Abstract
The question of why some types of organic matter are more persistent while others decompose quickly in soils has motivated a large amount of research in recent years. Persistence is commonly characterized as turnover or mean residence time of soil organic matter (SOM). However, turnover and residence times are ambiguous measures of persistence, because they could represent the concept of either age or transit time. To disambiguate these concepts and propose a metric to assess SOM persistence, we calculated age and transit time distributions for a wide range of soil organic carbon models. Furthermore, we show how age and transit time distributions can be obtained from a stochastic approach that takes a deterministic model of mass transfers among different pools and creates an equivalent stochastic model at the level of atoms. Using this approach we show the following: (1) Age distributions have relatively old mean values and long tails in relation to transit time distributions, suggesting that carbon stored in soils is on average much older than carbon in the release flux. (2) The difference between mean ages and mean transit times is large, with estimates of soil organic carbon persistence on the order of centuries or millennia when assessed using ages and on the order of decades when using transit or turnover times. (3) The age distribution is an appropriate metric to characterize persistence of SOM. An important implication of our analysis is that random chance is a factor that helps to explain why some organic matter persists for millennia in soil.
Keywords: soil carbon, time scales, biogeochemical cycling, carbon storage, soil models, model diagnostics
Key Points
Persistence of organic matter in soils can be characterized by age distributions of carbon
Stochastic processes can help to explain the persistence of soil organic matter
Carbon persists from centuries to millennia in soils when assessed with age distributions while for decades when assessed with transit time
1. Introduction
The observation that soil organic matter (SOM) persists in soils for a wide range of times independent of its quality has challenged previous views in SOM cycling research (Dungait et al., 2012; Gleixner, 2013; Kleber, 2010; Schmidt et al., 2011; Paul, 2016). It has been reported that easily degradable compounds can persist for years to decades in soils (Kiem & Kögel‐Knabner, 2003; Kleber, 2010), while highly aromatic organic compounds such as lignin or charcoal can degrade in a matter of years, much faster than previously believed (Gleixner et al., 1999; Heim & Schmidt, 2007; Kleber, 2010; Lehmann et al., 2015). To explain this variability, current research on mechanisms of SOM cycling and stabilization focus on physical and chemical protection rather than molecular structure to explain SOM persistence (Lehmann & Kleber, 2015; Schmidt et al., 2011).
The term persistence, however, has been vaguely defined. In most cases, persistence is associated with the turnover or mean residence time of carbon in SOM (Derrien & Amelung, 2011; Lehmann et al., 2015; Schmidt et al., 2011). These two terms are problematic to use as definitions of persistence for three main reasons: (1) They are ambiguously used in many studies, in some cases referring to the concept of age and in others to the concept of transit times (see below); (2) in some cases their calculation relies on the assumption of a single homogenous pool at steady state, while a range of time scales for C has been clearly reported (Trumbore, 2000, 2009); and (3) measures of central tendency do not provide information on the distribution of the maximum age that could be observed in a soil (Figure 1). This highlights the need for a more precise definition of SOM persistence.
Figure 1.
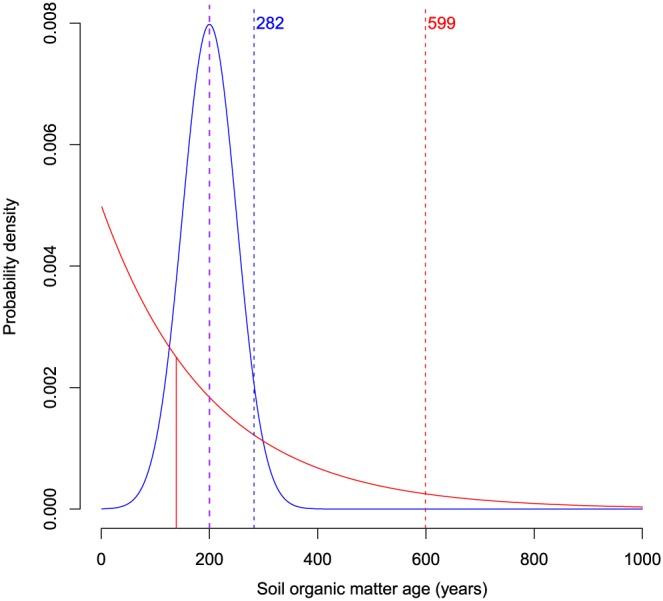
Soil organic matter age for two hypothetical soils with similar mean age but with different density functions. In both cases, the mean age is 200 years (purple vertical line), but for the exponentially distributed soil the 95% quantile Q 95=599 years, while for the normally distributed soil (blue line) Q 95=282 years. In asymmetric distributions with long tails such as the exponential or the phase type, the mean is more sensitive to the skewness than the median. In this example, the 50% quantile Q 50=139 years (vertical solid red line) for the exponential distribution.
Turnover and residence times are ambiguous terms because they may indicate concepts related to the age of carbon within the soil system or the age of carbon in the flux out of soils (e.g., CO2 respiration or dissolved organic carbon leaching; Sierra et al., 2017). Less ambiguous are the concepts of system age and transit times (Bolin & Rodhe, 1973; Bruun et al., 2004; Derrien & Amelung, 2011; Manzoni et al., 2009; Sierra et al., 2017). System age can be defined as the age of particles or atoms in a system, in this case a soil, since the time the atom entered the system until the time of observation. Transit time can be defined as the time elapsed since the atom entered until it leaves in the output flux. It is well established that both system age and transit time are equal in single‐pool systems at steady state. For systems with multiple pools that cycle at different time scales, these quantities are generally different.
System age and transit time can be characterized by a distribution that measures the proportion of atoms or particles in a soil that have less than a certain age (Bolin & Rodhe, 1973; Eriksson, 1971). These distributions can also be interpreted in a probabilistic sense, suggesting that the problem of persistence could also be approached from a stochastic point of view.
In this manuscript, we assess age and transit time distributions to determine which is most appropriate to characterize the persistence of SOM. We focus on carbon cycling, but the ideas and theory presented can be applied to any other biogeochemical element (Spohn & Sierra, 2018). To evaluate the applicability of these distributions, we calculate estimates of ages and transit times of carbon for a particular set of models, and estimates at the global scale using output from Earth system models. We take a mostly theoretical approach, starting from a general stochastic model for the definition of ages and using a set of examples from the literature to calculate ages and transit times at a set of single sites and at the global scale. Through this analysis, we show that (1) ages of carbon in soils can be explained by stochastic processes that can be directly linked to mechanisms of SOM (de)stabilization, (2) ages and transit times differ considerably in soils, with ages being much older than transit times and (3) across models, SOM age distributions have a long tail of old C, suggesting that a small fraction of SOM persists in soils for centuries to millennia. Finally, based on these findings, we suggest that age distributions are a strong indicator of SOM persistence in soils.
2. Conceptual Framework
2.1. A Stochastic Interpretation of SOM Dynamics
Soils are composed of a mixture of carbon atoms of mostly plant origin that have accumulated over a period of time. For each atom, the time elapsed since it entered the soil is defined here as age. If we were able to measure the age a of every single atom in a given amount of soil, we would be able to produce a frequency distribution of the ages of carbon. To be able to construct this frequency distribution, we would need to group C atoms in age bins of some size Δa and count how many atoms belong to each age class. If we make the size of these age bins very small, we would obtain a series of a + δ a intervals that define what is commonly known as a probability density function (pdf). This pdf measures the probability that a certain amount of carbon is below or above a certain age. Once we have a pdf of carbon ages, we can characterize the age structure of soil organic carbon (SOC) by simple measures such as the mean, the median, and quantiles (Figure 1).
