Abstract
Timing of menarche has largely been studied in the context of a secular trend. However, since mortality and fertility rates are fundamental demographic factors linked to a population’s developmental and reproductive characteristics, we expect that the timing of menarche, a precondition to reproduction, is also associated with these vital rates. We conduct an analysis of 89 countries and 21 demographic, socioeconomic, nutritional, and educational variables selected for their known influence on menarche. Model results predict that a country’s fertility and adult female mortality rates are significant predictors of mean age at menarche, while other covariates are not. Specifically, menarche is delayed in countries with high mortality and high fertility, which may be proxies for assessing overall environmental quality. We emphasize that, for a comprehensive understanding of the timing of menarche, it is critical to take into account both individual- and population-level influences.
Introduction
Menarche is a key life history event shaping the onset of a female’s reproductive career [1–8]. Unlike other pubertal characteristics that manifest gradually (e.g. breast and pubic hair development, fat deposition, and the adolescent growth spurt), menarche is a discreet event [6,9]. As such, it is a useful, but underrepresented, variable in demographic research [7].
The timing of menarche has largely been studied in the context of a secular trend. Over the last century, a general decline in the age at menarche has been well documented [1,4,10–17]. Many drivers have been proposed to explain this global phenomenon [4,10,11,14,16–20]. While age at menarche clearly has a genetic component [1,21,22], earlier ages at its onset are attributed to a variety of lifestyle and socioeconomic factors, including residence in a rural or urban setting, country economics (e.g. Gross Domestic Product (GDP)), family income, parental or sibling education, family size or exposure to environmental chemicals [23,24]. Nutritional and health factors that have been the focus of much research include energy intake, body mass index (BMI), consumption of particular macronutrients such as sugar or fat [25–28] or alcohol [29]; for a detailed overview of covariates and studies, see S1 Table.
While a breadth of factors likely contribute to variation in menarcheal age, most research has focused on individual responses. However, population characteristics also influence individuals [30–32]. For example, expectations about local mortality have been shown to shape fertility decisions [33–36]. In particular, among American and Canadian study subjects, an earlier onset of reproduction was associated with low neighborhood life expectancy [37] and perceptions of environmental uncertainty were shown to affect reproductive timing among low-income African American female teenagers [33]. In experimental studies, mortality perceptions influenced fertility preferences of student participants [38,39]. Thus, individuals appear to adjust their developmental and reproductive timing to their perception of future survival prospects in relation to local life expectancy and/or mortality rates [35–37]. As a consequence, this affects the average expression of the strategy within a population [40,41]. This suggests that a bidirectional relationship exists between individual responses and population-level characteristics [32]. Following on this, we suspect that mortality and fertility rates can be used as proxies for overall environmental quality and resource availability to which individuals respond in an adaptive way [33,36,37]. Here we consider population-level characteristics as an unexplored approach to the timing of menarche.
Mortality and fertility rates are fundamental demographic factors [42] that influence organisms’ life history [40,43,44]. As examples, Charnov and Berrigan [44] developed a mathematical model in which mortality rates across a range of mammalian taxa effectively predict growth and fertility rates. Promislow and Harvey [40] showed that mortality is the best predictor of variation in life history characteristics across various mammalian species. In humans, many within- and between-population studies have shown that mortality schedules influence differences in growth rates and reproductive strategies [5,36,41,43,45–49]. However, the association between mortality and fertility and the timing of menarche has received much less attention. As an exception, Walker et al. [49] found that in some small-scale societies, higher mortality rate is associated with faster development, earlier menarche, and age at first reproduction.
Moreover, research on menarcheal timing [4,49–52] has focused predominantly on a particular country, region, ethnic group or different kinds of subjects within populations, such as city residents, students or clinical patients [6,15,20,49,53–63]. Such research often has been conducted in high-income Western countries, which represent an exceptional and recent phenomenon contributing to human developmental and socio-cultural variation [64,65]. Despite great progress in understanding the causes of the timing of menarche at the individual level, population-level processes underlying global variation in menarcheal age remain understudied.
To fill this gap, this study investigates the timing of menarche using a comprehensive, global cross-country dataset (Fig 1) and explores the relationship between population-level characteristics and age at menarche. As far as we know, this is the only published compilation of such data. We predict that both a country’s 1) mortality rate and 2) fertility rate will influence mean age at menarche.
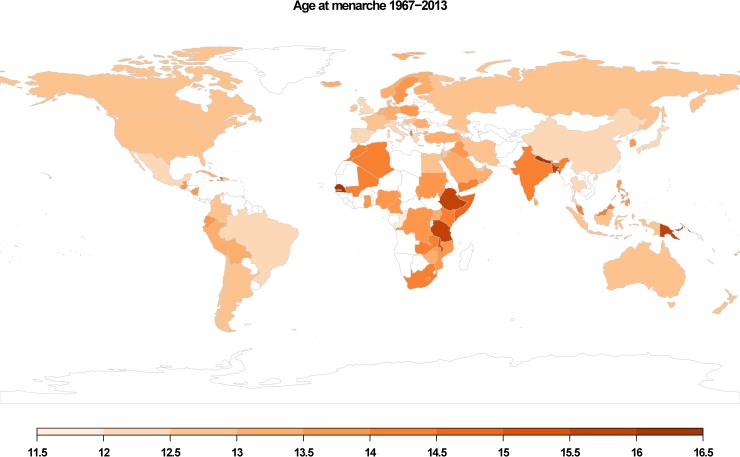
Fig 1. Global distribution of the dependent covariate mean age at menarche for 89 countries included in the sample.
Each country is plotted by mean age at menarche from a given year. Legend shows scaled ages at menarche.
Material and methods
Data collection
We assembled a database for 89 countries with mean menarcheal age data and 21 demographic, socioeconomic, nutritional, and educational covariates (Table 1 and S2 Table). The sample of countries was determined by the availability of menarcheal data, which were drawn from published sources. However, since no global database lists menarcheal age as a demographic variable [7], mean age at menarche for a country was established by the authors from previously published studies for a particular country or for a group of countries (e.g. Eveleth and Tanner [11]; Thomas et al. [52]). Included here are known menarcheal studies published between 1967 and 2013 (S2 Table).
