Abstract
We are developing assays to image tissue-specific reporter gene expression in living mice by using optical methods and positron emission tomography. Approaches for imaging reporter gene expression depend on robust levels of mRNA and reporter protein. Attempts to image reporter gene expression driven by weak promoters are often hampered by the poor transcriptional activity of such promoters. Most tissue-specific promoters are weak relative to stronger but constitutively expressing viral promoters. In this study, we have validated methods to enhance the transcriptional activity of the prostate-specific antigen promoter for imaging by using a two-step transcriptional amplification (TSTA) system. We used the TSTA system to amplify expression of firefly luciferase (fl) and mutant herpes simplex virus type 1 thymidine kinase (HSV1-sr39tk) in a prostate cancer cell line (LNCaP). We demonstrate ≈50-fold (fl) and ≈12-fold (HSV1-sr39tk) enhancement by using the two-step approach. The TSTA system is observed to retain tissue selectivity. A cooled charge-coupled device optical imaging system was used to visualize the amplified fl expression in living mice implanted with LNCaP cells transfected ex vivo. These imaging experiments reveal a ≈5-fold gain in imaging signal by using the TSTA system over the one-step system. The TSTA approach will be a valuable and generalizable tool to amplify and noninvasively image reporter gene expression in living animals by using tissue-specific promoters. The approaches validated should have important implications for study of gene therapy vectors, cell trafficking, transgenic models, as well as studying development of eukaryotic organisms.
Assays for imaging reporter gene expression are very useful for visualizing molecular events in living animals. Noninvasive imaging of reporter gene expression by using radionuclide and nonradionuclide techniques allows the monitoring of the location, magnitude, and time variation of gene expression in living animals and, in some cases, humans. Applications of reporter genes include: (i) optimization of gene therapy vectors that contain a reporter gene, (ii) imaging cell trafficking by marking cells with a reporter gene ex vivo, and (iii) monitoring endogenous gene expression through the use of reporter genes coupled to endogenous promoters in transgenic models. All of these applications should benefit from repetitive, noninvasive monitoring of reporter gene expression.
Methods to image reporter gene expression in living animals include positron emission tomography (PET) (1, 2), single photon emission computed tomography (3, 4), magnetic resonance imaging (5, 6), bioluminescent optical imaging with firefly luciferase (fl) (7–9), and fluorescence optical imaging with green fluorescent protein (10). A detailed review of the different approaches for imaging reporter genes is reported elsewhere (11).
The cooled charge-coupled device (CCD) camera offers a convenient, cost-effective, and reproducible method to image fl expression in small living animals (7–9). Recently, real-time monitoring of fl reporter gene expression in various tissues has been evaluated in rodents by using a cooled CCD camera (8). In this article, the authors have demonstrated that injection of adenovirus, AdHIV/fl (the fl expression is driven by the HIV–long-terminal repeat promoter) results in fl expression in the region over the testis in male mice. Transgenic mice with the human bone γ-carboxyglutamate protein promoter driving fl led to gene expression in bones and teeth. The use of tissue-specific promoters in these limited applications was possible, but in many applications specific promoters may not lead to sufficient levels of fl expression. In other cases, enhancement of observable signal may benefit from amplification.
The advantage of PET over optical approaches is the ability to obtain tomographic and quantitative information with high sensitivity. With appropriate corrections for photon attenuation, scatter, and object size, concentrations of radiolabeled tracers can be reliably estimated (12). Furthermore, unlike optical methods, small animal imaging with microPET (13) directly translates to human studies with clinical PET scanners. We have previously developed methods to monitor adenoviral-mediated herpes simplex virus type 1 thymidine kinase (HSV1-tk) expression in vivo by using 18F-labeled acycloguanosine analogs and microPET (1, 14). We have studied cell lines stably transfected ex vivo with various PET reporter genes and implanted in mice for imaging with microPET (15, 16). We also have validated the use of a mutant HSV1-sr39tk with enhanced imaging sensitivity (17). In our earlier approaches, the expression of the PET reporter gene was driven by relatively strong promoters [e.g., cytomegalovirus (CMV)] that achieve a constitutive, high level of transcription. In many therapeutic applications, targeted approaches to enhance safety and specificity are highly desirable. To this end, transcriptional targeting by using tissue-specific promoters to limit expression of potential cytotoxic transgenes to the tissue of interest has been frequently used (18–20). In applications where a relatively weak, cellular promoter drives the expression of a reporter gene, one has to contend with weaker transcriptional activity that, in turn, limits the ability to image reporter gene expression in vivo. In such cases, it is essential to find ways to enhance the transcriptional activity of such promoters. Several potential methods can be used to increase levels of reporter protein. These include (i) increased transcription by using chimeric promoters that retain tissue specificity (20, 21), (ii) enhancement at the posttranscriptional level (22–24), or (iii) transcriptional amplification approaches discussed next.
