Abstract
The possible role of ultraviolet light in the formation of cataract is not well understood. In this study, guinea pigs were exposed to a chronic, low level of UVA light (0.5 mW cm−2, 340–410 nm wavelength, peak at 365 nm) for 4–5 months. It is known that the lens of the guinea pig possesses unusually high levels of the UVA chromophore NADPH. In a preliminary analysis, it was found that isolated guinea pig corneas transmitted 70–90 % of 340–400 nm light, and that UVA radiation was able to penetrate deep into the nucleus of the guinea pig lens, where it was absorbed. Exposure of guinea pigs to UVA in vivo produced a 60 % inactivation of lens epithelial catalase; however, analysis by transmission electron microscopy (TEM) showed no apparent morphological effects on either the lens epithelium or the cortex. A number of UVA-induced effects were found in the nucleus of the guinea pig lens, but were observed either not at all or to a lesser extent in the cortex. The effects included an increase in light scattering (two-fold; slit-lamp examination), distention of intercellular spaces (TEM), an increase in lipid peroxidation (30–35 %; infrared spectroscopy), a decrease in GSH level (30 %), an increase in protein-thiol mixed disulfide levels (80 %), loss of water-soluble protein (20 %), an increase in the amount of protein disulfide (two-fold; two-dimensional diagonal electrophoresis), degradation of MIP26 (15 %) and loss of cytoskeletal proteins including actin, α- and β- tubulin, vimentin and α-actinin (60–100%). The results indicate that a 4–5 month exposure of guinea pigs to a biologically relevant level of UVA light produces deleterious effects on the central region of the lenses of the animals. UVA radiation, coupled presumably with the photoreactive UVA chromophore NADPH and trace amounts of o2 present in the lens nucleus, produced significant levels of oxidized products in the nuclear region over a five month period. The data demonstrate the potentially harmful nature of UVA light with respect to the lens, and highlight the importance of investigating a possible role for this type of radiation in the formation of human cataract.
Keywords: UVA, lens, cataract, animal model, oxidation, GSH, NADPH, oxygen, catalase, disulfide, cytoskeletal proteins, MIP26, lipid peroxidation
1. Introduction
The role of ultraviolet (UV) light in the development of human maturity-onset cataract is not well-understood. A number of epidemiological studies have linked UV exposure with the formation of cortical cataract, but only for the wavelengths of UVB (280–315 nm) radiation, not UVA (315–400 nm) (Taylor et al., 1988; Cruickshanks et al., 1992). The exclusion of UVA light as a cause of cataract has been challenged by Dillon (1999) who cited a possible flaw in the method that was used to distinguish UVB and UVA, and pointed out that the amount of UVB that reaches the surface of the lens is low compared to that for UVA. Epidemiologists have agreed that there is a need for additional work to determine the contribution of UVA to cataractogenesis (West and Duncan, 1999).
Although there is some support for a link between UV light and nuclear cataract (Zigman et al., 1979; Dolezal et al., 1989; Mohan et al., 1989; Wong et al., 1993), the general consensus among epidemiologists is that there is no such association (Taylor et al., 1988; Cruickshanks et al., 1992; West et al., 1998). This apparent lack of a UV/nuclear cataract relationship is puzzling for those lens researchers who believe that UVA light may contribute to this type of opacity (Zigler and Goosey, 1981; Linetsky and Ortwerth, 1997; Lee et al., 1999; Balasubramanian, 2000). It is known that a substantial amount of UVA light can reach the nucleus of the human lens where it is absorbed by endogenous chromophores (Dillon et al., 1999; Gaillard et al., 2000), and the water-insoluble fraction of old human lenses has been shown to contain a collection of protein-bound UVA sensitizers that can produce potentially damaging amounts of active species of oxygen (Linetsky and Ortwerth, 1995, 1997). There is general acceptance of the concept that oxidative stress is linked with the formation of nuclear cataract (reviewed in Giblin et al., 1995), which is caused in part by the higher level of susceptibility to oxidative damage that exists in the central region of the aging lens (Giblin, 2000; Truscott, 2000). That this stress may be associated with UVA light is suggested by reports that changes induced in lens crystallins by UVA radiation (or by singlet oxygen, which can be generated by light of this wavelength) have been found to be similar to those identified in crystallins from human nuclear cataracts (Zigler and Goosey, 1984; Balasubramanian et al., 1990; Ortwerth and Olesen, 1994).
A wealth of recent literature, much of it arising from research on the skin, has demonstrated the potentially damaging nature of UVA light (Tyrrell, 1991; Godar and Boer, 1992; Evelson et al., 1997; Gasparro, 2000); for example, UVA has been strongly implicated in photoaging of the skin (Hanson and Simon, 1998; Gasparro, 2000) and in the formation of malignant melanoma (Setlow et al., 1993; Moan et al., 1999; Westerdahl et al., 2000). It was formerly believed that UVA radiation was ‘safe’ for human exposure, but this is no longer the case (Godar and Boer, 1992). UVA, the major component of the UV solar spectrum that reaches the earth, is now considered to constitute a significant oxidative stress for human cells (Tyrrell, 1991). Zigman (2000) has compared the current situation in lens research to that which existed in clinical dermatology over 10 years ago, when only UVB was considered as a risk factor for skin damage. Since we know that, in contrast to UVB, a considerable amount of potentially damaging UVA light can penetrate deep into the human lens (Dillon et al., 1999; Gaillard et al., 2000), it is logical to carefully evaluate possible UVA-induced effects on the lens.
The purpose of the present study was to investigate lenticular effects following 4–5 months of exposure of guinea pigs to a physiologically relevant level of UVA light. Guinea pig lenses contain an unusually high level of NADPH (Zigler and Rao, 1991) which may act as a UVA chromophore in lenses of this species, comparable to the action of kynurenine and its related derivatives in the human lens (Dillon, 1991; Wood and Truscott, 1993; Bova et al., 2001). A goal of this work was to investigate whether UVA radiation could actually reach the interior of the guinea pig lens in vivo and cause some type of observable oxidative effect. In addition, it was hoped that the results would provide information regarding the controversy on whether or not sufficient oxygen is present in the lens center to cause damage (if UVA caused oxidation, it would have presumably required molecular oxygen for the generation of active species of oxygen). While the cortex of the guinea pig lens contains very high levels of GSH (over 20 mM), which would most likely be able to protect against UV damage, the nucleus contains low concentrations of this tripeptide (Giblin, 2000), making the lens center more susceptible to UVA-induced effects.
2. Materials and Methods
Male Hartley guinea pigs of two different ages were used in the studies. Most of the experiments were conducted with ‘retired breeder’ animals, initially 16–17 months old, purchased from Kuiper Rabbit Ranch (Indianapolis, IN, U.S.A.) or Elm Hill Breeding Labs (Chelmsford, MA, U.S.A.). One set of experiments used one month old animals obtained from Kingstar (Kingston, NH, U.S.A.). The lenses of the guinea pigs were examined carefully by slit-lamp biomicroscopy prior to the experiments, and animals with cortical or nuclear opacities were excluded. The diet used throughout the study was Purina Guinea Pig Chow 5025 (Purina Mills, Richmond, IN, U.S.A.), which contained 0.1 % ascorbic acid. All of the studies were approved by the Oakland University IACUC. The animals were killed by CO2 asphyxiation.
Optical Properties of the Guinea Pig Cornea and Lens
The measurement of the spectral transmission of isolated corneas and sectioned lenses using a fiberoptic spectrometer (PC 1000; Ocean Optics, Dunedin, FL, U.S.A.) has been described in detail previously (Dillon et al., 1999; Gaillard et al., 2000). Corneas were removed from enucleated eyes of 18 month old guinea pigs by using surgical scissors and cutting into the sclera close to the limbus. Each cornea was placed in a 1 cm path length cuvette filled with balanced salt solution (BSS). The top of the cuvette was sealed with Parafilm so that it could be placed in a horizontal position and light passed through the wall of the cuvette, and then through BSS, the cornea, BSS and the opposite wall of the cuvette to the receiving probe. Methods for sectioning decapsulated lenses into 0.25 mm sections at −10°C have been described (Gaillard et al., 2000). Each resultant slab was placed in a quartz cuvette having a 0.25 mm path length, and the cuvette was then covered and the section allowed to thaw prior to analysis. The fiberoptic spectrometer used the continuous output of a deuterium lamp (approximately 200–700 nm wavelength) as the light source and a high-sensitivity charge-coupled device (CCD) detector mounted on a card in an IBM Pentium 133 computer (IBM, Armonk, NY, U.S.A.). The CCD array detector was capable of collecting full-wavelength spectra with good signal-to-noise ratios at integration times as rapid as 1 msec. Thus, once the dark (0 % transmittance) and reference (100 % transmittance) spectra had been collected and stored, the actual transmission or absorption spectra could be obtained every few milliseconds. Data were analyzed and plotted using Origin 5·0 Microal, Northampton, MA, U.S.A.).
Exposure of Guinea Pigs to UVA Light In vivo
The procedures for exposing guinea pigs to UVA light were patterned after those previously described by Barron et al. (1987 and 1988). Irradiation of the animals was accomplished using Sylvania black light blue fluorescent lamps (F15T8/BLB, Osram Sylvania, Inc., Danvers, MA, U.S.A.) having a 340–410 nm wavelength spectral distribution, with maximum intensity at 365 nm (the power spectrum for the lamps is shown in Fig. 1). Two lamps were suspended from each cage top, such that the animals were never more than 5 cm away from the source. The intensity of the UVA light was measured using a UVX radiometer obtained from Ultra-Violet Products, Inc., (Upland, CA, U.S.A.). The irradiance on the corneas of the animals was approximately 0·5 mW cm−2. The UVA lamps were left on for 24 hr per day with no other lighting in the room. The temperature in the room was maintained at 20°C, and the temperature in each cage was 23–240°C. Guinea pigs housed in this way showed no adverse non-lens effects (no hair loss, skin lesions or corneal damage) except for a slight drying of the outer ear which was treated as needed with a moisturizing skinblock lotion (Coppertone SPF45, Schering-Plough, Inc., Memphis, TN, U.S.A.). Age-matched control animals were housed under normal lighting conditions. Control and experimental animals grew at the same rate over a five month period.
Fig. 1.