Table 1. Definitions and sources of the analysed covariates.
| Covariate | Definition | Reference |
|---|---|---|
| Demographic | ||
| Mean age at menarche | age of first menstruation | various sources (see S2 Table) |
| Life expectancy at birth | total (years) | [66] |
| Fertility rate | total (children per woman) | [66] |
| Adolescent fertility (15–19) | births per 1,000 women aged 15–19 | [66,67] |
| Maternal mortality ratio | modelled estimate, per 100,000 live births | [66,67] |
| Infant mortality | per 1,000 live births | [66] |
| Under-five mortality | per 1,000 live births | [66] |
| Adult female mortality | probability of female dying between the ages 15–60 | [67] |
| Adult male mortality | probability of male dying between the ages 15–60 | [67] |
| Total adult mortality | probability of dying between the ages 15–60 | average adult male/female mortality rate |
| Socioeconomic | ||
| Average size of household | average number of persons per household | various sources (see S1 Dataset) |
| Rural population | % of total population | [67] |
| Energy use | kg of oil | [67] |
| Fossil fuel energy consumption | % of total energy consumption | [67] |
| Gross domestic product (GDP) per capita | current US$ | [66] |
| Livestock production index | meat, milk, dairy, eggs, etc. | [67] |
| Food production index | food crops (except coffee and tea) | [67] |
| Nutritional | ||
| Energy consumption per capita | kcal/person/day | various sources (see S1 Dataset) |
| Sugar consumption | g/person/day | [66] |
| Body mass index (BMI) female | kg/m2 | [66] |
| Educational | ||
| Out of primary school, female | number of females not enrolled in either primary or secondary schools | [66] |
| Primary completion rate, female | % of females entrants in the last grade of primary education | [66] |
While we have conducted a thorough literature search and made the most of the global mean menarcheal age data available, the source studies on menarcheal age included in our sample (S2 Table) may not be representative of the country as a whole, and in most cases (72 out of 89) are necessarily derived from sub-populations specified by region, ethnic group or various socio-economic strata. Studies were considered nationally representative (17 out of 89) only when 1) authors stated it explicitly, 2) their data were derived from nation-wide surveys or 3) the data sampling locations chosen by authors covered roughly a country’s area (see S2 Table). Additionally, if more than one record for menarcheal age in a study was available for a country (Belgium, Brazil, Cameroon, China, Iraq, Jordan, Singapore, Sudan, and Uganda), an average was calculated. In case of two countries, Albania and United Kingdom, given years for the age at menarche in the sample represent average values calculated from time intervals (2000–05 in case of Albania and 1990–93 for the UK) over which data in both studies were collected. Mean menarcheal age for Belgium was taken from the study of Flemish girls and mean menarcheal age for Uganda reflects post-conflict state. However, in the latter study, authors report no effect of such stressful events on menarcheal age in their sample.
We selected 21 demographic, socioeconomic, nutritional, and educational covariates based on their demonstrated influence on the timing of menarche (Table 1 and S1 Table). Most covariates were available for most countries, and the year in which the covariate data were collected was matched as closely as possible to the year in which menarche data were collected or the study was published. When we were unable to do so, we used information gathered within five years prior to the reference year for menarcheal age, since the timing of menarche would be sensitive to prior not subsequent conditions. However, because data for average household size (N = 70), out of primary school (N = 56), and primary completion rate (N = 55) are lacking for many countries, we drew from a broader temporal period and employed an interval of ±5years. Reference years for average size of household for Norway and Singapore represent average values calculated from the two periods. The list of tested covariates with definitions and references is included in Table 1.
Data caveats
An ideal dataset to evaluate a causal relationship between population-level characteristics and age at menarche would have matched menarcheal data with regional populational traits collected at the same time period across multiple regions. To our knowledge, such data do not exist. While we do not have individual-level characteristics to link to menarcheal age, covariates added to the analysis here represent indicators of the general (e.g. nutritional, socio-economic, demographic) status of the population in which girls are maturing. Unfortunately, aggregate data and covariates chosen do not necessarily capture sufficient information regarding childhood and adolescence. We do feel, however, that we have carefully made the most of the age at menarche data available for a study that is mostly of explorative character. Because we are interested in age at menarche as an understudied but critical and discreet life history marker that is theoretically predicted to be sensitive to environmental circumstances, our goal is to first evaluate if such an association emerges between background mortality and fertility conditions. The results of this research will hopefully then generate predictions that can be evaluated with local, and individual level data.
Statistical procedure
The 21 covariates and the outcome variable (mean age at menarche) were tested for normality using the Shapiro-Wilk’s test and for homogeneity of variances using the Levene’s test (statistically significant at p<0.05). Covariates that did not fulfil the normality criterion were log- or square root- transformed and then tested for normality again. Since only two covariates were normally distributed (female BMI and sugar consumption), non-parametric tests were employed (S3 Table). Descriptive statistics of the sample therefore include measures for non-normally distributed data, such as inter-quartile range (IQR), median, and min. and max. values (S3 Table). To account for a possible spatial autocorrelation between countries due to similar environmental (e.g. climate) or economic (e.g. migration, type of economy) factors, we evaluated whether the observed value of a covariate in one country significantly depended on the value for the same covariate in neighbouring countries. We used centroids for each country to quantify the spatial autocorrelation using Mantel’s I coefficient for each variable’s class distance (S4 Table).
To obtain a general view of the data, associations between mean age at menarche and the 21 covariates were calculated using the Rho-Spearman’s rank correlation coefficients (p<0.05) (S5 Table). To select a refined set of covariates for a GLM model construction, we used a Partial Least Squares (PLS) regression with the mean age at menarche as a dependent covariate. Covariates with the highest values were selected (p<0.05). As we expected collinearity to occur among population-level covariates, we used PLS instead of a Prinicpal Component Analysis (PCA) because it is more sensitive test of autocorrelation. Moreover, because the full suite of covariates is not available for all countries (only 6 variables were available for all 89 countries), PLS is better suited to accomodate missing data than PCA.
