Abstract
Residents of Qidong, People's Republic of China, are at high risk for development of hepatocellular carcinoma, in part from consumption of foods contaminated with aflatoxins. Chlorophyllin, a mixture of semisynthetic, water-soluble derivatives of chlorophyll that is used as a food colorant and over-the-counter medicine, has been shown to be an effective inhibitor of aflatoxin hepatocarcinogenesis in animal models by blocking carcinogen bioavailability. In a randomized, double-blind, placebo-controlled chemoprevention trial, we tested whether chlorophyllin could alter the disposition of aflatoxin. One hundred and eighty healthy adults from Qidong were randomly assigned to ingest 100 mg of chlorophyllin or a placebo three times a day for 4 months. The primary endpoint was modulation of levels of aflatoxin-N7-guanine adducts in urine samples collected 3 months into the intervention measured by using sequential immunoaffinity chromatography and liquid chromatography–electrospray mass spectrometry. This aflatoxin–DNA adduct excretion product serves as a biomarker of the biologically effective dose of aflatoxin, and elevated levels are associated with increased risk of liver cancer. Adherence to the study protocol was outstanding, and no adverse events were reported. Aflatoxin-N7-guanine could be detected in 105 of 169 available samples. Chlorophyllin consumption at each meal led to an overall 55% reduction (P = 0.036) in median urinary levels of this aflatoxin biomarker compared with those taking placebo. Thus, prophylactic interventions with chlorophyllin or supplementation of diets with foods rich in chlorophylls may represent practical means to prevent the development of hepatocellular carcinoma or other environmentally induced cancers.
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide and results in more than 200,000 deaths annually in the People's Republic of China. HCC is the leading cause of cancer death in Qidong in eastern Jiangsu Province, People's Republic of China, and accounts for up to 10% of all adult deaths in some of the rural townships (1, 2). It has been postulated that chronic infection with hepatitis B virus and exposure to aflatoxins in the diet contribute to the extraordinarily high risk of HCC in Qidong (1, 2). Aflatoxins are potent hepatocarcinogens produced by fungi and are consistent contaminants of the food supply in this area, particularly in corn, peanuts, soy sauce, and fermented soy beans. Several nested case-control studies in nearby Shanghai have demonstrated a synergistic interaction between hepatitis B virus and aflatoxins for risk of HCC (3, 4). A similar chemical–viral interaction has been observed in Taiwan (5). From a public health perspective, these findings suggest that hepatitis virus vaccination programs and efforts to reduce aflatoxin exposures could have a major impact on the incidence of this disease. Indeed, a universal vaccination program against hepatitis B virus started a decade ago in Taiwan is now resulting in lower rates of HCC in children (6).
The extent of aflatoxin contamination in foods is a function of the ecology of molds and is not completely preventable. Secondary prevention programs, such as chemoprevention, may be useful in this setting. Cancer chemoprevention entails the use of natural or synthetic agents to retard, block, or reverse the carcinogenic process. Experimentally, aflatoxin-induced hepatocarcinogenesis can be inhibited by more than a score of different chemopreventive agents with multiple mechanisms of action (7). Recently, we reported that intervention with oltipraz, an effective inhibitor of experimental aflatoxin B1 (AFB1)-induced hepatocarcinogenesis, produced protective alterations in the excretion of metabolites of AFB1 in exposed individuals (8). While the clinical evaluation of the efficacy of oltipraz as a human chemopreventive agent continues, it is prudent to consider additional strategies for preventing the toxic and carcinogenic consequences of unavoidable exposures to aflatoxins. In particular, effective strategies that are completely safe, inexpensive, and mechanistically simple are highly desirable for use in the high-risk regions of developing countries.
Chlorophylls and their water-soluble salts (chlorophyllins) are constituents of the human diet and have been found to be effective anticarcinogens in several animal models (9). Chlorophyllin is a mixture of sodium-copper salts of chlorophyll that is marketed as an over-the-counter drug (Derifil) for controlling body, fecal, and urinary odor in geriatric and osteomy patients and as an accelerant in wound healing (10, 11). It is also used extensively as a food additive for coloration. Chlorophyllin is a potent antimutagen in a range of short-term genotoxicity assays in vitro and in vivo (9, 12). Mechanistic studies suggest that chlorophyllin can act as an “interceptor molecule” through the formation of tight molecular complexes with carcinogens such as AFB1 (13). Thus, chlorophyllin may diminish the bioavailability of dietary carcinogens by impeding their absorption and by shuttling them through the fecal stream, leading to reduced DNA adduct and tumor burden (14, 15). Chlorophyllin is most effective as an anticarcinogen in experimental models when given in large molar excess relative to the carcinogen at or around the time of carcinogen exposure. However, internal effects also may account for a portion of the anticarcinogenic spectrum of actions of chlorophyllin. For example, chlorophyllin is a potent inhibitor in vitro of cytochrome P450 enzymes involved in the bioactivation of several environmental carcinogens (16). Chlorophyllin also acts as an antioxidant to inhibit lipid peroxidation (17).