To arrive at a pdf of SOC ages, we need to take the atom perspective further. Consider a carbon atom that belongs to a dead plant part just entering the soil. This C atom will stay in the soil until something will happen; for instance, the plant part may be broken down by physical mechanisms or by macro‐organisms. The molecules that contain the atom may be transformed by exoenzymes, they might be absorbed by mineral surfaces, or they might be ingested by a microorganism. All these different possibilities are the well‐studied mechanisms of soil carbon (de)stabilization (Lehmann & Kleber, 2015; Schmidt et al., 2011; Sollins et al., 1996; von Lützow et al., 2008). In this context, however, we consider these mechanisms to set a probability on a C atom of changing its location and its state. Depending on the context, that is, to what macromolecule the atom belongs to, or the environment determined by factors such as pH, temperature, moisture, and redox potential, the probability of the C atom changing its state would be determined. However, we assume that none of these mechanisms operate at the age level. This means that mechanisms of (de)stabilization operate based on the physical, chemical, and biological properties of the organic matter but not on the age since it entered the soil. We assume that SOM processing is independent of age, and therefore atoms are processed randomly with respect to age but deterministically based on physical, chemical, and biological mechanisms.
Once the probabilities for the change of state of carbon atoms are determined, it is possible to use them to compute for how long a particle may stay in a specific state and how long it will take for the particle to leave the system (Metzler & Sierra, 2018). In the following section, we show how to perform these computations.
2.2. Computation of Age Distributions
To characterize SOM persistence using age distributions, it is necessary to use models that incorporate different (de)stabilization mechanisms. Over several decades, researchers have proposed a large variety of SOM models that account for biological, physical, and chemical mechanisms of SOM (de)stabilization (Manzoni & Porporato, 2009). A large number of these models can be represented as systems of linear differential equations (Sierra & Müller, 2015) of the form
| (1) |
where the rate of change of carbon over time in n different pools is represented by the n‐dimensional vector . Constant inputs of carbon to each soil pool are represented by the n‐dimensional vector u, and rates of carbon processing, stabilization, and destabilization are represented in the n × n dimensional matrix B. The diagonal entries of B contain the rates at which carbon is processed in each of the pools, and if we organize them from fast to slow from left to right, we can interpret all lower diagonal entries as the rates of carbon stabilization and the upper diagonal entries as the rates of carbon destabilization (Sierra & Müller, 2015; Sierra et al., 2011). By adding all rows from each column of B, one obtains a vector z with the fraction of the decomposed carbon that is released from the system either as respired or dissolved carbon. This vector can be computed as z T=−1 T·B, where T represents the transpose operator. At steady state, the model of equation (1) has the general solution x ∗=−B −1·u.
Metzler and Sierra (2018) showed that it is possible to build a stochastic model from the deterministic compartmental model of equation (1). The stochastic model corresponds to a continuous‐time absorbing Markov chain with transition rate matrix Q as
| (2) |
This transition rate matrix contains as elements the probabilities that carbon atoms move across different pools (B) and get released from the entire system (z T) to an absorbing state where the atom cannot return back to the system. This transition rate matrix also clearly establishes the relation between deterministic models of differential equations and stochastic models at the level of atoms and can be used to compute age and transit time distributions.
The age pdf for this type of linear models at steady state can be computed by the following expression (Metzler & Sierra, 2018):
| (3) |
where a is the random variable age, 1 T is the transpose of the n‐dimensional vector containing ones, e a·B is the matrix exponential computed for each value of a, and is the sum of the stocks of all pools at steady state.
The mean, that is, expected value, of the age pdf can be computed by the expression
| (4) |
2.3. Computation of Transit Time Distributions
Although we propose age distributions to characterize persistence of SOM, it is also useful to compute transit time distributions of SOM for comparison purposes and to get an idea of the age structure of the carbon that is released from soils.
The pdf of the transit time variable τ for models of the form of equation (1) is given by (Metzler & Sierra, 2018)
| (5) |
and the mean transit time by
| (6) |
It is important to note that the mean transit time can also be obtained dividing the stock of mass at steady state by either the input or the output flux. This metric is also called the turnover time, and it is used in many applications to characterize time scales of carbon cycling in ecosystems (Sierra et al., 2017).
Notice that the computation of the transit time density distribution and its mean depends directly on the elements of the matrix B and on the proportional distribution of the inputs as stated in the vector u/Σu (equations (5) and (6)). In other words, transit times depend on the cycling rates of each pool and the transfer rates among them as embedded in the compartmental matrix, and on how the external inputs are distributed to the different pools. However, transit times do not depend on the total amount of inputs to the system.
Ages however, depend on the cycling and transfer rates encoded in the compartmental matrix B and on the proportional distribution of the carbon stocks at steady state as expressed in x ∗/Σx ∗ (equations (3) and (4)). Since the steady state x ∗ does depend on the total amount of inputs, the age distribution and its mean do depend on the total amount of inputs and its distribution to different pools.
2.4. Computation of Quantiles
Quantiles for the age and transit time pdfs (equations (3) and (5)) are computed numerically by taking the inverse of their cumulative distribution function F(y) and finding the smallest y such that F(y)≥q, where q∈(0,1) are the cut points for which quantiles are desired; for example, q = 0.5 represents the Q 50 quantile (median) of the distribution.
2.5. Hypothetical Relation Between Ages and Transit Times
Following Bolin and Rodhe (1973), we examine the relations between ages and transit times of SOC, which fall into three categories: (I) the age and the transit time distributions are equal, (II) ages are younger than transit times, and (III) ages are older than transit times (Figure 2). The type I relation implies that the SOM system is well mixed and all carbon atoms in the soil have the same probability of being mineralized and released as CO2 or as dissolved organic carbon. When plotting ages versus transit times of carbon, type I systems would lie along the 1:1 line (Figure 2).
Figure 2.
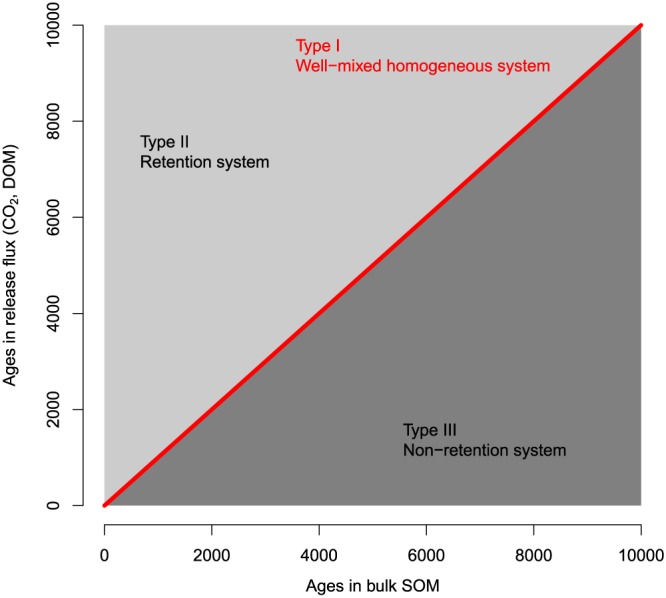
Possible relations between ages and transit times of organic matter in soils. Axes in arbitrary time units, for example, days, months, and years. DOM = dissolved organic matter; SOM = soil organic matter.