Having selected a set of covariates with the highest value of standardised coefficient in PLS regression (Table 2), we used GLM procedure to model the probability that mean age at menarche is a function of these predictor variables. GLM is appropriate in this case because it allows both dependent and independent response covariates to have a non-normal distribution. We used a stepwise (backward elimination) procedure and based our model selection on Akaike information criterion (AIC) scores. This index measures the quality of statistical models in an absolute sense by giving a relative estimate of the information lost when trading-off the goodness-of-fit and complexity (i.e. number of predictors) of the model. Therefore, the best-fit model was characterised by the lowest AIC value.
Table 2. Standardised coefficients in the PLS regression.
| Covariate | N | Standardized model coefficients |
|---|---|---|
| Demographic | ||
| Life expectancy at birth | 89 | -5.224* |
| Fertility rate | 89 | 0.972* |
| Adolescent fertility (15–19) | 89 | -0.432 |
| Maternal mortality ratio | 73 | -0.767 |
| Infant mortality | 87 | -4.438* |
| Under-five mortality | 87 | 4.069* |
| Adult female mortality | 87 | 0.877* |
| Adult male mortality | 87 | -0.899* |
| Total adult mortality | 87 | -0.014 |
| Socioeconomic | ||
| Average size of household | 70 | -0.607 |
| Rural population | 89 | -0.065 |
| Energy use | 79 | -0.607 |
| Fossil fuel energy consumption | 78 | -0.402 |
| Gross domestic product (GDP) per capita | 84 | -0.572 |
| Livestock production index | 89 | -0.101 |
| Food production index | 89 | -0.144 |
| Nutritional | ||
| Energy consumption per capita | 72 | 0.021 |
| Sugar consumption | 75 | 0.014 |
| Body mass index (BMI) female | 79 | 0.021 |
| Educational | ||
| Out of primary school female | 56 | 0.316 |
| Primary completion rate female | 55 | -0.470 |
Asterisk (*) indicates covariates selected for GLM analysis.
The descriptive statistics, spatial autocorrelation, GLM, and figure graphics were carried out in R software (version 3.5.1) [68] with ade4 package [69] for spatial autocorrelation test and rworldmap package [70] and DiagrammeR package for creating Figs 1 and 2, respectively. The Rho-Spearman correlation and PLS analyses were computed with SPSS software (version 22.0.0.1).
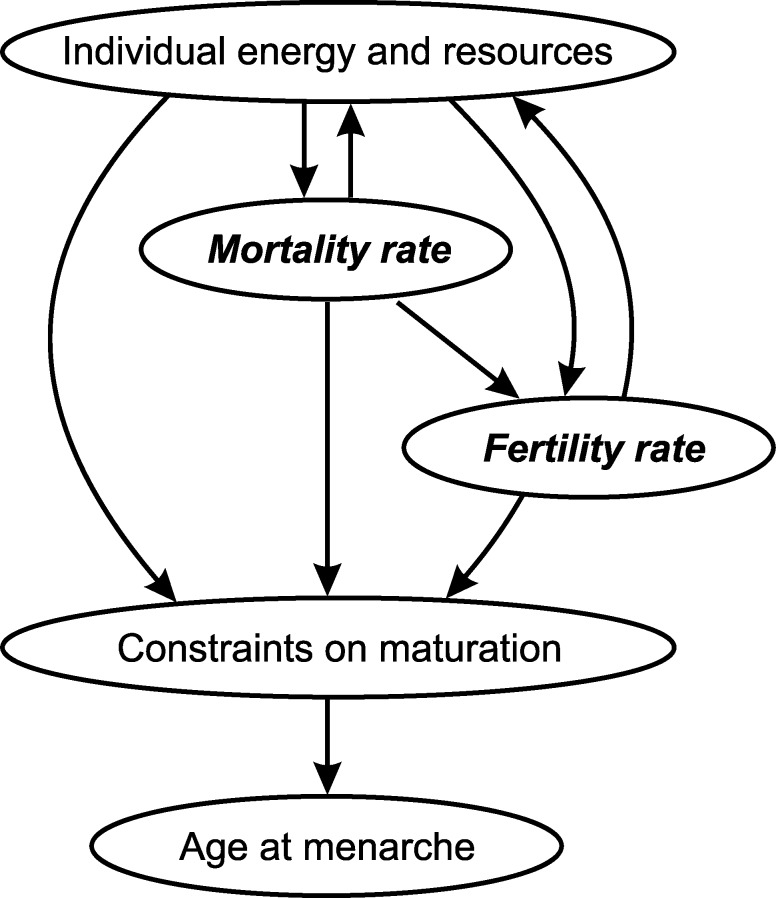
Fig 2. Graphical representation of the main findings.
Arrows depict bidirectional associations of individual- and population-level covariates with the timing of menarche. Circles filled with italic and bold point to the model results.
Results
Descriptive results
Univariate statistics including Interquartile range (IQR), median, min. and max. values for non-normally distributed data are summarized in S3 Table. The Mantel’s test for spatial autocorrelation between countries was non-significant for all 22 computed coefficients (including mean age at menarche), indicating no evidence for spatial autocorrelation (S4 Table). As expected, the Rho-Spearman correlation revealed that all demographic covariates, except life expectancy at birth correlating negatively (N = 89, SC = -0.681, p<0.001), are significantly positively correlated with mean age at menarche (S5 Table). In other words, menarche tends to occur later in countries with high rates of mortality and fertility and low life expectancy at birth (various measures; see Table 1). Except average household size (N = 70, SC = 0.453, p<0.001) and rural population (N = 89, SC = 0.652, p<0.001) correlating positively, all other socioeconomic and all nutritional factors were significantly negatively correlated with the age at menarche (S5 Table). For instance, earlier menarche is associated with countries with smaller families, higher percentage of urban populations, and/or higher GDP and energy consumption per capita and vice versa. The educational variables, female out of primary school and female primary completion rates, were positively (N = 56, SC = 0.596, p<0.001) and negatively (N = 55, SC = -0.532, p<0.001) significantly associated with mean age at menarche, respectively (S5 Table), suggesting that higher the degree of attained education, the earlier age at menarche.