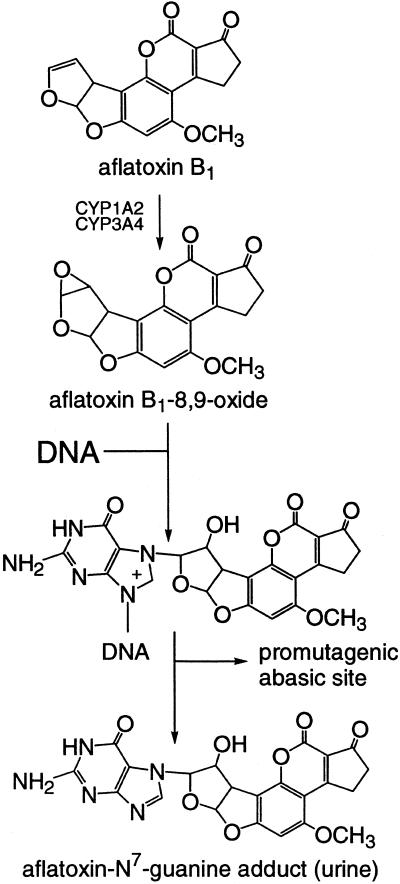
The efficacy of chlorophyllin as an antioxidant, antimutagen, and anticarcinogen in several models; its potential simple mechanism of molecular complexation; its widespread, low-cost availability; and its lack of any known toxicities prompted us to conduct a randomized, double-blind, placebo-controlled, chemoprevention trial in residents of Qidong, People's Republic of China, who are exposed to dietary aflatoxins and who are at high risk for subsequent development of HCC. The primary goal was to determine whether chlorophyllin could diminish the level of aflatoxin biomarkers, notably aflatoxin-N7-guanine, in biofluids collected from the study participants. As depicted in Fig. 1, this aflatoxin–DNA adduct excretion product serves as a biomarker of the biologically effective dose of aflatoxin and elevated levels are associated with increased risk of liver cancer.
Figure 1.
Pathway for formation of aflatoxin-N7-guanine after exposure to aflatoxin B1.
Materials and Methods
Study Design and Participants.
Adults in good general health without history of major chronic illnesses and with detectable serum aflatoxin–albumin adducts at baseline were randomized to two intervention arms: placebo and 100 mg of chlorophyllin administered three times daily. The trial included men and women and did not exclude hepatitis B surface antigen-positive individuals with normal liver function. Study participants were recruited from villages 4 and 5 (about 4,000 residents) in Daxin Township, Qidong, Jiangsu Province, People's Republic of China. Daxin is a rural farming community of ≈40,000 residents and is located at the mouth of the Changjiang (Yangtze) River. Village doctors identified potentially eligible residents and asked for volunteers to be screened for the trial. Five hundred and eleven individuals were screened at the Daxin Medical Clinic over 2.5 days in early July, 1997. Written informed consent was obtained from all participants. The protocol was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions, the Shanghai Municipal Health Bureau, and the Jiangsu Province Department of Health. A medical history, physical examination, liver ultrasound, electrocardiogram, and routine hematological, hepatic, and renal function tests were used to screen the individuals, aged 25–65 years, at the first baseline visit. Processing and evaluation of serum samples for clinical chemistry analyses were as described previously (18). Two hundred and fifteen eligible individuals from the screened group had detectable levels of the serum aflatoxin biomarker and were invited to a town meeting, where the investigators reviewed the study protocol. One hundred and eighty attendees reconfirmed their willingness to participate and were randomized by using a fixed randomization scheme with a block size of 20. Participants returned to the Daxin Medical Clinic on the first day of the study (August 3, 1997), where they were given their identification number and their first dose of study drug.
Study Protocol.
