Abstract
Helminths are highly prevalent metazoan parasites that infect over a billion of the world’s population. Hosts have evolved numerous mechanisms to drive the expulsion of these parasites via Th2-driven immunity, but these responses must be tightly controlled to prevent equally devastating immunopathology. However, mechanisms that regulate this balance are still unclear. Here we show that the vigorous Th2 immune response driven by the small intestinal helminth Trichinella spiralis, is associated with increased TGFβ signalling responses in CD4+ T-cells. Mechanistically, enhanced TGFβ signalling in CD4+ T-cells is dependent on dendritic cell-mediated TGFβ activation which requires expression of the integrin αvβ8. Importantly, mice lacking integrin αvβ8 on DCs had a delayed ability to expel a T. spiralis infection, indicating an important functional role for integrin αvβ8-mediated TGFβ activation in promoting parasite expulsion. In addition to maintaining regulatory T-cell responses, the CD4+ T-cell signalling of this pleiotropic cytokine induces a Th17 response which is crucial in promoting the intestinal muscle hypercontractility that drives worm expulsion. Collectively, these results provide novel insights into intestinal helminth expulsion beyond that of classical Th2 driven immunity, and highlight the importance of IL-17 in intestinal contraction which may aid therapeutics to numerous diseases of the intestine.
Author summary
Infection with intestinal parasitic worms is a major global health problem. We have therefore evolved means to drive the expulsion of these worms (known as helminths), based on protective (type 2) immune responses. However, if these immune responses are not regulated they can result in more harm than good. One protein that can be key in controlling immune responses is transforming growth factor beta (TGFβ). Using a model helminth which infects mice, we found that TGFβ was indeed signalling to the immune cells which can initiate the type 2 response, but rather than increasing the regulation of these T-cells it was driving a different inflammatory immune response (termed Th17). Interestingly, this Th17 response was important in expelling the parasite, as mice lacking the ability to activate the TGFβ protein, lacked Th17 responses and the ability to contract intestinal muscles and flush out the parasite. Our findings therefore provide new insights into how helminths are expelled and identify potential molecular targets for the prevention of helminth infection which affects billions of the world’s population in deprived communities.
Introduction
Human intestinal helminths infect more than 1 billion of the world’s population, often affecting the most deprived communities [1]. These parasites are one of the most prevalent Neglected Tropical Diseases worldwide bringing huge morbidities to the host population; sub-Saharan Africa alone is estimated to lose 2.3 million disability-adjusted life-years annually [2]. Notwithstanding this hugely successful colonisation, we have evolved numerous Th2-driven mechanisms of parasite expulsion [3–8], which must be tightly regulated to avoid potential immunopathology, such as uncontrolled fibrosis and barrier dysfunction, as seen in ulcerative colitis [9].
The small intestinal helminth Trichinella spiralis is the leading causative agent of trichinosis, which globally exhibits burdens of around 12 million [10], equivalent to kinetoplastid-caused infections such as Leishmania sp. and Trypanosoma cruzi [11]. The life cycle consists of the release of larvae from nurse cells following pepsin digestion of contaminated meat in the stomach, prior to migration and swift development into adults in the small intestine. Male and female adults mate to produce new born larvae which migrate via the blood and lymph to the striated muscle where they form new nurse cells. Mouse models have demonstrated that infection produces a strong CD4+ T-cell [12,13] and type 2 cytokine [14–16] driven transient inflammation culminating in worm expulsion around day 15 post-infection (p.i.) in C57BL/6 mice. IL-9 driven mastocytosis [17] is key in T. spiralis expulsion [18–20], driving the degradation of epithelial tight junctions via the release of mast cell proteases during degranulation [21,22]. The resulting increase in luminal fluid, works in combination with Th2 driven alterations of enhanced intestinal propulsive activity. IL-13 and IL-4, signalling via signal transducer and activator of transcription factor 6 (STAT6) [23] on smooth muscle cells [24], allow jejunal muscle hypercontractility [23–25]. Despite the potential for immunopathology in terms of intestinal barrier weakening and exposure to luminal commensals, in combination these pathways produce the “weep and sweep’ mechanism [26], to drive out the enteric stage of infection with only short-lived pathology.
In comparison to other helminths, T. spiralis infection produces a robust Th2 response with evident pathology in terms of weight loss prior to intestinal worm expulsion [27], while the following encapsulation of new born larvae within the striated muscle is associated with a general malaise. Previous work has demonstrated the importance of the pluripotent cytokine TGFβ in the chronic muscular phase of the parasite life cycle [28], but the role of this complex cytokine during the intestinal phase remains unclear. Given the fundamental importance of TGFβ in regulating many aspects of T-cell biology [29] we chose to investigate the mechanistic function of TGFβ signalling in regulating the potential pathological immune response during T. spiralis enteric infection.
Here, we demonstrate that mice infected with T. spiralis, display enhanced TGFβ signalling in intestinal CD4+ T-cells which drives Th17 induction, as opposed to an increased regulatory T-cell (Treg) response. We find that the expression of integrin αvβ8 on dendritic cells (DCs), previously shown to be key in activating TGFβ and maintaining Tregs during intestinal homeostasis [30,31], is essential for the induction of TGFβ signalling in CD4+ T-cells and the generation of Th17 cells during infection. Importantly, mice lacking integrin αvβ8 on DCs (Itgb8 (Cd11c-cre)) have a delayed ability to expel the intestinal stage of the infection, despite an equivalent Th2 response to wild-type controls. Utilising the DEREG system for Treg ablation [32] demonstrates an essential requirement of Tregs for parasite expulsion, yet the adoptive transfer of Tregs into Itgb8 (Cd11c-cre) mice suggests that the reduced Treg level seen is not responsible for the delayed parasite expulsion in this model. Instead, we show that the Th17 response promotes intestinal contractility and the “sweep” mechanism of parasite expulsion. Our results therefore provide novel insights into the role of TGFβ during intestinal helminth infection, contributing greater understanding to mechanisms of helminth expulsion and potentially enteric diseases encompassing muscle hypercontractility.
Results
Small intestinal helminth infection with Trichinella spiralis results in increased TGFβ signalling in CD4+ T-cells, inducing Th17 rather than Foxp3+ regulatory T-cells
Expulsion of the small intestinal helminth T. spiralis is associated with a strong and acute T-helper 2 (Th2) CD4+ T-cell response, around one week p.i. in mice ([12–16] and S1A Fig). Mice develop a biphasic morbidity in parallel to the enteritis and myositis of infection [27], indicating a need to regulate this strong inflammatory response. We investigated the mechanistic role of the pluripotent cytokine TGFβ, which regulates many aspects of innate and adaptive immunity including T-cells [29], during the potential pathological immune response during T. spiralis enteric infection.
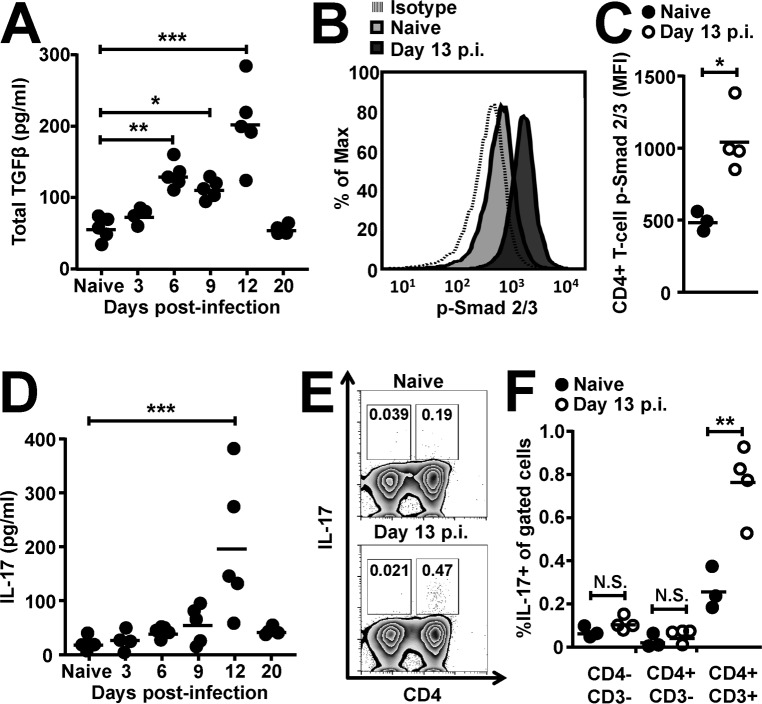
Wild-type C57BL/6 mice were infected with 300 T. spiralis larvae and followed throughout the time course of infection. We analysed parasite-specific cytokine production from mesenteric lymph node (mLN) cell preparations and saw a significant increase in TGFβ secretion in parallel to enhanced Th2 responses (IL-13, IL-9 and IL-4 production) at day 6 p.i. (Fig 1A and S1A Fig). Interestingly, in contrast to the reduction in IL-4, IL-9 and IL-13 cytokine release later in infection (S1A Fig), we saw a stronger, secondary peak of TGFβ at day 12 p.i. (Fig 1A). As TGFβ is produced as a latent cytokine requiring activation, we examined phosphorylation of Smad 2/3 (p-Smad2/3), which is the initial signalling event triggered by engagement of active TGFβ with its receptor. We saw significantly increased p-Smad2/3 levels in CD4+ T cells at day 13 p.i. in the small intestinal lamina propria (SILP) intestinal niche of the parasite (Fig 1B and 1C), indicating enhanced activation of TGFβ.
Fig 1. Infection with the small intestinal helminth T. spiralis increases TGFβ signalling in CD4+ T-cells producing late Th17 cell induction.
Wild-type C57BL/6 mice were infected with 300 T. spiralis larvae and examined at the indicated time points. (A) Total TGFβ cytokine levels from T. spiralis antigen-stimulated mLN cells across the time-course of intestinal infection, determined via ELISA. (B) Representative flow cytometry plots and (C) mean fluorescence intensities for p-Smad 2/3 staining in small intestinal lamina propria CD4+ T-cells from uninfected and day 13 post-infected mice. (D) IL-17 cytokine levels from T. spiralis antigen-stimulated mLN cells across the time-course of intestinal infection, determined via cytometric bead array. (E) Representative flow cytometry plots of total CD45+ small intestinal lamina propria cells and (F) percentage IL-17 expression in small intestinal lamina propria CD4-CD3-, CD4+CD3- and CD4+CD3+ gated cells from uninfected and day 13 post-infected mice. Data (n = 3–5 mice per group) are from two independent experiments performed.*, P<0.05; **, P<0.01; ***, P<0.005; N.S., not significant via Dunnett’s multiple comparison following ANOVA (A) and (D) or student’s t-test (C) and (F) for the indicated comparisons between groups.
