Abstract
As of 2017, the Centers for Disease Control and Prevention (CDC) HIV testing guidelines recommend that those at increased risk for HIV are tested two to four times per year. Evidence-based interventions that promote frequent and repeated testing remain sparse. We conducted a systematic review to: (1) identify frequent testing interventions; and (2) determine which were successful in increasing frequent testing rates. We searched PubMed, PsycInfo, Web of Science, Embase, and CINAHL for peer-reviewed articles published between January 1, 2010 and September 30, 2017. Ten studies met inclusion criteria. Operationalization of frequent HIV testing varied widely across studies. Four interventions involved text message reminders for HIV testing, three involved community-based testing, two self-testing, and one rapid testing. Text message reminder interventions were most successful in increasing rates of frequent HIV testing. Future research should standardize frequent testing measurement to allow for more robust comparisons of intervention efficacy.
Keywords: HIV testing, repeat HIV testing, community-based testing, HIV self-testing, SMS reminders
RESUMEN
A partir de 2017, las directrices de los Centros para el Control y Prevención de Enfermedades (CDC, por sus siglas en Ingles) recomiendan que las personas con mayor riesgo para el VIH se realicen la prueba del VIH de dos a cuatro veces al año. Las intervenciones basadas en evidencia que promueven realizarse pruebas frecuentes y repetidas siguen escasas. Realizamos una revisión sistemática para: (1) identificar intervenciones que promuevan pruebas frecuentas, y (2) determinar cuales tuvieron éxito en incrementar las tasas pruebas frecuentes. Se realizaron búsquedas en PubMed, PsycInfo, Web of Science, Embase, y CINAHL para identificar artículos revisados por pares publicados entre el 1 de enero de 2010 y el 30 de septiembre de 2017. Diez estudios cumplieron los criterios de inclusión. La operacionalización de pruebas frecuentes del VIH varió ampliamente entre los estudios. Cuatro de las intervenciones incluyeron el uso de recordatorios por mensajes de texto para las pruebas de VIH, tres involucraron el uso de pruebas de VIH en comunidades, dos incluyeron el uso de la auto-prueba y una intervención incluyó el uso de pruebas rápidas. Las intervenciones usando recordatorios por mensajes de texto fueron más exitosas en incrementar las tasas de realizarse pruebas frecuentes. Las investigaciones futuras deberían de estandarizar la medición de pruebas frecuentes para permitir mejores comparaciones de eficacia de intervenciones.
INTRODUCTION
In 2017, the Centers for Disease Control and Prevention (CDC) released updated HIV testing guidelines for men who have sex with men (MSM). These guidelines included the original 2006 recommendation that all asymptomatic, sexually active MSM should receive an HIV test at least once a year [1]. Although it was determined that research does not yet support recommending testing more than once a year, the 2017 CDC recommendations encourage medical providers to screen patients at increased risk for HIV, such as MSM, two to four times per year [1]. Frequent HIV testing among those at high risk for HIV, including MSM who engage in HIV-related risk behaviors and/or reside in communities with high HIV prevalence [1], is a primary component of the national “Treatment as Prevention” strategy for ending the HIV epidemic [2, 3]. Early HIV detection and treatment initiation can facilitate faster progression to viral suppression that reduces morbidity and mortality among individuals living with HIV [2, 4] and decreases the likelihood of transmission to others [5]. In 2016, males comprised approximately three quarters (76%) of all concurrent HIV and AIDS diagnoses in the U.S., demonstrating that many men are not testing frequently enough for early initiation of HIV treatment [6].
Although policies are in place to support yearly HIV testing among the general population [7], few policies or programs facilitate implementation of the CDC’s recommendations for more frequent HIV testing among those at greatest risk for HIV, such as MSM with behavioral and community risk factors [8–13]. Some interventions have sought to improve general HIV testing rates among MSM [14–19]. According to a recent review, leveraging social networks, particularly in regions of the world where homosexuality is highly stigmatized, is an effective strategy to increase overall HIV testing rates among MSM [15]. Still, evidence-based interventions that promote frequent and repeat testing more than once a year among individuals at high risk for HIV remain sparse [5].
To address the need for greater knowledge of effective and efficacious interventions to increase rates of frequent, repeat HIV testing to more than once a year (instead of one-off testing), in line with the recently-released CDC guidelines for MSM, the objectives of this systematic review were to: (1) identify interventions that have sought to promote frequent (i.e., more than yearly) HIV testing; and (2) determine which interventions were and were not successful in increasing frequent testing rates. For recent overviews of interventions examining uptake of HIV testing more broadly, see work by Campbell et al. [15], Lorenc et al. [20], and Conserve et al. [21].
METHODS
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22].
Eligibility criteria
Articles evaluating HIV testing interventions were eligible for inclusion in the systematic review if they: a) were in English; b) reported on the outcomes of an intervention; and c) included a measure of frequent HIV testing as a specific outcome. We operationalized “frequent” HIV testing as receiving at least two HIV tests within a year of enrollment in an intervention. To ensure that we captured all relevant studies that have addressed frequent testing, we expanded our definition beyond the CDC recommendation of testing two to four times per year: we also defined it as having received at least one HIV test prior to the intervention (up to a year prior to the study period) and at least one additional test within a year following the intervention. We excluded studies that simply described whether or not participants received a single HIV test post-intervention as these studies did not measure frequent, repeat testing. Because of the limited number of studies reporting on frequent testing outcomes, we included those that described interventions both for MSM and for other populations.
Finally, we limited our search to articles published in peer-reviewed journals between January 1, 2010 and September 30, 2017. On July 13, 2010, the Obama administration released the National HIV/AIDS Strategy for the United States [23]. The first goal of the strategy was to decrease HIV incidence [23], with the initial step being to locate and test individuals whose HIV status remained unknown [24]. Because the release of the National HIV/AIDS Strategy marked a renewed focus on the national HIV epidemic [25] and increased attention to the need for enhanced HIV testing strategies to fully implement CDC testing guidelines [23–25], we limited our search to articles published in and after 2010. An additional search of articles from 1996 (when highly active antiretroviral therapy [HAART] became available [26]) and the end of 2009 supported this time frame; the search did not identify any additional articles that fit our search criteria (described in further detail below).
Articles were excluded based on the following criteria: a) irrelevant to the topic of HIV testing (e.g., focused on tuberculosis; the impact of circumcision on HIV transmission; etc.); b) the study sample was already living with HIV; c) the intervention was limited to addressing prevention of mother-to-child HIV transmission or was implemented solely in antenatal or pediatric settings; d) the study was observational, and did not describe an intervention’s impact on testing frequency; e) systematic reviews, literature reviews, or meta-analyses; f) the article outlined policy guidelines or was a policy statement related to HIV testing; or g) the article reported on acceptability or preliminary efficacy studies.