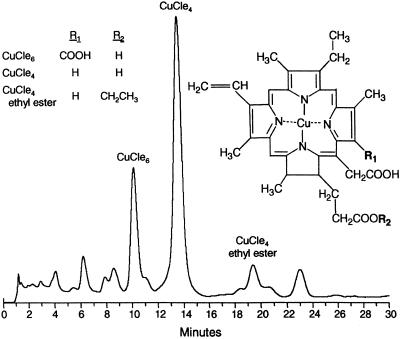
Chlorophyllin (Derifil) and placebo tablets containing 100 mg of chlorophyllin copper complex or excipient only, respectively, were provided by Rystan (Little Falls, NJ). Fig. 2 indicates that the chlorophyllin preparation used in this study was a mixture of sodium-copper salts of chlorophyll consisting principally of copper chlorins e6 and e4. Bioassay in rainbow trout demonstrated that this lot of Derifil tablets provided a degree of protection against carcinogen–DNA adduction and tumor yield comparable to other chlorophyllin preparations of similar chlorin content (G.S.B., unpublished observations). Masking for the clinical trial was achieved by coating all tablets to a dark-green–black color. The U.S. Food and Drug Administration Advisory Review Panel on over-the-counter drugs has suggested that chlorophyllin is safe in oral doses up to 100 mg three times a day. In practice, three chlorophyllin or placebo tablets were distributed daily in the late afternoon to each participant by the village doctors in a reusable, individually coded pill packet. Participants were instructed to take one tablet 20 min before each meal. Each day, the used pill packet was exchanged for a filled one. Compliance was determined by pill counts of the returned packets.
Figure 2.
HPLC tracing (A626) of the copper chlorin constituents of the Derifil used in the intervention. Analysis was conducted as described in ref. 19.
Urine and blood samples collected throughout the 16-week intervention provided the basis for monitoring toxicities and measuring aflatoxin biomarkers. Two consecutive, overnight, 12-hour urine collections were obtained on Tuesday and Wednesday mornings every 4 weeks. A chem-strip urinalysis was performed on the first sample of each monthly collection. Blood samples were collected at baseline, just before administration of the first tablet, and at 4-week intervals on Sundays thereafter for the duration of the intervention. Serum alanine aminotransferase activities were determined in Qidong on all collected samples. Aliquots of urine and serum were stored at −80°C. Portions of each sample were shipped frozen by airfreight to Baltimore. Serum samples from week 1 (baseline) and week 16 (study termination) collections were transferred immediately after collection to a clinical laboratory (Hagerstown Medical Laboratory, Hagerstown, MD) for comprehensive blood chemistry analyses.
Urinary levels of aflatoxin-N7-guanine were determined as described by Walton et al. (20). Briefly, 25 ml of urine was acidified, spiked with 1.5 ng of aflatoxin B2 (AFB2) as an internal standard, and loaded onto a preconditioned Waters Oasis HLB column. After elution of aflatoxins with methanol, samples were dried, reconstituted in water, and loaded onto an aflatoxin-specific preparative mAb immunoaffinity column. Aflatoxins were eluted from the immunoaffinity column with 70% DMSO/water and concentrated under an argon stream to a final volume of 30 μl. One-half of this concentrate then was injected onto a Thermoquest Finnigan LCQ liquid chromatography/electrospray ionization mass spectrometer (LC/ESI-MS/MS) system to measure aflatoxin-N7-guanine and internal standard AFB2 levels in the immunoaffinity processed urine. Standard curves were prepared from authentic aflatoxin-N7-guanine and AFB2. The limit for detection of the nucleic acid adduct (signal-to-noise ratio < 3) was ≈0.5 pg (about 1 fmol). Samples were processed in blocks of 20 to match the randomization scheme. Aflatoxin-N7-guanine and AFB2 were quantified by measuring the mass area of specific MS/MS daughter fragments MH+ 152.1 and 259.1 derived from the parent ions of m/z 480.1 and 315.1, respectively. Urinary levels of aflatoxin-N7-guanine were adjusted by the sample-specific recoveries of the internal standard AFB2, which averaged 48%. All values were normalized to creatinine levels in the original urinary aliquots measured at Hagerstown Medical Laboratories.
Statistical Analysis.
The distribution of the urinary levels of aflatoxin-N7-guanine was highly skewed and influenced by a large number of values below the limit of detection (0.02 pg/mg creatinine). Therefore, a nonparametric median test was used to compare the median biomarker levels of the two treatment arms. A second analysis was conducted to compare the geometric means of the samples above the limit of detection. Reported P values are two-tailed.
Results
Enrollment and Comparability of Intervention Arms.