TGFβ signalling in CD4+ T-cells can result in the induction of Th17 [33–35], Th9 [36,37] or peripheral Treg subsets [38], depending on co-stimulatory signals and the surrounding cytokine milieu. Although we did not see any significant increase in IL-9 secretion at day 12 p.i. (S1A Fig), nor increase in the percentage of IL-9 expressing mLN CD4+ T-cells (S1B Fig) or Foxp3 expression in small intestinal CD4+ T-cells around this time-point (S1C and S1D Fig), we did see a significant increase in IL-17 secretion at day 12 p.i.in parallel to the secondary peak of TGFβ production (Fig 1D). This increase in IL-17 production was also concomitant with a significant increase in IL-6 (S1A Fig), which can synergise with TGFβ to drive Th17 cell induction [39]. Indeed, on performing intracellular flow cytometry we identified CD4+ cells as the source of the IL-17 produced during this infection (Fig 1E), with additional gating showing significant increases in IL-17 seen within the CD4+CD3+ T-cell gated population during infection (Fig 1F).
These data indicate that TGFβ signalling in CD4+ T-cells is induced during the enteric stage of T. spiralis infection and is associated with Th17 cell induction subsequent to the classical Th2 response.
Expression of the TGFβ-activating integrin αvβ8 by DCs propagates TGFβ signalling in CD4+ T-cells and expulsion of enteric T. spiralis infection
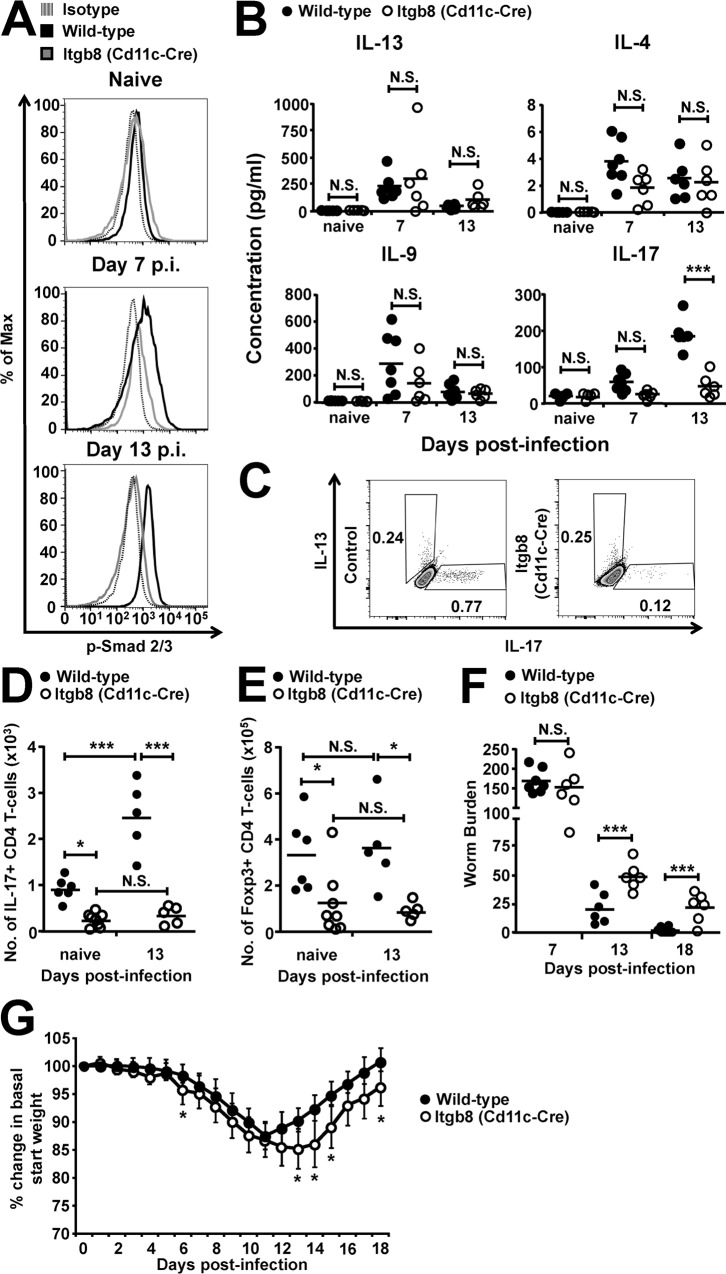
We next sought to determine the mechanisms responsible for enhanced TGFβ signalling during T. spiralis infection. The requirement for the activation of latent TGFβ prior to function [40] led us to investigate the potential for integrin αvβ8, a key activator of latent TGFβ in the intestine expressed by dendritic cells (DCs) [30,31,41], to be responsible for the enhanced signalling seen in CD4+ T-cells. To this end, we analysed T-cell responses following infection with 300 T. spiralis larvae in mice lacking integrin αvβ8 on DCs (Itgb8 (CD11c-Cre) mice [30]) and wild type littermate controls. We found that the increase in TGFβ signalling observed in CD4+ T-cells during T.spiralis infection was significantly reduced in Itgb8 (CD11c-Cre) mice, with pSmad2/3 levels remaining similar to those observed in uninfected mice (Fig 2A). Interestingly, this lack of TGFβ signalling in CD4+ T-cells did not affect the classical Th2, Th9 nor Th1 immune cytokine responses during the time-course of infection, with no significant difference observed in parasite specific IL-13, 4, 9 (Fig 2B) and IFNγ (S2A Fig) production from mLN antigen restimulation. This was also reflected in the similar IgG response seen at day 18 post-infection (S2B Fig), which is a key indicator of Th1/2 balance, and IL-9 expression in mLN CD4+ T-cells at day 13 post-infection (S2C Fig). However, IL-17 production was significantly reduced at day 13 p.i., in both mLN restimulations (Fig 2B) as well as from small intestinal lamina propria CD4+ T-cells (Fig 2C), which were also observed to produce similar IL-13 levels (Fig 2C). Indeed, beyond the previously reported initial baseline differences in intestinal Th17 cells in Itgb8 (CD11c-Cre) mice ([30] and (Fig 3D)), total small intestinal lamina propria IL-17+ CD4+ T-cell numbers failed to significantly increase following infection at day 13 p.i. in Itgb8 (CD11c-Cre) mice as compared to wild-types (Fig 2D). Interestingly, we also observed a significant reduction in small intestinal lamina propria Foxp3+ regulatory T-cells at rest in the Itgb8 (CD11c-Cre) mice, with neither wild-type or Itgb8 (CD11c-Cre) mice Treg numbers altering during enteric T. spiralis infection (Fig 2E). Thus, during enteric T. spiralis infection, enhanced TGFβ activation by integrin αvβ8 on DCs is important in triggering infection-induced TGFβ signalling pathways in CD4+ T-cells, driving Th17 cells, and maintaining Treg numbers during homeostasis.
Fig 2. Mice lacking the TGFβ-activating integrin αvβ8 on DCs have delayed expulsion of the small intestinal helminth T. spiralis.
Wild-type and Itgb8 (CD11c-cre) mice were infected with 300 T. spiralis larvae and examined at the indicated time-points post-infection. (A) Representative flow cytometry plots for p-Smad 2/3 staining in small intestinal lamina propria CD4+ T-cells. (B) IL-13, IL-4, IL-9, and IL-17 cytokine levels from T. spiralis antigen-stimulated mLN cells from wild-type and Itgb8 (CD11c-cre) mice, determined via ELISA. (C) Representative flow cytometry plots for intracellular IL-17 and IL-13 expression in small intestinal lamina propria CD4+ T-cells isolated from wild-type and Itgb8 (CD11c-cre) mice at day 13 post-infection. Number of (D) IL-17+ and (E) Foxp3+ CD4 T-cells in the small intestinal lamina propria of wild-type and Itgb8 (CD11c-cre) mice, assessed via flow cytometry. (F) Worm burdens from wild-type and Itgb8 (CD11c-cre) mice at days 7, 13 and 18 p.i. (G) Percentage change in basal start weight in wild-type and Itgb8 (CD11c-cre) mice over the course of infection. Data (n = 6–10 mice per group) are from two independent experiments performed. *, P<0.05; ***, P<0.005; N.S., not significant via Bonferonni’s multiple comparison following ANOVA (B), (D), and (E) or student’s t-test (F)and (G) for the indicated comparisons between groups.
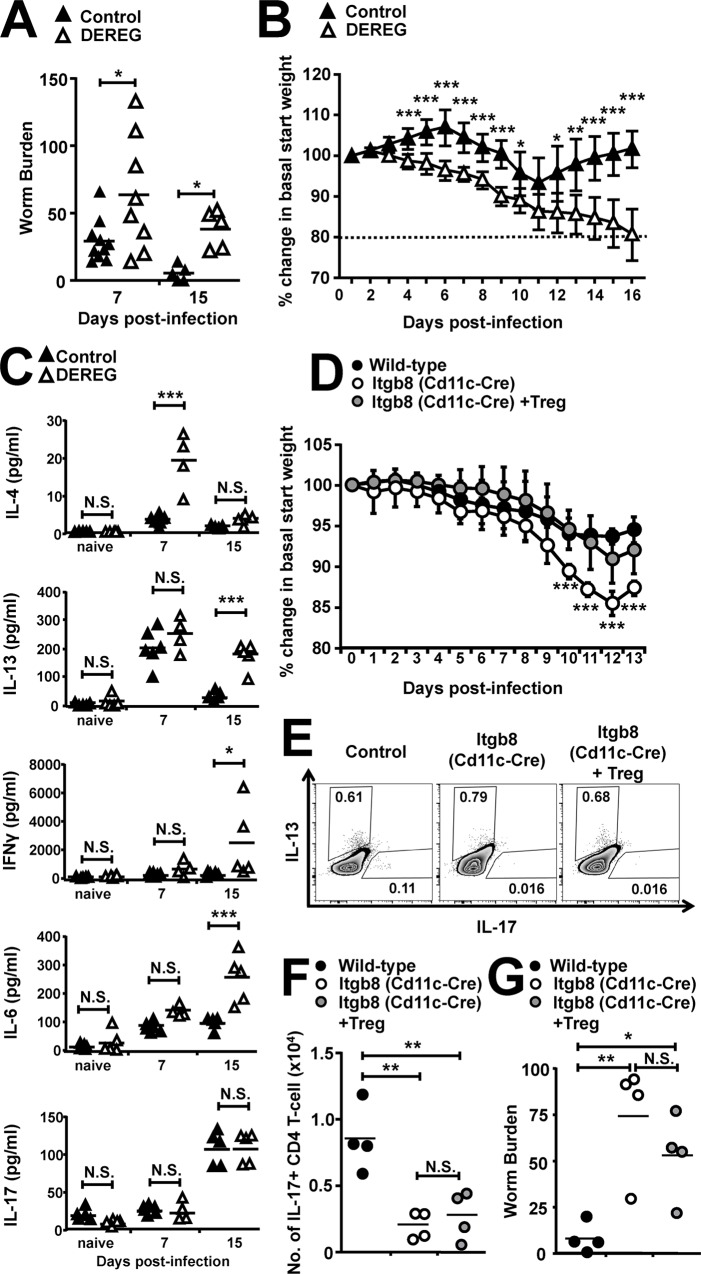
Fig 3. Depletion of Foxp3+ Tregs during T. spiralis infection results in extreme morbidity and delayed helminth expulsion, but the immune kinetics and delayed expulsion seen in mice lacking the TGFβ-activating integrin αvβ8 on DCs are independent of Tregs.