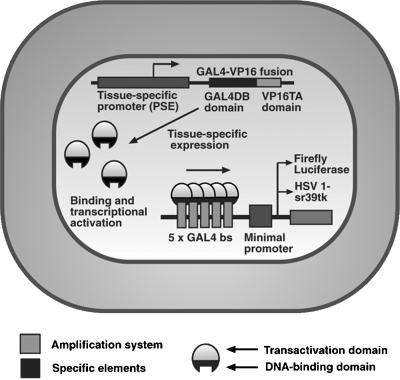
One of the amplification approaches referred to as a two-step transcriptional amplification (activation) (TSTA) approach that can potentially be used to augment the transcriptional activity of cellular promoters uses the GAL4-VP16 fusion protein (25, 26) (Fig. 1). This approach is also referred to as a recombinant transcriptional activation approach (27). The yeast GAL4 gene expression system is one of the most widely studied eukaryotic transcriptional regulatory systems (28).
Figure 1.
Schematic diagram of the TSTA system. The first step involves the tissue-specific (e.g., PSE) expression of the GAL4-VP16 fusion protein. In the second step, GAL4-VP16, in turn, drives target gene expression under the control of GAL4 response elements in a minimal promoter [shown are five GAL4 binding sites (bs)]. Transcription of the reporter gene, either fl or HSV1-sr39tk, leads to reporter protein, which in turn leads to a detectable signal in the presence of the appropriate reporter probe. The use of the GAL4-VP16 fusion protein can potentially lead to amplified levels of the reporter protein (FL or HSV1-sr39TK) and therefore an increase in imaging signal.
We are presently evaluating methods to noninvasively image reporter gene expression driven by cellular promoters. We chose to evaluate the prostate-specific antigen promoter (named PSE in this article) as it has been well characterized, determined to be tissue-specific, and is expressed after androgen administration in cell culture and in vivo (29). In this study, we chose to take advantage of the strong transactivating properties of the GAL4-VP16 fusion protein to achieve prostate-specific amplification by using the PSE promoter to drive expression of fl or HSV1-sr39tk in an LNCaP prostate cancer cell line. We also validated the system for monitoring reporter gene expression in living mice implanted with LNCaP cells transiently expressing fl by using a cooled CCD optical imaging system.
Materials and Methods
TSTA Experimental Strategy.
The HSV1 VP16 immediate early transactivator contains a highly potent activation domain that, when fused to the GAL4 DNA binding domain, elicits a robust response on a GAL4-responsive promoter (referred to as a minimal promoter in this work) bearing multiple tandem copies of the 17 bp near consensus DNA binding site (25, 26). The VP16 activation domain amino acids 411–490 can be subdivided further into N- and C-terminal subdomains. The C-terminal domain is sometimes toxic to cells and elicits a transcriptional inhibitory phenomenon called squelching (30). GAL4-VP16 is a fusion of the N-terminal portion of the VP16 activation domain from amino acids 413–454 and the GAL4 DNA binding domain amino acids 1–147. It is a potent activator that squelches less effectively than the intact activation domain.
Chemicals.
Methyltrienolone (R1881) was purchased from NEN. Tfx-50 transfection reagent and luciferase assay kit were purchased from Promega. D-Luciferin for use with in vivo fl imaging was obtained from Xenogen (Alameda, CA). [8-3H]Penciclovir (17.6 Ci/mmol) was purchased from Moravek Biochemicals (Brea, CA). Testosterone pellets (0.007 mg, 6-h release) were obtained from Innovative Research of America. Matrigel was purchased from BD Biosciences (Bedford, MA).
Cell Culture.
The human prostate cancer cells, LNCaP (provided by C. Sawyers, University of California, Los Angeles), were grown in RPMI 1640 supplemented with 10% FBS and 1% penicillin/streptomycin solution. The C6 rat glioma cells were kindly provided by Margaret Black (Washington State University, Pullman) and were grown in deficient DMEM, supplemented with 5% FBS and 1% penicillin/streptomycin/L-glutamine. HeLa cells (American Type Culture Collection) were grown in DMEM with 10% FBS and 1% penicillin/streptomycin.
Construction of Plasmids/Vectors.