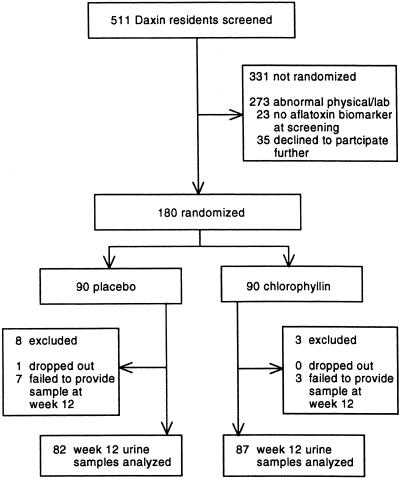
As indicated in Fig. 3, 180 individuals, representing 100% of the recruitment goal, were randomized into the two intervention arms. The intervention groups did not differ significantly (P > 0.05) by age, gender, hepatitis B surface antigen status, and baseline levels of aflatoxin–albumin adduct levels (Table 1). For most parameters, these distributions represented the screened population. The percentage of hepatitis B surface antigen-positive participants (4.4%) was half that of the screened population (8.2%), reflecting the accompanying altered liver function often observed in individuals infected with hepatitis B virus.
Figure 3.
Chlorophyllin chemoprevention trial profile.
Table 1.
Demographic distribution of screened population and enrolled participants by treatment
| Screened population (n = 511) | Total enrolled (n = 180) | Treatment group
|
||
|---|---|---|---|---|
| Placebo (n = 90) | Chlorophyllin (n = 90) | |||
| Gender* | ||||
| Male | 172 (33.7) | 49 (27.2) | 30 (33.3) | 19 (21.1) |
| Female | 339 (66.3) | 131 (72.8) | 60 (66.7) | 71 (78.9) |
| Age, years† | 44 (35, 51) | 42 (34, 49) | 43 (35, 49) | 41 (33, 49) |
| Body mass index† | 22.9 (20.8, 25.2) | 22.4 (20.7, 24.2) | 22.4 (20.7, 24.7) | 22.4 (20.8, 23.9) |
| Hepatitis B surface antigen, positive* | 42 (8.2) | 8 (4.4) | 3 (3.3) | 5 (5.6) |
| Aflatoxin–albumin adduct,†‡ pmol/mg protein | 0.41 (0.17, 0.77) | 0.50 (0.30, 0.87) | 0.55 (0.32, 0.90) | 0.47 (0.29, 0.84) |
n (%).
Median (interquartile range).
Two hundred and thirty-eight individuals were screened for levels of aflatoxin–albumin adducts.
Compliance and Data Collection Completeness.
Adherence to the study protocol was outstanding. Only one person, who was randomized to the placebo arm, dropped out of the study. Overall, 97% of the tablets were consumed during the study. Participants took all three tablets per day 94% of the time (placebo = 93.3%; chlorophyllin = 94.8%). Although quite infrequent, the preponderance of skipped administrations occurred during the middle of the day, when study participants were away from their homes at their work sites. Blood and urine samples were to be collected from each participant over the duration of the study. Adherence to this aspect of the protocol also was excellent, with ≈92% contributing all blood samples and 95% contributing all urine samples. Although chlorophyllin is not thought to be readily absorbed, a minor component of the formulation, copper chlorin e4 ethyl ester, was found regularly in the sera of participants receiving active drug, but not placebo (19). These results are consistent with the high level of compliance in the study cohort determined by pill counts. No adverse events were reported in the study group. As anticipated, some participants reported dark-green-colored feces.
Characterization of Aflatoxin-N7-Guanine in Human Urine by Mass Spectrometry.
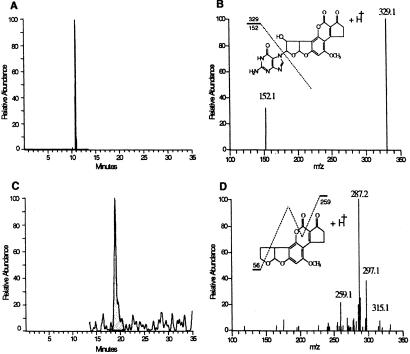
Fig. 4 shows the chromatographic profiles of aflatoxin-N7-guanine isolated from human urine by using LC-MS/MS. As shown in Fig. 4A, the retention time for aflatoxin-N7-guanine is 10.9 min. Fig. 4B illustrates the MS/MS spectra obtained for urinary aflatoxin-N7-guanine and reveals two major daughter ions at MH+ 329.1 and 152.1, reflecting the cleavage of this nucleic acid adduct (m/z 480.1) into 9-hydroxy-aflatoxin and guanine-positive ions. The signal-to-noise ratio was increased substantially by using the selectivity of MS/MS compared with monitoring for the parent ion at m/z 480.1. Thus, monitoring of the fragment ion at 152.1 was used for quantifying levels of aflatoxin-N7-guanine in the clinical trial samples. The retention time of the spiked internal standard AFB2 is 20.6 min (Fig. 4C). Shown in Fig. 4D is the MS/MS fragmentation pattern for AFB2. The minor daughter ion of MH+ 259.1 was used for quantifying AFB2 content because of interfering peaks in human urine for the major ions of 315.2 and 287.2. The 259 daughter ion of AFB2 has been characterized (21).