Search Strategy
The MeSH terms “HIV interventions/diagnosis” and the key word “HIV test” were combined using the Boolean operator and with the following key words: (intervention, evaluation, program, programme, promote, promotion, promoting, promotes) and (repeat, routine, frequent, revisit, re-attend, follow-up, re-test, increase). Key words in parentheses were joined with the operator or. The search strategy was implemented in the databases PubMed, PsycInfo, Web of Science, Embase, and CINAHL using a date range of January 1, 2010 through September 30, 2017. See Table 1 for the PubMed search string.
Table 1.
PubMed Search String
| (HIV infections/diagnosis [MeSH] OR HIV test* [TIAB]) AND (intervention [TIAB] OR evaluation [TIAB] OR program [TIAB] OR programme [TIAB] OR promote [TIAB] OR promotion [TIAB] OR promoting [TIAB] OR promotes [TIAB]) AND (repeat* [TIAB] OR routine* [TIAB] OR frequen* [TIAB] OR revisit* [TIAB] OR re-visit* [TIAB] OR reattend* [TIAB] or re-attend* [TIAB] OR follow-up [TIAB] OR follow up [TIAB] followup [TIAB]OR re-test* [TIAB] OR retest* [TIAB] OR increase* [TIAB]) |
Notes: MeSH = Medical Subject Heading; TIAB = title, abstract key word search
Additionally, to ensure that we were not overlooking relevant studies published prior to 2010, we conducted a supplemental search of systematic reviews and meta-analyses of HIV testing interventions published between January 1, 1996 (when HAART became available [26]) through December 31, 2009 in PubMed. We used the search string, (“hiv”[All Fields] AND testing[All Fields]) AND (Meta-Analysis[ptyp] OR systematic[sb]) NOT guideline[ptyp] AND (“1996/01/01”[PDAT]: “2009/12/31”[PDAT]), which returned 167 results. Two of the study authors (MPW and SS) each independently reviewed the titles, abstracts, and references of half the articles. Through this initial review, we identified 46 articles that were not relevant, 50 that reviewed studies of people already living with HIV, 13 regarding studies of maternal-to-child HIV transmission, 38 describing studies regarding HIV in general but not HIV testing (e.g., prevalence monitoring; guidelines for conducting meta-analyses on HIV prevention; partner notification studies, etc.), two books, and one duplicate reference. We identified 17 reviews of HIV testing intervention studies and then reviewed the full text of each of those articles. Of those, none ultimately reported on interventions that included frequent HIV testing outcomes. Reviews included, for example, studies on the impact of HIV testing to reduce sexual risk behavior, best practices for HIV testing among people with mental health problems, etc. Given that our supplemental review did not identify any additional studies that reported frequent, repeated testing outcomes, we retained our original review time frame of January 1, 2010 through September 30, 2017.
Study Selection
The search results were uploaded into Endnote and de-duplicated. Subsequently, four reviewers (MPW, AG, AR, and SS) each independently read the titles of a quarter of the identified articles and removed items that were not relevant to the aims of the review. Next, the four reviewers each independently read a quarter of the remaining article abstracts and selected all articles that, based on the abstract review, appeared to fit the eligibility criteria specified above. All four reviewers then read the full text of all articles potentially eligible for inclusion in the review and removed those that did not meet the specific eligibility criteria.
Data Extraction
Data were extracted for the year of publication, country in which the study took place, study population(s) (e.g., MSM, sexual health clinic patients, etc.), sample size, study design, method of intervention delivery (e.g., short message service [SMS; i.e., text message reminders], HIV self-testing, etc.), operationalization of frequent HIV testing, whether rates of frequent HIV testing increased post-intervention, and areas of potential bias and confounding.
Methodological Quality Assessment of Included Studies
To assess methodological quality of the reviewed studies, we used the Quality Assessment Tool for Quantitative studies from the Effective Public Health Practice Project [27, 28]. This tool has been used widely in literature reviews assessing randomized clinical trials on HIV research [29, 30], and is recommended by the Cochrane Health Promotion and Public Health Field [31, 32]. MPW, AR, and SS each independently conducted the quality assessment for all 10 studies, reviewed any discrepancies in ratings, and discussed the ratings until agreement was reached. Studies were rated on the following components: selection bias, study design, confounding, blinding, data collection, withdrawals and drop-outs. Each study was then assigned an overall rating of “strong”, “moderate”, or “weak” depending on quality scores for each of the individual components. A study received a “strong” overall rating when none of the individual components had been rated as “weak”. To receive a “moderate” overall rating, a study could receive no more than one weak rating on any of the individual components. A “weak” overall rating was given for studies that had at least two weak ratings for individual components. A summary of the quality assessment ratings for each of the 10 articles is included in Table 2.
Table 2.
Quality assessment of 10 Studies of Interventions to Promote Frequent HIV Testing
| Primary Author | Publication Year | Selection Bias | Study Design | Confounders | Blinding | Data Collection Method | Withdrawals and Drop-Outs | Overall Quality Rating |
|---|---|---|---|---|---|---|---|---|
| Anand | 2017 | 3 | 2 (Cohort) | 3 | 3 | 1 | 3 | 3 |
| Bourne | 2011 | 2 | 2 (Cohort Analytic) | 1 | 2 | 1 | NA | 1 |
| Brunie | 2016 | 2 | 1 (RCT) | 1 | 2 | 1 | 2 | 1 |
| Burton | 2013 | 2 | 2 (Cohort Analytic) | 1 | 3 | 1 | NA | 2 |
| Jamil | 2017 | 2 | 1 (RCT) | 1 | 2 | 1 | 1 | 1 |
| Kawichai | 2012 | 2 | 1 (RCT) | 1 | 3 | 1 | NA | 2 |
| Mugo | 2016 | 2 | 1 (RCT) | 1 | 3 | 1 | 1 | 2 |
| Nyatsanza | 2016 | 2 | 2 (Cohort Analytic) | 3 | 3 | 1 | NA | 3 |
| Read | 2013 | 2 | 1 (RCT) | 1 | 3 | 1 | 1 | 2 |
| Sweat | 2011 | 2 | 1 (RCT) | 3 | 3 | 1 | NA | 3 |
Notes: RCT = randomized controlled trial, NA = not applicable.
Key: 1 = Strong, 2 = Moderate, 3 = Weak, NA = Not relevant to the study.