The parental construct, PSE, derived from plasmid PSAR2.4k-PCPSA-P-Lux (29), consisted of a 2.4-kb enhancer fragment (−5322 to −2855) and the proximal promoter region from −541 to +12, upstream of an fl reporter gene. PSE was chosen to be the baseline construct because the 2.4-kb enhancer fragment generates the maximal transcriptional and androgen-responsive activity, comparable to the entire 6-kb regulatory region of the prostate-specific antigen gene (20, 31). The construction of PSE plasmid has been described (20). To construct pBS-PSEGAL4VP16, the HindIII to XbaI GAL4VP16 fragment was excised from pSP72-SV40-GAL4VP16 and inserted into PSE plasmid. The minimal promoter, G5E4TATA (G5E4T) contained templates bearing five 17-bp GAL4 binding sites positioned 23 bases from the TATA box of the E4 gene of adenovirus (32). The G5E4T-fl construct was made by PCR amplification of the relevant GAL4 sites and E4TATA from the pGEM3 G5E4T vector by using an upstream primer with a SacI site attached (cccgagctcatttaggtgacactatag) and a downstream primer with a XhoI site attached (cccctcgagacaccactcgacacggcacc). The PCR fragments were digested with SacI and XhoI and cloned into the pGL3 basic vector. The G5E4T-sr39tk plasmid was constructed by excising the G5E4T fragment from pSP72-G5E4T-CAT and cloning into pcDNA3.1 vector. The G5E4T sequence was PCR-amplified by using primer pair 5′-gactagatctacagcttgcatgcctgcag-3′ and 5′-gactgctagctcgacacggcacca-3′ and cloned into the BglII/NheI sites upstream of sr39tk in the main vector, pcDNA3.1. For the sake of convenience, PSEGAL4-VP16 is abbreviated as PG, and the reporter plasmid G5E4T-fl is abbreviated as L5. The reporter template G5E4T-sr39tk is referred to as T5. The constructs PSE-fl, PSE-HSV1-sr39tk, and SV40-GAL4-VP16 are abbreviated as PL, PT, and SG, respectively.
Cell Transfections and Enzyme Assays.
On day 1, LNCaP cells were plated in 6-well plates in RPMI 1640 containing charcoal-stripped FBS. Transient transfections were performed 24 h later by using Tfx-50 transfection reagent (Promega). Each transfection mix consisted of 0.5 μg of the effector and reporter plasmids or reporter plasmid alone. One hour after transfection, methyltrienolone (R1881) in ethanol was added to the medium at a concentration of 1 nM/well, and the cells were incubated for 48 h. For FL activities (fl refers to the gene and FL to the enzyme), the cells were harvested and assayed for FL activity by using the dual-reporter luciferase assay system (Promega) and a luminometer (Lumat 9507, Berthold, Germany) with an integration time of 10 s. LNCaP cells were also transfected by using CMV-fl plasmid (as a positive control). For the TK assay, the cells were harvested 48 h after transfection and assayed for HSV1-sr39TK enzyme activity (tk refers to the gene and TK to the enzyme) as described (14). For androgen inducibility experiments, LNCaP cells were transiently transfected with PG and T5 constructs in the presence of different concentrations of androgen. After 48 h, the cells were assayed for HSV1-sr39TK activity.
In Vivo Optical Imaging of fl Expression Using a Cooled CCD Camera.
LNCaP cells were transiently transfected with the effector and reporter plasmids or reporter plasmid alone as described earlier. The cells were harvested 48 h after transfection and resuspended in PBS. An aliquot of 1 × 106 cells was mixed with matrigel and injected i.p. in female nude mice. Five minutes after injection of the cells, the mice were anesthetized (ketamine–xylazine, 4:1), and 200 μl of D-luciferin (15 mg/ml) was injected (i.p.) 10 min before imaging. After the first scan, the mice were s.c. implanted with 0.007-mg sustained release testosterone pellets and imaged again after 24 h and 48 h. A total of five mice were used for the experiment, and one mouse in each group did not receive the androgen pellet.
The mice were imaged by using a cooled CCD camera (Xenogen IVIS, Alameda, CA) with an acquisition time of 5 min. The animals were placed supine in a light-tight chamber, and a gray scale reference image was obtained under low-level illumination. Photons emitted from cells implanted in the mice were collected and integrated for a period of 5 min. Images were obtained by using living image software (Xenogen) and igor image analysis software (WaveMetrics, Lake Oswego, OR). For quantitation of measured light, regions of interest were drawn over the peritoneal region, and maximum relative light units (RLU) per min were obtained as validated (9).
Results
TSTA System Mediates Prostate-Specific Amplification of fl Expression in LNCaP Cells and Demonstrates Cell-Type Specificity.