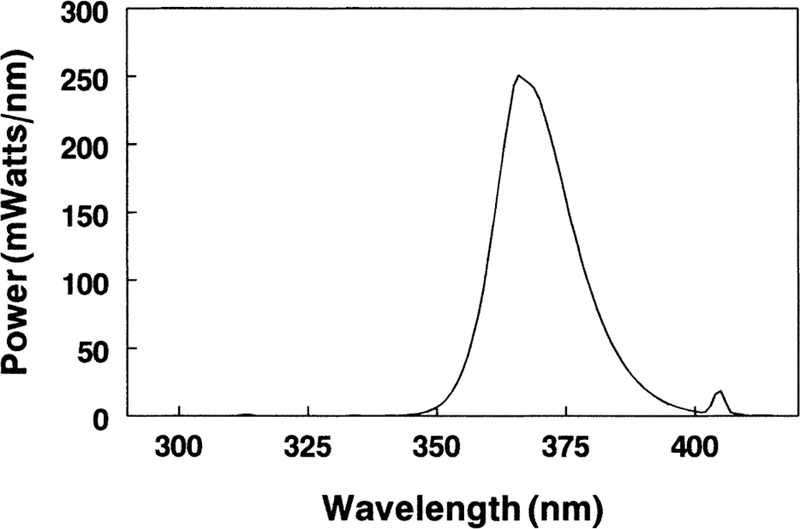
The power spectrum for Sylvania black light blue fluorescent lamps (F15T8/BLB) used in the study (spectrum supplied by Sylvania). The y-axis value of the curve at a given wavelength x is the total m-Watts of power radiated by the lamp in a one nm wide bandwidth centered on the given wavelength x. The wavelength range was from 340 to 410 nm with a maximum emission peak at 365 nm.
The transparency of lenses of control and UVA-exposed guinea pigs was assessed by a single observer (L.-R. Lin, M.D.) using a Zeiss slit-lamp photomicroscope following induction of full mydriasis with tropicamide (1%) and phenylephrine (10%). The results were documented by photography. Lens nuclear light scatter was quantified by using negatives of the slit-lamp photographs and measuring gray scale levels in the lens cortical and nuclear regions. The negatives were scanned onto a Kodak Digital Science compact disc, and image analysis performed using the UTHSCSA Image Tool program (developed at the University of Texas Health Science Center, San Antonio, TX, U.S.A.). Six areas each were measured in the cross-section of the lens cortex and nucleus; areas that were obviously the result of a reflection of the flash were avoided. The six values for each region were averaged and the results were expressed as the percent increase in scatter (brightness) for the lens nucleus compared to that present in the cortex of the same lens. Identical determinations were made for negatives corresponding to control and UVA-exposed animals.
Electron Microscopy
Details for the analysis of guinea pig lens epithelium, cortex and nucleus by transmission electron microscopy (TEM) have been described in detail previously (Giblin et al., 1995). The cited article describes the methods used for fixation, embedding and producing thin sections which were stained with uranyl acetate and lead citrate, and observed with an ISI LEM 2000 transmission electron microscope. The region of the nucleus that was examined was at a site close to the lens center.
Biochemical Analyses
For most of the biochemical analyses, isolated lenses were frozen immediately in dry ice and then stored at −80°C or in liquid N2 until use. Frozen lenses were divided into cortex and nucleus with the use of a cork borer. The nucleus (the inner region) accounted for approximately 25 % of the total weight; the rest of the lens was taken as cortex. Levels of reduced glutathione (GSH) were measured in deproteinized supernatants of lens cortical and nuclear homogenates with the use of Ellman’s reagent (Cui and Lou, 1993). Concentrations of protein-bound glutathione (PSSG) and protein-bound cysteine (PSSC) were determined in trichloroacetic acid (TCA) precipitates of lens cortex and nucleus as described previously (Giblin et al., 1995). Quantitation of PSSG and PSSC was conducted by following the method of Lou and Dickerson (1992). Protein-bound, non-protein thiols that were released from the samples as glutathione sulfonic acid or cysteic acid were separated using a Dionex anion-exchange amino acid analyzer (Dionex, Sunnyvale, CA, U.S.A.). The ninhydrin color reaction of these amino acids was quantified by monitoring their absorbance at a wavelength of 570 nm. Levels of lipid oxidation products in control and experimental lenses were measured with the use of Fourier-transform infrared (FTIR) analysis as described in a recent article (Borchman et al., 2000). The activity of catalase was measured polarographically in lens capsule-epithelial supernatants with the use of a Clark oxygen electrode contained in a Gilson 5/6H Oxygraph (Gilson Medical Electronics, Middleton, WI, U.S.A.) calibrated from 0 to 100% O2 (Giblin et al., 1990). Each capsule-epithelium was homogenized in 1 ml of cold 100 mM Na/K phosphate buffer, pH 7·2; after centrifugation of the homogenate, a 0–8 ml aliquot of the supernatant was analysed for activity of the enzyme in the presence of 10 mM H2O2.
For determining levels of water-soluble (WS) protein in the lens cortex and nucleus, the tissues were homogenized in 0·02 m phosphate buffer, pH 7.0, containing 2 mM EDTA. WS proteins were isolated in the supernatant following centrifugation of the homogenate at 12 000 g for 20 min. Protein concentration was determined with Pierce BCA Protein Assay Reagent using bovine serum albumin as the protein standard. The procedures for analysing urea-insoluble (UI) protein of lens cortex and nucleus by two-dimensional diagonal electrophoresis have been described in detail previously (Padgaonkar et al., 1989), including homogenization of the tissues in a N2-atmosphere in buffer containing EDTA and iodoacetamide to prevent artifactual oxidation of -SH groups. Similarly, the procedures for SDS-PAGE analysis of cytoskeletal proteins in the lens urea-soluble (US) protein fraction and MIP26 in the NaOH-washed, UI fraction have been fully-presented in a previous article (Padgaonkar et al., 1999).
3. Results
As a first step in the study, the spectral transmission and absorption characteristics of isolated guinea pig corneas and lenses were investigated. The light used in the in vivo study (i.e. the light that was incident on the corneas of the guinea pigs) had wavelengths between 340 and 410 nm, with a peak at 365 nm (Fig. 1). The isolated guinea pig cornea was found to transmit no light with wavelengths lower than 285 nm (Fig. 2). About 25% of incident 300 nm light passed through the cornea, while 70–90% of 340–400 nm light was transmitted. For the peak wavelength of 365 nm that was used in the in vivo study, 85 % of that light passed through the cornea. Fig. 3 shows a cumulative absorption spectrum for 0·25 mm slices of an isolated guinea pig lens for incident light between 300 and 400 nm wavelengths, with units on the y-axis expressed as percent of light transmitted to the next layer. The data indicate that almost all incident 300 nm wavelength light was absorbed in the first two layers of the lens (i.e. in the superficial cortex); only 0·5% of 300 nm light that reached the second lens layer was passed on to the third. In contrast, about 50% of 365 nm light that reached layer 2 was transmitted to layer 3. Indeed, 365 nm light was able to reach deep into the nucleus of the guinea pig lens and be absorbed. At layer 8, a region located 2·0 mm into the 5 mm diameter lens, about 5 % of incident 365 nm light was passed on to the next layer.
Fig. 2.
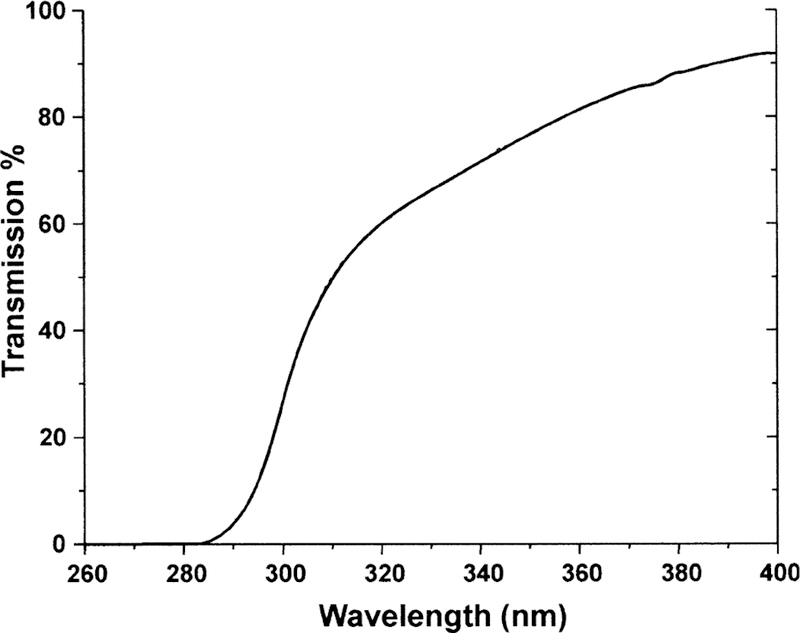
The transmission spectra for an isolated cornea of an 18 month old guinea pig. Note that light having a wavelength <285 nm is not transmitted and that 85 % of 365 nm light is transmitted.
Fig. 3.
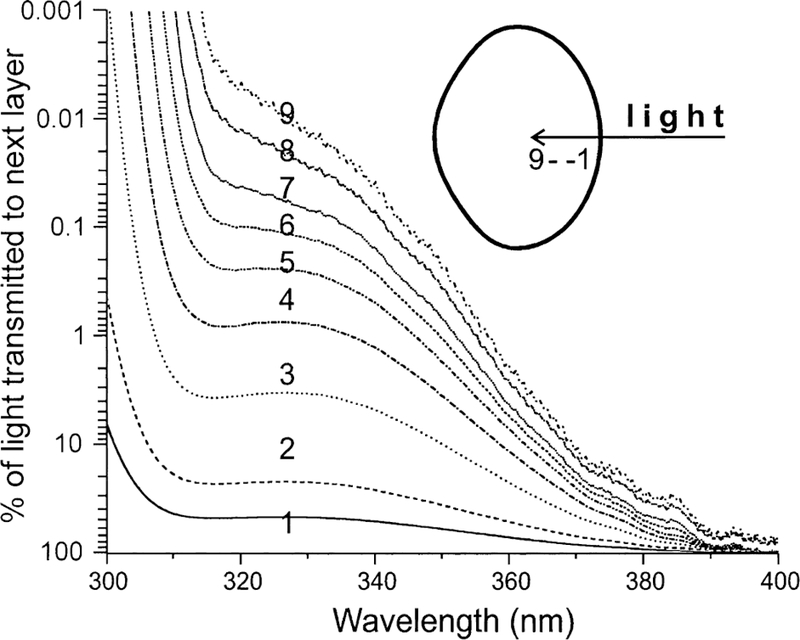
Cumulative absorption spectra along the visual axis of an isolated 18 month old guinea pig lens. The spectra were calculated from individual absorption spectra of 0·25 mm sections made from the anterior to the posterior of the lens. Each of the nine absorption spectra consists of the absorption of that slice plus the sum total of all preceding slices. The diameter of the lens was 5·0 mm. The final spectrum (number 9) represents a section located a distance of 2·25 mm or 45% into the lens. Note that, for 300 nm wavelength light, almost all of the light is absorbed within the first two sections; however, for 365 nm light, about 5 % of the light reaching section 8 (near the lens center) is transmitted to the next section.