Model results
Based on the PLS analysis, the following six covariates with the highest scores were selected as predictors for GLM: life expectancy at birth, fertility rate, infant mortality, under-five mortality, adult female mortality, and adult male mortality (Tables 2 and 3). The stepwise GLM procedure showed that the model containing mean age at menarche as a dependent covariate and fertility rate (SE = 0.074, t-value = 2.651, p<0.009) and adult female mortality rate (SE = 0.001, t-value = 2.209, p<0.029) as predictor covariates explained the most of the variation in menarcheal age (variation explained is 41.38%; Table 3). The best-fit GLM model was characterised by the lowest AIC score (203.81). Model estimates and p values show that fertility rate has a greater influence on the mean age at menarche than adult female mortality rate.
Table 3. General linear model of the effects of fertility rate and adult female mortality on the mean age at menarche calculated for 89 countries.
| Covariate | Estimate | Standard error | t-value | p-value |
|---|---|---|---|---|
| Intercept | 12.277 | 0.181 | 67.673 | <0.001 |
| Fertility rate | 0.196 | 0.074 | 2.651 | 0.009 |
| Adult female mortality | 0.003 | 0.001 | 2.209 | 0.029 |
AIC = 203.81
Discussion
The results reveal two major findings. First, of the 21 covariates, fertility rate (children per woman) and adult female mortality rate (the probability of female dying between the ages 15–60) are the best predictors of variation in the timing of menarche across countries in our sample (Table 3). Although improvements in socioeconomic, health, and nutritional conditions are factors responsible for a shift to an earlier age at menarche at the individual level, our model results show that at the population level, the timing of menarche is strongly associated with fertility and mortality rates. Second, fertility (N = 89, SC = 0.681, p<0.001) and adult female mortality rates (N = 89, SC = 0.685, p<0.001) correlate positively with menarche (S5 Table), indicating that both high fertility and high mortality are associated with later menarche. As we argue below, our results are not mutually exclusive with previous findings but offer a perspective on how factors at the individual level can be translated into and viewed from the population level and vice versa. We discuss our findings in light of life history theory.
Life history theory explains variation across species in the timing and energy allocated to life events [34]. It posits that because an organism’s time and resources are limited, trade-offs are made whether to invest in maintenance (basic somatic functions to survive) versus growth or reproduction [43,44]. Selection pressures from mortality and fertility significantly influence a specie’s and a population’s life history strategies [40,43,44,47,49]. In particular, average age at first birth has been the focus of many theoretical and empirical studies. Because menarche is an important gateway event that initiates reproductive potential, background (i.e. population-level) mortality and fertility rates, although understudied, likely impact variation in its timing as well.
With respect to mortality, while a particular life history strategy may be optimized through energy or time allocation trade-offs, extrinsic mortality–an unavoidable source of mortality not under the control of an organism shared by all individuals in the population–persists [43,49,68,69]. Life history theory predicts that individuals will respond to high mortality environments by adjusting their developmental and reproductive strategies. At the level of species, high mortality is associated with faster growth, earlier maturity and reproduction, and shorter lifespans [40,43,44]. Low mortality, on the other hand, is associated with greater somatic investment, slower growth and maturation, longer lifespan and thus later reproductive competence [40,43,44,70].
Expectations about the relationship between mortality and other life history characteristics have been applied to both individual allocation decisions and to within- and between-populational differenes [41,43,44,71,72]. Age at menarche within and between populations varies greatly across time and space [11,41], and is correlated with many genetic, biological, and environmental variables. While we know that age at menarche is influenced by a constellation of individual factors, mortality rate also may be an important predictor of a population’s mean age at menarche.
However, the few comparable studies investigating the effect of various mortality rates on the timing of menarche show mixed results. For example, in some small-scale societies, such as Pumé and Hiwi of Venezuela, who live in high mortality environments, menarche occurs early [49,73–75], a finding consistent with Charnov’s life history perspective. Similarly, low life expectancy, which is usually correlated with high infant mortality, is associated with early menarche [35,37]. In studies using aggregated data, Danker-Hopfe [51] found no association between either life expectancy or infant mortality and age at menarche in a sample of 19 European countries. On the other hand, Thomas et al. [52] found in the sample of 67 countries that low life expectancy predicts later menarche. Ellison [50] reports similar findings; in a sample of 37 human populations, high infant mortality positively correlates with later menarche.
Although our results likewise demonstrate a strong relationship between mortality rate and mean age at menarche, it is not in the theoretically expected direction. Rather, in our sample of countries, high mortality, and specifically adult female mortality, is associated with later menarche. This finding contrasts with Charnov’s prediction and other empirical studies [49,76–78], which show that high mortality rates are associated with faster maturity.
One possible explanation is that aggregated data can produce opposite associations to those at the individual level [30]. Another possible explanation why age at menarche is later under high-mortality conditions is becasue life history models assume that conditions are constant, and thus at equilibrium [79]. However, real, not predicted, living conditions can change substantially over a relatively short period of time which, coupled with dynamic socio-economic and political changes, particularly in developing countries, may cause that life history predictions do not necessarily hold [80]. For example, although Anderson [80] in the sample of sub-Saharan African countries found significant associations of life expectancy with other variables studied, the direction was the opposite of what should be expected from life history theory. It could also explain why we did not find evidence for spatial autocorrelation in our data spanning from 1967 to 2013. Although our results could suffer from these methodological limitations, there are a number of reasons why high mortality might be associated with later age at menarche at the population level.
In particular, girls growing up in challenging environments may adaptively assess their futures and adjust their maturation pace based on the population around them [33,37]. If others are dying, high mortality rates may function as a cue that conditions are not favourable and to mature more quickly. Thus, population characteristics, or rates, i.e. the probability of dying, may serve as signals of current and/or future conditions and life expectations to which an individual’s decisions are density dependent and based on what others are doing in the population [32,34,37].
