 6 h. After first flatus, participants were provided with semi‐solid food, and gradually introduced to solid food. After 12 h postoperatively, participants were asked to change to a reclining position (conventional feeding). | |
| Outcomes | Time to first flatus and time to defecation. Additional outcomes: time to initiation of lactation, breast filling, exclusive breastfeeding, halitosis, maternal psychological measures, infant weight gain, defecation and sleep patterns. |
|
| Notes | No study protocol available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of the study did not allow blinding after assignment of the intervention postoperatively. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus or stools. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | NA. No objective outcomes were reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses, all women randomised were included. |
| Selective reporting (reporting bias) | Unclear risk | Time to flatus and defecation not pre‐specified in Methods but reported in Results; no protocol available. |
| Other bias | Unclear risk | No information available. |
Luo 2010.
| Methods | RCT, single hospital, China. | |
| Participants | 300 women, all primiparas without previous abdominal surgeries, undergoing CS under epidural anaesthesia. No information on type of CS (elective or non‐elective). | |
| Interventions | Intervention group (N = 150): chewing gum 4 times/d (2‐4 pieces each time) for 10‐15 minutes per session, (time chewing = 40‐60 min/d) from 2 h‐3 d after CS. Control group (N = 150): early feeding protocol (semi‐solid food after 6 h, normal diet after passage of flatus). | |
| Outcomes | Time to first flatus and passage of faeces and also bowel sound (although this last was not prespecified as an outcome in Methods). Additional outcomes: incidence of abdominal distension, dry mouth, bad breath, incision pain. |
|
| Notes | No study protocol available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of the study did not allow blinding after assignment of the intervention postoperatively. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus or stools. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information about blinding of outcome assessors for time to first bowel sounds. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses, all randomised participants included in results. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. Bowel sound was reported in Results but was not a pre‐specified outcome. |
| Other bias | Unclear risk | No information available. |
Rashad 2013.
| Methods | RCT conducted in King Khalid General Hospital, Kingdom of Saudi Arabia. | |
| Participants | 60 women. Most (80%) were multiparas, having an emergency CS (65%) under general anaesthesia (70%). No significant differences between groups for these characteristics. No information on previous CS. | |
| Interventions |
Intervention group (N = 30): "chew one stick of sugarless gum for 30 minutes, three times/day as soon as they are awake and return from the operating theatre to the ward." Time spent chewing = 90 min/d. "The researcher provided each woman with required amount of gum sticks". Control group (N = 30): "followed the postoperative hospital routine". No details on type of feeding protocol. |
|
| Outcomes | Time to first flatus, time to defecation, time to first bowel sounds. Additional outcomes: time of feeling the first intestinal movement. |
|
| Notes | No study protocol available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information: "They were then randomly assigned into two equal groups of 30; study and control". |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of intervention did not allow blinding of women and personnel. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus or stools. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information about blinding of outcome assessors for time to first bowel sounds. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed. No losses. |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in Methods were reported in Results. No study protocol available. |
| Other bias | High risk | Duration of surgery (> 45 minutes) was significantly longer in intervention than in control group: 40% versus 13.3%, P = 0.02. |
Satij 2006.
| Methods | RCT, Virginia Commonwealth University, USA. | |
| Participants | 32 women who underwent CS; no information on parity, previous CS, anaesthesia or type of CS (elective or non‐elective). | |
| Interventions |
Intervention group (N = 15): women in the gum‐chewing group chewed gum (no information on type) "3 times/d as soon as they recovered from anaesthesia till the time they passed flatus or defecated". Time spent chewing per day = impossible to assess because there is no information on duration of each session. Control group (N = 17): no information on type of feeding protocol. |
|
| Outcomes | "Return to bowel function" (no clear definition) and tolerance of gum chewing. | |
| Notes | This study was published only as a congress abstract. We wrote to the study author for additional information but got no response. We used only the data on tolerance for this review No study protocol available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information: "Thirty‐two patients who underwent cesarean delivery were randomly assigned to a gum‐chewing group or a control group". |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of intervention did not allow blinding of women and personnel. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to tolerance to gum. Authors were unclear about the meaning of "return to bowel function" (whether this was bowel sounds or passage of flatus or stools). |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Unclear about the meaning of "return to bowel function" (whether this was bowel sounds or passage of flatus or stools). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes are pre‐specified in Methods section; no protocol available for this study. |
| Other bias | Unclear risk | No information available. |
Shang 2010.