We next sought evidence that UVA light in vivo could reach the epithelium of the guinea pig lens and produce an observable effect. For this study, we measured the activity of catalase which is known to be particularly susceptible to UVA-induced inactivation (Kramer and Ames, 1987; Zigman et al., 1996). Lens capsule-epithelia of guinea pigs exposed to UVA light for five months contained nearly 60% less activity of catalase, compared to age-matched controls (Fig. 4). However, a separate analysis of the lens epithelial and cortical regions of UVA-exposed guinea pigs, using TEM, showed no observable damage induced by the UVA light (Fig. 5(A) and (B)); the epithelial and cortical fiber cell morphology and organization were normal. In addition, whole mount preparations of fixed lenses (Reddan et al., 1995) indicated no evidence for cytotoxicity in the UVA-exposed lens epithelial cells (data not shown).
Fig. 4.
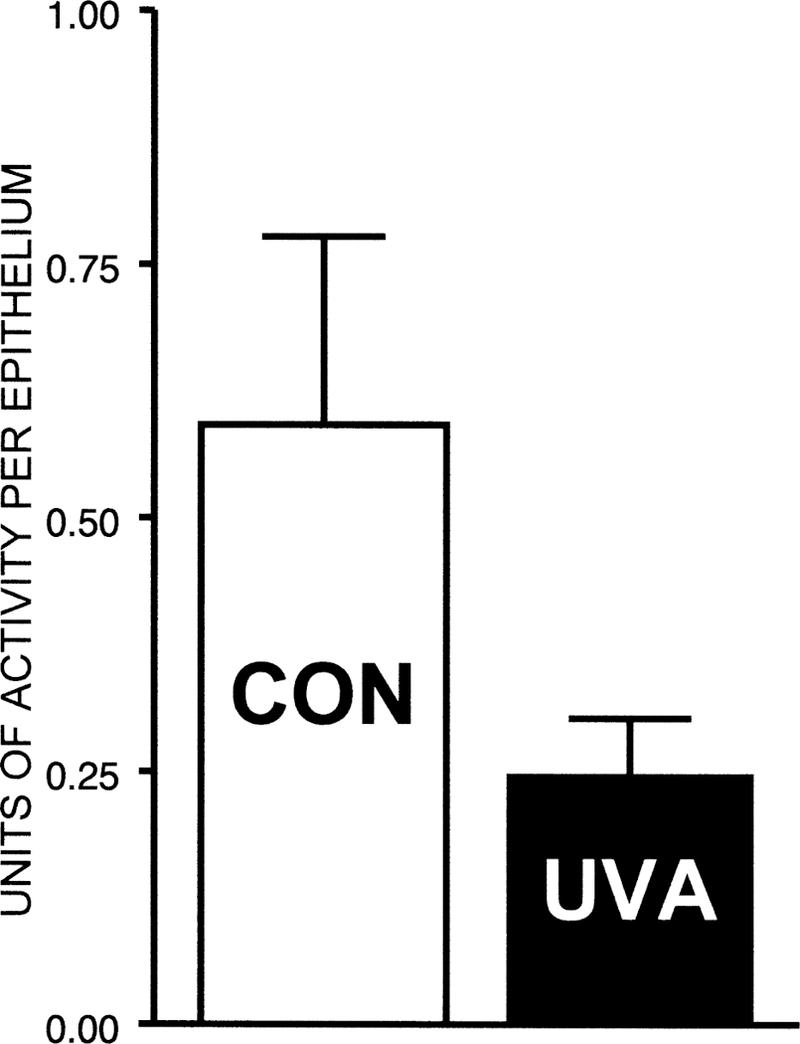
Effect of UVA light in vivo on the activity of catalase in guinea pig lens capsule-epithelium. The animals (initially 18 months old) were exposed to UVA light for five months. One unit of catalase activity corresponds to the conversion of 1 gmol of H2O2 per min at room temperature under optimal conditions in the presence of 10 mM H2O2. Results are expressed as means (±s.d. for four experiments. P = 0·01 for the difference between experimental and age-matched control values.
Fig. 5.
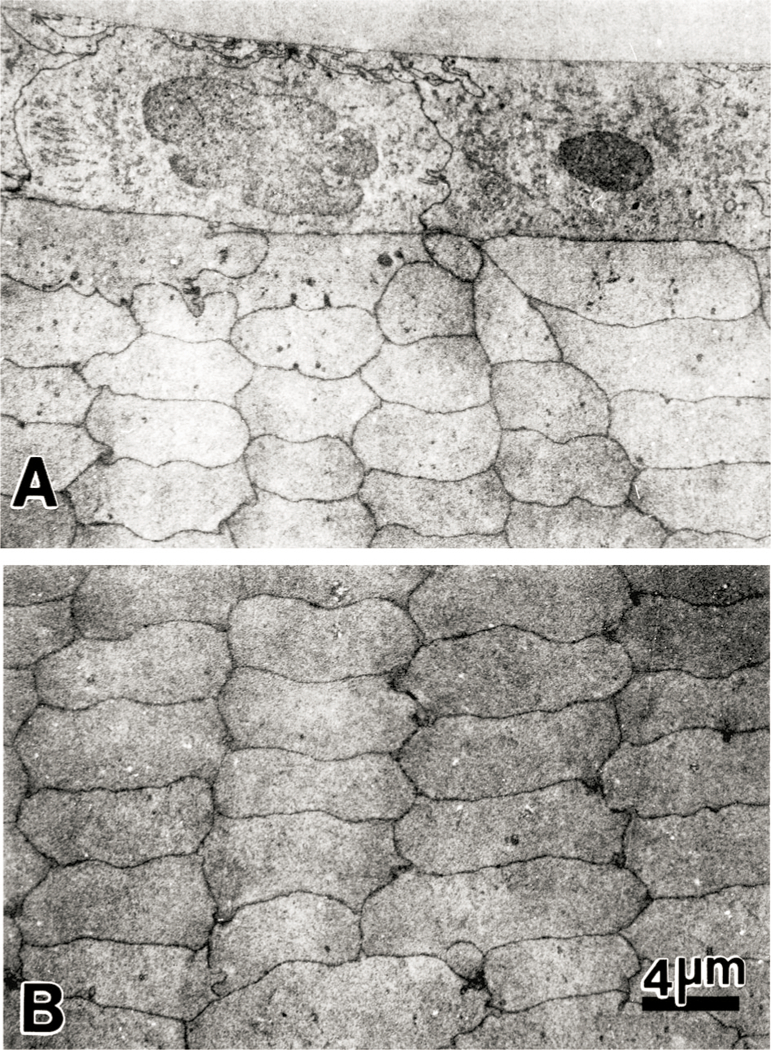
Transmission electron micrographs of the anterior polar region of the lens of a 23 month old guinea pig that had been exposed to UVA light for five months. (A) The epithelial cells appear normal and exhibit no alterations in cellular morphology or cellular organization. (B) Cortical fibers at the anterior pole also show normal organization and morphology. A and B: ×2500.
Levels of nuclear light scattering (NLS) in the lenses of guinea pigs were evaluated with the use of slit-lamp biomicroscopy. Increased NLS was evident in lenses of animals exposed to UVA light for five months, compared to age-matched controls (Fig. 6). The UVA-induced increase in NLS can be described as a nuclear haze in which there was a significantly enhanced demarcation between the lens nucleus and cortex, compared to the control. The lens nucleus of the UVA-exposed guinea pig also appeared to be larger (and the cortex narrower) than that of the controls. However, the loss of nuclear transparency in the lenses of the animals never developed into a distinct cataract; the increased scatter could only be observed with use of the direct optical portion of the slit-lamp, not by using transillumination or by examination of the isolated lens under a microscope. The UVA-induced increase in lens NLS was quantified by scanning negatives of slit-lamp photographs and measuring gray scale levels in the lens cortical and nuclear regions, as described in Materials and Methods. Based on a comparison of gray scale levels, a two-fold increase in lens NLS was observed for the UVA-exposed guinea pigs, compared to age-matched controls (Table I).
Fig. 6.
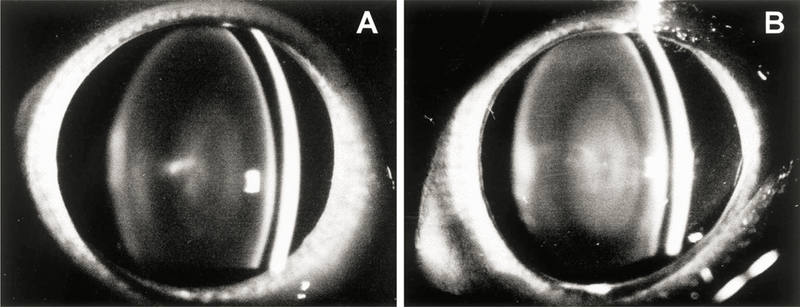
Slit-lamp biomicroscopy photographs of guinea pig eyes. (A) The eye of a 23 month old control animal (B) The eye of a 23 month old animal after five months of exposure to UVA light. Note the increased level of lens nuclear light scattering compared to the control.
Table I.
Effect of UVA light in vivo on the percent increase in gray scale level from the guinea pig lens cortex to the nucleus*
| Percent increase in gray level | |
|---|---|
| Control (10) | 14 ± 4 |
| UVA (4) | 28 ± 5 |
| P = 0·002 |
Guinea pigs were exposed to UVA light for five months and the eyes photographed using slit lamp biomicroscopy (Fig. 6). The percent increase in gray scale level from the lens cortex to the nucleus was determined as described in Materials and Methods for experimental and age-matched control animals (23 months old). Results are expressed as means ±s.d. The number of experiments is in parentheses.
Transmission electron microscopy was used to examine the nuclear regions of control (not exposed to UVA light) and experimental lenses from 23 month old guinea pigs after five months of exposure to UVA (Fig. 7). Lenses of control animals showed normal nuclear fiber cell morphology and organization (Fig. 7(A)). The intercellular spaces between neighboring fibers were uniform and without distension. In comparison, lenses from animals exposed to UVA light for five months showed consistent morphological alterations, specifically in the intercellular spaces between neighboring fiber cells (Fig. 7(B) and (C)). The intercellular spaces were unevenly distended, and this was more pronounced at the Y junctions formed between fibers. At these sites, projections and indentations between adjacent fibers were also evident.
Fig. 7.