RESULTS
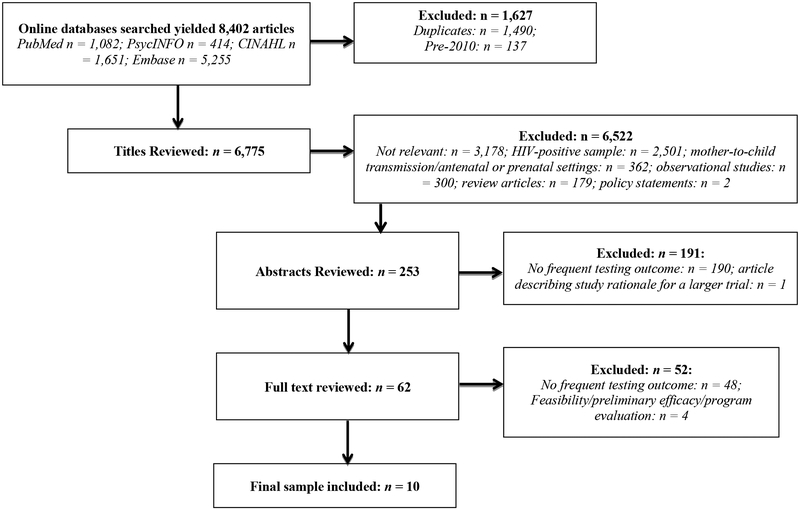
We identified 8,402 articles using the specified search criteria (Embase: n = 5,255; CINAHL: n = 1,651; PubMed: n = 1,082; PsycINFO: n = 414). 1,490 citations were duplicates and were removed. Also excluded were 137 articles that were published before 2010 but ended up in the initial search despite having set the publication date criteria to 2010 and later. The titles of 6,775 remaining citations were reviewed and 6,522 were excluded for the following reasons: not relevant: n = 3,178; HIV-positive sample: n = 2,501; mother-to-child transmission/antenatal or prenatal settings: n = 362; observational studies: n = 300; review articles: n = 179; policy statements: n = 2. Of the 253 abstracts reviewed, 190 were excluded because the study did not report on a frequent testing outcome. One additional article was excluded because it simply reported on the rationale for a larger study. We identified 62 articles as potentially appropriate, and reviewed the full text of each article. Of those 62, 48 were subsequently excluded because the study did not include a frequent testing outcome; four were excluded due to reporting on feasibility or preliminary efficacy studies or program evaluations.
Of the preliminary efficacy studies, one involved a sample of 130 young MSM who participated in an online intervention promoting HIV testing; however, whether or not the study measured frequent HIV testing was unclear. Participants were asked at baseline to report their most recent HIV test and HIV testing outcome was measured as receipt of an additional HIV test within 30 days of study baseline. The amount of time between the “most recent HIV test” reported as baseline and the subsequent HIV test was not reported [33]. The final sample included 10 studies (see Figure 1).
Figure 1.
Systematic Search Procedure and Results
Table 3 provides details regarding the 10 studies included in the review. Three took place in Australia [34–36], two in the United Kingdom [37, 38], two in Thailand [39, 40], and the remaining three in Kenya [41], Uganda [42], and a combination of Tanzania, Zimbabwe, and Thailand [43]. Of the five studies where the intervention significantly increased frequent HIV testing rates, three were in resource-rich countries [34, 35, 38] and two were in resource-limited countries [41, 42]. Five of the 10 studies either focused exclusively on MSM [34–36] or included MSM in their broader samples [38, 39]. Only one study examined outcome moderators (differences in frequent testing rates between recent and non-recent testers) and did not observe any differences between the two groups [35].
Table 3.
Characteristics of 10 Studies of Interventions to Promote Frequent HIV Testing
| Primary Author (Year) | Study Location | Study Population (Sample Size) | Intervention | Frequent Testing Indicator | Frequent/Repeat Testing Results | Significant Difference in Frequent Testing Rates Attributable to Intervention? |
|---|---|---|---|---|---|---|
| Anand (2017) | Thailand | MSM and transgender women (n = 186) | Arm 1: Electronic health record prompts plus clinic-based HTC (standard HTC); Arm 2: Online pre-test counseling plus clinic- based testing (Hybrid HTC); Arm 3: Online HTC, online supervised HIV self-testing, online post-test counseling (eHTC) | Scheduled follow-up HIV tests via electronic health record system | Revisited electronic health records to schedule next clinic-based HIV test: Arm 1: 51.2%; Arm 2:
66.7%; Arm 3: 0% (p < .001) Arm 3 (eHTC) participants had lower odds of revisiting (Odds Ratio 0.14, 95% CI: 0.03–0.67, p = 0.01) vs. Arm 1 (standard HTC) and Arm 2 (hybrid HTC) (Odds Ratio 0.10, 95% CI: 0.02–0.44, p = 0.003). |
Yes (lower HIV testing rates in the self-testing group) |
| Bourne (2011) | Australia | MSM (n =3551) | Text message reminder for HIV testing vs. those tested during the same timeframe and did not receive a reminder vs. those tested during the pre-text message time period | First HIV/STI test in study period and the subsequent test within 9 months of the first (only included HIV negative men). | Greater odds of HIV/STI re-testing among the text message vs. the comparison group (aOR: 4.4, 95% CI: 3.5–5.5) and vs. the pre-text message group (aOR: 3.1, 95% CI: 2.5–3.8) | Yes |
| Brunie (2016) | Uganda | Family planning clinic clients (n = 137 in the intervention group; 119 in the controlgroup) | HTC integrated into existing family planning clinics vs. family planning clinics without HTC | Number of tests in the past 12 months | 57.4% in clinics with integrated HTC (intervention) tested at least 3 times in a year vs. 38.7% in clinics without HTC (control); (p = 0.043) | Yes |
| Burton (2014) | United Kingdom (England) | Sexual health clinic participants at higher risk for HIV/STIs (n = 539) | Text message reminders vs. no text message reminders | Re-attendance for subsequent testing within 4 months of the initial visit | Intervention: 33% re-attended (95% CI: 28–39); Control: 35% re-attended (95% CI: 29–40) (p = 0.78) | No |
| Jamil (2017) | Australia | Gay and bisexual men (n = 362) | Free HIV self-testing plus clinic- based testing vs. standard clinic- based testingj | The mean number of HIV tests within the past year, including facility-based and selftests; recent testers: < 2 years; non-recent testers: > 2 years | Self-testing group: Overall 4.0 tests per year (95% CI: 3.7–4.3) vs. standard care group:
1.9 tests per year (95% CI: 1.7–2.2). (RR: 2.08, 95% CI: 1.82–2.38, p <
.0001). Recent testers (RR 199, 95% CI: 1.73–2.29; p < .0001); non-recent testers (RR 3.95, 95% CI: 2.30–6.78; p < .0001). |
Yes |
| Kawichai (2012) | Thailand | General population (intervention n = 44,477; participated in VCT n = 17.785) | Mobile VCT and “edutainment” | Number of participants who repeated testing after previously testing with the project (% of all VCT clients) | Proportion of those who received more than one test rose over time (Round 1: n = 0 repeat testers; Round 2: n = 37 (4.0% of VCT clients); Round 3: n = 56 (7.2%); Round 4: n = 116 (8.1%); Round 5: n = 161 (9.4%); Round 6: n = 461 (11.9); Round 7: n = 1611 (20.2%) | Did not report on whether or not changes in proportion of repeat testers from round to round were statistically significant |