DEREG mice were treated every 2 days with 200 ng diphtheria toxin or PBS (Control) 2 days prior to infection with 300 T. spiralis larvae and examined at the indicated time-points post-infection. (A) Worm burdens from control and DEREG mice at days 7 and 15 following infection. (B) Percentage change in basal start weight in control and DEREG mice during time course of infection, dashed line indicates point of morbidity sacrifice threshold. (C) IL-4, IL-13, IFNγ, IL-6 and IL-17 cytokine levels from T. spiralis antigen-stimulated mLN cells from control and DEREG mice at different time-points post-infection, determined via CBA. Data (n = 4–11 mice per group) are from two independent experiments performed. Wild-type, Itgb8 (CD11c-cre) and Itgb8 (CD11c-cre) mice adoptively transferred with 1x106 Tregs were infected with 300 T. spiralis larvae 2 days following cell transfer and examined at the indicated time-points post-infection. (D) Percentage change in basal start weight in wild-type, Itgb8 (CD11c-cre) and Itgb8 (CD11c-cre) mice adoptively transferred with Tregs during time course of infection. (E) Representative flow cytometry plots for intracellular IL-17 and IL-13 expression in mLN CD4+ T-cells isolated from wild-type, Itgb8 (CD11c-cre) and Itgb8 (CD11c-cre) mice adoptively transferred with Tregs, at day 13 post-infection. (F) Number of IL-17+ CD4 T-cells in the mLN of wild-type, Itgb8 (CD11c-cre) and Itgb8 (CD11c-cre) mice adoptively transferred with Tregs, at day 13 post-infection, assessed via flow cytometry. (G) Worm burdens from wild-type, Itgb8 (CD11c-cre) and Itgb8 (CD11c-cre) mice adoptively transferred with Tregs, at day 13 following infection. Data (n = 4 mice per group) are from two independent experiments performed.*, P<0.05; **, P<0.01; ***, P<0.005; N.S., not significant via Bonferonni’s multiple comparison following ANOVA (C), (F) and (G), and student’s t-test (A), (B) and (D) for indicated comparisons between groups.
Strikingly, and despite the maintained Th2 and Th9 response in Itgb8 (CD11c-Cre) mice, we observed a significant delay in worm expulsion and exacerbated weight loss (Fig 2F and 2G) following infection, as compared to wild-type mice. This delay was not associated with differences in other proposed mechanisms involved in helminth expulsion, with no significant difference in crypt/villus architecture (S2D Fig), goblet cell hyperplasia [42] (S2E Fig), mastocytosis [18–20] and associated MMCP-1 production [21,22] (S2F and S2G Fig) or RELMβ expression [43] (S2H Fig) between wild-type and Itgb8 (CD11c-Cre) mice. Collectively these data indicate that despite the maintenance of a Th2 response in Itgb8 (CD11c-Cre) mice, TGFβ activation by integrin αvβ8 on DCs is essential for triggering TGFβ signalling pathways in CD4+ T-cells and promoting parasite expulsion.
Foxp3+ Tregs are required for efficient T. spiralis worm expulsion, but their adoptive transfer does not rescue Th17 cell numbers or helminth expulsion in mice lacking the TGFβ-activating integrin αvβ8 on DCs
We next focussed on uncovering the mechanisms responsible for the delayed expulsion of the small intestinal helminth T. spiralis from mice lacking the TGFβ activating integrin αvβ8 on DCs. Given the stark baseline reduction in small intestinal Foxp3+ Tregs in Itgb8 (CD11c-Cre) mice (Fig 2E), we utilised the DEREG mouse model, which allows specific ablation of Foxp3+ Tregs by injection of diphtheria toxin [32], to directly test the functional role of Foxp3+ Tregs during infection. DEREG mice treated with diphtheria toxin had successful complete depletion of Foxp3-GFP+ cells during the time course of the experiment, although we did see grow back of non-GFP Foxp3+ cells (S3A Fig), which have previously been demonstrated to possess no inhibitory function [44]. We found that worm burdens in DEREG mice recapitulated the delayed expulsion seen in Itgb8 (CD11c-Cre) mice, with significantly increased worm burdens observed at day 7 and 15 p.i. (Fig 3A). Furthermore, as in Itgb8 (CD11c-Cre) mice, a heightened weight loss was apparent, but this took on differing kinetics, with mice presenting with sustained significant weight loss from day 4 p.i. in DEREG mice versus day 13 p.i. in Itgb8 (CD11c-Cre) mice (Fig 3B versus Fig 2G). Moreover, this weight loss in infected DEREG mice did not recede, despite attempts to rehydrate the animals with saline, resulting in mice reaching the threshold for humane end-point and the cessation of the experiments at day 15 p.i.(Fig 3B).
To try and decipher reasons behind this extreme morbidity, we examined parasite-specific cytokine responses following mLN antigen restimulation. In stark contrast to Itgb8 (CD11c-Cre) mice, we observed significant increases in IL-4 production at day 7 p.i.; while IL-13 and IFNγ increased at day 15 p.i. (Fig 3C). Interestingly no differences were seen in parasite–specific IgG antibody nor MMCP-1 production, as compared to untreated control mice, indicating no overall imbalance in the Th1/Th2 paradigm (S3B and S3C Fig). Recent publications have discovered an essential role for Foxp3+ Tregs in eliminating the small intestinal helminth Heligmosoides polygyrus, with Treg depletion associated with delayed worm expulsion following an uncontrolled “cytokine storm” [45]. We therefore looked at other pro-inflammatory cytokines and we did indeed see a significant increase in IL-6 at day 15 p.i. (Fig 3C). Importantly, we did not see the reduction in IL-17 later in infection in DEREG mice, as seen in Itgb8 (CD11c-Cre) mice (Fig 2B vs. Fig 3C).
Despite the clear evidence demonstrating a complete lack of Tregs could mediate worm expulsion and weight loss during T. spiralis infection, we next asked if the adoptive transfer of Tregs to Itgb8 (CD11c-Cre) mice was sufficient to rescue worm expulsion kinetics. Despite the successful restoration of small intestinal lamina propria Foxp3+ cells (S3D and S3E Fig) resulting in augmented percentage weight (Fig 3D), we saw no alteration in IL-13 or IL-17 production in Treg treated Itgb8 (CD11c-Cre) mice (Fig 3E and 3F), culminating in similar delayed expulsion as in untreated Itgb8 (CD11c-Cre) mice (Fig 3G).
Collectively, these data suggest Foxp3+ Tregs are an important cell type in the context of T. spiralis infection and are required for efficient expulsion of small intestinal helminths via inhibiting runaway inflammation, as well as modulating weight loss pathology. However, given the increased Th1 and Th2 cytokines but maintenance of IL-17 production in the DEREG system and the failure of Treg adoptive transfer to rescue Itgb8 (CD11c-Cre) delayed worm expulsion, this mechanism seems not to be solely responsible for the phenotype displayed in T. spiralis infected Itgb8 (CD11c-Cre) mice.
IL-17 drives intestinal muscle hypercontractility during T. spiralis infection
Given that the adoptive transfer of Tregs into Itgb8 (CD11c-Cre) mice restored weight loss kinetics but not worm expulsion, coupled with the strong Th2 response and accompanying effector mechanisms seen in infected Itgb8 (CD11c-Cre) mice, we next examined a role for the altered Th17 cell population during this infection. We hypothesised that IL-17 may influence muscle hypercontractility rather than mastocytosis induced luminal fluid increases, hence the “sweep” but not the “weep” aspect during expulsion of the enteric phase of T. spiralis.
To investigate the individual importance of IL-17 in T. spiralis infection, we blocked IL-17 from day 7 p.i. in C57BL/6 mice via antibody depletion. Although we saw no significant difference in weight or worm burdens when IL-17 was depleted from day 7 p.i. (Fig 4A and 4B), we did see a significant reduction in in vivo transit time in the small intestine, as measured by the transit of orally gavaged carmine dye (Fig 4C and 4D). Importantly, the depletion of IL-17 did not impinge on the CD4+ mLN T-cell production of IL-13 (or IFNγ) (S4A and S4B Fig), suggesting that alterations in transit time were possibly due to the absence of IL-17, rather than a follow-on effect of reduced Th2 cytokines known to induce small intestinal hypercontractility [23,24]. We next isolated jejunal smooth muscle and confirmed the expression of the IL-17ra via qPCR both at rest and following infection with T. spiralis (Fig 4E). This suggested the potential for IL-17 to directly influence intestinal smooth muscle contraction.
Fig 4. IL-17 drives intestinal muscle hypercontractility during T. spiralis infection and ex vivo via the ROCK signalling pathway.
C57BL/6 mice were infected with 300 T. spiralis larvae and treated with 100μg of anti-IL-17 or control antibody (Bio-X-Cell) every 3 days from day 7 post-infection. (A) Percentage change in basal start weight in control and α-IL-17 treated mice over the course of infection. (B) Worm burdens from control and α-IL-17 treated mice at days 13 and 18 p.i. Chow was removed 12 hrs prior to sacrifice at day 13 and mice received 200μls carmine red in methylcellulose 20 minutes before sacrifice. (C) Representative macroscopic images, arrow indicates front of dye and scale bar = 1 cm, and combined data (D). Data (n = 5 mice per group) are from two independent experiments performed. (E) Expression of IL-17ra in isolated jejunal muscle layer at rest and day 13 p.i, via qPCR relative to HPRT housekeeping gene. (F) Isolated jejunal strips from C57BL/6 wild-type mice were incubated in media with/without the addition of 10ng/ml rIL-17 for 6 hours prior to measuring longitudinal muscle tension generated in response to carbachol (10-6M) in an isolated tissue bath and (G) with/without the prior addition of the COX-2 and ROCK inhibitors celecoxib (10μM) and Y-27632 (10μM) and STAT6 inhibitor AS1517499 (100nm). Data (n = 3–5 mice per group) are from two independent experiments performed. *, P<0.05; **, P<0.01; ***, P<0.005; N.S., not significant via Bonferonni’s multiple comparison following ANOVA (G) and student’s t-test (A), (B), (D), (E) and (F) for indicated comparisons between groups.
To investigate this hypothesis, we first incubated isolated jejunal strips of intestine from wild-type mice with or without rIL-17 prior to assessing longitudinal muscular tension ex vivo generated in response to stimulation with carbachol. Treatment with rIL-17 produced a significant increase in tension (Fig 4F), indicating IL-17 could promote tension and therefore potentially drive parasite expulsion. We next asked what downstream pathways could be responsible for transposing the IL-17 signal, with COX-2 and STAT6 pathways previously being shown to drive TGFβ and IL-4/13 intestinal contraction respectively, following T.spiralis infection [24,46]. To this end, we repeated ex vivo contraction experiments with prior exposure to inhibitors for both pathways, but detected no alteration in the hypercontraction response to carbachol following rIL-17 incubation (Fig 4G). Previous studies have demonstrated that Rho kinase signalling is emerging as an important mediator of intestinal smooth muscle contraction [47], with IL-13 and TNFα driving smooth muscle contraction via the small GTPase, RhoA via STAT6 and NF-κβ signalling respectively [48]. We therefore targeted the RhoA downstream effector kinases via prior exposure to a ROCK pathway inhibitor, and observed an inhibition of the ability of IL-17 to produce significant hypercontraction in response to carbachol (Fig 4G).