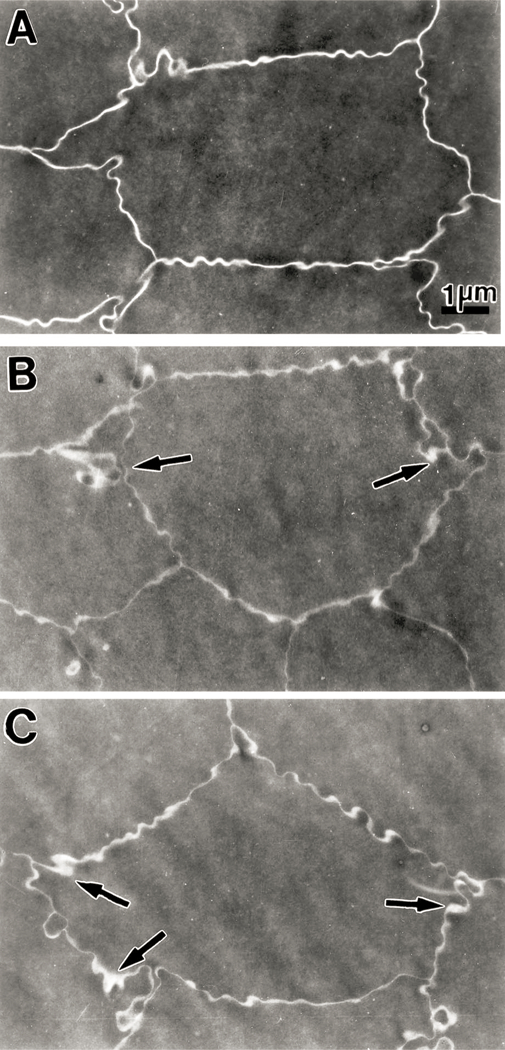
Transmission electron micrographs of the nuclear region (close to the center of the nucleus) of lenses from 23 month old guinea pigs. Micrograph A is from a section of a lens from a control animal (not exposed to UVA light) showing a uniform intercellular space between neighboring fiber cells. Micrographs B and C are from sections of a lens from an animal exposed to UVA light for five months. There is an observable distention of intercellular space between adjacent fiber cells; this is more pronounced at the Y junctions between neighboring fiber cells (arrows). Moreover, some projections and indentations between fibers are also observable at these junctions. A, B and C: × 9000.
As a means of further investigating effects on lens fiber cell membranes of guinea pigs exposed to UVA light, we used Fourier-transform infrared spectroscopy to measure levels of lens lipid oxidation products. When lipids become oxidized, cis double bonds of the hydrophobic chains rearrange to form trans double bonds (Borchman and Yappert, 1998). Guinea pigs exposed to UVA light for five months showed a 35 % decrease in the level of lens nuclear cis double bonds compared to controls (P < 0·01, Fig. 8(A)). No significant effects on the level of cis double bonds were evident in the cortex (P < 0·1). The intensity of OH stretching bands (3600–3100 cm−1) reflects the degree of oxidation present in a lipid sample (Dugan et al., 1949), as well as the amount of hydroxyl-containing lipids such as sphingolipids and cholesterol- The level of lipid hydroxyl was elevated by 30 % in the lens nucleus of UVA-exposed guinea pigs (P = 0·04, Fig. 8(B)). Surprisingly, and possibly due to some type of UVA-induced lipid compositional change, the UVA light produced a 35 % decrease in lens cortical hydroxyl (P < 0·01).
Fig. 8.
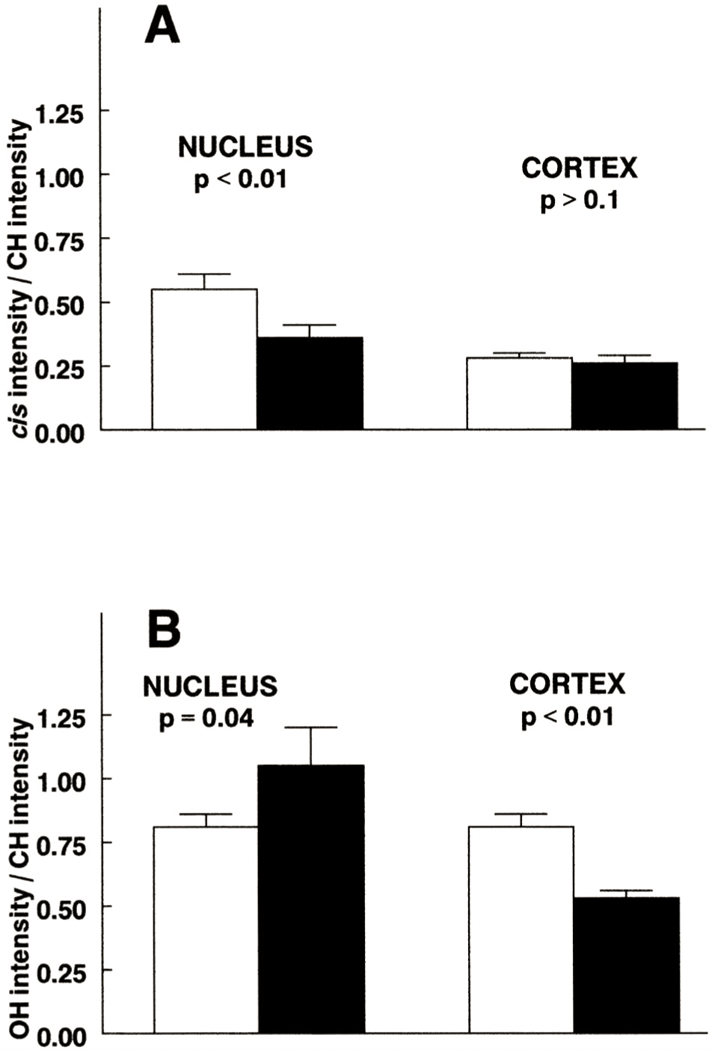
Effect of UVA light in vivo on the intensity of the cis C=C (A) and OH (B) stretching bands for lipid of the guinea pig lens. The animals (initially 18 months old) were exposed to UVA (solid bars) light for five months. Values were ratioed to the CH symmetric stretching-band intensity to quantitate the amount of oxidation relative to the amount of lipid. Results are expressed as means ±SEM with n = 23–28 for aged-matched controls (open bars) and n = 8–10 for experimental.
An analysis of the concentrations of GSH present in lens cortex and nucleus was conducted after 4–5 months of exposure of guinea pigs to UVA light (Table II). The level of cortical GSH was found to be high in both control and experimental lenses (14–15 µmol g−1 wet weight, or nearly 23 mM). Compared to the cortex, the concentration of GSH in the nucleus of the control lens was relatively low (2·4 µmol g−1 wet weight), about 16 % of the cortical value. The UVA exposure produced a small, but significant (P = 0·03), decrease of 6 % in the level of cortical GSH, compared to control (Table II). The UVA-induced loss of GSH in the lens nucleus was greater than that observed for the cortex, approaching nearly 30 % (P = 0·01). Concentrations of the protein-thiol mixed disulfides PSSG and PSSC were measured in the same lenses that were used for the analyses of GSH. The UVA light was found to produce a 67% increase in the level of cortical PSSG (P = 0·01), with no effect on the concentration of cortical PSSC. Levels of nuclear PSSG and PSSC were both increased by 80% (P = 0·01 and 0·0001, respectively) by exposure of the animals to UVA light for 4–5 months.
Table II.
Levels of reduced glutathione (GSH), protein bound glutathione (PSSG) and protein bound cysteine (PSSC) in lenses of guinea pigs exposed to UVA light for 4–5 months*
| GSH | PSSG | PSSC | |
|---|---|---|---|
| Cortex | |||
| Control | 15·3 ± 1·4 | 0·03 ± 0·01 | 0·02 ± 0·01 |
| UVA | 14·4 ± 0·8 | 0·05 ± 0·03 | 0·02 ± 0·01 |
| P = 0·03 | P = 0·01 | P > 0·5 | |
| Nucleus | |||
| Control | 2·4 ± 0·8 | 0·10 ± 0·07 | 0·24 ± 0·05 |
| UVA | 1·7 ± 0·5 | 0·18 ± 0·07 | 0·43 ± 0·12 |
| P = 0·01 | P = 0·01 | P = 0·0001 | |
Two separate studies consisting of four and five month exposures to UVA light were conducted, and the data were pooled. Measurements were made with trichloroacetaic acid precipitates. The units are µmol/g wet weight and the results are expressed as means ± s.d. with an n of 11–14. Aged-matched control animals (22 and 23 months old) were housed under normal lighting conditions.
The level of WS protein in the lens nucleus of UVA-exposed guinea pigs decreased by 20% compared to controls (P < 0·001, Table III); however, no significant protein loss occurred in the cortex. Two dimensional diagonal electrophoresis was used to investigate disulfide-crosslinking of proteins in the UI fraction of guinea pig lenses (Fig. 9). In this technique, crosslinked proteins are indicated by the presence of spots to the left of the diagonal in the electrophoretic profile. The spots correspond to polypeptides which remain disulfide-linked in the first dimension of electrophoresis, but become reduced in the second dimension after treatment with mercaptoethanol. Scanning of the electrophoretic profiles indicated an approximate doubling in the amount of PSSP in the lens nucleus of the UVA-exposed guinea pig, at molecular weights of 22, 28 and 38 kDa, compared to the age-matched control (Fig. 9(B) compared to Fig. 9(A)). An increase in protein to the right of the diagonal at molecular weights of 26 and 38 kDa was also evident for the experimental nucleus compared to the control. This effect is caused by a slower migration of protein in the second dimension following reduction of disulfide. Reduction of either intramolecular (rather than intermolecular) disulfide or protein-thiol mixed disulfide can produce this result. In contrast to the experimental nucleus, no increase in protein spots to either the left or right of the diagonal were observed for either the control or experimental cortex (Fig. 9(C) and (D)).
Table III.
Levels of water-soluble protein in lenses of guinea pigs exposed to UVA light for five months*
| water-soluble protein mg/g wet wt. | ||
|---|---|---|
| Nucleus | Cortex | |
| Control | 343 + 28 | 320 ± 28 |
| UVA | 276 ± 22 | 318 ± 15 |
| P < 0·001 | P > 0·5 | |
Results are expressed as means ± S.D. with an n of 6. Aged-matched control animals (23 months old) were housed under normal lighting conditions.
Fig. 9.
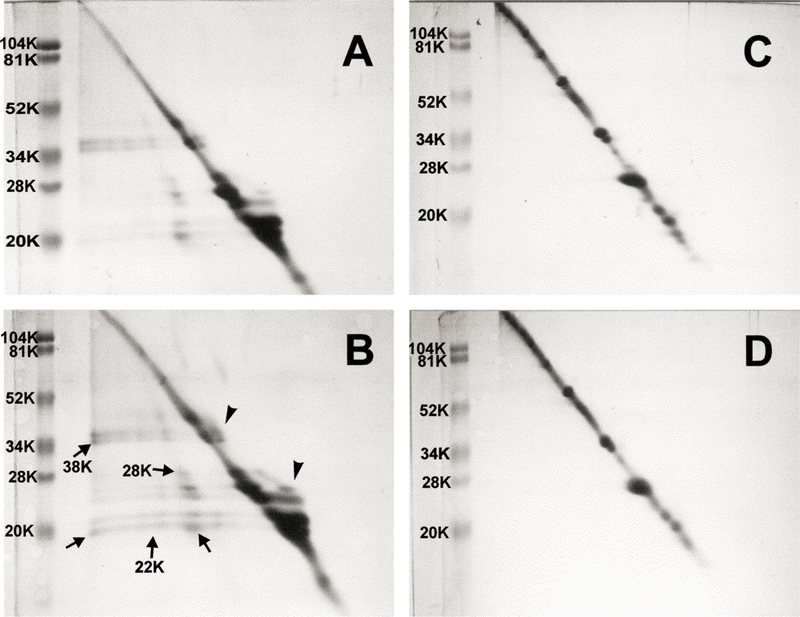
Two-dimensional diagonal SDS-PAGE patterns of urea-insoluble protein fractions of guinea pig lens nucleus and cortex. Samples of 120 µg protein were run on tube gels in the first dimension without prior treatment with mercaptoethanol. The tube gels were incubated for 30 min in buffer containing mercaptoethanol and applied on the second dimension. The presence of off-diagonal spots (arrows) indicates disulfide-crosslinked proteins. Also note the protein which appears to the right of the diagonal (arrowheads). (A) Lens nucleus from a 23 month old control animal. (B) Lens nucleus from a 23 month old animal after five months of exposure to UVA light. (C) Lens cortex from a 23 month old control animal. (D) Lens cortex from a 23 month old animal after five months of exposure to UVA light.
