Abstract
Background
Despite the increased morbidity and mortality of radical cystectomy (RC) in elderly individuals with bladder cancer, numerous studies have demonstrated that surgery can provide a survival benefit. We sought to better identify those patients at substantial risk of post-operative mortality.
Methods
We evaluated 220 consecutive patients age 75 years and older treated with RC for bladder cancer at a single institution from 2000-2008. The analytic cohort was comprised of 169 patients with complete pre-operative data. A Cox proportional hazards model was used to determine the value of pre-cystectomy clinical information in predicting 90-day survival post RC. These results were used to create a nomogram predicting the probability of 90-day survival after RC. The model was then subjected to 200 bootstrap resamples for internal validation.
Results
A total of 28/220 patients (12.7%) died within 90 days of surgery. Older age (HR 2.30, 95%CI 1.22-4.32) and lower preoperative albumin (HR 2.50, 95%CI 1.40-4.45) were significant predictors of 90-day mortality. A nomogram based on age, clinical stage, Charlson comorbidity index, and albumin predicting the likelihood of 90-day mortality with an accuracy of 75% was developed. Internal validation demonstrated a bootstrap-adjusted c-index of 71%.
Conclusion
We developed a nomogram which provides individualized risk estimations predicting the probability of 90-day mortality, potentially enhancing pre-operative counseling and providing clinicians with an added tool to individualize treatment decisions in this challenging patient population. These data suggest that albumin is a strong predictor of postoperative mortality and demonstrate the importance of assessing this variable prior to surgery.
Keywords: Bladder Cancer, Cystectomy, Elderly, Nutrition, Age, Albumin, Nomogram
Introduction
With one in five Americans expected be age 65 or older by the year 2030, there is an increasing need to focus on improving the medical care of older individuals.1 Nearly 75% of all bladder cancers occur in this population, and, therefore, age-related factors in the management of bladder cancer are of particular concern.2 In the elderly—generally defined as those 75 years or older—bladder cancer is the fifth-leading cancer diagnosis, and these individuals account for approximately 40% of new bladder cancers.2 Longstanding questions exist regarding the safety and efficacy of radical cystectomy (RC) in this population for the treatment of muscle-invasive and high-risk non-muscle-invasive bladder cancer. Although some evidence suggests that RC may improve survival in elderly patients, there are no direct comparisons between RC and more conservative treatment options, such as endoscopic resection or radiotherapy.3-5 Careful patient selection is imperative to a thoughtful surgical strategy, as there is significant potential for morbidity and/or mortality. Numerous studies have demonstrated that RC can be relatively safely performed in select elderly patients with invasive bladder cancer;6-9 however, balancing the treatment of elderly individuals with bladder cancer against the potential risks of surgery is a challenge. Despite this, there are currently no objective criteria for identifying elderly patients in whom RC can be safely performed.
While clinical decision-making relies largely on subjective judgment, there are limits and inherent biases associated with these judgments.10 Predictive modeling can substantially augment clinical decision-making by decreasing biases and improving the accuracy of clinical counseling. In particular, individualized modeling in the form of multivariate nomograms have been shown to benefit clinical decision-making in prostate cancer and to aid in outcomes prediction post-RC.11-14 We hypothesized that predictive modeling could assist preoperative risk assessment in a medically complex elderly population. With individualized nomograms as an added tool in clinical decision-making, it may be possible to more accurately assess the safety of RC in the elderly. In this study, we sought to identify clinical predictors of 90-day mortality after RC in patients 75 years and older and develop a nomogram to help individualize treatment decisions in this challenging patient population.
Methods
We performed a retrospective cohort study of 220 consecutive patients age 75 years or older who underwent RC for urothelial carcinoma of the bladder at Vanderbilt University Medical Center (VUMC) between January 2000 and June 2008. RC was performed and post-operative care administered as previously described according to the clinical care pathway by Lowrance et al.15 Pathologic specimens were evaluated by a staff surgical pathologist and staged according to the 2002 American Joint Committee on Cancer guidelines.16 Clinical, pathological, and outcome data were collected prospectively and were supplemented by review of the medical records. Institutional Review Board approval was obtained for the creation of a prospective database and for retrospective analysis of this patient population. All patients underwent RC for pure or mixed urothelial carcinoma of the bladder. In general, elderly patients with non-muscle-invasive disease underwent radical cystectomy only after failure of intravesical therapy. Complete patient data was available in 169 of the 220 patients, and these patients represented our analytic cohort for the multivariate analysis.