Collectively, these data show that, although not solely sufficient for worm expulsion or altered weight loss, IL-17 has direct effects on small intestinal hypercontractility, acting via the ROCK signalling pathway, and could potentially be responsible for the delayed expulsion seen in T. spiralis infected Itgb8 (CD11c-Cre) mice.
rIL-17 treatment following T. spiralis infection rescues intestinal muscle hypercontractility and worm expulsion in mice lacking the TGFβ-activating integrin αvβ8 on DCs
Given the role of IL-17 in driving small intestinal contraction, we tested whether the reduced levels of parasite specific IL-17 production seen in Itgb8 (CD11c-Cre) mice were responsible for delayed worm expulsion via a reduced small intestinal hypercontractility. To this end, we examined if we could rescue delayed expulsion in these mice via treatment with recombinant IL-17. Treatment with rIL-17 from day 9 p.i. completely restored the weight loss kinetics (Fig 5A) to levels seen in wild-type mice. This rescue of weight loss following rIL-17 treatment was not associated with any changes in parasite-specific IL-4, IL-13 or IFNγ cytokine production (Fig 5B), nor in parasite specific IgG responses (S5A Fig).
Fig 5. rIL-17 treatment following T. spiralis infection restores worm expulsion in mice lacking the TGFβ-activating integrin αvβ8 on DCs via rescuing intestinal muscle hypercontractility.
Wild-type and Itgb8 (CD11c-cre) mice were infected with 300 T. spiralis larvae and treated with PBS or 2ug of recombinant IL-17 every 3 days from day 9 post-infection and examined at the indicated time-points post-infection. (A) Percentage change in basal start weight in wild-type and Itgb8 (CD11c-cre) PBS or rIL-17 treated mice over the course of infection. (B) IL-4, 13 and IFNγ cytokine levels from T. spiralis antigen-stimulated mLN cells at day 13 post-infection, determined via ELISA. (C) Jejunal longitudinal muscle tension generated in response to carbachol (10-6M) from wild-type and Itgb8 (CD11c-cre) mice PBS or rIL-17 treated, intestinal contraction was examined in an isolated tissue bath at time points indicated. Wild-type and Itgb8 (CD11c-cre) mice were infected with 300 T. spiralis larvae and treated with PBS, 2ug of recombinant IL-17 every 3 days from day 9 post-infection or adoptively transferred with 1x106 Tregs 2 days prior to infection and examined at the indicated time-points post-infection. Chow was removed 12 hrs prior to sacrifice at day 13 and mice received 200μls carmine red in methylcellulose 20 minutes before sacrifice. (D) Representative macroscopic images, arrow indicates front of dye and scale bar = 1 cm, and combined mean data of dye front (E). (F) Worm burdens from wild-type and Itgb8 (CD11c-cre) PBS or rIL-17 treated mice at days 13 and 18 following infection. Data (n = 4–8 mice per group) are from two-three independent experiments performed. *, P<0.05; **, P<0.01; ***, P<0.005; N.S., not significant via Bonferonni’s multiple comparison following ANOVA (B), (C), (E) and (F) and student’s t-test (A) for indicated comparisons between groups.
Next, we examined isolated longitudinal muscle tension between jejunal samples from wild-type and Itgb8 (CD11c-Cre) mice. Although there was no differences in tension either at baseline nor following carbachol treatment in naïve mice (S5B Fig and Fig 5C), following infection Itgb8 (CD11c-Cre) mice failed to significantly increase jejunal tension in response to stimulation with carbachol at day 13 p.i., as seen in in wild-type infected mice (Fig 5C and [23–25]). Moreover, the treatment of infected Itgb8 (CD11c-Cre) mice with rIL-17 rescued this muscular tension to wild-type levels ex vivo (Fig 5C). Next, we examined in vivo contraction in the small intestine and despite no alteration at base line (Fig 5E and S5C Fig), we saw significantly delayed transit time following infection in Itgb8 (CD11c-Cre) mice, which was again rescued via the addition of rIL-17, but could not be restored by the adoptive transfer of Tregs (Fig 5D and 5E). Strikingly, in parallel to this recued small intestinal contraction, treatment with rIL-17 from day 9 p.i. completely restored the worm burden kinetics in infected Itgb8 (CD11c-Cre) mice (Fig 5F) to levels seen in wild-type mice.
In sum, these data indicate that TGFβ activation by integrin αvβ8 on DCs is essential for triggering TGFβ signalling pathways in CD4+ T-cells allowing the maintenance of Tregs and induction of Th17 cells during T. spiralis infection. Tregs play a key role in mediating weight loss and aiding helminth expulsion via inhibiting runaway inflammation, while Th17 produced IL-17 contributes to enhanced muscular “sweep” tension promoting parasite expulsion.
Discussion
We have evolved immune driven mechanisms to allow the expulsion of intestinal helminths, with the “weep and sweep” supplied by increased intestinal epithelial permeability and muscle contraction [21–25] essential during T. spiralis infection. In most cases these expulsion mechanisms rely on Th2 cytokines resulting in minimal host damage indicating an essential role for regulation to avoid immunopathology; however the pathways and mechanisms involved remain unclear. Our data now indicate an essential role for TGFβ, activated via DC expressed integrin αvβ8, in parasite expulsion via the maintenance of Tregs and induction of Th17 cells, as opposed to simply immuno-regulation. Using the small intestinal dwelling helminth T. spiralis, we observed increased TGFβ signalling in CD4+ T-cells and production of Th17 cells late in infection. Mechanistically, we find that enhanced TGFβ signalling in T-cells occurs via expression of the TGFβ-activating integrin αvβ8 on DCs and that DC-specific lack of this integrin results in increased weight loss and delayed worm expulsion, despite the occurrence of the “classical” Th2 response. The total ablation of Tregs, in the DEREG model, demonstrates a role for this cell in aiding helminth expulsion via inhibiting runaway inflammation, while their adoptive transfer into Itgb8 (CD11c-Cre) mice indicates a key role in mediating infection induced weight loss. Moreover, Itgb8 (CD11c-Cre) mice lack intestinal hypercontractility that can be rescued via treatment with recombinant IL-17, fully restoring both weight loss and worm expulsion kinetics. We have therefore identified a novel, non-Th2 based, mechanistic pathway that could potentially be targeted to treat helminth infection and contractile diseases of the intestine.
Previously, TGFβ signalling within T-cells has been shown to play an important role in downregulating Th2 responses via downregulation of the key transcription factor GATA-3 [49,50]. Indeed, we have previously shown that enhanced TGFβ signalling in T-cells during chronic Th1-induced Trichuris muris infection also occurs via expression of the TGFβ-activating integrin αvβ8 on DCs. Moreover the lack of this integrin on DCs completely protects mice from T. muris infection due to an enhanced protective Th2 response in this model of large intestinal infection [51]. However, here, we did not see any alteration in parasite-specific Th2 responses associated with delayed parasite expulsion, nor any increase in IFNγ production in T. spiralis infected Itgb8 (CD11c-Cre) mice. These data may represent tissue-specific effects of TGFβ activation in the small and large intestine, or more likely that it is mechanistically difficult to surpass the robust Th2 driven cytokine response seen during a normal T. spiralis infection.
Instead we saw a lack of IL-17 production at day 13p.i. in mice lacking the TGFβ-activating integrin αvβ8 on DCs, accompanying an unaltered Th1/Th2 balance. ILC3s are known as important producers of IL-17 at mucosal barriers [52]; however, it appeared that the IL-17+ population was found within the CD3/CD4+ T-cell pool, therefore likely bona-fide Th17 cells. Increased TGFβ release is seen in human DCs following treatment with T. spiralis antigen [53], although these DCs go on to favour a Th2 rather than a Th17 response, indicating that other cellular populations or subsets are producing cytokines which favour Th17 induction during in vivo infection.
Along with TGFβ, numerous cytokines are involved in Th17 induction, including IL-6, IL-21, IL-1β and IL-23 (reviewed in [39]). The production of IL-6 specifically at day 13p.i. is likely to be driving the Th17 induction [54] and possibly explains why we saw minimal IL-17 production corresponding with the initial peak of TGFβ at day 6 p.i. The source of IL-6 remains elusive, but Th17 induction via DC produced TGFβ relies on IL-6 production from a CD301b DC population during intranasal infection [55], indicating a possible DC source. Overall, it will be interesting to define what cytokines and from which cells are involved in inducing the Th17 seen during T. spiralis infection. Furthermore, it is interesting to postulate the antigen specificity in the system. The data displayed are based on parasite-specific cytokine responses as well as PMA/ionomycin re-stimulation and, given helminths directly influence the intestinal microbiome [56,57], it remains to be seen if Th17 responses to bacterial antigens would influence the outcome to T. spiralis infection.
Our initial hypothesis to explain the delayed parasite expulsion was based on the previous finding that TGFβ-activating integrin αvβ8 is key in Treg development, as mice lacking the integrin on DCs have reduced Foxp3+ Tregs within the colonic lamina propria [30]. We therefore predicted that a possible reduction in Tregs in the small intestine of Itgb8 (CD11c-Cre) mice could be playing a role in the delayed expulsion seen during T. spiralis infection. Indeed, recent publications have demonstrated a requirement for Tregs for efficient helminth expulsion in the small intestinal H. polygyrus model [45]. Of note previous findings have demonstrated that H. polygyrus produces a TGFβ mimic which acts as an immunomodulatory agent aiding chronicity [58], while our results suggest host TGFβ promotes expulsion of T. spiralis, as in our hands T. spiralis antigens have no TGFβ like properties [59]. This disparity could possibly be explained by the differing tissue localisation of the helminths during establishment, sub-mucosal versus epithelial niches or the local cytokine milieu, as H. polygyrus infection suppresses IL17 production [60]. However, the demonstration of reduced Tregs within the small intestinal lamina propria of Itgb8 (CD11c-Cre) mice, coupled with the delayed expulsion and increased weight loss in Treg depleted DEREG mice was initially indicative that reduced Treg numbers were solely responsible for the phenotype seen in Itgb8 (CD11c-Cre) mice. However, the extreme morbidity and mixed cytokine production observed, with no difference in IL-17 production, supported the previous hypothesise of “immunological chaos” in these mice. These results, coupled with the failure to rescue intestinal hypercontractility and worm expulsion kinetics when Itgb8 (CD11c-Cre) had been successfully adoptively transferred with Tregs, pointed towards additional mechanisms involved in T. spiralis delayed expulsion in Itgb8 (CD11c-Cre) mice. Adoptive transfer of Tregs was sufficient to return weight loss to wild-type levels, which has previously been shown to be mediated by the peptide hormone cholecystokinin [27]. It will therefore be of interest to examine any potential for Tregs to interact with production of cholecystokinin from enteroendocrine cells, given the recent interest in the immunoendocrine axis [61].