We next investigated effects of UVA light on the levels of MIP26 in the guinea pig lens. For this analysis, lens nuclear and cortical UI pellets were washed once with 0.1 M NaOH prior to SDS-PAGE (Padgaonkar et al., 1999). Scanning of the resultant electrophoretic profile (Fig. 10) indicated that five months of UVA exposure produced a 15 % decrease in the amount of lens nuclear MIP26, compared to the age-matched control. In addition, bands of degraded MIP at molecular weights of 24 kD and 22 kDa were apparent in the gel. In contrast to the results for the lens nucleus, no UVA-induced loss of MIP26 was observed for the lens cortex (Fig. 10).
Fig. 10.
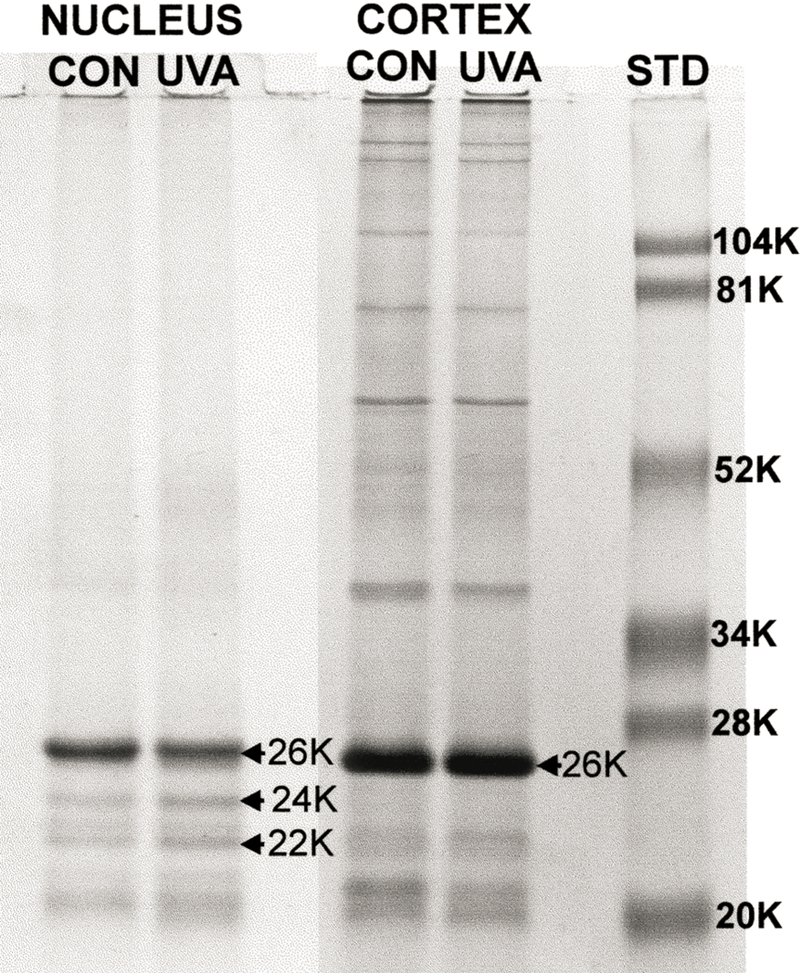
SDS-PAGE for lens nuclear and cortical urea-insoluble proteins from 23 month old control guinea pigs and 23 month old guinea pigs which had been exposed to UVA light for five months. For each analysis, 15 µg of protein was applied to the gel. For the nucleus, note the UVA-induced loss of MIP26, as well as the increased formation of MIP24 and MIP22. Far right: MW markers.
In the final study, we investigated the effect of in vivo UVA exposure on the levels of cytoskeletal proteins present in guinea pig lenses. For this work, we exposed younger animals, initially one month old, to UVA light for five months, and then used SDS-PAGE to analyse proteins present in the US nuclear and cortical protein fractions. Scanning of the electrophoretic profiles showed that the UVA light caused a 60–100 % loss of nuclear proteins at bands with molecular weights of 43 (60% loss), 51 (60% loss), 58 (78 % loss) and 90 (100% loss) kDa (Fig. 11). Based on prior immunoblot procedures conducted with guinea pig lenses (Padgaonkar et al., 1999), it was determined that the four bands corresponded to the cytoskeletal proteins actin, β-tubulin, α-tubulin and/or vimentin (the proteins co-migrate at 58 kDa), and α-actinin, respectively. No comparable UVA-induced loss of cortical cytoskeletal proteins was observed (Fig. 11).
Fig. 11.
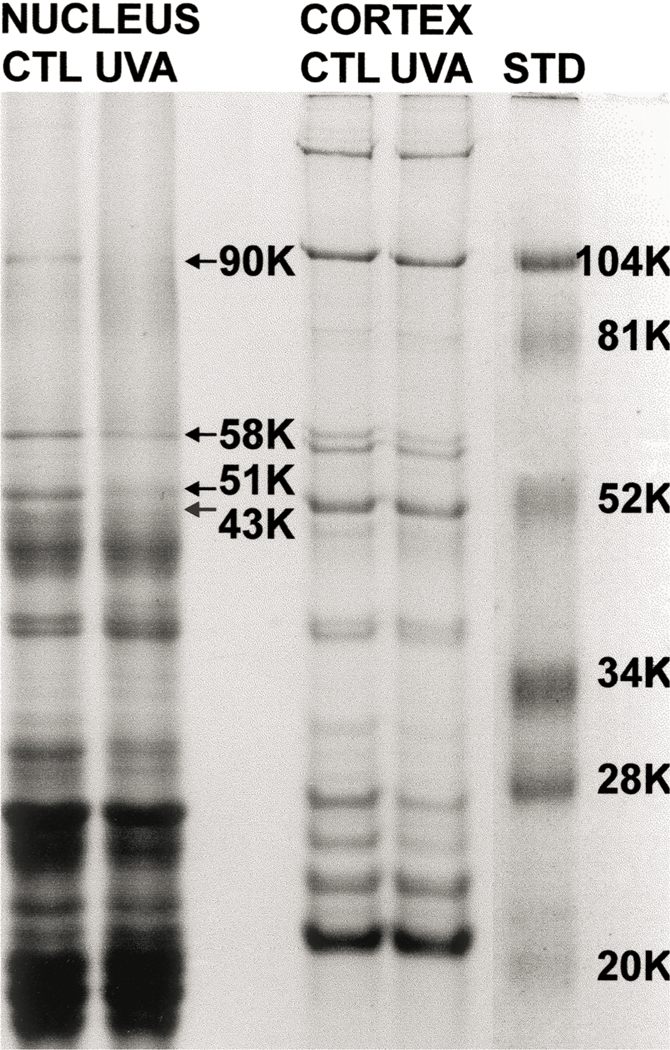
SDS-PAGE for lens nuclear and cortical urea-soluble proteins from 6 month old control guinea pigs and 6 month old guinea pigs which had been exposed to UVA light for five months. Arrows indicate the changes in band densities for four nuclear cytoskeletal proteins. The amount of protein applied to the gel was 40 and 20 µg for nuclear and cortical samples, respectively. Far right: MW markers.
4. Discussion
We have shown that a 4–5 month exposure of older guinea pigs to a low, biologically relevant level of UVA light produces deleterious effects on the central region of the lenses of the animals. The intensity of the UVA light used in the study, 0.5 mW cm−2, is about one-tenth the level of ambient UVA present in sunlight in New York City at noon on a clear summer day (Merriam, 1996). The present model was patterned after that used by Barron et al. (1987 and 1988) who treated weanling guinea pigs with UVA light for nine months. Those investigators described a nuclear haze present in the lenses of the treated animals and showed, with the use of Raman spectroscopy, a loss of -SH and an increase in -SS- in the experimental lens nucleus, compared to controls. Similar to the present study, the authors observed the UVA-induced effects primarily in the lens nucleus. In a separate investigation (Bergbauer et al., 1991), a 15 month exposure of guinea pigs to UVA light produced, surprisingly, a distinct yellowing of the animals’ lenses. The yellowing, along with an increase in the level of -SS-, could be prevented in the contralateral lens of an animal with the use of a UV-blocking contact lens. In our study, we were not able to detect any yellowing of guinea pig lenses after five months of exposure of the animals to UVA. The studies described above confirm that a physiological level of UVA light can indeed be harmful to the lens.
Our finding of a primary localization of UVA-induced effects in the nucleus of the guinea pig lens is in contrast to similar studies conducted with squirrels. Zigman et al. (1991) produced cataractous changes in the central epithelium and subcapsular cortex of lenses of squirrels exposed to UVA light for one year. Relatively, fewer changes were seen in thedeep cortex and nucleus. We found no morphological effects on either the lens epithelium or cortex of guinea pigs treated with UVA light for five months. Reasons for this marked difference in the two studies are not clear. The previous investigation used a different type of UVA lamp than we did, BL compared to BLB, and the UVA irradiance on the eyes of the animals, 6·8 mW cm−2, although still within the physiological range, was about 10 times higher than ours. The higher level of UVA light could have led to a more complete inactivation of lens epithelial catalase and Na-K ATPase (see below), possibly resulting in epithelial and cortical damage. The disparity in the results between UVA-exposed squirrels and guinea pigs cannot be attributed to any differences in lens GSH levels for the two species; both animals contain substantially higher levels of the tripeptide in the lens cortex than in the nucleus. Also, lenses of the squirrel, in contrast to the guinea pig, and similar to human, contain kynurenine-type compounds which act as UVA chromophores (Zigman and Paxhia, 1988). Kynurenine derivatives are believed to be photoprotective in the lens (Dillon et al., 1990; Wood and Truscott, 1993), in contrast to photoreactive NADPH, and thus may have been able to protect the squirrel lens nucleus from UVA-induced damage. The results of Zigman et al. (1991) are actually more in line with epidemiological findings that show more of a link of UV light with human cortical cataracts than with nuclear opacities (see Introduction).