Patient information including age, sex, race, pre-operative albumin, Charlson comorbidity index (CCI), clinical stage, pathologic stage, and type of urinary diversion were obtained through patient charts. Pre-operative serum albumin was obtained close to the time of surgery, and patients did not receive any specific therapies based on serum album levels. Vital status was ascertained through the VUMC cancer registry, the Social Security Death Index, and patient charts. Patients were censored at the date of last follow-up or date of death up to August 1, 2009.
Statistical analysis
The primary end-point for this study was 90-day mortality. Cox univariate and multivariate regression was performed to determine predictors of 90-day mortality. Hazard ratios (HR) are presented along with their 95% confidence intervals (95% CI). Kaplan-Meier survival curves were generated to compare the unadjusted 90-day mortality by age and pre-operative albumin. Multivariate Cox regression coefficients were used to generate the prognostic nomogram, and the concordance index (c-index) was assessed as a measure of the accuracy of the model.17 The model was then subjected to 200 bootstrap resamples for internal validation. All statistical analyses were conducted using Stata software, Version 11 (Stata, Inc., College Station, TX) and R version 2.10.1 (R Development Core Team, 2008).
Results
The median age of the complete cohort and the analytic cohort were both 78.8 years (IQR 76.9-82.0 years). No patients were lost to follow-up within the 90-day period of this study. An overview of the characteristics of the study cohort is given in Table 1. The majority of patients were male, and 30/169 patients (18%) with a pre-operative serum albumin had an albumin level ≤3.7 g/dL, representing the lowest quartile of patients. A total of 28/220 patients (12.7%) in the complete cohort and 18/169 patients (10.7%) in the analytic cohort died within 90 days of surgery. The 51 patients excluded from the analytic cohort due to incomplete pre-operative information did not differ statistically from the final cohort in terms of age, race, clinical stage, or pathologic stage; however, the excluded patients were more likely to undergo continent urinary diversion (9 [18%] vs. 11 [7%] patients, p=0.016). There were 10 (19%) deaths within 90 days among the excluded patients, which was not statistically different from the analytic cohort (log rank p=0.14).