| Mugo (2016) | Kenya | Young adults (n = 410) | Text message Reminders vs. standard appointment card vs. appointment card plus text message plus in-person reminder for those without a phone (enhanced reminder) | Proportion of participants coming to a follow-up visit for repeat testing within two weeks of the scheduled appointment | Intention-to-treat analysis: 41% attendance for control group vs. 59% enhanced group, RR: 1.4 (95% CI: 1.2–1.7). As-treated analysis: 42% control group vs. 58% intervention group, RR: 1.4 (95% CI: 1.1–1.7). | Yes |
| Nyatsanza (2016) | United Kingdom (England) | Patients at risk for STIs based on the following criteria: presented to clinic with acute STI or for emergency contraception, or commercial sex workers (CSWs), MSM (n = 539) | Personalized text message reminders (i.e., name and clinic contact information) vs. standard text message reminder | Re-attendance at the clinic for routine STI/HIV testing: if subsequent visit was within 4 months of “the end of a previous clinical episode” | Total re-attendance: 56% (95% CI: 50–62%) intervention; 33% control (95% CI: 28–39%) (p = 0.0001) | Yes |
| Read (2013) | Australia | MSM (n = 400) | HIV rapid testing via finger prick vs. standard testing via venipuncture | Frequency (“incidence rate”) of HIV testing over 18 months, measured as number of tests per person year, not including tests done at enrollment. | Mean of 1.63 tests per year in the rapid test arm vs. mean of 1.42 tests per year in standard
test arm (incidence RR: 1.15, 95% CI: 0.06–1.38, p = 0.12). In a post hoc test analysis, included only first HIV test post-enrollment and observed 1.32 tests per year in rapid test arm vs. 1.01 per year in standard test arm (Incidence RR: 1.32, 95% CI: 1.05–1.65, p = 0.02). After excluding first HIV test post-enrollment, observed 1.86 tests per year in rapid test arm vs. 1.83 tests per year in standard arm (RR: 1.01, 95% CI: 0.86–1.20, p = 0.90). Did not observe sustained increases over time. |
No |
| Sweat (2011) | Tanzania, Zimbabwe, and Thailand | Community samples (Tanzania n = 2920; Zimbabwe n = 6039; Thailand n = 12082) | Community-based VCT vs. standard clinic-based VCT | More than one test by Project Accept at community-based VCT and venues within data collection period (2006–2009). | Within the intervention group in Tanzania, in the first year, repeat testing approached 35%,
fluctuated from 15–20% over time. Within the intervention group in Thailand & Zimbabwe - consistent increase in repeat testing, reaching 28% of those tested for HIV by end of intervention. By the end of the intervention, about 40% of those getting tested at community-based VCT sites were repeat HIV testers. |
No |
Notes: MSM = men who have sex with men, HTC = HIV testing and counseling, aOR = Adjusted Odds Ratio, CI = Confidence Interval, VCT = Voluntary counseling and testing, RR = Risk Ratio
Quality Assessment
Overall, three studies received a “weak” rating [38, 39, 43], four were assigned a “moderate” rating [36, 37, 40, 41], and three were rated as “strong” [34, 35, 42]. In terms of selection bias, only one study received a “weak” rating [39], while the rest were rated as “moderate” [34–38, 40–43]. Most of these studies were rated this way because study samples were largely enrolled from within established health clinics rather than being representative samples. Most of the studies were randomized controlled trials (RCTs) [35, 36, 40–43] and thus were rated as “strong” for study design. Of the four rated as “moderate”, one was a cohort study [39] and the other three had a cohort analytic design [34, 37, 38]. Seven of the ten studies were rated as a “strong” for control of confounders [34–37, 40–42] and the remaining three as “weak” [38, 39, 43]. Most studies received a “weak” rating for blinding, with both assessors and participants being aware of the study group assignment [36–41, 43], while three were assigned a “moderate” rating [34, 35, 42]. All studies were rated as “strong” for data collection methods given that HIV testing rates were generally collected from electronic health records and not based on self-report [34–43]. Finally, withdrawals and drop-outs were not relevant for half of the studies given that they did not include follow-up assessments [34, 37, 38, 40, 43]. Of the other five studies, one was rated as weak with less than 60% of participants following up [39], one as moderare [42], and the other three as strong with over 80% completing the study [35, 36, 41].
Measurement of “Frequent” HIV Testing
Despite our broadened definition of “frequent” HIV testing, all studies included in the review reported on frequent testing results that fit within the CDC recommendation that those with higher HIV risk test two to four times per year. We observed that operationalization of HIV testing frequency varied widely from study to study, thus limiting our ability to compare intervention impact across studies. For example, although five studies defined HIV testing frequency in terms of whether patients returned to the study clinic for subsequent HIV testing post-intervention [37, 38, 40, 41, 43], they differed in their measurement of “returned to the clinic.” Some measured the timeframe between clinic visits [37, 38], one assessed the proportion of patients attending a follow-up visit for repeat testing within two weeks of their scheduled appointment [41], while still others determined the proportion of participants who had more than one test at study venues within the full study period [40, 43]. Another study assessed time frame between testing events in terms of whether participants had an additional HIV test within nine months of the first [34], without specifically asking where participants obtained the test. Three other studies assessed testing frequency in terms of the number of HIV tests received either in the past year [35, 42] or the past 18 months [36]. Finally, one study defined repeat HIV testing as whether participants scheduled a follow-up HIV test in the electronic health record system that was part of the study [39].
Intervention Methods
Four of the reviewed studies reported on interventions involving text message reminders for participants to get tested for HIV [34, 37, 38, 41]. Additional interventions included self-testing [35, 39]; rapid testing [36]; and community-based testing [40, 42, 43] (see Table 3).