We have recently identified activated Tregs as expressing the TGFβ-activating integrin αvβ8 [62] which in the presence of IL-6 allows Tregs to induce Th17 cells in a GARP-dependent process [63]. It was therefore possible that the reduced small intestinal Treg numbers seen in Itgb8 (CD11c-Cre) mice were also responsible for the reduction in Th17 induction during T. spiralis infection. However, given that Treg depleted DEREG mice still mounted similar IL-17 responses as infected controls and the adoptive transfer of Tregs into Itgb8 (CD11c-Cre) mice failed to rescue Th17 numbers, the delayed parasite expulsion and reduced Th17 induction appears independent of Treg activation of TGFβ, and directly dependent on DCs.
We began to examine several other mechanisms of helminth expulsion, and saw no changes in goblet cell kinetics or mastocytosis. Mucosal mast cells are also under the control of TGFβ, with the cytokine controlling mast cell expression of the gut homing integrin alphaE and MMCP-1 [64], essential for the weep aspect of T. spiralis expulsion20, 21. It is therefore surprising that both mastocytosis and release of MMCP-1 appeared normal in Itgb8 (CD11c-Cre) mice. This may reflect alternative cell-specific mechanisms for the activation of TGFβ, with the active cytokine signalling within the local cellular environment, such as the T cell synapse via DC expressed αvβ8. This hypothesised high level of control is perhaps unsurprising given the multiple pathways that TGFβ drives. Indeed, previous studies have demonstrated that epithelial expression of the TGFβ –activating integrin αvβ6 is essential for mast cell hyperplasia and MMCP-1 release during small intestinal helminth infection [65]. Moreover, epithelial cell specific αvβ6 null mice demonstrated abnormal mastocytosis and MMCP-1 expression [66] linked with reduced expression of the intestinal homing integrin alphaE [67]. Collectively, this supports the context specific integrin activation of TGFβ, allowing distinct and tight control of this pleiotropic cytokine.
Finally, after we observed rIL-17 treatment was able to rescue weight loss and expulsion kinetics in T. spiralis infected Itgb8 (CD11c-Cre) mice, we investigated the possibility for IL-17 driving parasite expulsion. Indeed, late acting Th17 cells would prove beneficial in aspects of immunity and repair to helminth infection, with IL-17 driving Paneth cell antimicrobial peptide production [68] and IgA secretion [69]. This may be another important role of Th17 induction during T. spiralis infection, as microbial dysbiosis is a hallmark of intestinal helminth infection [57] and the microbiota also plays important roles in Th17 cell induction [39]. Although the data presented here was gained from co-housed littermate controls, it is interesting to speculate on how the microbiome may alter intestinal contraction via the induction of Th17 cells. Alternatively, IL-17 can have direct effects on nematode behaviour [70] and epithelial permeability; TGFβ activation by αvβ8 integrin has been shown to be important for increased alveolar permeability in acute respiratory distress syndrome [71]. Although we saw no changes at the microscopic level in infected Itgb8 (CD11c-Cre) mice, including goblet cells and RELMβ expression, Th17 production of IL-22 is related to goblet cell hyperplasia and enhanced worm expulsion [72]. Taking these potential mechanisms into account, and given the minimal effect of extra-intestinal larvae on muscle function at this timepoint [73], we examined the possibility of alterations in jejunal contractility as a possible role for the delayed expulsion, concentrating on a possible role for IL-17 as an expulsion mechanism.
Gut contraction during T. spiralis infection has previously been shown to be driven by Th2 cytokines and TGFβ, acting via STAT6 and COX-2 respectively [24,46]. Although we saw no changes in Th2 responses in our model, the reduced gut levels of active TGFβ seen in infected Itgb8 (CD11c-Cre) mice, could be involved directly in the reduced contraction seen. However, we observed a significant effect of rIL-17 on baseline gut contraction, reinforcing data from other investigators [74], that was independent of COX-2, as well as a complete rescue during infection by the addition of rIL-17, but not Tregs; making it unlikely that TGFβ was directly responsible for contractility differences. Previous studies have demonstrated that Rho kinase signalling is emerging as an important mediator of intestinal smooth muscle contraction [47], and may play a role during pathophysiology [75]. Moreover, there is precedent within the mucosal barrier of the lung, for αvβ8 dependent Th17 induction driving smooth muscle contraction via NF-κβ and the ROCK2 signalling cascade, with Itgb8 (CD11c-Cre) mice protected from airway hyper-responsiveness in response to house dust mite and ovalbumin sensitization and challenge [76]. Indeed, inhibiting the ROCK pathway, rather than STAT6, prevented hypercontractility of small intestinal muscle in response to IL-17 indicating a potential similar mechanism ex vivo. However, it remains likely that Th2 cytokines and IL-17 may interact during the intestinal hypercontractility response to T. spiralis infection in vivo, with IL-17 previously shown to enhance IL-13 driven STAT6 intracellular responses in mouse and human lung epithelial cells [77].
Collectively, these data support a novel role for IL-17 in driving the intestinal contraction and augmenting the expulsion of T. spiralis. The inhibition of IL-17 during T. spiralis infection in wild-type mice further supports a key role for this cytokine in infection induced hypercontractility, but it must be noted that worm expulsion was unaltered when compared to vehicle treated animals. These data, when coupled with the complete rescue of weight, contractility and worm expulsion seen in IL-17 treated Itgb8 (CD11c-Cre) mice, suggests an additional facet, possibly reduced intestinal Tregs, that further promotes the key role of IL-17 within the Itgb8 (CD11c-Cre) model. An important question remains as to what regulates the strong Th2 response seen during T. spiralis infection. Although we did see some increased morbidity in terms of weight loss during the infection of Itgb8 (CD11c-Cre) mice, our adoptive transfer experiments suggest this is most likely due to the decreased Treg population and possibly the increased worm burden phenotype seen. As discussed earlier, activation of TGFβ via other mechanisms in a cell specific context may be responsible, or it may be a combination of several factors; as seen by the dual roles of IL-10 and TGFβ seen in T. spiralis nurse cell immunopathology [28]. Indeed IL-10 has previously been shown to be essential in avoiding fatal immunopathology in response to the microbiota during another epithelial dwelling helminth, Trichuris muris [78]. Tregs are likely to play a role, and are often associated with helminth infection, but we are reliant on more subtle approaches to remove distinct Treg subsets, as our results confirm global depletion as being detrimental to mouse survival by failing to regulate the majority of inflammatory pathways [45].
In summary, we have highlighted an important cellular and molecular pathway by which the DC expressed TGFβ-activating integrin αvβ8, maintains intestinal Tregs and drives the induction of Th17 cells late during infection with the small intestinal helminth T. spiralis. Tregs are essential for mediating infection induced weight loss, while the resulting Th17 produced IL-17 mediates the contraction of jejunal muscle via ROCK signalling aiding the “weep and sweep” mechanism of helminth expulsion. Thus, we have identified the molecular mechanism maintaining Tregs and driving Th17 induction and helminth expulsion, beyond the classical Th2 responses. Additionally, whether the Th17 pathway can be harnessed therapeutically in other parasitic diseases or pathologies encompassing muscle hypercontractility should be a focus of further studies.
Materials and methods
Animals
C57BL/6 mice were purchased from Harlan Laboratories. Mice lacking integrin αvβ8 on DCs via expression of a conditional floxed allele of β8 integrin in combination with CD11c-Cre (Itgb8 (CD11c-Cre) mice) [30] and DEREG mice [32], all on a C57BL/6 background, have been previously described and were bred in house. For Itgb8 (CD11c-Cre) mice transgene negative littermate controls were used in all experiments. For DEREG mice transgene positive littermates were treated with PBS for controls. All experiments were on male, age-matched mice maintained in specific pathogen-free conditions at the University of Manchester and used at 6 to 12 weeks of age.
Ethics statement
All animal experiments were performed under the regulations of the Home Office Scientific Procedures Act (1986), specifically under the project licence PPL 40/3633. The project licence was approved by both the Home Office and the local ethics committee of the University of Manchester. Animal euthanasia occurred using approved schedule 1 methods.
Trichinella spiralis infection
The maintenance, infection and recovery of T. spiralis were carried out as previously described [79]. Mice were orally infected with 300 larvae and individually weighed on a daily basis. Worm burdens were assessed by counting the number of worms present in the small intestine as described previously [79].
Treg and IL-17 depletion and treatment
Foxp3+ Tregs were depleted in DEREG mice as described [32], via i.p. injection of 200 ng diphtheria toxin (Merck) every 2 days from 2 days prior to infection. IL-17 was blocked via i.p. injection of 100μgs of anti-IL-17α (17F3) or IgG1 isotype control (MOPC-21) (BioXCell) from day 7 p.i. and every 3 days following. For Treg treatment, cells were isolated via Treg isolation kit (Miltenyi) according to manufacturer’s instructions. Cells were assessed as >95% Foxp3+ and mice were adoptively transferred with 1x106 Tregs prior to infection. For IL-17 treatment, 2ug of recombinant IL-17 (Peprotech) was injected i.p. every 3 days from day 9 post-infection. In both gain of function treatments control animals received PBS vehicle injections at identical time points.
Flow cytometry staining
Spleens and mesenteric lymph nodes (mLNs) were removed from mice and disaggregated through a 100 μm sieve. Small intestines were excised and lamina propria lymphocytes (SILP) were prepared essentially as described [80] with slight modification in the tissue digestion step (digestion medium used was RPMI with 10% Foetal calf serum, 0.1% w/v collagenase type I and Dispase II (both Invitrogen), and tissue was digested for 30 min at 37°C). Cell suspensions were blocked with anti-FcγR antibody (clone 24G2; eBioscience) before labelling with antibodies specific for CD3 (eBio500A2), CD4 (clone GK1.5; eBioscience), Foxp3 (clone FJK-16s; eBioscience), IL-13 (clone eBiol13A; eBioscience), IFNγ (clone XMG1.2; eBioscience), IL-17(eBio17B7; eBioscience), IL-9 (RM9A4e; Biolegend) or p-Smad 2/3 (Santa Cruz). For intracellular cytokine analysis cells were incubated for 12 hours with 1x Cell stimulation cocktail (plus protein inhibitors) (ebioscience). Cells were then stained with antibodies using the eBioscience Foxp3 permibilization kit according to the manufacturer's instructions. For pSmad2/3 staining, an Alexa Fluor 594-labelled donkey anti-goat secondary antibody was used (Invitrogen). All samples were analysed on a FACS LSRII.
Cell re-stimulation
mLN and SILP cells were prepared as described above before incubating with 50μg/ml T. spiralis antigen for 24 hours in media (RPMI-1640, 10% FCS, 100U/ml Pen/strp, 5%NEAA, L-glutamine and HEPES, 0.05 mM β-mercaptoethanol (SIGMA)). Cell-free supernatants were analysed for cytokine production via cytometric bead array (BD) or paired ELISA antibodies (anti-IFNγ, clone XMG1.2 and R4-6A2; anti-IL-13, clone eBio13A and eBio1316H; anti-IL-4, clone 11B1and BVD6-2462, anti-IL-17 clone eBio17CK15A5 and eBio17B7; (eBioscience)). For TGFβ analysis samples were acid-activated prior to detection on a mouse TGF-beta 1 DuoSet ELISA (R and D Systems).