Table 1.
Distribution of patients according to clinical and pathological variables. The analytic cohort was comprised of all patients with complete preoperative information.
| Complete cohort (n=220) | Analytic cohort (n=169) | |||
|---|---|---|---|---|
| Characteristic | n | %* | n | % |
| Age | ||||
| <80 | 117 | 53% | 105 | 62% |
| ≤80 | 103 | 47% | 64 | 38% |
| Race | ||||
| White | 208 | 95% | 159 | 94% |
| Non-white | 11 | 5% | 10 | 6% |
| Gender | ||||
| Male | 161 | 73% | 122 | 72% |
| Female | 59 | 27% | 47 | 28% |
| Pre-operative albumin (g/dL) | ||||
| ≤3.7 | 30 | 14% | 30 | 18% |
| 3.71-4.09 | 50 | 23% | 50 | 30% |
| 4.1-4.4 | 45 | 20% | 45 | 27% |
| >4.4 | 44 | 20% | 44 | 26% |
| CCI | ||||
| 0-1 | 109 | 50% | 80 | 47% |
| 2-3 | 74 | 34% | 60 | 36% |
| >3 | 37 | 17% | 29 | 17% |
| Clinical stage | ||||
| Non-muscle invasive | 77 | 35% | 65 | 38% |
| Muscle invasive | 143 | 65% | 104 | 62% |
| Neoadjuvant chemotherapy | ||||
| Yes | 2 | 1% | 2 | 1% |
| No | 218 | 99% | 167 | 99% |
| Pathologic T stage | ||||
| T0, Ta, Tis, T1 | 53 | 24% | 40 | 24% |
| T2 | 54 | 25% | 43 | 25% |
| T3 | 76 | 35% | 60 | 36% |
| T4 | 37 | 17% | 26 | 15% |
| Pathologic lymph node status | ||||
| Negative | 136 | 62% | 134 | 79% |
| Positive | 43 | 20% | 34 | 20% |
| Urinary diversion | ||||
| Ileal conduit | 199 | 90% | 157 | 93% |
| Neobladder | 20 | 9% | 11 | 7% |
Percentages may not add up to 100 due to missing data
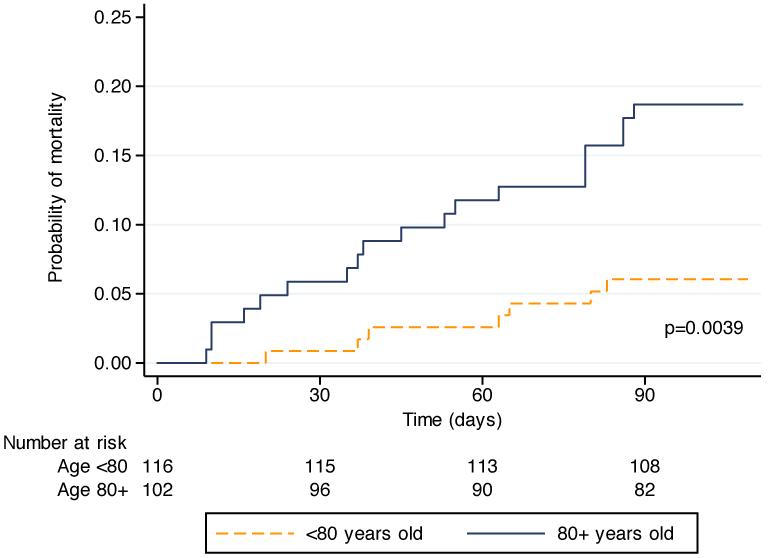
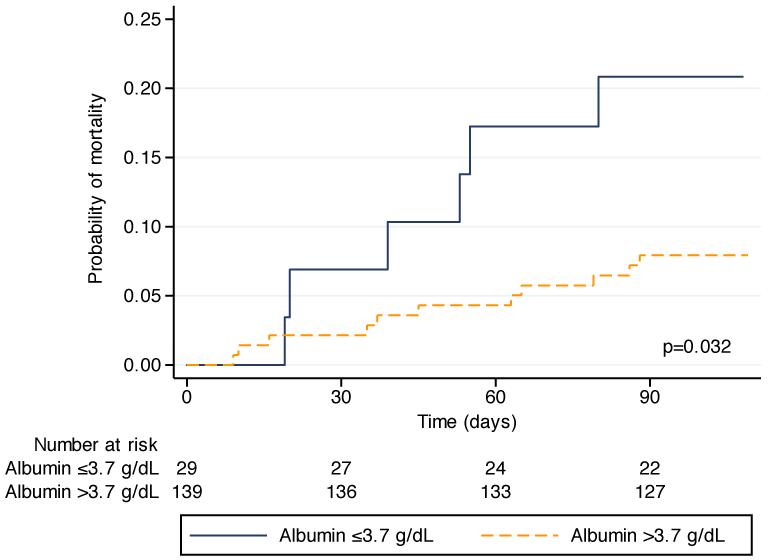
Univariate Cox regression analysis demonstrated that age and pre-operative albumin were significant predictors of 90-day mortality (Table 2). In the Kaplan-Meier analysis for age (Figure 1), patients under 80 years had a significantly lower probability of death within 90 days than those who were 80 years or older (log rank p=0.0039). The Kaplan-Meier analysis for albumin (Figure 2) shows a similar difference, as those patients with a pre-operative serum albumin ≤3.7 g/dL were significantly more likely to die within 90 days than those patients with an albumin >3.7 g/dL (log rank p=0.032). Spearman’s rank correlation test was performed to assess for a correlation between albumin and age, and no correlation was present (p=0.77).