Text Message Reminders for HIV Testing
Of the studies that employed text message reminder interventions, three resulted in significantly increased rates of frequent HIV testing [34, 38, 41]. Of those, two involved a cohort analytic design [34, 38], and another involved a RCT [41]. One cohort analytic study compared rates of getting an additional HIV test among MSM tested at a sexual health clinic in 2010 who had received an HIV testing text message reminder vs. rates among MSM who tested in 2008 and had not received a text message reminder [34]. A significantly greater proportion of participants who received the reminder re-tested for HIV within 9 months of the first test compared to those who did not receive the reminder [34]. The second cohort analytic study compared sexual health clinic patients at high risk for HIV (diagnosed with STIs; commercial sex workers; women receiving emergency contraception; MSM) who received a text message reminder tailored with their first name and methods for contacting the clinic, vs. those who received a generic text message with no names or clinic information [38]. A significantly larger proportion of those who received a tailored reminder re-attended the clinic for a subsequent HIV test [38]. In the RCT study, the HIV testing text message and phone call reminders plus an appointment card significantly increased HIV re-testing among 18–29 year-old clinic attendees compared to patients who only received a standard appointment card [41] (See Table 3 for further details).
Finally, a fourth cohort analytic study compared a 2012 patient sample who received text message reminders to a 2011 patient sample from before implementation of the text message program. No significant differences were observed between the two samples (Table 3) [37].
Self-Testing for HIV
Two studies involved HIV self-testing strategies, where participants could test for HIV at home rather than in a clinic-based setting. Both studies observed significant changes in frequent testing following the self-testing intervention [35, 39]; however, the self-testing component positively influenced frequent HIV testing in only one study [35].
The first study involved a RCT for MSM in Australia comparing free self-testing kits (participants were provided with a minimum of four to a maximum of 12 kits) plus clinic-based confirmatory testing and counseling following a positive result vs. standard clinic-based testing. Those in the self-testing group had significantly more tests in a one-year period than those in the standard clinic-based testing group [35].
The second HIV self-testing study involved a three-arm cohort design comparing HIV testing rates among MSM and transgender women in Thailand who received one of three interventions: Arm 1 participants received online counseling to electronically schedule clinic-based HIV testing and counseling (HTC) appointments; Arm 2 received online pretest counseling and online scheduling for clinic-based HIV testing appointments; and Arm 3 received online pre- and post-test counseling plus online supervised self-testing for HIV. Participants were assigned to each arm according to their stated choice; participants in the self-testing arm (Arm 3) were significantly less likely than those in the other two arms to receive a subsequent HIV test [39].
Community-Based HIV Testing
Of the three studies involving community-based interventions to promote HIV testing [40, 42, 43], only one found that the intervention had a significant impact on frequent HIV testing rates [42]. Of those studies that had no significant effect on HIV testing frequency, the first was implemented across 10 communities in Tanzania, eight in Zimbabwe, and 14 in Thailand. Participants were randomly assigned to receive testing in traditional clinic sites or testing in community-based sites (e.g., markets, transportation hubs, tents, caravans, community centers, or temples [44]) in addition to standard clinic-based testing [43]. The second was another RCT implemented in Thailand across 14 communities in six different districts within the northern Chiang Mai province, with seven communities randomly assigned to the intervention and seven to the control condition. Intervention communities received community mobilization and mobile voluntary testing and counseling (MVTC) conducted across seven rounds, followed by implementation of “edutainment” events (i.e., entertainment combined with HIV education and onsite testing). Although the proportion of those receiving more than one HIV test increased over time, no tests of significance were reported [40].
In the community-based intervention study that observed significant improvements in HIV testing frequency post-intervention, patients attending family planning clinics in Uganda were compared in terms of those receiving services in clinics vs. those without integrated, onsite HTC. Patients attending clinics with integrated HTC were significantly more likely to receive three or more tests in a one-year period compared to those in the control group [42].
Rapid HIV Testing
One study described a non-blinded RCT in which MSM attending a public sexual health clinic in Australia were randomly assigned to receive either rapid HIV testing administered via finger stick, or standard HIV testing via intravenous blood draw [36]. Study findings showed no significant differences in frequency of HIV testing between the intervention and the control conditions (measured as the number of tests per person-year over an 18-month follow-up).
DICUSSION
We systematically reviewed interventions that promoted frequent, repeated HIV testing. Regular HIV testing among groups at high risk for HIV is an important strategy in efforts to end the HIV epidemic. Frequent testing can facilitate improved identification of those living with HIV to accelerate ART initiation for better short- and long-term outcomes and to reduce transmission likelihood [2, 4, 5]. Frequent testing among individuals who are seronegative but at high risk for HIV also provides opportunities for linkage to HIV prevention such as pre-exposure prophylaxis (PrEP) initiation [45]. Of the 10 studies included in the review, five documented significant increases in frequency of HIV testing among participants in the intervention vs. control groups [34, 35, 38, 41, 42]. These included three studies involving text message reminders [34, 38, 41]; one that included self-testing components [35]; and one that involved community-based HTC [42], which are discussed in more detail below.
Text Message Reminders
Of the four studies that involved text message reminder interventions [34, 37, 38, 41], three observed statistically significant increases in HIV testing post-intervention [34, 38, 41]. Text message reminder strategies differed across studies, ranging from a template that allowed staff to generate reminders from the electronic medical records [34], to adding text message reminders plus phone calls as a follow-up to handing out standard appointment cards [41], to sending out text messages personalized with patients’ first names and clinic contact information [38].
The three text message-based studies in our review that successfully increased HIV testing frequency [34, 38, 41] demonstrate that text message reminders hold the potential to be a cost-effective and accessible means of improving HIV testing frequency among populations most at risk for HIV acquisition. Although minimal research has examined whether text message reminders impact rates of frequent HIV testing, studies have documented that personalized text message reminders are both an effective and acceptable means of enhancing patient usage of and attendance at a range of other health care services [46–48]. For example, one RCT found that automated, personalized text messages significantly improved patient attendance at follow-up appointments after release from an emergency room [46]. Similar to Nyatsanza and colleague’s aforementioned study [38], the text message reminders to emergency room patients included specific information about appointment date and time, clinic location, and hours of operation [46]. A meta-analysis also observed that text message reminders significantly increased rates of attending scheduled health care appointments, and that such reminders have become a progressively successful strategy to boost appointment attendance in more recent years [47]. Given that about 67% of the world’s population had cell phones in 2017 [49], text message reminders have the potential to reach patients who may be difficult to contact by other means.