| Methods | Prospective RCT, Linyi Women and Children's Hospital, Shandong Province, China. | |
| Participants | 388 randomised, but only 386 women undergoing CS under spinal anaesthesia were reported. 2 women had incomplete data and were excluded. Median parity 2, ranging from 1‐4, no information on proportion of women with a previous CS; included both elective or non‐elective CS. Mean age: 29.4 ± 5.4 and 29.9 ± 6.4 years (intervention group and control groups). Inclusion criteria: women 19‐44 years undergoing CS. Exclusion criteria: women with "pre‐existing gastrointestinal disorders, such as peptic ulcer, hiatus hernia, irritable bowel syndrome, or esophagitis, and those with an intraoperative blood loss exceeding 500 mL". |
|
| Interventions |
Intervention group (N = 195): chewed sugar‐free peppermint‐flavoured gum "for least half an hour" 3 times/d (total time chewing = at least 90 min/d) as soon as returning from the operating theatre, until the time they defecated or were discharged. No information on compliance to gum‐chewing. Control group (N = 191): nil‐by‐mouth until passage of flatus (conventional protocol). |
|
| Outcomes | Time to first passage of flatus, to first bowel sounds, defecation, rate of mild ileus (mild anorexia, abdominal cramps, non‐persistent nausea or vomiting), rate of severe ileus (abdominal distention, > 4 episodes vomiting/24 hours, intolerance to oral fluids, need for abdominal X‐rays or nasograstric decompression), duration of hospital stay, use of antiemetics/analgesics, tolerance of gum chewing. Additional outcome: time to lactation. |
|
| Notes | No study protocol available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to one of two groups by consecutive opening of sequentially numbered, opaque, sealed envelopes. Envelope randomisation was performed by a computer‐generated code using the blocked randomisation method." |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned to one of two groups by consecutive opening of sequentially numbered, opaque, sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The obstetricians involved in the intraoperative care of the participants were blinded to the assigned group. The nature of intervention did not allow blinding of participants. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus or stools or satisfaction. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | For time to first bowel sounds, ileus, use of additional analgesics/antiemetics and duration of hospital stay. "The patients' postoperative progress was assessed by an independent investigator (investigator B) who was blinded to the assigned treatment. Participants were taught not to reveal to the surgeon, surgical team, nurse or investigators to which arm they had been randomised." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop out rate: 0.5% (2/388 randomised). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes proposed in Methods were reported in Results. No protocol available for this study. |
| Other bias | Unclear risk | No information available. |
Wang 2011.
| Methods | RCT, Fourth Hospital of Suzhou University, China. | |
| Participants | 300 nulliparous women undergoing elective CS under combined epidural and spinal anaesthesia were randomly divided into chewing‐gum group (150) and control group (150). Inclusion criteria: "first baby, ability to chew, intraoperative epidural anaesthesia plus spinal surgery, use of patient‐controlled analgesia pump". Exclusion criteria: "severe pregnancy‐induced hypertension, pre‐eclampsia or eclampsia, intraoperative intraperitoneal adhesions intraoperative complications, bleeding, surgical time > 2 hours, postoperative fever, need blood transfusion and reoperation". 34 women excluded in CS group (22.7%) and 33 (22%) in control group due to complications (e.g. transfusion, fever, adhesions, surgery > 2 hours), leaving 233 women in analyses. Mean age: 25.9 ± 5.0 years (intervention group), 26.7 ± 4.2 years (control group). |
|
| Interventions |
Intervention group (N = 116): chewed 1 xylitol sugar‐free gum for 15 min at 2‐h intervals, from 2 h after surgery during the day time until first flatus. Time spent chewing gum > 60 min/d, based on mean time to first passage of flatus. Control group (N = 117): no food/beverage; water or liquid feed was provided after first bowel sound (conventional feeding). |
|
| Outcomes | Time to first flatus, time to first bowel sounds. Additional outcome: motilin levels in peripheral blood samples after CS. | |
| Notes | No study protocol available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information: "...were randomly divided into chewing gum group (150) and control group (150)". |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of intervention did not allow blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information on blinding of assessors evaluating time to first bowel sounds. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses balanced but higher than 20% in both groups: 22.7% and 22% in intervention and control groups, respectively. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes proposed in Methods were reported in Results. No study protocol available. |
| Other bias | Unclear risk | No information available. |
Zamora 2012.
| Methods | RCT, The Philippines. | |
| Participants | "53 women who underwent an emergency or elective CS under regional anaesthesia...". No information on parity or previous CS. | |
| Interventions |
Intervention group (N = 18): "...chewed 2 pellets of sugarless gum at 12 hours post operation for 15 minutes then advanced to sips of clear liquids at 16 hours post operation; soft boiled egg, tea and crackers after 24 hours post operation; soft diet once with passage of flatus and regular diet once with bowel movement." Duration of gum chewing per day = unclear. Control group (N = 35): "...nothing per mouth for 16 hours post operation then advanced to sips of clear liquids; soft boiled egg, tea and crackers after 24 hours post operation; soft diet once with passage of flatus and regular diet once with bowel movement". |
|
| Outcomes | Time to first passage of flatus, first bowel movement and length of postoperative hospital stay. Rate of ileus also reported in Results, although not pre‐specified in Methods. | |
| Notes | This study was published only as a congress abstract. We wrote to study authors for additional information but got no reply. No study protocol available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nature of intervention did not allow blinding of participants. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants were unable to be adequately blinded in relation to passage of flatus or stools. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information on blinding of assessors in relation to ileus or hospital discharge. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in Methods were reported in Results. No study protocol available. |
| Other bias | Unclear risk | No information available. |
CS: caesarean section RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cevik 2016 | The study is a quasi‐randomised trial because the method described by the authors to randomise the participants did not ensure that all women had the same chance of being included into 1 of the 3 arms of the study (chewing gum, exercise and control). Since quasi‐randomised studies were not eligible for inclusion in this review, we excluded it. |
| Sahin 2015 | The study, described by the authors as a randomised trial, has 7 arms which tested 3 interventions (chewing gum, oral hydration or exercise) alone or in combination with each other. However, the 8th arm of the study, the control group (women who received usual care after CS) consisted of women whose doctors did not allow them to be included in the trial. Since the control group (which was essential to our comparisons) was not randomised, we did not include this study. |
Characteristics of ongoing studies [ordered by study ID]
Abd‐El‐Maeboud 2010.