The peak wavelength of light used in this study, 365 nm, was absorbed throughout the anterior region of the guinea pig lens and into the nucleus (Fig. 3). The major chromophore for the light was most likely NADPH which absorbs from 300 to 400 nm with a peak at 340 nm (Bergmeyer, 1974), and is known to be present in the guinea pig lens at a high level of nearly 2 mM, primarily bound to zeta crystallin (Rao and Zigler, 1990). Our results suggest that NADPH may be acting as a UVA sensitizer in the guinea pig lens, since exposure of the tissue to UVA in vivo produced deleterious effects. The absorption of UVA by NADPH is known to cause the formation of active species of O2, including superoxide anion (Cunningham et al., 1985) and H2O2 (Czochralska et al., 1984). Photoexcitation of NADPH has been linked to damaging effects observed in cultured lens epithelial cells exposed to UVA light (Atherton et al., 1999). It is clear that by absorbing UVA light, lenticular NADPH would assist in protecting the retina from UVA-induced potential damage. Some investigators have proposed that NADPH would also protect the lens from photodamage (Krishna et al., 1991; Rao and Zigler, 1992); however, the results of this study do not support such a conclusion. It is of interest that the lenses of camels which also contain zeta crystalline (Garland et al., 1991) and, presumably, high levels of NADPH, have been reported to develop senile nuclear cataract (Duhaiman, 1988).
We found the enzyme catalase to be inactivated by 60 % in the lens epithelia of guinea pigs following five months of UVA exposure (Fig. 4). Catalase is highly susceptible to UVA-induced inhibition (Kramer and Ames, 1987; Zigman et al., 1996; Zigman et al., 2000), and because of its tightly bound heme and NADPH groups, it has been referred to as an endogenous cellular photosensitizer for UVA radiation (Kramer and Ames, 1987). Apparently, the remaining 40 % activity of catalase in the lenses of UVA-exposed animals was sufficient to protect the lens epithelial cells against damage, since no morphological effects were observed in the anterior region of the lenses (Fig. 5). UVA-induced damage to cultured lens epithelial cells, at a 1·25 mW cm−2 level of the light, has been associated with a 95 % inactivation of catalase (Zigman et al., 1995). Na-K ATPase activity, which was not analyzed in the present study, is another lens epithelial enzyme that is preferentially affected by UVA light (Zigman et al., 1995; Dovrat and Weinreb, 1999).
Although the cortex of the guinea pig lens absorbed much more of the incident UVA light than did the nucleus (Fig. 3), deleterious effects of the in vivo UVA exposure were observed primarily in the nucleus. This observation is undoubtedly linked with the seven-fold higher level of GSH present in the cortex, compared to the nucleus (Table II). The relative concentrations of NADPH in the guinea pig lens cortex and nucleus are not known; however, the level of NADPH in the rabbit lens nucleus is less than one-half that in the cortex (Giblin and Reddy, 1980). The ability of GSH to protect against damaging effects of UVA radiation is well-documented (Tyrrell and Pidoux, 1988; Godar et al., 1993; Tobi et al., 2000). The fact that GSH protects against UVA, but does not absorb the light directly, implicates a UVA-induced generation of active oxygen species. However, GSH is a better scavenger of free radicals when it is present at concentrations > 5 mM; at levels close to 1 mM, GSH can actually aid in the generation of radical species (Rowley and Halliwell, 1982). The guinea pig lens cortex, which possesses a level of GSH > 20 mM, is well-protected against UVA light. However, in contrast, the cortex of the aging human lens, as well as its nucleus, have GSH levels < 3 mM (Truscott and Augusteyn, 1977b; Lou andDickerson, 1992; Bova et al., 2001), similar to that of the guinea pig lens nucleus, and might thus be susceptible to UVA-induced damage. Analyses of human nuclear cataracts have revealed nuclear GSH levels of about 1 mM or less, depending on the severity (Truscott and Augusteyn, 1977b; Lou et al., 1999), indicating an increased likelihood for oxidative damage in that region.
The two-fold increase in the concentrations of PSSG and PSSC in the lens nucleus of the UVA-exposed animals (Table II) provides clear evidence of the ability of UVA radiation to produce oxidative stress in the center of the lens. A similar loss of GSH and increase in mixed disulfide levels have been shown to correlate with increases in the color and density of human nuclear cataracts (Truscott and Augusteyn, 1977b; Lou et al., 1999). The production of lens nuclear PSSG and PSSC in this UVA model was less pronounced than that observed following extended treatment of guinea pigs with hyperbaric oxygen (Giblin et al., 1995). It was not possible in the present study to fully account for the observed 0·7 µmol g−1 wet weight decrease in lens nuclear GSH (Table II) by a corresponding increase in nuclear PSSG (0·08 µmol g−1 wet weight). It is possible that oxidized glutathione (GSSG), which was not analyzed in this study, accumulated in the lens nuclei in this model, or that UVA light was able to induce a degradation of GSH. Increases in the levels of lens PSSG and PSSC have also been observed following the exposure of squirrels to UVA light (Zigman et al., 1991). However, similar to the finding that loss of lens transparency occurred more in the cortex than in the nucleus of the squirrel/UVA model, as described above, increases in the levels of mixed disulfides were also more pronounced in the lens cortex of the UVA-exposed squirrel, than in the nucleus.
UVA light in vivo was shown to oxidize guinea pig lens nuclear lipids (Fig. 8) and affect the morphology of lens nuclear membranes (Fig. 7). The observed increase in intercellular space between nuclear fiber cells would presumably contribute to the increased level of nuclear light scattering seen in the lenses of the UVA-exposed animals (Fig. 6). Similar to the results for PSSG and PSSC, the UVA-induced effects on lens nuclear lipids and membranes were not as severe as those observed after five months of treatment of guinea pigs with hyperbaric oxygen (Giblin et al., 1995; Borchman et al., 2000). The ability of UVA light to oxidize lipids and damage membranes has been well-documented by previous in vitro studies (Chamberlain and Moss, 1987; Bose et al., 1989; Tyrrell, 1991). There is considerable evidence to support the involvement of singlet oxygen and catalytic metals in the oxidation of lipids by UVA (Vile and Tyrrell, 1995; Shih and Hu, 1999; Tyrrell, 2000). Although we do not know whether UVA light in this study was able to generate singlet oxygen within the guinea pig lens, there are some data to indicate that absorption of UVA by NADPH can produce singlet oxygen (Czochralska et al., 1984), in addition to the superoxide anion and H202 which were mentioned earlier. Singlet oxygen has also been found to be the major active species of oxygen produced by the UVA irradiation of human lens proteins (Linetsky and Ortwerth, 1997). Indeed, UVA has been shown to peroxidize lipids even in the absence of a photosensitizer (Bose et al., 1989), indicating that singlet oxygen might be generated through direct UVA excitation of molecular oxygen.
Our combined results showing UVA-induced increases in PSSG and PSSC (Table II), PSSP (Fig. 9) and oxidized lipids (Fig. 8) in the guinea pig lens nucleus provide supportive evidence for the presence of sufficient molecular oxygen within the lens center to produce oxidative stress. It has been argued by some investigators, including Harding (1991), that the oxygen tension present within the lens nucleus would be too low to support oxidation. However, the formation of UVA-induced oxidized products in the lens nucleus, as documented in this study, would be expected to require molecular oxygen. Most UVA-mediated biological events have been shown to be dependent on the presence of oxygen (Tyrrell, 2000). We have shown that a low level of UVA radiation coupled with presumably trace amounts of O2 in the lens nucleus can, over five months of time, produce a significant increase in the levels of oxidized products.
It was shown that up to a 100% loss of the cytoskeletal proteins actin, α- and β-tubulins, vimentin and α-actinin occurred in the lens nucleus, but not in the cortex, of one month old guinea pigs exposed to UVA light for five months (Fig. 11). This is the first in vivo study to link UVA light with a loss of lens cytoskeletal proteins; a similar loss of these proteins is known to take place in the aging lens, particularly in the nucleus as cortical fibers compact into the central region (Alcala, 1985). The fact that oxidative stress in vivo also causes this type of effect on lens cytoskeletal proteins (Padgaonkar et al., 1999) provides further support for the belief that UVA can generate active species of oxygen intracellularly. A number of studies with cultured cells have demonstrated the susceptibility of the cytoskeleton to damage by UVA radiation, including damage to microtubules and microfilaments (Godar and Boer, 1992; Malorni et al., 1994; Rafferty et al., 1997). An investigation conducted with Chinese hamster fibroblasts (Banrud et al., 1999) found that while biologically-relevant fluences of UVA were able to disintegrate actin filaments in the cells, even lethal fluences of UVB light had no effect on the proteins. Studies with cultured lenses have also indicated distinct damaging effects of UVA light on cytoskeletal proteins (Rafferty et al., 1993; Zigman et al., 1996; Weinreb et al., 2001); however, these studies investigated only damage to proteins present in the lens epithelium and cortex. The fluences of UVA light used in the cultured lens work were at least 5–10 times higher than ours, which may be why the previous investigators were able to induce UVA damage in the epithelium and cortex, whereas we could not. High levels of GSH present in the epithelium and cortex of the guinea pig lens were apparently able to protect cytoskeletal proteins against the 0·5 mW cm−2 level of UVA used in the study; however, light that was able to reach the nucleus caused cytoskeletal damage as a result of lower levels of GSH.
The observed UVA-induced loss of WS protein and the degradation of MIP26, occurring in the lens nucleus, but not the cortex (Table III and Fig. 10), provide additional evidence to link UVA light with an acceleration of aging in the lens. Light of this wave length is known to cause photoaging of the skin (Hanson and Simon, 1998). Loss of WS protein in the lens nucleus is one of the most common features of lens aging and is, indeed, a hallmark in the formation of nuclear cataract (Young, 1991; Truscott and Augusteyn, 1977a). The 20% decrease in the level of nuclear WS protein observed in this study, after five months of UVA exposure, is comparable to that seen in guinea pigs after two and half months of intermittent hyperbaric oxygen treatment (Giblin et al., 1995). Degradation of MIP26 to MIP22, is also a well-recognized event in the aging lens (Roy, 1979), and is significantly more prevalent in the nucleus than the cortex (Horwitz et al., 1979). Weinreb et al. (2001) exposed cultured bovine lenses to UVA light and also reported a degradation of MIP26, occurring, however, in the cortex of the lenses (the nucleus was not analysed); this prior study used a UVA fluence that was about 20 times that of our in vivo model which may have produced the cortical effects.