The positive findings from the three text message-based studies [34, 38, 41] included in this review should be interpreted with caution. First, in one study, text message reminders only served to boost HIV testing frequency in conjunction with phone calls and/or in-person reminders [41]. In the study that compared personalized to standard text message reminders, texts were not compared to other methods of reminders such as phone calls or appointment cards [38]. For both studies, it is thus unclear whether the significant increases in HIV testing were a result of text message reminders on their own or of other aspects of the intervention in combination with text message reminders. Moreover, in the study that employed text message reminders but did not observe a significant impact of the intervention on HIV testing frequency, groups from two different time periods were compared and participants were deemed high risk for HIV based on non-mutually-exclusive risk categories [37]; therefore, it is unclear whether the text message reminder intervention would have been successful had the intervention and control groups been more comparable.
Furthermore, text message reminder strategies should be implemented with consideration for whether patients have consistent access to private cell phones, and are comfortable with text messaging as a mode of communication in general and with their healthcare provider. Additional factors may include recognizing that patient contact information may be incomplete or inaccurate, that reminders must be appropriately timed (e.g., time of day and proximity to visit date), that patients must be able to read and comprehend text-based reminders, and that patients may be less likely to communicate the need for appointment re-scheduling if reminded via text message vs. by a phone call [50]. Attention should also be given to personalizing text message reminders with more than just the patient’s first name [50] (e.g., with the physician’s name [51] and name of institution), particularly for reaching groups at higher risk for adverse health outcomes such as HIV [50].
More research would also be needed to assess acceptability and feasibility of sending text message reminders specifically related to frequent HIV testing. Confidentiality may be a key concern given that text messages may sometimes be visible even when one’s phone is locked. When reminders are placed via phone call, those making the call do not typically leave messages due to concerns for confidentiality [50]; thus, further research is necessary to determine how to implement specific protections of confidentiality when text message reminder systems are employed to boost frequent HIV testing.
HIV self-testing
Of the studies that involved HIV self-testing components [35, 39], only one found that a self-testing intervention significantly increased HIV testing frequency [35]. HIV self-testing appears to be acceptable among populations at high risk for HIV, including MSM. For example, MSM have cited convenience and privacy as benefits of self-testing [52]. Despite these positive aspects, concerns remain regarding the absence of pre- and post-test counseling, mistakes in administering self-tests, and misreading test results [52, 53]. A recent systematic review found that offering free HIV self-testing kits through online platforms like dating apps can address financial, confidentiality, and transportation barriers to obtaining HIV testing [53]; however, the review also highlighted the importance of incorporating free self-testing kits as one component of broader HIV prevention to temper previously-mentioned self-testing concerns. Self-testing interventions should also facilitate early linkage to and ongoing retention in HIV care by promoting and enabling connections to confirmatory testing and HIV care immediately following a preliminary positive result with a HIV self-test kit [53].
Though seemingly effective in improving frequent HIV testing rates, HIV self-testing may be further limited depending upon local, state, and federal policies. For example, in nations where young people must obtain authorization from parents before they can test for HIV, self-testing may not be feasible [53]. As of 2017, 18 states in the U.S. do not explicitly permit minors to receive an HIV test without parental permission [54]. Furthermore, even where HIV self-tests are permitted for all patient populations, they may not be readily accessible; self-test kits cost an average of $40 in the U.S. and are not widely sold in pharmacies [55]. Knowledge of self-test availability may also vary by level of education [56]. In sum, the evidence to date suggests that HIV self-testing may be an acceptable and feasible strategy for improving frequency of HIV testing, but should be part of larger HIV initiatives that support linkage to care and prevention. This strategy should account for laws and policies that could pose obstacles to successful self-testing.
Community-Based Interventions
Of the three studies that included community-based interventions [40, 42, 43], only one found that the intervention significantly improved frequent HIV testing rates [42]. This study was somewhat different from the other two community-based interventions in that it involved integrating HTC into an existing community-based health clinic setting. Such an intervention may have been more effective in increasing HIV testing rates given that clients likely already had a relationship with the existing clinic. Recent research has also demonstrated that providing testing in general health care settings may reduce stigma associated with HIV testing [15]. To fully understand whether community-based testing interventions would serve to increase rates of frequent HIV testing, more research would be necessary, particularly in high-resource settings.
Limitations
The current study is not without limitations. First, we were limited in our ability to compare study findings given the heterogeneity across definitions of frequent HIV testing. Second, only three of the included studies received a “strong” rating for overall study quality. Four of the 10 studies had cohort or cohort analytic designs and thus lacked strong comparison groups. Third, our review was limited to peer-reviewed literature in English, and therefore could have overlooked unpublished studies or gray literature, and studies written in other languages. Finally, the included studies lack potential generalizability. The interventions that targeted general populations rather than focusing on individuals at high risk for HIV may not be generalizable to populations with elevated HIV risk. Four of the studies occurred in resource-limited countries, including Thailand, Uganda, Kenya, Tanzania, Zimbabwe [39–43], and thus may not be generalizable to high-resource countries such as the United States.
CONCLUSION
In this systematic review, we identified 10 studies that assessed interventions aimed at improving HIV testing frequency, five of which demonstrated statistically significant increases in testing frequency among the intervention groups. Given the wide variability of how frequent or repeat testing was defined across studies, we suggest that international public health bodies such as the World Health Organization adopt frequent testing recommendations in line with those of the CDC. The CDC guidelines could be used to create a standard measure of frequent HIV testing, such as an individual receiving at least two tests in a one-year period. Future research, regardless of location, could thus consistently model frequent testing measurement after unified international HIV testing recommendations. Although gathering consistent, reliable, and comparable data on testing frequency from populations at risk for HIV may be challenging, strategies for improvement could include validating self-report with medical record reviews, especially across sites; asking patients about testing history at more frequent intervals to improve recall; and accessing pharmacy and online vendor records of self-testing.
Of the reviewed studies, we found that text message reminders appeared to be particularly promising for improving frequency of HIV testing. As digital communication becomes increasingly prevalent and face-to-face communication progressively challenging or obsolete, technology-assisted interventions that require less ongoing personnel involvement may be especially timely. Additionally, text message reminders could potentially be supplemented with follow-up phone calls and in-person outreach to ensure that all individuals at risk for HIV receive optimal care and treatment.