Histology
Intestinal tissue was fixed in Carnoy’s solution and embedded in wax prior to mast or goblet cell staining via toludine blue or Schiff's reagent, respectively. Following antigen retrieval, RELMβ was labelled via primary antibody 1:400 (Abcam-ab11429) followed by detection with an Elite ABC HRP Kit (Vectastain) according to manufacturer’s instructions. After mounting, positive cells were enumerated in 20 randomly selected villus crypt units (VCU) and results presented as mean number of positive cells/20 VCU (± S.D.). Lengths of villus/crypts were enumerated via image J.
Serum antibody and MMCP-1
Serum was obtained from blood at the time of sacrifice via centrifugation at 15000×g. Parasite specific IgG1 and IgG2a assessed via 5 μg/ml T. spiralis antigen coated ELISA plates in 0.05 M carbonate/bicarbonate buffer, pH 9.6. IgG1 and IgG2a were detected using biotinylated rat-anti mouse antibodies (Pharmingen, UK and Serotec, UK respectively) diluted in PBS-Tween and visulaised using streptavidin peroxidase and ABTS substrate prior to being read 405nm on a VersaMax microplate reader (Molecular devices, UK). Mouse mast cell protease-1 assessed via ELISA according to manufacturer’s instructions (Moredun).
Intestinal contraction
Ex vivo intestinal contraction was measured as previously described [81]. Briefly, 3cm isolated jejunal strips were placed in oxygenated (95%O2-5%CO2) Krebs solution and surgical silk was used to hang the tissue longitudinally in an isolated tissue bath (Radnoti). Tissues were equilibrated for 30mins at 37°C under tension (1g), prior to baseline and carbachol (10-6M) response readouts being measured. The maximum force generated by the tissue was assessed (AD Instruments and Labchart Reader 8) and expressed in milligrams after normalising for cross sectional area [81]. In some cases, jejunal tissue was incubated in 10ng/ml rIL-17 for 6 hours in medium (Leibovitz’s L-15, 10% FCS, 100 U/ml Pen/strep, 50mg/ml gentamicin, 5% NEAA, L-glutamine and HEPES, 0.05 mM β-mercaptoethanol),following 2 hour treatment with 10μM celecoxib (COX-2 inhibitor), 100nM AS1517499 (STAT6 inhibitor) or 10uM Y-27632 (ROCK inhibitor) (Sigma) prior to measuring longitudinal muscle tension generated in response to carbachol (10-6M).
In vivo intestinal contraction was assessed via a 12 hour fast prior to gavage of 200μl of 6% carmine red dye (Sigma) in 0.5% methylcellulose 400c.p. (Sigma) before measuring distance of dye front, confirmed via tissue blotting, and gut length precisely 20mins later.
Quantitative polymerase chain reaction
Total RNA was purified from small intestinal isolated jejunal muscle strips using Trizol reagent according to the manufacturer’s instructions (ThermoFischer). RNA was reverse transcribed using oligo(dT) primers and complementary DNA for specific genes detected using a SYBR Green qPCR Kit (Roche). Gene expression was normalized to HPRT levels. IL-17ra Forward-5’ CAAGTTTCACTGGTGCTGCC; IL-17ra Reverse-5’ TAGTCTGCAACTGGCTTGGG; HPRT Forward-5’ GCGTCGTGATTAGCGATGATGAAC; HRPT Reverse-5’ GAGCAAGTCTTTCAGTCCTGTCCA.
Statistics
Results are expressed as mean ± S.D.. Where statistics are quoted, two experimental groups were compared via the Student’s t test for non-parametric data. Three or more groups were compared with ANOVA, with Dunnett’s or Bonferroni’s post-test as indicated. A p value of <0.05 was considered statistically significant. *, P<0.05; **, P<0.01; or ***, P<0.005 for indicated comparisons, error bars represent SD of means.
Supporting information
Wild-type C57BL/6 mice were infected with 300 T. spiralis larvae and examined at the indicated time points. (A) IL-4, 13, 6 and 9 cytokine levels from T. spiralis antigen-stimulated mLN cells across the time-course of intestinal infection, determined via cytometric bead array. (B) Representative flow cytometry plots of percentage IL-9 expression in mLN CD4+ T-cells from uninfected and day 13 post-infected mice. (C) Representative flow cytometry plots and (D) Percentage Foxp3 expression in small intestinal lamina propria CD4+ T-cells from uninfected and day 13 post-infected mice. Data (n = 3–5 mice per group) are from two independent experiments performed. *, P<0.05; **, P<0.01; ***, P<0.005; N.S., not significant via Dunnet’s multiple comparison following ANOVA (A) or student’s t-test (D) for the indicated comparisons between groups.
(TIF)
Wild-type and Itgb8 (CD11c-cre) mice were infected with 300 T. spiralis larvae and examined at the indicated time-points post-infection. (A) IFNγ cytokine levels from T. spiralis antigen-stimulated mLN cells from wild-type and Itgb8 (CD11c-cre) mice, determined via ELISA. (B) Parasite-specific serum IgG1 and IgG2a levels in wild-type and Itgb8 (CD11c-cre) mice at day 18 post-infection. (C) Number of IL-9+ CD4 T-cells in the mLN of wild-type and Itgb8 (CD11c-cre) mice at day 13 p.i., assessed via flow cytometry. (D) Villus/crypt lengths assessed via examination of 20 randomly selected VCU in wild-type and Itgb8 (CD11c-cre) mice following infection, quantified via ImageJ software. Number of (E) goblet and (F) mast cells/20 VCU accessed via periodic acid-Schiff’s and toluidine blue histology staining respectively from wild-type and Itgb8 (CD11c-cre) mice. (G) Serum MMCP-1 levels from wild-type and Itgb8 (CD11c-cre) mice following infection, obtained via ELISA. (H) RELMβ+ cells/20VCU from wild-type and Itgb8 (CD11c-cre) mice assessed via immunohistochemistry. All data (n = 4–10 mice per group) are from two independent experiments performed.*, P<0.05; **, P<0.01; ***, P<0.005; N.S., not significant via Bonferonni’s multiple comparison following ANOVA (A), (D), (E-H) or student’s t-test (B),and (C) for the indicated comparisons between groups.
(TIF)
DEREG mice were treated every 2 days with 200 ng diphtheria toxin or PBS (Control) 2 days prior to infection with 300 T. spiralis larvae and examined at the indicated time-points post-infection. (A) The percentage of Foxp3+ CD4 T-cells in the mLN, as assessed via flow cytometry antibody staining and/or Foxp3-GFP reporter. (B) Parasite-specific serum IgG1 and IgG2a levels in Control and DEREG mice at day 15 post-infection, obtained via ELISA. (C) Serum MMCP-1 levels from Control and DEREG mice following infection, obtained via ELISA. Data (n = 4–9 mice per group) are from two independent experiments performed. Wild-type, Itgb8 (CD11c-cre) and Itgb8 (CD11c-cre) mice were adoptively transferred with 1x106 Tregs were infected with 300 T. spiralis larvae 2 days following cell transfer. Representative flow cytometry plots (D) and (E) percentage Foxp3 expression in small intestinal lamina propria CD4+ T-cells from day 13 post-infection. Data (n = 4 mice per group) are from two independent experiments performed. **, P<0.01; ***, P<0.005; N.S., not significant via Dunnet’s multiple comparison following ANOVA (A) and (E), Bonferonni’s multiple comparison following ANOVA (C) and student’s t-test (B) for indicated comparisons between groups.
(TIF)
C57BL/6 mice were infected with 300 T. spiralis larvae and treated with 100μg of anti-IL-17 or control antibody (Bio-X-Cell) every 3 days from day 7 post-infection. (A) Number of mLN IFNγ and IL-13 positive CD4+ T-cells and (B) representative flow cytometry plots. Data (n = 5 mice per group) are from two independent experiments performed. N.S., not significant via student’s t-test for indicated comparisons between groups.
(TIF)
Wild-type and Itgb8 (CD11c-cre) mice were infected with 300 T. spiralis larvae and treated with PBS or 2ug of recombinant IL-17 every 3 days from day 9 post-infection and examined at the indicated time-points post-infection. (A) Parasite-specific serum IgG1 and IgG2a levels in wild-type and Itgb8 (CD11c-cre) PBS or rIL-17 treated mice at day 18 following infection, obtained via ELISA. (B) Base line jejunal longitudinal muscle tension in naïve wild-type and Itgb8 (CD11c-cre) mice in an isolated tissue bath. Chow was removed 12 hrs prior to sacrifice at day 13 and mice received 200μls carmine red in methylcellulose 20 minutes before sacrifice. (C) Representative macroscopic images of wild-type and Itgb8 (CD11c-cre) naïve mice, arrow indicates front of dye and scale bar = 1 cm. Data (n = 4 mice per group) are from two independent experiments performed. N.S., not significant via Bonferonni’s (A) multiple comparison following ANOVA and student’s t-test (B) for indicated comparisons between groups.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
JJW was supported by a Wellcome Trust Grant; https://wellcome.ac.uk, Award Number: 209087/Z/17/Z. MAT received a Medical Research Grant, https://mrc.ukri.org, Award Number: MR/M00242X/1. The Wellcome Trust Centre for Cell-Matrix Research is supported by Wellcome Trust core funding, https://wellcome.ac.uk, Award Number: 203128/Z/16/Z. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McCarty TR, Turkeltaub JA, Hotez PJ (2014) Global progress towards eliminating gastrointestinal helminth infections. Curr Opin Gastroenterol 30: 18–24. 10.1097/MOG.0000000000000025 [DOI] [PubMed] [Google Scholar]
- 2.Lo NC, Addiss DG, Hotez PJ, King CH, Stothard JR, et al. (2017) A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: the time is now. Lancet Infect Dis 17: e64–e69. 10.1016/S1473-3099(16)30535-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bancroft AJ, Artis D, Donaldson DD, Sypek JP, Grencis RK (2000) Gastrointestinal nematode expulsion in IL-4 knockout mice is IL-13 dependent. European Journal of Immunology 30: 2083–2091. [DOI] [PubMed] [Google Scholar]
- 4.Bancroft AJ, McKenzie AN, Grencis RK (1998) A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol 160: 3453–3461. [PubMed] [Google Scholar]
- 5.Jackson JA, Turner JD, Rentoul L, Faulkner H, Behnke JM, et al. (2004) T helper cell type 2 responsiveness predicts future susceptibility to gastrointestinal nematodes in humans. Journal of Infectious Diseases 190: 1804–1811. 10.1086/425014 [DOI] [PubMed] [Google Scholar]
- 6.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie ANJ (1999) Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. Journal of Experimental Medicine 189: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban JF Jr., Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, et al. (1998) IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8: 255–264. [DOI] [PubMed] [Google Scholar]
- 8.Urban JF, Schopf L, Morris SC, Orekhova T, Madden KB, et al. (2000) Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. Journal of Immunology 164: 2046–2052. [DOI] [PubMed] [Google Scholar]
- 9.Mannon P, Reinisch W (2012) Interleukin 13 and its role in gut defence and inflammation. Gut 61: 1765–1773. 10.1136/gutjnl-2012-303461 [DOI] [PubMed] [Google Scholar]
- 10.Ortega-Pierres G, Vaquero-Vera A, Fonseca-Linan R, Bermudez-Cruz RM, Arguello-Garcia R (2015) Induction of protection in murine experimental models against Trichinella spiralis: an up-to-date review. J Helminthol 89: 526–539. 10.1017/S0022149X15000140 [DOI] [PubMed] [Google Scholar]