Still, based on the nature of aggregated data, it is difficult to interpret why adult female mortality among other mortality measures (e.g. infant mortality) in the model (Tables 2 and 3), is the best predictor of mean age at menarche. It has been found, however, that in some natural fertility populations, i.e. populations that do not use parity-specific birth control, child’s nutritional status and survival prospects are, unlike patrilineal kin, dependent on the presence of a living mother and other matrilineal kin, such as maternal grandmothers and/or older sisters [81]. It is possible that adult female mortality rate may also reflect the presence/absence of matrilineal kin which could explain the association we found at the population level.
Finally, life history predictions are in specific reference to extrinsic mortality (see above). However, mortality can also be care- and resource- dependent [68,82], and risk individually variable, particularly if associated with nutrition and social resources, such as access to health care [36]. Under these circumstances, girls may be energetically constrained from early development (e.g. Eveleth and Tanner [11]). Poor environments characterized by low energy availability and high mortality may favor slower growth, later maturity and delayed menarche [49]. For example, while Hiwi (Venezuela), Pumé (Venezuela), and Baka (African pygmies, Cameroon) live in high mortality environments due to infectious diseases and parasites, they have fast life histories, including early menarche. In contrast, the Ju/’hoansi (Botswana/Namibia) and Gainj and Asai (Papua New Guinea), also live in high mortality conditions but mostly due to the risk of starvation and have slow development, later menarche, and later reproduction [49]. Thus, mortality rate may be an indirect proxy for current and/or future conditions to which individuals respond in an adaptive way adjusting their developmental and reproductive patterns [36]. How individuals adjust, and its effect on population means, may importantly depend on the level of resource-dependent mortality.
Mortality (defined as life expectancy, infant mortality or a general rate) thus appears 1) to be an important predictor of the age at menarche both at the population and individual level (but see Kyweluk et al. [64]), and 2) to be variably positive or negative. We caution against deriving any causal inferences and simple interpretations that country-level data predict a positive relationship between mortality and age at menarche, while individual-level data predict a negative relationship. Rather both might be the case, depending on the nature and/or source of mortality.
Fertility rate is another strong predictor of menarcheal age (Table 3), with menarche occurring later in high-fertility countries. Following on resource-dependent mortality, one interpretation of this finding may be that high fertility increases energetic demands on mothers to provide sufficient resources for dependent offspring, which may result in competition for resources among siblings and with adults [83]. For example, in a longitudinal study using aggregated data for rural communities of Bangladesh, declining fertility rate led to significantly lower child mortality rates [84]. Presence of younger sibling/s in the household increases competition for food and other family resources. At the population level, lower fertility means more public resources available per each offspring [84]. This is particularly true in market-based economies, in contrast to traditional labor-based economies where the number of children is associated with increased household production and greater wealth [85]. If, however, energy consumption per offspring decreases with increasing fertility, it may result in slower growth and later ages at menarche [86] (but see Kramer et al. [85]). We also report significant negative correlations between our nutritional covariates and age at menarche (S5 Table).
Individual-level studies on the relationship between menarche and both family size and number of siblings may support this assertion [1,20,54,62,87,88]. For instance, family size and birth order had the strongest effect on menarcheal age in the sample of Portuguese university students with menarche occurring later with increasing number of children in the family [89]. In another study of three Polish provinces (Kraków, Opole, and Nowy Sącz) that differed in the degree of urbanization, family size was among the most significant socioeconomic variables predicting menarcheal age [54]. Particularly, in the least urbanized province, Nowy Sącz (less than 35% of population live in cities), family size was the only significant predictor of menarcheal age [54]. It is likely that pre-reproductive girls allocate substantial amount of resources to a direct care of their younger siblings, which, resulting in increased energy expenditure of girls, and eventually decreased energy consumption per capita, could be mirrored in later ages at menarche.
Taken together, the timing of menarche is likely a result of an optimization of trade-offs resulting from energetic constraints on growth and mortality pressure for an onset of fertility (Fig 2). An example of such a case could be seen in low mortality/low fertility countries, in which, unlike in high mortality/high fertility countries, sufficient amounts of energy drive menarche into earlier ages, but age at first reproduction is, perhaps due to (perceived or real) predictability or stability of the environment and resources postponed into later ages. Future research could focus on exploring the interactions, in terms of a length of an interval, between the timing of menarche and age at first reproduction along the dimensions of mortality and fertility within the context of demographic transition.