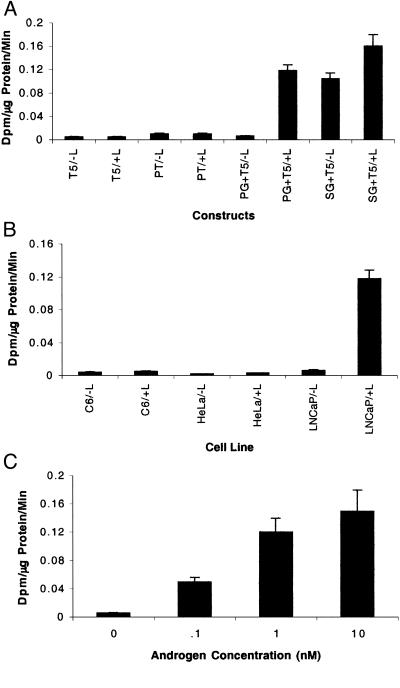
In transient transfection into LNCaP cells, when GAL4-VP16 was placed under the control of the PSE promoter, it activated poorly in the absence of androgen, but the response in the presence of androgen was significantly higher than that observed by using the reporter template alone (L5) (Fig. 2A). The maximal level of fl expression is observed by using five binding sites upstream of the adenovirus E4 promoter (L5) on the reporter template vs. one or two binding sites (data not shown). The TSTA system-driven amplification of fl expression in the presence of androgen is similar to the SG-activated expression of a GAL4-responsive fl reporter in the absence and presence of androgen stimulation (−L/+L, Fig. 2A). These results validate the concept that the tissue-specific, ligand-responsive PSE promoter could be substituted for a strong viral one to elicit similar levels of GAL4-VP16 functional activity. When CMV-fl was compared with the TSTA system, the FL activity by using the TSTA system was observed to be 2- to 3-fold lower than that driven by the CMV promoter (data not shown). A comparison of fl expression by using both the one-step and the TSTA systems reveals a ≈50-fold gain (P < 0.01) with the TSTA system when compared with the one-step system (Fig. 2A). To determine whether the TSTA system-mediated amplification is restricted to prostate cells alone, we studied the levels of fl expression in two nonprostate cell lines, C6 and HeLa. Firefly luciferase reporter gene expression after androgen administration is minimal in both the cell lines tested, indicating the tissue-specific nature of the PSE promoter (Fig. 2B). The results indicate a strong stimulatory effect on transcription exerted by the GAL4-VP16 fusion protein while maintaining tissue specificity.
Figure 2.
(A) TSTA system-mediated amplification of fl expression. LNCaP cells were transiently transfected in the absence (−L) and presence (+L) of androgen with (i) reporter construct alone (L5), (ii) PL (one-step), (iii) PG/L5 (two-step), and (iv) SG/L5 (control) constructs. The cells were harvested 48 h after transfection and assayed for FL activity. The error bars represent SEM for triplicate measurements. (B) Cell-type specificity of the TSTA system (fl). C6, HeLa, and LNCaP cells were transiently transfected in the absence (−L) and presence (+L) of androgen with effector construct, PG, and reporter construct, L5. The cells were harvested 48 h after transfection and assayed for FL activity. The error bars represent SEM for triplicate measurements.
TSTA System Mediates Prostate-Specific Amplification of HSV1-sr39tk Expression in LNCaP Cells, Demonstrates Cell-Type Specificity, and Expression Increases with Androgen Dose.
To test the HSV1-sr39tk reporter system, we used the T5 reporter construct with PG for transient transfections in LNCaP cells. The results with HSV1-sr39tk were similar to that observed by using fl. After androgen treatment, HSV1-sr39tk expression mediated by the TSTA system is significantly greater (≈24-fold) (P < 0.01) when compared with the expression in cells transfected with the reporter template only (T5) (Fig. 3A). Further, the TSTA system-mediated amplification of HSV1-sr39tk expression levels are ≈12-fold greater than those driven by PT alone (one-step) (Fig. 3A) in the presence of androgen. Although the gain in amplification for HSV1-sr39tk is lower than fl, it is nevertheless highly significant (P < 0.01). To determine whether the TSTA system-mediated reporter gene amplification is cell-type specific, we studied the levels of HSV1-sr39tk expression in two nonprostate cell lines, C6 and HeLa. HSV1-sr39tk expression level after androgen administration is minimal in both the cell lines tested, demonstrating that the TSTA system-mediated activation from the PSE promoter is highly cell-type specific (Fig. 3B). We further evaluated the effects of androgen response in LNCaP cells by using HSV1-sr39tk. In the absence of androgen, HSV1-sr39tk expression levels are similar to the values obtained by using the reporter template alone (minimal). A concentration-dependent increase in HSV1-sr39TK activity is observed with increasing androgen concentration, indicating that androgen treatment greatly enhances the TSTA system-mediated activation from the PSE promoter (Fig. 3C).
Figure 3.
(A) TSTA system-mediated amplification of HSV1-sr39tk expression. LNCaP cells were transiently transfected in the absence (−L) and presence (+L) of androgen with (i) reporter construct alone (T5), (ii) PT (one-step), (iii) PG/T5 (two-step), and (iv) SG/T5 (control) constructs. The cells were harvested 48 h after transfection and assayed for HSV1-sr39TK activity. The error bars represent SEM for triplicate measurements. (B) Cell-type specificity of the TSTA system (HSV1-sr39tk). C6, HeLa, and LNCaP cells were transiently transfected in the absence (−L) and presence (+L) of androgen with the effector construct, PG, and reporter construct, T5. The cells were harvested 48 h after transfection and assayed for HSV1-sr39TK activity. The error bars represent SEM for triplicate measurements. (C) Effect of androgen concentration on HSV1-sr39tk expression. LNCaP cells were transiently transfected in the absence (−L) and presence (+L) of androgen with PG and T5. The androgen concentration varied from 0.1 to 10 nM. The cells were harvested 48 h after transfection and assayed for HSV1-sr39TK activity. The error bars represent SEM for triplicate measurements.