| Trial name or title | Gum chewing and the return of bowel motility after CS under regional anaesthesia. |
| Methods | A prospective RCT with pregnant women aged 16‐45 years. |
| Participants | 48 women undergoing elective CS, aged 16‐45 years, at Ain Shams University Hospitals (Egypt). |
| Interventions | Following CS women will be randomised to 2 groups: group 1 (24 women) "...will receive 1 stick of sugarless non‐sweetened gum (Samarah Foods, Cairo, Egypt) for 15 min every two hours after surgery until the passage of flatus or bowel movement". Group 2 (24 women) "...will receive traditional postoperative management, with oral intake of clear fluids and soft foods allowed after the passage of flatus and regular diet after bowel movement". |
| Outcomes | Primary outcomes: time to interval of first hearing of normal intestinal sound, to first passage of flatus, to first bowel movement, and to discharge from the hospital. Secondary outcomes: recording of postoperative tolerance of gum chewing and postoperative complications. Occurrence of mild ileus symptoms or postoperative paralytic ileus. |
| Starting date | February 2010. |
| Contact information | Prof Karim Abd‐El‐Maeboud. |
| Notes | Recruitment completed. ISRCTN83008008. |
El‐Sharkawy 2015.
| Trial name or title | Gum chewing and bowel motility in patients undergoing cesarean section. Kasr el Ainy Experience. A Randomized Controlled Trial. |
| Methods | RCT, "conducted on 162 patients undergoing caesarean section in The Obstetrics & Gynecology Department 'kasr el ainy teaching hospital'." The women were randomised into 2 groups: group A (81 women) that received gum and group B (81 women) that had traditional management. |
| Participants | Women undergoing CS, aged 18‐35 years. Inclusion criteria: spinal anaesthesia and Pfannenstiel incision. Exclusion criteria: history of drug consumption especially opioids; water and electrolyte disturbances; pancreatitis; peritonitis; history of abdominal surgery except cesarean section; no willingness to co‐operate; severe intra and post‐operative complications; inability to chew gum; diabetes; pre‐eclampsia; prolonged rupture of membranes; hypothyroidism; muscular and neurological disorders. |
| Interventions | Intervention group: "Group A (81 Women) received one stick of sugarless gum (Samara for food & Chocolates Product Company) "S.A.E.", for 15 minutes every 2 hours after surgery until defecation" Control group: "Group B (81 Women) had traditional management (oral intake of clear fluid allowed after passage of flatus and regular diet with the passage of bowel movement." "Each woman in both groups was examined abdominally using a stethoscope to detect the intestinal movement every 4 hours and asked to report immediately the time of either passing flatus or stool." |
| Outcomes | Primary outcomes: the first bowel sounds (hours); the first passage of flatus (hours); the first defection (hours); and the first auscultation of regular bowel sounds in hours. Secondary outcomes: patient satisfaction. |
| Starting date | August 2015. |
| Contact information | Mohamed El‐Sharkawy. |
| Notes | ClinicalTrials.gov Identifier: NCT02533830. |
Ellaithy 2015.
| Trial name or title | Chewing gum to stimulate intestinal motility after CS. |
| Methods | RCT, in King Faisal Military hospital, Saudi Arabia. |
| Participants | All women undergoing elective CS, aged 20‐35 years. Exclusion criteria: "...emergency cesarean section, multifetal pregnancy, polyhydramnios, medical disorders, abnormal placentation, past history of bowel injury or operation, any complications that will increase operative duration such as uterine artery injury or uterine extension". |
| Interventions | 450 women will be divided into 3 groups: group A (150 women) "...will receive sugarless gum after their operating room discharge by 2 hours for at least half an hour at 2 hours interval"; group B (150 women) "...will receive traditional management of starting oral fluid after operating room discharge by 6 hours and hearing intestinal sounds on second day before initiating full regular diet"; and group C (150 women), "...as control group, they will not receive neither gum nor oral fluids". |
| Outcomes | Primary outcomes: passage of stools. Secondary outcomes: passage of flatus. |
| Starting date | March 2015. |
| Contact information | Mohamed I Ellaithy, MD. |
| Notes | ClinicalTrials.gov Identifier: NCT02386748. |
Kamalimanesh 2015.