Table 2.
Cox univariate and multivariate regression analysis for 90-day mortality.
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | CI | p | HR | CI | p | |
| Age (76.9, 81.8) | 2.15 | 1.41-3.29 | <0.001 | 2.30 | 1.22-4.32 | 0.010 |
|
| ||||||
| Charlson comorbidity index (0-3) | 1.47 | 0.77-2.83 | 0.25 | 1.30 | 0.53-3.18 | 0.56 |
|
| ||||||
| Clinical stage (muscle invasive) | 1.48 | 0.63-3.54 | 0.37 | 1.55 | 0.55-4.34 | 0.41 |
|
| ||||||
| Pre-operative albumin (4.4, 3.7) | 2.17 | 1.33-3.57 | 0.002 | 2.50 | 1.40-4.45 | 0.002 |
Figure 1.
Kaplan-Meier analysis of mortality up to 90 days from surgery in patients 80 years and older compared to those under 80 years of age (log rank p<0.0039).
Figure 2.
Kaplan-Meier analysis of mortality up to 90 days from surgery in patients with a preoperative serum albumin >3.7 g/dL compared to those with an albumin ≤3.7 g/dL (log rank p=0.0032).
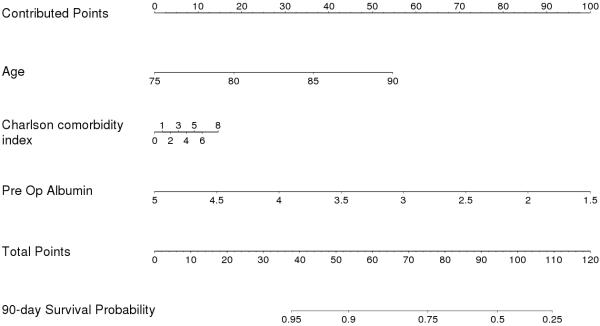
We performed a multivariate analysis with age, CCI, clinical stage, and pre-operative albumin as covariates. In this model, both age and pre-operative albumin remained significant predictors of 90-day mortality (Table 3). Based on this model, a nomogram predicting 90-day mortality was created (Figure 3). The c-index for the model was 0.75, indicative of 75% accuracy. After 200 bootstrap resamples for internal validation, the adjusted c-index was 0.71.
Figure 3.
Preoperative nomogram predicting 90-day mortality in patients ≥75 years of age undergoing radical cystectomy. Points are calculated independently for each of the four variables (“contributed points”), and the sum of the points (“total points”) is used to calculate the 90-day survival probability. For example, an 83 year-old patient with muscle-invasive cancer, CCI of 2, and preoperative albumin of 2.9 g/dL (100 points) would have a 90-day survival probability of 50%.
Discussion
Although several studies have evaluated short-term outcomes in elderly individuals undergoing RC, this is the first report we are aware of to identify pre-operative predictors of post-operative outcome in this patient population. Isbarn et al.,18 in a population based study, reported 90-day mortality rates of 1.2% for those under 60 years of age, 2.3% for patients 61-68 years old, 5.8% for patients 69-83 years old, 7.9% for patients 84-88 years old, and 14.3% for those over 88 years of age. Among patients 75 years or older, thirty-day mortality rates have generally been reported in the range of 3-4%, and 90-day mortality rates are typically in the range of 7-11%.8,19-21 Complication rates are similarly high, ranging from 28-64% in the elderly population. However, 90-day complication rates may be up to 64% in a general cystectomy population.8,19-22 These data suggest that radical cystectomy may be appropriately considered in select elderly patients with bladder cancer.