To fully understand the impact of text message reminders on HIV testing frequency, future research could examine text message reminders in the context of RCTs with frequent HIV testing as a specific outcome. Such research could also explore the cost-effectiveness of text message reminders (in terms of both staff effort and financial costs) vs. other forms of communication. Additionally, given that the CDC recommendations regarding frequent testing are based on HIV in the U.S., more research is needed in the U.S. given that existing studies of frequent testing interventions have primarily been implemented in other countries. Further research may also be needed to more broadly examine HIV self-testing and community-based testing strategies. Ensuring that all people at risk for HIV receive frequent, routine HIV testing would contribute to addressing the goals of the National U.S. HIV/AIDS Strategy [57] by promoting a more synchronized national approach to ending the HIV/AIDS epidemic.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health, National Institute of Mental Health, R21-MH103032 (Sandfort & Tsoi), T32-MH019139 (Sandfort), and P30-MH43520 (Remien). Dr. Margaret Paschen-Wolff and Dr. Anisha Gandhi were supported by a training grant (T32 MH019139; PI: Theodorus Sandfort, Ph.D.) from the National Institute of Mental Health at the HIV Center for Clinical and Behavioral Studies at the NY State Psychiatric Institute and Columbia University (P30-MH43520; Center Principal Investigator: Robert Remien, Ph.D.). The authors wish to acknowledge and thank Javier López Rios, MPH for the Spanish translation of the abstract.
Footnotes
Conflict of Interest: The authors (Paschen-Wolff, Restar, Gandhi, Serafino, and Sandfort) declare that they have no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.DiNenno EA, Prejean J, Irwin K, et al. Recommendations for HIV screening of gay, bisexual, and other men who have sex with men—United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(31):830–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hull MW, Wu Z, Montaner JS. Optimizing the engagement of care cascade: A critical step to maximize the impact of HIV treatment as prevention. Curr Opin HIV AIDS. 2012;7(6):579–86. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Holmes C, Padian N, et al. HIV treatment as prevention: How scientific discovery occurred and translated rapidly into policy for the global response. Health Aff. 2012;31(7):1439–49. [DOI] [PubMed] [Google Scholar]
- 5.Lucas A, Armbruster B. The cost-effectiveness of expanded HIV screening in the United States. AIDS. 2013;27(5):795–801. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. HIV Surveillance Report, 2016. Vol. 28; 2017. Available from: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hivsurveillance-report-2016-vol-28.pdf.
- 7.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Morb Mortal Wkly Rep. 2006;55(14):1–17. [PubMed] [Google Scholar]
- 8.Burns F, Hart G. Increased HIV testing in men who have sex with men. Br Med J. 2012;344(e501):1–2. [DOI] [PubMed] [Google Scholar]
- 9.Knox J, Sandfort T, Yi H, Reddy V, Maimane S. Social vulnerability and HIV testing among South African men who have sex with men. Int J STD AIDS. 2011;22(12):709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips AN, Cambiano V, Nakagawa F, et al. Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: Analysis of an extensively documented epidemic. PLoS One. 2013;8(e55312):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stekler JD, Golden MR. Learning from the missed opportunities for HIV testing. Sex Transm Infect. 2009;85(1):2–3. [DOI] [PubMed] [Google Scholar]
- 12.Williamson LM, Flowers P, Knussen C, Hart GJ. HIV testing trends among gay men in Scotland, UK (1996–2005): Implications for HIV testing policies and prevention. Sex Transm Infect. 2009;85(7):550–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson DP, Regan DG, Heymer K-J, Jin F, Prestage GP, Grulich AE. Serosorting may increase the risk of HIV acquisition among men who have sex with men. Sex Transm Dis. 2010;37(1):13–7. [DOI] [PubMed] [Google Scholar]
- 14.Blas MM. Effect of an online video-based intervention to increase HIV testing in gay-identified and non-gay-identified men who have sex with men in Peru. PLoS One. 2008;5(5):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell CK, Lippman SA, Moss N, Lightfoot M. Strategies to increase HIV testing among MSM: A synthesis of the literature. AIDS Behav. 2018:1–26. [DOI] [PubMed] [Google Scholar]
- 16.Champenois K, Le Gall J-M, Jacquemin C, et al. ANRS–COM’TEST: Description of a community-based HIV testing intervention in non-medical settings for men who have sex with men. BMJ Open. 2012;2(e000693):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenc T, Marrero-Guillamón I, Llewellyn A, et al. HIV testing among men who have sex with men (MSM): Systematic review of qualitative evidence. Health Educ Behav. 2011;26(5):834–46. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes SD, Vissman AT, Stowers J, et al. A CBPR partnership increases HIV testing among men who have sex with men (MSM): Outcome findings from a pilot test of the CyBER/testing internet intervention. Health Educ Behav. 2011;38(3):311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei C, Herrick A, Raymond HF, Anglemyer A, Gerbase A, Noar SM. Social marketing interventions to increase HIV/STI testing uptake among men who have sex with men and male- to- female transgender women. Cochrane Libr. 2011(9):CD009337. [DOI] [PubMed] [Google Scholar]
- 20.Lorenc T, Marrero-Guillamón I, Aggleton P, et al. Promoting the uptake of HIV testing among men who have sex with men: Systematic review of effectiveness and cost-effectiveness. Sex Transm Infect. 2011;87(4):272–8. [DOI] [PubMed] [Google Scholar]
- 21.Conserve DF, Jennings L, Aguiar C, Shin G, Handler L, Maman S. Systematic review of mobile health behavioural interventions to improve uptake of HIV testing for vulnerable and key populations. J Telemed Telecare. 2017;23(2):347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 23.White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States. Washington, DC: The White House; 2010. Available from: https://obamawhitehouse.archives.gov/sites/default/files/uploads/NHAS.pdf. [Google Scholar]
- 24.Yehia B, Frank I. Battling AIDS in America: An evaluation of the National HIV/AIDS Strategy. Am J Public Health. 2011;101(9):e4–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millett GA, Crowley JS, Koh H, et al. A way forward: The National HIV/AIDS Strategy and reducing HIV incidence in the United States. 2010;55:S144–S7. [DOI] [PubMed] [Google Scholar]
- 26.Ammassari A, Murri R, Pezzotti P, et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. 2001;28(5):445–9. [DOI] [PubMed] [Google Scholar]
- 27.Effective Public Health Practice Project. Quality assessment tool for quantitative studies 1998. Available from: https://merst.ca/ephpp/.