| Trial name or title | The effect of chewing gum on the bloating after of CS. |
| Methods | Study conducted in Iran. "Double blinded randomized clinical trial which took place between April 2015 to June 2015 in Sabzevar Mobini Hospital, Sabzevar, Iran". A total of 80 pregnant women who undergo CS (elective or urgent) will be enrolled in this study and randomly divided into 2 groups (Case and Control group). |
| Participants | Women undergoing CS (elective or urgent). Inclusion criteria: "...nullipara; lack of previous abdominal surgery; lack of pre‐eclampsia, diabetes and cardiovascular disease; being able to chew gum; Pfannenstiel incision and uterine transverse incision; using general anaesthesia". Exclusion criteria: "...women at risk of aspiration and ileus (radiation history, anastomosis and who are over sedated); women who are not willing to continue the study; surgical complication such as uterine atonia; using intravenous antibiotics for more than 4 doses after surgery; surgical complication at the time of the surgery such as severe adhesions, blood transfusion, damaging the intestine or bladder; women who were discharged by their own decision; surgery duration more than 90 minutes; using magnesium sulfate (because of possible risk of nausea); history of any inflammatory or obstructive intestine disease; intestine manipulation or damage". |
| Interventions | "One to four hours after the surgery, the patients are encouraged to chew gum each six hours for about 15 minutes. The control group will be monitored by usual surgery ward care. Study data will be gathered by observation and filling the questionnaire. Auscultation of intestinal sounds in both groups was documented every hour till complete return of bowel sounds. A researcher who is not aware of the study will perform the auscultation." |
| Outcomes | Primary outcomes: time of first auscultation of bowel sounds; time of sensation of first bowel movement; time of the first gas passage; time of the first defecation. Secondary outcomes: duration of hospital stay; pain. |
| Starting date | May 2015. |
| Contact information | Batool Kamalimanesh. |
| Notes | Center name: Sabzevar Shahidan Mobini Hospital. Country: Islamic Republic Of Iran. IRCT registration number: IRCT2015041221710N1. |
Yilmaz 2015.
| Trial name or title | Sisli Ethal hospital study. |
| Methods | Study conducted in Turkey. "This study is a randomised controlled trial that included 150 pregnant women undergoing caesarean in Sisli Ethal Training and Research Hospital, Department of Obstetrics and Gynecology, Istanbul, Turkey. It was carried out in the period from October 1, 2015 to December 31, 2015." After operation, the participants are going to be randomly assigned into 2 groups (gum group and control group) by using a sealed enveloped system. |
| Participants | Women undergoing caesarean section. Inclusion criteria: pregnant women who is at least 18 years of age; pregnant women whose body mass index is under 35. Exclusion criteria: "caesarean hysterectomy; surgical management of severe postpartum haemorrhage; previous bowel surgery; women with history of drug consumption, especially opioids; water and electrolyte disturbances; pancreatitis or peritonitis; inability to chew gum; diabetes, pregnancies accompanied by coagulopathy like pre‐eclampsia, hypothyroidism, muscular and neurological disorders; postoperative admission to intensive care unit; history of abdominal surgery except cesarean section; history of postoperative ileus; women with drains". |
| Interventions | "The patients in the study group, will chew one sugarless gum for 30 minutes in postoperative 3., 5. and 7. hours. The control group will be followed without chew gum. Both patient groups will be mobilized in postoperative 6 hour and 8 hour after the operation, juicy food will be given. It will be introduced to solid foods after bowel movements and gas output occurs." |
| Outcomes | Primary outcomes: Postoperative time to return to active bowel movements [Time Frame: up to 48 hours]. Secondary outcomes: Postoperative infection rates and early discharge [Time Frame: up to 48 hours]. |
| Starting date | October 2015. |
| Contact information | Fatma YAZICI YILMAZ. |
| Notes | ClinicalTrials.gov Identifier:NCT02497794. |
CS: caesarean section RCT: randomised controlled trial
Differences between protocol and review
We changed the cut‐off of time spent chewing gum per day from less than one hour versus one hour or more (originally proposed in the protocol) to "up to one hour" versus "more than one hour" because all trials had at least one hour per day of gum chewing.
We added two additional subgroup analyses for the primary outcomes: type of caesarean section (CS) (elective versus non‐elective) and time when gum‐chewing started after the CS (immediately versus two hours after, versus six or more hours after), to further explore heterogeneity in the meta‐analyses.
We have used GRADE to evaluate the quality of the body of evidence and have include Table 1.
Contributions of authors
Edna Pereira Gomes de Morais (EPGM), Vivian S Vasconcelos (VSV) and Alexsandra S Pedrosa (ASP) drafted the protocol with the support of Cristiane R Macedo for the search strategy and the assistance of Rachel Riera (RR) and Gustavo Porfírio (GP), under the supervision of Maria Regina Torloni (MRT). The data extraction was performed by EPGM and VSV, with the supervision of MRT, GP and RR. Quality assessment was performed by EPGM and ASP, with the supervision of MRT and RR. The meta‐analyses and assessment of the quality of the evidence were performed by EPGM, RR and MRT, under the supervision of GP. The final text was drafted by EPGM, under the supervision of MRT, with the contribution and critical assessment of all authors.