There is also evidence to support the need for aggressive surgical management in elderly individuals. Stroumbakis et al.5 reported a 25-month median survival in octogenarians undergoing cystectomy—a survival advantage that would not be expected for patients with untreated muscle-invasive bladder cancer. Additionally, 50% of patients in this series died of their disease, demonstrating the lethality of bladder cancer despite their advanced age and medical comorbidities. In a recent population-based series, Lughezzani and colleagues compared cancer-specific mortality to other-cause mortality after RC within specific age groups.4 In 70-79 year-old patients with node-negative muscle-invasive disease, 5-year cancer-specific mortality ranged from 29-43% while other-cause mortality was only 15-18%. Similarly, patients 80 years and older had a cancer-specific mortality of 39-54% compared to 21-25% for other-cause mortality. Furthermore, Donat et al.9 showed that disease-specific mortality after RC is similar between octogenarians and those under 80. While only a prospective study could adequately assess the efficacy of RC compared to alternative treatment strategies, these data provide further evidence that muscle-invasive bladder cancer is often fatal in elderly individuals and support the use of aggressive treatment in appropriately selected patients.
Given the morbidity and mortality associated with RC in this population, we sought to identify pre-operative characteristics in these individuals that may help with appropriate selection of patients. As has been previously observed, when postoperative mortality is assessed out to 90 days, RC is a high-risk procedure in elderly patients. Here, we report a 90-day mortality rate of 12.7% in our overall population. This mortality rate may be a reflection of the high proportion of patients age 80 years and older and our preference for aggressive surgical management of these patients at VUMC. Age and pre-operative serum albumin ≤3.7 g/d were the strongest predictors of early mortality. We were able to construct a nomogram with 71-75% accuracy for predicting 90-day mortality based on age, pre-operative albumin, clinical stage, and CCI. In this model, pre-operative serum albumin was the variable most strongly associated with 90-day mortality, accounting for a 2.5-fold increase in the risk of 90-day mortality for a 0.7 g/dL decrease in serum albumin. While CCI and clinical stage were not independently associated with this elderly patient cohort, their inclusion in the nomogram provided the best fit for the final model.
Numerous studies within the cardiac and general surgery literature have demonstrated an association between albumin and post-operative morbidity and mortality.23-26 While albumin may be a marker of nutritional status, it is also influenced by a number of other factors, such as inflammation and stress. It has a long half-life (20 days) and therefore reflects the influence of these systemic factors over time. In a report on both medical and surgical inpatients, albumin was the only predictor of in-hospital mortality in a model that adjusted for numerous clinical parameters, including overall nutritional status.27 In a retrospective evaluation of all patients undergoing RC at VUMC from 2000-2008, we found nutritional deficiency—defined by pre-operative weight loss, low BMI, and/or hypoalbuminemia—to be a significant predictor of both 90-day mortality and overall survival.28 Additionally, in a large population-based series of patients undergoing RC, Hollenbeck et al.29 found a significant association between low pre-operative albumin and increased 90-day mortality.
Although we report a nomogram developed from a post-operative cohort, it is likely to be applicable in the pre-operative setting based on at least two factors. First, only pre-operative variables were included in the multivariate model. Second, given that RC has been the preferred management approach for elderly patients with muscle-invasive bladder cancer at our institution, only limited numbers of patients generally opt for less aggressive treatment. We do not know the clinical parameters of those patients who underwent alternative treatment, but it is likely that they would represent an even higher risk population. It is important to note, however, that this nomogram predicts 90-day mortality only for those patients who elect surgery.
This study has a number of limitations. The end point of 90-day mortality is a useful, objective endpoint for assessing the risks associated with RC in the elderly, however the number of deaths limits the number of covariates that can be included in the multivariate model. Specifically, there is a concern regarding “overfitting” the model due to the relatively low event rate. We have tried to minimize this by limiting the number of independent variables included in the model to four (age, albumin, CCI, and stage). However, other authors have suggested an event per variable ratio of 10:1 to prevent overfitting of the model.30 To this end, additional study is needed to validate the model in a larger cohort. Moreover, while we controlled for available clinically and statistically significant confounders in our database, unmeasured confounders may have affected the results. Additionally, given the age of the study population, few patients received neoadjuvant chemotherapy, making it impossible to assess the performance of this factor in the model predicting 90-day mortality. It is also important to note that a number of patients in the complete cohort were excluded because they did not have a pre-operative albumin, although the characteristics of these patients were similar to the analytic cohort. While there were more neobladders in the excluded group, possibly indicative of a somewhat healthier subset, the 90-day mortality rates were similar in the excluded and included groups. Therefore, we don’t believe exclusion of these patients affected the study’s results. Although it would be desirable to also evaluate the cause of death in this study, cause of death is difficult to classify and was not reliably available for these patients. Given that overall mortality is a more accurate outcome than disease-specific mortality, particularly within the 90-day period after surgery, overall mortality was the sole outcome measure in this study. Finally, and most importantly, the nomogram was internally validated by bootstrapping—external validation of this model is necessary before it can be recommended for clinical application.