- 11.Beaumier CM, Gillespie PM, Hotez PJ, Bottazzi ME (2013) New vaccines for neglected parasitic diseases and dengue. Transl Res 162: 144–155. 10.1016/j.trsl.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 12.Grencis RK, Lee TDG, Wakelin D (1985) Adoptive Transfer of Immunity to Trichinella-Spiralis in Mice—Generation of Effective Cells by Different Life-Cycle Stages. International Journal for Parasitology 15: 195–202. [DOI] [PubMed] [Google Scholar]
- 13.Ramaswamy K, Goodman RE, Bell RG (1994) Cytokine profile of protective anti-Trichinella spiralis CD4+ OX22- and non-protective CD4+ OX22+ thoracic duct cells in rats: secretion of IL-4 alone does not determine protective capacity. Parasite Immunology 16: 435–445. [DOI] [PubMed] [Google Scholar]
- 14.Finkelman FD, SheaDonohue T, Goldhill J, Sullivan CA, Morris SC, et al. (1997) Cytokine regulation of host defense against parasitic gastrointestinal nematodes: Lessons from studies with rodent models. Annual Review of Immunology 15: 505–533. 10.1146/annurev.immunol.15.1.505 [DOI] [PubMed] [Google Scholar]
- 15.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, et al. (2004) Interleukin-4-and interleukin-13-mediated host protection against intestinal nematode parasites. Immunological Reviews 201: 139–155. 10.1111/j.0105-2896.2004.00192.x [DOI] [PubMed] [Google Scholar]
- 16.Grencis RK, Hultner L, Else KJ (1991) Host Protective Immunity to Trichinella-Spiralis in Mice—Activation of Th-Cell Subsets and Lymphokine Secretion in Mice Expressing Different Response Phenotypes. Immunology 74: 329–332. [PMC free article] [PubMed] [Google Scholar]
- 17.Faulkner H, Humphreys N, Renauld JC, VanSnick J, Grencis R (1997) Interleukin-9 is involved in host protective immunity to intestinal nematode infection. European Journal of Immunology 27: 2536–2540. 10.1002/eji.1830271011 [DOI] [PubMed] [Google Scholar]
- 18.Alizadeh H, Murrell KD (1984) The intestinal mast cell response to Trichinella spiralis infection in mast cell-deficient w/wv mice. J Parasitol 70: 767–773. [PubMed] [Google Scholar]
- 19.Grencis RK, Else KJ, Huntley JF, Nishikawa SI (1993) The Invivo Role of Stem-Cell Factor (C-Kit Ligand) on Mastocytosis and Host Protective Immunity to the Intestinal Nematode Trichinella-Spiralis in Mice. Parasite Immunology 15: 55–59. [DOI] [PubMed] [Google Scholar]
- 20.Kamiya M, Oku Y, Itayama H, Ohbayashi M (1985) Prolonged expulsion of adult Trichinella spiralis and eosinophil infiltration in mast cell-deficient W/Wv mice. Journal of Helminthology 59: 233–239. [DOI] [PubMed] [Google Scholar]
- 21.Knight PA, Brown JK, Pemberton AD (2008) Innate immune response mechanisms in the intestinal epithelium: potential roles for mast cells and goblet cells in the expulsion of adult Trichinella spiralis. Parasitology 135: 655–670. 10.1017/S0031182008004319 [DOI] [PubMed] [Google Scholar]
- 22.McDermott JR, Bartram RE, Knight PA, Miller HRP, Garrod DR, et al. (2003) Mast cells disrupt epithelial barrier function during enteric nematode infection. Proceedings of the National Academy of Sciences of the United States of America 100: 7761–7766. 10.1073/pnas.1231488100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan WI, Vallance BA, Blennerhasset PA, Deng Y, Verdu EF, et al. (2001) Critical role for signal transducer and activator of transcription factor 6 in mediating intestinal muscle hypercontractility and worm expulsion in Trichinella spiralis-infected mice. Infection and Immunity 69: 838–844. 10.1128/IAI.69.2.838-844.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiho H, Blennerhassett P, Deng YK, Collins SM (2002) Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. American Journal of Physiology-Gastrointestinal and Liver Physiology 282: G226–G232. 10.1152/ajpgi.2002.282.2.G226 [DOI] [PubMed] [Google Scholar]
- 25.Khan WI, Collins SM (2006) Gut motor function: immunological control in enteric infection and inflammation. Clinical and Experimental Immunology 143: 389–397. 10.1111/j.1365-2249.2005.02979.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray M, Jarrett WFH, Jennings FW (1971) Mast cells and macromolecular leak in intestinal immunological reactions. The influence of sex of rats infected with Nippostrongylus brasiliensis. Immunology 21: 17–31. [PMC free article] [PubMed] [Google Scholar]
- 27.Worthington JJ, Samuelson LC, Grencis RK, McLaughlin JT (2013) Adaptive immunity alters distinct host feeding pathways during nematode induced inflammation, a novel mechanism in parasite expulsion. PLoS Pathog 9: e1003122 10.1371/journal.ppat.1003122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beiting DF, Gagliardo LF, Hesse M, Bliss SK, Meskill D, et al. (2006) Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells and TGF-beta(1). American Journal of Tropical Medicine and Hygiene 75: 323–323. [DOI] [PubMed] [Google Scholar]
- 29.Kelly A, Houston SA, Sherwood E, Casulli J, Travis MA (2017) Regulation of Innate and Adaptive Immunity by TGFbeta. Adv Immunol 134: 137–233. 10.1016/bs.ai.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 30.Travis MA, Reizis B, Melton AC, Masteller E, Tang QZ, et al. (2007) Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449: 361–365. 10.1038/nature06110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worthington JJ, Czajkowska BI, Melton AC, Travis MA (2011) Intestinal dendritic cells specialize to activate transforming growth factor-beta and induce Foxp3+ regulatory T cells via integrin alphavbeta8. Gastroenterology 141: 1802–1812. 10.1053/j.gastro.2011.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, et al. (2007) Selective depletion of Foxp3(+) regulatory T cells induces a scurfy-like disease. Journal of Experimental Medicine 204: 57–63. 10.1084/jem.20061852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettelli E, Carrier YJ, Gao WD, Korn T, Strom TB, et al. (2006) Reciprocal developmental pathways for the generation of pathogenic effector T(H)17 and regulatory T cells. Nature 441: 235–238. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- 34.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B (2006) TGF beta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24: 179–189. 10.1016/j.immuni.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 35.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, et al. (2006) Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441: 231–234. 10.1038/nature04754 [DOI] [PubMed] [Google Scholar]
- 36.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, et al. (2008) IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol 9: 1347–1355. 10.1038/ni.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, et al. (2008) Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol 9: 1341–1346. 10.1038/ni.1659 [DOI] [PubMed] [Google Scholar]
- 38.Chen WJ, Jin WW, Hardegen N, Lei KJ, Li L, et al. (2003) Conversion of peripheral CD4(+)CD25(-) naive T cells to CD4(+)CD25(+) regulatory T cells by TGF-β induction of transcription factor Foxp3. Journal of Experimental Medicine 198: 1875–1886. 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burkett PR, Meyer zu Horste G, Kuchroo VK (2015) Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest 125: 2211–2219. 10.1172/JCI78085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Worthington JJ, Klementowicz JE, Travis MA (2011) TGFβ: a sleeping giant awoken by integrins. Trends in Biochemical Sciences 36: 47–54. 10.1016/j.tibs.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 41.Paidassi H, Acharya M, Zhang A, Mukhopadhyay S, Kwon M, et al. (2011) Preferential expression of integrin alphavbeta8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology 141: 1813–1820. 10.1053/j.gastro.2011.06.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasnain SZ, Gallagher AL, Grencis RK, Thornton DJ (2013) A new role for mucins in immunity: insights from gastrointestinal nematode infection. Int J Biochem Cell Biol 45: 364–374. 10.1016/j.biocel.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 43.Artis D, Mei LW, Keilbaugh SA, He WM, Brenes M, et al. (2004) RELM beta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proceedings of the National Academy of Sciences of the United States of America 101: 13596–13600. 10.1073/pnas.0404034101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lahl K, Sparwasser T (2011) In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. Methods Mol Biol 707: 157–172. 10.1007/978-1-61737-979-6_10 [DOI] [PubMed] [Google Scholar]
- 45.Smith KA, Filbey KJ, Reynolds LA, Hewitson JP, Harcus Y, et al. (2016) Low-level regulatory T-cell activity is essential for functional type-2 effector immunity to expel gastrointestinal helminths. Mucosal Immunol 9: 428–443. 10.1038/mi.2015.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akiho H, Deng MK, Blennerhassett P, Kanbayashi H, Collins SM (2005) Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology 129: 131–141. [DOI] [PubMed] [Google Scholar]
- 47.Murthy KS (2006) Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374. 10.1146/annurev.physiol.68.040504.094707 [DOI] [PubMed] [Google Scholar]
- 48.Goto K, Chiba Y, Matsusue K, Hattori Y, Maitani Y, et al. (2010) The proximal STAT6 and NF-kappaB sites are responsible for IL-13- and TNF-alpha-induced RhoA transcriptions in human bronchial smooth muscle cells. Pharmacol Res 61: 466–472. 10.1016/j.phrs.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorelik L, Fields PE, Flavell RA (2000) Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. Journal of Immunology 165: 4773–4777. [DOI] [PubMed] [Google Scholar]
- 50.Heath VL, Murphy EE, Crain C, Tomlinson MG, O'Garra A (2000) TGF-beta 1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. European Journal of Immunology 30: 2639–2649. [DOI] [PubMed] [Google Scholar]
- 51.Worthington JJ, Klementowicz JE, Rahman S, Czajkowska BI, Smedley C, et al. (2013) Loss of the TGFbeta-activating integrin alphavbeta8 on dendritic cells protects mice from chronic intestinal parasitic infection via control of type 2 immunity. PLoS Pathog 9: e1003675 10.1371/journal.ppat.1003675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Withers DR, Hepworth MR (2017) Group 3 Innate Lymphoid Cells: Communications Hubs of the Intestinal Immune System. Front Immunol 8: 1298 10.3389/fimmu.2017.01298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ilic N, Gruden-Movsesijan A, Cvetkovic J, Tomic S, Vucevic DB, et al. (2018) Trichinella spiralis Excretory-Secretory Products Induce Tolerogenic Properties in Human Dendritic Cells via Toll-Like Receptors 2 and 4. Front Immunol 9: 11 10.3389/fimmu.2018.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, et al. (2007) IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8: 967–974. 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]