Figure 4.
Liquid chromatography–electrospray mass spectrometry of aflatoxin-N7-guanine isolated from the urine (0.32 pg/mg creatinine) of a study participant. (A) Chromatogram of the HPLC separation of aflatoxin-N7-guanine after isolation from urine by immunoaffinity chromatography. The profile illustrates the elution of the daughter ion 152.1 MH+ formed from the fragmentation of the parent compound, aflatoxin-N7-guanine (m/z 480.1) at 10.9 min. (B) Two major fragmentation ions obtained from urinary aflatoxin-N7-guanine. (C) HPLC elution of the daughter ion 259.1 MH+ fragmented from the spiked internal standard, aflatoxin B2 (m/z 315.1), which elutes at 20.6 min. (D) Fragmentation pattern for aflatoxin B2.
Diminution of Urinary Aflatoxin-N7-Guanine by Chlorophyllin.
The study was designed to have the statistical power to evaluate biomarker modulation in a single, cross-sectional analysis. Such an approach is necessary because of the short half-life of urinary aflatoxin metabolites. Therefore, analyses were conducted on 169 urine samples collected during week 12. Week 12 was judged a priori as most likely to reveal possible treatment-related effects on aflatoxin-N7-guanine levels because measurements of the concentration of copper chlorin e4 ethyl ester in the sera of study participants indicated that steady-state levels (≈3 μM) were reached after 8–12 weeks of intervention (19). Although samples were collected at other time points for safety monitoring, they have not been analyzed for aflatoxin biomarkers. Aflatoxin-N7-guanine could be detected in 105 (62%) of the 169 available urine samples from the week 12 collection. No significant difference in the percentage of detectable values was seen between treatment arms: 63.4% (53 of 87 samples) of placebo and 60.9% (52 of 82 samples) of chlorophyllin samples. The relatively high percentage of nondetectable values (≈38%) may reflect both insufficient analytical sensitivity in which low exposure occurred and a likely prospect that up to one-third of the study participants did not ingest aflatoxins in meals consumed the day before the urine samples were collected. Because of the short biological half-life (≈8 h) of aflatoxin-N7-guanine, measurement of this biomarker reflects only very recent exposures. By contrast, measures of aflatoxin–albumin adduct levels in serum, as were used for eligibility screening, integrate exposures over a month or more because of the longer half-life (≈21 days) of circulating albumin.
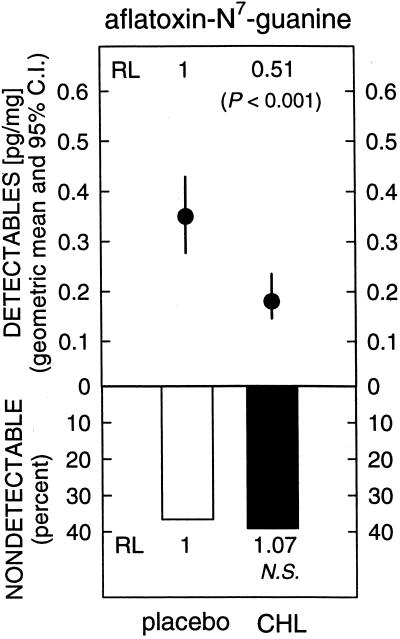
Table 2 shows the distributions of the levels of aflatoxin-N7-guanine in the two intervention arms. The median level of aflatoxin-N7-guanine in the urine of participants receiving placebo was 0.20 pg/mg creatinine, with a range of nondetectable to 4.10 pg/mg. Administration of 100 mg of chlorophyllin three times a day for 12 weeks led to a statistically significant (P = 0.036) 55% reduction in the urinary excretion of aflatoxin-N7-guanine (median, 0.09 pg/mg; range, nondetectable to 0.98 pg/mg). No differences were seen by gender (data not shown). As shown in Fig. 5, when the data were restricted to those with detectable values, the geometric mean level of aflatoxin-N7-guanine adduct in the urine of participants receiving placebo was 0.35 (0.28, 0.44; 95% confidence interval), and for those receiving chlorophyllin it was 0.18 (0.15, 0.24; 95% confidence interval). This 49% reduction in urinary aflatoxin–DNA adduct biomarker levels among participants with detectable levels was highly significant (P < 0.001).