If the transit time is longer than the age (type II), the system retains carbon for a relatively long time before it is released. This type of behavior is common in systems in which physical transport or recycling are dominant mechanisms, for example, when a physical transport pathway moves carbon atoms sequentially before they are released, or when carbon is continuously recycled by microorganisms and only released outside the system after a long time. In these cases, the age of atoms in the release flux is older than the age of atoms stored inside the system, and a plot of ages versus transit times would occupy the region above the 1:1 line (Figure 2). We can also think about the type II relation as representative of a sequential process with multiple steps in the degradation of organic matter, in which fresh OM is transformed into multiple products before it is mineralized and released from the soil in a final step. Or alternatively, we could also think of soil as a chromatography column with new C added at the top and oldest C released from the bottom in dissolved form.
Finally, for a type III relation, ages are older than transit times and occupy the lower region below the 1:1 line (Figure 2). A relationship in this region implies that most carbon atoms enter and leave the system in a relatively short time, but those atoms that remain in the system stay there for a long time. This situation can happen when input and output fluxes occur at relatively close distance between each other, and storage is physically separated from them, or when specific mechanisms favor the processing and release of material close to the input source (Bolin & Rodhe, 1973).
3. Methods
Equations (3) to (6) as well as additional formulas for the quantiles of these distributions described in Metzler and Sierra (2018) were implemented in the R package SoilR (Sierra et al., 2014).
We applied these formulas to a variety of SOC models reported in the literature (Table 1). We selected popular SOC models such as RothC and Century (Jenkinson et al., 1990; Parton et al., 1987), using reference parameter values reported in their original publications, that is, parameter values that are not affected by the temperature and moisture modifiers implemented in these models. For RothC, we excluded its inert pool because it has a decomposition rate equal to 0 and therefore the age is infinitely large at steady state and the matrix B is not invertible (Metzler & Sierra, 2018). We also selected the Yasso07 model because it has been parameterized using an extensive data set of litter decomposition experiments (Tuomi et al., 2009), and it has been implemented as part of the MPI Earth system model (Goll et al., 2015). Also, the Introductory Carbon Balance Model (ICBM) model was selected due to its simplicity and yet enough complexity to represent stabilization of SOC (Andren & Katterer, 1997).
Table 1.
Models Used for the Calculation of Age and Transit Time Distributions
| Model | Description | Number of pools | Reference |
|---|---|---|---|
| RothC | Rothamsted soil carbon model. | 4 | Jenkinson et al. (1990) |
| This implementation excludes the | |||
| inert pool. | |||
| Century | Implementation of the Century | 5 | Parton et al. (1987) |
| model as originally described for | |||
| temperate grasslands | |||
| Yasso07 | Model of litter decomposition | 5 | Tuomi et al. (2009) |
| parameterized from a global‐scale | |||
| database | |||
| ICBM | Introductory carbon model, | 2 | Andren and Katterer (1997) |
| parameters for an experimental treatment | |||
| without N and straw additions, | |||
| interpreted as a control treatment | |||
| CLM4cn‐Needleleaf | Community land model version 4 | 8 | Wieder et al. (2014) |
| with parameterization for needleleaf | |||
| evergreen forest | |||
| CLM4cn‐Deciduous | Community land model version 4 | 8 | Wieder et al. (2014) |
| with parameterization | |||
| deciduous forest | |||
| CLM4cn‐Tropical | Community land model version 4 | 8 | Wieder et al. (2014) |
| with parameterization for tropical | |||
| forest | |||
| CESM | Reduced complexity model | 3 | He et al. (2016) |
| obtained from the output of the | |||
| CESM model | |||
| IPSL | Reduced complexity model | 3 | He et al. (2016) |
| obtained from the output of the IPSL model | |||
| MRI | Reduced complexity model | 3 | He et al. (2016) |
| obtained from the output of the MRI model |
Note. Their matrix representation can be found in Appendix A. ICBM = Introductory Carbon Balance Model; CLM4 = Community Land Model version 4; CESM = Community Earth System Model; IPSL = L'Institut Pierre‐Simon Laplace Coupled Model; MRI = Meteorological Research Institute Earth System Model.
In addition, we selected a set of models used for global‐scale applications. First, we selected three different parameterizations of the Community Land Model version 4 (CLM4cn), for needleleaf, deciduous, and tropical forest plant functional types (PFTs). These parameterizations differ mostly on the amount and distribution of C inputs to the soil (Wieder et al., 2014). Second, we used three versions of reduced complexity models constructed from the output of three Earth system models (He et al., 2016): the Community Earth System Model, version 1‐Biogeochemistry (CESM1‐BGC, here CESM), L'Institut Pierre‐Simon Laplace Coupled Model, version 5, coupled with NEMO, low resolution (IPSL‐CM5A‐LR, here IPSL), and the Meteorological Research Institute Earth System Model, version 1 (MRI‐ESM1, here MRI). For these reduced complexity models, we had sets of parameter values for a fixed three‐pool structure and for each individual grid cell, corrected to match radiocarbon values observed in a data set of soil radiocarbon profiles as reported in He et al. (2016). We used the global mean of these parameter values to compare age and transit time distributions among all models and used the complete set of gridded values to calculate mean and 50% and 95% quantiles of the age and transit time distributions. For each model, we plotted the relation between age and transit time and tested whether relation type I, II, or III (Figure 2) holds.
4. Results
4.1. Age Distributions of SOC in Models
Despite large differences in mean ages, the shape of the age distribution was relatively similar across models. All models produced age distributions of SOC in which most of the carbon is stored in relatively young ages, but with relatively long tails of old C (Figure 3). The mean ages with corresponding 50% and 95% quantiles from these distributions presented large differences among models, from a few decades to thousands of years (Table 2). The lower values for the mean and quantiles of the age distributions were obtained for the CLM4cn model with parameters for the needleleaf PFT, while the highest values for the mean and quantiles of the distributions were obtained for the average parameter values of the IPSL model (Table 2).
Figure 3.
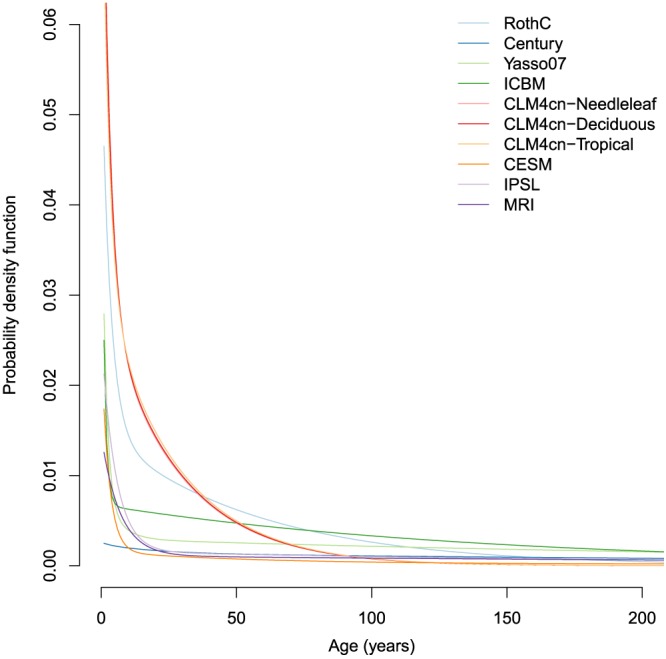
Age density distribution functions of soil organic carbon calculated for the models described in Table (1). ICBM = Introductory Carbon Balance Model; CLM4 = Community Land Model version 4; CESM = Community Earth System Model; IPSL = L'Institut Pierre‐Simon Laplace Coupled Model; MRI = Meteorological Research Institute Earth System Model.