TSTA System Mediates Prostate-Specific Amplification of fl Expression in Vivo.
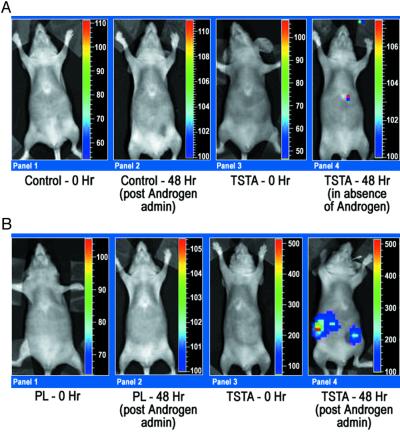
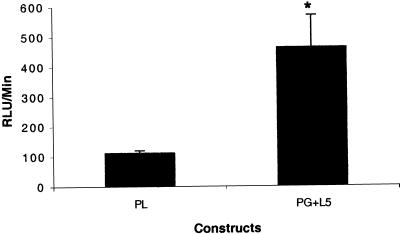
To further test the utility of the TSTA system in vivo, we injected transiently transfected LNCaP cells (transfected with PG and L5 plasmids, L5 plasmid alone, and PL plasmid) i.p. in female nude mice. Optical CCD imaging of all mice 15 min after injection of transfected LNCaP cells reveals basal levels of fl expression (RLU/min < 100). The mice were scanned again 24 h and 48 h after the implantation of androgen pellets. The results of the 24-h imaging were similar to those observed at 48 h. Forty-eight hours after pellet implantation, the control mice (L5 plasmid alone) again show minimal levels of fl expression (RLU/min < 100) (Fig. 4A). In the mice representing the TSTA system, fl expression is observed to be much higher (RLU/min ≈500) when compared with the control and the one-step system (RLU/min < 100) (Fig. 4B). Mice representing the TSTA system that did not receive androgen pellets displayed basal levels of fl expression at 48 h (Fig. 4A). This is indicative of the specificity of androgen in activating the PSE promoter in vivo. The results were found to be reproducible between experimental groups. The induction of transcriptional activation upon androgen administration across five mice in each of two groups is illustrated in Fig. 5. The TSTA system-mediated amplification in fl expression shows a ≈5-fold gain when compared with the one-step system (P < 0.05). These results demonstrate the ability to image in vivo the expression of fl driven by a weak, tissue-specific promoter in a mammalian system by using the TSTA approach. The TSTA system mediates tissue-specific expression of fl in a mammalian system.
Figure 4.
(A) In vivo optical CCD imaging of mice carrying transiently transfected LNCaP cells for control studies. All images shown are the visible light image superimposed on the optical CCD image with a scale in RLU/min as shown. (Panel 1) LNCaP cells were transiently transfected with only the L5 vector (control) and cells implanted i.p. The animal was then imaged after injection of d-luciferin (i.p.). (Panel 2) The same animal as in Panel 1 was imaged 48 h after implantation of androgen pellet by readministering d-luciferin (i.p.). (Panel 3) LNCaP cells were transiently transfected with the PG and L5 vectors and cells implanted i.p. The mouse was imaged after i.p. injection of d-luciferin. (Panel 4) The same animal imaged in Panel 3 was reimaged in the absence of androgen 48 h later after i.p. injection of d-luciferin. (B) In vivo optical CCD imaging of mice carrying transiently transfected LNCaP cells for comparison of one-step and TSTA. All images shown are the visible light image superimposed on the optical CCD image with a scale in RLU/min as shown. (Panel 1) LNCaP cells were transiently transfected with only the one-step PL vector and cells implanted i.p. The animal was then imaged after injection of d-luciferin (i.p.). (Panel 2) The same mouse in Panel 1 was reimaged with i.p. injection of d-luciferin 48 h after implantation of an androgen pellet. (Panel 3) LNCaP cells were transiently transfected with the PG and L5 vectors (TSTA system) and cells implanted i.p. The mouse was then imaged after i.p. injection of d-luciferin. (Panel 4) The same animal imaged in Panel 3 was reimaged with i.p. d-luciferin 48 h after implantation of an androgen pellet.
Figure 5.
Comparison of transcriptional activation in vivo using the TSTA system versus the one-step system. Shown are results of mean ± SEM RLU/min in five nude mice in each group. The first group had LNCaP cells transiently transfected with PL (one-step), and the second group had LNCaP cells transiently transfected with PG and L5 vectors (two-step). The cells were implanted i.p. in the mice. The mice were then implanted with androgen pellets and imaged again after 24 and 48 h. There is a significant difference (* P < 0.05) between the two groups.