Declarations of interest
Edna Pereira Gomes Morais: none known Rachel Riera: none known Gustavo JM Porfírio: none known Cristiane R Macedo: none known Vivian Sarmento Vasconcelos: none known Alexsandra de Souza Pedrosa: none known Maria R Torloni: none known
New
References
References to studies included in this review
Abasi 2014 {published data only}
- Abasi Z, Alavi F, Salehian M, Rashidi Fakari F, Taherpour M, Farazmand T, et al. An investigation on the effect of chewing gum on gastrointestinal function after cesarean operation. Journal of Urmia Nursing & Midwifery Faculty 2014;12(3):214‐20. [Google Scholar]
Abd‐El‐Maeboud 2009 {published data only}
- Abd‐El‐Maeboud KH, Ibrahim MI, Shalaby DA, Fikry MF. Gum chewing stimulates early return of bowel motility after caesarean section. BJOG: an international journal of obstetrics and gynaecology 2009;116(10):1334‐9. [DOI] [PubMed] [Google Scholar]
Ajuzieogu 2014 {published data only}
- Ajuzieogu OV, Amucheazi A, Ezike HA, Achi J, Abam DS. The efficacy of chewing gum on postoperative ileus following cesarean section in Enugu, South East Nigeria: a randomized controlled clinical trial. Nigerian Journal of Clinical Practice 2014;17(6):739‐42. [DOI] [PubMed] [Google Scholar]
Akhlaghi 2008 {published data only}
- Akhlaghi F, Pourjavad M, Mansouri A, Tara F, Vahedian M. Effect of gum chewing on prevention of post cesarean ileus. Journal of HAYAT 2008;14(2):35‐40. [Google Scholar]
Dehcheshmeh 2011 {published data only}
- Dehcheshmeh FS. Effect of early initiation of oral feeding and gum chewing on the return of bowel function in elective cesarean‐delivery. en.search.irct.ir/view/3086 Date first received: 3 July 2010.
- Dehcheshmeh FS, Salehian T, Gangi F, Beigi M. The effect of chewing sugar free gum after elective cesarean‐delivery on return of bowel function in primiparous women. Qom University of Medical Sciences Journal 2011;4(4):16‐20. [Google Scholar]
Garshasbi 2010 {published data only}
- Garshasbi A, Behboudi S. The effect of gum chewing on postoperative ileus after cesarean section. Society for Obstetric Anesthesia and Perinatology 42nd Annual Meeting; 2010 May 12‐16, San Antonio, Texas, USA. 2010.
Jakkaew 2013 {published data only}
- Charoenkwan K. The effects of gum chewing on bowel function recovery following cesarean section: randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01131416 Date first received: 25 May 2010.
- Jakkaew B, Charoenkwan K. Effects of gum chewing on recovery of bowel function following cesarean section: a randomized controlled trial. Archives of Gynecology and Obstetrics 2013;288(2):255‐60. [DOI] [PubMed] [Google Scholar]
Kafali 2010 {published data only}
- Kafali H, Duvan CI, Gozdemir E, Simavli S, Onaran Y, Keskin E. Influence of gum chewing on postoperative bowel activity after cesarean section. Gynecologic and Obstetric Investigation 2010;69(2):84‐7. [DOI] [PubMed] [Google Scholar]
Ledari 2012 {published data only}
- Ledari FM, Barat S, Amiri FN, Delavar MA, Banihosseini SZ, Khafri S. Effect of gum chewing after cesarean‐delivery on return of bowel function. Journal of Babol University of Medical Sciences 2012;14(3):19‐24. [Google Scholar]
- Ledari FM, Barat S, Delavar MA. Chewing gum has stimulatory effects on bowel function in patients undergoing cesarean section: a randomized controlled trial. Bosnian Journal of Basic Medical Sciences 2012;12(4):265‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledari FM, Barat S, Delavar MA, Banihosini SZ, Khafri S. Chewing sugar‐free gum reduces ileus after cesarean section in nulliparous women: a randomized clinical trial. Iranian Red Crescent Medical Journal 2013;15(4):330‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsenzadeh F. Evaluation the effect of gum chewing on the return of bowel function in cesarean–delivery patients. en.search.irct.ir/view/3844 Date first received: 6 March 2011.
Liang 2007 {published data only}
- Liang J, Gao T, Han W, Zhang Y, Liu S, Dai Q. The clinical observation of enhancing recovery of gastrointestinal function after cesarean section by gum chewing. Journal of Tongji University (Medical Science) 2007;28(2):81‐3. [Google Scholar]
Lu 2010 {published data only}
- Lu L, Zhao A. “False eat” diet promote cesarean section with recovery of gastrointestinal function. Cinical study of functional recovery and lactation. Journal of Nurses Training 2010;25(23):2158‐9. [Google Scholar]
Luo 2010 {published data only}
- Luo S, Wu C, Yang X, Lei L, Deng H, Li H. Effect of chewing gum after cesarean section on restoration of gastrointestinal function. China Journal of Modern Nursing 2010;16(24):2948‐9. [Google Scholar]
Rashad 2013 {published data only}
- Rashad WAE, Yousef SAAL. Effect of sugarless gum chewing on intestinal movement after cesarean section. Life Science Journal 2013;10(4):3257–61. [Google Scholar]
Satij 2006 {published data only}
- Satij B. Evaluation of gum chewing on the return of bowel functions in cesarean‐delivery patients (abstract). Obstetrics & Gynecology 2006;107(4 Suppl):10S. [Google Scholar]
Shang 2010 {published data only}
- Shang H, Yang Y, Tong X, Zhang L, Fang A, Hong L. Gum chewing slightly enhances early recovery from postoperative ileus after cesarean section: results of a prospective, randomized, controlled trial. American Journal of Perinatology 2010;27(5):387‐91. [DOI] [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Wang X, Ren Y, Qin X, Dai X. Influence of sham feeding on motilin and evacuating time after accepting cesarean section. Chinese Nursing Research 2011;25(8):682‐3. [Google Scholar]
Zamora 2012 {published data only}
- Zamora BBB, Kalalo RE. Gum chewing versus traditional feeding on the early return of bowel motility after cesarean delivery: a prospective randomized controlled trial. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S525. [Google Scholar]
References to studies excluded from this review
Cevik 2016 {published data only}
- Cevik SA, Baser M. Effect of bed exercises and gum chewing on abdominal sounds, flatulence and early discharge in the early period after caesarean section. Journal of Clinical Nursing 2016;25(9‐10):1416‐25. [DOI] [PubMed] [Google Scholar]
Sahin 2015 {published data only}
- Sahin E, Terzioglu F. The effect of gum chewing, early oral hydration, and early mobilization on intestinal motility after cesarean birth. Worldviews on Evidence‐Based Nursing 2015;12(6):380‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Abd‐El‐Maeboud 2010 {published data only}
- Abd‐El‐Maeboud K. Gum chewing and the return of bowel motility after caesarean section under regional anaesthesia. isrctn.com/ISRCTN83008008 Date first received: 18 February 2010.