How the results of this study might relate to the development of human maturity-onset cataract is not entirely clear. The guinea pig lens contains 100 times higher levels of the UVA chromophore NADPH than the human lens (Zigler and Rao, 1991); however, the human lens is known to contain other UVA chromophores (Dillon, 1991; Wood and Truscott, 1993; Linetsky and Ortwerth, 1997), and can absorb UVA light deep into the nucleus (Gaillard et al., 2000). A key point, as mentioned earlier, may be that, in contrast to NADPH, which is photoreactive, the UVA chromophore kynurenine and its derivatives present in human (as well as squirrel) lenses, are ‘benign photosensitizers’ believed to act as protective UV filters in the lens (Dillon et al., 1990; Wood and Truscott, 1993). The most abundant of the kynurenine UV filters in the young human lens is 3-hydro-xykynurenine glucoside at a level of 0·75 mM (Bova et al., 2001), which compares to nearly 2 mM for the level of NADPH in the guinea pig lens (Rao and Zigler, 1990). Our results have indicated, indirectly, the presence of a sufficient level of O2 in the center of the guinea pig lens to participate in UVA-induced damage; however, whether this is also true for the human lens, is not known. The level of O2 in the center of the rabbit lens has been measured using an in vivo fluorescent dye technique, and found to be 1·5–2·0 % (Liang et al., 2002). It is clear from the present study that under certain conditions, UVA light can be damaging to the lens and induce a loss of transparency. This potential for damage makes it important to continue to investigate a possible role for UVA radiation in the formation of human cataract.
Acknowledgements
The authors thank Ann Dunlop for the long term care of the guinea pigs, and we appreciate the help of students Amy Komendera and Chris Supina with setting up the animal cages for UVA light exposure. We also thank Dr John Reddan for the preparation of lens epithelial whole mounts. The work was supported by Natural Institutes of Health Research Grants EY0202 7 (F.J.G.), EY00484 (V.N.R.), EY05230 (Core Grant for Vision Research) and a grant from Research to Prevent Blindness (Kellogg Eye Center). The study was part of the Cooperative Cataract Research Group (CCRG) program.
References
- Alcala JMH (1985). Biochemistry of lens plasma membranes and cytoskeleton. In The Ocular Lens: Structure, Function, and Pathology (Maisel H, Ed.) pp. 169–222. Marcel Dekker: New York. [Google Scholar]
- Atherton SJ, Lambert C, Schultz J, Williams N and Zigman S (1999). Fluorescence studies of lens epithelial cells and their constituents. Photochem. Photobiol 70, 823–8. [PubMed] [Google Scholar]
- Balasubramanian D (2000). Ultraviolet radiation and cataract. J. Ocul. Pharmacol. Ther 16, 285–97. [DOI] [PubMed] [Google Scholar]
- Balasubramanian D, Du S and Zigler JS (1990). The reaction of singlet oxygen with proteins, with special reference to crystallins. Photochem. Photobiol 52, 761–8. [DOI] [PubMed] [Google Scholar]
- Banrud H, Moan J and Berg K (1999). Early induction of binucleated cells by ultraviolet A (UVA) radiation: a possible role of microfilaments. Photochem. Photobiol 70, 199–205. [PubMed] [Google Scholar]
- Barron BC, Yu NT and Kuck JF Jr. (1987). Tryptophan Raman/457·9-nm-excited fluorescence of intact guinea pig lenses in aging and ultraviolet light. Invest. Ophthalmol. Vis. Sci 28, 815–21. [PubMed] [Google Scholar]
- Barron BC, Yu NT and Kuck JF Jr. (1988). Raman spectroscopic evaluation of aging and long-wave UV exposure in the guinea pig lens: a possible model for human aging. Exp. Eye Res 46, 249–58. [DOI] [PubMed] [Google Scholar]
- Bergbauer KL, Kuck JF Jr., Su KC and Yu N-T (1991). Use of a UV-blocking contact lens in evaluation of UV-induced damage to the guinea pig lens. Int. Contact Lens Clin 18, 182–6. [Google Scholar]
- Bergmeyer HU (1974). General Introduction. In Methods of Enzymatic Analysis (Bergmeyer HU, Ed.) pp. 103–21. Verlag Chemie Weinheim: London. [Google Scholar]
- Borchman D and Yappert MC (1998). Age-related lipid oxidation in human lenses. Invest. Ophthalmol. Vis. Sci 39, 1053–8. [PubMed] [Google Scholar]
- Borchman D, Giblin FJ, Leverenz VR, Reddy VN, Lin LR, Yappert MC, Tang D and Li L (2000). Impact of aging and hyperbaric oxygen in vivo on guinea pig lens lipids and nuclear light scatter. Invest. Ophthalmol. Vis. Sci 41, 3061–73. [PubMed] [Google Scholar]
- Bose B, Agarwal S and Chatterjee SN (1989). UV-A induced lipid peroxidation in liposomal membrane. Radiat. Environ. Biophys 28, 59–65. [DOI] [PubMed] [Google Scholar]
- Bova LM, Sweeney MH, Jamie JF and Truscott RJ (2001). Major changes in human ocular UV protection with age. Invest. Ophthalmol. Vis. Sci 42, 200–5. [PubMed] [Google Scholar]
- Chamberlain J and Moss SH (1987). Lipid peroxidation and other membrane damage produced in Escherichia coli K1060 by near-UV radiation and deuterium oxide. Photochem. Photobiol 45, 625–30. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Klein BE and Klein R (1992). Ultraviolet light exposure and lens opacities: the Beaver Dam Eye Study. Am. J. Public Health 82, 1658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui XL and Lou MF (1993). The effect and recovery of long-term H2O2 exposure on lens morphology and biochemistry. Exp. Eye Res 57, 157–67. [DOI] [PubMed] [Google Scholar]
- Cunningham ML, Johnson JS, Giovanazzi SM and Peak MJ (1985). Photosensitized production of superoxide anion by monochromatic (290–405 nm) ultraviolet irradiation of NADH and NADPH coenzymes. Photochem. Photobiol 42, 125–8. [DOI] [PubMed] [Google Scholar]
- Czochralska B, Kawczynski W, Bartosz G and Shugar D (1984). Oxidation of excited-state NADH and NAD dimer in aqueous medium involvement of O2– as a mediator in the presence of oxygen. Biochim. Biophys. Acta 801, 403–9. [Google Scholar]
- Dillon J (1991). The photophysics and photobiology of the eye. J. Photochem. Photobiol. B 10, 23–40. [DOI] [PubMed] [Google Scholar]
- Dillon J (1999). Sunlight exposure and cataract. JAMA 281, 229. [PubMed] [Google Scholar]
- Dillon J, Wang RH and Atherton SJ (1990). Photochemical and photophysical studies on human lens constituents. Photochem. Photobiol 52, 849–54. [DOI] [PubMed] [Google Scholar]
- Dillon J, Zheng L, Merriam JC and Gaillard ER (1999). The optical properties of the anterior segment of the eye: implications for cortical cataract. Exp. Eye Res 68, 785–95. [DOI] [PubMed] [Google Scholar]
- Dolezal JM, Perkins ES and Wallace RB (1989). Sunlight, skin sensitivity, and senile cataract. Am. J. Epidemiol 129, 559–68. [DOI] [PubMed] [Google Scholar]
- Dovrat A and Weinreb O (1999). Effects of UV-A radiation on lens epithelial NaK-ATPase in organ culture. Invest. Ophthalmol. Vis. Sci 40, 1616–20. [PubMed] [Google Scholar]
- Dugan LR, Beadle BW and Henick AS (1949). An infrared absorption study of autoxidized methyl linoleate. J. Am. Oil Chem. Soc 26, 681–5. [Google Scholar]
- Duhaiman AS (1988). Purification and some properties of camel lens crystallins. Curr. Eye Res 7, 871–6. [DOI] [PubMed] [Google Scholar]
- Evelson P, Ordonez CP, Llesuy S and Boveris A (1997). Oxidative stress and in vivo chemiluminescence in mouse skin exposed to UVA radiation. J. Photochem. Photobiol. B 38, 215–9. [DOI] [PubMed] [Google Scholar]
- Gaillard ER, Zheng L, Merriam JC and Dillon J (2000). Age-related changes in the absorption characteristics of the primate lens. Invest. Ophthalmol. Vis. Sci 41,1454–9. [PubMed] [Google Scholar]
- Garland D, Rao PV, Del Corso A, Mura U and Zigler JS Jr. (1991). zeta-Crystallin is a major protein in the lens of Camelus dromedarius. Arch. Biochem. Biophys 285,134–6. [DOI] [PubMed] [Google Scholar]
- Gasparro FP (2000). Sunscreens, skin photobiology, and skin cancer: the need for UVA protection and evaluation of efficacy. Environ. Health Perspect 108(Supplement 1): 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin FJ (2000). Glutathione: a vital lens antioxidant. J. Ocul. Pharmacol. Ther 16, 121–35. [DOI] [PubMed] [Google Scholar]
- Giblin FJ and Reddy VN (1980). Pyridine nucleotides in ocular tissues as determined by the cycling assay. Exp. Eye Res 31, 601–9. [DOI] [PubMed] [Google Scholar]
- Giblin FJ, Reddan JR, Schrimscher L, Dziedzic DC and Reddy VN (1990). The relative roles of the glutathione redox cycle and catalase in the detoxification of H2O2 by cultured rabbit lens epithelial cells. Exp. Eye Res 50, 795–804. [DOI] [PubMed] [Google Scholar]
- Giblin FJ, Padgaonkar VA, Leverenz VR, Lin LR, Lou MF, Unakar NJ, Dang L, Dickerson JE Jr. and Reddy VN (1995). Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp. Eye Res 60, 219–35. [DOI] [PubMed] [Google Scholar]
- Godar DE and Boer JZ (1992). UVA1-induced anuclear damage in mammaIian cells. In Biological Responses to Ultraviolet A Radiation (Urbach F, Ed.) pp. 65–73. Valdenmar Publishing: Overland Park, KS. [Google Scholar]
- Godar DE, Thomas DP, Miller SA and Lee W (1993). Long-wavelength UVA radiation induces oxidative stress, cytoskeletal damage and hemolysis. Photochem. Photobiol 57, 1018–26. [DOI] [PubMed] [Google Scholar]
- Hanson KM and Simon JD (1998). Epidermal trans-urocanic acid and the UV-A-induced photoaging of the skin. Proc. Nat. Acad. Sci. U.S.A 95, 105 76–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J (1991). Cataract: Biochemistry, Epidemiology and Pharmacology, pp 180–1. Chapman and Hall: New York. [Google Scholar]
- Horwitz J, Robertson NP, Wong MM, Zigler JS and Kinoshita JH (1979). Some properties of lens plasma membrane polypeptides isolated from normal human lenses. Exp. Eye Res 28, 359–65. [DOI] [PubMed] [Google Scholar]