Discussion
We have previously demonstrated that adenoviral-mediated HSV1-tk expression can be noninvasively imaged by using acycloguanosine analogs and microPET (1, 14). We have been evaluating methods to noninvasively image reporter gene expression driven by weak promoters such as the PSE promoter. Several of the known weak promoters also demonstrate tissue specificity (33). In the present study, we describe a strategy to overcome the weak transcriptional activity of the PSE promoter for use in tissue-specific imaging applications. We used a two-step transcriptional amplification (activation) approach where, in the presence of androgen, a relatively weak PSE promoter activates a GAL4-VP16 fusion protein, which, in turn, drives reporter gene expression under the control of GAL4 response elements in a minimal promoter.
In the present study, we observed the TSTA system-mediated amplification of fl expression to be 50-fold higher than the one-step system in LNCaP cells in the presence of androgen. We have also demonstrated the TSTA system to be highly tissue-specific. Similar results were obtained when we changed the reporter gene from fl to the HSV1-sr39tk, although the fold gain in HSV1-sr39tk expression levels was lower than fl. The differences in degree of amplification between fl and HSV1-sr39tk may be attributed to different levels of mRNA amplification and differences in the two enzymes to act on their corresponding substrates. The TSTA system, however, does lead to a statistically significant increase in amplification for both the fl and HSV1-sr39tk in a tissue-specific manner.
The TSTA system-mediated activation of the PSE promoter depends highly on the levels of androgen as evidenced by the dose-dependent increase in HSV1-sr39tk expression. The system seems to function in a continuous manner (as opposed to a binary on/off fashion). There also does not seem to be a threshold effect. As more androgen becomes available, levels of GAL4-VP16 fusion protein increase, thereby increasing the ability to bind to GAL4 binding sites on the reporter template, resulting in greater levels of fl and therefore greater imaging signal. Finally, although we did not see full saturation, higher levels of GAL4-VP16 have previously been reported to inhibit transcription (referred to as squelching) (30, 34, 35).
We further tested the utility of the GAL4-VP16 induction system in vivo to noninvasively image tissue-specific amplification of reporter gene expression. To validate this system in vivo, several issues needed consideration. These included the development of (i) LNCaP and nonprostate cell lines stably expressing both the effector and reporter constructs and (ii) construction of adenoviral vectors containing the two components of the TSTA system. Both of these approaches require considerable time before they can be tested in vivo. To expedite the process of in vivo evaluation, we injected transiently transfected LNCaP cells (using fl as the reporter gene in the absence of androgen) in female nude mice. The mice were imaged by using a sensitive cooled CCD camera before and after implantation of testosterone pellets. All mice displayed basal levels of fl expression in the absence of androgen. Twenty-four to 48 h after androgen administration, the mice representing the TSTA system showed a significantly greater level of fl expression when compared with the control and one-step mice. We observed similar high levels of induction across several mice with a ≈5-fold gain for the TSTA system over the one-step system. In fact, in the absence of the TSTA system, the fl expression was close to background, and therefore cells could not be imaged. These initial results are aimed at noninvasively imaging reporter gene expression in a mammalian system by augmenting the transcriptional activity of a weak promoter by using the TSTA system. The level of TSTA amplification in vivo depends on the pharmacokinetics of androgen availability to cells, and it is possible that the in vivo signal can potentially be higher than observed based on the imaging time after androgen induction. Future studies will need to address the exact correlation between levels of androgen in blood and the levels of induction in vivo.
Fang et al. (36) have examined the functionality of the GAL4-VP16 transactivator to evaluate phosphoglycerate kinase (PGK) promoter activities in vivo by using adenoviral vectors. Nettelbeck et al. (33) have used recombinant transcriptional activation to establish a positive feedback loop initiated by transcriptional activation from a von Willebrand factor promoter. The GAL4-VP16 fusion protein was used to achieve target gene amplification from a prostate-specific antigen promoter (37). Both of these approaches were targeted at increasing the expression of transgenes for use in cancer gene therapy protocols. Further, the GAL4-VP16 responsive TSTA system has been recently used in conjunction with zebrafish tissue-specific promoters with green fluorescent protein reporters (35). In this study, the GAL4-VP16 was injected transiently into fish embryos that developed with minimal toxicity, permitting imaging of specific tissue in adult fish.
It is important that any reporter gene-imaging approach not significantly perturb the cells/animal models being studied. There is potential for the GAL4-VP16 system to be toxic to cells (35, 38). In zebrafish, low levels of injected GAL4-VP16 were apparently not deleterious to development, whereas higher levels were (35). However, in the current study, only transiently transfected cells were used, so toxicity is not practical to detect. Future studies with stable cell lines, gene therapy vectors, and transgenic animal models will require strict characterization of potential toxicities of the TSTA approach. It may be the case that levels of GAL4-VP16 will need to be modulated to strike a balance between amplification and any potential toxicity.