Ellaithy 2015 {published data only}
- Ellaithy M. Chewing gums to stimulate intestinal motility after cesarean section. clinicaltrials.gov/ct2/show/NCT02386748 Date first received: 5 March 2015.
El‐Sharkawy 2015 {published data only}
- El‐Sharkawy M. Gum chewing and bowel motility in patients undergoing cesarean section. Kasr el Ainy experience. A randomized controlled trial. clinicaltrials.gov/ct2/show/study/NCT02533830 Date first received: 21 August 2015.
Kamalimanesh 2015 {published data only}
- Kamalimanesh, B. The effect of chewing gum on the bloating after of cesarean section. en.search.irct.ir/view/22960 Date first received: 28 June 2015.
Yilmaz 2015 {published data only}
- Yilmaz F. Efficacy and effectiveness of gum chewing on bowel function after cesarean section: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02497794 Date first received: 28 June 2015.
Additional references
Artinyan 2008
- Artinyan A, Nunoo‐Mensah JW, Balasubramaniam S, Gauderman J, Essani R, Gonzalez‐Ruiz C, et al. Prolonged postoperative ileus‐definition, risk factors, and predictors after surgery. World Journal of Surgery 2008;32(7):1495‐500. [DOI] [PubMed] [Google Scholar]
Asao 2002
- Asao T, Kuwano H, Nakamura J‐I, Morinaga N, Hirayama I, Ide M. Gum chewing enhances early recovery from postoperative ileus after laparoscopic colectomy. Journal of the American College of Surgeons 2002;195(1):30‐2. [DOI] [PubMed] [Google Scholar]
Bauer 2004
- Bauer AJ, Boesckystaens GE. Mechanisms of postoperative ileus. Neurogastroenterology & Motility 2004;16:54‐60. [DOI] [PubMed] [Google Scholar]
Behm 2003
- Behm B, Stollman N. Postoperative ileus: etiologies and interventions. Clinical Gastroenterology and Hepatology 2003;1:71‐80. [DOI] [PubMed] [Google Scholar]
Betrán 2016
- Betrán AP, Ye J, Moller AB, Zhang J, Gülmezoglu AM, Torloni MR. The increasing trend in caesarean section rates: global, regional and national estimates: 1990‐2014. PLoS ONE 2016;11(2):e0148343. [DOI: 10.1371/journal.pone.0148343] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 1996
- Christensen LV, Tran KT, Mohamed SE. Gum chewing and jaw muscle fatigue and pains. Journal of Oral Rehabilitation 1996;23(6):424‐37. [DOI] [PubMed] [Google Scholar]
Condon 1995
- Condon RE, Cowles VE, Ferraz AA, Carilli S, Carlson ME, Ludwig K, et al. Human colonic smooth muscle electrical activity during and after recovery from postoperative ileus. American Journal of Physiology 1995;269:G408‐G417. [DOI] [PubMed] [Google Scholar]
Craciunas 2014
- Craciunas L, Sajid MS, Ahmed AS. Chewing gum in preventing postoperative ileus in women undergoing caesarean section: a systematic review and meta‐analysis of randomised controlled trials. BJOG: an international journal of obstetrics and gynaecology 2014;121(7):793‐9. [DOI] [PubMed] [Google Scholar]
Delaney 2004
- Delaney CP. Clinical perspective on postoperative ileus and the effect of opiates. Neurogastroenterology & Motility 2004;16 Suppl 2:61‐6. [DOI] [PubMed] [Google Scholar]
Deshpande 2008
- Deshpande A, Jadad AR. The impact of polyol‐containing gums on dental caries: a systematic review of original randomized controlled trials and observational studies. Journal of the American Dental Association 2008;139(1):1602‐14. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2009
- Fitzgerald JE, Ahmed I. Systematic review and meta‐analysis of chewing‐gum therapy in the reduction of postoperative paralytic ileus following gastrointestinal surgery. World Journal of Surgery 2009;33(12):2557‐66. [DOI] [PubMed] [Google Scholar]
Griffiths 2007
- Griffiths PD, Watson H. Chewing gum for postoperative ileus. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006506] [DOI] [Google Scholar]
Gustaitis 1998
- Gustaitis J. The stick history of chewing gum. American History 1998;33(4):30. [Google Scholar]
Hendrickson 1990
- Hendrickson R. Since 1928 it's been boom and bust with bubble gum. Smithsonian 1990;21(4):74. [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ho 2014
- Ho YM, Smith SR, Pockney P, Lim P, Attia J. A meta‐analysis on the effect of sham feeding following colectomy: should gum chewing be included in enhanced recovery after surgery protocols?. Diseases of the Colon & Rectum 2014;57(1):115‐26. [DOI] [PubMed] [Google Scholar]