Table 2.
Mean and 50% and 95% Quantiles (Q 50 and Q 95) of the Age and Transit Time Distributions for All Models. Units in Years.
| Model | Mean age | Age Q 50 | Age Q 95 | Mean transit time | Transit time Q 50 | Transit time Q 95 |
|---|---|---|---|---|---|---|
| RothC | 49.70 | 30.80 | 163.70 | 9.30 | 0.40 | 58.40 |
| Century | 4,082.40 | 814.50 | 17,981.40 | 382.50 | 49.20 | 1,027.60 |
| Yasso07 | 275.40 | 180.80 | 878.60 | 22.50 | 1.50 | 91.20 |
| ICBM | 134.30 | 90.70 | 416.00 | 18.70 | 0.90 | 130.50 |
| CLM4cn‐Needleleaf | 22.50 | 13.10 | 76.00 | 6.20 | 0.80 | 35.30 |
| CLM4cn‐Deciduous | 22.80 | 13.40 | 76.50 | 6.10 | 0.60 | 35.10 |
| CLM4cn‐Tropical | 23.50 | 14.40 | 77.40 | 5.80 | 0.30 | 34.90 |
| CESM | 4,210.60 | 2,647.50 | 13,933.10 | 41.30 | 2.10 | 18.80 |
| IPSL | 8,942.60 | 1,202.40 | 39,236.50 | 39.40 | 4.00 | 49.90 |
| MRI | 7,554.00 | 1,252.20 | 32,846.50 | 69.50 | 5.30 | 158.40 |
Note. ICBM = Introductory Carbon Balance Model; CLM4 = Community Land Model version 4; CESM = Community Earth System Model; IPSL = L'Institut Pierre‐Simon Laplace Coupled Model; MRI = Meteorological Research Institute Earth System Model.
Interestingly, relatively similar distributions were obtained within two groups of models, the reduced complexity models obtained from the three Earth system models (CESM, IPSL, and MRI) and the three parameterizations of CLM4cn. For the latter group, these results suggest that different parameterizations of carbon inputs for the PFTs in this model have little influence on the SOC age distribution and therefore on the predicted persistence of organic matter.
The Century and RothC models presented large differences in their age distributions, even though they are commonly considered as relatively similar models. One important reason that explains this difference is that we excluded the inert pool from RothC for the computations. This pool is, by definition, considered to not decompose or exchange material with other pools. Its decomposition rate is equal to 0, and therefore the age of the material is infinitely large at steady state ( ). After removing this pool from the calculations, the mean age is approximately 50 years with an upper 95% quantile of about 164 years.
For all models, the median of the age distribution (Age Q 50) was always lower than the mean age. Given that the age distributions have long tails (Figure 3), the mean age is more sensitive to the skewness of the distribution than the median. As a measure of central tendency, the median may be a better metric to characterize the age of these distributions.
4.2. Transit Time Distributions for All Models
The transit time distributions obtained for all models showed that most of the carbon leaving the soil is of relatively young ages (Figure 4). Compared to the age distributions, there were less differences in the shapes of the transit time distributions among models. However, there were still large differences in the mean and the quantiles of the transit time distributions, which vary from a few years to a few centuries (Table 2). Although the lowest mean transit time was predicted by the CLM4cn model, the lowest value of the 95% quantile was predicted by the CESM Earth system model. The highest mean and 95% quantile of the transit time distributions was predicted by the Century model.
Figure 4.
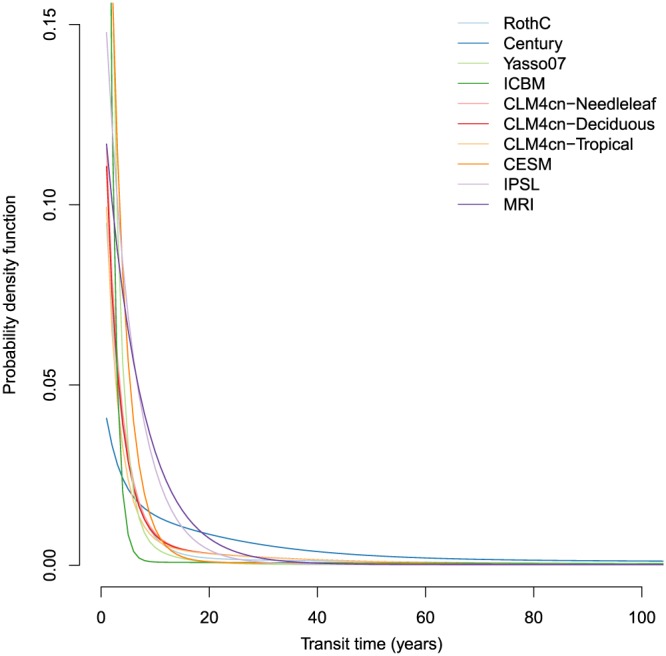
Transit time density distribution functions of soil organic carbon calculated for the models described in Table (1). ICBM = Introductory Carbon Balance Model; CLM4 = Community Land Model version 4; CESM = Community Earth System Model; IPSL = L'Institut Pierre‐Simon Laplace Coupled Model; MRI = Meteorological Research Institute Earth System Model.
The median of the transit time distribution (Q 50) was always much lower than the mean transit time, reflecting the fact that most of the output flux is dominated by carbon of very young age in all models but with a very small contribution of extremely old carbon. Given the long tails of these transit time distributions, the mean transit times were biased toward older values for characterizing central tendency. This bias was extreme for the CESM model where the mean transit time was even older than the 95% quantile of the distribution (Table 2). For characterizing central tendency of the transit time distribution, the median transit time may be a better metric than the mean.
4.3. Relation Between Age and Transit Time
Overall, the age distribution for each model is very different from the transit time distribution. To analyze the relation between these two distributions for all models, we plotted their sequence of quantiles, from 5% to 95%, in 5% increments (Figure 5). Two important results emerge from this analysis. First, for all models and across the entire quantile sequence, ages are always older than transit times. This gives strong support to the hypothetical relation of type III, and, although clear from the multiple‐pool structure of all models, it rejects the idea that SOC in these models behaves as a single well‐mixed reservoir or as a retention system in which material is sequentially processed or highly recycled before it leaves the system. This type III relation is also consistent with recent research that suggests that multiple biological, physical, and chemical mechanisms contribute to the (de)stabilization of SOM and that the probability of a carbon atom being mineralized at any given time would depend on the combined effect of these mechanisms without a specific progression of degradation steps or sequential physical movement (Lehmann & Kleber, 2015).
Figure 5.