Table 2.
Effect of chlorophyllin on urinary excretion of aflatoxin-N7-guanine
| Treatment assignment | n | Level of aflatoxin-N7- guanine, pg adduct/mg creatinine | P |
|---|---|---|---|
| Placebo, tid | 82 | 0.20 (<LOD–4.10)* | |
| Chlorophyllin, tid | 87 | 0.09 (<LOD–0.98) | 0.036 |
tid, 3 times daily; LOD, limit of detection.
Median (range).
Figure 5.
Geometric means and 95% confidence intervals for the distribution of detectable values for excreted amounts of aflatoxin-N7-guanine 12 weeks after thrice-daily administration of placebo or chlorophyllin (CHL, 100 mg) tablets. RL, relative level; NS, not significant.
Discussion
Sensitive and specific methods continue to be developed for detecting and quantifying levels of covalent adducts of several important classes of carcinogens with cellular DNA at ambient levels of exposure. Such biomarkers are of major interest because they are direct products of damage to critical molecular targets and reflect integration of toxicokinetic factors of absorption, distribution, and metabolism. These adduct biomarkers can be used to document environmental or occupational exposures to carcinogens. Of potentially greater importance, they also can be used to rapidly assess the efficacy of preventive interventions such as exposure abatement and chemoprevention in high-risk cohorts (22). AFB1 undergoes cytochrome P450-catalyzed oxidation to aflatoxin-8,9-epoxide, which can form DNA adducts through covalent binding to the N7 atom of guanine. Aflatoxin-N7-guanine yields predominantly G → T transversion mutations in mutational reporter assays (23). Several independent analyses of mutational patterns in the tumor suppressor gene p53 in HCC occurring in populations in China and Africa exposed to aflatoxins showed high frequencies of G → T transversions, with a clustering at codon 249 (24). Although efficient promutagenic lesions, aflatoxin-N7-guanine adducts are chemically unstable and undergo spontaneous depurination to yield a modified guanine base that can be measured in the urine of aflatoxin-exposed mammals (25). Dose-dependent increases in the levels of aflatoxin-N7-guanine in urine of rats after acute exposure to AFB1 have been observed (25).
Studies conducted in Guangxi Autonomous Region, an area in southern People's Republic of China with a high incidence of liver cancer, determined both dietary intake of aflatoxin and levels of adduct biomarkers over a 1-week period (26). Urinary aflatoxin-N7-guanine showed a dose-dependent relationship with aflatoxin intake. Two nested, case-control studies in Shanghai have examined the relationship between HCC and markers for aflatoxin and hepatitis B virus (3, 4). A highly significant increase in the relative risk (RR = 3.4) of HCC was seen when urinary aflatoxins were detected. The RR for people who tested positive for hepatitis B virus surface antigen was about 7, but individuals positive for both chemical and viral markers had a RR of 59 (3). When individual aflatoxin metabolites were examined for association with HCC, aflatoxin-N7-guanine in urine was shown to result in a 2- to 3-fold higher risk for developing liver cancer than did other metabolites (4). Thus, a relationship between presence of aflatoxin-N7-guanine in urine and risk for HCC in humans has been established.
It is possible to modify risk for hepatocarcinogenesis induced by aflatoxin in animals by using chemopreventive interventions with phenolic antioxidants, 1,2-dithiole-3-thiones, and other agents when they are administered simultaneously with the carcinogen (7). Because risk can be attenuated while AFB1 exposure is held constant, these animal models are useful experimental systems for examining the relationships between aflatoxin biomarkers in biological fluids and cancer risk. Oltipraz [4-methyl-5-(2-pyrazinyl)-1,2-dithiole-3-thione] is a particularly effective inhibitor of aflatoxin hepatocarcinogenesis in rats (27). Accompanying molecular dosimetry studies indicated that levels of aflatoxin-N7-guanine adducts in liver and urine of rats fed oltipraz were reduced. Although strong concordance exists between target tissue and excreted levels of aflatoxin-N7-guanine adducts in experimental models, this biomarker typically underestimates the chemopreventive efficacy of an intervention when compared with reductions in tumor burden (28).