Hochner 2015
- Hochner H, Tenfelde SM, Ahmad WA, Liebergall‐Wischnitzer M. Gum chewing and gastrointestinal function following caesarean delivery: a systematic review and meta‐analysis. Journal of Clinical Nursing 2015;241:1795‐804. [DOI: 10.1111/jocn.12836] [DOI] [PubMed] [Google Scholar]
Holte 2000
- Holte K, Kehlet H. Postoperative ileus: a preventable event. British Journal of Surgery 2000;87(11):1480‐93. [DOI] [PubMed] [Google Scholar]
Huang 2015
- Huang H‐P, He M. Usefulness of chewing gum for recovering intestinal function after cesarean delivery: a systematic review and meta‐analysis of randomized controlled trials. Taiwanese Journal of Obstetrics & Gynecology 2015;54:116‐21. [http://dx.doi.org/10.1016/j.tjog.2014.10.004] [DOI] [PubMed] [Google Scholar]
Iyer 2009
- Iyer S, Saunders WB, Stemkowski S. Economic burden of postoperative ileus associated with colectomy in the United States. Journal of Managed Care & Specialty Pharmacy 2009;15(6):485‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jepsen 1989
- Jepsen JM, Skoubo‐Kristensen E, Elsborg L. Rectosigmoid motility response to sham feeding in irritable bowel syndrome. Evidence of a cephalic phase. Scandinavian Journal of Gastroenterology 1989;24(1):53‐6. [DOI] [PubMed] [Google Scholar]
Johnson 2009
- Johnson MD, Wassh RM. Current therapies to shorten postoperative ileus. Cleveland Clinic Journal of Medicine 2009;76(11):641‐8. [DOI] [PubMed] [Google Scholar]
Keenahan 2014
- Keenahan M. Does gum chewing prevent postoperative paralytic ileus?. Nursing 2014;44(6):1‐2. [DOI] [PubMed] [Google Scholar]
Kehlet 2001
- Kehlet H, Holte K. Review of postoperative ileus. American Journal of Surgery 2001;182 Suppl:3S‐10S. [DOI] [PubMed] [Google Scholar]
Kellow 1999
- Kellow JE, Delvaux M, Azpiroz F, Camilleri M, Quigley EM, Thompson DG. Principles of applied neurogastroenterology: physiology/motility‐sensation. Gut 1999;45(Suppl 2):II17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konturek 1986
- Konturek SJ, Thor P. Relation between duodenal alkaline secretion and motility in fasted and sham‐fed dogs. American Journal of Physiology 1986;251:G591‐6. [DOI] [PubMed] [Google Scholar]
Kramer 1996
- Kramer RL, Someren JK, Qualls CR, Curet LB. Postoperative management of cesarean patients: the effect of immediate feeding on the incidence of ileus. Obstetrics & Gynecology 1996;88:29‐32. [DOI] [PubMed] [Google Scholar]
Lafon 2012
- Lafon C, Lawson L. Gum chewing as a strategy to reduce the duration of postoperative ileus. Gastrointestinal Nursing 2012;10(3):17‐22. [Google Scholar]
LaRosa 1993
- LaRosa JA, Saywell RM Jr, Zollinger TW, Oser TL, Erner BK, McClain E. The incidence of adynamic ileus in postcesarean patients. Patient‐controlled analgesia versus intramuscular analgesia. Journal of Reproductive Medicine 1993;38(4):293‐300. [PubMed] [Google Scholar]
Li 2013
- Li S, Liu Y, Peng Q, Xie L, Wang J, Qin X. Chewing gum reduces postoperative ileus following abdominal surgery: a meta‐analysis of 17 randomized controlled trials. Journal of Gastroenterology and Hepatology 2013;28:1122–32. [DOI] [PubMed] [Google Scholar]
Livingston 1990
- Livingston EH, Passaro EP Jr. Postoperative ileus. Digestive Diseases and Sciences 1990;35(1):121‐32. [DOI] [PubMed] [Google Scholar]
Luckey 2003
- Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Archives of Surgery 2003;138(2):206‐14. [DOI] [PubMed] [Google Scholar]
Mangesi 2002
- Mangesi L, Hofmeyr GJ. Early compared with delayed oral fluids and food after caesarean section. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattei 2006
- Mattei P, Rombeau JL. Review of the pathophysiology and management of postoperative ileus. World Journal of Surgery 2006;30:1382‐91. [DOI] [PubMed] [Google Scholar]
Matthews 2009
- Matthews JP. Chicle: the Chewing Gum of the Americas, from the Ancient Maya to William Wrigley. Arizona: The Arizona Board of Regents, 2009. [Google Scholar]
Milgrom 2006
- Milgrom P, Ly KA, Roberts MC, Rothen M, Mueller G, Yamaguchi DK. Mutans streptococci dose response to xylitol chewing gum. Journal of Dental Research 2006;85(2):177‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parnaby 2009