Conclusion
This is the first study we are aware of to utilize pre-operative variables to identify predictors of 90-day mortality and develop a nomogram to assist with pre-operative decision making in elderly individuals undergoing RC. Elderly patients with muscle-invasive or high-risk non-muscle-invasive bladder cancer represent a unique and challenging subset of patients. Weighing the surgical risks versus the potential oncologic benefits is particularly complex in these individuals, and pre-operative counseling needs to convey these facets as accurately as possible. Instruments that are developed and validated from within this subset of bladder cancer patients are going to offer the greatest accuracy for predicting key outcomes in patients 75 years and older undergoing RC. Of particular importance, these data demonstrate the significant role pre-operative serum albumin plays as a predictor of early mortality, emphasizing the need to assess this parameter prior to surgery. We are now beginning a prospective study to assess the impact of pre-operative nutritional status on functional recovery after radical cystectomy.
Given the lethality of muscle-invasive bladder cancer in elderly patients, RC has gained widespread acceptance as a treatment option for these individuals. Hopefully, with further assessment of post-operative outcomes, we will improve our ability to accurately counsel and select patients for radical surgery. Optimally, this nomogram may also allow for individualized alterations in the post-operative clinical care pathway that may ultimately reduce the 90-day mortality, thus improving the quality of care for this select patient population.
Acknowledgements
Supported in part by Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, National Institutes of Health.
Bibliography
- 1.Vincent GK, Velkoff VA, U.S. Census Bureau The Older Population in the United States: 2010 to 2050. 2010 http://www.census.gov/prod/2010pubs/p25-1138.pdf.
- 2.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94:2766. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 3.Hollenbeck BK, Miller DC, Taub D, et al. Aggressive treatment for bladder cancer is associated with improved overall survival among patients 80 years old or older. Urology. 2004;64:292. doi: 10.1016/j.urology.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Lughezzani G, Sun M, Shariat SF, et al. A population-based competing-risks analysis of the survival of patients treated with radical cystectomy for bladder cancer. Cancer. 2011;117:103. doi: 10.1002/cncr.25345. [DOI] [PubMed] [Google Scholar]
- 5.Stroumbakis N, Herr HW, Cookson MS, et al. Radical cystectomy in the octogenarian. J Urol. 1997;158:2113. doi: 10.1016/s0022-5347(01)68171-0. [DOI] [PubMed] [Google Scholar]
- 6.Chang SS, Alberts G, Cookson MS, et al. Radical cystectomy is safe in elderly patients at high risk. J Urol. 2001;166:938. [PubMed] [Google Scholar]
- 7.Clark PE, Stein JP, Groshen SG, et al. Radical cystectomy in the elderly: Comparison of survival between younger and older patients. Cancer. 2005;103:546. doi: 10.1002/cncr.20805. [DOI] [PubMed] [Google Scholar]
- 8.Soulie M, Straub M, Game X, et al. A multicenter study of the morbidity of radical cystectomy in select elderly patients with bladder cancer. J Urol. 2002;167:1325. [PubMed] [Google Scholar]
- 9.Donat SM, Siegrist T, Cronin A, et al. Radical cystectomy in octogenarians--does morbidity outweigh the potential survival benefits? J Urol. 2010;183:2171. doi: 10.1016/j.juro.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Elstein AS. Heuristics and biases: selected errors in clinical reasoning. Acad Med. 1999;74:791. doi: 10.1097/00001888-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol. 2006;24:3967. doi: 10.1200/JCO.2005.05.3884. [DOI] [PubMed] [Google Scholar]