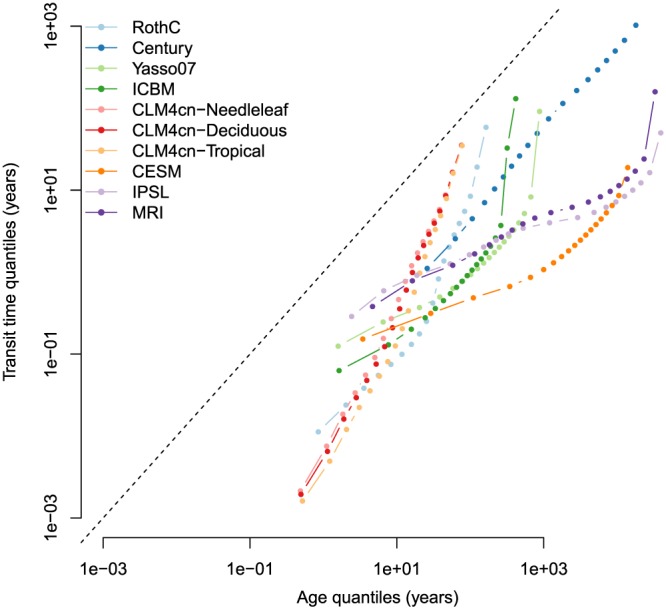
Sequence of quantiles, from 5% to 95% by 5% increments, for the age and transit time distributions of soil organic carbon calculated for the models of Table (1). Dashed line represents the 1:1 line. Notice that axes are in logarithmic scale. ICBM = Introductory Carbon Balance Model; CLM4 = Community Land Model version 4; CESM = Community Earth System Model; IPSL = L'Institut Pierre‐Simon Laplace Coupled Model; MRI = Meteorological Research Institute Earth System Model.
The second main result is that variability in the initial part of the distributions (5% quantile) is much larger for transit times than for ages. This can be observed in Figure (5) as a larger spread along the vertical direction toward the left part of the graph. On the contrary, the variability is much larger in terms of the 95% quantile of the age than of the transit time distributions. In other words, the upper tail of the distributions is much more variable for age than for transit time.
4.4. Global Distribution of SOC Age and Transit Time
The three reduced complexity Earth system models showed a global distribution of mean and median ages as well as mean and median transit times that support the type III relation across all grid cells (Figures 6 and 7 and supporting information figures). On a global scale, the spread of mean ages was much larger than the spread of mean transit times, suggesting that the age of SOC has a large degree of geographic variability, much larger than what can be estimated from mean transit times alone. Similarly, the median age had a larger variability than the median transit time (Figure 7).
Figure 6.
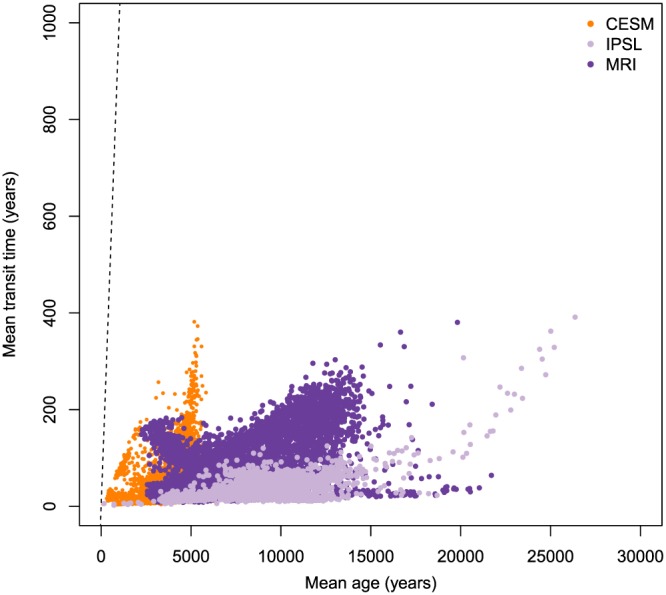
Mean age and mean transit times of soil organic carbon predicted for each grid cell by the three versions of the reduced complexity Earth system models CESM, IPSL, and MRI, corrected by observations of radiocarbon in soil profiles as described in He et al. (2016). Dashed line represents the 1:1 line. CESM = Community Earth System Model; IPSL = L'Institut Pierre‐Simon Laplace Coupled Model; MRI = Meteorological Research Institute Earth System Model.
Figure 7.
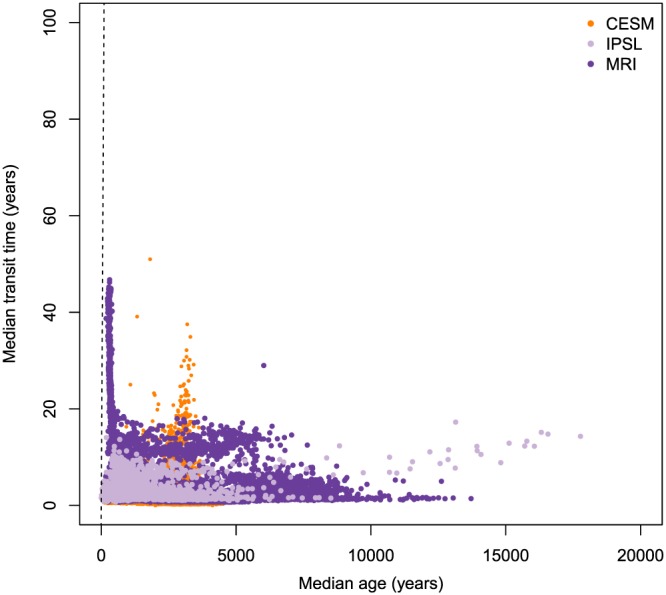
Median age and median transit times (Q 50) of soil organic carbon predicted for each grid cell by the three versions of the reduced complexity Earth system models CESM, IPSL, and MRI, corrected by observations of radiocarbon in soil profiles as described in He et al. (2016). Dashed line represents the 1:1 line. CESM = Community Earth System Model; IPSL = L'Institut Pierre‐Simon Laplace Coupled Model; MRI = Meteorological Research Institute Earth System Model.
The average of the mean age across all grid cells for the CESM, IPSL, and MRI models was 4,162, 8,916, and 7,312 years, respectively. However, mean ages can be as high as 25,000 years for some grid cells (see supporting information figures for details on the spatial distribution). The average of the median ages was much lower than the average of the mean age, which were 2,582, 1,488, and 1,887 years, respectively. The average of the 95% quantiles across all grid cells for the CESM, IPSL, and MRI models were 13,867, 39,056, and 31,802 years, respectively, but for some grid cells the 95% quantile was as high as 80,000 years.
These mean ages and quantiles of the age distributions suggest that some SOC persists on a millennial time scale across global soils. These values contrast with estimates of mean transit times across grid cells. For the CESM, IPSL, and MRI models, average mean transit times across grid cells were 40, 39, and 67 years, with average 50% quantiles as 2, 4, and 6 years, and 95% quantiles as 26, 56, and 157 years, respectively. This difference between age and transit time at the global scale confirms that using transit times as a metric of the persistence of SOM yields much different results than an age‐based approach.