Conclusions
This study investigated population-level influences on the timing of menarche. We found that (adult female) mortality and fertility rates have significant effect on the variation in mean menarcheal age across countries in our sample, which is consistent with general life history framework. It is clear that the energetic and social resources available to an individual have a direct influence on the timing of menarche. At the population level, if this is reflected in mortality and fertility rates, the latter may act as proxies for assessing overall quality of the environment. This, in turn, can exert their indirect effects on the individual timing of menarche (Fig 2). Thus, for a comprehensive understanding of the timing of menarche it is critical to take into account both individual- and population-level influences. We assert that a population-level age at menarche can be another important demographic variable.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLS)
Acknowledgments
We would like to thank to Emma Nelson, Lajos Rózsa, Piotr Tryjanowski, and Jakub Kosicki for their thoughtful comments.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
MH and GŠ received funding from 'EC, Employment, Social Affairs & Inclusion, European Social Fund (ESF); grant number: OPV ITMS: 26110230119; URL: http://ec.europa.eu/social/main.jsp?catId=325&langId=en' and 'Cultural and Educational Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic (KEGA)'; grant number: KEGA: 001PU-4/2017; URL: https://www.minedu.sk/kulturna-a-edukacna-grantova-agentura-msvvas-sr-kega/. GŠ was supported by Faculty of Science funds, University of South Bohemia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zacharias L, Wurtman RJ. Age at menarche: genetic and environmental influences. N Engl J Med. 1969;280(16):868–75. 10.1056/NEJM196904172801606 [DOI] [PubMed] [Google Scholar]
- 2.Presser HB. Age at menarche, socio‐sexual behavior, and fertility. Soc Biol. 1978;25(2):94–101. [DOI] [PubMed] [Google Scholar]
- 3.Sandler DP, Wilcox AJ, Horney LF. Age at menarche and subsequent reproductive events. Am J Epidemiol. 1984;119(5):765–74. [DOI] [PubMed] [Google Scholar]
- 4.de Muinck Keizer-Schrama SMPF, Mul D. Trends in pubertal development in Europe. APMIS. 2001;109(S103):S164–70. [Google Scholar]
- 5.Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychol Bull. 2004;130(6):920–58. 10.1037/0033-2909.130.6.920 [DOI] [PubMed] [Google Scholar]
- 6.Rah JH, Shamim AA, Arju UT, Labrique AB, Rashid M, Christian P. Age of onset, nutritional determinants, and seasonal variations in menarche in rural Bangladesh. J Heal Popul Nutr. 2009;27(6):802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer M. Menarche: a missing indicator in population health from low-income countries. Public Health Rep]. 2013;128(5):399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehmann A, Scheffler C. What does the mean menarcheal age mean?–An analysis of temporal pattern in variability in a historical swiss population from the 19th and 20th centuries. Am J Hum Biol. 2016;28(5):705–13. 10.1002/ajhb.22854 [DOI] [PubMed] [Google Scholar]
- 9.Amir D, Jordan MR, Bribiescas RG. A longitudinal assessment of associations between adolescent environment, adversity perception, and economic status on fertility and age of menarche. PLoS One. 2016;11(6):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyshak G, Frisch RE. Evidence for a secular trend in age of menarche. N Engl J Med. 1982;306(17):1033–5. 10.1056/NEJM198204293061707 [DOI] [PubMed] [Google Scholar]
- 11.Eveleth PB, Tanner JM. Worldwide variation in human growth. 2nd ed New York, Cambridge University Press; 1990. [Google Scholar]
- 12.Meyer F, Moisan J, Marcoux D, Bouchard C. Dietary and physical determinants of menarche. Epidemiology. 1990;1(5):377–81. [DOI] [PubMed] [Google Scholar]
- 13.Herman-Giddens ME. The decline in the age of menarche in the United States: Should we be concerned? Journal of Adolescent Health. 2007;40(3):201–3. 10.1016/j.jadohealth.2006.12.019 [DOI] [PubMed] [Google Scholar]
- 14.Rokade SA, Mane AK. A study of age at menarche, the secular trend and factors associated with it. Internet J Biol Anthropol. 2009;3(2). [Google Scholar]
- 15.Tomova A, Genov N, Kumanov F, Robeva R. [Menarche in Bulgarian–secular trend in twenty century]. Akush Ginekol (Sofiia). 2009;48(3):10–4. [PubMed] [Google Scholar]
- 16.Prentice S, Fulford AJ, Jarjou LMA, Goldberg GR, Prentice A. Evidence for a downward secular trend in age of menarche in a rural Gambian population. Ann Hum Biol. 2010;37(5):717–21. 10.3109/03014461003727606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris DH, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. Secular trends in age at menarche in women in the UK born 1908–93: Results from the breakthrough generations study. Paediatr Perinat Epidemiol. 2011;25(4):394–400. 10.1111/j.1365-3016.2011.01202.x [DOI] [PubMed] [Google Scholar]
- 18.Wellens R, Malina RM, Beunen G, Lefevre J. Age at menarche in Flemish girls: current status and secular change in the 20th century. Ann Hum Biol. 1990;17(2):145–52. [DOI] [PubMed] [Google Scholar]
- 19.Pasquet P, Manguelle-Dicoum Biyong A, Rikong-Adie H, Befidi-Mengue R, Garba M-T, Froment A. Age at menarche and urbanization in Cameroon: current status and secular trends. Ann Hum Biol. 1999;26(1):89–97. [DOI] [PubMed] [Google Scholar]
- 20.Rebacz E. Age at menarche in schoolgirls from Tanzania in light of socioeconomic and sociodemographic conditioning. Coll Antropol. 2009;33(1):23–9. [PubMed] [Google Scholar]
- 21.Kaprio J, Rimpelä A, Winter T, Viken RJ, Rimpelä M, Rose RJ. Common genetic influences on BMI and age at menarche. Hum Biol. 1995;67(5):739–53. [PubMed] [Google Scholar]