- 55.Linehan JL, Dileepan T, Kashem SW, Kaplan DH, Cleary P, et al. (2015) Generation of Th17 cells in response to intranasal infection requires TGF-beta1 from dendritic cells and IL-6 from CD301b+ dendritic cells. Proceedings of the National Academy of Sciences of the United States of America 112: 12782–12787. 10.1073/pnas.1513532112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holm JB, Sorobetea D, Kiilerich P, Ramayo-Caldas Y, Estelle J, et al. (2015) Chronic Trichuris muris Infection Decreases Diversity of the Intestinal Microbiota and Concomitantly Increases the Abundance of Lactobacilli. PLoS One 10: e0125495 10.1371/journal.pone.0125495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houlden A, Hayes KS, Bancroft AJ, Worthington JJ, Wang P, et al. (2015) Chronic Trichuris muris Infection in C57BL/6 Mice Causes Significant Changes in Host Microbiota and Metabolome: Effects Reversed by Pathogen Clearance. PLoS One 10: e0125945 10.1371/journal.pone.0125945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, et al. (2010) Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J Exp Med 207: 2331–2341. 10.1084/jem.20101074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilic N, Worthington JJ, Gruden-Movsesijan A, Travis MA, Sofronic-Milosavljevic L, et al. (2011) Trichinella spiralis antigens prime mixed Th1/Th2 response but do not induce de novo generation of Foxp3+ T cells in vitro. Parasite Immunol 33: 572–582. 10.1111/j.1365-3024.2011.01322.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elliott DE, Metwali A, Leung J, Setiawan T, Blum AM, et al. (2008) Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production. J Immunol 181: 2414–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Worthington JJ, Reimann F, Gribble FM (2017) Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. [DOI] [PubMed] [Google Scholar]
- 62.Worthington JJ, Kelly A, Smedley C, Bauche D, Campbell S, et al. (2015) Integrin alphavbeta8-Mediated TGF-beta Activation by Effector Regulatory T Cells Is Essential for Suppression of T-Cell-Mediated Inflammation. Immunity 42: 903–915. 10.1016/j.immuni.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edwards JP, Thornton AM, Shevach EM (2014) Release of active TGF-beta1 from the latent TGF-beta1/GARP complex on T regulatory cells is mediated by integrin beta8. J Immunol 193: 2843–2849. 10.4049/jimmunol.1401102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright SH, Brown J, Knight PA, Thornton EM, Kilshaw PJ, et al. (2002) Transforming growth factor-beta1 mediates coexpression of the integrin subunit alphaE and the chymase mouse mast cell protease-1 during the early differentiation of bone marrow-derived mucosal mast cell homologues. Clin Exp Allergy 32: 315–324. [DOI] [PubMed] [Google Scholar]
- 65.Knight PA, Wright SH, Brown JK, Huang X, Sheppard D, et al. (2002) Enteric expression of the integrin alpha(v)beta(6) is essential for nematode-induced mucosal mast cell hyperplasia and expression of the granule chymase, mouse mast cell protease-1. Am J Pathol 161: 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Knight PA, Brown JK, Wright SH, Thornton EM, Pate JA, et al. (2007) Aberrant mucosal mast cell protease expression in the enteric epithelium of nematode-infected mice lacking the integrin alpha(v)beta(6), a transforming growth factor-beta(1) activator. American Journal of Pathology 171: 1237–1248. 10.2353/ajpath.2007.061245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown JK, Knight PA, Pemberton AD, Wright SH, Pate JA, et al. (2004) Expression of integrin-alphaE by mucosal mast cells in the intestinal epithelium and its absence in nematode-infected mice lacking the transforming growth factor-beta1-activating integrin alphavbeta6. Am J Pathol 165: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paerewijck O, Maertens B, Dreesen L (2017) Interleukin-17 receptor A (IL-17RA) as a central regulator of the protective immune response against Giardia. Science Reports 7: 8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dann SM, Manthey CF, Le C, Miyamoto Y, Gima L, et al. (2015) IL-17A promotes protective IgA responses and expression of other potential effectors against the lumen-dwelling enteric parasite Giardia. Exp Parasitol 156: 68–78. 10.1016/j.exppara.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen C, Itakura E, Nelson GM, Sheng M, Laurent P, et al. (2017) IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses. Nature 542: 43–48. 10.1038/nature20818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li JT, Melton AC, Su G, Hamm DE, LaFemina M, et al. (2015) Unexpected Role for Adaptive alphabetaTh17 Cells in Acute Respiratory Distress Syndrome. J Immunol 195: 87–95. 10.4049/jimmunol.1500054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turner JE, Stockinger B, Helmby H (2013) IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog 9: e1003698 10.1371/journal.ppat.1003698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park MK, Kang YJ, Jo JO, Baek KW, Yu HS, et al. (2018) Effect of Muscle Strength by Trichinella spiralis Infection during Chronic Phase. Int J Med Sci 15: 802–807. 10.7150/ijms.23497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu Y, Wang W, Tong J, Pan Q, Long Y, et al. (2009) Th17: a new participant in gut dysfunction in mice infected with Trichinella spiralis. Mediators Inflamm 2009: 517052 10.1155/2009/517052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rattan S, Phillips BR, Maxwell PJt (2010) RhoA/Rho-kinase: pathophysiologic and therapeutic implications in gastrointestinal smooth muscle tone and relaxation. Gastroenterology 138: 13-18.e11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, et al. (2012) IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 18: 547–554. 10.1038/nm.2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall SL, Baker T, Lajoie S, Richgels PK, Yang Y, et al. (2017) IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription 6 activation. J Allergy Clin Immunol 139: 462–471.e414. 10.1016/j.jaci.2016.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schopf LR, Hoffmann KF, Cheever AW, Urban JF, Wynn TA (2002) IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. Journal of Immunology 168: 2383–2392. [DOI] [PubMed] [Google Scholar]
- 79.Wakelin D, Lloyd M (1976) Accelerated expulsion of adult Trichinella spiralis in mice given lymphoid cells and serum from infected donors. Parasitology 72: 307–315. [DOI] [PubMed] [Google Scholar]
- 80.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, et al. (2007) Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nature Protocols 2: 2307–2311. 10.1038/nprot.2007.315 [DOI] [PubMed] [Google Scholar]
- 81.Vallance BA, Blennerhassett PA, Collins SM (1997) Increased intestinal muscle contractility and worm expulsion in nematode-infected mice. Am J Physiol 272: G321–327. 10.1152/ajpgi.1997.272.2.G321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wild-type C57BL/6 mice were infected with 300 T. spiralis larvae and examined at the indicated time points. (A) IL-4, 13, 6 and 9 cytokine levels from T. spiralis antigen-stimulated mLN cells across the time-course of intestinal infection, determined via cytometric bead array. (B) Representative flow cytometry plots of percentage IL-9 expression in mLN CD4+ T-cells from uninfected and day 13 post-infected mice. (C) Representative flow cytometry plots and (D) Percentage Foxp3 expression in small intestinal lamina propria CD4+ T-cells from uninfected and day 13 post-infected mice. Data (n = 3–5 mice per group) are from two independent experiments performed. *, P<0.05; **, P<0.01; ***, P<0.005; N.S., not significant via Dunnet’s multiple comparison following ANOVA (A) or student’s t-test (D) for the indicated comparisons between groups.
(TIF)
Wild-type and Itgb8 (CD11c-cre) mice were infected with 300 T. spiralis larvae and examined at the indicated time-points post-infection. (A) IFNγ cytokine levels from T. spiralis antigen-stimulated mLN cells from wild-type and Itgb8 (CD11c-cre) mice, determined via ELISA. (B) Parasite-specific serum IgG1 and IgG2a levels in wild-type and Itgb8 (CD11c-cre) mice at day 18 post-infection. (C) Number of IL-9+ CD4 T-cells in the mLN of wild-type and Itgb8 (CD11c-cre) mice at day 13 p.i., assessed via flow cytometry. (D) Villus/crypt lengths assessed via examination of 20 randomly selected VCU in wild-type and Itgb8 (CD11c-cre) mice following infection, quantified via ImageJ software. Number of (E) goblet and (F) mast cells/20 VCU accessed via periodic acid-Schiff’s and toluidine blue histology staining respectively from wild-type and Itgb8 (CD11c-cre) mice. (G) Serum MMCP-1 levels from wild-type and Itgb8 (CD11c-cre) mice following infection, obtained via ELISA. (H) RELMβ+ cells/20VCU from wild-type and Itgb8 (CD11c-cre) mice assessed via immunohistochemistry. All data (n = 4–10 mice per group) are from two independent experiments performed.*, P<0.05; **, P<0.01; ***, P<0.005; N.S., not significant via Bonferonni’s multiple comparison following ANOVA (A), (D), (E-H) or student’s t-test (B),and (C) for the indicated comparisons between groups.
(TIF)
DEREG mice were treated every 2 days with 200 ng diphtheria toxin or PBS (Control) 2 days prior to infection with 300 T. spiralis larvae and examined at the indicated time-points post-infection. (A) The percentage of Foxp3+ CD4 T-cells in the mLN, as assessed via flow cytometry antibody staining and/or Foxp3-GFP reporter. (B) Parasite-specific serum IgG1 and IgG2a levels in Control and DEREG mice at day 15 post-infection, obtained via ELISA. (C) Serum MMCP-1 levels from Control and DEREG mice following infection, obtained via ELISA. Data (n = 4–9 mice per group) are from two independent experiments performed. Wild-type, Itgb8 (CD11c-cre) and Itgb8 (CD11c-cre) mice were adoptively transferred with 1x106 Tregs were infected with 300 T. spiralis larvae 2 days following cell transfer. Representative flow cytometry plots (D) and (E) percentage Foxp3 expression in small intestinal lamina propria CD4+ T-cells from day 13 post-infection. Data (n = 4 mice per group) are from two independent experiments performed. **, P<0.01; ***, P<0.005; N.S., not significant via Dunnet’s multiple comparison following ANOVA (A) and (E), Bonferonni’s multiple comparison following ANOVA (C) and student’s t-test (B) for indicated comparisons between groups.
(TIF)
C57BL/6 mice were infected with 300 T. spiralis larvae and treated with 100μg of anti-IL-17 or control antibody (Bio-X-Cell) every 3 days from day 7 post-infection. (A) Number of mLN IFNγ and IL-13 positive CD4+ T-cells and (B) representative flow cytometry plots. Data (n = 5 mice per group) are from two independent experiments performed. N.S., not significant via student’s t-test for indicated comparisons between groups.
(TIF)
Wild-type and Itgb8 (CD11c-cre) mice were infected with 300 T. spiralis larvae and treated with PBS or 2ug of recombinant IL-17 every 3 days from day 9 post-infection and examined at the indicated time-points post-infection. (A) Parasite-specific serum IgG1 and IgG2a levels in wild-type and Itgb8 (CD11c-cre) PBS or rIL-17 treated mice at day 18 following infection, obtained via ELISA. (B) Base line jejunal longitudinal muscle tension in naïve wild-type and Itgb8 (CD11c-cre) mice in an isolated tissue bath. Chow was removed 12 hrs prior to sacrifice at day 13 and mice received 200μls carmine red in methylcellulose 20 minutes before sacrifice. (C) Representative macroscopic images of wild-type and Itgb8 (CD11c-cre) naïve mice, arrow indicates front of dye and scale bar = 1 cm. Data (n = 4 mice per group) are from two independent experiments performed. N.S., not significant via Bonferonni’s (A) multiple comparison following ANOVA and student’s t-test (B) for indicated comparisons between groups.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.