5. Discussion
5.1. Age Distributions as a Measure of SOC Persistence
We computed age and transit time pdfs with corresponding mean and quantiles to assess the time that SOC persists in soils. Our results show that transit time distributions differ substantially from age distributions for all models. This is due to the fact that the transit time distribution characterizes the age structure of the output flux from the system, whereas the age distribution characterizes the age structure of the carbon stored within the system. Taken together, these two distributions provide important insights into soil C dynamics. However, based on an assessment of these distributions, we suggest the age distribution as a more appropriate characterization of SOM persistence in soils for two reasons: (1) Across all models, we found carbon age distributions with a very long tail of old C that represent storage in slow pools. This suggests that a fraction of SOM persists in soils on time scales of centuries to millennia. (2) Transit times represent the carbon that enters the soil and gets quickly processed and released, with small contributions from carbon that has been stored in slow cycling pools. As a result, transit times do not provide a good indication of how long C persists in soils.
The difference between age and transit time distributions is very important to consider in the context of the debate of SOM persistence (Dungait et al., 2012; Lehmann & Kleber, 2015; Schmidt et al., 2011). If persistence is ambiguously defined as age, residence, or turnover time, we may not be able to appropriately study how mechanisms of (de)stabilization contribute to the long‐term storage of carbon in soils. For instance, if we characterize persistence as the turnover time from models (stock over flux) and compare against radiocarbon‐derived ages measured in bulk soils, we would be comparing transit times versus ages, which obviously would yield contradictory results. For this reason, we advocate a more precise definition of SOM persistence and consider the age distribution of C as an adequate metric for this purpose.
5.2. How Long Does Carbon Persist in Soils?
Our results showed that age distributions depend on both model structure and the specific set of parameters in each model. The influence of model structure can be further decomposed between the relative split of the inputs to the different pools and the rates and transfers of carbon among pools. Furthermore, persistence is a model‐derived quantity and cannot be obtained by measurements alone. Radiocarbon is a useful tracer that can help to assess persistence (Trumbore, 2000; 2009), but to be able to translate isotopic ratios to ages, one must use a model (Trumbore et al., 2016). The choice of model (structure and parameters) would therefore influence the estimates of persistence that could be predicted for a particular soil.
The set of models we analyzed suggest that carbon persists on the order of centuries to millennia in soils. These time scales contrast with estimates of turnover or mean residence times of soil carbon, which are generally on the order of decades (Carvalhais et al., 2014; Wang et al., 2018). This apparent contradiction can be easily explained by the fact that the stock to flux ratio approximates the mean transit time and not the mean age. Furthermore, we found that the mean ages and mean transit times are not good metrics to characterize the central tendency of the distributions. Instead, the medians of these distributions better provide an idea of the central tendency of time scales of carbon cycling.
Geographically, ages are highly variable according to the reduced complexity models we analyzed. They are much more variable than transit times and can reach 95% quantiles of up to 80,000 years. A future research challenge is to study specific mechanisms that contribute to SOC persistence and how they change across biomes or environmental gradients. Radiocarbon, in combination with models, is a promising tool for this purpose. The radiocarbon value of bulk soil is a good indicator of the age of SOC, while radiocarbon in respired CO2 or leached in DOC is a good indicator of SOC transit time. If they both are measured across large environmental gradients, we may be able to better explain how different environmental drivers affect the time at which SOM persists.
Radiocarbon measurements, however, have a few caveats that must be taken into account for their interpretation. For example, radiocarbon in SOM can also be influenced by the incorporation of fossil C derived from parent rocks into soil organisms and therefore into organic matter (Seifert et al., 2012). Radiocarbon in SOM can also reflect the time spent by C in vegetation pools such as tree stems, and thus the derived age from these measurements must account for this time lag.
5.3. Is Random Chance a Relevant Factor in Explaining SOC Persistence?
The obtained pdfs of SOC age suggest that a stochastic interpretation of persistence is feasible. In a previous study (Metzler & Sierra, 2018), we found that deterministic pool models expressed as systems of differential equations such as those studied here have stochastic counterparts that correspond to the concept of continuous‐time absorbing Markov chains (see also correspondence between equations (1) and (2)). The rates that are used to represent decomposition of entire masses in the deterministic case can be interpreted as probabilities at the atom level. Given this duality in interpretation, we can consider random chance at the atomic level as a relevant factor in explaining SOM persistence. This interpretation is an important implication of our approach.
This stochastic interpretation does not mean that carbon atoms randomly persist in soils. On the contrary, the probability that an atom persists depends directly on the mechanisms of SOM (de)stabilization. These mechanisms set fixed probabilities for the atoms to move among different pools, to get consumed by microorganisms, or to leave the soil system in gas, liquid, or solid phase.
In our framework, we assume that these probabilities are based on physical, chemical, and biological processes, but not on age itself. For example, we assume that microbes consume an organic substrate based on the chemical or physical properties of the substrate, but not on the age of the carbon in the substrate. In this way, carbon consumption by microorganisms is random with respect to age, but not with respect to its chemical properties or its physical location within the soil matrix. If this is not the case, we would have to study how microorganisms can detect the age of a carbon substrate and make a decision to consume this substrate with respect to age. We consider this case unlikely and not supported by previous literature on soil microbial ecology.
Random chance is therefore a relevant factor that helps to explain why some carbon atoms persist for centuries or millennia in soils. It is a factor that has not been previously discussed in the literature of soil carbon (de)stabilization mechanisms (Dungait et al., 2012; Lehmann & Kleber, 2015; Schmidt et al., 2011), but we think it is key to explaining observations and interpreting model results. Some authors have emphasized the need to consider the probability that enzyme and substrates meet and the probability that the product can be consumed by microorganisms (Don et al., 2013; Gleixner, 2013; Spohn & Kuzyakov, 2014), and many previously proposed models have considered stochastic processes in modeling decomposition (Manzoni & Porporato, 2009). These stochastic interpretations of decomposition are important and must be considered in the context of the dual interpretation of models, at the atomic level from a stochastic point of view and at the deterministic level from a macroscale perspective.
5.4. Implications for Global Change Studies
Ages and transit time distributions are concepts that can be used beyond the realm of soil carbon (de)stabilization mechanisms to address other questions relevant for global change studies. For instance, they could be used to assess the effect of global change factors such as increased atmospheric CO2 concentrations and air temperature change on the time scale of ecosystem carbon cycling (Lu et al., 2018). As we showed here, these distributions are also very useful to compare models with diverse structures and parameterizations. In particular, the quantiles of these distributions can be very helpful in predicting how models predict the behavior of carbon of different ages, something that it is not possible to determine from mean ages or mean transit times alone. Furthermore, the bias in the means of the distributions we identified here advocate for approaches that characterize the central tendency of the age and transit time using the 50% quantile (median), which can only be obtained if the pdf of the distributions is known.
Thompson and Randerson (1999) also describe the utility of impulse response functions and storage response functions for a number of global change applications such as model intercomparisons and validation as well as to convolve these functions and obtain isotope dynamics. These functions are equivalent to the transit time distribution and the system age distribution, respectively (Metzler & Sierra, 2018). Therefore, it is possible to use the transit time and system age distributions to directly model the isotopic disequilibrium of carbon in global soils as well as radiocarbon dynamics. Taking advantage of the radiocarbon bomb spike, transit time distributions can be used to assess the speed at which this tracer of the global carbon cycle has been assimilated in soils worldwide.