- Parnaby CN, MacDonald AJ, Jenkins JT. Sham feed or sham? A meta‐analysis of randomized clinical trials assessing the effect of gum chewing on gut function after elective colorectal surgery. International Journal of Colorectal Disease 2009;24(5):585‐92. [DOI] [PubMed] [Google Scholar]
Ravry 1980
- Ravry MJR. Dietetic food diarrhea. JAMA 1980;244(3):270. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Short 2015
- Short V, Herbert G, Perry R, Atkinson C, Ness AR, Penfold C, et al. Chewing gum for postoperative recovery of gastrointestinal function. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD006506.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soffer 1992
- Soffer EE, Adrian TE. Effect of meal composition and sham feeding on duodenojejunal motility in humans. Digestive Diseases and Sciences 1992;37:1009‐14. [DOI] [PubMed] [Google Scholar]
Soriano 1996
- Soriano D, Dulitzki M, Kridar N, Barkai G, Mashiach S, Seidman DS. Early oral feeding after cesarean delivery. International Journal of Gynecology and Obstetrics 1996;87:1006‐8. [DOI] [PubMed] [Google Scholar]
Stern 1989
- Stern RM, Crawford HE, Stewart WR, Vasey MW, Koch KL. Sham feeding. Cephalic‐vagal influences on gastric myoelectric activity. Digestive Diseases and Sciences 1989;34(4):521‐7. [DOI] [PubMed] [Google Scholar]
Tabrizi 2014
- Tabrizi R, Karagah T, Aliabadi E, Hoseini SA. Does gum chewing increase the prevalence of temporomandibular disorders in individuals with gum chewing habits?. Journal of Craniofacial Surgery 2014;25(5):1818‐21. [DOI] [PubMed] [Google Scholar]
Tandeter 2009
- Tandeter H. Hypothesis: hexitols in chewing gum may play a role in reducing postoperative ileus. Medical Hypotheses 2009;72(1):39‐40. [DOI] [PubMed] [Google Scholar]
Vasquez 2009
- Vasquez W, Hernandez AV, Garcia‐Sabrido JL. Is gum chewing useful for ileus after elective colorectal surgery? A systematic review and meta‐analysis of randomized clinical trials. Journal of Gastrointestinal Surgery 2009;13(4):649‐56. [DOI] [PubMed] [Google Scholar]
Vather 2013
- Vather R, Trivedi S, Bisset I. Defining postoperative Ileus: results of a systematic review and global survey. Journal of Gastrointestinal Surgery 2013;17(5):962‐72. [DOI] [PubMed] [Google Scholar]
Waldhausen 1990
- Waldhausen JH, Shaffrey ME, Skenderis BS 2nd, Jones RS, Schirmer BD. Gastrointestinal myoelectric and clinical patterns of recovery after laparotomy. Annals of Surgery 1990;211:777‐84; discussion 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallström 2014
- Wallström A, Frisman GH. Facilitating early recovery of bowel motility after colorectal surgery: a systematic review. Journal of Clinical Nursing 2014;23(1‐2):24‐44. [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang XJ, Chi P. Effect of chewing gum on the promotion of intestinal function recovery after colorectal surgery: a meta‐analysis. Zhonghua Wei Chang Wai Ke Za Zhi 2013;16(11):1078‐83. [PubMed] [Google Scholar]
Watemberg 2014
- Watemberg N, Matar M, Har‐Gil M, Mahajnah M. The influence of excessive chewing gum use on headache frequency and severity among adolescents. Pediatric Neurology 2014;50(2014):69‐72. [DOI] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Global health observatory data repository. Maternal health service: births by caesarean section. apps.who.int/gho/data/node.main.HE‐ 1571?lang=en accessed 13 July 2014.
Yeh 2009
- Yeh YC, Klinger EV, Reddy P. Pharmacologic options to prevent postoperative ileus. Annals of Pharmacotherapy 2009;43(9):1474‐85. [DOI] [PubMed] [Google Scholar]
Yin 2013
- Yin Z, Sun J, Liu T, Zhu Y, Peng S, Wang J. Gum chewing: another simple potential method for more rapid improvement of postoperative gastrointestinal function. Digestion 2013;87(2):67‐74. [DOI] [PubMed] [Google Scholar]
Zhu 2014
- Zhu YP, Wang WJ, Zhang SL, Dai B, Ye DW. Effects of gum chewing on postoperative bowel motility after caesarean section: a meta‐analysis of randomised controlled trials. BJOG: an international journal of obstetrics and gynaecology 2014;121(7):787‐92. [DOI] [PubMed] [Google Scholar]