- 22.Perry JRB, Stolk L, Franceschini N, Lunetta KL, Zhai G, McArdle PF, et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat Genet. 2009;41(6):648–50. 10.1038/ng.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper RL, Stoker TE, Tyrey L, Goldman JM, McElroy WK. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol Sci. 2000;53(2):297–307. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson-Dickman E, Lee MM. The influence of endocrine disruptors on pubertal timing. Curr Opin Endocrinol Diabetes Obes. 2009;16(1):25–30. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez A, Kissinger DG, Phillips RI. A hypothesis on the etiological role of diet on age of menarche. Med Hypotheses. 1981;7(11):1339–45. [DOI] [PubMed] [Google Scholar]
- 26.Moisan J, Meyer F, Gingras S. Diet and age at menarche. Cancer Causes Control. 1990;1(2):149–54. [DOI] [PubMed] [Google Scholar]
- 27.Moisan J, Meyer F, Gingras S. A nested case-control study of the correlates of early menarche. Am J Epidemiol. 1990;132(5):953–61. [DOI] [PubMed] [Google Scholar]
- 28.Maclure M, Travis LB, Willett W, Macmahon B. A prospective cohort study of nutrient intake and age at menarche. Am J Clin Nutr. 1991;54(4):649–56. 10.1093/ajcn/54.4.649 [DOI] [PubMed] [Google Scholar]
- 29.Deardorff J, Gonzales NA, Christopher FS, Roosa MW, Millsap RE. Early puberty and adolescent pregnancy: the influence of alcohol use. Pediatrics. 2005;116(6):1451–6. 10.1542/peds.2005-0542 [DOI] [PubMed] [Google Scholar]
- 30.Gove WR, Hughes M. Reexamining the ecological fallacy: A study in which aggregate data are critical in investigating pathological effects of living alone. Soc Forces. 1980;58(4):1157–77. [Google Scholar]
- 31.Schwartz S. The fallacy of ecological fallacy: the potencial misuse of a concept and consequences. Am J Public Health. 1994;84(5):819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce N. The ecological fallacy strikes back. J Epidemiol Community Health. 2000;54(5):326–7. 10.1136/jech.54.5.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geronimus AT. What teen mothers know. Hum Nat. 1996;7(4):323–52. 10.1007/BF02732898 [DOI] [PubMed] [Google Scholar]
- 34.Hill EM, Ross LT, Low BS. The role of future unpredictability in human risk-taking. Hum Nat. 1997;8(4):287–325. 10.1007/BF02913037 [DOI] [PubMed] [Google Scholar]
- 35.Chisholm JS, Quinlivan JA, Petersen RW, Coall DA. Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Hum Nature, Fall. 2005;16(3):233–65. [DOI] [PubMed] [Google Scholar]
- 36.Caudell MA, Quinlan RJ. Resource availability, mortality, and fertility: A path analytic approach to global life-history variation. Hum Biol. 2012;84(2):101–25. 10.3378/027.084.0201 [DOI] [PubMed] [Google Scholar]
- 37.Wilson M, Daly M. Life expectancy, economic inequality, homicide, and reproductive timing in Chicago neighbourhoods. BMJ. 1997;314(7089):1271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fritsche I, Jonas E, Fischer P, Koranyi N, Berger N, Fleischmann B. Mortality salience and the desire for offspring. J Exp Soc Psychol. 2007;43(5):753–62. [Google Scholar]
- 39.Mathews P, Sear R. Life after death: An investigation into how mortality perceptions influence fertility preferences using evidence from an internet-based experiment. J Evol Psychol. 2008;6(3):155–72. [Google Scholar]
- 40.Promislow DEL, Harvey PH. Living fast and dying young: A comparative analysis of life-history variation among mammals. J Zool. 1990;220(3):417–37. [Google Scholar]
- 41.Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Hum Nat. 2009;20(2):204–68. 10.1007/s12110-009-9063-7 [DOI] [PubMed] [Google Scholar]
- 42.Caldwell J, Caldwell P, Caldwell B, Eversley D, Fix AG, Howell N, et al. Anthropology and Demography: The mutual reinforcement of speculation and research [and comments and reply]. Curr Anthropol. 1987;28(1):25–43. [Google Scholar]
- 43.Stearns SC. The evolution of life histories. Oxford University Press; 1992. 249 p. [Google Scholar]
- 44.Charnov EL, Berrigan D. Why do female primates have such long lifespans and so few babies? Or life in the slow lane. Evol Anthropol Issues, News, Rev. 1993;1(6):191–4. [Google Scholar]
- 45.Ellison PT. Human ovarian function and reproductive ecology: new hypotheses. Am Anthropol. 1990;92(4):933–52. [Google Scholar]
- 46.Ellison PT, Panter-Brick C, Lipson SF, O’Rourke MT. The ecological context of human ovarian function. Hum Reprod. 1993;8(12):2248–58. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan HS, Lancaster JB. An evolutionary and ecological analysis of human fertility, mating patterns, and parental investment In: Wachter KW, Bulatao RA, editors. Offspring: Human fertility behavior in biodemographic perspective. Washington, DC: National Academies Press; 2003. p. 170–223. [PubMed] [Google Scholar]
- 48.Gluckman PD, Hanson MA. Evolution, development and timing of puberty. Trends Endocrinol Metab. 2006;17(1):7–12. 10.1016/j.tem.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 49.Walker R, Gurven M, Hill K, Migliano A, Chagnon N, De Souza R, et al. Growth rates and life histories in twenty-two small-scale societies. Am J Hum Biol. 2006;18(3):295–311. 10.1002/ajhb.20510 [DOI] [PubMed] [Google Scholar]
- 50.Ellison PT. Morbidity, mortality, and menarche. Hum Biol. 1981;53(4):635–43. [PubMed] [Google Scholar]
- 51.Danker-Hopfe H. Menarcheal age in Europe. Yearb Phys Anthropol. 1986;29:81–112. [Google Scholar]
- 52.Thomas F, Renaud F, Benefice E, Meeus T de, Guegan JF. International variability of ages at menarche and menopause: Patterns and main determinants. Hum Biol. 2001;73(2):271–90. [DOI] [PubMed] [Google Scholar]
- 53.Ammari FL, Ajlouni HK, Ajlouni KM. Age at menarche in Jordanian girls. Saudi Med J. 2004;25(2):244–5. [PubMed] [Google Scholar]
- 54.Wronka I, Pawlińska-Chmara R. Menarcheal age and socio-economic factors in Poland. Ann Hum Biol. 2005;32(5):630–8. 10.1080/03014460500204478 [DOI] [PubMed] [Google Scholar]
- 55.Zegeye DT, Megabiaw B, Mulu A. Age at menarche and the menstrual pattern of secondary school adolescents in northwest Ethiopia. BMC Womens Health. 2009;9(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bata MS. Age at menarche, menstrual patterns, and menstrual characteristics in Jordanian adolescent girls. Int J Gynecol Obstet. 2012;119(3):281–3. [DOI] [PubMed] [Google Scholar]