The approaches validated in the current study should lead to better vectors for imaging gene therapy, study of tumor growth and regression following pharmacological intervention, as well as development of transgenic models with enhanced reporter gene expression. Applications and extensions of the TSTA approach include: (i) imaging PET reporter genes (e.g., HSV1-sr39tk) and other in vivo reporter genes; (ii) enhancing reporter gene expression by modifying the regulatory components of the PSE promoter and building a single vector that incorporates both components of the TSTA system; (iii) replacement of the PSE promoter with other weak promoters, thereby enabling one to target site-specific genes in vivo; (iv) the study of multiple endogenous genes by driving expression of GAL-4-A from one promoter and B-VP-16 from a separate promoter where genes A and B can be chosen so that their respective proteins interact (this procedure should allow the expression of a reporter gene if and only if both promoters are activated); (v) imaging of protein–protein interactions in vivo using inducible two-hybrid mammalian expression systems; and (vi) amplification of both therapeutic and reporter genes by modifying the reporter template.
Acknowledgments
We thank David Stout, Khoi Nguyen, and Xiaoman Lewis for technical assistance. This work is supported in part by National Institutes of Health Grants P50 CA86306 (to S.G.), R0–1 CA82214 (to S.G.), and SAIRP R24 CA92865 (to S.G.), Department of Energy Contract DE-FC03-87ER60615 (to S.G.), and CaP Cure (to S.G. and M.C.).
Abbreviations
- CCD
charge-coupled device
- PET
positron emission tomography
- TSTA
two-step transcriptional amplification (activation)
- CMV
cytomegalovirus
- PSE
prostate-specific antigen
- RLU
relative light units
- fl
firefly luciferase reporter gene
- HSV1
herpes simplex virus type 1
- tk
thymidine kinase
- FL
firefly luciferase enzyme
- HSV1-sr39tk
mutant HSV1 thymidine kinase reporter gene
- HSV1-sr39TK
mutant HSV1 thymidine kinase enzyme
References
- 1.Gambhir S S, Barrio J R, Phelps M E, Iyer M, Namavari M, Satyamurthy N, Wu L, Green L A, Bauer E, MacLaren D C, et al. Proc Natl Acad Sci USA. 1999;96:2333–2338. doi: 10.1073/pnas.96.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tjuvajev J G, Avril N, Oku T, Sasajima T, Miyagawa T, Joshi R, Safer M, Beattie B, DiResta G, Daghighian F, et al. Cancer Res. 1998;58:4333–4341. [PubMed] [Google Scholar]
- 3.Tjuvajev J G, Finn R, Watanabe K, Joshi R, Oku T, Kennedy J, Beattie B, Koutcher J, Larson S, Blasberg R G. Cancer Res. 1996;56:4087–4095. [PubMed] [Google Scholar]
- 4.Zinn K R, Buchsbaum D J, Chaudhari T R, Mountz J M, Grizzle W E, Rogers B E. J Nucl Med. 2000;41:887–895. [PubMed] [Google Scholar]
- 5.Louie A H, Ahrens E T, Rothbacher U, Moats R, Jacobs R E, Fraser S E, Meade T J. Nat Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 6.Weissleder R, Moore A, Mahmood U, Bhorade R, Benveniste H, Chiocca A, Basilion J P. Nat Med. 2000;6:351–355. doi: 10.1038/73219. [DOI] [PubMed] [Google Scholar]
- 7.Contag P R, Olomu I N, Stevenson D K, Contag C H. Nat Med. 1998;4:245–247. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]
- 8.Honigman A, Zeira E, Ohana P, Abramovitz R, Tavor E, Bar I, Zilbermman Y, Rabinovsky R, Gazit D, Joseph A, et al. Mol Ther. 2001;4:239–249. doi: 10.1006/mthe.2001.0437. [DOI] [PubMed] [Google Scholar]
- 9.Wu J C, Sundaresan G, Iyer M, Gambhir S S. Mol Ther. 2001;4:297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- 10.Yang M, Baranov E, Li X M, Wang J W, Jiang P, Li L, Moossa A R, Penman S, Hoffman R M. Proc Natl Acad Sci USA. 2001;98:2616–2621. doi: 10.1073/pnas.051626698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray P, Bauer E, Iyer M, Barrio J R, Satyamurthy N, Phelps M E, Herschman H, Gambhir S S. Semin Nucl Med. 2001;31:312–320. doi: 10.1053/snuc.2001.26209. [DOI] [PubMed] [Google Scholar]