6. Summary and Conclusions
Persistence of SOM can be characterized using the age distribution of carbon in soils. It provides information on the proportional distribution of carbon across the age range and offers insights on the maximum ages of carbon that could be observed in a soil. The age distribution differs from the transit time distribution, which provides information on the age of carbon in the output flux. Since metrics such as turnover or residence time are commonly calculated using approaches such as dividing stocks over fluxes, and since these metrics are in most cases indicative of the transit time rather than the age, persistence of SOM cannot be reliably estimated from these metrics. Instead, approaches to estimate soil carbon age such as isotopic measurements on the bulk SOM and soil fractions should better approximate the age distribution of carbon and the associated concept of persistence.
At the carbon atom level, random chance is an important factor that helps to explain persistence of SOM. We can interpret mechanisms of stabilization and destabilization of SOM as processes that determine the probability of carbon atoms to change state, moving across different pools in the soil or being consumed and mineralized by microorganisms. We assume that organic matter processing does not depend on the age of the carbon but rather on physical, chemical, and biological processes that set probabilities of carbon processing. The emergent outcome is a mix of carbon of different ages but with defined age distributions. We believe it is important to include random chance in the discussion of understanding persistence of organic matter in soils.
Radiocarbon measurements in bulk soils are important to understand SOC persistence. Our current best estimates of SOC in models corrected by radiocarbon measurements (He et al., 2016) indicate that SOC persists for centuries to millennia. This differs from estimates based on turnover times that are on the order of decades, but this difference can be explained by the fact that turnover times are indicative of transit times rather than age.
Both age and transit time distributions are useful concepts to integrate the diversity of mechanisms that control organic matter cycling. There is still a long way to determine how different interacting mechanisms produce different age and transit time distributions in soils. More efforts to integrate measurements with models should help us to integrate these different processes and understand why organic matter persists in soils for millennia.
Supporting information
Supporting Information S1
Acknowledgments
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement 695101). Funding was also provided by the Max Planck Institute for Biogeochemistry and the German Research Foundation (Emmy Noether Programme, SI 1953/2‐1). We also would like to thank H. Metzler for helpful discussions on stochastics and numerical implementations. All data and code required to reproduce the results presented here are available at https://git.bgc-jena.mpg.de/csierra/Persistence.git.
Appendix 1.
1.1.
A1. Matrix Representation of Analyzed Models
Each model was represented as a compartmental system of the form of equation (1). Below, we present the specific form of the matrix B and the vector u with the parameter values used for each model.
A1.1. RothC
| (A1) |
A.2. Century
| (A2) |
A1.3. Yasso07
| (A3) |
A1.4. ICBM
| (A4) |
A1.5. CLM4cn
This model contains one single B matrix for all plant functional types (PFTs):
| (A5) |
For the needleleaf, deciduous, and tropical PFTs, the vector u is given by, respectively,
| (A6) |
For the following reduced complexity models, the average of parameters across all grid cells is given by the following matrices.
A1.6. CESM
| (A7) |
A1.7. IPSL
| (A8) |
A1.8. MRI
| (A9) |
Sierra, C. A. , Hoyt, A. M. , He, Y. , & Trumbore, S. E. (2018). Soil organic matter persistence as a stochastic process: Age and transit time distributions of carbon in soils. Global Biogeochemical Cycles, 32, 1574–1588. 10.1029/2018GB005950
References
- Andren, O. , & Katterer, T. (1997). ICBM: The introductory carbon balance model for exploration of soil carbon balances. Ecological Applications, 7(4), 1226–1236. [Google Scholar]
- Bolin, B. , & Rodhe, H. (1973). A note on the concepts of age distribution and transit time in natural reservoirs. Tellus, 25(1), 58–62. [Google Scholar]
- Bruun, S. , Six, J. , & Jensen, L. S. (2004). Estimating vital statistics and age distributions of measurable soil organic carbon fractions based on their pathway of formation and radiocarbon content. Journal of Theoretical Biology, 230(2), 241–250. [DOI] [PubMed] [Google Scholar]
- Carvalhais, N. , Forkel, M. , Khomik, M. , Bellarby, J. , Jung, M. , Migliavacca, M. , Mu, M. , Saatchi, S. , Santoro, M. , Thurner, M. , Weber, U. , Ahrens, B. , Beer, C. , Cescatti, A. , Randerson, J. T. , Reichstein, M. (2014). Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature, 514, 213–217. [DOI] [PubMed] [Google Scholar]
- Derrien, D. , & Amelung, W. (2011). Computing the mean residence time of soil carbon fractions using stable isotopes: Impacts of the model framework. European Journal of Soil Science, 62(2), 237–252. [Google Scholar]
- Don, A. , Rödenbeck, C. , & Gleixner, G. (2013). Unexpected control of soil carbon turnover by soil carbon concentration. Environmental Chemistry Letters, 11(4), 407–413. 10.1007/s10311-013-0433-3 [DOI] [Google Scholar]
- Dungait, J. A. J. , Hopkins, D. W. , Gregory, A. S. , & Whitmore, A. P. (2012). Soil organic matter turnover is governed by accessibility not recalcitrance. Global Change Biology, 18(6), 1781–1796. 10.1111/j.1365-2486.2012.02665.x [DOI] [Google Scholar]
- Eriksson, E. (1971). Compartment models and reservoir theory. Annual Review of Ecology and Systematics, 2(1), 67–84. [Google Scholar]
- Gleixner, G. (2013). Soil organic matter dynamics: A biological perspective derived from the use of compound‐specific isotopes studies. Ecological Research, 28(5), 683–695. 10.1007/s11284-012-1022-9 [DOI] [Google Scholar]
- Gleixner, G. , Bol, R. , & Balesdent, J. (1999). Molecular insight into soil carbon turnover. Rapid Communications in Mass Spectrometry, 13(13), 1278–1283. [DOI] [PubMed] [Google Scholar]
- Goll, D. S. , Brovkin, V. , Liski, J. , Raddatz, T. , Thum, T. , & Todd‐Brown, K. E. O. (2015). Strong dependence of CO2 emissions from anthropogenic land cover change on initial land cover and soil carbon parametrization. Global Biogeochemical Cycles, 29, 1511–1523. 10.1002/2014GB004988, 2014GB004988. [DOI] [Google Scholar]
- He, Y. , Trumbore, S. E. , Torn, M. S. , Harden, J. W. , Vaughn, L. J. S. , Allison, S. D. , & Randerson, J. T. (2016). Radiocarbon constraints imply reduced carbon uptake by soils during the 21st century. Science, 353(6306), 1419–1424. 10.1126/science.aad4273 [DOI] [PubMed] [Google Scholar]