- 57.Mpora BO, Piloya T, Awor S, Ngwiri T, Laigong P, Mworozi EA, et al. Age at menarche in relation to nutritional status and critical life events among rural and urban secondary school girls in post-conflict Northern Uganda. BMC Womens Health. 2014;14(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bogin B, Wall M, Macvean RB. Longitudinal analysis of adolescent growth of ladino and Mayan school children in Guatemala: Effects of environment and sex. Am J Phys Anthropol. 1992;89(4):447–57. 10.1002/ajpa.1330890406 [DOI] [PubMed] [Google Scholar]
- 59.Abioye-Kuteyi EA, Ojofeitimi EO, Aina OI, Kio F, Aluko Y, Mosuro O. The influence of socioeconomic and nutritional status on menarche in Nigerian school girls. Nutr Health. 1997;11(3):185–95. 10.1177/026010609701100304 [DOI] [PubMed] [Google Scholar]
- 60.Chowdhury S, Shahabuddin AKM, Seal AJ, Talukder KK, Hassan Q, Begum RA, et al. Nutritional status and age at menarche in a rural area of Bangladesh. Ann Hum Biol. 2000;27(3):249–56. [DOI] [PubMed] [Google Scholar]
- 61.Pawloski LR. Growth and development of adolescent girls from the Segou Region of Mali (West Africa). Am J Phys Anthropol. 2002;117(4):364–72. 10.1002/ajpa.10037 [DOI] [PubMed] [Google Scholar]
- 62.Padez C. Age at menarche of schoolgirls in Maputo, Mozambique. Ann Hum Biol. 2003;30(4):487–95. [DOI] [PubMed] [Google Scholar]
- 63.Allal N, Sear R, Prentice M, Mace R. An evolutionary model of stature, age at first birth and reproductive success in Gambian women. Proc Biol Sci. 2004;271(1538):465–70. 10.1098/rspb.2003.2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kyweluk MA, Georgiev A V., Borja JB, Gettler LT, Kuzawa CW. Menarcheal timing is accelerated by favorable nutrition but unrelated to developmental cues of mortality or familial instability in Cebu, Philippines. Evol Hum Behav. 2018;39(1):76–81. [Google Scholar]
- 65.Henrich J, Heine SJ, Norenzayan A. Most people are not WEIRD. Nature. 2010;466:29 10.1038/466029a [DOI] [PubMed] [Google Scholar]
- 66.Gapminder. Gapminder Data Bank. 2016. [cited 2018 Oct 9]. Available from: https://www.gapminder.org/data/
- 67.World development indicators. Washington, D.C.: The World Bank. [cited 2018 Oct 9]. Available from: https://data.worldbank.org/indicator
- 68.R Core Team. R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria: 2012. [Google Scholar]
- 69.Dray S, Dufour AB. The ade4 Package: Implementing the duality diagram for ecologists. J Stat Softw. 2007;22(4). [Google Scholar]
- 70.South A. rworldmap: A new R package for mapping global data. R Journal. 2011;3(1). [Google Scholar]
- 71.Quinlan RJ. Human parental effort and environmental risk. Proc Biol Sci. 2007;274(1606):121–5. 10.1098/rspb.2006.3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Placek CD, Quinlan RJ. Adolescent fertility and risky environments: a population-level perspective across the lifespan. Proc R Soc B Biol Sci. 2012;279(1744):4003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kramer KL. Early sexual maturity among Pumé foragers of Venezuela: Fitness implications of teen motherhood. Am J Phys Anthropol. 2008;136(3):338–50. 10.1002/ajpa.20817 [DOI] [PubMed] [Google Scholar]
- 74.Kramer KL, Greaves RD. Synchrony between growth and reproductive patterns in human females: Early investment in growth among Pumé foragers. Am J Phys Anthropol. 2010;141(2):235–44. 10.1002/ajpa.21139 [DOI] [PubMed] [Google Scholar]
- 75.Kramer KL, Greaves RD, Ellison PT. Early reproductive maturity among Pumé foragers: Implications of a pooled energy model to fast life histories. Am J Hum Biol. 2009;21(4):430–7. 10.1002/ajhb.20930 [DOI] [PubMed] [Google Scholar]
- 76.Quinlan RJ. Extrinsic mortality effects on reproductive strategies in a Caribbean community. Hum Nat. 2010;21(2):124–39. [Google Scholar]
- 77.Polak M, Starmer WT. Parasite-induced risk of mortality elevates reproductive effort in male Drosophila. Proc Biol Sci. 1998;265(July):2197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stearns SC, Koella JC. The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution. 1986;40(5):893–913. 10.1111/j.1558-5646.1986.tb00560.x [DOI] [PubMed] [Google Scholar]
- 79.Low BS, Hazel A, Parker N, Welch KB. Influences on women’s reproductive lives: Unexpected ecological underpinnings. Cross-Cultural Res. 2008;42(3):201–19. [Google Scholar]
- 80.Anderson KG. Life expectancy and the timing of life history events in developing countries. Hum Nat. 2010;21(2):103–23. [Google Scholar]
- 81.Sear R, Steele F, McGregor IA, Mace R. The effects of kin on child mortality in rural Gambia. Demography. 2002;39(1):43–63. [DOI] [PubMed] [Google Scholar]
- 82.Pennington R, Harpending H. Fitness and fertility among Kalahari!Kung. Am J Phys Anthropol. 1988;77(3):303–19. 10.1002/ajpa.1330770304 [DOI] [PubMed] [Google Scholar]
- 83.Gurven M, Walker RS. Energetic demand of multiple dependents and the evolution of slow human growth. Proc R Soc B. 2006;273(1588):835–41. 10.1098/rspb.2005.3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.LeGrand TK, Phillips JF. The effect of fertility reductions on infant and child mortality: Evidence from matlab in rural Bangladesh. Popul Stud (NY). 1996;50(1):51–68. [Google Scholar]
- 85.Kramer KL, Veile A, Otárola-Castillo E. Sibling competition & growth tradeoffs. Biological vs. statistical significance. PLoS One. 2016;11(3):e0150126 10.1371/journal.pone.0150126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gurven M, Walker R. Energetic demand of multiple dependents and the evolution of slow human growth. Proc R Soc B Biol Sci. 2006;273(1588):835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Štukovský R, Valšik JA, Bulai-Ştirbu M. Family size and menarcheal age in Constanza, Roumania. Hum Biol. 1967;39(3):277–83. [Google Scholar]
- 88.Malina RM. Menarche in atheletes: A synthesis and hypothesis. Ann Hum Biol. 1983;10(1):1–24. [DOI] [PubMed] [Google Scholar]
- 89.Padez C. Social background and age at menarche in Portuguese university students: A note on the secular changes in Portugal. Am J Hum Biol. 2003;15(3):415–27. 10.1002/ajhb.10159 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLS)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.