- 28.Thomas B, Ciliska D, Dobbins M, Micucci S, Worldviews on Evidence- Based Nursing. A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. 2004;1(3):176–84. [DOI] [PubMed] [Google Scholar]
- 29.Genberg BL, Shangani S, Sabatino K, et al. Improving engagement in the HIV care cascade: A systematic review of interventions involving people living with HIV/AIDS as peers. AIDS Behav. 2016;20(10):2452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shangani S, Escudero D, Kirwa K, Harrison A, Marshall B, Operario D. Effectiveness of peer-led interventions to increase HIV testing among men who have sex with men: A systematic review and meta-analysis. AIDS Care. 2017;29(8):1003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson N, Waters E. Criteria for the systematic review of health promotion and public health interventions. Health Promot Int. 2005;20(4):367–74. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong R, Waters E, Jackson N, et al. Guidelines for systematic reviews of health promotion and public health interventions. Version 2 Australia: Melbourne University; 2007. Available from: http://ph.cochrane.org/sites/ph.cochrane.org/files/public/uploads/Guidelines%20HP_PH%20reviews.pdf. [Google Scholar]
- 33.Bauermeister JA, Pingel ES, Jadwin-Cakmak L, et al. Acceptability and preliminary efficacy of a tailored online HIV/STI testing intervention for young men who have sex with men: the Get Connected! program. AIDS Behav. 2015;19(10):1860–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourne C, Knight V, Guy R, Wand H, Lu H, McNulty A. Short message service reminder intervention doubles sexually transmitted infection/HIV re-testing rates among men who have sex with men. Sex Transm Infect. 2011;87(3):229–31. [DOI] [PubMed] [Google Scholar]
- 35.Jamil MS, Prestage G, Fairley CK, et al. Effect of availability of HIV self-testing on HIV testing frequency in gay and bisexual men at high risk of infection (FORTH): A waiting-list randomised controlled trial. Lancet HIV. 2017;4(6):e241–e50. [DOI] [PubMed] [Google Scholar]
- 36.Read TR, Hocking JS, Bradshaw CS, et al. Provision of rapid HIV tests within a health service and frequency of HIV testing among men who have sex with men: randomised controlled trial. BMJ. 2013;347(f5086):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton J, Brook G, McSorley J, Murphy S. The utility of short message service (SMS) texts to remind patients at higher risk of STIs and HIV to reattend for testing: a controlled before and after study. Sex Transm Infect. 2014;90:11–3. [DOI] [PubMed] [Google Scholar]
- 38.Nyatsanza F, McSorley J, Murphy S, Brook G. ‘It’s all in the message’: The utility of personalised short message service (SMS) texts to remind patients at higher risk of STIs and HIV to reattend for testing—a repeat before and after study. Sex Transm Infect. 2016;92:393–5. [DOI] [PubMed] [Google Scholar]
- 39.Anand T, Nitpolprasert C, Kerr SJ, et al. Implementation of an online HIV prevention and treatment cascade in Thai men who have sex with men and transgender women using Adam’s Love Electronic Health Record system. J Virus Erad. 2017;3(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawichai S, Celentano D, Srithanaviboonchai K, et al. NIMH Project Accept (HPTN 043) HIV/AIDS community mobilization (CM) to promote mobile HIV voluntary counseling and testing (MVCT) in rural communities in Northern Thailand: Modifications by experience. AIDS Behav. 2012;16(5):1227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mugo PM, Wahome EW, Gichuru EN, et al. Effect of text message, phone call, and in-person appointment reminders on uptake of repeat HIV testing among outpatients screened for acute HIV infection in Kenya: A randomized controlled trial. PLoS One. 2016;11(4):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunie A, Wamala-Mucheri P, Akol A, Mercer S, Chen M. Expanding HIV testing and counselling into communities: Feasibility, acceptability, and effects of an integrated family planning/HTC service delivery model by Village Health Teams in Uganda. Health Policy Plan. 2016;31(8):1050–7. [DOI] [PubMed] [Google Scholar]
- 43.Sweat M, Morin S, Celentano D, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): A randomised study. Lancet Infect Dis. 2011;11(7):525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khumalo-Sakutukwa G, Morin SF, Fritz K, et al. Project Accept (HPTN 043): A community-based intervention to reduce HIV incidence in populations at risk for HIV in sub-Saharan Africa and Thailand. J Acquir Immune Defic Syndr. 2008;49(4):422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNairy ML, El-Sadr WM. A paradigm shift: Focus on the HIV prevention continuum. Clin Infect Dis. 2014;59(Suppl 1):S12–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arora S, Burner E, Terp S, et al. Improving attendance at post–emergency department follow- up via automated text message appointment reminders: A randomized controlled trial. Acad Emerg Med. 2015;22(1):31–7. [DOI] [PubMed] [Google Scholar]
- 47.Boksmati N, Butler-Henderson K, Anderson K, Sahama T. The effectiveness of SMS reminders on appointment attendance: A meta-analysis. J Med Syst. 2016;40(4):1–10. [DOI] [PubMed] [Google Scholar]
- 48.Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: A systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14(1):1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawers P 5 billion people now have a mobile phone connection, according to GSMA data. 2017. Available from: https://venturebeat.com/2017/06/13/5-billion-people-now-have-a-mobile-phone-connection-according-to-gsma-data/.
- 50.McLean SM, Booth A, Gee M, et al. Appointment reminder systems are effective but not optimal: Results of a systematic review and evidence synthesis employing realist principles. Patient Prefer Adherence. 2016;10:479–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel S, Hemmige V, Street RL Jr, Viswanath K, Arya M. Activating racial and ethnic minorities to engage in preventive health: Patient preferences for health reminders. J Particip Med. 2017;9:e8. [PMC free article] [PubMed] [Google Scholar]
- 52.Figueroa C, Johnson C, Verster A, Baggaley R. Attitudes and acceptability on HIV self-testing among key populations: A literature review. AIDS Behav. 2015;19(11):1949–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LeGrand S, Muessig KE, Horvath KJ, Rosengren AL, Hightow-Weidman LB. Using technology to support HIV self-testing among MSM. Curr Opin HIV AIDS. 2017;12(5):425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guttmacher Institute. Minors’ access to STI services. 2018. Available from: https://www.guttmacher.org/state-policy/explore/minors-access-sti-services.
- 55.Myers JE, Davis OYE-S, Weinstein ER, et al. Availability, accessibility, and price of rapid HIV self-tests, New York City pharmacies, summer 2013. AIDS Behav. 2017;21(2):515–24. [DOI] [PubMed] [Google Scholar]
- 56.Flowers P, Riddell J, Park C, et al. Preparedness for use of the rapid result HIV self- test by gay men and other men who have sex with men (MSM): A mixed methods exploratory study among MSM and those involved in HIV prevention and care. HIV Med. 2017;18(4):245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.U.S. Department of Health and Human Services. National HIV/AIDS Strategy: Updated to 2020. 2017. Available from: https://www.hiv.gov/federal-response/national-hiv-aids-strategy/nhas-update.