- Heim, A. , & Schmidt, M. W. I. (2007). Lignin turnover in arable soil and grassland analysed with two different labelling approaches. European Journal of Soil Science, 58(3), 599–608. 10.1111/j.1365-2389.2006.00848.x [DOI] [Google Scholar]
- Jenkinson, D. S. , Andrew, S. P. S. , Lynch, J. M. , Goss, M. J. , & Tinker, P. B. (1990). The turnover of organic carbon and nitrogen in soil [and discussion]. Philosophical Transactions: Biological Sciences, 329(1255), 361–368. [Google Scholar]
- Kiem, R. , & Kögel‐Knabner, I. (2003). Contribution of lignin and polysaccharides to the refractory carbon pool in C‐depleted arable soils. Soil Biology and Biochemistry, 35(1), 101–118. 10.1016/S0038-0717(02)00242-0 [DOI] [Google Scholar]
- Kleber, M. (2010). What is recalcitrant soil organic matter? Environmental Chemistry, 7(4), 320–332. [Google Scholar]
- Lehmann, J. , Abiven, S. , Kleber, M. , Pan, G. , Singh, B. P. , Sohi, S. P. , & Zimmerman, A. R. (2015). Persistence of biochar in soil In Lehmann J. & Joseph S. (Eds.), Biochar for environmental management: Science, technology and implementation (pp. 235–282). Abingdon, UK: Routledge. [Google Scholar]
- Lehmann, J. , & Kleber, M. (2015). The contentious nature of soil organic matter. Nature, 528, 60–8. [DOI] [PubMed] [Google Scholar]
- Lu, X. , Wang, Y.‐P. , Luo, Y. , & Jiang, L. (2018). Ecosystem carbon transit versus turnover times in response to climate warming and rising atmospheric CO2 concentration. Biogeosciences Discussions, 2018, 1–22. 10.5194/bg-2018-175 [DOI] [Google Scholar]
- Manzoni, S. , Katul, G. G. , & Porporato, A. (2009). Analysis of soil carbon transit times and age distributions using network theories. Journal of Geophysical Research, 114, G04025 10.1029/2009JG001070 [DOI] [Google Scholar]
- Manzoni, S. , & Porporato, A. (2009). Soil Carbon and nitrogen mineralization: Theory and models across scales. Soil Biology and Biochemistry, 41(7), 1355–1379. 10.1016/j.soilbio.2009.02.031 [DOI] [Google Scholar]
- Metzler, H. , & Sierra, C. A. (2018). Linear autonomous compartmental models as continuous‐time Markov chains: Transit‐time and age distributions. Mathematical Geosciences, 50(1), 1–34. 10.1007/s11004-017-9690-1 [DOI] [Google Scholar]
- Parton, W. J. , Schimel, D. S. , Cole, C. V. , & Ojima, D. S. (1987). Analysis of factors controlling soil organic matter levels in great plains grasslands. Soil Science Society of America Journal, 51(5), 1173–1179. [Google Scholar]
- Paul, E. A. (2016). The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biology and Biochemistry, 98, 109–126. 10.1016/j.soilbio.2016.04.001 [DOI] [Google Scholar]
- Schmidt, M. W. I. , Torn, M. S. , Abiven, S. , Dittmar, T. , Guggenberger, G. , Janssens, I. A. , Kleber, M. , Kogel‐Knabner, I. , Lehmann, J. , Manning, D. A. C. , Nannipieri, P. , Rasse, D. P. , Weiner, S. , Trumbore, S. E. (2011). Persistence of soil organic matter as an ecosystem property. Nature, 478(7367), 49–56. [DOI] [PubMed] [Google Scholar]
- Seifert, A.‐G. , Trumbore, S. , Xu, X. , Zhang, D. , & Gleixner, G. (2012). Variable effects of plant colonization on black slate uptake into microbial PLFAs. Geochimica et Cosmochimica Acta, 106, 391–403. [Google Scholar]
- Sierra, C. A. , Harmon, M. E. , & Perakis, S. S. (2011). Decomposition of heterogeneous organic matter and its long‐term stabilization in soils. Ecological Monographs, 81(4), 619–634. 10.1890/11-0811.1 [DOI] [Google Scholar]
- Sierra, C. A. , & Müller, M. (2015). A general mathematical framework for representing soil organic matter dynamics. Ecological Monographs, 85, 505–524. 10.1890/15-0361.1 [DOI] [Google Scholar]
- Sierra, C. A. , Müller, M. , Metzler, H. , Manzoni, S. , & Trumbore, S. E. (2017). The muddle of ages, turnover, transit, and residence times in the carbon cycle. Global Change Biology, 23(5), 1763–1773. 10.1111/gcb.13556 [DOI] [PubMed] [Google Scholar]
- Sierra, C. A. , Müller, M. , & Trumbore, S. E. (2014). Modeling radiocarbon dynamics in soils: SoilR version 1.1. Geoscientific Model Development, 7(5), 1919–1931. 10.5194/gmd-7-1919-2014 [DOI] [Google Scholar]
- Sollins, P. , Homann, P. , & Caldwell, B. A. (1996). Stabilization and destabilization of soil organic matter: Mechanisms and controls. Geoderma, 74(1‐2), 65–105. 10.1016/S0016-7061(96)00036-5 [DOI] [Google Scholar]
- Spohn, M. , & Kuzyakov, Y. (2014). Spatial and temporal dynamics of hotspots of enzyme activity in soil as affected by living and dead roots—A soil zymography analysis. Plant and Soil, 379(1), 67–77. 10.1007/s11104-014-2041-9 [DOI] [Google Scholar]
- Spohn, M. , & Sierra, C. A. (2018). How long do elements cycle in terrestrial ecosystems? Biogeochemistry, 139(1), 69–83. 10.1007/s10533-018-0452-z [DOI] [Google Scholar]
- Thompson, M. V. , & Randerson, J. T. (1999). Impulse response functions of terrestrial carbon cycle models: Method and application. Global Change Biology, 5(4), 371–394. 10.1046/j.1365-2486.1999.00235.X [DOI] [Google Scholar]
- Trumbore, S. (2000). Age of soil organic matter and soil respiration: Radiocarbon constraints on belowground c dynamics. Ecological Applications, 10(2), 399–411. [Google Scholar]
- Trumbore, S. (2009). Radiocarbon and soil carbon dynamics. Annual Review of Earth and Planetary Sciences, 37(1), 47–66. [Google Scholar]
- Trumbore, S. E. , Sierra, C. A. , & Hicks Pries, C. E. (2016). Radiocarbon nomenclature, theory, models, and interpretation: Measuring age, determining cycling rates, and tracing source pools In Trumbore S. E, Sierra C. A, & C. E. Hicks Pries (Eds.), Radiocarbon and climate change: Mechanisms, applications and laboratory techniques (pp. 45–82). Switzerland: Springer International Publishing. [Google Scholar]
- Tuomi, M. , Thum, T. , Järvinen, H. , Fronzek, S. , Berg, B. , Harmon, M. , Trofymow, J. , Sevanto, S. , Liski, J. (2009). Leaf litter decomposition—Estimates of global variability based on Yasso07 model. Ecological Modelling, 220(23), 3362–3371. 10.1016/j.ecolmodel.2009.05.016 [DOI] [Google Scholar]
- von Lützow, M. , Kögel‐Knabner, I. , Ludwig, B. , Matzner, E. , Flessa, H. , Ekschmitt, K. , Guggenberger, G. , Marschner, B. , Kalbitz, K. (2008). Stabilization mechanisms of organic matter in four temperate soils: Development and application of a conceptual model. Journal of Plant Nutrition and Soil Science, 171(1), 111–124. [Google Scholar]
- Wang, J. , Sun, J. , Xia, J. , He, N. , Li, M. , & Niu, S. (2018). Soil and vegetation carbon turnover times from tropical to boreal forests. Functional Ecology, 32(1), 71–82. 10.1111/1365-2435.12914 [DOI] [Google Scholar]
- Wieder, W. R. , Boehnert, J. , & Bonan, G. B. (2014). Evaluating soil biogeochemistry parameterizations in Earth system models with observations. Global Biogeochemical Cycles, 28, 211–222. 10.1002/2013GB004665 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1