Chlorophyllin also modulates levels of aflatoxin biomarkers in animals. Concurrent oral treatment of rats with 100 mg of chlorophyllin and 10 μg of AFB1 engendered 45–50% reductions in the levels of aflatoxin–DNA adducts in liver and overall excretion of aflatoxin equivalents in urine (14). Specific measurements of urinary aflatoxin-N7-guanine were not undertaken in these studies. The 5,000:1 molar excess of chlorophyllin to AFB1 used in the rodent experiments is about 1/10 the ratio used in the clinical trial. We estimated daily aflatoxin intake in residents of Daxin Township during the oltipraz clinical trial in 1995 to approximately 1–2 μg/day (8). With an assigned daily intake of 300 mg of chlorophyllin, the molar ratio of chlorophyllin to AFB1 for the clinical trial participants was about 50,000–100,000:1. The observed 55% reduction in median excretion of aflatoxin-N7-guanine in the participants consuming chlorophyllin compared with placebo is consistent with the experimental modeling. A similar (49%) but more statistically significant reduction in geometric mean levels of urinary aflatoxin-N7-guanine was observed among those individuals with detectable levels of the biomarker as opposed to all study participants. Loeb has predicted that a 2-fold reduction in mutation rates, as might be anticipated from this decline in DNA adduct burden, could prolong the time between initiation and clinical manifestation of cancer from 20 or more years to 40 or more years (29). Given that the median age of diagnosis for HCC in Qidong is under 50 (1), such a delay could have a major impact upon health in this and other high-risk areas. Follow-up studies using biomarkers more tightly linked to increased risk of HCC, i.e., further along the causal pathway, will be required to confirm this expectation. The development of short oligonucleotide mass analyses methods to detect mutant p53 sequences in DNA obtained from plasma samples is one such approach under evaluation (30).
The results of the chlorophyllin intervention trial suggest that a prescriptive approach by using a safe, over-the-counter medicine can impact favorably upon the toxicokinetics of unavoidable exposures to aflatoxins and other classes of environmental carcinogens. Recent findings indicate that natural chlorophylls also exert protective effects against sequelae of carcinogen exposure in animals (31). Thus, supplementation of diets with foods rich in chlorophylls may be an effective approach to chemoprevention and yet even simpler to implement in many regions of the world.
Acknowledgments
We thank Mr. Herbert Wagner and Dr. Anil Patel of Rystan (Integra LifeSciences) for the generous contribution of placebo and chlorophyllin tablets. We also thank the staff of the Daxin Medical Clinic, the village doctors, and the residents of Daxin Township for their participation; Kevin Kensler for data entry; Xia He for assistance; and Martin Abeloff, Nancy Davidson, and Alvaro Muñoz for reviewing the manuscript. This study was supported by U.S. Public Health Service Grants P01 ES06052 and P30 ES03819.
Abbreviations
- HCC
hepatocellular carcinoma
- AFB1
aflatoxin B1
- AFB2
aflatoxin B2
References
- 1.Zhu Y-R, Chen J-G, Huang X Y. In: Primary Liver Cancer. Tang Z Y, editor. Berlin: Springer; 1989. pp. 204–222. [Google Scholar]
- 2.Yu S-Z. J Gastroenterol Hepatol. 1995;10:674–682. doi: 10.1111/j.1440-1746.1995.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 3.Ross R K, Yuan J-M, Yu M C, Wogan G N, Qian G S, Tu J T, Groopman J D, Gao Y T, Henderson B E. Lancet. 1992;339:943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 4.Qian G-S, Ross R K, Yu M C, Yuan J M, Gao Y T, Henderson B E, Wogan G N, Groopman J D. Cancer Epidemiol Biomarkers Prevent. 1994;3:3–10. [PubMed] [Google Scholar]