- 12.Karakiewicz PI, Shariat SF, Palapattu GS, et al. Nomogram for predicting disease recurrence after radical cystectomy for transitional cell carcinoma of the bladder. J Urol. 2006;176:1354. doi: 10.1016/j.juro.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Shariat SF, Karakiewicz PI, Palapattu GS, et al. Nomograms provide improved accuracy for predicting survival after radical cystectomy. Clin Cancer Res. 2006;12:6663. doi: 10.1158/1078-0432.CCR-06-0372. [DOI] [PubMed] [Google Scholar]
- 14.Ross PL, Gerigk C, Gonen M, et al. Comparisons of nomograms and urologists’ predictions in prostate cancer. Semin Urol Oncol. 2002;20:82. doi: 10.1053/suro.2002.32490. [DOI] [PubMed] [Google Scholar]
- 15.Lowrance WT, Rumohr JA, Chang SS, et al. Contemporary open radical cystectomy: analysis of perioperative outcomes. J Urol. 2008;179:1313. doi: 10.1016/j.juro.2007.11.084. [DOI] [PubMed] [Google Scholar]
- 16.Green F, Page D, Flemming I, et al. AJCC Cancer Staging Manual. 6th ed Springer; New York: 2002. [Google Scholar]
- 17.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Isbarn H, Jeldres C, Zini L, et al. A population based assessment of perioperative mortality after cystectomy for bladder cancer. J Urol. 2009;182:70. doi: 10.1016/j.juro.2009.02.120. [DOI] [PubMed] [Google Scholar]
- 19.Game X, Soulie M, Seguin P, et al. Radical cystectomy in patients older than 75 years: assessment of morbidity and mortality. Eur Urol. 2001;39:525. doi: 10.1159/000052498. [DOI] [PubMed] [Google Scholar]
- 20.Zebic N, Weinknecht S, Kroepfl D. Radical cystectomy in patients aged > or = 75 years: an updated review of patients treated with curative and palliative intent. BJU Int. 2005;95:1211. doi: 10.1111/j.1464-410X.2005.05507.x. [DOI] [PubMed] [Google Scholar]
- 21.Mendiola FP, Zorn KC, Gofrit ON, et al. Cystectomy in the ninth decade: operative results and long-term survival outcomes. Can J Urol. 2007;14:3628. [PubMed] [Google Scholar]
- 22.Shabsigh A, Korets R, Vora K, et al. Defining Early Morbidity of Radical Cystectomy for Patients with Bladder Cancer Using a Standardized Reporting Methodology. European Urology. 2009;55:164. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160. doi: 10.1016/0002-9610(80)90246-9. [DOI] [PubMed] [Google Scholar]
- 24.Detsky AS, Baker JP, O’Rourke K, et al. Predicting nutrition-associated complications for patients undergoing gastrointestinal surgery. JPEN J Parenter Enteral Nutr. 1987;11:440. doi: 10.1177/0148607187011005440. [DOI] [PubMed] [Google Scholar]
- 25.Gibbs J, Cull W, Henderson W, et al. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 26.Rady MY, Ryan T, Starr NJ. Clinical characteristics of preoperative hypoalbuminemia predict outcome of cardiovascular surgery. JPEN J Parenter Enteral Nutr. 1997;21:81. doi: 10.1177/014860719702100281. [DOI] [PubMed] [Google Scholar]
- 27.Beghetto MG, Luft VC, Mello ED, et al. Accuracy of nutritional assessment tools for predicting adverse hospital outcomes. Nutr Hosp. 2009;24:56. [PubMed] [Google Scholar]
- 28.Gregg JR, Cookson MS, Phillips S, et al. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. 2011;185:90. doi: 10.1016/j.juro.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollenbeck BK, Miller DC, Taub DA, et al. The effects of adjusting for case mix on mortality and length of stay following radical cystectomy. The Journal of Urology. 2006;176:1363. doi: 10.1016/j.juro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Peduzzi P, Concato J, Feinstein AR, et al. IMportance of events per independent variable in proportional hazards regression analysis. II. Accuracy. J Clin Epidemiol. 1995;48:1503. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]