- 12.Cherry S R, Gambhir S S. Inst Lab Anim Res J. 2001;42:219–232. [Google Scholar]
- 13.Cherry S R, Shao Y, Silverman R W, Meadors K, Siegel S, Chatziioannou A, Young J W, Jones W F, Moyers J C, Newport D, et al. IEEE Trans Nucl Sci. 1997;44:1161–1166. [Google Scholar]
- 14.Gambhir S S, Barrio J, Wu L, Iyer M, Namavari M, Satyamurthy N, Bauer E, Parrish C, MacLaren D, Borghei A, et al. J Nucl Med. 1998;39:2003–2011. [PubMed] [Google Scholar]
- 15.MacLaren D C, Gambhir S S, Satyamurthy N, Barrio J R, Sharfstein S, Toyokuni T, Wu L, Berk A J, Cherry S R, Phelps M E, Herschman H R. Gene Ther. 1999;6:785–791. doi: 10.1038/sj.gt.3300877. [DOI] [PubMed] [Google Scholar]
- 16.Iyer M, Barrio J R, Namavari M, Bauer E, Satyamurthy N, Nguyen K, Toyokuni T, Phelps M E, Herschman H R, Gambhir S S. J Nucl Med. 2001;42:96–105. [PubMed] [Google Scholar]
- 17.Gambhir S S, Bauer E, Black M E, Liang Q, Kokoris M S, Barrio J R, Iyer M, Namavari M, Satyamurthy N, Green L A, et al. Proc Natl Acad Sci USA. 2000;97:2785–2790. doi: 10.1073/pnas.97.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson L M, Swaminathan S. Gene Ther. 1999;6:854–864. doi: 10.1038/sj.gt.3300909. [DOI] [PubMed] [Google Scholar]
- 19.Bui L A, Butterfield L H, Kim J Y, Ribas A, Seu P, Lau R, Glaspy J A, McBride W H, Economou J S. Hum Gene Ther. 1997;8:2169–2170. doi: 10.1089/hum.1997.8.18-2173. [DOI] [PubMed] [Google Scholar]
- 20.Wu L, Matherly J, Smallwood A, Belldegrun A S, Carey M. Gene Ther. 2001;8:1416–1426. doi: 10.1038/sj.gt.3301549. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Eastman E M, Schwartz R J, Draghia-Akli R. Nat Biotechnol. 1999;17:241–245. doi: 10.1038/6981. [DOI] [PubMed] [Google Scholar]
- 22.Choi T, Huang M, Gorman C, Jaenisch R. Mol Cell Biol. 1991;11:3070–3074. doi: 10.1128/mcb.11.6.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donello J E, Loeb J E, Hope T J. J Virol. 1998;12:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henley D C, Wir J P. Virus Res. 1991;20:121–132. doi: 10.1016/0168-1702(91)90104-4. [DOI] [PubMed] [Google Scholar]
- 25.Emami K H, Carey M. EMBO J. 1992;11:5005–5012. doi: 10.1002/j.1460-2075.1992.tb05607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadowski I, Ma J, Triezenberg S J, Ptashne M. Nature (London) 1988;335:563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- 27.Nettelbeck D M, Jerome V, Muller R. Trends Genet. 2000;16:174–181. doi: 10.1016/s0168-9525(99)01950-2. [DOI] [PubMed] [Google Scholar]
- 28.Sadowski I. Genet Eng. 1995;17:119–148. [PubMed] [Google Scholar]
- 29.Pang S, Dannull J, Kaboo R, Xie Y, Tso C L, Michel K, deKernion J B, Belldegrun A S. Cancer Res. 1997;57:495–499. [PubMed] [Google Scholar]
- 30.Gill G, Ptashne M. Nature (London) 1988;334:721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- 31.Cleutjens K B, van der Korput H A, Ehren-van Eekelen C C, van Rooij H C, Faber P W, Trapman J. Mol Endocrinol. 1997;11:148–161. doi: 10.1210/mend.11.2.9883. [DOI] [PubMed] [Google Scholar]
- 32.Carey M, Leatherwood J, Ptashne M. Science. 1990;247:710–712. doi: 10.1126/science.2405489. [DOI] [PubMed] [Google Scholar]
- 33.Nettelbeck D M, Jerome V, Muller R. Gene Ther. 1998;5:1656–1664. doi: 10.1038/sj.gt.3300778. [DOI] [PubMed] [Google Scholar]
- 34.Carey M, Lin Y S, Green M R, Ptashne M. Nature (London) 1990;345:361–364. doi: 10.1038/345361a0. [DOI] [PubMed] [Google Scholar]
- 35.Koster R W, Fraser S E. Dev Biol. 2001;233:329–346. doi: 10.1006/dbio.2001.0242. [DOI] [PubMed] [Google Scholar]
- 36.Fang B, Ji L, Bouvet M, Roth J A. J Biol Chem. 1998;273:4972–4975. doi: 10.1074/jbc.273.9.4972. [DOI] [PubMed] [Google Scholar]
- 37.Segawa T, Takebayashi H, Kakehi Y, Yoshida O, Narumiya S, Kakizuka A. Cancer Res. 1998;58:2282–2287. [PubMed] [Google Scholar]
- 38.Braselmann S, Graninger P, Busslinger M. Proc Natl Acad Sci USA. 1993;90:1657–1661. doi: 10.1073/pnas.90.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]