- 5.Wang L-Y, Hatch M, Chen C-J, Levin B, You S L, Lu S N, Wu M H, Wu W P, Wang L W. Int J Cancer. 1996;67:620–625. doi: 10.1002/(SICI)1097-0215(19960904)67:5<620::AID-IJC5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Chang M H, Chen C J, Lai M S, Hsu H M, Wu T C, Kong M S, Liang D C, Shau W Y, Chen D S. New Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 7.Kensler T W, Davis E F, Bolton M G. In: The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance. Eaton D L, Groopman J D, editors. San Diego: Academic; 1994. pp. 281–306. [Google Scholar]
- 8.Wang J S, Shen X, He X, Zhu Y R, Zhang B C, Wang J B, Qian G S, Kuang S Y, Zarba A, Egner P A, et al. J Natl Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- 9.Dashwood R, Negishi T, Hayatsu H, Breinholt V, Hendricks J, Bailey G. Mutat Res. 1998;399:245–253. doi: 10.1016/s0027-5107(97)00259-5. [DOI] [PubMed] [Google Scholar]
- 10.Kephart J C. Econ Bot. 1955;9:3–38. [Google Scholar]
- 11.Young R W, Bergei J S. J Am Geriat Soc. 1980;24:46–47. doi: 10.1111/j.1532-5415.1980.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 12.Negishi T, Rai H, Hayatsu H. Mutat Res. 1997;376:97–100. doi: 10.1016/s0027-5107(97)00030-4. [DOI] [PubMed] [Google Scholar]
- 13.Breinholt V, Schimerlik M, Dashwood R, Bailey G. Chem Res Toxicol. 1995;8:506–514. doi: 10.1021/tx00046a004. [DOI] [PubMed] [Google Scholar]
- 14.Kensler T W, Groopman J D, Roebuck B D. Mutat Res. 1998;402:165–172. doi: 10.1016/s0027-5107(97)00294-7. [DOI] [PubMed] [Google Scholar]
- 15.Breinholt V, Arbogast D, Leveland P, Pereira C, Dashwood R, Hendricks J, Bailey G. Toxicol Appl Pharmacol. 1999;158:141–151. doi: 10.1006/taap.1999.8696. [DOI] [PubMed] [Google Scholar]
- 16.Yun C Y, Hye G J, Jhoun J W, Guengerich F P. Carcinogenesis. 1995;16:1437–1440. doi: 10.1093/carcin/16.6.1437. [DOI] [PubMed] [Google Scholar]
- 17.Kamat J P, Boloor K K, Devasagayam P A. Biochim Biophys Acta. 2000;1487:113–127. doi: 10.1016/s1388-1981(00)00088-3. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson L P, Zhang B C, Zhu Y R, Wang J B, Wu Y, Zhang Q N, Yu L Y, Qian G S, Kuang S Y, Li Y F, et al. Cancer Epidemiol Biomarkers Prev. 1997;6:257–265. [PubMed] [Google Scholar]
- 19.Egner P A, Stansbury K H, Snyder E P, Rogers M E, Hintz P A, Kensler T W. Chem Res Toxicol. 2000;13:900–906. doi: 10.1021/tx000069k. [DOI] [PubMed] [Google Scholar]
- 20.Walton M, Egner P, Scholl P F, Walker J, Kensler T, Groopman J D. Chem Res Toxicol. 2001;14:919–926. doi: 10.1021/tx010063a. [DOI] [PubMed] [Google Scholar]
- 21.Plattner R D, Bennett G A, Stubblefield R D. J Assoc Off Anal Chem. 1984;67:734–738. [PubMed] [Google Scholar]
- 22.Kensler T W, Groopman J D, Wogan G N. IARC Sci Publ. 1996;139:237–248. [PubMed] [Google Scholar]
- 23.Foster P L, Eisenstadt E, Miller J H. Proc Natl Acad Sci USA. 1983;80:2695–2698. doi: 10.1073/pnas.80.9.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollstein M, Shomer B, Greenblatt M, Soussi T, Hovig E, Montesano R, Harris C C. Nucleic Acids Res. 1996;24:141–146. doi: 10.1093/nar/24.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett R A, Essigmann J M, Wogan G N. Cancer Res. 1981;41:650–654. [PubMed] [Google Scholar]
- 26.Groopman J D, Zhu J-Q, Donahue P R, Pikul A, Zhang L-S, Chen J-S, Wogan G N. Cancer Res. 1992;52:45–52. [PubMed] [Google Scholar]
- 27.Roebuck B D, Liu Y L, Rogers A R, Groopman J D, Kensler T W. Cancer Res. 1992;51:5501–5506. [PubMed] [Google Scholar]
- 28.Kensler T W, Groopman J D, Sutter T R, Curphey T J, Roebuck B D. Chem Res Toxicol. 1999;12:113–126. doi: 10.1021/tx980185b. [DOI] [PubMed] [Google Scholar]
- 29.Loeb L A. Cancer Res. 2001;61:3230–3239. [PubMed] [Google Scholar]
- 30.Jackson P E, Qian G S, Friesen M D, Zhu Y R, Lu P, Wang J B, Wu Y, Kensler T W, Vogelstein B, Groopman J D. Cancer Res. 2001;61:33–35. [PubMed] [Google Scholar]
- 31.Hartig U, Bailey G S. Carcinogenesis. 1998;19:1323–1326. doi: 10.1093/carcin/19.7.1323. [DOI] [PubMed] [Google Scholar]