- Kramer GF and Ames BN (1987). Oxidative mechanisms of toxicity of low-intensity near-UV light in Salmonella typhimurium. J. Bacteriol 169, 2259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna CM, Uppuluri S, Riesz P, Zigler JS Jr. and Balasubramanian D (1991). A study of the photodynamic efficiencies of some eye lens constituents. Photochem. Photobiol 54, 51–8. [DOI] [PubMed] [Google Scholar]
- Lee KW, Meyer N and Ortwerth BJ (1999). Chromatographic comparison of the UVA sensitizers present in brunescent cataracts and in calf lens proteins ascorbylated in vitro. Exp. Eye Res 69, 3 75–84. [DOI] [PubMed] [Google Scholar]
- Liang J, Barazabetto I, Zheng L and Dillon J (2002). Oxygen tension in rabbit vitreous and lens. Assoc. Res. Vis. Ophthal Abstracts 2002. [Google Scholar]
- Linetsky M and Ortwerth BJ (1995). The generation of hydrogen peroxide by the UVA irradiation of human lens proteins. Photochem. Photobiol 62, 87–93. [DOI] [PubMed] [Google Scholar]
- Linetsky M and Ortwerth BJ (1997). Quantitation of the singlet oxygen produced by UVA irradiation of human lens proteins. Photochem. Photobiol. B 65, 522–9. [DOI] [PubMed] [Google Scholar]
- Lou MF and Dickerson JE (1992). Protein-thiol mixed disulfides in human lens. Exp. Eye Res 55, 889–96. [DOI] [PubMed] [Google Scholar]
- Lou MF, Dickerson JE Jr., Tung WH, Wolfe JK and Chylack LT Jr. (1999). Correlation of nuclear color and opalescence with protein S-thiolation in human lenses. Exp. Eye Res 68, 547–52. [DOI] [PubMed] [Google Scholar]
- Malorni W, Donelli G, Straface E, Santini MT, Paradisi S and Giacomoni PU (1994). Both UVA and UVB induce cytoskeIeton-dependent surface blebbing in epidermoid cells. J. Photochem. Photobiol 26, 265–70. [DOI] [PubMed] [Google Scholar]
- Merriam JC (1996). The concentration of light in the human lens. Trans. Am. Ophthalmol. Soc 94, 803–918. [PMC free article] [PubMed] [Google Scholar]
- Moan J, Dahlback A and Setlow RB (1999). Epidemiological support for an hypothesis for melanoma induction indicating a role for UVA radiation. Photochem. Photobiol 70, 243–7. [PubMed] [Google Scholar]
- Mohan M, Sperduto RD, Angra SK, Milton RC, Mathur RL, Underwood BA, Jaffery N, Pandya CB, Chhabra VK and Vajpayee RB (1989). India-US case-control study of age-related cataracts. India-US Case- Control Study Group. Arch. Ophthalmol 107, 670–6 (published erratum appears in Arch Ophthalmol 1989:107:1288). [DOI] [PubMed] [Google Scholar]
- Ortwerth BJ and Olesen PR (1994). UVA photolysis using the protein-bound sensitizers present in human lens. Photochem. Photobiol 60, 53–60. [DOI] [PubMed] [Google Scholar]
- Padgaonkar V, Giblin FJ and Reddy VN (1989). Disulfide cross-linking of urea-insoluble proteins in rabbit lenses treated with hyperbaric oxygen. Exp. Eye Res 49, 887–99. [DOI] [PubMed] [Google Scholar]
- Padgaonkar VA, Lin LR, Leverenz VR, Rinke A, Reddy VN and Giblin FJ (1999). Hyperbaric oxygen in vivo accelerates the loss of cytoskeletal proteins and MIP26 in guinea pig lens nucleus. Exp. Eye Res 68, 493–504. [DOI] [PubMed] [Google Scholar]
- Rafferty NS, Zigman S, McDaniel T and Scholz DL (1993). Near-UV radiation disrupts filamentous actin in lens epithelial cells. Cell Motil. Cytoskeleton 26, 40–8. [DOI] [PubMed] [Google Scholar]
- Rafferty NS, Rafferty KA and Zigman S (1997). Comparative response to UV irradiation of cytoskeletal elements in rabbit and skate lens epithelial cells. Curr. Eye Res 16, 310–9. [DOI] [PubMed] [Google Scholar]
- Rao PV and Zigler JS (1990). Extremely high levels of NADPH in guinea pig lens: correlation with zeta-crystallin concentration. Biochem. Biophys. Res. Commun 167, 1221–8. [DOI] [PubMed] [Google Scholar]
- Rao CM and Zigler JS (1992). Levels of reduced pyridine nucleotides and lens photodamage. Photochem. Photobiol 56, 523–8. [DOI] [PubMed] [Google Scholar]
- Reddan JR, Giblin FJ, Dziedzic DC, Wirebaugh BM and Peters JL (1995). Hydrogen peroxide affects specific epithelial subpopulations in cultured rabbit lenses. Invest. Ophthalmol. Vis. Sci 36, 289–99. [PubMed] [Google Scholar]
- Rowley DA and Halliwell B (1982). Superoxide-dependent formation of hydroxyl radicals in the presence of thiol compounds. FEBS Lett 138, 33–6. [DOI] [PubMed] [Google Scholar]
- Roy D (1979). Age dependent changes in the abundance of the major polypeptides of human lens membrane. Biochem. Biophys. Res. Commun 88, 30–6. [DOI] [PubMed] [Google Scholar]
- Setlow RB, Grist E, Thompson K and Woodhead AD (1993). Wavelengths effective in induction of malignant melanoma. Proc. Nat. Acad. Sci. U.S.A 90, 6666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih MK and Hu ML (1999). Relative roles of metal ions and singlet oxygen in UVA-induced liposomal peroxidation. J. Inorg. Biochem 77, 225–30. [Google Scholar]
- Taylor HR, West SK, Rosenthal FS, Munoz B, Newland HS, Abbey H and Emmett EA (1988). Effect of ultraviolet radiation on cataract formation. N. Engl. J. Med 319, 1429–33. [DOI] [PubMed] [Google Scholar]
- Tobi SE, Paul N and McMillan TJ (2000). Glutathione modulates the level of free radicals produced in UVA-irradiated cells. J. Photochem. Photobiol. B 57, 102–12. [DOI] [PubMed] [Google Scholar]
- Truscott RJ (2000). Age-related nuclear cataract: A lens transport problem. Ophthal. Res 32, 185–94. [DOI] [PubMed] [Google Scholar]
- Truscott RJ and Augusteyn RC (1977a). Changes in human lens proteins during nuclear cataract formation. Exp. Eye Res 24, 159–70. [DOI] [PubMed] [Google Scholar]
- Truscott RJ and Augusteyn RC (1977b). The state of sulphydryl groups in normal and cataractous human lenses. Exp. Eye Res 25, 139–48. [DOI] [PubMed] [Google Scholar]
- Tyrrell RM (1991). UVA (320–380 nm) radiation as an oxidative stress. In Oxidative Stress, Oxidants and Antioxidants (Sies H, Ed.) pp. 57–83. Academic Press: San Diego, CA. [Google Scholar]
- Tyrrell RM (2000). Role for singlet oxygen in biological effects of ultraviolet A radiation. Methods Enzymol 319, 290–6. [DOI] [PubMed] [Google Scholar]
- Tyrrell RM and Pidoux M (1988). Correlation between endogenous glutathione content and sensitivity of cultured human skin cells to radiation at defined wavelengths in the solar ultraviolet range. Photochem. Photobiol 47, 405–12. [DOI] [PubMed] [Google Scholar]
- Vile GF and Tyrrell RM (1995). UVA radiation-induced oxidative damage to lipids and proteins in vitro and in human skin fibroblasts is dependent on iron and singlet oxygen. Free Radic. Biol. Med 18, 721–30. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Dovrat A, Dunia I, Benedetti EL and Bloemendal H (2001). UV-A-related alterations of young and adult lens water-insoluble alpha-crystallin, plasma membranous and cytoskeletal proteins. Eur. J. Biochem 268, 536–43. [DOI] [PubMed] [Google Scholar]
- West SK and Duncan DD (1999). Sunlight exposure and cataract (letter to editor). JAMA 281, 230.9918474 [Google Scholar]
- West SK, Duncan DD, Munoz B, Rubin GS, Fried LP, Bandeen-Roche K and Schein OD (1998). Sunlight exposure and risk of lens opacities in a population-based study: the Salisbury Eye Evaluation project. JAMA 280, 714–8. [DOI] [PubMed] [Google Scholar]
- Westerdahl J, Ingvar C, Masback A, Jonsson N and Olsson H (2000). Risk of cutaneous malignant melanoma in relation to use of sunbeds: further evidence for UV-A carcinogenicity. Br. J. Cancer 82, 1593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L, Ho SC, Coggon D, Cruddas AM, Hwang CH, Ho CP, Robertshaw AM and MacDonald DM (1993). Sunlight exposure, antioxidant status, and cataract in Hong Kong fishermen. J. Epidemiol. Commun. Health 47, 46–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AM and Truscott RJ (1993). UV filters in human lenses: tryptophan catabolism. Exp. Eye Res 56, 317–25. [DOI] [PubMed] [Google Scholar]
- Young RW (1991). Age-related cataract pp. 47 Oxford Press: New York. [Google Scholar]
- Zigler JS and Goosey JD (1981). Photosensitized oxidation in the ocular lens: evidence for photosensitizers endogenous to the human lens. Photochem. Photobiol 33, 869–74. [DOI] [PubMed] [Google Scholar]
- Zigler JS and Goosey JD (1984). Singlet oxygen as a possible factor in human senile nuclear cataract development. Curr. Eye Res 3, 59–65. [DOI] [PubMed] [Google Scholar]
- Zigler JS and Rao PV (1991). Enzyme/crystallins and extremely high pyridine nucleotide levels in the eye lens. FASEB J 5, 223–5. [DOI] [PubMed] [Google Scholar]
- Zigman S (2000). Lens UVA photobiology. J. Ocul. Pharmacol. Ther 16, 161–5. [DOI] [PubMed] [Google Scholar]
- Zigman S and Paxhia T (1988). The nature and properties of squirrel lens yellow pigment. Exp. Eye Res 47, 819–24. [DOI] [PubMed] [Google Scholar]
- Zigman S, Datiles M and Torczynski E (1979). Sunlight and human cataracts. Invest. Ophthalmol. Vis. Sci 18, 462–7. [PubMed] [Google Scholar]
- Zigman S, Paxhia T, McDaniel T, Lou MF and Yu NT (1991). Effect of chronic near-ultraviolet radiation on the gray squirrel lens in vivo. Invest. Ophthalmol. Vis. Sci 32, 1723–32. [PubMed] [Google Scholar]
- Zigman S, McDaniel T, Schultz JB, Reddan J and Meydani M (1995). Damage to cultured lens epithelial cells of squirrels and rabbits by UV-A (99·9 %) plus UV-B (0·1 %) radiation and alpha tocopherol protection. Molec. Cell Biochem 143, 35–46. [DOI] [PubMed] [Google Scholar]
- Zigman S, Reddan J, Schultz JB and McDaniel T (1996). Structural and functional changes in catalase induced by near-UV radiation. Photochem. Photobiol 63, 818–24. [DOI] [PubMed] [Google Scholar]
- Zigman S, McDaniel T, Schultz J and Reddan J (2000). Effects of intermittent UVA exposure on cultured lens epithelial cells. Curr. Eye Res 20, 95–100. [PubMed] [Google Scholar]


